User login
AAD president shares legal tips
NEWPORT BEACH, CALIF. – Over the past 20 years, the number of medical malpractice claims against dermatologists has remained steady, in the ballpark of 86-123 per year, according to Abel Torres, MD, JD.
In fact, a study that used claims data from the Physician Insurers Association of America between 1985 and 2008 revealed that dermatology ranked 19th among 28 medical specialties evaluated. “The bad news is we’re not ranked 28th, so we’re still getting sued,” said Dr. Torres, professor and chairman of dermatology at Loma Linda (Calif.) University and professor of dermatology at Case Western Reserve University, Cleveland. In the study, 2,704 of 239,756 (1.1%) closed claims in this time period involved dermatologists; only 29% of the claims that involved dermatologists resulted in a payment for the plaintiff, with a median and average indemnity of $35,000 and $137,538, respectively (J Am Acad Dermatol. 2012 Jan;66[1]:78-85).
Speaking at the annual meeting of the Pacific Dermatologic Association, Dr. Torres, who is also current president of the American Academy of Dermatology, said that communication breakdowns between health care providers and patients account for more than 80% of medical errors and adverse events. In addition, ineffective communication can lead to below-average scores on Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), the Clinician and Group Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS), and other surveys, impacting public scrutiny, reputation, referrals, patient retention, and loyalty, and pay for performance. “Physicians who are on the lower third of communication on surveys tend to have 110% more lawsuits than everybody else,” he said.
Dr. Torres underscored the importance of obtaining verbal or written consent with patients prior to performing dermatologic procedures. “You want to discuss material risk, that is, What’s the likely significant risk? What’s a viable alternative? Do you think it’s viable? And if not, be prepared with data to support that. And what’s your rationale of benefit?” he said.
In a study that evaluated informed consent in dermatologic surgery, 85 patients undergoing Mohs surgery were given verbal and written instructions, including information about the potential for 10 possible complications (Dermatol Surg. 2003;29[9]:952-5). The researchers asked the patients to recall the 10 complications at 20 minutes and at 1 week after the informed consent process. The overall group retention rates for both time periods were 27% and 24%, respectively.
“The reality is, people are nervous,” said Dr. Torres, who was not involved with the study. “They’re not focusing well and may not be listening very carefully to you. So when you give them informed consent you need to help them focus on the important issues. Be cautious about diluting the discussion with so much information that they’re not getting the message.”
Lawsuits involving the use of lasers in dermatology are on the rise, according to one study that examined trends in legal cases secondary to cutaneous laser surgery over a 28-year period that peaked in 2010 (JAMA Dermatol. 2013;149[2]:188-93). It found that laser hair removal was the most commonly litigated procedure (63%), followed by lack of informed consent (53%). Nearly half of the cases favored the defendant and the mean indemnity payment was $380,719.
This is in contrast with commonly reported litigation trends where a majority of the cases favor the defendant. The study also found that nearly 40% of cases involved a nonphysician operator. “So you need to be extra careful when you’re dealing with laser procedures, or you’re dealing with nonphysician operators or extenders,” Dr. Torres noted. “In that regard, know the rules in your state; make sure that you’re clear on them. Make sure that the people who are going to [perform the procedures] are appropriately trained. Provide an adequate degree of supervision to make sure that the proper procedure is being followed, especially as it relates to informed consent.”
Another liability risk for clinicians is failing to follow up with patients. “As a doctor, you may have the responsibility to make sure the patient actually saw the specialist and that their reports were acted upon,” Dr. Torres said. “The law requires that you interact with the specialist in the patients’ best interest. Also, if you refer a patient to another doctor and you have a reason to think that [doctor is] incompetent, you may be held accountable. Referring to the wrong specialist can be a pitfall.”
Dermatologists may at times also be liable for providing interpreter services for patients, no matter the size of their office or the number of employees on their payroll. He recommended that physicians explore whether the Canopy Medical Translator APP, a technology that enables clinicians to communicate with patients in 15 languages, can prove useful to them. Funded by the National Institutes of Health, the technology can be run on any device that runs on iOS or Android. “It can take phrases you have and translate them, or translate phrases that patients have to you,” Dr. Torres said.
In his clinical experience, dermatologists can protect themselves from a legal standpoint by maintaining honesty with the patient; showing kindness and concern at each encounter; validating the patient’s complaints about complications without conveying blame; avoiding isolating the patient after a complication; having a remedy planned for the complication, and seeing and communicating with the patient frequently.
When things go wrong, he offered the “AAA” mnemonic: Always acknowledge a complaint and express empathy; make sure someone you designate is easily accessible to the patient making a complaint, and avoid premature conclusions or comments. “Why? Because you want to maintain honesty with your patients, you want to show kindness and concern and validate patients’ emotions,” Dr. Torres said. “In other words, treat them as you would like to be treated.”
