User login
It Started With a Bug Bite (He Thinks)
An 81-year-old man is brought in by his wife for evaluation of a very itchy rash on his bilateral lower tibial areas. He says the problem started about six months ago, after a spate of summer yardwork during which he sustained what he assumed was a bug bite. It was itchy, so he scratched it.
Of course, in the way of most itches, the scratching offered temporary relief, after which the itching resumed. The patient tried any number of OTC products, including rubbing alcohol, hydrogen peroxide, tea tree oil, several different essential oils, and triple-antibiotic cream and ointment. The worse the itching became, the more products he applied—all to no avail.
The patient describes his health as otherwise decent. He does have type 2 diabetes, which he says is in good control.
EXAMINATION
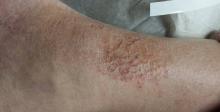
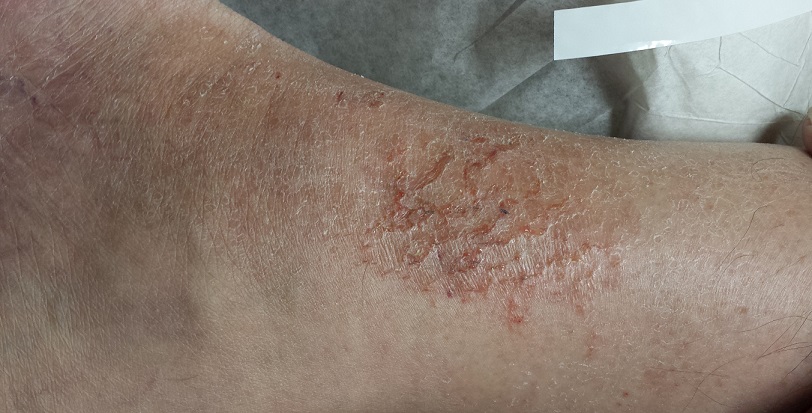
The lower anterior tibial areas of both legs are covered by a scaly red rash. The left leg is more heavily affected, and obvious edema can be seen distal to the rash on that leg. The surface scales of the rash have a polygonal look, resembling a dried lake bed or finely cracked porcelain. The edges of the cracks turn upward, resulting in a rough feel on palpation.
The patient’s skin is quite dry in general but otherwise within normal limits.
What is the diagnosis?
DISCUSSION
Leg skin is unique in many respects. For one thing, it’s down there, where gravity takes and often holds blood and other fluids that might not accumulate elsewhere. It’s also a long trip for blood to get out to the extremities and often a longer return trip.
Leg skin is also remarkable because it has far fewer sebaceous glands than the scalp, face, and chest, which means it tends to be quite dry. This is especially true in older patients already prone to dry skin and in those who seldom moisturize to counteract this problem (in other words, men!).
This is why most patients with asteatotic eczema (AE) are men. Also known as eczema craquele and xerotic eczema, AE is especially common in the dry winter months, when long, hot showers are so appealing, as are wearing warmer clothes and sleeping under heavy covers.
Patients with AE, including this one, often make matters worse by applying a multiplicity of contactants. The edema noted in the exam, although due to the AE, also served to worsen the condition by making the skin tighter and drier still.
At this point, the problem often starts to take on aspects of lichen simplex chronicus, in which more scratching leads to more itching (and then more scratching, and so on). Clearly, what this patient needed (and got) was a definitive diagnosis and a treatment plan dictated by that diagnosis.
AE can be a challenge to treat, but the first step is to help the patient understand the nature of the problem and his role in the solution. The patient also needs to stop applying nonprescribed/recommended contactants, which don’t help and may exacerbate the problem.
To achieve relief, the patient can soak the leg with wet compresses for 10 minutes, remove excess water, and then apply a medium-strength steroid ointment (eg, triamcinolone 0.5%) in a thin but thorough coat. The area can then be covered with an occlusive covering, such as saran wrap, taped in place. This should be left on all night. During the day, the patient should apply only petroleum jelly to the affected area.
This approach will take 90% of patients out of the crisis stage. After a week or two, attention must shift to preventing recurrence, with generous use of emollients such as petroleum jelly. The patient should also be instructed to avoid using harsh (colored, scented, high pH) soaps, switch to shorter, relatively cool showers, and stop using anything but his hand to wash with (ie, no washcloths or loofahs).
For nondiabetic patients with severe AE that persists despite these measures, an intramuscular injection of a glucocorticoid (eg, triamcinolone 40 - 60 mg) can work wonders.
TAKE-HOME LEARNING POINTS
• Asteatotic eczema (AE), also called xerotic eczema or eczema craquele, is quite common, especially on the lower legs of older men.
• The particular rash of AE is said to resemble the cracked surface of a porcelain vessel.
• AE is often accompanied by edema distal to the rash.
• A topical steroid ointment applied to water-soaked skin, held in place overnight with an occlusive dressing, usually takes the patient out of the crisis phase.
• Prevention is then directed at avoiding drying of the affected areas.
An 81-year-old man is brought in by his wife for evaluation of a very itchy rash on his bilateral lower tibial areas. He says the problem started about six months ago, after a spate of summer yardwork during which he sustained what he assumed was a bug bite. It was itchy, so he scratched it.
Of course, in the way of most itches, the scratching offered temporary relief, after which the itching resumed. The patient tried any number of OTC products, including rubbing alcohol, hydrogen peroxide, tea tree oil, several different essential oils, and triple-antibiotic cream and ointment. The worse the itching became, the more products he applied—all to no avail.
The patient describes his health as otherwise decent. He does have type 2 diabetes, which he says is in good control.
EXAMINATION
The lower anterior tibial areas of both legs are covered by a scaly red rash. The left leg is more heavily affected, and obvious edema can be seen distal to the rash on that leg. The surface scales of the rash have a polygonal look, resembling a dried lake bed or finely cracked porcelain. The edges of the cracks turn upward, resulting in a rough feel on palpation.
The patient’s skin is quite dry in general but otherwise within normal limits.
What is the diagnosis?
DISCUSSION
Leg skin is unique in many respects. For one thing, it’s down there, where gravity takes and often holds blood and other fluids that might not accumulate elsewhere. It’s also a long trip for blood to get out to the extremities and often a longer return trip.
Leg skin is also remarkable because it has far fewer sebaceous glands than the scalp, face, and chest, which means it tends to be quite dry. This is especially true in older patients already prone to dry skin and in those who seldom moisturize to counteract this problem (in other words, men!).
This is why most patients with asteatotic eczema (AE) are men. Also known as eczema craquele and xerotic eczema, AE is especially common in the dry winter months, when long, hot showers are so appealing, as are wearing warmer clothes and sleeping under heavy covers.
Patients with AE, including this one, often make matters worse by applying a multiplicity of contactants. The edema noted in the exam, although due to the AE, also served to worsen the condition by making the skin tighter and drier still.
At this point, the problem often starts to take on aspects of lichen simplex chronicus, in which more scratching leads to more itching (and then more scratching, and so on). Clearly, what this patient needed (and got) was a definitive diagnosis and a treatment plan dictated by that diagnosis.
AE can be a challenge to treat, but the first step is to help the patient understand the nature of the problem and his role in the solution. The patient also needs to stop applying nonprescribed/recommended contactants, which don’t help and may exacerbate the problem.
To achieve relief, the patient can soak the leg with wet compresses for 10 minutes, remove excess water, and then apply a medium-strength steroid ointment (eg, triamcinolone 0.5%) in a thin but thorough coat. The area can then be covered with an occlusive covering, such as saran wrap, taped in place. This should be left on all night. During the day, the patient should apply only petroleum jelly to the affected area.
This approach will take 90% of patients out of the crisis stage. After a week or two, attention must shift to preventing recurrence, with generous use of emollients such as petroleum jelly. The patient should also be instructed to avoid using harsh (colored, scented, high pH) soaps, switch to shorter, relatively cool showers, and stop using anything but his hand to wash with (ie, no washcloths or loofahs).
For nondiabetic patients with severe AE that persists despite these measures, an intramuscular injection of a glucocorticoid (eg, triamcinolone 40 - 60 mg) can work wonders.
TAKE-HOME LEARNING POINTS
• Asteatotic eczema (AE), also called xerotic eczema or eczema craquele, is quite common, especially on the lower legs of older men.
• The particular rash of AE is said to resemble the cracked surface of a porcelain vessel.
• AE is often accompanied by edema distal to the rash.
• A topical steroid ointment applied to water-soaked skin, held in place overnight with an occlusive dressing, usually takes the patient out of the crisis phase.
• Prevention is then directed at avoiding drying of the affected areas.
An 81-year-old man is brought in by his wife for evaluation of a very itchy rash on his bilateral lower tibial areas. He says the problem started about six months ago, after a spate of summer yardwork during which he sustained what he assumed was a bug bite. It was itchy, so he scratched it.
Of course, in the way of most itches, the scratching offered temporary relief, after which the itching resumed. The patient tried any number of OTC products, including rubbing alcohol, hydrogen peroxide, tea tree oil, several different essential oils, and triple-antibiotic cream and ointment. The worse the itching became, the more products he applied—all to no avail.
The patient describes his health as otherwise decent. He does have type 2 diabetes, which he says is in good control.
EXAMINATION
The lower anterior tibial areas of both legs are covered by a scaly red rash. The left leg is more heavily affected, and obvious edema can be seen distal to the rash on that leg. The surface scales of the rash have a polygonal look, resembling a dried lake bed or finely cracked porcelain. The edges of the cracks turn upward, resulting in a rough feel on palpation.
The patient’s skin is quite dry in general but otherwise within normal limits.
What is the diagnosis?
DISCUSSION
Leg skin is unique in many respects. For one thing, it’s down there, where gravity takes and often holds blood and other fluids that might not accumulate elsewhere. It’s also a long trip for blood to get out to the extremities and often a longer return trip.
Leg skin is also remarkable because it has far fewer sebaceous glands than the scalp, face, and chest, which means it tends to be quite dry. This is especially true in older patients already prone to dry skin and in those who seldom moisturize to counteract this problem (in other words, men!).
This is why most patients with asteatotic eczema (AE) are men. Also known as eczema craquele and xerotic eczema, AE is especially common in the dry winter months, when long, hot showers are so appealing, as are wearing warmer clothes and sleeping under heavy covers.
Patients with AE, including this one, often make matters worse by applying a multiplicity of contactants. The edema noted in the exam, although due to the AE, also served to worsen the condition by making the skin tighter and drier still.
At this point, the problem often starts to take on aspects of lichen simplex chronicus, in which more scratching leads to more itching (and then more scratching, and so on). Clearly, what this patient needed (and got) was a definitive diagnosis and a treatment plan dictated by that diagnosis.
AE can be a challenge to treat, but the first step is to help the patient understand the nature of the problem and his role in the solution. The patient also needs to stop applying nonprescribed/recommended contactants, which don’t help and may exacerbate the problem.
To achieve relief, the patient can soak the leg with wet compresses for 10 minutes, remove excess water, and then apply a medium-strength steroid ointment (eg, triamcinolone 0.5%) in a thin but thorough coat. The area can then be covered with an occlusive covering, such as saran wrap, taped in place. This should be left on all night. During the day, the patient should apply only petroleum jelly to the affected area.
This approach will take 90% of patients out of the crisis stage. After a week or two, attention must shift to preventing recurrence, with generous use of emollients such as petroleum jelly. The patient should also be instructed to avoid using harsh (colored, scented, high pH) soaps, switch to shorter, relatively cool showers, and stop using anything but his hand to wash with (ie, no washcloths or loofahs).
For nondiabetic patients with severe AE that persists despite these measures, an intramuscular injection of a glucocorticoid (eg, triamcinolone 40 - 60 mg) can work wonders.
TAKE-HOME LEARNING POINTS
• Asteatotic eczema (AE), also called xerotic eczema or eczema craquele, is quite common, especially on the lower legs of older men.
• The particular rash of AE is said to resemble the cracked surface of a porcelain vessel.
• AE is often accompanied by edema distal to the rash.
• A topical steroid ointment applied to water-soaked skin, held in place overnight with an occlusive dressing, usually takes the patient out of the crisis phase.
• Prevention is then directed at avoiding drying of the affected areas.
Consensus document issued on hematology research priorities for Europe
A consensus document that summarizes the status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical needs in Europe has been published in the February 2016 issue of Haematologica.
