User login
Drug produces responses in ‘challenging’ patients

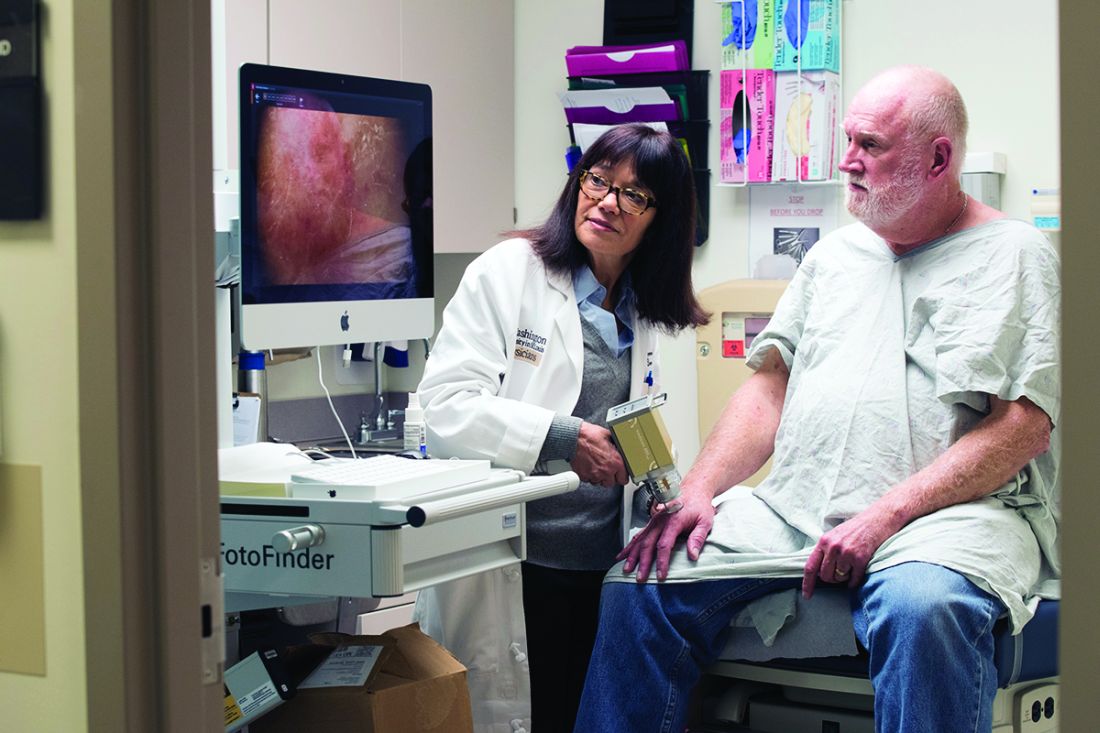
© Todd Buchanan 2016
SAN DIEGO—The oral BCL-2 inhibitor venetoclax can produce high objective response rates (ORRs) in chronic lymphocytic leukemia (CLL) patients who have failed treatment with at least one B-cell receptor inhibitor, according to investigators.
In a phase 2 study, venetoclax produced an ORR of 67% among all patients enrolled.
The drug produced a 70% ORR among patients who had failed treatment with ibrutinib and a 62% ORR among patients who had failed idelalisib.
“This represents the first prospective study in this patient population and does demonstrate high rates of durable responses, certainly making [venetoclax] a very viable option for a challenging group of patients to treat,” said study investigator Jeffrey Jones, MD, of The Ohio State University in Columbus.
Dr Jones presented results from this trial at the 2016 ASH Annual Meeting (abstract 637*). This study is sponsored by AbbVie in collaboration with Genentech/Roche.
The trial enrolled patients with CLL who relapsed after or were refractory to ibrutinib (arm A) or idelalisib (arm B). At the time of the data cut-off, 64 patients had been enrolled and treated with venetoclax, including 43 patients in arm A and 21 in arm B.
Patients received venetoclax via a recommended dose-titration schedule—20 mg once daily in week 1, 50 mg daily in week 2, 100 mg daily in week 3, 200 mg daily in week 4, and 400 mg daily from week 5 onward. Patients continued to receive the drug until disease progression or unacceptable toxicity.
To mitigate the risk of tumor lysis syndrome (TLS), patients received prophylaxis with uric acid lowering agents and hydration starting at least 72 hours before the first dose of venetoclax.
Patients with a high tumor burden were hospitalized for the first 20 mg dose and the first 50 mg dose, and they received intravenous hydration and rasburicase. Laboratory values were monitored at the first dose and all dose increases.
Patient characteristics: Arm A
Among patients who had failed ibrutinib, the median age was 66 (range, 48-80). Forty-nine percent of the patients had del(17p), and 35% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 4 (range, 1-12). All patients had received ibrutinib, but 9% had also received idelalisib. Ninety-one percent of patients were refractory to ibrutinib, and 5% were refractory to idelalisib.
The median time on ibrutinib was 17 months (range, 1-56), and the median time on idelalisib was 10 months (range, 2-31).
Patient characteristics: Arm B
Among patients who had failed idelalisib, the median age was 68 (range, 56-85). Ten percent of patients had del(17p), and 52% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 3 (range, 1-11). All patients had received idelalisib, but 24% had also received ibrutinib. Sixty-seven percent of patients were refractory to idelalisib, and 10% were refractory to ibrutinib.
The median time on idelalisib was 8 months (range, 1-27), and the median time on ibrutinib was 6 months (range, 2-11).
Results: Arm A
The median time on study in arm A was 13 months (range, 0.1-18). Eighteen patients in this arm discontinued the study—12 due to disease progression, 3 due to adverse events (AEs), 2 due to stem cell transplant, and 1 patient withdrew consent.
The ORR was 70% according to an independent review committee (IRC) and 67% according to investigators.
The rate of complete response (CR) was 0%, and the rate of CR with incomplete bone marrow recovery (CRi) was 2% according to the IRC. According to investigators, the CR rate was 5%, and the CRi rate was 2%.
Sixty-seven percent of patients had a partial response (PR) according to the IRC, and 56% had a PR according to investigators.
Results: Arm B
The median time on study in arm B was 9 months (range, 1.3-16). Four patients in this arm discontinued the study—3 related to disease progression and 1 for an “other” reason.
The ORR was 62% according to the IRC and 57% according to investigators.
The rate of CR/CRi was 0% according to the IRC. According to investigators, the CR rate was 10%, and the CRi rate was 5%.
Sixty-two percent of patients had a PR according to the IRC, and 43% had a PR according to investigators.
Results: Overall
The ORR was 67% according to the IRC and 64% according to investigators.
