User login
Case Studies in Toxicology: The Perilous Pursuit of Perfection
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
When it's beneficial to defer dialysis
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
THE CASE
A 94-year-old Hispanic man with hypertension, congestive heart failure (CHF), anemia of chronic disease, and end-stage renal disease (ESRD) presented to our facility with weakness and shortness of breath. We diagnosed a CHF exacerbation. Initially, he exhibited some respiratory distress that required observation in the coronary care unit and bi-level positive airway pressure therapy to maintain oxygen saturation. Our patient was then moved to a step-down unit where his primary caregiver, his granddaughter, told the medical team that he was limited at home in some of his instrumental activities of daily living. Specifically, he was unable to prepare meals or manage his finances on his own.
Nephrology was consulted for consideration of hemodialysis (HD) because our patient’s creatinine on admission was 7.2 mg/dL (normal for men is 0.7-1.3 mg/dL) and his estimated glomerular filtration rate (GFR) was 7 mL/min (normal is 90-120 mL/min). The patient’s family was conflicted over whether or not to start HD. Palliative Care was consulted to help establish goals of care.
A decision is made. In light of the patient’s limited functional status and his expressed desire to stay at home with his family and receive limited medical care there, the Nephrology and Palliative Care teams recommended delaying HD despite the patient’s worsening renal function. The patient was discharged home with home care services, and he and the family were instructed to follow up with Nephrology for supportive renal management.
DISCUSSION
The decision to delay HD in patients with ESRD is a difficult one that requires shared decision-making between patients and medical providers. Palliative Care consultation services are often involved in this process.
Recent literature supports an “intent-to-defer” based on an evaluation of the patient’s functionality. This represents a paradigm shift from the previous “intent-to-start-early” treatment strategy. In fact, rather than starting early, the Canadian Society of Nephrology recommends delaying initiation of HD in patients with a GFR <15 mL/min.1 Close monitoring of these patients by both a primary care physician and nephrologist is essential.
When considering initiation of HD, it’s important to look at the overall benefit of this intervention in light of the patient’s mortality risk and quality of life. Many patients who receive HD—especially the elderly—report that it takes more than 6 hours to recover following a dialysis treatment.2
Not surprisingly, depression is common in elderly HD patients. Compared to their younger cohorts, older HD patients have a 62% increased risk of developing depression.3 Also, patients who are considered frail and are receiving HD have more than 3 times the mortality risk within one year than those who are not (hazard ratio=3.42; 95% confidence interval, 2.45-4.76).4 (The researchers’ definition of frailty included poor self-reported physical function, exhaustion/fatigue, low physical activity, and undernutrition.4)
Functional status. Although a patient’s age should not be a limiting factor for HD referral, functional status should be considered. Patients with limited functionality and significant dependence have an increased risk of death during the first year of HD.5
Palliative approach gains acceptance. It is becoming more accepted within the nephrology community to consider a palliative approach to patients with ESRD. Organizations such as the Renal Physicians Association recommend effective prognostication, early advanced care planning, forgoing HD in patients with a poor prognosis, and involving Palliative Care early in the decision-making process.6 Aligning the patient’s goals of care with the appropriate treatment method—particularly in patients with comorbid conditions—is an important practice when caring for those with limited life expectancy and functionality.7
THE TAKEAWAY
Intent-to-defer HD may be a preferred strategy when caring for many patients with ESRD. Taking into consideration a patient’s comorbidities and functional status, while considering mortality risk and quality of life are essential. Involving palliative care and nephrology specialists can help patients and families understand HD and make an educated decision regarding when to start it.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
1. Nesrallah GE, Mustafa RA, Clark WF, et al; Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112-117.
2. Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86-94.
3. Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6:1651-1662.
4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967.
5. Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012-1021.
6. Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
7. Grubbs V, Moss AH, Cohen LM, et al; Dialysis Advisory Group of the American Society of Nephrology. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203-2209.
Weight loss • fatigue • joint pain • Dx?
THE CASE
A 49-year-old Mexican immigrant woman was admitted to the hospital with a 5-month history of fatigue and a 30-pound unintentional weight loss. She was also experiencing arthralgia, swelling, and stiffness in her hands and feet that was worse in the morning. The patient, who was obese and suffered from type 2 diabetes and hypertension, said that at the onset of her illness 5 months earlier, she’d experienced approximately 2 weeks of night sweats and a few days of fever.
A month before being admitted to the hospital, she’d been seen in our southern New Mexico family medicine office. Her recent history of fever, joint symptoms, and weight loss raised concerns of an insidious infection, a new-onset rheumatologic condition, or an occult malignancy.
Initial lab tests revealed leukopenia (white blood cell count, 3200/mcL), microcytic anemia (hemoglobin, 9.4 g/dL), and an elevated erythrocyte sedimentation rate of 30 mm/hr (normal range, 0-20 mm/hr). A rheumatoid factor test was negative, and her thyroid, kidney, and liver function tests were all normal.
More testing… The patient frequently traveled between New Mexico and her hometown of Chihuahua, Mexico, but there had been no recent changes in her diet or environmental exposures. She denied drinking any unpasteurized milk in Chihuahua. But based on her travel history, we ordered enzyme-linked immunosorbent assay (ELISA) antibody titers for Brucella, immunoglobulin G, and immunoglobulin M, which all came back negative. Additionally, we ordered an abdominal and pelvic ultrasound and a chest x-ray that were nondiagnostic. Given the patient’s weight loss and anemia, we referred her to a gastroenterologist for upper and lower gastrointestinal endoscopic evaluations. Unfortunately, the patient was uninsured and did not go to see the gastroenterologist.
A month after seeing us, our patient’s fatigue, lack of appetite, and joint pain became debilitating and she was admitted to the hospital for further evaluation, including a consultation with an oncologist.
THE DIAGNOSIS
During our patient’s 6-day hospital stay, a bone scintigraphy showed a focus of uptake in her left parietal bone and computed tomography scans of her chest, abdomen, and pelvis revealed a left thyroid nodule, as well as multiple noncalcified pulmonary nodules. Blood cultures were also obtained.
Despite the initial negative antibody tests, the blood cultures drawn in the hospital revealed the presence of Brucella melitensis, and we diagnosed brucellosis in this patient.
DISCUSSION
Brucella melitensis is one of the 4 recognized, land-based species of the Brucella genus that can cause disease in humans. Goats, sheep, and camels are natural hosts of B melitensis and consumption of their unpasteurized, infected milk and milk products (especially soft cheeses, ice cream, milk, and butter) leads to human disease. (Once hospitalized, our patient admitted to frequently eating unpasteurized goat cheese from Chihuahua. The only other person in her household that ate the cheese was her 26-year-old daughter, who was also experiencing similar symptoms.)
Brucellosis can also result from inhaling infected, aerosolized material; therefore, individuals whose occupations involve close work with host animals or work in laboratories with the bacteria have an increased risk of infection.1 Due to the risk of acquiring the infection via inhalation, brucellosis is considered a bioterrorism threat.2 Additionally, there have been reports of human-to-human transmission via sexual intercourse, transplacental infection, blood and bone marrow transfusion, and breastfeeding.3
B melitensis is the cause of the majority of Brucella-related illnesses in the world, though symptoms of infection are similar among the different Brucella species. The pathogen can affect almost all organ systems after the initial 2- to 4-week incubation period. Symptoms of brucellosis can be highly variable, although fever is consistently present.1 Other red flags include arthritis (usually affecting the peripheral joints, the sacroiliac joints, and the lower spine), epididymo-orchitis, and hepatitis resulting in transaminase elevation. Abscess formation can be seen in the liver, spleen, and other organs.
Less common but more ominous complications include central nervous system infections and abscesses, endocarditis, and pulmonary infections. Endocarditis and the resulting aortic valve involvement is the major cause of mortality.1Brucella-related uveitis, thyroiditis, nephritis, vasculitis, and acalculous cholecystitis have also been reported.4-9
Rare in the United States. Pasteurization of dairy products and mass vaccination of livestock make Brucella infection rare in the United States. While there have only been 80 to 139 cases of brucellosis reported per year in the United States since 1993, it remains a persistent threat. International travel is common from the United States to the Middle East and other parts of the world where brucellosis is endemic.
Additionally, infection of livestock with Brucella remains widespread in Mexico and the consumption of unpasteurized Mexican dairy products from goats and sheep remains a high-risk activity for acquiring the disease.10 Consequently, Texas and California account for more than half of the brucellosis diagnoses in the United States. However, in 2010, cases were reported in 25 other states and the District of Columbia.11
Repeat serology tests are preferred for confirming the Dx
It is interesting that our patient’s initial Brucella serology by ELISA was negative, because it was ordered months after her initial symptoms. Antibodies should be seen within a month of symptom onset. The Centers for Disease Control and Prevention (CDC) recommends taking 2 serum samples to establish a serologic diagnosis of brucellosis. The first should be drawn within 7 days of symptom onset and the second should be taken 2 to 4 weeks later. A greater than 4-fold rise in the antibody titer confirms the diagnosis. While ELISA is an acceptable serologic test, the CDC recommends using a serum tube agglutination test called the Brucella microagglutination test (BMAT).12 Repeat serology was not performed on our patient because the diagnosis had been confirmed by blood culture.
A combination of antibiotics is the recommended treatment
Treatment of brucellosis should include a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin 600 mg/d for an all-oral regimen.13 Doxycycline 100 mg twice a day is preferred due to fewer gastrointestinal adverse effects than tetracycline. Relapse is not uncommon (10%) and usually occurs within one year of completing the antibiotics.8 However, there is a case report of a patient having reactivated brucellosis manifested as acalculous cholecystitis 28 years after completing antibiotics.8
Our patient was started on oral doxycycline 100 mg twice a day and oral rifampin 600 mg/d for 6 weeks. Within days of starting the antibiotics, her joint symptoms and fatigue rapidly abated and her appetite returned. Follow-up radiological testing was not performed after her initial hospital studies due to her lack of financial resources.
The patient’s daughter had also been experiencing night sweats, chills, malaise, anorexia, joint pains, weight loss, and alopecia over the previous 2 months. Her blood cultures were positive for B melitensis as well, and she was started on the same antibiotic regimen as her mother. The daughter was also seen in our clinic by another physician and improved quickly within a week of starting treatment.
Both our patient and her daughter remained symptom-free 6 years after treatment.
THE TAKEAWAY
Brucellosis is rare in the United States, but international travel to endemic areas is commonplace and consumption of unpasteurized Mexican dairy products from goats and sheep is widespread. Brucellosis has a wide range of symptoms, but a prompt diagnosis by an ELISA or BMAT serologic test and appropriate treatment can avoid morbidity and mortality. Treatment includes a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin.
1. Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med. 2005;352:2325-2336.
2. Centers for Disease Control and Prevention (CDC). Suspected brucellosis case prompts investigation of possible bioterrorismrelated activity—New Hampshire and Massachusetts, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:509-512.
3. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis. 2014;20:126-130.
4. Rolando I, Vilchez G, Olarte L, et al. Brucellar uveitis: intraocular fluids and biopsy studies. Int J Infect Dis. 2009;13:e206-e211.
5. Azizi F, Katchoui A. Brucella infection of the thyroid gland. Thyroid. 1996;6:461-463.
6. Siegelmann N, Abraham AS, Rudensky B, et al. Brucellosis with nephrotic syndrome, nephritis and IgA nephropathy. Postgrad Med J. 1992;68:834-836.
7. Tanyel E, Tasdelen Fisgin N, Yildiz L, et al. Panniculitis as the initial manifestation of brucellosis: a case report. Am J Dermatopathol. 2008;30:169-171.
8. Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis. 2010;16:2021-2022.
9. Dhand A, Ross JJ. Implantable cardioverter-defibrillator infection due to Brucella melitensis: case report and review of brucellosis of cardiac devices. Clin Infect Dis. 2007;44:e37-e39.
10. Solorio-Rivera JL, Segura-Correa JC, Sánchez-Gil LG. Seroprevalence of and risk factors for brucellosis of goats in herds of Michoacan, Mexico. Prev Vet Med. 2007;82:282-290.
11. Centers for Disease Control and Prevention. Brucellosis surveillance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/resources/surveillance.html. Accessed October 30, 2015.
12. Centers for Disease Control and Prevention. Serology. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/clinicians/serology.html. Accessed October 30, 2015.
13. Corbel MJ. Brucellosis in humans and animals. World Health Organization (WHO);2006:36-41. Available at: http://www.who.int/csr/resources/publications/Brucellosis.pdf. Accessed November 2, 2015.
THE CASE
A 49-year-old Mexican immigrant woman was admitted to the hospital with a 5-month history of fatigue and a 30-pound unintentional weight loss. She was also experiencing arthralgia, swelling, and stiffness in her hands and feet that was worse in the morning. The patient, who was obese and suffered from type 2 diabetes and hypertension, said that at the onset of her illness 5 months earlier, she’d experienced approximately 2 weeks of night sweats and a few days of fever.
A month before being admitted to the hospital, she’d been seen in our southern New Mexico family medicine office. Her recent history of fever, joint symptoms, and weight loss raised concerns of an insidious infection, a new-onset rheumatologic condition, or an occult malignancy.
Initial lab tests revealed leukopenia (white blood cell count, 3200/mcL), microcytic anemia (hemoglobin, 9.4 g/dL), and an elevated erythrocyte sedimentation rate of 30 mm/hr (normal range, 0-20 mm/hr). A rheumatoid factor test was negative, and her thyroid, kidney, and liver function tests were all normal.
More testing… The patient frequently traveled between New Mexico and her hometown of Chihuahua, Mexico, but there had been no recent changes in her diet or environmental exposures. She denied drinking any unpasteurized milk in Chihuahua. But based on her travel history, we ordered enzyme-linked immunosorbent assay (ELISA) antibody titers for Brucella, immunoglobulin G, and immunoglobulin M, which all came back negative. Additionally, we ordered an abdominal and pelvic ultrasound and a chest x-ray that were nondiagnostic. Given the patient’s weight loss and anemia, we referred her to a gastroenterologist for upper and lower gastrointestinal endoscopic evaluations. Unfortunately, the patient was uninsured and did not go to see the gastroenterologist.
A month after seeing us, our patient’s fatigue, lack of appetite, and joint pain became debilitating and she was admitted to the hospital for further evaluation, including a consultation with an oncologist.
THE DIAGNOSIS
During our patient’s 6-day hospital stay, a bone scintigraphy showed a focus of uptake in her left parietal bone and computed tomography scans of her chest, abdomen, and pelvis revealed a left thyroid nodule, as well as multiple noncalcified pulmonary nodules. Blood cultures were also obtained.
Despite the initial negative antibody tests, the blood cultures drawn in the hospital revealed the presence of Brucella melitensis, and we diagnosed brucellosis in this patient.
DISCUSSION
Brucella melitensis is one of the 4 recognized, land-based species of the Brucella genus that can cause disease in humans. Goats, sheep, and camels are natural hosts of B melitensis and consumption of their unpasteurized, infected milk and milk products (especially soft cheeses, ice cream, milk, and butter) leads to human disease. (Once hospitalized, our patient admitted to frequently eating unpasteurized goat cheese from Chihuahua. The only other person in her household that ate the cheese was her 26-year-old daughter, who was also experiencing similar symptoms.)
Brucellosis can also result from inhaling infected, aerosolized material; therefore, individuals whose occupations involve close work with host animals or work in laboratories with the bacteria have an increased risk of infection.1 Due to the risk of acquiring the infection via inhalation, brucellosis is considered a bioterrorism threat.2 Additionally, there have been reports of human-to-human transmission via sexual intercourse, transplacental infection, blood and bone marrow transfusion, and breastfeeding.3
B melitensis is the cause of the majority of Brucella-related illnesses in the world, though symptoms of infection are similar among the different Brucella species. The pathogen can affect almost all organ systems after the initial 2- to 4-week incubation period. Symptoms of brucellosis can be highly variable, although fever is consistently present.1 Other red flags include arthritis (usually affecting the peripheral joints, the sacroiliac joints, and the lower spine), epididymo-orchitis, and hepatitis resulting in transaminase elevation. Abscess formation can be seen in the liver, spleen, and other organs.
Less common but more ominous complications include central nervous system infections and abscesses, endocarditis, and pulmonary infections. Endocarditis and the resulting aortic valve involvement is the major cause of mortality.1Brucella-related uveitis, thyroiditis, nephritis, vasculitis, and acalculous cholecystitis have also been reported.4-9
Rare in the United States. Pasteurization of dairy products and mass vaccination of livestock make Brucella infection rare in the United States. While there have only been 80 to 139 cases of brucellosis reported per year in the United States since 1993, it remains a persistent threat. International travel is common from the United States to the Middle East and other parts of the world where brucellosis is endemic.
Additionally, infection of livestock with Brucella remains widespread in Mexico and the consumption of unpasteurized Mexican dairy products from goats and sheep remains a high-risk activity for acquiring the disease.10 Consequently, Texas and California account for more than half of the brucellosis diagnoses in the United States. However, in 2010, cases were reported in 25 other states and the District of Columbia.11
Repeat serology tests are preferred for confirming the Dx
It is interesting that our patient’s initial Brucella serology by ELISA was negative, because it was ordered months after her initial symptoms. Antibodies should be seen within a month of symptom onset. The Centers for Disease Control and Prevention (CDC) recommends taking 2 serum samples to establish a serologic diagnosis of brucellosis. The first should be drawn within 7 days of symptom onset and the second should be taken 2 to 4 weeks later. A greater than 4-fold rise in the antibody titer confirms the diagnosis. While ELISA is an acceptable serologic test, the CDC recommends using a serum tube agglutination test called the Brucella microagglutination test (BMAT).12 Repeat serology was not performed on our patient because the diagnosis had been confirmed by blood culture.
A combination of antibiotics is the recommended treatment
Treatment of brucellosis should include a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin 600 mg/d for an all-oral regimen.13 Doxycycline 100 mg twice a day is preferred due to fewer gastrointestinal adverse effects than tetracycline. Relapse is not uncommon (10%) and usually occurs within one year of completing the antibiotics.8 However, there is a case report of a patient having reactivated brucellosis manifested as acalculous cholecystitis 28 years after completing antibiotics.8
Our patient was started on oral doxycycline 100 mg twice a day and oral rifampin 600 mg/d for 6 weeks. Within days of starting the antibiotics, her joint symptoms and fatigue rapidly abated and her appetite returned. Follow-up radiological testing was not performed after her initial hospital studies due to her lack of financial resources.
The patient’s daughter had also been experiencing night sweats, chills, malaise, anorexia, joint pains, weight loss, and alopecia over the previous 2 months. Her blood cultures were positive for B melitensis as well, and she was started on the same antibiotic regimen as her mother. The daughter was also seen in our clinic by another physician and improved quickly within a week of starting treatment.
Both our patient and her daughter remained symptom-free 6 years after treatment.
THE TAKEAWAY
Brucellosis is rare in the United States, but international travel to endemic areas is commonplace and consumption of unpasteurized Mexican dairy products from goats and sheep is widespread. Brucellosis has a wide range of symptoms, but a prompt diagnosis by an ELISA or BMAT serologic test and appropriate treatment can avoid morbidity and mortality. Treatment includes a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin.
THE CASE
A 49-year-old Mexican immigrant woman was admitted to the hospital with a 5-month history of fatigue and a 30-pound unintentional weight loss. She was also experiencing arthralgia, swelling, and stiffness in her hands and feet that was worse in the morning. The patient, who was obese and suffered from type 2 diabetes and hypertension, said that at the onset of her illness 5 months earlier, she’d experienced approximately 2 weeks of night sweats and a few days of fever.
A month before being admitted to the hospital, she’d been seen in our southern New Mexico family medicine office. Her recent history of fever, joint symptoms, and weight loss raised concerns of an insidious infection, a new-onset rheumatologic condition, or an occult malignancy.
Initial lab tests revealed leukopenia (white blood cell count, 3200/mcL), microcytic anemia (hemoglobin, 9.4 g/dL), and an elevated erythrocyte sedimentation rate of 30 mm/hr (normal range, 0-20 mm/hr). A rheumatoid factor test was negative, and her thyroid, kidney, and liver function tests were all normal.
More testing… The patient frequently traveled between New Mexico and her hometown of Chihuahua, Mexico, but there had been no recent changes in her diet or environmental exposures. She denied drinking any unpasteurized milk in Chihuahua. But based on her travel history, we ordered enzyme-linked immunosorbent assay (ELISA) antibody titers for Brucella, immunoglobulin G, and immunoglobulin M, which all came back negative. Additionally, we ordered an abdominal and pelvic ultrasound and a chest x-ray that were nondiagnostic. Given the patient’s weight loss and anemia, we referred her to a gastroenterologist for upper and lower gastrointestinal endoscopic evaluations. Unfortunately, the patient was uninsured and did not go to see the gastroenterologist.
