User login
Two conflicting aims collide when choosing initial empiric therapy for patients with a potential life‐threatening infection. On the one hand, the clinical picture and seriousness of the suspected infectionsometimes with a multi‐drug resistant (MDR) pathogenpoint to the need for immediate empiric therapy with a broad‐spectrum regimen covering the most likely pathogens. This getting it right the first time approach1 is clearly a reasonable one given the significant negative impact of inappropriate or inadequate initial therapy on patient outcomes and costs,24 and the apparent inability to remedy the initial error by subsequent antimicrobial regimen adjustment.57 On the other hand, use of a broad‐spectrum regimen increases the risk of emergent antimicrobial‐resistant pathogens, with potential harm for the immediate patient and all subsequent patients who become exposed and infected with the resistant pathogen. Hence, the aim of optimizing initial empiric therapy comes into conflict with an important aim of antimicrobial stewardship, namely, to use antimicrobials in a manner that does not excessively promote development or selection of antimicrobial‐resistant pathogens.
The de‐escalation strategy is an approach that attempts to balance these conflicting aims by providing optimal initial patient management without inordinately promoting development of antimicrobial resistance. As discussed more fully in the corresponding supplement article by Dr Syndman, the first part of this strategy involves collecting cultures from suitable patients prior to initiating broad‐spectrum empiric antimicrobial therapy designed to cover the most likely pathogenic microorganisms, based on local patterns of prevalence and susceptibility, and the presence of risk factors for infection with drug‐resistant species.810 The second critical step involves modification of initial empiric therapy (when warranted) based on clinical status and when culture results are available.810 In this manner, the initial broad‐spectrum regimen can often be streamlined or de‐escalated to a more narrow‐spectrum regimen or, in some cases, terminated when negative cultures suggest no infection. Frequently, initial combination therapy can be replaced by monotherapy targeting the pathogenic organism identified in culture. Sometimes culture results indicate that initial empiric therapy was inappropriate/emnadequate and requires replacement or other modification. Thus, by modifying empiric antimicrobial therapy on the basis of culture results and clinical criteria, the de‐escalation strategy enables more effective targeting of the causative pathogen(s), elimination of redundant therapy, a decrease in antimicrobial pressure for emergence of resistance, and cost savings.10, 11 Decreasing the number of antimicrobial agents and/or the spectrum of coverage is also expected to decrease the risk of adverse events, drugdrug interactions, and Clostridium difficile‐associated disease.12, 13 A number of studies have demonstrated that de‐escalation of initially appropriate therapy can be successfully accomplished with either improved outcomes14, 15 or with comparable effectiveness as continued initial therapy,1618 but with reduced antimicrobial exposure and costs.19
The timing of streamlining or other modification of initial empiric therapy typically occurs when microbiological culture results become available. Assuming blood or other relevant tissue cultures were obtained prior to initiating empiric therapy, this means de‐escalation or other modifications of initial therapy generally occurs 24 days after hospitalization and/or the beginning of empiric therapy. If rapid diagnostic tests are used to identify or rule out particular pathogens, then de‐escalation may occur slightly sooner. In addition to culture results, observation of the patient in the hospital setting and improved clarity as to his or her clinical status also affect the decision about whether and how to modify the initial empiric antimicrobial regimen. The clinical scenario of the patient and his or her response to initial antimicrobial therapy is also typically clearer by day 3 of antibiotic therapy. If, for some reason, cultures were not obtained prior to beginning empiric therapy, then observations of clinical status and consideration of patient risk factors for resistant pathogens become predominant in the decision‐making process. With respect to the timing of culture attainment, this should occur prior to beginning antimicrobial therapy, because therapy may reduce culture yield and result in false negative or other misleading findings.20, 21
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
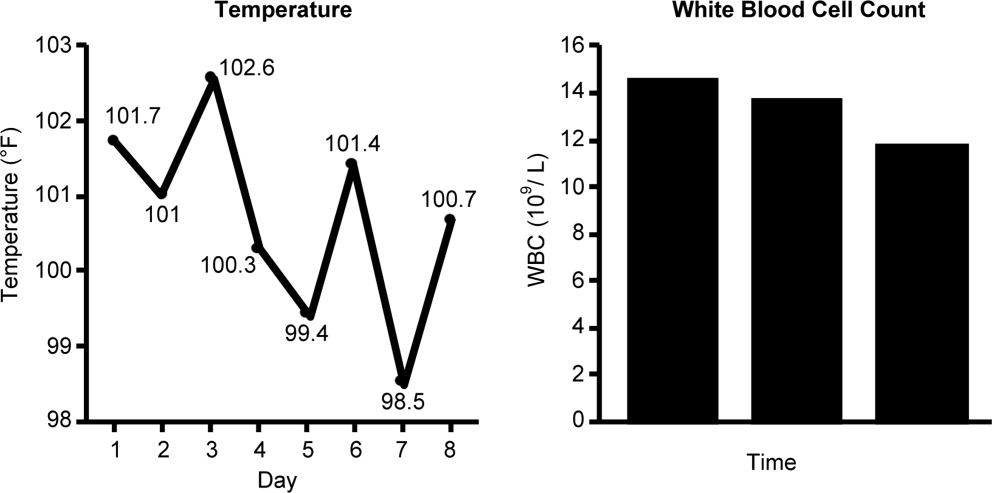
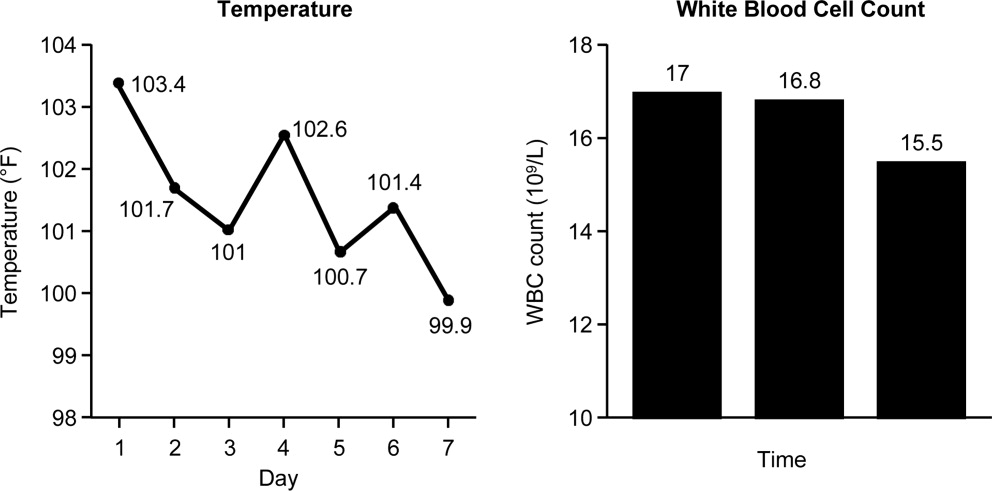
Case 1 is a 72‐year‐old woman admitted with findings consistent with healthcare‐associated pneumonia (HCAP). Empiric therapy was initiated with vancomycin and piperacillin/tazobactam. Figure 1 provides the laboratory (white blood cell [WBC] counts) and body temperature data for the patient since she entered the hospital and began empiric antibiotic therapy 3 days earlier. The WBC counts suggest the patient is responding to the antibiotic regimen, as demonstrated by a progressive reduction over the time period. However, her counts were still elevated above normal at last measurement, suggesting an incompletely resolved infection at this time. In addition, the patient is still coughing, but has less sputum production, and has some energy to get up and move around. Crackles are apparent at the right lung base. The patient's fever curve has trended down, but still shows notable fever spikes, with a temperature maximum of 101.4F for the past 24 hours. Her blood pressure (135/84 mmHg), pulse (74 bpm), and respiratory rate (14 breaths per minute) are normal, with slightly decreased oxygen saturation (94%) on room air, although improved from initial examination 3 days earlier (92%). The blood culture shows no growth; the sputum culture simply shows oropharyngeal flora. In other words, the culture results have not isolated a causative pathogen. In addition to vancomycin and piperacillin/tazobactam, the patient continues to receive her usual medications for a past history of myocardial infarction (low‐dose aspirin, metoprolol) and hypertension (enalapril, furosemide).
HCAP is a common infection often requiring initial empiric therapy with a broad‐spectrum regimen that covers possible involvement of resistant bacteria. As such, HCAP frequently provides excellent opportunities for de‐escalation. Figure 2 presents the general strategy from the 2005 American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) guidelines for the management of HCAP, hospital‐acquired pneumonia (HAP), or ventilator‐associated pneumonia (VAP).22 According to the guidelines, HCAP, HAP, and VAP should be similarly managed. Broad‐spectrum initial empiric antibiotic therapy is recommended for patients with late‐onset disease or those with risk factors for MDR pathogens (including high prevalence of resistance based on local antibiograms), while limited‐spectrum antibiotic therapy is recommended for all other patients. Note that consideration of de‐escalation or streamlining of initial therapy begins 2‐3 days after initiation of therapy. Data that should be reviewed prior to instituting de‐escalation include blood cultures and respiratory cultures, as well as the clinical status of the patient. The adequacy of respiratory samples used for culturing should factor into the decision‐making process. For example, in patients who are not intubated or mechanically ventilated, it can be challenging to obtain a quality respiratory specimen for culture. If clinicians are uncertain as to the quality of the respiratory specimen that was cultured, then de‐escalation decisions should be based more on the clinical status of the patient.
