User login
Ebola research update: July 2016
The struggle to defeat Ebola virus disease continues globally, although that effort may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
A study in the Journal of Infectious Diseases showed that responding to the challenges of confronting both the Ebola and Lassa fever viruses in West Africa will require continued investments in the development of countermeasures (e.g., vaccines, therapeutic agents, and diagnostic assays), infrastructure, and human resources.
The Partnership for Research on Ebola Virus in Liberia (PREVAIL), a U.S.-Liberia joint Clinical Research Partnership, has opened PREVAIL IV, a treatment trial for men who have survived Ebola virus disease (EVD) but continue to have evidence of Ebola virus genetic material, RNA, in their semen.
A report in Morbidity and Mortality Weekly Report (MMWR) revealed that the Sierra Leone Ministry of Health and Sanitation was able to prevent the transmission of Ebola virus within and beyond a family cluster by identifying contacts of a deceased woman who tested positive for the virus, monitoring them for Ebola signs and symptoms, ensuring timely treatment for anyone with Ebola, and conducting an epidemiologic investigation to identify the source of infection.
Lumbar punctures in three Ebola patients, presenting with behavioral modifications with ideation slowing and aggressiveness, found Ebola virus in all cerebrospinal fluids. The authors said this discovery contributes to the discussion of the concept of a specific Ebola virus encephalitis.
Researchers examined antibodies from Ebola or Marburg disease survivors 1-14 years post recovery and found persistent levels of antibodies to glycoprotein, recombinant nucleoprotein, and viral protein 40. The authors said survival of infection caused by one species imparted cross-reactive antibody responses to other filoviruses.
Yisheng Biopharma Co., a company focusing on biological vaccines and pharmaceutical products, and the United States Army Medical Research Institute of Infectious Diseases announced preliminary positive animal results of an Ebola virus-like particle–based vaccine in combination with PIKA adjuvant, a Toll-like receptor 3 (TLR3) agonist.
The U.S. Centers for Disease Control and Prevention released a supplement to MMWR chronicling the major aspects of the agency’s response to the 2014-2016 Ebola epidemic in West Africa. The reports summarized the CDC’s work, primarily during the first year and a half of the epidemic.
A study in eLife employed new human and animal occurrence data to expand upon the way in which potential bat EVD reservoir species are incorporated into a zoonotic niche map. The authors stated that it represents the most up-to-date estimate of the extent of EVD zoonotic risk in Africa.
New research in PLoS Neglected Tropical Diseases spotlights several potential host species and geographical regions as high-probability targets for future surveillance of Ebola and other filoviruses.
Soligenix Inc., a biopharmaceutical company focused on developing and commercializing products to treat rare diseases, announced positive preliminary proof-of-concept results on the development of a heat stable subunit Ebola vaccine.
A systematic review published in the American Journal of Infection Control of existing research pertinent to EVD and social media found that the utility of social media research – particularly focusing on Twitter and YouTube – to public health practitioners is warranted.
Governments and global health care organizations have a moral imperative to provide the required medical care to Ebola virus disease survivors, but must also seize the opportunity to collect evidence necessary to inform future guidelines for effective and safe medical care, researchers stated in a study published in the Journal of Hospital Infection.
The pharmaceutical manufacturer Merck announced that its investigational vaccine for Ebola Zaire, V920 was granted Breakthrough Therapy Designation by the Food and Drug Administration, and that the European Medicines Agency has granted the vaccine PRIME (PRIority MEdicines) status.
A study in the Journal of Infectious Diseases did not find strong evidence supporting respiratory or fomite-associated transmission of EVD in West Africa.
Investigators demonstrated the immunogenicity and protective efficacy of FILORAB1, a recombinant, bivalent, inactivated rabies virus–based Ebola virus vaccine, in rhesus and cynomolgus monkeys.
Cellular polyamines and hypusination are required for Ebolavirus gene expression and replication, a finding with implications for development of Ebola therapeutics, according to a study published in mBio.
The QuickNavi-Ebola immunochromatography (IC) assay is expected to be a useful tool for rapid diagnosis of Ebola virus disease, according to a study in The Journal of Infectious Diseases.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although that effort may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
A study in the Journal of Infectious Diseases showed that responding to the challenges of confronting both the Ebola and Lassa fever viruses in West Africa will require continued investments in the development of countermeasures (e.g., vaccines, therapeutic agents, and diagnostic assays), infrastructure, and human resources.
The Partnership for Research on Ebola Virus in Liberia (PREVAIL), a U.S.-Liberia joint Clinical Research Partnership, has opened PREVAIL IV, a treatment trial for men who have survived Ebola virus disease (EVD) but continue to have evidence of Ebola virus genetic material, RNA, in their semen.
A report in Morbidity and Mortality Weekly Report (MMWR) revealed that the Sierra Leone Ministry of Health and Sanitation was able to prevent the transmission of Ebola virus within and beyond a family cluster by identifying contacts of a deceased woman who tested positive for the virus, monitoring them for Ebola signs and symptoms, ensuring timely treatment for anyone with Ebola, and conducting an epidemiologic investigation to identify the source of infection.
Lumbar punctures in three Ebola patients, presenting with behavioral modifications with ideation slowing and aggressiveness, found Ebola virus in all cerebrospinal fluids. The authors said this discovery contributes to the discussion of the concept of a specific Ebola virus encephalitis.
Researchers examined antibodies from Ebola or Marburg disease survivors 1-14 years post recovery and found persistent levels of antibodies to glycoprotein, recombinant nucleoprotein, and viral protein 40. The authors said survival of infection caused by one species imparted cross-reactive antibody responses to other filoviruses.
Yisheng Biopharma Co., a company focusing on biological vaccines and pharmaceutical products, and the United States Army Medical Research Institute of Infectious Diseases announced preliminary positive animal results of an Ebola virus-like particle–based vaccine in combination with PIKA adjuvant, a Toll-like receptor 3 (TLR3) agonist.
The U.S. Centers for Disease Control and Prevention released a supplement to MMWR chronicling the major aspects of the agency’s response to the 2014-2016 Ebola epidemic in West Africa. The reports summarized the CDC’s work, primarily during the first year and a half of the epidemic.
A study in eLife employed new human and animal occurrence data to expand upon the way in which potential bat EVD reservoir species are incorporated into a zoonotic niche map. The authors stated that it represents the most up-to-date estimate of the extent of EVD zoonotic risk in Africa.
New research in PLoS Neglected Tropical Diseases spotlights several potential host species and geographical regions as high-probability targets for future surveillance of Ebola and other filoviruses.
Soligenix Inc., a biopharmaceutical company focused on developing and commercializing products to treat rare diseases, announced positive preliminary proof-of-concept results on the development of a heat stable subunit Ebola vaccine.
A systematic review published in the American Journal of Infection Control of existing research pertinent to EVD and social media found that the utility of social media research – particularly focusing on Twitter and YouTube – to public health practitioners is warranted.
Governments and global health care organizations have a moral imperative to provide the required medical care to Ebola virus disease survivors, but must also seize the opportunity to collect evidence necessary to inform future guidelines for effective and safe medical care, researchers stated in a study published in the Journal of Hospital Infection.
The pharmaceutical manufacturer Merck announced that its investigational vaccine for Ebola Zaire, V920 was granted Breakthrough Therapy Designation by the Food and Drug Administration, and that the European Medicines Agency has granted the vaccine PRIME (PRIority MEdicines) status.
A study in the Journal of Infectious Diseases did not find strong evidence supporting respiratory or fomite-associated transmission of EVD in West Africa.
Investigators demonstrated the immunogenicity and protective efficacy of FILORAB1, a recombinant, bivalent, inactivated rabies virus–based Ebola virus vaccine, in rhesus and cynomolgus monkeys.
