User login
Utilization of the ICF-CY for the Classification of Therapeutic Objectives in the Treatment of Spasticity in Children with Cerebral Palsy
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, [email protected].
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, [email protected].
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, [email protected].
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
Reducing Hospital Readmissions for CHF Patients through Pre-Discharge Simulation-Based Learning
From North Mississippi Health Services, Tupelo, MS (Drs. Greer and Fagan), and the University of Colorado, Denver, CO (Dr. Coleman).
Abstract
- Objective: To describe the self-care college, an innovative initiative designed to reduce hospital readmissions for congestive heart failure (CHF) patients.
- Methods: CHF patients at North Mississippi Medical Center are asked to participate in a “self-care college” prior to discharge. Participants rotate through 3 learning stations: weight, diet and medications. At each station, they are asked to perform the tasks they will be required to do at home. By engaging patients in the learning process, they are activated to assume responsibility for their care. This approach has the added advantage of providing a feedback loop, allowing the health care team to “road test” the proposed care plan to determine the likelihood that the patient (and family caregivers) will be able to execute following discharge.
- Results: Since the self-care college was implemented in 2011, the 30-day readmission rate for CHF patients at NMMC has been reduced from 16.8% to 12.85%. There has also been a reduction in the observed to expected CHF readmissions ratio, from 0.90 to 0.71.
- Conclusion: Although the self-care college targets CHF patients, it is likely that this type of initiative could be applied for rural patients with other chronic illnesses, such as asthma, COPD, and diabetes. It is a relatively simple and inexpensive program (approximately $30,000 per year, primarily in personnel expenses, or roughly the cost of 3 hospital readmissions) that does not require sophisticated technology or equipment, and could easily be replicated in health care settings across the country.
Congestive heart failure (CHF) is a chronic and costly condition that affects approximately 5.1 million people in the United States, with an additional 670,000 diagnosed yearly [1]. Heart failure is the most common cause of hospitalization among adults over 65. Nearly 25% of patients hospitalized with heart failure are readmitted within 30 days [2].
Medical management of people living with CHF and other chronic illnesses presents a challenge for health care providers. Due to their often complex medical conditions and limited opportunities to learn self-management skills, patients in rural areas with CHF are at increased risk for complications and hospital readmission [3]. Many approaches have been considered to reduce heart failure readmissions, including efforts to improve self-management skills. Initiatives that engage patients in the process of learning to self manage their illness may activate them to assume responsibility for their care.
North Mississippi Health Services (NMHS) is an integrated regional health care organization with over 5000 employees that serves more than 700,000 residents of 24 primarily rural counties in north Mississippi and northwest Alabama. The flagship of the NMHS system is North Mississippi Medical Center (NMMC), a 650-bed regional referral center in Tupelo. NMHS is one of the largest rural health systems in the United States, and the statistics for its service area reflect these challenges: the prevalence and age-adjusted mortality rates for most chronic illnesses exceed those for the nation as well as for Mississippi, which itself historically ranks at or near the bottom of almost all health status indicators [4–6]. On average, 800 patients with CHF are discharged annually from NMHS’s hospitals, and more than 2900 patients diagnosed with CHF are active NMMC clinic patients.
NHMS is addressing these challenges through a series of innovative quality improvement initiatives. NMHS’s newest initiative is the CHF self-care college. In this paper, we describe the initiative, its implementation, and evaluation to date.
Self-Care College
Background
The idea for the self-care college grew out of discussions with Nurse Link coaches, registered nurses employed by NMHS, who call CHF patients at their homes following discharge. The first call, within 48 hours following discharge, is to reconcile medications, conduct patient education, and confirm follow-up appointments. Three subsequent weekly calls focus on additional education and recognizing “red flags ” utilizing the IHI “teach back” method, in which patients are asked to restate instructions or concepts in their own words. During regular biweekly meetings with physicians to monitor patient progress, Nurse Link coaches observed that many patients (and in some cases, their caregivers) had difficulty following their discharge instructions. In particular, patients did not understand how to properly weigh themselves, how and when to take their medications, or how to ensure their diet met physicians’ guidelines. Although patients were being provided with written and oral instructions as part of the discharge process and through post-discharge follow-up communications, they did not properly implement those instructions once they returned home.
A multidisciplinary team consisting of NMHS physician leaders and representatives from pharmacy, dietary, physical therapy, cardiac rehabilitation, nursing, and case management met to brainstorm ways to overcome this challenge. What emerged from these discussions was the idea for a simulation-based learning experience for patients prior to discharge.
Simulation-based learning is not a new concept. It has been utilized for many years in aviation, health care, and the military as a way to train people in high-risk professions, using realistic scenarios in a controlled environment, without risk to participants. Participants receive immediate feedback from trained instructors as to whether they are performing critical functions properly, providing an opportunity to practice areas in which there is a need to improve technique, speed, or implementation of actions in the correct order. It has been proven to be a highly effective type of learning experience that results in better retention of skills, both cognitive and procedural, and it reduces preventable adverse events [7]. Simulation-based learning in medicine has traditionally been limited to clinician education, where providers practice on computerized patient simulators or other substitutes for live patients. To our knowledge, the concept of simulation learning has not been extended to patient education initiatives.
Simulation-based learning would actively engage patients in learning the necessary self-care skills rather than being passive recipients of information. As the self-care college team often says, “You don’t learn to ride a bike by reading a book; neither should you be asked how to manage CHF by reading a pamphlet.”
Learning Stations
Participants in the self-care college rotate sequentially through 3 learning stations: weight, diet and medications. The main location for the self-care college is a conference room on the cardiac unit of NMMC. At each station, patients are asked to perform the tasks they will be required to do at home. If they cannot complete the task, the deficit is recognized and addressed. This might include referring the patient to home health care, ensuring that a Nurse Link coach contacts him or his caregiver to reiterate medication instructions or ensuring that his case manager refers him to appropriate social services. Although no formal cognitive assessment is conducted, if the team perceives that the patient has a cognitive impairment that could prevent him from being able to perform self-care activities, this information is relayed to the case manager.
At the weight station, a physical therapist or cardiac rehabilitation professional stresses the importance of weighing daily and has the patient demonstrate weighing himself, providing feedback if necessary, to ensure that each patient knows how to properly weigh himself. If the patient does not own a scale, or needs an adaptive scale (such as one with extra large numbers or one that “talks”) and is financially unable to purchase one, he is given one to take home.
At the diet station, a registered dietitian asks the patient what he eats on a typical day, and he is given helpful dietary choices based on his responses. A display at this station provides sample food labels from some common foods, so that patients can see where and how to locate important nutrition information, such as sodium content. The dietitian also discusses fluid restriction and provides the patient and/or caregiver with a written copy of dietary recommendations. In the words of one self-care college patient, “I had to push that salt shaker away, but I also learned that salt comes in cans and boxes. I learned to read food labels for sodium content and to stay away from processed foods.”
At the medication station, a pharmacist reviews the patient’s heart failure medications, has the patient simulate how he will obtain, organize, and remember to take his medications at home, offers feedback and instruction, and answers questions. The pharmacist also provides the patient with a 7-day medication planner for home use and has the patient demonstrate completing the planner.
After the patient has been through the 3 learning stations, a Nurse Link coach enrolls him in the 4-week call-back program. In addition, home health care representatives are available to discuss the benefits of home health to help manage their CHF at home. Finally, each patient receives a CHF self-care college folder, with educational materials including a weight log/calendar; information on smoking cessation, medications, and prescription assistance; a personal health record; control zones for CHF management; red flags and warning signs/symptoms to report; and when to call the doctor.
When the patient has completed the self-care college, the self-care college team “huddles” to ensure that the patient is adequately prepared to transfer to their next health care destination. If not, recommendations are made to their provider to ensure a smooth transition. Family members and/or caregivers are encouraged to participate in the self-care college experience whenever possible and are included in the huddle.
Implementation
Prior to implementing the self-care college, the team identified 4 major challenges and developed strategies to address them. In many cases, strategies were effective in addressing more than one challenge.
- Coordinating the allocation of resources among different departments: as with any new initiative, finding time in everyone’s schedule to accommodate additional tasks is a challenge. In order to ensure that the self-care college was streamlined into everyone’s schedule, the team determined a set time of day that it would take place.
- Gaining buy-in from referring physicians: because referrals from physicians would be critical to the success of the self-care college, the team spent significant time meeting face-to-face with physicians to explain the reason for the program and how it would be implemented. In almost every case, physicians enthusiastically agreed to refer appropriate patients to the self-care college. Although NMHS operates in a fee-for-service environment (and physicians therefore are not financially incentivized to reduce readmissions), it has a strong culture of compassion and caring, focused on innovation, vision, and performance results. Physician buy-in was also facilitated by rolling out the program one floor at a time, so that the team and the physicians could become comfortable with the process. The nurses and case managers on each unit were educated about the program and could prompt the physician to consider placing a referral to the program if warranted.
- Logistical issues in getting the patients to the self-care college room: many CHF patients have significant mobility challenges, and the team discovered that it was not always possible for the patient to be transported to the room where the self-care college was set up, particularly as the program expanded into different wings of the medical center. As a result of feedback from patients and staff regarding the logistical issues around transporting patients to the college, the team developed a mobile version that is brought directly to the patient’s room. A cart holds scales, patient folders, medication planners, and all the tools necessary to present the program. Each member of the team rotates into the room to present their piece of the program. In addition to ensuring that patient mobility issues were not an obstacle to participation, developing the mobile program made the most efficient use of the team’s time in serving these patients, and no patient has been turned away due to having reached capacity at the stationary self-care college.
- Completing the self-care college in a timely fashion: In order to make most efficient use of time (for both the team and the patient), the content for each station was designed to last no more than 15 minutes on average. We have also worked with physicians to encourage referrals prior to the day of discharge, so that patients can be scheduled efficiently.
Program Evaluation
Because the self-care college is one of several initiatives being implemented by NMHS with a focus on reducing readmissions for CHF patients, it is difficult to identify the specific effect of the self-care college on readmissions. However, since implementation in 2011, we have seen a relative rate reduction in CHF readmissions of approximately 23%, and a reduction in the observed to expected CHF readmissions ratio from 0.90 to 0.70.
In addition, referrals have steadily increased since the program began, which suggests that physicians are confident in the program and its ability to improve outcomes.
Beyond the quantifiable measures available to us, comments from patients indicate that the self-care college is improving the quality of life for many of our patients. Two patients noted the following:
“I felt like I wasn’t just thrown out there by myself...I was scared because I didn’t know anything about this disease. The program let me know I wasn’t alone.”
“I eat much differently. I am learning to eat less and eat the right foods...I check my blood sugar every day now, and I weigh myself every day. I know if I weigh more than 244 pounds, I need to call someone.”
While patient and physician feedback has been very positive as far as the effectiveness in teaching patients important self-care skills, we discovered another benefit: not only does the self-care college give patients hands-on practice with skills they will need and the opportunity to ask questions, the team has an opportunity to observe patients actually performing self-care activities, ask the patient questions about how they will follow their discharge instructions, and evaluate whether they are ready to be discharged. Given the distances that many of these patients travel to receive care in the hospital, having insight into their capability prior to discharge is an important advantage.
For example, a patient completing the weight module was having difficulty reading the numbers on the scales due to poor visual acuity, which had not been otherwise noted in his hospital records. The team was able to fit him for a scale with large numbers. In other cases, we have found patients who are unable to identify low-sodium foods. To help them meet dietary guidelines, the dietitian uses a food prop to show them how to read and understand the Nutrition Facts label and then discusses alternative food choices with them. At the medication station, patients bring in all the medications they are currently taking and are asked to identify when, how, and why they take each medication. Frequently, we find that patients do not understand the instructions on the label or that they have duplicate medications because one is a generic and another is a brand name. We can provide the patient with a medication planner that helps ensure their medications are taken properly.
Lessons Learned
As with any new initiative, the self-care college team learned important lessons throughout the implementation process. Chief among these was that flexibility is critical to success. We listened to feedback from patients, physicians, and hospital staff and modified the program to ensure that it was integrated as seamlessly as possible into everyone’s schedule. Feedback was obtained through a variety of methods, including medical staff meetings, discussions with patients and their family members, and feedback from Nurse Link coaches. Feedback led to a number of changes, including development of the mobile self-care college and changing the timing from the day of discharge to the day prior to avoid conflicts with other day-of-discharge activities.
An additional lesson learned, which was actually a process of learning, was how important it is for self-care college team members to be active listeners. As opposed to the didactic approach, where clinicians provide instructions to patients, the self-care college team learned to ask questions of the patients and to actively listen to the responses, filling in the gaps where necessary. Interestingly, we found that this was also a learning process for the patients, many of whom are unaccustomed to engaging in dialogue with their doctors and to being active participants in their health care. They were not all initially comfortable with the concept of simulation, but our staff learned different ways to introduce patients to it, so that ultimately most seemed to enjoy the program.
Take-Away Points
For health care organizations considering implementing a self-care college or similar initiative, we offer a few key points:
- Consider the benefits beyond reducing readmissions: at NMHS, we have found that the self-care college has positively impacted patient satisfaction. For the past 2 years, our HCAHPS scores have consistently been well above the top performance threshold, a top quartile performer in Premier’s quality database (Premier, Inc., a health care performance improvement alliance of approximately 3000 U.S. hospitals). While it is difficult to correlate patient satisfaction scores with any one initiative, we hear from patients, physicians, and nursing staff that the self-care college greatly increases effective communication between provider and patient. We have also found that some of our biggest advocates are now the cardiologists who refer patients.
- Analyze your operational readiness: this is a low-tech but high-touch program. While it requires a minimal financial investment, it does require strong organizational leadership and staff buy-in to make it successful. Nursing staff are likely to buy into the program because they will not have to deliver discharge education to patients in addition to the many other responsibilities they have. Administrators should see that patient satisfaction will improve and readmissions will decrease. Ultimately, it is up to the program “champion” to make it clear to key stakeholders what the advantages are, and to include them in the process of developing the self-care college.
- This is the future of medicine: The self-care college is just one example of a team-based approach to medicine. Most of the disciplines on our team did not know each other prior to the program. We now have established a line of communication that permeates throughout the hospital to the outpatient setting.
Based on our success with the CHF self-care college, the next logical step will be to create self-care colleges for other common disease states, such as asthma/COPD or diabetes. However, while the value of this model for patient education has clearly been demonstrated, the team has also contemplated its application for staff training. Many large hospitals already use patient simulation manikins in nursing education, but the cost of this high-tech equipment is out of reach for many smaller, community hospitals. The possibility to create low-cost, low-tech simulation training experiences for clinicians similar to that provided by self-care college for patients bears examination.
Corresponding author: Lee Greer, MD, MBA, 830 S. Gloster St., Tupelo, MS 38801, [email protected].
Financial disclosures: None.
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239.
2. Hospital compare (Internet). Baltimore: Centers for Medicare and Medicaid Services; 2014. Available at www.medicare.gov/hospitalcompare.
