User login
Radioactive Iodine Scintiphotos of a Man With Thyroid Cancer
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
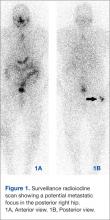
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
Reorganizing a Hospital Ward
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
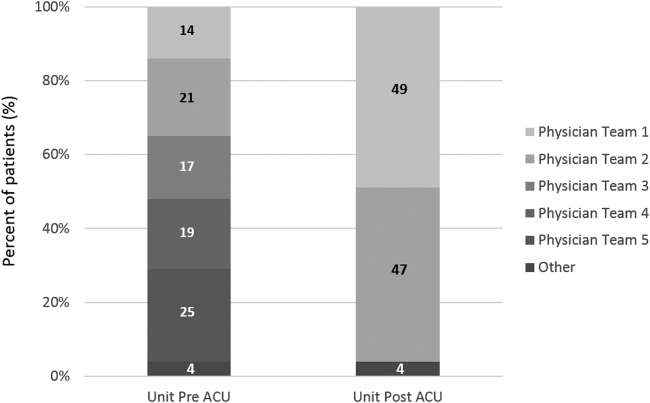
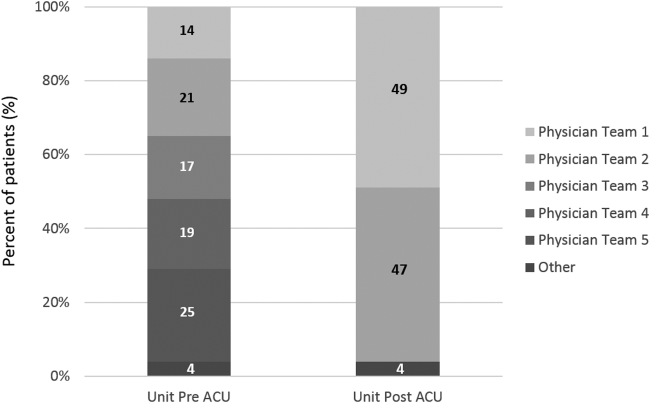
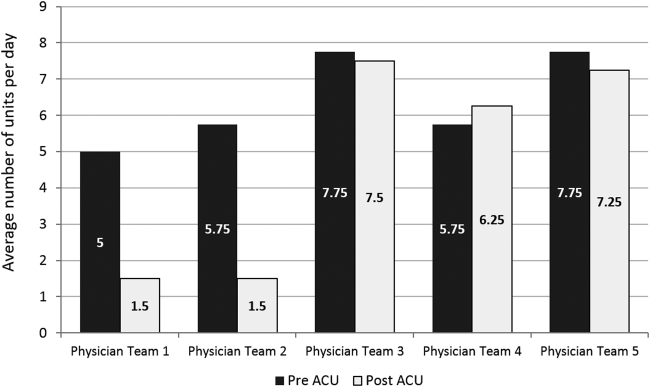
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.
Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
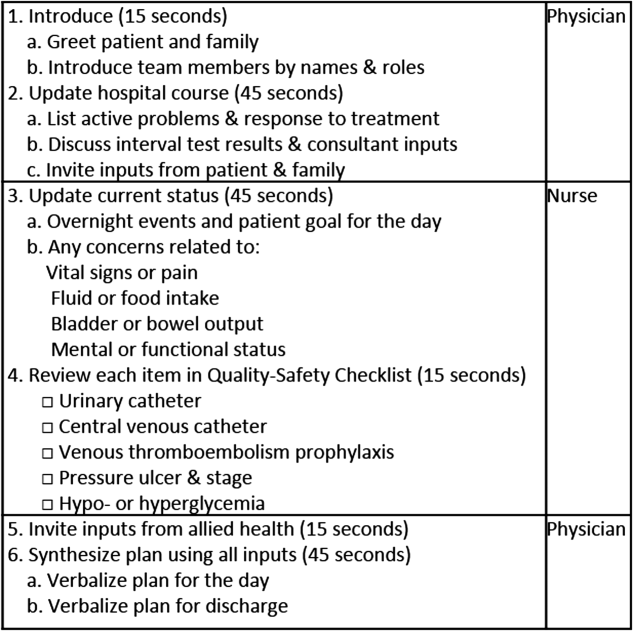
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]
Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.


Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]

Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.


Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]

Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
Telephone CPR training boosts cardiac arrest survival
CHICAGO – Systematic implementation of a comprehensive telephone CPR bundle of care targeting EMS dispatch services resulted in substantial improvements in rates of survival to hospital discharge with good neurologic outcomes in patients with out-of-hospital cardiac arrest in a major Arizona statewide public health initiative.
How big was the intervention’s impact? The rate of survival to hospital discharge showed a 33% relative increase compared to preintervention, and survival with a favorable Cerebral Performance Category score of 0 or 1 increased by 42%, Dr. Bentley J. Bobrow reported at the American Heart Association Scientific Sessions.
“These results suggest that when deliberately implemented and measured, telephone CPR is a targeted, effective method to increase bystander CPR and survival on a vast scale with minimal capital expense. This is why we believe telephone CPR along with public training may be the most efficient way to move the needle on cardiac arrest survival,” declared Dr. Bobrow, professor of emergency medicine at the University of Arizona College of Medicine-Phoenix Campus and chair of the AHA Basic Life Support Subcommittee.
