User login
BEST PRACTICES IN: Extended-Regimen Oral Contraception:Modifying the Hormone-Free Interval
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
A supplement to Ob.Gyn. News. This supplement was sponsored by TEVA Women's Health.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• History of the Hormone-Free Interval
• Physiologic Effects of a Modified Hormone-Free Interval
• Safety and Efficacy of Extended-Regimen Oral Contraception
Faculty/Faculty Disclosure
David J. Portman, MD
Director and Principal Investigator
Columbus Center for Women's
Health Research
Columbus, Ohio
Dr. Portman is a consultant to Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim GmbH, GlaxoSmithKline plc, and TEVA Women's Health. He has received funding for clinical grants from Bayer, Boehringer Ingelheim, Depomed, Inc., Pfizer Inc., TEVA Women's Health, and Warner Chilcott.
Clinical Perspectives on the Role of Hormone Therapy in Menopausal Management
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is based on physician interviews. It is supported by Kenwood Therapeutics, a division of Bradley Pharmaceuticals, Inc.

To view the supplement, click the image above.
Topic Highlights
• Menopause and Hormone Therapy
• Current Recommendations for Postmenopausal Hormone Therapy
• Clinical Trial of Transdermal Estrogen
• Other Considerations of Transdermal Hormone Therapy
Faculty/Faculty Disclosures
Copyright © 2007 by Elsevier Inc.
Heparin-Coated Stent Graft Gave High SFA Patency
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the International Symposium on Endovascular Therapy (ISET) 2012.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said.
Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II). The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
The new findings show that the Viabahn heparin-coated stent graft is comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Gary M. Ansel, clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. who was a coinvestigator on the pivotal trials for both devices. He added that such stenting should become the new standard of treatment.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision. http://www.cookmedical.com/zilverptx/index.html
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease.
Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound.
The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of stenting treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was considered "markedly oversized," according to Dr. Saxon.
A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status.
Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
While the results of the VIPER trial are exciting, our enthusiasm must be tempered by the relatively small size of the study and the even smaller size of the subset achieving the most remarkable results. We have been here before and to state that these heparin-coated stent-grafts are better than everything else [for treating SFA stenoses], and are the new standards clearly ignores the merits of some other therapies like the good old-fashioned saphenous vein bypass. This oversight is particularly important when one considers that the average age of the patients in this study was only 66. Call me old fashioned, or call me old-school, but 1-year follow up hasn’t quite convinced me. Sliced bread might still be good for something.
While I don’t doubt that this technology will have a place in SFA treatment, maybe even very prominent role, I, for one, will still be selective with its use. Finally, having removed a couple of heparin-bonded devices from HIT patients along the way, I can tell you that they aren’t for everyone.
Dr. Mark D. Morasch is a vascular surgeon at St. Vincent Healthcare’s Heart and Vascular Center, Billings Mont., and an associate medical editor of Vascular Specialist.
While the results of the VIPER trial are exciting, our enthusiasm must be tempered by the relatively small size of the study and the even smaller size of the subset achieving the most remarkable results. We have been here before and to state that these heparin-coated stent-grafts are better than everything else [for treating SFA stenoses], and are the new standards clearly ignores the merits of some other therapies like the good old-fashioned saphenous vein bypass. This oversight is particularly important when one considers that the average age of the patients in this study was only 66. Call me old fashioned, or call me old-school, but 1-year follow up hasn’t quite convinced me. Sliced bread might still be good for something.
While I don’t doubt that this technology will have a place in SFA treatment, maybe even very prominent role, I, for one, will still be selective with its use. Finally, having removed a couple of heparin-bonded devices from HIT patients along the way, I can tell you that they aren’t for everyone.
Dr. Mark D. Morasch is a vascular surgeon at St. Vincent Healthcare’s Heart and Vascular Center, Billings Mont., and an associate medical editor of Vascular Specialist.
While the results of the VIPER trial are exciting, our enthusiasm must be tempered by the relatively small size of the study and the even smaller size of the subset achieving the most remarkable results. We have been here before and to state that these heparin-coated stent-grafts are better than everything else [for treating SFA stenoses], and are the new standards clearly ignores the merits of some other therapies like the good old-fashioned saphenous vein bypass. This oversight is particularly important when one considers that the average age of the patients in this study was only 66. Call me old fashioned, or call me old-school, but 1-year follow up hasn’t quite convinced me. Sliced bread might still be good for something.
