User login
TauRx still rooting for its methylene blue AD drug, despite controversial study results
SAN DIEGO – An anti-tau compound that has stirred scientific controversy for 8 years will continue along its developmental pathway at a much lower dose, despite yet another study that has Alzheimer’s researchers scratching their heads.
The drug, dubbed LMTM, is a derivative of the dye methylene blue. Its most recent phase III study, reported at the Clinical Trials on Alzheimer’s Disease conference, found that 100 mg twice a day conferred no cognitive or functional benefit upon patients with mild AD, compared with a control dose of 4 mg.
Some significant differences, however, did emerge in two prespecified subanalyses of the 4-mg control group. Patients who took the low dose, intended to be a placebo comparator, did better than those on the high dose – but only if they were not taking any standard symptomatic AD medications.
Based on these findings, TauRx, which is developing LMTM, will abandon the 100-mg dose and refocus on the 4-mg dose, said Claude Wischik, MD, chairman and chief executive officer of the Singapore-based company.
“I think it looks effective and there’s no advantage to going to a higher dose,” Dr. Wischik said in an interview. “The 100-mg dose doesn’t offer anything above the 4-mg dose, and we saw more dropouts in the higher-dose group. We will go forward with a new trial using 4 mg.”
The new commitment to 4 mg turns LMTM’s prior development trajectory on its head, as nothing lower than 75 mg has been investigated in a phase III study. The 4-mg control dose was used as a placebo stand-in, since LMTM colors urine blue or green. The low dose was considered biologically inactive and used to maintain the study blind.
Dr. Wischik has been investigating LMTM as a tau anti-aggregant for 10 years, first publicly reporting clinical data in 2008. LMTM has never posted significant cognitive or functional benefits in any primary analysis. Instead, it has moved forward based on a series of subanalyses that showed significant or near-significant benefits in smaller, meticulously constructed subgroups – conclusions that critics have called questionable at best. The most recent of these examined the drug’s effect in patients with mild to moderate disease and was presented last July at the Alzheimer’s Association’s International Conference (AAIC).
That study also didn’t meet its primary endpoints in the overall cohort of 891 patients, but TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who were not taking memantine or cholinesterase inhibitors.
Among these patients, those taking 75 mg twice daily declined 6 points less on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) than those taking 4 mg. Those taking 125 mg twice daily declined 6 points less than the 4-mg group. On the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL), patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 7 points higher than did the placebo group.
At AAIC, researchers suggested that the monotherapy groups could have had a less aggressive disease course, or might not have had Alzheimer’s disease at all. Others complained about the unorthodox grouping of control patients in the subgroup analysis.
It was after digesting these data that TauRx investigators changed the statistical analysis of the current study, then in its final months, from a randomized trial to a cohort analysis. This was done before data lockdown, but it was still a dramatic shift from the original study design.
“The primary analysis was changed to essentially analyze this as a cohort study,” said Lon Schneider, MD, who presented the results at CTAD. “The comparisons of interest were patients taking 100 mg twice a day who were not on [symptomatic treatment], compared to the original control group of 4 mg. The other comparison was the 4-mg group not on cholinesterase inhibitors to the 4-mg group that was on them.”
The 18-month trial randomized 800 patients with mild AD to 100 mg LMTM twice daily or to 4 mg twice daily. Patients were drawn from two global regions: Canada and the United States, and eastern Europe and Australia.
The study group was a typical one, with a mean age of 70 years and a mean Mini Mental State Exam score of 22. The mean ADAS-cog score was 17. Most (80%) were taking a cholinesterase inhibitor, memantine, or both; 20% were naive to these medications.
Primary endpoints were the ADAS-cog11, ADCS-ADL, and left ventricular volume. Secondary outcomes included the Mini Mental State Exam and Neuropsychiatric Index.
In the primary analysis as the trial was created and conducted, LMTM 100 mg/twice daily did not confer any benefit, compared with the control 4-mg dose. The decline curves were virtually superimposable in the ADAS-cog score, ADCS-ADL score, and in loss of left ventricular volume.
This same nonsignificant pattern occurred in all the secondary endpoints, which Dr. Schneider did not show.
The cohort analyses stratified patients according to whether they were taking any cholinesterase inhibitor or memantine, or both, at baseline. That was where some differences did emerge.
The first compared the entire 4-mg cohort to the subset of patients taking 100 mg as monotherapy (absent any symptomatic medications). Both the 100-mg and 4-mg groups declined linearly on all measures, but compared to the 4 mg group the 100 mg group experienced about a 3-point benefit on both the ADAS-cog and ADS-ADL measures.* The 100-mg group also experienced significantly more dropouts (45% vs. 23%), with 16% of those being due to adverse events.
The second analysis compared the two 4-mg groups: those taking LMTM as monotherapy and those taking it in combination with standard AD medications. Again, both groups declined, but that decline was attenuated in the monotherapy group, with a 4-point advantage in the ADAS-cog and nearly a 5-point advantage in the ADS-ADL. The 3-cc ventricular volume advantage was seen as well.
Again, Dr. Schneider said, these results were recapitulated in the secondary endpoints, which he did not show.
The trial seems to upend TauRx’s earlier firm contention that the previously tested higher doses slow cognitive and functional decline – a view Dr. Wischik clung to after the July data were released. Dr. Schneider attempted to address this by suggesting that “the 4-mg dose may not have been as inactive as the developer thought.”
However, he noted, another possibility is that the patients who took the 4-mg dose but not the symptomatic drugs “may have had a more benign course of disease, compared to those taking cholinesterase inhibitors and memantine.”
This new study has now aroused the same criticism levied last summer. Maria Carrillo, PhD, chief scientific officer of the Alzheimer’s Association, was blunt in her assessment.
“The results of post hoc analyses, even when preplanned, are not valid and could be spurious,” she said. “They may mean nothing. As a field, we have been lured into rabbit holes in the past due to post hoc analyses and wasted too much time and way too much money. Of course, companies can do what they want as next steps in trials if they have the financial backing to do so.”
Dr. Wischik, however, said both the July data and the new data clearly justify taking the 4-mg dose forward in a randomized, placebo-controlled study.
“It’s really very exciting that we got exactly the same results now as we did in the post-hoc analysis [of the July data],” he said in an interview. “We predicted these results based on what we saw, we changed the statistical analysis, and we got the predicted results. This has nothing to do with data scouring.”
The question of whether the monotherapy patients are fundamentally different from those taking standard AD drugs is a valid one, he admitted. “We can’t avoid that criticism until we do another study where people who are not on any AD treatment are randomized.”
Dr. Wischik was then asked whether it would be difficult to recruit an entire cohort of patients with mild Alzheimer’s who are willing to forego approved symptomatic medications while in such a study. He did not think that would be problematic.
“Twenty percent of our cohort was already in that slot,” he said. “This practice pattern is determined somewhat by geography and somewhat by the type of clinician treating the patient. People also go on the drugs and then come off for various reasons. But even in the U.S., only 55% of Alzheimer’s patients are taking them.”
Dr. Wischik didn’t mention the problem of finding an appropriate placebo for such a study. If indeed the 4-mg dose is biologically active, such a placebo would have to be demonstrably inert, as well as provide the appropriate urine color to keep the blinding unbroken.
“That’s a challenge,” Dr. Schneider said.
Dr. Schneider was a coinvestigator on the LMTM phase III program. He has disclosed financial relationships with numerous pharmaceutical companies.
Correction, 12/12/16: An earlier version of this article misstated the results of this study.
[email protected]
On Twitter @Alz_Gal
There are several reasons why this kind of analysis defies scientific credibility.
The term “monotherapy” is really a euphemism for substandard care before the study. These patients on monotherapy were not selected to be so. These were people with mild to moderate AD dementia who should have been on memantine or a cholinesterase inhibitor and were not, for unknown reasons. They represent a health care bias, and on that fact alone this comparison should not have even been mentioned. Calling it monotherapy is an attempt to distract from the fact that this was an indication bias that defines this group.
The claim that this analysis was done before the database lock is true in principal. But because the investigators had already seen this result in their previous study, which was identically designed, they cannot really claim this was truly an ad hoc analysis. They already knew what they were going to see.
In July, the investigators claimed that the 100-mg dose was effective in monotherapy. They have been convinced over the entire course of development that the 4-mg dose was ineffective. Now they are retracting that. To me, this apparent wild goose chase for any kind of effect trivializes the entire process of a clinical trial.
What I believe we are observing here is a profound placebo effect that can occur when people who have been getting substandard care are put in a clinical trial and exposed to good care.
David Knopman, MD, is a clinical neurologist at the Mayo Clinic, Rochester, Minn., and a member of the Alzheimer’s Association Medical and Scientific Advisory Council.
There are several reasons why this kind of analysis defies scientific credibility.
The term “monotherapy” is really a euphemism for substandard care before the study. These patients on monotherapy were not selected to be so. These were people with mild to moderate AD dementia who should have been on memantine or a cholinesterase inhibitor and were not, for unknown reasons. They represent a health care bias, and on that fact alone this comparison should not have even been mentioned. Calling it monotherapy is an attempt to distract from the fact that this was an indication bias that defines this group.
The claim that this analysis was done before the database lock is true in principal. But because the investigators had already seen this result in their previous study, which was identically designed, they cannot really claim this was truly an ad hoc analysis. They already knew what they were going to see.
In July, the investigators claimed that the 100-mg dose was effective in monotherapy. They have been convinced over the entire course of development that the 4-mg dose was ineffective. Now they are retracting that. To me, this apparent wild goose chase for any kind of effect trivializes the entire process of a clinical trial.
What I believe we are observing here is a profound placebo effect that can occur when people who have been getting substandard care are put in a clinical trial and exposed to good care.
David Knopman, MD, is a clinical neurologist at the Mayo Clinic, Rochester, Minn., and a member of the Alzheimer’s Association Medical and Scientific Advisory Council.
There are several reasons why this kind of analysis defies scientific credibility.
The term “monotherapy” is really a euphemism for substandard care before the study. These patients on monotherapy were not selected to be so. These were people with mild to moderate AD dementia who should have been on memantine or a cholinesterase inhibitor and were not, for unknown reasons. They represent a health care bias, and on that fact alone this comparison should not have even been mentioned. Calling it monotherapy is an attempt to distract from the fact that this was an indication bias that defines this group.
The claim that this analysis was done before the database lock is true in principal. But because the investigators had already seen this result in their previous study, which was identically designed, they cannot really claim this was truly an ad hoc analysis. They already knew what they were going to see.
In July, the investigators claimed that the 100-mg dose was effective in monotherapy. They have been convinced over the entire course of development that the 4-mg dose was ineffective. Now they are retracting that. To me, this apparent wild goose chase for any kind of effect trivializes the entire process of a clinical trial.
What I believe we are observing here is a profound placebo effect that can occur when people who have been getting substandard care are put in a clinical trial and exposed to good care.
David Knopman, MD, is a clinical neurologist at the Mayo Clinic, Rochester, Minn., and a member of the Alzheimer’s Association Medical and Scientific Advisory Council.
SAN DIEGO – An anti-tau compound that has stirred scientific controversy for 8 years will continue along its developmental pathway at a much lower dose, despite yet another study that has Alzheimer’s researchers scratching their heads.