Telephone CPR (T-CPR) entails the provision of CPR instruction to bystanders who have called 911 regarding an out of hospital cardiac arrest (OHCA). It’s well established that bystander CPR commenced before EMS personnel arrive on the scene doubles or even triples OHCA survival, but it is provided in only about one-third of OHCA events. And while T-CPR is independently associated with increased rates of bystander CPR as well as patient survival, its utilization varies widely throughout the country and few EMS services measure performance.
Dr. Bobrow reported on an ambitious undertaking that involved systematic training in T-CPR for dispatchers, 911 managers, and medical directors at all nine of the regional emergency dispatch centers in Arizona, which together with 190 EMS agencies and 40 cardiac care hospitals participate in a statewide resuscitation program.
The training was designed to implement the latest AHA guidelines on T-CPR (Circulation 2012;125:648-55). The program entailed a half-day in-person training session plus completion of a 1-hour web-based interactive video. The protocol emphasizes asking two key questions of the 911 caller: “Is the patient conscious?” and “Is the patient breathing normally?” If the response is no to both, the dispatcher is to start issuing bystander CPR instructions without delay – no further questions – and continue the coaching until EMS personnel arrive on the scene to take over.
A core aspect of the T-CPR bundle is performance measurement for quality improvement, with auditing of 911 calls to learn the time from the start of the call to the bystander’s first chest compression and five other key performance metrics. Feedback is provided to the 911 call center regarding system- and case-level performance reports in a continuing education, quality improvement process. Individual dispatchers are singled out for exemplary performance, Dr. Bobrow explained.
He presented a prospective before-and-after study conducted at the three EMS dispatch centers serving Arizona’s Maricopa County, home to two-thirds of the state’s population. The study entailed auditing nearly 6,000 911 calls, each averaging 6.5 minutes in length. After excluding calls where CPR wasn’t indicated or the OHCA involved a patient less than 8 years old, investigators were left with two groups for comparison comprised of 1,289 pre- and 2,330 post-intervention events.
The improvements in process and clinical outcomes were dramatic. In 2012, after introduction of the T-CPR training program, the bystander CPR rate crossed the 50% threshold for the first time ever in Maricopa County. The rate of survival of OHCA to hospital discharge improved from 8.3% to 11%, a highly statistically significant 33% relative increase. Survival with a Cerebral Performance Category score of 0 or 1 climbed from 5.5% to 7.8%, a 42% relative increase. In a multivariate analysis adjusted for potential confounders, the adjusted odds ratio for survival of OHCA was 2.25-fold greater for all cases after implementation of the T-CPR program and similarly increased for arrests of cardiac origin.
Dr. Bobrow observed that this was not a randomized trial, which he considered would be both unethical and impractical.
“We controlled for known risk factors and confounders, and while we cannot prove that better outcomes resulted directly from the process improvements, the two are independently associated in this controlled study,” said the emergency physician, who is medical director of the Bureau of Emergency Medical Services and Trauma Systems at the Arizona Dept. of Health Services.
Audience members rose to praise the “fantastic” achievements in Arizona and ask why they’re not having similar success rates in their own districts, given that the AHA guidelines are readily available.
“A lot of places say they’re doing this,” Dr. Bobrow replied, “but when they realize what ‘this’ is, they understand that they really weren’t doing it in this type of depth. When we showed them the data on how marginal their performance was, I think that really made the difference.”
“I think that once most dispatch centers really understand the issues and their importance and the power that they have, and you can engage them, I’m confident that people would see the same changes,” he added.
His study was honored as the best oral abstract presentation at the AHA resuscitation science symposium.
Dr. Bobrow reported serving as co-principal investigator of the HeartRescue Project, funded by Medtronic Philanthropy.
CHICAGO – Systematic implementation of a comprehensive telephone CPR bundle of care targeting EMS dispatch services resulted in substantial improvements in rates of survival to hospital discharge with good neurologic outcomes in patients with out-of-hospital cardiac arrest in a major Arizona statewide public health initiative.
How big was the intervention’s impact? The rate of survival to hospital discharge showed a 33% relative increase compared to preintervention, and survival with a favorable Cerebral Performance Category score of 0 or 1 increased by 42%, Dr. Bentley J. Bobrow reported at the American Heart Association Scientific Sessions.
“These results suggest that when deliberately implemented and measured, telephone CPR is a targeted, effective method to increase bystander CPR and survival on a vast scale with minimal capital expense. This is why we believe telephone CPR along with public training may be the most efficient way to move the needle on cardiac arrest survival,” declared Dr. Bobrow, professor of emergency medicine at the University of Arizona College of Medicine-Phoenix Campus and chair of the AHA Basic Life Support Subcommittee.
Telephone CPR (T-CPR) entails the provision of CPR instruction to bystanders who have called 911 regarding an out of hospital cardiac arrest (OHCA). It’s well established that bystander CPR commenced before EMS personnel arrive on the scene doubles or even triples OHCA survival, but it is provided in only about one-third of OHCA events. And while T-CPR is independently associated with increased rates of bystander CPR as well as patient survival, its utilization varies widely throughout the country and few EMS services measure performance.
