User login
FDA approves drug for refractory Glanzmann’s Thrombasthenia

Credit: Piotr Bodzek
The US Food and Drug Administration (FDA) has approved a recombinant factor VIIa product (NovoSeven® RT) for the treatment of bleeding episodes and for perioperative management in patients with Glanzmann’s Thrombasthenia (GT) who are refractory to platelet transfusions and may or may not have antibodies to platelets.
GT is a rare genetic bleeding disorder with limited treatment options. Patients with GT have a lifelong susceptibility toward bleeding episodes.
The condition, which affects 1 in 1 million people globally, occurs because certain surface proteins on platelets are missing or nonfunctional, significantly impacting the blood’s ability to form strong clots.
Patients with GT typically receive platelet transfusions when experiencing severe bleeding or when undergoing surgical procedures. However, some patients do not respond well, or at all, to platelet transfusions.
The FDA approved NovoSeven® RT for these patients based on evidence collected from the global Glanzmann’s Thrombasthenia Registry and the Hemostasis & Thrombosis Research Society Registry.
The data included 92 GT patients treated with NovoSeven® RT for 266 severe bleeding episodes and 77 GT patients treated with NovoSeven® RT for 160 surgical and other invasive procedures.
The treatment was successful in 94.4% of bleeding episodes and 99.4% of surgical procedures, based on a review of the data by independent hematology experts.
Among 140 patients treated for 518 bleeding episodes, surgeries, or traumatic injuries, the following adverse events were reported: deep vein thrombosis (n=1), headache (n=2), fever (n=2), nausea (n=1), and dyspnea (n=1).
NovoSeven® RT is also approved in the European Union for the treatment of bleeding episodes in patients with GT. The product is marketed by NovoNordisk. ![]()

Credit: Piotr Bodzek
The US Food and Drug Administration (FDA) has approved a recombinant factor VIIa product (NovoSeven® RT) for the treatment of bleeding episodes and for perioperative management in patients with Glanzmann’s Thrombasthenia (GT) who are refractory to platelet transfusions and may or may not have antibodies to platelets.
GT is a rare genetic bleeding disorder with limited treatment options. Patients with GT have a lifelong susceptibility toward bleeding episodes.
The condition, which affects 1 in 1 million people globally, occurs because certain surface proteins on platelets are missing or nonfunctional, significantly impacting the blood’s ability to form strong clots.
Patients with GT typically receive platelet transfusions when experiencing severe bleeding or when undergoing surgical procedures. However, some patients do not respond well, or at all, to platelet transfusions.
The FDA approved NovoSeven® RT for these patients based on evidence collected from the global Glanzmann’s Thrombasthenia Registry and the Hemostasis & Thrombosis Research Society Registry.
The data included 92 GT patients treated with NovoSeven® RT for 266 severe bleeding episodes and 77 GT patients treated with NovoSeven® RT for 160 surgical and other invasive procedures.
The treatment was successful in 94.4% of bleeding episodes and 99.4% of surgical procedures, based on a review of the data by independent hematology experts.
Among 140 patients treated for 518 bleeding episodes, surgeries, or traumatic injuries, the following adverse events were reported: deep vein thrombosis (n=1), headache (n=2), fever (n=2), nausea (n=1), and dyspnea (n=1).
NovoSeven® RT is also approved in the European Union for the treatment of bleeding episodes in patients with GT. The product is marketed by NovoNordisk. ![]()

Credit: Piotr Bodzek
The US Food and Drug Administration (FDA) has approved a recombinant factor VIIa product (NovoSeven® RT) for the treatment of bleeding episodes and for perioperative management in patients with Glanzmann’s Thrombasthenia (GT) who are refractory to platelet transfusions and may or may not have antibodies to platelets.
GT is a rare genetic bleeding disorder with limited treatment options. Patients with GT have a lifelong susceptibility toward bleeding episodes.
The condition, which affects 1 in 1 million people globally, occurs because certain surface proteins on platelets are missing or nonfunctional, significantly impacting the blood’s ability to form strong clots.
Patients with GT typically receive platelet transfusions when experiencing severe bleeding or when undergoing surgical procedures. However, some patients do not respond well, or at all, to platelet transfusions.
The FDA approved NovoSeven® RT for these patients based on evidence collected from the global Glanzmann’s Thrombasthenia Registry and the Hemostasis & Thrombosis Research Society Registry.
The data included 92 GT patients treated with NovoSeven® RT for 266 severe bleeding episodes and 77 GT patients treated with NovoSeven® RT for 160 surgical and other invasive procedures.
The treatment was successful in 94.4% of bleeding episodes and 99.4% of surgical procedures, based on a review of the data by independent hematology experts.
Among 140 patients treated for 518 bleeding episodes, surgeries, or traumatic injuries, the following adverse events were reported: deep vein thrombosis (n=1), headache (n=2), fever (n=2), nausea (n=1), and dyspnea (n=1).
NovoSeven® RT is also approved in the European Union for the treatment of bleeding episodes in patients with GT. The product is marketed by NovoNordisk. ![]()
‘Herculean study’ reveals key regulators of malaria

lab’s mosquito insectory
The University of Nottingham
A researcher who battled malaria infection as a child is now fighting the disease in her lab and has made a discovery that may bring us closer to successfully disrupting the malaria parasite life-cycle.
Rita Tewari, PhD, of The University of Nottingham in the UK, and her colleagues have completed what she calls a “Herculean study” investigating the roles that 30 protein phosphatases and 72 kinases play as the malaria parasite develops.
Dr Tewari and her colleagues reported the results of this study in Cell Host and Microbe.
“This latest study identifies how protein phosphatases regulate parasite development and differentiation,” she said. “Our research provides a systematic functional analysis for all the 30 phosphatases in Plasmodium berghei, the parasite responsible for causing malaria in rodents.”
“These enzymes work in tandem with the protein kinases identified by the same team in a complementary study carried out in 2010. If we can find out what proteins are essential for these parasites to develop and divide, maybe we can target those proteins and arrest them with drugs or vaccines.”