The drug, dubbed LMTM, is a derivative of the dye methylene blue. Its most recent phase III study, reported at the Clinical Trials on Alzheimer’s Disease conference, found that 100 mg twice a day conferred no cognitive or functional benefit upon patients with mild AD, compared with a control dose of 4 mg.
Some significant differences, however, did emerge in two prespecified subanalyses of the 4-mg control group. Patients who took the low dose, intended to be a placebo comparator, did better than those on the high dose – but only if they were not taking any standard symptomatic AD medications.
Based on these findings, TauRx, which is developing LMTM, will abandon the 100-mg dose and refocus on the 4-mg dose, said Claude Wischik, MD, chairman and chief executive officer of the Singapore-based company.
“I think it looks effective and there’s no advantage to going to a higher dose,” Dr. Wischik said in an interview. “The 100-mg dose doesn’t offer anything above the 4-mg dose, and we saw more dropouts in the higher-dose group. We will go forward with a new trial using 4 mg.”
The new commitment to 4 mg turns LMTM’s prior development trajectory on its head, as nothing lower than 75 mg has been investigated in a phase III study. The 4-mg control dose was used as a placebo stand-in, since LMTM colors urine blue or green. The low dose was considered biologically inactive and used to maintain the study blind.
Dr. Wischik has been investigating LMTM as a tau anti-aggregant for 10 years, first publicly reporting clinical data in 2008. LMTM has never posted significant cognitive or functional benefits in any primary analysis. Instead, it has moved forward based on a series of subanalyses that showed significant or near-significant benefits in smaller, meticulously constructed subgroups – conclusions that critics have called questionable at best. The most recent of these examined the drug’s effect in patients with mild to moderate disease and was presented last July at the Alzheimer’s Association’s International Conference (AAIC).
That study also didn’t meet its primary endpoints in the overall cohort of 891 patients, but TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who were not taking memantine or cholinesterase inhibitors.
Among these patients, those taking 75 mg twice daily declined 6 points less on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) than those taking 4 mg. Those taking 125 mg twice daily declined 6 points less than the 4-mg group. On the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL), patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 7 points higher than did the placebo group.
At AAIC, researchers suggested that the monotherapy groups could have had a less aggressive disease course, or might not have had Alzheimer’s disease at all. Others complained about the unorthodox grouping of control patients in the subgroup analysis.
It was after digesting these data that TauRx investigators changed the statistical analysis of the current study, then in its final months, from a randomized trial to a cohort analysis. This was done before data lockdown, but it was still a dramatic shift from the original study design.
“The primary analysis was changed to essentially analyze this as a cohort study,” said Lon Schneider, MD, who presented the results at CTAD. “The comparisons of interest were patients taking 100 mg twice a day who were not on [symptomatic treatment], compared to the original control group of 4 mg. The other comparison was the 4-mg group not on cholinesterase inhibitors to the 4-mg group that was on them.”
The 18-month trial randomized 800 patients with mild AD to 100 mg LMTM twice daily or to 4 mg twice daily. Patients were drawn from two global regions: Canada and the United States, and eastern Europe and Australia.
The study group was a typical one, with a mean age of 70 years and a mean Mini Mental State Exam score of 22. The mean ADAS-cog score was 17. Most (80%) were taking a cholinesterase inhibitor, memantine, or both; 20% were naive to these medications.
Primary endpoints were the ADAS-cog11, ADCS-ADL, and left ventricular volume. Secondary outcomes included the Mini Mental State Exam and Neuropsychiatric Index.
In the primary analysis as the trial was created and conducted, LMTM 100 mg/twice daily did not confer any benefit, compared with the control 4-mg dose. The decline curves were virtually superimposable in the ADAS-cog score, ADCS-ADL score, and in loss of left ventricular volume.
This same nonsignificant pattern occurred in all the secondary endpoints, which Dr. Schneider did not show.
The cohort analyses stratified patients according to whether they were taking any cholinesterase inhibitor or memantine, or both, at baseline. That was where some differences did emerge.
The first compared the entire 4-mg cohort to the subset of patients taking 100 mg as monotherapy (absent any symptomatic medications). Both the 100-mg and 4-mg groups declined linearly on all measures, but compared to the 4 mg group the 100 mg group experienced about a 3-point benefit on both the ADAS-cog and ADS-ADL measures.* The 100-mg group also experienced significantly more dropouts (45% vs. 23%), with 16% of those being due to adverse events.
The second analysis compared the two 4-mg groups: those taking LMTM as monotherapy and those taking it in combination with standard AD medications. Again, both groups declined, but that decline was attenuated in the monotherapy group, with a 4-point advantage in the ADAS-cog and nearly a 5-point advantage in the ADS-ADL. The 3-cc ventricular volume advantage was seen as well.
Again, Dr. Schneider said, these results were recapitulated in the secondary endpoints, which he did not show.
The trial seems to upend TauRx’s earlier firm contention that the previously tested higher doses slow cognitive and functional decline – a view Dr. Wischik clung to after the July data were released. Dr. Schneider attempted to address this by suggesting that “the 4-mg dose may not have been as inactive as the developer thought.”
However, he noted, another possibility is that the patients who took the 4-mg dose but not the symptomatic drugs “may have had a more benign course of disease, compared to those taking cholinesterase inhibitors and memantine.”
This new study has now aroused the same criticism levied last summer. Maria Carrillo, PhD, chief scientific officer of the Alzheimer’s Association, was blunt in her assessment.
“The results of post hoc analyses, even when preplanned, are not valid and could be spurious,” she said. “They may mean nothing. As a field, we have been lured into rabbit holes in the past due to post hoc analyses and wasted too much time and way too much money. Of course, companies can do what they want as next steps in trials if they have the financial backing to do so.”
Dr. Wischik, however, said both the July data and the new data clearly justify taking the 4-mg dose forward in a randomized, placebo-controlled study.
“It’s really very exciting that we got exactly the same results now as we did in the post-hoc analysis [of the July data],” he said in an interview. “We predicted these results based on what we saw, we changed the statistical analysis, and we got the predicted results. This has nothing to do with data scouring.”
The question of whether the monotherapy patients are fundamentally different from those taking standard AD drugs is a valid one, he admitted. “We can’t avoid that criticism until we do another study where people who are not on any AD treatment are randomized.”
Dr. Wischik was then asked whether it would be difficult to recruit an entire cohort of patients with mild Alzheimer’s who are willing to forego approved symptomatic medications while in such a study. He did not think that would be problematic.
“Twenty percent of our cohort was already in that slot,” he said. “This practice pattern is determined somewhat by geography and somewhat by the type of clinician treating the patient. People also go on the drugs and then come off for various reasons. But even in the U.S., only 55% of Alzheimer’s patients are taking them.”
Dr. Wischik didn’t mention the problem of finding an appropriate placebo for such a study. If indeed the 4-mg dose is biologically active, such a placebo would have to be demonstrably inert, as well as provide the appropriate urine color to keep the blinding unbroken.
“That’s a challenge,” Dr. Schneider said.
Dr. Schneider was a coinvestigator on the LMTM phase III program. He has disclosed financial relationships with numerous pharmaceutical companies.
Correction, 12/12/16: An earlier version of this article misstated the results of this study.
[email protected]
On Twitter @Alz_Gal
SAN DIEGO – An anti-tau compound that has stirred scientific controversy for 8 years will continue along its developmental pathway at a much lower dose, despite yet another study that has Alzheimer’s researchers scratching their heads.
The drug, dubbed LMTM, is a derivative of the dye methylene blue. Its most recent phase III study, reported at the Clinical Trials on Alzheimer’s Disease conference, found that 100 mg twice a day conferred no cognitive or functional benefit upon patients with mild AD, compared with a control dose of 4 mg.
Some significant differences, however, did emerge in two prespecified subanalyses of the 4-mg control group. Patients who took the low dose, intended to be a placebo comparator, did better than those on the high dose – but only if they were not taking any standard symptomatic AD medications.
Based on these findings, TauRx, which is developing LMTM, will abandon the 100-mg dose and refocus on the 4-mg dose, said Claude Wischik, MD, chairman and chief executive officer of the Singapore-based company.
“I think it looks effective and there’s no advantage to going to a higher dose,” Dr. Wischik said in an interview. “The 100-mg dose doesn’t offer anything above the 4-mg dose, and we saw more dropouts in the higher-dose group. We will go forward with a new trial using 4 mg.”
The new commitment to 4 mg turns LMTM’s prior development trajectory on its head, as nothing lower than 75 mg has been investigated in a phase III study. The 4-mg control dose was used as a placebo stand-in, since LMTM colors urine blue or green. The low dose was considered biologically inactive and used to maintain the study blind.
Dr. Wischik has been investigating LMTM as a tau anti-aggregant for 10 years, first publicly reporting clinical data in 2008. LMTM has never posted significant cognitive or functional benefits in any primary analysis. Instead, it has moved forward based on a series of subanalyses that showed significant or near-significant benefits in smaller, meticulously constructed subgroups – conclusions that critics have called questionable at best. The most recent of these examined the drug’s effect in patients with mild to moderate disease and was presented last July at the Alzheimer’s Association’s International Conference (AAIC).
That study also didn’t meet its primary endpoints in the overall cohort of 891 patients, but TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who were not taking memantine or cholinesterase inhibitors.
Among these patients, those taking 75 mg twice daily declined 6 points less on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) than those taking 4 mg. Those taking 125 mg twice daily declined 6 points less than the 4-mg group. On the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL), patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 7 points higher than did the placebo group.
At AAIC, researchers suggested that the monotherapy groups could have had a less aggressive disease course, or might not have had Alzheimer’s disease at all. Others complained about the unorthodox grouping of control patients in the subgroup analysis.
It was after digesting these data that TauRx investigators changed the statistical analysis of the current study, then in its final months, from a randomized trial to a cohort analysis. This was done before data lockdown, but it was still a dramatic shift from the original study design.
“The primary analysis was changed to essentially analyze this as a cohort study,” said Lon Schneider, MD, who presented the results at CTAD. “The comparisons of interest were patients taking 100 mg twice a day who were not on [symptomatic treatment], compared to the original control group of 4 mg. The other comparison was the 4-mg group not on cholinesterase inhibitors to the 4-mg group that was on them.”
The 18-month trial randomized 800 patients with mild AD to 100 mg LMTM twice daily or to 4 mg twice daily. Patients were drawn from two global regions: Canada and the United States, and eastern Europe and Australia.
The study group was a typical one, with a mean age of 70 years and a mean Mini Mental State Exam score of 22. The mean ADAS-cog score was 17. Most (80%) were taking a cholinesterase inhibitor, memantine, or both; 20% were naive to these medications.
Primary endpoints were the ADAS-cog11, ADCS-ADL, and left ventricular volume. Secondary outcomes included the Mini Mental State Exam and Neuropsychiatric Index.
In the primary analysis as the trial was created and conducted, LMTM 100 mg/twice daily did not confer any benefit, compared with the control 4-mg dose. The decline curves were virtually superimposable in the ADAS-cog score, ADCS-ADL score, and in loss of left ventricular volume.
This same nonsignificant pattern occurred in all the secondary endpoints, which Dr. Schneider did not show.
The cohort analyses stratified patients according to whether they were taking any cholinesterase inhibitor or memantine, or both, at baseline. That was where some differences did emerge.