Forty-five percent of patient samples analyzed (14/31) demonstrated minimal residual disease (MRD) negativity in the peripheral blood between weeks 24 and 48. Five patients with sustained MRD negativity had bone marrow evaluations, and 1 was MRD negative.
At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival for all patients was 80%.
“Venetoclax has been well-tolerated,” Dr Jones noted. “The toxicity profile in this study is consistent with previous reports. Most of the toxicity has been cytopenias, which can be managed with dose adjustments or supportive care interventions, such as G-CSF.”
All 64 patients experienced an AE. Common AEs were neutropenia (58%), thrombocytopenia (44%), diarrhea (42%), nausea (41%), anemia (36%), fatigue (31%), decreased white blood cell count (22%), and hyperphosphatemia (22%).
Eighty-three percent of patients had grade 3/4 AEs, including neutropenia (45%), thrombocytopenia (28%), anemia (22%), decreased white blood cell count (13%), febrile neutropenia (11%), and pneumonia (11%).
Fifty-three percent of patients had serious AEs, including febrile neutropenia (9%), pneumonia (8%), multi-organ failure (3%), septic shock (3%), and increased potassium (3%).
There were no cases of clinical TLS. However, 1 patient with high tumor burden met Howard criteria for laboratory TLS. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—The oral BCL-2 inhibitor venetoclax can produce high objective response rates (ORRs) in chronic lymphocytic leukemia (CLL) patients who have failed treatment with at least one B-cell receptor inhibitor, according to investigators.
In a phase 2 study, venetoclax produced an ORR of 67% among all patients enrolled.
The drug produced a 70% ORR among patients who had failed treatment with ibrutinib and a 62% ORR among patients who had failed idelalisib.
“This represents the first prospective study in this patient population and does demonstrate high rates of durable responses, certainly making [venetoclax] a very viable option for a challenging group of patients to treat,” said study investigator Jeffrey Jones, MD, of The Ohio State University in Columbus.
Dr Jones presented results from this trial at the 2016 ASH Annual Meeting (abstract 637*). This study is sponsored by AbbVie in collaboration with Genentech/Roche.
The trial enrolled patients with CLL who relapsed after or were refractory to ibrutinib (arm A) or idelalisib (arm B). At the time of the data cut-off, 64 patients had been enrolled and treated with venetoclax, including 43 patients in arm A and 21 in arm B.
Patients received venetoclax via a recommended dose-titration schedule—20 mg once daily in week 1, 50 mg daily in week 2, 100 mg daily in week 3, 200 mg daily in week 4, and 400 mg daily from week 5 onward. Patients continued to receive the drug until disease progression or unacceptable toxicity.
To mitigate the risk of tumor lysis syndrome (TLS), patients received prophylaxis with uric acid lowering agents and hydration starting at least 72 hours before the first dose of venetoclax.
Patients with a high tumor burden were hospitalized for the first 20 mg dose and the first 50 mg dose, and they received intravenous hydration and rasburicase. Laboratory values were monitored at the first dose and all dose increases.
Patient characteristics: Arm A
Among patients who had failed ibrutinib, the median age was 66 (range, 48-80). Forty-nine percent of the patients had del(17p), and 35% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 4 (range, 1-12). All patients had received ibrutinib, but 9% had also received idelalisib. Ninety-one percent of patients were refractory to ibrutinib, and 5% were refractory to idelalisib.
The median time on ibrutinib was 17 months (range, 1-56), and the median time on idelalisib was 10 months (range, 2-31).
Patient characteristics: Arm B
Among patients who had failed idelalisib, the median age was 68 (range, 56-85). Ten percent of patients had del(17p), and 52% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 3 (range, 1-11). All patients had received idelalisib, but 24% had also received ibrutinib. Sixty-seven percent of patients were refractory to idelalisib, and 10% were refractory to ibrutinib.
The median time on idelalisib was 8 months (range, 1-27), and the median time on ibrutinib was 6 months (range, 2-11).
Results: Arm A
The median time on study in arm A was 13 months (range, 0.1-18). Eighteen patients in this arm discontinued the study—12 due to disease progression, 3 due to adverse events (AEs), 2 due to stem cell transplant, and 1 patient withdrew consent.
The ORR was 70% according to an independent review committee (IRC) and 67% according to investigators.
The rate of complete response (CR) was 0%, and the rate of CR with incomplete bone marrow recovery (CRi) was 2% according to the IRC. According to investigators, the CR rate was 5%, and the CRi rate was 2%.
Sixty-seven percent of patients had a partial response (PR) according to the IRC, and 56% had a PR according to investigators.
Results: Arm B
The median time on study in arm B was 9 months (range, 1.3-16). Four patients in this arm discontinued the study—3 related to disease progression and 1 for an “other” reason.
The ORR was 62% according to the IRC and 57% according to investigators.
The rate of CR/CRi was 0% according to the IRC. According to investigators, the CR rate was 10%, and the CRi rate was 5%.
Sixty-two percent of patients had a PR according to the IRC, and 43% had a PR according to investigators.
Results: Overall
The ORR was 67% according to the IRC and 64% according to investigators.
Forty-five percent of patient samples analyzed (14/31) demonstrated minimal residual disease (MRD) negativity in the peripheral blood between weeks 24 and 48. Five patients with sustained MRD negativity had bone marrow evaluations, and 1 was MRD negative.
At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival for all patients was 80%.
“Venetoclax has been well-tolerated,” Dr Jones noted. “The toxicity profile in this study is consistent with previous reports. Most of the toxicity has been cytopenias, which can be managed with dose adjustments or supportive care interventions, such as G-CSF.”
All 64 patients experienced an AE. Common AEs were neutropenia (58%), thrombocytopenia (44%), diarrhea (42%), nausea (41%), anemia (36%), fatigue (31%), decreased white blood cell count (22%), and hyperphosphatemia (22%).
Eighty-three percent of patients had grade 3/4 AEs, including neutropenia (45%), thrombocytopenia (28%), anemia (22%), decreased white blood cell count (13%), febrile neutropenia (11%), and pneumonia (11%).
Fifty-three percent of patients had serious AEs, including febrile neutropenia (9%), pneumonia (8%), multi-organ failure (3%), septic shock (3%), and increased potassium (3%).
There were no cases of clinical TLS. However, 1 patient with high tumor burden met Howard criteria for laboratory TLS. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—The oral BCL-2 inhibitor venetoclax can produce high objective response rates (ORRs) in chronic lymphocytic leukemia (CLL) patients who have failed treatment with at least one B-cell receptor inhibitor, according to investigators.
In a phase 2 study, venetoclax produced an ORR of 67% among all patients enrolled.