Cellular polyamines and hypusination are required for Ebolavirus gene expression and replication, a finding with implications for development of Ebola therapeutics, according to a study published in mBio.
The QuickNavi-Ebola immunochromatography (IC) assay is expected to be a useful tool for rapid diagnosis of Ebola virus disease, according to a study in The Journal of Infectious Diseases.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although that effort may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
A study in the Journal of Infectious Diseases showed that responding to the challenges of confronting both the Ebola and Lassa fever viruses in West Africa will require continued investments in the development of countermeasures (e.g., vaccines, therapeutic agents, and diagnostic assays), infrastructure, and human resources.
The Partnership for Research on Ebola Virus in Liberia (PREVAIL), a U.S.-Liberia joint Clinical Research Partnership, has opened PREVAIL IV, a treatment trial for men who have survived Ebola virus disease (EVD) but continue to have evidence of Ebola virus genetic material, RNA, in their semen.
A report in Morbidity and Mortality Weekly Report (MMWR) revealed that the Sierra Leone Ministry of Health and Sanitation was able to prevent the transmission of Ebola virus within and beyond a family cluster by identifying contacts of a deceased woman who tested positive for the virus, monitoring them for Ebola signs and symptoms, ensuring timely treatment for anyone with Ebola, and conducting an epidemiologic investigation to identify the source of infection.
Lumbar punctures in three Ebola patients, presenting with behavioral modifications with ideation slowing and aggressiveness, found Ebola virus in all cerebrospinal fluids. The authors said this discovery contributes to the discussion of the concept of a specific Ebola virus encephalitis.
Researchers examined antibodies from Ebola or Marburg disease survivors 1-14 years post recovery and found persistent levels of antibodies to glycoprotein, recombinant nucleoprotein, and viral protein 40. The authors said survival of infection caused by one species imparted cross-reactive antibody responses to other filoviruses.
Yisheng Biopharma Co., a company focusing on biological vaccines and pharmaceutical products, and the United States Army Medical Research Institute of Infectious Diseases announced preliminary positive animal results of an Ebola virus-like particle–based vaccine in combination with PIKA adjuvant, a Toll-like receptor 3 (TLR3) agonist.
The U.S. Centers for Disease Control and Prevention released a supplement to MMWR chronicling the major aspects of the agency’s response to the 2014-2016 Ebola epidemic in West Africa. The reports summarized the CDC’s work, primarily during the first year and a half of the epidemic.
A study in eLife employed new human and animal occurrence data to expand upon the way in which potential bat EVD reservoir species are incorporated into a zoonotic niche map. The authors stated that it represents the most up-to-date estimate of the extent of EVD zoonotic risk in Africa.
New research in PLoS Neglected Tropical Diseases spotlights several potential host species and geographical regions as high-probability targets for future surveillance of Ebola and other filoviruses.
Soligenix Inc., a biopharmaceutical company focused on developing and commercializing products to treat rare diseases, announced positive preliminary proof-of-concept results on the development of a heat stable subunit Ebola vaccine.
A systematic review published in the American Journal of Infection Control of existing research pertinent to EVD and social media found that the utility of social media research – particularly focusing on Twitter and YouTube – to public health practitioners is warranted.
Governments and global health care organizations have a moral imperative to provide the required medical care to Ebola virus disease survivors, but must also seize the opportunity to collect evidence necessary to inform future guidelines for effective and safe medical care, researchers stated in a study published in the Journal of Hospital Infection.
The pharmaceutical manufacturer Merck announced that its investigational vaccine for Ebola Zaire, V920 was granted Breakthrough Therapy Designation by the Food and Drug Administration, and that the European Medicines Agency has granted the vaccine PRIME (PRIority MEdicines) status.
A study in the Journal of Infectious Diseases did not find strong evidence supporting respiratory or fomite-associated transmission of EVD in West Africa.
Investigators demonstrated the immunogenicity and protective efficacy of FILORAB1, a recombinant, bivalent, inactivated rabies virus–based Ebola virus vaccine, in rhesus and cynomolgus monkeys.
Cellular polyamines and hypusination are required for Ebolavirus gene expression and replication, a finding with implications for development of Ebola therapeutics, according to a study published in mBio.
The QuickNavi-Ebola immunochromatography (IC) assay is expected to be a useful tool for rapid diagnosis of Ebola virus disease, according to a study in The Journal of Infectious Diseases.
On Twitter @richpizzi
Work Intensity and IWPUT
After reading the July 2016 column on global periods and Current Procedural Terminology (CPT) code 99024,1 you may be wondering why you get paid what you do and how the procedure and visits all link together, which is associated with work intensity.
When CPT codes are given a value, the determination of the value of the work is performed via a survey process carried out by specialties for presentation to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which is used by the Centers for Medicare & Medicaid Services (CMS) to help determine relative value units (RVUs) that determine payment. The work RVU (wRVU) is typically around half of the total RVU for each CPT code. The value is based on multiple factors including the time to perform the service, the technical skill needed, the physical effort involved, mental effort and judgment, and stress under which the physician works due to the potential risks to the patient.2 A series of instruments and calculations have been used to determine a value called intraservice work per unit of time (IWPUT), which is used to examine the intraservice (skin-to-skin) work of a procedure relative to similar procedures.
Calculating the IWPUT
To determine the IWPUT value of a procedure, a formula is used to subtract all the preservice and postservice work and look at what is left based on the total RVUs for the procedure, which can be mathematically presented using the following construct: total wRVUs (the complete work you provide in performing the service) is the sum of preservice work (eg, evaluation and management [E&M] services, preparatory work [eg, scrub, dress, wait]), intraservice (skin-to-skin) work, immediate postservice work (eg, dressings, prescriptions, instructions given by the physician), and postoperative work at E&M visits (eg, hospital days, discharge day, global follow-up visits).
All of these activities defined as E&M services are simply subtracted from the total wRVU, while wRVUs for preservice and postservice work that is not linked to a CPT global period are calculated by simply subtracting the product of each specified time by their intensity (eg, day prior evaluation, same day evaluation, and immediate post have an intensity of 0.0224, while scrub/preparation has an intensity of 0.0081),3 leaving you with intraservice (skin-to-skin) work. This intraservice work is divided by the intraservice time to give you IWPUT. For more information on the concept as well as the process and controversies, an excellent review is available from the CMS.4
Understanding the IWPUT
The procedure with the highest IWPUT value in all of medicine is an emergency endotracheal intubation (CPT code 31500), which has a value of 0.4061.5 The procedure is short and intense, and if it fails, the patient is dead. All other procedures have lower IWPUT values. For example, a small malignant excision on the trunk, arms, or legs (CPT code 11600) has an IWPUT of 0.0324, while a laparoscopic cholecystectomy with exploration of the common duct (CPT code 47564) has an IWPUT of 0.0737.5 These small values have been the drivers behind much of the Relative Value Scale Update Committee’s valuation process for more than a decade. Some specialists who perform mostly 90-day global procedures wanted IWPUT to be the critical validation factor in the process, which led to problems for the first few years of this century. It may seem obvious that if there are 2 ways to fix a broken leg, the more complex one would likely have a higher IWPUT. Because IWPUT is a pure number with no values attached, this assumption would seem reasonable. If we compare a malignant excision to a benign one, we would expect higher intensity for the malignant one, as we are going deeper and have more concerns about clear margins and recurrences. Within a group of similar procedures, these pure numbers can be useful to validate a proposed value. More wRVUs in a shorter time period would result in a higher IWPUT; however, anomalies arise. There are eleven 000 global period CPT codes, ten 010 codes, and one hundred ninety 090 codes with negative IWPUTs, implying the skin-to-skin work has a value less than 0, which is an illogical conclusion. The more logical conclusion is that the codes are overloaded with preservice and postservice times. The real travesty is when one begins to compare apples to oranges—glaucoma surgery to belly surgery, endoscopy to skin surgery, or any other comparison you can come up with—taking a number that can be used to evaluate intensity between similar procedures and generalizing across all procedures, a concept that has never been validated. The wRVUs themselves define the relativity, but in many instances the IWPUT has been used in the process to justify forcing values lower based on cross-specialty comparisons, which may lead some to think we need better measures, as has been reported in the literature.6-8 Reform likely will happen, but for now we must work within the constraints of this tiny number, the IWPUT.
