User login
Cosmetic Corner: Dermatologists Weigh in on Self-tanners
To improve patient care and outcomes, leading dermatologists offered their recommendations on self-tanners. Consideration must be given to:
- Anthelios 50 Mineral Tinted
La Roche-Posay Laboratoire Dermatologique
Recommended by Gary Goldenberg, MD, New York, New York
- St. Tropez Self Tan products
PZ Cussons Beauty LLP
“It helps to produce an even and natural-looking skin tone.”—Anthony M. Rossi, MD, New York, New York
- Sun-Free Self-Tanning Formula
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Sunless Tanning Towelette
Sun Bum
“This product is easy to use. Make sure to use it in conjunction with a broad-spectrum sunscreen.”—Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening products, and athlete’s foot treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on self-tanners. Consideration must be given to:
- Anthelios 50 Mineral Tinted
La Roche-Posay Laboratoire Dermatologique
Recommended by Gary Goldenberg, MD, New York, New York
- St. Tropez Self Tan products
PZ Cussons Beauty LLP
“It helps to produce an even and natural-looking skin tone.”—Anthony M. Rossi, MD, New York, New York
- Sun-Free Self-Tanning Formula
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Sunless Tanning Towelette
Sun Bum
“This product is easy to use. Make sure to use it in conjunction with a broad-spectrum sunscreen.”—Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening products, and athlete’s foot treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on self-tanners. Consideration must be given to:
- Anthelios 50 Mineral Tinted
La Roche-Posay Laboratoire Dermatologique
Recommended by Gary Goldenberg, MD, New York, New York
- St. Tropez Self Tan products
PZ Cussons Beauty LLP
“It helps to produce an even and natural-looking skin tone.”—Anthony M. Rossi, MD, New York, New York
- Sun-Free Self-Tanning Formula
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Sunless Tanning Towelette
Sun Bum
“This product is easy to use. Make sure to use it in conjunction with a broad-spectrum sunscreen.”—Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening products, and athlete’s foot treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Sonovaginography bests negative ‘sliding sign’ in predicting deep infiltrating endometriosis