He reported having no financial disclosures.
NEWPORT BEACH, CALIF. – Over the past 20 years, the number of medical malpractice claims against dermatologists has remained steady, in the ballpark of 86-123 per year, according to Abel Torres, MD, JD.
In fact, a study that used claims data from the Physician Insurers Association of America between 1985 and 2008 revealed that dermatology ranked 19th among 28 medical specialties evaluated. “The bad news is we’re not ranked 28th, so we’re still getting sued,” said Dr. Torres, professor and chairman of dermatology at Loma Linda (Calif.) University and professor of dermatology at Case Western Reserve University, Cleveland. In the study, 2,704 of 239,756 (1.1%) closed claims in this time period involved dermatologists; only 29% of the claims that involved dermatologists resulted in a payment for the plaintiff, with a median and average indemnity of $35,000 and $137,538, respectively (J Am Acad Dermatol. 2012 Jan;66[1]:78-85).
Speaking at the annual meeting of the Pacific Dermatologic Association, Dr. Torres, who is also current president of the American Academy of Dermatology, said that communication breakdowns between health care providers and patients account for more than 80% of medical errors and adverse events. In addition, ineffective communication can lead to below-average scores on Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), the Clinician and Group Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS), and other surveys, impacting public scrutiny, reputation, referrals, patient retention, and loyalty, and pay for performance. “Physicians who are on the lower third of communication on surveys tend to have 110% more lawsuits than everybody else,” he said.
Dr. Torres underscored the importance of obtaining verbal or written consent with patients prior to performing dermatologic procedures. “You want to discuss material risk, that is, What’s the likely significant risk? What’s a viable alternative? Do you think it’s viable? And if not, be prepared with data to support that. And what’s your rationale of benefit?” he said.
In a study that evaluated informed consent in dermatologic surgery, 85 patients undergoing Mohs surgery were given verbal and written instructions, including information about the potential for 10 possible complications (Dermatol Surg. 2003;29[9]:952-5). The researchers asked the patients to recall the 10 complications at 20 minutes and at 1 week after the informed consent process. The overall group retention rates for both time periods were 27% and 24%, respectively.
“The reality is, people are nervous,” said Dr. Torres, who was not involved with the study. “They’re not focusing well and may not be listening very carefully to you. So when you give them informed consent you need to help them focus on the important issues. Be cautious about diluting the discussion with so much information that they’re not getting the message.”
Lawsuits involving the use of lasers in dermatology are on the rise, according to one study that examined trends in legal cases secondary to cutaneous laser surgery over a 28-year period that peaked in 2010 (JAMA Dermatol. 2013;149[2]:188-93). It found that laser hair removal was the most commonly litigated procedure (63%), followed by lack of informed consent (53%). Nearly half of the cases favored the defendant and the mean indemnity payment was $380,719.
This is in contrast with commonly reported litigation trends where a majority of the cases favor the defendant. The study also found that nearly 40% of cases involved a nonphysician operator. “So you need to be extra careful when you’re dealing with laser procedures, or you’re dealing with nonphysician operators or extenders,” Dr. Torres noted. “In that regard, know the rules in your state; make sure that you’re clear on them. Make sure that the people who are going to [perform the procedures] are appropriately trained. Provide an adequate degree of supervision to make sure that the proper procedure is being followed, especially as it relates to informed consent.”
Another liability risk for clinicians is failing to follow up with patients. “As a doctor, you may have the responsibility to make sure the patient actually saw the specialist and that their reports were acted upon,” Dr. Torres said. “The law requires that you interact with the specialist in the patients’ best interest. Also, if you refer a patient to another doctor and you have a reason to think that [doctor is] incompetent, you may be held accountable. Referring to the wrong specialist can be a pitfall.”
Dermatologists may at times also be liable for providing interpreter services for patients, no matter the size of their office or the number of employees on their payroll. He recommended that physicians explore whether the Canopy Medical Translator APP, a technology that enables clinicians to communicate with patients in 15 languages, can prove useful to them. Funded by the National Institutes of Health, the technology can be run on any device that runs on iOS or Android. “It can take phrases you have and translate them, or translate phrases that patients have to you,” Dr. Torres said.
In his clinical experience, dermatologists can protect themselves from a legal standpoint by maintaining honesty with the patient; showing kindness and concern at each encounter; validating the patient’s complaints about complications without conveying blame; avoiding isolating the patient after a complication; having a remedy planned for the complication, and seeing and communicating with the patient frequently.
