User login
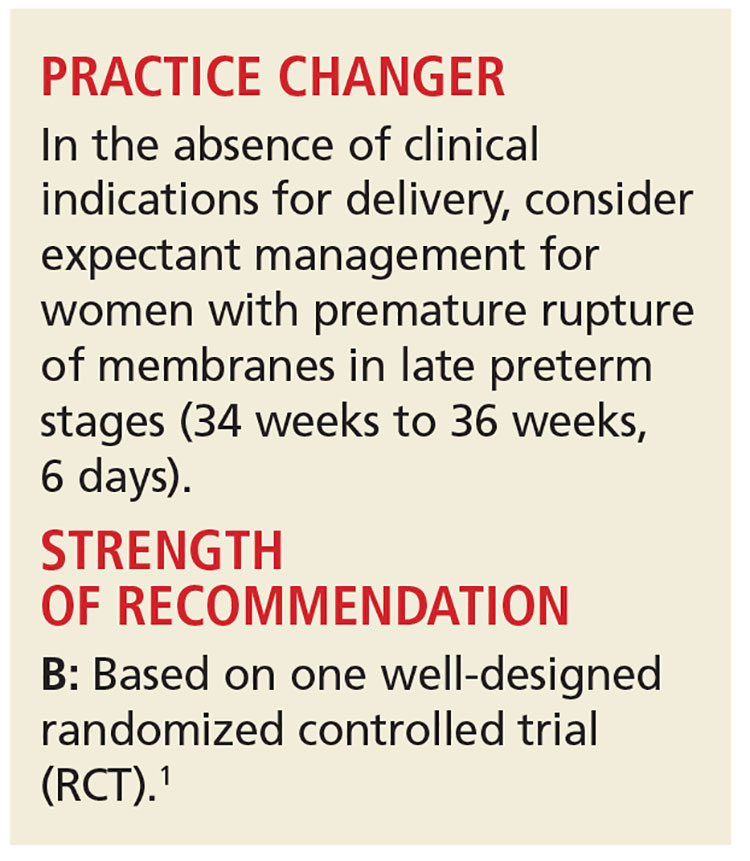
Deliver or Wait with Late Preterm Membrane Rupture?
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.

A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.

A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
Self-management May Provide Some Relief for Patients with Intellectual Disabilities and Epilepsy
Self-management techniques may help patients with epilepsy and intellectual disabilities suggests a recent review of the medical literature. Michelle Dannenberg and associates found that, while the research on self-management intervention is very limited, 5 high quality pilot and randomized controlled feasibility studies did suggest that such interventions have the potential to improve patients’ knowledge base, reduce the frequency of seizures, and improve their quality of life.
Dannenberg M, Mengoni SE, Gates B et al. Self-management interventions for epilepsy in people with intellectual disabilities: A scoping review. Seizure.2016; 41:16-25.
Self-management techniques may help patients with epilepsy and intellectual disabilities suggests a recent review of the medical literature. Michelle Dannenberg and associates found that, while the research on self-management intervention is very limited, 5 high quality pilot and randomized controlled feasibility studies did suggest that such interventions have the potential to improve patients’ knowledge base, reduce the frequency of seizures, and improve their quality of life.
Dannenberg M, Mengoni SE, Gates B et al. Self-management interventions for epilepsy in people with intellectual disabilities: A scoping review. Seizure.2016; 41:16-25.
Self-management techniques may help patients with epilepsy and intellectual disabilities suggests a recent review of the medical literature. Michelle Dannenberg and associates found that, while the research on self-management intervention is very limited, 5 high quality pilot and randomized controlled feasibility studies did suggest that such interventions have the potential to improve patients’ knowledge base, reduce the frequency of seizures, and improve their quality of life.
Dannenberg M, Mengoni SE, Gates B et al. Self-management interventions for epilepsy in people with intellectual disabilities: A scoping review. Seizure.2016; 41:16-25.
SCN8A Mutations Linked to Epilepsy Variants and Developmental Delay
Pathogenic variants of the SCN8A gene may contribute to a variety of epilepsy types, as well as nonseizure neurodevelopmental disorders, according a recent genetic analysis. Five variants of the gene called sodium channel alpha subunit 8, which codes for the ion pore region of the voltage-gated sodium channel, were detected in the genetic sequencing data from 275 epilepsy panels performed by the Emory Genetics Laboratory. Four of the 5 affected individuals had epilepsy and developmental delay/intellectual disability. The fifth patient had a less severe form of epilepsy that did not impair their cognitive abilities.
Butler KM, da Silva C, Shafir Y et al. De novo and inherited SCN8A epilepsy mutations detected by gene panel analysis. Epilepsy Res. 2016;129:17-25.
Pathogenic variants of the SCN8A gene may contribute to a variety of epilepsy types, as well as nonseizure neurodevelopmental disorders, according a recent genetic analysis. Five variants of the gene called sodium channel alpha subunit 8, which codes for the ion pore region of the voltage-gated sodium channel, were detected in the genetic sequencing data from 275 epilepsy panels performed by the Emory Genetics Laboratory. Four of the 5 affected individuals had epilepsy and developmental delay/intellectual disability. The fifth patient had a less severe form of epilepsy that did not impair their cognitive abilities.
Butler KM, da Silva C, Shafir Y et al. De novo and inherited SCN8A epilepsy mutations detected by gene panel analysis. Epilepsy Res. 2016;129:17-25.
Pathogenic variants of the SCN8A gene may contribute to a variety of epilepsy types, as well as nonseizure neurodevelopmental disorders, according a recent genetic analysis. Five variants of the gene called sodium channel alpha subunit 8, which codes for the ion pore region of the voltage-gated sodium channel, were detected in the genetic sequencing data from 275 epilepsy panels performed by the Emory Genetics Laboratory. Four of the 5 affected individuals had epilepsy and developmental delay/intellectual disability. The fifth patient had a less severe form of epilepsy that did not impair their cognitive abilities.
Butler KM, da Silva C, Shafir Y et al. De novo and inherited SCN8A epilepsy mutations detected by gene panel analysis. Epilepsy Res. 2016;129:17-25.