Dr. Bobrow reported on an ambitious undertaking that involved systematic training in T-CPR for dispatchers, 911 managers, and medical directors at all nine of the regional emergency dispatch centers in Arizona, which together with 190 EMS agencies and 40 cardiac care hospitals participate in a statewide resuscitation program.
The training was designed to implement the latest AHA guidelines on T-CPR (Circulation 2012;125:648-55). The program entailed a half-day in-person training session plus completion of a 1-hour web-based interactive video. The protocol emphasizes asking two key questions of the 911 caller: “Is the patient conscious?” and “Is the patient breathing normally?” If the response is no to both, the dispatcher is to start issuing bystander CPR instructions without delay – no further questions – and continue the coaching until EMS personnel arrive on the scene to take over.
A core aspect of the T-CPR bundle is performance measurement for quality improvement, with auditing of 911 calls to learn the time from the start of the call to the bystander’s first chest compression and five other key performance metrics. Feedback is provided to the 911 call center regarding system- and case-level performance reports in a continuing education, quality improvement process. Individual dispatchers are singled out for exemplary performance, Dr. Bobrow explained.
He presented a prospective before-and-after study conducted at the three EMS dispatch centers serving Arizona’s Maricopa County, home to two-thirds of the state’s population. The study entailed auditing nearly 6,000 911 calls, each averaging 6.5 minutes in length. After excluding calls where CPR wasn’t indicated or the OHCA involved a patient less than 8 years old, investigators were left with two groups for comparison comprised of 1,289 pre- and 2,330 post-intervention events.
The improvements in process and clinical outcomes were dramatic. In 2012, after introduction of the T-CPR training program, the bystander CPR rate crossed the 50% threshold for the first time ever in Maricopa County. The rate of survival of OHCA to hospital discharge improved from 8.3% to 11%, a highly statistically significant 33% relative increase. Survival with a Cerebral Performance Category score of 0 or 1 climbed from 5.5% to 7.8%, a 42% relative increase. In a multivariate analysis adjusted for potential confounders, the adjusted odds ratio for survival of OHCA was 2.25-fold greater for all cases after implementation of the T-CPR program and similarly increased for arrests of cardiac origin.
Dr. Bobrow observed that this was not a randomized trial, which he considered would be both unethical and impractical.
“We controlled for known risk factors and confounders, and while we cannot prove that better outcomes resulted directly from the process improvements, the two are independently associated in this controlled study,” said the emergency physician, who is medical director of the Bureau of Emergency Medical Services and Trauma Systems at the Arizona Dept. of Health Services.
Audience members rose to praise the “fantastic” achievements in Arizona and ask why they’re not having similar success rates in their own districts, given that the AHA guidelines are readily available.
“A lot of places say they’re doing this,” Dr. Bobrow replied, “but when they realize what ‘this’ is, they understand that they really weren’t doing it in this type of depth. When we showed them the data on how marginal their performance was, I think that really made the difference.”
“I think that once most dispatch centers really understand the issues and their importance and the power that they have, and you can engage them, I’m confident that people would see the same changes,” he added.
His study was honored as the best oral abstract presentation at the AHA resuscitation science symposium.
Dr. Bobrow reported serving as co-principal investigator of the HeartRescue Project, funded by Medtronic Philanthropy.
CHICAGO – Systematic implementation of a comprehensive telephone CPR bundle of care targeting EMS dispatch services resulted in substantial improvements in rates of survival to hospital discharge with good neurologic outcomes in patients with out-of-hospital cardiac arrest in a major Arizona statewide public health initiative.
How big was the intervention’s impact? The rate of survival to hospital discharge showed a 33% relative increase compared to preintervention, and survival with a favorable Cerebral Performance Category score of 0 or 1 increased by 42%, Dr. Bentley J. Bobrow reported at the American Heart Association Scientific Sessions.
“These results suggest that when deliberately implemented and measured, telephone CPR is a targeted, effective method to increase bystander CPR and survival on a vast scale with minimal capital expense. This is why we believe telephone CPR along with public training may be the most efficient way to move the needle on cardiac arrest survival,” declared Dr. Bobrow, professor of emergency medicine at the University of Arizona College of Medicine-Phoenix Campus and chair of the AHA Basic Life Support Subcommittee.
Telephone CPR (T-CPR) entails the provision of CPR instruction to bystanders who have called 911 regarding an out of hospital cardiac arrest (OHCA). It’s well established that bystander CPR commenced before EMS personnel arrive on the scene doubles or even triples OHCA survival, but it is provided in only about one-third of OHCA events. And while T-CPR is independently associated with increased rates of bystander CPR as well as patient survival, its utilization varies widely throughout the country and few EMS services measure performance.
Dr. Bobrow reported on an ambitious undertaking that involved systematic training in T-CPR for dispatchers, 911 managers, and medical directors at all nine of the regional emergency dispatch centers in Arizona, which together with 190 EMS agencies and 40 cardiac care hospitals participate in a statewide resuscitation program.
