User login
A quarter of hypertensive Medicare enrollees are nonadherent
Over 26% of Medicare part D enrollees aged 65 years and over are not taking their antihypertensive drugs properly, according to a report published online Sept. 13 in MMWR.
An analysis of 2014 data showed that 4.9 million hypertensive Medicare patients were taking an incorrect dose of their medication or were not taking it at all, reported Matthew Ritchey, DPT of the Centers for Disease Control and Prevention’s division for heart disease and stroke prevention, and his associates (MMWR. 2016 Sep 13:65).
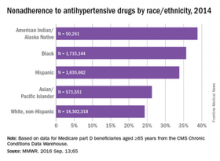
Nonadherence rates varied considerably by race and ethnicity, with American Indians/Alaska Natives the highest at 39%, followed by blacks at 36%, Hispanics at 34%, Asian/Pacific Islanders at 26%, and white non-Hispanics at 24%, the investigators noted.
The analysis included 18.5 million part D beneficiaries who filled two or more antihypertensive prescriptions in the same therapeutic class on different dates within a period of more than 90 days in 2014.
Over 26% of Medicare part D enrollees aged 65 years and over are not taking their antihypertensive drugs properly, according to a report published online Sept. 13 in MMWR.
An analysis of 2014 data showed that 4.9 million hypertensive Medicare patients were taking an incorrect dose of their medication or were not taking it at all, reported Matthew Ritchey, DPT of the Centers for Disease Control and Prevention’s division for heart disease and stroke prevention, and his associates (MMWR. 2016 Sep 13:65).
Nonadherence rates varied considerably by race and ethnicity, with American Indians/Alaska Natives the highest at 39%, followed by blacks at 36%, Hispanics at 34%, Asian/Pacific Islanders at 26%, and white non-Hispanics at 24%, the investigators noted.
The analysis included 18.5 million part D beneficiaries who filled two or more antihypertensive prescriptions in the same therapeutic class on different dates within a period of more than 90 days in 2014.
Over 26% of Medicare part D enrollees aged 65 years and over are not taking their antihypertensive drugs properly, according to a report published online Sept. 13 in MMWR.
An analysis of 2014 data showed that 4.9 million hypertensive Medicare patients were taking an incorrect dose of their medication or were not taking it at all, reported Matthew Ritchey, DPT of the Centers for Disease Control and Prevention’s division for heart disease and stroke prevention, and his associates (MMWR. 2016 Sep 13:65).
Nonadherence rates varied considerably by race and ethnicity, with American Indians/Alaska Natives the highest at 39%, followed by blacks at 36%, Hispanics at 34%, Asian/Pacific Islanders at 26%, and white non-Hispanics at 24%, the investigators noted.
The analysis included 18.5 million part D beneficiaries who filled two or more antihypertensive prescriptions in the same therapeutic class on different dates within a period of more than 90 days in 2014.
FROM MMWR
Quality or convenience: Pick one
A 35-year-old male with a 5-year-history of a changing mole on his back sends a picture of the lesion to a telemedicine website for advice. The photo reveals a black nodule. The clinician replies, advising the patient that the lesion is benign.
To most dermatologists, the above scenario would occur only in a bizarre nightmare, never in real life. In real life, if the words “black” and “nodule” are used to describe a lesion, they are followed by the verb “biopsy.” Most dermatologists would recognize this as a high-risk growth and recommend additional investigation.
Unfortunately, a recent study of direct-to-consumer (DTC) telemedicine in JAMA Dermatology showed that 21% of the time, the patient was wrongfully reassured that a lesion was benign (JAMA Dermatol. 2016;152[7]:768-75). The study examined how 16 DTC telemedicine websites and apps handled six standardized dermatology cases designed to test the quality of the services. While some provided good care, others missed important diagnoses such as syphilis, eczema herpeticum, and melanoma. If these cases had been actual patients, the consequences for such mistakes could have been dire.
“The services failed to ask simple, relevant questions of patients about their symptoms, leading them to repeatedly miss important diagnoses,” Jack Resneck Jr., MD, a dermatologist at the University of California, San Francisco, and lead author of the study, told the Wall Street Journal.
The study is timely, as telemedicine is accelerating explosively. The low cost of connectivity, viable business models, and changing consumer behaviors are fueling its rocket growth. Startups in digital health and telemedicine have raised over $700 million already this year, indicating that there is more fuel to be burned and more money to be made.
DTC telemedicine describes the model when a patient sends photos directly to a clinician without a prior history with that provider. A teleconsultation, in contrast, is an interaction between two doctors. In DTC, the episode of care is usually isolated from the patient’s record, and the information is not transferred to the primary care physician. Patients pay a fee, which can range from $1.59 to $250.
Advocates of DTC cite its low cost and extraordinary convenience as arguments for its adoption. However, these disconnected visits are notable exceptions to the current trend toward better care coordination and information sharing among providers.
Quality is also a concern. Although consumers were often promised answers from board-certified physicians, the JAMA Dermatology study was unable to verify this in many cases. The researchers also found that nondermatologists, physician extenders, and physicians practicing in India were often the providers, facts that were not obvious to users.
Worse, the study found both the quality of the diagnoses and the recommendations were poor. All the providers missed the cases of syphilis and most missed eczema herpeticum. Risks of prescription medications were not disclosed two-thirds of the time. Worse yet, three services mistakenly advised that a nodular melanoma did not need further treatment. Had these been real patients, such wrongful recommendations could have resulted in deaths.
In an effort to ensure safety and reliability for consumers, the American Telemedicine Association has begun credentialing telemedicine providers. Such credentials are not required, however, and consumers are likely to be unaware of which providers have or have not met this standard. The American Academy of Dermatology addresses DTC teledermatology in its position statement, updated in 2016: “Dermatologists providing direct-to-patient teledermatology must make every effort to collect accurate, complete, and quality clinical information. When appropriate, the dermatologist may wish to contact the primary care providers or other specialists to obtain additional corroborating information.”
Currently, patients remain on their own in choosing telemedicine and other digital health services: caveat emptor. Do they want quality and convenient care? For now, it seems, they must pick only one.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. He is @Dermdoc on Twitter. Write to him at [email protected].
A 35-year-old male with a 5-year-history of a changing mole on his back sends a picture of the lesion to a telemedicine website for advice. The photo reveals a black nodule. The clinician replies, advising the patient that the lesion is benign.
To most dermatologists, the above scenario would occur only in a bizarre nightmare, never in real life. In real life, if the words “black” and “nodule” are used to describe a lesion, they are followed by the verb “biopsy.” Most dermatologists would recognize this as a high-risk growth and recommend additional investigation.
Unfortunately, a recent study of direct-to-consumer (DTC) telemedicine in JAMA Dermatology showed that 21% of the time, the patient was wrongfully reassured that a lesion was benign (JAMA Dermatol. 2016;152[7]:768-75). The study examined how 16 DTC telemedicine websites and apps handled six standardized dermatology cases designed to test the quality of the services. While some provided good care, others missed important diagnoses such as syphilis, eczema herpeticum, and melanoma. If these cases had been actual patients, the consequences for such mistakes could have been dire.
“The services failed to ask simple, relevant questions of patients about their symptoms, leading them to repeatedly miss important diagnoses,” Jack Resneck Jr., MD, a dermatologist at the University of California, San Francisco, and lead author of the study, told the Wall Street Journal.
The study is timely, as telemedicine is accelerating explosively. The low cost of connectivity, viable business models, and changing consumer behaviors are fueling its rocket growth. Startups in digital health and telemedicine have raised over $700 million already this year, indicating that there is more fuel to be burned and more money to be made.
DTC telemedicine describes the model when a patient sends photos directly to a clinician without a prior history with that provider. A teleconsultation, in contrast, is an interaction between two doctors. In DTC, the episode of care is usually isolated from the patient’s record, and the information is not transferred to the primary care physician. Patients pay a fee, which can range from $1.59 to $250.
Advocates of DTC cite its low cost and extraordinary convenience as arguments for its adoption. However, these disconnected visits are notable exceptions to the current trend toward better care coordination and information sharing among providers.
Quality is also a concern. Although consumers were often promised answers from board-certified physicians, the JAMA Dermatology study was unable to verify this in many cases. The researchers also found that nondermatologists, physician extenders, and physicians practicing in India were often the providers, facts that were not obvious to users.
Worse, the study found both the quality of the diagnoses and the recommendations were poor. All the providers missed the cases of syphilis and most missed eczema herpeticum. Risks of prescription medications were not disclosed two-thirds of the time. Worse yet, three services mistakenly advised that a nodular melanoma did not need further treatment. Had these been real patients, such wrongful recommendations could have resulted in deaths.
In an effort to ensure safety and reliability for consumers, the American Telemedicine Association has begun credentialing telemedicine providers. Such credentials are not required, however, and consumers are likely to be unaware of which providers have or have not met this standard. The American Academy of Dermatology addresses DTC teledermatology in its position statement, updated in 2016: “Dermatologists providing direct-to-patient teledermatology must make every effort to collect accurate, complete, and quality clinical information. When appropriate, the dermatologist may wish to contact the primary care providers or other specialists to obtain additional corroborating information.”
Currently, patients remain on their own in choosing telemedicine and other digital health services: caveat emptor. Do they want quality and convenient care? For now, it seems, they must pick only one.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. He is @Dermdoc on Twitter. Write to him at [email protected].
A 35-year-old male with a 5-year-history of a changing mole on his back sends a picture of the lesion to a telemedicine website for advice. The photo reveals a black nodule. The clinician replies, advising the patient that the lesion is benign.