When to Perform Invasive EEG on Surgical Candidates With Epilepsy
The precise indications for intracranial electroencephalography (IEEG) remain unresolved and vary among epilepsy surgical centers. The International League Against Epilepsy has issued recommendations on the diagnostic usefulness of IEEG that discuss the application of a variety of modalities and that provide a consensus among experts on its efficacy, safety, ease, and cost benefits. The goal of the guidelines is to reduce over- and underuse of IEEE while at the same time allowing flexibility among the epilepsy centers that perform the procedure.
Jayakar P, Gotman J, Harvey AS et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016;57(11):1735-1747.
The precise indications for intracranial electroencephalography (IEEG) remain unresolved and vary among epilepsy surgical centers. The International League Against Epilepsy has issued recommendations on the diagnostic usefulness of IEEG that discuss the application of a variety of modalities and that provide a consensus among experts on its efficacy, safety, ease, and cost benefits. The goal of the guidelines is to reduce over- and underuse of IEEE while at the same time allowing flexibility among the epilepsy centers that perform the procedure.
Jayakar P, Gotman J, Harvey AS et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016;57(11):1735-1747.
The precise indications for intracranial electroencephalography (IEEG) remain unresolved and vary among epilepsy surgical centers. The International League Against Epilepsy has issued recommendations on the diagnostic usefulness of IEEG that discuss the application of a variety of modalities and that provide a consensus among experts on its efficacy, safety, ease, and cost benefits. The goal of the guidelines is to reduce over- and underuse of IEEE while at the same time allowing flexibility among the epilepsy centers that perform the procedure.
Jayakar P, Gotman J, Harvey AS et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016;57(11):1735-1747.
A Case of Intractable Epilepsy and Obstructive Sleep Apnea
Nancy Foldvary-Schaefer, DO, MS
Sleep Disorders and Epilepsy Center, Neurological Institute, Cleveland Clinic, Cleveland, OH
Madeleine Grigg-Damberger, MD
University of New Mexico School of Medicine, Department of Neurology, University of New Mexico, Albuquerque, NM
A 25-year-old, right-handed man with focal epilepsy since age 15 years presented to the epilepsy clinic. His seizures consisted of a visual change that was “like seeing bubbles through a microscope” and lightheadedness that progressed to unresponsiveness with staring, lip smacking, and unintelligible speech lasting 1 to 2 minutes; this was followed by tiredness for several hours. Focal seizures occurred several times per week and secondary generalization once per month despite trials of multiple antiepileptic drugs (AEDs). Birth and development were unremarkable. The patient had had 2 concussions with brief loss of consciousness in early childhood. Neurologic examination was normal.
Video electroencephalogram (EEG) monitoring showed left parieto-occipital spikes (95%) and generalized spike-and-wave complexes (5%). One typical seizure with secondary generalization was recorded as having a generalized, maximal, left-hemisphere EEG pattern. Ictal single-photon emission computed tomography (SPECT; 25-second injection) revealed left temporoparietal hyperfusion. Two subclinical seizures from the left parieto-occipital region were also recorded. Mild bilateral hippocampal atrophy and left hippocampal dysmorphism were seen on magnetic resonance imaging (MRI). Fluorodeoxyglucose (FDG)-positron emission tomography (PET) demonstrated left posterior temporoparietal hypometabolism. The patient underwent intracranial monitoring, which recorded spikes over the left temporo-occipital region, but no seizures were captured. He was discharged with a recommendation to consider vagus nerve stimulation (VNS) therapy.
The patient reported snoring, frequent nocturnal awakenings, and dozing while in sedentary situations. He had a body mass index of 30 kg/m2, a circumference of 42 cm, and oropharyngeal crowding. He had had a tonsillectomy in childhood, had gained 14 kg in the last 6 months following job loss, and reported feeling depressed. An overnight polysomnogram (PSG) was performed to detect suspected obstructive sleep apnea (OSA). It showed he had moderate OSA (17 obstructive events per hour of sleep) and oxygen desaturation to 67%. Continuous positive airway pressure (CPAP) therapy eliminated snoring and respiratory events and normalized oxygen saturation. The patient was started on CPAP at home, and the frequency of his seizures decreased without further adjustment of his AEDs. Months later, implantation of the VNS device was performed, and he became seizure free for 8 years despite a lead fracture and a battery failure. After 10 years of VNS and CPAP therapy, the patient had recurrence of daytime sleepiness and rare breakthrough seizures, prompting repeat CPAP titration resulting in airway pressure increase. He remains seizure free.
Diagnosis: Intractable focal epilepsy with comorbid obstructive sleep apnea responsive to CPAP and VNS therapy
Questions and Answers:
1. How common are sleep disorders in people with epilepsy?
Sleep disturbances are 2 to 3 times more common in people with epilepsy than in age-matched controls, with insomnia and OSA being the most common. The prevalence of OSA in adults with epilepsy exceeds 40%, with 16% of cases having moderate to severe OSA. Treatment of OSA in patients with epilepsy, using CPAP therapy in adults or tonsillectomy in children, has been shown to reduce seizures, alleviate daytime sleepiness, and improve sleep quality, depressive symptoms, and quality of life. Treatment of moderate to severe OSA has been shown to reduce the risk of hypertension, type 2 diabetes, obesity, cardiac arrhythmia, heart failure, myocardial infarction, stroke, and sudden death during sleep. This patient’s seizures began to improve after CPAP was initiated even before VNS device implantation and remained controlled despite 2 lapses in VNS therapy over time.
2. Why is PSG recommended in patients undergoing VNS implantation?
Emergence or worsening of apneas and hypopneas coinciding with VNS activation has been reported in adults and children with epilepsy. The underlying mechanisms are unclear, with both central and peripheral mechanisms proposed. A clinical diagnosis of OSA results in up to one-third of adult cases. Lower stimulating frequencies or prolonging off-time may prevent OSA exacerbations. The VNS device manufacturer recommends screening for OSA and consideration of PSG prior to and following VNS device implantation.
3. How can epilepsy providers screen patients with epilepsy for OSA?
Clinical instruments such as the STOP-BANG Questionnaire (Snoring, Tiredness/fatigue/sleepiness, Observed apnea, high blood Pressure, BMI >35kg/m2, Age >50 years, Neck circumference >40 cm [>16 in], and male Gender) can help identify patients with high probability of OSA. Patients with 3 or more positive responses on the STOP-BANG Questionnaire are considered high risk. High-risk patients should be referred for PSG for diagnostic confirmation.
