User login
VIDEO: DNA-derived Zika vaccine in humans holds promise
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
BALTIMORE – Hope is high right now for a genetically engineered Zika virus vaccine currently being tested in humans.
“If we saw something, even early on, that we were concerned about, we would stop the trial and re-evaluate it. And that hasn’t happened, so that’s good news,” said Kathleen M. Neuzil, MD, lead investigator of the trial and a professor at the University of Maryland in Baltimore, where she is also director of the Center for Vaccine Development.
Testing of the DNA-based Zika vaccine began in early August. Now that Congress has approved a $1.1 billion funding package, Dr. Neuzil said investigators can rest assured that there is enough funding for the duration of this trial, which ultimately could take 2 years or longer.
Sponsored by the National Institutes of Health, phase I of the trial is being conducted at three U.S. sites, including the University of Maryland’s Vaccine Development Center, the NIH Clinical Center in Bethesda, Md., and Emory University in Atlanta.
Safety and tolerability results from all three sites are expected by year’s end and will inform how to construct phase II, currently set to begin in early 2017. That phase is expected to include a much larger population than the 80 current participants, although the exact numbers are still to be determined, Dr. Neuzil said. Also still unknown is whether phase II will be conducted internationally.
If the vaccine proves effective, it will be a high point in the annals of maternal-fetal medicine, said Christopher Harman, MD, chair of obstetrics and gynecology and director of the division of maternal-fetal medicine at the University of Maryland. “It’s going to become a story where the power of modern medicine has defeated an epidemic that had enormous potential for harm.”
Dr. Harman also predicted that a viable vaccine, combined with infectious disease prevention services globally, will mitigate Zika’s threat to the population, particularly developing fetuses.
In the meantime, there needs to be continued public education about preventing the spread of Zika, as well as rigorous attention to family planning, Dr. Harman said.
“Planned pregnancy is essential,” he said. “This puts the burden on the couple or the woman. Don’t get pregnant if you’re in an endemic area.”
For more about the vaccine trial and how clinicians can counsel patients about the threat of Zika, watch this video recorded at the University of Maryland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
Cord blood product granted breakthrough designation