Born and raised in Delhi, India, Dr Tewari had malaria 7 times as a child. She now leads her own malaria research lab at The University of Nottingham, complete with her own mosquito insectary.
It has taken her team, together with collaborators at Imperial College London, 8 years to identify every one of the protein phosphatases and protein kinases responsible for malaria parasite development.
Protein kinases and phosphatases are crucial for many stages of the malaria parasite lifecycle. And Dr Tewari’s group has been investigating protein kinases and phosphatases to better understand the basic developmental biology of malaria parasites.
Using a number of molecular cell biology and biochemical techniques, the researchers found that 16 of the 30 phosphatase genes they identified could not be knocked out. This suggests some of these genes could be future drug targets, as their presence is critical to parasite growth.
“Interestingly, out of the genes that could be knocked out [14], 6 were found to be crucial for sexual development and, hence, could be drug targets for parasite transmission to and from the mosquito,” Dr Tewari said.
“The research gathered here using the mouse malaria parasite can be directly related to the human malaria parasite, as many of the genes share a very similar homology, and symptoms of the diseases are very similar.” ![]()

lab’s mosquito insectory
The University of Nottingham
A researcher who battled malaria infection as a child is now fighting the disease in her lab and has made a discovery that may bring us closer to successfully disrupting the malaria parasite life-cycle.
Rita Tewari, PhD, of The University of Nottingham in the UK, and her colleagues have completed what she calls a “Herculean study” investigating the roles that 30 protein phosphatases and 72 kinases play as the malaria parasite develops.
Dr Tewari and her colleagues reported the results of this study in Cell Host and Microbe.
“This latest study identifies how protein phosphatases regulate parasite development and differentiation,” she said. “Our research provides a systematic functional analysis for all the 30 phosphatases in Plasmodium berghei, the parasite responsible for causing malaria in rodents.”
“These enzymes work in tandem with the protein kinases identified by the same team in a complementary study carried out in 2010. If we can find out what proteins are essential for these parasites to develop and divide, maybe we can target those proteins and arrest them with drugs or vaccines.”
Born and raised in Delhi, India, Dr Tewari had malaria 7 times as a child. She now leads her own malaria research lab at The University of Nottingham, complete with her own mosquito insectary.
It has taken her team, together with collaborators at Imperial College London, 8 years to identify every one of the protein phosphatases and protein kinases responsible for malaria parasite development.
Protein kinases and phosphatases are crucial for many stages of the malaria parasite lifecycle. And Dr Tewari’s group has been investigating protein kinases and phosphatases to better understand the basic developmental biology of malaria parasites.
Using a number of molecular cell biology and biochemical techniques, the researchers found that 16 of the 30 phosphatase genes they identified could not be knocked out. This suggests some of these genes could be future drug targets, as their presence is critical to parasite growth.
“Interestingly, out of the genes that could be knocked out [14], 6 were found to be crucial for sexual development and, hence, could be drug targets for parasite transmission to and from the mosquito,” Dr Tewari said.
“The research gathered here using the mouse malaria parasite can be directly related to the human malaria parasite, as many of the genes share a very similar homology, and symptoms of the diseases are very similar.” ![]()

lab’s mosquito insectory
The University of Nottingham
A researcher who battled malaria infection as a child is now fighting the disease in her lab and has made a discovery that may bring us closer to successfully disrupting the malaria parasite life-cycle.
Rita Tewari, PhD, of The University of Nottingham in the UK, and her colleagues have completed what she calls a “Herculean study” investigating the roles that 30 protein phosphatases and 72 kinases play as the malaria parasite develops.
Dr Tewari and her colleagues reported the results of this study in Cell Host and Microbe.
“This latest study identifies how protein phosphatases regulate parasite development and differentiation,” she said. “Our research provides a systematic functional analysis for all the 30 phosphatases in Plasmodium berghei, the parasite responsible for causing malaria in rodents.”
“These enzymes work in tandem with the protein kinases identified by the same team in a complementary study carried out in 2010. If we can find out what proteins are essential for these parasites to develop and divide, maybe we can target those proteins and arrest them with drugs or vaccines.”
Born and raised in Delhi, India, Dr Tewari had malaria 7 times as a child. She now leads her own malaria research lab at The University of Nottingham, complete with her own mosquito insectary.
It has taken her team, together with collaborators at Imperial College London, 8 years to identify every one of the protein phosphatases and protein kinases responsible for malaria parasite development.
Protein kinases and phosphatases are crucial for many stages of the malaria parasite lifecycle. And Dr Tewari’s group has been investigating protein kinases and phosphatases to better understand the basic developmental biology of malaria parasites.
Using a number of molecular cell biology and biochemical techniques, the researchers found that 16 of the 30 phosphatase genes they identified could not be knocked out. This suggests some of these genes could be future drug targets, as their presence is critical to parasite growth.
“Interestingly, out of the genes that could be knocked out [14], 6 were found to be crucial for sexual development and, hence, could be drug targets for parasite transmission to and from the mosquito,” Dr Tewari said.
“The research gathered here using the mouse malaria parasite can be directly related to the human malaria parasite, as many of the genes share a very similar homology, and symptoms of the diseases are very similar.” ![]()
Product News: 07 2014
CoolSculptingZeltiq Aesthetics, Inc, introduces the CoolSmooth applicator and obtains US Food and Drug Administration clearance for the CoolSculpting procedure to treat the thigh area. The CoolSmooth applicator is designed for fat reduction of the outer thigh. It is a flat applicator that features nonvacuum-based cooling to easily treat nonpinchable fat bulges, offering physicians the ability to optimize patient outcomes and expand CoolSculpting treatment areas. The CoolSculpting procedure previously was cleared for noninvasive fat reduction in the abdomen and flank; now the thigh area (inner and outer thighs) can be treated with the entire suite of applicators. For more information, visit www.coolsculpting.com.
