User login
Necrotizing Fasciitis Caused by Cryptococcus gattii
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
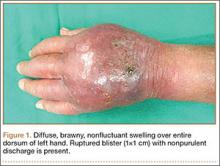
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
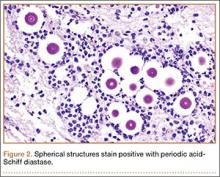
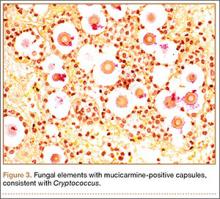
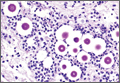
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
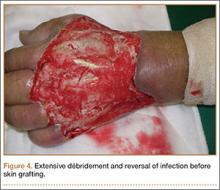
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
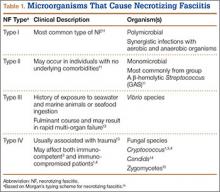
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
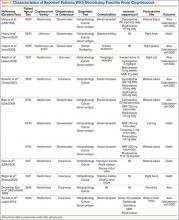
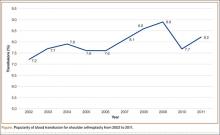
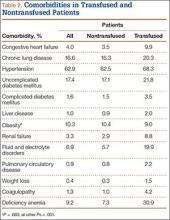
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Functional Knee Outcomes in Infrapatellar and Suprapatellar Tibial Nailing: Does Approach Matter?
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
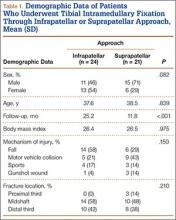
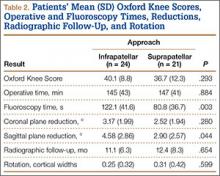
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
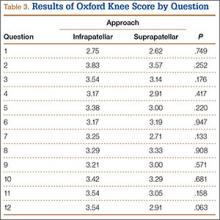
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
With an incidence of 75,000 per year in the United States alone, fractures of the tibial shaft are among the most common long-bone fractures.1 Diaphyseal tibial fractures present a unique treatment challenge because of complications, including nonunion, malunion, and the potential for an open injury. Intramedullary fixation of these fractures has long been the standard of care, allowing for early mobilization, shorter time to weight-bearing, and high union rates.2-4
The classic infrapatellar approach to intramedullary nailing involves placing the knee in hyperflexion over a bump or radiolucent triangle and inserting the nail through a longitudinal incision in line with the fibers of the patellar tendon. Deforming muscle forces often cause proximal-third tibial fractures and segmental fractures to fall into valgus and procurvatum. To counter these deforming forces, orthopedic surgeons have used some novel surgical approaches, including use of blocking screws5 and a parapatellar approach that could be used with the knee in semi-extended position.6 Anterior knee pain has been reported as a common complication of tibial nailing (reported incidence, 56%).7 In a prospective randomized controlled study, Toivanen and colleagues8 found no difference in incidence of knee pain between patellar tendon splitting and parapatellar approaches.
Techniques have been developed to insert the nail through a semi-extended suprapatellar approach to facilitate intraoperative imaging, allow easier access to starting-site position, and counter deforming forces. Although outcomes of traditional infrapatellar nailing have been well documented, there is a paucity of literature on outcomes of using a suprapatellar approach. Splitting the quadriceps tendon causes scar tissue to form superior to the patella versus the anterior knee, which may reduce flexion-related pain or kneeling pain.9 The infrapatellar nerve is also well protected with this approach.
We conducted a study to determine differences in functional knee pain in patients who underwent either traditional infrapatellar nailing or suprapatellar nailing. We hypothesized that there would be no difference in functional knee scores between these approaches and that, when compared with the infrapatellar approach, the suprapatellar approach would result in improved postoperative reduction and reduced intraoperative fluoroscopy time.
Materials and Methods
This study was approved by our institutional review board. We searched our level I trauma center’s database for Current Procedural Terminology (CPT) code 27759 to identify all patients who had a tibial shaft fracture fixed with an intramedullary implant between January 2009 and February 2013. Radiographs, operative reports, and inpatient records were reviewed. Patients older than 18 years at time of injury and patients with an isolated tibial shaft fracture (Orthopaedic Trauma Association type 42 A-C) surgically fixed with an intramedullary nail through either a traditional infrapatellar approach or a suprapatellar approach were included in the study. Exclusion criteria were required fasciotomy, Gustilo type 3B or 3C open fracture, prior knee surgery, additional orthopedic injury, and preexisting radiographic evidence of degenerative joint disease.