While I don’t doubt that this technology will have a place in SFA treatment, maybe even very prominent role, I, for one, will still be selective with its use. Finally, having removed a couple of heparin-bonded devices from HIT patients along the way, I can tell you that they aren’t for everyone.
Dr. Mark D. Morasch is a vascular surgeon at St. Vincent Healthcare’s Heart and Vascular Center, Billings Mont., and an associate medical editor of Vascular Specialist.
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the International Symposium on Endovascular Therapy (ISET) 2012.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said.
Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II). The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
The new findings show that the Viabahn heparin-coated stent graft is comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Gary M. Ansel, clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. who was a coinvestigator on the pivotal trials for both devices. He added that such stenting should become the new standard of treatment.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision. http://www.cookmedical.com/zilverptx/index.html
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease.
Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound.
The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of stenting treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was considered "markedly oversized," according to Dr. Saxon.
A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status.
Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the International Symposium on Endovascular Therapy (ISET) 2012.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said.
Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II). The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
The new findings show that the Viabahn heparin-coated stent graft is comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Gary M. Ansel, clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. who was a coinvestigator on the pivotal trials for both devices. He added that such stenting should become the new standard of treatment.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision. http://www.cookmedical.com/zilverptx/index.html
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease.
Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound.
The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of stenting treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was considered "markedly oversized," according to Dr. Saxon.
A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status.
Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
Major Finding: At 12-month follow-up, superficial femoral arteries treated with a heparin-coated stent graft had a 74% primary patency rate.
Data Source: Results are from VIPER, a single-arm study of 119 patients with long SFA lesions enrolled at 11 U.S. centers.
Disclosures: The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
Veith's Viewpoint: The Vascular Disease Paradox
Vascular patients who most need treatment are often difficult and risky to treat by open operation or an endovascular intervention. In contrast, patients who least need invasive treatment or need it not at all usually have lesions that are easy to treat with good results. Examples are patients with advanced gangrene versus those with intermittent claudication; symptomatic patients with carotid artery stenosis versus those who are asymptomatic; patients with large abdominal aortic aneurysms versus those with small lesions.
This paradoxical situation occurs because most vascular lesions, particularly those associated with atherosclerosis, are in their early phases benign, cause minimal or no symptoms, and are unassociated with widespread vascular disease.
Moreover, many of these lesions, particularly with statins and other good medical therapy, will remain stable for long periods. These early lesions are technically easy to treat invasively, although such treatment may provide little or no benefit to the patient.
In contrast, only when lesions advance, become more complex and are associated with a widely diseased arterial system do they become threatening to life, limb, and the brain. These latter lesions are usually much harder to treat both by transcatheter or open operative techniques. This situation gives rise to several consequences relating to physician judgment, procedural outcomes, physician and institutional incomes, health care costs and ethical considerations.
Everyone in the vascular field should recognize and face these issues.
To help do so, let us examine some of these issues as they relate to the common problem of carotid bifurcation stenosis. High grade stenosis at this site, even when asymptomatic, can cause some strokes. Level 1 evidence from the so-called landmark asymptomatic trials (ACAS, ACST), which randomized patients from 1990-2003, showed significant stroke prevention from carotid endarterectomy (CEA) compared to medical treatment.
However, the benefit was slight (stroke rates were reduced from about 2% per year to about 1% per year), and there have been substantial improvements in medical treatments over the last decade to prevent strokes with statins and other measures. Carotid artery stenting (CAS) has also become a commonly used treatment to prevent strokes in asymptomatic carotid stenosis patients, although there is no convincing evidence that such treatment is more effective than current medical treatment in most if not all of these patients.
In addition, there is no solid evidence that CEA in asymptomatic patients prevents strokes more effectively than current medical treatment. Yet in the United States, 70%-90% of CEAs and 70%-96% of CAS procedures are performed on asymptomatic patients.
Should this be and how does the vascular disease paradox relate to this situation?
