User login
Optimal duration of DAPT still unclear

Photo by Sage Ross
A systematic review of published evidence has failed to elucidate the optimal duration of dual antiplatelet therapy (DAPT) in patients who have a drug-eluting stent.
The data showed that patients who received DAPT for a longer period had a small reduction in myocardial infarction as well as a small increase in major bleeding and an even smaller increase in all-cause mortality, compared to patients who received DAPT for a shorter period.
Frederick A. Spencer, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues reported these findings in Annals of Internal Medicine.
The team searched databases for trials of DAPT published from 1996 to March 2015. They identified 9 randomized, controlled trials including a total of 29,531 patients. There was complete data for 28,808 patients who had coronary artery disease and received DAPT after drug-eluting stent placement.
In 4 of the trials, patients were randomized to DAPT when they received their stent. Patients in the shorter-duration arm received DAPT for 3 to 6 months, and patients in the longer-duration arm received DAPT for 12 to 24 months.
In a fifth study, patients were randomized to DAPT at stent placement, but thrombotic events occurring during the first 6 months (when both arms received DAPT) were excluded.
In the 4 remaining trials, patients were randomized to DAPT 6 months or more after stent placement. Patients in the shorter-duration arm received DAPT for 6 to 18 months, and patients in the longer-duration arm received DAPT for 12 to 42 months.
Analyzing data from these trials together, Dr Spencer and his colleagues found moderate-quality evidence suggesting that receiving DAPT for a longer period decreased the risk of myocardial infarction (risk ratio [RR]=0.73) but increased the risk of mortality (RR=1.19).
The team also said there was high-quality evidence suggesting that longer-duration DAPT increased the risk of major bleeding (RR=1.63).
Receiving DAPT for a longer period was associated with approximately 8 fewer myocardial infarctions per 1000 patients per year, 6 more major bleeding events per 1000 patients per year, and 2 more deaths per 1000 patients per year, when compared to shorter-duration DAPT.
Because these differences are small, Dr Spencer and his colleagues said the duration of DAPT therapy should probably be based on patient preference, following a discussion of the potential risks and benefits. ![]()

Photo by Sage Ross
A systematic review of published evidence has failed to elucidate the optimal duration of dual antiplatelet therapy (DAPT) in patients who have a drug-eluting stent.
The data showed that patients who received DAPT for a longer period had a small reduction in myocardial infarction as well as a small increase in major bleeding and an even smaller increase in all-cause mortality, compared to patients who received DAPT for a shorter period.
Frederick A. Spencer, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues reported these findings in Annals of Internal Medicine.
The team searched databases for trials of DAPT published from 1996 to March 2015. They identified 9 randomized, controlled trials including a total of 29,531 patients. There was complete data for 28,808 patients who had coronary artery disease and received DAPT after drug-eluting stent placement.
In 4 of the trials, patients were randomized to DAPT when they received their stent. Patients in the shorter-duration arm received DAPT for 3 to 6 months, and patients in the longer-duration arm received DAPT for 12 to 24 months.
In a fifth study, patients were randomized to DAPT at stent placement, but thrombotic events occurring during the first 6 months (when both arms received DAPT) were excluded.
In the 4 remaining trials, patients were randomized to DAPT 6 months or more after stent placement. Patients in the shorter-duration arm received DAPT for 6 to 18 months, and patients in the longer-duration arm received DAPT for 12 to 42 months.
Analyzing data from these trials together, Dr Spencer and his colleagues found moderate-quality evidence suggesting that receiving DAPT for a longer period decreased the risk of myocardial infarction (risk ratio [RR]=0.73) but increased the risk of mortality (RR=1.19).
The team also said there was high-quality evidence suggesting that longer-duration DAPT increased the risk of major bleeding (RR=1.63).
Receiving DAPT for a longer period was associated with approximately 8 fewer myocardial infarctions per 1000 patients per year, 6 more major bleeding events per 1000 patients per year, and 2 more deaths per 1000 patients per year, when compared to shorter-duration DAPT.
Because these differences are small, Dr Spencer and his colleagues said the duration of DAPT therapy should probably be based on patient preference, following a discussion of the potential risks and benefits. ![]()

Photo by Sage Ross
A systematic review of published evidence has failed to elucidate the optimal duration of dual antiplatelet therapy (DAPT) in patients who have a drug-eluting stent.
The data showed that patients who received DAPT for a longer period had a small reduction in myocardial infarction as well as a small increase in major bleeding and an even smaller increase in all-cause mortality, compared to patients who received DAPT for a shorter period.
Frederick A. Spencer, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues reported these findings in Annals of Internal Medicine.
The team searched databases for trials of DAPT published from 1996 to March 2015. They identified 9 randomized, controlled trials including a total of 29,531 patients. There was complete data for 28,808 patients who had coronary artery disease and received DAPT after drug-eluting stent placement.
In 4 of the trials, patients were randomized to DAPT when they received their stent. Patients in the shorter-duration arm received DAPT for 3 to 6 months, and patients in the longer-duration arm received DAPT for 12 to 24 months.
In a fifth study, patients were randomized to DAPT at stent placement, but thrombotic events occurring during the first 6 months (when both arms received DAPT) were excluded.
In the 4 remaining trials, patients were randomized to DAPT 6 months or more after stent placement. Patients in the shorter-duration arm received DAPT for 6 to 18 months, and patients in the longer-duration arm received DAPT for 12 to 42 months.
Analyzing data from these trials together, Dr Spencer and his colleagues found moderate-quality evidence suggesting that receiving DAPT for a longer period decreased the risk of myocardial infarction (risk ratio [RR]=0.73) but increased the risk of mortality (RR=1.19).
The team also said there was high-quality evidence suggesting that longer-duration DAPT increased the risk of major bleeding (RR=1.63).
Receiving DAPT for a longer period was associated with approximately 8 fewer myocardial infarctions per 1000 patients per year, 6 more major bleeding events per 1000 patients per year, and 2 more deaths per 1000 patients per year, when compared to shorter-duration DAPT.
Because these differences are small, Dr Spencer and his colleagues said the duration of DAPT therapy should probably be based on patient preference, following a discussion of the potential risks and benefits. ![]()
Teaching Effectiveness in HM
Hospital medicine (HM), which is the fastest growing medical specialty in the United States, includes more than 40,000 healthcare providers.[1] Hospitalists include practitioners from a variety of medical specialties, including internal medicine and pediatrics, and professional backgrounds such as physicians, nurse practitioners. and physician assistants.[2, 3] Originally defined as specialists of inpatient medicine, hospitalists must diagnose and manage a wide variety of clinical conditions, coordinate transitions of care, provide perioperative management to surgical patients, and contribute to quality improvement and hospital administration.[4, 5]
With the evolution of the HM, the need for effective continuing medical education (CME) has become increasingly important. Courses make up the largest percentage of CME activity types,[6] which also include regularly scheduled lecture series, internet materials, and journal‐related CME. Successful CME courses require educational content that matches the learning needs of its participants.[7] In 2006, the Society for Hospital Medicine (SHM) developed core competencies in HM to guide educators in identifying professional practice gaps for useful CME.[8] However, knowing a population's characteristics and learning needs is a key first step to recognizing a practice gap.[9] Understanding these components is important to ensuring that competencies in the field of HM remain relevant to address existing practice gaps.[10] Currently, little is known about the demographic characteristics of participants in HM CME.
Research on the characteristics of effective clinical teachers in medicine has revealed the importance of establishing a positive learning climate, asking questions, diagnosing learners needs, giving feedback, utilizing established teaching frameworks, and developing a personalized philosophy of teaching.[11] Within CME, research has generally demonstrated that courses lead to improvements in lower level outcomes,[12] such as satisfaction and learning, yet higher level outcomes such as behavior change and impacts on patients are inconsistent.[13, 14, 15] Additionally, we have shown that participant reflection on CME is enhanced by presenters who have prior teaching experience and higher teaching effectiveness scores, by the use of audience participation and by incorporating relevant content.[16, 17] Despite the existence of research on CME in general, we are not aware of prior studies regarding characteristics of effective CME in the field of HM.
To better understand and improve the quality of HM CME, we sought to describe the characteristics of participants at a large, national HM CME course, and to identify associations between characteristics of presentations and CME teaching effectiveness (CMETE) scores using a previously validated instrument.
METHODS
Study Design and Participants
This cross‐sectional study included all participants (n=368) and presenters (n=29) at the Mayo Clinic Hospital Medicine Managing Complex Patients (MCP) course in October 2014. MCP is a CME course designed for hospitalists (defined as those who spend most of their professional practice caring for hospitalized patients) and provides up to 24.5 American Medical Association Physician's Recognition Award category 1 credits. The course took place over 4 days and consisted of 32 didactic presentations, which comprised the context for data collection for this study. The structure of the course day consisted of early and late morning sessions, each made up of 3 to 5 presentations, followed by a question and answer session with presenters and a 15‐minute break. The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Independent Variables: Characteristics of Participants and Presentations
Demographic characteristics of participants were obtained through anonymous surveys attached to CME teaching effectiveness forms. Variables included participant sex, professional degree, self‐identified hospitalist, medical specialty, geographic practice location, age, years in practice/level of training, practice setting, American Board of Internal Medicine (ABIM) certification of Focused Practice in Hospital Medicine, number of CME credits earned, and number of CME programs attended in the past year. These variables were selected in an effort to describe potentially relevant demographics of a national cohort of HM CME participants.
Presentation variables included use of clinical cases, audience response system (ARS), number of slides, defined goals/objectives, summary slide and presentation length in minutes, and are supported by previous CME effectiveness research.[16, 17, 18, 19]
Outcome Variable: CME Teaching Effectiveness Scores
The CMETE scores for this study were obtained from an instrument described in our previous research.[16] The instrument contains 7 items on 5‐point scales (range: strongly disagree to strongly agree) that address speaker clarity and organization, relevant content, use of case examples, effective slides, interactive learning methods (eg, audience response), use of supporting evidence, appropriate amount of content, and summary of key points. Additionally, the instrument includes 2 open‐ended questions: (1) What did the speaker do well? (Please describe specific behaviors and examples) and (2) What could the speaker improve on? (Please describe specific behaviors and examples). Validity evidence for CMETE scores included factor analysis demonstrating a unidimensional model for measuring presenter feedback, along with excellent internal consistency and inter‐rater reliability.[16]
Data Analysis
A CMETE score per presentation from each attendee was calculated as the average over the 7 instrument items. A composite presentation‐level CMETE score was then computed as the average overall score within each presentation. CMETE scores were summarized using means and standard deviations (SDs). The overall CMETE scores were compared by presentation characteristics using Kruskal‐Wallis tests. To illustrate the size of observed differences, Cohen effect sizes are presented as the average difference between groups divided by the common SD. All analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC).
RESULTS
There were 32 presentations during the MCP conference in 2014. A total of 277 (75.2%) out of 368 participants completed the survey. This yielded 7947 CMETE evaluations for analysis, with an average of 28.7 per person (median: 31, interquartile range: 2732, range: 632).
Demographic characteristics of course participants are listed in Table 1. Participants (number, %), described themselves as hospitalists (181, 70.4%), ABIM certified with HM focus (48, 18.8%), physicians with MD or MBBS degrees (181, 70.4%), nurse practitioners or physician assistants (52; 20.2%), and in practice 20 years (73, 28%). The majority of participants (148, 58.3%) worked in private practice, whereas only 63 (24.8%) worked in academic settings.
| Variable | No. of Attendees (%), N=277 |
|---|---|
| |
| Sex | |
| Unknown | 22 |
| Male | 124 (48.6%) |
| Female | 131 (51.4%) |
| Age | |
| Unknown | 17 |
| 2029 years | 11 (4.2%) |
| 3039 years | 83 (31.9%) |
| 4049 years | 61 (23.5%) |
| 5059 years | 56 (21.5%) |
| 6069 years | 38 (14.6%) |
| 70+ years | 11 (4.2%) |
| Professional degree | |
| Unknown | 20 |
| MD/MBBS | 181 (70.4%) |
| DO | 23 (8.9%) |
| NP | 28 (10.9%) |
| PA | 24 (9.3%) |
| Other | 1 (0.4%) |
| Medical specialty | |
| Unknown | 26 |
| Internal medicine | 149 (59.4%) |
| Family medicine | 47 (18.7%) |
| IM subspecialty | 14 (5.6%) |
| Other | 41 (16.3%) |
| Geographic location | |
| Unknown | 16 |
| Western US | 48 (18.4%) |
| Northeastern US | 33 (12.6%) |
| Midwestern US | 98 (37.5%) |
| Southern US | 40 (15.3%) |
| Canada | 13 (5.0%) |
| Other | 29 (11.1%) |
| Years of practice/training | |
| Unknown | 16 |
| Currently in training | 1 (0.4%) |
| Practice 04 years | 68 (26.1%) |
| Practice 59 years | 55 (21.1%) |
| Practice 1019 years | 64 (24.5%) |
| Practice 20+ years | 73 (28.0%) |
| Practice setting | |
| Unknown | 23 |
| Academic | 63 (24.8%) |
| Privateurban | 99 (39.0%) |
| Privaterural | 49 (19.3%) |
| Other | 43 (16.9%) |
| ABIM certification HM | |
| Unknown | 22 |
| Yes | 48 (18.8%) |
| No | 207 (81.2%) |
| Hospitalist | |
| Unknown | 20 |
| Yes | 181 (70.4%) |
| No | 76 (29.6%) |
| CME credits claimed | |
| Unknown | 20 |
| 024 | 54 (21.0%) |
| 2549 | 105 (40.9%) |
| 5074 | 61 (23.7%) |
| 7599 | 15 (5.8%) |
| 100+ | 22 (8.6%) |
| CME programs attended | |
| Unknown | 18 |
| 0 | 38 (14.7%) |
| 12 | 166 (64.1%) |
| 35 | 52 (20.1%) |
| 6+ | 3 (1.2%) |
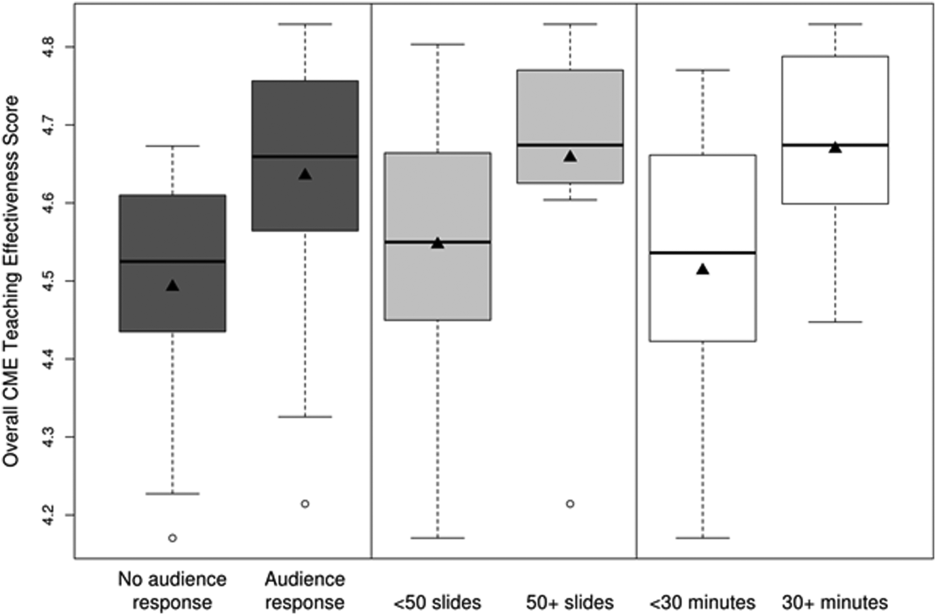
CMETE scores (mean [SD]) were significantly associated with the use of ARS (4.64 [0.16]) vs no ARS (4.49 [0.16]; P=0.01, Table 2, Figure 1), longer presentations (30 minutes: 4.67 [0.13] vs <30 minutes: 4.51 [0.18]; P=0.02), and larger number of slides (50: 4.66 [0.17] vs <50: 4.55 [0.17]; P=0.04). There were no significant associations between CMETE scores and use of clinical cases, defined goals, or summary slides.
| Presentation Variable | No. (%) | Mean Score | Standard Deviation | P Value |
|---|---|---|---|---|
| Use of clinical cases | ||||
| Yes | 28 (87.5%) | 4.60 | 0.18 | 0.14 |
| No | 4 (12.5%) | 4.49 | 0.14 | |
| Audience response system | ||||
| Yes | 20 (62.5%) | 4.64 | 0.16 | 0.01 |
| No | 12 (37.5%) | 4.49 | 0.16 | |
| No. of slides | ||||
| 50 | 10 (31.3%) | 4.66 | 0.17 | 0.04 |
| <50 | 22 (68.8%) | 4.55 | 0.17 | |
| Defined goals/objectives | ||||
| Yes | 29 (90.6%) | 4.58 | 0.18 | 0.87 |
| No | 3 (9.4%) | 4.61 | 0.17 | |
| Summary slide | ||||
| Yes | 22 (68.8%) | 4.56 | 0.18 | 0.44 |
| No | 10 (31.3%) | 4.62 | 0.15 | |
| Presentation length | ||||
| 30 minutes | 14 (43.8%) | 4.67 | 0.13 | 0.02 |
| <30 minutes | 18 (56.3%) | 4.51 | 0.18 |
The magnitude of score differences observed in this study are substantial when considered in terms of Cohen's effect sizes. For number of slides, the effect size is 0.65, for audience response the effect size is 0.94, and for presentation length the effect size is approximately 1. According to Cohen, effect sizes of 0.5 to 0.8 are moderate, and effect sizes >0.8 are large. Consequently, the effect sizes of our observed differences are moderate to large.[20, 21]
DISCUSSION
To our knowledge, this is the first study to measure associations between validated teaching effectiveness scores and characteristics of presentations in HM CME. We found that the use of ARS and longer presentations were associated with significantly higher CMETE scores. Our findings have implications for HM CME course directors and presenters as they attempt to develop methods to improve the quality of CME.
CME participants in our study crossed a wide range of ages and experience, which is consistent with national surveys of hospitalists.[22, 23] Interestingly, however, nearly 1 in 3 participants trained in a specialty other than internal medicine. Additionally, the professional degrees of participants were diverse, with 20% of participants having trained as nurse practitioners or physician assistants. These findings are at odds with an early national survey of inpatient practitioners,[22] but consistent with recent literature that the diversity of training backgrounds among hospitalists is increasing as the field of HM evolves.[24] Hospital medicine CME providers will need to be cognizant of these demographic changes as they work to identify practice gaps and apply appropriate educational methods.
The use of an ARS allows for increased participation and engagement among lecture attendees, which in turn promotes active learning.[25, 26, 27] The association of higher teaching scores with the use of ARS is consistent with previous research in other CME settings such as clinical round tables and medical grand rounds.[17, 28] As it pertains to HM specifically, our findings also build upon a recent study by Sehgal et al., which reported on the novel use of bedside CME to enhance interactive learning and discussion among hospitalists, and which was viewed favorably by course participants.[29]
The reasons why longer presentations in our study were linked to higher CMETE scores are not entirely clear, as previous CME research has failed to demonstrate this relationship.[18] One possibility is that course participants prefer learning from presentations that provide granular, content‐rich information. Another possibility may be that characteristics of effective presenters who gave longer presentations and that were not measured in this study, such as presenter experience and expertise, were responsible for the observed increase in CMETE scores. Yet another possibility is that effective presentations were longer due to the use of ARS, which was also associated with better CMETE scores. This explanation may be plausible because the ARS requires additional slides and provides opportunities for audience interaction, which may lengthen the duration of any given presentation.
This study has several limitations. This was a single CME conference sponsored by a large academic medical center, which may limit generalizability, especially to smaller conferences in community settings. However, the audience was large and diverse in terms of participants experiences, practice settings, professional backgrounds, and geographic locations. Furthermore, the demographic characteristics of hospitalists at our course appear very similar to a recently reported national cross‐section of hospitalist groups.[30] Second, this is a cross‐sectional study without a comparison group. Nonetheless, a systematic review showed that most published education research studies involved single‐group designs without comparison groups.[31] Last, the outcomes of the study include attitudes and objectively measured presenter behaviors such as the use of ARS, but not patient‐related outcomes. Nonetheless, evidence indicates that the majority of medical education research does not present outcomes beyond knowledge,[31] and it has been noted that behavior‐related outcomes strike the ideal balance between feasibility and rigor.[32, 33] Finally, the instrument used in this study to measure teaching effectiveness is supported by prior validity evidence.[16]
In summary, we found that hospital medicine CME presentations, which are longer and use audience responses, are associated with greater teaching effectiveness ratings by CME course participants. These findings build upon previous CME research and suggest that CME course directors and presenters should strive to incorporate opportunities that promote audience engagement and participation. Additionally, this study adds to the existing validity of evidence for the CMETE assessment tool. We believe that future research should explore potential associations between teacher effectiveness and patient‐related outcomes, and determine whether course content that reflects the SHM core competencies improves CME teaching effectiveness scores.
Disclosure
Nothing to report.
- Society of Hospital Medicine. 2013/2014 press kit. Available at: http://www.hospitalmedicine.org/Web/Media_Center/Web/Media_Center/Media_Center.aspx?hkey=e26ceba7-ba93-4e50-8eb1-1ccc75d6f0fd. Accessed May 18, 2015.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33:9–10.
- , , , et al. The evolving role of the pediatric nurse practitioner in hospital medicine. J Hosp Med. 2014;9:261–265.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/Web/About_SHM/Hospitalist_Definition/Web/About_SHM/Industry/Hospital_Medicine_Hospital_Definition.aspx. Accessed February 16, 2015.
- Accreditation Council for Continuing Medical Education. 2013 annual report data executive summary. Available at: http://www.accme.org/sites/default/files/630_2013_Annual_Report_20140715_0.pdf. Accessed February 16, 2015.
- . The anatomy of an outstanding CME meeting. J Am Coll Radiol. 2005;2:534–540.
- , , , , . How to use The Core Competencies in Hospital Medicine: a framework for curriculum development. J Hosp Med. 2006;1:57–67.
- , , , , , . Perspective: a practical approach to defining professional practice gaps for continuing medical education. Acad Med. 2012;87:582–585.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):148–156.
- , . Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84:339–344.
- , . Developing scholarly projects in education: a primer for medical teachers. Med Teach. 2007;29:210–218.
- , . A meta‐analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27:6–15.
- , , . Achieving desired results and improved outcomes: integrating planning and assessment throughout learning activities. J Contin Educ Health Prof. 2009;29:1–15.
- , . Effectiveness of continuing medical education: updated synthesis of systematic reviews. Available at: http://www.accme.org/sites/default/files/652_20141104_Effectiveness_of_Continuing_Medical_Education_Cervero_and_Gaines.pdf. Accessed March 25, 2015.
- , , , et al. Improving participant feedback to continuing medical education presenters in internal medicine: a mixed‐methods study. J Gen Intern Med. 2012;27:425–431.
- , , , et al. Measuring faculty reflection on medical grand rounds at Mayo Clinic: associations with teaching experience, clinical exposure, and presenter effectiveness. Mayo Clin Proc. 2013;88:277–284.
- , , , . Successful lecturing: a prospective study to validate attributes of the effective medical lecture. J Gen Intern Med. 2000;15:366–371.
- , , , , , . A standardized approach to assessing physician expectations and perceptions of continuing medical education. J Contin Educ Health Prof. 2007;27:173–182.
- . Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977.
- . Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
- , , , . Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130:343–349.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27:28–36.
- , , , et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9:615–620.
- , . A primer on audience response systems: current applications and future considerations. Am J Pharm Educ. 2008;72:77.
- , , . Continuing medical education: AMEE education guide no 35. Med Teach. 2008;30:652–666.
- . Clickers in the large classroom: current research and best‐practice tips. CBE Life Sci Educ. 2007;6:9–20.
- , , . Evaluation of an audience response system for the continuing education of health professionals. J Contin Educ Health Prof. 2003;23:109–115.
- , , . Bringing continuing medical education to the bedside: the University of California, San Francisco Hospitalist Mini‐College. J Hosp Med. 2014;9:129–134.
- Society of Hospital Medicine. 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , , , , . Association between funding and quality of published medical education research. JAMA. 2007;298:1002–1009.
- . Mind the gap: some reasons why medical education research is different from health services research. Med Educ. 2001;35:319–320.
- , . Reflections on experimental research in medical education. Adv Health Sci Educ Theory Pract. 2010;15:455–464.
Hospital medicine (HM), which is the fastest growing medical specialty in the United States, includes more than 40,000 healthcare providers.[1] Hospitalists include practitioners from a variety of medical specialties, including internal medicine and pediatrics, and professional backgrounds such as physicians, nurse practitioners. and physician assistants.[2, 3] Originally defined as specialists of inpatient medicine, hospitalists must diagnose and manage a wide variety of clinical conditions, coordinate transitions of care, provide perioperative management to surgical patients, and contribute to quality improvement and hospital administration.[4, 5]
With the evolution of the HM, the need for effective continuing medical education (CME) has become increasingly important. Courses make up the largest percentage of CME activity types,[6] which also include regularly scheduled lecture series, internet materials, and journal‐related CME. Successful CME courses require educational content that matches the learning needs of its participants.[7] In 2006, the Society for Hospital Medicine (SHM) developed core competencies in HM to guide educators in identifying professional practice gaps for useful CME.[8] However, knowing a population's characteristics and learning needs is a key first step to recognizing a practice gap.[9] Understanding these components is important to ensuring that competencies in the field of HM remain relevant to address existing practice gaps.[10] Currently, little is known about the demographic characteristics of participants in HM CME.
Research on the characteristics of effective clinical teachers in medicine has revealed the importance of establishing a positive learning climate, asking questions, diagnosing learners needs, giving feedback, utilizing established teaching frameworks, and developing a personalized philosophy of teaching.[11] Within CME, research has generally demonstrated that courses lead to improvements in lower level outcomes,[12] such as satisfaction and learning, yet higher level outcomes such as behavior change and impacts on patients are inconsistent.[13, 14, 15] Additionally, we have shown that participant reflection on CME is enhanced by presenters who have prior teaching experience and higher teaching effectiveness scores, by the use of audience participation and by incorporating relevant content.[16, 17] Despite the existence of research on CME in general, we are not aware of prior studies regarding characteristics of effective CME in the field of HM.
To better understand and improve the quality of HM CME, we sought to describe the characteristics of participants at a large, national HM CME course, and to identify associations between characteristics of presentations and CME teaching effectiveness (CMETE) scores using a previously validated instrument.
METHODS
Study Design and Participants
This cross‐sectional study included all participants (n=368) and presenters (n=29) at the Mayo Clinic Hospital Medicine Managing Complex Patients (MCP) course in October 2014. MCP is a CME course designed for hospitalists (defined as those who spend most of their professional practice caring for hospitalized patients) and provides up to 24.5 American Medical Association Physician's Recognition Award category 1 credits. The course took place over 4 days and consisted of 32 didactic presentations, which comprised the context for data collection for this study. The structure of the course day consisted of early and late morning sessions, each made up of 3 to 5 presentations, followed by a question and answer session with presenters and a 15‐minute break. The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Independent Variables: Characteristics of Participants and Presentations
Demographic characteristics of participants were obtained through anonymous surveys attached to CME teaching effectiveness forms. Variables included participant sex, professional degree, self‐identified hospitalist, medical specialty, geographic practice location, age, years in practice/level of training, practice setting, American Board of Internal Medicine (ABIM) certification of Focused Practice in Hospital Medicine, number of CME credits earned, and number of CME programs attended in the past year. These variables were selected in an effort to describe potentially relevant demographics of a national cohort of HM CME participants.
Presentation variables included use of clinical cases, audience response system (ARS), number of slides, defined goals/objectives, summary slide and presentation length in minutes, and are supported by previous CME effectiveness research.[16, 17, 18, 19]
Outcome Variable: CME Teaching Effectiveness Scores
The CMETE scores for this study were obtained from an instrument described in our previous research.[16] The instrument contains 7 items on 5‐point scales (range: strongly disagree to strongly agree) that address speaker clarity and organization, relevant content, use of case examples, effective slides, interactive learning methods (eg, audience response), use of supporting evidence, appropriate amount of content, and summary of key points. Additionally, the instrument includes 2 open‐ended questions: (1) What did the speaker do well? (Please describe specific behaviors and examples) and (2) What could the speaker improve on? (Please describe specific behaviors and examples). Validity evidence for CMETE scores included factor analysis demonstrating a unidimensional model for measuring presenter feedback, along with excellent internal consistency and inter‐rater reliability.[16]
Data Analysis
A CMETE score per presentation from each attendee was calculated as the average over the 7 instrument items. A composite presentation‐level CMETE score was then computed as the average overall score within each presentation. CMETE scores were summarized using means and standard deviations (SDs). The overall CMETE scores were compared by presentation characteristics using Kruskal‐Wallis tests. To illustrate the size of observed differences, Cohen effect sizes are presented as the average difference between groups divided by the common SD. All analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC).
RESULTS
There were 32 presentations during the MCP conference in 2014. A total of 277 (75.2%) out of 368 participants completed the survey. This yielded 7947 CMETE evaluations for analysis, with an average of 28.7 per person (median: 31, interquartile range: 2732, range: 632).
Demographic characteristics of course participants are listed in Table 1. Participants (number, %), described themselves as hospitalists (181, 70.4%), ABIM certified with HM focus (48, 18.8%), physicians with MD or MBBS degrees (181, 70.4%), nurse practitioners or physician assistants (52; 20.2%), and in practice 20 years (73, 28%). The majority of participants (148, 58.3%) worked in private practice, whereas only 63 (24.8%) worked in academic settings.
| Variable | No. of Attendees (%), N=277 |
|---|---|
| |
| Sex | |
| Unknown | 22 |
| Male | 124 (48.6%) |
| Female | 131 (51.4%) |
| Age | |
| Unknown | 17 |
| 2029 years | 11 (4.2%) |
| 3039 years | 83 (31.9%) |
| 4049 years | 61 (23.5%) |
| 5059 years | 56 (21.5%) |
| 6069 years | 38 (14.6%) |
| 70+ years | 11 (4.2%) |
| Professional degree | |
| Unknown | 20 |
| MD/MBBS | 181 (70.4%) |
| DO | 23 (8.9%) |
| NP | 28 (10.9%) |
| PA | 24 (9.3%) |
| Other | 1 (0.4%) |
| Medical specialty | |
| Unknown | 26 |
| Internal medicine | 149 (59.4%) |
| Family medicine | 47 (18.7%) |
| IM subspecialty | 14 (5.6%) |
| Other | 41 (16.3%) |
| Geographic location | |
| Unknown | 16 |
| Western US | 48 (18.4%) |
| Northeastern US | 33 (12.6%) |
| Midwestern US | 98 (37.5%) |
| Southern US | 40 (15.3%) |
| Canada | 13 (5.0%) |
| Other | 29 (11.1%) |
| Years of practice/training | |
| Unknown | 16 |
| Currently in training | 1 (0.4%) |
| Practice 04 years | 68 (26.1%) |
| Practice 59 years | 55 (21.1%) |
| Practice 1019 years | 64 (24.5%) |
| Practice 20+ years | 73 (28.0%) |
| Practice setting | |
| Unknown | 23 |
| Academic | 63 (24.8%) |
| Privateurban | 99 (39.0%) |
| Privaterural | 49 (19.3%) |
| Other | 43 (16.9%) |
| ABIM certification HM | |
| Unknown | 22 |
| Yes | 48 (18.8%) |
| No | 207 (81.2%) |
| Hospitalist | |
| Unknown | 20 |
| Yes | 181 (70.4%) |
| No | 76 (29.6%) |
| CME credits claimed | |
| Unknown | 20 |
| 024 | 54 (21.0%) |
| 2549 | 105 (40.9%) |
| 5074 | 61 (23.7%) |
| 7599 | 15 (5.8%) |
| 100+ | 22 (8.6%) |
| CME programs attended | |
| Unknown | 18 |
| 0 | 38 (14.7%) |
| 12 | 166 (64.1%) |
| 35 | 52 (20.1%) |
| 6+ | 3 (1.2%) |
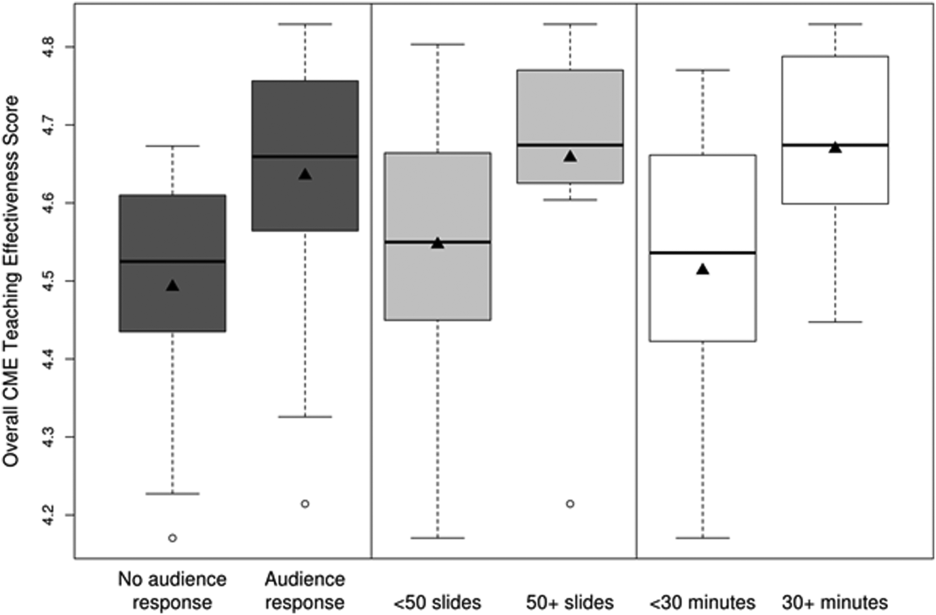
CMETE scores (mean [SD]) were significantly associated with the use of ARS (4.64 [0.16]) vs no ARS (4.49 [0.16]; P=0.01, Table 2, Figure 1), longer presentations (30 minutes: 4.67 [0.13] vs <30 minutes: 4.51 [0.18]; P=0.02), and larger number of slides (50: 4.66 [0.17] vs <50: 4.55 [0.17]; P=0.04). There were no significant associations between CMETE scores and use of clinical cases, defined goals, or summary slides.
| Presentation Variable | No. (%) | Mean Score | Standard Deviation | P Value |
|---|---|---|---|---|
| Use of clinical cases | ||||
| Yes | 28 (87.5%) | 4.60 | 0.18 | 0.14 |
| No | 4 (12.5%) | 4.49 | 0.14 | |
| Audience response system | ||||
| Yes | 20 (62.5%) | 4.64 | 0.16 | 0.01 |
| No | 12 (37.5%) | 4.49 | 0.16 | |
| No. of slides | ||||
| 50 | 10 (31.3%) | 4.66 | 0.17 | 0.04 |
| <50 | 22 (68.8%) | 4.55 | 0.17 | |
| Defined goals/objectives | ||||
| Yes | 29 (90.6%) | 4.58 | 0.18 | 0.87 |
| No | 3 (9.4%) | 4.61 | 0.17 | |
| Summary slide | ||||
| Yes | 22 (68.8%) | 4.56 | 0.18 | 0.44 |
| No | 10 (31.3%) | 4.62 | 0.15 | |
| Presentation length | ||||
| 30 minutes | 14 (43.8%) | 4.67 | 0.13 | 0.02 |
| <30 minutes | 18 (56.3%) | 4.51 | 0.18 |