When things go wrong, he offered the “AAA” mnemonic: Always acknowledge a complaint and express empathy; make sure someone you designate is easily accessible to the patient making a complaint, and avoid premature conclusions or comments. “Why? Because you want to maintain honesty with your patients, you want to show kindness and concern and validate patients’ emotions,” Dr. Torres said. “In other words, treat them as you would like to be treated.”
He reported having no financial disclosures.
NEWPORT BEACH, CALIF. – Over the past 20 years, the number of medical malpractice claims against dermatologists has remained steady, in the ballpark of 86-123 per year, according to Abel Torres, MD, JD.
In fact, a study that used claims data from the Physician Insurers Association of America between 1985 and 2008 revealed that dermatology ranked 19th among 28 medical specialties evaluated. “The bad news is we’re not ranked 28th, so we’re still getting sued,” said Dr. Torres, professor and chairman of dermatology at Loma Linda (Calif.) University and professor of dermatology at Case Western Reserve University, Cleveland. In the study, 2,704 of 239,756 (1.1%) closed claims in this time period involved dermatologists; only 29% of the claims that involved dermatologists resulted in a payment for the plaintiff, with a median and average indemnity of $35,000 and $137,538, respectively (J Am Acad Dermatol. 2012 Jan;66[1]:78-85).
Speaking at the annual meeting of the Pacific Dermatologic Association, Dr. Torres, who is also current president of the American Academy of Dermatology, said that communication breakdowns between health care providers and patients account for more than 80% of medical errors and adverse events. In addition, ineffective communication can lead to below-average scores on Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), the Clinician and Group Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS), and other surveys, impacting public scrutiny, reputation, referrals, patient retention, and loyalty, and pay for performance. “Physicians who are on the lower third of communication on surveys tend to have 110% more lawsuits than everybody else,” he said.
Dr. Torres underscored the importance of obtaining verbal or written consent with patients prior to performing dermatologic procedures. “You want to discuss material risk, that is, What’s the likely significant risk? What’s a viable alternative? Do you think it’s viable? And if not, be prepared with data to support that. And what’s your rationale of benefit?” he said.
In a study that evaluated informed consent in dermatologic surgery, 85 patients undergoing Mohs surgery were given verbal and written instructions, including information about the potential for 10 possible complications (Dermatol Surg. 2003;29[9]:952-5). The researchers asked the patients to recall the 10 complications at 20 minutes and at 1 week after the informed consent process. The overall group retention rates for both time periods were 27% and 24%, respectively.
“The reality is, people are nervous,” said Dr. Torres, who was not involved with the study. “They’re not focusing well and may not be listening very carefully to you. So when you give them informed consent you need to help them focus on the important issues. Be cautious about diluting the discussion with so much information that they’re not getting the message.”
Lawsuits involving the use of lasers in dermatology are on the rise, according to one study that examined trends in legal cases secondary to cutaneous laser surgery over a 28-year period that peaked in 2010 (JAMA Dermatol. 2013;149[2]:188-93). It found that laser hair removal was the most commonly litigated procedure (63%), followed by lack of informed consent (53%). Nearly half of the cases favored the defendant and the mean indemnity payment was $380,719.
This is in contrast with commonly reported litigation trends where a majority of the cases favor the defendant. The study also found that nearly 40% of cases involved a nonphysician operator. “So you need to be extra careful when you’re dealing with laser procedures, or you’re dealing with nonphysician operators or extenders,” Dr. Torres noted. “In that regard, know the rules in your state; make sure that you’re clear on them. Make sure that the people who are going to [perform the procedures] are appropriately trained. Provide an adequate degree of supervision to make sure that the proper procedure is being followed, especially as it relates to informed consent.”
Another liability risk for clinicians is failing to follow up with patients. “As a doctor, you may have the responsibility to make sure the patient actually saw the specialist and that their reports were acted upon,” Dr. Torres said. “The law requires that you interact with the specialist in the patients’ best interest. Also, if you refer a patient to another doctor and you have a reason to think that [doctor is] incompetent, you may be held accountable. Referring to the wrong specialist can be a pitfall.”
Dermatologists may at times also be liable for providing interpreter services for patients, no matter the size of their office or the number of employees on their payroll. He recommended that physicians explore whether the Canopy Medical Translator APP, a technology that enables clinicians to communicate with patients in 15 languages, can prove useful to them. Funded by the National Institutes of Health, the technology can be run on any device that runs on iOS or Android. “It can take phrases you have and translate them, or translate phrases that patients have to you,” Dr. Torres said.
In his clinical experience, dermatologists can protect themselves from a legal standpoint by maintaining honesty with the patient; showing kindness and concern at each encounter; validating the patient’s complaints about complications without conveying blame; avoiding isolating the patient after a complication; having a remedy planned for the complication, and seeing and communicating with the patient frequently.