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for NiCord®, a product in development as a graft modality for patients undergoing hematopoietic stem cell transplant to treat high-risk hematologic malignancies.
NiCord consists of cells from a single unit of umbilical cord blood cultured in nicotinamide (a vitamin B derivative) and cytokines that are typically used for expansion (thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor).
Data from the pilot and phase 1/2 studies of NiCord have suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
Additional research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
The phase 3 registration study of NiCord is scheduled to begin before the end of this year.
In addition to breakthrough designation, NiCord also has orphan designation from the FDA as a treatment for patients with acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, or myelodysplastic syndromes.
NiCord is being developed by Gamida Cell.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for NiCord®, a product in development as a graft modality for patients undergoing hematopoietic stem cell transplant to treat high-risk hematologic malignancies.
NiCord consists of cells from a single unit of umbilical cord blood cultured in nicotinamide (a vitamin B derivative) and cytokines that are typically used for expansion (thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor).
Data from the pilot and phase 1/2 studies of NiCord have suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
Additional research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
The phase 3 registration study of NiCord is scheduled to begin before the end of this year.
In addition to breakthrough designation, NiCord also has orphan designation from the FDA as a treatment for patients with acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, or myelodysplastic syndromes.
NiCord is being developed by Gamida Cell.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for NiCord®, a product in development as a graft modality for patients undergoing hematopoietic stem cell transplant to treat high-risk hematologic malignancies.
NiCord consists of cells from a single unit of umbilical cord blood cultured in nicotinamide (a vitamin B derivative) and cytokines that are typically used for expansion (thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor).
Data from the pilot and phase 1/2 studies of NiCord have suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
Additional research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
The phase 3 registration study of NiCord is scheduled to begin before the end of this year.
In addition to breakthrough designation, NiCord also has orphan designation from the FDA as a treatment for patients with acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, or myelodysplastic syndromes.
NiCord is being developed by Gamida Cell.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
Surveys Are Not the Most Effective Way to Improve Patient Satisfaction
What started with a car dealership survey has become a near avalanche of surveys from my credit card, bank, airlines, hotels, and other businesses. Each starts by assuring me that it will take only a minute or two to complete the survey, but if I completed every survey sent my way, it would add up to a significant amount of time. So I’ve stopped responding to nearly all of them, not so much as a form of protest but as part of my overall time-management efforts.
I imagine many of our patients see surveys from hospitals and other healthcare providers similarly: just another one to add to the pile. Patients in their 80s and 90s—a significant portion of hospitalist patients—probably interact a lot less with companies that send satisfaction surveys and so might be more attentive to ones from healthcare organizations. But I suspect that a reasonable portion of older patients rely on a family member to complete them, and this person, often a son or daughter, probably does get a lot of similar surveys. Surely, survey fatigue is influencing the results at least a little.
Healthcare Surveys: HCAHPS
For all the surveying going on, I find it pretty difficult to use HCAHPS results to guide patient-satisfaction improvement efforts. Sure, I can see how individual doctors or different physician groups score compared to one another and try to model my behaviors after the high performers. That is a really valuable thing to do, but it doesn’t get to the granular level I’d like.
One would hope the three physician-specific HCAHPS questions would support drilling down to more actionable information. But every hospitalist group I’ve seen always has the same pattern, scoring from lowest to highest as follows:
- How often did doctors explain things in a way you could understand?
- How often did doctors listen carefully to you?
- How often did doctors treat you with courtesy and respect?
So I don’t think the difference in scores on these questions is very useful in guiding improvement efforts.
Looking beyond HCAHPS
For a few years, our hospitalist group added a very short survey to the brochure describing the practice. I still think that was good idea to ensure accurate attribution and more granular information, but it didn’t yield much value in practice because of a low response rate. Ultimately, we stopped using it because of our hospital risk manager’s concern any such survey could be construed as “coaching” patients in their HCAHPS responses, something the Centers for Medicare & Medicaid Services forbids.
Mark Rudolph, MD, vice president of physician development and patient experience at Sound Physicians, told me about their experience with their employed RNs using tablet computers to survey every patient the day following hospital admission (i.e., while patients were still in the hospital). It seems to me this could be a really valuable tool to provide very granular feedback at the outset of a patient stay when there is still time to address areas in which the patient is less satisfied. They found that for about 30% of patients, the survey uncovered something that could be fixed, such as providing another blanket, determining what time a test was likely to be done, etc. I bet for most patients the fact that a nurse cared enough to ask how things are going and try to remedy problems improved their HCAPHS scores.
Yet after some experience with this approach, Sound Physicians found that, for a number of reasons, this wasn’t as valuable as hoped. They now survey a smaller sample of patients and sometimes adjust the questions based on the known or suspected strengths and weaknesses of individual providers. For one doctor, for example, the survey might ask whether the doctor spent enough time with the patient; for another, it might ask if the doctor spoke clearly, etc.
Dr. Rudolph thinks having someone such as the lead hospitalist observe the doctor while on rounds might ultimately prove more valuable than administering a survey. It will be interesting to see how his group and others around the country evolve their approach to better understand each provider’s strengths and weaknesses and most effective ways to improve patient satisfaction.
How to Improve Patient Satisfaction?
In my April 2012 column, I wrote about several things for hospitalists to consider including in their patient-satisfaction improvement plan. And, of course, there are a lot of additional sources of ideas available just by searching the Internet.
I find it difficult to consistently implement a bundle of multiple different habits, such as always sitting or always rounding with the patient’s bedside nurse, etc. I acknowledge these are proven valuable strategies to improve scores, but I still find it hard to do them consistently.
For some of us, it might be better to pick one thing to focus on. And while I don’t have research data to prove it, I think the single most valuable thing to improve patient satisfaction with hospitalists is to phone patients after discharge. It isn’t as difficult as most assume, and it often leads patients (or the family member you reach) to thank you profusely for the call. I think hospitalists can really benefit from more expressions of gratitude from patients and families, and these calls often provide it.
I’ve learned a few lessons about making post-discharge calls that are detailed in my August 2012 column. TH

