User login
Low transformation rate in nodular lymphocyte–predominant Hodgkin lymphoma
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
FROM BLOOD
Key clinical point: The risk of transformation to diffuse large B-cell lymphoma is low in patients with nodular lymphocyte–predominant Hodgkin lymphoma.
Major finding: Only 7.6% of cases transformed over a median of 16 years of follow-up, and transformation did not worsen overall survival.
Data source: A prospective single-center study of 222 consecutive adults with NLPHL.
Disclosures: The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
DAAs, HCV-positive livers could reduce transplant waiting list
BARCELONA – Using direct-acting antiviral therapy and livers from HCV-positive donors are separate approaches that could help reduce waiting lists and ensure that patients with HCV in most need get a liver transplant, according to data from two studies presented at the International Liver Congress.
In a retrospective European cohort study, 33% of HCV-positive patients with decompensated cirrhosis who were awaiting a transplant were no longer considered to be in urgent need and almost 20% could be removed from the list altogether 60 weeks after starting treatment with direct-acting antivirals (DAAs).
Dr. Luca Belli of Niguarda Hospital, Milan, who presented the findings at the meeting, cautioned that while the results were encouraging, it is not clear how long the clinical improvement will last. “It will be critical to assess the long-term risks of death, further redeterioration, and developments of hepatocellular carcinoma more specifically, as all these factors still need to be verified,” he said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
Meanwhile, data from a large analysis of all solid transplant recipients in the United States showed that using livers from HCV-positive donors in HCV-positive recipients was associated with long-term patient outcomes similar to outcomes of using livers from non–HCV-negative donors in HCV-positive recipients, with no difference in mortality or graft survival.
“Over the past 2 decades, the use of HCV-positive organs for liver transplantation has tripled in the United States,” said Maria Stepanova, Ph.D., a senior biostatistician for Inova Health System in Falls Church, Va., who presented her findings at the meeting.
“Despite this, the medium- to long-term outcomes of HCV-positive liver transplant recipients transplanted from HCV-positive donors were not affected by HCV-positivity of a donor,” added Dr. Stepanova, who also works at the Center for Outcomes Research in Liver Disease in Washington, D.C.
Dr. Zobair Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, and coauthor of the study, said during a press briefing that this does not mean that livers from HCV-positive donor could be used in HCV-negative recipients. The reason for that is that it would be causing an acute infection regardless of whether or not antiviral treatment is available and “at this point the evidence is not there,” he said.
Dr. Laurent Castera of Hôpital Beaujon in Paris, and Dr. Tom Hemming Karlsen of Oslo University Hospital Rikshospitalet in Norway commented on the significance of these data in press releases issued by the EASL. Both experts, who were not involved in the studies, noted the findings could help take the strain off the liver transplant list in the future.
“Treating patients with direct-acting antiviral therapy could result in those with a more pressing need for a liver transplant receiving the donation they need, potentially reducing the number of deaths that occur on the waiting list,” Dr. Castera said.
“With the number of people waiting for a liver transplant expected to rise, the study results should give hope over the coming years for those on the waiting list,” Dr. Karlsen said. Referring to the U.S. study, he said the results “clearly demonstrate a greater opportunity for use of HCV-positive livers over the coming years due to their comparable outcomes with healthy livers.”
The European study presented by Dr. Belli involved 134 patients with HCV and decompensated cirrhosis but without hepatocellular carcinoma listed for liver transplant between February 2014 and February 2015 at 11 centers in Austria, Italy, and France. Of these patients, 103 had been treated with DAAs while on the transplant list; approximately half had been treated with a single DAA (sofosbuvir plus ribavirin for 24-48 weeks) and half with two DAAs (sofosbuvir with either daclatasvir or ledipasvir for 12-24 weeks).
The primary endpoint was the probability of being “inactivated” and then “delisted.” Inactivated meant that there was clinical improvement resulting in patients being put “on hold,” and “delisted” was defined as patients being taken off the transplant list after a variable period of inactivation.
The median age of patients in the study was 54 years and 68% were male. Just under 50% of patients had a low (less than 16) MELD score, around 37% had a MELD score of 16-20, and 13% had a MELD score of more than 20. Around 45% had Child-Pugh B and 55% had Child-Pugh C cirrhosis. Two-thirds had medically controlled and 26% had medically uncontrolled ascites, and 46% had medically controlled and 1% had medically uncontrolled hepatic encephalopathy.
Good virologic efficacy was seen with both single- and dual-agent DAA therapy, with rapid virologic responses of 61% and 67% and early virologic responses of 98% and 98%. Of the 52 patients given single-agent DAA treatment, 22 had a transplant and one patient had a posttransplant relapse. Of the remaining 30 patients on the transplant list, four relapsed. Nineteen of 51 patients given dual-DAA therapy were transplanted and there was one relapse, with no relapses in the 32 patients who remained on the transplant list.
After about 60 weeks of follow-up, one in three patients were “inactivated” and not considered in urgent need of a transplant, but inactivation occurred as early as 12 weeks after DAA therapy, Dr. Belli observed.
Inactivation was associated with a 3.3 decrease in MELD score, a 2-point reduction in Child-Turcotte-Pugh score, and a 0.5-g/dL increase in serum albumin after 24 weeks. There was also regression or improvement in ascites and hepatic encephalopathy in most patients. The median time of delisting was 48 weeks.
These results suggest that there could be a wider role for DAA therapy even in the sickest of HCV-positive patients who are urgently awaiting a liver transplant. Another approach to increase the number of people receiving a transplant is to use HCV-positive livers. The practice has been increasing over the years, but it is not known if the approach is safe and if the risks outweigh the benefits.
To investigate, Dr. Stepanova and associates obtained data from the Scientific Registry of Transplant Recipients (SRTR) on all liver transplants performed in the United States between 1995 and 2003 involving HCV-positive patients. A total of 37,317 records were found, of which 33,668 had data on the donors’ HCV status and on the recipients’ mortality status. Of these, 1,930 (5.7%) had received a liver from an HCV-positive donor. Dr. Stepanova noted that there had been an increase in the percentage of patients who had received an HCV-positive liver, from less than 3% in 1995 to more than 9% in 2013.
Compared with HCV-negative donors, HCV-positive donors were older, were more likely to have a history of drug abuse, and more likely to be non–heart beating at the time of procurement.
The HCV-positive liver recipients also tended to be older, to be of African-American ethnicity, and to have liver cancer but lower MELD scores than those who received an HCV-negative liver. “So these are patients that cannot wait for a long time so maybe elect to use an HCV-positive donor,” Dr. Younossi said.
Adjusted hazard ratios (aHR) showed no statistically significant difference in posttransplant survival or posttransplant graft loss between HCV-positive and HCV-negative livers; aHR were a respective 1.03 and 0.905, with tight 95% confidence intervals. However, a more recent year of transplant did appear to suggest a possible advantage of using an HCV-positive donor liver in an HCV-positive patient, with lower mortality (aHR = 0.978 per year, P less than .0001) and graft failure (aHR = 0.960 per year, P less than .0001) rates.
While the use of HCV-positive livers in HCV-positive recipients was felt to be “reasonably safe,” these findings “cannot be used in support of indiscriminate use of HCV-positive donors,” Dr. Stepanova observed. Further studies are needed to establish criteria on which to select donors that would provide patients with the best possible risk-to-benefit ratio, she said.
Further avenues for research would also be to see if HCV-positive livers could be given to HCV-negative patients and if genotype matters. It would also need to be seen if posttransplantation antiviral treatment would be necessary.
Dr. Belli has received research support from Gilead, AbbVie and BMS and acted as a consultant to Gilead. Dr. Stepanova did not have conflicts of interest to disclose. Dr. Younossi has acted as a consultant to BMS, AbbVie, Gilead, GlaxoSmithKline, and Intercept. Dr. Castera and Dr. Karlsen had no conflicts of interest to disclose.
BARCELONA – Using direct-acting antiviral therapy and livers from HCV-positive donors are separate approaches that could help reduce waiting lists and ensure that patients with HCV in most need get a liver transplant, according to data from two studies presented at the International Liver Congress.
In a retrospective European cohort study, 33% of HCV-positive patients with decompensated cirrhosis who were awaiting a transplant were no longer considered to be in urgent need and almost 20% could be removed from the list altogether 60 weeks after starting treatment with direct-acting antivirals (DAAs).
Dr. Luca Belli of Niguarda Hospital, Milan, who presented the findings at the meeting, cautioned that while the results were encouraging, it is not clear how long the clinical improvement will last. “It will be critical to assess the long-term risks of death, further redeterioration, and developments of hepatocellular carcinoma more specifically, as all these factors still need to be verified,” he said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
Meanwhile, data from a large analysis of all solid transplant recipients in the United States showed that using livers from HCV-positive donors in HCV-positive recipients was associated with long-term patient outcomes similar to outcomes of using livers from non–HCV-negative donors in HCV-positive recipients, with no difference in mortality or graft survival.
“Over the past 2 decades, the use of HCV-positive organs for liver transplantation has tripled in the United States,” said Maria Stepanova, Ph.D., a senior biostatistician for Inova Health System in Falls Church, Va., who presented her findings at the meeting.
“Despite this, the medium- to long-term outcomes of HCV-positive liver transplant recipients transplanted from HCV-positive donors were not affected by HCV-positivity of a donor,” added Dr. Stepanova, who also works at the Center for Outcomes Research in Liver Disease in Washington, D.C.
Dr. Zobair Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, and coauthor of the study, said during a press briefing that this does not mean that livers from HCV-positive donor could be used in HCV-negative recipients. The reason for that is that it would be causing an acute infection regardless of whether or not antiviral treatment is available and “at this point the evidence is not there,” he said.
Dr. Laurent Castera of Hôpital Beaujon in Paris, and Dr. Tom Hemming Karlsen of Oslo University Hospital Rikshospitalet in Norway commented on the significance of these data in press releases issued by the EASL. Both experts, who were not involved in the studies, noted the findings could help take the strain off the liver transplant list in the future.
“Treating patients with direct-acting antiviral therapy could result in those with a more pressing need for a liver transplant receiving the donation they need, potentially reducing the number of deaths that occur on the waiting list,” Dr. Castera said.
“With the number of people waiting for a liver transplant expected to rise, the study results should give hope over the coming years for those on the waiting list,” Dr. Karlsen said. Referring to the U.S. study, he said the results “clearly demonstrate a greater opportunity for use of HCV-positive livers over the coming years due to their comparable outcomes with healthy livers.”
The European study presented by Dr. Belli involved 134 patients with HCV and decompensated cirrhosis but without hepatocellular carcinoma listed for liver transplant between February 2014 and February 2015 at 11 centers in Austria, Italy, and France. Of these patients, 103 had been treated with DAAs while on the transplant list; approximately half had been treated with a single DAA (sofosbuvir plus ribavirin for 24-48 weeks) and half with two DAAs (sofosbuvir with either daclatasvir or ledipasvir for 12-24 weeks).