“For the first time, hematologists in Europe came together to develop a road map to guide hematology research in Europe,” Professor Andreas Engert, chair of the European Hematology Association’s Research Roadmap Task Force, said in a written statement. “Hematology ... must focus and collaborate to be efficient and remain successful in improving patient outcomes.”
Some 300 experts from over 20 countries in Europe helped to draft the road map. A wide variety of stakeholders, such as national hematology societies, patient organizations, hematology trial groups, and other European organizations, were consulted to comment on the final draft.
“The document reflects the views of the hematological research community in Europe, Professor Tony Green, president of the European Hematology Association (EHA), noted in the statement. “This is crucial if we want to convince policy makers to support the realization of this important research.”
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations – quite a few of which involve hematology researchers, Europe faces increased health expenditures while budgets are limited,” Professor Ulrich Jäger, chair of the EHA European Affairs Committee, said in the statement. “So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. ... Now it is up to the policy makers in the EU to deliver, too.”
You may find the full article in Haematologica 2016 Jan. doi: 10.3324/haematol.2015.136739.
In a time of restricted federal budgets, research funding becomes somewhat of a luxury. Yet, research and innovation are the primary movers of change and progress, both of which are needed to drive growth to ease budget restrictions. In order to ensure that precious resources are allocated to the most promising endeavors, federal governments establish bureaucracies charged with the task of allocating funding to the “best” proposals. Unfortunately, the task of defining “best” is imprecise and largely subjective.
To address this systemic deficiency and to improve the efficient allocation of resources, the scientific community often provides guidance to funding agencies to help choose among competing proposals. In 2015, the American Society of Hematology (ASH) announced its “Agenda for Hematology Research.” The agenda included recommendations to prioritize funding to projects in the following domains: genomic profiling and chemical biology, immunologic treatments of hematologic malignancies, genome editing and gene therapy, stem cell biology and regenerative medicine, epigenetic mechanisms, and venous thromboembolic disease.

|
Dr. Matt Kalyacio |
More recently, the European Hematology Association has published its Roadmap for European Hematology Research. Ostensibly similar to the ASH Agenda, the EHA document is a much more detailed policy statement that calls out the most promising research opportunities across the nine major components of hematology: normal hematopoiesis, malignant lymphoid disorders, malignant myeloid disease, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation and other cell based therapies.
I find the two documents complementary in that the ASH Agenda is more accessible to grant reviewers and funding agencies, while the EHA Roadmap seems more directed to the scientific community. Whether a grant writer or a grant reviewer, these documents should help researchers focus their applications on preferred projects and help reviewers prioritize proposals.
While laudable in their goals globally, such consensus documents place much faith in the knowable future and less in the unknowable, disruptive future. Researchers with innovative ideas that do not fall into the prioritizations set forth by the community at large might find themselves struggling for resources. This unintended consequence of consensus building risks the loss of inspired science on the altar of groupthink. The “moonshot” championed by Vice-President Biden will be more likely to succeed when consensus science allows for novel approaches that have yet to be revealed.
Dr. Matt Kalaycio is the editor-in-chief of Hematology News and chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute, Cleveland. Leave your comments on our website or write to Dr. Kalaycio at [email protected].
In a time of restricted federal budgets, research funding becomes somewhat of a luxury. Yet, research and innovation are the primary movers of change and progress, both of which are needed to drive growth to ease budget restrictions. In order to ensure that precious resources are allocated to the most promising endeavors, federal governments establish bureaucracies charged with the task of allocating funding to the “best” proposals. Unfortunately, the task of defining “best” is imprecise and largely subjective.
To address this systemic deficiency and to improve the efficient allocation of resources, the scientific community often provides guidance to funding agencies to help choose among competing proposals. In 2015, the American Society of Hematology (ASH) announced its “Agenda for Hematology Research.” The agenda included recommendations to prioritize funding to projects in the following domains: genomic profiling and chemical biology, immunologic treatments of hematologic malignancies, genome editing and gene therapy, stem cell biology and regenerative medicine, epigenetic mechanisms, and venous thromboembolic disease.

|
Dr. Matt Kalyacio |
More recently, the European Hematology Association has published its Roadmap for European Hematology Research. Ostensibly similar to the ASH Agenda, the EHA document is a much more detailed policy statement that calls out the most promising research opportunities across the nine major components of hematology: normal hematopoiesis, malignant lymphoid disorders, malignant myeloid disease, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation and other cell based therapies.
I find the two documents complementary in that the ASH Agenda is more accessible to grant reviewers and funding agencies, while the EHA Roadmap seems more directed to the scientific community. Whether a grant writer or a grant reviewer, these documents should help researchers focus their applications on preferred projects and help reviewers prioritize proposals.
While laudable in their goals globally, such consensus documents place much faith in the knowable future and less in the unknowable, disruptive future. Researchers with innovative ideas that do not fall into the prioritizations set forth by the community at large might find themselves struggling for resources. This unintended consequence of consensus building risks the loss of inspired science on the altar of groupthink. The “moonshot” championed by Vice-President Biden will be more likely to succeed when consensus science allows for novel approaches that have yet to be revealed.
Dr. Matt Kalaycio is the editor-in-chief of Hematology News and chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute, Cleveland. Leave your comments on our website or write to Dr. Kalaycio at [email protected].
In a time of restricted federal budgets, research funding becomes somewhat of a luxury. Yet, research and innovation are the primary movers of change and progress, both of which are needed to drive growth to ease budget restrictions. In order to ensure that precious resources are allocated to the most promising endeavors, federal governments establish bureaucracies charged with the task of allocating funding to the “best” proposals. Unfortunately, the task of defining “best” is imprecise and largely subjective.
To address this systemic deficiency and to improve the efficient allocation of resources, the scientific community often provides guidance to funding agencies to help choose among competing proposals. In 2015, the American Society of Hematology (ASH) announced its “Agenda for Hematology Research.” The agenda included recommendations to prioritize funding to projects in the following domains: genomic profiling and chemical biology, immunologic treatments of hematologic malignancies, genome editing and gene therapy, stem cell biology and regenerative medicine, epigenetic mechanisms, and venous thromboembolic disease.

|
Dr. Matt Kalyacio |
More recently, the European Hematology Association has published its Roadmap for European Hematology Research. Ostensibly similar to the ASH Agenda, the EHA document is a much more detailed policy statement that calls out the most promising research opportunities across the nine major components of hematology: normal hematopoiesis, malignant lymphoid disorders, malignant myeloid disease, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation and other cell based therapies.
I find the two documents complementary in that the ASH Agenda is more accessible to grant reviewers and funding agencies, while the EHA Roadmap seems more directed to the scientific community. Whether a grant writer or a grant reviewer, these documents should help researchers focus their applications on preferred projects and help reviewers prioritize proposals.
While laudable in their goals globally, such consensus documents place much faith in the knowable future and less in the unknowable, disruptive future. Researchers with innovative ideas that do not fall into the prioritizations set forth by the community at large might find themselves struggling for resources. This unintended consequence of consensus building risks the loss of inspired science on the altar of groupthink. The “moonshot” championed by Vice-President Biden will be more likely to succeed when consensus science allows for novel approaches that have yet to be revealed.
Dr. Matt Kalaycio is the editor-in-chief of Hematology News and chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute, Cleveland. Leave your comments on our website or write to Dr. Kalaycio at [email protected].
A consensus document that summarizes the status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical needs in Europe has been published in the February 2016 issue of Haematologica.
“For the first time, hematologists in Europe came together to develop a road map to guide hematology research in Europe,” Professor Andreas Engert, chair of the European Hematology Association’s Research Roadmap Task Force, said in a written statement. “Hematology ... must focus and collaborate to be efficient and remain successful in improving patient outcomes.”
Some 300 experts from over 20 countries in Europe helped to draft the road map. A wide variety of stakeholders, such as national hematology societies, patient organizations, hematology trial groups, and other European organizations, were consulted to comment on the final draft.
“The document reflects the views of the hematological research community in Europe, Professor Tony Green, president of the European Hematology Association (EHA), noted in the statement. “This is crucial if we want to convince policy makers to support the realization of this important research.”
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations – quite a few of which involve hematology researchers, Europe faces increased health expenditures while budgets are limited,” Professor Ulrich Jäger, chair of the EHA European Affairs Committee, said in the statement. “So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. ... Now it is up to the policy makers in the EU to deliver, too.”
You may find the full article in Haematologica 2016 Jan. doi: 10.3324/haematol.2015.136739.
A consensus document that summarizes the status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical needs in Europe has been published in the February 2016 issue of Haematologica.
“For the first time, hematologists in Europe came together to develop a road map to guide hematology research in Europe,” Professor Andreas Engert, chair of the European Hematology Association’s Research Roadmap Task Force, said in a written statement. “Hematology ... must focus and collaborate to be efficient and remain successful in improving patient outcomes.”
Some 300 experts from over 20 countries in Europe helped to draft the road map. A wide variety of stakeholders, such as national hematology societies, patient organizations, hematology trial groups, and other European organizations, were consulted to comment on the final draft.
“The document reflects the views of the hematological research community in Europe, Professor Tony Green, president of the European Hematology Association (EHA), noted in the statement. “This is crucial if we want to convince policy makers to support the realization of this important research.”
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations – quite a few of which involve hematology researchers, Europe faces increased health expenditures while budgets are limited,” Professor Ulrich Jäger, chair of the EHA European Affairs Committee, said in the statement. “So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. ... Now it is up to the policy makers in the EU to deliver, too.”
You may find the full article in Haematologica 2016 Jan. doi: 10.3324/haematol.2015.136739.
Cyclical hypofractionated radiotherapy technique for palliative treatment of locally advanced head and neck cancer: institutional experience and review of palliative regimens
Background Effective palliation in patients with locally advanced head and neck cancer is important. Cyclical hypofractionated radiotherapy (Quad Shot) is a short-course palliative regimen with good patient compliance, low rates of acute toxicity, and delayed late fibrosis.
Objective To review use of the Quad Shot technique at our institution in order to quantify the palliative response in locally advanced head and neck cancer.
Methods The medical records of 70 patients with head and neck squamous cell carcinoma who had been treated with the Quad Shot technique were analyzed retrospectively (36 had been treated with intensity-modulated radiation therapy and 34 with 3-D conformal radiotherapy). They had received cyclical hypofractionated radiotherapy administrated as 14.8 Gy in 4 fractions over 2 days, twice daily, repeated every 3 weeks for a total of 3 cycles. The total prescribed dose was 44.4 Gy. Primary endpoints were improvement in pain using a verbal numeric pain rating scale (range 1-10, 10 being severe pain) and dysphagia using the Food Intake Level Scale, and the secondary endpoints included overall survival (OS), local regional recurrence-free survival (LRRFS), progression-free survival (PFS) and time to progression.
Results Pain response occurred in 61% of the patients. The mean pain scores decreased significantly from pre to post treatment (5.81 to 2.55, P = .009). The mean initial dysphagia score improved from 2.20 to 4.77 55 (P = .045). 26% of patients developed mucositis (≤ grade 2), with 9% developing grade 3-level mucositis. 12 patients had tumor recurrence. The estimated 1-year PFS was 20.7%. The median survival was 3.85 months with an estimated 1-year OS of 22.6%. Pain response (hazard ratio [HR], 2.69; 95% confidence index [CI], I.552-1.77) and completion of all 3 cycles (HR, 1.71; 95% CI, 1.003-2.907) were predictive for improved OS.
Limitations This study is a retrospective analysis.
Conclusion Quad Shot is an appropriate palliative regimen for locally advanced head and neck cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Background Effective palliation in patients with locally advanced head and neck cancer is important. Cyclical hypofractionated radiotherapy (Quad Shot) is a short-course palliative regimen with good patient compliance, low rates of acute toxicity, and delayed late fibrosis.
Objective To review use of the Quad Shot technique at our institution in order to quantify the palliative response in locally advanced head and neck cancer.
Methods The medical records of 70 patients with head and neck squamous cell carcinoma who had been treated with the Quad Shot technique were analyzed retrospectively (36 had been treated with intensity-modulated radiation therapy and 34 with 3-D conformal radiotherapy). They had received cyclical hypofractionated radiotherapy administrated as 14.8 Gy in 4 fractions over 2 days, twice daily, repeated every 3 weeks for a total of 3 cycles. The total prescribed dose was 44.4 Gy. Primary endpoints were improvement in pain using a verbal numeric pain rating scale (range 1-10, 10 being severe pain) and dysphagia using the Food Intake Level Scale, and the secondary endpoints included overall survival (OS), local regional recurrence-free survival (LRRFS), progression-free survival (PFS) and time to progression.