The magnitude of score differences observed in this study are substantial when considered in terms of Cohen's effect sizes. For number of slides, the effect size is 0.65, for audience response the effect size is 0.94, and for presentation length the effect size is approximately 1. According to Cohen, effect sizes of 0.5 to 0.8 are moderate, and effect sizes >0.8 are large. Consequently, the effect sizes of our observed differences are moderate to large.[20, 21]
DISCUSSION
To our knowledge, this is the first study to measure associations between validated teaching effectiveness scores and characteristics of presentations in HM CME. We found that the use of ARS and longer presentations were associated with significantly higher CMETE scores. Our findings have implications for HM CME course directors and presenters as they attempt to develop methods to improve the quality of CME.
CME participants in our study crossed a wide range of ages and experience, which is consistent with national surveys of hospitalists.[22, 23] Interestingly, however, nearly 1 in 3 participants trained in a specialty other than internal medicine. Additionally, the professional degrees of participants were diverse, with 20% of participants having trained as nurse practitioners or physician assistants. These findings are at odds with an early national survey of inpatient practitioners,[22] but consistent with recent literature that the diversity of training backgrounds among hospitalists is increasing as the field of HM evolves.[24] Hospital medicine CME providers will need to be cognizant of these demographic changes as they work to identify practice gaps and apply appropriate educational methods.
The use of an ARS allows for increased participation and engagement among lecture attendees, which in turn promotes active learning.[25, 26, 27] The association of higher teaching scores with the use of ARS is consistent with previous research in other CME settings such as clinical round tables and medical grand rounds.[17, 28] As it pertains to HM specifically, our findings also build upon a recent study by Sehgal et al., which reported on the novel use of bedside CME to enhance interactive learning and discussion among hospitalists, and which was viewed favorably by course participants.[29]
The reasons why longer presentations in our study were linked to higher CMETE scores are not entirely clear, as previous CME research has failed to demonstrate this relationship.[18] One possibility is that course participants prefer learning from presentations that provide granular, content‐rich information. Another possibility may be that characteristics of effective presenters who gave longer presentations and that were not measured in this study, such as presenter experience and expertise, were responsible for the observed increase in CMETE scores. Yet another possibility is that effective presentations were longer due to the use of ARS, which was also associated with better CMETE scores. This explanation may be plausible because the ARS requires additional slides and provides opportunities for audience interaction, which may lengthen the duration of any given presentation.
This study has several limitations. This was a single CME conference sponsored by a large academic medical center, which may limit generalizability, especially to smaller conferences in community settings. However, the audience was large and diverse in terms of participants experiences, practice settings, professional backgrounds, and geographic locations. Furthermore, the demographic characteristics of hospitalists at our course appear very similar to a recently reported national cross‐section of hospitalist groups.[30] Second, this is a cross‐sectional study without a comparison group. Nonetheless, a systematic review showed that most published education research studies involved single‐group designs without comparison groups.[31] Last, the outcomes of the study include attitudes and objectively measured presenter behaviors such as the use of ARS, but not patient‐related outcomes. Nonetheless, evidence indicates that the majority of medical education research does not present outcomes beyond knowledge,[31] and it has been noted that behavior‐related outcomes strike the ideal balance between feasibility and rigor.[32, 33] Finally, the instrument used in this study to measure teaching effectiveness is supported by prior validity evidence.[16]
In summary, we found that hospital medicine CME presentations, which are longer and use audience responses, are associated with greater teaching effectiveness ratings by CME course participants. These findings build upon previous CME research and suggest that CME course directors and presenters should strive to incorporate opportunities that promote audience engagement and participation. Additionally, this study adds to the existing validity of evidence for the CMETE assessment tool. We believe that future research should explore potential associations between teacher effectiveness and patient‐related outcomes, and determine whether course content that reflects the SHM core competencies improves CME teaching effectiveness scores.
Disclosure
Nothing to report.
Hospital medicine (HM), which is the fastest growing medical specialty in the United States, includes more than 40,000 healthcare providers.[1] Hospitalists include practitioners from a variety of medical specialties, including internal medicine and pediatrics, and professional backgrounds such as physicians, nurse practitioners. and physician assistants.[2, 3] Originally defined as specialists of inpatient medicine, hospitalists must diagnose and manage a wide variety of clinical conditions, coordinate transitions of care, provide perioperative management to surgical patients, and contribute to quality improvement and hospital administration.[4, 5]
With the evolution of the HM, the need for effective continuing medical education (CME) has become increasingly important. Courses make up the largest percentage of CME activity types,[6] which also include regularly scheduled lecture series, internet materials, and journal‐related CME. Successful CME courses require educational content that matches the learning needs of its participants.[7] In 2006, the Society for Hospital Medicine (SHM) developed core competencies in HM to guide educators in identifying professional practice gaps for useful CME.[8] However, knowing a population's characteristics and learning needs is a key first step to recognizing a practice gap.[9] Understanding these components is important to ensuring that competencies in the field of HM remain relevant to address existing practice gaps.[10] Currently, little is known about the demographic characteristics of participants in HM CME.
Research on the characteristics of effective clinical teachers in medicine has revealed the importance of establishing a positive learning climate, asking questions, diagnosing learners needs, giving feedback, utilizing established teaching frameworks, and developing a personalized philosophy of teaching.[11] Within CME, research has generally demonstrated that courses lead to improvements in lower level outcomes,[12] such as satisfaction and learning, yet higher level outcomes such as behavior change and impacts on patients are inconsistent.[13, 14, 15] Additionally, we have shown that participant reflection on CME is enhanced by presenters who have prior teaching experience and higher teaching effectiveness scores, by the use of audience participation and by incorporating relevant content.[16, 17] Despite the existence of research on CME in general, we are not aware of prior studies regarding characteristics of effective CME in the field of HM.
To better understand and improve the quality of HM CME, we sought to describe the characteristics of participants at a large, national HM CME course, and to identify associations between characteristics of presentations and CME teaching effectiveness (CMETE) scores using a previously validated instrument.
METHODS
Study Design and Participants
This cross‐sectional study included all participants (n=368) and presenters (n=29) at the Mayo Clinic Hospital Medicine Managing Complex Patients (MCP) course in October 2014. MCP is a CME course designed for hospitalists (defined as those who spend most of their professional practice caring for hospitalized patients) and provides up to 24.5 American Medical Association Physician's Recognition Award category 1 credits. The course took place over 4 days and consisted of 32 didactic presentations, which comprised the context for data collection for this study. The structure of the course day consisted of early and late morning sessions, each made up of 3 to 5 presentations, followed by a question and answer session with presenters and a 15‐minute break. The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Independent Variables: Characteristics of Participants and Presentations
Demographic characteristics of participants were obtained through anonymous surveys attached to CME teaching effectiveness forms. Variables included participant sex, professional degree, self‐identified hospitalist, medical specialty, geographic practice location, age, years in practice/level of training, practice setting, American Board of Internal Medicine (ABIM) certification of Focused Practice in Hospital Medicine, number of CME credits earned, and number of CME programs attended in the past year. These variables were selected in an effort to describe potentially relevant demographics of a national cohort of HM CME participants.
Presentation variables included use of clinical cases, audience response system (ARS), number of slides, defined goals/objectives, summary slide and presentation length in minutes, and are supported by previous CME effectiveness research.[16, 17, 18, 19]
Outcome Variable: CME Teaching Effectiveness Scores
The CMETE scores for this study were obtained from an instrument described in our previous research.[16] The instrument contains 7 items on 5‐point scales (range: strongly disagree to strongly agree) that address speaker clarity and organization, relevant content, use of case examples, effective slides, interactive learning methods (eg, audience response), use of supporting evidence, appropriate amount of content, and summary of key points. Additionally, the instrument includes 2 open‐ended questions: (1) What did the speaker do well? (Please describe specific behaviors and examples) and (2) What could the speaker improve on? (Please describe specific behaviors and examples). Validity evidence for CMETE scores included factor analysis demonstrating a unidimensional model for measuring presenter feedback, along with excellent internal consistency and inter‐rater reliability.[16]
Data Analysis
A CMETE score per presentation from each attendee was calculated as the average over the 7 instrument items. A composite presentation‐level CMETE score was then computed as the average overall score within each presentation. CMETE scores were summarized using means and standard deviations (SDs). The overall CMETE scores were compared by presentation characteristics using Kruskal‐Wallis tests. To illustrate the size of observed differences, Cohen effect sizes are presented as the average difference between groups divided by the common SD. All analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC).
RESULTS
There were 32 presentations during the MCP conference in 2014. A total of 277 (75.2%) out of 368 participants completed the survey. This yielded 7947 CMETE evaluations for analysis, with an average of 28.7 per person (median: 31, interquartile range: 2732, range: 632).
Demographic characteristics of course participants are listed in Table 1. Participants (number, %), described themselves as hospitalists (181, 70.4%), ABIM certified with HM focus (48, 18.8%), physicians with MD or MBBS degrees (181, 70.4%), nurse practitioners or physician assistants (52; 20.2%), and in practice 20 years (73, 28%). The majority of participants (148, 58.3%) worked in private practice, whereas only 63 (24.8%) worked in academic settings.
| Variable | No. of Attendees (%), N=277 |
|---|---|
| |
| Sex | |
| Unknown | 22 |
| Male | 124 (48.6%) |
| Female | 131 (51.4%) |
| Age | |
| Unknown | 17 |
| 2029 years | 11 (4.2%) |
| 3039 years | 83 (31.9%) |
| 4049 years | 61 (23.5%) |
| 5059 years | 56 (21.5%) |
| 6069 years | 38 (14.6%) |
| 70+ years | 11 (4.2%) |
| Professional degree | |
| Unknown | 20 |
| MD/MBBS | 181 (70.4%) |
| DO | 23 (8.9%) |
| NP | 28 (10.9%) |
| PA | 24 (9.3%) |
| Other | 1 (0.4%) |
| Medical specialty | |
| Unknown | 26 |
| Internal medicine | 149 (59.4%) |
| Family medicine | 47 (18.7%) |
| IM subspecialty | 14 (5.6%) |
| Other | 41 (16.3%) |
| Geographic location | |
| Unknown | 16 |
| Western US | 48 (18.4%) |
| Northeastern US | 33 (12.6%) |
| Midwestern US | 98 (37.5%) |
| Southern US | 40 (15.3%) |
| Canada | 13 (5.0%) |
| Other | 29 (11.1%) |
| Years of practice/training | |
| Unknown | 16 |
| Currently in training | 1 (0.4%) |
| Practice 04 years | 68 (26.1%) |
| Practice 59 years | 55 (21.1%) |
| Practice 1019 years | 64 (24.5%) |
| Practice 20+ years | 73 (28.0%) |
| Practice setting | |
| Unknown | 23 |
| Academic | 63 (24.8%) |
| Privateurban | 99 (39.0%) |
| Privaterural | 49 (19.3%) |
| Other | 43 (16.9%) |
| ABIM certification HM | |
| Unknown | 22 |
| Yes | 48 (18.8%) |
| No | 207 (81.2%) |
| Hospitalist | |
| Unknown | 20 |
| Yes | 181 (70.4%) |
| No | 76 (29.6%) |
| CME credits claimed | |
| Unknown | 20 |
| 024 | 54 (21.0%) |
| 2549 | 105 (40.9%) |
| 5074 | 61 (23.7%) |
| 7599 | 15 (5.8%) |
| 100+ | 22 (8.6%) |
| CME programs attended | |
| Unknown | 18 |
| 0 | 38 (14.7%) |
| 12 | 166 (64.1%) |
| 35 | 52 (20.1%) |
| 6+ | 3 (1.2%) |
CMETE scores (mean [SD]) were significantly associated with the use of ARS (4.64 [0.16]) vs no ARS (4.49 [0.16]; P=0.01, Table 2, Figure 1), longer presentations (30 minutes: 4.67 [0.13] vs <30 minutes: 4.51 [0.18]; P=0.02), and larger number of slides (50: 4.66 [0.17] vs <50: 4.55 [0.17]; P=0.04). There were no significant associations between CMETE scores and use of clinical cases, defined goals, or summary slides.
| Presentation Variable | No. (%) | Mean Score | Standard Deviation | P Value |
|---|---|---|---|---|
| Use of clinical cases | ||||
| Yes | 28 (87.5%) | 4.60 | 0.18 | 0.14 |
| No | 4 (12.5%) | 4.49 | 0.14 | |
| Audience response system | ||||
| Yes | 20 (62.5%) | 4.64 | 0.16 | 0.01 |
| No | 12 (37.5%) | 4.49 | 0.16 | |
| No. of slides | ||||
| 50 | 10 (31.3%) | 4.66 | 0.17 | 0.04 |
| <50 | 22 (68.8%) | 4.55 | 0.17 | |
| Defined goals/objectives | ||||
| Yes | 29 (90.6%) | 4.58 | 0.18 | 0.87 |
| No | 3 (9.4%) | 4.61 | 0.17 | |
| Summary slide | ||||
| Yes | 22 (68.8%) | 4.56 | 0.18 | 0.44 |
| No | 10 (31.3%) | 4.62 | 0.15 | |
| Presentation length | ||||
| 30 minutes | 14 (43.8%) | 4.67 | 0.13 | 0.02 |
| <30 minutes | 18 (56.3%) | 4.51 | 0.18 |

The magnitude of score differences observed in this study are substantial when considered in terms of Cohen's effect sizes. For number of slides, the effect size is 0.65, for audience response the effect size is 0.94, and for presentation length the effect size is approximately 1. According to Cohen, effect sizes of 0.5 to 0.8 are moderate, and effect sizes >0.8 are large. Consequently, the effect sizes of our observed differences are moderate to large.[20, 21]
DISCUSSION
To our knowledge, this is the first study to measure associations between validated teaching effectiveness scores and characteristics of presentations in HM CME. We found that the use of ARS and longer presentations were associated with significantly higher CMETE scores. Our findings have implications for HM CME course directors and presenters as they attempt to develop methods to improve the quality of CME.
CME participants in our study crossed a wide range of ages and experience, which is consistent with national surveys of hospitalists.[22, 23] Interestingly, however, nearly 1 in 3 participants trained in a specialty other than internal medicine. Additionally, the professional degrees of participants were diverse, with 20% of participants having trained as nurse practitioners or physician assistants. These findings are at odds with an early national survey of inpatient practitioners,[22] but consistent with recent literature that the diversity of training backgrounds among hospitalists is increasing as the field of HM evolves.[24] Hospital medicine CME providers will need to be cognizant of these demographic changes as they work to identify practice gaps and apply appropriate educational methods.
The use of an ARS allows for increased participation and engagement among lecture attendees, which in turn promotes active learning.[25, 26, 27] The association of higher teaching scores with the use of ARS is consistent with previous research in other CME settings such as clinical round tables and medical grand rounds.[17, 28] As it pertains to HM specifically, our findings also build upon a recent study by Sehgal et al., which reported on the novel use of bedside CME to enhance interactive learning and discussion among hospitalists, and which was viewed favorably by course participants.[29]
The reasons why longer presentations in our study were linked to higher CMETE scores are not entirely clear, as previous CME research has failed to demonstrate this relationship.[18] One possibility is that course participants prefer learning from presentations that provide granular, content‐rich information. Another possibility may be that characteristics of effective presenters who gave longer presentations and that were not measured in this study, such as presenter experience and expertise, were responsible for the observed increase in CMETE scores. Yet another possibility is that effective presentations were longer due to the use of ARS, which was also associated with better CMETE scores. This explanation may be plausible because the ARS requires additional slides and provides opportunities for audience interaction, which may lengthen the duration of any given presentation.
This study has several limitations. This was a single CME conference sponsored by a large academic medical center, which may limit generalizability, especially to smaller conferences in community settings. However, the audience was large and diverse in terms of participants experiences, practice settings, professional backgrounds, and geographic locations. Furthermore, the demographic characteristics of hospitalists at our course appear very similar to a recently reported national cross‐section of hospitalist groups.[30] Second, this is a cross‐sectional study without a comparison group. Nonetheless, a systematic review showed that most published education research studies involved single‐group designs without comparison groups.[31] Last, the outcomes of the study include attitudes and objectively measured presenter behaviors such as the use of ARS, but not patient‐related outcomes. Nonetheless, evidence indicates that the majority of medical education research does not present outcomes beyond knowledge,[31] and it has been noted that behavior‐related outcomes strike the ideal balance between feasibility and rigor.[32, 33] Finally, the instrument used in this study to measure teaching effectiveness is supported by prior validity evidence.[16]
In summary, we found that hospital medicine CME presentations, which are longer and use audience responses, are associated with greater teaching effectiveness ratings by CME course participants. These findings build upon previous CME research and suggest that CME course directors and presenters should strive to incorporate opportunities that promote audience engagement and participation. Additionally, this study adds to the existing validity of evidence for the CMETE assessment tool. We believe that future research should explore potential associations between teacher effectiveness and patient‐related outcomes, and determine whether course content that reflects the SHM core competencies improves CME teaching effectiveness scores.
Disclosure
Nothing to report.
- Society of Hospital Medicine. 2013/2014 press kit. Available at: http://www.hospitalmedicine.org/Web/Media_Center/Web/Media_Center/Media_Center.aspx?hkey=e26ceba7-ba93-4e50-8eb1-1ccc75d6f0fd. Accessed May 18, 2015.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33:9–10.
- , , , et al. The evolving role of the pediatric nurse practitioner in hospital medicine. J Hosp Med. 2014;9:261–265.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/Web/About_SHM/Hospitalist_Definition/Web/About_SHM/Industry/Hospital_Medicine_Hospital_Definition.aspx. Accessed February 16, 2015.
- Accreditation Council for Continuing Medical Education. 2013 annual report data executive summary. Available at: http://www.accme.org/sites/default/files/630_2013_Annual_Report_20140715_0.pdf. Accessed February 16, 2015.
- . The anatomy of an outstanding CME meeting. J Am Coll Radiol. 2005;2:534–540.
- , , , , . How to use The Core Competencies in Hospital Medicine: a framework for curriculum development. J Hosp Med. 2006;1:57–67.
- , , , , , . Perspective: a practical approach to defining professional practice gaps for continuing medical education. Acad Med. 2012;87:582–585.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):148–156.
- , . Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84:339–344.
- , . Developing scholarly projects in education: a primer for medical teachers. Med Teach. 2007;29:210–218.
- , . A meta‐analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27:6–15.
- , , . Achieving desired results and improved outcomes: integrating planning and assessment throughout learning activities. J Contin Educ Health Prof. 2009;29:1–15.
- , . Effectiveness of continuing medical education: updated synthesis of systematic reviews. Available at: http://www.accme.org/sites/default/files/652_20141104_Effectiveness_of_Continuing_Medical_Education_Cervero_and_Gaines.pdf. Accessed March 25, 2015.
- , , , et al. Improving participant feedback to continuing medical education presenters in internal medicine: a mixed‐methods study. J Gen Intern Med. 2012;27:425–431.
- , , , et al. Measuring faculty reflection on medical grand rounds at Mayo Clinic: associations with teaching experience, clinical exposure, and presenter effectiveness. Mayo Clin Proc. 2013;88:277–284.
- , , , . Successful lecturing: a prospective study to validate attributes of the effective medical lecture. J Gen Intern Med. 2000;15:366–371.
- , , , , , . A standardized approach to assessing physician expectations and perceptions of continuing medical education. J Contin Educ Health Prof. 2007;27:173–182.
- . Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977.
- . Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
- , , , . Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130:343–349.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27:28–36.
- , , , et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9:615–620.
- , . A primer on audience response systems: current applications and future considerations. Am J Pharm Educ. 2008;72:77.
- , , . Continuing medical education: AMEE education guide no 35. Med Teach. 2008;30:652–666.
- . Clickers in the large classroom: current research and best‐practice tips. CBE Life Sci Educ. 2007;6:9–20.
- , , . Evaluation of an audience response system for the continuing education of health professionals. J Contin Educ Health Prof. 2003;23:109–115.
- , , . Bringing continuing medical education to the bedside: the University of California, San Francisco Hospitalist Mini‐College. J Hosp Med. 2014;9:129–134.
- Society of Hospital Medicine. 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , , , , . Association between funding and quality of published medical education research. JAMA. 2007;298:1002–1009.
- . Mind the gap: some reasons why medical education research is different from health services research. Med Educ. 2001;35:319–320.
- , . Reflections on experimental research in medical education. Adv Health Sci Educ Theory Pract. 2010;15:455–464.
- Society of Hospital Medicine. 2013/2014 press kit. Available at: http://www.hospitalmedicine.org/Web/Media_Center/Web/Media_Center/Media_Center.aspx?hkey=e26ceba7-ba93-4e50-8eb1-1ccc75d6f0fd. Accessed May 18, 2015.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33:9–10.
- , , , et al. The evolving role of the pediatric nurse practitioner in hospital medicine. J Hosp Med. 2014;9:261–265.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/Web/About_SHM/Hospitalist_Definition/Web/About_SHM/Industry/Hospital_Medicine_Hospital_Definition.aspx. Accessed February 16, 2015.
- Accreditation Council for Continuing Medical Education. 2013 annual report data executive summary. Available at: http://www.accme.org/sites/default/files/630_2013_Annual_Report_20140715_0.pdf. Accessed February 16, 2015.
- . The anatomy of an outstanding CME meeting. J Am Coll Radiol. 2005;2:534–540.
- , , , , . How to use The Core Competencies in Hospital Medicine: a framework for curriculum development. J Hosp Med. 2006;1:57–67.
- , , , , , . Perspective: a practical approach to defining professional practice gaps for continuing medical education. Acad Med. 2012;87:582–585.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):148–156.
- , . Proposal for a collaborative approach to clinical teaching. Mayo Clin Proc. 2009;84:339–344.
- , . Developing scholarly projects in education: a primer for medical teachers. Med Teach. 2007;29:210–218.
- , . A meta‐analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27:6–15.
- , , . Achieving desired results and improved outcomes: integrating planning and assessment throughout learning activities. J Contin Educ Health Prof. 2009;29:1–15.
- , . Effectiveness of continuing medical education: updated synthesis of systematic reviews. Available at: http://www.accme.org/sites/default/files/652_20141104_Effectiveness_of_Continuing_Medical_Education_Cervero_and_Gaines.pdf. Accessed March 25, 2015.
- , , , et al. Improving participant feedback to continuing medical education presenters in internal medicine: a mixed‐methods study. J Gen Intern Med. 2012;27:425–431.
- , , , et al. Measuring faculty reflection on medical grand rounds at Mayo Clinic: associations with teaching experience, clinical exposure, and presenter effectiveness. Mayo Clin Proc. 2013;88:277–284.
- , , , . Successful lecturing: a prospective study to validate attributes of the effective medical lecture. J Gen Intern Med. 2000;15:366–371.
- , , , , , . A standardized approach to assessing physician expectations and perceptions of continuing medical education. J Contin Educ Health Prof. 2007;27:173–182.
- . Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977.
- . Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
- , , , . Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130:343–349.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27:28–36.
- , , , et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9:615–620.
- , . A primer on audience response systems: current applications and future considerations. Am J Pharm Educ. 2008;72:77.
- , , . Continuing medical education: AMEE education guide no 35. Med Teach. 2008;30:652–666.
- . Clickers in the large classroom: current research and best‐practice tips. CBE Life Sci Educ. 2007;6:9–20.
- , , . Evaluation of an audience response system for the continuing education of health professionals. J Contin Educ Health Prof. 2003;23:109–115.
- , , . Bringing continuing medical education to the bedside: the University of California, San Francisco Hospitalist Mini‐College. J Hosp Med. 2014;9:129–134.
- Society of Hospital Medicine. 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , , , , . Association between funding and quality of published medical education research. JAMA. 2007;298:1002–1009.
- . Mind the gap: some reasons why medical education research is different from health services research. Med Educ. 2001;35:319–320.
- , . Reflections on experimental research in medical education. Adv Health Sci Educ Theory Pract. 2010;15:455–464.
© 2015 Society of Hospital Medicine
Frequently Admitted Patients
The national healthcare improvement paradigm is shifting toward a more comprehensive, value‐focused, and patient‐centered approach. Reducing hospital readmissions has become a focal point as a policy strategy to improve care quality while reducing cost. Section 3025 of the Affordable Care Act mandated the Centers for Medicare and Medicaid Services to make progressive reductions in Medicare payments to hospitals that have higher than expected readmission rates for 3 conditions (heart failure, acute myocardial infarction, and pneumonia), and expanding to include chronic obstructive pulmonary disease and total hip and knee arthroplasty in 2015.[1] In response, hospitals and systems are developing and implementing programs that coordinate care beyond hospital walls to reduce readmissions and healthcare costs.[2, 3] However, patients are readmitted for a variety of reasons, and programs that address the needs of some may not address the distinct needs of others. Understanding the characteristics of patients with frequent readmissions will permit the well‐informed creation of solutions specific to this population to reduce cost, free resources, and provide better care.
Although a solid body of literature already exists that describes the characteristics of patients who frequently visit the emergency department (ED),[4, 5, 6, 7, 8, 9, 10, 11, 12] it is not clear to what extent these characteristics also apply to patients with frequent hospital admissions. Frequent ED visitors have been found to be largely insured (85%) although with over‐representation of public insurance, and to be heavy users of the healthcare system overall.[6] A high disease burden associated with multiple chronic conditions has been found to predict frequent ED use.[4, 9, 11, 12] Some characteristics may vary by location; for example, alcohol abuse and psychiatric morbidity have been found to be associated with frequent ED use in New York and San Francisco, but it is not clear to what extent they are a factor in less urban areas.[4, 6, 12]
Several previous studies have investigated the characteristics of frequently admitted patients at single sites.[13, 14, 15, 16] Nguyen et al. (2013) studied patients with the highest costs and the most admissions at a large academic medical center in San Francisco.[13] High admit patients were defined as those responsible for the top decile of admissions, and were grouped into equal‐sized high‐ and low‐cost cohorts. The high‐admission/high‐cost group represented 5% of all patients, 25% of all costs, and 16% of all admissions. These patients were hospitalized primarily for medical conditions (78%) and had a high 30‐day readmission rate (47%). The high‐admission/low‐cost group accounted for 5% of all patients, 12% of all admissions, and 7% of all costs. These patients were also predominantly admitted for medical conditions (87%), with the most common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions.[13]
Hwa (2012) conducted an analysis of 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco.[14] These patients represented just 1% of all patients, but 13% of readmissions. Fifty‐five percent of these patients had a psychiatric diagnosis, and 52% had chronic pain. Ninety percent had a primary care physician in the hospital system, 100% were insured either privately or publicly, and 93% had housing, although for 17% housing was described as marginal.[14]
In a third study, Boonyasai et al. (2012) identified 76 patients with 82 readmissions at a Baltimore, Maryland, hospital and classified them as isolated (1 readmission per 6‐month period) or serial (more than 1 readmission per 6‐month period) readmissions.[15] Patients with serial readmissions accounted for 70% of the total. Isolated readmissions were most likely to be related to suboptimal quality of care and care coordination, whereas serial readmissions were more likely to result from disease progression, psychiatric illness, and substance abuse.[15]
All of these studies were conducted at single‐site academic medical centers serving inner city populations. We undertook this study to identify patient and hospital‐level characteristics of frequently admitted patients in a broad sample of 101 US academic medical centers to determine whether previously reported findings are generalizable, and to identify characteristics of frequently admitted patients that can inform interventions designed to meet the needs of this relatively small but resource‐intensive group of patients.
METHODS
All data were obtained from the University HealthSystem Consortium (UHC) (Chicago, IL) Clinical Data Base/Resource Manager (CDB), a large administrative database to which UHC principal members submit comprehensive administrative data files. UHC's principal members include approximately 120 US academic medical centers delivering tertiary and quaternary care, with an average of 647 acute care beds. The CDB includes primary and secondary diagnoses using International Classification of Diseases, Ninth Revision (ICD‐9)[17] codes.
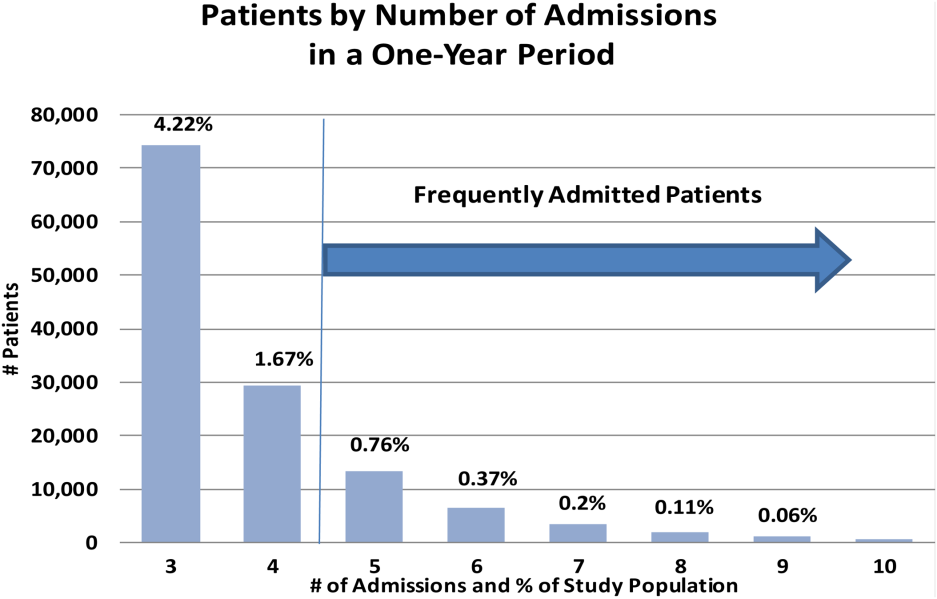
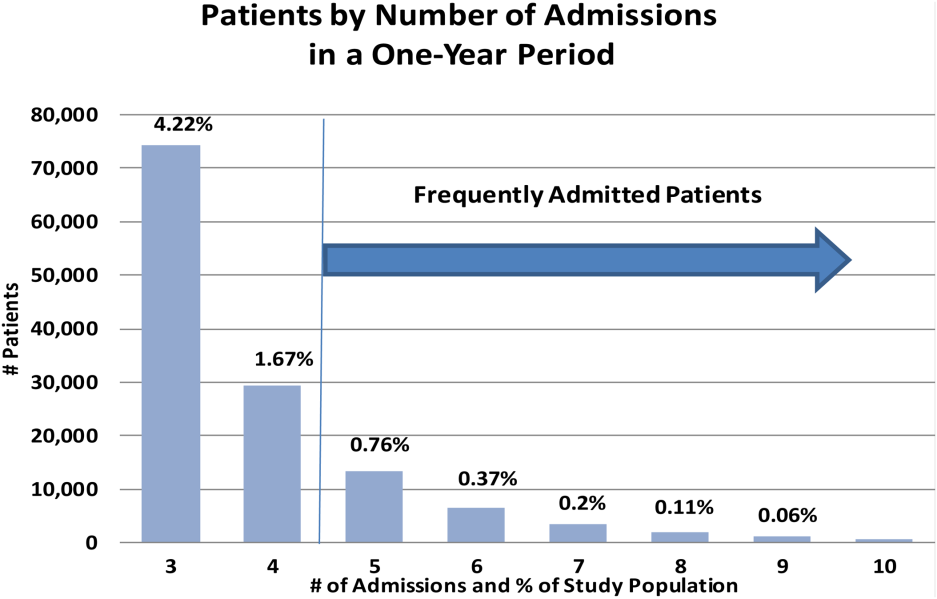
The data of 101 academic medical centers with complete datasets for the study period (October 1, 2011, to September 30, 2012) were included in this analysis. Frequently admitted patients were defined as patients admitted 5 or more times to the same facility in a 12‐month period; all admissions were included, even those more than 30 days apart. This definition was established based on a naturally occurring break in the frequency distribution (Figure 1) and our intention to focus on the unique characteristics of patients at the far right of the distribution. We excluded obstetric (MDC 14, ICD‐9)[17] admissions and pediatric (<18 years of age at index admission) patients, as well as admissions with principal diagnoses for chemotherapy (ICD‐9 diagnosis codes v5811v5812), dialysis (ICD‐9 diagnosis codes v560v568), and rehabilitation (ICD‐9 diagnosis codes v570v579), which are typically planned. The Agency for Healthcare Research and Quality (AHRQ) comorbidity software was used to identify comorbid conditions,[18, 19] and a score based on the Elixhauser comorbidity measures was calculated using a modified acuity point system.[20] For comparisons based on safety net status, we used a definition of payer mix being 25% Medicaid or uninsured.
Our analyses included patient demographics, admission source and discharge status, clinical diagnoses, procedures, and comorbidities, cost, and length of stay. Patients defined as frequently admitted were compared in aggregate to all other hospitalized patients (all other admissions).
To evaluate associations, we used [2] tests for categorical variables and t tests for continuous variables. When comparing the non‐normally distributed comorbidities of the control group to the normally distributed comorbidities of the frequently admitted patients, we performed a Kruskal‐Wallis test on the medians.
RESULTS
During a 1‐year period (October 1, 2011, to September 30, 2012), 1,758,027 patients were admitted 2,388,124 times at 101 academic medical centers. Of these, 28,291 patients had 5 or more admissions during this period, resulting in 180,185 admissions. These frequently admitted patients represented 1.6% of all patients (Figure 1) and 7.6% of all inpatient admissions. By comparison, nonfrequently admitted patients were admitted once (79%), twice (14%), 3 times (4%), or 4 times (2%).
Among hospitals, the volume and impact of frequently admitted patients varied widely. The frequently admitted patient population ranged from 64 patients (0.7% of all patients) to 785 patients (3.5%), with an average of 280 patients (1.6%). To look for differences that might explain this range, we compared hospitals in the top and bottom deciles with respect to geographic region and to safety net status, but found no significant or meaningful differences. The average number of admissions per patient was 6.4, with a range of 5 to 76. Days per patient ranged from 5 to 434 days, with an average of 42. The average patient‐day percentage (frequently admitted patient days/total patient days) was 8.4%, and ranged from 3.2% to 15.4%.
Frequently admitted patients were more likely to be younger than all other patients (71.9% under the age of 65 years, as compared with 65.3% of all other patients (P<0.001)). They were also more likely to have either Medicaid or no healthcare insurance (27.6% compared with 21.6%, P<0.001), although nearly three‐quarters had either private insurance or Medicare coverage.
Eighty‐four percent of frequently admitted patient admissions were to medical services (vs 58% of all other patients (P<0.001)). The admission status for these patients was much less likely to be elective (9.1% of frequently admitted patient admissions vs 26.6% of all other patients' admissions [P<0.001]). Frequently admitted patients were more likely to be discharged to a skilled nursing facility (9.3% vs 8.4%, [P<0.001]) or with home health services (19.7% vs 13.4% [P<0.001]).
The 10 most common primary diagnoses for patient admissions are shown in Table 1. No single primary diagnosis accounted for a large share of the admissions of these patients; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4% of admissions. The 10 most common diagnoses accounted for <20% of all admissions. The remainder of the diagnoses was spread over more than 3000 diagnosis codes; only about 300 codes had more than 100 admissions each.
| Primary Diagnoses | Secondary Diagnoses | Principal Procedures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other Patient Rank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other PatientRank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | |||
| ||||||||||
| Sickle cell disease with crisis | 3.97% (7,152) | 0.002% (5,887) | 63 | Hypertension NOS | 31.39% (56,556) | 40.04% (884,045) | 1 | Hemodialysis | 6.32% (11,380) | 1.08% (23,871) |
| Septicemia NOS | 2.58% (4,652) | 1.87% (41,369) | 1 | Hyperlipidemia NOS | 24.47% (44,089) | 25.94% (572,760) | 2 | Packed cell transfusion | 4.49% (8.091) | 1.57% (34,669) |
| Acute and chronic systolic heart failure | 2.06% (3,708) | 0.81% (17,802) | 12 | Congestive heart failure NOS | 22.86% (41,197) | 11.82% (260,944) | 8 | Percutaneous abdominal drainage | 2.42% (4,366) | 0.86% (18,974) |
| Acute kidney failure NOS | 2.04% (3,680) | 1.16% (25,528) | 6 | Esophageal reflux | 21.19% (38,184) | 17.32% (382,511) | 3 | Venous catheter NEC | 2.13% (3,843) | 0.89% (19,718) |
| Obstructive chronic bronchitis with exacerbation | 1.76% (3,180) | 0.68% (14,957) | 14 | Diabetes mellitus NOS uncomplicated | 20.39% (36,743) | 16.75% (369,808) | 4 | Central venous catheter placement with guidewire | 2.13% (3,834)) | 0.83% (18,307) |
| Pneumonia organism NOS | 1.72% (3,091) | 1.29% (28,468) | 4 | Tobacco use disorder | 16.98% (30,604) | 16.71% (368,880) | 5 | Continuous invasive mechanical ventilation <96 consecutive hours | 1.38% (2,480) | 0.7% (15,441) |
| Urinary tract infection NOS | 1.63% (2,939) | 0.86% (19,069) | 9 | History of tobacco use | 16.89% (30,439) | 14.77% (326,026) | 6 | Noninvasive mechanical ventilation | 1.3% (2,345) | 0.58% (12,899) |
| Acute pancreatitis | 1.23% (2,212) | 0.73% (16,168) | 13 | Coronary atherosclerosis native vessel | 16.12% (29,040) | 12.88% (284,487) | 7 | Small intestine endoscopy NEC | 1.26% (2.265) | 0.7% (15,480) |
| Acute and chronic diastolic heart failure | 1.22% (2,190) | 0.48% (10,600) | 22 | Depressive disorder | 15.42% (27,785) | 10.34% (228,347) | 10 | Heart ultrasound | 1.11% (1,997) | 1.37% (30,161) |
| Complication of kidney transplant | 1.08% (1,944) | 0.42% (9,354) | 28 | Acute kidney failure NOS | 13.8% (24,859) | 9.37%% (206,951) | 12 | Esophagogastroduodenoscopy with closed biopsy | 1.09% (1,963) | 0.8% (17,644) |
Secondary diagnoses were mainly chronic conditions, including hypertension, hyperlipidemia, esophageal reflux, and diabetes mellitus type 2 (Table 1.) Combined, congestive heart failure and diabetes mellitus accounted for 43.3% of the secondary diagnoses of admissions of frequently admitted patients, but for only 28.6% of other patients. Acute kidney failure was more common in frequently admitted patients (13.8% vs 9.4% [P<0.001]). Psychiatric disorders accounted for <1% of primary diagnoses for both frequently admitted patients and all other patients. As a secondary diagnosis, depressive disorder appeared in the top 10 for both groups, although more commonly for frequently admitted patients (15.4% vs 10.3% [P<0.001]).
The most commonly performed principal procedures are also shown in Table 1. These include hemodialysis (6.32%) and packed cell transfusion (4.49%), nonoperating room procedures associated with chronic medical conditions.
Comorbidities were compared using the AHRQ comorbidity software.[18, 19] Comorbid conditions were counted once per patient, regardless of the number of admissions in which the condition was coded. Frequently admitted patients have a significantly higher mean number of comorbidities: 7.1 compared to 2.5 for all other patients (P<0.001; Figure 2). In an additional analysis using the Elixhauser comorbidity measures to determine acuity scores, the mean scores were 13.1 for frequently admitted patients and 3.17 for all others (P<0.001). The most common comorbidities were hypertension (74%), fluid and electrolyte disorders (73%), and deficiency anemias (66%). The only behavioral health comorbidity that affected more than a quarter of frequently admitted patients was depression (40% as compared to 13% for all others).
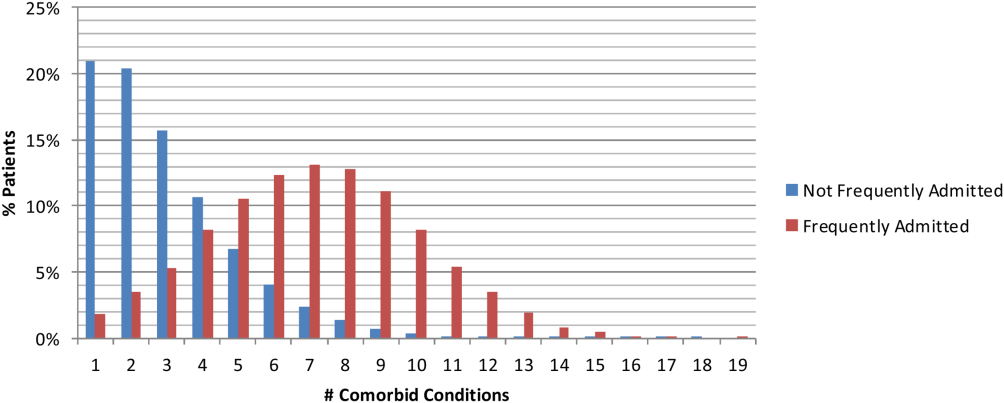
Additionally, frequently admitted patients were significantly more likely to have comorbidities of psychosis (18% vs 5% [P<0.001]), alcohol abuse (16% vs 7% [P<0.001]), and drug abuse (20% vs 7% [P<0.001]). Among hospitals, these comorbidities ranged widely: psychosis (3% 48%); alcohol abuse (3%46%); and drug abuse (3%58%). Hospitals with the highest rates (top decile) of frequently admitted patients with alcohol and drug abuse comorbidities were more likely to be safety net hospitals than those in the lowest decile (P<0.05 for each independently), but no such difference was found regarding rates of patients with psychosis.
Although the frequently admitted patient population accounted for only 1.6% of patients, they accounted for an average of 8.4% of all bed days and 7.1% of direct cost. The average cost per day was $1746, compared to $2144 for all other patients (Table 2).
| Length of Stay, Days | Direct Cost | % Total Bed Days | Cost/Day | All Other Patients Cost/Day | Difference | |
|---|---|---|---|---|---|---|
| Minimum | 1.0 | 2.3% | 3.2% | $809 | $1,005 | $(196) |
| Maximum | 86.8 | 14.1% | 15.4% | $3,208 | $4,070 | $(862) |
| Mean | 6.7 | 7.1% | 8.4% | $1,746 | $2,144 | $(398) |
| Median | 5.5 | 7.0% | 8.3% | $1,703 | $2,112 | $(410) |
DISCUSSION
An extensive analysis of the characteristics of frequently admitted patients at 101 US academic medical centers, from October 1, 2011 to September 30, 2012, revealed that these patients comprised 1.6% of all patients, but accounted for 8% of all admissions and 7% of direct costs. Relative to all other hospitalized patients, frequently admitted patients were likely to be younger, of lower socioeconomic status, in poorer health, and more often affected by mental health or substance abuse conditions that may mediate their health behaviors. However, the prevalence of patients with psychiatric or behavior conditions varied widely among hospitals, and hospitals with the highest rates of patients with substance abuse comorbidities were more likely to be safety net hospitals. Frequently admitted patients' diagnoses and procedures suggest that their admissions were related to complex chronic diseases; more than three‐quarters were admitted to medicine services, and their average length of stay was nearly 7 days. No single primary diagnosis accounted for a predominant share of their admissions; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4%. The cost of their care was lower than that of other patients, reflecting the preponderance of their admissions to medicine service lines.
In many ways, frequently admitted patients seem similar to frequent ED visitors. Their visits were driven by a high disease burden associated with multiple chronic conditions, and they were heavy users of the healthcare system overall.[4, 6] The majority of both groups were insured, although there was over‐representation of public insurance.[6] As with frequent ED users, some frequently admitted patients are affected by psychiatric morbidity and substance abuse.[4, 12]
Our results in some ways confirmed, and in some ways differed from, findings of prior studies of patients with frequent hospital admissions. Although each study performed to date has defined the population differently, comparison of findings is useful. Our population was similar to the high‐admission groups identified by Nguyen et al. (patients responsible for the top decile of admissions).[13] These patients were also predominantly admitted for medical conditions, with common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions. However, the median length of stay (3 days for the high‐admission/low‐cost group and 5 days for the high‐admission/high‐cost group) was lower than that of our population (5.5 days).
Hwa, who studied 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco,[14] found that 55% of frequently admitted patients had a psychiatric diagnosis, higher than our patient population. Our findings are similar to those of Boonyasai et al.[15] whose serial readmitters had admissions resulting from disease progression, psychiatric illness, and substance abuse.
Our more nationally representative analysis documented a wide range of patient volumes and clinical characteristics, including psychiatric and substance abuse comorbidities, across study hospitals. It demonstrates that different approachesand resourcesare needed to meet the needs of these varied groups of patients. Each hospital must identify, evaluate, and understand its own population of frequently admitted patients to create well‐informed solutions to prevent repeat hospitalization for these patients.
Our ability to create a distinctive picture of the population of frequently admitted patients in US academic medical centers is based on access to an expansive dataset that captures complete diagnostic and demographic information on the universe of patients admitted to our member hospitals. The availability of clinical and administrative data for the entire population of patients permits both an accurate description of patient characteristics and a standardized comparison of groups. All data conform to accepted formats and definitions; their validity is universally recognized by contributing database participants.
Limitations
There are several important limitations to our study. First, patients with 5 or more admissions in 1 year may be undercounted. The UHC Clinical Data Base/Resource Manager only captures readmissions to a single facility; admissions of any patient admitted to more than 1 hospital, even within the UHC membership, cannot be determined. This could have a particularly strong effect on our ability to detect admissions of patients with acute episodes related to psychiatric illness or substance abuse, as they may be more likely to present to multiple or specialty hospitals. Additionally, readmission rates vary among UHC‐member hospitals, based to some extent on geography and the availability of alternative settings of care.
It is possible that surveillance bias played a role in our finding that frequently admitted patients have a significantly higher mean number of comorbidities; each admission presents an opportunity to document additional comorbid conditions. Psychiatric conditions may be underdocumented in medical settings in academic medical centers, where the focus is often on acute medical conditions. Additionally, certain data elements that we believe are central to understanding the characteristics of frequently admitted patients are not part of the UHC Clinical Data Base/Resource Manager and were therefore not a part of our analysis. These highly influential upstream determinants of health include documentation of a primary care physician, housing status, and access to services at discharge.
CONCLUSION
The valuable information reported from analysis of nearly 2 million patients in the UHC Clinical Data Base/Resource Manager can be used to better understand the characteristics of frequently admitted patients. This important cohort of individuals has complex care needs that often result in hospitalization, but may be amenable to solutions that allow patients to remain in their communities. By understanding the demographic, social, and medical characteristics of these patients, hospitals can develop and implement solutions that address the needs of this small group of patients who consume a highly disproportionate share of healthcare resources.
Acknowledgements
The authors acknowledge the contributions of Samuel F. Hohmann, PhD, and Ryan Carroll, MBA, who provided expert statistical analyses and generous assistance in the completion of this article.
Disclosure: Nothing to report.
- Centers for Medicare 21(9):117–120.
- , , , . The influence of a postdischarge intervention on reducing hospital readmissions in a Medicare population. Popul Health Manag. 2013;16(5):310–316.
- , . Dispelling an urban legend: frequent emergency department users have substantial burden of disease. Health Aff (Millwood). 2013;32:2099–2108.
- , , , et al. Effectiveness of interventions targeting frequent users of emergency departments: a systematic review. Ann Emerg Med. 2011;58:41–52.
- , . Frequent users of emergency departments: the myths, the data, and the policy implications. Ann Emerg Med. 2010;20(10):1–8.
- , , , . Development and validation of a model for predicting emergency admissions over the next year. Arch Intern Med. 2008;168:1416–1422.
- , , , et al. A comparison of frequent and infrequent visitors to an urban emergency department. J Emerg Med. 2008;38:115–121.
- , . Frequent users of Massachusetts emergency departments: a statewide analysis. Ann Emerg Med. 2006;48:9–16.
- , , , et al. A descriptive study of heavy emergency department users at an academic emergency department reveals heavy users have better access to care than average users. J Emerg Nurs. 2005;31:139–144.
- , , . Predictors and outcomes of frequent emergency department users. Acad Emerg Med. 2003;10:320–328.
- , , . Epidemiologic analysis of an urban, public emergency department's frequent users. Acad Emerg Med. 2000;7:637–646.
- , , , . What's cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J Hosp Med. 2013;8:665–671.
- . Characteristics of a frequently readmitted patient population on an inpatient medical service. Abstract presented at: Society of Hospital Medicine Annual Meeting, April 1– 4, 2012; San Diego, CA.
- , , , , . Characteristics of isolated and serial rehospitalizations suggest a need for different types of improvement strategies [abstract] J Hosp Med. 2012;7(suppl 2):513.
- , , , , . An intervention to improve care and reduce costs for high‐risk patients with frequent hospital admissions: a pilot study. BMC Health Serv Res. 2011;11:270–279.
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD‐9). Available at: http://www.cdc.gov/nchs/icd/icd9.htm. Accessed February 18, 2015.
- Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed February 18, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633.
The national healthcare improvement paradigm is shifting toward a more comprehensive, value‐focused, and patient‐centered approach. Reducing hospital readmissions has become a focal point as a policy strategy to improve care quality while reducing cost. Section 3025 of the Affordable Care Act mandated the Centers for Medicare and Medicaid Services to make progressive reductions in Medicare payments to hospitals that have higher than expected readmission rates for 3 conditions (heart failure, acute myocardial infarction, and pneumonia), and expanding to include chronic obstructive pulmonary disease and total hip and knee arthroplasty in 2015.[1] In response, hospitals and systems are developing and implementing programs that coordinate care beyond hospital walls to reduce readmissions and healthcare costs.[2, 3] However, patients are readmitted for a variety of reasons, and programs that address the needs of some may not address the distinct needs of others. Understanding the characteristics of patients with frequent readmissions will permit the well‐informed creation of solutions specific to this population to reduce cost, free resources, and provide better care.
Although a solid body of literature already exists that describes the characteristics of patients who frequently visit the emergency department (ED),[4, 5, 6, 7, 8, 9, 10, 11, 12] it is not clear to what extent these characteristics also apply to patients with frequent hospital admissions. Frequent ED visitors have been found to be largely insured (85%) although with over‐representation of public insurance, and to be heavy users of the healthcare system overall.[6] A high disease burden associated with multiple chronic conditions has been found to predict frequent ED use.[4, 9, 11, 12] Some characteristics may vary by location; for example, alcohol abuse and psychiatric morbidity have been found to be associated with frequent ED use in New York and San Francisco, but it is not clear to what extent they are a factor in less urban areas.[4, 6, 12]
Several previous studies have investigated the characteristics of frequently admitted patients at single sites.[13, 14, 15, 16] Nguyen et al. (2013) studied patients with the highest costs and the most admissions at a large academic medical center in San Francisco.[13] High admit patients were defined as those responsible for the top decile of admissions, and were grouped into equal‐sized high‐ and low‐cost cohorts. The high‐admission/high‐cost group represented 5% of all patients, 25% of all costs, and 16% of all admissions. These patients were hospitalized primarily for medical conditions (78%) and had a high 30‐day readmission rate (47%). The high‐admission/low‐cost group accounted for 5% of all patients, 12% of all admissions, and 7% of all costs. These patients were also predominantly admitted for medical conditions (87%), with the most common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions.[13]
Hwa (2012) conducted an analysis of 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco.[14] These patients represented just 1% of all patients, but 13% of readmissions. Fifty‐five percent of these patients had a psychiatric diagnosis, and 52% had chronic pain. Ninety percent had a primary care physician in the hospital system, 100% were insured either privately or publicly, and 93% had housing, although for 17% housing was described as marginal.[14]
In a third study, Boonyasai et al. (2012) identified 76 patients with 82 readmissions at a Baltimore, Maryland, hospital and classified them as isolated (1 readmission per 6‐month period) or serial (more than 1 readmission per 6‐month period) readmissions.[15] Patients with serial readmissions accounted for 70% of the total. Isolated readmissions were most likely to be related to suboptimal quality of care and care coordination, whereas serial readmissions were more likely to result from disease progression, psychiatric illness, and substance abuse.[15]
All of these studies were conducted at single‐site academic medical centers serving inner city populations. We undertook this study to identify patient and hospital‐level characteristics of frequently admitted patients in a broad sample of 101 US academic medical centers to determine whether previously reported findings are generalizable, and to identify characteristics of frequently admitted patients that can inform interventions designed to meet the needs of this relatively small but resource‐intensive group of patients.
METHODS
All data were obtained from the University HealthSystem Consortium (UHC) (Chicago, IL) Clinical Data Base/Resource Manager (CDB), a large administrative database to which UHC principal members submit comprehensive administrative data files. UHC's principal members include approximately 120 US academic medical centers delivering tertiary and quaternary care, with an average of 647 acute care beds. The CDB includes primary and secondary diagnoses using International Classification of Diseases, Ninth Revision (ICD‐9)[17] codes.
The data of 101 academic medical centers with complete datasets for the study period (October 1, 2011, to September 30, 2012) were included in this analysis. Frequently admitted patients were defined as patients admitted 5 or more times to the same facility in a 12‐month period; all admissions were included, even those more than 30 days apart. This definition was established based on a naturally occurring break in the frequency distribution (Figure 1) and our intention to focus on the unique characteristics of patients at the far right of the distribution. We excluded obstetric (MDC 14, ICD‐9)[17] admissions and pediatric (<18 years of age at index admission) patients, as well as admissions with principal diagnoses for chemotherapy (ICD‐9 diagnosis codes v5811v5812), dialysis (ICD‐9 diagnosis codes v560v568), and rehabilitation (ICD‐9 diagnosis codes v570v579), which are typically planned. The Agency for Healthcare Research and Quality (AHRQ) comorbidity software was used to identify comorbid conditions,[18, 19] and a score based on the Elixhauser comorbidity measures was calculated using a modified acuity point system.[20] For comparisons based on safety net status, we used a definition of payer mix being 25% Medicaid or uninsured.

