User login
Sigma-1 agonist presses forward after positive results in small Alzheimer’s trial
SAN DIEGO – A novel Alzheimer’s disease drug candidate appeared to stabilize cognition and function over 57 weeks in a small, early-phase, open-label trial.
Patients with mild to moderate Alzheimer’s who took ANAVEX 2-73, an agonist of the sigma-1 receptor, experienced virtually no decline on either the Mini-Mental State Examination (MMSE) or the Alzheimer’s Disease Cooperative Study–activities of daily living (ADCS-ADL) functional scale. These findings correlated with significant improvements in the P300 evoked potential test – an electrophysiologic measure sometimes used to approximate synaptic connectivity and cortical processing speed.
The findings must be interpreted cautiously. The phase IIa study was designed to assess safety and tolerability; cognitive and functional endpoints were secondary. It comprised only 32 patients at baseline, 25 of whom completed both the 5-week, randomized, dose-finding, crossover trial and the 52-week, open-label, extension study. There was no placebo comparator. Instead, the study used three different sets of historical control data taken from other Alzheimer’s studies.
Nevertheless, the positive results are enough to propel ANAVEX 2-73 forward. The company will continue to treat and follow the extension study cohort, and plans to launch a placebo-controlled study in 2017, said Dr. Macfarlane, head of clinical governance for The Dementia Centre in Melbourne.
The 5-week, randomized, dose-finding, crossover trial started one group of patients on 30 or 50 mg/day oral ANAVEX 2-73 for 11 days after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 11 days of 3 mg/day or 5 mg/day intravenously. A second group first received 11 days of 3 mg/day or 5 mg/day ANAVEX 2-73 intravenously after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 30 or 50 mg/day oral ANAVEX 2-73 for 11 days. This was followed by a 52-week, open-label, extension trial of 10-50 mg/day orally, titrating each patient to the maximum tolerated dose. The extension phase was originally planned to last 6 months, but patients and caregivers wanted to continue on the medication, so the company extended it to 12 months. It is ongoing.
The sigma-1 receptor targeted by ANAVEX 2-73 is found on neurons and glia in many areas of the central nervous system. It modulates a number of processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke.
The sigma-1 receptor also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
In preclinical testing, ANAVEX 2-73 showed an additional cognitive property, seeming to display a cognition-enhancing effect in both wild-type and AD model mice.
The mean age of the patients in the extension study was 71 years. The median MMSE score was 20.5. Most patients (78%) were taking a stable dose of acetylcholinesterase inhibitor. During the extension phase, they were titrated to the maximum tolerated dose; 14 mg was the minimum dose necessary to achieve a therapeutic effect and keep the MMSE stable, but Dr. Macfarlane didn’t discuss detailed dosing.
The primary endpoints were safety, tolerability, and pharmacokinetics. The exploratory measures included the P300 electroencephalogram, MMSE score, the Computerized Cogstate Alzheimer’s Battery, and the ADCS-ADL. The Hamilton Depression (HAM-D) Scale was also employed as a neuropsychiatric symptom measure.
The cohort had low baseline depression scores, with a mean score of 2 on the HAM-D. By study’s end, that had decreased to a mean of 1 point. The biggest change was seen in insomnia; all patients who endorsed it at baseline reported it gone by 12 weeks into treatment.
Patients also reported improvements in their ability to work or do other activities, in anxiety, agitation, hypochondriasis, and insight.
The P300 wave amplitude showed a small initial bump from about 6 to 7 microvolts by 4 weeks, and then a dip back down to about 6 microvolts until about week 32. Thereafter it steadily improved, landing at around 8 microvolts by 57 weeks – a level usually seen in healthy age-matched controls. There was a significant separation from the P300 decline seen in a matched historical Alzheimer’s cohort, which dropped to about 4 microvolts over a 52-week period while patients were taking donepezil.
The study employed a second historical control group in another cognitive assessment using the Computerized Cogstate Alzheimer’s Battery. All subjects in the large Australian prospective cohort study, called AIBL (Australian Imaging, Biomarkers & Lifestyle Flagship Study of Ageing), were taking standard of care Alzheimer’s drugs. Compared with that cohort, the ANAVEX 2-73 group experienced benefits in processing speed, attention, and working memory, which became statistically significant at week 31 and continued to grow.
At 57 weeks, the mean MMSE score was stable, hovering around the baseline of 20. The ADCS-ADL declined slightly, from a mean of around 70 to around 65.
Finally, the investigators used yet another historical cohort as a comparator in a statistical analysis of projected cognitive and functional benefit. Compared with a pooled, placebo-arm, cohort study conducted by the Alzheimer Disease Cooperative Study Group over 12 months, ANAVEX 2-73 would have been associated with 1.8-point bump in score on the MMSE (P less than .016) and a 4-point benefit on the ADCS-ADL (P less than .019).
“The MMSE declined 45% less and the ADCS-ADL declined 56% less than what we would have expected from the historical control data,” Dr. Macfarlane said. “This is not only statistically significant, but clearly clinically meaningful for patients.”
Nearly all patients (98%) had some sort of adverse event, but most of them were mild transitory dizziness or headache; 76% of the events were grade 1, and 2% were grade 2. There were no serious adverse events. Three subjects dropped out because of adverse events (delirium, dizziness, and a combination of confusion, disorientation, and lethargy). There were no problematic interactions between the study drug and any standard of care AD medications.
Dr. Macfarlane has no financial interest in ANAVEX 2-73. He reported consultancies with Eli Lilly, Janssen-Cilag, and Lundbeck.
[email protected]
On Twitter @alz_gal
SAN DIEGO – A novel Alzheimer’s disease drug candidate appeared to stabilize cognition and function over 57 weeks in a small, early-phase, open-label trial.
Patients with mild to moderate Alzheimer’s who took ANAVEX 2-73, an agonist of the sigma-1 receptor, experienced virtually no decline on either the Mini-Mental State Examination (MMSE) or the Alzheimer’s Disease Cooperative Study–activities of daily living (ADCS-ADL) functional scale. These findings correlated with significant improvements in the P300 evoked potential test – an electrophysiologic measure sometimes used to approximate synaptic connectivity and cortical processing speed.
The findings must be interpreted cautiously. The phase IIa study was designed to assess safety and tolerability; cognitive and functional endpoints were secondary. It comprised only 32 patients at baseline, 25 of whom completed both the 5-week, randomized, dose-finding, crossover trial and the 52-week, open-label, extension study. There was no placebo comparator. Instead, the study used three different sets of historical control data taken from other Alzheimer’s studies.
Nevertheless, the positive results are enough to propel ANAVEX 2-73 forward. The company will continue to treat and follow the extension study cohort, and plans to launch a placebo-controlled study in 2017, said Dr. Macfarlane, head of clinical governance for The Dementia Centre in Melbourne.
The 5-week, randomized, dose-finding, crossover trial started one group of patients on 30 or 50 mg/day oral ANAVEX 2-73 for 11 days after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 11 days of 3 mg/day or 5 mg/day intravenously. A second group first received 11 days of 3 mg/day or 5 mg/day ANAVEX 2-73 intravenously after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 30 or 50 mg/day oral ANAVEX 2-73 for 11 days. This was followed by a 52-week, open-label, extension trial of 10-50 mg/day orally, titrating each patient to the maximum tolerated dose. The extension phase was originally planned to last 6 months, but patients and caregivers wanted to continue on the medication, so the company extended it to 12 months. It is ongoing.
The sigma-1 receptor targeted by ANAVEX 2-73 is found on neurons and glia in many areas of the central nervous system. It modulates a number of processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke.
The sigma-1 receptor also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
In preclinical testing, ANAVEX 2-73 showed an additional cognitive property, seeming to display a cognition-enhancing effect in both wild-type and AD model mice.
The mean age of the patients in the extension study was 71 years. The median MMSE score was 20.5. Most patients (78%) were taking a stable dose of acetylcholinesterase inhibitor. During the extension phase, they were titrated to the maximum tolerated dose; 14 mg was the minimum dose necessary to achieve a therapeutic effect and keep the MMSE stable, but Dr. Macfarlane didn’t discuss detailed dosing.
