User login
Mosquitos can sniff out malaria-infected mice

Credit: James Gathany
Scientists have found evidence to suggest that malaria parasites change the body odor of their host to attract hungry mosquitos.
The team observed an increase in mosquito attraction to malaria-infected mice, compared to healthy controls.
And the infected mice exhibited elevations in certain components of their natural scent, which suggests the malaria parasite changes the characteristics of its host’s body odor to make the host more attractive to mosquitos.
These findings appear in Proceedings of the National Academy of Sciences.
The researchers found that mice infected with Plasmodium chabaudii were more attractive to Anopheles stephensi mosquitos than uninfected control mice. And the attraction corresponded to an overall elevation in scent emissions from the infected mice.
However, malaria infection did not appear to trigger the expression of unique scent components. Instead, it seems the malaria pathogens alter the levels of compounds already present in the scent of uninfected mice.
“There appears to be an overall elevation of several compounds that are attractive to mosquitos,” said study author Consuelo De Moraes, PhD, of the Swiss Federal Institute of Technology in Zürich (ETH Zürich).
“Since mosquitos probably don’t benefit from feeding on infected people, it may make sense for the pathogen to exaggerate existing odor cues that the insects are already using for host location,” added study author Mark Mescher, PhD, also of ETH Zürich.
What the researchers found most surprising is the fact that the malaria infection leaves its mark on body odor long-term. Even when infected mice no longer had symptoms, their body odor showed they were carriers of the pathogen.
However, not all stages of disease smelled the same. The team found the scent profile of the acutely ill differed from the profile in mice exhibiting later stages of malaria infection.
Although the findings from this study cannot be directly translated to human malaria, they suggest similar effects might be involved in the attraction of mosquitos to infected people. Drs Mescher and De Moraes are currently investigating this possibility through additional research involving human subjects in Africa. ![]()

Credit: James Gathany
Scientists have found evidence to suggest that malaria parasites change the body odor of their host to attract hungry mosquitos.
The team observed an increase in mosquito attraction to malaria-infected mice, compared to healthy controls.
And the infected mice exhibited elevations in certain components of their natural scent, which suggests the malaria parasite changes the characteristics of its host’s body odor to make the host more attractive to mosquitos.
These findings appear in Proceedings of the National Academy of Sciences.
The researchers found that mice infected with Plasmodium chabaudii were more attractive to Anopheles stephensi mosquitos than uninfected control mice. And the attraction corresponded to an overall elevation in scent emissions from the infected mice.
However, malaria infection did not appear to trigger the expression of unique scent components. Instead, it seems the malaria pathogens alter the levels of compounds already present in the scent of uninfected mice.
“There appears to be an overall elevation of several compounds that are attractive to mosquitos,” said study author Consuelo De Moraes, PhD, of the Swiss Federal Institute of Technology in Zürich (ETH Zürich).
“Since mosquitos probably don’t benefit from feeding on infected people, it may make sense for the pathogen to exaggerate existing odor cues that the insects are already using for host location,” added study author Mark Mescher, PhD, also of ETH Zürich.
What the researchers found most surprising is the fact that the malaria infection leaves its mark on body odor long-term. Even when infected mice no longer had symptoms, their body odor showed they were carriers of the pathogen.
However, not all stages of disease smelled the same. The team found the scent profile of the acutely ill differed from the profile in mice exhibiting later stages of malaria infection.
Although the findings from this study cannot be directly translated to human malaria, they suggest similar effects might be involved in the attraction of mosquitos to infected people. Drs Mescher and De Moraes are currently investigating this possibility through additional research involving human subjects in Africa. ![]()

Credit: James Gathany
Scientists have found evidence to suggest that malaria parasites change the body odor of their host to attract hungry mosquitos.
The team observed an increase in mosquito attraction to malaria-infected mice, compared to healthy controls.
And the infected mice exhibited elevations in certain components of their natural scent, which suggests the malaria parasite changes the characteristics of its host’s body odor to make the host more attractive to mosquitos.
These findings appear in Proceedings of the National Academy of Sciences.
The researchers found that mice infected with Plasmodium chabaudii were more attractive to Anopheles stephensi mosquitos than uninfected control mice. And the attraction corresponded to an overall elevation in scent emissions from the infected mice.
However, malaria infection did not appear to trigger the expression of unique scent components. Instead, it seems the malaria pathogens alter the levels of compounds already present in the scent of uninfected mice.
“There appears to be an overall elevation of several compounds that are attractive to mosquitos,” said study author Consuelo De Moraes, PhD, of the Swiss Federal Institute of Technology in Zürich (ETH Zürich).
“Since mosquitos probably don’t benefit from feeding on infected people, it may make sense for the pathogen to exaggerate existing odor cues that the insects are already using for host location,” added study author Mark Mescher, PhD, also of ETH Zürich.
What the researchers found most surprising is the fact that the malaria infection leaves its mark on body odor long-term. Even when infected mice no longer had symptoms, their body odor showed they were carriers of the pathogen.