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Plasma energy ablation yields pregnancy rates similar to cystectomy
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
AT THE AAGL GLOBAL CONGRESS
An Update on Management of Syndesmosis Injury: A National US Database Study
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
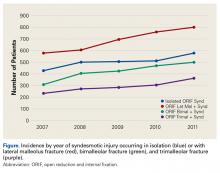
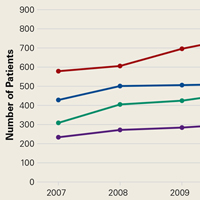
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Topical Imiquimod Clears Invasive Melanoma
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
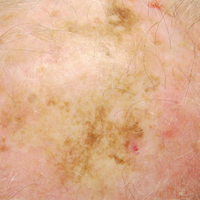
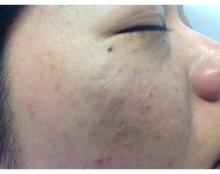
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.
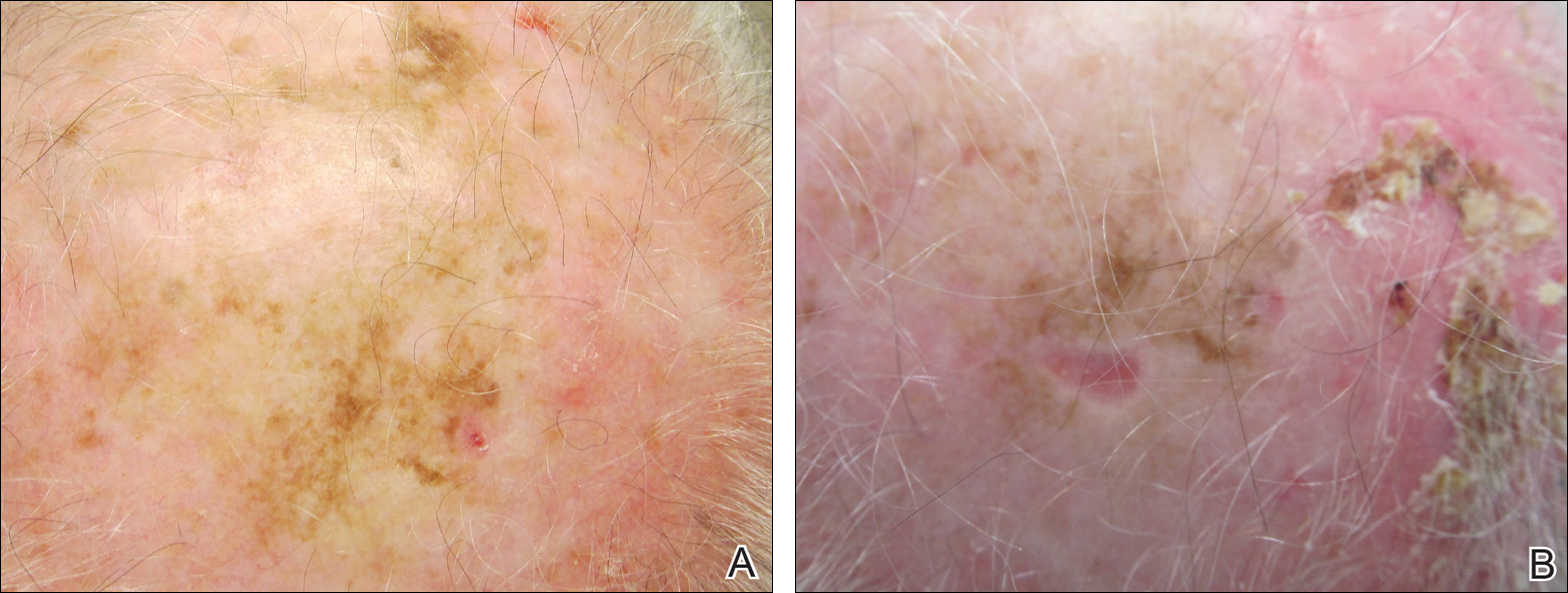
Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7
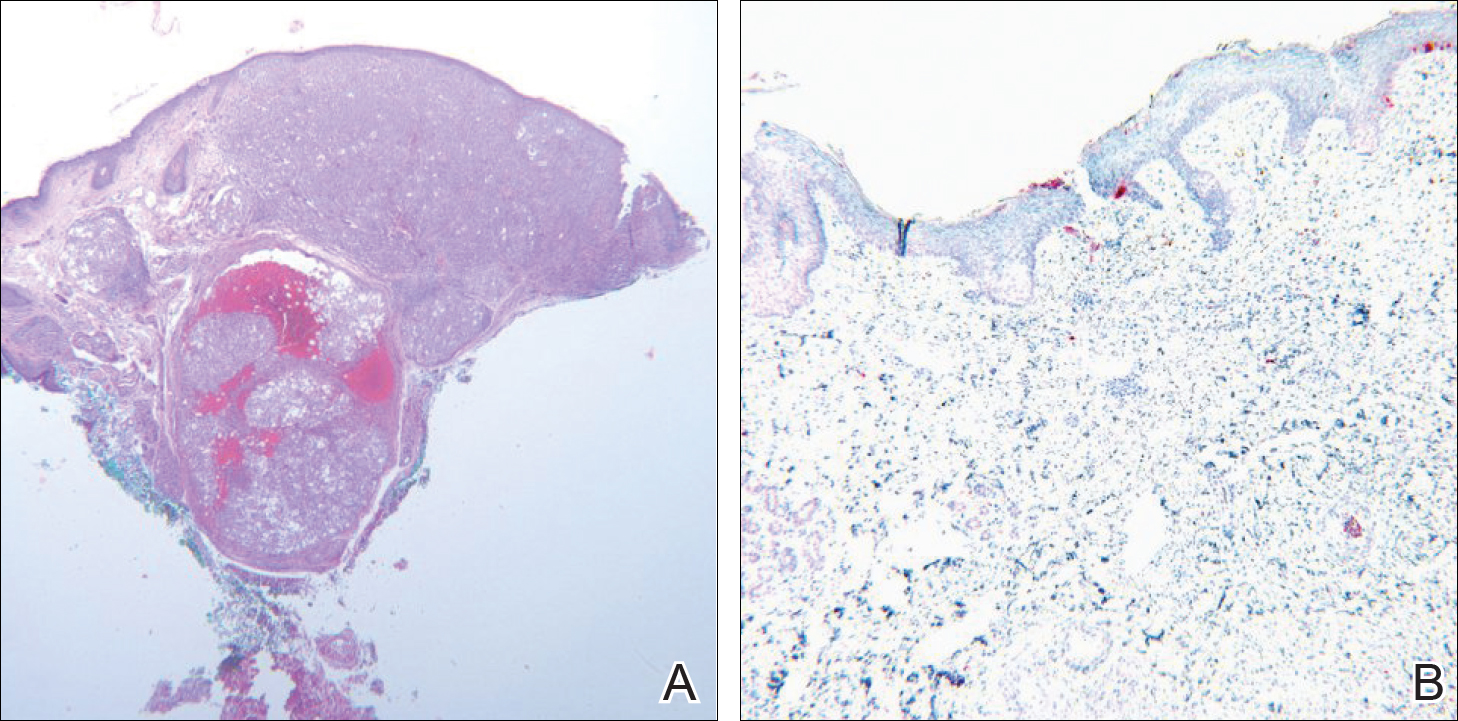
Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Practice Points
- Topical imiquimod may clear invasive melanoma as well as melanoma in situ.
- Further study is required to confirm the role of topical imiquimod in melanoma treatment.
MRI useful to distinguish between PACNS and neurosarcoidosis
WASHINGTON – MRI can help to differentiate between neurosarcoidosis and primary angiitis of the central nervous system, according to a single-center study comparing the two conditions.
Patients with neurosarcoidosis were significantly more likely to display spinal cord, basal meningeal, and cranial nerve involvements than were patients with primary angiitis of the central nervous system (PACNS), making MRI an efficient tool in distinguishing between the two.
Dr. Saygin and her coinvestigators at the Cleveland Clinic recruited 34 patients with PACNS and 42 patients with neurosarcoidosis, all of whom had brain and/or spinal cord MRIs performed close to the time of presentation. The average age was 45.6 years in the PACNS group and 44.1 years in the neurosarcoidosis group. The MRIs were blindly reviewed by two neuroradiologists who examined and recorded data on pachymeninges, leptomeninges, basal meninges, cranial nerves, cerebral gray and white matter, and the spinal cord itself. The sites, presence, patterns, localization, and lateral involvement for these were noted, as well as any mass effect, parenchymal hemorrhaging, and ventriculomegaly.