The primary endpoint was the probability of being “inactivated” and then “delisted.” Inactivated meant that there was clinical improvement resulting in patients being put “on hold,” and “delisted” was defined as patients being taken off the transplant list after a variable period of inactivation.
The median age of patients in the study was 54 years and 68% were male. Just under 50% of patients had a low (less than 16) MELD score, around 37% had a MELD score of 16-20, and 13% had a MELD score of more than 20. Around 45% had Child-Pugh B and 55% had Child-Pugh C cirrhosis. Two-thirds had medically controlled and 26% had medically uncontrolled ascites, and 46% had medically controlled and 1% had medically uncontrolled hepatic encephalopathy.
Good virologic efficacy was seen with both single- and dual-agent DAA therapy, with rapid virologic responses of 61% and 67% and early virologic responses of 98% and 98%. Of the 52 patients given single-agent DAA treatment, 22 had a transplant and one patient had a posttransplant relapse. Of the remaining 30 patients on the transplant list, four relapsed. Nineteen of 51 patients given dual-DAA therapy were transplanted and there was one relapse, with no relapses in the 32 patients who remained on the transplant list.
After about 60 weeks of follow-up, one in three patients were “inactivated” and not considered in urgent need of a transplant, but inactivation occurred as early as 12 weeks after DAA therapy, Dr. Belli observed.
Inactivation was associated with a 3.3 decrease in MELD score, a 2-point reduction in Child-Turcotte-Pugh score, and a 0.5-g/dL increase in serum albumin after 24 weeks. There was also regression or improvement in ascites and hepatic encephalopathy in most patients. The median time of delisting was 48 weeks.
These results suggest that there could be a wider role for DAA therapy even in the sickest of HCV-positive patients who are urgently awaiting a liver transplant. Another approach to increase the number of people receiving a transplant is to use HCV-positive livers. The practice has been increasing over the years, but it is not known if the approach is safe and if the risks outweigh the benefits.
To investigate, Dr. Stepanova and associates obtained data from the Scientific Registry of Transplant Recipients (SRTR) on all liver transplants performed in the United States between 1995 and 2003 involving HCV-positive patients. A total of 37,317 records were found, of which 33,668 had data on the donors’ HCV status and on the recipients’ mortality status. Of these, 1,930 (5.7%) had received a liver from an HCV-positive donor. Dr. Stepanova noted that there had been an increase in the percentage of patients who had received an HCV-positive liver, from less than 3% in 1995 to more than 9% in 2013.
Compared with HCV-negative donors, HCV-positive donors were older, were more likely to have a history of drug abuse, and more likely to be non–heart beating at the time of procurement.
The HCV-positive liver recipients also tended to be older, to be of African-American ethnicity, and to have liver cancer but lower MELD scores than those who received an HCV-negative liver. “So these are patients that cannot wait for a long time so maybe elect to use an HCV-positive donor,” Dr. Younossi said.
Adjusted hazard ratios (aHR) showed no statistically significant difference in posttransplant survival or posttransplant graft loss between HCV-positive and HCV-negative livers; aHR were a respective 1.03 and 0.905, with tight 95% confidence intervals. However, a more recent year of transplant did appear to suggest a possible advantage of using an HCV-positive donor liver in an HCV-positive patient, with lower mortality (aHR = 0.978 per year, P less than .0001) and graft failure (aHR = 0.960 per year, P less than .0001) rates.
While the use of HCV-positive livers in HCV-positive recipients was felt to be “reasonably safe,” these findings “cannot be used in support of indiscriminate use of HCV-positive donors,” Dr. Stepanova observed. Further studies are needed to establish criteria on which to select donors that would provide patients with the best possible risk-to-benefit ratio, she said.
Further avenues for research would also be to see if HCV-positive livers could be given to HCV-negative patients and if genotype matters. It would also need to be seen if posttransplantation antiviral treatment would be necessary.
Dr. Belli has received research support from Gilead, AbbVie and BMS and acted as a consultant to Gilead. Dr. Stepanova did not have conflicts of interest to disclose. Dr. Younossi has acted as a consultant to BMS, AbbVie, Gilead, GlaxoSmithKline, and Intercept. Dr. Castera and Dr. Karlsen had no conflicts of interest to disclose.
BARCELONA – Using direct-acting antiviral therapy and livers from HCV-positive donors are separate approaches that could help reduce waiting lists and ensure that patients with HCV in most need get a liver transplant, according to data from two studies presented at the International Liver Congress.
In a retrospective European cohort study, 33% of HCV-positive patients with decompensated cirrhosis who were awaiting a transplant were no longer considered to be in urgent need and almost 20% could be removed from the list altogether 60 weeks after starting treatment with direct-acting antivirals (DAAs).
Dr. Luca Belli of Niguarda Hospital, Milan, who presented the findings at the meeting, cautioned that while the results were encouraging, it is not clear how long the clinical improvement will last. “It will be critical to assess the long-term risks of death, further redeterioration, and developments of hepatocellular carcinoma more specifically, as all these factors still need to be verified,” he said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
Meanwhile, data from a large analysis of all solid transplant recipients in the United States showed that using livers from HCV-positive donors in HCV-positive recipients was associated with long-term patient outcomes similar to outcomes of using livers from non–HCV-negative donors in HCV-positive recipients, with no difference in mortality or graft survival.
“Over the past 2 decades, the use of HCV-positive organs for liver transplantation has tripled in the United States,” said Maria Stepanova, Ph.D., a senior biostatistician for Inova Health System in Falls Church, Va., who presented her findings at the meeting.
“Despite this, the medium- to long-term outcomes of HCV-positive liver transplant recipients transplanted from HCV-positive donors were not affected by HCV-positivity of a donor,” added Dr. Stepanova, who also works at the Center for Outcomes Research in Liver Disease in Washington, D.C.
Dr. Zobair Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, and coauthor of the study, said during a press briefing that this does not mean that livers from HCV-positive donor could be used in HCV-negative recipients. The reason for that is that it would be causing an acute infection regardless of whether or not antiviral treatment is available and “at this point the evidence is not there,” he said.
Dr. Laurent Castera of Hôpital Beaujon in Paris, and Dr. Tom Hemming Karlsen of Oslo University Hospital Rikshospitalet in Norway commented on the significance of these data in press releases issued by the EASL. Both experts, who were not involved in the studies, noted the findings could help take the strain off the liver transplant list in the future.
“Treating patients with direct-acting antiviral therapy could result in those with a more pressing need for a liver transplant receiving the donation they need, potentially reducing the number of deaths that occur on the waiting list,” Dr. Castera said.
“With the number of people waiting for a liver transplant expected to rise, the study results should give hope over the coming years for those on the waiting list,” Dr. Karlsen said. Referring to the U.S. study, he said the results “clearly demonstrate a greater opportunity for use of HCV-positive livers over the coming years due to their comparable outcomes with healthy livers.”
The European study presented by Dr. Belli involved 134 patients with HCV and decompensated cirrhosis but without hepatocellular carcinoma listed for liver transplant between February 2014 and February 2015 at 11 centers in Austria, Italy, and France. Of these patients, 103 had been treated with DAAs while on the transplant list; approximately half had been treated with a single DAA (sofosbuvir plus ribavirin for 24-48 weeks) and half with two DAAs (sofosbuvir with either daclatasvir or ledipasvir for 12-24 weeks).
The primary endpoint was the probability of being “inactivated” and then “delisted.” Inactivated meant that there was clinical improvement resulting in patients being put “on hold,” and “delisted” was defined as patients being taken off the transplant list after a variable period of inactivation.
The median age of patients in the study was 54 years and 68% were male. Just under 50% of patients had a low (less than 16) MELD score, around 37% had a MELD score of 16-20, and 13% had a MELD score of more than 20. Around 45% had Child-Pugh B and 55% had Child-Pugh C cirrhosis. Two-thirds had medically controlled and 26% had medically uncontrolled ascites, and 46% had medically controlled and 1% had medically uncontrolled hepatic encephalopathy.
Good virologic efficacy was seen with both single- and dual-agent DAA therapy, with rapid virologic responses of 61% and 67% and early virologic responses of 98% and 98%. Of the 52 patients given single-agent DAA treatment, 22 had a transplant and one patient had a posttransplant relapse. Of the remaining 30 patients on the transplant list, four relapsed. Nineteen of 51 patients given dual-DAA therapy were transplanted and there was one relapse, with no relapses in the 32 patients who remained on the transplant list.
After about 60 weeks of follow-up, one in three patients were “inactivated” and not considered in urgent need of a transplant, but inactivation occurred as early as 12 weeks after DAA therapy, Dr. Belli observed.
Inactivation was associated with a 3.3 decrease in MELD score, a 2-point reduction in Child-Turcotte-Pugh score, and a 0.5-g/dL increase in serum albumin after 24 weeks. There was also regression or improvement in ascites and hepatic encephalopathy in most patients. The median time of delisting was 48 weeks.
These results suggest that there could be a wider role for DAA therapy even in the sickest of HCV-positive patients who are urgently awaiting a liver transplant. Another approach to increase the number of people receiving a transplant is to use HCV-positive livers. The practice has been increasing over the years, but it is not known if the approach is safe and if the risks outweigh the benefits.
To investigate, Dr. Stepanova and associates obtained data from the Scientific Registry of Transplant Recipients (SRTR) on all liver transplants performed in the United States between 1995 and 2003 involving HCV-positive patients. A total of 37,317 records were found, of which 33,668 had data on the donors’ HCV status and on the recipients’ mortality status. Of these, 1,930 (5.7%) had received a liver from an HCV-positive donor. Dr. Stepanova noted that there had been an increase in the percentage of patients who had received an HCV-positive liver, from less than 3% in 1995 to more than 9% in 2013.
Compared with HCV-negative donors, HCV-positive donors were older, were more likely to have a history of drug abuse, and more likely to be non–heart beating at the time of procurement.
The HCV-positive liver recipients also tended to be older, to be of African-American ethnicity, and to have liver cancer but lower MELD scores than those who received an HCV-negative liver. “So these are patients that cannot wait for a long time so maybe elect to use an HCV-positive donor,” Dr. Younossi said.
Adjusted hazard ratios (aHR) showed no statistically significant difference in posttransplant survival or posttransplant graft loss between HCV-positive and HCV-negative livers; aHR were a respective 1.03 and 0.905, with tight 95% confidence intervals. However, a more recent year of transplant did appear to suggest a possible advantage of using an HCV-positive donor liver in an HCV-positive patient, with lower mortality (aHR = 0.978 per year, P less than .0001) and graft failure (aHR = 0.960 per year, P less than .0001) rates.