The primary endpoints were safety, tolerability, and pharmacokinetics. The exploratory measures included the P300 electroencephalogram, MMSE score, the Computerized Cogstate Alzheimer’s Battery, and the ADCS-ADL. The Hamilton Depression (HAM-D) Scale was also employed as a neuropsychiatric symptom measure.
The cohort had low baseline depression scores, with a mean score of 2 on the HAM-D. By study’s end, that had decreased to a mean of 1 point. The biggest change was seen in insomnia; all patients who endorsed it at baseline reported it gone by 12 weeks into treatment.
Patients also reported improvements in their ability to work or do other activities, in anxiety, agitation, hypochondriasis, and insight.
The P300 wave amplitude showed a small initial bump from about 6 to 7 microvolts by 4 weeks, and then a dip back down to about 6 microvolts until about week 32. Thereafter it steadily improved, landing at around 8 microvolts by 57 weeks – a level usually seen in healthy age-matched controls. There was a significant separation from the P300 decline seen in a matched historical Alzheimer’s cohort, which dropped to about 4 microvolts over a 52-week period while patients were taking donepezil.
The study employed a second historical control group in another cognitive assessment using the Computerized Cogstate Alzheimer’s Battery. All subjects in the large Australian prospective cohort study, called AIBL (Australian Imaging, Biomarkers & Lifestyle Flagship Study of Ageing), were taking standard of care Alzheimer’s drugs. Compared with that cohort, the ANAVEX 2-73 group experienced benefits in processing speed, attention, and working memory, which became statistically significant at week 31 and continued to grow.
At 57 weeks, the mean MMSE score was stable, hovering around the baseline of 20. The ADCS-ADL declined slightly, from a mean of around 70 to around 65.
Finally, the investigators used yet another historical cohort as a comparator in a statistical analysis of projected cognitive and functional benefit. Compared with a pooled, placebo-arm, cohort study conducted by the Alzheimer Disease Cooperative Study Group over 12 months, ANAVEX 2-73 would have been associated with 1.8-point bump in score on the MMSE (P less than .016) and a 4-point benefit on the ADCS-ADL (P less than .019).
“The MMSE declined 45% less and the ADCS-ADL declined 56% less than what we would have expected from the historical control data,” Dr. Macfarlane said. “This is not only statistically significant, but clearly clinically meaningful for patients.”
Nearly all patients (98%) had some sort of adverse event, but most of them were mild transitory dizziness or headache; 76% of the events were grade 1, and 2% were grade 2. There were no serious adverse events. Three subjects dropped out because of adverse events (delirium, dizziness, and a combination of confusion, disorientation, and lethargy). There were no problematic interactions between the study drug and any standard of care AD medications.
Dr. Macfarlane has no financial interest in ANAVEX 2-73. He reported consultancies with Eli Lilly, Janssen-Cilag, and Lundbeck.
[email protected]
On Twitter @alz_gal
SAN DIEGO – A novel Alzheimer’s disease drug candidate appeared to stabilize cognition and function over 57 weeks in a small, early-phase, open-label trial.
Patients with mild to moderate Alzheimer’s who took ANAVEX 2-73, an agonist of the sigma-1 receptor, experienced virtually no decline on either the Mini-Mental State Examination (MMSE) or the Alzheimer’s Disease Cooperative Study–activities of daily living (ADCS-ADL) functional scale. These findings correlated with significant improvements in the P300 evoked potential test – an electrophysiologic measure sometimes used to approximate synaptic connectivity and cortical processing speed.
The findings must be interpreted cautiously. The phase IIa study was designed to assess safety and tolerability; cognitive and functional endpoints were secondary. It comprised only 32 patients at baseline, 25 of whom completed both the 5-week, randomized, dose-finding, crossover trial and the 52-week, open-label, extension study. There was no placebo comparator. Instead, the study used three different sets of historical control data taken from other Alzheimer’s studies.
Nevertheless, the positive results are enough to propel ANAVEX 2-73 forward. The company will continue to treat and follow the extension study cohort, and plans to launch a placebo-controlled study in 2017, said Dr. Macfarlane, head of clinical governance for The Dementia Centre in Melbourne.
The 5-week, randomized, dose-finding, crossover trial started one group of patients on 30 or 50 mg/day oral ANAVEX 2-73 for 11 days after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 11 days of 3 mg/day or 5 mg/day intravenously. A second group first received 11 days of 3 mg/day or 5 mg/day ANAVEX 2-73 intravenously after an initial 2-day, single-dose, pharmacokinetic analysis, followed by an 11-day washout period, and then 30 or 50 mg/day oral ANAVEX 2-73 for 11 days. This was followed by a 52-week, open-label, extension trial of 10-50 mg/day orally, titrating each patient to the maximum tolerated dose. The extension phase was originally planned to last 6 months, but patients and caregivers wanted to continue on the medication, so the company extended it to 12 months. It is ongoing.
The sigma-1 receptor targeted by ANAVEX 2-73 is found on neurons and glia in many areas of the central nervous system. It modulates a number of processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke.
The sigma-1 receptor also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
In preclinical testing, ANAVEX 2-73 showed an additional cognitive property, seeming to display a cognition-enhancing effect in both wild-type and AD model mice.
The mean age of the patients in the extension study was 71 years. The median MMSE score was 20.5. Most patients (78%) were taking a stable dose of acetylcholinesterase inhibitor. During the extension phase, they were titrated to the maximum tolerated dose; 14 mg was the minimum dose necessary to achieve a therapeutic effect and keep the MMSE stable, but Dr. Macfarlane didn’t discuss detailed dosing.
The primary endpoints were safety, tolerability, and pharmacokinetics. The exploratory measures included the P300 electroencephalogram, MMSE score, the Computerized Cogstate Alzheimer’s Battery, and the ADCS-ADL. The Hamilton Depression (HAM-D) Scale was also employed as a neuropsychiatric symptom measure.
The cohort had low baseline depression scores, with a mean score of 2 on the HAM-D. By study’s end, that had decreased to a mean of 1 point. The biggest change was seen in insomnia; all patients who endorsed it at baseline reported it gone by 12 weeks into treatment.
Patients also reported improvements in their ability to work or do other activities, in anxiety, agitation, hypochondriasis, and insight.
The P300 wave amplitude showed a small initial bump from about 6 to 7 microvolts by 4 weeks, and then a dip back down to about 6 microvolts until about week 32. Thereafter it steadily improved, landing at around 8 microvolts by 57 weeks – a level usually seen in healthy age-matched controls. There was a significant separation from the P300 decline seen in a matched historical Alzheimer’s cohort, which dropped to about 4 microvolts over a 52-week period while patients were taking donepezil.
The study employed a second historical control group in another cognitive assessment using the Computerized Cogstate Alzheimer’s Battery. All subjects in the large Australian prospective cohort study, called AIBL (Australian Imaging, Biomarkers & Lifestyle Flagship Study of Ageing), were taking standard of care Alzheimer’s drugs. Compared with that cohort, the ANAVEX 2-73 group experienced benefits in processing speed, attention, and working memory, which became statistically significant at week 31 and continued to grow.
At 57 weeks, the mean MMSE score was stable, hovering around the baseline of 20. The ADCS-ADL declined slightly, from a mean of around 70 to around 65.
Finally, the investigators used yet another historical cohort as a comparator in a statistical analysis of projected cognitive and functional benefit. Compared with a pooled, placebo-arm, cohort study conducted by the Alzheimer Disease Cooperative Study Group over 12 months, ANAVEX 2-73 would have been associated with 1.8-point bump in score on the MMSE (P less than .016) and a 4-point benefit on the ADCS-ADL (P less than .019).
“The MMSE declined 45% less and the ADCS-ADL declined 56% less than what we would have expected from the historical control data,” Dr. Macfarlane said. “This is not only statistically significant, but clearly clinically meaningful for patients.”
Nearly all patients (98%) had some sort of adverse event, but most of them were mild transitory dizziness or headache; 76% of the events were grade 1, and 2% were grade 2. There were no serious adverse events. Three subjects dropped out because of adverse events (delirium, dizziness, and a combination of confusion, disorientation, and lethargy). There were no problematic interactions between the study drug and any standard of care AD medications.
Dr. Macfarlane has no financial interest in ANAVEX 2-73. He reported consultancies with Eli Lilly, Janssen-Cilag, and Lundbeck.