Nancy Foldvary-Schaefer, DO, MS
Sleep Disorders and Epilepsy Center, Neurological Institute, Cleveland Clinic, Cleveland, OH
Madeleine Grigg-Damberger, MD
University of New Mexico School of Medicine, Department of Neurology, University of New Mexico, Albuquerque, NM
A 25-year-old, right-handed man with focal epilepsy since age 15 years presented to the epilepsy clinic. His seizures consisted of a visual change that was “like seeing bubbles through a microscope” and lightheadedness that progressed to unresponsiveness with staring, lip smacking, and unintelligible speech lasting 1 to 2 minutes; this was followed by tiredness for several hours. Focal seizures occurred several times per week and secondary generalization once per month despite trials of multiple antiepileptic drugs (AEDs). Birth and development were unremarkable. The patient had had 2 concussions with brief loss of consciousness in early childhood. Neurologic examination was normal.
Video electroencephalogram (EEG) monitoring showed left parieto-occipital spikes (95%) and generalized spike-and-wave complexes (5%). One typical seizure with secondary generalization was recorded as having a generalized, maximal, left-hemisphere EEG pattern. Ictal single-photon emission computed tomography (SPECT; 25-second injection) revealed left temporoparietal hyperfusion. Two subclinical seizures from the left parieto-occipital region were also recorded. Mild bilateral hippocampal atrophy and left hippocampal dysmorphism were seen on magnetic resonance imaging (MRI). Fluorodeoxyglucose (FDG)-positron emission tomography (PET) demonstrated left posterior temporoparietal hypometabolism. The patient underwent intracranial monitoring, which recorded spikes over the left temporo-occipital region, but no seizures were captured. He was discharged with a recommendation to consider vagus nerve stimulation (VNS) therapy.
The patient reported snoring, frequent nocturnal awakenings, and dozing while in sedentary situations. He had a body mass index of 30 kg/m2, a circumference of 42 cm, and oropharyngeal crowding. He had had a tonsillectomy in childhood, had gained 14 kg in the last 6 months following job loss, and reported feeling depressed. An overnight polysomnogram (PSG) was performed to detect suspected obstructive sleep apnea (OSA). It showed he had moderate OSA (17 obstructive events per hour of sleep) and oxygen desaturation to 67%. Continuous positive airway pressure (CPAP) therapy eliminated snoring and respiratory events and normalized oxygen saturation. The patient was started on CPAP at home, and the frequency of his seizures decreased without further adjustment of his AEDs. Months later, implantation of the VNS device was performed, and he became seizure free for 8 years despite a lead fracture and a battery failure. After 10 years of VNS and CPAP therapy, the patient had recurrence of daytime sleepiness and rare breakthrough seizures, prompting repeat CPAP titration resulting in airway pressure increase. He remains seizure free.
Diagnosis: Intractable focal epilepsy with comorbid obstructive sleep apnea responsive to CPAP and VNS therapy
Questions and Answers:
1. How common are sleep disorders in people with epilepsy?
Sleep disturbances are 2 to 3 times more common in people with epilepsy than in age-matched controls, with insomnia and OSA being the most common. The prevalence of OSA in adults with epilepsy exceeds 40%, with 16% of cases having moderate to severe OSA. Treatment of OSA in patients with epilepsy, using CPAP therapy in adults or tonsillectomy in children, has been shown to reduce seizures, alleviate daytime sleepiness, and improve sleep quality, depressive symptoms, and quality of life. Treatment of moderate to severe OSA has been shown to reduce the risk of hypertension, type 2 diabetes, obesity, cardiac arrhythmia, heart failure, myocardial infarction, stroke, and sudden death during sleep. This patient’s seizures began to improve after CPAP was initiated even before VNS device implantation and remained controlled despite 2 lapses in VNS therapy over time.
2. Why is PSG recommended in patients undergoing VNS implantation?
Emergence or worsening of apneas and hypopneas coinciding with VNS activation has been reported in adults and children with epilepsy. The underlying mechanisms are unclear, with both central and peripheral mechanisms proposed. A clinical diagnosis of OSA results in up to one-third of adult cases. Lower stimulating frequencies or prolonging off-time may prevent OSA exacerbations. The VNS device manufacturer recommends screening for OSA and consideration of PSG prior to and following VNS device implantation.
3. How can epilepsy providers screen patients with epilepsy for OSA?
Clinical instruments such as the STOP-BANG Questionnaire (Snoring, Tiredness/fatigue/sleepiness, Observed apnea, high blood Pressure, BMI >35kg/m2, Age >50 years, Neck circumference >40 cm [>16 in], and male Gender) can help identify patients with high probability of OSA. Patients with 3 or more positive responses on the STOP-BANG Questionnaire are considered high risk. High-risk patients should be referred for PSG for diagnostic confirmation.
Nancy Foldvary-Schaefer, DO, MS
Sleep Disorders and Epilepsy Center, Neurological Institute, Cleveland Clinic, Cleveland, OH
Madeleine Grigg-Damberger, MD
University of New Mexico School of Medicine, Department of Neurology, University of New Mexico, Albuquerque, NM
A 25-year-old, right-handed man with focal epilepsy since age 15 years presented to the epilepsy clinic. His seizures consisted of a visual change that was “like seeing bubbles through a microscope” and lightheadedness that progressed to unresponsiveness with staring, lip smacking, and unintelligible speech lasting 1 to 2 minutes; this was followed by tiredness for several hours. Focal seizures occurred several times per week and secondary generalization once per month despite trials of multiple antiepileptic drugs (AEDs). Birth and development were unremarkable. The patient had had 2 concussions with brief loss of consciousness in early childhood. Neurologic examination was normal.
Video electroencephalogram (EEG) monitoring showed left parieto-occipital spikes (95%) and generalized spike-and-wave complexes (5%). One typical seizure with secondary generalization was recorded as having a generalized, maximal, left-hemisphere EEG pattern. Ictal single-photon emission computed tomography (SPECT; 25-second injection) revealed left temporoparietal hyperfusion. Two subclinical seizures from the left parieto-occipital region were also recorded. Mild bilateral hippocampal atrophy and left hippocampal dysmorphism were seen on magnetic resonance imaging (MRI). Fluorodeoxyglucose (FDG)-positron emission tomography (PET) demonstrated left posterior temporoparietal hypometabolism. The patient underwent intracranial monitoring, which recorded spikes over the left temporo-occipital region, but no seizures were captured. He was discharged with a recommendation to consider vagus nerve stimulation (VNS) therapy.
