User login
New ELISA better differentiates chronic, acute hepatitis E infection
While real-time polymerase chain reaction (PCR) testing is the standard test for hepatitis E virus infection, an anti-HEV-specific enzyme-linked immunosorbent assay (ELISA) is more effective than is PCR at distinguishing between acute and chronic HEV infections.
“A clinical challenge in the management of immunocompromised patients with HEV infection is to differentiate between the acute and chronic course of infection [as] approximately 60% of infected immunocompromised individuals experience a chronic infection, which might require treatment with ribavirin,” said lead author Patrick Behrendt, Dr.med., of Hannover (Germany) Medical School.
The anti-HEV antigen (Ag) ELISA has recently become commercially available, making it an even bigger matter of interest. Dr. Behrendt and his coinvestigators analyzed sera from 18 patients with acute and 21 patients with chronic HEV, whose samples were collected between 2008 and 2015. The sera were retrospectively analyzed via both real-time PCR and ELISA to compare the efficacy of each in identifying HEV – more specifically, HEV genotype 3 – in each subject. For the ELISA analysis, 100 mcL of serum were added to each well of an ELISA plate, which was subsequently incubated at 37 degrees Celsius for 1 hour.
The researchers analyzed sera from four individuals with chronic HEV using serial dilutions to compare sensitivity of real-time PCR and ELISA. In three out of those four sera (75%), ELISA showed negative results due to HEV RNA levels of fewer than 10,000 copies/mL; on the other hand, real-time PCR showed a “linear reduction in levels,” indicating that it is the better option in detecting HEV genotype 3 (J Infect Dis. 2016 May 27;214[3]:361-8).
ELISA was also used on 20 chronic HEV patients and 17 acute HEV patients to determine its sensitivity at distinguishing between the two types of infection. In this cohort, only 64.7% of RNA-positive patients were deemed positive according to the ELISA testing, while ELISA results were positive in all of the cases of chronic HEV infections. None of the patients with chronic infection registered a false-negative, which indicates “a high reliability of the assay for this cohort,” according to the investigators.
“Comparison of chronically infected individuals with acutely infected patients revealed drastically increased ODs in chronically infected patients, while most of the positive samples from acutely infected patients displayed significantly lower values [which] led to a sensitivity of 100% (20 of 20) [ELISA] results for chronically HEV infected individuals,” the authors concluded. “Our study demonstrates that [ELISA] has a sensitivity of 65% and a specificity of 92% in detecting an ongoing HEV infection in a real-life cohort.”
The German Center for Infection Research, the Helmholtz Center for Infection Research, and the German Ministry for Education and Research funded the study. The researchers reported no relevant financial disclosures.
While real-time polymerase chain reaction (PCR) testing is the standard test for hepatitis E virus infection, an anti-HEV-specific enzyme-linked immunosorbent assay (ELISA) is more effective than is PCR at distinguishing between acute and chronic HEV infections.
“A clinical challenge in the management of immunocompromised patients with HEV infection is to differentiate between the acute and chronic course of infection [as] approximately 60% of infected immunocompromised individuals experience a chronic infection, which might require treatment with ribavirin,” said lead author Patrick Behrendt, Dr.med., of Hannover (Germany) Medical School.
The anti-HEV antigen (Ag) ELISA has recently become commercially available, making it an even bigger matter of interest. Dr. Behrendt and his coinvestigators analyzed sera from 18 patients with acute and 21 patients with chronic HEV, whose samples were collected between 2008 and 2015. The sera were retrospectively analyzed via both real-time PCR and ELISA to compare the efficacy of each in identifying HEV – more specifically, HEV genotype 3 – in each subject. For the ELISA analysis, 100 mcL of serum were added to each well of an ELISA plate, which was subsequently incubated at 37 degrees Celsius for 1 hour.
The researchers analyzed sera from four individuals with chronic HEV using serial dilutions to compare sensitivity of real-time PCR and ELISA. In three out of those four sera (75%), ELISA showed negative results due to HEV RNA levels of fewer than 10,000 copies/mL; on the other hand, real-time PCR showed a “linear reduction in levels,” indicating that it is the better option in detecting HEV genotype 3 (J Infect Dis. 2016 May 27;214[3]:361-8).
ELISA was also used on 20 chronic HEV patients and 17 acute HEV patients to determine its sensitivity at distinguishing between the two types of infection. In this cohort, only 64.7% of RNA-positive patients were deemed positive according to the ELISA testing, while ELISA results were positive in all of the cases of chronic HEV infections. None of the patients with chronic infection registered a false-negative, which indicates “a high reliability of the assay for this cohort,” according to the investigators.
“Comparison of chronically infected individuals with acutely infected patients revealed drastically increased ODs in chronically infected patients, while most of the positive samples from acutely infected patients displayed significantly lower values [which] led to a sensitivity of 100% (20 of 20) [ELISA] results for chronically HEV infected individuals,” the authors concluded. “Our study demonstrates that [ELISA] has a sensitivity of 65% and a specificity of 92% in detecting an ongoing HEV infection in a real-life cohort.”
The German Center for Infection Research, the Helmholtz Center for Infection Research, and the German Ministry for Education and Research funded the study. The researchers reported no relevant financial disclosures.
While real-time polymerase chain reaction (PCR) testing is the standard test for hepatitis E virus infection, an anti-HEV-specific enzyme-linked immunosorbent assay (ELISA) is more effective than is PCR at distinguishing between acute and chronic HEV infections.
“A clinical challenge in the management of immunocompromised patients with HEV infection is to differentiate between the acute and chronic course of infection [as] approximately 60% of infected immunocompromised individuals experience a chronic infection, which might require treatment with ribavirin,” said lead author Patrick Behrendt, Dr.med., of Hannover (Germany) Medical School.
The anti-HEV antigen (Ag) ELISA has recently become commercially available, making it an even bigger matter of interest. Dr. Behrendt and his coinvestigators analyzed sera from 18 patients with acute and 21 patients with chronic HEV, whose samples were collected between 2008 and 2015. The sera were retrospectively analyzed via both real-time PCR and ELISA to compare the efficacy of each in identifying HEV – more specifically, HEV genotype 3 – in each subject. For the ELISA analysis, 100 mcL of serum were added to each well of an ELISA plate, which was subsequently incubated at 37 degrees Celsius for 1 hour.
The researchers analyzed sera from four individuals with chronic HEV using serial dilutions to compare sensitivity of real-time PCR and ELISA. In three out of those four sera (75%), ELISA showed negative results due to HEV RNA levels of fewer than 10,000 copies/mL; on the other hand, real-time PCR showed a “linear reduction in levels,” indicating that it is the better option in detecting HEV genotype 3 (J Infect Dis. 2016 May 27;214[3]:361-8).
ELISA was also used on 20 chronic HEV patients and 17 acute HEV patients to determine its sensitivity at distinguishing between the two types of infection. In this cohort, only 64.7% of RNA-positive patients were deemed positive according to the ELISA testing, while ELISA results were positive in all of the cases of chronic HEV infections. None of the patients with chronic infection registered a false-negative, which indicates “a high reliability of the assay for this cohort,” according to the investigators.
“Comparison of chronically infected individuals with acutely infected patients revealed drastically increased ODs in chronically infected patients, while most of the positive samples from acutely infected patients displayed significantly lower values [which] led to a sensitivity of 100% (20 of 20) [ELISA] results for chronically HEV infected individuals,” the authors concluded. “Our study demonstrates that [ELISA] has a sensitivity of 65% and a specificity of 92% in detecting an ongoing HEV infection in a real-life cohort.”
The German Center for Infection Research, the Helmholtz Center for Infection Research, and the German Ministry for Education and Research funded the study. The researchers reported no relevant financial disclosures.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: An anti-HEV-specific enzyme-linked immunosorbent assay (ELISA) is more effective at identifying acute versus chronic HEV infection than is HEV RNA real-time PCR.
Major finding: ELISA detected significantly higher levels of HEV Ag in chronically infected subjects than in acutely affected ones, but was less sensitive at detecting HEV infection overall than real-time PCR.
Data source: Retrospective cohort study of 21 chronic and 18 acute HEV patients from 2008-2015.
Disclosures: The German Center for Infection Research, the Helmholtz Center for Infection Research, and the German Ministry for Education and Research funded the study. The researchers reported no relevant financial disclosures.
Colon cancer
Colorectal cancer (CRC) screening has become a focal point of the practice of gastroenterology. Successful screening leads to a reduction in cancer mortality and is one of the most impactful means by which we improve population health.
Rick Boland, MD, of the Baylor Center of Gastrointestinal Cancer Research in Dallas reminded us at the annual Digestive Disease Week that carcinogenesis in colorectal cancer is a multistep process and described several pathways of which clinicians should be aware. Germline mutations (inherited) vs. somatic mutations (acquired) may be driver mutations that confer selection advantage and become targets for therapy in contrast to passenger mutations that are not mechanistic targets. Germline mutations should be considered when there is a family history of CRC, especially in a young patient, and are characteristic of familial adenomatous polyposis (APC), attenuated polyposis (MYH-MUTYH), and nonadenomatous polyposis syndromes such as Peutz-Jeghers (STK11).
