User login
Two Home Health Agencies Reduce Readmissions Among Heart Failure Patients Using a Quality Improvement Approach
From Collaborative Healthcare Strategies, Lexington, MA (Dr. Boutwell), and the National Home Health Corporation, Scarsdale, NY.
Abstract
- Objective: To describe a quality improvement initiative implemented by 2 home health care agencies to reduce readmissions.
- Methods: The agencies reviewed their data and identified best practices for reducing acute hospital transfers among their high-risk heart failure patients, focusing on the first 14 days of the care episode. Care intensity was increased during the first 3 days, and an active surveillance approach was used during the first 2 weeks. Training for staff, called “Heart Failure Boot Camp,” was introduced and made part of new employee orientation.
- Results: The 30-day rehospitalization rate was reduced from 31% to 17%.
- Conclusion: A data-driven transitional care model can lead to reductions in 30-day readmissions among high-risk patients receiving home health care.
Hospital readmissions are frequent, costly, and can be a marker of poorly coordinated postdischarge care [1]. Since the passage of the Affordable Care Act in 2010, reducing readmissions has become a national priority, as reflected in the emergence of accountable care organizations, bundled payments, and readmission penalties for hospitals, among other efforts. With the increasing attention on reducing readmissions, home health care agencies are under pressure to identify opportunities to improve performance. Two affiliated home health care agencies in Massachusetts and Connecticut made reducing their all-cause 30-day readmission rate a strategic priority. In this article, we describe their approach.
Setting
New England Home Care (NEHC, Cromwell, CT) is a regional agency that serves 7 of the 8 counties in Connecticut and over 7400 patients annually. Medical Resources Home Health (Newton, MA) is a regional agency that serves 8 of the 12 counties in Massachusetts and 2000 patients annually. These home health agencies are both owned by National Home Health Care Corporation (Scarsdale, NY) but are independently managed, with separate staff and regional market differences.
Data Analysis
Opportunities for Improvement The data analysis highlighted several immediate opportunities for improvement. First, the data showed that the first 3 days of the home health episode are a period of increased risk. Thus, intensification of care—and successful first contacts—during the first 3 days of the home health episode was an important target goal. Similarly, the first 14 days were a period of increased risk, suggesting the potential benefit of home health staff proactively monitoring patients during this time to identify clinical or other needs early in the effort to avert a hospitalization. In addition, while respiratory symptoms were among the top reasons for acute care hospitalization within 30 days of episode initiation, many other diagnoses were also implicated. Nine conditions comprised 80% of coded reasons for hospitalization, suggesting that vigilance around respiratory complaints is important but cannot be the exclusive focus of symptom management in this population.
Approach The agencies started this performance improvement initiative by focusing on one patient subgroup (heart failure patients) served by one of the agencies (NEHC). New approaches were tried, and effective approaches were codified through training, on-the job coaching, and performance feedback to front-line clinicians. This approach facilitated the spread of these changes to the second agency, and subsequently both agencies expanded their focus beyond heart failure to other common diagnoses in the home health population.
Changes to Standard Care Practices Based on identified opportunities for improvement, the agency incorporated modifications to standard care utilizing existing resources and within the construct of a certified Medicare home health episode (Medicare’s required specific services, assessments, and other activities that a home health agency must provide in order to bill for an episode). First, working with managers and front-line clinicians, they focused on establishing successful contact during the first 3 days of an episode of care. Front-line staff reported that some patients did not respond to the first attempt to establish contact, and staff thought that it indicated that the patient did not want home health care. The agency designed a new initial contact protocol to increase the likelihood of a successful first contact with the patient. Second, staff increased the frequency of contact in the first 3 days of the episode, either through home visits or phone calls. Increased contact served to allowed the home health staff to get to know the patient and have more points of reference upon which to identify whether symptoms were developing or changing. In addition, increase initial frequency served to increase the comfort and confidence that the patient and their family had in the agency. Third, in the context of increasing the frequency and effectiveness of contacts in the first 14 days, home care staff were trained to adopt an “active surveillance” approach. This staff development and re-training initiative instructed staff on practices to increase their awareness and recognition of patient needs or changing circumstances, outside the specific problem-defined focus area(s) of the home health episode. Frequent contact in the first 14 days creates an opportunity for home health staff to intervene proactively in the confusion or symptoms that lead patients or their families to call 911. Fourth, home care staff received professional development training specifically focused on heart failure, called “Heart Failure Boot Camp.” This training provided a review and update on the clinical management best practices for home care for heart failure patients. This training was conducted by the agency’s staff clinical educator at each local field office. Once all the agencies’ existing staff were trained, the training materials were included in new employee orientation. Rate of acute care hospitalizations was tracked and reports were provided to each local field office on a quarterly basis. Agency leadership included review of these data with local field office managers in their existing management meetings to reinforce the importance of this initiative for the agency at the highest levels. As the efforts to reduce hospitalizations evolved to include patients with conditions other than heart failure, the agencies developed a readmission risk assessment that included the number of medications ( < 5; 6–10; 11–15; 16–20; or > 20) and number of hospitalizations within the past 12 months. Staff could act upon the risk elements identified to reduce each individual patient’s risk of readmission.
Adding Enhanced Service to Standard Care: The Transitional Care Liaison
ollowing the implementation of the above changes to standard home health care practices, the agencies subsequently deployed full-time transitional care liaisons at local hospitals. This was a new role, requiring newly dedicated resources for the position. The role is modeled upon the Care Transitions Intervention [2],which emphasizes the value of initiating transitional care during the hospitalization. In this new role, the hospital-based home care liaisons establish a relationship with the patient, schedule immediate follow-up, review medications and the plan to obtain new medications, and review the plan of care in the hospital, prior to the transition home. On-site transitional care liaisons greatly facilitate clinical collaboration, allowing the “receiving” provider to request clarifications prior to discharge. On-site relationships also enable formal and informal mutual improvements in the transition process. An unanticipated benefit to this collaboration is that the agencies are able to identify some high-utilizing patients who are served by several area hospitals. Thus the agencies were able to add to the list of highest-risk patients for some hospitals that were otherwise unidentified.
