User login
FDA grants breakthrough therapy status to rituximab for pemphigus vulgaris
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
A history of asthma exacerbations predicts future exacerbations
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
Smoking During Pregnancy May Damage Offspring’s Retinal Nerve Fiber Layer
Retinal nerve fiber layer defects are a defining feature of optic neuropathies and have been implicated in several neurodegenerative disorders, including multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease. Both maternal smoking during pregnancy and low birth weight have been implicated in impaired development of the retina.
The Copenhagen Child Cohort 2000 Eye Study
Mr. Ashina and colleagues sought to investigate the associations of maternal smoking during pregnancy and low birth weight with retinal nerve fiber layer thickness in preadolescent children. They examined data from a prospective, population-based, birth cohort study that included all children (n = 6,090) born in 2000 in Copenhagen. Maternal smoking data were collected through parental interviews. Birth weight, pregnancy, and medical history data were obtained from the Danish Medical Birth Registry. As a follow-up, the researchers performed eye examinations on 1,406 of these children from May 1, 2011, to October 31, 2012, when the children were age 11 or 12.
Of the 1,406 children in the study, 1,323 were included in the analysis. Mean age was 11.7. Nearly half of the children (47.8%) were boys. The mean retinal nerve fiber layer thickness was 104 mm. In 227 children whose mothers had smoked during pregnancy, the peripapillary retinal nerve fiber layer was 5.7 mm thinner than in children whose mothers had not smoked during pregnancy, after adjusting for age, sex, birth weight, height, body weight, Tanner stage of pubertal development, axial length, and spherical equivalent refractive error. In children with low birth weight (ie, < 2,500 g), the retinal nerve fiber layer was 3.5 mm thinner than in children with normal birth weight, after adjustment for all variables.
“The results of this study add evidence to existing recommendations to avoid smoking during pregnancy and support measures that promote maternal and fetal health,” the researchers said.
A Public Health Message
In an invited commentary that accompanied the study, Christopher Kai-Shun Leung, MD, from the Department of Ophthalmology and Visual Sciences at Hong Kong Eye Hospital and Chinese University of Hong Kong in Kowloon, said that “although a difference of 5 to 6 mm in average circumpapillary retinal nerve fiber layer thickness is unlikely to translate into a detectable difference in visual function in children aged 12 to 13 years, the risk of subsequent development of visual impairment should not be overlooked.” Furthermore, he noted, “whether a thinner retinal nerve fiber layer in the children of mothers who smoked during pregnancy w
—Glenn S. Williams
Suggested Reading
Ashina H, Li XQ, Olsen EM, et al. Association of maternal smoking during pregnancy and birth weight with retinal nerve fiber layer thickness in children aged 11 or 12 years: The Copenhagen Child Cohort 2000 Eye Study. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Leung CK. Evaluation of retinal nerve fiber layer thinning with Fourier-domain optical coherence tomography. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Retinal nerve fiber layer defects are a defining feature of optic neuropathies and have been implicated in several neurodegenerative disorders, including multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease. Both maternal smoking during pregnancy and low birth weight have been implicated in impaired development of the retina.
The Copenhagen Child Cohort 2000 Eye Study
Mr. Ashina and colleagues sought to investigate the associations of maternal smoking during pregnancy and low birth weight with retinal nerve fiber layer thickness in preadolescent children. They examined data from a prospective, population-based, birth cohort study that included all children (n = 6,090) born in 2000 in Copenhagen. Maternal smoking data were collected through parental interviews. Birth weight, pregnancy, and medical history data were obtained from the Danish Medical Birth Registry. As a follow-up, the researchers performed eye examinations on 1,406 of these children from May 1, 2011, to October 31, 2012, when the children were age 11 or 12.
Of the 1,406 children in the study, 1,323 were included in the analysis. Mean age was 11.7. Nearly half of the children (47.8%) were boys. The mean retinal nerve fiber layer thickness was 104 mm. In 227 children whose mothers had smoked during pregnancy, the peripapillary retinal nerve fiber layer was 5.7 mm thinner than in children whose mothers had not smoked during pregnancy, after adjusting for age, sex, birth weight, height, body weight, Tanner stage of pubertal development, axial length, and spherical equivalent refractive error. In children with low birth weight (ie, < 2,500 g), the retinal nerve fiber layer was 3.5 mm thinner than in children with normal birth weight, after adjustment for all variables.
“The results of this study add evidence to existing recommendations to avoid smoking during pregnancy and support measures that promote maternal and fetal health,” the researchers said.
