User login
Lipid Screening in Young Adults Still Unsettled
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
FROM ANNALS OF INTERNAL MEDICINE
Lipid screening in young adults still unsettled
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
The value of screening young adults for dyslipidemia remains unknown because there is still no direct evidence regarding the benefits and harms in this patient population, according to an update of the 2008 U.S. Preventive Services Task Force recommendations on lipid screening, which was published online August 8 in Annals of Internal Medicine.
In 2008, the USPSTF also could find no direct evidence regarding asymptomatic men and women aged 21-39 years, and thus could make no recommendation for or against lipid screening “because of the low likelihood of identifying lipid levels high enough to justify treatment.” Now the organization has undertaken an extensive review of all English-language articles released since then, searching the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, Medline, reference lists, and ClinicalTrials.gov, said Roger Chou, MD, lead author of the update and director of the Pacific Northwest Evidence-Based Practice Center, Oregon Health & Science University, Portland, and his associates.
They were unable to find any randomized trials, cohort studies, or case-control studies comparing lipid screening against no lipid screening, dyslipidemia treatment against no treatment, or immediate against delayed treatment that assessed mortality, cardiovascular outcomes, or harms in this age group.
Some health organizations recommend starting dyslipidemia screening in asymptomatic adults at age 20, while others don’t recommend the practice until age 35-40 for men and age 50 for women. The 2014 American College of Cardiology/American Heart Association guideline on assessing CV risk deems it “reasonable” to assess traditional risk factors including lipids beginning at age 20, Dr. Chou and his associates said (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0946).
However, the potential adverse effects of statin therapy that is initiated in young adulthood and continued for decades haven’t been well studied, they noted.
In addition, some experts advocate lipid screening to identify young adults who have familial hypercholesterolemia. But this condition has such a low prevalence (estimated at only 1 in 500 people), and affected patients have such a low rate of coronary heat disease events before age 40 (approximately 10%) that the potential benefits of screening for this reason are very limited, the investigators added.
This study was supported by the Agency for Healthcare Research and Quality.
FROM ANNALS OF INTERNAL MEDICINE
Elbasvir-grazoprevir works effectively against HCV despite current drug use
A 12-week course of elbasvir plus grazoprevir proved effective against hepatitis C virus in 301 patients who were receiving opioid agonist therapy for injectable drug addiction, according to a report published online August 8 in Annals of Internal Medicine.
This patient population showed excellent treatment adherence and achieved sustained virologic response (SVR) rates of approximately 90%, even though most participants continued to use injectable drugs during the study. These findings demonstrate that current drug users can achieve HCV treatment outcomes that are comparable to those in the general HCV population, and “suggest that access to interferon-free direct-acting antiviral therapy should be expanded to patients receiving opioid agonist therapy, including the removal of drug use-based restrictions,” said Gregory J. Dore, MD, of the Kirby Institute, University of New South Wales, Sydney, and his associates.
They assessed elbasvir-grazoprevir (Zepatier) treatment in patients with untreated chronic HCV who were aged 18 and older and had been receiving opioid agonist therapy with methadone, buprenorphine, or buprenorphine-naloxone for at least 3 months at facilities in 13 countries. The study participants were randomly assigned in double-blind fashion to receive either immediate active treatment for 12 weeks (201 patients) or matching placebo for 12 weeks followed by 4 weeks of follow-up followed by deferred open-label active treatment for 12 weeks (100 control subjects).
All patients were followed for 6 months after they completed active treatment. All underwent frequent urine testing for illegal drugs, and more than half of both study groups tested positive for at least one drug during active treatment and follow-up. Illegal drug use was stable throughout the study period and did not affect either treatment adherence or efficacy. Nearly every participant showed excellent adherence (over 95%) to elbasvir-grazoprevir.
The primary efficacy endpoint, the SVR rate immediately after active treatment, was 91.5% in the immediate-treatment group and 89.5% in the deferred-treatment group. Both rates are substantially higher than the historical reference SVR rate of 67%, Dr. Dore and his associates noted (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0816).
Elbasvir-grazoprevir was equally effective against the GT1a, GT1b, and GT4 strains of HCV, but was less effective against the GT6 strain, which occurs primarily in China and Southeast Asia. It also was effective across important subgroups of patients, including those who had cirrhosis and those who carried HCV variants associated with drug resistance.
Regarding treatment safety, the rate of serious adverse events was low in both study groups (3.5% and 4.0%, respectively), and only one event in each group was deemed to be related to elbasvir-grazoprevir.
The incidence of reinfection for the 24-week period after successful treatment was 4.6 per 100 person-years, and it often was attributed to continuing use of contaminated needles or sexual contact with an infected partner. The effect of reinfection is “of considerable clinical and public health interest,” and will be further examined during a 3-year extension of the follow-up of this study, the investigators said.
“Of interest, 3 of the 6 patients categorized as having HCV reinfection subsequently had undetectable HCV-RNA levels without additional HCV treatment, indicating that not all reinfection cases develop viral persistence,” they added.
This study was funded primarily by Merck, which markets Zepatier. Dr. Dore reported ties to AbbVie, Merck, Bristol-Myers Squibb, Janssen, Roche, Gilead, GlaxoSmithKline, and Abbott, and his associates reported ties to numerous industry sources.
A 12-week course of elbasvir plus grazoprevir proved effective against hepatitis C virus in 301 patients who were receiving opioid agonist therapy for injectable drug addiction, according to a report published online August 8 in Annals of Internal Medicine.
This patient population showed excellent treatment adherence and achieved sustained virologic response (SVR) rates of approximately 90%, even though most participants continued to use injectable drugs during the study. These findings demonstrate that current drug users can achieve HCV treatment outcomes that are comparable to those in the general HCV population, and “suggest that access to interferon-free direct-acting antiviral therapy should be expanded to patients receiving opioid agonist therapy, including the removal of drug use-based restrictions,” said Gregory J. Dore, MD, of the Kirby Institute, University of New South Wales, Sydney, and his associates.
They assessed elbasvir-grazoprevir (Zepatier) treatment in patients with untreated chronic HCV who were aged 18 and older and had been receiving opioid agonist therapy with methadone, buprenorphine, or buprenorphine-naloxone for at least 3 months at facilities in 13 countries. The study participants were randomly assigned in double-blind fashion to receive either immediate active treatment for 12 weeks (201 patients) or matching placebo for 12 weeks followed by 4 weeks of follow-up followed by deferred open-label active treatment for 12 weeks (100 control subjects).
All patients were followed for 6 months after they completed active treatment. All underwent frequent urine testing for illegal drugs, and more than half of both study groups tested positive for at least one drug during active treatment and follow-up. Illegal drug use was stable throughout the study period and did not affect either treatment adherence or efficacy. Nearly every participant showed excellent adherence (over 95%) to elbasvir-grazoprevir.
The primary efficacy endpoint, the SVR rate immediately after active treatment, was 91.5% in the immediate-treatment group and 89.5% in the deferred-treatment group. Both rates are substantially higher than the historical reference SVR rate of 67%, Dr. Dore and his associates noted (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0816).
Elbasvir-grazoprevir was equally effective against the GT1a, GT1b, and GT4 strains of HCV, but was less effective against the GT6 strain, which occurs primarily in China and Southeast Asia. It also was effective across important subgroups of patients, including those who had cirrhosis and those who carried HCV variants associated with drug resistance.
Regarding treatment safety, the rate of serious adverse events was low in both study groups (3.5% and 4.0%, respectively), and only one event in each group was deemed to be related to elbasvir-grazoprevir.
The incidence of reinfection for the 24-week period after successful treatment was 4.6 per 100 person-years, and it often was attributed to continuing use of contaminated needles or sexual contact with an infected partner. The effect of reinfection is “of considerable clinical and public health interest,” and will be further examined during a 3-year extension of the follow-up of this study, the investigators said.
“Of interest, 3 of the 6 patients categorized as having HCV reinfection subsequently had undetectable HCV-RNA levels without additional HCV treatment, indicating that not all reinfection cases develop viral persistence,” they added.
This study was funded primarily by Merck, which markets Zepatier. Dr. Dore reported ties to AbbVie, Merck, Bristol-Myers Squibb, Janssen, Roche, Gilead, GlaxoSmithKline, and Abbott, and his associates reported ties to numerous industry sources.
A 12-week course of elbasvir plus grazoprevir proved effective against hepatitis C virus in 301 patients who were receiving opioid agonist therapy for injectable drug addiction, according to a report published online August 8 in Annals of Internal Medicine.
This patient population showed excellent treatment adherence and achieved sustained virologic response (SVR) rates of approximately 90%, even though most participants continued to use injectable drugs during the study. These findings demonstrate that current drug users can achieve HCV treatment outcomes that are comparable to those in the general HCV population, and “suggest that access to interferon-free direct-acting antiviral therapy should be expanded to patients receiving opioid agonist therapy, including the removal of drug use-based restrictions,” said Gregory J. Dore, MD, of the Kirby Institute, University of New South Wales, Sydney, and his associates.