What started with a car dealership survey has become a near avalanche of surveys from my credit card, bank, airlines, hotels, and other businesses. Each starts by assuring me that it will take only a minute or two to complete the survey, but if I completed every survey sent my way, it would add up to a significant amount of time. So I’ve stopped responding to nearly all of them, not so much as a form of protest but as part of my overall time-management efforts.
I imagine many of our patients see surveys from hospitals and other healthcare providers similarly: just another one to add to the pile. Patients in their 80s and 90s—a significant portion of hospitalist patients—probably interact a lot less with companies that send satisfaction surveys and so might be more attentive to ones from healthcare organizations. But I suspect that a reasonable portion of older patients rely on a family member to complete them, and this person, often a son or daughter, probably does get a lot of similar surveys. Surely, survey fatigue is influencing the results at least a little.
Healthcare Surveys: HCAHPS
For all the surveying going on, I find it pretty difficult to use HCAHPS results to guide patient-satisfaction improvement efforts. Sure, I can see how individual doctors or different physician groups score compared to one another and try to model my behaviors after the high performers. That is a really valuable thing to do, but it doesn’t get to the granular level I’d like.
One would hope the three physician-specific HCAHPS questions would support drilling down to more actionable information. But every hospitalist group I’ve seen always has the same pattern, scoring from lowest to highest as follows:
- How often did doctors explain things in a way you could understand?
- How often did doctors listen carefully to you?
- How often did doctors treat you with courtesy and respect?
So I don’t think the difference in scores on these questions is very useful in guiding improvement efforts.
Looking beyond HCAHPS
For a few years, our hospitalist group added a very short survey to the brochure describing the practice. I still think that was good idea to ensure accurate attribution and more granular information, but it didn’t yield much value in practice because of a low response rate. Ultimately, we stopped using it because of our hospital risk manager’s concern any such survey could be construed as “coaching” patients in their HCAHPS responses, something the Centers for Medicare & Medicaid Services forbids.
Mark Rudolph, MD, vice president of physician development and patient experience at Sound Physicians, told me about their experience with their employed RNs using tablet computers to survey every patient the day following hospital admission (i.e., while patients were still in the hospital). It seems to me this could be a really valuable tool to provide very granular feedback at the outset of a patient stay when there is still time to address areas in which the patient is less satisfied. They found that for about 30% of patients, the survey uncovered something that could be fixed, such as providing another blanket, determining what time a test was likely to be done, etc. I bet for most patients the fact that a nurse cared enough to ask how things are going and try to remedy problems improved their HCAPHS scores.
Yet after some experience with this approach, Sound Physicians found that, for a number of reasons, this wasn’t as valuable as hoped. They now survey a smaller sample of patients and sometimes adjust the questions based on the known or suspected strengths and weaknesses of individual providers. For one doctor, for example, the survey might ask whether the doctor spent enough time with the patient; for another, it might ask if the doctor spoke clearly, etc.
Dr. Rudolph thinks having someone such as the lead hospitalist observe the doctor while on rounds might ultimately prove more valuable than administering a survey. It will be interesting to see how his group and others around the country evolve their approach to better understand each provider’s strengths and weaknesses and most effective ways to improve patient satisfaction.
How to Improve Patient Satisfaction?
In my April 2012 column, I wrote about several things for hospitalists to consider including in their patient-satisfaction improvement plan. And, of course, there are a lot of additional sources of ideas available just by searching the Internet.
I find it difficult to consistently implement a bundle of multiple different habits, such as always sitting or always rounding with the patient’s bedside nurse, etc. I acknowledge these are proven valuable strategies to improve scores, but I still find it hard to do them consistently.
For some of us, it might be better to pick one thing to focus on. And while I don’t have research data to prove it, I think the single most valuable thing to improve patient satisfaction with hospitalists is to phone patients after discharge. It isn’t as difficult as most assume, and it often leads patients (or the family member you reach) to thank you profusely for the call. I think hospitalists can really benefit from more expressions of gratitude from patients and families, and these calls often provide it.
I’ve learned a few lessons about making post-discharge calls that are detailed in my August 2012 column. TH

What started with a car dealership survey has become a near avalanche of surveys from my credit card, bank, airlines, hotels, and other businesses. Each starts by assuring me that it will take only a minute or two to complete the survey, but if I completed every survey sent my way, it would add up to a significant amount of time. So I’ve stopped responding to nearly all of them, not so much as a form of protest but as part of my overall time-management efforts.
I imagine many of our patients see surveys from hospitals and other healthcare providers similarly: just another one to add to the pile. Patients in their 80s and 90s—a significant portion of hospitalist patients—probably interact a lot less with companies that send satisfaction surveys and so might be more attentive to ones from healthcare organizations. But I suspect that a reasonable portion of older patients rely on a family member to complete them, and this person, often a son or daughter, probably does get a lot of similar surveys. Surely, survey fatigue is influencing the results at least a little.
Healthcare Surveys: HCAHPS
For all the surveying going on, I find it pretty difficult to use HCAHPS results to guide patient-satisfaction improvement efforts. Sure, I can see how individual doctors or different physician groups score compared to one another and try to model my behaviors after the high performers. That is a really valuable thing to do, but it doesn’t get to the granular level I’d like.
One would hope the three physician-specific HCAHPS questions would support drilling down to more actionable information. But every hospitalist group I’ve seen always has the same pattern, scoring from lowest to highest as follows:
- How often did doctors explain things in a way you could understand?
- How often did doctors listen carefully to you?
- How often did doctors treat you with courtesy and respect?
So I don’t think the difference in scores on these questions is very useful in guiding improvement efforts.
Looking beyond HCAHPS
For a few years, our hospitalist group added a very short survey to the brochure describing the practice. I still think that was good idea to ensure accurate attribution and more granular information, but it didn’t yield much value in practice because of a low response rate. Ultimately, we stopped using it because of our hospital risk manager’s concern any such survey could be construed as “coaching” patients in their HCAHPS responses, something the Centers for Medicare & Medicaid Services forbids.
Mark Rudolph, MD, vice president of physician development and patient experience at Sound Physicians, told me about their experience with their employed RNs using tablet computers to survey every patient the day following hospital admission (i.e., while patients were still in the hospital). It seems to me this could be a really valuable tool to provide very granular feedback at the outset of a patient stay when there is still time to address areas in which the patient is less satisfied. They found that for about 30% of patients, the survey uncovered something that could be fixed, such as providing another blanket, determining what time a test was likely to be done, etc. I bet for most patients the fact that a nurse cared enough to ask how things are going and try to remedy problems improved their HCAPHS scores.
Yet after some experience with this approach, Sound Physicians found that, for a number of reasons, this wasn’t as valuable as hoped. They now survey a smaller sample of patients and sometimes adjust the questions based on the known or suspected strengths and weaknesses of individual providers. For one doctor, for example, the survey might ask whether the doctor spent enough time with the patient; for another, it might ask if the doctor spoke clearly, etc.
Dr. Rudolph thinks having someone such as the lead hospitalist observe the doctor while on rounds might ultimately prove more valuable than administering a survey. It will be interesting to see how his group and others around the country evolve their approach to better understand each provider’s strengths and weaknesses and most effective ways to improve patient satisfaction.
How to Improve Patient Satisfaction?
In my April 2012 column, I wrote about several things for hospitalists to consider including in their patient-satisfaction improvement plan. And, of course, there are a lot of additional sources of ideas available just by searching the Internet.
I find it difficult to consistently implement a bundle of multiple different habits, such as always sitting or always rounding with the patient’s bedside nurse, etc. I acknowledge these are proven valuable strategies to improve scores, but I still find it hard to do them consistently.
For some of us, it might be better to pick one thing to focus on. And while I don’t have research data to prove it, I think the single most valuable thing to improve patient satisfaction with hospitalists is to phone patients after discharge. It isn’t as difficult as most assume, and it often leads patients (or the family member you reach) to thank you profusely for the call. I think hospitalists can really benefit from more expressions of gratitude from patients and families, and these calls often provide it.
I’ve learned a few lessons about making post-discharge calls that are detailed in my August 2012 column. TH