DalvanceDurata Therapeutics, Inc, obtains US Food and Drug Administration approval for Dalvance (dalbavan-cin), an intravenous antibiotic for the treatment of adult patients with acute bacterial skin and skin structure infections caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Streptococcus pyogenes. Dalvance is a second-generation semisynthetic lipoglycopeptide. It is administered in a 2-dose regimen of 1000 mg followed 1 week later by 500 mg. Each dose is administered over 30 minutes. Dalvance provides physicians with a treatment option that moves beyond the standard daily or twice-daily intravenous antibiotic infusions. Exercise caution in patients with known hypersensitivity to glycopeptides. For more information, visit www.dalvance.com.
excel HR Laser SystemCutera, Inc, introduces the excel HR laser system for hair removal. excel HR is a dual-wavelength laser system that combines the high-power 755-nm alexandrite and the 1064-nm Nd:YAG with sapphire contact cooling to effectively target deep follicular structures and deliver energy more efficiently. The result is enhanced efficacy using less fluence with improved patient comfort. excel HR has received 510(k) clearance by the US Food and Drug Administration. For more information, visit www.cutera.com/excelhr.
JubliaValeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration approval for Jublia (efinaconazole solution 10%) for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. This quick-drying solution is applied daily to the nail with a bottle that has a built-in flow-through brush applicator. There are no concerns for systemic side effects such as drug-drug interactions or acute liver injury. For more information, visit www.jubliarx.com.
SitavigInnocutis launches Sitavig (acyclovir) 50-mg buccal tablets for herpes labialis in the United States. Sitavig uses a proprietary Lauriad delivery system that consists of a tablet that sticks to the patient’s gum, above the canine tooth on the side of the lip that is infected with a cold sore, then dissolves to provide sustained release of medicine. The tablet is tasteless and odorless. Sitavig is user-friendly; patients can eat and drink normally once the tablet adheres to the gum, usually within a few minutes. The application once per episode is unique compared to other systemic and topical treatments. Sitavig is licensed from BioAlliance Pharma. For more information, visit www.innocutis.com.
SivextroCubist Pharmaceuticals announces US Food and Drug Administration approval of Sivextro (tedizolid phosphate) for the treatment of acute bacterial skin and skin structure infections in adults caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. It is available as an intravenous infusion over 1 hour or as a 200-mg tablet administered once daily. Both methods offer an effective 6-day course of therapy. Sivextro allows physicians to transition patients from intravenous to oral treatment; oral administration provides the opportunity for outpatient care. For more information, visit www.sivextro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
CoolSculptingZeltiq Aesthetics, Inc, introduces the CoolSmooth applicator and obtains US Food and Drug Administration clearance for the CoolSculpting procedure to treat the thigh area. The CoolSmooth applicator is designed for fat reduction of the outer thigh. It is a flat applicator that features nonvacuum-based cooling to easily treat nonpinchable fat bulges, offering physicians the ability to optimize patient outcomes and expand CoolSculpting treatment areas. The CoolSculpting procedure previously was cleared for noninvasive fat reduction in the abdomen and flank; now the thigh area (inner and outer thighs) can be treated with the entire suite of applicators. For more information, visit www.coolsculpting.com.
DalvanceDurata Therapeutics, Inc, obtains US Food and Drug Administration approval for Dalvance (dalbavan-cin), an intravenous antibiotic for the treatment of adult patients with acute bacterial skin and skin structure infections caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Streptococcus pyogenes. Dalvance is a second-generation semisynthetic lipoglycopeptide. It is administered in a 2-dose regimen of 1000 mg followed 1 week later by 500 mg. Each dose is administered over 30 minutes. Dalvance provides physicians with a treatment option that moves beyond the standard daily or twice-daily intravenous antibiotic infusions. Exercise caution in patients with known hypersensitivity to glycopeptides. For more information, visit www.dalvance.com.
excel HR Laser SystemCutera, Inc, introduces the excel HR laser system for hair removal. excel HR is a dual-wavelength laser system that combines the high-power 755-nm alexandrite and the 1064-nm Nd:YAG with sapphire contact cooling to effectively target deep follicular structures and deliver energy more efficiently. The result is enhanced efficacy using less fluence with improved patient comfort. excel HR has received 510(k) clearance by the US Food and Drug Administration. For more information, visit www.cutera.com/excelhr.
JubliaValeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration approval for Jublia (efinaconazole solution 10%) for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. This quick-drying solution is applied daily to the nail with a bottle that has a built-in flow-through brush applicator. There are no concerns for systemic side effects such as drug-drug interactions or acute liver injury. For more information, visit www.jubliarx.com.
SitavigInnocutis launches Sitavig (acyclovir) 50-mg buccal tablets for herpes labialis in the United States. Sitavig uses a proprietary Lauriad delivery system that consists of a tablet that sticks to the patient’s gum, above the canine tooth on the side of the lip that is infected with a cold sore, then dissolves to provide sustained release of medicine. The tablet is tasteless and odorless. Sitavig is user-friendly; patients can eat and drink normally once the tablet adheres to the gum, usually within a few minutes. The application once per episode is unique compared to other systemic and topical treatments. Sitavig is licensed from BioAlliance Pharma. For more information, visit www.innocutis.com.
SivextroCubist Pharmaceuticals announces US Food and Drug Administration approval of Sivextro (tedizolid phosphate) for the treatment of acute bacterial skin and skin structure infections in adults caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. It is available as an intravenous infusion over 1 hour or as a 200-mg tablet administered once daily. Both methods offer an effective 6-day course of therapy. Sivextro allows physicians to transition patients from intravenous to oral treatment; oral administration provides the opportunity for outpatient care. For more information, visit www.sivextro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
CoolSculptingZeltiq Aesthetics, Inc, introduces the CoolSmooth applicator and obtains US Food and Drug Administration clearance for the CoolSculpting procedure to treat the thigh area. The CoolSmooth applicator is designed for fat reduction of the outer thigh. It is a flat applicator that features nonvacuum-based cooling to easily treat nonpinchable fat bulges, offering physicians the ability to optimize patient outcomes and expand CoolSculpting treatment areas. The CoolSculpting procedure previously was cleared for noninvasive fat reduction in the abdomen and flank; now the thigh area (inner and outer thighs) can be treated with the entire suite of applicators. For more information, visit www.coolsculpting.com.