Unilateral cranial nerve involvement appeared on MRI in 71% of neurosarcoidosis patients, compared with 3.0% for PACNS. Neurosarcoidosis patients also had consistently higher rates of involvement of the spinal cord (cervical, 62.5% vs. 0%; thoracic, 45.8% vs. 0%) as well as basal meninges (basal cistern, 21.6% vs. 0%; brain stem, 27.0% vs. 3.0%).
However, there was no significant difference between PACNS and neurosarcoidosis patients in terms of the pachymeningeal and leptomeningeal involvement, pituitary/sella turcica involvement, and mass effect, the latter of which was seen in 20% of PACNS patients and 14% of those with neurosarcoidosis.
The relatively small sample size of 76 patients is a limitation of the study, Dr. Saygin said.
No funding source was disclosed for this study. Dr. Saygin did not report any relevant financial disclosures.
WASHINGTON – MRI can help to differentiate between neurosarcoidosis and primary angiitis of the central nervous system, according to a single-center study comparing the two conditions.
Patients with neurosarcoidosis were significantly more likely to display spinal cord, basal meningeal, and cranial nerve involvements than were patients with primary angiitis of the central nervous system (PACNS), making MRI an efficient tool in distinguishing between the two.
Dr. Saygin and her coinvestigators at the Cleveland Clinic recruited 34 patients with PACNS and 42 patients with neurosarcoidosis, all of whom had brain and/or spinal cord MRIs performed close to the time of presentation. The average age was 45.6 years in the PACNS group and 44.1 years in the neurosarcoidosis group. The MRIs were blindly reviewed by two neuroradiologists who examined and recorded data on pachymeninges, leptomeninges, basal meninges, cranial nerves, cerebral gray and white matter, and the spinal cord itself. The sites, presence, patterns, localization, and lateral involvement for these were noted, as well as any mass effect, parenchymal hemorrhaging, and ventriculomegaly.
Unilateral cranial nerve involvement appeared on MRI in 71% of neurosarcoidosis patients, compared with 3.0% for PACNS. Neurosarcoidosis patients also had consistently higher rates of involvement of the spinal cord (cervical, 62.5% vs. 0%; thoracic, 45.8% vs. 0%) as well as basal meninges (basal cistern, 21.6% vs. 0%; brain stem, 27.0% vs. 3.0%).
However, there was no significant difference between PACNS and neurosarcoidosis patients in terms of the pachymeningeal and leptomeningeal involvement, pituitary/sella turcica involvement, and mass effect, the latter of which was seen in 20% of PACNS patients and 14% of those with neurosarcoidosis.
The relatively small sample size of 76 patients is a limitation of the study, Dr. Saygin said.
No funding source was disclosed for this study. Dr. Saygin did not report any relevant financial disclosures.
WASHINGTON – MRI can help to differentiate between neurosarcoidosis and primary angiitis of the central nervous system, according to a single-center study comparing the two conditions.
Patients with neurosarcoidosis were significantly more likely to display spinal cord, basal meningeal, and cranial nerve involvements than were patients with primary angiitis of the central nervous system (PACNS), making MRI an efficient tool in distinguishing between the two.
Dr. Saygin and her coinvestigators at the Cleveland Clinic recruited 34 patients with PACNS and 42 patients with neurosarcoidosis, all of whom had brain and/or spinal cord MRIs performed close to the time of presentation. The average age was 45.6 years in the PACNS group and 44.1 years in the neurosarcoidosis group. The MRIs were blindly reviewed by two neuroradiologists who examined and recorded data on pachymeninges, leptomeninges, basal meninges, cranial nerves, cerebral gray and white matter, and the spinal cord itself. The sites, presence, patterns, localization, and lateral involvement for these were noted, as well as any mass effect, parenchymal hemorrhaging, and ventriculomegaly.
Unilateral cranial nerve involvement appeared on MRI in 71% of neurosarcoidosis patients, compared with 3.0% for PACNS. Neurosarcoidosis patients also had consistently higher rates of involvement of the spinal cord (cervical, 62.5% vs. 0%; thoracic, 45.8% vs. 0%) as well as basal meninges (basal cistern, 21.6% vs. 0%; brain stem, 27.0% vs. 3.0%).
However, there was no significant difference between PACNS and neurosarcoidosis patients in terms of the pachymeningeal and leptomeningeal involvement, pituitary/sella turcica involvement, and mass effect, the latter of which was seen in 20% of PACNS patients and 14% of those with neurosarcoidosis.
The relatively small sample size of 76 patients is a limitation of the study, Dr. Saygin said.
No funding source was disclosed for this study. Dr. Saygin did not report any relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Unilateral cranial nerve involvement appeared on MRI in 71% of neurosarcoidosis patients, compared with 3.0% for PACNS.
Data source: Prospective cohort study of 34 PACNS patients and 42 neurosarcoidosis patients.
Disclosures: No relevant financial disclosures were reported.
Big data ready to revolutionize infectious diseases research
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF INFECTIOUS DISEASES
Cardiovascular comorbidities common in patients with epilepsy
HOUSTON – Adults with epilepsy reported five of the six most common cardiovascular diseases more often than did adults without epilepsy, according to results from the 2013 National Health Interview Survey.
“Often, neurologists are busy treating the seizure, making sure the patient has proper treatment, but they often don’t have the time to look at these conditions that can cause deaths a lot more commonly than things like sudden unexpected death from epilepsy,” lead study author Matthew Zack, MD, said in an interview at the annual meeting of the American Epilepsy Society. “We’re not talking about mortality here, but it’s important for doctors to be aware of the fact that patients with epilepsy have increased risk for conditions like heart attacks, high blood pressure, and stroke.”