Vascular surgeons and interventionalists from all specialties want to continue to intervene on most of these asymptomatic carotid stenosis patients for several reasons. These include gratifyingly good outcomes from treating these usually simple, low-risk asymptomatic lesions and provision of income to physicians and hospitals.
These good outcomes also provide many accessory benefits to the treating physicians and surgeons by improving their overall results, a desirable goal in view of looming audits and pay-for-performance incentives. Also, increasing case numbers help practitioners to meet credentialing requirements.
However, there are negatives to continuing to perform large numbers of invasive treatments on asymptomatic carotid stenosis patients. One is the high cost to our health care system of providing these large numbers of invasive treatments largely to a group of patients who will derive little or no benefit. Another is the possibility that more patients will be harmed than helped.
Clearly what is needed are better ways to detect the asymptomatic patient at high risk for having a stroke so only those patients can be treated invasively. Although no such method is universally accepted, there are glimmers of hope that one or more will be proven effective.
Also needed are trials to establish the effectiveness of current medical therapy for stroke prevention in patients with asymptomatic carotid stenosis. However, such trials will not be simple to design because of the benign nature of most asymptomatic lesions. Thus, such trials will have to be conducted in patients selected to have more risky lesions.
Until such information is available, all practitioners should exercise restraint in treating patients with asymptomatic carotid stenosis invasively. They should not be seduced into treating simply because of the ease of treatment or the good outcomes – the vascular disease paradox. It is risk-benefit ratio that is more important. Physicians and surgeons should recognize that the landmark trials on this subject are now outdated, and should restrict such invasive treatment in some way to fewer patients than in the past – perhaps those with an increasing or very high grade (pinhole) stenosis or a contralateral occlusion.
Finally, it should be noted that a proposal to provide reimbursement for performing CAS on standard and low-risk carotid stenosis patients, including asymptomatic patients, is currently being considered by Medicare.
It is likely that some support for this proposal stems from the facts that lesions in such patients are easy to treat and the results of treatment are excellent. This is the vascular disease paradox which should be recognized and dealt with by all in the field.n
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
Vascular patients who most need treatment are often difficult and risky to treat by open operation or an endovascular intervention. In contrast, patients who least need invasive treatment or need it not at all usually have lesions that are easy to treat with good results. Examples are patients with advanced gangrene versus those with intermittent claudication; symptomatic patients with carotid artery stenosis versus those who are asymptomatic; patients with large abdominal aortic aneurysms versus those with small lesions.
This paradoxical situation occurs because most vascular lesions, particularly those associated with atherosclerosis, are in their early phases benign, cause minimal or no symptoms, and are unassociated with widespread vascular disease.
Moreover, many of these lesions, particularly with statins and other good medical therapy, will remain stable for long periods. These early lesions are technically easy to treat invasively, although such treatment may provide little or no benefit to the patient.
In contrast, only when lesions advance, become more complex and are associated with a widely diseased arterial system do they become threatening to life, limb, and the brain. These latter lesions are usually much harder to treat both by transcatheter or open operative techniques. This situation gives rise to several consequences relating to physician judgment, procedural outcomes, physician and institutional incomes, health care costs and ethical considerations.
Everyone in the vascular field should recognize and face these issues.
To help do so, let us examine some of these issues as they relate to the common problem of carotid bifurcation stenosis. High grade stenosis at this site, even when asymptomatic, can cause some strokes. Level 1 evidence from the so-called landmark asymptomatic trials (ACAS, ACST), which randomized patients from 1990-2003, showed significant stroke prevention from carotid endarterectomy (CEA) compared to medical treatment.
However, the benefit was slight (stroke rates were reduced from about 2% per year to about 1% per year), and there have been substantial improvements in medical treatments over the last decade to prevent strokes with statins and other measures. Carotid artery stenting (CAS) has also become a commonly used treatment to prevent strokes in asymptomatic carotid stenosis patients, although there is no convincing evidence that such treatment is more effective than current medical treatment in most if not all of these patients.
In addition, there is no solid evidence that CEA in asymptomatic patients prevents strokes more effectively than current medical treatment. Yet in the United States, 70%-90% of CEAs and 70%-96% of CAS procedures are performed on asymptomatic patients.
Should this be and how does the vascular disease paradox relate to this situation?
