User login
Early Warning System Boosts Sepsis Detection, Care
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
A recent study published in the Journal of Hospital Medicine reports on an early warning and response system (EWRS) for sepsis used in all three hospitals within the Philadelphia-based University of Pennsylvania Health System (UPHS) for three-month spans in 2012 and 2013. The system integrates laboratory values and vital signs into patients EHRs and establishes a threshold for triggering the alert.
After implementing the EWRS, at-risk patients received faster care for sepsis and/or were transferred to the ICU more quickly, says lead author Craig A. Umscheid, MD, MSCE, director of the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Study authors also note that quicker care suggested reduced mortality from sepsis as well.
"Whenever a patient triggered the alert, their probability of mortality was much higher than patients who didn't trigger the alert," Dr. Umscheid says. "I think what makes our study unique compared to other studies that have tried to predict sepsis is that beyond just creating a prediction rule for sepsis, we actually implemented it into a clinical care setting, alerted providers in real time, and then those providers changed their care based on the prediction."
More than 90% of care teams arrived at the bedside when they received an alert. "Meaning that they saw some value in the alert, and the infrastructure that we put in place was able to mobilize the team and get them to the bedside within 30 minutes," Dr. Umscheid adds. "We saw an increase in sepsis antibiotics used, and we saw an increase in fluid boluses within six hours.”
As many as 3 million cases of severe sepsis occur in the U.S. annually, and 750,000 result in deaths, according to the study. The high number of cases has led to several efforts to create better clinical practices for sepsis patients.
"Sepsis is arguably one of the most, if not the most important, causes of preventable mortality in the inpatient setting," Dr. Umscheid says. "One thing that we thought we could do better was identify sepsis cases earlier so that we could provide early antibiotics and fluids."
Visit our website for more information on identifying and treating sepsis.
Hospitalist Adds County Coroner to His Résumé
Hospitalists have taken positions in every corner of healthcare: the C-suite, hospital administration, and even nominee for U.S. surgeon general.
Now, add county coroner to the list.
This month, hospitalist Adam Duckett, MD, was elected coroner for Cayuga County, N.Y., whose county seat of Auburn is about 30 miles west of Syracuse. Dr. Duckett, who had never run for public office, is a hospitalist at Auburn Community Hospital and serves as a board member for Hospice of the Finger Lakes.
The Hospitalist spoke with him about his new post, which might make him the only hospitalist/coroner in the country.
Question: HM is a time-consuming job. Why take time out for public service?
Answer: I believe everyone owes a debt of service to their community, and I felt that this was one that I would enjoy.
Q: What skills from HM apply to your new position?
A: The majority of unattended deaths in our county are related to long-standing medical illness. Because of this, I feel that in order to understand how somebody may have died, you must first know how they lived. I believe my role as a hospitalist enables me to review medical records and determine if the medical history provides enough information to determine a cause of death.
Q: What skills from your hospice care experience apply?
A: My role as a hospitalist has given me valuable insight in helping families cope with the loss of a loved one by providing explanations as to why somebody might have passed. It’s very important for a family to understand why a loved one died before they can accept it, and it’s very rewarding to help families through this process.
Get involved in public policy via SHM's advocacy home page. TH
Visit our website for more information about community involvement.
Hospitalists have taken positions in every corner of healthcare: the C-suite, hospital administration, and even nominee for U.S. surgeon general.
Now, add county coroner to the list.
This month, hospitalist Adam Duckett, MD, was elected coroner for Cayuga County, N.Y., whose county seat of Auburn is about 30 miles west of Syracuse. Dr. Duckett, who had never run for public office, is a hospitalist at Auburn Community Hospital and serves as a board member for Hospice of the Finger Lakes.
The Hospitalist spoke with him about his new post, which might make him the only hospitalist/coroner in the country.
Question: HM is a time-consuming job. Why take time out for public service?
Answer: I believe everyone owes a debt of service to their community, and I felt that this was one that I would enjoy.
Q: What skills from HM apply to your new position?
A: The majority of unattended deaths in our county are related to long-standing medical illness. Because of this, I feel that in order to understand how somebody may have died, you must first know how they lived. I believe my role as a hospitalist enables me to review medical records and determine if the medical history provides enough information to determine a cause of death.
Q: What skills from your hospice care experience apply?
A: My role as a hospitalist has given me valuable insight in helping families cope with the loss of a loved one by providing explanations as to why somebody might have passed. It’s very important for a family to understand why a loved one died before they can accept it, and it’s very rewarding to help families through this process.
Get involved in public policy via SHM's advocacy home page. TH
Visit our website for more information about community involvement.
Hospitalists have taken positions in every corner of healthcare: the C-suite, hospital administration, and even nominee for U.S. surgeon general.
Now, add county coroner to the list.
This month, hospitalist Adam Duckett, MD, was elected coroner for Cayuga County, N.Y., whose county seat of Auburn is about 30 miles west of Syracuse. Dr. Duckett, who had never run for public office, is a hospitalist at Auburn Community Hospital and serves as a board member for Hospice of the Finger Lakes.
The Hospitalist spoke with him about his new post, which might make him the only hospitalist/coroner in the country.
Question: HM is a time-consuming job. Why take time out for public service?
Answer: I believe everyone owes a debt of service to their community, and I felt that this was one that I would enjoy.
Q: What skills from HM apply to your new position?