3. Health disparities—a rural-urban chartbook. Columbia, SC: South Carolina Rural Health Research Center; 2008.
4. America’s health rankings [Internet]. Minnetonka: United Health Foundation; 2014. Available at www.americashealthrankings.org/MS.
5. County health profiles 2007 [Internet]. Jackson: Mississippi State Department of Health; 2009. Available at msdh.ms.gov/msdhsite/_static/31,0,299,463.html.
6. County Health rankings and roadmaps [Internet]. Madison: University of Wisconsin Population Health Institute; 2014. Available at www.countyhealthrankings.org.
7. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. OJIN: Online J Iss Nurs 2013; 18(2):Manuscript 6.
From North Mississippi Health Services, Tupelo, MS (Drs. Greer and Fagan), and the University of Colorado, Denver, CO (Dr. Coleman).
Abstract
- Objective: To describe the self-care college, an innovative initiative designed to reduce hospital readmissions for congestive heart failure (CHF) patients.
- Methods: CHF patients at North Mississippi Medical Center are asked to participate in a “self-care college” prior to discharge. Participants rotate through 3 learning stations: weight, diet and medications. At each station, they are asked to perform the tasks they will be required to do at home. By engaging patients in the learning process, they are activated to assume responsibility for their care. This approach has the added advantage of providing a feedback loop, allowing the health care team to “road test” the proposed care plan to determine the likelihood that the patient (and family caregivers) will be able to execute following discharge.
- Results: Since the self-care college was implemented in 2011, the 30-day readmission rate for CHF patients at NMMC has been reduced from 16.8% to 12.85%. There has also been a reduction in the observed to expected CHF readmissions ratio, from 0.90 to 0.71.
- Conclusion: Although the self-care college targets CHF patients, it is likely that this type of initiative could be applied for rural patients with other chronic illnesses, such as asthma, COPD, and diabetes. It is a relatively simple and inexpensive program (approximately $30,000 per year, primarily in personnel expenses, or roughly the cost of 3 hospital readmissions) that does not require sophisticated technology or equipment, and could easily be replicated in health care settings across the country.
Congestive heart failure (CHF) is a chronic and costly condition that affects approximately 5.1 million people in the United States, with an additional 670,000 diagnosed yearly [1]. Heart failure is the most common cause of hospitalization among adults over 65. Nearly 25% of patients hospitalized with heart failure are readmitted within 30 days [2].
Medical management of people living with CHF and other chronic illnesses presents a challenge for health care providers. Due to their often complex medical conditions and limited opportunities to learn self-management skills, patients in rural areas with CHF are at increased risk for complications and hospital readmission [3]. Many approaches have been considered to reduce heart failure readmissions, including efforts to improve self-management skills. Initiatives that engage patients in the process of learning to self manage their illness may activate them to assume responsibility for their care.
North Mississippi Health Services (NMHS) is an integrated regional health care organization with over 5000 employees that serves more than 700,000 residents of 24 primarily rural counties in north Mississippi and northwest Alabama. The flagship of the NMHS system is North Mississippi Medical Center (NMMC), a 650-bed regional referral center in Tupelo. NMHS is one of the largest rural health systems in the United States, and the statistics for its service area reflect these challenges: the prevalence and age-adjusted mortality rates for most chronic illnesses exceed those for the nation as well as for Mississippi, which itself historically ranks at or near the bottom of almost all health status indicators [4–6]. On average, 800 patients with CHF are discharged annually from NMHS’s hospitals, and more than 2900 patients diagnosed with CHF are active NMMC clinic patients.
NHMS is addressing these challenges through a series of innovative quality improvement initiatives. NMHS’s newest initiative is the CHF self-care college. In this paper, we describe the initiative, its implementation, and evaluation to date.
Self-Care College
Background
The idea for the self-care college grew out of discussions with Nurse Link coaches, registered nurses employed by NMHS, who call CHF patients at their homes following discharge. The first call, within 48 hours following discharge, is to reconcile medications, conduct patient education, and confirm follow-up appointments. Three subsequent weekly calls focus on additional education and recognizing “red flags ” utilizing the IHI “teach back” method, in which patients are asked to restate instructions or concepts in their own words. During regular biweekly meetings with physicians to monitor patient progress, Nurse Link coaches observed that many patients (and in some cases, their caregivers) had difficulty following their discharge instructions. In particular, patients did not understand how to properly weigh themselves, how and when to take their medications, or how to ensure their diet met physicians’ guidelines. Although patients were being provided with written and oral instructions as part of the discharge process and through post-discharge follow-up communications, they did not properly implement those instructions once they returned home.
A multidisciplinary team consisting of NMHS physician leaders and representatives from pharmacy, dietary, physical therapy, cardiac rehabilitation, nursing, and case management met to brainstorm ways to overcome this challenge. What emerged from these discussions was the idea for a simulation-based learning experience for patients prior to discharge.
Simulation-based learning is not a new concept. It has been utilized for many years in aviation, health care, and the military as a way to train people in high-risk professions, using realistic scenarios in a controlled environment, without risk to participants. Participants receive immediate feedback from trained instructors as to whether they are performing critical functions properly, providing an opportunity to practice areas in which there is a need to improve technique, speed, or implementation of actions in the correct order. It has been proven to be a highly effective type of learning experience that results in better retention of skills, both cognitive and procedural, and it reduces preventable adverse events [7]. Simulation-based learning in medicine has traditionally been limited to clinician education, where providers practice on computerized patient simulators or other substitutes for live patients. To our knowledge, the concept of simulation learning has not been extended to patient education initiatives.
Simulation-based learning would actively engage patients in learning the necessary self-care skills rather than being passive recipients of information. As the self-care college team often says, “You don’t learn to ride a bike by reading a book; neither should you be asked how to manage CHF by reading a pamphlet.”
Learning Stations
Participants in the self-care college rotate sequentially through 3 learning stations: weight, diet and medications. The main location for the self-care college is a conference room on the cardiac unit of NMMC. At each station, patients are asked to perform the tasks they will be required to do at home. If they cannot complete the task, the deficit is recognized and addressed. This might include referring the patient to home health care, ensuring that a Nurse Link coach contacts him or his caregiver to reiterate medication instructions or ensuring that his case manager refers him to appropriate social services. Although no formal cognitive assessment is conducted, if the team perceives that the patient has a cognitive impairment that could prevent him from being able to perform self-care activities, this information is relayed to the case manager.
At the weight station, a physical therapist or cardiac rehabilitation professional stresses the importance of weighing daily and has the patient demonstrate weighing himself, providing feedback if necessary, to ensure that each patient knows how to properly weigh himself. If the patient does not own a scale, or needs an adaptive scale (such as one with extra large numbers or one that “talks”) and is financially unable to purchase one, he is given one to take home.
At the diet station, a registered dietitian asks the patient what he eats on a typical day, and he is given helpful dietary choices based on his responses. A display at this station provides sample food labels from some common foods, so that patients can see where and how to locate important nutrition information, such as sodium content. The dietitian also discusses fluid restriction and provides the patient and/or caregiver with a written copy of dietary recommendations. In the words of one self-care college patient, “I had to push that salt shaker away, but I also learned that salt comes in cans and boxes. I learned to read food labels for sodium content and to stay away from processed foods.”
At the medication station, a pharmacist reviews the patient’s heart failure medications, has the patient simulate how he will obtain, organize, and remember to take his medications at home, offers feedback and instruction, and answers questions. The pharmacist also provides the patient with a 7-day medication planner for home use and has the patient demonstrate completing the planner.
After the patient has been through the 3 learning stations, a Nurse Link coach enrolls him in the 4-week call-back program. In addition, home health care representatives are available to discuss the benefits of home health to help manage their CHF at home. Finally, each patient receives a CHF self-care college folder, with educational materials including a weight log/calendar; information on smoking cessation, medications, and prescription assistance; a personal health record; control zones for CHF management; red flags and warning signs/symptoms to report; and when to call the doctor.
When the patient has completed the self-care college, the self-care college team “huddles” to ensure that the patient is adequately prepared to transfer to their next health care destination. If not, recommendations are made to their provider to ensure a smooth transition. Family members and/or caregivers are encouraged to participate in the self-care college experience whenever possible and are included in the huddle.
Implementation
Prior to implementing the self-care college, the team identified 4 major challenges and developed strategies to address them. In many cases, strategies were effective in addressing more than one challenge.
- Coordinating the allocation of resources among different departments: as with any new initiative, finding time in everyone’s schedule to accommodate additional tasks is a challenge. In order to ensure that the self-care college was streamlined into everyone’s schedule, the team determined a set time of day that it would take place.
- Gaining buy-in from referring physicians: because referrals from physicians would be critical to the success of the self-care college, the team spent significant time meeting face-to-face with physicians to explain the reason for the program and how it would be implemented. In almost every case, physicians enthusiastically agreed to refer appropriate patients to the self-care college. Although NMHS operates in a fee-for-service environment (and physicians therefore are not financially incentivized to reduce readmissions), it has a strong culture of compassion and caring, focused on innovation, vision, and performance results. Physician buy-in was also facilitated by rolling out the program one floor at a time, so that the team and the physicians could become comfortable with the process. The nurses and case managers on each unit were educated about the program and could prompt the physician to consider placing a referral to the program if warranted.
- Logistical issues in getting the patients to the self-care college room: many CHF patients have significant mobility challenges, and the team discovered that it was not always possible for the patient to be transported to the room where the self-care college was set up, particularly as the program expanded into different wings of the medical center. As a result of feedback from patients and staff regarding the logistical issues around transporting patients to the college, the team developed a mobile version that is brought directly to the patient’s room. A cart holds scales, patient folders, medication planners, and all the tools necessary to present the program. Each member of the team rotates into the room to present their piece of the program. In addition to ensuring that patient mobility issues were not an obstacle to participation, developing the mobile program made the most efficient use of the team’s time in serving these patients, and no patient has been turned away due to having reached capacity at the stationary self-care college.
- Completing the self-care college in a timely fashion: In order to make most efficient use of time (for both the team and the patient), the content for each station was designed to last no more than 15 minutes on average. We have also worked with physicians to encourage referrals prior to the day of discharge, so that patients can be scheduled efficiently.
Program Evaluation
Because the self-care college is one of several initiatives being implemented by NMHS with a focus on reducing readmissions for CHF patients, it is difficult to identify the specific effect of the self-care college on readmissions. However, since implementation in 2011, we have seen a relative rate reduction in CHF readmissions of approximately 23%, and a reduction in the observed to expected CHF readmissions ratio from 0.90 to 0.70.
In addition, referrals have steadily increased since the program began, which suggests that physicians are confident in the program and its ability to improve outcomes.
Beyond the quantifiable measures available to us, comments from patients indicate that the self-care college is improving the quality of life for many of our patients. Two patients noted the following:
“I felt like I wasn’t just thrown out there by myself...I was scared because I didn’t know anything about this disease. The program let me know I wasn’t alone.”
“I eat much differently. I am learning to eat less and eat the right foods...I check my blood sugar every day now, and I weigh myself every day. I know if I weigh more than 244 pounds, I need to call someone.”
While patient and physician feedback has been very positive as far as the effectiveness in teaching patients important self-care skills, we discovered another benefit: not only does the self-care college give patients hands-on practice with skills they will need and the opportunity to ask questions, the team has an opportunity to observe patients actually performing self-care activities, ask the patient questions about how they will follow their discharge instructions, and evaluate whether they are ready to be discharged. Given the distances that many of these patients travel to receive care in the hospital, having insight into their capability prior to discharge is an important advantage.
For example, a patient completing the weight module was having difficulty reading the numbers on the scales due to poor visual acuity, which had not been otherwise noted in his hospital records. The team was able to fit him for a scale with large numbers. In other cases, we have found patients who are unable to identify low-sodium foods. To help them meet dietary guidelines, the dietitian uses a food prop to show them how to read and understand the Nutrition Facts label and then discusses alternative food choices with them. At the medication station, patients bring in all the medications they are currently taking and are asked to identify when, how, and why they take each medication. Frequently, we find that patients do not understand the instructions on the label or that they have duplicate medications because one is a generic and another is a brand name. We can provide the patient with a medication planner that helps ensure their medications are taken properly.
Lessons Learned
As with any new initiative, the self-care college team learned important lessons throughout the implementation process. Chief among these was that flexibility is critical to success. We listened to feedback from patients, physicians, and hospital staff and modified the program to ensure that it was integrated as seamlessly as possible into everyone’s schedule. Feedback was obtained through a variety of methods, including medical staff meetings, discussions with patients and their family members, and feedback from Nurse Link coaches. Feedback led to a number of changes, including development of the mobile self-care college and changing the timing from the day of discharge to the day prior to avoid conflicts with other day-of-discharge activities.
An additional lesson learned, which was actually a process of learning, was how important it is for self-care college team members to be active listeners. As opposed to the didactic approach, where clinicians provide instructions to patients, the self-care college team learned to ask questions of the patients and to actively listen to the responses, filling in the gaps where necessary. Interestingly, we found that this was also a learning process for the patients, many of whom are unaccustomed to engaging in dialogue with their doctors and to being active participants in their health care. They were not all initially comfortable with the concept of simulation, but our staff learned different ways to introduce patients to it, so that ultimately most seemed to enjoy the program.
Take-Away Points
For health care organizations considering implementing a self-care college or similar initiative, we offer a few key points:
- Consider the benefits beyond reducing readmissions: at NMHS, we have found that the self-care college has positively impacted patient satisfaction. For the past 2 years, our HCAHPS scores have consistently been well above the top performance threshold, a top quartile performer in Premier’s quality database (Premier, Inc., a health care performance improvement alliance of approximately 3000 U.S. hospitals). While it is difficult to correlate patient satisfaction scores with any one initiative, we hear from patients, physicians, and nursing staff that the self-care college greatly increases effective communication between provider and patient. We have also found that some of our biggest advocates are now the cardiologists who refer patients.
- Analyze your operational readiness: this is a low-tech but high-touch program. While it requires a minimal financial investment, it does require strong organizational leadership and staff buy-in to make it successful. Nursing staff are likely to buy into the program because they will not have to deliver discharge education to patients in addition to the many other responsibilities they have. Administrators should see that patient satisfaction will improve and readmissions will decrease. Ultimately, it is up to the program “champion” to make it clear to key stakeholders what the advantages are, and to include them in the process of developing the self-care college.
- This is the future of medicine: The self-care college is just one example of a team-based approach to medicine. Most of the disciplines on our team did not know each other prior to the program. We now have established a line of communication that permeates throughout the hospital to the outpatient setting.
Based on our success with the CHF self-care college, the next logical step will be to create self-care colleges for other common disease states, such as asthma/COPD or diabetes. However, while the value of this model for patient education has clearly been demonstrated, the team has also contemplated its application for staff training. Many large hospitals already use patient simulation manikins in nursing education, but the cost of this high-tech equipment is out of reach for many smaller, community hospitals. The possibility to create low-cost, low-tech simulation training experiences for clinicians similar to that provided by self-care college for patients bears examination.
Corresponding author: Lee Greer, MD, MBA, 830 S. Gloster St., Tupelo, MS 38801, [email protected].