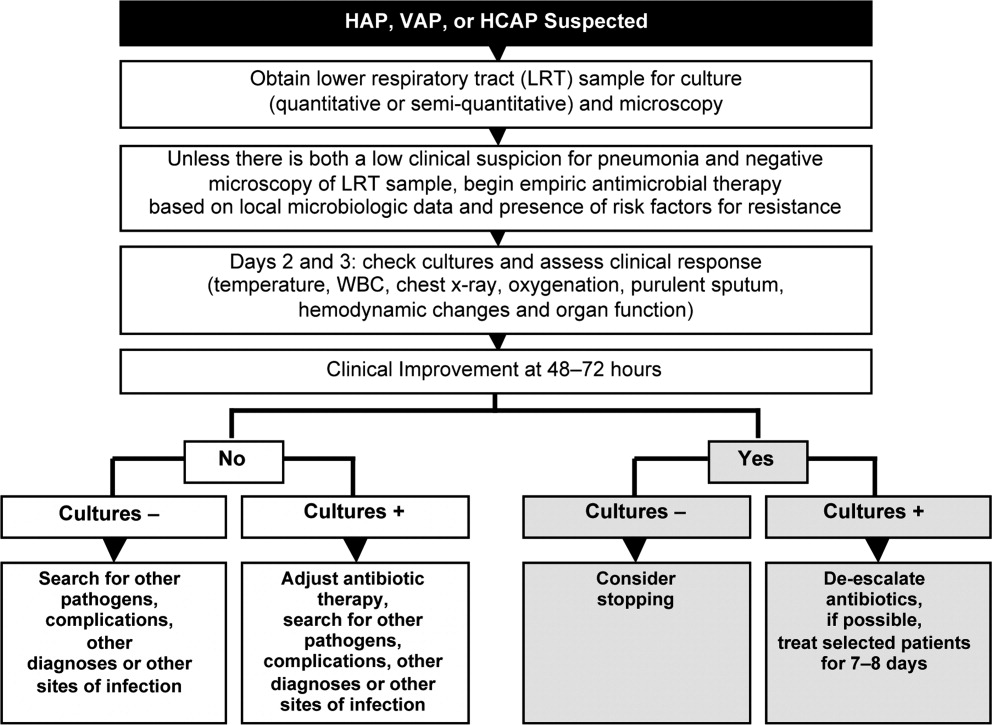
The clinical status of the patient, 2 days after beginning treatment, and culture results are critical in guiding the de‐escalation process.9, 22 The ATS/IDSA guidelines recommend serial assessments of clinical parameters to define the response to initial empiric therapy. If the therapy regimen is effective, an improvement in clinical response should be apparent within 2‐3 days of its initiation.22 Hence, no change in antimicrobial therapy should be undertaken before 3 days, unless there is evidence of rapid deterioration in clinical status or infectious diseases experts recommend a change. With respect to culture results, failure to isolate a group of MDR pathogens for which initial broad‐spectrum empiric therapy was selected affords an opportunity to now streamline therapy or treat with a more narrow‐spectrum regimen.9 Similarly, isolation of a particular pathogen can guide treatment modifications (when necessary), while a negative culture raises the possibility of terminating antimicrobial therapy, provided the culture was collected before initiating therapy. Confidence in this latter decision is bolstered when the patient exhibits rapid improvement in clinical status that is backed by radiographic resolution of lung abnormalities, or an alternative diagnosis has been established for which antimicrobial therapy is not indicated.9
At this stage in the process3 days after initiating empiric therapy, and with culture results in hand and evidence of clinical improvementthe first decision or question is whether antimicrobial therapy can be stopped altogether, ie, do the current data suggest a noninfectious diagnosis (eg, pulmonary embolism, atelectasis) or that bacterial pneumonia is unlikely or has resolved. A 2000 study by Singh et al. highlighted the feasibility of using operational criteria in the form of clinical pulmonary infection score (CPIS) to decide whether to terminate or shorten the duration of initial empiric antibiotic therapy for suspected VAP.23 More specifically, patients with pulmonary infiltrates but a low likelihood of pneumonia (CPIS 6) were randomized to receive either standard antibiotic therapy or ciprofloxacin monotherapy. The situation was re‐evaluated at 3 days, and ciprofloxacin therapy was discontinued if the CPIS remained 6. Results showed no difference in mortality between the ciprofloxacin and standard therapy groups, despite shorter duration of therapy for the former, together with lower antimicrobial exposure and costs for the ciprofloxacin group. (Use of the CPIS to shorten the duration of empiric therapy and limit antimicrobial exposure is discussed in greater detail in the corresponding article in this supplement by Dr File.) Having said that, the case study before us describes a patient with pneumonia by clinical criteria who has responded to broad‐spectrum therapy. Alternative noninfectious diagnoses are not apparent, and even though cultures have returned without significant growth, the patient should continue to receive antimicrobial treatment. The question now is whether to de‐escalate/streamline to a more narrow‐spectrum regimen, or continue the current one.
De‐escalation often targets antimicrobials that provide unnecessarily broad coverage, eg, those with antipseudomonal activity (particularly antipseudomonal carbapenems) and/or agents with activity against methicillin‐resistant Staphylococcus aureus (MRSA). In the absence of definitive culture results isolating a particular pathogen(s), decisions regarding which antibiotics to stop or change often depends, in large part, on patient characteristics (eg, history of prior infection with resistant pathogens, as well as drug allergies or renal insufficiency) and local antibiograms indicating the prevalence and antimicrobial susceptibility of different pneumonia pathogens in the hospital at large or particular wards within the hospital. However, negative culture results can also be useful in guiding subsequent therapy decisions or modifications. In the present case, MRSA was not grown from any cultures, and there was no evidence of Gram‐positive cocci clusters with Gram staining. This suggests that vancomycin should be stopped, and antimicrobial therapy continued with a single antibiotic or antibiotic product that does include MRSA coverage. The question then is whether to continue piperacillin/tazobactam or replace it with another antibiotic.
Because Pseudomonas aeruginosa was not isolated, the clinician might consider streamlining piperacillin/tazobactam to an antibiotic with less pseudomonal and anaerobic coverage, possibly a nonpseudomonal third‐generation cephalosporin or nonpseudomonal carbapenem, such as ertapenem. Given the activity of piperacillin/tazobactam against aerobic Gram‐positive and Gram‐negative pathogens, continuing piperacillin‐tazobactam as single‐agent therapy would also be a viable alternative. However, in the spirit of stewardship and lack of need for pseudomonal coverage, a decision was made to replace piperacillin/tazobactam with ceftriaxone. Ceftriaxone is a nonpseudomonal third‐generation cephalosporin with activity against most other Gram‐negative bacteria. Note that in this case, only oropharyngeal flora grew from the respiratory culture, and the blood culture was negative. However, if a pathogen had grown from either respiratory or blood cultures, then single‐agent therapy could have been used to target that specific pathogen. For example, if Klebsiella spp susceptible to ceftriaxone was isolated from the respiratory culture, then ceftriaxone would have been the obvious choice. If MRSA was isolated, then vancomycin (or another appropriate active agent, such as linezolid or clindamycin) could be administered as a single agent.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with a diverticular abscess and walled off perforation. Interventional radiology inserts a drain, and the patient is treated with ciprofloxacin plus metronidazole. This regimen is consistent with guidelines from the Surgical Infection Society and IDSA for initial empiric treatment of complicated intra‐abdominal infection of mild‐to‐moderate severity.24 On day 3 following hospital admission and initiation of empiric therapy, the patient seems to show treatment response, as evidenced by downward trends in body temperature and WBC count (Figure 3). However, although the body temperature measures are trending in the right direction, there is still concern about continuing fever spikes and fever at last measure (100.9F). In addition, the WBC count is still elevated, though improving. The patient's blood pressure has normalized (112/72 mmHg vs 84/58 mmHg at admission), and oxygen saturation (98%) measures are normal. The patient's lungs are clear, and her abdominal examination results are improving, though there is still some diffuse tenderness. Microbiological data show blood cultures with no growth, and isolation of Gram‐negative rods from cultures of the abdominal abscess.
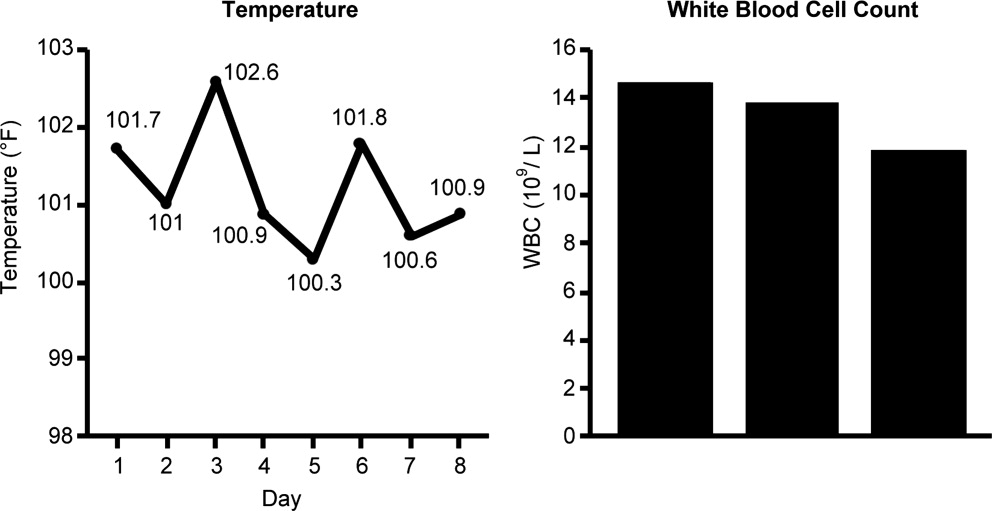
We now have preliminary microbiological data for a patient who remains febrile and has continuing abdominal tenderness, but who is otherwise clinically stable. Can her antimicrobial regimen be de‐escalated at this point, based on what is currently known? When managing a patient after the first 3 or 4 days of empiric treatment, it is important to realize that the patient's condition with regards to infection might reflect issues unrelated to inadequate antimicrobial coverage. If the patient's clinical status has not improved, or if he or she remains febrile even 3 or 4 days into therapy, the clinician should not automatically assume the lack of improvement is due to antibiotic failure. At this point, it is important to consider possible nonantibiotic causes of persistent clinical abnormalities and fever, and for the case here, one possibility is inadequate abscess drainage. The patient should be evaluated with abdominal imaging to ascertain whether the abscess is being adequately drained. With respect to antimicrobial therapy, the patient's blood pressure has stabilized, and her fever is trending downward. In many cases, a lingering fever such as the one observed here, in the context of improving WBC counts and clinical stabilization, may reflect inadequate mechanical drainage of the abscess. Certainly the antimicrobial therapy should not be broadened at this time, and consideration should be given to de‐escalation based on the available microbiological data.
If a type of pathogenic organism is preliminarily identified from culture, but the exact identification of the organism is pending, adjustments of therapy can still be made. Adjustments can also be made based on what is not growing. In this case, the abscess culture has grown Gram‐negative rods, but no Gram‐positive organisms. Hence, continued coverage of Gram‐negative organisms is warranted. In addition, anaerobes often will not readily grow in clinical cultures, and because anaerobes are frequent co‐pathogens, it is appropriate to continue to provide anaerobic coverage. Based on this information, continuation of both ciprofloxacin (for aerobic Gram‐negative coverage) and metronidazole (to cover for anaerobic bacteria) is appropriate in the present case. In other words, the initial empiric therapy should be continued until subsequent culture identifies a particular pathogen, at which time the therapy can be streamlined.
Now, 1 day later (day 4 of hospital admission and empiric therapy), the patient's clinical status is essentially unchangedexcept for a spike in fever to 103.2F. The WBC count is unchanged. Moreover, additional abscess culture data are available, showing definitive identification of an extended‐spectrum ‐lactamase (ESBL)‐producing Escherichia coli organism. The blood culture is still negative. The first observation is that ESBL‐producing E coli is a relatively unusual pathogen in a community‐based infection. However, the patient here did have risk factors for antibiotic‐resistant pathogens, notably prior antimicrobial therapy as an outpatient. It is also important to recognize that community‐acquired infections with ESBL‐producing bacteria (mostly isolated from the urinary tract) have been reported in many parts of the world, and even in some parts of the United States.25
Based on these additional microbiological data, the patient was switched to treatment with ertapenem, a nonpseudomonal carbapenem with activity against ESBL‐producing Enterobacteriaceae.26 In addition, ertapenem, and other carbapenems, have excellent activity against anaerobes,26 and it is prudent to continue coverage for anaerobes even though anaerobes were not grown in the culture. As mentioned above, these organisms are difficult to grow in clinical culture, and they are common pathogens or co‐pathogens in intra‐abdominal infections. Carbapenems are widely regarded as the antimicrobials of choice for treatment of serious, invasive infections with ESBL‐producing bacteria.27 Furthermore, by choosing a nonpseudomonal carbapenem, compared with an antipseudomonal carbapenem, the new antibiotic regimen provides coverage of the isolated ESBL‐producing E coli organismas well as covering possible anaerobe involvementwithout exposing host bacteria to unnecessarily broad antipseudomonal activity. Cephalosporins, monobactams, and fluoroquinolones are generally not active against ESBL‐producing Enterobacteriaceae, and ‐lactam/‐lactamase inhibitor combinations (eg, ampicillin/sulbactam, piperacillin/tazobactam) do not have reliable activity in serious, high inoculum infections caused by ESBL‐producing Enterobacteriaceae.27
CASE 3: CENTRAL LINE‐ASSOCIATED BLOODSTREAM INFECTION
Case 3 is a 56‐year‐old man who presented to the hospital emergency department with status epilepticus. He was intubated, had a central line placed in the internal jugular vein, and was admitted to the intensive care unit (ICU). The seizure was successfully broken by aggressive treatment with repeated intravenous dosing of lorazepam and loading with fosphenytoin. Empiric antibiotic therapy was initiated with vancomycin and piperacillin/tazobactam on day 5, after spiking a fever of 103.4F. No clear source of the fever was identified. While in the ICU with a central line in place, 2 sets of blood cultures were drawn. Now on hospital day 6, the patient is still spiking fever, although the fever trend appears to be decreasing. The patient is hemodynamically stable, with no other abnormal findings (besides persistent fever) on physical examination. WBC count remains elevated, and both sets of blood cultures are notable for growth of Gram‐positive cocci.