A: The majority of unattended deaths in our county are related to long-standing medical illness. Because of this, I feel that in order to understand how somebody may have died, you must first know how they lived. I believe my role as a hospitalist enables me to review medical records and determine if the medical history provides enough information to determine a cause of death.
Q: What skills from your hospice care experience apply?
A: My role as a hospitalist has given me valuable insight in helping families cope with the loss of a loved one by providing explanations as to why somebody might have passed. It’s very important for a family to understand why a loved one died before they can accept it, and it’s very rewarding to help families through this process.
Get involved in public policy via SHM's advocacy home page. TH
Visit our website for more information about community involvement.
PoCUS for Hospitalists
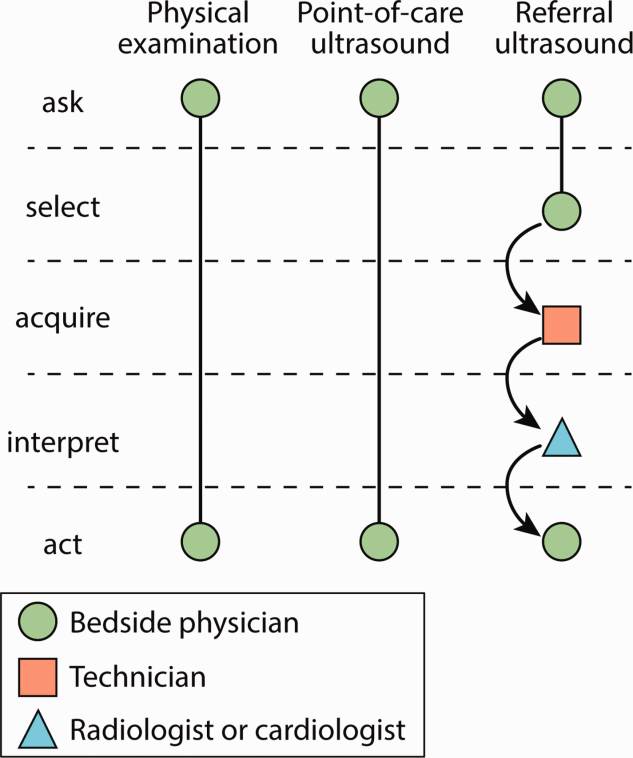
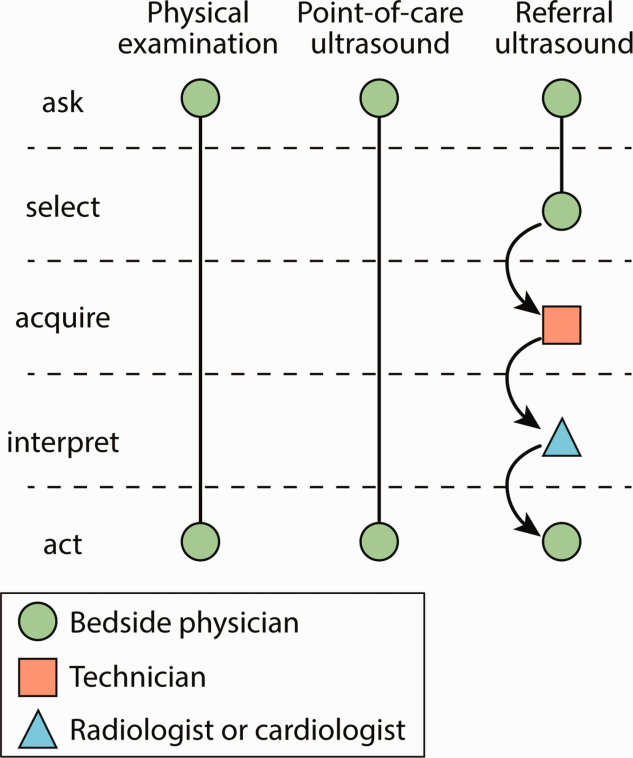
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]
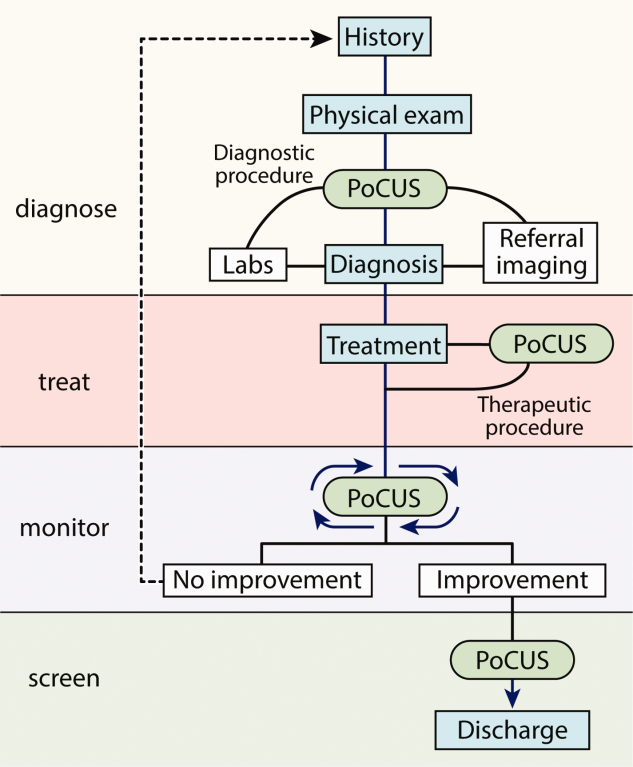
The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.
ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]

The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.

ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]

The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.

ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
© 2014 Society of Hospital Medicine
Medication Reconciliation Perspectives
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
Medication reconciliation, when performed well, effectively identifies discrepancies and reduces medication errors in the hospital setting.[1, 2, 3] This process involves 4 major steps: (1) obtain and document a comprehensive medication history on admission, (2) compare the medication history to medication orders in the hospital and identify and resolve discrepancies, (3) provide the patient with a written list of discharge medications, and (4) educate the patient about their discharge medication regimen.[4, 5, 6]
However, medication reconciliation has been challenging to implement given difficulties with accurate medication information, patients' ability to communicate or remember, and clinician's not having enough time, motivation, or clear roles.[5, 7, 8, 9, 10, 11] Lack of role clarity is generally a barrier to quality improvement; therefore, we studied the perceptions of physicians, nurses, and pharmacists about their roles and responsibilities in completing inpatient medication reconciliation.
METHODS
We independently surveyed attending and resident physicians, nurses, and pharmacists at the University of California San Francisco (UCSF) Medical Center via email who were actively caring for hospitalized patients in April 2010. We collected data on demographics, roles on specific tasks in the medication reconciliation process from admission through discharge, and attitudes and barriers toward medication reconciliation and health information technology systems. Responses to questions used a 4‐point Likert scale. We calculated frequencies and proportions, and used the Fisher exact test to evaluate differences in role agreement for specific medication reconciliation tasks.
RESULTS
Of 256 active clinicians, 78 completed the survey (30.5% overall response rate) providing care in various hospital services (medicine, surgery, cardiology, neurology, pediatrics, obstetrics/gynecology). We received responses from 7 attending physicians (16% response rate), 14 resident physicians (19% response rate), 35 nurses (43% response rate), and 22 pharmacists (43% response rate). Most clinicians worked more than 5 years at UCSF, except residents (14 years).
Overall agreement was poor to fair on whose primary role it was for specific medication reconciliation tasks from admission through discharge (Table 1). Clinicians mainly agreed that it was a physician's responsibility to decide which medications should be continued or discontinued on admission and discharge, although agreement between attending and resident physicians varied. Fisher exact test revealed significant differences in agreement among attending and resident physicians, nurses, and pharmacists to obtain and document a medication history on admission (P=0.001), provide a list of the discharge medications (P0.001), or educate patients on the postdischarge medication regimen (P0.001). For these tasks, the physician, nurse, pharmacist or a combination of these clinicians (multiple category) were each identified to be responsible.
| Response to who is responsible | |||||
|---|---|---|---|---|---|
| Clinician | Attending | Resident | Nurse | Pharmacist | Multiple* |
| |||||
| A. On admission, obtaining and documenting the patient's medication history (P=0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 14 (100%) | 0 | 0 | 0 |
| Nurse | 6 (17%) | 20 (57%) | 5 (14%) | 2 (6%) | 2 (6%) |
| Pharmacist | 1 (5%) | 9 (41%) | 0 | 10 (45%) | 2 (9%) |
| B. On admission, deciding which medications will be continued or discontinued (P=0.027) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 3 (21%) | 11 (79%) | 0 | 0 | 0 |
| Nurse | 12 (34%) | 22 (63%) | 0 | 0 | 1 (3%) |
| Pharmacist | 4 (18%) | 15 (68%) | 0 | 2 (9%) | 1 (5%) |
| C. On discharge, deciding which medications will be continued or discontinued (P=0.123) | |||||
| Attending | 6 (86%) | 1 (14%) | 0 | 0 | 0 |
| Resident | 5 (36%) | 9 (64%) | 0 | 0 | 0 |
| Nurse | 10 (29%) | 15 (43%) | 1 (3%) | 1 (3%) | 8 (23%) |
| Pharmacist | 5 (23%) | 12 (55%) | 1 (5%) | 0 | 4 (18%) |
| D. On discharge, providing a list of the discharge medications to the patient (P0.001) | |||||
| Attending | 1 (14%) | 6 (86%) | 0 | 0 | 0 |
| Resident | 0 | 13 (93%) | 0 | 1 (7%) | 0 |
| Nurse | 2 (6%) | 22 (63%) | 3 (11%) | 6 (17%) | 2 (6%) |
| Pharmacist | 0 | 4 (18%) | 2 (9%) | 14 (64%) | 2 (9%) |
| E. On discharge, educating the patient on the postdischarge medication regimen (P0.001) | |||||
| Attending | 1 (14%) | 4 (57%) | 1 (14%) | 1 (14%) | 0 |
| Resident | 0 | 4 (29%) | 8 (57%) | 2 (14%) | 0 |
| Nurse | 0 | 2 (6%) | 23 (66%) | 8 (23%) | 2 (6%) |
| Pharmacist | 0 | 0 | 3 (14%) | 14 (64%) | 5 (23%) |
Most clinicians believed that maintaining a patient's list of medications improves patient care (94%100% agreement). However, when asked whether clinicians other than yourself should be responsible for an accurate medication list, most nurses (73%) and pharmacists (52%) agreed with this statement compared to resident (50%) and attending physicians (29%). Most clinicians agreed that information technology systems for reconciling medications were complicated, and that patients who do not know their medications, accessing outside medical records, working with inaccurate lists, or nonEnglish‐speaking patients are barriers to reconciliation.