Financial disclosures: None.
From North Mississippi Health Services, Tupelo, MS (Drs. Greer and Fagan), and the University of Colorado, Denver, CO (Dr. Coleman).
Abstract
- Objective: To describe the self-care college, an innovative initiative designed to reduce hospital readmissions for congestive heart failure (CHF) patients.
- Methods: CHF patients at North Mississippi Medical Center are asked to participate in a “self-care college” prior to discharge. Participants rotate through 3 learning stations: weight, diet and medications. At each station, they are asked to perform the tasks they will be required to do at home. By engaging patients in the learning process, they are activated to assume responsibility for their care. This approach has the added advantage of providing a feedback loop, allowing the health care team to “road test” the proposed care plan to determine the likelihood that the patient (and family caregivers) will be able to execute following discharge.
- Results: Since the self-care college was implemented in 2011, the 30-day readmission rate for CHF patients at NMMC has been reduced from 16.8% to 12.85%. There has also been a reduction in the observed to expected CHF readmissions ratio, from 0.90 to 0.71.
- Conclusion: Although the self-care college targets CHF patients, it is likely that this type of initiative could be applied for rural patients with other chronic illnesses, such as asthma, COPD, and diabetes. It is a relatively simple and inexpensive program (approximately $30,000 per year, primarily in personnel expenses, or roughly the cost of 3 hospital readmissions) that does not require sophisticated technology or equipment, and could easily be replicated in health care settings across the country.
Congestive heart failure (CHF) is a chronic and costly condition that affects approximately 5.1 million people in the United States, with an additional 670,000 diagnosed yearly [1]. Heart failure is the most common cause of hospitalization among adults over 65. Nearly 25% of patients hospitalized with heart failure are readmitted within 30 days [2].
Medical management of people living with CHF and other chronic illnesses presents a challenge for health care providers. Due to their often complex medical conditions and limited opportunities to learn self-management skills, patients in rural areas with CHF are at increased risk for complications and hospital readmission [3]. Many approaches have been considered to reduce heart failure readmissions, including efforts to improve self-management skills. Initiatives that engage patients in the process of learning to self manage their illness may activate them to assume responsibility for their care.
North Mississippi Health Services (NMHS) is an integrated regional health care organization with over 5000 employees that serves more than 700,000 residents of 24 primarily rural counties in north Mississippi and northwest Alabama. The flagship of the NMHS system is North Mississippi Medical Center (NMMC), a 650-bed regional referral center in Tupelo. NMHS is one of the largest rural health systems in the United States, and the statistics for its service area reflect these challenges: the prevalence and age-adjusted mortality rates for most chronic illnesses exceed those for the nation as well as for Mississippi, which itself historically ranks at or near the bottom of almost all health status indicators [4–6]. On average, 800 patients with CHF are discharged annually from NMHS’s hospitals, and more than 2900 patients diagnosed with CHF are active NMMC clinic patients.
NHMS is addressing these challenges through a series of innovative quality improvement initiatives. NMHS’s newest initiative is the CHF self-care college. In this paper, we describe the initiative, its implementation, and evaluation to date.
Self-Care College
Background
The idea for the self-care college grew out of discussions with Nurse Link coaches, registered nurses employed by NMHS, who call CHF patients at their homes following discharge. The first call, within 48 hours following discharge, is to reconcile medications, conduct patient education, and confirm follow-up appointments. Three subsequent weekly calls focus on additional education and recognizing “red flags ” utilizing the IHI “teach back” method, in which patients are asked to restate instructions or concepts in their own words. During regular biweekly meetings with physicians to monitor patient progress, Nurse Link coaches observed that many patients (and in some cases, their caregivers) had difficulty following their discharge instructions. In particular, patients did not understand how to properly weigh themselves, how and when to take their medications, or how to ensure their diet met physicians’ guidelines. Although patients were being provided with written and oral instructions as part of the discharge process and through post-discharge follow-up communications, they did not properly implement those instructions once they returned home.
A multidisciplinary team consisting of NMHS physician leaders and representatives from pharmacy, dietary, physical therapy, cardiac rehabilitation, nursing, and case management met to brainstorm ways to overcome this challenge. What emerged from these discussions was the idea for a simulation-based learning experience for patients prior to discharge.
Simulation-based learning is not a new concept. It has been utilized for many years in aviation, health care, and the military as a way to train people in high-risk professions, using realistic scenarios in a controlled environment, without risk to participants. Participants receive immediate feedback from trained instructors as to whether they are performing critical functions properly, providing an opportunity to practice areas in which there is a need to improve technique, speed, or implementation of actions in the correct order. It has been proven to be a highly effective type of learning experience that results in better retention of skills, both cognitive and procedural, and it reduces preventable adverse events [7]. Simulation-based learning in medicine has traditionally been limited to clinician education, where providers practice on computerized patient simulators or other substitutes for live patients. To our knowledge, the concept of simulation learning has not been extended to patient education initiatives.
Simulation-based learning would actively engage patients in learning the necessary self-care skills rather than being passive recipients of information. As the self-care college team often says, “You don’t learn to ride a bike by reading a book; neither should you be asked how to manage CHF by reading a pamphlet.”
Learning Stations
Participants in the self-care college rotate sequentially through 3 learning stations: weight, diet and medications. The main location for the self-care college is a conference room on the cardiac unit of NMMC. At each station, patients are asked to perform the tasks they will be required to do at home. If they cannot complete the task, the deficit is recognized and addressed. This might include referring the patient to home health care, ensuring that a Nurse Link coach contacts him or his caregiver to reiterate medication instructions or ensuring that his case manager refers him to appropriate social services. Although no formal cognitive assessment is conducted, if the team perceives that the patient has a cognitive impairment that could prevent him from being able to perform self-care activities, this information is relayed to the case manager.
At the weight station, a physical therapist or cardiac rehabilitation professional stresses the importance of weighing daily and has the patient demonstrate weighing himself, providing feedback if necessary, to ensure that each patient knows how to properly weigh himself. If the patient does not own a scale, or needs an adaptive scale (such as one with extra large numbers or one that “talks”) and is financially unable to purchase one, he is given one to take home.
At the diet station, a registered dietitian asks the patient what he eats on a typical day, and he is given helpful dietary choices based on his responses. A display at this station provides sample food labels from some common foods, so that patients can see where and how to locate important nutrition information, such as sodium content. The dietitian also discusses fluid restriction and provides the patient and/or caregiver with a written copy of dietary recommendations. In the words of one self-care college patient, “I had to push that salt shaker away, but I also learned that salt comes in cans and boxes. I learned to read food labels for sodium content and to stay away from processed foods.”
At the medication station, a pharmacist reviews the patient’s heart failure medications, has the patient simulate how he will obtain, organize, and remember to take his medications at home, offers feedback and instruction, and answers questions. The pharmacist also provides the patient with a 7-day medication planner for home use and has the patient demonstrate completing the planner.
After the patient has been through the 3 learning stations, a Nurse Link coach enrolls him in the 4-week call-back program. In addition, home health care representatives are available to discuss the benefits of home health to help manage their CHF at home. Finally, each patient receives a CHF self-care college folder, with educational materials including a weight log/calendar; information on smoking cessation, medications, and prescription assistance; a personal health record; control zones for CHF management; red flags and warning signs/symptoms to report; and when to call the doctor.
When the patient has completed the self-care college, the self-care college team “huddles” to ensure that the patient is adequately prepared to transfer to their next health care destination. If not, recommendations are made to their provider to ensure a smooth transition. Family members and/or caregivers are encouraged to participate in the self-care college experience whenever possible and are included in the huddle.
Implementation
Prior to implementing the self-care college, the team identified 4 major challenges and developed strategies to address them. In many cases, strategies were effective in addressing more than one challenge.
- Coordinating the allocation of resources among different departments: as with any new initiative, finding time in everyone’s schedule to accommodate additional tasks is a challenge. In order to ensure that the self-care college was streamlined into everyone’s schedule, the team determined a set time of day that it would take place.
- Gaining buy-in from referring physicians: because referrals from physicians would be critical to the success of the self-care college, the team spent significant time meeting face-to-face with physicians to explain the reason for the program and how it would be implemented. In almost every case, physicians enthusiastically agreed to refer appropriate patients to the self-care college. Although NMHS operates in a fee-for-service environment (and physicians therefore are not financially incentivized to reduce readmissions), it has a strong culture of compassion and caring, focused on innovation, vision, and performance results. Physician buy-in was also facilitated by rolling out the program one floor at a time, so that the team and the physicians could become comfortable with the process. The nurses and case managers on each unit were educated about the program and could prompt the physician to consider placing a referral to the program if warranted.
- Logistical issues in getting the patients to the self-care college room: many CHF patients have significant mobility challenges, and the team discovered that it was not always possible for the patient to be transported to the room where the self-care college was set up, particularly as the program expanded into different wings of the medical center. As a result of feedback from patients and staff regarding the logistical issues around transporting patients to the college, the team developed a mobile version that is brought directly to the patient’s room. A cart holds scales, patient folders, medication planners, and all the tools necessary to present the program. Each member of the team rotates into the room to present their piece of the program. In addition to ensuring that patient mobility issues were not an obstacle to participation, developing the mobile program made the most efficient use of the team’s time in serving these patients, and no patient has been turned away due to having reached capacity at the stationary self-care college.
- Completing the self-care college in a timely fashion: In order to make most efficient use of time (for both the team and the patient), the content for each station was designed to last no more than 15 minutes on average. We have also worked with physicians to encourage referrals prior to the day of discharge, so that patients can be scheduled efficiently.
Program Evaluation
Because the self-care college is one of several initiatives being implemented by NMHS with a focus on reducing readmissions for CHF patients, it is difficult to identify the specific effect of the self-care college on readmissions. However, since implementation in 2011, we have seen a relative rate reduction in CHF readmissions of approximately 23%, and a reduction in the observed to expected CHF readmissions ratio from 0.90 to 0.70.
In addition, referrals have steadily increased since the program began, which suggests that physicians are confident in the program and its ability to improve outcomes.
Beyond the quantifiable measures available to us, comments from patients indicate that the self-care college is improving the quality of life for many of our patients. Two patients noted the following:
“I felt like I wasn’t just thrown out there by myself...I was scared because I didn’t know anything about this disease. The program let me know I wasn’t alone.”
“I eat much differently. I am learning to eat less and eat the right foods...I check my blood sugar every day now, and I weigh myself every day. I know if I weigh more than 244 pounds, I need to call someone.”
While patient and physician feedback has been very positive as far as the effectiveness in teaching patients important self-care skills, we discovered another benefit: not only does the self-care college give patients hands-on practice with skills they will need and the opportunity to ask questions, the team has an opportunity to observe patients actually performing self-care activities, ask the patient questions about how they will follow their discharge instructions, and evaluate whether they are ready to be discharged. Given the distances that many of these patients travel to receive care in the hospital, having insight into their capability prior to discharge is an important advantage.
For example, a patient completing the weight module was having difficulty reading the numbers on the scales due to poor visual acuity, which had not been otherwise noted in his hospital records. The team was able to fit him for a scale with large numbers. In other cases, we have found patients who are unable to identify low-sodium foods. To help them meet dietary guidelines, the dietitian uses a food prop to show them how to read and understand the Nutrition Facts label and then discusses alternative food choices with them. At the medication station, patients bring in all the medications they are currently taking and are asked to identify when, how, and why they take each medication. Frequently, we find that patients do not understand the instructions on the label or that they have duplicate medications because one is a generic and another is a brand name. We can provide the patient with a medication planner that helps ensure their medications are taken properly.
Lessons Learned
As with any new initiative, the self-care college team learned important lessons throughout the implementation process. Chief among these was that flexibility is critical to success. We listened to feedback from patients, physicians, and hospital staff and modified the program to ensure that it was integrated as seamlessly as possible into everyone’s schedule. Feedback was obtained through a variety of methods, including medical staff meetings, discussions with patients and their family members, and feedback from Nurse Link coaches. Feedback led to a number of changes, including development of the mobile self-care college and changing the timing from the day of discharge to the day prior to avoid conflicts with other day-of-discharge activities.
An additional lesson learned, which was actually a process of learning, was how important it is for self-care college team members to be active listeners. As opposed to the didactic approach, where clinicians provide instructions to patients, the self-care college team learned to ask questions of the patients and to actively listen to the responses, filling in the gaps where necessary. Interestingly, we found that this was also a learning process for the patients, many of whom are unaccustomed to engaging in dialogue with their doctors and to being active participants in their health care. They were not all initially comfortable with the concept of simulation, but our staff learned different ways to introduce patients to it, so that ultimately most seemed to enjoy the program.
Take-Away Points
For health care organizations considering implementing a self-care college or similar initiative, we offer a few key points:
- Consider the benefits beyond reducing readmissions: at NMHS, we have found that the self-care college has positively impacted patient satisfaction. For the past 2 years, our HCAHPS scores have consistently been well above the top performance threshold, a top quartile performer in Premier’s quality database (Premier, Inc., a health care performance improvement alliance of approximately 3000 U.S. hospitals). While it is difficult to correlate patient satisfaction scores with any one initiative, we hear from patients, physicians, and nursing staff that the self-care college greatly increases effective communication between provider and patient. We have also found that some of our biggest advocates are now the cardiologists who refer patients.
- Analyze your operational readiness: this is a low-tech but high-touch program. While it requires a minimal financial investment, it does require strong organizational leadership and staff buy-in to make it successful. Nursing staff are likely to buy into the program because they will not have to deliver discharge education to patients in addition to the many other responsibilities they have. Administrators should see that patient satisfaction will improve and readmissions will decrease. Ultimately, it is up to the program “champion” to make it clear to key stakeholders what the advantages are, and to include them in the process of developing the self-care college.
- This is the future of medicine: The self-care college is just one example of a team-based approach to medicine. Most of the disciplines on our team did not know each other prior to the program. We now have established a line of communication that permeates throughout the hospital to the outpatient setting.
Based on our success with the CHF self-care college, the next logical step will be to create self-care colleges for other common disease states, such as asthma/COPD or diabetes. However, while the value of this model for patient education has clearly been demonstrated, the team has also contemplated its application for staff training. Many large hospitals already use patient simulation manikins in nursing education, but the cost of this high-tech equipment is out of reach for many smaller, community hospitals. The possibility to create low-cost, low-tech simulation training experiences for clinicians similar to that provided by self-care college for patients bears examination.
Corresponding author: Lee Greer, MD, MBA, 830 S. Gloster St., Tupelo, MS 38801, [email protected].
Financial disclosures: None.
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239.
2. Hospital compare (Internet). Baltimore: Centers for Medicare and Medicaid Services; 2014. Available at www.medicare.gov/hospitalcompare.
3. Health disparities—a rural-urban chartbook. Columbia, SC: South Carolina Rural Health Research Center; 2008.
4. America’s health rankings [Internet]. Minnetonka: United Health Foundation; 2014. Available at www.americashealthrankings.org/MS.