Results Pain response occurred in 61% of the patients. The mean pain scores decreased significantly from pre to post treatment (5.81 to 2.55, P = .009). The mean initial dysphagia score improved from 2.20 to 4.77 55 (P = .045). 26% of patients developed mucositis (≤ grade 2), with 9% developing grade 3-level mucositis. 12 patients had tumor recurrence. The estimated 1-year PFS was 20.7%. The median survival was 3.85 months with an estimated 1-year OS of 22.6%. Pain response (hazard ratio [HR], 2.69; 95% confidence index [CI], I.552-1.77) and completion of all 3 cycles (HR, 1.71; 95% CI, 1.003-2.907) were predictive for improved OS.
Limitations This study is a retrospective analysis.
Conclusion Quad Shot is an appropriate palliative regimen for locally advanced head and neck cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Background Effective palliation in patients with locally advanced head and neck cancer is important. Cyclical hypofractionated radiotherapy (Quad Shot) is a short-course palliative regimen with good patient compliance, low rates of acute toxicity, and delayed late fibrosis.
Objective To review use of the Quad Shot technique at our institution in order to quantify the palliative response in locally advanced head and neck cancer.
Methods The medical records of 70 patients with head and neck squamous cell carcinoma who had been treated with the Quad Shot technique were analyzed retrospectively (36 had been treated with intensity-modulated radiation therapy and 34 with 3-D conformal radiotherapy). They had received cyclical hypofractionated radiotherapy administrated as 14.8 Gy in 4 fractions over 2 days, twice daily, repeated every 3 weeks for a total of 3 cycles. The total prescribed dose was 44.4 Gy. Primary endpoints were improvement in pain using a verbal numeric pain rating scale (range 1-10, 10 being severe pain) and dysphagia using the Food Intake Level Scale, and the secondary endpoints included overall survival (OS), local regional recurrence-free survival (LRRFS), progression-free survival (PFS) and time to progression.
Results Pain response occurred in 61% of the patients. The mean pain scores decreased significantly from pre to post treatment (5.81 to 2.55, P = .009). The mean initial dysphagia score improved from 2.20 to 4.77 55 (P = .045). 26% of patients developed mucositis (≤ grade 2), with 9% developing grade 3-level mucositis. 12 patients had tumor recurrence. The estimated 1-year PFS was 20.7%. The median survival was 3.85 months with an estimated 1-year OS of 22.6%. Pain response (hazard ratio [HR], 2.69; 95% confidence index [CI], I.552-1.77) and completion of all 3 cycles (HR, 1.71; 95% CI, 1.003-2.907) were predictive for improved OS.
Limitations This study is a retrospective analysis.
Conclusion Quad Shot is an appropriate palliative regimen for locally advanced head and neck cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Warfarin is best for anticoagulation in prosthetic heart valve pregnancies
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
SNOWMASS, COLO. – How would you manage anticoagulation in a newly pregnant 23-year-old with a mechanical heart valve who has been on warfarin at 3 mg/day?
A) Weight-adjusted low-molecular-weight heparin during the first trimester, then warfarin in the second and third until switching to unfractionated heparin for delivery.
B) Low-molecular-weight heparin throughout pregnancy.
C) Warfarin throughout pregnancy.
D) Unfractionated heparin in the first trimester, warfarin in the second and third until returning to unfractionated heparin peridelivery.
The correct answer, according to both the ACC/AHA guidelines (Circulation. 2014 Jun 10;129[23]:e521-643) and European Society of Cardiology guidelines (Eur Heart J. 2011 Dec;32[24]:3147-97), is C in women who are on 5 mg/day of warfarin or less.
“Oral anticoagulants throughout pregnancy are much better for the mother, and this is where the guidelines have moved,” Dr. Carole A. Warnes said at the Annual Cardiovascular Conference at Snowmass.
Both sets of guidelines give a class I recommendation to warfarin during the second and third trimesters, because the risk of warfarin embryopathy is confined to weeks 6-12. During the first trimester, warfarin at 5 mg/day or less gets a class IIa rating – making it preferable to unfractionated or low-molecular-weight heparin – because heparin is a far less effective anticoagulant. Plus, multiple small studies indicate the risk of embryopathy is low – roughly 1%-2% – when the mother is on warfarin at 5 mg/day or less.
In a woman on more than 5 mg/day of warfarin, the risk of warfarin embryopathy is about 6%, so the guidelines recommend replacing the drug with heparin during weeks 6-12.
“It’s not a walk in the park,” said Dr. Warnes, director of the Snowmass conference and professor of medicine at the Mayo Clinic in Rochester, Minn.
The major concern in using heparin for anticoagulation in pregnancy is valve thrombosis. It doubles the risk.
“Pregnancy is the most prothrombotic state there is,” she said. “It’s not like managing a patient through a hip replacement or prostate surgery. Women with a mechanical prosthetic valve should be managed by a heart valve team with expertise in treatment during pregnancy.”
The alternatives to warfarin are adjusted-dose unfractionated heparin, which must be given in a continuous intravenous infusion with meticulous monitoring of activated partial thromboplastin time, or twice-daily low-molecular-weight heparin with dose adjustment by weight and maintenance of a target anti–Factor Xa level of 1.0-1.2 IU/mL.
“If you use low-molecular-weight heparin, you’re going to be seeing that patient every week to monitor anti–Factor Xa 4-6 hours post injection. You’ll find it’s not that easy to stay in the sweet spot, with excellent anticoagulation without an increased risk of maternal thromboembolism, or at the other extreme, fetal bleeding. What might look initially as a relatively easy strategy with a lot of appeal turns out to entail considerable risk,” Dr. Warnes said.
This was underscored in a cautionary report by highly experienced University of Toronto investigators. In their series of 23 pregnancies in 17 women with mechanical heart valves on low-molecular-weight heparin throughout pregnancy with careful monitoring, there was one maternal thromboembolic event resulting in maternal and fetal death despite a documented therapeutic anti–Factor Xa level (Am J Cardiol. 2009 Nov 1;104[9]:1259-63).
Although warfarin is clearly the better anticoagulant for the mother, the fetus pays the price. This was highlighted in a recent report from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) that compared pregnancy outcomes in 212 patients with a mechanical heart valve, 134 with a tissue valve, and 2,620 women without a prosthetic heart valve. Use of warfarin or another vitamin K antagonist in the first trimester was associated with a higher rate of miscarriage than heparin – 28.6% vs. 9.2% – as well as a 7.1% incidence of late fetal death, compared with just 0.7% with heparin.
On the other hand, the mechanical valve thrombosis rate was 4.7%, with half of those serious events occurring during the first trimester in patients after they’d been switched to heparin (Circulation. 2015 Jul 14;132[2]:132-42).
Hemorrhagic events occurred in 23.1% of mothers with a mechanical heart valve, 5.1% of those with a bioprosthetic valve, and 4.9% of patients without a prosthetic valve. A point worth incorporating into prepregnancy patient counseling, Dr. Warnes noted, is that only 58% of ROPAC participants with a mechanical heart valve had an uncomplicated pregnancy with a live birth, in contrast to 79% of those with a tissue valve and 78% of controls.
Because warfarin crosses the placenta, and it takes about a week for the fetus to eliminate the drug following maternal discontinuation, the guidelines recommend stopping warfarin at about week 36 and changing to a continuous infusion of dose-adjusted unfractionated heparin peridelivery. The heparin should be stopped for as short a time as possible before delivery and resumed 6-12 hours post delivery in order to protect against valve thrombosis.
Of course, opting for a bioprosthetic rather than a mechanical heart valve avoids all these difficult anticoagulation-related issues. But it poses a different serious problem: The younger the patient at the time of tissue valve implantation, the greater the risk of rapid calcification and structural valve deterioration. Indeed, among patients who are age 16-39 when they receive a bioprosthetic valve, the rate of structural valve deterioration is 50% at 10 years and 90% at 15 years.
“There is no ideal valve prosthesis. If you elect a tissue prosthesis, you have to discuss the risk of reoperation in that young woman,” Dr. Warnes advised.
Recent data from the Society of Thoracic Surgeons database indicate the mortality associated with redo elective aortic valve replacement in a 35-year-old woman with no comorbidities averages 1.63%, with a 2% mortality rate for redo mitral valve replacement.
Dr. Warnes reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Migraines more severe in PNES patients than in epilepsy patients
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
FROM SEIZURE
Cariprazine for schizophrenia and bipolar I disorder
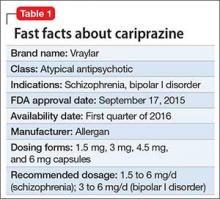
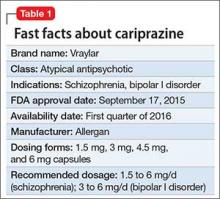
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
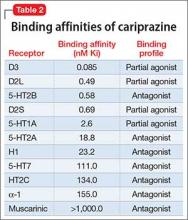
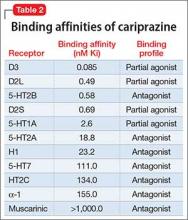
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
Cariprazine for schizophrenia and bipolar I disorder
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
Cariprazine is a newly approved (September 2015) dopamine D3/D2 receptor partial agonist with higher affinity for the D3 receptor than for D2. The drug is FDA-indicated for treating schizophrenia and bipolar I disorder (BD I)1,2 (Table 1). In clinical trials, cariprazine alleviated symptoms of schizophrenia and mixed and manic symptoms of BD I, with minimal effect on metabolic parameters, the prolactin level, and cardiac conduction.
Clinical implications
Despite numerous developments in pharmacotherapeutics, people with schizophrenia or bipolar disorder continue to struggle with residual symptoms or endure treatments that produce adverse effects (AEs). In particular, metabolic issues, sedation, and cognitive impairment plague many current treatment options for these disorders.
Receptor blocking. As a dopamine D3-preferring D3/D2 partial agonist, cariprazine offers an alternative to antipsychotics that preferentially modulate D2 receptors. First-generation (typical) antipsychotics block D2 receptors; atypical antipsychotics block D2 receptors and 5-HT2A receptors. Dopamine partial agonists aripiprazole and brexpiprazole are D2-preferring, with minimal D3 effects. In contrast, cariprazine has a 6-fold to 8-fold higher affinity for D3 receptors than for D2 receptors, and has specificity for the D3 receptor that is 3 to 10 times higher than what aripiprazole has for the D3 receptor3-5 (Table 2).
Use in schizophrenia. Recommended dosage range is 1.5 to 6 mg/d. In Phase-III clinical trials, dosages of 3 to 9 mg/d produced significant improvement on the Positive and Negative Symptom Scale (PANSS) and on the Clinical Global Impression scale. Higher dosages (6 to 9 mg/d) showed early separation from placebo—by the end of Week 1—but carried a dosage-related risk of AEs, leading the FDA to recommend 6 mg/d as the maximum dosage.1,6-8
Use in manic or mixed episodes of BD I. Recommended dosage range is 3 to 6 mg/d. In clinical trials, dosages in the range of 3 to 12 mg/d were effective for acute manic or mixed symptoms; significant improvement in the Young Mania Rating Scale (YMRS) score was seen as early as Day 4. Dosages >6 mg/d yielded no additional benefit and were associated with increased risk of AEs.9-12
Pharmacologic profile, adverse effects. Cariprazine has a pharmacologic profile consistent with the generally favorable metabolic profile and lack of anticholinergic effects seen in clinical trials. In short- and long-term trials, the drug had minimal effects on prolactin, blood pressure, and cardiac conduction.13
Across clinical trials for both disorders, akathisia and parkinsonism were among more common AEs of cariprazine. Both AEs were usually mild, resulting in relatively few premature discontinuations from trials. Parkinsonism appeared somewhat dosage-related; akathisia had no clear relationship to dosage.
How it works
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by:
- decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as mesolimbic regions in schizophrenia or mania
- simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as the prefrontal cortex in schizophrenia
- exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
- simultaneously
Cariprazine has higher binding affinity for dopamine D3 receptors (Ki 0.085 nM) than for D2L receptors (Ki 0.49 nM) and D2S receptors (Ki 0.69 nM). The drug also has strong affinity for serotonin receptor 5-HT2B; moderate affinity for 5-HT1A; and lower affinity for 5-HT2A, histamine H1, and 5-HT7 receptors. Cariprazine has little or no affinity for adrenergic or cholinergic receptors.14In patients with schizophrenia, as measured on PET scanning, a dosage of 1.5 mg/d yielded 69% to 75% D2/D3 receptor occupancy. A dosage of 3 mg/d yielded >90% occupancy.
