User login
Gray matter of first-degree bipolar-patient relatives same as general population
Unaffected first-degree relatives of bipolar disorder patients show no differences in gray-matter volume compared with other healthy adults, Dr. Fabiano G. Nery of the University of São Paulo and colleagues reported.
Investigators took magnetic resonance images of the brains of 25 patients with bipolar disorder, 23 unaffected relatives, and 27 healthy controls recruited from outpatient facilities at the university and the local community. The total gray-matter volume from images was 646.64 mL plus or minus 71.87 among bipolar disorder patients, 645.97 mL plus or minus 48.20 in unaffected relatives, and 637.87 mL plus or minus 62.50 in healthy controls, indicating no significant differences. Bipolar disorder patients, however, had reduced gray-matter volumes in the bilateral thalamus, compared with healthy controls.
This finding was present after controlling for possible confounding effects of age and gender, suggesting that the thalamus “may be involved in the neurocircuitry responsible for the clinical manifestations of” bipolar disorder, they wrote.
“These results suggest that there is no structural endophenotype for [bipolar disorder] and support the role of the thalamus in the pathophysiology of” bipolar disorder, the authors noted.
Read the article in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.09.005).
Unaffected first-degree relatives of bipolar disorder patients show no differences in gray-matter volume compared with other healthy adults, Dr. Fabiano G. Nery of the University of São Paulo and colleagues reported.
Investigators took magnetic resonance images of the brains of 25 patients with bipolar disorder, 23 unaffected relatives, and 27 healthy controls recruited from outpatient facilities at the university and the local community. The total gray-matter volume from images was 646.64 mL plus or minus 71.87 among bipolar disorder patients, 645.97 mL plus or minus 48.20 in unaffected relatives, and 637.87 mL plus or minus 62.50 in healthy controls, indicating no significant differences. Bipolar disorder patients, however, had reduced gray-matter volumes in the bilateral thalamus, compared with healthy controls.
This finding was present after controlling for possible confounding effects of age and gender, suggesting that the thalamus “may be involved in the neurocircuitry responsible for the clinical manifestations of” bipolar disorder, they wrote.
“These results suggest that there is no structural endophenotype for [bipolar disorder] and support the role of the thalamus in the pathophysiology of” bipolar disorder, the authors noted.
Read the article in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.09.005).
Unaffected first-degree relatives of bipolar disorder patients show no differences in gray-matter volume compared with other healthy adults, Dr. Fabiano G. Nery of the University of São Paulo and colleagues reported.
Investigators took magnetic resonance images of the brains of 25 patients with bipolar disorder, 23 unaffected relatives, and 27 healthy controls recruited from outpatient facilities at the university and the local community. The total gray-matter volume from images was 646.64 mL plus or minus 71.87 among bipolar disorder patients, 645.97 mL plus or minus 48.20 in unaffected relatives, and 637.87 mL plus or minus 62.50 in healthy controls, indicating no significant differences. Bipolar disorder patients, however, had reduced gray-matter volumes in the bilateral thalamus, compared with healthy controls.
This finding was present after controlling for possible confounding effects of age and gender, suggesting that the thalamus “may be involved in the neurocircuitry responsible for the clinical manifestations of” bipolar disorder, they wrote.
“These results suggest that there is no structural endophenotype for [bipolar disorder] and support the role of the thalamus in the pathophysiology of” bipolar disorder, the authors noted.
Read the article in Psychiatry Research: Neuroimaging (doi: 10.1016/j.pscychresns.2015.09.005).
FROM PSYCHIATRY RESEARCH: NEUROIMAGING
Top Trends at Society of Hospital Medicine for 2016
Ready or not, 2016 is almost here, and SHM is gearing up for another year jam packed with exciting, enriching opportunities for hospitalists and their teams. Here are 10 things to have on your radar as we head into the new year.
1 Hospital Medicine 2016: March 6–9, 2016
This year’s annual meeting in San Diego promises to be the biggest yet, with new tracks in Co-Management/Perioperative Medicine, Health IT for Hospitalists, and Post-Acute Care—and opportunities to connect and collaborate with a vibrant community of hospital medicine professionals from around the nation. Register before early bird rates end on Jan. 11, 2016!
2 The Year of the Hospitalist
In celebration of the 20-year anniversary of the coining of the word “hospitalist,” SHM is preparing for a yearlong series of special events, contests, and opportunities. Follow SHM on Twitter at @SHMLive, and visit Hospital Medicine for the latest news!
3 Get Engaged with Public Policy
Healthcare legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Sign up for the Grassroots Network now to stay updated on developments in healthcare policy, share your experiences with healthcare programs, and even participate in policy forums.
4 Fight the Resistance Campaign to Promote Antibiotic Stewardship
SHM is partnering with the CDC to change hospital culture and, in turn, change antibiotic prescription behaviors to prevent antibiotic resistance. Learn how you can be a part of the campaign, and download the campaign posters—featuring striking designs inspired by 1940s propaganda posters.
5 2016 State of Hospital Medicine Survey
The 2016 State of Hospital Medicine survey will take place January through March, with the release of the report scheduled for September. The survey consists of comprehensive, current information crucial to understanding the hospital medicine landscape and making better decisions in the hospital. Visit SHM’s website to find out how you can participate.
6 Expansion of the Quality Improvement Portfolio
SHM’s Center for Hospital Innovation and Improvement continues to develop guides, toolkits, and programs to meet the evolving needs of hospital-based clinicians and improve the care of hospitalized patients. New additions to the portfolio include resources for VTE, chronic heart failure, delirium, anemia, and end-of-life care.
7 SHM Student Hospitalist Scholar Grant Applications
Student members of SHM could be eligible to apply for an SHM Student Hospitalist Scholar Grant, including funding to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other hospital medicine-related fields. The deadline to apply is Feb. 15, 2016.