Bloodstream infection is a serious condition in hospitalized patients that is associated with significant morbidity and mortality.28 Patients with suspected bloodstream infection typically receive empiric broad‐spectrum antimicrobial therapy, and are thus good candidates for de‐escalation based on subsequent clinical status and blood culture results. Because of the seriousness of bloodstream infection, healthcare workers are sometimes hesitant to de‐escalate initial empiric therapy, even when cultures isolate a pathogen susceptible to narrower‐spectrum agents, particularly if the patient appears to be improving on such therapy. This is true for various serious hospital or healthcare‐associated infections,16, 29 but particularly for bloodstream infections. Moreover, when central line‐associated bloodstream infection (CLABSI) is suspected, the most important initial intervention is to remove the infected central venous catheter. For a patient with a short‐term catheter and a CLABSI due to Gram‐negative bacilli, S aureus (which appears to be a likely pathogen for the case patient here), enterococci, fungi, or mycobacteria, the 2009 IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal.30 Catheter removal is even more important than antibiotic coverage; this point cannot be stressed enough. In some extreme cases, when the line cannot be removed for clinical reasons, antibiotic lock therapy can be used to supplement systemic antimicrobial therapy.30 This involves instilling a high antibiotic solution into the catheter lumen for a period of time in order to sterilize the lumen and prevent biofilm formation.31
The first step taken for the patient here was to remove the central venous catheter. Then, turning to the preliminary culture data, there is evidence for Gram‐positive cocci in the patient's blood. The blood culture did not grow any Gram‐negative organisms. Gram‐positive cocci (coagulase‐negative staphylococci, S aureus [methicillin‐susceptible or MRSA]) are the most common causes of CLABSI.32 Can the physician de‐escalate antibiotic therapy in this patient with CLABSI based on the preliminary information? Yes. The information is solid enough to suggest removal of the catheter which was providing coverage for Gram‐negative bacteria (piperacillin/tazobactam), while continuing vancomycin for coverage of possible MRSA, pending further review, ie, until the Gram‐positive cocci are speciated. Rapid diagnostic methods, including polymerase chain reaction (PCR) and nucleic acid probes, can be used to provide more information about certain pathogens (such as MRSA33, 34) before final culture and susceptibility results are available, but these are not routinely available in many clinical microbiology laboratories. Furthermore, these newer technologies remain fairly expensive.
Revisiting the patient 1 day later (hospital day 7), after narrowing the initial combination antibiotic regimen to vancomycin monotherapy, the physical examination indicates the patient is clinically stable, with continued improvement in fever and WBC count (Figure 4). Blood culture analysis now isolates methicillin‐susceptible S aureus (MSSA). Methicillin resistance mediates resistance to all ‐lactams, including carbapenems, greatly limiting treatment options. Vancomycin is the most commonly utilized antibiotic for the treatment of MRSA, and the recent clinical practice guidelines from the IDSA recommend either vancomycin or daptomycin for management of MRSA bacteremia in adult patients.35 However, antistaphylococcal penicillins and first‐generation cephalosporins are the antibiotics of choice for MSSA infections, and particularly for MSSA bloodstream infections.
The activity provided by vancomycin (or daptomycin) is overly broad if MSSA is involved, and importantly, it is not as effective as treatment with an antistaphylococcal penicillin or first‐generation cephalosporin. A recent study by Stryjewski et al., of hemodialysis patients with MSSA bacteremia, reported a higher proportion of treatment failure with vancomycin versus first‐generation cephalosporin therapy (31% vs 13%; P = 0.02).36 Furthermore, multivariate analysis identified vancomycin (vs first‐generation cephalosporin) use as a significant independent predictor of treatment failure (odds ratio [OR], 3.53; 95% confidence interval [CI], 1.1513.45; P = 0.04). Similarly, Chang et al. reported nafcillin, an antistaphylococcal penicillin, was superior to vancomycin in preventing bacteriologic failure (persistent failure and/or relapse) in patients with MSSA bacteremia (0% vs 19%; P = 0.058), and used multivariate analysis to identify vancomycin as a significant independent predictor of relapse (OR, 6.5; 95% CI, 1.052.8; P 0.05).37 Another recent study by Lodise et al. reported that initial empiric therapy with vancomycin for endocarditis caused by MSSA was associated with a higher infection‐related mortality rate than initial empiric therapy with a ‐lactam‐containing regimen (39% vs 11%; P = 0.005).38 The negative impact of initial treatment with vancomycin persisted even in patients switched to a ‐lactam therapy after culture results became available.
Hence, if a patient is being treated with vancomycin for a bloodstream (or other) infection due to MSSA, the therapy is suboptimal. In such a scenariowhich corresponds to that for the case patient herevancomycin should be discontinued and replaced with an antistaphylococcal penicillin or first‐generation cephalosporin. Many times, clinicians are resistant to terminating vancomycin and de‐escalating to antistaphylococcal penicillin/first‐generation cephalosporin therapy in a patient with bacteremia who is apparently responding to vancomycin. However, as the studies just reviewed make clear, not only is vancomycin treatment overly broad for the circumstance, it is also suboptimal and does not represent best clinical practice or patient care. Furthermore, continuing vancomycin in this situation unnecessarily exposes the patient to possible renal toxicity, particularly when aggressive dosing or prolonged vancomycin treatment is involved.39 Because of these issues and concerns, case 3 was de‐escalated from vancomycin to cefazolin, a first‐generation cephalosporin. One word of caution, however, is that there is some controversy over using cefazolin in patients with S aureus native valve endocarditis, given the possibility of a Type A ‐lactamase‐producing species causing cefazolin degradation.40 As a result, the clinician should first rule out endocarditis in the patient here before proceeding with cefazolin therapy. Another alternative would be to use an antistaphylococcal penicillin, such as nafcillin.
Finally, when dealing with bacteremia, and particularly when dealing with a possible CLABSI, the issue of potential culture contamination needs to be seriously considered and answered. Treating an actual infection, not what appears to be an infection because of culture contamination, is particularly important when dealing with possible CLABSI, because coagulase‐negative staphylococci (CoNS) are the most common cause of these types of infections,32 and CoNS are also frequent blood‐culture contaminants.41 Therefore, one needs to determine whether a blood culture growing a CoNS represents true bacteremia or simply contaminationwhich will obviously impact de‐escalation decisions.
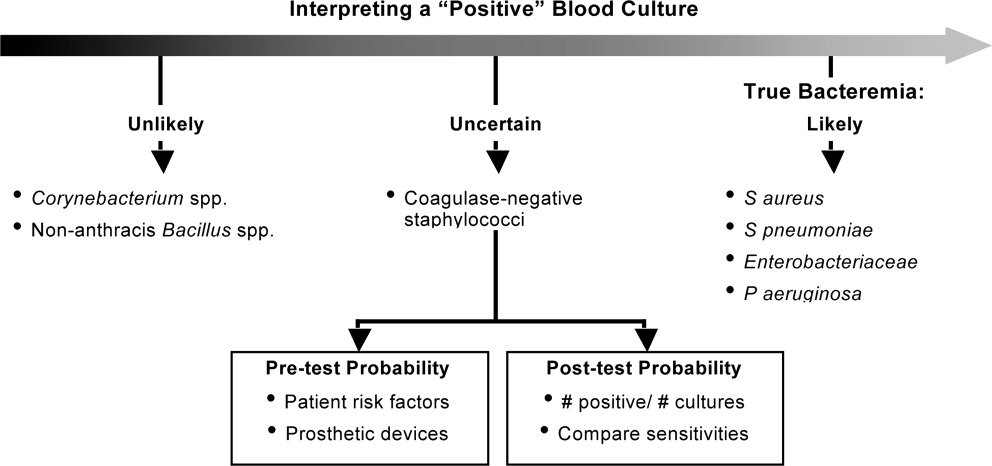
In addition, when determining whether a blood culture is truly positive and clinically significant, it is important to consider whether the isolated pathogens are unlikely to be contaminants, likely to be contaminants, or the situation is unclear. A 2000 study by Kim et al.42 suggested that, among patients with 2 positive blood cultures for CoNS, routine identification of CoNS species and genotyping selected isolates using pulsed‐field gel electrophoresis may improve the process of discriminating contaminants from pathogens. Various additional factors need to be weighed when trying to interpret CoNS blood culture results, including patient risk factors, presence of prosthetic devices, number of blood cultures and number positive, and the antimicrobial sensitivity patterns of different isolates. For example, if the sensitivity patterns of 2 CoNS strains isolated from a patient are the same, the likelihood is increased that they represent true pathogens rather than contaminants. Figure 5 presents a schematic of this general approach.42
CONCLUSIONS
De‐escalation is a critical component of antimicrobial stewardship. As the prevalence of antimicrobial resistance grows in the hospital and community, de‐escalation will have an increasingly important role in limiting the further emergence of antimicrobial resistance. Pneumonia, intra‐abdominal infection, and bloodstream infection are commonly managed in the hospital setting. Each of these infection types presents excellent opportunities for de‐escalation, and each presents unique challenges and caveats. Concerted efforts must be made by clinicians and stewardship personnel to de‐escalate as soon as possible, based on culture results and clinical status. Although not discussed here, successful de‐escalation programs utilize structured process, guidelines, and algorithms to consistently implement de‐escalation efforts. These tools of implementation are more fully discussed in the corresponding article in this supplement by Dr Rosenberg.
- .Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time.Drugs.2003;63:2157–2168.
- ,,, et al.Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study.J Antimicrob Chemother.2008;61:436–441.
- ,,,,,.Predictors of 30‐day mortality and hospital costs in patients with ventilator‐associated pneumonia attributed to potentially antibiotic‐resistant gram‐negative bacteria.Chest.2008;134:281–287.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,,,.Emergence of antibiotic‐resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy.Clin Infect Dis.1996;23:486–494.
- ,,,,.Antimicrobial therapy escalation and hospital mortality among patients with health‐care‐associated pneumonia: a single‐center experience.Chest.2008;134:963–968.
- ,.De‐escalation in lower respiratory tract infections.Curr Opin Pulm Med.2006;12:364–368.
- .The importance of de‐escalating antimicrobial therapy in patients with ventilator‐associated pneumonia.Semin Respir Crit Care Med.2006;27:45–50.
- .Impact of antibiotic resistance in gram‐negative bacilli on empirical and definitive antibiotic therapy.Clin Infect Dis.2008;47(suppl 1):S14–S20.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- Bad Bugs, No Drugs: As Antibiotic R46:155–164.
- ,,, et al.De‐escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate.Intensive Care Med.2007;33:1533–1540.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,, et al.Empiric broad‐spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study.Crit Care.2006;10:R78.
- ,,,.Does de‐escalation of antibiotic therapy for ventilator‐associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?J Trauma.2009;66:1343–1348.
- ,,, et al.Ventilator‐associated pneumonia: breaking the vicious circle of antibiotic overuse.Crit Care Med.2007;35:379–385; quiz 386.
- ,,,,.Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs.J Antimicrob Chemother.2006;57:326–330.
- ,,,,.Diagnostic yield of blood cultures from antibiotic‐naive and antibiotically treated patients with haematological malignancies and high‐risk neutropenia.Scand J Infect Dis.2009;41:650–655.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,.Molecular characteristics of extended‐spectrum beta‐lactamase‐producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX‐M‐15 in community hospitals.Int J Antimicrob Agents.2010;36:19–23.
- ,.Ertapenem: a review of its use in the treatment of bacterial infections.Drugs.2005;65:2151–2178.
- .Infections with extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: changing epidemiology and drug treatment choices.Drugs.2010;70:313–333.