Outcomes
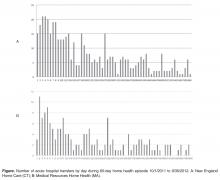
The 30-day rehospitalization rate for NEHC was 31% during the baseline period (9/30/2008 to 6/30/2010). The quarterly variability in readmission rates was high, ranging from 20% to 42% in any given quarter. Following the start of the performance improvement initiative in Quarter 3 of 2010, and through the most recent quarter for which data are available (Quarter 3 2013) the quarterly 30-day readmission rates demonstrated decreased variability (15% to 22%) and the 30-day readmission rate was reduced to 17%—a 45% reduction in rehospitalizations. The agencies’ rehospitalization rates are lower than local benchmarks [1].
Lessons Learned
The agencies experience with this initiative has led to several lessons learned that may be of interest to other agencies and providers looking to design and implement care models to reduce rehospitalizations.
First, it was essential to supplement our knowledge of best practices from the literature and industry experts with an examination of our own data. In addition to examining rates of rehospitalization, we were able to identify patients at highest risk so we could intensify services early for this group.
Second, the 2 agencies participating in this effort are affiliated but independently managed, with separate staff and regional market differences. We were pleased to learn that a common service delivery model could be successfully implemented in both agencies. This suggests that this structured care delivery improvement approach can be replicated in other organizations and local contexts.
Third, by no means was the staff development and retraining a “one and done” effort; continuous reinforcement of the rationale for the practice change and the protocols for optimizing engagement to reduce rehospitalizations was required.
Finally, the performance improvement initiative started with an initial focus on heart failure patients. Over the course of the Initiative we named the series of practice changes our “Healthy@Home” model of care. As we expanded our focus to all patients at high risk of readmission (as identified by our risk assessment score), we learned that staff thought the Healthy@Home practice changes only applied to heart failure patients. This required re-messaging with the staff and regional supervisors.
Conclusion
As rehospitalizations continue to be a prominent measure of quality, cost, and patient experience and a measure for which hospitals and post-acute and community-based providers are either penalized or rewarded, there is a growing awareness of the many factors outside the walls of the hospital that determine whether a patient will return within a defined period of time. Many home health agencies see this as an opportune moment to highlight the critical role they play in care transitions across settings and over time. In this article, we describe the experience of 2 affiliated regional home health care agencies as they engaged in a structured performance improvement effort to reduce readmissions among their high-risk patients. This effort involving data analysis, identification of locally relevant opportunities for improvement, modifications to standard care utilizing existing resources, adding a new service, and expansion beyond the initial target population to all patients at high risk of readmission. This 3-year effort has resulted in a substantial and sustained reduction in rehospitalization rates.
1. Masspro, Massachusetts’ Quality Improvement Organization. State of the state: readmissions in Massachusetts January 1 2009-September 30 2013. Accessed 14 April 2014 at www.masspro.org/files/tools/12_13_state_of_the_state.pdf.
2. Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention. Arch Intern Med 2006;166:1822–8.
From Collaborative Healthcare Strategies, Lexington, MA (Dr. Boutwell), and the National Home Health Corporation, Scarsdale, NY.
Abstract
- Objective: To describe a quality improvement initiative implemented by 2 home health care agencies to reduce readmissions.
- Methods: The agencies reviewed their data and identified best practices for reducing acute hospital transfers among their high-risk heart failure patients, focusing on the first 14 days of the care episode. Care intensity was increased during the first 3 days, and an active surveillance approach was used during the first 2 weeks. Training for staff, called “Heart Failure Boot Camp,” was introduced and made part of new employee orientation.
- Results: The 30-day rehospitalization rate was reduced from 31% to 17%.
- Conclusion: A data-driven transitional care model can lead to reductions in 30-day readmissions among high-risk patients receiving home health care.
Hospital readmissions are frequent, costly, and can be a marker of poorly coordinated postdischarge care [1]. Since the passage of the Affordable Care Act in 2010, reducing readmissions has become a national priority, as reflected in the emergence of accountable care organizations, bundled payments, and readmission penalties for hospitals, among other efforts. With the increasing attention on reducing readmissions, home health care agencies are under pressure to identify opportunities to improve performance. Two affiliated home health care agencies in Massachusetts and Connecticut made reducing their all-cause 30-day readmission rate a strategic priority. In this article, we describe their approach.
Setting
New England Home Care (NEHC, Cromwell, CT) is a regional agency that serves 7 of the 8 counties in Connecticut and over 7400 patients annually. Medical Resources Home Health (Newton, MA) is a regional agency that serves 8 of the 12 counties in Massachusetts and 2000 patients annually. These home health agencies are both owned by National Home Health Care Corporation (Scarsdale, NY) but are independently managed, with separate staff and regional market differences.
Data Analysis
Opportunities for Improvement The data analysis highlighted several immediate opportunities for improvement. First, the data showed that the first 3 days of the home health episode are a period of increased risk. Thus, intensification of care—and successful first contacts—during the first 3 days of the home health episode was an important target goal. Similarly, the first 14 days were a period of increased risk, suggesting the potential benefit of home health staff proactively monitoring patients during this time to identify clinical or other needs early in the effort to avert a hospitalization. In addition, while respiratory symptoms were among the top reasons for acute care hospitalization within 30 days of episode initiation, many other diagnoses were also implicated. Nine conditions comprised 80% of coded reasons for hospitalization, suggesting that vigilance around respiratory complaints is important but cannot be the exclusive focus of symptom management in this population.
Approach The agencies started this performance improvement initiative by focusing on one patient subgroup (heart failure patients) served by one of the agencies (NEHC). New approaches were tried, and effective approaches were codified through training, on-the job coaching, and performance feedback to front-line clinicians. This approach facilitated the spread of these changes to the second agency, and subsequently both agencies expanded their focus beyond heart failure to other common diagnoses in the home health population.