5. County health profiles 2007 [Internet]. Jackson: Mississippi State Department of Health; 2009. Available at msdh.ms.gov/msdhsite/_static/31,0,299,463.html.
6. County Health rankings and roadmaps [Internet]. Madison: University of Wisconsin Population Health Institute; 2014. Available at www.countyhealthrankings.org.
7. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. OJIN: Online J Iss Nurs 2013; 18(2):Manuscript 6.
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239.
2. Hospital compare (Internet). Baltimore: Centers for Medicare and Medicaid Services; 2014. Available at www.medicare.gov/hospitalcompare.
3. Health disparities—a rural-urban chartbook. Columbia, SC: South Carolina Rural Health Research Center; 2008.
4. America’s health rankings [Internet]. Minnetonka: United Health Foundation; 2014. Available at www.americashealthrankings.org/MS.
5. County health profiles 2007 [Internet]. Jackson: Mississippi State Department of Health; 2009. Available at msdh.ms.gov/msdhsite/_static/31,0,299,463.html.
6. County Health rankings and roadmaps [Internet]. Madison: University of Wisconsin Population Health Institute; 2014. Available at www.countyhealthrankings.org.
7. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. OJIN: Online J Iss Nurs 2013; 18(2):Manuscript 6.
Improving Functional Outcomes in Patients with Intermittent Claudication
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
From the University of York, York, UK, and the University Hospital of Angers, Angers, France.
Abstract
- Objective: To provide an overview of therapies for improving functional outcomes in individuals with intermittent claudication due to lower-limb peripheral arterial disease (PAD).
- Methods: Literature review.
- Results: Treatment approaches that aim to improve functional outcomes (and walking performance specifically) in individuals with intermittent claudication include exercise training, lower-limb revascularization, and prescription of various drugs, including peripheral vasodilators. Supervised exercise training, particularly that which involves walking as the main exercise modality, is an effective treatment for improving walking performance in individuals with intermittent claudication; however, few supervised exercise programs exist specifically for these patients, limiting access to this therapy. Consequently, most patients with intermittent claudication do not participate in supervised exercise. The evidence for the effectiveness of unsupervised exercise programs is currently weak and mixed, and lack of motivation and pain have been cited as major barriers to participation in self-managed exercise. Lower-limb revascularization procedures (angioplasty or bypass surgery) can improve walking performance; however, such procedures are not feasible for some patients (eg, in the case of extensive multi-segmental disease) and are invasive and expensive. Medications used to treat PAD-related functional impairment (eg, cilostazol, pentoxifylline, inositol nicotinate, and naftidrofuryl oxalate [not approved in the US]) all have limited efficacy.
- Conclusion: Supervised walking exercise is a cheap and effective approach for improving walking performance in individuals with intermittent claudication. Therefore, efforts should be made to provide patients with access to a supervised exercise program, or to promote self-managed walking when supervised exercise is not available or practical.
Peripheral arterial disease (PAD) is a chronic cardiovascular disease characterised by atherosclerotic narrowing or occlusion of the arteries supplying the legs. It is highly prevalent in older adults, affecting around 20% of adults aged > 70 years [1,2]. Around 10% to 35% of patients report the typical symptoms of intermittent claudication, which is specifically defined as lower-limb discomfort or pain on exertion that is relieved within 10 minutes of rest; however, a further 30% to 40% report other, atypical lower-limb symptoms [3]. Intermittent claudication impairs quality of life by limiting ambulation and activities of daily living [4] and is associated with a several-fold increased risk of cardiovascular and all-cause mortality compared with age-matched healthy controls [5,6]. The treatment of individuals with intermittent claudication has 2 main objectives: secondary prevention of cardiovascular disease and improvement of functional status (and, in turn, quality of life) [3,7,8]. The former objective is usually pursued through prescribing various medications to help manage cardiovascular risk factors (eg, antiplatelets, HMG-CoA reductase inhibitors, antihypertensive and antidiabetic medication) and promoting lifestyle changes such as smoking cessation, increased physical activity, and consumption of a healthy diet. This review focuses on the latter objective by providing an overview of the evidence for different treatments to improve functional outcomes in individuals with intermittent claudication. Patients with PAD often present with multiple comorbidities that may have independent adverse effects on functional capacity (eg, osteoarthritis, chronic heart failure, chronic obstructive pulmonary disease) [9]; therefore, concomitant treatment of comorbidities should be considered when attempting to optimize the functional status of patients.
Assessing Function Outcomes
Functional capacity is a multidimensional construct that represents the highest level of activity that a person may reach at a given moment in a standardized environment [10]. It can encompass one’s ability to perform work-related activities (eg, lifting, static work), activities of daily living (eg, walking, climbing stairs, standing up from a chair), and other exercise-related activities (eg, walking, cycling, weight lifting). Given that the primary functional limitation in intermittent claudication is walking impairment, most functional capacity evaluations in this population focus on walking capacity as the outcome of interest. In terms of walking impairment, individuals with intermittent claudication have poorer walking endurance and slower walking velocity compared to individuals without PAD [4]. People with intermittent claudication may reduce their walking activity to avoid leg symptoms. Thus, clinicians should not equate stabilization or improvement in intermittent claudication with stabilization or improvement in walking performance [11].
There are several methods for assessing walking capacity in individuals with intermittent claudication. Treadmill walking tests are commonly used. Following a transatlantic conference on clinical trials guidelines in PAD [12], two internationally accepted treadmill protocols were recommended: (1) constant-pace treadmill protocol (constant walking speed of 3.2 km·h–1 at 10%–12% gradient), and (2) incremental treadmill protocol (starting horizontally at a constant speed of 3.2 km·h–1, but with the gradient increasing in pre-defined steps (eg, 2%) at pre-defined time intervals (eg, every 2 minutes). The main variables measured during treadmill testing are (1) time to the onset of claudication pain (ie, claudication onset time), and (2) peak walking time, at which point patients request to stop, usually because of intolerable claudication pain [13]. The latter measure is used most frequently in clinical trials as the primary outcome. Previous terms for these variables include pain-free walking distance/time and maximum walking distance/time, respectively.
The 6-minute walk test is an alternative to treadmill testing that is highly reproducible, valid, and sensitive to change in patients with claudication [14,15]. Advantages of this test include the lack of need for special equipment and that it provides a better approximation of community walking compared to treadmill walking in older patients [16,17]. More recently, global positioning system technology has been used to provide an objective assessment of walking capacity under free-living conditions in patients with intermittent claudication [17,18]. This may provide a useful method for physicians who do not have a treadmill and have trouble performing a 6-minute walk test (eg, due to space limitations); however, the validity and reliability of this method is dependent on patients adhering to standardized instructions for conducting a self-managed walking assessment in the community.
Self-reported walking capacity, assessed using standardized questionnaires, can provide a convenient alternative to objective measurement procedures. Various questionnaires have been proposed, of which the Walking Impairment Questionnaire (WIQ) is the most widely used. The WIQ, which was proposed over 20 years ago to standardize the estimation of walking limitation by patient interview [19], involves 14 items with 5 possible items for each item. The 14 items are divided into 3 sub-scales: a distance sub-scale (7 items), a speed sub-scale (4 items), and a stair-climbing sub-scale (3 items). It has been translated into several languages [20–22] and has been shown to be responsive to various treatment modalities [23,24]. Recently, a new shorter questionnaire has been proposed for estimating walking capacity in intermittent claudication, the Walking Estimated Limitation Calculated by History (WELCH) questionnaire [25,26]. Patients are required to report the maximum duration (8 possible responses ranging from “impossible” to “3 hours or more”) they can walk at 3 different speeds (ranging “slow” to “fast”), as well as what their normal walking speed is in comparison to their friends, relatives, and people of a similar age. Compared to the WIQ, the WELCH is shorter, suffers fewer errors when self-completed, provides comparable correlation with treadmill walking capacity data, and can be easily scored without a calculator or computer spreadsheet [25,27,28]. Further research is needed to assess its responsiveness to various interventions. Many other generic and disease-specific questionnaires have been proposed for assessing functional status and quality of life in claudication patients; an extensive review of these questionnaires can be found elsewhere [29]. In our opinion, very few questionnaires besides the WIQ and WELCH are useful for the routine assessment of patients’ walking limitation.
Several tests have been used to assess other aspects of functional capacity in patients with PAD, such as 4-meter walking speed, time to rise from a seated position 5 times, and standing balance (23). Although the inclusion of such measures may provide a more complete picture of a patient’s functional status than by assessing walking capacity alone, given the important of walking impairment in these patients and the predominant focus on this in the literature, the following sections on different treatments will focus solely on walking outcomes.
Treatments
Supervised Exercise Training
There is a considerable body of evidence to support a beneficial effect of supervised exercise training on walking performance in individuals with intermittent claudication. As such, supervised exercise training is recommended as a first-line therapy in clinical guidelines throughout the world [3,7,8]. Several systematic reviews and meta-analyses have attempted to quantify the effects of supervised exercise programs on walking performance [30–34]. For example, Fakhry et al [31] conducted a meta-analysis of 25 randomized controlled trials from 1966 to 2012,
Exercise programs comprise several components, including the mode and intensity of exercise, the duration and frequency of exercise sessions, the length of the program, and the level of supervision. Although few studies have directly compared different exercise regimes, some meta-analyses and systematic reviews have been conducted in an attempt to identify the program components that are the best predictors of improvement in walking distances [31,34,36–39]. For example, the meta-analysis of Gardner and Poehlman [36], which synthesized data from 21 randomized and nonrandomized exercise studies conducted between 1966 and 1993, indicated that claudication pain endpoint, program length, and mode of exercise explained 87% of the variance in improvements in maximum walking distance. Specifically, walking exercise appeared about twice as effective compared with other exercise modalities, walking to near-maximal leg pain was about 3 times more effective than walking to the point of claudication onset, and programs of at least 6 months' duration were about twice as effective as shorter programs. In contrast, the more contemporary synthesis of Fakhry et al [31] found that none of their predefined exercise components were independently associated with improvements in walking distances. Although walking programs are beneficial and frequently recommended
The role of supervision has attracted much interest in recent years. Currently, clinical guidelines recommend supervised exercise as a primary therapy for people with PAD, but not unsupervised exercise because of insufficient supporting evidence [3,7,8]. Unfortunately, most patients with intermittent claudication do not participate in supervised exercise training because of issues such as limited provision and patients being unable or unwilling to travel regularly to an exercise center [42–44]. Therefore, exercise is usually promoted in the form of “go home and walk” advice, but several studies have demonstrated this to have limited efficacy [41,45]. This has prompted researchers to develop and evaluate home-based exercise programs (HEPs), which are structured interventions that include at least one recognized behavior change technique [46] to promote self-managed walking. Recent reviews suggest that HEPs have superior effects on walking distance compared with basic advice to walk more, but inferior effects when compared with supervised exercise training [34,47]. However, most of the HEPs included in those reviews were poorly defined and failed to address patients’ knowledge gaps and uncertainty around the disease process and the role of walking, which is likely critical for providing impetus to behaviour change [48]. Recent trials that have included HEPs that have a clear theoretical underpinning and evidence-based behavior change techniques such as goal-setting, self-monitoring, and barrier identification and problem-solving have shown promising results and therefore may offer a pragmatic approach to promoting self-managed exercise in patients who are unwilling or unable to engage in supervised exercise training [45,49,50].
Safety Considerations
The risk of adverse cardiovascular and physiologic responses during exercise training is higher in patients with cardiovascular disease; therefore, to minimize the risk of exercise-related adverse events, patients with intermittent claudication should be evaluated clinically before initiating an exercise program. Patients should ideally perform a standard treadmill exercise test, with 12-lead electro-cardiographic monitoring if available, before a therapeutic exercise program is initiated [7], to determine that there are no untoward cardiovascular responses during exercise. It will also provide information about claudication thresholds and heart rate and blood pressure responses for establishing an exercise prescription. In best practice it is generally recommended that heart rate, exertion and ischemic symptoms are always monitored, given that an improvement in exercise tolerance might unmask myocardial ischemia. Patients should be counselled that although walking with claudication pain can improve walking distances and will not cause lasting harm, exercising with cardiac ischemia is not desirable and that if they experience chest pain they should stop exercising and, if it persists, contact a doctor or paramedic immediately. Proper foot care is also important, especially in those with diabetes mellitus, to prevent blisters and possible infections, which might in some cases develop into arterial ulcers. Daily inspection of the toes and plantar surfaces of the feet is therefore essential for early detection of any abnormality. Patients should be advised to return to their physician/general practitioner immediately if any changes occur in their feet.
Pharmacologic Therapies
In the UK, 4 drugs are licensed for the symptomatic relief of intermittent claudication: pentoxifylline, inositol nicotinate, cilostazol, and naftidrofuryl oxalate (in the US, naftidrofuryl oxalate is not FDA approved, and inositol is labeled GRAS [generally regarded as safe]). Pentoxifylline (Trental 400, Sanofi-Aventis) is an oral peripheral vasodilator derived from methylxanthine. To date, most studies have found no significant difference in walking distances between pentoxifylline and placebo groups, and a recent meta-analysis suggested that pentoxifylline only increased maximum walking distance by 11% (95% credible interval, –1 to 24%) relative to placebo [51]. Inositol nicotinate (Hexopal, Genus Pharmaceuticals) is an oral peripheral vasodilator that slows the release of nicotinic acid. A recent Health Technology Assessment highlighted that there have only been a few trials of this drug in claudication patients, and that the available data show limited efficacy [52]. It is also relatively expensive and has potential side effects of nausea/vomiting, skin rashes, and headache. Cilostazol (Pletal, Otsuka Pharmaceuticals) is an oral phosphodiesterase type 3 inhibitor, which is reported to have both antiplatelet and vasodilator effects [53]. In a systematic review and meta-analysis of drug therapies for intermittent claudication, Momsen et al reported a dose-dependent positive effect of cilostazol, with mean differences for maximum walking distance of 36 m (95% CI, 30 to 41 m) and 70 m (95% CI, 47 to 93), respectively, for 50 and 100 mg doses taken twice daily [50]. In a separate review, cilostazol was shown to increase maximum walking distance by 25% relative to placebo (95% credible interval, 20 to 114%), and pain-free walking distance by 13% [52]. Naftidrofuryl oxalate (Praxilene, Merck Serono) is an oral peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine 2 (5-HT2) receptors. The meta-analysis of Stevens et al, which included 2 trials of naftidrofuryl oxalate for claudication, indicated that this drug increased maximum walking distance by 60% (95% credible interval, 20 to 114%) and pain-free walking distance by 49% (95% credible interval, 23 to 81%) relative to placebo [51]. Comparative analyses indicated that the improvements were of a greater magnitude than those observed with pentoxifylline and cilostazol. An economic evaluation also suggested that naftidrofuryl oxalate “dominated” cilostazol and pentoxifylline, and has an incremental cost per QALY (quality-adjusted life-years) gained of around $9720 compared with no vasoactive drug [52]. However, Hong and Mackey recently concluded that the clinical data for both naftidrofuryl and cilostazol are plagued by flaws related to lack of protocol standardization, objective endpoints, and strict eligibility criteria in study subjects, making identification of a true treatment effect difficult [54].