To most dermatologists, the above scenario would occur only in a bizarre nightmare, never in real life. In real life, if the words “black” and “nodule” are used to describe a lesion, they are followed by the verb “biopsy.” Most dermatologists would recognize this as a high-risk growth and recommend additional investigation.
Unfortunately, a recent study of direct-to-consumer (DTC) telemedicine in JAMA Dermatology showed that 21% of the time, the patient was wrongfully reassured that a lesion was benign (JAMA Dermatol. 2016;152[7]:768-75). The study examined how 16 DTC telemedicine websites and apps handled six standardized dermatology cases designed to test the quality of the services. While some provided good care, others missed important diagnoses such as syphilis, eczema herpeticum, and melanoma. If these cases had been actual patients, the consequences for such mistakes could have been dire.
“The services failed to ask simple, relevant questions of patients about their symptoms, leading them to repeatedly miss important diagnoses,” Jack Resneck Jr., MD, a dermatologist at the University of California, San Francisco, and lead author of the study, told the Wall Street Journal.
The study is timely, as telemedicine is accelerating explosively. The low cost of connectivity, viable business models, and changing consumer behaviors are fueling its rocket growth. Startups in digital health and telemedicine have raised over $700 million already this year, indicating that there is more fuel to be burned and more money to be made.
DTC telemedicine describes the model when a patient sends photos directly to a clinician without a prior history with that provider. A teleconsultation, in contrast, is an interaction between two doctors. In DTC, the episode of care is usually isolated from the patient’s record, and the information is not transferred to the primary care physician. Patients pay a fee, which can range from $1.59 to $250.
Advocates of DTC cite its low cost and extraordinary convenience as arguments for its adoption. However, these disconnected visits are notable exceptions to the current trend toward better care coordination and information sharing among providers.
Quality is also a concern. Although consumers were often promised answers from board-certified physicians, the JAMA Dermatology study was unable to verify this in many cases. The researchers also found that nondermatologists, physician extenders, and physicians practicing in India were often the providers, facts that were not obvious to users.
Worse, the study found both the quality of the diagnoses and the recommendations were poor. All the providers missed the cases of syphilis and most missed eczema herpeticum. Risks of prescription medications were not disclosed two-thirds of the time. Worse yet, three services mistakenly advised that a nodular melanoma did not need further treatment. Had these been real patients, such wrongful recommendations could have resulted in deaths.
In an effort to ensure safety and reliability for consumers, the American Telemedicine Association has begun credentialing telemedicine providers. Such credentials are not required, however, and consumers are likely to be unaware of which providers have or have not met this standard. The American Academy of Dermatology addresses DTC teledermatology in its position statement, updated in 2016: “Dermatologists providing direct-to-patient teledermatology must make every effort to collect accurate, complete, and quality clinical information. When appropriate, the dermatologist may wish to contact the primary care providers or other specialists to obtain additional corroborating information.”
Currently, patients remain on their own in choosing telemedicine and other digital health services: caveat emptor. Do they want quality and convenient care? For now, it seems, they must pick only one.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. He is @Dermdoc on Twitter. Write to him at [email protected].
Four factors raise risk of post-TAVR endocarditis
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Four factors – younger patient age, male sex, diabetes, and moderate to severe residual aortic regurgitation – are associated with a significantly increased risk of infective endocarditis after transcatheter aortic valve replacement, according to a report published online Sept. 13 in JAMA.
Until now, data pertaining to endocarditis following TAVR “have been limited to case reports and relatively small series with limited follow-up,” said Ander Regueiro, MD, of Laval University, Quebec City, and his associates.
They performed a retrospective analysis of data in a large international registry of TAVR cases to better characterize post-TAVR endocarditis.
Dr. Regueiro and his colleagues focused on 20,006 TAVR procedures done at 47 medical centers in Europe, North America, and South America during a 10-year period. The median time to symptom onset was 5.3 months after the procedure.
Infective endocarditis was definitively diagnosed in 250 of these cases. This incidence is similar to that reported for endocarditis following surgical aortic valve replacement, indicating that TAVR is no less predisposing to endocarditis despite being a less invasive approach.
The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who did not (HR, 0.97). The reason for this association is unclear, but it is possible that younger patients chosen for TAVR because of their prohibitive surgical risk carry a higher burden of comorbidity than do older patients. Similarly, 62% of endocarditis cases arose in men (HR, 1.69), and sex differences in comorbid conditions may explain the higher risk among men.
More patients who developed endocarditis had diabetes (41.7%), compared with those who did not develop endocarditis (30%), for an HR of 1.52. And patients who had moderate to severe residual aortic regurgitation after TAVR also were at much higher risk for endocarditis than were those who did not (HR, 2.05), the investigators noted (JAMA. 2016 Sep 13;316[10]:1083-92).
In contrast, factors that were not associated with endocarditis risk included chronic pulmonary disease, type of valve (self-expandable or balloon-expandable), and setting of the procedure (catheterization lab vs. operating room).
The bacteria that most commonly caused infective endocarditis were Enterococci species (24.6% of cases), Staphylococcus aureus (23.8%), and coagulase-negative staphylococci (16.8%). This should be taken into consideration when selecting antibiotics for prophylaxis before TAVR and when choosing empirical antibiotics for treatment while waiting for blood culture results, wrote Dr. Regueiro and his associates.
“This information may help clinicians identify patients at higher risk [for endocarditis] and aid in implementing appropriate preventive measures,” they noted.
This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Four factors raise the risk that patients undergoing transcatheter aortic valve replacement will develop infective endocarditis.
Major finding: The mean age of patients who developed post-TAVR endocarditis was 78.9 years, compared with 81.8 years for those who didn’t (HR, 0.97).
Data source: A retrospective analysis of data in an international registry involving 20,006 patients who underwent TAVR at 47 medical centers during a 10-year period.
Disclosures: This study was supported by a grant from the Alfonso Martin Escudero Foundation. Dr. Regueiro reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Is It All in the Eye of the Beholder? Comparing Pulmonologists’ and Radiologists’ Performance
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
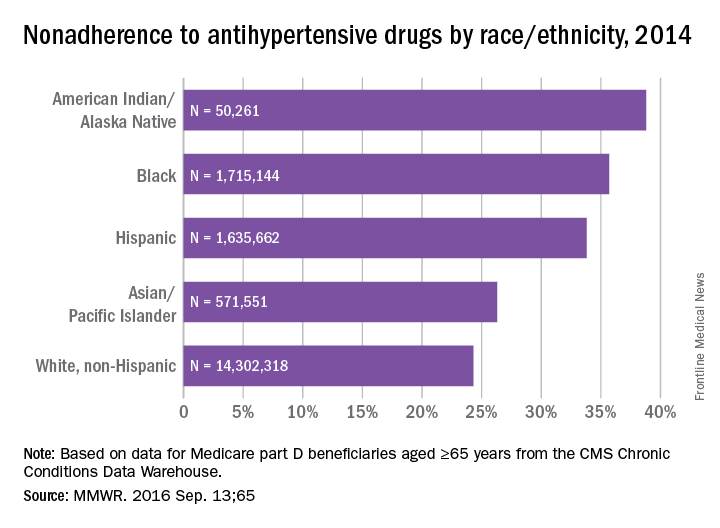
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
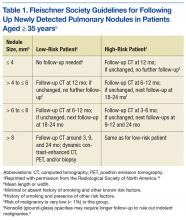
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
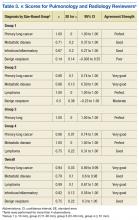
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Who’s the Best Candidate for Endovascular Treatment?
Patients who have had ischemic strokes often receive endovascular treatment along with tissue plasminogen activator (T-PA) to break up clots in the brain, but bleeding is a serious risk. T-PA has a distinct window of effectiveness, but less is known about endovascular treatment. A new imaging method may help resolve that by identifying stroke patients who are not likely to benefit from endovascular surgery.
Related: Percutaneous Endovascular Treatment of Subclavian Steal Syndrome
Researchers from the National Institute of Neurological Disorders and Stroke, Bethesda, MD; Stanford University, Palo Alto, CA; University of Melbourne, Australia, collected brain scans from more than 100 patients before they underwent endovascular therapy, within 12 hours of the stroke. Using a new method of image processing, the researchers got detailed measurements on just how much a stroke disrupts the blood-brain barrier and combined those measurements with findings from the DEFUSE-2 study (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution).
They found that large degrees of blood-brain barrier disruption were associated with severe bleeding following endovascular surgery. Extensive breakdown of the blood-brain barrier was associated with parenchymal hematoma, which 24 of the 100 patients in the study experienced. The study also showed a link between the location of blood-brain barrier damage and posttreatment bleeding.
Related: Standard vs Intensive Emergency Stroke Treatment
“The biggest impact of this research is that information from MRI scans routinely collected at a number of research hospitals and stroke centers can inform treating physicians on the risk of bleeding,” said one of the study authors, Richard Leigh, MD, a scientist with the National Institute of Neurological Disorders and Stroke.
Patients who have had ischemic strokes often receive endovascular treatment along with tissue plasminogen activator (T-PA) to break up clots in the brain, but bleeding is a serious risk. T-PA has a distinct window of effectiveness, but less is known about endovascular treatment. A new imaging method may help resolve that by identifying stroke patients who are not likely to benefit from endovascular surgery.