They assessed elbasvir-grazoprevir (Zepatier) treatment in patients with untreated chronic HCV who were aged 18 and older and had been receiving opioid agonist therapy with methadone, buprenorphine, or buprenorphine-naloxone for at least 3 months at facilities in 13 countries. The study participants were randomly assigned in double-blind fashion to receive either immediate active treatment for 12 weeks (201 patients) or matching placebo for 12 weeks followed by 4 weeks of follow-up followed by deferred open-label active treatment for 12 weeks (100 control subjects).
All patients were followed for 6 months after they completed active treatment. All underwent frequent urine testing for illegal drugs, and more than half of both study groups tested positive for at least one drug during active treatment and follow-up. Illegal drug use was stable throughout the study period and did not affect either treatment adherence or efficacy. Nearly every participant showed excellent adherence (over 95%) to elbasvir-grazoprevir.
The primary efficacy endpoint, the SVR rate immediately after active treatment, was 91.5% in the immediate-treatment group and 89.5% in the deferred-treatment group. Both rates are substantially higher than the historical reference SVR rate of 67%, Dr. Dore and his associates noted (Ann Intern Med. 2016 Aug 8. doi: 10.7326/M16-0816).
Elbasvir-grazoprevir was equally effective against the GT1a, GT1b, and GT4 strains of HCV, but was less effective against the GT6 strain, which occurs primarily in China and Southeast Asia. It also was effective across important subgroups of patients, including those who had cirrhosis and those who carried HCV variants associated with drug resistance.
Regarding treatment safety, the rate of serious adverse events was low in both study groups (3.5% and 4.0%, respectively), and only one event in each group was deemed to be related to elbasvir-grazoprevir.
The incidence of reinfection for the 24-week period after successful treatment was 4.6 per 100 person-years, and it often was attributed to continuing use of contaminated needles or sexual contact with an infected partner. The effect of reinfection is “of considerable clinical and public health interest,” and will be further examined during a 3-year extension of the follow-up of this study, the investigators said.
“Of interest, 3 of the 6 patients categorized as having HCV reinfection subsequently had undetectable HCV-RNA levels without additional HCV treatment, indicating that not all reinfection cases develop viral persistence,” they added.
This study was funded primarily by Merck, which markets Zepatier. Dr. Dore reported ties to AbbVie, Merck, Bristol-Myers Squibb, Janssen, Roche, Gilead, GlaxoSmithKline, and Abbott, and his associates reported ties to numerous industry sources.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: A 12-week course of elbasvir plus grazoprevir was effective against HCV in patients receiving opioid agonist therapy for injectable drug addiction.
Major finding: The primary efficacy endpoint, the SVR rate after active treatment, was 91.5% with immediate treatment and 89.5% with deferred treatment.
Data source: An international, randomized, placebo-controlled, double-blind trial involving 301 patients followed for 6 months.
Disclosures: This study was funded primarily by Merck, which markets Zepatier. Dr. Dore reported ties to AbbVie, Merck, Bristol-Myers Squibb, Janssen, Roche, Gilead, GlaxoSmithKline, and Abbott, and his associates reported ties to numerous industry sources.
Skip SNL biopsy for desmoplastic melanoma
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
AT AHNS 2016
Key clinical point: Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Major finding: SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Data source: Retrospective case-control study with 92 patients
Disclosures: There was no outside funding for the work, and the presenter had no disclosures.
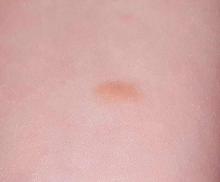
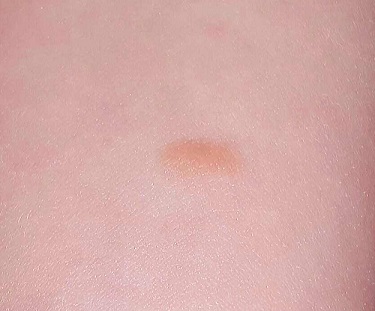
The Birthmark That Wasn't
A 3-year-old girl is brought to dermatology by her mother, who requests evaluation of a lesion that manifested shortly after the child’s birth. The family’s primary care provider has always dismissed her lesion as a birthmark, but her parents are concerned by its tendency to abruptly change for no apparent reason. It swells up, becomes itchy and red, and then returns to normal within minutes.
According to her parents, the patient is otherwise quite healthy. She has never experienced any breathing problems and is not atopic.
EXAMINATION
The lesion—an orange, 2-cm, round nodule with a smooth surface—is located on the volar aspect of her forearm. When forcefully stroked, it immediately begins to swell and redden, resembling a wheal. The patient verifies that the lesion itches when touched but is not tender. Before the examination ends, the lesion returns to normal.
Her type I skin is otherwise unremarkable.
What is the diagnosis?
DISCUSSION
Mastocytoma is the term for this localized accumulation of mast cells. When traumatized, histamine (among other active components) is released, causing sudden swelling and itching.
Mast cells are normal white cells involved in the natural function of the immune system, but they can be involved in rare but serious pathologic processes. For example, some children develop dozens of lesions (a condition called urticarial pigmentosa); under extreme circumstances, they can release enough histamine to induce shock and even respiratory distress.
Rarely, mast cells can undergo malignant transformation in the bone marrow, leading to mast cell leukemia. Another rare complication is mastocytosis, in which organs and tissue are invaded by mast cells, destroying the function of the organs and causing problems related to histamine release.
While simple, benign mastocytomas are common and easy to identify visually, skin biopsy is the key to diagnosing this family of diseases. With a small, stable lesion such as this patient’s, spontaneous resolution alleviates the need for treatment. Surgical removal does serve as a permanent cure, however, and as an accurate way to distinguish mastocytoma from mast cell tumors and juvenile xanthogranuloma—the other items in the differential.
TAKE-HOME LEARNING POINTS
• Mastocytomas are benign lesions composed of mast cells.
• Present at or soon after birth, mastocytomas are usually orangish brown and round to oval, and measure, on average, 1 to 3 cm.
• When traumatized by friction, they swell due to the release of histamine from the mast cells. They return to normal within minutes to hours.
• Mastocytomas only rarely require removal, since they are benign and resolve on their own.
A 3-year-old girl is brought to dermatology by her mother, who requests evaluation of a lesion that manifested shortly after the child’s birth. The family’s primary care provider has always dismissed her lesion as a birthmark, but her parents are concerned by its tendency to abruptly change for no apparent reason. It swells up, becomes itchy and red, and then returns to normal within minutes.
According to her parents, the patient is otherwise quite healthy. She has never experienced any breathing problems and is not atopic.
EXAMINATION
The lesion—an orange, 2-cm, round nodule with a smooth surface—is located on the volar aspect of her forearm. When forcefully stroked, it immediately begins to swell and redden, resembling a wheal. The patient verifies that the lesion itches when touched but is not tender. Before the examination ends, the lesion returns to normal.
Her type I skin is otherwise unremarkable.
What is the diagnosis?
DISCUSSION
Mastocytoma is the term for this localized accumulation of mast cells. When traumatized, histamine (among other active components) is released, causing sudden swelling and itching.
Mast cells are normal white cells involved in the natural function of the immune system, but they can be involved in rare but serious pathologic processes. For example, some children develop dozens of lesions (a condition called urticarial pigmentosa); under extreme circumstances, they can release enough histamine to induce shock and even respiratory distress.
Rarely, mast cells can undergo malignant transformation in the bone marrow, leading to mast cell leukemia. Another rare complication is mastocytosis, in which organs and tissue are invaded by mast cells, destroying the function of the organs and causing problems related to histamine release.
While simple, benign mastocytomas are common and easy to identify visually, skin biopsy is the key to diagnosing this family of diseases. With a small, stable lesion such as this patient’s, spontaneous resolution alleviates the need for treatment. Surgical removal does serve as a permanent cure, however, and as an accurate way to distinguish mastocytoma from mast cell tumors and juvenile xanthogranuloma—the other items in the differential.
TAKE-HOME LEARNING POINTS
• Mastocytomas are benign lesions composed of mast cells.
• Present at or soon after birth, mastocytomas are usually orangish brown and round to oval, and measure, on average, 1 to 3 cm.
• When traumatized by friction, they swell due to the release of histamine from the mast cells. They return to normal within minutes to hours.
• Mastocytomas only rarely require removal, since they are benign and resolve on their own.
A 3-year-old girl is brought to dermatology by her mother, who requests evaluation of a lesion that manifested shortly after the child’s birth. The family’s primary care provider has always dismissed her lesion as a birthmark, but her parents are concerned by its tendency to abruptly change for no apparent reason. It swells up, becomes itchy and red, and then returns to normal within minutes.