A month after seeing us, our patient’s fatigue, lack of appetite, and joint pain became debilitating and she was admitted to the hospital for further evaluation, including a consultation with an oncologist.
THE DIAGNOSIS
During our patient’s 6-day hospital stay, a bone scintigraphy showed a focus of uptake in her left parietal bone and computed tomography scans of her chest, abdomen, and pelvis revealed a left thyroid nodule, as well as multiple noncalcified pulmonary nodules. Blood cultures were also obtained.
Despite the initial negative antibody tests, the blood cultures drawn in the hospital revealed the presence of Brucella melitensis, and we diagnosed brucellosis in this patient.
DISCUSSION
Brucella melitensis is one of the 4 recognized, land-based species of the Brucella genus that can cause disease in humans. Goats, sheep, and camels are natural hosts of B melitensis and consumption of their unpasteurized, infected milk and milk products (especially soft cheeses, ice cream, milk, and butter) leads to human disease. (Once hospitalized, our patient admitted to frequently eating unpasteurized goat cheese from Chihuahua. The only other person in her household that ate the cheese was her 26-year-old daughter, who was also experiencing similar symptoms.)
Brucellosis can also result from inhaling infected, aerosolized material; therefore, individuals whose occupations involve close work with host animals or work in laboratories with the bacteria have an increased risk of infection.1 Due to the risk of acquiring the infection via inhalation, brucellosis is considered a bioterrorism threat.2 Additionally, there have been reports of human-to-human transmission via sexual intercourse, transplacental infection, blood and bone marrow transfusion, and breastfeeding.3
B melitensis is the cause of the majority of Brucella-related illnesses in the world, though symptoms of infection are similar among the different Brucella species. The pathogen can affect almost all organ systems after the initial 2- to 4-week incubation period. Symptoms of brucellosis can be highly variable, although fever is consistently present.1 Other red flags include arthritis (usually affecting the peripheral joints, the sacroiliac joints, and the lower spine), epididymo-orchitis, and hepatitis resulting in transaminase elevation. Abscess formation can be seen in the liver, spleen, and other organs.
Less common but more ominous complications include central nervous system infections and abscesses, endocarditis, and pulmonary infections. Endocarditis and the resulting aortic valve involvement is the major cause of mortality.1Brucella-related uveitis, thyroiditis, nephritis, vasculitis, and acalculous cholecystitis have also been reported.4-9
Rare in the United States. Pasteurization of dairy products and mass vaccination of livestock make Brucella infection rare in the United States. While there have only been 80 to 139 cases of brucellosis reported per year in the United States since 1993, it remains a persistent threat. International travel is common from the United States to the Middle East and other parts of the world where brucellosis is endemic.
Additionally, infection of livestock with Brucella remains widespread in Mexico and the consumption of unpasteurized Mexican dairy products from goats and sheep remains a high-risk activity for acquiring the disease.10 Consequently, Texas and California account for more than half of the brucellosis diagnoses in the United States. However, in 2010, cases were reported in 25 other states and the District of Columbia.11
Repeat serology tests are preferred for confirming the Dx
It is interesting that our patient’s initial Brucella serology by ELISA was negative, because it was ordered months after her initial symptoms. Antibodies should be seen within a month of symptom onset. The Centers for Disease Control and Prevention (CDC) recommends taking 2 serum samples to establish a serologic diagnosis of brucellosis. The first should be drawn within 7 days of symptom onset and the second should be taken 2 to 4 weeks later. A greater than 4-fold rise in the antibody titer confirms the diagnosis. While ELISA is an acceptable serologic test, the CDC recommends using a serum tube agglutination test called the Brucella microagglutination test (BMAT).12 Repeat serology was not performed on our patient because the diagnosis had been confirmed by blood culture.
A combination of antibiotics is the recommended treatment
Treatment of brucellosis should include a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin 600 mg/d for an all-oral regimen.13 Doxycycline 100 mg twice a day is preferred due to fewer gastrointestinal adverse effects than tetracycline. Relapse is not uncommon (10%) and usually occurs within one year of completing the antibiotics.8 However, there is a case report of a patient having reactivated brucellosis manifested as acalculous cholecystitis 28 years after completing antibiotics.8
Our patient was started on oral doxycycline 100 mg twice a day and oral rifampin 600 mg/d for 6 weeks. Within days of starting the antibiotics, her joint symptoms and fatigue rapidly abated and her appetite returned. Follow-up radiological testing was not performed after her initial hospital studies due to her lack of financial resources.
The patient’s daughter had also been experiencing night sweats, chills, malaise, anorexia, joint pains, weight loss, and alopecia over the previous 2 months. Her blood cultures were positive for B melitensis as well, and she was started on the same antibiotic regimen as her mother. The daughter was also seen in our clinic by another physician and improved quickly within a week of starting treatment.
Both our patient and her daughter remained symptom-free 6 years after treatment.
THE TAKEAWAY
Brucellosis is rare in the United States, but international travel to endemic areas is commonplace and consumption of unpasteurized Mexican dairy products from goats and sheep is widespread. Brucellosis has a wide range of symptoms, but a prompt diagnosis by an ELISA or BMAT serologic test and appropriate treatment can avoid morbidity and mortality. Treatment includes a tetracycline for at least 6 weeks in combination with an aminoglycoside or rifampin.
1. Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med. 2005;352:2325-2336.
2. Centers for Disease Control and Prevention (CDC). Suspected brucellosis case prompts investigation of possible bioterrorismrelated activity—New Hampshire and Massachusetts, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:509-512.
3. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis. 2014;20:126-130.
4. Rolando I, Vilchez G, Olarte L, et al. Brucellar uveitis: intraocular fluids and biopsy studies. Int J Infect Dis. 2009;13:e206-e211.
5. Azizi F, Katchoui A. Brucella infection of the thyroid gland. Thyroid. 1996;6:461-463.
6. Siegelmann N, Abraham AS, Rudensky B, et al. Brucellosis with nephrotic syndrome, nephritis and IgA nephropathy. Postgrad Med J. 1992;68:834-836.
7. Tanyel E, Tasdelen Fisgin N, Yildiz L, et al. Panniculitis as the initial manifestation of brucellosis: a case report. Am J Dermatopathol. 2008;30:169-171.
8. Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis. 2010;16:2021-2022.
9. Dhand A, Ross JJ. Implantable cardioverter-defibrillator infection due to Brucella melitensis: case report and review of brucellosis of cardiac devices. Clin Infect Dis. 2007;44:e37-e39.
10. Solorio-Rivera JL, Segura-Correa JC, Sánchez-Gil LG. Seroprevalence of and risk factors for brucellosis of goats in herds of Michoacan, Mexico. Prev Vet Med. 2007;82:282-290.
11. Centers for Disease Control and Prevention. Brucellosis surveillance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/resources/surveillance.html. Accessed October 30, 2015.
12. Centers for Disease Control and Prevention. Serology. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/clinicians/serology.html. Accessed October 30, 2015.
13. Corbel MJ. Brucellosis in humans and animals. World Health Organization (WHO);2006:36-41. Available at: http://www.who.int/csr/resources/publications/Brucellosis.pdf. Accessed November 2, 2015.
1. Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med. 2005;352:2325-2336.
2. Centers for Disease Control and Prevention (CDC). Suspected brucellosis case prompts investigation of possible bioterrorismrelated activity—New Hampshire and Massachusetts, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:509-512.
3. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis. 2014;20:126-130.
4. Rolando I, Vilchez G, Olarte L, et al. Brucellar uveitis: intraocular fluids and biopsy studies. Int J Infect Dis. 2009;13:e206-e211.
5. Azizi F, Katchoui A. Brucella infection of the thyroid gland. Thyroid. 1996;6:461-463.
6. Siegelmann N, Abraham AS, Rudensky B, et al. Brucellosis with nephrotic syndrome, nephritis and IgA nephropathy. Postgrad Med J. 1992;68:834-836.
7. Tanyel E, Tasdelen Fisgin N, Yildiz L, et al. Panniculitis as the initial manifestation of brucellosis: a case report. Am J Dermatopathol. 2008;30:169-171.
8. Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis. 2010;16:2021-2022.
9. Dhand A, Ross JJ. Implantable cardioverter-defibrillator infection due to Brucella melitensis: case report and review of brucellosis of cardiac devices. Clin Infect Dis. 2007;44:e37-e39.
10. Solorio-Rivera JL, Segura-Correa JC, Sánchez-Gil LG. Seroprevalence of and risk factors for brucellosis of goats in herds of Michoacan, Mexico. Prev Vet Med. 2007;82:282-290.
11. Centers for Disease Control and Prevention. Brucellosis surveillance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/resources/surveillance.html. Accessed October 30, 2015.
12. Centers for Disease Control and Prevention. Serology. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/brucellosis/clinicians/serology.html. Accessed October 30, 2015.
13. Corbel MJ. Brucellosis in humans and animals. World Health Organization (WHO);2006:36-41. Available at: http://www.who.int/csr/resources/publications/Brucellosis.pdf. Accessed November 2, 2015.
Masters of complexity
While some physicians prefer a narrower clinic focus, the wide variety and complexity of the care provided by family physicians is precisely what drew many of us to the specialty. We enjoy caring for patients of all ages who see us for the diagnosis and treatment of acute illnesses, management of chronic diseases, preventive care, and behavioral and mental health concerns.
The quantity, variety, and complexity of the care we provide has been well-documented in the literature. In a study performed by the Wisconsin Research Network,1 29 family physicians reported addressing an average of 3.05 problems per patient during 572 office visits. A chart review confirmed physician self-report: 2.82 problems were recorded on average for each visit. For patients older than age 65, an average of 3.88 problems were addressed at each visit. For patients with diabetes, the average was 4.60.
Using data from the 2000 National Ambulatory Medical Care Survey, Katerndahl et al2 estimated the complexity of patient encounters in family practice, cardiology, and psychiatry. They used a formula that took into account the per-patient number of reasons for each visit, diagnoses, and body systems examined, and the variability and diversity of each of these factors. After adjusting the results for length of visit, they found the complexity of care provided per hour by family physicians was 33% higher than that of cardiologists and 5 times higher than that of psychiatrists.
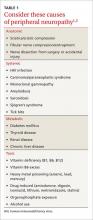
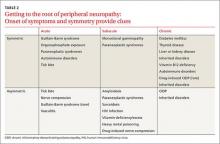
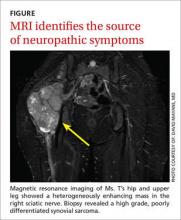
The topics in this issue are a terrific illustration of the breadth of family medicine. Peripheral neuropathy, which we see almost daily, is difficult to diagnose and treat. Prolotherapy is an up-and-coming dextrose injection therapy for tendinopathies and joint pain that shows promise, and patients are likely to start asking us about it. Home apnea monitors are used frequently for newborns—but how effective are they, and when can you tell parents to discontinue their use?