However, not all stages of disease smelled the same. The team found the scent profile of the acutely ill differed from the profile in mice exhibiting later stages of malaria infection.
Although the findings from this study cannot be directly translated to human malaria, they suggest similar effects might be involved in the attraction of mosquitos to infected people. Drs Mescher and De Moraes are currently investigating this possibility through additional research involving human subjects in Africa. ![]()
Study validates drug’s efficacy in CLL/SLL
MILAN—Results of the phase 3 RESONATE trial suggest ibrutinib can improve response and survival rates in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), when compared to ofatumumab.
Ibrutinib conferred these benefits irrespective of baseline clinical characteristics or molecular features, including 17p deletion.
Atrial fibrillation and bleeding-related events were more common with ibrutinib. But the rate of serious adverse events was similar between the treatment arms.
About 86% of patients remained on ibrutinib at last analysis, and roughly 29% of patients initially randomized to ofatumumab crossed over to the ibrutinib arm after disease progression.
“This study undoubtedly confirms that ibrutinib is a very effective agent—as a single-agent—in relapsed CLL patients,” said investigator Peter Hillmen, MD, PhD, of The Leeds Teaching Hospitals in the UK.
Dr Hillmen presented these results at the 19th Congress of the European Hematology Association (EHA) as abstract S693. The RESONATE trial was sponsored by Pharmacyclics and Janssen, the companies developing ibrutinib.
The trial included 391 patients with relapsed or refractory CLL/SLL. They were randomized to receive oral ibrutinib at 420 mg once daily until progression or unacceptable toxicity (n=195) or intravenous ofatumumab at an initial dose of 300 mg, followed by 11 doses of 2000 mg (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57).
The median age in both treatment arms was 67. Overall, roughly 50% of patients had received 3 or more prior therapies, including purine analogs, alkylating agents, and anti-CD20 antibodies. The proportion of patients with del17p was similar between the treatment arms—32% in the ibrutinib arm and 33% in the ofatumumab arm.
Response and survival
At the time of interim analysis, patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm, according to an independent review committee.
In addition, ibrutinib significantly prolonged progression-free survival (PFS). The median PFS was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The improvement in PFS represents a 78% reduction in the risk of progression or death.
Dr Hillmen noted that PFS favored ibrutinib regardless of baseline characteristics such as refractoriness to purine analogs, del17p, age, gender, Rai stage, bulky disease, number of prior treatments, del11q, B2 microglobulin, and IgVH mutation status.
Ibrutinib significantly prolonged overall survival (OS) as well. The median OS was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049). The improvement in OS represents a 56% reduction in the risk of death in patients treated with ibrutinib.
Adverse events
Dr Hillmen pointed out that the median treatment duration was 8.6 months for ibrutinib and 5.3 months for ofatumumab, and this difference confounds the assessment of side effects.
Nevertheless, nearly all patients in both treatment arms experienced adverse events—99% in the ibrutinib arm and 98% in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation of any grade was more common in the ibrutinib arm (n=10) than in the ofatumumab arm (n=1), but 5 of the ibrutinib-treated patients had a prior history of atrial fibrillation. Bleeding-related events were also more common with ibrutinib (44% vs 12%), as were diarrhea (48% vs 18%) and arthralgia (17% vs 7%).
Events more common in the ofatumumab arm included infusion-related reactions (28% vs 0%), peripheral sensory neuropathy (13% vs 4%), urticaria (6% vs 1%), night sweats (13% vs 5%), and pruritus (9% vs 4%). ![]()
MILAN—Results of the phase 3 RESONATE trial suggest ibrutinib can improve response and survival rates in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), when compared to ofatumumab.
Ibrutinib conferred these benefits irrespective of baseline clinical characteristics or molecular features, including 17p deletion.
Atrial fibrillation and bleeding-related events were more common with ibrutinib. But the rate of serious adverse events was similar between the treatment arms.
About 86% of patients remained on ibrutinib at last analysis, and roughly 29% of patients initially randomized to ofatumumab crossed over to the ibrutinib arm after disease progression.
“This study undoubtedly confirms that ibrutinib is a very effective agent—as a single-agent—in relapsed CLL patients,” said investigator Peter Hillmen, MD, PhD, of The Leeds Teaching Hospitals in the UK.
Dr Hillmen presented these results at the 19th Congress of the European Hematology Association (EHA) as abstract S693. The RESONATE trial was sponsored by Pharmacyclics and Janssen, the companies developing ibrutinib.
The trial included 391 patients with relapsed or refractory CLL/SLL. They were randomized to receive oral ibrutinib at 420 mg once daily until progression or unacceptable toxicity (n=195) or intravenous ofatumumab at an initial dose of 300 mg, followed by 11 doses of 2000 mg (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57).
The median age in both treatment arms was 67. Overall, roughly 50% of patients had received 3 or more prior therapies, including purine analogs, alkylating agents, and anti-CD20 antibodies. The proportion of patients with del17p was similar between the treatment arms—32% in the ibrutinib arm and 33% in the ofatumumab arm.
Response and survival
At the time of interim analysis, patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm, according to an independent review committee.