8 Innovative Additions to SHM’s Digital Learning Offerings
In addition to SHM SPARK, an MOC preparation tool for the Focused Practice in Hospital Medicine exam, and SHMConsults, online modules for consultative and perioperative medicine, look for new SHM Learning Portal activities in 2016, like “Management of Postoperative Atrial Fibrillation,” “Managing Pain in Postoperative Patients: What the Hospitalist Needs to Know,” and “Perioperative Bridging of Anticoagulant Theory.”
9 Get #SHeMpowered on Social Media
Have a success story to share about how SHM helped you advance your career or enhance patient care in your hospital? Maybe you improved your clinical skills at the annual meeting or improved care transitions with Project BOOST? Shout it from the rooftops—tweet @SHMLive and use the hashtag #SHeMpowered. If you haven’t followed SHM on Twitter, head over to @SHMLive.
10 “Are You Number 15,000?”
This is a question you want to say “yes” to! SHM is poised to welcome its 15,000th member in early 2016 as part of the Year of the Hospitalist. The 15,000th member will receive an assortment of exciting prizes, including complimentary registration to Hospital Medicine 2016 in San Diego. Know someone who is interested in joining? Spread the word!
Brett Radler is SHM’s communications coordinator.
Ready or not, 2016 is almost here, and SHM is gearing up for another year jam packed with exciting, enriching opportunities for hospitalists and their teams. Here are 10 things to have on your radar as we head into the new year.
1 Hospital Medicine 2016: March 6–9, 2016
This year’s annual meeting in San Diego promises to be the biggest yet, with new tracks in Co-Management/Perioperative Medicine, Health IT for Hospitalists, and Post-Acute Care—and opportunities to connect and collaborate with a vibrant community of hospital medicine professionals from around the nation. Register before early bird rates end on Jan. 11, 2016!
2 The Year of the Hospitalist
In celebration of the 20-year anniversary of the coining of the word “hospitalist,” SHM is preparing for a yearlong series of special events, contests, and opportunities. Follow SHM on Twitter at @SHMLive, and visit Hospital Medicine for the latest news!
3 Get Engaged with Public Policy
Healthcare legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Sign up for the Grassroots Network now to stay updated on developments in healthcare policy, share your experiences with healthcare programs, and even participate in policy forums.
4 Fight the Resistance Campaign to Promote Antibiotic Stewardship
SHM is partnering with the CDC to change hospital culture and, in turn, change antibiotic prescription behaviors to prevent antibiotic resistance. Learn how you can be a part of the campaign, and download the campaign posters—featuring striking designs inspired by 1940s propaganda posters.
5 2016 State of Hospital Medicine Survey
The 2016 State of Hospital Medicine survey will take place January through March, with the release of the report scheduled for September. The survey consists of comprehensive, current information crucial to understanding the hospital medicine landscape and making better decisions in the hospital. Visit SHM’s website to find out how you can participate.
6 Expansion of the Quality Improvement Portfolio
SHM’s Center for Hospital Innovation and Improvement continues to develop guides, toolkits, and programs to meet the evolving needs of hospital-based clinicians and improve the care of hospitalized patients. New additions to the portfolio include resources for VTE, chronic heart failure, delirium, anemia, and end-of-life care.
7 SHM Student Hospitalist Scholar Grant Applications
Student members of SHM could be eligible to apply for an SHM Student Hospitalist Scholar Grant, including funding to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other hospital medicine-related fields. The deadline to apply is Feb. 15, 2016.
8 Innovative Additions to SHM’s Digital Learning Offerings
In addition to SHM SPARK, an MOC preparation tool for the Focused Practice in Hospital Medicine exam, and SHMConsults, online modules for consultative and perioperative medicine, look for new SHM Learning Portal activities in 2016, like “Management of Postoperative Atrial Fibrillation,” “Managing Pain in Postoperative Patients: What the Hospitalist Needs to Know,” and “Perioperative Bridging of Anticoagulant Theory.”
9 Get #SHeMpowered on Social Media
Have a success story to share about how SHM helped you advance your career or enhance patient care in your hospital? Maybe you improved your clinical skills at the annual meeting or improved care transitions with Project BOOST? Shout it from the rooftops—tweet @SHMLive and use the hashtag #SHeMpowered. If you haven’t followed SHM on Twitter, head over to @SHMLive.
10 “Are You Number 15,000?”
This is a question you want to say “yes” to! SHM is poised to welcome its 15,000th member in early 2016 as part of the Year of the Hospitalist. The 15,000th member will receive an assortment of exciting prizes, including complimentary registration to Hospital Medicine 2016 in San Diego. Know someone who is interested in joining? Spread the word!
Brett Radler is SHM’s communications coordinator.
Ready or not, 2016 is almost here, and SHM is gearing up for another year jam packed with exciting, enriching opportunities for hospitalists and their teams. Here are 10 things to have on your radar as we head into the new year.
1 Hospital Medicine 2016: March 6–9, 2016
This year’s annual meeting in San Diego promises to be the biggest yet, with new tracks in Co-Management/Perioperative Medicine, Health IT for Hospitalists, and Post-Acute Care—and opportunities to connect and collaborate with a vibrant community of hospital medicine professionals from around the nation. Register before early bird rates end on Jan. 11, 2016!
2 The Year of the Hospitalist
In celebration of the 20-year anniversary of the coining of the word “hospitalist,” SHM is preparing for a yearlong series of special events, contests, and opportunities. Follow SHM on Twitter at @SHMLive, and visit Hospital Medicine for the latest news!
3 Get Engaged with Public Policy
Healthcare legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Sign up for the Grassroots Network now to stay updated on developments in healthcare policy, share your experiences with healthcare programs, and even participate in policy forums.
4 Fight the Resistance Campaign to Promote Antibiotic Stewardship
SHM is partnering with the CDC to change hospital culture and, in turn, change antibiotic prescription behaviors to prevent antibiotic resistance. Learn how you can be a part of the campaign, and download the campaign posters—featuring striking designs inspired by 1940s propaganda posters.
5 2016 State of Hospital Medicine Survey
The 2016 State of Hospital Medicine survey will take place January through March, with the release of the report scheduled for September. The survey consists of comprehensive, current information crucial to understanding the hospital medicine landscape and making better decisions in the hospital. Visit SHM’s website to find out how you can participate.
