User login
Antireflux surgery in the proton pump inhibitor era
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
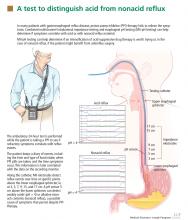
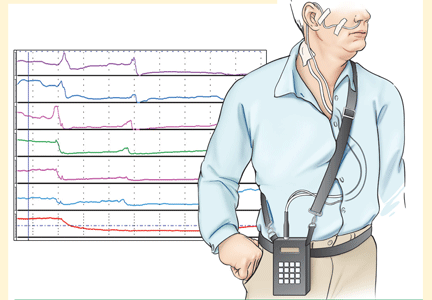
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
KEY POINTS
- If a PPI in twice-daily doses fails to relieve GERD symptoms, a pH study combined with multichannel intraluminal impedance testing can help in deciding whether to try surgery.
- Antireflux surgery can be considered for erosive esophagitis that does not resolve with drug therapy, for volume regurgitation (particularly if it occurs at night or if there is a risk of aspiration), and for patients who need lifelong treatment for reflux but have had a serious adverse event related to PPI therapy.
- Studies are needed to directly compare medical and surgical therapy in patients with extraesophageal manifestations of GERD and refractory symptoms, a difficult group of patients.
- Drugs that inhibit transient relaxation of the lower esophageal sphincter are under investigation, as are minimally invasive procedures to manipulate the physical barrier to reflux.
Factor V Leiden: How great is the risk of venous thromboembolism?
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
KEY POINTS
- The pathogenesis of venous thromboembolism is complex and multifactorial, often reflecting the interplay between environmental, clinical, and genetic factors.
- Factor V Leiden increases the risk of venous thromboembolism but by itself does not appear to increase the risk of arterial thrombosis.
- Often, people with factor V Leiden may have additional risk factors that increase the rate of venous clots, such as older age, surgery, obesity, immobility, prolonged travel, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy.
- General measures and precautions are needed to minimize the risk of venous thromboembolism in people with the factor V Leiden mutation, especially when modifiable factors are present, such as obesity and long periods of immobilization.
Purple urine in a woman with chronic kidney disease
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
Purpuric lesion on the elbow
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
A 75-year-old man was admitted to the hospital with new-onset atrial fibrillation. He underwent rate control, and a heparin infusion was started. Warfarin (Coumadin) 10 mg was added on the second hospital day. Two days later, the heparin infusion was discontinued when the international normalized ratio (INR) was in the therapeutic range.
Q: Which is the most likely diagnosis?
- Pyoderma gangrenosum
- Cutaneous vasculitis
- Warfarin-induced skin necrosis
- Ecthyma gangrenosum
- Dermatitis herpetiformis
A: The most likely diagnosis is warfarin-induced skin necrosis, a rare paradoxical complication that occurs in 0.01% to 0.1% of patients receiving this drug.1 Microthrombosis leads to necrosis of the skin and subcutaneous tissues, arising within 2 to 10 days after the start of anticoagulation therapy, although in rare cases it can occur months to years later.2,3
The most common risk factors include the unopposed use of warfarin (ie, unopposed by heparin at the start of therapy), using higher doses of warfarin during the initiation of anticoagulation, and inadequate overlap with an effective parenteral anticoagulant. Patients with protein C or S deficiency, heparin-induced thrombocytopenia,4 resistance to activated protein C, antithrombin deficiency, and lupus anticoagulant have also been reported to be at risk.
The most common sites affected are areas with high subcutaneous fat content, such as the abdomen, thighs, breasts, and buttocks. Skin presentations can vary from dermal plaques to petechial lesions, which rapidly progress to well-demarcated, bluish-black, painful lesions and eventually to hemorrhagic bullae and necrosis.1
At the start of warfarin therapy, the levels of protein C and factor VII (with half-lives of 5 to 8 hours) fall faster than those of other vitamin-K-dependent factors (ie, factors II, IX, and X). This causes a transient imbalance in procoagulant and anticoagulant pathways favoring thrombosis of the microvasculature, with resulting necrosis. Patients with hereditary protein C deficiency are at higher risk.5
Histologic review of lesions often shows venous thrombosis and diffuse necrosis of the dermis and subcutaneous tissue.2
Promptly stopping the warfarin and choosing alternative anticoagulation may help prevent further progression of this condition. Wound care, debridement, and sometimes skin grafting may be necessary, depending on the extent of the lesions. A rechallenge with warfarin is often difficult, but cases have been reported in which treatment was resumed without adverse consequences.6 Avoiding large loading doses of warfarin, gradually increasing doses over an extended period (about 10 days),3 and starting warfarin with a heparin bridge for at least 5 days (which was not done in this patient) would prevent the condition.
Early recognition, differentiation, and diagnosis are essential to minimize morbidity and to prevent death.
CASE CONTINUED
Warfarin was discontinued once the patient developed the skin lesions. He received vitamin K and fresh frozen plasma to normalize his INR, and he was started on a heparin infusion, after which the lesions began to heal. The patient refused a skin biopsy. Platelet counts remained stable during his hospital course. Protein C levels were not checked, given his recent use of warfarin. He was started on dabigatran (Pradaxa) and was discharged a week later.
THE OTHER DIAGNOSTIC CHOICES
Pyoderma gangrenosum is an uncommon ulcerative skin condition often associated with autoimmune disease. It usually starts at the site of a minor injury, more commonly on the legs, and gradually progresses to a painful ulcer.
Cutaneous vasculitis is an inflammation of small blood vessels characterized by palpable purpura. The lesions can resemble urticaria, petechia, or erythema multiforme. It is commonly associated with infection, drug therapy, inflammatory disease, and malignancy.
Ecthyma gangrenosum is an infection of skin caused by Pseudomonas aeruginosa. Usually, it presents as hemorrhagic pustules or infarct-like areas with surrounding erythema that evolve into necrotic ulcers surrounded by erythema.
Dermatitis herpetiformis is a chronic skin condition, presenting with fluid-filled blisters and commonly involving the neck, back, scalp, and elbows. This condition is associated with celiac disease, and the lesions are extremely pruritic.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.
- Nazarian RM, Van Cott EM, Zembowicz A, Duncan LM. Warfarin-induced skin necrosis. J Am Acad Dermatol 2009; 61:325–332.
- Ward CT, Chavalitanonda N. Atypical warfarin-induced skin necrosis. Pharmacotherapy 2006; 26:1175–1179.
- Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272.
- Warkentin TE, Sikov WM, Lillicrap DP. Multicentric warfarin-induced skin necrosis complicating heparin-induced thrombocytopenia. Am J Hematol 1999; 62:44–48.
- Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg 2000; 53:624–626.
- Jillella AP, Lutcher CL. Reinstituting warfarin in patients who develop warfarin skin necrosis. Am J Hematol 1996; 52:117–119.
Examine before ordering: An algorithm unchanged by new tests
We rheumatologists may have inadvertently encouraged this practice. We teach about the prevalence of specific autoantibodies in patients with specific, accurately diagnosed autoimmune disorders as opposed to that in the general population (ie, the test’s sensitivity and specificity). But that is different than using a test to diagnose a specific disease in an ill patient with a heretofore undiagnosed condition (ie, the test’s predictive value). When I ask trainees or nonrheumatologists, “Why order all those tests?” the response I often get is that they thought the rheumatologist would want them when he or she was consulted. The fact that I also see our rheumatology fellows requesting the same tests before fully evaluating the patient clinically suggests that we have not done a great job at explaining the clinical utility and limitations of these tests. A serologic test should be used to strengthen or refute the clinician’s preliminary diagnosis, depending on the test’s specificity and sensitivity. It should not be used to generate a diagnosis.