In addition to surgical approach, demographic data, including body mass index (BMI), age, sex, and mechanism of injury, were documented from the medical record. Each patient was contacted by telephone by an investigator blinded to surgical exposure, and the 12-item Oxford Knee Score (OKS) questionnaire was administered (Figure). Operative time, quality of reduction on postoperative radiographs, and intraoperative fluoroscopy time were compared between the 2 approaches. We determined quality of reduction by measuring the angle between the line perpendicular to the tibial plateau and plafond on both the anteroposterior and lateral postoperative radiographs. Rotation was determined by measuring displacement of the fracture by cortical widths. The infrapatellar and suprapatellar groups were statistically analyzed with an unpaired, 2-tailed Student t test. Categorical variables between groups were analyzed with the χ2 test or, when expected values in a cell were less than 5, the Fisher exact test.
We then conducted an a priori power analysis to determine the appropriate sample size. To detect the reported minimally clinically important difference in the OKS of 5.2,10 estimating an approximate 20% larger patient population in the infrapatellar group, we would need to enroll 24 infrapatellar patients and 20 suprapatellar patients to achieve a power of 0.80 with a type I error rate of 0.05.11 This analysis is also based on an estimated OKS standard deviation of 6, which has been reported in several studies.12,13
Results
We identified 176 patients who had the CPT code for intramedullary fixation of a tibial shaft fracture between January 2009 and February 2013. After analysis of radiographs and medical records, 82 patients met the inclusion criteria. Thirty-six (45%) of the original 82 patients were lost to follow-up after attempts to contact them by telephone. One patient refused to participate in the study. Twenty-four patients underwent traditional infrapatellar nailing, and 21 patients had a suprapatellar nail placed with approach-specific instrumentation. Nine patients had an open fracture. There was no significant difference between the groups in terms of sex, age, BMI, mechanism of injury, or operative time (Table 1). There was also no difference (P = .210) in fracture location between groups (0 proximal-third, 14 midshaft, 10 distal-third vs 3 proximal-third, 10 midshaft, 8 distal-third). Mean age was 37.6 years (range, 20-65 years) for the infrapatellar group and 38.5 years (range, 18-68 years) for the suprapatellar group (P = .839). Mean follow-up was significantly (P < .001) shorter for the suprapatellar group (12 mo; range, 3-33 mo) than for the infrapatellar group (25 mo; range, 4-43 mo).
Mean OKS (maximum, 48 points) was 40.1 (range, 11-48) for the infrapatellar group and 36.7 (range, 2-48) for the suprapatellar group (P = .293). Table 2 summarizes the data. Radiographic reduction in the sagittal plane was improved (P = .044) in the suprapatellar group (2.90°) compared with the infrapatellar group (4.58°). There was no difference in rotational malreduction (0.31 vs 0.25 cortical width; P = .599) or in reduction in the coronal plane (2.52° vs 3.17°; P = .280). All patients in both groups maintained radiographic reduction within 5° in any plane throughout follow-up. There was no difference (P = .654) in radiographic follow-up between the infrapatellar group (11 mo) and the suprapatellar group (12 mo). The 1 nonunion in the suprapatellar group required return to the operating room for exchange intramedullary nailing. The suprapatellar approach required less (P = .003) operative fluoroscopy time (80.8 s; range, 46-180 s) than the standard infrapatellar approach (122.1 s; range, 71-240 s). Two patients in the suprapatellar group and 8 in the infrapatellar group did not have their fluoroscopy time recorded in the operative report.
Discussion
We have described the first retrospective cohort-comparison study of functional knee scores associated with traditional infrapatellar nailing and suprapatellar nailing. Although much has been written about the incidence of anterior knee pain with use of a patellar splitting or parapatellar approach, the clinical effects of knee pain after use of suprapatellar nails are yet to be addressed. In a cadaveric study, Gelbke and colleagues14 found higher mean patellofemoral pressures and higher peak contact pressures with a suprapatellar approach. These numbers, however, were still far below the threshold for chondrocyte damage, and that study is yet to be clinically validated. Our data showed no difference in OKS between the 2 groups. Despite being intra-articular, approach-specific instrumentation may protect the trochlea and patellar cartilage.