6 Expansion of the Quality Improvement Portfolio
SHM’s Center for Hospital Innovation and Improvement continues to develop guides, toolkits, and programs to meet the evolving needs of hospital-based clinicians and improve the care of hospitalized patients. New additions to the portfolio include resources for VTE, chronic heart failure, delirium, anemia, and end-of-life care.
7 SHM Student Hospitalist Scholar Grant Applications
Student members of SHM could be eligible to apply for an SHM Student Hospitalist Scholar Grant, including funding to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other hospital medicine-related fields. The deadline to apply is Feb. 15, 2016.
8 Innovative Additions to SHM’s Digital Learning Offerings
In addition to SHM SPARK, an MOC preparation tool for the Focused Practice in Hospital Medicine exam, and SHMConsults, online modules for consultative and perioperative medicine, look for new SHM Learning Portal activities in 2016, like “Management of Postoperative Atrial Fibrillation,” “Managing Pain in Postoperative Patients: What the Hospitalist Needs to Know,” and “Perioperative Bridging of Anticoagulant Theory.”
9 Get #SHeMpowered on Social Media
Have a success story to share about how SHM helped you advance your career or enhance patient care in your hospital? Maybe you improved your clinical skills at the annual meeting or improved care transitions with Project BOOST? Shout it from the rooftops—tweet @SHMLive and use the hashtag #SHeMpowered. If you haven’t followed SHM on Twitter, head over to @SHMLive.
10 “Are You Number 15,000?”
This is a question you want to say “yes” to! SHM is poised to welcome its 15,000th member in early 2016 as part of the Year of the Hospitalist. The 15,000th member will receive an assortment of exciting prizes, including complimentary registration to Hospital Medicine 2016 in San Diego. Know someone who is interested in joining? Spread the word!
Brett Radler is SHM’s communications coordinator.
ICD-10 Flexibility Helps Transition to New Coding Systems, Principles, Payer Policy Requirements
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
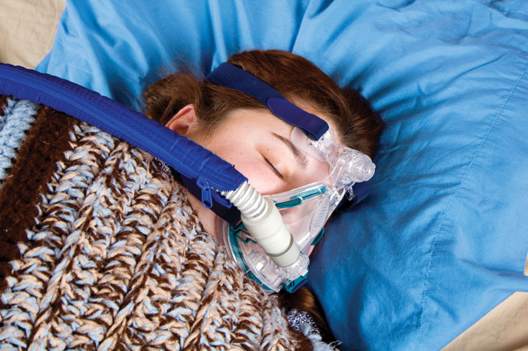
CPAP, oral devices reduced blood pressure in sleep apnea
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
FROM JAMA
Key clinical point: Continuous positive airway pressure and mandibular advancement devices both achieve similar reductions in blood pressure in individuals with obstructive sleep apnea.
Major finding: CPAP use was associated with a mean systolic blood pressure reduction of 2.5 mm Hg, and MADs were associated with a 2.1 mm Hg reduction, compared with inactive controls.
Data source: A systematic review and meta-analysis of 51 studies involving 4,888 patients.
Disclosures: The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
EREFS value has diagnostic utility for eosinophilic esophagitis
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The Eosinophilic Esophagitis Endoscopic Reference Score is highly predictive of eosinophilic esophagitis and responsiveness to treatment.
Major finding: A model containing all five components of the EREFS system as categorical variables had an AUC of 0.946.
Data source: A prospective study of 211 adults undergoing upper endoscopy to investigate esophageal dysfunction.
Disclosures: The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
Prolotherapy: Can it help your patient?
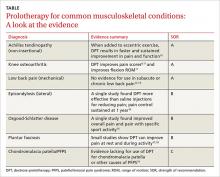
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
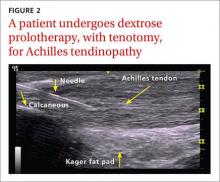
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
Hospitalist Enjoys Mentoring Residents on Patient Care Practices
From 2003 to 2007, Joshua LaBrin, MD, FACP, SFHM, completed his residency in the University of Pittsburgh’s internal medicine/pediatrics program. In his chief resident year, he began to look at fellowships.
So he started thinking about who he considered role models in medicine.
“And they were the hospitalists,” Dr. LaBrin says. “They were the ones on the wards. I was able to see their compassion for their patients, their ability to teach and mentor residents such as myself.
“Those are the people I looked up to, and that’s who I wanted to be.”
And with that, his career in HM began. A fellowship in the specialty followed at the Mayo Clinic in Rochester, Minn. Then a teaching position there. Then one at Vanderbilt University School of Medicine in Nashville. And last year, he became an academic hospitalist and assistant professor at the University of Utah School of Medicine.
Now, he’s one of seven new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: What got you involved in medicine in the first place?
Answer: I remember my pediatrician … putting me at ease during the visits, and then when I was in high school and had appendicitis and had to go through surgery. ... Each of the physicians caring for me left a different impact and served as an inspiration for me to follow in their footsteps.
Q: What was it about those who treated you that struck a nerve?
A: The fact that they cared for me and were able to either keep me well or get me better. The surgeons helped me through a pretty scary part in my life. [They] treated me as a person, rather than just another patient, even meeting me where I was at at that point. That was the kind of thing that resonated with me, and I wanted to do that for people as well.
Q: Did you have a mentor?

A: I had more than one mentor in residency and fellowship where I was able to really learn many lessons from them. And I think among the things that I learned, beyond hospital medicine, was the value of work-life balance. I’ve definitely been able to talk with them, even beyond fellowship, as I have moved and changed positions. That’s something I’ve been able to reflect on regularly. Especially as my family has grown, I continue to communicate with them, so I have always been grateful for their mentorship.
Q: Have you looked to mentor others?
A: That’s one of the things I’ve definitely enjoyed. I have been given opportunities to provide mentorship for new faculty or for residents, and it’s been a humbling experience and it’s been an honor to be able to do that.
