User login
Ovarian hyperstimulation syndrome as a complication of molar pregnancy
An 18-year-old woman, pregnant for the third time, presented to the emergency department with constant vaginal bleeding and intermittent cramping for the past 3 weeks. Her last menstrual period was 14 weeks and 2 days ago. In her previous two pregnancies, she had given birth to one living child and had had one miscarriage.
Physical examination suggested that her uterus was bigger than expected for the gestational age, measuring 23 cm from the symphysis pubis to the uterine fundus. Ultrasonography in the obstetrics service revealed a “snowstorm” appearance strongly suggestive of molar pregnancy. Her level of beta human chorionic gonadotropin (beta-hCG) was greater than 1,125,000 mIU/mL (reference range for 14 weeks of pregnancy 18,300–137,000). Dilation and curettage was performed, and pathologic study confirmed molar pregnancy.
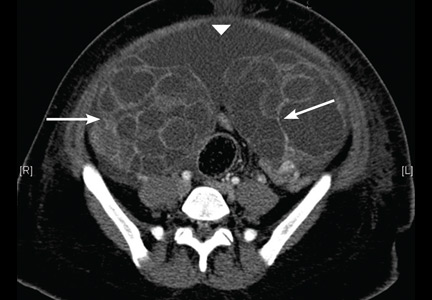
On the 6th day after the procedure, she returned to the emergency department with progressive abdominal pain, distention, and nausea. Her blood urea nitrogen level was 8 mg/dL (reference range 5–20 mg/dL) and her serum creatinine level was 0.5 mg/dL (0.5–0.9). Computed tomography of the abdomen and pelvis demonstrated an enlarged and bulky uterus with heterogeneous enhancement. The ovaries were greatly enlarged with multiple cysts, and massive ascites was noted in the abdomen (Figures 1 and 2). These findings confirmed the diagnosis of ovarian hyperstimulation syndrome (OHSS).
OVARIAN HYPERSTIMULATION SYNDROME
OHSS is enlargement of the ovaries associated with fluid shifts secondary to ovulation induction therapy with clomiphene citrate or hCG.1 In its mild form, it is a common complication, seen in 5% to 10% of patients undergoing ovulation induction; the moderate form is reported in 2% to 4% of patients undergoing ovulation induction, and the severe form in 0.1% to 0.5%.2 It may also occur spontaneously after pregnancy or with any condition that leads to a rise in hCG levels.
Factors associated with a high risk of developing OHSS include young age, low body weight, polycystic ovary syndrome, a high serum estradiol level, and a history of OHSS.3,4
In our patient, OHSS was secondary to molar pregnancy and markedly elevated hCG levels. Hydatidiform mole or molar pregnancy is a cystic swelling of the chorionic villi and proliferation of the trophoblastic epithelium. Elevated circulating hCG is thought to lead to ovarian enlargement and multiple cysts; this stimulates the ovaries to secrete vasoactive substances, increasing vascular permeability, leading to fluid shifts and the accumulation of extravascular fluid, resulting in renal failure, hypovolemic shock, ascites, and pleural and pericardial effusions.5 This acute shift produces hypovolemia, which may result in multiple organ failure, hemoconcentration (hematocrit > 45%), thrombosis, and disseminated intravascular coagulation from the increased viscosity of the blood.
GRADING OF OHSS IS BASED ON SYMPTOMS, TEST RESULTS, IMAGING
The severity of OHSS is classified as mild, moderate, or severe, with further grading as follows5,6:
Mild OHSS
- Grade 1: abdominal distention and discomfort.
- Grade 2: features of grade 1, plus nausea and vomiting, with or without diarrhea, and ovarian size of 5 to 12 cm.
Moderate OHSS
- Grade 3: mild OHSS with imaging evidence of ascites.
Severe OHSS
- Grade 4: moderate OHSS plus clinical evidence of ascites, with or without hydrothorax.
- Grade 5: all of the above plus hypovolemia, hemoconcentration (hematocrit > 45%), coagulation abnormalities, and oliguria.
- Grade 6: all the features of grades 1 to 4 plus hypovolemia, hemoconcentration (hematocrit > 55%), anuria, renal failure, venous thrombosis, and adult respiratory distress syndrome. This can be life-threatening and may require hospitalization.
TREATMENT
Treatment is generally conservative and includes management of ascites and pleural effusion and supportive care.
Mild OHSS can be treated on an outpatient basis with bed rest, oral analgesics, limited oral intake, and avoidance of vaginal intercourse, and usually resolves in 10 to 14 days. Moderate and severe OHSS require bed rest and aggressive fluid resuscitation. OHSS in patients with renal failure, relentless hemoconcentration, or thrombovascular accident can be life-threatening and may require intensive-care monitoring.
Paracentesis may be performed if tension ascites and oliguria or anuria develop.2 Prophylactic anticoagulation with warfarin, heparin, or low-molecular-weight heparin is indicated in women with a high tendency for thrombotic events who develop moderate to severe OHSS.3,4
Surgical intervention may be necessary in patients with ectopic pregnancy, ovarian torsion, or ruptured ovarian cyst.
Our patient was treated conservatively with supportive care and experienced a full recovery.
- Arora R, Merhi ZO, Khulpateea N, Roth D, Minkoff H. Ovarian hyperstimulation syndrome after a molar pregnancy evacuation. Fertil Steril 2008; 90:1197.e5–e7.
- Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol 2012; 10:32.
- Mor YS, Schenker JG. Ovarian hyperstimulation syndrome and thrombotic events. Am J Reprod Immunol 2014; 72:541–548.
- Practice Committee of American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril 2008; 90(suppl):S188–S193.
- Whelan JG 3rd, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril 2000; 73:883–896.
- Golan A, Weissman A. Symposium: update on prediction and management of OHSS. A modern classification of OHSS. Reprod Biomed Online 2009; 19:28–32.
An 18-year-old woman, pregnant for the third time, presented to the emergency department with constant vaginal bleeding and intermittent cramping for the past 3 weeks. Her last menstrual period was 14 weeks and 2 days ago. In her previous two pregnancies, she had given birth to one living child and had had one miscarriage.
Physical examination suggested that her uterus was bigger than expected for the gestational age, measuring 23 cm from the symphysis pubis to the uterine fundus. Ultrasonography in the obstetrics service revealed a “snowstorm” appearance strongly suggestive of molar pregnancy. Her level of beta human chorionic gonadotropin (beta-hCG) was greater than 1,125,000 mIU/mL (reference range for 14 weeks of pregnancy 18,300–137,000). Dilation and curettage was performed, and pathologic study confirmed molar pregnancy.
On the 6th day after the procedure, she returned to the emergency department with progressive abdominal pain, distention, and nausea. Her blood urea nitrogen level was 8 mg/dL (reference range 5–20 mg/dL) and her serum creatinine level was 0.5 mg/dL (0.5–0.9). Computed tomography of the abdomen and pelvis demonstrated an enlarged and bulky uterus with heterogeneous enhancement. The ovaries were greatly enlarged with multiple cysts, and massive ascites was noted in the abdomen (Figures 1 and 2). These findings confirmed the diagnosis of ovarian hyperstimulation syndrome (OHSS).
OVARIAN HYPERSTIMULATION SYNDROME
OHSS is enlargement of the ovaries associated with fluid shifts secondary to ovulation induction therapy with clomiphene citrate or hCG.1 In its mild form, it is a common complication, seen in 5% to 10% of patients undergoing ovulation induction; the moderate form is reported in 2% to 4% of patients undergoing ovulation induction, and the severe form in 0.1% to 0.5%.2 It may also occur spontaneously after pregnancy or with any condition that leads to a rise in hCG levels.
Factors associated with a high risk of developing OHSS include young age, low body weight, polycystic ovary syndrome, a high serum estradiol level, and a history of OHSS.3,4
In our patient, OHSS was secondary to molar pregnancy and markedly elevated hCG levels. Hydatidiform mole or molar pregnancy is a cystic swelling of the chorionic villi and proliferation of the trophoblastic epithelium. Elevated circulating hCG is thought to lead to ovarian enlargement and multiple cysts; this stimulates the ovaries to secrete vasoactive substances, increasing vascular permeability, leading to fluid shifts and the accumulation of extravascular fluid, resulting in renal failure, hypovolemic shock, ascites, and pleural and pericardial effusions.5 This acute shift produces hypovolemia, which may result in multiple organ failure, hemoconcentration (hematocrit > 45%), thrombosis, and disseminated intravascular coagulation from the increased viscosity of the blood.
GRADING OF OHSS IS BASED ON SYMPTOMS, TEST RESULTS, IMAGING
The severity of OHSS is classified as mild, moderate, or severe, with further grading as follows5,6:
Mild OHSS
- Grade 1: abdominal distention and discomfort.
- Grade 2: features of grade 1, plus nausea and vomiting, with or without diarrhea, and ovarian size of 5 to 12 cm.
Moderate OHSS
- Grade 3: mild OHSS with imaging evidence of ascites.
Severe OHSS
- Grade 4: moderate OHSS plus clinical evidence of ascites, with or without hydrothorax.
- Grade 5: all of the above plus hypovolemia, hemoconcentration (hematocrit > 45%), coagulation abnormalities, and oliguria.
- Grade 6: all the features of grades 1 to 4 plus hypovolemia, hemoconcentration (hematocrit > 55%), anuria, renal failure, venous thrombosis, and adult respiratory distress syndrome. This can be life-threatening and may require hospitalization.
TREATMENT
Treatment is generally conservative and includes management of ascites and pleural effusion and supportive care.
Mild OHSS can be treated on an outpatient basis with bed rest, oral analgesics, limited oral intake, and avoidance of vaginal intercourse, and usually resolves in 10 to 14 days. Moderate and severe OHSS require bed rest and aggressive fluid resuscitation. OHSS in patients with renal failure, relentless hemoconcentration, or thrombovascular accident can be life-threatening and may require intensive-care monitoring.
Paracentesis may be performed if tension ascites and oliguria or anuria develop.2 Prophylactic anticoagulation with warfarin, heparin, or low-molecular-weight heparin is indicated in women with a high tendency for thrombotic events who develop moderate to severe OHSS.3,4
Surgical intervention may be necessary in patients with ectopic pregnancy, ovarian torsion, or ruptured ovarian cyst.
Our patient was treated conservatively with supportive care and experienced a full recovery.
An 18-year-old woman, pregnant for the third time, presented to the emergency department with constant vaginal bleeding and intermittent cramping for the past 3 weeks. Her last menstrual period was 14 weeks and 2 days ago. In her previous two pregnancies, she had given birth to one living child and had had one miscarriage.
Physical examination suggested that her uterus was bigger than expected for the gestational age, measuring 23 cm from the symphysis pubis to the uterine fundus. Ultrasonography in the obstetrics service revealed a “snowstorm” appearance strongly suggestive of molar pregnancy. Her level of beta human chorionic gonadotropin (beta-hCG) was greater than 1,125,000 mIU/mL (reference range for 14 weeks of pregnancy 18,300–137,000). Dilation and curettage was performed, and pathologic study confirmed molar pregnancy.
On the 6th day after the procedure, she returned to the emergency department with progressive abdominal pain, distention, and nausea. Her blood urea nitrogen level was 8 mg/dL (reference range 5–20 mg/dL) and her serum creatinine level was 0.5 mg/dL (0.5–0.9). Computed tomography of the abdomen and pelvis demonstrated an enlarged and bulky uterus with heterogeneous enhancement. The ovaries were greatly enlarged with multiple cysts, and massive ascites was noted in the abdomen (Figures 1 and 2). These findings confirmed the diagnosis of ovarian hyperstimulation syndrome (OHSS).
OVARIAN HYPERSTIMULATION SYNDROME
OHSS is enlargement of the ovaries associated with fluid shifts secondary to ovulation induction therapy with clomiphene citrate or hCG.1 In its mild form, it is a common complication, seen in 5% to 10% of patients undergoing ovulation induction; the moderate form is reported in 2% to 4% of patients undergoing ovulation induction, and the severe form in 0.1% to 0.5%.2 It may also occur spontaneously after pregnancy or with any condition that leads to a rise in hCG levels.
Factors associated with a high risk of developing OHSS include young age, low body weight, polycystic ovary syndrome, a high serum estradiol level, and a history of OHSS.3,4
In our patient, OHSS was secondary to molar pregnancy and markedly elevated hCG levels. Hydatidiform mole or molar pregnancy is a cystic swelling of the chorionic villi and proliferation of the trophoblastic epithelium. Elevated circulating hCG is thought to lead to ovarian enlargement and multiple cysts; this stimulates the ovaries to secrete vasoactive substances, increasing vascular permeability, leading to fluid shifts and the accumulation of extravascular fluid, resulting in renal failure, hypovolemic shock, ascites, and pleural and pericardial effusions.5 This acute shift produces hypovolemia, which may result in multiple organ failure, hemoconcentration (hematocrit > 45%), thrombosis, and disseminated intravascular coagulation from the increased viscosity of the blood.
GRADING OF OHSS IS BASED ON SYMPTOMS, TEST RESULTS, IMAGING
The severity of OHSS is classified as mild, moderate, or severe, with further grading as follows5,6:
Mild OHSS
- Grade 1: abdominal distention and discomfort.
- Grade 2: features of grade 1, plus nausea and vomiting, with or without diarrhea, and ovarian size of 5 to 12 cm.
Moderate OHSS
- Grade 3: mild OHSS with imaging evidence of ascites.
Severe OHSS
- Grade 4: moderate OHSS plus clinical evidence of ascites, with or without hydrothorax.
- Grade 5: all of the above plus hypovolemia, hemoconcentration (hematocrit > 45%), coagulation abnormalities, and oliguria.
- Grade 6: all the features of grades 1 to 4 plus hypovolemia, hemoconcentration (hematocrit > 55%), anuria, renal failure, venous thrombosis, and adult respiratory distress syndrome. This can be life-threatening and may require hospitalization.
TREATMENT
Treatment is generally conservative and includes management of ascites and pleural effusion and supportive care.
Mild OHSS can be treated on an outpatient basis with bed rest, oral analgesics, limited oral intake, and avoidance of vaginal intercourse, and usually resolves in 10 to 14 days. Moderate and severe OHSS require bed rest and aggressive fluid resuscitation. OHSS in patients with renal failure, relentless hemoconcentration, or thrombovascular accident can be life-threatening and may require intensive-care monitoring.
Paracentesis may be performed if tension ascites and oliguria or anuria develop.2 Prophylactic anticoagulation with warfarin, heparin, or low-molecular-weight heparin is indicated in women with a high tendency for thrombotic events who develop moderate to severe OHSS.3,4
Surgical intervention may be necessary in patients with ectopic pregnancy, ovarian torsion, or ruptured ovarian cyst.
Our patient was treated conservatively with supportive care and experienced a full recovery.
- Arora R, Merhi ZO, Khulpateea N, Roth D, Minkoff H. Ovarian hyperstimulation syndrome after a molar pregnancy evacuation. Fertil Steril 2008; 90:1197.e5–e7.
- Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol 2012; 10:32.
- Mor YS, Schenker JG. Ovarian hyperstimulation syndrome and thrombotic events. Am J Reprod Immunol 2014; 72:541–548.
- Practice Committee of American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril 2008; 90(suppl):S188–S193.
- Whelan JG 3rd, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril 2000; 73:883–896.
- Golan A, Weissman A. Symposium: update on prediction and management of OHSS. A modern classification of OHSS. Reprod Biomed Online 2009; 19:28–32.
- Arora R, Merhi ZO, Khulpateea N, Roth D, Minkoff H. Ovarian hyperstimulation syndrome after a molar pregnancy evacuation. Fertil Steril 2008; 90:1197.e5–e7.
- Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol 2012; 10:32.
- Mor YS, Schenker JG. Ovarian hyperstimulation syndrome and thrombotic events. Am J Reprod Immunol 2014; 72:541–548.
- Practice Committee of American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril 2008; 90(suppl):S188–S193.
- Whelan JG 3rd, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril 2000; 73:883–896.
- Golan A, Weissman A. Symposium: update on prediction and management of OHSS. A modern classification of OHSS. Reprod Biomed Online 2009; 19:28–32.
A female liver transplant recipient asks: Can I become pregnant?
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
Yes, pregnancy is possible, but not immediately after transplant, and it involves risks. Appropriate management and multidisciplinary care are necessary to optimize the outcomes.
HOW LONG SHOULD PREGNANCY BE POSTPONED?
Hypogonadism and amenorrhea are common and multifactorial in women with end-stage liver disease. Hypogonadotrophic hypogonadism, elevated estrogen level, and malnutrition all contribute to the problem.1 However, most premenopausal women experience a return of their menstrual cycle, and possibly of fertility, within the first 10 months after liver transplant,2,3 after which pregnancy is possible.