[email protected]
On Twitter @alz_gal
AT CTAD
Key clinical point:
Major finding: At 57 weeks, the mean MMSE score stayed around the baseline of 20. The ADCS-ADL declined slightly, from about 70 to 65.
Data source: A phase IIa study comprising 32 patients, 25 of whom completed 57 weeks of treatment.
Disclosures: Dr. Macfarlane has no financial ties with Anavex Life Sciences, which is developing the drug. He reported consultancies with Eli Lilly, Janssen-Cilag, and Lundbeck.
Macrolide monotherapy works in some NTM lung disease
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
“In this study by Koh et al., it is gratifying that most patients had a favorable microbiologic outcome. It is also somewhat surprising that only two patients developed acquired macrolide resistant M. abscessus subsp massiliense isolates. While the absolute number is low, for those two individuals, the consequences of developing macrolide resistance are far from trivial. They have transitioned from having a mycobacterial infection that is relatively easy to treat effectively to a mycobacterial infection that is not,” David E. Griffith, MD, FCCP, and Timothy R. Aksamit, MD, FCCP, wrote in an editorial published in the December issue of CHEST (Chest. 2016 Dec;150[6];1177-8).
The authors noted that they “enthusiastically applaud and acknowledge the prolific and consistently excellent work done by the group in South Korea, but we cannot endorse the widespread adoption of macrolide monotherapy for” this patient group. “In our view, the risk/benefit balance of this approach does not favor macrolide monotherapy even though the majority of patients in this study were adequately treated.”
Dr. Griffith is professor of medicine at University of Texas Health Science Center, Tyler, and Dr. Aksamit is a consultant on pulmonary disease and critical care medicine at the Mayo Clinic, Rochester, Minn. They disclosed no conflicts of interest.
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
Patients with cystic fibrosis or bronchiectasis and one form of Mycobacterium abscessus disease can be successfully treated with long-term oral macrolide monotherapy following short-term intravenous combination antibiotic therapy, a Korean research team has shown.
The M. abscessus complex is implicated in between a fifth and half of all cases of lung disease caused by nontuberculous mycobacteria (NTM). Though treatment is notoriously difficult and prolonged in all NTM lung disease, one subspecies of M. abscessus – M. massiliense – lacks the active gene needed for developing resistance to macrolide-based antibiotics, making it potentially more readily treated.
In research published in CHEST, Won-Jung Koh, MD, of Samsung Medical Center and Sungkyunkwan University in Seoul, South Korea, and colleagues, sought to determine the optimal treatment protocol for patients with massiliense disease (Chest. 2016 Dec;150[6]:1211-21). They identified 71 patients with massiliense disease who had initiated antibiotic treatment between January 2007 and December 2012. These patients were part of an ongoing prospective cohort study on NTM lung disease. The first 28 patients in the study were hospitalized for 4 weeks and treated with intravenous amikacin and cefoxitin along with oral clarithromycin and a fluoroquinolone. Following discharge these patients remained on the oral agents for 24 months.
Two years into the study, the protocol changed, and the next 43 patients were treated with a 2-week course of intravenous amikacin and cefoxitin along with the oral agents. In some patients, azithromycin, which came into use in Korea for NTM lung disease in 2011, replaced a fluoroquinolone. After discharge, all patients stayed on the oral macrolides (with seven also taking a fluoroquinolone) until their sputum cultures were negative for 12 months.
For the patients treated for 4 weeks, the response rates after 12 months of treatment were 89% for symptoms, 79% for computed tomography, and 100% for negative sputum cultures. In the patients treated for 2 weeks, they were 100%, 91%, and 91%, respectively. None of these differences between the two groups were statistically significant. Median total treatment duration, however, was significantly shorter – by nearly a year – in the 2-week plus macrolide monotherapy group than in the other group of patients (15.2 months vs. 23.9 months, P less than .001).
Acquired macrolide resistance developed in two patients in the group who received a 2-week course of intravenous amikacin and cefoxitin along with the oral agents, including one case of high-level clarithromycin resistance. Genotyping revealed reinfection with different strains of M. massiliense.
“[Oral] macrolide therapy after an initial 2-week course of combination antibiotics, rather than long-term parenteral antibiotics, might be effective in most patients with M. massiliense lung disease,” Dr. Koh and colleagues wrote, noting that their study’s nonrandomized single-site design was a limitation, and that multicenter randomized trials would be needed “to assess the efficacy” of the findings.
The Korean government funded Dr. Koh and colleagues’ study. None of the authors disclosed conflicts of interest.
FROM CHEST
Key clinical point: A short course of intravenous antibiotics followed by oral macrolides may be effective at treating lung disease caused by the massiliense subspecies of M. abscessus.
Major finding: Of 43 patients receiving 2 weeks of combination antibiotics followed by a year of oral macrolides, 39 (91%) converted to negative sputum cultures before 12 months.
Data source: A prospective cohort study enrolling 71 patients at a single treatment center in Korea.
Disclosures: The Korean government sponsored the study and investigators disclosed no conflicts of interest.
XR version of Synjardy gets FDA’s nod
for use in the management of blood sugar in adults with type 2 diabetes. The drug, to be marketed as Synjardy XR by Boehringer Ingelheim and Eli Lilly, is intended for use with a diet and exercise program.
Its standard release formulation was approved in August 2015.
FDA approved the extended-release version on Dec. 12. The updated label includes a boxed warning regarding the risk of metformin-associated lactic acidosis
Empagliflozin recently received an FDA indication as a medication for use in reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease, the first drug for type 2 diabetes to do so.
The label includes information of the drug’s four doses, all of which contain 1,000 mg of metformin plus empagliflozin in strengths of 5 mg, 10 mg, 12.5 mg, and 25 mg.
Among patients who are already taking metformin and plan to begin a regimen of Synjardy XR, their best choice is to switch to the formulation containing a similar daily dose of metformin and a total daily dose of 10 mg empagliflozin. Those already on empagliflozin should switch to Synjardy XR containing the same daily dose of empagliflozin and a total daily dose of 1,000 mg metformin. Dosing may be adjusted upward on the basis of effectiveness and tolerability up to a maximum of 25 mg empagliflozin/2,000 mg metformin, taken once daily with a meal in the morning.
Synjardy XR is contraindicated in patients with an estimated glomerular filtration rate of less than 45 mL/min per 1.73 m2. Renal function should be assessed in patients with impaired renal function before taking Synjardy XR.
Several clinical trials tested coadministration of metformin and empagliflozin. In the largest, 637 patients with type 2 diabetes who were inadequately controlled (hemoglobin A1c of 7%-10%) with 1,500 mg metformin were randomized to receive placebo, 10 mg empagliflozin, or 25 mg empagliflozin for 24 weeks. Both the 10-mg and 25-mg dosages of empagliflozin resulted in significant reductions compared with placebo in HbA1c (–0.7% and –0.8%, respectively) fasting glucose (–26 mg/dL and –29 mg/dL), and body weight (–2 kg and –2.5 kg).
The single-pill formulation of Synjardy XR was not tested in clinical trials for efficacy; however, several trials performed in healthy subjects showed bioequivalence of the fixed dose combination, compared with separate pills.
[email protected]
for use in the management of blood sugar in adults with type 2 diabetes. The drug, to be marketed as Synjardy XR by Boehringer Ingelheim and Eli Lilly, is intended for use with a diet and exercise program.
Its standard release formulation was approved in August 2015.
FDA approved the extended-release version on Dec. 12. The updated label includes a boxed warning regarding the risk of metformin-associated lactic acidosis
Empagliflozin recently received an FDA indication as a medication for use in reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease, the first drug for type 2 diabetes to do so.
The label includes information of the drug’s four doses, all of which contain 1,000 mg of metformin plus empagliflozin in strengths of 5 mg, 10 mg, 12.5 mg, and 25 mg.
Among patients who are already taking metformin and plan to begin a regimen of Synjardy XR, their best choice is to switch to the formulation containing a similar daily dose of metformin and a total daily dose of 10 mg empagliflozin. Those already on empagliflozin should switch to Synjardy XR containing the same daily dose of empagliflozin and a total daily dose of 1,000 mg metformin. Dosing may be adjusted upward on the basis of effectiveness and tolerability up to a maximum of 25 mg empagliflozin/2,000 mg metformin, taken once daily with a meal in the morning.