Q: In terms of personal satisfaction, what do you enjoy most about your job?
A: I really enjoy working with the trainees, walking with them in their development, seeing them on the wards caring for inpatients and thinking more about issues germane to their patients. Seeing them grow as doctors, as physicians. That’s something I don’t think I’ll ever get tired of doing.
Q: No job is perfect. What do you like least about your job?
A: The most frustrating issue for me is when you are caring for patients and you’re really unable to provide an ideal discharge plan for them due to a financial or technical issue. For example, the three-midnight rule with Medicare sometimes limits you in what you are able to provide for your patients ... so I think that’s probably one of the frustrating things.
Q: What are the biggest changes you’d like to see in the field?
A: One of the big things I have seen over this past year is the whole MOC (Maintenance of Certification) path debate. As nebulous as it has been for internal medicine, in general, I think it is even more so for hospitalists. So having a kind of clear, more practical path for hospitalists would be one of the biggest changes I would like to see. I think the current discussion is healthy. I think it provides a good forum for, hopefully, some positive changes, and I hope that also translates into positive changes for hospitalists as well.
Q: Academic HM can be a real time crunch, between clinical care and classes. How do you balance?
A: Balancing patient care with teaching is one of the big struggles that educators find nowadays, and I think you have to get creative. Obviously, the goal for me as an educator when I am on the wards is to help the trainees take better care of their patients.
So the more I can find ways to be able to provide great patient care, but do it in a way that also either allows the residents or students to learn to do that themselves or to be able to model that for them, then debrief with them afterwards, the more I can achieve that goal.
I have started to learn there are many different ways to be able to do that. And so being able to go into the day as prepared as you can be, and try to have a good plan in place with also plan B and plan C, if necessary, depending on how the day goes, I think is what has helped me.
From 2003 to 2007, Joshua LaBrin, MD, FACP, SFHM, completed his residency in the University of Pittsburgh’s internal medicine/pediatrics program. In his chief resident year, he began to look at fellowships.
So he started thinking about who he considered role models in medicine.
“And they were the hospitalists,” Dr. LaBrin says. “They were the ones on the wards. I was able to see their compassion for their patients, their ability to teach and mentor residents such as myself.
“Those are the people I looked up to, and that’s who I wanted to be.”
And with that, his career in HM began. A fellowship in the specialty followed at the Mayo Clinic in Rochester, Minn. Then a teaching position there. Then one at Vanderbilt University School of Medicine in Nashville. And last year, he became an academic hospitalist and assistant professor at the University of Utah School of Medicine.
Now, he’s one of seven new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: What got you involved in medicine in the first place?
Answer: I remember my pediatrician … putting me at ease during the visits, and then when I was in high school and had appendicitis and had to go through surgery. ... Each of the physicians caring for me left a different impact and served as an inspiration for me to follow in their footsteps.
Q: What was it about those who treated you that struck a nerve?
A: The fact that they cared for me and were able to either keep me well or get me better. The surgeons helped me through a pretty scary part in my life. [They] treated me as a person, rather than just another patient, even meeting me where I was at at that point. That was the kind of thing that resonated with me, and I wanted to do that for people as well.
Q: Did you have a mentor?

A: I had more than one mentor in residency and fellowship where I was able to really learn many lessons from them. And I think among the things that I learned, beyond hospital medicine, was the value of work-life balance. I’ve definitely been able to talk with them, even beyond fellowship, as I have moved and changed positions. That’s something I’ve been able to reflect on regularly. Especially as my family has grown, I continue to communicate with them, so I have always been grateful for their mentorship.
Q: Have you looked to mentor others?
A: That’s one of the things I’ve definitely enjoyed. I have been given opportunities to provide mentorship for new faculty or for residents, and it’s been a humbling experience and it’s been an honor to be able to do that.
Q: In terms of personal satisfaction, what do you enjoy most about your job?
A: I really enjoy working with the trainees, walking with them in their development, seeing them on the wards caring for inpatients and thinking more about issues germane to their patients. Seeing them grow as doctors, as physicians. That’s something I don’t think I’ll ever get tired of doing.
Q: No job is perfect. What do you like least about your job?
A: The most frustrating issue for me is when you are caring for patients and you’re really unable to provide an ideal discharge plan for them due to a financial or technical issue. For example, the three-midnight rule with Medicare sometimes limits you in what you are able to provide for your patients ... so I think that’s probably one of the frustrating things.
Q: What are the biggest changes you’d like to see in the field?
A: One of the big things I have seen over this past year is the whole MOC (Maintenance of Certification) path debate. As nebulous as it has been for internal medicine, in general, I think it is even more so for hospitalists. So having a kind of clear, more practical path for hospitalists would be one of the biggest changes I would like to see. I think the current discussion is healthy. I think it provides a good forum for, hopefully, some positive changes, and I hope that also translates into positive changes for hospitalists as well.
Q: Academic HM can be a real time crunch, between clinical care and classes. How do you balance?
A: Balancing patient care with teaching is one of the big struggles that educators find nowadays, and I think you have to get creative. Obviously, the goal for me as an educator when I am on the wards is to help the trainees take better care of their patients.
So the more I can find ways to be able to provide great patient care, but do it in a way that also either allows the residents or students to learn to do that themselves or to be able to model that for them, then debrief with them afterwards, the more I can achieve that goal.
I have started to learn there are many different ways to be able to do that. And so being able to go into the day as prepared as you can be, and try to have a good plan in place with also plan B and plan C, if necessary, depending on how the day goes, I think is what has helped me.
From 2003 to 2007, Joshua LaBrin, MD, FACP, SFHM, completed his residency in the University of Pittsburgh’s internal medicine/pediatrics program. In his chief resident year, he began to look at fellowships.
So he started thinking about who he considered role models in medicine.
“And they were the hospitalists,” Dr. LaBrin says. “They were the ones on the wards. I was able to see their compassion for their patients, their ability to teach and mentor residents such as myself.
“Those are the people I looked up to, and that’s who I wanted to be.”