Compared to NHIS respondents without epilepsy, those with any epilepsy reported significantly more hypertension (36.4% vs. 30.2%), angina pectoris (3.9% vs. 2.0%), heart attack (5.2% vs. 3.3%), other heart condition/diseases (11.8% vs. 7.4%), and stroke (12.2% vs. 2.6%; P less than .01 for all associations). “We knew that persons with epilepsy often have a preexisting stroke, but we didn’t expect this for hypertension,” Dr. Zack said. “Hypertension is a risk factor for cardiovascular disease, but we also know that persons with epilepsy tend to smoke cigarettes a little bit more often than the general population. They also tend not to exercise or have physical activity. It had been a recommendation that they not participate in physical activity because it was thought to evoke seizures. But in fact, other follow-up studies have shown that’s not true, and that persons who engage in physical activity can often lessen the effects of epilepsy.”
Women with epilepsy reported significantly higher rates of three CVD conditions, compared with women who did not have epilepsy: hypertension (36.4% vs. 29.6%), angina pectoris (3.9% vs. 1.7%), and stroke (14.1% vs. 2.6%; P less than .01 for all associations). However, men with epilepsy reported only significantly more stroke, compared with men who did not have epilepsy (10.1% vs. 2.7%; P less than .01). Reasons for the differences observed between genders remain unclear and will require further analysis, Dr. Zack said. He acknowledged the self-reported nature of the NHIS as a chief limitation of the analysis. “We don’t have measurements of high blood pressure, we just have the person’s report that the doctor told them they had high blood pressure,” he said.
Dr. Zack reported having no financial disclosures.
HOUSTON – Adults with epilepsy reported five of the six most common cardiovascular diseases more often than did adults without epilepsy, according to results from the 2013 National Health Interview Survey.
“Often, neurologists are busy treating the seizure, making sure the patient has proper treatment, but they often don’t have the time to look at these conditions that can cause deaths a lot more commonly than things like sudden unexpected death from epilepsy,” lead study author Matthew Zack, MD, said in an interview at the annual meeting of the American Epilepsy Society. “We’re not talking about mortality here, but it’s important for doctors to be aware of the fact that patients with epilepsy have increased risk for conditions like heart attacks, high blood pressure, and stroke.”
Compared to NHIS respondents without epilepsy, those with any epilepsy reported significantly more hypertension (36.4% vs. 30.2%), angina pectoris (3.9% vs. 2.0%), heart attack (5.2% vs. 3.3%), other heart condition/diseases (11.8% vs. 7.4%), and stroke (12.2% vs. 2.6%; P less than .01 for all associations). “We knew that persons with epilepsy often have a preexisting stroke, but we didn’t expect this for hypertension,” Dr. Zack said. “Hypertension is a risk factor for cardiovascular disease, but we also know that persons with epilepsy tend to smoke cigarettes a little bit more often than the general population. They also tend not to exercise or have physical activity. It had been a recommendation that they not participate in physical activity because it was thought to evoke seizures. But in fact, other follow-up studies have shown that’s not true, and that persons who engage in physical activity can often lessen the effects of epilepsy.”
Women with epilepsy reported significantly higher rates of three CVD conditions, compared with women who did not have epilepsy: hypertension (36.4% vs. 29.6%), angina pectoris (3.9% vs. 1.7%), and stroke (14.1% vs. 2.6%; P less than .01 for all associations). However, men with epilepsy reported only significantly more stroke, compared with men who did not have epilepsy (10.1% vs. 2.7%; P less than .01). Reasons for the differences observed between genders remain unclear and will require further analysis, Dr. Zack said. He acknowledged the self-reported nature of the NHIS as a chief limitation of the analysis. “We don’t have measurements of high blood pressure, we just have the person’s report that the doctor told them they had high blood pressure,” he said.
Dr. Zack reported having no financial disclosures.
HOUSTON – Adults with epilepsy reported five of the six most common cardiovascular diseases more often than did adults without epilepsy, according to results from the 2013 National Health Interview Survey.
“Often, neurologists are busy treating the seizure, making sure the patient has proper treatment, but they often don’t have the time to look at these conditions that can cause deaths a lot more commonly than things like sudden unexpected death from epilepsy,” lead study author Matthew Zack, MD, said in an interview at the annual meeting of the American Epilepsy Society. “We’re not talking about mortality here, but it’s important for doctors to be aware of the fact that patients with epilepsy have increased risk for conditions like heart attacks, high blood pressure, and stroke.”
Compared to NHIS respondents without epilepsy, those with any epilepsy reported significantly more hypertension (36.4% vs. 30.2%), angina pectoris (3.9% vs. 2.0%), heart attack (5.2% vs. 3.3%), other heart condition/diseases (11.8% vs. 7.4%), and stroke (12.2% vs. 2.6%; P less than .01 for all associations). “We knew that persons with epilepsy often have a preexisting stroke, but we didn’t expect this for hypertension,” Dr. Zack said. “Hypertension is a risk factor for cardiovascular disease, but we also know that persons with epilepsy tend to smoke cigarettes a little bit more often than the general population. They also tend not to exercise or have physical activity. It had been a recommendation that they not participate in physical activity because it was thought to evoke seizures. But in fact, other follow-up studies have shown that’s not true, and that persons who engage in physical activity can often lessen the effects of epilepsy.”