The training was designed to implement the latest AHA guidelines on T-CPR (Circulation 2012;125:648-55). The program entailed a half-day in-person training session plus completion of a 1-hour web-based interactive video. The protocol emphasizes asking two key questions of the 911 caller: “Is the patient conscious?” and “Is the patient breathing normally?” If the response is no to both, the dispatcher is to start issuing bystander CPR instructions without delay – no further questions – and continue the coaching until EMS personnel arrive on the scene to take over.
A core aspect of the T-CPR bundle is performance measurement for quality improvement, with auditing of 911 calls to learn the time from the start of the call to the bystander’s first chest compression and five other key performance metrics. Feedback is provided to the 911 call center regarding system- and case-level performance reports in a continuing education, quality improvement process. Individual dispatchers are singled out for exemplary performance, Dr. Bobrow explained.
He presented a prospective before-and-after study conducted at the three EMS dispatch centers serving Arizona’s Maricopa County, home to two-thirds of the state’s population. The study entailed auditing nearly 6,000 911 calls, each averaging 6.5 minutes in length. After excluding calls where CPR wasn’t indicated or the OHCA involved a patient less than 8 years old, investigators were left with two groups for comparison comprised of 1,289 pre- and 2,330 post-intervention events.
The improvements in process and clinical outcomes were dramatic. In 2012, after introduction of the T-CPR training program, the bystander CPR rate crossed the 50% threshold for the first time ever in Maricopa County. The rate of survival of OHCA to hospital discharge improved from 8.3% to 11%, a highly statistically significant 33% relative increase. Survival with a Cerebral Performance Category score of 0 or 1 climbed from 5.5% to 7.8%, a 42% relative increase. In a multivariate analysis adjusted for potential confounders, the adjusted odds ratio for survival of OHCA was 2.25-fold greater for all cases after implementation of the T-CPR program and similarly increased for arrests of cardiac origin.
Dr. Bobrow observed that this was not a randomized trial, which he considered would be both unethical and impractical.
“We controlled for known risk factors and confounders, and while we cannot prove that better outcomes resulted directly from the process improvements, the two are independently associated in this controlled study,” said the emergency physician, who is medical director of the Bureau of Emergency Medical Services and Trauma Systems at the Arizona Dept. of Health Services.
Audience members rose to praise the “fantastic” achievements in Arizona and ask why they’re not having similar success rates in their own districts, given that the AHA guidelines are readily available.
“A lot of places say they’re doing this,” Dr. Bobrow replied, “but when they realize what ‘this’ is, they understand that they really weren’t doing it in this type of depth. When we showed them the data on how marginal their performance was, I think that really made the difference.”
“I think that once most dispatch centers really understand the issues and their importance and the power that they have, and you can engage them, I’m confident that people would see the same changes,” he added.
His study was honored as the best oral abstract presentation at the AHA resuscitation science symposium.
Dr. Bobrow reported serving as co-principal investigator of the HeartRescue Project, funded by Medtronic Philanthropy.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Adoption of the most recent AHA guidelines on telephone CPR by EMS dispatchers will lead to vast improvement in survival for patients with out-of-hospital cardiac arrest.
Major finding: Following implementation of an Arizona statewide program to improve telephone CPR by 911 dispatchers to bystanders at the scene of out-of-hospital cardiac arrest, survival to hospital discharge climbed from 8.3% to 11.0%.
Data source: This was a prospective study comparing the outcomes of 1,289 calls to Arizona 911 centers regarding out-of-hospital cardiac arrests before introduction of a comprehensive statewide telephone CPR program to 2,330 calls received post-intervention.
Disclosures: The study was financially supported by Medtronic Philanthropy as part of the HeartRescue Project. The presenter is co-principal investigator of the project.
IL-6 inhibitor helps prevent GVHD

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()
Will US lift ban on MSM blood donation?

Credit: Daniel Gay
A committee that advises the US Department of Health and Human Services (HHS) has recommended changing the policy that prevents men who have sex with men (MSM) from donating blood.
The Advisory Committee on Blood and Tissue Safety and Availability decided MSM should be allowed to donate blood if they have abstained from sex for a year.
A group of advisers to the US Food and Drug Administration (FDA) will consider this recommendation in a meeting on December 2.
The HHS advisory committee plans to meet on December 5 to discuss establishing a donor transfusion-transmissible infection-monitoring system. The HHS has said such a system should be put in place before lifting the lifetime ban on MSM blood donation.
Should the FDA decide to change its policy, the US would follow other countries that have lifted the lifetime ban in recent years.
For instance, MSM in the UK and Australia are now allowed to donate blood if they have been celibate for a year, MSM in Canada must be celibate for 5 years, and MSM in South Africa can donate if they have been celibate or in a monogamous relationship for 6 months.
The safety of the blood supply
Lifting the lifetime ban on MSM blood donors may raise concerns about the safety of the blood supply, with transfusion recipients worrying they will have a greater risk of contracting HIV.
Although donated blood is tested for HIV, there is an 11-day window in which current tests cannot detect the virus in people who just contracted it. And MSM are more severely affected by HIV than any other group in the US, according to the Centers for Disease Control and Prevention.