DISCUSSION
We found fair agreement among clinicians that physicians were responsible for reconciling medications on admission and discharge. However, attending and resident physicians each believed it was their primary responsibility, respectively, suggesting the need for better communication between each other. We found poor agreement among clinicians about whose primary role it was to perform the other main steps of medication reconciliation including obtaining and documenting a medication history, and providing a medication list and educating the patient at discharge. For these tasks, there was more confusion among physicians, nurses, and pharmacists. Our findings highlight the need for better role clarity and good communication among team members, particularly at discharge.
Nearly all clinicians agreed that updating patients' medication lists improves patient care. However, most nurses and pharmacists preferred that physicians be responsible for updating information and reconciling medications. They also noted a number of patient‐related and information system barriers to effective reconciliation as others have identified.[7, 8, 9, 10, 11] Although standardizing medication information reporting and implementing technology that can integrate medical records to create, update, and share information between patients and providers can help streamline the medication reconciliation process,[4, 5, 7, 8, 12] these procedures are unlikely to be effective unless good interprofessional communication, role clarity, and clinician understanding of how the system works are in place.
When this study was conducted, our institution's policy required that medication reconciliation be completed, but no specific roles or standard work documents existed. Since then, we have clarified the role of the physician to be responsible for completing medication reconciliation with ancillary help from nurses, pharmacists, and other clinicians, particularly when obtaining a medication history and preparing the patient for discharge. This role clarity has led to focused training and standard work guide documents as guidance to clinicians in different hospital settings about expectations and how to complete medication reconciliation. Clearly, no single reconciliation workflow process will meet the needs of all hospitals. However, it is crucial that interprofessional teams are established with clearly defined roles and responsibilities, and how these roles and responsibilities may change in various situations or services.[8]
Our study had several limitations. We surveyed 1 academic medical center, thus limiting the generalizability of our findings to other organizations or settings. Our small sample size and low response rate could be susceptible to selection bias. However, our findings are similar to other studies.[7, 10, 11] Finally, we included clinicians practicing on various services throughout our hospital, and the local medication reconciliation process could have contributed to the poor agreement. Nonetheless, differences in perceived roles and attitudes for completing medication reconciliation were observed.
In conclusion, lack of agreement among clinicians about their specific roles and responsibilities in the medication reconciliation process exists, and this may result in incomplete reconciliation, inefficiency, duplication of work, and possibly more confusion about a patient's medication regimen. Clinically meaningful and efficient medication reconciliation requires interprofessional teamwork with clear roles and responsibilities, good communication and better information reporting, and tracking systems to successfully combine the steps of medication reconciliation and ensure patient safety.[8, 12]
Disclosures: Funded by research grant NHLBI R01 HL086473 to Dr. Auerbach, and through UCSF‐ CTSI grant number KL2 RR024130 to Dr. Lee from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Lee had full access to all study data and takes responsibility for data integrity and data analysis accuracy. The authors report no conflicts of interest.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447.
- Institute for Healthcare Improvement. How‐to Guide: Prevent Adverse Drug Events (Medication Reconciliation). Available at: www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventAdverseDrugEvents.aspx. Accessed March 22, 2014.
- The Joint Commission. National patient safety goals effective January 1, 2014. Hospital Accreditation Program. Available at: http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. Accessed March 22, 2014.
- Agency for Healthcare Research and Quality. Introduction: medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/match/matchintro.html. Updated August 2012. Accessed March 22, 2014.
- , , , , . Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , , . How reliable are patient‐completed medication reconciliation forms compared with pharmacy lists? Am J Emerg Med. 2012;30(7):1048–1054.
- , , , , . Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hosp Med. 2011;6(6):329–337.
- , , , . Medication reconciliation: a qualitative analysis of clinicians' perceptions. Res Social Adm Pharm. 2013;9(4):419–430.
- , . Improving care transitions: optimizing medication reconciliation. J Am Pharm Assoc (2003). 2012;52(4):e43–e52.
Repeat BMD screening not helpful for women under 65
Postmenopausal women without osteoporosis on their first bone mineral density test are unlikely to fracture before age 65, and are therefore unlikely to benefit from regular or repeated screening before that age, according to an analysis of results from a large cohort study.
“Our data can help inform a BMD testing interval for postmenopausal women who are screened before age 65 years. Using the more conservative time estimates for major osteoporotic fracture, clinicians might allow women aged 50 to 54 years without osteoporosis on their first BMD test to wait 10 years for their next test. Similarly, women aged 60 to 64 years without osteoporosis on their first BMD test might wait until after age 65 years for their next test,” Dr. Margaret Lee Gourlay of the University of North Carolina, Chapel Hill, and her associates wrote in their analysis.
Dr. Gourlay and her coinvestigators on the larger Women’s Health Initiative cohort study looked at data from 4,068 postmenopausal women between the ages of 50 and 64 years. None of the women had prior hip or vertebral fractures or received antifracture treatment; they underwent baseline bone mineral density testing between 1993 and 2005. Fracture follow-up continued through 2012 (Menopause 2014 [doi: 10.1097/gme.0000000000000356]).
Among women with a normal BMD on first screening, the estimated time for 1% of those aged 50-54 years to have a hip or clinical vertebral fracture was 12.8 years. Among women aged 60-64 years, the time to fracture was 7.6 years, Dr. Gourlay and her colleagues found.
For the 8.5% of women in the cohort (all ages) with osteoporosis at baseline (n = 344), the age-adjusted time to hip or vertebral fracture was only 3 years.