It seems that vaccine recommendations change every year, and this issue’s Practice Alert covers the latest on meningococcal immunization. This month’s PURL answers the question: Do we really need to “bridge” patients with atrial fibrillation from warfarin to low molecular weight heparin immediately before and after a surgical procedure?
The authors and editors of The Journal of Family Practice constantly strive to bring you the most up-to-date information to support your work as a master of the complexity of primary care practice.
1. Beasley JW, Hankey TH, Erickson R, et al. How many problems do family physicians manage at each encounter? A WReN study. Ann Fam Med. 2004;2:405-410.
2. Katerndahl D, Wood R, Jaén CR. Family medicine outpatient encounters are more complex than those of cardiology and psychiatry. J Am Board Fam Med. 2011;24:6-15.
While some physicians prefer a narrower clinic focus, the wide variety and complexity of the care provided by family physicians is precisely what drew many of us to the specialty. We enjoy caring for patients of all ages who see us for the diagnosis and treatment of acute illnesses, management of chronic diseases, preventive care, and behavioral and mental health concerns.
The quantity, variety, and complexity of the care we provide has been well-documented in the literature. In a study performed by the Wisconsin Research Network,1 29 family physicians reported addressing an average of 3.05 problems per patient during 572 office visits. A chart review confirmed physician self-report: 2.82 problems were recorded on average for each visit. For patients older than age 65, an average of 3.88 problems were addressed at each visit. For patients with diabetes, the average was 4.60.
Using data from the 2000 National Ambulatory Medical Care Survey, Katerndahl et al2 estimated the complexity of patient encounters in family practice, cardiology, and psychiatry. They used a formula that took into account the per-patient number of reasons for each visit, diagnoses, and body systems examined, and the variability and diversity of each of these factors. After adjusting the results for length of visit, they found the complexity of care provided per hour by family physicians was 33% higher than that of cardiologists and 5 times higher than that of psychiatrists.
The topics in this issue are a terrific illustration of the breadth of family medicine. Peripheral neuropathy, which we see almost daily, is difficult to diagnose and treat. Prolotherapy is an up-and-coming dextrose injection therapy for tendinopathies and joint pain that shows promise, and patients are likely to start asking us about it. Home apnea monitors are used frequently for newborns—but how effective are they, and when can you tell parents to discontinue their use?
It seems that vaccine recommendations change every year, and this issue’s Practice Alert covers the latest on meningococcal immunization. This month’s PURL answers the question: Do we really need to “bridge” patients with atrial fibrillation from warfarin to low molecular weight heparin immediately before and after a surgical procedure?
The authors and editors of The Journal of Family Practice constantly strive to bring you the most up-to-date information to support your work as a master of the complexity of primary care practice.
While some physicians prefer a narrower clinic focus, the wide variety and complexity of the care provided by family physicians is precisely what drew many of us to the specialty. We enjoy caring for patients of all ages who see us for the diagnosis and treatment of acute illnesses, management of chronic diseases, preventive care, and behavioral and mental health concerns.
The quantity, variety, and complexity of the care we provide has been well-documented in the literature. In a study performed by the Wisconsin Research Network,1 29 family physicians reported addressing an average of 3.05 problems per patient during 572 office visits. A chart review confirmed physician self-report: 2.82 problems were recorded on average for each visit. For patients older than age 65, an average of 3.88 problems were addressed at each visit. For patients with diabetes, the average was 4.60.
Using data from the 2000 National Ambulatory Medical Care Survey, Katerndahl et al2 estimated the complexity of patient encounters in family practice, cardiology, and psychiatry. They used a formula that took into account the per-patient number of reasons for each visit, diagnoses, and body systems examined, and the variability and diversity of each of these factors. After adjusting the results for length of visit, they found the complexity of care provided per hour by family physicians was 33% higher than that of cardiologists and 5 times higher than that of psychiatrists.
The topics in this issue are a terrific illustration of the breadth of family medicine. Peripheral neuropathy, which we see almost daily, is difficult to diagnose and treat. Prolotherapy is an up-and-coming dextrose injection therapy for tendinopathies and joint pain that shows promise, and patients are likely to start asking us about it. Home apnea monitors are used frequently for newborns—but how effective are they, and when can you tell parents to discontinue their use?
It seems that vaccine recommendations change every year, and this issue’s Practice Alert covers the latest on meningococcal immunization. This month’s PURL answers the question: Do we really need to “bridge” patients with atrial fibrillation from warfarin to low molecular weight heparin immediately before and after a surgical procedure?
The authors and editors of The Journal of Family Practice constantly strive to bring you the most up-to-date information to support your work as a master of the complexity of primary care practice.
1. Beasley JW, Hankey TH, Erickson R, et al. How many problems do family physicians manage at each encounter? A WReN study. Ann Fam Med. 2004;2:405-410.
2. Katerndahl D, Wood R, Jaén CR. Family medicine outpatient encounters are more complex than those of cardiology and psychiatry. J Am Board Fam Med. 2011;24:6-15.
1. Beasley JW, Hankey TH, Erickson R, et al. How many problems do family physicians manage at each encounter? A WReN study. Ann Fam Med. 2004;2:405-410.
2. Katerndahl D, Wood R, Jaén CR. Family medicine outpatient encounters are more complex than those of cardiology and psychiatry. J Am Board Fam Med. 2011;24:6-15.
Causes of peripheral neuropathy: Diabetes and beyond
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
Home apnea monitors—when to discontinue use
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
A young man with an unusual cause of palpitations
A 23-year-old man presents to the emergency department with the sudden onset of palpitations, lightheadedness, and dyspnea, accompanied by weakness and nausea, which started earlier in the evening. He estimates that he has experienced 15 similar episodes, lasting minutes to hours, since the age of 16, with the last one 3 years ago. These episodes typically end by themselves or with self-induced vomiting and lying supine. The current episode did not resolve with these maneuvers.
He has never received medical attention for these symptoms. He has no chest pain, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, or syncope. He has had no recent illness, contacts with sick people, or travel.
The patient’s history includes a “childhood heart murmur,” which resolved, and also mild asthma. He is otherwise healthy but has not received regular medical care. He used to play competitive soccer but quit because playing made his symptoms of dyspnea on exertion and palpitations much worse.
He uses marijuana frequently and alcohol occasionally. He does not smoke tobacco or use other recreational drugs. Other than infrequent use of albuterol, he does not take any prescription or over-the-counter medications. He has no allergies. He knows of no family history of arrhythmia or sudden cardiac death.
Physical examination. On initial examination, his temperature is 36.4°C (97.5°F), heart rate 230 bpm, systolic blood pressure 60 mm Hg, respiratory rate 30 breaths per minute, oxygen saturation 100% while breathing room air, and body mass index 25 kg/m2.
He is awake, anxious, and appears ill. He speaks only in short sentences. A focused cardiac examination reveals a regular tachycardia with no appreciable murmur or extra heart sounds; the apical impulse is not displaced. His lungs are clear. His abdomen is soft and nontender. He has 2+ pulses on a scale of 0 to 4+, with no peripheral edema.
His initial electrocardiogram (ECG) (Figure 1) shows a heart rate of 260 bpm and a regular wide complex tachycardia, defined as a rate greater than 100 bpm and a QRS complex wider than 0.12 seconds.
FOCUS ON REGULAR WIDE COMPLEX TACHYCARDIA
1. Which of the following is not in the differential diagnosis of regular wide complex tachycardia?
- Monomorphic ventricular tachycardia
- Orthodromic atrioventricular reentrant tachycardia
- Antidromic atrioventricular reentrant tachycardia
- Sinus tachycardia with bundle branch block
Orthodromic atrioventricular reentrant tachycardia is not in the differential diagnosis.
Wide complex tachycardia can occur when the impulse originates outside the normal conduction system or when there is abnormal ventricular activation through the atrioventricular (AV) node and His-Purkinje system.
The main distinction to make when diagnosing the cause of a wide complex tachycardia is between the following:
Monomorphic ventricular tachycardia, which originates from a single ventricular focus that depolarizes the adjacent myocardium in a stepwise fashion, causing a wide QRS complex that does not begin in the native conduction system, and
Sinus tachycardia with bundle branch block, ie, supraventricular tachycardia with aberrant conduction within the normal conduction system.
Three different conduction patterns are seen with atrioventricular reentrant tachycardia (Figure 2):
Sinus depolarization (Figure 2), in which the atrial impulse travels down the AV node, and the accessory pathway can be hidden and not contribute to the surface ECG.
Orthodromic atrioventricular reentrant tachycardia (Figure2), in which the depolarizing impulse travels antegrade down the AV node, then propagates from the ventricle back to the atria via the accessory pathway, resulting in a narrow QRS.
Antidromic atrioventricular reentrant tachycardia (Figure 2), in which the depolarization travels antegrade down the accessory pathway then propagates from the ventricle back to the atria via the AV node, resulting in a wide complex QRS with a delta wave.
Important features of the patient’s electrocardiogram (Figure 1) are consistent with antidromic atrioventricular reentrant tachycardia:
- “Buried” retrograde P waves, which are best seen in the continuous strip of lead II as a positive deflection notching in the negative nadir of the wave
- The PR segment is short, suggesting retrograde atrial depolarization
- The P wave is followed by a slow slurred upstroke (delta wave), best seen in lead I.
Treatment depends on diagnosis
Distinguishing supraventricular tachycardia from ventricular tachycardia is important, as the treatments differ. Supraventricular tachycardia is treated with adenosine, calcium channel blockers, and beta-blockers, which are not only ineffective for ventricular tachycardia, but rarely may precipitate hemodynamic deterioration.
Also important is distinguishing pre-excitation atrial fibrillation from other types of supraventricular tachycardia with aberrancy, because the nodal blockade used to treat other causes of the condition may worsen the tachycardia via the accessory pathway. If pre-excitation atrial fibrillation is suspected on the basis of an irregular wide complex tachycardia with delta waves on ECG, then procainamide—a sodium channel blocker that affects the cardiac action potential and prolongs the refractory period of the accessory pathway—can be used to help control the arrhythmia.1
Brugada criteria aid diagnosis
In 1991, Brugada et al2 devised an algorithm to differentiate ventricular tachycardia from supraventricular tachycardia with aberrancy in the setting of regular wide complex tachycardia (Figure 3). It has a sensitivity of 98.7% and a specificity of 96.5% for diagnosing ventricular tachycardia and 96.5% sensitivity and 98.7% specificity for diagnosing supraventricular tachycardia with aberrant conduction. Using the algorithm, only 11 (ie, 2%) of the 544 tachycardias in their study were misclassified.2–4
The Brugada algorithm consists of four criteria, with the presence of any leading to a diagnosis of ventricular tachycardia:
- Absence of an RS complex in all precordial leads (the QRS complexes in precordial leads have all negative or all positive deflections).