Related: Percutaneous Endovascular Treatment of Subclavian Steal Syndrome
Researchers from the National Institute of Neurological Disorders and Stroke, Bethesda, MD; Stanford University, Palo Alto, CA; University of Melbourne, Australia, collected brain scans from more than 100 patients before they underwent endovascular therapy, within 12 hours of the stroke. Using a new method of image processing, the researchers got detailed measurements on just how much a stroke disrupts the blood-brain barrier and combined those measurements with findings from the DEFUSE-2 study (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution).
They found that large degrees of blood-brain barrier disruption were associated with severe bleeding following endovascular surgery. Extensive breakdown of the blood-brain barrier was associated with parenchymal hematoma, which 24 of the 100 patients in the study experienced. The study also showed a link between the location of blood-brain barrier damage and posttreatment bleeding.
Related: Standard vs Intensive Emergency Stroke Treatment
“The biggest impact of this research is that information from MRI scans routinely collected at a number of research hospitals and stroke centers can inform treating physicians on the risk of bleeding,” said one of the study authors, Richard Leigh, MD, a scientist with the National Institute of Neurological Disorders and Stroke.
Patients who have had ischemic strokes often receive endovascular treatment along with tissue plasminogen activator (T-PA) to break up clots in the brain, but bleeding is a serious risk. T-PA has a distinct window of effectiveness, but less is known about endovascular treatment. A new imaging method may help resolve that by identifying stroke patients who are not likely to benefit from endovascular surgery.
Related: Percutaneous Endovascular Treatment of Subclavian Steal Syndrome
Researchers from the National Institute of Neurological Disorders and Stroke, Bethesda, MD; Stanford University, Palo Alto, CA; University of Melbourne, Australia, collected brain scans from more than 100 patients before they underwent endovascular therapy, within 12 hours of the stroke. Using a new method of image processing, the researchers got detailed measurements on just how much a stroke disrupts the blood-brain barrier and combined those measurements with findings from the DEFUSE-2 study (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution).
They found that large degrees of blood-brain barrier disruption were associated with severe bleeding following endovascular surgery. Extensive breakdown of the blood-brain barrier was associated with parenchymal hematoma, which 24 of the 100 patients in the study experienced. The study also showed a link between the location of blood-brain barrier damage and posttreatment bleeding.
Related: Standard vs Intensive Emergency Stroke Treatment
“The biggest impact of this research is that information from MRI scans routinely collected at a number of research hospitals and stroke centers can inform treating physicians on the risk of bleeding,” said one of the study authors, Richard Leigh, MD, a scientist with the National Institute of Neurological Disorders and Stroke.
The State of Hospital Medicine Is Strong
Editor's Note: Listen to Dr. Smith share more of his views on the State of Hospital Medicine report.
2016 is the “Year of the Hospitalist,” a sobriquet meant as a proud nod to the specialty’s maturation as a fixture in hospitals across the country. Hospital medicine is no longer the new kid on the block as it has assumed care for the vast majority of hospitalized patients nationwide.
One could understand then if the ever-rising salaries hospitalists have commanded for 20 years might have finally plateaued, particularly as tightening budgets have C-suite administrators looking to trim costs.
Think again.
“Growth suggests that there is still a huge demand,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee. “The demand for hospitalists still exceeds the supply, and so recruiting hospitalists, particularly to nonurban areas, is really challenging and is requiring more money.”
The SoHM is a biennial partnership between SHM and MGMA that provides HM group leaders and rank-and-file hospitalists a litany of benchmarks for salaries, workloads, and everything that informs those two topics. Call it the specialty’s empirical roadmap.
“Often, compensation information relative to staffing information is proprietary, so hospitalists are in a position where they are dependent upon their hospital stakeholders to have access to this information, but they are also the same stakeholders with whom they negotiate their contracts,” says G. Randy Smith, MD, MS, FRCP(Edin), SFHM, an assistant professor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago and a member of SHM’s Practice Analysis Committee. “The State of Hospital Medicine report by SHM provides an opportunity for hospitalists to have an independent view of the compensation and workforce distribution factors that can impact negotiations with their hospital stakeholders. It’s a very powerful tool.”
Compensation Data
Rachel Lovins, MD, SFHM, CPE, voraciously reads every SoHM report because she uses its keynote compensation data to benchmark what she should pay her staff.
“I make a promise to my group,” says Dr. Lovins, chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. “I promise them that we will always be at or above what is standard for our areas. So for me, I have to look every time.”
Promises like that are getting more expensive to keep.
Hospitalists in the East region earn a median compensation of $245,977, up 3.1% from the $238,676 figure reported in 2014. But the East, where the bulk of the country’s population lives and where competition for hospitalists is typically lessened, is by far the lowest-paid region.
Hospitalists in the South continue to earn the most, with a median compensation of $301,833, up a whopping 16.9% from $258,020 from 2013. Hospitalists in the West earned a median of $275,658 (up 10.3% from $249,894), while Midwestern hospitalists saw a median compensation of $285,213 (up 8.9% from $261,868).
In addition to year-over-year growth, HM is also seeing outsized growth as compared with family medicine physicians, according to MGMA data. From 2011 to 2015, family medicine hospitalists saw an average compensation increase of 4.7%, bringing the average salary to $285,213. During the same period, family medicine physicians who are not hospitalists and don’t practice obstetrics saw an average annual compensation increase of only 3%, bringing the average salary to $230,456.
“The hospitalist can save the hospital considerable amounts of money because of their ability to better manage the patient and improve the quality of care at the same time,” says David Gans, MSHA, FACMPE, MGMA’s senior fellow of industry affairs. “Hospitals, they have recognized that, and therefore, there is considerable competition for recruiting and retaining hospitalists.”
To that end, 96.3% of HM groups (HMGs) received financial support in addition to their professional fee revenue. That’s up from 89% of HMGs that relied last year on their host hospitals. The median support is $157,535 per full-time employee (FTE), up just 1%. Correspondingly, SoHM reported 8.5% of HMGs received enough income from professional fee revenue to cover expenses, up from 6% two years ago.
Industry watchers predicted that, in two years, fee revenue would have to rise to offset hospitals’ inability to pay. The early returns seem to show that bearing out.
“We’re pretty close to that breaking point,” Flores says. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”
Productivity Stalls
While compensation continues to climb, productivity flattened out in this year’s report.
Median relative value units (RVUs) dipped slightly from the figure reported in 2014, to 4,252 from 4,297. But the tally is still ahead of 2012’s total of 4,159. Median collection-to-work RVUs also ticked down from 2014’s tally, to $50.29 from $51.50 in 2013.
Flores largely attributes the falling metrics of productivity to the evolution of HMGs that have standardized their scheduling to the point that most HMGs now offer vacation time.
“So the number of groups that are working 182 days is fewer, and we see a lot more groups that are working something like 168 days or 172 days,” she says. “And if a hospitalist works fewer shifts, even if they see the same number of patients per shift, they’re going to generate less productivity over the course of the year, so that’s part of it.”
Andrew White, MD, SFHM, director of the HM service at the University of Washington Medical Center in Seattle, says the report’s value is in avoiding a myopic approach to how HMGs operate. For example, RVUs are an important metric of productivity, but not all shifts should be expected to produce the same.
For example, it’d be valuable to use the report to see how hard your nocturnists are working compared with other sites, says Dr. White, also a member of the Practice Analysis Committee.
“The fundamental issue with working at night is that not everybody wants to do it, and so you have to recognize that it’s a pain to do and you have to either pay those people more, have them work less, or acknowledge that they’re going to be less productive,” he says. “We use the survey to assess all three of those things and then can work with our nocturnists to reach an agreement about a fair approach to their job structure that’s actually informed by national benchmarks. That process has helped us to pick, for example, how many nights per year they should work or what their salaries should look like compared to the day hospitalists.”
Dr. White says that because the report is comprehensive and includes broad participation, he’s able to use it as a benchmark to make hiring and service structure decisions.
“It also helps me to keep abreast of some trends that may be occurring in the broader workplace that we aren’t participating in but maybe should be or should be thinking about,” he says.
The report’s subsections are also critical for comparing one HMG to others, Dr. White says.
“Obviously, there is the aggregate data there to look at the average program,” he says. “But really as a hospitalist group leader, you want to know what are other programs like mine doing, and it allows you to drill down into that data.”
Survey Limitations
Any hospitalist worth their weight in scrubs knows that any medical study is only as good as its limitations. And while SoHM is a trove of valuable data information, Flores always cautions against taking data points as gospel.
“People should understand what the numbers are telling us, what goes into those numbers, and take them not with a grain of salt but take them for what they are,” she says.
For example, Flores says, look at productivity metrics per shift. Day shifts have traditionally driven that figure, and those shifts are typically busy. But night shifts have fewer patients and less productivity.
“So as more and more hospitals get 24-hour in-house coverage and have doctors working low-productivity night shifts, that [productivity] number might fall,” she says.
That sort of nuanced analysis of productivity can’t be found anywhere else, says Dr. Lovins.
These are “data that we don’t normally get from our administration,” she says, “information on things like staffing and patient loads, and how much more the director makes than the people that work for the director, and how much more nighttime people make than daytime people make. There is no other way for me to get that information, and it’s very important to make sure that our program is fair.”
Aside from fair, the data points are essential talking points as HMGs negotiate contracts and other arrangements with their administrators.
“It’s a reference point so that everybody feels like we’re using data from a national source that everyone can agree upon as fair,” says Dr. White.
In Dr. White’s case, he doesn’t have many local academic programs to benchmark against. And comparing to private, for-profit hospitals isn’t the proverbial apples-to-apples comparison. Having vetted regional and national figures for comparison is incredibly valuable, particularly since he doesn’t have to compile the data.