Microsatellite instability is an alternative pathway to cancer. Hereditary nonpolyposis colorectal cancer (Lynch syndrome) is representative of this pathway with common abnormalities in mismatch repair genes such as MLH1, MSH2, MSH6, or PMS2. Lynch syndrome patients are BRAF and CIMP negative. This is in contrast to a more recent pathway to cancer that arises from sessile serrated polyps that also display microsatellite instability, but are BRAF and CIMP positive.
The clinical importance of understanding the pathway through which cancer develops in an individual patient is that depending on the mechanism of tumorigenesis, different treatment strategies can prevent or treat cancer. For example, exciting novel therapies are being developed for microsatellite unstable cancers using immune checkpoint inhibitors. Aspirin can reduce CRC mortality, especially tumors that overexpress COX2 or harbor PIK3CA mutations, and have demonstrated benefit even postoperatively in patients receiving surgical resection of CRC.
I gave a talk focused on current guidelines for reducing mortality among individuals at average risk for developing CRC. The U.S. Preventive Services Task Force updated their recommendations this year and chose to focus on highlighting the importance of CRC screening between the ages of 50 and 75 years, tailored screening for those between the ages of 76 and 85 years, and stopping screening after age 85 years. However, the statement does not recommend specific tests and instead lists competing strategies of annual fecal immunochemical test (FIT) or highly sensitive fecal occult blood test (FOBT) with or without flexible sigmoidoscopy every 10 years, flexible sigmoidoscopy every 5 years, colonoscopy every 10 years, CT colonography every 5 years, and FIT-DNA every 1 or 3 years. The FIT-DNA adds a separate FIT to a stool exam for KRAS mutations, NDRG4 and BMP3 methylation, and beta-actin. There are no studies to determine whether CRC mortality is reduced, but one study demonstrated a higher sensitivity for CRC with the combined FIT-DNA, compared with FIT alone, but a lower specificity. The FDA also recently approved a blood-based CRC screening test that detects circulating methylated septin 9 gene DNA. Septin 9 is a gene that codes for GTP-binding protein and acts as a tumor suppressor that can be inactivated by methylation.
Irrespective of which strategy is pursued, colonoscopy is the final common pathway. Quality is important because there remains a disappointing rate of CRC after colonoscopy. Prior work has demonstrated significant differences in the adenoma detection rate (ADR) between endoscopists in the same practice. A recent Kaiser study found that for every 1% increase in the ADR there is a 3% decrease in the rate of cancer developing after colonoscopy.
Disruptive technology is emerging that may displace gastroenterologists from primary endoscopic screening. A self-propelled disposable colonoscope (Aer-O-Scope) has been developed that uses pneumatic pressure to advance a tethered camera through the colonic lumen guided by a joystick. Sedation is not required and the device does not need manual advancement, facilitating use by nongastroenterologists.
Uri Ladabaum, MD, of Stanford (Calif.) University presented the clinical impact of screening for hereditary and familial CRC. Lynch syndrome is characterized by a family history of cancer in at least three individuals spanning at least two generations including one with early cancer development. He reminded us that Lynch syndrome cancers are not limited to CRC but also include uterine, ovarian, gastric, small intestinal, urinary, biliary, pancreatic, and neurologic tumors. Microsatellite instability is likely present, mismatch repair gene abnormalities should be sought, and screening should include colonoscopy every 1-2 years. Aspirin chemoprophylaxis should be recommended, as should prophylactic hysterectomy and oophorectomy among affected women due to the uterine and ovarian cancer risk.
Polyposis syndromes constitute a separate group of hereditary cancers that includes familial adenomatous polyposis (FAP) arising from defects in the APC gene that may present as florid or attenuated FAP, and MYH-associated polyposis. If a patient presents with more than 10 adenomas, a polyposis syndrome should be considered. More rare syndromes include Cowden, juvenile polyposis, and Peutz-Jeghers.
Douglas Rex, MD, of Indiana University, Indianapolis, provided an array of highly clinically relevant tips to improve screening colonoscopy quality, which has been shown to reduce CRC mortality. Some of the basic concepts include using split-dose preparations and high-definition endoscopes, measuring and reporting adenoma detection rates, and using water immersion with carbon dioxide instead of air insufflation. Special attention was given to the diagnosis and removal of sessile lesions of the colon. Dr. Rex revealed many of his “tricks” to achieving high-quality colonoscopy, which include retroflexion to access polyps behind folds, using a stiff snare, and attaching a cap to the end of the colonoscope in order to facilitate visualization of polyps and assist in the mucosal resection of sessile lesions. Dr. Rex also presented data to support his recommendation to use “avulsion” with cold snare and forceps while limiting coagulative ablation for residual polyp tissue.
Finally, Rajeev Jain, MD, of Dallas presented the rapidly evolving area of reimbursement reform including the Medicare Access and CHIP Reauthorization Act (MACRA) that is driving payment away from fee-for-service and towards bundled payments, accountable care networks, and patient-centered medical homes. Key to this reform is the documentation and reporting of quality for procedural and cognitive patient encounters. There are limited performance measures specific to gastroenterology and hepatology, which currently include measures to both document appropriate CRC screening and reduce colonoscopy overuse, inflammatory bowel disease management, and hepatitis C management. However, there are many cost-cutting measures that can also be reported by gastroenterologists to fulfill these requirements. The Physician Quality Reporting System requires at least nine measures for at least 50% of Medicare patients in order to avoid penalties. It is not enough to track and document quality measures – results must also be reported through CMS-approved entities called qualified clinical data registries.
Dr. Jain described the movement toward payment bundles in which a single reimbursement is provided for services rendered by all providers and sites across a single episode of care. Screening colonoscopy was used as an example of how this could impact gastroenterology: a single payment would be provided for the preprocedure evaluation and education, the procedure itself (including endoscopy, anesthesia, pathology, facility), and postprocedure follow-up. Additional payments would not be provided for additional services such as repeating the colonoscopy because of a poor preparation or to treat postpolypectomy bleeding. Overall, health care reimbursement is moving to recognize value (quality/cost), reduce variation in care, and transfer more of the financial risk to health care providers and systems.
Dr. Inadomi, division of gastroenterology, department of medicine, University of Washington School of Medicine, department of health services, University of Washington School of Public Health, Seattle. He is on the clinical advisory committee for ChemImage and on the scientific advisory board for Epigenomics.
Colorectal cancer (CRC) screening has become a focal point of the practice of gastroenterology. Successful screening leads to a reduction in cancer mortality and is one of the most impactful means by which we improve population health.
Rick Boland, MD, of the Baylor Center of Gastrointestinal Cancer Research in Dallas reminded us at the annual Digestive Disease Week that carcinogenesis in colorectal cancer is a multistep process and described several pathways of which clinicians should be aware. Germline mutations (inherited) vs. somatic mutations (acquired) may be driver mutations that confer selection advantage and become targets for therapy in contrast to passenger mutations that are not mechanistic targets. Germline mutations should be considered when there is a family history of CRC, especially in a young patient, and are characteristic of familial adenomatous polyposis (APC), attenuated polyposis (MYH-MUTYH), and nonadenomatous polyposis syndromes such as Peutz-Jeghers (STK11).
Microsatellite instability is an alternative pathway to cancer. Hereditary nonpolyposis colorectal cancer (Lynch syndrome) is representative of this pathway with common abnormalities in mismatch repair genes such as MLH1, MSH2, MSH6, or PMS2. Lynch syndrome patients are BRAF and CIMP negative. This is in contrast to a more recent pathway to cancer that arises from sessile serrated polyps that also display microsatellite instability, but are BRAF and CIMP positive.
The clinical importance of understanding the pathway through which cancer develops in an individual patient is that depending on the mechanism of tumorigenesis, different treatment strategies can prevent or treat cancer. For example, exciting novel therapies are being developed for microsatellite unstable cancers using immune checkpoint inhibitors. Aspirin can reduce CRC mortality, especially tumors that overexpress COX2 or harbor PIK3CA mutations, and have demonstrated benefit even postoperatively in patients receiving surgical resection of CRC.
I gave a talk focused on current guidelines for reducing mortality among individuals at average risk for developing CRC. The U.S. Preventive Services Task Force updated their recommendations this year and chose to focus on highlighting the importance of CRC screening between the ages of 50 and 75 years, tailored screening for those between the ages of 76 and 85 years, and stopping screening after age 85 years. However, the statement does not recommend specific tests and instead lists competing strategies of annual fecal immunochemical test (FIT) or highly sensitive fecal occult blood test (FOBT) with or without flexible sigmoidoscopy every 10 years, flexible sigmoidoscopy every 5 years, colonoscopy every 10 years, CT colonography every 5 years, and FIT-DNA every 1 or 3 years. The FIT-DNA adds a separate FIT to a stool exam for KRAS mutations, NDRG4 and BMP3 methylation, and beta-actin. There are no studies to determine whether CRC mortality is reduced, but one study demonstrated a higher sensitivity for CRC with the combined FIT-DNA, compared with FIT alone, but a lower specificity. The FDA also recently approved a blood-based CRC screening test that detects circulating methylated septin 9 gene DNA. Septin 9 is a gene that codes for GTP-binding protein and acts as a tumor suppressor that can be inactivated by methylation.