Although the OKS questionnaire was originally developed and widely validated to describe clinical outcomes of total knee arthroplasty,15,16 it has also been evaluated for other interventions, including viscosupplementation injections17 and high tibial osteotomy.18 We used the OKS questionnaire in our study because it is simple to administer by telephone and is not as cumbersome as the Knee Society Score or the Western Ontario and McMaster Universities Osteoarthritis Index. It is also more specific to the knee than generalized outcome measures used in trauma, such as the Short Form 36 (SF-36). Sanders and colleagues19 reported excellent tibial alignment, radiographic union, and knee range of motion using semi-extended tibial nailing with a suprapatellar approach. For outcome measures, they used the Lysholm Knee Score and the SF-36. Our clinical and radiographic results confirmed their finding—that the semi-extended suprapatellar approach is an option for tibial nailing.
OKS results by question (Table 3) showed that the infrapatellar group had less pain walking down stairs. This result approached statistical significance (P = .063). As surgeons at our institution began using the suprapatellar approach only during the final 2 years of the study period, mean follow-up was significantly (P < .001) less than for the infrapatellar group (12 vs 25 mo). Although there was no statistically significant difference in reduction quality on anteroposterior radiographs, the suprapatellar approach had improved (P = .044) reduction on lateral radiographs (2.90° vs 4.58°).
Although operative time did not differ between our 2 groups, significantly (P = .003) less fluoroscopy time was required for suprapatellar nails (80.8 s) than for infrapatellar nails (122.1 s). Positioning the knee in the semi-extended position offers easier access for fluoroscopy and less radiation exposure for the patient. Placing the nail in extension also helps eliminate the deforming forces that cause malreduction of proximal tibial shaft or segmental fractures. However, our study was limited in that only 2 surgeons at our institution used the suprapatellar approach, and both were fellowship-trained in orthopedic traumatology. This situation could have introduced bias into the interpretation of fluoroscopy data, as these surgeons may have been more comfortable with the procedure and less likely to use fluoroscopy. Both surgeons also performed infrapatellar nailing during the study period, and there was no statistical difference in fracture patterns between the groups, thus minimizing bias.
This study was retrospective but had several strengths. Sample size met the prestudy power analysis to determine a minimally clinically important difference in OKS results. The investigator who administered the telephone survey was blinded to surgical approach. This study was also the first clinical study to compare outcomes of infrapatellar and suprapatellar nailing. However, the study’s follow-up rate was a weakness. The patient population at our academic, urban, level I trauma center is transient. We lost 36 patients (45%) to follow-up; their telephone numbers in the hospital records likely changed since surgery, and we could not contact these patients.
Conclusion
Our retrospective cohort study found no difference in OKS between traditional infrapatellar nailing and suprapatellar nailing for diaphyseal tibia fractures. Suprapatellar nails require less fluoroscopy time and may show improved radiographic reduction in the sagittal plane. Although further study is needed, the suprapatellar entry portal appears to be a safe alternative for tibial nailing with use of appropriate instrumentation.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
1. Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
2. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced isolated fractures of the tibial shaft treated with either a cast or intramedullary nailing. An outcome analysis of matched pairs of patients. J Bone Joint Surg Am. 1997;79(9):1336-1341.
3. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
4. Alho A, Benterud JG, Høgevold HE, Ekeland A, Strømsøe K. Comparison of functional bracing and locked intramedullary nailing in the treatment of displaced tibial shaft fractures. Clin Orthop Relat Res. 1992;(277):243-250.
5. Ricci WM, O’Boyle M, Borrelli J, Bellabarba C, Sanders R. Fractures of the proximal third of the tibial shaft treated with intramedullary nails and blocking screws. J Orthop Trauma. 2001;15(4):264-270.
6. Tornetta P 3rd, Collins E. Semiextended position of intramedullary nailing of the proximal tibia. Clin Orthop Relat Res. 1996;(328):185-189.
7. Court-Brown CM, Gustilo T, Shaw AD. Knee pain after intramedullary tibial nailing: its incidence, etiology, and outcome. J Orthop Trauma. 1997;11(2):103-105.
8. Toivanen JA, Väistö O, Kannus P, Latvala K, Honkonen SE, Järvinen MJ. Anterior knee pain after intramedullary nailing of fractures of the tibial shaft. A prospective, randomized study comparing two different nail-insertion techniques. J Bone Joint Surg Am. 2002;84(4):580-585.
9. Morandi M, Banka T, Gairarsa GP, et al. Intramedullary nailing of tibial fractures: review of surgical techniques and description of a percutaneous lateral suprapatellar approach. Orthopaedics. 2010;33(3):172-179.