Bovine heparin may be comparable to porcine heparin

A new study suggests that heparin derived from cattle may be as effective as heparin derived from pigs, but more research is needed.
Investigators found differences in the structural and molecular characteristics of bovine and porcine heparins, but these differences did not appear to have a significant effect on the drugs’ anticoagulant properties in vitro.
Still, the researchers said it is not clear if these differences affect the drugs’ activity in humans.
Jawed Fareed, PhD, of Loyola University Chicago Stritch School of Medicine in Illinois, and his colleagues conducted this research and reported the results in Clinical and Applied Thrombosis/Hemostasis.
Heparin used in the US now comes from the intestines of pigs that are slaughtered for meat. Seventy-five percent of the heparin used in the US comes from China, which is the world’s largest pork producer.
As heparin is a widely used drug that, in certain cases, has no medical alternatives, the US healthcare system could be vulnerable to fluctuations in the crude heparin supply, according to the US Food and Drug Administration’s Center for Drug Evaluation and Research.
Therefore, the center is evaluating reintroducing bovine heparin to the market in order to avoid shortages, particularly in case the porcine heparin supply is contaminated, as occurred in 2008.
Bovine heparin was voluntarily withdrawn from the market during the 1980s due to worries that it could, in rare cases, cause thrombocytopenia or be contaminated with bovine spongiform encephalopathy (mad cow disease).
According to Dr Fareed, those fears have been put to rest by advancements in manufacturing and quality control that have resulted in purer forms of heparin. Still, some experts remain skeptical about bovine heparin.
With this in mind, Dr Fareed and his colleagues compared individually manufactured batches of bovine heparin (historical samples from a variety of manufacturers) with individually manufactured batches of porcine heparin (current samples from 2 different manufacturers).
The investigators studied heparins isolated from pig intestines (PI), bovine lungs (BL), and bovine intestines (BI).
They found the BL, BI, and PI heparins all produced a concentration-dependent anticoagulant effect. And there was no significant difference in the anti-Xa and anti-IIa potencies of the BL, BI, and PI heparins.
The researchers noted that there were significant structural differences between PI and BI heparins, and it isn’t clear whether these differences would have an impact in patients.
Likewise, although the BL heparin samples were “highly similar” to the PI heparin samples, the BL heparins had a lower molecular weight. And the investigators said it was unclear if this would adversely affect the BL heparins’ pharmacology.
Furthermore, the researchers noted that there was greater variability among the BL heparins than the PI heparins. However, they said this could be due to the multiple historic manufacturers of BL heparins.
The investigators concluded that more research is needed to examine these possibilities. ![]()

A new study suggests that heparin derived from cattle may be as effective as heparin derived from pigs, but more research is needed.
Investigators found differences in the structural and molecular characteristics of bovine and porcine heparins, but these differences did not appear to have a significant effect on the drugs’ anticoagulant properties in vitro.
Still, the researchers said it is not clear if these differences affect the drugs’ activity in humans.
Jawed Fareed, PhD, of Loyola University Chicago Stritch School of Medicine in Illinois, and his colleagues conducted this research and reported the results in Clinical and Applied Thrombosis/Hemostasis.
Heparin used in the US now comes from the intestines of pigs that are slaughtered for meat. Seventy-five percent of the heparin used in the US comes from China, which is the world’s largest pork producer.
As heparin is a widely used drug that, in certain cases, has no medical alternatives, the US healthcare system could be vulnerable to fluctuations in the crude heparin supply, according to the US Food and Drug Administration’s Center for Drug Evaluation and Research.
Therefore, the center is evaluating reintroducing bovine heparin to the market in order to avoid shortages, particularly in case the porcine heparin supply is contaminated, as occurred in 2008.
Bovine heparin was voluntarily withdrawn from the market during the 1980s due to worries that it could, in rare cases, cause thrombocytopenia or be contaminated with bovine spongiform encephalopathy (mad cow disease).
According to Dr Fareed, those fears have been put to rest by advancements in manufacturing and quality control that have resulted in purer forms of heparin. Still, some experts remain skeptical about bovine heparin.
With this in mind, Dr Fareed and his colleagues compared individually manufactured batches of bovine heparin (historical samples from a variety of manufacturers) with individually manufactured batches of porcine heparin (current samples from 2 different manufacturers).
The investigators studied heparins isolated from pig intestines (PI), bovine lungs (BL), and bovine intestines (BI).
They found the BL, BI, and PI heparins all produced a concentration-dependent anticoagulant effect. And there was no significant difference in the anti-Xa and anti-IIa potencies of the BL, BI, and PI heparins.
The researchers noted that there were significant structural differences between PI and BI heparins, and it isn’t clear whether these differences would have an impact in patients.
Likewise, although the BL heparin samples were “highly similar” to the PI heparin samples, the BL heparins had a lower molecular weight. And the investigators said it was unclear if this would adversely affect the BL heparins’ pharmacology.
Furthermore, the researchers noted that there was greater variability among the BL heparins than the PI heparins. However, they said this could be due to the multiple historic manufacturers of BL heparins.
The investigators concluded that more research is needed to examine these possibilities. ![]()