When things go wrong, he offered the “AAA” mnemonic: Always acknowledge a complaint and express empathy; make sure someone you designate is easily accessible to the patient making a complaint, and avoid premature conclusions or comments. “Why? Because you want to maintain honesty with your patients, you want to show kindness and concern and validate patients’ emotions,” Dr. Torres said. “In other words, treat them as you would like to be treated.”
He reported having no financial disclosures.
EXPERT ANALYSIS AT PDA 2016
Delirium ABCDEF Bundle Program Implementation Toolkit Now Available
The Baylor Research Institute and SHM joined forces to provide a new resource to help accelerate adoption of a specific set of patient safety practices (collectively termed the ABCDEF bundle) to mitigate delirium in the ICU. This guide will allow you to impact care at both the individual patient and the institutional levels. It is intended for the broad, multidisciplinary spectrum of personnel involved in hospital-based quality improvement and patient safety efforts, ranging from frontline care providers to executive leaders. View the toolkit and download the guide at www.hospitalmedicine.org/delirium.
The Baylor Research Institute and SHM joined forces to provide a new resource to help accelerate adoption of a specific set of patient safety practices (collectively termed the ABCDEF bundle) to mitigate delirium in the ICU. This guide will allow you to impact care at both the individual patient and the institutional levels. It is intended for the broad, multidisciplinary spectrum of personnel involved in hospital-based quality improvement and patient safety efforts, ranging from frontline care providers to executive leaders. View the toolkit and download the guide at www.hospitalmedicine.org/delirium.
The Baylor Research Institute and SHM joined forces to provide a new resource to help accelerate adoption of a specific set of patient safety practices (collectively termed the ABCDEF bundle) to mitigate delirium in the ICU. This guide will allow you to impact care at both the individual patient and the institutional levels. It is intended for the broad, multidisciplinary spectrum of personnel involved in hospital-based quality improvement and patient safety efforts, ranging from frontline care providers to executive leaders. View the toolkit and download the guide at www.hospitalmedicine.org/delirium.
Become an SHM Ambassador for a Chance at Free Registration to HM17
- A $35 credit toward 2017–2018 dues when recruiting 1 new member.
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members.
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members.
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members.
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to Hospital Medicine 2017 in Las Vegas.
- A $35 credit toward 2017–2018 dues when recruiting 1 new member.
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members.
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members.
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members.
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to Hospital Medicine 2017 in Las Vegas.
- A $35 credit toward 2017–2018 dues when recruiting 1 new member.
- A $50 credit toward 2017–2018 dues when recruiting 2–4 new members.
- A $75 credit toward 2017–2018 dues when recruiting 5–9 new members.
- A $125 credit toward 2017–2018 dues when recruiting 10+ new members.
For each member recruited, referrers will receive one entry into a grand-prize drawing to receive complimentary registration to Hospital Medicine 2017 in Las Vegas.
Improving communication between cancer pts and docs

©ASCO/Todd Buchanan 2016
SAN FRANCISCO—Results of the VOICE study showed that training advanced cancer patients and their oncologists on how to communicate resulted in more clinically meaningful discussions between the parties.
However, these discussions did not significantly improve patients’ understanding of their prognosis, have a significant impact on their quality of life or end-of-life care, or significantly improve the
patient-physician relationship.
Ronald Epstein, MD, of the University of Rochester in New York, and his colleagues reported results from this study in JAMA Oncology and at the 2016 Palliative Care in Oncology Symposium (abstract 2).
The VOICE study included 265 patients with stage 3 or 4 cancer, 130 of whom received communication training. As part of the training, patients received a booklet Dr Epstein’s team wrote called “My Cancer Care: What Now? What Next? What I Prefer.”
The patients and their caregivers also met with social workers or nurses to discuss commonly asked questions and how to express their fears, for example, or how to be assertive and state their preferences.
Of the 38 oncologists studied, 19 received communication training. This included mock office sessions with actors (known as standardized patients), video training, and individualized feedback.
Later, the researchers audio-recorded real sessions between the oncologists and patients, then asked both groups to fill out questionnaires. The team coded the interactions and matched the scores to the goals of the training.
Results
The study’s primary endpoint was a composite of 4 communication measures—engaging patients in consultations, responding to emotions, informing patients about prognosis and treatment choices, and balanced framing of information.
The researchers found that communication training resulted in a significant improvement in this endpoint (P=0.02).
“We have shown, in the first large study of its kind, that it is possible to change the conversation in advanced cancer,” Dr Epstein said. “This is a huge first step.”
However, when Dr Epstein and his colleagues looked at the individual components of the endpoint, only the engaging measure was significantly different between the intervention and control groups.
