User login
Verify Your Liability Coverage before Taking that New Job
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at [email protected].
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at [email protected].
Does your employer provide your medical malpractice insurance coverage? Are you looking for new employment? Are you in the market to purchase a professional malpractice insurance policy? Are you planning to retire soon?
If you answered “yes” to any of these questions, you likely will confront the concept of “tail” insurance at some point in your medical career.
Now is the time to dust off your employment agreement and professional liability insurance policy and review what happens in the event a lawsuit is filed against you after you leave your current employer. This means paying special attention to whether your professional liability insurance policy provides for claims-made or occurrence-based coverage, and, if it’s the former, who is responsible for purchasing tail coverage.
When Do I Need Tail Coverage?
Tail insurance issues frequently arise when a physician leaves his or her place of employment, whether due to switching jobs, retirement, or a buyout of a physician’s ownership interest. If the physician is leaving an employer that has claims-made professional liability insurance, the physician’s insurance coverage might not be seamless. Instead, tail or similar coverage is required.
Claims-made coverage protects a physician for professional negligence, as long as a two-part test is met: First, the physician must have the claims-made coverage in place when the negligent act occurs (with employer No. 1); second, the physician must be covered by the same carrier when he or she is notified of the claim while employed by employer No. 2. If either test is not satisfied, the current claims-made insurance policy will not provide coverage to the physician in the event a lawsuit is filed for an act of negligence that took place while employed by employer No. 1. Alternatively, some employers offer “nose” coverage from its insurance carrier, which will cover negligent acts that might have occurred during your current job. The vast majority of professional liability insurance policies written for medical practice groups are for claims-made coverage.
If, however, an employer has occurrence-based professional liability insurance, the departing physician’s insurance coverage is seamless and no tail insurance is required.
Example A
Here is a common example of what happens when a physician leaves an employer with claims-made professional liability coverage:
An employer maintains claims-made professional liability insurance coverage for its physicians with ABC Insurance Co. A physician decides to leave his or her current employer and accepts employment by a new employer, which maintains claims-made coverage with XYZ Insurance Co.
Within a few months of the physician’s new employment, a medical malpractice lawsuit is filed by a patient for medical treatment the patient received when the physician was employed by the former employer. By leaving the former employer, the departing physician automatically fails the two-part test for claims-made coverage, as the second prong is not satisfied. Therefore, even though the physician has liability coverage through the new employer, this insurance policy will not cover the lawsuit described above.
Unless the physician has tail insurance (or nose coverage) to cover lawsuits related to the former employment, a gap in liability coverage will exist. If claims-made insurance is the benefit you have received in your employment agreement, it is imperative that you understand that tail coverage is necessary when you leave.
However, if a physician leaves and a) is subsequently employed within the same state and b) stays insured by the same insurance carrier, then the insurance carrier will provide continuous coverage and no tail insurance policy is needed.
Who Pays the Premium?
If the physician will need tail coverage, the next critical question is, Who pays for such coverage? Even though tail coverage comes into effect when a physician leaves an employer, tail coverage should be addressed before the physician informs the employer of their departure; an even better approach would be while the employment agreement is negotiated. Payment of tail coverage should be defined in the physician’s employment agreement.
In terms of payment for the coverage, there are several options. First, the cost of tail coverage can be attributed 100% to either physician or employer. In specialties for which recruitment of new physicians is challenging (i.e. HM), employers are more likely to pay a substantial portion, if not all, of the cost as a benefit or inducement.
Second, the physician can connect the payment of tail coverage to the manner in which employment is terminated. For example, if the physician terminates the agreement for cause or if the employer terminates the physician’s employment without cause, the employer could be required to pay for the tail insurance. Alternatively, if the physician terminates the agreement without cause or if the employer terminates the physician’s employment with cause, the physician could be required to pay for the tail coverage. Frequently, physician employment agreements require physicians to pay for tail coverage if the physician violates a restrictive covenant (e.g. non-competition).
A third option is to split the cost of tail insurance between the former employer and the physician based on a percentage, or to include a vesting schedule, for example, such that the former employer pays one-third of the coverage if employment ends in the second year, two-thirds of the coverage if employment ends in the third year, and 100% of the coverage if employment ends in the fourth year or later.
Whatever arrangement the parties agree upon should be included in the physician’s employment agreement in order to prevent an expensive surprise.
Review Your Policy
Now that you have an understanding of claims-made coverage, occurrence-based coverage and tail insurance, it’s time to review your insurance policy. When reviewing your current policy, look for answers to the following important questions:
- Is your policy claims-made or occurrence-based?
- Does your insurance policy only cover professional negligence claims? Does your policy also cover claims of unprofessional conduct reported to state medical licensing boards? Does your policy also cover medical staff bylaw disputes and state licensing matters?
- How is loss defined? “Pure loss” is coverage for the amount awarded to the plaintiff; “ultimate net loss” covers what pure loss covers, plus attorneys’ fees and costs.
- What procedures do you need to follow in order to properly notify the insurance carrier of a claim? Are you precluded from full coverage if you fail to properly report?
- What does the “duty to defend” provision cover? Will you be reimbursed for lost wages for your time in court? What services will be provided as part of your defense?
- What does the “consent to settle” provision say? If a settlement is negotiated between the plaintiff (patient) and the insurance company and the physician does not consent to the settlement, is the physician responsible for the ongoing defense costs and the amount of any verdict in excess of the recommended settlement amount?
It is important to both understand your insurance policy and what your employment agreement says about the policy. If you will be responsible for purchasing a tail policy at the end of your current employment, you should be well aware—and financially prepared—for this post-employment responsibility. Make sure your tail is not left exposed.
Steven M. Harris, Esq., is a nationally recognized healthcare attorney and a member of the law firm McDonald Hopkins LLC in Chicago. Write to him at [email protected].
Nigerian-Born Hospitalist Steers Career Down Path of Administrative Challenges
In some ways, Femi Adewunmi, MD, MBA, CPE, SFHM, seemed destined to become a physician. He grew up in a medical family—his mother is an orthodontist; his father is an obstetrician/gynecologist. As a child, he often spent holidays visiting patients at the hospital where his dad worked. He grew to appreciate medicine as a noble profession, and when he reached his teens, he never seriously considered another career path.
“There were times when I was in medical school, dreading having to study for the numerous tests and exams, when I wished I had someone I could have blamed my decision to go to medical school on,” says Dr. Adewunmi, a native of Nigeria who has practiced as a hospitalist in the U.S. since 2003. “But no one pushed me to do it. It was something I always looked forward to doing, and I’m very glad I stuck with it.”
Dr. Adewunmi has only become more passionate about his work since then. His experience as a front-line hospitalist laid the foundation for a series of leadership roles, first directing the HM program at Johnston Memorial Hospital in Smithfield, N.C., and now as regional chief medical officer for Sound Physicians, which provides inpatient services to more than 70 hospitals nationally.
“I really want to be a good physician executive,” he says. “It’s definitely a case of ‘The more you learn, the more you realize how little you know.’ I still have a lot to learn, but I’m looking forward to the challenge.”
When did you decide to go into HM?
During residency, I realized I loved taking care of patients in the hospital, both along the wards and in the ICU. I enjoyed my outpatient clinics but found myself looking for any reason I could to stay in the hospital caring for patients. I was interested in patient safety and I was doing a little bit of utilization review, so I also felt it would give me a great overall perspective of the healthcare system.
What about leading the hospitalist program at Johnston Memorial appealed to you?
I enjoyed clinical medicine, and I still do, but I was looking to do more. I wanted to make an impact at a systems level, and I knew, to do that, I eventually had to gain some leadership experience.
What is the most valuable lesson you learned in that role?
Understanding that change doesn’t happen instantaneously. For instance, as a clinician, you sometimes admit patients with congestive heart failure. You diagnose correctly, treat appropriately, and in a few days, the patients do better and go home. You get pretty swift gratification. Administration is much different. You put processes in place and it could take weeks or several quarters before you start to see the effects of the changes you implement.
What appealed to you about moving from a single-site leadership position at Johnston to a regional position with Sound?
I wanted to continue evolving. I wanted more of a challenge and was seeking opportunities where I would have operational responsibility—overseeing performance improvement in quality, satisfaction, and financial performance for several programs. In addition, I wanted to be accountable for physician development, recruiting, negotiations, and the whole gamut of business development. It was the next logical step in my career.
Why did you pursue an MBA?
I’d made that decision just before I got into medical school. I recall first thinking about it after a conversation I had with my father as a teenager. When I told him that I had made up my mind to study medicine, he said, “You should consider getting an MBA as well. Your generation is going to need to have business experience and expertise, and be better in that area than our generation was.” It’s been invaluable for me in terms of preparing me for handling the business side of medicine, including ways to make operations more efficient and to reduce costs without compromising the quality of care provided.
You have worked in both hospital-employed and privately contracted HM programs. Do you prefer one model?
In general, the larger organizations tend to have an advantage in that they have established protocols and processes that work and have been refined over time. Couple that with the economies of scale they enjoy, as we move into an era of value-based purchasing, it’s becoming harder for the smaller community-based hospital to do that as well. That said, I have seen local hospital-run programs that function really well and have administrative support, so there is definitely enough room for both models.
You were in the inaugural FHM class. What did that recognition mean to you?
I saw it as validation of how we were starting to mature as a specialty and as recognition of a commitment to being a hospitalist, not just an internist. I never practiced outpatient medicine. I went straight from residency to hospitalist medicine. That’s how I identify myself, and I was happy to see that physicians specializing in hospital medicine were starting to get recognized.
What is your biggest professional reward?
The satisfaction from knowing you’re making a difference—not just by the care you provide one-on-one to your patients, but also knowing you’re contributing at a systems level or a population level because you’re making decisions and trying to redefine processes that actually could impact a much larger cohort.
What is your biggest professional challenge?
Trying to find enough hours in the day to do all that needs to be done.
What is next for you professionally?
I enjoy having varied opportunities and being involved in many different aspects of operations. That’s what attracted me to a larger company such as Sound Physicians, and I see myself staying in that type of role. Down the road, I’d love to be able to take some of my knowledge to Nigeria and find a way to help develop and shape the healthcare sector back home.
Why would that mean so much to you?
It would be a chance to give back. We still have people dying from largely preventable diseases, and our healthcare system is not what it should be. We don’t have enough physicians for the population, and most of the physicians are in urban areas.
Close to half of the members of my graduating medical school class are either in the U.S., Europe, Asia, or South Africa.
That type of brain drain has a tremendous effect over several decades. That’s a lot of talent outside the country, and we need that back home.
Mark Leiser is a freelance writer in New Jersey.
In some ways, Femi Adewunmi, MD, MBA, CPE, SFHM, seemed destined to become a physician. He grew up in a medical family—his mother is an orthodontist; his father is an obstetrician/gynecologist. As a child, he often spent holidays visiting patients at the hospital where his dad worked. He grew to appreciate medicine as a noble profession, and when he reached his teens, he never seriously considered another career path.
“There were times when I was in medical school, dreading having to study for the numerous tests and exams, when I wished I had someone I could have blamed my decision to go to medical school on,” says Dr. Adewunmi, a native of Nigeria who has practiced as a hospitalist in the U.S. since 2003. “But no one pushed me to do it. It was something I always looked forward to doing, and I’m very glad I stuck with it.”
Dr. Adewunmi has only become more passionate about his work since then. His experience as a front-line hospitalist laid the foundation for a series of leadership roles, first directing the HM program at Johnston Memorial Hospital in Smithfield, N.C., and now as regional chief medical officer for Sound Physicians, which provides inpatient services to more than 70 hospitals nationally.
“I really want to be a good physician executive,” he says. “It’s definitely a case of ‘The more you learn, the more you realize how little you know.’ I still have a lot to learn, but I’m looking forward to the challenge.”
When did you decide to go into HM?
During residency, I realized I loved taking care of patients in the hospital, both along the wards and in the ICU. I enjoyed my outpatient clinics but found myself looking for any reason I could to stay in the hospital caring for patients. I was interested in patient safety and I was doing a little bit of utilization review, so I also felt it would give me a great overall perspective of the healthcare system.
What about leading the hospitalist program at Johnston Memorial appealed to you?
I enjoyed clinical medicine, and I still do, but I was looking to do more. I wanted to make an impact at a systems level, and I knew, to do that, I eventually had to gain some leadership experience.
