User login
Decompressive brain surgery carries high complication risk
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: The high risk of complications associated with decompressive hemicraniectomy for malignant middle cerebral artery infarction warrants appropriate counseling and individualized therapeutic decision-making.
Major finding: The in-hospital and late complication rates associated with decompressive hemicraniectomy for malignant middle cerebral artery infarction were 88.1% and 89.5%, respectively.
Data source: Retrospective, observational, single-center study of 48 patients who underwent decompressive hemicrainiectomy between 2008 and 2014.
Disclosures: Dr. Pledl reported no conflicts.
EHA: Inotuzumab rallies against refractory/relapsed ALL
VIENNA – The investigational agent inotuzumab ozagamicin more than doubled complete remission rates compared with standard therapy in relapsed or refractory acute lymphoblastic leukemia, preliminary results from the INO-VATE study show.
The co-primary endpoint of complete remission or CR with incomplete hematologic recovery (CRi) by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care (SOC) (P < .0001).
Significantly more CR/CRi responders treated with inotuzumab were minimal residual disease (MRD)-negative by multicolor flow cytometry (78.4% vs. 28.1%; P < .0001), Dr. Daniel DeAngelo reported in a late-breaking abstract (LBA2073) at the annual congress of the European Hematology Association.
“The fact that the response rate was astronomically high with a high MRD-negative status really allows this or this should be an opportunity for patients with relapsed/refractory disease,” he said in an interview.
Inotuzumab ozagamicin is an investigational anti-CD22 antibody conjugated to calicheamicin, an antitumor antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Previous phase II studies reported strong initial antitumor activity and safety with inotuzumab in relapsed or refractory ALL, Dr. DeAngelo, of the Dana Farber Cancer Institute in Boston, said.
The ongoing phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or SOC: either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once CR/CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients from the SOC arm who refused to start treatment.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Data for the co-primary endpoint of overall survival in all 326 patients are still blinded and not expected to mature until 2016, Dr. DeAngelo said.
CR/CRi analyses significantly favored inotuzumab in all stratification factors and baseline factors including peripheral blasts and CD22 expression. Cytogenetics are still being evaluated, but 11 of 14 (79%) patients with Philadelphia-positive karyotype achieved a CR or CRi, he said.
Median duration of remission among responders was 4.6 months in the inotuzumab arm and 3.1 months in the SOC arm (hazard ratio, 0.55; P = .016).
Safety assessed in 259 patients who received at least one dose of study drug showed similar incidence of grade 3 or higher adverse events in the inotuzumab and SOC arms (91% vs. 95%). There were 2 fatal events in the SOC arm and 4 in the inotuzumab arm: 2 veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS), both after poststudy transplant, 1 intestinal ischemia/septic shock, and 1 acute respiratory distress syndrome as a terminal event of pneumonia. In multivariate analysis, dual alkylator conditioning was the only significant covariate of VOD/SOS (P = .039), Dr. DeAngelo said.
An audience member chided the author for the short duration of remission, but session co-moderator Dr. Anthony Moorman, of Newcastle University, Newcastle upon Tyne, England, said it is not that concerning because of the aggressive nature of ALL.
“For all patients that have relapsed or refractory adult ALL, their responses are incredibly low. So any kind of complete remission is a major achievement in this patient population, especially if they are refractory or relapse after tyrosine kinase inhibitors or Philadelphia-positive,” he said in an interview.
“When you have an active agent that works with relapsed refractory disease, in this case leukemia, the goal is to move it up front,” Dr. DeAngelo told this publication.
Indeed, updated results presented at the meeting from M.D. Anderson Cancer Center of frontline inotuzumab added to low-intensity chemotherapy (Mini-hyper CVD) in elderly ALL patients were “provocative,” he added. CR rates reached 97% in the study, according to the abstract (S114).
VIENNA – The investigational agent inotuzumab ozagamicin more than doubled complete remission rates compared with standard therapy in relapsed or refractory acute lymphoblastic leukemia, preliminary results from the INO-VATE study show.
The co-primary endpoint of complete remission or CR with incomplete hematologic recovery (CRi) by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care (SOC) (P < .0001).
Significantly more CR/CRi responders treated with inotuzumab were minimal residual disease (MRD)-negative by multicolor flow cytometry (78.4% vs. 28.1%; P < .0001), Dr. Daniel DeAngelo reported in a late-breaking abstract (LBA2073) at the annual congress of the European Hematology Association.
“The fact that the response rate was astronomically high with a high MRD-negative status really allows this or this should be an opportunity for patients with relapsed/refractory disease,” he said in an interview.
Inotuzumab ozagamicin is an investigational anti-CD22 antibody conjugated to calicheamicin, an antitumor antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Previous phase II studies reported strong initial antitumor activity and safety with inotuzumab in relapsed or refractory ALL, Dr. DeAngelo, of the Dana Farber Cancer Institute in Boston, said.
The ongoing phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or SOC: either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once CR/CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients from the SOC arm who refused to start treatment.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Data for the co-primary endpoint of overall survival in all 326 patients are still blinded and not expected to mature until 2016, Dr. DeAngelo said.
CR/CRi analyses significantly favored inotuzumab in all stratification factors and baseline factors including peripheral blasts and CD22 expression. Cytogenetics are still being evaluated, but 11 of 14 (79%) patients with Philadelphia-positive karyotype achieved a CR or CRi, he said.
Median duration of remission among responders was 4.6 months in the inotuzumab arm and 3.1 months in the SOC arm (hazard ratio, 0.55; P = .016).
Safety assessed in 259 patients who received at least one dose of study drug showed similar incidence of grade 3 or higher adverse events in the inotuzumab and SOC arms (91% vs. 95%). There were 2 fatal events in the SOC arm and 4 in the inotuzumab arm: 2 veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS), both after poststudy transplant, 1 intestinal ischemia/septic shock, and 1 acute respiratory distress syndrome as a terminal event of pneumonia. In multivariate analysis, dual alkylator conditioning was the only significant covariate of VOD/SOS (P = .039), Dr. DeAngelo said.
An audience member chided the author for the short duration of remission, but session co-moderator Dr. Anthony Moorman, of Newcastle University, Newcastle upon Tyne, England, said it is not that concerning because of the aggressive nature of ALL.
“For all patients that have relapsed or refractory adult ALL, their responses are incredibly low. So any kind of complete remission is a major achievement in this patient population, especially if they are refractory or relapse after tyrosine kinase inhibitors or Philadelphia-positive,” he said in an interview.
“When you have an active agent that works with relapsed refractory disease, in this case leukemia, the goal is to move it up front,” Dr. DeAngelo told this publication.
Indeed, updated results presented at the meeting from M.D. Anderson Cancer Center of frontline inotuzumab added to low-intensity chemotherapy (Mini-hyper CVD) in elderly ALL patients were “provocative,” he added. CR rates reached 97% in the study, according to the abstract (S114).
VIENNA – The investigational agent inotuzumab ozagamicin more than doubled complete remission rates compared with standard therapy in relapsed or refractory acute lymphoblastic leukemia, preliminary results from the INO-VATE study show.
The co-primary endpoint of complete remission or CR with incomplete hematologic recovery (CRi) by independent review was achieved by 80.7% of patients treated with inotuzumab and 33.3% treated with standard of care (SOC) (P < .0001).
Significantly more CR/CRi responders treated with inotuzumab were minimal residual disease (MRD)-negative by multicolor flow cytometry (78.4% vs. 28.1%; P < .0001), Dr. Daniel DeAngelo reported in a late-breaking abstract (LBA2073) at the annual congress of the European Hematology Association.
“The fact that the response rate was astronomically high with a high MRD-negative status really allows this or this should be an opportunity for patients with relapsed/refractory disease,” he said in an interview.
Inotuzumab ozagamicin is an investigational anti-CD22 antibody conjugated to calicheamicin, an antitumor antibiotic. CD22 is expressed on the surface of about 90% of B-cell ALL cells.
Previous phase II studies reported strong initial antitumor activity and safety with inotuzumab in relapsed or refractory ALL, Dr. DeAngelo, of the Dana Farber Cancer Institute in Boston, said.
The ongoing phase III trial randomized 326 patients with relapsed/refractory CD22-positive ALL due for salvage 1 or 2 therapy to inotuzumab or SOC: either the FLAG regimen (fludarabine (Fludara)/cytarabine (Ara-C)/granulocyte colony-stimulating factor), Ara-C plus mitoxantrone (Novantrone), or high-dose Ara-C. The starting dose for inotuzumab was 1.8 mg/m2/cycle and was reduced to 1.5 mg/m2/cycle once CR/CRi was achieved. Patients were stratified by duration of first remission, salvage 1 or 2, and age.
The first 218 randomized patients were included in the intention-to-treat CR/CRi analysis, which was modified after excluding 13 patients from the SOC arm who refused to start treatment.
The patients’ median age was 47 years (ranging up to 79 years), two-thirds were salvage 1, and more than half had a remission duration of less than 12 months, an adverse prognostic feature.
Data for the co-primary endpoint of overall survival in all 326 patients are still blinded and not expected to mature until 2016, Dr. DeAngelo said.
CR/CRi analyses significantly favored inotuzumab in all stratification factors and baseline factors including peripheral blasts and CD22 expression. Cytogenetics are still being evaluated, but 11 of 14 (79%) patients with Philadelphia-positive karyotype achieved a CR or CRi, he said.
Median duration of remission among responders was 4.6 months in the inotuzumab arm and 3.1 months in the SOC arm (hazard ratio, 0.55; P = .016).
Safety assessed in 259 patients who received at least one dose of study drug showed similar incidence of grade 3 or higher adverse events in the inotuzumab and SOC arms (91% vs. 95%). There were 2 fatal events in the SOC arm and 4 in the inotuzumab arm: 2 veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS), both after poststudy transplant, 1 intestinal ischemia/septic shock, and 1 acute respiratory distress syndrome as a terminal event of pneumonia. In multivariate analysis, dual alkylator conditioning was the only significant covariate of VOD/SOS (P = .039), Dr. DeAngelo said.
An audience member chided the author for the short duration of remission, but session co-moderator Dr. Anthony Moorman, of Newcastle University, Newcastle upon Tyne, England, said it is not that concerning because of the aggressive nature of ALL.
“For all patients that have relapsed or refractory adult ALL, their responses are incredibly low. So any kind of complete remission is a major achievement in this patient population, especially if they are refractory or relapse after tyrosine kinase inhibitors or Philadelphia-positive,” he said in an interview.
“When you have an active agent that works with relapsed refractory disease, in this case leukemia, the goal is to move it up front,” Dr. DeAngelo told this publication.
Indeed, updated results presented at the meeting from M.D. Anderson Cancer Center of frontline inotuzumab added to low-intensity chemotherapy (Mini-hyper CVD) in elderly ALL patients were “provocative,” he added. CR rates reached 97% in the study, according to the abstract (S114).
AT THE EHA CONGRESS
Key clinical point: Inotuzumab ozagamicin shows promise as a new treatment option for relapsed or refractory acute lymphoblastic leukemia.
Major finding: The rate of complete remission or CR with incomplete hematologic recovery was 80.7% with inotuzumab vs. 28.1% with standard of care (P < .0001).
Data source: Randomized, phase III study in the first 218 of 326 patients.
Disclosures: Pfizer sponsored the study and funded editorial assistance supplied by Complete Heathcare Communications. Dr. De Angelo reported research support from Sigma Tau and consulting for Novartis, Sigma Tau, Bristol-Myers Squibb, Amgen, and Pfizer.
EHA: Dasatinib gets early edge over imatinib in CML
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
AT THE EHA CONGRESS
Key clinical point: Dasatinib provides more molecular responses than imatinib, but no survival advantage at 2 years in the first-line treatment of chronic-phase chronic myeloid leukemia.
Major finding: More patients receiving dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 6%; P = .02).
Data source: Randomized, phase III trial in 814 patients with newly diagnosed chronic myeloid leukemia in chronic phase.
Disclosures: Bristol-Myers Squibb sponsored the study. Dr. O’Brien reported honoraria and research funding from Ariad Pharmaceuticals, Bristol-Myers Squibb, Novartis, and Pfizer.
Viral protein protects EBV-infected B cells
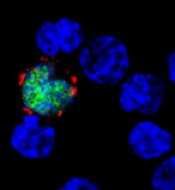
expresses the ligand (red)
that activates NKG2D, while
uninfected cells (blue) do not
Benjamin Chaigne-Delalande
A study published in PLOS Pathogens sheds new light on why the immune system cannot eliminate Epstein-Barr virus (EBV) or the risk of cancer associated with the virus.
Researchers investigated the immune system’s response against EBV, focusing on the role of LMP2A.
This viral protein is present in latently infected B cells and in many EBV-associated cancers, which have somehow escaped detection and elimination by the immune system.
Andreas Moosmann, PhD, of the Helmholtz-Zentrum in Munich, Germany, and his colleagues studied an engineered EBV virus that cannot make LMP2A and compared this mutant virus with the normal one.
The researchers infected human B cells with normal and LMP2A-deficient EBV. Because EBV transforms these cells, the team was able to examine lymphoblastic cell lines that contained either virus.
They found that LMP2A counteracts the recognition of EBV-infected B cells by EBV-specific, CD8+ killer T cells. In contrast, EBV-transformed cells without LMP2A are more efficiently identified, and the T cells’ ability to recognize and kill the EBV-infected B cells is enhanced.
The researchers examined the mechanism underlying the LMP2A-mediated evasion and found several ways in which it interferes with the recognition of EBV-infected cells.
First, LMP2A reduced the levels of several EBV proteins whose fragments are recognized by CD8+ T cells on the surface of the cell targeted for killing.
Second, LMP2A disturbs the expression of cellular molecules on infected B cells that interact with NKG2D, a host molecule on the surface of CD8+ T cells that aids their activation, thereby weakening the immune response against EBV-infected cells.
The researchers said these results suggest a functional immunomodulatory effect for the EBV protein LMP2A and show that LMP2A mediates the partial escape of infected B cells from recognition by CD8+ T cells.
The team also said similar immune evasion mechanisms may operate in different types of LMP2A-expressing cancers caused by EBV. ![]()

expresses the ligand (red)
that activates NKG2D, while
uninfected cells (blue) do not
Benjamin Chaigne-Delalande
A study published in PLOS Pathogens sheds new light on why the immune system cannot eliminate Epstein-Barr virus (EBV) or the risk of cancer associated with the virus.
Researchers investigated the immune system’s response against EBV, focusing on the role of LMP2A.
This viral protein is present in latently infected B cells and in many EBV-associated cancers, which have somehow escaped detection and elimination by the immune system.
Andreas Moosmann, PhD, of the Helmholtz-Zentrum in Munich, Germany, and his colleagues studied an engineered EBV virus that cannot make LMP2A and compared this mutant virus with the normal one.
The researchers infected human B cells with normal and LMP2A-deficient EBV. Because EBV transforms these cells, the team was able to examine lymphoblastic cell lines that contained either virus.
They found that LMP2A counteracts the recognition of EBV-infected B cells by EBV-specific, CD8+ killer T cells. In contrast, EBV-transformed cells without LMP2A are more efficiently identified, and the T cells’ ability to recognize and kill the EBV-infected B cells is enhanced.
The researchers examined the mechanism underlying the LMP2A-mediated evasion and found several ways in which it interferes with the recognition of EBV-infected cells.
First, LMP2A reduced the levels of several EBV proteins whose fragments are recognized by CD8+ T cells on the surface of the cell targeted for killing.
Second, LMP2A disturbs the expression of cellular molecules on infected B cells that interact with NKG2D, a host molecule on the surface of CD8+ T cells that aids their activation, thereby weakening the immune response against EBV-infected cells.
The researchers said these results suggest a functional immunomodulatory effect for the EBV protein LMP2A and show that LMP2A mediates the partial escape of infected B cells from recognition by CD8+ T cells.
The team also said similar immune evasion mechanisms may operate in different types of LMP2A-expressing cancers caused by EBV. ![]()

expresses the ligand (red)
that activates NKG2D, while
uninfected cells (blue) do not
Benjamin Chaigne-Delalande
A study published in PLOS Pathogens sheds new light on why the immune system cannot eliminate Epstein-Barr virus (EBV) or the risk of cancer associated with the virus.
Researchers investigated the immune system’s response against EBV, focusing on the role of LMP2A.
This viral protein is present in latently infected B cells and in many EBV-associated cancers, which have somehow escaped detection and elimination by the immune system.
Andreas Moosmann, PhD, of the Helmholtz-Zentrum in Munich, Germany, and his colleagues studied an engineered EBV virus that cannot make LMP2A and compared this mutant virus with the normal one.