The patient reported snoring, frequent nocturnal awakenings, and dozing while in sedentary situations. He had a body mass index of 30 kg/m2, a circumference of 42 cm, and oropharyngeal crowding. He had had a tonsillectomy in childhood, had gained 14 kg in the last 6 months following job loss, and reported feeling depressed. An overnight polysomnogram (PSG) was performed to detect suspected obstructive sleep apnea (OSA). It showed he had moderate OSA (17 obstructive events per hour of sleep) and oxygen desaturation to 67%. Continuous positive airway pressure (CPAP) therapy eliminated snoring and respiratory events and normalized oxygen saturation. The patient was started on CPAP at home, and the frequency of his seizures decreased without further adjustment of his AEDs. Months later, implantation of the VNS device was performed, and he became seizure free for 8 years despite a lead fracture and a battery failure. After 10 years of VNS and CPAP therapy, the patient had recurrence of daytime sleepiness and rare breakthrough seizures, prompting repeat CPAP titration resulting in airway pressure increase. He remains seizure free.
Diagnosis: Intractable focal epilepsy with comorbid obstructive sleep apnea responsive to CPAP and VNS therapy
Questions and Answers:
1. How common are sleep disorders in people with epilepsy?
Sleep disturbances are 2 to 3 times more common in people with epilepsy than in age-matched controls, with insomnia and OSA being the most common. The prevalence of OSA in adults with epilepsy exceeds 40%, with 16% of cases having moderate to severe OSA. Treatment of OSA in patients with epilepsy, using CPAP therapy in adults or tonsillectomy in children, has been shown to reduce seizures, alleviate daytime sleepiness, and improve sleep quality, depressive symptoms, and quality of life. Treatment of moderate to severe OSA has been shown to reduce the risk of hypertension, type 2 diabetes, obesity, cardiac arrhythmia, heart failure, myocardial infarction, stroke, and sudden death during sleep. This patient’s seizures began to improve after CPAP was initiated even before VNS device implantation and remained controlled despite 2 lapses in VNS therapy over time.
2. Why is PSG recommended in patients undergoing VNS implantation?
Emergence or worsening of apneas and hypopneas coinciding with VNS activation has been reported in adults and children with epilepsy. The underlying mechanisms are unclear, with both central and peripheral mechanisms proposed. A clinical diagnosis of OSA results in up to one-third of adult cases. Lower stimulating frequencies or prolonging off-time may prevent OSA exacerbations. The VNS device manufacturer recommends screening for OSA and consideration of PSG prior to and following VNS device implantation.
3. How can epilepsy providers screen patients with epilepsy for OSA?
Clinical instruments such as the STOP-BANG Questionnaire (Snoring, Tiredness/fatigue/sleepiness, Observed apnea, high blood Pressure, BMI >35kg/m2, Age >50 years, Neck circumference >40 cm [>16 in], and male Gender) can help identify patients with high probability of OSA. Patients with 3 or more positive responses on the STOP-BANG Questionnaire are considered high risk. High-risk patients should be referred for PSG for diagnostic confirmation.
Narrow band imaging could expand endometriosis detection
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Only topical lidocaine consistently eased infant vaccine pain
During infant vaccinations, only liposomal lidocaine provided consistent pain relief as part of a pain intervention, based on data from 352 infants.
“There is a dearth of data regarding the relative effects of combined interventions [for infants’ pain during vaccination] and their effectiveness over time,” wrote Anna Taddio, Ph.D., of the University of Toronto and her colleagues.
The researchers randomized 352 infants to one of four pain relief regimens to be used for all vaccinations during the first year of life: placebo control, parent-directed video education about infant soothing, the video plus oral sucrose, and the video plus sucrose plus topical lidocaine. The primary outcome of infant distress was assessed using the Modified Behavioural Pain Scale.
Overall, the mean pain scores were significantly lower in the video/sucrose/lidocaine group, compared with all other groups, and there were no significant differences in scores among the other groups.
“The observed treatment effect, albeit above the a priori threshold value set for clinical significance, may not be sufficiently compelling to clinicians to alter clinical practice, particularly in light of the short-lived nature of the pain,” the researchers said. However, “given that vaccination pain is iatrogenic and most infants were distressed despite the use of cointerventions, consideration should be given to adding lidocaine to reduce the burden of pain.”
Dr. Taddio disclosed a research grant from Pfizer, and materials for the study were supplied by Natus and Ferndale.
The findings were published online Dec. 12 in the Canadian Medical Association Journal (CMAJ 2016. doi: 10.1503/ cmaj.160542). Read the full study here.
During infant vaccinations, only liposomal lidocaine provided consistent pain relief as part of a pain intervention, based on data from 352 infants.
“There is a dearth of data regarding the relative effects of combined interventions [for infants’ pain during vaccination] and their effectiveness over time,” wrote Anna Taddio, Ph.D., of the University of Toronto and her colleagues.
The researchers randomized 352 infants to one of four pain relief regimens to be used for all vaccinations during the first year of life: placebo control, parent-directed video education about infant soothing, the video plus oral sucrose, and the video plus sucrose plus topical lidocaine. The primary outcome of infant distress was assessed using the Modified Behavioural Pain Scale.
Overall, the mean pain scores were significantly lower in the video/sucrose/lidocaine group, compared with all other groups, and there were no significant differences in scores among the other groups.
“The observed treatment effect, albeit above the a priori threshold value set for clinical significance, may not be sufficiently compelling to clinicians to alter clinical practice, particularly in light of the short-lived nature of the pain,” the researchers said. However, “given that vaccination pain is iatrogenic and most infants were distressed despite the use of cointerventions, consideration should be given to adding lidocaine to reduce the burden of pain.”
Dr. Taddio disclosed a research grant from Pfizer, and materials for the study were supplied by Natus and Ferndale.
The findings were published online Dec. 12 in the Canadian Medical Association Journal (CMAJ 2016. doi: 10.1503/ cmaj.160542). Read the full study here.
During infant vaccinations, only liposomal lidocaine provided consistent pain relief as part of a pain intervention, based on data from 352 infants.