Search for an understanding of action continues. The relative contribution of D3 partial agonism, compared with D2 partial agonism, is a subject of ongoing basic scientific and clinical research. D3 is an autoreceptor that (1) controls phasic, but not tonic, activity of dopamine nerve cells and (2) mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists.5,12 In animal studies, D3-preferring agents have been shown to exert pro-cognitive effects and improve anhedonic symptoms.
Pharmacokinetics
Cariprazine is a once-daily medication with a relatively long half-life that can be taken with or without food. Dosages of 3 to 12 mg/d yield a fairly linear, dose-proportional increase in plasma concentration. The peak serum concentration for cariprazine is 3 to 4 hours under fasting conditions; taking the drug with food causes a slight delay in absorption but does not have a significant effect on the area under the curve. Mean half-life for cariprazine is 2 to 5 days over a dosage range of 1.5 to 12.5 mg/d in otherwise healthy adults with schizophrenia.1
Cariprazine is metabolized primarily by cytochrome P450 (CYP) 3A4. It is a weak inhibitor of CYP2D6 and CYP3A4.1 Hepatic metabolism of cariprazine produces 2 active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), both of which are equipotent to cariprazine. After multiple dose administration, mean cariprazine and DCAR levels reach steady state in 1 to 2 weeks; DDCAR, in 4 to 8 weeks. The systemic exposure and serum levels of DDCAR are roughly 3-fold greater than cariprazine because of the longer elimination half-life of DDCAR.1
Efficacy in schizophrenia
The efficacy of cariprazine in schizophrenia was established by 3 six-week, randomized, placebo-controlled trials. Two trials were fixed-dosage; a third used 2 flexible dosage ranges. The primary efficacy measure was change from baseline in the total score of the PANSS at the end of Week 6, compared with placebo. In all trials, patients were adults (age 18 to 60) who met DSM-IV-TR criteria for schizophrenia and had a PANSS score between 80 and 120 at screening and baseline.
Study 1 (n = 711) compared dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d with placebo.7 All cariprazine dosages and an active control (risperdone) were superior to placebo in reducing symptoms of schizophrenia, as measured by the PANSS. The placebo-subtracted differences on PANSS score at 6 weeks for dosages of 1.5 mg/d, 3 mg/d, and 4.5 mg/d were –7.6, –8.8, –10.4, respectively (significant at 95% CI).
Study 2 (n = 151) compared 3 mg/d and 6 mg/d dosages of cariprazine with placebo.1 Both dosages and an active control (aripiprazole) were superior to placebo in reducing PANSS scores. Placebo-subtracted differences on PANSS score at 6 weeks for dosages of 3 mg/d and 6 mg/day were –6.0, –8.8, respectively (significant at 95% CI).
Study 3 (n = 147) was a fixed-flexible dosage trial comparing cariprazine, 3 to 6 mg/d and 6 to 9 mg/d dosage ranges, to placebo.8 Both ranges were superior to placebo in reducing symptoms on PANSS. Placebo-subtracted differences from placebo on PANSS at 6 weeks for cariprazine 3 to 6 or 6 to 9 mg/d were –6.8, –9.9, respectively (significant at 95% CI).
These trials established the efficacy of cariprazine for acute schizophrenia at dosages ranging from 1.5 to 9 mg/d. Although there was a modest trend toward higher efficacy at higher dosages, there was a dose-related increase in certain adverse reactions (extrapyramidal symptoms [EPS]) at dosages >6 mg/d.1
Efficacy in bipolar disorder
The efficacy of cariprazine for acute treatment of manic or mixed episodes of BD I was established in 3 randomized, placebo-controlled, flexibly dosed 3-week trials. In all trials, patients were adults (age 18 to 65) who met DSM-IV-TR criteria for BD I with manic or mixed episodes and with or without psychotic features (YMRS score, ≥20). The primary efficacy measure in the 3 trials was a change from baseline in the total YMRS score at the end of Week 3, compared with placebo.
Study 1 (n = 492) compared 2 flexibly dosed ranges of cariprazine (3 to 6 mg/d and 6 to 12 mg/d) with placebo.10 Both dosage ranges were superior to placebo in reducing mixed and manic symptoms, as measured by reduction in the total YMRS score. Placebo-subtracted differences in YMRS scores from placebo at Week 3 for cariprazine 3 to 6 mg/d and 6 to 12 mg/d were –6.1, –5.9, respectively (significant at 95% CI). The higher range offered no additional advantage over the lower range.
Study 2 (n = 235) compared flexibly dosed cariprazine, 3 to 12 mg/d, to placebo.11 Cariprazine was superior to placebo in reducing bipolar symptoms as measured by the YMRS. The difference between cariprazine 3 to 12 mg/d and placebo on the YMRS score at Week 3 was –6.1 (significant at 95% CI).
Study 3 (n = 310) compared flexibly dosed cariprazine, 3 to 12 mg/d, with placebo.15 Again, cariprazine was superior to placebo in reducing the YMRS score at Week 3: difference, –4.3 (significant at 95% CI).
These trials establish the efficacy of cariprazine in treating acute mania or mixed BD I episodes at dosages ranging from 3 to 12 mg/d. Dosages >6 mg/d did not offer additional benefit over lower dosages, and resulted in a dosage-related increase in EPS at dosages >6 mg/d.16
Tolerability
Cariprazine generally was well tolerated in short-term trials for schizophrenia and BD I. The only treatment-emergent adverse event reported for at least 1 treatment group in all trials at a rate of ≥10%, and at least twice the rate seen with placebo was akathisia. Adverse events reported at a lower rate than placebo included EPS (particularly parkinsonism), restlessness, headache, insomnia, fatigue, and gastrointestinal distress. The discontinuation rate due to AEs for treatment groups and placebo-treated patients generally was similar. In schizophrenia Study 3, for example, the discontinuation rate due to AEs was 13% for placebo; 14% for cariprazine, 3 to 6 mg/d; and 13% for cariprazine, 6 to 9 mg/d.1 48-Week open-label safety study. Patients with schizophrenia received open-label cariprazine for as long as 48 weeks.7 Serious adverse events were reported in 12.9%, including 1 death (suicide); exacerbation of symptoms of schizophrenia (4.3%); and psychosis (2.2%). Treatment-emergent adverse events reported in at least 10% of patients included akathisia (14.0%), insomnia (14.0%), and weight gain (11.8%). The mean change in laboratory values, blood pressure, pulse rate, and electrocardiographic parameters was clinically insignificant.
Other studies. In a 16-week, open-label extension study of patients with BD I, the major tolerability issue was akathisia. This AE developed in 37% of patients and led to a 5% withdrawal rate.12
In short- and long-term studies for either indication, the effect of the drug on metabolic parameters appears to be small. In studies with active controls, potentially significant weight gain (>7%) was greater for aripiprazole and risperidone than for cariprazine.6,7 The effect on the prolactin level was minimal. There do not appear to be clinically meaningful changes in laboratory values, vital signs, or QT interval.
Unique clinical issues
Preferential binding. Cariprazine is the third dopamine partial agonist approved for use in the United States; unlike the other 2—aripiprazole and brexpiprazole—cariprazine shows preference for D3 receptors over D2 receptors. The exact clinical impact of a preference for D3 and the drug’s partial agonism of 5-HT1A has not been fully elucidated.
EPS, including akathisia and parkinsonism, were among common adverse events. Both were usually mild, with 0.5% of schizophrenia patients and 2% of BD I patients dropping out of trials because of any type of EPS-related AEs.
Why Rx? On a practical medical level, reasons to prescribe cariprazine likely include:
- minimal effect on prolactin
- relative lack of effect on metabolic parameters, including weight (cariprazine showed less weight gain than risperidone or aripiprazole control arms in trials).
Dosing
The recommended dosage of cariprazine for schizophrenia ranges from 1.5 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
The recommended dosages of cariprazine for mixed and manic episodes of BD I range from 3 to 6 mg/d. The recommended starting dosage is 1.5 mg/d, which can be increased to 3 mg on Day 2, with further upward dosage adjustments of 1.5 to 3 mg/d, based on clinical response and tolerability.1
Other key aspects of dosing to keep in mind:
- Because of the long half-life and 2 equipotent active metabolites of cariprazine, any changes made to the dosage will not be reflected fully in the serum level for 2 weeks.
- Administering the drug with food slightly delays, but does not affect, the extent of absorption.
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 inhibitor; the recommended starting dosage of cariprazine is 1.5 mg every other day with a maximum dosage of 3 mg/d when it is administered concomitantly with a strong CYP3A4 inhibitor.
- Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4 inducer, this practice is not recommended.1
- Because the drug is metabolized primarily by CYP3A4, dosage adjustment is required in the presence of a CYP3A4 Because data are not available regarding concomitant use of cariprazine with a strong CYP3A4
Contraindications
Cariprazine carries a FDA black-box warning of increased mortality in older patients who have dementia-related psychosis, as other atypical antipsychotics do. Clinical trials produced few data about the use of cariprazine in geriatric patients; no data exist about use in the pediatric population.1
Metabolic, prolactin, and cardiac concerns about cariprazine appeared favorably minor in Phase-III and long-term safety trials. Concomitant use of cariprazine with any strong inducer of CYP3A4 has not been studied, and is not recommended. Dosage reduction is recommended when using cariprazine concomitantly with a CYP3A4 inhibitor.1
In conclusion
The puzzle in neuropsychiatry has always been to find ways to produce different effects in different brain regions—with a single drug. Cariprazine’s particular binding profile—higher affinity and higher selectivity for D3 receptors than for D2 receptors compared with either aripiprazole or brexpiprazole—may secure a role for it in managing psychosis and mood disorders.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
1. Vraylar [package insert]. Parsippany, NJ: Actavis Pharma, Inc.; 2015.
2. McCormack PL, Cariprazine: first global approval. Drugs. 2015;75(17):2035-2043.
3. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
4. Potkin, S, Keator, D, Mukherjee J, et al. P. 1. E 028 dopamine D3 and D2 receptor occupancy of cariprazine in schizophrenic patients. Eur Neuropsychopharmacology. 2009;19(suppl 3):S316.
5. Veselinovicˇ T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13(11):1141-1159.
6. Cutler A, Mokliatchouk O, Laszlovszky I, et al. Cariprazine in acute schizophrenia: a fixed-dose phase III, randomized, double-blind, placebo- and active-controlled trial. Abstract presented at: 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, CA.
7. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457.
8. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373.
9. Bose A, Starace A, Lu, K, et al. Cariprazine in the treatment of acute mania in bipolar disorder: a double-blind, placebo-controlled, phase III trial. Poster presented at: 16th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists; April 21-24, 2013; Colorado Springs, CO.
10. Calabrese JR, Keck PE Jr, Starace A, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284-292.
11. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75.
12. Ketter, T. A phase III, open-label, 16-week study of flexibly dosed cariprazine in 402 patients with bipolar I disorder. Presented at: 53rd Annual Meeting of the New Clinical Drug Evaluation Unit; May 28-31, 2013; Hollywood, FL.
13. Bose A, Li D, Migliore R. The efficacy and safety of the novel antipsychotic cariprazine in the acute exacerbation of schizophrenia. Poster presented at: 50th Annual Meeting of the New Clinical Drug Evaluation Unit; June 14-17, 2010; Boca Raton, FL.
14. Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193-206.
15. Sachs GS, Greenberg WM, Starace A, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296-302.
16. Vieta E, Durgam S, Lu K, et al. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Eur Neuropsycholpharmacol. 2015;25(11):1882-1891.
The newest ‘rage’: disruptive mood dysregulation disorder
Outbursts by children when frustrated or when asked to “do something they don’t want to do” are among the most common behavioral complaints voiced by parents. But behavioral outbursts, beyond the typical tantrums of children up to age 4 years, can be signs of very severe mental health disorders and are the most common reason for psychiatric admission (50%-60%).
While behavioral dysregulation is undeniably a huge problem for families, there has been an unreasonable 40-fold rise in diagnosis of bipolar disorder from 1994 to 2003, and 48% were prescribed atypical neuroleptics – medications with serious side effects. In response to this overdiagnosis as bipolar disorder, in 2013 the DSM-5 created a new diagnosis called disruptive mood dysregulation disorder (DMDD) to differentiate children who experience explosive outbursts who have a different outcome. This new classification includes children aged 6-12 years with persistent irritability most of the time, nearly every day, lasting at least 12 months and starting before age 10 years. DMDD diagnosis is not used after age 18 years.