The researchers infected human B cells with normal and LMP2A-deficient EBV. Because EBV transforms these cells, the team was able to examine lymphoblastic cell lines that contained either virus.
They found that LMP2A counteracts the recognition of EBV-infected B cells by EBV-specific, CD8+ killer T cells. In contrast, EBV-transformed cells without LMP2A are more efficiently identified, and the T cells’ ability to recognize and kill the EBV-infected B cells is enhanced.
The researchers examined the mechanism underlying the LMP2A-mediated evasion and found several ways in which it interferes with the recognition of EBV-infected cells.
First, LMP2A reduced the levels of several EBV proteins whose fragments are recognized by CD8+ T cells on the surface of the cell targeted for killing.
Second, LMP2A disturbs the expression of cellular molecules on infected B cells that interact with NKG2D, a host molecule on the surface of CD8+ T cells that aids their activation, thereby weakening the immune response against EBV-infected cells.
The researchers said these results suggest a functional immunomodulatory effect for the EBV protein LMP2A and show that LMP2A mediates the partial escape of infected B cells from recognition by CD8+ T cells.
The team also said similar immune evasion mechanisms may operate in different types of LMP2A-expressing cancers caused by EBV. ![]()
DDW: Early ERCP reduces LOS, costs of acute pancreatitis without cholangitis
WASHINGTON – When it’s given early in the course of treatment for patients who have acute pancreatitis and biliary obstruction without cholangitis, endoscopic retrograde cholangiopancreatography (ECRP) is associated with shorter lengths of stay, reductions in infectious complications, and substantially lower costs.
In more than 10,000 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction with cholangitis, ECRP performed on the day of admission or the next day was associated with a significantly lower risk for septicemia than ECRP performed after the first full day of hospitalization, and hospitalization costs were nearly $20,000 lower when the procedure was done early in the course of care, reported Dr. Raxitkimar Jinjuvadia from the Henry Ford Hospital in Detroit.
“Even though the inpatient mortality did not differ among the early and late groups, early ERCP has significant other benefits in reducing health care resources utilization,” he said at the annual Digestive Disease Week.
The study’s use of a large, nationally representative hospital sample supports and adds weight to current guidelines on the use of ERCP from the American Society for Gastrointestinal Endoscopy (ASGE).
“We suggest that early ERCP should be encouraged for patients with acute biliary pancreatitis with biliary obstruction and without cholangitis,” he stated.
The investigators took a retrospective stroll through data from the National Inpatient Sample (NIS) database for the year 2011. From among approximately 8 million hospitalizations in about 1000 hospitals in the United States, they identified patients who presented with acute pancreatitis, choledocholithiasis, biliary obstruction, and cholangitis, as determined by International Classification of Disease, 9th Revision (ICD-9) codes.
The cohort included 10,364 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction without cholangitis. The mean age of patients was 57.2 years, 66.4% were white and 62.2% were female. In all, 58.9% of patients underwent ERCP at some point during their hospitalizations, with 48.6% receiving it early (day 0 or day 1).
Patients who had ERCP had a significantly lower rate of inpatient deaths compared with those who did not have the procedure (0.5% vs, 1.7%, P < .001), but the timing of the procedure, the primary endpoint, did not make a difference in in-hospital mortality.
However, when the authors looked at secondary outcomes, they found that early ERCP was associated with significantly lower rates of septicemia (4.0% vs 7.2%, P < .001), shorter mean length of stay (5.2 vs. 8.0 days, P < .001) and lower costs ($52,400 vs $71,736, P <. 001).
In multivariable modeling adjusted for age, sex, race and Elixhauser comorbidities, independent risk factors for in-hospital mortality were age (adjusted odds ratio [aOR], 1.04, 95% confidence interval [CI] 1.03-1.06), Elixhauser comorbidities (aOR 1.17, 95% CI, 1.06-1.29), ERCP during hospitalization (aOR 0.30, 95% CI 0.19-0.47), and septicemia (aOR 13.5, 95% CI, 8.75-20.75).
As noted before, early ERCP was not an independent risk factor for inpatient mortality.
Dr. Jinjuvadia said that the study was limited by potential biases related to ICD-9 coding; the lack of lab values, imaging, or treatment data; and the fact that the database does not include data from Veterans Affairs hospitals.
WASHINGTON – When it’s given early in the course of treatment for patients who have acute pancreatitis and biliary obstruction without cholangitis, endoscopic retrograde cholangiopancreatography (ECRP) is associated with shorter lengths of stay, reductions in infectious complications, and substantially lower costs.
In more than 10,000 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction with cholangitis, ECRP performed on the day of admission or the next day was associated with a significantly lower risk for septicemia than ECRP performed after the first full day of hospitalization, and hospitalization costs were nearly $20,000 lower when the procedure was done early in the course of care, reported Dr. Raxitkimar Jinjuvadia from the Henry Ford Hospital in Detroit.
“Even though the inpatient mortality did not differ among the early and late groups, early ERCP has significant other benefits in reducing health care resources utilization,” he said at the annual Digestive Disease Week.
The study’s use of a large, nationally representative hospital sample supports and adds weight to current guidelines on the use of ERCP from the American Society for Gastrointestinal Endoscopy (ASGE).
“We suggest that early ERCP should be encouraged for patients with acute biliary pancreatitis with biliary obstruction and without cholangitis,” he stated.
The investigators took a retrospective stroll through data from the National Inpatient Sample (NIS) database for the year 2011. From among approximately 8 million hospitalizations in about 1000 hospitals in the United States, they identified patients who presented with acute pancreatitis, choledocholithiasis, biliary obstruction, and cholangitis, as determined by International Classification of Disease, 9th Revision (ICD-9) codes.
The cohort included 10,364 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction without cholangitis. The mean age of patients was 57.2 years, 66.4% were white and 62.2% were female. In all, 58.9% of patients underwent ERCP at some point during their hospitalizations, with 48.6% receiving it early (day 0 or day 1).
Patients who had ERCP had a significantly lower rate of inpatient deaths compared with those who did not have the procedure (0.5% vs, 1.7%, P < .001), but the timing of the procedure, the primary endpoint, did not make a difference in in-hospital mortality.
However, when the authors looked at secondary outcomes, they found that early ERCP was associated with significantly lower rates of septicemia (4.0% vs 7.2%, P < .001), shorter mean length of stay (5.2 vs. 8.0 days, P < .001) and lower costs ($52,400 vs $71,736, P <. 001).
In multivariable modeling adjusted for age, sex, race and Elixhauser comorbidities, independent risk factors for in-hospital mortality were age (adjusted odds ratio [aOR], 1.04, 95% confidence interval [CI] 1.03-1.06), Elixhauser comorbidities (aOR 1.17, 95% CI, 1.06-1.29), ERCP during hospitalization (aOR 0.30, 95% CI 0.19-0.47), and septicemia (aOR 13.5, 95% CI, 8.75-20.75).
As noted before, early ERCP was not an independent risk factor for inpatient mortality.
Dr. Jinjuvadia said that the study was limited by potential biases related to ICD-9 coding; the lack of lab values, imaging, or treatment data; and the fact that the database does not include data from Veterans Affairs hospitals.
WASHINGTON – When it’s given early in the course of treatment for patients who have acute pancreatitis and biliary obstruction without cholangitis, endoscopic retrograde cholangiopancreatography (ECRP) is associated with shorter lengths of stay, reductions in infectious complications, and substantially lower costs.
In more than 10,000 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction with cholangitis, ECRP performed on the day of admission or the next day was associated with a significantly lower risk for septicemia than ECRP performed after the first full day of hospitalization, and hospitalization costs were nearly $20,000 lower when the procedure was done early in the course of care, reported Dr. Raxitkimar Jinjuvadia from the Henry Ford Hospital in Detroit.
“Even though the inpatient mortality did not differ among the early and late groups, early ERCP has significant other benefits in reducing health care resources utilization,” he said at the annual Digestive Disease Week.
The study’s use of a large, nationally representative hospital sample supports and adds weight to current guidelines on the use of ERCP from the American Society for Gastrointestinal Endoscopy (ASGE).
“We suggest that early ERCP should be encouraged for patients with acute biliary pancreatitis with biliary obstruction and without cholangitis,” he stated.
The investigators took a retrospective stroll through data from the National Inpatient Sample (NIS) database for the year 2011. From among approximately 8 million hospitalizations in about 1000 hospitals in the United States, they identified patients who presented with acute pancreatitis, choledocholithiasis, biliary obstruction, and cholangitis, as determined by International Classification of Disease, 9th Revision (ICD-9) codes.
The cohort included 10,364 hospitalizations related to acute pancreatitis with choledocholithiasis/biliary obstruction without cholangitis. The mean age of patients was 57.2 years, 66.4% were white and 62.2% were female. In all, 58.9% of patients underwent ERCP at some point during their hospitalizations, with 48.6% receiving it early (day 0 or day 1).
Patients who had ERCP had a significantly lower rate of inpatient deaths compared with those who did not have the procedure (0.5% vs, 1.7%, P < .001), but the timing of the procedure, the primary endpoint, did not make a difference in in-hospital mortality.
However, when the authors looked at secondary outcomes, they found that early ERCP was associated with significantly lower rates of septicemia (4.0% vs 7.2%, P < .001), shorter mean length of stay (5.2 vs. 8.0 days, P < .001) and lower costs ($52,400 vs $71,736, P <. 001).
In multivariable modeling adjusted for age, sex, race and Elixhauser comorbidities, independent risk factors for in-hospital mortality were age (adjusted odds ratio [aOR], 1.04, 95% confidence interval [CI] 1.03-1.06), Elixhauser comorbidities (aOR 1.17, 95% CI, 1.06-1.29), ERCP during hospitalization (aOR 0.30, 95% CI 0.19-0.47), and septicemia (aOR 13.5, 95% CI, 8.75-20.75).
As noted before, early ERCP was not an independent risk factor for inpatient mortality.
Dr. Jinjuvadia said that the study was limited by potential biases related to ICD-9 coding; the lack of lab values, imaging, or treatment data; and the fact that the database does not include data from Veterans Affairs hospitals.
AT DDW® 2015
Key clinical point: ECRP early in the course of hospitalization reduced adverse events, length of stay, and costs.
Major finding: For patients with acute pancreatitis with biliary obstruction without cholangitis, early ERCP was associated with a 4.0% rate of septicemia, compared with 7.2% for ERCP after the first day.
Data source: Retrospective review of inpatient data on 10,364 hospitalizations.
Disclosures: The study funding source was not disclosed. Dr. Jinjuvadia reported having no relevant disclosures.
Panobinostat combos can treat rel/ref MM

©ASCO/Rodney White
CHICAGO—Combination regimens including the histone deacetylase inhibitor panobinostat can produce durable responses and prolong progression-free survival (PFS) in patients with relapsed/refractory multiple myeloma (MM), according to research presented at the 2015 ASCO Annual Meeting.
In a phase 2 trial, panobinostat plus lenalidomide and dexamethasone produced durable responses, even in high-risk, lenalidomide-refractory MM patients.
In a phase 3 trial, panobinostat in combination with bortezomib and dexamethasone led to a 7.8-month improvement in median PFS over placebo-bortezomib-dexamethasone in patients with relapsed or relapsed and refractory MM who had received 2 or more prior regimens.
Both studies were sponsored by Novartis, the company developing panobinostat.
PANORAMA-1 substudy
PANORAMA-1 was a phase 3, randomized, double-blind, placebo-controlled trial of 768 MM patients. Overall, panobinostat in combination with bortezomib and dexamethasone led to a clinically relevant and statistically significant increase in PFS of about 4 months compared to placebo-bortezomib-dexamethasone.
At ASCO, Jesús San Miguel, MD, of Clínica Universidad de Navarra in Pamplona, Spain, presented the results of an exploratory analysis of 147 patients in this trial (abstract 8526*).
The patients had relapsed or relapsed and refractory MM and had received 2 or more prior regimens, including bortezomib and an immunomodulatory agent (IMiD).
Disease and treatment characteristics were as follows:
| Panobinostat
(n=73) |
Placebo (n=74) | |
| Disease
characteristics, n (%) |
||
| Relapsed | 39 (53) | 30 (41) |
| Relapsed/refractory | 34 (47) | 43 (58) |
| Prior
therapies, n (%) |
||
| Bortezomib | 73 (100) | 74 (100) |
| Lenalidomide | 28 (38) | 37 (50) |
| Thalidomide | 63 (86) | 50 (68) |
| Bortezomib
+ lenalidomide |
28 (38) | 37 (50) |
| Bortezomib
+ dexamethasone |
69 (95) | 74 (100) |
| Prior
autologous transplant, n (%) |
54 (74) | 47 (64) |
| Median
prior lines of therapy (range) |
3 (2-4) | 3 (2-3) |
The median PFS was 12.5 months in the panobinostat arm, compared to 4.7 months in the placebo arm. Treatment with panobinostat also led to an increase in complete/near complete response rates (21.9% vs 8.1%) and overall response rate (58.9% vs 39.2%).
Common grade 3/4 non-hematologic adverse events in the panobinostat arm and placebo arm, respectively, included diarrhea (33.3% vs 15.1%), asthenia/fatigue (26.4% vs 13.7%), and peripheral neuropathy (16.7% vs 6.8%).
The most common grade 3/4 hematologic abnormalities in the panobinostat arm and placebo arm, respectively, were thrombocytopenia (68.1% vs 44.4%), lymphopenia (48.6% vs 49.3%), and neutropenia (40.3% vs 16.4%).
The percentage of on-treatment deaths was similar between the treatment arms (6.9% vs 6.8%).
“These data provide physicians with a better understanding of the clinical use of panobinostat, an HDAC inhibitor, a promising new drug class for this difficult-to-treat patient population with a high unmet need,” Dr San Miguel said.
Phase 2 trial
Ajai Chari, MD, of Mount Sinai Medical Center in New York, presented the results of a phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in patients with relapsed/refractory MM (abstract 8528*).
There were 20 evaluable patients with a median age of 64 (range, 51-75). They had received a median of 3 prior therapies (range, 1-10). Prior regimens were as follows:
| Prior
therapy |
Exposed/Refractory, n (%) |
| Dexamethasone | 20 (100)/9
(45) |
| Thalidomide | 6 (30)/2
(10) |
| Lenalidomide |
20 (100)/15 (75) |
| Pomalidomide | 7 (35)/7
(35) |
| Bortezomib | 20 (100)/9
(45) |
| Carfilzomib | 6 (30)/6
(30) |
| Autologous
transplant |
15 (75) |
For this study, patients received panobinostat (20 mg on days 1, 3, 5, 15, 17, and 19), lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, and 15).
The overall response rate was 45%. This included 1 complete response, 3 very good partial responses, 5 partial responses, and 8 minimal responses. Two patients had stable disease, and 1 progressed.
Among lenalidomide-refractory patients (n=16), the overall response rate was 38%. This included 3 very good partial responses, 3 partial responses, and 7 minimal responses. Two patients had stable disease, and 1 progressed.
The median PFS was 6.5 months overall and among lenalidomide-refractory patients.
Grade 3/4 toxicities were primarily hematologic, including neutropenia (55%), thrombocytopenia (40%), and anemia (5%). Grade 3/4 non-hematologic adverse events included infections (n=4), diarrhea (n=3), pulmonary emboli (n=2), neck pain (n=1), QTc prolongation (n=1), fatigue (n=1), and weight loss (n=1).
“In relapsed/refractory MM patients, panobinostat in combination with lenalidomide and dexamethasone demonstrated durable responses comparable to other recently approved agents, even in lenalidomide-refractory patients with high-risk molecular findings,” Dr Chari said.
“In notable contrast to PANORAMA-1 results, this completely oral regimen is well-tolerated, with no grade 3/4 [gastrointestinal] toxicities and primarily expected hematologic toxicities.” ![]()
*Information in the abstract differs from that presented at the meeting.

©ASCO/Rodney White
CHICAGO—Combination regimens including the histone deacetylase inhibitor panobinostat can produce durable responses and prolong progression-free survival (PFS) in patients with relapsed/refractory multiple myeloma (MM), according to research presented at the 2015 ASCO Annual Meeting.
In a phase 2 trial, panobinostat plus lenalidomide and dexamethasone produced durable responses, even in high-risk, lenalidomide-refractory MM patients.
In a phase 3 trial, panobinostat in combination with bortezomib and dexamethasone led to a 7.8-month improvement in median PFS over placebo-bortezomib-dexamethasone in patients with relapsed or relapsed and refractory MM who had received 2 or more prior regimens.
Both studies were sponsored by Novartis, the company developing panobinostat.