So with these concerns, why would we invite a paper encouraging the use of the relatively new anti-cyclic citrullinated peptide (anti-CCP) test to evaluate patients with possible rheumatoid arthritis (Bose and Calabrese)?
As discussed in that paper, this test has characteristics that are useful when evaluating patients with polyarthritis compatible with the diagnosis of rheumatoid arthritis. Specifically, this test, unlike the traditional test for rheumatoid factor, can help discern whether the arthritis is a reaction to an infection like hepatitis C or endocarditis. Like rheumatoid factor, anti-CCP may precede the appearance of clinically meaningful arthritis and helps to predict prognosis in established rheumatoid arthritis. But, like other serologic tests, the anti-CCP test cannot supplant the listening ears and examining fingers of the clinician in establishing the pretest likelihood of the diagnosis. Clinical evaluation must precede laboratory testing.
We rheumatologists may have inadvertently encouraged this practice. We teach about the prevalence of specific autoantibodies in patients with specific, accurately diagnosed autoimmune disorders as opposed to that in the general population (ie, the test’s sensitivity and specificity). But that is different than using a test to diagnose a specific disease in an ill patient with a heretofore undiagnosed condition (ie, the test’s predictive value). When I ask trainees or nonrheumatologists, “Why order all those tests?” the response I often get is that they thought the rheumatologist would want them when he or she was consulted. The fact that I also see our rheumatology fellows requesting the same tests before fully evaluating the patient clinically suggests that we have not done a great job at explaining the clinical utility and limitations of these tests. A serologic test should be used to strengthen or refute the clinician’s preliminary diagnosis, depending on the test’s specificity and sensitivity. It should not be used to generate a diagnosis.
So with these concerns, why would we invite a paper encouraging the use of the relatively new anti-cyclic citrullinated peptide (anti-CCP) test to evaluate patients with possible rheumatoid arthritis (Bose and Calabrese)?
As discussed in that paper, this test has characteristics that are useful when evaluating patients with polyarthritis compatible with the diagnosis of rheumatoid arthritis. Specifically, this test, unlike the traditional test for rheumatoid factor, can help discern whether the arthritis is a reaction to an infection like hepatitis C or endocarditis. Like rheumatoid factor, anti-CCP may precede the appearance of clinically meaningful arthritis and helps to predict prognosis in established rheumatoid arthritis. But, like other serologic tests, the anti-CCP test cannot supplant the listening ears and examining fingers of the clinician in establishing the pretest likelihood of the diagnosis. Clinical evaluation must precede laboratory testing.
We rheumatologists may have inadvertently encouraged this practice. We teach about the prevalence of specific autoantibodies in patients with specific, accurately diagnosed autoimmune disorders as opposed to that in the general population (ie, the test’s sensitivity and specificity). But that is different than using a test to diagnose a specific disease in an ill patient with a heretofore undiagnosed condition (ie, the test’s predictive value). When I ask trainees or nonrheumatologists, “Why order all those tests?” the response I often get is that they thought the rheumatologist would want them when he or she was consulted. The fact that I also see our rheumatology fellows requesting the same tests before fully evaluating the patient clinically suggests that we have not done a great job at explaining the clinical utility and limitations of these tests. A serologic test should be used to strengthen or refute the clinician’s preliminary diagnosis, depending on the test’s specificity and sensitivity. It should not be used to generate a diagnosis.
So with these concerns, why would we invite a paper encouraging the use of the relatively new anti-cyclic citrullinated peptide (anti-CCP) test to evaluate patients with possible rheumatoid arthritis (Bose and Calabrese)?
As discussed in that paper, this test has characteristics that are useful when evaluating patients with polyarthritis compatible with the diagnosis of rheumatoid arthritis. Specifically, this test, unlike the traditional test for rheumatoid factor, can help discern whether the arthritis is a reaction to an infection like hepatitis C or endocarditis. Like rheumatoid factor, anti-CCP may precede the appearance of clinically meaningful arthritis and helps to predict prognosis in established rheumatoid arthritis. But, like other serologic tests, the anti-CCP test cannot supplant the listening ears and examining fingers of the clinician in establishing the pretest likelihood of the diagnosis. Clinical evaluation must precede laboratory testing.
Should I order an anti-CCP antibody test to diagnose rheumatoid arthritis?
Yes. Testing for anti-cyclic citrullinated peptide (anti-CCP) antibody can help diagnose rheumatoid arthritis (RA) because it is a highly specific test.
For many years, the diagnosis of RA has been based on the presentation of symmetrical small- and large-joint polyarthritis that spares the lower spine, further supported by the presence of characteristic joint damage on radiography and an elevated rheumatoid factor while also excluding clinical mimics. However, rheumatoid factor is often not detected early in RA, and detection of rheumatoid factor is not specific for RA. Testing for anti-CCP antibody can provide additional information and, in some cases, enable earlier and more specific diagnosis.
An important advance in our understanding of the pathogenesis of RA and in improving our ability to diagnose it early is the recognition that RA patients often produce autoantibodies directed against proteins and peptides containing the amino acid citrulline. Citrulline is generated in an inflammatory environment by the modification of the amino acid arginine by the enzyme peptidylarginine deiminase. Antibodies against cyclic citrulline are generated by patients with a certain genetic makeup, although citrulline can be detected in inflammatory tissues in conditions other than RA (without the antibody).
Anti-CCP antibody has been found in sera up to 10 years before the onset of joint symptoms in patients who later develop RA and may appear somewhat earlier than rheumatoid factor.1 From 10% to 15% of RA patients remain seronegative for rheumatoid factor throughout the disease course.
INFORMAL GUIDELINES FOR ANTI-CCP ANTIBODY TESTING
The role of anti-CCP antibody testing in the management of RA is still being defined, but we suggest several informal guidelines.
Anti-CCP antibody testing can help interpret the significance of an inexplicably high rheumatoid factor titer in the absence of classic RA. In such situations, a negative anti-CCP antibody test suggests a nonrheumatic disorder such as hepatitis C virus infection or endocarditis, whereas a positive anti-CCP antibody test is more consistent with early or even preclinical RA since this test, unlike rheumatoid factor testing, is generally negative in the setting of infection.
However, in a patient who has documented RA and who is seropositive for rheumatoid factor, anti-CCP antibody testing has limited value, as the information it provides may be redundant. In a patient with a low to intermediate probability for RA and with a negative or low level of rheumatoid factor, a positive anti-CCP antibody test helps confirm the diagnosis. Rheumatoid factor positivity and anti-CCP antibody positivity are each associated with more severe RA. Neither test varies with the activity of RA.
Finally, in smokers with a particular genotype, the presence of anti-CCP antibody predicts a particularly worse course for RA.
THE ROLE OF RHEUMATOID FACTOR TESTING
Rheumatoid factor, first described in 1940,4 is an antibody against the Fc portion of immunoglobulin G. The cutoff value for positivity varies by laboratory but is usually greater than 45 IU/mL by enzyme-linked immunosorbent assay or laser nephelometry, or greater than 1:80 by latex fixation. However, serum titers or serum levels expressed as “IU/mL” cannot accurately be compared between laboratories; instead, when using tests for rheumatoid factor, physicians should refer to specificity and sensitivity measurements for each analyzing laboratory.