Vascular surgeons and interventionalists from all specialties want to continue to intervene on most of these asymptomatic carotid stenosis patients for several reasons. These include gratifyingly good outcomes from treating these usually simple, low-risk asymptomatic lesions and provision of income to physicians and hospitals.
These good outcomes also provide many accessory benefits to the treating physicians and surgeons by improving their overall results, a desirable goal in view of looming audits and pay-for-performance incentives. Also, increasing case numbers help practitioners to meet credentialing requirements.
However, there are negatives to continuing to perform large numbers of invasive treatments on asymptomatic carotid stenosis patients. One is the high cost to our health care system of providing these large numbers of invasive treatments largely to a group of patients who will derive little or no benefit. Another is the possibility that more patients will be harmed than helped.
Clearly what is needed are better ways to detect the asymptomatic patient at high risk for having a stroke so only those patients can be treated invasively. Although no such method is universally accepted, there are glimmers of hope that one or more will be proven effective.
Also needed are trials to establish the effectiveness of current medical therapy for stroke prevention in patients with asymptomatic carotid stenosis. However, such trials will not be simple to design because of the benign nature of most asymptomatic lesions. Thus, such trials will have to be conducted in patients selected to have more risky lesions.
Until such information is available, all practitioners should exercise restraint in treating patients with asymptomatic carotid stenosis invasively. They should not be seduced into treating simply because of the ease of treatment or the good outcomes – the vascular disease paradox. It is risk-benefit ratio that is more important. Physicians and surgeons should recognize that the landmark trials on this subject are now outdated, and should restrict such invasive treatment in some way to fewer patients than in the past – perhaps those with an increasing or very high grade (pinhole) stenosis or a contralateral occlusion.
Finally, it should be noted that a proposal to provide reimbursement for performing CAS on standard and low-risk carotid stenosis patients, including asymptomatic patients, is currently being considered by Medicare.
It is likely that some support for this proposal stems from the facts that lesions in such patients are easy to treat and the results of treatment are excellent. This is the vascular disease paradox which should be recognized and dealt with by all in the field.n
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
Vascular patients who most need treatment are often difficult and risky to treat by open operation or an endovascular intervention. In contrast, patients who least need invasive treatment or need it not at all usually have lesions that are easy to treat with good results. Examples are patients with advanced gangrene versus those with intermittent claudication; symptomatic patients with carotid artery stenosis versus those who are asymptomatic; patients with large abdominal aortic aneurysms versus those with small lesions.
This paradoxical situation occurs because most vascular lesions, particularly those associated with atherosclerosis, are in their early phases benign, cause minimal or no symptoms, and are unassociated with widespread vascular disease.
Moreover, many of these lesions, particularly with statins and other good medical therapy, will remain stable for long periods. These early lesions are technically easy to treat invasively, although such treatment may provide little or no benefit to the patient.
In contrast, only when lesions advance, become more complex and are associated with a widely diseased arterial system do they become threatening to life, limb, and the brain. These latter lesions are usually much harder to treat both by transcatheter or open operative techniques. This situation gives rise to several consequences relating to physician judgment, procedural outcomes, physician and institutional incomes, health care costs and ethical considerations.
Everyone in the vascular field should recognize and face these issues.
To help do so, let us examine some of these issues as they relate to the common problem of carotid bifurcation stenosis. High grade stenosis at this site, even when asymptomatic, can cause some strokes. Level 1 evidence from the so-called landmark asymptomatic trials (ACAS, ACST), which randomized patients from 1990-2003, showed significant stroke prevention from carotid endarterectomy (CEA) compared to medical treatment.
However, the benefit was slight (stroke rates were reduced from about 2% per year to about 1% per year), and there have been substantial improvements in medical treatments over the last decade to prevent strokes with statins and other measures. Carotid artery stenting (CAS) has also become a commonly used treatment to prevent strokes in asymptomatic carotid stenosis patients, although there is no convincing evidence that such treatment is more effective than current medical treatment in most if not all of these patients.
In addition, there is no solid evidence that CEA in asymptomatic patients prevents strokes more effectively than current medical treatment. Yet in the United States, 70%-90% of CEAs and 70%-96% of CAS procedures are performed on asymptomatic patients.
Should this be and how does the vascular disease paradox relate to this situation?