What is the most valuable lesson you learned in that role?
Understanding that change doesn’t happen instantaneously. For instance, as a clinician, you sometimes admit patients with congestive heart failure. You diagnose correctly, treat appropriately, and in a few days, the patients do better and go home. You get pretty swift gratification. Administration is much different. You put processes in place and it could take weeks or several quarters before you start to see the effects of the changes you implement.
What appealed to you about moving from a single-site leadership position at Johnston to a regional position with Sound?
I wanted to continue evolving. I wanted more of a challenge and was seeking opportunities where I would have operational responsibility—overseeing performance improvement in quality, satisfaction, and financial performance for several programs. In addition, I wanted to be accountable for physician development, recruiting, negotiations, and the whole gamut of business development. It was the next logical step in my career.
Why did you pursue an MBA?
I’d made that decision just before I got into medical school. I recall first thinking about it after a conversation I had with my father as a teenager. When I told him that I had made up my mind to study medicine, he said, “You should consider getting an MBA as well. Your generation is going to need to have business experience and expertise, and be better in that area than our generation was.” It’s been invaluable for me in terms of preparing me for handling the business side of medicine, including ways to make operations more efficient and to reduce costs without compromising the quality of care provided.
You have worked in both hospital-employed and privately contracted HM programs. Do you prefer one model?
In general, the larger organizations tend to have an advantage in that they have established protocols and processes that work and have been refined over time. Couple that with the economies of scale they enjoy, as we move into an era of value-based purchasing, it’s becoming harder for the smaller community-based hospital to do that as well. That said, I have seen local hospital-run programs that function really well and have administrative support, so there is definitely enough room for both models.
You were in the inaugural FHM class. What did that recognition mean to you?
I saw it as validation of how we were starting to mature as a specialty and as recognition of a commitment to being a hospitalist, not just an internist. I never practiced outpatient medicine. I went straight from residency to hospitalist medicine. That’s how I identify myself, and I was happy to see that physicians specializing in hospital medicine were starting to get recognized.
What is your biggest professional reward?
The satisfaction from knowing you’re making a difference—not just by the care you provide one-on-one to your patients, but also knowing you’re contributing at a systems level or a population level because you’re making decisions and trying to redefine processes that actually could impact a much larger cohort.
What is your biggest professional challenge?
Trying to find enough hours in the day to do all that needs to be done.
What is next for you professionally?
I enjoy having varied opportunities and being involved in many different aspects of operations. That’s what attracted me to a larger company such as Sound Physicians, and I see myself staying in that type of role. Down the road, I’d love to be able to take some of my knowledge to Nigeria and find a way to help develop and shape the healthcare sector back home.
Why would that mean so much to you?
It would be a chance to give back. We still have people dying from largely preventable diseases, and our healthcare system is not what it should be. We don’t have enough physicians for the population, and most of the physicians are in urban areas.
Close to half of the members of my graduating medical school class are either in the U.S., Europe, Asia, or South Africa.
That type of brain drain has a tremendous effect over several decades. That’s a lot of talent outside the country, and we need that back home.
Mark Leiser is a freelance writer in New Jersey.
In some ways, Femi Adewunmi, MD, MBA, CPE, SFHM, seemed destined to become a physician. He grew up in a medical family—his mother is an orthodontist; his father is an obstetrician/gynecologist. As a child, he often spent holidays visiting patients at the hospital where his dad worked. He grew to appreciate medicine as a noble profession, and when he reached his teens, he never seriously considered another career path.
“There were times when I was in medical school, dreading having to study for the numerous tests and exams, when I wished I had someone I could have blamed my decision to go to medical school on,” says Dr. Adewunmi, a native of Nigeria who has practiced as a hospitalist in the U.S. since 2003. “But no one pushed me to do it. It was something I always looked forward to doing, and I’m very glad I stuck with it.”
Dr. Adewunmi has only become more passionate about his work since then. His experience as a front-line hospitalist laid the foundation for a series of leadership roles, first directing the HM program at Johnston Memorial Hospital in Smithfield, N.C., and now as regional chief medical officer for Sound Physicians, which provides inpatient services to more than 70 hospitals nationally.
“I really want to be a good physician executive,” he says. “It’s definitely a case of ‘The more you learn, the more you realize how little you know.’ I still have a lot to learn, but I’m looking forward to the challenge.”
When did you decide to go into HM?
During residency, I realized I loved taking care of patients in the hospital, both along the wards and in the ICU. I enjoyed my outpatient clinics but found myself looking for any reason I could to stay in the hospital caring for patients. I was interested in patient safety and I was doing a little bit of utilization review, so I also felt it would give me a great overall perspective of the healthcare system.
What about leading the hospitalist program at Johnston Memorial appealed to you?
I enjoyed clinical medicine, and I still do, but I was looking to do more. I wanted to make an impact at a systems level, and I knew, to do that, I eventually had to gain some leadership experience.
What is the most valuable lesson you learned in that role?
Understanding that change doesn’t happen instantaneously. For instance, as a clinician, you sometimes admit patients with congestive heart failure. You diagnose correctly, treat appropriately, and in a few days, the patients do better and go home. You get pretty swift gratification. Administration is much different. You put processes in place and it could take weeks or several quarters before you start to see the effects of the changes you implement.
What appealed to you about moving from a single-site leadership position at Johnston to a regional position with Sound?
I wanted to continue evolving. I wanted more of a challenge and was seeking opportunities where I would have operational responsibility—overseeing performance improvement in quality, satisfaction, and financial performance for several programs. In addition, I wanted to be accountable for physician development, recruiting, negotiations, and the whole gamut of business development. It was the next logical step in my career.
Why did you pursue an MBA?
I’d made that decision just before I got into medical school. I recall first thinking about it after a conversation I had with my father as a teenager. When I told him that I had made up my mind to study medicine, he said, “You should consider getting an MBA as well. Your generation is going to need to have business experience and expertise, and be better in that area than our generation was.” It’s been invaluable for me in terms of preparing me for handling the business side of medicine, including ways to make operations more efficient and to reduce costs without compromising the quality of care provided.
You have worked in both hospital-employed and privately contracted HM programs. Do you prefer one model?
In general, the larger organizations tend to have an advantage in that they have established protocols and processes that work and have been refined over time. Couple that with the economies of scale they enjoy, as we move into an era of value-based purchasing, it’s becoming harder for the smaller community-based hospital to do that as well. That said, I have seen local hospital-run programs that function really well and have administrative support, so there is definitely enough room for both models.
You were in the inaugural FHM class. What did that recognition mean to you?
I saw it as validation of how we were starting to mature as a specialty and as recognition of a commitment to being a hospitalist, not just an internist. I never practiced outpatient medicine. I went straight from residency to hospitalist medicine. That’s how I identify myself, and I was happy to see that physicians specializing in hospital medicine were starting to get recognized.
What is your biggest professional reward?
The satisfaction from knowing you’re making a difference—not just by the care you provide one-on-one to your patients, but also knowing you’re contributing at a systems level or a population level because you’re making decisions and trying to redefine processes that actually could impact a much larger cohort.
What is your biggest professional challenge?
Trying to find enough hours in the day to do all that needs to be done.
What is next for you professionally?
I enjoy having varied opportunities and being involved in many different aspects of operations. That’s what attracted me to a larger company such as Sound Physicians, and I see myself staying in that type of role. Down the road, I’d love to be able to take some of my knowledge to Nigeria and find a way to help develop and shape the healthcare sector back home.
Why would that mean so much to you?
It would be a chance to give back. We still have people dying from largely preventable diseases, and our healthcare system is not what it should be. We don’t have enough physicians for the population, and most of the physicians are in urban areas.
Close to half of the members of my graduating medical school class are either in the U.S., Europe, Asia, or South Africa.
That type of brain drain has a tremendous effect over several decades. That’s a lot of talent outside the country, and we need that back home.
Mark Leiser is a freelance writer in New Jersey.
Time-based billing allows hospitalists to avoid
Providers typically rely on the “key components” (history, exam, medical decision-making) when documenting in the medical record. However, there are instances when the majority of the encounter constitutes counseling/coordination of care (C/CC). Physicians might only document a brief history and exam, or nothing at all. Utilizing time-based billing principles allows a physician to disregard the “key component” requirements and select a visit level reflective of this effort.
For example, a 64-year-old female is hospitalized with newly diagnosed diabetes and requires extensive counseling regarding disease management, lifestyle modification, and medication regime, as well as coordination of care for outpatient programs and services. The hospitalist reviews some of the pertinent information with the patient and leaves the room to coordinate the patient’s ongoing care (25 minutes). The hospitalist then asks a resident to assist with the remaining counseling efforts (20 minutes). Code 99232 (inpatient visit, 25 minutes total visit time) would be appropriate to report.
Counseling, Coordination of Care
Time may be used as the determining factor for the visit level, if more than 50% of the total visit time involves C/CC.1 Time is not used for visit-level selection if C/CC is minimal or absent from the patient encounter. Total visit time is acknowledged as the physician’s face-to-face (i.e. bedside) time combined with time spent on the unit/floor reviewing data, obtaining relevant patient information, and discussing the individual case with other involved healthcare providers.
Time associated with activities performed outside of the patient’s unit/floor is not considered when calculating total visit time. Time associated with teaching students/interns also is excluded; only the attending physician’s time counts.
When the requirements have been met, the physician selects the visit level that corresponds with the documented total visit time (see Table 1). In the scenario above, the visit level is chosen based on the attending physician’s documented time (25 minutes). The resident’s time cannot be included.
Documentation Requirements
Physicians must document the interaction during the patient encounter: history and exam, if updated or performed; discussion points; and patient response, if applicable. The medical record entry must contain both the C/CC time and the total visit time.2 “Total visit time=35 minutes; >50% spent counseling/coordinating care” or “20 of 35 minutes spent counseling/coordinating care.”
A payor may prefer one documentation style over another. It is always best to ask about the payor’s policy and review local documentation standards to ensure compliance.
Family Discussions
Physicians are always involved in family discussions. It is appropriate to count this as C/CC time. In the event that the family discussion takes place without the patient present, only count this as C/CC time if:
- The patient is unable or clinically incompetent to participate in discussions;
- The time is spent on the unit/floor with the family members or surrogate decision-makers obtaining a medical history, reviewing the patient’s condition or prognosis, or discussing treatment or limitation(s) of treatment; and
- The conversation bears directly on the management of the patient.4
The medical record should reflect these criteria. Do not consider the time if the discussion takes place in an area outside of the patient’s unit/floor, or if the time is spent counseling family members through their grieving process.
It is not uncommon for the family discussion to take place later in the day, after the physician has made earlier rounds. If the earlier encounter involved C/CC, the physician would report the cumulative time spent for that service date. If the earlier encounter was a typical patient evaluation (i.e. history update and physical) and management service (i.e. care plan review/revision), this second encounter might be regarded as a prolonged care service.
Prolonged Care
Prolonged care codes exist for both outpatient and inpatient services. A hospitalists’ focus involves the inpatient code series:
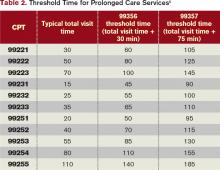
99356: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, first hour; and
99357: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, each additional 30 minutes.
Code 99356 is reported during the first hour of prolonged services, after the initial 30 minutes is reached; code 99357 is reported for each additional 30 minutes of prolonged care beyond the first hour, after the first 15 minutes of each additional segment. Both are “add on” codes and cannot be reported alone on a claim form; a “primary” code must be reported. Similarly, 99357 cannot be reported without 99356, and 99356 must be reported with one of the following inpatient service (primary) codes: 99218-99220, 99221-99223, 99231-99233, 99251-99255, 99304-99310. Only one unit of 99356 may be reported per patient per physician group per day, whereas multiple units of 99357 may be reported in a single day.
The CPT definition of prolonged care varies from that of the Centers for Medicare & Medicaid Services (CMS). Since 2009, CPT recognizes the total duration spent by a physician on a given date, even if the time spent by the physician on that date is not continuous; the time involves both face-to-face time and unit/floor time.5 CMS only attributes direct face-to-face time between the physician and the patient toward prolonged care billing. Time spent reviewing charts or discussion of a patient with house medical staff, waiting for test results, waiting for changes in the patient’s condition, waiting for end of a therapy session, or waiting for use of facilities cannot be billed as prolonged services.5 This is in direct opposition to its policy for C/CC services, and makes prolonged care services inefficient.