Dr. Gourlay and her colleagues also estimated times to major osteoporotic fracture for 3% of the cohort, finding that it took 11.5 years for women aged 50-54 years to sustain a hip, clinical vertebral, proximal humerus, or wrist fracture, compared with 8.6 years for women who were 60-64 years at baseline. For women who had osteoporosis at baseline, the age-adjusted time for 3% to have an osteoporotic fracture was 2.5 years.
The researchers acknowledged as limitations of the study the fact that time estimates were based only on transitions to major fracture; that the full benefits and risks of screening, including cost-effectiveness, were not analyzed; and that the study was not powered to determine fracture risk in subgroups defined by individual risk factors.
Nonetheless, the results suggest that deferred repeat screening can be safe for postmenopausal women aged 50 years and older with normal BMD results at baseline, Dr. Gourlay and her coauthors wrote.
The study was funded by the National Institutes of Health. One of Dr. Gourlay’s coauthors is a consultant for MSD, and another reported receiving recent funding from Bone Ultrasound Finland.
This post hoc analysis of the Women’s Health Initiative cohort pursues the question of how frequently we should repeat BMD assessment on women under age 65 with normal baseline BMD. The study offers meaningful insight into how minuscule the fracture and osteoporosis risks are for women younger than 65 who have normal BMD at baseline. The younger cohort in this large study did not fracture or develop osteoporosis under surveillance to any significant extent.
While guidelines advise that women 65 and older be screened, as should younger postmenopausal women with risk factors, in clinical practice this often means that younger postmenopausal women with normal baseline BMD will enter into “autopilot” and undergo testing every 2 years. For young postmenopausal women with healthy BMD, we don’t need to fall into this default of biannual assessment. Clinicians could consider safely deferring a follow-up BMD test for young postmenopausal women with documented normal BMD for a few years, and for some even until age 65.
Nonetheless, clinicians should be mindful that this observational study was carried out in an asymptomatic group of women, and that the clinical picture should always guide the decision-making process on when and how often to screen.
Importantly, this study does not provide us any guidance regarding a young postmenopausal patient who has had a change in health status, such as a newly diagnosed autoimmune disease necessitating treatment with oral steroids, or after discontinuation of systemic menopausal hormone therapy, for whom repeating BMD assessment within 2 years or even 1 year of the initial study can be clinically justified despite evidence of normal BMD on her baseline scan.
Lubna Pal, M.D., is an associate director of obstetrics, gynecology, and reproductive sciences as well as director of the Menopause Program at Yale University in New Haven, Conn.
This post hoc analysis of the Women’s Health Initiative cohort pursues the question of how frequently we should repeat BMD assessment on women under age 65 with normal baseline BMD. The study offers meaningful insight into how minuscule the fracture and osteoporosis risks are for women younger than 65 who have normal BMD at baseline. The younger cohort in this large study did not fracture or develop osteoporosis under surveillance to any significant extent.
While guidelines advise that women 65 and older be screened, as should younger postmenopausal women with risk factors, in clinical practice this often means that younger postmenopausal women with normal baseline BMD will enter into “autopilot” and undergo testing every 2 years. For young postmenopausal women with healthy BMD, we don’t need to fall into this default of biannual assessment. Clinicians could consider safely deferring a follow-up BMD test for young postmenopausal women with documented normal BMD for a few years, and for some even until age 65.
Nonetheless, clinicians should be mindful that this observational study was carried out in an asymptomatic group of women, and that the clinical picture should always guide the decision-making process on when and how often to screen.
Importantly, this study does not provide us any guidance regarding a young postmenopausal patient who has had a change in health status, such as a newly diagnosed autoimmune disease necessitating treatment with oral steroids, or after discontinuation of systemic menopausal hormone therapy, for whom repeating BMD assessment within 2 years or even 1 year of the initial study can be clinically justified despite evidence of normal BMD on her baseline scan.
Lubna Pal, M.D., is an associate director of obstetrics, gynecology, and reproductive sciences as well as director of the Menopause Program at Yale University in New Haven, Conn.
This post hoc analysis of the Women’s Health Initiative cohort pursues the question of how frequently we should repeat BMD assessment on women under age 65 with normal baseline BMD. The study offers meaningful insight into how minuscule the fracture and osteoporosis risks are for women younger than 65 who have normal BMD at baseline. The younger cohort in this large study did not fracture or develop osteoporosis under surveillance to any significant extent.
While guidelines advise that women 65 and older be screened, as should younger postmenopausal women with risk factors, in clinical practice this often means that younger postmenopausal women with normal baseline BMD will enter into “autopilot” and undergo testing every 2 years. For young postmenopausal women with healthy BMD, we don’t need to fall into this default of biannual assessment. Clinicians could consider safely deferring a follow-up BMD test for young postmenopausal women with documented normal BMD for a few years, and for some even until age 65.
Nonetheless, clinicians should be mindful that this observational study was carried out in an asymptomatic group of women, and that the clinical picture should always guide the decision-making process on when and how often to screen.
Importantly, this study does not provide us any guidance regarding a young postmenopausal patient who has had a change in health status, such as a newly diagnosed autoimmune disease necessitating treatment with oral steroids, or after discontinuation of systemic menopausal hormone therapy, for whom repeating BMD assessment within 2 years or even 1 year of the initial study can be clinically justified despite evidence of normal BMD on her baseline scan.