Other studies have investigated the functional effects of drugs that are commonly used to reduce the risk of cardiovascular events in patients with PAD, including antiplatelet, antihypertensive and lipid-lowering agents. The meta-analysis of Momsen et al assessed the effects of antiplatelet agents on walking distances in intermittent claudication [55]. The included studies involved 5 different drugs (ticlopidine, cloricromene, mesoglycan, indobufen and defibrotide), and while some studies did not show a statistically significant benefit of antiplatelet therapy, the pooled estimate showed a modest increase in maximum walking distance favoring treatment of 59 m (95% CI, 37 to 81 m). The same paper also assessed the effects of 4 lipid-lowering drugs: atorvastatin, simvastatin, policosanol, and avasimibe [55]. Despite variable results according to the specific drug used, the effect estimates favored lipid-lowering agents in all studies and was statistically significant in all but one study. The pooled effect estimate was in favor of intervention, with a clinically relevant increase in maximum walking distance of 163 m (95% CI, 83 to 242 m). Two recent meta-analyses have also reviewed the functional effects of ACE inhibitors in patients with intermittent claudication [56,57], and although data are conflicting, a recent large trial of 212 patients reported that ramipril increased claudication onset time by 75 seconds (95% CI, 60 to 89 seconds) and peak walking time by 255 seconds (215 to 295 seconds) [58]. These changes were independent of the small change in blood pressure that occurred with ramipril treatment.
In summary, while some drugs have been shown to improve walking performance in patients with intermittent claudication, the effect has tended to be modest at best and smaller than that observed with supervised exercise training. Momsen et al concluded that statins probably have the greatest functional benefits [55], and clinical guidelines recommend that all patients with PAD should receive statin therapy [3,7,8], irrespective of its effect on functional status. The UK clinical guidelines recommend considering using naftidrofuryl oxalate for the treatment of claudication, but only when supervised exercise has not worked and revascularization is not feasible or declined by the patient [8]. The ACC/AHA guidelines state that a therapeutic trial of cilostazol should be considered in all patients with lifestyle-limiting claudication in the absence of heart failure [7].
Lower-Limb Revascularization
Intermittent claudication can also be treated using endovascular procedures (angioplasty ± stent placement) or bypass surgery, both of which constitute a relatively more direct means of addressing the problem since they target the arterial lesions causing claudication. Trials of revascularization in PAD have typically focused on vessel/graft patency as the primary outcome, with less emphasis placed on functional endpoints [59]. Despite this, it is clear that successful revascularization rapidly improves walking performance [60,61], whereas noticeable improvements with supervised exercise training can take several weeks to occur (assuming good adherence) [62]. Long-term comparisons of lower-limb revascularization with alternative treatment modalities for people with intermittent claudication are scarce. Recently, Fakhry et al [63] reported the long-term clinical effectiveness of supervised exercise therapy and endovascular revascularization from a randomized trial of 151 patients. After 7 years, the treatment strategies were similarly effective in improving functional performance and quality of life; however, the total number of endovascular and surgical interventions (primary and secondary) was substantially higher in the revascularization group, which will have resulted in significantly higher health care costs in this group. Furthermore, given that supervised exercise training costs substantially less than any revascularization procedure, it is not surprising that economic analyses indicate supervised exercise training as being more cost-effective [64,65]. This is reflected in clinical guidelines, which promote supervised exercise training as the first-line therapy [3,7,8]. In the UK, NICE recommends that clinicians should only offer angioplasty for treating people with intermittent claudication when advice on the benefits of modifying risk factors has been reinforced, a supervised exercise program has not led to a satisfactory improvement in symptoms, and imaging has confirmed that angioplasty is suitable for the person [8]. Bypass surgery for treating people with severe lifestyle-limiting intermittent claudication is only recommended when angioplasty has been unsuccessful or is unsuitable, and imaging has confirmed that bypass surgery is appropriate for the person. Overall, from a technical point of view during revascularization, there is no strong evidence to support that differences in clinical outcomes are observed as a function of technical choices of anastomoses in aortobifemoral bypasses [66] or kind of angioplasty in femoropopliteal lesions [67].
Potential Alternative Therapeutic Approaches
Several non-drug, non-exercise, and non-revascularization approaches have been investigated for their impact on claudication-related functional impairment, including (but not limited to) acupuncture, biofeedback, chelation therapy, CO2-applications, and the dietary supplements Allium sativum (garlic), Ginkgo biloba, omega-3 fatty acids, Padma 28, Vitamin E, and carnitine supplementation. In a recent systematic review, Delaney et al highlighted that most of the 8 parallel-group randomized controlled trials of propionyl-L-carnitine supplementation (600 to 3000 mg administered orally) demonstrated improvements in walking performance between 31 and 54 m greater than placebo for pain-free walking distance and between 9 and 86 m greater than placebo for maximum walking distance [68]. Propionyl-L-carnitine has been postulated to improve walking distance by improving endothelial function, and increasing total carnitine content in the ischemic muscle, which improves muscle metabolism and stimulates oxidative phosphorylation resulting in a decrease in plasma lactate concentration on exercise [68]. In a systematic review of these complementary therapies for PAD from 2005 [69], Pittler and Ernst concluded that there was some evidence for a beneficial effect of Ginkgo biloba and Padma 28 in claudication patients; however, recent meta-analyses have concluded that there is no evidence that Ginkgo biloba produces clinically meaningful improvements in walking distances [70], and that further well-designed research is required to determine the true effects of Padma 28 [71]. None of the other complementary treatment options have sufficient supporting evidence for them to be proposed as a routine approach [72–75]. Last, a few small studies have indicated that intermittent pneumatic compression (IPC) interventions can improve walking distances in people with intermittent claudication [76–78]. To date, IPC has received limited use in the clinical setting due to issues of cost and constraint; however, modern technology has permitted the development of portable systems to be made readily available for affordable at-home use. Adequately powered randomized controlled trials and economic evaluations are required to clarify the role of IPC for improving functional outcomes in intermittent claudication.
Conclusion
Intermittent claudication, the main symptom of mild-to-moderate PAD, is common in older adults. Individuals with intermittent claudication have reduced walking endurance and slower walking speed compared to individuals without PAD, and impairments in walking can reduce patients’ quality of life. There are several therapeutic options for improving walking performance in intermittent claudication, none of which are without limitations. Lower-limb revascularization procedures (angioplasty, bypass surgery) are invasive and have limited durability, and the medications approved for claudication-related functional impairment have limited efficacy. Supervised walking exercise can substantially improve walking performance; however, most patients do not participate in a supervised program due to issues of availability, awareness and access. Therefore, efforts should be made to provide patients with access to a supervised exercise program and encouragement to attend, or to promote self-managed walking when supervised exercise is not available or practical.
Corresponding author: Dr Garry A. Tew, York Trials Unit, Dept. of Health Sciences, University of York, York, YO10 5DD, UK, [email protected].
Financial disclosures: None.
Author contributions: conception and design, GAT, PA; drafting of article, GAT, PA; critical revision of the article, GAT, PA.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.
1. Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92.
2. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71;510–5.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
4. Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–26.
5. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14.
6. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
8. Layden J, Michaels J, Bermingham S, et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012;345:e4947.
9. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105.
10. Soer R, van der Schans CP, Groothoff JW, et al. Towards consensus in operational definitions in functional capacity evaluation: a Delphi Survey. J Occup Rehabil 2008;18:389–400.
11. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep 2013;15:347.
12. Labs KH, Dormandy JA, Jaeger KA, et al. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation 1999;100:e75–81.
13. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation 2008;118:2826–9.
14. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11.
15. McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61–8.
16. McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008;48:1231–7, 7.e1.
17. Tew G, Copeland R, Le Faucheur A, et al. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227–34.
18. Le Faucheur A, Abraham P, Jaquinandi V, et al. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117:897–904.
19. Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990;2:142–52.
20. Verspaget M, Nicolaï SP, Kruidenier LM, et al. Validation of the Dutch version of the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2009;37:56–61.
21. Yan BP, Lau JY, Yu CM, et al. Chinese translation and validation of the Walking Impairment Questionnaire in patients with peripheral artery disease. Vasc Med 2011;16:167–72.
22. Collins TC, Suarez-Almazor M, Petersen NJ, O'Malley KJ. A Spanish translation of the Walking Impairment Questionnaire was validated for patients with peripheral arterial disease. J Clin Epidemiol 2004;57:1305–15.
23. McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74.
24. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9.
25. Ouedraogo N, Chanut M, Aubourg M, et al. Development and evaluation of the Walking Estimated-Limitation Calculated by History questionnaire in patients with claudication. J Vasc Surg 2013;58:981–8.
26. Tew GA, Nawaz S, Humphreys L, et al. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med 2014;19:27–32.
27. Mahe G, Ouedraogo N, Vasseur M, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg 2011;41:104–9.
28. Ouedraogo N, Mahe G, Marchand J, et al. Validation of a new simple questionnaire to "estimate ambulation capacity by history" (EACH) in patients with claudication. J Vasc Surg 2011;54:133–8.
29. Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011;53:1410–21.
30. Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg 2007;34:1–9.
31. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012;56:1132–42.
32. Fokkenrood HJ, Bendermacher BL, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263.
33. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990.
34. Gommans LN, Saarloos R, Schelting MR, et al. Editor's choice--The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. Eur J Vasc Endovasc Surg 2014;48:169–84.
35. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123:87–97.
36. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–80.
37. Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med 2004;34:983–1003.
38. Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1–12.
39. Lauret GJ, Fakhry F, Fokkenrood HJ, et al. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD009638.
40. Zwierska I, Walker RD, Choksy SA, et al. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 2005;42:1122–30.
41. Tew G, Nawaz S, Zwierska I, Saxton JM. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci (Lond) 2009;117:405–13.
42. Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:233–9.
43. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;44:569–75.
44. Popplewell MA, Bradbury AW. Why do health systems not fund supervised exercise programmes for intermittent claudication? Eur J Vasc Endovasc Surg. 2014 Aug 28. [Epub ahead of print]
45. Cunningham MA, Swanson V, O'Carroll RE, et al. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. Br J Surg 2012;99:49–56.
46. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–98.
47. Al-Jundi W, Madbak K, Beard JD, et al. Systematic review of home-based exercise programmes for individuals with intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:690–706.
48. Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5–10.
49. McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65.
50. Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123:491–8.
51. Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630–8.
52. Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess 2011;15:1–210.
53. Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992;20:900–6.
54. Hong H, Mackey WC. The limits of evidence in drug approval and availability: a case study of cilostazol and naftidrofuryl for the treatment of intermittent claudication. Clin Ther 2014;36:1290–301.
55. Momsen AH, Jensen MB, Norager CB, et al. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463–74.
56. Shahin Y, Mazari F, Chetter I. Do angiotensin converting enzyme inhibitors improve walking distance in patients with symptomatic lower limb arterial disease? A systematic review and meta-analysis of randomised controlled trials. Int J Surg 2011;9:209–13.
57. Hunter MR, Cahoon WD, Lowe DK. Angiotensin-converting enzyme inhibitors for intermittent claudication associated with peripheral arterial disease. Ann Pharmacother 2013;47:1552–7.
58. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 2013;309:453–60.
59. Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation 2013;127:1241–50.
60. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg 2011;54:1511–21.
61. Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one year results of the IRONIC Trial. Circulation 2014 Aug. [Epub ahead of print]
62. Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg 2012;55:1346–54.
63. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013;100:1164–71.
64. Fokkenrood HJ, Scheltinga MR, Koelemay MJ, et al. Significant savings with a stepped care model for treatment of patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;48:423–9.
65. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008;48:1472–80.
66. Ameli FM, Stein M, Aro L, et al. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg 1991;34:243–6.
67. Cejna M, Thurnher S, Illiasch H, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol 2001;12:23–31.
68. Delaney CL, Spark JI, Thomas J, et al. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229:1–9.
69. Pittler MH, Ernst E. Complementary therapies for peripheral arterial disease: systematic review. Atherosclerosis 2005;181:1–7.
70. Nicolaï SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev 2009 (2):CD006888.
71. Morling JR, Maxwell H, Stewart M. Padma 28 for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD007371.
72. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013;7:CD003833.
73. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev 2000;2:CD000987.
74. Jepson RG, Kleijnen J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2013;4:CD000095.
75. Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev 2002;4:CD002785.
76. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg 2005;30:164–75.
77. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241:431–41.
78. de Haro J, Acin F, Florez A, et al. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 2010;51:857–62.
VIDEO: Hepatitis C screening recommendations falling on deaf ears
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2014
Hypopigmented Facial Papules on the Cheeks
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).
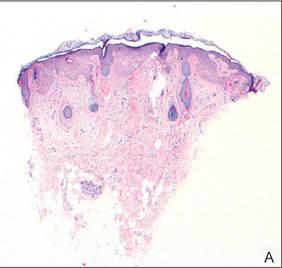
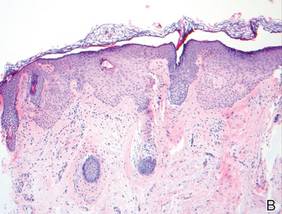
Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).


Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).


Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
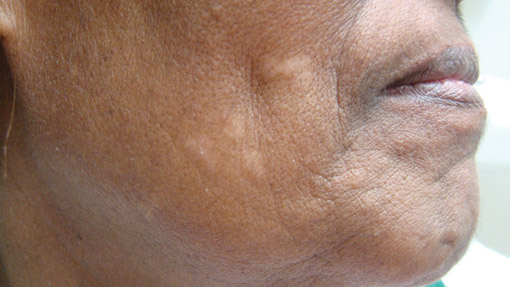
A 73-year-old woman presented with multiple mildly pruritic, hypopigmented, thin papules involving both cheeks of 5 months’ duration. The patient had no improvement with ketoconazole cream 2% and hydrocortisone cream 1% used daily for 1 month for presumed tinea versicolor. Physical examination revealed 10 ill-defined, 2- to 5-mm, round and oval, smooth hypopigmented, slightly raised papules located on the lower aspect of both cheeks.
Are Non-Nutritive Sweetened Beverages Comparable to Water in Weight Loss Trials?
Study Overview
Objective. To compare the efficacy of non-nutritive sweetened beverages (NNS) or water for weight loss during a 12-week behavioral weight loss treatment program.
Study design. 2-arm equivalence randomized clinical trial.
Setting and participants. Participants were recruited at the University of Colorado and Temple University. A total of 506 participants were screened and 308 were enrolled in the study. Inclusion criteria included being weight stable within 10 pounds in the 6 months prior to the trial, engaging in fewer than 300 min of physical activity per week and consuming at least 3 NNS beverages per week. Exclusion criteria included pregnancy, diabetes, cardiovascular disease, uncontrolled hypertension, and the use of medications affecting metabolism or weight. Participants also had physician approval stating they were in good health and could handle the nutrition and exercise requirements of the trial. Participants were randomly assigned to a NNS or water treatment arm using a computer-generated randomization that equally distributed men and women between the 2 groups. Participants had to be willing to discontinue consumption of NNS beverages for the duration of the 1-year study if they were randomized to the water-only group.