Obtaining the IWPUT
You are probably wondering, “How can I learn the IWPUT for the codes I use?” You probably do not want or need to other than to gain an understanding of how they have been misused. Purchase a subscription to the Resource-Based Relative Value Scale (RBRVS) DataManager Online or access the data for free through the CMS website (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html) by downloading the CY 2017 PFS Proposed Rule Addenda, which gives you total RVUs for all CPT codes, and the CY 2017 PFS Proposed Rule Physician Time, which gives you the preservice, intraservice, and postservice times for all CPT codes.
Using CPT code 11600 as an example, the total wRVU is 1.63, while preservice evaluation time is 10 minutes, intraservice time is 10 minutes, and immediate postservice time is 5 minutes. There is also 1 global follow-up visit, a CPT 99213, built in with a wRVU of 0.97 that determines the total value of the code. Using the IWPUT formula, we have the following: {1.63 – [(10 × 0.0224) + (5 × 0.0224) + 0.97]} / 10 = 0.0324.
These data also are useful if you are negotiating a contract based on RVUs, so learn a bit more about why you get paid what you are paid.
The Importance of IWPUT
Those interested in the academic discourse behind IWPUT should consult the literature,3,9 which is open source and freely available, but for now we will concentrate on why IWPUT is an important concept. As global periods are reevaluated under the Medicare Access and CHIP Reauthorization Act of 2015,10 the removal of global period visits will cause changes in the IWPUT value of codes, which will make them outliers and therefore targeted for resurvey and revaluation. The intent of the CMS is to cut reimbursement under our fee-for-service system, so there will be pain for physicians who have flourished under the current policy. To avoid inappropriate decreases in reimbursement, we should all keep accurate records of which global postoperative services are indeed provided, which leads us back to CPT code 99024. If it is not tracked, then it may not be seen as having been done. So be sure to use it.
Remember that if you do what you document, document what you do, and report medically necessary CPT codes, you should have nothing to worry about for now.
- Siegel DM. Global visits, 99024, and MACRA: 3 things you should think about and lose sleep over but probably do not. Cutis. 2016;98:43-44, 46.
- Overview of the RBRVS. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/medicare/the-resource-based-relative-value-scale/overview-of-rbrvs.page. Accessed July 14, 2016.
- Mabry CD, McCann BC, Harris JA, et al. The use of intraservice work per unit of time (IWPUT) and the building block method (BBM) for the calculation of surgical work. Ann Surg. 2005;241:929-938; discussion 938-940.
- Wynn BO, Burgette LF, Mulcahy AW, et al. Development of a Model for the Validation of Work Relative Value Units for the Medicare Physician Fee Schedule. Santa Monica, CA: RAND Corporation; 2015. http://www.rand.org/content/dam/rand/pubs/research_reports/RR600/RR662/RAND_RR662.pdf. Accessed July 18, 2016.
- RBRVS DataManager Online. American Medical Association. https://commerce.ama-assn .org/store/catalog/productDetail.jsp?product_ id=prod280002&navAction=push. Accessed July 20, 2016.
- Horner RD, Szaflarski JP, Ying J, et al. Physician work intensity among medical specialties: emerging evidence on its magnitude and composition. Med Care. 2011;49:1007-1111.
- Jacobson CJ Jr, Bolon S, Elder N, et al. Temporal and subjective work demands in office-based patient care: an exploration of the dimensions of physician work intensity. Med Care. 2011;49:52-58.
- Horner RD, Szaflarski JP, Jacobson CJ, et al. Clinical work intensity among physician specialties: how might we assess it? what do we find? Med Care. 2011;49:108-113.
- Zwolak RM, Trout HH 3rd. Vascular surgery and the Resource-based Relative Value Scale five-year review. J Vasc Surg. 1997;25:1077-1086.
- Medicare Access and CHIP Reauthorization Act of 2015, HR 2, 114th Cong, 1st Sess (2015).
After reading the July 2016 column on global periods and Current Procedural Terminology (CPT) code 99024,1 you may be wondering why you get paid what you do and how the procedure and visits all link together, which is associated with work intensity.
When CPT codes are given a value, the determination of the value of the work is performed via a survey process carried out by specialties for presentation to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which is used by the Centers for Medicare & Medicaid Services (CMS) to help determine relative value units (RVUs) that determine payment. The work RVU (wRVU) is typically around half of the total RVU for each CPT code. The value is based on multiple factors including the time to perform the service, the technical skill needed, the physical effort involved, mental effort and judgment, and stress under which the physician works due to the potential risks to the patient.2 A series of instruments and calculations have been used to determine a value called intraservice work per unit of time (IWPUT), which is used to examine the intraservice (skin-to-skin) work of a procedure relative to similar procedures.
Calculating the IWPUT
To determine the IWPUT value of a procedure, a formula is used to subtract all the preservice and postservice work and look at what is left based on the total RVUs for the procedure, which can be mathematically presented using the following construct: total wRVUs (the complete work you provide in performing the service) is the sum of preservice work (eg, evaluation and management [E&M] services, preparatory work [eg, scrub, dress, wait]), intraservice (skin-to-skin) work, immediate postservice work (eg, dressings, prescriptions, instructions given by the physician), and postoperative work at E&M visits (eg, hospital days, discharge day, global follow-up visits).
All of these activities defined as E&M services are simply subtracted from the total wRVU, while wRVUs for preservice and postservice work that is not linked to a CPT global period are calculated by simply subtracting the product of each specified time by their intensity (eg, day prior evaluation, same day evaluation, and immediate post have an intensity of 0.0224, while scrub/preparation has an intensity of 0.0081),3 leaving you with intraservice (skin-to-skin) work. This intraservice work is divided by the intraservice time to give you IWPUT. For more information on the concept as well as the process and controversies, an excellent review is available from the CMS.4
Understanding the IWPUT
The procedure with the highest IWPUT value in all of medicine is an emergency endotracheal intubation (CPT code 31500), which has a value of 0.4061.5 The procedure is short and intense, and if it fails, the patient is dead. All other procedures have lower IWPUT values. For example, a small malignant excision on the trunk, arms, or legs (CPT code 11600) has an IWPUT of 0.0324, while a laparoscopic cholecystectomy with exploration of the common duct (CPT code 47564) has an IWPUT of 0.0737.5 These small values have been the drivers behind much of the Relative Value Scale Update Committee’s valuation process for more than a decade. Some specialists who perform mostly 90-day global procedures wanted IWPUT to be the critical validation factor in the process, which led to problems for the first few years of this century. It may seem obvious that if there are 2 ways to fix a broken leg, the more complex one would likely have a higher IWPUT. Because IWPUT is a pure number with no values attached, this assumption would seem reasonable. If we compare a malignant excision to a benign one, we would expect higher intensity for the malignant one, as we are going deeper and have more concerns about clear margins and recurrences. Within a group of similar procedures, these pure numbers can be useful to validate a proposed value. More wRVUs in a shorter time period would result in a higher IWPUT; however, anomalies arise. There are eleven 000 global period CPT codes, ten 010 codes, and one hundred ninety 090 codes with negative IWPUTs, implying the skin-to-skin work has a value less than 0, which is an illogical conclusion. The more logical conclusion is that the codes are overloaded with preservice and postservice times. The real travesty is when one begins to compare apples to oranges—glaucoma surgery to belly surgery, endoscopy to skin surgery, or any other comparison you can come up with—taking a number that can be used to evaluate intensity between similar procedures and generalizing across all procedures, a concept that has never been validated. The wRVUs themselves define the relativity, but in many instances the IWPUT has been used in the process to justify forcing values lower based on cross-specialty comparisons, which may lead some to think we need better measures, as has been reported in the literature.6-8 Reform likely will happen, but for now we must work within the constraints of this tiny number, the IWPUT.