DalvanceDurata Therapeutics, Inc, obtains US Food and Drug Administration approval for Dalvance (dalbavan-cin), an intravenous antibiotic for the treatment of adult patients with acute bacterial skin and skin structure infections caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Streptococcus pyogenes. Dalvance is a second-generation semisynthetic lipoglycopeptide. It is administered in a 2-dose regimen of 1000 mg followed 1 week later by 500 mg. Each dose is administered over 30 minutes. Dalvance provides physicians with a treatment option that moves beyond the standard daily or twice-daily intravenous antibiotic infusions. Exercise caution in patients with known hypersensitivity to glycopeptides. For more information, visit www.dalvance.com.
excel HR Laser SystemCutera, Inc, introduces the excel HR laser system for hair removal. excel HR is a dual-wavelength laser system that combines the high-power 755-nm alexandrite and the 1064-nm Nd:YAG with sapphire contact cooling to effectively target deep follicular structures and deliver energy more efficiently. The result is enhanced efficacy using less fluence with improved patient comfort. excel HR has received 510(k) clearance by the US Food and Drug Administration. For more information, visit www.cutera.com/excelhr.
JubliaValeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration approval for Jublia (efinaconazole solution 10%) for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. This quick-drying solution is applied daily to the nail with a bottle that has a built-in flow-through brush applicator. There are no concerns for systemic side effects such as drug-drug interactions or acute liver injury. For more information, visit www.jubliarx.com.
SitavigInnocutis launches Sitavig (acyclovir) 50-mg buccal tablets for herpes labialis in the United States. Sitavig uses a proprietary Lauriad delivery system that consists of a tablet that sticks to the patient’s gum, above the canine tooth on the side of the lip that is infected with a cold sore, then dissolves to provide sustained release of medicine. The tablet is tasteless and odorless. Sitavig is user-friendly; patients can eat and drink normally once the tablet adheres to the gum, usually within a few minutes. The application once per episode is unique compared to other systemic and topical treatments. Sitavig is licensed from BioAlliance Pharma. For more information, visit www.innocutis.com.
SivextroCubist Pharmaceuticals announces US Food and Drug Administration approval of Sivextro (tedizolid phosphate) for the treatment of acute bacterial skin and skin structure infections in adults caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. It is available as an intravenous infusion over 1 hour or as a 200-mg tablet administered once daily. Both methods offer an effective 6-day course of therapy. Sivextro allows physicians to transition patients from intravenous to oral treatment; oral administration provides the opportunity for outpatient care. For more information, visit www.sivextro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Retail me not!
In an era where everything around us has moved toward an "I need it now and I need it fast" attitude, the face of medicine also has changed. The days of patients wanting to wait to see their physician have faded, and now there is a demand for a quick fix so they can keep their already hectic lives moving.
Retail clinics also have thrown a curve ball to practicing physicians because now patients can get their fast medicine right along with their fast food, all at the corner strip mall.
The Internet also has changed how office visits run. Now physicians spend a lot of time explaining diagnoses that patients have found during their exhaustive research of their symptoms, or dispelling erroneous information that has been found on the Internet. This adds to time per patient, as well as distrust. Patients are now smarter, busier, and more likely to have chronic illnesses, so how does medicine keep up with the times?
As physicians, we must remember that our expertise as medical doctors is to rule in and rule out serious diseases. The "bread and butter" of any medical practice is likely easy to identify and treat, but where the expertise comes in is how to distinguish minor acute illness from life-threatening or potentially chronic illness. Many disease states are efficiently diagnosed only because the patient presents with further complaints that put the entire picture together. How is that achieved when patients fast-track through "minute clinics"?
Experience is also golden. If you have practiced long enough, you have had your share of surprises and know that "oh, it’s nothing" is the diagnosis only after all the "somethings " have been ruled out. For example, in adolescent medicine I commonly get the complaints of abdominal pain and anxiety. So when a patient presents with ongoing complaints of abdominal pain with no other clinical signs of disease, there is a temptation to assume it is just the anxiety. But experience teaches you that viral hepatitis, appendicitis, or urological disorders could be the underlying problem.
Another lesson that is taught by experience is how children express themselves. I recently saw an adolescent who had a minor trauma where he was struck in the chest with a basketball. He subsequently complained of chest pain, but kept saying, "I feel like I’m going to die." His mother was insistent that this was just his already diagnosed anxiety, and that he would settle down. But stating he "felt like he was going to die" was such an unusual complaint for a child that I was prompted to do an EKG, which revealed a viral myocarditis. Although this may have been identified in an express clinic, knowing the patient helped in expediting the diagnosis.
As physicians, we must educate and ensure that our patients feel they are getting the best care by sticking with someone who knows them. We have to accept that patients have options, so if we are going to keep their business, we have to work more efficiently, form relationships, and provide good care. Many practices have moved toward a concierge service, where a fee is charged for immediate appointments or telephone access. Utilization of a nurse practitioner can allow you to run your office more efficiently to manage the more acute illnesses, shorten the wait times, and maximize patient visits.
Retail clinics are here to stay. If we are going to keep private practices afloat, we have to make the visit worth the wait!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
In an era where everything around us has moved toward an "I need it now and I need it fast" attitude, the face of medicine also has changed. The days of patients wanting to wait to see their physician have faded, and now there is a demand for a quick fix so they can keep their already hectic lives moving.
Retail clinics also have thrown a curve ball to practicing physicians because now patients can get their fast medicine right along with their fast food, all at the corner strip mall.
The Internet also has changed how office visits run. Now physicians spend a lot of time explaining diagnoses that patients have found during their exhaustive research of their symptoms, or dispelling erroneous information that has been found on the Internet. This adds to time per patient, as well as distrust. Patients are now smarter, busier, and more likely to have chronic illnesses, so how does medicine keep up with the times?