Our analyses included patient demographics, admission source and discharge status, clinical diagnoses, procedures, and comorbidities, cost, and length of stay. Patients defined as frequently admitted were compared in aggregate to all other hospitalized patients (all other admissions).
To evaluate associations, we used [2] tests for categorical variables and t tests for continuous variables. When comparing the non‐normally distributed comorbidities of the control group to the normally distributed comorbidities of the frequently admitted patients, we performed a Kruskal‐Wallis test on the medians.
RESULTS
During a 1‐year period (October 1, 2011, to September 30, 2012), 1,758,027 patients were admitted 2,388,124 times at 101 academic medical centers. Of these, 28,291 patients had 5 or more admissions during this period, resulting in 180,185 admissions. These frequently admitted patients represented 1.6% of all patients (Figure 1) and 7.6% of all inpatient admissions. By comparison, nonfrequently admitted patients were admitted once (79%), twice (14%), 3 times (4%), or 4 times (2%).
Among hospitals, the volume and impact of frequently admitted patients varied widely. The frequently admitted patient population ranged from 64 patients (0.7% of all patients) to 785 patients (3.5%), with an average of 280 patients (1.6%). To look for differences that might explain this range, we compared hospitals in the top and bottom deciles with respect to geographic region and to safety net status, but found no significant or meaningful differences. The average number of admissions per patient was 6.4, with a range of 5 to 76. Days per patient ranged from 5 to 434 days, with an average of 42. The average patient‐day percentage (frequently admitted patient days/total patient days) was 8.4%, and ranged from 3.2% to 15.4%.
Frequently admitted patients were more likely to be younger than all other patients (71.9% under the age of 65 years, as compared with 65.3% of all other patients (P<0.001)). They were also more likely to have either Medicaid or no healthcare insurance (27.6% compared with 21.6%, P<0.001), although nearly three‐quarters had either private insurance or Medicare coverage.
Eighty‐four percent of frequently admitted patient admissions were to medical services (vs 58% of all other patients (P<0.001)). The admission status for these patients was much less likely to be elective (9.1% of frequently admitted patient admissions vs 26.6% of all other patients' admissions [P<0.001]). Frequently admitted patients were more likely to be discharged to a skilled nursing facility (9.3% vs 8.4%, [P<0.001]) or with home health services (19.7% vs 13.4% [P<0.001]).
The 10 most common primary diagnoses for patient admissions are shown in Table 1. No single primary diagnosis accounted for a large share of the admissions of these patients; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4% of admissions. The 10 most common diagnoses accounted for <20% of all admissions. The remainder of the diagnoses was spread over more than 3000 diagnosis codes; only about 300 codes had more than 100 admissions each.
| Primary Diagnoses | Secondary Diagnoses | Principal Procedures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other Patient Rank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other PatientRank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | |||
| ||||||||||
| Sickle cell disease with crisis | 3.97% (7,152) | 0.002% (5,887) | 63 | Hypertension NOS | 31.39% (56,556) | 40.04% (884,045) | 1 | Hemodialysis | 6.32% (11,380) | 1.08% (23,871) |
| Septicemia NOS | 2.58% (4,652) | 1.87% (41,369) | 1 | Hyperlipidemia NOS | 24.47% (44,089) | 25.94% (572,760) | 2 | Packed cell transfusion | 4.49% (8.091) | 1.57% (34,669) |
| Acute and chronic systolic heart failure | 2.06% (3,708) | 0.81% (17,802) | 12 | Congestive heart failure NOS | 22.86% (41,197) | 11.82% (260,944) | 8 | Percutaneous abdominal drainage | 2.42% (4,366) | 0.86% (18,974) |
| Acute kidney failure NOS | 2.04% (3,680) | 1.16% (25,528) | 6 | Esophageal reflux | 21.19% (38,184) | 17.32% (382,511) | 3 | Venous catheter NEC | 2.13% (3,843) | 0.89% (19,718) |
| Obstructive chronic bronchitis with exacerbation | 1.76% (3,180) | 0.68% (14,957) | 14 | Diabetes mellitus NOS uncomplicated | 20.39% (36,743) | 16.75% (369,808) | 4 | Central venous catheter placement with guidewire | 2.13% (3,834)) | 0.83% (18,307) |
| Pneumonia organism NOS | 1.72% (3,091) | 1.29% (28,468) | 4 | Tobacco use disorder | 16.98% (30,604) | 16.71% (368,880) | 5 | Continuous invasive mechanical ventilation <96 consecutive hours | 1.38% (2,480) | 0.7% (15,441) |
| Urinary tract infection NOS | 1.63% (2,939) | 0.86% (19,069) | 9 | History of tobacco use | 16.89% (30,439) | 14.77% (326,026) | 6 | Noninvasive mechanical ventilation | 1.3% (2,345) | 0.58% (12,899) |
| Acute pancreatitis | 1.23% (2,212) | 0.73% (16,168) | 13 | Coronary atherosclerosis native vessel | 16.12% (29,040) | 12.88% (284,487) | 7 | Small intestine endoscopy NEC | 1.26% (2.265) | 0.7% (15,480) |
| Acute and chronic diastolic heart failure | 1.22% (2,190) | 0.48% (10,600) | 22 | Depressive disorder | 15.42% (27,785) | 10.34% (228,347) | 10 | Heart ultrasound | 1.11% (1,997) | 1.37% (30,161) |
| Complication of kidney transplant | 1.08% (1,944) | 0.42% (9,354) | 28 | Acute kidney failure NOS | 13.8% (24,859) | 9.37%% (206,951) | 12 | Esophagogastroduodenoscopy with closed biopsy | 1.09% (1,963) | 0.8% (17,644) |
Secondary diagnoses were mainly chronic conditions, including hypertension, hyperlipidemia, esophageal reflux, and diabetes mellitus type 2 (Table 1.) Combined, congestive heart failure and diabetes mellitus accounted for 43.3% of the secondary diagnoses of admissions of frequently admitted patients, but for only 28.6% of other patients. Acute kidney failure was more common in frequently admitted patients (13.8% vs 9.4% [P<0.001]). Psychiatric disorders accounted for <1% of primary diagnoses for both frequently admitted patients and all other patients. As a secondary diagnosis, depressive disorder appeared in the top 10 for both groups, although more commonly for frequently admitted patients (15.4% vs 10.3% [P<0.001]).
The most commonly performed principal procedures are also shown in Table 1. These include hemodialysis (6.32%) and packed cell transfusion (4.49%), nonoperating room procedures associated with chronic medical conditions.
Comorbidities were compared using the AHRQ comorbidity software.[18, 19] Comorbid conditions were counted once per patient, regardless of the number of admissions in which the condition was coded. Frequently admitted patients have a significantly higher mean number of comorbidities: 7.1 compared to 2.5 for all other patients (P<0.001; Figure 2). In an additional analysis using the Elixhauser comorbidity measures to determine acuity scores, the mean scores were 13.1 for frequently admitted patients and 3.17 for all others (P<0.001). The most common comorbidities were hypertension (74%), fluid and electrolyte disorders (73%), and deficiency anemias (66%). The only behavioral health comorbidity that affected more than a quarter of frequently admitted patients was depression (40% as compared to 13% for all others).

Additionally, frequently admitted patients were significantly more likely to have comorbidities of psychosis (18% vs 5% [P<0.001]), alcohol abuse (16% vs 7% [P<0.001]), and drug abuse (20% vs 7% [P<0.001]). Among hospitals, these comorbidities ranged widely: psychosis (3% 48%); alcohol abuse (3%46%); and drug abuse (3%58%). Hospitals with the highest rates (top decile) of frequently admitted patients with alcohol and drug abuse comorbidities were more likely to be safety net hospitals than those in the lowest decile (P<0.05 for each independently), but no such difference was found regarding rates of patients with psychosis.
Although the frequently admitted patient population accounted for only 1.6% of patients, they accounted for an average of 8.4% of all bed days and 7.1% of direct cost. The average cost per day was $1746, compared to $2144 for all other patients (Table 2).
| Length of Stay, Days | Direct Cost | % Total Bed Days | Cost/Day | All Other Patients Cost/Day | Difference | |
|---|---|---|---|---|---|---|
| Minimum | 1.0 | 2.3% | 3.2% | $809 | $1,005 | $(196) |
| Maximum | 86.8 | 14.1% | 15.4% | $3,208 | $4,070 | $(862) |
| Mean | 6.7 | 7.1% | 8.4% | $1,746 | $2,144 | $(398) |
| Median | 5.5 | 7.0% | 8.3% | $1,703 | $2,112 | $(410) |
DISCUSSION
An extensive analysis of the characteristics of frequently admitted patients at 101 US academic medical centers, from October 1, 2011 to September 30, 2012, revealed that these patients comprised 1.6% of all patients, but accounted for 8% of all admissions and 7% of direct costs. Relative to all other hospitalized patients, frequently admitted patients were likely to be younger, of lower socioeconomic status, in poorer health, and more often affected by mental health or substance abuse conditions that may mediate their health behaviors. However, the prevalence of patients with psychiatric or behavior conditions varied widely among hospitals, and hospitals with the highest rates of patients with substance abuse comorbidities were more likely to be safety net hospitals. Frequently admitted patients' diagnoses and procedures suggest that their admissions were related to complex chronic diseases; more than three‐quarters were admitted to medicine services, and their average length of stay was nearly 7 days. No single primary diagnosis accounted for a predominant share of their admissions; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4%. The cost of their care was lower than that of other patients, reflecting the preponderance of their admissions to medicine service lines.
In many ways, frequently admitted patients seem similar to frequent ED visitors. Their visits were driven by a high disease burden associated with multiple chronic conditions, and they were heavy users of the healthcare system overall.[4, 6] The majority of both groups were insured, although there was over‐representation of public insurance.[6] As with frequent ED users, some frequently admitted patients are affected by psychiatric morbidity and substance abuse.[4, 12]
Our results in some ways confirmed, and in some ways differed from, findings of prior studies of patients with frequent hospital admissions. Although each study performed to date has defined the population differently, comparison of findings is useful. Our population was similar to the high‐admission groups identified by Nguyen et al. (patients responsible for the top decile of admissions).[13] These patients were also predominantly admitted for medical conditions, with common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions. However, the median length of stay (3 days for the high‐admission/low‐cost group and 5 days for the high‐admission/high‐cost group) was lower than that of our population (5.5 days).
Hwa, who studied 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco,[14] found that 55% of frequently admitted patients had a psychiatric diagnosis, higher than our patient population. Our findings are similar to those of Boonyasai et al.[15] whose serial readmitters had admissions resulting from disease progression, psychiatric illness, and substance abuse.
Our more nationally representative analysis documented a wide range of patient volumes and clinical characteristics, including psychiatric and substance abuse comorbidities, across study hospitals. It demonstrates that different approachesand resourcesare needed to meet the needs of these varied groups of patients. Each hospital must identify, evaluate, and understand its own population of frequently admitted patients to create well‐informed solutions to prevent repeat hospitalization for these patients.
Our ability to create a distinctive picture of the population of frequently admitted patients in US academic medical centers is based on access to an expansive dataset that captures complete diagnostic and demographic information on the universe of patients admitted to our member hospitals. The availability of clinical and administrative data for the entire population of patients permits both an accurate description of patient characteristics and a standardized comparison of groups. All data conform to accepted formats and definitions; their validity is universally recognized by contributing database participants.
Limitations
There are several important limitations to our study. First, patients with 5 or more admissions in 1 year may be undercounted. The UHC Clinical Data Base/Resource Manager only captures readmissions to a single facility; admissions of any patient admitted to more than 1 hospital, even within the UHC membership, cannot be determined. This could have a particularly strong effect on our ability to detect admissions of patients with acute episodes related to psychiatric illness or substance abuse, as they may be more likely to present to multiple or specialty hospitals. Additionally, readmission rates vary among UHC‐member hospitals, based to some extent on geography and the availability of alternative settings of care.
It is possible that surveillance bias played a role in our finding that frequently admitted patients have a significantly higher mean number of comorbidities; each admission presents an opportunity to document additional comorbid conditions. Psychiatric conditions may be underdocumented in medical settings in academic medical centers, where the focus is often on acute medical conditions. Additionally, certain data elements that we believe are central to understanding the characteristics of frequently admitted patients are not part of the UHC Clinical Data Base/Resource Manager and were therefore not a part of our analysis. These highly influential upstream determinants of health include documentation of a primary care physician, housing status, and access to services at discharge.
CONCLUSION
The valuable information reported from analysis of nearly 2 million patients in the UHC Clinical Data Base/Resource Manager can be used to better understand the characteristics of frequently admitted patients. This important cohort of individuals has complex care needs that often result in hospitalization, but may be amenable to solutions that allow patients to remain in their communities. By understanding the demographic, social, and medical characteristics of these patients, hospitals can develop and implement solutions that address the needs of this small group of patients who consume a highly disproportionate share of healthcare resources.
Acknowledgements
The authors acknowledge the contributions of Samuel F. Hohmann, PhD, and Ryan Carroll, MBA, who provided expert statistical analyses and generous assistance in the completion of this article.
Disclosure: Nothing to report.
The national healthcare improvement paradigm is shifting toward a more comprehensive, value‐focused, and patient‐centered approach. Reducing hospital readmissions has become a focal point as a policy strategy to improve care quality while reducing cost. Section 3025 of the Affordable Care Act mandated the Centers for Medicare and Medicaid Services to make progressive reductions in Medicare payments to hospitals that have higher than expected readmission rates for 3 conditions (heart failure, acute myocardial infarction, and pneumonia), and expanding to include chronic obstructive pulmonary disease and total hip and knee arthroplasty in 2015.[1] In response, hospitals and systems are developing and implementing programs that coordinate care beyond hospital walls to reduce readmissions and healthcare costs.[2, 3] However, patients are readmitted for a variety of reasons, and programs that address the needs of some may not address the distinct needs of others. Understanding the characteristics of patients with frequent readmissions will permit the well‐informed creation of solutions specific to this population to reduce cost, free resources, and provide better care.
Although a solid body of literature already exists that describes the characteristics of patients who frequently visit the emergency department (ED),[4, 5, 6, 7, 8, 9, 10, 11, 12] it is not clear to what extent these characteristics also apply to patients with frequent hospital admissions. Frequent ED visitors have been found to be largely insured (85%) although with over‐representation of public insurance, and to be heavy users of the healthcare system overall.[6] A high disease burden associated with multiple chronic conditions has been found to predict frequent ED use.[4, 9, 11, 12] Some characteristics may vary by location; for example, alcohol abuse and psychiatric morbidity have been found to be associated with frequent ED use in New York and San Francisco, but it is not clear to what extent they are a factor in less urban areas.[4, 6, 12]
Several previous studies have investigated the characteristics of frequently admitted patients at single sites.[13, 14, 15, 16] Nguyen et al. (2013) studied patients with the highest costs and the most admissions at a large academic medical center in San Francisco.[13] High admit patients were defined as those responsible for the top decile of admissions, and were grouped into equal‐sized high‐ and low‐cost cohorts. The high‐admission/high‐cost group represented 5% of all patients, 25% of all costs, and 16% of all admissions. These patients were hospitalized primarily for medical conditions (78%) and had a high 30‐day readmission rate (47%). The high‐admission/low‐cost group accounted for 5% of all patients, 12% of all admissions, and 7% of all costs. These patients were also predominantly admitted for medical conditions (87%), with the most common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions.[13]
Hwa (2012) conducted an analysis of 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco.[14] These patients represented just 1% of all patients, but 13% of readmissions. Fifty‐five percent of these patients had a psychiatric diagnosis, and 52% had chronic pain. Ninety percent had a primary care physician in the hospital system, 100% were insured either privately or publicly, and 93% had housing, although for 17% housing was described as marginal.[14]
In a third study, Boonyasai et al. (2012) identified 76 patients with 82 readmissions at a Baltimore, Maryland, hospital and classified them as isolated (1 readmission per 6‐month period) or serial (more than 1 readmission per 6‐month period) readmissions.[15] Patients with serial readmissions accounted for 70% of the total. Isolated readmissions were most likely to be related to suboptimal quality of care and care coordination, whereas serial readmissions were more likely to result from disease progression, psychiatric illness, and substance abuse.[15]
All of these studies were conducted at single‐site academic medical centers serving inner city populations. We undertook this study to identify patient and hospital‐level characteristics of frequently admitted patients in a broad sample of 101 US academic medical centers to determine whether previously reported findings are generalizable, and to identify characteristics of frequently admitted patients that can inform interventions designed to meet the needs of this relatively small but resource‐intensive group of patients.
METHODS
All data were obtained from the University HealthSystem Consortium (UHC) (Chicago, IL) Clinical Data Base/Resource Manager (CDB), a large administrative database to which UHC principal members submit comprehensive administrative data files. UHC's principal members include approximately 120 US academic medical centers delivering tertiary and quaternary care, with an average of 647 acute care beds. The CDB includes primary and secondary diagnoses using International Classification of Diseases, Ninth Revision (ICD‐9)[17] codes.
The data of 101 academic medical centers with complete datasets for the study period (October 1, 2011, to September 30, 2012) were included in this analysis. Frequently admitted patients were defined as patients admitted 5 or more times to the same facility in a 12‐month period; all admissions were included, even those more than 30 days apart. This definition was established based on a naturally occurring break in the frequency distribution (Figure 1) and our intention to focus on the unique characteristics of patients at the far right of the distribution. We excluded obstetric (MDC 14, ICD‐9)[17] admissions and pediatric (<18 years of age at index admission) patients, as well as admissions with principal diagnoses for chemotherapy (ICD‐9 diagnosis codes v5811v5812), dialysis (ICD‐9 diagnosis codes v560v568), and rehabilitation (ICD‐9 diagnosis codes v570v579), which are typically planned. The Agency for Healthcare Research and Quality (AHRQ) comorbidity software was used to identify comorbid conditions,[18, 19] and a score based on the Elixhauser comorbidity measures was calculated using a modified acuity point system.[20] For comparisons based on safety net status, we used a definition of payer mix being 25% Medicaid or uninsured.

Our analyses included patient demographics, admission source and discharge status, clinical diagnoses, procedures, and comorbidities, cost, and length of stay. Patients defined as frequently admitted were compared in aggregate to all other hospitalized patients (all other admissions).
To evaluate associations, we used [2] tests for categorical variables and t tests for continuous variables. When comparing the non‐normally distributed comorbidities of the control group to the normally distributed comorbidities of the frequently admitted patients, we performed a Kruskal‐Wallis test on the medians.
RESULTS
During a 1‐year period (October 1, 2011, to September 30, 2012), 1,758,027 patients were admitted 2,388,124 times at 101 academic medical centers. Of these, 28,291 patients had 5 or more admissions during this period, resulting in 180,185 admissions. These frequently admitted patients represented 1.6% of all patients (Figure 1) and 7.6% of all inpatient admissions. By comparison, nonfrequently admitted patients were admitted once (79%), twice (14%), 3 times (4%), or 4 times (2%).
Among hospitals, the volume and impact of frequently admitted patients varied widely. The frequently admitted patient population ranged from 64 patients (0.7% of all patients) to 785 patients (3.5%), with an average of 280 patients (1.6%). To look for differences that might explain this range, we compared hospitals in the top and bottom deciles with respect to geographic region and to safety net status, but found no significant or meaningful differences. The average number of admissions per patient was 6.4, with a range of 5 to 76. Days per patient ranged from 5 to 434 days, with an average of 42. The average patient‐day percentage (frequently admitted patient days/total patient days) was 8.4%, and ranged from 3.2% to 15.4%.
Frequently admitted patients were more likely to be younger than all other patients (71.9% under the age of 65 years, as compared with 65.3% of all other patients (P<0.001)). They were also more likely to have either Medicaid or no healthcare insurance (27.6% compared with 21.6%, P<0.001), although nearly three‐quarters had either private insurance or Medicare coverage.
Eighty‐four percent of frequently admitted patient admissions were to medical services (vs 58% of all other patients (P<0.001)). The admission status for these patients was much less likely to be elective (9.1% of frequently admitted patient admissions vs 26.6% of all other patients' admissions [P<0.001]). Frequently admitted patients were more likely to be discharged to a skilled nursing facility (9.3% vs 8.4%, [P<0.001]) or with home health services (19.7% vs 13.4% [P<0.001]).
The 10 most common primary diagnoses for patient admissions are shown in Table 1. No single primary diagnosis accounted for a large share of the admissions of these patients; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4% of admissions. The 10 most common diagnoses accounted for <20% of all admissions. The remainder of the diagnoses was spread over more than 3000 diagnosis codes; only about 300 codes had more than 100 admissions each.
| Primary Diagnoses | Secondary Diagnoses | Principal Procedures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other Patient Rank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | All Other PatientRank | Frequently Admitted Patient Admissions, N=180,185 | All Other Patient Admissions, N=2,207,939 | |||
| ||||||||||
| Sickle cell disease with crisis | 3.97% (7,152) | 0.002% (5,887) | 63 | Hypertension NOS | 31.39% (56,556) | 40.04% (884,045) | 1 | Hemodialysis | 6.32% (11,380) | 1.08% (23,871) |
| Septicemia NOS | 2.58% (4,652) | 1.87% (41,369) | 1 | Hyperlipidemia NOS | 24.47% (44,089) | 25.94% (572,760) | 2 | Packed cell transfusion | 4.49% (8.091) | 1.57% (34,669) |
| Acute and chronic systolic heart failure | 2.06% (3,708) | 0.81% (17,802) | 12 | Congestive heart failure NOS | 22.86% (41,197) | 11.82% (260,944) | 8 | Percutaneous abdominal drainage | 2.42% (4,366) | 0.86% (18,974) |
| Acute kidney failure NOS | 2.04% (3,680) | 1.16% (25,528) | 6 | Esophageal reflux | 21.19% (38,184) | 17.32% (382,511) | 3 | Venous catheter NEC | 2.13% (3,843) | 0.89% (19,718) |
| Obstructive chronic bronchitis with exacerbation | 1.76% (3,180) | 0.68% (14,957) | 14 | Diabetes mellitus NOS uncomplicated | 20.39% (36,743) | 16.75% (369,808) | 4 | Central venous catheter placement with guidewire | 2.13% (3,834)) | 0.83% (18,307) |
| Pneumonia organism NOS | 1.72% (3,091) | 1.29% (28,468) | 4 | Tobacco use disorder | 16.98% (30,604) | 16.71% (368,880) | 5 | Continuous invasive mechanical ventilation <96 consecutive hours | 1.38% (2,480) | 0.7% (15,441) |
| Urinary tract infection NOS | 1.63% (2,939) | 0.86% (19,069) | 9 | History of tobacco use | 16.89% (30,439) | 14.77% (326,026) | 6 | Noninvasive mechanical ventilation | 1.3% (2,345) | 0.58% (12,899) |
| Acute pancreatitis | 1.23% (2,212) | 0.73% (16,168) | 13 | Coronary atherosclerosis native vessel | 16.12% (29,040) | 12.88% (284,487) | 7 | Small intestine endoscopy NEC | 1.26% (2.265) | 0.7% (15,480) |
| Acute and chronic diastolic heart failure | 1.22% (2,190) | 0.48% (10,600) | 22 | Depressive disorder | 15.42% (27,785) | 10.34% (228,347) | 10 | Heart ultrasound | 1.11% (1,997) | 1.37% (30,161) |
| Complication of kidney transplant | 1.08% (1,944) | 0.42% (9,354) | 28 | Acute kidney failure NOS | 13.8% (24,859) | 9.37%% (206,951) | 12 | Esophagogastroduodenoscopy with closed biopsy | 1.09% (1,963) | 0.8% (17,644) |
Secondary diagnoses were mainly chronic conditions, including hypertension, hyperlipidemia, esophageal reflux, and diabetes mellitus type 2 (Table 1.) Combined, congestive heart failure and diabetes mellitus accounted for 43.3% of the secondary diagnoses of admissions of frequently admitted patients, but for only 28.6% of other patients. Acute kidney failure was more common in frequently admitted patients (13.8% vs 9.4% [P<0.001]). Psychiatric disorders accounted for <1% of primary diagnoses for both frequently admitted patients and all other patients. As a secondary diagnosis, depressive disorder appeared in the top 10 for both groups, although more commonly for frequently admitted patients (15.4% vs 10.3% [P<0.001]).
The most commonly performed principal procedures are also shown in Table 1. These include hemodialysis (6.32%) and packed cell transfusion (4.49%), nonoperating room procedures associated with chronic medical conditions.
Comorbidities were compared using the AHRQ comorbidity software.[18, 19] Comorbid conditions were counted once per patient, regardless of the number of admissions in which the condition was coded. Frequently admitted patients have a significantly higher mean number of comorbidities: 7.1 compared to 2.5 for all other patients (P<0.001; Figure 2). In an additional analysis using the Elixhauser comorbidity measures to determine acuity scores, the mean scores were 13.1 for frequently admitted patients and 3.17 for all others (P<0.001). The most common comorbidities were hypertension (74%), fluid and electrolyte disorders (73%), and deficiency anemias (66%). The only behavioral health comorbidity that affected more than a quarter of frequently admitted patients was depression (40% as compared to 13% for all others).