- An RS interval in at least one precordial lead of at least 100 ms (the interval is measured from the onset of R to the nadir of the S wave).
- AV dissociation, as determined by the existence of P waves marching out independent of the QRS complexes, capture beats (narrow QRS complexes resulting from the rare occasion when an intrinsic P wave conducts down the native pathway), or fusion beats (combined capture beat and ventricular beat, resulting in a different morphology than most of the wide QRS complexes present).
- Leads V1, V2, and V6 satisfying the classic morphologic criteria for ventricular tachycardia.
If none of these criteria are met, supraventricular tachycardia is diagnosed.
In our patient, we can further confirm the diagnosis of antidromic atrioventricular reentrant tachycardia by using Brugada criteria to exclude ventricular tachycardia (Figure 3): the ECG (Figure 1) shows an RS complex in multiple precordial leads, the maximum RS interval is less than 100 ms in the precordial leads, there is no evidence of AV dissociation (lead II in the continuous strip shows buried P waves associated with QRS), and morphologic criteria are not met for ventricular tachycardia in leads V1, V2, and V6.
IS CARDIOVERSION NEEDED?
According to the American Heart Association guidelines for advanced cardiopulmonary life support, patients with tachyarrhythmias who are hemodynamically unstable should undergo cardioversion immediately.5
Our patient, who has a heart rate faster than 200 bpm and a systolic blood pressure of only 60 mm Hg, undergoes synchronized cardioversion in the emergency department. Immediately afterward, his ECG (Figure 4) demonstrates sinus rhythm with pre-excitation consistent with type B Wolff-Parkinson-White syndrome.
Once he is hemodynamically stable, a more thorough physical examination is performed. Examination of the head, ears, eyes, nose, and throat is unremarkable. He has no jugular venous distention or carotid bruits. His lungs are clear to auscultation bilaterally, without wheezes. His cardiac examination shows a regular rate and rhythm, normal first and second heart sounds, and no murmurs, rubs, or gallops.
WHICH DIAGNOSTIC STUDIES ARE NEEDED?
Laboratory tests
In an otherwise healthy young patient presenting with an arrhythmia, the initial laboratory workup should focus on a precipitating illness or a disease state that may incite an arrhythmia.
Our patient is evaluated for infection or septic shock (white blood cell count with differential), anemia (hemoglobin), thyrotoxicosis (thyroid-stimulating hormone and free thyroxine levels), drug abuse (urine toxicology screen), and cardiac syndromes including structural heart disease and myocardial injury (cardiac enzymes and B-type natriuretic peptide).6
His initial laboratory tests show normal electrolyte levels and renal function, leukocytosis with a white blood cell count of 15.6 × 109/L (normal 4.0–10.0), mildly elevated thyroid-stimulating hormone, and a negative urine toxicology screen.
Transthoracic echocardiography
For a young patient presenting with pre-excitation on ECG and hemodynamic instability, transthoracic echocardiography to evaluate chamber size and look for structural abnormalities is a reasonable option.
Our patient undergoes transthoracic echocardiography, which demonstrates normal left ventricular size and function with a left ventricular ejection fraction of 69%, moderate right atrial enlargement, and mild right ventricular enlargement (Figure 5). The septal leaflet of the tricuspid valve is apically displaced, and there is mild regurgitation.
DIAGNOSIS: EBSTEIN ANOMALY
These findings are consistent with Ebstein anomaly. It can be recognized on transthoracic echocardiography as adherence of the septal and posterior tricuspid valve leaflets to the myocardium due to failure of the tissue to detach during embryogenesis, apical displacement of the annulus, right atrial enlargement, and right ventricular enlargement.7–10 Apical displacement of the tricuspid valve is a hallmark finding and must be more than 20 mm or 8 mm/m2 of body surface area to make the diagnosis.11–13 ECG often demonstrates right atrial enlargement, first-degree atrial ventricular block, and right bundle branch block.
Ebstein anomaly is a rare embryonic developmental abnormality of the tricuspid valve. It occurs in 1 to 5 of 200,000 live births, accounting for approximately 0.5% of all congenital heart disease.14,15 Most cases are sporadic and result from failure of the ventricle to delaminate during embryogenesis of the tricuspid valve, resulting in apical displacement of either the septal, posterior, or, very rarely, anterior leaflet of the tricuspid valve.7,8 The prevalence is higher in infants whose mothers took lithium during early pregnancy.16
2. Which of the following is not a common finding associated with Ebstein anomaly?
- Apical displacement of the septal leaflet of the tricuspid valve
- Wolff-Parkinson-White syndrome
- Accessory bypass tract
- Tachyarrhythmias
- Increased risk of sudden death
- Left-sided heart failure
The answer is left-sided heart failure. Ebstein anomaly is associated with increased risk of tachyarrhythmias, right-sided heart failure, and sudden death.7,8,17,18 In Ebstein anomaly, the tricuspid valve forms closer to the apex, so the part of the right ventricle that is superior to the displaced tricuspid valve functions as the right atrium, thus the term “atrialized” right ventricle. These abnormalities create an environment for accessory pathways, most commonly type B Wolff-Parkinson-White syndrome.19 Biventricular dysfunction can occur in rare severe cases.7,8,18
Our patient is found to have an accessory tract-mediated antidromic atrioventricular reentrant tachycardia in the setting of Wolff-Parkinson-White syndrome and Ebstein anomaly. This is further confirmed with an electrophysiology study demonstrating a right posterior accessory pathway.
TREATMENT FOR EBSTEIN ANOMALY
3. Which treatment is advised for Ebstein anomaly?
- Observation alone
- Standard heart failure medications
- Radiofrequency catheter ablation
- Tricuspid valve repair or replacement
- Biventricular reconstruction
- Heart transplant
The answer is all of the above. Observation alone is advised for patients with mild symptoms, no evidence of right-to-left shunting, and only mild cardiomegaly. Medical management includes an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, a beta-blocker, and diuretics. Radiofrequency catheter ablation is the first-line therapy for patients with symptomatic Wolff-Parkinson-White syndrome.20 A patient who develops worsening right-sided heart failure, cyanosis, paradoxical emboli, or frequent tachyarrhythmias should be considered for corrective surgery, which may include tricuspid valve repair or replacement, or biventricular reconstruction.7,8,21 Cardiac transplant is reserved for severe cases.8
On hospital day 4, our patient undergoes successful radiofrequency catheter ablation without complications. At follow-up 3 months later, he continues to do well, with resolution of his symptoms and no further evidence of pre-excitation. His postprocedure ECG no longer shows delta waves.
TAKE-HOME POINTS
- For a patient with regular wide complex tachycardia, the first step is to assess hemodynamic stability. If the patient is hemodynamically unstable, emergent cardioversion is indicated.
- The differential diagnosis for regular wide complex tachycardia includes supraventricular tachycardia with aberrancy (orthodromic atrioventricular reentrant tachycardia, antidromic atrioventricular reentrant tachycardia, atrial tachycardia), and ventricular tachycardia.
- When pre-excited atrial fibrillation is suspected, AV nodal blocking agents should be avoided, as they may worsen tachyarrhythmia. Sodium channel blockers such as procainamide can help slow down the conduction of the accessory pathway.
- Ebstein anomaly is diagnosed on transthoracic echocardiography as apical displacement of the tricuspid valve resulting in atrialization of the right ventricle.
- Patients with Ebstein anomaly have a higher risk of death from right-sided heart failure and tachyarrhythmias, most commonly type B Wolff-Parkinson-White syndrome.
- Ebstein anomaly is medically managed with standard heart failure medications, including neurohormonal blockade therapies.
- Patients with Ebstein anomaly and cyanosis require surgical intervention with either valve repair or replacement.
Acknowledgment: We thank Dr. William Collins for his contribution in reviewing the manuscript and his technical expertise in developing some of the figures.
- January CT, Wann L, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991; 83:1649–1659.
- Alzand BS, Crijns HJ. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace 2011; 13:465–472.
- Wellens HJ, Bar FW, Lie KI. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med 1978; 64:27–33.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122(suppl 3):S640–S656.
- Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011; 306:2248–2254.
- Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein’s anomaly - review of a multifaceted congenital cardiac condition. Swiss Med Wkly 2005; 135:269–281.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation 2007; 115:277–285.
- Oechslin E, Buchholz S, Jenni R. Ebstein’s anomaly in adults: Doppler-echocardiographic evaluation. Thorac Cardiovasc Surg 2000; 48:209–213.
- Ali SK, Nimeri NA. Clinical and echocardiographic features of Ebstein’s malformation in Sudanese patients. Cardiol Young 2006; 16:147–151.
- Edwards WD. Embryology and pathologic features of Ebstein’s anomaly. Prog Pediatr Cardiol 1993; 2:5–15.
- Shiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two dimensional echocardiographic spectrum of Ebstein’s anomaly: detailed anatomic assessment. J Am Coll Cardiol 1984; 3:356–370.
- Gussenhoven EJ, Stewart PA, Becker AE, Essed CE, Ligtvoet KM, De Villeneuve VH. “Offsetting” of the septal tricuspid leaflet in normal hearts and in hearts with Ebstein’s anomaly. Anatomic and echographic correlation. Am J Cardiol 1984; 54:172–176.
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med 2000; 342:334–342.
- Report of the New England Regional Infant Cardiac Program. Pediatrics 1980; 65:375–461.
- Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150.
- Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart 2008; 94:237–243.
- Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence. An international co-operative study of 505 cases. Br Heart J 1974; 36:417–427.
- Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010; 31:229–223.
- Tischenko A, Fox DJ, Yee R, et al. When should we recommend catheter ablation for patients with the Wolff-Parkinson-White syndrome? Curr Opin Cardiol 2008; 23:32–37.
- Misaki T, Watanabe G, Iwa T, et al. Surgical treatment of patients with Wolff-Parkinson-White syndrome and associated Ebstein’s anomaly. J Thorac Cardiovasc Surg 1995; 110:1702–1707.