As physicians, we must remember that our expertise as medical doctors is to rule in and rule out serious diseases. The "bread and butter" of any medical practice is likely easy to identify and treat, but where the expertise comes in is how to distinguish minor acute illness from life-threatening or potentially chronic illness. Many disease states are efficiently diagnosed only because the patient presents with further complaints that put the entire picture together. How is that achieved when patients fast-track through "minute clinics"?
Experience is also golden. If you have practiced long enough, you have had your share of surprises and know that "oh, it’s nothing" is the diagnosis only after all the "somethings " have been ruled out. For example, in adolescent medicine I commonly get the complaints of abdominal pain and anxiety. So when a patient presents with ongoing complaints of abdominal pain with no other clinical signs of disease, there is a temptation to assume it is just the anxiety. But experience teaches you that viral hepatitis, appendicitis, or urological disorders could be the underlying problem.
Another lesson that is taught by experience is how children express themselves. I recently saw an adolescent who had a minor trauma where he was struck in the chest with a basketball. He subsequently complained of chest pain, but kept saying, "I feel like I’m going to die." His mother was insistent that this was just his already diagnosed anxiety, and that he would settle down. But stating he "felt like he was going to die" was such an unusual complaint for a child that I was prompted to do an EKG, which revealed a viral myocarditis. Although this may have been identified in an express clinic, knowing the patient helped in expediting the diagnosis.
As physicians, we must educate and ensure that our patients feel they are getting the best care by sticking with someone who knows them. We have to accept that patients have options, so if we are going to keep their business, we have to work more efficiently, form relationships, and provide good care. Many practices have moved toward a concierge service, where a fee is charged for immediate appointments or telephone access. Utilization of a nurse practitioner can allow you to run your office more efficiently to manage the more acute illnesses, shorten the wait times, and maximize patient visits.
Retail clinics are here to stay. If we are going to keep private practices afloat, we have to make the visit worth the wait!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
In an era where everything around us has moved toward an "I need it now and I need it fast" attitude, the face of medicine also has changed. The days of patients wanting to wait to see their physician have faded, and now there is a demand for a quick fix so they can keep their already hectic lives moving.
Retail clinics also have thrown a curve ball to practicing physicians because now patients can get their fast medicine right along with their fast food, all at the corner strip mall.
The Internet also has changed how office visits run. Now physicians spend a lot of time explaining diagnoses that patients have found during their exhaustive research of their symptoms, or dispelling erroneous information that has been found on the Internet. This adds to time per patient, as well as distrust. Patients are now smarter, busier, and more likely to have chronic illnesses, so how does medicine keep up with the times?
As physicians, we must remember that our expertise as medical doctors is to rule in and rule out serious diseases. The "bread and butter" of any medical practice is likely easy to identify and treat, but where the expertise comes in is how to distinguish minor acute illness from life-threatening or potentially chronic illness. Many disease states are efficiently diagnosed only because the patient presents with further complaints that put the entire picture together. How is that achieved when patients fast-track through "minute clinics"?
Experience is also golden. If you have practiced long enough, you have had your share of surprises and know that "oh, it’s nothing" is the diagnosis only after all the "somethings " have been ruled out. For example, in adolescent medicine I commonly get the complaints of abdominal pain and anxiety. So when a patient presents with ongoing complaints of abdominal pain with no other clinical signs of disease, there is a temptation to assume it is just the anxiety. But experience teaches you that viral hepatitis, appendicitis, or urological disorders could be the underlying problem.
Another lesson that is taught by experience is how children express themselves. I recently saw an adolescent who had a minor trauma where he was struck in the chest with a basketball. He subsequently complained of chest pain, but kept saying, "I feel like I’m going to die." His mother was insistent that this was just his already diagnosed anxiety, and that he would settle down. But stating he "felt like he was going to die" was such an unusual complaint for a child that I was prompted to do an EKG, which revealed a viral myocarditis. Although this may have been identified in an express clinic, knowing the patient helped in expediting the diagnosis.
As physicians, we must educate and ensure that our patients feel they are getting the best care by sticking with someone who knows them. We have to accept that patients have options, so if we are going to keep their business, we have to work more efficiently, form relationships, and provide good care. Many practices have moved toward a concierge service, where a fee is charged for immediate appointments or telephone access. Utilization of a nurse practitioner can allow you to run your office more efficiently to manage the more acute illnesses, shorten the wait times, and maximize patient visits.
Retail clinics are here to stay. If we are going to keep private practices afloat, we have to make the visit worth the wait!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
Adaptability is the surgeon’s best friend
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
Shame
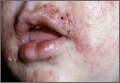
At 16, Eddie is tall and athletic. He’s been treating closed comedones on his forehead and chest off and on for a couple of years.
"I hope you can help him," his mother says. "There are days when he won’t go to school, he’s so embarrassed."
Really?
If you sat people down and asked them to list what people hate about their bodies, they wouldn’t come close to guessing what we dermatologists run into every day. Here are some cases I’ve seen lately. I’m sure you can easily come up with your own examples.
• A 50-year-old attorney with a wart on the dorsum of his right hand wanted me to take it off. "I sign a lot of documents," he said," and I’d prefer that clients remember me for my legal skills, not the wart on my hand."
• A 36-year-old waiter with a picker’s nodule over the proximal interphalangeal joint of his left fifth finger said I just had to get rid of it. "I’m a waiter," he said. "This is killing my tips."
• A 36-year-old woman gave up yoga a year ago, because she was sure the woman on the adjacent mat was disgusted by her plantar warts.
• A sprightly retiree, age 88(!), insisted on paying out of pocket to remove dermatosis papulosa nigra lesions from her face. Her explanation? "My children want me to stay at home, but I want to get out and be social!"
Self-consciousness doesn’t require lesions. For instance, I saw an 11-year-old last month with widespread atopic dermatitis, the kind that’s lifelong, miserably itchy, and hard to control. Yet she wasn’t even being treated. Why had she come now?
"What bothers you most about this?" I asked her.
"This brown patch on my neck," she said.
