User login
Three Ways to Improve Quality of Patient Care in Your Hospital
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Pre-Order Your State of Hospital Medicine Report Now
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
Code-H Interactive Tool Helps Hospital Medicine Groups with Coding
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Society of Hospital Medicine Leadership Academy Provides Practical Tips, Hands-On Collaboration, Networking for Hospitalists
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.
Medicare Rule Change Raises Stakes for Hospital Discharge Planning
When she presents information to hospitalists about the little-known revision to Medicare’s condition of participation for discharge planning by hospitals, most hospitalists have no idea what Amy Boutwell, MD, MPP, is talking about. Even hospitalists who are active in their institutions’ efforts to improve transitions of care out of the hospital setting are unaware of the change, which was published in the Centers for Medicare & Medicaid Services’ Transmittal 87 and became effective July 19, 2013.
“I just don’t hear hospital professionals talking about it,” says Dr. Boutwell, a hospitalist at Newton-Wellesley Hospital and president of Collaborative Healthcare Strategies in Lexington, Mass. “When I say, ‘There are new rules of the road for discharge planning and evaluation,’ many are not aware of it.”
The revised condition states that the hospital must have a discharge planning process that applies to all patients—not just Medicare beneficiaries. Not every patient needs to have a written discharge plan—although this is recommended—but all patients should be screened and, if indicated, evaluated at an early stage of their hospitalization for risk of adverse post-discharge outcomes. Observation patients are not included in this requirement.
The discharge plan is different from a discharge summary document, which must be completed by the inpatient attending physician, not the hospital, and is not directly addressed in the regulation. The regulation does address the need for transfer of essential information to the next provider of care and says the hospital should have a written policy and procedure in place for discharge planning. The policy and procedure should be developed with input from medical staff and approved by the hospital’s governing body.
Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation.
—Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST
Transmittal 87 represents the first major update of the discharge planning regulation (Standard 482.43) and accompanying interpretive guidelines in more than a decade, Dr. Boutwell says. It consolidates and reorganizes 24 “tags” of regulatory language down to 13 and contains blue advisory boxes recommending best practices in discharge planning, drawn from the suggestions of a technical expert panel convened by CMS.
That panel included many of the country’s recognized thought leaders on improving care transitions, such as Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST; Eric Coleman, MD, MPH, head of the University of Colorado’s division of health care policy and research and creator of the widely-adopted Care Transitions Program (caretransitions.org), and Dr. Boutwell, co-founder of the STAAR initiative (www.ihi.org/engage/Initiatives/completed/STAAR).
The new condition raises baseline expectations for discharge planning and elevates care transitions efforts from a quality improvement issue to the realm of regulatory compliance, Dr. Boutwell says.
“This goes way beyond case review,” she adds. “It represents an evolution from discharge planning case by case to a system for improving transitions of care [for the hospital]. I’m impressed.”
The recommendations are consistent with best practices promoted by Project BOOST, STAAR, Project RED [Re-Engineered Discharge], and other national quality initiatives for improving care transitions.
“Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation,” Dr. Williams says.
In the Byzantine structure of federal regulations, Medicare’s conditions of participation are the regulations providers must meet in order to participate in the Medicare program and bill for their services. Condition-level citations, if not resolved, can cause hospitals to be decertified from Medicare. The accompanying interpretive guidelines, with survey protocols, are the playbook to help state auditors and providers know how to interpret and apply the language of the regulations. The suggestions and examples of best practices contained in the new condition are not required of hospitals but, if followed, could increase their likelihood of achieving better patient outcomes and staying in compliance with the regulations on surveys.
“If hospitals were to actually implement all of the CMS advisory practice recommendations contained in this 35-page document, they’d be in really good shape for effectively managing transitions of care,” says Teresa L. Hamblin, RN, MS, a CMS consultant with Joint Commission Resources. “The government has provided robust practice recommendations that are a model for what hospitals can do. I’d advise doing your best to implement these recommendations. Check your current processes using this detailed document for reference.”
Discharge planning starts at admission, Hamblin says. If the hospitalist assumes that responsibility, it becomes easier to leave a paper trail in the patient’s chart. Other important lessons for hospitalists include participation in a multidisciplinary approach to discharge planning (i.e., interdisciplinary rounding) and development of policies and procedures in this area.
“If the hospital has not elected to do a discharge plan on every patient, request this for your own patients and recommend it as a policy,” Hamblin says. “Go the extra mile, making follow-up appointments for your patients, filling prescriptions in house, and calling the patient 24 to 72 hours after discharge.”
Weekend coverage, when case managers typically are not present, is a particular challenge in care transitions.
“Encourage your hospital to provide reliable weekend coverage for discharge planning. Involve the nurses,” Hamblin says. “Anything the hospitalist can do to help the hospital close this gap is important.”
Larry Beresford is a freelance writer in Alameda, Calif.
When she presents information to hospitalists about the little-known revision to Medicare’s condition of participation for discharge planning by hospitals, most hospitalists have no idea what Amy Boutwell, MD, MPP, is talking about. Even hospitalists who are active in their institutions’ efforts to improve transitions of care out of the hospital setting are unaware of the change, which was published in the Centers for Medicare & Medicaid Services’ Transmittal 87 and became effective July 19, 2013.
“I just don’t hear hospital professionals talking about it,” says Dr. Boutwell, a hospitalist at Newton-Wellesley Hospital and president of Collaborative Healthcare Strategies in Lexington, Mass. “When I say, ‘There are new rules of the road for discharge planning and evaluation,’ many are not aware of it.”
The revised condition states that the hospital must have a discharge planning process that applies to all patients—not just Medicare beneficiaries. Not every patient needs to have a written discharge plan—although this is recommended—but all patients should be screened and, if indicated, evaluated at an early stage of their hospitalization for risk of adverse post-discharge outcomes. Observation patients are not included in this requirement.
The discharge plan is different from a discharge summary document, which must be completed by the inpatient attending physician, not the hospital, and is not directly addressed in the regulation. The regulation does address the need for transfer of essential information to the next provider of care and says the hospital should have a written policy and procedure in place for discharge planning. The policy and procedure should be developed with input from medical staff and approved by the hospital’s governing body.
Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation.
—Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST
Transmittal 87 represents the first major update of the discharge planning regulation (Standard 482.43) and accompanying interpretive guidelines in more than a decade, Dr. Boutwell says. It consolidates and reorganizes 24 “tags” of regulatory language down to 13 and contains blue advisory boxes recommending best practices in discharge planning, drawn from the suggestions of a technical expert panel convened by CMS.
That panel included many of the country’s recognized thought leaders on improving care transitions, such as Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST; Eric Coleman, MD, MPH, head of the University of Colorado’s division of health care policy and research and creator of the widely-adopted Care Transitions Program (caretransitions.org), and Dr. Boutwell, co-founder of the STAAR initiative (www.ihi.org/engage/Initiatives/completed/STAAR).
The new condition raises baseline expectations for discharge planning and elevates care transitions efforts from a quality improvement issue to the realm of regulatory compliance, Dr. Boutwell says.
“This goes way beyond case review,” she adds. “It represents an evolution from discharge planning case by case to a system for improving transitions of care [for the hospital]. I’m impressed.”
The recommendations are consistent with best practices promoted by Project BOOST, STAAR, Project RED [Re-Engineered Discharge], and other national quality initiatives for improving care transitions.
“Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation,” Dr. Williams says.
In the Byzantine structure of federal regulations, Medicare’s conditions of participation are the regulations providers must meet in order to participate in the Medicare program and bill for their services. Condition-level citations, if not resolved, can cause hospitals to be decertified from Medicare. The accompanying interpretive guidelines, with survey protocols, are the playbook to help state auditors and providers know how to interpret and apply the language of the regulations. The suggestions and examples of best practices contained in the new condition are not required of hospitals but, if followed, could increase their likelihood of achieving better patient outcomes and staying in compliance with the regulations on surveys.
“If hospitals were to actually implement all of the CMS advisory practice recommendations contained in this 35-page document, they’d be in really good shape for effectively managing transitions of care,” says Teresa L. Hamblin, RN, MS, a CMS consultant with Joint Commission Resources. “The government has provided robust practice recommendations that are a model for what hospitals can do. I’d advise doing your best to implement these recommendations. Check your current processes using this detailed document for reference.”
Discharge planning starts at admission, Hamblin says. If the hospitalist assumes that responsibility, it becomes easier to leave a paper trail in the patient’s chart. Other important lessons for hospitalists include participation in a multidisciplinary approach to discharge planning (i.e., interdisciplinary rounding) and development of policies and procedures in this area.
“If the hospital has not elected to do a discharge plan on every patient, request this for your own patients and recommend it as a policy,” Hamblin says. “Go the extra mile, making follow-up appointments for your patients, filling prescriptions in house, and calling the patient 24 to 72 hours after discharge.”
Weekend coverage, when case managers typically are not present, is a particular challenge in care transitions.
“Encourage your hospital to provide reliable weekend coverage for discharge planning. Involve the nurses,” Hamblin says. “Anything the hospitalist can do to help the hospital close this gap is important.”
Larry Beresford is a freelance writer in Alameda, Calif.
When she presents information to hospitalists about the little-known revision to Medicare’s condition of participation for discharge planning by hospitals, most hospitalists have no idea what Amy Boutwell, MD, MPP, is talking about. Even hospitalists who are active in their institutions’ efforts to improve transitions of care out of the hospital setting are unaware of the change, which was published in the Centers for Medicare & Medicaid Services’ Transmittal 87 and became effective July 19, 2013.
“I just don’t hear hospital professionals talking about it,” says Dr. Boutwell, a hospitalist at Newton-Wellesley Hospital and president of Collaborative Healthcare Strategies in Lexington, Mass. “When I say, ‘There are new rules of the road for discharge planning and evaluation,’ many are not aware of it.”
The revised condition states that the hospital must have a discharge planning process that applies to all patients—not just Medicare beneficiaries. Not every patient needs to have a written discharge plan—although this is recommended—but all patients should be screened and, if indicated, evaluated at an early stage of their hospitalization for risk of adverse post-discharge outcomes. Observation patients are not included in this requirement.
The discharge plan is different from a discharge summary document, which must be completed by the inpatient attending physician, not the hospital, and is not directly addressed in the regulation. The regulation does address the need for transfer of essential information to the next provider of care and says the hospital should have a written policy and procedure in place for discharge planning. The policy and procedure should be developed with input from medical staff and approved by the hospital’s governing body.
Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation.
—Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST
Transmittal 87 represents the first major update of the discharge planning regulation (Standard 482.43) and accompanying interpretive guidelines in more than a decade, Dr. Boutwell says. It consolidates and reorganizes 24 “tags” of regulatory language down to 13 and contains blue advisory boxes recommending best practices in discharge planning, drawn from the suggestions of a technical expert panel convened by CMS.
That panel included many of the country’s recognized thought leaders on improving care transitions, such as Mark Williams, MD, FACP, SFHM, principal investigator of SHM’s Project BOOST; Eric Coleman, MD, MPH, head of the University of Colorado’s division of health care policy and research and creator of the widely-adopted Care Transitions Program (caretransitions.org), and Dr. Boutwell, co-founder of the STAAR initiative (www.ihi.org/engage/Initiatives/completed/STAAR).
The new condition raises baseline expectations for discharge planning and elevates care transitions efforts from a quality improvement issue to the realm of regulatory compliance, Dr. Boutwell says.
“This goes way beyond case review,” she adds. “It represents an evolution from discharge planning case by case to a system for improving transitions of care [for the hospital]. I’m impressed.”
The recommendations are consistent with best practices promoted by Project BOOST, STAAR, Project RED [Re-Engineered Discharge], and other national quality initiatives for improving care transitions.
“Any hospitalist participating with a hospital QI team involved in Project BOOST is helping their hospital comply with this condition of participation,” Dr. Williams says.
In the Byzantine structure of federal regulations, Medicare’s conditions of participation are the regulations providers must meet in order to participate in the Medicare program and bill for their services. Condition-level citations, if not resolved, can cause hospitals to be decertified from Medicare. The accompanying interpretive guidelines, with survey protocols, are the playbook to help state auditors and providers know how to interpret and apply the language of the regulations. The suggestions and examples of best practices contained in the new condition are not required of hospitals but, if followed, could increase their likelihood of achieving better patient outcomes and staying in compliance with the regulations on surveys.
“If hospitals were to actually implement all of the CMS advisory practice recommendations contained in this 35-page document, they’d be in really good shape for effectively managing transitions of care,” says Teresa L. Hamblin, RN, MS, a CMS consultant with Joint Commission Resources. “The government has provided robust practice recommendations that are a model for what hospitals can do. I’d advise doing your best to implement these recommendations. Check your current processes using this detailed document for reference.”
Discharge planning starts at admission, Hamblin says. If the hospitalist assumes that responsibility, it becomes easier to leave a paper trail in the patient’s chart. Other important lessons for hospitalists include participation in a multidisciplinary approach to discharge planning (i.e., interdisciplinary rounding) and development of policies and procedures in this area.
“If the hospital has not elected to do a discharge plan on every patient, request this for your own patients and recommend it as a policy,” Hamblin says. “Go the extra mile, making follow-up appointments for your patients, filling prescriptions in house, and calling the patient 24 to 72 hours after discharge.”
Weekend coverage, when case managers typically are not present, is a particular challenge in care transitions.
“Encourage your hospital to provide reliable weekend coverage for discharge planning. Involve the nurses,” Hamblin says. “Anything the hospitalist can do to help the hospital close this gap is important.”
Larry Beresford is a freelance writer in Alameda, Calif.
SGR Reform, ICD-10 Implementation Delays Frustrate Hospitalists, Physicians
Congress has once again delayed implementation of draconian Medicare cuts tied to the sustainable growth rate (SGR) formula. It was the 17th temporary patch applied to the ailing physician reimbursement program, so the decision caused little surprise.
But with the same legislation—the Protecting Access to Medicare Act of 2014—being used to delay the long-awaited debut of ICD-10, many hospitalists and physicians couldn’t help but wonder whether billing and coding would now be as much of a political football as the SGR fix.1
The upshot: It doesn’t seem that way.
“I think it’s two separate issues,” says Phyllis “PJ” Floyd, RN, BSN, MBA, NE-BC, CCA, director of health information services and clinical documentation improvement at Medical University of South Carolina (MUSC) in Charleston, S.C. “The fact that it was all in one bill, I don’t know that it was well thought out as much as it was, ‘Let’s put the ICD-10 in here at the same time.’
“It was just a few sentences, and then it wasn’t even brought up in the discussion on the floor.”
Four policy wonks interviewed by The Hospitalist concurred that while tying the ICD-10 delay to the SGR issue was an unexpected and frustrating development, the coding system likely will be implemented in the relative short term. Meanwhile, a long-term resolution of the SGR dilemma remains much more elusive.