Irrespective of which strategy is pursued, colonoscopy is the final common pathway. Quality is important because there remains a disappointing rate of CRC after colonoscopy. Prior work has demonstrated significant differences in the adenoma detection rate (ADR) between endoscopists in the same practice. A recent Kaiser study found that for every 1% increase in the ADR there is a 3% decrease in the rate of cancer developing after colonoscopy.
Disruptive technology is emerging that may displace gastroenterologists from primary endoscopic screening. A self-propelled disposable colonoscope (Aer-O-Scope) has been developed that uses pneumatic pressure to advance a tethered camera through the colonic lumen guided by a joystick. Sedation is not required and the device does not need manual advancement, facilitating use by nongastroenterologists.
Uri Ladabaum, MD, of Stanford (Calif.) University presented the clinical impact of screening for hereditary and familial CRC. Lynch syndrome is characterized by a family history of cancer in at least three individuals spanning at least two generations including one with early cancer development. He reminded us that Lynch syndrome cancers are not limited to CRC but also include uterine, ovarian, gastric, small intestinal, urinary, biliary, pancreatic, and neurologic tumors. Microsatellite instability is likely present, mismatch repair gene abnormalities should be sought, and screening should include colonoscopy every 1-2 years. Aspirin chemoprophylaxis should be recommended, as should prophylactic hysterectomy and oophorectomy among affected women due to the uterine and ovarian cancer risk.
Polyposis syndromes constitute a separate group of hereditary cancers that includes familial adenomatous polyposis (FAP) arising from defects in the APC gene that may present as florid or attenuated FAP, and MYH-associated polyposis. If a patient presents with more than 10 adenomas, a polyposis syndrome should be considered. More rare syndromes include Cowden, juvenile polyposis, and Peutz-Jeghers.
Douglas Rex, MD, of Indiana University, Indianapolis, provided an array of highly clinically relevant tips to improve screening colonoscopy quality, which has been shown to reduce CRC mortality. Some of the basic concepts include using split-dose preparations and high-definition endoscopes, measuring and reporting adenoma detection rates, and using water immersion with carbon dioxide instead of air insufflation. Special attention was given to the diagnosis and removal of sessile lesions of the colon. Dr. Rex revealed many of his “tricks” to achieving high-quality colonoscopy, which include retroflexion to access polyps behind folds, using a stiff snare, and attaching a cap to the end of the colonoscope in order to facilitate visualization of polyps and assist in the mucosal resection of sessile lesions. Dr. Rex also presented data to support his recommendation to use “avulsion” with cold snare and forceps while limiting coagulative ablation for residual polyp tissue.
Finally, Rajeev Jain, MD, of Dallas presented the rapidly evolving area of reimbursement reform including the Medicare Access and CHIP Reauthorization Act (MACRA) that is driving payment away from fee-for-service and towards bundled payments, accountable care networks, and patient-centered medical homes. Key to this reform is the documentation and reporting of quality for procedural and cognitive patient encounters. There are limited performance measures specific to gastroenterology and hepatology, which currently include measures to both document appropriate CRC screening and reduce colonoscopy overuse, inflammatory bowel disease management, and hepatitis C management. However, there are many cost-cutting measures that can also be reported by gastroenterologists to fulfill these requirements. The Physician Quality Reporting System requires at least nine measures for at least 50% of Medicare patients in order to avoid penalties. It is not enough to track and document quality measures – results must also be reported through CMS-approved entities called qualified clinical data registries.
Dr. Jain described the movement toward payment bundles in which a single reimbursement is provided for services rendered by all providers and sites across a single episode of care. Screening colonoscopy was used as an example of how this could impact gastroenterology: a single payment would be provided for the preprocedure evaluation and education, the procedure itself (including endoscopy, anesthesia, pathology, facility), and postprocedure follow-up. Additional payments would not be provided for additional services such as repeating the colonoscopy because of a poor preparation or to treat postpolypectomy bleeding. Overall, health care reimbursement is moving to recognize value (quality/cost), reduce variation in care, and transfer more of the financial risk to health care providers and systems.
Dr. Inadomi, division of gastroenterology, department of medicine, University of Washington School of Medicine, department of health services, University of Washington School of Public Health, Seattle. He is on the clinical advisory committee for ChemImage and on the scientific advisory board for Epigenomics.
Colorectal cancer (CRC) screening has become a focal point of the practice of gastroenterology. Successful screening leads to a reduction in cancer mortality and is one of the most impactful means by which we improve population health.
Rick Boland, MD, of the Baylor Center of Gastrointestinal Cancer Research in Dallas reminded us at the annual Digestive Disease Week that carcinogenesis in colorectal cancer is a multistep process and described several pathways of which clinicians should be aware. Germline mutations (inherited) vs. somatic mutations (acquired) may be driver mutations that confer selection advantage and become targets for therapy in contrast to passenger mutations that are not mechanistic targets. Germline mutations should be considered when there is a family history of CRC, especially in a young patient, and are characteristic of familial adenomatous polyposis (APC), attenuated polyposis (MYH-MUTYH), and nonadenomatous polyposis syndromes such as Peutz-Jeghers (STK11).
Microsatellite instability is an alternative pathway to cancer. Hereditary nonpolyposis colorectal cancer (Lynch syndrome) is representative of this pathway with common abnormalities in mismatch repair genes such as MLH1, MSH2, MSH6, or PMS2. Lynch syndrome patients are BRAF and CIMP negative. This is in contrast to a more recent pathway to cancer that arises from sessile serrated polyps that also display microsatellite instability, but are BRAF and CIMP positive.
The clinical importance of understanding the pathway through which cancer develops in an individual patient is that depending on the mechanism of tumorigenesis, different treatment strategies can prevent or treat cancer. For example, exciting novel therapies are being developed for microsatellite unstable cancers using immune checkpoint inhibitors. Aspirin can reduce CRC mortality, especially tumors that overexpress COX2 or harbor PIK3CA mutations, and have demonstrated benefit even postoperatively in patients receiving surgical resection of CRC.
I gave a talk focused on current guidelines for reducing mortality among individuals at average risk for developing CRC. The U.S. Preventive Services Task Force updated their recommendations this year and chose to focus on highlighting the importance of CRC screening between the ages of 50 and 75 years, tailored screening for those between the ages of 76 and 85 years, and stopping screening after age 85 years. However, the statement does not recommend specific tests and instead lists competing strategies of annual fecal immunochemical test (FIT) or highly sensitive fecal occult blood test (FOBT) with or without flexible sigmoidoscopy every 10 years, flexible sigmoidoscopy every 5 years, colonoscopy every 10 years, CT colonography every 5 years, and FIT-DNA every 1 or 3 years. The FIT-DNA adds a separate FIT to a stool exam for KRAS mutations, NDRG4 and BMP3 methylation, and beta-actin. There are no studies to determine whether CRC mortality is reduced, but one study demonstrated a higher sensitivity for CRC with the combined FIT-DNA, compared with FIT alone, but a lower specificity. The FDA also recently approved a blood-based CRC screening test that detects circulating methylated septin 9 gene DNA. Septin 9 is a gene that codes for GTP-binding protein and acts as a tumor suppressor that can be inactivated by methylation.
Irrespective of which strategy is pursued, colonoscopy is the final common pathway. Quality is important because there remains a disappointing rate of CRC after colonoscopy. Prior work has demonstrated significant differences in the adenoma detection rate (ADR) between endoscopists in the same practice. A recent Kaiser study found that for every 1% increase in the ADR there is a 3% decrease in the rate of cancer developing after colonoscopy.
Disruptive technology is emerging that may displace gastroenterologists from primary endoscopic screening. A self-propelled disposable colonoscope (Aer-O-Scope) has been developed that uses pneumatic pressure to advance a tethered camera through the colonic lumen guided by a joystick. Sedation is not required and the device does not need manual advancement, facilitating use by nongastroenterologists.
Uri Ladabaum, MD, of Stanford (Calif.) University presented the clinical impact of screening for hereditary and familial CRC. Lynch syndrome is characterized by a family history of cancer in at least three individuals spanning at least two generations including one with early cancer development. He reminded us that Lynch syndrome cancers are not limited to CRC but also include uterine, ovarian, gastric, small intestinal, urinary, biliary, pancreatic, and neurologic tumors. Microsatellite instability is likely present, mismatch repair gene abnormalities should be sought, and screening should include colonoscopy every 1-2 years. Aspirin chemoprophylaxis should be recommended, as should prophylactic hysterectomy and oophorectomy among affected women due to the uterine and ovarian cancer risk.
Polyposis syndromes constitute a separate group of hereditary cancers that includes familial adenomatous polyposis (FAP) arising from defects in the APC gene that may present as florid or attenuated FAP, and MYH-associated polyposis. If a patient presents with more than 10 adenomas, a polyposis syndrome should be considered. More rare syndromes include Cowden, juvenile polyposis, and Peutz-Jeghers.