- ,.Bloodstream infection in the ICU.Infect Dis Clin North Am.2009;23:557–569.
- ,,,,.Insights from the Society of Infectious Diseases Pharmacists on antimicrobial stewardship guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America.Pharmacotherapy.2009;29:593–607.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 Update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials.Clin Infect Dis.2008;47:83–93.
- ,.Catheter‐related bloodstream infections: catheter management according to pathogen.Int J Antimicrob Agents.2010;36(suppl 2):S26–S32.
- ,,,.Detection of methicillin‐resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth‐enriched culture in an area with a low prevalence of MRSA infections.J Clin Microbiol.2010;48:3882–3887.
- ,,,,.Clinical validation of the molecular BD GeneOhm StaphSR assay for direct detection of Staphylococcus aureus and methicillin‐resistant Staphylococcus aureus in positive blood cultures.J Clin Microbiol.2007;45:2191–2196.
- ,,, et al.Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children.Clin Infect Dis.2011;52:e18–e55.
- ,,, et al.Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia.Clin Infect Dis.2007;44:190–196.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,,,.Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus.Antimicrob Agents Chemother.2007;51:3731–3733.
- ,,,,.A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia.Clin Ther.2007;29:1107–1115.
- ,,.Relapse of type A beta‐lactamase‐producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue.Clin Infect Dis.2003;37:1194–1198.
- ,.The origin of coagulase‐negative staphylococci isolated from blood cultures.J Hosp Infect.1995;30:217–223.
- ,,, et al.Determining the significance of coagulase‐negative staphylococci isolated from blood cultures at a community hospital: a role for species and strain identification.Infect Control Hosp Epidemiol.2000;21:213–217.
Two conflicting aims collide when choosing initial empiric therapy for patients with a potential life‐threatening infection. On the one hand, the clinical picture and seriousness of the suspected infectionsometimes with a multi‐drug resistant (MDR) pathogenpoint to the need for immediate empiric therapy with a broad‐spectrum regimen covering the most likely pathogens. This getting it right the first time approach1 is clearly a reasonable one given the significant negative impact of inappropriate or inadequate initial therapy on patient outcomes and costs,24 and the apparent inability to remedy the initial error by subsequent antimicrobial regimen adjustment.57 On the other hand, use of a broad‐spectrum regimen increases the risk of emergent antimicrobial‐resistant pathogens, with potential harm for the immediate patient and all subsequent patients who become exposed and infected with the resistant pathogen. Hence, the aim of optimizing initial empiric therapy comes into conflict with an important aim of antimicrobial stewardship, namely, to use antimicrobials in a manner that does not excessively promote development or selection of antimicrobial‐resistant pathogens.
The de‐escalation strategy is an approach that attempts to balance these conflicting aims by providing optimal initial patient management without inordinately promoting development of antimicrobial resistance. As discussed more fully in the corresponding supplement article by Dr Syndman, the first part of this strategy involves collecting cultures from suitable patients prior to initiating broad‐spectrum empiric antimicrobial therapy designed to cover the most likely pathogenic microorganisms, based on local patterns of prevalence and susceptibility, and the presence of risk factors for infection with drug‐resistant species.810 The second critical step involves modification of initial empiric therapy (when warranted) based on clinical status and when culture results are available.810 In this manner, the initial broad‐spectrum regimen can often be streamlined or de‐escalated to a more narrow‐spectrum regimen or, in some cases, terminated when negative cultures suggest no infection. Frequently, initial combination therapy can be replaced by monotherapy targeting the pathogenic organism identified in culture. Sometimes culture results indicate that initial empiric therapy was inappropriate/emnadequate and requires replacement or other modification. Thus, by modifying empiric antimicrobial therapy on the basis of culture results and clinical criteria, the de‐escalation strategy enables more effective targeting of the causative pathogen(s), elimination of redundant therapy, a decrease in antimicrobial pressure for emergence of resistance, and cost savings.10, 11 Decreasing the number of antimicrobial agents and/or the spectrum of coverage is also expected to decrease the risk of adverse events, drugdrug interactions, and Clostridium difficile‐associated disease.12, 13 A number of studies have demonstrated that de‐escalation of initially appropriate therapy can be successfully accomplished with either improved outcomes14, 15 or with comparable effectiveness as continued initial therapy,1618 but with reduced antimicrobial exposure and costs.19
The timing of streamlining or other modification of initial empiric therapy typically occurs when microbiological culture results become available. Assuming blood or other relevant tissue cultures were obtained prior to initiating empiric therapy, this means de‐escalation or other modifications of initial therapy generally occurs 24 days after hospitalization and/or the beginning of empiric therapy. If rapid diagnostic tests are used to identify or rule out particular pathogens, then de‐escalation may occur slightly sooner. In addition to culture results, observation of the patient in the hospital setting and improved clarity as to his or her clinical status also affect the decision about whether and how to modify the initial empiric antimicrobial regimen. The clinical scenario of the patient and his or her response to initial antimicrobial therapy is also typically clearer by day 3 of antibiotic therapy. If, for some reason, cultures were not obtained prior to beginning empiric therapy, then observations of clinical status and consideration of patient risk factors for resistant pathogens become predominant in the decision‐making process. With respect to the timing of culture attainment, this should occur prior to beginning antimicrobial therapy, because therapy may reduce culture yield and result in false negative or other misleading findings.20, 21
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
Case 1 is a 72‐year‐old woman admitted with findings consistent with healthcare‐associated pneumonia (HCAP). Empiric therapy was initiated with vancomycin and piperacillin/tazobactam. Figure 1 provides the laboratory (white blood cell [WBC] counts) and body temperature data for the patient since she entered the hospital and began empiric antibiotic therapy 3 days earlier. The WBC counts suggest the patient is responding to the antibiotic regimen, as demonstrated by a progressive reduction over the time period. However, her counts were still elevated above normal at last measurement, suggesting an incompletely resolved infection at this time. In addition, the patient is still coughing, but has less sputum production, and has some energy to get up and move around. Crackles are apparent at the right lung base. The patient's fever curve has trended down, but still shows notable fever spikes, with a temperature maximum of 101.4F for the past 24 hours. Her blood pressure (135/84 mmHg), pulse (74 bpm), and respiratory rate (14 breaths per minute) are normal, with slightly decreased oxygen saturation (94%) on room air, although improved from initial examination 3 days earlier (92%). The blood culture shows no growth; the sputum culture simply shows oropharyngeal flora. In other words, the culture results have not isolated a causative pathogen. In addition to vancomycin and piperacillin/tazobactam, the patient continues to receive her usual medications for a past history of myocardial infarction (low‐dose aspirin, metoprolol) and hypertension (enalapril, furosemide).

HCAP is a common infection often requiring initial empiric therapy with a broad‐spectrum regimen that covers possible involvement of resistant bacteria. As such, HCAP frequently provides excellent opportunities for de‐escalation. Figure 2 presents the general strategy from the 2005 American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) guidelines for the management of HCAP, hospital‐acquired pneumonia (HAP), or ventilator‐associated pneumonia (VAP).22 According to the guidelines, HCAP, HAP, and VAP should be similarly managed. Broad‐spectrum initial empiric antibiotic therapy is recommended for patients with late‐onset disease or those with risk factors for MDR pathogens (including high prevalence of resistance based on local antibiograms), while limited‐spectrum antibiotic therapy is recommended for all other patients. Note that consideration of de‐escalation or streamlining of initial therapy begins 2‐3 days after initiation of therapy. Data that should be reviewed prior to instituting de‐escalation include blood cultures and respiratory cultures, as well as the clinical status of the patient. The adequacy of respiratory samples used for culturing should factor into the decision‐making process. For example, in patients who are not intubated or mechanically ventilated, it can be challenging to obtain a quality respiratory specimen for culture. If clinicians are uncertain as to the quality of the respiratory specimen that was cultured, then de‐escalation decisions should be based more on the clinical status of the patient.

The clinical status of the patient, 2 days after beginning treatment, and culture results are critical in guiding the de‐escalation process.9, 22 The ATS/IDSA guidelines recommend serial assessments of clinical parameters to define the response to initial empiric therapy. If the therapy regimen is effective, an improvement in clinical response should be apparent within 2‐3 days of its initiation.22 Hence, no change in antimicrobial therapy should be undertaken before 3 days, unless there is evidence of rapid deterioration in clinical status or infectious diseases experts recommend a change. With respect to culture results, failure to isolate a group of MDR pathogens for which initial broad‐spectrum empiric therapy was selected affords an opportunity to now streamline therapy or treat with a more narrow‐spectrum regimen.9 Similarly, isolation of a particular pathogen can guide treatment modifications (when necessary), while a negative culture raises the possibility of terminating antimicrobial therapy, provided the culture was collected before initiating therapy. Confidence in this latter decision is bolstered when the patient exhibits rapid improvement in clinical status that is backed by radiographic resolution of lung abnormalities, or an alternative diagnosis has been established for which antimicrobial therapy is not indicated.9
At this stage in the process3 days after initiating empiric therapy, and with culture results in hand and evidence of clinical improvementthe first decision or question is whether antimicrobial therapy can be stopped altogether, ie, do the current data suggest a noninfectious diagnosis (eg, pulmonary embolism, atelectasis) or that bacterial pneumonia is unlikely or has resolved. A 2000 study by Singh et al. highlighted the feasibility of using operational criteria in the form of clinical pulmonary infection score (CPIS) to decide whether to terminate or shorten the duration of initial empiric antibiotic therapy for suspected VAP.23 More specifically, patients with pulmonary infiltrates but a low likelihood of pneumonia (CPIS 6) were randomized to receive either standard antibiotic therapy or ciprofloxacin monotherapy. The situation was re‐evaluated at 3 days, and ciprofloxacin therapy was discontinued if the CPIS remained 6. Results showed no difference in mortality between the ciprofloxacin and standard therapy groups, despite shorter duration of therapy for the former, together with lower antimicrobial exposure and costs for the ciprofloxacin group. (Use of the CPIS to shorten the duration of empiric therapy and limit antimicrobial exposure is discussed in greater detail in the corresponding article in this supplement by Dr File.) Having said that, the case study before us describes a patient with pneumonia by clinical criteria who has responded to broad‐spectrum therapy. Alternative noninfectious diagnoses are not apparent, and even though cultures have returned without significant growth, the patient should continue to receive antimicrobial treatment. The question now is whether to de‐escalate/streamline to a more narrow‐spectrum regimen, or continue the current one.
De‐escalation often targets antimicrobials that provide unnecessarily broad coverage, eg, those with antipseudomonal activity (particularly antipseudomonal carbapenems) and/or agents with activity against methicillin‐resistant Staphylococcus aureus (MRSA). In the absence of definitive culture results isolating a particular pathogen(s), decisions regarding which antibiotics to stop or change often depends, in large part, on patient characteristics (eg, history of prior infection with resistant pathogens, as well as drug allergies or renal insufficiency) and local antibiograms indicating the prevalence and antimicrobial susceptibility of different pneumonia pathogens in the hospital at large or particular wards within the hospital. However, negative culture results can also be useful in guiding subsequent therapy decisions or modifications. In the present case, MRSA was not grown from any cultures, and there was no evidence of Gram‐positive cocci clusters with Gram staining. This suggests that vancomycin should be stopped, and antimicrobial therapy continued with a single antibiotic or antibiotic product that does include MRSA coverage. The question then is whether to continue piperacillin/tazobactam or replace it with another antibiotic.