Lubna Pal, M.D., is an associate director of obstetrics, gynecology, and reproductive sciences as well as director of the Menopause Program at Yale University in New Haven, Conn.
Postmenopausal women without osteoporosis on their first bone mineral density test are unlikely to fracture before age 65, and are therefore unlikely to benefit from regular or repeated screening before that age, according to an analysis of results from a large cohort study.
“Our data can help inform a BMD testing interval for postmenopausal women who are screened before age 65 years. Using the more conservative time estimates for major osteoporotic fracture, clinicians might allow women aged 50 to 54 years without osteoporosis on their first BMD test to wait 10 years for their next test. Similarly, women aged 60 to 64 years without osteoporosis on their first BMD test might wait until after age 65 years for their next test,” Dr. Margaret Lee Gourlay of the University of North Carolina, Chapel Hill, and her associates wrote in their analysis.
Dr. Gourlay and her coinvestigators on the larger Women’s Health Initiative cohort study looked at data from 4,068 postmenopausal women between the ages of 50 and 64 years. None of the women had prior hip or vertebral fractures or received antifracture treatment; they underwent baseline bone mineral density testing between 1993 and 2005. Fracture follow-up continued through 2012 (Menopause 2014 [doi: 10.1097/gme.0000000000000356]).
Among women with a normal BMD on first screening, the estimated time for 1% of those aged 50-54 years to have a hip or clinical vertebral fracture was 12.8 years. Among women aged 60-64 years, the time to fracture was 7.6 years, Dr. Gourlay and her colleagues found.
For the 8.5% of women in the cohort (all ages) with osteoporosis at baseline (n = 344), the age-adjusted time to hip or vertebral fracture was only 3 years.
Dr. Gourlay and her colleagues also estimated times to major osteoporotic fracture for 3% of the cohort, finding that it took 11.5 years for women aged 50-54 years to sustain a hip, clinical vertebral, proximal humerus, or wrist fracture, compared with 8.6 years for women who were 60-64 years at baseline. For women who had osteoporosis at baseline, the age-adjusted time for 3% to have an osteoporotic fracture was 2.5 years.
The researchers acknowledged as limitations of the study the fact that time estimates were based only on transitions to major fracture; that the full benefits and risks of screening, including cost-effectiveness, were not analyzed; and that the study was not powered to determine fracture risk in subgroups defined by individual risk factors.
Nonetheless, the results suggest that deferred repeat screening can be safe for postmenopausal women aged 50 years and older with normal BMD results at baseline, Dr. Gourlay and her coauthors wrote.
The study was funded by the National Institutes of Health. One of Dr. Gourlay’s coauthors is a consultant for MSD, and another reported receiving recent funding from Bone Ultrasound Finland.
Postmenopausal women without osteoporosis on their first bone mineral density test are unlikely to fracture before age 65, and are therefore unlikely to benefit from regular or repeated screening before that age, according to an analysis of results from a large cohort study.
“Our data can help inform a BMD testing interval for postmenopausal women who are screened before age 65 years. Using the more conservative time estimates for major osteoporotic fracture, clinicians might allow women aged 50 to 54 years without osteoporosis on their first BMD test to wait 10 years for their next test. Similarly, women aged 60 to 64 years without osteoporosis on their first BMD test might wait until after age 65 years for their next test,” Dr. Margaret Lee Gourlay of the University of North Carolina, Chapel Hill, and her associates wrote in their analysis.
Dr. Gourlay and her coinvestigators on the larger Women’s Health Initiative cohort study looked at data from 4,068 postmenopausal women between the ages of 50 and 64 years. None of the women had prior hip or vertebral fractures or received antifracture treatment; they underwent baseline bone mineral density testing between 1993 and 2005. Fracture follow-up continued through 2012 (Menopause 2014 [doi: 10.1097/gme.0000000000000356]).
Among women with a normal BMD on first screening, the estimated time for 1% of those aged 50-54 years to have a hip or clinical vertebral fracture was 12.8 years. Among women aged 60-64 years, the time to fracture was 7.6 years, Dr. Gourlay and her colleagues found.
For the 8.5% of women in the cohort (all ages) with osteoporosis at baseline (n = 344), the age-adjusted time to hip or vertebral fracture was only 3 years.
Dr. Gourlay and her colleagues also estimated times to major osteoporotic fracture for 3% of the cohort, finding that it took 11.5 years for women aged 50-54 years to sustain a hip, clinical vertebral, proximal humerus, or wrist fracture, compared with 8.6 years for women who were 60-64 years at baseline. For women who had osteoporosis at baseline, the age-adjusted time for 3% to have an osteoporotic fracture was 2.5 years.
The researchers acknowledged as limitations of the study the fact that time estimates were based only on transitions to major fracture; that the full benefits and risks of screening, including cost-effectiveness, were not analyzed; and that the study was not powered to determine fracture risk in subgroups defined by individual risk factors.
Nonetheless, the results suggest that deferred repeat screening can be safe for postmenopausal women aged 50 years and older with normal BMD results at baseline, Dr. Gourlay and her coauthors wrote.
The study was funded by the National Institutes of Health. One of Dr. Gourlay’s coauthors is a consultant for MSD, and another reported receiving recent funding from Bone Ultrasound Finland.
FROM MENOPAUSE
Key clinical point: Women under age 65 without risk factors whose first BMD screen is normal are not likely to benefit from repeat screening.