A new study suggests that heparin derived from cattle may be as effective as heparin derived from pigs, but more research is needed.
Investigators found differences in the structural and molecular characteristics of bovine and porcine heparins, but these differences did not appear to have a significant effect on the drugs’ anticoagulant properties in vitro.
Still, the researchers said it is not clear if these differences affect the drugs’ activity in humans.
Jawed Fareed, PhD, of Loyola University Chicago Stritch School of Medicine in Illinois, and his colleagues conducted this research and reported the results in Clinical and Applied Thrombosis/Hemostasis.
Heparin used in the US now comes from the intestines of pigs that are slaughtered for meat. Seventy-five percent of the heparin used in the US comes from China, which is the world’s largest pork producer.
As heparin is a widely used drug that, in certain cases, has no medical alternatives, the US healthcare system could be vulnerable to fluctuations in the crude heparin supply, according to the US Food and Drug Administration’s Center for Drug Evaluation and Research.
Therefore, the center is evaluating reintroducing bovine heparin to the market in order to avoid shortages, particularly in case the porcine heparin supply is contaminated, as occurred in 2008.
Bovine heparin was voluntarily withdrawn from the market during the 1980s due to worries that it could, in rare cases, cause thrombocytopenia or be contaminated with bovine spongiform encephalopathy (mad cow disease).
According to Dr Fareed, those fears have been put to rest by advancements in manufacturing and quality control that have resulted in purer forms of heparin. Still, some experts remain skeptical about bovine heparin.
With this in mind, Dr Fareed and his colleagues compared individually manufactured batches of bovine heparin (historical samples from a variety of manufacturers) with individually manufactured batches of porcine heparin (current samples from 2 different manufacturers).
The investigators studied heparins isolated from pig intestines (PI), bovine lungs (BL), and bovine intestines (BI).
They found the BL, BI, and PI heparins all produced a concentration-dependent anticoagulant effect. And there was no significant difference in the anti-Xa and anti-IIa potencies of the BL, BI, and PI heparins.
The researchers noted that there were significant structural differences between PI and BI heparins, and it isn’t clear whether these differences would have an impact in patients.
Likewise, although the BL heparin samples were “highly similar” to the PI heparin samples, the BL heparins had a lower molecular weight. And the investigators said it was unclear if this would adversely affect the BL heparins’ pharmacology.
Furthermore, the researchers noted that there was greater variability among the BL heparins than the PI heparins. However, they said this could be due to the multiple historic manufacturers of BL heparins.
The investigators concluded that more research is needed to examine these possibilities. ![]()
FDA says ROCKET AF trial results hold up

The US Food and Drug Administration (FDA) has concluded that results of the ROCKET AF trial were not significantly affected by accuracy issues with the Alere INRatio
Monitor System.
ROCKET AF was the trial used to support the 2011 approval of rivaroxaban (Xarelto) in patients with nonvalvular atrial fibrillation, and the Alere INRatio Monitor System was used to measure international normalized ratios (INRs) in the warfarin arm of the trial.
Critics have questioned the results of ROCKET AF because the INRatio system (INRatio Monitor or INRatio2 Monitor and INRatio Test Strips) has been shown to give falsely low test results.
If the system provided falsely low INR values for patients in the warfarin arm of ROCKET AF, investigators may have given those patients incorrect doses of warfarin. This could increase the risk of bleeding in those patients and give a false impression of the comparative safety of rivaroxaban.
In addition to increasing the risk of bleeding, over-anticoagulation in the warfarin arm might have reduced the rate of ischemic stroke for these patients. This might have distorted the study’s efficacy results in favor of warfarin.
Because the INRatio system was shown to be defective, it was recalled for certain patients in December 2014 and was withdrawn from the US market in July 2016.
Janssen Pharmaceuticals, Inc., the company developing rivaroxaban in cooperation with Bayer, informed the FDA of the issues with the INRatio system, and its possible impact on ROCKET AF, in September 2015.
In response, the FDA conducted a variety of analyses to assess the impact the INRatio system had on the results of ROCKET AF. Janssen also performed its own analyses.
The FDA said the results of these analyses, which are available on the FDA website, suggest the INRatio system had minimal effects on strokes and bleeding in ROCKET AF.
The FDA therefore concluded that rivaroxaban is a safe and effective alternative to warfarin for patients with nonvalvular atrial fibrillation, no changes in rivaroxaban labeling are warranted, and no other major regulatory action should be taken with respect to rivaroxaban.
This corresponds to the opinion of the European Medicine’s Agency, which released a statement earlier this year saying the defect with the INRatio system does not change the overall conclusions of ROCKET AF.
Still, the issues with rivaroxaban are not fully resolved, as a recent investigation published in The BMJ suggested that Janssen and Bayer knew about concerns regarding the accuracy of the INRatio system while ROCKET AF was underway but allowed the system to be used in the trial anyway.
The BMJ said the companies also neglected to mention these concerns to regulatory authorities prior to rivaroxaban’s approval and later failed to notify regulators about the recall of the INRatio system, and its potential impact on ROCKET AF, in a timely manner.
In addition, Janssen, which was responsible for conducting ROCKET AF, did not tell regulators about a safety program the company launched during the trial to address concerns about the INRatio system. ![]()