“For about 12 hours, I felt relief about the ICD-10 [being delayed], and then I just realized, it’s still coming, presumably,” says John Nelson, MD, MHM, a co-founder and past president of SHM and medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash. “[It’s] like a patient who needs surgery and finds out it’s canceled for the day and he’ll have it tomorrow. Well, that’s good for right now, but [he] still has to face this eventually.”
“Doc-Pay” Fix Near?
Congress’ recent decision to delay both an SGR fix and the ICD-10 are troubling to some hospitalists and others for different reasons.
The SGR extension through this year’s end means that physicians do not face a 24% cut to physician payments under Medicare. SHM has long lobbied against temporary patches to the SGR, repeatedly backing legislation that would once and for all scrap the formula and replace it with something sustainable.
The SGR formula was first crafted in 1997, but the now often-delayed cuts were a byproduct of the federal sequester that was included in the Budget Control Act of 2011. At the time, the massive reduction to Medicare payments was tied to political brinksmanship over the country’s debt ceiling. The cuts were implemented as a doomsday scenario that was never likely to actually happen, but despite negotiations over the past three years, no long-term compromise can be found. Paying for the reform remains the main stumbling block.
“I think, this year, Congress was as close as it’s been in a long time to enacting a serious fix, aided by the agreement of major professional societies like the American College of Physicians and American College of Surgeons,” says David Howard, PhD, an associate professor in the department of health policy and management at the Rollins School of Public Health at Emory University in Atlanta. “They were all on board with this solution. ... Who knows, maybe if the economic situation continues to improve [and] tax revenues continue to go up...that will create a more favorable environment for compromise.”
Dr. Howard adds that while Congress might be close to a solution in theory, agreement on how to offset the roughly $100 billion in costs “is just very difficult.” That is why the healthcare professor is pessimistic that a long-term fix is truly at hand.
“The places where Congress might have looked for savings to offset the cost of the doc fix, such as hospital reimbursement rates or payment rates to Medicare Advantage plans—those are exactly the areas that the Affordable Care Act is targeting to pay for insurance expansion,” Dr. Howard adds. “So those areas of savings are not going to be available to offset the cost of the doc fix.”
ICD-10 Delays “Unfair”
The medical coding conundrum presents a different set of issues. The delay in transitioning healthcare providers from the ICD-9 medical coding classification system to the more complicated ICD-10 means the upgraded system is now against an Oct. 1, 2015, deadline. This comes after the Centers for Medicare & Medicaid Services (CMS) already pushed back the original implementation date for ICD-10 by one year.
SHM Public Policy Committee member Joshua Lenchus, DO, RPh, FACP, SFHM, says he thinks most doctors are content with the delay, particularly in light of some estimates that show that only about 20% of physicians “have actually initiated the ICD-10 transition.” But he also notes that it’s unfair to the health systems that have prepared for ICD-10.
“ICD-9 has a little more than 14,000 diagnostic codes and nearly 4,000 procedural codes. That is to be contrasted to ICD-10, which has more than 68,000 diagnostic codes ... and over 72,000 procedural codes,” Dr. Lenchus says. “So, it is not surprising that many take solace in the delay.”
–Dr. Lenchus
Dr. Nelson says the level of frustration for hospitalists is growing; however, the level of disruption for hospitals and health systems is reaching a boiling point.
“Of course, in some places, hospitalists may be the physician lead on ICD-10 efforts, so [they are] very much wrapped up in the problem of ‘What do we do now?’”
The answer, at least to the Coalition for ICD-10, a group of medical/technology trade groups, is to fight to ensure that the delays go no further. In an April letter to CMS Administrator Marilyn Tavenner, the coalition made that case, noting that in 2012, “CMS estimated the cost to the healthcare industry of a one-year delay to be as much as $6.6 billion, or approximately 30% of the $22 billion that CMS estimated had been invested or budgeted for ICD-10 implementation.”2
The letter went on to explain that the disruption and cost will grow each time the ICD-10 deadline is pushed.
“Furthermore, as CMS stated in 2012, implementation costs will continue to increase considerably with every year of a delay,” according to the letter. “The lost opportunity costs of failing to move to a more effective code set also continue to climb every year.”
Stay Engaged, Switch Gears
One of Floyd’s biggest concerns is that the ICD-10 implementation delays will affect physician engagement. The hospitalist groups at MUSC began training for ICD-10 in January 2013; however, the preparation and training were geared toward a 2014 implementation.
“You have to switch gears a little bit,” she says. “What we plan to do now is begin to do heavy auditing, and then from those audits we can give real-time feedback on what we’re doing well and what we’re not doing well. So I think that will be a method for engagement.”
She urges hospitalists, practice leaders, and informatics professionals to discuss ICD-10 not as a theoretical application, but as one tied to reimbursement that will have major impact in the years ahead. To that end, the American Health Information Management Association highlights the fact that the new coding system will result in higher-quality data that can improve performance measures, provide “increased sensitivity” to reimbursement methodologies, and help with stronger public health surveillance.3
“A lot of physicians see this as a hospital issue, and I think that’s why they shy away,” Floyd says. “Now there are some physicians who are interested in how well the hospital does, but the other piece is that it does affect things like [reduced] risk of mortality [and] comparison of data worldwide—those are things that we just have to continue to reiterate … and give them real examples.”
Richard Quinn is a freelance writer in New Jersey.
References
- Govtrack. H.R. 4302: Protecting Access to Medicare Act of 2014. https://www.govtrack.us/congress/bills/113/hr4302. Accessed June 5, 2014.
- Coalition for ICD. Letter to CMS Administrator Tavenner, April 11, 2014. http://coalitionforicd10.wordpress.com/2014/03/26/letter-from-the-coalition-for-icd-10. Accessed June 5, 2014.
- American Health Information Management Association. ICD-10-CM/PCS Transition: Planning and Preparation Checklist. http://journal.ahima.org/wp-content/uploads/ICD10-checklist.pdf. Accessed June 5, 2014.
Congress has once again delayed implementation of draconian Medicare cuts tied to the sustainable growth rate (SGR) formula. It was the 17th temporary patch applied to the ailing physician reimbursement program, so the decision caused little surprise.
But with the same legislation—the Protecting Access to Medicare Act of 2014—being used to delay the long-awaited debut of ICD-10, many hospitalists and physicians couldn’t help but wonder whether billing and coding would now be as much of a political football as the SGR fix.1
The upshot: It doesn’t seem that way.
“I think it’s two separate issues,” says Phyllis “PJ” Floyd, RN, BSN, MBA, NE-BC, CCA, director of health information services and clinical documentation improvement at Medical University of South Carolina (MUSC) in Charleston, S.C. “The fact that it was all in one bill, I don’t know that it was well thought out as much as it was, ‘Let’s put the ICD-10 in here at the same time.’
“It was just a few sentences, and then it wasn’t even brought up in the discussion on the floor.”
Four policy wonks interviewed by The Hospitalist concurred that while tying the ICD-10 delay to the SGR issue was an unexpected and frustrating development, the coding system likely will be implemented in the relative short term. Meanwhile, a long-term resolution of the SGR dilemma remains much more elusive.
“For about 12 hours, I felt relief about the ICD-10 [being delayed], and then I just realized, it’s still coming, presumably,” says John Nelson, MD, MHM, a co-founder and past president of SHM and medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash. “[It’s] like a patient who needs surgery and finds out it’s canceled for the day and he’ll have it tomorrow. Well, that’s good for right now, but [he] still has to face this eventually.”
“Doc-Pay” Fix Near?
Congress’ recent decision to delay both an SGR fix and the ICD-10 are troubling to some hospitalists and others for different reasons.
The SGR extension through this year’s end means that physicians do not face a 24% cut to physician payments under Medicare. SHM has long lobbied against temporary patches to the SGR, repeatedly backing legislation that would once and for all scrap the formula and replace it with something sustainable.
The SGR formula was first crafted in 1997, but the now often-delayed cuts were a byproduct of the federal sequester that was included in the Budget Control Act of 2011. At the time, the massive reduction to Medicare payments was tied to political brinksmanship over the country’s debt ceiling. The cuts were implemented as a doomsday scenario that was never likely to actually happen, but despite negotiations over the past three years, no long-term compromise can be found. Paying for the reform remains the main stumbling block.
“I think, this year, Congress was as close as it’s been in a long time to enacting a serious fix, aided by the agreement of major professional societies like the American College of Physicians and American College of Surgeons,” says David Howard, PhD, an associate professor in the department of health policy and management at the Rollins School of Public Health at Emory University in Atlanta. “They were all on board with this solution. ... Who knows, maybe if the economic situation continues to improve [and] tax revenues continue to go up...that will create a more favorable environment for compromise.”
Dr. Howard adds that while Congress might be close to a solution in theory, agreement on how to offset the roughly $100 billion in costs “is just very difficult.” That is why the healthcare professor is pessimistic that a long-term fix is truly at hand.
“The places where Congress might have looked for savings to offset the cost of the doc fix, such as hospital reimbursement rates or payment rates to Medicare Advantage plans—those are exactly the areas that the Affordable Care Act is targeting to pay for insurance expansion,” Dr. Howard adds. “So those areas of savings are not going to be available to offset the cost of the doc fix.”
ICD-10 Delays “Unfair”
The medical coding conundrum presents a different set of issues. The delay in transitioning healthcare providers from the ICD-9 medical coding classification system to the more complicated ICD-10 means the upgraded system is now against an Oct. 1, 2015, deadline. This comes after the Centers for Medicare & Medicaid Services (CMS) already pushed back the original implementation date for ICD-10 by one year.
SHM Public Policy Committee member Joshua Lenchus, DO, RPh, FACP, SFHM, says he thinks most doctors are content with the delay, particularly in light of some estimates that show that only about 20% of physicians “have actually initiated the ICD-10 transition.” But he also notes that it’s unfair to the health systems that have prepared for ICD-10.
“ICD-9 has a little more than 14,000 diagnostic codes and nearly 4,000 procedural codes. That is to be contrasted to ICD-10, which has more than 68,000 diagnostic codes ... and over 72,000 procedural codes,” Dr. Lenchus says. “So, it is not surprising that many take solace in the delay.”
–Dr. Lenchus
Dr. Nelson says the level of frustration for hospitalists is growing; however, the level of disruption for hospitals and health systems is reaching a boiling point.
“Of course, in some places, hospitalists may be the physician lead on ICD-10 efforts, so [they are] very much wrapped up in the problem of ‘What do we do now?’”
The answer, at least to the Coalition for ICD-10, a group of medical/technology trade groups, is to fight to ensure that the delays go no further. In an April letter to CMS Administrator Marilyn Tavenner, the coalition made that case, noting that in 2012, “CMS estimated the cost to the healthcare industry of a one-year delay to be as much as $6.6 billion, or approximately 30% of the $22 billion that CMS estimated had been invested or budgeted for ICD-10 implementation.”2
The letter went on to explain that the disruption and cost will grow each time the ICD-10 deadline is pushed.
“Furthermore, as CMS stated in 2012, implementation costs will continue to increase considerably with every year of a delay,” according to the letter. “The lost opportunity costs of failing to move to a more effective code set also continue to climb every year.”
Stay Engaged, Switch Gears
One of Floyd’s biggest concerns is that the ICD-10 implementation delays will affect physician engagement. The hospitalist groups at MUSC began training for ICD-10 in January 2013; however, the preparation and training were geared toward a 2014 implementation.
“You have to switch gears a little bit,” she says. “What we plan to do now is begin to do heavy auditing, and then from those audits we can give real-time feedback on what we’re doing well and what we’re not doing well. So I think that will be a method for engagement.”
She urges hospitalists, practice leaders, and informatics professionals to discuss ICD-10 not as a theoretical application, but as one tied to reimbursement that will have major impact in the years ahead. To that end, the American Health Information Management Association highlights the fact that the new coding system will result in higher-quality data that can improve performance measures, provide “increased sensitivity” to reimbursement methodologies, and help with stronger public health surveillance.3
“A lot of physicians see this as a hospital issue, and I think that’s why they shy away,” Floyd says. “Now there are some physicians who are interested in how well the hospital does, but the other piece is that it does affect things like [reduced] risk of mortality [and] comparison of data worldwide—those are things that we just have to continue to reiterate … and give them real examples.”
Richard Quinn is a freelance writer in New Jersey.
References
- Govtrack. H.R. 4302: Protecting Access to Medicare Act of 2014. https://www.govtrack.us/congress/bills/113/hr4302. Accessed June 5, 2014.
- Coalition for ICD. Letter to CMS Administrator Tavenner, April 11, 2014. http://coalitionforicd10.wordpress.com/2014/03/26/letter-from-the-coalition-for-icd-10. Accessed June 5, 2014.
- American Health Information Management Association. ICD-10-CM/PCS Transition: Planning and Preparation Checklist. http://journal.ahima.org/wp-content/uploads/ICD10-checklist.pdf. Accessed June 5, 2014.
Congress has once again delayed implementation of draconian Medicare cuts tied to the sustainable growth rate (SGR) formula. It was the 17th temporary patch applied to the ailing physician reimbursement program, so the decision caused little surprise.
But with the same legislation—the Protecting Access to Medicare Act of 2014—being used to delay the long-awaited debut of ICD-10, many hospitalists and physicians couldn’t help but wonder whether billing and coding would now be as much of a political football as the SGR fix.1
The upshot: It doesn’t seem that way.
“I think it’s two separate issues,” says Phyllis “PJ” Floyd, RN, BSN, MBA, NE-BC, CCA, director of health information services and clinical documentation improvement at Medical University of South Carolina (MUSC) in Charleston, S.C. “The fact that it was all in one bill, I don’t know that it was well thought out as much as it was, ‘Let’s put the ICD-10 in here at the same time.’
“It was just a few sentences, and then it wasn’t even brought up in the discussion on the floor.”
Four policy wonks interviewed by The Hospitalist concurred that while tying the ICD-10 delay to the SGR issue was an unexpected and frustrating development, the coding system likely will be implemented in the relative short term. Meanwhile, a long-term resolution of the SGR dilemma remains much more elusive.
“For about 12 hours, I felt relief about the ICD-10 [being delayed], and then I just realized, it’s still coming, presumably,” says John Nelson, MD, MHM, a co-founder and past president of SHM and medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash. “[It’s] like a patient who needs surgery and finds out it’s canceled for the day and he’ll have it tomorrow. Well, that’s good for right now, but [he] still has to face this eventually.”
“Doc-Pay” Fix Near?
Congress’ recent decision to delay both an SGR fix and the ICD-10 are troubling to some hospitalists and others for different reasons.
The SGR extension through this year’s end means that physicians do not face a 24% cut to physician payments under Medicare. SHM has long lobbied against temporary patches to the SGR, repeatedly backing legislation that would once and for all scrap the formula and replace it with something sustainable.
The SGR formula was first crafted in 1997, but the now often-delayed cuts were a byproduct of the federal sequester that was included in the Budget Control Act of 2011. At the time, the massive reduction to Medicare payments was tied to political brinksmanship over the country’s debt ceiling. The cuts were implemented as a doomsday scenario that was never likely to actually happen, but despite negotiations over the past three years, no long-term compromise can be found. Paying for the reform remains the main stumbling block.