Synjardy XR is contraindicated in patients with an estimated glomerular filtration rate of less than 45 mL/min per 1.73 m2. Renal function should be assessed in patients with impaired renal function before taking Synjardy XR.
Several clinical trials tested coadministration of metformin and empagliflozin. In the largest, 637 patients with type 2 diabetes who were inadequately controlled (hemoglobin A1c of 7%-10%) with 1,500 mg metformin were randomized to receive placebo, 10 mg empagliflozin, or 25 mg empagliflozin for 24 weeks. Both the 10-mg and 25-mg dosages of empagliflozin resulted in significant reductions compared with placebo in HbA1c (–0.7% and –0.8%, respectively) fasting glucose (–26 mg/dL and –29 mg/dL), and body weight (–2 kg and –2.5 kg).
The single-pill formulation of Synjardy XR was not tested in clinical trials for efficacy; however, several trials performed in healthy subjects showed bioequivalence of the fixed dose combination, compared with separate pills.
[email protected]
for use in the management of blood sugar in adults with type 2 diabetes. The drug, to be marketed as Synjardy XR by Boehringer Ingelheim and Eli Lilly, is intended for use with a diet and exercise program.
Its standard release formulation was approved in August 2015.
FDA approved the extended-release version on Dec. 12. The updated label includes a boxed warning regarding the risk of metformin-associated lactic acidosis
Empagliflozin recently received an FDA indication as a medication for use in reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease, the first drug for type 2 diabetes to do so.
The label includes information of the drug’s four doses, all of which contain 1,000 mg of metformin plus empagliflozin in strengths of 5 mg, 10 mg, 12.5 mg, and 25 mg.
Among patients who are already taking metformin and plan to begin a regimen of Synjardy XR, their best choice is to switch to the formulation containing a similar daily dose of metformin and a total daily dose of 10 mg empagliflozin. Those already on empagliflozin should switch to Synjardy XR containing the same daily dose of empagliflozin and a total daily dose of 1,000 mg metformin. Dosing may be adjusted upward on the basis of effectiveness and tolerability up to a maximum of 25 mg empagliflozin/2,000 mg metformin, taken once daily with a meal in the morning.
Synjardy XR is contraindicated in patients with an estimated glomerular filtration rate of less than 45 mL/min per 1.73 m2. Renal function should be assessed in patients with impaired renal function before taking Synjardy XR.
Several clinical trials tested coadministration of metformin and empagliflozin. In the largest, 637 patients with type 2 diabetes who were inadequately controlled (hemoglobin A1c of 7%-10%) with 1,500 mg metformin were randomized to receive placebo, 10 mg empagliflozin, or 25 mg empagliflozin for 24 weeks. Both the 10-mg and 25-mg dosages of empagliflozin resulted in significant reductions compared with placebo in HbA1c (–0.7% and –0.8%, respectively) fasting glucose (–26 mg/dL and –29 mg/dL), and body weight (–2 kg and –2.5 kg).
The single-pill formulation of Synjardy XR was not tested in clinical trials for efficacy; however, several trials performed in healthy subjects showed bioequivalence of the fixed dose combination, compared with separate pills.
[email protected]
Cooling device reduces breast cancer–related alopecia during chemotherapy
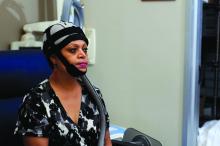
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
AT SABCS 2016
Key clinical point:
Major finding: 50.5% of treated patients experienced no more than grade 1 alopecia (less than 50% hair loss), compared with 0% of control subjects.
Data source: The prospective, randomized SCALP trial involving 182 women.
Disclosures: Dr. Nangia reported receiving research funding from Paxman, sponsor of the study, to her institution.
FDA affirms bladder cancer warning with diabetes drug
Though a 10-year epidemiologic study did not find an increased risk of bladder cancer with pioglitazone use, the Food and Drug Administration has chosen to affirm the warning on the label of the type 2 diabetes drug following an updated review of several studies.
The FDA issued a warning about the possible risk of bladder cancer based on interim results from the 10-year epidemiologic study in 2010, and it changed the labels of pioglitazone-containing medicines in 2011 to include warnings about this risk.
There was a modest trend toward higher risk with increasing duration of use, but the trend was not statistically significant. Compared with the interim 5-year results, these final 10-year results found weaker associations that were not statistically significant.
The directions of the associations, however, remained unchanged. Based on these findings and other reviewed studies with conflicting results, the FDA has concluded that use of pioglitazone may be linked to an increased risk of bladder cancer.
The labels of pioglitazone-containing medicines already contain warnings about this risk, but the FDA has now approved label updates to describe the additional studies reviewed.
Read the FDA update and data summary here.
[email protected]
On Twitter @nikolaideslaura
Though a 10-year epidemiologic study did not find an increased risk of bladder cancer with pioglitazone use, the Food and Drug Administration has chosen to affirm the warning on the label of the type 2 diabetes drug following an updated review of several studies.
The FDA issued a warning about the possible risk of bladder cancer based on interim results from the 10-year epidemiologic study in 2010, and it changed the labels of pioglitazone-containing medicines in 2011 to include warnings about this risk.
There was a modest trend toward higher risk with increasing duration of use, but the trend was not statistically significant. Compared with the interim 5-year results, these final 10-year results found weaker associations that were not statistically significant.
The directions of the associations, however, remained unchanged. Based on these findings and other reviewed studies with conflicting results, the FDA has concluded that use of pioglitazone may be linked to an increased risk of bladder cancer.
The labels of pioglitazone-containing medicines already contain warnings about this risk, but the FDA has now approved label updates to describe the additional studies reviewed.
Read the FDA update and data summary here.
[email protected]
On Twitter @nikolaideslaura
Though a 10-year epidemiologic study did not find an increased risk of bladder cancer with pioglitazone use, the Food and Drug Administration has chosen to affirm the warning on the label of the type 2 diabetes drug following an updated review of several studies.
The FDA issued a warning about the possible risk of bladder cancer based on interim results from the 10-year epidemiologic study in 2010, and it changed the labels of pioglitazone-containing medicines in 2011 to include warnings about this risk.
There was a modest trend toward higher risk with increasing duration of use, but the trend was not statistically significant. Compared with the interim 5-year results, these final 10-year results found weaker associations that were not statistically significant.
The directions of the associations, however, remained unchanged. Based on these findings and other reviewed studies with conflicting results, the FDA has concluded that use of pioglitazone may be linked to an increased risk of bladder cancer.
The labels of pioglitazone-containing medicines already contain warnings about this risk, but the FDA has now approved label updates to describe the additional studies reviewed.
Read the FDA update and data summary here.
[email protected]
On Twitter @nikolaideslaura
HIV research update: Late November 2016
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
There has been a significant increase over time in the proportion of deliveries to women living with HIV aged 40 years and older, which has implications for pregnancy management, according to a study in HIV Medicine.
HIV-positive older adults have higher cystatin C levels than do HIV-negative older adults, according to a recent study, and cystatin C may be associated with neurocognitive impairment in this population, particularly if they use tenofovir.
A low Infant and Child Feeding Index, dietary diversity, and food consistency scores were likely associated with increased risk of diarrhea and acute respiratory infection among HIV-exposed infants in Tanzania, according to a study in JAIDS.
A Pediatric HIV/AIDS Cohort Study of HIV-exposed, uninfected U.S. children found growth was above average, although maternal tenofovir use was not associated with lower length or head circumference at 2 years of age, as hypothesized.
Cerebrovascular endothelial dysfunction associated with HIV infection may be most relevant for individuals with less traditional vascular risk, such as those with lower cholesterol, according to a study published in JAIDS.
Antiretroviral therapy during pregnancy was not associated with preterm birth, a recent study found, and it may in fact be protective against severe adverse outcomes accompanying extremely to very preterm birth.
A large majority of HIV-negative gay and bisexual men were appropriate candidates for PrEP, yet less than 1 in 10 were using and adherent to PrEP, according to a study in JAIDS. The findings highlight the need for interventions tailored to address the unique barriers men face at each stage of the care cascade.
Men who have sex with men remain a high risk group for HIV infection in a low prevalence setting, according to a study in Istanbul, and thus represent a key target population for diagnostic and therapeutic interventions.