“There is a dearth of data regarding the relative effects of combined interventions [for infants’ pain during vaccination] and their effectiveness over time,” wrote Anna Taddio, Ph.D., of the University of Toronto and her colleagues.
The researchers randomized 352 infants to one of four pain relief regimens to be used for all vaccinations during the first year of life: placebo control, parent-directed video education about infant soothing, the video plus oral sucrose, and the video plus sucrose plus topical lidocaine. The primary outcome of infant distress was assessed using the Modified Behavioural Pain Scale.
Overall, the mean pain scores were significantly lower in the video/sucrose/lidocaine group, compared with all other groups, and there were no significant differences in scores among the other groups.
“The observed treatment effect, albeit above the a priori threshold value set for clinical significance, may not be sufficiently compelling to clinicians to alter clinical practice, particularly in light of the short-lived nature of the pain,” the researchers said. However, “given that vaccination pain is iatrogenic and most infants were distressed despite the use of cointerventions, consideration should be given to adding lidocaine to reduce the burden of pain.”
Dr. Taddio disclosed a research grant from Pfizer, and materials for the study were supplied by Natus and Ferndale.
The findings were published online Dec. 12 in the Canadian Medical Association Journal (CMAJ 2016. doi: 10.1503/ cmaj.160542). Read the full study here.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Gene therapy to boost levodopa conversion enzyme shows benefit in Parkinson’s
Patients with advanced Parkinson’s disease were able to reduce daily levodopa doses in phase Ib testing of an experimental gene therapy from Voyager Therapeutics of Cambridge, Mass.
The company packed the gene for aromatic L-amino acid decarboxylase (AADC) – the enzyme that converts levodopa to dopamine – into a benign adenovirus capsid, then injected it directly into the putamen of 10 patients under MRI-guidance. The idea was to counter the decline in AADC as Parkinson’s progresses. For now, the name of the AADC vector is VY-AADC01.
Due to symptom improvement, cohort 1 patients were able to reduce daily levodopa and related medications by 14%, and cohort 2 patients by 34%, at 6 months. The “reduction in oral medication was generally maintained at 12 months,” the company said in an announcement. The results have not been published in a peer-reviewed journal.
“VY-AADC01 treatment prolonged the duration and markedly increased the motor symptom response to levodopa measured following a controlled intravenous infusion of levodopa [at] 6 months ... when compared to baseline.” There were no serious treatment-related adverse events, and patients went home within 2 days, Voyager said.
“We are excited about the data,” but “we think we have to be as good [as deep brain stimulation]; ideally, we’d obviously like to be better,” Voyager President and CEO Steven Paul, MD, said in a conference call Dec. 7. “We are optimistic that we can at least be equivalent with a onetime treatment, no indwelling hardware, no stimulators, and possibly even beat [deep brain stimulation], but that’s to be determined.” A placebo-controlled trial is scheduled to begin in late 2017 “so we can establish this really does work well against placebo.”
At 6 months, cohort 1 patients had a 15.6-point, off-medication improvement on the 56-point motor exam section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III), which further improved to 16.4 points over baseline at 12 months. Cohort 2 patient improved 17.8 points at 6 months, but declined to 14.3 points over baseline at 12 months.
On medication, cohort 1 worsened 1.6 points on the UPDRS-III at 6 months, and remained there at month 12. Cohort 2 improved 9.6 points over baseline at 6 months, and kept the gain at 1 year.
Cohort 1 lost 0.3 hours in diary on-time over baseline at 6 months, but gained 1.6 hours at 12 months, a 16% improvement. Cohort 2 had a 2.2-hour increase at 6 months, and a further increase to 4.1 hours at 1 year, a 43% improvement over baseline.
Patients had Parkinson’s for an average of 10 years, after being diagnosed at a mean age of 58 years.
Voyager’s stock jumped more than 20% when the results were announced, but lost much of the gain in subsequent trading; VY-AADC01 is one of several gene therapies under development for Parkinson’s.
Patients with advanced Parkinson’s disease were able to reduce daily levodopa doses in phase Ib testing of an experimental gene therapy from Voyager Therapeutics of Cambridge, Mass.
The company packed the gene for aromatic L-amino acid decarboxylase (AADC) – the enzyme that converts levodopa to dopamine – into a benign adenovirus capsid, then injected it directly into the putamen of 10 patients under MRI-guidance. The idea was to counter the decline in AADC as Parkinson’s progresses. For now, the name of the AADC vector is VY-AADC01.
Due to symptom improvement, cohort 1 patients were able to reduce daily levodopa and related medications by 14%, and cohort 2 patients by 34%, at 6 months. The “reduction in oral medication was generally maintained at 12 months,” the company said in an announcement. The results have not been published in a peer-reviewed journal.
“VY-AADC01 treatment prolonged the duration and markedly increased the motor symptom response to levodopa measured following a controlled intravenous infusion of levodopa [at] 6 months ... when compared to baseline.” There were no serious treatment-related adverse events, and patients went home within 2 days, Voyager said.
“We are excited about the data,” but “we think we have to be as good [as deep brain stimulation]; ideally, we’d obviously like to be better,” Voyager President and CEO Steven Paul, MD, said in a conference call Dec. 7. “We are optimistic that we can at least be equivalent with a onetime treatment, no indwelling hardware, no stimulators, and possibly even beat [deep brain stimulation], but that’s to be determined.” A placebo-controlled trial is scheduled to begin in late 2017 “so we can establish this really does work well against placebo.”
At 6 months, cohort 1 patients had a 15.6-point, off-medication improvement on the 56-point motor exam section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III), which further improved to 16.4 points over baseline at 12 months. Cohort 2 patient improved 17.8 points at 6 months, but declined to 14.3 points over baseline at 12 months.
On medication, cohort 1 worsened 1.6 points on the UPDRS-III at 6 months, and remained there at month 12. Cohort 2 improved 9.6 points over baseline at 6 months, and kept the gain at 1 year.
Cohort 1 lost 0.3 hours in diary on-time over baseline at 6 months, but gained 1.6 hours at 12 months, a 16% improvement. Cohort 2 had a 2.2-hour increase at 6 months, and a further increase to 4.1 hours at 1 year, a 43% improvement over baseline.
Patients had Parkinson’s for an average of 10 years, after being diagnosed at a mean age of 58 years.