The drug produced a 70% ORR among patients who had failed treatment with ibrutinib and a 62% ORR among patients who had failed idelalisib.
“This represents the first prospective study in this patient population and does demonstrate high rates of durable responses, certainly making [venetoclax] a very viable option for a challenging group of patients to treat,” said study investigator Jeffrey Jones, MD, of The Ohio State University in Columbus.
Dr Jones presented results from this trial at the 2016 ASH Annual Meeting (abstract 637*). This study is sponsored by AbbVie in collaboration with Genentech/Roche.
The trial enrolled patients with CLL who relapsed after or were refractory to ibrutinib (arm A) or idelalisib (arm B). At the time of the data cut-off, 64 patients had been enrolled and treated with venetoclax, including 43 patients in arm A and 21 in arm B.
Patients received venetoclax via a recommended dose-titration schedule—20 mg once daily in week 1, 50 mg daily in week 2, 100 mg daily in week 3, 200 mg daily in week 4, and 400 mg daily from week 5 onward. Patients continued to receive the drug until disease progression or unacceptable toxicity.
To mitigate the risk of tumor lysis syndrome (TLS), patients received prophylaxis with uric acid lowering agents and hydration starting at least 72 hours before the first dose of venetoclax.
Patients with a high tumor burden were hospitalized for the first 20 mg dose and the first 50 mg dose, and they received intravenous hydration and rasburicase. Laboratory values were monitored at the first dose and all dose increases.
Patient characteristics: Arm A
Among patients who had failed ibrutinib, the median age was 66 (range, 48-80). Forty-nine percent of the patients had del(17p), and 35% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 4 (range, 1-12). All patients had received ibrutinib, but 9% had also received idelalisib. Ninety-one percent of patients were refractory to ibrutinib, and 5% were refractory to idelalisib.
The median time on ibrutinib was 17 months (range, 1-56), and the median time on idelalisib was 10 months (range, 2-31).
Patient characteristics: Arm B
Among patients who had failed idelalisib, the median age was 68 (range, 56-85). Ten percent of patients had del(17p), and 52% had bulky nodal disease (5 cm or greater).
The median number of prior treatments was 3 (range, 1-11). All patients had received idelalisib, but 24% had also received ibrutinib. Sixty-seven percent of patients were refractory to idelalisib, and 10% were refractory to ibrutinib.
The median time on idelalisib was 8 months (range, 1-27), and the median time on ibrutinib was 6 months (range, 2-11).
Results: Arm A
The median time on study in arm A was 13 months (range, 0.1-18). Eighteen patients in this arm discontinued the study—12 due to disease progression, 3 due to adverse events (AEs), 2 due to stem cell transplant, and 1 patient withdrew consent.
The ORR was 70% according to an independent review committee (IRC) and 67% according to investigators.
The rate of complete response (CR) was 0%, and the rate of CR with incomplete bone marrow recovery (CRi) was 2% according to the IRC. According to investigators, the CR rate was 5%, and the CRi rate was 2%.
Sixty-seven percent of patients had a partial response (PR) according to the IRC, and 56% had a PR according to investigators.
Results: Arm B
The median time on study in arm B was 9 months (range, 1.3-16). Four patients in this arm discontinued the study—3 related to disease progression and 1 for an “other” reason.
The ORR was 62% according to the IRC and 57% according to investigators.
The rate of CR/CRi was 0% according to the IRC. According to investigators, the CR rate was 10%, and the CRi rate was 5%.
Sixty-two percent of patients had a PR according to the IRC, and 43% had a PR according to investigators.
Results: Overall
The ORR was 67% according to the IRC and 64% according to investigators.
Forty-five percent of patient samples analyzed (14/31) demonstrated minimal residual disease (MRD) negativity in the peripheral blood between weeks 24 and 48. Five patients with sustained MRD negativity had bone marrow evaluations, and 1 was MRD negative.
At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival for all patients was 80%.
“Venetoclax has been well-tolerated,” Dr Jones noted. “The toxicity profile in this study is consistent with previous reports. Most of the toxicity has been cytopenias, which can be managed with dose adjustments or supportive care interventions, such as G-CSF.”
All 64 patients experienced an AE. Common AEs were neutropenia (58%), thrombocytopenia (44%), diarrhea (42%), nausea (41%), anemia (36%), fatigue (31%), decreased white blood cell count (22%), and hyperphosphatemia (22%).
Eighty-three percent of patients had grade 3/4 AEs, including neutropenia (45%), thrombocytopenia (28%), anemia (22%), decreased white blood cell count (13%), febrile neutropenia (11%), and pneumonia (11%).
Fifty-three percent of patients had serious AEs, including febrile neutropenia (9%), pneumonia (8%), multi-organ failure (3%), septic shock (3%), and increased potassium (3%).
There were no cases of clinical TLS. However, 1 patient with high tumor burden met Howard criteria for laboratory TLS. ![]()
*Information presented at the meeting differs from the abstract.
New Zika cases in pregnant women continue to drop
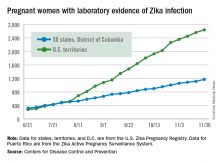
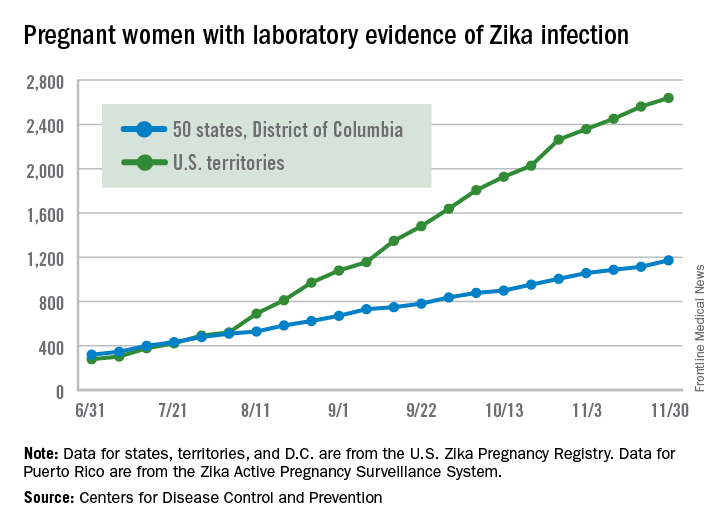
There were 136 new cases of pregnant women with laboratory evidence of Zika infection reported during the 2-week period ending Nov. 30, along with four liveborn infants with Zika-related birth defects, according to the Centers for Disease Control and Prevention.