PANORAMA-1 substudy
PANORAMA-1 was a phase 3, randomized, double-blind, placebo-controlled trial of 768 MM patients. Overall, panobinostat in combination with bortezomib and dexamethasone led to a clinically relevant and statistically significant increase in PFS of about 4 months compared to placebo-bortezomib-dexamethasone.
At ASCO, Jesús San Miguel, MD, of Clínica Universidad de Navarra in Pamplona, Spain, presented the results of an exploratory analysis of 147 patients in this trial (abstract 8526*).
The patients had relapsed or relapsed and refractory MM and had received 2 or more prior regimens, including bortezomib and an immunomodulatory agent (IMiD).
Disease and treatment characteristics were as follows:
| Panobinostat
(n=73) |
Placebo (n=74) | |
| Disease
characteristics, n (%) |
||
| Relapsed | 39 (53) | 30 (41) |
| Relapsed/refractory | 34 (47) | 43 (58) |
| Prior
therapies, n (%) |
||
| Bortezomib | 73 (100) | 74 (100) |
| Lenalidomide | 28 (38) | 37 (50) |
| Thalidomide | 63 (86) | 50 (68) |
| Bortezomib
+ lenalidomide |
28 (38) | 37 (50) |
| Bortezomib
+ dexamethasone |
69 (95) | 74 (100) |
| Prior
autologous transplant, n (%) |
54 (74) | 47 (64) |
| Median
prior lines of therapy (range) |
3 (2-4) | 3 (2-3) |
The median PFS was 12.5 months in the panobinostat arm, compared to 4.7 months in the placebo arm. Treatment with panobinostat also led to an increase in complete/near complete response rates (21.9% vs 8.1%) and overall response rate (58.9% vs 39.2%).
Common grade 3/4 non-hematologic adverse events in the panobinostat arm and placebo arm, respectively, included diarrhea (33.3% vs 15.1%), asthenia/fatigue (26.4% vs 13.7%), and peripheral neuropathy (16.7% vs 6.8%).
The most common grade 3/4 hematologic abnormalities in the panobinostat arm and placebo arm, respectively, were thrombocytopenia (68.1% vs 44.4%), lymphopenia (48.6% vs 49.3%), and neutropenia (40.3% vs 16.4%).
The percentage of on-treatment deaths was similar between the treatment arms (6.9% vs 6.8%).
“These data provide physicians with a better understanding of the clinical use of panobinostat, an HDAC inhibitor, a promising new drug class for this difficult-to-treat patient population with a high unmet need,” Dr San Miguel said.
Phase 2 trial
Ajai Chari, MD, of Mount Sinai Medical Center in New York, presented the results of a phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in patients with relapsed/refractory MM (abstract 8528*).
There were 20 evaluable patients with a median age of 64 (range, 51-75). They had received a median of 3 prior therapies (range, 1-10). Prior regimens were as follows:
| Prior
therapy |
Exposed/Refractory, n (%) |
| Dexamethasone | 20 (100)/9
(45) |
| Thalidomide | 6 (30)/2
(10) |
| Lenalidomide |
20 (100)/15 (75) |
| Pomalidomide | 7 (35)/7
(35) |
| Bortezomib | 20 (100)/9
(45) |
| Carfilzomib | 6 (30)/6
(30) |
| Autologous
transplant |
15 (75) |
For this study, patients received panobinostat (20 mg on days 1, 3, 5, 15, 17, and 19), lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, and 15).
The overall response rate was 45%. This included 1 complete response, 3 very good partial responses, 5 partial responses, and 8 minimal responses. Two patients had stable disease, and 1 progressed.
Among lenalidomide-refractory patients (n=16), the overall response rate was 38%. This included 3 very good partial responses, 3 partial responses, and 7 minimal responses. Two patients had stable disease, and 1 progressed.
The median PFS was 6.5 months overall and among lenalidomide-refractory patients.
Grade 3/4 toxicities were primarily hematologic, including neutropenia (55%), thrombocytopenia (40%), and anemia (5%). Grade 3/4 non-hematologic adverse events included infections (n=4), diarrhea (n=3), pulmonary emboli (n=2), neck pain (n=1), QTc prolongation (n=1), fatigue (n=1), and weight loss (n=1).
“In relapsed/refractory MM patients, panobinostat in combination with lenalidomide and dexamethasone demonstrated durable responses comparable to other recently approved agents, even in lenalidomide-refractory patients with high-risk molecular findings,” Dr Chari said.
“In notable contrast to PANORAMA-1 results, this completely oral regimen is well-tolerated, with no grade 3/4 [gastrointestinal] toxicities and primarily expected hematologic toxicities.” ![]()
*Information in the abstract differs from that presented at the meeting.

©ASCO/Rodney White
CHICAGO—Combination regimens including the histone deacetylase inhibitor panobinostat can produce durable responses and prolong progression-free survival (PFS) in patients with relapsed/refractory multiple myeloma (MM), according to research presented at the 2015 ASCO Annual Meeting.
In a phase 2 trial, panobinostat plus lenalidomide and dexamethasone produced durable responses, even in high-risk, lenalidomide-refractory MM patients.
In a phase 3 trial, panobinostat in combination with bortezomib and dexamethasone led to a 7.8-month improvement in median PFS over placebo-bortezomib-dexamethasone in patients with relapsed or relapsed and refractory MM who had received 2 or more prior regimens.
Both studies were sponsored by Novartis, the company developing panobinostat.
PANORAMA-1 substudy
PANORAMA-1 was a phase 3, randomized, double-blind, placebo-controlled trial of 768 MM patients. Overall, panobinostat in combination with bortezomib and dexamethasone led to a clinically relevant and statistically significant increase in PFS of about 4 months compared to placebo-bortezomib-dexamethasone.
At ASCO, Jesús San Miguel, MD, of Clínica Universidad de Navarra in Pamplona, Spain, presented the results of an exploratory analysis of 147 patients in this trial (abstract 8526*).
The patients had relapsed or relapsed and refractory MM and had received 2 or more prior regimens, including bortezomib and an immunomodulatory agent (IMiD).
Disease and treatment characteristics were as follows:
| Panobinostat
(n=73) |
Placebo (n=74) | |
| Disease
characteristics, n (%) |
||
| Relapsed | 39 (53) | 30 (41) |
| Relapsed/refractory | 34 (47) | 43 (58) |
| Prior
therapies, n (%) |
||
| Bortezomib | 73 (100) | 74 (100) |
| Lenalidomide | 28 (38) | 37 (50) |
| Thalidomide | 63 (86) | 50 (68) |
| Bortezomib
+ lenalidomide |
28 (38) | 37 (50) |
| Bortezomib
+ dexamethasone |
69 (95) | 74 (100) |
| Prior
autologous transplant, n (%) |
54 (74) | 47 (64) |
| Median
prior lines of therapy (range) |
3 (2-4) | 3 (2-3) |
The median PFS was 12.5 months in the panobinostat arm, compared to 4.7 months in the placebo arm. Treatment with panobinostat also led to an increase in complete/near complete response rates (21.9% vs 8.1%) and overall response rate (58.9% vs 39.2%).
Common grade 3/4 non-hematologic adverse events in the panobinostat arm and placebo arm, respectively, included diarrhea (33.3% vs 15.1%), asthenia/fatigue (26.4% vs 13.7%), and peripheral neuropathy (16.7% vs 6.8%).
The most common grade 3/4 hematologic abnormalities in the panobinostat arm and placebo arm, respectively, were thrombocytopenia (68.1% vs 44.4%), lymphopenia (48.6% vs 49.3%), and neutropenia (40.3% vs 16.4%).
The percentage of on-treatment deaths was similar between the treatment arms (6.9% vs 6.8%).
“These data provide physicians with a better understanding of the clinical use of panobinostat, an HDAC inhibitor, a promising new drug class for this difficult-to-treat patient population with a high unmet need,” Dr San Miguel said.
Phase 2 trial
Ajai Chari, MD, of Mount Sinai Medical Center in New York, presented the results of a phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in patients with relapsed/refractory MM (abstract 8528*).
There were 20 evaluable patients with a median age of 64 (range, 51-75). They had received a median of 3 prior therapies (range, 1-10). Prior regimens were as follows:
| Prior
therapy |
Exposed/Refractory, n (%) |
| Dexamethasone | 20 (100)/9
(45) |
| Thalidomide | 6 (30)/2
(10) |
| Lenalidomide |
20 (100)/15 (75) |
| Pomalidomide | 7 (35)/7
(35) |
| Bortezomib | 20 (100)/9
(45) |
| Carfilzomib | 6 (30)/6
(30) |
| Autologous
transplant |
15 (75) |
For this study, patients received panobinostat (20 mg on days 1, 3, 5, 15, 17, and 19), lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, and 15).
The overall response rate was 45%. This included 1 complete response, 3 very good partial responses, 5 partial responses, and 8 minimal responses. Two patients had stable disease, and 1 progressed.
Among lenalidomide-refractory patients (n=16), the overall response rate was 38%. This included 3 very good partial responses, 3 partial responses, and 7 minimal responses. Two patients had stable disease, and 1 progressed.
The median PFS was 6.5 months overall and among lenalidomide-refractory patients.
Grade 3/4 toxicities were primarily hematologic, including neutropenia (55%), thrombocytopenia (40%), and anemia (5%). Grade 3/4 non-hematologic adverse events included infections (n=4), diarrhea (n=3), pulmonary emboli (n=2), neck pain (n=1), QTc prolongation (n=1), fatigue (n=1), and weight loss (n=1).
“In relapsed/refractory MM patients, panobinostat in combination with lenalidomide and dexamethasone demonstrated durable responses comparable to other recently approved agents, even in lenalidomide-refractory patients with high-risk molecular findings,” Dr Chari said.
“In notable contrast to PANORAMA-1 results, this completely oral regimen is well-tolerated, with no grade 3/4 [gastrointestinal] toxicities and primarily expected hematologic toxicities.” ![]()
*Information in the abstract differs from that presented at the meeting.
OSA and Outcomes in Ward Patients
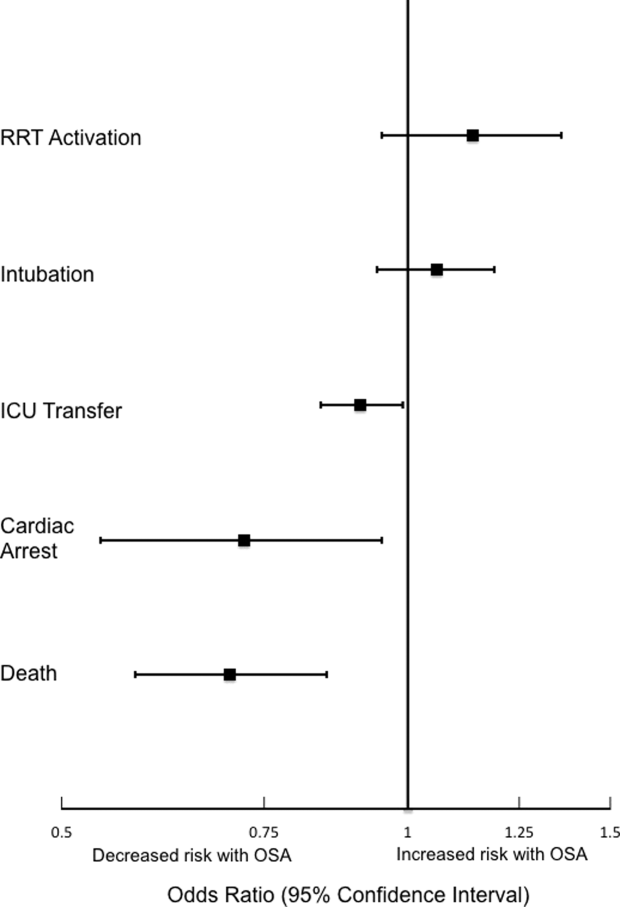
Obstructive sleep apnea (OSA) is an increasingly prevalent condition characterized by intermittent airway obstruction during sleep, which leads to hypoxemia, hypercapnia, and fragmented sleep. The current prevalence estimates of moderate to severe OSA (apnea‐hypopnea index 15, measured as events/hour) in middle‐aged adults are approximately 13% in men and 6% in women.[1] OSA is a well‐described independent risk factor for long‐term neurocognitive, cardiovascular, and cerebrovascular morbidity and mortality.[2, 3, 4, 5, 6]
Recent studies have also identified OSA as an independent risk factor for adverse perioperative outcomes, including endotracheal intubation, intensive care unit (ICU) transfer, and increased length of stay.[7, 8, 9, 10, 11] Paradoxically, despite an increase in the risk of complications, several of these studies did not find an association between in‐hospital death and OSA even after controlling for potential confounders.[9, 10, 11] Furthermore, a recent study of patients hospitalized for pneumonia reported increased rates of clinical deterioration and mechanical ventilation, but also lower odds of inpatient mortality in patients with OSA.[12]
These studies may have been limited by the absence of physiologic data, which prevented controlling for severity of illness. It is also unclear whether these previously described associations between OSA and adverse clinical outcomes hold true for general hospital inpatients. OSA may be worsened by medications frequently used in hospitals, such as narcotics and benzodiazepines. Opiate use contributes to both central and obstructive sleep apneas,[13, 14] and benzodiazepines are known to produce airway smooth muscle relaxation and can cause respiratory depression.[15] In fact, the use of benzodiazepines has been implicated in the unmasking of OSA in patients with previously undiagnosed sleep‐disordered breathing.[16] These findings suggest mechanisms by which OSA could contribute to an increased risk in hospital ward patients for rapid response team (RRT) activation, ICU transfer, cardiac arrest, and in‐hospital death.
The aim of this study was to determine the independent association between OSA and in‐hospital mortality in ward patients. We also aimed to investigate the association of OSA with clinical deterioration on the wards, while controlling for patient characteristics, initial physiology, and severity of illness.
MATERIALS AND METHODS
Setting and Study Population
This observational cohort study was performed at an academic tertiary care medical center with approximately 500 beds. Data were obtained from all adult patients hospitalized on the wards between November 1, 2008 and October 1, 2013. Our hospital has utilized an RRT, led by a critical care nurse and respiratory therapist with hospitalist and pharmacist consultation available upon request, since 2008. This team is separate from the team that responds to a cardiac arrest. Criteria for RRT activation include tachypnea, tachycardia, hypotension, and staff worry, but specific vital sign thresholds are not specified.
The study analyzed deidentified data from the hospital's Clinical Research Data Warehouse, which is maintained by the Center for Research Informatics at The University of Chicago. The study protocol was approved by the University of Chicago Institutional Review Board (IRB #16995A).
Data Collection
Patient age, sex, race, body mass index (BMI), and location prior to ward admission (ie, whether they were admitted from the emergency department, transferred from the ICU, or directly admitted from clinic or home) were collected. Patients who underwent surgery during their admission were identified using the hospital's admission‐transfer‐discharge database. In addition, routinely collected vital signs (eg, respiratory rate, blood pressure, heart rate) were obtained from the electronic health record (Epic, Verona, WI). To determine severity of illness, the first set of vital signs measured on hospital presentation were utilized to calculate the cardiac arrest risk triage (CART) score, a vital‐signbased early warning score we previously developed and validated for predicting adverse events in our population.[17] The CART score ranges from 0 to 57, with points assigned for abnormalities in respiratory rate, heart rate, diastolic blood pressure, and age. If any vital sign was missing, the next available measurement was pulled into the set. If any vital sign remained missing after this change, the median value for that particular location (ie, wards, ICU, or emergency department) was imputed as previously described.[18, 19]
Patients with OSA were identified by the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes using inpatient and outpatient medical records: 278.03, 327.20, 327.23, 327.29, 780.51, 780.53, and 780.57 (Table 1). Data on other patient comorbidities, including coronary artery disease, congestive heart failure, arrhythmias, uncomplicated and complicated diabetes mellitus, hypertension, and cerebrovascular disease were collected using specific ICD‐9‐CM codes from both inpatient and outpatient records. Information on insurance payer was also collected from the hospital's billing database. Insurance payers were grouped into the following categories: private payer, Medicare/Medicaid, and no insurance. Patients with both public and private payers were counted as being privately insured.
| Diagnosis Code | Description | % of Sleep Apnea Diagnosesa |
|---|---|---|
| ||
| 327.23 | Obstructive sleep apnea | 65.6 |
| 780.57 | Unspecified sleep apnea | 19.4 |
| 780.53 | Hypersomnia with sleep apnea, unspecified | 11.7 |
| 780.51 | Insomnia with sleep apnea, unspecified | 1.5 |
| 327.2 | Organic sleep apnea, unspecified | 0.2 |
| 278.03 | Obesity hypoventilation syndrome | 1.7 |
Outcomes
The primary outcome of the study was in‐hospital mortality. Secondary outcomes included length of stay, RRT activation, transfer to the ICU, endotracheal intubation, cardiac arrest (defined as a loss of pulse with attempted resuscitation) on the wards, and a composite outcome of RRT activation, ICU transfer, and death. Because cardiac arrests on the wards result either in death or ICU transfer following successful resuscitation, this variable was omitted from the composite outcome. Cardiac arrests were identified using a prospectively validated quality improvement database that has been described previously.[20] ICU transfer was identified using the hospital's admission‐transfer‐discharge database. Only the index cardiac arrest, intubation, RRT, or ICU transfer for each admission was used in the study, but more than 1 type of outcome could occur for each patient (eg, a patient who died following an unsuccessful resuscitation attempt would count as both a cardiac arrest and a death).