Changes to Standard Care Practices Based on identified opportunities for improvement, the agency incorporated modifications to standard care utilizing existing resources and within the construct of a certified Medicare home health episode (Medicare’s required specific services, assessments, and other activities that a home health agency must provide in order to bill for an episode). First, working with managers and front-line clinicians, they focused on establishing successful contact during the first 3 days of an episode of care. Front-line staff reported that some patients did not respond to the first attempt to establish contact, and staff thought that it indicated that the patient did not want home health care. The agency designed a new initial contact protocol to increase the likelihood of a successful first contact with the patient. Second, staff increased the frequency of contact in the first 3 days of the episode, either through home visits or phone calls. Increased contact served to allowed the home health staff to get to know the patient and have more points of reference upon which to identify whether symptoms were developing or changing. In addition, increase initial frequency served to increase the comfort and confidence that the patient and their family had in the agency. Third, in the context of increasing the frequency and effectiveness of contacts in the first 14 days, home care staff were trained to adopt an “active surveillance” approach. This staff development and re-training initiative instructed staff on practices to increase their awareness and recognition of patient needs or changing circumstances, outside the specific problem-defined focus area(s) of the home health episode. Frequent contact in the first 14 days creates an opportunity for home health staff to intervene proactively in the confusion or symptoms that lead patients or their families to call 911. Fourth, home care staff received professional development training specifically focused on heart failure, called “Heart Failure Boot Camp.” This training provided a review and update on the clinical management best practices for home care for heart failure patients. This training was conducted by the agency’s staff clinical educator at each local field office. Once all the agencies’ existing staff were trained, the training materials were included in new employee orientation. Rate of acute care hospitalizations was tracked and reports were provided to each local field office on a quarterly basis. Agency leadership included review of these data with local field office managers in their existing management meetings to reinforce the importance of this initiative for the agency at the highest levels. As the efforts to reduce hospitalizations evolved to include patients with conditions other than heart failure, the agencies developed a readmission risk assessment that included the number of medications ( < 5; 6–10; 11–15; 16–20; or > 20) and number of hospitalizations within the past 12 months. Staff could act upon the risk elements identified to reduce each individual patient’s risk of readmission.
Adding Enhanced Service to Standard Care: The Transitional Care Liaison
ollowing the implementation of the above changes to standard home health care practices, the agencies subsequently deployed full-time transitional care liaisons at local hospitals. This was a new role, requiring newly dedicated resources for the position. The role is modeled upon the Care Transitions Intervention [2],which emphasizes the value of initiating transitional care during the hospitalization. In this new role, the hospital-based home care liaisons establish a relationship with the patient, schedule immediate follow-up, review medications and the plan to obtain new medications, and review the plan of care in the hospital, prior to the transition home. On-site transitional care liaisons greatly facilitate clinical collaboration, allowing the “receiving” provider to request clarifications prior to discharge. On-site relationships also enable formal and informal mutual improvements in the transition process. An unanticipated benefit to this collaboration is that the agencies are able to identify some high-utilizing patients who are served by several area hospitals. Thus the agencies were able to add to the list of highest-risk patients for some hospitals that were otherwise unidentified.
Outcomes
The 30-day rehospitalization rate for NEHC was 31% during the baseline period (9/30/2008 to 6/30/2010). The quarterly variability in readmission rates was high, ranging from 20% to 42% in any given quarter. Following the start of the performance improvement initiative in Quarter 3 of 2010, and through the most recent quarter for which data are available (Quarter 3 2013) the quarterly 30-day readmission rates demonstrated decreased variability (15% to 22%) and the 30-day readmission rate was reduced to 17%—a 45% reduction in rehospitalizations. The agencies’ rehospitalization rates are lower than local benchmarks [1].
Lessons Learned
The agencies experience with this initiative has led to several lessons learned that may be of interest to other agencies and providers looking to design and implement care models to reduce rehospitalizations.
First, it was essential to supplement our knowledge of best practices from the literature and industry experts with an examination of our own data. In addition to examining rates of rehospitalization, we were able to identify patients at highest risk so we could intensify services early for this group.
Second, the 2 agencies participating in this effort are affiliated but independently managed, with separate staff and regional market differences. We were pleased to learn that a common service delivery model could be successfully implemented in both agencies. This suggests that this structured care delivery improvement approach can be replicated in other organizations and local contexts.
Third, by no means was the staff development and retraining a “one and done” effort; continuous reinforcement of the rationale for the practice change and the protocols for optimizing engagement to reduce rehospitalizations was required.
Finally, the performance improvement initiative started with an initial focus on heart failure patients. Over the course of the Initiative we named the series of practice changes our “Healthy@Home” model of care. As we expanded our focus to all patients at high risk of readmission (as identified by our risk assessment score), we learned that staff thought the Healthy@Home practice changes only applied to heart failure patients. This required re-messaging with the staff and regional supervisors.
Conclusion
As rehospitalizations continue to be a prominent measure of quality, cost, and patient experience and a measure for which hospitals and post-acute and community-based providers are either penalized or rewarded, there is a growing awareness of the many factors outside the walls of the hospital that determine whether a patient will return within a defined period of time. Many home health agencies see this as an opportune moment to highlight the critical role they play in care transitions across settings and over time. In this article, we describe the experience of 2 affiliated regional home health care agencies as they engaged in a structured performance improvement effort to reduce readmissions among their high-risk patients. This effort involving data analysis, identification of locally relevant opportunities for improvement, modifications to standard care utilizing existing resources, adding a new service, and expansion beyond the initial target population to all patients at high risk of readmission. This 3-year effort has resulted in a substantial and sustained reduction in rehospitalization rates.
From Collaborative Healthcare Strategies, Lexington, MA (Dr. Boutwell), and the National Home Health Corporation, Scarsdale, NY.