“I think, this year, Congress was as close as it’s been in a long time to enacting a serious fix, aided by the agreement of major professional societies like the American College of Physicians and American College of Surgeons,” says David Howard, PhD, an associate professor in the department of health policy and management at the Rollins School of Public Health at Emory University in Atlanta. “They were all on board with this solution. ... Who knows, maybe if the economic situation continues to improve [and] tax revenues continue to go up...that will create a more favorable environment for compromise.”
Dr. Howard adds that while Congress might be close to a solution in theory, agreement on how to offset the roughly $100 billion in costs “is just very difficult.” That is why the healthcare professor is pessimistic that a long-term fix is truly at hand.
“The places where Congress might have looked for savings to offset the cost of the doc fix, such as hospital reimbursement rates or payment rates to Medicare Advantage plans—those are exactly the areas that the Affordable Care Act is targeting to pay for insurance expansion,” Dr. Howard adds. “So those areas of savings are not going to be available to offset the cost of the doc fix.”
ICD-10 Delays “Unfair”
The medical coding conundrum presents a different set of issues. The delay in transitioning healthcare providers from the ICD-9 medical coding classification system to the more complicated ICD-10 means the upgraded system is now against an Oct. 1, 2015, deadline. This comes after the Centers for Medicare & Medicaid Services (CMS) already pushed back the original implementation date for ICD-10 by one year.
SHM Public Policy Committee member Joshua Lenchus, DO, RPh, FACP, SFHM, says he thinks most doctors are content with the delay, particularly in light of some estimates that show that only about 20% of physicians “have actually initiated the ICD-10 transition.” But he also notes that it’s unfair to the health systems that have prepared for ICD-10.
“ICD-9 has a little more than 14,000 diagnostic codes and nearly 4,000 procedural codes. That is to be contrasted to ICD-10, which has more than 68,000 diagnostic codes ... and over 72,000 procedural codes,” Dr. Lenchus says. “So, it is not surprising that many take solace in the delay.”
–Dr. Lenchus
Dr. Nelson says the level of frustration for hospitalists is growing; however, the level of disruption for hospitals and health systems is reaching a boiling point.
“Of course, in some places, hospitalists may be the physician lead on ICD-10 efforts, so [they are] very much wrapped up in the problem of ‘What do we do now?’”
The answer, at least to the Coalition for ICD-10, a group of medical/technology trade groups, is to fight to ensure that the delays go no further. In an April letter to CMS Administrator Marilyn Tavenner, the coalition made that case, noting that in 2012, “CMS estimated the cost to the healthcare industry of a one-year delay to be as much as $6.6 billion, or approximately 30% of the $22 billion that CMS estimated had been invested or budgeted for ICD-10 implementation.”2
The letter went on to explain that the disruption and cost will grow each time the ICD-10 deadline is pushed.
“Furthermore, as CMS stated in 2012, implementation costs will continue to increase considerably with every year of a delay,” according to the letter. “The lost opportunity costs of failing to move to a more effective code set also continue to climb every year.”
Stay Engaged, Switch Gears
One of Floyd’s biggest concerns is that the ICD-10 implementation delays will affect physician engagement. The hospitalist groups at MUSC began training for ICD-10 in January 2013; however, the preparation and training were geared toward a 2014 implementation.
“You have to switch gears a little bit,” she says. “What we plan to do now is begin to do heavy auditing, and then from those audits we can give real-time feedback on what we’re doing well and what we’re not doing well. So I think that will be a method for engagement.”
She urges hospitalists, practice leaders, and informatics professionals to discuss ICD-10 not as a theoretical application, but as one tied to reimbursement that will have major impact in the years ahead. To that end, the American Health Information Management Association highlights the fact that the new coding system will result in higher-quality data that can improve performance measures, provide “increased sensitivity” to reimbursement methodologies, and help with stronger public health surveillance.3
“A lot of physicians see this as a hospital issue, and I think that’s why they shy away,” Floyd says. “Now there are some physicians who are interested in how well the hospital does, but the other piece is that it does affect things like [reduced] risk of mortality [and] comparison of data worldwide—those are things that we just have to continue to reiterate … and give them real examples.”
Richard Quinn is a freelance writer in New Jersey.
References
- Govtrack. H.R. 4302: Protecting Access to Medicare Act of 2014. https://www.govtrack.us/congress/bills/113/hr4302. Accessed June 5, 2014.
- Coalition for ICD. Letter to CMS Administrator Tavenner, April 11, 2014. http://coalitionforicd10.wordpress.com/2014/03/26/letter-from-the-coalition-for-icd-10. Accessed June 5, 2014.
- American Health Information Management Association. ICD-10-CM/PCS Transition: Planning and Preparation Checklist. http://journal.ahima.org/wp-content/uploads/ICD10-checklist.pdf. Accessed June 5, 2014.
Hospital employment or physician-led ACO?
Primary care physicians around the country are facing the largest decision of their lives: Do I stay independent and maybe form an accountable care organization with other independent physicians, or do I become an employee of a hospital or health system?
As accountable care is taking hold, new data may alter historic thinking on this "bet-the-practice" question.
Tired of being overworked, undersatisfied, and overwhelmed with growing regulatory requirements, many primary care physicians have sought the security and strength of hospital employment. They say the pressures to invest in technology, billing, coding, and continued reimbursement pressures are just too great.
Yet, the majority of these physicians miss their days of self-employed autonomy, are on average less productive, and worry that the clocks on their compensation guarantees are ticking down.
Most of the moves by your colleagues, and perhaps you, to hospital employment have been defensive. It was just no longer feasible to stay afloat in the current fee-for-service system. You cannot work any harder, faster, or cheaper. You can no longer spend satisfactory time with your patients.
On the other hand, some of you may have joined a hospital or health system to be proactive and gain a solid platform to prepare for the new value-based payment era.
You may have envisioned being integrated with a critical mass of like-minded physicians and facilities, aided by advanced population management tools and a strong balance sheet, and all linked together on the hospital’s health information technology platform. You read that primary care should be in a leadership position and financially incentivized in any accountable care organization – including a hospital’s. Independent physicians could theoretically form ACOs, too, but lack the up-front capital, know-how, and any spare intellectual bandwidth to do so.
So, from a strategic perspective, becoming employed with other physicians by a health system seemed the way to go.
The pace has quickened of health care’s movement away from fee for service or "pay for volume" to payment for better outcomes at lower overall costs, or "pay for value." The factors that applied to the decision to become employed in the fee-for-service era may be yielding to those in the accountable care era sooner than anticipated.
Independent physician-led ACOs appear to be adapting better than hospitals to this change. Although much better prepared fiscally, hospitals are conflicted, or at least hesitant, to make this switch, because much of the savings comes from avoidable admissions and readmissions. On the other hand, emerging data and experience are showing that physician-led ACOs can be very successful.
There are some very integrated and successful hospital-led ACOs or other value-delivery hospital/physician models. In fact, I believe that if the hospital is willing to right-size and truly commit to value, it can be the most successful model.
However, many physicians signed volume-only physician work relative value unit (wRVU) compensation formulas in their hospital employment agreements, with no incentive payments for value. They have not been involved as partners, much less leaders, in any ACO planning. Even though the fee-for-service days are waning and strains are showing for many hospitals that are not adapting, for many employed physicians, the pace of preparedness for the accountable care era has been disappointing.
New data show that while most of the early ACOs in the Medicare Shared Savings Program were hospital led, there are now more physician-led ACOs than any other. At the same time, early results of some modest primary care–only ACOs have been exciting. The rural primary care physician ACO previously reported on in this column, Rio Grande Valley Health Alliance in McAllen, Tex., is preliminarily looking at 90th-percentile quality results and more than $500,000 in (unofficial) savings per physician in their first year under the Medicare Shared Savings Program.
In fact, in a May 14, 2014, article in JAMA, its authors stated: "Even though most adult primary care physicians may not realize it, they each can be seen as a chief executive officer (CEO) in charge of approximately $10 million in annual revenue" (JAMA 2014;311:1855-6). They noted that primary care receives only 5% of that spending, but can control much of the average of $5,000 in annual spending of their 2,000 or so patients. The independent physician-led Palm Beach ACO is cited as an example, with $22 million in savings their first year. The authors recommend physician-led ACOs as the best way to leverage that "CEO" power.
These new success lessons are being learned and need to be shared. Primary care physicians need to understand that the risk of change is now much less than the risk of maintaining the status quo. You need transparency regarding the realities of all your choices, including hospital employment and physician ACOs.
As readers of this column know, I heartily endorse the trend recognized in the JAMA article: "[A]n increasing number of primary care physicians see physician-led ACOs as a powerful opportunity to retain their autonomy and make a positive difference for their patient – as well as their practices’ bottom lines."
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Primary care physicians around the country are facing the largest decision of their lives: Do I stay independent and maybe form an accountable care organization with other independent physicians, or do I become an employee of a hospital or health system?
As accountable care is taking hold, new data may alter historic thinking on this "bet-the-practice" question.
Tired of being overworked, undersatisfied, and overwhelmed with growing regulatory requirements, many primary care physicians have sought the security and strength of hospital employment. They say the pressures to invest in technology, billing, coding, and continued reimbursement pressures are just too great.
Yet, the majority of these physicians miss their days of self-employed autonomy, are on average less productive, and worry that the clocks on their compensation guarantees are ticking down.
Most of the moves by your colleagues, and perhaps you, to hospital employment have been defensive. It was just no longer feasible to stay afloat in the current fee-for-service system. You cannot work any harder, faster, or cheaper. You can no longer spend satisfactory time with your patients.
On the other hand, some of you may have joined a hospital or health system to be proactive and gain a solid platform to prepare for the new value-based payment era.
You may have envisioned being integrated with a critical mass of like-minded physicians and facilities, aided by advanced population management tools and a strong balance sheet, and all linked together on the hospital’s health information technology platform. You read that primary care should be in a leadership position and financially incentivized in any accountable care organization – including a hospital’s. Independent physicians could theoretically form ACOs, too, but lack the up-front capital, know-how, and any spare intellectual bandwidth to do so.
So, from a strategic perspective, becoming employed with other physicians by a health system seemed the way to go.
The pace has quickened of health care’s movement away from fee for service or "pay for volume" to payment for better outcomes at lower overall costs, or "pay for value." The factors that applied to the decision to become employed in the fee-for-service era may be yielding to those in the accountable care era sooner than anticipated.
Independent physician-led ACOs appear to be adapting better than hospitals to this change. Although much better prepared fiscally, hospitals are conflicted, or at least hesitant, to make this switch, because much of the savings comes from avoidable admissions and readmissions. On the other hand, emerging data and experience are showing that physician-led ACOs can be very successful.
There are some very integrated and successful hospital-led ACOs or other value-delivery hospital/physician models. In fact, I believe that if the hospital is willing to right-size and truly commit to value, it can be the most successful model.
However, many physicians signed volume-only physician work relative value unit (wRVU) compensation formulas in their hospital employment agreements, with no incentive payments for value. They have not been involved as partners, much less leaders, in any ACO planning. Even though the fee-for-service days are waning and strains are showing for many hospitals that are not adapting, for many employed physicians, the pace of preparedness for the accountable care era has been disappointing.
New data show that while most of the early ACOs in the Medicare Shared Savings Program were hospital led, there are now more physician-led ACOs than any other. At the same time, early results of some modest primary care–only ACOs have been exciting. The rural primary care physician ACO previously reported on in this column, Rio Grande Valley Health Alliance in McAllen, Tex., is preliminarily looking at 90th-percentile quality results and more than $500,000 in (unofficial) savings per physician in their first year under the Medicare Shared Savings Program.
In fact, in a May 14, 2014, article in JAMA, its authors stated: "Even though most adult primary care physicians may not realize it, they each can be seen as a chief executive officer (CEO) in charge of approximately $10 million in annual revenue" (JAMA 2014;311:1855-6). They noted that primary care receives only 5% of that spending, but can control much of the average of $5,000 in annual spending of their 2,000 or so patients. The independent physician-led Palm Beach ACO is cited as an example, with $22 million in savings their first year. The authors recommend physician-led ACOs as the best way to leverage that "CEO" power.
These new success lessons are being learned and need to be shared. Primary care physicians need to understand that the risk of change is now much less than the risk of maintaining the status quo. You need transparency regarding the realities of all your choices, including hospital employment and physician ACOs.
As readers of this column know, I heartily endorse the trend recognized in the JAMA article: "[A]n increasing number of primary care physicians see physician-led ACOs as a powerful opportunity to retain their autonomy and make a positive difference for their patient – as well as their practices’ bottom lines."
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Primary care physicians around the country are facing the largest decision of their lives: Do I stay independent and maybe form an accountable care organization with other independent physicians, or do I become an employee of a hospital or health system?
As accountable care is taking hold, new data may alter historic thinking on this "bet-the-practice" question.
Tired of being overworked, undersatisfied, and overwhelmed with growing regulatory requirements, many primary care physicians have sought the security and strength of hospital employment. They say the pressures to invest in technology, billing, coding, and continued reimbursement pressures are just too great.
Yet, the majority of these physicians miss their days of self-employed autonomy, are on average less productive, and worry that the clocks on their compensation guarantees are ticking down.
Most of the moves by your colleagues, and perhaps you, to hospital employment have been defensive. It was just no longer feasible to stay afloat in the current fee-for-service system. You cannot work any harder, faster, or cheaper. You can no longer spend satisfactory time with your patients.
On the other hand, some of you may have joined a hospital or health system to be proactive and gain a solid platform to prepare for the new value-based payment era.
You may have envisioned being integrated with a critical mass of like-minded physicians and facilities, aided by advanced population management tools and a strong balance sheet, and all linked together on the hospital’s health information technology platform. You read that primary care should be in a leadership position and financially incentivized in any accountable care organization – including a hospital’s. Independent physicians could theoretically form ACOs, too, but lack the up-front capital, know-how, and any spare intellectual bandwidth to do so.
So, from a strategic perspective, becoming employed with other physicians by a health system seemed the way to go.
The pace has quickened of health care’s movement away from fee for service or "pay for volume" to payment for better outcomes at lower overall costs, or "pay for value." The factors that applied to the decision to become employed in the fee-for-service era may be yielding to those in the accountable care era sooner than anticipated.
Independent physician-led ACOs appear to be adapting better than hospitals to this change. Although much better prepared fiscally, hospitals are conflicted, or at least hesitant, to make this switch, because much of the savings comes from avoidable admissions and readmissions. On the other hand, emerging data and experience are showing that physician-led ACOs can be very successful.
There are some very integrated and successful hospital-led ACOs or other value-delivery hospital/physician models. In fact, I believe that if the hospital is willing to right-size and truly commit to value, it can be the most successful model.
However, many physicians signed volume-only physician work relative value unit (wRVU) compensation formulas in their hospital employment agreements, with no incentive payments for value. They have not been involved as partners, much less leaders, in any ACO planning. Even though the fee-for-service days are waning and strains are showing for many hospitals that are not adapting, for many employed physicians, the pace of preparedness for the accountable care era has been disappointing.