While the use of HCV-positive livers in HCV-positive recipients was felt to be “reasonably safe,” these findings “cannot be used in support of indiscriminate use of HCV-positive donors,” Dr. Stepanova observed. Further studies are needed to establish criteria on which to select donors that would provide patients with the best possible risk-to-benefit ratio, she said.
Further avenues for research would also be to see if HCV-positive livers could be given to HCV-negative patients and if genotype matters. It would also need to be seen if posttransplantation antiviral treatment would be necessary.
Dr. Belli has received research support from Gilead, AbbVie and BMS and acted as a consultant to Gilead. Dr. Stepanova did not have conflicts of interest to disclose. Dr. Younossi has acted as a consultant to BMS, AbbVie, Gilead, GlaxoSmithKline, and Intercept. Dr. Castera and Dr. Karlsen had no conflicts of interest to disclose.
AT THE INTERNATIONAL LIVER CONGRESS 2016
Key clinical point: Using direct-acting antiviral (DAA) therapy and HCV-positive livers could help reduce the strain on liver transplant lists.
Major finding: One in three HCV-positive patients treated with DAAs were considered nonurgent cases and one in five could be delisted. HCV-positive livers were associated with similar graft and host survival.
Data source: A retrospective European cohort study of 103 patients with decompensated cirrhosis who were treated with DAAs while awaiting liver transplant and an analysis of more than 33,000 HCV patients awaiting a liver transplant in the United States.
Disclosures: Dr. Belli has received research support from Gilead, AbbVie, and BMS and acted as a consultant to Gilead. Dr. Stepanova did not have conflicts of interest to disclose. Dr. Younossi has acted as a consultant to BMS, AbbVie, Gilead, GlaxoSmithKline, and Intercept. Dr. Castera and Dr. Karlsen had no conflicts of interest to disclose.
Doppler ultrasound headset performs well at spotting sports-related concussion
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
VANCOUVER – A new transcranial Doppler platform that analyzes subtle changes in the cerebral blood flow waveform performed well in detecting sports-related concussion in a cohort study of 238 Los Angeles high school athletes.
The investigational headset device was able to differentiate between those with and without a recent concussion 83% of the time, investigators reported at the annual meeting of the American Academy of Neurology. In contrast, traditional transcranial Doppler analysis detected a recent concussion only 50%-60% of the time.
“Over the last few years, there has been growing evidence that cerebral hemodynamics are altered following sports-related concussion,” senior author Robert Hamilton, Ph.D., cofounder and chief science officer of Neural Analytics in Los Angeles, commented in a session and interview.
Most studies in this area have used MRI or traditional transcranial Doppler analysis, he said. However, the former is costly, time consuming, and not portable, and the latter has not proven very accurate.
As traditional Doppler analysis disregards the majority of waveform data, Dr. Hamilton and his colleagues developed an advanced platform that uses machine learning to analyze the entire shape of the cerebral blood flow velocity waveform through quantitative cerebral hemodynamics.
They compared the advanced analysis with traditional analysis among 69 high school athletes in contact sports who had sustained a concussion an average of 6 days earlier and a control group of 169 unaffected age-matched high school athletes from contact and noncontact sports.
Both groups had bilateral monitoring of blood flow in the middle cerebral artery with transcranial Doppler while they followed a standard cerebrovascular reactivity protocol that included rest and breath holding.
Results showed that for differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%. (Sensitivity was 72%, specificity was 82%, and overall accuracy was 80%.)
In comparison, the area under the curve was substantially lower for the traditional analysis measures: It was 55% for mean velocity (100% sensitivity, 0% specificity, 76% accuracy), 52% for the pulsatility index (86% sensitivity, 23% specificity, 61% accuracy), and 60% for the cerebrovascular reactivity index (51% sensitivity, 68% specificity, 64% accuracy).
“Unfortunately, concussion diagnostics and management today are basically subjective,” Dr. Hamilton commented. The advanced analysis may therefore improve the situation by providing objective evidence of blood flow dysfunction after injury.
The new analysis platform “is easy to use and portable, and [testing] can be done very quickly, within 5 minutes,” he noted. “The nice thing is it can be done on the sideline, in the emergency room, or in a doctor’s office.”
The investigators will next use the advanced analysis to track recovery of blood flow regulation after sports-related concussion and will compare its performance with that of additional modalities, such as MRI, according to Dr. Hamilton. Furthermore, they are testing it in various other populations: adolescents, college athletes, and members of the military.
“Ultimately, blood flow dysfunction is also important in a wide variety of conditions, such as stroke and dementia,” he pointed out. “So those are conditions that we are looking at to study this year and moving forward in the future.”
The research was supported by the National Institutes of Health and the National Science Foundation.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Advanced transcranial Doppler analysis may improve identification of athletes with concussion at the point of care.
Major finding: For differentiating between athletes who did and did not have concussion, the advanced analysis had an area under the receiver operating characteristic curve of 83%.
Data source: A cohort study of 69 concussed and 169 nonconcussed high school athletes.
Disclosures: Dr. Hamilton disclosed that he is a cofounder and chief science officer of Neural Analytics. The study was supported by the National Institutes of Health and the National Science Foundation.
Product News: 05 2016
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Sexual Orientation and Cancer Risk
Young people in sexual minorities are at higher risk of cancer because they engage in risky behavior more often, say researchers from City University of New York, Harvard, Boston’s Children’s Hospital, and San Diego State University.
The researchers analyzed data from 9,958 participants in the national Growing Up Today Study (1999-2010). The study participants were the children of the women in the Nurses’ Health Study II; those women were invited in 1996 to enroll their 9- to 14-year-old children. Of the participants, 84.5% reported being “completely” heterosexual, 12.1% were “mostly” heterosexual, 1.8% were lesbian or gay, and 1.6% were bisexual.
Related: Native Americans Address LGBT Health Issues
The researchers measured responses about tobacco and alcohol, diet and physical activity, exposure to ultraviolet radiation, and sexually transmitted infections.
Compared with completely heterosexual women, lesbian, bisexual, and mostly heterosexual women more frequently engaged in multiple cancer-related risk behaviors. For instance, they were more likely to have smoked, to be overweight, and to have been physically inactive in the previous year. Bisexual and mostly heterosexual women were more likely to have had a sexually transmitted infection. Interestingly, heterosexual women were more likely to have used a tanning booth ≥ 10 times in the previous year.
Compared with heterosexual men, sexual-minority women were also more often engaged in risky behaviors. The differences between gay/bisexual men and heterosexual men were less marked, although gay men more often vomited to control their weight, compared with heterosexual men, and had a higher prevalence of STIs.
The literature, the researchers note, tends to focus on “ever or never” behavior. They were mindful, they say, that exposure to a potential carcinogen usually must occur over time, and that the likelihood of cancer increases with exposure, which is why they focused on assessing frequent engagement in each cancer-related risk behavior, long term. Their findings indicated that sexual minorities, relative to heterosexuals, are at risk for cancer through multiple risk behaviors—“concerning,” they add, because the “additive or synergistic effect of another cancer-related risk behavior may provoke or exacerbate a determinant of cancer: chronic inflammation.”
Source:
Rosario M, Li F, Wypij D, et al. Am J Public Health. 2016;106(4):698-706.
doi: 10.2105/AJPH.2015.302977.
Young people in sexual minorities are at higher risk of cancer because they engage in risky behavior more often, say researchers from City University of New York, Harvard, Boston’s Children’s Hospital, and San Diego State University.
The researchers analyzed data from 9,958 participants in the national Growing Up Today Study (1999-2010). The study participants were the children of the women in the Nurses’ Health Study II; those women were invited in 1996 to enroll their 9- to 14-year-old children. Of the participants, 84.5% reported being “completely” heterosexual, 12.1% were “mostly” heterosexual, 1.8% were lesbian or gay, and 1.6% were bisexual.
Related: Native Americans Address LGBT Health Issues
The researchers measured responses about tobacco and alcohol, diet and physical activity, exposure to ultraviolet radiation, and sexually transmitted infections.
Compared with completely heterosexual women, lesbian, bisexual, and mostly heterosexual women more frequently engaged in multiple cancer-related risk behaviors. For instance, they were more likely to have smoked, to be overweight, and to have been physically inactive in the previous year. Bisexual and mostly heterosexual women were more likely to have had a sexually transmitted infection. Interestingly, heterosexual women were more likely to have used a tanning booth ≥ 10 times in the previous year.
Compared with heterosexual men, sexual-minority women were also more often engaged in risky behaviors. The differences between gay/bisexual men and heterosexual men were less marked, although gay men more often vomited to control their weight, compared with heterosexual men, and had a higher prevalence of STIs.
The literature, the researchers note, tends to focus on “ever or never” behavior. They were mindful, they say, that exposure to a potential carcinogen usually must occur over time, and that the likelihood of cancer increases with exposure, which is why they focused on assessing frequent engagement in each cancer-related risk behavior, long term. Their findings indicated that sexual minorities, relative to heterosexuals, are at risk for cancer through multiple risk behaviors—“concerning,” they add, because the “additive or synergistic effect of another cancer-related risk behavior may provoke or exacerbate a determinant of cancer: chronic inflammation.”
Source:
Rosario M, Li F, Wypij D, et al. Am J Public Health. 2016;106(4):698-706.
doi: 10.2105/AJPH.2015.302977.
Young people in sexual minorities are at higher risk of cancer because they engage in risky behavior more often, say researchers from City University of New York, Harvard, Boston’s Children’s Hospital, and San Diego State University.
The researchers analyzed data from 9,958 participants in the national Growing Up Today Study (1999-2010). The study participants were the children of the women in the Nurses’ Health Study II; those women were invited in 1996 to enroll their 9- to 14-year-old children. Of the participants, 84.5% reported being “completely” heterosexual, 12.1% were “mostly” heterosexual, 1.8% were lesbian or gay, and 1.6% were bisexual.
Related: Native Americans Address LGBT Health Issues
The researchers measured responses about tobacco and alcohol, diet and physical activity, exposure to ultraviolet radiation, and sexually transmitted infections.
Compared with completely heterosexual women, lesbian, bisexual, and mostly heterosexual women more frequently engaged in multiple cancer-related risk behaviors. For instance, they were more likely to have smoked, to be overweight, and to have been physically inactive in the previous year. Bisexual and mostly heterosexual women were more likely to have had a sexually transmitted infection. Interestingly, heterosexual women were more likely to have used a tanning booth ≥ 10 times in the previous year.
Compared with heterosexual men, sexual-minority women were also more often engaged in risky behaviors. The differences between gay/bisexual men and heterosexual men were less marked, although gay men more often vomited to control their weight, compared with heterosexual men, and had a higher prevalence of STIs.