The first compared the entire 4-mg cohort to the subset of patients taking 100 mg as monotherapy (absent any symptomatic medications). Both the 100-mg and 4-mg groups declined linearly on all measures, but compared to the 4 mg group the 100 mg group experienced about a 3-point benefit on both the ADAS-cog and ADS-ADL measures.* The 100-mg group also experienced significantly more dropouts (45% vs. 23%), with 16% of those being due to adverse events.
The second analysis compared the two 4-mg groups: those taking LMTM as monotherapy and those taking it in combination with standard AD medications. Again, both groups declined, but that decline was attenuated in the monotherapy group, with a 4-point advantage in the ADAS-cog and nearly a 5-point advantage in the ADS-ADL. The 3-cc ventricular volume advantage was seen as well.
Again, Dr. Schneider said, these results were recapitulated in the secondary endpoints, which he did not show.
The trial seems to upend TauRx’s earlier firm contention that the previously tested higher doses slow cognitive and functional decline – a view Dr. Wischik clung to after the July data were released. Dr. Schneider attempted to address this by suggesting that “the 4-mg dose may not have been as inactive as the developer thought.”
However, he noted, another possibility is that the patients who took the 4-mg dose but not the symptomatic drugs “may have had a more benign course of disease, compared to those taking cholinesterase inhibitors and memantine.”
This new study has now aroused the same criticism levied last summer. Maria Carrillo, PhD, chief scientific officer of the Alzheimer’s Association, was blunt in her assessment.
“The results of post hoc analyses, even when preplanned, are not valid and could be spurious,” she said. “They may mean nothing. As a field, we have been lured into rabbit holes in the past due to post hoc analyses and wasted too much time and way too much money. Of course, companies can do what they want as next steps in trials if they have the financial backing to do so.”
Dr. Wischik, however, said both the July data and the new data clearly justify taking the 4-mg dose forward in a randomized, placebo-controlled study.
“It’s really very exciting that we got exactly the same results now as we did in the post-hoc analysis [of the July data],” he said in an interview. “We predicted these results based on what we saw, we changed the statistical analysis, and we got the predicted results. This has nothing to do with data scouring.”
The question of whether the monotherapy patients are fundamentally different from those taking standard AD drugs is a valid one, he admitted. “We can’t avoid that criticism until we do another study where people who are not on any AD treatment are randomized.”
Dr. Wischik was then asked whether it would be difficult to recruit an entire cohort of patients with mild Alzheimer’s who are willing to forego approved symptomatic medications while in such a study. He did not think that would be problematic.
“Twenty percent of our cohort was already in that slot,” he said. “This practice pattern is determined somewhat by geography and somewhat by the type of clinician treating the patient. People also go on the drugs and then come off for various reasons. But even in the U.S., only 55% of Alzheimer’s patients are taking them.”
Dr. Wischik didn’t mention the problem of finding an appropriate placebo for such a study. If indeed the 4-mg dose is biologically active, such a placebo would have to be demonstrably inert, as well as provide the appropriate urine color to keep the blinding unbroken.
“That’s a challenge,” Dr. Schneider said.
Dr. Schneider was a coinvestigator on the LMTM phase III program. He has disclosed financial relationships with numerous pharmaceutical companies.
Correction, 12/12/16: An earlier version of this article misstated the results of this study.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: After saying that the placebo dose of drug effected cognitive and clinical benefit in a cohort analysis comparing it to 100 mg twice daily, the company will further develop LMTM in a 4-mg dose.
Data source: The cohort analysis involved 891 patients with mild Alzheimer’s.
Disclosures: Dr. Wischik is the founder and president of Singapore-based TauRx. Dr. Schneider is an investigator in the drug’s phase III trial and has reported financial relationships with numerous pharmaceutical companies.
Thinking Outside the DRG Box
When choosing quality improvement activities, hospitalists have no shortage of choices. In this column, I offer a strategic guide for hospitalists as they assess where best to spend their energy as the shift to value-based care progresses. This includes the introduction of MACRA, the landmark new payment program for doctors and other clinicians (aka the Medicare Access and CHIP Reauthorization Act of 2015), with its incentives for participation in alternative payment models.
Since 1983, Medicare has reimbursed hospitals using a lump-sum payment known as a diagnosis-related group, or DRG. Since then, hospitals have focused a good deal of their energy on removing needless expenses from the hospitalization to improve their bottom line, recognizing the DRG payment they receive is relatively fixed. To this end, a major strategy has been to use hospitalists to decrease length of stay and “right size” the utilization of in-hospital tests and treatments.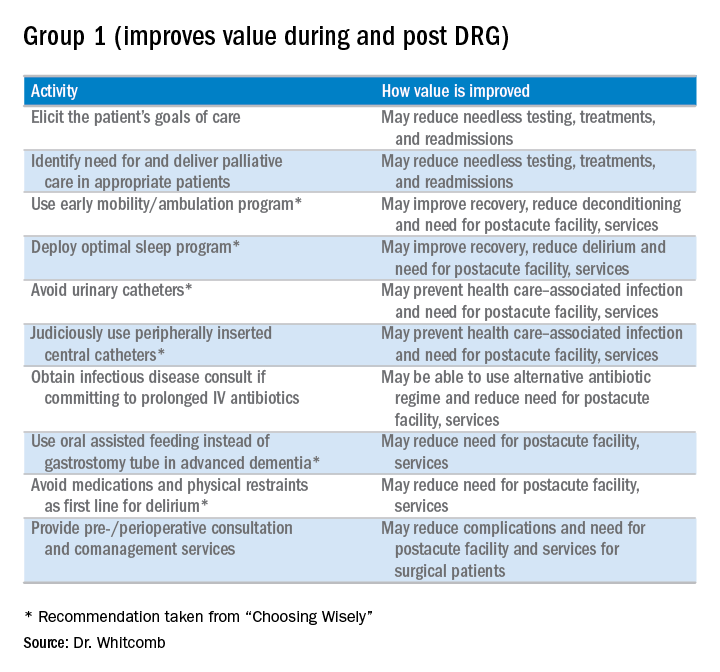
However, things are changing as we enter the era of alternative payment models such as accountable care organizations (ACOs) and bundled payments. The lens Medicare (and, to a great extent, commercial payors) peers through to assess inpatient hospital costs is the DRG payment amount. Beyond that, Medicare has little visibility into the actual costs hospitals incur. Since hospital spending equates to the payment amount for a DRG, it becomes apparent that the incremental opportunity for hospitalists to improve value (quality divided by cost) in alternative payment models stems from payments outside the DRG. Such payments include those related to the post-acute period such as nursing and rehabilitation facilities, readmissions, and part B activity (e.g., consultants and outpatient tests).
What does this mean for hospitalists? MACRA begins in 2019, but initial payments will be based on 2017 performance. The associated advantage of participating in an “advanced alternative payment model” where there is accountability for care beyond the hospitalization is that hospitalists will be rewarded for taking costs out of the post-acute time period.
To be clear, hospitalists should remain agents of in-hospital efficiency and quality. After all, that is how we add value to the hospitals in which we practice. All things being equal, however, hospitalists should focus on practices that will improve value beyond the four walls of the hospital.
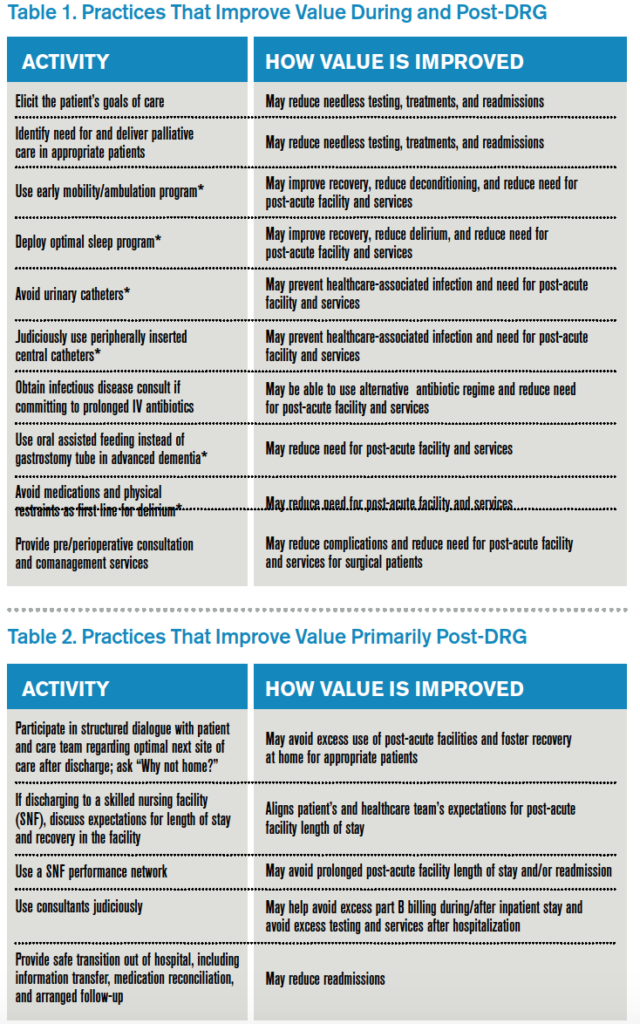
Here is my shortlist of these practices. While there is crossover between the categories, I divide the practices into those that improve value during the DRG period and also post-DRG and those that improve value primarily post-DRG (thanks to Choosing Wisely for contributing to the recommendations with an asterisk1):
Thinking outside the DRG box will require an adjustment to the approach taken by hospitalists because the current demands are often more than enough for a day’s work. Hospitalists will be called upon to innovate and fashion better approaches to care. This will require support by other members of the healthcare team so hospitalists can work smarter, not harder, to meet the requirements of a changing healthcare system. A prerequisite is better payment models that align financial incentives so that providing higher-value care is sustainable and appropriately rewarded.

Reference
Clinician lists. Choosing Wisely website. Accessed October 25, 2016.
When choosing quality improvement activities, hospitalists have no shortage of choices. In this column, I offer a strategic guide for hospitalists as they assess where best to spend their energy as the shift to value-based care progresses. This includes the introduction of MACRA, the landmark new payment program for doctors and other clinicians (aka the Medicare Access and CHIP Reauthorization Act of 2015), with its incentives for participation in alternative payment models.
Since 1983, Medicare has reimbursed hospitals using a lump-sum payment known as a diagnosis-related group, or DRG. Since then, hospitals have focused a good deal of their energy on removing needless expenses from the hospitalization to improve their bottom line, recognizing the DRG payment they receive is relatively fixed. To this end, a major strategy has been to use hospitalists to decrease length of stay and “right size” the utilization of in-hospital tests and treatments.
However, things are changing as we enter the era of alternative payment models such as accountable care organizations (ACOs) and bundled payments. The lens Medicare (and, to a great extent, commercial payors) peers through to assess inpatient hospital costs is the DRG payment amount. Beyond that, Medicare has little visibility into the actual costs hospitals incur. Since hospital spending equates to the payment amount for a DRG, it becomes apparent that the incremental opportunity for hospitalists to improve value (quality divided by cost) in alternative payment models stems from payments outside the DRG. Such payments include those related to the post-acute period such as nursing and rehabilitation facilities, readmissions, and part B activity (e.g., consultants and outpatient tests).
What does this mean for hospitalists? MACRA begins in 2019, but initial payments will be based on 2017 performance. The associated advantage of participating in an “advanced alternative payment model” where there is accountability for care beyond the hospitalization is that hospitalists will be rewarded for taking costs out of the post-acute time period.