The US Food and Drug Administration (FDA) has concluded that results of the ROCKET AF trial were not significantly affected by accuracy issues with the Alere INRatio
Monitor System.
ROCKET AF was the trial used to support the 2011 approval of rivaroxaban (Xarelto) in patients with nonvalvular atrial fibrillation, and the Alere INRatio Monitor System was used to measure international normalized ratios (INRs) in the warfarin arm of the trial.
Critics have questioned the results of ROCKET AF because the INRatio system (INRatio Monitor or INRatio2 Monitor and INRatio Test Strips) has been shown to give falsely low test results.
If the system provided falsely low INR values for patients in the warfarin arm of ROCKET AF, investigators may have given those patients incorrect doses of warfarin. This could increase the risk of bleeding in those patients and give a false impression of the comparative safety of rivaroxaban.
In addition to increasing the risk of bleeding, over-anticoagulation in the warfarin arm might have reduced the rate of ischemic stroke for these patients. This might have distorted the study’s efficacy results in favor of warfarin.
Because the INRatio system was shown to be defective, it was recalled for certain patients in December 2014 and was withdrawn from the US market in July 2016.
Janssen Pharmaceuticals, Inc., the company developing rivaroxaban in cooperation with Bayer, informed the FDA of the issues with the INRatio system, and its possible impact on ROCKET AF, in September 2015.
In response, the FDA conducted a variety of analyses to assess the impact the INRatio system had on the results of ROCKET AF. Janssen also performed its own analyses.
The FDA said the results of these analyses, which are available on the FDA website, suggest the INRatio system had minimal effects on strokes and bleeding in ROCKET AF.
The FDA therefore concluded that rivaroxaban is a safe and effective alternative to warfarin for patients with nonvalvular atrial fibrillation, no changes in rivaroxaban labeling are warranted, and no other major regulatory action should be taken with respect to rivaroxaban.
This corresponds to the opinion of the European Medicine’s Agency, which released a statement earlier this year saying the defect with the INRatio system does not change the overall conclusions of ROCKET AF.
Still, the issues with rivaroxaban are not fully resolved, as a recent investigation published in The BMJ suggested that Janssen and Bayer knew about concerns regarding the accuracy of the INRatio system while ROCKET AF was underway but allowed the system to be used in the trial anyway.
The BMJ said the companies also neglected to mention these concerns to regulatory authorities prior to rivaroxaban’s approval and later failed to notify regulators about the recall of the INRatio system, and its potential impact on ROCKET AF, in a timely manner.
In addition, Janssen, which was responsible for conducting ROCKET AF, did not tell regulators about a safety program the company launched during the trial to address concerns about the INRatio system. ![]()