A 23-year-old man presents to the emergency department with the sudden onset of palpitations, lightheadedness, and dyspnea, accompanied by weakness and nausea, which started earlier in the evening. He estimates that he has experienced 15 similar episodes, lasting minutes to hours, since the age of 16, with the last one 3 years ago. These episodes typically end by themselves or with self-induced vomiting and lying supine. The current episode did not resolve with these maneuvers.
He has never received medical attention for these symptoms. He has no chest pain, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, or syncope. He has had no recent illness, contacts with sick people, or travel.
The patient’s history includes a “childhood heart murmur,” which resolved, and also mild asthma. He is otherwise healthy but has not received regular medical care. He used to play competitive soccer but quit because playing made his symptoms of dyspnea on exertion and palpitations much worse.
He uses marijuana frequently and alcohol occasionally. He does not smoke tobacco or use other recreational drugs. Other than infrequent use of albuterol, he does not take any prescription or over-the-counter medications. He has no allergies. He knows of no family history of arrhythmia or sudden cardiac death.
Physical examination. On initial examination, his temperature is 36.4°C (97.5°F), heart rate 230 bpm, systolic blood pressure 60 mm Hg, respiratory rate 30 breaths per minute, oxygen saturation 100% while breathing room air, and body mass index 25 kg/m2.
He is awake, anxious, and appears ill. He speaks only in short sentences. A focused cardiac examination reveals a regular tachycardia with no appreciable murmur or extra heart sounds; the apical impulse is not displaced. His lungs are clear. His abdomen is soft and nontender. He has 2+ pulses on a scale of 0 to 4+, with no peripheral edema.
His initial electrocardiogram (ECG) (Figure 1) shows a heart rate of 260 bpm and a regular wide complex tachycardia, defined as a rate greater than 100 bpm and a QRS complex wider than 0.12 seconds.
FOCUS ON REGULAR WIDE COMPLEX TACHYCARDIA
1. Which of the following is not in the differential diagnosis of regular wide complex tachycardia?
- Monomorphic ventricular tachycardia
- Orthodromic atrioventricular reentrant tachycardia
- Antidromic atrioventricular reentrant tachycardia
- Sinus tachycardia with bundle branch block
Orthodromic atrioventricular reentrant tachycardia is not in the differential diagnosis.
Wide complex tachycardia can occur when the impulse originates outside the normal conduction system or when there is abnormal ventricular activation through the atrioventricular (AV) node and His-Purkinje system.
The main distinction to make when diagnosing the cause of a wide complex tachycardia is between the following:
Monomorphic ventricular tachycardia, which originates from a single ventricular focus that depolarizes the adjacent myocardium in a stepwise fashion, causing a wide QRS complex that does not begin in the native conduction system, and
Sinus tachycardia with bundle branch block, ie, supraventricular tachycardia with aberrant conduction within the normal conduction system.
Three different conduction patterns are seen with atrioventricular reentrant tachycardia (Figure 2):
Sinus depolarization (Figure 2), in which the atrial impulse travels down the AV node, and the accessory pathway can be hidden and not contribute to the surface ECG.
Orthodromic atrioventricular reentrant tachycardia (Figure2), in which the depolarizing impulse travels antegrade down the AV node, then propagates from the ventricle back to the atria via the accessory pathway, resulting in a narrow QRS.
Antidromic atrioventricular reentrant tachycardia (Figure 2), in which the depolarization travels antegrade down the accessory pathway then propagates from the ventricle back to the atria via the AV node, resulting in a wide complex QRS with a delta wave.
Important features of the patient’s electrocardiogram (Figure 1) are consistent with antidromic atrioventricular reentrant tachycardia:
- “Buried” retrograde P waves, which are best seen in the continuous strip of lead II as a positive deflection notching in the negative nadir of the wave
- The PR segment is short, suggesting retrograde atrial depolarization
- The P wave is followed by a slow slurred upstroke (delta wave), best seen in lead I.
Treatment depends on diagnosis
Distinguishing supraventricular tachycardia from ventricular tachycardia is important, as the treatments differ. Supraventricular tachycardia is treated with adenosine, calcium channel blockers, and beta-blockers, which are not only ineffective for ventricular tachycardia, but rarely may precipitate hemodynamic deterioration.
Also important is distinguishing pre-excitation atrial fibrillation from other types of supraventricular tachycardia with aberrancy, because the nodal blockade used to treat other causes of the condition may worsen the tachycardia via the accessory pathway. If pre-excitation atrial fibrillation is suspected on the basis of an irregular wide complex tachycardia with delta waves on ECG, then procainamide—a sodium channel blocker that affects the cardiac action potential and prolongs the refractory period of the accessory pathway—can be used to help control the arrhythmia.1
Brugada criteria aid diagnosis
In 1991, Brugada et al2 devised an algorithm to differentiate ventricular tachycardia from supraventricular tachycardia with aberrancy in the setting of regular wide complex tachycardia (Figure 3). It has a sensitivity of 98.7% and a specificity of 96.5% for diagnosing ventricular tachycardia and 96.5% sensitivity and 98.7% specificity for diagnosing supraventricular tachycardia with aberrant conduction. Using the algorithm, only 11 (ie, 2%) of the 544 tachycardias in their study were misclassified.2–4
The Brugada algorithm consists of four criteria, with the presence of any leading to a diagnosis of ventricular tachycardia:
- Absence of an RS complex in all precordial leads (the QRS complexes in precordial leads have all negative or all positive deflections).
- An RS interval in at least one precordial lead of at least 100 ms (the interval is measured from the onset of R to the nadir of the S wave).
- AV dissociation, as determined by the existence of P waves marching out independent of the QRS complexes, capture beats (narrow QRS complexes resulting from the rare occasion when an intrinsic P wave conducts down the native pathway), or fusion beats (combined capture beat and ventricular beat, resulting in a different morphology than most of the wide QRS complexes present).
- Leads V1, V2, and V6 satisfying the classic morphologic criteria for ventricular tachycardia.
If none of these criteria are met, supraventricular tachycardia is diagnosed.
In our patient, we can further confirm the diagnosis of antidromic atrioventricular reentrant tachycardia by using Brugada criteria to exclude ventricular tachycardia (Figure 3): the ECG (Figure 1) shows an RS complex in multiple precordial leads, the maximum RS interval is less than 100 ms in the precordial leads, there is no evidence of AV dissociation (lead II in the continuous strip shows buried P waves associated with QRS), and morphologic criteria are not met for ventricular tachycardia in leads V1, V2, and V6.
IS CARDIOVERSION NEEDED?
According to the American Heart Association guidelines for advanced cardiopulmonary life support, patients with tachyarrhythmias who are hemodynamically unstable should undergo cardioversion immediately.5
Our patient, who has a heart rate faster than 200 bpm and a systolic blood pressure of only 60 mm Hg, undergoes synchronized cardioversion in the emergency department. Immediately afterward, his ECG (Figure 4) demonstrates sinus rhythm with pre-excitation consistent with type B Wolff-Parkinson-White syndrome.
Once he is hemodynamically stable, a more thorough physical examination is performed. Examination of the head, ears, eyes, nose, and throat is unremarkable. He has no jugular venous distention or carotid bruits. His lungs are clear to auscultation bilaterally, without wheezes. His cardiac examination shows a regular rate and rhythm, normal first and second heart sounds, and no murmurs, rubs, or gallops.
WHICH DIAGNOSTIC STUDIES ARE NEEDED?
Laboratory tests
In an otherwise healthy young patient presenting with an arrhythmia, the initial laboratory workup should focus on a precipitating illness or a disease state that may incite an arrhythmia.
Our patient is evaluated for infection or septic shock (white blood cell count with differential), anemia (hemoglobin), thyrotoxicosis (thyroid-stimulating hormone and free thyroxine levels), drug abuse (urine toxicology screen), and cardiac syndromes including structural heart disease and myocardial injury (cardiac enzymes and B-type natriuretic peptide).6
His initial laboratory tests show normal electrolyte levels and renal function, leukocytosis with a white blood cell count of 15.6 × 109/L (normal 4.0–10.0), mildly elevated thyroid-stimulating hormone, and a negative urine toxicology screen.
Transthoracic echocardiography
For a young patient presenting with pre-excitation on ECG and hemodynamic instability, transthoracic echocardiography to evaluate chamber size and look for structural abnormalities is a reasonable option.
Our patient undergoes transthoracic echocardiography, which demonstrates normal left ventricular size and function with a left ventricular ejection fraction of 69%, moderate right atrial enlargement, and mild right ventricular enlargement (Figure 5). The septal leaflet of the tricuspid valve is apically displaced, and there is mild regurgitation.
DIAGNOSIS: EBSTEIN ANOMALY
These findings are consistent with Ebstein anomaly. It can be recognized on transthoracic echocardiography as adherence of the septal and posterior tricuspid valve leaflets to the myocardium due to failure of the tissue to detach during embryogenesis, apical displacement of the annulus, right atrial enlargement, and right ventricular enlargement.7–10 Apical displacement of the tricuspid valve is a hallmark finding and must be more than 20 mm or 8 mm/m2 of body surface area to make the diagnosis.11–13 ECG often demonstrates right atrial enlargement, first-degree atrial ventricular block, and right bundle branch block.
Ebstein anomaly is a rare embryonic developmental abnormality of the tricuspid valve. It occurs in 1 to 5 of 200,000 live births, accounting for approximately 0.5% of all congenital heart disease.14,15 Most cases are sporadic and result from failure of the ventricle to delaminate during embryogenesis of the tricuspid valve, resulting in apical displacement of either the septal, posterior, or, very rarely, anterior leaflet of the tricuspid valve.7,8 The prevalence is higher in infants whose mothers took lithium during early pregnancy.16
2. Which of the following is not a common finding associated with Ebstein anomaly?
- Apical displacement of the septal leaflet of the tricuspid valve
- Wolff-Parkinson-White syndrome
- Accessory bypass tract
- Tachyarrhythmias
- Increased risk of sudden death
- Left-sided heart failure
The answer is left-sided heart failure. Ebstein anomaly is associated with increased risk of tachyarrhythmias, right-sided heart failure, and sudden death.7,8,17,18 In Ebstein anomaly, the tricuspid valve forms closer to the apex, so the part of the right ventricle that is superior to the displaced tricuspid valve functions as the right atrium, thus the term “atrialized” right ventricle. These abnormalities create an environment for accessory pathways, most commonly type B Wolff-Parkinson-White syndrome.19 Biventricular dysfunction can occur in rare severe cases.7,8,18
Our patient is found to have an accessory tract-mediated antidromic atrioventricular reentrant tachycardia in the setting of Wolff-Parkinson-White syndrome and Ebstein anomaly. This is further confirmed with an electrophysiology study demonstrating a right posterior accessory pathway.