The literature, the researchers note, tends to focus on “ever or never” behavior. They were mindful, they say, that exposure to a potential carcinogen usually must occur over time, and that the likelihood of cancer increases with exposure, which is why they focused on assessing frequent engagement in each cancer-related risk behavior, long term. Their findings indicated that sexual minorities, relative to heterosexuals, are at risk for cancer through multiple risk behaviors—“concerning,” they add, because the “additive or synergistic effect of another cancer-related risk behavior may provoke or exacerbate a determinant of cancer: chronic inflammation.”
Source:
Rosario M, Li F, Wypij D, et al. Am J Public Health. 2016;106(4):698-706.
doi: 10.2105/AJPH.2015.302977.
Opioid reform legislation passes House committee
The House Energy & Commerce Committee has passed a comprehensive package of bills designed to curb the nation’s opioid epidemic.
Eleven opioid-related bills passed the full committee by voice vote on April 27 and April 28. Key provisions of the legislation would:
• Create an interagency task force to review best practices for pain management and prescribing.
• Require annual updates of federal opioid-prescribing guidelines.
• Authorize grants to test coprescribing opioids with buprenorphine or naloxone.
• Limit the number of pills prescribed.
• Increase the number of patients that a qualified addiction treatment specialist could see annually.
• Require an FDA advisory committee to review any new opioid proposed without abuse-deterrent properties.
• Require a detailed assessment of currently available inpatient and outpatient treatment beds.
• Prohibit the sale dextromethorphan-containing products to minors.
The full Senate also has a package of opioid-related bills to consider. On March 17, the Senate Committee on Health, Education, Labor and Pensions moved similar legislation to the Senate floor, including bills that would increase addiction patient panels, require coprescribing, and mandate insurance coverage of addiction treatment as required by current mental health parity laws.
Earlier this year, in a near unanimous vote, the Senate passed the Comprehensive Addiction and Recovery Act, which calls for the creation of a federal pain management best practices interagency task force. No funding was attached to the legislation, however, and companion legislation remains in committee in the House.
Although the opioid bills had bipartisan support in the Energy & Commerce Committee, rancor may yet surface. During mark-up, three amendments were defeated mostly along party lines. The amendments would have increased the number of patients each qualified provider can treat with buprenorphine to a variety of levels – one amendment called for a maximum of 250 patients while others called for as many as 300 or 500. Supporters of the amendments said higher numbers would ensure treatment for many more patients while opponents expressed concern about sacrificing quality of care for quantity.
Another defeated amendment called for a $1 billion appropriation for increased opioid treatment, echoing President Obama’s call earlier this year. Opponents painted the proposal as “fiscally irresponsible.”
At press time, the House had not scheduled consideration on the opioid bills.
On Twitter @whitneymcknight
The House Energy & Commerce Committee has passed a comprehensive package of bills designed to curb the nation’s opioid epidemic.
Eleven opioid-related bills passed the full committee by voice vote on April 27 and April 28. Key provisions of the legislation would:
• Create an interagency task force to review best practices for pain management and prescribing.
• Require annual updates of federal opioid-prescribing guidelines.
• Authorize grants to test coprescribing opioids with buprenorphine or naloxone.
• Limit the number of pills prescribed.
• Increase the number of patients that a qualified addiction treatment specialist could see annually.
• Require an FDA advisory committee to review any new opioid proposed without abuse-deterrent properties.
• Require a detailed assessment of currently available inpatient and outpatient treatment beds.
• Prohibit the sale dextromethorphan-containing products to minors.
The full Senate also has a package of opioid-related bills to consider. On March 17, the Senate Committee on Health, Education, Labor and Pensions moved similar legislation to the Senate floor, including bills that would increase addiction patient panels, require coprescribing, and mandate insurance coverage of addiction treatment as required by current mental health parity laws.
Earlier this year, in a near unanimous vote, the Senate passed the Comprehensive Addiction and Recovery Act, which calls for the creation of a federal pain management best practices interagency task force. No funding was attached to the legislation, however, and companion legislation remains in committee in the House.
Although the opioid bills had bipartisan support in the Energy & Commerce Committee, rancor may yet surface. During mark-up, three amendments were defeated mostly along party lines. The amendments would have increased the number of patients each qualified provider can treat with buprenorphine to a variety of levels – one amendment called for a maximum of 250 patients while others called for as many as 300 or 500. Supporters of the amendments said higher numbers would ensure treatment for many more patients while opponents expressed concern about sacrificing quality of care for quantity.
Another defeated amendment called for a $1 billion appropriation for increased opioid treatment, echoing President Obama’s call earlier this year. Opponents painted the proposal as “fiscally irresponsible.”
At press time, the House had not scheduled consideration on the opioid bills.
On Twitter @whitneymcknight
The House Energy & Commerce Committee has passed a comprehensive package of bills designed to curb the nation’s opioid epidemic.
Eleven opioid-related bills passed the full committee by voice vote on April 27 and April 28. Key provisions of the legislation would:
• Create an interagency task force to review best practices for pain management and prescribing.
• Require annual updates of federal opioid-prescribing guidelines.
• Authorize grants to test coprescribing opioids with buprenorphine or naloxone.
• Limit the number of pills prescribed.
• Increase the number of patients that a qualified addiction treatment specialist could see annually.
• Require an FDA advisory committee to review any new opioid proposed without abuse-deterrent properties.
• Require a detailed assessment of currently available inpatient and outpatient treatment beds.
• Prohibit the sale dextromethorphan-containing products to minors.
The full Senate also has a package of opioid-related bills to consider. On March 17, the Senate Committee on Health, Education, Labor and Pensions moved similar legislation to the Senate floor, including bills that would increase addiction patient panels, require coprescribing, and mandate insurance coverage of addiction treatment as required by current mental health parity laws.
Earlier this year, in a near unanimous vote, the Senate passed the Comprehensive Addiction and Recovery Act, which calls for the creation of a federal pain management best practices interagency task force. No funding was attached to the legislation, however, and companion legislation remains in committee in the House.
Although the opioid bills had bipartisan support in the Energy & Commerce Committee, rancor may yet surface. During mark-up, three amendments were defeated mostly along party lines. The amendments would have increased the number of patients each qualified provider can treat with buprenorphine to a variety of levels – one amendment called for a maximum of 250 patients while others called for as many as 300 or 500. Supporters of the amendments said higher numbers would ensure treatment for many more patients while opponents expressed concern about sacrificing quality of care for quantity.
Another defeated amendment called for a $1 billion appropriation for increased opioid treatment, echoing President Obama’s call earlier this year. Opponents painted the proposal as “fiscally irresponsible.”
At press time, the House had not scheduled consideration on the opioid bills.
On Twitter @whitneymcknight
FROM A HOUSE ENERGY & COMMERCE COMMITTEE HEARING
Training impacted performance of surgical quality measures
INDIAN WELLS, CALIF. – Surgeons with fellowship training in female pelvic medicine and reconstructive surgery were significantly more likely to perform proposed quality measures at the time of hysterectomy for pelvic organ prolapse, compared with those who lack such training, a single-center study showed.
“The Physician Quality Reporting System was instituted as part of recent health care reform, with the aim of improving the reporting of quality measures, with the overall goal of improving the quality of care provided to patients throughout all areas of medicine,” Dr. Emily Adams-Piper said at the annual scientific meeting of the Society of Gynecologic Surgeons. “While there are many types of quality measures, including outcome measures and patient satisfaction measures, process measures may be the most directly applicable for the practicing clinician, because they provide recommended actions during specific patient encounters that can guide practice.”
Dr. Adams-Piper, a resident physician in the division of urogynecology at the University of California, Irvine, and her associates set out to investigate the use of proposed quality measures at the time of hysterectomy for pelvic organ prolapse (POP) among women receiving care from Southern California Permanente Medical Group, a large HMO.
They wanted to know if training background affected the rate of performance of four different quality measures related to hysterectomy for POP: offering conservative treatment prior to the surgical treatment of POP, quantitative assessment of POP with either a Baden-Walker or a POP-Q exam, apical support procedure performed at the time of hysterectomy for prolapse, and performance of intraoperative cystoscopy.
Patients who underwent hysterectomy for POP in 2008 were eligible for the study. The researchers reviewed electronic medical records for clinical and demographic data and categorized surgeons by their level of training.
“They were considered fellowship trained if they had pursued additional formal subspecialty training in female pelvic medicine and reconstructive surgery,” Dr. Adams-Piper explained. “Surgeons were considered grandfathered if they subsequently took the FPMRS [Female Pelvic Medicine and Reconstructive Surgery] boards when they became available in 2013. Surgeons were considered generalist if they fit into neither of these two categories and completed a residency in ob.gyn.”
Chi-squared tests were used to compare demographics and performance of the proposed quality measures. Of the 662 hysterectomies performed in 2008, 328 were included in the final analysis. The mean patient age was 60 years, the mean parity was 2.9, and the mean body mass index was 27.9 kg/m2.
Overall performance of the four proposed quality measures was high, ranging from 82%-87%. More than half of quality assessments (58%) were performed with the POP-Q exam, while the majority of apical support procedures were uterosacral ligament vault suspensions (67%), followed by sacrocolpopexy (18%), McCall culdoplasty (12%), and sacrospinous ligament fixation (3%).
When categorized by training, fellowship-trained surgeons performed 133 hysterectomies, “grandfathered” surgeons performed 55, and generalist gynecologic surgeons performed 140. Fellowship-trained surgeons performed each of the four proposed quality measures more often than did grandfathered surgeons, who performed them more often than generalist gynecologic surgeons did.
Specifically, conservative treatment was offered by 94% of fellowship-trained surgeons, 87% of grandfathered surgeons, and 76% of generalist gynecologic surgeons (P = .0002). Qualitative preoperative assessment of POP was performed by 99% of fellowship-trained surgeons, 93% of grandfathered surgeons, and 73% of generalist gynecologic surgeons (three-way comparison reached statistical significance, with a P less than .0001).
Apical repair was performed by 96% of fellowship-trained surgeons, 82% of grandfathered surgeons, and 69% of generalist gynecologic surgeons (P less than .0001). Finally, cystoscopy was performed by 98% of fellowship-trained surgeons, 91% of grandfathered surgeons, and 72% of generalist gynecologic surgeons (P less than .0001).
When the researchers evaluated the cumulative performance of all measures in the same patient, fellowship-trained surgeons had the highest rates (89%, compared with 62% of grandfathered surgeons, and 39% of generalist gynecologic surgeons; P less than .0001).
“When we looked at the patient characteristics and their distribution across the surgeon training backgrounds, we found no significant differences in the age, BMI, gravidity, or parity of the subjects that underwent surgeries with the three groups,” Dr. Adams-Piper said.
She acknowledged certain limitations of the study, including the fact that it reflects clinical practice in a single health care delivery system, it relied on prior documentation, and it evaluated data from 2008.