Medicare also identifies “threshold” time (see Table 2). The total physician visit time must exceed the time requirements associated with the “primary” codes by a 30-minute threshold (e.g. 99221+99356=30 minutes+30 minutes=60 minutes threshold time). The physician must document the total face-to-face time spent in separate notes throughout the day or, more realistically, in one cumulative note.
When two providers from the same group and same specialty perform services on the same date (e.g. physician A saw the patient during morning rounds, and physician B spoke with the patient/family in the afternoon), only one physician can report the cumulative service.6 As always, query payors for coverage, because some non-Medicare insurers do not recognize these codes.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare National Coverage Determinations Manual: Chapter 1, Section 70.1. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/ncd103c1_Part1.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.15.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011:7-21.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
Providers typically rely on the “key components” (history, exam, medical decision-making) when documenting in the medical record. However, there are instances when the majority of the encounter constitutes counseling/coordination of care (C/CC). Physicians might only document a brief history and exam, or nothing at all. Utilizing time-based billing principles allows a physician to disregard the “key component” requirements and select a visit level reflective of this effort.
For example, a 64-year-old female is hospitalized with newly diagnosed diabetes and requires extensive counseling regarding disease management, lifestyle modification, and medication regime, as well as coordination of care for outpatient programs and services. The hospitalist reviews some of the pertinent information with the patient and leaves the room to coordinate the patient’s ongoing care (25 minutes). The hospitalist then asks a resident to assist with the remaining counseling efforts (20 minutes). Code 99232 (inpatient visit, 25 minutes total visit time) would be appropriate to report.
Counseling, Coordination of Care
Time may be used as the determining factor for the visit level, if more than 50% of the total visit time involves C/CC.1 Time is not used for visit-level selection if C/CC is minimal or absent from the patient encounter. Total visit time is acknowledged as the physician’s face-to-face (i.e. bedside) time combined with time spent on the unit/floor reviewing data, obtaining relevant patient information, and discussing the individual case with other involved healthcare providers.
Time associated with activities performed outside of the patient’s unit/floor is not considered when calculating total visit time. Time associated with teaching students/interns also is excluded; only the attending physician’s time counts.
When the requirements have been met, the physician selects the visit level that corresponds with the documented total visit time (see Table 1). In the scenario above, the visit level is chosen based on the attending physician’s documented time (25 minutes). The resident’s time cannot be included.
Documentation Requirements
Physicians must document the interaction during the patient encounter: history and exam, if updated or performed; discussion points; and patient response, if applicable. The medical record entry must contain both the C/CC time and the total visit time.2 “Total visit time=35 minutes; >50% spent counseling/coordinating care” or “20 of 35 minutes spent counseling/coordinating care.”
A payor may prefer one documentation style over another. It is always best to ask about the payor’s policy and review local documentation standards to ensure compliance.
Family Discussions
Physicians are always involved in family discussions. It is appropriate to count this as C/CC time. In the event that the family discussion takes place without the patient present, only count this as C/CC time if:
- The patient is unable or clinically incompetent to participate in discussions;
- The time is spent on the unit/floor with the family members or surrogate decision-makers obtaining a medical history, reviewing the patient’s condition or prognosis, or discussing treatment or limitation(s) of treatment; and
- The conversation bears directly on the management of the patient.4
The medical record should reflect these criteria. Do not consider the time if the discussion takes place in an area outside of the patient’s unit/floor, or if the time is spent counseling family members through their grieving process.
It is not uncommon for the family discussion to take place later in the day, after the physician has made earlier rounds. If the earlier encounter involved C/CC, the physician would report the cumulative time spent for that service date. If the earlier encounter was a typical patient evaluation (i.e. history update and physical) and management service (i.e. care plan review/revision), this second encounter might be regarded as a prolonged care service.
Prolonged Care
Prolonged care codes exist for both outpatient and inpatient services. A hospitalists’ focus involves the inpatient code series:
99356: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, first hour; and
99357: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, each additional 30 minutes.
Code 99356 is reported during the first hour of prolonged services, after the initial 30 minutes is reached; code 99357 is reported for each additional 30 minutes of prolonged care beyond the first hour, after the first 15 minutes of each additional segment. Both are “add on” codes and cannot be reported alone on a claim form; a “primary” code must be reported. Similarly, 99357 cannot be reported without 99356, and 99356 must be reported with one of the following inpatient service (primary) codes: 99218-99220, 99221-99223, 99231-99233, 99251-99255, 99304-99310. Only one unit of 99356 may be reported per patient per physician group per day, whereas multiple units of 99357 may be reported in a single day.
The CPT definition of prolonged care varies from that of the Centers for Medicare & Medicaid Services (CMS). Since 2009, CPT recognizes the total duration spent by a physician on a given date, even if the time spent by the physician on that date is not continuous; the time involves both face-to-face time and unit/floor time.5 CMS only attributes direct face-to-face time between the physician and the patient toward prolonged care billing. Time spent reviewing charts or discussion of a patient with house medical staff, waiting for test results, waiting for changes in the patient’s condition, waiting for end of a therapy session, or waiting for use of facilities cannot be billed as prolonged services.5 This is in direct opposition to its policy for C/CC services, and makes prolonged care services inefficient.
Medicare also identifies “threshold” time (see Table 2). The total physician visit time must exceed the time requirements associated with the “primary” codes by a 30-minute threshold (e.g. 99221+99356=30 minutes+30 minutes=60 minutes threshold time). The physician must document the total face-to-face time spent in separate notes throughout the day or, more realistically, in one cumulative note.
When two providers from the same group and same specialty perform services on the same date (e.g. physician A saw the patient during morning rounds, and physician B spoke with the patient/family in the afternoon), only one physician can report the cumulative service.6 As always, query payors for coverage, because some non-Medicare insurers do not recognize these codes.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare National Coverage Determinations Manual: Chapter 1, Section 70.1. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/ncd103c1_Part1.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.15.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011:7-21.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
Providers typically rely on the “key components” (history, exam, medical decision-making) when documenting in the medical record. However, there are instances when the majority of the encounter constitutes counseling/coordination of care (C/CC). Physicians might only document a brief history and exam, or nothing at all. Utilizing time-based billing principles allows a physician to disregard the “key component” requirements and select a visit level reflective of this effort.
For example, a 64-year-old female is hospitalized with newly diagnosed diabetes and requires extensive counseling regarding disease management, lifestyle modification, and medication regime, as well as coordination of care for outpatient programs and services. The hospitalist reviews some of the pertinent information with the patient and leaves the room to coordinate the patient’s ongoing care (25 minutes). The hospitalist then asks a resident to assist with the remaining counseling efforts (20 minutes). Code 99232 (inpatient visit, 25 minutes total visit time) would be appropriate to report.
Counseling, Coordination of Care
Time may be used as the determining factor for the visit level, if more than 50% of the total visit time involves C/CC.1 Time is not used for visit-level selection if C/CC is minimal or absent from the patient encounter. Total visit time is acknowledged as the physician’s face-to-face (i.e. bedside) time combined with time spent on the unit/floor reviewing data, obtaining relevant patient information, and discussing the individual case with other involved healthcare providers.
Time associated with activities performed outside of the patient’s unit/floor is not considered when calculating total visit time. Time associated with teaching students/interns also is excluded; only the attending physician’s time counts.
When the requirements have been met, the physician selects the visit level that corresponds with the documented total visit time (see Table 1). In the scenario above, the visit level is chosen based on the attending physician’s documented time (25 minutes). The resident’s time cannot be included.
Documentation Requirements
Physicians must document the interaction during the patient encounter: history and exam, if updated or performed; discussion points; and patient response, if applicable. The medical record entry must contain both the C/CC time and the total visit time.2 “Total visit time=35 minutes; >50% spent counseling/coordinating care” or “20 of 35 minutes spent counseling/coordinating care.”
A payor may prefer one documentation style over another. It is always best to ask about the payor’s policy and review local documentation standards to ensure compliance.
Family Discussions
Physicians are always involved in family discussions. It is appropriate to count this as C/CC time. In the event that the family discussion takes place without the patient present, only count this as C/CC time if:
- The patient is unable or clinically incompetent to participate in discussions;
- The time is spent on the unit/floor with the family members or surrogate decision-makers obtaining a medical history, reviewing the patient’s condition or prognosis, or discussing treatment or limitation(s) of treatment; and
- The conversation bears directly on the management of the patient.4
The medical record should reflect these criteria. Do not consider the time if the discussion takes place in an area outside of the patient’s unit/floor, or if the time is spent counseling family members through their grieving process.
It is not uncommon for the family discussion to take place later in the day, after the physician has made earlier rounds. If the earlier encounter involved C/CC, the physician would report the cumulative time spent for that service date. If the earlier encounter was a typical patient evaluation (i.e. history update and physical) and management service (i.e. care plan review/revision), this second encounter might be regarded as a prolonged care service.
Prolonged Care
Prolonged care codes exist for both outpatient and inpatient services. A hospitalists’ focus involves the inpatient code series:
99356: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, first hour; and
99357: Prolonged service in the inpatient or observation setting, requiring unit/floor time beyond the usual service, each additional 30 minutes.
Code 99356 is reported during the first hour of prolonged services, after the initial 30 minutes is reached; code 99357 is reported for each additional 30 minutes of prolonged care beyond the first hour, after the first 15 minutes of each additional segment. Both are “add on” codes and cannot be reported alone on a claim form; a “primary” code must be reported. Similarly, 99357 cannot be reported without 99356, and 99356 must be reported with one of the following inpatient service (primary) codes: 99218-99220, 99221-99223, 99231-99233, 99251-99255, 99304-99310. Only one unit of 99356 may be reported per patient per physician group per day, whereas multiple units of 99357 may be reported in a single day.
The CPT definition of prolonged care varies from that of the Centers for Medicare & Medicaid Services (CMS). Since 2009, CPT recognizes the total duration spent by a physician on a given date, even if the time spent by the physician on that date is not continuous; the time involves both face-to-face time and unit/floor time.5 CMS only attributes direct face-to-face time between the physician and the patient toward prolonged care billing. Time spent reviewing charts or discussion of a patient with house medical staff, waiting for test results, waiting for changes in the patient’s condition, waiting for end of a therapy session, or waiting for use of facilities cannot be billed as prolonged services.5 This is in direct opposition to its policy for C/CC services, and makes prolonged care services inefficient.
Medicare also identifies “threshold” time (see Table 2). The total physician visit time must exceed the time requirements associated with the “primary” codes by a 30-minute threshold (e.g. 99221+99356=30 minutes+30 minutes=60 minutes threshold time). The physician must document the total face-to-face time spent in separate notes throughout the day or, more realistically, in one cumulative note.
When two providers from the same group and same specialty perform services on the same date (e.g. physician A saw the patient during morning rounds, and physician B spoke with the patient/family in the afternoon), only one physician can report the cumulative service.6 As always, query payors for coverage, because some non-Medicare insurers do not recognize these codes.
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1B. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare National Coverage Determinations Manual: Chapter 1, Section 70.1. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/ncd103c1_Part1.pdf. Accessed Jan. 8, 2012.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.15.1C. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011:7-21.
- Centers for Medicare & Medicaid Services (CMS). Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 8, 2012.
Hospitalists Provide Leadership as Unit Medical Directors
A project to formalize “local leadership models”—partnering leadership teams comprising a hospitalist and a nurse manager on each participating unit—at the University of Michigan Health System helped to redefine the role of unit medical director and led to allocating sufficient time (15% to 20% of an FTE) for hospitalists to fill that role. The process also led to a joint role description for the physician and nurse leaders.
“In our organization, we had the medical director concept in place previously, but things were missing, with no direct accountability, no dedicated effort, and lack of clarity on reporting,” explains hospitalist Christopher Kim, MD, MBA, lead author of an article about the project published in the American Journal of Medical Quality.1 “We learned from other organizations, and one of the first things we learned was to make sure we hired the right person as medical director. We need an energetic, enthusiastic physician who can reach out to nurses and bridge gaps in communication and coordination of care.”
The clinical partnership model was piloted on five units—four adult and one pediatric—and has since been adopted by eight others. The physician/nurse leaders work on such issues as improving care transitions, reducing pressure ulcers and catheter-related urinary tract infections, developing multi-disciplinary rounding on the units, and sharing quality data with staff.