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and 2 statistics, as appropriate. Unadjusted logistic regression models were fit to estimate the change in odds of each adverse event and a composite outcome of any event for patient admissions with OSA compared to those without OSA. Adjusted logistic regression models were then fit for each outcome to control for patient characteristics (age, sex, BMI, insurance status, and individual comorbidities), location immediately prior to ward admission, and admission severity of illness (as measured by CART score). In the adjusted model, CART score, age, and number of comorbidities were entered linearly, with the addition of squared terms for age and CART score, as these variables showed nonlinear associations with the outcomes of interest. Race, surgical status, insurance payer, location prior to ward, and BMI (underweight, <18.5 kg/m2; normal weight, 18.524.9 kg/m2; overweight, 25.029.9 kg/m2; obese, 3039.9 kg/m2; and severely obese, (40 kg/m2) were modeled as categorical variables.
Given that an individual patient could experience multiple hospitalizations during the study period, we performed a sensitivity analysis of all adjusted and unadjusted models using a single randomly selected hospitalization for each unique patient. In addition, we performed a sensitivity analysis of all patients who were not admitted to the ICU prior to their ward stay. Finally, we performed subgroup analyses of all unadjusted and adjusted models for each BMI category and surgical status.
All tests of significance used a 2‐sided P value <0.05. Statistical analyses were completed using Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
During the study period, 93,676 patient admissions from 53,150 unique patients resulted in the occurrence of 1,069 RRT activations, 6,305 ICU transfers, and 1,239 in‐hospital deaths. Within our sample, 40,034 patients had at least 1 inpatient record and at least 1 outpatient record. OSA diagnosis was present in 5,625 patients (10.6% of the total sample), with 4,748 patients having an OSA diagnosis code entered during a hospitalization, 2,143 with an OSA diagnosis code entered during an outpatient encounter, and 877 with both inpatient and outpatient diagnosis codes. These patients identified as having OSA contributed 12,745 (13.6%) hospital admissions.
Patients with an OSA diagnosis were more likely to be older (median age 59 years [interquartile range 4968] vs 55 years [3868]), male (49% vs 42%), overweight or obese (88% vs 62%), and more likely to carry diagnoses of diabetes (53.8% vs 25.5%), hypertension (45.3% vs 18.2%), arrhythmias (44.4% vs 26.7%), coronary artery disease (46.8% vs 23.5%), heart failure (35.8% vs 13.5%), and cerebrovascular disease (13.5% vs 8.1%) than patients without an OSA diagnosis (all comparisons significant, P < 0.001) (Table 2).
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Age, y, median (IQR) | 59 (4968) | 55 (3868) | <0.001 |
| Female, n (%) | 6,514 (51%) | 47,202 (58%) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 4,205 (33%) | 30,119 (37%) | |
| Black/African American | 7,024 (55%) | 38,561 (48%) | |
| Asian | 561 (4.4%) | 3,419 (4.2%) | |
| American Indian or Native Alaskan | 20 (0.2%) | 113 (0.1%) | |
| More than 1 race | 127 (1%) | 843 (1%) | |
| Race unknown | 808 (6%) | 7,876 (10%) | |
| Insurance status, n (%) | <0.001 | ||
| Private | 4,484 (35%) | 32,467 (40%) | |
| Medicare/Medicaid | 8,201 (64%) | 42,208 (58%) | |
| Uninsured | 53 (0.4%) | 1,190 (1%) | |
| Unknown | 4 (<0.1%) | 16 (<0.1%) | |
| Location prior to wards, n (%) | <0.001 | ||
| ICU | 1,400 (11%) | 8,065 (10%) | |
| Emergency department | 4,633 (36%) | 25,170 (31%) | |
| Ambulatory admission | 6,712 (53%) | 47,696 (59%) | |
| Body mass index, kg/m2, n (%) | <0.001 | ||
| Normal (18.525) | 1,431 (11%) | 26,560 (33%) | |
| Underweight (<18.5) | 122 (1%) | 4,256 (5%) | |
| Overweight (2530) | 2,484 (20%) | 23,761 (29%) | |
| Obese (3040) | 4,959 (39%) | 19,132 (24%) | |
| Severely obese (40) | 3,745 (29%) | 7,171 (9%) | |
| Initial cardiac arrest risk triage score, median (IQR) | 4 (09) | 4 (09) | <0.001 |
| Underwent surgery, n (%) | 4,482 (35%) | 28,843 (36%) | 0.3 |
| Comorbidities | |||
| Number of comorbidities, median (IQR) | 2 (14) | 1 (02) | <0.001 |
| Arrhythmia | 5,659 (44%) | 21,581 (27%) | <0.001 |
| Diabetes mellitus | 6,855 (54%) | 20,641 (26%) | <0.001 |
| Hypertension | 5,777 (45%) | 14,728 (18%) | <0.001 |
| Coronary artery disease | 5,958 (47%) | 18,979 (23%) | <0.001 |
| Cerebrovascular accident | 1,725 (14%) | 6,556 (8%) | <0.001 |
| Congestive heart failure | 4,559 (36%) | 10,919 (13%) | <0.001 |
Complications and Adverse Outcomes
In the unadjusted analyses, the overall incidence of adverse outcomes was higher among patient admissions with a diagnosis of OSA compared to those without OSA (Table 3). Those with OSA were more likely to experience RRT activation (1.5% vs 1.1%), ICU transfer (8% vs 7%), and endotracheal intubation (3.9% vs 2.9%) than those without OSA diagnoses (P < 0.001 for all comparisons). There was no significant difference in the incidence of cardiac arrest between the 2 groups, nor was there a significant difference in length of stay. Unadjusted inpatient mortality for OSA patient admissions was lower than that for non‐OSA hospitalizations (1.1% vs 1.4%, P < 0.05). A diagnosis of OSA was associated with increased unadjusted odds for RRT activation (odds ratio [OR]: 1.36 [1.16‐1.59]) and ICU transfer (OR: 1.28 [1.20‐1.38]). However, after controlling for confounders, OSA was not associated with increased odds for RRT activation (OR: 1.14 [0.95‐1.36]) or intubation (OR: 1.06 [0.94‐1.19]), and was associated with slightly decreased odds for ICU transfer (OR: 0.91 [0.84‐0.99]) (Figure 1). Those with OSA had decreased adjusted odds of cardiac arrest (OR: 0.72 [0.55‐0.95]) compared to those without OSA. OSA was also associated with decreased odds of in‐hospital mortality before (OR: 0.83 [0.70‐0.99]) and after (OR: 0.70 [0.58‐0.85]) controlling for confounders.
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Outcomes, n (%) | |||
| Composite outcomea | 1,137 (9%) | 5,792 (7%) | <0.001 |
| In‐hospital death | 144 (1.1%) | 1,095 (1.4%) | 0.04 |
| Rapid response team call | 188 (1.5%) | 881 (1.1%) | <0.001 |
| ICU transfer | 1,045 (8%) | 5,260 (7%) | <0.001 |
| Cardiac arrest | 413 (0.5%) | 73 (0.6%) | 0.36 |
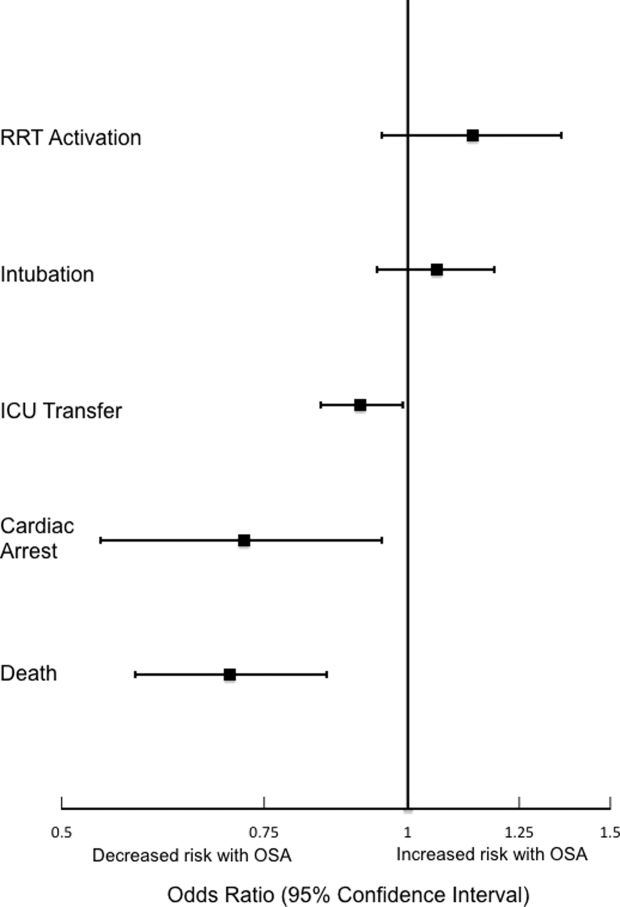
Sensitivity Analyses
The sensitivity analysis involving 1 randomly selected hospitalization per patient included a total of 53,150 patients. The results were similar to the main analysis, with adjusted odds of 1.01 (0.77‐1.32) for RRT activation, 0.86 (0.76‐0.96) for ICU transfer, and 0.69 (0.53‐0.89) for inpatient mortality. An additional sensitivity analysis included only patients who were not admitted to the ICU prior to their ward stay. This analysis included 84,211 hospitalizations and demonstrated similar findings, with adjusted odds of 0.70 for in‐hospital mortality (0.57‐0.87). Adjusted odds for RRT activation (OR: 1.12 [0.92‐1.37]) and ICU transfer (OR: 0.88 [0.81‐0.96] were also similar to the results of our main analysis.
Subgroup Analyses
Surgical and Nonsurgical Patients
Subgroup analyses of surgical versus nonsurgical patients (Figure 2) revealed similarly decreased adjusted odds of in‐hospital death for OSA patients in both groups (surgical OR: 0.69 [0.49‐0.97]; nonsurgical OR: 0.72 [0.58‐0.91]). Surgical patients with OSA diagnoses had decreased adjusted odds for ICU transfer (surgical OR: 0.82 [0.73‐0.92], but this finding was not seen in nonsurgical patients (OR: 1.03 [0.92‐1.15]).
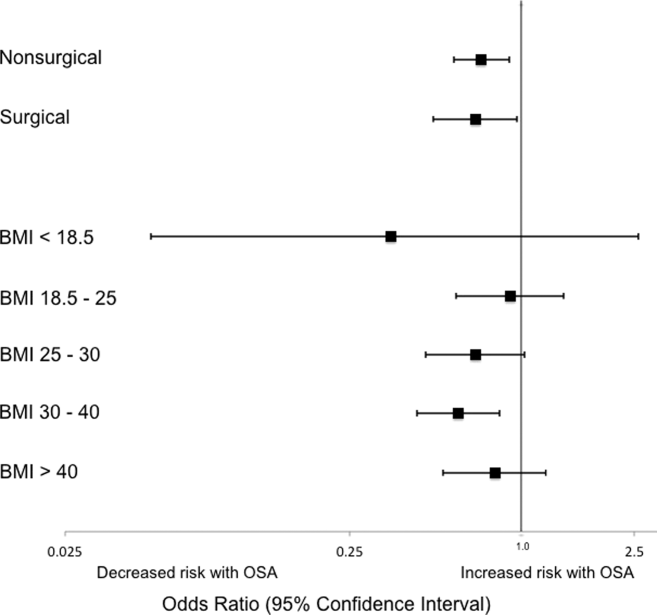
Patients Stratified by BMI
Examination across BMI categories (Figure 2) showed a significant decrease in adjusted odds of death for OSA patients with BMI 30 to 40 kg/m2 (OR: 0.60 [0.43‐0.84]). A nonsignificant decrease in adjusted odds of death was seen for OSA patients in all other groups. Adjusted odds ratios for the risk of RRT activation and ICU transfer in OSA patients within the different BMI categories were not statistically significant.
DISCUSSION
In this large observational single‐center cohort study, we found that OSA was associated with increased odds of adverse events, such as ICU transfers and RRT calls, but this risk was no longer present after adjusting for demographics, comorbidities, and presenting vital signs. Interestingly, we also found that patients with OSA had decreased adjusted odds for cardiac arrest and mortality. This mortality finding was robust to multiple sensitivity analyses and subgroup analyses. These results have significant implications for our understanding of the short‐term risks of sleep‐disordered breathing in hospitalized patients, and suggest the possibility that OSA is associated with a protective effect with regard to inpatient mortality.
Our findings are in line with other recent work in this area. In 2 large observational cohorts of surgical populations drawn from the nationally representative Nationwide Inpatient Sample administrative database, our group reported decreased odds of in‐hospital postoperative mortality in OSA patients.[10, 11] Using the same Nationwide Inpatient Sample, Lindenauer et al. showed that among inpatients hospitalized with pneumonia, OSA diagnosis was associated with increased rates of clinical deterioration but lower rates of inpatient mortality.[12] Although these 3 studies have identified decreased inpatient mortality among certain surgical populations and patients hospitalized with pneumonia, they are limited by using administrative databases that do not provide specific data on vital signs, presenting physiology, BMI, or race. Another important limitation of the Nationwide Inpatient Sample is the lack of any information on RRT activations and ICU transfers. Moreover, the database does not include information on outpatient diagnoses, which may have led to a significantly lower prevalence of OSA than expected in these studies. Despite the important methodological differences, our study corroborates this finding among a diverse cohort of hospitalized patients. Unlike these previous studies of postoperative patients or those hospitalized with pneumonia, we did not find an increased risk of adverse events associated with OSA after controlling for potential confounders.
The decreased mortality seen in OSA patients could be explained by these patients receiving more vigilant care, showing earlier signs of deterioration, or displaying more easily treatable forms of distress than patients without OSA. For example, earlier identification of deterioration could lead to earlier interventions, which could decrease inpatient mortality. In 2 studies of postsurgical patients,[10, 11] those with OSA diagnosis who developed respiratory failure were intubated earlier and received mechanical ventilation for a shorter period of time, suggesting that the cause of respiratory failure was rapidly reversible (eg, upper airway complications due to oversedation or excessive analgesia). However, we did not find increased adjusted odds of RRT activation or ICU transfer for OSA patients in our study, and so it is less likely that earlier recognition of decompensation occurred in our sample. In addition, our hospital did not have standardized practices for monitoring or managing OSA patients during the study period, which makes systematic early recognition of clinical deterioration among the OSA population in our study less likely.
Alternatively, there may be a true physiologic phenomenon providing a short‐term mortality benefit in those with OSA. It has been observed that patients with obesity (but without severe obesity) often have better outcomes after acute illness, whether by earlier or more frequent contact with medical care or heightened levels of metabolic reserve.[21, 22] However, our findings of decreased mortality for OSA patients remained even after controlling for BMI. An additional important possibility to consider is ischemic preconditioning, a well‐described phenomenon in which episodes of sublethal ischemia confer protection on tissues from subsequent ischemia/reperfusion damage.[23] Ischemic preconditioning has been demonstrated in models of cardiac and neural tissue[24, 25, 26] and has been shown to enhance stem cell survival by providing resistance to necrosis and lending functional benefits to heart, brain, and kidney models after transplantation.[25, 26, 27, 28, 29, 30, 31] The fundamentals of this concept may have applications in transplant and cardiac surgery,[32, 33] in the management of acute coronary syndromes and stroke,[32, 34, 35] and in athletic training and performance.[35, 36] Although OSA has been associated with long‐term cardiovascular morbidity and mortality,[2, 3, 4, 5, 6] the intermittent hypoxemia OSA patients experience could actually improve their ability to survive clinical deterioration in the short‐term (ie, during a hospitalization).