“From this study we can conclude that perioperative practice patterns differ by surgeon training background,” she said. “However, in order for the proposed quality measures to be clinically meaningful, they must be correlated with patient-centered outcomes.”
Dr. Adams-Piper reported having no financial disclosures. The meeting was jointly sponsored by the American College of Surgeons.
INDIAN WELLS, CALIF. – Surgeons with fellowship training in female pelvic medicine and reconstructive surgery were significantly more likely to perform proposed quality measures at the time of hysterectomy for pelvic organ prolapse, compared with those who lack such training, a single-center study showed.
“The Physician Quality Reporting System was instituted as part of recent health care reform, with the aim of improving the reporting of quality measures, with the overall goal of improving the quality of care provided to patients throughout all areas of medicine,” Dr. Emily Adams-Piper said at the annual scientific meeting of the Society of Gynecologic Surgeons. “While there are many types of quality measures, including outcome measures and patient satisfaction measures, process measures may be the most directly applicable for the practicing clinician, because they provide recommended actions during specific patient encounters that can guide practice.”
Dr. Adams-Piper, a resident physician in the division of urogynecology at the University of California, Irvine, and her associates set out to investigate the use of proposed quality measures at the time of hysterectomy for pelvic organ prolapse (POP) among women receiving care from Southern California Permanente Medical Group, a large HMO.
They wanted to know if training background affected the rate of performance of four different quality measures related to hysterectomy for POP: offering conservative treatment prior to the surgical treatment of POP, quantitative assessment of POP with either a Baden-Walker or a POP-Q exam, apical support procedure performed at the time of hysterectomy for prolapse, and performance of intraoperative cystoscopy.
Patients who underwent hysterectomy for POP in 2008 were eligible for the study. The researchers reviewed electronic medical records for clinical and demographic data and categorized surgeons by their level of training.
“They were considered fellowship trained if they had pursued additional formal subspecialty training in female pelvic medicine and reconstructive surgery,” Dr. Adams-Piper explained. “Surgeons were considered grandfathered if they subsequently took the FPMRS [Female Pelvic Medicine and Reconstructive Surgery] boards when they became available in 2013. Surgeons were considered generalist if they fit into neither of these two categories and completed a residency in ob.gyn.”
Chi-squared tests were used to compare demographics and performance of the proposed quality measures. Of the 662 hysterectomies performed in 2008, 328 were included in the final analysis. The mean patient age was 60 years, the mean parity was 2.9, and the mean body mass index was 27.9 kg/m2.
Overall performance of the four proposed quality measures was high, ranging from 82%-87%. More than half of quality assessments (58%) were performed with the POP-Q exam, while the majority of apical support procedures were uterosacral ligament vault suspensions (67%), followed by sacrocolpopexy (18%), McCall culdoplasty (12%), and sacrospinous ligament fixation (3%).
When categorized by training, fellowship-trained surgeons performed 133 hysterectomies, “grandfathered” surgeons performed 55, and generalist gynecologic surgeons performed 140. Fellowship-trained surgeons performed each of the four proposed quality measures more often than did grandfathered surgeons, who performed them more often than generalist gynecologic surgeons did.
Specifically, conservative treatment was offered by 94% of fellowship-trained surgeons, 87% of grandfathered surgeons, and 76% of generalist gynecologic surgeons (P = .0002). Qualitative preoperative assessment of POP was performed by 99% of fellowship-trained surgeons, 93% of grandfathered surgeons, and 73% of generalist gynecologic surgeons (three-way comparison reached statistical significance, with a P less than .0001).
Apical repair was performed by 96% of fellowship-trained surgeons, 82% of grandfathered surgeons, and 69% of generalist gynecologic surgeons (P less than .0001). Finally, cystoscopy was performed by 98% of fellowship-trained surgeons, 91% of grandfathered surgeons, and 72% of generalist gynecologic surgeons (P less than .0001).
When the researchers evaluated the cumulative performance of all measures in the same patient, fellowship-trained surgeons had the highest rates (89%, compared with 62% of grandfathered surgeons, and 39% of generalist gynecologic surgeons; P less than .0001).
“When we looked at the patient characteristics and their distribution across the surgeon training backgrounds, we found no significant differences in the age, BMI, gravidity, or parity of the subjects that underwent surgeries with the three groups,” Dr. Adams-Piper said.
She acknowledged certain limitations of the study, including the fact that it reflects clinical practice in a single health care delivery system, it relied on prior documentation, and it evaluated data from 2008.
“From this study we can conclude that perioperative practice patterns differ by surgeon training background,” she said. “However, in order for the proposed quality measures to be clinically meaningful, they must be correlated with patient-centered outcomes.”
Dr. Adams-Piper reported having no financial disclosures. The meeting was jointly sponsored by the American College of Surgeons.
INDIAN WELLS, CALIF. – Surgeons with fellowship training in female pelvic medicine and reconstructive surgery were significantly more likely to perform proposed quality measures at the time of hysterectomy for pelvic organ prolapse, compared with those who lack such training, a single-center study showed.
“The Physician Quality Reporting System was instituted as part of recent health care reform, with the aim of improving the reporting of quality measures, with the overall goal of improving the quality of care provided to patients throughout all areas of medicine,” Dr. Emily Adams-Piper said at the annual scientific meeting of the Society of Gynecologic Surgeons. “While there are many types of quality measures, including outcome measures and patient satisfaction measures, process measures may be the most directly applicable for the practicing clinician, because they provide recommended actions during specific patient encounters that can guide practice.”
Dr. Adams-Piper, a resident physician in the division of urogynecology at the University of California, Irvine, and her associates set out to investigate the use of proposed quality measures at the time of hysterectomy for pelvic organ prolapse (POP) among women receiving care from Southern California Permanente Medical Group, a large HMO.
They wanted to know if training background affected the rate of performance of four different quality measures related to hysterectomy for POP: offering conservative treatment prior to the surgical treatment of POP, quantitative assessment of POP with either a Baden-Walker or a POP-Q exam, apical support procedure performed at the time of hysterectomy for prolapse, and performance of intraoperative cystoscopy.
Patients who underwent hysterectomy for POP in 2008 were eligible for the study. The researchers reviewed electronic medical records for clinical and demographic data and categorized surgeons by their level of training.
“They were considered fellowship trained if they had pursued additional formal subspecialty training in female pelvic medicine and reconstructive surgery,” Dr. Adams-Piper explained. “Surgeons were considered grandfathered if they subsequently took the FPMRS [Female Pelvic Medicine and Reconstructive Surgery] boards when they became available in 2013. Surgeons were considered generalist if they fit into neither of these two categories and completed a residency in ob.gyn.”
Chi-squared tests were used to compare demographics and performance of the proposed quality measures. Of the 662 hysterectomies performed in 2008, 328 were included in the final analysis. The mean patient age was 60 years, the mean parity was 2.9, and the mean body mass index was 27.9 kg/m2.
Overall performance of the four proposed quality measures was high, ranging from 82%-87%. More than half of quality assessments (58%) were performed with the POP-Q exam, while the majority of apical support procedures were uterosacral ligament vault suspensions (67%), followed by sacrocolpopexy (18%), McCall culdoplasty (12%), and sacrospinous ligament fixation (3%).
When categorized by training, fellowship-trained surgeons performed 133 hysterectomies, “grandfathered” surgeons performed 55, and generalist gynecologic surgeons performed 140. Fellowship-trained surgeons performed each of the four proposed quality measures more often than did grandfathered surgeons, who performed them more often than generalist gynecologic surgeons did.
Specifically, conservative treatment was offered by 94% of fellowship-trained surgeons, 87% of grandfathered surgeons, and 76% of generalist gynecologic surgeons (P = .0002). Qualitative preoperative assessment of POP was performed by 99% of fellowship-trained surgeons, 93% of grandfathered surgeons, and 73% of generalist gynecologic surgeons (three-way comparison reached statistical significance, with a P less than .0001).
Apical repair was performed by 96% of fellowship-trained surgeons, 82% of grandfathered surgeons, and 69% of generalist gynecologic surgeons (P less than .0001). Finally, cystoscopy was performed by 98% of fellowship-trained surgeons, 91% of grandfathered surgeons, and 72% of generalist gynecologic surgeons (P less than .0001).
When the researchers evaluated the cumulative performance of all measures in the same patient, fellowship-trained surgeons had the highest rates (89%, compared with 62% of grandfathered surgeons, and 39% of generalist gynecologic surgeons; P less than .0001).
“When we looked at the patient characteristics and their distribution across the surgeon training backgrounds, we found no significant differences in the age, BMI, gravidity, or parity of the subjects that underwent surgeries with the three groups,” Dr. Adams-Piper said.
She acknowledged certain limitations of the study, including the fact that it reflects clinical practice in a single health care delivery system, it relied on prior documentation, and it evaluated data from 2008.
“From this study we can conclude that perioperative practice patterns differ by surgeon training background,” she said. “However, in order for the proposed quality measures to be clinically meaningful, they must be correlated with patient-centered outcomes.”
Dr. Adams-Piper reported having no financial disclosures. The meeting was jointly sponsored by the American College of Surgeons.
AT SGS 2016
Key clinical point: The level of surgical training impacted performance of proposed quality measures at the time of hysterectomy for pelvic organ prolapse.
Major finding: Fellowship-trained surgeons in female pelvic medicine and reconstructive surgery were significantly more likely to perform proposed quality measures relating to hysterectomy for pelvic organ prolapse at 89%, compared with 39% of generalist gynecologic surgeons.
Data source: A review 328 hysterectomies performed in 2008 by surgeons in a Southern California HMO.
Disclosures: Dr. Adams-Piper reported having no financial disclosures.
Scans show high prevalence of TBI among symptomatic retired NFL players
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
VANCOUVER – Many retired National Football League (NFL) players seeking care for neurocognitive symptoms have MRI evidence of traumatic brain injury, according to the largest study of this issue among living players.
Data reported at the annual meeting of the American Academy of Neurology show that 43% of a cohort of 40 symptomatic NFL retirees had abnormal results on diffusion tensor MRI and 30% had evidence of traumatic axonal injury on conventional MRI.
The likelihood of abnormal diffusion tensor MRI results was correlated, albeit weakly, with the length of the player’s NFL career, but not with the number of concussions sustained.
“It appears that subconcussive hits – that is, the cumulative effects and longer playing careers – place retired alumni at risk for abnormal diffusion tensor MRI,” commented lead author Dr. Francis X. Conidi, director of the Florida Center for Headache & Sports Neurology in Palm Beach and team neurologist for the Florida Panthers of the National Hockey League.
“This could be a possible link to chronic traumatic encephalopathy, as consensus is you need to have repetitive head trauma,” he proposed. “Or this could be a separate entity whereby, in NFL players, the symptoms we are seeing are actually related to the traumatic brain injury itself and [in a subset] with some genetic predisposition, they will go on to have progressive neurological decline.”