“If I had to go call all those group leaders and figure out what they were doing, it would be pretty exhausting,” he says.
Alternative Payment Models
Dr. Smith says that one area where the report will become even more valuable over the next few years is addressing alternative payment models (APMs). In particular, HM leaders say they’re excited about being drivers in one of the largest APMs: the Bundled Payments for Care Improvement (BCPI) initiative. In short, the program covers 48 defined episodes of care, including medical and surgical, that could begin three days prior to admission and stretch 30, 60, or 90 days post-discharge.
Dr. Smith thinks it’s still a bit too early to see from the report how APMs have affected compensation.
“We’re still relatively in the early days of bundled-payment models, so in that regard, the State of Hospital Medicine Report still represents very much a starting point with regard to where hospital medicine groups will find themselves as they start to encounter challenges,” he says.
Perhaps more important, Gans doesn’t expect that the maturation of APMs will result in decreased compensation for hospitalists.
“In a hospital environment where the hospital is being reimbursed a set amount for a complete hospital admission and follow-up care and potential readmissions, that is an episodic payment already,” he says. “Consequently, the incentive is there today to better manage the patient and to attain the care coordination and care management necessary for that patient to be discharged and not readmitted.”
In fact, the SHM/MGMA data tell him that the basic economic theory of supply and demand continues to drive hospitalist compensation even 20 years after the field was given its name. He says rising compensation, even as more practices look to hire nurse practitioners or physician assistants as less expensive alternatives, shows no sign of letting up.
“I think demand will continue to be there,” Gans adds. “There may be in the long run some lessening of demand for hospitalists, but I don’t see that for years.”
Richard Quinn is a freelance writer in New Jersey.
Editor's Note: Listen to Dr. Smith share more of his views on the State of Hospital Medicine report.
2016 is the “Year of the Hospitalist,” a sobriquet meant as a proud nod to the specialty’s maturation as a fixture in hospitals across the country. Hospital medicine is no longer the new kid on the block as it has assumed care for the vast majority of hospitalized patients nationwide.
One could understand then if the ever-rising salaries hospitalists have commanded for 20 years might have finally plateaued, particularly as tightening budgets have C-suite administrators looking to trim costs.
Think again.
“Growth suggests that there is still a huge demand,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee. “The demand for hospitalists still exceeds the supply, and so recruiting hospitalists, particularly to nonurban areas, is really challenging and is requiring more money.”
The SoHM is a biennial partnership between SHM and MGMA that provides HM group leaders and rank-and-file hospitalists a litany of benchmarks for salaries, workloads, and everything that informs those two topics. Call it the specialty’s empirical roadmap.
“Often, compensation information relative to staffing information is proprietary, so hospitalists are in a position where they are dependent upon their hospital stakeholders to have access to this information, but they are also the same stakeholders with whom they negotiate their contracts,” says G. Randy Smith, MD, MS, FRCP(Edin), SFHM, an assistant professor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago and a member of SHM’s Practice Analysis Committee. “The State of Hospital Medicine report by SHM provides an opportunity for hospitalists to have an independent view of the compensation and workforce distribution factors that can impact negotiations with their hospital stakeholders. It’s a very powerful tool.”
Compensation Data
Rachel Lovins, MD, SFHM, CPE, voraciously reads every SoHM report because she uses its keynote compensation data to benchmark what she should pay her staff.
“I make a promise to my group,” says Dr. Lovins, chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. “I promise them that we will always be at or above what is standard for our areas. So for me, I have to look every time.”
Promises like that are getting more expensive to keep.
Hospitalists in the East region earn a median compensation of $245,977, up 3.1% from the $238,676 figure reported in 2014. But the East, where the bulk of the country’s population lives and where competition for hospitalists is typically lessened, is by far the lowest-paid region.
Hospitalists in the South continue to earn the most, with a median compensation of $301,833, up a whopping 16.9% from $258,020 from 2013. Hospitalists in the West earned a median of $275,658 (up 10.3% from $249,894), while Midwestern hospitalists saw a median compensation of $285,213 (up 8.9% from $261,868).
In addition to year-over-year growth, HM is also seeing outsized growth as compared with family medicine physicians, according to MGMA data. From 2011 to 2015, family medicine hospitalists saw an average compensation increase of 4.7%, bringing the average salary to $285,213. During the same period, family medicine physicians who are not hospitalists and don’t practice obstetrics saw an average annual compensation increase of only 3%, bringing the average salary to $230,456.
“The hospitalist can save the hospital considerable amounts of money because of their ability to better manage the patient and improve the quality of care at the same time,” says David Gans, MSHA, FACMPE, MGMA’s senior fellow of industry affairs. “Hospitals, they have recognized that, and therefore, there is considerable competition for recruiting and retaining hospitalists.”
To that end, 96.3% of HM groups (HMGs) received financial support in addition to their professional fee revenue. That’s up from 89% of HMGs that relied last year on their host hospitals. The median support is $157,535 per full-time employee (FTE), up just 1%. Correspondingly, SoHM reported 8.5% of HMGs received enough income from professional fee revenue to cover expenses, up from 6% two years ago.
Industry watchers predicted that, in two years, fee revenue would have to rise to offset hospitals’ inability to pay. The early returns seem to show that bearing out.
“We’re pretty close to that breaking point,” Flores says. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”
Productivity Stalls
While compensation continues to climb, productivity flattened out in this year’s report.
Median relative value units (RVUs) dipped slightly from the figure reported in 2014, to 4,252 from 4,297. But the tally is still ahead of 2012’s total of 4,159. Median collection-to-work RVUs also ticked down from 2014’s tally, to $50.29 from $51.50 in 2013.
Flores largely attributes the falling metrics of productivity to the evolution of HMGs that have standardized their scheduling to the point that most HMGs now offer vacation time.
“So the number of groups that are working 182 days is fewer, and we see a lot more groups that are working something like 168 days or 172 days,” she says. “And if a hospitalist works fewer shifts, even if they see the same number of patients per shift, they’re going to generate less productivity over the course of the year, so that’s part of it.”
Andrew White, MD, SFHM, director of the HM service at the University of Washington Medical Center in Seattle, says the report’s value is in avoiding a myopic approach to how HMGs operate. For example, RVUs are an important metric of productivity, but not all shifts should be expected to produce the same.
For example, it’d be valuable to use the report to see how hard your nocturnists are working compared with other sites, says Dr. White, also a member of the Practice Analysis Committee.
“The fundamental issue with working at night is that not everybody wants to do it, and so you have to recognize that it’s a pain to do and you have to either pay those people more, have them work less, or acknowledge that they’re going to be less productive,” he says. “We use the survey to assess all three of those things and then can work with our nocturnists to reach an agreement about a fair approach to their job structure that’s actually informed by national benchmarks. That process has helped us to pick, for example, how many nights per year they should work or what their salaries should look like compared to the day hospitalists.”
Dr. White says that because the report is comprehensive and includes broad participation, he’s able to use it as a benchmark to make hiring and service structure decisions.
“It also helps me to keep abreast of some trends that may be occurring in the broader workplace that we aren’t participating in but maybe should be or should be thinking about,” he says.
The report’s subsections are also critical for comparing one HMG to others, Dr. White says.
“Obviously, there is the aggregate data there to look at the average program,” he says. “But really as a hospitalist group leader, you want to know what are other programs like mine doing, and it allows you to drill down into that data.”
Survey Limitations
Any hospitalist worth their weight in scrubs knows that any medical study is only as good as its limitations. And while SoHM is a trove of valuable data information, Flores always cautions against taking data points as gospel.
“People should understand what the numbers are telling us, what goes into those numbers, and take them not with a grain of salt but take them for what they are,” she says.
For example, Flores says, look at productivity metrics per shift. Day shifts have traditionally driven that figure, and those shifts are typically busy. But night shifts have fewer patients and less productivity.
“So as more and more hospitals get 24-hour in-house coverage and have doctors working low-productivity night shifts, that [productivity] number might fall,” she says.
That sort of nuanced analysis of productivity can’t be found anywhere else, says Dr. Lovins.
These are “data that we don’t normally get from our administration,” she says, “information on things like staffing and patient loads, and how much more the director makes than the people that work for the director, and how much more nighttime people make than daytime people make. There is no other way for me to get that information, and it’s very important to make sure that our program is fair.”
Aside from fair, the data points are essential talking points as HMGs negotiate contracts and other arrangements with their administrators.
“It’s a reference point so that everybody feels like we’re using data from a national source that everyone can agree upon as fair,” says Dr. White.
In Dr. White’s case, he doesn’t have many local academic programs to benchmark against. And comparing to private, for-profit hospitals isn’t the proverbial apples-to-apples comparison. Having vetted regional and national figures for comparison is incredibly valuable, particularly since he doesn’t have to compile the data.
“If I had to go call all those group leaders and figure out what they were doing, it would be pretty exhausting,” he says.
Alternative Payment Models
Dr. Smith says that one area where the report will become even more valuable over the next few years is addressing alternative payment models (APMs). In particular, HM leaders say they’re excited about being drivers in one of the largest APMs: the Bundled Payments for Care Improvement (BCPI) initiative. In short, the program covers 48 defined episodes of care, including medical and surgical, that could begin three days prior to admission and stretch 30, 60, or 90 days post-discharge.
Dr. Smith thinks it’s still a bit too early to see from the report how APMs have affected compensation.
“We’re still relatively in the early days of bundled-payment models, so in that regard, the State of Hospital Medicine Report still represents very much a starting point with regard to where hospital medicine groups will find themselves as they start to encounter challenges,” he says.