Intervention. The study was designed to include a 12-week weight loss phase followed by a 9-month maintenance phase. All participants received a cognitive-behavioral weight loss intervention called The Colorado Weigh. The program involved weekly hour-long group meetings led by registered dieticians or clinical psychologists. Groups were split by research arm and participants were taught about different weight loss strategies including self-monitoring, portion sizes, and physical activity. Participants were weighed at each meeting. The group curriculum was the same for both arms of the study except in the type of beverage they were encouraged to consume.
Participants were given individual energy targets based on their estimated resting metabolic rate (RMR), determined by using a Tanita Model TBF-300A bioelectrical impedance device that assesses body composition. Group leaders adjusted these targets as needed for participants in order to achieve a goal weight loss of 1 to 2 pounds per week. Physical activity targets were set to increase each participant’s typical physical activity by 10 minutes a week with a final target of 60 minutes a day, 6 days a week. Participants filled out daily exercise logs. Additionally, physical activity was assessed by the use of a Body Media armband that participants wore weeks 1 and 12.
Participants in the NNS group were asked to consume at least 24 fluid ounces of NNS beverage per day. Their water consumption was not limited. A beverage was considered NNS if it had less than 5 kcal per 8-ounce serving, was pre-mixed, and contained non-nutritive sweeteners. Participants in the water-only group were asked to drink at least 24 ounces of water a day and not drink any NNS beverages. They were allowed to eat foods that contained NNS but could not intentionally add NNS to beverages such as coffee. Participants in both groups were asked to record their beverage intake daily. Participants were given manufacturers’ coupons for bottled water or NNS beverages.
Main outcome measures. The primary outcomes were weight loss at 12 weeks (weight loss period) and at 1 year (weight loss maintenance). All assessments were conducted at baseline and after 12 weeks. This was designed as an equivalence trial, and the authors’ hypothesis was that there would be no clinically meaningful difference in weight change between the 2 groups. The authors pre-specified that the bounds of equivalence would be 1.7 kg. Waist circumference was recorded in addition to height and weight. Participant’s blood pressure was also recorded and blood samples were collected to measure lipids and glucose. Urine samples were collected to measure urine osmolality. Participants completed questionnaires at baseline and 12 weeks to assess changes in perceived hunger.
Results. A total of 308 patients were randomized following baseline assessment but 5 did not begin treatment. 279 of the remaining 303 participants completed the full 12-week weight loss phase of the study. The dropout rate in the water group was 10% compared to 5.8% in the NNS group, but this was not statistically significant. 80% of participants were female, 68% were white, and 27% African American. There were no significant differences at baseline in age, gender, race/ethnicity or other measures between the water-only and NNS groups. There was no significant difference in adherence to the beverage requirements between the 2 groups (96.6% in the NNS group and 95.7% in the water-only group), and similarly group attendance did not differ between the 2 groups (90.8% for NNS and 89.7% for water-only).
The mean weight loss difference between the water and NNS groups was –1.85 kg (90% confidence interval [CI], –1.12 to –2.58 kg). Because the lower confidence limit of –2.58 kg was outside the equivalence limit set in the hypothesis, the 2 treatments were not considered equivalent and paired comparisons were carried out. Analysis done using an intention-to-treat scheme indicated that the weight loss in the NNS group (5.95 kg ± 3.94 kg) was significantly higher than the weight loss in the water-only group (4.09 ± 3.74 kg, P < 0.001). 43.0% of participants in the water-only group lost > 5% of their body weight and 64.3% of participants in the NNS group lost > 5% of their body weight (P < 0.001).
After 12 weeks of treatment there was no significant difference between the 2 groups in changes in waist circumference, blood pressure, HDL, triglycerides, or urine osmolality. Reductions in total cholesterol and LDL were significantly greater in the NNS group than the water group. There were no significant changes in physical activity between the 2 groups as measured by the exercise logs or the Body Media armbands. There was a statistically significant difference in hunger between the 2 groups (P = 0.013): participants in the water group reported increased hunger, while participants in the NNS group reported a slight decrease in hunger.
Conclusion. Participants who drank at least 3 servings of NNS beverages a day at baseline lost more weight during a behavioral weight loss program when they continued to drink NNS beverages than participants who were asked to cut NNS beverages and drink only water. The study was designed as an equivalence trial but paired comparisons showed a significant difference in weight loss between the 2 groups.
Commentary
Obesity is a major public health concern in the United States and drinking sugar-sweetened beverages has been indicated as a significant contributing factor. Consumption of sugar-sweetened beverages increased considerably from 1994 to 2004 [1]. Fortunately, there is strong evidence that decreasing the consumption of sugar sweetened beverages can lead to weight loss [2]. Most studies look at the effect of replacing sugar-sweetened beverages with water [3] and, in fact, increased consumption of water has been shown to aid weight loss [4]. The relationship between diet drinks and obesity, however, has been a source of controversy. Since NNS beverages contain little to no calories they are a logical replacement for sugar-sweetened beverages, but observational studies have shown a positive correlation between diet drinks and obesity [5,6] as well as type 2 diabetes [7]. Additionally, a recent study by Suez et al [8] found that consumption of artificial sweeteners affects the gut microbiota and increases glucose intolerance. However this correlation may not be causal; NNS beverage consumption may be higher in overweight individuals. A study by Tate et al [9,10] looked at replacing sugar-sweetened beverages with water or artificially sweetened beverages and found no significant difference in weight loss between the 2 groups. However, the Tate et al study used beverage replacement as the primary intervention. This experiment by Peters et al is unique because it tested the hypothesis that NNS is equivalent to water alone when combined with a structured weight loss program. Their results reject the equivalence hypothesis and suggest that NNS beverages facilitate weight loss for patients already consuming them.
Strengths of this study included the use of a randomized, equivalence design. The study also examined secondary outcomes (eg, waist circumference, lipids, and urine osmolality) that helped reinforce that participants consuming NNS were able to lose weight without compromising their health. Further, they measured hunger and found that participants in the NNS beverage group had decreased hunger while those in the water group had increased hunger, which points to a potential mechanism for their findings.
However, the potential for bias in this study is concerning. One major weakness is that all the participants were initially regular drinkers of NNS beverages. The authors never explain why consuming 3 NNS beverages per week was an inclusion criteria. Participants in the water group had to change their behavior to abstain from NNS beverages and this may have impacted results. More concerning, this study was fully funded by the American Beverage Association, who has an obvious interest in promoting NNS beverage consumption. Finally, the authors mention that 5 participants dropped out after randomization but before the start of treatment and were excluded from the study after baseline assessment. The authors do not provide information about group allocation or if the participants knew which group they were assigned to, calling into question the integrity of the intention-to-treat design.
Applications for Clinical Practice
For patients who already drink NNS beverages and are motivated to lose weight, these results support continued use. However, it is unclear how NNS beverages impact weight loss efforts for patients who do not currently drink them. Further, since other studies have shown potential harm of NNS beverages [6–8], more studies are needed to better elucidate their health effects.
—Susan Creighton and Melanie Jay, MD, MS
1. Bleich SN, Wang YC, Wang Y. Increasing consumption of sugar-sweetened beverages among US adults—1988-1994 to 1999-2004. Am J Clin Nutr 2009;89;372–81.
2. Hu FB. Resolved—there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obesity Rev 2013;14;606–19.
3. Stokey JD, Constant F, Gardner CD. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity 2007;15;3013–22.
4. Vij VA, Joshi AS. Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants. J Nat Sci Biol Med 2014;340–4.
5. Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433–40.
6. Fowler SP, Williams K, Resendez RG. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008;16:1894–900.
7. Nettleton JA, Lutsey PL, Wang Y. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94.
8. Suez J, Korem T, Zeevi D. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514:181–6.
9. Tate DF, Turner-McGrievy G, Lyons E. Replacing caloric beverages with water or diet beverages for weight loss in adults—main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63.
10. Piernas C, Tate DF, Wang X. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2013;97:604–11.
Study Overview
Objective. To compare the efficacy of non-nutritive sweetened beverages (NNS) or water for weight loss during a 12-week behavioral weight loss treatment program.
Study design. 2-arm equivalence randomized clinical trial.
Setting and participants. Participants were recruited at the University of Colorado and Temple University. A total of 506 participants were screened and 308 were enrolled in the study. Inclusion criteria included being weight stable within 10 pounds in the 6 months prior to the trial, engaging in fewer than 300 min of physical activity per week and consuming at least 3 NNS beverages per week. Exclusion criteria included pregnancy, diabetes, cardiovascular disease, uncontrolled hypertension, and the use of medications affecting metabolism or weight. Participants also had physician approval stating they were in good health and could handle the nutrition and exercise requirements of the trial. Participants were randomly assigned to a NNS or water treatment arm using a computer-generated randomization that equally distributed men and women between the 2 groups. Participants had to be willing to discontinue consumption of NNS beverages for the duration of the 1-year study if they were randomized to the water-only group.
Intervention. The study was designed to include a 12-week weight loss phase followed by a 9-month maintenance phase. All participants received a cognitive-behavioral weight loss intervention called The Colorado Weigh. The program involved weekly hour-long group meetings led by registered dieticians or clinical psychologists. Groups were split by research arm and participants were taught about different weight loss strategies including self-monitoring, portion sizes, and physical activity. Participants were weighed at each meeting. The group curriculum was the same for both arms of the study except in the type of beverage they were encouraged to consume.
Participants were given individual energy targets based on their estimated resting metabolic rate (RMR), determined by using a Tanita Model TBF-300A bioelectrical impedance device that assesses body composition. Group leaders adjusted these targets as needed for participants in order to achieve a goal weight loss of 1 to 2 pounds per week. Physical activity targets were set to increase each participant’s typical physical activity by 10 minutes a week with a final target of 60 minutes a day, 6 days a week. Participants filled out daily exercise logs. Additionally, physical activity was assessed by the use of a Body Media armband that participants wore weeks 1 and 12.
Participants in the NNS group were asked to consume at least 24 fluid ounces of NNS beverage per day. Their water consumption was not limited. A beverage was considered NNS if it had less than 5 kcal per 8-ounce serving, was pre-mixed, and contained non-nutritive sweeteners. Participants in the water-only group were asked to drink at least 24 ounces of water a day and not drink any NNS beverages. They were allowed to eat foods that contained NNS but could not intentionally add NNS to beverages such as coffee. Participants in both groups were asked to record their beverage intake daily. Participants were given manufacturers’ coupons for bottled water or NNS beverages.
Main outcome measures. The primary outcomes were weight loss at 12 weeks (weight loss period) and at 1 year (weight loss maintenance). All assessments were conducted at baseline and after 12 weeks. This was designed as an equivalence trial, and the authors’ hypothesis was that there would be no clinically meaningful difference in weight change between the 2 groups. The authors pre-specified that the bounds of equivalence would be 1.7 kg. Waist circumference was recorded in addition to height and weight. Participant’s blood pressure was also recorded and blood samples were collected to measure lipids and glucose. Urine samples were collected to measure urine osmolality. Participants completed questionnaires at baseline and 12 weeks to assess changes in perceived hunger.
Results. A total of 308 patients were randomized following baseline assessment but 5 did not begin treatment. 279 of the remaining 303 participants completed the full 12-week weight loss phase of the study. The dropout rate in the water group was 10% compared to 5.8% in the NNS group, but this was not statistically significant. 80% of participants were female, 68% were white, and 27% African American. There were no significant differences at baseline in age, gender, race/ethnicity or other measures between the water-only and NNS groups. There was no significant difference in adherence to the beverage requirements between the 2 groups (96.6% in the NNS group and 95.7% in the water-only group), and similarly group attendance did not differ between the 2 groups (90.8% for NNS and 89.7% for water-only).
The mean weight loss difference between the water and NNS groups was –1.85 kg (90% confidence interval [CI], –1.12 to –2.58 kg). Because the lower confidence limit of –2.58 kg was outside the equivalence limit set in the hypothesis, the 2 treatments were not considered equivalent and paired comparisons were carried out. Analysis done using an intention-to-treat scheme indicated that the weight loss in the NNS group (5.95 kg ± 3.94 kg) was significantly higher than the weight loss in the water-only group (4.09 ± 3.74 kg, P < 0.001). 43.0% of participants in the water-only group lost > 5% of their body weight and 64.3% of participants in the NNS group lost > 5% of their body weight (P < 0.001).
After 12 weeks of treatment there was no significant difference between the 2 groups in changes in waist circumference, blood pressure, HDL, triglycerides, or urine osmolality. Reductions in total cholesterol and LDL were significantly greater in the NNS group than the water group. There were no significant changes in physical activity between the 2 groups as measured by the exercise logs or the Body Media armbands. There was a statistically significant difference in hunger between the 2 groups (P = 0.013): participants in the water group reported increased hunger, while participants in the NNS group reported a slight decrease in hunger.
Conclusion. Participants who drank at least 3 servings of NNS beverages a day at baseline lost more weight during a behavioral weight loss program when they continued to drink NNS beverages than participants who were asked to cut NNS beverages and drink only water. The study was designed as an equivalence trial but paired comparisons showed a significant difference in weight loss between the 2 groups.
Commentary
Obesity is a major public health concern in the United States and drinking sugar-sweetened beverages has been indicated as a significant contributing factor. Consumption of sugar-sweetened beverages increased considerably from 1994 to 2004 [1]. Fortunately, there is strong evidence that decreasing the consumption of sugar sweetened beverages can lead to weight loss [2]. Most studies look at the effect of replacing sugar-sweetened beverages with water [3] and, in fact, increased consumption of water has been shown to aid weight loss [4]. The relationship between diet drinks and obesity, however, has been a source of controversy. Since NNS beverages contain little to no calories they are a logical replacement for sugar-sweetened beverages, but observational studies have shown a positive correlation between diet drinks and obesity [5,6] as well as type 2 diabetes [7]. Additionally, a recent study by Suez et al [8] found that consumption of artificial sweeteners affects the gut microbiota and increases glucose intolerance. However this correlation may not be causal; NNS beverage consumption may be higher in overweight individuals. A study by Tate et al [9,10] looked at replacing sugar-sweetened beverages with water or artificially sweetened beverages and found no significant difference in weight loss between the 2 groups. However, the Tate et al study used beverage replacement as the primary intervention. This experiment by Peters et al is unique because it tested the hypothesis that NNS is equivalent to water alone when combined with a structured weight loss program. Their results reject the equivalence hypothesis and suggest that NNS beverages facilitate weight loss for patients already consuming them.
Strengths of this study included the use of a randomized, equivalence design. The study also examined secondary outcomes (eg, waist circumference, lipids, and urine osmolality) that helped reinforce that participants consuming NNS were able to lose weight without compromising their health. Further, they measured hunger and found that participants in the NNS beverage group had decreased hunger while those in the water group had increased hunger, which points to a potential mechanism for their findings.