To be diagnosed, the child has to have frequent, severe temper outbursts “grossly out of proportion” to the situation, averaging at least three times per week. The outbursts can be verbal or physical aggression to people, things, or themselves. While tantrums can be severe in children with delayed development, for the DMDD diagnosis these behaviors must be inconsistent with developmental level and must occur in at least two settings, and in one setting it must be severe. While outbursts are common, only half of children in one study of severe tantrum behavior in 5- to 9-year-olds also had the required persistent irritability.
If this does sound a lot like bipolar disorder so far, you are right. So what is different? DMDD has a prevalence of 2%-5% and occurs mostly in boys, whereas bipolar disorder affects boys and girls equally and affects less than 1% prior to adolescence.
The key features distinguishing DMDD from bipolar disorder are lack of an episodic nature to the irritability and lack of mania. Irritability in DMDD has to be persistently present with breaks of no more than 3 consecutive months in the defining 12-month period. There also cannot be any more than 1 day of the elevated mood features of mania or hypomania. Identifying mania is the hardest part, even in diagnosing adult bipolar, where it occurs only 1% of the year, much less in children who are generally lively! Hypomania, while less intense than mania, is when the person is energetic, talkative, and confident to an extreme extent, often with a flight of creative ideas. Excitement over birthdays or Christmas specifically does not count! So getting this history has to be done carefully, generally by a mental health professional, to make the distinction.
Interestingly, DMDD is not diagnosed when outbursts and irritability are better explained by autism spectrum disorder, separation anxiety disorder, or PTSD. To me, these exclusions point out the importance of sorting out the “set conditions” for all problematic behaviors, not always an easy task. Symptoms of autism in high functioning individuals can be quite subtle. Was the upset from change in a rigid routine known only to the child? Were sensory stimuli such as loud noises intolerable to this child? Was a nonverbal signal of a peer mistaken as a threat? While violent outbursts precipitated by these factors would still be considered “grossly out of proportion to the situation” for a typical child, they are not uncommon in atypical children. Similarly, children with separation anxiety disorder experience a high level of threat from even thinking about being apart from their caregivers, setting them up for alarm by situations other children would not find difficult.
The American Academy of Pediatrics emphasizes the need to assess all children for a history of psychological trauma. Traumas are quite common, and their sequelae affect many aspects of the child’s life; in the case of outbursts, it is emotional resilience that is impaired. As for all DSM-5 diagnoses, DMDD is not diagnosed when the irritability is due to physiological effects of a substance (e.g. steroids) or another medical or neurological disorder. Children with chronic pain conditions such as rheumatoid arthritis or sickle cell usually cope remarkably well, but when they don’t, their irritability should not be considered a mental health disorder. More commonly, sleep debt can produce chronic irritability and always should be assessed.
When coaching families about outbursts, I work to help them recognize that the child is not just angry, but very distressed. While “typical” tantrums last 1-5 minutes and show a rise then decline in intensity of the anger and distress, anger outbursts are longer and have an initial short and rapid burst of anger that then declines over the duration of the outburst, and with a steady but lower level of distress throughout.
The option to hug and verbally console the child’s distress is sometimes effective and does not reinforce the behavior unless the parent also yields to demands. But once outbursts begin, I liken them to a bomb going off – there is no intervention possible then. Instead, the task of the family, and over time that of the child, is to recognize and better manage the triggers.
Dr. Ross Greene, in his book, “The Explosive Child: A New Approach for Understanding and Parenting Easily Frustrated, Chronically Inflexible Children,” asserts that the child’s anger and distress can be interpreted as frustration from a gap in skills. This has treatment implications for identifying, educating about, and ameliorating the child’s weaknesses (deficits in understanding, communication, emotion regulation, flexibility or performance; or excess jealousy or hypersensitivity), and coaching parents to recognize, acknowledge, and avoid stressing these areas, if possible. I coach families to give points to the child for progressive little steps toward being able to recognize, verbalize, and inhibit outbursts with a reward system for the points. This helps put the parents and child “on the same team” in working on improving these skills.
Research on children with DMDD indicates that they show less positive affect when winning a “fixed” video game and are less able to suppress negative affect when losing. (Don’t forget to examine the role of real video games as precipitants of tantrums and contingently remove them!) Threshold for upset is lower and the degree of the upsets less well handled by children with DMDD.
In another study, when presented with a series of ambiguous facial expressions, children with DMDD were more likely to see anger in the faces than were controls. One hopeful result was that they could be taught to shift their perceptions significantly away from seeing anger, also reducing irritability and resulting in functional MRI changes. Such hostile bias attribution (tending to see threat) is well known to predispose to aggression. Cognitive behavioral therapy, the most effective counseling intervention, similarly teaches children to rethink their own negative thoughts before acting.
If irritability and rages were not enough, most children with DMDD have other psychiatric disorders; 39% having two, and 51% three or more (J Child Adolesc Psychopharmacol. 2013 Nov;23[9]:588-96). If not for the DMDD diagnosis, 82% would meet criteria for oppositional defiant disorder (ODD). The other common comorbidities are attention-deficit/hyperactivity disorder (ADHD) (74.5%), anxiety disorders (49.0%), and depression that is not major depressive disorder (MDD)(33.3%). When MDD is present, that diagnosis takes precedence. One cannot diagnose ODD, intermittent explosive disorder, or bipolar disorder along with DMDD, conditions from which it is intended to differentiate. Each of these comorbid disorders can be difficult to manage alone much less in combination, making DMDD a disorder deserving diagnosis and treatment by a mental health professional.
One of the main reasons DMDD was created is that children with these features go on to depressive or anxiety disorder in adolescence, not bipolar disorder.
While there is no treatment specific to DMDD, the depression component and prognosis suggest use of SSRIs, in addition to psychosocial therapies, and stimulants for the comorbid ADHD. Unfortunately, these two classes of medication are relatively contraindicated in bipolar disorder because they can lead to treatment-induced episodic mania (TEM). TEM occurs twice as often with antidepressants compared with stimulants (44% vs. 18%) in children with bipolar disorder (J Affect Disord. 2004 Oct 1;82[1]:149-58). Getting the diagnosis correct is, therefore, of great importance when medication is considered.
Approaches such as behavior modification, family therapy, and inpatient treatment can be effective for chronic irritability and aggression. Stimulant treatment of comorbid ADHD can decrease aggression and irritability. Alpha agonists such as guanfacine or clonidine also can help. In cases of partial improvement, adding either risperidone or divalproex may further decrease aggression in ADHD. In refractory aggression, risperidone has the best evidence. The Affective Reactivity Index or Outburst Monitoring Scale can be helpful in assessing severity and monitoring outcomes.
While a prognosis for depression rather than bipolar disorder sounds like a plus, in a longitudinal study, adults who had DMDD as children had worse outcomes, including being more likely to have adverse health outcomes (smoking, sexually transmitted infection), police contact, and low educational attainment, and being more likely to live in poverty, compared with controls who had other psychiatric disorders. While DMDD is a new and different diagnosis, it is similar to bipolar in having a potential course of life disruption, dangerous behaviors, suicide risk, and hospitalization.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline. E-mail her at [email protected].
Outbursts by children when frustrated or when asked to “do something they don’t want to do” are among the most common behavioral complaints voiced by parents. But behavioral outbursts, beyond the typical tantrums of children up to age 4 years, can be signs of very severe mental health disorders and are the most common reason for psychiatric admission (50%-60%).
While behavioral dysregulation is undeniably a huge problem for families, there has been an unreasonable 40-fold rise in diagnosis of bipolar disorder from 1994 to 2003, and 48% were prescribed atypical neuroleptics – medications with serious side effects. In response to this overdiagnosis as bipolar disorder, in 2013 the DSM-5 created a new diagnosis called disruptive mood dysregulation disorder (DMDD) to differentiate children who experience explosive outbursts who have a different outcome. This new classification includes children aged 6-12 years with persistent irritability most of the time, nearly every day, lasting at least 12 months and starting before age 10 years. DMDD diagnosis is not used after age 18 years.
To be diagnosed, the child has to have frequent, severe temper outbursts “grossly out of proportion” to the situation, averaging at least three times per week. The outbursts can be verbal or physical aggression to people, things, or themselves. While tantrums can be severe in children with delayed development, for the DMDD diagnosis these behaviors must be inconsistent with developmental level and must occur in at least two settings, and in one setting it must be severe. While outbursts are common, only half of children in one study of severe tantrum behavior in 5- to 9-year-olds also had the required persistent irritability.
If this does sound a lot like bipolar disorder so far, you are right. So what is different? DMDD has a prevalence of 2%-5% and occurs mostly in boys, whereas bipolar disorder affects boys and girls equally and affects less than 1% prior to adolescence.
The key features distinguishing DMDD from bipolar disorder are lack of an episodic nature to the irritability and lack of mania. Irritability in DMDD has to be persistently present with breaks of no more than 3 consecutive months in the defining 12-month period. There also cannot be any more than 1 day of the elevated mood features of mania or hypomania. Identifying mania is the hardest part, even in diagnosing adult bipolar, where it occurs only 1% of the year, much less in children who are generally lively! Hypomania, while less intense than mania, is when the person is energetic, talkative, and confident to an extreme extent, often with a flight of creative ideas. Excitement over birthdays or Christmas specifically does not count! So getting this history has to be done carefully, generally by a mental health professional, to make the distinction.
Interestingly, DMDD is not diagnosed when outbursts and irritability are better explained by autism spectrum disorder, separation anxiety disorder, or PTSD. To me, these exclusions point out the importance of sorting out the “set conditions” for all problematic behaviors, not always an easy task. Symptoms of autism in high functioning individuals can be quite subtle. Was the upset from change in a rigid routine known only to the child? Were sensory stimuli such as loud noises intolerable to this child? Was a nonverbal signal of a peer mistaken as a threat? While violent outbursts precipitated by these factors would still be considered “grossly out of proportion to the situation” for a typical child, they are not uncommon in atypical children. Similarly, children with separation anxiety disorder experience a high level of threat from even thinking about being apart from their caregivers, setting them up for alarm by situations other children would not find difficult.
The American Academy of Pediatrics emphasizes the need to assess all children for a history of psychological trauma. Traumas are quite common, and their sequelae affect many aspects of the child’s life; in the case of outbursts, it is emotional resilience that is impaired. As for all DSM-5 diagnoses, DMDD is not diagnosed when the irritability is due to physiological effects of a substance (e.g. steroids) or another medical or neurological disorder. Children with chronic pain conditions such as rheumatoid arthritis or sickle cell usually cope remarkably well, but when they don’t, their irritability should not be considered a mental health disorder. More commonly, sleep debt can produce chronic irritability and always should be assessed.
When coaching families about outbursts, I work to help them recognize that the child is not just angry, but very distressed. While “typical” tantrums last 1-5 minutes and show a rise then decline in intensity of the anger and distress, anger outbursts are longer and have an initial short and rapid burst of anger that then declines over the duration of the outburst, and with a steady but lower level of distress throughout.
The option to hug and verbally console the child’s distress is sometimes effective and does not reinforce the behavior unless the parent also yields to demands. But once outbursts begin, I liken them to a bomb going off – there is no intervention possible then. Instead, the task of the family, and over time that of the child, is to recognize and better manage the triggers.
Dr. Ross Greene, in his book, “The Explosive Child: A New Approach for Understanding and Parenting Easily Frustrated, Chronically Inflexible Children,” asserts that the child’s anger and distress can be interpreted as frustration from a gap in skills. This has treatment implications for identifying, educating about, and ameliorating the child’s weaknesses (deficits in understanding, communication, emotion regulation, flexibility or performance; or excess jealousy or hypersensitivity), and coaching parents to recognize, acknowledge, and avoid stressing these areas, if possible. I coach families to give points to the child for progressive little steps toward being able to recognize, verbalize, and inhibit outbursts with a reward system for the points. This helps put the parents and child “on the same team” in working on improving these skills.
Research on children with DMDD indicates that they show less positive affect when winning a “fixed” video game and are less able to suppress negative affect when losing. (Don’t forget to examine the role of real video games as precipitants of tantrums and contingently remove them!) Threshold for upset is lower and the degree of the upsets less well handled by children with DMDD.