Of course, deferring MSM donation for a year would allow enough time for HIV to be strong enough for tests to detect the virus. However, that assumes that donors are telling the truth about their sexual practices.
A study of more than 1000 MSM in Britain showed that 11% had donated blood after having penetrative sex with a man, and 3% had done so in the past 12 months, despite the lifetime ban on MSM blood donation. The study was conducted before the UK lifted the ban.
Still, study investigators said the results supported lifting the lifetime ban on MSM because men who did not comply with the ban generally said they would comply with a 1-year deferral period.
The AABB, America’s Blood Centers, and the American Red Cross have said they support a 1-year deferral period for MSM who want to donate blood in the US.
“This change in policy would align the donor deferral period for MSM with criteria for other activities that may pose a similar risk of transfusion-transmissible infections,” the groups said.
“We believe the current FDA indefinite blood donation deferral for a man who has sex with another man since 1977 is medically and scientifically unwarranted. The blood banking community strongly supports the use of rational, scientifically based deferral periods that are applied fairly and consistently among blood donors who engage in similar-risk activities.”
A report by the Williams Institute suggested that, if the FDA were to lift the ban on MSM completely, an additional 360,600 men could donate 615,300 additional pints of blood each year.
On the other hand, an HHS report suggested that the US supply of blood units is already surpassing demand. The report showed that 15.7 million units of whole blood and red blood cells were collected in 2011, and the total number of units transfused was 13.8 million. ![]()

Credit: Daniel Gay
A committee that advises the US Department of Health and Human Services (HHS) has recommended changing the policy that prevents men who have sex with men (MSM) from donating blood.
The Advisory Committee on Blood and Tissue Safety and Availability decided MSM should be allowed to donate blood if they have abstained from sex for a year.
A group of advisers to the US Food and Drug Administration (FDA) will consider this recommendation in a meeting on December 2.
The HHS advisory committee plans to meet on December 5 to discuss establishing a donor transfusion-transmissible infection-monitoring system. The HHS has said such a system should be put in place before lifting the lifetime ban on MSM blood donation.
Should the FDA decide to change its policy, the US would follow other countries that have lifted the lifetime ban in recent years.
For instance, MSM in the UK and Australia are now allowed to donate blood if they have been celibate for a year, MSM in Canada must be celibate for 5 years, and MSM in South Africa can donate if they have been celibate or in a monogamous relationship for 6 months.
The safety of the blood supply
Lifting the lifetime ban on MSM blood donors may raise concerns about the safety of the blood supply, with transfusion recipients worrying they will have a greater risk of contracting HIV.
Although donated blood is tested for HIV, there is an 11-day window in which current tests cannot detect the virus in people who just contracted it. And MSM are more severely affected by HIV than any other group in the US, according to the Centers for Disease Control and Prevention.
Of course, deferring MSM donation for a year would allow enough time for HIV to be strong enough for tests to detect the virus. However, that assumes that donors are telling the truth about their sexual practices.
A study of more than 1000 MSM in Britain showed that 11% had donated blood after having penetrative sex with a man, and 3% had done so in the past 12 months, despite the lifetime ban on MSM blood donation. The study was conducted before the UK lifted the ban.
Still, study investigators said the results supported lifting the lifetime ban on MSM because men who did not comply with the ban generally said they would comply with a 1-year deferral period.
The AABB, America’s Blood Centers, and the American Red Cross have said they support a 1-year deferral period for MSM who want to donate blood in the US.
“This change in policy would align the donor deferral period for MSM with criteria for other activities that may pose a similar risk of transfusion-transmissible infections,” the groups said.
“We believe the current FDA indefinite blood donation deferral for a man who has sex with another man since 1977 is medically and scientifically unwarranted. The blood banking community strongly supports the use of rational, scientifically based deferral periods that are applied fairly and consistently among blood donors who engage in similar-risk activities.”
A report by the Williams Institute suggested that, if the FDA were to lift the ban on MSM completely, an additional 360,600 men could donate 615,300 additional pints of blood each year.
On the other hand, an HHS report suggested that the US supply of blood units is already surpassing demand. The report showed that 15.7 million units of whole blood and red blood cells were collected in 2011, and the total number of units transfused was 13.8 million. ![]()

Credit: Daniel Gay
A committee that advises the US Department of Health and Human Services (HHS) has recommended changing the policy that prevents men who have sex with men (MSM) from donating blood.
The Advisory Committee on Blood and Tissue Safety and Availability decided MSM should be allowed to donate blood if they have abstained from sex for a year.
A group of advisers to the US Food and Drug Administration (FDA) will consider this recommendation in a meeting on December 2.
The HHS advisory committee plans to meet on December 5 to discuss establishing a donor transfusion-transmissible infection-monitoring system. The HHS has said such a system should be put in place before lifting the lifetime ban on MSM blood donation.
Should the FDA decide to change its policy, the US would follow other countries that have lifted the lifetime ban in recent years.
For instance, MSM in the UK and Australia are now allowed to donate blood if they have been celibate for a year, MSM in Canada must be celibate for 5 years, and MSM in South Africa can donate if they have been celibate or in a monogamous relationship for 6 months.