In addition, ibrutinib significantly prolonged progression-free survival (PFS). The median PFS was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The improvement in PFS represents a 78% reduction in the risk of progression or death.
Dr Hillmen noted that PFS favored ibrutinib regardless of baseline characteristics such as refractoriness to purine analogs, del17p, age, gender, Rai stage, bulky disease, number of prior treatments, del11q, B2 microglobulin, and IgVH mutation status.
Ibrutinib significantly prolonged overall survival (OS) as well. The median OS was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049). The improvement in OS represents a 56% reduction in the risk of death in patients treated with ibrutinib.
Adverse events
Dr Hillmen pointed out that the median treatment duration was 8.6 months for ibrutinib and 5.3 months for ofatumumab, and this difference confounds the assessment of side effects.
Nevertheless, nearly all patients in both treatment arms experienced adverse events—99% in the ibrutinib arm and 98% in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation of any grade was more common in the ibrutinib arm (n=10) than in the ofatumumab arm (n=1), but 5 of the ibrutinib-treated patients had a prior history of atrial fibrillation. Bleeding-related events were also more common with ibrutinib (44% vs 12%), as were diarrhea (48% vs 18%) and arthralgia (17% vs 7%).
Events more common in the ofatumumab arm included infusion-related reactions (28% vs 0%), peripheral sensory neuropathy (13% vs 4%), urticaria (6% vs 1%), night sweats (13% vs 5%), and pruritus (9% vs 4%). ![]()
MILAN—Results of the phase 3 RESONATE trial suggest ibrutinib can improve response and survival rates in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), when compared to ofatumumab.
Ibrutinib conferred these benefits irrespective of baseline clinical characteristics or molecular features, including 17p deletion.
Atrial fibrillation and bleeding-related events were more common with ibrutinib. But the rate of serious adverse events was similar between the treatment arms.
About 86% of patients remained on ibrutinib at last analysis, and roughly 29% of patients initially randomized to ofatumumab crossed over to the ibrutinib arm after disease progression.
“This study undoubtedly confirms that ibrutinib is a very effective agent—as a single-agent—in relapsed CLL patients,” said investigator Peter Hillmen, MD, PhD, of The Leeds Teaching Hospitals in the UK.
Dr Hillmen presented these results at the 19th Congress of the European Hematology Association (EHA) as abstract S693. The RESONATE trial was sponsored by Pharmacyclics and Janssen, the companies developing ibrutinib.
The trial included 391 patients with relapsed or refractory CLL/SLL. They were randomized to receive oral ibrutinib at 420 mg once daily until progression or unacceptable toxicity (n=195) or intravenous ofatumumab at an initial dose of 300 mg, followed by 11 doses of 2000 mg (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57).
The median age in both treatment arms was 67. Overall, roughly 50% of patients had received 3 or more prior therapies, including purine analogs, alkylating agents, and anti-CD20 antibodies. The proportion of patients with del17p was similar between the treatment arms—32% in the ibrutinib arm and 33% in the ofatumumab arm.
Response and survival
At the time of interim analysis, patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm, according to an independent review committee.
In addition, ibrutinib significantly prolonged progression-free survival (PFS). The median PFS was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The improvement in PFS represents a 78% reduction in the risk of progression or death.
Dr Hillmen noted that PFS favored ibrutinib regardless of baseline characteristics such as refractoriness to purine analogs, del17p, age, gender, Rai stage, bulky disease, number of prior treatments, del11q, B2 microglobulin, and IgVH mutation status.
Ibrutinib significantly prolonged overall survival (OS) as well. The median OS was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049). The improvement in OS represents a 56% reduction in the risk of death in patients treated with ibrutinib.
Adverse events
Dr Hillmen pointed out that the median treatment duration was 8.6 months for ibrutinib and 5.3 months for ofatumumab, and this difference confounds the assessment of side effects.
Nevertheless, nearly all patients in both treatment arms experienced adverse events—99% in the ibrutinib arm and 98% in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation of any grade was more common in the ibrutinib arm (n=10) than in the ofatumumab arm (n=1), but 5 of the ibrutinib-treated patients had a prior history of atrial fibrillation. Bleeding-related events were also more common with ibrutinib (44% vs 12%), as were diarrhea (48% vs 18%) and arthralgia (17% vs 7%).
Events more common in the ofatumumab arm included infusion-related reactions (28% vs 0%), peripheral sensory neuropathy (13% vs 4%), urticaria (6% vs 1%), night sweats (13% vs 5%), and pruritus (9% vs 4%). ![]()
Quality Initiatives Earn Low Marks
More than 70% of people who responded to a survey at The-Hospitalist.org had a negative opinion about how local and national quality initiatives (QI) have impacted their ability to care for hospitalized patients.
Survey respondents were asked to gauge the effectiveness of core measures, Physician Quality Reporting System (PQRS) reporting, and clinical reminders. A combined 38% of respondents said that QI measures produced little benefit for their patients or rarely addressed patients' acute issues. Another 21% of respondents labeled QI measures as "distractions," and 12% said QI measures affected their productivity.