In another study, when presented with a series of ambiguous facial expressions, children with DMDD were more likely to see anger in the faces than were controls. One hopeful result was that they could be taught to shift their perceptions significantly away from seeing anger, also reducing irritability and resulting in functional MRI changes. Such hostile bias attribution (tending to see threat) is well known to predispose to aggression. Cognitive behavioral therapy, the most effective counseling intervention, similarly teaches children to rethink their own negative thoughts before acting.
If irritability and rages were not enough, most children with DMDD have other psychiatric disorders; 39% having two, and 51% three or more (J Child Adolesc Psychopharmacol. 2013 Nov;23[9]:588-96). If not for the DMDD diagnosis, 82% would meet criteria for oppositional defiant disorder (ODD). The other common comorbidities are attention-deficit/hyperactivity disorder (ADHD) (74.5%), anxiety disorders (49.0%), and depression that is not major depressive disorder (MDD)(33.3%). When MDD is present, that diagnosis takes precedence. One cannot diagnose ODD, intermittent explosive disorder, or bipolar disorder along with DMDD, conditions from which it is intended to differentiate. Each of these comorbid disorders can be difficult to manage alone much less in combination, making DMDD a disorder deserving diagnosis and treatment by a mental health professional.
One of the main reasons DMDD was created is that children with these features go on to depressive or anxiety disorder in adolescence, not bipolar disorder.
While there is no treatment specific to DMDD, the depression component and prognosis suggest use of SSRIs, in addition to psychosocial therapies, and stimulants for the comorbid ADHD. Unfortunately, these two classes of medication are relatively contraindicated in bipolar disorder because they can lead to treatment-induced episodic mania (TEM). TEM occurs twice as often with antidepressants compared with stimulants (44% vs. 18%) in children with bipolar disorder (J Affect Disord. 2004 Oct 1;82[1]:149-58). Getting the diagnosis correct is, therefore, of great importance when medication is considered.
Approaches such as behavior modification, family therapy, and inpatient treatment can be effective for chronic irritability and aggression. Stimulant treatment of comorbid ADHD can decrease aggression and irritability. Alpha agonists such as guanfacine or clonidine also can help. In cases of partial improvement, adding either risperidone or divalproex may further decrease aggression in ADHD. In refractory aggression, risperidone has the best evidence. The Affective Reactivity Index or Outburst Monitoring Scale can be helpful in assessing severity and monitoring outcomes.
While a prognosis for depression rather than bipolar disorder sounds like a plus, in a longitudinal study, adults who had DMDD as children had worse outcomes, including being more likely to have adverse health outcomes (smoking, sexually transmitted infection), police contact, and low educational attainment, and being more likely to live in poverty, compared with controls who had other psychiatric disorders. While DMDD is a new and different diagnosis, it is similar to bipolar in having a potential course of life disruption, dangerous behaviors, suicide risk, and hospitalization.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline. E-mail her at [email protected].
Outbursts by children when frustrated or when asked to “do something they don’t want to do” are among the most common behavioral complaints voiced by parents. But behavioral outbursts, beyond the typical tantrums of children up to age 4 years, can be signs of very severe mental health disorders and are the most common reason for psychiatric admission (50%-60%).
While behavioral dysregulation is undeniably a huge problem for families, there has been an unreasonable 40-fold rise in diagnosis of bipolar disorder from 1994 to 2003, and 48% were prescribed atypical neuroleptics – medications with serious side effects. In response to this overdiagnosis as bipolar disorder, in 2013 the DSM-5 created a new diagnosis called disruptive mood dysregulation disorder (DMDD) to differentiate children who experience explosive outbursts who have a different outcome. This new classification includes children aged 6-12 years with persistent irritability most of the time, nearly every day, lasting at least 12 months and starting before age 10 years. DMDD diagnosis is not used after age 18 years.
To be diagnosed, the child has to have frequent, severe temper outbursts “grossly out of proportion” to the situation, averaging at least three times per week. The outbursts can be verbal or physical aggression to people, things, or themselves. While tantrums can be severe in children with delayed development, for the DMDD diagnosis these behaviors must be inconsistent with developmental level and must occur in at least two settings, and in one setting it must be severe. While outbursts are common, only half of children in one study of severe tantrum behavior in 5- to 9-year-olds also had the required persistent irritability.
If this does sound a lot like bipolar disorder so far, you are right. So what is different? DMDD has a prevalence of 2%-5% and occurs mostly in boys, whereas bipolar disorder affects boys and girls equally and affects less than 1% prior to adolescence.
The key features distinguishing DMDD from bipolar disorder are lack of an episodic nature to the irritability and lack of mania. Irritability in DMDD has to be persistently present with breaks of no more than 3 consecutive months in the defining 12-month period. There also cannot be any more than 1 day of the elevated mood features of mania or hypomania. Identifying mania is the hardest part, even in diagnosing adult bipolar, where it occurs only 1% of the year, much less in children who are generally lively! Hypomania, while less intense than mania, is when the person is energetic, talkative, and confident to an extreme extent, often with a flight of creative ideas. Excitement over birthdays or Christmas specifically does not count! So getting this history has to be done carefully, generally by a mental health professional, to make the distinction.
Interestingly, DMDD is not diagnosed when outbursts and irritability are better explained by autism spectrum disorder, separation anxiety disorder, or PTSD. To me, these exclusions point out the importance of sorting out the “set conditions” for all problematic behaviors, not always an easy task. Symptoms of autism in high functioning individuals can be quite subtle. Was the upset from change in a rigid routine known only to the child? Were sensory stimuli such as loud noises intolerable to this child? Was a nonverbal signal of a peer mistaken as a threat? While violent outbursts precipitated by these factors would still be considered “grossly out of proportion to the situation” for a typical child, they are not uncommon in atypical children. Similarly, children with separation anxiety disorder experience a high level of threat from even thinking about being apart from their caregivers, setting them up for alarm by situations other children would not find difficult.
The American Academy of Pediatrics emphasizes the need to assess all children for a history of psychological trauma. Traumas are quite common, and their sequelae affect many aspects of the child’s life; in the case of outbursts, it is emotional resilience that is impaired. As for all DSM-5 diagnoses, DMDD is not diagnosed when the irritability is due to physiological effects of a substance (e.g. steroids) or another medical or neurological disorder. Children with chronic pain conditions such as rheumatoid arthritis or sickle cell usually cope remarkably well, but when they don’t, their irritability should not be considered a mental health disorder. More commonly, sleep debt can produce chronic irritability and always should be assessed.
When coaching families about outbursts, I work to help them recognize that the child is not just angry, but very distressed. While “typical” tantrums last 1-5 minutes and show a rise then decline in intensity of the anger and distress, anger outbursts are longer and have an initial short and rapid burst of anger that then declines over the duration of the outburst, and with a steady but lower level of distress throughout.
The option to hug and verbally console the child’s distress is sometimes effective and does not reinforce the behavior unless the parent also yields to demands. But once outbursts begin, I liken them to a bomb going off – there is no intervention possible then. Instead, the task of the family, and over time that of the child, is to recognize and better manage the triggers.
Dr. Ross Greene, in his book, “The Explosive Child: A New Approach for Understanding and Parenting Easily Frustrated, Chronically Inflexible Children,” asserts that the child’s anger and distress can be interpreted as frustration from a gap in skills. This has treatment implications for identifying, educating about, and ameliorating the child’s weaknesses (deficits in understanding, communication, emotion regulation, flexibility or performance; or excess jealousy or hypersensitivity), and coaching parents to recognize, acknowledge, and avoid stressing these areas, if possible. I coach families to give points to the child for progressive little steps toward being able to recognize, verbalize, and inhibit outbursts with a reward system for the points. This helps put the parents and child “on the same team” in working on improving these skills.
Research on children with DMDD indicates that they show less positive affect when winning a “fixed” video game and are less able to suppress negative affect when losing. (Don’t forget to examine the role of real video games as precipitants of tantrums and contingently remove them!) Threshold for upset is lower and the degree of the upsets less well handled by children with DMDD.
In another study, when presented with a series of ambiguous facial expressions, children with DMDD were more likely to see anger in the faces than were controls. One hopeful result was that they could be taught to shift their perceptions significantly away from seeing anger, also reducing irritability and resulting in functional MRI changes. Such hostile bias attribution (tending to see threat) is well known to predispose to aggression. Cognitive behavioral therapy, the most effective counseling intervention, similarly teaches children to rethink their own negative thoughts before acting.
If irritability and rages were not enough, most children with DMDD have other psychiatric disorders; 39% having two, and 51% three or more (J Child Adolesc Psychopharmacol. 2013 Nov;23[9]:588-96). If not for the DMDD diagnosis, 82% would meet criteria for oppositional defiant disorder (ODD). The other common comorbidities are attention-deficit/hyperactivity disorder (ADHD) (74.5%), anxiety disorders (49.0%), and depression that is not major depressive disorder (MDD)(33.3%). When MDD is present, that diagnosis takes precedence. One cannot diagnose ODD, intermittent explosive disorder, or bipolar disorder along with DMDD, conditions from which it is intended to differentiate. Each of these comorbid disorders can be difficult to manage alone much less in combination, making DMDD a disorder deserving diagnosis and treatment by a mental health professional.
One of the main reasons DMDD was created is that children with these features go on to depressive or anxiety disorder in adolescence, not bipolar disorder.
While there is no treatment specific to DMDD, the depression component and prognosis suggest use of SSRIs, in addition to psychosocial therapies, and stimulants for the comorbid ADHD. Unfortunately, these two classes of medication are relatively contraindicated in bipolar disorder because they can lead to treatment-induced episodic mania (TEM). TEM occurs twice as often with antidepressants compared with stimulants (44% vs. 18%) in children with bipolar disorder (J Affect Disord. 2004 Oct 1;82[1]:149-58). Getting the diagnosis correct is, therefore, of great importance when medication is considered.
Approaches such as behavior modification, family therapy, and inpatient treatment can be effective for chronic irritability and aggression. Stimulant treatment of comorbid ADHD can decrease aggression and irritability. Alpha agonists such as guanfacine or clonidine also can help. In cases of partial improvement, adding either risperidone or divalproex may further decrease aggression in ADHD. In refractory aggression, risperidone has the best evidence. The Affective Reactivity Index or Outburst Monitoring Scale can be helpful in assessing severity and monitoring outcomes.
While a prognosis for depression rather than bipolar disorder sounds like a plus, in a longitudinal study, adults who had DMDD as children had worse outcomes, including being more likely to have adverse health outcomes (smoking, sexually transmitted infection), police contact, and low educational attainment, and being more likely to live in poverty, compared with controls who had other psychiatric disorders. While DMDD is a new and different diagnosis, it is similar to bipolar in having a potential course of life disruption, dangerous behaviors, suicide risk, and hospitalization.
Dr. Howard is assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline. E-mail her at [email protected].
The Starbucks generation
Iced, Half-Caff, Ristretto, Venti, 4-Pump, Sugar Free, Cinnamon, Dolce Soy Skinny Latte. Or Non-Fat Frappuccino with Extra Whipped Cream and Chocolate Sauce. Sorry, let me simplify: Triple, Venti, Soy, No Foam Latte. If you’re thinking I am speaking a foreign language, just ask a teen and they likely will be able to translate for you. This is normal Starbucks lingo. If you’re not a coffee drinker, you’re likely completely lost, but for those of us who live by the bean, it’s the language of love, caffeine love.
Twenty years ago, the thought of a teen drinking coffee was unheard of. Sure, soda has been there for decades, and in many cultures tea is a common drink, but today many kids are lining up at Starbucks for the caffeine jolt, and the new drinks such as 5-Hour Energy, Jolt, and Red Bull are making this generation the most sleep-deprived ever.
But how bad is caffeine for teens? The average adult consumes approximately 300 mg of caffeine per day,1 which is equivalent to 2-4 cups of coffee; this is considered to be a moderate intake. But the average teen intake of caffeine is not limited to just coffee. Many are consuming their favorite drink from Starbucks, then a few cans of soda, chocolate candy, and maybe even an energy drink – all before the school day is over. When we consider the content of caffeine in these products, the intake of caffeine is staggering.
For example, the average soda contains 35-55 mg of caffeine. Energy drinks such as Red Bull, Amp, and Monster contain approximately 150 mg of caffeine. A tall (small) Starbucks coffee also contains about 150 mg of caffeine, and when we increase the size to a grande, then we are looking at 320 mg.2,4 Simple math will show that the average teen likely has excessive intake of caffeine, resulting in adverse health effects.