The safety of the blood supply
Lifting the lifetime ban on MSM blood donors may raise concerns about the safety of the blood supply, with transfusion recipients worrying they will have a greater risk of contracting HIV.
Although donated blood is tested for HIV, there is an 11-day window in which current tests cannot detect the virus in people who just contracted it. And MSM are more severely affected by HIV than any other group in the US, according to the Centers for Disease Control and Prevention.
Of course, deferring MSM donation for a year would allow enough time for HIV to be strong enough for tests to detect the virus. However, that assumes that donors are telling the truth about their sexual practices.
A study of more than 1000 MSM in Britain showed that 11% had donated blood after having penetrative sex with a man, and 3% had done so in the past 12 months, despite the lifetime ban on MSM blood donation. The study was conducted before the UK lifted the ban.
Still, study investigators said the results supported lifting the lifetime ban on MSM because men who did not comply with the ban generally said they would comply with a 1-year deferral period.
The AABB, America’s Blood Centers, and the American Red Cross have said they support a 1-year deferral period for MSM who want to donate blood in the US.
“This change in policy would align the donor deferral period for MSM with criteria for other activities that may pose a similar risk of transfusion-transmissible infections,” the groups said.
“We believe the current FDA indefinite blood donation deferral for a man who has sex with another man since 1977 is medically and scientifically unwarranted. The blood banking community strongly supports the use of rational, scientifically based deferral periods that are applied fairly and consistently among blood donors who engage in similar-risk activities.”
A report by the Williams Institute suggested that, if the FDA were to lift the ban on MSM completely, an additional 360,600 men could donate 615,300 additional pints of blood each year.
On the other hand, an HHS report suggested that the US supply of blood units is already surpassing demand. The report showed that 15.7 million units of whole blood and red blood cells were collected in 2011, and the total number of units transfused was 13.8 million. ![]()
Kidney donors at greater risk of preeclampsia, gestational hypertension
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
FROM KIDNEY WEEK 2014
Key clinical point: Information on an increased risk for preeclampsia and gestational hypertension should be included in clinical practice guidelines and in informed-consent processes for potential kidney donors and their recipients.
Major finding: Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy.
Data source: Retrospective cohort study of 85 kidney donors who were matched on a 1:6 ratio with 510 healthy nondonors and followed for a median of 10.9 years.
Disclosures:Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. The meeting was sponsored by the American Society of Nephrology.
Extended use of oral anticoagulants reduces VTE recurrence
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
Key clinical point: All of the NOACs provide a net clinical benefit for reducing VTE recurrence.
Major finding: The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban.
Data source: An analysis of data from three clinical trials, including a total of 5,035 patients.
Disclosures: Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
Shrink Rap News: The surprisingly high cost of Abilify
Recently, I gave a patient a prescription for Abilify. I wrote for 30 tablets of the lowest dose, 2 mg. While I knew it was expensive, I was shocked when the patient returned a few days later and told me it had cost $1,100 to fill the prescription; he not yet met the deductible for his health insurance and he had paid cash for the medication.
According to Medscape (and Twitter, too), Abilify is the medication that grosses more money than any other pharmaceutical in the United States. In the 12-month period from July 2013 to June 2014, sales of Abilify totaled $7.2 billion. An atypical antipsychotic medication that is widely marketed to TV viewers as an augmenting agent to treat major depression, Abilify is the 14th most-prescribed medication in the United States. If you’re wondering, the most prescribed medications are Synthroid, Crestor, and Nexium. Abilify has been available in this country since 2002, initially with an indication for schizophrenia. Since then, indications have expanded to include bipolar disorder, irritability in autism, as well as augmentation for major depression.
Still, $1,100 for 30 tablets? I wondered if the high cost was attributed to where the patient went – a boutique independent pharmacy. I decided to make some calls to local pharmacists (see the table), and queried druggists at CVS, Walmart, and Lykos, an independent pharmacy in Towson, Md. I also checked with a Walmart in Vermont to see if the prices were the same in another part of the country, and they were. Let me share with you what I learned.
For the three pharmacies I called, a single 2-mg tablet of Abilify cost between $30 and $33, so the cost was less than the $1,100 my patient paid. There is no discount for buying in bulk, and the price per pill stays virtually the same whether a patient buys 1 pill, 30 pills, or 90 pills. I checked with two pharmacies, and the price for a tablet is the same for the dosages of 2 mg, 5 mg, 10 mg, and 15 mg. The price rises to $38-$47 per pill for the 20-mg and 30-mg dose.
As physicians in a health care system where resources are limited, it is incumbent upon us to at least consider the cost of the tests and treatments we order, but we often have no way of knowing what these costs actually are. Was I missing something? Is everyone else aware that Abilify is this costly? I did a quick survey of a handful of psychiatrists by text message (please don’t count this as science), requesting a guess for the cost of a single 2-mg tablet of Abilify. The responses I received ranged from $7 to $20, and a lone respondent answered $40. For the most part, the cost of medications remains opaque to the prescriber.