Abstract
- Objective: To describe a quality improvement initiative implemented by 2 home health care agencies to reduce readmissions.
- Methods: The agencies reviewed their data and identified best practices for reducing acute hospital transfers among their high-risk heart failure patients, focusing on the first 14 days of the care episode. Care intensity was increased during the first 3 days, and an active surveillance approach was used during the first 2 weeks. Training for staff, called “Heart Failure Boot Camp,” was introduced and made part of new employee orientation.
- Results: The 30-day rehospitalization rate was reduced from 31% to 17%.
- Conclusion: A data-driven transitional care model can lead to reductions in 30-day readmissions among high-risk patients receiving home health care.
Hospital readmissions are frequent, costly, and can be a marker of poorly coordinated postdischarge care [1]. Since the passage of the Affordable Care Act in 2010, reducing readmissions has become a national priority, as reflected in the emergence of accountable care organizations, bundled payments, and readmission penalties for hospitals, among other efforts. With the increasing attention on reducing readmissions, home health care agencies are under pressure to identify opportunities to improve performance. Two affiliated home health care agencies in Massachusetts and Connecticut made reducing their all-cause 30-day readmission rate a strategic priority. In this article, we describe their approach.
Setting
New England Home Care (NEHC, Cromwell, CT) is a regional agency that serves 7 of the 8 counties in Connecticut and over 7400 patients annually. Medical Resources Home Health (Newton, MA) is a regional agency that serves 8 of the 12 counties in Massachusetts and 2000 patients annually. These home health agencies are both owned by National Home Health Care Corporation (Scarsdale, NY) but are independently managed, with separate staff and regional market differences.
Data Analysis
Opportunities for Improvement The data analysis highlighted several immediate opportunities for improvement. First, the data showed that the first 3 days of the home health episode are a period of increased risk. Thus, intensification of care—and successful first contacts—during the first 3 days of the home health episode was an important target goal. Similarly, the first 14 days were a period of increased risk, suggesting the potential benefit of home health staff proactively monitoring patients during this time to identify clinical or other needs early in the effort to avert a hospitalization. In addition, while respiratory symptoms were among the top reasons for acute care hospitalization within 30 days of episode initiation, many other diagnoses were also implicated. Nine conditions comprised 80% of coded reasons for hospitalization, suggesting that vigilance around respiratory complaints is important but cannot be the exclusive focus of symptom management in this population.
Approach The agencies started this performance improvement initiative by focusing on one patient subgroup (heart failure patients) served by one of the agencies (NEHC). New approaches were tried, and effective approaches were codified through training, on-the job coaching, and performance feedback to front-line clinicians. This approach facilitated the spread of these changes to the second agency, and subsequently both agencies expanded their focus beyond heart failure to other common diagnoses in the home health population.
Changes to Standard Care Practices Based on identified opportunities for improvement, the agency incorporated modifications to standard care utilizing existing resources and within the construct of a certified Medicare home health episode (Medicare’s required specific services, assessments, and other activities that a home health agency must provide in order to bill for an episode). First, working with managers and front-line clinicians, they focused on establishing successful contact during the first 3 days of an episode of care. Front-line staff reported that some patients did not respond to the first attempt to establish contact, and staff thought that it indicated that the patient did not want home health care. The agency designed a new initial contact protocol to increase the likelihood of a successful first contact with the patient. Second, staff increased the frequency of contact in the first 3 days of the episode, either through home visits or phone calls. Increased contact served to allowed the home health staff to get to know the patient and have more points of reference upon which to identify whether symptoms were developing or changing. In addition, increase initial frequency served to increase the comfort and confidence that the patient and their family had in the agency. Third, in the context of increasing the frequency and effectiveness of contacts in the first 14 days, home care staff were trained to adopt an “active surveillance” approach. This staff development and re-training initiative instructed staff on practices to increase their awareness and recognition of patient needs or changing circumstances, outside the specific problem-defined focus area(s) of the home health episode. Frequent contact in the first 14 days creates an opportunity for home health staff to intervene proactively in the confusion or symptoms that lead patients or their families to call 911. Fourth, home care staff received professional development training specifically focused on heart failure, called “Heart Failure Boot Camp.” This training provided a review and update on the clinical management best practices for home care for heart failure patients. This training was conducted by the agency’s staff clinical educator at each local field office. Once all the agencies’ existing staff were trained, the training materials were included in new employee orientation. Rate of acute care hospitalizations was tracked and reports were provided to each local field office on a quarterly basis. Agency leadership included review of these data with local field office managers in their existing management meetings to reinforce the importance of this initiative for the agency at the highest levels. As the efforts to reduce hospitalizations evolved to include patients with conditions other than heart failure, the agencies developed a readmission risk assessment that included the number of medications ( < 5; 6–10; 11–15; 16–20; or > 20) and number of hospitalizations within the past 12 months. Staff could act upon the risk elements identified to reduce each individual patient’s risk of readmission.
Adding Enhanced Service to Standard Care: The Transitional Care Liaison
ollowing the implementation of the above changes to standard home health care practices, the agencies subsequently deployed full-time transitional care liaisons at local hospitals. This was a new role, requiring newly dedicated resources for the position. The role is modeled upon the Care Transitions Intervention [2],which emphasizes the value of initiating transitional care during the hospitalization. In this new role, the hospital-based home care liaisons establish a relationship with the patient, schedule immediate follow-up, review medications and the plan to obtain new medications, and review the plan of care in the hospital, prior to the transition home. On-site transitional care liaisons greatly facilitate clinical collaboration, allowing the “receiving” provider to request clarifications prior to discharge. On-site relationships also enable formal and informal mutual improvements in the transition process. An unanticipated benefit to this collaboration is that the agencies are able to identify some high-utilizing patients who are served by several area hospitals. Thus the agencies were able to add to the list of highest-risk patients for some hospitals that were otherwise unidentified.