Perhaps more important, Gans doesn’t expect that the maturation of APMs will result in decreased compensation for hospitalists.
“In a hospital environment where the hospital is being reimbursed a set amount for a complete hospital admission and follow-up care and potential readmissions, that is an episodic payment already,” he says. “Consequently, the incentive is there today to better manage the patient and to attain the care coordination and care management necessary for that patient to be discharged and not readmitted.”
In fact, the SHM/MGMA data tell him that the basic economic theory of supply and demand continues to drive hospitalist compensation even 20 years after the field was given its name. He says rising compensation, even as more practices look to hire nurse practitioners or physician assistants as less expensive alternatives, shows no sign of letting up.
“I think demand will continue to be there,” Gans adds. “There may be in the long run some lessening of demand for hospitalists, but I don’t see that for years.”
Richard Quinn is a freelance writer in New Jersey.
Editor's Note: Listen to Dr. Smith share more of his views on the State of Hospital Medicine report.
2016 is the “Year of the Hospitalist,” a sobriquet meant as a proud nod to the specialty’s maturation as a fixture in hospitals across the country. Hospital medicine is no longer the new kid on the block as it has assumed care for the vast majority of hospitalized patients nationwide.
One could understand then if the ever-rising salaries hospitalists have commanded for 20 years might have finally plateaued, particularly as tightening budgets have C-suite administrators looking to trim costs.
Think again.
“Growth suggests that there is still a huge demand,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee. “The demand for hospitalists still exceeds the supply, and so recruiting hospitalists, particularly to nonurban areas, is really challenging and is requiring more money.”
The SoHM is a biennial partnership between SHM and MGMA that provides HM group leaders and rank-and-file hospitalists a litany of benchmarks for salaries, workloads, and everything that informs those two topics. Call it the specialty’s empirical roadmap.
“Often, compensation information relative to staffing information is proprietary, so hospitalists are in a position where they are dependent upon their hospital stakeholders to have access to this information, but they are also the same stakeholders with whom they negotiate their contracts,” says G. Randy Smith, MD, MS, FRCP(Edin), SFHM, an assistant professor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago and a member of SHM’s Practice Analysis Committee. “The State of Hospital Medicine report by SHM provides an opportunity for hospitalists to have an independent view of the compensation and workforce distribution factors that can impact negotiations with their hospital stakeholders. It’s a very powerful tool.”
Compensation Data
Rachel Lovins, MD, SFHM, CPE, voraciously reads every SoHM report because she uses its keynote compensation data to benchmark what she should pay her staff.
“I make a promise to my group,” says Dr. Lovins, chief of hospital medicine and vice chair of the Department of Medicine at Middlesex Hospital in Middletown, Conn. “I promise them that we will always be at or above what is standard for our areas. So for me, I have to look every time.”
Promises like that are getting more expensive to keep.
Hospitalists in the East region earn a median compensation of $245,977, up 3.1% from the $238,676 figure reported in 2014. But the East, where the bulk of the country’s population lives and where competition for hospitalists is typically lessened, is by far the lowest-paid region.
Hospitalists in the South continue to earn the most, with a median compensation of $301,833, up a whopping 16.9% from $258,020 from 2013. Hospitalists in the West earned a median of $275,658 (up 10.3% from $249,894), while Midwestern hospitalists saw a median compensation of $285,213 (up 8.9% from $261,868).
In addition to year-over-year growth, HM is also seeing outsized growth as compared with family medicine physicians, according to MGMA data. From 2011 to 2015, family medicine hospitalists saw an average compensation increase of 4.7%, bringing the average salary to $285,213. During the same period, family medicine physicians who are not hospitalists and don’t practice obstetrics saw an average annual compensation increase of only 3%, bringing the average salary to $230,456.
“The hospitalist can save the hospital considerable amounts of money because of their ability to better manage the patient and improve the quality of care at the same time,” says David Gans, MSHA, FACMPE, MGMA’s senior fellow of industry affairs. “Hospitals, they have recognized that, and therefore, there is considerable competition for recruiting and retaining hospitalists.”
To that end, 96.3% of HM groups (HMGs) received financial support in addition to their professional fee revenue. That’s up from 89% of HMGs that relied last year on their host hospitals. The median support is $157,535 per full-time employee (FTE), up just 1%. Correspondingly, SoHM reported 8.5% of HMGs received enough income from professional fee revenue to cover expenses, up from 6% two years ago.
Industry watchers predicted that, in two years, fee revenue would have to rise to offset hospitals’ inability to pay. The early returns seem to show that bearing out.
“We’re pretty close to that breaking point,” Flores says. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”
Productivity Stalls
While compensation continues to climb, productivity flattened out in this year’s report.
Median relative value units (RVUs) dipped slightly from the figure reported in 2014, to 4,252 from 4,297. But the tally is still ahead of 2012’s total of 4,159. Median collection-to-work RVUs also ticked down from 2014’s tally, to $50.29 from $51.50 in 2013.
Flores largely attributes the falling metrics of productivity to the evolution of HMGs that have standardized their scheduling to the point that most HMGs now offer vacation time.
“So the number of groups that are working 182 days is fewer, and we see a lot more groups that are working something like 168 days or 172 days,” she says. “And if a hospitalist works fewer shifts, even if they see the same number of patients per shift, they’re going to generate less productivity over the course of the year, so that’s part of it.”
Andrew White, MD, SFHM, director of the HM service at the University of Washington Medical Center in Seattle, says the report’s value is in avoiding a myopic approach to how HMGs operate. For example, RVUs are an important metric of productivity, but not all shifts should be expected to produce the same.
For example, it’d be valuable to use the report to see how hard your nocturnists are working compared with other sites, says Dr. White, also a member of the Practice Analysis Committee.
“The fundamental issue with working at night is that not everybody wants to do it, and so you have to recognize that it’s a pain to do and you have to either pay those people more, have them work less, or acknowledge that they’re going to be less productive,” he says. “We use the survey to assess all three of those things and then can work with our nocturnists to reach an agreement about a fair approach to their job structure that’s actually informed by national benchmarks. That process has helped us to pick, for example, how many nights per year they should work or what their salaries should look like compared to the day hospitalists.”
Dr. White says that because the report is comprehensive and includes broad participation, he’s able to use it as a benchmark to make hiring and service structure decisions.
“It also helps me to keep abreast of some trends that may be occurring in the broader workplace that we aren’t participating in but maybe should be or should be thinking about,” he says.
The report’s subsections are also critical for comparing one HMG to others, Dr. White says.
“Obviously, there is the aggregate data there to look at the average program,” he says. “But really as a hospitalist group leader, you want to know what are other programs like mine doing, and it allows you to drill down into that data.”
Survey Limitations
Any hospitalist worth their weight in scrubs knows that any medical study is only as good as its limitations. And while SoHM is a trove of valuable data information, Flores always cautions against taking data points as gospel.
“People should understand what the numbers are telling us, what goes into those numbers, and take them not with a grain of salt but take them for what they are,” she says.
For example, Flores says, look at productivity metrics per shift. Day shifts have traditionally driven that figure, and those shifts are typically busy. But night shifts have fewer patients and less productivity.
“So as more and more hospitals get 24-hour in-house coverage and have doctors working low-productivity night shifts, that [productivity] number might fall,” she says.
That sort of nuanced analysis of productivity can’t be found anywhere else, says Dr. Lovins.
These are “data that we don’t normally get from our administration,” she says, “information on things like staffing and patient loads, and how much more the director makes than the people that work for the director, and how much more nighttime people make than daytime people make. There is no other way for me to get that information, and it’s very important to make sure that our program is fair.”
Aside from fair, the data points are essential talking points as HMGs negotiate contracts and other arrangements with their administrators.
“It’s a reference point so that everybody feels like we’re using data from a national source that everyone can agree upon as fair,” says Dr. White.
In Dr. White’s case, he doesn’t have many local academic programs to benchmark against. And comparing to private, for-profit hospitals isn’t the proverbial apples-to-apples comparison. Having vetted regional and national figures for comparison is incredibly valuable, particularly since he doesn’t have to compile the data.
“If I had to go call all those group leaders and figure out what they were doing, it would be pretty exhausting,” he says.
Alternative Payment Models
Dr. Smith says that one area where the report will become even more valuable over the next few years is addressing alternative payment models (APMs). In particular, HM leaders say they’re excited about being drivers in one of the largest APMs: the Bundled Payments for Care Improvement (BCPI) initiative. In short, the program covers 48 defined episodes of care, including medical and surgical, that could begin three days prior to admission and stretch 30, 60, or 90 days post-discharge.
Dr. Smith thinks it’s still a bit too early to see from the report how APMs have affected compensation.
“We’re still relatively in the early days of bundled-payment models, so in that regard, the State of Hospital Medicine Report still represents very much a starting point with regard to where hospital medicine groups will find themselves as they start to encounter challenges,” he says.
Perhaps more important, Gans doesn’t expect that the maturation of APMs will result in decreased compensation for hospitalists.
“In a hospital environment where the hospital is being reimbursed a set amount for a complete hospital admission and follow-up care and potential readmissions, that is an episodic payment already,” he says. “Consequently, the incentive is there today to better manage the patient and to attain the care coordination and care management necessary for that patient to be discharged and not readmitted.”
In fact, the SHM/MGMA data tell him that the basic economic theory of supply and demand continues to drive hospitalist compensation even 20 years after the field was given its name. He says rising compensation, even as more practices look to hire nurse practitioners or physician assistants as less expensive alternatives, shows no sign of letting up.