According to her parents, the patient is otherwise quite healthy. She has never experienced any breathing problems and is not atopic.
EXAMINATION
The lesion—an orange, 2-cm, round nodule with a smooth surface—is located on the volar aspect of her forearm. When forcefully stroked, it immediately begins to swell and redden, resembling a wheal. The patient verifies that the lesion itches when touched but is not tender. Before the examination ends, the lesion returns to normal.
Her type I skin is otherwise unremarkable.
What is the diagnosis?
DISCUSSION
Mastocytoma is the term for this localized accumulation of mast cells. When traumatized, histamine (among other active components) is released, causing sudden swelling and itching.
Mast cells are normal white cells involved in the natural function of the immune system, but they can be involved in rare but serious pathologic processes. For example, some children develop dozens of lesions (a condition called urticarial pigmentosa); under extreme circumstances, they can release enough histamine to induce shock and even respiratory distress.
Rarely, mast cells can undergo malignant transformation in the bone marrow, leading to mast cell leukemia. Another rare complication is mastocytosis, in which organs and tissue are invaded by mast cells, destroying the function of the organs and causing problems related to histamine release.
While simple, benign mastocytomas are common and easy to identify visually, skin biopsy is the key to diagnosing this family of diseases. With a small, stable lesion such as this patient’s, spontaneous resolution alleviates the need for treatment. Surgical removal does serve as a permanent cure, however, and as an accurate way to distinguish mastocytoma from mast cell tumors and juvenile xanthogranuloma—the other items in the differential.
TAKE-HOME LEARNING POINTS
• Mastocytomas are benign lesions composed of mast cells.
• Present at or soon after birth, mastocytomas are usually orangish brown and round to oval, and measure, on average, 1 to 3 cm.
• When traumatized by friction, they swell due to the release of histamine from the mast cells. They return to normal within minutes to hours.
• Mastocytomas only rarely require removal, since they are benign and resolve on their own.
Primary care’s rising role in behavioral health requires specialty partnerships
Amid rising suicide rates and a raging opioid crisis, the key to improving behavioral health care services nationally is to structure primary care practices for collaborative care, according to experts.
“A number of studies have shown that trying to train primary care doctors in mental health and substance abuse treatment does not change outcomes, so I would hope we don’t waste money on doing that,” said Henry Harbin, MD, a psychiatrist and senior health care policy analyst for the Kennedy Forum, while speaking as a panelist in a National Institute of Health Care Management Foundation webinar about mental health care service gaps.
Instead, the most evidence-based approach to caring for those with mental health needs is the collaborative care model, in which a person’s physical and mental needs are treated in one setting, said Dr. Harbin and his copanelists.
Between 2009 and 2013, the United States spent more on mental disorders than on any other health condition – about $200 billion, according to data published earlier this year. Heart conditions were second, at just under $150 billion.
When there is a comorbid mental illness, treatment costs more than double or triple for patients with a medical condition, according to Substance Abuse and Mental Health Services Administration data. For example, the cost of treating a patient with diabetes alone is about $9,500. Adding a mental illness to the mix pushes treatment costs to just under $37,000 annually.
But data from research such as the Improving Mood: Promoting Access to Collaborative Treatment (IMPACT) study show that treating the whole person saves money. In that study, it was shown that $522 invested in collaborative care resulted in a net savings of $3,363 4 years later – about a $6.50 return on every $1 spent.
Components of collaborative care
In general, the components of collaborative care emphasize the use of measurement-based care tools. Those tools range from screening for various mental health conditions to systematic use of symptom rating scales, patient registries, and clinical decision-making algorithms. Applying these kinds of metrics-driven protocols can help curb the “clinical inertia, even in specialty care,” that can happen by relying on clinical judgment alone, said Glenda Wrenn, MD, an assistant professor of psychiatry and behavioral sciences at the Morehouse School of Medicine, Atlanta, and a webinar panelist.
“We’re actually really poor at detecting when our patients are going off track,” Dr. Wrenn said, citing a statistic that only about a fifth of patients whose symptom severity is increasing are detected by physicians who do not use measurement-based tools.
“That’s true for specialty providers who have the additional training, and so it’s especially true for those who do not have that kind of diagnostic training,” cautioned Dr. Wrenn, who is also the behavioral health director at the Satcher Health Leadership Institute at Morehouse.
The American Psychiatry Association also now offers training courses for psychiatrists interested in working with primary care practices. However, colocation of mental health specialists with primary care practices is not always necessary, according to John Fortney, PhD, director of population health at the Advancing Integrated Mental Health Solutions Center at the University of Washington, Seattle, and a webinar panelist. Once measurement-based tools are in place, “most first-line treatment of mental illnesses can be handled by the primary care physician without any help,” he said.
Dr. Fortney has conducted numerous studies of integrating behavioral health into primary care, including the use of telemedicine services. He advocates a stepwise approach to treatment once a patient’s needs are determined to be beyond the basic level of care.
An ad hoc consultation usually from an offsite mental health specialist would be the second step, Dr. Fortney noted, progressing as needed through an onsite intervention by a specialist, collaborative care with targeted treatment, and finally an outside referral to specialty care.
Legislative support
The pressure on the health care system to respond to the nation’s rampant rates of opioid abuse and overdose deaths coincides with a dramatic overhaul of regulations for how physicians who participate in Medicaid are measured and paid, along with recently proposed changes to the Physician Fee Schedule.
Together, these reforms call not only for more metric-based care, but, if finalized in their entirety, would create payment codes specifically for a collaborative, team-based approach to mental health care – including addiction treatment – through the use of coordinated services by primary care practitioners, behavioral health care managers, and psychiatric consultants.
Meanwhile, President Barack Obama recently signed into law a comprehensive package of opioid abuse–related reforms. Once finalized, the reforms will expand and support primary care’s use of medication-assisted therapies to combat opioid addiction and overdose, and extend prescribing privileges for buprenorphine and related therapies to practitioners and physician assistants. The new law also strengthens prescription drug monitoring and disposal programs.
In March, the American Board of Medical Specialties helped elevate addiction medicine’s clinical status with the announcement that it will recognize addiction medicine as a subspecialty, sponsored by the American Board of Preventive Medicine. Although no date has been announced for the first certification exam, the ABMS move was reinforced by a recent Obama administration regulatory change that nearly triples the number of patients addiction specialists can see annually.
Pressure is also mounting on primary care providers to play a more active role in reversing the highest suicide rates in 3 decades. Although the U.S. Preventive Services Task Force concluded in 2014 the evidence is insufficient enough to endorse screening for suicide risk, study data show that in the month prior to their death by suicide, nearly half of people had seen their primary care provider at least once.
Partly in response to these data, the federal Center for Integrated Health Solutions has created a resource center for suicide prevention in primary care.
Partnerships inevitable
In an environment where high care costs directly impact reimbursement, physicians and insurers alike are motivated to “aggressively” seek an integrated approach to care, according to webinar panelist Charles Gross, PhD, vice president for behavioral health in Anthem Blue Cross Blue Shield’s government affairs division.
“Doctors should be compensated for the hard work necessary to integrate care,” Dr. Gross said. “It’s not inexpensive. And also, they should be compensated for patient outcomes.”
Increasingly, provider membership contracts are tailored to a practice’s patient panel, the practice’s current level of integration, and its overall objectives, reinforced by metrics and the implementation of measurement-based care. Bundled care codes and other coding strategies are also being developed to support integrated care, according to Dr. Gross.
Whether it is through telemedicine, colocation, or developing referral networks, primary care physicians are in a position now where they must partner with mental health specialists.
“I don’t think more [training] on how to diagnose and treat mental health and substance abuse is going to be effective in primary care,” Dr. Gross cautioned. “That’s why we’ve really come down firmly on the side of the collaborative care model, based on the IMPACT study. That’s where we’re going.”
On Twitter @whitneymcknight
Amid rising suicide rates and a raging opioid crisis, the key to improving behavioral health care services nationally is to structure primary care practices for collaborative care, according to experts.
“A number of studies have shown that trying to train primary care doctors in mental health and substance abuse treatment does not change outcomes, so I would hope we don’t waste money on doing that,” said Henry Harbin, MD, a psychiatrist and senior health care policy analyst for the Kennedy Forum, while speaking as a panelist in a National Institute of Health Care Management Foundation webinar about mental health care service gaps.
Instead, the most evidence-based approach to caring for those with mental health needs is the collaborative care model, in which a person’s physical and mental needs are treated in one setting, said Dr. Harbin and his copanelists.
Between 2009 and 2013, the United States spent more on mental disorders than on any other health condition – about $200 billion, according to data published earlier this year. Heart conditions were second, at just under $150 billion.