Outcomes
The 30-day rehospitalization rate for NEHC was 31% during the baseline period (9/30/2008 to 6/30/2010). The quarterly variability in readmission rates was high, ranging from 20% to 42% in any given quarter. Following the start of the performance improvement initiative in Quarter 3 of 2010, and through the most recent quarter for which data are available (Quarter 3 2013) the quarterly 30-day readmission rates demonstrated decreased variability (15% to 22%) and the 30-day readmission rate was reduced to 17%—a 45% reduction in rehospitalizations. The agencies’ rehospitalization rates are lower than local benchmarks [1].
Lessons Learned
The agencies experience with this initiative has led to several lessons learned that may be of interest to other agencies and providers looking to design and implement care models to reduce rehospitalizations.
First, it was essential to supplement our knowledge of best practices from the literature and industry experts with an examination of our own data. In addition to examining rates of rehospitalization, we were able to identify patients at highest risk so we could intensify services early for this group.
Second, the 2 agencies participating in this effort are affiliated but independently managed, with separate staff and regional market differences. We were pleased to learn that a common service delivery model could be successfully implemented in both agencies. This suggests that this structured care delivery improvement approach can be replicated in other organizations and local contexts.
Third, by no means was the staff development and retraining a “one and done” effort; continuous reinforcement of the rationale for the practice change and the protocols for optimizing engagement to reduce rehospitalizations was required.
Finally, the performance improvement initiative started with an initial focus on heart failure patients. Over the course of the Initiative we named the series of practice changes our “Healthy@Home” model of care. As we expanded our focus to all patients at high risk of readmission (as identified by our risk assessment score), we learned that staff thought the Healthy@Home practice changes only applied to heart failure patients. This required re-messaging with the staff and regional supervisors.
Conclusion
As rehospitalizations continue to be a prominent measure of quality, cost, and patient experience and a measure for which hospitals and post-acute and community-based providers are either penalized or rewarded, there is a growing awareness of the many factors outside the walls of the hospital that determine whether a patient will return within a defined period of time. Many home health agencies see this as an opportune moment to highlight the critical role they play in care transitions across settings and over time. In this article, we describe the experience of 2 affiliated regional home health care agencies as they engaged in a structured performance improvement effort to reduce readmissions among their high-risk patients. This effort involving data analysis, identification of locally relevant opportunities for improvement, modifications to standard care utilizing existing resources, adding a new service, and expansion beyond the initial target population to all patients at high risk of readmission. This 3-year effort has resulted in a substantial and sustained reduction in rehospitalization rates.
1. Masspro, Massachusetts’ Quality Improvement Organization. State of the state: readmissions in Massachusetts January 1 2009-September 30 2013. Accessed 14 April 2014 at www.masspro.org/files/tools/12_13_state_of_the_state.pdf.
2. Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention. Arch Intern Med 2006;166:1822–8.
1. Masspro, Massachusetts’ Quality Improvement Organization. State of the state: readmissions in Massachusetts January 1 2009-September 30 2013. Accessed 14 April 2014 at www.masspro.org/files/tools/12_13_state_of_the_state.pdf.
2. Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention. Arch Intern Med 2006;166:1822–8.
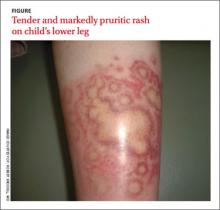
Young girl with lower leg rash
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
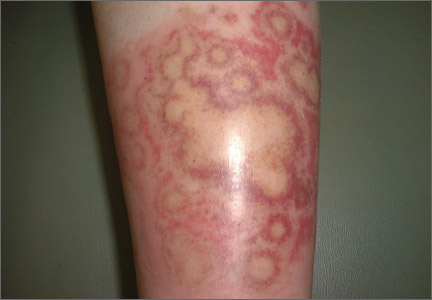
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.
English Ability and Glycemic Control in Latinos with Diabetes
Study Overview
Objective. To determine if there is an association between self-reported English language ability and glycemic control in Latinos with type 2 diabetes.
Design. Descriptive correlational study using data from a larger cross-sectional study.
Setting and participants. 167 adults with diabetes who self-identified as Latino or Hispanic recruited at clinics in the Chicago area from May 2004 to May 2006. The dataset was collected using face-to-face interviews with diabetic patients aged ≥ 18 years. All participants attended clinics affiliated with an academic medical center or physician offices affiliated with a suburban hospital. Patients with type 1 diabetes and those with < 17 points on the Mini-Mental State Examination were excluded. English speaking ability was categorized as speaking English “not at all,” “not well,” “well,” or “very well” based on patient self-report. A multivariable logistic regression model was used to examine the predictive relationship between English language skills and HbA1c levels, with covariates selected if they were significantly correlated with English language ability. The final regression model accounted for age, sex, education, annual income, health insurance status, duration of diabetes, birth in the United States, and years in the United States.
Main outcome measure. HbA1c ≥ 7.0% as captured by chart review.
Main results. Of the 167 patients, 38% reported speaking English very well, 21% reported speaking well, 26% reported speaking not very well, and 14% did not speak English at all. Reflecting immigration-sensitive patterns, patients who spoke English very well were younger and more likely to have graduated high school and have an annual income over $25,000 per year. Comorbidities and complications did not differ by English speaking ability except for diabetic eye disease, which was was more prevalent among those who did not speak English at all (42%, p = 0.04). Whether speaking ability was treated as a continuous or dichotomous variable, HbA1c levels formed a U-shaped curve: those who spoke English very well (odds ratio [OR] 2.32, 95% CI, 1.00–5.41) or not at all (OR 4.11, 95% CI 1.35–12.54) had higher odds of having an elevated HbA1c than those who spoke English well, although this was only statistically significant for those who spoke no English. In adjusted analyses, the U-shaped curve persisted with the highest odds among those who spoke English very well (OR 3.20, 95% CI 1.05–9.79) or not at all (OR 4.95, 95% CI 1.29–18.92).
Conclusion. The relationship between English speaking ability and diabetes management is more complex than previously described. Interventions aimed at improving diabetes outcomes may need to be tailored to specific subgroups within the Latino population.