Because Pseudomonas aeruginosa was not isolated, the clinician might consider streamlining piperacillin/tazobactam to an antibiotic with less pseudomonal and anaerobic coverage, possibly a nonpseudomonal third‐generation cephalosporin or nonpseudomonal carbapenem, such as ertapenem. Given the activity of piperacillin/tazobactam against aerobic Gram‐positive and Gram‐negative pathogens, continuing piperacillin‐tazobactam as single‐agent therapy would also be a viable alternative. However, in the spirit of stewardship and lack of need for pseudomonal coverage, a decision was made to replace piperacillin/tazobactam with ceftriaxone. Ceftriaxone is a nonpseudomonal third‐generation cephalosporin with activity against most other Gram‐negative bacteria. Note that in this case, only oropharyngeal flora grew from the respiratory culture, and the blood culture was negative. However, if a pathogen had grown from either respiratory or blood cultures, then single‐agent therapy could have been used to target that specific pathogen. For example, if Klebsiella spp susceptible to ceftriaxone was isolated from the respiratory culture, then ceftriaxone would have been the obvious choice. If MRSA was isolated, then vancomycin (or another appropriate active agent, such as linezolid or clindamycin) could be administered as a single agent.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with a diverticular abscess and walled off perforation. Interventional radiology inserts a drain, and the patient is treated with ciprofloxacin plus metronidazole. This regimen is consistent with guidelines from the Surgical Infection Society and IDSA for initial empiric treatment of complicated intra‐abdominal infection of mild‐to‐moderate severity.24 On day 3 following hospital admission and initiation of empiric therapy, the patient seems to show treatment response, as evidenced by downward trends in body temperature and WBC count (Figure 3). However, although the body temperature measures are trending in the right direction, there is still concern about continuing fever spikes and fever at last measure (100.9F). In addition, the WBC count is still elevated, though improving. The patient's blood pressure has normalized (112/72 mmHg vs 84/58 mmHg at admission), and oxygen saturation (98%) measures are normal. The patient's lungs are clear, and her abdominal examination results are improving, though there is still some diffuse tenderness. Microbiological data show blood cultures with no growth, and isolation of Gram‐negative rods from cultures of the abdominal abscess.

We now have preliminary microbiological data for a patient who remains febrile and has continuing abdominal tenderness, but who is otherwise clinically stable. Can her antimicrobial regimen be de‐escalated at this point, based on what is currently known? When managing a patient after the first 3 or 4 days of empiric treatment, it is important to realize that the patient's condition with regards to infection might reflect issues unrelated to inadequate antimicrobial coverage. If the patient's clinical status has not improved, or if he or she remains febrile even 3 or 4 days into therapy, the clinician should not automatically assume the lack of improvement is due to antibiotic failure. At this point, it is important to consider possible nonantibiotic causes of persistent clinical abnormalities and fever, and for the case here, one possibility is inadequate abscess drainage. The patient should be evaluated with abdominal imaging to ascertain whether the abscess is being adequately drained. With respect to antimicrobial therapy, the patient's blood pressure has stabilized, and her fever is trending downward. In many cases, a lingering fever such as the one observed here, in the context of improving WBC counts and clinical stabilization, may reflect inadequate mechanical drainage of the abscess. Certainly the antimicrobial therapy should not be broadened at this time, and consideration should be given to de‐escalation based on the available microbiological data.
If a type of pathogenic organism is preliminarily identified from culture, but the exact identification of the organism is pending, adjustments of therapy can still be made. Adjustments can also be made based on what is not growing. In this case, the abscess culture has grown Gram‐negative rods, but no Gram‐positive organisms. Hence, continued coverage of Gram‐negative organisms is warranted. In addition, anaerobes often will not readily grow in clinical cultures, and because anaerobes are frequent co‐pathogens, it is appropriate to continue to provide anaerobic coverage. Based on this information, continuation of both ciprofloxacin (for aerobic Gram‐negative coverage) and metronidazole (to cover for anaerobic bacteria) is appropriate in the present case. In other words, the initial empiric therapy should be continued until subsequent culture identifies a particular pathogen, at which time the therapy can be streamlined.
Now, 1 day later (day 4 of hospital admission and empiric therapy), the patient's clinical status is essentially unchangedexcept for a spike in fever to 103.2F. The WBC count is unchanged. Moreover, additional abscess culture data are available, showing definitive identification of an extended‐spectrum ‐lactamase (ESBL)‐producing Escherichia coli organism. The blood culture is still negative. The first observation is that ESBL‐producing E coli is a relatively unusual pathogen in a community‐based infection. However, the patient here did have risk factors for antibiotic‐resistant pathogens, notably prior antimicrobial therapy as an outpatient. It is also important to recognize that community‐acquired infections with ESBL‐producing bacteria (mostly isolated from the urinary tract) have been reported in many parts of the world, and even in some parts of the United States.25
Based on these additional microbiological data, the patient was switched to treatment with ertapenem, a nonpseudomonal carbapenem with activity against ESBL‐producing Enterobacteriaceae.26 In addition, ertapenem, and other carbapenems, have excellent activity against anaerobes,26 and it is prudent to continue coverage for anaerobes even though anaerobes were not grown in the culture. As mentioned above, these organisms are difficult to grow in clinical culture, and they are common pathogens or co‐pathogens in intra‐abdominal infections. Carbapenems are widely regarded as the antimicrobials of choice for treatment of serious, invasive infections with ESBL‐producing bacteria.27 Furthermore, by choosing a nonpseudomonal carbapenem, compared with an antipseudomonal carbapenem, the new antibiotic regimen provides coverage of the isolated ESBL‐producing E coli organismas well as covering possible anaerobe involvementwithout exposing host bacteria to unnecessarily broad antipseudomonal activity. Cephalosporins, monobactams, and fluoroquinolones are generally not active against ESBL‐producing Enterobacteriaceae, and ‐lactam/‐lactamase inhibitor combinations (eg, ampicillin/sulbactam, piperacillin/tazobactam) do not have reliable activity in serious, high inoculum infections caused by ESBL‐producing Enterobacteriaceae.27
CASE 3: CENTRAL LINE‐ASSOCIATED BLOODSTREAM INFECTION
Case 3 is a 56‐year‐old man who presented to the hospital emergency department with status epilepticus. He was intubated, had a central line placed in the internal jugular vein, and was admitted to the intensive care unit (ICU). The seizure was successfully broken by aggressive treatment with repeated intravenous dosing of lorazepam and loading with fosphenytoin. Empiric antibiotic therapy was initiated with vancomycin and piperacillin/tazobactam on day 5, after spiking a fever of 103.4F. No clear source of the fever was identified. While in the ICU with a central line in place, 2 sets of blood cultures were drawn. Now on hospital day 6, the patient is still spiking fever, although the fever trend appears to be decreasing. The patient is hemodynamically stable, with no other abnormal findings (besides persistent fever) on physical examination. WBC count remains elevated, and both sets of blood cultures are notable for growth of Gram‐positive cocci.
Bloodstream infection is a serious condition in hospitalized patients that is associated with significant morbidity and mortality.28 Patients with suspected bloodstream infection typically receive empiric broad‐spectrum antimicrobial therapy, and are thus good candidates for de‐escalation based on subsequent clinical status and blood culture results. Because of the seriousness of bloodstream infection, healthcare workers are sometimes hesitant to de‐escalate initial empiric therapy, even when cultures isolate a pathogen susceptible to narrower‐spectrum agents, particularly if the patient appears to be improving on such therapy. This is true for various serious hospital or healthcare‐associated infections,16, 29 but particularly for bloodstream infections. Moreover, when central line‐associated bloodstream infection (CLABSI) is suspected, the most important initial intervention is to remove the infected central venous catheter. For a patient with a short‐term catheter and a CLABSI due to Gram‐negative bacilli, S aureus (which appears to be a likely pathogen for the case patient here), enterococci, fungi, or mycobacteria, the 2009 IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal.30 Catheter removal is even more important than antibiotic coverage; this point cannot be stressed enough. In some extreme cases, when the line cannot be removed for clinical reasons, antibiotic lock therapy can be used to supplement systemic antimicrobial therapy.30 This involves instilling a high antibiotic solution into the catheter lumen for a period of time in order to sterilize the lumen and prevent biofilm formation.31
The first step taken for the patient here was to remove the central venous catheter. Then, turning to the preliminary culture data, there is evidence for Gram‐positive cocci in the patient's blood. The blood culture did not grow any Gram‐negative organisms. Gram‐positive cocci (coagulase‐negative staphylococci, S aureus [methicillin‐susceptible or MRSA]) are the most common causes of CLABSI.32 Can the physician de‐escalate antibiotic therapy in this patient with CLABSI based on the preliminary information? Yes. The information is solid enough to suggest removal of the catheter which was providing coverage for Gram‐negative bacteria (piperacillin/tazobactam), while continuing vancomycin for coverage of possible MRSA, pending further review, ie, until the Gram‐positive cocci are speciated. Rapid diagnostic methods, including polymerase chain reaction (PCR) and nucleic acid probes, can be used to provide more information about certain pathogens (such as MRSA33, 34) before final culture and susceptibility results are available, but these are not routinely available in many clinical microbiology laboratories. Furthermore, these newer technologies remain fairly expensive.
Revisiting the patient 1 day later (hospital day 7), after narrowing the initial combination antibiotic regimen to vancomycin monotherapy, the physical examination indicates the patient is clinically stable, with continued improvement in fever and WBC count (Figure 4). Blood culture analysis now isolates methicillin‐susceptible S aureus (MSSA). Methicillin resistance mediates resistance to all ‐lactams, including carbapenems, greatly limiting treatment options. Vancomycin is the most commonly utilized antibiotic for the treatment of MRSA, and the recent clinical practice guidelines from the IDSA recommend either vancomycin or daptomycin for management of MRSA bacteremia in adult patients.35 However, antistaphylococcal penicillins and first‐generation cephalosporins are the antibiotics of choice for MSSA infections, and particularly for MSSA bloodstream infections.

The activity provided by vancomycin (or daptomycin) is overly broad if MSSA is involved, and importantly, it is not as effective as treatment with an antistaphylococcal penicillin or first‐generation cephalosporin. A recent study by Stryjewski et al., of hemodialysis patients with MSSA bacteremia, reported a higher proportion of treatment failure with vancomycin versus first‐generation cephalosporin therapy (31% vs 13%; P = 0.02).36 Furthermore, multivariate analysis identified vancomycin (vs first‐generation cephalosporin) use as a significant independent predictor of treatment failure (odds ratio [OR], 3.53; 95% confidence interval [CI], 1.1513.45; P = 0.04). Similarly, Chang et al. reported nafcillin, an antistaphylococcal penicillin, was superior to vancomycin in preventing bacteriologic failure (persistent failure and/or relapse) in patients with MSSA bacteremia (0% vs 19%; P = 0.058), and used multivariate analysis to identify vancomycin as a significant independent predictor of relapse (OR, 6.5; 95% CI, 1.052.8; P 0.05).37 Another recent study by Lodise et al. reported that initial empiric therapy with vancomycin for endocarditis caused by MSSA was associated with a higher infection‐related mortality rate than initial empiric therapy with a ‐lactam‐containing regimen (39% vs 11%; P = 0.005).38 The negative impact of initial treatment with vancomycin persisted even in patients switched to a ‐lactam therapy after culture results became available.
Hence, if a patient is being treated with vancomycin for a bloodstream (or other) infection due to MSSA, the therapy is suboptimal. In such a scenariowhich corresponds to that for the case patient herevancomycin should be discontinued and replaced with an antistaphylococcal penicillin or first‐generation cephalosporin. Many times, clinicians are resistant to terminating vancomycin and de‐escalating to antistaphylococcal penicillin/first‐generation cephalosporin therapy in a patient with bacteremia who is apparently responding to vancomycin. However, as the studies just reviewed make clear, not only is vancomycin treatment overly broad for the circumstance, it is also suboptimal and does not represent best clinical practice or patient care. Furthermore, continuing vancomycin in this situation unnecessarily exposes the patient to possible renal toxicity, particularly when aggressive dosing or prolonged vancomycin treatment is involved.39 Because of these issues and concerns, case 3 was de‐escalated from vancomycin to cefazolin, a first‐generation cephalosporin. One word of caution, however, is that there is some controversy over using cefazolin in patients with S aureus native valve endocarditis, given the possibility of a Type A ‐lactamase‐producing species causing cefazolin degradation.40 As a result, the clinician should first rule out endocarditis in the patient here before proceeding with cefazolin therapy. Another alternative would be to use an antistaphylococcal penicillin, such as nafcillin.