When there is a comorbid mental illness, treatment costs more than double or triple for patients with a medical condition, according to Substance Abuse and Mental Health Services Administration data. For example, the cost of treating a patient with diabetes alone is about $9,500. Adding a mental illness to the mix pushes treatment costs to just under $37,000 annually.
But data from research such as the Improving Mood: Promoting Access to Collaborative Treatment (IMPACT) study show that treating the whole person saves money. In that study, it was shown that $522 invested in collaborative care resulted in a net savings of $3,363 4 years later – about a $6.50 return on every $1 spent.
Components of collaborative care
In general, the components of collaborative care emphasize the use of measurement-based care tools. Those tools range from screening for various mental health conditions to systematic use of symptom rating scales, patient registries, and clinical decision-making algorithms. Applying these kinds of metrics-driven protocols can help curb the “clinical inertia, even in specialty care,” that can happen by relying on clinical judgment alone, said Glenda Wrenn, MD, an assistant professor of psychiatry and behavioral sciences at the Morehouse School of Medicine, Atlanta, and a webinar panelist.
“We’re actually really poor at detecting when our patients are going off track,” Dr. Wrenn said, citing a statistic that only about a fifth of patients whose symptom severity is increasing are detected by physicians who do not use measurement-based tools.
“That’s true for specialty providers who have the additional training, and so it’s especially true for those who do not have that kind of diagnostic training,” cautioned Dr. Wrenn, who is also the behavioral health director at the Satcher Health Leadership Institute at Morehouse.
The American Psychiatry Association also now offers training courses for psychiatrists interested in working with primary care practices. However, colocation of mental health specialists with primary care practices is not always necessary, according to John Fortney, PhD, director of population health at the Advancing Integrated Mental Health Solutions Center at the University of Washington, Seattle, and a webinar panelist. Once measurement-based tools are in place, “most first-line treatment of mental illnesses can be handled by the primary care physician without any help,” he said.
Dr. Fortney has conducted numerous studies of integrating behavioral health into primary care, including the use of telemedicine services. He advocates a stepwise approach to treatment once a patient’s needs are determined to be beyond the basic level of care.
An ad hoc consultation usually from an offsite mental health specialist would be the second step, Dr. Fortney noted, progressing as needed through an onsite intervention by a specialist, collaborative care with targeted treatment, and finally an outside referral to specialty care.
Legislative support
The pressure on the health care system to respond to the nation’s rampant rates of opioid abuse and overdose deaths coincides with a dramatic overhaul of regulations for how physicians who participate in Medicaid are measured and paid, along with recently proposed changes to the Physician Fee Schedule.
Together, these reforms call not only for more metric-based care, but, if finalized in their entirety, would create payment codes specifically for a collaborative, team-based approach to mental health care – including addiction treatment – through the use of coordinated services by primary care practitioners, behavioral health care managers, and psychiatric consultants.
Meanwhile, President Barack Obama recently signed into law a comprehensive package of opioid abuse–related reforms. Once finalized, the reforms will expand and support primary care’s use of medication-assisted therapies to combat opioid addiction and overdose, and extend prescribing privileges for buprenorphine and related therapies to practitioners and physician assistants. The new law also strengthens prescription drug monitoring and disposal programs.
In March, the American Board of Medical Specialties helped elevate addiction medicine’s clinical status with the announcement that it will recognize addiction medicine as a subspecialty, sponsored by the American Board of Preventive Medicine. Although no date has been announced for the first certification exam, the ABMS move was reinforced by a recent Obama administration regulatory change that nearly triples the number of patients addiction specialists can see annually.
Pressure is also mounting on primary care providers to play a more active role in reversing the highest suicide rates in 3 decades. Although the U.S. Preventive Services Task Force concluded in 2014 the evidence is insufficient enough to endorse screening for suicide risk, study data show that in the month prior to their death by suicide, nearly half of people had seen their primary care provider at least once.
Partly in response to these data, the federal Center for Integrated Health Solutions has created a resource center for suicide prevention in primary care.
Partnerships inevitable
In an environment where high care costs directly impact reimbursement, physicians and insurers alike are motivated to “aggressively” seek an integrated approach to care, according to webinar panelist Charles Gross, PhD, vice president for behavioral health in Anthem Blue Cross Blue Shield’s government affairs division.
“Doctors should be compensated for the hard work necessary to integrate care,” Dr. Gross said. “It’s not inexpensive. And also, they should be compensated for patient outcomes.”
Increasingly, provider membership contracts are tailored to a practice’s patient panel, the practice’s current level of integration, and its overall objectives, reinforced by metrics and the implementation of measurement-based care. Bundled care codes and other coding strategies are also being developed to support integrated care, according to Dr. Gross.
Whether it is through telemedicine, colocation, or developing referral networks, primary care physicians are in a position now where they must partner with mental health specialists.
“I don’t think more [training] on how to diagnose and treat mental health and substance abuse is going to be effective in primary care,” Dr. Gross cautioned. “That’s why we’ve really come down firmly on the side of the collaborative care model, based on the IMPACT study. That’s where we’re going.”
On Twitter @whitneymcknight
Amid rising suicide rates and a raging opioid crisis, the key to improving behavioral health care services nationally is to structure primary care practices for collaborative care, according to experts.
“A number of studies have shown that trying to train primary care doctors in mental health and substance abuse treatment does not change outcomes, so I would hope we don’t waste money on doing that,” said Henry Harbin, MD, a psychiatrist and senior health care policy analyst for the Kennedy Forum, while speaking as a panelist in a National Institute of Health Care Management Foundation webinar about mental health care service gaps.
Instead, the most evidence-based approach to caring for those with mental health needs is the collaborative care model, in which a person’s physical and mental needs are treated in one setting, said Dr. Harbin and his copanelists.
Between 2009 and 2013, the United States spent more on mental disorders than on any other health condition – about $200 billion, according to data published earlier this year. Heart conditions were second, at just under $150 billion.
When there is a comorbid mental illness, treatment costs more than double or triple for patients with a medical condition, according to Substance Abuse and Mental Health Services Administration data. For example, the cost of treating a patient with diabetes alone is about $9,500. Adding a mental illness to the mix pushes treatment costs to just under $37,000 annually.
But data from research such as the Improving Mood: Promoting Access to Collaborative Treatment (IMPACT) study show that treating the whole person saves money. In that study, it was shown that $522 invested in collaborative care resulted in a net savings of $3,363 4 years later – about a $6.50 return on every $1 spent.
Components of collaborative care
In general, the components of collaborative care emphasize the use of measurement-based care tools. Those tools range from screening for various mental health conditions to systematic use of symptom rating scales, patient registries, and clinical decision-making algorithms. Applying these kinds of metrics-driven protocols can help curb the “clinical inertia, even in specialty care,” that can happen by relying on clinical judgment alone, said Glenda Wrenn, MD, an assistant professor of psychiatry and behavioral sciences at the Morehouse School of Medicine, Atlanta, and a webinar panelist.
“We’re actually really poor at detecting when our patients are going off track,” Dr. Wrenn said, citing a statistic that only about a fifth of patients whose symptom severity is increasing are detected by physicians who do not use measurement-based tools.
“That’s true for specialty providers who have the additional training, and so it’s especially true for those who do not have that kind of diagnostic training,” cautioned Dr. Wrenn, who is also the behavioral health director at the Satcher Health Leadership Institute at Morehouse.
The American Psychiatry Association also now offers training courses for psychiatrists interested in working with primary care practices. However, colocation of mental health specialists with primary care practices is not always necessary, according to John Fortney, PhD, director of population health at the Advancing Integrated Mental Health Solutions Center at the University of Washington, Seattle, and a webinar panelist. Once measurement-based tools are in place, “most first-line treatment of mental illnesses can be handled by the primary care physician without any help,” he said.
Dr. Fortney has conducted numerous studies of integrating behavioral health into primary care, including the use of telemedicine services. He advocates a stepwise approach to treatment once a patient’s needs are determined to be beyond the basic level of care.
An ad hoc consultation usually from an offsite mental health specialist would be the second step, Dr. Fortney noted, progressing as needed through an onsite intervention by a specialist, collaborative care with targeted treatment, and finally an outside referral to specialty care.
Legislative support
The pressure on the health care system to respond to the nation’s rampant rates of opioid abuse and overdose deaths coincides with a dramatic overhaul of regulations for how physicians who participate in Medicaid are measured and paid, along with recently proposed changes to the Physician Fee Schedule.
Together, these reforms call not only for more metric-based care, but, if finalized in their entirety, would create payment codes specifically for a collaborative, team-based approach to mental health care – including addiction treatment – through the use of coordinated services by primary care practitioners, behavioral health care managers, and psychiatric consultants.