And with that, his career in HM began. A fellowship in the specialty followed at the Mayo Clinic in Rochester, Minn. Then a teaching position there. Then one at Vanderbilt University School of Medicine in Nashville. And last year, he became an academic hospitalist and assistant professor at the University of Utah School of Medicine.
Now, he’s one of seven new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: What got you involved in medicine in the first place?
Answer: I remember my pediatrician … putting me at ease during the visits, and then when I was in high school and had appendicitis and had to go through surgery. ... Each of the physicians caring for me left a different impact and served as an inspiration for me to follow in their footsteps.
Q: What was it about those who treated you that struck a nerve?
A: The fact that they cared for me and were able to either keep me well or get me better. The surgeons helped me through a pretty scary part in my life. [They] treated me as a person, rather than just another patient, even meeting me where I was at at that point. That was the kind of thing that resonated with me, and I wanted to do that for people as well.
Q: Did you have a mentor?

A: I had more than one mentor in residency and fellowship where I was able to really learn many lessons from them. And I think among the things that I learned, beyond hospital medicine, was the value of work-life balance. I’ve definitely been able to talk with them, even beyond fellowship, as I have moved and changed positions. That’s something I’ve been able to reflect on regularly. Especially as my family has grown, I continue to communicate with them, so I have always been grateful for their mentorship.
Q: Have you looked to mentor others?
A: That’s one of the things I’ve definitely enjoyed. I have been given opportunities to provide mentorship for new faculty or for residents, and it’s been a humbling experience and it’s been an honor to be able to do that.
Q: In terms of personal satisfaction, what do you enjoy most about your job?
A: I really enjoy working with the trainees, walking with them in their development, seeing them on the wards caring for inpatients and thinking more about issues germane to their patients. Seeing them grow as doctors, as physicians. That’s something I don’t think I’ll ever get tired of doing.
Q: No job is perfect. What do you like least about your job?
A: The most frustrating issue for me is when you are caring for patients and you’re really unable to provide an ideal discharge plan for them due to a financial or technical issue. For example, the three-midnight rule with Medicare sometimes limits you in what you are able to provide for your patients ... so I think that’s probably one of the frustrating things.
Q: What are the biggest changes you’d like to see in the field?
A: One of the big things I have seen over this past year is the whole MOC (Maintenance of Certification) path debate. As nebulous as it has been for internal medicine, in general, I think it is even more so for hospitalists. So having a kind of clear, more practical path for hospitalists would be one of the biggest changes I would like to see. I think the current discussion is healthy. I think it provides a good forum for, hopefully, some positive changes, and I hope that also translates into positive changes for hospitalists as well.
Q: Academic HM can be a real time crunch, between clinical care and classes. How do you balance?
A: Balancing patient care with teaching is one of the big struggles that educators find nowadays, and I think you have to get creative. Obviously, the goal for me as an educator when I am on the wards is to help the trainees take better care of their patients.
So the more I can find ways to be able to provide great patient care, but do it in a way that also either allows the residents or students to learn to do that themselves or to be able to model that for them, then debrief with them afterwards, the more I can achieve that goal.
I have started to learn there are many different ways to be able to do that. And so being able to go into the day as prepared as you can be, and try to have a good plan in place with also plan B and plan C, if necessary, depending on how the day goes, I think is what has helped me.
Modified Valsalva Better than Standard Maneuver to Restore Sinus Rhythm
Clinical question: Does a postural modification to the Valsalva maneuver improve its effectiveness?
Background: The Valsalva maneuver, often used to treat supraventricular tachycardia, is rarely successful. A modification to the maneuver to increase relaxation phase venous return and vagal stimulation could improve its efficacy.
Study design: Multicenter, randomized controlled trial (RCT).
Setting: Ten emergency departments in England.
Synopsis: Four hundred thirty-three patients with stable supraventricular tachycardia (excluding atrial fibrillation or flutter) were randomized to use the Valsalva maneuver (control) or modified Valsalva maneuver (intervention). In the control group, strain was standardized using a manometer (40 mm Hg for 15 seconds). In the intervention group, patients underwent the same maneuver, followed by lying supine with passive leg raise to 45 degrees for 15 seconds. Participants could repeat the maneuver if it was initially unsuccessful. Randomization was stratified by center.
Using an intention-to-treat analysis, 43% of the intervention group achieved the primary outcome of sinus rhythm one minute after straining, compared with 17% of the control group (P<0.0001). The intervention group was less likely to receive adenosine (50% vs. 69%, P=0.0002) or any emergency, anti-arrhythmic treatment (80% vs. 57%, P<0.0001).
No significant differences were seen in hospital admissions, length of ED stay, or adverse events between groups.
Bottom line: In patients with stable supraventricular tachycardia, modifying the Valsalva maneuver is significantly more effective in restoring sinus rhythm.
Citation: Appelboam A, Reuben A, Mann C, et al. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial [published online ahead of print August 24, 2015]. Lancet. doi: 10.1016/S0140-6736(15)61485-4.
Clinical question: Does a postural modification to the Valsalva maneuver improve its effectiveness?
Background: The Valsalva maneuver, often used to treat supraventricular tachycardia, is rarely successful. A modification to the maneuver to increase relaxation phase venous return and vagal stimulation could improve its efficacy.
Study design: Multicenter, randomized controlled trial (RCT).
Setting: Ten emergency departments in England.
Synopsis: Four hundred thirty-three patients with stable supraventricular tachycardia (excluding atrial fibrillation or flutter) were randomized to use the Valsalva maneuver (control) or modified Valsalva maneuver (intervention). In the control group, strain was standardized using a manometer (40 mm Hg for 15 seconds). In the intervention group, patients underwent the same maneuver, followed by lying supine with passive leg raise to 45 degrees for 15 seconds. Participants could repeat the maneuver if it was initially unsuccessful. Randomization was stratified by center.