Vascular surgeons and interventionalists from all specialties want to continue to intervene on most of these asymptomatic carotid stenosis patients for several reasons. These include gratifyingly good outcomes from treating these usually simple, low-risk asymptomatic lesions and provision of income to physicians and hospitals.
These good outcomes also provide many accessory benefits to the treating physicians and surgeons by improving their overall results, a desirable goal in view of looming audits and pay-for-performance incentives. Also, increasing case numbers help practitioners to meet credentialing requirements.
However, there are negatives to continuing to perform large numbers of invasive treatments on asymptomatic carotid stenosis patients. One is the high cost to our health care system of providing these large numbers of invasive treatments largely to a group of patients who will derive little or no benefit. Another is the possibility that more patients will be harmed than helped.
Clearly what is needed are better ways to detect the asymptomatic patient at high risk for having a stroke so only those patients can be treated invasively. Although no such method is universally accepted, there are glimmers of hope that one or more will be proven effective.
Also needed are trials to establish the effectiveness of current medical therapy for stroke prevention in patients with asymptomatic carotid stenosis. However, such trials will not be simple to design because of the benign nature of most asymptomatic lesions. Thus, such trials will have to be conducted in patients selected to have more risky lesions.
Until such information is available, all practitioners should exercise restraint in treating patients with asymptomatic carotid stenosis invasively. They should not be seduced into treating simply because of the ease of treatment or the good outcomes – the vascular disease paradox. It is risk-benefit ratio that is more important. Physicians and surgeons should recognize that the landmark trials on this subject are now outdated, and should restrict such invasive treatment in some way to fewer patients than in the past – perhaps those with an increasing or very high grade (pinhole) stenosis or a contralateral occlusion.
Finally, it should be noted that a proposal to provide reimbursement for performing CAS on standard and low-risk carotid stenosis patients, including asymptomatic patients, is currently being considered by Medicare.
It is likely that some support for this proposal stems from the facts that lesions in such patients are easy to treat and the results of treatment are excellent. This is the vascular disease paradox which should be recognized and dealt with by all in the field.n
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
BEST PRACTICES IN: Prenatal Screening & Cord Blood Banking
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by PerkinElmer.
•Topics
•Faculty/Faculty Disclosure

To view the supplement, click the image above.
Topics
• Introduction
• Screening for Fetal Chromosomal Abnormalities
• UCB Banking
• Conclusions
Faculty/Faculty Disclosure
Joanne Stone, MD
Director, Perinatal Ultrasound
Division Director, Maternal Fetal Medicine
Department of Obstetrics, Gynecology, and Reproductive Medicine
Mt. Sinai School of Medicine
New York, NY
University of California, San Diego
La Jolla, California
Dr. Stone has nothing to disclose.
Copyright © 2009 by Elsevier Inc.
Cervical Cancer Screening in the Era of Improved Technology and HPV Vaccines
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement was supported by CYTYC Corporation.
The articles are based on interviews with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Introduction
Mark H. Einstein, MD, MS, Chair
Director, Clinical Research
Division of Gynecologic Oncology
Department of Obstetrics and Gynecology and Women's Health
Montefiore Medical Center
Bronx, N.Y.
Dr Einstein has received clinical grants from GlaxoSmithKline, Merck & Co., Inc., and Tigris Pharmaceuticals, Inc.
Milestones in Cervical Cancer Detection and Prevention: Significance in Clinical Practice
Mark H. Einstein, MD, MS, Chair
Improving HSIL and Glandular Disease Detection: What the Recent Data Show
Richard Lozano, MD
Director of Cytology
Pathology and Cytology Laboratories, Inc.
Lexington, Ky.
Dr Lozano has nothing to disclose.
and
Harold J. Sauer, MD, FACOG
Associate Professor and Acting Chair
Department of Obstetrics and Gynecology and Reproductive Biology
Michigan State University
Lansing
Dr Sauer has nothing to disclose.