Meanwhile, President Barack Obama recently signed into law a comprehensive package of opioid abuse–related reforms. Once finalized, the reforms will expand and support primary care’s use of medication-assisted therapies to combat opioid addiction and overdose, and extend prescribing privileges for buprenorphine and related therapies to practitioners and physician assistants. The new law also strengthens prescription drug monitoring and disposal programs.
In March, the American Board of Medical Specialties helped elevate addiction medicine’s clinical status with the announcement that it will recognize addiction medicine as a subspecialty, sponsored by the American Board of Preventive Medicine. Although no date has been announced for the first certification exam, the ABMS move was reinforced by a recent Obama administration regulatory change that nearly triples the number of patients addiction specialists can see annually.
Pressure is also mounting on primary care providers to play a more active role in reversing the highest suicide rates in 3 decades. Although the U.S. Preventive Services Task Force concluded in 2014 the evidence is insufficient enough to endorse screening for suicide risk, study data show that in the month prior to their death by suicide, nearly half of people had seen their primary care provider at least once.
Partly in response to these data, the federal Center for Integrated Health Solutions has created a resource center for suicide prevention in primary care.
Partnerships inevitable
In an environment where high care costs directly impact reimbursement, physicians and insurers alike are motivated to “aggressively” seek an integrated approach to care, according to webinar panelist Charles Gross, PhD, vice president for behavioral health in Anthem Blue Cross Blue Shield’s government affairs division.
“Doctors should be compensated for the hard work necessary to integrate care,” Dr. Gross said. “It’s not inexpensive. And also, they should be compensated for patient outcomes.”
Increasingly, provider membership contracts are tailored to a practice’s patient panel, the practice’s current level of integration, and its overall objectives, reinforced by metrics and the implementation of measurement-based care. Bundled care codes and other coding strategies are also being developed to support integrated care, according to Dr. Gross.
Whether it is through telemedicine, colocation, or developing referral networks, primary care physicians are in a position now where they must partner with mental health specialists.
“I don’t think more [training] on how to diagnose and treat mental health and substance abuse is going to be effective in primary care,” Dr. Gross cautioned. “That’s why we’ve really come down firmly on the side of the collaborative care model, based on the IMPACT study. That’s where we’re going.”
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AN NIHCM FOUNDATION WEBINAR
Hospital safety culture may influence surgical outcomes
SAN DIEGO – Hospital safety culture may positively influence certain surgical patient outcomes, results from a study of 56 Illinois hospitals demonstrated.
“Efforts to improve awareness of safety and quality improvement principles should be encouraged at both the surgical system and hospital levels,” David D. Odell, MD, said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “Safety culture itself is a concept [that] is increasingly viewed as important in the delivery of high-quality care. Yet in the surgical world, very little is known about how hospital culture actually influences outcomes for our patients.”
Dr. Odell, a thoracic surgeon at Northwestern Memorial Hospital, Chicago, discussed results from a study by the Illinois Surgical Quality Improvement Collaborative, a group of Illinois hospitals working together to improve the quality of surgical care in the state. Participants in the Collaborative include 56 hospitals, including all academic medical centers in the state, as well as 11 rural hospitals. Combined, these facilities perform 60% of general surgery operations in the state and 80% of all complex operations, impacting more than 600,000 patients each year.
In an effort to evaluate the relationship between hospital safety culture and surgical patient outcomes, Dr. Odell and his associates invited staff of Collaborative members to complete the Safety Attitudes Questionnaire (SAQ), a 56-item validated tool for assessment of hospital culture. Domains focused on were teamwork, communication, engagement, and leadership. The SAQ was given to administrators, staff, and front-line providers “to measure safety culture across all levels of the hospital,” Dr. Odell said. Percent positive responses were calculated at the hospital level for each of the eight domains to calculate a composite measure of safety. The researchers measured the impact of safety culture by assessing positive SAQ response rates. Outcome variables of interest were morbidity, mortality, death or serious morbidity, and readmission. Hospital-level risk-adjusted event rates and linear regression models were used to assess the impact of safety culture while controlling for teaching status, rural location, trauma center designation, hospital control (management), and the annual surgical volume.
Of the 49 participating hospitals represented in the survey responses, 49% had an Accreditation Council for Graduate Medical Education (ACGME)-accredited residency program, 12% were rural, 61% provided trauma care, 35% had a religious affiliation, 57% were “other” not-for-profit, and the mean total surgical volume was 11,412 cases.
Dr. Odell reported that by domain, SAQ responses were most positive for operating room safety and lowest for hospital management. “That doesn’t necessarily reflect the management’s outcomes only, but the views of those who took the survey toward management,” he said.
When the researchers evaluated the impact of a more-positive safety culture on the risk-adjusted outcome measures, they observed a statistically significant impact on morbidity following surgery (P = .02). The trend was similar although not statistically significant for death/serious morbidity (P = .08), mortality (P =. 20), or readmission (P = .68).
Dr. Odell acknowledged certain limitations of the study, including its retrospective design and the fact that the SAQ is a subjective assessment tool. “Not all [staff invited] were surveyed,” he added. “We sent out just under 1,400 surveys and we had a response rate of 44%.”
Staff from participating institutions of the Collaborative meet on a semiannual basis to share ideas, celebrate successes and learn from each other’s experiences, Dr. Odell said. Ongoing efforts to improve safety culture include fostering opportunities for mentorship in quality improvement and process improvement endeavors, as well as the provision of educational materials targeted at all levels of hospital staff “so that we can get everyone thinking and speaking the same language when it comes to quality improvement,” he said.
The Collaborative is funded by Blue Cross Blue Shield of Illinois. Dr. Odell reported having no financial disclosures.
SAN DIEGO – Hospital safety culture may positively influence certain surgical patient outcomes, results from a study of 56 Illinois hospitals demonstrated.
“Efforts to improve awareness of safety and quality improvement principles should be encouraged at both the surgical system and hospital levels,” David D. Odell, MD, said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “Safety culture itself is a concept [that] is increasingly viewed as important in the delivery of high-quality care. Yet in the surgical world, very little is known about how hospital culture actually influences outcomes for our patients.”
Dr. Odell, a thoracic surgeon at Northwestern Memorial Hospital, Chicago, discussed results from a study by the Illinois Surgical Quality Improvement Collaborative, a group of Illinois hospitals working together to improve the quality of surgical care in the state. Participants in the Collaborative include 56 hospitals, including all academic medical centers in the state, as well as 11 rural hospitals. Combined, these facilities perform 60% of general surgery operations in the state and 80% of all complex operations, impacting more than 600,000 patients each year.
In an effort to evaluate the relationship between hospital safety culture and surgical patient outcomes, Dr. Odell and his associates invited staff of Collaborative members to complete the Safety Attitudes Questionnaire (SAQ), a 56-item validated tool for assessment of hospital culture. Domains focused on were teamwork, communication, engagement, and leadership. The SAQ was given to administrators, staff, and front-line providers “to measure safety culture across all levels of the hospital,” Dr. Odell said. Percent positive responses were calculated at the hospital level for each of the eight domains to calculate a composite measure of safety. The researchers measured the impact of safety culture by assessing positive SAQ response rates. Outcome variables of interest were morbidity, mortality, death or serious morbidity, and readmission. Hospital-level risk-adjusted event rates and linear regression models were used to assess the impact of safety culture while controlling for teaching status, rural location, trauma center designation, hospital control (management), and the annual surgical volume.
Of the 49 participating hospitals represented in the survey responses, 49% had an Accreditation Council for Graduate Medical Education (ACGME)-accredited residency program, 12% were rural, 61% provided trauma care, 35% had a religious affiliation, 57% were “other” not-for-profit, and the mean total surgical volume was 11,412 cases.
Dr. Odell reported that by domain, SAQ responses were most positive for operating room safety and lowest for hospital management. “That doesn’t necessarily reflect the management’s outcomes only, but the views of those who took the survey toward management,” he said.
When the researchers evaluated the impact of a more-positive safety culture on the risk-adjusted outcome measures, they observed a statistically significant impact on morbidity following surgery (P = .02). The trend was similar although not statistically significant for death/serious morbidity (P = .08), mortality (P =. 20), or readmission (P = .68).
Dr. Odell acknowledged certain limitations of the study, including its retrospective design and the fact that the SAQ is a subjective assessment tool. “Not all [staff invited] were surveyed,” he added. “We sent out just under 1,400 surveys and we had a response rate of 44%.”
Staff from participating institutions of the Collaborative meet on a semiannual basis to share ideas, celebrate successes and learn from each other’s experiences, Dr. Odell said. Ongoing efforts to improve safety culture include fostering opportunities for mentorship in quality improvement and process improvement endeavors, as well as the provision of educational materials targeted at all levels of hospital staff “so that we can get everyone thinking and speaking the same language when it comes to quality improvement,” he said.
The Collaborative is funded by Blue Cross Blue Shield of Illinois. Dr. Odell reported having no financial disclosures.