In transplant recipients of childbearing age, the need for preconception counseling and family planning should be emphasized. The timing, potential risks, and outcomes of pregnancy, and the importance of coordinated prenatal and perinatal care should be addressed.4 The National Transplant Pregnancy Registry guidelines recommend postponing conception until:
- At least 1 year has elapsed after transplant
- Graft function is stable
- Medical comorbidities such as diabetes and hypertension are well controlled
- Immunosuppression is at a low maintenance level.3
Strong evidence suggests that an appropriate liver transplant-conception interval reduces adverse maternal and fetal outcomes. In particular, the risks of a low birth weight, graft rejection, and loss during pregnancy are significantly decreased.3 Therefore, contraception must be initiated after transplant before any sexual activity, with no preference as to the form of protection used.
Limited data demonstrate the safety and efficacy of combined oral contraceptives and transdermal contraceptive patches in stable solid-organ recipients.5,6 Estrogen-containing contraceptives should, however, be avoided in recurrent liver disease after transplant because of the risk of increased hepatic toxicity.
MANAGING RISKS ASSOCIATED WITH PREGNANCY
Physicians should be alert to the possibility of a pregnancy. Early diagnosis allows the optimization of management and outcomes, as complications are increased in this population of expectant mothers.7
Well-known risks to the expectant liver transplant recipient include hypertension and preeclampsia.8 Moreover, infants born to these patients have a higher risk of prematurity and low birth weight.3,7,9 However, rates of neonatal or maternal deaths and birth defects do not differ significantly from those seen in the general population. Graft rejection is a potential complication, with rates varying between 0% and 20% in different studies.3
Multidisciplinary care is therefore crucial during these high-risk pregnancies.10 An obstetrician, a hepatologist, and a perinatalogist should collaborate to maximize outcomes.11 Frequent evaluations, preferably 2 weeks apart, are suggested for the serial assessment of fetal growth.
Furthermore, daily monitoring of the blood pressure and aggressive management of hypertension are recommended. Methyldopa appears to be the drug treatment of choice.12
Close monitoring of graft function and liver biopsy in suspected graft rejection are of essence as well.3 Routine screening for urinary tract infection, cytomegalovirus and toxoplasmosis infections, gestational diabetes, and preeclampsia should also be undertaken.
MANAGING IMMUNOSUPPRESSION IN THE PREGNANT PATIENT
The choice of immunosuppression is ideally made before pregnancy. All immunosuppressive drugs cross the placenta. Thus, in theory, all agents carry risks of teratogenicity and fetal loss. However, immunosuppression is crucial in avoiding rejection. Furthermore, the use of appropriate immunosuppressive regimens prevents negative outcomes. Drugs are classified as class A (safest to use in pregnancy), through classes B, C, D, and X.
Tacrolimus (class C) monotherapy appears to be safe, with attention to the maintenance of therapeutic levels throughout pregnancy. Allograft function and tacrolimus serum levels need to be monitored because of the change in the volume of drug distribution. Cyclosporine (a pregnancy class C drug), prednisone (class B), and azathioprine (class D) are also reasonable options and may also be used if judged necessary.13
Mycophenolic acid and mTOR (mammalian target of rapamycin) inhibitors such as sirolimus and everolimus are significantly teratogenic and should be avoided in pregnant women. They are more commonly associated with spontaneous abortion, structural abnormalities, and birth defects than other immunosuppressive drugs, especially if taken in the early stages of pregnancy. Cleft lip and palate, absent auditory canals, and microtia have been reported.2,13
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
- Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol 1995; 132:444–449.
- Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996; 62:476–479.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2009; 103–122.
- Parolin MB, Coelho JC, Urbanetz AA, Pampuch M. Contraception and pregnancy after liver transplantation: an update overview. Arq Gastroenterol 2009; 46:154–158. In Portuguese.
- Paulen ME, Folger SG, Curtis KM, Jamieson DJ. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 2010; 82:102–112.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl 2010; 16:56–63.
- Heneghan MA, Selzner M, Yoshida EM, Mullhaupt B. Pregnancy and sexual function in liver transplantation. J Hepatol 2008; 49:507–519.
- Ho JK, Ko HH, Schaeffer DF, et al. Sexual health after orthotopic liver transplantation. Liver Transpl 2006; 12:1478–1484.
- Jabiry-Zieniewicz Z, Dabrowski FA, Pietrzak B, Wielgos M. Pregnancy complications after liver transplantation. Int J Gynaecol Obstet 2015; 128:27–29.
- Parhar KS, Gibson PS, Coffin CS. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can J Gastroenterol 2012; 26:621–626.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82:1698–1702.
Are we causing anemia by ordering unnecessary blood tests?
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
A serpiginous, itchy rash on the foot
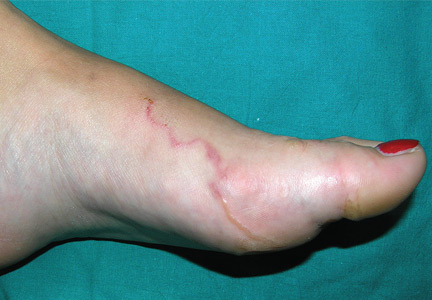
A 22-year-old woman presented with a serpiginous, erythematous, itchy lesion on her left foot (Figure 1), 10 days after returning from a beach holiday in Tanzania. She noted that the site of the lesion kept changing. Her medical history was otherwise unremarkable.
Based on the patient’s recent travel, the pattern of the lesion, the intense pruritus, and the lack of other symptoms, the lesion was diagnosed as cutaneous larva migrans. We applied cryosurgery and prescribed thiabendazole 10% cream twice daily for 2 weeks. The lesions resolved completely after 2 weeks.
BEACH WORM
Cutaneous larva migrans—also known as migrant linear epidermitis, beach worm, migrant helminthiasis, dermatitis serpiginosa, creeping eruption, or sand worm—is a zoodermatosis caused by cutaneous penetration of helminth larvae, usually parasites of the small intestines of cats and dogs.1–3 This eruption is usually seen in tropical and subtropical climates, such as Central America, South America, Africa, and even the southeastern parts of the United States, although with the ease of travel to the tropics its incidence could well be increasing on return to the home countries.1,2 The disease is endemic along the southeastern Atlantic coast of North America, in the Gulf of Mexico, in the Caribbean, and on the coast of Uruguay.
Common species
The most common cause is Ancylostoma braziliense, with less common species being Ancylostoma caninum, Uncinaria stenocephala, and Bunostomum phlebotomum.4 The larvae may cause a nonspecific dermatitis at the site of penetration where the skin has been in contact with infected soil, commonly the feet, hands, or buttocks. From the point where larvae penetrate, they gradually form linear tunnels with an irregular and capricious path, advancing at a rate of a few millimeters a day.3 There may be a single path, as in our patient, or hundreds or even a thousand in cases of massive infection.
The larvae rarely affect other organs, and systemic manifestations such as migratory pulmonary infiltrates and peripheral eosinophilia (Loeffler syndrome) are rarely seen. Larva currens, caused by the rapid-moving parasitic roundworm Strongyloides stercoralis, generally manifests on the buttocks or the perianal region and lasts only a few hours.
Key diagnostic features
The diagnosis of cutaneous larva migrans is based on the clinical history and on the serpiginous and migratory pattern of the lesions. However, eczematization and secondary infection may make recognition of these features more difficult.3 Biopsy of the lesions usually does not help identify the larvae, since they advance in front of the path.
TREATMENT OPTIONS
Although the disease is self-limiting, treatment is usually recommended because of intense pruritus and the risk of bacterial infection. With no treatment, the number of lesions falls by 33% after 1 week, 54% after 2 weeks, 71% after 3 weeks, and 81% after 4 weeks according to Katz et al.5 Other reports mention that 25% to 33% of the larvae die every 4 weeks.5
Treatment is topical or systemic, depending on the extent and location of the lesions. Systemic treatment is preferred for widespread or multiple lesions or lesions located near the eye, but use is limited due to a high incidence of adverse effects.
The drugs of choice are albendazole 400 mg/day for 3 days, ivermectin 0.2 mg/kg in a single dose, or thiabendazole 25 mg/kg/day, divided into two doses for 5 days. If there are few lesions, as in our patient, thiabendazole ointment or 10% cream may be applied to the entire lesion, slightly in front of the leading point of advance of the lesion.1,6 However, thiabendazole is not available in the United States.
Adverse effects of ivermectin include fever, pruritus, and skin rash. Albendazole may cause abdominal pain, dizziness, headache, fever, nausea, vomiting, hair loss, bone marrow suppression, agranulocytosis, aplastic anemia, or elevation of liver enzyme levels. Thiabendazole may cause delirium, diarrhea, hallucinations, loss of appetite, numbness, nausea, and central nervous system toxicity.
- Kalil CLPV, Webber A. Zoodermatoses. In: Ramos e Silva M, Castro MCR, editors. Fundamentos de Dermatologia. Rio de Janeiro: Atheneu; 2010:1055–1057.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol 2001; 145:434–437.
- Meotti CD, Plates G, Nogueira LL, et al. Cutaneous larva migrans on the scalp: atypical presentation of a common disease. An Bras Dermatol 2014; 89:332–333.
- Karthikeyan K, Thappa DM. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2002; 68:252–258.
- Katz R, Ziegler J, Blank H. The natural course of creeping eruption and treatment with thiabendazole. Arch Dermatol 1965; 91:420–424.
- Upendra Y, Mahajan VK, Mehta KS, Chauhan PS, Chander B. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2013; 79:418–419.
A 22-year-old woman presented with a serpiginous, erythematous, itchy lesion on her left foot (Figure 1), 10 days after returning from a beach holiday in Tanzania. She noted that the site of the lesion kept changing. Her medical history was otherwise unremarkable.
Based on the patient’s recent travel, the pattern of the lesion, the intense pruritus, and the lack of other symptoms, the lesion was diagnosed as cutaneous larva migrans. We applied cryosurgery and prescribed thiabendazole 10% cream twice daily for 2 weeks. The lesions resolved completely after 2 weeks.
BEACH WORM
Cutaneous larva migrans—also known as migrant linear epidermitis, beach worm, migrant helminthiasis, dermatitis serpiginosa, creeping eruption, or sand worm—is a zoodermatosis caused by cutaneous penetration of helminth larvae, usually parasites of the small intestines of cats and dogs.1–3 This eruption is usually seen in tropical and subtropical climates, such as Central America, South America, Africa, and even the southeastern parts of the United States, although with the ease of travel to the tropics its incidence could well be increasing on return to the home countries.1,2 The disease is endemic along the southeastern Atlantic coast of North America, in the Gulf of Mexico, in the Caribbean, and on the coast of Uruguay.
Common species
The most common cause is Ancylostoma braziliense, with less common species being Ancylostoma caninum, Uncinaria stenocephala, and Bunostomum phlebotomum.4 The larvae may cause a nonspecific dermatitis at the site of penetration where the skin has been in contact with infected soil, commonly the feet, hands, or buttocks. From the point where larvae penetrate, they gradually form linear tunnels with an irregular and capricious path, advancing at a rate of a few millimeters a day.3 There may be a single path, as in our patient, or hundreds or even a thousand in cases of massive infection.
The larvae rarely affect other organs, and systemic manifestations such as migratory pulmonary infiltrates and peripheral eosinophilia (Loeffler syndrome) are rarely seen. Larva currens, caused by the rapid-moving parasitic roundworm Strongyloides stercoralis, generally manifests on the buttocks or the perianal region and lasts only a few hours.
Key diagnostic features
The diagnosis of cutaneous larva migrans is based on the clinical history and on the serpiginous and migratory pattern of the lesions. However, eczematization and secondary infection may make recognition of these features more difficult.3 Biopsy of the lesions usually does not help identify the larvae, since they advance in front of the path.
TREATMENT OPTIONS
Although the disease is self-limiting, treatment is usually recommended because of intense pruritus and the risk of bacterial infection. With no treatment, the number of lesions falls by 33% after 1 week, 54% after 2 weeks, 71% after 3 weeks, and 81% after 4 weeks according to Katz et al.5 Other reports mention that 25% to 33% of the larvae die every 4 weeks.5
Treatment is topical or systemic, depending on the extent and location of the lesions. Systemic treatment is preferred for widespread or multiple lesions or lesions located near the eye, but use is limited due to a high incidence of adverse effects.
The drugs of choice are albendazole 400 mg/day for 3 days, ivermectin 0.2 mg/kg in a single dose, or thiabendazole 25 mg/kg/day, divided into two doses for 5 days. If there are few lesions, as in our patient, thiabendazole ointment or 10% cream may be applied to the entire lesion, slightly in front of the leading point of advance of the lesion.1,6 However, thiabendazole is not available in the United States.
Adverse effects of ivermectin include fever, pruritus, and skin rash. Albendazole may cause abdominal pain, dizziness, headache, fever, nausea, vomiting, hair loss, bone marrow suppression, agranulocytosis, aplastic anemia, or elevation of liver enzyme levels. Thiabendazole may cause delirium, diarrhea, hallucinations, loss of appetite, numbness, nausea, and central nervous system toxicity.
A 22-year-old woman presented with a serpiginous, erythematous, itchy lesion on her left foot (Figure 1), 10 days after returning from a beach holiday in Tanzania. She noted that the site of the lesion kept changing. Her medical history was otherwise unremarkable.
Based on the patient’s recent travel, the pattern of the lesion, the intense pruritus, and the lack of other symptoms, the lesion was diagnosed as cutaneous larva migrans. We applied cryosurgery and prescribed thiabendazole 10% cream twice daily for 2 weeks. The lesions resolved completely after 2 weeks.
BEACH WORM
Cutaneous larva migrans—also known as migrant linear epidermitis, beach worm, migrant helminthiasis, dermatitis serpiginosa, creeping eruption, or sand worm—is a zoodermatosis caused by cutaneous penetration of helminth larvae, usually parasites of the small intestines of cats and dogs.1–3 This eruption is usually seen in tropical and subtropical climates, such as Central America, South America, Africa, and even the southeastern parts of the United States, although with the ease of travel to the tropics its incidence could well be increasing on return to the home countries.1,2 The disease is endemic along the southeastern Atlantic coast of North America, in the Gulf of Mexico, in the Caribbean, and on the coast of Uruguay.
Common species
The most common cause is Ancylostoma braziliense, with less common species being Ancylostoma caninum, Uncinaria stenocephala, and Bunostomum phlebotomum.4 The larvae may cause a nonspecific dermatitis at the site of penetration where the skin has been in contact with infected soil, commonly the feet, hands, or buttocks. From the point where larvae penetrate, they gradually form linear tunnels with an irregular and capricious path, advancing at a rate of a few millimeters a day.3 There may be a single path, as in our patient, or hundreds or even a thousand in cases of massive infection.
The larvae rarely affect other organs, and systemic manifestations such as migratory pulmonary infiltrates and peripheral eosinophilia (Loeffler syndrome) are rarely seen. Larva currens, caused by the rapid-moving parasitic roundworm Strongyloides stercoralis, generally manifests on the buttocks or the perianal region and lasts only a few hours.
Key diagnostic features
The diagnosis of cutaneous larva migrans is based on the clinical history and on the serpiginous and migratory pattern of the lesions. However, eczematization and secondary infection may make recognition of these features more difficult.3 Biopsy of the lesions usually does not help identify the larvae, since they advance in front of the path.
TREATMENT OPTIONS
Although the disease is self-limiting, treatment is usually recommended because of intense pruritus and the risk of bacterial infection. With no treatment, the number of lesions falls by 33% after 1 week, 54% after 2 weeks, 71% after 3 weeks, and 81% after 4 weeks according to Katz et al.5 Other reports mention that 25% to 33% of the larvae die every 4 weeks.5
Treatment is topical or systemic, depending on the extent and location of the lesions. Systemic treatment is preferred for widespread or multiple lesions or lesions located near the eye, but use is limited due to a high incidence of adverse effects.
The drugs of choice are albendazole 400 mg/day for 3 days, ivermectin 0.2 mg/kg in a single dose, or thiabendazole 25 mg/kg/day, divided into two doses for 5 days. If there are few lesions, as in our patient, thiabendazole ointment or 10% cream may be applied to the entire lesion, slightly in front of the leading point of advance of the lesion.1,6 However, thiabendazole is not available in the United States.
Adverse effects of ivermectin include fever, pruritus, and skin rash. Albendazole may cause abdominal pain, dizziness, headache, fever, nausea, vomiting, hair loss, bone marrow suppression, agranulocytosis, aplastic anemia, or elevation of liver enzyme levels. Thiabendazole may cause delirium, diarrhea, hallucinations, loss of appetite, numbness, nausea, and central nervous system toxicity.