Only 28% of respondents thought that QI have improved inpatient care, just 2% more than those who found "little benefit" to them (26%), indicating that 54% of respondents were nearly evenly split on whether QI measures directly benefit patients.
Felix Aguirre, MD, FHM, vice president of medical affairs for IPC: The Hospitalist Company and a member of SHM's Performance Measurement and Reporting Committee (PMRC), says while certain core measures, such as PQRS reporting, may not address the specific needs of all hospital patients, it does not make them unsuccessful.
"I think measures do improve care, even if it's not for my patients, [then] for the global population of patients," Dr. Aguirre says. "We're not moving the needle quickly by treating my patients; we're moving the needle slowly, but surely, by treating all patients."
PMRC chair Gregory B. Seymann, MD, SFHM, clinical professor and chief of the division of hospital medicine at University of California San Diego Health Sciences, says the variety of QI measures included in the survey may explain the difference in opinions.
"There are multiple different practice arrangements among the general population of hospitalists and thus many different ways an individual respondent might interact with the measures," Dr. Seymann says. TH
Visit our website for more information on quality initiatives.
More than 70% of people who responded to a survey at The-Hospitalist.org had a negative opinion about how local and national quality initiatives (QI) have impacted their ability to care for hospitalized patients.
Survey respondents were asked to gauge the effectiveness of core measures, Physician Quality Reporting System (PQRS) reporting, and clinical reminders. A combined 38% of respondents said that QI measures produced little benefit for their patients or rarely addressed patients' acute issues. Another 21% of respondents labeled QI measures as "distractions," and 12% said QI measures affected their productivity.
Only 28% of respondents thought that QI have improved inpatient care, just 2% more than those who found "little benefit" to them (26%), indicating that 54% of respondents were nearly evenly split on whether QI measures directly benefit patients.
Felix Aguirre, MD, FHM, vice president of medical affairs for IPC: The Hospitalist Company and a member of SHM's Performance Measurement and Reporting Committee (PMRC), says while certain core measures, such as PQRS reporting, may not address the specific needs of all hospital patients, it does not make them unsuccessful.
"I think measures do improve care, even if it's not for my patients, [then] for the global population of patients," Dr. Aguirre says. "We're not moving the needle quickly by treating my patients; we're moving the needle slowly, but surely, by treating all patients."
PMRC chair Gregory B. Seymann, MD, SFHM, clinical professor and chief of the division of hospital medicine at University of California San Diego Health Sciences, says the variety of QI measures included in the survey may explain the difference in opinions.
"There are multiple different practice arrangements among the general population of hospitalists and thus many different ways an individual respondent might interact with the measures," Dr. Seymann says. TH
Visit our website for more information on quality initiatives.
More than 70% of people who responded to a survey at The-Hospitalist.org had a negative opinion about how local and national quality initiatives (QI) have impacted their ability to care for hospitalized patients.
Survey respondents were asked to gauge the effectiveness of core measures, Physician Quality Reporting System (PQRS) reporting, and clinical reminders. A combined 38% of respondents said that QI measures produced little benefit for their patients or rarely addressed patients' acute issues. Another 21% of respondents labeled QI measures as "distractions," and 12% said QI measures affected their productivity.
Only 28% of respondents thought that QI have improved inpatient care, just 2% more than those who found "little benefit" to them (26%), indicating that 54% of respondents were nearly evenly split on whether QI measures directly benefit patients.
Felix Aguirre, MD, FHM, vice president of medical affairs for IPC: The Hospitalist Company and a member of SHM's Performance Measurement and Reporting Committee (PMRC), says while certain core measures, such as PQRS reporting, may not address the specific needs of all hospital patients, it does not make them unsuccessful.
"I think measures do improve care, even if it's not for my patients, [then] for the global population of patients," Dr. Aguirre says. "We're not moving the needle quickly by treating my patients; we're moving the needle slowly, but surely, by treating all patients."
PMRC chair Gregory B. Seymann, MD, SFHM, clinical professor and chief of the division of hospital medicine at University of California San Diego Health Sciences, says the variety of QI measures included in the survey may explain the difference in opinions.
"There are multiple different practice arrangements among the general population of hospitalists and thus many different ways an individual respondent might interact with the measures," Dr. Seymann says. TH
Visit our website for more information on quality initiatives.
Inhaled Corticosteroids Increase Risk of Serious Pneumonia in Patients with COPD
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
New clinical practice guidelines on pheochromocytomas
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
AT ICE/ENDO 2014
Frostbite on the Hand of a Homeless Man
To the Editor:
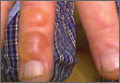
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

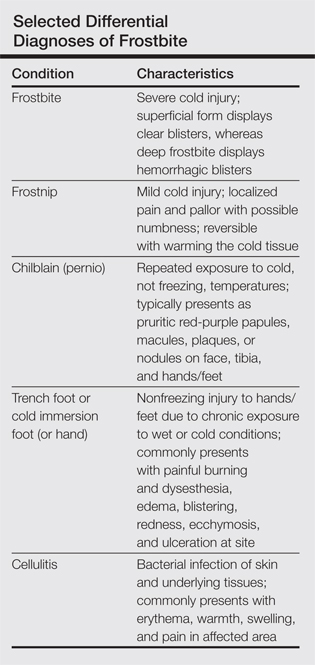
Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.
Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
2014 Update on infectious disease
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
Does prenatal acetaminophen exposure increase the risk of behavioral problems in the child?
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
Survey: ObGyns’ salaries rose slightly in 2013
The 2014 Medscape Compensation Report surveyed more than 24,000 physicians in 25 specialties. Five percent of respondents were ObGyns, whose mean income rose slightly to $243,000 in 2013 from $242,000 in 2012, up from $220,000 in 2011.1–3 The highest ObGyn earners lived in the Great Lakes and North Central regions.1
Survey findings
Men make more than women. In 2013, male ObGyns reported earning $256,000; female ObGyns reported $229,000 in mean income. However, women felt more satisfied with their salary (47% of women vs 38% of men). Regardless of gender, ObGyns were slightly less happy with their income than all physicians (50% satisfied).1
Among all female physicians, more were employed than self-employed; the opposite was true for male physicians.4 Half of all graduating physicians are now female, and demographics show that 62% of all female physicians are younger than age 45.1
Practice settings are key to income. Sixty percent of ObGyns indicated they would choose medicine again as a career; 43% would choose their own specialty. However, only 25% of ObGyns would make the same decision about practice setting.1
In 2013, employed and self-employed ObGyns reported nearly the same mean income: $243,000 versus $246,000, respectively. However, when broken down by specific practice setting, the highest earners were ObGyns who worked for health-care organizations, at $273,000. Additional 2013 mean earnings ranked by work setting were1:
- multispecialty office-based group practices, $271,000
- single-specialty office-based group practices, $255,000
- hospitals, $228,000
- solo office-based practices, $212,000
- outpatient clinics, $207,000.
In 2013, 49% of employed physicians worked in hospitals or in groups owned by a hospital, while 21% were employed by private groups. Other employment situations included community health centers, corporate laboratories, correction institutions, military bases, and nursing homes.4
ACO participation grows. In 2013, 37% of ObGyns either participated in an Accountable Care Organization (ACO) or planned on joining an ACO within the next year.1 This was an increase from 25% in 2012.2,3
In the most recent report, 2% chose concierge practices (also known as direct primary care) and 5% opted for cash-only practices.1 In 2012, only 1% of ObGyns opted for concierge practices, and 3% for cash-only practices.2,3
Related article: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Employment over private practice? In 2013, physicians were enticed to seek employment by the financial challenges of private practice (38%); not having to be concerned about administrative issues (29%); and working shorter and more regular hours (19%). Other reported benefits of employment were academic opportunities, better life−work balance, more vacation time, and no loss of income during vacation. More than half (53%) of employed physicians who were previously self-employed felt that patient care was superior now that they were employed, and 37% thought it was about the same.4
Related article: Mean income for ObGyns increased in 2012. Deborah Reale (News for your Practice; August 2013)
Career satisfaction
ObGyns were close to the bottom among all physicians (48%) when it came to overall career satisfaction, tied with nephrologists, surgeons, and pulmonologists. The most satisfied physicians were dermatologists (65%); the least satisfied were plastic surgeons (45%).1
What drives you? In 2013, more ObGyns (41%) than all physicians (33%) reported that the most rewarding part of their job was their relationships with patients. Thirty percent of ObGyns chose being good at their jobs; 8% chose making good money; and 2% found nothing rewarding about the job.1
How much patient time do you spend? The majority (58%) of ObGyns reported spending more than 40 hours per week with patients and 16 minutes or less (66%) per patient.1 In 2012, 60% of ObGyn respondents reported spending 16 minutes or less per patient.2,3
Anticipating the effects of the Affordable Care Act
Under the Affordable Care Act (ACA), an organization’s revenue will still be determined largely by the volume generated by physicians. The percentage of ObGyns who saw 50 to 124 patients per week increased from 57% in 2012 to 69% in 2013 (TABLE).1,2
In 2013, 53% of ObGyns still were undecided about health-insurance exchange participation—the same percentage as all survey respondents. Among ObGyns, 30% would participate, and 17% would not participate.1
Related article: As the Affordable Care Act comes of age, a look behind the headlines. Lucia DiVenere, MA (Practice Management; January 2014)
Almost half (49%) of ObGyns expect their income under the ACA to decrease. About 45% of ObGyns did not foresee any change, and 5% believed their incomes would increase (1% didn’t know) under the ACA. ObGyns also anticipated a higher workload, a decline in quality of patient care and access, and reduced ability to make decisions.1
Almost one-third of ObGyns dropped poorly paying insurers. In 2013, 29% of ObGyns said they regularly drop insurers who pay poorly, but 46% said they keep their insurers year after year. In 2012, 26% of ObGyns said they drop insurers who pay the least or create the most trouble; 29% said they keep all insurers.2,3 Private insurance paid for 63% of patient visits to ObGyns in 2013.1
Fewer ObGyns indicated they would see Medicare and Medicaid patients. In 2013, 20% of self-employed and 5% of employed ObGyns said that they plan to stop taking new Medicare or Medicaid patients. More employed (72%) than self-employed (46%) ObGyns reported that they would continue seeing new and current Medicare and Medicaid patients.1
Related article: Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander. Robert L. Barbieri, MD (Editorial; October 2011)
In 2012, 15% of ObGyn respondents planned to stop taking new Medicare or Medicaid patients, but 53% of ObGyn respondents said they would continue to see current patients and would take on new Medicare or Medicaid patients.2,3
TELL US WHAT YOU THINK! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
- Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
- Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
- Kane L. Employed vs self-employed: Who is better off? Medscape Web site. http://www.medscape.com/features/slideshow/public/employed-doctors. Published March 11, 2014. Accessed June 2, 2014.