SAN DIEGO – Hospital safety culture may positively influence certain surgical patient outcomes, results from a study of 56 Illinois hospitals demonstrated.
“Efforts to improve awareness of safety and quality improvement principles should be encouraged at both the surgical system and hospital levels,” David D. Odell, MD, said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “Safety culture itself is a concept [that] is increasingly viewed as important in the delivery of high-quality care. Yet in the surgical world, very little is known about how hospital culture actually influences outcomes for our patients.”
Dr. Odell, a thoracic surgeon at Northwestern Memorial Hospital, Chicago, discussed results from a study by the Illinois Surgical Quality Improvement Collaborative, a group of Illinois hospitals working together to improve the quality of surgical care in the state. Participants in the Collaborative include 56 hospitals, including all academic medical centers in the state, as well as 11 rural hospitals. Combined, these facilities perform 60% of general surgery operations in the state and 80% of all complex operations, impacting more than 600,000 patients each year.
In an effort to evaluate the relationship between hospital safety culture and surgical patient outcomes, Dr. Odell and his associates invited staff of Collaborative members to complete the Safety Attitudes Questionnaire (SAQ), a 56-item validated tool for assessment of hospital culture. Domains focused on were teamwork, communication, engagement, and leadership. The SAQ was given to administrators, staff, and front-line providers “to measure safety culture across all levels of the hospital,” Dr. Odell said. Percent positive responses were calculated at the hospital level for each of the eight domains to calculate a composite measure of safety. The researchers measured the impact of safety culture by assessing positive SAQ response rates. Outcome variables of interest were morbidity, mortality, death or serious morbidity, and readmission. Hospital-level risk-adjusted event rates and linear regression models were used to assess the impact of safety culture while controlling for teaching status, rural location, trauma center designation, hospital control (management), and the annual surgical volume.
Of the 49 participating hospitals represented in the survey responses, 49% had an Accreditation Council for Graduate Medical Education (ACGME)-accredited residency program, 12% were rural, 61% provided trauma care, 35% had a religious affiliation, 57% were “other” not-for-profit, and the mean total surgical volume was 11,412 cases.
Dr. Odell reported that by domain, SAQ responses were most positive for operating room safety and lowest for hospital management. “That doesn’t necessarily reflect the management’s outcomes only, but the views of those who took the survey toward management,” he said.
When the researchers evaluated the impact of a more-positive safety culture on the risk-adjusted outcome measures, they observed a statistically significant impact on morbidity following surgery (P = .02). The trend was similar although not statistically significant for death/serious morbidity (P = .08), mortality (P =. 20), or readmission (P = .68).
Dr. Odell acknowledged certain limitations of the study, including its retrospective design and the fact that the SAQ is a subjective assessment tool. “Not all [staff invited] were surveyed,” he added. “We sent out just under 1,400 surveys and we had a response rate of 44%.”
Staff from participating institutions of the Collaborative meet on a semiannual basis to share ideas, celebrate successes and learn from each other’s experiences, Dr. Odell said. Ongoing efforts to improve safety culture include fostering opportunities for mentorship in quality improvement and process improvement endeavors, as well as the provision of educational materials targeted at all levels of hospital staff “so that we can get everyone thinking and speaking the same language when it comes to quality improvement,” he said.
The Collaborative is funded by Blue Cross Blue Shield of Illinois. Dr. Odell reported having no financial disclosures.
EXPERT ANALYSIS AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: A positive hospital safety culture significantly impacted morbidity following surgery.
Major finding: When the researchers evaluated the impact of a positive safety culture on risk-adjusted outcome measures, they observed a statistically significant impact on morbidity following surgery (P = .02).
Data source: A retrospective study by the Illinois Surgical Quality Improvement Collaborative, a group of 56 hospitals in the state.
Disclosures: The Collaborative is funded by Blue Cross Blue Shield of Illinois. Dr. Odell reported having no financial disclosures.
Revised axial spondyloarthritis classification criteria remain elusive
DENVER – The revised axial spondyloarthritis classification criteria that U.S. rheumatologists say they desperately need remain elusive, with no firm path to creation.
The 2009 classification criteria for axial spondyloarthritis (SpA) developed by the Assessment of Spondyloarthritis international Society (ASAS) was a landmark in creating a definition for axial SpA to use when enrolling patients into clinical trials and to also potentially use for diagnosis.
But the 2009 classification criteria also have several shortcomings, especially from a U.S. perspective, that have limited the utility of the criteria in U.S. practice and studies.
Several years of discussions among North American rheumatologists about the classification criteria have produced consensus about what is wrong with them: The clinical elements of the criteria are not specific enough with sensitivity and specificity rates that are each about 80%-85%; the imaging component of the criteria is not totally up to date and as one example only considers osteitis and not other MRI abnormalities such as fatty metaplasia and T1 structural lesions; the criteria were entirely based on results from studies with Europeans, making their applicability to other patient types uncertain; and some terminology in the criteria are confusing, Liron Caplan, MD, said during a discussion of the criteria at the annual meeting of the of the Spondyloarthritis Research and Treatment Network.
But while the SpA experts who gathered at the meeting and who have also been discussing this issue since 2013 are of one mind on the problems, agreement on exactly how to solve them remains unresolved.
“Everything is still on the table,” said Dr. Caplan, a rheumatologist and director of the SpA program at the University of Colorado in Aurora.
A year ago, at SPARTAN’s prior annual meeting, circumstances looked on track for a quicker resolution. In a vote of the membership at the 2015 gathering, 64% backed creation of new classification criteria, and Dr. Caplan proposed quickly preparing a research proposal to present in early 2016 to the American College of Rheumatology and the European League Against Rheumatism for funding.
But in the ensuing year, the effort got mired in discussions on how to perform the study needed to gather the evidence base for criteria revisions. Some progress occurred, most notably an agreement between SPARTAN officials and the ASAS leadership to work together on the revision, said Atul A. Deodhar, MD, professor of medicine and medical director of the rheumatology clinics at the Oregon Health & Science University in Portland.
The SPARTAN and ASAS representatives also agreed on several specifics of what’s needed, including a weighting formula for the 10 different clinical criteria of the SpA classification. Currently, in individuals with low back pain lasting 3 months or longer at an age of onset younger than 45 years, any one of these 10 clinical criteria can create a case of SpA either if combined with MRI or radiographic evidence of sacroiliitis, or without any imaging if the individual is HLA-B27 positive and fulfills two items from the 10-item list of clinical criteria.
It’s the lack of radiographic imaging confirmation, so-called “nonradiographic SpA,” that generates the greatest concern about below-par specificity and that may be helped by a weighting adjustment that varies the classification power of each of the 10 clinical items. A more ideal balance might be criteria with sensitivity reduced from the current level to about 70% and with specificity rising to about 90%, Dr. Caplan suggested.
The keystone of any revision will be a large, new study with SpA patients from various locations. Dr. Caplan emphasized that “no firm decisions have yet been made” regarding the study's name or research focus.
On Twitter @mitchelzoler
This article was updated August 11, 2016.
DENVER – The revised axial spondyloarthritis classification criteria that U.S. rheumatologists say they desperately need remain elusive, with no firm path to creation.
The 2009 classification criteria for axial spondyloarthritis (SpA) developed by the Assessment of Spondyloarthritis international Society (ASAS) was a landmark in creating a definition for axial SpA to use when enrolling patients into clinical trials and to also potentially use for diagnosis.
But the 2009 classification criteria also have several shortcomings, especially from a U.S. perspective, that have limited the utility of the criteria in U.S. practice and studies.
Several years of discussions among North American rheumatologists about the classification criteria have produced consensus about what is wrong with them: The clinical elements of the criteria are not specific enough with sensitivity and specificity rates that are each about 80%-85%; the imaging component of the criteria is not totally up to date and as one example only considers osteitis and not other MRI abnormalities such as fatty metaplasia and T1 structural lesions; the criteria were entirely based on results from studies with Europeans, making their applicability to other patient types uncertain; and some terminology in the criteria are confusing, Liron Caplan, MD, said during a discussion of the criteria at the annual meeting of the of the Spondyloarthritis Research and Treatment Network.
But while the SpA experts who gathered at the meeting and who have also been discussing this issue since 2013 are of one mind on the problems, agreement on exactly how to solve them remains unresolved.
“Everything is still on the table,” said Dr. Caplan, a rheumatologist and director of the SpA program at the University of Colorado in Aurora.
A year ago, at SPARTAN’s prior annual meeting, circumstances looked on track for a quicker resolution. In a vote of the membership at the 2015 gathering, 64% backed creation of new classification criteria, and Dr. Caplan proposed quickly preparing a research proposal to present in early 2016 to the American College of Rheumatology and the European League Against Rheumatism for funding.
