User login
FDA clears test to detect bacteria in platelets

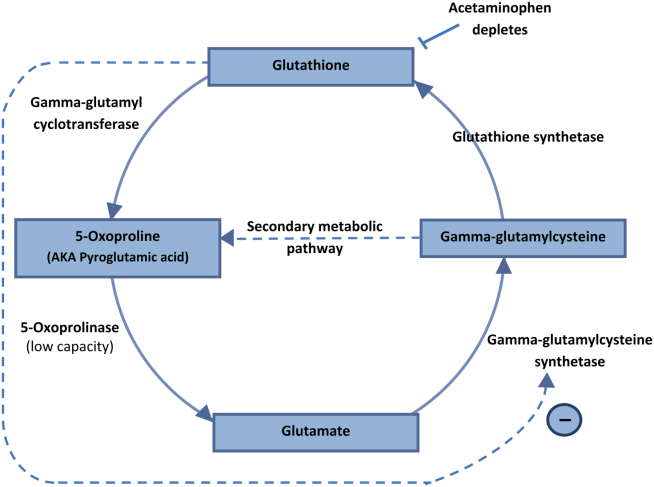
The US Food and Drug Administration (FDA) has expanded the authorized use of Verax Biomedical’s Platelet PGD Test, which detects bacteria in platelets intended for transfusion.
The FDA previously approved the test for leukocyte-reduced apheresis platelets (in 2007) and platelets derived from whole blood (in 2009).
Now, the test has been approved for pre-storage pooled platelets and apheresis platelets in platelet additive solution C (PAS-C) and plasma.
This makes the Platelet PGD Test the only rapid test on the market that can check every commonly distributed platelet type in the US, according to Verax Biomedical.
About the test
The Platelet PGD Test is an immunoassay used on the day of transfusion at the point of care—a hospital or transfusion service—to detect bacterial contamination in platelets to be transfused.
The test consists of a disposable plastic cartridge and 3 pretreatment reagents. To use, the tester pretreats a freshly collected platelet sample (500µL) and applies it to the sample well on the test cartridge.
Lights on the cartridge change from yellow to blue-violet when the test is ready to be interpreted, which is typically about 20 minutes after the sample is applied to the cartridge. The lights confirm that the appropriate volume of a sample was added and the testing is complete.
If the test is positive, a pink line will appear in 1 of the 2 windows on the cartridge. One window represents Gram-positive bacteria and the other Gram-negative. Non-reactive samples will have no line in either window.
Now that the FDA has expanded the indications for the Platelet PGD Test, it can be used as a quality control test for pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced whole-blood-derived platelets suspended in plasma that are pooled within 4 hours of transfusion.
The test can also be used within 24 hours of transfusion as a safety measure following testing with a growth-based, quality control test cleared by the FDA. For this indication, the Platelet PGD Test can be used with:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in PAS-C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced whole-blood-derived platelets suspended in plasma.
In studies conducted by Verax Biomedical (described in the summary document here), the Platelet PGD Test successfully detected bacteria in pre-storage pools of whole-blood derived platelets suspended in plasma and leukocyte-reduced apheresis platelets suspended in plasma or PAS-C and plasma. ![]()

The US Food and Drug Administration (FDA) has expanded the authorized use of Verax Biomedical’s Platelet PGD Test, which detects bacteria in platelets intended for transfusion.
The FDA previously approved the test for leukocyte-reduced apheresis platelets (in 2007) and platelets derived from whole blood (in 2009).
Now, the test has been approved for pre-storage pooled platelets and apheresis platelets in platelet additive solution C (PAS-C) and plasma.
This makes the Platelet PGD Test the only rapid test on the market that can check every commonly distributed platelet type in the US, according to Verax Biomedical.
About the test
The Platelet PGD Test is an immunoassay used on the day of transfusion at the point of care—a hospital or transfusion service—to detect bacterial contamination in platelets to be transfused.
The test consists of a disposable plastic cartridge and 3 pretreatment reagents. To use, the tester pretreats a freshly collected platelet sample (500µL) and applies it to the sample well on the test cartridge.
Lights on the cartridge change from yellow to blue-violet when the test is ready to be interpreted, which is typically about 20 minutes after the sample is applied to the cartridge. The lights confirm that the appropriate volume of a sample was added and the testing is complete.
If the test is positive, a pink line will appear in 1 of the 2 windows on the cartridge. One window represents Gram-positive bacteria and the other Gram-negative. Non-reactive samples will have no line in either window.
Now that the FDA has expanded the indications for the Platelet PGD Test, it can be used as a quality control test for pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced whole-blood-derived platelets suspended in plasma that are pooled within 4 hours of transfusion.
The test can also be used within 24 hours of transfusion as a safety measure following testing with a growth-based, quality control test cleared by the FDA. For this indication, the Platelet PGD Test can be used with:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in PAS-C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced whole-blood-derived platelets suspended in plasma.
In studies conducted by Verax Biomedical (described in the summary document here), the Platelet PGD Test successfully detected bacteria in pre-storage pools of whole-blood derived platelets suspended in plasma and leukocyte-reduced apheresis platelets suspended in plasma or PAS-C and plasma. ![]()

The US Food and Drug Administration (FDA) has expanded the authorized use of Verax Biomedical’s Platelet PGD Test, which detects bacteria in platelets intended for transfusion.
The FDA previously approved the test for leukocyte-reduced apheresis platelets (in 2007) and platelets derived from whole blood (in 2009).
Now, the test has been approved for pre-storage pooled platelets and apheresis platelets in platelet additive solution C (PAS-C) and plasma.
This makes the Platelet PGD Test the only rapid test on the market that can check every commonly distributed platelet type in the US, according to Verax Biomedical.
About the test
The Platelet PGD Test is an immunoassay used on the day of transfusion at the point of care—a hospital or transfusion service—to detect bacterial contamination in platelets to be transfused.
The test consists of a disposable plastic cartridge and 3 pretreatment reagents. To use, the tester pretreats a freshly collected platelet sample (500µL) and applies it to the sample well on the test cartridge.
Lights on the cartridge change from yellow to blue-violet when the test is ready to be interpreted, which is typically about 20 minutes after the sample is applied to the cartridge. The lights confirm that the appropriate volume of a sample was added and the testing is complete.
If the test is positive, a pink line will appear in 1 of the 2 windows on the cartridge. One window represents Gram-positive bacteria and the other Gram-negative. Non-reactive samples will have no line in either window.
Now that the FDA has expanded the indications for the Platelet PGD Test, it can be used as a quality control test for pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced whole-blood-derived platelets suspended in plasma that are pooled within 4 hours of transfusion.
The test can also be used within 24 hours of transfusion as a safety measure following testing with a growth-based, quality control test cleared by the FDA. For this indication, the Platelet PGD Test can be used with:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in PAS-C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced whole-blood-derived platelets suspended in plasma.
In studies conducted by Verax Biomedical (described in the summary document here), the Platelet PGD Test successfully detected bacteria in pre-storage pools of whole-blood derived platelets suspended in plasma and leukocyte-reduced apheresis platelets suspended in plasma or PAS-C and plasma. ![]()
Team says delayed cord clamping can’t hurt
Photo by Meutia Chaerani
and Indradi Soemardjan
New research suggests that delayed umbilical cord clamping in full-term infants may confer some minor long-term benefits and, at the very least, does not pose any harm.
Delayed clamping did not appear to have a significant effect on most of the mental and physical measures assessed in the study.
It was associated with improved scores in fine-motor skills and social skills at age 4, but these effects only occurred in boys.
Researchers reported these results in JAMA Pediatrics alongside a related editorial.
Previous research has shown that delaying umbilical cord clamping by 2 to 3 minutes after delivery allows fetal blood remaining in the placental circulation to be transfused back to the newborn, and this is associated with improved iron status at 4 to 6 months of age.
However, there is a lack of knowledge regarding the long-term effects of delayed clamping. So policymakers have been hesitant about making clear recommendations regarding cord clamping in full-term infants.
To gain more insight, Ola Andersson, MD, PhD, of Uppsala University in Sweden, and his colleagues performed follow-up assessments of 263 children who were previously enrolled in a randomized trial of cord clamping in full-term infants born in a Swedish hospital.
The team assessed the effects of delayed cord clamping on childhood development at age 4. Delayed clamping (n=141) was defined as occurring 3 or more minutes after delivery, and early clamping (n=122) was defined as occurring 10 seconds or fewer after delivery.
The researchers evaluated child behavior and development using parents’ responses on the Ages and Stages Questionnaire, Third Edition (ASQ), which is used to assess communication, motor skills, and other measures; and the Strengths and Difficulties Questionnaire, which is used to score children’s emotional difficulties, hyperactivity, and other difficulties.
A blinded psychologist also assessed children’s scores on the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III), which is used to assess IQ and similar measures, and the Movement Assessment Battery for Children (Movement ABC), which is used to assess manual dexterity and similar measures.
The researchers found no significant differences between the delayed and early clamping groups with regard to results on the WPPSI-III or the Movement ABC.
However, delayed clamping was associated with a significant improvement over early clamping in ASQ personal-social scores (adjusted mean difference [AMD]=2.8, P=0.006), fine-motor scores (AMD=2.1, P=0.03), and the Strengths and Difficulties Questionnaire prosocial subscale (AMD=0.5, P=0.05).
When the researchers assessed the children according to sex, they found that significant improvements associated with delayed clamping were only present in males.
Males in the delayed clamping group had significantly higher mean scores in tasks involving fine-motor function, including the WPPSI-III processing-speed quotient (AMD=4.2, P=0.02), the Movement ABC bicycle-trail task (AMD=0.8, P=0.03), and fine-motor scores on the ASQ (AMD=4.7, P=0.01). These boys also had significantly higher personal-social scores on the ASQ (AMD=4.9, P=0.004).
The researchers concluded that, although delayed cord clamping and early clamping resulted in similar overall neurodevelopment and behavior among 4-year-old children, there were differences in this study. And this suggests there are some positive, and no harmful, long-term effects of delayed cord clamping. ![]()

Photo by Meutia Chaerani
and Indradi Soemardjan
New research suggests that delayed umbilical cord clamping in full-term infants may confer some minor long-term benefits and, at the very least, does not pose any harm.
Delayed clamping did not appear to have a significant effect on most of the mental and physical measures assessed in the study.
It was associated with improved scores in fine-motor skills and social skills at age 4, but these effects only occurred in boys.
Researchers reported these results in JAMA Pediatrics alongside a related editorial.
Previous research has shown that delaying umbilical cord clamping by 2 to 3 minutes after delivery allows fetal blood remaining in the placental circulation to be transfused back to the newborn, and this is associated with improved iron status at 4 to 6 months of age.
However, there is a lack of knowledge regarding the long-term effects of delayed clamping. So policymakers have been hesitant about making clear recommendations regarding cord clamping in full-term infants.
To gain more insight, Ola Andersson, MD, PhD, of Uppsala University in Sweden, and his colleagues performed follow-up assessments of 263 children who were previously enrolled in a randomized trial of cord clamping in full-term infants born in a Swedish hospital.
The team assessed the effects of delayed cord clamping on childhood development at age 4. Delayed clamping (n=141) was defined as occurring 3 or more minutes after delivery, and early clamping (n=122) was defined as occurring 10 seconds or fewer after delivery.
The researchers evaluated child behavior and development using parents’ responses on the Ages and Stages Questionnaire, Third Edition (ASQ), which is used to assess communication, motor skills, and other measures; and the Strengths and Difficulties Questionnaire, which is used to score children’s emotional difficulties, hyperactivity, and other difficulties.
A blinded psychologist also assessed children’s scores on the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III), which is used to assess IQ and similar measures, and the Movement Assessment Battery for Children (Movement ABC), which is used to assess manual dexterity and similar measures.
The researchers found no significant differences between the delayed and early clamping groups with regard to results on the WPPSI-III or the Movement ABC.
However, delayed clamping was associated with a significant improvement over early clamping in ASQ personal-social scores (adjusted mean difference [AMD]=2.8, P=0.006), fine-motor scores (AMD=2.1, P=0.03), and the Strengths and Difficulties Questionnaire prosocial subscale (AMD=0.5, P=0.05).
When the researchers assessed the children according to sex, they found that significant improvements associated with delayed clamping were only present in males.
Males in the delayed clamping group had significantly higher mean scores in tasks involving fine-motor function, including the WPPSI-III processing-speed quotient (AMD=4.2, P=0.02), the Movement ABC bicycle-trail task (AMD=0.8, P=0.03), and fine-motor scores on the ASQ (AMD=4.7, P=0.01). These boys also had significantly higher personal-social scores on the ASQ (AMD=4.9, P=0.004).
The researchers concluded that, although delayed cord clamping and early clamping resulted in similar overall neurodevelopment and behavior among 4-year-old children, there were differences in this study. And this suggests there are some positive, and no harmful, long-term effects of delayed cord clamping. ![]()

Photo by Meutia Chaerani
and Indradi Soemardjan
New research suggests that delayed umbilical cord clamping in full-term infants may confer some minor long-term benefits and, at the very least, does not pose any harm.
Delayed clamping did not appear to have a significant effect on most of the mental and physical measures assessed in the study.
It was associated with improved scores in fine-motor skills and social skills at age 4, but these effects only occurred in boys.
Researchers reported these results in JAMA Pediatrics alongside a related editorial.
Previous research has shown that delaying umbilical cord clamping by 2 to 3 minutes after delivery allows fetal blood remaining in the placental circulation to be transfused back to the newborn, and this is associated with improved iron status at 4 to 6 months of age.
However, there is a lack of knowledge regarding the long-term effects of delayed clamping. So policymakers have been hesitant about making clear recommendations regarding cord clamping in full-term infants.
To gain more insight, Ola Andersson, MD, PhD, of Uppsala University in Sweden, and his colleagues performed follow-up assessments of 263 children who were previously enrolled in a randomized trial of cord clamping in full-term infants born in a Swedish hospital.
The team assessed the effects of delayed cord clamping on childhood development at age 4. Delayed clamping (n=141) was defined as occurring 3 or more minutes after delivery, and early clamping (n=122) was defined as occurring 10 seconds or fewer after delivery.