Around 50% of patients with RA become positive for rheumatoid factor in the first 6 months, and 85% become positive over the first 2 years. Also, rheumatoid factor testing suffers from low specificity, since it can be detected (although sometimes in low levels) in a variety of infectious and inflammatory conditions, such as bacterial endocarditis, malaria, tuberculosis, osteomyelitis, hepatitis C (with or without cryoglobulinemia), Sjögren syndrome, systemic lupus erythematosus, primary biliary cirrhosis, postvaccination arthropathy, and aging.
Current detection methods cannot differentiate between naturally occurring, transiently induced, and RA-associated rheumatoid factor. The levels are generally higher in RA than in many non-RA disorders, but significant overlap occurs. Rheumatoid factor positivity serves as a marker of poor prognosis, predicting generally more aggressive, erosive disease, and it is correlated with extra-articular manifestations such as rheumatoid nodules and lung involvement.
The classification criteria for RA published in 2010 by the American College of Rheumatology and the European League Against Rheumatism provide references for the measurement of rheumatoid factor: “low-level positive” refers to values less than or equal to three times the upper limit of normal for a particular laboratory; “high-level positive” refers to values more than three times the upper limit of normal.5 This is an attempt to provide a clinically useful benchmark for the measurement of rheumatoid factor, the values of which may vary between laboratories.
STUDIES COMPARING THE TWO TESTS
Several studies have evaluated the utility and validity of anti-CCP antibody testing vs rheumatoid factor testing.
In a study of 826 US veterans with RA,6 75% tested positive for anti-CCP antibody and 80% were positive for rheumatoid factor. It was found that a higher anti-CCP antibody titer was associated with increased disease activity and inversely correlated with remission, especially in those also positive for rheumatoid factor.6
In another study,1 in which blood samples from 79 patients with RA who had been blood donors were analyzed, 39 patients (49.4%) were positive for either rheumatoid factor or anti-CCP antibody, or both, a median of 4.5 years (range 0.1 to 13.8 years) before the onset of RA symptoms; 32 patients (40.5%) became positive for anti-CCP antibody before symptom onset.
Whiting et al,7 in a systematic review of 151 studies, showed that anti-CCP antibody testing had greater specificity than rheumatoid factor testing (96% vs 86%), with similar sensitivity (56% vs 58%)—most notably in eight cohort studies of patients with early RA.7 In the 15 cohort studies analyzed, the test was found to have a positive likelihood ratio of 12.7 and a negative likelihood ratio of 0.45, supporting this as a test of high positive predictive value for RA.
In view of the evidence from these studies, it is not surprising that the 2010 collaborative classification of RA of the American College of Rheumatology and the European League Against Rheumatism places equal weight on anti-CCP antibody testing and rheumatoid factor testing in the early diagnosis of RA.5
GENETICS AND THE PROGNOSIS OF RHEUMATOID ARTHRITIS
In recent years, there has been a growing recognition that the pathogenesis of RA in patients who are seropositive for rheumatoid factor or anti-CCP antibody is different from the pathogenesis of RA in patients who are seronegative for rheumatoid factor and anti-CCP antibody. This may help us guide therapy.
Patients positive for rheumatoid factor or anti-CCP antibody who have a specific allelic subset of a region of the immune-response gene DRB1*04 appear to be highly vulnerable to smoking as an environmental trigger or to worsening RA.8
Patients positive for anti-CCP antibody tend also to have severe joint destruction and, hence, have a worse prognosis. Kaltenhäuser et al9 found that determining the presence of the shared epitope (an RA-specific genetic marker) and positivity for anti-CCP antibody facilitates prediction of the disease course and prognosis.9
Studies have shown that patients with confirmed RA who test positive for anti-CCP antibody may also have more-severe extraarticular manifestations. Recent studies have found anti-CCP antibody positivity in 15.7% to 17.5% of patients with psoriatic arthritis and in 85% of patients with RA. Patients with psoriatic arthritis who were positive for anti-CCP antibody had more joints that were tender and swollen, erosive arthritis, deformities, and functional impairment of peripheral joints.10,11
THE COST DIFFERENCE IS TRIVIAL IN THE LONG RUN
Cost is the major differentiating factor between rheumatoid factor testing and anti-CCP antibody testing. Rheumatoid factor testing costs around $43, and anti-CCP antibody testing costs $102 in the reference laboratory at Cleveland Clinic. However, the difference in cost is trivial, since this is only a one-time cost, whereas the information anti-CCP antibody testing provides can have a major impact on predicting the prognosis and determining the choice of therapy for a disease associated with high direct and indirect costs over a lifetime. Also, Medicare and other insurers would likely reimburse for anti-CCP antibody testing as long as it was associated with a related diagnosis such as arthralgia or arthritis.
Given that there will be a small number of patients with confirmed RA who will be negative for rheumatoid factor yet positive for anti-CCP antibody, one can support ordering both tests in tandem in a patient whom you strongly suspect of having RA. Or, at $100, one could make the argument that it would be cost-effective to order anti-CCP antibody testing only if rheumatoid factor testing is negative.
Testing for rheumatoid factor and anti-CCP antibody should not be done serially to assess treatment response or disease activity in these patients: these markers do not vary with inflammatory activity or disappear with clinical “remission.”
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–386.
- Egerer K, Feist E, Burmester GR. The serological diagnosis of rheumatoid arthritis: antibodies to citrullinated antigens. Dtsch Arztebl Int 2009; 106:159–163.
- Conrad K, Roggenbuck D, Reinhold D, Dörner T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun Rev 2010; 9:431–435.
- Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. 1939. APMIS 2007; 115:422–438.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581.
- Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010; 69:1292–1297.
- Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010; 152:456–464;W155–W166.
- van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 2008; 1143:268–285.
- Kaltenhäuser S, Pierer M, Arnold S, et al. Antibodies against cyclic citrullinated peptide are associated with the DRB1 shared epitope and predict joint erosion in rheumatoid arthritis. Rheumatology (Oxford) 2007; 46:100–104.
- Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol 2005; 32:511–515.
- Abdel Fattah NS, Hassan HE, Galal ZA, El Okda el SE. Assessment of anti-cyclic citrullinated peptide in psoriatic arthritis. BMC Res Notes 2009; 2:44.
Yes. Testing for anti-cyclic citrullinated peptide (anti-CCP) antibody can help diagnose rheumatoid arthritis (RA) because it is a highly specific test.
For many years, the diagnosis of RA has been based on the presentation of symmetrical small- and large-joint polyarthritis that spares the lower spine, further supported by the presence of characteristic joint damage on radiography and an elevated rheumatoid factor while also excluding clinical mimics. However, rheumatoid factor is often not detected early in RA, and detection of rheumatoid factor is not specific for RA. Testing for anti-CCP antibody can provide additional information and, in some cases, enable earlier and more specific diagnosis.
An important advance in our understanding of the pathogenesis of RA and in improving our ability to diagnose it early is the recognition that RA patients often produce autoantibodies directed against proteins and peptides containing the amino acid citrulline. Citrulline is generated in an inflammatory environment by the modification of the amino acid arginine by the enzyme peptidylarginine deiminase. Antibodies against cyclic citrulline are generated by patients with a certain genetic makeup, although citrulline can be detected in inflammatory tissues in conditions other than RA (without the antibody).
Anti-CCP antibody has been found in sera up to 10 years before the onset of joint symptoms in patients who later develop RA and may appear somewhat earlier than rheumatoid factor.1 From 10% to 15% of RA patients remain seronegative for rheumatoid factor throughout the disease course.
INFORMAL GUIDELINES FOR ANTI-CCP ANTIBODY TESTING
The role of anti-CCP antibody testing in the management of RA is still being defined, but we suggest several informal guidelines.