Douglas Rex, MD, of Indiana University, Indianapolis, provided an array of highly clinically relevant tips to improve screening colonoscopy quality, which has been shown to reduce CRC mortality. Some of the basic concepts include using split-dose preparations and high-definition endoscopes, measuring and reporting adenoma detection rates, and using water immersion with carbon dioxide instead of air insufflation. Special attention was given to the diagnosis and removal of sessile lesions of the colon. Dr. Rex revealed many of his “tricks” to achieving high-quality colonoscopy, which include retroflexion to access polyps behind folds, using a stiff snare, and attaching a cap to the end of the colonoscope in order to facilitate visualization of polyps and assist in the mucosal resection of sessile lesions. Dr. Rex also presented data to support his recommendation to use “avulsion” with cold snare and forceps while limiting coagulative ablation for residual polyp tissue.
Finally, Rajeev Jain, MD, of Dallas presented the rapidly evolving area of reimbursement reform including the Medicare Access and CHIP Reauthorization Act (MACRA) that is driving payment away from fee-for-service and towards bundled payments, accountable care networks, and patient-centered medical homes. Key to this reform is the documentation and reporting of quality for procedural and cognitive patient encounters. There are limited performance measures specific to gastroenterology and hepatology, which currently include measures to both document appropriate CRC screening and reduce colonoscopy overuse, inflammatory bowel disease management, and hepatitis C management. However, there are many cost-cutting measures that can also be reported by gastroenterologists to fulfill these requirements. The Physician Quality Reporting System requires at least nine measures for at least 50% of Medicare patients in order to avoid penalties. It is not enough to track and document quality measures – results must also be reported through CMS-approved entities called qualified clinical data registries.
Dr. Jain described the movement toward payment bundles in which a single reimbursement is provided for services rendered by all providers and sites across a single episode of care. Screening colonoscopy was used as an example of how this could impact gastroenterology: a single payment would be provided for the preprocedure evaluation and education, the procedure itself (including endoscopy, anesthesia, pathology, facility), and postprocedure follow-up. Additional payments would not be provided for additional services such as repeating the colonoscopy because of a poor preparation or to treat postpolypectomy bleeding. Overall, health care reimbursement is moving to recognize value (quality/cost), reduce variation in care, and transfer more of the financial risk to health care providers and systems.
Dr. Inadomi, division of gastroenterology, department of medicine, University of Washington School of Medicine, department of health services, University of Washington School of Public Health, Seattle. He is on the clinical advisory committee for ChemImage and on the scientific advisory board for Epigenomics.
AT DDW 2016
Abstracts Presented at the 2016 AVAHO Annual Meeting
Veterans’ keratinocyte carcinoma, actinic keratosis care cost $356 million in 2012
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
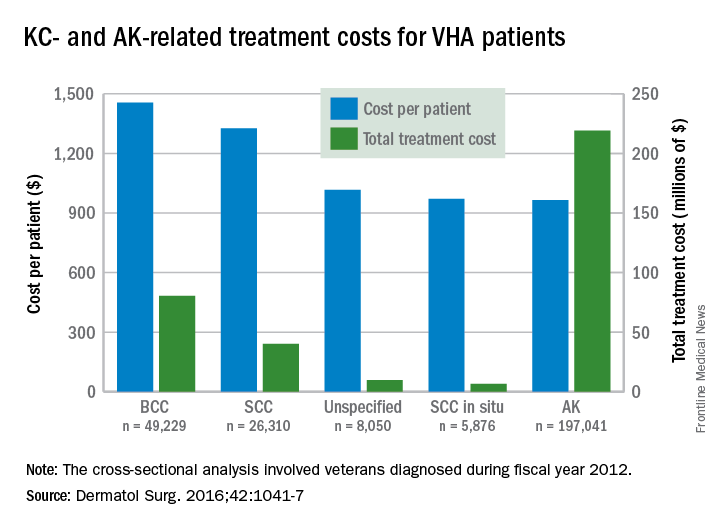
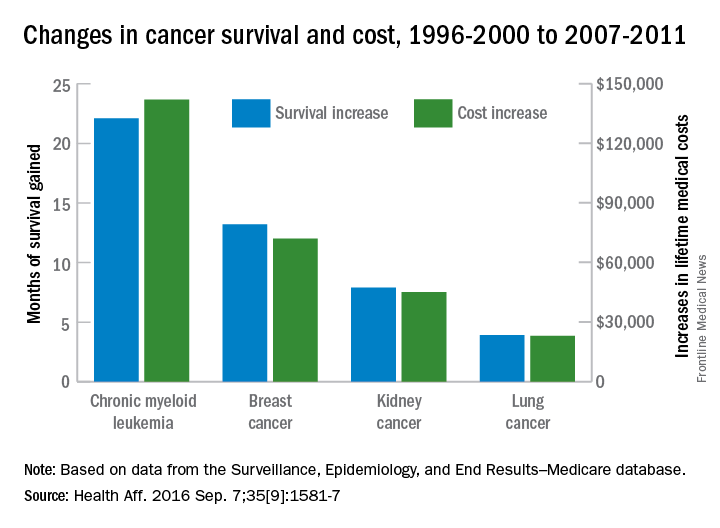
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).
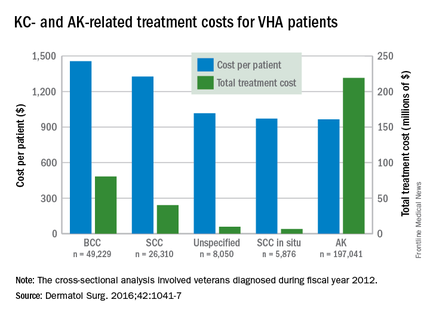
The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
FROM DERMATOLOGIC SURGERY
ANTARCTIC results chill enthusiasm for platelet monitoring
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
ROME – Measuring platelet function in order to tailor antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention for acute coronary syndromes did not improve their clinical outcomes in the randomized ANTARCTIC trial, Gilles Montalescot, MD, reported at the annual congress of the European Society of Cardiology.
“We found absolutely no benefit for this strategy of adjustment of antiplatelet therapy based upon platelet function testing. The study was completely neutral on all types of endpoints, ischemic as well as bleeding,” said Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Pitié-Salpêtrière Hospital.
This was a disappointing result in what was the largest-ever randomized clinical trial involving PCI in elderly patients, he said. This was a high-risk population, not only by virtue of everyone being over age 75 years, but because they all presented with ACS. Indeed, one-third of ANTARCTIC participants underwent primary PCI for ST-segment elevation myocardial infarction.
ANTARCTIC (Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged Over 75 Years to Reduce the Composite of Bleeding, Stent Thrombosis, and Ischemic Complications) was carried out as a follow-up to the earlier ARCTIC randomized trial, also conducted by Dr. Montalescot and his coinvestigators. Like ANTARCTIC, ARCTIC, too, showed no clinical benefit for platelet function testing in order to adjust antiplatelet therapy (N Engl J Med. 2012;367:2100-9). At the time, ARCTIC’s critics argued that this individualized strategy didn’t achieve the expected improved outcomes because the trial was conducted in low-risk, stable patients undergoing elective scheduled PCI. In contrast, if there was ever a high-risk population in which platelet function testing and tailored antiplatelet therapy should work, it was in the very high-risk ANTARCTIC population, he said.
ANTARCTIC included 877 elderly patients undergoing urgent PCI for ACS who were placed on low-dose aspirin and randomized to standard antiplatelet therapy with prasugrel (Effient) at 5 mg/day, the European approved dose for long-term maintenance therapy in elderly patients, or to tailored antiplatelet therapy.
Patients in the tailored therapy arm received prasugrel at 5 mg/day for the first 14 days, then underwent platelet function testing with the VerifyNow P2Y12 system. If they demonstrated high on-drug platelet activity, defined as at least 208 P2Y12 reaction units (PRU), their prasugrel was bumped up to 10 mg/day. If their PRU measurement was in what is considered the optimal range for quelling ischemia without promoting bleeding – that is, less than 208 but more than 85 PRU – they remained on prasugrel at 5 mg/day. And if they scored less than 85 PRU, exposing them to excess bleeding risk due to high suppression of platelets, they were switched to clopidogrel (Plavix) at 75 mg/day, a less potent antiplatelet regimen.
Two weeks after their first platelet function measurement, participants in the tailored therapy arm returned for a second round of platelet activity testing, with their antiplatelet regimen once again being adjusted on the basis of the results.
The primary study endpoint was net clinical benefit over a 12-month follow-up period. This was defined as the composite of cardiovascular death, MI, stroke, urgent revascularization, stent thrombosis, and Bleeding Academic Research Consortium (BARC) types 2, 3, or 5. This composite endpoint occurred in 27.6% of the platelet monitoring group and a near-identical 27.8% of conventionally managed patients.