Obtaining the IWPUT
You are probably wondering, “How can I learn the IWPUT for the codes I use?” You probably do not want or need to other than to gain an understanding of how they have been misused. Purchase a subscription to the Resource-Based Relative Value Scale (RBRVS) DataManager Online or access the data for free through the CMS website (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html) by downloading the CY 2017 PFS Proposed Rule Addenda, which gives you total RVUs for all CPT codes, and the CY 2017 PFS Proposed Rule Physician Time, which gives you the preservice, intraservice, and postservice times for all CPT codes.
Using CPT code 11600 as an example, the total wRVU is 1.63, while preservice evaluation time is 10 minutes, intraservice time is 10 minutes, and immediate postservice time is 5 minutes. There is also 1 global follow-up visit, a CPT 99213, built in with a wRVU of 0.97 that determines the total value of the code. Using the IWPUT formula, we have the following: {1.63 – [(10 × 0.0224) + (5 × 0.0224) + 0.97]} / 10 = 0.0324.
These data also are useful if you are negotiating a contract based on RVUs, so learn a bit more about why you get paid what you are paid.
The Importance of IWPUT
Those interested in the academic discourse behind IWPUT should consult the literature,3,9 which is open source and freely available, but for now we will concentrate on why IWPUT is an important concept. As global periods are reevaluated under the Medicare Access and CHIP Reauthorization Act of 2015,10 the removal of global period visits will cause changes in the IWPUT value of codes, which will make them outliers and therefore targeted for resurvey and revaluation. The intent of the CMS is to cut reimbursement under our fee-for-service system, so there will be pain for physicians who have flourished under the current policy. To avoid inappropriate decreases in reimbursement, we should all keep accurate records of which global postoperative services are indeed provided, which leads us back to CPT code 99024. If it is not tracked, then it may not be seen as having been done. So be sure to use it.
Remember that if you do what you document, document what you do, and report medically necessary CPT codes, you should have nothing to worry about for now.
After reading the July 2016 column on global periods and Current Procedural Terminology (CPT) code 99024,1 you may be wondering why you get paid what you do and how the procedure and visits all link together, which is associated with work intensity.
When CPT codes are given a value, the determination of the value of the work is performed via a survey process carried out by specialties for presentation to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which is used by the Centers for Medicare & Medicaid Services (CMS) to help determine relative value units (RVUs) that determine payment. The work RVU (wRVU) is typically around half of the total RVU for each CPT code. The value is based on multiple factors including the time to perform the service, the technical skill needed, the physical effort involved, mental effort and judgment, and stress under which the physician works due to the potential risks to the patient.2 A series of instruments and calculations have been used to determine a value called intraservice work per unit of time (IWPUT), which is used to examine the intraservice (skin-to-skin) work of a procedure relative to similar procedures.
Calculating the IWPUT
To determine the IWPUT value of a procedure, a formula is used to subtract all the preservice and postservice work and look at what is left based on the total RVUs for the procedure, which can be mathematically presented using the following construct: total wRVUs (the complete work you provide in performing the service) is the sum of preservice work (eg, evaluation and management [E&M] services, preparatory work [eg, scrub, dress, wait]), intraservice (skin-to-skin) work, immediate postservice work (eg, dressings, prescriptions, instructions given by the physician), and postoperative work at E&M visits (eg, hospital days, discharge day, global follow-up visits).
All of these activities defined as E&M services are simply subtracted from the total wRVU, while wRVUs for preservice and postservice work that is not linked to a CPT global period are calculated by simply subtracting the product of each specified time by their intensity (eg, day prior evaluation, same day evaluation, and immediate post have an intensity of 0.0224, while scrub/preparation has an intensity of 0.0081),3 leaving you with intraservice (skin-to-skin) work. This intraservice work is divided by the intraservice time to give you IWPUT. For more information on the concept as well as the process and controversies, an excellent review is available from the CMS.4
Understanding the IWPUT
The procedure with the highest IWPUT value in all of medicine is an emergency endotracheal intubation (CPT code 31500), which has a value of 0.4061.5 The procedure is short and intense, and if it fails, the patient is dead. All other procedures have lower IWPUT values. For example, a small malignant excision on the trunk, arms, or legs (CPT code 11600) has an IWPUT of 0.0324, while a laparoscopic cholecystectomy with exploration of the common duct (CPT code 47564) has an IWPUT of 0.0737.5 These small values have been the drivers behind much of the Relative Value Scale Update Committee’s valuation process for more than a decade. Some specialists who perform mostly 90-day global procedures wanted IWPUT to be the critical validation factor in the process, which led to problems for the first few years of this century. It may seem obvious that if there are 2 ways to fix a broken leg, the more complex one would likely have a higher IWPUT. Because IWPUT is a pure number with no values attached, this assumption would seem reasonable. If we compare a malignant excision to a benign one, we would expect higher intensity for the malignant one, as we are going deeper and have more concerns about clear margins and recurrences. Within a group of similar procedures, these pure numbers can be useful to validate a proposed value. More wRVUs in a shorter time period would result in a higher IWPUT; however, anomalies arise. There are eleven 000 global period CPT codes, ten 010 codes, and one hundred ninety 090 codes with negative IWPUTs, implying the skin-to-skin work has a value less than 0, which is an illogical conclusion. The more logical conclusion is that the codes are overloaded with preservice and postservice times. The real travesty is when one begins to compare apples to oranges—glaucoma surgery to belly surgery, endoscopy to skin surgery, or any other comparison you can come up with—taking a number that can be used to evaluate intensity between similar procedures and generalizing across all procedures, a concept that has never been validated. The wRVUs themselves define the relativity, but in many instances the IWPUT has been used in the process to justify forcing values lower based on cross-specialty comparisons, which may lead some to think we need better measures, as has been reported in the literature.6-8 Reform likely will happen, but for now we must work within the constraints of this tiny number, the IWPUT.
Obtaining the IWPUT
You are probably wondering, “How can I learn the IWPUT for the codes I use?” You probably do not want or need to other than to gain an understanding of how they have been misused. Purchase a subscription to the Resource-Based Relative Value Scale (RBRVS) DataManager Online or access the data for free through the CMS website (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html) by downloading the CY 2017 PFS Proposed Rule Addenda, which gives you total RVUs for all CPT codes, and the CY 2017 PFS Proposed Rule Physician Time, which gives you the preservice, intraservice, and postservice times for all CPT codes.
Using CPT code 11600 as an example, the total wRVU is 1.63, while preservice evaluation time is 10 minutes, intraservice time is 10 minutes, and immediate postservice time is 5 minutes. There is also 1 global follow-up visit, a CPT 99213, built in with a wRVU of 0.97 that determines the total value of the code. Using the IWPUT formula, we have the following: {1.63 – [(10 × 0.0224) + (5 × 0.0224) + 0.97]} / 10 = 0.0324.
These data also are useful if you are negotiating a contract based on RVUs, so learn a bit more about why you get paid what you are paid.