- Kalil CLPV, Webber A. Zoodermatoses. In: Ramos e Silva M, Castro MCR, editors. Fundamentos de Dermatologia. Rio de Janeiro: Atheneu; 2010:1055–1057.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol 2001; 145:434–437.
- Meotti CD, Plates G, Nogueira LL, et al. Cutaneous larva migrans on the scalp: atypical presentation of a common disease. An Bras Dermatol 2014; 89:332–333.
- Karthikeyan K, Thappa DM. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2002; 68:252–258.
- Katz R, Ziegler J, Blank H. The natural course of creeping eruption and treatment with thiabendazole. Arch Dermatol 1965; 91:420–424.
- Upendra Y, Mahajan VK, Mehta KS, Chauhan PS, Chander B. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2013; 79:418–419.
- Kalil CLPV, Webber A. Zoodermatoses. In: Ramos e Silva M, Castro MCR, editors. Fundamentos de Dermatologia. Rio de Janeiro: Atheneu; 2010:1055–1057.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol 2001; 145:434–437.
- Meotti CD, Plates G, Nogueira LL, et al. Cutaneous larva migrans on the scalp: atypical presentation of a common disease. An Bras Dermatol 2014; 89:332–333.
- Karthikeyan K, Thappa DM. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2002; 68:252–258.
- Katz R, Ziegler J, Blank H. The natural course of creeping eruption and treatment with thiabendazole. Arch Dermatol 1965; 91:420–424.
- Upendra Y, Mahajan VK, Mehta KS, Chauhan PS, Chander B. Cutaneous larva migrans. Indian J Dermatol Venereol Leprol 2013; 79:418–419.
LGBT care: There has been progress
McNamara and Ng, in this issue of the Journal, discuss the psychosocial and clinical aspects of caring for people who are lesbian, gay, bisexual, or transgender (LGBT), a community that numbers more than 9 million in the United States. Choices for health maintenance and screening are influenced by the patient’s age, sexual practices, comorbidities, and in some transgender patients by current and previous therapeutic hormone manipulations. Although these medical decisions must be personalized, many are informed by existing guidelines for the general population and thus do not represent a dramatic departure from decision-making in other patients.
More difficult is acquiring the requisite understanding and appreciation of the special challenges each LGBT patient brings to the office visit. We need to understand these challenges to provide medically appropriate, compassionate, patient-centered care.
McNamara and Ng suggest simple acts of kindness and consideration to increase the comfort of this group of patients who historically, for a myriad of complex reasons, have not been uniformly made to feel comfortable receiving routine care in our medical system. A tectonic shift has taken place in the way society at large perceives and interacts with members of the LGBT community. Large pockets of intolerance and lack of understanding certainly still exist. But I want to believe that as a medical community, we have changed dramatically over the past several decades.
Early in my medical career, as the AIDS epidemic expanded from unexplained Pneumocystis carinii (now jirovecii) and fungal infections and virulent Kaposi sarcoma to include Guillain-Barré syndrome, central nervous system tumors and infections, and so much more, I watched the gay community rally around specific doctors and medical centers—and avoid others. It was more than just the perception that some hospitals were “gay-friendly.” Care was more compassionate, supportive, and thorough at some hospitals than others. I well recall having difficulty finding a neurosurgeon willing to biopsy a brain mass in one of my patients with AIDS, and finding an orthopedic surgeon willing to manage an infected hip prosthesis in another one. Fast forward 3 decades, and now in a different hospital I am managing gout in a patient who is infected with HIV, is in apparent remission without detectable virus on highly active antiretroviral therapy, and recently received a heart transplant.
As controversies continue to swirl and external acts of discrimination continue to impact the LGBT community on many fronts—bathroom laws, spousal rights, child adoption, gay political leaders, xenophobia, and even hate crimes—it is encouraging to read a matter-of-fact, practical approach to “best practices” in LGBT care. No hype. No judgment. Just compassionate, appropriate medical care.
The journey isn’t over, but there has been progress.
McNamara and Ng, in this issue of the Journal, discuss the psychosocial and clinical aspects of caring for people who are lesbian, gay, bisexual, or transgender (LGBT), a community that numbers more than 9 million in the United States. Choices for health maintenance and screening are influenced by the patient’s age, sexual practices, comorbidities, and in some transgender patients by current and previous therapeutic hormone manipulations. Although these medical decisions must be personalized, many are informed by existing guidelines for the general population and thus do not represent a dramatic departure from decision-making in other patients.
More difficult is acquiring the requisite understanding and appreciation of the special challenges each LGBT patient brings to the office visit. We need to understand these challenges to provide medically appropriate, compassionate, patient-centered care.
McNamara and Ng suggest simple acts of kindness and consideration to increase the comfort of this group of patients who historically, for a myriad of complex reasons, have not been uniformly made to feel comfortable receiving routine care in our medical system. A tectonic shift has taken place in the way society at large perceives and interacts with members of the LGBT community. Large pockets of intolerance and lack of understanding certainly still exist. But I want to believe that as a medical community, we have changed dramatically over the past several decades.
Early in my medical career, as the AIDS epidemic expanded from unexplained Pneumocystis carinii (now jirovecii) and fungal infections and virulent Kaposi sarcoma to include Guillain-Barré syndrome, central nervous system tumors and infections, and so much more, I watched the gay community rally around specific doctors and medical centers—and avoid others. It was more than just the perception that some hospitals were “gay-friendly.” Care was more compassionate, supportive, and thorough at some hospitals than others. I well recall having difficulty finding a neurosurgeon willing to biopsy a brain mass in one of my patients with AIDS, and finding an orthopedic surgeon willing to manage an infected hip prosthesis in another one. Fast forward 3 decades, and now in a different hospital I am managing gout in a patient who is infected with HIV, is in apparent remission without detectable virus on highly active antiretroviral therapy, and recently received a heart transplant.
As controversies continue to swirl and external acts of discrimination continue to impact the LGBT community on many fronts—bathroom laws, spousal rights, child adoption, gay political leaders, xenophobia, and even hate crimes—it is encouraging to read a matter-of-fact, practical approach to “best practices” in LGBT care. No hype. No judgment. Just compassionate, appropriate medical care.
The journey isn’t over, but there has been progress.
McNamara and Ng, in this issue of the Journal, discuss the psychosocial and clinical aspects of caring for people who are lesbian, gay, bisexual, or transgender (LGBT), a community that numbers more than 9 million in the United States. Choices for health maintenance and screening are influenced by the patient’s age, sexual practices, comorbidities, and in some transgender patients by current and previous therapeutic hormone manipulations. Although these medical decisions must be personalized, many are informed by existing guidelines for the general population and thus do not represent a dramatic departure from decision-making in other patients.
More difficult is acquiring the requisite understanding and appreciation of the special challenges each LGBT patient brings to the office visit. We need to understand these challenges to provide medically appropriate, compassionate, patient-centered care.
McNamara and Ng suggest simple acts of kindness and consideration to increase the comfort of this group of patients who historically, for a myriad of complex reasons, have not been uniformly made to feel comfortable receiving routine care in our medical system. A tectonic shift has taken place in the way society at large perceives and interacts with members of the LGBT community. Large pockets of intolerance and lack of understanding certainly still exist. But I want to believe that as a medical community, we have changed dramatically over the past several decades.
Early in my medical career, as the AIDS epidemic expanded from unexplained Pneumocystis carinii (now jirovecii) and fungal infections and virulent Kaposi sarcoma to include Guillain-Barré syndrome, central nervous system tumors and infections, and so much more, I watched the gay community rally around specific doctors and medical centers—and avoid others. It was more than just the perception that some hospitals were “gay-friendly.” Care was more compassionate, supportive, and thorough at some hospitals than others. I well recall having difficulty finding a neurosurgeon willing to biopsy a brain mass in one of my patients with AIDS, and finding an orthopedic surgeon willing to manage an infected hip prosthesis in another one. Fast forward 3 decades, and now in a different hospital I am managing gout in a patient who is infected with HIV, is in apparent remission without detectable virus on highly active antiretroviral therapy, and recently received a heart transplant.
As controversies continue to swirl and external acts of discrimination continue to impact the LGBT community on many fronts—bathroom laws, spousal rights, child adoption, gay political leaders, xenophobia, and even hate crimes—it is encouraging to read a matter-of-fact, practical approach to “best practices” in LGBT care. No hype. No judgment. Just compassionate, appropriate medical care.
The journey isn’t over, but there has been progress.
Best practices in LGBT care: A guide for primary care physicians
Primary care physicians are very likely to encounter lesbian, gay, bisexual, and transgender (LGBT) patients in their practice, and must be able to provide informed, appropriate, and culturally sensitive care.
Approximately 9 million people in the United States identify as lesbian, gay, or bisexual, and 700,000 adults are transgender.1 In the 2013 National Health Interview Survey,2 which queried 34,557 adults about their sexual orientation, 2.3% reported being lesbian, gay, or bisexual, with only slight differences according to age or sex: of those ages 18 through 44, 1.9% were gay or lesbian and 1.1% were bisexual; of those ages 65 and over, 0.7% were gay or lesbian and 0.2% were bisexual. By sex, 0.9% of women vs 0.4% of men identified as bisexual.2
This article identifies and corrects common myths about LGBT care, addresses disparities in healthcare access, and outlines a step-by-step approach for delivering comprehensive care to LGBT patients.
MYTHS ABOUT LGBT CARE
Myth #1: L = G = B = T
Although LGBT is a commonly used term, each group described by the abbreviation has its own unique healthcare needs. For example, lesbian and bisexual women are more likely than heterosexual women to smoke, and gay men are at increased risk for human immunodeficiency virus (HIV) and other sexually transmitted infections.3,4 Transgender persons have high rates of suicide.5
Primary care of the LGBT patient needs to be individualized but also informed by the knowledge of distinct risks and behaviors associated with particular groups.
Myth #2: Sexual orientation = sexual activity
Sexual identity correlates closely but not completely with sexual behavior; individuals may engage in same-sex behavior but not identify as lesbian, gay, or bisexual.6,7 Many women who identify as lesbian have previously had sex with men, and men may have had same-sex encounters but consider themselves heterosexual.8,9
Since the risk of certain infections is related to sexual activity, providers should query patients about their sexual partners and practices in an open, nonjudgmental way, and avoid labeling patients solely according to sexual orientation. Table 1 suggests questions to use when interviewing patients.
Myth #3: Sexual orientation = gender identity
Gender identity describes a person’s inherent sense of being a woman, man, or of neither gender, whereas sexual orientation refers to how a person identifies their physical and emotional attraction to others.10,11 Conflating the two concepts can alienate patients, lead to incorrect assumptions, and result in an underestimation of an individual’s risk of sexually transmitted diseases.
Using questions such as “Are you sexually active with men, women, or both?” or “When you are sexually active, what parts of your body do you use?” with all patients, regardless of gender identity, will facilitate open and honest conversations that allow for appropriate counseling and risk assessment. Table 2 lists commonly used gender-identity terms.
Myth #4: LGBT people have the same access to healthcare as heterosexual people
People who identify as lesbian, gay, bisexual, or transgender experience significant disparities in access to healthcare compared with cisgender heterosexual people. For example, lesbian women are less likely to receive the human papillomavirus vaccine, cervical cancer screening, and mammograms, and men in same-sex relationships are twice as likely to have unmet medical needs.8,12 In a national survey,5 19% of transgender individuals reported that they had been refused healthcare. Among 152 transgender adults who described their experiences with the healthcare system, 7% reported receiving substandard care.13
We can eliminate these disparities by creating a welcoming environment for all patients (Table 3), and also by being aware of the specific services that should be offered to LGBT individuals.
ADDRESSING THE NEEDS OF LGBT PATIENTS
Outlined here is an office-based approach for addressing the unique clinical concerns of adult LGBT patients. Not all of these issues need to or should be addressed at the first visit, and the sequence in which these steps are accomplished may vary.
Step #1: Screen for mental health disorders
Lesbian, bisexual, and gay people are more likely to experience depression and anxiety. According to the results of a large meta-analysis,14 the prevalence of these conditions is 1.5 times higher in this population than in heterosexual people. Risk may vary according to group, with gay and bisexual men experiencing a higher lifetime prevalence of anxiety and depression than lesbian and bisexual women.15 Suicidal attempts are also more common in gay and bisexual men, who have a lifetime risk four times higher than that of heterosexual men.14
The risk of suicide is even higher among transgender people: 41% of surveyed transgender adults reported that they had attempted suicide, with higher rates in younger individuals.5 Risk factors include experiences of harassment or physical or sexual violence, as well as poverty, low education level, and unemployment.5 The risk of suicide in transgender people who served in the military is 20 times higher than that in the general veteran population.16
It is imperative to routinely screen LGBT patients for anxiety, depression, and suicidality and to refer them to mental health providers who are sensitive to LGBT patients’ needs and concerns. Screening tools such as the Patient Health Questionnaire-2 (PHQ2), PHQ9A, PHQ9, and Generalized Anxiety Disorder 7-item scale (GAD7) are useful in screening patients for depression and anxiety in addition to mnemonics such as SIGECAPS (sleep, interest, guilt, energy, concentration, appetite, psychomotor, suicidal thoughts or ideation).17
Although the same screening tools are used in cisgender heterosexual patients, factors contributing to the experience of depression or anxiety may be directly related to gender identity, gender expression, or sexual orientation. In a 2001 study, more lesbian, gay, and bisexual people reported lifetime and day-to-day experiences with discrimination than heterosexual people, and approximately 42% attributed this in part or in total to their sexual orientation.18
Step #2: Assess for substance use
Substance use is also more common in LGBT people. Lesbian and bisexual women have higher rates of tobacco abuse, exposure to second-hand smoke, and alcohol and drug dependence.3,14 In one study, compared with heterosexual individuals, the odds of lifetime alcohol and substance use disorder was three times higher in lesbian women, and the odds of lifetime drug-use disorder was 1.6 times higher in gay men.19
In a survey of transgender people, 30% reported using tobacco compared with 20% of the US adult population, and 8% reported using alcohol or drugs to cope with mistreatment and bias.5 In a study of transgender women in San Francisco, 58% used alcohol and 43% used substances, including marijuana, methamphetamine, and crack cocaine. Substance use significantly increased the odds of testing positive for HIV.20
Clinicians should carefully question LGBT patients about their use of alcohol, tobacco, and other substances and provide counseling and assistance with cessation. Several LGBT-specific resources can be used to aid patients in their efforts, and referral to substance abuse groups that are welcoming to LGBT people may increase cessation rates.19,21
Step #3: Offer appropriate screening services
Human papillomavirus (HPV). Like heterosexual women, lesbian and bisexual women are at risk of HPV infection, which is associated with cervical cancer and genital warts.8 HPV can be transmitted in several ways, including skin-to-skin and digital-to-genital contact, as well as penile-vaginal intercourse. Lesbian and bisexual women may have acquired HPV from previous male sexual partners or from female-to-female transmission.8 In a study comparing cervical cancer screening results among lesbian, bisexual, and heterosexual women, there was no significant difference in the odds for Papanicolaou (Pap) test abnormalities and only a minor decrease in the odds of HPV infection.22 Lesbian and bisexual women should receive Pap and HPV testing according to current guidelines.
Other sexually transmitted infections, including herpes simplex virus 1, herpes simplex virus 2, Trichomonas vaginalis, syphilis, and hepatitis A, can be passed between female partners; risk may vary according to sexual practices.23 Thus, providers should not assume that lesbian women are at low risk of these infections and should screen according to current guidelines.
The US Centers for Disease Control and Prevention (CDC) recommends annual screening for Chlamydia infection for all women under age 25, as well as those at increased risk for this infection (ie, those with a new sex partner or multiple sex partners).24
Breast cancer. Studies reveal that lesbian and bisexual women are less likely to receive mammograms, and they may have several risk factors that increase their risk for breast cancer, including overweight, obesity, and excessive alcohol intake.12,18,25 Providers should discuss the risks and benefits of mammography and offer this screening service at appropriate intervals.
Screening in men who have sex with men
Men who have sex with men are at increased risk for several sexually transmitted infections, including HIV, syphilis, gonorrhea, Chlamydia, anal HPV, and hepatitis B and C.4,9 The CDC recommends annual sexual health screening that includes serologic testing for HIV and syphilis, and urine, rectal, or pharyngeal testing for gonorrhea and Chlamydia according to sexual practices.24
In contrast, routine screening for anal HPV is not currently recommended because we lack data demonstrating that screening reduces mortality rates from anal carcinoma.24,26 Nevertheless, the CDC acknowledges that some clinicians may choose to perform anal Pap testing in patients who are at high risk, and guidelines from the New York City Department of Health and Mental Hygiene suggest annual anal Pap testing in HIV-positive men who have sex with men.27
According to the results of a systematic review,28 a significant proportion of transgender women reported sexual practices that increased their risk for sexually transmitted infections, and 27.7% tested positive for HIV infection. In contrast, rates of HIV and risk behaviors were much lower among transgender men. Risk may be heightened in transgender women who have not had sexual reassignment surgery and who engage in insertive anal, vaginal, or oral intercourse.28 An awareness of an individual patient’s current anatomy and sexual practices is essential for providing appropriate counseling about sexually transmitted infections.