The most concerning adverse effect is sleep deprivation.5 Physiologically, the circadian rhythm of adolescents changes and decreases the secretion of melatonin naturally, making it more difficult for them to fall asleep. With the addition of caffeine in high amounts, this is making falling asleep a greater challenge. Sleep deprivation leads to daytime sleepiness and inattention, resulting in learning issues.
Other ill effects found in some studies is that consumption of more than 220 mg of caffeine per day is associated with increased impulsivity, sensation seeking, and risk-taking behaviors.2 In people who are predisposed to arrhythmias, excessive intake can result in the onset of arrhythmias. Nervousness and jitteriness are other common effects.
Another consideration beyond the direct effects of caffeine is that it is usually coupled with sugary substances like those found in syrups used in Starbucks drinks, chocolate candy, and energy drinks. This has led to concerns of obesity as well as dental decay.
Now, when caffeine is taken in small to moderate amounts, less than 300 mg, there are health benefits. It certainly does improve concentration by attaching to the adenosine receptors that block the adenosine effect of sedation on the brain. This leads to improved concentration, memory retention, auditory vigilance, and reaction time.
Recent studies have shown that caffeine in moderate amounts can protect against Alzheimer’s, and is linked to a small decreased risk of cancer and liver disease. Coffee drinkers have also shown a moderate decrease in Parkinson’s disease and stroke.3,6
Regardless of the benefits of caffeine, the American Academy of Pediatrics has been very clear that it does not recommend caffeine in children. In its 2011 guideline,7 the extra calories, the risk of impulsive behavior, and sleep deprivation far outweighed any benefit that caffeine would have. It is critical that physicians educate their patients about foods that contain caffeine and the cumulative effect these foods have on their well-being, now and in the future.
References
1. “Trends in Caffeine Intake Among US Children and Adolescents” (Pediatrics 2014;133:386-93).
2. Caffeine Consumption Among Children and Adolescents. National Council on Strength and Fitness.
3. What is it about coffee? Harvard Health Letter (www.health.harvard.edu/staying-healthy/what-is-it-about-coffee).
4. Caffeine counts. American Physiological Association (Monitor on Psychology. 2001 June;32[6]).
5. J Pediatrics. 2011 March;158(3):508-9.
6. J Alzheimers Dis. 2010;20(suppl 1):s167-74.
7. Pediatrics 2011 June;127(6):1182-9
Dr. Pearce is a pediatrician in Frankfort, Ill.
Iced, Half-Caff, Ristretto, Venti, 4-Pump, Sugar Free, Cinnamon, Dolce Soy Skinny Latte. Or Non-Fat Frappuccino with Extra Whipped Cream and Chocolate Sauce. Sorry, let me simplify: Triple, Venti, Soy, No Foam Latte. If you’re thinking I am speaking a foreign language, just ask a teen and they likely will be able to translate for you. This is normal Starbucks lingo. If you’re not a coffee drinker, you’re likely completely lost, but for those of us who live by the bean, it’s the language of love, caffeine love.
Twenty years ago, the thought of a teen drinking coffee was unheard of. Sure, soda has been there for decades, and in many cultures tea is a common drink, but today many kids are lining up at Starbucks for the caffeine jolt, and the new drinks such as 5-Hour Energy, Jolt, and Red Bull are making this generation the most sleep-deprived ever.
But how bad is caffeine for teens? The average adult consumes approximately 300 mg of caffeine per day,1 which is equivalent to 2-4 cups of coffee; this is considered to be a moderate intake. But the average teen intake of caffeine is not limited to just coffee. Many are consuming their favorite drink from Starbucks, then a few cans of soda, chocolate candy, and maybe even an energy drink – all before the school day is over. When we consider the content of caffeine in these products, the intake of caffeine is staggering.
For example, the average soda contains 35-55 mg of caffeine. Energy drinks such as Red Bull, Amp, and Monster contain approximately 150 mg of caffeine. A tall (small) Starbucks coffee also contains about 150 mg of caffeine, and when we increase the size to a grande, then we are looking at 320 mg.2,4 Simple math will show that the average teen likely has excessive intake of caffeine, resulting in adverse health effects.
The most concerning adverse effect is sleep deprivation.5 Physiologically, the circadian rhythm of adolescents changes and decreases the secretion of melatonin naturally, making it more difficult for them to fall asleep. With the addition of caffeine in high amounts, this is making falling asleep a greater challenge. Sleep deprivation leads to daytime sleepiness and inattention, resulting in learning issues.
Other ill effects found in some studies is that consumption of more than 220 mg of caffeine per day is associated with increased impulsivity, sensation seeking, and risk-taking behaviors.2 In people who are predisposed to arrhythmias, excessive intake can result in the onset of arrhythmias. Nervousness and jitteriness are other common effects.
Another consideration beyond the direct effects of caffeine is that it is usually coupled with sugary substances like those found in syrups used in Starbucks drinks, chocolate candy, and energy drinks. This has led to concerns of obesity as well as dental decay.
Now, when caffeine is taken in small to moderate amounts, less than 300 mg, there are health benefits. It certainly does improve concentration by attaching to the adenosine receptors that block the adenosine effect of sedation on the brain. This leads to improved concentration, memory retention, auditory vigilance, and reaction time.
Recent studies have shown that caffeine in moderate amounts can protect against Alzheimer’s, and is linked to a small decreased risk of cancer and liver disease. Coffee drinkers have also shown a moderate decrease in Parkinson’s disease and stroke.3,6
Regardless of the benefits of caffeine, the American Academy of Pediatrics has been very clear that it does not recommend caffeine in children. In its 2011 guideline,7 the extra calories, the risk of impulsive behavior, and sleep deprivation far outweighed any benefit that caffeine would have. It is critical that physicians educate their patients about foods that contain caffeine and the cumulative effect these foods have on their well-being, now and in the future.
References
1. “Trends in Caffeine Intake Among US Children and Adolescents” (Pediatrics 2014;133:386-93).
2. Caffeine Consumption Among Children and Adolescents. National Council on Strength and Fitness.
3. What is it about coffee? Harvard Health Letter (www.health.harvard.edu/staying-healthy/what-is-it-about-coffee).
4. Caffeine counts. American Physiological Association (Monitor on Psychology. 2001 June;32[6]).
5. J Pediatrics. 2011 March;158(3):508-9.
6. J Alzheimers Dis. 2010;20(suppl 1):s167-74.
7. Pediatrics 2011 June;127(6):1182-9
Dr. Pearce is a pediatrician in Frankfort, Ill.
Iced, Half-Caff, Ristretto, Venti, 4-Pump, Sugar Free, Cinnamon, Dolce Soy Skinny Latte. Or Non-Fat Frappuccino with Extra Whipped Cream and Chocolate Sauce. Sorry, let me simplify: Triple, Venti, Soy, No Foam Latte. If you’re thinking I am speaking a foreign language, just ask a teen and they likely will be able to translate for you. This is normal Starbucks lingo. If you’re not a coffee drinker, you’re likely completely lost, but for those of us who live by the bean, it’s the language of love, caffeine love.
Twenty years ago, the thought of a teen drinking coffee was unheard of. Sure, soda has been there for decades, and in many cultures tea is a common drink, but today many kids are lining up at Starbucks for the caffeine jolt, and the new drinks such as 5-Hour Energy, Jolt, and Red Bull are making this generation the most sleep-deprived ever.
But how bad is caffeine for teens? The average adult consumes approximately 300 mg of caffeine per day,1 which is equivalent to 2-4 cups of coffee; this is considered to be a moderate intake. But the average teen intake of caffeine is not limited to just coffee. Many are consuming their favorite drink from Starbucks, then a few cans of soda, chocolate candy, and maybe even an energy drink – all before the school day is over. When we consider the content of caffeine in these products, the intake of caffeine is staggering.
For example, the average soda contains 35-55 mg of caffeine. Energy drinks such as Red Bull, Amp, and Monster contain approximately 150 mg of caffeine. A tall (small) Starbucks coffee also contains about 150 mg of caffeine, and when we increase the size to a grande, then we are looking at 320 mg.2,4 Simple math will show that the average teen likely has excessive intake of caffeine, resulting in adverse health effects.
The most concerning adverse effect is sleep deprivation.5 Physiologically, the circadian rhythm of adolescents changes and decreases the secretion of melatonin naturally, making it more difficult for them to fall asleep. With the addition of caffeine in high amounts, this is making falling asleep a greater challenge. Sleep deprivation leads to daytime sleepiness and inattention, resulting in learning issues.
Other ill effects found in some studies is that consumption of more than 220 mg of caffeine per day is associated with increased impulsivity, sensation seeking, and risk-taking behaviors.2 In people who are predisposed to arrhythmias, excessive intake can result in the onset of arrhythmias. Nervousness and jitteriness are other common effects.
Another consideration beyond the direct effects of caffeine is that it is usually coupled with sugary substances like those found in syrups used in Starbucks drinks, chocolate candy, and energy drinks. This has led to concerns of obesity as well as dental decay.
Now, when caffeine is taken in small to moderate amounts, less than 300 mg, there are health benefits. It certainly does improve concentration by attaching to the adenosine receptors that block the adenosine effect of sedation on the brain. This leads to improved concentration, memory retention, auditory vigilance, and reaction time.
Recent studies have shown that caffeine in moderate amounts can protect against Alzheimer’s, and is linked to a small decreased risk of cancer and liver disease. Coffee drinkers have also shown a moderate decrease in Parkinson’s disease and stroke.3,6
Regardless of the benefits of caffeine, the American Academy of Pediatrics has been very clear that it does not recommend caffeine in children. In its 2011 guideline,7 the extra calories, the risk of impulsive behavior, and sleep deprivation far outweighed any benefit that caffeine would have. It is critical that physicians educate their patients about foods that contain caffeine and the cumulative effect these foods have on their well-being, now and in the future.
References
1. “Trends in Caffeine Intake Among US Children and Adolescents” (Pediatrics 2014;133:386-93).
2. Caffeine Consumption Among Children and Adolescents. National Council on Strength and Fitness.
3. What is it about coffee? Harvard Health Letter (www.health.harvard.edu/staying-healthy/what-is-it-about-coffee).
4. Caffeine counts. American Physiological Association (Monitor on Psychology. 2001 June;32[6]).
5. J Pediatrics. 2011 March;158(3):508-9.
6. J Alzheimers Dis. 2010;20(suppl 1):s167-74.
7. Pediatrics 2011 June;127(6):1182-9
Dr. Pearce is a pediatrician in Frankfort, Ill.
Hernia repair: Studies don’t support use of higher-cost biological mesh
The jury is still out on the costs and efficacy of biological mesh implants for abdominal wall hernia repair, according to a systematic review of the literature.
Of 20 articles that met search criteria, only 3 were comparative studies and none was a randomized clinical trial. In fact, most were case series involving convenience samples of patients at single institutions, Dr. Sergio Huerta of the University of Texas Southwestern Medical Center, Dallas, and his colleagues reported online Jan. 27 in JAMA Surgery.
The authors used multiple electronic databases to identify articles published between 1948 and June 30, 2015, on the use of biological mesh materials for reinforcement of the abdominal wall for hernia repair. Included were 14 articles that described outcomes with human acellular dermal matrix, 2 that reported results for porcine collagen intestinal submucosa derivatives, 3 that reported on porcine acelluar collagen skin derivatives, and 1 that described results for bovine pericardium.
Several problems were noted with respect to the studies, including widely varying follow-up time, operative technique, types of mesh used, and patient selection criteria. Also, outcome measures were not reported consistently across studies.
In addition, 16 of the 20 studies that met search criteria did not report investigator conflicts of interest, the authors reported (JAMA Surg. 2016 Jan 27. doi: 10.10001/jamasurg.2015.5234).
Notably, all the meshes used in the studies were approved by the Food and Drug Administration and were considered to be comparable to a group of nonbiological predicate devices, which cost up to 25% less than the biological equivalents, they noted.
“We were unable to find any evidence that supported the use of expensive biological material relative to low-cost synthetic mesh. In fact, with one exception, the biological materials became commercially available by showing that these materials were equivalent to low-cost established synthetic mesh material in an FDA 510(k) approval process. This process does not require phase 0 through IV clinical trials as required for drugs or biological agents,” they wrote, noting that the one material that bypassed the 510(k) process (Alloderm) was not required to demonstrate equivalence because it was classified as human transplanted tissue.