The researchers evaluated child behavior and development using parents’ responses on the Ages and Stages Questionnaire, Third Edition (ASQ), which is used to assess communication, motor skills, and other measures; and the Strengths and Difficulties Questionnaire, which is used to score children’s emotional difficulties, hyperactivity, and other difficulties.
A blinded psychologist also assessed children’s scores on the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III), which is used to assess IQ and similar measures, and the Movement Assessment Battery for Children (Movement ABC), which is used to assess manual dexterity and similar measures.
The researchers found no significant differences between the delayed and early clamping groups with regard to results on the WPPSI-III or the Movement ABC.
However, delayed clamping was associated with a significant improvement over early clamping in ASQ personal-social scores (adjusted mean difference [AMD]=2.8, P=0.006), fine-motor scores (AMD=2.1, P=0.03), and the Strengths and Difficulties Questionnaire prosocial subscale (AMD=0.5, P=0.05).
When the researchers assessed the children according to sex, they found that significant improvements associated with delayed clamping were only present in males.
Males in the delayed clamping group had significantly higher mean scores in tasks involving fine-motor function, including the WPPSI-III processing-speed quotient (AMD=4.2, P=0.02), the Movement ABC bicycle-trail task (AMD=0.8, P=0.03), and fine-motor scores on the ASQ (AMD=4.7, P=0.01). These boys also had significantly higher personal-social scores on the ASQ (AMD=4.9, P=0.004).
The researchers concluded that, although delayed cord clamping and early clamping resulted in similar overall neurodevelopment and behavior among 4-year-old children, there were differences in this study. And this suggests there are some positive, and no harmful, long-term effects of delayed cord clamping. ![]()
Histone variant may contribute to lymphoma

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()
Improving Patient Satisfaction
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
© 2015 Society of Hospital Medicine
More Than a Mnemonic
A 51‐year‐old man presented to the emergency department after 1 day of progressive dyspnea and increasing confusion.
Acute dyspnea most commonly stems from a cardiac or pulmonary disorder such as heart failure, acute coronary syndrome, pneumonia, pulmonary embolism, or exacerbations of asthma or chronic obstructive pulmonary disease. Less frequent cardiopulmonary considerations include pericardial or pleural effusion, pneumothorax, aspiration, and upper airway obstruction. Dyspnea might also be the initial manifestation of profound anemia or metabolic acidosis.
The presence of confusion suggests either a severe presentation of any of the aforementioned possibilities (with confusion resulting from hypoxia, hypercapnia, or hypotension); a multiorgan illness such as sepsis, malignancy, thromboembolic disease, vasculitis, thyroid dysfunction, or toxic ingestion; or a metabolic derangement related to the underlying cause of dyspnea (for example, hypercalcemia or hyponatremia associated with lung cancer).
Twelve hours prior to presentation, he started to have visual hallucinations. He denied fever, chills, cough, chest discomfort, palpitations, weight gain, headache, neck pain, or weakness.
Visual hallucinations could result from a toxic‐metabolic encephalopathy, such as drug overdose or withdrawal, liver or kidney failure, or hypoxia. A structural brain abnormality may also manifest with visual hallucination. Acute onset at age 51 and the absence of auditory hallucinations argue against a neurodegenerative illness and a primary psychiatric disturbance, respectively.
Episodic hallucinations would support the possibility of seizures, monocular hallucinations would point to a retinal or ocular problem, and a description of yellow‐green hue would suggest a side effect of digoxin.
His past medical history was remarkable for diet‐controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic low back pain. His medications included metoprolol tartrate 25 mg twice daily, omeprazole 40 mg daily, baclofen 15 mg twice daily, oxycodone 30 mg 3 times daily, and hydrocodone 10 mg/acetaminophen 325 mg, 2 tablets 3 times daily as needed for back pain. He was a smoker with a 30 pack‐year history. He had a history of alcohol and cocaine use, but denied any recent substance use. He had no known history of obstructive pulmonary disease.
The patient takes 3 medications well known to cause confusion and hallucinations (oxycodone, hydrocodone, and baclofen), especially when they accumulate due to excessive ingestion or impaired clearance. Although these medications may suppress ventilatory drive, dyspnea would not be a common presenting complaint. He has risk factors for ischemic heart disease and cardiomyopathy, and his smoking history raises the possibility of malignancy.
On exam, the patient's temperature was 94.4C, heart rate 128 beats per minute, respiration rate 28 breaths per minute, blood pressure 155/63 mm Hg, and oxygen saturation 100% while breathing ambient air. The patient was cachectic and appeared in moderate respiratory distress. His pupils were equal and reactive to light, and extraocular movements were intact. He did not have scleral icterus, or cervical or clavicular lymphadenopathy. His oropharynx was negative for erythema, edema, or exudate. His cardiovascular exam revealed a regular tachycardia without rubs or diastolic gallops. There was a 2/6 systolic murmur heard best at left sternal border, without radiation. He did not have jugular venous distention. His pulmonary exam was notable for tachypnea but with normal vesicular breath sounds throughout. He did not have stridor, wheezing, rhonchi, or rales. His abdomen had normal bowel tones and was soft without tenderness, distention, or organomegaly. His extremities were warm, revealed normal pulses, and no edema was present. His joints were cool to palpation, without effusion. On neurologic exam, he was oriented to person and place and able to answer yes/no questions, but unable to provide detailed history. His speech was fluent. His motor exam was without focal deficits. His skin was without any notable lesions.
The constellation of findings does not point to a specific toxidrome. The finding of warm extremities in a hypothermic patient suggests heat loss due to inappropriate peripheral vasodilation. In the absence of vasodilators or features of aortic insufficiency, sepsis becomes a leading consideration. Infection could result in hypothermia and altered sensorium, and accompanying lactic acidosis could trigger tachypnea.
Shortly after admission, he became more somnolent and developed progressive respiratory distress, requiring intubation. Arterial blood gas revealed a pH of 6.93, PaCO2 20 mm Hg, PaO2 127 mm Hg, and HCO3 5 mEq/L. Other laboratory results included a lactate of 4.1 mmol/L, blood urea nitrogen 49 mg/dL, creatinine 2.3 mg/dL (0.8 at 1 month prior), sodium level of 143 mmol/L, chloride of 106 mmol/L, and bicarbonate level of 5 mg/dL. His aspartate aminotransferase was 34 IU/L, alanine transaminase was 28 IU/L, total bilirubin was 0.6 mg/dL, International Normalized Ratio was 1.3. A complete blood count revealed a white blood cell count of 23,000/L, hemoglobin of 10.6 g/dL, and platelet count of 454,000/L. A urinalysis was unremarkable. Cultures of blood, urine, and sputum were collected. Head computed tomography was negative.
This patient has a combined anion gap and nongap metabolic acidosis, as well as respiratory alkalosis. Although his acute kidney failure could produce these 2 types of metabolic acidosis, the modest elevation of the serum creatinine is not commensurate with such profound acidosis. Similarly, sepsis without hypotension or more striking elevation in lactate levels would not account for the entirety of the acidosis. Severe diabetic ketoacidosis can result in profound metabolic acidosis, and marked hyperglycemia or hyperosmolarity could result in somnolence; however, his diabetes has been controlled without medication and there is no obvious precipitant for an episode of ketoacidosis.
Remaining causes of anion gap acidosis include ingestion of methanol, ethylene glycol, ethanol, or salicylates. A careful history of ingestions and medications from witnesses including any prehospital personnel might suggest a source of intoxication. Absent this information, the hypothermia favors an ingestion of an alcohol over salicylates, and the lack of urine crystals and the presence of prominent visual hallucinations would point more toward methanol poisoning than ethylene glycol. A serum osmolarity measurement would allow determination of the osmolar gap, which would be elevated in the setting of methanol or ethylene glycol poisoning. If he were this ill from ethanol, I would have expected to see evidence of hepatotoxicity.
I would administer sodium bicarbonate to reverse the acidosis and to promote renal clearance of salicylates, methanol, ethylene glycol, and their metabolites. Orogastric decontamination with activated charcoal should be considered. If the osmolar gap is elevated, I would also administer intravenous fomepizole to attempt to reverse methanol or ethylene glycol poisoning. I would not delay treatment while waiting for these serum levels to return.
Initial serologic toxicology performed in the emergency department revealed negative ethanol, salicylates, and ketones. His osmolar gap was 13 mOsm/kg. His acetaminophen level was 69 g/mL (normal 120 g/mL). A creatinine phosphokinase was 84 IU/L and myoglobin was 93 ng/mL. His subsequent serum toxicology screen was negative for methanol, ethylene glycol, isopropranol, and hippuric acid. Urine toxicology was positive for opiates, but negative for amphetamine, benzodiazepine, cannabinoid, and cocaine.
Serum and urine ketone assays typically involve the nitroprusside reaction and detect acetoacetate, but not ‐hydroxybutyrate, and can lead to negative test results early in diabetic or alcoholic ketoacidosis. However, the normal ethanol level argues against alcoholic ketoacidosis. Rare causes of elevated anion gap acidosis include toluene toxicity, acetaminophen poisoning, and ingestion of other alcohols. Toluene is metabolized to hippuric acid, and acetaminophen toxicity and associated glutathione depletion can lead to 5‐oxoproline accumulation, producing an anion gap. Patients who abuse alcohol are at risk for acetaminophen toxicity even at doses considered normal. However, this degree of encephalopathy would be unusual for acetaminophen toxicity unless liver failure had developed or unless there was another ingestion that might alter sensorium. Furthermore, the elevated osmolar gap is not a feature of acetaminophen poisoning. I would monitor liver enzyme tests and consider a serum ammonia level, but would not attribute the entire picture to acetaminophen.
The combination of elevated anion gap with an elevated osmolar gap narrows the diagnostic possibilities. Ingestion of several alcohols (ethanol, methanol, ethylene glycol, diethylene glycol) or toluene could produce these abnormalities. Of note, the osmolar gap is typically most markedly elevated early in methanol and ethylene glycol ingestions, and then as the parent compound is metabolized, the osmolar gap closes and the accumulation of metabolites produces the anion gap. Hallucinations are more common with methanol and toluene, and renal failure is more typical of ethylene glycol or toluene. The lack of oxalate crystalluria does not exclude ethylene glycol poisoning. Unfortunately, urine testing for oxalate crystals or fluorescein examination are neither sensitive nor specific enough to diagnosis ethylene glycol toxicity reliably. In most hospitals, assays used for serum testing for alcohols are insensitive, and require confirmation with gas chromatography performed at a specialty lab.
Additional history might reveal the likely culprit or culprits. Inhalant abuse including huffing would point to toluene or organic acid exposure. Solvent ingestion (eg, antifreeze, brake fluid) would suggest methanol or ethylene glycol. Absent this history, I remain suspicious for poisoning with methanol or ethylene glycol and would consider empiric treatment after urgent consultation with a medical toxicologist. A careful ophthalmologic exam might demonstrate characteristic features of methanol poisoning. Serum samples should be sent to a regional lab for analysis for alcohols and organic acids.
He was admitted to the intensive care unit, and empiric antibiotics started. He was empirically started on N‐acetylcysteine and sodium bicarbonate drips. However, his acidemia persisted and he required hemodialysis, which was initiated 12 hours after initial presentation. His acidemia and mental status quickly improved after hemodialysis. He was extubated on hospital day 2 and no longer required hemodialysis.
The differential diagnosis at this point consists of 3 main possibilities: ingestion of methanol, ethylene glycol, or inhalant abuse such as from toluene. The normal hippuric acid level points away from toluene, whereas serum levels can be misleading in the alcohol poisonings. Other discriminating features to consider include exposure history and unique clinical aspects. In this patient, an exposure history is lacking, but 4 clinical features stand out: visual hallucinations, acute kidney injury, mild lactic acidosis, and rapid improvement with hemodialysis. Both ethylene glycol and methanol toxicity may produce a mild lactic acidosis by increasing hepatic metabolism of pyruvate to lactate, and both are rapidly cleared by dialysis. Although it is tempting to place methanol at the top of the list of possibilities due to the report of visual hallucinations, the subjective visual complaints without objective exam corollaries (loss of visual acuity, abnormal pupillary reflexes, or optic disc hyperemia) are nonspecific and might be provoked by alcohol or an inhalant. Furthermore, the acute renal failure is much more typical of ethylene glycol, and thus I would consider ethylene glycol as being the more likely of the ingestions. Coingestion of multiple alcohols is a possibility, but it would be statistically less likely. Confirmation of ethylene glycol poisoning would consist of further insight into his exposures and measurement of levels using gas chromatography.
A urine sample from his emergency department presentation was sent to an outside lab for organic acid levels. Based on high clinical suspicion for 5‐oxoprolinemia (pyroglutamic acidemia) the patient was counseled to avoid any acetaminophen. His primary care provider was informed of this and acetaminophen was added as an adverse drug reaction. The patient left against medical advice soon after extubation. Following discharge, his 5‐oxoproline (pyroglutamic acid) level returned markedly elevated at greater than 10,000 mmol/mol creatinine (200 times the upper limit of normal).
Elevations in 5‐oxoproline levels in this patient most likely stem from glutathione depletion related to chronic acetaminophen use. Alcohol use and malnutrition may have heightened this patient's susceptibility. Despite the common occurrence of acetaminophen use in alcohol abusers or the malnourished, the rarity of severe 5‐oxoproline toxicity suggests unknown factors may be present in predisposed individuals, or under‐recognition. Although acetaminophen‐induced hepatotoxicity may occur along with 5‐oxoprolinemia, this does not always occur.
Several features led me away from this syndrome. First, its rarity lowered my pretest probability. Second, the lack of exposure history and details about the serum assays, specifically whether the measurements were confirmed by gas chromatography, reduced my confidence in eliminating more common ingestions. Third, several aspects proved to be less useful discriminating features: the mild elevation in osmolar gap, renal failure, and hallucinations, which in retrospect proved to be nonspecific.
The patient admitted that he had a longstanding use of acetaminophen in addition to using his girlfriend's acetaminophen‐hydrocodone. He had significant weight loss of over 50 pounds over the previous year, which he attributed to poor appetite. On further chart review, he had been admitted 3 times with a similar clinical presentation and recovered quickly with intensive and supportive care, with no etiology found at those times. He had 2 subsequent hospital admissions for altered mental status and respiratory failure, and his final hospitalization resulted in cardiac arrest and death.