The CDC did not report new totals for pregnant women and pregnancy outcomes for the week ending Nov. 23, so the most recent data release covers the 2-week period from Nov. 17-30. That 2-week total was barely more than the 124 reported for the week ending Nov. 10.
The four infants born with Zika-related birth defects were all born in the 50 states and D.C., as the CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika. There were no new pregnancy losses with Zika-related birth defects in the states/D.C., so that number remains at five, while the total number of liveborn infants with Zika-related birth defects is now 32, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 136 new cases of pregnant women with laboratory evidence of Zika infection reported during the 2-week period ending Nov. 30, along with four liveborn infants with Zika-related birth defects, according to the Centers for Disease Control and Prevention.
The CDC did not report new totals for pregnant women and pregnancy outcomes for the week ending Nov. 23, so the most recent data release covers the 2-week period from Nov. 17-30. That 2-week total was barely more than the 124 reported for the week ending Nov. 10.
The four infants born with Zika-related birth defects were all born in the 50 states and D.C., as the CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika. There were no new pregnancy losses with Zika-related birth defects in the states/D.C., so that number remains at five, while the total number of liveborn infants with Zika-related birth defects is now 32, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 136 new cases of pregnant women with laboratory evidence of Zika infection reported during the 2-week period ending Nov. 30, along with four liveborn infants with Zika-related birth defects, according to the Centers for Disease Control and Prevention.
The CDC did not report new totals for pregnant women and pregnancy outcomes for the week ending Nov. 23, so the most recent data release covers the 2-week period from Nov. 17-30. That 2-week total was barely more than the 124 reported for the week ending Nov. 10.
The four infants born with Zika-related birth defects were all born in the 50 states and D.C., as the CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika. There were no new pregnancy losses with Zika-related birth defects in the states/D.C., so that number remains at five, while the total number of liveborn infants with Zika-related birth defects is now 32, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
VIDEO: No improvement in pCR with dual ER and HER2 inhibition
SAN ANTONIO – Breast cancers that are positive for the estrogen receptor (ER) and human epidermal growth factor receptor–2 (HER2) are less likely than ER-negative/HER2-positive tumors to respond to dual anti-HER2 therapy, suggesting that the estrogen receptor may act as a pathway of resistance to anti-HER2 treatment.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-52 trial was designed to test the hypothesis that concurrent inhibition of both ER and HER2 added to chemotherapy with a platinum compound and a taxane will overcome resistance to treatment and improve pathologic complete response (pCR) rates in patients with ER-positive/HER2-positive breast cancer.
In a video interview at the San Antonio Breast Cancer Symposium, Mothaffar F. Rimawi, MD, discusses the trial results, which failed to show a significant difference in pCR rates between women who received chemotherapy with estrogen deprivation or chemotherapy alone. However, the trial still provided important information about the interplay between hormonal and HER2 receptors, and may inform future clinical trials examining reduction in tumor burden as a prognostic measure, says Dr. Rimawi from the Breast Center at Baylor College of Medicine, Houston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Breast cancers that are positive for the estrogen receptor (ER) and human epidermal growth factor receptor–2 (HER2) are less likely than ER-negative/HER2-positive tumors to respond to dual anti-HER2 therapy, suggesting that the estrogen receptor may act as a pathway of resistance to anti-HER2 treatment.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-52 trial was designed to test the hypothesis that concurrent inhibition of both ER and HER2 added to chemotherapy with a platinum compound and a taxane will overcome resistance to treatment and improve pathologic complete response (pCR) rates in patients with ER-positive/HER2-positive breast cancer.
In a video interview at the San Antonio Breast Cancer Symposium, Mothaffar F. Rimawi, MD, discusses the trial results, which failed to show a significant difference in pCR rates between women who received chemotherapy with estrogen deprivation or chemotherapy alone. However, the trial still provided important information about the interplay between hormonal and HER2 receptors, and may inform future clinical trials examining reduction in tumor burden as a prognostic measure, says Dr. Rimawi from the Breast Center at Baylor College of Medicine, Houston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Breast cancers that are positive for the estrogen receptor (ER) and human epidermal growth factor receptor–2 (HER2) are less likely than ER-negative/HER2-positive tumors to respond to dual anti-HER2 therapy, suggesting that the estrogen receptor may act as a pathway of resistance to anti-HER2 treatment.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-52 trial was designed to test the hypothesis that concurrent inhibition of both ER and HER2 added to chemotherapy with a platinum compound and a taxane will overcome resistance to treatment and improve pathologic complete response (pCR) rates in patients with ER-positive/HER2-positive breast cancer.
In a video interview at the San Antonio Breast Cancer Symposium, Mothaffar F. Rimawi, MD, discusses the trial results, which failed to show a significant difference in pCR rates between women who received chemotherapy with estrogen deprivation or chemotherapy alone. However, the trial still provided important information about the interplay between hormonal and HER2 receptors, and may inform future clinical trials examining reduction in tumor burden as a prognostic measure, says Dr. Rimawi from the Breast Center at Baylor College of Medicine, Houston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
VIDEO: Tips & Strategies for the Hospital Medicine Job Search
Dr. Thomas Frederickson, Dr. Benjamin Frizner, and Dr. Darlene Tad-y are all experienced at hiring and mentoring hospitalists at all career stages. They offer tips and strategies for assessing opportunity and negotiating your ideal HM job.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Thomas Frederickson, Dr. Benjamin Frizner, and Dr. Darlene Tad-y are all experienced at hiring and mentoring hospitalists at all career stages. They offer tips and strategies for assessing opportunity and negotiating your ideal HM job.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Thomas Frederickson, Dr. Benjamin Frizner, and Dr. Darlene Tad-y are all experienced at hiring and mentoring hospitalists at all career stages. They offer tips and strategies for assessing opportunity and negotiating your ideal HM job.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1967 at a glance
• The Early and Periodic Screening, Diagnostic and Treatment (EPSDT) program was enacted as the child health component of Medicaid. Children under 21 years enrolled in Medicaid are entitled to EPSDT benefits, and states must cover a “broad array of preventive and treatment services.”
• By 1967, all states had laws requiring that physicians report child abuse.
• Field trials of experimental respiratory syncytial virus vaccines were performed but stalled. Currently, 65 RSV vaccines are being studied, and 13 are in phase III trials.
• The World Health Association declared a war on smallpox with a worldwide vaccination campaign. Twelve years later the disease was eradicated.
• The Public Health Service issued the second Surgeon General’s report on the health consequences of smoking.
• The first heart transplant was performed by Christian Barnard.
• The Early and Periodic Screening, Diagnostic and Treatment (EPSDT) program was enacted as the child health component of Medicaid. Children under 21 years enrolled in Medicaid are entitled to EPSDT benefits, and states must cover a “broad array of preventive and treatment services.”