Of note, 42% of patients in the actively monitored group were within the target platelet inhibition range when tested 14 days into the study. At study’s end, 55% of patients remained on prasugrel at 5 mg/day, 39% were on clopidogrel at 75 mg/day, and less than 4% were on prasugrel at 10 mg/day. Thus, most patients who underwent a dose adjustment on the basis of their VerifyNow results were downgraded to a less-potent antiplatelet regimen. Very few required enhanced platelet suppression in the form of 10 mg/day of prasugrel.
“Platelet function monitoring is difficult to use. Patients have to come back twice to be monitored. It’s costly. It’s time consuming. And platelet function monitoring clearly does not help,” the cardiologist said.
The ANTARCTIC results will likely lead to a revision of the American and European guidelines, which currently give a class IIb/level of evidence C recommendation for platelet function testing in high-risk situations.
“There is a huge literature showing that platelet reactivity affects clinical outcomes,” Dr. Montalescot continued. “One hypothesis now is that platelet reactivity may be only a marker of risk; you can modify it, but that has no impact on patient outcomes. We may be in the same situation here as with HDL cholesterol, for example.”
Discussant Steen Dalby Kristensen, MD, noted that ANTARCTIC is just the latest in a slew of negative randomized clinical trials of individualized antiplatelet therapy for coronary artery disease. In addition to ARCTIC, others include GRAVITAS, TRIGGER PCI, and ASCET. One study, the German/Austrian TROPICAL ACS trial, remains ongoing.
“It really is an intriguing concept that many of us have been fascinated by for years: to identify the sweet spot where, by measuring platelet aggregation and maybe changing the therapy, we can find just the right balance between bleeding and ischemia. The ANTARCTIC results are quite disappointing for platelet-monitoring enthusiasts. Is the whole concept wrong?” said Dr. Kristensen, professor of cardiology and head of the cardiovascular research center at Aarhus (Denmark) University Hospital.
“I think even more disappointing for me than the lack of impact on ischemic events was the bleeding. I would have anticipated that maybe bleeding could be avoided by adjusting the dose, but this was not the case,” he added.
But Stephan Gielen, MD, saw a silver lining in the negative results for ANTARCTIC.
“From my perspective as a clinical interventionalist, I’m happy that you ended up in the way you did. Putting things positively, this study confirms the safety of dual-platelet inhibition with prasugrel at the reduced dose of 5 mg in an elderly population. There is no need to go to the trouble of monitoring platelet function even in this elderly population, which I think for clinical practice is a good message,” said Dr. Gielen of Detmold (Germany) Hospital, who cochaired a press conference where Dr. Montalescot presented the ANTARCTIC results.
Simultaneously with Dr. Montalescot’s presentation at ESC 2016 in Rome, the ANTARCTIC results were published online (Lancet. 2016 Aug 26. doi: 10.1016/S0140-6736(16)31323-X).
ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
AT THE ESC CONGRESS 2016
Key clinical point: Researchers have just about given up on the notion that monitoring platelet function in order to individualize antiplatelet therapy in patients undergoing PCI provides any clinical benefit.
Major finding: Individualized antiplatelet therapy based upon serial measurements of platelet function did not result in improved outcomes in elderly patients undergoing PCI for acute coronary syndrome (27.6% in the platelet monitoring group and 27.8% in conventionally managed patients).
Data source: ANTARCTIC was an open-label, blinded-endpoint randomized trial of tailored versus standard antiplatelet therapy in 877 elderly patients undergoing PCI for ACS.
Disclosures: ANTARCTIC was funded by Eli Lilly, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and the French Foundation for Heart Research. The presenter reported receiving research grants from and/or serving as a consultant to those organizations and numerous others.
Getting prior lab results is worth the effort
Many patients we see need blood work as part of their evaluation. Although labs are cheap compared with other tests we order, they can still be frustrating to get.
It’s not hard to order them. Ordering any test is pretty easy.
But I hate duplicating tests. Patients often say they just had labs done, which “were all fine,” but that tells me nothing. For all I know, it was a lipid panel and PSA, entirely unrelated to what I’m seeing them for.
Occasionally, they bring labs in with them, or I’ve gotten them in advance, but usually I’m working blind.
Back when I was new to practice, I just ordered everything I wanted. I figured it was easier than trying to get the previous ones. I think we all do that sometimes. And there’s kind of an ivory-tower mentality we all have early in our careers that “I’m the doctor, and I’ll do what I want.”
I quickly learned that often backfires. If the same labs were done recently, many insurance companies won’t pay for them ... and the patients get a bill. Then they call my office and complain. It didn’t take me long to realize this approach was a waste of their time, money, and blood.
So now I always ask if they’ve had labs done since the symptoms started. If the answer is yes, I’ll call or fax the other doctor to get them. This can take (depending on the other office) a few hours to days. But the majority of outpatient neurology is nonurgent, and a extra few days usually doesn’t matter in the things I treat.
When I get the labs, it’s easy to make some quick notes on what was done and what still needs to be checked. I scribble out a lab order, mail or fax it, and have my staff notify the patient the ball is rolling. It’s not hard.
Patients appreciate it. I’m saving them time, blood, money, and maybe even a venipuncture. I get the tests I want, still in a timely fashion. It also keeps insurance costs down for all of us.
Obviously, there are some cases where urgency has to take priority. But for the majority of them, duplicating tests needlessly is a bad idea for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Many patients we see need blood work as part of their evaluation. Although labs are cheap compared with other tests we order, they can still be frustrating to get.
It’s not hard to order them. Ordering any test is pretty easy.
But I hate duplicating tests. Patients often say they just had labs done, which “were all fine,” but that tells me nothing. For all I know, it was a lipid panel and PSA, entirely unrelated to what I’m seeing them for.
Occasionally, they bring labs in with them, or I’ve gotten them in advance, but usually I’m working blind.
Back when I was new to practice, I just ordered everything I wanted. I figured it was easier than trying to get the previous ones. I think we all do that sometimes. And there’s kind of an ivory-tower mentality we all have early in our careers that “I’m the doctor, and I’ll do what I want.”
I quickly learned that often backfires. If the same labs were done recently, many insurance companies won’t pay for them ... and the patients get a bill. Then they call my office and complain. It didn’t take me long to realize this approach was a waste of their time, money, and blood.
So now I always ask if they’ve had labs done since the symptoms started. If the answer is yes, I’ll call or fax the other doctor to get them. This can take (depending on the other office) a few hours to days. But the majority of outpatient neurology is nonurgent, and a extra few days usually doesn’t matter in the things I treat.
When I get the labs, it’s easy to make some quick notes on what was done and what still needs to be checked. I scribble out a lab order, mail or fax it, and have my staff notify the patient the ball is rolling. It’s not hard.
Patients appreciate it. I’m saving them time, blood, money, and maybe even a venipuncture. I get the tests I want, still in a timely fashion. It also keeps insurance costs down for all of us.
Obviously, there are some cases where urgency has to take priority. But for the majority of them, duplicating tests needlessly is a bad idea for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Many patients we see need blood work as part of their evaluation. Although labs are cheap compared with other tests we order, they can still be frustrating to get.
It’s not hard to order them. Ordering any test is pretty easy.
But I hate duplicating tests. Patients often say they just had labs done, which “were all fine,” but that tells me nothing. For all I know, it was a lipid panel and PSA, entirely unrelated to what I’m seeing them for.
Occasionally, they bring labs in with them, or I’ve gotten them in advance, but usually I’m working blind.
Back when I was new to practice, I just ordered everything I wanted. I figured it was easier than trying to get the previous ones. I think we all do that sometimes. And there’s kind of an ivory-tower mentality we all have early in our careers that “I’m the doctor, and I’ll do what I want.”
I quickly learned that often backfires. If the same labs were done recently, many insurance companies won’t pay for them ... and the patients get a bill. Then they call my office and complain. It didn’t take me long to realize this approach was a waste of their time, money, and blood.
So now I always ask if they’ve had labs done since the symptoms started. If the answer is yes, I’ll call or fax the other doctor to get them. This can take (depending on the other office) a few hours to days. But the majority of outpatient neurology is nonurgent, and a extra few days usually doesn’t matter in the things I treat.
When I get the labs, it’s easy to make some quick notes on what was done and what still needs to be checked. I scribble out a lab order, mail or fax it, and have my staff notify the patient the ball is rolling. It’s not hard.
Patients appreciate it. I’m saving them time, blood, money, and maybe even a venipuncture. I get the tests I want, still in a timely fashion. It also keeps insurance costs down for all of us.
Obviously, there are some cases where urgency has to take priority. But for the majority of them, duplicating tests needlessly is a bad idea for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hidradenitis suppurativa is more common than you might think
NEWPORT BEACH, CALIF. – For decades, hidradenitis suppurativa (HS) has been characterized as a rare disorder, but recent evidence from the medical dermatology literature suggests that between 0.4% and 4% of the population is affected, with a predominance in female and black individuals.
At the annual meeting of the Pacific Dermatologic Association, Haley Naik, MD, characterized those estimates as “astounding.” There is also diagnostic delay that ranges from 5 to 14 years in Western populations, said Dr. Naik, of the department of dermatology at the University of California, San Francisco (Br J Dermatol. 2015 Dec;173[6]:1546-9). “These patients are repeatedly interacting with the health care system and they’re not getting the correct diagnosis and therefore they’re not getting effective therapy for the management of their disease,” she said. “We can begin to tackle this problem by educating ourselves and our colleagues about the best ways to diagnose these patients.”