Voyager’s stock jumped more than 20% when the results were announced, but lost much of the gain in subsequent trading; VY-AADC01 is one of several gene therapies under development for Parkinson’s.
Patients with advanced Parkinson’s disease were able to reduce daily levodopa doses in phase Ib testing of an experimental gene therapy from Voyager Therapeutics of Cambridge, Mass.
The company packed the gene for aromatic L-amino acid decarboxylase (AADC) – the enzyme that converts levodopa to dopamine – into a benign adenovirus capsid, then injected it directly into the putamen of 10 patients under MRI-guidance. The idea was to counter the decline in AADC as Parkinson’s progresses. For now, the name of the AADC vector is VY-AADC01.
Due to symptom improvement, cohort 1 patients were able to reduce daily levodopa and related medications by 14%, and cohort 2 patients by 34%, at 6 months. The “reduction in oral medication was generally maintained at 12 months,” the company said in an announcement. The results have not been published in a peer-reviewed journal.
“VY-AADC01 treatment prolonged the duration and markedly increased the motor symptom response to levodopa measured following a controlled intravenous infusion of levodopa [at] 6 months ... when compared to baseline.” There were no serious treatment-related adverse events, and patients went home within 2 days, Voyager said.
“We are excited about the data,” but “we think we have to be as good [as deep brain stimulation]; ideally, we’d obviously like to be better,” Voyager President and CEO Steven Paul, MD, said in a conference call Dec. 7. “We are optimistic that we can at least be equivalent with a onetime treatment, no indwelling hardware, no stimulators, and possibly even beat [deep brain stimulation], but that’s to be determined.” A placebo-controlled trial is scheduled to begin in late 2017 “so we can establish this really does work well against placebo.”
At 6 months, cohort 1 patients had a 15.6-point, off-medication improvement on the 56-point motor exam section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III), which further improved to 16.4 points over baseline at 12 months. Cohort 2 patient improved 17.8 points at 6 months, but declined to 14.3 points over baseline at 12 months.
On medication, cohort 1 worsened 1.6 points on the UPDRS-III at 6 months, and remained there at month 12. Cohort 2 improved 9.6 points over baseline at 6 months, and kept the gain at 1 year.
Cohort 1 lost 0.3 hours in diary on-time over baseline at 6 months, but gained 1.6 hours at 12 months, a 16% improvement. Cohort 2 had a 2.2-hour increase at 6 months, and a further increase to 4.1 hours at 1 year, a 43% improvement over baseline.
Patients had Parkinson’s for an average of 10 years, after being diagnosed at a mean age of 58 years.
Voyager’s stock jumped more than 20% when the results were announced, but lost much of the gain in subsequent trading; VY-AADC01 is one of several gene therapies under development for Parkinson’s.
Key clinical point:
Major finding: Cohort 1 patients were able to reduce daily levodopa and related medications by 14%, and cohort 2 patients by 34%, at 6 months.
Data source: Phase Ib testing in 10 advanced Parkinson’s disease patients.
Disclosures: The study was funded and conducted by Voyager Therapeutics. The company announced the results; they have not been published in a peer-reviewed journal.
Two-test sequence identifies sporadic Creutzfeldt-Jakob with 100% sensitivity, specificity
The sequential use of two diagnostic tests – one assessing cerebrospinal fluid and the other assessing nasal swabs – identifies sporadic Creutzfeldt-Jakob disease with virtually 100% sensitivity and specificity, according to a report published online Dec. 12 in JAMA Neurology.
“Patients with sporadic CJD show great variability in clinical signs and pathologic lesions,” which makes distinguishing the disorder from other neurodegenerative abnormalities challenging. Researchers assessed the performance of two diagnostic tests in a case-control study involving 86 patients referred because of clinical suspicion of CJD, 81 control subjects who had other neurologic disorders, and 23 control subjects who had no neurologic disorders. The final diagnoses of the 86 case patients were established either at postmortem examination or when a definite alternative diagnosis was made, said Matilde Bongianni, PhD, of the department of neurosciences, biomedicine, and movement sciences at the University of Verona (Italy) and associates.
The first diagnostic test was a second-generation real-time quaking-induced conversion (RT-QuIC) assay that detects femtograms of an abnormal CJD-specific prion protein in the cerebrospinal fluid (CSF), which has been reported to have a diagnostic sensitivity of 96%. The second diagnostic test applied the RT-QuIC to brushings from the olfactory mucosa using a soft, flocked nasal swab rather than the more invasive and painful cytobrush, which has been reported to have a diagnostic sensitivity of 97%.
By using these two tests sequentially, the investigators were able to identify all cases of CJD and distinguish affected patients from all the control subjects. Alternative diagnoses were immediately pursued in the 17 case patients in whom CJD was ruled out, and 5 of them were successfully treated for other abnormalities, the investigators said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4614).
“Our results suggest that the application of RT-QuIC testing will improve the accuracy and speed of sporadic CJD diagnosis compared with internationally recognized antemortem diagnostic criteria,” Dr. Bongianni and associates wrote.
“A positive RT-QuIC finding in the CSF of patients with progressive neurologic signs should be sufficient to establish a diagnosis of probable CJD and would make olfactory mucosa sampling unnecessary. However, when the RT-QuIC CSF finding is negative or lumbar puncture is not feasible, olfactory mucosa sampling would become necessary to confirm prion disease or to divert to alternative diagnoses,” they added.
The study was supported by the Alliance Biosecure Foundation, the University of Verona, and the National Institute of Allergy and Infectious Diseases. The investigators reported having no relevant financial disclosures.
At last we have a truly practical, inexpensive, and accurate procedure for diagnosing sporadic CJD, thanks to the impeccably conducted and convincing work of Dr. Bongianni and associates.
Testing the CSF and then the olfactory mucosa using the RT-QuIC assay should soon become the standard approach for diagnosing CJD, possibly replacing current CSF tests for 14-3-3 and tau proteins. This assay also could be extended to an even larger patient population if it is included in routine laboratory examinations of all patients who have mental/behavioral or cerebellar signs, regardless of their presumed diagnosis.
Because only four of eight mutation-positive symptomatic patients with genetic forms of prion disease tested positive, there may be some future cases of sporadic CJD with false-negative test results or it may be the case that genetic forms have a different timing and spread of misfolded prion protein through the CSF and nasal mucosa.