Although the cohort was quite young, only 39 years old on average, some had likely played football since youth, and that has implications for prevention, Dr. Conidi added in an interview. “One thing we need to consider is limiting the amount of contact that these people receive on a cumulative basis, starting when they are young and starting in practice, because that’s where most of the contact occurs,” he recommended.
“It is important to note that diffusion tensor imaging is not a routine part of a brain MRI study,” session moderator Dr. José E. Cavazos, professor of neurology and assistant dean at the University of Texas Health Science Center in San Antonio, said in comments provided by email. “The significant correlation between duration of years played and abnormalities in diffusion tensor imaging is of concern given the popularity of the sport.”
“The next step is to replicate the findings, but more importantly, it is to find surrogate markers for early detection for these abnormalities, aiming to intervene (sideline) those players at greater risk for developing cognitive deficits or other impairments,” he added.
In the study, the retired players had a battery of neuropsychological and imaging examinations and tests over a period of 2 days. They were classified as having abnormal diffusion tensor MRI results if they had fractional anisotropy (FA) values at least 2.5 standard deviations below those of age-matched peers in a normative database for specific regions of interest in the brain.
The players ranged in age from 27 to 56 years. On average, they had played 7 years in the NFL and sustained eight concussions during that time. Most had retired in the past 5 years.
Results showed that, overall, 43% had abnormal diffusion tensor MRI results, Dr. Conidi reported. Prevalence, however, varied according to player position: It was highest for defensive linemen (64%) and wide receivers (60%); intermediate for running backs (43%), defensive backs (33%), and offensive linemen (29%); and lowest for quarterbacks (0%) and linebackers (0%).
The number of years played was significantly correlated with abnormal results (P = .049), but the number of concussions was not.
In other findings, sizable proportions of the players had significant abnormalities in attention and concentration (43%), executive function (54%), learning and memory (46%), spatial and perceptual function (24%), and language (5%).
“These guys have played these positions probably since they were young. This isn’t just NFL. We don’t make any claims that professional football caused this,” Dr. Conidi emphasized.
As for future research, the investigators plan to undertake PET scanning to assess clinical and laboratory evidence of Alzheimer’s disease, study sleep pathology, and look for tau protein (a marker for chronic traumatic encephalopathy) in cerebrospinal fluid. Additionally, they will assess treatment outcomes.
The study is not without limitations, Dr. Conidi acknowledged. “With every study that has ever been done on these guys, it is a skewed population: They are coming to us and they are looking to be evaluated,” he elaborated. “The other issue is we don’t have a normative comparison database for the neuropsychological testing.”
Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: MRI findings suggest that traumatic brain injury is prevalent among symptomatic retired NFL players.
Major finding: Overall, 43% of the players had abnormal diffusion tensor MRI results and 30% had evidence of traumatic axonal injury on conventional MRI.
Data source: A prospective cohort study of 40 retired NFL players who sought care for neurocognitive symptoms.
Disclosures: Dr. Conidi disclosed that he is a consultant for the NFL, NHL, USTA, PGA, and NCAA and that he receives research support from the Seeing Stars Foundation.
The Impact of Fellowship Training on Scholarly Productivity in Academic Dermatology
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).
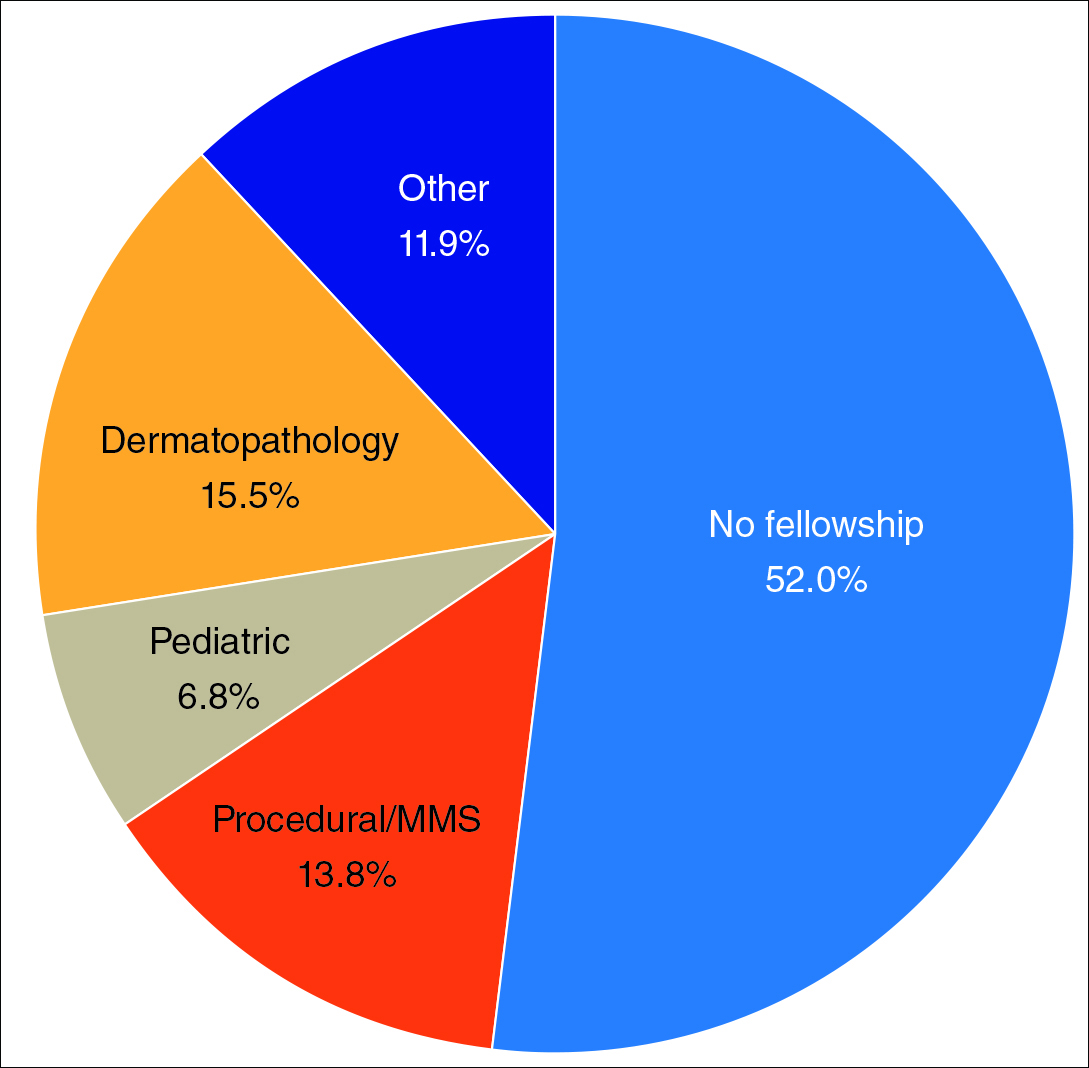
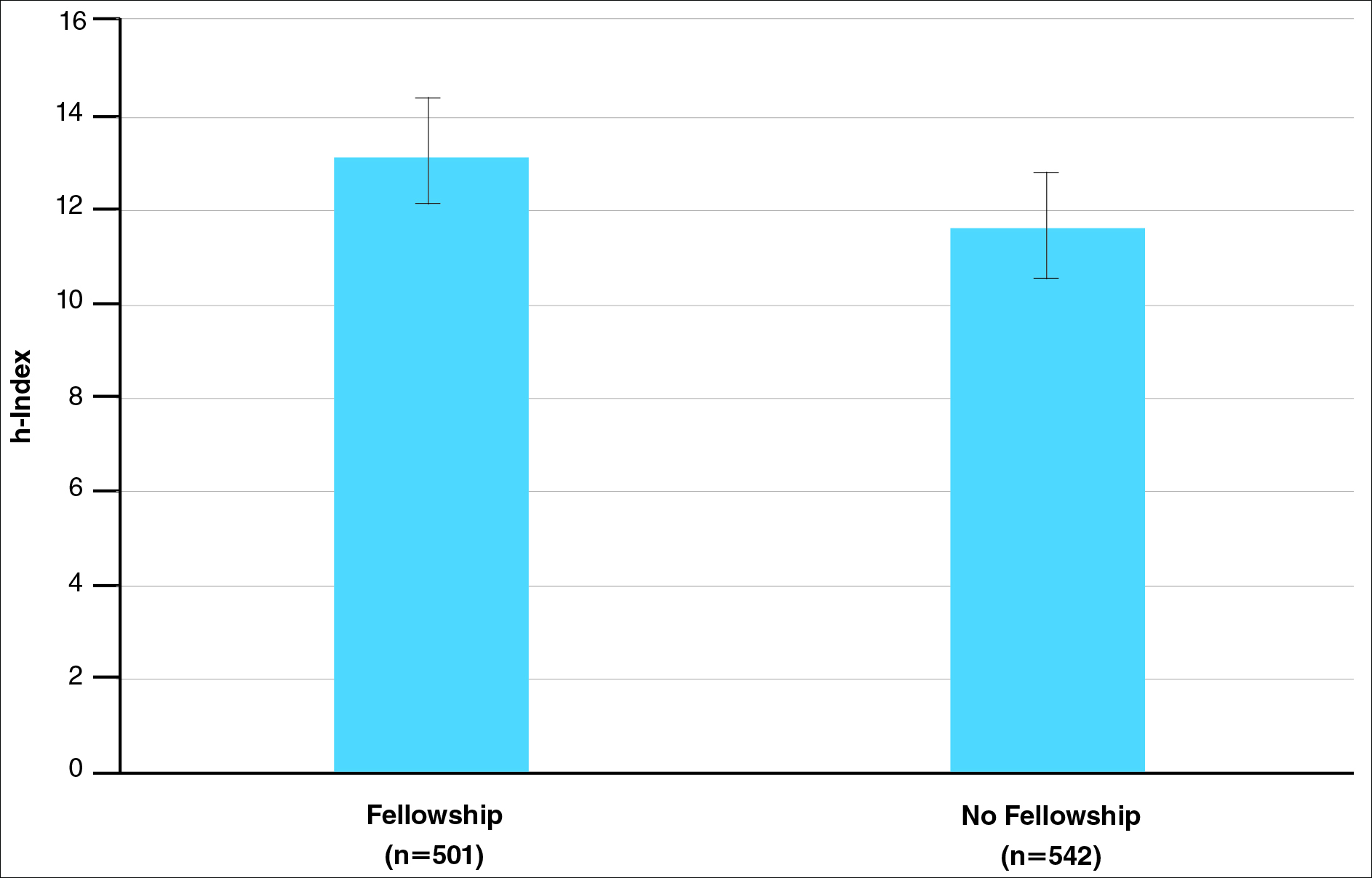
There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.