Using an intention-to-treat analysis, 43% of the intervention group achieved the primary outcome of sinus rhythm one minute after straining, compared with 17% of the control group (P<0.0001). The intervention group was less likely to receive adenosine (50% vs. 69%, P=0.0002) or any emergency, anti-arrhythmic treatment (80% vs. 57%, P<0.0001).
No significant differences were seen in hospital admissions, length of ED stay, or adverse events between groups.
Bottom line: In patients with stable supraventricular tachycardia, modifying the Valsalva maneuver is significantly more effective in restoring sinus rhythm.
Citation: Appelboam A, Reuben A, Mann C, et al. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial [published online ahead of print August 24, 2015]. Lancet. doi: 10.1016/S0140-6736(15)61485-4.
Clinical question: Does a postural modification to the Valsalva maneuver improve its effectiveness?
Background: The Valsalva maneuver, often used to treat supraventricular tachycardia, is rarely successful. A modification to the maneuver to increase relaxation phase venous return and vagal stimulation could improve its efficacy.
Study design: Multicenter, randomized controlled trial (RCT).
Setting: Ten emergency departments in England.
Synopsis: Four hundred thirty-three patients with stable supraventricular tachycardia (excluding atrial fibrillation or flutter) were randomized to use the Valsalva maneuver (control) or modified Valsalva maneuver (intervention). In the control group, strain was standardized using a manometer (40 mm Hg for 15 seconds). In the intervention group, patients underwent the same maneuver, followed by lying supine with passive leg raise to 45 degrees for 15 seconds. Participants could repeat the maneuver if it was initially unsuccessful. Randomization was stratified by center.
Using an intention-to-treat analysis, 43% of the intervention group achieved the primary outcome of sinus rhythm one minute after straining, compared with 17% of the control group (P<0.0001). The intervention group was less likely to receive adenosine (50% vs. 69%, P=0.0002) or any emergency, anti-arrhythmic treatment (80% vs. 57%, P<0.0001).
No significant differences were seen in hospital admissions, length of ED stay, or adverse events between groups.
Bottom line: In patients with stable supraventricular tachycardia, modifying the Valsalva maneuver is significantly more effective in restoring sinus rhythm.
Citation: Appelboam A, Reuben A, Mann C, et al. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial [published online ahead of print August 24, 2015]. Lancet. doi: 10.1016/S0140-6736(15)61485-4.
CHA2DS2-Vasc Score Modestly Predicts Stroke, Thromboembolism, Death
Clinical question: For patients with heart failure (HF), with and without concurrent Afib (AF), does the CHA2DS2-VASc score predict ischemic stroke, thromboembolism, and death?
Background: Factors in the CHA2DS2-VASc score predict increased risk of stroke, thromboembolism, and death, regardless of whether AF is present. It is unknown if this score can identify subgroups of patients with HF, with and without AF, at particularly high or low risk of these events.
Study design: Prospective, cohort study.
Setting: Three Danish registries, 2000-2012.
Synopsis: Among 42,987 patients 50 years and older with incident HF not on anticoagulation, the absolute risk of stroke among patients without AF was 1.5% per year or higher with a CHA2DS2-VASc score of two or higher. The absolute risk of stroke was 4% or higher at five years. Risks were higher in the 21.9% of patients with AF. The CHA2DS2-VASc score modestly predicted endpoints and had an approximately 90% negative predictive value for stroke, thromboembolism, and death at one-year follow-up, regardless of whether or not AF was present.
In this large study, HF patients in a non-diverse population were studied, and some patients may have had undiagnosed AF. Functional status and ejection fraction in patients with HF could not be categorized; however, reported five-year results may be generalizable to patients with chronic HF. Select patients with HF without AF, who have two or more factors of the score besides HF, might benefit from anticoagulation.
Bottom line: The CHA2DS2-VASc score modestly predicts stroke, thromboembolism, and death among patients with HF, but further studies are needed to determine its clinical usefulness.
Citation: Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314(10):1030-1038.
Clinical question: For patients with heart failure (HF), with and without concurrent Afib (AF), does the CHA2DS2-VASc score predict ischemic stroke, thromboembolism, and death?
Background: Factors in the CHA2DS2-VASc score predict increased risk of stroke, thromboembolism, and death, regardless of whether AF is present. It is unknown if this score can identify subgroups of patients with HF, with and without AF, at particularly high or low risk of these events.
Study design: Prospective, cohort study.
Setting: Three Danish registries, 2000-2012.
Synopsis: Among 42,987 patients 50 years and older with incident HF not on anticoagulation, the absolute risk of stroke among patients without AF was 1.5% per year or higher with a CHA2DS2-VASc score of two or higher. The absolute risk of stroke was 4% or higher at five years. Risks were higher in the 21.9% of patients with AF. The CHA2DS2-VASc score modestly predicted endpoints and had an approximately 90% negative predictive value for stroke, thromboembolism, and death at one-year follow-up, regardless of whether or not AF was present.
In this large study, HF patients in a non-diverse population were studied, and some patients may have had undiagnosed AF. Functional status and ejection fraction in patients with HF could not be categorized; however, reported five-year results may be generalizable to patients with chronic HF. Select patients with HF without AF, who have two or more factors of the score besides HF, might benefit from anticoagulation.
Bottom line: The CHA2DS2-VASc score modestly predicts stroke, thromboembolism, and death among patients with HF, but further studies are needed to determine its clinical usefulness.
Citation: Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314(10):1030-1038.
Clinical question: For patients with heart failure (HF), with and without concurrent Afib (AF), does the CHA2DS2-VASc score predict ischemic stroke, thromboembolism, and death?
Background: Factors in the CHA2DS2-VASc score predict increased risk of stroke, thromboembolism, and death, regardless of whether AF is present. It is unknown if this score can identify subgroups of patients with HF, with and without AF, at particularly high or low risk of these events.
Study design: Prospective, cohort study.
Setting: Three Danish registries, 2000-2012.