Paul Brown, MD, is a retired senior investigator at the Laboratory of Central Nervous System Studies at the National Institutes of Health, Bethesda, Md. He reported having no relevant financial disclosures. Dr. Brown made these remarks in an accompanying editorial (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4877).
At last we have a truly practical, inexpensive, and accurate procedure for diagnosing sporadic CJD, thanks to the impeccably conducted and convincing work of Dr. Bongianni and associates.
Testing the CSF and then the olfactory mucosa using the RT-QuIC assay should soon become the standard approach for diagnosing CJD, possibly replacing current CSF tests for 14-3-3 and tau proteins. This assay also could be extended to an even larger patient population if it is included in routine laboratory examinations of all patients who have mental/behavioral or cerebellar signs, regardless of their presumed diagnosis.
Because only four of eight mutation-positive symptomatic patients with genetic forms of prion disease tested positive, there may be some future cases of sporadic CJD with false-negative test results or it may be the case that genetic forms have a different timing and spread of misfolded prion protein through the CSF and nasal mucosa.
Paul Brown, MD, is a retired senior investigator at the Laboratory of Central Nervous System Studies at the National Institutes of Health, Bethesda, Md. He reported having no relevant financial disclosures. Dr. Brown made these remarks in an accompanying editorial (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4877).
At last we have a truly practical, inexpensive, and accurate procedure for diagnosing sporadic CJD, thanks to the impeccably conducted and convincing work of Dr. Bongianni and associates.
Testing the CSF and then the olfactory mucosa using the RT-QuIC assay should soon become the standard approach for diagnosing CJD, possibly replacing current CSF tests for 14-3-3 and tau proteins. This assay also could be extended to an even larger patient population if it is included in routine laboratory examinations of all patients who have mental/behavioral or cerebellar signs, regardless of their presumed diagnosis.
Because only four of eight mutation-positive symptomatic patients with genetic forms of prion disease tested positive, there may be some future cases of sporadic CJD with false-negative test results or it may be the case that genetic forms have a different timing and spread of misfolded prion protein through the CSF and nasal mucosa.
Paul Brown, MD, is a retired senior investigator at the Laboratory of Central Nervous System Studies at the National Institutes of Health, Bethesda, Md. He reported having no relevant financial disclosures. Dr. Brown made these remarks in an accompanying editorial (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4877).
The sequential use of two diagnostic tests – one assessing cerebrospinal fluid and the other assessing nasal swabs – identifies sporadic Creutzfeldt-Jakob disease with virtually 100% sensitivity and specificity, according to a report published online Dec. 12 in JAMA Neurology.
“Patients with sporadic CJD show great variability in clinical signs and pathologic lesions,” which makes distinguishing the disorder from other neurodegenerative abnormalities challenging. Researchers assessed the performance of two diagnostic tests in a case-control study involving 86 patients referred because of clinical suspicion of CJD, 81 control subjects who had other neurologic disorders, and 23 control subjects who had no neurologic disorders. The final diagnoses of the 86 case patients were established either at postmortem examination or when a definite alternative diagnosis was made, said Matilde Bongianni, PhD, of the department of neurosciences, biomedicine, and movement sciences at the University of Verona (Italy) and associates.
The first diagnostic test was a second-generation real-time quaking-induced conversion (RT-QuIC) assay that detects femtograms of an abnormal CJD-specific prion protein in the cerebrospinal fluid (CSF), which has been reported to have a diagnostic sensitivity of 96%. The second diagnostic test applied the RT-QuIC to brushings from the olfactory mucosa using a soft, flocked nasal swab rather than the more invasive and painful cytobrush, which has been reported to have a diagnostic sensitivity of 97%.
By using these two tests sequentially, the investigators were able to identify all cases of CJD and distinguish affected patients from all the control subjects. Alternative diagnoses were immediately pursued in the 17 case patients in whom CJD was ruled out, and 5 of them were successfully treated for other abnormalities, the investigators said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4614).
“Our results suggest that the application of RT-QuIC testing will improve the accuracy and speed of sporadic CJD diagnosis compared with internationally recognized antemortem diagnostic criteria,” Dr. Bongianni and associates wrote.
“A positive RT-QuIC finding in the CSF of patients with progressive neurologic signs should be sufficient to establish a diagnosis of probable CJD and would make olfactory mucosa sampling unnecessary. However, when the RT-QuIC CSF finding is negative or lumbar puncture is not feasible, olfactory mucosa sampling would become necessary to confirm prion disease or to divert to alternative diagnoses,” they added.
The study was supported by the Alliance Biosecure Foundation, the University of Verona, and the National Institute of Allergy and Infectious Diseases. The investigators reported having no relevant financial disclosures.
The sequential use of two diagnostic tests – one assessing cerebrospinal fluid and the other assessing nasal swabs – identifies sporadic Creutzfeldt-Jakob disease with virtually 100% sensitivity and specificity, according to a report published online Dec. 12 in JAMA Neurology.
“Patients with sporadic CJD show great variability in clinical signs and pathologic lesions,” which makes distinguishing the disorder from other neurodegenerative abnormalities challenging. Researchers assessed the performance of two diagnostic tests in a case-control study involving 86 patients referred because of clinical suspicion of CJD, 81 control subjects who had other neurologic disorders, and 23 control subjects who had no neurologic disorders. The final diagnoses of the 86 case patients were established either at postmortem examination or when a definite alternative diagnosis was made, said Matilde Bongianni, PhD, of the department of neurosciences, biomedicine, and movement sciences at the University of Verona (Italy) and associates.
The first diagnostic test was a second-generation real-time quaking-induced conversion (RT-QuIC) assay that detects femtograms of an abnormal CJD-specific prion protein in the cerebrospinal fluid (CSF), which has been reported to have a diagnostic sensitivity of 96%. The second diagnostic test applied the RT-QuIC to brushings from the olfactory mucosa using a soft, flocked nasal swab rather than the more invasive and painful cytobrush, which has been reported to have a diagnostic sensitivity of 97%.
By using these two tests sequentially, the investigators were able to identify all cases of CJD and distinguish affected patients from all the control subjects. Alternative diagnoses were immediately pursued in the 17 case patients in whom CJD was ruled out, and 5 of them were successfully treated for other abnormalities, the investigators said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.4614).