Women with epilepsy reported significantly higher rates of three CVD conditions, compared with women who did not have epilepsy: hypertension (36.4% vs. 29.6%), angina pectoris (3.9% vs. 1.7%), and stroke (14.1% vs. 2.6%; P less than .01 for all associations). However, men with epilepsy reported only significantly more stroke, compared with men who did not have epilepsy (10.1% vs. 2.7%; P less than .01). Reasons for the differences observed between genders remain unclear and will require further analysis, Dr. Zack said. He acknowledged the self-reported nature of the NHIS as a chief limitation of the analysis. “We don’t have measurements of high blood pressure, we just have the person’s report that the doctor told them they had high blood pressure,” he said.
Dr. Zack reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: Compared to respondents without epilepsy, those with any epilepsy reported significantly more hypertension (36.4% vs. 30.2%), angina pectoris (3.9% vs. 2.0%), heart attack (5.2% vs. 3.3%), other heart condition/diseases (11.8% vs. 7.4%), and stroke (12.2% vs. 2.6%; P less than .01 for all associations).
Data source: Responses from 587 adults who identified themselves as having epilepsy on the 2013 U.S. National Health Interview Survey, and 33,946 adults who did not have epilepsy.
Disclosures: Dr. Zack reported having no financial disclosures.
The hidden vascular component to melasma
So lets revisit this topic. We hate melasma. It is recalcitrant, resistant, and often recurs. But how many of us dermatologists look at melasma with a dermatoscope? A minority? And probably even fewer of us biopsy melasma. Are we missing the many, many cases of vascular or erythematotelangiectatic melasma and just treating melasma as a pigment problem instead of treating it as a vascular problem?
We know that melasma develops because of increased melanin production, not an increased number of melanocytes, but the underlying cause of increased melanogenesis is not fully understood. Several recent studies suggest that the increased vascularity in melasma skin is the underlying etiology. Understanding the way endogeneous and exogeneous stimuli such as sex hormones, oral contraceptives, ultraviolet irradiation, and visible light stimulate inflammation in the dermis, leading to the release of various mediators that stimulate angiogenesis and the activation of melanocytes, will help us improve the treatment of this relentless disease.
Similarly, elevated estrogen and progesterone in pregnancy or with oral contraceptive use is known to stimulate melasma. Melanocytes express estrogen receptors and estradiol increases the level of TRP-2, which stimulates melanocytes to produce melanin. Recent literature has shown that the number of blood vessels, vessel size, and vessel density also are greater in lesional melasma skin than in perilesional skin. In addition, immunohistochemical staining has shown an increased level of factor VIIIa-related antigen in blood vessels in melasma skin, compared with perilesional normal skin.
Unfortunately, the treatment of vascular melasma is very difficult. Lasers such as the pulsed dye laser that help skin vascularity can trigger worsening melanogenesis through dermal inflammation. The melanin cap overlying the melanocyte nucleus also can mask the underlying vascularity and make laser treatments more difficult. The isolated treatment of epidermal pigment also may be ineffective and transient.
By targeting the vessels in addition to the pigment, we will get improved clinical results and fewer relapses. We suggest that melasma be treated conservatively, not aggressively. It should be treated as an inflammatory process. Patients with melasma also have a slightly abnormal skin barrier, so we should be hesitant in using deep lasers, radio frequency, and aggressive chemical peels. Topical preparations – particularly triple-combination bleaching agents, retinoids, and nonhydroquinone skin lighteners – should be used sparingly and always in combination with treatments targeting skin vascularity.
References
J Dermatol Sci. 2007 May;46(2):111-6.
Exp Dermatol. 2005 Aug;14(8):625-33.
Clin Exp Dermatol. 2008;33(3):305-8.
J Eur Acad Dermatol Venereol. 2009 Nov;23(11):1254-62.
Ann Dermatol. 2010 Nov;22(4):373-8.
J Eur Acad Dermatol Venereol. 2013 Jan;27 Suppl 1:5-6.
Am J Clin Dermatol. 2013 Oct;14(5):359-76.
J Am Acad Dermatol. 2014 Feb;70(2):369-73.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
So lets revisit this topic. We hate melasma. It is recalcitrant, resistant, and often recurs. But how many of us dermatologists look at melasma with a dermatoscope? A minority? And probably even fewer of us biopsy melasma. Are we missing the many, many cases of vascular or erythematotelangiectatic melasma and just treating melasma as a pigment problem instead of treating it as a vascular problem?
We know that melasma develops because of increased melanin production, not an increased number of melanocytes, but the underlying cause of increased melanogenesis is not fully understood. Several recent studies suggest that the increased vascularity in melasma skin is the underlying etiology. Understanding the way endogeneous and exogeneous stimuli such as sex hormones, oral contraceptives, ultraviolet irradiation, and visible light stimulate inflammation in the dermis, leading to the release of various mediators that stimulate angiogenesis and the activation of melanocytes, will help us improve the treatment of this relentless disease.
Similarly, elevated estrogen and progesterone in pregnancy or with oral contraceptive use is known to stimulate melasma. Melanocytes express estrogen receptors and estradiol increases the level of TRP-2, which stimulates melanocytes to produce melanin. Recent literature has shown that the number of blood vessels, vessel size, and vessel density also are greater in lesional melasma skin than in perilesional skin. In addition, immunohistochemical staining has shown an increased level of factor VIIIa-related antigen in blood vessels in melasma skin, compared with perilesional normal skin.