New data show that while most of the early ACOs in the Medicare Shared Savings Program were hospital led, there are now more physician-led ACOs than any other. At the same time, early results of some modest primary care–only ACOs have been exciting. The rural primary care physician ACO previously reported on in this column, Rio Grande Valley Health Alliance in McAllen, Tex., is preliminarily looking at 90th-percentile quality results and more than $500,000 in (unofficial) savings per physician in their first year under the Medicare Shared Savings Program.
In fact, in a May 14, 2014, article in JAMA, its authors stated: "Even though most adult primary care physicians may not realize it, they each can be seen as a chief executive officer (CEO) in charge of approximately $10 million in annual revenue" (JAMA 2014;311:1855-6). They noted that primary care receives only 5% of that spending, but can control much of the average of $5,000 in annual spending of their 2,000 or so patients. The independent physician-led Palm Beach ACO is cited as an example, with $22 million in savings their first year. The authors recommend physician-led ACOs as the best way to leverage that "CEO" power.
These new success lessons are being learned and need to be shared. Primary care physicians need to understand that the risk of change is now much less than the risk of maintaining the status quo. You need transparency regarding the realities of all your choices, including hospital employment and physician ACOs.
As readers of this column know, I heartily endorse the trend recognized in the JAMA article: "[A]n increasing number of primary care physicians see physician-led ACOs as a powerful opportunity to retain their autonomy and make a positive difference for their patient – as well as their practices’ bottom lines."
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
23-year-old woman being treated for opioid dependence, unexpected weight gain
THE CASE
We were treating a 23-year-old woman in our clinic for opioid dependence. She had begun using hydrocodone/acetaminophen, oxycodone, and heroin at age 17. Her parents and relatives had a history of alcohol and drug addiction and her brother had died from a heroin overdose.
The patient was taking buprenorphine/naloxone 12 mg/3 mg daily. She attended weekly counseling sessions at a community outreach center. We explained to her the potentially dangerous effects of buprenorphine/naloxone. Urine toxicology was negative for substances other than buprenorphine/naloxone.
Over 8 months, our patient gained 33 pounds and began wearing loose clothing to her appointments. When we asked her about it, she said that she had been “eating more bagels” lately.
What the patient wasn’t telling us was that she was pregnant. (We learned of her pregnancy only after she delivered.) In addition, she didn’t disclose to her obstetrician (OB) that she was taking buprenorphine/naloxone until she was nearly full term. At that point, the OB consulted maternal fetal medicine, and the buprenorphine/naloxone was continued through delivery. The patient had an uncomplicated spontaneous vaginal delivery of an 8.19 lb girl with an APGAR score of 8 at 1 minute and 8 again at 5 minutes.
Concerned about neonatal abstinence syndrome (NAS), which is characterized by tremors, increased body tone, feeding intolerance, vomiting, sweating, and fever, the healthcare team used the NAS scoring system to assess the newborn’s need for pharmacologic therapy. The newborn’s score at birth was 16/45. It then dropped to 11/45 indicating that she was experiencing mild withdrawal, but her symptoms—grunting, tachycardia, increased tone, tremors, irritability, and sweating—suggested she was experiencing severe withdrawal. The infant remained hospitalized for 29 days and received oral morphine titrated to her NAS score. The drug regimen for treatment/tapering was oral morphine given at 0.1 mg/kg/dose every 4 hours. This dose was lowered by 10% each time her NAS score was <8. At discharge, the infant’s NAS score had decreased to 3/45.
After discharge, the mother admitted to us that she concealed her pregnancy because she was afraid of being placed on methadone. She said she didn’t want to have to go to a clinic to receive the medication.
Continued good health. The child has since reached all of her developmental milestones appropriately and has normal height and weight.
DISCUSSION
Opioid abuse is an increasing cause of morbidity and mortality. In the United States, the number of deaths from opioid overdose is approaching that of motor vehicle accidents: approximately 100 deaths a day.1
The use of opioids by a pregnant woman can cause intrauterine growth retardation and preterm delivery.2 It also can result in withdrawal symptoms in the newborn,3 necessitating treatment guided by the NAS score. This score takes into consideration the metabolic, respiratory, central nervous system, and gastrointestinal symptoms of the infant at specified time intervals.4
Treatment options. For nonpregnant patients, opioid dependence typically is treated with methadone, an opioid agonist or buprenorphine, a partial opioid agonist; buprenorphine usually is prescribed as a combination medication that also contains naloxone, an opioid antagonist.
While methadone must be prescribed through licensed clinics, physicians meeting specific qualifications can prescribe buprenorphine or buprenorphine/naloxone in the office setting.5 Studies have supported the effectiveness of buprenorphine, alone or in combination with naloxone, in discouraging illicit opioid use.6-8
When the patient is pregnant… Methadone is the current standard of care for opioid-dependent patients who become pregnant.9 Buprenorphine/naloxone is currently a US Food and Drug Administration category C drug. However, recent studies have demonstrated the safety of buprenorphine without naloxone during pregnancy.10,11
The incidence and severity of NAS following treatment with buprenorphine is less than or comparable to methadone maintenance.10,11 The NAS score of 11 recorded in our patient’s case was comparable to those reported by Jones et al,9 who found neonates of women on buprenorphine had an average maximum NAS score of 11 and those on methadone had a maximum of 12.8.
Higher birth weights have been found for infants in the buprenorphine group. One study noted a mean birth weight of 6.48 lb in a methadone group vs 7.17 lb in a buprenorphine group, a statistically significant difference.11 The birth weight of our patient’s daughter (8.19 lb) was higher than those reported in studies of women receiving buprenorphine and methadone.11,12
Hospital stays were shorter for neonates exposed to buprenorphine when compared to methadone.12 When methadone was used as maintenance therapy their hospital stays were between 8.1 and 19.7 days. On average, buprenorphine-exposed neonates were hospitalized between 6.8 and 10 days.9,11,12
THE TAKEAWAY
Physicians who prescribe or care for women who receive buprenorphine need to remain alert for the possibility of pregnancy. Assess your patient’s weight at each appointment. If you suspect she has become pregnant, address the issue with the patient and obtain consent for a pregnancy test. Although buprenorphine is a category C drug, patients who become pregnant should be made aware that several studies have found that buprenorphine can be used safely and effectively during pregnancy9-12 and it may be an option to continue the medication through delivery.
Because naloxone can trigger withdrawal symptoms in a fetus if a mother uses illicit opioids while pregnant, we recommend that naloxone be discontinued once pregnancy is discovered.
1. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Moral Wkly Rep. 2011;60:1487-1492.
2. Dattel BJ. Substance abuse in pregnancy. Semin Perinatol. 1990;14:179-187.
3. Kassim Z, Greenough A. Neonatal abstinence syndrome: Identification and management. Curr Paediatrics. 2006;16:172-175.
4. Finnegan LP, Kandall SR. Maternal and neonatal effects of drug dependence in pregnancy. In: Lowinson J, Ruiz P, Millman RB, et al, eds. Substance Abuse: A Comprehensive Textbook. 2nd ed. Baltimore, MD: Williams & Wilkins; 1992.
5. US Department of Health and Human Services. Drug Addiction Treatment Act of 2000. US Department of Health and Human Services Web site. Available at: http://buprenorphine.samhsa.gov/fulllaw.html. Accessed June 4, 2014.
6. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
7. Bell J, Byron G, Gibson A, et al. A pilot study of buprenorphine-naloxone combination tablet (Suboxone) in treatment of opioid dependence. Drug and Alcohol Rev. 2004;23:311-317.
8. Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106:56-60.
9. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320-2331.
10. Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70(2 suppl):S87-S101.
11. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69-78.
12. Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1-10.
THE CASE
We were treating a 23-year-old woman in our clinic for opioid dependence. She had begun using hydrocodone/acetaminophen, oxycodone, and heroin at age 17. Her parents and relatives had a history of alcohol and drug addiction and her brother had died from a heroin overdose.
The patient was taking buprenorphine/naloxone 12 mg/3 mg daily. She attended weekly counseling sessions at a community outreach center. We explained to her the potentially dangerous effects of buprenorphine/naloxone. Urine toxicology was negative for substances other than buprenorphine/naloxone.
Over 8 months, our patient gained 33 pounds and began wearing loose clothing to her appointments. When we asked her about it, she said that she had been “eating more bagels” lately.
What the patient wasn’t telling us was that she was pregnant. (We learned of her pregnancy only after she delivered.) In addition, she didn’t disclose to her obstetrician (OB) that she was taking buprenorphine/naloxone until she was nearly full term. At that point, the OB consulted maternal fetal medicine, and the buprenorphine/naloxone was continued through delivery. The patient had an uncomplicated spontaneous vaginal delivery of an 8.19 lb girl with an APGAR score of 8 at 1 minute and 8 again at 5 minutes.
Concerned about neonatal abstinence syndrome (NAS), which is characterized by tremors, increased body tone, feeding intolerance, vomiting, sweating, and fever, the healthcare team used the NAS scoring system to assess the newborn’s need for pharmacologic therapy. The newborn’s score at birth was 16/45. It then dropped to 11/45 indicating that she was experiencing mild withdrawal, but her symptoms—grunting, tachycardia, increased tone, tremors, irritability, and sweating—suggested she was experiencing severe withdrawal. The infant remained hospitalized for 29 days and received oral morphine titrated to her NAS score. The drug regimen for treatment/tapering was oral morphine given at 0.1 mg/kg/dose every 4 hours. This dose was lowered by 10% each time her NAS score was <8. At discharge, the infant’s NAS score had decreased to 3/45.
After discharge, the mother admitted to us that she concealed her pregnancy because she was afraid of being placed on methadone. She said she didn’t want to have to go to a clinic to receive the medication.
Continued good health. The child has since reached all of her developmental milestones appropriately and has normal height and weight.
DISCUSSION
Opioid abuse is an increasing cause of morbidity and mortality. In the United States, the number of deaths from opioid overdose is approaching that of motor vehicle accidents: approximately 100 deaths a day.1
The use of opioids by a pregnant woman can cause intrauterine growth retardation and preterm delivery.2 It also can result in withdrawal symptoms in the newborn,3 necessitating treatment guided by the NAS score. This score takes into consideration the metabolic, respiratory, central nervous system, and gastrointestinal symptoms of the infant at specified time intervals.4
Treatment options. For nonpregnant patients, opioid dependence typically is treated with methadone, an opioid agonist or buprenorphine, a partial opioid agonist; buprenorphine usually is prescribed as a combination medication that also contains naloxone, an opioid antagonist.
While methadone must be prescribed through licensed clinics, physicians meeting specific qualifications can prescribe buprenorphine or buprenorphine/naloxone in the office setting.5 Studies have supported the effectiveness of buprenorphine, alone or in combination with naloxone, in discouraging illicit opioid use.6-8
When the patient is pregnant… Methadone is the current standard of care for opioid-dependent patients who become pregnant.9 Buprenorphine/naloxone is currently a US Food and Drug Administration category C drug. However, recent studies have demonstrated the safety of buprenorphine without naloxone during pregnancy.10,11
The incidence and severity of NAS following treatment with buprenorphine is less than or comparable to methadone maintenance.10,11 The NAS score of 11 recorded in our patient’s case was comparable to those reported by Jones et al,9 who found neonates of women on buprenorphine had an average maximum NAS score of 11 and those on methadone had a maximum of 12.8.
Higher birth weights have been found for infants in the buprenorphine group. One study noted a mean birth weight of 6.48 lb in a methadone group vs 7.17 lb in a buprenorphine group, a statistically significant difference.11 The birth weight of our patient’s daughter (8.19 lb) was higher than those reported in studies of women receiving buprenorphine and methadone.11,12
Hospital stays were shorter for neonates exposed to buprenorphine when compared to methadone.12 When methadone was used as maintenance therapy their hospital stays were between 8.1 and 19.7 days. On average, buprenorphine-exposed neonates were hospitalized between 6.8 and 10 days.9,11,12
THE TAKEAWAY
Physicians who prescribe or care for women who receive buprenorphine need to remain alert for the possibility of pregnancy. Assess your patient’s weight at each appointment. If you suspect she has become pregnant, address the issue with the patient and obtain consent for a pregnancy test. Although buprenorphine is a category C drug, patients who become pregnant should be made aware that several studies have found that buprenorphine can be used safely and effectively during pregnancy9-12 and it may be an option to continue the medication through delivery.
Because naloxone can trigger withdrawal symptoms in a fetus if a mother uses illicit opioids while pregnant, we recommend that naloxone be discontinued once pregnancy is discovered.
THE CASE
We were treating a 23-year-old woman in our clinic for opioid dependence. She had begun using hydrocodone/acetaminophen, oxycodone, and heroin at age 17. Her parents and relatives had a history of alcohol and drug addiction and her brother had died from a heroin overdose.
The patient was taking buprenorphine/naloxone 12 mg/3 mg daily. She attended weekly counseling sessions at a community outreach center. We explained to her the potentially dangerous effects of buprenorphine/naloxone. Urine toxicology was negative for substances other than buprenorphine/naloxone.
Over 8 months, our patient gained 33 pounds and began wearing loose clothing to her appointments. When we asked her about it, she said that she had been “eating more bagels” lately.
What the patient wasn’t telling us was that she was pregnant. (We learned of her pregnancy only after she delivered.) In addition, she didn’t disclose to her obstetrician (OB) that she was taking buprenorphine/naloxone until she was nearly full term. At that point, the OB consulted maternal fetal medicine, and the buprenorphine/naloxone was continued through delivery. The patient had an uncomplicated spontaneous vaginal delivery of an 8.19 lb girl with an APGAR score of 8 at 1 minute and 8 again at 5 minutes.
Concerned about neonatal abstinence syndrome (NAS), which is characterized by tremors, increased body tone, feeding intolerance, vomiting, sweating, and fever, the healthcare team used the NAS scoring system to assess the newborn’s need for pharmacologic therapy. The newborn’s score at birth was 16/45. It then dropped to 11/45 indicating that she was experiencing mild withdrawal, but her symptoms—grunting, tachycardia, increased tone, tremors, irritability, and sweating—suggested she was experiencing severe withdrawal. The infant remained hospitalized for 29 days and received oral morphine titrated to her NAS score. The drug regimen for treatment/tapering was oral morphine given at 0.1 mg/kg/dose every 4 hours. This dose was lowered by 10% each time her NAS score was <8. At discharge, the infant’s NAS score had decreased to 3/45.
After discharge, the mother admitted to us that she concealed her pregnancy because she was afraid of being placed on methadone. She said she didn’t want to have to go to a clinic to receive the medication.
Continued good health. The child has since reached all of her developmental milestones appropriately and has normal height and weight.