But in the ensuing year, the effort got mired in discussions on how to perform the study needed to gather the evidence base for criteria revisions. Some progress occurred, most notably an agreement between SPARTAN officials and the ASAS leadership to work together on the revision, said Atul A. Deodhar, MD, professor of medicine and medical director of the rheumatology clinics at the Oregon Health & Science University in Portland.
The SPARTAN and ASAS representatives also agreed on several specifics of what’s needed, including a weighting formula for the 10 different clinical criteria of the SpA classification. Currently, in individuals with low back pain lasting 3 months or longer at an age of onset younger than 45 years, any one of these 10 clinical criteria can create a case of SpA either if combined with MRI or radiographic evidence of sacroiliitis, or without any imaging if the individual is HLA-B27 positive and fulfills two items from the 10-item list of clinical criteria.
It’s the lack of radiographic imaging confirmation, so-called “nonradiographic SpA,” that generates the greatest concern about below-par specificity and that may be helped by a weighting adjustment that varies the classification power of each of the 10 clinical items. A more ideal balance might be criteria with sensitivity reduced from the current level to about 70% and with specificity rising to about 90%, Dr. Caplan suggested.
The keystone of any revision will be a large, new study with SpA patients from various locations. Dr. Caplan emphasized that “no firm decisions have yet been made” regarding the study's name or research focus.
On Twitter @mitchelzoler
This article was updated August 11, 2016.
DENVER – The revised axial spondyloarthritis classification criteria that U.S. rheumatologists say they desperately need remain elusive, with no firm path to creation.
The 2009 classification criteria for axial spondyloarthritis (SpA) developed by the Assessment of Spondyloarthritis international Society (ASAS) was a landmark in creating a definition for axial SpA to use when enrolling patients into clinical trials and to also potentially use for diagnosis.
But the 2009 classification criteria also have several shortcomings, especially from a U.S. perspective, that have limited the utility of the criteria in U.S. practice and studies.
Several years of discussions among North American rheumatologists about the classification criteria have produced consensus about what is wrong with them: The clinical elements of the criteria are not specific enough with sensitivity and specificity rates that are each about 80%-85%; the imaging component of the criteria is not totally up to date and as one example only considers osteitis and not other MRI abnormalities such as fatty metaplasia and T1 structural lesions; the criteria were entirely based on results from studies with Europeans, making their applicability to other patient types uncertain; and some terminology in the criteria are confusing, Liron Caplan, MD, said during a discussion of the criteria at the annual meeting of the of the Spondyloarthritis Research and Treatment Network.
But while the SpA experts who gathered at the meeting and who have also been discussing this issue since 2013 are of one mind on the problems, agreement on exactly how to solve them remains unresolved.
“Everything is still on the table,” said Dr. Caplan, a rheumatologist and director of the SpA program at the University of Colorado in Aurora.
A year ago, at SPARTAN’s prior annual meeting, circumstances looked on track for a quicker resolution. In a vote of the membership at the 2015 gathering, 64% backed creation of new classification criteria, and Dr. Caplan proposed quickly preparing a research proposal to present in early 2016 to the American College of Rheumatology and the European League Against Rheumatism for funding.
But in the ensuing year, the effort got mired in discussions on how to perform the study needed to gather the evidence base for criteria revisions. Some progress occurred, most notably an agreement between SPARTAN officials and the ASAS leadership to work together on the revision, said Atul A. Deodhar, MD, professor of medicine and medical director of the rheumatology clinics at the Oregon Health & Science University in Portland.
The SPARTAN and ASAS representatives also agreed on several specifics of what’s needed, including a weighting formula for the 10 different clinical criteria of the SpA classification. Currently, in individuals with low back pain lasting 3 months or longer at an age of onset younger than 45 years, any one of these 10 clinical criteria can create a case of SpA either if combined with MRI or radiographic evidence of sacroiliitis, or without any imaging if the individual is HLA-B27 positive and fulfills two items from the 10-item list of clinical criteria.
It’s the lack of radiographic imaging confirmation, so-called “nonradiographic SpA,” that generates the greatest concern about below-par specificity and that may be helped by a weighting adjustment that varies the classification power of each of the 10 clinical items. A more ideal balance might be criteria with sensitivity reduced from the current level to about 70% and with specificity rising to about 90%, Dr. Caplan suggested.
The keystone of any revision will be a large, new study with SpA patients from various locations. Dr. Caplan emphasized that “no firm decisions have yet been made” regarding the study's name or research focus.
On Twitter @mitchelzoler
This article was updated August 11, 2016.
EXPERT ANALYSIS FROM THE 2016 SPARTAN ANNUAL MEETING
Salvage RT may reduce risk of prostate cancer metastasis even at low PSA levels
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
Increasing prostate-specific antigen (PSA) levels prior to salvage radiotherapy were significantly associated with an increased risk of distant metastasis and cause-specific mortality but not overall survival among men with detectable PSA following radical prostatectomy, according to a report published in the Journal of Clinical Oncology.
Investigators retrospectively studied 1,106 consecutive patients with surgically staged prostate cancer who received salvage radiotherapy (SRT) and who had a documented PSA of 0.1 ng/mL or greater. A total of 208 patients developed distant metastasis during median follow-up of 8.9 years.
In multivariate analysis, each doubling of pre-SRT PSA was associated with a 32% increased risk of distant metastasis (P less than .001), reported Bradley J. Stish, MD, and his associates at the Mayo Clinic (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3425).
Each pre-SRT PSA doubling also significantly increased the relative risk of cause-specific mortality (HR, 1.40; P less than .001) but not overall survival (HR, 1.12; P = .02).
More advanced tumor stages, higher Gleason scores, and higher PSA levels prior to salvage radiotherapy were significantly associated with an increased risk of biochemical recurrence. Salvage radiotherapy dose greater than 68 Gy and use of androgen suppression were associated with reduced risk of biochemical recurrence. Overall survival was 92.9% (95% confidence interval, 91.3%-94.5%) at 5 years and 77.3% (95% CI, 74.2%-80.5%) at 10 years. The cause-specific mortality rates were 3.0% (95% CI, 1.9%-4.0%) at 5 years and 10.4% (95% CI, 8.0%-12.6%) at 10 years.
“When taken together, these findings provide strong evidence supporting the clinical benefits attributable to early [salvage radiotherapy] in men with detectable PSA after [radical prostatectomy],” investigators wrote.
Beginning salvage radiotherapy treatment at the lowest PSA level is “most beneficial for long-term therapeutic efficacy,” they added.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Salvage radiotherapy should not be delayed by prolonged monitoring of detectable post radical prostatectomy PSA levels.
Major finding: A multivariate analysis revealed that prior to salvage radiotherapy, each doubling of prostate specific antigen was significantly associated with an increased risk of distant metastasis (hazard ratio, 1.32; 95% CI, 1.19-1.46; P less than .001) and cause-specific mortality (HR, 1.40; 95% CI, 1.21-1.63, P less than .001).
Data source: A retrospective study of 1,106 men with detectable prostate-specific antigen levels following radical prostatectomy for prostate cancer.
Disclosures: The Mayo Clinic funded this study. Six of the investigators had no disclosures to report; one investigator reported holding stock or ownership interest in Pfizer.
ACS NSQIP Geriatric Surgery Pilot Collaborative: Evaluating variables for inclusion
SAN DIEGO – A project to assess geriatric-specific variables for inclusion in the American College of Surgeons/National Surgical Quality Improvement Program National Conference is underway, according to Thomas N. Robinson, MD.
Dr. Robinson discussed results from the ACS-NSQIP Geriatric Surgery Pilot Collaborative, an effort launched in January 2014 with the ultimate goal of evaluating specific geriatric variables for incorporation into the ACS NSQIP set of essential variables collected by all participating hospitals.
Dr. Robinson, professor of surgery at the University of Colorado, Denver, said that 23 clinical sites in the United States are currently studying the following geriatric-specific variables in surgery patients aged 65 and older:
• Origin from home with support (to determine baseline functional status: lives alone at home, lives with support in home, origin status not from home).
• Discharge functional health status (ability to perform activities of daily living).
• Discharge with/without services (to capture care needs upon discharge).
• Preoperative use of a mobility aid.
• Preoperative history of prior falls.
• Postoperative history of pressure ulcer.
• Fall risk on discharge.
• New mobility aid on discharge.
• History of dementia.
• Competency status on admission.
• Postoperative delirium (yes or no).
• Hospice care on admission (yes or no).
• Do Not Resuscitate (DNR) order in place on admission (yes or no).
• DNR order during hospitalization (yes or no).
• Setting where DNR order was placed.
• Postoperative palliative care consult (yes or no).
• 30-day postoperative outcomes: functional health status (ability to perform activities of daily living), physical function compared with baseline, and living location.