‘Screen what you have’
When considering screening for breast, cervical, and prostate cancer, providers should consider an individual patient’s surgical history and hormonal status. “Screen what you have” is an easy rule to help both patients and providers remember which services to consider.
Transgender men who have not had a mastectomy should discuss the risks and benefits of breast cancer screening and consider mammography as recommended by the American Cancer Society.29 Similarly, cervical cancer screening should be performed according to current guidelines, although providers should be aware that this examination can cause significant anxiety and emotional distress for the patient.30
In transgender women, guidelines for breast cancer screening for those who were previously or currently treated with hormones are lacking. The University of California-San Francisco Center of Excellence for Transgender Health recommends mammography for patients over age 50 with additional risk factors (family history, obesity, estrogen and progestin use for more than 5 years).31 Transgender women should be counseled about the risks and benefits of prostate cancer screening.
Step #4: Immunize, and promote healthy behaviors
Table 4 outlines the screening services, immunizations, and health behavior promotions that should be offered to LGBT patients.
Vaccinations. LGBT individuals should be routinely offered HPV vaccination through age 26, according to current guidelines.24 Immunization against hepatitis A and B is also recommended for men who have sex with men, if they are not already immune.24 Meningococcal vaccine should be given to men who have sex with men if they have an additional medical, occupational, or lifestyle risk factor.32
Physical activity should be encouraged, especially in lesbian and bisexual women, who are more likely to be overweight and obese.25 In a recent study,33 gay, lesbian, and bisexual youths (ages 12–22) reported 1.21 to 2.62 fewer hours of moderate or vigorous physical activity per week than their “completely heterosexual” counterparts, and were 46% to 76% less likely to participate in team sports, in part due to concerns about gender nonconformity. On the other hand, results from a recent national survey of adults ages 18 through 64 found no significant differences in physical activity according to sexual orientation.
Providers should address patients’ perceived barriers to participating in exercise programs.2
Preexposure prophylaxis against HIV. A growing number of patients and health providers are asking about preexposure prophylaxis for HIV infection. The initial CDC recommendations for the daily use of emtricitabine-tenofovir were restricted to gay and bisexual men and men who have sex with men in serodiscordant relationships or in situations where the HIV status of the patient’s partner was unknown.34 Since then, the CDC has expanded the groups who may benefit from preexposure prophylaxis.35 Assessment of the patient’s ability to adhere to a daily oral medication regimen is central to its success. Patients should be screened for hepatitis, HIV, and renal and liver function before starting emtricitabine-tenofovir and should have these tests repeated at 3-month intervals if pre-exposure prophylaxis is continued.
Step #5: Initiate or continue hormone therapy for transgender individuals
Hormone therapy often improves the quality of life for patients who desire to have their physical appearance align more closely with their gender identity.29 Moreover, abruptly stopping hormone therapy can have significant psychological consequences.36
Clinicians should feel comfortable starting hormone therapy for patients who have been diagnosed with gender dysphoria by a mental health professional, can demonstrate knowledge about and outcomes of hormone therapy, and have lived as a member of the desired gender (“real-life experience”) for at least 3 months, and preferably 12 months.29 More recently, some practitioners have advocated prescribing hormone therapy for patients without the requirement for real-life experience or a formal letter from a mental health professional recommending hormonal therapy.37 However, mental healthcare is recommended for any patient with moderate to severe mental health conditions, especially if not treated at the time of presentation.37
Providers should continue hormone therapy for patients who are already receiving it, while being aware of the appropriate treatment goals and monitoring parameters. The two main principles of hormone therapy for transgender patients are to reduce endogenous hormone levels and their associated sex characteristics and replace with hormones of the preferred sex.29 Doses and formulations are similar to those used for treatment of hypogonadism. This topic has been reviewed by Spack.10
The only absolute contraindications to hormone therapy are estrogen- or testosterone-responsive tumors. Otherwise, hormone therapy can be initiated or continued with the patient’s informed consent about its benefits and risks.
Estrogen therapy may increase the risk of thromboembolic disease, coronary artery disease, cerebrovascular disease, severe migraine headaches, liver dysfunction, and macroprolactinoma.29 In a cross-sectional study of 100 transgender patients receiving hormone therapy, 12% of transgender women experienced a thromboembolic or cardiovascular event after an average of 11 years of treatment.38 However, many of these patients had additional risk factors for these events, such as smoking. In contrast, results from a recent systematic review39 indicated a much lower rate of venous thromboembolism among transgender women receiving estrogen therapy (1.7%–6.3%). Use of transdermal estrogen may minimize the likelihood of thromboembolic disease, and cessation of hormonal care in the perioperative period is advisable, especially for procedures with greater risk of venous thromboembolism.39
Transgender men are at risk of erythrocytosis (hematocrit > 50%) as a result of testosterone therapy. Although current guidelines indicate that testosterone may increase the risk of breast or uterine cancer, results from a recent systematic review40 indicate that the overall cancer incidence in transgender men is not higher than in natal controls. Both estrogen and testosterone therapy increase insulin resistance and fasting glucose levels, whereas only estrogen increases triglyceride concentrations.40
For transgender women, estrogen levels should be maintained in the normal range for cisgender women of reproductive age (< 200 pg/mL), and testosterone levels should be suppressed to less than 55 ng/dL. Goal testosterone levels for transgender men are between 320 and 1,000 ng/dL and should be measured at intervals specific to the preparation used (ie, measured midway between injections for individuals treated with testosterone cypionate). Estradiol levels should be less than 50 ng/dL.29 Transgender women and men should have estradiol and testosterone levels measured quarterly during the first year of treatment, and then every 6 to 12 months thereafter once goal levels are achieved.
Additional monitoring for transgender women includes measuring serum prolactin at baseline and after 12 months of therapy, and serum electrolytes for those taking spironolactone as antiandrogen therapy. Complete blood cell counts and liver function tests should be done every 3 months during the first year of testosterone therapy for transgender men, and then one to two times per year.29 Reference laboratory values for the patient’s affirmed gender should be used to assess response to therapy as well as effects on end-organ function.
The marked suppression of endogenous hormone levels that occurs during therapy may have adverse effects on the bone mineral density of both transgender women and men. Clinicians should assess patients’ baseline risk for osteoporotic fracture at the time hormone therapy is started and consider bone mineral density testing if appropriate. For those at low risk for fracture, current guidelines recommend screening for osteoporosis starting at age 60.29
Providers should counsel patients who have recently initiated hormone therapy that some changes may occur gradually over time. While transgender women will notice a decrease in libido and spontaneous erections within the first 3 months of therapy, breast growth begins approximately 3 to 6 months after treatment is started. Similarly, for transgender men, fat redistribution occurs during the first 6 months of treatment, but facial and body hair growth occur more slowly and are at maximum 4 to 5 years after starting hormone therapy.29 Amenorrhea typically occurs 1 to 6 months after starting hormonal therapy for transgender men.
Some patients may be interested in surgery to continue their physical transformation to the desired sex. Patients who have used hormone therapy and participated in a real-life experience or otherwise completed social transition by living as the affirmed gender for 12 months are considered eligible for surgery if they can demonstrate a good understanding of the cost, potential complications, and expected recovery time of the procedure. Guidelines also recommend that the patient demonstrate progress in work, family, and interpersonal issues regarding their new gender.29 Available surgical options include breast augmentation, orchiectomy and penectomy, and vaginoplasty, clitoroplasty, and vulvoplasty for transgender women. Feminizing procedures include voice surgery, thyroid cartilage reduction, and facial feminization surgery. Transgender men may choose to have mastectomy, hysterectomy and salpingo-oophorectomy, vaginectomy, scrotoplasty and testicular implant placement, and implantation of a penile prosthesis. Additional virilizing surgeries include voice surgery and pectoral implants.41
Step #6: Screen for intimate partner violence
Intimate partner violence refers to physical, sexual, and psychological harm by a current or former partner or spouse, and it can occur in gay and lesbian relationships. In 2000, a National Violence Against Women survey found that 21.5% of men and 35.4% of women who reported living with a same-sex partner had experienced physical abuse.42 More recent studies confirm rates similar to those in heterosexual relationships. In an online study,43 11.8% of men who have sex with men reported physical violence from a current male partner, and about 4% reported experiencing coerced sex.
Intimate partner violence is uniquely challenging for LGBT people. In addition to the commonly described methods an abuser uses to maintain power and control, forced disclosure or “outing”—publicly revealing someone’s sexual orientation or gender identity—may result in additional psychological violence and harm. Survivors of intimate partner violence who are in same-gender intimate relationships often find that obtaining services through the police, judicial, and social services systems is challenging. Survivors may be required to disclose their sexual orientation or gender identity as part of filing a report or judicial order to obtain help or protection from the abuser. Many male and transgender survivors of intimate partner violence are unable to access traditional shelters. Female survivors may find that their same-sex abusers have the same access to resources and shelters that they do.
Intimate partner violence is associated with negative physical and mental health outcomes. Physical injuries such as bruises, fractures, and burns are some of the more obvious harms survivors sustain. However, the negative psychological impact on survivors cannot be overstated. LGBT individuals are at greater risk of depression and substance abuse as a result of intimate partner violence than their cisgender heterosexual counterparts. The stress resulting from stigmatization and discrimination can be exacerbated by intimate partner violence.44 This can be seen in health outcomes of HIV-positive men who have sex with men, in whom abuse predicts interruptions in care, more advanced HIV disease, and HIV-associated hospitalizations.45
We recommend that providers screen all LGBT patients for intimate partner violence. One commonly used tool is the Partner Violence Screen, which consists of three gender-neutral questions:
- Have you been hit, kicked, punched, or otherwise hurt by someone in the past year? If so, by whom?
- Do you feel safe in your current relationship?
- Is there a partner from a previous relationship who is making you feel unsafe now?
Like other screening tools for intimate partner violence, the Partner Violence Screen is more specific than sensitive.46 Screening and discussions about intimate partner violence should be performed in a private, confidential manner while the patient is alone.
Providers who care for LGBT patients need to be aware of not only the medical and mental health sequelae of intimate partner violence but also the social and legal issues facing survivors. Familiarity with the available community resources and their limitations can better facilitate trust and patient care for those affected by intimate partner violence. In one study, the most frequent requests for assistance from sexual and gender minority survivors were for counseling, safe housing, legal assistance, and assistance navigating the medical system.47 Providers should refer patients to LGBT-focused resources in their community as available, and when no such resources exist, initiate contact with standard domestic violence services, with patient consent, to ask about a program’s ability to assist survivors of LGBT intimate partner violence.
IN A NUTSHELL
Optimizing the care of LGBT patients requires developing both clinical and cultural competency.
Initial steps for creating an inclusive and welcoming clinical environment include becoming familiar with local resources for LGBT patients (support groups, substance and alcohol cessation groups, mental health providers; see sidebar), providing education and training for support staff and nurses, and establishing gender-neutral bathrooms. Waiting areas should include literature relevant to LGBT patients and signage that is relevant to all patients, including gender-nonconforming individuals. Providers should offer all patients universal HIV screening initially and at clinically appropriate intervals and discuss preexposure prophylaxis with emtricitabine-tenofovir for at-risk individuals.
For transgender patients, addressing them by their preferred name and pronouns is central to building rapport. General health maintenance is the same for transgender patients as for cisgender patients and can be guided by the adage “screen what you have.” Hormonal care can be offered using an informed consent method consistent with the World Professional Association for Transgender Health Standards of Care.48 Guidelines exist to assist providers in initiation and maintenance of hormonal care. Cross-gender hormonal therapy is initiated with low-dose medication that is gradually increased over time, with a goal of approximating the pubertal changes of the desired gender over a 2- to 3-year period. Some, but not all, patients may pursue various surgical procedures as part of their gender affirmation process.
- Gates GJ. How many people are lesbian, gay, bisexual, or transgender? Williams Institute. 2011. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 19, 2016.
- Ward BW, Dahlhamer JM, Galinsky AM, Joesti SS. Sexual orientation and health among U.S. Adults: National Health Interview Survey, 2013. Natl Health Stat Report 2014; 77:1–10.
- Cochran SD, Bandiera FC, Mays VM. Sexual orientation-related differences in tobacco use and secondhand smoke exposure among US adults aged 20-59 years: 2003–2010 National Health and Nutrition Examination surveys. Am J Publ Health 2013; 103:1837–1844.
- Mayer KH. Sexually transmitted diseases in men who have sex with men. Clin Infect Disease 2011; 53:S79–S83.
- Grant JM, Mottet LA, Tanis J, Herman JL, Harrison J, Keisling M. National transgender discrimination survey report on health and health care. www.thetaskforce.org/static_html/downloads/reports/reports/ntds_report_on_health.pdf. Accessed May 19, 2016.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425.
- Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2009 National Survey of Family Growth. Natl Health Stat Report 2011; 36:1–36.
- Agenor M, Peitzmeier S, Gordon AR, Haneuse S, Potter JE, Austin SB. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med 2015; 163:99–106.
- Ard KL, Makadon HJ. Improving the health care of lesbian, gay, bisexual and transgender (LGBT) people: understanding and eliminating health disparities. www.lgbthealtheducation.org/wp-content/uploads/12-054_LGBTHealtharticle_v3_07-09-12.pdf. Accessed May 19, 2016.
- Spack NP. Management of transgenderism. JAMA 2013; 309:474–484.
- National LGBT Health Education Center. Achieving health equity for lesbian, gay, bisexual, and transgender (LGBT) people, Module 1. www.lgbthealtheducation.org/wp-content/uploads/Achieving-Health-Equity-for-LGBT-People-1.pdf. Accessed May 19, 2016.
- Buchmueller T, Carpenter CS. Disparities in health Insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000–2007. Am J Public Health 2010; 100:489–496.
- Kosenko K, Rintamaki L, Raney S, Maness K. Transgender patient perceptions of stigma in health care contexts. Med Care 2013; 51:819–822.
- King M, Semlyen J, Tai SS, et al. A systematic review of mental disorder, suicide, and deliberate self-harm in lesbian, gay, and bisexual people. BMC Psychiatry 2008; 8:70.
- Bostwick WB, Boyd CJ, Hughes TL, McCabe SE. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health 2010; 100:468–475.
- Blosnich JR, Brown GR, Shipherd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing Veterans Health Administration care. Am J Public Health 2013: 103:e27–e32.
- Maurer DM. Screening for depression. Am Fam Physician 2012; 85:139–144.
- Mays VM, Cochran SD. Mental health correlates of perceived discrimination among lesbian, gay, and bisexual adults in the United States. Am J Pub Health 2001; 91:1869–1875.
- McCabe SE, West BT, Hughes TL, Boyd CJ. Sexual orientation and substance abuse treatment utilization in the United States: results from a national survey. J Subst Abuse Treat 2013; 44:4–12.
- Santos GM, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev 2014; 33:287–295.
- National LGBT Tobacco Control Network. www.lgbttobacco.org. Accessed May 19, 2016.
- Massad LS, Xie X, Minkoff H, et al. Abnormal Pap tests and human papillomavirus infections among HIV infected and uninfected women who have sex with women. J Low Genit Tract Dis 2014; 18:50–56.
- Gorgos LM, Marrazzo JM. Sexually transmitted infections among women who have sex with women. Clin Infect Dis 2011; 53:S84–S91.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR 2015; 64(3):1–138.
- Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual-minority women: evidence from population-based data. Am J Public Health 2007; 97:1134–1140.
- Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS 2013; 24:843–851.
- New York City Department of Health and Mental Hygiene. Preventing sexually transmitted infections. 2013; 32(4):19–27. www.nyc.gov/html/doh/html/data/chi32-4_screening.html. Accessed May 19, 2016.
- Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav 2008; 12:1–17.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3133.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients may have high prevalence of unsatisfactory paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med 2014; 29:778–784.
- UCSF Center of Excellence for Transgender Health. Primary care protocol for transgender patient care. http://transhealth.ucsf.edu/protocols. Accessed May 19, 2016.
- Centers for Disease Control and Prevention. Recommended adult immunization schedule United States—2015. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed May 19, 2016.
- Calzo JP, Roberts AL, Corliss HL, Blood EA, Kroshus E, Austin SB. Physical activity disparities in heterosexual and sexual minority youth ages 12–22 years old: roles of childhood gender nonconformity and athletic self-esteem. Ann Behav Med 2014; 47:17–27.
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR 2012; 61:586–589.
- Centers for Disease Control and Prevention and US Public Health Service. Preexposure prophlyaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed May 19, 2016.