Unfortunately, the treatment of vascular melasma is very difficult. Lasers such as the pulsed dye laser that help skin vascularity can trigger worsening melanogenesis through dermal inflammation. The melanin cap overlying the melanocyte nucleus also can mask the underlying vascularity and make laser treatments more difficult. The isolated treatment of epidermal pigment also may be ineffective and transient.
By targeting the vessels in addition to the pigment, we will get improved clinical results and fewer relapses. We suggest that melasma be treated conservatively, not aggressively. It should be treated as an inflammatory process. Patients with melasma also have a slightly abnormal skin barrier, so we should be hesitant in using deep lasers, radio frequency, and aggressive chemical peels. Topical preparations – particularly triple-combination bleaching agents, retinoids, and nonhydroquinone skin lighteners – should be used sparingly and always in combination with treatments targeting skin vascularity.
References
J Dermatol Sci. 2007 May;46(2):111-6.
Exp Dermatol. 2005 Aug;14(8):625-33.
Clin Exp Dermatol. 2008;33(3):305-8.
J Eur Acad Dermatol Venereol. 2009 Nov;23(11):1254-62.
Ann Dermatol. 2010 Nov;22(4):373-8.
J Eur Acad Dermatol Venereol. 2013 Jan;27 Suppl 1:5-6.
Am J Clin Dermatol. 2013 Oct;14(5):359-76.
J Am Acad Dermatol. 2014 Feb;70(2):369-73.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
So lets revisit this topic. We hate melasma. It is recalcitrant, resistant, and often recurs. But how many of us dermatologists look at melasma with a dermatoscope? A minority? And probably even fewer of us biopsy melasma. Are we missing the many, many cases of vascular or erythematotelangiectatic melasma and just treating melasma as a pigment problem instead of treating it as a vascular problem?
We know that melasma develops because of increased melanin production, not an increased number of melanocytes, but the underlying cause of increased melanogenesis is not fully understood. Several recent studies suggest that the increased vascularity in melasma skin is the underlying etiology. Understanding the way endogeneous and exogeneous stimuli such as sex hormones, oral contraceptives, ultraviolet irradiation, and visible light stimulate inflammation in the dermis, leading to the release of various mediators that stimulate angiogenesis and the activation of melanocytes, will help us improve the treatment of this relentless disease.
Similarly, elevated estrogen and progesterone in pregnancy or with oral contraceptive use is known to stimulate melasma. Melanocytes express estrogen receptors and estradiol increases the level of TRP-2, which stimulates melanocytes to produce melanin. Recent literature has shown that the number of blood vessels, vessel size, and vessel density also are greater in lesional melasma skin than in perilesional skin. In addition, immunohistochemical staining has shown an increased level of factor VIIIa-related antigen in blood vessels in melasma skin, compared with perilesional normal skin.
Unfortunately, the treatment of vascular melasma is very difficult. Lasers such as the pulsed dye laser that help skin vascularity can trigger worsening melanogenesis through dermal inflammation. The melanin cap overlying the melanocyte nucleus also can mask the underlying vascularity and make laser treatments more difficult. The isolated treatment of epidermal pigment also may be ineffective and transient.
By targeting the vessels in addition to the pigment, we will get improved clinical results and fewer relapses. We suggest that melasma be treated conservatively, not aggressively. It should be treated as an inflammatory process. Patients with melasma also have a slightly abnormal skin barrier, so we should be hesitant in using deep lasers, radio frequency, and aggressive chemical peels. Topical preparations – particularly triple-combination bleaching agents, retinoids, and nonhydroquinone skin lighteners – should be used sparingly and always in combination with treatments targeting skin vascularity.
References
J Dermatol Sci. 2007 May;46(2):111-6.
Exp Dermatol. 2005 Aug;14(8):625-33.
Clin Exp Dermatol. 2008;33(3):305-8.
J Eur Acad Dermatol Venereol. 2009 Nov;23(11):1254-62.
Ann Dermatol. 2010 Nov;22(4):373-8.
J Eur Acad Dermatol Venereol. 2013 Jan;27 Suppl 1:5-6.
Am J Clin Dermatol. 2013 Oct;14(5):359-76.
J Am Acad Dermatol. 2014 Feb;70(2):369-73.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
Digital Doctor
There were many books I read in 2016 that I found relevant and helpful for the practice of medicine as modern physicians. Here were some of my favorites:
”How to Have a Good Day” by Caroline Webb (Crown Business, 2016).
With media headlines screaming about the surge in physician burnout, now is a good time to read “How to Have a Good Day.” The author, Caroline Webb, an Oxford- and Cambridge-trained economist, management consultant, and executive coach, shows how little tweaks in behavior can garner big results in both your workday productivity and your overall well-being. Her science-based techniques are wonderfully practical. She teaches you how to be more effective at work by establishing smart habits, setting appropriate priorities, and pumping up your workplace enthusiasm. She also shows you a path to stronger workplace relationships by learning how to better manage conflict, build rapport, and get the most out of others. My copy is dog-eared. I hope yours will be too.
In this expansively researched book, Nancy Tomes, Ph.D., professor of history at the State University of New York, Stony Brook, documents the formation and consequences of the consumer economy in American medicine. Unlike other medical historians, who claim that patient consumerism emerged in the 1970s, Dr. Tomes argues that it developed “a full century earlier.” She dismantles the American ideal of the “golden age” of Norman Rockwell doctors and “deferential patients.” In eminently readable prose, Dr. Tomes analyzes numerous developments that have disrupted the doctor-patient relationship, including the growth of drug stores, the ever-changing medical insurance industry, and direct-to-consumer advertising. A skilled historian and writer, she doesn’t provide tidy answers to messy questions. She acknowledges that, despite many technological and social advances, particularly patient empowerment, there is no “magic bullet” to cure what ails our current medical system. This is a good choice for those looking to put digital medicine into historical context.