In 2015, a group of European researchers published proposed diagnostic criteria for hidradenitis suppurativa (Dermatology. 2015;231[2]:184-90). The criteria comprise three components: typical lesions (double-ended comedones, active inflammatory cysts and nodules, abscesses, follicularly-based papules and pustules, fistula and sinus formation, and scarring); typical distribution (in intertriginous areas such as the axilla and the buttocks), and chronicity (they suggest that patients must have at least two episodes of the disease over a 6-month period). “In addition to satisfying these three criteria, they also suggest that there are some secondary criteria that can be used to help make this diagnosis: a family history of [hidradenitis suppurativa] and absence of pathogens at lesional sites,” Dr. Naik said. “These criteria are a practical framework in which we can begin to educate our colleagues about this diagnosis, but they should not lead us to believe that hidradenitis suppurativa is a phenotypically homogenous disease.”
There are multiple phenotypes of HS, including comedonal, nodular/cystic, and ulcerative. Typical sites that are involved are the axilla, groin, and buttocks. “There is also a subset of patients who are typically thin and male who have a gluteal predominant, or exclusive presentation of their disease,” Dr. Naik said. “In my experience, these patients tend to have severe and progressive disease.” Atypical sites include the postauricular skin, the trunk, and the extremities.
In a cross-sectional study, researchers in France found that HS patients fall into one of three categories. Those in category 1 had involvement of breasts, axilla, and hypertrophic scars; those in category 2 had involvement of the breasts, axilla, ears, chest, back, follicular lesions, and acne, and tended to have a family history of HS; those in category 3 had gluteal involvement with prominent papules and folliculitis (J Invest Dermatol. 2013 Jun;133[6]:1506-11). “Ideally, we hope this type of phenotyping will facilitate phenotype-genotype correlation, and eventually help in understanding disease course of these patients leading to optimized therapeutic options,” Dr. Naik said. “We’re just at the tip of the iceberg here in learning about these various disease subtypes.”
In 2015, the tumor necrosis factor blocker adalimumab (Humira) became the first treatment approved by the Food and Drug Administration for HS, for moderate to severe disease in adults. “The problem is, the dosing and the dose frequency of adalimumab is fixed, so if your patient is only partially responding to the therapy, you don’t have much wiggle room in terms of trying to titrate this medication,” Dr. Naik said. “In that situation the agent of choice to switch to is infliximab.”
Another biologic agent, the interleukin-1 receptor antagonist anakinra (Kineret), also shows promise. Results from a small randomized study of 20 patients found that 7 of 10 patients in the treatment group reported 80% improvement in their disease, compared with 3 out of 10 in the placebo group (JAMA Dermatol. 2016;152[1]:52-9). “Further work needs to be done in this area to understand the efficacy of anakinra for HS,” she said. “I certainly wouldn’t consider anakinra a first- or second-line therapy for the management of HS, but it’s an option for patients who have refractory disease.”
In addition to experiencing fistula and sinus formation, scarring, and wound contractures, HS patients are at risk for developing a host of complications, including lymphedema, peripheral neuropathy, squamous cell carcinoma, chronic pain, anemia, hypoproteinemia, amyloidosis, nephrotic syndrome, and uveitis. According to Dr. Naik, surgical management of HS can be considered in patients who have localized severe disease in which the risks of long-term immunosuppressive therapy outweigh the benefits. “In patients who have chronic refractory disease, surgical management can be particularly helpful,” she said. “In those who have fistula or sinus formation, significant scarring, or range of motion limitation from wound contractures, medical management can only go so far, because the medication can’t really get to those areas.”
Dr. Naik concluded her presentation by emphasizing the role dermatologists can play in helping patients address comorbidities that can accompany HS, from obesity and lipid abnormalities, to depression and arthritis. “We as dermatologists may not be in a position to manage these patients’ comorbidities over the long term, but we are in a position to encourage them to seek additional medical attention and to communicate with our colleagues when appropriate to help facilitate multidisciplinary management of this disease,” she said. “I routinely pull on the sleeves of my surgical and medical colleagues to get help with my most complicated HS patients.”
Dr. Naik reported having no financial disclosures.
NEWPORT BEACH, CALIF. – For decades, hidradenitis suppurativa (HS) has been characterized as a rare disorder, but recent evidence from the medical dermatology literature suggests that between 0.4% and 4% of the population is affected, with a predominance in female and black individuals.
At the annual meeting of the Pacific Dermatologic Association, Haley Naik, MD, characterized those estimates as “astounding.” There is also diagnostic delay that ranges from 5 to 14 years in Western populations, said Dr. Naik, of the department of dermatology at the University of California, San Francisco (Br J Dermatol. 2015 Dec;173[6]:1546-9). “These patients are repeatedly interacting with the health care system and they’re not getting the correct diagnosis and therefore they’re not getting effective therapy for the management of their disease,” she said. “We can begin to tackle this problem by educating ourselves and our colleagues about the best ways to diagnose these patients.”
In 2015, a group of European researchers published proposed diagnostic criteria for hidradenitis suppurativa (Dermatology. 2015;231[2]:184-90). The criteria comprise three components: typical lesions (double-ended comedones, active inflammatory cysts and nodules, abscesses, follicularly-based papules and pustules, fistula and sinus formation, and scarring); typical distribution (in intertriginous areas such as the axilla and the buttocks), and chronicity (they suggest that patients must have at least two episodes of the disease over a 6-month period). “In addition to satisfying these three criteria, they also suggest that there are some secondary criteria that can be used to help make this diagnosis: a family history of [hidradenitis suppurativa] and absence of pathogens at lesional sites,” Dr. Naik said. “These criteria are a practical framework in which we can begin to educate our colleagues about this diagnosis, but they should not lead us to believe that hidradenitis suppurativa is a phenotypically homogenous disease.”
There are multiple phenotypes of HS, including comedonal, nodular/cystic, and ulcerative. Typical sites that are involved are the axilla, groin, and buttocks. “There is also a subset of patients who are typically thin and male who have a gluteal predominant, or exclusive presentation of their disease,” Dr. Naik said. “In my experience, these patients tend to have severe and progressive disease.” Atypical sites include the postauricular skin, the trunk, and the extremities.
In a cross-sectional study, researchers in France found that HS patients fall into one of three categories. Those in category 1 had involvement of breasts, axilla, and hypertrophic scars; those in category 2 had involvement of the breasts, axilla, ears, chest, back, follicular lesions, and acne, and tended to have a family history of HS; those in category 3 had gluteal involvement with prominent papules and folliculitis (J Invest Dermatol. 2013 Jun;133[6]:1506-11). “Ideally, we hope this type of phenotyping will facilitate phenotype-genotype correlation, and eventually help in understanding disease course of these patients leading to optimized therapeutic options,” Dr. Naik said. “We’re just at the tip of the iceberg here in learning about these various disease subtypes.”
In 2015, the tumor necrosis factor blocker adalimumab (Humira) became the first treatment approved by the Food and Drug Administration for HS, for moderate to severe disease in adults. “The problem is, the dosing and the dose frequency of adalimumab is fixed, so if your patient is only partially responding to the therapy, you don’t have much wiggle room in terms of trying to titrate this medication,” Dr. Naik said. “In that situation the agent of choice to switch to is infliximab.”
Another biologic agent, the interleukin-1 receptor antagonist anakinra (Kineret), also shows promise. Results from a small randomized study of 20 patients found that 7 of 10 patients in the treatment group reported 80% improvement in their disease, compared with 3 out of 10 in the placebo group (JAMA Dermatol. 2016;152[1]:52-9). “Further work needs to be done in this area to understand the efficacy of anakinra for HS,” she said. “I certainly wouldn’t consider anakinra a first- or second-line therapy for the management of HS, but it’s an option for patients who have refractory disease.”
In addition to experiencing fistula and sinus formation, scarring, and wound contractures, HS patients are at risk for developing a host of complications, including lymphedema, peripheral neuropathy, squamous cell carcinoma, chronic pain, anemia, hypoproteinemia, amyloidosis, nephrotic syndrome, and uveitis. According to Dr. Naik, surgical management of HS can be considered in patients who have localized severe disease in which the risks of long-term immunosuppressive therapy outweigh the benefits. “In patients who have chronic refractory disease, surgical management can be particularly helpful,” she said. “In those who have fistula or sinus formation, significant scarring, or range of motion limitation from wound contractures, medical management can only go so far, because the medication can’t really get to those areas.”
Dr. Naik concluded her presentation by emphasizing the role dermatologists can play in helping patients address comorbidities that can accompany HS, from obesity and lipid abnormalities, to depression and arthritis. “We as dermatologists may not be in a position to manage these patients’ comorbidities over the long term, but we are in a position to encourage them to seek additional medical attention and to communicate with our colleagues when appropriate to help facilitate multidisciplinary management of this disease,” she said. “I routinely pull on the sleeves of my surgical and medical colleagues to get help with my most complicated HS patients.”