“Take UTIs or pressure ulcers; we’re all familiar with recommended practice, but how it gets played out on the units varies. If team leaders understand this, they can champion the processes and
create an educational push for them,” Dr. Kim says. “Those organizations that have done this well cite higher staff satisfaction as a result.”
Study results show that the initial five units were “among the highest-performing units in our facility on satisfaction,” he adds. “It’s an exciting opportunity to bring change processes necessary to build a local clinical care environment that will improve the overall experience of the patient.”
Reference
A project to formalize “local leadership models”—partnering leadership teams comprising a hospitalist and a nurse manager on each participating unit—at the University of Michigan Health System helped to redefine the role of unit medical director and led to allocating sufficient time (15% to 20% of an FTE) for hospitalists to fill that role. The process also led to a joint role description for the physician and nurse leaders.
“In our organization, we had the medical director concept in place previously, but things were missing, with no direct accountability, no dedicated effort, and lack of clarity on reporting,” explains hospitalist Christopher Kim, MD, MBA, lead author of an article about the project published in the American Journal of Medical Quality.1 “We learned from other organizations, and one of the first things we learned was to make sure we hired the right person as medical director. We need an energetic, enthusiastic physician who can reach out to nurses and bridge gaps in communication and coordination of care.”
The clinical partnership model was piloted on five units—four adult and one pediatric—and has since been adopted by eight others. The physician/nurse leaders work on such issues as improving care transitions, reducing pressure ulcers and catheter-related urinary tract infections, developing multi-disciplinary rounding on the units, and sharing quality data with staff.
“Take UTIs or pressure ulcers; we’re all familiar with recommended practice, but how it gets played out on the units varies. If team leaders understand this, they can champion the processes and
create an educational push for them,” Dr. Kim says. “Those organizations that have done this well cite higher staff satisfaction as a result.”
Study results show that the initial five units were “among the highest-performing units in our facility on satisfaction,” he adds. “It’s an exciting opportunity to bring change processes necessary to build a local clinical care environment that will improve the overall experience of the patient.”
Reference
A project to formalize “local leadership models”—partnering leadership teams comprising a hospitalist and a nurse manager on each participating unit—at the University of Michigan Health System helped to redefine the role of unit medical director and led to allocating sufficient time (15% to 20% of an FTE) for hospitalists to fill that role. The process also led to a joint role description for the physician and nurse leaders.
“In our organization, we had the medical director concept in place previously, but things were missing, with no direct accountability, no dedicated effort, and lack of clarity on reporting,” explains hospitalist Christopher Kim, MD, MBA, lead author of an article about the project published in the American Journal of Medical Quality.1 “We learned from other organizations, and one of the first things we learned was to make sure we hired the right person as medical director. We need an energetic, enthusiastic physician who can reach out to nurses and bridge gaps in communication and coordination of care.”
The clinical partnership model was piloted on five units—four adult and one pediatric—and has since been adopted by eight others. The physician/nurse leaders work on such issues as improving care transitions, reducing pressure ulcers and catheter-related urinary tract infections, developing multi-disciplinary rounding on the units, and sharing quality data with staff.
“Take UTIs or pressure ulcers; we’re all familiar with recommended practice, but how it gets played out on the units varies. If team leaders understand this, they can champion the processes and
create an educational push for them,” Dr. Kim says. “Those organizations that have done this well cite higher staff satisfaction as a result.”
Study results show that the initial five units were “among the highest-performing units in our facility on satisfaction,” he adds. “It’s an exciting opportunity to bring change processes necessary to build a local clinical care environment that will improve the overall experience of the patient.”
Reference
First Set of CMS Advisors Includes Hospitalists
In January, the Centers for Medicare & Medicaid Services (CMS) selected 73 professionals as the initial set of advisors for its Innovation Center (http://innovations.cms.gov/). The advisors include 37 physicians, as well as some nurses and health administrators. Each advisor will receive six months of intensive training in quality-improvement (QI) methods and health systems research in order to deepen skills that could help drive improvements in patient care across the system.
Each of the 920 applicants named a project they wanted to pursue at their home institution; many already are involved in quality activities, says Fran Griffin, the program coordinator. CMS hopes that advisors will become “change agents” and mentors to others within their organizations and communities, she adds. “But we are clear that we are not funding research. We want people to come and be educated, and we want to know if they are learning these skills and applying what they learn in real time,” Griffin says.
Advisors will participate in four in-person meetings, the first of which was held in January, as well as four conference calls or webinars each month. The Innovation Center aims to eventually bring 200 advisors on board, with a second cycle of applications and selections expected later this spring.Funded by the Affordable Care Act, the program provides a stipend of up to $20,000 to the advisor’s institution to free up 10 hours a week for training and to complete their projects. Of the initial set of advisors, at least two are hospitalists: Stephen Liu, MD, MPH, FACPM, of Dartmouth-Hitchcock Medical Center in Hanover, N.H., and Jason Stein, MD, SFHM, director of the clinical research program at Emory School of Medicine in Atlanta. Topics pursued by the advisors include unnecessary hospital readmissions, improving care transitions, chronic disease management, and the development of medical homes outside the hospital.
Dr. Liu’s proposed project is to re-engineer and improve geriatric inpatient stays to help preserve patients’ functional status. “Overall, I had a great experience at the first meeting of the advisors,” Dr. Liu says. “It was great to discuss the challenges and opportunities for improvement at each of the different settings represented, and to learn that many of the challenges are similar to those we face in the inpatient setting, such as communication with primary-care providers, transitions of care, and avoiding complications from hospitalizations.”
For more information or to receive email updates, visit www.innovations.cms.gov/initiatives.
In January, the Centers for Medicare & Medicaid Services (CMS) selected 73 professionals as the initial set of advisors for its Innovation Center (http://innovations.cms.gov/). The advisors include 37 physicians, as well as some nurses and health administrators. Each advisor will receive six months of intensive training in quality-improvement (QI) methods and health systems research in order to deepen skills that could help drive improvements in patient care across the system.
Each of the 920 applicants named a project they wanted to pursue at their home institution; many already are involved in quality activities, says Fran Griffin, the program coordinator. CMS hopes that advisors will become “change agents” and mentors to others within their organizations and communities, she adds. “But we are clear that we are not funding research. We want people to come and be educated, and we want to know if they are learning these skills and applying what they learn in real time,” Griffin says.
Advisors will participate in four in-person meetings, the first of which was held in January, as well as four conference calls or webinars each month. The Innovation Center aims to eventually bring 200 advisors on board, with a second cycle of applications and selections expected later this spring.Funded by the Affordable Care Act, the program provides a stipend of up to $20,000 to the advisor’s institution to free up 10 hours a week for training and to complete their projects. Of the initial set of advisors, at least two are hospitalists: Stephen Liu, MD, MPH, FACPM, of Dartmouth-Hitchcock Medical Center in Hanover, N.H., and Jason Stein, MD, SFHM, director of the clinical research program at Emory School of Medicine in Atlanta. Topics pursued by the advisors include unnecessary hospital readmissions, improving care transitions, chronic disease management, and the development of medical homes outside the hospital.
Dr. Liu’s proposed project is to re-engineer and improve geriatric inpatient stays to help preserve patients’ functional status. “Overall, I had a great experience at the first meeting of the advisors,” Dr. Liu says. “It was great to discuss the challenges and opportunities for improvement at each of the different settings represented, and to learn that many of the challenges are similar to those we face in the inpatient setting, such as communication with primary-care providers, transitions of care, and avoiding complications from hospitalizations.”
For more information or to receive email updates, visit www.innovations.cms.gov/initiatives.
In January, the Centers for Medicare & Medicaid Services (CMS) selected 73 professionals as the initial set of advisors for its Innovation Center (http://innovations.cms.gov/). The advisors include 37 physicians, as well as some nurses and health administrators. Each advisor will receive six months of intensive training in quality-improvement (QI) methods and health systems research in order to deepen skills that could help drive improvements in patient care across the system.
Each of the 920 applicants named a project they wanted to pursue at their home institution; many already are involved in quality activities, says Fran Griffin, the program coordinator. CMS hopes that advisors will become “change agents” and mentors to others within their organizations and communities, she adds. “But we are clear that we are not funding research. We want people to come and be educated, and we want to know if they are learning these skills and applying what they learn in real time,” Griffin says.
Advisors will participate in four in-person meetings, the first of which was held in January, as well as four conference calls or webinars each month. The Innovation Center aims to eventually bring 200 advisors on board, with a second cycle of applications and selections expected later this spring.Funded by the Affordable Care Act, the program provides a stipend of up to $20,000 to the advisor’s institution to free up 10 hours a week for training and to complete their projects. Of the initial set of advisors, at least two are hospitalists: Stephen Liu, MD, MPH, FACPM, of Dartmouth-Hitchcock Medical Center in Hanover, N.H., and Jason Stein, MD, SFHM, director of the clinical research program at Emory School of Medicine in Atlanta. Topics pursued by the advisors include unnecessary hospital readmissions, improving care transitions, chronic disease management, and the development of medical homes outside the hospital.
Dr. Liu’s proposed project is to re-engineer and improve geriatric inpatient stays to help preserve patients’ functional status. “Overall, I had a great experience at the first meeting of the advisors,” Dr. Liu says. “It was great to discuss the challenges and opportunities for improvement at each of the different settings represented, and to learn that many of the challenges are similar to those we face in the inpatient setting, such as communication with primary-care providers, transitions of care, and avoiding complications from hospitalizations.”
For more information or to receive email updates, visit www.innovations.cms.gov/initiatives.
Understanding Physicians’ Attitudes toward Safety Culture
Results from a survey to assess physicians’ and medical trainees’ perceptions and attitudes about the culture of patient safety at the University of California at San Francisco (UCSF) Medical Center were reported at HM11 in Dallas by Patrick Kneeland, MD, who has since moved to Providence Regional Medical Center’s Everett Clinic in Seattle, where he co-chairs the Medical Quality Review Committee.
“We were interested in perceptions about what most determines a safety culture within a hospital,” and about differences and similarities between faculty, fellows, and residents, Dr. Kneeland explains. A positive safety culture is essential to enhancing patient safety, and it requires support and commitment at multiple levels.
Dr. Kneeland and colleagues used an established, validated instrument, the federal Agency for Healthcare Research and Quality’s “Hospital Survey on Patient Safety Culture,” which is used by hospitals to assess their staffs’ attitudes toward safety. But the UCSF team
modified the instrument to include additional survey dimensions, such as trainee supervision, event disclosure to patients, and physician-to-physician handoffs.1 Of 290 physicians surveyed in UCSF’s Department of Medicine, 53% completed the survey.
“What was surprising from our survey was the overall high degree of agreement, but with some interesting differences,” Dr. Kneeland explains. In terms of the overall rating of safety culture, on a 1-to-5 scale with five being the highest, fellows rated the safety culture the highest, followed by faculty, and then residents. “Even though, across the board, 70 percent or more said adverse events should be disclosed to patients, only half of the trainees felt encouraged to do so, and half felt there is some danger in doing so,” he says.
Findings led to a major educational initiative around error disclosure, and to having the chief residents openly discuss overnight adverse patient events at morning rounds. The goal is to make event reporting part of customary practice. UCSF plans to repeat the survey in five years, using the initial results as a benchmark, Dr. Kneeland adds.
For more information or to request a copy of the modified survey, email Dr. Kneeland at [email protected].
Larry Beresford is a freelance writer in Oakland, Calif.
Reference
Results from a survey to assess physicians’ and medical trainees’ perceptions and attitudes about the culture of patient safety at the University of California at San Francisco (UCSF) Medical Center were reported at HM11 in Dallas by Patrick Kneeland, MD, who has since moved to Providence Regional Medical Center’s Everett Clinic in Seattle, where he co-chairs the Medical Quality Review Committee.
“We were interested in perceptions about what most determines a safety culture within a hospital,” and about differences and similarities between faculty, fellows, and residents, Dr. Kneeland explains. A positive safety culture is essential to enhancing patient safety, and it requires support and commitment at multiple levels.
Dr. Kneeland and colleagues used an established, validated instrument, the federal Agency for Healthcare Research and Quality’s “Hospital Survey on Patient Safety Culture,” which is used by hospitals to assess their staffs’ attitudes toward safety. But the UCSF team
modified the instrument to include additional survey dimensions, such as trainee supervision, event disclosure to patients, and physician-to-physician handoffs.1 Of 290 physicians surveyed in UCSF’s Department of Medicine, 53% completed the survey.