10. Bohm ER, Loucks L, Tan QE, et al. Determining minimum clinically important difference and targeted clinical improvement values for the Oxford 12. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; 2012; San Francisco, CA.
11. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128.
12. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
13. Jenny JY, Diesinger Y. The Oxford Knee Score: compared performance before and after knee replacement. Orthop Traumatol Surg Res. 2012;98(4):409-412.
14. Gelbke MK, Coombs D, Powell S, et al. Suprapatellar versus infra-patellar intramedullary nail insertion of the tibia: a cadaveric model for comparison of patellofemoral contact pressures and forces. J Orthop Trauma. 2010;24(11):665-671.
15. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63-69.
16. Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Translation and validation of the Oxford-12 item knee score for use in Sweden. Acta Orthop Scand. 2000;71(3):268-274.
17. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57-62.
18. Weale AE, Lee AS, MacEachern AG. High tibial osteotomy using a dynamic axial external fixator. Clin Orthop Relat Res. 2001;(382):154-167.
19. Sanders RW, DiPasquale TG, Jordan CJ, Arrington JA, Sagi HC. Semiextended intramedullary nailing of the tibia using a suprapatellar approach: radiographic results and clinical outcomes at a minimum of 12 months follow-up. J Orthop Trauma. 2014;28(suppl 8):S29-S39.
ADT linked to increased risk of Alzheimer’s disease
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients who underwent ADT for prostate cancer had significantly increased risk of future Alzheimer’s disease diagnosis.
Major finding: ADT use was significantly associated with Alzheimer’s disease risk, with an HR of 1.88 by propensity score–matched Cox regression analysis (95% CI, 1.10-3.20; P = .021).
Data source: The electronic medical record analysis evaluated 16,888 patients with prostate cancer. Of 2,397 patients who received ADT, 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years.
Disclosures: Research was supported by grants from the National Institutes of Health, National Library of Medicine, and National Institute of General Medical Sciences, which owns the patent by Stanford on data mining techniques. Dr. Nead reported having no disclosures.
US National Practice Patterns in Ambulatory Operative Management of Lateral Epicondylitis
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
VIDEO: Novel GBT440 improves blood parameters in sickle cell disease
ORLANDO – A novel small molecule agent improved hematologic parameters and was associated with significant reduction in sickling of red blood cells in patients with sickle cell disease.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation.
In an interview, Dr. Claire Hemmaway of Queens Hospital in Romford, England, discusses early results from a phase I/II randomized, double-blind, placebo-controlled, parallel-group trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – A novel small molecule agent improved hematologic parameters and was associated with significant reduction in sickling of red blood cells in patients with sickle cell disease.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation.
In an interview, Dr. Claire Hemmaway of Queens Hospital in Romford, England, discusses early results from a phase I/II randomized, double-blind, placebo-controlled, parallel-group trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – A novel small molecule agent improved hematologic parameters and was associated with significant reduction in sickling of red blood cells in patients with sickle cell disease.
The drug was shown to increase hemoglobin in both healthy volunteers and patients, reduce reticulocytosis, and improve biomarkers of hemolysis and inflammation.
In an interview, Dr. Claire Hemmaway of Queens Hospital in Romford, England, discusses early results from a phase I/II randomized, double-blind, placebo-controlled, parallel-group trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
Total Knee Arthroplasty in Hemophilic Arthropathy
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Prevalence of Low Bone Mineral Density in Younger Versus Older Women With Distal Radius Fractures
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
Analysis of Predictors and Outcomes of Allogeneic Blood Transfusion After Shoulder Arthroplasty
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
Listen Now: Highlights of the December 2015 issue of The Hospitalist
Highlights from the December 2015 issue of The Hospitalist include a look at ways hospitals can better care for unassigned or uninsured patients, how electronic health record implementation has contributed to physician burnout, and our key clinical question examines strategies for secondary stroke prevention.
Highlights from the December 2015 issue of The Hospitalist include a look at ways hospitals can better care for unassigned or uninsured patients, how electronic health record implementation has contributed to physician burnout, and our key clinical question examines strategies for secondary stroke prevention.
Highlights from the December 2015 issue of The Hospitalist include a look at ways hospitals can better care for unassigned or uninsured patients, how electronic health record implementation has contributed to physician burnout, and our key clinical question examines strategies for secondary stroke prevention.
Orthopedic Practice Patterns Relating to Anterior Cruciate Ligament Reconstruction in Elite Athletes
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.