DISCUSSION
5‐Oxoprolinemia is a rare, but potentially lethal cause of severe anion gap metabolic acidosis.[1, 2] The mechanism is thought to be impairment of glutathione metabolism, in the context of other predisposing factors. This can be a congenital error of metabolism, or can be acquired and exacerbated by acetaminophen use. Ingestion of acetaminophen leads to glutathione depletion, which in turn may precipitate accumulation of pyroglutamic acid and subsequent anion gap metabolic acidosis (Figure 1). Additional risk factors that may predispose patients to this condition include malnutrition, renal insufficiency, concurrent infection, and female gender.[1, 2, 3]
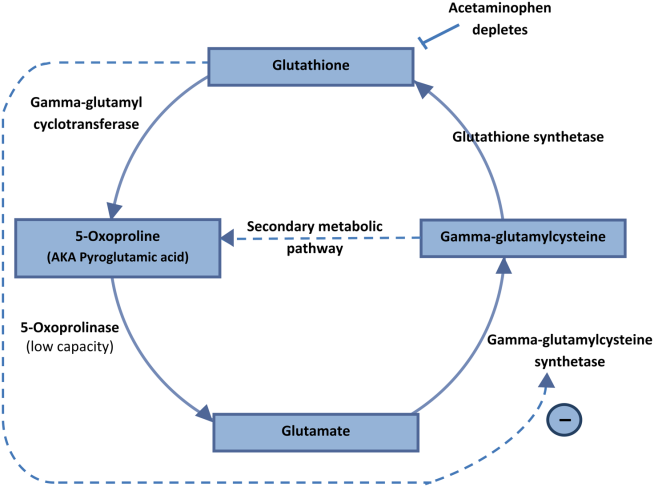
The diagnosis of 5‐oxoprolinemia is made via urine or serum organic acid analysis, testing routinely performed in pediatric populations when screening for congenital metabolic disorders. The pathophysiology suggests that obtaining a urine sample early in presentation, when acidosis is greatest, would lead to the highest 5‐oxoproline levels and best chance for diagnosis. Case patients have had normal levels prior to and in convalescent phases after the acute episode.[4] Given the long turnaround time for lab testing, presumptive diagnosis and treatment may be necessary.
Treatment of 5‐oxoprolinemia is primarily supportive, aimed at the metabolic acidosis. Fluid resuscitation and bicarbonate therapy are reasonable temporizing measures. Hemodialysis can clear 5‐oxoproline and may be indicated in severe acidosis.[5] Furthermore, the proposed pathophysiology suggests that administration of N‐acetylcysteine (NAC) may help to address the underlying process, but there are no trials to support a specific dosing regimen. However, given the fulminant presentation and common competing concern for acetaminophen toxicity, it is reasonable to initiate NAC aimed at treatment for possible acetaminophen overdose. Prevention of recurrence includes avoidance of acetaminophen, and counseling the patient to avoid acetaminophen in prescription combination medications and over‐the‐counter preparations.
Recent regulatory changes regarding acetaminophen/opioid combinations may reduce the incidence of 5‐oxoprolinemia. The US Food and Drug Administration has taken action to reduce adverse effects from acetaminophen exposure by limiting the amount of acetaminophen in opioid combination pills from 500 mg to a maximum of 325 mg per pill. This is aimed at preventing hepatotoxicity from ingestion of higher‐than‐recommended doses. However, clinicians should remember that 5‐oxoprolinemia can result from ingestion of acetaminophen at therapeutic levels.
Given its rare incidence, low clinical suspicion, and transient nature of confirmatory testing, it is likely this remains an underdiagnosed syndrome. In the case discussed, subsequent chart review demonstrated 5 previous admissions in multiple hospitals for severe transient anion gap acidosis. The likelihood that 5‐oxoprolinemia was missed in each of these cases supports a lack of awareness of this syndrome. In this patient, the discussant appropriately identified the possibility of 5‐oxoproline toxicity, but felt ethylene glycol ingestion was more likely. As this case underscores, a cornerstone in the management of suspected ingestions is empiric treatment for the most likely etiologies. Here, treatment for acetaminophen overdose and for methanol or ethylene glycol were warranted, and fortunately also addressed the rarer possibility of 5‐oxoproline toxicity.
The mnemonic MUDPILES is commonly used to identify possible causes of life‐threatening anion gap metabolic acidosis, as such heuristics have benefits in rapidly generating a differential diagnosis to guide initial evaluation. Given the fact that the traditional letter P (paraldehyde) in MUDPILES is no longer clinically utilized, some authors have suggested replacing this with pyroglutamic acid (a synonym of 5‐oxoproline). Such a change may help providers who have ruled out other causes of a high anion gap metabolic acidosis, facilitating diagnosis of this life‐threatening syndrome. In any case, clinicians must be mindful that simple memory aids may mislead clinicians, and a complete differential diagnosis may require more than a mnemonic.
TEACHING POINTS
- Acetaminophen use, even at therapeutic levels, can lead to 5‐oxoprolinemia, a potentially lethal anion gap metabolic acidosis.
- 5‐oxoprolinemia is likely related to glutathione depletion, worsened by acetaminophen, malnutrition, renal insufficiency, female gender, and infection. This implies theoretical benefit from administration of NAC for glutathione repletion.
- Mnemonics can be useful, but have limitations by way of oversimplification. This case suggests that changing the letter P in MUDPILES from paraldehyde to pyroglutamic acid could reduce underdiagnosis.
Disclosure: Nothing to report.
- , , , , . 5‐oxoprolinemia causing elevated anion gap metabolic acidosis in the setting of acetaminophen use. J Emerg Med. 2012;43(1):54–57.
- , , , . What is the clinical significance of 5‐oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure? Clin Toxicol (Phila). 2013;51(9):817–827.
- , , , , . Increased anion gap metabolic acidosis as a result of 5‐oxoproline (pyroglutamic acid): a role for acetaminophen. Clin J Am Soc Nephrol. 2006;1(3):441–447.
- , , , et al. Recurrent high anion gap metabolic acidosis secondary to 5‐oxoproline (pyroglutamic acid). Am J Kidney Dis. 2005;46(1):e4–e10.
- , , . Profound metabolic acidosis from pyroglutamic acidemia: an underappreciated cause of high anion gap metabolic acidosis. CJEM. 2010;12(5):449–452.
A 51‐year‐old man presented to the emergency department after 1 day of progressive dyspnea and increasing confusion.
Acute dyspnea most commonly stems from a cardiac or pulmonary disorder such as heart failure, acute coronary syndrome, pneumonia, pulmonary embolism, or exacerbations of asthma or chronic obstructive pulmonary disease. Less frequent cardiopulmonary considerations include pericardial or pleural effusion, pneumothorax, aspiration, and upper airway obstruction. Dyspnea might also be the initial manifestation of profound anemia or metabolic acidosis.
The presence of confusion suggests either a severe presentation of any of the aforementioned possibilities (with confusion resulting from hypoxia, hypercapnia, or hypotension); a multiorgan illness such as sepsis, malignancy, thromboembolic disease, vasculitis, thyroid dysfunction, or toxic ingestion; or a metabolic derangement related to the underlying cause of dyspnea (for example, hypercalcemia or hyponatremia associated with lung cancer).
Twelve hours prior to presentation, he started to have visual hallucinations. He denied fever, chills, cough, chest discomfort, palpitations, weight gain, headache, neck pain, or weakness.
Visual hallucinations could result from a toxic‐metabolic encephalopathy, such as drug overdose or withdrawal, liver or kidney failure, or hypoxia. A structural brain abnormality may also manifest with visual hallucination. Acute onset at age 51 and the absence of auditory hallucinations argue against a neurodegenerative illness and a primary psychiatric disturbance, respectively.
Episodic hallucinations would support the possibility of seizures, monocular hallucinations would point to a retinal or ocular problem, and a description of yellow‐green hue would suggest a side effect of digoxin.
His past medical history was remarkable for diet‐controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic low back pain. His medications included metoprolol tartrate 25 mg twice daily, omeprazole 40 mg daily, baclofen 15 mg twice daily, oxycodone 30 mg 3 times daily, and hydrocodone 10 mg/acetaminophen 325 mg, 2 tablets 3 times daily as needed for back pain. He was a smoker with a 30 pack‐year history. He had a history of alcohol and cocaine use, but denied any recent substance use. He had no known history of obstructive pulmonary disease.
The patient takes 3 medications well known to cause confusion and hallucinations (oxycodone, hydrocodone, and baclofen), especially when they accumulate due to excessive ingestion or impaired clearance. Although these medications may suppress ventilatory drive, dyspnea would not be a common presenting complaint. He has risk factors for ischemic heart disease and cardiomyopathy, and his smoking history raises the possibility of malignancy.
On exam, the patient's temperature was 94.4C, heart rate 128 beats per minute, respiration rate 28 breaths per minute, blood pressure 155/63 mm Hg, and oxygen saturation 100% while breathing ambient air. The patient was cachectic and appeared in moderate respiratory distress. His pupils were equal and reactive to light, and extraocular movements were intact. He did not have scleral icterus, or cervical or clavicular lymphadenopathy. His oropharynx was negative for erythema, edema, or exudate. His cardiovascular exam revealed a regular tachycardia without rubs or diastolic gallops. There was a 2/6 systolic murmur heard best at left sternal border, without radiation. He did not have jugular venous distention. His pulmonary exam was notable for tachypnea but with normal vesicular breath sounds throughout. He did not have stridor, wheezing, rhonchi, or rales. His abdomen had normal bowel tones and was soft without tenderness, distention, or organomegaly. His extremities were warm, revealed normal pulses, and no edema was present. His joints were cool to palpation, without effusion. On neurologic exam, he was oriented to person and place and able to answer yes/no questions, but unable to provide detailed history. His speech was fluent. His motor exam was without focal deficits. His skin was without any notable lesions.
The constellation of findings does not point to a specific toxidrome. The finding of warm extremities in a hypothermic patient suggests heat loss due to inappropriate peripheral vasodilation. In the absence of vasodilators or features of aortic insufficiency, sepsis becomes a leading consideration. Infection could result in hypothermia and altered sensorium, and accompanying lactic acidosis could trigger tachypnea.
Shortly after admission, he became more somnolent and developed progressive respiratory distress, requiring intubation. Arterial blood gas revealed a pH of 6.93, PaCO2 20 mm Hg, PaO2 127 mm Hg, and HCO3 5 mEq/L. Other laboratory results included a lactate of 4.1 mmol/L, blood urea nitrogen 49 mg/dL, creatinine 2.3 mg/dL (0.8 at 1 month prior), sodium level of 143 mmol/L, chloride of 106 mmol/L, and bicarbonate level of 5 mg/dL. His aspartate aminotransferase was 34 IU/L, alanine transaminase was 28 IU/L, total bilirubin was 0.6 mg/dL, International Normalized Ratio was 1.3. A complete blood count revealed a white blood cell count of 23,000/L, hemoglobin of 10.6 g/dL, and platelet count of 454,000/L. A urinalysis was unremarkable. Cultures of blood, urine, and sputum were collected. Head computed tomography was negative.
This patient has a combined anion gap and nongap metabolic acidosis, as well as respiratory alkalosis. Although his acute kidney failure could produce these 2 types of metabolic acidosis, the modest elevation of the serum creatinine is not commensurate with such profound acidosis. Similarly, sepsis without hypotension or more striking elevation in lactate levels would not account for the entirety of the acidosis. Severe diabetic ketoacidosis can result in profound metabolic acidosis, and marked hyperglycemia or hyperosmolarity could result in somnolence; however, his diabetes has been controlled without medication and there is no obvious precipitant for an episode of ketoacidosis.
Remaining causes of anion gap acidosis include ingestion of methanol, ethylene glycol, ethanol, or salicylates. A careful history of ingestions and medications from witnesses including any prehospital personnel might suggest a source of intoxication. Absent this information, the hypothermia favors an ingestion of an alcohol over salicylates, and the lack of urine crystals and the presence of prominent visual hallucinations would point more toward methanol poisoning than ethylene glycol. A serum osmolarity measurement would allow determination of the osmolar gap, which would be elevated in the setting of methanol or ethylene glycol poisoning. If he were this ill from ethanol, I would have expected to see evidence of hepatotoxicity.
I would administer sodium bicarbonate to reverse the acidosis and to promote renal clearance of salicylates, methanol, ethylene glycol, and their metabolites. Orogastric decontamination with activated charcoal should be considered. If the osmolar gap is elevated, I would also administer intravenous fomepizole to attempt to reverse methanol or ethylene glycol poisoning. I would not delay treatment while waiting for these serum levels to return.
Initial serologic toxicology performed in the emergency department revealed negative ethanol, salicylates, and ketones. His osmolar gap was 13 mOsm/kg. His acetaminophen level was 69 g/mL (normal 120 g/mL). A creatinine phosphokinase was 84 IU/L and myoglobin was 93 ng/mL. His subsequent serum toxicology screen was negative for methanol, ethylene glycol, isopropranol, and hippuric acid. Urine toxicology was positive for opiates, but negative for amphetamine, benzodiazepine, cannabinoid, and cocaine.
Serum and urine ketone assays typically involve the nitroprusside reaction and detect acetoacetate, but not ‐hydroxybutyrate, and can lead to negative test results early in diabetic or alcoholic ketoacidosis. However, the normal ethanol level argues against alcoholic ketoacidosis. Rare causes of elevated anion gap acidosis include toluene toxicity, acetaminophen poisoning, and ingestion of other alcohols. Toluene is metabolized to hippuric acid, and acetaminophen toxicity and associated glutathione depletion can lead to 5‐oxoproline accumulation, producing an anion gap. Patients who abuse alcohol are at risk for acetaminophen toxicity even at doses considered normal. However, this degree of encephalopathy would be unusual for acetaminophen toxicity unless liver failure had developed or unless there was another ingestion that might alter sensorium. Furthermore, the elevated osmolar gap is not a feature of acetaminophen poisoning. I would monitor liver enzyme tests and consider a serum ammonia level, but would not attribute the entire picture to acetaminophen.