• By 1967, all states had laws requiring that physicians report child abuse.
• Field trials of experimental respiratory syncytial virus vaccines were performed but stalled. Currently, 65 RSV vaccines are being studied, and 13 are in phase III trials.
• The World Health Association declared a war on smallpox with a worldwide vaccination campaign. Twelve years later the disease was eradicated.
• The Public Health Service issued the second Surgeon General’s report on the health consequences of smoking.
• The first heart transplant was performed by Christian Barnard.
• The Early and Periodic Screening, Diagnostic and Treatment (EPSDT) program was enacted as the child health component of Medicaid. Children under 21 years enrolled in Medicaid are entitled to EPSDT benefits, and states must cover a “broad array of preventive and treatment services.”
• By 1967, all states had laws requiring that physicians report child abuse.
• Field trials of experimental respiratory syncytial virus vaccines were performed but stalled. Currently, 65 RSV vaccines are being studied, and 13 are in phase III trials.
• The World Health Association declared a war on smallpox with a worldwide vaccination campaign. Twelve years later the disease was eradicated.
• The Public Health Service issued the second Surgeon General’s report on the health consequences of smoking.
• The first heart transplant was performed by Christian Barnard.
A look back at 1967
As Pediatric News celebrates 50 years of publication, we’re taking a look back at our first year: 1967.
A review of the 1967 issues of the journal Pediatrics offers a snapshot of the state of the science and some surprising similarities with pediatric medicine today.
A commentary in the November issue entitled “Pediatrics at a Crossroad” described an accelerating trend toward pediatric group practice and noted that full-time hospital employment of pediatricians was an emerging pattern of pediatric practice. The author, Richard Smith, MD, of the University of Florida, Gainesville, expressed concern about certain practices, such as the wide employment of antibiotics for uncomplicated respiratory infections and “routine use of gamma globulin in community hospital nurseries supposedly to treat sepsis or its regular injection in putatively allergic children.” He also was concerned about a quality gap in pediatric specialty education (Pediatrics. 1967 Nov;40[5]:783-7).
Articles of interest in the infectious disease area included an article demonstrating the response of infants to trivalent polio vaccine (Dec;40[6]:980-5); a new antibody test for rubella (Dec;40[5]:787-8, 789-97); and a live attenuated mumps vaccine (Dec;40[5]:798-803). And as we deal with emerging infectious diseases on a large scale, a fatal case of dengue hemorrhagic fever was reported in an American child (Dec;40[5]:804-7).
In a commentary, Starkey D. Davis, MD, and Ralph J. Wedgwood, MD, of the University of Washington, Seattle, said that the annual tuberculosis infection rate was low and falling, and that new cases were decreasing. Sufficient beds were available to isolate infectious cases, and effective chemotherapy made most cases noninfectious quickly. Isoniazid was cheap and effective, and it decreased complications of asymptomatic primary tuberculosis in children by 85%. “Pediatricians can expect to see tuberculosis in children become a medical curiosity in this country as infection rates continue to fall, if isoniazid prophylaxis is energetically used,” they predicted (Jun;39[6]:809-10).
D. Holdaway of the Royal Victoria Infirmary in Newcastle Upon Tyne, England, and associates reported on 211 children with acute bronchiolitis and 295 controls with nonrespiratory illness. Of the children with bronchiolitis, 59% had respiratory syncytial virus (RSV), compared with 1% of the controls, which confirmed the etiological significance of RSV in bronchiolitis, the researchers said. They also maintained that “oxygen is vitally important in bronchiolitis and there is little conclusive evidence that any other therapy is consistently or even occasionally useful” (Jun;39[6]:924-8).
In a commentary, Leon Eisenberg, MD, of the division of child psychiatry at the Johns Hopkins Hospital, Baltimore, called for more training in child development for pediatricians (May;39[5];645-7). Today, pediatricians are calling for more training in child psychiatry.
A report by the American Academy of Pediatrics Committee on Nutrition on obesity in childhood was prescient. They said, despite much research, that “our ignorance concerning the etiology, pathogenesis, and treatment is remarkable” (Sep;40[3]:455-67). The committee acknowledged that morbidity and mortality for diabetes and cardiovascular disease were higher in obese adults than in those of average weight, and that obese children tended to remain obese as adults. The committee also wrote that no treatments had achieved more than minor success of weight reduction. It suggested prevention of weight gain as the likely best approach, with initiation by the pediatrician in high-risk families – advice that still holds true today 50 years later.
Throughout 2017, Pediatric News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including changes in child psychiatry, pediatric dermatology, and infectious disease medicine, changes in residency training, and the transformation of the well-child visit. Look for these articles and more special features on the pages of Pediatric News and here online.
As Pediatric News celebrates 50 years of publication, we’re taking a look back at our first year: 1967.
A review of the 1967 issues of the journal Pediatrics offers a snapshot of the state of the science and some surprising similarities with pediatric medicine today.
A commentary in the November issue entitled “Pediatrics at a Crossroad” described an accelerating trend toward pediatric group practice and noted that full-time hospital employment of pediatricians was an emerging pattern of pediatric practice. The author, Richard Smith, MD, of the University of Florida, Gainesville, expressed concern about certain practices, such as the wide employment of antibiotics for uncomplicated respiratory infections and “routine use of gamma globulin in community hospital nurseries supposedly to treat sepsis or its regular injection in putatively allergic children.” He also was concerned about a quality gap in pediatric specialty education (Pediatrics. 1967 Nov;40[5]:783-7).
Articles of interest in the infectious disease area included an article demonstrating the response of infants to trivalent polio vaccine (Dec;40[6]:980-5); a new antibody test for rubella (Dec;40[5]:787-8, 789-97); and a live attenuated mumps vaccine (Dec;40[5]:798-803). And as we deal with emerging infectious diseases on a large scale, a fatal case of dengue hemorrhagic fever was reported in an American child (Dec;40[5]:804-7).
In a commentary, Starkey D. Davis, MD, and Ralph J. Wedgwood, MD, of the University of Washington, Seattle, said that the annual tuberculosis infection rate was low and falling, and that new cases were decreasing. Sufficient beds were available to isolate infectious cases, and effective chemotherapy made most cases noninfectious quickly. Isoniazid was cheap and effective, and it decreased complications of asymptomatic primary tuberculosis in children by 85%. “Pediatricians can expect to see tuberculosis in children become a medical curiosity in this country as infection rates continue to fall, if isoniazid prophylaxis is energetically used,” they predicted (Jun;39[6]:809-10).