”Visual Intelligence: Sharpen Your Perception, Change Your Life” by Amy E. Herman (Eamon Dolan/Houghton Mifflin Harcourt, 2016).
How do we see? Not terribly well, according to Amy E. Herman. A lawyer and art historian, Ms. Herman has developed a course called “The Art of Perception” that has helped professionals from physicians to police officers hone their observation and communication skills. The course happens to be based on the class for students and residents of dermatology given by the eminent dermatologist Irwin Braverman, MD. A basic tenet of the book is that even highly intelligent professionals often experience “blindness.” She writes: “For no physiological reason, sometimes we fail to see something that’s in our direct line of sight. We overlook things when they are unexpected or too familiar, when they blend in, and when they are too aberrant or abhorrent to imagine.” Fortunately, correcting such “inattentional blindness” isn’t a gift, it’s a skill that improves with practice. The key is to acknowledge the “blind spots” in our perception and to become more detail oriented. Enter the art. Through analyzing dozens of works of art, Ms. Herman shows you how to “see what matters,” encouraging you to notice every detail and, equally important, every missing detail. The result: Over time, attention to detail becomes second nature, helping you to become more observant and fully present both in clinic and at home.
”When Breath Becomes Air” by Paul Kalanithi, MD (Penguin Random House, 2016).
This posthumously published memoir by Paul Kalanithi, MD, is tormentingly beautiful. In May 2013, the 36-year-old neurosurgeon was diagnosed with stage IV lung cancer; he was a nonsmoker. In a blink, Dr. Kalanithi would don the patient’s gown and relinquish his role as healer. “Instead of being the pastoral figure aiding a life transition, I found myself the sheep, lost and confused. Severe illness wasn’t life-altering, it was life-shattering,” he writes. Dr. Kalanithi battles the cancer valiantly for 2 years, during which time he writes this book and has a child with his wife, Lucy, also a doctor. As someone with a BA and MA in literature from Stanford and a MPhil from Cambridge, his writing is eloquent and compelling, in the vein of Atul Gawande, MD, and Jerome Groopman, MD. He voices his readers’ thoughts perfectly when he writes, “My life had been building potential, potential that would now go unrealized. I had planned to do so much, and I had come so close.” We ache for him, for his unrealized contributions to the worlds of medicine and literature, and for his loved ones. Yet, as Lucy writes in the epilogue, “this book is a new way for him to help others, a contribution only he could make.” When Breath Becomes Air will leave you both deeply saddened and deeply inspired.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.
There were many books I read in 2016 that I found relevant and helpful for the practice of medicine as modern physicians. Here were some of my favorites:
”How to Have a Good Day” by Caroline Webb (Crown Business, 2016).
With media headlines screaming about the surge in physician burnout, now is a good time to read “How to Have a Good Day.” The author, Caroline Webb, an Oxford- and Cambridge-trained economist, management consultant, and executive coach, shows how little tweaks in behavior can garner big results in both your workday productivity and your overall well-being. Her science-based techniques are wonderfully practical. She teaches you how to be more effective at work by establishing smart habits, setting appropriate priorities, and pumping up your workplace enthusiasm. She also shows you a path to stronger workplace relationships by learning how to better manage conflict, build rapport, and get the most out of others. My copy is dog-eared. I hope yours will be too.
In this expansively researched book, Nancy Tomes, Ph.D., professor of history at the State University of New York, Stony Brook, documents the formation and consequences of the consumer economy in American medicine. Unlike other medical historians, who claim that patient consumerism emerged in the 1970s, Dr. Tomes argues that it developed “a full century earlier.” She dismantles the American ideal of the “golden age” of Norman Rockwell doctors and “deferential patients.” In eminently readable prose, Dr. Tomes analyzes numerous developments that have disrupted the doctor-patient relationship, including the growth of drug stores, the ever-changing medical insurance industry, and direct-to-consumer advertising. A skilled historian and writer, she doesn’t provide tidy answers to messy questions. She acknowledges that, despite many technological and social advances, particularly patient empowerment, there is no “magic bullet” to cure what ails our current medical system. This is a good choice for those looking to put digital medicine into historical context.
”Visual Intelligence: Sharpen Your Perception, Change Your Life” by Amy E. Herman (Eamon Dolan/Houghton Mifflin Harcourt, 2016).
How do we see? Not terribly well, according to Amy E. Herman. A lawyer and art historian, Ms. Herman has developed a course called “The Art of Perception” that has helped professionals from physicians to police officers hone their observation and communication skills. The course happens to be based on the class for students and residents of dermatology given by the eminent dermatologist Irwin Braverman, MD. A basic tenet of the book is that even highly intelligent professionals often experience “blindness.” She writes: “For no physiological reason, sometimes we fail to see something that’s in our direct line of sight. We overlook things when they are unexpected or too familiar, when they blend in, and when they are too aberrant or abhorrent to imagine.” Fortunately, correcting such “inattentional blindness” isn’t a gift, it’s a skill that improves with practice. The key is to acknowledge the “blind spots” in our perception and to become more detail oriented. Enter the art. Through analyzing dozens of works of art, Ms. Herman shows you how to “see what matters,” encouraging you to notice every detail and, equally important, every missing detail. The result: Over time, attention to detail becomes second nature, helping you to become more observant and fully present both in clinic and at home.