Additionally, frequently admitted patients were significantly more likely to have comorbidities of psychosis (18% vs 5% [P<0.001]), alcohol abuse (16% vs 7% [P<0.001]), and drug abuse (20% vs 7% [P<0.001]). Among hospitals, these comorbidities ranged widely: psychosis (3% 48%); alcohol abuse (3%46%); and drug abuse (3%58%). Hospitals with the highest rates (top decile) of frequently admitted patients with alcohol and drug abuse comorbidities were more likely to be safety net hospitals than those in the lowest decile (P<0.05 for each independently), but no such difference was found regarding rates of patients with psychosis.
Although the frequently admitted patient population accounted for only 1.6% of patients, they accounted for an average of 8.4% of all bed days and 7.1% of direct cost. The average cost per day was $1746, compared to $2144 for all other patients (Table 2).
| Length of Stay, Days | Direct Cost | % Total Bed Days | Cost/Day | All Other Patients Cost/Day | Difference | |
|---|---|---|---|---|---|---|
| Minimum | 1.0 | 2.3% | 3.2% | $809 | $1,005 | $(196) |
| Maximum | 86.8 | 14.1% | 15.4% | $3,208 | $4,070 | $(862) |
| Mean | 6.7 | 7.1% | 8.4% | $1,746 | $2,144 | $(398) |
| Median | 5.5 | 7.0% | 8.3% | $1,703 | $2,112 | $(410) |
DISCUSSION
An extensive analysis of the characteristics of frequently admitted patients at 101 US academic medical centers, from October 1, 2011 to September 30, 2012, revealed that these patients comprised 1.6% of all patients, but accounted for 8% of all admissions and 7% of direct costs. Relative to all other hospitalized patients, frequently admitted patients were likely to be younger, of lower socioeconomic status, in poorer health, and more often affected by mental health or substance abuse conditions that may mediate their health behaviors. However, the prevalence of patients with psychiatric or behavior conditions varied widely among hospitals, and hospitals with the highest rates of patients with substance abuse comorbidities were more likely to be safety net hospitals. Frequently admitted patients' diagnoses and procedures suggest that their admissions were related to complex chronic diseases; more than three‐quarters were admitted to medicine services, and their average length of stay was nearly 7 days. No single primary diagnosis accounted for a predominant share of their admissions; the most common diagnosis, sickle cell disease with crisis, accounted for only about 4%. The cost of their care was lower than that of other patients, reflecting the preponderance of their admissions to medicine service lines.
In many ways, frequently admitted patients seem similar to frequent ED visitors. Their visits were driven by a high disease burden associated with multiple chronic conditions, and they were heavy users of the healthcare system overall.[4, 6] The majority of both groups were insured, although there was over‐representation of public insurance.[6] As with frequent ED users, some frequently admitted patients are affected by psychiatric morbidity and substance abuse.[4, 12]
Our results in some ways confirmed, and in some ways differed from, findings of prior studies of patients with frequent hospital admissions. Although each study performed to date has defined the population differently, comparison of findings is useful. Our population was similar to the high‐admission groups identified by Nguyen et al. (patients responsible for the top decile of admissions).[13] These patients were also predominantly admitted for medical conditions, with common admitting diagnoses representing respiratory, gastrointestinal, and cardiovascular conditions. However, the median length of stay (3 days for the high‐admission/low‐cost group and 5 days for the high‐admission/high‐cost group) was lower than that of our population (5.5 days).
Hwa, who studied 29 patients admitted 6 or more times in 1 year to an inpatient medical service in San Francisco,[14] found that 55% of frequently admitted patients had a psychiatric diagnosis, higher than our patient population. Our findings are similar to those of Boonyasai et al.[15] whose serial readmitters had admissions resulting from disease progression, psychiatric illness, and substance abuse.
Our more nationally representative analysis documented a wide range of patient volumes and clinical characteristics, including psychiatric and substance abuse comorbidities, across study hospitals. It demonstrates that different approachesand resourcesare needed to meet the needs of these varied groups of patients. Each hospital must identify, evaluate, and understand its own population of frequently admitted patients to create well‐informed solutions to prevent repeat hospitalization for these patients.
Our ability to create a distinctive picture of the population of frequently admitted patients in US academic medical centers is based on access to an expansive dataset that captures complete diagnostic and demographic information on the universe of patients admitted to our member hospitals. The availability of clinical and administrative data for the entire population of patients permits both an accurate description of patient characteristics and a standardized comparison of groups. All data conform to accepted formats and definitions; their validity is universally recognized by contributing database participants.
Limitations
There are several important limitations to our study. First, patients with 5 or more admissions in 1 year may be undercounted. The UHC Clinical Data Base/Resource Manager only captures readmissions to a single facility; admissions of any patient admitted to more than 1 hospital, even within the UHC membership, cannot be determined. This could have a particularly strong effect on our ability to detect admissions of patients with acute episodes related to psychiatric illness or substance abuse, as they may be more likely to present to multiple or specialty hospitals. Additionally, readmission rates vary among UHC‐member hospitals, based to some extent on geography and the availability of alternative settings of care.
It is possible that surveillance bias played a role in our finding that frequently admitted patients have a significantly higher mean number of comorbidities; each admission presents an opportunity to document additional comorbid conditions. Psychiatric conditions may be underdocumented in medical settings in academic medical centers, where the focus is often on acute medical conditions. Additionally, certain data elements that we believe are central to understanding the characteristics of frequently admitted patients are not part of the UHC Clinical Data Base/Resource Manager and were therefore not a part of our analysis. These highly influential upstream determinants of health include documentation of a primary care physician, housing status, and access to services at discharge.
CONCLUSION
The valuable information reported from analysis of nearly 2 million patients in the UHC Clinical Data Base/Resource Manager can be used to better understand the characteristics of frequently admitted patients. This important cohort of individuals has complex care needs that often result in hospitalization, but may be amenable to solutions that allow patients to remain in their communities. By understanding the demographic, social, and medical characteristics of these patients, hospitals can develop and implement solutions that address the needs of this small group of patients who consume a highly disproportionate share of healthcare resources.
Acknowledgements
The authors acknowledge the contributions of Samuel F. Hohmann, PhD, and Ryan Carroll, MBA, who provided expert statistical analyses and generous assistance in the completion of this article.
Disclosure: Nothing to report.
- Centers for Medicare 21(9):117–120.
- , , , . The influence of a postdischarge intervention on reducing hospital readmissions in a Medicare population. Popul Health Manag. 2013;16(5):310–316.
- , . Dispelling an urban legend: frequent emergency department users have substantial burden of disease. Health Aff (Millwood). 2013;32:2099–2108.
- , , , et al. Effectiveness of interventions targeting frequent users of emergency departments: a systematic review. Ann Emerg Med. 2011;58:41–52.
- , . Frequent users of emergency departments: the myths, the data, and the policy implications. Ann Emerg Med. 2010;20(10):1–8.
- , , , . Development and validation of a model for predicting emergency admissions over the next year. Arch Intern Med. 2008;168:1416–1422.
- , , , et al. A comparison of frequent and infrequent visitors to an urban emergency department. J Emerg Med. 2008;38:115–121.
- , . Frequent users of Massachusetts emergency departments: a statewide analysis. Ann Emerg Med. 2006;48:9–16.
- , , , et al. A descriptive study of heavy emergency department users at an academic emergency department reveals heavy users have better access to care than average users. J Emerg Nurs. 2005;31:139–144.
- , , . Predictors and outcomes of frequent emergency department users. Acad Emerg Med. 2003;10:320–328.
- , , . Epidemiologic analysis of an urban, public emergency department's frequent users. Acad Emerg Med. 2000;7:637–646.
- , , , . What's cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J Hosp Med. 2013;8:665–671.
- . Characteristics of a frequently readmitted patient population on an inpatient medical service. Abstract presented at: Society of Hospital Medicine Annual Meeting, April 1– 4, 2012; San Diego, CA.
- , , , , . Characteristics of isolated and serial rehospitalizations suggest a need for different types of improvement strategies [abstract] J Hosp Med. 2012;7(suppl 2):513.
- , , , , . An intervention to improve care and reduce costs for high‐risk patients with frequent hospital admissions: a pilot study. BMC Health Serv Res. 2011;11:270–279.
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD‐9). Available at: http://www.cdc.gov/nchs/icd/icd9.htm. Accessed February 18, 2015.
- Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed February 18, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633.
- Centers for Medicare 21(9):117–120.
- , , , . The influence of a postdischarge intervention on reducing hospital readmissions in a Medicare population. Popul Health Manag. 2013;16(5):310–316.
- , . Dispelling an urban legend: frequent emergency department users have substantial burden of disease. Health Aff (Millwood). 2013;32:2099–2108.
- , , , et al. Effectiveness of interventions targeting frequent users of emergency departments: a systematic review. Ann Emerg Med. 2011;58:41–52.
- , . Frequent users of emergency departments: the myths, the data, and the policy implications. Ann Emerg Med. 2010;20(10):1–8.
- , , , . Development and validation of a model for predicting emergency admissions over the next year. Arch Intern Med. 2008;168:1416–1422.
- , , , et al. A comparison of frequent and infrequent visitors to an urban emergency department. J Emerg Med. 2008;38:115–121.
- , . Frequent users of Massachusetts emergency departments: a statewide analysis. Ann Emerg Med. 2006;48:9–16.
- , , , et al. A descriptive study of heavy emergency department users at an academic emergency department reveals heavy users have better access to care than average users. J Emerg Nurs. 2005;31:139–144.
- , , . Predictors and outcomes of frequent emergency department users. Acad Emerg Med. 2003;10:320–328.
- , , . Epidemiologic analysis of an urban, public emergency department's frequent users. Acad Emerg Med. 2000;7:637–646.
- , , , . What's cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J Hosp Med. 2013;8:665–671.
- . Characteristics of a frequently readmitted patient population on an inpatient medical service. Abstract presented at: Society of Hospital Medicine Annual Meeting, April 1– 4, 2012; San Diego, CA.
- , , , , . Characteristics of isolated and serial rehospitalizations suggest a need for different types of improvement strategies [abstract] J Hosp Med. 2012;7(suppl 2):513.
- , , , , . An intervention to improve care and reduce costs for high‐risk patients with frequent hospital admissions: a pilot study. BMC Health Serv Res. 2011;11:270–279.
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD‐9). Available at: http://www.cdc.gov/nchs/icd/icd9.htm. Accessed February 18, 2015.
- Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. Comorbidity software, version 3.7. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed February 18, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633.
© 2015 The Authors Journal of Hospital Medicine published by Wiley Periodicals, Inc. on behalf of Society of Hospital Medicine
CKD Awareness in Hospitalized Patients
Chronic kidney disease (CKD) affects over 13% of the US population and is associated with increased morbidity, mortality, and healthcare costs.[1] However, only 10% of individuals with CKD are aware of their diagnoses.[2] Even in those with stage 5 CKD, only 60% of individuals are aware of their CKD.[3] To our knowledge, no work has examined CKD awareness in a hospitalized patient population.
Patient awareness of their CKD diagnosis is important because progression of kidney disease can be slowed by patient self‐management of diabetes and hypertension.[4] Patient awareness of CKD may also increase acceptance of preend‐stage renal disease (ESRD) patient education and nephrology referral, which have been shown to delay CKD progression and improve clinical status at dialysis initiation.[5] However, only 60% of patients with advanced CKD have visited a nephrologist in the past year or have seen a nephrologist prior to dialysis initiation.[1]
The hospital is an important site for patient education and linkage to outpatient care for patients with CKD.[6] The hospital serves high‐risk patients who may not be well connected to outpatient care or who have lesswell‐controlled disease.[6, 7] Thus, hospitalization represents an opportunity to identify existing CKD and to use a multidisciplinary approach to preventative care, patient education, and patient‐provider planning for renal replacement therapy needs. In our cross‐sectional study in an urban, minority‐serving hospital, we sought to determine what patient factors were associated with hospitalized patients correctly self‐identifying as having CKD.
METHODS
Subjects and Data
We used data from the University of Chicago Hospitalist Project, a study of hospitalized patient outcomes.[8] Within 48 hours of hospitalization, all general medicine patients or their proxies are approached to enroll. During one‐on‐one inpatient interviews, a trained research assistant obtains demographic, health status, and healthcare utilization information. Participants consent for study staff to review their medical records. More than 80% of general medicine patients enroll.
We obtained data on 1234 general medicine patients discharged between January 1, 2012 and March 31, 2013 with an International Classification of Diseases, Ninth Revision (ICD‐9) code for chronic kidney disease (ICD‐9 codes 585.0585.5, 585.9) in their first 20 admission diagnoses. These codes are highly specific for CKD but have lower sensitivity.[9] We excluded all patients with a history of transplant (996.81, V42.0, n=90) or ESRD (585.6, n=416). We excluded repeat admissions during the study period (n=138). Our final sample included 590 unique patients with ICD‐9 diagnosis of CKD without ESRD.
Demographic, Clinical, and Health Service Utilization Characteristics
Our outcome was CKD awareness, the patient's correct self‐report of kidney disease. Patients selected their chronic medical conditions from a list read to them and were specifically asked if they had kidney problems. Demographic characteristics including age, gender, race/ethnicity, marital status, and education were also obtained. Healthcare utilization variables included how often the patient saw their primary medical care provider in the past year and whether patients had a prior hospitalization in the last year.
Health status variables such as mental status, diabetes, hypertension, and CKD stage were also assessed. Mental status was quantified using the telephone version of the Mini‐Mental State Examination (MMSE), scored from 1 to 22.10 We defined diabetes as ICD‐9 codes 250.0250.00, and hypertension as ICD‐9 codes 401.0, 401.9, 403, 405.09, 405.19, 405.91, 405.99, or by patient self‐report. CKD stage was based on the estimated glomerular filtration rate (eGFR) from the medical record using Kidney Disease Outcomes Quality Initiative guidelines.[11] We used the mode of the eGFR to calculate the appropriate CKD stage for those with more than 1 eGFR value from the hospitalization (576/590, 98%). The eGFR was calculated by the modified Modification of Diet in Renal Disease equation: (GFR (mL/min/1.73 m2)=175 (Screatinine)1.154 (Age)0.203 (0.742 if female) x (1.212 if African American)) recommended by the National Kidney Disease Education Program.[12]
Analysis
We used logistic regression to analyze the influence of the demographic, clinical, and healthcare utilization covariates on the likelihood of a patient reporting kidney problems. For the multivariate analysis, we sequentially added variables in a step‐wise fashion. We adjusted for (1) demographic factors: gender, race, ethnicity, marital status, and education; (2) eGFR‐calculated CKD stage and comorbidities: diabetes, hypertension, and mental status; and (3) healthcare utilization in the last 12 months: any hospitalizations and the number of visits to a health provider.
RESULTS
Patient Characteristics and Bivariable Association With Patient CKD Self‐Report
Table 1 shows demographic, clinical, and health service use characteristics for 590 patients with ICD‐9 coded CKD. In the bivariable model in Table 1, age, race, marital status and comorbidities, were associated with patient self‐report of CKD. Patients older than 80 years with physician‐identified CKD through ICD‐9 coding had 57% lower odds of reporting CKD than their younger counterparts. Patients of other races (nonwhite, non‐African American), married patients, and those with CKD stages 4 and 5 were much more likely to correctly self‐report CKD. Patients with higher MMSE score, diabetes, or hypertension had greater odds of CKD self‐report (all P0.05).
| Total, N=590 | Bivariable Odds Ratio (95% CI) | Multivariable With Stepwise Addition (95% CI) | |||
|---|---|---|---|---|---|
| Model 1, Demographic Factors | Model 2, Plus CKD Stage and Other Comorbidities | Model 3, Plus Health Service Use | |||
| |||||
| Female | 312 (52.98%) | 1.21 (0.85‐1.70) | 1.64 (1.09‐2.47)* | 1.28 (0.79‐2.06) | 1.36 (0.75‐2.48) |
| Age, y | |||||
| Below 54 | 138 (23.4%) | REF | REF | REF | REF |
| 5466 | 150 (24.4%) | 0.96 (0.59‐1.54) | 0.95 (0.56‐1.59) | 1.01 (0.56‐1.81) | 0.83 (0.41‐1.68) |
| 6779 | 161 (27.3%) | 0.77 (0.48‐1.23) | 0.67 (0.39‐1.15) | 0.66 (0.35‐1.23) | 0.72(0.34‐1.52) |
| Above 80 | 141 (23.9%) | 0.43 (0.26‐0.74) | 0.32 (0.17‐0.60) | 0.42 (0.20‐0.91)* | 0.37 (0.15‐0.93)* |
| Race | |||||
| African American | 449 (82.1%) | REF | REF | REF | REF |
| White | 75 (13.7%) | 1.47 (0.89‐2.42) | 1.53 (0.86‐2.71) | 1.56 (0.78‐3.12) | 1.08 (0.47‐2.49) |
| Other | 23 (4.2%) | 3.62 (1.53‐8.54) | 5.29 (1.78‐15.73) | 6.19 (1.72‐22.29) | 11.63 (1.80‐75.21)* |
| Ethnicity (Hispanic is reference group) | 21 (4.0%) | 0.97 (0.38‐2.44) | 0.43 (0.13‐1.45) | 0.71 (0.16‐3.09) | 0.46 (0.06‐3.57) |
| Married | 171 (32.6%) | 1.49 (1.03‐2.18)* | 1.32 (0.85‐2.04) | 1.20 (0.73‐1.98) | 1.77 (0.94‐3.33) |
| Education | |||||
| Less than high school | 128 (25.2%) | REF | REF | REF | REF |
| High school grad/some college | 296 (58.2%) | 1.05 (0.68‐1.63) | 0.93 (0.58‐1.49) | 0.95 (0.54‐1.67) | 0.85 (0.42‐1.72) |
| College grad or higher | 85 (16.7%) | 1.13 (0.64‐2.01) | 0.96 (0.51‐1.78) | 1.07 (0.51‐2.23) | 0.69 (0.27‐1.77) |
| CKD stage (eGFR calculated, mode) | |||||
| 12 | 115 (20.0%) | 0.55 (0.32‐0.95)* | 0.52 (0.27‐1.01) | 0.28 (0.11‐0.69) | |
| 3 | 300 (52.1%) | REF | REF | REF | |
| 4 | 112 (19.4%) | 2.43 (1.55‐3.81) | 2.69 (1.52‐4.76) | 3.07 (1.56‐6.07) | |
| 5 | 49 (8.5%) | 4.50 (2.39‐8.48) | 3.94 (1.81‐8.56) | 5.16 (1.85‐14.41) | |
| Diabetes (ICD‐9 coded or self‐report)‖ | 292 (49.5%) | 1.52 (1.07‐2.15)* | 1.54 (0.97‐2.45) | 1.26 (0.71‐2.26) | |
| Hypertension (ICD‐9 coded or self‐report) | 436 (73.9%) | 3.36 (2.09‐5.41) | 1.53 (0.80‐2.90) | 1.26 (0.57‐2.81) | |
| Mini‐Mental State Exam score# | 19.7 (2.5) | 1.13 (1.03‐1.23) | 1.09 (0.98‐1.22) | 1.22 (1.06‐1.42) | |
| Hospitalized in last 12 months | 253 (46.9%) | 1.50 (1.05‐2.13)* | 1.12 (0.65‐1.95) | ||
| No. of visits to health provider | |||||
| Once/year or less | 80 (18.4%) | REF | REF | ||
| 23 times/year | 58 (13.3%) | 0.80 (0.40‐1.60) | 0.44 (0.17‐1.16) | ||
| 4+ times/year | 298 (68.4%) | 0.60 (0.36‐0.99)* | 0.38 (0.19‐0.74) | ||
Multivariable Associations With Patient CKD Self‐Report
Age, race, and CKD stage remained consistently associated with CKD self‐report, although the magnitude of the effects (odds ratios [ORs]) varied across models (Table 1). Across all the models, patients older than 80 years were still significantly less likely than younger patients to self‐report CKD (ORs ranging from 0.32 to 0.42, all P0.05). Patients classified as being of other race were found to have a 5.29 to 11.63 greater odds of CKD self‐report than African American patients (all P0.05).
Patients with CKD stage 4 and 5 were more likely to self‐report than patients with CKD stage 3 (ORs ranging from 2.693.07 for stage 4 and 3.945.16 for stage 5, P0.05). In the final model, every unit increase in MMSE score increased the odds of CKD self‐report by 22%. In addition, patients who saw their health provider 4 or more times per year were 62% less likely to self‐report CKD than patients who saw their provider 1 or fewer times per year (OR: 0.38, P0.05).
A large proportion of patients (68.6%) were CKD unspecified by ICD‐9 codes, and only 27% of the unspecified group reported having CKD (Table 2). Examining the eGFR CKD stage of the CKD unspecified group showed that 22.8% were eGFR‐determined CKD stage 1 to 2, 57.1% were CKD stage 3, 14.8% were CKD stage 4, and 5.3% were CKD stage 5. Patients had 2 to 3 times greater odds of correct CKD self‐report if physicians had correctly identified their CKD stage (bivariable OR: 2.42, 95% confidence interval [CI]: 1.57‐3.72, multivariable OR: 3.22, 95% CI: 0.99‐10.46) (analysis not shown).
| CKD Stage* | Physician (ICD‐9) Coded | eGFR Calculated | eGFR Coded With ICD‐9 Correct |
|---|---|---|---|
| |||
| Unspecified | 110 (27.2%) | ||
| 12 | 5 (31.2%) | 20 (17.4%) | 0 |
| 3 | 25 (27.2%) | 83 (27.7%) | 17 (29.8%) |
| 4 | 40 (63.5%) | 54 (48.2%) | 25 (69.4%) |
| 5 | 11 (78.6%) | 31 (63.3%) | 10 (83.3%) |
DISCUSSION
Although prior work has examined CKD awareness in the general population and in high‐risk cohorts,[2, 3, 13] this is the first study examining CKD awareness in an urban, underserved hospitalized population. We found that overall patients' CKD awareness was low (32%), but increased as high as 63% for CKD stage 5, even after controlling for patient demographic, clinical characteristics, and healthcare use. Our overall rate of CKD awareness was higher than prior studies overall and at lower CKD stages.[2, 3, 13] Our work is consistent with prior literature that shows increasing CKD awareness with advancing CKD stage.[2, 3, 13]
Older patients (>80 years) had lower awareness of CKD. Older patients are more likely to have a near normal creatinine, despite a markedly reduced eGFR, so their CKD may go unnoticed.[14] Even with appropriate recognition, providers may also feel like their CKD is unlikely to progress to ESRD, given its stability and/or their competing risk of death.[15] Finally, older hospitalized patients may also be less likely to report a CKD diagnosis due to difficulty in recall due to denial, dementia, or delirium.
One limitation is that our case‐finding for CKD was physician ICD‐9 coding, which is highly specific but not sensitive.[9] The majority of patients with physician‐identified CKD were CKD unspecified, perhaps due to poor coding, physician underdocumentation, or physician under‐recognition of CKD stage. Although only 27% of the CKD unspecified group correctly self‐identified as having CKD, over 75% were found to be CKD stage 3 or higher, which should trigger additional monitoring or care based on guidelines.[10] In addition, despite statistical significance, we may not be able to make meaningful inferences about our small other group (nonwhite, non‐African American). Our sample was from 1 hospitalan urban, academic, tertiary care center with a large proportion of African American patientswhich may limit generalizability. The multivariable model will need to be tested in other populations for reproducibility.
Our study significantly contributes to the literature by examining patient awareness of CKD in a high‐risk, urban, hospitalized minority population. Other study strengths include use of basic demographic information, as well as survey and laboratory data for a richer examination of the associations between patient factors and CKD awareness.
CONCLUSION
Hospitalized patients with CKD have a low CKD awareness. Patient awareness of their CKD is increased with physician documentation of CKD severity. Patient awareness of their CKD must be coupled with provider awareness and CKD documentation to link patients to multidisciplinary CKD education and care to slow CKD progression and reduce associated cardiovascular and metabolic complications. Further work is needed across hospitals to determineand improveCKD awareness among both patients and providers.
Disclosures
Dr. Saunders was supported by Pilot and Feasibility Funding from the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949). Dr. Chin was supported by NIDDK K24 DK071933. Dr. Meltzer was supported by NIA T35 AG029795. Dr. Saunders had full access to all of the study data and takes responsibility for the integrity of the data and accuracy of the data analysis. An abstract of this article was presented at the Society of Hospital Medicine Annual Meeting in Las Vegas, Nevada in March 2014 and at the Society of General Internal Medicine Annual Meeting in San Diego, California in April 2014. The authors report no conflicts of interest.
- United States Renal Data System. Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; 2012.
- , , , et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Neph. 2011;6(8):1838–1844.
- , , , et al. Comparison of CKD awareness in a screening population using the Modification of Diet in Renal Disease (MDRD) Study and CKD Epidemiology Collaboration (CKD‐EPI) equations. Am J Kidney Dis. 2011;57(3 suppl 2):S17–S23.
- , , , et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis. 2000;36(3):646–661.
- , , , et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality—a controlled cohort study based on the NKFDOQI guidelines. Nephrol Dial Transplant. 2009;24(11):3426–3433.
- , , . Pre‐dialysis hospital use and late referrals in incident dialysis patients in England: a retrospective cohort study. Nephrol Dial Transplant. 2015;30(1):124–129.
- , , , et al. Do hospitals that provide heart failure patient education prior to discharge also promote continuity of care? A report from OPTIMIZE‐HF. J Card Fail. 2006;12(6 suppl):S111.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , et al. Failure of ICD‐9‐CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41(2):564–580.
- , , , . Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40(7):697–702.
- National Kidney Foundation. Kidney disease outcomes quality initiative guidelines 2002. Available at: http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/toc.htm. Accessed October 9, 2014.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470.
- , , , et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53(2):238–247.
- , , , , , . Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55(6):816–823.
- , , , et al. Prediction, Progression, and Outcomes of Chronic Kidney Disease in Older Adults. J Am Soc Neph. 2009;20(6):1199–1209.
Chronic kidney disease (CKD) affects over 13% of the US population and is associated with increased morbidity, mortality, and healthcare costs.[1] However, only 10% of individuals with CKD are aware of their diagnoses.[2] Even in those with stage 5 CKD, only 60% of individuals are aware of their CKD.[3] To our knowledge, no work has examined CKD awareness in a hospitalized patient population.
Patient awareness of their CKD diagnosis is important because progression of kidney disease can be slowed by patient self‐management of diabetes and hypertension.[4] Patient awareness of CKD may also increase acceptance of preend‐stage renal disease (ESRD) patient education and nephrology referral, which have been shown to delay CKD progression and improve clinical status at dialysis initiation.[5] However, only 60% of patients with advanced CKD have visited a nephrologist in the past year or have seen a nephrologist prior to dialysis initiation.[1]
The hospital is an important site for patient education and linkage to outpatient care for patients with CKD.[6] The hospital serves high‐risk patients who may not be well connected to outpatient care or who have lesswell‐controlled disease.[6, 7] Thus, hospitalization represents an opportunity to identify existing CKD and to use a multidisciplinary approach to preventative care, patient education, and patient‐provider planning for renal replacement therapy needs. In our cross‐sectional study in an urban, minority‐serving hospital, we sought to determine what patient factors were associated with hospitalized patients correctly self‐identifying as having CKD.
METHODS
Subjects and Data
We used data from the University of Chicago Hospitalist Project, a study of hospitalized patient outcomes.[8] Within 48 hours of hospitalization, all general medicine patients or their proxies are approached to enroll. During one‐on‐one inpatient interviews, a trained research assistant obtains demographic, health status, and healthcare utilization information. Participants consent for study staff to review their medical records. More than 80% of general medicine patients enroll.
We obtained data on 1234 general medicine patients discharged between January 1, 2012 and March 31, 2013 with an International Classification of Diseases, Ninth Revision (ICD‐9) code for chronic kidney disease (ICD‐9 codes 585.0585.5, 585.9) in their first 20 admission diagnoses. These codes are highly specific for CKD but have lower sensitivity.[9] We excluded all patients with a history of transplant (996.81, V42.0, n=90) or ESRD (585.6, n=416). We excluded repeat admissions during the study period (n=138). Our final sample included 590 unique patients with ICD‐9 diagnosis of CKD without ESRD.
Demographic, Clinical, and Health Service Utilization Characteristics
Our outcome was CKD awareness, the patient's correct self‐report of kidney disease. Patients selected their chronic medical conditions from a list read to them and were specifically asked if they had kidney problems. Demographic characteristics including age, gender, race/ethnicity, marital status, and education were also obtained. Healthcare utilization variables included how often the patient saw their primary medical care provider in the past year and whether patients had a prior hospitalization in the last year.
Health status variables such as mental status, diabetes, hypertension, and CKD stage were also assessed. Mental status was quantified using the telephone version of the Mini‐Mental State Examination (MMSE), scored from 1 to 22.10 We defined diabetes as ICD‐9 codes 250.0250.00, and hypertension as ICD‐9 codes 401.0, 401.9, 403, 405.09, 405.19, 405.91, 405.99, or by patient self‐report. CKD stage was based on the estimated glomerular filtration rate (eGFR) from the medical record using Kidney Disease Outcomes Quality Initiative guidelines.[11] We used the mode of the eGFR to calculate the appropriate CKD stage for those with more than 1 eGFR value from the hospitalization (576/590, 98%). The eGFR was calculated by the modified Modification of Diet in Renal Disease equation: (GFR (mL/min/1.73 m2)=175 (Screatinine)1.154 (Age)0.203 (0.742 if female) x (1.212 if African American)) recommended by the National Kidney Disease Education Program.[12]
Analysis
We used logistic regression to analyze the influence of the demographic, clinical, and healthcare utilization covariates on the likelihood of a patient reporting kidney problems. For the multivariate analysis, we sequentially added variables in a step‐wise fashion. We adjusted for (1) demographic factors: gender, race, ethnicity, marital status, and education; (2) eGFR‐calculated CKD stage and comorbidities: diabetes, hypertension, and mental status; and (3) healthcare utilization in the last 12 months: any hospitalizations and the number of visits to a health provider.
RESULTS
Patient Characteristics and Bivariable Association With Patient CKD Self‐Report
Table 1 shows demographic, clinical, and health service use characteristics for 590 patients with ICD‐9 coded CKD. In the bivariable model in Table 1, age, race, marital status and comorbidities, were associated with patient self‐report of CKD. Patients older than 80 years with physician‐identified CKD through ICD‐9 coding had 57% lower odds of reporting CKD than their younger counterparts. Patients of other races (nonwhite, non‐African American), married patients, and those with CKD stages 4 and 5 were much more likely to correctly self‐report CKD. Patients with higher MMSE score, diabetes, or hypertension had greater odds of CKD self‐report (all P0.05).
| Total, N=590 | Bivariable Odds Ratio (95% CI) | Multivariable With Stepwise Addition (95% CI) | |||
|---|---|---|---|---|---|
| Model 1, Demographic Factors | Model 2, Plus CKD Stage and Other Comorbidities | Model 3, Plus Health Service Use | |||
| |||||
| Female | 312 (52.98%) | 1.21 (0.85‐1.70) | 1.64 (1.09‐2.47)* | 1.28 (0.79‐2.06) | 1.36 (0.75‐2.48) |
| Age, y | |||||
| Below 54 | 138 (23.4%) | REF | REF | REF | REF |
| 5466 | 150 (24.4%) | 0.96 (0.59‐1.54) | 0.95 (0.56‐1.59) | 1.01 (0.56‐1.81) | 0.83 (0.41‐1.68) |
| 6779 | 161 (27.3%) | 0.77 (0.48‐1.23) | 0.67 (0.39‐1.15) | 0.66 (0.35‐1.23) | 0.72(0.34‐1.52) |
| Above 80 | 141 (23.9%) | 0.43 (0.26‐0.74) | 0.32 (0.17‐0.60) | 0.42 (0.20‐0.91)* | 0.37 (0.15‐0.93)* |
| Race | |||||
| African American | 449 (82.1%) | REF | REF | REF | REF |
| White | 75 (13.7%) | 1.47 (0.89‐2.42) | 1.53 (0.86‐2.71) | 1.56 (0.78‐3.12) | 1.08 (0.47‐2.49) |
| Other | 23 (4.2%) | 3.62 (1.53‐8.54) | 5.29 (1.78‐15.73) | 6.19 (1.72‐22.29) | 11.63 (1.80‐75.21)* |
| Ethnicity (Hispanic is reference group) | 21 (4.0%) | 0.97 (0.38‐2.44) | 0.43 (0.13‐1.45) | 0.71 (0.16‐3.09) | 0.46 (0.06‐3.57) |
| Married | 171 (32.6%) | 1.49 (1.03‐2.18)* | 1.32 (0.85‐2.04) | 1.20 (0.73‐1.98) | 1.77 (0.94‐3.33) |
| Education | |||||
| Less than high school | 128 (25.2%) | REF | REF | REF | REF |
| High school grad/some college | 296 (58.2%) | 1.05 (0.68‐1.63) | 0.93 (0.58‐1.49) | 0.95 (0.54‐1.67) | 0.85 (0.42‐1.72) |
| College grad or higher | 85 (16.7%) | 1.13 (0.64‐2.01) | 0.96 (0.51‐1.78) | 1.07 (0.51‐2.23) | 0.69 (0.27‐1.77) |
| CKD stage (eGFR calculated, mode) | |||||
| 12 | 115 (20.0%) | 0.55 (0.32‐0.95)* | 0.52 (0.27‐1.01) | 0.28 (0.11‐0.69) | |
| 3 | 300 (52.1%) | REF | REF | REF | |
| 4 | 112 (19.4%) | 2.43 (1.55‐3.81) | 2.69 (1.52‐4.76) | 3.07 (1.56‐6.07) | |
| 5 | 49 (8.5%) | 4.50 (2.39‐8.48) | 3.94 (1.81‐8.56) | 5.16 (1.85‐14.41) | |
| Diabetes (ICD‐9 coded or self‐report)‖ | 292 (49.5%) | 1.52 (1.07‐2.15)* | 1.54 (0.97‐2.45) | 1.26 (0.71‐2.26) | |
| Hypertension (ICD‐9 coded or self‐report) | 436 (73.9%) | 3.36 (2.09‐5.41) | 1.53 (0.80‐2.90) | 1.26 (0.57‐2.81) | |
| Mini‐Mental State Exam score# | 19.7 (2.5) | 1.13 (1.03‐1.23) | 1.09 (0.98‐1.22) | 1.22 (1.06‐1.42) | |
| Hospitalized in last 12 months | 253 (46.9%) | 1.50 (1.05‐2.13)* | 1.12 (0.65‐1.95) | ||
| No. of visits to health provider | |||||
| Once/year or less | 80 (18.4%) | REF | REF | ||
| 23 times/year | 58 (13.3%) | 0.80 (0.40‐1.60) | 0.44 (0.17‐1.16) | ||
| 4+ times/year | 298 (68.4%) | 0.60 (0.36‐0.99)* | 0.38 (0.19‐0.74) | ||
Multivariable Associations With Patient CKD Self‐Report
Age, race, and CKD stage remained consistently associated with CKD self‐report, although the magnitude of the effects (odds ratios [ORs]) varied across models (Table 1). Across all the models, patients older than 80 years were still significantly less likely than younger patients to self‐report CKD (ORs ranging from 0.32 to 0.42, all P0.05). Patients classified as being of other race were found to have a 5.29 to 11.63 greater odds of CKD self‐report than African American patients (all P0.05).
Patients with CKD stage 4 and 5 were more likely to self‐report than patients with CKD stage 3 (ORs ranging from 2.693.07 for stage 4 and 3.945.16 for stage 5, P0.05). In the final model, every unit increase in MMSE score increased the odds of CKD self‐report by 22%. In addition, patients who saw their health provider 4 or more times per year were 62% less likely to self‐report CKD than patients who saw their provider 1 or fewer times per year (OR: 0.38, P0.05).
A large proportion of patients (68.6%) were CKD unspecified by ICD‐9 codes, and only 27% of the unspecified group reported having CKD (Table 2). Examining the eGFR CKD stage of the CKD unspecified group showed that 22.8% were eGFR‐determined CKD stage 1 to 2, 57.1% were CKD stage 3, 14.8% were CKD stage 4, and 5.3% were CKD stage 5. Patients had 2 to 3 times greater odds of correct CKD self‐report if physicians had correctly identified their CKD stage (bivariable OR: 2.42, 95% confidence interval [CI]: 1.57‐3.72, multivariable OR: 3.22, 95% CI: 0.99‐10.46) (analysis not shown).
| CKD Stage* | Physician (ICD‐9) Coded | eGFR Calculated | eGFR Coded With ICD‐9 Correct |
|---|---|---|---|
| |||
| Unspecified | 110 (27.2%) | ||
| 12 | 5 (31.2%) | 20 (17.4%) | 0 |
| 3 | 25 (27.2%) | 83 (27.7%) | 17 (29.8%) |
| 4 | 40 (63.5%) | 54 (48.2%) | 25 (69.4%) |
| 5 | 11 (78.6%) | 31 (63.3%) | 10 (83.3%) |
DISCUSSION
Although prior work has examined CKD awareness in the general population and in high‐risk cohorts,[2, 3, 13] this is the first study examining CKD awareness in an urban, underserved hospitalized population. We found that overall patients' CKD awareness was low (32%), but increased as high as 63% for CKD stage 5, even after controlling for patient demographic, clinical characteristics, and healthcare use. Our overall rate of CKD awareness was higher than prior studies overall and at lower CKD stages.[2, 3, 13] Our work is consistent with prior literature that shows increasing CKD awareness with advancing CKD stage.[2, 3, 13]
Older patients (>80 years) had lower awareness of CKD. Older patients are more likely to have a near normal creatinine, despite a markedly reduced eGFR, so their CKD may go unnoticed.[14] Even with appropriate recognition, providers may also feel like their CKD is unlikely to progress to ESRD, given its stability and/or their competing risk of death.[15] Finally, older hospitalized patients may also be less likely to report a CKD diagnosis due to difficulty in recall due to denial, dementia, or delirium.
One limitation is that our case‐finding for CKD was physician ICD‐9 coding, which is highly specific but not sensitive.[9] The majority of patients with physician‐identified CKD were CKD unspecified, perhaps due to poor coding, physician underdocumentation, or physician under‐recognition of CKD stage. Although only 27% of the CKD unspecified group correctly self‐identified as having CKD, over 75% were found to be CKD stage 3 or higher, which should trigger additional monitoring or care based on guidelines.[10] In addition, despite statistical significance, we may not be able to make meaningful inferences about our small other group (nonwhite, non‐African American). Our sample was from 1 hospitalan urban, academic, tertiary care center with a large proportion of African American patientswhich may limit generalizability. The multivariable model will need to be tested in other populations for reproducibility.
Our study significantly contributes to the literature by examining patient awareness of CKD in a high‐risk, urban, hospitalized minority population. Other study strengths include use of basic demographic information, as well as survey and laboratory data for a richer examination of the associations between patient factors and CKD awareness.
CONCLUSION
Hospitalized patients with CKD have a low CKD awareness. Patient awareness of their CKD is increased with physician documentation of CKD severity. Patient awareness of their CKD must be coupled with provider awareness and CKD documentation to link patients to multidisciplinary CKD education and care to slow CKD progression and reduce associated cardiovascular and metabolic complications. Further work is needed across hospitals to determineand improveCKD awareness among both patients and providers.
Disclosures
Dr. Saunders was supported by Pilot and Feasibility Funding from the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949). Dr. Chin was supported by NIDDK K24 DK071933. Dr. Meltzer was supported by NIA T35 AG029795. Dr. Saunders had full access to all of the study data and takes responsibility for the integrity of the data and accuracy of the data analysis. An abstract of this article was presented at the Society of Hospital Medicine Annual Meeting in Las Vegas, Nevada in March 2014 and at the Society of General Internal Medicine Annual Meeting in San Diego, California in April 2014. The authors report no conflicts of interest.
Chronic kidney disease (CKD) affects over 13% of the US population and is associated with increased morbidity, mortality, and healthcare costs.[1] However, only 10% of individuals with CKD are aware of their diagnoses.[2] Even in those with stage 5 CKD, only 60% of individuals are aware of their CKD.[3] To our knowledge, no work has examined CKD awareness in a hospitalized patient population.
Patient awareness of their CKD diagnosis is important because progression of kidney disease can be slowed by patient self‐management of diabetes and hypertension.[4] Patient awareness of CKD may also increase acceptance of preend‐stage renal disease (ESRD) patient education and nephrology referral, which have been shown to delay CKD progression and improve clinical status at dialysis initiation.[5] However, only 60% of patients with advanced CKD have visited a nephrologist in the past year or have seen a nephrologist prior to dialysis initiation.[1]
The hospital is an important site for patient education and linkage to outpatient care for patients with CKD.[6] The hospital serves high‐risk patients who may not be well connected to outpatient care or who have lesswell‐controlled disease.[6, 7] Thus, hospitalization represents an opportunity to identify existing CKD and to use a multidisciplinary approach to preventative care, patient education, and patient‐provider planning for renal replacement therapy needs. In our cross‐sectional study in an urban, minority‐serving hospital, we sought to determine what patient factors were associated with hospitalized patients correctly self‐identifying as having CKD.
METHODS
Subjects and Data
We used data from the University of Chicago Hospitalist Project, a study of hospitalized patient outcomes.[8] Within 48 hours of hospitalization, all general medicine patients or their proxies are approached to enroll. During one‐on‐one inpatient interviews, a trained research assistant obtains demographic, health status, and healthcare utilization information. Participants consent for study staff to review their medical records. More than 80% of general medicine patients enroll.
We obtained data on 1234 general medicine patients discharged between January 1, 2012 and March 31, 2013 with an International Classification of Diseases, Ninth Revision (ICD‐9) code for chronic kidney disease (ICD‐9 codes 585.0585.5, 585.9) in their first 20 admission diagnoses. These codes are highly specific for CKD but have lower sensitivity.[9] We excluded all patients with a history of transplant (996.81, V42.0, n=90) or ESRD (585.6, n=416). We excluded repeat admissions during the study period (n=138). Our final sample included 590 unique patients with ICD‐9 diagnosis of CKD without ESRD.
Demographic, Clinical, and Health Service Utilization Characteristics
Our outcome was CKD awareness, the patient's correct self‐report of kidney disease. Patients selected their chronic medical conditions from a list read to them and were specifically asked if they had kidney problems. Demographic characteristics including age, gender, race/ethnicity, marital status, and education were also obtained. Healthcare utilization variables included how often the patient saw their primary medical care provider in the past year and whether patients had a prior hospitalization in the last year.
Health status variables such as mental status, diabetes, hypertension, and CKD stage were also assessed. Mental status was quantified using the telephone version of the Mini‐Mental State Examination (MMSE), scored from 1 to 22.10 We defined diabetes as ICD‐9 codes 250.0250.00, and hypertension as ICD‐9 codes 401.0, 401.9, 403, 405.09, 405.19, 405.91, 405.99, or by patient self‐report. CKD stage was based on the estimated glomerular filtration rate (eGFR) from the medical record using Kidney Disease Outcomes Quality Initiative guidelines.[11] We used the mode of the eGFR to calculate the appropriate CKD stage for those with more than 1 eGFR value from the hospitalization (576/590, 98%). The eGFR was calculated by the modified Modification of Diet in Renal Disease equation: (GFR (mL/min/1.73 m2)=175 (Screatinine)1.154 (Age)0.203 (0.742 if female) x (1.212 if African American)) recommended by the National Kidney Disease Education Program.[12]
Analysis
We used logistic regression to analyze the influence of the demographic, clinical, and healthcare utilization covariates on the likelihood of a patient reporting kidney problems. For the multivariate analysis, we sequentially added variables in a step‐wise fashion. We adjusted for (1) demographic factors: gender, race, ethnicity, marital status, and education; (2) eGFR‐calculated CKD stage and comorbidities: diabetes, hypertension, and mental status; and (3) healthcare utilization in the last 12 months: any hospitalizations and the number of visits to a health provider.
RESULTS
Patient Characteristics and Bivariable Association With Patient CKD Self‐Report
Table 1 shows demographic, clinical, and health service use characteristics for 590 patients with ICD‐9 coded CKD. In the bivariable model in Table 1, age, race, marital status and comorbidities, were associated with patient self‐report of CKD. Patients older than 80 years with physician‐identified CKD through ICD‐9 coding had 57% lower odds of reporting CKD than their younger counterparts. Patients of other races (nonwhite, non‐African American), married patients, and those with CKD stages 4 and 5 were much more likely to correctly self‐report CKD. Patients with higher MMSE score, diabetes, or hypertension had greater odds of CKD self‐report (all P0.05).
| Total, N=590 | Bivariable Odds Ratio (95% CI) | Multivariable With Stepwise Addition (95% CI) | |||
|---|---|---|---|---|---|
| Model 1, Demographic Factors | Model 2, Plus CKD Stage and Other Comorbidities | Model 3, Plus Health Service Use | |||
| |||||
| Female | 312 (52.98%) | 1.21 (0.85‐1.70) | 1.64 (1.09‐2.47)* | 1.28 (0.79‐2.06) | 1.36 (0.75‐2.48) |
| Age, y | |||||
| Below 54 | 138 (23.4%) | REF | REF | REF | REF |
| 5466 | 150 (24.4%) | 0.96 (0.59‐1.54) | 0.95 (0.56‐1.59) | 1.01 (0.56‐1.81) | 0.83 (0.41‐1.68) |
| 6779 | 161 (27.3%) | 0.77 (0.48‐1.23) | 0.67 (0.39‐1.15) | 0.66 (0.35‐1.23) | 0.72(0.34‐1.52) |
| Above 80 | 141 (23.9%) | 0.43 (0.26‐0.74) | 0.32 (0.17‐0.60) | 0.42 (0.20‐0.91)* | 0.37 (0.15‐0.93)* |
| Race | |||||
| African American | 449 (82.1%) | REF | REF | REF | REF |
| White | 75 (13.7%) | 1.47 (0.89‐2.42) | 1.53 (0.86‐2.71) | 1.56 (0.78‐3.12) | 1.08 (0.47‐2.49) |
| Other | 23 (4.2%) | 3.62 (1.53‐8.54) | 5.29 (1.78‐15.73) | 6.19 (1.72‐22.29) | 11.63 (1.80‐75.21)* |
| Ethnicity (Hispanic is reference group) | 21 (4.0%) | 0.97 (0.38‐2.44) | 0.43 (0.13‐1.45) | 0.71 (0.16‐3.09) | 0.46 (0.06‐3.57) |
| Married | 171 (32.6%) | 1.49 (1.03‐2.18)* | 1.32 (0.85‐2.04) | 1.20 (0.73‐1.98) | 1.77 (0.94‐3.33) |
| Education | |||||
| Less than high school | 128 (25.2%) | REF | REF | REF | REF |
| High school grad/some college | 296 (58.2%) | 1.05 (0.68‐1.63) | 0.93 (0.58‐1.49) | 0.95 (0.54‐1.67) | 0.85 (0.42‐1.72) |
| College grad or higher | 85 (16.7%) | 1.13 (0.64‐2.01) | 0.96 (0.51‐1.78) | 1.07 (0.51‐2.23) | 0.69 (0.27‐1.77) |
| CKD stage (eGFR calculated, mode) | |||||
| 12 | 115 (20.0%) | 0.55 (0.32‐0.95)* | 0.52 (0.27‐1.01) | 0.28 (0.11‐0.69) | |
| 3 | 300 (52.1%) | REF | REF | REF | |
| 4 | 112 (19.4%) | 2.43 (1.55‐3.81) | 2.69 (1.52‐4.76) | 3.07 (1.56‐6.07) | |
| 5 | 49 (8.5%) | 4.50 (2.39‐8.48) | 3.94 (1.81‐8.56) | 5.16 (1.85‐14.41) | |
| Diabetes (ICD‐9 coded or self‐report)‖ | 292 (49.5%) | 1.52 (1.07‐2.15)* | 1.54 (0.97‐2.45) | 1.26 (0.71‐2.26) | |
| Hypertension (ICD‐9 coded or self‐report) | 436 (73.9%) | 3.36 (2.09‐5.41) | 1.53 (0.80‐2.90) | 1.26 (0.57‐2.81) | |
| Mini‐Mental State Exam score# | 19.7 (2.5) | 1.13 (1.03‐1.23) | 1.09 (0.98‐1.22) | 1.22 (1.06‐1.42) | |
| Hospitalized in last 12 months | 253 (46.9%) | 1.50 (1.05‐2.13)* | 1.12 (0.65‐1.95) | ||
| No. of visits to health provider | |||||
| Once/year or less | 80 (18.4%) | REF | REF | ||
| 23 times/year | 58 (13.3%) | 0.80 (0.40‐1.60) | 0.44 (0.17‐1.16) | ||
| 4+ times/year | 298 (68.4%) | 0.60 (0.36‐0.99)* | 0.38 (0.19‐0.74) | ||
Multivariable Associations With Patient CKD Self‐Report
Age, race, and CKD stage remained consistently associated with CKD self‐report, although the magnitude of the effects (odds ratios [ORs]) varied across models (Table 1). Across all the models, patients older than 80 years were still significantly less likely than younger patients to self‐report CKD (ORs ranging from 0.32 to 0.42, all P0.05). Patients classified as being of other race were found to have a 5.29 to 11.63 greater odds of CKD self‐report than African American patients (all P0.05).
Patients with CKD stage 4 and 5 were more likely to self‐report than patients with CKD stage 3 (ORs ranging from 2.693.07 for stage 4 and 3.945.16 for stage 5, P0.05). In the final model, every unit increase in MMSE score increased the odds of CKD self‐report by 22%. In addition, patients who saw their health provider 4 or more times per year were 62% less likely to self‐report CKD than patients who saw their provider 1 or fewer times per year (OR: 0.38, P0.05).
A large proportion of patients (68.6%) were CKD unspecified by ICD‐9 codes, and only 27% of the unspecified group reported having CKD (Table 2). Examining the eGFR CKD stage of the CKD unspecified group showed that 22.8% were eGFR‐determined CKD stage 1 to 2, 57.1% were CKD stage 3, 14.8% were CKD stage 4, and 5.3% were CKD stage 5. Patients had 2 to 3 times greater odds of correct CKD self‐report if physicians had correctly identified their CKD stage (bivariable OR: 2.42, 95% confidence interval [CI]: 1.57‐3.72, multivariable OR: 3.22, 95% CI: 0.99‐10.46) (analysis not shown).
| CKD Stage* | Physician (ICD‐9) Coded | eGFR Calculated | eGFR Coded With ICD‐9 Correct |
|---|---|---|---|
| |||
| Unspecified | 110 (27.2%) | ||
| 12 | 5 (31.2%) | 20 (17.4%) | 0 |
| 3 | 25 (27.2%) | 83 (27.7%) | 17 (29.8%) |
| 4 | 40 (63.5%) | 54 (48.2%) | 25 (69.4%) |
| 5 | 11 (78.6%) | 31 (63.3%) | 10 (83.3%) |
DISCUSSION
Although prior work has examined CKD awareness in the general population and in high‐risk cohorts,[2, 3, 13] this is the first study examining CKD awareness in an urban, underserved hospitalized population. We found that overall patients' CKD awareness was low (32%), but increased as high as 63% for CKD stage 5, even after controlling for patient demographic, clinical characteristics, and healthcare use. Our overall rate of CKD awareness was higher than prior studies overall and at lower CKD stages.[2, 3, 13] Our work is consistent with prior literature that shows increasing CKD awareness with advancing CKD stage.[2, 3, 13]
Older patients (>80 years) had lower awareness of CKD. Older patients are more likely to have a near normal creatinine, despite a markedly reduced eGFR, so their CKD may go unnoticed.[14] Even with appropriate recognition, providers may also feel like their CKD is unlikely to progress to ESRD, given its stability and/or their competing risk of death.[15] Finally, older hospitalized patients may also be less likely to report a CKD diagnosis due to difficulty in recall due to denial, dementia, or delirium.
One limitation is that our case‐finding for CKD was physician ICD‐9 coding, which is highly specific but not sensitive.[9] The majority of patients with physician‐identified CKD were CKD unspecified, perhaps due to poor coding, physician underdocumentation, or physician under‐recognition of CKD stage. Although only 27% of the CKD unspecified group correctly self‐identified as having CKD, over 75% were found to be CKD stage 3 or higher, which should trigger additional monitoring or care based on guidelines.[10] In addition, despite statistical significance, we may not be able to make meaningful inferences about our small other group (nonwhite, non‐African American). Our sample was from 1 hospitalan urban, academic, tertiary care center with a large proportion of African American patientswhich may limit generalizability. The multivariable model will need to be tested in other populations for reproducibility.
Our study significantly contributes to the literature by examining patient awareness of CKD in a high‐risk, urban, hospitalized minority population. Other study strengths include use of basic demographic information, as well as survey and laboratory data for a richer examination of the associations between patient factors and CKD awareness.
CONCLUSION
Hospitalized patients with CKD have a low CKD awareness. Patient awareness of their CKD is increased with physician documentation of CKD severity. Patient awareness of their CKD must be coupled with provider awareness and CKD documentation to link patients to multidisciplinary CKD education and care to slow CKD progression and reduce associated cardiovascular and metabolic complications. Further work is needed across hospitals to determineand improveCKD awareness among both patients and providers.
Disclosures
Dr. Saunders was supported by Pilot and Feasibility Funding from the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949). Dr. Chin was supported by NIDDK K24 DK071933. Dr. Meltzer was supported by NIA T35 AG029795. Dr. Saunders had full access to all of the study data and takes responsibility for the integrity of the data and accuracy of the data analysis. An abstract of this article was presented at the Society of Hospital Medicine Annual Meeting in Las Vegas, Nevada in March 2014 and at the Society of General Internal Medicine Annual Meeting in San Diego, California in April 2014. The authors report no conflicts of interest.
- United States Renal Data System. Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; 2012.
- , , , et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Neph. 2011;6(8):1838–1844.
- , , , et al. Comparison of CKD awareness in a screening population using the Modification of Diet in Renal Disease (MDRD) Study and CKD Epidemiology Collaboration (CKD‐EPI) equations. Am J Kidney Dis. 2011;57(3 suppl 2):S17–S23.
- , , , et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis. 2000;36(3):646–661.
- , , , et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality—a controlled cohort study based on the NKFDOQI guidelines. Nephrol Dial Transplant. 2009;24(11):3426–3433.
- , , . Pre‐dialysis hospital use and late referrals in incident dialysis patients in England: a retrospective cohort study. Nephrol Dial Transplant. 2015;30(1):124–129.
- , , , et al. Do hospitals that provide heart failure patient education prior to discharge also promote continuity of care? A report from OPTIMIZE‐HF. J Card Fail. 2006;12(6 suppl):S111.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , et al. Failure of ICD‐9‐CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41(2):564–580.
- , , , . Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40(7):697–702.
- National Kidney Foundation. Kidney disease outcomes quality initiative guidelines 2002. Available at: http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/toc.htm. Accessed October 9, 2014.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470.
- , , , et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53(2):238–247.
- , , , , , . Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55(6):816–823.
- , , , et al. Prediction, Progression, and Outcomes of Chronic Kidney Disease in Older Adults. J Am Soc Neph. 2009;20(6):1199–1209.
- United States Renal Data System. Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; 2012.
- , , , et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Neph. 2011;6(8):1838–1844.
- , , , et al. Comparison of CKD awareness in a screening population using the Modification of Diet in Renal Disease (MDRD) Study and CKD Epidemiology Collaboration (CKD‐EPI) equations. Am J Kidney Dis. 2011;57(3 suppl 2):S17–S23.
- , , , et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis. 2000;36(3):646–661.
- , , , et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality—a controlled cohort study based on the NKFDOQI guidelines. Nephrol Dial Transplant. 2009;24(11):3426–3433.
- , , . Pre‐dialysis hospital use and late referrals in incident dialysis patients in England: a retrospective cohort study. Nephrol Dial Transplant. 2015;30(1):124–129.
- , , , et al. Do hospitals that provide heart failure patient education prior to discharge also promote continuity of care? A report from OPTIMIZE‐HF. J Card Fail. 2006;12(6 suppl):S111.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , et al. Failure of ICD‐9‐CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41(2):564–580.
- , , , . Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40(7):697–702.
- National Kidney Foundation. Kidney disease outcomes quality initiative guidelines 2002. Available at: http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/toc.htm. Accessed October 9, 2014.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470.
- , , , et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53(2):238–247.
- , , , , , . Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55(6):816–823.
- , , , et al. Prediction, Progression, and Outcomes of Chronic Kidney Disease in Older Adults. J Am Soc Neph. 2009;20(6):1199–1209.
Peripheral Administration of VM
Vasoactive medications (VMs) are often required to improve hemodynamic function in patients with shock. They are usually given through central venous catheter (CVC) access, primarily out of concern that extravasation of peripheral intravenous (PIV) access may result in local tissue injury due to the vasoconstrictive effect of the VM. However, insertion of CVC is associated with a variety of mechanical complications and risk of central lineassociated bacteremia. To examine the feasibility and safety of using VM via PIV access, we report on the administration of VM in the form of norepinephrine, dopamine, and phenylephrine via PIV access, with the rationale that this would be a method of reducing the need of CVC use. Our hypotheses are that VM via PIV access is both feasible and safe.
MATERIAL AND METHODS
Study Design
This was a single‐arm, consecutive‐patient study conducted from September 2012 to June 2014. The study site was an 18‐bed medical intensive care unit (MICU) staffed by full‐time attendings, fellows, and residents at the Long Island Jewish Medical Center, which is an 827‐bed tertiary care teaching hospital that is part of the North ShoreLong Island Jewish Health System. The primary outcome measure was the rate of local tissue injury resulting from use of VM via PIV access. The study was approved by the hospital institutional review board (study #13583A), which waived requirement for informed consent.
Protocol for Administration of VM via PIV Access
In cooperation with the Department of Pharmacy, medical and nursing staff developed a written protocol for administration of VM via PIV access. The protocol was reviewed and approved by the hospital pharmacy and therapeutics committee and the MICU nursing leadership. The MICU nursing staff received in‐service training before rollout of the protocol, which included training on the recognition of PIV access extravasation and the type of line that could be used. The MICU housestaff teams were given specific instructions concerning the protocol during their MICU rotations. A summary of the requirements for PIV access for VM use is summarized in Table 1.
|
| Vein diameter >4 mm measured with ultrasonography |
| Position of PIV access documented to be in the vein with ultrasonography before starting infusion of VM |
| Upper extremity only, contralateral to the blood pressure cuff |
| Intravenous line size 20 gauge or 18 gauge |
| No hand, wrist, or antecubital fossa PIV access position |
| Blood return from the PIV access prior to VM administration |
| Assessment of PIV access function every 2 hours as per nursing protocol |
| Immediate alert by nursing staff to the medical team if line extravasation, with prompt initiation of local treatment |
| 72 hours maximum duration of PIV access use |
Patient Management
The decision to initiate treatment with VM was made by the clinical management team. The standard concentrations of VM for use via PIV access were: norepinephrine 8 mg or 16 mg/250 mL normal saline, dopamine 400 mg or 800 mg/250 mL D5W, and phenylephrine 80 mg or 160 mg/500 mL normal saline. If the attending or fellow in charge of the case decided that VM should be administered through PIV access, peripheral access was established that conformed to the requirements of the protocol, and VM was administered via PIV access for as long as there was clinical indication or until PIV access suitable for VM administration was no longer feasible. If the patient received VM via PIV access, a second PIV access site was established in case of failure of the primary PIV site. If no PIV access could be inserted, the patient received CVC access. The decision to use VM via CVC access was made by the clinical management team, as was the type, dose, and duration of the VM use via PIV access or CVC access. Vasopressin was not used via PIV access. Dobutamine was used via PIV access but not recorded in our results, as it has no ‐mediated vasoconstrictor effect. Dobutamine was not used concomitantly with other vasoactive medication through the same PIV access. If PIV access was not established using standard technique by nursing staff, medical residents or critical care fellows inserted PIV access using real‐time ultrasound guidance. The PIV access use for VM could also be used for other medications providing they were compatible with the VM. Only 1 type of VM was infused through the PIV access.
As indicated in Table 1, the nursing staff examined the PIV access site every 2 hours and checked that blood could be aspirated from the line. The aspiration of the line requires several seconds of discontinuation of VM use, which we considered to have no clinical relevance. If the nursing staff identified extravasation of the PIV access site through which VM was infusing, they notified the medical housestaff, who promptly initiated treatment with local injection of phentolamine and local application of nitroglycerin paste as described in Table 2. The extravasation site was examined for tissue injury on a shift basis by the nursing staff, and on bedside rounds by the attending and fellow for at least 48 hours following PIV access removal. Tissue injury was defined as any erythema, blistering, skin breakdown, or necrosis in the site of extravasation.
|
| 1. The VM via PIV infusion is stopped immediately. |
| 2. Residual medication is aspirated through the PIV access, and the catheter is removed. |
| 3. The extent of the extravasation is outlined to provide a baseline for monitoring. |
| 4. Two vials, each containing 5 mg of phentolamine, are reconstituted with 5 mL of normal saline per vial to yield a final concentration of 1 mg/mL. |
| 5. The phentolamine solution is injected in 0.5‐ to 1‐mL aliquots in 5 separate injections around the leading edge of the extravasation, using separate 25‐gauge or 27‐gauge needles for each injection. |
| 6. Nitroglycerin paste (2.5 cm) is applied to the area of extravasation. |
| 7. A medication occurrence report is filled out for review by the quality committee. |
Data were collected prospectively by an investigator (J.C.‐G.) and entered into a standard data‐collection sheet for quality and safety assessment for the initial 13 months of the study. In the subsequent 7 months of observation, data were collected from retrospective chart review. The initial 13 months of data collection were performed as an ongoing safety analysis project; the subsequent 7‐month review was performed as an additional quality assessment project. The deidentified data included patient demographics, patient disease characteristics, use of VM, and VM via PIV access complications.
Statistical Analysis
The statistical analysis was performed using SPSS 21 (Statistical Package for the Social Sciences; IBM, Armonk, NY). Continuous variables are presented as meanstandard deviation.
RESULTS
Characteristics of patients who received VM via PIV access are presented in Table 3. During the study period, there were 2462 admissions to the MICU, and 267 CVCs were inserted by the MICU team, 170 of which were triple‐lumen catheters and 97 were large‐gauge catheters for hemodialysis or plasmapheresis. Of the total admissions, 953 cases received VM; 783/953 (82%) received VM via PIV access, and 170/953 received VM via CVC access (18%). For VM use, an 18‐gauge PIV catheter was used in 192/783 (25%), a 20‐gauge catheter was used in 590/783 (75%), and a 22‐gauge catheter was used in 1/783 of interventions. Catheter length was 30 mm, 45 mm, or 48 mm depending on availability. The 22‐gauge catheter, which was a deviation from standard protocol, infiltrated shortly following insertion. We did not formally record the anatomic position of the PIV access in the standard data‐collection sheet; anecdotally, the majority of PIV accesses were placed in the upper arm basilic or cephalic vein. The duration of VM via PIV access was 4922 hours. Central intravenous access was required in 95/734 (13%) of patients who initially had VM via PIV access. These catheters are included in the 170 triple‐lumen CVCs that were inserted by the MICU team during the study period. The type and highest dose of VM administered via PIV access are presented in Table 4.
| Total Study Group | |
|---|---|
| |
| No. of patients | 734 |
| Age, y | 7215 |
| Gender | |
| Male | 398 (54%) |
| Female | 336 (46%) |
| SAPS II score | 7515 |
| Patients on mechanical ventilation | 235 (32%) |
| Patients on hemodialysis | 90 (12%) |
| MICU mortality | 177 (23%) |
| Use of VM via PIV access | 783 |
| Extravasations of VM via PIV access | 19 (2%) |
| Total MICU admissions during study period | 2,462 |
| |
| Norepinephrine | |
| Interventions | 506 |
| Dose, g/kg/min, meanSD | 0.700.23 |
| PIV access extravasations | 16 |
| Dopamine | |
| Interventions | 101 |
| Dose, g/kg/min, meanSD | 12.75.23 |
| PIV access extravasations | 3 |
| Phenylephrine | |
| Interventions | 176 |
| Dose, g/kg/min, meanSD | 3.251.69 |
| PIV access extravasations | 0 |
A total of 734 patients received VM via PIV access during the 20‐month study period; 49 of these patients required 2 or more PIV access insertions, as the initial and/or subsequent site timed out at 72 hours, resulting in a total of 783 separate interventions. Infiltration of the PIV access site occurred in 19/783 (2%) of interventions. All of them were identified by nursing staff with prompt response using local injection of phentolamine and application of nitroglycerin paste at the site of the extravasation. There was no tissue injury at the site of VM extravasation. Sixteen of the extravasations occurred with norepinephrine infusions and 3 with dopamine infusions. There were no infections of the PIV access sites used for VM. Use of phentolamine and nitroglycerin paste was not associated with hypotension, as defined as mean arterial pressure less than 65 mm Hg.
DISCUSSION
Our study demonstrates that administration of VM via PIV access is feasible, carries a low rate of complications, and offers an alternative to CVC access. There are several elements that may have allowed safe use of VM via PIV access. We developed a protocol that involved a multidisciplinary team. The hospital pharmacy performed an extensive literature search and formulated the initial protocol with the MICU attending staff. The protocol was then subjected to iterative process improvement by a hospital committee and nursing leadership in the MICU. Before program rollout, the MICU nursing staff were educated and trained to use the protocol. This was a key component of the program, as the nurses were responsible for many of the line insertions, line maintenance, and identification of infiltration. Although we did not perform any formal measurement of the impact of PIV access use on nursing workflow, we note that leadership and frontline nurses have been enthusiastic about the implementation of VM via PIV access. The MICU housestaff teams were given an in‐service instruction concerning the importance of prompt initiation of local treatment in case of infiltration of the PIV access site. Specific elements of the protocol that may have improved safety were the use of ultrasonography to insert difficult PIV access and confirmation of all PIV access insertions using ultrasonography by the MICU housestaff. The requirement for frequent checks of PIV access function, prompt recognition of infiltration, and specific antidote to extravasation were important elements of safety. The low rate of PIV access extravasation (2%) may be related to the use of ultrasonography to guide PIV access insertion in patients who had challenging anatomy (eg, obesity, edema, recreational drug use, history of multiple PIV insertions), and ultrasonography was used to check that the PIV access was well positioned before VM infusion.
There were early literature reports that subcutaneous extravasation of catecholamines could result in local ischemic injury both in human patients and animal models.[1, 2, 3, 4, 5] Local phentolamine injection has been identified as a specific antidote to block the local ischemic injury.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] More recently, there have been anecdotal reports showing that local application of nitroglycerin paste blocks ischemic injury in the pediatric population.[5, 16, 17] With this information, our protocol included the requirement of prompt treatment of local extravasation with phentolamine and nitroglycerin paste at the site of VM via PIV access extravasation. In theory, both phentolamine and nitroglycerin might cause hypotension. In our study, administration of both phentolamine and nitroglycerin paste was not associated with more hypotension nor did it increase requirements for VM.
Multilumen small bore CVCs may be used for several reasons, some of which need to be reconsidered. First, before introduction of the VM via PIV access protocol, a common indication for triple lumen CVC insertion in our MICU was the perception that VM could only be administered through CVC access, for fear of local tissue injury should extravasation of the VM occur through the PIV access site. Our results indicate that VM use is not an automatic indication for CVC insertion. Second, a possible indication for CVC insertion is to measure central venous pressure for the purpose of guiding volume resuscitation in patients with hemodynamic failure. As the utility of central venous pressure monitoring has been called into serious question,[18, 19, 20] we do not consider this indication for CVC use to be valid. Third, CVC access may be required due to anatomic constraints (ie, there is no suitable PIV site). Fourth, there may be need for such a large number of intravenous medications that PIV access cannot support. Fifth, there is occasional situation where the patient requires use of medications where extravasation of PIV access would cause local tissue injury without local antidote (eg, certain chemotherapeutic agents). The continued need for CVC access in some patients is reflected in the finding that 13% of our study patients who received VM via PIV access eventually required triple‐lumen CVC insertion. However, our results indicate that the rate of CVC use may be reduced by using PIV access for VM administration.
Our study has some methodological limitations. Study design was single center and observational. The focus of this study was to examine the safety of VM via PIV access. We cannot comment on its effectiveness, indications, or influence on patient outcome nor on why some patients required CVC insertion whereas others did not. The decision to administer VM was made by the clinical team, as was the route of its administration and concentration, without any input from the investigators. We did not collect data on who performed the PIV access insertion (medical or nursing staff), demographics, and disease characteristics of the CVC group, nor to what extent ultrasonography was used to guide PIV insertion. We did not attempt to define whether there were any factors that identified risk for PIV access extravasation, nor did we evaluate for any differences between the PIV and CVC group in terms of demographics and disease characteristics. Lacking a control group, we cannot say definitively that VM via PIV access is safer than VM via CVC. Being a single‐center study, it is not possible to say that the results are transferable to another clinical environment; this applies particularly to the use of ultrasonography, which is a user‐dependent skill. We cannot determine which, if any, component of the protocol was responsible for the safe use of VM via PIV access. The rate of PIV access extravasation was low, so it is possible that a larger sample size is required to identify incidents of tissue necrosis from extravasation of VM delivered via PIV access despite the use of local antidote.
CONCLUSIONS
The delivery of VM via PIV access is safe and feasible. Tto reduce the risk of extravasation leading to possible local tissue injury, we developed a protocol that emphasized close cooperation between the nursing and medical staff, routine use of ultrasonography, rapid identification of extravasation of the PIV access, and prompt response to local extravasation of VM using phentolamine and nitroglycerin paste. This approach offers a means of reducing CVC use, in both intensive care unit (ICU) and non‐ICU settings, including hospital wards and emergency departments. Clinicians should no longer consider administration of norepinephrine, dopamine, or phenylephrine to be an automatic indication for CVC access. This study focused on the safety of VM administered via PIV access, with emphasis on local complications related to extravasation, and should be considered a preliminary single‐center study that demonstrates that administration of certain vasoactive medications may not universally require central venous access. A broader study regarding assessment of safety and efficacy will require a multicenter design.
Disclosures
J.C.‐G., K.F.S., Y.G.B., M.N., S.J.K., and P.H.M. participated in the study design, statistical review, and manuscript writing. J.C.‐G. is the guarantor of the article, taking responsibility for the integrity of the work as a whole from inception to published article. This work is original, and all authors meet the criteria for authorship, including acceptance of responsibility for the scientific content of the article. This article is not under consideration in any other journal, and all of the authors have read and approved the content of the article. No potential conflict of interest exists with any companies or organizations whose products or services are discussed in this article. This article has not been funded by the National Institutes of Health, the Wellcome Trust, or their agencies. All financial support of the study was derived from the Division of Pulmonary, Critical Care and Sleep Medicine at North ShoreLong Island Jewish Medical Center, New Hyde Park, New York.
- , , . Cutaneous necrosis due to norepinephrine. II. Mechanism and prevention. Ann Surg. 1958;147:44–50.
- , , . Pedal gangrene associated with the use of dopamine. N Engl J Med. 1975;293:591.
- , . Gangrene aggravation after use of dopamine [letter]. JAMA. 1976;235:2812.
- , . Dopamine gangrene [letter]. N Engl J Med. 1976;294:114.
- , . Extravasation injury associated with low‐dose dopamine. Ann Pharmacother. 1998;32:545–548.
- . Use of phenytolamine to prevent necrosis due to levarterenol. JAMA. 1957;163:1477–1479.
- . Phentolamine hydrochloride in prevention of cutaneous necrosis due to levarterenol. JAMA. 1959;170:1916–1917.
- , . Avoidance of vascular complications associated with the use of dopamine. Can Anaesth Soc J. 1977;24:727–733.
- . Management of intravenous extravasations. Infusion. 1984;6:77–79.
- , . Acute management of dopamine infiltration injury with Regitine. Plast Reconstr Surg. 1987;80:610–612.
- . High dose phentolamine for extravasation of pressors [letter]. Clin Pharm. 1989;8:689.
- , . Phentolamine reversal of epinephrine‐induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3:193–195.
- , , . Phentolamine use in a neonate for the prevention of dermal necrosis caused by dopamine: a case report. J Perinatol. 2001;21:324–326.
- , , , , . Images in vascular medicine: rapid epinephrine 'reversal' with phentolamine following accidental autoinjector inoculation. Vasc Med. 2011;16:215–216.
- , . Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014 8;48:870–886.
- , . Reversal of dopamine extravasation injury with topical nitroglycerin ointment. Plast Reconstr Surg. 1989;84:811–813.
- , . Treatment of peripheral tissue ischemia with topical nitroglycerin ointment in neonates. J Pediatr. 1992;121:980–983.
- , , . Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178.
- , . Does the central venous pressure predict fluid responsiveness? An updated meta‐analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370:1683–1693.
Vasoactive medications (VMs) are often required to improve hemodynamic function in patients with shock. They are usually given through central venous catheter (CVC) access, primarily out of concern that extravasation of peripheral intravenous (PIV) access may result in local tissue injury due to the vasoconstrictive effect of the VM. However, insertion of CVC is associated with a variety of mechanical complications and risk of central lineassociated bacteremia. To examine the feasibility and safety of using VM via PIV access, we report on the administration of VM in the form of norepinephrine, dopamine, and phenylephrine via PIV access, with the rationale that this would be a method of reducing the need of CVC use. Our hypotheses are that VM via PIV access is both feasible and safe.
MATERIAL AND METHODS
Study Design
This was a single‐arm, consecutive‐patient study conducted from September 2012 to June 2014. The study site was an 18‐bed medical intensive care unit (MICU) staffed by full‐time attendings, fellows, and residents at the Long Island Jewish Medical Center, which is an 827‐bed tertiary care teaching hospital that is part of the North ShoreLong Island Jewish Health System. The primary outcome measure was the rate of local tissue injury resulting from use of VM via PIV access. The study was approved by the hospital institutional review board (study #13583A), which waived requirement for informed consent.
Protocol for Administration of VM via PIV Access
In cooperation with the Department of Pharmacy, medical and nursing staff developed a written protocol for administration of VM via PIV access. The protocol was reviewed and approved by the hospital pharmacy and therapeutics committee and the MICU nursing leadership. The MICU nursing staff received in‐service training before rollout of the protocol, which included training on the recognition of PIV access extravasation and the type of line that could be used. The MICU housestaff teams were given specific instructions concerning the protocol during their MICU rotations. A summary of the requirements for PIV access for VM use is summarized in Table 1.
|
| Vein diameter >4 mm measured with ultrasonography |
| Position of PIV access documented to be in the vein with ultrasonography before starting infusion of VM |
| Upper extremity only, contralateral to the blood pressure cuff |
| Intravenous line size 20 gauge or 18 gauge |
| No hand, wrist, or antecubital fossa PIV access position |
| Blood return from the PIV access prior to VM administration |
| Assessment of PIV access function every 2 hours as per nursing protocol |
| Immediate alert by nursing staff to the medical team if line extravasation, with prompt initiation of local treatment |
| 72 hours maximum duration of PIV access use |
Patient Management
The decision to initiate treatment with VM was made by the clinical management team. The standard concentrations of VM for use via PIV access were: norepinephrine 8 mg or 16 mg/250 mL normal saline, dopamine 400 mg or 800 mg/250 mL D5W, and phenylephrine 80 mg or 160 mg/500 mL normal saline. If the attending or fellow in charge of the case decided that VM should be administered through PIV access, peripheral access was established that conformed to the requirements of the protocol, and VM was administered via PIV access for as long as there was clinical indication or until PIV access suitable for VM administration was no longer feasible. If the patient received VM via PIV access, a second PIV access site was established in case of failure of the primary PIV site. If no PIV access could be inserted, the patient received CVC access. The decision to use VM via CVC access was made by the clinical management team, as was the type, dose, and duration of the VM use via PIV access or CVC access. Vasopressin was not used via PIV access. Dobutamine was used via PIV access but not recorded in our results, as it has no ‐mediated vasoconstrictor effect. Dobutamine was not used concomitantly with other vasoactive medication through the same PIV access. If PIV access was not established using standard technique by nursing staff, medical residents or critical care fellows inserted PIV access using real‐time ultrasound guidance. The PIV access use for VM could also be used for other medications providing they were compatible with the VM. Only 1 type of VM was infused through the PIV access.
As indicated in Table 1, the nursing staff examined the PIV access site every 2 hours and checked that blood could be aspirated from the line. The aspiration of the line requires several seconds of discontinuation of VM use, which we considered to have no clinical relevance. If the nursing staff identified extravasation of the PIV access site through which VM was infusing, they notified the medical housestaff, who promptly initiated treatment with local injection of phentolamine and local application of nitroglycerin paste as described in Table 2. The extravasation site was examined for tissue injury on a shift basis by the nursing staff, and on bedside rounds by the attending and fellow for at least 48 hours following PIV access removal. Tissue injury was defined as any erythema, blistering, skin breakdown, or necrosis in the site of extravasation.
|
| 1. The VM via PIV infusion is stopped immediately. |
| 2. Residual medication is aspirated through the PIV access, and the catheter is removed. |
| 3. The extent of the extravasation is outlined to provide a baseline for monitoring. |
| 4. Two vials, each containing 5 mg of phentolamine, are reconstituted with 5 mL of normal saline per vial to yield a final concentration of 1 mg/mL. |
| 5. The phentolamine solution is injected in 0.5‐ to 1‐mL aliquots in 5 separate injections around the leading edge of the extravasation, using separate 25‐gauge or 27‐gauge needles for each injection. |
| 6. Nitroglycerin paste (2.5 cm) is applied to the area of extravasation. |
| 7. A medication occurrence report is filled out for review by the quality committee. |
Data were collected prospectively by an investigator (J.C.‐G.) and entered into a standard data‐collection sheet for quality and safety assessment for the initial 13 months of the study. In the subsequent 7 months of observation, data were collected from retrospective chart review. The initial 13 months of data collection were performed as an ongoing safety analysis project; the subsequent 7‐month review was performed as an additional quality assessment project. The deidentified data included patient demographics, patient disease characteristics, use of VM, and VM via PIV access complications.
Statistical Analysis
The statistical analysis was performed using SPSS 21 (Statistical Package for the Social Sciences; IBM, Armonk, NY). Continuous variables are presented as meanstandard deviation.
RESULTS
Characteristics of patients who received VM via PIV access are presented in Table 3. During the study period, there were 2462 admissions to the MICU, and 267 CVCs were inserted by the MICU team, 170 of which were triple‐lumen catheters and 97 were large‐gauge catheters for hemodialysis or plasmapheresis. Of the total admissions, 953 cases received VM; 783/953 (82%) received VM via PIV access, and 170/953 received VM via CVC access (18%). For VM use, an 18‐gauge PIV catheter was used in 192/783 (25%), a 20‐gauge catheter was used in 590/783 (75%), and a 22‐gauge catheter was used in 1/783 of interventions. Catheter length was 30 mm, 45 mm, or 48 mm depending on availability. The 22‐gauge catheter, which was a deviation from standard protocol, infiltrated shortly following insertion. We did not formally record the anatomic position of the PIV access in the standard data‐collection sheet; anecdotally, the majority of PIV accesses were placed in the upper arm basilic or cephalic vein. The duration of VM via PIV access was 4922 hours. Central intravenous access was required in 95/734 (13%) of patients who initially had VM via PIV access. These catheters are included in the 170 triple‐lumen CVCs that were inserted by the MICU team during the study period. The type and highest dose of VM administered via PIV access are presented in Table 4.
| Total Study Group | |
|---|---|
| |
| No. of patients | 734 |
| Age, y | 7215 |
| Gender | |
| Male | 398 (54%) |
| Female | 336 (46%) |
| SAPS II score | 7515 |
| Patients on mechanical ventilation | 235 (32%) |
| Patients on hemodialysis | 90 (12%) |
| MICU mortality | 177 (23%) |
| Use of VM via PIV access | 783 |
| Extravasations of VM via PIV access | 19 (2%) |
| Total MICU admissions during study period | 2,462 |
| |
| Norepinephrine | |
| Interventions | 506 |
| Dose, g/kg/min, meanSD | 0.700.23 |
| PIV access extravasations | 16 |
| Dopamine | |
| Interventions | 101 |
| Dose, g/kg/min, meanSD | 12.75.23 |
| PIV access extravasations | 3 |
| Phenylephrine | |
| Interventions | 176 |
| Dose, g/kg/min, meanSD | 3.251.69 |
| PIV access extravasations | 0 |
A total of 734 patients received VM via PIV access during the 20‐month study period; 49 of these patients required 2 or more PIV access insertions, as the initial and/or subsequent site timed out at 72 hours, resulting in a total of 783 separate interventions. Infiltration of the PIV access site occurred in 19/783 (2%) of interventions. All of them were identified by nursing staff with prompt response using local injection of phentolamine and application of nitroglycerin paste at the site of the extravasation. There was no tissue injury at the site of VM extravasation. Sixteen of the extravasations occurred with norepinephrine infusions and 3 with dopamine infusions. There were no infections of the PIV access sites used for VM. Use of phentolamine and nitroglycerin paste was not associated with hypotension, as defined as mean arterial pressure less than 65 mm Hg.
DISCUSSION
Our study demonstrates that administration of VM via PIV access is feasible, carries a low rate of complications, and offers an alternative to CVC access. There are several elements that may have allowed safe use of VM via PIV access. We developed a protocol that involved a multidisciplinary team. The hospital pharmacy performed an extensive literature search and formulated the initial protocol with the MICU attending staff. The protocol was then subjected to iterative process improvement by a hospital committee and nursing leadership in the MICU. Before program rollout, the MICU nursing staff were educated and trained to use the protocol. This was a key component of the program, as the nurses were responsible for many of the line insertions, line maintenance, and identification of infiltration. Although we did not perform any formal measurement of the impact of PIV access use on nursing workflow, we note that leadership and frontline nurses have been enthusiastic about the implementation of VM via PIV access. The MICU housestaff teams were given an in‐service instruction concerning the importance of prompt initiation of local treatment in case of infiltration of the PIV access site. Specific elements of the protocol that may have improved safety were the use of ultrasonography to insert difficult PIV access and confirmation of all PIV access insertions using ultrasonography by the MICU housestaff. The requirement for frequent checks of PIV access function, prompt recognition of infiltration, and specific antidote to extravasation were important elements of safety. The low rate of PIV access extravasation (2%) may be related to the use of ultrasonography to guide PIV access insertion in patients who had challenging anatomy (eg, obesity, edema, recreational drug use, history of multiple PIV insertions), and ultrasonography was used to check that the PIV access was well positioned before VM infusion.
There were early literature reports that subcutaneous extravasation of catecholamines could result in local ischemic injury both in human patients and animal models.[1, 2, 3, 4, 5] Local phentolamine injection has been identified as a specific antidote to block the local ischemic injury.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] More recently, there have been anecdotal reports showing that local application of nitroglycerin paste blocks ischemic injury in the pediatric population.[5, 16, 17] With this information, our protocol included the requirement of prompt treatment of local extravasation with phentolamine and nitroglycerin paste at the site of VM via PIV access extravasation. In theory, both phentolamine and nitroglycerin might cause hypotension. In our study, administration of both phentolamine and nitroglycerin paste was not associated with more hypotension nor did it increase requirements for VM.
Multilumen small bore CVCs may be used for several reasons, some of which need to be reconsidered. First, before introduction of the VM via PIV access protocol, a common indication for triple lumen CVC insertion in our MICU was the perception that VM could only be administered through CVC access, for fear of local tissue injury should extravasation of the VM occur through the PIV access site. Our results indicate that VM use is not an automatic indication for CVC insertion. Second, a possible indication for CVC insertion is to measure central venous pressure for the purpose of guiding volume resuscitation in patients with hemodynamic failure. As the utility of central venous pressure monitoring has been called into serious question,[18, 19, 20] we do not consider this indication for CVC use to be valid. Third, CVC access may be required due to anatomic constraints (ie, there is no suitable PIV site). Fourth, there may be need for such a large number of intravenous medications that PIV access cannot support. Fifth, there is occasional situation where the patient requires use of medications where extravasation of PIV access would cause local tissue injury without local antidote (eg, certain chemotherapeutic agents). The continued need for CVC access in some patients is reflected in the finding that 13% of our study patients who received VM via PIV access eventually required triple‐lumen CVC insertion. However, our results indicate that the rate of CVC use may be reduced by using PIV access for VM administration.
Our study has some methodological limitations. Study design was single center and observational. The focus of this study was to examine the safety of VM via PIV access. We cannot comment on its effectiveness, indications, or influence on patient outcome nor on why some patients required CVC insertion whereas others did not. The decision to administer VM was made by the clinical team, as was the route of its administration and concentration, without any input from the investigators. We did not collect data on who performed the PIV access insertion (medical or nursing staff), demographics, and disease characteristics of the CVC group, nor to what extent ultrasonography was used to guide PIV insertion. We did not attempt to define whether there were any factors that identified risk for PIV access extravasation, nor did we evaluate for any differences between the PIV and CVC group in terms of demographics and disease characteristics. Lacking a control group, we cannot say definitively that VM via PIV access is safer than VM via CVC. Being a single‐center study, it is not possible to say that the results are transferable to another clinical environment; this applies particularly to the use of ultrasonography, which is a user‐dependent skill. We cannot determine which, if any, component of the protocol was responsible for the safe use of VM via PIV access. The rate of PIV access extravasation was low, so it is possible that a larger sample size is required to identify incidents of tissue necrosis from extravasation of VM delivered via PIV access despite the use of local antidote.
CONCLUSIONS
The delivery of VM via PIV access is safe and feasible. Tto reduce the risk of extravasation leading to possible local tissue injury, we developed a protocol that emphasized close cooperation between the nursing and medical staff, routine use of ultrasonography, rapid identification of extravasation of the PIV access, and prompt response to local extravasation of VM using phentolamine and nitroglycerin paste. This approach offers a means of reducing CVC use, in both intensive care unit (ICU) and non‐ICU settings, including hospital wards and emergency departments. Clinicians should no longer consider administration of norepinephrine, dopamine, or phenylephrine to be an automatic indication for CVC access. This study focused on the safety of VM administered via PIV access, with emphasis on local complications related to extravasation, and should be considered a preliminary single‐center study that demonstrates that administration of certain vasoactive medications may not universally require central venous access. A broader study regarding assessment of safety and efficacy will require a multicenter design.
Disclosures
J.C.‐G., K.F.S., Y.G.B., M.N., S.J.K., and P.H.M. participated in the study design, statistical review, and manuscript writing. J.C.‐G. is the guarantor of the article, taking responsibility for the integrity of the work as a whole from inception to published article. This work is original, and all authors meet the criteria for authorship, including acceptance of responsibility for the scientific content of the article. This article is not under consideration in any other journal, and all of the authors have read and approved the content of the article. No potential conflict of interest exists with any companies or organizations whose products or services are discussed in this article. This article has not been funded by the National Institutes of Health, the Wellcome Trust, or their agencies. All financial support of the study was derived from the Division of Pulmonary, Critical Care and Sleep Medicine at North ShoreLong Island Jewish Medical Center, New Hyde Park, New York.
Vasoactive medications (VMs) are often required to improve hemodynamic function in patients with shock. They are usually given through central venous catheter (CVC) access, primarily out of concern that extravasation of peripheral intravenous (PIV) access may result in local tissue injury due to the vasoconstrictive effect of the VM. However, insertion of CVC is associated with a variety of mechanical complications and risk of central lineassociated bacteremia. To examine the feasibility and safety of using VM via PIV access, we report on the administration of VM in the form of norepinephrine, dopamine, and phenylephrine via PIV access, with the rationale that this would be a method of reducing the need of CVC use. Our hypotheses are that VM via PIV access is both feasible and safe.
MATERIAL AND METHODS
Study Design
This was a single‐arm, consecutive‐patient study conducted from September 2012 to June 2014. The study site was an 18‐bed medical intensive care unit (MICU) staffed by full‐time attendings, fellows, and residents at the Long Island Jewish Medical Center, which is an 827‐bed tertiary care teaching hospital that is part of the North ShoreLong Island Jewish Health System. The primary outcome measure was the rate of local tissue injury resulting from use of VM via PIV access. The study was approved by the hospital institutional review board (study #13583A), which waived requirement for informed consent.
Protocol for Administration of VM via PIV Access
In cooperation with the Department of Pharmacy, medical and nursing staff developed a written protocol for administration of VM via PIV access. The protocol was reviewed and approved by the hospital pharmacy and therapeutics committee and the MICU nursing leadership. The MICU nursing staff received in‐service training before rollout of the protocol, which included training on the recognition of PIV access extravasation and the type of line that could be used. The MICU housestaff teams were given specific instructions concerning the protocol during their MICU rotations. A summary of the requirements for PIV access for VM use is summarized in Table 1.
|
| Vein diameter >4 mm measured with ultrasonography |
| Position of PIV access documented to be in the vein with ultrasonography before starting infusion of VM |
| Upper extremity only, contralateral to the blood pressure cuff |
| Intravenous line size 20 gauge or 18 gauge |
| No hand, wrist, or antecubital fossa PIV access position |
| Blood return from the PIV access prior to VM administration |
| Assessment of PIV access function every 2 hours as per nursing protocol |
| Immediate alert by nursing staff to the medical team if line extravasation, with prompt initiation of local treatment |
| 72 hours maximum duration of PIV access use |
Patient Management
The decision to initiate treatment with VM was made by the clinical management team. The standard concentrations of VM for use via PIV access were: norepinephrine 8 mg or 16 mg/250 mL normal saline, dopamine 400 mg or 800 mg/250 mL D5W, and phenylephrine 80 mg or 160 mg/500 mL normal saline. If the attending or fellow in charge of the case decided that VM should be administered through PIV access, peripheral access was established that conformed to the requirements of the protocol, and VM was administered via PIV access for as long as there was clinical indication or until PIV access suitable for VM administration was no longer feasible. If the patient received VM via PIV access, a second PIV access site was established in case of failure of the primary PIV site. If no PIV access could be inserted, the patient received CVC access. The decision to use VM via CVC access was made by the clinical management team, as was the type, dose, and duration of the VM use via PIV access or CVC access. Vasopressin was not used via PIV access. Dobutamine was used via PIV access but not recorded in our results, as it has no ‐mediated vasoconstrictor effect. Dobutamine was not used concomitantly with other vasoactive medication through the same PIV access. If PIV access was not established using standard technique by nursing staff, medical residents or critical care fellows inserted PIV access using real‐time ultrasound guidance. The PIV access use for VM could also be used for other medications providing they were compatible with the VM. Only 1 type of VM was infused through the PIV access.
As indicated in Table 1, the nursing staff examined the PIV access site every 2 hours and checked that blood could be aspirated from the line. The aspiration of the line requires several seconds of discontinuation of VM use, which we considered to have no clinical relevance. If the nursing staff identified extravasation of the PIV access site through which VM was infusing, they notified the medical housestaff, who promptly initiated treatment with local injection of phentolamine and local application of nitroglycerin paste as described in Table 2. The extravasation site was examined for tissue injury on a shift basis by the nursing staff, and on bedside rounds by the attending and fellow for at least 48 hours following PIV access removal. Tissue injury was defined as any erythema, blistering, skin breakdown, or necrosis in the site of extravasation.
|
| 1. The VM via PIV infusion is stopped immediately. |
| 2. Residual medication is aspirated through the PIV access, and the catheter is removed. |
| 3. The extent of the extravasation is outlined to provide a baseline for monitoring. |
| 4. Two vials, each containing 5 mg of phentolamine, are reconstituted with 5 mL of normal saline per vial to yield a final concentration of 1 mg/mL. |
| 5. The phentolamine solution is injected in 0.5‐ to 1‐mL aliquots in 5 separate injections around the leading edge of the extravasation, using separate 25‐gauge or 27‐gauge needles for each injection. |
| 6. Nitroglycerin paste (2.5 cm) is applied to the area of extravasation. |
| 7. A medication occurrence report is filled out for review by the quality committee. |
Data were collected prospectively by an investigator (J.C.‐G.) and entered into a standard data‐collection sheet for quality and safety assessment for the initial 13 months of the study. In the subsequent 7 months of observation, data were collected from retrospective chart review. The initial 13 months of data collection were performed as an ongoing safety analysis project; the subsequent 7‐month review was performed as an additional quality assessment project. The deidentified data included patient demographics, patient disease characteristics, use of VM, and VM via PIV access complications.
Statistical Analysis
The statistical analysis was performed using SPSS 21 (Statistical Package for the Social Sciences; IBM, Armonk, NY). Continuous variables are presented as meanstandard deviation.
RESULTS
Characteristics of patients who received VM via PIV access are presented in Table 3. During the study period, there were 2462 admissions to the MICU, and 267 CVCs were inserted by the MICU team, 170 of which were triple‐lumen catheters and 97 were large‐gauge catheters for hemodialysis or plasmapheresis. Of the total admissions, 953 cases received VM; 783/953 (82%) received VM via PIV access, and 170/953 received VM via CVC access (18%). For VM use, an 18‐gauge PIV catheter was used in 192/783 (25%), a 20‐gauge catheter was used in 590/783 (75%), and a 22‐gauge catheter was used in 1/783 of interventions. Catheter length was 30 mm, 45 mm, or 48 mm depending on availability. The 22‐gauge catheter, which was a deviation from standard protocol, infiltrated shortly following insertion. We did not formally record the anatomic position of the PIV access in the standard data‐collection sheet; anecdotally, the majority of PIV accesses were placed in the upper arm basilic or cephalic vein. The duration of VM via PIV access was 4922 hours. Central intravenous access was required in 95/734 (13%) of patients who initially had VM via PIV access. These catheters are included in the 170 triple‐lumen CVCs that were inserted by the MICU team during the study period. The type and highest dose of VM administered via PIV access are presented in Table 4.
| Total Study Group | |
|---|---|
| |
| No. of patients | 734 |
| Age, y | 7215 |
| Gender | |
| Male | 398 (54%) |
| Female | 336 (46%) |
| SAPS II score | 7515 |
| Patients on mechanical ventilation | 235 (32%) |
| Patients on hemodialysis | 90 (12%) |
| MICU mortality | 177 (23%) |
| Use of VM via PIV access | 783 |
| Extravasations of VM via PIV access | 19 (2%) |
| Total MICU admissions during study period | 2,462 |
| |
| Norepinephrine | |
| Interventions | 506 |
| Dose, g/kg/min, meanSD | 0.700.23 |
| PIV access extravasations | 16 |
| Dopamine | |
| Interventions | 101 |
| Dose, g/kg/min, meanSD | 12.75.23 |
| PIV access extravasations | 3 |
| Phenylephrine | |
| Interventions | 176 |
| Dose, g/kg/min, meanSD | 3.251.69 |
| PIV access extravasations | 0 |
A total of 734 patients received VM via PIV access during the 20‐month study period; 49 of these patients required 2 or more PIV access insertions, as the initial and/or subsequent site timed out at 72 hours, resulting in a total of 783 separate interventions. Infiltration of the PIV access site occurred in 19/783 (2%) of interventions. All of them were identified by nursing staff with prompt response using local injection of phentolamine and application of nitroglycerin paste at the site of the extravasation. There was no tissue injury at the site of VM extravasation. Sixteen of the extravasations occurred with norepinephrine infusions and 3 with dopamine infusions. There were no infections of the PIV access sites used for VM. Use of phentolamine and nitroglycerin paste was not associated with hypotension, as defined as mean arterial pressure less than 65 mm Hg.
DISCUSSION
Our study demonstrates that administration of VM via PIV access is feasible, carries a low rate of complications, and offers an alternative to CVC access. There are several elements that may have allowed safe use of VM via PIV access. We developed a protocol that involved a multidisciplinary team. The hospital pharmacy performed an extensive literature search and formulated the initial protocol with the MICU attending staff. The protocol was then subjected to iterative process improvement by a hospital committee and nursing leadership in the MICU. Before program rollout, the MICU nursing staff were educated and trained to use the protocol. This was a key component of the program, as the nurses were responsible for many of the line insertions, line maintenance, and identification of infiltration. Although we did not perform any formal measurement of the impact of PIV access use on nursing workflow, we note that leadership and frontline nurses have been enthusiastic about the implementation of VM via PIV access. The MICU housestaff teams were given an in‐service instruction concerning the importance of prompt initiation of local treatment in case of infiltration of the PIV access site. Specific elements of the protocol that may have improved safety were the use of ultrasonography to insert difficult PIV access and confirmation of all PIV access insertions using ultrasonography by the MICU housestaff. The requirement for frequent checks of PIV access function, prompt recognition of infiltration, and specific antidote to extravasation were important elements of safety. The low rate of PIV access extravasation (2%) may be related to the use of ultrasonography to guide PIV access insertion in patients who had challenging anatomy (eg, obesity, edema, recreational drug use, history of multiple PIV insertions), and ultrasonography was used to check that the PIV access was well positioned before VM infusion.
There were early literature reports that subcutaneous extravasation of catecholamines could result in local ischemic injury both in human patients and animal models.[1, 2, 3, 4, 5] Local phentolamine injection has been identified as a specific antidote to block the local ischemic injury.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] More recently, there have been anecdotal reports showing that local application of nitroglycerin paste blocks ischemic injury in the pediatric population.[5, 16, 17] With this information, our protocol included the requirement of prompt treatment of local extravasation with phentolamine and nitroglycerin paste at the site of VM via PIV access extravasation. In theory, both phentolamine and nitroglycerin might cause hypotension. In our study, administration of both phentolamine and nitroglycerin paste was not associated with more hypotension nor did it increase requirements for VM.
Multilumen small bore CVCs may be used for several reasons, some of which need to be reconsidered. First, before introduction of the VM via PIV access protocol, a common indication for triple lumen CVC insertion in our MICU was the perception that VM could only be administered through CVC access, for fear of local tissue injury should extravasation of the VM occur through the PIV access site. Our results indicate that VM use is not an automatic indication for CVC insertion. Second, a possible indication for CVC insertion is to measure central venous pressure for the purpose of guiding volume resuscitation in patients with hemodynamic failure. As the utility of central venous pressure monitoring has been called into serious question,[18, 19, 20] we do not consider this indication for CVC use to be valid. Third, CVC access may be required due to anatomic constraints (ie, there is no suitable PIV site). Fourth, there may be need for such a large number of intravenous medications that PIV access cannot support. Fifth, there is occasional situation where the patient requires use of medications where extravasation of PIV access would cause local tissue injury without local antidote (eg, certain chemotherapeutic agents). The continued need for CVC access in some patients is reflected in the finding that 13% of our study patients who received VM via PIV access eventually required triple‐lumen CVC insertion. However, our results indicate that the rate of CVC use may be reduced by using PIV access for VM administration.
Our study has some methodological limitations. Study design was single center and observational. The focus of this study was to examine the safety of VM via PIV access. We cannot comment on its effectiveness, indications, or influence on patient outcome nor on why some patients required CVC insertion whereas others did not. The decision to administer VM was made by the clinical team, as was the route of its administration and concentration, without any input from the investigators. We did not collect data on who performed the PIV access insertion (medical or nursing staff), demographics, and disease characteristics of the CVC group, nor to what extent ultrasonography was used to guide PIV insertion. We did not attempt to define whether there were any factors that identified risk for PIV access extravasation, nor did we evaluate for any differences between the PIV and CVC group in terms of demographics and disease characteristics. Lacking a control group, we cannot say definitively that VM via PIV access is safer than VM via CVC. Being a single‐center study, it is not possible to say that the results are transferable to another clinical environment; this applies particularly to the use of ultrasonography, which is a user‐dependent skill. We cannot determine which, if any, component of the protocol was responsible for the safe use of VM via PIV access. The rate of PIV access extravasation was low, so it is possible that a larger sample size is required to identify incidents of tissue necrosis from extravasation of VM delivered via PIV access despite the use of local antidote.
CONCLUSIONS
The delivery of VM via PIV access is safe and feasible. Tto reduce the risk of extravasation leading to possible local tissue injury, we developed a protocol that emphasized close cooperation between the nursing and medical staff, routine use of ultrasonography, rapid identification of extravasation of the PIV access, and prompt response to local extravasation of VM using phentolamine and nitroglycerin paste. This approach offers a means of reducing CVC use, in both intensive care unit (ICU) and non‐ICU settings, including hospital wards and emergency departments. Clinicians should no longer consider administration of norepinephrine, dopamine, or phenylephrine to be an automatic indication for CVC access. This study focused on the safety of VM administered via PIV access, with emphasis on local complications related to extravasation, and should be considered a preliminary single‐center study that demonstrates that administration of certain vasoactive medications may not universally require central venous access. A broader study regarding assessment of safety and efficacy will require a multicenter design.
Disclosures
J.C.‐G., K.F.S., Y.G.B., M.N., S.J.K., and P.H.M. participated in the study design, statistical review, and manuscript writing. J.C.‐G. is the guarantor of the article, taking responsibility for the integrity of the work as a whole from inception to published article. This work is original, and all authors meet the criteria for authorship, including acceptance of responsibility for the scientific content of the article. This article is not under consideration in any other journal, and all of the authors have read and approved the content of the article. No potential conflict of interest exists with any companies or organizations whose products or services are discussed in this article. This article has not been funded by the National Institutes of Health, the Wellcome Trust, or their agencies. All financial support of the study was derived from the Division of Pulmonary, Critical Care and Sleep Medicine at North ShoreLong Island Jewish Medical Center, New Hyde Park, New York.
- , , . Cutaneous necrosis due to norepinephrine. II. Mechanism and prevention. Ann Surg. 1958;147:44–50.
- , , . Pedal gangrene associated with the use of dopamine. N Engl J Med. 1975;293:591.
- , . Gangrene aggravation after use of dopamine [letter]. JAMA. 1976;235:2812.
- , . Dopamine gangrene [letter]. N Engl J Med. 1976;294:114.
- , . Extravasation injury associated with low‐dose dopamine. Ann Pharmacother. 1998;32:545–548.
- . Use of phenytolamine to prevent necrosis due to levarterenol. JAMA. 1957;163:1477–1479.
- . Phentolamine hydrochloride in prevention of cutaneous necrosis due to levarterenol. JAMA. 1959;170:1916–1917.
- , . Avoidance of vascular complications associated with the use of dopamine. Can Anaesth Soc J. 1977;24:727–733.
- . Management of intravenous extravasations. Infusion. 1984;6:77–79.
- , . Acute management of dopamine infiltration injury with Regitine. Plast Reconstr Surg. 1987;80:610–612.
- . High dose phentolamine for extravasation of pressors [letter]. Clin Pharm. 1989;8:689.
- , . Phentolamine reversal of epinephrine‐induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3:193–195.
- , , . Phentolamine use in a neonate for the prevention of dermal necrosis caused by dopamine: a case report. J Perinatol. 2001;21:324–326.
- , , , , . Images in vascular medicine: rapid epinephrine 'reversal' with phentolamine following accidental autoinjector inoculation. Vasc Med. 2011;16:215–216.
- , . Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014 8;48:870–886.
- , . Reversal of dopamine extravasation injury with topical nitroglycerin ointment. Plast Reconstr Surg. 1989;84:811–813.
- , . Treatment of peripheral tissue ischemia with topical nitroglycerin ointment in neonates. J Pediatr. 1992;121:980–983.
- , , . Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178.
- , . Does the central venous pressure predict fluid responsiveness? An updated meta‐analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370:1683–1693.
- , , . Cutaneous necrosis due to norepinephrine. II. Mechanism and prevention. Ann Surg. 1958;147:44–50.
- , , . Pedal gangrene associated with the use of dopamine. N Engl J Med. 1975;293:591.
- , . Gangrene aggravation after use of dopamine [letter]. JAMA. 1976;235:2812.
- , . Dopamine gangrene [letter]. N Engl J Med. 1976;294:114.
- , . Extravasation injury associated with low‐dose dopamine. Ann Pharmacother. 1998;32:545–548.
- . Use of phenytolamine to prevent necrosis due to levarterenol. JAMA. 1957;163:1477–1479.
- . Phentolamine hydrochloride in prevention of cutaneous necrosis due to levarterenol. JAMA. 1959;170:1916–1917.
- , . Avoidance of vascular complications associated with the use of dopamine. Can Anaesth Soc J. 1977;24:727–733.
- . Management of intravenous extravasations. Infusion. 1984;6:77–79.
- , . Acute management of dopamine infiltration injury with Regitine. Plast Reconstr Surg. 1987;80:610–612.
- . High dose phentolamine for extravasation of pressors [letter]. Clin Pharm. 1989;8:689.
- , . Phentolamine reversal of epinephrine‐induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3:193–195.
- , , . Phentolamine use in a neonate for the prevention of dermal necrosis caused by dopamine: a case report. J Perinatol. 2001;21:324–326.
- , , , , . Images in vascular medicine: rapid epinephrine 'reversal' with phentolamine following accidental autoinjector inoculation. Vasc Med. 2011;16:215–216.
- , . Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014 8;48:870–886.
- , . Reversal of dopamine extravasation injury with topical nitroglycerin ointment. Plast Reconstr Surg. 1989;84:811–813.
- , . Treatment of peripheral tissue ischemia with topical nitroglycerin ointment in neonates. J Pediatr. 1992;121:980–983.
- , , . Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178.
- , . Does the central venous pressure predict fluid responsiveness? An updated meta‐analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370:1683–1693.
© 2015 Society of Hospital Medicine
Variation in Printed Handoff Documents
Handoffs among hospital providers are highly error prone and can result in serious morbidity and mortality. Best practices for verbal handoffs have been described[1, 2, 3, 4] and include conducting verbal handoffs face to face, providing opportunities for questions, having the receiver perform a readback, as well as specific content recommendations including action items. Far less research has focused on best practices for printed handoff documents,[5, 6] despite the routine use of written handoff tools as a reference by on‐call physicians.[7, 8] Erroneous or outdated information on the written handoff can mislead on‐call providers, potentially leading to serious medical errors.
In their most basic form, printed handoff documents list patients for whom a provider is responsible. Typically, they also contain demographic information, reason for hospital admission, and a task list for each patient. They may also contain more detailed information on patient history, hospital course, and/or care plan, and may vary among specialties.[9] They come in various forms, ranging from index cards with handwritten notes, to word‐processor or spreadsheet documents, to printed documents that are autopopulated from the electronic health record (EHR).[2] Importantly, printed handoff documents supplement the verbal handoff by allowing receivers to follow along as patients are presented. The concurrent use of written and verbal handoffs may improve retention of clinical information as compared with either alone.[10, 11]
The Joint Commission requires an institutional approach to patient handoffs.[12] The requirements state that handoff communication solutions should take a standardized form, but they do not provide details regarding what data elements should be included in printed or verbal handoffs. Accreditation Council for Graduate Medical Education Common Program Requirements likewise require that residents must become competent in patient handoffs[13] but do not provide specific details or measurement tools. Absent widely accepted guidelines, decisions regarding which elements to include in printed handoff documents are currently made at an individual or institutional level.
The I‐PASS study is a federally funded multi‐institutional project that demonstrated a decrease in medical errors and preventable adverse events after implementation of a standardized resident handoff bundle.[14, 15] The I‐PASS Study Group developed a bundle of handoff interventions, beginning with a handoff and teamwork training program (based in part on TeamSTEPPS [Team Strategies and Tools to Enhance Performance and Patient Safety]),[16] a novel verbal mnemonic, I‐PASS (Illness Severity, Patient Summary, Action List, Situation Awareness and Contingency Planning, and Synthesis by Receiver),[17] and changes to the verbal handoff process, in addition to several other elements.
We hypothesized that developing a standardized printed handoff template would reinforce the handoff training and enhance the value of the verbal handoff process changes. Given the paucity of data on best printed handoff practices, however, we first conducted a needs assessment to identify which data elements were currently contained in printed handoffs across sites, and to allow an expert panel to make recommendations for best practices.
METHODS
I‐PASS Study sites included 9 pediatric residency programs at academic medical centers from across North America. Programs were identified through professional networks and invited to participate. The nonintensive care unit hospitalist services at these medical centers are primarily staffed by residents and medical students with attending supervision. At 1 site, nurse practitioners also participate in care. Additional details about study sites can be found in the study descriptions previously published.[14, 15] All sites received local institutional review board approval.
We began by inviting members of the I‐PASS Education Executive Committee (EEC)[14] to build a collective, comprehensive list of possible data elements for printed handoff documents. This committee included pediatric residency program directors, pediatric hospitalists, education researchers, health services researchers, and patient safety experts. We obtained sample handoff documents from pediatric hospitalist services at each of 9 institutions in the United States and Canada (with protected health information redacted). We reviewed these sample handoff documents to characterize their format and to determine what discrete data elements appeared in each site's printed handoff document. Presence or absence of each data element across sites was tabulated. We also queried sites to determine the feasibility of including elements that were not presently included.
Subsequently, I‐PASS site investigators led structured group interviews at participating sites to gather additional information about handoff practices at each site. These structured group interviews included diverse representation from residents, faculty, and residency program leadership, as well as hospitalists and medical students, to ensure the comprehensive acquisition of information regarding site‐specific characteristics. Each group provided answers to a standardized set of open‐ended questions that addressed current practices, handoff education, simulation use, team structure, and the nature of current written handoff tools, if applicable, at each site. One member of the structured group interview served as a scribe and created a document that summarized the content of the structured group interview meeting and answers to the standardized questions.
Consensus on Content
The initial data collection also included a multivote process[18] of the full I‐PASS EEC to help prioritize data elements. Committee members brainstormed a list of all possible data elements for a printed handoff document. Each member (n=14) was given 10 votes to distribute among the elements. Committee members could assign more than 1 vote to an element to emphasize its importance.
The results of this process as well as the current data elements included in each printed handoff tool were reviewed by a subgroup of the I‐PASS EEC. These expert panel members participated in a series of conference calls during which they tabulated categorical information, reviewed narrative comments, discussed existing evidence, and conducted simple content analysis to identify areas of concordance or discordance. Areas of discordance were discussed by the committee. Disagreements were resolved with group consensus with attention to published evidence or best practices, if available.
Elements were divided into those that were essential (unanimous consensus, no conflicting literature) and those that were recommended (majority supported inclusion of element, no conflicting literature). Ratings were assigned using the American College of Cardiology/American Heart Association framework for practice guidelines,[19] in which each element is assigned a classification (I=effective, II=conflicting evidence/opinion, III=not effective) and a level of evidence to support that classification (A=multiple large randomized controlled trials, B=single randomized trial, or nonrandomized studies, C=expert consensus).
The expert panel reached consensus, through active discussion, on a list of data elements that should be included in an ideal printed handoff document. Elements were chosen based on perceived importance, with attention to published best practices[1, 16] and the multivoting results. In making recommendations, consideration was given to whether data elements could be electronically imported into the printed handoff document from the EHR, or whether they would be entered manually. The potential for serious medical errors due to possible errors in manual entry of data was an important aspect of recommendations made. The list of candidate elements was then reviewed by a larger group of investigators from the I‐PASS Education Executive Committee and Coordinating Council for additional input.
The panel asked site investigators from each participating hospital to gather data on the feasibility of redesigning the printed handoff at that hospital to include each recommended element. Site investigators reported whether each element was already included, possible to include but not included currently, or not currently possible to include within that site's printed handoff tool. Site investigators also reported how data elements were populated in their handoff documents, with options including: (1) autopopulated from administrative data (eg, pharmacy‐entered medication list, demographic data entered by admitting office), (2) autoimported from physicians' free‐text entries elsewhere in the EHR (eg, progress notes), (3) free text entered specifically for the printed handoff, or (4) not applicable (element cannot be included).
RESULTS
Nine programs (100%) provided data on the structure and contents of their printed handoff documents. We found wide variation in structure across the 9 sites. Three sites used a word‐processorbased document that required manual entry of all data elements. The other 6 institutions had a direct link with the EHR to enable autopopulation of between 10 and 20 elements on the printed handoff document.
The content of written handoff documents, as well as the sources of data included in them (present or future), likewise varied substantially across sites (Table 1). Only 4 data elements (name, age, weight, and a list of medications) were universally included at all 9 sites. Among the 6 institutions that linked the printed handoff to the EHR, there was also substantial variation in which elements were autoimported. Only 7 elements were universally autoimported at these 6 sites: patient name, medical record number, room number, weight, date of birth, age, and date of admission. Two elements from the original brainstorming were not presently included in any sites' documents (emergency contact and primary language).
| Data Elements | Sites With Data Element Included at Initial Needs Assessment (Out of Nine Sites) | Data Source (Current or Anticipated) | ||
|---|---|---|---|---|
| Autoimported* | Manually Entered | Not Applicable | ||
| ||||
| Name | 9 | 6 | 3 | 0 |
| Medical record number | 8 | 6 | 3 | 0 |
| Room number | 8 | 6 | 3 | 0 |
| Allergies | 6 | 4 | 5 | 0 |
| Weight | 9 | 6 | 3 | 0 |
| Age | 9 | 6 | 3 | 0 |
| Date of birth | 6 | 6 | 3 | 0 |
| Admission date | 8 | 6 | 3 | 0 |
| Attending name | 5 | 4 | 5 | 0 |
| Team/service | 7 | 4 | 5 | 0 |
| Illness severity | 1 | 0 | 9 | 0 |
| Patient summary | 8 | 0 | 9 | 0 |
| Action items | 8 | 0 | 9 | 0 |
| Situation monitoring/contingency plan | 5 | 0 | 9 | 0 |
| Medication name | 9 | 4 | 5 | 0 |
| Medication name and dose/route/frequency | 4 | 4 | 5 | 0 |
| Code status | 2 | 2 | 7 | 0 |
| Labs | 6 | 5 | 4 | 0 |
| Access | 2 | 2 | 7 | 0 |
| Ins/outs | 2 | 4 | 4 | 1 |
| Primary language | 0 | 3 | 6 | 0 |
| Vital signs | 3 | 4 | 4 | 1 |
| Emergency contact | 0 | 2 | 7 | 0 |
| Primary care provider | 4 | 4 | 5 | 0 |
Nine institutions (100%) conducted structured group interviews, ranging in size from 4 to 27 individuals with a median of 5 participants. The documents containing information from each site were provided to the authors. The authors then tabulated categorical information, reviewed narrative comments to understand current institutional practices, and conducted simple content analysis to identify areas of concordance or discordance, particularly with respect to data elements and EHR usage. Based on the results of the printed handoff document review and structured group interviews, with additional perspectives provided by the I‐PASS EEC, the expert panel came to consensus on a list of 23 elements that should be included in printed handoff documents, including 15 essential data elements and 8 additional recommended elements (Table 2).
|
| Essential Elements |
| Patient identifiers |
| Patient name (class I, level of evidence C) |
| Medical record number (class I, level of evidence C) |
| Date of birth (class I, level of evidence C) |
| Hospital service identifiers |
| Attending name (class I, level of evidence C) |
| Team/service (class I, level of evidence C) |
| Room number (class I, level of evidence C) |
| Admission date (class I, level of evidence C) |
| Age (class I, level of evidence C) |
| Weight (class I, level of evidence C) |
| Illness severity (class I, level of evidence B)[20, 21] |
| Patient summary (class I, level of evidence B)[21, 22] |
| Action items (class I, level of evidence B) [21, 22] |
| Situation awareness/contingency planning (class I, level of evidence B) [21, 22] |
| Allergies (class I, level of evidence C) |
| Medications |
| Autopopulation of medications (class I, level of evidence B)[22, 23, 24] |
| Free‐text entry of medications (class IIa, level of evidence C) |
| Recommended elements |
| Primary language (class IIa, level of evidence C) |
| Emergency contact (class IIa, level of evidence C) |
| Primary care provider (class IIa, level of evidence C) |
| Code status (class IIb, level of evidence C) |
| Labs (class IIa, level of evidence C) |
| Access (class IIa, level of evidence C) |
| Ins/outs (class IIa, level of evidence C) |
| Vital signs (class IIa, level of evidence C) |
Evidence ratings[19] of these elements are included. Several elements are classified as I‐B (effective, nonrandomized studies) based on either studies of individual elements, or greater than 1 study of bundled elements that could reasonably be extrapolated. These include Illness severity,[20, 21] patient summary,[21, 22] action items[21, 22] (to do lists), situation awareness and contingency plan,[21, 22] and medications[22, 23, 24] with attention to importing from the EHR. Medications entered as free text were classified as IIa‐C because of risk and potential significance of errors; in particular there was concern that transcription errors, errors of omission, or errors of commission could potentially lead to patient harms. The remaining essential elements are classified as I‐C (effective, expert consensus). Of note, date of birth was specifically included as a patient identifier, distinct from age, which was felt to be useful as a descriptor (often within a one‐liner or as part of the patient summary).
The 8 recommended elements were elements for which there was not unanimous agreement on inclusion, but the majority of the panel felt they should be included. These elements were classified as IIa‐C, with 1 exception. Code status generated significant controversy among the group. After extensive discussion among the group and consideration of safety, supervision, educational, and pediatric‐specific considerations, all members of the group agreed on the categorization as a recommended element; it is classified as IIb‐C.
All members of the group agreed that data elements should be directly imported from the EHR whenever possible. Finally, members agreed that the elements that make up the I‐PASS mnemonic (illness severity, patient summary, action items, situation awareness/contingency planning) should be listed in that order whenever possible. A sample I‐PASS‐compliant printed handoff document is shown Figure 1.