Commentary
Immigrant health is complex and language is an understudied factor in health transitions of those who migrate for new lives or temporary work. For Latinos, migration abroad was once thought to improve health, but a recent systematic review by Teruya et al [1] suggests that the migration experience has a wide variety of effects on health, many of which can be negative.
The notion that English fluency confers health care benefits is questionable, as the authors state. Those unfamiliar with the acculturation literature might think that English speaking ability is a good marker of acculturation, but recent research on the subject suggests otherwise. Acculturation is a complex phenomenon that cannot be measured or gauged by a single variable [2–5]. Among the many factors influencing acculturation, the migration experience and country of origin will play a major role in acculturation and how it occurs in the arrival country. Health care providers seeking to understand the complexity of acculturation better to improve care for their immigrant patients would benefit from examining the extensive social science literature on the subject. The results of this study suggest that providers should not take for granted someone’s English speaking ability as a marker of acculturation and thus assume that their health outcomes would be equivalent to native born populations.
This study has number of weaknesses. The main concern is that the study did not consider a number of important health service delivery factors. The researchers did not assess for the number of visits the patient had with appropriate interpretation services, whether or not there were language concordant visits between patients and providers (limited English proficiency patients are more likely to form consistent service relationships with language concordant providers [6–10]), or whether the patient had diabetes education classes or individual counseling sessions to facilitate self-management. These service-based factors could potentially explain some of the results seen. The small sample size, age of the data in the study, and failure to distinguish the country of origin of the Latino patients are other weaknesses.
Applications for Clinical Practice
Providers can improve their clinical practice with limited English proficiency Latino patients with diabetes by being more sensitive to the potential effects of language on diabetes outcomes in this population. The results suggest that providers should not assume that a Latino patient’s English language skills mean that they are better at self-managing their diabetes and will have better outcomes. Asking patients about their country of origin and migration experiences may help differentiate the effects of language in concert with other potentially confounding variables that can help elucidate the effects of language on diabetes related outcomes.
—Allison Squires, PhD, RN
1. Teruya SA, Bazargan-Hejazi S. The immigrant and Hispanic paradoxes: a systematic review of their predictions and effects. Hisp J Behav Sci 2013 Sep 5;35:486–509.
2. Rudmin FW. Phenomenology of acculturation: retrospective reports from the Philippines, Japan, Quebec, and Norway. Cult Psychol 2010;16:313–32.
3. Matsunaga M, Hecht ML, Elek E, Ndiaye K. Ethnic identity development and acculturation: a longitudinal analysis of Mexican-heritage youth in the Southwest United States. J Cross Cult Psychol 2010;41:410–27.
4. Siatkowski A. Hispanic acculturation: a concept analysis. J Transcult Nurs 2007;18:316–23.
5. Horevitz E, Organista KC. The Mexican health paradox: expanding the explanatory power of the acculturation construct. Hisp J Behav Sci 2012;35:3–34.
6. Gany F, Leng J, Shapiro E, et al. Patient satisfaction with different interpreting methods: a randomized controlled trial. J Gen Intern Med 2007;22 Suppl 2:312–8.
7. Grover A, Deakyne S, Bajaj L, Roosevelt GE. Comparison of throughput times for limited English proficiency patient visits in the emergency department between different interpreter modalities. J Immigr Minor Health 2012;14:602–7.
8. Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med 2007;22 Suppl 2:324–30.
9. Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res 2007;42:727–54.
10. Arauz Boudreau AD, Fluet CF, Reuland CP, et al. Associations of providers’ language and cultural skills with Latino parents’ perceptions of well-child care. Acad Pediatr 2010;10:172–8.
Study Overview
Objective. To determine if there is an association between self-reported English language ability and glycemic control in Latinos with type 2 diabetes.
Design. Descriptive correlational study using data from a larger cross-sectional study.
Setting and participants. 167 adults with diabetes who self-identified as Latino or Hispanic recruited at clinics in the Chicago area from May 2004 to May 2006. The dataset was collected using face-to-face interviews with diabetic patients aged ≥ 18 years. All participants attended clinics affiliated with an academic medical center or physician offices affiliated with a suburban hospital. Patients with type 1 diabetes and those with < 17 points on the Mini-Mental State Examination were excluded. English speaking ability was categorized as speaking English “not at all,” “not well,” “well,” or “very well” based on patient self-report. A multivariable logistic regression model was used to examine the predictive relationship between English language skills and HbA1c levels, with covariates selected if they were significantly correlated with English language ability. The final regression model accounted for age, sex, education, annual income, health insurance status, duration of diabetes, birth in the United States, and years in the United States.
Main outcome measure. HbA1c ≥ 7.0% as captured by chart review.
Main results. Of the 167 patients, 38% reported speaking English very well, 21% reported speaking well, 26% reported speaking not very well, and 14% did not speak English at all. Reflecting immigration-sensitive patterns, patients who spoke English very well were younger and more likely to have graduated high school and have an annual income over $25,000 per year. Comorbidities and complications did not differ by English speaking ability except for diabetic eye disease, which was was more prevalent among those who did not speak English at all (42%, p = 0.04). Whether speaking ability was treated as a continuous or dichotomous variable, HbA1c levels formed a U-shaped curve: those who spoke English very well (odds ratio [OR] 2.32, 95% CI, 1.00–5.41) or not at all (OR 4.11, 95% CI 1.35–12.54) had higher odds of having an elevated HbA1c than those who spoke English well, although this was only statistically significant for those who spoke no English. In adjusted analyses, the U-shaped curve persisted with the highest odds among those who spoke English very well (OR 3.20, 95% CI 1.05–9.79) or not at all (OR 4.95, 95% CI 1.29–18.92).
Conclusion. The relationship between English speaking ability and diabetes management is more complex than previously described. Interventions aimed at improving diabetes outcomes may need to be tailored to specific subgroups within the Latino population.