Finally, when dealing with bacteremia, and particularly when dealing with a possible CLABSI, the issue of potential culture contamination needs to be seriously considered and answered. Treating an actual infection, not what appears to be an infection because of culture contamination, is particularly important when dealing with possible CLABSI, because coagulase‐negative staphylococci (CoNS) are the most common cause of these types of infections,32 and CoNS are also frequent blood‐culture contaminants.41 Therefore, one needs to determine whether a blood culture growing a CoNS represents true bacteremia or simply contaminationwhich will obviously impact de‐escalation decisions.
In addition, when determining whether a blood culture is truly positive and clinically significant, it is important to consider whether the isolated pathogens are unlikely to be contaminants, likely to be contaminants, or the situation is unclear. A 2000 study by Kim et al.42 suggested that, among patients with 2 positive blood cultures for CoNS, routine identification of CoNS species and genotyping selected isolates using pulsed‐field gel electrophoresis may improve the process of discriminating contaminants from pathogens. Various additional factors need to be weighed when trying to interpret CoNS blood culture results, including patient risk factors, presence of prosthetic devices, number of blood cultures and number positive, and the antimicrobial sensitivity patterns of different isolates. For example, if the sensitivity patterns of 2 CoNS strains isolated from a patient are the same, the likelihood is increased that they represent true pathogens rather than contaminants. Figure 5 presents a schematic of this general approach.42

CONCLUSIONS
De‐escalation is a critical component of antimicrobial stewardship. As the prevalence of antimicrobial resistance grows in the hospital and community, de‐escalation will have an increasingly important role in limiting the further emergence of antimicrobial resistance. Pneumonia, intra‐abdominal infection, and bloodstream infection are commonly managed in the hospital setting. Each of these infection types presents excellent opportunities for de‐escalation, and each presents unique challenges and caveats. Concerted efforts must be made by clinicians and stewardship personnel to de‐escalate as soon as possible, based on culture results and clinical status. Although not discussed here, successful de‐escalation programs utilize structured process, guidelines, and algorithms to consistently implement de‐escalation efforts. These tools of implementation are more fully discussed in the corresponding article in this supplement by Dr Rosenberg.
Two conflicting aims collide when choosing initial empiric therapy for patients with a potential life‐threatening infection. On the one hand, the clinical picture and seriousness of the suspected infectionsometimes with a multi‐drug resistant (MDR) pathogenpoint to the need for immediate empiric therapy with a broad‐spectrum regimen covering the most likely pathogens. This getting it right the first time approach1 is clearly a reasonable one given the significant negative impact of inappropriate or inadequate initial therapy on patient outcomes and costs,24 and the apparent inability to remedy the initial error by subsequent antimicrobial regimen adjustment.57 On the other hand, use of a broad‐spectrum regimen increases the risk of emergent antimicrobial‐resistant pathogens, with potential harm for the immediate patient and all subsequent patients who become exposed and infected with the resistant pathogen. Hence, the aim of optimizing initial empiric therapy comes into conflict with an important aim of antimicrobial stewardship, namely, to use antimicrobials in a manner that does not excessively promote development or selection of antimicrobial‐resistant pathogens.
The de‐escalation strategy is an approach that attempts to balance these conflicting aims by providing optimal initial patient management without inordinately promoting development of antimicrobial resistance. As discussed more fully in the corresponding supplement article by Dr Syndman, the first part of this strategy involves collecting cultures from suitable patients prior to initiating broad‐spectrum empiric antimicrobial therapy designed to cover the most likely pathogenic microorganisms, based on local patterns of prevalence and susceptibility, and the presence of risk factors for infection with drug‐resistant species.810 The second critical step involves modification of initial empiric therapy (when warranted) based on clinical status and when culture results are available.810 In this manner, the initial broad‐spectrum regimen can often be streamlined or de‐escalated to a more narrow‐spectrum regimen or, in some cases, terminated when negative cultures suggest no infection. Frequently, initial combination therapy can be replaced by monotherapy targeting the pathogenic organism identified in culture. Sometimes culture results indicate that initial empiric therapy was inappropriate/emnadequate and requires replacement or other modification. Thus, by modifying empiric antimicrobial therapy on the basis of culture results and clinical criteria, the de‐escalation strategy enables more effective targeting of the causative pathogen(s), elimination of redundant therapy, a decrease in antimicrobial pressure for emergence of resistance, and cost savings.10, 11 Decreasing the number of antimicrobial agents and/or the spectrum of coverage is also expected to decrease the risk of adverse events, drugdrug interactions, and Clostridium difficile‐associated disease.12, 13 A number of studies have demonstrated that de‐escalation of initially appropriate therapy can be successfully accomplished with either improved outcomes14, 15 or with comparable effectiveness as continued initial therapy,1618 but with reduced antimicrobial exposure and costs.19
The timing of streamlining or other modification of initial empiric therapy typically occurs when microbiological culture results become available. Assuming blood or other relevant tissue cultures were obtained prior to initiating empiric therapy, this means de‐escalation or other modifications of initial therapy generally occurs 24 days after hospitalization and/or the beginning of empiric therapy. If rapid diagnostic tests are used to identify or rule out particular pathogens, then de‐escalation may occur slightly sooner. In addition to culture results, observation of the patient in the hospital setting and improved clarity as to his or her clinical status also affect the decision about whether and how to modify the initial empiric antimicrobial regimen. The clinical scenario of the patient and his or her response to initial antimicrobial therapy is also typically clearer by day 3 of antibiotic therapy. If, for some reason, cultures were not obtained prior to beginning empiric therapy, then observations of clinical status and consideration of patient risk factors for resistant pathogens become predominant in the decision‐making process. With respect to the timing of culture attainment, this should occur prior to beginning antimicrobial therapy, because therapy may reduce culture yield and result in false negative or other misleading findings.20, 21
CASE 1: HEALTHCARE‐ASSOCIATED PNEUMONIA
Case 1 is a 72‐year‐old woman admitted with findings consistent with healthcare‐associated pneumonia (HCAP). Empiric therapy was initiated with vancomycin and piperacillin/tazobactam. Figure 1 provides the laboratory (white blood cell [WBC] counts) and body temperature data for the patient since she entered the hospital and began empiric antibiotic therapy 3 days earlier. The WBC counts suggest the patient is responding to the antibiotic regimen, as demonstrated by a progressive reduction over the time period. However, her counts were still elevated above normal at last measurement, suggesting an incompletely resolved infection at this time. In addition, the patient is still coughing, but has less sputum production, and has some energy to get up and move around. Crackles are apparent at the right lung base. The patient's fever curve has trended down, but still shows notable fever spikes, with a temperature maximum of 101.4F for the past 24 hours. Her blood pressure (135/84 mmHg), pulse (74 bpm), and respiratory rate (14 breaths per minute) are normal, with slightly decreased oxygen saturation (94%) on room air, although improved from initial examination 3 days earlier (92%). The blood culture shows no growth; the sputum culture simply shows oropharyngeal flora. In other words, the culture results have not isolated a causative pathogen. In addition to vancomycin and piperacillin/tazobactam, the patient continues to receive her usual medications for a past history of myocardial infarction (low‐dose aspirin, metoprolol) and hypertension (enalapril, furosemide).

HCAP is a common infection often requiring initial empiric therapy with a broad‐spectrum regimen that covers possible involvement of resistant bacteria. As such, HCAP frequently provides excellent opportunities for de‐escalation. Figure 2 presents the general strategy from the 2005 American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) guidelines for the management of HCAP, hospital‐acquired pneumonia (HAP), or ventilator‐associated pneumonia (VAP).22 According to the guidelines, HCAP, HAP, and VAP should be similarly managed. Broad‐spectrum initial empiric antibiotic therapy is recommended for patients with late‐onset disease or those with risk factors for MDR pathogens (including high prevalence of resistance based on local antibiograms), while limited‐spectrum antibiotic therapy is recommended for all other patients. Note that consideration of de‐escalation or streamlining of initial therapy begins 2‐3 days after initiation of therapy. Data that should be reviewed prior to instituting de‐escalation include blood cultures and respiratory cultures, as well as the clinical status of the patient. The adequacy of respiratory samples used for culturing should factor into the decision‐making process. For example, in patients who are not intubated or mechanically ventilated, it can be challenging to obtain a quality respiratory specimen for culture. If clinicians are uncertain as to the quality of the respiratory specimen that was cultured, then de‐escalation decisions should be based more on the clinical status of the patient.

The clinical status of the patient, 2 days after beginning treatment, and culture results are critical in guiding the de‐escalation process.9, 22 The ATS/IDSA guidelines recommend serial assessments of clinical parameters to define the response to initial empiric therapy. If the therapy regimen is effective, an improvement in clinical response should be apparent within 2‐3 days of its initiation.22 Hence, no change in antimicrobial therapy should be undertaken before 3 days, unless there is evidence of rapid deterioration in clinical status or infectious diseases experts recommend a change. With respect to culture results, failure to isolate a group of MDR pathogens for which initial broad‐spectrum empiric therapy was selected affords an opportunity to now streamline therapy or treat with a more narrow‐spectrum regimen.9 Similarly, isolation of a particular pathogen can guide treatment modifications (when necessary), while a negative culture raises the possibility of terminating antimicrobial therapy, provided the culture was collected before initiating therapy. Confidence in this latter decision is bolstered when the patient exhibits rapid improvement in clinical status that is backed by radiographic resolution of lung abnormalities, or an alternative diagnosis has been established for which antimicrobial therapy is not indicated.9
At this stage in the process3 days after initiating empiric therapy, and with culture results in hand and evidence of clinical improvementthe first decision or question is whether antimicrobial therapy can be stopped altogether, ie, do the current data suggest a noninfectious diagnosis (eg, pulmonary embolism, atelectasis) or that bacterial pneumonia is unlikely or has resolved. A 2000 study by Singh et al. highlighted the feasibility of using operational criteria in the form of clinical pulmonary infection score (CPIS) to decide whether to terminate or shorten the duration of initial empiric antibiotic therapy for suspected VAP.23 More specifically, patients with pulmonary infiltrates but a low likelihood of pneumonia (CPIS 6) were randomized to receive either standard antibiotic therapy or ciprofloxacin monotherapy. The situation was re‐evaluated at 3 days, and ciprofloxacin therapy was discontinued if the CPIS remained 6. Results showed no difference in mortality between the ciprofloxacin and standard therapy groups, despite shorter duration of therapy for the former, together with lower antimicrobial exposure and costs for the ciprofloxacin group. (Use of the CPIS to shorten the duration of empiric therapy and limit antimicrobial exposure is discussed in greater detail in the corresponding article in this supplement by Dr File.) Having said that, the case study before us describes a patient with pneumonia by clinical criteria who has responded to broad‐spectrum therapy. Alternative noninfectious diagnoses are not apparent, and even though cultures have returned without significant growth, the patient should continue to receive antimicrobial treatment. The question now is whether to de‐escalate/streamline to a more narrow‐spectrum regimen, or continue the current one.
De‐escalation often targets antimicrobials that provide unnecessarily broad coverage, eg, those with antipseudomonal activity (particularly antipseudomonal carbapenems) and/or agents with activity against methicillin‐resistant Staphylococcus aureus (MRSA). In the absence of definitive culture results isolating a particular pathogen(s), decisions regarding which antibiotics to stop or change often depends, in large part, on patient characteristics (eg, history of prior infection with resistant pathogens, as well as drug allergies or renal insufficiency) and local antibiograms indicating the prevalence and antimicrobial susceptibility of different pneumonia pathogens in the hospital at large or particular wards within the hospital. However, negative culture results can also be useful in guiding subsequent therapy decisions or modifications. In the present case, MRSA was not grown from any cultures, and there was no evidence of Gram‐positive cocci clusters with Gram staining. This suggests that vancomycin should be stopped, and antimicrobial therapy continued with a single antibiotic or antibiotic product that does include MRSA coverage. The question then is whether to continue piperacillin/tazobactam or replace it with another antibiotic.