DISCUSSION
We identified substantial variability in the structure and content of printed handoff documents used by 9 pediatric hospitalist teaching services, reflective of a lack of standardization. We found that institutional printed handoff documents shared some demographic elements (eg, name, room, medical record number) but also varied in clinical content (eg, vital signs, lab tests, code status). Our expert panel developed a list of 15 essential and 8 recommended data elements for printed handoff documents. Although this is a large number of fields, the majority of the essential fields were already included by most sites, and many are basic demographic identifiers. Illness severity is the 1 essential field that was not routinely included; however, including this type of overview is consistently recommended[2, 4] and supported by evidence,[20, 21] and contributes to building a shared mental model.[16] We recommend the categories of stable/watcher/unstable.[17]
Several prior single‐center studies have found that introducing a printed handoff document can lead to improvements in workflow, communication, and patient safety. In an early study, Petersen et al.[25] showed an association between use of a computerized sign‐out program and reduced odds of preventable adverse events during periods of cross‐coverage. Wayne et al.[26] reported fewer perceived inaccuracies in handoff documents as well as improved clarity at the time of transfer, supporting the role for standardization. Van Eaton et al.[27] demonstrated rapid uptake and desirability of a computerized handoff document, which combined autoimportation of information from an EHR with resident‐entered patient details, reflecting the importance of both data sources. In addition, they demonstrated improvements in both the rounding and sign‐out processes.[28]
Two studies specifically reported the increased use of specific fields after implementation. Payne et al. implemented a Web‐based handoff tool and documented significant increases in the number of handoffs containing problem lists, medication lists, and code status, accompanied by perceived improvements in quality of handoffs and fewer near‐miss events.[24] Starmer et al. found that introduction of a resident handoff bundle that included a printed handoff tool led to reduction in medical errors and adverse events.[22] The study group using the tool populated 11 data elements more often after implementation, and introduction of this printed handoff tool in particular was associated with reductions in written handoff miscommunications. Neither of these studies included subanalysis to indicate which data elements may have been most important.
In contrast to previous single‐institution studies, our recommendations for a printed handoff template come from evaluations of tools and discussions with front line providers across 9 institutions. We had substantial overlap with data elements recommended by Van Eaton et al.[27] However, there were several areas in which we did not have overlap with published templates including weight, ins/outs, primary language, emergency contact information, or primary care provider. Other published handoff tools have been highly specialized (eg, for cardiac intensive care) or included many fewer data elements than our group felt were essential. These differences may reflect the unique aspects of caring for pediatric patients (eg, need for weights) and the absence of defined protocols for many pediatric conditions. In addition, the level of detail needed for contingency planning may vary between teaching and nonteaching services.
Resident physicians may provide valuable information in the development of standardized handoff documents. Clark et al.,[29] at Virginia Mason University, utilized resident‐driven continuous quality improvement processes including real‐time feedback to implement an electronic template. They found that engagement of both senior leaders and front‐line users was an important component of their success in uptake. Our study utilized residents as essential members of structured group interviews to ensure that front‐line users' needs were represented as recommendations for a printed handoff tool template were developed.
As previously described,[17] our study group had identified several key data elements that should be included in verbal handoffs: illness severity, a patient summary, a discrete action list, situation awareness/contingency planning, and a synthesis by receiver. With consideration of the multivoting results as well as known best practices,[1, 4, 12] the expert panel for this study agreed that each of these elements should also be highlighted in the printed template to ensure consistency between the printed document and the verbal handoff, and to have each reinforce the other. On the printed handoff tool, the final S in the I‐PASS mnemonic (synthesis by receiver) cannot be prepopulated, but considering the importance of this step,[16, 30, 31, 32] it should be printed as synthesis by receiver to serve as a text‐reminder to both givers and receivers.
The panel also felt, however, that the printed handoff document should provide additional background information not routinely included in a verbal handoff. It should serve as a reference tool both at the time of verbal handoff and throughout the day and night, and therefore should include more comprehensive information than is necessary or appropriate to convey during the verbal handoff. We identified 10 data elements that are essential in a printed handoff document in addition to the I‐PASS elements (Table 2).
Patient demographic data elements, as well as team assignments and attending physician, were uniformly supported for inclusion. The medication list was viewed as essential; however, the panel also recognized the potential for medical errors due to inaccuracies in the medication list. In particular, there was concern that including all fields of a medication order (drug, dose, route, frequency) would result in handoffs containing a high proportion of inaccurate information, particularly for complex patients whose medication regimens may vary over the course of hospitalization. Therefore, the panel agreed that if medication lists were entered manually, then only the medication name should be included as they did not wish to perpetuate inaccurate or potentially harmful information. If medication lists were autoimported from an EHR, then they should include drug name, dose, route, and frequency if possible.
In the I‐PASS study,[15] all institutions implemented printed handoff documents that included fields for the essential data elements. After implementation, there was a significant increase in completion of all essential fields. Although there is limited evidence to support any individual data element, increased usage of these elements was associated with the overall study finding of decreased rates of medical errors and preventable adverse events.
EHRs have the potential to help standardize printed handoff documents[5, 6, 33, 34, 35]; all participants in our study agreed that printed handoff documents should ideally be linked with the EHR and should autoimport data wherever appropriate. Manually populated (eg, word processor‐ or spreadsheet‐based) handoff tools have important limitations, particularly related to the potential for typographical errors as well as accidental omission of data fields, and lead to unnecessary duplication of work (eg, re‐entering data already included in a progress note) that can waste providers' time. It was also acknowledged that word processor‐ or spreadsheet‐based documents may have flexibility that is lacking in EHR‐based handoff documents. For example, formatting can more easily be adjusted to increase the number of patients per printed page. As technology advances, printed documents may be phased out in favor of EHR‐based on‐screen reports, which by their nature would be more accurate due to real‐time autoupdates.
In making recommendations about essential versus recommended items for inclusion in the printed handoff template, the only data element that generated controversy among our experts was code status. Some felt that it should be included as an essential element, whereas others did not. We believe that this was unique to our practice in pediatric hospital ward settings, as codes in most pediatric ward settings are rare. Among the concerns expressed with including code status for all patients were that residents might assume patients were full‐code without verifying. The potential inaccuracy created by this might have severe implications. Alternatively, residents might feel obligated to have code discussions with all patients regardless of severity of illness, which may be inappropriate in a pediatric population. Several educators expressed concerns about trainees having unsupervised code‐status conversations with families of pediatric patients. Conversely, although codes are rare in pediatric ward settings, concerns were raised that not including code status could be problematic during these rare but critically important events. Other fields, such as weight, might have less relevance for an adult population in which emergency drug doses are standardized.
Limitations
Our study has several limitations. We only collected data from hospitalist services at pediatric sites. It is likely that providers in other specialties would have specific data elements they felt were essential (eg, postoperative day, code status). Our methodology was expert consensus based, driven by data collection from sites that were already participating in the I‐PASS study. Although the I‐PASS study demonstrated decreased rates of medical errors and preventable adverse events with inclusion of these data elements as part of a bundle, future research will be required to evaluate whether some of these items are more important than others in improving written communication and ultimately patient safety. In spite of these limitations, our work represents an important starting point for the development of standards for written handoff documents that should be used in patient handoffs, particularly those generated from EHRs.
CONCLUSIONS
In this article we describe the results of a needs assessment that informed expert consensus‐based recommendations for data elements to include in a printed handoff document. We recommend that pediatric programs include the elements identified as part of a standardized written handoff tool. Although many of these elements are also applicable to other specialties, future work should be conducted to adapt the printed handoff document elements described here for use in other specialties and settings. Future studies should work to validate the importance of these elements, studying the manner in which their inclusion affects the quality of written handoffs, and ultimately patient safety.
Acknowledgements
Members of the I‐PASS Study Education Executive Committee who contributed to this manuscript include: Boston Children's Hospital/Harvard Medical School (primary site) (Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA. Theodore C. Sectish, MD. Lisa L. Tse, BA). Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine (Jennifer K. O'Toole, MD, MEd). Doernbecher Children's Hospital/Oregon Health and Science University (Amy J. Starmer, MD, MPH). Hospital for Sick Children/University of Toronto (Zia Bismilla, MD. Maitreya Coffey, MD). Lucile Packard Children's Hospital/Stanford University (Lauren A. Destino, MD. Jennifer L. Everhart, MD. Shilpa J. Patel, MD [currently at Kapi'olani Children's Hospital/University of Hawai'i School of Medicine]). National Capital Consortium (Jennifer H. Hepps, MD. Joseph O. Lopreiato, MD, MPH. Clifton E. Yu, MD). Primary Children's Medical Center/University of Utah (James F. Bale, Jr., MD. Adam T. Stevenson, MD). St. Louis Children's Hospital/Washington University (F. Sessions Cole, MD). St. Christopher's Hospital for Children/Drexel University College of Medicine (Sharon Calaman, MD. Nancy D. Spector, MD). Benioff Children's Hospital/University of California San Francisco School of Medicine (Glenn Rosenbluth, MD. Daniel C. West, MD).
Additional I‐PASS Study Group members who contributed to this manuscript include April D. Allen, MPA, MA (Heller School for Social Policy and Management, Brandeis University, previously affiliated with Boston Children's Hospital), Madelyn D. Kahana, MD (The Children's Hospital at Montefiore/Albert Einstein College of Medicine, previously affiliated with Lucile Packard Children's Hospital/Stanford University), Robert S. McGregor, MD (Akron Children's Hospital/Northeast Ohio Medical University, previously affiliated with St. Christopher's Hospital for Children/Drexel University), and John S. Webster, MD, MBA, MS (Webster Healthcare Consulting Inc., formerly of the Department of Defense).
Members of the I‐PASS Study Group include individuals from the institutions listed below as follows: Boston Children's Hospital/Harvard Medical School (primary site): April D. Allen, MPA, MA (currently at Heller School for Social Policy and Management, Brandeis University), Angela M. Feraco, MD, Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA, Theodore C. Sectish, MD, Lisa L. Tse, BA. Brigham and Women's Hospital (data coordinating center): Anuj K. Dalal, MD, Carol A. Keohane, BSN, RN, Stuart Lipsitz, PhD, Jeffrey M. Rothschild, MD, MPH, Matt F. Wien, BS, Catherine S. Yoon, MS, Katherine R. Zigmont, BSN, RN. Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine: Javier Gonzalez del Rey, MD, MEd, Jennifer K. O'Toole, MD, MEd, Lauren G. Solan, MD. Doernbecher Children's Hospital/Oregon Health and Science University: Megan E. Aylor, MD, Amy J. Starmer, MD, MPH, Windy Stevenson, MD, Tamara Wagner, MD. Hospital for Sick Children/University of Toronto: Zia Bismilla, MD, Maitreya Coffey, MD, Sanjay Mahant, MD, MSc. Lucile Packard Children's Hospital/Stanford University: Rebecca L. Blankenburg, MD, MPH, Lauren A. Destino, MD, Jennifer L. Everhart, MD, Madelyn Kahana, MD, Shilpa J. Patel, MD (currently at Kapi'olani Children's Hospital/University of Hawaii School of Medicine). National Capital Consortium: Jennifer H. Hepps, MD, Joseph O. Lopreiato, MD, MPH, Clifton E. Yu, MD. Primary Children's Hospital/University of Utah: James F. Bale, Jr., MD, Jaime Blank Spackman, MSHS, CCRP, Rajendu Srivastava, MD, FRCP(C), MPH, Adam Stevenson, MD. St. Louis Children's Hospital/Washington University: Kevin Barton, MD, Kathleen Berchelmann, MD, F. Sessions Cole, MD, Christine Hrach, MD, Kyle S. Schultz, MD, Michael P. Turmelle, MD, Andrew J. White, MD. St. Christopher's Hospital for Children/Drexel University: Sharon Calaman, MD, Bronwyn D. Carlson, MD, Robert S. McGregor, MD (currently at Akron Children's Hospital/Northeast Ohio Medical University), Vahideh Nilforoshan, MD, Nancy D. Spector, MD. and Benioff Children's Hospital/University of California San Francisco School of Medicine: Glenn Rosenbluth, MD, Daniel C. West, MD. Dorene Balmer, PhD, RD, Carol L. Carraccio, MD, MA, Laura Degnon, CAE, and David McDonald, and Alan Schwartz PhD serve the I‐PASS Study Group as part of the IIPE. Karen M. Wilson, MD, MPH serves the I‐PASS Study Group as part of the advisory board from the PRIS Executive Council. John Webster served the I‐PASS Study Group and Education Executive Committee as a representative from TeamSTEPPS.
Disclosures: The I‐PASS Study was primarily supported by the US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation (1R18AE000029‐01). The opinions and conclusions expressed herein are solely those of the author(s) and should not be constructed as representing the opinions or policy of any agency of the federal government. Developed with input from the Initiative for Innovation in Pediatric Education and the Pediatric Research in Inpatient Settings Network (supported by the Children's Hospital Association, the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine). A. J. S. was supported by the Agency for Healthcare Research and Quality/Oregon Comparative Effectiveness Research K12 Program (1K12HS019456‐01). Additional funding for the I‐PASS Study was provided by the Medical Research Foundation of Oregon, Physician Services Incorporated Foundation (Ontario, Canada), and Pfizer (unrestricted medical education grant to N.D.S.). C.P.L, A.J.S. were supported by the Oregon Comparative Effectiveness Research K12 Program (1K12HS019456 from the Agency for Healthcare Research and Quality). A.J.S. was also supported by the Medical Research Foundation of Oregon. The authors report no conflicts of interest.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , . Development and implementation of an oral sign‐out skills curriculum. J Gen Intern Med. 2007;22(10):1470–1474.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . A systematic review of the literature on the evaluation of handoff tools: implications for research and practice. J Am Med Inform Assoc. 2014;21(1):154–162.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Sign‐out snapshot: cross‐sectional evaluation of written sign‐outs among specialties. BMJ Qual Saf. 2014;23(1):66–72.
- , , , . An experimental comparison of handover methods. Ann R Coll Surg Engl. 2007;89(3):298–300.
- , , , . Pilot study to show the loss of important data in nursing handover. Br J Nurs. 2005;14(20):1090–1093.
- The Joint Commission. Hospital Accreditation Standards 2015: Joint Commission Resources; 2015:PC.02.02.01.
- Accreditation Council for Graduate Medical Education. Common Program Requirements. 2013; http://acgme.org/acgmeweb/tabid/429/ProgramandInstitutionalAccreditation/CommonProgramRequirements.aspx. Accessed May 11, 2015.
- , , , . Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim. Pediatrics. 2010;126(4):619–622.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- US Department of Health and Human Services. Agency for Healthcare Research and Quality. TeamSTEPPS website. Available at: http://teamstepps.ahrq.gov/. Accessed July 12, 2013.
- , , , , , . I‐PASS, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . The Team Handbook. 3rd ed. Middleton, WI: Oriel STAT A MATRIX; 2010.
- ACC/AHA Task Force on Practice Guidelines. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines. Available at: http://my.americanheart.org/idc/groups/ahamah‐public/@wcm/@sop/documents/downloadable/ucm_319826.pdf. Published June 2010. Accessed January 11, 2015.
- , , , et al. Effect of illness severity and comorbidity on patient safety and adverse events. Am J Med Qual. 2012;27(1):48–57.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , , . Medication discrepancies in resident sign‐outs and their potential to harm. J Gen Intern Med. 2007;22(12):1751–1755.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , , . A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , . Template for success: using a resident‐designed sign‐out template in the handover of patient care. J Surg Educ. 2011;68(1):52–57.
- , , , , , . Read‐back improves information transfer in simulated clinical crises. BMJ Qual Saf. 2014;23(12):989–993.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- , , , , , . Improving patient safety by repeating (read‐back) telephone reports of critical information. Am J Clin Pathol. 2004;121(6):801–803.
- , , , , . Content overlap in nurse and physician handoff artifacts and the potential role of electronic health records: a systematic review. J Biomed Inform. 2011;44(4):704–712.
- , , , . Clinical summarization capabilities of commercially‐available and internally‐developed electronic health records. Appl Clin Inform. 2012;3(1):80–93.
- , . An analysis and recommendations for multidisciplinary computerized handoff applications in hospitals. AMIA Annu Symp Proc. 2011;2011:588–597.
Handoffs among hospital providers are highly error prone and can result in serious morbidity and mortality. Best practices for verbal handoffs have been described[1, 2, 3, 4] and include conducting verbal handoffs face to face, providing opportunities for questions, having the receiver perform a readback, as well as specific content recommendations including action items. Far less research has focused on best practices for printed handoff documents,[5, 6] despite the routine use of written handoff tools as a reference by on‐call physicians.[7, 8] Erroneous or outdated information on the written handoff can mislead on‐call providers, potentially leading to serious medical errors.
In their most basic form, printed handoff documents list patients for whom a provider is responsible. Typically, they also contain demographic information, reason for hospital admission, and a task list for each patient. They may also contain more detailed information on patient history, hospital course, and/or care plan, and may vary among specialties.[9] They come in various forms, ranging from index cards with handwritten notes, to word‐processor or spreadsheet documents, to printed documents that are autopopulated from the electronic health record (EHR).[2] Importantly, printed handoff documents supplement the verbal handoff by allowing receivers to follow along as patients are presented. The concurrent use of written and verbal handoffs may improve retention of clinical information as compared with either alone.[10, 11]
The Joint Commission requires an institutional approach to patient handoffs.[12] The requirements state that handoff communication solutions should take a standardized form, but they do not provide details regarding what data elements should be included in printed or verbal handoffs. Accreditation Council for Graduate Medical Education Common Program Requirements likewise require that residents must become competent in patient handoffs[13] but do not provide specific details or measurement tools. Absent widely accepted guidelines, decisions regarding which elements to include in printed handoff documents are currently made at an individual or institutional level.
The I‐PASS study is a federally funded multi‐institutional project that demonstrated a decrease in medical errors and preventable adverse events after implementation of a standardized resident handoff bundle.[14, 15] The I‐PASS Study Group developed a bundle of handoff interventions, beginning with a handoff and teamwork training program (based in part on TeamSTEPPS [Team Strategies and Tools to Enhance Performance and Patient Safety]),[16] a novel verbal mnemonic, I‐PASS (Illness Severity, Patient Summary, Action List, Situation Awareness and Contingency Planning, and Synthesis by Receiver),[17] and changes to the verbal handoff process, in addition to several other elements.
We hypothesized that developing a standardized printed handoff template would reinforce the handoff training and enhance the value of the verbal handoff process changes. Given the paucity of data on best printed handoff practices, however, we first conducted a needs assessment to identify which data elements were currently contained in printed handoffs across sites, and to allow an expert panel to make recommendations for best practices.
METHODS
I‐PASS Study sites included 9 pediatric residency programs at academic medical centers from across North America. Programs were identified through professional networks and invited to participate. The nonintensive care unit hospitalist services at these medical centers are primarily staffed by residents and medical students with attending supervision. At 1 site, nurse practitioners also participate in care. Additional details about study sites can be found in the study descriptions previously published.[14, 15] All sites received local institutional review board approval.
We began by inviting members of the I‐PASS Education Executive Committee (EEC)[14] to build a collective, comprehensive list of possible data elements for printed handoff documents. This committee included pediatric residency program directors, pediatric hospitalists, education researchers, health services researchers, and patient safety experts. We obtained sample handoff documents from pediatric hospitalist services at each of 9 institutions in the United States and Canada (with protected health information redacted). We reviewed these sample handoff documents to characterize their format and to determine what discrete data elements appeared in each site's printed handoff document. Presence or absence of each data element across sites was tabulated. We also queried sites to determine the feasibility of including elements that were not presently included.
Subsequently, I‐PASS site investigators led structured group interviews at participating sites to gather additional information about handoff practices at each site. These structured group interviews included diverse representation from residents, faculty, and residency program leadership, as well as hospitalists and medical students, to ensure the comprehensive acquisition of information regarding site‐specific characteristics. Each group provided answers to a standardized set of open‐ended questions that addressed current practices, handoff education, simulation use, team structure, and the nature of current written handoff tools, if applicable, at each site. One member of the structured group interview served as a scribe and created a document that summarized the content of the structured group interview meeting and answers to the standardized questions.
Consensus on Content
The initial data collection also included a multivote process[18] of the full I‐PASS EEC to help prioritize data elements. Committee members brainstormed a list of all possible data elements for a printed handoff document. Each member (n=14) was given 10 votes to distribute among the elements. Committee members could assign more than 1 vote to an element to emphasize its importance.
The results of this process as well as the current data elements included in each printed handoff tool were reviewed by a subgroup of the I‐PASS EEC. These expert panel members participated in a series of conference calls during which they tabulated categorical information, reviewed narrative comments, discussed existing evidence, and conducted simple content analysis to identify areas of concordance or discordance. Areas of discordance were discussed by the committee. Disagreements were resolved with group consensus with attention to published evidence or best practices, if available.
Elements were divided into those that were essential (unanimous consensus, no conflicting literature) and those that were recommended (majority supported inclusion of element, no conflicting literature). Ratings were assigned using the American College of Cardiology/American Heart Association framework for practice guidelines,[19] in which each element is assigned a classification (I=effective, II=conflicting evidence/opinion, III=not effective) and a level of evidence to support that classification (A=multiple large randomized controlled trials, B=single randomized trial, or nonrandomized studies, C=expert consensus).
The expert panel reached consensus, through active discussion, on a list of data elements that should be included in an ideal printed handoff document. Elements were chosen based on perceived importance, with attention to published best practices[1, 16] and the multivoting results. In making recommendations, consideration was given to whether data elements could be electronically imported into the printed handoff document from the EHR, or whether they would be entered manually. The potential for serious medical errors due to possible errors in manual entry of data was an important aspect of recommendations made. The list of candidate elements was then reviewed by a larger group of investigators from the I‐PASS Education Executive Committee and Coordinating Council for additional input.
The panel asked site investigators from each participating hospital to gather data on the feasibility of redesigning the printed handoff at that hospital to include each recommended element. Site investigators reported whether each element was already included, possible to include but not included currently, or not currently possible to include within that site's printed handoff tool. Site investigators also reported how data elements were populated in their handoff documents, with options including: (1) autopopulated from administrative data (eg, pharmacy‐entered medication list, demographic data entered by admitting office), (2) autoimported from physicians' free‐text entries elsewhere in the EHR (eg, progress notes), (3) free text entered specifically for the printed handoff, or (4) not applicable (element cannot be included).
RESULTS
Nine programs (100%) provided data on the structure and contents of their printed handoff documents. We found wide variation in structure across the 9 sites. Three sites used a word‐processorbased document that required manual entry of all data elements. The other 6 institutions had a direct link with the EHR to enable autopopulation of between 10 and 20 elements on the printed handoff document.
The content of written handoff documents, as well as the sources of data included in them (present or future), likewise varied substantially across sites (Table 1). Only 4 data elements (name, age, weight, and a list of medications) were universally included at all 9 sites. Among the 6 institutions that linked the printed handoff to the EHR, there was also substantial variation in which elements were autoimported. Only 7 elements were universally autoimported at these 6 sites: patient name, medical record number, room number, weight, date of birth, age, and date of admission. Two elements from the original brainstorming were not presently included in any sites' documents (emergency contact and primary language).
| Data Elements | Sites With Data Element Included at Initial Needs Assessment (Out of Nine Sites) | Data Source (Current or Anticipated) | ||
|---|---|---|---|---|
| Autoimported* | Manually Entered | Not Applicable | ||
| ||||
| Name | 9 | 6 | 3 | 0 |
| Medical record number | 8 | 6 | 3 | 0 |
| Room number | 8 | 6 | 3 | 0 |
| Allergies | 6 | 4 | 5 | 0 |
| Weight | 9 | 6 | 3 | 0 |
| Age | 9 | 6 | 3 | 0 |
| Date of birth | 6 | 6 | 3 | 0 |
| Admission date | 8 | 6 | 3 | 0 |
| Attending name | 5 | 4 | 5 | 0 |
| Team/service | 7 | 4 | 5 | 0 |
| Illness severity | 1 | 0 | 9 | 0 |
| Patient summary | 8 | 0 | 9 | 0 |
| Action items | 8 | 0 | 9 | 0 |
| Situation monitoring/contingency plan | 5 | 0 | 9 | 0 |
| Medication name | 9 | 4 | 5 | 0 |
| Medication name and dose/route/frequency | 4 | 4 | 5 | 0 |
| Code status | 2 | 2 | 7 | 0 |
| Labs | 6 | 5 | 4 | 0 |
| Access | 2 | 2 | 7 | 0 |
| Ins/outs | 2 | 4 | 4 | 1 |
| Primary language | 0 | 3 | 6 | 0 |
| Vital signs | 3 | 4 | 4 | 1 |
| Emergency contact | 0 | 2 | 7 | 0 |
| Primary care provider | 4 | 4 | 5 | 0 |
Nine institutions (100%) conducted structured group interviews, ranging in size from 4 to 27 individuals with a median of 5 participants. The documents containing information from each site were provided to the authors. The authors then tabulated categorical information, reviewed narrative comments to understand current institutional practices, and conducted simple content analysis to identify areas of concordance or discordance, particularly with respect to data elements and EHR usage. Based on the results of the printed handoff document review and structured group interviews, with additional perspectives provided by the I‐PASS EEC, the expert panel came to consensus on a list of 23 elements that should be included in printed handoff documents, including 15 essential data elements and 8 additional recommended elements (Table 2).
|
| Essential Elements |
| Patient identifiers |
| Patient name (class I, level of evidence C) |
| Medical record number (class I, level of evidence C) |
| Date of birth (class I, level of evidence C) |
| Hospital service identifiers |
| Attending name (class I, level of evidence C) |
| Team/service (class I, level of evidence C) |
| Room number (class I, level of evidence C) |
| Admission date (class I, level of evidence C) |
| Age (class I, level of evidence C) |
| Weight (class I, level of evidence C) |
| Illness severity (class I, level of evidence B)[20, 21] |
| Patient summary (class I, level of evidence B)[21, 22] |
| Action items (class I, level of evidence B) [21, 22] |
| Situation awareness/contingency planning (class I, level of evidence B) [21, 22] |
| Allergies (class I, level of evidence C) |
| Medications |
| Autopopulation of medications (class I, level of evidence B)[22, 23, 24] |
| Free‐text entry of medications (class IIa, level of evidence C) |
| Recommended elements |
| Primary language (class IIa, level of evidence C) |
| Emergency contact (class IIa, level of evidence C) |
| Primary care provider (class IIa, level of evidence C) |
| Code status (class IIb, level of evidence C) |
| Labs (class IIa, level of evidence C) |
| Access (class IIa, level of evidence C) |
| Ins/outs (class IIa, level of evidence C) |
| Vital signs (class IIa, level of evidence C) |
Evidence ratings[19] of these elements are included. Several elements are classified as I‐B (effective, nonrandomized studies) based on either studies of individual elements, or greater than 1 study of bundled elements that could reasonably be extrapolated. These include Illness severity,[20, 21] patient summary,[21, 22] action items[21, 22] (to do lists), situation awareness and contingency plan,[21, 22] and medications[22, 23, 24] with attention to importing from the EHR. Medications entered as free text were classified as IIa‐C because of risk and potential significance of errors; in particular there was concern that transcription errors, errors of omission, or errors of commission could potentially lead to patient harms. The remaining essential elements are classified as I‐C (effective, expert consensus). Of note, date of birth was specifically included as a patient identifier, distinct from age, which was felt to be useful as a descriptor (often within a one‐liner or as part of the patient summary).
The 8 recommended elements were elements for which there was not unanimous agreement on inclusion, but the majority of the panel felt they should be included. These elements were classified as IIa‐C, with 1 exception. Code status generated significant controversy among the group. After extensive discussion among the group and consideration of safety, supervision, educational, and pediatric‐specific considerations, all members of the group agreed on the categorization as a recommended element; it is classified as IIb‐C.
All members of the group agreed that data elements should be directly imported from the EHR whenever possible. Finally, members agreed that the elements that make up the I‐PASS mnemonic (illness severity, patient summary, action items, situation awareness/contingency planning) should be listed in that order whenever possible. A sample I‐PASS‐compliant printed handoff document is shown Figure 1.