D. Holdaway of the Royal Victoria Infirmary in Newcastle Upon Tyne, England, and associates reported on 211 children with acute bronchiolitis and 295 controls with nonrespiratory illness. Of the children with bronchiolitis, 59% had respiratory syncytial virus (RSV), compared with 1% of the controls, which confirmed the etiological significance of RSV in bronchiolitis, the researchers said. They also maintained that “oxygen is vitally important in bronchiolitis and there is little conclusive evidence that any other therapy is consistently or even occasionally useful” (Jun;39[6]:924-8).
In a commentary, Leon Eisenberg, MD, of the division of child psychiatry at the Johns Hopkins Hospital, Baltimore, called for more training in child development for pediatricians (May;39[5];645-7). Today, pediatricians are calling for more training in child psychiatry.
A report by the American Academy of Pediatrics Committee on Nutrition on obesity in childhood was prescient. They said, despite much research, that “our ignorance concerning the etiology, pathogenesis, and treatment is remarkable” (Sep;40[3]:455-67). The committee acknowledged that morbidity and mortality for diabetes and cardiovascular disease were higher in obese adults than in those of average weight, and that obese children tended to remain obese as adults. The committee also wrote that no treatments had achieved more than minor success of weight reduction. It suggested prevention of weight gain as the likely best approach, with initiation by the pediatrician in high-risk families – advice that still holds true today 50 years later.
Throughout 2017, Pediatric News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including changes in child psychiatry, pediatric dermatology, and infectious disease medicine, changes in residency training, and the transformation of the well-child visit. Look for these articles and more special features on the pages of Pediatric News and here online.
As Pediatric News celebrates 50 years of publication, we’re taking a look back at our first year: 1967.
A review of the 1967 issues of the journal Pediatrics offers a snapshot of the state of the science and some surprising similarities with pediatric medicine today.
A commentary in the November issue entitled “Pediatrics at a Crossroad” described an accelerating trend toward pediatric group practice and noted that full-time hospital employment of pediatricians was an emerging pattern of pediatric practice. The author, Richard Smith, MD, of the University of Florida, Gainesville, expressed concern about certain practices, such as the wide employment of antibiotics for uncomplicated respiratory infections and “routine use of gamma globulin in community hospital nurseries supposedly to treat sepsis or its regular injection in putatively allergic children.” He also was concerned about a quality gap in pediatric specialty education (Pediatrics. 1967 Nov;40[5]:783-7).
Articles of interest in the infectious disease area included an article demonstrating the response of infants to trivalent polio vaccine (Dec;40[6]:980-5); a new antibody test for rubella (Dec;40[5]:787-8, 789-97); and a live attenuated mumps vaccine (Dec;40[5]:798-803). And as we deal with emerging infectious diseases on a large scale, a fatal case of dengue hemorrhagic fever was reported in an American child (Dec;40[5]:804-7).
In a commentary, Starkey D. Davis, MD, and Ralph J. Wedgwood, MD, of the University of Washington, Seattle, said that the annual tuberculosis infection rate was low and falling, and that new cases were decreasing. Sufficient beds were available to isolate infectious cases, and effective chemotherapy made most cases noninfectious quickly. Isoniazid was cheap and effective, and it decreased complications of asymptomatic primary tuberculosis in children by 85%. “Pediatricians can expect to see tuberculosis in children become a medical curiosity in this country as infection rates continue to fall, if isoniazid prophylaxis is energetically used,” they predicted (Jun;39[6]:809-10).
D. Holdaway of the Royal Victoria Infirmary in Newcastle Upon Tyne, England, and associates reported on 211 children with acute bronchiolitis and 295 controls with nonrespiratory illness. Of the children with bronchiolitis, 59% had respiratory syncytial virus (RSV), compared with 1% of the controls, which confirmed the etiological significance of RSV in bronchiolitis, the researchers said. They also maintained that “oxygen is vitally important in bronchiolitis and there is little conclusive evidence that any other therapy is consistently or even occasionally useful” (Jun;39[6]:924-8).
In a commentary, Leon Eisenberg, MD, of the division of child psychiatry at the Johns Hopkins Hospital, Baltimore, called for more training in child development for pediatricians (May;39[5];645-7). Today, pediatricians are calling for more training in child psychiatry.
A report by the American Academy of Pediatrics Committee on Nutrition on obesity in childhood was prescient. They said, despite much research, that “our ignorance concerning the etiology, pathogenesis, and treatment is remarkable” (Sep;40[3]:455-67). The committee acknowledged that morbidity and mortality for diabetes and cardiovascular disease were higher in obese adults than in those of average weight, and that obese children tended to remain obese as adults. The committee also wrote that no treatments had achieved more than minor success of weight reduction. It suggested prevention of weight gain as the likely best approach, with initiation by the pediatrician in high-risk families – advice that still holds true today 50 years later.
Throughout 2017, Pediatric News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including changes in child psychiatry, pediatric dermatology, and infectious disease medicine, changes in residency training, and the transformation of the well-child visit. Look for these articles and more special features on the pages of Pediatric News and here online.
Addition of calcipotriene to 5-FU increases efficacy, tolerability as AK treatment
A combined formulation of calcipotriol and 5-fluorouracil (5-FU) outperformed 5-FU alone in reducing the number of actinic keratoses (AKs), with a shorter treatment course and less inflammation than typically seen with 5-FU alone, researchers reported in a study published online in November.
5-FU is effective, but it produces crusting and significant irritation, and is temporarily disfiguring, creating discomfort and inconvenience that often leads to poor patient compliance with treatment.
After demonstrating that the combined treatment reduces AKs in mice, they conducted the study of 131 patients with AKs, randomized to treatment with a cream containing 5% 5-FU and 0.005% calcipotriol, or Vaseline plus 5% 5-FU alone. Participants applied the treatments twice per day for 4 days.
Eight weeks after treatment, the combination group had a mean 87.8% reduction in the number of AKs on the face, compared with 26.3% of the 5-FU controls. The treatment group also had better responses on the scalp (a mean 76.4% reduction in AKs versus 5.7%), right upper extremity (68.8% versus 9.6%), and left upper extremity (79% versus 16.3%). All differences were statistically significant (P less than .0001 for all comparisons).
“The greater efficacy of calcipotriol plus 5-FU versus Vaseline plus 5-FU treatment in eliminating actinic keratoses remained highly significant after controlling for the baseline actinic keratosis count, age, and sex of the participants,” they wrote (J Clin Invest. 2016 Nov 21. pii: 89820. doi: 10.1172/JCI89820).
Significantly more of those in the combination group has skin redness during treatment, and 39% experienced a burning sensation on treated skin, compared with 13% of the 5-FU treated group. The rate of scaling and itching of treated skin during treatment was similar, and no patients had crusting or wounding of the treated skin.