DISCUSSION
Opioid abuse is an increasing cause of morbidity and mortality. In the United States, the number of deaths from opioid overdose is approaching that of motor vehicle accidents: approximately 100 deaths a day.1
The use of opioids by a pregnant woman can cause intrauterine growth retardation and preterm delivery.2 It also can result in withdrawal symptoms in the newborn,3 necessitating treatment guided by the NAS score. This score takes into consideration the metabolic, respiratory, central nervous system, and gastrointestinal symptoms of the infant at specified time intervals.4
Treatment options. For nonpregnant patients, opioid dependence typically is treated with methadone, an opioid agonist or buprenorphine, a partial opioid agonist; buprenorphine usually is prescribed as a combination medication that also contains naloxone, an opioid antagonist.
While methadone must be prescribed through licensed clinics, physicians meeting specific qualifications can prescribe buprenorphine or buprenorphine/naloxone in the office setting.5 Studies have supported the effectiveness of buprenorphine, alone or in combination with naloxone, in discouraging illicit opioid use.6-8
When the patient is pregnant… Methadone is the current standard of care for opioid-dependent patients who become pregnant.9 Buprenorphine/naloxone is currently a US Food and Drug Administration category C drug. However, recent studies have demonstrated the safety of buprenorphine without naloxone during pregnancy.10,11
The incidence and severity of NAS following treatment with buprenorphine is less than or comparable to methadone maintenance.10,11 The NAS score of 11 recorded in our patient’s case was comparable to those reported by Jones et al,9 who found neonates of women on buprenorphine had an average maximum NAS score of 11 and those on methadone had a maximum of 12.8.
Higher birth weights have been found for infants in the buprenorphine group. One study noted a mean birth weight of 6.48 lb in a methadone group vs 7.17 lb in a buprenorphine group, a statistically significant difference.11 The birth weight of our patient’s daughter (8.19 lb) was higher than those reported in studies of women receiving buprenorphine and methadone.11,12
Hospital stays were shorter for neonates exposed to buprenorphine when compared to methadone.12 When methadone was used as maintenance therapy their hospital stays were between 8.1 and 19.7 days. On average, buprenorphine-exposed neonates were hospitalized between 6.8 and 10 days.9,11,12
THE TAKEAWAY
Physicians who prescribe or care for women who receive buprenorphine need to remain alert for the possibility of pregnancy. Assess your patient’s weight at each appointment. If you suspect she has become pregnant, address the issue with the patient and obtain consent for a pregnancy test. Although buprenorphine is a category C drug, patients who become pregnant should be made aware that several studies have found that buprenorphine can be used safely and effectively during pregnancy9-12 and it may be an option to continue the medication through delivery.
Because naloxone can trigger withdrawal symptoms in a fetus if a mother uses illicit opioids while pregnant, we recommend that naloxone be discontinued once pregnancy is discovered.
1. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Moral Wkly Rep. 2011;60:1487-1492.
2. Dattel BJ. Substance abuse in pregnancy. Semin Perinatol. 1990;14:179-187.
3. Kassim Z, Greenough A. Neonatal abstinence syndrome: Identification and management. Curr Paediatrics. 2006;16:172-175.
4. Finnegan LP, Kandall SR. Maternal and neonatal effects of drug dependence in pregnancy. In: Lowinson J, Ruiz P, Millman RB, et al, eds. Substance Abuse: A Comprehensive Textbook. 2nd ed. Baltimore, MD: Williams & Wilkins; 1992.
5. US Department of Health and Human Services. Drug Addiction Treatment Act of 2000. US Department of Health and Human Services Web site. Available at: http://buprenorphine.samhsa.gov/fulllaw.html. Accessed June 4, 2014.
6. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
7. Bell J, Byron G, Gibson A, et al. A pilot study of buprenorphine-naloxone combination tablet (Suboxone) in treatment of opioid dependence. Drug and Alcohol Rev. 2004;23:311-317.
8. Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106:56-60.
9. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320-2331.
10. Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70(2 suppl):S87-S101.
11. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69-78.
12. Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1-10.
1. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Moral Wkly Rep. 2011;60:1487-1492.
2. Dattel BJ. Substance abuse in pregnancy. Semin Perinatol. 1990;14:179-187.
3. Kassim Z, Greenough A. Neonatal abstinence syndrome: Identification and management. Curr Paediatrics. 2006;16:172-175.
4. Finnegan LP, Kandall SR. Maternal and neonatal effects of drug dependence in pregnancy. In: Lowinson J, Ruiz P, Millman RB, et al, eds. Substance Abuse: A Comprehensive Textbook. 2nd ed. Baltimore, MD: Williams & Wilkins; 1992.
5. US Department of Health and Human Services. Drug Addiction Treatment Act of 2000. US Department of Health and Human Services Web site. Available at: http://buprenorphine.samhsa.gov/fulllaw.html. Accessed June 4, 2014.
6. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
7. Bell J, Byron G, Gibson A, et al. A pilot study of buprenorphine-naloxone combination tablet (Suboxone) in treatment of opioid dependence. Drug and Alcohol Rev. 2004;23:311-317.
8. Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106:56-60.
9. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320-2331.
10. Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70(2 suppl):S87-S101.
11. Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69-78.
12. Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1-10.
Can yoga reduce symptoms of anxiety and depression?
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.
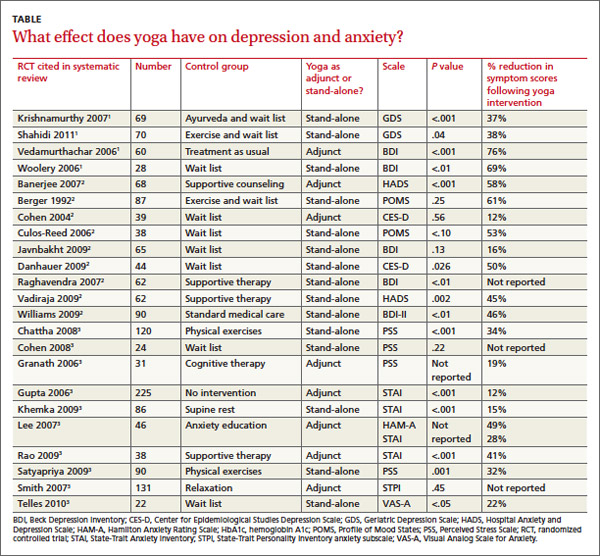
Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.

Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
Yes, yoga can reduce symptoms of anxiety and depression (strength of recommendation [SOR]: B, systematic reviews of randomized controlled trials [RCTs] with significant heterogeneity). Across multiple RCTs using varied yoga interventions and diverse study populations, yoga typically improves overall symptom scores for anxiety and depression by about 40%, both by itself and as an adjunctive treatment. It produces no reported harmful side effects.
EVIDENCE SUMMARY
Across 3 systematic reviews of yoga for depression, anxiety, and stress, yoga produced overall reductions of symptoms between 12% and 76%, with an average of 39% net reduction in symptom scores across measures (TABLE).1-3 The RCTs included in the systematic reviews were too heterogeneous to allow quantitative analyses of effect sizes.
Yoga found to significantly reduce depression symptoms
Two 2012 systematic reviews of yoga for depression evaluated 13 RCTs with a total of 782 participants, ages 18 to 80 years with mild to moderate depression. In the 12 RCTs that reported gender, 82% of participants were female; in 6 RCTs a total of 313 patients had cancer.1,2
The RCTs compared yoga to wait-list controls, counseling, education, exercise, or usual care. They evaluated yoga both as a stand-alone intervention and an adjunct to usual care. Yoga sessions varied from 1 hour weekly to 90 minutes daily over 2 to 24 weeks and included physical postures, relaxation, and breathing techniques.
Eight moderate- to high-quality RCTs with a total of 483 participants reported statistically significant reductions in depression symptoms in the yoga groups compared with control groups. In 3 RCTs, yoga was equivalent to wait-list controls; 2 RCTs showed results equivalent to exercise and superior to wait-list controls.
Yoga alleviates anxiety and stress without adverse effects
A 2012 systematic review of yoga for stress and anxiety evaluated 10 RCTs with a total of 813 heterogeneous participants, ages 18 to 76 years, including pregnant women, breast cancer patients, flood survivors, healthy volunteers, patients with chronic illnesses, perimenopausal women, adults with metabolic syndrome, and people working in finance, all with a range of anxiety and stress symptoms.3 The RCTs compared yoga, as an adjunctive or stand-alone treatment, with wait-list controls, relaxation, therapy, anxiety education, rest, or exercise. Yoga regimens varied from a single 20-minute session to 16 weeks of daily 1-hour sessions, with most regimens lasting 6 to 10 weeks.
Of the 10 RCTs reviewed, 7 moderate- to high-quality studies with a total of 627 participants found statistically significant reductions in anxiety and stress in yoga groups compared with control groups. Of the remaining 3 studies, 1 found yoga equivalent to cognitive therapy; 1 found a nonsignificant benefit for yoga compared with wait-list controls; and 1 found no improvement with either yoga or relaxation.

Study limitations included a range of symptom severity, variable type and length of yoga, lack of participant blinding, wait-list rather than active-treatment controls, and a lack of consistent long-term follow-up data. The RCTs didn’t report any adverse effects of yoga, and yoga is considered safe when taught by a competent instructor.3,4
RECOMMENDATIONS
The Institute for Clinical Systems Improvement and the Canadian Network for Mood and Anxiety Treatments recommend yoga as an effective adjunctive treatment to decrease the severity of depression symptoms.5,6
The Veterans Health Administration and the US Department of Defense recommend yoga as a potential adjunctive treatment to manage the hyperarousal symptoms of post-traumatic stress disorder (PTSD).7
The Work Loss Data Institute recommends yoga as an intervention for workers compensation conditions including occupational stress, major depressive disorder, PTSD, and other mental disorders.8
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
1. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117.
2. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
3. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
4. Brown RP, Gerbarg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II—clinical applications and guidelines. J Altern Complement Med. 2005;11:711-717.
5. Mitchell J, Trangle M, Degnan B, et al. Institute for Clinical Systems Improvement. Adult depression primary care. Available at: https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. Updated September 2013. Accessed March 6, 2014.
6. Ravindran AV, Lam RW, Filteau MJ, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord. 2009;117(suppl 1):S54-S64.
7. US Department of Veterans Affairs. VA/DoD clinical practice guideline for management of post-traumatic stress disorder and acute stress reaction. US Department of Veterans Affairs Web site. Available at: http://www.healthquality.va.gov/ptsd/. Accessed March 6, 2014.
8. Agency for Healthcare Research and Quality. Mental illness & stress. Agency for Healthcare Research and Quality Web site. Available at: http://www.guideline.gov/content.aspx?id=47588. Updated May 2011. Accessed June 17, 2014
Evidence-based answers from the Family Physicians Inquiries Network
Health Risks Associated with Tattoos and Body Piercing
From the West Virginia University School of Medicine, Morgantown, WV.
Abstract
- Objective: To review the health risks associated with tattoos and body piercing.
- Methods: Review of the literature.
- Results: Tattooing and piercing have become increasingly popular practices in the United States. There are important physical and behavioral risks associated with these forms of body modification. The most common complications from tattooing include skin infections and allergic reactions. Minor complications such as infection and bleeding occur frequently with piercings, but major complications have also been reported. Tattoos and piercings appear to be a marker for risk-taking behavior.
- Conclusion: Clinicians should understand the potential complications of these procedures and be able to counsel patients on how to reduce their health risks.
Tattoos and piercings are ancient practices of body modification that have gained widespread acceptance in modern society, particularly among young adults. Tattoos involve the insertion of colored pigment into the dermal layer of the skin with the goal of creating a permanent marking. They are commonly applied using an electrically powered handheld tattoo machine that moves a needle up and down to inject ink through the epidermis and deposit a drop of ink into the dermis [1]. The cells of the dermis are more stable compared with those of the epidermis, so the ink will mostly stay in place for a person’s lifetime [2].
Body piercing is defined as the insertion of a needle or specially designed piercing gun to create a fistula-like opening into either cartilage or skin for the introduction of decorative ornaments [3]. These ornaments can include jewelry, plastic or wood plugs, beads, or pearls. Body piercing has been part of ritualistic or cultural practices for centuries but is rapidly becoming a worldwide mainstream fashion trend, especially among young women aged 17 to 25 years [4]. Ear piercing has become so well accepted that most studies of body piercing do not include earlobe piercing [5].
There are important physical and behavioral risks associated with body modification. In this paper, we review the epidemiology, potential complications, and behavioral factors associated with tattoos and piercings.
Epidemiology
Tattoos are increasingly popular, with a 2012 Harris poll indicating that 1 in 5 adults has at least 1 tattoo [6], up from 14% in 2008 [7]. In the 2012 poll, adults aged 30 to 39 are most likely to have a tattoo (38%) compared to both those younger (30% of those 25–29 and 22% of those 18–24) and older (27% of those 40–49, 11% of those 50–64 and just 5% of those 65 and older). Women are slightly more likely than men, for the first time since this question was first asked, to have a tattoo (23% versus 19% in men). Current US body piercing rates are approximately 36% [3]. Body piercing is more popular among women than in men [8]. Among adolescents, body piercing is performed considerably earlier than tattooing [9]. The head area is the favored location for piercing, while the most common location for a tattoo is the limb [10].
Associated Health Risks
Tattoos
The most common complications that result from tattooing are skin infections and allergic reactions to the tattoo ink. Extensive skin puncturing can result in bleeding and prolonged leaking of serosanguinous fluid. Pyodermal infections can include temporary inflammation at the site of needle puncture, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis [11]. Unsterile equipment and needles can transmit infectious diseases such as hepatitis [2]. Human immunodeficiency virus is theoretically transmissible this way, but no cases of HIV infection caused by tattooing have been documented [12].
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo ink, especially red ink, but also to chromium in green ink, cadmium in yellow ink, and cobalt in blue ink [13]. The reaction can manifest as either allergic contact dermatitis or photo-allergic dermatitis. Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma. Other possible but less common conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy [12].
A recent outbreak of nontuburculous nycobacterial infection was linked to contaminated tattoo inks [14]. Contamination can occur during the manufacturing process due to use of contaminated ingredients or when inks are diluted with nonsterile water by tattoo artists [15]. Investigations of 22 cases of tattoo-associated NTM skin infections in 4 states that occurred during 2011–2012 found that ink was contaminated with NTM before use [16]. M. chelonae, one of several disease-causing NTM species, can cause lung disease, joint infection, eye problems and other organ infections. These infections can be difficult to diagnose and can require treatment lasting 6 months or more [15].
Tattoo laws and regulations vary by state. The inks and ink colorings (pigments) used for tattoos are subject to regulation by the US Food and Drug Administration as cosmetics and color additives. However, the FDA has not traditionally enforced its authority over tattoo inks. The FDA encourages reporting of tattoo-associated complications to its MedWatch program (www.fda.gov/Safety/MedWatch/).
Malignancies reported to develop within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, and dermatofibrosarcoma protuberans [17,18]. These malignancies may be coincidental, but carcinogenicity of the tattoo is as yet unknown. Another concern is that a malignancy within a tattoo is more difficult to identify on skin examination [11,17].