To be clear, hospitalists should remain agents of in-hospital efficiency and quality. After all, that is how we add value to the hospitals in which we practice. All things being equal, however, hospitalists should focus on practices that will improve value beyond the four walls of the hospital.
Here is my shortlist of these practices. While there is crossover between the categories, I divide the practices into those that improve value during the DRG period and also post-DRG and those that improve value primarily post-DRG (thanks to Choosing Wisely for contributing to the recommendations with an asterisk1):
Thinking outside the DRG box will require an adjustment to the approach taken by hospitalists because the current demands are often more than enough for a day’s work. Hospitalists will be called upon to innovate and fashion better approaches to care. This will require support by other members of the healthcare team so hospitalists can work smarter, not harder, to meet the requirements of a changing healthcare system. A prerequisite is better payment models that align financial incentives so that providing higher-value care is sustainable and appropriately rewarded.

Reference
Clinician lists. Choosing Wisely website. Accessed October 25, 2016.
When choosing quality improvement activities, hospitalists have no shortage of choices. In this column, I offer a strategic guide for hospitalists as they assess where best to spend their energy as the shift to value-based care progresses. This includes the introduction of MACRA, the landmark new payment program for doctors and other clinicians (aka the Medicare Access and CHIP Reauthorization Act of 2015), with its incentives for participation in alternative payment models.
Since 1983, Medicare has reimbursed hospitals using a lump-sum payment known as a diagnosis-related group, or DRG. Since then, hospitals have focused a good deal of their energy on removing needless expenses from the hospitalization to improve their bottom line, recognizing the DRG payment they receive is relatively fixed. To this end, a major strategy has been to use hospitalists to decrease length of stay and “right size” the utilization of in-hospital tests and treatments.
However, things are changing as we enter the era of alternative payment models such as accountable care organizations (ACOs) and bundled payments. The lens Medicare (and, to a great extent, commercial payors) peers through to assess inpatient hospital costs is the DRG payment amount. Beyond that, Medicare has little visibility into the actual costs hospitals incur. Since hospital spending equates to the payment amount for a DRG, it becomes apparent that the incremental opportunity for hospitalists to improve value (quality divided by cost) in alternative payment models stems from payments outside the DRG. Such payments include those related to the post-acute period such as nursing and rehabilitation facilities, readmissions, and part B activity (e.g., consultants and outpatient tests).
What does this mean for hospitalists? MACRA begins in 2019, but initial payments will be based on 2017 performance. The associated advantage of participating in an “advanced alternative payment model” where there is accountability for care beyond the hospitalization is that hospitalists will be rewarded for taking costs out of the post-acute time period.
To be clear, hospitalists should remain agents of in-hospital efficiency and quality. After all, that is how we add value to the hospitals in which we practice. All things being equal, however, hospitalists should focus on practices that will improve value beyond the four walls of the hospital.
Here is my shortlist of these practices. While there is crossover between the categories, I divide the practices into those that improve value during the DRG period and also post-DRG and those that improve value primarily post-DRG (thanks to Choosing Wisely for contributing to the recommendations with an asterisk1):
Thinking outside the DRG box will require an adjustment to the approach taken by hospitalists because the current demands are often more than enough for a day’s work. Hospitalists will be called upon to innovate and fashion better approaches to care. This will require support by other members of the healthcare team so hospitalists can work smarter, not harder, to meet the requirements of a changing healthcare system. A prerequisite is better payment models that align financial incentives so that providing higher-value care is sustainable and appropriately rewarded.

Reference
Clinician lists. Choosing Wisely website. Accessed October 25, 2016.
Agent exhibits activity in relapsed/refractory AML

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()

acute myeloid leukemia
SAN DIEGO—A next-generation DNA hypomethylating agent has demonstrated clinical activity and an acceptable safety profile in relapsed/refractory acute myeloid leukemia (AML), according to researchers.
The agent, guadecitabine, produced a composite complete response (CRc) rate of 23% in a phase 2 study.
CRc was observed in all patient subgroups and was associated with longer survival, regardless of whether patients went on to receive a transplant.
Based on these results, researchers are initiating a phase 3 trial of the drug in relapsed/refractory AML.
Naval Daver, MD, of the University of Texas MD Anderson Cancer Center in Houston, presented the phase 2 results at the 2016 ASH Annual Meeting (abstract 904). The study was sponsored by Astex Pharmaceuticals.
Guadecitabine (formerly SGI-110) is a hypomethylating dinucleotide of decitabine and deoxyguanosine that is resistant to cytidine deaminase degradation. It is administered as a small volume subcutaneous injection, which results in extended decitabine exposure.
“Rapid metabolization, elimination shortens the in vivo exposure and may limit the efficacy of decitabine,” Dr Daver noted. “Guadecitabine was engineered to improve the in vivo levels . . . and the efficacy of decitabine by blocking the rapid elimination.”
In the phase 2 trial, Dr Daver and his colleagues investigated guadecitabine in 103 patients with relapsed/refractory AML. The patients’ median age was 60 (range, 22-82), and 60% were male. Eighty-six percent of patients had an ECOG performance status of 0-1, and 41% had poor-risk cytogenetics.
The median number of prior therapies was 2 (range, 1-7). All patients had received prior chemotherapy, 85% had received prior induction with 7+3 (a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days), and 18% had a prior hematopoietic stem cell transplant (HSCT).
Fifty-three percent of patients had a CR to first induction, and 47% were primary refractory.
Treatment
The researchers tested 2 different doses and schedules of guadecitabine. In the first cohort (5-day regimen), 50 patients were randomized (1:1) to either 60 mg/m2/day (n=24) or 90 mg/m2/day (n=26) on days 1-5.
In the second cohort (10-day regimen), 53 patients were assigned to treatment with 60 mg/m2/day on days 1-5 and days 8-12 for up to 4 cycles, followed by 60 mg/m2/day on days 1-5 in subsequent cycles.
Cycles were scheduled every 28 days for both regimens. Dose reductions and delays were allowed based on response and tolerability. And patients remained on treatment as long as they continued to benefit without unacceptable toxicity.
Response
The study’s primary endpoint was the CRc rate, which consisted of CR plus CR with incomplete platelet recovery (CRp) plus CR with incomplete neutrophil recovery (CRi).
The CRc rate was 16% in the 5-day cohort and 30% in the 10-day cohort. The CR rate was 6% and 19%, respectively. The CRp rate was 2% and 7%, respectively. And the CRi rate was 8% and 4%, respectively.
There was a trend toward a higher CR/CRc rate with the 10-day regimen (P=0.074 and 0.106, respectively).
There was no significant difference in CRc according to patient age (65 and older vs younger than 65), cytogenetics, prior HSCT, response to induction, or time from last therapy (less than 6 months vs 6 months or more).
However, the CRc rate was significantly lower for patients with an ECOG performance status of 2 than for those with a status of 0-1 (P<0.001).
Survival
For the entire study cohort, the median overall survival (OS) was 6.6 months, the 1-year OS was 28%, and the 2-year OS was 19%.
The median OS was 7.1 months with the 10-day regimen and 5.7 months with the 5-day regimen. This difference was not significant (P=0.51).
The median OS was not reached for patients who achieved a CR or for those who achieved a CRp plus a CRi. For patients who did not achieve a CRc, the median OS was 5.6 months (P<0.01).
The median OS was not reached for patients who had a CRc, whether or not they received a subsequent HSCT. There was no significant difference between patients who received an HSCT post-guadecitabine and those who did not (P=0.87).
Likewise, there was no significant difference in OS according to patient age, prior HSCT, or response to induction.
However, OS was significantly worse for patients with an ECOG performance status of 2 (P<0.001), those with poor-risk cytogenetics (P<0.001), and those for whom 6 months or more had elapsed since their last therapy (P=0.015).
Safety
Common grade 3 or higher adverse events (regardless of the relationship to therapy) were febrile neutropenia (60%), pneumonia (36%), thrombocytopenia (36%), anemia (31%), neutropenia (19%), and sepsis (16%).
The 30-day mortality rate was 3.9%, and the 60-day mortality rate was 11.7%. ![]()
After full data release, experts say failed Alzheimer’s trial EXPEDITION 3 offers hopeful signals
SAN DIEGO – Solanezumab may have not have slowed the relentless march of Alzheimer’s disease (AD), but it was a valuable proving ground of the amyloid hypothesis, experts said during a wide-ranging discussion of Lilly’s failed EXPEDITION 3 trial.
Lilly representatives and EXPEDITION investigators released the study’s full results at the Clinical Trials in Alzheimer ’s disease meeting. – findings that should be read as tremendously encouraging rather than a defeat, according to Paul Aisen, MD, director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, who was also an EXPEDITION 3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION 3, but in its predecessors EXPEDITION and EXPEDITION 2.
“This is not a refutation of the amyloid hypothesis but a confirmation of it.”
Nevertheless, the trial must be read as a failed one, he admitted. There was no statistically significant separation between solanezumab and placebo on the ADAS-Cog14, a combined assessment of cognition and function that was the study’s primary endpoint. The active group experienced 11% less cognitive decline than did the placebo group, but the p-value remained tantalizingly below the level of significance, at 0.095.
“But what if solanezumab had hit at 0.05 instead of 0.095?” Dr. Aisen asked. “In fact, it would still be a small effect size,” which would have thrown into question the drug’s clinical utility. “Going into this, we thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key secondaries. Overall the effect size looks to be about 12%-13%, and that’s just too small.”
EXPEDITION 3 was the last of a triad of solanezumab studies, all of which posted intriguing signals of cognitive and functional benefit in patients with mild-moderate AD. It was based on subgroup analyses of EXPEDITION 1 and 2, both of which failed to meet their primary endpoints. But when researchers pooled the mild patients from both studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted EXPEDITION 3 to come as close to recreating those findings as possible but still stumbled over results that were numerically positive for the antibody but not statistically significant or clinically meaningful.
Lawrence S. Honig, MD, professor of neurology at Columbia University Medical Center, N.Y., and principal investigator of the EXPEDITION 3 study, presented the study’s full results to a packed audience on Dec. 8.
The study comprised about 2,000 patients with imaging-confirmed amyloid brain plaques and mild-moderate AD. They were randomized to placebo or monthly injections of 400 mg solanezumab for 80 weeks. The global study was conducted in 11 countries and 210 study sites.
Dr. Honig detailed the key secondary endpoints of cognition and function, and also revealed biomarker data.
While the ADAS-Cog failed to meet statistical significance, changes in the Mini Mental State Exam score did, with a 13% slowing of decline compared to placebo (P = .014). There was also a significant 5% difference in the Clinical Dementia Rating scale-sum of boxes test (P = .004).
Outcomes were mixed in measures of function. The Alzheimer’s Disease Cooperative Study activities of daily living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, posted significant results with 15% and 14% differences, respectively, relative to placebo (P = .009 and .019, respectively).
But results on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant, with only a 7% separation from placebo and a P value of .140.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab did what it was supposed to: bind soluble amyloid beta. This resulted in a 500- to 800-fold increase in the protein in plasma relative to placebo. There were no changes in amyloid brain plaques as measured by PET imaging, but this was no surprise, Dr. Aisen said, since the antibody doesn’t recognize fibrillar amyloid.