“Our results suggest that the application of RT-QuIC testing will improve the accuracy and speed of sporadic CJD diagnosis compared with internationally recognized antemortem diagnostic criteria,” Dr. Bongianni and associates wrote.
“A positive RT-QuIC finding in the CSF of patients with progressive neurologic signs should be sufficient to establish a diagnosis of probable CJD and would make olfactory mucosa sampling unnecessary. However, when the RT-QuIC CSF finding is negative or lumbar puncture is not feasible, olfactory mucosa sampling would become necessary to confirm prion disease or to divert to alternative diagnoses,” they added.
The study was supported by the Alliance Biosecure Foundation, the University of Verona, and the National Institute of Allergy and Infectious Diseases. The investigators reported having no relevant financial disclosures.
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: By using the RT-QuIC assay first on cerebrospinal fluid samples and then on olfactory mucosal swabbings, all 69 cases of CJD and prion disease were readily distinguished from all 17 cases of other neurologic disorders and from all 104 control subjects.
Data source: A case-control study involving 86 adults with clinical diagnoses of suspected, possible, or probable sporadic CJD; 81 control subjects with other neurologic disorders; and 23 control subjects with no neurologic disorders.
Disclosures: This study was supported by the Alliance Biosecure Foundation, the University of Verona, and the National Institute of Allergy and Infectious Diseases. Dr. Bongianni and her associates reported having no relevant financial disclosures.
Statins protect against Alzheimer’s in most patients
Statins taken to reduce cholesterol also protect most patients against Alzheimer’s disease, but the decrease in risk varies across different statins and by the patient’s gender and race/ethnicity, according to a report published Dec. 12 in JAMA Neurology.
In particular, none of the statins assessed in this study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, and her associates at the University of Southern California, Los Angeles.
The study population included 310,240 non-Hispanic white people, 32,658 Hispanic people, 32,278 non-Hispanic black people, and 24,803 people of Asian, Native American, other, or unknown race/ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin and atorvastatin, which are both lipophilic, and pravastatin and rosuvastatin, which are both hydrophilic. Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up.
Study participants who were exposed to higher statin levels during the 2-year exposure period were 10% less likely to receive an Alzheimer’s diagnosis during follow-up than were those exposed to lower levels of statins, across all four statins. High exposure to statins reduced the risk of Alzheimer’s among women of all races (hazard ratio, 0.85) and men of all races (HR, 0.88), reflecting 15% and 12% decreases, respectively.
This association, however, varied across gender and race/ethnicity. Statins decreased the risk of Alzheimer’s the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s among black men, Dr. Zissimopoulos and her associates said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.3783). Simvastatin significantly decreased the risk of Alzheimer’s among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin only decreased the risk of Alzheimer’s significantly among white women.
These findings suggest that “certain patients, facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program. Dr. Zissimopoulos and her associates reported having no relevant financial disclosures.
Statins taken to reduce cholesterol also protect most patients against Alzheimer’s disease, but the decrease in risk varies across different statins and by the patient’s gender and race/ethnicity, according to a report published Dec. 12 in JAMA Neurology.
In particular, none of the statins assessed in this study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, and her associates at the University of Southern California, Los Angeles.
The study population included 310,240 non-Hispanic white people, 32,658 Hispanic people, 32,278 non-Hispanic black people, and 24,803 people of Asian, Native American, other, or unknown race/ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin and atorvastatin, which are both lipophilic, and pravastatin and rosuvastatin, which are both hydrophilic. Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up.
Study participants who were exposed to higher statin levels during the 2-year exposure period were 10% less likely to receive an Alzheimer’s diagnosis during follow-up than were those exposed to lower levels of statins, across all four statins. High exposure to statins reduced the risk of Alzheimer’s among women of all races (hazard ratio, 0.85) and men of all races (HR, 0.88), reflecting 15% and 12% decreases, respectively.
This association, however, varied across gender and race/ethnicity. Statins decreased the risk of Alzheimer’s the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s among black men, Dr. Zissimopoulos and her associates said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.3783). Simvastatin significantly decreased the risk of Alzheimer’s among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin only decreased the risk of Alzheimer’s significantly among white women.
These findings suggest that “certain patients, facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program. Dr. Zissimopoulos and her associates reported having no relevant financial disclosures.
Statins taken to reduce cholesterol also protect most patients against Alzheimer’s disease, but the decrease in risk varies across different statins and by the patient’s gender and race/ethnicity, according to a report published Dec. 12 in JAMA Neurology.
In particular, none of the statins assessed in this study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, and her associates at the University of Southern California, Los Angeles.
The study population included 310,240 non-Hispanic white people, 32,658 Hispanic people, 32,278 non-Hispanic black people, and 24,803 people of Asian, Native American, other, or unknown race/ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin and atorvastatin, which are both lipophilic, and pravastatin and rosuvastatin, which are both hydrophilic. Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up.
Study participants who were exposed to higher statin levels during the 2-year exposure period were 10% less likely to receive an Alzheimer’s diagnosis during follow-up than were those exposed to lower levels of statins, across all four statins. High exposure to statins reduced the risk of Alzheimer’s among women of all races (hazard ratio, 0.85) and men of all races (HR, 0.88), reflecting 15% and 12% decreases, respectively.
This association, however, varied across gender and race/ethnicity. Statins decreased the risk of Alzheimer’s the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s among black men, Dr. Zissimopoulos and her associates said (JAMA Neurol. 2016 Dec 12. doi: 10.1001/jamaneurol.2016.3783). Simvastatin significantly decreased the risk of Alzheimer’s among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin only decreased the risk of Alzheimer’s significantly among white women.
These findings suggest that “certain patients, facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program. Dr. Zissimopoulos and her associates reported having no relevant financial disclosures.
FROM JAMA NEUROLOGY
Key clinical point: Statins taken to reduce cholesterol also protect most patients against Alzheimer’s disease, but the decrease in risk varies across different statins and according to the patient’s gender and race/ethnicity.
Major finding: Statins decreased the risk of Alzheimer’s the most among Hispanic men (hazard ratio, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s among black men.
Data source: A retrospective, longitudinal analysis of medical and pharmacy claims for approximately 400,000 Medicare beneficiaries from 2006 to 2013.
Disclosures: This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program. Dr. Zissimopoulos and her associates reported having no relevant financial disclosures.