Communication training had no significant effect on the patient-physician relationship, patients’ quality of life, or healthcare utilization at the end of life.
Likewise, communication training had no significant effect on patients’ understanding of their prognosis, which was assessed by the discordance in 2-year survival estimates and curability estimates between patients and physicians.
“We need to try harder to communicate well so that it’s harder to miscommunicate,” Dr Epstein said. “Simply having the conversation is not enough. The quality of the conversation will influence a mutual understanding between patients and their oncologists.”
The researchers said a limitation of this study may have been the timing of the training, which was only provided once and not timed to key decision points during patients’ trajectories. The effects of the training may have waned over the months, especially as the cancer progressed.
“We need to embed communication interventions into the fabric of everyday clinical care,” Dr Epstein said. “This does not take a lot of time, but, in our audio-recordings, there was precious little dialogue that reaffirmed the human experience and the needs of patients. The next step is to make good communication the rule, not the exception, so that cancer patients’ voices can be heard.” ![]()

©ASCO/Todd Buchanan 2016
SAN FRANCISCO—Results of the VOICE study showed that training advanced cancer patients and their oncologists on how to communicate resulted in more clinically meaningful discussions between the parties.
However, these discussions did not significantly improve patients’ understanding of their prognosis, have a significant impact on their quality of life or end-of-life care, or significantly improve the
patient-physician relationship.
Ronald Epstein, MD, of the University of Rochester in New York, and his colleagues reported results from this study in JAMA Oncology and at the 2016 Palliative Care in Oncology Symposium (abstract 2).
The VOICE study included 265 patients with stage 3 or 4 cancer, 130 of whom received communication training. As part of the training, patients received a booklet Dr Epstein’s team wrote called “My Cancer Care: What Now? What Next? What I Prefer.”
The patients and their caregivers also met with social workers or nurses to discuss commonly asked questions and how to express their fears, for example, or how to be assertive and state their preferences.
Of the 38 oncologists studied, 19 received communication training. This included mock office sessions with actors (known as standardized patients), video training, and individualized feedback.
Later, the researchers audio-recorded real sessions between the oncologists and patients, then asked both groups to fill out questionnaires. The team coded the interactions and matched the scores to the goals of the training.
Results
The study’s primary endpoint was a composite of 4 communication measures—engaging patients in consultations, responding to emotions, informing patients about prognosis and treatment choices, and balanced framing of information.
The researchers found that communication training resulted in a significant improvement in this endpoint (P=0.02).
“We have shown, in the first large study of its kind, that it is possible to change the conversation in advanced cancer,” Dr Epstein said. “This is a huge first step.”
However, when Dr Epstein and his colleagues looked at the individual components of the endpoint, only the engaging measure was significantly different between the intervention and control groups.
Communication training had no significant effect on the patient-physician relationship, patients’ quality of life, or healthcare utilization at the end of life.
Likewise, communication training had no significant effect on patients’ understanding of their prognosis, which was assessed by the discordance in 2-year survival estimates and curability estimates between patients and physicians.
“We need to try harder to communicate well so that it’s harder to miscommunicate,” Dr Epstein said. “Simply having the conversation is not enough. The quality of the conversation will influence a mutual understanding between patients and their oncologists.”
The researchers said a limitation of this study may have been the timing of the training, which was only provided once and not timed to key decision points during patients’ trajectories. The effects of the training may have waned over the months, especially as the cancer progressed.
“We need to embed communication interventions into the fabric of everyday clinical care,” Dr Epstein said. “This does not take a lot of time, but, in our audio-recordings, there was precious little dialogue that reaffirmed the human experience and the needs of patients. The next step is to make good communication the rule, not the exception, so that cancer patients’ voices can be heard.” ![]()

©ASCO/Todd Buchanan 2016
SAN FRANCISCO—Results of the VOICE study showed that training advanced cancer patients and their oncologists on how to communicate resulted in more clinically meaningful discussions between the parties.
However, these discussions did not significantly improve patients’ understanding of their prognosis, have a significant impact on their quality of life or end-of-life care, or significantly improve the
patient-physician relationship.
Ronald Epstein, MD, of the University of Rochester in New York, and his colleagues reported results from this study in JAMA Oncology and at the 2016 Palliative Care in Oncology Symposium (abstract 2).
The VOICE study included 265 patients with stage 3 or 4 cancer, 130 of whom received communication training. As part of the training, patients received a booklet Dr Epstein’s team wrote called “My Cancer Care: What Now? What Next? What I Prefer.”
The patients and their caregivers also met with social workers or nurses to discuss commonly asked questions and how to express their fears, for example, or how to be assertive and state their preferences.
Of the 38 oncologists studied, 19 received communication training. This included mock office sessions with actors (known as standardized patients), video training, and individualized feedback.
Later, the researchers audio-recorded real sessions between the oncologists and patients, then asked both groups to fill out questionnaires. The team coded the interactions and matched the scores to the goals of the training.
Results
The study’s primary endpoint was a composite of 4 communication measures—engaging patients in consultations, responding to emotions, informing patients about prognosis and treatment choices, and balanced framing of information.
The researchers found that communication training resulted in a significant improvement in this endpoint (P=0.02).
“We have shown, in the first large study of its kind, that it is possible to change the conversation in advanced cancer,” Dr Epstein said. “This is a huge first step.”
However, when Dr Epstein and his colleagues looked at the individual components of the endpoint, only the engaging measure was significantly different between the intervention and control groups.
Communication training had no significant effect on the patient-physician relationship, patients’ quality of life, or healthcare utilization at the end of life.
Likewise, communication training had no significant effect on patients’ understanding of their prognosis, which was assessed by the discordance in 2-year survival estimates and curability estimates between patients and physicians.
“We need to try harder to communicate well so that it’s harder to miscommunicate,” Dr Epstein said. “Simply having the conversation is not enough. The quality of the conversation will influence a mutual understanding between patients and their oncologists.”
The researchers said a limitation of this study may have been the timing of the training, which was only provided once and not timed to key decision points during patients’ trajectories. The effects of the training may have waned over the months, especially as the cancer progressed.
“We need to embed communication interventions into the fabric of everyday clinical care,” Dr Epstein said. “This does not take a lot of time, but, in our audio-recordings, there was precious little dialogue that reaffirmed the human experience and the needs of patients. The next step is to make good communication the rule, not the exception, so that cancer patients’ voices can be heard.” ![]()
Ticagrelor slashes first stroke risk after MI
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
ROME – Adding ticagrelor at 60 mg twice daily in patients on low-dose aspirin due to a prior MI reduced their risk of a first stroke by 25% in a secondary analysis of the landmark PEGASUS-TIMI 54 trial, Marc P. Bonaca, MD, reported at the annual congress of the European Society of Cardiology.
PEGASUS-TIMI 54 was a randomized, double-blind, placebo-controlled clinical trial conducted in more than 21,000 stable patients on low-dose aspirin with a history of an acute MI 1-3 years earlier. The significant reduction in secondary cardiovascular events seen in this study during a median 33 months of follow-up (N Engl J Med. 2015 May 7;372[19]:1791-800) led to approval of ticagrelor (Brilinta) at 60 mg twice daily for long-term secondary prevention.
But while PEGASUS-TIMI 54 was a secondary prevention study in terms of cardiovascular events, it was actually a primary prevention study in terms of stroke, since patients with a history of stroke weren’t eligible for enrollment. And in this trial, recipients of ticagrelor at 50 mg twice daily experienced a 25% reduction in the risk of stroke relative to placebo, from 1.94% at 3 years to 1.47%. This benefit was driven by fewer ischemic strokes, with no increase in hemorrhagic strokes seen with ticagrelor. And therein lies a clinical take home point: “When evaluating the overall benefits and risks of long-term ticagrelor in patients with prior MI, stroke reduction should also be considered,” according to Dr. Bonaca of Brigham and Women’s Hospital, Boston.
All strokes were adjudicated and subclassified by a blinded central committee. A total of 213 stroke events occurred during follow-up: 81% ischemic, 7% hemorrhagic, 4% ischemic with hemorrhagic conversion, and 8% unknown; 18% of the strokes were fatal. Another 15% resulted in moderate or severe disability at 30 days. All PEGASUS-TIMI 54 participants were on aspirin and more than 90% were on statin therapy.
The strokes that occurred in patients on ticagrelor were generally less severe than in controls. The risk of having a modified Rankin score of 3-6, which encompasses outcomes ranging from moderate disability to death, was reduced by 43% in stroke patients on ticagrelor relative to those on placebo, the cardiologist continued.
To ensure that the stroke benefit with ticagrelor seen in PEGASUS-TIMI 54 wasn’t a fluke, Dr. Bonaca and his coinvestigators performed a meta-analysis of four placebo-controlled randomized trials of more intensive versus less intensive antiplatelet therapy in nearly 45,000 participants with coronary disease in the CHARISMA, DAPT, PEGASUS-TIMI 54, and TRA 2*P-TIMI 50 trials. A total of 532 strokes occurred in this enlarged analysis. More intensive antiplatelet therapy – typically adding a second drug to low-dose aspirin – resulted in a 34% reduction in ischemic stroke, compared with low-dose aspirin and placebo.