Rarely, tattoos or permanent makeup might cause swelling or burning in the affected areas during magnetic resonance imaging (MRI) exams. The metallic ferric acid pigments used in tattoos can conduct heat on the skin during MRI, resulting in traumatic burns [19]. This has also been reported to occur with tattoos with nonferrous pigments. In some cases, tattoo pigments can interfere with the quality of the image, such as when a person with permanent eyeliner has an MRI of the eye [19].
Patients may self-administer tattoos using sewing needles, forks, paper clips, or pens, and colorants may include charcoal, soot, mascara, or ink. The use of unprofessional tattooists and piercers, who often have limited knowledge of health and hygiene precautions, is more likely to lead to complications [10].
Piercings
While most body piercings are not problematic, the potential for localized infections as well as associated systemic disease is present as long as the piercing site remains open [20]. Bacterial skin infections at or near the site are the most commonly reported complication of body piercings, with causative organisms primarily consisting of Staphylococcus, group A beta-hemolytic Streptococcus, and Pseudomonas [21]. Contributing to the health risks of piercing is the reluctance of patients to seek qualified medical intervention when initial site irritation, pain, or oozing occurs [21].
Systemic infections have been reported. More than 25 infective endocarditis cases in the past decade have come from tongue, navel, earlobe, lower lip, and nipple piercings [21]. Infective endocarditis should be considered in individuals with a new piercing (ie, up to 4 months), with or without a history of congenital heart disease, who present with unexplained fever, night chills, weakness, myalgia, arthralgia, lethargy, or malaise [22]. General complications include allergic contact dermatitis (eg, from nickel or latex), bleeding, scarring and keloid formation, nerve damage, and interference with medical procedures such as intubation and blood/organ donation [20].
Oral piercings may lead to difficulty speaking and eating, excessive salivation, and dental problems. Oral and nasal piercings may be aspirated or become embedded, requiring surgical removal. Tongue piercing, usually performed without anesthesia, may cause damage to teeth and gums, including dental fractures [23] and changes in chewing and speech. Because of the tongue’s vascular nature, prolonged bleeding can result if vessels are punctured during the piercing procedure. In addition, the technique for inserting tongue jewelry may abrade or fracture anterior dentition, and digital manipulation of the jewelry can significantly increase the potential for infection [24]. In fact, complications arising from oral piercing are so numerous—and in some cases life-threatening—that the American Dental Association has issued a formal statement opposing the practice [24].
Behavioral Risk
Several studies have found that those with body modifications engaged in earlier or more frequent sexual activity and had a greater number of sexual partners [26–28]. A study by Deschesnes and colleagues [29] reported that certain “externalized” risk behaviors were more commonly associated with tattooed and pierced youth than with their unmodified counterparts, including the use of drugs, gang affiliation, school truancy, and problem gambling. Other studies of high school youth have found that tattooing was significantly and independently associated with other high-risk behaviors, including sexual intercourse, binge drinking, smoking, marijuana use, gang membership, truancy, and school failure [30]. However, a survey of college students found that, compared to individuals with no body art, individuals with 1 tattoo and less than 4 piercings had no greater likelihood to engage in high-risk behaviors [31].
Conversely, some researchers have attempted to show a positive association for body modification. In a study of women with eating disorders, the authors suggested that body piercing could be seen as reflecting a positive attitude towards the body, an expression of self-care [32]. In addition, people with piercings are more likely to give attention to their physical appearance and are less likely to be overweight than people without piercings [33].
Stirn and Hinz [34] concluded that most people who partake in body modification clearly do not do it because they have any psychological problems. However, for a few, modifications may be utilized as a convenient means to either realize psychopathological inclinations, such as self-injury, or to overcome psychological traumas. The prevalence of self-injury is unknown, though it is believed to be a growing problem. While self-injury is believed to be a low-lethality behavior, teens who hurt themselves are at increased risk for suicide related to their underlying anxiety or depression. Moreover, self-harmers report that they often had their skin tattooed or body pierced to help overcome a negative experience, or simply to experience physical pain. Another clue that self-harm and piercing/tattooing might in some cases be linked derives from the fact that many of the self-harmers said they had ceased cutting themselves after obtaining their first piercing or tattoo [35]. The increasing incidence makes deliberate self-harm a problem that all health care providers dealing with adolescents are likely to encounter.
Given the link between body modification and “externalized risk behaviors” in young people, tattooing and body piercing may serve as clinical markers for health care professionals, potentially identifying those who may be involved in activities that hinder their health and development [29]. For example, closer examination of teens who wear long sleeves or clothing inappropriate for weather could reveal cuts, burns, carvings, or bruises that are self-inflicted.
Patients are more likely to discuss the issue of body art if the clinician does not speak or act judgmentally [1]. Practitioners who are concerned that their tattooed patient might be self-injuring or engaging in other risky behaviors should invite a discussion with the patient, perhaps using general terms, such as “Sometimes people may get involved in self-injury and don’t know where to turn for help. I will try to help you if you are ever worried about that.” As always, if the clinician suspects a patient is engaged in self-harming activities, an immediate referral should be made for mental health evaluation and any necessary intervention.
Preventatively, clinicians should provide targeted and repeated education to transmit the message of effective decision-making and evaluation of risks to children in the early elementary grades, since some students start to obtain body art as early as fifth grade.
Regret and Removal
Over time, many individuals regret getting tattoos and wish to have them removed [35]. In some cases, delayed complications, like the development of allergic, hypersensitivity, or granulomatous reactions, require tattoo removal. On average, tattoo regret occurs 14 years after tattooing and by the age of 40 years.
Removal is more painful and laborious than the tattooing itself, and complete removal, with no scarring, is often not possible. The American Society for Dermatologic Surgery reports that in 2011, its doctors performed nearly 100,000 tattoo removal procedures, up from the 86,000 performed in 2010 [36]. Pulsed lasers have been used to remove tattoos for more than 20 years. With this procedure, pulses of high-intensity laser energy pass through the epidermis and are selectively absorbed by the tattoo pigment. The laser breaks the pigment into smaller particles, which may be metabolized or excreted by the body, or transported to and stored in lymph nodes or other tissues [37].
Other removal techniques include dermabrasion and surgical excision. Do-it-yourself tattoo removal ointments and creams can be purchased online, but they have not been approved by the FDA and there is no clinical evidence that they work. Furthermore, tattoo removal ointments and creams may cause unexpected reactions, such as rashes, burning, scarring, or changes in skin pigmentation [37].
Clinician’s Role
Body art provides a window into an individual’s uniqueness, and acknowledging body modifications can help build trust and develop the physician/patient relationship. The health professional armed with knowledge about body modifications can forge more functional relationships, obtain critical historical information, and provide better treatment and referral for this population [5].
Having a clinician who is a trusted, nonjudgmental source of information and intervention for patients who choose body art will reduce the health risk of complications associated with tattooing and piercing [1]. Unfortunately, only 14% of the population identify health care professionals as a common resource for information on body modification. Instead, young adults endorsed friends (82%), body piercing shops (61%), and tattoo shops (51%) as their top information sources. Clinicians should help patients make informed decisions about body art and counsel them about the importance of universal precautions [35].
Corresponding author: Susannah Grimm Poe, EdD, 176 Gilboa Rd., Fairmont, WV 26554, [email protected].
Financial disclosures: None.
1. Schmidt R, Armstrong M. Tattooing in adolescents and adults. Up to Date 2013. Available at www.uptodate.com.
2. US Food and Drug Administration. Tattoos and permanent makeup. 2009. Available at www.fda.gov/Cosmetics/ProductandIngredientSafety/ProductInformation/ucm108530.htm.
3. Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol 2007;25:398-406.
4. Schorzman CM, Gold MA, Downs JS, Murray PJ. Body art: attitudes and practices regarding body piercing among urban undergraduates. J Am Osteopath Assoc 2007;107:432-8.
5. Urdang, M, Mallek, J, Mallon, W. Tattoos and piercings: a review for the emergency physician. West J Emerg Med 2011;12:393–8.
6. Braverman S. One in five US adults now has a tattoo. New York: Harris Interactive; 2012.
7. Corso RA. Nationwide Harris poll. New York: Harris Interactive; 2008.
8. Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol 2006;55:413–21.
9. Antoszewski B, Sitek A, Fijałkowska M, et al. Tattooing and body piercing--what motivates you to do it? Int J Soc Psychiatry. 2010;56:471–9.
10. Quaranta A, Napoli C, Fasano F, et al. Body piercing and tattoos: a survey on young adults’ knowledge of the risks and practices in body art. BMC Public Health 2011;11:774.
11. Glassy C, Glassy M, Aldasoiuqi A. Tattooing: medical uses and problems. Clev Clin J Med 2012;79:761–70.
12. Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992;13:7–17.
13. Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
14. Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. N Engl J Med 2012;367:1020–4.
15. CDC. The hidden dangers of getting inked. Available at http://blogs.cdc.gov/publichealthmatters/2012/08/the-hidden-dangers-of-getting-inked.
16. LeBlanc PM, Hollinger KA, Klontz KC. Tattoo ink-related infections—awareness, diagnosis, reporting, and prevention. N Engl J Med 2012;367:985–7.
17. Shinohara MM, Nguyen J, Gardner J, Rosenbach M, Elenitsas R. The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol 2012;39:1110–8.
18. Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011;10:837–42.
19. Tope WD, Shellock FG. Magnetic resonance imaging and permanent cosmetics (tattoos): survey of complications and adverse events. J Magn Reson Imaging 2002;15:180–4.
20. Holbrook, J, Minocha J, Laumann A. Body piercing: complications and prevention of health risks. Am J Clin Dermatol 2012;13:1–17.
21. Hogan L, Armstrong ML. Body piercing: more than skin deep. Skin therapy letter. Available at www.skintherapyletter.com/2009/14.7/2.html.
22. Raja SG, Shad SK, Dreyfus GD. Body piercing: a rare cause of mitral valve endocarditis. J Heart Valve Dis 2004;13:854.
23. Botchway C, Kuc I. Tongue piercing and associated tooth fracture. J Can Dent Assoc 1998;64:803–5.
24. American Dental Association. Statement on intraoral/perioral piercing and tongue splitting. 2012. Available at www.ada.org/prof/resources/positions/statements/piercing.asp.
25. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet 2003;361:1205–15.
26. Koch R, Roberts AE, Armstrong ML, Owen DC. Body art, deviance, and American college students. Soc Sci J 2010;47:151–61.
27. Gueguen N. Tattoos, piercings, and sexual activity. Social Behav Personality 2012;40:1543–7.
28. Skegg K, Nada-Raja S, Paul C, et al. Body piercing, personality, and sexual behavior. Arch Sex Behav 2007; 36:47–54.
29. Deschesnes M, Fines P, Demers S. Are tattooing and body piercing indicators of risk-taking behaviours among high school students? J Adolesc 2006; 29:379–93.
30. Roberts TA, Ryan SA. Tattooing and high-risk behavior in adolescents. Pediatrics 2002;110:1058–63.
31. Owen DC, Armstrong ML, Koch JR, Roberts AE. College students with body art: well-being or high-risk behavior? J Psychosoc Nurs Ment Health Serv 2013;51:20–8.
32. Claes L, Vandereycken W, Vertommen H. Self-care versus self-harm: piercing, tattooing, and self-injuring in eating disorders. Eur Eat Disord Rev 2004;13:11–18.
33. Hicinbothem J, Gonsalves S, Lester D. Body modification and suicidal behavior. Death Stud 2006; 30:351–63.
34. Stirn A, Hinz A. Tattoos, body piercings, and self-injury: Is there a connection? Psychotherapy Res 2008;18:326–33.
35. Burris K, Kim K. Tattoo removal. Clin Dermatol 2007;25:388–92.
36. American Society for Dermatologic Surgery. Unwanted tattoos. Available at www.asds.net/tattooremovalinformation.aspx.
37. US Food and Drug Administration. Inked and regretful: removing tattoos. 2013. Available at www.fda.gov/ForConsumers/ucm336842.htm.
38. Meltzer DI. Complications of body piercing. Am Fam Physician 2005;72:2029–34.
From the West Virginia University School of Medicine, Morgantown, WV.
Abstract
- Objective: To review the health risks associated with tattoos and body piercing.
- Methods: Review of the literature.
- Results: Tattooing and piercing have become increasingly popular practices in the United States. There are important physical and behavioral risks associated with these forms of body modification. The most common complications from tattooing include skin infections and allergic reactions. Minor complications such as infection and bleeding occur frequently with piercings, but major complications have also been reported. Tattoos and piercings appear to be a marker for risk-taking behavior.
- Conclusion: Clinicians should understand the potential complications of these procedures and be able to counsel patients on how to reduce their health risks.
Tattoos and piercings are ancient practices of body modification that have gained widespread acceptance in modern society, particularly among young adults. Tattoos involve the insertion of colored pigment into the dermal layer of the skin with the goal of creating a permanent marking. They are commonly applied using an electrically powered handheld tattoo machine that moves a needle up and down to inject ink through the epidermis and deposit a drop of ink into the dermis [1]. The cells of the dermis are more stable compared with those of the epidermis, so the ink will mostly stay in place for a person’s lifetime [2].
Body piercing is defined as the insertion of a needle or specially designed piercing gun to create a fistula-like opening into either cartilage or skin for the introduction of decorative ornaments [3]. These ornaments can include jewelry, plastic or wood plugs, beads, or pearls. Body piercing has been part of ritualistic or cultural practices for centuries but is rapidly becoming a worldwide mainstream fashion trend, especially among young women aged 17 to 25 years [4]. Ear piercing has become so well accepted that most studies of body piercing do not include earlobe piercing [5].
There are important physical and behavioral risks associated with body modification. In this paper, we review the epidemiology, potential complications, and behavioral factors associated with tattoos and piercings.
Epidemiology
Tattoos are increasingly popular, with a 2012 Harris poll indicating that 1 in 5 adults has at least 1 tattoo [6], up from 14% in 2008 [7]. In the 2012 poll, adults aged 30 to 39 are most likely to have a tattoo (38%) compared to both those younger (30% of those 25–29 and 22% of those 18–24) and older (27% of those 40–49, 11% of those 50–64 and just 5% of those 65 and older). Women are slightly more likely than men, for the first time since this question was first asked, to have a tattoo (23% versus 19% in men). Current US body piercing rates are approximately 36% [3]. Body piercing is more popular among women than in men [8]. Among adolescents, body piercing is performed considerably earlier than tattooing [9]. The head area is the favored location for piercing, while the most common location for a tattoo is the limb [10].
Associated Health Risks
Tattoos
The most common complications that result from tattooing are skin infections and allergic reactions to the tattoo ink. Extensive skin puncturing can result in bleeding and prolonged leaking of serosanguinous fluid. Pyodermal infections can include temporary inflammation at the site of needle puncture, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis [11]. Unsterile equipment and needles can transmit infectious diseases such as hepatitis [2]. Human immunodeficiency virus is theoretically transmissible this way, but no cases of HIV infection caused by tattooing have been documented [12].