Weighing the Costs and Benefits: Technologic Advances in Cervical Cancer Screening
Warner K. Huh, MD, FACOG, FACS
Assistant Professor
Division of Gynecologic Oncology
University of Alabama at Birmingham
Dr Huh has received clinical grants from 3M Pharmaceuticals, Cytyc Corporation, GlaxoSmithKline, MGI PHARMA, Merck, Roche Molecular Systems, and Tigris Pharmaceuticals. He is a consultant to GlaxoSmithKline, MGI PHARMA, Roche Molecular Systems, and mtm laboratories AG.
Efficacy of HPV Screening Versus Liquid-Based Cervical Cytology and Imaging: What the Data Really Show
Michael Karram, MD, FACOG
President and Medical Director
Seven Hills Women's Health Centers
Cincinnati, Ohio
Dr Karram has nothing to disclose.
and
Michael L. Krychman, MD
Associate Clinical Attending
Sexual Medicine Program
Memorial Sloan-Kettering Cancer Center
New York, N.Y.
Dr Krychman is a consultant to Cytyc.
Copyright © 2007 by Elsevier Inc.
CLINICAL UPDATE: Women's Health and Nutrition: Demographic Challenges
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
A supplement to Ob.Gyn. News.
This supplement is supported by Xanodyne Pharmaceuticals, Inc.
•Topic Highlights
•Faculty/Faculty Disclosures

To view the supplement, click the image above.
Topic Highlights
• Nutritional Gaps for Women in the United States
• Role of Obstetricians and Gynecologists in Women's Health Care
Faculty/Faculty Disclosures
Linda D. Bradley MD
Vice Chair, Obstetrics
Gynecology & Women's Health Institute
Cleveland Clinic
Cleveland, OH
Dr. Bradley is a consultant for Xanodyne Pharmaceuticals, Inc.
Beth Reardon, MS, RD, LDN
Integrative Nutritionist
Duke Integrative Nutrition
Durham, NC
Dr. Reardon has nothing to disclose.
John M. Thorp, Jr., MD
McAllister Distinguished Professor
Department of Obstetrics and Gynecology
University of North Carolina at Chapel Hill
Chapel Hill, NC
Dr. Thorp has nothing to disclose.
Barbara A. Underwood, PhD
Adjunct Professor of Nutrition
Columbia University
Institute of Human Nutrition
New York, NY
Dr. Underwood has nothing to disclose.
Fernando E. Viteri, MD, ScD
Professor (Emeritus)
Department of Nutritional Sciences and Toxicology
University of California
Berkeley, CA
and
Scientist
Children's Hospital
Oakland Research Institute
Oakland, CA
Dr. Viteri has received clinical grant funding from the University of California Institute for Mexico and the United States.
Copyright © 2009 by Elsevier Inc.
The Changing Landscape of Cervical Cancer Screening and Implications for the Clinician
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
A supplement to Ob.Gyn. News.
This educational supplement was supported by an educational grant from CYTYC Corporation.
The articles are based on clinical dialogues with the faculty.

To view the supplement, click the image above.
Topic Highlights/Faculty
Implications of Computer-Assisted Cervical Screening for the Ob.Gyn. Clinician
Co-Chairs:
Randall K. Gibb, MD
Assistant Professor, Division of Gynecologic Oncology
Washington University School of Medicine
St. Louis, Mo.
Thomas J. Herzog, MD
Director, Division of Gynecologic Oncology
Columbia University College of Physicians and Surgeons
New York, N.Y.
Comparison of Manual and Image-Directed Screening of Liquid-Based Cervical Cytology in a Large Metropolitan Cytology Practice
James R. Lingle, MD
Lingle, Gore, and Harding, P.C.
Englewood, Colo.
Fern S. Miller, MSN, CT(ASCP)
Cytology Manager, Cytology Department
Metropolitan Pathologists
Denver, Colo.
Performance of a Computer-Assisted Imaging System in Detecting High-Grade Squamous Intraepithelial Lesions
Bruce R. Dziura, MD
Chief of Pathology
New England Pathology Associates
Mercy Medical Center
Springfield, Mass
Timothy Kelly Fitzpatrick, MD
Attending Physician
Mercy Medical Center
Springfield, Mass.
Evaluation of a Computer-Assisted Imaging System in Diagnosing Uncommon Malignancies
Andrea E. Dawson, MD
Staff Pathologist
Cleveland Clinic Foundation
Cleveland, Ohio
Holly L. Thacker, MD
Director, Women's Health Center
Cleveland Clinic Foundation
Cleveland, Ohio
The faculty report they have nothing to disclose.