Dr. Naik reported having no financial disclosures.
NEWPORT BEACH, CALIF. – For decades, hidradenitis suppurativa (HS) has been characterized as a rare disorder, but recent evidence from the medical dermatology literature suggests that between 0.4% and 4% of the population is affected, with a predominance in female and black individuals.
At the annual meeting of the Pacific Dermatologic Association, Haley Naik, MD, characterized those estimates as “astounding.” There is also diagnostic delay that ranges from 5 to 14 years in Western populations, said Dr. Naik, of the department of dermatology at the University of California, San Francisco (Br J Dermatol. 2015 Dec;173[6]:1546-9). “These patients are repeatedly interacting with the health care system and they’re not getting the correct diagnosis and therefore they’re not getting effective therapy for the management of their disease,” she said. “We can begin to tackle this problem by educating ourselves and our colleagues about the best ways to diagnose these patients.”
In 2015, a group of European researchers published proposed diagnostic criteria for hidradenitis suppurativa (Dermatology. 2015;231[2]:184-90). The criteria comprise three components: typical lesions (double-ended comedones, active inflammatory cysts and nodules, abscesses, follicularly-based papules and pustules, fistula and sinus formation, and scarring); typical distribution (in intertriginous areas such as the axilla and the buttocks), and chronicity (they suggest that patients must have at least two episodes of the disease over a 6-month period). “In addition to satisfying these three criteria, they also suggest that there are some secondary criteria that can be used to help make this diagnosis: a family history of [hidradenitis suppurativa] and absence of pathogens at lesional sites,” Dr. Naik said. “These criteria are a practical framework in which we can begin to educate our colleagues about this diagnosis, but they should not lead us to believe that hidradenitis suppurativa is a phenotypically homogenous disease.”
There are multiple phenotypes of HS, including comedonal, nodular/cystic, and ulcerative. Typical sites that are involved are the axilla, groin, and buttocks. “There is also a subset of patients who are typically thin and male who have a gluteal predominant, or exclusive presentation of their disease,” Dr. Naik said. “In my experience, these patients tend to have severe and progressive disease.” Atypical sites include the postauricular skin, the trunk, and the extremities.
In a cross-sectional study, researchers in France found that HS patients fall into one of three categories. Those in category 1 had involvement of breasts, axilla, and hypertrophic scars; those in category 2 had involvement of the breasts, axilla, ears, chest, back, follicular lesions, and acne, and tended to have a family history of HS; those in category 3 had gluteal involvement with prominent papules and folliculitis (J Invest Dermatol. 2013 Jun;133[6]:1506-11). “Ideally, we hope this type of phenotyping will facilitate phenotype-genotype correlation, and eventually help in understanding disease course of these patients leading to optimized therapeutic options,” Dr. Naik said. “We’re just at the tip of the iceberg here in learning about these various disease subtypes.”
In 2015, the tumor necrosis factor blocker adalimumab (Humira) became the first treatment approved by the Food and Drug Administration for HS, for moderate to severe disease in adults. “The problem is, the dosing and the dose frequency of adalimumab is fixed, so if your patient is only partially responding to the therapy, you don’t have much wiggle room in terms of trying to titrate this medication,” Dr. Naik said. “In that situation the agent of choice to switch to is infliximab.”
Another biologic agent, the interleukin-1 receptor antagonist anakinra (Kineret), also shows promise. Results from a small randomized study of 20 patients found that 7 of 10 patients in the treatment group reported 80% improvement in their disease, compared with 3 out of 10 in the placebo group (JAMA Dermatol. 2016;152[1]:52-9). “Further work needs to be done in this area to understand the efficacy of anakinra for HS,” she said. “I certainly wouldn’t consider anakinra a first- or second-line therapy for the management of HS, but it’s an option for patients who have refractory disease.”