“I think demand will continue to be there,” Gans adds. “There may be in the long run some lessening of demand for hospitalists, but I don’t see that for years.”
Richard Quinn is a freelance writer in New Jersey.
Drug granted fast track designation for MF

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to Resimmune® (A-dmDT390-bisFv[UCHT1]) for the treatment of mycosis fungoides (MF).
Resimmune is an anti-CD3 immunotoxin that targets and transiently depletes a high percentage of malignant and normal T cells.
Researchers believe this high rate of T-cell depletion may “reset” a patient’s immune system, leading to immunomodulation. However, the safety and efficacy of Resimmune has not been established.
Resimmune is being developed by Angimmune LLC.
Resimmune research
The FDA’s fast track designation for Resimmune is based on results from a phase 1 trial, which were published in haematologica last year.
The trial included 25 patients with cutaneous T-cell lymphoma (CTCL)—17 with stage IB-IIB disease and 8 with stage III-IV disease. The patients received Resimmune at varying doses—between 2.5 µg/kg and 11.25 µg/kg.
Safety
Drug-related adverse events for the entire study cohort (n=30) included:
- Grade 2-3 AST/ALT elevations (n=6)
- Grade 2 chills (n=10)
- Grade 5 EBV infection (n=1)
- Grade 3 EBV/CMV infection (n=5)
- Grade 2 fever (n=6)
- Grade 5 heart failure (n=2)
- Grade 2-3 hypoalbuminemia (n=11)
- Grade 2 hypomagnesemia (n=1)
- Grade 3 hypophosphatemia (n=3)
- Grade 2-4 hypotension (n=3)
- Grade 4 hypoxia (n=1)
- Grade 4 liver failure (n=1)
- Grade 4 metabolic acidosis (n=1)
- Grade 3 supraventricular tachycardia (n=2)
- Grade 3-4 uremia (n=3)
- Grade 2-4 vascular leak syndrome (n=5).
Efficacy and phase 2
Nine of the CTCL patients (36%) responded to Resimmune, and 4 (16%) had complete responses (CRs). The duration of CR was more than 38 months for 1 patient, more than 60 months for another, and more than 72 months for 2 patients.
Of the 5 partial responses, 2 lasted 3 months, 1 lasted more than 3 months, 1 lasted more than 6 months, and the longest lasted 14 months.
The researchers said this trial revealed a subgroup of CTCL patients with a very high response rate.
When they excluded patients whose mSWAT scores never exceeded 50 and who never had lymph node involvement or stage III disease, the researchers were left with 9 patients. This subgroup had an overall response rate of 89% and a CR rate of 50% (at 6 months of follow-up).
“Results from our phase 1 trial demonstrated a particularly robust response among certain patients with early stage disease, and the randomized phase 2 trial is designed to further explore this potential therapeutic benefit,” said David Neville, MD, president and chief scientific officer of Angimmune.
The objective of this phase 2 trial is to document the incidence of CRs with Resimmune compared to oral vorinostat after a maximum of 12 months of treatment. The trial will enroll patients with stage IB/IIB MF with mSWAT < 50 who have never had lymphoid disease or a prior hematopoietic stem cell transplant.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to Resimmune® (A-dmDT390-bisFv[UCHT1]) for the treatment of mycosis fungoides (MF).
Resimmune is an anti-CD3 immunotoxin that targets and transiently depletes a high percentage of malignant and normal T cells.
Researchers believe this high rate of T-cell depletion may “reset” a patient’s immune system, leading to immunomodulation. However, the safety and efficacy of Resimmune has not been established.
Resimmune is being developed by Angimmune LLC.
Resimmune research
The FDA’s fast track designation for Resimmune is based on results from a phase 1 trial, which were published in haematologica last year.
The trial included 25 patients with cutaneous T-cell lymphoma (CTCL)—17 with stage IB-IIB disease and 8 with stage III-IV disease. The patients received Resimmune at varying doses—between 2.5 µg/kg and 11.25 µg/kg.
Safety
Drug-related adverse events for the entire study cohort (n=30) included:
- Grade 2-3 AST/ALT elevations (n=6)
- Grade 2 chills (n=10)
- Grade 5 EBV infection (n=1)
- Grade 3 EBV/CMV infection (n=5)
- Grade 2 fever (n=6)
- Grade 5 heart failure (n=2)
- Grade 2-3 hypoalbuminemia (n=11)
- Grade 2 hypomagnesemia (n=1)
- Grade 3 hypophosphatemia (n=3)
- Grade 2-4 hypotension (n=3)
- Grade 4 hypoxia (n=1)
- Grade 4 liver failure (n=1)
- Grade 4 metabolic acidosis (n=1)
- Grade 3 supraventricular tachycardia (n=2)
- Grade 3-4 uremia (n=3)
- Grade 2-4 vascular leak syndrome (n=5).
Efficacy and phase 2
Nine of the CTCL patients (36%) responded to Resimmune, and 4 (16%) had complete responses (CRs). The duration of CR was more than 38 months for 1 patient, more than 60 months for another, and more than 72 months for 2 patients.
Of the 5 partial responses, 2 lasted 3 months, 1 lasted more than 3 months, 1 lasted more than 6 months, and the longest lasted 14 months.
The researchers said this trial revealed a subgroup of CTCL patients with a very high response rate.
When they excluded patients whose mSWAT scores never exceeded 50 and who never had lymph node involvement or stage III disease, the researchers were left with 9 patients. This subgroup had an overall response rate of 89% and a CR rate of 50% (at 6 months of follow-up).
“Results from our phase 1 trial demonstrated a particularly robust response among certain patients with early stage disease, and the randomized phase 2 trial is designed to further explore this potential therapeutic benefit,” said David Neville, MD, president and chief scientific officer of Angimmune.
The objective of this phase 2 trial is to document the incidence of CRs with Resimmune compared to oral vorinostat after a maximum of 12 months of treatment. The trial will enroll patients with stage IB/IIB MF with mSWAT < 50 who have never had lymphoid disease or a prior hematopoietic stem cell transplant.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to Resimmune® (A-dmDT390-bisFv[UCHT1]) for the treatment of mycosis fungoides (MF).
Resimmune is an anti-CD3 immunotoxin that targets and transiently depletes a high percentage of malignant and normal T cells.
Researchers believe this high rate of T-cell depletion may “reset” a patient’s immune system, leading to immunomodulation. However, the safety and efficacy of Resimmune has not been established.
Resimmune is being developed by Angimmune LLC.
Resimmune research
The FDA’s fast track designation for Resimmune is based on results from a phase 1 trial, which were published in haematologica last year.
The trial included 25 patients with cutaneous T-cell lymphoma (CTCL)—17 with stage IB-IIB disease and 8 with stage III-IV disease. The patients received Resimmune at varying doses—between 2.5 µg/kg and 11.25 µg/kg.
Safety
Drug-related adverse events for the entire study cohort (n=30) included:
- Grade 2-3 AST/ALT elevations (n=6)
- Grade 2 chills (n=10)
- Grade 5 EBV infection (n=1)
- Grade 3 EBV/CMV infection (n=5)
- Grade 2 fever (n=6)
- Grade 5 heart failure (n=2)
- Grade 2-3 hypoalbuminemia (n=11)
- Grade 2 hypomagnesemia (n=1)
- Grade 3 hypophosphatemia (n=3)
- Grade 2-4 hypotension (n=3)
- Grade 4 hypoxia (n=1)
- Grade 4 liver failure (n=1)
- Grade 4 metabolic acidosis (n=1)
- Grade 3 supraventricular tachycardia (n=2)
- Grade 3-4 uremia (n=3)
- Grade 2-4 vascular leak syndrome (n=5).
Efficacy and phase 2
Nine of the CTCL patients (36%) responded to Resimmune, and 4 (16%) had complete responses (CRs). The duration of CR was more than 38 months for 1 patient, more than 60 months for another, and more than 72 months for 2 patients.
Of the 5 partial responses, 2 lasted 3 months, 1 lasted more than 3 months, 1 lasted more than 6 months, and the longest lasted 14 months.
The researchers said this trial revealed a subgroup of CTCL patients with a very high response rate.
When they excluded patients whose mSWAT scores never exceeded 50 and who never had lymph node involvement or stage III disease, the researchers were left with 9 patients. This subgroup had an overall response rate of 89% and a CR rate of 50% (at 6 months of follow-up).
“Results from our phase 1 trial demonstrated a particularly robust response among certain patients with early stage disease, and the randomized phase 2 trial is designed to further explore this potential therapeutic benefit,” said David Neville, MD, president and chief scientific officer of Angimmune.
The objective of this phase 2 trial is to document the incidence of CRs with Resimmune compared to oral vorinostat after a maximum of 12 months of treatment. The trial will enroll patients with stage IB/IIB MF with mSWAT < 50 who have never had lymphoid disease or a prior hematopoietic stem cell transplant.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()
Studies reveal potential therapeutic targets for resistant AML

Image by NIGMS
A pair of studies suggest the DNA replication checkpoint pathway could be targeted to treat resistant acute myeloid leukemia (AML).
One study indicated that the kinase CHK1, which coordinates the DNA damage response and cell-cycle checkpoint response, might be targeted to overcome cytarabine resistance in AML.
The other study suggested that ATR, a kinase that activates CHK1, and ATM, a second upstream kinase in the DNA damage response, might be therapeutic targets in MLL-rearranged AML.
Both studies were published in Science Sigaling.