“What was surprising from our survey was the overall high degree of agreement, but with some interesting differences,” Dr. Kneeland explains. In terms of the overall rating of safety culture, on a 1-to-5 scale with five being the highest, fellows rated the safety culture the highest, followed by faculty, and then residents. “Even though, across the board, 70 percent or more said adverse events should be disclosed to patients, only half of the trainees felt encouraged to do so, and half felt there is some danger in doing so,” he says.
Findings led to a major educational initiative around error disclosure, and to having the chief residents openly discuss overnight adverse patient events at morning rounds. The goal is to make event reporting part of customary practice. UCSF plans to repeat the survey in five years, using the initial results as a benchmark, Dr. Kneeland adds.
For more information or to request a copy of the modified survey, email Dr. Kneeland at [email protected].
Larry Beresford is a freelance writer in Oakland, Calif.
Reference
Results from a survey to assess physicians’ and medical trainees’ perceptions and attitudes about the culture of patient safety at the University of California at San Francisco (UCSF) Medical Center were reported at HM11 in Dallas by Patrick Kneeland, MD, who has since moved to Providence Regional Medical Center’s Everett Clinic in Seattle, where he co-chairs the Medical Quality Review Committee.
“We were interested in perceptions about what most determines a safety culture within a hospital,” and about differences and similarities between faculty, fellows, and residents, Dr. Kneeland explains. A positive safety culture is essential to enhancing patient safety, and it requires support and commitment at multiple levels.
Dr. Kneeland and colleagues used an established, validated instrument, the federal Agency for Healthcare Research and Quality’s “Hospital Survey on Patient Safety Culture,” which is used by hospitals to assess their staffs’ attitudes toward safety. But the UCSF team
modified the instrument to include additional survey dimensions, such as trainee supervision, event disclosure to patients, and physician-to-physician handoffs.1 Of 290 physicians surveyed in UCSF’s Department of Medicine, 53% completed the survey.
“What was surprising from our survey was the overall high degree of agreement, but with some interesting differences,” Dr. Kneeland explains. In terms of the overall rating of safety culture, on a 1-to-5 scale with five being the highest, fellows rated the safety culture the highest, followed by faculty, and then residents. “Even though, across the board, 70 percent or more said adverse events should be disclosed to patients, only half of the trainees felt encouraged to do so, and half felt there is some danger in doing so,” he says.
Findings led to a major educational initiative around error disclosure, and to having the chief residents openly discuss overnight adverse patient events at morning rounds. The goal is to make event reporting part of customary practice. UCSF plans to repeat the survey in five years, using the initial results as a benchmark, Dr. Kneeland adds.
For more information or to request a copy of the modified survey, email Dr. Kneeland at [email protected].
Larry Beresford is a freelance writer in Oakland, Calif.
Reference
By the Numbers: 8.3%
8.3%1 in 12 adults ages 21 and older Discharged from the hospital to the community were readmitted within 30 days, according to the National Institute for Health Care Reform. One in 3 (32.9%) were readmitted within one year, suggesting that a significant number of patients remain at risk for readmission far beyond the typically measured 30-day window.
8.3%1 in 12 adults ages 21 and older Discharged from the hospital to the community were readmitted within 30 days, according to the National Institute for Health Care Reform. One in 3 (32.9%) were readmitted within one year, suggesting that a significant number of patients remain at risk for readmission far beyond the typically measured 30-day window.
8.3%1 in 12 adults ages 21 and older Discharged from the hospital to the community were readmitted within 30 days, according to the National Institute for Health Care Reform. One in 3 (32.9%) were readmitted within one year, suggesting that a significant number of patients remain at risk for readmission far beyond the typically measured 30-day window.
WIN WHITCOMB: CMS Core Measures Program a Win-Win for Hospitalists
EDITOR’S NOTE: This month, we introduce a new column, “On the Horizon: Quality, Systems, Safety.” Herein, author Win Whitcomb, MD, MHM, one of SHM’s founders and medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., will deliver his views on all things quality and monitor the major issues affecting hospitalists today. As a companion to Dr. Whitcomb’s perspectives, you’ll find “The View from the Center.” “The View” will on occasion provide readers with news and details of how SHM’s Center for Hospital Innovation and Improvement (“the center”) is addressing implementation of healthcare reform, planning programs to improve quality and safety of care, and influencing decisions that will affect hospital medicine for years to come.
Burke Kealey, MD, SFHM, SHM board member and medical director with HealthPartners in Minneapolis, once remarked, “The core measures program is one of the greatest gifts hospital medicine has been given.” Scoring high on the Centers for Medicare & Medicaid Services (CMS) core measures has been a no-brainer for many hospitalist programs over the years; this success has allowed hospitalists to distinguish themselves from traditional PCPs in the hospital.
Looking back, many of us saw the huge opportunity created by the core measures a decade ago. What could be so hard about writing for a flu shot or ordering an echocardiogram? We joined teams, and put systems in place to ensure high performance and, ahem, figured out how to jump through documentation hoops. (Who disputes that quality improvement is two parts better care, one part managing the medical record?)
The result? A bonanza for hospitalists (as overachievers) in the process measures known as the CMS core measures. Admittedly, some of us have struggled more than others in achieving high performance on some of the measures. For example, we couldn’t for the life of us figure out how to excel in “discharge instructions” for heart failure patients at my hospital because we stunk at medication reconciliation. And, being the team sport that QI is, some of these struggles have been beyond hospitalists’ influence.
Well, times are changing, and a good number of core measures (the CMS Inpatient Quality Reporting, or IQR, Program) recently have been retired or suspended. Table 1 outlines the retired or suspended CMS measures; The Joint Commission is retiring many, but not all, of the same measures. To clarify, CMS uses Hospital IQR measures for reporting on the public website hospitalcompare.hhs.gov and, beginning in 2013, a subset will make up part of the value-based purchasing program (see “Value-Based Purchasing Raises the Stakes,” May 2011, p. 1, or visit www.hospitalmedicine.org/vbp). For the commission, the measures are used as part of the survey process for hospital accreditation.
Timeline
The changes described in Table 1 denote measures that will be retired or suspended from the FY14 Hospital IQR measure set, and were reflected in hospital discharges effective Jan. 1, 2012. In other words, the changes are reflected in CMS’ collection of data from hospitals as of this year.
A few words of explanation of the table terms: “AMI,” of course, stands for acute myocardial infarction, “HF” is heart failure, and “PN” is pneumonia.
“Retire” means just that. Let’s hope so, and not what Michael Jordan meant when calling it quits the first time to try out baseball.
“Suspended” means CMS is retaining the measure in the IQR program but is not collecting data until such time that evidence shows hospital performance has unacceptably declined. Win’s word: Hard to know exactly what that means. It scares me enough that my hospital will continue data collection for internal purposes and not take our eye off the ball regarding performance.
“IMM” is short for global immunization and means that influenza and pneumococcal vaccine administration will be tracked on all hospital patients, not just those with pneumonia, who meet the age and high-risk criteria. Win’s word: You read that correctly. All inpatients require these vaccinations if they meet the criteria.
“Accountability measures” became part of The Joint Commission’s accreditation process Jan. 1. According to the commission’s website, they refer to a subset of core measures resulting in “the greatest positive impact on patient outcomes when hospitals demonstrate improvement on them.” Each accountability measure must meet these four criteria:
RESEARCH: There is strong evidence that compliance with this process of care improves outcomes.
PROXIMITY: The process being measured is “close” to the outcome (i.e. relatively few steps between the process and outcome).
ACCURACY: The measure accurately reflects that the process actually has been provided. Win’s word: In = “aspirin on arrival” and “ACE/ARB for LVSD.” Out = “smoking cessation counseling.” For smoking cessation, who knows if this was actually provided or the box was simply checked?
ADVERSE EFFECTS: The measure minimizes adverse effects. Win’s word: The “initial antibiotic timing” measure probably reduced diagnostic accuracy of pneumonia. See ya.
The other core measures will remain in their current form for now. Going forward, the IQR program provides hospitalists new opportunities to shine. These went into effect Jan. 1:
- Admit decision time to ED departure for admitted patients;
- Median time from ED arrival to ED departure for admitted patients; and
- Catheter-associated UTI rates.
As with all the measures, working in teams will be essential to success in these new measures. So go out there and hit a few more home runs (or at least a base hit or two). The season is well underway.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder of SHM and an expert in quality improvement and hospitalist practice management. Write to him at [email protected].
EDITOR’S NOTE: This month, we introduce a new column, “On the Horizon: Quality, Systems, Safety.” Herein, author Win Whitcomb, MD, MHM, one of SHM’s founders and medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., will deliver his views on all things quality and monitor the major issues affecting hospitalists today. As a companion to Dr. Whitcomb’s perspectives, you’ll find “The View from the Center.” “The View” will on occasion provide readers with news and details of how SHM’s Center for Hospital Innovation and Improvement (“the center”) is addressing implementation of healthcare reform, planning programs to improve quality and safety of care, and influencing decisions that will affect hospital medicine for years to come.
Burke Kealey, MD, SFHM, SHM board member and medical director with HealthPartners in Minneapolis, once remarked, “The core measures program is one of the greatest gifts hospital medicine has been given.” Scoring high on the Centers for Medicare & Medicaid Services (CMS) core measures has been a no-brainer for many hospitalist programs over the years; this success has allowed hospitalists to distinguish themselves from traditional PCPs in the hospital.
Looking back, many of us saw the huge opportunity created by the core measures a decade ago. What could be so hard about writing for a flu shot or ordering an echocardiogram? We joined teams, and put systems in place to ensure high performance and, ahem, figured out how to jump through documentation hoops. (Who disputes that quality improvement is two parts better care, one part managing the medical record?)
The result? A bonanza for hospitalists (as overachievers) in the process measures known as the CMS core measures. Admittedly, some of us have struggled more than others in achieving high performance on some of the measures. For example, we couldn’t for the life of us figure out how to excel in “discharge instructions” for heart failure patients at my hospital because we stunk at medication reconciliation. And, being the team sport that QI is, some of these struggles have been beyond hospitalists’ influence.
Well, times are changing, and a good number of core measures (the CMS Inpatient Quality Reporting, or IQR, Program) recently have been retired or suspended. Table 1 outlines the retired or suspended CMS measures; The Joint Commission is retiring many, but not all, of the same measures. To clarify, CMS uses Hospital IQR measures for reporting on the public website hospitalcompare.hhs.gov and, beginning in 2013, a subset will make up part of the value-based purchasing program (see “Value-Based Purchasing Raises the Stakes,” May 2011, p. 1, or visit www.hospitalmedicine.org/vbp). For the commission, the measures are used as part of the survey process for hospital accreditation.
Timeline
The changes described in Table 1 denote measures that will be retired or suspended from the FY14 Hospital IQR measure set, and were reflected in hospital discharges effective Jan. 1, 2012. In other words, the changes are reflected in CMS’ collection of data from hospitals as of this year.
A few words of explanation of the table terms: “AMI,” of course, stands for acute myocardial infarction, “HF” is heart failure, and “PN” is pneumonia.
“Retire” means just that. Let’s hope so, and not what Michael Jordan meant when calling it quits the first time to try out baseball.
“Suspended” means CMS is retaining the measure in the IQR program but is not collecting data until such time that evidence shows hospital performance has unacceptably declined. Win’s word: Hard to know exactly what that means. It scares me enough that my hospital will continue data collection for internal purposes and not take our eye off the ball regarding performance.
“IMM” is short for global immunization and means that influenza and pneumococcal vaccine administration will be tracked on all hospital patients, not just those with pneumonia, who meet the age and high-risk criteria. Win’s word: You read that correctly. All inpatients require these vaccinations if they meet the criteria.
“Accountability measures” became part of The Joint Commission’s accreditation process Jan. 1. According to the commission’s website, they refer to a subset of core measures resulting in “the greatest positive impact on patient outcomes when hospitals demonstrate improvement on them.” Each accountability measure must meet these four criteria:
RESEARCH: There is strong evidence that compliance with this process of care improves outcomes.
PROXIMITY: The process being measured is “close” to the outcome (i.e. relatively few steps between the process and outcome).
ACCURACY: The measure accurately reflects that the process actually has been provided. Win’s word: In = “aspirin on arrival” and “ACE/ARB for LVSD.” Out = “smoking cessation counseling.” For smoking cessation, who knows if this was actually provided or the box was simply checked?