Because Pseudomonas aeruginosa was not isolated, the clinician might consider streamlining piperacillin/tazobactam to an antibiotic with less pseudomonal and anaerobic coverage, possibly a nonpseudomonal third‐generation cephalosporin or nonpseudomonal carbapenem, such as ertapenem. Given the activity of piperacillin/tazobactam against aerobic Gram‐positive and Gram‐negative pathogens, continuing piperacillin‐tazobactam as single‐agent therapy would also be a viable alternative. However, in the spirit of stewardship and lack of need for pseudomonal coverage, a decision was made to replace piperacillin/tazobactam with ceftriaxone. Ceftriaxone is a nonpseudomonal third‐generation cephalosporin with activity against most other Gram‐negative bacteria. Note that in this case, only oropharyngeal flora grew from the respiratory culture, and the blood culture was negative. However, if a pathogen had grown from either respiratory or blood cultures, then single‐agent therapy could have been used to target that specific pathogen. For example, if Klebsiella spp susceptible to ceftriaxone was isolated from the respiratory culture, then ceftriaxone would have been the obvious choice. If MRSA was isolated, then vancomycin (or another appropriate active agent, such as linezolid or clindamycin) could be administered as a single agent.
CASE 2: INTRA‐ABDOMINAL INFECTION (DIVERTICULITIS)
Case 2 is a 56‐year‐old woman who presents with a diverticular abscess and walled off perforation. Interventional radiology inserts a drain, and the patient is treated with ciprofloxacin plus metronidazole. This regimen is consistent with guidelines from the Surgical Infection Society and IDSA for initial empiric treatment of complicated intra‐abdominal infection of mild‐to‐moderate severity.24 On day 3 following hospital admission and initiation of empiric therapy, the patient seems to show treatment response, as evidenced by downward trends in body temperature and WBC count (Figure 3). However, although the body temperature measures are trending in the right direction, there is still concern about continuing fever spikes and fever at last measure (100.9F). In addition, the WBC count is still elevated, though improving. The patient's blood pressure has normalized (112/72 mmHg vs 84/58 mmHg at admission), and oxygen saturation (98%) measures are normal. The patient's lungs are clear, and her abdominal examination results are improving, though there is still some diffuse tenderness. Microbiological data show blood cultures with no growth, and isolation of Gram‐negative rods from cultures of the abdominal abscess.

We now have preliminary microbiological data for a patient who remains febrile and has continuing abdominal tenderness, but who is otherwise clinically stable. Can her antimicrobial regimen be de‐escalated at this point, based on what is currently known? When managing a patient after the first 3 or 4 days of empiric treatment, it is important to realize that the patient's condition with regards to infection might reflect issues unrelated to inadequate antimicrobial coverage. If the patient's clinical status has not improved, or if he or she remains febrile even 3 or 4 days into therapy, the clinician should not automatically assume the lack of improvement is due to antibiotic failure. At this point, it is important to consider possible nonantibiotic causes of persistent clinical abnormalities and fever, and for the case here, one possibility is inadequate abscess drainage. The patient should be evaluated with abdominal imaging to ascertain whether the abscess is being adequately drained. With respect to antimicrobial therapy, the patient's blood pressure has stabilized, and her fever is trending downward. In many cases, a lingering fever such as the one observed here, in the context of improving WBC counts and clinical stabilization, may reflect inadequate mechanical drainage of the abscess. Certainly the antimicrobial therapy should not be broadened at this time, and consideration should be given to de‐escalation based on the available microbiological data.
If a type of pathogenic organism is preliminarily identified from culture, but the exact identification of the organism is pending, adjustments of therapy can still be made. Adjustments can also be made based on what is not growing. In this case, the abscess culture has grown Gram‐negative rods, but no Gram‐positive organisms. Hence, continued coverage of Gram‐negative organisms is warranted. In addition, anaerobes often will not readily grow in clinical cultures, and because anaerobes are frequent co‐pathogens, it is appropriate to continue to provide anaerobic coverage. Based on this information, continuation of both ciprofloxacin (for aerobic Gram‐negative coverage) and metronidazole (to cover for anaerobic bacteria) is appropriate in the present case. In other words, the initial empiric therapy should be continued until subsequent culture identifies a particular pathogen, at which time the therapy can be streamlined.
Now, 1 day later (day 4 of hospital admission and empiric therapy), the patient's clinical status is essentially unchangedexcept for a spike in fever to 103.2F. The WBC count is unchanged. Moreover, additional abscess culture data are available, showing definitive identification of an extended‐spectrum ‐lactamase (ESBL)‐producing Escherichia coli organism. The blood culture is still negative. The first observation is that ESBL‐producing E coli is a relatively unusual pathogen in a community‐based infection. However, the patient here did have risk factors for antibiotic‐resistant pathogens, notably prior antimicrobial therapy as an outpatient. It is also important to recognize that community‐acquired infections with ESBL‐producing bacteria (mostly isolated from the urinary tract) have been reported in many parts of the world, and even in some parts of the United States.25
Based on these additional microbiological data, the patient was switched to treatment with ertapenem, a nonpseudomonal carbapenem with activity against ESBL‐producing Enterobacteriaceae.26 In addition, ertapenem, and other carbapenems, have excellent activity against anaerobes,26 and it is prudent to continue coverage for anaerobes even though anaerobes were not grown in the culture. As mentioned above, these organisms are difficult to grow in clinical culture, and they are common pathogens or co‐pathogens in intra‐abdominal infections. Carbapenems are widely regarded as the antimicrobials of choice for treatment of serious, invasive infections with ESBL‐producing bacteria.27 Furthermore, by choosing a nonpseudomonal carbapenem, compared with an antipseudomonal carbapenem, the new antibiotic regimen provides coverage of the isolated ESBL‐producing E coli organismas well as covering possible anaerobe involvementwithout exposing host bacteria to unnecessarily broad antipseudomonal activity. Cephalosporins, monobactams, and fluoroquinolones are generally not active against ESBL‐producing Enterobacteriaceae, and ‐lactam/‐lactamase inhibitor combinations (eg, ampicillin/sulbactam, piperacillin/tazobactam) do not have reliable activity in serious, high inoculum infections caused by ESBL‐producing Enterobacteriaceae.27
CASE 3: CENTRAL LINE‐ASSOCIATED BLOODSTREAM INFECTION
Case 3 is a 56‐year‐old man who presented to the hospital emergency department with status epilepticus. He was intubated, had a central line placed in the internal jugular vein, and was admitted to the intensive care unit (ICU). The seizure was successfully broken by aggressive treatment with repeated intravenous dosing of lorazepam and loading with fosphenytoin. Empiric antibiotic therapy was initiated with vancomycin and piperacillin/tazobactam on day 5, after spiking a fever of 103.4F. No clear source of the fever was identified. While in the ICU with a central line in place, 2 sets of blood cultures were drawn. Now on hospital day 6, the patient is still spiking fever, although the fever trend appears to be decreasing. The patient is hemodynamically stable, with no other abnormal findings (besides persistent fever) on physical examination. WBC count remains elevated, and both sets of blood cultures are notable for growth of Gram‐positive cocci.
Bloodstream infection is a serious condition in hospitalized patients that is associated with significant morbidity and mortality.28 Patients with suspected bloodstream infection typically receive empiric broad‐spectrum antimicrobial therapy, and are thus good candidates for de‐escalation based on subsequent clinical status and blood culture results. Because of the seriousness of bloodstream infection, healthcare workers are sometimes hesitant to de‐escalate initial empiric therapy, even when cultures isolate a pathogen susceptible to narrower‐spectrum agents, particularly if the patient appears to be improving on such therapy. This is true for various serious hospital or healthcare‐associated infections,16, 29 but particularly for bloodstream infections. Moreover, when central line‐associated bloodstream infection (CLABSI) is suspected, the most important initial intervention is to remove the infected central venous catheter. For a patient with a short‐term catheter and a CLABSI due to Gram‐negative bacilli, S aureus (which appears to be a likely pathogen for the case patient here), enterococci, fungi, or mycobacteria, the 2009 IDSA guidelines for management of intravascular catheter‐related infections recommend catheter removal.30 Catheter removal is even more important than antibiotic coverage; this point cannot be stressed enough. In some extreme cases, when the line cannot be removed for clinical reasons, antibiotic lock therapy can be used to supplement systemic antimicrobial therapy.30 This involves instilling a high antibiotic solution into the catheter lumen for a period of time in order to sterilize the lumen and prevent biofilm formation.31
The first step taken for the patient here was to remove the central venous catheter. Then, turning to the preliminary culture data, there is evidence for Gram‐positive cocci in the patient's blood. The blood culture did not grow any Gram‐negative organisms. Gram‐positive cocci (coagulase‐negative staphylococci, S aureus [methicillin‐susceptible or MRSA]) are the most common causes of CLABSI.32 Can the physician de‐escalate antibiotic therapy in this patient with CLABSI based on the preliminary information? Yes. The information is solid enough to suggest removal of the catheter which was providing coverage for Gram‐negative bacteria (piperacillin/tazobactam), while continuing vancomycin for coverage of possible MRSA, pending further review, ie, until the Gram‐positive cocci are speciated. Rapid diagnostic methods, including polymerase chain reaction (PCR) and nucleic acid probes, can be used to provide more information about certain pathogens (such as MRSA33, 34) before final culture and susceptibility results are available, but these are not routinely available in many clinical microbiology laboratories. Furthermore, these newer technologies remain fairly expensive.
Revisiting the patient 1 day later (hospital day 7), after narrowing the initial combination antibiotic regimen to vancomycin monotherapy, the physical examination indicates the patient is clinically stable, with continued improvement in fever and WBC count (Figure 4). Blood culture analysis now isolates methicillin‐susceptible S aureus (MSSA). Methicillin resistance mediates resistance to all ‐lactams, including carbapenems, greatly limiting treatment options. Vancomycin is the most commonly utilized antibiotic for the treatment of MRSA, and the recent clinical practice guidelines from the IDSA recommend either vancomycin or daptomycin for management of MRSA bacteremia in adult patients.35 However, antistaphylococcal penicillins and first‐generation cephalosporins are the antibiotics of choice for MSSA infections, and particularly for MSSA bloodstream infections.

The activity provided by vancomycin (or daptomycin) is overly broad if MSSA is involved, and importantly, it is not as effective as treatment with an antistaphylococcal penicillin or first‐generation cephalosporin. A recent study by Stryjewski et al., of hemodialysis patients with MSSA bacteremia, reported a higher proportion of treatment failure with vancomycin versus first‐generation cephalosporin therapy (31% vs 13%; P = 0.02).36 Furthermore, multivariate analysis identified vancomycin (vs first‐generation cephalosporin) use as a significant independent predictor of treatment failure (odds ratio [OR], 3.53; 95% confidence interval [CI], 1.1513.45; P = 0.04). Similarly, Chang et al. reported nafcillin, an antistaphylococcal penicillin, was superior to vancomycin in preventing bacteriologic failure (persistent failure and/or relapse) in patients with MSSA bacteremia (0% vs 19%; P = 0.058), and used multivariate analysis to identify vancomycin as a significant independent predictor of relapse (OR, 6.5; 95% CI, 1.052.8; P 0.05).37 Another recent study by Lodise et al. reported that initial empiric therapy with vancomycin for endocarditis caused by MSSA was associated with a higher infection‐related mortality rate than initial empiric therapy with a ‐lactam‐containing regimen (39% vs 11%; P = 0.005).38 The negative impact of initial treatment with vancomycin persisted even in patients switched to a ‐lactam therapy after culture results became available.