TREATMENT FOR EBSTEIN ANOMALY
3. Which treatment is advised for Ebstein anomaly?
- Observation alone
- Standard heart failure medications
- Radiofrequency catheter ablation
- Tricuspid valve repair or replacement
- Biventricular reconstruction
- Heart transplant
The answer is all of the above. Observation alone is advised for patients with mild symptoms, no evidence of right-to-left shunting, and only mild cardiomegaly. Medical management includes an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, a beta-blocker, and diuretics. Radiofrequency catheter ablation is the first-line therapy for patients with symptomatic Wolff-Parkinson-White syndrome.20 A patient who develops worsening right-sided heart failure, cyanosis, paradoxical emboli, or frequent tachyarrhythmias should be considered for corrective surgery, which may include tricuspid valve repair or replacement, or biventricular reconstruction.7,8,21 Cardiac transplant is reserved for severe cases.8
On hospital day 4, our patient undergoes successful radiofrequency catheter ablation without complications. At follow-up 3 months later, he continues to do well, with resolution of his symptoms and no further evidence of pre-excitation. His postprocedure ECG no longer shows delta waves.
TAKE-HOME POINTS
- For a patient with regular wide complex tachycardia, the first step is to assess hemodynamic stability. If the patient is hemodynamically unstable, emergent cardioversion is indicated.
- The differential diagnosis for regular wide complex tachycardia includes supraventricular tachycardia with aberrancy (orthodromic atrioventricular reentrant tachycardia, antidromic atrioventricular reentrant tachycardia, atrial tachycardia), and ventricular tachycardia.
- When pre-excited atrial fibrillation is suspected, AV nodal blocking agents should be avoided, as they may worsen tachyarrhythmia. Sodium channel blockers such as procainamide can help slow down the conduction of the accessory pathway.
- Ebstein anomaly is diagnosed on transthoracic echocardiography as apical displacement of the tricuspid valve resulting in atrialization of the right ventricle.
- Patients with Ebstein anomaly have a higher risk of death from right-sided heart failure and tachyarrhythmias, most commonly type B Wolff-Parkinson-White syndrome.
- Ebstein anomaly is medically managed with standard heart failure medications, including neurohormonal blockade therapies.
- Patients with Ebstein anomaly and cyanosis require surgical intervention with either valve repair or replacement.
Acknowledgment: We thank Dr. William Collins for his contribution in reviewing the manuscript and his technical expertise in developing some of the figures.
A 23-year-old man presents to the emergency department with the sudden onset of palpitations, lightheadedness, and dyspnea, accompanied by weakness and nausea, which started earlier in the evening. He estimates that he has experienced 15 similar episodes, lasting minutes to hours, since the age of 16, with the last one 3 years ago. These episodes typically end by themselves or with self-induced vomiting and lying supine. The current episode did not resolve with these maneuvers.
He has never received medical attention for these symptoms. He has no chest pain, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, or syncope. He has had no recent illness, contacts with sick people, or travel.
The patient’s history includes a “childhood heart murmur,” which resolved, and also mild asthma. He is otherwise healthy but has not received regular medical care. He used to play competitive soccer but quit because playing made his symptoms of dyspnea on exertion and palpitations much worse.
He uses marijuana frequently and alcohol occasionally. He does not smoke tobacco or use other recreational drugs. Other than infrequent use of albuterol, he does not take any prescription or over-the-counter medications. He has no allergies. He knows of no family history of arrhythmia or sudden cardiac death.
Physical examination. On initial examination, his temperature is 36.4°C (97.5°F), heart rate 230 bpm, systolic blood pressure 60 mm Hg, respiratory rate 30 breaths per minute, oxygen saturation 100% while breathing room air, and body mass index 25 kg/m2.
He is awake, anxious, and appears ill. He speaks only in short sentences. A focused cardiac examination reveals a regular tachycardia with no appreciable murmur or extra heart sounds; the apical impulse is not displaced. His lungs are clear. His abdomen is soft and nontender. He has 2+ pulses on a scale of 0 to 4+, with no peripheral edema.
His initial electrocardiogram (ECG) (Figure 1) shows a heart rate of 260 bpm and a regular wide complex tachycardia, defined as a rate greater than 100 bpm and a QRS complex wider than 0.12 seconds.
FOCUS ON REGULAR WIDE COMPLEX TACHYCARDIA
1. Which of the following is not in the differential diagnosis of regular wide complex tachycardia?
- Monomorphic ventricular tachycardia
- Orthodromic atrioventricular reentrant tachycardia
- Antidromic atrioventricular reentrant tachycardia
- Sinus tachycardia with bundle branch block
Orthodromic atrioventricular reentrant tachycardia is not in the differential diagnosis.
Wide complex tachycardia can occur when the impulse originates outside the normal conduction system or when there is abnormal ventricular activation through the atrioventricular (AV) node and His-Purkinje system.
The main distinction to make when diagnosing the cause of a wide complex tachycardia is between the following:
Monomorphic ventricular tachycardia, which originates from a single ventricular focus that depolarizes the adjacent myocardium in a stepwise fashion, causing a wide QRS complex that does not begin in the native conduction system, and
Sinus tachycardia with bundle branch block, ie, supraventricular tachycardia with aberrant conduction within the normal conduction system.
Three different conduction patterns are seen with atrioventricular reentrant tachycardia (Figure 2):
Sinus depolarization (Figure 2), in which the atrial impulse travels down the AV node, and the accessory pathway can be hidden and not contribute to the surface ECG.
Orthodromic atrioventricular reentrant tachycardia (Figure2), in which the depolarizing impulse travels antegrade down the AV node, then propagates from the ventricle back to the atria via the accessory pathway, resulting in a narrow QRS.
Antidromic atrioventricular reentrant tachycardia (Figure 2), in which the depolarization travels antegrade down the accessory pathway then propagates from the ventricle back to the atria via the AV node, resulting in a wide complex QRS with a delta wave.
Important features of the patient’s electrocardiogram (Figure 1) are consistent with antidromic atrioventricular reentrant tachycardia:
- “Buried” retrograde P waves, which are best seen in the continuous strip of lead II as a positive deflection notching in the negative nadir of the wave
- The PR segment is short, suggesting retrograde atrial depolarization
- The P wave is followed by a slow slurred upstroke (delta wave), best seen in lead I.
Treatment depends on diagnosis
Distinguishing supraventricular tachycardia from ventricular tachycardia is important, as the treatments differ. Supraventricular tachycardia is treated with adenosine, calcium channel blockers, and beta-blockers, which are not only ineffective for ventricular tachycardia, but rarely may precipitate hemodynamic deterioration.
Also important is distinguishing pre-excitation atrial fibrillation from other types of supraventricular tachycardia with aberrancy, because the nodal blockade used to treat other causes of the condition may worsen the tachycardia via the accessory pathway. If pre-excitation atrial fibrillation is suspected on the basis of an irregular wide complex tachycardia with delta waves on ECG, then procainamide—a sodium channel blocker that affects the cardiac action potential and prolongs the refractory period of the accessory pathway—can be used to help control the arrhythmia.1
Brugada criteria aid diagnosis
In 1991, Brugada et al2 devised an algorithm to differentiate ventricular tachycardia from supraventricular tachycardia with aberrancy in the setting of regular wide complex tachycardia (Figure 3). It has a sensitivity of 98.7% and a specificity of 96.5% for diagnosing ventricular tachycardia and 96.5% sensitivity and 98.7% specificity for diagnosing supraventricular tachycardia with aberrant conduction. Using the algorithm, only 11 (ie, 2%) of the 544 tachycardias in their study were misclassified.2–4
The Brugada algorithm consists of four criteria, with the presence of any leading to a diagnosis of ventricular tachycardia:
- Absence of an RS complex in all precordial leads (the QRS complexes in precordial leads have all negative or all positive deflections).
- An RS interval in at least one precordial lead of at least 100 ms (the interval is measured from the onset of R to the nadir of the S wave).
- AV dissociation, as determined by the existence of P waves marching out independent of the QRS complexes, capture beats (narrow QRS complexes resulting from the rare occasion when an intrinsic P wave conducts down the native pathway), or fusion beats (combined capture beat and ventricular beat, resulting in a different morphology than most of the wide QRS complexes present).
- Leads V1, V2, and V6 satisfying the classic morphologic criteria for ventricular tachycardia.
If none of these criteria are met, supraventricular tachycardia is diagnosed.
In our patient, we can further confirm the diagnosis of antidromic atrioventricular reentrant tachycardia by using Brugada criteria to exclude ventricular tachycardia (Figure 3): the ECG (Figure 1) shows an RS complex in multiple precordial leads, the maximum RS interval is less than 100 ms in the precordial leads, there is no evidence of AV dissociation (lead II in the continuous strip shows buried P waves associated with QRS), and morphologic criteria are not met for ventricular tachycardia in leads V1, V2, and V6.
IS CARDIOVERSION NEEDED?
According to the American Heart Association guidelines for advanced cardiopulmonary life support, patients with tachyarrhythmias who are hemodynamically unstable should undergo cardioversion immediately.5
Our patient, who has a heart rate faster than 200 bpm and a systolic blood pressure of only 60 mm Hg, undergoes synchronized cardioversion in the emergency department. Immediately afterward, his ECG (Figure 4) demonstrates sinus rhythm with pre-excitation consistent with type B Wolff-Parkinson-White syndrome.
Once he is hemodynamically stable, a more thorough physical examination is performed. Examination of the head, ears, eyes, nose, and throat is unremarkable. He has no jugular venous distention or carotid bruits. His lungs are clear to auscultation bilaterally, without wheezes. His cardiac examination shows a regular rate and rhythm, normal first and second heart sounds, and no murmurs, rubs, or gallops.
WHICH DIAGNOSTIC STUDIES ARE NEEDED?
Laboratory tests
In an otherwise healthy young patient presenting with an arrhythmia, the initial laboratory workup should focus on a precipitating illness or a disease state that may incite an arrhythmia.
Our patient is evaluated for infection or septic shock (white blood cell count with differential), anemia (hemoglobin), thyrotoxicosis (thyroid-stimulating hormone and free thyroxine levels), drug abuse (urine toxicology screen), and cardiac syndromes including structural heart disease and myocardial injury (cardiac enzymes and B-type natriuretic peptide).6
His initial laboratory tests show normal electrolyte levels and renal function, leukocytosis with a white blood cell count of 15.6 × 109/L (normal 4.0–10.0), mildly elevated thyroid-stimulating hormone, and a negative urine toxicology screen.