A Kenya-based study found there is an urgent need to implement HIV prevention and treatment interventions targeting young people and patients entering care with severe immunosuppression (CD4 cell counts less than 100 cells/mcL), to optimize the impact of HIV prevention, care, and treatment in resource scarce settings.
A recent Nigerian study found evidence of a rising tide of HIV infection in TB patients, in particular among single middle-aged women with low education.
Negative emotional states with significant severity were common among people living with HIV, particularly depression and anxiety, according to a study in HIV & AIDS Review.
Drug-drug interactions between direct-acting antivirals and antiretroviral therapy are frequently found among HIV/HCV-coinfected patients, according to a study in HIV Medicine.
Key pretreatment viral resistant-associated variants (RAVs) for hepatitis C protease inhibitors are present in a major portion of the HCV/HIV coinfected population prior to therapy, a recent study found.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
There has been a significant increase over time in the proportion of deliveries to women living with HIV aged 40 years and older, which has implications for pregnancy management, according to a study in HIV Medicine.
HIV-positive older adults have higher cystatin C levels than do HIV-negative older adults, according to a recent study, and cystatin C may be associated with neurocognitive impairment in this population, particularly if they use tenofovir.
A low Infant and Child Feeding Index, dietary diversity, and food consistency scores were likely associated with increased risk of diarrhea and acute respiratory infection among HIV-exposed infants in Tanzania, according to a study in JAIDS.
A Pediatric HIV/AIDS Cohort Study of HIV-exposed, uninfected U.S. children found growth was above average, although maternal tenofovir use was not associated with lower length or head circumference at 2 years of age, as hypothesized.
Cerebrovascular endothelial dysfunction associated with HIV infection may be most relevant for individuals with less traditional vascular risk, such as those with lower cholesterol, according to a study published in JAIDS.
Antiretroviral therapy during pregnancy was not associated with preterm birth, a recent study found, and it may in fact be protective against severe adverse outcomes accompanying extremely to very preterm birth.
A large majority of HIV-negative gay and bisexual men were appropriate candidates for PrEP, yet less than 1 in 10 were using and adherent to PrEP, according to a study in JAIDS. The findings highlight the need for interventions tailored to address the unique barriers men face at each stage of the care cascade.
Men who have sex with men remain a high risk group for HIV infection in a low prevalence setting, according to a study in Istanbul, and thus represent a key target population for diagnostic and therapeutic interventions.
A Kenya-based study found there is an urgent need to implement HIV prevention and treatment interventions targeting young people and patients entering care with severe immunosuppression (CD4 cell counts less than 100 cells/mcL), to optimize the impact of HIV prevention, care, and treatment in resource scarce settings.
A recent Nigerian study found evidence of a rising tide of HIV infection in TB patients, in particular among single middle-aged women with low education.
Negative emotional states with significant severity were common among people living with HIV, particularly depression and anxiety, according to a study in HIV & AIDS Review.
Drug-drug interactions between direct-acting antivirals and antiretroviral therapy are frequently found among HIV/HCV-coinfected patients, according to a study in HIV Medicine.
Key pretreatment viral resistant-associated variants (RAVs) for hepatitis C protease inhibitors are present in a major portion of the HCV/HIV coinfected population prior to therapy, a recent study found.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
There has been a significant increase over time in the proportion of deliveries to women living with HIV aged 40 years and older, which has implications for pregnancy management, according to a study in HIV Medicine.
HIV-positive older adults have higher cystatin C levels than do HIV-negative older adults, according to a recent study, and cystatin C may be associated with neurocognitive impairment in this population, particularly if they use tenofovir.
A low Infant and Child Feeding Index, dietary diversity, and food consistency scores were likely associated with increased risk of diarrhea and acute respiratory infection among HIV-exposed infants in Tanzania, according to a study in JAIDS.
A Pediatric HIV/AIDS Cohort Study of HIV-exposed, uninfected U.S. children found growth was above average, although maternal tenofovir use was not associated with lower length or head circumference at 2 years of age, as hypothesized.
Cerebrovascular endothelial dysfunction associated with HIV infection may be most relevant for individuals with less traditional vascular risk, such as those with lower cholesterol, according to a study published in JAIDS.
Antiretroviral therapy during pregnancy was not associated with preterm birth, a recent study found, and it may in fact be protective against severe adverse outcomes accompanying extremely to very preterm birth.
A large majority of HIV-negative gay and bisexual men were appropriate candidates for PrEP, yet less than 1 in 10 were using and adherent to PrEP, according to a study in JAIDS. The findings highlight the need for interventions tailored to address the unique barriers men face at each stage of the care cascade.
Men who have sex with men remain a high risk group for HIV infection in a low prevalence setting, according to a study in Istanbul, and thus represent a key target population for diagnostic and therapeutic interventions.
A Kenya-based study found there is an urgent need to implement HIV prevention and treatment interventions targeting young people and patients entering care with severe immunosuppression (CD4 cell counts less than 100 cells/mcL), to optimize the impact of HIV prevention, care, and treatment in resource scarce settings.
A recent Nigerian study found evidence of a rising tide of HIV infection in TB patients, in particular among single middle-aged women with low education.
Negative emotional states with significant severity were common among people living with HIV, particularly depression and anxiety, according to a study in HIV & AIDS Review.
Drug-drug interactions between direct-acting antivirals and antiretroviral therapy are frequently found among HIV/HCV-coinfected patients, according to a study in HIV Medicine.
Key pretreatment viral resistant-associated variants (RAVs) for hepatitis C protease inhibitors are present in a major portion of the HCV/HIV coinfected population prior to therapy, a recent study found.
[email protected]
On Twitter @richpizzi
Good response from CAR T cells with ‘safety switch’ for advanced ALL
SAN DIEGO – Anti-CD19 chimeric antigen receptor (CAR) T cells engineered with a “safety switch” yielded high rates of complete response and an acceptable toxicity profile in chemotherapy-resistant B cell acute lymphoblastic leukemia, according to a multicenter phase I/II trial.
Importantly, high tumor burden did not increase the risk of cytokine release syndrome, said Lung-Ji Chang, PhD, of Shenzhen (China) Genoimmune Medical Institute and the University of Florida in Gainesville. “This reliable, standardized CAR T-cell preparation protocol has now served more than 30 major medical centers in China,” he said at the annual meeting of the American Society of Hematology.
Anti-CD19 CAR T cells have shown dramatic potential for treating B-cell malignancies, but toxicities have been a concern. One potentially serious adverse reaction is cytokine release syndrome, in which patients develop marked rises in blood levels of several types of cytokines. Another problem is that anti-CD19 CAR T cells can trigger loss of CD19 B cells, ultimately leading to humoral deficiencies, Dr. Chang noted. Consequently, researchers have searched for ways to continue controlling the activity of CAR T cells even after infusing them into patients.
As part of that effort, Dr. Chang and his associates developed a standardized protocol for engineering next-generation anti-CD19 CAR T cells based on the established concept of a “safety switch.” After collecting T cells from patients with chemotherapy-resistant ALL, they used a lentiviral vector to transform them into CAR T cells with fusion proteins consisting of a proapoptotic molecule called caspase-9 that is linked to modified human FK506-binding proteins, or FKBP. The addition of iCaspase9-FKBP enables clinicians to induce CAR T cell apoptosis by treating patients with a synthetic dimerizer called AP1903.
Apoptosis occurs about 45 minutes after this drug is given, according to Dr. Chang. This “safety switch” also enables clinicians to eliminate anti-CD19 CAR T cells after tumor cells are eradicated so that patients can recover their humoral immunity. He and his associates further modified these anti-CD19 CAR T cells by introducing four intracellular signaling domains that are associated with T-cell activation, survival, and longevity, he said.
A total of 22 treatment centers helped test this approach in a phase I/II trial of 110 leukemia patients, about half of whom were children with a median age of 9 years. The median age of adults was 37 years, and the oldest patient was 70. Cancer types included Philadelphia chromosome–positive ALL, Philadelphia chromosome–negative ALL, and chronic myeloid leukemia with blast crisis. About a third of patients had bone marrow samples with at least 50% blasts, and a similar proportion had already undergone hematopoietic stem cell transplantation.