“What we expect to see with biomarkers differs based on the epitope targeted. Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble AB. Now how that helps AD is something of a debate, but it is important tor recognize that it does not attack plaques. Instead, by tying up monomeric AB, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma AB increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau, either in cerebrospinal fluid or imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was quite safe, with 17% of patient reporting an adverse event, compared to 19% of placebo patients. There were 9 deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
Although the data discussion was framed in the most hopeful light possible, no one on the panel attempted to massage it into a more clinically positive form. On a webcast in late November, Eric Siemers, MD, senior medical director of the Alzheimer’s Disease Global Development Team at Eli Lilly, said the company was disappointed but would not bring solanezumab forward for approval for mild or moderate AD patients. He echoed that sentiment during the panel discussion.
“We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress. So yes, we are very disappointed.”
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in a different population will go forward uninterrupted. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively health elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
There was also brief discussion of dosing. Some audience members suggested that a higher dose than EXPEDITION’s 400 mg might have bumped up efficacy, and asked if Lilly would reconsider the dosing schedule in the A4 and DIAN studies.
“There has been a lot of discussion around that,” said Dr. Siemers. “But it’s not as easy and straightforward as you think.”
Nevertheless, Dr. Aisen is excited about solanezumab’s potential in these trials that target the disease at its earliest phase, even before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive. Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of AD than even in the mild dementia stage.”
Maria Carrillo, PhD, chief science officer of the Alzheimer’s Association, said that EXPEDITION 3 is far from a path to nowhere. Instead, she urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder. This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach and to broaden the armamentarium our clinicians will need to combat this disease.”
“This is not a win, true. But it gets us a little closer to one.”
[email protected]
On Twitter @Alz_Gal
This new phase III trial of solanezumab reveals that the drug is not effective for mild Alzheimer’s disease patients, despite the hint that it was possibly effective based on post-hoc analyses of earlier studies with this drug.
The findings expose the hazards of such post-hoc analyses, typically done when the desired results are not observed, in the hope of squeezing lemonade from lemons. Although the subanalysis of mild AD patients in the earlier studies suggested a 34% slowing of cognitive decline as assessed by ADAS-Cog, an incremental slowing of 11% was seen in the new study that was not even statistically significant. While some secondary endpoints reached statistical significance, the slowing was so modest as to make no practical difference clinically.
The combination of all these clinical trial failures with the result of imaging studies that have shown amyloid deposition some 20 years before the expected onset of symptoms clearly tells us that antiamyloid agents should only be considered as potential prophylactics. By the time symptoms appear, disease progression is largely independent of amyloid and may be primarily tau-driven, spreading from neuron to neuron even when amyloid is effectively targeted by therapeutics. Even the A4 and DIAN studies are likely initiating treatment too late to make anything more than a modest effect with little practical value clinically. I am not suggesting that we drop amyloid as a target, only that we stop making these incremental changes in clinical trial design in the hope of getting a different result.
Michael S. Wolfe, PhD, is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no financial disclosures.
This new phase III trial of solanezumab reveals that the drug is not effective for mild Alzheimer’s disease patients, despite the hint that it was possibly effective based on post-hoc analyses of earlier studies with this drug.
The findings expose the hazards of such post-hoc analyses, typically done when the desired results are not observed, in the hope of squeezing lemonade from lemons. Although the subanalysis of mild AD patients in the earlier studies suggested a 34% slowing of cognitive decline as assessed by ADAS-Cog, an incremental slowing of 11% was seen in the new study that was not even statistically significant. While some secondary endpoints reached statistical significance, the slowing was so modest as to make no practical difference clinically.
The combination of all these clinical trial failures with the result of imaging studies that have shown amyloid deposition some 20 years before the expected onset of symptoms clearly tells us that antiamyloid agents should only be considered as potential prophylactics. By the time symptoms appear, disease progression is largely independent of amyloid and may be primarily tau-driven, spreading from neuron to neuron even when amyloid is effectively targeted by therapeutics. Even the A4 and DIAN studies are likely initiating treatment too late to make anything more than a modest effect with little practical value clinically. I am not suggesting that we drop amyloid as a target, only that we stop making these incremental changes in clinical trial design in the hope of getting a different result.
Michael S. Wolfe, PhD, is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no financial disclosures.
This new phase III trial of solanezumab reveals that the drug is not effective for mild Alzheimer’s disease patients, despite the hint that it was possibly effective based on post-hoc analyses of earlier studies with this drug.
The findings expose the hazards of such post-hoc analyses, typically done when the desired results are not observed, in the hope of squeezing lemonade from lemons. Although the subanalysis of mild AD patients in the earlier studies suggested a 34% slowing of cognitive decline as assessed by ADAS-Cog, an incremental slowing of 11% was seen in the new study that was not even statistically significant. While some secondary endpoints reached statistical significance, the slowing was so modest as to make no practical difference clinically.
The combination of all these clinical trial failures with the result of imaging studies that have shown amyloid deposition some 20 years before the expected onset of symptoms clearly tells us that antiamyloid agents should only be considered as potential prophylactics. By the time symptoms appear, disease progression is largely independent of amyloid and may be primarily tau-driven, spreading from neuron to neuron even when amyloid is effectively targeted by therapeutics. Even the A4 and DIAN studies are likely initiating treatment too late to make anything more than a modest effect with little practical value clinically. I am not suggesting that we drop amyloid as a target, only that we stop making these incremental changes in clinical trial design in the hope of getting a different result.
Michael S. Wolfe, PhD, is the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. He has no financial disclosures.
SAN DIEGO – Solanezumab may have not have slowed the relentless march of Alzheimer’s disease (AD), but it was a valuable proving ground of the amyloid hypothesis, experts said during a wide-ranging discussion of Lilly’s failed EXPEDITION 3 trial.
Lilly representatives and EXPEDITION investigators released the study’s full results at the Clinical Trials in Alzheimer ’s disease meeting. – findings that should be read as tremendously encouraging rather than a defeat, according to Paul Aisen, MD, director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, who was also an EXPEDITION 3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION 3, but in its predecessors EXPEDITION and EXPEDITION 2.
“This is not a refutation of the amyloid hypothesis but a confirmation of it.”
Nevertheless, the trial must be read as a failed one, he admitted. There was no statistically significant separation between solanezumab and placebo on the ADAS-Cog14, a combined assessment of cognition and function that was the study’s primary endpoint. The active group experienced 11% less cognitive decline than did the placebo group, but the p-value remained tantalizingly below the level of significance, at 0.095.
“But what if solanezumab had hit at 0.05 instead of 0.095?” Dr. Aisen asked. “In fact, it would still be a small effect size,” which would have thrown into question the drug’s clinical utility. “Going into this, we thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key secondaries. Overall the effect size looks to be about 12%-13%, and that’s just too small.”
EXPEDITION 3 was the last of a triad of solanezumab studies, all of which posted intriguing signals of cognitive and functional benefit in patients with mild-moderate AD. It was based on subgroup analyses of EXPEDITION 1 and 2, both of which failed to meet their primary endpoints. But when researchers pooled the mild patients from both studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted EXPEDITION 3 to come as close to recreating those findings as possible but still stumbled over results that were numerically positive for the antibody but not statistically significant or clinically meaningful.
Lawrence S. Honig, MD, professor of neurology at Columbia University Medical Center, N.Y., and principal investigator of the EXPEDITION 3 study, presented the study’s full results to a packed audience on Dec. 8.
The study comprised about 2,000 patients with imaging-confirmed amyloid brain plaques and mild-moderate AD. They were randomized to placebo or monthly injections of 400 mg solanezumab for 80 weeks. The global study was conducted in 11 countries and 210 study sites.
Dr. Honig detailed the key secondary endpoints of cognition and function, and also revealed biomarker data.
While the ADAS-Cog failed to meet statistical significance, changes in the Mini Mental State Exam score did, with a 13% slowing of decline compared to placebo (P = .014). There was also a significant 5% difference in the Clinical Dementia Rating scale-sum of boxes test (P = .004).
Outcomes were mixed in measures of function. The Alzheimer’s Disease Cooperative Study activities of daily living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, posted significant results with 15% and 14% differences, respectively, relative to placebo (P = .009 and .019, respectively).
But results on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant, with only a 7% separation from placebo and a P value of .140.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab did what it was supposed to: bind soluble amyloid beta. This resulted in a 500- to 800-fold increase in the protein in plasma relative to placebo. There were no changes in amyloid brain plaques as measured by PET imaging, but this was no surprise, Dr. Aisen said, since the antibody doesn’t recognize fibrillar amyloid.
“What we expect to see with biomarkers differs based on the epitope targeted. Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble AB. Now how that helps AD is something of a debate, but it is important tor recognize that it does not attack plaques. Instead, by tying up monomeric AB, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma AB increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau, either in cerebrospinal fluid or imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was quite safe, with 17% of patient reporting an adverse event, compared to 19% of placebo patients. There were 9 deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
Although the data discussion was framed in the most hopeful light possible, no one on the panel attempted to massage it into a more clinically positive form. On a webcast in late November, Eric Siemers, MD, senior medical director of the Alzheimer’s Disease Global Development Team at Eli Lilly, said the company was disappointed but would not bring solanezumab forward for approval for mild or moderate AD patients. He echoed that sentiment during the panel discussion.
“We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress. So yes, we are very disappointed.”
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in a different population will go forward uninterrupted. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively health elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
There was also brief discussion of dosing. Some audience members suggested that a higher dose than EXPEDITION’s 400 mg might have bumped up efficacy, and asked if Lilly would reconsider the dosing schedule in the A4 and DIAN studies.
“There has been a lot of discussion around that,” said Dr. Siemers. “But it’s not as easy and straightforward as you think.”
Nevertheless, Dr. Aisen is excited about solanezumab’s potential in these trials that target the disease at its earliest phase, even before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive. Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of AD than even in the mild dementia stage.”
Maria Carrillo, PhD, chief science officer of the Alzheimer’s Association, said that EXPEDITION 3 is far from a path to nowhere. Instead, she urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder. This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach and to broaden the armamentarium our clinicians will need to combat this disease.”
“This is not a win, true. But it gets us a little closer to one.”
[email protected]
On Twitter @Alz_Gal
SAN DIEGO – Solanezumab may have not have slowed the relentless march of Alzheimer’s disease (AD), but it was a valuable proving ground of the amyloid hypothesis, experts said during a wide-ranging discussion of Lilly’s failed EXPEDITION 3 trial.
Lilly representatives and EXPEDITION investigators released the study’s full results at the Clinical Trials in Alzheimer ’s disease meeting. – findings that should be read as tremendously encouraging rather than a defeat, according to Paul Aisen, MD, director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles.
“We have here a negative study that confirms a beneficial treatment,” said Dr. Aisen, who was also an EXPEDITION 3 investigator. “We have a treatment that engages its target, binds to soluble amyloid, and, by virtue of that mechanism, is slowing cognitive and functional decline,” not only in EXPEDITION 3, but in its predecessors EXPEDITION and EXPEDITION 2.
“This is not a refutation of the amyloid hypothesis but a confirmation of it.”
Nevertheless, the trial must be read as a failed one, he admitted. There was no statistically significant separation between solanezumab and placebo on the ADAS-Cog14, a combined assessment of cognition and function that was the study’s primary endpoint. The active group experienced 11% less cognitive decline than did the placebo group, but the p-value remained tantalizingly below the level of significance, at 0.095.