That’s right – not the itch, not the scratching, not the staying awake at night. What bothered her was the postinflammatory pigmentation on her neck that other kids would see and comment on.
She’s not alone. Another mother brought her 8-year-old daughter to see me. The girl’s eczema was being treated with nothing but moisturizer (which was "working, sort of").
"The reason we’re here," said Mom, "is that now she is starting to get self-conscious about the dark staining on her hand."
As doctors, we’re trained to think functionally so we can measure the ways disease impairs functionality: length of life, duration of fever, days out of work, oxygen saturation, percentage of involved body surface.
But you can’t measure shame. Nor can you predict what will produce it. Even after all these years, people surprise me all the time.
The secondary codes some insurers demand to cover wart treatment include pain, rapid growth, or bleeding. They do not include, "Clients are staring at my hand at real estate closings." Or, "This bump is reducing my tips." We could, of course, tell people not to mind being stared at, but they will not agree. They know how people look at them, and what parts of them they look at.
If any of us had a big welt over one eye, we might think twice before going to work, knowing that every patient and staff member are going to ask, "What bar were you in, and what does the other guy look like?" The teenager with the stain on her neck and the boy with the papules on his forehead and chest feel that way every morning.
Basically, nobody wants to stand out. If we do, we’d like it to be for some admirable quality. The truth is, we shouldn’t really take credit for being called handsome, smart, or healthy, but we do anyway. By the same token, it’s not our fault if we’re ill or different, but being singled out for either makes things worse – not functionally, just humanly.
Many years ago, a 15-year-old girl asked me to take off a mole from the top of her foot.
"The mole’s fine," I said. "Why do you want it off?"
"It’s embarrassing," she said.
"How?" I asked her. "Don’t you go to pools in the summer?"
"I stand with the one foot covering the spot on the other foot. Nobody has ever seen it."
Not everything people are embarrassed about can be removed with a cream or a hyfrecator. But shame is powerful, and we need to recognize it for what it is so we can address it if we can.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
At 16, Eddie is tall and athletic. He’s been treating closed comedones on his forehead and chest off and on for a couple of years.
"I hope you can help him," his mother says. "There are days when he won’t go to school, he’s so embarrassed."
Really?
If you sat people down and asked them to list what people hate about their bodies, they wouldn’t come close to guessing what we dermatologists run into every day. Here are some cases I’ve seen lately. I’m sure you can easily come up with your own examples.
• A 50-year-old attorney with a wart on the dorsum of his right hand wanted me to take it off. "I sign a lot of documents," he said," and I’d prefer that clients remember me for my legal skills, not the wart on my hand."
• A 36-year-old waiter with a picker’s nodule over the proximal interphalangeal joint of his left fifth finger said I just had to get rid of it. "I’m a waiter," he said. "This is killing my tips."
• A 36-year-old woman gave up yoga a year ago, because she was sure the woman on the adjacent mat was disgusted by her plantar warts.
• A sprightly retiree, age 88(!), insisted on paying out of pocket to remove dermatosis papulosa nigra lesions from her face. Her explanation? "My children want me to stay at home, but I want to get out and be social!"
Self-consciousness doesn’t require lesions. For instance, I saw an 11-year-old last month with widespread atopic dermatitis, the kind that’s lifelong, miserably itchy, and hard to control. Yet she wasn’t even being treated. Why had she come now?
"What bothers you most about this?" I asked her.
"This brown patch on my neck," she said.
That’s right – not the itch, not the scratching, not the staying awake at night. What bothered her was the postinflammatory pigmentation on her neck that other kids would see and comment on.
She’s not alone. Another mother brought her 8-year-old daughter to see me. The girl’s eczema was being treated with nothing but moisturizer (which was "working, sort of").
"The reason we’re here," said Mom, "is that now she is starting to get self-conscious about the dark staining on her hand."
As doctors, we’re trained to think functionally so we can measure the ways disease impairs functionality: length of life, duration of fever, days out of work, oxygen saturation, percentage of involved body surface.
But you can’t measure shame. Nor can you predict what will produce it. Even after all these years, people surprise me all the time.
The secondary codes some insurers demand to cover wart treatment include pain, rapid growth, or bleeding. They do not include, "Clients are staring at my hand at real estate closings." Or, "This bump is reducing my tips." We could, of course, tell people not to mind being stared at, but they will not agree. They know how people look at them, and what parts of them they look at.
If any of us had a big welt over one eye, we might think twice before going to work, knowing that every patient and staff member are going to ask, "What bar were you in, and what does the other guy look like?" The teenager with the stain on her neck and the boy with the papules on his forehead and chest feel that way every morning.
Basically, nobody wants to stand out. If we do, we’d like it to be for some admirable quality. The truth is, we shouldn’t really take credit for being called handsome, smart, or healthy, but we do anyway. By the same token, it’s not our fault if we’re ill or different, but being singled out for either makes things worse – not functionally, just humanly.
Many years ago, a 15-year-old girl asked me to take off a mole from the top of her foot.
"The mole’s fine," I said. "Why do you want it off?"
"It’s embarrassing," she said.
"How?" I asked her. "Don’t you go to pools in the summer?"
"I stand with the one foot covering the spot on the other foot. Nobody has ever seen it."
Not everything people are embarrassed about can be removed with a cream or a hyfrecator. But shame is powerful, and we need to recognize it for what it is so we can address it if we can.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
At 16, Eddie is tall and athletic. He’s been treating closed comedones on his forehead and chest off and on for a couple of years.
"I hope you can help him," his mother says. "There are days when he won’t go to school, he’s so embarrassed."
Really?
If you sat people down and asked them to list what people hate about their bodies, they wouldn’t come close to guessing what we dermatologists run into every day. Here are some cases I’ve seen lately. I’m sure you can easily come up with your own examples.
• A 50-year-old attorney with a wart on the dorsum of his right hand wanted me to take it off. "I sign a lot of documents," he said," and I’d prefer that clients remember me for my legal skills, not the wart on my hand."
• A 36-year-old waiter with a picker’s nodule over the proximal interphalangeal joint of his left fifth finger said I just had to get rid of it. "I’m a waiter," he said. "This is killing my tips."