The Importance of IWPUT
Those interested in the academic discourse behind IWPUT should consult the literature,3,9 which is open source and freely available, but for now we will concentrate on why IWPUT is an important concept. As global periods are reevaluated under the Medicare Access and CHIP Reauthorization Act of 2015,10 the removal of global period visits will cause changes in the IWPUT value of codes, which will make them outliers and therefore targeted for resurvey and revaluation. The intent of the CMS is to cut reimbursement under our fee-for-service system, so there will be pain for physicians who have flourished under the current policy. To avoid inappropriate decreases in reimbursement, we should all keep accurate records of which global postoperative services are indeed provided, which leads us back to CPT code 99024. If it is not tracked, then it may not be seen as having been done. So be sure to use it.
Remember that if you do what you document, document what you do, and report medically necessary CPT codes, you should have nothing to worry about for now.
- Siegel DM. Global visits, 99024, and MACRA: 3 things you should think about and lose sleep over but probably do not. Cutis. 2016;98:43-44, 46.
- Overview of the RBRVS. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/medicare/the-resource-based-relative-value-scale/overview-of-rbrvs.page. Accessed July 14, 2016.
- Mabry CD, McCann BC, Harris JA, et al. The use of intraservice work per unit of time (IWPUT) and the building block method (BBM) for the calculation of surgical work. Ann Surg. 2005;241:929-938; discussion 938-940.
- Wynn BO, Burgette LF, Mulcahy AW, et al. Development of a Model for the Validation of Work Relative Value Units for the Medicare Physician Fee Schedule. Santa Monica, CA: RAND Corporation; 2015. http://www.rand.org/content/dam/rand/pubs/research_reports/RR600/RR662/RAND_RR662.pdf. Accessed July 18, 2016.
- RBRVS DataManager Online. American Medical Association. https://commerce.ama-assn .org/store/catalog/productDetail.jsp?product_ id=prod280002&navAction=push. Accessed July 20, 2016.
- Horner RD, Szaflarski JP, Ying J, et al. Physician work intensity among medical specialties: emerging evidence on its magnitude and composition. Med Care. 2011;49:1007-1111.
- Jacobson CJ Jr, Bolon S, Elder N, et al. Temporal and subjective work demands in office-based patient care: an exploration of the dimensions of physician work intensity. Med Care. 2011;49:52-58.
- Horner RD, Szaflarski JP, Jacobson CJ, et al. Clinical work intensity among physician specialties: how might we assess it? what do we find? Med Care. 2011;49:108-113.
- Zwolak RM, Trout HH 3rd. Vascular surgery and the Resource-based Relative Value Scale five-year review. J Vasc Surg. 1997;25:1077-1086.
- Medicare Access and CHIP Reauthorization Act of 2015, HR 2, 114th Cong, 1st Sess (2015).
- Siegel DM. Global visits, 99024, and MACRA: 3 things you should think about and lose sleep over but probably do not. Cutis. 2016;98:43-44, 46.
- Overview of the RBRVS. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/medicare/the-resource-based-relative-value-scale/overview-of-rbrvs.page. Accessed July 14, 2016.
- Mabry CD, McCann BC, Harris JA, et al. The use of intraservice work per unit of time (IWPUT) and the building block method (BBM) for the calculation of surgical work. Ann Surg. 2005;241:929-938; discussion 938-940.
- Wynn BO, Burgette LF, Mulcahy AW, et al. Development of a Model for the Validation of Work Relative Value Units for the Medicare Physician Fee Schedule. Santa Monica, CA: RAND Corporation; 2015. http://www.rand.org/content/dam/rand/pubs/research_reports/RR600/RR662/RAND_RR662.pdf. Accessed July 18, 2016.
- RBRVS DataManager Online. American Medical Association. https://commerce.ama-assn .org/store/catalog/productDetail.jsp?product_ id=prod280002&navAction=push. Accessed July 20, 2016.
- Horner RD, Szaflarski JP, Ying J, et al. Physician work intensity among medical specialties: emerging evidence on its magnitude and composition. Med Care. 2011;49:1007-1111.
- Jacobson CJ Jr, Bolon S, Elder N, et al. Temporal and subjective work demands in office-based patient care: an exploration of the dimensions of physician work intensity. Med Care. 2011;49:52-58.
- Horner RD, Szaflarski JP, Jacobson CJ, et al. Clinical work intensity among physician specialties: how might we assess it? what do we find? Med Care. 2011;49:108-113.
- Zwolak RM, Trout HH 3rd. Vascular surgery and the Resource-based Relative Value Scale five-year review. J Vasc Surg. 1997;25:1077-1086.
- Medicare Access and CHIP Reauthorization Act of 2015, HR 2, 114th Cong, 1st Sess (2015).
Practice Points
- Intraservice work per unit of time (IWPUT) examines skin-to-skin work of a procedure.
- The removal of global period visits will cause changes in the IWPUT of codes.
Mayo Clinic tops hospital rankings for 2016-2017
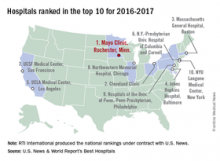
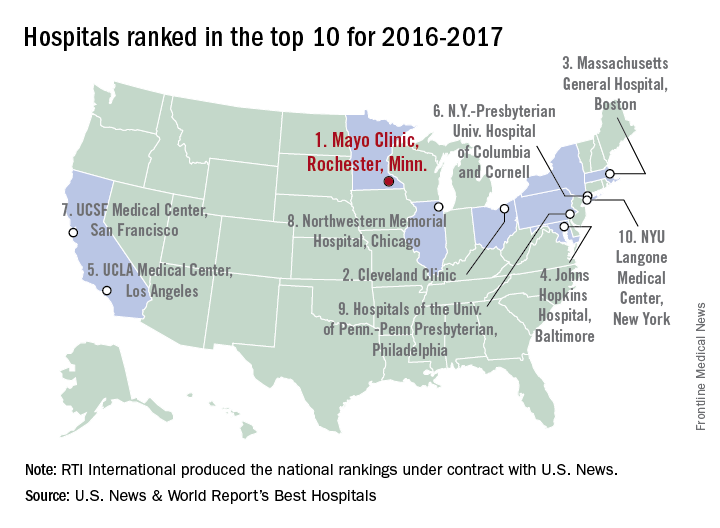
The Mayo Clinic in Rochester, Minn., was named the top hospital in the United States, according to the U.S. News & World Report Best Hospitals ranking for 2016-2017.
After finishing second to Massachusetts General Hospital in Boston last year, the Mayo Clinic regained the top spot it earned in 2014-2015. This year, the Cleveland Clinic finished second in the U.S. News Honor Roll, with Massachusetts General third, Johns Hopkins Hospital in Baltimore fourth, and the University of California, Los Angeles, Medical Center in fifth.
For 2016-2017, there were 20 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top among the 16 specialties included in the U.S. News rankings. This year, 153 hospitals did well enough to be nationally ranked in one or more specialties, which is up from the 144 that were ranked nationally in at least one specialty last year.
The 16 specialties used in the analysis are cancer, cardiology and heart surgery, diabetes and endocrinology, otolaryngology, gastroenterology and gastrointestinal surgery, geriatrics, gynecology, nephrology, neurology and neurosurgery, ophthalmology, orthopedics, psychiatry, pulmonology, rehabilitation, rheumatology, and urology.
This year’s ranking process initially included 4,667 nonfederal community hospitals. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
The Mayo Clinic in Rochester, Minn., was named the top hospital in the United States, according to the U.S. News & World Report Best Hospitals ranking for 2016-2017.
After finishing second to Massachusetts General Hospital in Boston last year, the Mayo Clinic regained the top spot it earned in 2014-2015. This year, the Cleveland Clinic finished second in the U.S. News Honor Roll, with Massachusetts General third, Johns Hopkins Hospital in Baltimore fourth, and the University of California, Los Angeles, Medical Center in fifth.