Anti-CCP antibody testing can help interpret the significance of an inexplicably high rheumatoid factor titer in the absence of classic RA. In such situations, a negative anti-CCP antibody test suggests a nonrheumatic disorder such as hepatitis C virus infection or endocarditis, whereas a positive anti-CCP antibody test is more consistent with early or even preclinical RA since this test, unlike rheumatoid factor testing, is generally negative in the setting of infection.
However, in a patient who has documented RA and who is seropositive for rheumatoid factor, anti-CCP antibody testing has limited value, as the information it provides may be redundant. In a patient with a low to intermediate probability for RA and with a negative or low level of rheumatoid factor, a positive anti-CCP antibody test helps confirm the diagnosis. Rheumatoid factor positivity and anti-CCP antibody positivity are each associated with more severe RA. Neither test varies with the activity of RA.
Finally, in smokers with a particular genotype, the presence of anti-CCP antibody predicts a particularly worse course for RA.
THE ROLE OF RHEUMATOID FACTOR TESTING
Rheumatoid factor, first described in 1940,4 is an antibody against the Fc portion of immunoglobulin G. The cutoff value for positivity varies by laboratory but is usually greater than 45 IU/mL by enzyme-linked immunosorbent assay or laser nephelometry, or greater than 1:80 by latex fixation. However, serum titers or serum levels expressed as “IU/mL” cannot accurately be compared between laboratories; instead, when using tests for rheumatoid factor, physicians should refer to specificity and sensitivity measurements for each analyzing laboratory.
Around 50% of patients with RA become positive for rheumatoid factor in the first 6 months, and 85% become positive over the first 2 years. Also, rheumatoid factor testing suffers from low specificity, since it can be detected (although sometimes in low levels) in a variety of infectious and inflammatory conditions, such as bacterial endocarditis, malaria, tuberculosis, osteomyelitis, hepatitis C (with or without cryoglobulinemia), Sjögren syndrome, systemic lupus erythematosus, primary biliary cirrhosis, postvaccination arthropathy, and aging.
Current detection methods cannot differentiate between naturally occurring, transiently induced, and RA-associated rheumatoid factor. The levels are generally higher in RA than in many non-RA disorders, but significant overlap occurs. Rheumatoid factor positivity serves as a marker of poor prognosis, predicting generally more aggressive, erosive disease, and it is correlated with extra-articular manifestations such as rheumatoid nodules and lung involvement.
The classification criteria for RA published in 2010 by the American College of Rheumatology and the European League Against Rheumatism provide references for the measurement of rheumatoid factor: “low-level positive” refers to values less than or equal to three times the upper limit of normal for a particular laboratory; “high-level positive” refers to values more than three times the upper limit of normal.5 This is an attempt to provide a clinically useful benchmark for the measurement of rheumatoid factor, the values of which may vary between laboratories.
STUDIES COMPARING THE TWO TESTS
Several studies have evaluated the utility and validity of anti-CCP antibody testing vs rheumatoid factor testing.
In a study of 826 US veterans with RA,6 75% tested positive for anti-CCP antibody and 80% were positive for rheumatoid factor. It was found that a higher anti-CCP antibody titer was associated with increased disease activity and inversely correlated with remission, especially in those also positive for rheumatoid factor.6
In another study,1 in which blood samples from 79 patients with RA who had been blood donors were analyzed, 39 patients (49.4%) were positive for either rheumatoid factor or anti-CCP antibody, or both, a median of 4.5 years (range 0.1 to 13.8 years) before the onset of RA symptoms; 32 patients (40.5%) became positive for anti-CCP antibody before symptom onset.
Whiting et al,7 in a systematic review of 151 studies, showed that anti-CCP antibody testing had greater specificity than rheumatoid factor testing (96% vs 86%), with similar sensitivity (56% vs 58%)—most notably in eight cohort studies of patients with early RA.7 In the 15 cohort studies analyzed, the test was found to have a positive likelihood ratio of 12.7 and a negative likelihood ratio of 0.45, supporting this as a test of high positive predictive value for RA.
In view of the evidence from these studies, it is not surprising that the 2010 collaborative classification of RA of the American College of Rheumatology and the European League Against Rheumatism places equal weight on anti-CCP antibody testing and rheumatoid factor testing in the early diagnosis of RA.5
GENETICS AND THE PROGNOSIS OF RHEUMATOID ARTHRITIS
In recent years, there has been a growing recognition that the pathogenesis of RA in patients who are seropositive for rheumatoid factor or anti-CCP antibody is different from the pathogenesis of RA in patients who are seronegative for rheumatoid factor and anti-CCP antibody. This may help us guide therapy.
Patients positive for rheumatoid factor or anti-CCP antibody who have a specific allelic subset of a region of the immune-response gene DRB1*04 appear to be highly vulnerable to smoking as an environmental trigger or to worsening RA.8
Patients positive for anti-CCP antibody tend also to have severe joint destruction and, hence, have a worse prognosis. Kaltenhäuser et al9 found that determining the presence of the shared epitope (an RA-specific genetic marker) and positivity for anti-CCP antibody facilitates prediction of the disease course and prognosis.9
Studies have shown that patients with confirmed RA who test positive for anti-CCP antibody may also have more-severe extraarticular manifestations. Recent studies have found anti-CCP antibody positivity in 15.7% to 17.5% of patients with psoriatic arthritis and in 85% of patients with RA. Patients with psoriatic arthritis who were positive for anti-CCP antibody had more joints that were tender and swollen, erosive arthritis, deformities, and functional impairment of peripheral joints.10,11
THE COST DIFFERENCE IS TRIVIAL IN THE LONG RUN
Cost is the major differentiating factor between rheumatoid factor testing and anti-CCP antibody testing. Rheumatoid factor testing costs around $43, and anti-CCP antibody testing costs $102 in the reference laboratory at Cleveland Clinic. However, the difference in cost is trivial, since this is only a one-time cost, whereas the information anti-CCP antibody testing provides can have a major impact on predicting the prognosis and determining the choice of therapy for a disease associated with high direct and indirect costs over a lifetime. Also, Medicare and other insurers would likely reimburse for anti-CCP antibody testing as long as it was associated with a related diagnosis such as arthralgia or arthritis.
Given that there will be a small number of patients with confirmed RA who will be negative for rheumatoid factor yet positive for anti-CCP antibody, one can support ordering both tests in tandem in a patient whom you strongly suspect of having RA. Or, at $100, one could make the argument that it would be cost-effective to order anti-CCP antibody testing only if rheumatoid factor testing is negative.
Testing for rheumatoid factor and anti-CCP antibody should not be done serially to assess treatment response or disease activity in these patients: these markers do not vary with inflammatory activity or disappear with clinical “remission.”
Yes. Testing for anti-cyclic citrullinated peptide (anti-CCP) antibody can help diagnose rheumatoid arthritis (RA) because it is a highly specific test.
For many years, the diagnosis of RA has been based on the presentation of symmetrical small- and large-joint polyarthritis that spares the lower spine, further supported by the presence of characteristic joint damage on radiography and an elevated rheumatoid factor while also excluding clinical mimics. However, rheumatoid factor is often not detected early in RA, and detection of rheumatoid factor is not specific for RA. Testing for anti-CCP antibody can provide additional information and, in some cases, enable earlier and more specific diagnosis.
An important advance in our understanding of the pathogenesis of RA and in improving our ability to diagnose it early is the recognition that RA patients often produce autoantibodies directed against proteins and peptides containing the amino acid citrulline. Citrulline is generated in an inflammatory environment by the modification of the amino acid arginine by the enzyme peptidylarginine deiminase. Antibodies against cyclic citrulline are generated by patients with a certain genetic makeup, although citrulline can be detected in inflammatory tissues in conditions other than RA (without the antibody).