DISCUSSION
We identified substantial variability in the structure and content of printed handoff documents used by 9 pediatric hospitalist teaching services, reflective of a lack of standardization. We found that institutional printed handoff documents shared some demographic elements (eg, name, room, medical record number) but also varied in clinical content (eg, vital signs, lab tests, code status). Our expert panel developed a list of 15 essential and 8 recommended data elements for printed handoff documents. Although this is a large number of fields, the majority of the essential fields were already included by most sites, and many are basic demographic identifiers. Illness severity is the 1 essential field that was not routinely included; however, including this type of overview is consistently recommended[2, 4] and supported by evidence,[20, 21] and contributes to building a shared mental model.[16] We recommend the categories of stable/watcher/unstable.[17]
Several prior single‐center studies have found that introducing a printed handoff document can lead to improvements in workflow, communication, and patient safety. In an early study, Petersen et al.[25] showed an association between use of a computerized sign‐out program and reduced odds of preventable adverse events during periods of cross‐coverage. Wayne et al.[26] reported fewer perceived inaccuracies in handoff documents as well as improved clarity at the time of transfer, supporting the role for standardization. Van Eaton et al.[27] demonstrated rapid uptake and desirability of a computerized handoff document, which combined autoimportation of information from an EHR with resident‐entered patient details, reflecting the importance of both data sources. In addition, they demonstrated improvements in both the rounding and sign‐out processes.[28]
Two studies specifically reported the increased use of specific fields after implementation. Payne et al. implemented a Web‐based handoff tool and documented significant increases in the number of handoffs containing problem lists, medication lists, and code status, accompanied by perceived improvements in quality of handoffs and fewer near‐miss events.[24] Starmer et al. found that introduction of a resident handoff bundle that included a printed handoff tool led to reduction in medical errors and adverse events.[22] The study group using the tool populated 11 data elements more often after implementation, and introduction of this printed handoff tool in particular was associated with reductions in written handoff miscommunications. Neither of these studies included subanalysis to indicate which data elements may have been most important.
In contrast to previous single‐institution studies, our recommendations for a printed handoff template come from evaluations of tools and discussions with front line providers across 9 institutions. We had substantial overlap with data elements recommended by Van Eaton et al.[27] However, there were several areas in which we did not have overlap with published templates including weight, ins/outs, primary language, emergency contact information, or primary care provider. Other published handoff tools have been highly specialized (eg, for cardiac intensive care) or included many fewer data elements than our group felt were essential. These differences may reflect the unique aspects of caring for pediatric patients (eg, need for weights) and the absence of defined protocols for many pediatric conditions. In addition, the level of detail needed for contingency planning may vary between teaching and nonteaching services.
Resident physicians may provide valuable information in the development of standardized handoff documents. Clark et al.,[29] at Virginia Mason University, utilized resident‐driven continuous quality improvement processes including real‐time feedback to implement an electronic template. They found that engagement of both senior leaders and front‐line users was an important component of their success in uptake. Our study utilized residents as essential members of structured group interviews to ensure that front‐line users' needs were represented as recommendations for a printed handoff tool template were developed.
As previously described,[17] our study group had identified several key data elements that should be included in verbal handoffs: illness severity, a patient summary, a discrete action list, situation awareness/contingency planning, and a synthesis by receiver. With consideration of the multivoting results as well as known best practices,[1, 4, 12] the expert panel for this study agreed that each of these elements should also be highlighted in the printed template to ensure consistency between the printed document and the verbal handoff, and to have each reinforce the other. On the printed handoff tool, the final S in the I‐PASS mnemonic (synthesis by receiver) cannot be prepopulated, but considering the importance of this step,[16, 30, 31, 32] it should be printed as synthesis by receiver to serve as a text‐reminder to both givers and receivers.
The panel also felt, however, that the printed handoff document should provide additional background information not routinely included in a verbal handoff. It should serve as a reference tool both at the time of verbal handoff and throughout the day and night, and therefore should include more comprehensive information than is necessary or appropriate to convey during the verbal handoff. We identified 10 data elements that are essential in a printed handoff document in addition to the I‐PASS elements (Table 2).
Patient demographic data elements, as well as team assignments and attending physician, were uniformly supported for inclusion. The medication list was viewed as essential; however, the panel also recognized the potential for medical errors due to inaccuracies in the medication list. In particular, there was concern that including all fields of a medication order (drug, dose, route, frequency) would result in handoffs containing a high proportion of inaccurate information, particularly for complex patients whose medication regimens may vary over the course of hospitalization. Therefore, the panel agreed that if medication lists were entered manually, then only the medication name should be included as they did not wish to perpetuate inaccurate or potentially harmful information. If medication lists were autoimported from an EHR, then they should include drug name, dose, route, and frequency if possible.
In the I‐PASS study,[15] all institutions implemented printed handoff documents that included fields for the essential data elements. After implementation, there was a significant increase in completion of all essential fields. Although there is limited evidence to support any individual data element, increased usage of these elements was associated with the overall study finding of decreased rates of medical errors and preventable adverse events.
EHRs have the potential to help standardize printed handoff documents[5, 6, 33, 34, 35]; all participants in our study agreed that printed handoff documents should ideally be linked with the EHR and should autoimport data wherever appropriate. Manually populated (eg, word processor‐ or spreadsheet‐based) handoff tools have important limitations, particularly related to the potential for typographical errors as well as accidental omission of data fields, and lead to unnecessary duplication of work (eg, re‐entering data already included in a progress note) that can waste providers' time. It was also acknowledged that word processor‐ or spreadsheet‐based documents may have flexibility that is lacking in EHR‐based handoff documents. For example, formatting can more easily be adjusted to increase the number of patients per printed page. As technology advances, printed documents may be phased out in favor of EHR‐based on‐screen reports, which by their nature would be more accurate due to real‐time autoupdates.
In making recommendations about essential versus recommended items for inclusion in the printed handoff template, the only data element that generated controversy among our experts was code status. Some felt that it should be included as an essential element, whereas others did not. We believe that this was unique to our practice in pediatric hospital ward settings, as codes in most pediatric ward settings are rare. Among the concerns expressed with including code status for all patients were that residents might assume patients were full‐code without verifying. The potential inaccuracy created by this might have severe implications. Alternatively, residents might feel obligated to have code discussions with all patients regardless of severity of illness, which may be inappropriate in a pediatric population. Several educators expressed concerns about trainees having unsupervised code‐status conversations with families of pediatric patients. Conversely, although codes are rare in pediatric ward settings, concerns were raised that not including code status could be problematic during these rare but critically important events. Other fields, such as weight, might have less relevance for an adult population in which emergency drug doses are standardized.
Limitations
Our study has several limitations. We only collected data from hospitalist services at pediatric sites. It is likely that providers in other specialties would have specific data elements they felt were essential (eg, postoperative day, code status). Our methodology was expert consensus based, driven by data collection from sites that were already participating in the I‐PASS study. Although the I‐PASS study demonstrated decreased rates of medical errors and preventable adverse events with inclusion of these data elements as part of a bundle, future research will be required to evaluate whether some of these items are more important than others in improving written communication and ultimately patient safety. In spite of these limitations, our work represents an important starting point for the development of standards for written handoff documents that should be used in patient handoffs, particularly those generated from EHRs.
CONCLUSIONS
In this article we describe the results of a needs assessment that informed expert consensus‐based recommendations for data elements to include in a printed handoff document. We recommend that pediatric programs include the elements identified as part of a standardized written handoff tool. Although many of these elements are also applicable to other specialties, future work should be conducted to adapt the printed handoff document elements described here for use in other specialties and settings. Future studies should work to validate the importance of these elements, studying the manner in which their inclusion affects the quality of written handoffs, and ultimately patient safety.
Acknowledgements
Members of the I‐PASS Study Education Executive Committee who contributed to this manuscript include: Boston Children's Hospital/Harvard Medical School (primary site) (Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA. Theodore C. Sectish, MD. Lisa L. Tse, BA). Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine (Jennifer K. O'Toole, MD, MEd). Doernbecher Children's Hospital/Oregon Health and Science University (Amy J. Starmer, MD, MPH). Hospital for Sick Children/University of Toronto (Zia Bismilla, MD. Maitreya Coffey, MD). Lucile Packard Children's Hospital/Stanford University (Lauren A. Destino, MD. Jennifer L. Everhart, MD. Shilpa J. Patel, MD [currently at Kapi'olani Children's Hospital/University of Hawai'i School of Medicine]). National Capital Consortium (Jennifer H. Hepps, MD. Joseph O. Lopreiato, MD, MPH. Clifton E. Yu, MD). Primary Children's Medical Center/University of Utah (James F. Bale, Jr., MD. Adam T. Stevenson, MD). St. Louis Children's Hospital/Washington University (F. Sessions Cole, MD). St. Christopher's Hospital for Children/Drexel University College of Medicine (Sharon Calaman, MD. Nancy D. Spector, MD). Benioff Children's Hospital/University of California San Francisco School of Medicine (Glenn Rosenbluth, MD. Daniel C. West, MD).
Additional I‐PASS Study Group members who contributed to this manuscript include April D. Allen, MPA, MA (Heller School for Social Policy and Management, Brandeis University, previously affiliated with Boston Children's Hospital), Madelyn D. Kahana, MD (The Children's Hospital at Montefiore/Albert Einstein College of Medicine, previously affiliated with Lucile Packard Children's Hospital/Stanford University), Robert S. McGregor, MD (Akron Children's Hospital/Northeast Ohio Medical University, previously affiliated with St. Christopher's Hospital for Children/Drexel University), and John S. Webster, MD, MBA, MS (Webster Healthcare Consulting Inc., formerly of the Department of Defense).
Members of the I‐PASS Study Group include individuals from the institutions listed below as follows: Boston Children's Hospital/Harvard Medical School (primary site): April D. Allen, MPA, MA (currently at Heller School for Social Policy and Management, Brandeis University), Angela M. Feraco, MD, Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA, Theodore C. Sectish, MD, Lisa L. Tse, BA. Brigham and Women's Hospital (data coordinating center): Anuj K. Dalal, MD, Carol A. Keohane, BSN, RN, Stuart Lipsitz, PhD, Jeffrey M. Rothschild, MD, MPH, Matt F. Wien, BS, Catherine S. Yoon, MS, Katherine R. Zigmont, BSN, RN. Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine: Javier Gonzalez del Rey, MD, MEd, Jennifer K. O'Toole, MD, MEd, Lauren G. Solan, MD. Doernbecher Children's Hospital/Oregon Health and Science University: Megan E. Aylor, MD, Amy J. Starmer, MD, MPH, Windy Stevenson, MD, Tamara Wagner, MD. Hospital for Sick Children/University of Toronto: Zia Bismilla, MD, Maitreya Coffey, MD, Sanjay Mahant, MD, MSc. Lucile Packard Children's Hospital/Stanford University: Rebecca L. Blankenburg, MD, MPH, Lauren A. Destino, MD, Jennifer L. Everhart, MD, Madelyn Kahana, MD, Shilpa J. Patel, MD (currently at Kapi'olani Children's Hospital/University of Hawaii School of Medicine). National Capital Consortium: Jennifer H. Hepps, MD, Joseph O. Lopreiato, MD, MPH, Clifton E. Yu, MD. Primary Children's Hospital/University of Utah: James F. Bale, Jr., MD, Jaime Blank Spackman, MSHS, CCRP, Rajendu Srivastava, MD, FRCP(C), MPH, Adam Stevenson, MD. St. Louis Children's Hospital/Washington University: Kevin Barton, MD, Kathleen Berchelmann, MD, F. Sessions Cole, MD, Christine Hrach, MD, Kyle S. Schultz, MD, Michael P. Turmelle, MD, Andrew J. White, MD. St. Christopher's Hospital for Children/Drexel University: Sharon Calaman, MD, Bronwyn D. Carlson, MD, Robert S. McGregor, MD (currently at Akron Children's Hospital/Northeast Ohio Medical University), Vahideh Nilforoshan, MD, Nancy D. Spector, MD. and Benioff Children's Hospital/University of California San Francisco School of Medicine: Glenn Rosenbluth, MD, Daniel C. West, MD. Dorene Balmer, PhD, RD, Carol L. Carraccio, MD, MA, Laura Degnon, CAE, and David McDonald, and Alan Schwartz PhD serve the I‐PASS Study Group as part of the IIPE. Karen M. Wilson, MD, MPH serves the I‐PASS Study Group as part of the advisory board from the PRIS Executive Council. John Webster served the I‐PASS Study Group and Education Executive Committee as a representative from TeamSTEPPS.
Disclosures: The I‐PASS Study was primarily supported by the US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation (1R18AE000029‐01). The opinions and conclusions expressed herein are solely those of the author(s) and should not be constructed as representing the opinions or policy of any agency of the federal government. Developed with input from the Initiative for Innovation in Pediatric Education and the Pediatric Research in Inpatient Settings Network (supported by the Children's Hospital Association, the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine). A. J. S. was supported by the Agency for Healthcare Research and Quality/Oregon Comparative Effectiveness Research K12 Program (1K12HS019456‐01). Additional funding for the I‐PASS Study was provided by the Medical Research Foundation of Oregon, Physician Services Incorporated Foundation (Ontario, Canada), and Pfizer (unrestricted medical education grant to N.D.S.). C.P.L, A.J.S. were supported by the Oregon Comparative Effectiveness Research K12 Program (1K12HS019456 from the Agency for Healthcare Research and Quality). A.J.S. was also supported by the Medical Research Foundation of Oregon. The authors report no conflicts of interest.
Handoffs among hospital providers are highly error prone and can result in serious morbidity and mortality. Best practices for verbal handoffs have been described[1, 2, 3, 4] and include conducting verbal handoffs face to face, providing opportunities for questions, having the receiver perform a readback, as well as specific content recommendations including action items. Far less research has focused on best practices for printed handoff documents,[5, 6] despite the routine use of written handoff tools as a reference by on‐call physicians.[7, 8] Erroneous or outdated information on the written handoff can mislead on‐call providers, potentially leading to serious medical errors.
In their most basic form, printed handoff documents list patients for whom a provider is responsible. Typically, they also contain demographic information, reason for hospital admission, and a task list for each patient. They may also contain more detailed information on patient history, hospital course, and/or care plan, and may vary among specialties.[9] They come in various forms, ranging from index cards with handwritten notes, to word‐processor or spreadsheet documents, to printed documents that are autopopulated from the electronic health record (EHR).[2] Importantly, printed handoff documents supplement the verbal handoff by allowing receivers to follow along as patients are presented. The concurrent use of written and verbal handoffs may improve retention of clinical information as compared with either alone.[10, 11]
The Joint Commission requires an institutional approach to patient handoffs.[12] The requirements state that handoff communication solutions should take a standardized form, but they do not provide details regarding what data elements should be included in printed or verbal handoffs. Accreditation Council for Graduate Medical Education Common Program Requirements likewise require that residents must become competent in patient handoffs[13] but do not provide specific details or measurement tools. Absent widely accepted guidelines, decisions regarding which elements to include in printed handoff documents are currently made at an individual or institutional level.
The I‐PASS study is a federally funded multi‐institutional project that demonstrated a decrease in medical errors and preventable adverse events after implementation of a standardized resident handoff bundle.[14, 15] The I‐PASS Study Group developed a bundle of handoff interventions, beginning with a handoff and teamwork training program (based in part on TeamSTEPPS [Team Strategies and Tools to Enhance Performance and Patient Safety]),[16] a novel verbal mnemonic, I‐PASS (Illness Severity, Patient Summary, Action List, Situation Awareness and Contingency Planning, and Synthesis by Receiver),[17] and changes to the verbal handoff process, in addition to several other elements.
We hypothesized that developing a standardized printed handoff template would reinforce the handoff training and enhance the value of the verbal handoff process changes. Given the paucity of data on best printed handoff practices, however, we first conducted a needs assessment to identify which data elements were currently contained in printed handoffs across sites, and to allow an expert panel to make recommendations for best practices.
METHODS
I‐PASS Study sites included 9 pediatric residency programs at academic medical centers from across North America. Programs were identified through professional networks and invited to participate. The nonintensive care unit hospitalist services at these medical centers are primarily staffed by residents and medical students with attending supervision. At 1 site, nurse practitioners also participate in care. Additional details about study sites can be found in the study descriptions previously published.[14, 15] All sites received local institutional review board approval.
We began by inviting members of the I‐PASS Education Executive Committee (EEC)[14] to build a collective, comprehensive list of possible data elements for printed handoff documents. This committee included pediatric residency program directors, pediatric hospitalists, education researchers, health services researchers, and patient safety experts. We obtained sample handoff documents from pediatric hospitalist services at each of 9 institutions in the United States and Canada (with protected health information redacted). We reviewed these sample handoff documents to characterize their format and to determine what discrete data elements appeared in each site's printed handoff document. Presence or absence of each data element across sites was tabulated. We also queried sites to determine the feasibility of including elements that were not presently included.
Subsequently, I‐PASS site investigators led structured group interviews at participating sites to gather additional information about handoff practices at each site. These structured group interviews included diverse representation from residents, faculty, and residency program leadership, as well as hospitalists and medical students, to ensure the comprehensive acquisition of information regarding site‐specific characteristics. Each group provided answers to a standardized set of open‐ended questions that addressed current practices, handoff education, simulation use, team structure, and the nature of current written handoff tools, if applicable, at each site. One member of the structured group interview served as a scribe and created a document that summarized the content of the structured group interview meeting and answers to the standardized questions.
Consensus on Content
The initial data collection also included a multivote process[18] of the full I‐PASS EEC to help prioritize data elements. Committee members brainstormed a list of all possible data elements for a printed handoff document. Each member (n=14) was given 10 votes to distribute among the elements. Committee members could assign more than 1 vote to an element to emphasize its importance.
The results of this process as well as the current data elements included in each printed handoff tool were reviewed by a subgroup of the I‐PASS EEC. These expert panel members participated in a series of conference calls during which they tabulated categorical information, reviewed narrative comments, discussed existing evidence, and conducted simple content analysis to identify areas of concordance or discordance. Areas of discordance were discussed by the committee. Disagreements were resolved with group consensus with attention to published evidence or best practices, if available.
Elements were divided into those that were essential (unanimous consensus, no conflicting literature) and those that were recommended (majority supported inclusion of element, no conflicting literature). Ratings were assigned using the American College of Cardiology/American Heart Association framework for practice guidelines,[19] in which each element is assigned a classification (I=effective, II=conflicting evidence/opinion, III=not effective) and a level of evidence to support that classification (A=multiple large randomized controlled trials, B=single randomized trial, or nonrandomized studies, C=expert consensus).
The expert panel reached consensus, through active discussion, on a list of data elements that should be included in an ideal printed handoff document. Elements were chosen based on perceived importance, with attention to published best practices[1, 16] and the multivoting results. In making recommendations, consideration was given to whether data elements could be electronically imported into the printed handoff document from the EHR, or whether they would be entered manually. The potential for serious medical errors due to possible errors in manual entry of data was an important aspect of recommendations made. The list of candidate elements was then reviewed by a larger group of investigators from the I‐PASS Education Executive Committee and Coordinating Council for additional input.
The panel asked site investigators from each participating hospital to gather data on the feasibility of redesigning the printed handoff at that hospital to include each recommended element. Site investigators reported whether each element was already included, possible to include but not included currently, or not currently possible to include within that site's printed handoff tool. Site investigators also reported how data elements were populated in their handoff documents, with options including: (1) autopopulated from administrative data (eg, pharmacy‐entered medication list, demographic data entered by admitting office), (2) autoimported from physicians' free‐text entries elsewhere in the EHR (eg, progress notes), (3) free text entered specifically for the printed handoff, or (4) not applicable (element cannot be included).
RESULTS
Nine programs (100%) provided data on the structure and contents of their printed handoff documents. We found wide variation in structure across the 9 sites. Three sites used a word‐processorbased document that required manual entry of all data elements. The other 6 institutions had a direct link with the EHR to enable autopopulation of between 10 and 20 elements on the printed handoff document.
The content of written handoff documents, as well as the sources of data included in them (present or future), likewise varied substantially across sites (Table 1). Only 4 data elements (name, age, weight, and a list of medications) were universally included at all 9 sites. Among the 6 institutions that linked the printed handoff to the EHR, there was also substantial variation in which elements were autoimported. Only 7 elements were universally autoimported at these 6 sites: patient name, medical record number, room number, weight, date of birth, age, and date of admission. Two elements from the original brainstorming were not presently included in any sites' documents (emergency contact and primary language).
| Data Elements | Sites With Data Element Included at Initial Needs Assessment (Out of Nine Sites) | Data Source (Current or Anticipated) | ||
|---|---|---|---|---|
| Autoimported* | Manually Entered | Not Applicable | ||
| ||||
| Name | 9 | 6 | 3 | 0 |
| Medical record number | 8 | 6 | 3 | 0 |
| Room number | 8 | 6 | 3 | 0 |
| Allergies | 6 | 4 | 5 | 0 |
| Weight | 9 | 6 | 3 | 0 |
| Age | 9 | 6 | 3 | 0 |
| Date of birth | 6 | 6 | 3 | 0 |
| Admission date | 8 | 6 | 3 | 0 |
| Attending name | 5 | 4 | 5 | 0 |
| Team/service | 7 | 4 | 5 | 0 |
| Illness severity | 1 | 0 | 9 | 0 |
| Patient summary | 8 | 0 | 9 | 0 |
| Action items | 8 | 0 | 9 | 0 |
| Situation monitoring/contingency plan | 5 | 0 | 9 | 0 |
| Medication name | 9 | 4 | 5 | 0 |
| Medication name and dose/route/frequency | 4 | 4 | 5 | 0 |
| Code status | 2 | 2 | 7 | 0 |
| Labs | 6 | 5 | 4 | 0 |
| Access | 2 | 2 | 7 | 0 |
| Ins/outs | 2 | 4 | 4 | 1 |
| Primary language | 0 | 3 | 6 | 0 |
| Vital signs | 3 | 4 | 4 | 1 |
| Emergency contact | 0 | 2 | 7 | 0 |
| Primary care provider | 4 | 4 | 5 | 0 |
Nine institutions (100%) conducted structured group interviews, ranging in size from 4 to 27 individuals with a median of 5 participants. The documents containing information from each site were provided to the authors. The authors then tabulated categorical information, reviewed narrative comments to understand current institutional practices, and conducted simple content analysis to identify areas of concordance or discordance, particularly with respect to data elements and EHR usage. Based on the results of the printed handoff document review and structured group interviews, with additional perspectives provided by the I‐PASS EEC, the expert panel came to consensus on a list of 23 elements that should be included in printed handoff documents, including 15 essential data elements and 8 additional recommended elements (Table 2).
|
| Essential Elements |
| Patient identifiers |
| Patient name (class I, level of evidence C) |
| Medical record number (class I, level of evidence C) |
| Date of birth (class I, level of evidence C) |
| Hospital service identifiers |
| Attending name (class I, level of evidence C) |
| Team/service (class I, level of evidence C) |
| Room number (class I, level of evidence C) |
| Admission date (class I, level of evidence C) |
| Age (class I, level of evidence C) |
| Weight (class I, level of evidence C) |
| Illness severity (class I, level of evidence B)[20, 21] |
| Patient summary (class I, level of evidence B)[21, 22] |
| Action items (class I, level of evidence B) [21, 22] |
| Situation awareness/contingency planning (class I, level of evidence B) [21, 22] |
| Allergies (class I, level of evidence C) |
| Medications |
| Autopopulation of medications (class I, level of evidence B)[22, 23, 24] |
| Free‐text entry of medications (class IIa, level of evidence C) |
| Recommended elements |
| Primary language (class IIa, level of evidence C) |
| Emergency contact (class IIa, level of evidence C) |
| Primary care provider (class IIa, level of evidence C) |
| Code status (class IIb, level of evidence C) |
| Labs (class IIa, level of evidence C) |
| Access (class IIa, level of evidence C) |
| Ins/outs (class IIa, level of evidence C) |
| Vital signs (class IIa, level of evidence C) |
Evidence ratings[19] of these elements are included. Several elements are classified as I‐B (effective, nonrandomized studies) based on either studies of individual elements, or greater than 1 study of bundled elements that could reasonably be extrapolated. These include Illness severity,[20, 21] patient summary,[21, 22] action items[21, 22] (to do lists), situation awareness and contingency plan,[21, 22] and medications[22, 23, 24] with attention to importing from the EHR. Medications entered as free text were classified as IIa‐C because of risk and potential significance of errors; in particular there was concern that transcription errors, errors of omission, or errors of commission could potentially lead to patient harms. The remaining essential elements are classified as I‐C (effective, expert consensus). Of note, date of birth was specifically included as a patient identifier, distinct from age, which was felt to be useful as a descriptor (often within a one‐liner or as part of the patient summary).
The 8 recommended elements were elements for which there was not unanimous agreement on inclusion, but the majority of the panel felt they should be included. These elements were classified as IIa‐C, with 1 exception. Code status generated significant controversy among the group. After extensive discussion among the group and consideration of safety, supervision, educational, and pediatric‐specific considerations, all members of the group agreed on the categorization as a recommended element; it is classified as IIb‐C.
All members of the group agreed that data elements should be directly imported from the EHR whenever possible. Finally, members agreed that the elements that make up the I‐PASS mnemonic (illness severity, patient summary, action items, situation awareness/contingency planning) should be listed in that order whenever possible. A sample I‐PASS‐compliant printed handoff document is shown Figure 1.