Major finding: Time to hip or vertebral fracture for 1% of women aged 50-54 years with normal BMD at baseline was 12.8 years, and 7.6 years for women aged 60-64 years, compared with 3 years for women aged 50-64 years with baseline osteoporosis.
Data source: An observational cohort of 4,068 women recruited as part of a larger trial cohort of postmenopausal women (n = 161,808) in the Women’s Health Initiative study.
Disclosures: The study was funded by the National Institutes of Health. One of Dr. Gourlay’s coauthors is a consultant for MSD, and another reported receiving recent funding from Bone Ultrasound Finland.
Up to 90% of gout hospitalizations might be preventable
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Gout patients are often admitted to the hospital via the emergency department; most of these admissions could be avoidable.
Major finding: Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission.
Data source: A retrospective analysis of 79 cases of gout at a single institution.
Disclosures: The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
Total thyroidectomy more likely with younger thyroid cancer patients
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Younger patients with differentiated thyroid cancer were more likely to undergo total thyroidectomy and receive radioactive iodine.
Major finding: Total or near-total thyroidectomy was slightly more common in patients younger than age 45 years, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients were also more likely to receive RAI (55% vs. 49%, P < .0001).
Data source: A study of 23,629 patients from the California Cancer Registry who were diagnosed with differentiated thyroid cancer between 2004 and 2011.
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
Noninsulinoma Pancreatogenous Hypoglycemia Syndrome Following Gastric Bypass Surgery
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
Fatal Family History Worries Young Man
ANSWER
The ECG shows normal sinus rhythm and left ventricular hypertrophy (LVH). LVH is indicated by high voltages in limb leads I and III (sum of R and S waves in leads I and III ≥ 25 mm) or in precordial leads V1, V5, and/or V6 (sum of V1 and either V5 or V6 ≥ 35 mm).
Subsequent work-up, including echocardiography and genetic testing, revealed a familial LVH.
ANSWER
The ECG shows normal sinus rhythm and left ventricular hypertrophy (LVH). LVH is indicated by high voltages in limb leads I and III (sum of R and S waves in leads I and III ≥ 25 mm) or in precordial leads V1, V5, and/or V6 (sum of V1 and either V5 or V6 ≥ 35 mm).
Subsequent work-up, including echocardiography and genetic testing, revealed a familial LVH.
ANSWER
The ECG shows normal sinus rhythm and left ventricular hypertrophy (LVH). LVH is indicated by high voltages in limb leads I and III (sum of R and S waves in leads I and III ≥ 25 mm) or in precordial leads V1, V5, and/or V6 (sum of V1 and either V5 or V6 ≥ 35 mm).
Subsequent work-up, including echocardiography and genetic testing, revealed a familial LVH.
A college student, 19, presents with increasing palpitations. Six months ago, when they began, they were rare and intermittent; now they occur daily, primarily at night. He has just received an athletic scholarship and worries that the palpitations may affect his ability to play. Furthermore, his older brother died of sudden cardiac death in high school, while playing football, and the patient is afraid this may happen to him too. He is in otherwise excellent health and has never been hospitalized. He takes no medications but has smoked marijuana a couple of times. He has not used performance enhancing drugs or homeopathic medications. A careful review of his family history reveals that two uncles, a brother, and a cousin died of sudden cardiac death. Their ages at the time of death were 42, 51, 17, and 54, respectively. Review of systems is unremarkable. Vital signs include a blood pressure of 108/62 mm Hg; pulse, 60 beats/min; and respiratory rate, 14 breaths/min-1. His weight is 179 lb and his height, 78 in. The physical exam reveals a tall, thin, well-developed young male in no distress. A comprehensive examination reveals no adverse findings. There are no palpitations heard or felt. Despite the lack of unusual physical findings, the patient’s family history concerns you. You decide to order an ECG and an echocardiogram. The ECG shows a ventricular rate of 61 beats/min; PR interval, 120 ms; QRS duration, 108 ms; QT/QTc interval, 430/432 ms; P axis, –25°; R axis, –14°; and T axis, 12°. What is your interpretation of this ECG—and is further work-up indicated?
Retired Tour Guide Intends to Maintain Her Tan
ANSWER
The correct answer is poikiloderma of Civatte (choice “c”), details of which are discussed below.
Poikiloderma vasculare atrophicans (choice “a”) can be an early indication of T-cell lymphoma but would probably not be chronic or confined to sun-exposed skin.
Several forms of lupus (choice “b”) can present with poikilodermatous skin changes, but these would probably not be chronic.
Dermatoheliosis (choice “d”) is the term for the collective effects of overexposure to the sun, of which poikiloderma of Civatte is but one example.
DISCUSSION
The French dermatologist Achille Civatte (1877-1956) first described this particular pattern of sun damage in 1923—about the same time that sunbathing became fashionable among the well-off in the post-WWI era. He noted the distinct combination of telangiectasias, hyperpigmentation, and epidermal atrophy affecting the bilateral neck and lower face, combined with sharply defined sparing of the portion of the anterior neck shaded by the chin. Poikiloderma of Civatte (PC) is extremely common, especially in middle-aged women and, as one might expect, in those with a history of excessive sun exposure over a period of many years.
Though sun-caused, PC is a purely cosmetic issue and does not lead to skin cancer. While it typically causes no symptoms, it does become more obvious with time. The changes are so gradual that others typically notice them before the patient becomes aware.