Anti-CCP antibody has been found in sera up to 10 years before the onset of joint symptoms in patients who later develop RA and may appear somewhat earlier than rheumatoid factor.1 From 10% to 15% of RA patients remain seronegative for rheumatoid factor throughout the disease course.
INFORMAL GUIDELINES FOR ANTI-CCP ANTIBODY TESTING
The role of anti-CCP antibody testing in the management of RA is still being defined, but we suggest several informal guidelines.
Anti-CCP antibody testing can help interpret the significance of an inexplicably high rheumatoid factor titer in the absence of classic RA. In such situations, a negative anti-CCP antibody test suggests a nonrheumatic disorder such as hepatitis C virus infection or endocarditis, whereas a positive anti-CCP antibody test is more consistent with early or even preclinical RA since this test, unlike rheumatoid factor testing, is generally negative in the setting of infection.
However, in a patient who has documented RA and who is seropositive for rheumatoid factor, anti-CCP antibody testing has limited value, as the information it provides may be redundant. In a patient with a low to intermediate probability for RA and with a negative or low level of rheumatoid factor, a positive anti-CCP antibody test helps confirm the diagnosis. Rheumatoid factor positivity and anti-CCP antibody positivity are each associated with more severe RA. Neither test varies with the activity of RA.
Finally, in smokers with a particular genotype, the presence of anti-CCP antibody predicts a particularly worse course for RA.
THE ROLE OF RHEUMATOID FACTOR TESTING
Rheumatoid factor, first described in 1940,4 is an antibody against the Fc portion of immunoglobulin G. The cutoff value for positivity varies by laboratory but is usually greater than 45 IU/mL by enzyme-linked immunosorbent assay or laser nephelometry, or greater than 1:80 by latex fixation. However, serum titers or serum levels expressed as “IU/mL” cannot accurately be compared between laboratories; instead, when using tests for rheumatoid factor, physicians should refer to specificity and sensitivity measurements for each analyzing laboratory.
Around 50% of patients with RA become positive for rheumatoid factor in the first 6 months, and 85% become positive over the first 2 years. Also, rheumatoid factor testing suffers from low specificity, since it can be detected (although sometimes in low levels) in a variety of infectious and inflammatory conditions, such as bacterial endocarditis, malaria, tuberculosis, osteomyelitis, hepatitis C (with or without cryoglobulinemia), Sjögren syndrome, systemic lupus erythematosus, primary biliary cirrhosis, postvaccination arthropathy, and aging.
Current detection methods cannot differentiate between naturally occurring, transiently induced, and RA-associated rheumatoid factor. The levels are generally higher in RA than in many non-RA disorders, but significant overlap occurs. Rheumatoid factor positivity serves as a marker of poor prognosis, predicting generally more aggressive, erosive disease, and it is correlated with extra-articular manifestations such as rheumatoid nodules and lung involvement.
The classification criteria for RA published in 2010 by the American College of Rheumatology and the European League Against Rheumatism provide references for the measurement of rheumatoid factor: “low-level positive” refers to values less than or equal to three times the upper limit of normal for a particular laboratory; “high-level positive” refers to values more than three times the upper limit of normal.5 This is an attempt to provide a clinically useful benchmark for the measurement of rheumatoid factor, the values of which may vary between laboratories.
STUDIES COMPARING THE TWO TESTS
Several studies have evaluated the utility and validity of anti-CCP antibody testing vs rheumatoid factor testing.
In a study of 826 US veterans with RA,6 75% tested positive for anti-CCP antibody and 80% were positive for rheumatoid factor. It was found that a higher anti-CCP antibody titer was associated with increased disease activity and inversely correlated with remission, especially in those also positive for rheumatoid factor.6
In another study,1 in which blood samples from 79 patients with RA who had been blood donors were analyzed, 39 patients (49.4%) were positive for either rheumatoid factor or anti-CCP antibody, or both, a median of 4.5 years (range 0.1 to 13.8 years) before the onset of RA symptoms; 32 patients (40.5%) became positive for anti-CCP antibody before symptom onset.
Whiting et al,7 in a systematic review of 151 studies, showed that anti-CCP antibody testing had greater specificity than rheumatoid factor testing (96% vs 86%), with similar sensitivity (56% vs 58%)—most notably in eight cohort studies of patients with early RA.7 In the 15 cohort studies analyzed, the test was found to have a positive likelihood ratio of 12.7 and a negative likelihood ratio of 0.45, supporting this as a test of high positive predictive value for RA.
In view of the evidence from these studies, it is not surprising that the 2010 collaborative classification of RA of the American College of Rheumatology and the European League Against Rheumatism places equal weight on anti-CCP antibody testing and rheumatoid factor testing in the early diagnosis of RA.5
GENETICS AND THE PROGNOSIS OF RHEUMATOID ARTHRITIS
In recent years, there has been a growing recognition that the pathogenesis of RA in patients who are seropositive for rheumatoid factor or anti-CCP antibody is different from the pathogenesis of RA in patients who are seronegative for rheumatoid factor and anti-CCP antibody. This may help us guide therapy.
Patients positive for rheumatoid factor or anti-CCP antibody who have a specific allelic subset of a region of the immune-response gene DRB1*04 appear to be highly vulnerable to smoking as an environmental trigger or to worsening RA.8
Patients positive for anti-CCP antibody tend also to have severe joint destruction and, hence, have a worse prognosis. Kaltenhäuser et al9 found that determining the presence of the shared epitope (an RA-specific genetic marker) and positivity for anti-CCP antibody facilitates prediction of the disease course and prognosis.9
Studies have shown that patients with confirmed RA who test positive for anti-CCP antibody may also have more-severe extraarticular manifestations. Recent studies have found anti-CCP antibody positivity in 15.7% to 17.5% of patients with psoriatic arthritis and in 85% of patients with RA. Patients with psoriatic arthritis who were positive for anti-CCP antibody had more joints that were tender and swollen, erosive arthritis, deformities, and functional impairment of peripheral joints.10,11
THE COST DIFFERENCE IS TRIVIAL IN THE LONG RUN
Cost is the major differentiating factor between rheumatoid factor testing and anti-CCP antibody testing. Rheumatoid factor testing costs around $43, and anti-CCP antibody testing costs $102 in the reference laboratory at Cleveland Clinic. However, the difference in cost is trivial, since this is only a one-time cost, whereas the information anti-CCP antibody testing provides can have a major impact on predicting the prognosis and determining the choice of therapy for a disease associated with high direct and indirect costs over a lifetime. Also, Medicare and other insurers would likely reimburse for anti-CCP antibody testing as long as it was associated with a related diagnosis such as arthralgia or arthritis.
Given that there will be a small number of patients with confirmed RA who will be negative for rheumatoid factor yet positive for anti-CCP antibody, one can support ordering both tests in tandem in a patient whom you strongly suspect of having RA. Or, at $100, one could make the argument that it would be cost-effective to order anti-CCP antibody testing only if rheumatoid factor testing is negative.
Testing for rheumatoid factor and anti-CCP antibody should not be done serially to assess treatment response or disease activity in these patients: these markers do not vary with inflammatory activity or disappear with clinical “remission.”
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–386.
- Egerer K, Feist E, Burmester GR. The serological diagnosis of rheumatoid arthritis: antibodies to citrullinated antigens. Dtsch Arztebl Int 2009; 106:159–163.
- Conrad K, Roggenbuck D, Reinhold D, Dörner T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun Rev 2010; 9:431–435.
- Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. 1939. APMIS 2007; 115:422–438.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581.
- Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010; 69:1292–1297.
- Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010; 152:456–464;W155–W166.
- van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 2008; 1143:268–285.
- Kaltenhäuser S, Pierer M, Arnold S, et al. Antibodies against cyclic citrullinated peptide are associated with the DRB1 shared epitope and predict joint erosion in rheumatoid arthritis. Rheumatology (Oxford) 2007; 46:100–104.
- Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol 2005; 32:511–515.
- Abdel Fattah NS, Hassan HE, Galal ZA, El Okda el SE. Assessment of anti-cyclic citrullinated peptide in psoriatic arthritis. BMC Res Notes 2009; 2:44.
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–386.
- Egerer K, Feist E, Burmester GR. The serological diagnosis of rheumatoid arthritis: antibodies to citrullinated antigens. Dtsch Arztebl Int 2009; 106:159–163.
- Conrad K, Roggenbuck D, Reinhold D, Dörner T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun Rev 2010; 9:431–435.
- Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. 1939. APMIS 2007; 115:422–438.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581.
- Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010; 69:1292–1297.
- Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010; 152:456–464;W155–W166.
- van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 2008; 1143:268–285.
- Kaltenhäuser S, Pierer M, Arnold S, et al. Antibodies against cyclic citrullinated peptide are associated with the DRB1 shared epitope and predict joint erosion in rheumatoid arthritis. Rheumatology (Oxford) 2007; 46:100–104.
- Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol 2005; 32:511–515.
- Abdel Fattah NS, Hassan HE, Galal ZA, El Okda el SE. Assessment of anti-cyclic citrullinated peptide in psoriatic arthritis. BMC Res Notes 2009; 2:44.
In reply: Parkinson disease
In Reply: I thank Dr. Keller for his thoughtful comments. They are most appreciated.
It is true that with availability of generic ropinirole and pramipexole, there are now cheaper alternatives to levodopa. Nonetheless, levodopa remains the cheapest and most efficacious medication for Parkinson disease to date. Whenever levodopa is compared head-to-head with any dopamine agonist, the general results remain consistent: levodopa affords better motor improvement with lesser side effects, but is more likely to lead to motor fluctuations, specifically dyskinesias. Therefore, in general, levodopa is the first choice in elderly patients where tolerability may be an issue, whereas a dopamine agonist may be the initial treatment of choice in younger Parkinson patients, who are able to tolerate the drug better and have a higher likelihood of developing dyskinesias.
It is a tougher task to determine which among the dopamine agonists is superior. The newer dopamine agonists have not been compared head-to-head. Therefore, it is practically a “coin toss” when selecting which dopamine agonist to try. Their mechanism of action (D2 and D3 receptor agonist activity) and frequency of intake (three times per day for generics; once daily for long-acting formulations), cost, and side effect profile are nearly identical, despite minor differences in their half-lives.
Regarding putative neuroprotective agents in Parkinson disease, indeed, isradipine is one of the medications currently undergoing investigation for its potential neuroprotective effect. While I personally have no objection to using it for a Parkinson disease patient who also happens to need an antihypertensive agent, I am more cautious about endorsing it as a neuroprotective agent until results of clinical trials have been released. Similarly, while a large epidemiologic study has shown that people who take ibuprofen are less likely to develop Parkinson disease, there has been no robust human trial that has shown the drug to slow the progression of Parkinson disease among patients who are already suffering from the disorder. Therefore, the current use of ibuprofen in Parkinson disease should be based more on its anti-inflammatory indications rather than its possible neuroprotective effect. Finally, we have shown, in a large, multicenter, global randomized controlled trial with a delayed-start design, that pramipexole is unlikely to possess any meaningful neuroprotective effect. Therefore, I am personally not that optimistic that dexpramipexole would demonstrate such an effect.
While in theory combining the use of catechol-O-methyltransferase (COMT) inhibitors and monoamine oxidase (MAO) type B inhibitors can synergistically work to inhibit the breakdown of other catecholamines and lead to adrenergic crisis when taken concomitantly, this has not been our experience. Perhaps it is because at recommended doses, the MAO inhibition is selective to type B (where receptors are more confined to the brain) and not type A (where receptors are more distributed throughout blood vessels, thereby having a higher likelihood of causing a hypertensive crisis as is seen in the use of nonselective MAO inhibitors). Therefore, at our center, we routinely use the two classes of agents concomitantly with minimal safety concerns.
In Reply: I thank Dr. Keller for his thoughtful comments. They are most appreciated.
It is true that with availability of generic ropinirole and pramipexole, there are now cheaper alternatives to levodopa. Nonetheless, levodopa remains the cheapest and most efficacious medication for Parkinson disease to date. Whenever levodopa is compared head-to-head with any dopamine agonist, the general results remain consistent: levodopa affords better motor improvement with lesser side effects, but is more likely to lead to motor fluctuations, specifically dyskinesias. Therefore, in general, levodopa is the first choice in elderly patients where tolerability may be an issue, whereas a dopamine agonist may be the initial treatment of choice in younger Parkinson patients, who are able to tolerate the drug better and have a higher likelihood of developing dyskinesias.
It is a tougher task to determine which among the dopamine agonists is superior. The newer dopamine agonists have not been compared head-to-head. Therefore, it is practically a “coin toss” when selecting which dopamine agonist to try. Their mechanism of action (D2 and D3 receptor agonist activity) and frequency of intake (three times per day for generics; once daily for long-acting formulations), cost, and side effect profile are nearly identical, despite minor differences in their half-lives.
Regarding putative neuroprotective agents in Parkinson disease, indeed, isradipine is one of the medications currently undergoing investigation for its potential neuroprotective effect. While I personally have no objection to using it for a Parkinson disease patient who also happens to need an antihypertensive agent, I am more cautious about endorsing it as a neuroprotective agent until results of clinical trials have been released. Similarly, while a large epidemiologic study has shown that people who take ibuprofen are less likely to develop Parkinson disease, there has been no robust human trial that has shown the drug to slow the progression of Parkinson disease among patients who are already suffering from the disorder. Therefore, the current use of ibuprofen in Parkinson disease should be based more on its anti-inflammatory indications rather than its possible neuroprotective effect. Finally, we have shown, in a large, multicenter, global randomized controlled trial with a delayed-start design, that pramipexole is unlikely to possess any meaningful neuroprotective effect. Therefore, I am personally not that optimistic that dexpramipexole would demonstrate such an effect.
While in theory combining the use of catechol-O-methyltransferase (COMT) inhibitors and monoamine oxidase (MAO) type B inhibitors can synergistically work to inhibit the breakdown of other catecholamines and lead to adrenergic crisis when taken concomitantly, this has not been our experience. Perhaps it is because at recommended doses, the MAO inhibition is selective to type B (where receptors are more confined to the brain) and not type A (where receptors are more distributed throughout blood vessels, thereby having a higher likelihood of causing a hypertensive crisis as is seen in the use of nonselective MAO inhibitors). Therefore, at our center, we routinely use the two classes of agents concomitantly with minimal safety concerns.
In Reply: I thank Dr. Keller for his thoughtful comments. They are most appreciated.
It is true that with availability of generic ropinirole and pramipexole, there are now cheaper alternatives to levodopa. Nonetheless, levodopa remains the cheapest and most efficacious medication for Parkinson disease to date. Whenever levodopa is compared head-to-head with any dopamine agonist, the general results remain consistent: levodopa affords better motor improvement with lesser side effects, but is more likely to lead to motor fluctuations, specifically dyskinesias. Therefore, in general, levodopa is the first choice in elderly patients where tolerability may be an issue, whereas a dopamine agonist may be the initial treatment of choice in younger Parkinson patients, who are able to tolerate the drug better and have a higher likelihood of developing dyskinesias.