- Feldman JL, Goldberg J. Transgender primary medical care: suggested guidelines for clinicians in British Columbia. www.cwhn.ca/en/node/27567. Accessed May 19, 2016.
- Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-noncomforming people, version 7. Int J Transgenderism 2011; 13:165–232.
- Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012; 9: 2641–2651.
- Asscheman H, T’Sjoen G, Lemaire A, et al. Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review. Andrologia 2014; 46:791–795.
- Weinand JD, Safer JD. Hormone therapy in transgender adults is safe with provider supervision. A review of hormone therapy sequelae for transgender individuals. J Clin Transl Endocrinol 2015; 2:55–60.
- Unger CA. Care of the transgender patient: the role of the gynecologist. Am J Obstet Gynecol 2014; 210:16–26.
- Tjaden P, Thoennes N. Extent, nature, and consequences of intimate partner violence: findings from the National Violence Against Women Survey. Washington, DC: US Department of Justice, National Institute of Justice; 2000. P. 29–31. Report No.: NCJ 181867.
- Stephenson R, Khosropour C, Sullivan P. Reporting of intimate partner violence among men who have sex with men in an online survey. West J Emerg Med 2010; 11:242–246.
- Chen PH, Jacobs A, Rovi SL. Intimate partner violence: IPV in the LGBT community. FP Essent 2013; 412:28–35.
- Siemieniuk R, Miller P, Woodman K, et al. Prevalence, clinical associations, and impact of intimate partner violence among HIV infected gay and bisexual men: a population based study. HIV Med 2013; 14:293–302.
- Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36:439–445.
- Ford CL, Slavin T, Hilton KL, Holt SL. Intimate partner violence prevention services and resources in Los Angeles: issues, needs, and challenges for assisting lesbian, gay, bisexual, and transgender clients. Health Promot Pract 2013; 14:841–849.
- World Professional Association for Transgender Health. www.wpath.org/site_page.cfm?pk_association_webpage_menu=1351. Accessed May 19, 2016.
Primary care physicians are very likely to encounter lesbian, gay, bisexual, and transgender (LGBT) patients in their practice, and must be able to provide informed, appropriate, and culturally sensitive care.
Approximately 9 million people in the United States identify as lesbian, gay, or bisexual, and 700,000 adults are transgender.1 In the 2013 National Health Interview Survey,2 which queried 34,557 adults about their sexual orientation, 2.3% reported being lesbian, gay, or bisexual, with only slight differences according to age or sex: of those ages 18 through 44, 1.9% were gay or lesbian and 1.1% were bisexual; of those ages 65 and over, 0.7% were gay or lesbian and 0.2% were bisexual. By sex, 0.9% of women vs 0.4% of men identified as bisexual.2
This article identifies and corrects common myths about LGBT care, addresses disparities in healthcare access, and outlines a step-by-step approach for delivering comprehensive care to LGBT patients.
MYTHS ABOUT LGBT CARE
Myth #1: L = G = B = T
Although LGBT is a commonly used term, each group described by the abbreviation has its own unique healthcare needs. For example, lesbian and bisexual women are more likely than heterosexual women to smoke, and gay men are at increased risk for human immunodeficiency virus (HIV) and other sexually transmitted infections.3,4 Transgender persons have high rates of suicide.5
Primary care of the LGBT patient needs to be individualized but also informed by the knowledge of distinct risks and behaviors associated with particular groups.
Myth #2: Sexual orientation = sexual activity
Sexual identity correlates closely but not completely with sexual behavior; individuals may engage in same-sex behavior but not identify as lesbian, gay, or bisexual.6,7 Many women who identify as lesbian have previously had sex with men, and men may have had same-sex encounters but consider themselves heterosexual.8,9
Since the risk of certain infections is related to sexual activity, providers should query patients about their sexual partners and practices in an open, nonjudgmental way, and avoid labeling patients solely according to sexual orientation. Table 1 suggests questions to use when interviewing patients.
Myth #3: Sexual orientation = gender identity
Gender identity describes a person’s inherent sense of being a woman, man, or of neither gender, whereas sexual orientation refers to how a person identifies their physical and emotional attraction to others.10,11 Conflating the two concepts can alienate patients, lead to incorrect assumptions, and result in an underestimation of an individual’s risk of sexually transmitted diseases.
Using questions such as “Are you sexually active with men, women, or both?” or “When you are sexually active, what parts of your body do you use?” with all patients, regardless of gender identity, will facilitate open and honest conversations that allow for appropriate counseling and risk assessment. Table 2 lists commonly used gender-identity terms.
Myth #4: LGBT people have the same access to healthcare as heterosexual people
People who identify as lesbian, gay, bisexual, or transgender experience significant disparities in access to healthcare compared with cisgender heterosexual people. For example, lesbian women are less likely to receive the human papillomavirus vaccine, cervical cancer screening, and mammograms, and men in same-sex relationships are twice as likely to have unmet medical needs.8,12 In a national survey,5 19% of transgender individuals reported that they had been refused healthcare. Among 152 transgender adults who described their experiences with the healthcare system, 7% reported receiving substandard care.13
We can eliminate these disparities by creating a welcoming environment for all patients (Table 3), and also by being aware of the specific services that should be offered to LGBT individuals.
ADDRESSING THE NEEDS OF LGBT PATIENTS
Outlined here is an office-based approach for addressing the unique clinical concerns of adult LGBT patients. Not all of these issues need to or should be addressed at the first visit, and the sequence in which these steps are accomplished may vary.
Step #1: Screen for mental health disorders
Lesbian, bisexual, and gay people are more likely to experience depression and anxiety. According to the results of a large meta-analysis,14 the prevalence of these conditions is 1.5 times higher in this population than in heterosexual people. Risk may vary according to group, with gay and bisexual men experiencing a higher lifetime prevalence of anxiety and depression than lesbian and bisexual women.15 Suicidal attempts are also more common in gay and bisexual men, who have a lifetime risk four times higher than that of heterosexual men.14
The risk of suicide is even higher among transgender people: 41% of surveyed transgender adults reported that they had attempted suicide, with higher rates in younger individuals.5 Risk factors include experiences of harassment or physical or sexual violence, as well as poverty, low education level, and unemployment.5 The risk of suicide in transgender people who served in the military is 20 times higher than that in the general veteran population.16
It is imperative to routinely screen LGBT patients for anxiety, depression, and suicidality and to refer them to mental health providers who are sensitive to LGBT patients’ needs and concerns. Screening tools such as the Patient Health Questionnaire-2 (PHQ2), PHQ9A, PHQ9, and Generalized Anxiety Disorder 7-item scale (GAD7) are useful in screening patients for depression and anxiety in addition to mnemonics such as SIGECAPS (sleep, interest, guilt, energy, concentration, appetite, psychomotor, suicidal thoughts or ideation).17
Although the same screening tools are used in cisgender heterosexual patients, factors contributing to the experience of depression or anxiety may be directly related to gender identity, gender expression, or sexual orientation. In a 2001 study, more lesbian, gay, and bisexual people reported lifetime and day-to-day experiences with discrimination than heterosexual people, and approximately 42% attributed this in part or in total to their sexual orientation.18
Step #2: Assess for substance use
Substance use is also more common in LGBT people. Lesbian and bisexual women have higher rates of tobacco abuse, exposure to second-hand smoke, and alcohol and drug dependence.3,14 In one study, compared with heterosexual individuals, the odds of lifetime alcohol and substance use disorder was three times higher in lesbian women, and the odds of lifetime drug-use disorder was 1.6 times higher in gay men.19
In a survey of transgender people, 30% reported using tobacco compared with 20% of the US adult population, and 8% reported using alcohol or drugs to cope with mistreatment and bias.5 In a study of transgender women in San Francisco, 58% used alcohol and 43% used substances, including marijuana, methamphetamine, and crack cocaine. Substance use significantly increased the odds of testing positive for HIV.20
Clinicians should carefully question LGBT patients about their use of alcohol, tobacco, and other substances and provide counseling and assistance with cessation. Several LGBT-specific resources can be used to aid patients in their efforts, and referral to substance abuse groups that are welcoming to LGBT people may increase cessation rates.19,21
Step #3: Offer appropriate screening services
Human papillomavirus (HPV). Like heterosexual women, lesbian and bisexual women are at risk of HPV infection, which is associated with cervical cancer and genital warts.8 HPV can be transmitted in several ways, including skin-to-skin and digital-to-genital contact, as well as penile-vaginal intercourse. Lesbian and bisexual women may have acquired HPV from previous male sexual partners or from female-to-female transmission.8 In a study comparing cervical cancer screening results among lesbian, bisexual, and heterosexual women, there was no significant difference in the odds for Papanicolaou (Pap) test abnormalities and only a minor decrease in the odds of HPV infection.22 Lesbian and bisexual women should receive Pap and HPV testing according to current guidelines.
Other sexually transmitted infections, including herpes simplex virus 1, herpes simplex virus 2, Trichomonas vaginalis, syphilis, and hepatitis A, can be passed between female partners; risk may vary according to sexual practices.23 Thus, providers should not assume that lesbian women are at low risk of these infections and should screen according to current guidelines.
The US Centers for Disease Control and Prevention (CDC) recommends annual screening for Chlamydia infection for all women under age 25, as well as those at increased risk for this infection (ie, those with a new sex partner or multiple sex partners).24
Breast cancer. Studies reveal that lesbian and bisexual women are less likely to receive mammograms, and they may have several risk factors that increase their risk for breast cancer, including overweight, obesity, and excessive alcohol intake.12,18,25 Providers should discuss the risks and benefits of mammography and offer this screening service at appropriate intervals.
Screening in men who have sex with men
Men who have sex with men are at increased risk for several sexually transmitted infections, including HIV, syphilis, gonorrhea, Chlamydia, anal HPV, and hepatitis B and C.4,9 The CDC recommends annual sexual health screening that includes serologic testing for HIV and syphilis, and urine, rectal, or pharyngeal testing for gonorrhea and Chlamydia according to sexual practices.24
In contrast, routine screening for anal HPV is not currently recommended because we lack data demonstrating that screening reduces mortality rates from anal carcinoma.24,26 Nevertheless, the CDC acknowledges that some clinicians may choose to perform anal Pap testing in patients who are at high risk, and guidelines from the New York City Department of Health and Mental Hygiene suggest annual anal Pap testing in HIV-positive men who have sex with men.27
According to the results of a systematic review,28 a significant proportion of transgender women reported sexual practices that increased their risk for sexually transmitted infections, and 27.7% tested positive for HIV infection. In contrast, rates of HIV and risk behaviors were much lower among transgender men. Risk may be heightened in transgender women who have not had sexual reassignment surgery and who engage in insertive anal, vaginal, or oral intercourse.28 An awareness of an individual patient’s current anatomy and sexual practices is essential for providing appropriate counseling about sexually transmitted infections.
‘Screen what you have’
When considering screening for breast, cervical, and prostate cancer, providers should consider an individual patient’s surgical history and hormonal status. “Screen what you have” is an easy rule to help both patients and providers remember which services to consider.
Transgender men who have not had a mastectomy should discuss the risks and benefits of breast cancer screening and consider mammography as recommended by the American Cancer Society.29 Similarly, cervical cancer screening should be performed according to current guidelines, although providers should be aware that this examination can cause significant anxiety and emotional distress for the patient.30
In transgender women, guidelines for breast cancer screening for those who were previously or currently treated with hormones are lacking. The University of California-San Francisco Center of Excellence for Transgender Health recommends mammography for patients over age 50 with additional risk factors (family history, obesity, estrogen and progestin use for more than 5 years).31 Transgender women should be counseled about the risks and benefits of prostate cancer screening.
Step #4: Immunize, and promote healthy behaviors
Table 4 outlines the screening services, immunizations, and health behavior promotions that should be offered to LGBT patients.
Vaccinations. LGBT individuals should be routinely offered HPV vaccination through age 26, according to current guidelines.24 Immunization against hepatitis A and B is also recommended for men who have sex with men, if they are not already immune.24 Meningococcal vaccine should be given to men who have sex with men if they have an additional medical, occupational, or lifestyle risk factor.32
Physical activity should be encouraged, especially in lesbian and bisexual women, who are more likely to be overweight and obese.25 In a recent study,33 gay, lesbian, and bisexual youths (ages 12–22) reported 1.21 to 2.62 fewer hours of moderate or vigorous physical activity per week than their “completely heterosexual” counterparts, and were 46% to 76% less likely to participate in team sports, in part due to concerns about gender nonconformity. On the other hand, results from a recent national survey of adults ages 18 through 64 found no significant differences in physical activity according to sexual orientation.
Providers should address patients’ perceived barriers to participating in exercise programs.2
Preexposure prophylaxis against HIV. A growing number of patients and health providers are asking about preexposure prophylaxis for HIV infection. The initial CDC recommendations for the daily use of emtricitabine-tenofovir were restricted to gay and bisexual men and men who have sex with men in serodiscordant relationships or in situations where the HIV status of the patient’s partner was unknown.34 Since then, the CDC has expanded the groups who may benefit from preexposure prophylaxis.35 Assessment of the patient’s ability to adhere to a daily oral medication regimen is central to its success. Patients should be screened for hepatitis, HIV, and renal and liver function before starting emtricitabine-tenofovir and should have these tests repeated at 3-month intervals if pre-exposure prophylaxis is continued.
Step #5: Initiate or continue hormone therapy for transgender individuals
Hormone therapy often improves the quality of life for patients who desire to have their physical appearance align more closely with their gender identity.29 Moreover, abruptly stopping hormone therapy can have significant psychological consequences.36
Clinicians should feel comfortable starting hormone therapy for patients who have been diagnosed with gender dysphoria by a mental health professional, can demonstrate knowledge about and outcomes of hormone therapy, and have lived as a member of the desired gender (“real-life experience”) for at least 3 months, and preferably 12 months.29 More recently, some practitioners have advocated prescribing hormone therapy for patients without the requirement for real-life experience or a formal letter from a mental health professional recommending hormonal therapy.37 However, mental healthcare is recommended for any patient with moderate to severe mental health conditions, especially if not treated at the time of presentation.37
Providers should continue hormone therapy for patients who are already receiving it, while being aware of the appropriate treatment goals and monitoring parameters. The two main principles of hormone therapy for transgender patients are to reduce endogenous hormone levels and their associated sex characteristics and replace with hormones of the preferred sex.29 Doses and formulations are similar to those used for treatment of hypogonadism. This topic has been reviewed by Spack.10
The only absolute contraindications to hormone therapy are estrogen- or testosterone-responsive tumors. Otherwise, hormone therapy can be initiated or continued with the patient’s informed consent about its benefits and risks.
Estrogen therapy may increase the risk of thromboembolic disease, coronary artery disease, cerebrovascular disease, severe migraine headaches, liver dysfunction, and macroprolactinoma.29 In a cross-sectional study of 100 transgender patients receiving hormone therapy, 12% of transgender women experienced a thromboembolic or cardiovascular event after an average of 11 years of treatment.38 However, many of these patients had additional risk factors for these events, such as smoking. In contrast, results from a recent systematic review39 indicated a much lower rate of venous thromboembolism among transgender women receiving estrogen therapy (1.7%–6.3%). Use of transdermal estrogen may minimize the likelihood of thromboembolic disease, and cessation of hormonal care in the perioperative period is advisable, especially for procedures with greater risk of venous thromboembolism.39
Transgender men are at risk of erythrocytosis (hematocrit > 50%) as a result of testosterone therapy. Although current guidelines indicate that testosterone may increase the risk of breast or uterine cancer, results from a recent systematic review40 indicate that the overall cancer incidence in transgender men is not higher than in natal controls. Both estrogen and testosterone therapy increase insulin resistance and fasting glucose levels, whereas only estrogen increases triglyceride concentrations.40
For transgender women, estrogen levels should be maintained in the normal range for cisgender women of reproductive age (< 200 pg/mL), and testosterone levels should be suppressed to less than 55 ng/dL. Goal testosterone levels for transgender men are between 320 and 1,000 ng/dL and should be measured at intervals specific to the preparation used (ie, measured midway between injections for individuals treated with testosterone cypionate). Estradiol levels should be less than 50 ng/dL.29 Transgender women and men should have estradiol and testosterone levels measured quarterly during the first year of treatment, and then every 6 to 12 months thereafter once goal levels are achieved.
Additional monitoring for transgender women includes measuring serum prolactin at baseline and after 12 months of therapy, and serum electrolytes for those taking spironolactone as antiandrogen therapy. Complete blood cell counts and liver function tests should be done every 3 months during the first year of testosterone therapy for transgender men, and then one to two times per year.29 Reference laboratory values for the patient’s affirmed gender should be used to assess response to therapy as well as effects on end-organ function.