Commentary
Immigrant health is complex and language is an understudied factor in health transitions of those who migrate for new lives or temporary work. For Latinos, migration abroad was once thought to improve health, but a recent systematic review by Teruya et al [1] suggests that the migration experience has a wide variety of effects on health, many of which can be negative.
The notion that English fluency confers health care benefits is questionable, as the authors state. Those unfamiliar with the acculturation literature might think that English speaking ability is a good marker of acculturation, but recent research on the subject suggests otherwise. Acculturation is a complex phenomenon that cannot be measured or gauged by a single variable [2–5]. Among the many factors influencing acculturation, the migration experience and country of origin will play a major role in acculturation and how it occurs in the arrival country. Health care providers seeking to understand the complexity of acculturation better to improve care for their immigrant patients would benefit from examining the extensive social science literature on the subject. The results of this study suggest that providers should not take for granted someone’s English speaking ability as a marker of acculturation and thus assume that their health outcomes would be equivalent to native born populations.
This study has number of weaknesses. The main concern is that the study did not consider a number of important health service delivery factors. The researchers did not assess for the number of visits the patient had with appropriate interpretation services, whether or not there were language concordant visits between patients and providers (limited English proficiency patients are more likely to form consistent service relationships with language concordant providers [6–10]), or whether the patient had diabetes education classes or individual counseling sessions to facilitate self-management. These service-based factors could potentially explain some of the results seen. The small sample size, age of the data in the study, and failure to distinguish the country of origin of the Latino patients are other weaknesses.
Applications for Clinical Practice
Providers can improve their clinical practice with limited English proficiency Latino patients with diabetes by being more sensitive to the potential effects of language on diabetes outcomes in this population. The results suggest that providers should not assume that a Latino patient’s English language skills mean that they are better at self-managing their diabetes and will have better outcomes. Asking patients about their country of origin and migration experiences may help differentiate the effects of language in concert with other potentially confounding variables that can help elucidate the effects of language on diabetes related outcomes.
—Allison Squires, PhD, RN
Study Overview
Objective. To determine if there is an association between self-reported English language ability and glycemic control in Latinos with type 2 diabetes.
Design. Descriptive correlational study using data from a larger cross-sectional study.
Setting and participants. 167 adults with diabetes who self-identified as Latino or Hispanic recruited at clinics in the Chicago area from May 2004 to May 2006. The dataset was collected using face-to-face interviews with diabetic patients aged ≥ 18 years. All participants attended clinics affiliated with an academic medical center or physician offices affiliated with a suburban hospital. Patients with type 1 diabetes and those with < 17 points on the Mini-Mental State Examination were excluded. English speaking ability was categorized as speaking English “not at all,” “not well,” “well,” or “very well” based on patient self-report. A multivariable logistic regression model was used to examine the predictive relationship between English language skills and HbA1c levels, with covariates selected if they were significantly correlated with English language ability. The final regression model accounted for age, sex, education, annual income, health insurance status, duration of diabetes, birth in the United States, and years in the United States.
Main outcome measure. HbA1c ≥ 7.0% as captured by chart review.
Main results. Of the 167 patients, 38% reported speaking English very well, 21% reported speaking well, 26% reported speaking not very well, and 14% did not speak English at all. Reflecting immigration-sensitive patterns, patients who spoke English very well were younger and more likely to have graduated high school and have an annual income over $25,000 per year. Comorbidities and complications did not differ by English speaking ability except for diabetic eye disease, which was was more prevalent among those who did not speak English at all (42%, p = 0.04). Whether speaking ability was treated as a continuous or dichotomous variable, HbA1c levels formed a U-shaped curve: those who spoke English very well (odds ratio [OR] 2.32, 95% CI, 1.00–5.41) or not at all (OR 4.11, 95% CI 1.35–12.54) had higher odds of having an elevated HbA1c than those who spoke English well, although this was only statistically significant for those who spoke no English. In adjusted analyses, the U-shaped curve persisted with the highest odds among those who spoke English very well (OR 3.20, 95% CI 1.05–9.79) or not at all (OR 4.95, 95% CI 1.29–18.92).
Conclusion. The relationship between English speaking ability and diabetes management is more complex than previously described. Interventions aimed at improving diabetes outcomes may need to be tailored to specific subgroups within the Latino population.
Commentary
Immigrant health is complex and language is an understudied factor in health transitions of those who migrate for new lives or temporary work. For Latinos, migration abroad was once thought to improve health, but a recent systematic review by Teruya et al [1] suggests that the migration experience has a wide variety of effects on health, many of which can be negative.
The notion that English fluency confers health care benefits is questionable, as the authors state. Those unfamiliar with the acculturation literature might think that English speaking ability is a good marker of acculturation, but recent research on the subject suggests otherwise. Acculturation is a complex phenomenon that cannot be measured or gauged by a single variable [2–5]. Among the many factors influencing acculturation, the migration experience and country of origin will play a major role in acculturation and how it occurs in the arrival country. Health care providers seeking to understand the complexity of acculturation better to improve care for their immigrant patients would benefit from examining the extensive social science literature on the subject. The results of this study suggest that providers should not take for granted someone’s English speaking ability as a marker of acculturation and thus assume that their health outcomes would be equivalent to native born populations.
This study has number of weaknesses. The main concern is that the study did not consider a number of important health service delivery factors. The researchers did not assess for the number of visits the patient had with appropriate interpretation services, whether or not there were language concordant visits between patients and providers (limited English proficiency patients are more likely to form consistent service relationships with language concordant providers [6–10]), or whether the patient had diabetes education classes or individual counseling sessions to facilitate self-management. These service-based factors could potentially explain some of the results seen. The small sample size, age of the data in the study, and failure to distinguish the country of origin of the Latino patients are other weaknesses.