Limitations of our study include its conduction at a single center, which may prevent generalization to populations different than ours. Furthermore, during the study period, our hospital did not have formal guidelines or standardized management or monitoring practices for patients with OSA. Additionally, practices for managing OSA may vary across institutions. Therefore, our results may not be generalizable to hospitals with such protocols in place. However, as mentioned above, similar findings have been noted in studies using large, nationally representative administrative databases. In addition, we identified OSA via ICD‐9‐CM codes, which are likely insensitive for estimating the true prevalence of OSA in our sample. Despite this, our reported OSA prevalence of over 10% falls within the prevalence range reported in large epidemiological studies.[37, 38, 39] Finally, we did not have data on polysomnograms or treatment received for patients with OSA, so we do not know the severity of OSA or adequacy of treatment for these patients.
Notwithstanding our limitations, our study has several strengths. First, we included a large number of hospitalized patients across a diverse range of medical and surgical ward admissions, which increases the generalizability of our results. We also addressed potential confounders by including a large number of comorbidities and controlling for severity of presenting physiology with the CART score. The CART score, which contains physiologic variables such as respiratory rate, heart rate, and diastolic blood pressure, is an accurate predictor of cardiac arrest, ICU transfer, and in‐hospital mortality in our population.[40] Finally, we were able to obtain information about these diagnoses from outpatient as well as inpatient data.
In conclusion, we found that after adjustment for important confounders, OSA was associated with a decrease in hospital mortality and cardiac arrest but not with other adverse events on the wards. These results may suggest a protective benefit from OSA with regard to mortality, an advantage that could be explained by ischemic preconditioning or a higher level of care or vigilance not reflected by the number of RRT activations or ICU transfers experienced by these patients. Further research is needed to confirm these findings across other populations, to investigate the physiologic pathways by which OSA may produce these effects, and to examine the mechanisms by which treatment of OSA could influence these outcomes.
Acknowledgements
The authors thank Nicole Babuskow for administrative support, as well as Brian Furner and Timothy Holper for assistance with data acquisition.
Disclosures: Study concept and design: P.L., D.P.E, B.M., M.C.; acquisition of data: P.L.; analysis and interpretation of data: all authors; first drafting of the manuscript: P.L.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: P.L., F.Z., M.C.; obtained funding: D.P.E., M.C.; administrative, technical, and material support: F.Z., D.P.E.; study supervision: D.P.E, B.M., M.C.; data access and responsibility: P.L. and M.C. had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek and Dr. Edelson are both supported by career development awards from the National Heart, Lung, and Blood Institute (K08 HL121080 and K23 HL097157, respectively). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics.
- , , , , , . Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- , , , . Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053.
- , , , . Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384.
- , , , , , . Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041.
- , , , , . Obstructive sleep apnea and risk of cardiovascular events and all‐cause mortality: a decade‐long historical cohort study. PLoS Med. 2014;11(2):e1001599.
- , , , , , . Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085.
- , , , , . Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441.
- , , , , , . Meta‐analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906.
- , , , et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407–418.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23(11):1842–1851.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903–914.
- , , , , , . Prevalence, treatment and outcomes associated with obstructive sleep apnea among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038.
- , , , et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8(1):e54807.
- , , , , . Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250–254.
- , , , et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65–69.
- , . Effect of flurazepam on sleep‐disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73(2):239–243.
- , , , , , . Derivation of a cardiac arrest prediction model using ward vital signs*. Crit Care Med. 2012;40(7):2102–2108.
- , , , , . Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards*. Crit Care Med. 2014;42(4):841–848.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , , , . Predicting cardiac arrest on the wards: a nested case‐control study. Chest. 2012;141(5):1170–1176.
- , , , , , . Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(4):306–311.
- , , , et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012(8);125:796–803.
- , , . Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136.
- , , , . Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–931.
- , , , et al. Transplantation of hypoxia‐preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808.
- , , , et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8(5):e62703.
- , . Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22.
- , , , et al. Ischemic pre‐conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53(19):1814–1822.
- , , , et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301(2):C362–C372.
- , , , et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670.
- , , , , . Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–645.
- , , . Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565.
- , , . Novel adjunctive treatments of myocardial infarction. World J Cardiol. 2014;6(6):434–443.
- , . Hypoxic‐preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11(2):669–686.
- , , , , . Ischemic preconditioning improves oxygen saturation and attenuates hypoxic pulmonary vasoconstriction at high altitude. High Alt Med Biol. 2014;15(2):155–161.
- , , , et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43(7):1280–1286.
- , , , . Obstructive sleep apnea‐hypopnea and related clinical features in a population‐based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689.
- , , , , , . The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328(17):1230–1235.
- , , . Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239.
- , , . Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758–1765.
Obstructive sleep apnea (OSA) is an increasingly prevalent condition characterized by intermittent airway obstruction during sleep, which leads to hypoxemia, hypercapnia, and fragmented sleep. The current prevalence estimates of moderate to severe OSA (apnea‐hypopnea index 15, measured as events/hour) in middle‐aged adults are approximately 13% in men and 6% in women.[1] OSA is a well‐described independent risk factor for long‐term neurocognitive, cardiovascular, and cerebrovascular morbidity and mortality.[2, 3, 4, 5, 6]
Recent studies have also identified OSA as an independent risk factor for adverse perioperative outcomes, including endotracheal intubation, intensive care unit (ICU) transfer, and increased length of stay.[7, 8, 9, 10, 11] Paradoxically, despite an increase in the risk of complications, several of these studies did not find an association between in‐hospital death and OSA even after controlling for potential confounders.[9, 10, 11] Furthermore, a recent study of patients hospitalized for pneumonia reported increased rates of clinical deterioration and mechanical ventilation, but also lower odds of inpatient mortality in patients with OSA.[12]
These studies may have been limited by the absence of physiologic data, which prevented controlling for severity of illness. It is also unclear whether these previously described associations between OSA and adverse clinical outcomes hold true for general hospital inpatients. OSA may be worsened by medications frequently used in hospitals, such as narcotics and benzodiazepines. Opiate use contributes to both central and obstructive sleep apneas,[13, 14] and benzodiazepines are known to produce airway smooth muscle relaxation and can cause respiratory depression.[15] In fact, the use of benzodiazepines has been implicated in the unmasking of OSA in patients with previously undiagnosed sleep‐disordered breathing.[16] These findings suggest mechanisms by which OSA could contribute to an increased risk in hospital ward patients for rapid response team (RRT) activation, ICU transfer, cardiac arrest, and in‐hospital death.
The aim of this study was to determine the independent association between OSA and in‐hospital mortality in ward patients. We also aimed to investigate the association of OSA with clinical deterioration on the wards, while controlling for patient characteristics, initial physiology, and severity of illness.
MATERIALS AND METHODS
Setting and Study Population
This observational cohort study was performed at an academic tertiary care medical center with approximately 500 beds. Data were obtained from all adult patients hospitalized on the wards between November 1, 2008 and October 1, 2013. Our hospital has utilized an RRT, led by a critical care nurse and respiratory therapist with hospitalist and pharmacist consultation available upon request, since 2008. This team is separate from the team that responds to a cardiac arrest. Criteria for RRT activation include tachypnea, tachycardia, hypotension, and staff worry, but specific vital sign thresholds are not specified.
The study analyzed deidentified data from the hospital's Clinical Research Data Warehouse, which is maintained by the Center for Research Informatics at The University of Chicago. The study protocol was approved by the University of Chicago Institutional Review Board (IRB #16995A).
Data Collection
Patient age, sex, race, body mass index (BMI), and location prior to ward admission (ie, whether they were admitted from the emergency department, transferred from the ICU, or directly admitted from clinic or home) were collected. Patients who underwent surgery during their admission were identified using the hospital's admission‐transfer‐discharge database. In addition, routinely collected vital signs (eg, respiratory rate, blood pressure, heart rate) were obtained from the electronic health record (Epic, Verona, WI). To determine severity of illness, the first set of vital signs measured on hospital presentation were utilized to calculate the cardiac arrest risk triage (CART) score, a vital‐signbased early warning score we previously developed and validated for predicting adverse events in our population.[17] The CART score ranges from 0 to 57, with points assigned for abnormalities in respiratory rate, heart rate, diastolic blood pressure, and age. If any vital sign was missing, the next available measurement was pulled into the set. If any vital sign remained missing after this change, the median value for that particular location (ie, wards, ICU, or emergency department) was imputed as previously described.[18, 19]
Patients with OSA were identified by the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes using inpatient and outpatient medical records: 278.03, 327.20, 327.23, 327.29, 780.51, 780.53, and 780.57 (Table 1). Data on other patient comorbidities, including coronary artery disease, congestive heart failure, arrhythmias, uncomplicated and complicated diabetes mellitus, hypertension, and cerebrovascular disease were collected using specific ICD‐9‐CM codes from both inpatient and outpatient records. Information on insurance payer was also collected from the hospital's billing database. Insurance payers were grouped into the following categories: private payer, Medicare/Medicaid, and no insurance. Patients with both public and private payers were counted as being privately insured.
| Diagnosis Code | Description | % of Sleep Apnea Diagnosesa |
|---|---|---|
| ||
| 327.23 | Obstructive sleep apnea | 65.6 |
| 780.57 | Unspecified sleep apnea | 19.4 |
| 780.53 | Hypersomnia with sleep apnea, unspecified | 11.7 |
| 780.51 | Insomnia with sleep apnea, unspecified | 1.5 |
| 327.2 | Organic sleep apnea, unspecified | 0.2 |
| 278.03 | Obesity hypoventilation syndrome | 1.7 |
Outcomes
The primary outcome of the study was in‐hospital mortality. Secondary outcomes included length of stay, RRT activation, transfer to the ICU, endotracheal intubation, cardiac arrest (defined as a loss of pulse with attempted resuscitation) on the wards, and a composite outcome of RRT activation, ICU transfer, and death. Because cardiac arrests on the wards result either in death or ICU transfer following successful resuscitation, this variable was omitted from the composite outcome. Cardiac arrests were identified using a prospectively validated quality improvement database that has been described previously.[20] ICU transfer was identified using the hospital's admission‐transfer‐discharge database. Only the index cardiac arrest, intubation, RRT, or ICU transfer for each admission was used in the study, but more than 1 type of outcome could occur for each patient (eg, a patient who died following an unsuccessful resuscitation attempt would count as both a cardiac arrest and a death).
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and 2 statistics, as appropriate. Unadjusted logistic regression models were fit to estimate the change in odds of each adverse event and a composite outcome of any event for patient admissions with OSA compared to those without OSA. Adjusted logistic regression models were then fit for each outcome to control for patient characteristics (age, sex, BMI, insurance status, and individual comorbidities), location immediately prior to ward admission, and admission severity of illness (as measured by CART score). In the adjusted model, CART score, age, and number of comorbidities were entered linearly, with the addition of squared terms for age and CART score, as these variables showed nonlinear associations with the outcomes of interest. Race, surgical status, insurance payer, location prior to ward, and BMI (underweight, <18.5 kg/m2; normal weight, 18.524.9 kg/m2; overweight, 25.029.9 kg/m2; obese, 3039.9 kg/m2; and severely obese, (40 kg/m2) were modeled as categorical variables.
Given that an individual patient could experience multiple hospitalizations during the study period, we performed a sensitivity analysis of all adjusted and unadjusted models using a single randomly selected hospitalization for each unique patient. In addition, we performed a sensitivity analysis of all patients who were not admitted to the ICU prior to their ward stay. Finally, we performed subgroup analyses of all unadjusted and adjusted models for each BMI category and surgical status.
All tests of significance used a 2‐sided P value <0.05. Statistical analyses were completed using Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
During the study period, 93,676 patient admissions from 53,150 unique patients resulted in the occurrence of 1,069 RRT activations, 6,305 ICU transfers, and 1,239 in‐hospital deaths. Within our sample, 40,034 patients had at least 1 inpatient record and at least 1 outpatient record. OSA diagnosis was present in 5,625 patients (10.6% of the total sample), with 4,748 patients having an OSA diagnosis code entered during a hospitalization, 2,143 with an OSA diagnosis code entered during an outpatient encounter, and 877 with both inpatient and outpatient diagnosis codes. These patients identified as having OSA contributed 12,745 (13.6%) hospital admissions.
Patients with an OSA diagnosis were more likely to be older (median age 59 years [interquartile range 4968] vs 55 years [3868]), male (49% vs 42%), overweight or obese (88% vs 62%), and more likely to carry diagnoses of diabetes (53.8% vs 25.5%), hypertension (45.3% vs 18.2%), arrhythmias (44.4% vs 26.7%), coronary artery disease (46.8% vs 23.5%), heart failure (35.8% vs 13.5%), and cerebrovascular disease (13.5% vs 8.1%) than patients without an OSA diagnosis (all comparisons significant, P < 0.001) (Table 2).
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Age, y, median (IQR) | 59 (4968) | 55 (3868) | <0.001 |
| Female, n (%) | 6,514 (51%) | 47,202 (58%) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 4,205 (33%) | 30,119 (37%) | |
| Black/African American | 7,024 (55%) | 38,561 (48%) | |
| Asian | 561 (4.4%) | 3,419 (4.2%) | |
| American Indian or Native Alaskan | 20 (0.2%) | 113 (0.1%) | |
| More than 1 race | 127 (1%) | 843 (1%) | |
| Race unknown | 808 (6%) | 7,876 (10%) | |
| Insurance status, n (%) | <0.001 | ||
| Private | 4,484 (35%) | 32,467 (40%) | |
| Medicare/Medicaid | 8,201 (64%) | 42,208 (58%) | |
| Uninsured | 53 (0.4%) | 1,190 (1%) | |
| Unknown | 4 (<0.1%) | 16 (<0.1%) | |
| Location prior to wards, n (%) | <0.001 | ||
| ICU | 1,400 (11%) | 8,065 (10%) | |
| Emergency department | 4,633 (36%) | 25,170 (31%) | |
| Ambulatory admission | 6,712 (53%) | 47,696 (59%) | |
| Body mass index, kg/m2, n (%) | <0.001 | ||
| Normal (18.525) | 1,431 (11%) | 26,560 (33%) | |
| Underweight (<18.5) | 122 (1%) | 4,256 (5%) | |
| Overweight (2530) | 2,484 (20%) | 23,761 (29%) | |
| Obese (3040) | 4,959 (39%) | 19,132 (24%) | |
| Severely obese (40) | 3,745 (29%) | 7,171 (9%) | |
| Initial cardiac arrest risk triage score, median (IQR) | 4 (09) | 4 (09) | <0.001 |
| Underwent surgery, n (%) | 4,482 (35%) | 28,843 (36%) | 0.3 |
| Comorbidities | |||
| Number of comorbidities, median (IQR) | 2 (14) | 1 (02) | <0.001 |
| Arrhythmia | 5,659 (44%) | 21,581 (27%) | <0.001 |
| Diabetes mellitus | 6,855 (54%) | 20,641 (26%) | <0.001 |
| Hypertension | 5,777 (45%) | 14,728 (18%) | <0.001 |
| Coronary artery disease | 5,958 (47%) | 18,979 (23%) | <0.001 |
| Cerebrovascular accident | 1,725 (14%) | 6,556 (8%) | <0.001 |
| Congestive heart failure | 4,559 (36%) | 10,919 (13%) | <0.001 |
Complications and Adverse Outcomes
In the unadjusted analyses, the overall incidence of adverse outcomes was higher among patient admissions with a diagnosis of OSA compared to those without OSA (Table 3). Those with OSA were more likely to experience RRT activation (1.5% vs 1.1%), ICU transfer (8% vs 7%), and endotracheal intubation (3.9% vs 2.9%) than those without OSA diagnoses (P < 0.001 for all comparisons). There was no significant difference in the incidence of cardiac arrest between the 2 groups, nor was there a significant difference in length of stay. Unadjusted inpatient mortality for OSA patient admissions was lower than that for non‐OSA hospitalizations (1.1% vs 1.4%, P < 0.05). A diagnosis of OSA was associated with increased unadjusted odds for RRT activation (odds ratio [OR]: 1.36 [1.16‐1.59]) and ICU transfer (OR: 1.28 [1.20‐1.38]). However, after controlling for confounders, OSA was not associated with increased odds for RRT activation (OR: 1.14 [0.95‐1.36]) or intubation (OR: 1.06 [0.94‐1.19]), and was associated with slightly decreased odds for ICU transfer (OR: 0.91 [0.84‐0.99]) (Figure 1). Those with OSA had decreased adjusted odds of cardiac arrest (OR: 0.72 [0.55‐0.95]) compared to those without OSA. OSA was also associated with decreased odds of in‐hospital mortality before (OR: 0.83 [0.70‐0.99]) and after (OR: 0.70 [0.58‐0.85]) controlling for confounders.