”When Breath Becomes Air” by Paul Kalanithi, MD (Penguin Random House, 2016).
This posthumously published memoir by Paul Kalanithi, MD, is tormentingly beautiful. In May 2013, the 36-year-old neurosurgeon was diagnosed with stage IV lung cancer; he was a nonsmoker. In a blink, Dr. Kalanithi would don the patient’s gown and relinquish his role as healer. “Instead of being the pastoral figure aiding a life transition, I found myself the sheep, lost and confused. Severe illness wasn’t life-altering, it was life-shattering,” he writes. Dr. Kalanithi battles the cancer valiantly for 2 years, during which time he writes this book and has a child with his wife, Lucy, also a doctor. As someone with a BA and MA in literature from Stanford and a MPhil from Cambridge, his writing is eloquent and compelling, in the vein of Atul Gawande, MD, and Jerome Groopman, MD. He voices his readers’ thoughts perfectly when he writes, “My life had been building potential, potential that would now go unrealized. I had planned to do so much, and I had come so close.” We ache for him, for his unrealized contributions to the worlds of medicine and literature, and for his loved ones. Yet, as Lucy writes in the epilogue, “this book is a new way for him to help others, a contribution only he could make.” When Breath Becomes Air will leave you both deeply saddened and deeply inspired.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.
There were many books I read in 2016 that I found relevant and helpful for the practice of medicine as modern physicians. Here were some of my favorites:
”How to Have a Good Day” by Caroline Webb (Crown Business, 2016).
With media headlines screaming about the surge in physician burnout, now is a good time to read “How to Have a Good Day.” The author, Caroline Webb, an Oxford- and Cambridge-trained economist, management consultant, and executive coach, shows how little tweaks in behavior can garner big results in both your workday productivity and your overall well-being. Her science-based techniques are wonderfully practical. She teaches you how to be more effective at work by establishing smart habits, setting appropriate priorities, and pumping up your workplace enthusiasm. She also shows you a path to stronger workplace relationships by learning how to better manage conflict, build rapport, and get the most out of others. My copy is dog-eared. I hope yours will be too.
In this expansively researched book, Nancy Tomes, Ph.D., professor of history at the State University of New York, Stony Brook, documents the formation and consequences of the consumer economy in American medicine. Unlike other medical historians, who claim that patient consumerism emerged in the 1970s, Dr. Tomes argues that it developed “a full century earlier.” She dismantles the American ideal of the “golden age” of Norman Rockwell doctors and “deferential patients.” In eminently readable prose, Dr. Tomes analyzes numerous developments that have disrupted the doctor-patient relationship, including the growth of drug stores, the ever-changing medical insurance industry, and direct-to-consumer advertising. A skilled historian and writer, she doesn’t provide tidy answers to messy questions. She acknowledges that, despite many technological and social advances, particularly patient empowerment, there is no “magic bullet” to cure what ails our current medical system. This is a good choice for those looking to put digital medicine into historical context.
”Visual Intelligence: Sharpen Your Perception, Change Your Life” by Amy E. Herman (Eamon Dolan/Houghton Mifflin Harcourt, 2016).
How do we see? Not terribly well, according to Amy E. Herman. A lawyer and art historian, Ms. Herman has developed a course called “The Art of Perception” that has helped professionals from physicians to police officers hone their observation and communication skills. The course happens to be based on the class for students and residents of dermatology given by the eminent dermatologist Irwin Braverman, MD. A basic tenet of the book is that even highly intelligent professionals often experience “blindness.” She writes: “For no physiological reason, sometimes we fail to see something that’s in our direct line of sight. We overlook things when they are unexpected or too familiar, when they blend in, and when they are too aberrant or abhorrent to imagine.” Fortunately, correcting such “inattentional blindness” isn’t a gift, it’s a skill that improves with practice. The key is to acknowledge the “blind spots” in our perception and to become more detail oriented. Enter the art. Through analyzing dozens of works of art, Ms. Herman shows you how to “see what matters,” encouraging you to notice every detail and, equally important, every missing detail. The result: Over time, attention to detail becomes second nature, helping you to become more observant and fully present both in clinic and at home.
”When Breath Becomes Air” by Paul Kalanithi, MD (Penguin Random House, 2016).
This posthumously published memoir by Paul Kalanithi, MD, is tormentingly beautiful. In May 2013, the 36-year-old neurosurgeon was diagnosed with stage IV lung cancer; he was a nonsmoker. In a blink, Dr. Kalanithi would don the patient’s gown and relinquish his role as healer. “Instead of being the pastoral figure aiding a life transition, I found myself the sheep, lost and confused. Severe illness wasn’t life-altering, it was life-shattering,” he writes. Dr. Kalanithi battles the cancer valiantly for 2 years, during which time he writes this book and has a child with his wife, Lucy, also a doctor. As someone with a BA and MA in literature from Stanford and a MPhil from Cambridge, his writing is eloquent and compelling, in the vein of Atul Gawande, MD, and Jerome Groopman, MD. He voices his readers’ thoughts perfectly when he writes, “My life had been building potential, potential that would now go unrealized. I had planned to do so much, and I had come so close.” We ache for him, for his unrealized contributions to the worlds of medicine and literature, and for his loved ones. Yet, as Lucy writes in the epilogue, “this book is a new way for him to help others, a contribution only he could make.” When Breath Becomes Air will leave you both deeply saddened and deeply inspired.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]. He has no disclosures related to this column.