• A 36-year-old woman gave up yoga a year ago, because she was sure the woman on the adjacent mat was disgusted by her plantar warts.
• A sprightly retiree, age 88(!), insisted on paying out of pocket to remove dermatosis papulosa nigra lesions from her face. Her explanation? "My children want me to stay at home, but I want to get out and be social!"
Self-consciousness doesn’t require lesions. For instance, I saw an 11-year-old last month with widespread atopic dermatitis, the kind that’s lifelong, miserably itchy, and hard to control. Yet she wasn’t even being treated. Why had she come now?
"What bothers you most about this?" I asked her.
"This brown patch on my neck," she said.
That’s right – not the itch, not the scratching, not the staying awake at night. What bothered her was the postinflammatory pigmentation on her neck that other kids would see and comment on.
She’s not alone. Another mother brought her 8-year-old daughter to see me. The girl’s eczema was being treated with nothing but moisturizer (which was "working, sort of").
"The reason we’re here," said Mom, "is that now she is starting to get self-conscious about the dark staining on her hand."
As doctors, we’re trained to think functionally so we can measure the ways disease impairs functionality: length of life, duration of fever, days out of work, oxygen saturation, percentage of involved body surface.
But you can’t measure shame. Nor can you predict what will produce it. Even after all these years, people surprise me all the time.
The secondary codes some insurers demand to cover wart treatment include pain, rapid growth, or bleeding. They do not include, "Clients are staring at my hand at real estate closings." Or, "This bump is reducing my tips." We could, of course, tell people not to mind being stared at, but they will not agree. They know how people look at them, and what parts of them they look at.
If any of us had a big welt over one eye, we might think twice before going to work, knowing that every patient and staff member are going to ask, "What bar were you in, and what does the other guy look like?" The teenager with the stain on her neck and the boy with the papules on his forehead and chest feel that way every morning.
Basically, nobody wants to stand out. If we do, we’d like it to be for some admirable quality. The truth is, we shouldn’t really take credit for being called handsome, smart, or healthy, but we do anyway. By the same token, it’s not our fault if we’re ill or different, but being singled out for either makes things worse – not functionally, just humanly.
Many years ago, a 15-year-old girl asked me to take off a mole from the top of her foot.
"The mole’s fine," I said. "Why do you want it off?"
"It’s embarrassing," she said.
"How?" I asked her. "Don’t you go to pools in the summer?"
"I stand with the one foot covering the spot on the other foot. Nobody has ever seen it."
Not everything people are embarrassed about can be removed with a cream or a hyfrecator. But shame is powerful, and we need to recognize it for what it is so we can address it if we can.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Devices and Topical Agents for Rosacea Management
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
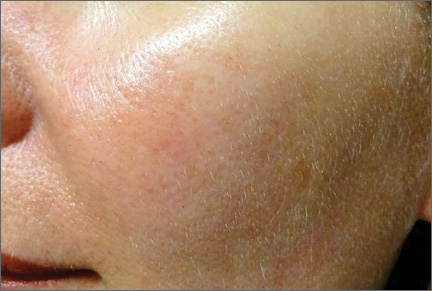
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.
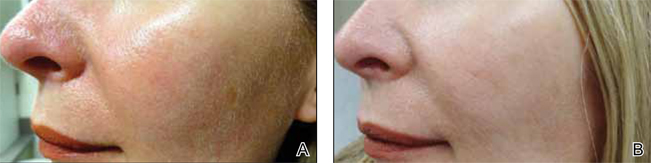
According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Practice Points
- Rosacea patients should be advised on appropriate skin care.
- Purpuric settings of the pulsed dye laser may be more effective in treating rosacea-associated erythema.
- Topical brimodine tartrate can control facial erythema, but patients should be warned of the potential risk for rebound erythema.
ACP pelvic guidelines could lead to care variations
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
On Twitter @MaryEllenNY
Neonatal and Infantile Acne Vulgaris: An Update
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8
Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23

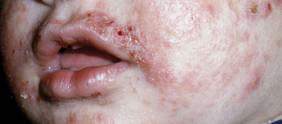
Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Practice Points
- Infantile acne needs to be recognized and treated.
- Acne in early childhood may represent virilization.
Inhibitor improves survival in older AML patients

Credit: Rhoda Baer
Adding the Plk1 inhibitor volasertib to chemotherapy can prolong survival in older patients with previously untreated acute myeloid leukemia (AML), researchers have reported in Blood.
In a phase 2 study, AML patients aged 65 or older who were ineligible for intensive induction therapy had higher response and survival rates when they received volasertib plus low-dose cytarabine (LDAC), compared to LDAC alone.
However, adverse events, such as febrile neutropenia and infections, were more common with volasertib.
“These clinical trial results . . . are important and have informed future research for this rare disease, where new treatment options are greatly needed,” said study author Hartmut Döhner, MD, of the University Hospital Ulm in Germany.
“The established approach to treat younger AML patients is an intensive chemotherapy regimen, [but] older patients often cannot tolerate these chemotherapy doses and have very limited treatment options.”
To test volasertib as a potential option, the researchers enrolled and treated 87 patients with previously untreated AML who were ineligible for intensive induction therapy. Their median age was 75 years.
Patients received LDAC at 20 mg BID subcutaneously on days 1 through 10 (n=45) or LDAC plus volasertib at 350 mg intravenously on days 1 and 15, every 4 weeks (n=42). Overall, patient demographics and baseline disease characteristics were balanced between the treatment arms.
The response rate (complete response or complete response with incomplete blood count recovery) was more than doubled for patients receiving volasertib and LDAC compared to LDAC alone. The rates were 31% (13/42) and 13.3% (6/45), respectively (odds ratio, 2.91; P=0.052).
Responses in patients receiving volasertib and LDAC were observed across all genetic groups, including 5 of 14 patients with adverse genetics.
Remissions with the combination treatment appeared to be more durable than those observed with LDAC alone. The median relapse-free survival was 18.5 months and 10.0 months, respectively.