“It was incredibly well tolerated. There wasn’t as much discomfort or crusting to where people had to stop. And patients who had used 5-FU in the past preferred this shorter treatment course as well as the type and amount of inflammation they had,” compared with their previous experience, Lynn Cornelius, MD, professor and chief of dermatology, Washington University, Saint Louis, said in an interview. “And it was more efficacious,” added Dr. Cornelius, who was one of the study authors.
The trial was investigator initiated. Two authors received grants from the American Skin Association, the Dermatology Foundation, the Burroughs Wellcome Fund, the American Philosophical Society, the La Roche-Posay Research Foundation, and the National Institutes of Health; three investigators were supported by an NIH grant. Dr. Cornelius reported having no financial disclosures.
A combined formulation of calcipotriol and 5-fluorouracil (5-FU) outperformed 5-FU alone in reducing the number of actinic keratoses (AKs), with a shorter treatment course and less inflammation than typically seen with 5-FU alone, researchers reported in a study published online in November.
5-FU is effective, but it produces crusting and significant irritation, and is temporarily disfiguring, creating discomfort and inconvenience that often leads to poor patient compliance with treatment.
After demonstrating that the combined treatment reduces AKs in mice, they conducted the study of 131 patients with AKs, randomized to treatment with a cream containing 5% 5-FU and 0.005% calcipotriol, or Vaseline plus 5% 5-FU alone. Participants applied the treatments twice per day for 4 days.
Eight weeks after treatment, the combination group had a mean 87.8% reduction in the number of AKs on the face, compared with 26.3% of the 5-FU controls. The treatment group also had better responses on the scalp (a mean 76.4% reduction in AKs versus 5.7%), right upper extremity (68.8% versus 9.6%), and left upper extremity (79% versus 16.3%). All differences were statistically significant (P less than .0001 for all comparisons).
“The greater efficacy of calcipotriol plus 5-FU versus Vaseline plus 5-FU treatment in eliminating actinic keratoses remained highly significant after controlling for the baseline actinic keratosis count, age, and sex of the participants,” they wrote (J Clin Invest. 2016 Nov 21. pii: 89820. doi: 10.1172/JCI89820).
Significantly more of those in the combination group has skin redness during treatment, and 39% experienced a burning sensation on treated skin, compared with 13% of the 5-FU treated group. The rate of scaling and itching of treated skin during treatment was similar, and no patients had crusting or wounding of the treated skin.
“It was incredibly well tolerated. There wasn’t as much discomfort or crusting to where people had to stop. And patients who had used 5-FU in the past preferred this shorter treatment course as well as the type and amount of inflammation they had,” compared with their previous experience, Lynn Cornelius, MD, professor and chief of dermatology, Washington University, Saint Louis, said in an interview. “And it was more efficacious,” added Dr. Cornelius, who was one of the study authors.
The trial was investigator initiated. Two authors received grants from the American Skin Association, the Dermatology Foundation, the Burroughs Wellcome Fund, the American Philosophical Society, the La Roche-Posay Research Foundation, and the National Institutes of Health; three investigators were supported by an NIH grant. Dr. Cornelius reported having no financial disclosures.
A combined formulation of calcipotriol and 5-fluorouracil (5-FU) outperformed 5-FU alone in reducing the number of actinic keratoses (AKs), with a shorter treatment course and less inflammation than typically seen with 5-FU alone, researchers reported in a study published online in November.
5-FU is effective, but it produces crusting and significant irritation, and is temporarily disfiguring, creating discomfort and inconvenience that often leads to poor patient compliance with treatment.
After demonstrating that the combined treatment reduces AKs in mice, they conducted the study of 131 patients with AKs, randomized to treatment with a cream containing 5% 5-FU and 0.005% calcipotriol, or Vaseline plus 5% 5-FU alone. Participants applied the treatments twice per day for 4 days.
Eight weeks after treatment, the combination group had a mean 87.8% reduction in the number of AKs on the face, compared with 26.3% of the 5-FU controls. The treatment group also had better responses on the scalp (a mean 76.4% reduction in AKs versus 5.7%), right upper extremity (68.8% versus 9.6%), and left upper extremity (79% versus 16.3%). All differences were statistically significant (P less than .0001 for all comparisons).
“The greater efficacy of calcipotriol plus 5-FU versus Vaseline plus 5-FU treatment in eliminating actinic keratoses remained highly significant after controlling for the baseline actinic keratosis count, age, and sex of the participants,” they wrote (J Clin Invest. 2016 Nov 21. pii: 89820. doi: 10.1172/JCI89820).
Significantly more of those in the combination group has skin redness during treatment, and 39% experienced a burning sensation on treated skin, compared with 13% of the 5-FU treated group. The rate of scaling and itching of treated skin during treatment was similar, and no patients had crusting or wounding of the treated skin.
“It was incredibly well tolerated. There wasn’t as much discomfort or crusting to where people had to stop. And patients who had used 5-FU in the past preferred this shorter treatment course as well as the type and amount of inflammation they had,” compared with their previous experience, Lynn Cornelius, MD, professor and chief of dermatology, Washington University, Saint Louis, said in an interview. “And it was more efficacious,” added Dr. Cornelius, who was one of the study authors.
The trial was investigator initiated. Two authors received grants from the American Skin Association, the Dermatology Foundation, the Burroughs Wellcome Fund, the American Philosophical Society, the La Roche-Posay Research Foundation, and the National Institutes of Health; three investigators were supported by an NIH grant. Dr. Cornelius reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL INVESTIGATION
Key clinical point:
Major finding: The combination cream reduced the number of AKs on the face by 87.8%, compared with 5-FU alone.
Data source: A randomized, placebo controlled trial of 131 subjects with AKs.
Disclosures: The trial was investigator initiated. Two authors received grants from the American Skin Association, the Dermatology Foundation, the Burroughs Wellcome Fund, the American Philosophical Society, the La Roche-Posay Research Foundation, and the National Institutes of Health; three investigators were supported by an NIH grant. Dr. Cornelius reported having no financial disclosures.
Make the Diagnosis - December 2016
Acute generalized exanthematous pustulosis (AGEP)
Acute generalized exanthematous pustulosis (AGEP) is a fairly rare condition, with a reported incidence of one to five cases per million people per year. AGEP affects both sexes, but with a slight female predominance. Although the median age of occurrence is 56 years, people of all ages can develop AGEP. In approximately 90% of cases, AGEP is caused by a drug reaction, with the most common culprits being antibiotics, diltiazem, and antimalarials.