The US Food and Drug Administration (FDA) has concluded that results of the ROCKET AF trial were not significantly affected by accuracy issues with the Alere INRatio
Monitor System.
ROCKET AF was the trial used to support the 2011 approval of rivaroxaban (Xarelto) in patients with nonvalvular atrial fibrillation, and the Alere INRatio Monitor System was used to measure international normalized ratios (INRs) in the warfarin arm of the trial.
Critics have questioned the results of ROCKET AF because the INRatio system (INRatio Monitor or INRatio2 Monitor and INRatio Test Strips) has been shown to give falsely low test results.
If the system provided falsely low INR values for patients in the warfarin arm of ROCKET AF, investigators may have given those patients incorrect doses of warfarin. This could increase the risk of bleeding in those patients and give a false impression of the comparative safety of rivaroxaban.
In addition to increasing the risk of bleeding, over-anticoagulation in the warfarin arm might have reduced the rate of ischemic stroke for these patients. This might have distorted the study’s efficacy results in favor of warfarin.
Because the INRatio system was shown to be defective, it was recalled for certain patients in December 2014 and was withdrawn from the US market in July 2016.
Janssen Pharmaceuticals, Inc., the company developing rivaroxaban in cooperation with Bayer, informed the FDA of the issues with the INRatio system, and its possible impact on ROCKET AF, in September 2015.
In response, the FDA conducted a variety of analyses to assess the impact the INRatio system had on the results of ROCKET AF. Janssen also performed its own analyses.
The FDA said the results of these analyses, which are available on the FDA website, suggest the INRatio system had minimal effects on strokes and bleeding in ROCKET AF.
The FDA therefore concluded that rivaroxaban is a safe and effective alternative to warfarin for patients with nonvalvular atrial fibrillation, no changes in rivaroxaban labeling are warranted, and no other major regulatory action should be taken with respect to rivaroxaban.
This corresponds to the opinion of the European Medicine’s Agency, which released a statement earlier this year saying the defect with the INRatio system does not change the overall conclusions of ROCKET AF.
Still, the issues with rivaroxaban are not fully resolved, as a recent investigation published in The BMJ suggested that Janssen and Bayer knew about concerns regarding the accuracy of the INRatio system while ROCKET AF was underway but allowed the system to be used in the trial anyway.
The BMJ said the companies also neglected to mention these concerns to regulatory authorities prior to rivaroxaban’s approval and later failed to notify regulators about the recall of the INRatio system, and its potential impact on ROCKET AF, in a timely manner.
In addition, Janssen, which was responsible for conducting ROCKET AF, did not tell regulators about a safety program the company launched during the trial to address concerns about the INRatio system. ![]()
Plasmapheresis system cleared by FDA

Photo from Business Wire
The US Food and Drug Administration (FDA) has granted 510(k) clearance for the Aurora™ Xi Plasmapheresis System.
The system collects plasma from donated blood and returns the remaining blood components to the donor.
The Aurora Xi Plasmapheresis System features a proprietary filtration-separation method that enables faster collection of source plasma, according to Fresenius Kabi, the company marketing the system.
“The system helps to improve plasma center efficiency and the overall experience for operators and donors,” said Dean Gregory, president, medical devices, Fresenius Kabi USA.
“Faster collection times mean more throughput for our customers, helping maximize the volumes of plasma they collect while assuring a good experience for plasma donors.”
Plasma collected via the Aurora Xi Plasmapheresis System can be used to treat bleeding disorders, burn victims, human immune deficiencies, and other chronic or genetic disorders.
The plasma can also be used to manufacture therapies such as albumin and intravenous immunoglobulin. ![]()

Photo from Business Wire
The US Food and Drug Administration (FDA) has granted 510(k) clearance for the Aurora™ Xi Plasmapheresis System.
The system collects plasma from donated blood and returns the remaining blood components to the donor.
The Aurora Xi Plasmapheresis System features a proprietary filtration-separation method that enables faster collection of source plasma, according to Fresenius Kabi, the company marketing the system.
“The system helps to improve plasma center efficiency and the overall experience for operators and donors,” said Dean Gregory, president, medical devices, Fresenius Kabi USA.
“Faster collection times mean more throughput for our customers, helping maximize the volumes of plasma they collect while assuring a good experience for plasma donors.”
Plasma collected via the Aurora Xi Plasmapheresis System can be used to treat bleeding disorders, burn victims, human immune deficiencies, and other chronic or genetic disorders.
The plasma can also be used to manufacture therapies such as albumin and intravenous immunoglobulin. ![]()