The median event-free survival was prolonged in patients receiving volasertib as well. Their event-free survival was 5.6 months, compared to 2.3 months for patients who received LDAC alone (hazard ratio 0.57,
P=0.021).
Patients who received volasertib also experienced improvements in overall survival. The median overall survival was 8.0 months for the volasertib arm and 5.2 months for the LDAC-alone arm (hazard ratio 0.63; P=0.047).
Patients receiving volasertib and LDAC had higher rates of adverse events than patients in the LDAC-alone arm. Events of note included grade 3 febrile neutropenia (38% vs 7%), grade 3 infections (38% vs 7%) and grade 3 gastrointestinal events (21% vs 7%).
Based on these results, researchers are now investigating volasertib in combination with LDAC in a randomized, double-blind, phase 3 trial for AML called POLO-AML-2. ![]()

Credit: Rhoda Baer
Adding the Plk1 inhibitor volasertib to chemotherapy can prolong survival in older patients with previously untreated acute myeloid leukemia (AML), researchers have reported in Blood.
In a phase 2 study, AML patients aged 65 or older who were ineligible for intensive induction therapy had higher response and survival rates when they received volasertib plus low-dose cytarabine (LDAC), compared to LDAC alone.
However, adverse events, such as febrile neutropenia and infections, were more common with volasertib.
“These clinical trial results . . . are important and have informed future research for this rare disease, where new treatment options are greatly needed,” said study author Hartmut Döhner, MD, of the University Hospital Ulm in Germany.
“The established approach to treat younger AML patients is an intensive chemotherapy regimen, [but] older patients often cannot tolerate these chemotherapy doses and have very limited treatment options.”
To test volasertib as a potential option, the researchers enrolled and treated 87 patients with previously untreated AML who were ineligible for intensive induction therapy. Their median age was 75 years.
Patients received LDAC at 20 mg BID subcutaneously on days 1 through 10 (n=45) or LDAC plus volasertib at 350 mg intravenously on days 1 and 15, every 4 weeks (n=42). Overall, patient demographics and baseline disease characteristics were balanced between the treatment arms.
The response rate (complete response or complete response with incomplete blood count recovery) was more than doubled for patients receiving volasertib and LDAC compared to LDAC alone. The rates were 31% (13/42) and 13.3% (6/45), respectively (odds ratio, 2.91; P=0.052).
Responses in patients receiving volasertib and LDAC were observed across all genetic groups, including 5 of 14 patients with adverse genetics.
Remissions with the combination treatment appeared to be more durable than those observed with LDAC alone. The median relapse-free survival was 18.5 months and 10.0 months, respectively.
The median event-free survival was prolonged in patients receiving volasertib as well. Their event-free survival was 5.6 months, compared to 2.3 months for patients who received LDAC alone (hazard ratio 0.57,
P=0.021).
Patients who received volasertib also experienced improvements in overall survival. The median overall survival was 8.0 months for the volasertib arm and 5.2 months for the LDAC-alone arm (hazard ratio 0.63; P=0.047).
Patients receiving volasertib and LDAC had higher rates of adverse events than patients in the LDAC-alone arm. Events of note included grade 3 febrile neutropenia (38% vs 7%), grade 3 infections (38% vs 7%) and grade 3 gastrointestinal events (21% vs 7%).
Based on these results, researchers are now investigating volasertib in combination with LDAC in a randomized, double-blind, phase 3 trial for AML called POLO-AML-2. ![]()

Credit: Rhoda Baer
Adding the Plk1 inhibitor volasertib to chemotherapy can prolong survival in older patients with previously untreated acute myeloid leukemia (AML), researchers have reported in Blood.
In a phase 2 study, AML patients aged 65 or older who were ineligible for intensive induction therapy had higher response and survival rates when they received volasertib plus low-dose cytarabine (LDAC), compared to LDAC alone.
However, adverse events, such as febrile neutropenia and infections, were more common with volasertib.
“These clinical trial results . . . are important and have informed future research for this rare disease, where new treatment options are greatly needed,” said study author Hartmut Döhner, MD, of the University Hospital Ulm in Germany.
“The established approach to treat younger AML patients is an intensive chemotherapy regimen, [but] older patients often cannot tolerate these chemotherapy doses and have very limited treatment options.”
To test volasertib as a potential option, the researchers enrolled and treated 87 patients with previously untreated AML who were ineligible for intensive induction therapy. Their median age was 75 years.
Patients received LDAC at 20 mg BID subcutaneously on days 1 through 10 (n=45) or LDAC plus volasertib at 350 mg intravenously on days 1 and 15, every 4 weeks (n=42). Overall, patient demographics and baseline disease characteristics were balanced between the treatment arms.
The response rate (complete response or complete response with incomplete blood count recovery) was more than doubled for patients receiving volasertib and LDAC compared to LDAC alone. The rates were 31% (13/42) and 13.3% (6/45), respectively (odds ratio, 2.91; P=0.052).
Responses in patients receiving volasertib and LDAC were observed across all genetic groups, including 5 of 14 patients with adverse genetics.
Remissions with the combination treatment appeared to be more durable than those observed with LDAC alone. The median relapse-free survival was 18.5 months and 10.0 months, respectively.
The median event-free survival was prolonged in patients receiving volasertib as well. Their event-free survival was 5.6 months, compared to 2.3 months for patients who received LDAC alone (hazard ratio 0.57,
P=0.021).
Patients who received volasertib also experienced improvements in overall survival. The median overall survival was 8.0 months for the volasertib arm and 5.2 months for the LDAC-alone arm (hazard ratio 0.63; P=0.047).
Patients receiving volasertib and LDAC had higher rates of adverse events than patients in the LDAC-alone arm. Events of note included grade 3 febrile neutropenia (38% vs 7%), grade 3 infections (38% vs 7%) and grade 3 gastrointestinal events (21% vs 7%).
Based on these results, researchers are now investigating volasertib in combination with LDAC in a randomized, double-blind, phase 3 trial for AML called POLO-AML-2. ![]()