However, the potential for bias in this study is concerning. One major weakness is that all the participants were initially regular drinkers of NNS beverages. The authors never explain why consuming 3 NNS beverages per week was an inclusion criteria. Participants in the water group had to change their behavior to abstain from NNS beverages and this may have impacted results. More concerning, this study was fully funded by the American Beverage Association, who has an obvious interest in promoting NNS beverage consumption. Finally, the authors mention that 5 participants dropped out after randomization but before the start of treatment and were excluded from the study after baseline assessment. The authors do not provide information about group allocation or if the participants knew which group they were assigned to, calling into question the integrity of the intention-to-treat design.
Applications for Clinical Practice
For patients who already drink NNS beverages and are motivated to lose weight, these results support continued use. However, it is unclear how NNS beverages impact weight loss efforts for patients who do not currently drink them. Further, since other studies have shown potential harm of NNS beverages [6–8], more studies are needed to better elucidate their health effects.
—Susan Creighton and Melanie Jay, MD, MS
Study Overview
Objective. To compare the efficacy of non-nutritive sweetened beverages (NNS) or water for weight loss during a 12-week behavioral weight loss treatment program.
Study design. 2-arm equivalence randomized clinical trial.
Setting and participants. Participants were recruited at the University of Colorado and Temple University. A total of 506 participants were screened and 308 were enrolled in the study. Inclusion criteria included being weight stable within 10 pounds in the 6 months prior to the trial, engaging in fewer than 300 min of physical activity per week and consuming at least 3 NNS beverages per week. Exclusion criteria included pregnancy, diabetes, cardiovascular disease, uncontrolled hypertension, and the use of medications affecting metabolism or weight. Participants also had physician approval stating they were in good health and could handle the nutrition and exercise requirements of the trial. Participants were randomly assigned to a NNS or water treatment arm using a computer-generated randomization that equally distributed men and women between the 2 groups. Participants had to be willing to discontinue consumption of NNS beverages for the duration of the 1-year study if they were randomized to the water-only group.
Intervention. The study was designed to include a 12-week weight loss phase followed by a 9-month maintenance phase. All participants received a cognitive-behavioral weight loss intervention called The Colorado Weigh. The program involved weekly hour-long group meetings led by registered dieticians or clinical psychologists. Groups were split by research arm and participants were taught about different weight loss strategies including self-monitoring, portion sizes, and physical activity. Participants were weighed at each meeting. The group curriculum was the same for both arms of the study except in the type of beverage they were encouraged to consume.
Participants were given individual energy targets based on their estimated resting metabolic rate (RMR), determined by using a Tanita Model TBF-300A bioelectrical impedance device that assesses body composition. Group leaders adjusted these targets as needed for participants in order to achieve a goal weight loss of 1 to 2 pounds per week. Physical activity targets were set to increase each participant’s typical physical activity by 10 minutes a week with a final target of 60 minutes a day, 6 days a week. Participants filled out daily exercise logs. Additionally, physical activity was assessed by the use of a Body Media armband that participants wore weeks 1 and 12.
Participants in the NNS group were asked to consume at least 24 fluid ounces of NNS beverage per day. Their water consumption was not limited. A beverage was considered NNS if it had less than 5 kcal per 8-ounce serving, was pre-mixed, and contained non-nutritive sweeteners. Participants in the water-only group were asked to drink at least 24 ounces of water a day and not drink any NNS beverages. They were allowed to eat foods that contained NNS but could not intentionally add NNS to beverages such as coffee. Participants in both groups were asked to record their beverage intake daily. Participants were given manufacturers’ coupons for bottled water or NNS beverages.
Main outcome measures. The primary outcomes were weight loss at 12 weeks (weight loss period) and at 1 year (weight loss maintenance). All assessments were conducted at baseline and after 12 weeks. This was designed as an equivalence trial, and the authors’ hypothesis was that there would be no clinically meaningful difference in weight change between the 2 groups. The authors pre-specified that the bounds of equivalence would be 1.7 kg. Waist circumference was recorded in addition to height and weight. Participant’s blood pressure was also recorded and blood samples were collected to measure lipids and glucose. Urine samples were collected to measure urine osmolality. Participants completed questionnaires at baseline and 12 weeks to assess changes in perceived hunger.
Results. A total of 308 patients were randomized following baseline assessment but 5 did not begin treatment. 279 of the remaining 303 participants completed the full 12-week weight loss phase of the study. The dropout rate in the water group was 10% compared to 5.8% in the NNS group, but this was not statistically significant. 80% of participants were female, 68% were white, and 27% African American. There were no significant differences at baseline in age, gender, race/ethnicity or other measures between the water-only and NNS groups. There was no significant difference in adherence to the beverage requirements between the 2 groups (96.6% in the NNS group and 95.7% in the water-only group), and similarly group attendance did not differ between the 2 groups (90.8% for NNS and 89.7% for water-only).
The mean weight loss difference between the water and NNS groups was –1.85 kg (90% confidence interval [CI], –1.12 to –2.58 kg). Because the lower confidence limit of –2.58 kg was outside the equivalence limit set in the hypothesis, the 2 treatments were not considered equivalent and paired comparisons were carried out. Analysis done using an intention-to-treat scheme indicated that the weight loss in the NNS group (5.95 kg ± 3.94 kg) was significantly higher than the weight loss in the water-only group (4.09 ± 3.74 kg, P < 0.001). 43.0% of participants in the water-only group lost > 5% of their body weight and 64.3% of participants in the NNS group lost > 5% of their body weight (P < 0.001).
After 12 weeks of treatment there was no significant difference between the 2 groups in changes in waist circumference, blood pressure, HDL, triglycerides, or urine osmolality. Reductions in total cholesterol and LDL were significantly greater in the NNS group than the water group. There were no significant changes in physical activity between the 2 groups as measured by the exercise logs or the Body Media armbands. There was a statistically significant difference in hunger between the 2 groups (P = 0.013): participants in the water group reported increased hunger, while participants in the NNS group reported a slight decrease in hunger.
Conclusion. Participants who drank at least 3 servings of NNS beverages a day at baseline lost more weight during a behavioral weight loss program when they continued to drink NNS beverages than participants who were asked to cut NNS beverages and drink only water. The study was designed as an equivalence trial but paired comparisons showed a significant difference in weight loss between the 2 groups.
Commentary
Obesity is a major public health concern in the United States and drinking sugar-sweetened beverages has been indicated as a significant contributing factor. Consumption of sugar-sweetened beverages increased considerably from 1994 to 2004 [1]. Fortunately, there is strong evidence that decreasing the consumption of sugar sweetened beverages can lead to weight loss [2]. Most studies look at the effect of replacing sugar-sweetened beverages with water [3] and, in fact, increased consumption of water has been shown to aid weight loss [4]. The relationship between diet drinks and obesity, however, has been a source of controversy. Since NNS beverages contain little to no calories they are a logical replacement for sugar-sweetened beverages, but observational studies have shown a positive correlation between diet drinks and obesity [5,6] as well as type 2 diabetes [7]. Additionally, a recent study by Suez et al [8] found that consumption of artificial sweeteners affects the gut microbiota and increases glucose intolerance. However this correlation may not be causal; NNS beverage consumption may be higher in overweight individuals. A study by Tate et al [9,10] looked at replacing sugar-sweetened beverages with water or artificially sweetened beverages and found no significant difference in weight loss between the 2 groups. However, the Tate et al study used beverage replacement as the primary intervention. This experiment by Peters et al is unique because it tested the hypothesis that NNS is equivalent to water alone when combined with a structured weight loss program. Their results reject the equivalence hypothesis and suggest that NNS beverages facilitate weight loss for patients already consuming them.
Strengths of this study included the use of a randomized, equivalence design. The study also examined secondary outcomes (eg, waist circumference, lipids, and urine osmolality) that helped reinforce that participants consuming NNS were able to lose weight without compromising their health. Further, they measured hunger and found that participants in the NNS beverage group had decreased hunger while those in the water group had increased hunger, which points to a potential mechanism for their findings.
However, the potential for bias in this study is concerning. One major weakness is that all the participants were initially regular drinkers of NNS beverages. The authors never explain why consuming 3 NNS beverages per week was an inclusion criteria. Participants in the water group had to change their behavior to abstain from NNS beverages and this may have impacted results. More concerning, this study was fully funded by the American Beverage Association, who has an obvious interest in promoting NNS beverage consumption. Finally, the authors mention that 5 participants dropped out after randomization but before the start of treatment and were excluded from the study after baseline assessment. The authors do not provide information about group allocation or if the participants knew which group they were assigned to, calling into question the integrity of the intention-to-treat design.
Applications for Clinical Practice
For patients who already drink NNS beverages and are motivated to lose weight, these results support continued use. However, it is unclear how NNS beverages impact weight loss efforts for patients who do not currently drink them. Further, since other studies have shown potential harm of NNS beverages [6–8], more studies are needed to better elucidate their health effects.
—Susan Creighton and Melanie Jay, MD, MS
1. Bleich SN, Wang YC, Wang Y. Increasing consumption of sugar-sweetened beverages among US adults—1988-1994 to 1999-2004. Am J Clin Nutr 2009;89;372–81.
2. Hu FB. Resolved—there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obesity Rev 2013;14;606–19.
3. Stokey JD, Constant F, Gardner CD. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity 2007;15;3013–22.
4. Vij VA, Joshi AS. Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants. J Nat Sci Biol Med 2014;340–4.
5. Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433–40.
6. Fowler SP, Williams K, Resendez RG. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008;16:1894–900.
7. Nettleton JA, Lutsey PL, Wang Y. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94.
8. Suez J, Korem T, Zeevi D. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514:181–6.
9. Tate DF, Turner-McGrievy G, Lyons E. Replacing caloric beverages with water or diet beverages for weight loss in adults—main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63.
10. Piernas C, Tate DF, Wang X. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2013;97:604–11.
1. Bleich SN, Wang YC, Wang Y. Increasing consumption of sugar-sweetened beverages among US adults—1988-1994 to 1999-2004. Am J Clin Nutr 2009;89;372–81.
2. Hu FB. Resolved—there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obesity Rev 2013;14;606–19.
3. Stokey JD, Constant F, Gardner CD. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity 2007;15;3013–22.
4. Vij VA, Joshi AS. Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants. J Nat Sci Biol Med 2014;340–4.
5. Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433–40.
6. Fowler SP, Williams K, Resendez RG. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008;16:1894–900.
7. Nettleton JA, Lutsey PL, Wang Y. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94.
8. Suez J, Korem T, Zeevi D. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514:181–6.
9. Tate DF, Turner-McGrievy G, Lyons E. Replacing caloric beverages with water or diet beverages for weight loss in adults—main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63.
10. Piernas C, Tate DF, Wang X. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2013;97:604–11.
VIDEO: Most baby boomers didn’t know their hep C status
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2014
Telehealth as an Alternative to Traditional, In-Person Diabetes Self-Management Support
Study Overview
Objective. To investigate the feasibility and effectiveness of administering diabetes self-management support (DSMS) via telephone or secure messaging.
Design. Prospective, longitudinal quasi-experimental study.
Setting and participants. Participants (n = 150) who had previously completed diabetes self-management education (DSME) received follow-up DSMS in 1 of 3 self-selected ways: a one-time in-person visit, 3 brief visits by telephone, or via secure messaging via the electronic health record. The (usual care) in-person group (n = 47) received 1 follow-up appointment at the patient’s request with a certified diabetes educator (CDE) within 3 to 6 months of DSME completion. The telephone group (n = 44) was given follow-up phone appointments with a CDE, each lasting approximately 20 minutes, at 3, 6, and 9 months post-DSME. The secure message group (n = 59) received follow-up messages via the patient portal from a CDE at 3, 6, and 9 months post-DSME. At each interval, patients received 3 messages, an initial one followed by 2 structured replies. Motivational interviewing techniques were used in all 3 groups to identify barriers to achieving behavior goals and solutions.
Main outcome measures. Behavior goal measures, feasibility measures, and physiologic measures at 9 months’ post DSME. Behavior goal achievement was measured using a survey that asked patients to rate their achievement regarding the following AADE7 goals: healthy eating, being active, self-monitoring, taking medications, problem solving, reducing risks, and healthy coping. Goals are rated on a scale from 0 to 10, with a rating ≥ 7 considered successful completion. Feasibility to integrate this technology into a DSME platform was assessed by comparing the number of attempts to contact patients with the number of contacts achieved; also calculated was intervention completion, mean time spent with the CDE, and total cost of each visit. Physiologic measures included HbA1C and LDL levels collected through medical record review.
Results. There were no statistically significant differences between groups with respect to any of the primary outcomes. Behavioral goals were achieved by 59% of the in-person group, 73% of the telephone group, and 77% of the secure message group . Mean goal achievement for all 3 groups combined improved from 6.2 ± 2.4 to 7.2 ± 1.8 (P < 0.05). Overall, 70.3% ± 0.46% achieved behavioral goals, with no difference among groups. In terms of feasibility, at 3 months the contact success rate was 39%, 46%, and 29% in the in-person, telephone, and secure message groups, respectively. At 6 months, the contact success rate was 47% in the phone group versus 32% in the secure message group. At 9 months, the contact success rate was 35% in the phone group versus 21% in the secure message group. Sixty-two participants (41%) completed the intervention per protocol: 51% of in-person patients, 47% of phone patients, and 28% of secure message patients (P < 0.02). Visits lasted and cost, on average, 60 minutes and $50.00, 45.3 minutes and $37.75, and 17.8 minutes (P < 0.05) and $14.83 for the in-person, telephone, and secure message groups, respectively. There was no difference in HbA1c among groups. Overall, HbA1c decreased by −0.88% ± 1.63 (P < 0.05) from baseline to 9 months. Change in LDL was not significant, and neither were there statistical differences among groups.
Conclusion. Diabetes follow-up care delivered via telephone and secure messaging is feasible. Using either of these methods results in similar outcomes compared with the traditional in-person visit, while requiring less staff time.
Commentary
Diabetes mellitus is a growing epidemic in the United States, affecting nearly 10% of American adults [1]. The disease is associated with multiple, potentially fatal complications, including heart disease, stroke, kidney failure, and limb amputation [1]. Studies show that ongoing diabetes self-management education (DSME) can result in lifestyle and behavioral changes that improve glycemic control, ultimately reducing the risk of complications [2,3]. However, traditional follow-up care and education for patients with diabetes requires considerable time on the part of patients and providers, and is both costly and resource-intensive [4]. The use of telehealth to educate and monitor patients with diabetes is a growing phenomenon. Theoretically, telehealth enables providers to reach greater subsets of the population who may not otherwise be able to consult with a doctor or nurse regularly. However, little is known about the overall effectiveness of telehealth compared with regular office visits with respect to diabetes and patient outcomes.
This study investigated the feasibility of using telephone and secure message methods to deliver ongoing DSMS after the completion of an existing DSME program. The results suggest that there is no difference in behavioral goal achievement, feasibility, and clinical outcomes among usual care and intervention groups.