If I had to do it again, I still would have prescribed Abilify to this particular patient. I would have suggested he buy only a few tablets to start, and I would have prescribed the 5-mg dose and recommended splitting the pills to halve the cost. In a December 2006 article in Current Psychiatry, “Pros and cons of pill splitting,” Dr. Rakesh Jain and Dr. Shailesh Jain note that it is safe to divide Abilify tablets. Filling only a few tablets seems like the prudent thing to do with such an expensive medication, at least until it is clear that it is tolerable to the patient, but as we know, filling less than a month’s supply often creates hurdles and increased copays when health insurance is paying for the prescription. And with requirements for preauthorization, I’m not certain if it’s even possible for a patient to take home just a few to try.
When I informed the psychiatrists I queried that Abilify costs $30-$33 per 2-mg dose, they expressed their surprise. One friend, however, put it most aptly with her reply of simply, “Good grief.”
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
Recently, I gave a patient a prescription for Abilify. I wrote for 30 tablets of the lowest dose, 2 mg. While I knew it was expensive, I was shocked when the patient returned a few days later and told me it had cost $1,100 to fill the prescription; he not yet met the deductible for his health insurance and he had paid cash for the medication.
According to Medscape (and Twitter, too), Abilify is the medication that grosses more money than any other pharmaceutical in the United States. In the 12-month period from July 2013 to June 2014, sales of Abilify totaled $7.2 billion. An atypical antipsychotic medication that is widely marketed to TV viewers as an augmenting agent to treat major depression, Abilify is the 14th most-prescribed medication in the United States. If you’re wondering, the most prescribed medications are Synthroid, Crestor, and Nexium. Abilify has been available in this country since 2002, initially with an indication for schizophrenia. Since then, indications have expanded to include bipolar disorder, irritability in autism, as well as augmentation for major depression.
Still, $1,100 for 30 tablets? I wondered if the high cost was attributed to where the patient went – a boutique independent pharmacy. I decided to make some calls to local pharmacists (see the table), and queried druggists at CVS, Walmart, and Lykos, an independent pharmacy in Towson, Md. I also checked with a Walmart in Vermont to see if the prices were the same in another part of the country, and they were. Let me share with you what I learned.

For the three pharmacies I called, a single 2-mg tablet of Abilify cost between $30 and $33, so the cost was less than the $1,100 my patient paid. There is no discount for buying in bulk, and the price per pill stays virtually the same whether a patient buys 1 pill, 30 pills, or 90 pills. I checked with two pharmacies, and the price for a tablet is the same for the dosages of 2 mg, 5 mg, 10 mg, and 15 mg. The price rises to $38-$47 per pill for the 20-mg and 30-mg dose.
As physicians in a health care system where resources are limited, it is incumbent upon us to at least consider the cost of the tests and treatments we order, but we often have no way of knowing what these costs actually are. Was I missing something? Is everyone else aware that Abilify is this costly? I did a quick survey of a handful of psychiatrists by text message (please don’t count this as science), requesting a guess for the cost of a single 2-mg tablet of Abilify. The responses I received ranged from $7 to $20, and a lone respondent answered $40. For the most part, the cost of medications remains opaque to the prescriber.
If I had to do it again, I still would have prescribed Abilify to this particular patient. I would have suggested he buy only a few tablets to start, and I would have prescribed the 5-mg dose and recommended splitting the pills to halve the cost. In a December 2006 article in Current Psychiatry, “Pros and cons of pill splitting,” Dr. Rakesh Jain and Dr. Shailesh Jain note that it is safe to divide Abilify tablets. Filling only a few tablets seems like the prudent thing to do with such an expensive medication, at least until it is clear that it is tolerable to the patient, but as we know, filling less than a month’s supply often creates hurdles and increased copays when health insurance is paying for the prescription. And with requirements for preauthorization, I’m not certain if it’s even possible for a patient to take home just a few to try.
When I informed the psychiatrists I queried that Abilify costs $30-$33 per 2-mg dose, they expressed their surprise. One friend, however, put it most aptly with her reply of simply, “Good grief.”
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
Recently, I gave a patient a prescription for Abilify. I wrote for 30 tablets of the lowest dose, 2 mg. While I knew it was expensive, I was shocked when the patient returned a few days later and told me it had cost $1,100 to fill the prescription; he not yet met the deductible for his health insurance and he had paid cash for the medication.
According to Medscape (and Twitter, too), Abilify is the medication that grosses more money than any other pharmaceutical in the United States. In the 12-month period from July 2013 to June 2014, sales of Abilify totaled $7.2 billion. An atypical antipsychotic medication that is widely marketed to TV viewers as an augmenting agent to treat major depression, Abilify is the 14th most-prescribed medication in the United States. If you’re wondering, the most prescribed medications are Synthroid, Crestor, and Nexium. Abilify has been available in this country since 2002, initially with an indication for schizophrenia. Since then, indications have expanded to include bipolar disorder, irritability in autism, as well as augmentation for major depression.
Still, $1,100 for 30 tablets? I wondered if the high cost was attributed to where the patient went – a boutique independent pharmacy. I decided to make some calls to local pharmacists (see the table), and queried druggists at CVS, Walmart, and Lykos, an independent pharmacy in Towson, Md. I also checked with a Walmart in Vermont to see if the prices were the same in another part of the country, and they were. Let me share with you what I learned.