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Outcomes, n (%) | |||
| Composite outcomea | 1,137 (9%) | 5,792 (7%) | <0.001 |
| In‐hospital death | 144 (1.1%) | 1,095 (1.4%) | 0.04 |
| Rapid response team call | 188 (1.5%) | 881 (1.1%) | <0.001 |
| ICU transfer | 1,045 (8%) | 5,260 (7%) | <0.001 |
| Cardiac arrest | 413 (0.5%) | 73 (0.6%) | 0.36 |

Sensitivity Analyses
The sensitivity analysis involving 1 randomly selected hospitalization per patient included a total of 53,150 patients. The results were similar to the main analysis, with adjusted odds of 1.01 (0.77‐1.32) for RRT activation, 0.86 (0.76‐0.96) for ICU transfer, and 0.69 (0.53‐0.89) for inpatient mortality. An additional sensitivity analysis included only patients who were not admitted to the ICU prior to their ward stay. This analysis included 84,211 hospitalizations and demonstrated similar findings, with adjusted odds of 0.70 for in‐hospital mortality (0.57‐0.87). Adjusted odds for RRT activation (OR: 1.12 [0.92‐1.37]) and ICU transfer (OR: 0.88 [0.81‐0.96] were also similar to the results of our main analysis.
Subgroup Analyses
Surgical and Nonsurgical Patients
Subgroup analyses of surgical versus nonsurgical patients (Figure 2) revealed similarly decreased adjusted odds of in‐hospital death for OSA patients in both groups (surgical OR: 0.69 [0.49‐0.97]; nonsurgical OR: 0.72 [0.58‐0.91]). Surgical patients with OSA diagnoses had decreased adjusted odds for ICU transfer (surgical OR: 0.82 [0.73‐0.92], but this finding was not seen in nonsurgical patients (OR: 1.03 [0.92‐1.15]).

Patients Stratified by BMI
Examination across BMI categories (Figure 2) showed a significant decrease in adjusted odds of death for OSA patients with BMI 30 to 40 kg/m2 (OR: 0.60 [0.43‐0.84]). A nonsignificant decrease in adjusted odds of death was seen for OSA patients in all other groups. Adjusted odds ratios for the risk of RRT activation and ICU transfer in OSA patients within the different BMI categories were not statistically significant.
DISCUSSION
In this large observational single‐center cohort study, we found that OSA was associated with increased odds of adverse events, such as ICU transfers and RRT calls, but this risk was no longer present after adjusting for demographics, comorbidities, and presenting vital signs. Interestingly, we also found that patients with OSA had decreased adjusted odds for cardiac arrest and mortality. This mortality finding was robust to multiple sensitivity analyses and subgroup analyses. These results have significant implications for our understanding of the short‐term risks of sleep‐disordered breathing in hospitalized patients, and suggest the possibility that OSA is associated with a protective effect with regard to inpatient mortality.
Our findings are in line with other recent work in this area. In 2 large observational cohorts of surgical populations drawn from the nationally representative Nationwide Inpatient Sample administrative database, our group reported decreased odds of in‐hospital postoperative mortality in OSA patients.[10, 11] Using the same Nationwide Inpatient Sample, Lindenauer et al. showed that among inpatients hospitalized with pneumonia, OSA diagnosis was associated with increased rates of clinical deterioration but lower rates of inpatient mortality.[12] Although these 3 studies have identified decreased inpatient mortality among certain surgical populations and patients hospitalized with pneumonia, they are limited by using administrative databases that do not provide specific data on vital signs, presenting physiology, BMI, or race. Another important limitation of the Nationwide Inpatient Sample is the lack of any information on RRT activations and ICU transfers. Moreover, the database does not include information on outpatient diagnoses, which may have led to a significantly lower prevalence of OSA than expected in these studies. Despite the important methodological differences, our study corroborates this finding among a diverse cohort of hospitalized patients. Unlike these previous studies of postoperative patients or those hospitalized with pneumonia, we did not find an increased risk of adverse events associated with OSA after controlling for potential confounders.
The decreased mortality seen in OSA patients could be explained by these patients receiving more vigilant care, showing earlier signs of deterioration, or displaying more easily treatable forms of distress than patients without OSA. For example, earlier identification of deterioration could lead to earlier interventions, which could decrease inpatient mortality. In 2 studies of postsurgical patients,[10, 11] those with OSA diagnosis who developed respiratory failure were intubated earlier and received mechanical ventilation for a shorter period of time, suggesting that the cause of respiratory failure was rapidly reversible (eg, upper airway complications due to oversedation or excessive analgesia). However, we did not find increased adjusted odds of RRT activation or ICU transfer for OSA patients in our study, and so it is less likely that earlier recognition of decompensation occurred in our sample. In addition, our hospital did not have standardized practices for monitoring or managing OSA patients during the study period, which makes systematic early recognition of clinical deterioration among the OSA population in our study less likely.
Alternatively, there may be a true physiologic phenomenon providing a short‐term mortality benefit in those with OSA. It has been observed that patients with obesity (but without severe obesity) often have better outcomes after acute illness, whether by earlier or more frequent contact with medical care or heightened levels of metabolic reserve.[21, 22] However, our findings of decreased mortality for OSA patients remained even after controlling for BMI. An additional important possibility to consider is ischemic preconditioning, a well‐described phenomenon in which episodes of sublethal ischemia confer protection on tissues from subsequent ischemia/reperfusion damage.[23] Ischemic preconditioning has been demonstrated in models of cardiac and neural tissue[24, 25, 26] and has been shown to enhance stem cell survival by providing resistance to necrosis and lending functional benefits to heart, brain, and kidney models after transplantation.[25, 26, 27, 28, 29, 30, 31] The fundamentals of this concept may have applications in transplant and cardiac surgery,[32, 33] in the management of acute coronary syndromes and stroke,[32, 34, 35] and in athletic training and performance.[35, 36] Although OSA has been associated with long‐term cardiovascular morbidity and mortality,[2, 3, 4, 5, 6] the intermittent hypoxemia OSA patients experience could actually improve their ability to survive clinical deterioration in the short‐term (ie, during a hospitalization).
Limitations of our study include its conduction at a single center, which may prevent generalization to populations different than ours. Furthermore, during the study period, our hospital did not have formal guidelines or standardized management or monitoring practices for patients with OSA. Additionally, practices for managing OSA may vary across institutions. Therefore, our results may not be generalizable to hospitals with such protocols in place. However, as mentioned above, similar findings have been noted in studies using large, nationally representative administrative databases. In addition, we identified OSA via ICD‐9‐CM codes, which are likely insensitive for estimating the true prevalence of OSA in our sample. Despite this, our reported OSA prevalence of over 10% falls within the prevalence range reported in large epidemiological studies.[37, 38, 39] Finally, we did not have data on polysomnograms or treatment received for patients with OSA, so we do not know the severity of OSA or adequacy of treatment for these patients.
Notwithstanding our limitations, our study has several strengths. First, we included a large number of hospitalized patients across a diverse range of medical and surgical ward admissions, which increases the generalizability of our results. We also addressed potential confounders by including a large number of comorbidities and controlling for severity of presenting physiology with the CART score. The CART score, which contains physiologic variables such as respiratory rate, heart rate, and diastolic blood pressure, is an accurate predictor of cardiac arrest, ICU transfer, and in‐hospital mortality in our population.[40] Finally, we were able to obtain information about these diagnoses from outpatient as well as inpatient data.
In conclusion, we found that after adjustment for important confounders, OSA was associated with a decrease in hospital mortality and cardiac arrest but not with other adverse events on the wards. These results may suggest a protective benefit from OSA with regard to mortality, an advantage that could be explained by ischemic preconditioning or a higher level of care or vigilance not reflected by the number of RRT activations or ICU transfers experienced by these patients. Further research is needed to confirm these findings across other populations, to investigate the physiologic pathways by which OSA may produce these effects, and to examine the mechanisms by which treatment of OSA could influence these outcomes.
Acknowledgements
The authors thank Nicole Babuskow for administrative support, as well as Brian Furner and Timothy Holper for assistance with data acquisition.
Disclosures: Study concept and design: P.L., D.P.E, B.M., M.C.; acquisition of data: P.L.; analysis and interpretation of data: all authors; first drafting of the manuscript: P.L.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: P.L., F.Z., M.C.; obtained funding: D.P.E., M.C.; administrative, technical, and material support: F.Z., D.P.E.; study supervision: D.P.E, B.M., M.C.; data access and responsibility: P.L. and M.C. had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek and Dr. Edelson are both supported by career development awards from the National Heart, Lung, and Blood Institute (K08 HL121080 and K23 HL097157, respectively). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics.
Obstructive sleep apnea (OSA) is an increasingly prevalent condition characterized by intermittent airway obstruction during sleep, which leads to hypoxemia, hypercapnia, and fragmented sleep. The current prevalence estimates of moderate to severe OSA (apnea‐hypopnea index 15, measured as events/hour) in middle‐aged adults are approximately 13% in men and 6% in women.[1] OSA is a well‐described independent risk factor for long‐term neurocognitive, cardiovascular, and cerebrovascular morbidity and mortality.[2, 3, 4, 5, 6]
Recent studies have also identified OSA as an independent risk factor for adverse perioperative outcomes, including endotracheal intubation, intensive care unit (ICU) transfer, and increased length of stay.[7, 8, 9, 10, 11] Paradoxically, despite an increase in the risk of complications, several of these studies did not find an association between in‐hospital death and OSA even after controlling for potential confounders.[9, 10, 11] Furthermore, a recent study of patients hospitalized for pneumonia reported increased rates of clinical deterioration and mechanical ventilation, but also lower odds of inpatient mortality in patients with OSA.[12]
These studies may have been limited by the absence of physiologic data, which prevented controlling for severity of illness. It is also unclear whether these previously described associations between OSA and adverse clinical outcomes hold true for general hospital inpatients. OSA may be worsened by medications frequently used in hospitals, such as narcotics and benzodiazepines. Opiate use contributes to both central and obstructive sleep apneas,[13, 14] and benzodiazepines are known to produce airway smooth muscle relaxation and can cause respiratory depression.[15] In fact, the use of benzodiazepines has been implicated in the unmasking of OSA in patients with previously undiagnosed sleep‐disordered breathing.[16] These findings suggest mechanisms by which OSA could contribute to an increased risk in hospital ward patients for rapid response team (RRT) activation, ICU transfer, cardiac arrest, and in‐hospital death.
The aim of this study was to determine the independent association between OSA and in‐hospital mortality in ward patients. We also aimed to investigate the association of OSA with clinical deterioration on the wards, while controlling for patient characteristics, initial physiology, and severity of illness.
MATERIALS AND METHODS
Setting and Study Population
This observational cohort study was performed at an academic tertiary care medical center with approximately 500 beds. Data were obtained from all adult patients hospitalized on the wards between November 1, 2008 and October 1, 2013. Our hospital has utilized an RRT, led by a critical care nurse and respiratory therapist with hospitalist and pharmacist consultation available upon request, since 2008. This team is separate from the team that responds to a cardiac arrest. Criteria for RRT activation include tachypnea, tachycardia, hypotension, and staff worry, but specific vital sign thresholds are not specified.
The study analyzed deidentified data from the hospital's Clinical Research Data Warehouse, which is maintained by the Center for Research Informatics at The University of Chicago. The study protocol was approved by the University of Chicago Institutional Review Board (IRB #16995A).
Data Collection
Patient age, sex, race, body mass index (BMI), and location prior to ward admission (ie, whether they were admitted from the emergency department, transferred from the ICU, or directly admitted from clinic or home) were collected. Patients who underwent surgery during their admission were identified using the hospital's admission‐transfer‐discharge database. In addition, routinely collected vital signs (eg, respiratory rate, blood pressure, heart rate) were obtained from the electronic health record (Epic, Verona, WI). To determine severity of illness, the first set of vital signs measured on hospital presentation were utilized to calculate the cardiac arrest risk triage (CART) score, a vital‐signbased early warning score we previously developed and validated for predicting adverse events in our population.[17] The CART score ranges from 0 to 57, with points assigned for abnormalities in respiratory rate, heart rate, diastolic blood pressure, and age. If any vital sign was missing, the next available measurement was pulled into the set. If any vital sign remained missing after this change, the median value for that particular location (ie, wards, ICU, or emergency department) was imputed as previously described.[18, 19]
Patients with OSA were identified by the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes using inpatient and outpatient medical records: 278.03, 327.20, 327.23, 327.29, 780.51, 780.53, and 780.57 (Table 1). Data on other patient comorbidities, including coronary artery disease, congestive heart failure, arrhythmias, uncomplicated and complicated diabetes mellitus, hypertension, and cerebrovascular disease were collected using specific ICD‐9‐CM codes from both inpatient and outpatient records. Information on insurance payer was also collected from the hospital's billing database. Insurance payers were grouped into the following categories: private payer, Medicare/Medicaid, and no insurance. Patients with both public and private payers were counted as being privately insured.
| Diagnosis Code | Description | % of Sleep Apnea Diagnosesa |
|---|---|---|
| ||
| 327.23 | Obstructive sleep apnea | 65.6 |
| 780.57 | Unspecified sleep apnea | 19.4 |
| 780.53 | Hypersomnia with sleep apnea, unspecified | 11.7 |
| 780.51 | Insomnia with sleep apnea, unspecified | 1.5 |
| 327.2 | Organic sleep apnea, unspecified | 0.2 |
| 278.03 | Obesity hypoventilation syndrome | 1.7 |
Outcomes
The primary outcome of the study was in‐hospital mortality. Secondary outcomes included length of stay, RRT activation, transfer to the ICU, endotracheal intubation, cardiac arrest (defined as a loss of pulse with attempted resuscitation) on the wards, and a composite outcome of RRT activation, ICU transfer, and death. Because cardiac arrests on the wards result either in death or ICU transfer following successful resuscitation, this variable was omitted from the composite outcome. Cardiac arrests were identified using a prospectively validated quality improvement database that has been described previously.[20] ICU transfer was identified using the hospital's admission‐transfer‐discharge database. Only the index cardiac arrest, intubation, RRT, or ICU transfer for each admission was used in the study, but more than 1 type of outcome could occur for each patient (eg, a patient who died following an unsuccessful resuscitation attempt would count as both a cardiac arrest and a death).
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and 2 statistics, as appropriate. Unadjusted logistic regression models were fit to estimate the change in odds of each adverse event and a composite outcome of any event for patient admissions with OSA compared to those without OSA. Adjusted logistic regression models were then fit for each outcome to control for patient characteristics (age, sex, BMI, insurance status, and individual comorbidities), location immediately prior to ward admission, and admission severity of illness (as measured by CART score). In the adjusted model, CART score, age, and number of comorbidities were entered linearly, with the addition of squared terms for age and CART score, as these variables showed nonlinear associations with the outcomes of interest. Race, surgical status, insurance payer, location prior to ward, and BMI (underweight, <18.5 kg/m2; normal weight, 18.524.9 kg/m2; overweight, 25.029.9 kg/m2; obese, 3039.9 kg/m2; and severely obese, (40 kg/m2) were modeled as categorical variables.
Given that an individual patient could experience multiple hospitalizations during the study period, we performed a sensitivity analysis of all adjusted and unadjusted models using a single randomly selected hospitalization for each unique patient. In addition, we performed a sensitivity analysis of all patients who were not admitted to the ICU prior to their ward stay. Finally, we performed subgroup analyses of all unadjusted and adjusted models for each BMI category and surgical status.
All tests of significance used a 2‐sided P value <0.05. Statistical analyses were completed using Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
During the study period, 93,676 patient admissions from 53,150 unique patients resulted in the occurrence of 1,069 RRT activations, 6,305 ICU transfers, and 1,239 in‐hospital deaths. Within our sample, 40,034 patients had at least 1 inpatient record and at least 1 outpatient record. OSA diagnosis was present in 5,625 patients (10.6% of the total sample), with 4,748 patients having an OSA diagnosis code entered during a hospitalization, 2,143 with an OSA diagnosis code entered during an outpatient encounter, and 877 with both inpatient and outpatient diagnosis codes. These patients identified as having OSA contributed 12,745 (13.6%) hospital admissions.
Patients with an OSA diagnosis were more likely to be older (median age 59 years [interquartile range 4968] vs 55 years [3868]), male (49% vs 42%), overweight or obese (88% vs 62%), and more likely to carry diagnoses of diabetes (53.8% vs 25.5%), hypertension (45.3% vs 18.2%), arrhythmias (44.4% vs 26.7%), coronary artery disease (46.8% vs 23.5%), heart failure (35.8% vs 13.5%), and cerebrovascular disease (13.5% vs 8.1%) than patients without an OSA diagnosis (all comparisons significant, P < 0.001) (Table 2).