The combination of elevated anion gap with an elevated osmolar gap narrows the diagnostic possibilities. Ingestion of several alcohols (ethanol, methanol, ethylene glycol, diethylene glycol) or toluene could produce these abnormalities. Of note, the osmolar gap is typically most markedly elevated early in methanol and ethylene glycol ingestions, and then as the parent compound is metabolized, the osmolar gap closes and the accumulation of metabolites produces the anion gap. Hallucinations are more common with methanol and toluene, and renal failure is more typical of ethylene glycol or toluene. The lack of oxalate crystalluria does not exclude ethylene glycol poisoning. Unfortunately, urine testing for oxalate crystals or fluorescein examination are neither sensitive nor specific enough to diagnosis ethylene glycol toxicity reliably. In most hospitals, assays used for serum testing for alcohols are insensitive, and require confirmation with gas chromatography performed at a specialty lab.
Additional history might reveal the likely culprit or culprits. Inhalant abuse including huffing would point to toluene or organic acid exposure. Solvent ingestion (eg, antifreeze, brake fluid) would suggest methanol or ethylene glycol. Absent this history, I remain suspicious for poisoning with methanol or ethylene glycol and would consider empiric treatment after urgent consultation with a medical toxicologist. A careful ophthalmologic exam might demonstrate characteristic features of methanol poisoning. Serum samples should be sent to a regional lab for analysis for alcohols and organic acids.
He was admitted to the intensive care unit, and empiric antibiotics started. He was empirically started on N‐acetylcysteine and sodium bicarbonate drips. However, his acidemia persisted and he required hemodialysis, which was initiated 12 hours after initial presentation. His acidemia and mental status quickly improved after hemodialysis. He was extubated on hospital day 2 and no longer required hemodialysis.
The differential diagnosis at this point consists of 3 main possibilities: ingestion of methanol, ethylene glycol, or inhalant abuse such as from toluene. The normal hippuric acid level points away from toluene, whereas serum levels can be misleading in the alcohol poisonings. Other discriminating features to consider include exposure history and unique clinical aspects. In this patient, an exposure history is lacking, but 4 clinical features stand out: visual hallucinations, acute kidney injury, mild lactic acidosis, and rapid improvement with hemodialysis. Both ethylene glycol and methanol toxicity may produce a mild lactic acidosis by increasing hepatic metabolism of pyruvate to lactate, and both are rapidly cleared by dialysis. Although it is tempting to place methanol at the top of the list of possibilities due to the report of visual hallucinations, the subjective visual complaints without objective exam corollaries (loss of visual acuity, abnormal pupillary reflexes, or optic disc hyperemia) are nonspecific and might be provoked by alcohol or an inhalant. Furthermore, the acute renal failure is much more typical of ethylene glycol, and thus I would consider ethylene glycol as being the more likely of the ingestions. Coingestion of multiple alcohols is a possibility, but it would be statistically less likely. Confirmation of ethylene glycol poisoning would consist of further insight into his exposures and measurement of levels using gas chromatography.
A urine sample from his emergency department presentation was sent to an outside lab for organic acid levels. Based on high clinical suspicion for 5‐oxoprolinemia (pyroglutamic acidemia) the patient was counseled to avoid any acetaminophen. His primary care provider was informed of this and acetaminophen was added as an adverse drug reaction. The patient left against medical advice soon after extubation. Following discharge, his 5‐oxoproline (pyroglutamic acid) level returned markedly elevated at greater than 10,000 mmol/mol creatinine (200 times the upper limit of normal).
Elevations in 5‐oxoproline levels in this patient most likely stem from glutathione depletion related to chronic acetaminophen use. Alcohol use and malnutrition may have heightened this patient's susceptibility. Despite the common occurrence of acetaminophen use in alcohol abusers or the malnourished, the rarity of severe 5‐oxoproline toxicity suggests unknown factors may be present in predisposed individuals, or under‐recognition. Although acetaminophen‐induced hepatotoxicity may occur along with 5‐oxoprolinemia, this does not always occur.
Several features led me away from this syndrome. First, its rarity lowered my pretest probability. Second, the lack of exposure history and details about the serum assays, specifically whether the measurements were confirmed by gas chromatography, reduced my confidence in eliminating more common ingestions. Third, several aspects proved to be less useful discriminating features: the mild elevation in osmolar gap, renal failure, and hallucinations, which in retrospect proved to be nonspecific.
The patient admitted that he had a longstanding use of acetaminophen in addition to using his girlfriend's acetaminophen‐hydrocodone. He had significant weight loss of over 50 pounds over the previous year, which he attributed to poor appetite. On further chart review, he had been admitted 3 times with a similar clinical presentation and recovered quickly with intensive and supportive care, with no etiology found at those times. He had 2 subsequent hospital admissions for altered mental status and respiratory failure, and his final hospitalization resulted in cardiac arrest and death.
DISCUSSION
5‐Oxoprolinemia is a rare, but potentially lethal cause of severe anion gap metabolic acidosis.[1, 2] The mechanism is thought to be impairment of glutathione metabolism, in the context of other predisposing factors. This can be a congenital error of metabolism, or can be acquired and exacerbated by acetaminophen use. Ingestion of acetaminophen leads to glutathione depletion, which in turn may precipitate accumulation of pyroglutamic acid and subsequent anion gap metabolic acidosis (Figure 1). Additional risk factors that may predispose patients to this condition include malnutrition, renal insufficiency, concurrent infection, and female gender.[1, 2, 3]

The diagnosis of 5‐oxoprolinemia is made via urine or serum organic acid analysis, testing routinely performed in pediatric populations when screening for congenital metabolic disorders. The pathophysiology suggests that obtaining a urine sample early in presentation, when acidosis is greatest, would lead to the highest 5‐oxoproline levels and best chance for diagnosis. Case patients have had normal levels prior to and in convalescent phases after the acute episode.[4] Given the long turnaround time for lab testing, presumptive diagnosis and treatment may be necessary.
Treatment of 5‐oxoprolinemia is primarily supportive, aimed at the metabolic acidosis. Fluid resuscitation and bicarbonate therapy are reasonable temporizing measures. Hemodialysis can clear 5‐oxoproline and may be indicated in severe acidosis.[5] Furthermore, the proposed pathophysiology suggests that administration of N‐acetylcysteine (NAC) may help to address the underlying process, but there are no trials to support a specific dosing regimen. However, given the fulminant presentation and common competing concern for acetaminophen toxicity, it is reasonable to initiate NAC aimed at treatment for possible acetaminophen overdose. Prevention of recurrence includes avoidance of acetaminophen, and counseling the patient to avoid acetaminophen in prescription combination medications and over‐the‐counter preparations.
Recent regulatory changes regarding acetaminophen/opioid combinations may reduce the incidence of 5‐oxoprolinemia. The US Food and Drug Administration has taken action to reduce adverse effects from acetaminophen exposure by limiting the amount of acetaminophen in opioid combination pills from 500 mg to a maximum of 325 mg per pill. This is aimed at preventing hepatotoxicity from ingestion of higher‐than‐recommended doses. However, clinicians should remember that 5‐oxoprolinemia can result from ingestion of acetaminophen at therapeutic levels.
Given its rare incidence, low clinical suspicion, and transient nature of confirmatory testing, it is likely this remains an underdiagnosed syndrome. In the case discussed, subsequent chart review demonstrated 5 previous admissions in multiple hospitals for severe transient anion gap acidosis. The likelihood that 5‐oxoprolinemia was missed in each of these cases supports a lack of awareness of this syndrome. In this patient, the discussant appropriately identified the possibility of 5‐oxoproline toxicity, but felt ethylene glycol ingestion was more likely. As this case underscores, a cornerstone in the management of suspected ingestions is empiric treatment for the most likely etiologies. Here, treatment for acetaminophen overdose and for methanol or ethylene glycol were warranted, and fortunately also addressed the rarer possibility of 5‐oxoproline toxicity.
The mnemonic MUDPILES is commonly used to identify possible causes of life‐threatening anion gap metabolic acidosis, as such heuristics have benefits in rapidly generating a differential diagnosis to guide initial evaluation. Given the fact that the traditional letter P (paraldehyde) in MUDPILES is no longer clinically utilized, some authors have suggested replacing this with pyroglutamic acid (a synonym of 5‐oxoproline). Such a change may help providers who have ruled out other causes of a high anion gap metabolic acidosis, facilitating diagnosis of this life‐threatening syndrome. In any case, clinicians must be mindful that simple memory aids may mislead clinicians, and a complete differential diagnosis may require more than a mnemonic.
TEACHING POINTS
- Acetaminophen use, even at therapeutic levels, can lead to 5‐oxoprolinemia, a potentially lethal anion gap metabolic acidosis.
- 5‐oxoprolinemia is likely related to glutathione depletion, worsened by acetaminophen, malnutrition, renal insufficiency, female gender, and infection. This implies theoretical benefit from administration of NAC for glutathione repletion.
- Mnemonics can be useful, but have limitations by way of oversimplification. This case suggests that changing the letter P in MUDPILES from paraldehyde to pyroglutamic acid could reduce underdiagnosis.
Disclosure: Nothing to report.
A 51‐year‐old man presented to the emergency department after 1 day of progressive dyspnea and increasing confusion.
Acute dyspnea most commonly stems from a cardiac or pulmonary disorder such as heart failure, acute coronary syndrome, pneumonia, pulmonary embolism, or exacerbations of asthma or chronic obstructive pulmonary disease. Less frequent cardiopulmonary considerations include pericardial or pleural effusion, pneumothorax, aspiration, and upper airway obstruction. Dyspnea might also be the initial manifestation of profound anemia or metabolic acidosis.
The presence of confusion suggests either a severe presentation of any of the aforementioned possibilities (with confusion resulting from hypoxia, hypercapnia, or hypotension); a multiorgan illness such as sepsis, malignancy, thromboembolic disease, vasculitis, thyroid dysfunction, or toxic ingestion; or a metabolic derangement related to the underlying cause of dyspnea (for example, hypercalcemia or hyponatremia associated with lung cancer).
Twelve hours prior to presentation, he started to have visual hallucinations. He denied fever, chills, cough, chest discomfort, palpitations, weight gain, headache, neck pain, or weakness.
Visual hallucinations could result from a toxic‐metabolic encephalopathy, such as drug overdose or withdrawal, liver or kidney failure, or hypoxia. A structural brain abnormality may also manifest with visual hallucination. Acute onset at age 51 and the absence of auditory hallucinations argue against a neurodegenerative illness and a primary psychiatric disturbance, respectively.
Episodic hallucinations would support the possibility of seizures, monocular hallucinations would point to a retinal or ocular problem, and a description of yellow‐green hue would suggest a side effect of digoxin.
His past medical history was remarkable for diet‐controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic low back pain. His medications included metoprolol tartrate 25 mg twice daily, omeprazole 40 mg daily, baclofen 15 mg twice daily, oxycodone 30 mg 3 times daily, and hydrocodone 10 mg/acetaminophen 325 mg, 2 tablets 3 times daily as needed for back pain. He was a smoker with a 30 pack‐year history. He had a history of alcohol and cocaine use, but denied any recent substance use. He had no known history of obstructive pulmonary disease.
The patient takes 3 medications well known to cause confusion and hallucinations (oxycodone, hydrocodone, and baclofen), especially when they accumulate due to excessive ingestion or impaired clearance. Although these medications may suppress ventilatory drive, dyspnea would not be a common presenting complaint. He has risk factors for ischemic heart disease and cardiomyopathy, and his smoking history raises the possibility of malignancy.
On exam, the patient's temperature was 94.4C, heart rate 128 beats per minute, respiration rate 28 breaths per minute, blood pressure 155/63 mm Hg, and oxygen saturation 100% while breathing ambient air. The patient was cachectic and appeared in moderate respiratory distress. His pupils were equal and reactive to light, and extraocular movements were intact. He did not have scleral icterus, or cervical or clavicular lymphadenopathy. His oropharynx was negative for erythema, edema, or exudate. His cardiovascular exam revealed a regular tachycardia without rubs or diastolic gallops. There was a 2/6 systolic murmur heard best at left sternal border, without radiation. He did not have jugular venous distention. His pulmonary exam was notable for tachypnea but with normal vesicular breath sounds throughout. He did not have stridor, wheezing, rhonchi, or rales. His abdomen had normal bowel tones and was soft without tenderness, distention, or organomegaly. His extremities were warm, revealed normal pulses, and no edema was present. His joints were cool to palpation, without effusion. On neurologic exam, he was oriented to person and place and able to answer yes/no questions, but unable to provide detailed history. His speech was fluent. His motor exam was without focal deficits. His skin was without any notable lesions.
The constellation of findings does not point to a specific toxidrome. The finding of warm extremities in a hypothermic patient suggests heat loss due to inappropriate peripheral vasodilation. In the absence of vasodilators or features of aortic insufficiency, sepsis becomes a leading consideration. Infection could result in hypothermia and altered sensorium, and accompanying lactic acidosis could trigger tachypnea.
Shortly after admission, he became more somnolent and developed progressive respiratory distress, requiring intubation. Arterial blood gas revealed a pH of 6.93, PaCO2 20 mm Hg, PaO2 127 mm Hg, and HCO3 5 mEq/L. Other laboratory results included a lactate of 4.1 mmol/L, blood urea nitrogen 49 mg/dL, creatinine 2.3 mg/dL (0.8 at 1 month prior), sodium level of 143 mmol/L, chloride of 106 mmol/L, and bicarbonate level of 5 mg/dL. His aspartate aminotransferase was 34 IU/L, alanine transaminase was 28 IU/L, total bilirubin was 0.6 mg/dL, International Normalized Ratio was 1.3. A complete blood count revealed a white blood cell count of 23,000/L, hemoglobin of 10.6 g/dL, and platelet count of 454,000/L. A urinalysis was unremarkable. Cultures of blood, urine, and sputum were collected. Head computed tomography was negative.