For 2016-2017, there were 20 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top among the 16 specialties included in the U.S. News rankings. This year, 153 hospitals did well enough to be nationally ranked in one or more specialties, which is up from the 144 that were ranked nationally in at least one specialty last year.
The 16 specialties used in the analysis are cancer, cardiology and heart surgery, diabetes and endocrinology, otolaryngology, gastroenterology and gastrointestinal surgery, geriatrics, gynecology, nephrology, neurology and neurosurgery, ophthalmology, orthopedics, psychiatry, pulmonology, rehabilitation, rheumatology, and urology.
This year’s ranking process initially included 4,667 nonfederal community hospitals. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
The Mayo Clinic in Rochester, Minn., was named the top hospital in the United States, according to the U.S. News & World Report Best Hospitals ranking for 2016-2017.
After finishing second to Massachusetts General Hospital in Boston last year, the Mayo Clinic regained the top spot it earned in 2014-2015. This year, the Cleveland Clinic finished second in the U.S. News Honor Roll, with Massachusetts General third, Johns Hopkins Hospital in Baltimore fourth, and the University of California, Los Angeles, Medical Center in fifth.
For 2016-2017, there were 20 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top among the 16 specialties included in the U.S. News rankings. This year, 153 hospitals did well enough to be nationally ranked in one or more specialties, which is up from the 144 that were ranked nationally in at least one specialty last year.
The 16 specialties used in the analysis are cancer, cardiology and heart surgery, diabetes and endocrinology, otolaryngology, gastroenterology and gastrointestinal surgery, geriatrics, gynecology, nephrology, neurology and neurosurgery, ophthalmology, orthopedics, psychiatry, pulmonology, rehabilitation, rheumatology, and urology.
This year’s ranking process initially included 4,667 nonfederal community hospitals. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
Daratumumab Effective in Combo Regimen
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Managing Patients Undergoing Cosmetic Procedures: Report From the AAD Meeting
Ensuring patients have a realistic expectation of cosmetic procedures starts with good communication between the physician and patient. Dr. Emmy Graber describes how to have a happy patient and focuses on conversations with patients regarding downtime, side effects, and treatment results. She also addresses the financial consequences of cosmetic procedures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Ensuring patients have a realistic expectation of cosmetic procedures starts with good communication between the physician and patient. Dr. Emmy Graber describes how to have a happy patient and focuses on conversations with patients regarding downtime, side effects, and treatment results. She also addresses the financial consequences of cosmetic procedures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Ensuring patients have a realistic expectation of cosmetic procedures starts with good communication between the physician and patient. Dr. Emmy Graber describes how to have a happy patient and focuses on conversations with patients regarding downtime, side effects, and treatment results. She also addresses the financial consequences of cosmetic procedures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Project BOOST Improves Care Transitions in Northern Arizona
Learn more by visiting www.hospitalmedicine.org/BOOST and clicking on “BOOST Results” to read the full case study.
Learn more by visiting www.hospitalmedicine.org/BOOST and clicking on “BOOST Results” to read the full case study.
Learn more by visiting www.hospitalmedicine.org/BOOST and clicking on “BOOST Results” to read the full case study.
SHM Can Help Your Hospital with Opioid Monitoring
Recruitment is under way for 10 hospitals to participate in a one-year mentored-implementation program related to opioid monitoring. SHM will be assigning two mentors to guide them through:
- Needs assessment
- Formal selection of data collection measures
- Data collection
- The implementation of key interventions to enhance safety for patients in the hospital who are prescribed opioid medications
The program will include monthly calls, a site visit with the SHM mentors, and a formal assessment of program implementation. Is your hospital interested in participating? Visit www.hospitalmedicine.org/RADEO to learn more and complete the form.
Recruitment is under way for 10 hospitals to participate in a one-year mentored-implementation program related to opioid monitoring. SHM will be assigning two mentors to guide them through:
- Needs assessment
- Formal selection of data collection measures
- Data collection
- The implementation of key interventions to enhance safety for patients in the hospital who are prescribed opioid medications
The program will include monthly calls, a site visit with the SHM mentors, and a formal assessment of program implementation. Is your hospital interested in participating? Visit www.hospitalmedicine.org/RADEO to learn more and complete the form.
Recruitment is under way for 10 hospitals to participate in a one-year mentored-implementation program related to opioid monitoring. SHM will be assigning two mentors to guide them through:
- Needs assessment
- Formal selection of data collection measures
- Data collection
- The implementation of key interventions to enhance safety for patients in the hospital who are prescribed opioid medications
The program will include monthly calls, a site visit with the SHM mentors, and a formal assessment of program implementation. Is your hospital interested in participating? Visit www.hospitalmedicine.org/RADEO to learn more and complete the form.
Stable INRs uncommon with long-term warfarin, study suggests

Results of a large study suggest most patients taking warfarin long-term do not maintain stable international normalized ratio (INR) values.
Researchers analyzed data from more than 3700 patients and found that 74% did not have stable INRs during the first 6 months of analysis.
Of the patients who did have stable INRs during this period, only 34% maintained stable INRs over the subsequent year.
Sean D. Pokorney, MD, of Duke University Medical Center in Durham, North Carolina, and his colleagues reported these findings in JAMA.
The researchers analyzed data from a prospective registry of patients with atrial fibrillation treated at 176 clinics. The patients were enrolled from June 2010 through August 2011 and followed for 3 years through November 2014.
Patients receiving warfarin at study entry with 3 or more INR values in the first 6 months and 6 or more in the subsequent year were included.
Of 10,132 registry patients, 6383 were not taking warfarin or had insufficient INR values and were excluded.
So there were 3749 eligible patients taking warfarin. Their average age was 75. Forty-three percent of patients were female, and 91% self-identified as white.
The patients’ median time from their first warfarin prescription to baseline was 3.9 years (range, 1.5-7.5 years). Thirty-seven percent of patients were also taking aspirin, and 5% were taking clopidogrel.
Results
INR stability was defined as 80% or more INRs in the therapeutic range (2.0-3.0).
Twenty-six percent of the patients met this definition during the first 6 months, and 34% of these patients maintained stable INRs over the subsequent year.
Ten percent of all patients had 100% of their INR values in the therapeutic range during the first 6 months. Of these patients, 37% met the definition of stability over the subsequent year.
Of the patients who had 80% or more of their INR values in the therapeutic range at baseline, 36% had 1 or more well-out-of-range INRs in the following year.
Of the patients with 100% of their baseline INR values in the therapeutic range, 33% had 1 or more well-out-of-range INRs in the subsequent year.
The researchers said these results suggest warfarin stability is difficult to predict, and this study challenges the notion that patients who have done well taking warfarin should continue taking warfarin. ![]()

Results of a large study suggest most patients taking warfarin long-term do not maintain stable international normalized ratio (INR) values.
Researchers analyzed data from more than 3700 patients and found that 74% did not have stable INRs during the first 6 months of analysis.
Of the patients who did have stable INRs during this period, only 34% maintained stable INRs over the subsequent year.
Sean D. Pokorney, MD, of Duke University Medical Center in Durham, North Carolina, and his colleagues reported these findings in JAMA.
The researchers analyzed data from a prospective registry of patients with atrial fibrillation treated at 176 clinics. The patients were enrolled from June 2010 through August 2011 and followed for 3 years through November 2014.
Patients receiving warfarin at study entry with 3 or more INR values in the first 6 months and 6 or more in the subsequent year were included.
Of 10,132 registry patients, 6383 were not taking warfarin or had insufficient INR values and were excluded.
So there were 3749 eligible patients taking warfarin. Their average age was 75. Forty-three percent of patients were female, and 91% self-identified as white.
The patients’ median time from their first warfarin prescription to baseline was 3.9 years (range, 1.5-7.5 years). Thirty-seven percent of patients were also taking aspirin, and 5% were taking clopidogrel.