ADVERSE EFFECTS: The measure minimizes adverse effects. Win’s word: The “initial antibiotic timing” measure probably reduced diagnostic accuracy of pneumonia. See ya.
The other core measures will remain in their current form for now. Going forward, the IQR program provides hospitalists new opportunities to shine. These went into effect Jan. 1:
- Admit decision time to ED departure for admitted patients;
- Median time from ED arrival to ED departure for admitted patients; and
- Catheter-associated UTI rates.
As with all the measures, working in teams will be essential to success in these new measures. So go out there and hit a few more home runs (or at least a base hit or two). The season is well underway.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder of SHM and an expert in quality improvement and hospitalist practice management. Write to him at [email protected].
EDITOR’S NOTE: This month, we introduce a new column, “On the Horizon: Quality, Systems, Safety.” Herein, author Win Whitcomb, MD, MHM, one of SHM’s founders and medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., will deliver his views on all things quality and monitor the major issues affecting hospitalists today. As a companion to Dr. Whitcomb’s perspectives, you’ll find “The View from the Center.” “The View” will on occasion provide readers with news and details of how SHM’s Center for Hospital Innovation and Improvement (“the center”) is addressing implementation of healthcare reform, planning programs to improve quality and safety of care, and influencing decisions that will affect hospital medicine for years to come.
Burke Kealey, MD, SFHM, SHM board member and medical director with HealthPartners in Minneapolis, once remarked, “The core measures program is one of the greatest gifts hospital medicine has been given.” Scoring high on the Centers for Medicare & Medicaid Services (CMS) core measures has been a no-brainer for many hospitalist programs over the years; this success has allowed hospitalists to distinguish themselves from traditional PCPs in the hospital.
Looking back, many of us saw the huge opportunity created by the core measures a decade ago. What could be so hard about writing for a flu shot or ordering an echocardiogram? We joined teams, and put systems in place to ensure high performance and, ahem, figured out how to jump through documentation hoops. (Who disputes that quality improvement is two parts better care, one part managing the medical record?)
The result? A bonanza for hospitalists (as overachievers) in the process measures known as the CMS core measures. Admittedly, some of us have struggled more than others in achieving high performance on some of the measures. For example, we couldn’t for the life of us figure out how to excel in “discharge instructions” for heart failure patients at my hospital because we stunk at medication reconciliation. And, being the team sport that QI is, some of these struggles have been beyond hospitalists’ influence.
Well, times are changing, and a good number of core measures (the CMS Inpatient Quality Reporting, or IQR, Program) recently have been retired or suspended. Table 1 outlines the retired or suspended CMS measures; The Joint Commission is retiring many, but not all, of the same measures. To clarify, CMS uses Hospital IQR measures for reporting on the public website hospitalcompare.hhs.gov and, beginning in 2013, a subset will make up part of the value-based purchasing program (see “Value-Based Purchasing Raises the Stakes,” May 2011, p. 1, or visit www.hospitalmedicine.org/vbp). For the commission, the measures are used as part of the survey process for hospital accreditation.
Timeline
The changes described in Table 1 denote measures that will be retired or suspended from the FY14 Hospital IQR measure set, and were reflected in hospital discharges effective Jan. 1, 2012. In other words, the changes are reflected in CMS’ collection of data from hospitals as of this year.
A few words of explanation of the table terms: “AMI,” of course, stands for acute myocardial infarction, “HF” is heart failure, and “PN” is pneumonia.
“Retire” means just that. Let’s hope so, and not what Michael Jordan meant when calling it quits the first time to try out baseball.
“Suspended” means CMS is retaining the measure in the IQR program but is not collecting data until such time that evidence shows hospital performance has unacceptably declined. Win’s word: Hard to know exactly what that means. It scares me enough that my hospital will continue data collection for internal purposes and not take our eye off the ball regarding performance.
“IMM” is short for global immunization and means that influenza and pneumococcal vaccine administration will be tracked on all hospital patients, not just those with pneumonia, who meet the age and high-risk criteria. Win’s word: You read that correctly. All inpatients require these vaccinations if they meet the criteria.
“Accountability measures” became part of The Joint Commission’s accreditation process Jan. 1. According to the commission’s website, they refer to a subset of core measures resulting in “the greatest positive impact on patient outcomes when hospitals demonstrate improvement on them.” Each accountability measure must meet these four criteria:
RESEARCH: There is strong evidence that compliance with this process of care improves outcomes.
PROXIMITY: The process being measured is “close” to the outcome (i.e. relatively few steps between the process and outcome).
ACCURACY: The measure accurately reflects that the process actually has been provided. Win’s word: In = “aspirin on arrival” and “ACE/ARB for LVSD.” Out = “smoking cessation counseling.” For smoking cessation, who knows if this was actually provided or the box was simply checked?
ADVERSE EFFECTS: The measure minimizes adverse effects. Win’s word: The “initial antibiotic timing” measure probably reduced diagnostic accuracy of pneumonia. See ya.
The other core measures will remain in their current form for now. Going forward, the IQR program provides hospitalists new opportunities to shine. These went into effect Jan. 1:
- Admit decision time to ED departure for admitted patients;
- Median time from ED arrival to ED departure for admitted patients; and
- Catheter-associated UTI rates.
As with all the measures, working in teams will be essential to success in these new measures. So go out there and hit a few more home runs (or at least a base hit or two). The season is well underway.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder of SHM and an expert in quality improvement and hospitalist practice management. Write to him at [email protected].
The Society of Hospital Medicine's Physician Editors Contribute in Ways Large, Small, Significant
Mark and Jeff set the bar: What are we going to do for hospital medicine?
“Write an article, about a thousand words long. Just make sure you turn it in on time every month.” These were verbatim the instructions I received from the editorial staff about a year ago before I started writing this monthly column in The Hospitalist. Most hospitalists, even those in academia, don’t write an article every month. In fact, for most hospitalists, it’s probably been years, possibly decades, since they have penned a piece for publication. The last probably was turned in for a grade.
Well, the good news was that I was given carte blanche to write about topics of my choice. I thought that was a good idea until it came time to write my first column. Yikes! What do I write about? It certainly was easier in school when I was told I had to write about specific topics─say, why Napoleon scapegoated Snowball in Orwell’s “Animal Farm.” Now, not only did I have to write, but I also had to come up with the topic.
Partly because of my obligation to The Hospitalist, I have developed great admiration for interesting, prolific writers. What else did I learn from writing this column? I found out that it is hard to write in a vacuum. Although I knew the editorial staff would be proofreading my language and grammar, I didn’t expect them to give me feedback about content or style.
Soon after my first column, I started putting my email address at the end of the column. Some of the best feedback I received this year as SHM president came because I listed my email address with this column. Secretly, I am hoping that future SHM presidents will do the same and that SHM members will take advantage of the opportunity to communicate with their president.
There is a lesson to be learned here: In order to improve, all of us need consistent, timely, constructive feedback. I have enjoyed writing this monthly column and thank everyone at SHM and The Hospitalist for this opportunity. It was not always easy coming up with a topic or finding the time to string together coherent ideas on a keyboard.
As challenging as it has been at times for me, I think about what Jeff Glasheen has done for the past five years as physician editor of The Hospitalist. Not only did Jeff produce a monthly column five times longer than I did, he also worked tirelessly with the editorial staff to shape, expand, and improve the content of The Hospitalist. Under his leadership, we finally have a professional magazine with content that the advertisers can be proud of. He did this on top of his day job as chief of the hospitalist program at University of Colorado Denver. I won’t elaborate on his multiple other SHM commitments, which including roles as course director of the Academic Hospitalist Academy and chair of the Academic Hospitalist Committee. By the way, Jeff was HM12 course director, too. I’d like to take this opportunity to thank him for his service.
Jeff has been a tireless advocate not only for SHM, but for our profession. Enjoy Jeff’s column this month (see "The End," p. 52). I don’t know ahead of time what he will write about, but rest assured, it will be timely and interesting. This will be Jeff’s final column as physician editor of The Hospitalist. He recently made the decision to step down and hand over the reins to another incredibly talented hospitalist, Danielle Scheurer.
Luckily, Jeff is not one to rest on his laurels. He will be joining the editorial staff of the Journal of Hospital Medicine. Jeff has been the face of The Hospitalist for so long that it will be hard to think of The Hospitalist without him. Danielle, I’m sure, will do a great job. She also knows she has massive shoes to fill.
What I just said about Jeff can and should be said also for Mark Williams, who recently stepped down as JHM’s editor in chief. As the first editor of our field’s preeminent scientific journal, Mark set a high standard. His successor, Andy Auerbach, will be challenged to surpass this high standard. Like Jeff, Mark’s leadership of JHM was only the tip of the iceberg. Mark has done virtually everything there is do at SHM, including serving as an annual meeting course director, SHM board member, and SHM president. Mark is a recognized national leader in transitions of care and was instrumental in development of SHM’s Project BOOST. All of us owe Mark and Jeff a tremendous debt of gratitude. I am a big fan of both of them and feel fortunate to know both HM leaders.
My expectation is that Mark and Jeff, like others who have been so influential in our field—Jeff Wiese, Tina Budnitz, and Larry Wellikson among them—will one day join the Wachters, Nelsons, and Whitcombs as Masters in Hospital Medicine. The views and ideas in The Hospitalist and JHM formulated and shaped the ideas of this young profession. Over the past few years, nobody’s voice has been louder than those of our editors, Mark Williams and Jeff Glasheen.
The field of hospital medicine and our professional society, the Society of Hospital Medicine, has been blessed with visionary leaders, hard-working volunteers, and a talented staff in its first 15 years. They have laid a solid foundation for our field. HM and SHM will continue to grow only if we are able to produce high-value care for our patients. Our ability to increase healthcare value will be based on our ability to improve the quality of care for patients inside and outside the hospital. Individuals like Mark and Jeff have shown us the way. The challenge is for individuals like you and me to help them carry the torch over the next decade and beyond.
I welcome your feedback. Email me at [email protected]; catch me on Twitter: @_JosephLi; or contact me via LinkedIn at Joseph Li.
Dr. Li is president of SHM.
Mark and Jeff set the bar: What are we going to do for hospital medicine?
“Write an article, about a thousand words long. Just make sure you turn it in on time every month.” These were verbatim the instructions I received from the editorial staff about a year ago before I started writing this monthly column in The Hospitalist. Most hospitalists, even those in academia, don’t write an article every month. In fact, for most hospitalists, it’s probably been years, possibly decades, since they have penned a piece for publication. The last probably was turned in for a grade.
Well, the good news was that I was given carte blanche to write about topics of my choice. I thought that was a good idea until it came time to write my first column. Yikes! What do I write about? It certainly was easier in school when I was told I had to write about specific topics─say, why Napoleon scapegoated Snowball in Orwell’s “Animal Farm.” Now, not only did I have to write, but I also had to come up with the topic.
Partly because of my obligation to The Hospitalist, I have developed great admiration for interesting, prolific writers. What else did I learn from writing this column? I found out that it is hard to write in a vacuum. Although I knew the editorial staff would be proofreading my language and grammar, I didn’t expect them to give me feedback about content or style.
Soon after my first column, I started putting my email address at the end of the column. Some of the best feedback I received this year as SHM president came because I listed my email address with this column. Secretly, I am hoping that future SHM presidents will do the same and that SHM members will take advantage of the opportunity to communicate with their president.
There is a lesson to be learned here: In order to improve, all of us need consistent, timely, constructive feedback. I have enjoyed writing this monthly column and thank everyone at SHM and The Hospitalist for this opportunity. It was not always easy coming up with a topic or finding the time to string together coherent ideas on a keyboard.
As challenging as it has been at times for me, I think about what Jeff Glasheen has done for the past five years as physician editor of The Hospitalist. Not only did Jeff produce a monthly column five times longer than I did, he also worked tirelessly with the editorial staff to shape, expand, and improve the content of The Hospitalist. Under his leadership, we finally have a professional magazine with content that the advertisers can be proud of. He did this on top of his day job as chief of the hospitalist program at University of Colorado Denver. I won’t elaborate on his multiple other SHM commitments, which including roles as course director of the Academic Hospitalist Academy and chair of the Academic Hospitalist Committee. By the way, Jeff was HM12 course director, too. I’d like to take this opportunity to thank him for his service.