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo ink, especially red ink, but also to chromium in green ink, cadmium in yellow ink, and cobalt in blue ink [13]. The reaction can manifest as either allergic contact dermatitis or photo-allergic dermatitis. Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma. Other possible but less common conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy [12].
A recent outbreak of nontuburculous nycobacterial infection was linked to contaminated tattoo inks [14]. Contamination can occur during the manufacturing process due to use of contaminated ingredients or when inks are diluted with nonsterile water by tattoo artists [15]. Investigations of 22 cases of tattoo-associated NTM skin infections in 4 states that occurred during 2011–2012 found that ink was contaminated with NTM before use [16]. M. chelonae, one of several disease-causing NTM species, can cause lung disease, joint infection, eye problems and other organ infections. These infections can be difficult to diagnose and can require treatment lasting 6 months or more [15].
Tattoo laws and regulations vary by state. The inks and ink colorings (pigments) used for tattoos are subject to regulation by the US Food and Drug Administration as cosmetics and color additives. However, the FDA has not traditionally enforced its authority over tattoo inks. The FDA encourages reporting of tattoo-associated complications to its MedWatch program (www.fda.gov/Safety/MedWatch/).
Malignancies reported to develop within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, and dermatofibrosarcoma protuberans [17,18]. These malignancies may be coincidental, but carcinogenicity of the tattoo is as yet unknown. Another concern is that a malignancy within a tattoo is more difficult to identify on skin examination [11,17].
Rarely, tattoos or permanent makeup might cause swelling or burning in the affected areas during magnetic resonance imaging (MRI) exams. The metallic ferric acid pigments used in tattoos can conduct heat on the skin during MRI, resulting in traumatic burns [19]. This has also been reported to occur with tattoos with nonferrous pigments. In some cases, tattoo pigments can interfere with the quality of the image, such as when a person with permanent eyeliner has an MRI of the eye [19].
Patients may self-administer tattoos using sewing needles, forks, paper clips, or pens, and colorants may include charcoal, soot, mascara, or ink. The use of unprofessional tattooists and piercers, who often have limited knowledge of health and hygiene precautions, is more likely to lead to complications [10].
Piercings
While most body piercings are not problematic, the potential for localized infections as well as associated systemic disease is present as long as the piercing site remains open [20]. Bacterial skin infections at or near the site are the most commonly reported complication of body piercings, with causative organisms primarily consisting of Staphylococcus, group A beta-hemolytic Streptococcus, and Pseudomonas [21]. Contributing to the health risks of piercing is the reluctance of patients to seek qualified medical intervention when initial site irritation, pain, or oozing occurs [21].
Systemic infections have been reported. More than 25 infective endocarditis cases in the past decade have come from tongue, navel, earlobe, lower lip, and nipple piercings [21]. Infective endocarditis should be considered in individuals with a new piercing (ie, up to 4 months), with or without a history of congenital heart disease, who present with unexplained fever, night chills, weakness, myalgia, arthralgia, lethargy, or malaise [22]. General complications include allergic contact dermatitis (eg, from nickel or latex), bleeding, scarring and keloid formation, nerve damage, and interference with medical procedures such as intubation and blood/organ donation [20].
Oral piercings may lead to difficulty speaking and eating, excessive salivation, and dental problems. Oral and nasal piercings may be aspirated or become embedded, requiring surgical removal. Tongue piercing, usually performed without anesthesia, may cause damage to teeth and gums, including dental fractures [23] and changes in chewing and speech. Because of the tongue’s vascular nature, prolonged bleeding can result if vessels are punctured during the piercing procedure. In addition, the technique for inserting tongue jewelry may abrade or fracture anterior dentition, and digital manipulation of the jewelry can significantly increase the potential for infection [24]. In fact, complications arising from oral piercing are so numerous—and in some cases life-threatening—that the American Dental Association has issued a formal statement opposing the practice [24].
Behavioral Risk
Several studies have found that those with body modifications engaged in earlier or more frequent sexual activity and had a greater number of sexual partners [26–28]. A study by Deschesnes and colleagues [29] reported that certain “externalized” risk behaviors were more commonly associated with tattooed and pierced youth than with their unmodified counterparts, including the use of drugs, gang affiliation, school truancy, and problem gambling. Other studies of high school youth have found that tattooing was significantly and independently associated with other high-risk behaviors, including sexual intercourse, binge drinking, smoking, marijuana use, gang membership, truancy, and school failure [30]. However, a survey of college students found that, compared to individuals with no body art, individuals with 1 tattoo and less than 4 piercings had no greater likelihood to engage in high-risk behaviors [31].
Conversely, some researchers have attempted to show a positive association for body modification. In a study of women with eating disorders, the authors suggested that body piercing could be seen as reflecting a positive attitude towards the body, an expression of self-care [32]. In addition, people with piercings are more likely to give attention to their physical appearance and are less likely to be overweight than people without piercings [33].
Stirn and Hinz [34] concluded that most people who partake in body modification clearly do not do it because they have any psychological problems. However, for a few, modifications may be utilized as a convenient means to either realize psychopathological inclinations, such as self-injury, or to overcome psychological traumas. The prevalence of self-injury is unknown, though it is believed to be a growing problem. While self-injury is believed to be a low-lethality behavior, teens who hurt themselves are at increased risk for suicide related to their underlying anxiety or depression. Moreover, self-harmers report that they often had their skin tattooed or body pierced to help overcome a negative experience, or simply to experience physical pain. Another clue that self-harm and piercing/tattooing might in some cases be linked derives from the fact that many of the self-harmers said they had ceased cutting themselves after obtaining their first piercing or tattoo [35]. The increasing incidence makes deliberate self-harm a problem that all health care providers dealing with adolescents are likely to encounter.
Given the link between body modification and “externalized risk behaviors” in young people, tattooing and body piercing may serve as clinical markers for health care professionals, potentially identifying those who may be involved in activities that hinder their health and development [29]. For example, closer examination of teens who wear long sleeves or clothing inappropriate for weather could reveal cuts, burns, carvings, or bruises that are self-inflicted.
Patients are more likely to discuss the issue of body art if the clinician does not speak or act judgmentally [1]. Practitioners who are concerned that their tattooed patient might be self-injuring or engaging in other risky behaviors should invite a discussion with the patient, perhaps using general terms, such as “Sometimes people may get involved in self-injury and don’t know where to turn for help. I will try to help you if you are ever worried about that.” As always, if the clinician suspects a patient is engaged in self-harming activities, an immediate referral should be made for mental health evaluation and any necessary intervention.
Preventatively, clinicians should provide targeted and repeated education to transmit the message of effective decision-making and evaluation of risks to children in the early elementary grades, since some students start to obtain body art as early as fifth grade.
Regret and Removal
Over time, many individuals regret getting tattoos and wish to have them removed [35]. In some cases, delayed complications, like the development of allergic, hypersensitivity, or granulomatous reactions, require tattoo removal. On average, tattoo regret occurs 14 years after tattooing and by the age of 40 years.
Removal is more painful and laborious than the tattooing itself, and complete removal, with no scarring, is often not possible. The American Society for Dermatologic Surgery reports that in 2011, its doctors performed nearly 100,000 tattoo removal procedures, up from the 86,000 performed in 2010 [36]. Pulsed lasers have been used to remove tattoos for more than 20 years. With this procedure, pulses of high-intensity laser energy pass through the epidermis and are selectively absorbed by the tattoo pigment. The laser breaks the pigment into smaller particles, which may be metabolized or excreted by the body, or transported to and stored in lymph nodes or other tissues [37].
Other removal techniques include dermabrasion and surgical excision. Do-it-yourself tattoo removal ointments and creams can be purchased online, but they have not been approved by the FDA and there is no clinical evidence that they work. Furthermore, tattoo removal ointments and creams may cause unexpected reactions, such as rashes, burning, scarring, or changes in skin pigmentation [37].
Clinician’s Role
Body art provides a window into an individual’s uniqueness, and acknowledging body modifications can help build trust and develop the physician/patient relationship. The health professional armed with knowledge about body modifications can forge more functional relationships, obtain critical historical information, and provide better treatment and referral for this population [5].
Having a clinician who is a trusted, nonjudgmental source of information and intervention for patients who choose body art will reduce the health risk of complications associated with tattooing and piercing [1]. Unfortunately, only 14% of the population identify health care professionals as a common resource for information on body modification. Instead, young adults endorsed friends (82%), body piercing shops (61%), and tattoo shops (51%) as their top information sources. Clinicians should help patients make informed decisions about body art and counsel them about the importance of universal precautions [35].
Corresponding author: Susannah Grimm Poe, EdD, 176 Gilboa Rd., Fairmont, WV 26554, [email protected].
Financial disclosures: None.
From the West Virginia University School of Medicine, Morgantown, WV.
Abstract
- Objective: To review the health risks associated with tattoos and body piercing.
- Methods: Review of the literature.
- Results: Tattooing and piercing have become increasingly popular practices in the United States. There are important physical and behavioral risks associated with these forms of body modification. The most common complications from tattooing include skin infections and allergic reactions. Minor complications such as infection and bleeding occur frequently with piercings, but major complications have also been reported. Tattoos and piercings appear to be a marker for risk-taking behavior.
- Conclusion: Clinicians should understand the potential complications of these procedures and be able to counsel patients on how to reduce their health risks.
Tattoos and piercings are ancient practices of body modification that have gained widespread acceptance in modern society, particularly among young adults. Tattoos involve the insertion of colored pigment into the dermal layer of the skin with the goal of creating a permanent marking. They are commonly applied using an electrically powered handheld tattoo machine that moves a needle up and down to inject ink through the epidermis and deposit a drop of ink into the dermis [1]. The cells of the dermis are more stable compared with those of the epidermis, so the ink will mostly stay in place for a person’s lifetime [2].
Body piercing is defined as the insertion of a needle or specially designed piercing gun to create a fistula-like opening into either cartilage or skin for the introduction of decorative ornaments [3]. These ornaments can include jewelry, plastic or wood plugs, beads, or pearls. Body piercing has been part of ritualistic or cultural practices for centuries but is rapidly becoming a worldwide mainstream fashion trend, especially among young women aged 17 to 25 years [4]. Ear piercing has become so well accepted that most studies of body piercing do not include earlobe piercing [5].
There are important physical and behavioral risks associated with body modification. In this paper, we review the epidemiology, potential complications, and behavioral factors associated with tattoos and piercings.
Epidemiology
Tattoos are increasingly popular, with a 2012 Harris poll indicating that 1 in 5 adults has at least 1 tattoo [6], up from 14% in 2008 [7]. In the 2012 poll, adults aged 30 to 39 are most likely to have a tattoo (38%) compared to both those younger (30% of those 25–29 and 22% of those 18–24) and older (27% of those 40–49, 11% of those 50–64 and just 5% of those 65 and older). Women are slightly more likely than men, for the first time since this question was first asked, to have a tattoo (23% versus 19% in men). Current US body piercing rates are approximately 36% [3]. Body piercing is more popular among women than in men [8]. Among adolescents, body piercing is performed considerably earlier than tattooing [9]. The head area is the favored location for piercing, while the most common location for a tattoo is the limb [10].
Associated Health Risks
Tattoos
The most common complications that result from tattooing are skin infections and allergic reactions to the tattoo ink. Extensive skin puncturing can result in bleeding and prolonged leaking of serosanguinous fluid. Pyodermal infections can include temporary inflammation at the site of needle puncture, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis [11]. Unsterile equipment and needles can transmit infectious diseases such as hepatitis [2]. Human immunodeficiency virus is theoretically transmissible this way, but no cases of HIV infection caused by tattooing have been documented [12].
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo ink, especially red ink, but also to chromium in green ink, cadmium in yellow ink, and cobalt in blue ink [13]. The reaction can manifest as either allergic contact dermatitis or photo-allergic dermatitis. Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma. Other possible but less common conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy [12].
A recent outbreak of nontuburculous nycobacterial infection was linked to contaminated tattoo inks [14]. Contamination can occur during the manufacturing process due to use of contaminated ingredients or when inks are diluted with nonsterile water by tattoo artists [15]. Investigations of 22 cases of tattoo-associated NTM skin infections in 4 states that occurred during 2011–2012 found that ink was contaminated with NTM before use [16]. M. chelonae, one of several disease-causing NTM species, can cause lung disease, joint infection, eye problems and other organ infections. These infections can be difficult to diagnose and can require treatment lasting 6 months or more [15].
Tattoo laws and regulations vary by state. The inks and ink colorings (pigments) used for tattoos are subject to regulation by the US Food and Drug Administration as cosmetics and color additives. However, the FDA has not traditionally enforced its authority over tattoo inks. The FDA encourages reporting of tattoo-associated complications to its MedWatch program (www.fda.gov/Safety/MedWatch/).
Malignancies reported to develop within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, and dermatofibrosarcoma protuberans [17,18]. These malignancies may be coincidental, but carcinogenicity of the tattoo is as yet unknown. Another concern is that a malignancy within a tattoo is more difficult to identify on skin examination [11,17].
Rarely, tattoos or permanent makeup might cause swelling or burning in the affected areas during magnetic resonance imaging (MRI) exams. The metallic ferric acid pigments used in tattoos can conduct heat on the skin during MRI, resulting in traumatic burns [19]. This has also been reported to occur with tattoos with nonferrous pigments. In some cases, tattoo pigments can interfere with the quality of the image, such as when a person with permanent eyeliner has an MRI of the eye [19].
Patients may self-administer tattoos using sewing needles, forks, paper clips, or pens, and colorants may include charcoal, soot, mascara, or ink. The use of unprofessional tattooists and piercers, who often have limited knowledge of health and hygiene precautions, is more likely to lead to complications [10].
Piercings
While most body piercings are not problematic, the potential for localized infections as well as associated systemic disease is present as long as the piercing site remains open [20]. Bacterial skin infections at or near the site are the most commonly reported complication of body piercings, with causative organisms primarily consisting of Staphylococcus, group A beta-hemolytic Streptococcus, and Pseudomonas [21]. Contributing to the health risks of piercing is the reluctance of patients to seek qualified medical intervention when initial site irritation, pain, or oozing occurs [21].
Systemic infections have been reported. More than 25 infective endocarditis cases in the past decade have come from tongue, navel, earlobe, lower lip, and nipple piercings [21]. Infective endocarditis should be considered in individuals with a new piercing (ie, up to 4 months), with or without a history of congenital heart disease, who present with unexplained fever, night chills, weakness, myalgia, arthralgia, lethargy, or malaise [22]. General complications include allergic contact dermatitis (eg, from nickel or latex), bleeding, scarring and keloid formation, nerve damage, and interference with medical procedures such as intubation and blood/organ donation [20].