This study had a number of strengths, including a strong scientific background in support of research that examines telehealth options for diabetes management. The inclusion criteria were straightforward and appropriate for the targeted patient population: all participants were over 18 and had previously completed the DSME class; all participants in the phone group were required to have a working telephone line, while the secure message group participants were required to have internet access. However, there were some methodologic weaknesses, most of which were pointed out by the authors. These included (1) lack of randomization, (2) a high attrition rate, and (3) nonspecific outcome measures. In addition, participants were able to self-enroll into a category of their choice. The lack of randomization enabled selection bias and prohibits the authors from inferring a causal effect between DSMS and improved health outcomes. Attrition rates were also problematic in this study. Not only did 59% of enrolled participants fail to complete the intervention, but the overall contact success rates declined over time. Finally, the outcome measure for feasibility is poorly defined because the authors never provide a numerical measure limitation. External validity is limited by a largely Caucasian sample that is predominately female. Due to the weaknesses inherent in the study’s methodology, the findings should be interpreted with some degree of caution.
Applications for Clinical Practice
Given the potential for long-term complications from diabetes, the rising cost of health care services, and the overall shortage of medical and nursing personnel, alternative methods of patient follow-up are needed in the management of diabetes. Telehealth has the potential to reach a significant portion of the population that is receiving little or no care in rural and underserved areas in a convenient and less costly way than traditional care. Investigating which alternatives to usual care are effective for which patient groups will pave the way for resource optimization and cost-effectiveness. Providing DSME follow-up through telehealth methodologies may be an effective alternative to in-person visits. Additional research is needed to support the outcomes of this study and to determine the duration of DSMS that is needed to ensure sufficient diabetes self-management.
—Amy Burchard, BA, and Tina Sadarangani, MSN, ANP-BC, GNP-BC
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: US Department of Health and Human Services; 2011.
2. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–71.
3. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12.
4. Shani M, Sasson N, Lustman A, et al. Structured nursing follow-up: does it help in diabetes care? Isr J Health Policy Res 2014;3:27.
Study Overview
Objective. To investigate the feasibility and effectiveness of administering diabetes self-management support (DSMS) via telephone or secure messaging.
Design. Prospective, longitudinal quasi-experimental study.
Setting and participants. Participants (n = 150) who had previously completed diabetes self-management education (DSME) received follow-up DSMS in 1 of 3 self-selected ways: a one-time in-person visit, 3 brief visits by telephone, or via secure messaging via the electronic health record. The (usual care) in-person group (n = 47) received 1 follow-up appointment at the patient’s request with a certified diabetes educator (CDE) within 3 to 6 months of DSME completion. The telephone group (n = 44) was given follow-up phone appointments with a CDE, each lasting approximately 20 minutes, at 3, 6, and 9 months post-DSME. The secure message group (n = 59) received follow-up messages via the patient portal from a CDE at 3, 6, and 9 months post-DSME. At each interval, patients received 3 messages, an initial one followed by 2 structured replies. Motivational interviewing techniques were used in all 3 groups to identify barriers to achieving behavior goals and solutions.
Main outcome measures. Behavior goal measures, feasibility measures, and physiologic measures at 9 months’ post DSME. Behavior goal achievement was measured using a survey that asked patients to rate their achievement regarding the following AADE7 goals: healthy eating, being active, self-monitoring, taking medications, problem solving, reducing risks, and healthy coping. Goals are rated on a scale from 0 to 10, with a rating ≥ 7 considered successful completion. Feasibility to integrate this technology into a DSME platform was assessed by comparing the number of attempts to contact patients with the number of contacts achieved; also calculated was intervention completion, mean time spent with the CDE, and total cost of each visit. Physiologic measures included HbA1C and LDL levels collected through medical record review.
Results. There were no statistically significant differences between groups with respect to any of the primary outcomes. Behavioral goals were achieved by 59% of the in-person group, 73% of the telephone group, and 77% of the secure message group . Mean goal achievement for all 3 groups combined improved from 6.2 ± 2.4 to 7.2 ± 1.8 (P < 0.05). Overall, 70.3% ± 0.46% achieved behavioral goals, with no difference among groups. In terms of feasibility, at 3 months the contact success rate was 39%, 46%, and 29% in the in-person, telephone, and secure message groups, respectively. At 6 months, the contact success rate was 47% in the phone group versus 32% in the secure message group. At 9 months, the contact success rate was 35% in the phone group versus 21% in the secure message group. Sixty-two participants (41%) completed the intervention per protocol: 51% of in-person patients, 47% of phone patients, and 28% of secure message patients (P < 0.02). Visits lasted and cost, on average, 60 minutes and $50.00, 45.3 minutes and $37.75, and 17.8 minutes (P < 0.05) and $14.83 for the in-person, telephone, and secure message groups, respectively. There was no difference in HbA1c among groups. Overall, HbA1c decreased by −0.88% ± 1.63 (P < 0.05) from baseline to 9 months. Change in LDL was not significant, and neither were there statistical differences among groups.
Conclusion. Diabetes follow-up care delivered via telephone and secure messaging is feasible. Using either of these methods results in similar outcomes compared with the traditional in-person visit, while requiring less staff time.
Commentary
Diabetes mellitus is a growing epidemic in the United States, affecting nearly 10% of American adults [1]. The disease is associated with multiple, potentially fatal complications, including heart disease, stroke, kidney failure, and limb amputation [1]. Studies show that ongoing diabetes self-management education (DSME) can result in lifestyle and behavioral changes that improve glycemic control, ultimately reducing the risk of complications [2,3]. However, traditional follow-up care and education for patients with diabetes requires considerable time on the part of patients and providers, and is both costly and resource-intensive [4]. The use of telehealth to educate and monitor patients with diabetes is a growing phenomenon. Theoretically, telehealth enables providers to reach greater subsets of the population who may not otherwise be able to consult with a doctor or nurse regularly. However, little is known about the overall effectiveness of telehealth compared with regular office visits with respect to diabetes and patient outcomes.
This study investigated the feasibility of using telephone and secure message methods to deliver ongoing DSMS after the completion of an existing DSME program. The results suggest that there is no difference in behavioral goal achievement, feasibility, and clinical outcomes among usual care and intervention groups.
This study had a number of strengths, including a strong scientific background in support of research that examines telehealth options for diabetes management. The inclusion criteria were straightforward and appropriate for the targeted patient population: all participants were over 18 and had previously completed the DSME class; all participants in the phone group were required to have a working telephone line, while the secure message group participants were required to have internet access. However, there were some methodologic weaknesses, most of which were pointed out by the authors. These included (1) lack of randomization, (2) a high attrition rate, and (3) nonspecific outcome measures. In addition, participants were able to self-enroll into a category of their choice. The lack of randomization enabled selection bias and prohibits the authors from inferring a causal effect between DSMS and improved health outcomes. Attrition rates were also problematic in this study. Not only did 59% of enrolled participants fail to complete the intervention, but the overall contact success rates declined over time. Finally, the outcome measure for feasibility is poorly defined because the authors never provide a numerical measure limitation. External validity is limited by a largely Caucasian sample that is predominately female. Due to the weaknesses inherent in the study’s methodology, the findings should be interpreted with some degree of caution.
Applications for Clinical Practice
Given the potential for long-term complications from diabetes, the rising cost of health care services, and the overall shortage of medical and nursing personnel, alternative methods of patient follow-up are needed in the management of diabetes. Telehealth has the potential to reach a significant portion of the population that is receiving little or no care in rural and underserved areas in a convenient and less costly way than traditional care. Investigating which alternatives to usual care are effective for which patient groups will pave the way for resource optimization and cost-effectiveness. Providing DSME follow-up through telehealth methodologies may be an effective alternative to in-person visits. Additional research is needed to support the outcomes of this study and to determine the duration of DSMS that is needed to ensure sufficient diabetes self-management.
—Amy Burchard, BA, and Tina Sadarangani, MSN, ANP-BC, GNP-BC
Study Overview
Objective. To investigate the feasibility and effectiveness of administering diabetes self-management support (DSMS) via telephone or secure messaging.
Design. Prospective, longitudinal quasi-experimental study.
Setting and participants. Participants (n = 150) who had previously completed diabetes self-management education (DSME) received follow-up DSMS in 1 of 3 self-selected ways: a one-time in-person visit, 3 brief visits by telephone, or via secure messaging via the electronic health record. The (usual care) in-person group (n = 47) received 1 follow-up appointment at the patient’s request with a certified diabetes educator (CDE) within 3 to 6 months of DSME completion. The telephone group (n = 44) was given follow-up phone appointments with a CDE, each lasting approximately 20 minutes, at 3, 6, and 9 months post-DSME. The secure message group (n = 59) received follow-up messages via the patient portal from a CDE at 3, 6, and 9 months post-DSME. At each interval, patients received 3 messages, an initial one followed by 2 structured replies. Motivational interviewing techniques were used in all 3 groups to identify barriers to achieving behavior goals and solutions.
Main outcome measures. Behavior goal measures, feasibility measures, and physiologic measures at 9 months’ post DSME. Behavior goal achievement was measured using a survey that asked patients to rate their achievement regarding the following AADE7 goals: healthy eating, being active, self-monitoring, taking medications, problem solving, reducing risks, and healthy coping. Goals are rated on a scale from 0 to 10, with a rating ≥ 7 considered successful completion. Feasibility to integrate this technology into a DSME platform was assessed by comparing the number of attempts to contact patients with the number of contacts achieved; also calculated was intervention completion, mean time spent with the CDE, and total cost of each visit. Physiologic measures included HbA1C and LDL levels collected through medical record review.
Results. There were no statistically significant differences between groups with respect to any of the primary outcomes. Behavioral goals were achieved by 59% of the in-person group, 73% of the telephone group, and 77% of the secure message group . Mean goal achievement for all 3 groups combined improved from 6.2 ± 2.4 to 7.2 ± 1.8 (P < 0.05). Overall, 70.3% ± 0.46% achieved behavioral goals, with no difference among groups. In terms of feasibility, at 3 months the contact success rate was 39%, 46%, and 29% in the in-person, telephone, and secure message groups, respectively. At 6 months, the contact success rate was 47% in the phone group versus 32% in the secure message group. At 9 months, the contact success rate was 35% in the phone group versus 21% in the secure message group. Sixty-two participants (41%) completed the intervention per protocol: 51% of in-person patients, 47% of phone patients, and 28% of secure message patients (P < 0.02). Visits lasted and cost, on average, 60 minutes and $50.00, 45.3 minutes and $37.75, and 17.8 minutes (P < 0.05) and $14.83 for the in-person, telephone, and secure message groups, respectively. There was no difference in HbA1c among groups. Overall, HbA1c decreased by −0.88% ± 1.63 (P < 0.05) from baseline to 9 months. Change in LDL was not significant, and neither were there statistical differences among groups.
Conclusion. Diabetes follow-up care delivered via telephone and secure messaging is feasible. Using either of these methods results in similar outcomes compared with the traditional in-person visit, while requiring less staff time.
Commentary
Diabetes mellitus is a growing epidemic in the United States, affecting nearly 10% of American adults [1]. The disease is associated with multiple, potentially fatal complications, including heart disease, stroke, kidney failure, and limb amputation [1]. Studies show that ongoing diabetes self-management education (DSME) can result in lifestyle and behavioral changes that improve glycemic control, ultimately reducing the risk of complications [2,3]. However, traditional follow-up care and education for patients with diabetes requires considerable time on the part of patients and providers, and is both costly and resource-intensive [4]. The use of telehealth to educate and monitor patients with diabetes is a growing phenomenon. Theoretically, telehealth enables providers to reach greater subsets of the population who may not otherwise be able to consult with a doctor or nurse regularly. However, little is known about the overall effectiveness of telehealth compared with regular office visits with respect to diabetes and patient outcomes.
This study investigated the feasibility of using telephone and secure message methods to deliver ongoing DSMS after the completion of an existing DSME program. The results suggest that there is no difference in behavioral goal achievement, feasibility, and clinical outcomes among usual care and intervention groups.
This study had a number of strengths, including a strong scientific background in support of research that examines telehealth options for diabetes management. The inclusion criteria were straightforward and appropriate for the targeted patient population: all participants were over 18 and had previously completed the DSME class; all participants in the phone group were required to have a working telephone line, while the secure message group participants were required to have internet access. However, there were some methodologic weaknesses, most of which were pointed out by the authors. These included (1) lack of randomization, (2) a high attrition rate, and (3) nonspecific outcome measures. In addition, participants were able to self-enroll into a category of their choice. The lack of randomization enabled selection bias and prohibits the authors from inferring a causal effect between DSMS and improved health outcomes. Attrition rates were also problematic in this study. Not only did 59% of enrolled participants fail to complete the intervention, but the overall contact success rates declined over time. Finally, the outcome measure for feasibility is poorly defined because the authors never provide a numerical measure limitation. External validity is limited by a largely Caucasian sample that is predominately female. Due to the weaknesses inherent in the study’s methodology, the findings should be interpreted with some degree of caution.
Applications for Clinical Practice
Given the potential for long-term complications from diabetes, the rising cost of health care services, and the overall shortage of medical and nursing personnel, alternative methods of patient follow-up are needed in the management of diabetes. Telehealth has the potential to reach a significant portion of the population that is receiving little or no care in rural and underserved areas in a convenient and less costly way than traditional care. Investigating which alternatives to usual care are effective for which patient groups will pave the way for resource optimization and cost-effectiveness. Providing DSME follow-up through telehealth methodologies may be an effective alternative to in-person visits. Additional research is needed to support the outcomes of this study and to determine the duration of DSMS that is needed to ensure sufficient diabetes self-management.
—Amy Burchard, BA, and Tina Sadarangani, MSN, ANP-BC, GNP-BC
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: US Department of Health and Human Services; 2011.
2. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–71.
3. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12.
4. Shani M, Sasson N, Lustman A, et al. Structured nursing follow-up: does it help in diabetes care? Isr J Health Policy Res 2014;3:27.
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: US Department of Health and Human Services; 2011.
2. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–71.
3. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12.
4. Shani M, Sasson N, Lustman A, et al. Structured nursing follow-up: does it help in diabetes care? Isr J Health Policy Res 2014;3:27.
Behavioral Health Problems in Medical Patients
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
Interferon-free regimen benefits HCV-infected liver transplant recipients
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
*Clarification, 11/11/14: A previous version of this story did not state that the data were presented by Dr. Mantry
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
*Clarification, 11/11/14: A previous version of this story did not state that the data were presented by Dr. Mantry
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
*Clarification, 11/11/14: A previous version of this story did not state that the data were presented by Dr. Mantry
Key clinical point: An oral, interferon-free drug combination produced a 97% sustained virologic response rate in liver transplant recipients with recurrent HCV infection.
Major finding: The primary efficacy endpoint, an SVR 12 weeks after completion of treatment, was 97% (33 of 34 patients).
Data source: An industry-sponsored, multicenter, open-label phase II trial involving 34 adults with chronic HCV infection despite liver transplantation.
Disclosures: This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Dr. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.