For the three pharmacies I called, a single 2-mg tablet of Abilify cost between $30 and $33, so the cost was less than the $1,100 my patient paid. There is no discount for buying in bulk, and the price per pill stays virtually the same whether a patient buys 1 pill, 30 pills, or 90 pills. I checked with two pharmacies, and the price for a tablet is the same for the dosages of 2 mg, 5 mg, 10 mg, and 15 mg. The price rises to $38-$47 per pill for the 20-mg and 30-mg dose.
As physicians in a health care system where resources are limited, it is incumbent upon us to at least consider the cost of the tests and treatments we order, but we often have no way of knowing what these costs actually are. Was I missing something? Is everyone else aware that Abilify is this costly? I did a quick survey of a handful of psychiatrists by text message (please don’t count this as science), requesting a guess for the cost of a single 2-mg tablet of Abilify. The responses I received ranged from $7 to $20, and a lone respondent answered $40. For the most part, the cost of medications remains opaque to the prescriber.
If I had to do it again, I still would have prescribed Abilify to this particular patient. I would have suggested he buy only a few tablets to start, and I would have prescribed the 5-mg dose and recommended splitting the pills to halve the cost. In a December 2006 article in Current Psychiatry, “Pros and cons of pill splitting,” Dr. Rakesh Jain and Dr. Shailesh Jain note that it is safe to divide Abilify tablets. Filling only a few tablets seems like the prudent thing to do with such an expensive medication, at least until it is clear that it is tolerable to the patient, but as we know, filling less than a month’s supply often creates hurdles and increased copays when health insurance is paying for the prescription. And with requirements for preauthorization, I’m not certain if it’s even possible for a patient to take home just a few to try.
When I informed the psychiatrists I queried that Abilify costs $30-$33 per 2-mg dose, they expressed their surprise. One friend, however, put it most aptly with her reply of simply, “Good grief.”
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
Protein discovery paves way for patient-specific HSCs

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()
Early Cancer Detection Helps Underserved Women
Nearly 60,000 breast and cervical cancers were caught and diagnosed between 1991 and 2011 through the CDC’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP).
The NBCCEDP is the only nationwide cancer screening program serving all 50 states, the District of Columbia, 5 U.S. territories, and 11 tribes or tribal organizations. In its first 20 years, the program served > 4.3 million women who might not otherwise have received preventive screenings. More than 10.7 million received mammograms and Pap tests.
More than 90% of the women in whom cancerous or precancerous lesions were detected received appropriate and timely follow-up care, according to a CDC report, published in an August 2014 supplement to Cancer. The supplement, National Breast and Cervical Cancer Early Detection Program: Two Decades of Service to Underserved Women, contains 13 new papers that evaluate aspects of the NBCCEDP, showing “consistent value” in the program, the CDC says, even beyond its original purpose of detecting cancers in underserved women.
This is the first time detailed information has been published about the program’s screening activities and other interventions. Partnerships with national organizations, community-based organizations, government agencies, tribes, health care systems, and professional organizations have played a “critical role” in achieving NBCCEDP goals, the CDC says.
Nearly 60,000 breast and cervical cancers were caught and diagnosed between 1991 and 2011 through the CDC’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP).
The NBCCEDP is the only nationwide cancer screening program serving all 50 states, the District of Columbia, 5 U.S. territories, and 11 tribes or tribal organizations. In its first 20 years, the program served > 4.3 million women who might not otherwise have received preventive screenings. More than 10.7 million received mammograms and Pap tests.
More than 90% of the women in whom cancerous or precancerous lesions were detected received appropriate and timely follow-up care, according to a CDC report, published in an August 2014 supplement to Cancer. The supplement, National Breast and Cervical Cancer Early Detection Program: Two Decades of Service to Underserved Women, contains 13 new papers that evaluate aspects of the NBCCEDP, showing “consistent value” in the program, the CDC says, even beyond its original purpose of detecting cancers in underserved women.
This is the first time detailed information has been published about the program’s screening activities and other interventions. Partnerships with national organizations, community-based organizations, government agencies, tribes, health care systems, and professional organizations have played a “critical role” in achieving NBCCEDP goals, the CDC says.
Nearly 60,000 breast and cervical cancers were caught and diagnosed between 1991 and 2011 through the CDC’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP).
The NBCCEDP is the only nationwide cancer screening program serving all 50 states, the District of Columbia, 5 U.S. territories, and 11 tribes or tribal organizations. In its first 20 years, the program served > 4.3 million women who might not otherwise have received preventive screenings. More than 10.7 million received mammograms and Pap tests.
More than 90% of the women in whom cancerous or precancerous lesions were detected received appropriate and timely follow-up care, according to a CDC report, published in an August 2014 supplement to Cancer. The supplement, National Breast and Cervical Cancer Early Detection Program: Two Decades of Service to Underserved Women, contains 13 new papers that evaluate aspects of the NBCCEDP, showing “consistent value” in the program, the CDC says, even beyond its original purpose of detecting cancers in underserved women.
This is the first time detailed information has been published about the program’s screening activities and other interventions. Partnerships with national organizations, community-based organizations, government agencies, tribes, health care systems, and professional organizations have played a “critical role” in achieving NBCCEDP goals, the CDC says.