Cytokine release syndrome affected 86% of patients with low or no tumor burden, but only 53% of patients with bone marrow blasts exceeding 5%, Dr. Chang reported. He emphasized that patients with high tumor burden were no more likely to develop moderate or severe cytokine release syndrome than were patients with little or no tumor burden (P = .3). Furthermore, among 17 patients with more than 80% bone marrow involvement, only three developed grade 3-4 cytokine release syndrome, while eight developed grade 1 cytokine release syndrome.
A total of 96 patients (87%) had a complete response to this CAR T cell regimen, including 51 children and 45 adults, Dr. Chang reported. Median overall survival was 222 days (range, 23-1,041 days), and 60% of patients lived at least 400 days after treatment. Patients survived a median of 115 days without relapsing (range, 0-455 days), and 55% ultimately relapsed. Age did not appear to predict relapse, he noted.
Kaplan-Meier curves revealed no major differences in rates of overall survival (OS) between adults and children at 400-day data cutoff, Dr. Chang said. However, patients with more than 50% blast cells in their bone marrow had significantly lower rates of survival (P = .02) than did patients with less advanced ALL. A lower T-cell dose predicted lower survival in children (P = .04), but not in adults. Dr. Chang and his colleagues now dose patients of all ages with 106 cells per kilogram, he said.
Survival was significantly more likely when CAR T cell recipients went on to allogeneic hematopoietic stem cell transplantation (P = .0002) than otherwise. Based on the findings, Dr. Chang particularly recommends this approach for highly chemotherapy-resistant disease with a high tumor burden. Among patients who relapsed, repeating CAR T cell therapy led to better survival than administering combination chemotherapy-tyrosine kinase inhibitor therapy (P = .01).
These safety and efficacy results suggest that CAR T cell immunotherapy can benefit patients if they have very high-burden leukemia, Dr. Chang concluded. Patients outcomes remained consistent across centers due to a “highly standardized CAR T cell preparation profile,” he said.
Dr. Chang did not report funding sources. He reported having no relevant conflicts of interest.
SAN DIEGO – Anti-CD19 chimeric antigen receptor (CAR) T cells engineered with a “safety switch” yielded high rates of complete response and an acceptable toxicity profile in chemotherapy-resistant B cell acute lymphoblastic leukemia, according to a multicenter phase I/II trial.
Importantly, high tumor burden did not increase the risk of cytokine release syndrome, said Lung-Ji Chang, PhD, of Shenzhen (China) Genoimmune Medical Institute and the University of Florida in Gainesville. “This reliable, standardized CAR T-cell preparation protocol has now served more than 30 major medical centers in China,” he said at the annual meeting of the American Society of Hematology.
Anti-CD19 CAR T cells have shown dramatic potential for treating B-cell malignancies, but toxicities have been a concern. One potentially serious adverse reaction is cytokine release syndrome, in which patients develop marked rises in blood levels of several types of cytokines. Another problem is that anti-CD19 CAR T cells can trigger loss of CD19 B cells, ultimately leading to humoral deficiencies, Dr. Chang noted. Consequently, researchers have searched for ways to continue controlling the activity of CAR T cells even after infusing them into patients.
As part of that effort, Dr. Chang and his associates developed a standardized protocol for engineering next-generation anti-CD19 CAR T cells based on the established concept of a “safety switch.” After collecting T cells from patients with chemotherapy-resistant ALL, they used a lentiviral vector to transform them into CAR T cells with fusion proteins consisting of a proapoptotic molecule called caspase-9 that is linked to modified human FK506-binding proteins, or FKBP. The addition of iCaspase9-FKBP enables clinicians to induce CAR T cell apoptosis by treating patients with a synthetic dimerizer called AP1903.
Apoptosis occurs about 45 minutes after this drug is given, according to Dr. Chang. This “safety switch” also enables clinicians to eliminate anti-CD19 CAR T cells after tumor cells are eradicated so that patients can recover their humoral immunity. He and his associates further modified these anti-CD19 CAR T cells by introducing four intracellular signaling domains that are associated with T-cell activation, survival, and longevity, he said.
A total of 22 treatment centers helped test this approach in a phase I/II trial of 110 leukemia patients, about half of whom were children with a median age of 9 years. The median age of adults was 37 years, and the oldest patient was 70. Cancer types included Philadelphia chromosome–positive ALL, Philadelphia chromosome–negative ALL, and chronic myeloid leukemia with blast crisis. About a third of patients had bone marrow samples with at least 50% blasts, and a similar proportion had already undergone hematopoietic stem cell transplantation.
Cytokine release syndrome affected 86% of patients with low or no tumor burden, but only 53% of patients with bone marrow blasts exceeding 5%, Dr. Chang reported. He emphasized that patients with high tumor burden were no more likely to develop moderate or severe cytokine release syndrome than were patients with little or no tumor burden (P = .3). Furthermore, among 17 patients with more than 80% bone marrow involvement, only three developed grade 3-4 cytokine release syndrome, while eight developed grade 1 cytokine release syndrome.
A total of 96 patients (87%) had a complete response to this CAR T cell regimen, including 51 children and 45 adults, Dr. Chang reported. Median overall survival was 222 days (range, 23-1,041 days), and 60% of patients lived at least 400 days after treatment. Patients survived a median of 115 days without relapsing (range, 0-455 days), and 55% ultimately relapsed. Age did not appear to predict relapse, he noted.
Kaplan-Meier curves revealed no major differences in rates of overall survival (OS) between adults and children at 400-day data cutoff, Dr. Chang said. However, patients with more than 50% blast cells in their bone marrow had significantly lower rates of survival (P = .02) than did patients with less advanced ALL. A lower T-cell dose predicted lower survival in children (P = .04), but not in adults. Dr. Chang and his colleagues now dose patients of all ages with 106 cells per kilogram, he said.
Survival was significantly more likely when CAR T cell recipients went on to allogeneic hematopoietic stem cell transplantation (P = .0002) than otherwise. Based on the findings, Dr. Chang particularly recommends this approach for highly chemotherapy-resistant disease with a high tumor burden. Among patients who relapsed, repeating CAR T cell therapy led to better survival than administering combination chemotherapy-tyrosine kinase inhibitor therapy (P = .01).
These safety and efficacy results suggest that CAR T cell immunotherapy can benefit patients if they have very high-burden leukemia, Dr. Chang concluded. Patients outcomes remained consistent across centers due to a “highly standardized CAR T cell preparation profile,” he said.
Dr. Chang did not report funding sources. He reported having no relevant conflicts of interest.
SAN DIEGO – Anti-CD19 chimeric antigen receptor (CAR) T cells engineered with a “safety switch” yielded high rates of complete response and an acceptable toxicity profile in chemotherapy-resistant B cell acute lymphoblastic leukemia, according to a multicenter phase I/II trial.
Importantly, high tumor burden did not increase the risk of cytokine release syndrome, said Lung-Ji Chang, PhD, of Shenzhen (China) Genoimmune Medical Institute and the University of Florida in Gainesville. “This reliable, standardized CAR T-cell preparation protocol has now served more than 30 major medical centers in China,” he said at the annual meeting of the American Society of Hematology.
Anti-CD19 CAR T cells have shown dramatic potential for treating B-cell malignancies, but toxicities have been a concern. One potentially serious adverse reaction is cytokine release syndrome, in which patients develop marked rises in blood levels of several types of cytokines. Another problem is that anti-CD19 CAR T cells can trigger loss of CD19 B cells, ultimately leading to humoral deficiencies, Dr. Chang noted. Consequently, researchers have searched for ways to continue controlling the activity of CAR T cells even after infusing them into patients.
As part of that effort, Dr. Chang and his associates developed a standardized protocol for engineering next-generation anti-CD19 CAR T cells based on the established concept of a “safety switch.” After collecting T cells from patients with chemotherapy-resistant ALL, they used a lentiviral vector to transform them into CAR T cells with fusion proteins consisting of a proapoptotic molecule called caspase-9 that is linked to modified human FK506-binding proteins, or FKBP. The addition of iCaspase9-FKBP enables clinicians to induce CAR T cell apoptosis by treating patients with a synthetic dimerizer called AP1903.
Apoptosis occurs about 45 minutes after this drug is given, according to Dr. Chang. This “safety switch” also enables clinicians to eliminate anti-CD19 CAR T cells after tumor cells are eradicated so that patients can recover their humoral immunity. He and his associates further modified these anti-CD19 CAR T cells by introducing four intracellular signaling domains that are associated with T-cell activation, survival, and longevity, he said.