The marked suppression of endogenous hormone levels that occurs during therapy may have adverse effects on the bone mineral density of both transgender women and men. Clinicians should assess patients’ baseline risk for osteoporotic fracture at the time hormone therapy is started and consider bone mineral density testing if appropriate. For those at low risk for fracture, current guidelines recommend screening for osteoporosis starting at age 60.29
Providers should counsel patients who have recently initiated hormone therapy that some changes may occur gradually over time. While transgender women will notice a decrease in libido and spontaneous erections within the first 3 months of therapy, breast growth begins approximately 3 to 6 months after treatment is started. Similarly, for transgender men, fat redistribution occurs during the first 6 months of treatment, but facial and body hair growth occur more slowly and are at maximum 4 to 5 years after starting hormone therapy.29 Amenorrhea typically occurs 1 to 6 months after starting hormonal therapy for transgender men.
Some patients may be interested in surgery to continue their physical transformation to the desired sex. Patients who have used hormone therapy and participated in a real-life experience or otherwise completed social transition by living as the affirmed gender for 12 months are considered eligible for surgery if they can demonstrate a good understanding of the cost, potential complications, and expected recovery time of the procedure. Guidelines also recommend that the patient demonstrate progress in work, family, and interpersonal issues regarding their new gender.29 Available surgical options include breast augmentation, orchiectomy and penectomy, and vaginoplasty, clitoroplasty, and vulvoplasty for transgender women. Feminizing procedures include voice surgery, thyroid cartilage reduction, and facial feminization surgery. Transgender men may choose to have mastectomy, hysterectomy and salpingo-oophorectomy, vaginectomy, scrotoplasty and testicular implant placement, and implantation of a penile prosthesis. Additional virilizing surgeries include voice surgery and pectoral implants.41
Step #6: Screen for intimate partner violence
Intimate partner violence refers to physical, sexual, and psychological harm by a current or former partner or spouse, and it can occur in gay and lesbian relationships. In 2000, a National Violence Against Women survey found that 21.5% of men and 35.4% of women who reported living with a same-sex partner had experienced physical abuse.42 More recent studies confirm rates similar to those in heterosexual relationships. In an online study,43 11.8% of men who have sex with men reported physical violence from a current male partner, and about 4% reported experiencing coerced sex.
Intimate partner violence is uniquely challenging for LGBT people. In addition to the commonly described methods an abuser uses to maintain power and control, forced disclosure or “outing”—publicly revealing someone’s sexual orientation or gender identity—may result in additional psychological violence and harm. Survivors of intimate partner violence who are in same-gender intimate relationships often find that obtaining services through the police, judicial, and social services systems is challenging. Survivors may be required to disclose their sexual orientation or gender identity as part of filing a report or judicial order to obtain help or protection from the abuser. Many male and transgender survivors of intimate partner violence are unable to access traditional shelters. Female survivors may find that their same-sex abusers have the same access to resources and shelters that they do.
Intimate partner violence is associated with negative physical and mental health outcomes. Physical injuries such as bruises, fractures, and burns are some of the more obvious harms survivors sustain. However, the negative psychological impact on survivors cannot be overstated. LGBT individuals are at greater risk of depression and substance abuse as a result of intimate partner violence than their cisgender heterosexual counterparts. The stress resulting from stigmatization and discrimination can be exacerbated by intimate partner violence.44 This can be seen in health outcomes of HIV-positive men who have sex with men, in whom abuse predicts interruptions in care, more advanced HIV disease, and HIV-associated hospitalizations.45
We recommend that providers screen all LGBT patients for intimate partner violence. One commonly used tool is the Partner Violence Screen, which consists of three gender-neutral questions:
- Have you been hit, kicked, punched, or otherwise hurt by someone in the past year? If so, by whom?
- Do you feel safe in your current relationship?
- Is there a partner from a previous relationship who is making you feel unsafe now?
Like other screening tools for intimate partner violence, the Partner Violence Screen is more specific than sensitive.46 Screening and discussions about intimate partner violence should be performed in a private, confidential manner while the patient is alone.
Providers who care for LGBT patients need to be aware of not only the medical and mental health sequelae of intimate partner violence but also the social and legal issues facing survivors. Familiarity with the available community resources and their limitations can better facilitate trust and patient care for those affected by intimate partner violence. In one study, the most frequent requests for assistance from sexual and gender minority survivors were for counseling, safe housing, legal assistance, and assistance navigating the medical system.47 Providers should refer patients to LGBT-focused resources in their community as available, and when no such resources exist, initiate contact with standard domestic violence services, with patient consent, to ask about a program’s ability to assist survivors of LGBT intimate partner violence.
IN A NUTSHELL
Optimizing the care of LGBT patients requires developing both clinical and cultural competency.
Initial steps for creating an inclusive and welcoming clinical environment include becoming familiar with local resources for LGBT patients (support groups, substance and alcohol cessation groups, mental health providers; see sidebar), providing education and training for support staff and nurses, and establishing gender-neutral bathrooms. Waiting areas should include literature relevant to LGBT patients and signage that is relevant to all patients, including gender-nonconforming individuals. Providers should offer all patients universal HIV screening initially and at clinically appropriate intervals and discuss preexposure prophylaxis with emtricitabine-tenofovir for at-risk individuals.
For transgender patients, addressing them by their preferred name and pronouns is central to building rapport. General health maintenance is the same for transgender patients as for cisgender patients and can be guided by the adage “screen what you have.” Hormonal care can be offered using an informed consent method consistent with the World Professional Association for Transgender Health Standards of Care.48 Guidelines exist to assist providers in initiation and maintenance of hormonal care. Cross-gender hormonal therapy is initiated with low-dose medication that is gradually increased over time, with a goal of approximating the pubertal changes of the desired gender over a 2- to 3-year period. Some, but not all, patients may pursue various surgical procedures as part of their gender affirmation process.
Primary care physicians are very likely to encounter lesbian, gay, bisexual, and transgender (LGBT) patients in their practice, and must be able to provide informed, appropriate, and culturally sensitive care.
Approximately 9 million people in the United States identify as lesbian, gay, or bisexual, and 700,000 adults are transgender.1 In the 2013 National Health Interview Survey,2 which queried 34,557 adults about their sexual orientation, 2.3% reported being lesbian, gay, or bisexual, with only slight differences according to age or sex: of those ages 18 through 44, 1.9% were gay or lesbian and 1.1% were bisexual; of those ages 65 and over, 0.7% were gay or lesbian and 0.2% were bisexual. By sex, 0.9% of women vs 0.4% of men identified as bisexual.2
This article identifies and corrects common myths about LGBT care, addresses disparities in healthcare access, and outlines a step-by-step approach for delivering comprehensive care to LGBT patients.
MYTHS ABOUT LGBT CARE
Myth #1: L = G = B = T
Although LGBT is a commonly used term, each group described by the abbreviation has its own unique healthcare needs. For example, lesbian and bisexual women are more likely than heterosexual women to smoke, and gay men are at increased risk for human immunodeficiency virus (HIV) and other sexually transmitted infections.3,4 Transgender persons have high rates of suicide.5
Primary care of the LGBT patient needs to be individualized but also informed by the knowledge of distinct risks and behaviors associated with particular groups.
Myth #2: Sexual orientation = sexual activity
Sexual identity correlates closely but not completely with sexual behavior; individuals may engage in same-sex behavior but not identify as lesbian, gay, or bisexual.6,7 Many women who identify as lesbian have previously had sex with men, and men may have had same-sex encounters but consider themselves heterosexual.8,9
Since the risk of certain infections is related to sexual activity, providers should query patients about their sexual partners and practices in an open, nonjudgmental way, and avoid labeling patients solely according to sexual orientation. Table 1 suggests questions to use when interviewing patients.
Myth #3: Sexual orientation = gender identity
Gender identity describes a person’s inherent sense of being a woman, man, or of neither gender, whereas sexual orientation refers to how a person identifies their physical and emotional attraction to others.10,11 Conflating the two concepts can alienate patients, lead to incorrect assumptions, and result in an underestimation of an individual’s risk of sexually transmitted diseases.
Using questions such as “Are you sexually active with men, women, or both?” or “When you are sexually active, what parts of your body do you use?” with all patients, regardless of gender identity, will facilitate open and honest conversations that allow for appropriate counseling and risk assessment. Table 2 lists commonly used gender-identity terms.
Myth #4: LGBT people have the same access to healthcare as heterosexual people
People who identify as lesbian, gay, bisexual, or transgender experience significant disparities in access to healthcare compared with cisgender heterosexual people. For example, lesbian women are less likely to receive the human papillomavirus vaccine, cervical cancer screening, and mammograms, and men in same-sex relationships are twice as likely to have unmet medical needs.8,12 In a national survey,5 19% of transgender individuals reported that they had been refused healthcare. Among 152 transgender adults who described their experiences with the healthcare system, 7% reported receiving substandard care.13
We can eliminate these disparities by creating a welcoming environment for all patients (Table 3), and also by being aware of the specific services that should be offered to LGBT individuals.
ADDRESSING THE NEEDS OF LGBT PATIENTS
Outlined here is an office-based approach for addressing the unique clinical concerns of adult LGBT patients. Not all of these issues need to or should be addressed at the first visit, and the sequence in which these steps are accomplished may vary.
Step #1: Screen for mental health disorders
Lesbian, bisexual, and gay people are more likely to experience depression and anxiety. According to the results of a large meta-analysis,14 the prevalence of these conditions is 1.5 times higher in this population than in heterosexual people. Risk may vary according to group, with gay and bisexual men experiencing a higher lifetime prevalence of anxiety and depression than lesbian and bisexual women.15 Suicidal attempts are also more common in gay and bisexual men, who have a lifetime risk four times higher than that of heterosexual men.14
The risk of suicide is even higher among transgender people: 41% of surveyed transgender adults reported that they had attempted suicide, with higher rates in younger individuals.5 Risk factors include experiences of harassment or physical or sexual violence, as well as poverty, low education level, and unemployment.5 The risk of suicide in transgender people who served in the military is 20 times higher than that in the general veteran population.16
It is imperative to routinely screen LGBT patients for anxiety, depression, and suicidality and to refer them to mental health providers who are sensitive to LGBT patients’ needs and concerns. Screening tools such as the Patient Health Questionnaire-2 (PHQ2), PHQ9A, PHQ9, and Generalized Anxiety Disorder 7-item scale (GAD7) are useful in screening patients for depression and anxiety in addition to mnemonics such as SIGECAPS (sleep, interest, guilt, energy, concentration, appetite, psychomotor, suicidal thoughts or ideation).17
Although the same screening tools are used in cisgender heterosexual patients, factors contributing to the experience of depression or anxiety may be directly related to gender identity, gender expression, or sexual orientation. In a 2001 study, more lesbian, gay, and bisexual people reported lifetime and day-to-day experiences with discrimination than heterosexual people, and approximately 42% attributed this in part or in total to their sexual orientation.18
Step #2: Assess for substance use
Substance use is also more common in LGBT people. Lesbian and bisexual women have higher rates of tobacco abuse, exposure to second-hand smoke, and alcohol and drug dependence.3,14 In one study, compared with heterosexual individuals, the odds of lifetime alcohol and substance use disorder was three times higher in lesbian women, and the odds of lifetime drug-use disorder was 1.6 times higher in gay men.19
In a survey of transgender people, 30% reported using tobacco compared with 20% of the US adult population, and 8% reported using alcohol or drugs to cope with mistreatment and bias.5 In a study of transgender women in San Francisco, 58% used alcohol and 43% used substances, including marijuana, methamphetamine, and crack cocaine. Substance use significantly increased the odds of testing positive for HIV.20
Clinicians should carefully question LGBT patients about their use of alcohol, tobacco, and other substances and provide counseling and assistance with cessation. Several LGBT-specific resources can be used to aid patients in their efforts, and referral to substance abuse groups that are welcoming to LGBT people may increase cessation rates.19,21
Step #3: Offer appropriate screening services
Human papillomavirus (HPV). Like heterosexual women, lesbian and bisexual women are at risk of HPV infection, which is associated with cervical cancer and genital warts.8 HPV can be transmitted in several ways, including skin-to-skin and digital-to-genital contact, as well as penile-vaginal intercourse. Lesbian and bisexual women may have acquired HPV from previous male sexual partners or from female-to-female transmission.8 In a study comparing cervical cancer screening results among lesbian, bisexual, and heterosexual women, there was no significant difference in the odds for Papanicolaou (Pap) test abnormalities and only a minor decrease in the odds of HPV infection.22 Lesbian and bisexual women should receive Pap and HPV testing according to current guidelines.
Other sexually transmitted infections, including herpes simplex virus 1, herpes simplex virus 2, Trichomonas vaginalis, syphilis, and hepatitis A, can be passed between female partners; risk may vary according to sexual practices.23 Thus, providers should not assume that lesbian women are at low risk of these infections and should screen according to current guidelines.
The US Centers for Disease Control and Prevention (CDC) recommends annual screening for Chlamydia infection for all women under age 25, as well as those at increased risk for this infection (ie, those with a new sex partner or multiple sex partners).24
Breast cancer. Studies reveal that lesbian and bisexual women are less likely to receive mammograms, and they may have several risk factors that increase their risk for breast cancer, including overweight, obesity, and excessive alcohol intake.12,18,25 Providers should discuss the risks and benefits of mammography and offer this screening service at appropriate intervals.
Screening in men who have sex with men
Men who have sex with men are at increased risk for several sexually transmitted infections, including HIV, syphilis, gonorrhea, Chlamydia, anal HPV, and hepatitis B and C.4,9 The CDC recommends annual sexual health screening that includes serologic testing for HIV and syphilis, and urine, rectal, or pharyngeal testing for gonorrhea and Chlamydia according to sexual practices.24
In contrast, routine screening for anal HPV is not currently recommended because we lack data demonstrating that screening reduces mortality rates from anal carcinoma.24,26 Nevertheless, the CDC acknowledges that some clinicians may choose to perform anal Pap testing in patients who are at high risk, and guidelines from the New York City Department of Health and Mental Hygiene suggest annual anal Pap testing in HIV-positive men who have sex with men.27
According to the results of a systematic review,28 a significant proportion of transgender women reported sexual practices that increased their risk for sexually transmitted infections, and 27.7% tested positive for HIV infection. In contrast, rates of HIV and risk behaviors were much lower among transgender men. Risk may be heightened in transgender women who have not had sexual reassignment surgery and who engage in insertive anal, vaginal, or oral intercourse.28 An awareness of an individual patient’s current anatomy and sexual practices is essential for providing appropriate counseling about sexually transmitted infections.
‘Screen what you have’
When considering screening for breast, cervical, and prostate cancer, providers should consider an individual patient’s surgical history and hormonal status. “Screen what you have” is an easy rule to help both patients and providers remember which services to consider.
Transgender men who have not had a mastectomy should discuss the risks and benefits of breast cancer screening and consider mammography as recommended by the American Cancer Society.29 Similarly, cervical cancer screening should be performed according to current guidelines, although providers should be aware that this examination can cause significant anxiety and emotional distress for the patient.30
In transgender women, guidelines for breast cancer screening for those who were previously or currently treated with hormones are lacking. The University of California-San Francisco Center of Excellence for Transgender Health recommends mammography for patients over age 50 with additional risk factors (family history, obesity, estrogen and progestin use for more than 5 years).31 Transgender women should be counseled about the risks and benefits of prostate cancer screening.
Step #4: Immunize, and promote healthy behaviors
Table 4 outlines the screening services, immunizations, and health behavior promotions that should be offered to LGBT patients.
Vaccinations. LGBT individuals should be routinely offered HPV vaccination through age 26, according to current guidelines.24 Immunization against hepatitis A and B is also recommended for men who have sex with men, if they are not already immune.24 Meningococcal vaccine should be given to men who have sex with men if they have an additional medical, occupational, or lifestyle risk factor.32
Physical activity should be encouraged, especially in lesbian and bisexual women, who are more likely to be overweight and obese.25 In a recent study,33 gay, lesbian, and bisexual youths (ages 12–22) reported 1.21 to 2.62 fewer hours of moderate or vigorous physical activity per week than their “completely heterosexual” counterparts, and were 46% to 76% less likely to participate in team sports, in part due to concerns about gender nonconformity. On the other hand, results from a recent national survey of adults ages 18 through 64 found no significant differences in physical activity according to sexual orientation.
Providers should address patients’ perceived barriers to participating in exercise programs.2
Preexposure prophylaxis against HIV. A growing number of patients and health providers are asking about preexposure prophylaxis for HIV infection. The initial CDC recommendations for the daily use of emtricitabine-tenofovir were restricted to gay and bisexual men and men who have sex with men in serodiscordant relationships or in situations where the HIV status of the patient’s partner was unknown.34 Since then, the CDC has expanded the groups who may benefit from preexposure prophylaxis.35 Assessment of the patient’s ability to adhere to a daily oral medication regimen is central to its success. Patients should be screened for hepatitis, HIV, and renal and liver function before starting emtricitabine-tenofovir and should have these tests repeated at 3-month intervals if pre-exposure prophylaxis is continued.