Oral piercings may lead to difficulty speaking and eating, excessive salivation, and dental problems. Oral and nasal piercings may be aspirated or become embedded, requiring surgical removal. Tongue piercing, usually performed without anesthesia, may cause damage to teeth and gums, including dental fractures [23] and changes in chewing and speech. Because of the tongue’s vascular nature, prolonged bleeding can result if vessels are punctured during the piercing procedure. In addition, the technique for inserting tongue jewelry may abrade or fracture anterior dentition, and digital manipulation of the jewelry can significantly increase the potential for infection [24]. In fact, complications arising from oral piercing are so numerous—and in some cases life-threatening—that the American Dental Association has issued a formal statement opposing the practice [24].
Behavioral Risk
Several studies have found that those with body modifications engaged in earlier or more frequent sexual activity and had a greater number of sexual partners [26–28]. A study by Deschesnes and colleagues [29] reported that certain “externalized” risk behaviors were more commonly associated with tattooed and pierced youth than with their unmodified counterparts, including the use of drugs, gang affiliation, school truancy, and problem gambling. Other studies of high school youth have found that tattooing was significantly and independently associated with other high-risk behaviors, including sexual intercourse, binge drinking, smoking, marijuana use, gang membership, truancy, and school failure [30]. However, a survey of college students found that, compared to individuals with no body art, individuals with 1 tattoo and less than 4 piercings had no greater likelihood to engage in high-risk behaviors [31].
Conversely, some researchers have attempted to show a positive association for body modification. In a study of women with eating disorders, the authors suggested that body piercing could be seen as reflecting a positive attitude towards the body, an expression of self-care [32]. In addition, people with piercings are more likely to give attention to their physical appearance and are less likely to be overweight than people without piercings [33].
Stirn and Hinz [34] concluded that most people who partake in body modification clearly do not do it because they have any psychological problems. However, for a few, modifications may be utilized as a convenient means to either realize psychopathological inclinations, such as self-injury, or to overcome psychological traumas. The prevalence of self-injury is unknown, though it is believed to be a growing problem. While self-injury is believed to be a low-lethality behavior, teens who hurt themselves are at increased risk for suicide related to their underlying anxiety or depression. Moreover, self-harmers report that they often had their skin tattooed or body pierced to help overcome a negative experience, or simply to experience physical pain. Another clue that self-harm and piercing/tattooing might in some cases be linked derives from the fact that many of the self-harmers said they had ceased cutting themselves after obtaining their first piercing or tattoo [35]. The increasing incidence makes deliberate self-harm a problem that all health care providers dealing with adolescents are likely to encounter.
Given the link between body modification and “externalized risk behaviors” in young people, tattooing and body piercing may serve as clinical markers for health care professionals, potentially identifying those who may be involved in activities that hinder their health and development [29]. For example, closer examination of teens who wear long sleeves or clothing inappropriate for weather could reveal cuts, burns, carvings, or bruises that are self-inflicted.
Patients are more likely to discuss the issue of body art if the clinician does not speak or act judgmentally [1]. Practitioners who are concerned that their tattooed patient might be self-injuring or engaging in other risky behaviors should invite a discussion with the patient, perhaps using general terms, such as “Sometimes people may get involved in self-injury and don’t know where to turn for help. I will try to help you if you are ever worried about that.” As always, if the clinician suspects a patient is engaged in self-harming activities, an immediate referral should be made for mental health evaluation and any necessary intervention.
Preventatively, clinicians should provide targeted and repeated education to transmit the message of effective decision-making and evaluation of risks to children in the early elementary grades, since some students start to obtain body art as early as fifth grade.
Regret and Removal
Over time, many individuals regret getting tattoos and wish to have them removed [35]. In some cases, delayed complications, like the development of allergic, hypersensitivity, or granulomatous reactions, require tattoo removal. On average, tattoo regret occurs 14 years after tattooing and by the age of 40 years.
Removal is more painful and laborious than the tattooing itself, and complete removal, with no scarring, is often not possible. The American Society for Dermatologic Surgery reports that in 2011, its doctors performed nearly 100,000 tattoo removal procedures, up from the 86,000 performed in 2010 [36]. Pulsed lasers have been used to remove tattoos for more than 20 years. With this procedure, pulses of high-intensity laser energy pass through the epidermis and are selectively absorbed by the tattoo pigment. The laser breaks the pigment into smaller particles, which may be metabolized or excreted by the body, or transported to and stored in lymph nodes or other tissues [37].
Other removal techniques include dermabrasion and surgical excision. Do-it-yourself tattoo removal ointments and creams can be purchased online, but they have not been approved by the FDA and there is no clinical evidence that they work. Furthermore, tattoo removal ointments and creams may cause unexpected reactions, such as rashes, burning, scarring, or changes in skin pigmentation [37].
Clinician’s Role
Body art provides a window into an individual’s uniqueness, and acknowledging body modifications can help build trust and develop the physician/patient relationship. The health professional armed with knowledge about body modifications can forge more functional relationships, obtain critical historical information, and provide better treatment and referral for this population [5].
Having a clinician who is a trusted, nonjudgmental source of information and intervention for patients who choose body art will reduce the health risk of complications associated with tattooing and piercing [1]. Unfortunately, only 14% of the population identify health care professionals as a common resource for information on body modification. Instead, young adults endorsed friends (82%), body piercing shops (61%), and tattoo shops (51%) as their top information sources. Clinicians should help patients make informed decisions about body art and counsel them about the importance of universal precautions [35].
Corresponding author: Susannah Grimm Poe, EdD, 176 Gilboa Rd., Fairmont, WV 26554, [email protected].
Financial disclosures: None.
1. Schmidt R, Armstrong M. Tattooing in adolescents and adults. Up to Date 2013. Available at www.uptodate.com.
2. US Food and Drug Administration. Tattoos and permanent makeup. 2009. Available at www.fda.gov/Cosmetics/ProductandIngredientSafety/ProductInformation/ucm108530.htm.
3. Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol 2007;25:398-406.
4. Schorzman CM, Gold MA, Downs JS, Murray PJ. Body art: attitudes and practices regarding body piercing among urban undergraduates. J Am Osteopath Assoc 2007;107:432-8.
5. Urdang, M, Mallek, J, Mallon, W. Tattoos and piercings: a review for the emergency physician. West J Emerg Med 2011;12:393–8.
6. Braverman S. One in five US adults now has a tattoo. New York: Harris Interactive; 2012.
7. Corso RA. Nationwide Harris poll. New York: Harris Interactive; 2008.
8. Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol 2006;55:413–21.
9. Antoszewski B, Sitek A, Fijałkowska M, et al. Tattooing and body piercing--what motivates you to do it? Int J Soc Psychiatry. 2010;56:471–9.
10. Quaranta A, Napoli C, Fasano F, et al. Body piercing and tattoos: a survey on young adults’ knowledge of the risks and practices in body art. BMC Public Health 2011;11:774.
11. Glassy C, Glassy M, Aldasoiuqi A. Tattooing: medical uses and problems. Clev Clin J Med 2012;79:761–70.
12. Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992;13:7–17.
13. Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
14. Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. N Engl J Med 2012;367:1020–4.
15. CDC. The hidden dangers of getting inked. Available at http://blogs.cdc.gov/publichealthmatters/2012/08/the-hidden-dangers-of-getting-inked.
16. LeBlanc PM, Hollinger KA, Klontz KC. Tattoo ink-related infections—awareness, diagnosis, reporting, and prevention. N Engl J Med 2012;367:985–7.
17. Shinohara MM, Nguyen J, Gardner J, Rosenbach M, Elenitsas R. The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol 2012;39:1110–8.
18. Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011;10:837–42.
19. Tope WD, Shellock FG. Magnetic resonance imaging and permanent cosmetics (tattoos): survey of complications and adverse events. J Magn Reson Imaging 2002;15:180–4.
20. Holbrook, J, Minocha J, Laumann A. Body piercing: complications and prevention of health risks. Am J Clin Dermatol 2012;13:1–17.
21. Hogan L, Armstrong ML. Body piercing: more than skin deep. Skin therapy letter. Available at www.skintherapyletter.com/2009/14.7/2.html.
22. Raja SG, Shad SK, Dreyfus GD. Body piercing: a rare cause of mitral valve endocarditis. J Heart Valve Dis 2004;13:854.
23. Botchway C, Kuc I. Tongue piercing and associated tooth fracture. J Can Dent Assoc 1998;64:803–5.
24. American Dental Association. Statement on intraoral/perioral piercing and tongue splitting. 2012. Available at www.ada.org/prof/resources/positions/statements/piercing.asp.
25. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet 2003;361:1205–15.
26. Koch R, Roberts AE, Armstrong ML, Owen DC. Body art, deviance, and American college students. Soc Sci J 2010;47:151–61.
27. Gueguen N. Tattoos, piercings, and sexual activity. Social Behav Personality 2012;40:1543–7.
28. Skegg K, Nada-Raja S, Paul C, et al. Body piercing, personality, and sexual behavior. Arch Sex Behav 2007; 36:47–54.
29. Deschesnes M, Fines P, Demers S. Are tattooing and body piercing indicators of risk-taking behaviours among high school students? J Adolesc 2006; 29:379–93.
30. Roberts TA, Ryan SA. Tattooing and high-risk behavior in adolescents. Pediatrics 2002;110:1058–63.
31. Owen DC, Armstrong ML, Koch JR, Roberts AE. College students with body art: well-being or high-risk behavior? J Psychosoc Nurs Ment Health Serv 2013;51:20–8.
32. Claes L, Vandereycken W, Vertommen H. Self-care versus self-harm: piercing, tattooing, and self-injuring in eating disorders. Eur Eat Disord Rev 2004;13:11–18.
33. Hicinbothem J, Gonsalves S, Lester D. Body modification and suicidal behavior. Death Stud 2006; 30:351–63.
34. Stirn A, Hinz A. Tattoos, body piercings, and self-injury: Is there a connection? Psychotherapy Res 2008;18:326–33.
35. Burris K, Kim K. Tattoo removal. Clin Dermatol 2007;25:388–92.
36. American Society for Dermatologic Surgery. Unwanted tattoos. Available at www.asds.net/tattooremovalinformation.aspx.
37. US Food and Drug Administration. Inked and regretful: removing tattoos. 2013. Available at www.fda.gov/ForConsumers/ucm336842.htm.
38. Meltzer DI. Complications of body piercing. Am Fam Physician 2005;72:2029–34.
1. Schmidt R, Armstrong M. Tattooing in adolescents and adults. Up to Date 2013. Available at www.uptodate.com.
2. US Food and Drug Administration. Tattoos and permanent makeup. 2009. Available at www.fda.gov/Cosmetics/ProductandIngredientSafety/ProductInformation/ucm108530.htm.
3. Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol 2007;25:398-406.
4. Schorzman CM, Gold MA, Downs JS, Murray PJ. Body art: attitudes and practices regarding body piercing among urban undergraduates. J Am Osteopath Assoc 2007;107:432-8.
5. Urdang, M, Mallek, J, Mallon, W. Tattoos and piercings: a review for the emergency physician. West J Emerg Med 2011;12:393–8.
6. Braverman S. One in five US adults now has a tattoo. New York: Harris Interactive; 2012.
7. Corso RA. Nationwide Harris poll. New York: Harris Interactive; 2008.
8. Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol 2006;55:413–21.
9. Antoszewski B, Sitek A, Fijałkowska M, et al. Tattooing and body piercing--what motivates you to do it? Int J Soc Psychiatry. 2010;56:471–9.
10. Quaranta A, Napoli C, Fasano F, et al. Body piercing and tattoos: a survey on young adults’ knowledge of the risks and practices in body art. BMC Public Health 2011;11:774.
11. Glassy C, Glassy M, Aldasoiuqi A. Tattooing: medical uses and problems. Clev Clin J Med 2012;79:761–70.
12. Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992;13:7–17.
13. Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
14. Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. N Engl J Med 2012;367:1020–4.
15. CDC. The hidden dangers of getting inked. Available at http://blogs.cdc.gov/publichealthmatters/2012/08/the-hidden-dangers-of-getting-inked.
16. LeBlanc PM, Hollinger KA, Klontz KC. Tattoo ink-related infections—awareness, diagnosis, reporting, and prevention. N Engl J Med 2012;367:985–7.
17. Shinohara MM, Nguyen J, Gardner J, Rosenbach M, Elenitsas R. The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol 2012;39:1110–8.
18. Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011;10:837–42.
19. Tope WD, Shellock FG. Magnetic resonance imaging and permanent cosmetics (tattoos): survey of complications and adverse events. J Magn Reson Imaging 2002;15:180–4.
20. Holbrook, J, Minocha J, Laumann A. Body piercing: complications and prevention of health risks. Am J Clin Dermatol 2012;13:1–17.
21. Hogan L, Armstrong ML. Body piercing: more than skin deep. Skin therapy letter. Available at www.skintherapyletter.com/2009/14.7/2.html.
22. Raja SG, Shad SK, Dreyfus GD. Body piercing: a rare cause of mitral valve endocarditis. J Heart Valve Dis 2004;13:854.
23. Botchway C, Kuc I. Tongue piercing and associated tooth fracture. J Can Dent Assoc 1998;64:803–5.
24. American Dental Association. Statement on intraoral/perioral piercing and tongue splitting. 2012. Available at www.ada.org/prof/resources/positions/statements/piercing.asp.
25. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet 2003;361:1205–15.
26. Koch R, Roberts AE, Armstrong ML, Owen DC. Body art, deviance, and American college students. Soc Sci J 2010;47:151–61.
27. Gueguen N. Tattoos, piercings, and sexual activity. Social Behav Personality 2012;40:1543–7.
28. Skegg K, Nada-Raja S, Paul C, et al. Body piercing, personality, and sexual behavior. Arch Sex Behav 2007; 36:47–54.
29. Deschesnes M, Fines P, Demers S. Are tattooing and body piercing indicators of risk-taking behaviours among high school students? J Adolesc 2006; 29:379–93.
30. Roberts TA, Ryan SA. Tattooing and high-risk behavior in adolescents. Pediatrics 2002;110:1058–63.
31. Owen DC, Armstrong ML, Koch JR, Roberts AE. College students with body art: well-being or high-risk behavior? J Psychosoc Nurs Ment Health Serv 2013;51:20–8.
32. Claes L, Vandereycken W, Vertommen H. Self-care versus self-harm: piercing, tattooing, and self-injuring in eating disorders. Eur Eat Disord Rev 2004;13:11–18.
33. Hicinbothem J, Gonsalves S, Lester D. Body modification and suicidal behavior. Death Stud 2006; 30:351–63.
34. Stirn A, Hinz A. Tattoos, body piercings, and self-injury: Is there a connection? Psychotherapy Res 2008;18:326–33.
35. Burris K, Kim K. Tattoo removal. Clin Dermatol 2007;25:388–92.
36. American Society for Dermatologic Surgery. Unwanted tattoos. Available at www.asds.net/tattooremovalinformation.aspx.
37. US Food and Drug Administration. Inked and regretful: removing tattoos. 2013. Available at www.fda.gov/ForConsumers/ucm336842.htm.
38. Meltzer DI. Complications of body piercing. Am Fam Physician 2005;72:2029–34.