It is a tougher task to determine which among the dopamine agonists is superior. The newer dopamine agonists have not been compared head-to-head. Therefore, it is practically a “coin toss” when selecting which dopamine agonist to try. Their mechanism of action (D2 and D3 receptor agonist activity) and frequency of intake (three times per day for generics; once daily for long-acting formulations), cost, and side effect profile are nearly identical, despite minor differences in their half-lives.
Regarding putative neuroprotective agents in Parkinson disease, indeed, isradipine is one of the medications currently undergoing investigation for its potential neuroprotective effect. While I personally have no objection to using it for a Parkinson disease patient who also happens to need an antihypertensive agent, I am more cautious about endorsing it as a neuroprotective agent until results of clinical trials have been released. Similarly, while a large epidemiologic study has shown that people who take ibuprofen are less likely to develop Parkinson disease, there has been no robust human trial that has shown the drug to slow the progression of Parkinson disease among patients who are already suffering from the disorder. Therefore, the current use of ibuprofen in Parkinson disease should be based more on its anti-inflammatory indications rather than its possible neuroprotective effect. Finally, we have shown, in a large, multicenter, global randomized controlled trial with a delayed-start design, that pramipexole is unlikely to possess any meaningful neuroprotective effect. Therefore, I am personally not that optimistic that dexpramipexole would demonstrate such an effect.
While in theory combining the use of catechol-O-methyltransferase (COMT) inhibitors and monoamine oxidase (MAO) type B inhibitors can synergistically work to inhibit the breakdown of other catecholamines and lead to adrenergic crisis when taken concomitantly, this has not been our experience. Perhaps it is because at recommended doses, the MAO inhibition is selective to type B (where receptors are more confined to the brain) and not type A (where receptors are more distributed throughout blood vessels, thereby having a higher likelihood of causing a hypertensive crisis as is seen in the use of nonselective MAO inhibitors). Therefore, at our center, we routinely use the two classes of agents concomitantly with minimal safety concerns.
Parkinson disease
To the Editor: I have the following comments and questions regarding the excellent Medical Grand Rounds article on Parkinson disease by Dr. Fernandez in your January 2012 issue.1
The author mentions that when “cost may be of concern, levodopa is the preferred starting drug.”1 Generic versions of pramipexole and ropinirole are now available and have made these medications more affordable. For example, the cash price of generic ropinirole 5 mg was recently $66 for 100 tablets, comparable with generic carbidopa/levodopa (25/100 mg priced at $46 for 100 tablets.2 And even though the price of generic pramipexole was $240 for 90 tablets, seniors with Medicare Part D drug coverage can usually get any generic medication for a low copay.
When choosing a dopamine agonist, how does Dr. Fernandez decide between ropinirole and pramipexole (aside from the price difference noted above)? Pramipexole has a longer elimination half-life (8 to 12 hours) compared with ropinirole (6 hours).3 Does this imply a significantly longer effective dosing interval for pramipexole? Are there other significant clinical differences between these agents?
Isradipine (DynaCirc CR), a dihydropyridine calcium channel blocker, has shown promise as a neuroprotective agent for slowing the progression of Parkinson disease in epidemiologic and laboratory studies, as noted by the author. In addition, immediate-release isradipine, with its relatively short elimination half-life of 8 hours,3 may be well suited for treating Parkinson patients whose essential hypertension is complicated by episodes of orthostatic hypotension. It should be noted that dihydropyridines that do not cross the blood-brain barrier (such as amlodipine [Norvasc]) have shown no evidence of neuroprotection.
Ibuprofen is another drug that has fairly strong epidemiologic and laboratory evidence that it might be neuroprotective,4 although the other nonsteroidal anti-inflammatory drugs (NSAIDs) have proven disappointing as a class.5 Lacking any prospective randomized trials, the evidence is not strong enough to recommend ibuprofen solely for neuroprotection. Does Dr. Fernandez, however, consider it reasonable to suggest ibuprofen to Parkinson patients who need to take an NSAID for an approved indication (such as pain)?
Dexpramipexole has recently demonstrated great promise in a phase 3 clinical trial as a neuroprotective agent in amyotrophic lateral sclerosis.6 How does this compound relate to pramipexole, and does the author believe it may offer neuroprotection in other neurodegenerative diseases like Parkinson disease?
The author discusses the use of catechol-O-methyltransferase (COMT) inhibitors (such as Comtan and Tasmar) and the monoamine oxidase (MAO) type-B inhibitors rasagiline (Azilect) and selegiline (Eldepryl, Zelapar) for prolonging the effects of levodopa by slowing the breakdown of dopamine. However, it is important to note that it is contraindicated to prescribe both a COMT inhibitor and an MAO-B inhibitor, because these agents also inhibit the breakdown of other catecholamines and can lead to adrenergic crisis when taken concomitantly.
- Fernandez HH. Updates in the medical management of Parkinson disease. Cleve Clin J Med 2012; 79:28–35.
- Drugstore.com. www.Drugstore.com. Accessed February 5, 2012.
- PDR.net. www.PDR.net. Accessed February 25, 2012.
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011; 76:863–869.
- Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case-control study. BMJ 2011; 342:d198.
- Cudkowicz M, Bozik ME, Ingersoll EW, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 2011; 17:1652–1656.
To the Editor: I have the following comments and questions regarding the excellent Medical Grand Rounds article on Parkinson disease by Dr. Fernandez in your January 2012 issue.1
The author mentions that when “cost may be of concern, levodopa is the preferred starting drug.”1 Generic versions of pramipexole and ropinirole are now available and have made these medications more affordable. For example, the cash price of generic ropinirole 5 mg was recently $66 for 100 tablets, comparable with generic carbidopa/levodopa (25/100 mg priced at $46 for 100 tablets.2 And even though the price of generic pramipexole was $240 for 90 tablets, seniors with Medicare Part D drug coverage can usually get any generic medication for a low copay.
When choosing a dopamine agonist, how does Dr. Fernandez decide between ropinirole and pramipexole (aside from the price difference noted above)? Pramipexole has a longer elimination half-life (8 to 12 hours) compared with ropinirole (6 hours).3 Does this imply a significantly longer effective dosing interval for pramipexole? Are there other significant clinical differences between these agents?
Isradipine (DynaCirc CR), a dihydropyridine calcium channel blocker, has shown promise as a neuroprotective agent for slowing the progression of Parkinson disease in epidemiologic and laboratory studies, as noted by the author. In addition, immediate-release isradipine, with its relatively short elimination half-life of 8 hours,3 may be well suited for treating Parkinson patients whose essential hypertension is complicated by episodes of orthostatic hypotension. It should be noted that dihydropyridines that do not cross the blood-brain barrier (such as amlodipine [Norvasc]) have shown no evidence of neuroprotection.
Ibuprofen is another drug that has fairly strong epidemiologic and laboratory evidence that it might be neuroprotective,4 although the other nonsteroidal anti-inflammatory drugs (NSAIDs) have proven disappointing as a class.5 Lacking any prospective randomized trials, the evidence is not strong enough to recommend ibuprofen solely for neuroprotection. Does Dr. Fernandez, however, consider it reasonable to suggest ibuprofen to Parkinson patients who need to take an NSAID for an approved indication (such as pain)?
Dexpramipexole has recently demonstrated great promise in a phase 3 clinical trial as a neuroprotective agent in amyotrophic lateral sclerosis.6 How does this compound relate to pramipexole, and does the author believe it may offer neuroprotection in other neurodegenerative diseases like Parkinson disease?