“But what if solanezumab had hit at 0.05 instead of 0.095?” Dr. Aisen asked. “In fact, it would still be a small effect size,” which would have thrown into question the drug’s clinical utility. “Going into this, we thought we might see a 30% slowing of decline on the ADAS-Cog, and it was disappointing to only get 11%. But that is also what we saw on the key secondaries. Overall the effect size looks to be about 12%-13%, and that’s just too small.”
EXPEDITION 3 was the last of a triad of solanezumab studies, all of which posted intriguing signals of cognitive and functional benefit in patients with mild-moderate AD. It was based on subgroup analyses of EXPEDITION 1 and 2, both of which failed to meet their primary endpoints. But when researchers pooled the mild patients from both studies, they found that solanezumab was associated with a 34% slowing of cognitive decline on the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted EXPEDITION 3 to come as close to recreating those findings as possible but still stumbled over results that were numerically positive for the antibody but not statistically significant or clinically meaningful.
Lawrence S. Honig, MD, professor of neurology at Columbia University Medical Center, N.Y., and principal investigator of the EXPEDITION 3 study, presented the study’s full results to a packed audience on Dec. 8.
The study comprised about 2,000 patients with imaging-confirmed amyloid brain plaques and mild-moderate AD. They were randomized to placebo or monthly injections of 400 mg solanezumab for 80 weeks. The global study was conducted in 11 countries and 210 study sites.
Dr. Honig detailed the key secondary endpoints of cognition and function, and also revealed biomarker data.
While the ADAS-Cog failed to meet statistical significance, changes in the Mini Mental State Exam score did, with a 13% slowing of decline compared to placebo (P = .014). There was also a significant 5% difference in the Clinical Dementia Rating scale-sum of boxes test (P = .004).
Outcomes were mixed in measures of function. The Alzheimer’s Disease Cooperative Study activities of daily living (ADCS-ADL) and its related measure, the ADCS-ADL inventory instrumental items, posted significant results with 15% and 14% differences, respectively, relative to placebo (P = .009 and .019, respectively).
But results on the Functional Activities Questionnaire, an informant measure of more complex activities, were not significant, with only a 7% separation from placebo and a P value of .140.
Biomarkers trended the right way, Dr. Honig noted. Solanezumab did what it was supposed to: bind soluble amyloid beta. This resulted in a 500- to 800-fold increase in the protein in plasma relative to placebo. There were no changes in amyloid brain plaques as measured by PET imaging, but this was no surprise, Dr. Aisen said, since the antibody doesn’t recognize fibrillar amyloid.
“What we expect to see with biomarkers differs based on the epitope targeted. Solanezumab ignores plaques. It targets the middle of the peptide, binding to soluble AB. Now how that helps AD is something of a debate, but it is important tor recognize that it does not attack plaques. Instead, by tying up monomeric AB, it may change the dynamic exchange of various species of amyloid around plaques; the toxicity of amyloid is thought to reside as much in oligomeric species as in the fibrillar deposits. I see this [plasma AB increase] as confirming that it’s tying up monomeric amyloid species and that the result is a slowing of disease progression. I believe it is supportive of the amyloid hypothesis.”
Solanezumab had no significant effect on tau, either in cerebrospinal fluid or imaging, nor did it change the progression of ventricular enlargement, a marker of whole brain atrophy.
The antibody was quite safe, with 17% of patient reporting an adverse event, compared to 19% of placebo patients. There were 9 deaths in the solanezumab arm and 16 in the placebo arm; about 4% of each group discontinued treatment because of an adverse event.
Although the data discussion was framed in the most hopeful light possible, no one on the panel attempted to massage it into a more clinically positive form. On a webcast in late November, Eric Siemers, MD, senior medical director of the Alzheimer’s Disease Global Development Team at Eli Lilly, said the company was disappointed but would not bring solanezumab forward for approval for mild or moderate AD patients. He echoed that sentiment during the panel discussion.
“We didn’t expect this to be a cure for this disease, but we did hope it would be the first drug to slow its progress. So yes, we are very disappointed.”
He and Dr. Aisen confirmed, however, that two other trials using solanezumab in a different population will go forward uninterrupted. The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study) is investigating its effect in cognitively health elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
There was also brief discussion of dosing. Some audience members suggested that a higher dose than EXPEDITION’s 400 mg might have bumped up efficacy, and asked if Lilly would reconsider the dosing schedule in the A4 and DIAN studies.
“There has been a lot of discussion around that,” said Dr. Siemers. “But it’s not as easy and straightforward as you think.”
Nevertheless, Dr. Aisen is excited about solanezumab’s potential in these trials that target the disease at its earliest phase, even before cognitive symptoms develop. “I expect all antiamyloid treatments would work better when neurodegeneration is not extensive. Any of the antiamyloid antibodies would theoretically be more effective at a preclinical stage of AD than even in the mild dementia stage.”
Maria Carrillo, PhD, chief science officer of the Alzheimer’s Association, said that EXPEDITION 3 is far from a path to nowhere. Instead, she urged the research community, patients, and families to double down on their commitment to tackling the disease.
“These results stress the urgency for pushing forward harder. This is not a time to slow down. It’s a time to ramp up our efforts. This is not the time to sit back and say, ‘The amyloid hypothesis has been the wrong pathway and we need to drop it.’ But we also need to pursue other pathways, to broaden our approach and to broaden the armamentarium our clinicians will need to combat this disease.”
“This is not a win, true. But it gets us a little closer to one.”
[email protected]
On Twitter @Alz_Gal
EXPERT ANALYSIS AT CTAD
Spike in Colombian microcephaly cases linked to Zika infection early in pregnancy
Colombia experienced a fourfold increase in cases of microcephaly following the Zika virus outbreak in 2016, with temporal evidence suggesting that infection in the first months of pregnancy poses the greatest risk to the fetus of microcephaly.
From January 31 through mid-November 2016, there were 476 cases of microcephaly reported in Colombia, four times the rate of cases reported during the same period in 2015. In July 2016, there was a ninefold increase in Colombian microcephaly cases reported, compared with July 2015, according to data published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
Based on an average full-term gestation – because the 24-week period from the peak of Colombia’s Zika virus outbreak occurred simultaneously with the peak in reported microcephaly cases – there is a temporal suggestion that the greatest risk for microcephaly is associated with Zika virus infection during the first half of pregnancy, according to the authors of the report. Of the reported cases of microcephaly in 2016, 432 were live-born infants and 44 were pregnancy losses (MMWR. 2016 Dec 9. doi: 10.15585/mmwr.mm6549e1).
Of Colombia’s reported 105,000 cases of Zika virus occurring between August 9, 2015, and November 12, 2016, nearly 20,000 cases were in pregnant women, according to the Instituto Nacional de Salud.
The findings reinforce previous data indicating the correlation between early Zika virus-infection and microcephaly (N Engl J Med. 2016;374:1981-7. doi: 10.1056/NEJMsr1604338), although the report’s authors cautioned there are several confounders to these data, including that the surveillance was passive, not all reported cases of Zika virus infection were confirmed by a laboratory, and not all cases might have been reported.
The authors reported no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
Colombia experienced a fourfold increase in cases of microcephaly following the Zika virus outbreak in 2016, with temporal evidence suggesting that infection in the first months of pregnancy poses the greatest risk to the fetus of microcephaly.
From January 31 through mid-November 2016, there were 476 cases of microcephaly reported in Colombia, four times the rate of cases reported during the same period in 2015. In July 2016, there was a ninefold increase in Colombian microcephaly cases reported, compared with July 2015, according to data published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
Based on an average full-term gestation – because the 24-week period from the peak of Colombia’s Zika virus outbreak occurred simultaneously with the peak in reported microcephaly cases – there is a temporal suggestion that the greatest risk for microcephaly is associated with Zika virus infection during the first half of pregnancy, according to the authors of the report. Of the reported cases of microcephaly in 2016, 432 were live-born infants and 44 were pregnancy losses (MMWR. 2016 Dec 9. doi: 10.15585/mmwr.mm6549e1).
Of Colombia’s reported 105,000 cases of Zika virus occurring between August 9, 2015, and November 12, 2016, nearly 20,000 cases were in pregnant women, according to the Instituto Nacional de Salud.
The findings reinforce previous data indicating the correlation between early Zika virus-infection and microcephaly (N Engl J Med. 2016;374:1981-7. doi: 10.1056/NEJMsr1604338), although the report’s authors cautioned there are several confounders to these data, including that the surveillance was passive, not all reported cases of Zika virus infection were confirmed by a laboratory, and not all cases might have been reported.
The authors reported no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
Colombia experienced a fourfold increase in cases of microcephaly following the Zika virus outbreak in 2016, with temporal evidence suggesting that infection in the first months of pregnancy poses the greatest risk to the fetus of microcephaly.
From January 31 through mid-November 2016, there were 476 cases of microcephaly reported in Colombia, four times the rate of cases reported during the same period in 2015. In July 2016, there was a ninefold increase in Colombian microcephaly cases reported, compared with July 2015, according to data published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
Based on an average full-term gestation – because the 24-week period from the peak of Colombia’s Zika virus outbreak occurred simultaneously with the peak in reported microcephaly cases – there is a temporal suggestion that the greatest risk for microcephaly is associated with Zika virus infection during the first half of pregnancy, according to the authors of the report. Of the reported cases of microcephaly in 2016, 432 were live-born infants and 44 were pregnancy losses (MMWR. 2016 Dec 9. doi: 10.15585/mmwr.mm6549e1).
Of Colombia’s reported 105,000 cases of Zika virus occurring between August 9, 2015, and November 12, 2016, nearly 20,000 cases were in pregnant women, according to the Instituto Nacional de Salud.
The findings reinforce previous data indicating the correlation between early Zika virus-infection and microcephaly (N Engl J Med. 2016;374:1981-7. doi: 10.1056/NEJMsr1604338), although the report’s authors cautioned there are several confounders to these data, including that the surveillance was passive, not all reported cases of Zika virus infection were confirmed by a laboratory, and not all cases might have been reported.
The authors reported no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
FROM MMWR
Key clinical point:
Major finding: In 2016, a fourfold increase in microcephaly cases occurred in Colombia compared with 2015. Most cases coincided with women who were in their first trimester at the Zika outbreak’s inception.
Data source: National passive surveillance data of reported birth defects from Colombia’s Instituto Nacional de Salud.
Disclosures: The authors reported no relevant disclosures.
VIDEO: No effect of BRCA status on overall outcomes in early-onset breast cancer
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
Key clinical point:
Major finding: A significant 11% improvement in overall survival was seen among BRCA mutation carriers vs. noncarriers with triple-negative breast cancer.
Data source: The prospective POSH trial included 2,759 women with early-stage invasive breast cancer.
Disclosures: Dr. Eccles has been a consultant for AstraZeneca.
No boost in pCR from neoadjuvant estrogen deprivation
SAN ANTONIO – Although adding concurrent estrogen deprivation to a standard combination chemotherapy regimen for women with breast cancers positive for the estrogen receptors (ER+) and the human epidermal growth factor receptor–2 (HER2+) did not add to the already considerable toxicity, it also did not seem to add much benefit, according to an investigator in the NSABP B-52 trial.
Among 311 women with ER+/HER2+ tumors randomized to neoadjuvant therapy with docetaxel, carboplatin, trastuzumab (Herceptin), and pertuzumab (Perjeta) with or without estrogen deprivation therapy, there was no statistically significant difference in the primary endpoint of pathologic complete response (pCR) rates, reported Mothaffar F. Rimawi, MD, of the breast center at Baylor College of Medicine in Houston.