Results
INR stability was defined as 80% or more INRs in the therapeutic range (2.0-3.0).
Twenty-six percent of the patients met this definition during the first 6 months, and 34% of these patients maintained stable INRs over the subsequent year.
Ten percent of all patients had 100% of their INR values in the therapeutic range during the first 6 months. Of these patients, 37% met the definition of stability over the subsequent year.
Of the patients who had 80% or more of their INR values in the therapeutic range at baseline, 36% had 1 or more well-out-of-range INRs in the following year.
Of the patients with 100% of their baseline INR values in the therapeutic range, 33% had 1 or more well-out-of-range INRs in the subsequent year.
The researchers said these results suggest warfarin stability is difficult to predict, and this study challenges the notion that patients who have done well taking warfarin should continue taking warfarin. ![]()

Results of a large study suggest most patients taking warfarin long-term do not maintain stable international normalized ratio (INR) values.
Researchers analyzed data from more than 3700 patients and found that 74% did not have stable INRs during the first 6 months of analysis.
Of the patients who did have stable INRs during this period, only 34% maintained stable INRs over the subsequent year.
Sean D. Pokorney, MD, of Duke University Medical Center in Durham, North Carolina, and his colleagues reported these findings in JAMA.
The researchers analyzed data from a prospective registry of patients with atrial fibrillation treated at 176 clinics. The patients were enrolled from June 2010 through August 2011 and followed for 3 years through November 2014.
Patients receiving warfarin at study entry with 3 or more INR values in the first 6 months and 6 or more in the subsequent year were included.
Of 10,132 registry patients, 6383 were not taking warfarin or had insufficient INR values and were excluded.
So there were 3749 eligible patients taking warfarin. Their average age was 75. Forty-three percent of patients were female, and 91% self-identified as white.
The patients’ median time from their first warfarin prescription to baseline was 3.9 years (range, 1.5-7.5 years). Thirty-seven percent of patients were also taking aspirin, and 5% were taking clopidogrel.
Results
INR stability was defined as 80% or more INRs in the therapeutic range (2.0-3.0).
Twenty-six percent of the patients met this definition during the first 6 months, and 34% of these patients maintained stable INRs over the subsequent year.
Ten percent of all patients had 100% of their INR values in the therapeutic range during the first 6 months. Of these patients, 37% met the definition of stability over the subsequent year.
Of the patients who had 80% or more of their INR values in the therapeutic range at baseline, 36% had 1 or more well-out-of-range INRs in the following year.
Of the patients with 100% of their baseline INR values in the therapeutic range, 33% had 1 or more well-out-of-range INRs in the subsequent year.
The researchers said these results suggest warfarin stability is difficult to predict, and this study challenges the notion that patients who have done well taking warfarin should continue taking warfarin. ![]()
Device could be used to monitor antiplatelet therapy
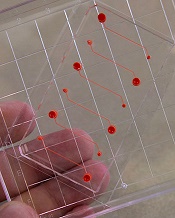
Photo courtesy of the
Wyss Institute at Harvard
A novel microfluidic device can be used to monitor thrombus formation and platelet function in patients receiving antiplatelet therapy, according to research published in Biomedical Microdevices.
The device is designed to mimic cellular and vascular flow conditions inside the human body.
Investigators say it demonstrates how endothelial cells contribute to hemostasis, even though it does not contain living endothelial cells.
The device contains microfluidic channels lined with chemically fixed human endothelial cells.
“It’s a bioinspired device that contains the endothelial function of a diseased patient without having actual living cells, and this greatly increases the robustness of the device,” explained study author Abhishek Jain, PhD, of Texas A&M University in College Station, Texas.
D Jain and his colleagues said their device retains the ability to modulate hemostasis under continuous flow in vitro, even after a few days of storage.
And the team successfully used the device to measure thrombus formation and platelet function in small amounts of whole blood from patients receiving antiplatelet therapy.
“Abnormal blood coagulation and platelet activation are major medical problems, and the ways we study them now are overly simplified,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston, Massachusetts.
“Clinicians currently do not have tools to monitor hemostasis that take into account physiologically important interactions between endothelial cells and flowing blood.”
In a previous study, Dr Ingber and his colleagues showed that recreating the physicality and blood flow of vasculature within microfluidic channels allowed them to predict precise times that blood might clot, with potential applications in real-time monitoring of patients receiving anticoagulants.
The group’s new device adds another layer of complexity by embedding the functionality of the vascular endothelium within a tool that might be manufactured, stored, and shipped for clinical use.
“This is one of the first examples of how a microfluidic cell culture system could have added value in clinical diagnostics,” said study author Andries van der Meer, PhD, of University of Twente in Enschede, Netherlands.
“Using chemically fixed tissue that is no longer alive offers a clear, low-risk path toward further testing and product development.” ![]()

Photo courtesy of the
Wyss Institute at Harvard
A novel microfluidic device can be used to monitor thrombus formation and platelet function in patients receiving antiplatelet therapy, according to research published in Biomedical Microdevices.
The device is designed to mimic cellular and vascular flow conditions inside the human body.
Investigators say it demonstrates how endothelial cells contribute to hemostasis, even though it does not contain living endothelial cells.
The device contains microfluidic channels lined with chemically fixed human endothelial cells.
“It’s a bioinspired device that contains the endothelial function of a diseased patient without having actual living cells, and this greatly increases the robustness of the device,” explained study author Abhishek Jain, PhD, of Texas A&M University in College Station, Texas.
D Jain and his colleagues said their device retains the ability to modulate hemostasis under continuous flow in vitro, even after a few days of storage.
And the team successfully used the device to measure thrombus formation and platelet function in small amounts of whole blood from patients receiving antiplatelet therapy.
“Abnormal blood coagulation and platelet activation are major medical problems, and the ways we study them now are overly simplified,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston, Massachusetts.
“Clinicians currently do not have tools to monitor hemostasis that take into account physiologically important interactions between endothelial cells and flowing blood.”
In a previous study, Dr Ingber and his colleagues showed that recreating the physicality and blood flow of vasculature within microfluidic channels allowed them to predict precise times that blood might clot, with potential applications in real-time monitoring of patients receiving anticoagulants.
The group’s new device adds another layer of complexity by embedding the functionality of the vascular endothelium within a tool that might be manufactured, stored, and shipped for clinical use.
“This is one of the first examples of how a microfluidic cell culture system could have added value in clinical diagnostics,” said study author Andries van der Meer, PhD, of University of Twente in Enschede, Netherlands.
“Using chemically fixed tissue that is no longer alive offers a clear, low-risk path toward further testing and product development.” ![]()

Photo courtesy of the
Wyss Institute at Harvard
A novel microfluidic device can be used to monitor thrombus formation and platelet function in patients receiving antiplatelet therapy, according to research published in Biomedical Microdevices.
The device is designed to mimic cellular and vascular flow conditions inside the human body.
Investigators say it demonstrates how endothelial cells contribute to hemostasis, even though it does not contain living endothelial cells.
The device contains microfluidic channels lined with chemically fixed human endothelial cells.
“It’s a bioinspired device that contains the endothelial function of a diseased patient without having actual living cells, and this greatly increases the robustness of the device,” explained study author Abhishek Jain, PhD, of Texas A&M University in College Station, Texas.
D Jain and his colleagues said their device retains the ability to modulate hemostasis under continuous flow in vitro, even after a few days of storage.
And the team successfully used the device to measure thrombus formation and platelet function in small amounts of whole blood from patients receiving antiplatelet therapy.
“Abnormal blood coagulation and platelet activation are major medical problems, and the ways we study them now are overly simplified,” said study author Donald Ingber, MD, PhD, of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston, Massachusetts.
“Clinicians currently do not have tools to monitor hemostasis that take into account physiologically important interactions between endothelial cells and flowing blood.”