The 2014 Medscape Compensation Report surveyed more than 24,000 physicians in 25 specialties. Five percent of respondents were ObGyns, whose mean income rose slightly to $243,000 in 2013 from $242,000 in 2012, up from $220,000 in 2011.1–3 The highest ObGyn earners lived in the Great Lakes and North Central regions.1
Survey findings
Men make more than women. In 2013, male ObGyns reported earning $256,000; female ObGyns reported $229,000 in mean income. However, women felt more satisfied with their salary (47% of women vs 38% of men). Regardless of gender, ObGyns were slightly less happy with their income than all physicians (50% satisfied).1
Among all female physicians, more were employed than self-employed; the opposite was true for male physicians.4 Half of all graduating physicians are now female, and demographics show that 62% of all female physicians are younger than age 45.1
Practice settings are key to income. Sixty percent of ObGyns indicated they would choose medicine again as a career; 43% would choose their own specialty. However, only 25% of ObGyns would make the same decision about practice setting.1
In 2013, employed and self-employed ObGyns reported nearly the same mean income: $243,000 versus $246,000, respectively. However, when broken down by specific practice setting, the highest earners were ObGyns who worked for health-care organizations, at $273,000. Additional 2013 mean earnings ranked by work setting were1:
- multispecialty office-based group practices, $271,000
- single-specialty office-based group practices, $255,000
- hospitals, $228,000
- solo office-based practices, $212,000
- outpatient clinics, $207,000.
In 2013, 49% of employed physicians worked in hospitals or in groups owned by a hospital, while 21% were employed by private groups. Other employment situations included community health centers, corporate laboratories, correction institutions, military bases, and nursing homes.4
ACO participation grows. In 2013, 37% of ObGyns either participated in an Accountable Care Organization (ACO) or planned on joining an ACO within the next year.1 This was an increase from 25% in 2012.2,3
In the most recent report, 2% chose concierge practices (also known as direct primary care) and 5% opted for cash-only practices.1 In 2012, only 1% of ObGyns opted for concierge practices, and 3% for cash-only practices.2,3
Related article: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Employment over private practice? In 2013, physicians were enticed to seek employment by the financial challenges of private practice (38%); not having to be concerned about administrative issues (29%); and working shorter and more regular hours (19%). Other reported benefits of employment were academic opportunities, better life−work balance, more vacation time, and no loss of income during vacation. More than half (53%) of employed physicians who were previously self-employed felt that patient care was superior now that they were employed, and 37% thought it was about the same.4
Related article: Mean income for ObGyns increased in 2012. Deborah Reale (News for your Practice; August 2013)
Career satisfaction
ObGyns were close to the bottom among all physicians (48%) when it came to overall career satisfaction, tied with nephrologists, surgeons, and pulmonologists. The most satisfied physicians were dermatologists (65%); the least satisfied were plastic surgeons (45%).1
What drives you? In 2013, more ObGyns (41%) than all physicians (33%) reported that the most rewarding part of their job was their relationships with patients. Thirty percent of ObGyns chose being good at their jobs; 8% chose making good money; and 2% found nothing rewarding about the job.1
How much patient time do you spend? The majority (58%) of ObGyns reported spending more than 40 hours per week with patients and 16 minutes or less (66%) per patient.1 In 2012, 60% of ObGyn respondents reported spending 16 minutes or less per patient.2,3
Anticipating the effects of the Affordable Care Act
Under the Affordable Care Act (ACA), an organization’s revenue will still be determined largely by the volume generated by physicians. The percentage of ObGyns who saw 50 to 124 patients per week increased from 57% in 2012 to 69% in 2013 (TABLE).1,2
In 2013, 53% of ObGyns still were undecided about health-insurance exchange participation—the same percentage as all survey respondents. Among ObGyns, 30% would participate, and 17% would not participate.1
Related article: As the Affordable Care Act comes of age, a look behind the headlines. Lucia DiVenere, MA (Practice Management; January 2014)
Almost half (49%) of ObGyns expect their income under the ACA to decrease. About 45% of ObGyns did not foresee any change, and 5% believed their incomes would increase (1% didn’t know) under the ACA. ObGyns also anticipated a higher workload, a decline in quality of patient care and access, and reduced ability to make decisions.1
Almost one-third of ObGyns dropped poorly paying insurers. In 2013, 29% of ObGyns said they regularly drop insurers who pay poorly, but 46% said they keep their insurers year after year. In 2012, 26% of ObGyns said they drop insurers who pay the least or create the most trouble; 29% said they keep all insurers.2,3 Private insurance paid for 63% of patient visits to ObGyns in 2013.1
Fewer ObGyns indicated they would see Medicare and Medicaid patients. In 2013, 20% of self-employed and 5% of employed ObGyns said that they plan to stop taking new Medicare or Medicaid patients. More employed (72%) than self-employed (46%) ObGyns reported that they would continue seeing new and current Medicare and Medicaid patients.1