Transthoracic echocardiography
For a young patient presenting with pre-excitation on ECG and hemodynamic instability, transthoracic echocardiography to evaluate chamber size and look for structural abnormalities is a reasonable option.
Our patient undergoes transthoracic echocardiography, which demonstrates normal left ventricular size and function with a left ventricular ejection fraction of 69%, moderate right atrial enlargement, and mild right ventricular enlargement (Figure 5). The septal leaflet of the tricuspid valve is apically displaced, and there is mild regurgitation.
DIAGNOSIS: EBSTEIN ANOMALY
These findings are consistent with Ebstein anomaly. It can be recognized on transthoracic echocardiography as adherence of the septal and posterior tricuspid valve leaflets to the myocardium due to failure of the tissue to detach during embryogenesis, apical displacement of the annulus, right atrial enlargement, and right ventricular enlargement.7–10 Apical displacement of the tricuspid valve is a hallmark finding and must be more than 20 mm or 8 mm/m2 of body surface area to make the diagnosis.11–13 ECG often demonstrates right atrial enlargement, first-degree atrial ventricular block, and right bundle branch block.
Ebstein anomaly is a rare embryonic developmental abnormality of the tricuspid valve. It occurs in 1 to 5 of 200,000 live births, accounting for approximately 0.5% of all congenital heart disease.14,15 Most cases are sporadic and result from failure of the ventricle to delaminate during embryogenesis of the tricuspid valve, resulting in apical displacement of either the septal, posterior, or, very rarely, anterior leaflet of the tricuspid valve.7,8 The prevalence is higher in infants whose mothers took lithium during early pregnancy.16
2. Which of the following is not a common finding associated with Ebstein anomaly?
- Apical displacement of the septal leaflet of the tricuspid valve
- Wolff-Parkinson-White syndrome
- Accessory bypass tract
- Tachyarrhythmias
- Increased risk of sudden death
- Left-sided heart failure
The answer is left-sided heart failure. Ebstein anomaly is associated with increased risk of tachyarrhythmias, right-sided heart failure, and sudden death.7,8,17,18 In Ebstein anomaly, the tricuspid valve forms closer to the apex, so the part of the right ventricle that is superior to the displaced tricuspid valve functions as the right atrium, thus the term “atrialized” right ventricle. These abnormalities create an environment for accessory pathways, most commonly type B Wolff-Parkinson-White syndrome.19 Biventricular dysfunction can occur in rare severe cases.7,8,18
Our patient is found to have an accessory tract-mediated antidromic atrioventricular reentrant tachycardia in the setting of Wolff-Parkinson-White syndrome and Ebstein anomaly. This is further confirmed with an electrophysiology study demonstrating a right posterior accessory pathway.
TREATMENT FOR EBSTEIN ANOMALY
3. Which treatment is advised for Ebstein anomaly?
- Observation alone
- Standard heart failure medications
- Radiofrequency catheter ablation
- Tricuspid valve repair or replacement
- Biventricular reconstruction
- Heart transplant
The answer is all of the above. Observation alone is advised for patients with mild symptoms, no evidence of right-to-left shunting, and only mild cardiomegaly. Medical management includes an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, a beta-blocker, and diuretics. Radiofrequency catheter ablation is the first-line therapy for patients with symptomatic Wolff-Parkinson-White syndrome.20 A patient who develops worsening right-sided heart failure, cyanosis, paradoxical emboli, or frequent tachyarrhythmias should be considered for corrective surgery, which may include tricuspid valve repair or replacement, or biventricular reconstruction.7,8,21 Cardiac transplant is reserved for severe cases.8
On hospital day 4, our patient undergoes successful radiofrequency catheter ablation without complications. At follow-up 3 months later, he continues to do well, with resolution of his symptoms and no further evidence of pre-excitation. His postprocedure ECG no longer shows delta waves.
TAKE-HOME POINTS
- For a patient with regular wide complex tachycardia, the first step is to assess hemodynamic stability. If the patient is hemodynamically unstable, emergent cardioversion is indicated.
- The differential diagnosis for regular wide complex tachycardia includes supraventricular tachycardia with aberrancy (orthodromic atrioventricular reentrant tachycardia, antidromic atrioventricular reentrant tachycardia, atrial tachycardia), and ventricular tachycardia.
- When pre-excited atrial fibrillation is suspected, AV nodal blocking agents should be avoided, as they may worsen tachyarrhythmia. Sodium channel blockers such as procainamide can help slow down the conduction of the accessory pathway.
- Ebstein anomaly is diagnosed on transthoracic echocardiography as apical displacement of the tricuspid valve resulting in atrialization of the right ventricle.
- Patients with Ebstein anomaly have a higher risk of death from right-sided heart failure and tachyarrhythmias, most commonly type B Wolff-Parkinson-White syndrome.
- Ebstein anomaly is medically managed with standard heart failure medications, including neurohormonal blockade therapies.
- Patients with Ebstein anomaly and cyanosis require surgical intervention with either valve repair or replacement.
Acknowledgment: We thank Dr. William Collins for his contribution in reviewing the manuscript and his technical expertise in developing some of the figures.
- January CT, Wann L, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991; 83:1649–1659.
- Alzand BS, Crijns HJ. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace 2011; 13:465–472.
- Wellens HJ, Bar FW, Lie KI. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med 1978; 64:27–33.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122(suppl 3):S640–S656.
- Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011; 306:2248–2254.
- Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein’s anomaly - review of a multifaceted congenital cardiac condition. Swiss Med Wkly 2005; 135:269–281.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation 2007; 115:277–285.
- Oechslin E, Buchholz S, Jenni R. Ebstein’s anomaly in adults: Doppler-echocardiographic evaluation. Thorac Cardiovasc Surg 2000; 48:209–213.
- Ali SK, Nimeri NA. Clinical and echocardiographic features of Ebstein’s malformation in Sudanese patients. Cardiol Young 2006; 16:147–151.
- Edwards WD. Embryology and pathologic features of Ebstein’s anomaly. Prog Pediatr Cardiol 1993; 2:5–15.
- Shiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two dimensional echocardiographic spectrum of Ebstein’s anomaly: detailed anatomic assessment. J Am Coll Cardiol 1984; 3:356–370.
- Gussenhoven EJ, Stewart PA, Becker AE, Essed CE, Ligtvoet KM, De Villeneuve VH. “Offsetting” of the septal tricuspid leaflet in normal hearts and in hearts with Ebstein’s anomaly. Anatomic and echographic correlation. Am J Cardiol 1984; 54:172–176.
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med 2000; 342:334–342.
- Report of the New England Regional Infant Cardiac Program. Pediatrics 1980; 65:375–461.
- Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150.
- Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart 2008; 94:237–243.
- Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence. An international co-operative study of 505 cases. Br Heart J 1974; 36:417–427.
- Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010; 31:229–223.
- Tischenko A, Fox DJ, Yee R, et al. When should we recommend catheter ablation for patients with the Wolff-Parkinson-White syndrome? Curr Opin Cardiol 2008; 23:32–37.
- Misaki T, Watanabe G, Iwa T, et al. Surgical treatment of patients with Wolff-Parkinson-White syndrome and associated Ebstein’s anomaly. J Thorac Cardiovasc Surg 1995; 110:1702–1707.
- January CT, Wann L, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991; 83:1649–1659.
- Alzand BS, Crijns HJ. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace 2011; 13:465–472.
- Wellens HJ, Bar FW, Lie KI. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med 1978; 64:27–33.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122(suppl 3):S640–S656.
- Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011; 306:2248–2254.
- Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein’s anomaly - review of a multifaceted congenital cardiac condition. Swiss Med Wkly 2005; 135:269–281.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation 2007; 115:277–285.
- Oechslin E, Buchholz S, Jenni R. Ebstein’s anomaly in adults: Doppler-echocardiographic evaluation. Thorac Cardiovasc Surg 2000; 48:209–213.
- Ali SK, Nimeri NA. Clinical and echocardiographic features of Ebstein’s malformation in Sudanese patients. Cardiol Young 2006; 16:147–151.
- Edwards WD. Embryology and pathologic features of Ebstein’s anomaly. Prog Pediatr Cardiol 1993; 2:5–15.
- Shiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two dimensional echocardiographic spectrum of Ebstein’s anomaly: detailed anatomic assessment. J Am Coll Cardiol 1984; 3:356–370.
- Gussenhoven EJ, Stewart PA, Becker AE, Essed CE, Ligtvoet KM, De Villeneuve VH. “Offsetting” of the septal tricuspid leaflet in normal hearts and in hearts with Ebstein’s anomaly. Anatomic and echographic correlation. Am J Cardiol 1984; 54:172–176.
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med 2000; 342:334–342.
- Report of the New England Regional Infant Cardiac Program. Pediatrics 1980; 65:375–461.
- Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150.
- Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart 2008; 94:237–243.
- Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence. An international co-operative study of 505 cases. Br Heart J 1974; 36:417–427.
- Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010; 31:229–223.
- Tischenko A, Fox DJ, Yee R, et al. When should we recommend catheter ablation for patients with the Wolff-Parkinson-White syndrome? Curr Opin Cardiol 2008; 23:32–37.
- Misaki T, Watanabe G, Iwa T, et al. Surgical treatment of patients with Wolff-Parkinson-White syndrome and associated Ebstein’s anomaly. J Thorac Cardiovasc Surg 1995; 110:1702–1707.
The new oral anticoagulants: Reasonable alternatives to warfarin
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
KEY POINTS
- The new oral anticoagulants have favorable pharmacologic properties and similar efficacy and safety as vitamin K antagonists.
- The new agents are indicated for preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation and preventing and treating deep vein thrombosis and pulmonary embolism (the indications regarding venous thromboembolism differ somewhat among agents).
- Except for dabigatran, lack of an antidote in case of bleeding or emergency surgery is a major drawback.
- Be cautious when using these drugs in patients with renal or liver disease and in those taking an inhibitor or inducer of the P-glycoprotein transporter or the cytochrome P450 enzymes.
Insulin pumps: Beyond basal-bolus
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
KEY POINTS
- Insulin pumps allow for more accurate insulin dosing than multiple daily injections, resulting in less drastic extremes in blood sugar.
- Insulin pumps allow for more individualized basal insulin coverage than long-acting injectable insulin.
- Both the patient and provider need a good understanding of insulin pump therapy for successful pump management.
Insulin pumps: Great devices, but you still have to press the button
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.