Transposing these types of skin changes to other locations would make them considerably more worrisome, specifically in the context of possible incipient T-cell lymphoma—one of the very few types of skin cancer that can take years to evolve into frank cancer. But the atrophy, telangiectasias, and discoloration signaling early cutaneous T-cell lymphoma are usually seen in non–sun-exposed skin, particularly in the waistline and groin.
Poikiloderma vasculare atrophicans is only one of several manifestations termed premycotic. This refers to mycosis fungoides, one of the two most common forms of T-cell lymphoma. Serial biopsy, sometimes over the span of several years, is often used to track such changes.
Pulsed light devices and certain types of lasers have been used successfully to treat PC. Our patient, however, declined treatment, declaring her firm intention to maintain “a healthy tan” year-round.
ANSWER
The correct answer is poikiloderma of Civatte (choice “c”), details of which are discussed below.
Poikiloderma vasculare atrophicans (choice “a”) can be an early indication of T-cell lymphoma but would probably not be chronic or confined to sun-exposed skin.
Several forms of lupus (choice “b”) can present with poikilodermatous skin changes, but these would probably not be chronic.
Dermatoheliosis (choice “d”) is the term for the collective effects of overexposure to the sun, of which poikiloderma of Civatte is but one example.
DISCUSSION
The French dermatologist Achille Civatte (1877-1956) first described this particular pattern of sun damage in 1923—about the same time that sunbathing became fashionable among the well-off in the post-WWI era. He noted the distinct combination of telangiectasias, hyperpigmentation, and epidermal atrophy affecting the bilateral neck and lower face, combined with sharply defined sparing of the portion of the anterior neck shaded by the chin. Poikiloderma of Civatte (PC) is extremely common, especially in middle-aged women and, as one might expect, in those with a history of excessive sun exposure over a period of many years.
Though sun-caused, PC is a purely cosmetic issue and does not lead to skin cancer. While it typically causes no symptoms, it does become more obvious with time. The changes are so gradual that others typically notice them before the patient becomes aware.
Transposing these types of skin changes to other locations would make them considerably more worrisome, specifically in the context of possible incipient T-cell lymphoma—one of the very few types of skin cancer that can take years to evolve into frank cancer. But the atrophy, telangiectasias, and discoloration signaling early cutaneous T-cell lymphoma are usually seen in non–sun-exposed skin, particularly in the waistline and groin.
Poikiloderma vasculare atrophicans is only one of several manifestations termed premycotic. This refers to mycosis fungoides, one of the two most common forms of T-cell lymphoma. Serial biopsy, sometimes over the span of several years, is often used to track such changes.
Pulsed light devices and certain types of lasers have been used successfully to treat PC. Our patient, however, declined treatment, declaring her firm intention to maintain “a healthy tan” year-round.
ANSWER
The correct answer is poikiloderma of Civatte (choice “c”), details of which are discussed below.
Poikiloderma vasculare atrophicans (choice “a”) can be an early indication of T-cell lymphoma but would probably not be chronic or confined to sun-exposed skin.
Several forms of lupus (choice “b”) can present with poikilodermatous skin changes, but these would probably not be chronic.
Dermatoheliosis (choice “d”) is the term for the collective effects of overexposure to the sun, of which poikiloderma of Civatte is but one example.
DISCUSSION
The French dermatologist Achille Civatte (1877-1956) first described this particular pattern of sun damage in 1923—about the same time that sunbathing became fashionable among the well-off in the post-WWI era. He noted the distinct combination of telangiectasias, hyperpigmentation, and epidermal atrophy affecting the bilateral neck and lower face, combined with sharply defined sparing of the portion of the anterior neck shaded by the chin. Poikiloderma of Civatte (PC) is extremely common, especially in middle-aged women and, as one might expect, in those with a history of excessive sun exposure over a period of many years.
Though sun-caused, PC is a purely cosmetic issue and does not lead to skin cancer. While it typically causes no symptoms, it does become more obvious with time. The changes are so gradual that others typically notice them before the patient becomes aware.
Transposing these types of skin changes to other locations would make them considerably more worrisome, specifically in the context of possible incipient T-cell lymphoma—one of the very few types of skin cancer that can take years to evolve into frank cancer. But the atrophy, telangiectasias, and discoloration signaling early cutaneous T-cell lymphoma are usually seen in non–sun-exposed skin, particularly in the waistline and groin.
Poikiloderma vasculare atrophicans is only one of several manifestations termed premycotic. This refers to mycosis fungoides, one of the two most common forms of T-cell lymphoma. Serial biopsy, sometimes over the span of several years, is often used to track such changes.
Pulsed light devices and certain types of lasers have been used successfully to treat PC. Our patient, however, declined treatment, declaring her firm intention to maintain “a healthy tan” year-round.
A 60-year-old woman is seen for complaints of skin changes on her neck that have slowly become more noticeable over a period of years. Although asymptomatic, these changes have been observed by others, who brought them to the patient’s attention. The patient worked as a tour guide in Arizona for 20 years, leading groups along desert trails to view native flora and fauna. During that time, she maintained a dark tan almost year-round, tanning easily and never using sunscreen. The patient has type III skin, bluish gray eyes, and light brown hair. Dark brown–to–red mottled pigmentary changes are seen on the sides of her neck; the central portion of the anterior neck is sharply spared. On closer inspection, many fine telangiectasias are noted in these same areas, as well as on the sun-exposed areas of the face. Aside from her skin changes, the patient claims to be quite healthy, with no joint pain, fever, or malaise.