“I think the most important lesson from B-52 is that when we hit the tumors with everything we’ve got, this is what we have. We need to start thinking about whether we can, essentially, start dialing down these treatments. Can we omit one of the chemotherapy agents, if not both, and see what happens,” Dr. Rimawi said in an interview.
Endocrine/chemo interplay?
The rationale for the trial was based on several previous findings, including the propensity for ER+/HER2+ tumors to be less responsive to dual anti-HER2 therapy with trastuzumab (Herceptin) and pertuzumab (Perjeta).
There is also evidence to suggest that the estrogen receptor may act as a pathway of resistance to anti-HER2 therapy, and evidence from older trials suggested that chemotherapy and endocrine therapy may have antagonistic effects, he said.
The investigators hypothesized that concurrent inhibition of ER and HER2 tumors with neoadjuvant chemotherapy consisting of docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) would not be antagonistic, and could overcome resistance to treatment as shown by improvements in pathologic complete response rates.
The investigators enrolled women with HER+ and ER+ and/or progresterone receptor–positive (PR+) invasive breast cancer diagnosed by core needle biopsy, stratified them by premenopausal or postmenopausal status, and then randomized them to receive chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for six cycles, with or without estrogen deprivation therapy. Postmenopausal women received an aromatase inhibitor, and premenopausal women received an aromatase inhibitor plus ovarian suppression with goserelin and a lutenizing hormone–releasing hormone agonist.
For the primary endpoint, overall rates of pCR in the breast and nodes were 41% among 154 patients treated with TCHP chemotherapy alone, vs. 46% for 157 women treated with TCHP and estrogen deprivation, a difference that was not statistically significant. The results were similar in an analysis stratified by menopausal status: 44% vs. 46%, respectively, in premenopausal women, and 38% vs. 45% in postmenopausal women. Neither comparison was statistically significant.
In addition, no overall or stratified differences were seen in terms of pCR in the breast alone, and no differences were seen in clinical complete response rates, at 68.1% vs. 73.9%, respectively.
Nearly 100% of patients in the TCHP chemotherapy alone arm had diarrhea, ranging from 42% with grade 1, to 34% with grade 2, to 23% with grade 3, to less than 1% with grade 4. The distribution of diarrhea severity was virtually identical among patients treated with TCHP plus estrogen deprivation, all of whom experienced some grade. Other common gastrointestinal side effects in each study arm included nausea, vomiting, and dehydration, occurring in nearly all patients with equal distribution of severity between the two arms.
Hematologic toxicities included anemia, hypokalemia, and febrile neutropenia, again distributed evenly in severity between the trial arms.
Alternative endpoint?
Dr. Rimawi said the pCR endpoint commonly used in clinical trials may not be ideal for studying therapy in this population, because it generally correlates with outcomes among women when tumors are ER negative,“but when they are ER positive, the absence of a pathologic complete response is not necessarily a bad thing.”
He said that residual cancer burden, or RCB, appears to be a better marker for prognosis than pCR in patients with ER+/HER2+ tumors.
“In this population we need a different metric, a metric that is not path CR,” agreed Carlos Arteaga, MD, professor of cancer biology and medicine, and coleader of the breast cancer research program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
“We need other metrics that are more objective and more correlated with long-term outcomes. Path CR is not,” said Dr. Arteaga, who moderated a briefing where NSABP B-52 data were discussed prior to their presentation in the general session.
Regarding the lack of statistical significance, Dr. Rimawi said it is possible that the effects of chemotherapy may have blunted the responses that might otherwise have been seen with the addition of estrogen deprivation.
“We believe that if we de-escalate treatment, we could see a higher response to the estrogen receptor inhibitor,” he said.
NSABP B-52 was supported by the National Cancer Institute and Genentech. Dr. Rimawi reported contracted research from Genentech and GlaxoSmithKline. Dr. Arteaga reported no disclosures relevant to the trial.
SAN ANTONIO – Although adding concurrent estrogen deprivation to a standard combination chemotherapy regimen for women with breast cancers positive for the estrogen receptors (ER+) and the human epidermal growth factor receptor–2 (HER2+) did not add to the already considerable toxicity, it also did not seem to add much benefit, according to an investigator in the NSABP B-52 trial.
Among 311 women with ER+/HER2+ tumors randomized to neoadjuvant therapy with docetaxel, carboplatin, trastuzumab (Herceptin), and pertuzumab (Perjeta) with or without estrogen deprivation therapy, there was no statistically significant difference in the primary endpoint of pathologic complete response (pCR) rates, reported Mothaffar F. Rimawi, MD, of the breast center at Baylor College of Medicine in Houston.
“I think the most important lesson from B-52 is that when we hit the tumors with everything we’ve got, this is what we have. We need to start thinking about whether we can, essentially, start dialing down these treatments. Can we omit one of the chemotherapy agents, if not both, and see what happens,” Dr. Rimawi said in an interview.
Endocrine/chemo interplay?
The rationale for the trial was based on several previous findings, including the propensity for ER+/HER2+ tumors to be less responsive to dual anti-HER2 therapy with trastuzumab (Herceptin) and pertuzumab (Perjeta).
There is also evidence to suggest that the estrogen receptor may act as a pathway of resistance to anti-HER2 therapy, and evidence from older trials suggested that chemotherapy and endocrine therapy may have antagonistic effects, he said.
The investigators hypothesized that concurrent inhibition of ER and HER2 tumors with neoadjuvant chemotherapy consisting of docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) would not be antagonistic, and could overcome resistance to treatment as shown by improvements in pathologic complete response rates.
The investigators enrolled women with HER+ and ER+ and/or progresterone receptor–positive (PR+) invasive breast cancer diagnosed by core needle biopsy, stratified them by premenopausal or postmenopausal status, and then randomized them to receive chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for six cycles, with or without estrogen deprivation therapy. Postmenopausal women received an aromatase inhibitor, and premenopausal women received an aromatase inhibitor plus ovarian suppression with goserelin and a lutenizing hormone–releasing hormone agonist.
For the primary endpoint, overall rates of pCR in the breast and nodes were 41% among 154 patients treated with TCHP chemotherapy alone, vs. 46% for 157 women treated with TCHP and estrogen deprivation, a difference that was not statistically significant. The results were similar in an analysis stratified by menopausal status: 44% vs. 46%, respectively, in premenopausal women, and 38% vs. 45% in postmenopausal women. Neither comparison was statistically significant.
In addition, no overall or stratified differences were seen in terms of pCR in the breast alone, and no differences were seen in clinical complete response rates, at 68.1% vs. 73.9%, respectively.
Nearly 100% of patients in the TCHP chemotherapy alone arm had diarrhea, ranging from 42% with grade 1, to 34% with grade 2, to 23% with grade 3, to less than 1% with grade 4. The distribution of diarrhea severity was virtually identical among patients treated with TCHP plus estrogen deprivation, all of whom experienced some grade. Other common gastrointestinal side effects in each study arm included nausea, vomiting, and dehydration, occurring in nearly all patients with equal distribution of severity between the two arms.
Hematologic toxicities included anemia, hypokalemia, and febrile neutropenia, again distributed evenly in severity between the trial arms.
Alternative endpoint?
Dr. Rimawi said the pCR endpoint commonly used in clinical trials may not be ideal for studying therapy in this population, because it generally correlates with outcomes among women when tumors are ER negative,“but when they are ER positive, the absence of a pathologic complete response is not necessarily a bad thing.”
He said that residual cancer burden, or RCB, appears to be a better marker for prognosis than pCR in patients with ER+/HER2+ tumors.
“In this population we need a different metric, a metric that is not path CR,” agreed Carlos Arteaga, MD, professor of cancer biology and medicine, and coleader of the breast cancer research program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
“We need other metrics that are more objective and more correlated with long-term outcomes. Path CR is not,” said Dr. Arteaga, who moderated a briefing where NSABP B-52 data were discussed prior to their presentation in the general session.
Regarding the lack of statistical significance, Dr. Rimawi said it is possible that the effects of chemotherapy may have blunted the responses that might otherwise have been seen with the addition of estrogen deprivation.
“We believe that if we de-escalate treatment, we could see a higher response to the estrogen receptor inhibitor,” he said.
NSABP B-52 was supported by the National Cancer Institute and Genentech. Dr. Rimawi reported contracted research from Genentech and GlaxoSmithKline. Dr. Arteaga reported no disclosures relevant to the trial.
SAN ANTONIO – Although adding concurrent estrogen deprivation to a standard combination chemotherapy regimen for women with breast cancers positive for the estrogen receptors (ER+) and the human epidermal growth factor receptor–2 (HER2+) did not add to the already considerable toxicity, it also did not seem to add much benefit, according to an investigator in the NSABP B-52 trial.
Among 311 women with ER+/HER2+ tumors randomized to neoadjuvant therapy with docetaxel, carboplatin, trastuzumab (Herceptin), and pertuzumab (Perjeta) with or without estrogen deprivation therapy, there was no statistically significant difference in the primary endpoint of pathologic complete response (pCR) rates, reported Mothaffar F. Rimawi, MD, of the breast center at Baylor College of Medicine in Houston.
“I think the most important lesson from B-52 is that when we hit the tumors with everything we’ve got, this is what we have. We need to start thinking about whether we can, essentially, start dialing down these treatments. Can we omit one of the chemotherapy agents, if not both, and see what happens,” Dr. Rimawi said in an interview.
Endocrine/chemo interplay?
The rationale for the trial was based on several previous findings, including the propensity for ER+/HER2+ tumors to be less responsive to dual anti-HER2 therapy with trastuzumab (Herceptin) and pertuzumab (Perjeta).
There is also evidence to suggest that the estrogen receptor may act as a pathway of resistance to anti-HER2 therapy, and evidence from older trials suggested that chemotherapy and endocrine therapy may have antagonistic effects, he said.
The investigators hypothesized that concurrent inhibition of ER and HER2 tumors with neoadjuvant chemotherapy consisting of docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) would not be antagonistic, and could overcome resistance to treatment as shown by improvements in pathologic complete response rates.
The investigators enrolled women with HER+ and ER+ and/or progresterone receptor–positive (PR+) invasive breast cancer diagnosed by core needle biopsy, stratified them by premenopausal or postmenopausal status, and then randomized them to receive chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for six cycles, with or without estrogen deprivation therapy. Postmenopausal women received an aromatase inhibitor, and premenopausal women received an aromatase inhibitor plus ovarian suppression with goserelin and a lutenizing hormone–releasing hormone agonist.
For the primary endpoint, overall rates of pCR in the breast and nodes were 41% among 154 patients treated with TCHP chemotherapy alone, vs. 46% for 157 women treated with TCHP and estrogen deprivation, a difference that was not statistically significant. The results were similar in an analysis stratified by menopausal status: 44% vs. 46%, respectively, in premenopausal women, and 38% vs. 45% in postmenopausal women. Neither comparison was statistically significant.
In addition, no overall or stratified differences were seen in terms of pCR in the breast alone, and no differences were seen in clinical complete response rates, at 68.1% vs. 73.9%, respectively.