This patient has a combined anion gap and nongap metabolic acidosis, as well as respiratory alkalosis. Although his acute kidney failure could produce these 2 types of metabolic acidosis, the modest elevation of the serum creatinine is not commensurate with such profound acidosis. Similarly, sepsis without hypotension or more striking elevation in lactate levels would not account for the entirety of the acidosis. Severe diabetic ketoacidosis can result in profound metabolic acidosis, and marked hyperglycemia or hyperosmolarity could result in somnolence; however, his diabetes has been controlled without medication and there is no obvious precipitant for an episode of ketoacidosis.
Remaining causes of anion gap acidosis include ingestion of methanol, ethylene glycol, ethanol, or salicylates. A careful history of ingestions and medications from witnesses including any prehospital personnel might suggest a source of intoxication. Absent this information, the hypothermia favors an ingestion of an alcohol over salicylates, and the lack of urine crystals and the presence of prominent visual hallucinations would point more toward methanol poisoning than ethylene glycol. A serum osmolarity measurement would allow determination of the osmolar gap, which would be elevated in the setting of methanol or ethylene glycol poisoning. If he were this ill from ethanol, I would have expected to see evidence of hepatotoxicity.
I would administer sodium bicarbonate to reverse the acidosis and to promote renal clearance of salicylates, methanol, ethylene glycol, and their metabolites. Orogastric decontamination with activated charcoal should be considered. If the osmolar gap is elevated, I would also administer intravenous fomepizole to attempt to reverse methanol or ethylene glycol poisoning. I would not delay treatment while waiting for these serum levels to return.
Initial serologic toxicology performed in the emergency department revealed negative ethanol, salicylates, and ketones. His osmolar gap was 13 mOsm/kg. His acetaminophen level was 69 g/mL (normal 120 g/mL). A creatinine phosphokinase was 84 IU/L and myoglobin was 93 ng/mL. His subsequent serum toxicology screen was negative for methanol, ethylene glycol, isopropranol, and hippuric acid. Urine toxicology was positive for opiates, but negative for amphetamine, benzodiazepine, cannabinoid, and cocaine.
Serum and urine ketone assays typically involve the nitroprusside reaction and detect acetoacetate, but not ‐hydroxybutyrate, and can lead to negative test results early in diabetic or alcoholic ketoacidosis. However, the normal ethanol level argues against alcoholic ketoacidosis. Rare causes of elevated anion gap acidosis include toluene toxicity, acetaminophen poisoning, and ingestion of other alcohols. Toluene is metabolized to hippuric acid, and acetaminophen toxicity and associated glutathione depletion can lead to 5‐oxoproline accumulation, producing an anion gap. Patients who abuse alcohol are at risk for acetaminophen toxicity even at doses considered normal. However, this degree of encephalopathy would be unusual for acetaminophen toxicity unless liver failure had developed or unless there was another ingestion that might alter sensorium. Furthermore, the elevated osmolar gap is not a feature of acetaminophen poisoning. I would monitor liver enzyme tests and consider a serum ammonia level, but would not attribute the entire picture to acetaminophen.
The combination of elevated anion gap with an elevated osmolar gap narrows the diagnostic possibilities. Ingestion of several alcohols (ethanol, methanol, ethylene glycol, diethylene glycol) or toluene could produce these abnormalities. Of note, the osmolar gap is typically most markedly elevated early in methanol and ethylene glycol ingestions, and then as the parent compound is metabolized, the osmolar gap closes and the accumulation of metabolites produces the anion gap. Hallucinations are more common with methanol and toluene, and renal failure is more typical of ethylene glycol or toluene. The lack of oxalate crystalluria does not exclude ethylene glycol poisoning. Unfortunately, urine testing for oxalate crystals or fluorescein examination are neither sensitive nor specific enough to diagnosis ethylene glycol toxicity reliably. In most hospitals, assays used for serum testing for alcohols are insensitive, and require confirmation with gas chromatography performed at a specialty lab.
Additional history might reveal the likely culprit or culprits. Inhalant abuse including huffing would point to toluene or organic acid exposure. Solvent ingestion (eg, antifreeze, brake fluid) would suggest methanol or ethylene glycol. Absent this history, I remain suspicious for poisoning with methanol or ethylene glycol and would consider empiric treatment after urgent consultation with a medical toxicologist. A careful ophthalmologic exam might demonstrate characteristic features of methanol poisoning. Serum samples should be sent to a regional lab for analysis for alcohols and organic acids.
He was admitted to the intensive care unit, and empiric antibiotics started. He was empirically started on N‐acetylcysteine and sodium bicarbonate drips. However, his acidemia persisted and he required hemodialysis, which was initiated 12 hours after initial presentation. His acidemia and mental status quickly improved after hemodialysis. He was extubated on hospital day 2 and no longer required hemodialysis.
The differential diagnosis at this point consists of 3 main possibilities: ingestion of methanol, ethylene glycol, or inhalant abuse such as from toluene. The normal hippuric acid level points away from toluene, whereas serum levels can be misleading in the alcohol poisonings. Other discriminating features to consider include exposure history and unique clinical aspects. In this patient, an exposure history is lacking, but 4 clinical features stand out: visual hallucinations, acute kidney injury, mild lactic acidosis, and rapid improvement with hemodialysis. Both ethylene glycol and methanol toxicity may produce a mild lactic acidosis by increasing hepatic metabolism of pyruvate to lactate, and both are rapidly cleared by dialysis. Although it is tempting to place methanol at the top of the list of possibilities due to the report of visual hallucinations, the subjective visual complaints without objective exam corollaries (loss of visual acuity, abnormal pupillary reflexes, or optic disc hyperemia) are nonspecific and might be provoked by alcohol or an inhalant. Furthermore, the acute renal failure is much more typical of ethylene glycol, and thus I would consider ethylene glycol as being the more likely of the ingestions. Coingestion of multiple alcohols is a possibility, but it would be statistically less likely. Confirmation of ethylene glycol poisoning would consist of further insight into his exposures and measurement of levels using gas chromatography.
A urine sample from his emergency department presentation was sent to an outside lab for organic acid levels. Based on high clinical suspicion for 5‐oxoprolinemia (pyroglutamic acidemia) the patient was counseled to avoid any acetaminophen. His primary care provider was informed of this and acetaminophen was added as an adverse drug reaction. The patient left against medical advice soon after extubation. Following discharge, his 5‐oxoproline (pyroglutamic acid) level returned markedly elevated at greater than 10,000 mmol/mol creatinine (200 times the upper limit of normal).
Elevations in 5‐oxoproline levels in this patient most likely stem from glutathione depletion related to chronic acetaminophen use. Alcohol use and malnutrition may have heightened this patient's susceptibility. Despite the common occurrence of acetaminophen use in alcohol abusers or the malnourished, the rarity of severe 5‐oxoproline toxicity suggests unknown factors may be present in predisposed individuals, or under‐recognition. Although acetaminophen‐induced hepatotoxicity may occur along with 5‐oxoprolinemia, this does not always occur.
Several features led me away from this syndrome. First, its rarity lowered my pretest probability. Second, the lack of exposure history and details about the serum assays, specifically whether the measurements were confirmed by gas chromatography, reduced my confidence in eliminating more common ingestions. Third, several aspects proved to be less useful discriminating features: the mild elevation in osmolar gap, renal failure, and hallucinations, which in retrospect proved to be nonspecific.
The patient admitted that he had a longstanding use of acetaminophen in addition to using his girlfriend's acetaminophen‐hydrocodone. He had significant weight loss of over 50 pounds over the previous year, which he attributed to poor appetite. On further chart review, he had been admitted 3 times with a similar clinical presentation and recovered quickly with intensive and supportive care, with no etiology found at those times. He had 2 subsequent hospital admissions for altered mental status and respiratory failure, and his final hospitalization resulted in cardiac arrest and death.
DISCUSSION
5‐Oxoprolinemia is a rare, but potentially lethal cause of severe anion gap metabolic acidosis.[1, 2] The mechanism is thought to be impairment of glutathione metabolism, in the context of other predisposing factors. This can be a congenital error of metabolism, or can be acquired and exacerbated by acetaminophen use. Ingestion of acetaminophen leads to glutathione depletion, which in turn may precipitate accumulation of pyroglutamic acid and subsequent anion gap metabolic acidosis (Figure 1). Additional risk factors that may predispose patients to this condition include malnutrition, renal insufficiency, concurrent infection, and female gender.[1, 2, 3]

The diagnosis of 5‐oxoprolinemia is made via urine or serum organic acid analysis, testing routinely performed in pediatric populations when screening for congenital metabolic disorders. The pathophysiology suggests that obtaining a urine sample early in presentation, when acidosis is greatest, would lead to the highest 5‐oxoproline levels and best chance for diagnosis. Case patients have had normal levels prior to and in convalescent phases after the acute episode.[4] Given the long turnaround time for lab testing, presumptive diagnosis and treatment may be necessary.
Treatment of 5‐oxoprolinemia is primarily supportive, aimed at the metabolic acidosis. Fluid resuscitation and bicarbonate therapy are reasonable temporizing measures. Hemodialysis can clear 5‐oxoproline and may be indicated in severe acidosis.[5] Furthermore, the proposed pathophysiology suggests that administration of N‐acetylcysteine (NAC) may help to address the underlying process, but there are no trials to support a specific dosing regimen. However, given the fulminant presentation and common competing concern for acetaminophen toxicity, it is reasonable to initiate NAC aimed at treatment for possible acetaminophen overdose. Prevention of recurrence includes avoidance of acetaminophen, and counseling the patient to avoid acetaminophen in prescription combination medications and over‐the‐counter preparations.
Recent regulatory changes regarding acetaminophen/opioid combinations may reduce the incidence of 5‐oxoprolinemia. The US Food and Drug Administration has taken action to reduce adverse effects from acetaminophen exposure by limiting the amount of acetaminophen in opioid combination pills from 500 mg to a maximum of 325 mg per pill. This is aimed at preventing hepatotoxicity from ingestion of higher‐than‐recommended doses. However, clinicians should remember that 5‐oxoprolinemia can result from ingestion of acetaminophen at therapeutic levels.
Given its rare incidence, low clinical suspicion, and transient nature of confirmatory testing, it is likely this remains an underdiagnosed syndrome. In the case discussed, subsequent chart review demonstrated 5 previous admissions in multiple hospitals for severe transient anion gap acidosis. The likelihood that 5‐oxoprolinemia was missed in each of these cases supports a lack of awareness of this syndrome. In this patient, the discussant appropriately identified the possibility of 5‐oxoproline toxicity, but felt ethylene glycol ingestion was more likely. As this case underscores, a cornerstone in the management of suspected ingestions is empiric treatment for the most likely etiologies. Here, treatment for acetaminophen overdose and for methanol or ethylene glycol were warranted, and fortunately also addressed the rarer possibility of 5‐oxoproline toxicity.
The mnemonic MUDPILES is commonly used to identify possible causes of life‐threatening anion gap metabolic acidosis, as such heuristics have benefits in rapidly generating a differential diagnosis to guide initial evaluation. Given the fact that the traditional letter P (paraldehyde) in MUDPILES is no longer clinically utilized, some authors have suggested replacing this with pyroglutamic acid (a synonym of 5‐oxoproline). Such a change may help providers who have ruled out other causes of a high anion gap metabolic acidosis, facilitating diagnosis of this life‐threatening syndrome. In any case, clinicians must be mindful that simple memory aids may mislead clinicians, and a complete differential diagnosis may require more than a mnemonic.
TEACHING POINTS
- Acetaminophen use, even at therapeutic levels, can lead to 5‐oxoprolinemia, a potentially lethal anion gap metabolic acidosis.
- 5‐oxoprolinemia is likely related to glutathione depletion, worsened by acetaminophen, malnutrition, renal insufficiency, female gender, and infection. This implies theoretical benefit from administration of NAC for glutathione repletion.
- Mnemonics can be useful, but have limitations by way of oversimplification. This case suggests that changing the letter P in MUDPILES from paraldehyde to pyroglutamic acid could reduce underdiagnosis.
Disclosure: Nothing to report.
- , , , , . 5‐oxoprolinemia causing elevated anion gap metabolic acidosis in the setting of acetaminophen use. J Emerg Med. 2012;43(1):54–57.
- , , , . What is the clinical significance of 5‐oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure? Clin Toxicol (Phila). 2013;51(9):817–827.
- , , , , . Increased anion gap metabolic acidosis as a result of 5‐oxoproline (pyroglutamic acid): a role for acetaminophen. Clin J Am Soc Nephrol. 2006;1(3):441–447.
- , , , et al. Recurrent high anion gap metabolic acidosis secondary to 5‐oxoproline (pyroglutamic acid). Am J Kidney Dis. 2005;46(1):e4–e10.
- , , . Profound metabolic acidosis from pyroglutamic acidemia: an underappreciated cause of high anion gap metabolic acidosis. CJEM. 2010;12(5):449–452.
- , , , , . 5‐oxoprolinemia causing elevated anion gap metabolic acidosis in the setting of acetaminophen use. J Emerg Med. 2012;43(1):54–57.
- , , , . What is the clinical significance of 5‐oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure? Clin Toxicol (Phila). 2013;51(9):817–827.
- , , , , . Increased anion gap metabolic acidosis as a result of 5‐oxoproline (pyroglutamic acid): a role for acetaminophen. Clin J Am Soc Nephrol. 2006;1(3):441–447.
- , , , et al. Recurrent high anion gap metabolic acidosis secondary to 5‐oxoproline (pyroglutamic acid). Am J Kidney Dis. 2005;46(1):e4–e10.
- , , . Profound metabolic acidosis from pyroglutamic acidemia: an underappreciated cause of high anion gap metabolic acidosis. CJEM. 2010;12(5):449–452.
Treatment of preschool ADHD
Attention deficit/hyperactivity disorder (ADHD) has been identified in children, and appropriate treatments studied now for over half a century. The vast majority of cases that present for treatment do so after the child starts school and concerns are raised about ability to manage academics. Yet, when asked when the symptoms first began, many parents will describe onset prior to the school years – in the preschool period. But identification of ADHD in preschoolers can be difficult because of the developmental changes that are ongoing during the period from 3 to 5 years. Many of the symptoms that one would attribute to ADHD, such as increased motor activity, inattention, and distractibility are commonplace in this age group. Furthermore, some behaviors commonly associated with ADHD, such as emotional lability and obstinacy, are nearly synonymous with being a preschooler. So, how is the diagnosis made? When is it appropriate to treat? And what would that treatment look like? The following case, where symptoms of preschool ADHD go beyond typical development, provides some guides for treatment based on the evolving literature regarding preschool ADHD.