DISCUSSION
We identified substantial variability in the structure and content of printed handoff documents used by 9 pediatric hospitalist teaching services, reflective of a lack of standardization. We found that institutional printed handoff documents shared some demographic elements (eg, name, room, medical record number) but also varied in clinical content (eg, vital signs, lab tests, code status). Our expert panel developed a list of 15 essential and 8 recommended data elements for printed handoff documents. Although this is a large number of fields, the majority of the essential fields were already included by most sites, and many are basic demographic identifiers. Illness severity is the 1 essential field that was not routinely included; however, including this type of overview is consistently recommended[2, 4] and supported by evidence,[20, 21] and contributes to building a shared mental model.[16] We recommend the categories of stable/watcher/unstable.[17]
Several prior single‐center studies have found that introducing a printed handoff document can lead to improvements in workflow, communication, and patient safety. In an early study, Petersen et al.[25] showed an association between use of a computerized sign‐out program and reduced odds of preventable adverse events during periods of cross‐coverage. Wayne et al.[26] reported fewer perceived inaccuracies in handoff documents as well as improved clarity at the time of transfer, supporting the role for standardization. Van Eaton et al.[27] demonstrated rapid uptake and desirability of a computerized handoff document, which combined autoimportation of information from an EHR with resident‐entered patient details, reflecting the importance of both data sources. In addition, they demonstrated improvements in both the rounding and sign‐out processes.[28]
Two studies specifically reported the increased use of specific fields after implementation. Payne et al. implemented a Web‐based handoff tool and documented significant increases in the number of handoffs containing problem lists, medication lists, and code status, accompanied by perceived improvements in quality of handoffs and fewer near‐miss events.[24] Starmer et al. found that introduction of a resident handoff bundle that included a printed handoff tool led to reduction in medical errors and adverse events.[22] The study group using the tool populated 11 data elements more often after implementation, and introduction of this printed handoff tool in particular was associated with reductions in written handoff miscommunications. Neither of these studies included subanalysis to indicate which data elements may have been most important.
In contrast to previous single‐institution studies, our recommendations for a printed handoff template come from evaluations of tools and discussions with front line providers across 9 institutions. We had substantial overlap with data elements recommended by Van Eaton et al.[27] However, there were several areas in which we did not have overlap with published templates including weight, ins/outs, primary language, emergency contact information, or primary care provider. Other published handoff tools have been highly specialized (eg, for cardiac intensive care) or included many fewer data elements than our group felt were essential. These differences may reflect the unique aspects of caring for pediatric patients (eg, need for weights) and the absence of defined protocols for many pediatric conditions. In addition, the level of detail needed for contingency planning may vary between teaching and nonteaching services.
Resident physicians may provide valuable information in the development of standardized handoff documents. Clark et al.,[29] at Virginia Mason University, utilized resident‐driven continuous quality improvement processes including real‐time feedback to implement an electronic template. They found that engagement of both senior leaders and front‐line users was an important component of their success in uptake. Our study utilized residents as essential members of structured group interviews to ensure that front‐line users' needs were represented as recommendations for a printed handoff tool template were developed.
As previously described,[17] our study group had identified several key data elements that should be included in verbal handoffs: illness severity, a patient summary, a discrete action list, situation awareness/contingency planning, and a synthesis by receiver. With consideration of the multivoting results as well as known best practices,[1, 4, 12] the expert panel for this study agreed that each of these elements should also be highlighted in the printed template to ensure consistency between the printed document and the verbal handoff, and to have each reinforce the other. On the printed handoff tool, the final S in the I‐PASS mnemonic (synthesis by receiver) cannot be prepopulated, but considering the importance of this step,[16, 30, 31, 32] it should be printed as synthesis by receiver to serve as a text‐reminder to both givers and receivers.
The panel also felt, however, that the printed handoff document should provide additional background information not routinely included in a verbal handoff. It should serve as a reference tool both at the time of verbal handoff and throughout the day and night, and therefore should include more comprehensive information than is necessary or appropriate to convey during the verbal handoff. We identified 10 data elements that are essential in a printed handoff document in addition to the I‐PASS elements (Table 2).
Patient demographic data elements, as well as team assignments and attending physician, were uniformly supported for inclusion. The medication list was viewed as essential; however, the panel also recognized the potential for medical errors due to inaccuracies in the medication list. In particular, there was concern that including all fields of a medication order (drug, dose, route, frequency) would result in handoffs containing a high proportion of inaccurate information, particularly for complex patients whose medication regimens may vary over the course of hospitalization. Therefore, the panel agreed that if medication lists were entered manually, then only the medication name should be included as they did not wish to perpetuate inaccurate or potentially harmful information. If medication lists were autoimported from an EHR, then they should include drug name, dose, route, and frequency if possible.
In the I‐PASS study,[15] all institutions implemented printed handoff documents that included fields for the essential data elements. After implementation, there was a significant increase in completion of all essential fields. Although there is limited evidence to support any individual data element, increased usage of these elements was associated with the overall study finding of decreased rates of medical errors and preventable adverse events.
EHRs have the potential to help standardize printed handoff documents[5, 6, 33, 34, 35]; all participants in our study agreed that printed handoff documents should ideally be linked with the EHR and should autoimport data wherever appropriate. Manually populated (eg, word processor‐ or spreadsheet‐based) handoff tools have important limitations, particularly related to the potential for typographical errors as well as accidental omission of data fields, and lead to unnecessary duplication of work (eg, re‐entering data already included in a progress note) that can waste providers' time. It was also acknowledged that word processor‐ or spreadsheet‐based documents may have flexibility that is lacking in EHR‐based handoff documents. For example, formatting can more easily be adjusted to increase the number of patients per printed page. As technology advances, printed documents may be phased out in favor of EHR‐based on‐screen reports, which by their nature would be more accurate due to real‐time autoupdates.
In making recommendations about essential versus recommended items for inclusion in the printed handoff template, the only data element that generated controversy among our experts was code status. Some felt that it should be included as an essential element, whereas others did not. We believe that this was unique to our practice in pediatric hospital ward settings, as codes in most pediatric ward settings are rare. Among the concerns expressed with including code status for all patients were that residents might assume patients were full‐code without verifying. The potential inaccuracy created by this might have severe implications. Alternatively, residents might feel obligated to have code discussions with all patients regardless of severity of illness, which may be inappropriate in a pediatric population. Several educators expressed concerns about trainees having unsupervised code‐status conversations with families of pediatric patients. Conversely, although codes are rare in pediatric ward settings, concerns were raised that not including code status could be problematic during these rare but critically important events. Other fields, such as weight, might have less relevance for an adult population in which emergency drug doses are standardized.
Limitations
Our study has several limitations. We only collected data from hospitalist services at pediatric sites. It is likely that providers in other specialties would have specific data elements they felt were essential (eg, postoperative day, code status). Our methodology was expert consensus based, driven by data collection from sites that were already participating in the I‐PASS study. Although the I‐PASS study demonstrated decreased rates of medical errors and preventable adverse events with inclusion of these data elements as part of a bundle, future research will be required to evaluate whether some of these items are more important than others in improving written communication and ultimately patient safety. In spite of these limitations, our work represents an important starting point for the development of standards for written handoff documents that should be used in patient handoffs, particularly those generated from EHRs.
CONCLUSIONS
In this article we describe the results of a needs assessment that informed expert consensus‐based recommendations for data elements to include in a printed handoff document. We recommend that pediatric programs include the elements identified as part of a standardized written handoff tool. Although many of these elements are also applicable to other specialties, future work should be conducted to adapt the printed handoff document elements described here for use in other specialties and settings. Future studies should work to validate the importance of these elements, studying the manner in which their inclusion affects the quality of written handoffs, and ultimately patient safety.
Acknowledgements
Members of the I‐PASS Study Education Executive Committee who contributed to this manuscript include: Boston Children's Hospital/Harvard Medical School (primary site) (Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA. Theodore C. Sectish, MD. Lisa L. Tse, BA). Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine (Jennifer K. O'Toole, MD, MEd). Doernbecher Children's Hospital/Oregon Health and Science University (Amy J. Starmer, MD, MPH). Hospital for Sick Children/University of Toronto (Zia Bismilla, MD. Maitreya Coffey, MD). Lucile Packard Children's Hospital/Stanford University (Lauren A. Destino, MD. Jennifer L. Everhart, MD. Shilpa J. Patel, MD [currently at Kapi'olani Children's Hospital/University of Hawai'i School of Medicine]). National Capital Consortium (Jennifer H. Hepps, MD. Joseph O. Lopreiato, MD, MPH. Clifton E. Yu, MD). Primary Children's Medical Center/University of Utah (James F. Bale, Jr., MD. Adam T. Stevenson, MD). St. Louis Children's Hospital/Washington University (F. Sessions Cole, MD). St. Christopher's Hospital for Children/Drexel University College of Medicine (Sharon Calaman, MD. Nancy D. Spector, MD). Benioff Children's Hospital/University of California San Francisco School of Medicine (Glenn Rosenbluth, MD. Daniel C. West, MD).
Additional I‐PASS Study Group members who contributed to this manuscript include April D. Allen, MPA, MA (Heller School for Social Policy and Management, Brandeis University, previously affiliated with Boston Children's Hospital), Madelyn D. Kahana, MD (The Children's Hospital at Montefiore/Albert Einstein College of Medicine, previously affiliated with Lucile Packard Children's Hospital/Stanford University), Robert S. McGregor, MD (Akron Children's Hospital/Northeast Ohio Medical University, previously affiliated with St. Christopher's Hospital for Children/Drexel University), and John S. Webster, MD, MBA, MS (Webster Healthcare Consulting Inc., formerly of the Department of Defense).
Members of the I‐PASS Study Group include individuals from the institutions listed below as follows: Boston Children's Hospital/Harvard Medical School (primary site): April D. Allen, MPA, MA (currently at Heller School for Social Policy and Management, Brandeis University), Angela M. Feraco, MD, Christopher P. Landrigan, MD, MPH, Elizabeth L. Noble, BA, Theodore C. Sectish, MD, Lisa L. Tse, BA. Brigham and Women's Hospital (data coordinating center): Anuj K. Dalal, MD, Carol A. Keohane, BSN, RN, Stuart Lipsitz, PhD, Jeffrey M. Rothschild, MD, MPH, Matt F. Wien, BS, Catherine S. Yoon, MS, Katherine R. Zigmont, BSN, RN. Cincinnati Children's Hospital Medical Center/University of Cincinnati College of Medicine: Javier Gonzalez del Rey, MD, MEd, Jennifer K. O'Toole, MD, MEd, Lauren G. Solan, MD. Doernbecher Children's Hospital/Oregon Health and Science University: Megan E. Aylor, MD, Amy J. Starmer, MD, MPH, Windy Stevenson, MD, Tamara Wagner, MD. Hospital for Sick Children/University of Toronto: Zia Bismilla, MD, Maitreya Coffey, MD, Sanjay Mahant, MD, MSc. Lucile Packard Children's Hospital/Stanford University: Rebecca L. Blankenburg, MD, MPH, Lauren A. Destino, MD, Jennifer L. Everhart, MD, Madelyn Kahana, MD, Shilpa J. Patel, MD (currently at Kapi'olani Children's Hospital/University of Hawaii School of Medicine). National Capital Consortium: Jennifer H. Hepps, MD, Joseph O. Lopreiato, MD, MPH, Clifton E. Yu, MD. Primary Children's Hospital/University of Utah: James F. Bale, Jr., MD, Jaime Blank Spackman, MSHS, CCRP, Rajendu Srivastava, MD, FRCP(C), MPH, Adam Stevenson, MD. St. Louis Children's Hospital/Washington University: Kevin Barton, MD, Kathleen Berchelmann, MD, F. Sessions Cole, MD, Christine Hrach, MD, Kyle S. Schultz, MD, Michael P. Turmelle, MD, Andrew J. White, MD. St. Christopher's Hospital for Children/Drexel University: Sharon Calaman, MD, Bronwyn D. Carlson, MD, Robert S. McGregor, MD (currently at Akron Children's Hospital/Northeast Ohio Medical University), Vahideh Nilforoshan, MD, Nancy D. Spector, MD. and Benioff Children's Hospital/University of California San Francisco School of Medicine: Glenn Rosenbluth, MD, Daniel C. West, MD. Dorene Balmer, PhD, RD, Carol L. Carraccio, MD, MA, Laura Degnon, CAE, and David McDonald, and Alan Schwartz PhD serve the I‐PASS Study Group as part of the IIPE. Karen M. Wilson, MD, MPH serves the I‐PASS Study Group as part of the advisory board from the PRIS Executive Council. John Webster served the I‐PASS Study Group and Education Executive Committee as a representative from TeamSTEPPS.
Disclosures: The I‐PASS Study was primarily supported by the US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation (1R18AE000029‐01). The opinions and conclusions expressed herein are solely those of the author(s) and should not be constructed as representing the opinions or policy of any agency of the federal government. Developed with input from the Initiative for Innovation in Pediatric Education and the Pediatric Research in Inpatient Settings Network (supported by the Children's Hospital Association, the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine). A. J. S. was supported by the Agency for Healthcare Research and Quality/Oregon Comparative Effectiveness Research K12 Program (1K12HS019456‐01). Additional funding for the I‐PASS Study was provided by the Medical Research Foundation of Oregon, Physician Services Incorporated Foundation (Ontario, Canada), and Pfizer (unrestricted medical education grant to N.D.S.). C.P.L, A.J.S. were supported by the Oregon Comparative Effectiveness Research K12 Program (1K12HS019456 from the Agency for Healthcare Research and Quality). A.J.S. was also supported by the Medical Research Foundation of Oregon. The authors report no conflicts of interest.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , . Development and implementation of an oral sign‐out skills curriculum. J Gen Intern Med. 2007;22(10):1470–1474.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . A systematic review of the literature on the evaluation of handoff tools: implications for research and practice. J Am Med Inform Assoc. 2014;21(1):154–162.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Sign‐out snapshot: cross‐sectional evaluation of written sign‐outs among specialties. BMJ Qual Saf. 2014;23(1):66–72.
- , , , . An experimental comparison of handover methods. Ann R Coll Surg Engl. 2007;89(3):298–300.
- , , , . Pilot study to show the loss of important data in nursing handover. Br J Nurs. 2005;14(20):1090–1093.
- The Joint Commission. Hospital Accreditation Standards 2015: Joint Commission Resources; 2015:PC.02.02.01.
- Accreditation Council for Graduate Medical Education. Common Program Requirements. 2013; http://acgme.org/acgmeweb/tabid/429/ProgramandInstitutionalAccreditation/CommonProgramRequirements.aspx. Accessed May 11, 2015.
- , , , . Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim. Pediatrics. 2010;126(4):619–622.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- US Department of Health and Human Services. Agency for Healthcare Research and Quality. TeamSTEPPS website. Available at: http://teamstepps.ahrq.gov/. Accessed July 12, 2013.
- , , , , , . I‐PASS, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . The Team Handbook. 3rd ed. Middleton, WI: Oriel STAT A MATRIX; 2010.
- ACC/AHA Task Force on Practice Guidelines. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines. Available at: http://my.americanheart.org/idc/groups/ahamah‐public/@wcm/@sop/documents/downloadable/ucm_319826.pdf. Published June 2010. Accessed January 11, 2015.
- , , , et al. Effect of illness severity and comorbidity on patient safety and adverse events. Am J Med Qual. 2012;27(1):48–57.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , , . Medication discrepancies in resident sign‐outs and their potential to harm. J Gen Intern Med. 2007;22(12):1751–1755.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , , . A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , . Template for success: using a resident‐designed sign‐out template in the handover of patient care. J Surg Educ. 2011;68(1):52–57.
- , , , , , . Read‐back improves information transfer in simulated clinical crises. BMJ Qual Saf. 2014;23(12):989–993.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- , , , , , . Improving patient safety by repeating (read‐back) telephone reports of critical information. Am J Clin Pathol. 2004;121(6):801–803.
- , , , , . Content overlap in nurse and physician handoff artifacts and the potential role of electronic health records: a systematic review. J Biomed Inform. 2011;44(4):704–712.
- , , , . Clinical summarization capabilities of commercially‐available and internally‐developed electronic health records. Appl Clin Inform. 2012;3(1):80–93.
- , . An analysis and recommendations for multidisciplinary computerized handoff applications in hospitals. AMIA Annu Symp Proc. 2011;2011:588–597.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , . Development and implementation of an oral sign‐out skills curriculum. J Gen Intern Med. 2007;22(10):1470–1474.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . A systematic review of the literature on the evaluation of handoff tools: implications for research and practice. J Am Med Inform Assoc. 2014;21(1):154–162.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Sign‐out snapshot: cross‐sectional evaluation of written sign‐outs among specialties. BMJ Qual Saf. 2014;23(1):66–72.
- , , , . An experimental comparison of handover methods. Ann R Coll Surg Engl. 2007;89(3):298–300.
- , , , . Pilot study to show the loss of important data in nursing handover. Br J Nurs. 2005;14(20):1090–1093.
- The Joint Commission. Hospital Accreditation Standards 2015: Joint Commission Resources; 2015:PC.02.02.01.
- Accreditation Council for Graduate Medical Education. Common Program Requirements. 2013; http://acgme.org/acgmeweb/tabid/429/ProgramandInstitutionalAccreditation/CommonProgramRequirements.aspx. Accessed May 11, 2015.
- , , , . Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim. Pediatrics. 2010;126(4):619–622.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- US Department of Health and Human Services. Agency for Healthcare Research and Quality. TeamSTEPPS website. Available at: http://teamstepps.ahrq.gov/. Accessed July 12, 2013.
- , , , , , . I‐PASS, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . The Team Handbook. 3rd ed. Middleton, WI: Oriel STAT A MATRIX; 2010.
- ACC/AHA Task Force on Practice Guidelines. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines. Available at: http://my.americanheart.org/idc/groups/ahamah‐public/@wcm/@sop/documents/downloadable/ucm_319826.pdf. Published June 2010. Accessed January 11, 2015.
- , , , et al. Effect of illness severity and comorbidity on patient safety and adverse events. Am J Med Qual. 2012;27(1):48–57.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , , . Medication discrepancies in resident sign‐outs and their potential to harm. J Gen Intern Med. 2007;22(12):1751–1755.
- , , , . Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925–932.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , , . A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , . Template for success: using a resident‐designed sign‐out template in the handover of patient care. J Surg Educ. 2011;68(1):52–57.
- , , , , , . Read‐back improves information transfer in simulated clinical crises. BMJ Qual Saf. 2014;23(12):989–993.
- , , , , . Interns overestimate the effectiveness of their hand‐off communication. Pediatrics. 2010;125(3):491–496.
- , , , , , . Improving patient safety by repeating (read‐back) telephone reports of critical information. Am J Clin Pathol. 2004;121(6):801–803.
- , , , , . Content overlap in nurse and physician handoff artifacts and the potential role of electronic health records: a systematic review. J Biomed Inform. 2011;44(4):704–712.
- , , , . Clinical summarization capabilities of commercially‐available and internally‐developed electronic health records. Appl Clin Inform. 2012;3(1):80–93.
- , . An analysis and recommendations for multidisciplinary computerized handoff applications in hospitals. AMIA Annu Symp Proc. 2011;2011:588–597.
© 2015 Society of Hospital Medicine
Herbs reduce fatigue in cancer patients