The number of surgery cases in the collaborative grew from 7,235 in the first 6 months of 2014 to 24,835 cases in the last 6 months of 2015. The top 10 operations were total joint arthroplasty (29%), colectomy (12%), spine (8%), hip fracture (7%), carotid endarterectomy (4%), hysterectomy (4%), lung resection (2%), open lower extremity bypass (2%), laparoscopic cholecystectomy (2%), and pancreatectomy (2%).
Dr. Robinson reported that the rate of preoperative dementia among cases studied in the collaborative was 10%. “The incorporation of dementia into a surgical dataset represents an important step forward in providing quality surgical care for the elderly,” he said. “Dementia is a global public health concern.” He went on to note that patients with dementia have a 2.5-fold increased risk of developing postoperative delirium, making it “the perfect place to start a quality project. One in three cases of delirium is preventable. In our data set, delirium is associated with a hospital stay that’s 4 days longer, an increased chance of requiring discharge to an institutional care facility, an increased chance of a serious complication, and a higher 30-day mortality.”
Simple, low-tech bedside interventions such as ambulating in the hall three times a day, orienting the person, having the person sleep at night rather than sleep during the day, and avoiding medications with high risk for adverse events in older adults can prevent postoperative delirium, Dr. Robinson said.
One way that the Geriatric Surgery Pilot Collaborative can improve the surgical care of older adults is by fostering quality programs initiated at the participating local hospitals. “Preserving function after hospital stays is a first major goal,” he said. Another strategy involves creating a multidisciplinary frailty assessment to aid with decision making and risk assessment. “This takes into consideration NSQIP variables such as function, nutrition, comorbidity burden, cognition, social vulnerability, and mobility,” he said. The final and ultimate goal of the geriatric surgery collaborative is to establish a foundation of quality measurement for the Coalition for Quality in Geriatric Surgery, a project initiated by the American College of Surgeons to systematically improve the surgical care of older adults.
Dr. Robinson reported having no relevant financial disclosures.
SAN DIEGO – A project to assess geriatric-specific variables for inclusion in the American College of Surgeons/National Surgical Quality Improvement Program National Conference is underway, according to Thomas N. Robinson, MD.
Dr. Robinson discussed results from the ACS-NSQIP Geriatric Surgery Pilot Collaborative, an effort launched in January 2014 with the ultimate goal of evaluating specific geriatric variables for incorporation into the ACS NSQIP set of essential variables collected by all participating hospitals.
Dr. Robinson, professor of surgery at the University of Colorado, Denver, said that 23 clinical sites in the United States are currently studying the following geriatric-specific variables in surgery patients aged 65 and older:
• Origin from home with support (to determine baseline functional status: lives alone at home, lives with support in home, origin status not from home).
• Discharge functional health status (ability to perform activities of daily living).
• Discharge with/without services (to capture care needs upon discharge).
• Preoperative use of a mobility aid.
• Preoperative history of prior falls.
• Postoperative history of pressure ulcer.
• Fall risk on discharge.
• New mobility aid on discharge.
• History of dementia.
• Competency status on admission.
• Postoperative delirium (yes or no).
• Hospice care on admission (yes or no).
• Do Not Resuscitate (DNR) order in place on admission (yes or no).
• DNR order during hospitalization (yes or no).
• Setting where DNR order was placed.
• Postoperative palliative care consult (yes or no).
• 30-day postoperative outcomes: functional health status (ability to perform activities of daily living), physical function compared with baseline, and living location.
The number of surgery cases in the collaborative grew from 7,235 in the first 6 months of 2014 to 24,835 cases in the last 6 months of 2015. The top 10 operations were total joint arthroplasty (29%), colectomy (12%), spine (8%), hip fracture (7%), carotid endarterectomy (4%), hysterectomy (4%), lung resection (2%), open lower extremity bypass (2%), laparoscopic cholecystectomy (2%), and pancreatectomy (2%).
Dr. Robinson reported that the rate of preoperative dementia among cases studied in the collaborative was 10%. “The incorporation of dementia into a surgical dataset represents an important step forward in providing quality surgical care for the elderly,” he said. “Dementia is a global public health concern.” He went on to note that patients with dementia have a 2.5-fold increased risk of developing postoperative delirium, making it “the perfect place to start a quality project. One in three cases of delirium is preventable. In our data set, delirium is associated with a hospital stay that’s 4 days longer, an increased chance of requiring discharge to an institutional care facility, an increased chance of a serious complication, and a higher 30-day mortality.”
Simple, low-tech bedside interventions such as ambulating in the hall three times a day, orienting the person, having the person sleep at night rather than sleep during the day, and avoiding medications with high risk for adverse events in older adults can prevent postoperative delirium, Dr. Robinson said.
One way that the Geriatric Surgery Pilot Collaborative can improve the surgical care of older adults is by fostering quality programs initiated at the participating local hospitals. “Preserving function after hospital stays is a first major goal,” he said. Another strategy involves creating a multidisciplinary frailty assessment to aid with decision making and risk assessment. “This takes into consideration NSQIP variables such as function, nutrition, comorbidity burden, cognition, social vulnerability, and mobility,” he said. The final and ultimate goal of the geriatric surgery collaborative is to establish a foundation of quality measurement for the Coalition for Quality in Geriatric Surgery, a project initiated by the American College of Surgeons to systematically improve the surgical care of older adults.
Dr. Robinson reported having no relevant financial disclosures.
SAN DIEGO – A project to assess geriatric-specific variables for inclusion in the American College of Surgeons/National Surgical Quality Improvement Program National Conference is underway, according to Thomas N. Robinson, MD.
Dr. Robinson discussed results from the ACS-NSQIP Geriatric Surgery Pilot Collaborative, an effort launched in January 2014 with the ultimate goal of evaluating specific geriatric variables for incorporation into the ACS NSQIP set of essential variables collected by all participating hospitals.
Dr. Robinson, professor of surgery at the University of Colorado, Denver, said that 23 clinical sites in the United States are currently studying the following geriatric-specific variables in surgery patients aged 65 and older:
• Origin from home with support (to determine baseline functional status: lives alone at home, lives with support in home, origin status not from home).
• Discharge functional health status (ability to perform activities of daily living).
• Discharge with/without services (to capture care needs upon discharge).
• Preoperative use of a mobility aid.
• Preoperative history of prior falls.
• Postoperative history of pressure ulcer.
• Fall risk on discharge.
• New mobility aid on discharge.
• History of dementia.
• Competency status on admission.
• Postoperative delirium (yes or no).
• Hospice care on admission (yes or no).
• Do Not Resuscitate (DNR) order in place on admission (yes or no).
• DNR order during hospitalization (yes or no).
• Setting where DNR order was placed.
• Postoperative palliative care consult (yes or no).
• 30-day postoperative outcomes: functional health status (ability to perform activities of daily living), physical function compared with baseline, and living location.
The number of surgery cases in the collaborative grew from 7,235 in the first 6 months of 2014 to 24,835 cases in the last 6 months of 2015. The top 10 operations were total joint arthroplasty (29%), colectomy (12%), spine (8%), hip fracture (7%), carotid endarterectomy (4%), hysterectomy (4%), lung resection (2%), open lower extremity bypass (2%), laparoscopic cholecystectomy (2%), and pancreatectomy (2%).
Dr. Robinson reported that the rate of preoperative dementia among cases studied in the collaborative was 10%. “The incorporation of dementia into a surgical dataset represents an important step forward in providing quality surgical care for the elderly,” he said. “Dementia is a global public health concern.” He went on to note that patients with dementia have a 2.5-fold increased risk of developing postoperative delirium, making it “the perfect place to start a quality project. One in three cases of delirium is preventable. In our data set, delirium is associated with a hospital stay that’s 4 days longer, an increased chance of requiring discharge to an institutional care facility, an increased chance of a serious complication, and a higher 30-day mortality.”
Simple, low-tech bedside interventions such as ambulating in the hall three times a day, orienting the person, having the person sleep at night rather than sleep during the day, and avoiding medications with high risk for adverse events in older adults can prevent postoperative delirium, Dr. Robinson said.
One way that the Geriatric Surgery Pilot Collaborative can improve the surgical care of older adults is by fostering quality programs initiated at the participating local hospitals. “Preserving function after hospital stays is a first major goal,” he said. Another strategy involves creating a multidisciplinary frailty assessment to aid with decision making and risk assessment. “This takes into consideration NSQIP variables such as function, nutrition, comorbidity burden, cognition, social vulnerability, and mobility,” he said. The final and ultimate goal of the geriatric surgery collaborative is to establish a foundation of quality measurement for the Coalition for Quality in Geriatric Surgery, a project initiated by the American College of Surgeons to systematically improve the surgical care of older adults.
Dr. Robinson reported having no relevant financial disclosures.
EXPERT ANALYSIS AT THE ACS NSQIP NATIONAL CONFERENCE