Case Summary
Johnny is a 4-year-old boy who was the product of a complicated pregnancy and delivery. Born at 35 weeks to a 17-year-old mother with a history of tobacco use disorder and depression, he spent several weeks in the special care nursery before leaving the hospital with his mother. His early temperament was described as being “difficult” with frequent episodes of colic and trouble establishing a sleep routine. His father had a history of conduct problems and school failure, and would come in and out of the family for the first 3 years. Lately, he had moved in with Johnny and his mother, and they were trying to “make a go of it.” Johnny had been slightly behind in his developmental milestones – particularly his language – but by 4 years he was able to speak in simple sentences, was able to name his colors, and had started copying circles and squares.
His parents bring Johnny in for an appointment that they made specifically to discuss his activity level and the question of ADHD, which has been brought up by multiple family members and his preschool teacher. They describe some behaviors that you have not heard about previously because they had assumed that “this is what boys did.” At age 3 years, he impulsively ran into the road after being told “no” and was nearly struck by a car. He continually tries to put things into the toaster, and they have had to get “industrial strength” plug covers because he tries to pry them off with a kitchen knife. On multiple occasions, his mother has locked herself in her bedroom because he wouldn’t stop talking to her and she couldn’t stand it anymore. When this happens, she checks often to make sure Johnny is safe, but then calls Johnny’s father home from his job as a delivery driver because she’s at her limit. In fact, Johnny’s father has been called to the preschool to bring Johnny home so many times that his father is in danger of losing his job. While Johnny appears to be a good athlete, he is often picked last for teams because he doesn’t pay attention in the game and likes to “play his own game” of tackling the other children. The stress of raising Johnny is weighing on the parents’ relationship, and Johnny’s father is considering moving out again. The parents ask for an assessment and treatment, preferably with medication.
Case Discussion
Johnny very likely has ADHD. However, to take appropriate caution in the diagnosis, one would consider that he needs to have six of nine criteria of inattention (being careless, difficulty sustaining attention, not listening, not following through, avoiding hard mental tasks, not organizing, losing important items, being easily distractible, and being forgetful) and/or six of nine criteria of hyperactivity/impulsivity (squirming/fidgeting, can’t stay seated, running or climbing excessively, can’t play quietly, “driven by a motor,” talking excessively, blurting out answers, not waiting his turn, and interrupting/intruding on others). As with school-aged ADHD, there need to be symptoms that are frequent (“often”) and that interfere with home, academic, or occupational function. One must take into account the base rate for these symptoms in preschoolers. For example, Willoughby and colleagues (J. Abnorm. Child Psychol. 2012;40:1301-12) demonstrated that at age 4 years, 26.3% of children fidget or squirm, 39.5% act as if “driven by a motor,” 46.3% talk excessively, 28.8% are easily distracted, and 25.4% have difficult waiting their turn. In fact, on average, a 4-year-old will have 1.3 inattentive items and 2.4 hyperactive-impulsive items. Still, Johnny seems to have more than his fair share. This can be validated by a) doing a careful evaluation over time using multiple informants, b) taking a family history, c) looking at developmental signs and ruling out other developmental disorders, d) making physical observations in the office (although these can be deceiving) and e) having the parents and others complete parent and caregiver checklists.
When asking parents and caregivers to complete checklists, it is crucial to make sure that these checklists look for symptoms other than just ADHD, because there are often co-occurring symptoms and disorders. These include oppositional defiant disorder, anxiety, obsessive compulsive disorder, depressive disorders, autism spectrum disorders, trauma, and learning/communication disorders. In fact, the Preschool ADHD Treatment Study (PATS) demonstrated that 71.5% of children with preschool ADHD had at least one other diagnosis and 29.7% had two or more (J. Child Adolesc. Psychopharmacol. 2007;17:563-80). Use of a broad-based instrument that captures all of these domains, in addition to attention, is warranted. In our clinic, we also assess the parents for psychopathology using the same instruments. The reason for this is, first, that family history increases the likelihood of an ADHD diagnosis and, perhaps more importantly, presence of family psychopathology makes treatment more difficult. This is because the treatment you will prescribe is going to actively involve the parents.
The treatment of choice for preschool ADHD, based on practice parameters and expert opinion, is to start with family-based behavioral treatments. There are now several empirically-based treatments that have shown efficacy for the symptoms of inattention and hyperactivity-impulsivity in preschoolers. These include Triple P (“Practitioner’s Manual for Enhanced Triple P” [Brisbane: Families International Publishing, 1998]), The Incredible Years (Webster-Stratton & Hancock, 1998), and the Revised New Forest Parent Program (Daley & Thompson, 2007), among others. If these are not available in your community, other options would be “Helping the noncompliant child: A clinician’s guide to effective parent training,” 2nd ed. (The Guilford Press: New York, 2003) or any other empirically-based parent training program. This is why it is critical to engage the parents in treatment and to refer them for treatment for their own psychopathology, if present. Furthermore, engaging the family in a program of wellness (freedom from substances, enhanced nutrition, avoidance of artificial food coloring, increased exercise), has less of a research base, but the available evidence is that it is helpful.
If medications become necessary because of safety concerns, there are few options that have a Food and Drug Administration indication. Those that do have an indication for disruptive behavior below the age of 5 years (haloperidol, dextroamphetamine, chlorpromazine, and risperidone) should not be considered as first line. The PATS study demonstrated the safety and efficacy of methylphenidate, but with optimal doses lower than those seen in school-aged children (0.7 mg/kg per day) and with increased numbers of adverse effects (11% discontinuing) (J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1284-93; J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1294-303).
Because of the increased amount of side effects, medication treatment cannot be considered as the first treatment. Treatment with nonstimulants is poorly studied. Any treatment with methylphenidate would be considered off-label prescribing, which must be done with great caution and, preferably, in consultation with a child and adolescent psychiatrist.
The diagnosis and management of ADHD in the very young is tricky, but possible. Doing a comprehensive evaluation with information from multiple informants, assessing and treating the parents for psychopathology, engaging the family in wellness, and starting with behavioral management is the way to go. If you feel that medication treatment is necessary for safety of the little ones, it’s best to consult, because none of the medications with FDA indication are likely to be the answer.
Dr. Althoff is associate professor of psychiatry, psychology, and pediatrics at the University of Vermont, Burlington. He is director of the division of behavioral genetics and conducts research on the development of self-regulation in children. Dr. Althoff receives no funding from pharmaceutical companies or industry. He has grant funding from the National Institute of General Medical Sciences and the Klingenstein Third Generation Foundation, and is employed, in part, by the nonprofit Research Center for Children, Youth, and Families that develops the Child Behavior Checklist and associated instruments. E-mail him at [email protected].
Attention deficit/hyperactivity disorder (ADHD) has been identified in children, and appropriate treatments studied now for over half a century. The vast majority of cases that present for treatment do so after the child starts school and concerns are raised about ability to manage academics. Yet, when asked when the symptoms first began, many parents will describe onset prior to the school years – in the preschool period. But identification of ADHD in preschoolers can be difficult because of the developmental changes that are ongoing during the period from 3 to 5 years. Many of the symptoms that one would attribute to ADHD, such as increased motor activity, inattention, and distractibility are commonplace in this age group. Furthermore, some behaviors commonly associated with ADHD, such as emotional lability and obstinacy, are nearly synonymous with being a preschooler. So, how is the diagnosis made? When is it appropriate to treat? And what would that treatment look like? The following case, where symptoms of preschool ADHD go beyond typical development, provides some guides for treatment based on the evolving literature regarding preschool ADHD.
Case Summary
Johnny is a 4-year-old boy who was the product of a complicated pregnancy and delivery. Born at 35 weeks to a 17-year-old mother with a history of tobacco use disorder and depression, he spent several weeks in the special care nursery before leaving the hospital with his mother. His early temperament was described as being “difficult” with frequent episodes of colic and trouble establishing a sleep routine. His father had a history of conduct problems and school failure, and would come in and out of the family for the first 3 years. Lately, he had moved in with Johnny and his mother, and they were trying to “make a go of it.” Johnny had been slightly behind in his developmental milestones – particularly his language – but by 4 years he was able to speak in simple sentences, was able to name his colors, and had started copying circles and squares.
His parents bring Johnny in for an appointment that they made specifically to discuss his activity level and the question of ADHD, which has been brought up by multiple family members and his preschool teacher. They describe some behaviors that you have not heard about previously because they had assumed that “this is what boys did.” At age 3 years, he impulsively ran into the road after being told “no” and was nearly struck by a car. He continually tries to put things into the toaster, and they have had to get “industrial strength” plug covers because he tries to pry them off with a kitchen knife. On multiple occasions, his mother has locked herself in her bedroom because he wouldn’t stop talking to her and she couldn’t stand it anymore. When this happens, she checks often to make sure Johnny is safe, but then calls Johnny’s father home from his job as a delivery driver because she’s at her limit. In fact, Johnny’s father has been called to the preschool to bring Johnny home so many times that his father is in danger of losing his job. While Johnny appears to be a good athlete, he is often picked last for teams because he doesn’t pay attention in the game and likes to “play his own game” of tackling the other children. The stress of raising Johnny is weighing on the parents’ relationship, and Johnny’s father is considering moving out again. The parents ask for an assessment and treatment, preferably with medication.
Case Discussion
Johnny very likely has ADHD. However, to take appropriate caution in the diagnosis, one would consider that he needs to have six of nine criteria of inattention (being careless, difficulty sustaining attention, not listening, not following through, avoiding hard mental tasks, not organizing, losing important items, being easily distractible, and being forgetful) and/or six of nine criteria of hyperactivity/impulsivity (squirming/fidgeting, can’t stay seated, running or climbing excessively, can’t play quietly, “driven by a motor,” talking excessively, blurting out answers, not waiting his turn, and interrupting/intruding on others). As with school-aged ADHD, there need to be symptoms that are frequent (“often”) and that interfere with home, academic, or occupational function. One must take into account the base rate for these symptoms in preschoolers. For example, Willoughby and colleagues (J. Abnorm. Child Psychol. 2012;40:1301-12) demonstrated that at age 4 years, 26.3% of children fidget or squirm, 39.5% act as if “driven by a motor,” 46.3% talk excessively, 28.8% are easily distracted, and 25.4% have difficult waiting their turn. In fact, on average, a 4-year-old will have 1.3 inattentive items and 2.4 hyperactive-impulsive items. Still, Johnny seems to have more than his fair share. This can be validated by a) doing a careful evaluation over time using multiple informants, b) taking a family history, c) looking at developmental signs and ruling out other developmental disorders, d) making physical observations in the office (although these can be deceiving) and e) having the parents and others complete parent and caregiver checklists.
When asking parents and caregivers to complete checklists, it is crucial to make sure that these checklists look for symptoms other than just ADHD, because there are often co-occurring symptoms and disorders. These include oppositional defiant disorder, anxiety, obsessive compulsive disorder, depressive disorders, autism spectrum disorders, trauma, and learning/communication disorders. In fact, the Preschool ADHD Treatment Study (PATS) demonstrated that 71.5% of children with preschool ADHD had at least one other diagnosis and 29.7% had two or more (J. Child Adolesc. Psychopharmacol. 2007;17:563-80). Use of a broad-based instrument that captures all of these domains, in addition to attention, is warranted. In our clinic, we also assess the parents for psychopathology using the same instruments. The reason for this is, first, that family history increases the likelihood of an ADHD diagnosis and, perhaps more importantly, presence of family psychopathology makes treatment more difficult. This is because the treatment you will prescribe is going to actively involve the parents.
The treatment of choice for preschool ADHD, based on practice parameters and expert opinion, is to start with family-based behavioral treatments. There are now several empirically-based treatments that have shown efficacy for the symptoms of inattention and hyperactivity-impulsivity in preschoolers. These include Triple P (“Practitioner’s Manual for Enhanced Triple P” [Brisbane: Families International Publishing, 1998]), The Incredible Years (Webster-Stratton & Hancock, 1998), and the Revised New Forest Parent Program (Daley & Thompson, 2007), among others. If these are not available in your community, other options would be “Helping the noncompliant child: A clinician’s guide to effective parent training,” 2nd ed. (The Guilford Press: New York, 2003) or any other empirically-based parent training program. This is why it is critical to engage the parents in treatment and to refer them for treatment for their own psychopathology, if present. Furthermore, engaging the family in a program of wellness (freedom from substances, enhanced nutrition, avoidance of artificial food coloring, increased exercise), has less of a research base, but the available evidence is that it is helpful.
If medications become necessary because of safety concerns, there are few options that have a Food and Drug Administration indication. Those that do have an indication for disruptive behavior below the age of 5 years (haloperidol, dextroamphetamine, chlorpromazine, and risperidone) should not be considered as first line. The PATS study demonstrated the safety and efficacy of methylphenidate, but with optimal doses lower than those seen in school-aged children (0.7 mg/kg per day) and with increased numbers of adverse effects (11% discontinuing) (J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1284-93; J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1294-303).
Because of the increased amount of side effects, medication treatment cannot be considered as the first treatment. Treatment with nonstimulants is poorly studied. Any treatment with methylphenidate would be considered off-label prescribing, which must be done with great caution and, preferably, in consultation with a child and adolescent psychiatrist.
The diagnosis and management of ADHD in the very young is tricky, but possible. Doing a comprehensive evaluation with information from multiple informants, assessing and treating the parents for psychopathology, engaging the family in wellness, and starting with behavioral management is the way to go. If you feel that medication treatment is necessary for safety of the little ones, it’s best to consult, because none of the medications with FDA indication are likely to be the answer.
Dr. Althoff is associate professor of psychiatry, psychology, and pediatrics at the University of Vermont, Burlington. He is director of the division of behavioral genetics and conducts research on the development of self-regulation in children. Dr. Althoff receives no funding from pharmaceutical companies or industry. He has grant funding from the National Institute of General Medical Sciences and the Klingenstein Third Generation Foundation, and is employed, in part, by the nonprofit Research Center for Children, Youth, and Families that develops the Child Behavior Checklist and associated instruments. E-mail him at [email protected].