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Age, y, median (IQR) | 59 (4968) | 55 (3868) | <0.001 |
| Female, n (%) | 6,514 (51%) | 47,202 (58%) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 4,205 (33%) | 30,119 (37%) | |
| Black/African American | 7,024 (55%) | 38,561 (48%) | |
| Asian | 561 (4.4%) | 3,419 (4.2%) | |
| American Indian or Native Alaskan | 20 (0.2%) | 113 (0.1%) | |
| More than 1 race | 127 (1%) | 843 (1%) | |
| Race unknown | 808 (6%) | 7,876 (10%) | |
| Insurance status, n (%) | <0.001 | ||
| Private | 4,484 (35%) | 32,467 (40%) | |
| Medicare/Medicaid | 8,201 (64%) | 42,208 (58%) | |
| Uninsured | 53 (0.4%) | 1,190 (1%) | |
| Unknown | 4 (<0.1%) | 16 (<0.1%) | |
| Location prior to wards, n (%) | <0.001 | ||
| ICU | 1,400 (11%) | 8,065 (10%) | |
| Emergency department | 4,633 (36%) | 25,170 (31%) | |
| Ambulatory admission | 6,712 (53%) | 47,696 (59%) | |
| Body mass index, kg/m2, n (%) | <0.001 | ||
| Normal (18.525) | 1,431 (11%) | 26,560 (33%) | |
| Underweight (<18.5) | 122 (1%) | 4,256 (5%) | |
| Overweight (2530) | 2,484 (20%) | 23,761 (29%) | |
| Obese (3040) | 4,959 (39%) | 19,132 (24%) | |
| Severely obese (40) | 3,745 (29%) | 7,171 (9%) | |
| Initial cardiac arrest risk triage score, median (IQR) | 4 (09) | 4 (09) | <0.001 |
| Underwent surgery, n (%) | 4,482 (35%) | 28,843 (36%) | 0.3 |
| Comorbidities | |||
| Number of comorbidities, median (IQR) | 2 (14) | 1 (02) | <0.001 |
| Arrhythmia | 5,659 (44%) | 21,581 (27%) | <0.001 |
| Diabetes mellitus | 6,855 (54%) | 20,641 (26%) | <0.001 |
| Hypertension | 5,777 (45%) | 14,728 (18%) | <0.001 |
| Coronary artery disease | 5,958 (47%) | 18,979 (23%) | <0.001 |
| Cerebrovascular accident | 1,725 (14%) | 6,556 (8%) | <0.001 |
| Congestive heart failure | 4,559 (36%) | 10,919 (13%) | <0.001 |
Complications and Adverse Outcomes
In the unadjusted analyses, the overall incidence of adverse outcomes was higher among patient admissions with a diagnosis of OSA compared to those without OSA (Table 3). Those with OSA were more likely to experience RRT activation (1.5% vs 1.1%), ICU transfer (8% vs 7%), and endotracheal intubation (3.9% vs 2.9%) than those without OSA diagnoses (P < 0.001 for all comparisons). There was no significant difference in the incidence of cardiac arrest between the 2 groups, nor was there a significant difference in length of stay. Unadjusted inpatient mortality for OSA patient admissions was lower than that for non‐OSA hospitalizations (1.1% vs 1.4%, P < 0.05). A diagnosis of OSA was associated with increased unadjusted odds for RRT activation (odds ratio [OR]: 1.36 [1.16‐1.59]) and ICU transfer (OR: 1.28 [1.20‐1.38]). However, after controlling for confounders, OSA was not associated with increased odds for RRT activation (OR: 1.14 [0.95‐1.36]) or intubation (OR: 1.06 [0.94‐1.19]), and was associated with slightly decreased odds for ICU transfer (OR: 0.91 [0.84‐0.99]) (Figure 1). Those with OSA had decreased adjusted odds of cardiac arrest (OR: 0.72 [0.55‐0.95]) compared to those without OSA. OSA was also associated with decreased odds of in‐hospital mortality before (OR: 0.83 [0.70‐0.99]) and after (OR: 0.70 [0.58‐0.85]) controlling for confounders.
| Characteristic | Patient Admissions With OSA Diagnoses, n = 12,745 | Patient Admissions Without OSA Diagnoses, n = 80,931 | P Value |
|---|---|---|---|
| |||
| Outcomes, n (%) | |||
| Composite outcomea | 1,137 (9%) | 5,792 (7%) | <0.001 |
| In‐hospital death | 144 (1.1%) | 1,095 (1.4%) | 0.04 |
| Rapid response team call | 188 (1.5%) | 881 (1.1%) | <0.001 |
| ICU transfer | 1,045 (8%) | 5,260 (7%) | <0.001 |
| Cardiac arrest | 413 (0.5%) | 73 (0.6%) | 0.36 |

Sensitivity Analyses
The sensitivity analysis involving 1 randomly selected hospitalization per patient included a total of 53,150 patients. The results were similar to the main analysis, with adjusted odds of 1.01 (0.77‐1.32) for RRT activation, 0.86 (0.76‐0.96) for ICU transfer, and 0.69 (0.53‐0.89) for inpatient mortality. An additional sensitivity analysis included only patients who were not admitted to the ICU prior to their ward stay. This analysis included 84,211 hospitalizations and demonstrated similar findings, with adjusted odds of 0.70 for in‐hospital mortality (0.57‐0.87). Adjusted odds for RRT activation (OR: 1.12 [0.92‐1.37]) and ICU transfer (OR: 0.88 [0.81‐0.96] were also similar to the results of our main analysis.
Subgroup Analyses
Surgical and Nonsurgical Patients
Subgroup analyses of surgical versus nonsurgical patients (Figure 2) revealed similarly decreased adjusted odds of in‐hospital death for OSA patients in both groups (surgical OR: 0.69 [0.49‐0.97]; nonsurgical OR: 0.72 [0.58‐0.91]). Surgical patients with OSA diagnoses had decreased adjusted odds for ICU transfer (surgical OR: 0.82 [0.73‐0.92], but this finding was not seen in nonsurgical patients (OR: 1.03 [0.92‐1.15]).

Patients Stratified by BMI
Examination across BMI categories (Figure 2) showed a significant decrease in adjusted odds of death for OSA patients with BMI 30 to 40 kg/m2 (OR: 0.60 [0.43‐0.84]). A nonsignificant decrease in adjusted odds of death was seen for OSA patients in all other groups. Adjusted odds ratios for the risk of RRT activation and ICU transfer in OSA patients within the different BMI categories were not statistically significant.
DISCUSSION
In this large observational single‐center cohort study, we found that OSA was associated with increased odds of adverse events, such as ICU transfers and RRT calls, but this risk was no longer present after adjusting for demographics, comorbidities, and presenting vital signs. Interestingly, we also found that patients with OSA had decreased adjusted odds for cardiac arrest and mortality. This mortality finding was robust to multiple sensitivity analyses and subgroup analyses. These results have significant implications for our understanding of the short‐term risks of sleep‐disordered breathing in hospitalized patients, and suggest the possibility that OSA is associated with a protective effect with regard to inpatient mortality.
Our findings are in line with other recent work in this area. In 2 large observational cohorts of surgical populations drawn from the nationally representative Nationwide Inpatient Sample administrative database, our group reported decreased odds of in‐hospital postoperative mortality in OSA patients.[10, 11] Using the same Nationwide Inpatient Sample, Lindenauer et al. showed that among inpatients hospitalized with pneumonia, OSA diagnosis was associated with increased rates of clinical deterioration but lower rates of inpatient mortality.[12] Although these 3 studies have identified decreased inpatient mortality among certain surgical populations and patients hospitalized with pneumonia, they are limited by using administrative databases that do not provide specific data on vital signs, presenting physiology, BMI, or race. Another important limitation of the Nationwide Inpatient Sample is the lack of any information on RRT activations and ICU transfers. Moreover, the database does not include information on outpatient diagnoses, which may have led to a significantly lower prevalence of OSA than expected in these studies. Despite the important methodological differences, our study corroborates this finding among a diverse cohort of hospitalized patients. Unlike these previous studies of postoperative patients or those hospitalized with pneumonia, we did not find an increased risk of adverse events associated with OSA after controlling for potential confounders.
The decreased mortality seen in OSA patients could be explained by these patients receiving more vigilant care, showing earlier signs of deterioration, or displaying more easily treatable forms of distress than patients without OSA. For example, earlier identification of deterioration could lead to earlier interventions, which could decrease inpatient mortality. In 2 studies of postsurgical patients,[10, 11] those with OSA diagnosis who developed respiratory failure were intubated earlier and received mechanical ventilation for a shorter period of time, suggesting that the cause of respiratory failure was rapidly reversible (eg, upper airway complications due to oversedation or excessive analgesia). However, we did not find increased adjusted odds of RRT activation or ICU transfer for OSA patients in our study, and so it is less likely that earlier recognition of decompensation occurred in our sample. In addition, our hospital did not have standardized practices for monitoring or managing OSA patients during the study period, which makes systematic early recognition of clinical deterioration among the OSA population in our study less likely.
Alternatively, there may be a true physiologic phenomenon providing a short‐term mortality benefit in those with OSA. It has been observed that patients with obesity (but without severe obesity) often have better outcomes after acute illness, whether by earlier or more frequent contact with medical care or heightened levels of metabolic reserve.[21, 22] However, our findings of decreased mortality for OSA patients remained even after controlling for BMI. An additional important possibility to consider is ischemic preconditioning, a well‐described phenomenon in which episodes of sublethal ischemia confer protection on tissues from subsequent ischemia/reperfusion damage.[23] Ischemic preconditioning has been demonstrated in models of cardiac and neural tissue[24, 25, 26] and has been shown to enhance stem cell survival by providing resistance to necrosis and lending functional benefits to heart, brain, and kidney models after transplantation.[25, 26, 27, 28, 29, 30, 31] The fundamentals of this concept may have applications in transplant and cardiac surgery,[32, 33] in the management of acute coronary syndromes and stroke,[32, 34, 35] and in athletic training and performance.[35, 36] Although OSA has been associated with long‐term cardiovascular morbidity and mortality,[2, 3, 4, 5, 6] the intermittent hypoxemia OSA patients experience could actually improve their ability to survive clinical deterioration in the short‐term (ie, during a hospitalization).
Limitations of our study include its conduction at a single center, which may prevent generalization to populations different than ours. Furthermore, during the study period, our hospital did not have formal guidelines or standardized management or monitoring practices for patients with OSA. Additionally, practices for managing OSA may vary across institutions. Therefore, our results may not be generalizable to hospitals with such protocols in place. However, as mentioned above, similar findings have been noted in studies using large, nationally representative administrative databases. In addition, we identified OSA via ICD‐9‐CM codes, which are likely insensitive for estimating the true prevalence of OSA in our sample. Despite this, our reported OSA prevalence of over 10% falls within the prevalence range reported in large epidemiological studies.[37, 38, 39] Finally, we did not have data on polysomnograms or treatment received for patients with OSA, so we do not know the severity of OSA or adequacy of treatment for these patients.
Notwithstanding our limitations, our study has several strengths. First, we included a large number of hospitalized patients across a diverse range of medical and surgical ward admissions, which increases the generalizability of our results. We also addressed potential confounders by including a large number of comorbidities and controlling for severity of presenting physiology with the CART score. The CART score, which contains physiologic variables such as respiratory rate, heart rate, and diastolic blood pressure, is an accurate predictor of cardiac arrest, ICU transfer, and in‐hospital mortality in our population.[40] Finally, we were able to obtain information about these diagnoses from outpatient as well as inpatient data.
In conclusion, we found that after adjustment for important confounders, OSA was associated with a decrease in hospital mortality and cardiac arrest but not with other adverse events on the wards. These results may suggest a protective benefit from OSA with regard to mortality, an advantage that could be explained by ischemic preconditioning or a higher level of care or vigilance not reflected by the number of RRT activations or ICU transfers experienced by these patients. Further research is needed to confirm these findings across other populations, to investigate the physiologic pathways by which OSA may produce these effects, and to examine the mechanisms by which treatment of OSA could influence these outcomes.
Acknowledgements
The authors thank Nicole Babuskow for administrative support, as well as Brian Furner and Timothy Holper for assistance with data acquisition.
Disclosures: Study concept and design: P.L., D.P.E, B.M., M.C.; acquisition of data: P.L.; analysis and interpretation of data: all authors; first drafting of the manuscript: P.L.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: P.L., F.Z., M.C.; obtained funding: D.P.E., M.C.; administrative, technical, and material support: F.Z., D.P.E.; study supervision: D.P.E, B.M., M.C.; data access and responsibility: P.L. and M.C. had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek and Dr. Edelson are both supported by career development awards from the National Heart, Lung, and Blood Institute (K08 HL121080 and K23 HL097157, respectively). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics.
- , , , , , . Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- , , , . Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053.
- , , , . Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384.
- , , , , , . Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041.
- , , , , . Obstructive sleep apnea and risk of cardiovascular events and all‐cause mortality: a decade‐long historical cohort study. PLoS Med. 2014;11(2):e1001599.
- , , , , , . Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085.
- , , , , . Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441.
- , , , , , . Meta‐analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906.
- , , , et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407–418.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23(11):1842–1851.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903–914.
- , , , , , . Prevalence, treatment and outcomes associated with obstructive sleep apnea among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038.
- , , , et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8(1):e54807.
- , , , , . Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250–254.
- , , , et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65–69.
- , . Effect of flurazepam on sleep‐disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73(2):239–243.
- , , , , , . Derivation of a cardiac arrest prediction model using ward vital signs*. Crit Care Med. 2012;40(7):2102–2108.
- , , , , . Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards*. Crit Care Med. 2014;42(4):841–848.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , , , . Predicting cardiac arrest on the wards: a nested case‐control study. Chest. 2012;141(5):1170–1176.
- , , , , , . Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(4):306–311.
- , , , et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012(8);125:796–803.
- , , . Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136.
- , , , . Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–931.
- , , , et al. Transplantation of hypoxia‐preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808.
- , , , et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8(5):e62703.
- , . Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22.
- , , , et al. Ischemic pre‐conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53(19):1814–1822.
- , , , et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301(2):C362–C372.
- , , , et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670.
- , , , , . Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–645.
- , , . Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565.
- , , . Novel adjunctive treatments of myocardial infarction. World J Cardiol. 2014;6(6):434–443.
- , . Hypoxic‐preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11(2):669–686.
- , , , , . Ischemic preconditioning improves oxygen saturation and attenuates hypoxic pulmonary vasoconstriction at high altitude. High Alt Med Biol. 2014;15(2):155–161.
- , , , et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43(7):1280–1286.
- , , , . Obstructive sleep apnea‐hypopnea and related clinical features in a population‐based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689.
- , , , , , . The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328(17):1230–1235.
- , , . Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239.
- , , . Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758–1765.
- , , , , , . Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- , , , . Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053.
- , , , . Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384.
- , , , , , . Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041.
- , , , , . Obstructive sleep apnea and risk of cardiovascular events and all‐cause mortality: a decade‐long historical cohort study. PLoS Med. 2014;11(2):e1001599.
- , , , , , . Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085.
- , , , , . Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441.
- , , , , , . Meta‐analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906.
- , , , et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407–418.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23(11):1842–1851.
- , , , , , . Sleep‐disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903–914.
- , , , , , . Prevalence, treatment and outcomes associated with obstructive sleep apnea among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038.
- , , , et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8(1):e54807.
- , , , , . Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250–254.
- , , , et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65–69.
- , . Effect of flurazepam on sleep‐disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73(2):239–243.
- , , , , , . Derivation of a cardiac arrest prediction model using ward vital signs*. Crit Care Med. 2012;40(7):2102–2108.
- , , , , . Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards*. Crit Care Med. 2014;42(4):841–848.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , , , . Predicting cardiac arrest on the wards: a nested case‐control study. Chest. 2012;141(5):1170–1176.
- , , , , , . Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(4):306–311.
- , , , et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012(8);125:796–803.
- , , . Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136.
- , , , . Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–931.
- , , , et al. Transplantation of hypoxia‐preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808.
- , , , et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8(5):e62703.
- , . Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22.
- , , , et al. Ischemic pre‐conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53(19):1814–1822.
- , , , et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301(2):C362–C372.
- , , , et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670.
- , , , , . Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–645.
- , , . Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565.
- , , . Novel adjunctive treatments of myocardial infarction. World J Cardiol. 2014;6(6):434–443.
- , . Hypoxic‐preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11(2):669–686.
- , , , , . Ischemic preconditioning improves oxygen saturation and attenuates hypoxic pulmonary vasoconstriction at high altitude. High Alt Med Biol. 2014;15(2):155–161.
- , , , et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43(7):1280–1286.