In a previous study, Dr Ingber and his colleagues showed that recreating the physicality and blood flow of vasculature within microfluidic channels allowed them to predict precise times that blood might clot, with potential applications in real-time monitoring of patients receiving anticoagulants.
The group’s new device adds another layer of complexity by embedding the functionality of the vascular endothelium within a tool that might be manufactured, stored, and shipped for clinical use.
“This is one of the first examples of how a microfluidic cell culture system could have added value in clinical diagnostics,” said study author Andries van der Meer, PhD, of University of Twente in Enschede, Netherlands.
“Using chemically fixed tissue that is no longer alive offers a clear, low-risk path toward further testing and product development.” ![]()
Odor-baited mosquito traps can fight malaria

Photo courtesy of the CDC
Solar-powered mosquito traps incorporating human odor can reduce the incidence of malaria, according to research published in The Lancet.
Researchers introduced these traps to homes on the Kenyan island of Rusinga.
The population of malaria-carrying mosquitoes declined by 42% in homes that had the traps.
And the prevalence of malaria was 30% lower among people living in houses with a trap than among those in houses without a trap.
“The objective of the trial on Rusinga Island in Lake Victoria was to investigate whether malaria mosquitoes can be captured and destroyed using traps with a lure so that the risk of new malaria infections is minimized,” explained study author Willem Takken, PhD, of Wageningen University and Research Centre in Wageningen, Netherlands.
The trial enrolled 34,041 participants. Each individual was assigned to a cluster, which consisted of 50 or 51 geographically contiguous households. There were 81 clusters in all.
The researchers installed their solar-powered, odor-baited mosquito trapping systems (SMoTS) in the various households, cluster by cluster, until all of the clusters had the traps.
During the roll-out period—between June 3, 2013, and May 16, 2015—SMoTS were installed in 4358 households.
The density of Anopheles mosquitoes was lower in the clusters with SMoTS than those without. The adjusted estimated effectiveness of the traps was 42.2%.
The densities of Anopheles funestus and Anopheles gambiae mosquitoes were lower in clusters with SMoTS than those without. The adjusted estimated effectiveness was 69.2% (P=0.005) and 10.8% (P=0.6), respectively.
The prevalence of malaria was 29.8% lower in clusters with SMoTS than those without (P<0.0001).
About 24% of people in clusters with SMoTS were positive for Plasmodium parasites (23.7%, 1552/6550), compared to about 35% of people in clusters without SMoTS (34.5%, 2002/5795).
“Ultimately, we want to eradicate malaria completely, in an environmentally friendly and sustainable manner,” Dr Takken said.
“As we use a natural lure—namely, human odor—in our approach, there is no negative impact on the environment, and it is very improbable that the mosquitoes will become ‘resistant’ to being captured. After all, the mosquitoes need their attraction to the lure in order to be able to survive.”
Dr Takken and his colleagues believe their SMoTS may also be able to combat dengue fever and the Zika virus. Aedes aegypti is a vector for these viruses, and this mosquito is attracted to the same humanized scent that attracts malaria-carrying mosquitoes. ![]()

Photo courtesy of the CDC
Solar-powered mosquito traps incorporating human odor can reduce the incidence of malaria, according to research published in The Lancet.
Researchers introduced these traps to homes on the Kenyan island of Rusinga.
The population of malaria-carrying mosquitoes declined by 42% in homes that had the traps.
And the prevalence of malaria was 30% lower among people living in houses with a trap than among those in houses without a trap.
“The objective of the trial on Rusinga Island in Lake Victoria was to investigate whether malaria mosquitoes can be captured and destroyed using traps with a lure so that the risk of new malaria infections is minimized,” explained study author Willem Takken, PhD, of Wageningen University and Research Centre in Wageningen, Netherlands.
The trial enrolled 34,041 participants. Each individual was assigned to a cluster, which consisted of 50 or 51 geographically contiguous households. There were 81 clusters in all.
The researchers installed their solar-powered, odor-baited mosquito trapping systems (SMoTS) in the various households, cluster by cluster, until all of the clusters had the traps.
During the roll-out period—between June 3, 2013, and May 16, 2015—SMoTS were installed in 4358 households.
The density of Anopheles mosquitoes was lower in the clusters with SMoTS than those without. The adjusted estimated effectiveness of the traps was 42.2%.
The densities of Anopheles funestus and Anopheles gambiae mosquitoes were lower in clusters with SMoTS than those without. The adjusted estimated effectiveness was 69.2% (P=0.005) and 10.8% (P=0.6), respectively.
The prevalence of malaria was 29.8% lower in clusters with SMoTS than those without (P<0.0001).
About 24% of people in clusters with SMoTS were positive for Plasmodium parasites (23.7%, 1552/6550), compared to about 35% of people in clusters without SMoTS (34.5%, 2002/5795).
“Ultimately, we want to eradicate malaria completely, in an environmentally friendly and sustainable manner,” Dr Takken said.
“As we use a natural lure—namely, human odor—in our approach, there is no negative impact on the environment, and it is very improbable that the mosquitoes will become ‘resistant’ to being captured. After all, the mosquitoes need their attraction to the lure in order to be able to survive.”
Dr Takken and his colleagues believe their SMoTS may also be able to combat dengue fever and the Zika virus. Aedes aegypti is a vector for these viruses, and this mosquito is attracted to the same humanized scent that attracts malaria-carrying mosquitoes. ![]()

Photo courtesy of the CDC
Solar-powered mosquito traps incorporating human odor can reduce the incidence of malaria, according to research published in The Lancet.
Researchers introduced these traps to homes on the Kenyan island of Rusinga.
The population of malaria-carrying mosquitoes declined by 42% in homes that had the traps.
And the prevalence of malaria was 30% lower among people living in houses with a trap than among those in houses without a trap.
“The objective of the trial on Rusinga Island in Lake Victoria was to investigate whether malaria mosquitoes can be captured and destroyed using traps with a lure so that the risk of new malaria infections is minimized,” explained study author Willem Takken, PhD, of Wageningen University and Research Centre in Wageningen, Netherlands.
The trial enrolled 34,041 participants. Each individual was assigned to a cluster, which consisted of 50 or 51 geographically contiguous households. There were 81 clusters in all.
The researchers installed their solar-powered, odor-baited mosquito trapping systems (SMoTS) in the various households, cluster by cluster, until all of the clusters had the traps.
During the roll-out period—between June 3, 2013, and May 16, 2015—SMoTS were installed in 4358 households.
The density of Anopheles mosquitoes was lower in the clusters with SMoTS than those without. The adjusted estimated effectiveness of the traps was 42.2%.
The densities of Anopheles funestus and Anopheles gambiae mosquitoes were lower in clusters with SMoTS than those without. The adjusted estimated effectiveness was 69.2% (P=0.005) and 10.8% (P=0.6), respectively.
The prevalence of malaria was 29.8% lower in clusters with SMoTS than those without (P<0.0001).
About 24% of people in clusters with SMoTS were positive for Plasmodium parasites (23.7%, 1552/6550), compared to about 35% of people in clusters without SMoTS (34.5%, 2002/5795).
“Ultimately, we want to eradicate malaria completely, in an environmentally friendly and sustainable manner,” Dr Takken said.
“As we use a natural lure—namely, human odor—in our approach, there is no negative impact on the environment, and it is very improbable that the mosquitoes will become ‘resistant’ to being captured. After all, the mosquitoes need their attraction to the lure in order to be able to survive.”
Dr Takken and his colleagues believe their SMoTS may also be able to combat dengue fever and the Zika virus. Aedes aegypti is a vector for these viruses, and this mosquito is attracted to the same humanized scent that attracts malaria-carrying mosquitoes. ![]()