Attention deficit/hyperactivity disorder (ADHD) has been identified in children, and appropriate treatments studied now for over half a century. The vast majority of cases that present for treatment do so after the child starts school and concerns are raised about ability to manage academics. Yet, when asked when the symptoms first began, many parents will describe onset prior to the school years – in the preschool period. But identification of ADHD in preschoolers can be difficult because of the developmental changes that are ongoing during the period from 3 to 5 years. Many of the symptoms that one would attribute to ADHD, such as increased motor activity, inattention, and distractibility are commonplace in this age group. Furthermore, some behaviors commonly associated with ADHD, such as emotional lability and obstinacy, are nearly synonymous with being a preschooler. So, how is the diagnosis made? When is it appropriate to treat? And what would that treatment look like? The following case, where symptoms of preschool ADHD go beyond typical development, provides some guides for treatment based on the evolving literature regarding preschool ADHD.
Case Summary
Johnny is a 4-year-old boy who was the product of a complicated pregnancy and delivery. Born at 35 weeks to a 17-year-old mother with a history of tobacco use disorder and depression, he spent several weeks in the special care nursery before leaving the hospital with his mother. His early temperament was described as being “difficult” with frequent episodes of colic and trouble establishing a sleep routine. His father had a history of conduct problems and school failure, and would come in and out of the family for the first 3 years. Lately, he had moved in with Johnny and his mother, and they were trying to “make a go of it.” Johnny had been slightly behind in his developmental milestones – particularly his language – but by 4 years he was able to speak in simple sentences, was able to name his colors, and had started copying circles and squares.
His parents bring Johnny in for an appointment that they made specifically to discuss his activity level and the question of ADHD, which has been brought up by multiple family members and his preschool teacher. They describe some behaviors that you have not heard about previously because they had assumed that “this is what boys did.” At age 3 years, he impulsively ran into the road after being told “no” and was nearly struck by a car. He continually tries to put things into the toaster, and they have had to get “industrial strength” plug covers because he tries to pry them off with a kitchen knife. On multiple occasions, his mother has locked herself in her bedroom because he wouldn’t stop talking to her and she couldn’t stand it anymore. When this happens, she checks often to make sure Johnny is safe, but then calls Johnny’s father home from his job as a delivery driver because she’s at her limit. In fact, Johnny’s father has been called to the preschool to bring Johnny home so many times that his father is in danger of losing his job. While Johnny appears to be a good athlete, he is often picked last for teams because he doesn’t pay attention in the game and likes to “play his own game” of tackling the other children. The stress of raising Johnny is weighing on the parents’ relationship, and Johnny’s father is considering moving out again. The parents ask for an assessment and treatment, preferably with medication.
Case Discussion
Johnny very likely has ADHD. However, to take appropriate caution in the diagnosis, one would consider that he needs to have six of nine criteria of inattention (being careless, difficulty sustaining attention, not listening, not following through, avoiding hard mental tasks, not organizing, losing important items, being easily distractible, and being forgetful) and/or six of nine criteria of hyperactivity/impulsivity (squirming/fidgeting, can’t stay seated, running or climbing excessively, can’t play quietly, “driven by a motor,” talking excessively, blurting out answers, not waiting his turn, and interrupting/intruding on others). As with school-aged ADHD, there need to be symptoms that are frequent (“often”) and that interfere with home, academic, or occupational function. One must take into account the base rate for these symptoms in preschoolers. For example, Willoughby and colleagues (J. Abnorm. Child Psychol. 2012;40:1301-12) demonstrated that at age 4 years, 26.3% of children fidget or squirm, 39.5% act as if “driven by a motor,” 46.3% talk excessively, 28.8% are easily distracted, and 25.4% have difficult waiting their turn. In fact, on average, a 4-year-old will have 1.3 inattentive items and 2.4 hyperactive-impulsive items. Still, Johnny seems to have more than his fair share. This can be validated by a) doing a careful evaluation over time using multiple informants, b) taking a family history, c) looking at developmental signs and ruling out other developmental disorders, d) making physical observations in the office (although these can be deceiving) and e) having the parents and others complete parent and caregiver checklists.
When asking parents and caregivers to complete checklists, it is crucial to make sure that these checklists look for symptoms other than just ADHD, because there are often co-occurring symptoms and disorders. These include oppositional defiant disorder, anxiety, obsessive compulsive disorder, depressive disorders, autism spectrum disorders, trauma, and learning/communication disorders. In fact, the Preschool ADHD Treatment Study (PATS) demonstrated that 71.5% of children with preschool ADHD had at least one other diagnosis and 29.7% had two or more (J. Child Adolesc. Psychopharmacol. 2007;17:563-80). Use of a broad-based instrument that captures all of these domains, in addition to attention, is warranted. In our clinic, we also assess the parents for psychopathology using the same instruments. The reason for this is, first, that family history increases the likelihood of an ADHD diagnosis and, perhaps more importantly, presence of family psychopathology makes treatment more difficult. This is because the treatment you will prescribe is going to actively involve the parents.
The treatment of choice for preschool ADHD, based on practice parameters and expert opinion, is to start with family-based behavioral treatments. There are now several empirically-based treatments that have shown efficacy for the symptoms of inattention and hyperactivity-impulsivity in preschoolers. These include Triple P (“Practitioner’s Manual for Enhanced Triple P” [Brisbane: Families International Publishing, 1998]), The Incredible Years (Webster-Stratton & Hancock, 1998), and the Revised New Forest Parent Program (Daley & Thompson, 2007), among others. If these are not available in your community, other options would be “Helping the noncompliant child: A clinician’s guide to effective parent training,” 2nd ed. (The Guilford Press: New York, 2003) or any other empirically-based parent training program. This is why it is critical to engage the parents in treatment and to refer them for treatment for their own psychopathology, if present. Furthermore, engaging the family in a program of wellness (freedom from substances, enhanced nutrition, avoidance of artificial food coloring, increased exercise), has less of a research base, but the available evidence is that it is helpful.
If medications become necessary because of safety concerns, there are few options that have a Food and Drug Administration indication. Those that do have an indication for disruptive behavior below the age of 5 years (haloperidol, dextroamphetamine, chlorpromazine, and risperidone) should not be considered as first line. The PATS study demonstrated the safety and efficacy of methylphenidate, but with optimal doses lower than those seen in school-aged children (0.7 mg/kg per day) and with increased numbers of adverse effects (11% discontinuing) (J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1284-93; J. Am. Acad. Child Adolesc. Psychiatry 2006;45:1294-303).
Because of the increased amount of side effects, medication treatment cannot be considered as the first treatment. Treatment with nonstimulants is poorly studied. Any treatment with methylphenidate would be considered off-label prescribing, which must be done with great caution and, preferably, in consultation with a child and adolescent psychiatrist.
The diagnosis and management of ADHD in the very young is tricky, but possible. Doing a comprehensive evaluation with information from multiple informants, assessing and treating the parents for psychopathology, engaging the family in wellness, and starting with behavioral management is the way to go. If you feel that medication treatment is necessary for safety of the little ones, it’s best to consult, because none of the medications with FDA indication are likely to be the answer.
Dr. Althoff is associate professor of psychiatry, psychology, and pediatrics at the University of Vermont, Burlington. He is director of the division of behavioral genetics and conducts research on the development of self-regulation in children. Dr. Althoff receives no funding from pharmaceutical companies or industry. He has grant funding from the National Institute of General Medical Sciences and the Klingenstein Third Generation Foundation, and is employed, in part, by the nonprofit Research Center for Children, Youth, and Families that develops the Child Behavior Checklist and associated instruments. E-mail him at [email protected].
Dexrazoxane Tx did not affect overall survival in pediatric leukemia and lymphoma
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Treatment with dexrazoxane was not associated with an increased risk for cancer relapse or death.
Major finding: For pediatric patients with leukemia and lymphoma, the cumulative incidence of relapse at 10 years was 16.1% with DRZ, compared with 19.1% without DRZ (difference, – 3.0%; 95% CI, – 7.9% to 0.2%); overall mortality was 12.8% with DRZ vs. 12.2% without DRZ (difference, – 0.6%; 95% CI, – 3.5% to 4.7%).
Data source: Aggregated Children’s Oncology Group trials enrolling 1,008 pediatric patients with leukemia or lymphoma who were randomized to receive doxorubicin with or without DRZ from 1996 to 2001.
Disclosures: The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
HRS: Meta-analyses strengthen obesity–atrial fib link
BOSTON– The already-firm evidence implicating obesity in boosting both the incidence and severity of atrial fibrillation grew even stronger with results from four meta-analyses that comprised 51 controlled studies involving a total of more than 600,000 people.
“We should pay more attention to using weight reduction strategies to prevent AF [atrial fibrillation] and to reduce its burden in patients with obesity and established AF,” Dr. Dennis H. Lau said at the annual scientific sessions of the Heart Rhythm Society.
Physicians are increasingly aware of the strong evidence linking obesity and atrial fibrillation incidence and severity, said Dr. Christine M. Albert, director of the Center for Arrhythmia Prevention at Brigham and Women’s Hospital, Boston. The existence of this link is “really important because it is something we can offer patients,” Dr. Albert said in an interview. Obesity interventions provide a way to intervene in patients with, or at risk for, atrial fibrillation that goes beyond atrial ablation and antiarrhythmic drugs to reduce symptoms and help patients feel better, she noted.
Dr. Lau and his associates reviewed the published medical literature through January 2012 and identified 51 studies that examined the link between obesity and AF in a total of 626,603 people.
They found 16 studies with 5,864 patients that assessed the link between obesity and AF recurrence following atrial ablation treatment and found a statistically significant 13% increased rate of recurrent AF for every 5-unit rise in body mass index, Dr. Lau reported.
They also identified 12 studies on the impact of obesity in 62,160 patients who underwent cardiac surgery that collectively showed a statistically significant 10% higher incidence of postoperative AF for every additional 5 BMI units.
The researchers found nine studies of the role of obesity in new-onset AF in cohort analyses with a total of 157,518 patients that showed an overall, statistically significant 29% rise in AF incidence for every 5 additional BMI units. And in 14 case-control studies with 401,061 patients, the rate of new-onset AF increased by a statistically significant 19% for every 5-unit rise in BMI.
These findings fit into an already substantial body of evidence documenting a significant link between obesity and AF, said Dr. Lau, director of the cardiac pacing unit at the Royal Adelaide (Australia) Hospital. For example, an analysis of 14,598 Americans enrolled in the Atherosclerosis Risk in Communities (ARIC) study found that 18% of the 1,520 new cases of AF that occurred in this cohort during an average 17 years of follow-up could be attributed to obesity or overweight (Circulation 2011;123:1501-8). Data collected from 34,309 women enrolled in the Women’s Health Study who had 834 cases of incident AF during an average 13-year follow-up showed that every 1-unit increase in BMI was linked to a 5% increased risk for AF, and that obese women had an overall 65% higher incidence of AF than did women with a normal BMI (J. Am Coll. Cardiol. 2010;55:2319-27).
And Dr. Lau and his associates recently published a review of 355 patients with AF and a baseline BMI of at least 27 kg/m2 who participated in a weight-management program. After 5 years, patients who lost at least 10% of their baseline weight had an 86% rate of arrhythmia-free survival, compared with a 40% rate in patients who either lost less than 3% of their baseline weight or gained weight. In a multivariate analysis, weight loss of at least 10% linked with a statistically significant sixfold increase in arrhythmia-free survival, compared with all the other patients in the analysis (J. Am. Coll. Cardiol. 2015;65:2159-69).
Dr. Lau also cited findings from animal studies by his group that point to a direct role for obesity, and specifically deposits of epicardial fat in causing AF. Their model uses overfed sheep, and his group found that a higher burden of epicardial fat leads to fat infiltration into the myocardium, including atrial tissue. “We postulate that this fat contributes to conduction heterogeneity, increased re-entry, increased susceptibility to AF, and increased duration of AF episodes,” he said.
“It’s quite clear that obesity itself is important because, for example, the sheep do not develop sleep apnea, and they have only marginally elevated blood pressures. Using this animal model, we are quite convinced that obesity itself is an important risk factor.”
Dr. Lau added that results from recent sheep studies showed that after previously obese sheep lose their excess weight their atrial abnormalities revert to normal.
On Twitter @mitchelzoler
BOSTON– The already-firm evidence implicating obesity in boosting both the incidence and severity of atrial fibrillation grew even stronger with results from four meta-analyses that comprised 51 controlled studies involving a total of more than 600,000 people.
“We should pay more attention to using weight reduction strategies to prevent AF [atrial fibrillation] and to reduce its burden in patients with obesity and established AF,” Dr. Dennis H. Lau said at the annual scientific sessions of the Heart Rhythm Society.
Physicians are increasingly aware of the strong evidence linking obesity and atrial fibrillation incidence and severity, said Dr. Christine M. Albert, director of the Center for Arrhythmia Prevention at Brigham and Women’s Hospital, Boston. The existence of this link is “really important because it is something we can offer patients,” Dr. Albert said in an interview. Obesity interventions provide a way to intervene in patients with, or at risk for, atrial fibrillation that goes beyond atrial ablation and antiarrhythmic drugs to reduce symptoms and help patients feel better, she noted.
Dr. Lau and his associates reviewed the published medical literature through January 2012 and identified 51 studies that examined the link between obesity and AF in a total of 626,603 people.
They found 16 studies with 5,864 patients that assessed the link between obesity and AF recurrence following atrial ablation treatment and found a statistically significant 13% increased rate of recurrent AF for every 5-unit rise in body mass index, Dr. Lau reported.
They also identified 12 studies on the impact of obesity in 62,160 patients who underwent cardiac surgery that collectively showed a statistically significant 10% higher incidence of postoperative AF for every additional 5 BMI units.
The researchers found nine studies of the role of obesity in new-onset AF in cohort analyses with a total of 157,518 patients that showed an overall, statistically significant 29% rise in AF incidence for every 5 additional BMI units. And in 14 case-control studies with 401,061 patients, the rate of new-onset AF increased by a statistically significant 19% for every 5-unit rise in BMI.