Step #5: Initiate or continue hormone therapy for transgender individuals
Hormone therapy often improves the quality of life for patients who desire to have their physical appearance align more closely with their gender identity.29 Moreover, abruptly stopping hormone therapy can have significant psychological consequences.36
Clinicians should feel comfortable starting hormone therapy for patients who have been diagnosed with gender dysphoria by a mental health professional, can demonstrate knowledge about and outcomes of hormone therapy, and have lived as a member of the desired gender (“real-life experience”) for at least 3 months, and preferably 12 months.29 More recently, some practitioners have advocated prescribing hormone therapy for patients without the requirement for real-life experience or a formal letter from a mental health professional recommending hormonal therapy.37 However, mental healthcare is recommended for any patient with moderate to severe mental health conditions, especially if not treated at the time of presentation.37
Providers should continue hormone therapy for patients who are already receiving it, while being aware of the appropriate treatment goals and monitoring parameters. The two main principles of hormone therapy for transgender patients are to reduce endogenous hormone levels and their associated sex characteristics and replace with hormones of the preferred sex.29 Doses and formulations are similar to those used for treatment of hypogonadism. This topic has been reviewed by Spack.10
The only absolute contraindications to hormone therapy are estrogen- or testosterone-responsive tumors. Otherwise, hormone therapy can be initiated or continued with the patient’s informed consent about its benefits and risks.
Estrogen therapy may increase the risk of thromboembolic disease, coronary artery disease, cerebrovascular disease, severe migraine headaches, liver dysfunction, and macroprolactinoma.29 In a cross-sectional study of 100 transgender patients receiving hormone therapy, 12% of transgender women experienced a thromboembolic or cardiovascular event after an average of 11 years of treatment.38 However, many of these patients had additional risk factors for these events, such as smoking. In contrast, results from a recent systematic review39 indicated a much lower rate of venous thromboembolism among transgender women receiving estrogen therapy (1.7%–6.3%). Use of transdermal estrogen may minimize the likelihood of thromboembolic disease, and cessation of hormonal care in the perioperative period is advisable, especially for procedures with greater risk of venous thromboembolism.39
Transgender men are at risk of erythrocytosis (hematocrit > 50%) as a result of testosterone therapy. Although current guidelines indicate that testosterone may increase the risk of breast or uterine cancer, results from a recent systematic review40 indicate that the overall cancer incidence in transgender men is not higher than in natal controls. Both estrogen and testosterone therapy increase insulin resistance and fasting glucose levels, whereas only estrogen increases triglyceride concentrations.40
For transgender women, estrogen levels should be maintained in the normal range for cisgender women of reproductive age (< 200 pg/mL), and testosterone levels should be suppressed to less than 55 ng/dL. Goal testosterone levels for transgender men are between 320 and 1,000 ng/dL and should be measured at intervals specific to the preparation used (ie, measured midway between injections for individuals treated with testosterone cypionate). Estradiol levels should be less than 50 ng/dL.29 Transgender women and men should have estradiol and testosterone levels measured quarterly during the first year of treatment, and then every 6 to 12 months thereafter once goal levels are achieved.
Additional monitoring for transgender women includes measuring serum prolactin at baseline and after 12 months of therapy, and serum electrolytes for those taking spironolactone as antiandrogen therapy. Complete blood cell counts and liver function tests should be done every 3 months during the first year of testosterone therapy for transgender men, and then one to two times per year.29 Reference laboratory values for the patient’s affirmed gender should be used to assess response to therapy as well as effects on end-organ function.
The marked suppression of endogenous hormone levels that occurs during therapy may have adverse effects on the bone mineral density of both transgender women and men. Clinicians should assess patients’ baseline risk for osteoporotic fracture at the time hormone therapy is started and consider bone mineral density testing if appropriate. For those at low risk for fracture, current guidelines recommend screening for osteoporosis starting at age 60.29
Providers should counsel patients who have recently initiated hormone therapy that some changes may occur gradually over time. While transgender women will notice a decrease in libido and spontaneous erections within the first 3 months of therapy, breast growth begins approximately 3 to 6 months after treatment is started. Similarly, for transgender men, fat redistribution occurs during the first 6 months of treatment, but facial and body hair growth occur more slowly and are at maximum 4 to 5 years after starting hormone therapy.29 Amenorrhea typically occurs 1 to 6 months after starting hormonal therapy for transgender men.
Some patients may be interested in surgery to continue their physical transformation to the desired sex. Patients who have used hormone therapy and participated in a real-life experience or otherwise completed social transition by living as the affirmed gender for 12 months are considered eligible for surgery if they can demonstrate a good understanding of the cost, potential complications, and expected recovery time of the procedure. Guidelines also recommend that the patient demonstrate progress in work, family, and interpersonal issues regarding their new gender.29 Available surgical options include breast augmentation, orchiectomy and penectomy, and vaginoplasty, clitoroplasty, and vulvoplasty for transgender women. Feminizing procedures include voice surgery, thyroid cartilage reduction, and facial feminization surgery. Transgender men may choose to have mastectomy, hysterectomy and salpingo-oophorectomy, vaginectomy, scrotoplasty and testicular implant placement, and implantation of a penile prosthesis. Additional virilizing surgeries include voice surgery and pectoral implants.41
Step #6: Screen for intimate partner violence
Intimate partner violence refers to physical, sexual, and psychological harm by a current or former partner or spouse, and it can occur in gay and lesbian relationships. In 2000, a National Violence Against Women survey found that 21.5% of men and 35.4% of women who reported living with a same-sex partner had experienced physical abuse.42 More recent studies confirm rates similar to those in heterosexual relationships. In an online study,43 11.8% of men who have sex with men reported physical violence from a current male partner, and about 4% reported experiencing coerced sex.
Intimate partner violence is uniquely challenging for LGBT people. In addition to the commonly described methods an abuser uses to maintain power and control, forced disclosure or “outing”—publicly revealing someone’s sexual orientation or gender identity—may result in additional psychological violence and harm. Survivors of intimate partner violence who are in same-gender intimate relationships often find that obtaining services through the police, judicial, and social services systems is challenging. Survivors may be required to disclose their sexual orientation or gender identity as part of filing a report or judicial order to obtain help or protection from the abuser. Many male and transgender survivors of intimate partner violence are unable to access traditional shelters. Female survivors may find that their same-sex abusers have the same access to resources and shelters that they do.
Intimate partner violence is associated with negative physical and mental health outcomes. Physical injuries such as bruises, fractures, and burns are some of the more obvious harms survivors sustain. However, the negative psychological impact on survivors cannot be overstated. LGBT individuals are at greater risk of depression and substance abuse as a result of intimate partner violence than their cisgender heterosexual counterparts. The stress resulting from stigmatization and discrimination can be exacerbated by intimate partner violence.44 This can be seen in health outcomes of HIV-positive men who have sex with men, in whom abuse predicts interruptions in care, more advanced HIV disease, and HIV-associated hospitalizations.45
We recommend that providers screen all LGBT patients for intimate partner violence. One commonly used tool is the Partner Violence Screen, which consists of three gender-neutral questions:
- Have you been hit, kicked, punched, or otherwise hurt by someone in the past year? If so, by whom?
- Do you feel safe in your current relationship?
- Is there a partner from a previous relationship who is making you feel unsafe now?
Like other screening tools for intimate partner violence, the Partner Violence Screen is more specific than sensitive.46 Screening and discussions about intimate partner violence should be performed in a private, confidential manner while the patient is alone.
Providers who care for LGBT patients need to be aware of not only the medical and mental health sequelae of intimate partner violence but also the social and legal issues facing survivors. Familiarity with the available community resources and their limitations can better facilitate trust and patient care for those affected by intimate partner violence. In one study, the most frequent requests for assistance from sexual and gender minority survivors were for counseling, safe housing, legal assistance, and assistance navigating the medical system.47 Providers should refer patients to LGBT-focused resources in their community as available, and when no such resources exist, initiate contact with standard domestic violence services, with patient consent, to ask about a program’s ability to assist survivors of LGBT intimate partner violence.
IN A NUTSHELL
Optimizing the care of LGBT patients requires developing both clinical and cultural competency.
Initial steps for creating an inclusive and welcoming clinical environment include becoming familiar with local resources for LGBT patients (support groups, substance and alcohol cessation groups, mental health providers; see sidebar), providing education and training for support staff and nurses, and establishing gender-neutral bathrooms. Waiting areas should include literature relevant to LGBT patients and signage that is relevant to all patients, including gender-nonconforming individuals. Providers should offer all patients universal HIV screening initially and at clinically appropriate intervals and discuss preexposure prophylaxis with emtricitabine-tenofovir for at-risk individuals.
For transgender patients, addressing them by their preferred name and pronouns is central to building rapport. General health maintenance is the same for transgender patients as for cisgender patients and can be guided by the adage “screen what you have.” Hormonal care can be offered using an informed consent method consistent with the World Professional Association for Transgender Health Standards of Care.48 Guidelines exist to assist providers in initiation and maintenance of hormonal care. Cross-gender hormonal therapy is initiated with low-dose medication that is gradually increased over time, with a goal of approximating the pubertal changes of the desired gender over a 2- to 3-year period. Some, but not all, patients may pursue various surgical procedures as part of their gender affirmation process.
- Gates GJ. How many people are lesbian, gay, bisexual, or transgender? Williams Institute. 2011. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 19, 2016.
- Ward BW, Dahlhamer JM, Galinsky AM, Joesti SS. Sexual orientation and health among U.S. Adults: National Health Interview Survey, 2013. Natl Health Stat Report 2014; 77:1–10.
- Cochran SD, Bandiera FC, Mays VM. Sexual orientation-related differences in tobacco use and secondhand smoke exposure among US adults aged 20-59 years: 2003–2010 National Health and Nutrition Examination surveys. Am J Publ Health 2013; 103:1837–1844.
- Mayer KH. Sexually transmitted diseases in men who have sex with men. Clin Infect Disease 2011; 53:S79–S83.
- Grant JM, Mottet LA, Tanis J, Herman JL, Harrison J, Keisling M. National transgender discrimination survey report on health and health care. www.thetaskforce.org/static_html/downloads/reports/reports/ntds_report_on_health.pdf. Accessed May 19, 2016.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425.
- Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2009 National Survey of Family Growth. Natl Health Stat Report 2011; 36:1–36.
- Agenor M, Peitzmeier S, Gordon AR, Haneuse S, Potter JE, Austin SB. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med 2015; 163:99–106.
- Ard KL, Makadon HJ. Improving the health care of lesbian, gay, bisexual and transgender (LGBT) people: understanding and eliminating health disparities. www.lgbthealtheducation.org/wp-content/uploads/12-054_LGBTHealtharticle_v3_07-09-12.pdf. Accessed May 19, 2016.
- Spack NP. Management of transgenderism. JAMA 2013; 309:474–484.
- National LGBT Health Education Center. Achieving health equity for lesbian, gay, bisexual, and transgender (LGBT) people, Module 1. www.lgbthealtheducation.org/wp-content/uploads/Achieving-Health-Equity-for-LGBT-People-1.pdf. Accessed May 19, 2016.
- Buchmueller T, Carpenter CS. Disparities in health Insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000–2007. Am J Public Health 2010; 100:489–496.
- Kosenko K, Rintamaki L, Raney S, Maness K. Transgender patient perceptions of stigma in health care contexts. Med Care 2013; 51:819–822.
- King M, Semlyen J, Tai SS, et al. A systematic review of mental disorder, suicide, and deliberate self-harm in lesbian, gay, and bisexual people. BMC Psychiatry 2008; 8:70.
- Bostwick WB, Boyd CJ, Hughes TL, McCabe SE. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health 2010; 100:468–475.
- Blosnich JR, Brown GR, Shipherd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing Veterans Health Administration care. Am J Public Health 2013: 103:e27–e32.
- Maurer DM. Screening for depression. Am Fam Physician 2012; 85:139–144.
- Mays VM, Cochran SD. Mental health correlates of perceived discrimination among lesbian, gay, and bisexual adults in the United States. Am J Pub Health 2001; 91:1869–1875.
- McCabe SE, West BT, Hughes TL, Boyd CJ. Sexual orientation and substance abuse treatment utilization in the United States: results from a national survey. J Subst Abuse Treat 2013; 44:4–12.
- Santos GM, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev 2014; 33:287–295.
- National LGBT Tobacco Control Network. www.lgbttobacco.org. Accessed May 19, 2016.
- Massad LS, Xie X, Minkoff H, et al. Abnormal Pap tests and human papillomavirus infections among HIV infected and uninfected women who have sex with women. J Low Genit Tract Dis 2014; 18:50–56.
- Gorgos LM, Marrazzo JM. Sexually transmitted infections among women who have sex with women. Clin Infect Dis 2011; 53:S84–S91.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR 2015; 64(3):1–138.
- Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual-minority women: evidence from population-based data. Am J Public Health 2007; 97:1134–1140.
- Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS 2013; 24:843–851.
- New York City Department of Health and Mental Hygiene. Preventing sexually transmitted infections. 2013; 32(4):19–27. www.nyc.gov/html/doh/html/data/chi32-4_screening.html. Accessed May 19, 2016.
- Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav 2008; 12:1–17.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3133.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients may have high prevalence of unsatisfactory paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med 2014; 29:778–784.
- UCSF Center of Excellence for Transgender Health. Primary care protocol for transgender patient care. http://transhealth.ucsf.edu/protocols. Accessed May 19, 2016.
- Centers for Disease Control and Prevention. Recommended adult immunization schedule United States—2015. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed May 19, 2016.
- Calzo JP, Roberts AL, Corliss HL, Blood EA, Kroshus E, Austin SB. Physical activity disparities in heterosexual and sexual minority youth ages 12–22 years old: roles of childhood gender nonconformity and athletic self-esteem. Ann Behav Med 2014; 47:17–27.
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR 2012; 61:586–589.
- Centers for Disease Control and Prevention and US Public Health Service. Preexposure prophlyaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed May 19, 2016.
- Feldman JL, Goldberg J. Transgender primary medical care: suggested guidelines for clinicians in British Columbia. www.cwhn.ca/en/node/27567. Accessed May 19, 2016.
- Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-noncomforming people, version 7. Int J Transgenderism 2011; 13:165–232.
- Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012; 9: 2641–2651.
- Asscheman H, T’Sjoen G, Lemaire A, et al. Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review. Andrologia 2014; 46:791–795.
- Weinand JD, Safer JD. Hormone therapy in transgender adults is safe with provider supervision. A review of hormone therapy sequelae for transgender individuals. J Clin Transl Endocrinol 2015; 2:55–60.
- Unger CA. Care of the transgender patient: the role of the gynecologist. Am J Obstet Gynecol 2014; 210:16–26.
- Tjaden P, Thoennes N. Extent, nature, and consequences of intimate partner violence: findings from the National Violence Against Women Survey. Washington, DC: US Department of Justice, National Institute of Justice; 2000. P. 29–31. Report No.: NCJ 181867.
- Stephenson R, Khosropour C, Sullivan P. Reporting of intimate partner violence among men who have sex with men in an online survey. West J Emerg Med 2010; 11:242–246.
- Chen PH, Jacobs A, Rovi SL. Intimate partner violence: IPV in the LGBT community. FP Essent 2013; 412:28–35.
- Siemieniuk R, Miller P, Woodman K, et al. Prevalence, clinical associations, and impact of intimate partner violence among HIV infected gay and bisexual men: a population based study. HIV Med 2013; 14:293–302.
- Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36:439–445.
- Ford CL, Slavin T, Hilton KL, Holt SL. Intimate partner violence prevention services and resources in Los Angeles: issues, needs, and challenges for assisting lesbian, gay, bisexual, and transgender clients. Health Promot Pract 2013; 14:841–849.
- World Professional Association for Transgender Health. www.wpath.org/site_page.cfm?pk_association_webpage_menu=1351. Accessed May 19, 2016.
- Gates GJ. How many people are lesbian, gay, bisexual, or transgender? Williams Institute. 2011. http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 19, 2016.
- Ward BW, Dahlhamer JM, Galinsky AM, Joesti SS. Sexual orientation and health among U.S. Adults: National Health Interview Survey, 2013. Natl Health Stat Report 2014; 77:1–10.
- Cochran SD, Bandiera FC, Mays VM. Sexual orientation-related differences in tobacco use and secondhand smoke exposure among US adults aged 20-59 years: 2003–2010 National Health and Nutrition Examination surveys. Am J Publ Health 2013; 103:1837–1844.