Synopsis: Among 42,987 patients 50 years and older with incident HF not on anticoagulation, the absolute risk of stroke among patients without AF was 1.5% per year or higher with a CHA2DS2-VASc score of two or higher. The absolute risk of stroke was 4% or higher at five years. Risks were higher in the 21.9% of patients with AF. The CHA2DS2-VASc score modestly predicted endpoints and had an approximately 90% negative predictive value for stroke, thromboembolism, and death at one-year follow-up, regardless of whether or not AF was present.
In this large study, HF patients in a non-diverse population were studied, and some patients may have had undiagnosed AF. Functional status and ejection fraction in patients with HF could not be categorized; however, reported five-year results may be generalizable to patients with chronic HF. Select patients with HF without AF, who have two or more factors of the score besides HF, might benefit from anticoagulation.
Bottom line: The CHA2DS2-VASc score modestly predicts stroke, thromboembolism, and death among patients with HF, but further studies are needed to determine its clinical usefulness.
Citation: Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314(10):1030-1038.
Residents’ Forum: 2015 Cardiac training experience from the perspective of a trainee
At the 62nd annual Southern Thoracic Surgical Association meeting in Orlando, Fla., there were several pervasive themes, including measurement of quality metrics in pulmonary, esophageal, and cardiac surgery. Piggybacking on the topic of quality, Dr. Asad A. Shah of Duke University, Durham, N.C., and colleagues identified something inherent to improving a surgical product: the quality of resident training.
In the presentation titled Characterizing the Operative Experience of Cardiothoracic Surgery Residents in the United States: What are Residents Really Doing in the Operating Room?, Dr. Shah and his group utilized data from the 2015 Thoracic Surgery Directors Association Survey (to which 356 trainees responded) in order to analyze specific critical steps that are being performed by each PGY level in both traditional (2- and 3- year) and I-6 integrated programs.
In I-6 programs, trainees routinely performed sternotomy by PGY1; harvested LIMA, cannulated, and performed proximal anastomoses by PGY3; and performed all aspects of CABG by PGY4. Fully 100% of I-6 residents reported being the operative surgeon for both coronary artery bypass grafting (CABG) and aortic valve replacement (AVR) compared to 94% and 86% for CABG in 2-year and 3-year programs (respectively), and 89% and 83% for AVR in 2-year and 3-year programs (respectively).
Few trainees reported experience with other cardiac surgeries as an operative surgeon. Likely because of this lack of experience, 42% of trainees reported the need for further fellowship training to become facile with most standard cardiac procedures.
When we discuss quality metrics, qualifying an educational experience is critical. I commend Dr. Shah and colleagues for a well-analyzed, thoughtful study using the results of a survey that we all take at the end of our annual in-service training exam.
Graded operative responsibility is shown to work well in I-6 training programs where ALL residents reported experience as operative surgeon for CABG and AVR. Interestingly, traditional residents did not have the same experience: It is possible that a truncated training program makes graded learning more difficult in this population group, despite superior surgical skill at entry into cardiothoracic training. Or is this a matter of poor reporting? The definition of “operating surgeon” is not used or interpreted in a standard way and may be incorrectly used by I-6 trainees who have no basis of comparison to relative operative experience in a general surgery program.
Conversely, traditional cardiothoracic residents may have a different barometer of what it means to be an operating surgeon, potentially under-qualifying their experience. Either way, it is difficult to truly objectify a survey, as all individuals will interpret their experience based on their personal learning environment. Dr. Shah’s team is accurate in alluding to the heterogeneity of this experience.
If the operative experience is perceived to be so different amongst trainees across programs, how do we as a society standardize education in order to graduate more competent and capable cardiothoracic surgeons? Sending trainees to boot camps and increasing utilization of simulation labs is one step. Additionally, 360-degree Accreditation Council for Graduate Medical Education–mandated evaluations may open communication avenues that didn’t exist before between mentor and mentee in the operating room and encourage more stepwise teaching. And how do we augment operating surgeon experience across the other cardiac categories (i.e. mitral valve repair, aorta, TAVR, etc)?
In order for the composite body of new graduates to report better national outcomes, we must standardize quality teaching between programs. It is simply not acceptable that half of trainees feel that advanced fellowships are necessary to reach comfort in standard cardiac cases.
The aforementioned study is a great first start, and the analysis should be extended to thoracic experience. Specifically, it would be interesting to perform the same analysis for thoracoscopic cases, as these also include steps that can be learned and mastered prior to doing a case skin to skin on the operating-surgeon side of the table. Standardizing education is difficult in cardiothoracic surgery, but Dr. Shah and colleagues begin an excellent conversation about the heterogeneous training experience that prepares some but fails others.
Dr. Shersher is a cardiothoracic surgeon at Rush University Medical Center, Chicago, and a resident medical editor for Thoracic Surgery News.
At the 62nd annual Southern Thoracic Surgical Association meeting in Orlando, Fla., there were several pervasive themes, including measurement of quality metrics in pulmonary, esophageal, and cardiac surgery. Piggybacking on the topic of quality, Dr. Asad A. Shah of Duke University, Durham, N.C., and colleagues identified something inherent to improving a surgical product: the quality of resident training.
In the presentation titled Characterizing the Operative Experience of Cardiothoracic Surgery Residents in the United States: What are Residents Really Doing in the Operating Room?, Dr. Shah and his group utilized data from the 2015 Thoracic Surgery Directors Association Survey (to which 356 trainees responded) in order to analyze specific critical steps that are being performed by each PGY level in both traditional (2- and 3- year) and I-6 integrated programs.
In I-6 programs, trainees routinely performed sternotomy by PGY1; harvested LIMA, cannulated, and performed proximal anastomoses by PGY3; and performed all aspects of CABG by PGY4. Fully 100% of I-6 residents reported being the operative surgeon for both coronary artery bypass grafting (CABG) and aortic valve replacement (AVR) compared to 94% and 86% for CABG in 2-year and 3-year programs (respectively), and 89% and 83% for AVR in 2-year and 3-year programs (respectively).
Few trainees reported experience with other cardiac surgeries as an operative surgeon. Likely because of this lack of experience, 42% of trainees reported the need for further fellowship training to become facile with most standard cardiac procedures.