Laure David, of La Ligue Contre Le Cancer in Toulouse, France, and colleagues conducted this study.
The researchers integrated gene expression data with survival data from 198 AML patients who were previously treated with cytarabine-based chemotherapy.
The data showed that patients with high expression of CHEK1, the gene that encodes CHK1, had poor survival. And patient cells with high CHK1 abundance were resistant to cytarabine.
However, when the researchers exposed the cells with high CHK1 abundance to the CHK1 inhibitor SCH900776, they observed “reduced clonogenic ability and progression of DNA replication in the presence of cytarabine.”
The team therefore concluded that treatment with a CHK1 inhibitor might overcome resistance to cytarabine-based treatments in AML. They also said that monitoring CHEK1 expression in AML patients could potentially predict outcomes and reveal patients who might benefit from treatment with a CHK1 inhibitor.
The second study was conducted by Isabel Morgado-Palacin, of the Spanish National Cancer Research Center in Madrid, and colleagues.
The researchers investigated ATR, which is the primary sensor of DNA replication stress, and ATM, a kinase that senses DNA double-strand breaks, as possible therapeutic targets in MLL-rearranged AML.
The team found that inhibiting ATR induced chromosomal breakage and death in murine AML-MLL cells in vitro. And inhibiting ATR in vivo reduced the tumor burden and prevented tumors from growing.
The researchers also found that inhibiting ATM prolonged survival in the mouse model of AML-MLL, which suggests that both ATR and ATM might be therapeutic targets in MLL-rearranged AML. ![]()

Image by NIGMS
A pair of studies suggest the DNA replication checkpoint pathway could be targeted to treat resistant acute myeloid leukemia (AML).
One study indicated that the kinase CHK1, which coordinates the DNA damage response and cell-cycle checkpoint response, might be targeted to overcome cytarabine resistance in AML.
The other study suggested that ATR, a kinase that activates CHK1, and ATM, a second upstream kinase in the DNA damage response, might be therapeutic targets in MLL-rearranged AML.
Both studies were published in Science Sigaling.
Laure David, of La Ligue Contre Le Cancer in Toulouse, France, and colleagues conducted this study.
The researchers integrated gene expression data with survival data from 198 AML patients who were previously treated with cytarabine-based chemotherapy.
The data showed that patients with high expression of CHEK1, the gene that encodes CHK1, had poor survival. And patient cells with high CHK1 abundance were resistant to cytarabine.
However, when the researchers exposed the cells with high CHK1 abundance to the CHK1 inhibitor SCH900776, they observed “reduced clonogenic ability and progression of DNA replication in the presence of cytarabine.”
The team therefore concluded that treatment with a CHK1 inhibitor might overcome resistance to cytarabine-based treatments in AML. They also said that monitoring CHEK1 expression in AML patients could potentially predict outcomes and reveal patients who might benefit from treatment with a CHK1 inhibitor.
The second study was conducted by Isabel Morgado-Palacin, of the Spanish National Cancer Research Center in Madrid, and colleagues.
The researchers investigated ATR, which is the primary sensor of DNA replication stress, and ATM, a kinase that senses DNA double-strand breaks, as possible therapeutic targets in MLL-rearranged AML.
The team found that inhibiting ATR induced chromosomal breakage and death in murine AML-MLL cells in vitro. And inhibiting ATR in vivo reduced the tumor burden and prevented tumors from growing.
The researchers also found that inhibiting ATM prolonged survival in the mouse model of AML-MLL, which suggests that both ATR and ATM might be therapeutic targets in MLL-rearranged AML. ![]()

Image by NIGMS
A pair of studies suggest the DNA replication checkpoint pathway could be targeted to treat resistant acute myeloid leukemia (AML).
One study indicated that the kinase CHK1, which coordinates the DNA damage response and cell-cycle checkpoint response, might be targeted to overcome cytarabine resistance in AML.
The other study suggested that ATR, a kinase that activates CHK1, and ATM, a second upstream kinase in the DNA damage response, might be therapeutic targets in MLL-rearranged AML.
Both studies were published in Science Sigaling.
Laure David, of La Ligue Contre Le Cancer in Toulouse, France, and colleagues conducted this study.
The researchers integrated gene expression data with survival data from 198 AML patients who were previously treated with cytarabine-based chemotherapy.
The data showed that patients with high expression of CHEK1, the gene that encodes CHK1, had poor survival. And patient cells with high CHK1 abundance were resistant to cytarabine.
However, when the researchers exposed the cells with high CHK1 abundance to the CHK1 inhibitor SCH900776, they observed “reduced clonogenic ability and progression of DNA replication in the presence of cytarabine.”
The team therefore concluded that treatment with a CHK1 inhibitor might overcome resistance to cytarabine-based treatments in AML. They also said that monitoring CHEK1 expression in AML patients could potentially predict outcomes and reveal patients who might benefit from treatment with a CHK1 inhibitor.
The second study was conducted by Isabel Morgado-Palacin, of the Spanish National Cancer Research Center in Madrid, and colleagues.
The researchers investigated ATR, which is the primary sensor of DNA replication stress, and ATM, a kinase that senses DNA double-strand breaks, as possible therapeutic targets in MLL-rearranged AML.
The team found that inhibiting ATR induced chromosomal breakage and death in murine AML-MLL cells in vitro. And inhibiting ATR in vivo reduced the tumor burden and prevented tumors from growing.
The researchers also found that inhibiting ATM prolonged survival in the mouse model of AML-MLL, which suggests that both ATR and ATM might be therapeutic targets in MLL-rearranged AML. ![]()
Drug warrants further investigation in ATLL, team says
Researchers say lenalidomide exhibited clinically meaningful antitumor activity and an acceptable safety profile in a phase 2 study of patients with relapsed adult T-cell leukemia/lymphoma (ATLL).
Of the 26 patients enrolled in the trial, 42% responded to lenalidomide, and 35% experienced serious adverse events (AEs).
Results from this trial were published in the Journal of Clinical Oncology. Data were previously presented at the 8th Annual T-cell Lymphoma Forum. The study was sponsored by Celgene K.K.
Patients and treatment
All 26 patients had relapsed ATLL—15 with the acute subtype, 7 with the lymphoma subtype, and 4 with the unfavorable chronic subtype. The patients’ median age was 68.5 (range, 53-81), all were Japanese, and 54% were male.
The median number of prior treatment regimens was 2 (range, 1-4). Thirty-five percent of patients had prior mogamulizumab (n=9), 35% had received LSG15 (n=3) or modified LSG15 (n=6), and 27% had prior CHOP (n=7).
The patients received lenalidomide at 25 mg per day, given continuously until disease progression or intolerability.
Results
Five patients (19%) were still receiving lenalidomide at the data cutoff, which was November 20, 2014.
Thirteen patients (50%) stopped treatment due to progression, 6 (23%) because of AEs, 1 (4%) due to investigator decision, and 1 (4%) because of concerns about the increased possibility of disease recurrence.
The median follow-up was 3.9 months. Eleven patients (42%) responded to lenalidomide, including 4 complete responses and 1 unconfirmed complete response.
The median duration of response was not reached (range, 0.5 months to not reached), and the mean duration of response was 5.2 months (range, 0 to 16.6 months).
The median progression-free survival was 3.8 months (range, 1.9 months to not reached), and the median overall survival was 20.3 months (range, 9.1 months to not reached).
Nine patients (35%) experienced serious AEs. The only serious AE that occurred in more than 1 patient was thrombocytopenia, which occurred in 2 patients.
The most frequent AEs of any grade were thrombocytopenia (77%), neutropenia (73%), lymphopenia (69%), anemia (54%), and leukopenia (50%).
The most frequent grade 3 or higher AEs were neutropenia (65%), leukopenia (38%), lymphopenia (38%), and thrombocytopenia (23%). These events were all manageable and reversible, according to the researchers.
Based on these results, the team concluded that lenalidomide warrants further investigation as a treatment for ATLL. ![]()

Researchers say lenalidomide exhibited clinically meaningful antitumor activity and an acceptable safety profile in a phase 2 study of patients with relapsed adult T-cell leukemia/lymphoma (ATLL).
Of the 26 patients enrolled in the trial, 42% responded to lenalidomide, and 35% experienced serious adverse events (AEs).
Results from this trial were published in the Journal of Clinical Oncology. Data were previously presented at the 8th Annual T-cell Lymphoma Forum. The study was sponsored by Celgene K.K.
Patients and treatment
All 26 patients had relapsed ATLL—15 with the acute subtype, 7 with the lymphoma subtype, and 4 with the unfavorable chronic subtype. The patients’ median age was 68.5 (range, 53-81), all were Japanese, and 54% were male.
The median number of prior treatment regimens was 2 (range, 1-4). Thirty-five percent of patients had prior mogamulizumab (n=9), 35% had received LSG15 (n=3) or modified LSG15 (n=6), and 27% had prior CHOP (n=7).
The patients received lenalidomide at 25 mg per day, given continuously until disease progression or intolerability.
Results
Five patients (19%) were still receiving lenalidomide at the data cutoff, which was November 20, 2014.
Thirteen patients (50%) stopped treatment due to progression, 6 (23%) because of AEs, 1 (4%) due to investigator decision, and 1 (4%) because of concerns about the increased possibility of disease recurrence.
The median follow-up was 3.9 months. Eleven patients (42%) responded to lenalidomide, including 4 complete responses and 1 unconfirmed complete response.
The median duration of response was not reached (range, 0.5 months to not reached), and the mean duration of response was 5.2 months (range, 0 to 16.6 months).
The median progression-free survival was 3.8 months (range, 1.9 months to not reached), and the median overall survival was 20.3 months (range, 9.1 months to not reached).
Nine patients (35%) experienced serious AEs. The only serious AE that occurred in more than 1 patient was thrombocytopenia, which occurred in 2 patients.
The most frequent AEs of any grade were thrombocytopenia (77%), neutropenia (73%), lymphopenia (69%), anemia (54%), and leukopenia (50%).
The most frequent grade 3 or higher AEs were neutropenia (65%), leukopenia (38%), lymphopenia (38%), and thrombocytopenia (23%). These events were all manageable and reversible, according to the researchers.
Based on these results, the team concluded that lenalidomide warrants further investigation as a treatment for ATLL. ![]()

Researchers say lenalidomide exhibited clinically meaningful antitumor activity and an acceptable safety profile in a phase 2 study of patients with relapsed adult T-cell leukemia/lymphoma (ATLL).
Of the 26 patients enrolled in the trial, 42% responded to lenalidomide, and 35% experienced serious adverse events (AEs).
Results from this trial were published in the Journal of Clinical Oncology. Data were previously presented at the 8th Annual T-cell Lymphoma Forum. The study was sponsored by Celgene K.K.
Patients and treatment
All 26 patients had relapsed ATLL—15 with the acute subtype, 7 with the lymphoma subtype, and 4 with the unfavorable chronic subtype. The patients’ median age was 68.5 (range, 53-81), all were Japanese, and 54% were male.
The median number of prior treatment regimens was 2 (range, 1-4). Thirty-five percent of patients had prior mogamulizumab (n=9), 35% had received LSG15 (n=3) or modified LSG15 (n=6), and 27% had prior CHOP (n=7).
The patients received lenalidomide at 25 mg per day, given continuously until disease progression or intolerability.
Results
Five patients (19%) were still receiving lenalidomide at the data cutoff, which was November 20, 2014.
Thirteen patients (50%) stopped treatment due to progression, 6 (23%) because of AEs, 1 (4%) due to investigator decision, and 1 (4%) because of concerns about the increased possibility of disease recurrence.
The median follow-up was 3.9 months. Eleven patients (42%) responded to lenalidomide, including 4 complete responses and 1 unconfirmed complete response.
The median duration of response was not reached (range, 0.5 months to not reached), and the mean duration of response was 5.2 months (range, 0 to 16.6 months).
The median progression-free survival was 3.8 months (range, 1.9 months to not reached), and the median overall survival was 20.3 months (range, 9.1 months to not reached).
Nine patients (35%) experienced serious AEs. The only serious AE that occurred in more than 1 patient was thrombocytopenia, which occurred in 2 patients.
The most frequent AEs of any grade were thrombocytopenia (77%), neutropenia (73%), lymphopenia (69%), anemia (54%), and leukopenia (50%).
The most frequent grade 3 or higher AEs were neutropenia (65%), leukopenia (38%), lymphopenia (38%), and thrombocytopenia (23%). These events were all manageable and reversible, according to the researchers.
Based on these results, the team concluded that lenalidomide warrants further investigation as a treatment for ATLL. ![]()
SpHb monitoring may reduce use of RBC transfusion

Photo by Elisa Amendola
HONG KONG—Continuous and noninvasive hemoglobin monitoring may reduce the excessive use of intraoperative red blood cell (RBC) transfusion, according to researchers.
The team conducted a retrospective study of patients who received intraoperative RBC transfusions at a single institution, comparing patients who had noninvasive and continuous monitoring of hemoglobin concentrations by spectrophotometry (SpHb) to those who did not.
The results showed a significantly lower mean RBC transfusion volume per 1 g of blood loss in the SpHb group than in the control group.
The researchers presented these findings at the 16th World Congress of Anaesthesiologists (abstract PR607).
The study included 371 patients who received intraoperative RBC transfusions between 2012 and 2014 at Fukushima Medical University in Japan.
The researchers compared 94 patients who had SpHb with the Radical-7® Pulse CO-Oximeter (a product of Masimo) to 277 patients who did not.
The team noted that measured SpHb values are similar to the Hb concentration values obtained by blood sampling, and the procedure allows for continuous monitoring of changes in SpHb levels over time.
The median blood loss during surgery was 1160 g in the SpHb group and 900 g in the control group.
There was no significant difference in the average RBC transfusion volume between the SpHb group and the control group—815 ± 819 mL and 785 ± 773 mL, respectively (P=0.75).
Likewise, there was no significant difference in the preoperative hemoglobin concentration in the SpHb group and the control group—10.4 ± 1.9 g/dL and 10.2 ± 2.4 g/dL, respectively (P=0.27).
However, the researchers did find that patients in the SpHb group had a significantly lower mean RBC transfusion volume per 1 g of blood loss when compared to controls—0.9 ± 1.0 mL/g blood loss and 2.4 ± 5.9 mL/g blood loss, respectively (P<0.01).
“This is the third study, published by different researchers on 3 continents (US1, Egypt2, and now Japan) that has shown that, in addition to other clinical tools, SpHb may be used to help clinicians make informed transfusion decisions during different types of surgery,” said Steven Barker, MD, PhD, chief science officer of Masimo.
Though Masimo’s Radical-7® Pulse CO-Oximeter was used in this study, the researchers declared no conflicts of interest. ![]()

Photo by Elisa Amendola
HONG KONG—Continuous and noninvasive hemoglobin monitoring may reduce the excessive use of intraoperative red blood cell (RBC) transfusion, according to researchers.
The team conducted a retrospective study of patients who received intraoperative RBC transfusions at a single institution, comparing patients who had noninvasive and continuous monitoring of hemoglobin concentrations by spectrophotometry (SpHb) to those who did not.
The results showed a significantly lower mean RBC transfusion volume per 1 g of blood loss in the SpHb group than in the control group.
The researchers presented these findings at the 16th World Congress of Anaesthesiologists (abstract PR607).
The study included 371 patients who received intraoperative RBC transfusions between 2012 and 2014 at Fukushima Medical University in Japan.
The researchers compared 94 patients who had SpHb with the Radical-7® Pulse CO-Oximeter (a product of Masimo) to 277 patients who did not.
The team noted that measured SpHb values are similar to the Hb concentration values obtained by blood sampling, and the procedure allows for continuous monitoring of changes in SpHb levels over time.
The median blood loss during surgery was 1160 g in the SpHb group and 900 g in the control group.
There was no significant difference in the average RBC transfusion volume between the SpHb group and the control group—815 ± 819 mL and 785 ± 773 mL, respectively (P=0.75).
Likewise, there was no significant difference in the preoperative hemoglobin concentration in the SpHb group and the control group—10.4 ± 1.9 g/dL and 10.2 ± 2.4 g/dL, respectively (P=0.27).
However, the researchers did find that patients in the SpHb group had a significantly lower mean RBC transfusion volume per 1 g of blood loss when compared to controls—0.9 ± 1.0 mL/g blood loss and 2.4 ± 5.9 mL/g blood loss, respectively (P<0.01).
“This is the third study, published by different researchers on 3 continents (US1, Egypt2, and now Japan) that has shown that, in addition to other clinical tools, SpHb may be used to help clinicians make informed transfusion decisions during different types of surgery,” said Steven Barker, MD, PhD, chief science officer of Masimo.
Though Masimo’s Radical-7® Pulse CO-Oximeter was used in this study, the researchers declared no conflicts of interest. ![]()

Photo by Elisa Amendola
HONG KONG—Continuous and noninvasive hemoglobin monitoring may reduce the excessive use of intraoperative red blood cell (RBC) transfusion, according to researchers.
The team conducted a retrospective study of patients who received intraoperative RBC transfusions at a single institution, comparing patients who had noninvasive and continuous monitoring of hemoglobin concentrations by spectrophotometry (SpHb) to those who did not.
The results showed a significantly lower mean RBC transfusion volume per 1 g of blood loss in the SpHb group than in the control group.
The researchers presented these findings at the 16th World Congress of Anaesthesiologists (abstract PR607).
The study included 371 patients who received intraoperative RBC transfusions between 2012 and 2014 at Fukushima Medical University in Japan.
The researchers compared 94 patients who had SpHb with the Radical-7® Pulse CO-Oximeter (a product of Masimo) to 277 patients who did not.
The team noted that measured SpHb values are similar to the Hb concentration values obtained by blood sampling, and the procedure allows for continuous monitoring of changes in SpHb levels over time.
The median blood loss during surgery was 1160 g in the SpHb group and 900 g in the control group.
There was no significant difference in the average RBC transfusion volume between the SpHb group and the control group—815 ± 819 mL and 785 ± 773 mL, respectively (P=0.75).
Likewise, there was no significant difference in the preoperative hemoglobin concentration in the SpHb group and the control group—10.4 ± 1.9 g/dL and 10.2 ± 2.4 g/dL, respectively (P=0.27).
However, the researchers did find that patients in the SpHb group had a significantly lower mean RBC transfusion volume per 1 g of blood loss when compared to controls—0.9 ± 1.0 mL/g blood loss and 2.4 ± 5.9 mL/g blood loss, respectively (P<0.01).
“This is the third study, published by different researchers on 3 continents (US1, Egypt2, and now Japan) that has shown that, in addition to other clinical tools, SpHb may be used to help clinicians make informed transfusion decisions during different types of surgery,” said Steven Barker, MD, PhD, chief science officer of Masimo.
Though Masimo’s Radical-7® Pulse CO-Oximeter was used in this study, the researchers declared no conflicts of interest. ![]()