Copyright © 2005 by International Medical News Group
Chronic Dysfunctional Uterine Bleeding: Identifying Patients and Helping Them Understand Their Treatment Options
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
A supplement to Ob.Gyn. News.
Supported by an educational grant from Gynecare Worldwide, a division of Ethicon, Inc., a Johnson & Johnson Company.
The articles in this supplement are based on clinical dialogues with the faculty.
•Contents
•Faculty/Faculty Disclosure Statement

To view the supplement, click the image above.
Contents
Introduction
Consequences of Heavy Menstrual Bleeding
Types, Patterns, and Causes of Abnormal Uterine Bleeding
• Evaluating the Endometrial Cavity
Treatment Options: Entering the Dialogue
• Medical Therapy
• Surgical Interventions
• Endometrial Ablation Procedures
Considering Cases:
• An Overweight Patient
• A Patient Who Prefers to Avoid Hysterectomy
• A Patient With Postsurgical HMB
Helping Patients Choose
Conclusion
Faculty/Faculty Disclosure Statement
Mary Jane Minkin, MD, FACOG, Chair
Clinical Professor
Department of Obstetrics and Gynecology
Yale University School of Medicine
New Haven, Conn.
Developed a Web site for Gynecare; Speaker's Bureau: Berlex, Inc.
Charles E. Miller, MD, FACOG