Nearly 100% of patients in the TCHP chemotherapy alone arm had diarrhea, ranging from 42% with grade 1, to 34% with grade 2, to 23% with grade 3, to less than 1% with grade 4. The distribution of diarrhea severity was virtually identical among patients treated with TCHP plus estrogen deprivation, all of whom experienced some grade. Other common gastrointestinal side effects in each study arm included nausea, vomiting, and dehydration, occurring in nearly all patients with equal distribution of severity between the two arms.
Hematologic toxicities included anemia, hypokalemia, and febrile neutropenia, again distributed evenly in severity between the trial arms.
Alternative endpoint?
Dr. Rimawi said the pCR endpoint commonly used in clinical trials may not be ideal for studying therapy in this population, because it generally correlates with outcomes among women when tumors are ER negative,“but when they are ER positive, the absence of a pathologic complete response is not necessarily a bad thing.”
He said that residual cancer burden, or RCB, appears to be a better marker for prognosis than pCR in patients with ER+/HER2+ tumors.
“In this population we need a different metric, a metric that is not path CR,” agreed Carlos Arteaga, MD, professor of cancer biology and medicine, and coleader of the breast cancer research program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
“We need other metrics that are more objective and more correlated with long-term outcomes. Path CR is not,” said Dr. Arteaga, who moderated a briefing where NSABP B-52 data were discussed prior to their presentation in the general session.
Regarding the lack of statistical significance, Dr. Rimawi said it is possible that the effects of chemotherapy may have blunted the responses that might otherwise have been seen with the addition of estrogen deprivation.
“We believe that if we de-escalate treatment, we could see a higher response to the estrogen receptor inhibitor,” he said.
NSABP B-52 was supported by the National Cancer Institute and Genentech. Dr. Rimawi reported contracted research from Genentech and GlaxoSmithKline. Dr. Arteaga reported no disclosures relevant to the trial.
AT SABCS 2016
Key clinical point: Estrogen deprivation added to chemotherapy and dual HER2 inhibition did not improve pathologic complete responses, compared with chemotherapy alone.
Major finding: The pathologic complete response rate for women with ER-positive/HER2-positive tumors was 41%, without estrogen deprivation, vs. 46% with estrogen deprivation (a nonsignficant difference).
Data source: A randomized phase III trial of neoadjuvant therapy in 311 women with ER+/HER2+ breast cancers.
Disclosures: NSABP-B52 was supported by the National Cancer Institute and Genentech. Dr. Rimawi reported contracted research from Genentech and GlaxoSmithKline. Dr. Arteaga reported no disclosures relevant to the trial.
Survey: Commonly delayed PCOS diagnosis suggests room for clinical improvement
Diagnosis of polycystic ovarian syndrome (PCOS) is commonly delayed, and this situation is a source of frustration for affected women, judging from findings from a survey of patients that was released early by the Journal of Clinical Endocrinology & Metabolism.
PCOS is not a difficult diagnosis to make. The disorder affected between 9% and 18% of women of reproductive age, and diagnosis requires the presence of two of the following three features: polycystic ovaries on ultrasound, biochemical/clinical hyperandrogenism, and/or oligomenorrhea/amenorrhea. Major gaps in early diagnosis of PCOS reflect the need for education and support for physicians and patients as “clear opportunities for improving patient experience,” noted Melanie Gibson-Helm, PhD, of Monash University, Melbourne, and her associates (J Clin Endo Metab. 2016. Dec 1. doi: 10.1210/jc.2016-2963).
A total of 1,385 women with a reported diagnosis of PCOS completed the survey, the largest international assessment of this issue. Participants were recruited via support group websites during 2015-2016. Most (64.8%) were aged 18-35 years of age at the time of the survey; 53% lived in North America, 42.2% in Europe, and 4.9% in other places.
About 34% reported waiting more than 2 years for a diagnosis, and 47.1% said they had seen more than three health care professionals before a diagnosis was established. More than half (52.5%) reported they didn’t receive any information about long-term PCOS complications or emotional counseling or support. About 35.2% said they were satisfied with their diagnosis experience, and 15.6% were satisfied with the information they received.
“The bottom line it’s easy to diagnose. There’s no reason why primary care providers and general practice physicians shouldn’t be able to evaluate those women,” she said. “These women are incredibly underserved.”
Amenorrhea and hirsutism should raise a red flag among physicians, coauthor Helena Teede, MBBS, PhD, said in an interview. Dr. Teede, president-elect of the Endocrine Society of Australia, is also on the faculty of Monash University.
The survey finding confirms a previous Australian study that noted PCOS diagnosis is often delayed, includes the involvement of a series of health professionals, and also leaves many women with unmet information needs.
For researchers, the findings were no surprise and reaffirmed what they said are inadequate responses to the disease from practitioners, with far-reaching changes needed, from improvements in diagnostic procedures to possible name changes for the condition to increase its understanding.
The study findings are intended to buttress an international initiative to improve diagnoses and education to not only meet women’s needs but also, it said, to “optimize early engagement with evidence-based management.”
This research received no direct funding. Dr Gibson-Helm and Dr. Teede are National Health & Medical Research Council Research Fellows; otherwise the authors have nothing to disclose.
Diagnosis of polycystic ovarian syndrome (PCOS) is commonly delayed, and this situation is a source of frustration for affected women, judging from findings from a survey of patients that was released early by the Journal of Clinical Endocrinology & Metabolism.
PCOS is not a difficult diagnosis to make. The disorder affected between 9% and 18% of women of reproductive age, and diagnosis requires the presence of two of the following three features: polycystic ovaries on ultrasound, biochemical/clinical hyperandrogenism, and/or oligomenorrhea/amenorrhea. Major gaps in early diagnosis of PCOS reflect the need for education and support for physicians and patients as “clear opportunities for improving patient experience,” noted Melanie Gibson-Helm, PhD, of Monash University, Melbourne, and her associates (J Clin Endo Metab. 2016. Dec 1. doi: 10.1210/jc.2016-2963).
A total of 1,385 women with a reported diagnosis of PCOS completed the survey, the largest international assessment of this issue. Participants were recruited via support group websites during 2015-2016. Most (64.8%) were aged 18-35 years of age at the time of the survey; 53% lived in North America, 42.2% in Europe, and 4.9% in other places.
About 34% reported waiting more than 2 years for a diagnosis, and 47.1% said they had seen more than three health care professionals before a diagnosis was established. More than half (52.5%) reported they didn’t receive any information about long-term PCOS complications or emotional counseling or support. About 35.2% said they were satisfied with their diagnosis experience, and 15.6% were satisfied with the information they received.
“The bottom line it’s easy to diagnose. There’s no reason why primary care providers and general practice physicians shouldn’t be able to evaluate those women,” she said. “These women are incredibly underserved.”
Amenorrhea and hirsutism should raise a red flag among physicians, coauthor Helena Teede, MBBS, PhD, said in an interview. Dr. Teede, president-elect of the Endocrine Society of Australia, is also on the faculty of Monash University.
The survey finding confirms a previous Australian study that noted PCOS diagnosis is often delayed, includes the involvement of a series of health professionals, and also leaves many women with unmet information needs.
For researchers, the findings were no surprise and reaffirmed what they said are inadequate responses to the disease from practitioners, with far-reaching changes needed, from improvements in diagnostic procedures to possible name changes for the condition to increase its understanding.
The study findings are intended to buttress an international initiative to improve diagnoses and education to not only meet women’s needs but also, it said, to “optimize early engagement with evidence-based management.”
This research received no direct funding. Dr Gibson-Helm and Dr. Teede are National Health & Medical Research Council Research Fellows; otherwise the authors have nothing to disclose.
Diagnosis of polycystic ovarian syndrome (PCOS) is commonly delayed, and this situation is a source of frustration for affected women, judging from findings from a survey of patients that was released early by the Journal of Clinical Endocrinology & Metabolism.
PCOS is not a difficult diagnosis to make. The disorder affected between 9% and 18% of women of reproductive age, and diagnosis requires the presence of two of the following three features: polycystic ovaries on ultrasound, biochemical/clinical hyperandrogenism, and/or oligomenorrhea/amenorrhea. Major gaps in early diagnosis of PCOS reflect the need for education and support for physicians and patients as “clear opportunities for improving patient experience,” noted Melanie Gibson-Helm, PhD, of Monash University, Melbourne, and her associates (J Clin Endo Metab. 2016. Dec 1. doi: 10.1210/jc.2016-2963).
A total of 1,385 women with a reported diagnosis of PCOS completed the survey, the largest international assessment of this issue. Participants were recruited via support group websites during 2015-2016. Most (64.8%) were aged 18-35 years of age at the time of the survey; 53% lived in North America, 42.2% in Europe, and 4.9% in other places.
About 34% reported waiting more than 2 years for a diagnosis, and 47.1% said they had seen more than three health care professionals before a diagnosis was established. More than half (52.5%) reported they didn’t receive any information about long-term PCOS complications or emotional counseling or support. About 35.2% said they were satisfied with their diagnosis experience, and 15.6% were satisfied with the information they received.
“The bottom line it’s easy to diagnose. There’s no reason why primary care providers and general practice physicians shouldn’t be able to evaluate those women,” she said. “These women are incredibly underserved.”
Amenorrhea and hirsutism should raise a red flag among physicians, coauthor Helena Teede, MBBS, PhD, said in an interview. Dr. Teede, president-elect of the Endocrine Society of Australia, is also on the faculty of Monash University.
The survey finding confirms a previous Australian study that noted PCOS diagnosis is often delayed, includes the involvement of a series of health professionals, and also leaves many women with unmet information needs.
For researchers, the findings were no surprise and reaffirmed what they said are inadequate responses to the disease from practitioners, with far-reaching changes needed, from improvements in diagnostic procedures to possible name changes for the condition to increase its understanding.
The study findings are intended to buttress an international initiative to improve diagnoses and education to not only meet women’s needs but also, it said, to “optimize early engagement with evidence-based management.”
This research received no direct funding. Dr Gibson-Helm and Dr. Teede are National Health & Medical Research Council Research Fellows; otherwise the authors have nothing to disclose.
AATS Resident Poster Competition Submission
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Deadline: January 20, 2017
Share:
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Deadline: January 20, 2017
Share:
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at The AATS Centennial.
The meeting will take place April 29 - May 3, 2017
Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
Deadline: January 20, 2017
Share:
“Honoring Our Mentors” Fellowship Open for Submission
The AATS Graham Foundation is calling for submission for its Denton A. Cooley “Honoring Our Mentors” fellowship.
Denton A. Cooley Fellowship
New! Provides a deserving CT surgeon resident or young postgraduate surgeon the opportunity to enrich his/her education during four weeks of study at the Texas Heart Institute and Baylor St. Luke’s Medical Center.
Deadline: December 30, 2016
Share:
The AATS Graham Foundation is calling for submission for its Denton A. Cooley “Honoring Our Mentors” fellowship.
Denton A. Cooley Fellowship
New! Provides a deserving CT surgeon resident or young postgraduate surgeon the opportunity to enrich his/her education during four weeks of study at the Texas Heart Institute and Baylor St. Luke’s Medical Center.
Deadline: December 30, 2016
Share:
The AATS Graham Foundation is calling for submission for its Denton A. Cooley “Honoring Our Mentors” fellowship.
Denton A. Cooley Fellowship
New! Provides a deserving CT surgeon resident or young postgraduate surgeon the opportunity to enrich his/her education during four weeks of study at the Texas Heart Institute and Baylor St. Luke’s Medical Center.
Deadline: December 30, 2016
Share: