User login
New-onset pediatric AD phenotype differs from adult AD
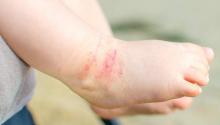
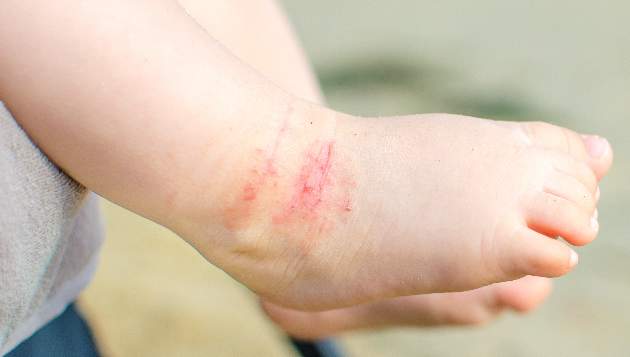
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Severe AD places a huge burden on patients, their families, and society in terms of health care dollars spent and lost work days. Considering the prevalence of AD, both families and dermatologists find it understandably frustrating that we have limited and often ineffective tools to treat severe AD in children. Change may be around the corner.
This study by Esaki et al. sheds critical light on the pathogenesis of early onset AD in children and we hope it will set the stage to revolutionize the treatment of AD using the paradigm of psoriasis as a model. Using lesional and nonlesional biopsies from 19 children under age 5 obtained during the first 6 months of onset of AD, Esaki et al. have demonstrated that children with AD have a multicytokine inflammatory infiltrate with Th17 predominance. This sets the stage for biologics focused on the Th17 pathway in these children, although multimodal therapy to address different cytokines may ultimately be required.
The investigators also found that children with AD had similar filaggrin expression compared to control children, implying that atopic dermatitis is at its heart an immunologic disorder rather than a barrier defect although we will likely continue to learn more about this fine balance.
As pediatric dermatologists on the front line caring for patients with severe AD, we welcome further studies and especially look forward to effective treatments for our patients who might finally experience relief of itch, clear skin, and a good night’s sleep.
A. Yasmine Kirkorian, MD, and Kalyani Marathe, MD, are pediatric dermatologists at Children’s National Health System, in the departments of dermatology and pediatrics at George Washington University, Washington, DC. They are on the editorial advisory board of Dermatology News. They had no disclosures.
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, according to an assessment of biopsy findings in infants and children.
The study findings have important therapeutic implications, especially in light of the fact that much of the work in this area has been based on adult biomarkers, reflecting “decades of disease activity and chronic use of immunosuppressants in adults,” the investigators reported. Little is known about alterations in early lesions in children, which limits the advancement of targeted therapies, Hitokazu Esaki, MD, of the Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported online in the Journal of Allergy and Clinical Immunology (2016 Sep 22. doi: 10.1016/j.jaci.2016.07.013).
To characterize early pediatric AD skin phenotype, the investigators assessed lesional and nonlesional biopsies from 19 children under age 5 years (mean, 1.3 years) within 6 months of moderate to severe disease onset, as well as those from age-matched controls and adults, and found that, compared with adult AD, early AD involves comparable or greater epidermal hyperplasia and cellular infiltration, similar strong activation of Th2 and Th22 axes, and some Th1 skewing.
In addition, early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD. Expression of filaggrin – an abundant barrier differentiation protein – was similar in AD and healthy children, whereas down-regulation is characteristic in adult AD, the investigators noted.
Nonlesional skin biopsies from the children showed both higher levels of inflammation and epidermal proliferation markers, they said.
The “surprising findings” of an early multicytokine response in new-onset pediatric AD, characterized by marked Th17, Th9, Th2, and Th22 activation, suggest that targeting of multiple cytokine axes may be needed in children with early-onset AD, one of the lead authors on the study, Emma Guttman-Yassky, MD, also of the Icahn School of Medicine at Mount Sinai, said in an interview.
Dr. Guttman-Yassky, who noted that the study was conducted in close collaboration with Amy S. Paller, MD, of Northwestern University, Chicago, explained that early AD, compared with adult AD, involves differential immune skewing and barrier responses with features that are in some ways comparable to those of psoriasis – particularly with respect to the consistently higher levels of Th17-related mediators in childhood AD, as psoriasis is considered a Th17-centered disease.
Further, the findings with respect to filaggrin represent another important aspect of the study, she said, noting that they represent a possible challenge to the notion that filaggrin is integral to disease elicitation and instigation of the “atopic march.”
The study findings may suggest novel targets for pediatric AD, and they also suggest a need for early immune intervention, not only to treat the AD, but also to prevent the atopic march, she said.
“These findings are likely to result in both different understanding of AD onset and distinct treatment approaches for infants and children,” she and her colleagues concluded.
This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Key clinical point: The skin phenotype of new-onset pediatric atopic dermatitis differs substantially from that in adult AD, which has important therapeutic implications, according to a study of biopsy findings in infants and children.
Major finding: Early AD involves significantly higher induction of Th17-related cytokines, compared with adult AD.
Data source: An analysis of biopsies from 19 children with AD.
Disclosures: This work was funded by a research grant from the LEO Foundation. Individual authors were supported by grants from the National Center for Advancing Translational Sciences and the National Institutes of Health Clinical and Translational Science Award program. Dr. Esaki reported having no disclosures. Dr. Guttman-Yassky reported financial relationships with numerous pharmaceutical companies.
University of Pennsylvania Orphan Disease Center Posts RFPs
Grants made possible by the “Million Dollar Bike Ride” and rare disease patient organizations are now available through the University of Pennsylvania Orphan Disease Center. September 16 is the deadline for letters of intent.
The diseases for which research funding is available are: adult polyglucosan body disease; ataxia-telangiectasia; Castleman disease; CFTR nonsense mutations; congenital hyperinsulinism; congenital muscular dystrophy; CRB1 degenerative retinal disease; nonsense mutations in cystic fibrosis; dyskeratosis congenital & telomere biology disorders; fibrous dysplasia/McCune Albright syndrome; generalized lymphatic anomaly and Gorham-Stout disease; Glut 1 deficiency; lymphangioleiomyomatosis; mucolipidosis type IV; mucopolysaccharidoses; Niemann Pick type C; Pitt Hopkins syndrome; RASopathies; Tay-Sachs, Sandhoff, GM1, or Canavan disease; and Snyder-Robinson syndrome.
Grants made possible by the “Million Dollar Bike Ride” and rare disease patient organizations are now available through the University of Pennsylvania Orphan Disease Center. September 16 is the deadline for letters of intent.
The diseases for which research funding is available are: adult polyglucosan body disease; ataxia-telangiectasia; Castleman disease; CFTR nonsense mutations; congenital hyperinsulinism; congenital muscular dystrophy; CRB1 degenerative retinal disease; nonsense mutations in cystic fibrosis; dyskeratosis congenital & telomere biology disorders; fibrous dysplasia/McCune Albright syndrome; generalized lymphatic anomaly and Gorham-Stout disease; Glut 1 deficiency; lymphangioleiomyomatosis; mucolipidosis type IV; mucopolysaccharidoses; Niemann Pick type C; Pitt Hopkins syndrome; RASopathies; Tay-Sachs, Sandhoff, GM1, or Canavan disease; and Snyder-Robinson syndrome.
Grants made possible by the “Million Dollar Bike Ride” and rare disease patient organizations are now available through the University of Pennsylvania Orphan Disease Center. September 16 is the deadline for letters of intent.
The diseases for which research funding is available are: adult polyglucosan body disease; ataxia-telangiectasia; Castleman disease; CFTR nonsense mutations; congenital hyperinsulinism; congenital muscular dystrophy; CRB1 degenerative retinal disease; nonsense mutations in cystic fibrosis; dyskeratosis congenital & telomere biology disorders; fibrous dysplasia/McCune Albright syndrome; generalized lymphatic anomaly and Gorham-Stout disease; Glut 1 deficiency; lymphangioleiomyomatosis; mucolipidosis type IV; mucopolysaccharidoses; Niemann Pick type C; Pitt Hopkins syndrome; RASopathies; Tay-Sachs, Sandhoff, GM1, or Canavan disease; and Snyder-Robinson syndrome.
OMA publishes pediatric obesity treatment guide
The Pediatric Obesity Algorithm – a free state-of-the-science management review from the Obesity Medicine Association – should help clinicians weed through the options for overweight kids, according to Texas pediatrician and lead author Suzanne Cuda, MD.
The group put the latest thinking into one place to serve as a handy guide, and plans to update it every 2 years. The idea was to present choices, not push particular approaches. “Many of my colleagues have expressed concern [that] they do not have the resources to provide the kind of support these kids need,” said Dr. Cuda, director of the weight management clinic at the Children’s Hospital of San Antonio and associate professor of pediatrics at the Baylor College of Medicine. Clinicians can find the guide at www.PediatricObesityAlgorithm.org.
The effort also will help clinicians prepare for American Board of Obesity Medicine certification, since it was “designed to address the content on the exam. A lot of tools out there for children [emphasize] prevention. Our starting point was children who are already overweight,“ she said.
The document covers risk factors, differential diagnoses, assessment, diet, appropriate activity levels, medications, surgery, comorbidity management, and other issues, often broken down by age and body mass index.
Nothing is particularly controversial, although some clinicians are reluctant to move beyond diet and exercise for kids, Dr. Cuda said.
The Obesity Medicine Association (OMA) was aware of that, and so was careful to note, for instance, which obesity medications are approved for pediatric use – orlistat (Xenical), metformin, and phentermine – and their real-world effect.
“We didn’t cover all the drugs out there” because many haven’t been tested in children, Dr. Cuda said. The group also highlights antiseizure and other drugs that put on weight.
The guide covers birth to adulthood. There can be signs of problems even before the first birthday, such as weight above the 95th percentile. In those cases, evidence supports exclusive breast feeding for as long as possible, and no more than 24 ounces per day in formula-fed children, with no cereal or media watching.
OMA hasn’t submitted its work for endorsement by other groups, so other associations haven’t signed onto it. “We didn’t want to prolong putting it out there to go through that whole process,” Dr. Cuda said.
Even so, some organizations are aware of the contents and support the effort. “It aligns with the resources we have already developed on this topic. These are core competencies ... all physicians should have, regardless of whether they are certified in obesity medicine,” said an American Academy of Pediatrics staff member.
Until recently, OMA was known as the American Society of Bariatric Physicians. It rebranded itself to avoid being mistaken for a bariatric surgery group.
Dr. Cuda and the other authors had no disclosures. There was no industry funding for the work.
The Pediatric Obesity Algorithm – a free state-of-the-science management review from the Obesity Medicine Association – should help clinicians weed through the options for overweight kids, according to Texas pediatrician and lead author Suzanne Cuda, MD.
The group put the latest thinking into one place to serve as a handy guide, and plans to update it every 2 years. The idea was to present choices, not push particular approaches. “Many of my colleagues have expressed concern [that] they do not have the resources to provide the kind of support these kids need,” said Dr. Cuda, director of the weight management clinic at the Children’s Hospital of San Antonio and associate professor of pediatrics at the Baylor College of Medicine. Clinicians can find the guide at www.PediatricObesityAlgorithm.org.
The effort also will help clinicians prepare for American Board of Obesity Medicine certification, since it was “designed to address the content on the exam. A lot of tools out there for children [emphasize] prevention. Our starting point was children who are already overweight,“ she said.
The document covers risk factors, differential diagnoses, assessment, diet, appropriate activity levels, medications, surgery, comorbidity management, and other issues, often broken down by age and body mass index.
Nothing is particularly controversial, although some clinicians are reluctant to move beyond diet and exercise for kids, Dr. Cuda said.
The Obesity Medicine Association (OMA) was aware of that, and so was careful to note, for instance, which obesity medications are approved for pediatric use – orlistat (Xenical), metformin, and phentermine – and their real-world effect.
“We didn’t cover all the drugs out there” because many haven’t been tested in children, Dr. Cuda said. The group also highlights antiseizure and other drugs that put on weight.
The guide covers birth to adulthood. There can be signs of problems even before the first birthday, such as weight above the 95th percentile. In those cases, evidence supports exclusive breast feeding for as long as possible, and no more than 24 ounces per day in formula-fed children, with no cereal or media watching.
OMA hasn’t submitted its work for endorsement by other groups, so other associations haven’t signed onto it. “We didn’t want to prolong putting it out there to go through that whole process,” Dr. Cuda said.
Even so, some organizations are aware of the contents and support the effort. “It aligns with the resources we have already developed on this topic. These are core competencies ... all physicians should have, regardless of whether they are certified in obesity medicine,” said an American Academy of Pediatrics staff member.
Until recently, OMA was known as the American Society of Bariatric Physicians. It rebranded itself to avoid being mistaken for a bariatric surgery group.
Dr. Cuda and the other authors had no disclosures. There was no industry funding for the work.
The Pediatric Obesity Algorithm – a free state-of-the-science management review from the Obesity Medicine Association – should help clinicians weed through the options for overweight kids, according to Texas pediatrician and lead author Suzanne Cuda, MD.
The group put the latest thinking into one place to serve as a handy guide, and plans to update it every 2 years. The idea was to present choices, not push particular approaches. “Many of my colleagues have expressed concern [that] they do not have the resources to provide the kind of support these kids need,” said Dr. Cuda, director of the weight management clinic at the Children’s Hospital of San Antonio and associate professor of pediatrics at the Baylor College of Medicine. Clinicians can find the guide at www.PediatricObesityAlgorithm.org.
The effort also will help clinicians prepare for American Board of Obesity Medicine certification, since it was “designed to address the content on the exam. A lot of tools out there for children [emphasize] prevention. Our starting point was children who are already overweight,“ she said.
The document covers risk factors, differential diagnoses, assessment, diet, appropriate activity levels, medications, surgery, comorbidity management, and other issues, often broken down by age and body mass index.
Nothing is particularly controversial, although some clinicians are reluctant to move beyond diet and exercise for kids, Dr. Cuda said.
The Obesity Medicine Association (OMA) was aware of that, and so was careful to note, for instance, which obesity medications are approved for pediatric use – orlistat (Xenical), metformin, and phentermine – and their real-world effect.
“We didn’t cover all the drugs out there” because many haven’t been tested in children, Dr. Cuda said. The group also highlights antiseizure and other drugs that put on weight.
The guide covers birth to adulthood. There can be signs of problems even before the first birthday, such as weight above the 95th percentile. In those cases, evidence supports exclusive breast feeding for as long as possible, and no more than 24 ounces per day in formula-fed children, with no cereal or media watching.
OMA hasn’t submitted its work for endorsement by other groups, so other associations haven’t signed onto it. “We didn’t want to prolong putting it out there to go through that whole process,” Dr. Cuda said.
Even so, some organizations are aware of the contents and support the effort. “It aligns with the resources we have already developed on this topic. These are core competencies ... all physicians should have, regardless of whether they are certified in obesity medicine,” said an American Academy of Pediatrics staff member.
Until recently, OMA was known as the American Society of Bariatric Physicians. It rebranded itself to avoid being mistaken for a bariatric surgery group.
Dr. Cuda and the other authors had no disclosures. There was no industry funding for the work.
FROM THE OBESITY MEDICINE ASSOCIATION
Empiric warfarin adjustment cut drug-drug interactions with antimicrobials
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: Clinical guidelines with empiric warfarin adjustments improved time within therapeutic range (TWTR) for inpatients on antimicrobials.
Major finding: In-hospital TWTR increased to 72% from 50% before implementation of clinical guidelines (P = .043).
Data source: Retrospective, single-center study of inpatients on warfarin and antimicrobial with potential for DDI before (n = 78) and after (n = 31) implementation of a comprehensive clinical guideline.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
Less pain, quicker discharge with post-TORS dexamethasone
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
AT AHNS 2016
Key clinical point: A longer course of dexamethasone is a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery.
Major finding: The dexamethasone group had significantly less pain on post-op day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up.
Data source: A randomized trial of 68 TORS patients.
Disclosures: There was no external funding for the study, and the lead investigator had no relevant financial disclosures.
Lenvatinib sparked or worsened hypertension in patients with RAI-resistant thyroid cancer
DENVER – Patients put on lenvatinib for the management of radioactive iodine–resistant differentiated thyroid cancer need to be taught how to monitor their blood pressure, be given a cuff with which to do so, and be called daily by someone on the medical staff for at least the first 2 weeks, according to Sina A. Jasim, MD, reporting on real world use of the drug since its approval in February 2015.
For now, oncologists prescribe and manage this drug. But as lenvatinib (Lenvima, Eisai) becomes more widely used, endocrinologists can expect to be the ones prescribing it sometimes and counseling patients in the practical aspects of using this drug, Dr. Jasim of the Mayo Clinic, Rochester, Minn., said in an interview.
It was with endocrinologists in mind that Dr. Jasim and her associates compiled postapproval data on adverse events and patient quality of life. To date, no such data – including those from Mayo – have been published, she said during her presentation at the American Thyroid Association’s annual meeting.
While lenvatinib seems to be a promising therapeutic agent, adverse events are common with its use and occur early. Patients treated with it at Mayo get called by someone on the medical staff daily for the first 2 weeks of therapy, are given a blood pressure cuff, and are taught how to use it. They also receive the cell phone number of a member of the medical staff to consult with about sudden symptoms.
This retrospective analysis involved 25 sequentially treated patients given lenvatinib for RAI-resistant differentiated thyroid cancer (14 papillary, 7 poorly differentiated, 3 Hürthle cell, and 1 follicular). While all had received RAI, 11 also had received radiotherapy, 8 had been given at least one other kinase inhibitor previously, and 3 had received two. Fourteen were on an antihypertensive medication at baseline.
All patients initiated lenvatinib at the full dose, but it was reduced in four patients because of old age, renal impairment, or prior colitis. Twenty-one patients developed adverse events within the first month of being on the drug. Hypertension occurred in 16. Six of these required either a raising of the dose of antihypertensive drug they were on at baseline or initiation of antihypertensive therapy.
Adverse events were pronounced enough that the lenvatinib dose had to be lowered in 11 within a median 33 days of starting the drug. Drug treatment had to be interrupted for at least 3 weeks in four patients (two cases of cholecystitis, one case of diverticulitis, and one case of skin lesions).
Patients reported that their quality of life was stable at 2 months, but that their fatigue was worse.
The mean duration of lenvatinib therapy was 6.5 months. Twenty patients are alive at the time of this report.
The study was sponsored by the Mayo Clinic. Dr. Jasim reported that she had no relevant financial disclosures.
DENVER – Patients put on lenvatinib for the management of radioactive iodine–resistant differentiated thyroid cancer need to be taught how to monitor their blood pressure, be given a cuff with which to do so, and be called daily by someone on the medical staff for at least the first 2 weeks, according to Sina A. Jasim, MD, reporting on real world use of the drug since its approval in February 2015.
For now, oncologists prescribe and manage this drug. But as lenvatinib (Lenvima, Eisai) becomes more widely used, endocrinologists can expect to be the ones prescribing it sometimes and counseling patients in the practical aspects of using this drug, Dr. Jasim of the Mayo Clinic, Rochester, Minn., said in an interview.
It was with endocrinologists in mind that Dr. Jasim and her associates compiled postapproval data on adverse events and patient quality of life. To date, no such data – including those from Mayo – have been published, she said during her presentation at the American Thyroid Association’s annual meeting.
While lenvatinib seems to be a promising therapeutic agent, adverse events are common with its use and occur early. Patients treated with it at Mayo get called by someone on the medical staff daily for the first 2 weeks of therapy, are given a blood pressure cuff, and are taught how to use it. They also receive the cell phone number of a member of the medical staff to consult with about sudden symptoms.
This retrospective analysis involved 25 sequentially treated patients given lenvatinib for RAI-resistant differentiated thyroid cancer (14 papillary, 7 poorly differentiated, 3 Hürthle cell, and 1 follicular). While all had received RAI, 11 also had received radiotherapy, 8 had been given at least one other kinase inhibitor previously, and 3 had received two. Fourteen were on an antihypertensive medication at baseline.
All patients initiated lenvatinib at the full dose, but it was reduced in four patients because of old age, renal impairment, or prior colitis. Twenty-one patients developed adverse events within the first month of being on the drug. Hypertension occurred in 16. Six of these required either a raising of the dose of antihypertensive drug they were on at baseline or initiation of antihypertensive therapy.
Adverse events were pronounced enough that the lenvatinib dose had to be lowered in 11 within a median 33 days of starting the drug. Drug treatment had to be interrupted for at least 3 weeks in four patients (two cases of cholecystitis, one case of diverticulitis, and one case of skin lesions).
Patients reported that their quality of life was stable at 2 months, but that their fatigue was worse.
The mean duration of lenvatinib therapy was 6.5 months. Twenty patients are alive at the time of this report.
The study was sponsored by the Mayo Clinic. Dr. Jasim reported that she had no relevant financial disclosures.
DENVER – Patients put on lenvatinib for the management of radioactive iodine–resistant differentiated thyroid cancer need to be taught how to monitor their blood pressure, be given a cuff with which to do so, and be called daily by someone on the medical staff for at least the first 2 weeks, according to Sina A. Jasim, MD, reporting on real world use of the drug since its approval in February 2015.
For now, oncologists prescribe and manage this drug. But as lenvatinib (Lenvima, Eisai) becomes more widely used, endocrinologists can expect to be the ones prescribing it sometimes and counseling patients in the practical aspects of using this drug, Dr. Jasim of the Mayo Clinic, Rochester, Minn., said in an interview.
It was with endocrinologists in mind that Dr. Jasim and her associates compiled postapproval data on adverse events and patient quality of life. To date, no such data – including those from Mayo – have been published, she said during her presentation at the American Thyroid Association’s annual meeting.
While lenvatinib seems to be a promising therapeutic agent, adverse events are common with its use and occur early. Patients treated with it at Mayo get called by someone on the medical staff daily for the first 2 weeks of therapy, are given a blood pressure cuff, and are taught how to use it. They also receive the cell phone number of a member of the medical staff to consult with about sudden symptoms.
This retrospective analysis involved 25 sequentially treated patients given lenvatinib for RAI-resistant differentiated thyroid cancer (14 papillary, 7 poorly differentiated, 3 Hürthle cell, and 1 follicular). While all had received RAI, 11 also had received radiotherapy, 8 had been given at least one other kinase inhibitor previously, and 3 had received two. Fourteen were on an antihypertensive medication at baseline.
All patients initiated lenvatinib at the full dose, but it was reduced in four patients because of old age, renal impairment, or prior colitis. Twenty-one patients developed adverse events within the first month of being on the drug. Hypertension occurred in 16. Six of these required either a raising of the dose of antihypertensive drug they were on at baseline or initiation of antihypertensive therapy.
Adverse events were pronounced enough that the lenvatinib dose had to be lowered in 11 within a median 33 days of starting the drug. Drug treatment had to be interrupted for at least 3 weeks in four patients (two cases of cholecystitis, one case of diverticulitis, and one case of skin lesions).
Patients reported that their quality of life was stable at 2 months, but that their fatigue was worse.
The mean duration of lenvatinib therapy was 6.5 months. Twenty patients are alive at the time of this report.
The study was sponsored by the Mayo Clinic. Dr. Jasim reported that she had no relevant financial disclosures.
AT THE ATA ANNUAL MEETING
Key clinical point: Hypertension appeared suddenly or worsened abruptly in more than half of one group of patients with RAI-resistant differentiated thyroid cancer after initiation of treatment with lenvatinib.
Major finding: Of 25 patients in whom lenvatinib was initiated, 21 developed adverse events in the first month. Of those were 16 who developed hypertension, often requiring dose reduction.
Data source: A retrospective report on 25 consecutively treated patients with RAI-resistant differentiated thyroid cancer, who were given lenvatinib between February 2015 and May 2016.
Disclosures: The study was sponsored by the Mayo Clinic. Dr. Jasim reported that she had no relevant financial disclosures.
Prospects brighten for an HIV vaccine
DURBAN, SOUTH AFRICA – A new optimism regarding the possibility of creating a safe and effective HIV preventive vaccine was very much in evidence at the 21st International AIDS Conference.
“The HIV vaccine field is open for business,” an exuberant Larry Corey, MD, declared in a plenary address highlighting recent major progress in HIV vaccine development.
Three extremely important HIV vaccine efficacy clinical trials testing diverse promising strategies are now either in progress or soon to start, noted Dr. Corey, professor of laboratory medicine and medicine at the University of Washington and emeritus director of the Fred Hutchinson Cancer Research Center in Seattle.
“We are finally moving the needle forward with human efficacy trials that are commensurate with the need for developing an HIV vaccine,” Dr. Corey said.
He emphasized that HIV is “still the world’s most pressing global health issue,” with more than 45,000 new infections occurring annually in the United States and more than 2 million annually worldwide. And while numerous nonvaccine prevention methods have been developed, they share a major limitation: Their extended effectiveness requires continuous adherence.
“With asymptomatic acquisition, prolonged subclinical infection, and sexual transmission, getting to an AIDS-free generation will require a vaccine,” Dr. Corey predicted.
After years of discouragingly negative HIV vaccine studies, researchers finally turned a corner in 2009 with the reported results of the U.S. Military HIV Research Program–led RV144 trial, commonly known as the Thai Trial, Anthony S. Fauci, MD, recalled in an interview.
The trial, which randomized more than 16,000 young adult Thais, showed a modest 31% efficacy at 3.5 years, but a more substantial and encouraging 60% efficacy at 12 months (N Engl J Med. 2009 Dec 3;361[23]:2209-20). More importantly, the Thai trial opened up a whole new avenue to HIV vaccine development.
“We thought the Thai vaccine would induce neutralizing antibodies, but it didn’t. Instead, it induced nonneutralizing antibodies against a component of the V1V2 loop region of the HIV envelope, which was associated with protection. So the good news about that study was that even though that vaccine wasn’t efficacious enough to make it a usable vaccine, it gave us something we could improve upon by using the same platform and enhancing the response to provide greater depth, breadth, and durability,” explained Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID).
The improved vaccine consists of a canarypox-based vaccine called ALVAC-HIV and a bivalent gp120 protein subunit vaccine with MF59, a different adjuvant from that used in the Thai trial, in an effort to achieve a more robust immune response. Also, the four-injection series studied in the Thai trial is now bolstered by a booster injection at the 12-month mark. This vaccine has been altered to be specific to HIV clade C, the predominant HIV subtype in southern Africa, where the bulk of new HIV infections occur.
At the AIDS 2016 conference, Linda-Gail Bekker, MD, presented the primary immunogenicity results from the HIV Vaccine Trials Network (HVTN) 100 trial, a phase 1/2, double-blind, placebo-controlled study of the improved version of the Thai trial vaccine, known as the Clade C ALVAC-(vCP2438) and bivalent subtype C gp120/MF59 vaccine, in 252 HIV-uninfected South African adults.
The ALVAC/protein vaccine achieved cellular and humoral immune responses that exceeded all four predetermined criteria as correlates of protection. As a result, a pivotal phase III, randomized, double-blind, placebo-controlled vaccine efficacy trial known as HVTN 702 got the green light to begin in November 2016 in 5,400 HIV-negative adults in South Africa. Participants will be assessed at 24 and 36 months of follow-up, announced Dr. Bekker, cochair of HVTN 702, International AIDS Society president-elect, and deputy director of the Desmond Tutu HIV Center in Cape Town, South Africa.
As a principal investigator in the NIAID-supported HIV Vaccine Trials Network, Dr. Corey has been deeply involved in the development of this vaccine. He also is chair of the ongoing HVTN 703 and 704 phase IIb trials, testing an entirely different vaccine approach. The hypothesis being tested in HVTN 703 and 704 is that a passively infused monoclonal antibody can protect against HIV infection in 2,400 men who have sex with men and transgender men in North and South America, as well as in 1,500 women in sub-Saharan Africa. Both studies began in spring 2016.
“Every card-carrying virologist feels this should work,” according to Dr. Corey.
The rationale for having two study populations is that investigators suspect the effects of the antibody may vary depending upon whether the route of HIV acquisition is rectal or vaginal, he explained.
The monoclonal antibody contains VRC01, which effectively blocks viral binding to CD4 cells. Study participants will receive an intravenous infusion of VRC01 at 10 or 30 mg/kg or placebo every 2 months. If the results are positive and a second-generation product and delivery system can be developed, antibody-mediated prevention could also have a major potential role in interrupting maternal to child transmission of HIV resulting from intrapartum exposure or breastfeeding.
Dr. Corey also highlighted a third strategy of HIV vaccine development, one at an earlier stage. Investigators at Johnson & Johnson, in collaboration with the NIAID, HVTN, and other partners, are pursuing a multi-clade approach, one designed to protect against all clades of HIV found around the world. This strategy entails first giving an adenovirus serotype 26–vectored vaccine to prime the immune system, following up with administration of several boosters containing mosaic inserts to increase the response. This vaccine is in phase I studies with no results yet.
Dr. Fauci is not sure which if any of these three approaches will yield a safe and effective vaccine for HIV prevention.
“It’s important to realize that this is a very difficult scientific challenge,” he said. “The body does not readily make an adequate immune response against HIV, unlike virtually any other viral infection. Even the serious ones that cause a degree of morbidity and mortality – smallpox, measles, rubella, polio – ultimately the body does make a good immune response and allows us to clear the virus and leaves us with protection against subsequent exposure to the same virus. We don’t have that advantage with HIV. So it’s going to be difficult to get a safe and effective HIV vaccine, but I think the scientific challenge is worth going after and there’s a reasonable chance we might get there.”
Dr. Corey, Dr. Fauci, and Dr. Bekker reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – A new optimism regarding the possibility of creating a safe and effective HIV preventive vaccine was very much in evidence at the 21st International AIDS Conference.
“The HIV vaccine field is open for business,” an exuberant Larry Corey, MD, declared in a plenary address highlighting recent major progress in HIV vaccine development.
Three extremely important HIV vaccine efficacy clinical trials testing diverse promising strategies are now either in progress or soon to start, noted Dr. Corey, professor of laboratory medicine and medicine at the University of Washington and emeritus director of the Fred Hutchinson Cancer Research Center in Seattle.
“We are finally moving the needle forward with human efficacy trials that are commensurate with the need for developing an HIV vaccine,” Dr. Corey said.
He emphasized that HIV is “still the world’s most pressing global health issue,” with more than 45,000 new infections occurring annually in the United States and more than 2 million annually worldwide. And while numerous nonvaccine prevention methods have been developed, they share a major limitation: Their extended effectiveness requires continuous adherence.
“With asymptomatic acquisition, prolonged subclinical infection, and sexual transmission, getting to an AIDS-free generation will require a vaccine,” Dr. Corey predicted.
After years of discouragingly negative HIV vaccine studies, researchers finally turned a corner in 2009 with the reported results of the U.S. Military HIV Research Program–led RV144 trial, commonly known as the Thai Trial, Anthony S. Fauci, MD, recalled in an interview.
The trial, which randomized more than 16,000 young adult Thais, showed a modest 31% efficacy at 3.5 years, but a more substantial and encouraging 60% efficacy at 12 months (N Engl J Med. 2009 Dec 3;361[23]:2209-20). More importantly, the Thai trial opened up a whole new avenue to HIV vaccine development.
“We thought the Thai vaccine would induce neutralizing antibodies, but it didn’t. Instead, it induced nonneutralizing antibodies against a component of the V1V2 loop region of the HIV envelope, which was associated with protection. So the good news about that study was that even though that vaccine wasn’t efficacious enough to make it a usable vaccine, it gave us something we could improve upon by using the same platform and enhancing the response to provide greater depth, breadth, and durability,” explained Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID).
The improved vaccine consists of a canarypox-based vaccine called ALVAC-HIV and a bivalent gp120 protein subunit vaccine with MF59, a different adjuvant from that used in the Thai trial, in an effort to achieve a more robust immune response. Also, the four-injection series studied in the Thai trial is now bolstered by a booster injection at the 12-month mark. This vaccine has been altered to be specific to HIV clade C, the predominant HIV subtype in southern Africa, where the bulk of new HIV infections occur.
At the AIDS 2016 conference, Linda-Gail Bekker, MD, presented the primary immunogenicity results from the HIV Vaccine Trials Network (HVTN) 100 trial, a phase 1/2, double-blind, placebo-controlled study of the improved version of the Thai trial vaccine, known as the Clade C ALVAC-(vCP2438) and bivalent subtype C gp120/MF59 vaccine, in 252 HIV-uninfected South African adults.
The ALVAC/protein vaccine achieved cellular and humoral immune responses that exceeded all four predetermined criteria as correlates of protection. As a result, a pivotal phase III, randomized, double-blind, placebo-controlled vaccine efficacy trial known as HVTN 702 got the green light to begin in November 2016 in 5,400 HIV-negative adults in South Africa. Participants will be assessed at 24 and 36 months of follow-up, announced Dr. Bekker, cochair of HVTN 702, International AIDS Society president-elect, and deputy director of the Desmond Tutu HIV Center in Cape Town, South Africa.
As a principal investigator in the NIAID-supported HIV Vaccine Trials Network, Dr. Corey has been deeply involved in the development of this vaccine. He also is chair of the ongoing HVTN 703 and 704 phase IIb trials, testing an entirely different vaccine approach. The hypothesis being tested in HVTN 703 and 704 is that a passively infused monoclonal antibody can protect against HIV infection in 2,400 men who have sex with men and transgender men in North and South America, as well as in 1,500 women in sub-Saharan Africa. Both studies began in spring 2016.
“Every card-carrying virologist feels this should work,” according to Dr. Corey.
The rationale for having two study populations is that investigators suspect the effects of the antibody may vary depending upon whether the route of HIV acquisition is rectal or vaginal, he explained.
The monoclonal antibody contains VRC01, which effectively blocks viral binding to CD4 cells. Study participants will receive an intravenous infusion of VRC01 at 10 or 30 mg/kg or placebo every 2 months. If the results are positive and a second-generation product and delivery system can be developed, antibody-mediated prevention could also have a major potential role in interrupting maternal to child transmission of HIV resulting from intrapartum exposure or breastfeeding.
Dr. Corey also highlighted a third strategy of HIV vaccine development, one at an earlier stage. Investigators at Johnson & Johnson, in collaboration with the NIAID, HVTN, and other partners, are pursuing a multi-clade approach, one designed to protect against all clades of HIV found around the world. This strategy entails first giving an adenovirus serotype 26–vectored vaccine to prime the immune system, following up with administration of several boosters containing mosaic inserts to increase the response. This vaccine is in phase I studies with no results yet.
Dr. Fauci is not sure which if any of these three approaches will yield a safe and effective vaccine for HIV prevention.
“It’s important to realize that this is a very difficult scientific challenge,” he said. “The body does not readily make an adequate immune response against HIV, unlike virtually any other viral infection. Even the serious ones that cause a degree of morbidity and mortality – smallpox, measles, rubella, polio – ultimately the body does make a good immune response and allows us to clear the virus and leaves us with protection against subsequent exposure to the same virus. We don’t have that advantage with HIV. So it’s going to be difficult to get a safe and effective HIV vaccine, but I think the scientific challenge is worth going after and there’s a reasonable chance we might get there.”
Dr. Corey, Dr. Fauci, and Dr. Bekker reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – A new optimism regarding the possibility of creating a safe and effective HIV preventive vaccine was very much in evidence at the 21st International AIDS Conference.
“The HIV vaccine field is open for business,” an exuberant Larry Corey, MD, declared in a plenary address highlighting recent major progress in HIV vaccine development.
Three extremely important HIV vaccine efficacy clinical trials testing diverse promising strategies are now either in progress or soon to start, noted Dr. Corey, professor of laboratory medicine and medicine at the University of Washington and emeritus director of the Fred Hutchinson Cancer Research Center in Seattle.
“We are finally moving the needle forward with human efficacy trials that are commensurate with the need for developing an HIV vaccine,” Dr. Corey said.
He emphasized that HIV is “still the world’s most pressing global health issue,” with more than 45,000 new infections occurring annually in the United States and more than 2 million annually worldwide. And while numerous nonvaccine prevention methods have been developed, they share a major limitation: Their extended effectiveness requires continuous adherence.
“With asymptomatic acquisition, prolonged subclinical infection, and sexual transmission, getting to an AIDS-free generation will require a vaccine,” Dr. Corey predicted.
After years of discouragingly negative HIV vaccine studies, researchers finally turned a corner in 2009 with the reported results of the U.S. Military HIV Research Program–led RV144 trial, commonly known as the Thai Trial, Anthony S. Fauci, MD, recalled in an interview.
The trial, which randomized more than 16,000 young adult Thais, showed a modest 31% efficacy at 3.5 years, but a more substantial and encouraging 60% efficacy at 12 months (N Engl J Med. 2009 Dec 3;361[23]:2209-20). More importantly, the Thai trial opened up a whole new avenue to HIV vaccine development.
“We thought the Thai vaccine would induce neutralizing antibodies, but it didn’t. Instead, it induced nonneutralizing antibodies against a component of the V1V2 loop region of the HIV envelope, which was associated with protection. So the good news about that study was that even though that vaccine wasn’t efficacious enough to make it a usable vaccine, it gave us something we could improve upon by using the same platform and enhancing the response to provide greater depth, breadth, and durability,” explained Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID).
The improved vaccine consists of a canarypox-based vaccine called ALVAC-HIV and a bivalent gp120 protein subunit vaccine with MF59, a different adjuvant from that used in the Thai trial, in an effort to achieve a more robust immune response. Also, the four-injection series studied in the Thai trial is now bolstered by a booster injection at the 12-month mark. This vaccine has been altered to be specific to HIV clade C, the predominant HIV subtype in southern Africa, where the bulk of new HIV infections occur.
At the AIDS 2016 conference, Linda-Gail Bekker, MD, presented the primary immunogenicity results from the HIV Vaccine Trials Network (HVTN) 100 trial, a phase 1/2, double-blind, placebo-controlled study of the improved version of the Thai trial vaccine, known as the Clade C ALVAC-(vCP2438) and bivalent subtype C gp120/MF59 vaccine, in 252 HIV-uninfected South African adults.
The ALVAC/protein vaccine achieved cellular and humoral immune responses that exceeded all four predetermined criteria as correlates of protection. As a result, a pivotal phase III, randomized, double-blind, placebo-controlled vaccine efficacy trial known as HVTN 702 got the green light to begin in November 2016 in 5,400 HIV-negative adults in South Africa. Participants will be assessed at 24 and 36 months of follow-up, announced Dr. Bekker, cochair of HVTN 702, International AIDS Society president-elect, and deputy director of the Desmond Tutu HIV Center in Cape Town, South Africa.
As a principal investigator in the NIAID-supported HIV Vaccine Trials Network, Dr. Corey has been deeply involved in the development of this vaccine. He also is chair of the ongoing HVTN 703 and 704 phase IIb trials, testing an entirely different vaccine approach. The hypothesis being tested in HVTN 703 and 704 is that a passively infused monoclonal antibody can protect against HIV infection in 2,400 men who have sex with men and transgender men in North and South America, as well as in 1,500 women in sub-Saharan Africa. Both studies began in spring 2016.
“Every card-carrying virologist feels this should work,” according to Dr. Corey.
The rationale for having two study populations is that investigators suspect the effects of the antibody may vary depending upon whether the route of HIV acquisition is rectal or vaginal, he explained.
The monoclonal antibody contains VRC01, which effectively blocks viral binding to CD4 cells. Study participants will receive an intravenous infusion of VRC01 at 10 or 30 mg/kg or placebo every 2 months. If the results are positive and a second-generation product and delivery system can be developed, antibody-mediated prevention could also have a major potential role in interrupting maternal to child transmission of HIV resulting from intrapartum exposure or breastfeeding.
Dr. Corey also highlighted a third strategy of HIV vaccine development, one at an earlier stage. Investigators at Johnson & Johnson, in collaboration with the NIAID, HVTN, and other partners, are pursuing a multi-clade approach, one designed to protect against all clades of HIV found around the world. This strategy entails first giving an adenovirus serotype 26–vectored vaccine to prime the immune system, following up with administration of several boosters containing mosaic inserts to increase the response. This vaccine is in phase I studies with no results yet.
Dr. Fauci is not sure which if any of these three approaches will yield a safe and effective vaccine for HIV prevention.
“It’s important to realize that this is a very difficult scientific challenge,” he said. “The body does not readily make an adequate immune response against HIV, unlike virtually any other viral infection. Even the serious ones that cause a degree of morbidity and mortality – smallpox, measles, rubella, polio – ultimately the body does make a good immune response and allows us to clear the virus and leaves us with protection against subsequent exposure to the same virus. We don’t have that advantage with HIV. So it’s going to be difficult to get a safe and effective HIV vaccine, but I think the scientific challenge is worth going after and there’s a reasonable chance we might get there.”
Dr. Corey, Dr. Fauci, and Dr. Bekker reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM AIDS 2016
Ebola research update: August 2016
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Plasmodium species parasitemia is associated with an increase in the probability of surviving Ebola virus infection, according to a study in Clinical Infectious Diseases. The authors said more research is needed to understand the molecular mechanism underlying this phenomenon and translate it into treatment options for Ebola virus infection.
A recent study found that a structured doffing protocol for health care providers wearing personal protective equipment, using a trained monitor and alcohol-based hand rub, protects against enveloped Ebola virus self-contamination. The authors noted that doffing protocols protective against all viruses need to incorporate highly effective glove and hand hygiene agents.
A reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for postmortem Ebola virus disease (EVD) testing was found useful as an EVD diagnostic testing method in the field or remote areas, according to a study in Guinea.
A structural study of the Ebola virus glycoprotein (GP) gene provides a detailed picture of the accessible Ebola virus glycoprotein landscape, according to investigators, and a structural basis to evaluate patient and vaccine antibody responses toward differently structured products of the GP gene.
A community-based strategy of social mobilization and community engagement was effective in case detection and reducing the extent of Ebola transmission in Sierra Leone, according to a report in Infectious Diseases of Poverty.
The Center of Excellence for Emerging Zoonotic and Animal Diseases, or CEEZAD, at Kansas State University, will use a $2.3 million grant from the U.S. Department of Defense to study the safety in livestock of a newly developed vaccine to protect humans from the Ebola Zaire virus.
An NIH study found that there is no difference in virus stability between the two strains of Zaire Ebola virus from the 1976 and 2013 outbreaks, and that viable virus can be recovered from an aerosol 180 minutes after it is generated.
According to a study in PLOS Medicine, the associations between war trauma and both EVD risk behaviors and EVD prevention behaviors may be mediated through two key mental health variables: depression and PTSD symptoms.
Inovio Pharmaceuticals announced its intention to more than double study enrollment to further characterize and identify in humans the most optimal immunization regimen using intradermal delivery of its preventive Ebola DNA vaccine.
Ordering clusters in a step-wedge Ebola virus vaccine trial based on the cluster’s underlying risk of infection, as predicted by a spatial model, can increase the statistical power of a stepped-wedge cluster study, according to a study in PLOS Neglected Tropical Diseases.
A Guinean study found that in survivors of EVD, CD16+ monocytes were activated during recovery, coincident with viral clearance, which suggests an important role of this cell subset in EVD pathophysiology.
Examining proactive vaccination strategies for Ebola prevention and response is critical to advancing development of vaccine production and administration technologies that could be instrumental to mitigating future Ebola outbreaks, according to an analysis in PLOS Neglected Tropical Diseases.
A study of the clinical features of Ebola virus disease in Sierra Leone showed that a better awareness of risk factors for death could be used to group EVD patients at greatest risk into dedicated wards with more intensive medical support.
Contact with individuals who died of EVD at home in rural communities in Liberia and Guinea resulted in more secondary infections than did contact with individuals admitted to Ebola treatment units, according to a study in Emerging Infectious Diseases.
A study of the 2014 Ebola virus disease outbreak in the Congo found that cycle threshold was a robust predictor of death, as were fever, hiccups, diarrhea, dyspnea, dehydration, disorientation, hematemesis, bloody feces during hospitalization, and anorexia in recent medical history.
A study published in Nature Microbiology demonstrated that researchers were able to protect nonhuman primates against a lethal Ebola Sudan infection when treatment began 4 days following infection.
Treatment of patients infected with Ebola virus disease in Sierra Leone with favipiravir (T-705) was associated with prolonged survival and markedly reduced viral load, according to a recent study, which makes a case for further randomized controlled trials of T-705 for treating EVD.
A study in the Journal of Infectious Diseases found that convalescent sera alone are not sufficient for providing 100% protection against lethal Zaire Ebola virus infection when administered at the onset of viremia.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Plasmodium species parasitemia is associated with an increase in the probability of surviving Ebola virus infection, according to a study in Clinical Infectious Diseases. The authors said more research is needed to understand the molecular mechanism underlying this phenomenon and translate it into treatment options for Ebola virus infection.
A recent study found that a structured doffing protocol for health care providers wearing personal protective equipment, using a trained monitor and alcohol-based hand rub, protects against enveloped Ebola virus self-contamination. The authors noted that doffing protocols protective against all viruses need to incorporate highly effective glove and hand hygiene agents.
A reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for postmortem Ebola virus disease (EVD) testing was found useful as an EVD diagnostic testing method in the field or remote areas, according to a study in Guinea.
A structural study of the Ebola virus glycoprotein (GP) gene provides a detailed picture of the accessible Ebola virus glycoprotein landscape, according to investigators, and a structural basis to evaluate patient and vaccine antibody responses toward differently structured products of the GP gene.
A community-based strategy of social mobilization and community engagement was effective in case detection and reducing the extent of Ebola transmission in Sierra Leone, according to a report in Infectious Diseases of Poverty.
The Center of Excellence for Emerging Zoonotic and Animal Diseases, or CEEZAD, at Kansas State University, will use a $2.3 million grant from the U.S. Department of Defense to study the safety in livestock of a newly developed vaccine to protect humans from the Ebola Zaire virus.
An NIH study found that there is no difference in virus stability between the two strains of Zaire Ebola virus from the 1976 and 2013 outbreaks, and that viable virus can be recovered from an aerosol 180 minutes after it is generated.
According to a study in PLOS Medicine, the associations between war trauma and both EVD risk behaviors and EVD prevention behaviors may be mediated through two key mental health variables: depression and PTSD symptoms.
Inovio Pharmaceuticals announced its intention to more than double study enrollment to further characterize and identify in humans the most optimal immunization regimen using intradermal delivery of its preventive Ebola DNA vaccine.
Ordering clusters in a step-wedge Ebola virus vaccine trial based on the cluster’s underlying risk of infection, as predicted by a spatial model, can increase the statistical power of a stepped-wedge cluster study, according to a study in PLOS Neglected Tropical Diseases.
A Guinean study found that in survivors of EVD, CD16+ monocytes were activated during recovery, coincident with viral clearance, which suggests an important role of this cell subset in EVD pathophysiology.
Examining proactive vaccination strategies for Ebola prevention and response is critical to advancing development of vaccine production and administration technologies that could be instrumental to mitigating future Ebola outbreaks, according to an analysis in PLOS Neglected Tropical Diseases.
A study of the clinical features of Ebola virus disease in Sierra Leone showed that a better awareness of risk factors for death could be used to group EVD patients at greatest risk into dedicated wards with more intensive medical support.
Contact with individuals who died of EVD at home in rural communities in Liberia and Guinea resulted in more secondary infections than did contact with individuals admitted to Ebola treatment units, according to a study in Emerging Infectious Diseases.
A study of the 2014 Ebola virus disease outbreak in the Congo found that cycle threshold was a robust predictor of death, as were fever, hiccups, diarrhea, dyspnea, dehydration, disorientation, hematemesis, bloody feces during hospitalization, and anorexia in recent medical history.
A study published in Nature Microbiology demonstrated that researchers were able to protect nonhuman primates against a lethal Ebola Sudan infection when treatment began 4 days following infection.
Treatment of patients infected with Ebola virus disease in Sierra Leone with favipiravir (T-705) was associated with prolonged survival and markedly reduced viral load, according to a recent study, which makes a case for further randomized controlled trials of T-705 for treating EVD.
A study in the Journal of Infectious Diseases found that convalescent sera alone are not sufficient for providing 100% protection against lethal Zaire Ebola virus infection when administered at the onset of viremia.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Plasmodium species parasitemia is associated with an increase in the probability of surviving Ebola virus infection, according to a study in Clinical Infectious Diseases. The authors said more research is needed to understand the molecular mechanism underlying this phenomenon and translate it into treatment options for Ebola virus infection.
A recent study found that a structured doffing protocol for health care providers wearing personal protective equipment, using a trained monitor and alcohol-based hand rub, protects against enveloped Ebola virus self-contamination. The authors noted that doffing protocols protective against all viruses need to incorporate highly effective glove and hand hygiene agents.
A reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for postmortem Ebola virus disease (EVD) testing was found useful as an EVD diagnostic testing method in the field or remote areas, according to a study in Guinea.
A structural study of the Ebola virus glycoprotein (GP) gene provides a detailed picture of the accessible Ebola virus glycoprotein landscape, according to investigators, and a structural basis to evaluate patient and vaccine antibody responses toward differently structured products of the GP gene.
A community-based strategy of social mobilization and community engagement was effective in case detection and reducing the extent of Ebola transmission in Sierra Leone, according to a report in Infectious Diseases of Poverty.
The Center of Excellence for Emerging Zoonotic and Animal Diseases, or CEEZAD, at Kansas State University, will use a $2.3 million grant from the U.S. Department of Defense to study the safety in livestock of a newly developed vaccine to protect humans from the Ebola Zaire virus.
An NIH study found that there is no difference in virus stability between the two strains of Zaire Ebola virus from the 1976 and 2013 outbreaks, and that viable virus can be recovered from an aerosol 180 minutes after it is generated.
According to a study in PLOS Medicine, the associations between war trauma and both EVD risk behaviors and EVD prevention behaviors may be mediated through two key mental health variables: depression and PTSD symptoms.
Inovio Pharmaceuticals announced its intention to more than double study enrollment to further characterize and identify in humans the most optimal immunization regimen using intradermal delivery of its preventive Ebola DNA vaccine.
Ordering clusters in a step-wedge Ebola virus vaccine trial based on the cluster’s underlying risk of infection, as predicted by a spatial model, can increase the statistical power of a stepped-wedge cluster study, according to a study in PLOS Neglected Tropical Diseases.
A Guinean study found that in survivors of EVD, CD16+ monocytes were activated during recovery, coincident with viral clearance, which suggests an important role of this cell subset in EVD pathophysiology.
Examining proactive vaccination strategies for Ebola prevention and response is critical to advancing development of vaccine production and administration technologies that could be instrumental to mitigating future Ebola outbreaks, according to an analysis in PLOS Neglected Tropical Diseases.
A study of the clinical features of Ebola virus disease in Sierra Leone showed that a better awareness of risk factors for death could be used to group EVD patients at greatest risk into dedicated wards with more intensive medical support.
Contact with individuals who died of EVD at home in rural communities in Liberia and Guinea resulted in more secondary infections than did contact with individuals admitted to Ebola treatment units, according to a study in Emerging Infectious Diseases.
A study of the 2014 Ebola virus disease outbreak in the Congo found that cycle threshold was a robust predictor of death, as were fever, hiccups, diarrhea, dyspnea, dehydration, disorientation, hematemesis, bloody feces during hospitalization, and anorexia in recent medical history.
A study published in Nature Microbiology demonstrated that researchers were able to protect nonhuman primates against a lethal Ebola Sudan infection when treatment began 4 days following infection.
Treatment of patients infected with Ebola virus disease in Sierra Leone with favipiravir (T-705) was associated with prolonged survival and markedly reduced viral load, according to a recent study, which makes a case for further randomized controlled trials of T-705 for treating EVD.
A study in the Journal of Infectious Diseases found that convalescent sera alone are not sufficient for providing 100% protection against lethal Zaire Ebola virus infection when administered at the onset of viremia.
On Twitter @richpizzi
Prenatal Tdap vaccination prevents occurrence, reduces severity of pertussis in infants
Prenatal Tdap vaccination prevents the occurrence of and reduces the severity of pertussis in infants, two retrospective cohort studies showed.
In 2012, the Advisory Committee on Immunization Practices recommended that pregnant women receive a Tdap vaccination during their third trimester of pregnancy to optimize the transfer of pertussis antibodies to the fetus. Since the committee’s recommendation, no studies to evaluate the effectiveness of this strategy have been conducted in the United States, Kathleen Winter of the California Department of Public Health and her associates reported (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw634) .
Therefore, Ms. Winter and her associates conducted two separate retrospective cohort studies: one to compare the effectiveness of prenatal versus postpartum Tdap vaccination in preventing pertussis and the second to investigate the effectiveness of prenatal Tdap vaccination on pertussis severity in infants.
For the comparison study, researchers identified 42,941 mothers who were vaccinated during pregnancy and 31,563 mothers who were vaccinated following delivery. The stage of pregnancy at the time of vaccination was documented for 42,218 of the mothers vaccinated prenatally, and 77% were vaccinated during the recommended window of 27-36 weeks’ gestation. For the remaining mothers, 14% received vaccinations before 27 weeks’ gestation, and 9% were vaccinated after 36 weeks’ gestation. Infants whose mothers received the Tdap vaccine at any point during pregnancy were less likely to develop pertussis before 8 weeks of age (odds ratio, 0.36; 95% confidence interval, 0.15-0.89) or 12 weeks of age (OR, 0.47; 95% CI, 0.24-0.92).
Moreover, infants whose mothers received the vaccine during 27-36 weeks’ gestation were less likely to develop pertussis than were infants whose mothers were vaccinated during pregnancy but outside the 27- to 36-week time frame (OR, 0.22; 95% CI, 0.08-0.63).
Overall, Tdap vaccination during 27-36 weeks’ gestation was 85% more effective in reducing pertussis in infants younger than 8 weeks old and 72% more effective in preventing pertussis in infants younger than 12 weeks old, compared with postpartum vaccination.
In a companion paper, researchers described the results of a separate retrospective cohort study, the “first known study demonstrating that prenatal Tdap vaccination reduces severity of disease in infants who are infected with pertussis,” according to Ms. Winter and her associates (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw633).
For this study, the researchers identified 420 infants born between January 2011 and December 2015 who reported with pertussis at less than 63 days of age and had known maternal vaccination status. Of those 420 infants, only 49 mothers (12%) received Tdap vaccination during pregnancy, and only 14 received Tdap during the recommended window of 27-36 weeks’ gestation.
Infants born to mothers who received the Tdap vaccine during pregnancy were significantly less likely to be hospitalized when they developed pertussis (OR, 0.4; 95% CI, 0.2-0.9), were less likely to be admitted to the intensive care unit (OR, 0.5; 95% CI, 0.2-1.2), and had shorter hospital stays (median, 3 days vs. 6 days; P = .019). Infants born to vaccinated mothers also were older when they developed pertussis and were less likely to display common pertussis symptoms: paroxysmal cough, apnea, cyanosis, and whoop.
“Prenatal Tdap vaccination was 58% effective in preventing hospitalizations in infants infected with pertussis,” the researchers wrote, adding that “prenatal Tdap vaccination of mothers is a critical strategy for reducing the morbidity and mortality from pertussis.”
On Twitter @jessnicolecraig
The two papers by Ms. Winter and her colleagues are remarkably important and of high clinical interest. In 2012, recognizing the significant morbidity and mortality of pertussis among infants in the first 2-3 months of life and the lack of a newborn vaccination for pertussis, the Advisory Committee on Immunization Practices (ACIP) recommended maternal vaccination at 27-36 weeks’ gestation to stem the tide of increasing pertussis infections in this vulnerable age group. The recommendation was based on immunogenicity studies that showed maternal vaccination at 27-36 weeks’ gestation boosted maternal antibodies to pertussis antigens in the acellular vaccines used and that the antibodies crossed the placenta in sufficient amounts such that protection in the newborn could be anticipated up to at least 3 months of age.

|
Dr. Michael E. Pichichero |
Winter et al. showed in these two companion papers that the ACIP recommendation was effective and excellent results have been achieved in California. I would expect the same results across the United States.
While this research is novel in the United States, the results could have been anticipated because two prior studies from the United Kingdom and Australia demonstrated the effectiveness of maternal Tdap vaccination. Although the study design was retrospective (a weakness, compared with a prospective study design), the findings are convincing.
More vaccines are being studied for possible maternal use during pregnancy. The hesitation of the “unknown risks” of maternal vaccination slowly are disappearing as more success stories like this one provide confidence in terms of the lack of side effects and the substantial benefit.
Dr. Michael E. Pichichero is a clinical professor in the department of pediatrics at the University of Rochester (N.Y.), a research professor at the Rochester Institute of Technology, and the director of research at Rochester General Hospital Research Institute. Dr. Pichichero said he had no relevant financial disclosures.
The two papers by Ms. Winter and her colleagues are remarkably important and of high clinical interest. In 2012, recognizing the significant morbidity and mortality of pertussis among infants in the first 2-3 months of life and the lack of a newborn vaccination for pertussis, the Advisory Committee on Immunization Practices (ACIP) recommended maternal vaccination at 27-36 weeks’ gestation to stem the tide of increasing pertussis infections in this vulnerable age group. The recommendation was based on immunogenicity studies that showed maternal vaccination at 27-36 weeks’ gestation boosted maternal antibodies to pertussis antigens in the acellular vaccines used and that the antibodies crossed the placenta in sufficient amounts such that protection in the newborn could be anticipated up to at least 3 months of age.

|
Dr. Michael E. Pichichero |
Winter et al. showed in these two companion papers that the ACIP recommendation was effective and excellent results have been achieved in California. I would expect the same results across the United States.
While this research is novel in the United States, the results could have been anticipated because two prior studies from the United Kingdom and Australia demonstrated the effectiveness of maternal Tdap vaccination. Although the study design was retrospective (a weakness, compared with a prospective study design), the findings are convincing.
More vaccines are being studied for possible maternal use during pregnancy. The hesitation of the “unknown risks” of maternal vaccination slowly are disappearing as more success stories like this one provide confidence in terms of the lack of side effects and the substantial benefit.
Dr. Michael E. Pichichero is a clinical professor in the department of pediatrics at the University of Rochester (N.Y.), a research professor at the Rochester Institute of Technology, and the director of research at Rochester General Hospital Research Institute. Dr. Pichichero said he had no relevant financial disclosures.
The two papers by Ms. Winter and her colleagues are remarkably important and of high clinical interest. In 2012, recognizing the significant morbidity and mortality of pertussis among infants in the first 2-3 months of life and the lack of a newborn vaccination for pertussis, the Advisory Committee on Immunization Practices (ACIP) recommended maternal vaccination at 27-36 weeks’ gestation to stem the tide of increasing pertussis infections in this vulnerable age group. The recommendation was based on immunogenicity studies that showed maternal vaccination at 27-36 weeks’ gestation boosted maternal antibodies to pertussis antigens in the acellular vaccines used and that the antibodies crossed the placenta in sufficient amounts such that protection in the newborn could be anticipated up to at least 3 months of age.

|
Dr. Michael E. Pichichero |
Winter et al. showed in these two companion papers that the ACIP recommendation was effective and excellent results have been achieved in California. I would expect the same results across the United States.
While this research is novel in the United States, the results could have been anticipated because two prior studies from the United Kingdom and Australia demonstrated the effectiveness of maternal Tdap vaccination. Although the study design was retrospective (a weakness, compared with a prospective study design), the findings are convincing.
More vaccines are being studied for possible maternal use during pregnancy. The hesitation of the “unknown risks” of maternal vaccination slowly are disappearing as more success stories like this one provide confidence in terms of the lack of side effects and the substantial benefit.
Dr. Michael E. Pichichero is a clinical professor in the department of pediatrics at the University of Rochester (N.Y.), a research professor at the Rochester Institute of Technology, and the director of research at Rochester General Hospital Research Institute. Dr. Pichichero said he had no relevant financial disclosures.
Prenatal Tdap vaccination prevents the occurrence of and reduces the severity of pertussis in infants, two retrospective cohort studies showed.
In 2012, the Advisory Committee on Immunization Practices recommended that pregnant women receive a Tdap vaccination during their third trimester of pregnancy to optimize the transfer of pertussis antibodies to the fetus. Since the committee’s recommendation, no studies to evaluate the effectiveness of this strategy have been conducted in the United States, Kathleen Winter of the California Department of Public Health and her associates reported (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw634) .
Therefore, Ms. Winter and her associates conducted two separate retrospective cohort studies: one to compare the effectiveness of prenatal versus postpartum Tdap vaccination in preventing pertussis and the second to investigate the effectiveness of prenatal Tdap vaccination on pertussis severity in infants.
For the comparison study, researchers identified 42,941 mothers who were vaccinated during pregnancy and 31,563 mothers who were vaccinated following delivery. The stage of pregnancy at the time of vaccination was documented for 42,218 of the mothers vaccinated prenatally, and 77% were vaccinated during the recommended window of 27-36 weeks’ gestation. For the remaining mothers, 14% received vaccinations before 27 weeks’ gestation, and 9% were vaccinated after 36 weeks’ gestation. Infants whose mothers received the Tdap vaccine at any point during pregnancy were less likely to develop pertussis before 8 weeks of age (odds ratio, 0.36; 95% confidence interval, 0.15-0.89) or 12 weeks of age (OR, 0.47; 95% CI, 0.24-0.92).
Moreover, infants whose mothers received the vaccine during 27-36 weeks’ gestation were less likely to develop pertussis than were infants whose mothers were vaccinated during pregnancy but outside the 27- to 36-week time frame (OR, 0.22; 95% CI, 0.08-0.63).
Overall, Tdap vaccination during 27-36 weeks’ gestation was 85% more effective in reducing pertussis in infants younger than 8 weeks old and 72% more effective in preventing pertussis in infants younger than 12 weeks old, compared with postpartum vaccination.
In a companion paper, researchers described the results of a separate retrospective cohort study, the “first known study demonstrating that prenatal Tdap vaccination reduces severity of disease in infants who are infected with pertussis,” according to Ms. Winter and her associates (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw633).
For this study, the researchers identified 420 infants born between January 2011 and December 2015 who reported with pertussis at less than 63 days of age and had known maternal vaccination status. Of those 420 infants, only 49 mothers (12%) received Tdap vaccination during pregnancy, and only 14 received Tdap during the recommended window of 27-36 weeks’ gestation.
Infants born to mothers who received the Tdap vaccine during pregnancy were significantly less likely to be hospitalized when they developed pertussis (OR, 0.4; 95% CI, 0.2-0.9), were less likely to be admitted to the intensive care unit (OR, 0.5; 95% CI, 0.2-1.2), and had shorter hospital stays (median, 3 days vs. 6 days; P = .019). Infants born to vaccinated mothers also were older when they developed pertussis and were less likely to display common pertussis symptoms: paroxysmal cough, apnea, cyanosis, and whoop.
“Prenatal Tdap vaccination was 58% effective in preventing hospitalizations in infants infected with pertussis,” the researchers wrote, adding that “prenatal Tdap vaccination of mothers is a critical strategy for reducing the morbidity and mortality from pertussis.”
On Twitter @jessnicolecraig
Prenatal Tdap vaccination prevents the occurrence of and reduces the severity of pertussis in infants, two retrospective cohort studies showed.
In 2012, the Advisory Committee on Immunization Practices recommended that pregnant women receive a Tdap vaccination during their third trimester of pregnancy to optimize the transfer of pertussis antibodies to the fetus. Since the committee’s recommendation, no studies to evaluate the effectiveness of this strategy have been conducted in the United States, Kathleen Winter of the California Department of Public Health and her associates reported (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw634) .
Therefore, Ms. Winter and her associates conducted two separate retrospective cohort studies: one to compare the effectiveness of prenatal versus postpartum Tdap vaccination in preventing pertussis and the second to investigate the effectiveness of prenatal Tdap vaccination on pertussis severity in infants.
For the comparison study, researchers identified 42,941 mothers who were vaccinated during pregnancy and 31,563 mothers who were vaccinated following delivery. The stage of pregnancy at the time of vaccination was documented for 42,218 of the mothers vaccinated prenatally, and 77% were vaccinated during the recommended window of 27-36 weeks’ gestation. For the remaining mothers, 14% received vaccinations before 27 weeks’ gestation, and 9% were vaccinated after 36 weeks’ gestation. Infants whose mothers received the Tdap vaccine at any point during pregnancy were less likely to develop pertussis before 8 weeks of age (odds ratio, 0.36; 95% confidence interval, 0.15-0.89) or 12 weeks of age (OR, 0.47; 95% CI, 0.24-0.92).
Moreover, infants whose mothers received the vaccine during 27-36 weeks’ gestation were less likely to develop pertussis than were infants whose mothers were vaccinated during pregnancy but outside the 27- to 36-week time frame (OR, 0.22; 95% CI, 0.08-0.63).
Overall, Tdap vaccination during 27-36 weeks’ gestation was 85% more effective in reducing pertussis in infants younger than 8 weeks old and 72% more effective in preventing pertussis in infants younger than 12 weeks old, compared with postpartum vaccination.
In a companion paper, researchers described the results of a separate retrospective cohort study, the “first known study demonstrating that prenatal Tdap vaccination reduces severity of disease in infants who are infected with pertussis,” according to Ms. Winter and her associates (Clin. Infect. Dis. 2016. doi: 10.1093/cid/ciw633).
For this study, the researchers identified 420 infants born between January 2011 and December 2015 who reported with pertussis at less than 63 days of age and had known maternal vaccination status. Of those 420 infants, only 49 mothers (12%) received Tdap vaccination during pregnancy, and only 14 received Tdap during the recommended window of 27-36 weeks’ gestation.
Infants born to mothers who received the Tdap vaccine during pregnancy were significantly less likely to be hospitalized when they developed pertussis (OR, 0.4; 95% CI, 0.2-0.9), were less likely to be admitted to the intensive care unit (OR, 0.5; 95% CI, 0.2-1.2), and had shorter hospital stays (median, 3 days vs. 6 days; P = .019). Infants born to vaccinated mothers also were older when they developed pertussis and were less likely to display common pertussis symptoms: paroxysmal cough, apnea, cyanosis, and whoop.
“Prenatal Tdap vaccination was 58% effective in preventing hospitalizations in infants infected with pertussis,” the researchers wrote, adding that “prenatal Tdap vaccination of mothers is a critical strategy for reducing the morbidity and mortality from pertussis.”
On Twitter @jessnicolecraig
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Prenatal Tdap vaccination prevents the occurrence of and reduces the severity of pertussis in infants.
Major finding: Tdap vaccination during 27-36 weeks’ gestation was 85% more effective in reducing pertussis in infants younger than 8 weeks old, compared with postpartum Tdap vaccination.
Data source: Two retrospective cohort studies.
Disclosures: The California Department of Public Health Immunization Branch funded this study. One investigator reported receiving financial compensation from GlaxoSmithKline and serving as a speaker for Sanofi Pasteur.
Influence of Diet in Acne Vulgaris and Atopic Dermatitis
When I am in clinic, I often get at least 3 to 4 inquiries each day from patients about the necessity for dietary restrictions or alterations as well as the benefits of these changes in limiting their dermatological disease processes. I usually am restricted in my response because the research rarely indicates benefits of one diet versus another; however, this discussion has recently become a heavily researched area as patients have come to value natural nonpharmaceutical approaches to their holistic care. In this article, a few dietary restrictions and supplements are reviewed that may have a beneficial effect in managing patients with acne vulgaris and atopic dermatitis.
Acne Vulgaris
In 1969 Fulton et al1 conducted one of the first few trials on acne and diet management. In this crossover, patient-blinded, interventional study, patients were divided into 2 subgroups (N=65): 1 adolescent patient with moderate acne was compared to 1 male prisoner given a chocolate bar for 4 weeks or a control bar with equivalent caloric index. The results indicated no change in acne vulgaris lesions based on either intervention; however, there were obvious deficiencies in the study including small sample size, inappropriate grouping of an adolescent patient versus a prisoner, and limited study period.1
Since then, multiple studies have been conducted with parallel participants, large sample sizes, and at least a 12-week study period. In 2005, Adebamowo et al2 studied 47,355 women using a validated food frequency questionnaire that determined the amount of dairy consumed, specifically skim milk. The study showed a positive link between increased dairy consumption and acne formation; however, again due to the retrospective analysis and recall bias, it is difficult to determine if a link can truly be noted between acne and dairy in this study.2
More recently, LaRosa et al3 conducted a study that included 225 participants aged 14 to 19 years. Excluding participants with lactose intolerance and current use of oral contraceptives and isotretinoin, the study placed 120 participants in the test group versus 105 participants in the control group. The study was conducted using 3 telephone interviews and a 24-hour diet recall technique. The results supported a link between acne and skim milk consumption. Again, although the studied relied on participant self-reports of diet and followed a case-control design, a possible association was suspected but not validated.3 A longitudinal, questionnaire-based population study performed by Ulvestad et al4 included 2489 patients. This study further evaluated recall of dairy product consumption at 15 to 16 years of age and then 3 years later acne severity was self-assessed and reported at 18 to 19 years of age. Overall, this evaluation indicated that a high intake of dairy products and acne in adolescence have been positively associated. However, it was another retrospective study with recall bias.4 In 2009 Melnick and Schmitz5 concluded that milk causes the body to elevate both insulin and insulinlike growth factor 1 levels. In another study by Melnick6 in 2011, a definitive link between increased insulin and insulinlike growth factor 1 signaling in promoting comedogenesis was reported. Given the few studies that show the potential link between dairy products and acne, this dairy-free diet can be considered as a diet recommendation for acne patients.
Atopic Dermatitis
A Cochrane review conducted in 2012 regarding dietary supplements as a treatment of atopic dermatitis evaluated randomized controlled trials (N=596). Supplementation with vitamin D, fish oil, olive oil, zinc sulfate, selenium, vitamin E, pyridoxine, sea buckthorn seed oil, hempseed oil, sunflower oil (linoleic acid), and docosahexaenoic acid were evaluated among all the studies reviewed for atopic dermatitis.7 Bronsnick et al8 conducted a review of evidence supporting vitamin supplementation and atopic dermatitis, and for the most part determined that the studies had insufficient evidence. The only positive correlation was noted with prebiotics and probiotics in another Cochrane review in 2013, which evaluated 4 studies with 1428 infants showing prebiotic supplementation reduced atopic dermatitis.9 In 2014 Panduru et al10 evaluated 16 studies in a meta-analysis that showed how probiotics were possibly beneficial in both general and high-risk atopic populations. Specifically, a subgroup analysis showed that Lactobacillus and Lactobacillus with Bifidobacterium also can be protective against atopic dermatitis.10 Lastly, diet avoidance in pregnancy or during lactation in infants up to 18 months of age did not have any effect on improving the infant’s atopic dermatitis based on a 2012 Cochrane review that included 952 participants.11
Conclusion
Overall, there are some benefits to dietary restrictions and supplementation as indicated by the studies reviewed here; however, the extent to which these changes contribute to disease manifestation has only been linked, not definitively proven. Randomized controlled trials with large sample sizes, double-blind studies, and appropriately controlled studies with comparative patient populations are difficult to obtain, as diet cannot be completely restrictive for every patient. Patients should be provided with the latest data supporting a possible link between dairy consumption and acne production as well as prebiotics or probiotics during pregnancy and at infancy to reduce the risk for atopic dermatitis with the caveat of association. That said, future studies might prove that dietary and environmental alterations may prevent disease progression or appearance far more than previously assumed.
- Fulton JE Jr, Plewaig G, Kligman AM. Effect of chocolate on acne vulgaris. JAMA. 1969;210: 2071-2074.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- LaRosa CL, Quach KA, Koons K, et al. Consumption of dairy in teenagers with and without acne. J Am Acad Dermatol. 2016;75:318-322.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online ahead of print July 16, 2016]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.13835.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop
Ser Pediatr Program. 2011;67:131-145. - Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part i. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Osborn DA, Sinn JKH. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013;2:CD006474.
- Panduru M, Panduru NM, Saˇlaˇvaˇstru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies [published online April 4, 2014]. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
When I am in clinic, I often get at least 3 to 4 inquiries each day from patients about the necessity for dietary restrictions or alterations as well as the benefits of these changes in limiting their dermatological disease processes. I usually am restricted in my response because the research rarely indicates benefits of one diet versus another; however, this discussion has recently become a heavily researched area as patients have come to value natural nonpharmaceutical approaches to their holistic care. In this article, a few dietary restrictions and supplements are reviewed that may have a beneficial effect in managing patients with acne vulgaris and atopic dermatitis.
Acne Vulgaris
In 1969 Fulton et al1 conducted one of the first few trials on acne and diet management. In this crossover, patient-blinded, interventional study, patients were divided into 2 subgroups (N=65): 1 adolescent patient with moderate acne was compared to 1 male prisoner given a chocolate bar for 4 weeks or a control bar with equivalent caloric index. The results indicated no change in acne vulgaris lesions based on either intervention; however, there were obvious deficiencies in the study including small sample size, inappropriate grouping of an adolescent patient versus a prisoner, and limited study period.1
Since then, multiple studies have been conducted with parallel participants, large sample sizes, and at least a 12-week study period. In 2005, Adebamowo et al2 studied 47,355 women using a validated food frequency questionnaire that determined the amount of dairy consumed, specifically skim milk. The study showed a positive link between increased dairy consumption and acne formation; however, again due to the retrospective analysis and recall bias, it is difficult to determine if a link can truly be noted between acne and dairy in this study.2
More recently, LaRosa et al3 conducted a study that included 225 participants aged 14 to 19 years. Excluding participants with lactose intolerance and current use of oral contraceptives and isotretinoin, the study placed 120 participants in the test group versus 105 participants in the control group. The study was conducted using 3 telephone interviews and a 24-hour diet recall technique. The results supported a link between acne and skim milk consumption. Again, although the studied relied on participant self-reports of diet and followed a case-control design, a possible association was suspected but not validated.3 A longitudinal, questionnaire-based population study performed by Ulvestad et al4 included 2489 patients. This study further evaluated recall of dairy product consumption at 15 to 16 years of age and then 3 years later acne severity was self-assessed and reported at 18 to 19 years of age. Overall, this evaluation indicated that a high intake of dairy products and acne in adolescence have been positively associated. However, it was another retrospective study with recall bias.4 In 2009 Melnick and Schmitz5 concluded that milk causes the body to elevate both insulin and insulinlike growth factor 1 levels. In another study by Melnick6 in 2011, a definitive link between increased insulin and insulinlike growth factor 1 signaling in promoting comedogenesis was reported. Given the few studies that show the potential link between dairy products and acne, this dairy-free diet can be considered as a diet recommendation for acne patients.
Atopic Dermatitis
A Cochrane review conducted in 2012 regarding dietary supplements as a treatment of atopic dermatitis evaluated randomized controlled trials (N=596). Supplementation with vitamin D, fish oil, olive oil, zinc sulfate, selenium, vitamin E, pyridoxine, sea buckthorn seed oil, hempseed oil, sunflower oil (linoleic acid), and docosahexaenoic acid were evaluated among all the studies reviewed for atopic dermatitis.7 Bronsnick et al8 conducted a review of evidence supporting vitamin supplementation and atopic dermatitis, and for the most part determined that the studies had insufficient evidence. The only positive correlation was noted with prebiotics and probiotics in another Cochrane review in 2013, which evaluated 4 studies with 1428 infants showing prebiotic supplementation reduced atopic dermatitis.9 In 2014 Panduru et al10 evaluated 16 studies in a meta-analysis that showed how probiotics were possibly beneficial in both general and high-risk atopic populations. Specifically, a subgroup analysis showed that Lactobacillus and Lactobacillus with Bifidobacterium also can be protective against atopic dermatitis.10 Lastly, diet avoidance in pregnancy or during lactation in infants up to 18 months of age did not have any effect on improving the infant’s atopic dermatitis based on a 2012 Cochrane review that included 952 participants.11
Conclusion
Overall, there are some benefits to dietary restrictions and supplementation as indicated by the studies reviewed here; however, the extent to which these changes contribute to disease manifestation has only been linked, not definitively proven. Randomized controlled trials with large sample sizes, double-blind studies, and appropriately controlled studies with comparative patient populations are difficult to obtain, as diet cannot be completely restrictive for every patient. Patients should be provided with the latest data supporting a possible link between dairy consumption and acne production as well as prebiotics or probiotics during pregnancy and at infancy to reduce the risk for atopic dermatitis with the caveat of association. That said, future studies might prove that dietary and environmental alterations may prevent disease progression or appearance far more than previously assumed.
When I am in clinic, I often get at least 3 to 4 inquiries each day from patients about the necessity for dietary restrictions or alterations as well as the benefits of these changes in limiting their dermatological disease processes. I usually am restricted in my response because the research rarely indicates benefits of one diet versus another; however, this discussion has recently become a heavily researched area as patients have come to value natural nonpharmaceutical approaches to their holistic care. In this article, a few dietary restrictions and supplements are reviewed that may have a beneficial effect in managing patients with acne vulgaris and atopic dermatitis.
Acne Vulgaris
In 1969 Fulton et al1 conducted one of the first few trials on acne and diet management. In this crossover, patient-blinded, interventional study, patients were divided into 2 subgroups (N=65): 1 adolescent patient with moderate acne was compared to 1 male prisoner given a chocolate bar for 4 weeks or a control bar with equivalent caloric index. The results indicated no change in acne vulgaris lesions based on either intervention; however, there were obvious deficiencies in the study including small sample size, inappropriate grouping of an adolescent patient versus a prisoner, and limited study period.1
Since then, multiple studies have been conducted with parallel participants, large sample sizes, and at least a 12-week study period. In 2005, Adebamowo et al2 studied 47,355 women using a validated food frequency questionnaire that determined the amount of dairy consumed, specifically skim milk. The study showed a positive link between increased dairy consumption and acne formation; however, again due to the retrospective analysis and recall bias, it is difficult to determine if a link can truly be noted between acne and dairy in this study.2
More recently, LaRosa et al3 conducted a study that included 225 participants aged 14 to 19 years. Excluding participants with lactose intolerance and current use of oral contraceptives and isotretinoin, the study placed 120 participants in the test group versus 105 participants in the control group. The study was conducted using 3 telephone interviews and a 24-hour diet recall technique. The results supported a link between acne and skim milk consumption. Again, although the studied relied on participant self-reports of diet and followed a case-control design, a possible association was suspected but not validated.3 A longitudinal, questionnaire-based population study performed by Ulvestad et al4 included 2489 patients. This study further evaluated recall of dairy product consumption at 15 to 16 years of age and then 3 years later acne severity was self-assessed and reported at 18 to 19 years of age. Overall, this evaluation indicated that a high intake of dairy products and acne in adolescence have been positively associated. However, it was another retrospective study with recall bias.4 In 2009 Melnick and Schmitz5 concluded that milk causes the body to elevate both insulin and insulinlike growth factor 1 levels. In another study by Melnick6 in 2011, a definitive link between increased insulin and insulinlike growth factor 1 signaling in promoting comedogenesis was reported. Given the few studies that show the potential link between dairy products and acne, this dairy-free diet can be considered as a diet recommendation for acne patients.
Atopic Dermatitis
A Cochrane review conducted in 2012 regarding dietary supplements as a treatment of atopic dermatitis evaluated randomized controlled trials (N=596). Supplementation with vitamin D, fish oil, olive oil, zinc sulfate, selenium, vitamin E, pyridoxine, sea buckthorn seed oil, hempseed oil, sunflower oil (linoleic acid), and docosahexaenoic acid were evaluated among all the studies reviewed for atopic dermatitis.7 Bronsnick et al8 conducted a review of evidence supporting vitamin supplementation and atopic dermatitis, and for the most part determined that the studies had insufficient evidence. The only positive correlation was noted with prebiotics and probiotics in another Cochrane review in 2013, which evaluated 4 studies with 1428 infants showing prebiotic supplementation reduced atopic dermatitis.9 In 2014 Panduru et al10 evaluated 16 studies in a meta-analysis that showed how probiotics were possibly beneficial in both general and high-risk atopic populations. Specifically, a subgroup analysis showed that Lactobacillus and Lactobacillus with Bifidobacterium also can be protective against atopic dermatitis.10 Lastly, diet avoidance in pregnancy or during lactation in infants up to 18 months of age did not have any effect on improving the infant’s atopic dermatitis based on a 2012 Cochrane review that included 952 participants.11
Conclusion
Overall, there are some benefits to dietary restrictions and supplementation as indicated by the studies reviewed here; however, the extent to which these changes contribute to disease manifestation has only been linked, not definitively proven. Randomized controlled trials with large sample sizes, double-blind studies, and appropriately controlled studies with comparative patient populations are difficult to obtain, as diet cannot be completely restrictive for every patient. Patients should be provided with the latest data supporting a possible link between dairy consumption and acne production as well as prebiotics or probiotics during pregnancy and at infancy to reduce the risk for atopic dermatitis with the caveat of association. That said, future studies might prove that dietary and environmental alterations may prevent disease progression or appearance far more than previously assumed.
- Fulton JE Jr, Plewaig G, Kligman AM. Effect of chocolate on acne vulgaris. JAMA. 1969;210: 2071-2074.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- LaRosa CL, Quach KA, Koons K, et al. Consumption of dairy in teenagers with and without acne. J Am Acad Dermatol. 2016;75:318-322.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online ahead of print July 16, 2016]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.13835.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop
Ser Pediatr Program. 2011;67:131-145. - Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part i. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Osborn DA, Sinn JKH. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013;2:CD006474.
- Panduru M, Panduru NM, Saˇlaˇvaˇstru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies [published online April 4, 2014]. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
- Fulton JE Jr, Plewaig G, Kligman AM. Effect of chocolate on acne vulgaris. JAMA. 1969;210: 2071-2074.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- LaRosa CL, Quach KA, Koons K, et al. Consumption of dairy in teenagers with and without acne. J Am Acad Dermatol. 2016;75:318-322.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online ahead of print July 16, 2016]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.13835.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop
Ser Pediatr Program. 2011;67:131-145. - Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part i. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Osborn DA, Sinn JKH. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013;2:CD006474.
- Panduru M, Panduru NM, Saˇlaˇvaˇstru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies [published online April 4, 2014]. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.