- Mayer KH. Sexually transmitted diseases in men who have sex with men. Clin Infect Disease 2011; 53:S79–S83.
- Grant JM, Mottet LA, Tanis J, Herman JL, Harrison J, Keisling M. National transgender discrimination survey report on health and health care. www.thetaskforce.org/static_html/downloads/reports/reports/ntds_report_on_health.pdf. Accessed May 19, 2016.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425.
- Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2009 National Survey of Family Growth. Natl Health Stat Report 2011; 36:1–36.
- Agenor M, Peitzmeier S, Gordon AR, Haneuse S, Potter JE, Austin SB. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med 2015; 163:99–106.
- Ard KL, Makadon HJ. Improving the health care of lesbian, gay, bisexual and transgender (LGBT) people: understanding and eliminating health disparities. www.lgbthealtheducation.org/wp-content/uploads/12-054_LGBTHealtharticle_v3_07-09-12.pdf. Accessed May 19, 2016.
- Spack NP. Management of transgenderism. JAMA 2013; 309:474–484.
- National LGBT Health Education Center. Achieving health equity for lesbian, gay, bisexual, and transgender (LGBT) people, Module 1. www.lgbthealtheducation.org/wp-content/uploads/Achieving-Health-Equity-for-LGBT-People-1.pdf. Accessed May 19, 2016.
- Buchmueller T, Carpenter CS. Disparities in health Insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000–2007. Am J Public Health 2010; 100:489–496.
- Kosenko K, Rintamaki L, Raney S, Maness K. Transgender patient perceptions of stigma in health care contexts. Med Care 2013; 51:819–822.
- King M, Semlyen J, Tai SS, et al. A systematic review of mental disorder, suicide, and deliberate self-harm in lesbian, gay, and bisexual people. BMC Psychiatry 2008; 8:70.
- Bostwick WB, Boyd CJ, Hughes TL, McCabe SE. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health 2010; 100:468–475.
- Blosnich JR, Brown GR, Shipherd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing Veterans Health Administration care. Am J Public Health 2013: 103:e27–e32.
- Maurer DM. Screening for depression. Am Fam Physician 2012; 85:139–144.
- Mays VM, Cochran SD. Mental health correlates of perceived discrimination among lesbian, gay, and bisexual adults in the United States. Am J Pub Health 2001; 91:1869–1875.
- McCabe SE, West BT, Hughes TL, Boyd CJ. Sexual orientation and substance abuse treatment utilization in the United States: results from a national survey. J Subst Abuse Treat 2013; 44:4–12.
- Santos GM, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev 2014; 33:287–295.
- National LGBT Tobacco Control Network. www.lgbttobacco.org. Accessed May 19, 2016.
- Massad LS, Xie X, Minkoff H, et al. Abnormal Pap tests and human papillomavirus infections among HIV infected and uninfected women who have sex with women. J Low Genit Tract Dis 2014; 18:50–56.
- Gorgos LM, Marrazzo JM. Sexually transmitted infections among women who have sex with women. Clin Infect Dis 2011; 53:S84–S91.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR 2015; 64(3):1–138.
- Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual-minority women: evidence from population-based data. Am J Public Health 2007; 97:1134–1140.
- Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS 2013; 24:843–851.
- New York City Department of Health and Mental Hygiene. Preventing sexually transmitted infections. 2013; 32(4):19–27. www.nyc.gov/html/doh/html/data/chi32-4_screening.html. Accessed May 19, 2016.
- Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav 2008; 12:1–17.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3133.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients may have high prevalence of unsatisfactory paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med 2014; 29:778–784.
- UCSF Center of Excellence for Transgender Health. Primary care protocol for transgender patient care. http://transhealth.ucsf.edu/protocols. Accessed May 19, 2016.
- Centers for Disease Control and Prevention. Recommended adult immunization schedule United States—2015. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed May 19, 2016.
- Calzo JP, Roberts AL, Corliss HL, Blood EA, Kroshus E, Austin SB. Physical activity disparities in heterosexual and sexual minority youth ages 12–22 years old: roles of childhood gender nonconformity and athletic self-esteem. Ann Behav Med 2014; 47:17–27.
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR 2012; 61:586–589.
- Centers for Disease Control and Prevention and US Public Health Service. Preexposure prophlyaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed May 19, 2016.
- Feldman JL, Goldberg J. Transgender primary medical care: suggested guidelines for clinicians in British Columbia. www.cwhn.ca/en/node/27567. Accessed May 19, 2016.
- Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-noncomforming people, version 7. Int J Transgenderism 2011; 13:165–232.
- Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012; 9: 2641–2651.
- Asscheman H, T’Sjoen G, Lemaire A, et al. Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review. Andrologia 2014; 46:791–795.
- Weinand JD, Safer JD. Hormone therapy in transgender adults is safe with provider supervision. A review of hormone therapy sequelae for transgender individuals. J Clin Transl Endocrinol 2015; 2:55–60.
- Unger CA. Care of the transgender patient: the role of the gynecologist. Am J Obstet Gynecol 2014; 210:16–26.
- Tjaden P, Thoennes N. Extent, nature, and consequences of intimate partner violence: findings from the National Violence Against Women Survey. Washington, DC: US Department of Justice, National Institute of Justice; 2000. P. 29–31. Report No.: NCJ 181867.
- Stephenson R, Khosropour C, Sullivan P. Reporting of intimate partner violence among men who have sex with men in an online survey. West J Emerg Med 2010; 11:242–246.
- Chen PH, Jacobs A, Rovi SL. Intimate partner violence: IPV in the LGBT community. FP Essent 2013; 412:28–35.
- Siemieniuk R, Miller P, Woodman K, et al. Prevalence, clinical associations, and impact of intimate partner violence among HIV infected gay and bisexual men: a population based study. HIV Med 2013; 14:293–302.
- Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36:439–445.
- Ford CL, Slavin T, Hilton KL, Holt SL. Intimate partner violence prevention services and resources in Los Angeles: issues, needs, and challenges for assisting lesbian, gay, bisexual, and transgender clients. Health Promot Pract 2013; 14:841–849.
- World Professional Association for Transgender Health. www.wpath.org/site_page.cfm?pk_association_webpage_menu=1351. Accessed May 19, 2016.
KEY POINTS
- Lesbian and bisexual women are at increased risk for overweight, obesity, tobacco use, and drug and alcohol use disorders. Clinicians should screen for these conditions regularly and provide appropriate referral.
- Annual screening for HIV, syphilis, Chlamydia, and gonorrhea should be offered to men who have sex with men.
- Pre-exposure prophylaxis against HIV infection may be appropriate for some at-risk individuals who can adhere to daily therapy.
- “Screen what you have” is a rule that can help physicians to consider the appropriate screening services for transgender individuals.
- Hormone therapy (estrogen and testosterone) can benefit transgender individuals who are changing their physical appearance to their affirmed gender.
In reply: Blood pressure targets
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
In Reply: We thank the readers for their important and insightful comments and questions.
Dr. Yilmaz raises the point that there was no mandate in the SPRINT trial to preferentially use any specific class of antihypertensive medications in either group. However, there was greater use of all drug classes (including diuretics and renin-angiotensin-aldosterone blockers) in the intensive-treatment group.1 (This information was included as a supplementary appendix in the main paper, and as Table 1 in our review.) Could this have contributed to the primary cardiovascular outcome benefit seen in the intensive-therapy group, largely driven by a decreased incidence of heart failure, or could it even have masked the symptoms of heart failure rather than preventing it2,3? While this is plausible, since the SPRINT trial was designed as a “treat to target” study and not as an antihypertensive medication efficacy study, it is difficult to conclusively answer the question of potential pleiotropic effects of antihypertensive medications influencing the trial results. The authors did not comment on this in the main paper, and we agree that further analysis would be helpful in exploring this important question.
Dr. Edwards raises the question whether antihypertensive therapy confers additional cardiovascular benefit over aggressive use of statins. Statin use in the SPRINT cohort (both intensive and standard groups) was low at baseline, despite this being a population at high cardiovascular risk.1 It is unclear whether treatment practices pertaining to lipid management could have changed during the course of the trial in participants within the SPRINT cohort, particularly after the new lipid guidelines were published. The recently published HOPE-3 trial indicated cardiovascular benefit with statins used as a primary prevention strategy in older persons with intermediate cardiovascular risk.4,5 Notably, outcomes with combination therapy in this trial using a statin plus antihypertensive therapy were not significantly better than with statin alone, except in the subgroup of participants who were in the upper third of systolic blood pressure levels, where combination appeared to benefit more. This study, of course, was done in a population with lower cardiovascular risk than in SPRINT, and the antihypertensive medications used (candesartan and hydrochlorothiazide) were not at maximal doses. There is also a question of whether use of chlorthalidone in HOPE-3 may have been more effective.
We agree with Dr. Edwards that this is an important question that merits further exploration, especially in the broader context of treatment based on cardiovascular risk.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Mancia G. The SPRINT trial: cons. J Am Coll Cardiol 2015 Dec 2. www.acc.org/latest-in-cardiology/articles/2015/12/01/10/04/the-sprint-trial-cons. Accessed May 18, 2016.
- Zanchetti A, Liu L, Mancia G, et al. Continuation of the ESH-CHL-SHOT trial after publication of the SPRINT: rationale for further study on blood pressure targets of antihypertensive treatment after stroke. J Hypertens 2016; 34:393–396.
- Yusuf S, Lonn E, Pais P, et al; HOPE-3 Investigators. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMoa1600177. Accessed May 19, 2016.
- Cushman WC, Goff DC Jr. More HOPE for prevention with statins. N Engl J Med 2016 Apr 2 [Epub ahead of print]. www.nejm.org/doi/full/10.1056/NEJMe1603504. Accessed May 19, 2016.
SHM Brief Cited in Supreme Court Decision on Abortion Restrictions
In their landmark decision in the Whole Woman’s Health v. Hellerstedt case that challenged abortion restrictions put into place in the state of Texas, U.S. Supreme Court justices specifically cited an amicus brief submitted by the Society of Hospital Medicine.
Getting cited is a pretty big deal! It means that justices not only used what SHM wrote to inform their opinion, but also shows they gave it particular weight.
On June 27 in a 5-3 vote, the Court struck down both the admitting privileges and ambulatory surgical center requirements of the Texas law, finding that neither of these requirements provided sufficient medical benefits to justify the burdens imposed.
The brief, submitted by SHM in conjunction with the Society of OB/GYN Hospitalists last January, provided the Court with material to clarify obsolete impressions about how inpatient care currently transpires, how provider-to-provider transitions occur, and how admitting privileges work in real life.
Texas partially justified their law on views about site-to-site patient handoffs as unsafe and not in keeping with current care standards. However, as geographically based providers (hospitalists) know, care no longer arises in that manner and handoffs are not just the norm, but customary and safe.
SHM, however, did not weigh in on Constitutional questions, and did not take any stance on moral or ethical matters. SHM’s membership has diverse beliefs, and a position on this topic would be inappropriate.
In part, the SHM brief noted that:
“Admitting privileges are appropriate for physicians who regularly admit patients. But requiring physicians who specialize in outpatient procedures with low incidence of post-procedure complications, whether that specialty is podiatry or gynecology, to maintain privileges serves no medical purpose, is inconsistent with modern medicine, and is unnecessary to ensure continuity of care. Having a hospitalist serve as the admitting or attending physician does not deprive patients of quality inpatient or outpatient services.”
SHM’s amicus brief provides procedural clarification and informs the Court of how hospitalists function, but SHM emphasizes that it has not taken an ideological or ethical stance regarding the issue. SHM applauds the diversity of its membership, in background and in belief.
Regardless of procedure, modern medicine now has a presence in multiple locations with varied disciplines coordinating care. Hospitalists and other providers know handoffs are safe with outcomes equivalent to, or exceeding, prior norms. SHM felt duty-bound to correct the record for future reference and the purpose of precedent.
In their landmark decision in the Whole Woman’s Health v. Hellerstedt case that challenged abortion restrictions put into place in the state of Texas, U.S. Supreme Court justices specifically cited an amicus brief submitted by the Society of Hospital Medicine.
Getting cited is a pretty big deal! It means that justices not only used what SHM wrote to inform their opinion, but also shows they gave it particular weight.
On June 27 in a 5-3 vote, the Court struck down both the admitting privileges and ambulatory surgical center requirements of the Texas law, finding that neither of these requirements provided sufficient medical benefits to justify the burdens imposed.
The brief, submitted by SHM in conjunction with the Society of OB/GYN Hospitalists last January, provided the Court with material to clarify obsolete impressions about how inpatient care currently transpires, how provider-to-provider transitions occur, and how admitting privileges work in real life.
Texas partially justified their law on views about site-to-site patient handoffs as unsafe and not in keeping with current care standards. However, as geographically based providers (hospitalists) know, care no longer arises in that manner and handoffs are not just the norm, but customary and safe.
SHM, however, did not weigh in on Constitutional questions, and did not take any stance on moral or ethical matters. SHM’s membership has diverse beliefs, and a position on this topic would be inappropriate.
In part, the SHM brief noted that:
“Admitting privileges are appropriate for physicians who regularly admit patients. But requiring physicians who specialize in outpatient procedures with low incidence of post-procedure complications, whether that specialty is podiatry or gynecology, to maintain privileges serves no medical purpose, is inconsistent with modern medicine, and is unnecessary to ensure continuity of care. Having a hospitalist serve as the admitting or attending physician does not deprive patients of quality inpatient or outpatient services.”
SHM’s amicus brief provides procedural clarification and informs the Court of how hospitalists function, but SHM emphasizes that it has not taken an ideological or ethical stance regarding the issue. SHM applauds the diversity of its membership, in background and in belief.
Regardless of procedure, modern medicine now has a presence in multiple locations with varied disciplines coordinating care. Hospitalists and other providers know handoffs are safe with outcomes equivalent to, or exceeding, prior norms. SHM felt duty-bound to correct the record for future reference and the purpose of precedent.
In their landmark decision in the Whole Woman’s Health v. Hellerstedt case that challenged abortion restrictions put into place in the state of Texas, U.S. Supreme Court justices specifically cited an amicus brief submitted by the Society of Hospital Medicine.
Getting cited is a pretty big deal! It means that justices not only used what SHM wrote to inform their opinion, but also shows they gave it particular weight.
On June 27 in a 5-3 vote, the Court struck down both the admitting privileges and ambulatory surgical center requirements of the Texas law, finding that neither of these requirements provided sufficient medical benefits to justify the burdens imposed.
The brief, submitted by SHM in conjunction with the Society of OB/GYN Hospitalists last January, provided the Court with material to clarify obsolete impressions about how inpatient care currently transpires, how provider-to-provider transitions occur, and how admitting privileges work in real life.
Texas partially justified their law on views about site-to-site patient handoffs as unsafe and not in keeping with current care standards. However, as geographically based providers (hospitalists) know, care no longer arises in that manner and handoffs are not just the norm, but customary and safe.
SHM, however, did not weigh in on Constitutional questions, and did not take any stance on moral or ethical matters. SHM’s membership has diverse beliefs, and a position on this topic would be inappropriate.
In part, the SHM brief noted that:
“Admitting privileges are appropriate for physicians who regularly admit patients. But requiring physicians who specialize in outpatient procedures with low incidence of post-procedure complications, whether that specialty is podiatry or gynecology, to maintain privileges serves no medical purpose, is inconsistent with modern medicine, and is unnecessary to ensure continuity of care. Having a hospitalist serve as the admitting or attending physician does not deprive patients of quality inpatient or outpatient services.”
SHM’s amicus brief provides procedural clarification and informs the Court of how hospitalists function, but SHM emphasizes that it has not taken an ideological or ethical stance regarding the issue. SHM applauds the diversity of its membership, in background and in belief.
Regardless of procedure, modern medicine now has a presence in multiple locations with varied disciplines coordinating care. Hospitalists and other providers know handoffs are safe with outcomes equivalent to, or exceeding, prior norms. SHM felt duty-bound to correct the record for future reference and the purpose of precedent.
Significant race and health care disparities exist among hospitalized psoriasis patients
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Permanent pacemaker in TAVR: Earlier implantation costs much less
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
AT EUROPCR 2016
Key clinical point: The incremental cost of permanent pacemaker implantation more than 24 hours after transcatheter aortic valve replacement is almost twice as great as if the pacemaker goes in within 24 hours.
Major finding: The mean cost of hospitalization for transcatheter aortic valve replacement without permanent pacemaker implication in Medicare patients in 2014 was $63,136, compared with $80,441 if they needed a pacemaker.
Data source: This was a retrospective study of the health care costs and lengths of stay for all 12,148 hospitalizations for transcatheter aortic valve replacement in the Medicare inpatient database for 2014.
Disclosures: The presenter is an employee of Edwards Lifesciences, which funded the study.