In addition to experiencing fistula and sinus formation, scarring, and wound contractures, HS patients are at risk for developing a host of complications, including lymphedema, peripheral neuropathy, squamous cell carcinoma, chronic pain, anemia, hypoproteinemia, amyloidosis, nephrotic syndrome, and uveitis. According to Dr. Naik, surgical management of HS can be considered in patients who have localized severe disease in which the risks of long-term immunosuppressive therapy outweigh the benefits. “In patients who have chronic refractory disease, surgical management can be particularly helpful,” she said. “In those who have fistula or sinus formation, significant scarring, or range of motion limitation from wound contractures, medical management can only go so far, because the medication can’t really get to those areas.”
Dr. Naik concluded her presentation by emphasizing the role dermatologists can play in helping patients address comorbidities that can accompany HS, from obesity and lipid abnormalities, to depression and arthritis. “We as dermatologists may not be in a position to manage these patients’ comorbidities over the long term, but we are in a position to encourage them to seek additional medical attention and to communicate with our colleagues when appropriate to help facilitate multidisciplinary management of this disease,” she said. “I routinely pull on the sleeves of my surgical and medical colleagues to get help with my most complicated HS patients.”
Dr. Naik reported having no financial disclosures.
EXPERT ANALYSIS AT PDA 2016
New anticancer drugs linked to increased costs, life expectancy
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
FROM HEALTH AFFAIRS
Rapidly Growing Scalp Nodule
Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).
Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.
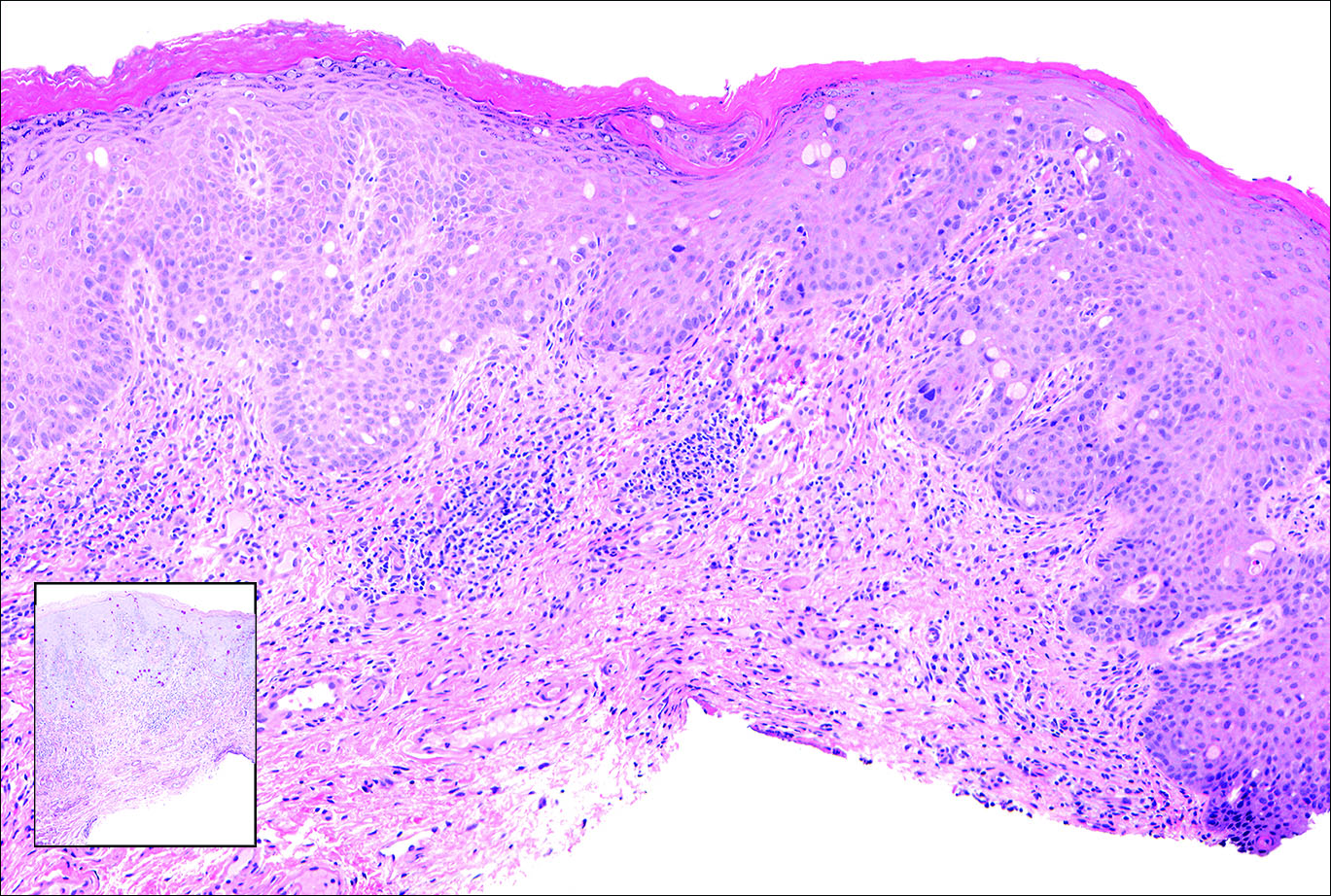
Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11
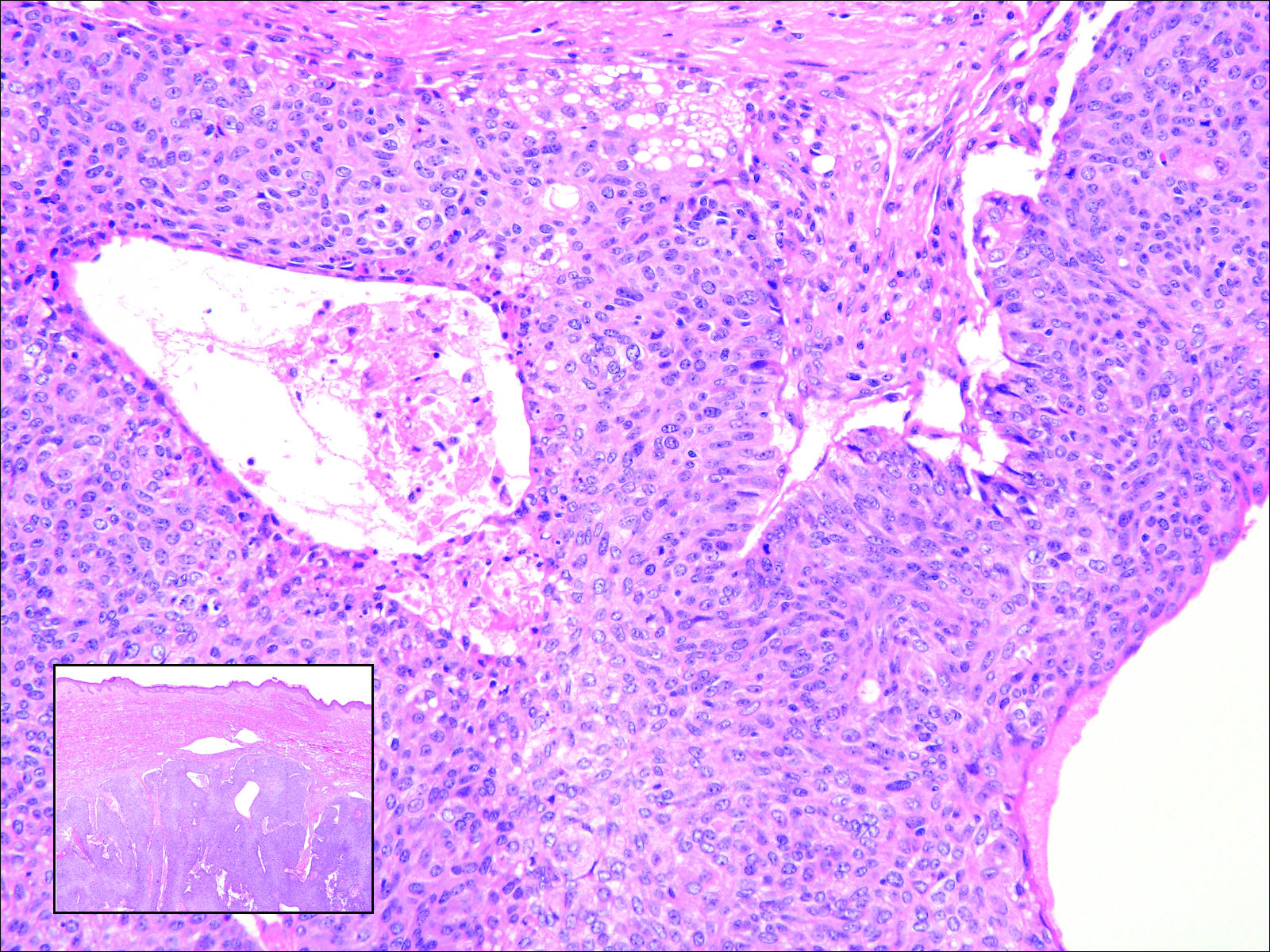
- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).

Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.


Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11

Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).

Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.


Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11

- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
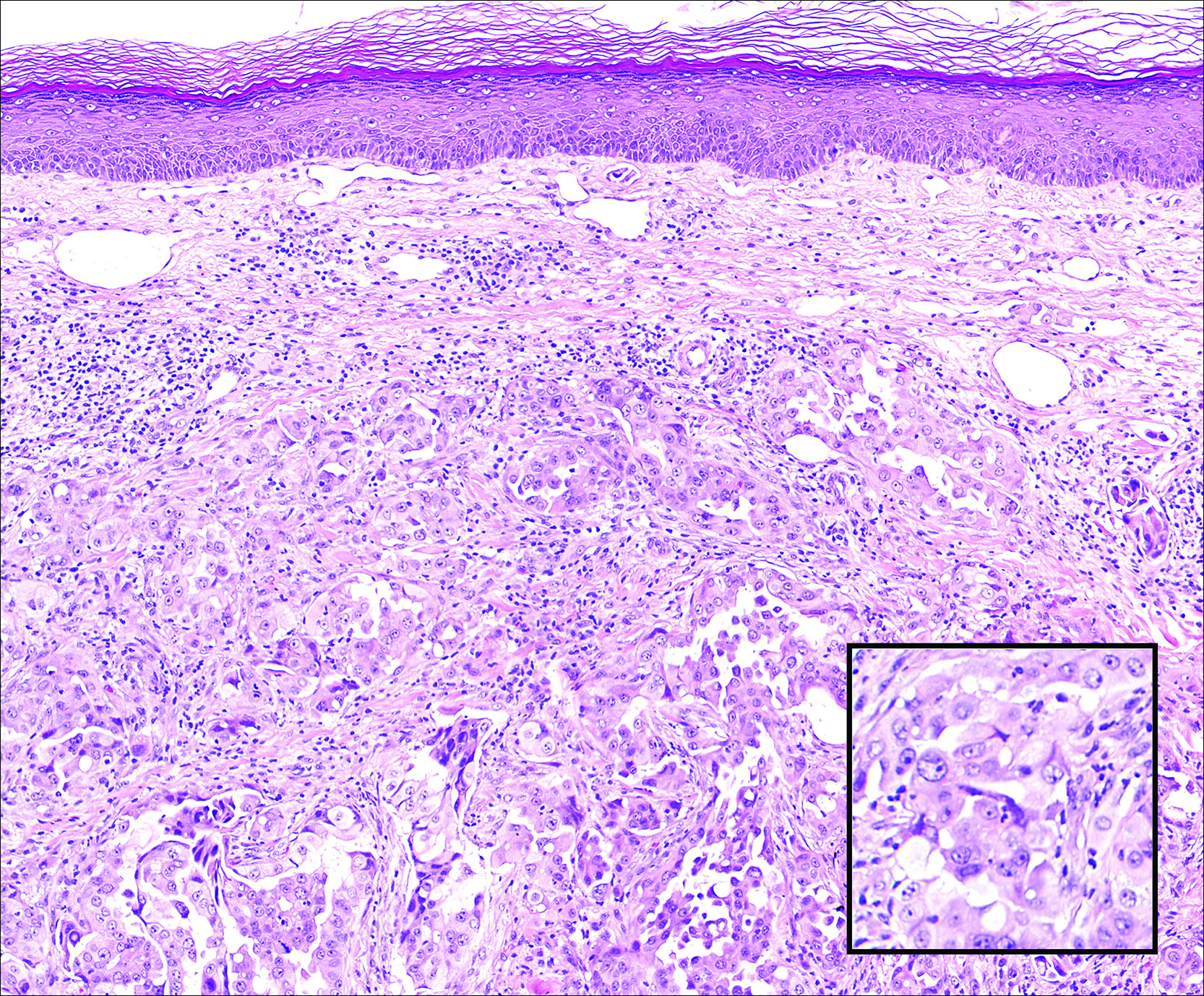
A 67-year-old woman with no history of malignancy presented with a scalp nodule. The photomicrograph showed atypical glands forming a subepidermal nodule with pleomorphic cells characterized by scant eosinophilic cytoplasm and large prominent nucleoli. Immunohistochemical analysis revealed diffuse thyroid transcription factor 1 and cytokeratin 7 positivity.
SVS Member Vice Chair of ABS
SVS member Dr. Spence M. Taylor has been elected vice chair of the American Board of Surgery for 2017-18. He will serve as chair in 2018-19. Dr. Taylor joined the ABS as a director in 2011, representing the Southern Surgical Association.
He is a member of the ABS Vascular Surgery Board and Credentials Committee.
For more information, read the ABS summer newsletter.
SVS member Dr. Spence M. Taylor has been elected vice chair of the American Board of Surgery for 2017-18. He will serve as chair in 2018-19. Dr. Taylor joined the ABS as a director in 2011, representing the Southern Surgical Association.
He is a member of the ABS Vascular Surgery Board and Credentials Committee.
For more information, read the ABS summer newsletter.
SVS member Dr. Spence M. Taylor has been elected vice chair of the American Board of Surgery for 2017-18. He will serve as chair in 2018-19. Dr. Taylor joined the ABS as a director in 2011, representing the Southern Surgical Association.
He is a member of the ABS Vascular Surgery Board and Credentials Committee.
For more information, read the ABS summer newsletter.