Fever, leukocytosis with an elevated neutrophil count ( greater than 7,000/microL), and mild eosinophilia are commonly observed during the acute phase of AGEP. Systemic involvement is fairly uncommon, with a study of 58 cases exhibiting organ involvement in 17% of patients. When systemic involvement was observed, hepatic, pulmonary, and renal dysfunction were most common. Hepatic dysfunction can result in elevated transaminase levels in some patients. Similarly, reduced creatinine clearance may be observed because of renal involvement.
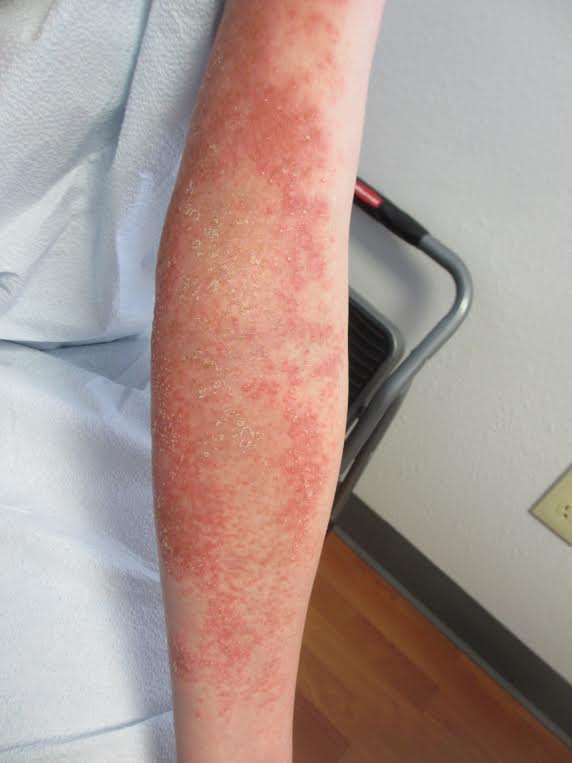
The management of AGEP involves removal of the offending agent. Antiseptic solutions and moist dressings may be used during the pustular phase to prevent infection. Emollients can aid in restoring skin barrier function during the desquamation phase. Antibiotics are not necessary prophylactically and should be avoided unless infection occurs. Pruritus and inflammation may be treated with topical corticosteroids in prolonged cases. Systemic corticosteroids are generally unwarranted as AGEP is a self-limited condition that is known to spontaneously resolve with the removal of the causative drug.
This case and photo were submitted by Natasha Cowan, BS, University of California, San Diego, and Brooke Resh Sateesh MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Acute generalized exanthematous pustulosis (AGEP)
Acute generalized exanthematous pustulosis (AGEP) is a fairly rare condition, with a reported incidence of one to five cases per million people per year. AGEP affects both sexes, but with a slight female predominance. Although the median age of occurrence is 56 years, people of all ages can develop AGEP. In approximately 90% of cases, AGEP is caused by a drug reaction, with the most common culprits being antibiotics, diltiazem, and antimalarials.
Fever, leukocytosis with an elevated neutrophil count ( greater than 7,000/microL), and mild eosinophilia are commonly observed during the acute phase of AGEP. Systemic involvement is fairly uncommon, with a study of 58 cases exhibiting organ involvement in 17% of patients. When systemic involvement was observed, hepatic, pulmonary, and renal dysfunction were most common. Hepatic dysfunction can result in elevated transaminase levels in some patients. Similarly, reduced creatinine clearance may be observed because of renal involvement.

The management of AGEP involves removal of the offending agent. Antiseptic solutions and moist dressings may be used during the pustular phase to prevent infection. Emollients can aid in restoring skin barrier function during the desquamation phase. Antibiotics are not necessary prophylactically and should be avoided unless infection occurs. Pruritus and inflammation may be treated with topical corticosteroids in prolonged cases. Systemic corticosteroids are generally unwarranted as AGEP is a self-limited condition that is known to spontaneously resolve with the removal of the causative drug.
This case and photo were submitted by Natasha Cowan, BS, University of California, San Diego, and Brooke Resh Sateesh MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Acute generalized exanthematous pustulosis (AGEP)
Acute generalized exanthematous pustulosis (AGEP) is a fairly rare condition, with a reported incidence of one to five cases per million people per year. AGEP affects both sexes, but with a slight female predominance. Although the median age of occurrence is 56 years, people of all ages can develop AGEP. In approximately 90% of cases, AGEP is caused by a drug reaction, with the most common culprits being antibiotics, diltiazem, and antimalarials.
Fever, leukocytosis with an elevated neutrophil count ( greater than 7,000/microL), and mild eosinophilia are commonly observed during the acute phase of AGEP. Systemic involvement is fairly uncommon, with a study of 58 cases exhibiting organ involvement in 17% of patients. When systemic involvement was observed, hepatic, pulmonary, and renal dysfunction were most common. Hepatic dysfunction can result in elevated transaminase levels in some patients. Similarly, reduced creatinine clearance may be observed because of renal involvement.

The management of AGEP involves removal of the offending agent. Antiseptic solutions and moist dressings may be used during the pustular phase to prevent infection. Emollients can aid in restoring skin barrier function during the desquamation phase. Antibiotics are not necessary prophylactically and should be avoided unless infection occurs. Pruritus and inflammation may be treated with topical corticosteroids in prolonged cases. Systemic corticosteroids are generally unwarranted as AGEP is a self-limited condition that is known to spontaneously resolve with the removal of the causative drug.
This case and photo were submitted by Natasha Cowan, BS, University of California, San Diego, and Brooke Resh Sateesh MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 27 year old female presented with pustules on her upper extremities and bilateral antecubital fossa. She also had superficial desquamation with underlying erythema on her torso. Five days prior to presentation, she underwent rhinoplasty for a deviated nasal septum. She received cefazolin during the surgery. One day post surgery, she developed lesions on her arms that then spread to her chest and back. She was treated in the emergency room with prednisone, famotidine (Pepcid), and diphenhydramine (Benadryl). She returned to the emergency department 4 days later and was treated with vancomycin, piperacillin/tazobactam, and clindamycin for possible toxic shock syndrome. She was admitted to the hospital and dermatology was consulted. Her liver function tests and eosinophil count were normal.
Landmark psychosocial guidelines for diabetes spark debate over the ideal vs. the practical
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
FROM DIABETES CARE
QUIZ: What is the Rate of Postoperative Atrial Fibrillation after Non-Cardiac Surgery?
[WpProQuiz 16]
[WpProQuiz_toplist 16]
[WpProQuiz 16]
[WpProQuiz_toplist 16]
[WpProQuiz 16]
[WpProQuiz_toplist 16]