Photo from Business Wire
The US Food and Drug Administration (FDA) has granted 510(k) clearance for the Aurora™ Xi Plasmapheresis System.
The system collects plasma from donated blood and returns the remaining blood components to the donor.
The Aurora Xi Plasmapheresis System features a proprietary filtration-separation method that enables faster collection of source plasma, according to Fresenius Kabi, the company marketing the system.
“The system helps to improve plasma center efficiency and the overall experience for operators and donors,” said Dean Gregory, president, medical devices, Fresenius Kabi USA.
“Faster collection times mean more throughput for our customers, helping maximize the volumes of plasma they collect while assuring a good experience for plasma donors.”
Plasma collected via the Aurora Xi Plasmapheresis System can be used to treat bleeding disorders, burn victims, human immune deficiencies, and other chronic or genetic disorders.
The plasma can also be used to manufacture therapies such as albumin and intravenous immunoglobulin. ![]()
Risk Factors for Early Readmission After Anatomical or Reverse Total Shoulder Arthroplasty
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
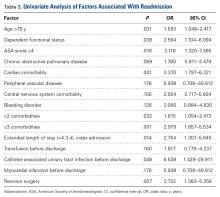
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.
Keeping Watch for Sepsis
Sepsis begins outside the hospital for 80% of patients, according to a recent CDC evaluation, reported in Vital Signs. CDC researchers who reviewed medical records of 246 adults and 79 children at 4 New York hospitals in Albany and Rochester found that 7 in 10 patients with sepsis had recently used health care services or had chronic diseases requiring frequent medical care.
Related: The Role of Procalcitonin in the Management of Infectious Diseases
Sepsis is most common in adults aged ≥ 65 years, infants < 1 year, people with weakened immune systems, or people with chronic conditions, such as diabetes. Nearly all the adults (97%) had at least 1 comorbidity, and 70% of children who developed sepsis had a health condition that may have put them at risk.
Although multiple infections and organisms were implicated, Staphylococcus aureus, Escherichia coli, and some types of Streptococcus were identified most often. Among adults with sepsis, 35% had a lung infection, 25% had a urinary tract infection, 11% had a gastrointestinal infection, and 11% had a skin infection.
Related: Mass Transit for Viruses
Most of the patients had recent interactions with the health care system before admission with sepsis, which likely reflects their vulnerability to infection, the researchers say, “it also suggests that health care facilities and providers could play a central role in sepsis prevention.” The CDC report advises the following for health care providers:
- Follow infection control requirements;
- Ensure that patients receive recommended vaccines (such as flu and pneumococcal);
- Educate patients and families, stressing the need to seek care if they see signs of severe infection or sepsis;
- “Think sepsis”—know the signs and symptoms and treat them early;
- Act fast—order tests to identify infection, start antibiotics and other care immediately; document dose, duration, and purpose; and
- Check patient progress frequently; reassess antibiotic therapy at 24 to 48 hours or sooner to change therapy if needed
Sepsis begins outside the hospital for 80% of patients, according to a recent CDC evaluation, reported in Vital Signs. CDC researchers who reviewed medical records of 246 adults and 79 children at 4 New York hospitals in Albany and Rochester found that 7 in 10 patients with sepsis had recently used health care services or had chronic diseases requiring frequent medical care.
Related: The Role of Procalcitonin in the Management of Infectious Diseases
Sepsis is most common in adults aged ≥ 65 years, infants < 1 year, people with weakened immune systems, or people with chronic conditions, such as diabetes. Nearly all the adults (97%) had at least 1 comorbidity, and 70% of children who developed sepsis had a health condition that may have put them at risk.
Although multiple infections and organisms were implicated, Staphylococcus aureus, Escherichia coli, and some types of Streptococcus were identified most often. Among adults with sepsis, 35% had a lung infection, 25% had a urinary tract infection, 11% had a gastrointestinal infection, and 11% had a skin infection.
Related: Mass Transit for Viruses
Most of the patients had recent interactions with the health care system before admission with sepsis, which likely reflects their vulnerability to infection, the researchers say, “it also suggests that health care facilities and providers could play a central role in sepsis prevention.” The CDC report advises the following for health care providers:
- Follow infection control requirements;
- Ensure that patients receive recommended vaccines (such as flu and pneumococcal);
- Educate patients and families, stressing the need to seek care if they see signs of severe infection or sepsis;
- “Think sepsis”—know the signs and symptoms and treat them early;
- Act fast—order tests to identify infection, start antibiotics and other care immediately; document dose, duration, and purpose; and
- Check patient progress frequently; reassess antibiotic therapy at 24 to 48 hours or sooner to change therapy if needed
Sepsis begins outside the hospital for 80% of patients, according to a recent CDC evaluation, reported in Vital Signs. CDC researchers who reviewed medical records of 246 adults and 79 children at 4 New York hospitals in Albany and Rochester found that 7 in 10 patients with sepsis had recently used health care services or had chronic diseases requiring frequent medical care.
Related: The Role of Procalcitonin in the Management of Infectious Diseases
Sepsis is most common in adults aged ≥ 65 years, infants < 1 year, people with weakened immune systems, or people with chronic conditions, such as diabetes. Nearly all the adults (97%) had at least 1 comorbidity, and 70% of children who developed sepsis had a health condition that may have put them at risk.
Although multiple infections and organisms were implicated, Staphylococcus aureus, Escherichia coli, and some types of Streptococcus were identified most often. Among adults with sepsis, 35% had a lung infection, 25% had a urinary tract infection, 11% had a gastrointestinal infection, and 11% had a skin infection.
Related: Mass Transit for Viruses
Most of the patients had recent interactions with the health care system before admission with sepsis, which likely reflects their vulnerability to infection, the researchers say, “it also suggests that health care facilities and providers could play a central role in sepsis prevention.” The CDC report advises the following for health care providers:
- Follow infection control requirements;
- Ensure that patients receive recommended vaccines (such as flu and pneumococcal);
- Educate patients and families, stressing the need to seek care if they see signs of severe infection or sepsis;
- “Think sepsis”—know the signs and symptoms and treat them early;
- Act fast—order tests to identify infection, start antibiotics and other care immediately; document dose, duration, and purpose; and
- Check patient progress frequently; reassess antibiotic therapy at 24 to 48 hours or sooner to change therapy if needed
The Highs and Lows of Medical Marijuana
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.
PHARMACOKINETICS
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10
A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.

PHARMACOKINETICS
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10

A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.

PHARMACOKINETICS
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10

A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
No rise in complications with concomitant gynecologic cancer, PFD surgery
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
AT PFD WEEK 2016
Key clinical point:
Major finding: Women who underwent concomitant surgeries had similar rates of infectious, pulmonary, and cardiac complications as those who underwent surgery only for gynecologic cancer, with all P-values exceeding .05.
Data source: A study of 23,501 gynecologic cancer patients in the ACS National Surgical Quality Improvement Program dataset.
Disclosures: Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.