Excluding from the meta-analysis the large subgroup of patients in TRA 2*P-TIMI 50 who were on triple-drug antiplatelet therapy, investigators were left with 32,348 participants in the four trials who were randomized to dual-antiplatelet therapy or monotherapy with aspirin. In this population, there was no increase in the risk of hemorrhagic stroke associated with more intensive antiplatelet therapy, according to Dr. Bonaca.
Session co-chair Keith A.A. Fox, MD, of the University of Edinburgh, noted that various studies have shown monotherapy with aspirin or another antiplatelet agent reduces stroke risk by about 15%, and now PEGASUS-TIMI 54 shows that ticagrelor plus aspirin decreases stroke risk by 25%. He posed a direct question: “How much is too much?”
“More and more antiplatelet therapy begets more bleeding, so I think that more than two agents may be approaching too much, although it really depends on what agents you’re using and in what dosages,” Dr. Bonaca replied.
He reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Simultaneous with Dr. Bonaca’s presentation at ESC 2016 in Rome, the new report from PEGASUS-TIMI 54 including the four-trial meta-analysis was published online (Circulation. 2016 Aug 30. doi: circulationaha.116.024637).
AT THE ESC CONGRESS 2016
Key clinical point: Ticagrelor reduced the risk of a first stroke by 25% in patients with a prior MI.
Major finding: Ticagrelor, at the approved dose of 60 mg twice daily for long-term secondary cardiovascular prevention, reduced the risk of a first stroke by 25% in patients with a prior MI.
Data source: This secondary analysis of a randomized, double-blind, placebo-controlled trial included 14,112 stable patients with a prior MI 1-3 years earlier who were randomized to ticagrelor at 60 mg twice daily or placebo and followed prospectively for a median of 33 months.
Disclosures: PEGASUS-TIMI 54 was supported by AstraZeneca. The presenter of the updated analysis reported serving as a consultant to AstraZeneca, Merck, and Bayer.
Trials offer lessons despite negative primary endpoints
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Conventional wisdom holds that for randomized, controlled trials, it’s all about the primary endpoint. If it’s negative or neutral, then none of the other results means much beyond “hypothesis generating.”
This strict-constructionist thinking has now been called into question. A recent article in the New England Journal of Medicine declared “an unreasonable yet widespread practice is the labeling of all randomized trials as either positive or negative on the basis of whether the P value for the primary outcome is less than .05. This view is overly simplistic.” (2016 Sept 1;375[9]:861-70).
The article, by the highly experienced and respected trialists Stuart J. Pocock, PhD, and Gregg W. Stone, MD, adds this: “If the primary outcome is negative, positive findings for secondary outcomes are usually considered to be hypothesis generating. Certainly, regulatory approval of a new drug is unlikely to follow. However, in some instances, secondary findings are compelling enough to affect guidelines and practice.”
This unconventional take from a pair of high-level trialists was especially timely given the buzz around the results from two studies reported at the European Society of Cardiology annual congress in late August, DANISH and NORSTENT.
The DANISH trial compared the impact of implantable cardioverter-defibrillators (ICDs) plus optimal care against optimal care without ICDs in 1,116 patients with nonischemic systolic heart failure. The primary outcome, all-cause death during more than 5 years of follow-up, was a relative 13% less with ICD use, a difference that was not statistically significant, and one secondary outcome, cardiovascular death, was cut by a relative 25% with ICD use, also not statistically significant.
But for the study’s second prespecified secondary endpoint of sudden cardiac death, treatment with ICDs cut the rate in half, compared with nonischemic heart failure patients who did not receive an ICD, a 4-percentage-point difference that was statistically significant.
And in a prespecified secondary analysis of the primary endpoint that broke down the study group by age, the two-thirds of patients younger than 68 years had a significant reduction in all-cause mortality with ICD use, a benefit not seen in patients aged 68 or older.
Discussion of the results at the meeting mainly focused on what meaning, if any, could be drawn from these strongly positive secondary outcomes in a trial neutral for its primary outcome.
“The ICDs did what they were supposed to, prevent sudden cardiac death,” said the lead investigator of the study, Lars Køber, MD. “As a principle I say don’t believe in a subgroup, but guidelines are often based on subgroup analyses.”
“The primary outcome was neutral, but the reduction in sudden cardiac death, the primary objective of an ICD, was significant, so an ICD should be taken into consideration,” commented Michel Komajda, MD, a discussant for the report.