- , , , . Obstructive sleep apnea‐hypopnea and related clinical features in a population‐based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689.
- , , , , , . The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med. 1993;328(17):1230–1235.
- , , . Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239.
- , , . Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758–1765.
© 2015 Society of Hospital Medicine
EHA: Venetoclax-rituxumab combo highly active in relapsed/refractory CLL
VIENNA – A daily dose of the investigational BCL-2 inhibitor venetoclax plus rituximab induced responses in 84% of patients with relapsed or refractory chronic lymphocytic leukemia in a phase Ib study.
Of the 49 patients, 20 (41%) achieved a complete response by standard assessment and 13 (27%) achieved a complete response with no evidence of residual disease on flow cytometry.
Moreover, six patients elected to stop venetoclax after achieving a complete response and, to date, only one has had recurrence of disease after 24 months without therapy, lead investigator Dr. Andrew W. Roberts reported at the annual congress of the European Hematology Association.
Not only were patients able to come off treatment and continue to remain in complete response, but responses were seen at the same frequencies across all classes of cytogenetic and molecular abnormalities, he noted.
“The greatest advance that this drug brings is for those patients who currently have a terrible prognosis with all other drugs that we now have,” Dr. Roberts of Royal Melbourne Hospital said in a press briefing.
“This is an important step forward in finding chemotherapy-free regimens in these vulnerable, elderly patients,” said press briefing moderator Dr. Anton Hagenbeek of the University Medical Center, Utrecht, the Netherlands.
Patients in the open-label, dose-escalation study had received a median of two prior lines of therapy (range, one to five) for chronic lymphocytic leukemia (CLL); their median age was 68 years (50-88 years). They began treatment with 20 mg or 50 mg venetoclax daily, increasing weekly to final cohort doses of 200 mg to 600 mg. Six cycles of monthly standard rituximab were added after the weekly lead-in phase.
CLL depends on high levels of B-cell lymphoma-2 (BCL-2) to stay alive. Venetoclax binds to and switches off the BCL-2 protein function, triggering the death of the CLL cell.
Grade 3 or 4 adverse events occurring in more than 10% of patients were neutropenia (51%), thrombocytopenia (16%), and anemia (14%). There was one treatment-emergent case of tumor lysis syndrome leading to death early in the trial. This phenomenon can occur when the CLL breaks down very quickly and, as a consequence, the study was redesigned and a lower starting dose is now used, Dr. Roberts said.
“That problem has been eliminated, but we still see a very large improvement in patients in a few weeks,” he said. “Other than that, there is a little bit of neutropenia, but that is very manageable.”
“So do you think you are curing patients with this approach?” Dr. Hagenbeek asked, to which Dr. Roberts replied, “Too early to say.”
Venetoclax is currently being evaluated in less heavily pretreated patients and a phase III trial is comparing the combination of venetoclax and rituximab with standard bendamustine chemotherapy plus rituximab, he said.
In May, the Food and Drug Administration granted venetoclax breakthrough therapy designation for use in relapsed or refractory chronic lymphocytic leukemia with a 17p deletion mutation.
AbbVie, which is developing venetoclax in partnership with Roche and Genentech, plans to submit regulatory applications for venetoclax to the FDA and the European Medicines Agency before the end of 2015.
On Twitter @pwendl
VIENNA – A daily dose of the investigational BCL-2 inhibitor venetoclax plus rituximab induced responses in 84% of patients with relapsed or refractory chronic lymphocytic leukemia in a phase Ib study.
Of the 49 patients, 20 (41%) achieved a complete response by standard assessment and 13 (27%) achieved a complete response with no evidence of residual disease on flow cytometry.
Moreover, six patients elected to stop venetoclax after achieving a complete response and, to date, only one has had recurrence of disease after 24 months without therapy, lead investigator Dr. Andrew W. Roberts reported at the annual congress of the European Hematology Association.
Not only were patients able to come off treatment and continue to remain in complete response, but responses were seen at the same frequencies across all classes of cytogenetic and molecular abnormalities, he noted.
“The greatest advance that this drug brings is for those patients who currently have a terrible prognosis with all other drugs that we now have,” Dr. Roberts of Royal Melbourne Hospital said in a press briefing.
“This is an important step forward in finding chemotherapy-free regimens in these vulnerable, elderly patients,” said press briefing moderator Dr. Anton Hagenbeek of the University Medical Center, Utrecht, the Netherlands.
Patients in the open-label, dose-escalation study had received a median of two prior lines of therapy (range, one to five) for chronic lymphocytic leukemia (CLL); their median age was 68 years (50-88 years). They began treatment with 20 mg or 50 mg venetoclax daily, increasing weekly to final cohort doses of 200 mg to 600 mg. Six cycles of monthly standard rituximab were added after the weekly lead-in phase.
CLL depends on high levels of B-cell lymphoma-2 (BCL-2) to stay alive. Venetoclax binds to and switches off the BCL-2 protein function, triggering the death of the CLL cell.
Grade 3 or 4 adverse events occurring in more than 10% of patients were neutropenia (51%), thrombocytopenia (16%), and anemia (14%). There was one treatment-emergent case of tumor lysis syndrome leading to death early in the trial. This phenomenon can occur when the CLL breaks down very quickly and, as a consequence, the study was redesigned and a lower starting dose is now used, Dr. Roberts said.
“That problem has been eliminated, but we still see a very large improvement in patients in a few weeks,” he said. “Other than that, there is a little bit of neutropenia, but that is very manageable.”
“So do you think you are curing patients with this approach?” Dr. Hagenbeek asked, to which Dr. Roberts replied, “Too early to say.”
Venetoclax is currently being evaluated in less heavily pretreated patients and a phase III trial is comparing the combination of venetoclax and rituximab with standard bendamustine chemotherapy plus rituximab, he said.
In May, the Food and Drug Administration granted venetoclax breakthrough therapy designation for use in relapsed or refractory chronic lymphocytic leukemia with a 17p deletion mutation.
AbbVie, which is developing venetoclax in partnership with Roche and Genentech, plans to submit regulatory applications for venetoclax to the FDA and the European Medicines Agency before the end of 2015.
On Twitter @pwendl
VIENNA – A daily dose of the investigational BCL-2 inhibitor venetoclax plus rituximab induced responses in 84% of patients with relapsed or refractory chronic lymphocytic leukemia in a phase Ib study.
Of the 49 patients, 20 (41%) achieved a complete response by standard assessment and 13 (27%) achieved a complete response with no evidence of residual disease on flow cytometry.
Moreover, six patients elected to stop venetoclax after achieving a complete response and, to date, only one has had recurrence of disease after 24 months without therapy, lead investigator Dr. Andrew W. Roberts reported at the annual congress of the European Hematology Association.
Not only were patients able to come off treatment and continue to remain in complete response, but responses were seen at the same frequencies across all classes of cytogenetic and molecular abnormalities, he noted.
“The greatest advance that this drug brings is for those patients who currently have a terrible prognosis with all other drugs that we now have,” Dr. Roberts of Royal Melbourne Hospital said in a press briefing.
“This is an important step forward in finding chemotherapy-free regimens in these vulnerable, elderly patients,” said press briefing moderator Dr. Anton Hagenbeek of the University Medical Center, Utrecht, the Netherlands.
Patients in the open-label, dose-escalation study had received a median of two prior lines of therapy (range, one to five) for chronic lymphocytic leukemia (CLL); their median age was 68 years (50-88 years). They began treatment with 20 mg or 50 mg venetoclax daily, increasing weekly to final cohort doses of 200 mg to 600 mg. Six cycles of monthly standard rituximab were added after the weekly lead-in phase.
CLL depends on high levels of B-cell lymphoma-2 (BCL-2) to stay alive. Venetoclax binds to and switches off the BCL-2 protein function, triggering the death of the CLL cell.
Grade 3 or 4 adverse events occurring in more than 10% of patients were neutropenia (51%), thrombocytopenia (16%), and anemia (14%). There was one treatment-emergent case of tumor lysis syndrome leading to death early in the trial. This phenomenon can occur when the CLL breaks down very quickly and, as a consequence, the study was redesigned and a lower starting dose is now used, Dr. Roberts said.
“That problem has been eliminated, but we still see a very large improvement in patients in a few weeks,” he said. “Other than that, there is a little bit of neutropenia, but that is very manageable.”
“So do you think you are curing patients with this approach?” Dr. Hagenbeek asked, to which Dr. Roberts replied, “Too early to say.”
Venetoclax is currently being evaluated in less heavily pretreated patients and a phase III trial is comparing the combination of venetoclax and rituximab with standard bendamustine chemotherapy plus rituximab, he said.
In May, the Food and Drug Administration granted venetoclax breakthrough therapy designation for use in relapsed or refractory chronic lymphocytic leukemia with a 17p deletion mutation.
AbbVie, which is developing venetoclax in partnership with Roche and Genentech, plans to submit regulatory applications for venetoclax to the FDA and the European Medicines Agency before the end of 2015.
On Twitter @pwendl
AT THE EHA CONGRESS
Key clinical point: Venetoclax plus rituximab is a highly active nonchemotherapy combination for patients with relapsed or refractory chronic lymphocytic leukemia.
Major finding: Overall, 84% of patients responded to venetoclax plus rituximab.
Data source: Phase Ib trial in 49 patients with relapsed or refractory chronic lymphocytic leukemia.
Disclosures: AbbVie sponsored the study. Dr. Roberts’ financial disclosures were not available at press time.
Pruritic Urticarial Papules and Plaques of Pregnancy Occurring Postpartum
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.

Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
Practice Points
- Pruritic urticarial papules and plaques of pregnancy (PUPPP) is an intensely pruritic eruption that typically affects women during the third trimester of pregnancy.
- Because clinical manifestations can vary, PUPPP should be considered in the differential diagnosis when patients present in the postpartum period with a pruritic eruption.
- Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.
Identifying melasma triggers
Melasma can be a very frustrating, remitting, and relapsing condition, particularly in the summer months. Often patients get good results with at-home and in-office treatments and return frustrated as the melasma frequently recurs. A thorough history can help identify melasma triggers.
Ask about exposure to:
1. Any heat source. You will be surprised by the answers. Examples include overhead work lights, overhead desk lamps, extensive cooking over an oven or a grill, lamps used to treat seasonal affective disorder, heating lamps, and hair dryers. Heat is a very common trigger for melasma as it increases vasodilation. Melasma is typically thought of as solely hyperpigmentation; however, vascular dilatation often occurs in the affected area. In addition, heat may lead to more inflammation, also stimulating melanocyte pigment production.
2. UV sources. These include computer screens, car side windows, sunroofs (even if the roof glass is closed, UV can penetrate the glass, so the sunroof shade also should be closed), and a window near an office desk or a window near a bed (UVA penetrates window glass).
3. Visible light sources. Examples are overhead lights at home and in office buildings. These lights increase pigmentation. Iron oxide in sunscreens helps block visible light.
4. Hormonal triggers. These include birth control pills, hormone-releasing intrauterine devices, hormone therapy, and vitamin supplements such as those used for pregnancy, nursing, and perimenopausal symptoms (such as black cohosh and dong quai).
5. Other triggers:• Scented or deodorant soaps, toiletries, cosmetics, or fragrances that may cause phototoxic reactions. These reactions may in turn trigger melasma, which may then persist.
• Sunglasses. This is the most common avoidable trigger. Aviator sunglasses or sunglasses with metal rims, or metal attached to the inside handle or rim absorb the heat when in the sun and/or when left in the car. The metal gets warm, and the heat transfers to the skin when the sunglasses are placed on the face. I ask every melasma patient to bring in all their sunglasses so I can check for metal on the rim or handles. This is a very common trigger, and patients are shocked after they observe that streaks of melasma can often follow the pattern of their sunglasses.
• Autoimmune thyroid disorders, chronic stress, or adrenal dysfunction.
• Triggers of melanocyte-stimulating hormone.
The history is crucial to long-term clearance of melasma. Asking questions to get to the source of the trigger often can help isolate the cause and help eliminate significant recurrences of melasma in skin of color patients.
Dr. Wesley and Dr. Talakoub are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month's column is by Dr. Talakoub.
Melasma can be a very frustrating, remitting, and relapsing condition, particularly in the summer months. Often patients get good results with at-home and in-office treatments and return frustrated as the melasma frequently recurs. A thorough history can help identify melasma triggers.
Ask about exposure to:
1. Any heat source. You will be surprised by the answers. Examples include overhead work lights, overhead desk lamps, extensive cooking over an oven or a grill, lamps used to treat seasonal affective disorder, heating lamps, and hair dryers. Heat is a very common trigger for melasma as it increases vasodilation. Melasma is typically thought of as solely hyperpigmentation; however, vascular dilatation often occurs in the affected area. In addition, heat may lead to more inflammation, also stimulating melanocyte pigment production.
2. UV sources. These include computer screens, car side windows, sunroofs (even if the roof glass is closed, UV can penetrate the glass, so the sunroof shade also should be closed), and a window near an office desk or a window near a bed (UVA penetrates window glass).
3. Visible light sources. Examples are overhead lights at home and in office buildings. These lights increase pigmentation. Iron oxide in sunscreens helps block visible light.
4. Hormonal triggers. These include birth control pills, hormone-releasing intrauterine devices, hormone therapy, and vitamin supplements such as those used for pregnancy, nursing, and perimenopausal symptoms (such as black cohosh and dong quai).
5. Other triggers:• Scented or deodorant soaps, toiletries, cosmetics, or fragrances that may cause phototoxic reactions. These reactions may in turn trigger melasma, which may then persist.
• Sunglasses. This is the most common avoidable trigger. Aviator sunglasses or sunglasses with metal rims, or metal attached to the inside handle or rim absorb the heat when in the sun and/or when left in the car. The metal gets warm, and the heat transfers to the skin when the sunglasses are placed on the face. I ask every melasma patient to bring in all their sunglasses so I can check for metal on the rim or handles. This is a very common trigger, and patients are shocked after they observe that streaks of melasma can often follow the pattern of their sunglasses.
• Autoimmune thyroid disorders, chronic stress, or adrenal dysfunction.
• Triggers of melanocyte-stimulating hormone.
The history is crucial to long-term clearance of melasma. Asking questions to get to the source of the trigger often can help isolate the cause and help eliminate significant recurrences of melasma in skin of color patients.
Dr. Wesley and Dr. Talakoub are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month's column is by Dr. Talakoub.
Melasma can be a very frustrating, remitting, and relapsing condition, particularly in the summer months. Often patients get good results with at-home and in-office treatments and return frustrated as the melasma frequently recurs. A thorough history can help identify melasma triggers.
Ask about exposure to:
1. Any heat source. You will be surprised by the answers. Examples include overhead work lights, overhead desk lamps, extensive cooking over an oven or a grill, lamps used to treat seasonal affective disorder, heating lamps, and hair dryers. Heat is a very common trigger for melasma as it increases vasodilation. Melasma is typically thought of as solely hyperpigmentation; however, vascular dilatation often occurs in the affected area. In addition, heat may lead to more inflammation, also stimulating melanocyte pigment production.
2. UV sources. These include computer screens, car side windows, sunroofs (even if the roof glass is closed, UV can penetrate the glass, so the sunroof shade also should be closed), and a window near an office desk or a window near a bed (UVA penetrates window glass).
3. Visible light sources. Examples are overhead lights at home and in office buildings. These lights increase pigmentation. Iron oxide in sunscreens helps block visible light.
4. Hormonal triggers. These include birth control pills, hormone-releasing intrauterine devices, hormone therapy, and vitamin supplements such as those used for pregnancy, nursing, and perimenopausal symptoms (such as black cohosh and dong quai).
5. Other triggers:• Scented or deodorant soaps, toiletries, cosmetics, or fragrances that may cause phototoxic reactions. These reactions may in turn trigger melasma, which may then persist.
• Sunglasses. This is the most common avoidable trigger. Aviator sunglasses or sunglasses with metal rims, or metal attached to the inside handle or rim absorb the heat when in the sun and/or when left in the car. The metal gets warm, and the heat transfers to the skin when the sunglasses are placed on the face. I ask every melasma patient to bring in all their sunglasses so I can check for metal on the rim or handles. This is a very common trigger, and patients are shocked after they observe that streaks of melasma can often follow the pattern of their sunglasses.
• Autoimmune thyroid disorders, chronic stress, or adrenal dysfunction.
• Triggers of melanocyte-stimulating hormone.
The history is crucial to long-term clearance of melasma. Asking questions to get to the source of the trigger often can help isolate the cause and help eliminate significant recurrences of melasma in skin of color patients.
Dr. Wesley and Dr. Talakoub are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month's column is by Dr. Talakoub.