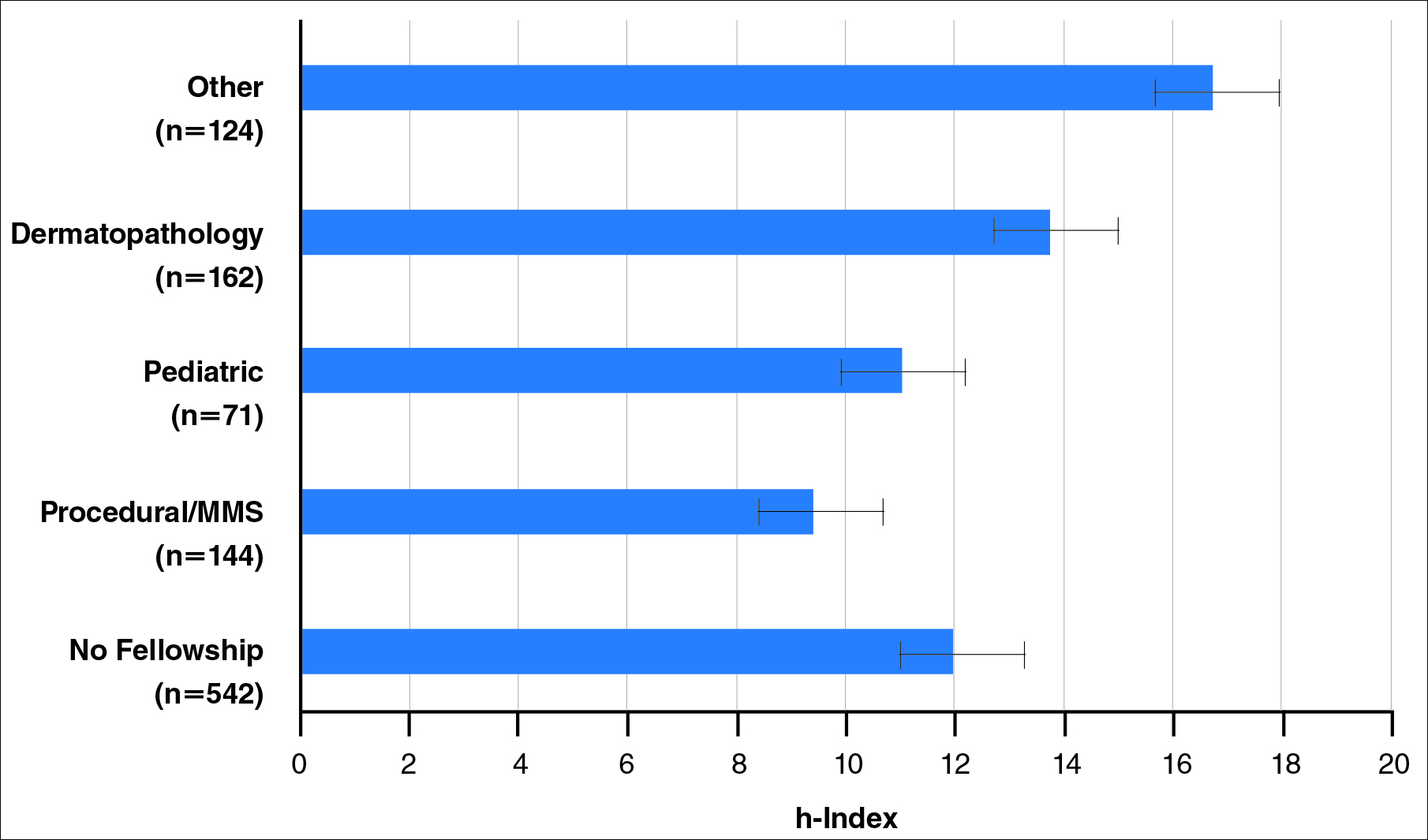
Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.
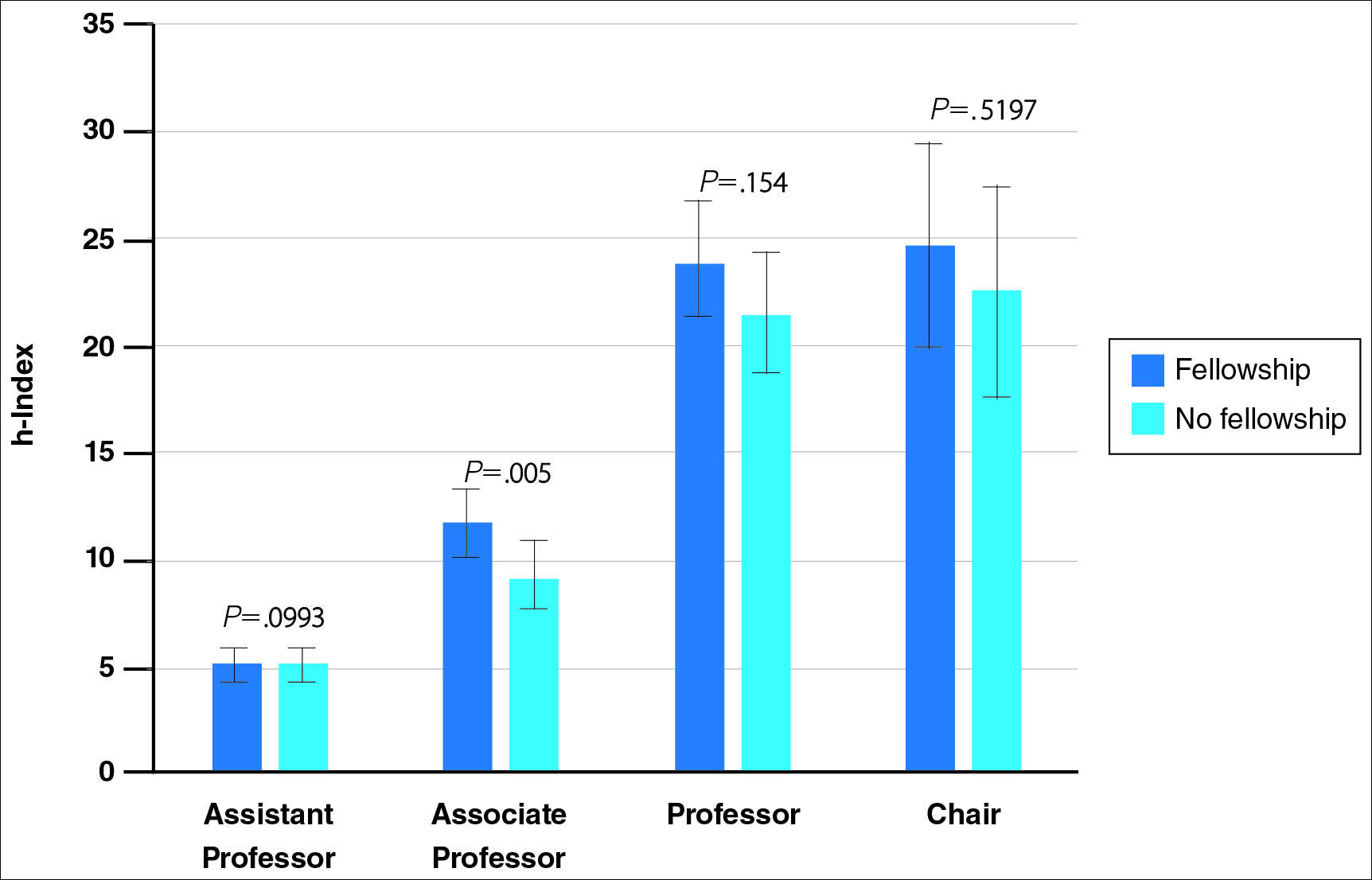
When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).
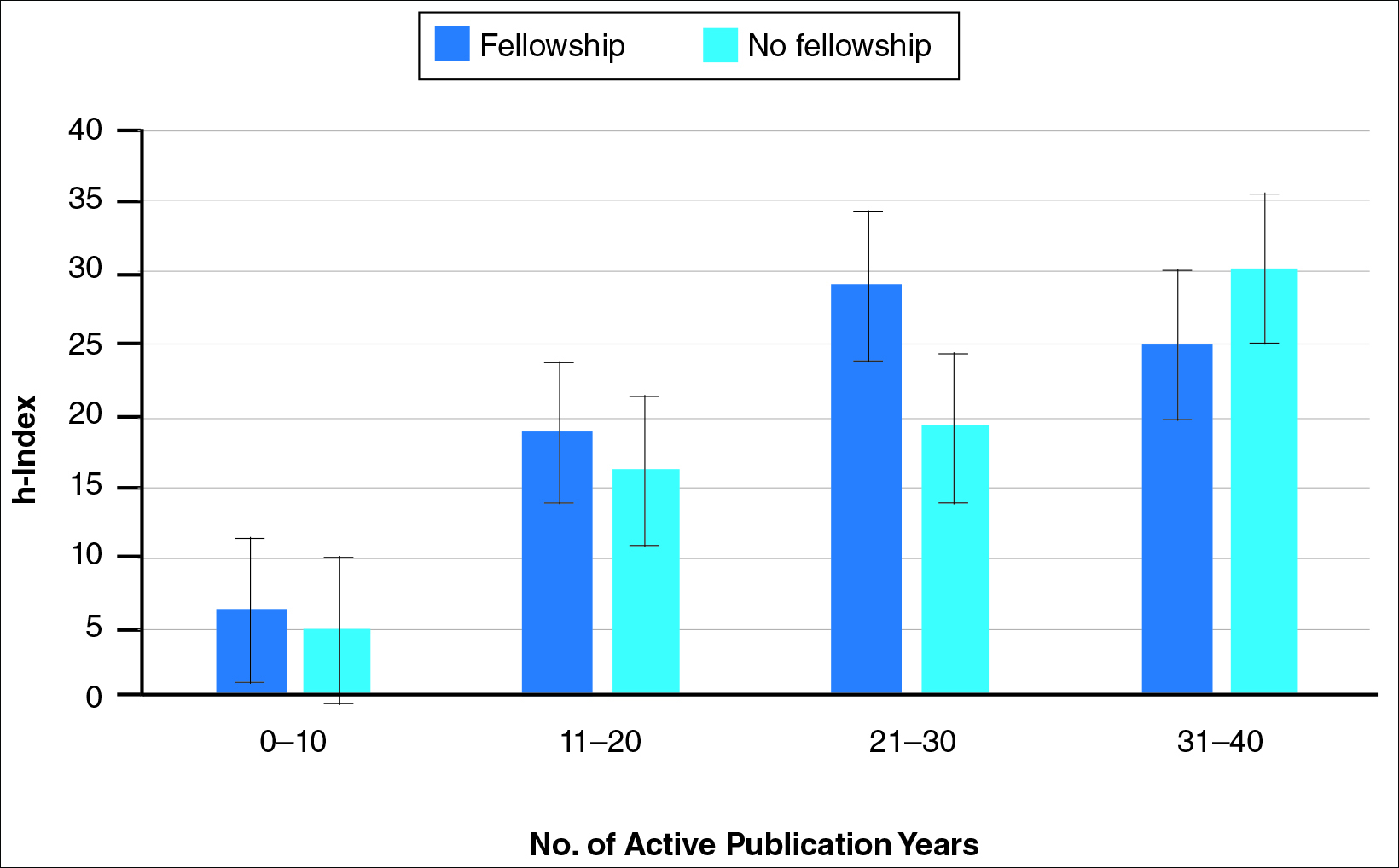
Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).