Jeff has been a tireless advocate not only for SHM, but for our profession. Enjoy Jeff’s column this month (see "The End," p. 52). I don’t know ahead of time what he will write about, but rest assured, it will be timely and interesting. This will be Jeff’s final column as physician editor of The Hospitalist. He recently made the decision to step down and hand over the reins to another incredibly talented hospitalist, Danielle Scheurer.
Luckily, Jeff is not one to rest on his laurels. He will be joining the editorial staff of the Journal of Hospital Medicine. Jeff has been the face of The Hospitalist for so long that it will be hard to think of The Hospitalist without him. Danielle, I’m sure, will do a great job. She also knows she has massive shoes to fill.
What I just said about Jeff can and should be said also for Mark Williams, who recently stepped down as JHM’s editor in chief. As the first editor of our field’s preeminent scientific journal, Mark set a high standard. His successor, Andy Auerbach, will be challenged to surpass this high standard. Like Jeff, Mark’s leadership of JHM was only the tip of the iceberg. Mark has done virtually everything there is do at SHM, including serving as an annual meeting course director, SHM board member, and SHM president. Mark is a recognized national leader in transitions of care and was instrumental in development of SHM’s Project BOOST. All of us owe Mark and Jeff a tremendous debt of gratitude. I am a big fan of both of them and feel fortunate to know both HM leaders.
My expectation is that Mark and Jeff, like others who have been so influential in our field—Jeff Wiese, Tina Budnitz, and Larry Wellikson among them—will one day join the Wachters, Nelsons, and Whitcombs as Masters in Hospital Medicine. The views and ideas in The Hospitalist and JHM formulated and shaped the ideas of this young profession. Over the past few years, nobody’s voice has been louder than those of our editors, Mark Williams and Jeff Glasheen.
The field of hospital medicine and our professional society, the Society of Hospital Medicine, has been blessed with visionary leaders, hard-working volunteers, and a talented staff in its first 15 years. They have laid a solid foundation for our field. HM and SHM will continue to grow only if we are able to produce high-value care for our patients. Our ability to increase healthcare value will be based on our ability to improve the quality of care for patients inside and outside the hospital. Individuals like Mark and Jeff have shown us the way. The challenge is for individuals like you and me to help them carry the torch over the next decade and beyond.
I welcome your feedback. Email me at [email protected]; catch me on Twitter: @_JosephLi; or contact me via LinkedIn at Joseph Li.
Dr. Li is president of SHM.
Mark and Jeff set the bar: What are we going to do for hospital medicine?
“Write an article, about a thousand words long. Just make sure you turn it in on time every month.” These were verbatim the instructions I received from the editorial staff about a year ago before I started writing this monthly column in The Hospitalist. Most hospitalists, even those in academia, don’t write an article every month. In fact, for most hospitalists, it’s probably been years, possibly decades, since they have penned a piece for publication. The last probably was turned in for a grade.
Well, the good news was that I was given carte blanche to write about topics of my choice. I thought that was a good idea until it came time to write my first column. Yikes! What do I write about? It certainly was easier in school when I was told I had to write about specific topics─say, why Napoleon scapegoated Snowball in Orwell’s “Animal Farm.” Now, not only did I have to write, but I also had to come up with the topic.
Partly because of my obligation to The Hospitalist, I have developed great admiration for interesting, prolific writers. What else did I learn from writing this column? I found out that it is hard to write in a vacuum. Although I knew the editorial staff would be proofreading my language and grammar, I didn’t expect them to give me feedback about content or style.
Soon after my first column, I started putting my email address at the end of the column. Some of the best feedback I received this year as SHM president came because I listed my email address with this column. Secretly, I am hoping that future SHM presidents will do the same and that SHM members will take advantage of the opportunity to communicate with their president.
There is a lesson to be learned here: In order to improve, all of us need consistent, timely, constructive feedback. I have enjoyed writing this monthly column and thank everyone at SHM and The Hospitalist for this opportunity. It was not always easy coming up with a topic or finding the time to string together coherent ideas on a keyboard.
As challenging as it has been at times for me, I think about what Jeff Glasheen has done for the past five years as physician editor of The Hospitalist. Not only did Jeff produce a monthly column five times longer than I did, he also worked tirelessly with the editorial staff to shape, expand, and improve the content of The Hospitalist. Under his leadership, we finally have a professional magazine with content that the advertisers can be proud of. He did this on top of his day job as chief of the hospitalist program at University of Colorado Denver. I won’t elaborate on his multiple other SHM commitments, which including roles as course director of the Academic Hospitalist Academy and chair of the Academic Hospitalist Committee. By the way, Jeff was HM12 course director, too. I’d like to take this opportunity to thank him for his service.
Jeff has been a tireless advocate not only for SHM, but for our profession. Enjoy Jeff’s column this month (see "The End," p. 52). I don’t know ahead of time what he will write about, but rest assured, it will be timely and interesting. This will be Jeff’s final column as physician editor of The Hospitalist. He recently made the decision to step down and hand over the reins to another incredibly talented hospitalist, Danielle Scheurer.
Luckily, Jeff is not one to rest on his laurels. He will be joining the editorial staff of the Journal of Hospital Medicine. Jeff has been the face of The Hospitalist for so long that it will be hard to think of The Hospitalist without him. Danielle, I’m sure, will do a great job. She also knows she has massive shoes to fill.
What I just said about Jeff can and should be said also for Mark Williams, who recently stepped down as JHM’s editor in chief. As the first editor of our field’s preeminent scientific journal, Mark set a high standard. His successor, Andy Auerbach, will be challenged to surpass this high standard. Like Jeff, Mark’s leadership of JHM was only the tip of the iceberg. Mark has done virtually everything there is do at SHM, including serving as an annual meeting course director, SHM board member, and SHM president. Mark is a recognized national leader in transitions of care and was instrumental in development of SHM’s Project BOOST. All of us owe Mark and Jeff a tremendous debt of gratitude. I am a big fan of both of them and feel fortunate to know both HM leaders.
My expectation is that Mark and Jeff, like others who have been so influential in our field—Jeff Wiese, Tina Budnitz, and Larry Wellikson among them—will one day join the Wachters, Nelsons, and Whitcombs as Masters in Hospital Medicine. The views and ideas in The Hospitalist and JHM formulated and shaped the ideas of this young profession. Over the past few years, nobody’s voice has been louder than those of our editors, Mark Williams and Jeff Glasheen.
The field of hospital medicine and our professional society, the Society of Hospital Medicine, has been blessed with visionary leaders, hard-working volunteers, and a talented staff in its first 15 years. They have laid a solid foundation for our field. HM and SHM will continue to grow only if we are able to produce high-value care for our patients. Our ability to increase healthcare value will be based on our ability to improve the quality of care for patients inside and outside the hospital. Individuals like Mark and Jeff have shown us the way. The challenge is for individuals like you and me to help them carry the torch over the next decade and beyond.
I welcome your feedback. Email me at [email protected]; catch me on Twitter: @_JosephLi; or contact me via LinkedIn at Joseph Li.
Dr. Li is president of SHM.
JEFF GLASHEEN: What's Been, What's Next, and a Hogan Update
By now you are no doubt aware that the world will soon end. And by soon, I don’t mean Dec. 21, 2012, as predicted by the Mayans—rather, July 21, the date the Weekly World News recently reported that the Earth will hurdle into the planet Nibiru, no doubt inconveniencing my son’s tee-ball game and upending my father’s 72nd annual failed attempt at pulling off the white T, black socks, and sandals look at the beach.
The tabloid also reports that Elvis was spotted pilfering a Vegas buffet (photos of inexplicably barren shrimp cocktail containers support their case), a manigator is loose in Alabama (heads up if you’re reading this in Mobile!), and that duck hunters mistakenly shot an angel (bad move angering the angels right before Doomsday).
OK, maybe you don’t believe that the world will end in 2012. But one thing that will end is my tenure as physician editor of The Hospitalist. The date: March 31, 2012. Yes, after nearly five years, I’m passing the baton and moving on before my editing career takes on an overweight, beefy side-burned, flowing-jumpsuit-in-Vegas course of its own.
And after editing and writing columns for 55 issues of the definitive publication in hospital medicine, what question do I get the most?
How’s Hogan?
History Through Headlines
Looking back at my columns, I now see that some themes developed. I touched on disease states (Spanish Flu Redux?), obesity (Fight the Losing Battle), electronic health records (Only Fools Rush In), patient satisfaction (Doctor Remodel), physician burnout (Left Turns), and healthcare reform (Get Well Now). I wrote about the births of my children, Greyson (Lesson of the Titanic) and Kaiya (Undercover, MD), Grey’s trip to the ED (Mind Games & Silence), and the myriad ways my father can irritate from the backseat of a car (Minivan, Major Lesson).
But over the years, I also increasingly wrote about patient safety (Bueller … Bueller?; Handless Employees; Designed to Harm; A Run For Safety), quality improvement (Something Interesting Happened; Quality Defined) and the need for hospitalists to lead these imperatives (Exceed Acceptable; Promise or Insanity?; Subsidy or Payment?; Fiddling As HM Burns).
And along the way, my monologue turned into a dialogue. I remember the first email that proved someone other than my coerced wife read my column. Then again, I shouldn’t have been surprised that my dad emailed to tell me to stop writing about him.
But then it happened again. And again. And again.
I was surprised to hear from so many readers. People wrote that they, too, had embarrassingly misdiagnosed their child’s croup as a life-threatening disorder, were struggling with burnout and balance, didn’t like the new ABIM recognition of focused practice in hospital medicine (Urban Legends; Certified Special), and, in the case of my chair of medicine, that they could not achieve my challenge (Transitions Telethon) to call every PCP on discharge for one week.
Readers wrote that I made them laugh, that a story touched them, or that they were angry. One was upset that I called childbirth a “miracle,” another that I was too forgiving about the new duty hours (Rise of the Napturnist), and my father that I still hadn’t stopped writing about him.
But none of this generated the interest that Hogan did.
What Would Hogan Want?
In August 2009, I wrote The Anvil of Indecision column about my dog, Hogan, and our experience with his incidentally discovered 5-cm lung mass. It was my first personal foray into end-of-life decision-making, and it came in the form of a 10-year-old Weimaraner. Hogan was present for many of the most important strata of my life—his rings counting my single-guy resident days, early hospitalist career, marriage, a few relocations, and the births of my kids. And along the way, he was the one constant, the glue that kept my life together.
Best friends are like that.
As I noted in that column, my wife and I struggled with “how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.” Not knowing if the mass was benign or malignant, should we work it up or just let nature sort it out? If we treated, should we diagnose and stage the tumor, blindly surgically remove it, or just give palliative chemotherapy? What if it was isolated and surgery would be curative? What if it was metastatic and surgery just added morbidity? What if this was benign and Hogan died in the operating room? What would Hogan want? Hogan trusted me to make the right decision.
Best friends are like that.
This was the easiest column I’d ever typed but the hardest I’d ever written. I wrote it in about an hour and then cried about it for two days. I was distraught, miserable, and unmoored in the way that only pet-lovers who have faced the demise of a loved one can understand.
I was truly confounded, and the act of writing was cathartic. It was my first venture beyond standard professional content. I spent two weeks deciding if I could really publish it. It was difficult, I found, to expose myself—to be vulnerable in this manner.
No one can beat you up for saying we should care about patient safety and improve quality. Dedicating an entire column to a dog? What if my peers, the society, my bosses found it immature or self-pitying? What if they didn’t get it?
Within days of publishing the column about Hogan, I’d received hundreds of comments from readers, most relating their similar experiences, all expressing support—by far more interest than I got about anything else I’d written.
Turns out, you got it.
What’s Next?
I wonder what my next 55 columns would have looked like. I surely would continue to discuss HM’s struggles to operationalize the quality and safety promise we hold. This should continue to be our singular goal.
I’d likely write about bundled payments and ACOs. I believe these are potential game-changers in much the same way the prospective payment system was in the 1980s. The latter laid the groundwork for hospitalists. Will new payment models prove a boon or a death knell?
I’d spill ink, no doubt, about the financing of HM groups. As hospital reimbursement fades, can hospitalist salaries be far behind? Will this push us toward more encounters and more shifts, leaving less time for meaningful process improvement work, less time for personal and professional balance, less satisfaction in our careers?