A total of 22 treatment centers helped test this approach in a phase I/II trial of 110 leukemia patients, about half of whom were children with a median age of 9 years. The median age of adults was 37 years, and the oldest patient was 70. Cancer types included Philadelphia chromosome–positive ALL, Philadelphia chromosome–negative ALL, and chronic myeloid leukemia with blast crisis. About a third of patients had bone marrow samples with at least 50% blasts, and a similar proportion had already undergone hematopoietic stem cell transplantation.
Cytokine release syndrome affected 86% of patients with low or no tumor burden, but only 53% of patients with bone marrow blasts exceeding 5%, Dr. Chang reported. He emphasized that patients with high tumor burden were no more likely to develop moderate or severe cytokine release syndrome than were patients with little or no tumor burden (P = .3). Furthermore, among 17 patients with more than 80% bone marrow involvement, only three developed grade 3-4 cytokine release syndrome, while eight developed grade 1 cytokine release syndrome.
A total of 96 patients (87%) had a complete response to this CAR T cell regimen, including 51 children and 45 adults, Dr. Chang reported. Median overall survival was 222 days (range, 23-1,041 days), and 60% of patients lived at least 400 days after treatment. Patients survived a median of 115 days without relapsing (range, 0-455 days), and 55% ultimately relapsed. Age did not appear to predict relapse, he noted.
Kaplan-Meier curves revealed no major differences in rates of overall survival (OS) between adults and children at 400-day data cutoff, Dr. Chang said. However, patients with more than 50% blast cells in their bone marrow had significantly lower rates of survival (P = .02) than did patients with less advanced ALL. A lower T-cell dose predicted lower survival in children (P = .04), but not in adults. Dr. Chang and his colleagues now dose patients of all ages with 106 cells per kilogram, he said.
Survival was significantly more likely when CAR T cell recipients went on to allogeneic hematopoietic stem cell transplantation (P = .0002) than otherwise. Based on the findings, Dr. Chang particularly recommends this approach for highly chemotherapy-resistant disease with a high tumor burden. Among patients who relapsed, repeating CAR T cell therapy led to better survival than administering combination chemotherapy-tyrosine kinase inhibitor therapy (P = .01).
These safety and efficacy results suggest that CAR T cell immunotherapy can benefit patients if they have very high-burden leukemia, Dr. Chang concluded. Patients outcomes remained consistent across centers due to a “highly standardized CAR T cell preparation profile,” he said.
Dr. Chang did not report funding sources. He reported having no relevant conflicts of interest.
AT ASH 2016
Key clinical point: Safety-engineered anti-CD19 autologous chimeric antigen receptor (CAR) T cells achieved good efficacy and adequate safety results in a multicenter study of children and adults with acute lymphoblastic leukemia.
Major finding: A total of 96 patients (87%) had a complete response, and median overall survival was 222 days. High tumor burden did not increase the risk of cytokine release syndrome.
Data source: A multicenter phase I/II study of 110 children and adults with ALL.
Disclosures: The researchers had no relevant financial disclosures.
T-Capsulotomy to Improve Visualization of the Peripheral Compartment and Repair
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Toxicity high for SBRT in centrally-located lung tumors
VIENNA – Stereotactic body radiotherapy (SBRT) proved too toxic for many of patients recruited into a multinational phase II trial with centrally-located lung tumors.
The majority of patients experienced some type of adverse effect, with 28% experiencing serious (grade 3-5) adverse effects.
“Our major concern now is that we had six cases of grade 5 bleedings,” Karen Lindberg, MD, said at the World Conference on Lung Cancer. “Tumor location seems to be a risk factor for bleeding,” she added, with five of the six cases seen in patients who had tumors close to a main bronchus (group A). The other case was in patient who had tumors close to a lobular bronchus (group B).
“The classical definition of a centrally-located lung tumor is a tumor residing within or touching an imaginary zone 2 cm around the proximal bronchial tree,” explained Dr. Lindberg of Karolinska University Hospital and the Karolinska Institutet in Stockholm, who presented the first results of the Nordic HILUS Trial.
“When we designed this study we wanted to look at very centrally located tumors, so we tightened up this definition to look at tumors that occurred within 1 cm around the proximal bronchial tree,” she said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
During the trial, SBRT was to be delivered at a dose of 7 Gray (Gy) in 8 fractions at 65%-75% isodose lines to ultra-centrally-located tumors. Dose constraints were stipulated for tumors situated very close to the spinal cord, contralateral main bronchus, and trachea, with some dosing recommendations on reducing the dose delivered to tumors that were ipsilateral to the main bronchus, or very close to the esophagus or heart.
A total of 74 patients with centrally-located, locally progressive tumors, which were less than 5 cm in size and due to non–small-cell lung cancer (NSCLC) or metastatic lung disease from another solid tumor, were recruited. Patients had to have a good performance status and life expectancy of 3 months.
Patients with brain metastases or tumors that reached through the wall of a main bronchus were excluded as were those who were taking any concomitant systemic therapy.
The mean age of the recruited patients was 71 years; 58 (78%) had NSCLC, of which 20 (27%) had adenomas and 19 (26%) had squamous cell cancers. Of those with secondary lung tumors, eight (11%), had primary renal cell carcinoma and four (5%) had colorectal cancer.
After a follow-up of 18.6 months, 31 (42%) patients had died.
The most common adverse events reported were grade 1-2 dyspnea, cough, and fatigue. However, there was a high rate of grade 3 (dyspnea, fatigue, pain, pneumonitis, and heart rhythm disturbance), 4 (pain, lung infection, fever, heart rhythm disturbance, fistula, and pneumothorax) and 5 toxicities (pneumonitis and bleeding).
Bleeding often occurred without warning and had an acute onset and toll.
Seven patients (six in group A and one in group B) may have suffered grade 5 side effects; six patients experienced lethal hemoptysis after a median of 15.5 months (2.5-21 months) and one patient suffered from a lethal pneumonitis 5 months post study treatment. The reintroduction of TKIs may also have played a role, Dr. Lindberg suggested.
SBRT for early stage NSCLC is very effective and “generates outstanding tumor control”, said Feng-Ming Kong, MD, of Indiana University, Indianapolis, who was invited to comment on the findings.
Most studies of SBRT have looked at peripherally-located tumors, however, so the study by the NORDIC Study Group provides valuable information on a more centrally-located approach. The big question of course is what caused the bleeding.
“What percentage of TKI patients had bleeding and how many of them had a TKI without bleeding?” Dr. Kong asked. Other questions around dosing remain: How much radiation was delivered to the great vessels such as the pulmonary artery, and how much of the dose hit critical structures? And, were tumors invading the pulmonary artery?
“SBRT may be applied for centrally-located tumors safely with other prescription regimens, she suggested, such as 10-11 Gy given in 5 fractions. That is assuming that “the dose limits of normal tissues are strictly controlled and the patients are carefully selected to exclude T4 diseases for adjacent organ invasion.”
The NORDIC SBRT Study Group conducted the study. Dr. Lindberg had no conflicts of interest to disclose. Dr. Kong has received research grants from the National Cancer Institute (part of the National Institutes of Health) and speakers honorarium and travel support from Varian Medical.
VIENNA – Stereotactic body radiotherapy (SBRT) proved too toxic for many of patients recruited into a multinational phase II trial with centrally-located lung tumors.
The majority of patients experienced some type of adverse effect, with 28% experiencing serious (grade 3-5) adverse effects.
“Our major concern now is that we had six cases of grade 5 bleedings,” Karen Lindberg, MD, said at the World Conference on Lung Cancer. “Tumor location seems to be a risk factor for bleeding,” she added, with five of the six cases seen in patients who had tumors close to a main bronchus (group A). The other case was in patient who had tumors close to a lobular bronchus (group B).
“The classical definition of a centrally-located lung tumor is a tumor residing within or touching an imaginary zone 2 cm around the proximal bronchial tree,” explained Dr. Lindberg of Karolinska University Hospital and the Karolinska Institutet in Stockholm, who presented the first results of the Nordic HILUS Trial.