After I wrote a news article about the DANISH report at ESC, I received an email from a reader who objected to spinning the results this way and insisted that no valid lessons can be drawn from the DANISH results because the study’s primary endpoint failed to show a statistical significance. This purist view misses the important, relevant lessons from the DANISH results. The DANISH trial was not designed to provide pivotal data for regulatory approval of ICDs in these patients. Rather, Dr. Køber and his associates designed DANISH to see whether ICD use in these patients could cut all-cause death over a fairly long follow-up. It was a very high bar and ICDs failed, but the deck was stacked against an ICD win. Enrolled patients averaged 64 years old at entry into the study, and they all had New York Heart Association class II or III heart failure. “The overall survival curves start to diverge, but then converge after 5 years because of the comorbidities and patients dying for other reasons,” Dr. Køber noted.
“The message is, in younger patients with less morbidity and more life expectancy, sudden cardiac death is a bigger problem, and they had a substantial drop in mortality” with ICD use, commented heart failure specialist Javed Butler, MD. “It’s very consistent with the way we think about providing ICD treatment to patients.”
In other words, the DANISH results showed that all patients with nonischemic systolic heart failure can’t expect to live substantially longer during extended follow-up if they get an ICD, because the cut in sudden cardiac death the devices provide eventually gets washed out by the many other risks for death these patients face. But younger, relatively healthier patients might very well see their reduced rate of sudden cardiac death translate into an overall mortality benefit even when they are followed for at least 5 years. That’s important information to help an individual patient decide whether to have an ICD placed, and an important message from the DANISH trial despite the neutral primary endpoint.
NORSTENT involved a similar scenario in a trial that addressed a totally different issue: Should patients with either stable or unstable coronary artery disease who are undergoing coronary stenting receive a drug-eluting stent (DES) or a bare metal stent (BMS)? The trial randomized 9,013 patients to receive either of the two stent types plus optimal medical therapy. The primary endpoint was the rate of all-cause death or nonfatal MI during 5 years of follow-up, and the results showed no statistically significant difference between the patients who received a DES and those who got a BMS (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
But for the secondary endpoint of repeat revascularizations performed during follow-up, the use of a DES cut the procedure rate by 3.3 percentage points, a 17% relative risk reduction that was statistically significant. The use of a DES also cut the stent thrombosis rate by 0.4 percentage points, a one-third relative drop in these events that was also statistically significant.
In short, despite the neutral primary endpoint for the trial, the results showed that drug-eluting stents did what they were designed to do relative to bare metal stents: cut the rate of target lesion restenosis and the need for repeat revascularization. Several interventional cardiologists who heard the results at the meeting said that the findings would not change their practice and that they would continue to use the DES as their default device for percutaneous coronary interventions. Although “the long-term benefit of contemporary DES over BMS was less than expected,” said Kaare H. Bønaa, MD, lead investigator for the NORSTENT trial, the secondary benefit of significantly reduced repeat revascularization and the very modest price difference that now exists between drug-eluting stents and bare metal stents means that many interventionalists will continue to use a DES for most patients.
The message from Dr. Pocock and Dr. Stone, underscored by the DANISH and NORSTENT results, is that large and well-run randomized trials can yield important evidence to inform practice that transcends a simple black or white statistical assessment of the primary endpoint.
On Twitter @mitchelzoler
Pediatric Dermatology Consult - September 2016
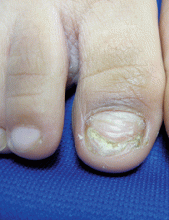
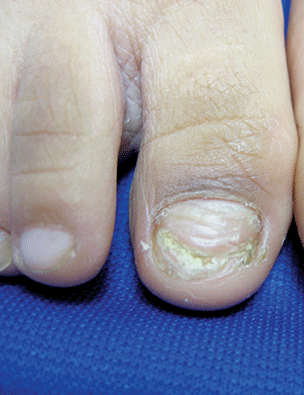
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
A 5-year-old boy presents to his physician for evaluation of "toenail issues" for at least 1 year. The family has noticed changes of the his right great toenail, which they thought might be due to "tight shoes," stating that the boy has been growing out of his shoes quickly. In the last 6 months, his mother has noted a "crumbly" nail with yellow discoloration. There has been no prior treatment, although his parents now are replacing his sneakers more regularly to allow him "room to grow." He has no history of toe swelling or pain. He is otherwise healthy, and he has no history of psoriasis or eczema. He has had no significant viral infections, although some children in his school did have hand, foot, and mouth disease several months ago. His mother states that her husband has athlete's foot, which has been treated with "creams and sprays." Physical exam The toenail of the right foot great toe has thickening of the distal part of the nail, with onycholysis (separation of the nail plate from the nail bed), yellow discoloration, and subungual debris. The right toe shows some chronic dystrophy. Other toenails appear normal, and the skin of the feet is otherwise unremarkable.
VIDEO: When geriatric depression turns psychotic
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
10 recommendations for the Cancer Moonshot
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
6 Tips for Community Hospitalists Initiating QI Projects
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.