Applications for Clinical Practice
Providers can improve their clinical practice with limited English proficiency Latino patients with diabetes by being more sensitive to the potential effects of language on diabetes outcomes in this population. The results suggest that providers should not assume that a Latino patient’s English language skills mean that they are better at self-managing their diabetes and will have better outcomes. Asking patients about their country of origin and migration experiences may help differentiate the effects of language in concert with other potentially confounding variables that can help elucidate the effects of language on diabetes related outcomes.
—Allison Squires, PhD, RN
1. Teruya SA, Bazargan-Hejazi S. The immigrant and Hispanic paradoxes: a systematic review of their predictions and effects. Hisp J Behav Sci 2013 Sep 5;35:486–509.
2. Rudmin FW. Phenomenology of acculturation: retrospective reports from the Philippines, Japan, Quebec, and Norway. Cult Psychol 2010;16:313–32.
3. Matsunaga M, Hecht ML, Elek E, Ndiaye K. Ethnic identity development and acculturation: a longitudinal analysis of Mexican-heritage youth in the Southwest United States. J Cross Cult Psychol 2010;41:410–27.
4. Siatkowski A. Hispanic acculturation: a concept analysis. J Transcult Nurs 2007;18:316–23.
5. Horevitz E, Organista KC. The Mexican health paradox: expanding the explanatory power of the acculturation construct. Hisp J Behav Sci 2012;35:3–34.
6. Gany F, Leng J, Shapiro E, et al. Patient satisfaction with different interpreting methods: a randomized controlled trial. J Gen Intern Med 2007;22 Suppl 2:312–8.
7. Grover A, Deakyne S, Bajaj L, Roosevelt GE. Comparison of throughput times for limited English proficiency patient visits in the emergency department between different interpreter modalities. J Immigr Minor Health 2012;14:602–7.
8. Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med 2007;22 Suppl 2:324–30.
9. Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res 2007;42:727–54.
10. Arauz Boudreau AD, Fluet CF, Reuland CP, et al. Associations of providers’ language and cultural skills with Latino parents’ perceptions of well-child care. Acad Pediatr 2010;10:172–8.
1. Teruya SA, Bazargan-Hejazi S. The immigrant and Hispanic paradoxes: a systematic review of their predictions and effects. Hisp J Behav Sci 2013 Sep 5;35:486–509.
2. Rudmin FW. Phenomenology of acculturation: retrospective reports from the Philippines, Japan, Quebec, and Norway. Cult Psychol 2010;16:313–32.
3. Matsunaga M, Hecht ML, Elek E, Ndiaye K. Ethnic identity development and acculturation: a longitudinal analysis of Mexican-heritage youth in the Southwest United States. J Cross Cult Psychol 2010;41:410–27.
4. Siatkowski A. Hispanic acculturation: a concept analysis. J Transcult Nurs 2007;18:316–23.
5. Horevitz E, Organista KC. The Mexican health paradox: expanding the explanatory power of the acculturation construct. Hisp J Behav Sci 2012;35:3–34.
6. Gany F, Leng J, Shapiro E, et al. Patient satisfaction with different interpreting methods: a randomized controlled trial. J Gen Intern Med 2007;22 Suppl 2:312–8.
7. Grover A, Deakyne S, Bajaj L, Roosevelt GE. Comparison of throughput times for limited English proficiency patient visits in the emergency department between different interpreter modalities. J Immigr Minor Health 2012;14:602–7.
8. Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med 2007;22 Suppl 2:324–30.
9. Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res 2007;42:727–54.
10. Arauz Boudreau AD, Fluet CF, Reuland CP, et al. Associations of providers’ language and cultural skills with Latino parents’ perceptions of well-child care. Acad Pediatr 2010;10:172–8.
Skip the compression stockings following DVT
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Do not recommend elastic compression stockings (ECS) to decrease the incidence of post-thrombotic syndrome (PTS) after deep vein thrombosis (DVT).1
Strength of recommendation
B: Based on a large, randomized controlled trial
Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
Illustrative case
A 56-year-old man comes to your clinic 3 days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin, 5 mg/d, with enoxaparin bridging, 120 mg/d. He has read about post-thrombotic syndrome (PTS) online and is very concerned about this possible side effect. He is asking about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by reducing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior studies suggested that using ECS can cut the incidence of PTS in half.6,7 However, these were small, single-center studies, and they were not placebo-controlled.6,7
STUDY SUMMARY: RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active vs placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (5-10 days of heparin and 3-6 months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of <6 months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30-40 mm Hg graduated) ECS or identical-looking placebo ECS with <5 mm Hg compression at the ankle for 2 years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from when they woke until they went to bed.
Participants were followed at one, 6, 12, 18, and 24 months. The primary outcome was the cumulative incidence of PTS diagnosed at 6 months or later using Ginsberg’s criteria of ipsilateral pain and swelling of at least 1 month’s duration.8 Secondary outcomes included severity of PTS, presence of leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively using a validated scale (the Villalta scale) for PTS severity and the 36-item Short Form Health Survey (SF-36) and the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) questionnaire to measure QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including body mass index (BMI), VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age was 55.4 years in the study group (standard deviation [SD] ± 15.3 years) and 54.8 years (SD ± 15.8 years) in the place- bo group. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both the active and placebo groups used the stockings; at 24 months, a little less than 70% of the participants in both groups continued to use the stockings. The percentage of people who used the stockings for at least 3 days a week was similar across both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group vs 12.7% in the placebo group, with a hazard ratio of 1.13 (95% confidence interval [CI], .73-1.76; P=.58). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on the outcomes. There was a marginal benefit for ECS for women (P=.047) over men, but this does not likely reflect a true difference because the CIs surrounding the hazard ratios for men and women overlapped and crossed the null value.
WHAT'S NEW: New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements for or against the use of ECS after DVT.
CAVEATS: High nonadherence rates might have affected the results
In both groups, adherence to the assigned intervention diminished throughout the study. Overall, approximately 95% of patients reported wearing their stockings at one month; this dropped to just under 70% by 2 years. Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the 2-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported in previous studies. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION: There are no barriers to ending this practice
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500- 509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.