Clinical Associate Professor
Department of Obstetrics and Gynecology
University of Illinois at Chicago
Clinical Associate
Department of Obstetrics and Gynecology
University of Chicago
Consultant: Gynecare Worldwide.
Malcolm G. Munro, MD, FRCS(c), FACOG
Professor
Department of Obstetrics and Gynecology
The David Geffen School of Medicine at UCLA
Los Angeles
Attending Staff
Department of Obstetrics and Gynecology
Kaiser Permanente Los Angeles Medical Center
Received Funding for Clinical Grants: Kaiser Research Foundation and Karl Storz Endoscopy-America, Inc.M
Consultant: Boston Scientific Corporation, Gynecare, and Karl Storz Endoscopy.
Robert K. Zurawin, MD, FACOG
Associate Professor
Department of Obstetrics and Gynecology
Baylor College of Medicine
Houston
Consultant/Speaker: Gynecare Worldwide.
Copyright © 2004 by International Medical News Group
Verbal Communication at Discharge
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
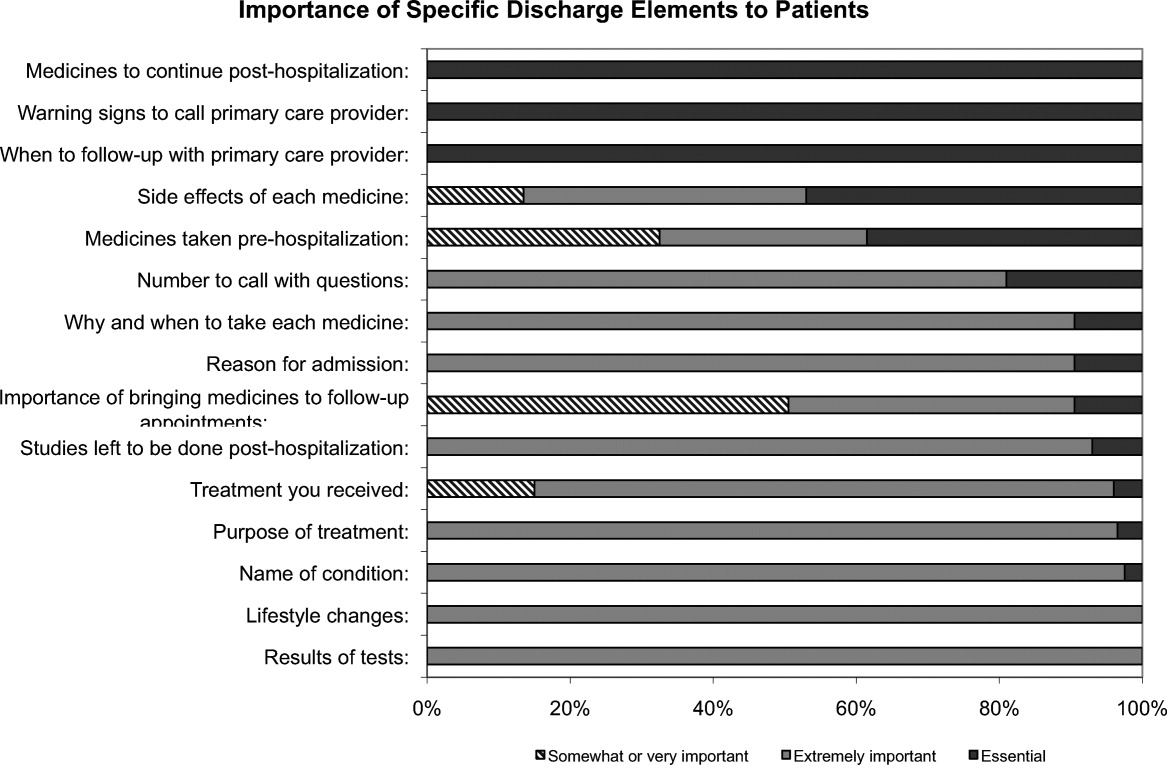
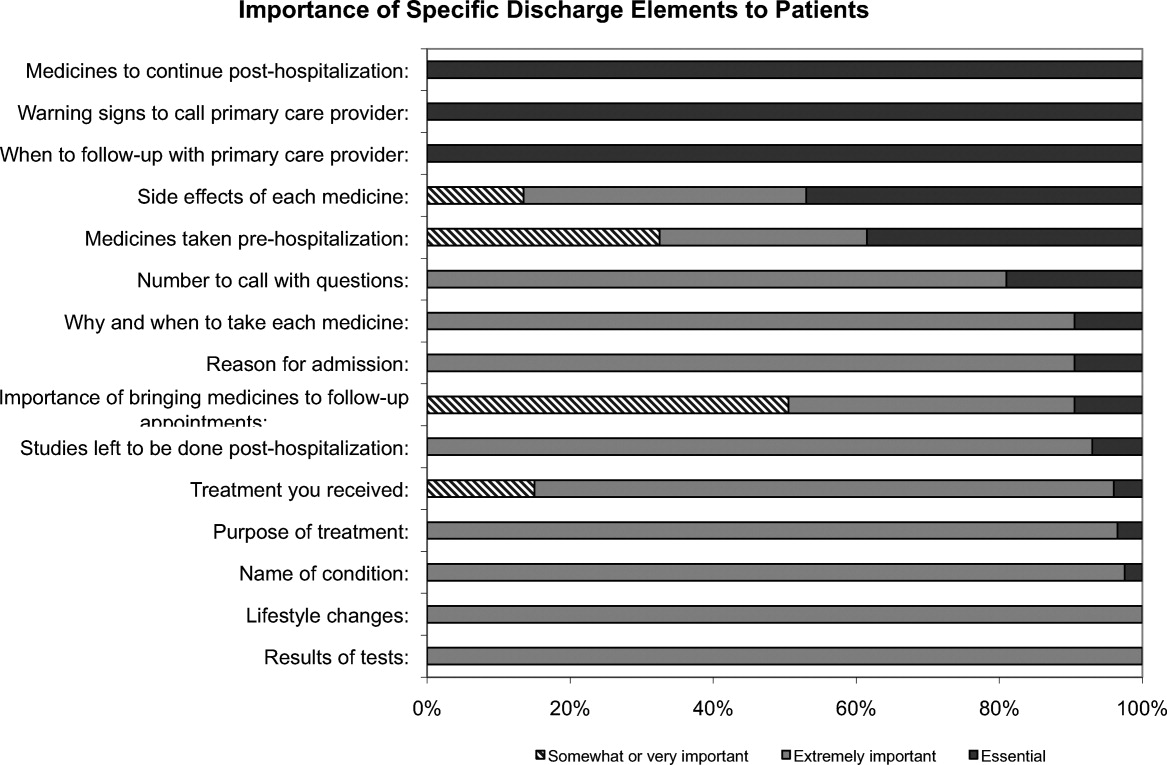
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.
The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.

The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.

The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.