The author discusses the use of catechol-O-methyltransferase (COMT) inhibitors (such as Comtan and Tasmar) and the monoamine oxidase (MAO) type-B inhibitors rasagiline (Azilect) and selegiline (Eldepryl, Zelapar) for prolonging the effects of levodopa by slowing the breakdown of dopamine. However, it is important to note that it is contraindicated to prescribe both a COMT inhibitor and an MAO-B inhibitor, because these agents also inhibit the breakdown of other catecholamines and can lead to adrenergic crisis when taken concomitantly.
To the Editor: I have the following comments and questions regarding the excellent Medical Grand Rounds article on Parkinson disease by Dr. Fernandez in your January 2012 issue.1
The author mentions that when “cost may be of concern, levodopa is the preferred starting drug.”1 Generic versions of pramipexole and ropinirole are now available and have made these medications more affordable. For example, the cash price of generic ropinirole 5 mg was recently $66 for 100 tablets, comparable with generic carbidopa/levodopa (25/100 mg priced at $46 for 100 tablets.2 And even though the price of generic pramipexole was $240 for 90 tablets, seniors with Medicare Part D drug coverage can usually get any generic medication for a low copay.
When choosing a dopamine agonist, how does Dr. Fernandez decide between ropinirole and pramipexole (aside from the price difference noted above)? Pramipexole has a longer elimination half-life (8 to 12 hours) compared with ropinirole (6 hours).3 Does this imply a significantly longer effective dosing interval for pramipexole? Are there other significant clinical differences between these agents?
Isradipine (DynaCirc CR), a dihydropyridine calcium channel blocker, has shown promise as a neuroprotective agent for slowing the progression of Parkinson disease in epidemiologic and laboratory studies, as noted by the author. In addition, immediate-release isradipine, with its relatively short elimination half-life of 8 hours,3 may be well suited for treating Parkinson patients whose essential hypertension is complicated by episodes of orthostatic hypotension. It should be noted that dihydropyridines that do not cross the blood-brain barrier (such as amlodipine [Norvasc]) have shown no evidence of neuroprotection.
Ibuprofen is another drug that has fairly strong epidemiologic and laboratory evidence that it might be neuroprotective,4 although the other nonsteroidal anti-inflammatory drugs (NSAIDs) have proven disappointing as a class.5 Lacking any prospective randomized trials, the evidence is not strong enough to recommend ibuprofen solely for neuroprotection. Does Dr. Fernandez, however, consider it reasonable to suggest ibuprofen to Parkinson patients who need to take an NSAID for an approved indication (such as pain)?
Dexpramipexole has recently demonstrated great promise in a phase 3 clinical trial as a neuroprotective agent in amyotrophic lateral sclerosis.6 How does this compound relate to pramipexole, and does the author believe it may offer neuroprotection in other neurodegenerative diseases like Parkinson disease?
The author discusses the use of catechol-O-methyltransferase (COMT) inhibitors (such as Comtan and Tasmar) and the monoamine oxidase (MAO) type-B inhibitors rasagiline (Azilect) and selegiline (Eldepryl, Zelapar) for prolonging the effects of levodopa by slowing the breakdown of dopamine. However, it is important to note that it is contraindicated to prescribe both a COMT inhibitor and an MAO-B inhibitor, because these agents also inhibit the breakdown of other catecholamines and can lead to adrenergic crisis when taken concomitantly.
- Fernandez HH. Updates in the medical management of Parkinson disease. Cleve Clin J Med 2012; 79:28–35.
- Drugstore.com. www.Drugstore.com. Accessed February 5, 2012.
- PDR.net. www.PDR.net. Accessed February 25, 2012.
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011; 76:863–869.
- Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case-control study. BMJ 2011; 342:d198.
- Cudkowicz M, Bozik ME, Ingersoll EW, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 2011; 17:1652–1656.
- Fernandez HH. Updates in the medical management of Parkinson disease. Cleve Clin J Med 2012; 79:28–35.
- Drugstore.com. www.Drugstore.com. Accessed February 5, 2012.
- PDR.net. www.PDR.net. Accessed February 25, 2012.
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011; 76:863–869.
- Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case-control study. BMJ 2011; 342:d198.
- Cudkowicz M, Bozik ME, Ingersoll EW, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 2011; 17:1652–1656.
In reply: Essential tremor, beta-blockers, calcium channel blockers
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
Essential tremor, beta-blockers, and calcium channel blockers
To the Editor: In their thorough review of essential tremor,1Drs. Abboud, Ahmed, and Fernandez make a statement that needs clarification. In their list of absolute contraindications to propranolol (Inderal), the authors include “concurrent use of a calcium channel blocker.” This warning applies only to the nondihydropyridine calcium channel blockers, which are diltiazem (Cardizem) and verapamil (Calan). These two medications slow the heart rate and generally should not be combined with beta-blockers such as propranolol unless the patient requires this combination to control tachycardia. Most calcium channel blockers are dihydropyridines, which include amlodipine (Norvasc), nifedipine (Procardia), felodipine (Plendil), nisoldipine (Sular), isradipine (DynaCirc CR), and nicardipine (Cardene). These agents do not slow the heart rate significantly and therefore can be used freely in combination with propranolol. Of course, the dose of the calcium channel blocker may need to be decreased because of the antihypertensive effect of propranolol.
- Abboud H, Ahmed A, Fernandez HH. Essential tremor: choosing the right management plan for your patient. Cleve Clin J Med 2011; 78:821–828.
To the Editor: In their thorough review of essential tremor,1Drs. Abboud, Ahmed, and Fernandez make a statement that needs clarification. In their list of absolute contraindications to propranolol (Inderal), the authors include “concurrent use of a calcium channel blocker.” This warning applies only to the nondihydropyridine calcium channel blockers, which are diltiazem (Cardizem) and verapamil (Calan). These two medications slow the heart rate and generally should not be combined with beta-blockers such as propranolol unless the patient requires this combination to control tachycardia. Most calcium channel blockers are dihydropyridines, which include amlodipine (Norvasc), nifedipine (Procardia), felodipine (Plendil), nisoldipine (Sular), isradipine (DynaCirc CR), and nicardipine (Cardene). These agents do not slow the heart rate significantly and therefore can be used freely in combination with propranolol. Of course, the dose of the calcium channel blocker may need to be decreased because of the antihypertensive effect of propranolol.
To the Editor: In their thorough review of essential tremor,1Drs. Abboud, Ahmed, and Fernandez make a statement that needs clarification. In their list of absolute contraindications to propranolol (Inderal), the authors include “concurrent use of a calcium channel blocker.” This warning applies only to the nondihydropyridine calcium channel blockers, which are diltiazem (Cardizem) and verapamil (Calan). These two medications slow the heart rate and generally should not be combined with beta-blockers such as propranolol unless the patient requires this combination to control tachycardia. Most calcium channel blockers are dihydropyridines, which include amlodipine (Norvasc), nifedipine (Procardia), felodipine (Plendil), nisoldipine (Sular), isradipine (DynaCirc CR), and nicardipine (Cardene). These agents do not slow the heart rate significantly and therefore can be used freely in combination with propranolol. Of course, the dose of the calcium channel blocker may need to be decreased because of the antihypertensive effect of propranolol.
- Abboud H, Ahmed A, Fernandez HH. Essential tremor: choosing the right management plan for your patient. Cleve Clin J Med 2011; 78:821–828.
- Abboud H, Ahmed A, Fernandez HH. Essential tremor: choosing the right management plan for your patient. Cleve Clin J Med 2011; 78:821–828.