When we discuss quality metrics, qualifying an educational experience is critical. I commend Dr. Shah and colleagues for a well-analyzed, thoughtful study using the results of a survey that we all take at the end of our annual in-service training exam.
Graded operative responsibility is shown to work well in I-6 training programs where ALL residents reported experience as operative surgeon for CABG and AVR. Interestingly, traditional residents did not have the same experience: It is possible that a truncated training program makes graded learning more difficult in this population group, despite superior surgical skill at entry into cardiothoracic training. Or is this a matter of poor reporting? The definition of “operating surgeon” is not used or interpreted in a standard way and may be incorrectly used by I-6 trainees who have no basis of comparison to relative operative experience in a general surgery program.
Conversely, traditional cardiothoracic residents may have a different barometer of what it means to be an operating surgeon, potentially under-qualifying their experience. Either way, it is difficult to truly objectify a survey, as all individuals will interpret their experience based on their personal learning environment. Dr. Shah’s team is accurate in alluding to the heterogeneity of this experience.
If the operative experience is perceived to be so different amongst trainees across programs, how do we as a society standardize education in order to graduate more competent and capable cardiothoracic surgeons? Sending trainees to boot camps and increasing utilization of simulation labs is one step. Additionally, 360-degree Accreditation Council for Graduate Medical Education–mandated evaluations may open communication avenues that didn’t exist before between mentor and mentee in the operating room and encourage more stepwise teaching. And how do we augment operating surgeon experience across the other cardiac categories (i.e. mitral valve repair, aorta, TAVR, etc)?
In order for the composite body of new graduates to report better national outcomes, we must standardize quality teaching between programs. It is simply not acceptable that half of trainees feel that advanced fellowships are necessary to reach comfort in standard cardiac cases.
The aforementioned study is a great first start, and the analysis should be extended to thoracic experience. Specifically, it would be interesting to perform the same analysis for thoracoscopic cases, as these also include steps that can be learned and mastered prior to doing a case skin to skin on the operating-surgeon side of the table. Standardizing education is difficult in cardiothoracic surgery, but Dr. Shah and colleagues begin an excellent conversation about the heterogeneous training experience that prepares some but fails others.
Dr. Shersher is a cardiothoracic surgeon at Rush University Medical Center, Chicago, and a resident medical editor for Thoracic Surgery News.
At the 62nd annual Southern Thoracic Surgical Association meeting in Orlando, Fla., there were several pervasive themes, including measurement of quality metrics in pulmonary, esophageal, and cardiac surgery. Piggybacking on the topic of quality, Dr. Asad A. Shah of Duke University, Durham, N.C., and colleagues identified something inherent to improving a surgical product: the quality of resident training.
In the presentation titled Characterizing the Operative Experience of Cardiothoracic Surgery Residents in the United States: What are Residents Really Doing in the Operating Room?, Dr. Shah and his group utilized data from the 2015 Thoracic Surgery Directors Association Survey (to which 356 trainees responded) in order to analyze specific critical steps that are being performed by each PGY level in both traditional (2- and 3- year) and I-6 integrated programs.
In I-6 programs, trainees routinely performed sternotomy by PGY1; harvested LIMA, cannulated, and performed proximal anastomoses by PGY3; and performed all aspects of CABG by PGY4. Fully 100% of I-6 residents reported being the operative surgeon for both coronary artery bypass grafting (CABG) and aortic valve replacement (AVR) compared to 94% and 86% for CABG in 2-year and 3-year programs (respectively), and 89% and 83% for AVR in 2-year and 3-year programs (respectively).
Few trainees reported experience with other cardiac surgeries as an operative surgeon. Likely because of this lack of experience, 42% of trainees reported the need for further fellowship training to become facile with most standard cardiac procedures.
When we discuss quality metrics, qualifying an educational experience is critical. I commend Dr. Shah and colleagues for a well-analyzed, thoughtful study using the results of a survey that we all take at the end of our annual in-service training exam.
Graded operative responsibility is shown to work well in I-6 training programs where ALL residents reported experience as operative surgeon for CABG and AVR. Interestingly, traditional residents did not have the same experience: It is possible that a truncated training program makes graded learning more difficult in this population group, despite superior surgical skill at entry into cardiothoracic training. Or is this a matter of poor reporting? The definition of “operating surgeon” is not used or interpreted in a standard way and may be incorrectly used by I-6 trainees who have no basis of comparison to relative operative experience in a general surgery program.
Conversely, traditional cardiothoracic residents may have a different barometer of what it means to be an operating surgeon, potentially under-qualifying their experience. Either way, it is difficult to truly objectify a survey, as all individuals will interpret their experience based on their personal learning environment. Dr. Shah’s team is accurate in alluding to the heterogeneity of this experience.
If the operative experience is perceived to be so different amongst trainees across programs, how do we as a society standardize education in order to graduate more competent and capable cardiothoracic surgeons? Sending trainees to boot camps and increasing utilization of simulation labs is one step. Additionally, 360-degree Accreditation Council for Graduate Medical Education–mandated evaluations may open communication avenues that didn’t exist before between mentor and mentee in the operating room and encourage more stepwise teaching. And how do we augment operating surgeon experience across the other cardiac categories (i.e. mitral valve repair, aorta, TAVR, etc)?
In order for the composite body of new graduates to report better national outcomes, we must standardize quality teaching between programs. It is simply not acceptable that half of trainees feel that advanced fellowships are necessary to reach comfort in standard cardiac cases.
The aforementioned study is a great first start, and the analysis should be extended to thoracic experience. Specifically, it would be interesting to perform the same analysis for thoracoscopic cases, as these also include steps that can be learned and mastered prior to doing a case skin to skin on the operating-surgeon side of the table. Standardizing education is difficult in cardiothoracic surgery, but Dr. Shah and colleagues begin an excellent conversation about the heterogeneous training experience that prepares some but fails others.
Dr. Shersher is a cardiothoracic surgeon at Rush University Medical Center, Chicago, and a resident medical editor for Thoracic Surgery News.