These findings fit into an already substantial body of evidence documenting a significant link between obesity and AF, said Dr. Lau, director of the cardiac pacing unit at the Royal Adelaide (Australia) Hospital. For example, an analysis of 14,598 Americans enrolled in the Atherosclerosis Risk in Communities (ARIC) study found that 18% of the 1,520 new cases of AF that occurred in this cohort during an average 17 years of follow-up could be attributed to obesity or overweight (Circulation 2011;123:1501-8). Data collected from 34,309 women enrolled in the Women’s Health Study who had 834 cases of incident AF during an average 13-year follow-up showed that every 1-unit increase in BMI was linked to a 5% increased risk for AF, and that obese women had an overall 65% higher incidence of AF than did women with a normal BMI (J. Am Coll. Cardiol. 2010;55:2319-27).
And Dr. Lau and his associates recently published a review of 355 patients with AF and a baseline BMI of at least 27 kg/m2 who participated in a weight-management program. After 5 years, patients who lost at least 10% of their baseline weight had an 86% rate of arrhythmia-free survival, compared with a 40% rate in patients who either lost less than 3% of their baseline weight or gained weight. In a multivariate analysis, weight loss of at least 10% linked with a statistically significant sixfold increase in arrhythmia-free survival, compared with all the other patients in the analysis (J. Am. Coll. Cardiol. 2015;65:2159-69).
Dr. Lau also cited findings from animal studies by his group that point to a direct role for obesity, and specifically deposits of epicardial fat in causing AF. Their model uses overfed sheep, and his group found that a higher burden of epicardial fat leads to fat infiltration into the myocardium, including atrial tissue. “We postulate that this fat contributes to conduction heterogeneity, increased re-entry, increased susceptibility to AF, and increased duration of AF episodes,” he said.
“It’s quite clear that obesity itself is important because, for example, the sheep do not develop sleep apnea, and they have only marginally elevated blood pressures. Using this animal model, we are quite convinced that obesity itself is an important risk factor.”
Dr. Lau added that results from recent sheep studies showed that after previously obese sheep lose their excess weight their atrial abnormalities revert to normal.
On Twitter @mitchelzoler
BOSTON– The already-firm evidence implicating obesity in boosting both the incidence and severity of atrial fibrillation grew even stronger with results from four meta-analyses that comprised 51 controlled studies involving a total of more than 600,000 people.
“We should pay more attention to using weight reduction strategies to prevent AF [atrial fibrillation] and to reduce its burden in patients with obesity and established AF,” Dr. Dennis H. Lau said at the annual scientific sessions of the Heart Rhythm Society.
Physicians are increasingly aware of the strong evidence linking obesity and atrial fibrillation incidence and severity, said Dr. Christine M. Albert, director of the Center for Arrhythmia Prevention at Brigham and Women’s Hospital, Boston. The existence of this link is “really important because it is something we can offer patients,” Dr. Albert said in an interview. Obesity interventions provide a way to intervene in patients with, or at risk for, atrial fibrillation that goes beyond atrial ablation and antiarrhythmic drugs to reduce symptoms and help patients feel better, she noted.
Dr. Lau and his associates reviewed the published medical literature through January 2012 and identified 51 studies that examined the link between obesity and AF in a total of 626,603 people.
They found 16 studies with 5,864 patients that assessed the link between obesity and AF recurrence following atrial ablation treatment and found a statistically significant 13% increased rate of recurrent AF for every 5-unit rise in body mass index, Dr. Lau reported.
They also identified 12 studies on the impact of obesity in 62,160 patients who underwent cardiac surgery that collectively showed a statistically significant 10% higher incidence of postoperative AF for every additional 5 BMI units.
The researchers found nine studies of the role of obesity in new-onset AF in cohort analyses with a total of 157,518 patients that showed an overall, statistically significant 29% rise in AF incidence for every 5 additional BMI units. And in 14 case-control studies with 401,061 patients, the rate of new-onset AF increased by a statistically significant 19% for every 5-unit rise in BMI.
These findings fit into an already substantial body of evidence documenting a significant link between obesity and AF, said Dr. Lau, director of the cardiac pacing unit at the Royal Adelaide (Australia) Hospital. For example, an analysis of 14,598 Americans enrolled in the Atherosclerosis Risk in Communities (ARIC) study found that 18% of the 1,520 new cases of AF that occurred in this cohort during an average 17 years of follow-up could be attributed to obesity or overweight (Circulation 2011;123:1501-8). Data collected from 34,309 women enrolled in the Women’s Health Study who had 834 cases of incident AF during an average 13-year follow-up showed that every 1-unit increase in BMI was linked to a 5% increased risk for AF, and that obese women had an overall 65% higher incidence of AF than did women with a normal BMI (J. Am Coll. Cardiol. 2010;55:2319-27).
And Dr. Lau and his associates recently published a review of 355 patients with AF and a baseline BMI of at least 27 kg/m2 who participated in a weight-management program. After 5 years, patients who lost at least 10% of their baseline weight had an 86% rate of arrhythmia-free survival, compared with a 40% rate in patients who either lost less than 3% of their baseline weight or gained weight. In a multivariate analysis, weight loss of at least 10% linked with a statistically significant sixfold increase in arrhythmia-free survival, compared with all the other patients in the analysis (J. Am. Coll. Cardiol. 2015;65:2159-69).
Dr. Lau also cited findings from animal studies by his group that point to a direct role for obesity, and specifically deposits of epicardial fat in causing AF. Their model uses overfed sheep, and his group found that a higher burden of epicardial fat leads to fat infiltration into the myocardium, including atrial tissue. “We postulate that this fat contributes to conduction heterogeneity, increased re-entry, increased susceptibility to AF, and increased duration of AF episodes,” he said.
“It’s quite clear that obesity itself is important because, for example, the sheep do not develop sleep apnea, and they have only marginally elevated blood pressures. Using this animal model, we are quite convinced that obesity itself is an important risk factor.”
Dr. Lau added that results from recent sheep studies showed that after previously obese sheep lose their excess weight their atrial abnormalities revert to normal.
On Twitter @mitchelzoler
AT HEART RHYTHM 2015
Key clinical point: Four meta-analyses that together included 51 controlled studies provide further evidence that obesity boosts the risk for new-onset atrial fibrillation.
Major finding: For every 5-unit rise in body mass index, the incidence of atrial fibrillation increased by 10%-29%.
Data source: Meta-analyses of 51 controlled studies involving a total of 626,603 people.
Disclosures: Dr. Lau and Dr. Albert had no relevant disclosures.
Subclinical hyperthyroidism linked to higher fracture risk
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
FROM JAMA
Key clinical point: Subclinical hyperthyroidism is associated with an increased risk of hip and other fractures.
Major finding: Individuals with subclinical hyperthyroidism had a 28% increase in their risk of any fracture compared to individuals with normal thyroid function.
Data source: A meta-analysis of 13 prospective cohort studies comprising 70,298 individuals.
Disclosures: The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants, and funding from a range of pharmaceutical companies.
Warfarin bridge therapy ups bleeding risk, with no reduction in VTE
Bridge therapy for warfarin patients undergoing invasive therapy is unnecessary for most, said investigators who found an increased risk of bleeding associated with the use of short-acting anticoagulant at the time of the procedure.
A retrospective cohort study of 1,812 procedures in 1,178 patients – most of whom were considered to be at low risk of venous thromboembolism recurrence – showed a 17-fold increase in the risk of clinically relevant bleeding in the group that received bridge anticoagulant therapy, compared with the group that didn’t (2.7% vs. 0.2%).
There was, however, no significant difference in the rate of recurrent venous thromboembolism between the bridge-therapy and non–bridge-therapy groups (0 vs. 3), and no deaths were observed in either group, according to an article published online May 26 (JAMA Intern. Med. [doi:10.1001/jamainternmed.2015.1843].
“Our results confirm and strengthen the findings of those previous studies and highlight the need for a risk categorization scheme that identifies patients at highest risk for recurrent VTE who may benefit from bridge therapy,” wrote Thomas Delate, Ph.D., from Kaiser Permanente Colorado, and coauthors.
The study was conducted and supported by Kaiser Permanente Colorado. One author reported consultancies with Astra-Zeneca, Boehringer-Ingelheim, Pfizer, and Sanofi.

|
| Dr. Daniel J. Brotman |
There are undoubtedly some patients at such high risk for recurrent venous thromboembolism that bridge therapy is a necessary evil, such as those with acute VTE in the preceding month and those with a prior pattern of brisk VTE recurrence during short-term interruption of anticoagulation therapy.
However, for the vast majority of patients receiving oral anticoagulants for VTE, it is probably safer to simply allow the oral anticoagulant to wash out before the procedure and, if indicated based on the type of surgery, to use routine prophylactic-dose anticoagulation therapy afterward.
Dr. Daniel J. Brotman and Dr. Michael B. Streiff are from Johns Hopkins University, Baltimore. These comments are taken from an accompanying editorial (JAMA Intern. Med. 2015 May 26 [doi:10.1001/jamainternmed.2015.1858]). Dr Streiff declared research funding from Bristol-Myers Squibb and Portola and consultancies for Boehringer-Ingelheim, Daiichi-Sankyo, Eisai, Janssen HealthCare, Pfizer, and Sanofi.

|
| Dr. Daniel J. Brotman |
There are undoubtedly some patients at such high risk for recurrent venous thromboembolism that bridge therapy is a necessary evil, such as those with acute VTE in the preceding month and those with a prior pattern of brisk VTE recurrence during short-term interruption of anticoagulation therapy.
However, for the vast majority of patients receiving oral anticoagulants for VTE, it is probably safer to simply allow the oral anticoagulant to wash out before the procedure and, if indicated based on the type of surgery, to use routine prophylactic-dose anticoagulation therapy afterward.
Dr. Daniel J. Brotman and Dr. Michael B. Streiff are from Johns Hopkins University, Baltimore. These comments are taken from an accompanying editorial (JAMA Intern. Med. 2015 May 26 [doi:10.1001/jamainternmed.2015.1858]). Dr Streiff declared research funding from Bristol-Myers Squibb and Portola and consultancies for Boehringer-Ingelheim, Daiichi-Sankyo, Eisai, Janssen HealthCare, Pfizer, and Sanofi.

|
| Dr. Daniel J. Brotman |
There are undoubtedly some patients at such high risk for recurrent venous thromboembolism that bridge therapy is a necessary evil, such as those with acute VTE in the preceding month and those with a prior pattern of brisk VTE recurrence during short-term interruption of anticoagulation therapy.
However, for the vast majority of patients receiving oral anticoagulants for VTE, it is probably safer to simply allow the oral anticoagulant to wash out before the procedure and, if indicated based on the type of surgery, to use routine prophylactic-dose anticoagulation therapy afterward.
Dr. Daniel J. Brotman and Dr. Michael B. Streiff are from Johns Hopkins University, Baltimore. These comments are taken from an accompanying editorial (JAMA Intern. Med. 2015 May 26 [doi:10.1001/jamainternmed.2015.1858]). Dr Streiff declared research funding from Bristol-Myers Squibb and Portola and consultancies for Boehringer-Ingelheim, Daiichi-Sankyo, Eisai, Janssen HealthCare, Pfizer, and Sanofi.
Bridge therapy for warfarin patients undergoing invasive therapy is unnecessary for most, said investigators who found an increased risk of bleeding associated with the use of short-acting anticoagulant at the time of the procedure.
A retrospective cohort study of 1,812 procedures in 1,178 patients – most of whom were considered to be at low risk of venous thromboembolism recurrence – showed a 17-fold increase in the risk of clinically relevant bleeding in the group that received bridge anticoagulant therapy, compared with the group that didn’t (2.7% vs. 0.2%).
There was, however, no significant difference in the rate of recurrent venous thromboembolism between the bridge-therapy and non–bridge-therapy groups (0 vs. 3), and no deaths were observed in either group, according to an article published online May 26 (JAMA Intern. Med. [doi:10.1001/jamainternmed.2015.1843].
“Our results confirm and strengthen the findings of those previous studies and highlight the need for a risk categorization scheme that identifies patients at highest risk for recurrent VTE who may benefit from bridge therapy,” wrote Thomas Delate, Ph.D., from Kaiser Permanente Colorado, and coauthors.
The study was conducted and supported by Kaiser Permanente Colorado. One author reported consultancies with Astra-Zeneca, Boehringer-Ingelheim, Pfizer, and Sanofi.
Bridge therapy for warfarin patients undergoing invasive therapy is unnecessary for most, said investigators who found an increased risk of bleeding associated with the use of short-acting anticoagulant at the time of the procedure.
A retrospective cohort study of 1,812 procedures in 1,178 patients – most of whom were considered to be at low risk of venous thromboembolism recurrence – showed a 17-fold increase in the risk of clinically relevant bleeding in the group that received bridge anticoagulant therapy, compared with the group that didn’t (2.7% vs. 0.2%).
There was, however, no significant difference in the rate of recurrent venous thromboembolism between the bridge-therapy and non–bridge-therapy groups (0 vs. 3), and no deaths were observed in either group, according to an article published online May 26 (JAMA Intern. Med. [doi:10.1001/jamainternmed.2015.1843].
“Our results confirm and strengthen the findings of those previous studies and highlight the need for a risk categorization scheme that identifies patients at highest risk for recurrent VTE who may benefit from bridge therapy,” wrote Thomas Delate, Ph.D., from Kaiser Permanente Colorado, and coauthors.
The study was conducted and supported by Kaiser Permanente Colorado. One author reported consultancies with Astra-Zeneca, Boehringer-Ingelheim, Pfizer, and Sanofi.
Key clinical point: Bridge therapy for warfarin patients undergoing invasive therapy is associated with an increased risk of bleeding without a reduction in thromboembolism risk.
Major finding: Patients given bridge therapy during invasive therapy had a 17-fold increase in the risk of clinically significant bleeding.
Data source: A retrospective cohort study of 1,812 procedures in 1,178 patients.
Disclosures: The study was conducted and supported by Kaiser Permanente Colorado. One author reported consultancies with AstraZeneca, Boehringer-Ingelheim, Pfizer, and Sanofi.