Hence, if a patient is being treated with vancomycin for a bloodstream (or other) infection due to MSSA, the therapy is suboptimal. In such a scenariowhich corresponds to that for the case patient herevancomycin should be discontinued and replaced with an antistaphylococcal penicillin or first‐generation cephalosporin. Many times, clinicians are resistant to terminating vancomycin and de‐escalating to antistaphylococcal penicillin/first‐generation cephalosporin therapy in a patient with bacteremia who is apparently responding to vancomycin. However, as the studies just reviewed make clear, not only is vancomycin treatment overly broad for the circumstance, it is also suboptimal and does not represent best clinical practice or patient care. Furthermore, continuing vancomycin in this situation unnecessarily exposes the patient to possible renal toxicity, particularly when aggressive dosing or prolonged vancomycin treatment is involved.39 Because of these issues and concerns, case 3 was de‐escalated from vancomycin to cefazolin, a first‐generation cephalosporin. One word of caution, however, is that there is some controversy over using cefazolin in patients with S aureus native valve endocarditis, given the possibility of a Type A ‐lactamase‐producing species causing cefazolin degradation.40 As a result, the clinician should first rule out endocarditis in the patient here before proceeding with cefazolin therapy. Another alternative would be to use an antistaphylococcal penicillin, such as nafcillin.
Finally, when dealing with bacteremia, and particularly when dealing with a possible CLABSI, the issue of potential culture contamination needs to be seriously considered and answered. Treating an actual infection, not what appears to be an infection because of culture contamination, is particularly important when dealing with possible CLABSI, because coagulase‐negative staphylococci (CoNS) are the most common cause of these types of infections,32 and CoNS are also frequent blood‐culture contaminants.41 Therefore, one needs to determine whether a blood culture growing a CoNS represents true bacteremia or simply contaminationwhich will obviously impact de‐escalation decisions.
In addition, when determining whether a blood culture is truly positive and clinically significant, it is important to consider whether the isolated pathogens are unlikely to be contaminants, likely to be contaminants, or the situation is unclear. A 2000 study by Kim et al.42 suggested that, among patients with 2 positive blood cultures for CoNS, routine identification of CoNS species and genotyping selected isolates using pulsed‐field gel electrophoresis may improve the process of discriminating contaminants from pathogens. Various additional factors need to be weighed when trying to interpret CoNS blood culture results, including patient risk factors, presence of prosthetic devices, number of blood cultures and number positive, and the antimicrobial sensitivity patterns of different isolates. For example, if the sensitivity patterns of 2 CoNS strains isolated from a patient are the same, the likelihood is increased that they represent true pathogens rather than contaminants. Figure 5 presents a schematic of this general approach.42

CONCLUSIONS
De‐escalation is a critical component of antimicrobial stewardship. As the prevalence of antimicrobial resistance grows in the hospital and community, de‐escalation will have an increasingly important role in limiting the further emergence of antimicrobial resistance. Pneumonia, intra‐abdominal infection, and bloodstream infection are commonly managed in the hospital setting. Each of these infection types presents excellent opportunities for de‐escalation, and each presents unique challenges and caveats. Concerted efforts must be made by clinicians and stewardship personnel to de‐escalate as soon as possible, based on culture results and clinical status. Although not discussed here, successful de‐escalation programs utilize structured process, guidelines, and algorithms to consistently implement de‐escalation efforts. These tools of implementation are more fully discussed in the corresponding article in this supplement by Dr Rosenberg.
- .Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time.Drugs.2003;63:2157–2168.
- ,,, et al.Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study.J Antimicrob Chemother.2008;61:436–441.
- ,,,,,.Predictors of 30‐day mortality and hospital costs in patients with ventilator‐associated pneumonia attributed to potentially antibiotic‐resistant gram‐negative bacteria.Chest.2008;134:281–287.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,,,.Emergence of antibiotic‐resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy.Clin Infect Dis.1996;23:486–494.
- ,,,,.Antimicrobial therapy escalation and hospital mortality among patients with health‐care‐associated pneumonia: a single‐center experience.Chest.2008;134:963–968.
- ,.De‐escalation in lower respiratory tract infections.Curr Opin Pulm Med.2006;12:364–368.
- .The importance of de‐escalating antimicrobial therapy in patients with ventilator‐associated pneumonia.Semin Respir Crit Care Med.2006;27:45–50.
- .Impact of antibiotic resistance in gram‐negative bacilli on empirical and definitive antibiotic therapy.Clin Infect Dis.2008;47(suppl 1):S14–S20.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- Bad Bugs, No Drugs: As Antibiotic R46:155–164.
- ,,, et al.De‐escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate.Intensive Care Med.2007;33:1533–1540.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,, et al.Empiric broad‐spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study.Crit Care.2006;10:R78.
- ,,,.Does de‐escalation of antibiotic therapy for ventilator‐associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?J Trauma.2009;66:1343–1348.
- ,,, et al.Ventilator‐associated pneumonia: breaking the vicious circle of antibiotic overuse.Crit Care Med.2007;35:379–385; quiz 386.
- ,,,,.Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs.J Antimicrob Chemother.2006;57:326–330.
- ,,,,.Diagnostic yield of blood cultures from antibiotic‐naive and antibiotically treated patients with haematological malignancies and high‐risk neutropenia.Scand J Infect Dis.2009;41:650–655.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,.Molecular characteristics of extended‐spectrum beta‐lactamase‐producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX‐M‐15 in community hospitals.Int J Antimicrob Agents.2010;36:19–23.
- ,.Ertapenem: a review of its use in the treatment of bacterial infections.Drugs.2005;65:2151–2178.
- .Infections with extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: changing epidemiology and drug treatment choices.Drugs.2010;70:313–333.
- ,.Bloodstream infection in the ICU.Infect Dis Clin North Am.2009;23:557–569.
- ,,,,.Insights from the Society of Infectious Diseases Pharmacists on antimicrobial stewardship guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America.Pharmacotherapy.2009;29:593–607.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 Update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials.Clin Infect Dis.2008;47:83–93.
- ,.Catheter‐related bloodstream infections: catheter management according to pathogen.Int J Antimicrob Agents.2010;36(suppl 2):S26–S32.
- ,,,.Detection of methicillin‐resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth‐enriched culture in an area with a low prevalence of MRSA infections.J Clin Microbiol.2010;48:3882–3887.
- ,,,,.Clinical validation of the molecular BD GeneOhm StaphSR assay for direct detection of Staphylococcus aureus and methicillin‐resistant Staphylococcus aureus in positive blood cultures.J Clin Microbiol.2007;45:2191–2196.
- ,,, et al.Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children.Clin Infect Dis.2011;52:e18–e55.
- ,,, et al.Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia.Clin Infect Dis.2007;44:190–196.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,,,.Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus.Antimicrob Agents Chemother.2007;51:3731–3733.
- ,,,,.A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia.Clin Ther.2007;29:1107–1115.
- ,,.Relapse of type A beta‐lactamase‐producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue.Clin Infect Dis.2003;37:1194–1198.
- ,.The origin of coagulase‐negative staphylococci isolated from blood cultures.J Hosp Infect.1995;30:217–223.
- ,,, et al.Determining the significance of coagulase‐negative staphylococci isolated from blood cultures at a community hospital: a role for species and strain identification.Infect Control Hosp Epidemiol.2000;21:213–217.
- .Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time.Drugs.2003;63:2157–2168.
- ,,, et al.Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study.J Antimicrob Chemother.2008;61:436–441.
- ,,,,,.Predictors of 30‐day mortality and hospital costs in patients with ventilator‐associated pneumonia attributed to potentially antibiotic‐resistant gram‐negative bacteria.Chest.2008;134:281–287.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,,,.Emergence of antibiotic‐resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy.Clin Infect Dis.1996;23:486–494.
- ,,,,.Antimicrobial therapy escalation and hospital mortality among patients with health‐care‐associated pneumonia: a single‐center experience.Chest.2008;134:963–968.
- ,.De‐escalation in lower respiratory tract infections.Curr Opin Pulm Med.2006;12:364–368.
- .The importance of de‐escalating antimicrobial therapy in patients with ventilator‐associated pneumonia.Semin Respir Crit Care Med.2006;27:45–50.
- .Impact of antibiotic resistance in gram‐negative bacilli on empirical and definitive antibiotic therapy.Clin Infect Dis.2008;47(suppl 1):S14–S20.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- Bad Bugs, No Drugs: As Antibiotic R46:155–164.
- ,,, et al.De‐escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate.Intensive Care Med.2007;33:1533–1540.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,, et al.Empiric broad‐spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study.Crit Care.2006;10:R78.
- ,,,.Does de‐escalation of antibiotic therapy for ventilator‐associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?J Trauma.2009;66:1343–1348.
- ,,, et al.Ventilator‐associated pneumonia: breaking the vicious circle of antibiotic overuse.Crit Care Med.2007;35:379–385; quiz 386.
- ,,,,.Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs.J Antimicrob Chemother.2006;57:326–330.
- ,,,,.Diagnostic yield of blood cultures from antibiotic‐naive and antibiotically treated patients with haematological malignancies and high‐risk neutropenia.Scand J Infect Dis.2009;41:650–655.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,, et al.Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America.Clin Infect Dis.2010;50:133–164.
- ,,.Molecular characteristics of extended‐spectrum beta‐lactamase‐producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX‐M‐15 in community hospitals.Int J Antimicrob Agents.2010;36:19–23.
- ,.Ertapenem: a review of its use in the treatment of bacterial infections.Drugs.2005;65:2151–2178.
- .Infections with extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae: changing epidemiology and drug treatment choices.Drugs.2010;70:313–333.
- ,.Bloodstream infection in the ICU.Infect Dis Clin North Am.2009;23:557–569.
- ,,,,.Insights from the Society of Infectious Diseases Pharmacists on antimicrobial stewardship guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America.Pharmacotherapy.2009;29:593–607.
- ,,, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 Update by the Infectious Diseases Society of America.Clin Infect Dis.2009;49:1–45.
- ,,,,,.Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials.Clin Infect Dis.2008;47:83–93.
- ,.Catheter‐related bloodstream infections: catheter management according to pathogen.Int J Antimicrob Agents.2010;36(suppl 2):S26–S32.
- ,,,.Detection of methicillin‐resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth‐enriched culture in an area with a low prevalence of MRSA infections.J Clin Microbiol.2010;48:3882–3887.
- ,,,,.Clinical validation of the molecular BD GeneOhm StaphSR assay for direct detection of Staphylococcus aureus and methicillin‐resistant Staphylococcus aureus in positive blood cultures.J Clin Microbiol.2007;45:2191–2196.
- ,,, et al.Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children.Clin Infect Dis.2011;52:e18–e55.
- ,,, et al.Use of vancomycin or first‐generation cephalosporins for the treatment of hemodialysis‐dependent patients with methicillin‐susceptible Staphylococcus aureus bacteremia.Clin Infect Dis.2007;44:190–196.
- ,,, et al.Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.Medicine (Baltimore).2003;82:333–339.
- ,,,.Impact of empirical‐therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin‐susceptible Staphylococcus aureus.Antimicrob Agents Chemother.2007;51:3731–3733.
- ,,,,.A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia.Clin Ther.2007;29:1107–1115.
- ,,.Relapse of type A beta‐lactamase‐producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue.Clin Infect Dis.2003;37:1194–1198.
- ,.The origin of coagulase‐negative staphylococci isolated from blood cultures.J Hosp Infect.1995;30:217–223.
- ,,, et al.Determining the significance of coagulase‐negative staphylococci isolated from blood cultures at a community hospital: a role for species and strain identification.Infect Control Hosp Epidemiol.2000;21:213–217.