I would wrestle with how we can attract the best and brightest to our field. Who will fill the next 20,000 hospitalist positions?
I would, no doubt, chronicle the tribulations of my kids (a record to be leveraged when I look to move in with them in 2040), send a few more barbs my father’s way (payback for the 1980s, Dad!), and deprecate a few of my future dimwitted moves.
And I might even devote a few more inches of column to Hogan.
Because, you see, Hogan is doing well. He’s cancer-free nearly three years after surgery and chemotherapy for metastatic pulmonary adenocarcinoma. I’ll skip the details and just say “thanks.”
Thanks for asking. Thanks for reading.
Dr. Glasheen is physician editor of The Hospitalist.
By now you are no doubt aware that the world will soon end. And by soon, I don’t mean Dec. 21, 2012, as predicted by the Mayans—rather, July 21, the date the Weekly World News recently reported that the Earth will hurdle into the planet Nibiru, no doubt inconveniencing my son’s tee-ball game and upending my father’s 72nd annual failed attempt at pulling off the white T, black socks, and sandals look at the beach.
The tabloid also reports that Elvis was spotted pilfering a Vegas buffet (photos of inexplicably barren shrimp cocktail containers support their case), a manigator is loose in Alabama (heads up if you’re reading this in Mobile!), and that duck hunters mistakenly shot an angel (bad move angering the angels right before Doomsday).
OK, maybe you don’t believe that the world will end in 2012. But one thing that will end is my tenure as physician editor of The Hospitalist. The date: March 31, 2012. Yes, after nearly five years, I’m passing the baton and moving on before my editing career takes on an overweight, beefy side-burned, flowing-jumpsuit-in-Vegas course of its own.
And after editing and writing columns for 55 issues of the definitive publication in hospital medicine, what question do I get the most?
How’s Hogan?
History Through Headlines
Looking back at my columns, I now see that some themes developed. I touched on disease states (Spanish Flu Redux?), obesity (Fight the Losing Battle), electronic health records (Only Fools Rush In), patient satisfaction (Doctor Remodel), physician burnout (Left Turns), and healthcare reform (Get Well Now). I wrote about the births of my children, Greyson (Lesson of the Titanic) and Kaiya (Undercover, MD), Grey’s trip to the ED (Mind Games & Silence), and the myriad ways my father can irritate from the backseat of a car (Minivan, Major Lesson).
But over the years, I also increasingly wrote about patient safety (Bueller … Bueller?; Handless Employees; Designed to Harm; A Run For Safety), quality improvement (Something Interesting Happened; Quality Defined) and the need for hospitalists to lead these imperatives (Exceed Acceptable; Promise or Insanity?; Subsidy or Payment?; Fiddling As HM Burns).
And along the way, my monologue turned into a dialogue. I remember the first email that proved someone other than my coerced wife read my column. Then again, I shouldn’t have been surprised that my dad emailed to tell me to stop writing about him.
But then it happened again. And again. And again.
I was surprised to hear from so many readers. People wrote that they, too, had embarrassingly misdiagnosed their child’s croup as a life-threatening disorder, were struggling with burnout and balance, didn’t like the new ABIM recognition of focused practice in hospital medicine (Urban Legends; Certified Special), and, in the case of my chair of medicine, that they could not achieve my challenge (Transitions Telethon) to call every PCP on discharge for one week.
Readers wrote that I made them laugh, that a story touched them, or that they were angry. One was upset that I called childbirth a “miracle,” another that I was too forgiving about the new duty hours (Rise of the Napturnist), and my father that I still hadn’t stopped writing about him.
But none of this generated the interest that Hogan did.
What Would Hogan Want?
In August 2009, I wrote The Anvil of Indecision column about my dog, Hogan, and our experience with his incidentally discovered 5-cm lung mass. It was my first personal foray into end-of-life decision-making, and it came in the form of a 10-year-old Weimaraner. Hogan was present for many of the most important strata of my life—his rings counting my single-guy resident days, early hospitalist career, marriage, a few relocations, and the births of my kids. And along the way, he was the one constant, the glue that kept my life together.
Best friends are like that.
As I noted in that column, my wife and I struggled with “how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.” Not knowing if the mass was benign or malignant, should we work it up or just let nature sort it out? If we treated, should we diagnose and stage the tumor, blindly surgically remove it, or just give palliative chemotherapy? What if it was isolated and surgery would be curative? What if it was metastatic and surgery just added morbidity? What if this was benign and Hogan died in the operating room? What would Hogan want? Hogan trusted me to make the right decision.
Best friends are like that.
This was the easiest column I’d ever typed but the hardest I’d ever written. I wrote it in about an hour and then cried about it for two days. I was distraught, miserable, and unmoored in the way that only pet-lovers who have faced the demise of a loved one can understand.
I was truly confounded, and the act of writing was cathartic. It was my first venture beyond standard professional content. I spent two weeks deciding if I could really publish it. It was difficult, I found, to expose myself—to be vulnerable in this manner.
No one can beat you up for saying we should care about patient safety and improve quality. Dedicating an entire column to a dog? What if my peers, the society, my bosses found it immature or self-pitying? What if they didn’t get it?
Within days of publishing the column about Hogan, I’d received hundreds of comments from readers, most relating their similar experiences, all expressing support—by far more interest than I got about anything else I’d written.
Turns out, you got it.
What’s Next?
I wonder what my next 55 columns would have looked like. I surely would continue to discuss HM’s struggles to operationalize the quality and safety promise we hold. This should continue to be our singular goal.
I’d likely write about bundled payments and ACOs. I believe these are potential game-changers in much the same way the prospective payment system was in the 1980s. The latter laid the groundwork for hospitalists. Will new payment models prove a boon or a death knell?
I’d spill ink, no doubt, about the financing of HM groups. As hospital reimbursement fades, can hospitalist salaries be far behind? Will this push us toward more encounters and more shifts, leaving less time for meaningful process improvement work, less time for personal and professional balance, less satisfaction in our careers?
I would wrestle with how we can attract the best and brightest to our field. Who will fill the next 20,000 hospitalist positions?
I would, no doubt, chronicle the tribulations of my kids (a record to be leveraged when I look to move in with them in 2040), send a few more barbs my father’s way (payback for the 1980s, Dad!), and deprecate a few of my future dimwitted moves.
And I might even devote a few more inches of column to Hogan.
Because, you see, Hogan is doing well. He’s cancer-free nearly three years after surgery and chemotherapy for metastatic pulmonary adenocarcinoma. I’ll skip the details and just say “thanks.”
Thanks for asking. Thanks for reading.
Dr. Glasheen is physician editor of The Hospitalist.
By now you are no doubt aware that the world will soon end. And by soon, I don’t mean Dec. 21, 2012, as predicted by the Mayans—rather, July 21, the date the Weekly World News recently reported that the Earth will hurdle into the planet Nibiru, no doubt inconveniencing my son’s tee-ball game and upending my father’s 72nd annual failed attempt at pulling off the white T, black socks, and sandals look at the beach.
The tabloid also reports that Elvis was spotted pilfering a Vegas buffet (photos of inexplicably barren shrimp cocktail containers support their case), a manigator is loose in Alabama (heads up if you’re reading this in Mobile!), and that duck hunters mistakenly shot an angel (bad move angering the angels right before Doomsday).
OK, maybe you don’t believe that the world will end in 2012. But one thing that will end is my tenure as physician editor of The Hospitalist. The date: March 31, 2012. Yes, after nearly five years, I’m passing the baton and moving on before my editing career takes on an overweight, beefy side-burned, flowing-jumpsuit-in-Vegas course of its own.
And after editing and writing columns for 55 issues of the definitive publication in hospital medicine, what question do I get the most?
How’s Hogan?
History Through Headlines
Looking back at my columns, I now see that some themes developed. I touched on disease states (Spanish Flu Redux?), obesity (Fight the Losing Battle), electronic health records (Only Fools Rush In), patient satisfaction (Doctor Remodel), physician burnout (Left Turns), and healthcare reform (Get Well Now). I wrote about the births of my children, Greyson (Lesson of the Titanic) and Kaiya (Undercover, MD), Grey’s trip to the ED (Mind Games & Silence), and the myriad ways my father can irritate from the backseat of a car (Minivan, Major Lesson).
But over the years, I also increasingly wrote about patient safety (Bueller … Bueller?; Handless Employees; Designed to Harm; A Run For Safety), quality improvement (Something Interesting Happened; Quality Defined) and the need for hospitalists to lead these imperatives (Exceed Acceptable; Promise or Insanity?; Subsidy or Payment?; Fiddling As HM Burns).
And along the way, my monologue turned into a dialogue. I remember the first email that proved someone other than my coerced wife read my column. Then again, I shouldn’t have been surprised that my dad emailed to tell me to stop writing about him.
But then it happened again. And again. And again.
I was surprised to hear from so many readers. People wrote that they, too, had embarrassingly misdiagnosed their child’s croup as a life-threatening disorder, were struggling with burnout and balance, didn’t like the new ABIM recognition of focused practice in hospital medicine (Urban Legends; Certified Special), and, in the case of my chair of medicine, that they could not achieve my challenge (Transitions Telethon) to call every PCP on discharge for one week.
Readers wrote that I made them laugh, that a story touched them, or that they were angry. One was upset that I called childbirth a “miracle,” another that I was too forgiving about the new duty hours (Rise of the Napturnist), and my father that I still hadn’t stopped writing about him.
But none of this generated the interest that Hogan did.
What Would Hogan Want?
In August 2009, I wrote The Anvil of Indecision column about my dog, Hogan, and our experience with his incidentally discovered 5-cm lung mass. It was my first personal foray into end-of-life decision-making, and it came in the form of a 10-year-old Weimaraner. Hogan was present for many of the most important strata of my life—his rings counting my single-guy resident days, early hospitalist career, marriage, a few relocations, and the births of my kids. And along the way, he was the one constant, the glue that kept my life together.
Best friends are like that.
As I noted in that column, my wife and I struggled with “how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.” Not knowing if the mass was benign or malignant, should we work it up or just let nature sort it out? If we treated, should we diagnose and stage the tumor, blindly surgically remove it, or just give palliative chemotherapy? What if it was isolated and surgery would be curative? What if it was metastatic and surgery just added morbidity? What if this was benign and Hogan died in the operating room? What would Hogan want? Hogan trusted me to make the right decision.
Best friends are like that.
This was the easiest column I’d ever typed but the hardest I’d ever written. I wrote it in about an hour and then cried about it for two days. I was distraught, miserable, and unmoored in the way that only pet-lovers who have faced the demise of a loved one can understand.
I was truly confounded, and the act of writing was cathartic. It was my first venture beyond standard professional content. I spent two weeks deciding if I could really publish it. It was difficult, I found, to expose myself—to be vulnerable in this manner.
No one can beat you up for saying we should care about patient safety and improve quality. Dedicating an entire column to a dog? What if my peers, the society, my bosses found it immature or self-pitying? What if they didn’t get it?
Within days of publishing the column about Hogan, I’d received hundreds of comments from readers, most relating their similar experiences, all expressing support—by far more interest than I got about anything else I’d written.
Turns out, you got it.
What’s Next?
I wonder what my next 55 columns would have looked like. I surely would continue to discuss HM’s struggles to operationalize the quality and safety promise we hold. This should continue to be our singular goal.
I’d likely write about bundled payments and ACOs. I believe these are potential game-changers in much the same way the prospective payment system was in the 1980s. The latter laid the groundwork for hospitalists. Will new payment models prove a boon or a death knell?
I’d spill ink, no doubt, about the financing of HM groups. As hospital reimbursement fades, can hospitalist salaries be far behind? Will this push us toward more encounters and more shifts, leaving less time for meaningful process improvement work, less time for personal and professional balance, less satisfaction in our careers?
I would wrestle with how we can attract the best and brightest to our field. Who will fill the next 20,000 hospitalist positions?
I would, no doubt, chronicle the tribulations of my kids (a record to be leveraged when I look to move in with them in 2040), send a few more barbs my father’s way (payback for the 1980s, Dad!), and deprecate a few of my future dimwitted moves.
And I might even devote a few more inches of column to Hogan.
Because, you see, Hogan is doing well. He’s cancer-free nearly three years after surgery and chemotherapy for metastatic pulmonary adenocarcinoma. I’ll skip the details and just say “thanks.”
Thanks for asking. Thanks for reading.
Dr. Glasheen is physician editor of The Hospitalist.