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()
Inhibitor promotes chemosensitization in CLL

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.
CHMP recommends drug for WM

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending that ibrutinib (Imbruvica) be approved to treat Waldenström’s macroglobulinemia (WM).
The CHMP is recommending the drug for use in WM patients who have received at least 1 prior therapy as well as previously untreated WM patients who are not suitable candidates for chemo-immunotherapy.
The European Commission will review this recommendation and should make a decision later this year.
Ibrutinib is already approved to treat WM in the US. The drug is also approved in the European Union, the US, and other countries to treat chronic lymphocytic leukemia and mantle cell lymphoma.
Janssen-Cilag International NV (Janssen) holds the marketing authorization for ibrutinib in Europe, and its affiliates market the drug in Europe and the rest of the world. In the US, ibrutinib is under joint development by Pharmacyclics and Janssen Biotech, Inc.
Phase 2 study
The CHMP’s recommendation for ibrutinib was based on a multicenter, phase 2 study in which researchers tested the drug in 63 patients with previously treated WM. Initial data showed an overall response rate of 87.3% in patients who received the drug for a median of 11.7 months.
Updated results from the study were published in NEJM in April. After a median treatment duration of 19.1 months, the overall response rate was 91%.
At 24 months, the estimated rate of progression-free survival was 69%, and the estimated rate of overall survival was 95%.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending that ibrutinib (Imbruvica) be approved to treat Waldenström’s macroglobulinemia (WM).
The CHMP is recommending the drug for use in WM patients who have received at least 1 prior therapy as well as previously untreated WM patients who are not suitable candidates for chemo-immunotherapy.
The European Commission will review this recommendation and should make a decision later this year.
Ibrutinib is already approved to treat WM in the US. The drug is also approved in the European Union, the US, and other countries to treat chronic lymphocytic leukemia and mantle cell lymphoma.
Janssen-Cilag International NV (Janssen) holds the marketing authorization for ibrutinib in Europe, and its affiliates market the drug in Europe and the rest of the world. In the US, ibrutinib is under joint development by Pharmacyclics and Janssen Biotech, Inc.
Phase 2 study
The CHMP’s recommendation for ibrutinib was based on a multicenter, phase 2 study in which researchers tested the drug in 63 patients with previously treated WM. Initial data showed an overall response rate of 87.3% in patients who received the drug for a median of 11.7 months.
Updated results from the study were published in NEJM in April. After a median treatment duration of 19.1 months, the overall response rate was 91%.
At 24 months, the estimated rate of progression-free survival was 69%, and the estimated rate of overall survival was 95%.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending that ibrutinib (Imbruvica) be approved to treat Waldenström’s macroglobulinemia (WM).
The CHMP is recommending the drug for use in WM patients who have received at least 1 prior therapy as well as previously untreated WM patients who are not suitable candidates for chemo-immunotherapy.
The European Commission will review this recommendation and should make a decision later this year.
Ibrutinib is already approved to treat WM in the US. The drug is also approved in the European Union, the US, and other countries to treat chronic lymphocytic leukemia and mantle cell lymphoma.
Janssen-Cilag International NV (Janssen) holds the marketing authorization for ibrutinib in Europe, and its affiliates market the drug in Europe and the rest of the world. In the US, ibrutinib is under joint development by Pharmacyclics and Janssen Biotech, Inc.
Phase 2 study
The CHMP’s recommendation for ibrutinib was based on a multicenter, phase 2 study in which researchers tested the drug in 63 patients with previously treated WM. Initial data showed an overall response rate of 87.3% in patients who received the drug for a median of 11.7 months.
Updated results from the study were published in NEJM in April. After a median treatment duration of 19.1 months, the overall response rate was 91%.
At 24 months, the estimated rate of progression-free survival was 69%, and the estimated rate of overall survival was 95%.
The most common grade 2-4 adverse events were neutropenia (22%) and thrombocytopenia (14%). Ibrutinib-related neutropenia and thrombocytopenia were reversible but required a dose reduction in 3 patients and treatment discontinuation in 4 patients.
Grade 2 or higher bleeding events occurred in 4 patients, and there were 15 infections considered possibly related to ibrutinib.
Treatment-related atrial fibrillation (AFib) occurred in 3 patients, all of whom had a prior history of paroxysmal AFib. AFib resolved when treatment was withheld, and all 3 patients were able to continue on therapy per protocol without an additional event. ![]()
APA: Predictive analytics and big data hold promise in mood disorders
TORONTO – “What if we could detect a mood episode before it happened?” It was with this question that Dr. Andrew A. Nierenberg began his talk on new advances in mood disorders research at the annual meeting of the American Psychiatric Association.
From predictive analytics to big data collaboration to therapeutic apps, Dr. Nierenberg led the audience through a tour of the now and near future.
One company in this space, Ginger.io, uses behavioral analytics to better understand patients’ changing social, mental, and physical health status. The data can then be fed quickly back to clinicians when intervention is warranted. The company’s app collects passive sensor data from patients’ smartphones about their movement, communication, and sleep patterns. Sophisticated analytical methods detect changes in behavior and predict people’s moods and actions.
“It’s a little creepy in some ways, but maybe not,” he said. “If you think about it, when people come to us in distress, it’s not at the very edge or beginning of a mood episode, but they’re deep into it [and that is] when we tend to intervene.”
When a patient is evaluated, he explained, the strength of the evaluation is dependent on accurate self-observation, and accurate storage and recall of the patient’s observations about their emotional states.
“Those are all problems for people with mood disorders,” Dr. Nierenberg said. “So, when we ask someone how they have been in the past week, we’re really getting a window into the past 3-6 hours. What these predictive analytics allow is real time data to look at what is actually happening with people.”
The question really being asked here, said Dr. Nierenberg, is whether it’s possible to see objective changes that are not among the information people are likely to report to their clinicians, that can predict a mood episode.
Harnessing technology
Big data also has come to mood disorders care in a big way. Large registries are being compiled for research purposes, and patient communities are growing that help patients cope with their conditions and help researchers collect huge amounts of data. Based on cognitive-behavioral therapy combined with relaxation and wellness techniques, we believe in holistic daily tools aimed at breaking the anxiety cycle. We’re not about quick fixes or false promises. We are about real progress, a day at a time.
According to its website, Big White Wall is an online community of people “who are anxious, down, or not coping who support and help each other by sharing what’s troubling them, guided by trained professionals.”
Other examples of these tech-based solutions are therapeutic apps and websites. Dr. Nierenberg mentioned just three: MoodGYM, Now Matters Now, and Pacifica, all of which are “cutting edge and evidence-based” and help patients manage their conditions.
• MoodGym is a free, interactive self-help program that provides cognitive-behavior therapy (CBT) training to help users prevent and cope with depression and anxiety.
• Now Matters Now is an online video-based program that uses “real” people, including suicide prevention researchers and clinicians, to teach coping skills such as mindfulness, paced breathing, and opposite action to individuals having suicidal thoughts. The skills taught are part of dialectical behavior therapy, or DBT, proven to be helpful for people considering suicide. Dr. Nierenberg called this community “quite extraordinary” and uniquely valuable, “because the majority of people who are having suicidal thoughts don’t have them when they’re in your office …”
• Pacifica is a self-help app for anxiety that uses CBT combined with relaxation and wellness techniques aimed at “breaking the anxiety cycle,” the company says.
‘A game changer’
The Patient Centered Outcomes Research Network (PCORnet.org) is “a game changer,” said Dr. Nierenberg. It is part of the Patient-Centered Outcomes Research Institute (PCORI), which is part of the Affordable Care Act, funded at about $500 million a year. One part of PCORnet.org is the Patient-Powered Research Networks, including a mood-focused network, moodnetwork.org.
“It allows the patients to choose how they want to be monitored, through self-report, but also gives them a voice in prioritizing research and research questions.” A goal is to transform research and mood disorder care by creating an infrastructure for both research and clinicians wanting to follow their patients and through prospective comparative effectiveness trials embedded within routine care.
The organizers hope to gather 50,000 patients in the network, a “wild and audacious goal,” admitted Dr. Nierenberg, who is the principal investigator of moodnetwork.org. PCORnet.org ultimately might cover 90 million people and truly be able to answer real-world questions in a way that most research today does not address, he added.
Dr. Nierenberg is a top researcher and educator from Massachusetts General Hospital and Harvard Medical School, Boston. In 2013, he won the prestigious Colvin Prize given by the Brain & Behavior Research Foundation for Outstanding Achievement in Mood Disorders Research.
Dr. Nierenberg reported working with several pharmaceutical companies in drug development.
TORONTO – “What if we could detect a mood episode before it happened?” It was with this question that Dr. Andrew A. Nierenberg began his talk on new advances in mood disorders research at the annual meeting of the American Psychiatric Association.
From predictive analytics to big data collaboration to therapeutic apps, Dr. Nierenberg led the audience through a tour of the now and near future.
One company in this space, Ginger.io, uses behavioral analytics to better understand patients’ changing social, mental, and physical health status. The data can then be fed quickly back to clinicians when intervention is warranted. The company’s app collects passive sensor data from patients’ smartphones about their movement, communication, and sleep patterns. Sophisticated analytical methods detect changes in behavior and predict people’s moods and actions.
“It’s a little creepy in some ways, but maybe not,” he said. “If you think about it, when people come to us in distress, it’s not at the very edge or beginning of a mood episode, but they’re deep into it [and that is] when we tend to intervene.”
When a patient is evaluated, he explained, the strength of the evaluation is dependent on accurate self-observation, and accurate storage and recall of the patient’s observations about their emotional states.
“Those are all problems for people with mood disorders,” Dr. Nierenberg said. “So, when we ask someone how they have been in the past week, we’re really getting a window into the past 3-6 hours. What these predictive analytics allow is real time data to look at what is actually happening with people.”
The question really being asked here, said Dr. Nierenberg, is whether it’s possible to see objective changes that are not among the information people are likely to report to their clinicians, that can predict a mood episode.
Harnessing technology
Big data also has come to mood disorders care in a big way. Large registries are being compiled for research purposes, and patient communities are growing that help patients cope with their conditions and help researchers collect huge amounts of data. Based on cognitive-behavioral therapy combined with relaxation and wellness techniques, we believe in holistic daily tools aimed at breaking the anxiety cycle. We’re not about quick fixes or false promises. We are about real progress, a day at a time.
According to its website, Big White Wall is an online community of people “who are anxious, down, or not coping who support and help each other by sharing what’s troubling them, guided by trained professionals.”
Other examples of these tech-based solutions are therapeutic apps and websites. Dr. Nierenberg mentioned just three: MoodGYM, Now Matters Now, and Pacifica, all of which are “cutting edge and evidence-based” and help patients manage their conditions.
• MoodGym is a free, interactive self-help program that provides cognitive-behavior therapy (CBT) training to help users prevent and cope with depression and anxiety.
• Now Matters Now is an online video-based program that uses “real” people, including suicide prevention researchers and clinicians, to teach coping skills such as mindfulness, paced breathing, and opposite action to individuals having suicidal thoughts. The skills taught are part of dialectical behavior therapy, or DBT, proven to be helpful for people considering suicide. Dr. Nierenberg called this community “quite extraordinary” and uniquely valuable, “because the majority of people who are having suicidal thoughts don’t have them when they’re in your office …”
• Pacifica is a self-help app for anxiety that uses CBT combined with relaxation and wellness techniques aimed at “breaking the anxiety cycle,” the company says.
‘A game changer’
The Patient Centered Outcomes Research Network (PCORnet.org) is “a game changer,” said Dr. Nierenberg. It is part of the Patient-Centered Outcomes Research Institute (PCORI), which is part of the Affordable Care Act, funded at about $500 million a year. One part of PCORnet.org is the Patient-Powered Research Networks, including a mood-focused network, moodnetwork.org.
“It allows the patients to choose how they want to be monitored, through self-report, but also gives them a voice in prioritizing research and research questions.” A goal is to transform research and mood disorder care by creating an infrastructure for both research and clinicians wanting to follow their patients and through prospective comparative effectiveness trials embedded within routine care.
The organizers hope to gather 50,000 patients in the network, a “wild and audacious goal,” admitted Dr. Nierenberg, who is the principal investigator of moodnetwork.org. PCORnet.org ultimately might cover 90 million people and truly be able to answer real-world questions in a way that most research today does not address, he added.
Dr. Nierenberg is a top researcher and educator from Massachusetts General Hospital and Harvard Medical School, Boston. In 2013, he won the prestigious Colvin Prize given by the Brain & Behavior Research Foundation for Outstanding Achievement in Mood Disorders Research.
Dr. Nierenberg reported working with several pharmaceutical companies in drug development.
TORONTO – “What if we could detect a mood episode before it happened?” It was with this question that Dr. Andrew A. Nierenberg began his talk on new advances in mood disorders research at the annual meeting of the American Psychiatric Association.
From predictive analytics to big data collaboration to therapeutic apps, Dr. Nierenberg led the audience through a tour of the now and near future.
One company in this space, Ginger.io, uses behavioral analytics to better understand patients’ changing social, mental, and physical health status. The data can then be fed quickly back to clinicians when intervention is warranted. The company’s app collects passive sensor data from patients’ smartphones about their movement, communication, and sleep patterns. Sophisticated analytical methods detect changes in behavior and predict people’s moods and actions.
“It’s a little creepy in some ways, but maybe not,” he said. “If you think about it, when people come to us in distress, it’s not at the very edge or beginning of a mood episode, but they’re deep into it [and that is] when we tend to intervene.”
When a patient is evaluated, he explained, the strength of the evaluation is dependent on accurate self-observation, and accurate storage and recall of the patient’s observations about their emotional states.
“Those are all problems for people with mood disorders,” Dr. Nierenberg said. “So, when we ask someone how they have been in the past week, we’re really getting a window into the past 3-6 hours. What these predictive analytics allow is real time data to look at what is actually happening with people.”
The question really being asked here, said Dr. Nierenberg, is whether it’s possible to see objective changes that are not among the information people are likely to report to their clinicians, that can predict a mood episode.
Harnessing technology
Big data also has come to mood disorders care in a big way. Large registries are being compiled for research purposes, and patient communities are growing that help patients cope with their conditions and help researchers collect huge amounts of data. Based on cognitive-behavioral therapy combined with relaxation and wellness techniques, we believe in holistic daily tools aimed at breaking the anxiety cycle. We’re not about quick fixes or false promises. We are about real progress, a day at a time.
According to its website, Big White Wall is an online community of people “who are anxious, down, or not coping who support and help each other by sharing what’s troubling them, guided by trained professionals.”
Other examples of these tech-based solutions are therapeutic apps and websites. Dr. Nierenberg mentioned just three: MoodGYM, Now Matters Now, and Pacifica, all of which are “cutting edge and evidence-based” and help patients manage their conditions.
• MoodGym is a free, interactive self-help program that provides cognitive-behavior therapy (CBT) training to help users prevent and cope with depression and anxiety.
• Now Matters Now is an online video-based program that uses “real” people, including suicide prevention researchers and clinicians, to teach coping skills such as mindfulness, paced breathing, and opposite action to individuals having suicidal thoughts. The skills taught are part of dialectical behavior therapy, or DBT, proven to be helpful for people considering suicide. Dr. Nierenberg called this community “quite extraordinary” and uniquely valuable, “because the majority of people who are having suicidal thoughts don’t have them when they’re in your office …”
• Pacifica is a self-help app for anxiety that uses CBT combined with relaxation and wellness techniques aimed at “breaking the anxiety cycle,” the company says.
‘A game changer’
The Patient Centered Outcomes Research Network (PCORnet.org) is “a game changer,” said Dr. Nierenberg. It is part of the Patient-Centered Outcomes Research Institute (PCORI), which is part of the Affordable Care Act, funded at about $500 million a year. One part of PCORnet.org is the Patient-Powered Research Networks, including a mood-focused network, moodnetwork.org.
“It allows the patients to choose how they want to be monitored, through self-report, but also gives them a voice in prioritizing research and research questions.” A goal is to transform research and mood disorder care by creating an infrastructure for both research and clinicians wanting to follow their patients and through prospective comparative effectiveness trials embedded within routine care.
The organizers hope to gather 50,000 patients in the network, a “wild and audacious goal,” admitted Dr. Nierenberg, who is the principal investigator of moodnetwork.org. PCORnet.org ultimately might cover 90 million people and truly be able to answer real-world questions in a way that most research today does not address, he added.
Dr. Nierenberg is a top researcher and educator from Massachusetts General Hospital and Harvard Medical School, Boston. In 2013, he won the prestigious Colvin Prize given by the Brain & Behavior Research Foundation for Outstanding Achievement in Mood Disorders Research.
Dr. Nierenberg reported working with several pharmaceutical companies in drug development.
EXPERT ANALYSIS FROM THE APA ANNUAL MEETING