“When we designed this study we wanted to look at very centrally located tumors, so we tightened up this definition to look at tumors that occurred within 1 cm around the proximal bronchial tree,” she said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
During the trial, SBRT was to be delivered at a dose of 7 Gray (Gy) in 8 fractions at 65%-75% isodose lines to ultra-centrally-located tumors. Dose constraints were stipulated for tumors situated very close to the spinal cord, contralateral main bronchus, and trachea, with some dosing recommendations on reducing the dose delivered to tumors that were ipsilateral to the main bronchus, or very close to the esophagus or heart.
A total of 74 patients with centrally-located, locally progressive tumors, which were less than 5 cm in size and due to non–small-cell lung cancer (NSCLC) or metastatic lung disease from another solid tumor, were recruited. Patients had to have a good performance status and life expectancy of 3 months.
Patients with brain metastases or tumors that reached through the wall of a main bronchus were excluded as were those who were taking any concomitant systemic therapy.
The mean age of the recruited patients was 71 years; 58 (78%) had NSCLC, of which 20 (27%) had adenomas and 19 (26%) had squamous cell cancers. Of those with secondary lung tumors, eight (11%), had primary renal cell carcinoma and four (5%) had colorectal cancer.
After a follow-up of 18.6 months, 31 (42%) patients had died.
The most common adverse events reported were grade 1-2 dyspnea, cough, and fatigue. However, there was a high rate of grade 3 (dyspnea, fatigue, pain, pneumonitis, and heart rhythm disturbance), 4 (pain, lung infection, fever, heart rhythm disturbance, fistula, and pneumothorax) and 5 toxicities (pneumonitis and bleeding).
Bleeding often occurred without warning and had an acute onset and toll.
Seven patients (six in group A and one in group B) may have suffered grade 5 side effects; six patients experienced lethal hemoptysis after a median of 15.5 months (2.5-21 months) and one patient suffered from a lethal pneumonitis 5 months post study treatment. The reintroduction of TKIs may also have played a role, Dr. Lindberg suggested.
SBRT for early stage NSCLC is very effective and “generates outstanding tumor control”, said Feng-Ming Kong, MD, of Indiana University, Indianapolis, who was invited to comment on the findings.
Most studies of SBRT have looked at peripherally-located tumors, however, so the study by the NORDIC Study Group provides valuable information on a more centrally-located approach. The big question of course is what caused the bleeding.
“What percentage of TKI patients had bleeding and how many of them had a TKI without bleeding?” Dr. Kong asked. Other questions around dosing remain: How much radiation was delivered to the great vessels such as the pulmonary artery, and how much of the dose hit critical structures? And, were tumors invading the pulmonary artery?
“SBRT may be applied for centrally-located tumors safely with other prescription regimens, she suggested, such as 10-11 Gy given in 5 fractions. That is assuming that “the dose limits of normal tissues are strictly controlled and the patients are carefully selected to exclude T4 diseases for adjacent organ invasion.”
The NORDIC SBRT Study Group conducted the study. Dr. Lindberg had no conflicts of interest to disclose. Dr. Kong has received research grants from the National Cancer Institute (part of the National Institutes of Health) and speakers honorarium and travel support from Varian Medical.
VIENNA – Stereotactic body radiotherapy (SBRT) proved too toxic for many of patients recruited into a multinational phase II trial with centrally-located lung tumors.
The majority of patients experienced some type of adverse effect, with 28% experiencing serious (grade 3-5) adverse effects.
“Our major concern now is that we had six cases of grade 5 bleedings,” Karen Lindberg, MD, said at the World Conference on Lung Cancer. “Tumor location seems to be a risk factor for bleeding,” she added, with five of the six cases seen in patients who had tumors close to a main bronchus (group A). The other case was in patient who had tumors close to a lobular bronchus (group B).
“The classical definition of a centrally-located lung tumor is a tumor residing within or touching an imaginary zone 2 cm around the proximal bronchial tree,” explained Dr. Lindberg of Karolinska University Hospital and the Karolinska Institutet in Stockholm, who presented the first results of the Nordic HILUS Trial.
“When we designed this study we wanted to look at very centrally located tumors, so we tightened up this definition to look at tumors that occurred within 1 cm around the proximal bronchial tree,” she said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
During the trial, SBRT was to be delivered at a dose of 7 Gray (Gy) in 8 fractions at 65%-75% isodose lines to ultra-centrally-located tumors. Dose constraints were stipulated for tumors situated very close to the spinal cord, contralateral main bronchus, and trachea, with some dosing recommendations on reducing the dose delivered to tumors that were ipsilateral to the main bronchus, or very close to the esophagus or heart.
A total of 74 patients with centrally-located, locally progressive tumors, which were less than 5 cm in size and due to non–small-cell lung cancer (NSCLC) or metastatic lung disease from another solid tumor, were recruited. Patients had to have a good performance status and life expectancy of 3 months.
Patients with brain metastases or tumors that reached through the wall of a main bronchus were excluded as were those who were taking any concomitant systemic therapy.
The mean age of the recruited patients was 71 years; 58 (78%) had NSCLC, of which 20 (27%) had adenomas and 19 (26%) had squamous cell cancers. Of those with secondary lung tumors, eight (11%), had primary renal cell carcinoma and four (5%) had colorectal cancer.
After a follow-up of 18.6 months, 31 (42%) patients had died.
The most common adverse events reported were grade 1-2 dyspnea, cough, and fatigue. However, there was a high rate of grade 3 (dyspnea, fatigue, pain, pneumonitis, and heart rhythm disturbance), 4 (pain, lung infection, fever, heart rhythm disturbance, fistula, and pneumothorax) and 5 toxicities (pneumonitis and bleeding).
Bleeding often occurred without warning and had an acute onset and toll.
Seven patients (six in group A and one in group B) may have suffered grade 5 side effects; six patients experienced lethal hemoptysis after a median of 15.5 months (2.5-21 months) and one patient suffered from a lethal pneumonitis 5 months post study treatment. The reintroduction of TKIs may also have played a role, Dr. Lindberg suggested.
SBRT for early stage NSCLC is very effective and “generates outstanding tumor control”, said Feng-Ming Kong, MD, of Indiana University, Indianapolis, who was invited to comment on the findings.
Most studies of SBRT have looked at peripherally-located tumors, however, so the study by the NORDIC Study Group provides valuable information on a more centrally-located approach. The big question of course is what caused the bleeding.
“What percentage of TKI patients had bleeding and how many of them had a TKI without bleeding?” Dr. Kong asked. Other questions around dosing remain: How much radiation was delivered to the great vessels such as the pulmonary artery, and how much of the dose hit critical structures? And, were tumors invading the pulmonary artery?
“SBRT may be applied for centrally-located tumors safely with other prescription regimens, she suggested, such as 10-11 Gy given in 5 fractions. That is assuming that “the dose limits of normal tissues are strictly controlled and the patients are carefully selected to exclude T4 diseases for adjacent organ invasion.”
The NORDIC SBRT Study Group conducted the study. Dr. Lindberg had no conflicts of interest to disclose. Dr. Kong has received research grants from the National Cancer Institute (part of the National Institutes of Health) and speakers honorarium and travel support from Varian Medical.
AT WCLC 2016
Key clinical point: Stereotactic body radiotherapy (SBRT) proved too toxic for many of patients recruited into a multinational phase II trial with centrally-located lung tumors.
Major finding: There was a high rate of grade 3, 4, and 5 toxicities, including six cases of grade 5 bleeding.
Data source: The phase II non-randomized HILUS trial of 74 patients with centrally-located lung tumors treated with SBRT.
Disclosures: The NORDIC SBRT Study Group conducted the study. Dr. Lindberg had no conflicts of interest to disclose. Dr. Kong has received research grants from the National Cancer Institute (part of the National Institutes of Health) and speakers honorarium and travel support from Varian Medical.
Multiple Keratoacanthomas Occurring in Surgical Margins and De Novo Treated With Intralesional Methotrexate
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.
Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Practice Points
- Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas but also may develop in areas of trauma including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.
- Intralesional methotrexate is a potential alternative to surgical treatment of KAs as a less invasive and less costly treatment modality with decreased morbidity for multiple KAs.
- Isotopic response refers to the occurrence of a new skin disorder arising at the site of another unrelated and already healed skin disease. Isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of injuries.