There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.

Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.

When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).

Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).


There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.

Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.

When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).

Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
Practice Points
- As residents decide whether to pursue fellowship training, it is important to consider the importance of fellowship completion for academic promotion and productivity.
- Although there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists, this difference is minimized when controlling for academic rank and publication range.
- Fellowships may provide more opportunity for structured research experiences but may not be necessary for successful careers in academic dermatology.
Fewer new lesions, side effects differentiate fingolimod from dimethyl fumarate
VANCOUVER – Multiple sclerosis patients discontinued treatment and relapsed earlier with dimethyl fumarate (Tecfidera) than with fingolimod (Gilenya), and had more gadolinium-enhancing lesions at 12 months, in a propensity score matching analysis involving 775 patients at the Cleveland Clinic.
“Based on these data, I now [favor] Gilenya over Tecfidera; Gilenya works a little bit better,” lead investigator and staff neurologist Carrie Hersh said at the annual meeting of the American Academy of Neurology.
The two drugs performed about equally in clinical trials, but Dr. Hersh and her colleagues said fingolimod seems to have the edge in clinical practice; they wanted to see if that hunch held up to scrutiny.
In the single-center cohort study, about 30% of the 458 dimethyl fumarate patients discontinued the drug after an average of 4 months, and about 14% relapsed within a year of starting it. About a quarter of the 317 fingolimod patients discontinued at an average of 6.5 months, and 11% relapsed. About 9% of dimethyl fumarate patients, but 6% of fingolimod patients, had new gadolinium-enhancing (GdE) brain lesions at 12 months.
A propensity score analysis was performed to control for confounders; patients were matched one to one for baseline demographics and clinical and MRI characteristics. Dimethyl fumarate patients were almost three times more likely than fingolimod patients to have new GdE lesions after a year (odds ratio, 2.90; 95% confidence interval, 1.24-6.57). They also had an earlier time to discontinuation (OR, 1.35; 95% CI, 1.05-1.74) and clinical relapse (OR, 1.64; 95% CI, 1.10-2.46). The study included patients with secondary progressive disease. Results were the same when the analysis was limited to relapsing-remitting multiple sclerosis.
The investigators concluded that “dimethyl fumarate and fingolimod have comparable annualized relapse rates, overall brain MRI activity, and discontinuation at 12 months.” However, “dimethyl fumarate may have greater GdE lesions and side effects early after treatment initiation, leading to early discontinuation and relapses.
“This makes sense from what we are seeing in the clinic. We know Tecfidera patients have tolerability issues,” especially with gastrointestinal and flushing events, “so they discontinue earlier or might not be as adherent, and so they relapse earlier. The new enhancing lesions might be a difference in efficacy,” Dr. Hersh said.
Patients treated with fingolimod were more likely to be white (91% vs. 83%), have a longer disease duration (16 vs. 14 years), have a higher proportion of relapsing-remitting disease (82% vs. 74%), and have more severe baseline brain lesion burden (15% vs. 8%). The subjects had tried interferon, glatiramer acetate (Copaxone), natalizumab (Tysabri), or other options before being switched to the study medications because of disease activity or intolerability. Patients were in their 40s, on average, and about 70% were women.
Data are now being collected for a 2-year analysis.
There was no industry funding for the work, and Dr. Hersh had no disclosures. Other investigators reported ties to both Novartis, the maker of Gilenya, and Biogen, the maker of Tecfidera.
VANCOUVER – Multiple sclerosis patients discontinued treatment and relapsed earlier with dimethyl fumarate (Tecfidera) than with fingolimod (Gilenya), and had more gadolinium-enhancing lesions at 12 months, in a propensity score matching analysis involving 775 patients at the Cleveland Clinic.
“Based on these data, I now [favor] Gilenya over Tecfidera; Gilenya works a little bit better,” lead investigator and staff neurologist Carrie Hersh said at the annual meeting of the American Academy of Neurology.
The two drugs performed about equally in clinical trials, but Dr. Hersh and her colleagues said fingolimod seems to have the edge in clinical practice; they wanted to see if that hunch held up to scrutiny.
In the single-center cohort study, about 30% of the 458 dimethyl fumarate patients discontinued the drug after an average of 4 months, and about 14% relapsed within a year of starting it. About a quarter of the 317 fingolimod patients discontinued at an average of 6.5 months, and 11% relapsed. About 9% of dimethyl fumarate patients, but 6% of fingolimod patients, had new gadolinium-enhancing (GdE) brain lesions at 12 months.
A propensity score analysis was performed to control for confounders; patients were matched one to one for baseline demographics and clinical and MRI characteristics. Dimethyl fumarate patients were almost three times more likely than fingolimod patients to have new GdE lesions after a year (odds ratio, 2.90; 95% confidence interval, 1.24-6.57). They also had an earlier time to discontinuation (OR, 1.35; 95% CI, 1.05-1.74) and clinical relapse (OR, 1.64; 95% CI, 1.10-2.46). The study included patients with secondary progressive disease. Results were the same when the analysis was limited to relapsing-remitting multiple sclerosis.
The investigators concluded that “dimethyl fumarate and fingolimod have comparable annualized relapse rates, overall brain MRI activity, and discontinuation at 12 months.” However, “dimethyl fumarate may have greater GdE lesions and side effects early after treatment initiation, leading to early discontinuation and relapses.
“This makes sense from what we are seeing in the clinic. We know Tecfidera patients have tolerability issues,” especially with gastrointestinal and flushing events, “so they discontinue earlier or might not be as adherent, and so they relapse earlier. The new enhancing lesions might be a difference in efficacy,” Dr. Hersh said.
Patients treated with fingolimod were more likely to be white (91% vs. 83%), have a longer disease duration (16 vs. 14 years), have a higher proportion of relapsing-remitting disease (82% vs. 74%), and have more severe baseline brain lesion burden (15% vs. 8%). The subjects had tried interferon, glatiramer acetate (Copaxone), natalizumab (Tysabri), or other options before being switched to the study medications because of disease activity or intolerability. Patients were in their 40s, on average, and about 70% were women.
Data are now being collected for a 2-year analysis.
There was no industry funding for the work, and Dr. Hersh had no disclosures. Other investigators reported ties to both Novartis, the maker of Gilenya, and Biogen, the maker of Tecfidera.
VANCOUVER – Multiple sclerosis patients discontinued treatment and relapsed earlier with dimethyl fumarate (Tecfidera) than with fingolimod (Gilenya), and had more gadolinium-enhancing lesions at 12 months, in a propensity score matching analysis involving 775 patients at the Cleveland Clinic.
“Based on these data, I now [favor] Gilenya over Tecfidera; Gilenya works a little bit better,” lead investigator and staff neurologist Carrie Hersh said at the annual meeting of the American Academy of Neurology.
The two drugs performed about equally in clinical trials, but Dr. Hersh and her colleagues said fingolimod seems to have the edge in clinical practice; they wanted to see if that hunch held up to scrutiny.
In the single-center cohort study, about 30% of the 458 dimethyl fumarate patients discontinued the drug after an average of 4 months, and about 14% relapsed within a year of starting it. About a quarter of the 317 fingolimod patients discontinued at an average of 6.5 months, and 11% relapsed. About 9% of dimethyl fumarate patients, but 6% of fingolimod patients, had new gadolinium-enhancing (GdE) brain lesions at 12 months.
A propensity score analysis was performed to control for confounders; patients were matched one to one for baseline demographics and clinical and MRI characteristics. Dimethyl fumarate patients were almost three times more likely than fingolimod patients to have new GdE lesions after a year (odds ratio, 2.90; 95% confidence interval, 1.24-6.57). They also had an earlier time to discontinuation (OR, 1.35; 95% CI, 1.05-1.74) and clinical relapse (OR, 1.64; 95% CI, 1.10-2.46). The study included patients with secondary progressive disease. Results were the same when the analysis was limited to relapsing-remitting multiple sclerosis.
The investigators concluded that “dimethyl fumarate and fingolimod have comparable annualized relapse rates, overall brain MRI activity, and discontinuation at 12 months.” However, “dimethyl fumarate may have greater GdE lesions and side effects early after treatment initiation, leading to early discontinuation and relapses.
“This makes sense from what we are seeing in the clinic. We know Tecfidera patients have tolerability issues,” especially with gastrointestinal and flushing events, “so they discontinue earlier or might not be as adherent, and so they relapse earlier. The new enhancing lesions might be a difference in efficacy,” Dr. Hersh said.
Patients treated with fingolimod were more likely to be white (91% vs. 83%), have a longer disease duration (16 vs. 14 years), have a higher proportion of relapsing-remitting disease (82% vs. 74%), and have more severe baseline brain lesion burden (15% vs. 8%). The subjects had tried interferon, glatiramer acetate (Copaxone), natalizumab (Tysabri), or other options before being switched to the study medications because of disease activity or intolerability. Patients were in their 40s, on average, and about 70% were women.
Data are now being collected for a 2-year analysis.
There was no industry funding for the work, and Dr. Hersh had no disclosures. Other investigators reported ties to both Novartis, the maker of Gilenya, and Biogen, the maker of Tecfidera.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Multiple sclerosis patients discontinued treatment and relapsed earlier when treated with dimethyl fumarate (Tecfidera) than with fingolimod (Gilenya), and had more gadolinium-enhancing lesions at 12 months.
Major finding: Dimethyl fumarate patients were almost three times more likely than fingolimod patients to have new GdE lesions after a year (OR, 2.90; 95% CI, 1.24-6.57). They also had an earlier time to discontinuation (OR, 1.35; 95% CI, 1.05-1.74) and clinical relapse (OR, 1.64; 95% CI, 1.10-2.46).
Data source: A propensity score matching analysis involving 775 multiple sclerosis patients at the Cleveland Clinic.
Disclosures: There was no industry funding for the work, and the lead investigator had no disclosures. Other investigators reported ties to both Novartis, the maker of Gilenya, and Biogen, the maker of Tecfidera.