Related article: Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander. Robert L. Barbieri, MD (Editorial; October 2011)
In 2012, 15% of ObGyn respondents planned to stop taking new Medicare or Medicaid patients, but 53% of ObGyn respondents said they would continue to see current patients and would take on new Medicare or Medicaid patients.2,3
TELL US WHAT YOU THINK! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
The 2014 Medscape Compensation Report surveyed more than 24,000 physicians in 25 specialties. Five percent of respondents were ObGyns, whose mean income rose slightly to $243,000 in 2013 from $242,000 in 2012, up from $220,000 in 2011.1–3 The highest ObGyn earners lived in the Great Lakes and North Central regions.1
Survey findings
Men make more than women. In 2013, male ObGyns reported earning $256,000; female ObGyns reported $229,000 in mean income. However, women felt more satisfied with their salary (47% of women vs 38% of men). Regardless of gender, ObGyns were slightly less happy with their income than all physicians (50% satisfied).1
Among all female physicians, more were employed than self-employed; the opposite was true for male physicians.4 Half of all graduating physicians are now female, and demographics show that 62% of all female physicians are younger than age 45.1
Practice settings are key to income. Sixty percent of ObGyns indicated they would choose medicine again as a career; 43% would choose their own specialty. However, only 25% of ObGyns would make the same decision about practice setting.1
In 2013, employed and self-employed ObGyns reported nearly the same mean income: $243,000 versus $246,000, respectively. However, when broken down by specific practice setting, the highest earners were ObGyns who worked for health-care organizations, at $273,000. Additional 2013 mean earnings ranked by work setting were1:
- multispecialty office-based group practices, $271,000
- single-specialty office-based group practices, $255,000
- hospitals, $228,000
- solo office-based practices, $212,000
- outpatient clinics, $207,000.
In 2013, 49% of employed physicians worked in hospitals or in groups owned by a hospital, while 21% were employed by private groups. Other employment situations included community health centers, corporate laboratories, correction institutions, military bases, and nursing homes.4
ACO participation grows. In 2013, 37% of ObGyns either participated in an Accountable Care Organization (ACO) or planned on joining an ACO within the next year.1 This was an increase from 25% in 2012.2,3
In the most recent report, 2% chose concierge practices (also known as direct primary care) and 5% opted for cash-only practices.1 In 2012, only 1% of ObGyns opted for concierge practices, and 3% for cash-only practices.2,3
Related article: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Employment over private practice? In 2013, physicians were enticed to seek employment by the financial challenges of private practice (38%); not having to be concerned about administrative issues (29%); and working shorter and more regular hours (19%). Other reported benefits of employment were academic opportunities, better life−work balance, more vacation time, and no loss of income during vacation. More than half (53%) of employed physicians who were previously self-employed felt that patient care was superior now that they were employed, and 37% thought it was about the same.4
Related article: Mean income for ObGyns increased in 2012. Deborah Reale (News for your Practice; August 2013)
Career satisfaction
ObGyns were close to the bottom among all physicians (48%) when it came to overall career satisfaction, tied with nephrologists, surgeons, and pulmonologists. The most satisfied physicians were dermatologists (65%); the least satisfied were plastic surgeons (45%).1
What drives you? In 2013, more ObGyns (41%) than all physicians (33%) reported that the most rewarding part of their job was their relationships with patients. Thirty percent of ObGyns chose being good at their jobs; 8% chose making good money; and 2% found nothing rewarding about the job.1
How much patient time do you spend? The majority (58%) of ObGyns reported spending more than 40 hours per week with patients and 16 minutes or less (66%) per patient.1 In 2012, 60% of ObGyn respondents reported spending 16 minutes or less per patient.2,3
Anticipating the effects of the Affordable Care Act
Under the Affordable Care Act (ACA), an organization’s revenue will still be determined largely by the volume generated by physicians. The percentage of ObGyns who saw 50 to 124 patients per week increased from 57% in 2012 to 69% in 2013 (TABLE).1,2
In 2013, 53% of ObGyns still were undecided about health-insurance exchange participation—the same percentage as all survey respondents. Among ObGyns, 30% would participate, and 17% would not participate.1
Related article: As the Affordable Care Act comes of age, a look behind the headlines. Lucia DiVenere, MA (Practice Management; January 2014)
Almost half (49%) of ObGyns expect their income under the ACA to decrease. About 45% of ObGyns did not foresee any change, and 5% believed their incomes would increase (1% didn’t know) under the ACA. ObGyns also anticipated a higher workload, a decline in quality of patient care and access, and reduced ability to make decisions.1