Biological mesh materials were introduced in the 1990s to minimize the risk of complications commonly seen with the use of synthetic mesh for abdominal wall hernia repair – one of the most common procedures performed by general surgeons, the authors explained.
“Because the outcomes for biological mesh material are perceived to be better than those for polymer-based prosthetic mesh replacement materials, the use of biological grafts increased exponentially without clear clinical evidence of efficacy,” they wrote.
The current review suggests that the evidence remains insufficient to determine whether cost and clinical benefits exist.
“It is generally assumed that FDA-approved drug or biological agents have been rigorously evaluated and that there is demonstrable safety and efficacy. This is not the case for 510(k) medical devices. Before using a new medical device, physicians should know the approval basis for the device and recognize that if it is a 510(k) device neither safety nor efficacy is ensured,” they said, adding that physicians should assume such devices are no better than predicate devices to which they are equivalent, and that “there can be no justification for purchasing a more expensive device when a lower-cost predicate device, which is equivalent, is available.”
Though limited by certain factors such as lack of access to detailed FDA information such as the specific criteria used to determine equivalence, and a lack of published literature on the full market penetration of biological mesh materials vs. nonbiological counterparts, the authors maintained that until evidence demonstrates superiority of biological materials, the expense associated with their use cannot be justified.
This study was supported by the Hudson-Penn Endowment fund at the University of Texas Southwestern Medical Center. The authors reported having no relevant financial disclosures.
Balancing the need for innovation against the practicalities of demonstrating clinical benefit for novel ideas is a fundamental problem in surgery – a problem highlighted by Heurta et al., Dr. Benjamin K. Poulose and his colleagues said in an editorial.
“This issue is particularly timely given an unsustainable trajectory of health care spending in the United States,” they wrote (JAMA Surg 2016 Jan 27. doi: 10.1001/jamasurg2015.5236).
Like the authors of the literature review, the editorial authors stressed the importance of understanding the limitations of the 510(k) process, and noted that surgeons should consider that the FDA sees them as “the group responsible for understanding and evaluating the data before using expensive medical devices.
“Understanding who is in charge of making sure that the devices that we use during surgery are safe and effective is critical. Likely, it will require a collaborative effort of the FDA, medical device companies, and physicians,” they wrote. Establishing a more formal system of postmarketing surveillance for higher-risk medical devices will benefit patients, they added. Current efforts suffer from reliance on self-reporting and lack of standardized data collection.
Postmarketing surveillance can be greatly improved by directly linking medical device approval with the support of high-quality registries, and the end result should provide transparent data for monitoring effectiveness and safety to drive value-based care and maintain innovation, they said.
Dr. Poulose is with Vanderbilt University Medical Center, Nashville, Tenn. Dr. Poulose reported receiving personal fees from Ariste Medical for consulting work, and research grants from Bard-Davol outside the submitted work.
Balancing the need for innovation against the practicalities of demonstrating clinical benefit for novel ideas is a fundamental problem in surgery – a problem highlighted by Heurta et al., Dr. Benjamin K. Poulose and his colleagues said in an editorial.
“This issue is particularly timely given an unsustainable trajectory of health care spending in the United States,” they wrote (JAMA Surg 2016 Jan 27. doi: 10.1001/jamasurg2015.5236).
Like the authors of the literature review, the editorial authors stressed the importance of understanding the limitations of the 510(k) process, and noted that surgeons should consider that the FDA sees them as “the group responsible for understanding and evaluating the data before using expensive medical devices.
“Understanding who is in charge of making sure that the devices that we use during surgery are safe and effective is critical. Likely, it will require a collaborative effort of the FDA, medical device companies, and physicians,” they wrote. Establishing a more formal system of postmarketing surveillance for higher-risk medical devices will benefit patients, they added. Current efforts suffer from reliance on self-reporting and lack of standardized data collection.
Postmarketing surveillance can be greatly improved by directly linking medical device approval with the support of high-quality registries, and the end result should provide transparent data for monitoring effectiveness and safety to drive value-based care and maintain innovation, they said.
Dr. Poulose is with Vanderbilt University Medical Center, Nashville, Tenn. Dr. Poulose reported receiving personal fees from Ariste Medical for consulting work, and research grants from Bard-Davol outside the submitted work.
Balancing the need for innovation against the practicalities of demonstrating clinical benefit for novel ideas is a fundamental problem in surgery – a problem highlighted by Heurta et al., Dr. Benjamin K. Poulose and his colleagues said in an editorial.
“This issue is particularly timely given an unsustainable trajectory of health care spending in the United States,” they wrote (JAMA Surg 2016 Jan 27. doi: 10.1001/jamasurg2015.5236).
Like the authors of the literature review, the editorial authors stressed the importance of understanding the limitations of the 510(k) process, and noted that surgeons should consider that the FDA sees them as “the group responsible for understanding and evaluating the data before using expensive medical devices.
“Understanding who is in charge of making sure that the devices that we use during surgery are safe and effective is critical. Likely, it will require a collaborative effort of the FDA, medical device companies, and physicians,” they wrote. Establishing a more formal system of postmarketing surveillance for higher-risk medical devices will benefit patients, they added. Current efforts suffer from reliance on self-reporting and lack of standardized data collection.
Postmarketing surveillance can be greatly improved by directly linking medical device approval with the support of high-quality registries, and the end result should provide transparent data for monitoring effectiveness and safety to drive value-based care and maintain innovation, they said.
Dr. Poulose is with Vanderbilt University Medical Center, Nashville, Tenn. Dr. Poulose reported receiving personal fees from Ariste Medical for consulting work, and research grants from Bard-Davol outside the submitted work.
The jury is still out on the costs and efficacy of biological mesh implants for abdominal wall hernia repair, according to a systematic review of the literature.
Of 20 articles that met search criteria, only 3 were comparative studies and none was a randomized clinical trial. In fact, most were case series involving convenience samples of patients at single institutions, Dr. Sergio Huerta of the University of Texas Southwestern Medical Center, Dallas, and his colleagues reported online Jan. 27 in JAMA Surgery.
The authors used multiple electronic databases to identify articles published between 1948 and June 30, 2015, on the use of biological mesh materials for reinforcement of the abdominal wall for hernia repair. Included were 14 articles that described outcomes with human acellular dermal matrix, 2 that reported results for porcine collagen intestinal submucosa derivatives, 3 that reported on porcine acelluar collagen skin derivatives, and 1 that described results for bovine pericardium.
Several problems were noted with respect to the studies, including widely varying follow-up time, operative technique, types of mesh used, and patient selection criteria. Also, outcome measures were not reported consistently across studies.
In addition, 16 of the 20 studies that met search criteria did not report investigator conflicts of interest, the authors reported (JAMA Surg. 2016 Jan 27. doi: 10.10001/jamasurg.2015.5234).
Notably, all the meshes used in the studies were approved by the Food and Drug Administration and were considered to be comparable to a group of nonbiological predicate devices, which cost up to 25% less than the biological equivalents, they noted.
“We were unable to find any evidence that supported the use of expensive biological material relative to low-cost synthetic mesh. In fact, with one exception, the biological materials became commercially available by showing that these materials were equivalent to low-cost established synthetic mesh material in an FDA 510(k) approval process. This process does not require phase 0 through IV clinical trials as required for drugs or biological agents,” they wrote, noting that the one material that bypassed the 510(k) process (Alloderm) was not required to demonstrate equivalence because it was classified as human transplanted tissue.
Biological mesh materials were introduced in the 1990s to minimize the risk of complications commonly seen with the use of synthetic mesh for abdominal wall hernia repair – one of the most common procedures performed by general surgeons, the authors explained.
“Because the outcomes for biological mesh material are perceived to be better than those for polymer-based prosthetic mesh replacement materials, the use of biological grafts increased exponentially without clear clinical evidence of efficacy,” they wrote.
The current review suggests that the evidence remains insufficient to determine whether cost and clinical benefits exist.
“It is generally assumed that FDA-approved drug or biological agents have been rigorously evaluated and that there is demonstrable safety and efficacy. This is not the case for 510(k) medical devices. Before using a new medical device, physicians should know the approval basis for the device and recognize that if it is a 510(k) device neither safety nor efficacy is ensured,” they said, adding that physicians should assume such devices are no better than predicate devices to which they are equivalent, and that “there can be no justification for purchasing a more expensive device when a lower-cost predicate device, which is equivalent, is available.”
Though limited by certain factors such as lack of access to detailed FDA information such as the specific criteria used to determine equivalence, and a lack of published literature on the full market penetration of biological mesh materials vs. nonbiological counterparts, the authors maintained that until evidence demonstrates superiority of biological materials, the expense associated with their use cannot be justified.
This study was supported by the Hudson-Penn Endowment fund at the University of Texas Southwestern Medical Center. The authors reported having no relevant financial disclosures.
The jury is still out on the costs and efficacy of biological mesh implants for abdominal wall hernia repair, according to a systematic review of the literature.
Of 20 articles that met search criteria, only 3 were comparative studies and none was a randomized clinical trial. In fact, most were case series involving convenience samples of patients at single institutions, Dr. Sergio Huerta of the University of Texas Southwestern Medical Center, Dallas, and his colleagues reported online Jan. 27 in JAMA Surgery.
The authors used multiple electronic databases to identify articles published between 1948 and June 30, 2015, on the use of biological mesh materials for reinforcement of the abdominal wall for hernia repair. Included were 14 articles that described outcomes with human acellular dermal matrix, 2 that reported results for porcine collagen intestinal submucosa derivatives, 3 that reported on porcine acelluar collagen skin derivatives, and 1 that described results for bovine pericardium.
Several problems were noted with respect to the studies, including widely varying follow-up time, operative technique, types of mesh used, and patient selection criteria. Also, outcome measures were not reported consistently across studies.
In addition, 16 of the 20 studies that met search criteria did not report investigator conflicts of interest, the authors reported (JAMA Surg. 2016 Jan 27. doi: 10.10001/jamasurg.2015.5234).
Notably, all the meshes used in the studies were approved by the Food and Drug Administration and were considered to be comparable to a group of nonbiological predicate devices, which cost up to 25% less than the biological equivalents, they noted.
“We were unable to find any evidence that supported the use of expensive biological material relative to low-cost synthetic mesh. In fact, with one exception, the biological materials became commercially available by showing that these materials were equivalent to low-cost established synthetic mesh material in an FDA 510(k) approval process. This process does not require phase 0 through IV clinical trials as required for drugs or biological agents,” they wrote, noting that the one material that bypassed the 510(k) process (Alloderm) was not required to demonstrate equivalence because it was classified as human transplanted tissue.
Biological mesh materials were introduced in the 1990s to minimize the risk of complications commonly seen with the use of synthetic mesh for abdominal wall hernia repair – one of the most common procedures performed by general surgeons, the authors explained.
“Because the outcomes for biological mesh material are perceived to be better than those for polymer-based prosthetic mesh replacement materials, the use of biological grafts increased exponentially without clear clinical evidence of efficacy,” they wrote.
The current review suggests that the evidence remains insufficient to determine whether cost and clinical benefits exist.
“It is generally assumed that FDA-approved drug or biological agents have been rigorously evaluated and that there is demonstrable safety and efficacy. This is not the case for 510(k) medical devices. Before using a new medical device, physicians should know the approval basis for the device and recognize that if it is a 510(k) device neither safety nor efficacy is ensured,” they said, adding that physicians should assume such devices are no better than predicate devices to which they are equivalent, and that “there can be no justification for purchasing a more expensive device when a lower-cost predicate device, which is equivalent, is available.”
Though limited by certain factors such as lack of access to detailed FDA information such as the specific criteria used to determine equivalence, and a lack of published literature on the full market penetration of biological mesh materials vs. nonbiological counterparts, the authors maintained that until evidence demonstrates superiority of biological materials, the expense associated with their use cannot be justified.
This study was supported by the Hudson-Penn Endowment fund at the University of Texas Southwestern Medical Center. The authors reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: The jury is still out on the costs and efficacy of biological mesh implants for abdominal wall hernia repair, and the expense associated with their use cannot be justified at this time, a systematic review of the literature suggested.
Major finding: Available evidence remains insufficient to determine whether cost and clinical benefits exist with the use of biological mesh for abdominal wall hernia repair.
Data source: A systematic review of the literature, yielding 20 eligible studies.
Disclosures: This study was supported by the Hudson-Penn Endowment fund at University of Texas Southwestern Medical Center. The authors reported having no relevant financial disclosures.