Almost one-third of ObGyns dropped poorly paying insurers. In 2013, 29% of ObGyns said they regularly drop insurers who pay poorly, but 46% said they keep their insurers year after year. In 2012, 26% of ObGyns said they drop insurers who pay the least or create the most trouble; 29% said they keep all insurers.2,3 Private insurance paid for 63% of patient visits to ObGyns in 2013.1
Fewer ObGyns indicated they would see Medicare and Medicaid patients. In 2013, 20% of self-employed and 5% of employed ObGyns said that they plan to stop taking new Medicare or Medicaid patients. More employed (72%) than self-employed (46%) ObGyns reported that they would continue seeing new and current Medicare and Medicaid patients.1
Related article: Medicare and Medicaid are on the brink of insolvency, and you’re not just a bystander. Robert L. Barbieri, MD (Editorial; October 2011)
In 2012, 15% of ObGyn respondents planned to stop taking new Medicare or Medicaid patients, but 53% of ObGyn respondents said they would continue to see current patients and would take on new Medicare or Medicaid patients.2,3
TELL US WHAT YOU THINK! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
- Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
- Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
- Kane L. Employed vs self-employed: Who is better off? Medscape Web site. http://www.medscape.com/features/slideshow/public/employed-doctors. Published March 11, 2014. Accessed June 2, 2014.
- Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
- Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
- Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
- Kane L. Employed vs self-employed: Who is better off? Medscape Web site. http://www.medscape.com/features/slideshow/public/employed-doctors. Published March 11, 2014. Accessed June 2, 2014.
HSCT regimen could be ‘transformative’ for SCD

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()

pre-HSCT (top) and post-HSCT
Credit: NIH Molecular and
Clinical Hematology Branch
In a small study, a nonmyeloablative hematopoietic stem cell transplant (HSCT) regimen reversed sickle cell disease (SCD) phenotype in a majority of adult patients, some of whom also had thalassemia.
Half of the patients were able to stop taking immunosuppressants and did not develop graft-vs-host disease (GVHD).
There were adverse events associated with the regimen, but the researchers believe it shows promise and could be “transformative” for patients with severe SCD.
The team described the regimen and its effects in JAMA.
Matthew M. Hsieh, MD, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, and his colleagues first explored a nonmyeloablative HSCT approach in a pilot group of 10 adults with severe SCD.
The regimen had few toxic effects, but all patients continued taking immunosuppression medication. The researchers have since revised the protocol to include an option to stop immunosuppression after 1 year in patients with donor CD3 engraftment of greater than 50% and normalization of hemoglobin.
In JAMA, the team described the outcomes for 20 additional patients with severe SCD, with or without thalassemia, along with updated results from the first 10 patients.
All 30 patients (ages 16-65 years) were enrolled in the study from July 2004 to October 2013. Two patients had heterozygous hemoglobin S and C, 1 patient had HbSβ+-thalassemia, 1 patient had HbSβ0- thalassemia, and 1 had transfusion-dependent β-thalassemia intermedia. The remaining patients had homozygous hemoglobin S.
Patients received alemtuzumab (1mg/kg in divided doses), total-body irradiation (300 cGy), sirolimus, and an infusion of unmanipulated, filgrastim-mobilized peripheral blood stem cells (5.5-31.7 × 106 cells/kg) from HLA-matched siblings.
There were 38 serious adverse events. The most common were pain-related (n=15), transplant-related infections (n=6), abdominal events (n=6), and toxic effects associated with sirolimus (n=5).
As of October 25, 2013, 29 patients were still alive, with a median follow-up of 3.4 years. Twenty-six patients (87%) had long-term stable donor engraftment without acute or chronic GVHD.
Hemoglobin levels improved after HSCT. At 1 year, 25 patients (83%) had full donor-type hemoglobin. Fifteen engrafted patients discontinued immunosuppression medication and did not develop GVHD.
“Typically, stem cell recipients must take immunosuppressants all their lives,” Dr Hsieh noted. “That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen.”
Hospitalization rates also decreased following HSCT. The average annual hospitalization rate was 3.2 the year before HSCT, 0.63 the first year after, 0.19 the second year after, and 0.11 the third year after transplant.
“One of the most debilitating effects of sickle cell disease is the often relentless pain,” Dr Hsieh pointed out. “Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain.”
Eleven patients were taking narcotics long-term at the time of transplant. During the week they were hospitalized and received their HSCT, the average narcotics use per week was 639 mg of intravenous morphine-equivalent dose. The dosage decreased to 140 mg at 6 months after the transplant.
“The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work, and long-term well-being,” said study author Griffin P. Rodgers, MD, director of the National Institute of Diabetes and Digestive and Kidney Diseases.
“This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem cell transplant or long-term use of immunosuppressants.” ![]()