A Public Health Message
In an invited commentary that accompanied the study, Christopher Kai-Shun Leung, MD, from the Department of Ophthalmology and Visual Sciences at Hong Kong Eye Hospital and Chinese University of Hong Kong in Kowloon, said that “although a difference of 5 to 6 mm in average circumpapillary retinal nerve fiber layer thickness is unlikely to translate into a detectable difference in visual function in children aged 12 to 13 years, the risk of subsequent development of visual impairment should not be overlooked.” Furthermore, he noted, “whether a thinner retinal nerve fiber layer in the children of mothers who smoked during pregnancy w
—Glenn S. Williams
Suggested Reading
Ashina H, Li XQ, Olsen EM, et al. Association of maternal smoking during pregnancy and birth weight with retinal nerve fiber layer thickness in children aged 11 or 12 years: The Copenhagen Child Cohort 2000 Eye Study. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Leung CK. Evaluation of retinal nerve fiber layer thinning with Fourier-domain optical coherence tomography. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Retinal nerve fiber layer defects are a defining feature of optic neuropathies and have been implicated in several neurodegenerative disorders, including multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease. Both maternal smoking during pregnancy and low birth weight have been implicated in impaired development of the retina.
The Copenhagen Child Cohort 2000 Eye Study
Mr. Ashina and colleagues sought to investigate the associations of maternal smoking during pregnancy and low birth weight with retinal nerve fiber layer thickness in preadolescent children. They examined data from a prospective, population-based, birth cohort study that included all children (n = 6,090) born in 2000 in Copenhagen. Maternal smoking data were collected through parental interviews. Birth weight, pregnancy, and medical history data were obtained from the Danish Medical Birth Registry. As a follow-up, the researchers performed eye examinations on 1,406 of these children from May 1, 2011, to October 31, 2012, when the children were age 11 or 12.
Of the 1,406 children in the study, 1,323 were included in the analysis. Mean age was 11.7. Nearly half of the children (47.8%) were boys. The mean retinal nerve fiber layer thickness was 104 mm. In 227 children whose mothers had smoked during pregnancy, the peripapillary retinal nerve fiber layer was 5.7 mm thinner than in children whose mothers had not smoked during pregnancy, after adjusting for age, sex, birth weight, height, body weight, Tanner stage of pubertal development, axial length, and spherical equivalent refractive error. In children with low birth weight (ie, < 2,500 g), the retinal nerve fiber layer was 3.5 mm thinner than in children with normal birth weight, after adjustment for all variables.
“The results of this study add evidence to existing recommendations to avoid smoking during pregnancy and support measures that promote maternal and fetal health,” the researchers said.
A Public Health Message
In an invited commentary that accompanied the study, Christopher Kai-Shun Leung, MD, from the Department of Ophthalmology and Visual Sciences at Hong Kong Eye Hospital and Chinese University of Hong Kong in Kowloon, said that “although a difference of 5 to 6 mm in average circumpapillary retinal nerve fiber layer thickness is unlikely to translate into a detectable difference in visual function in children aged 12 to 13 years, the risk of subsequent development of visual impairment should not be overlooked.” Furthermore, he noted, “whether a thinner retinal nerve fiber layer in the children of mothers who smoked during pregnancy w
—Glenn S. Williams
Suggested Reading
Ashina H, Li XQ, Olsen EM, et al. Association of maternal smoking during pregnancy and birth weight with retinal nerve fiber layer thickness in children aged 11 or 12 years: The Copenhagen Child Cohort 2000 Eye Study. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Leung CK. Evaluation of retinal nerve fiber layer thinning with Fourier-domain optical coherence tomography. JAMA Ophthalmol. 2017 March 2 [Epub ahead of print].
Treating polycystic ovary syndrome: Start using dual medical therapy
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.
Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
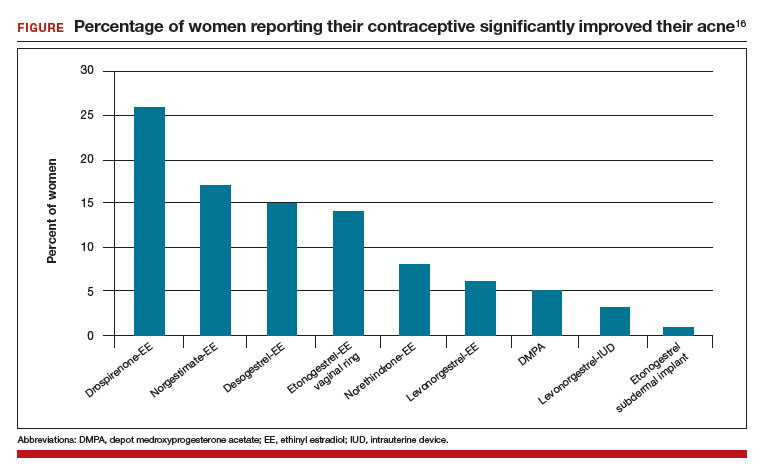
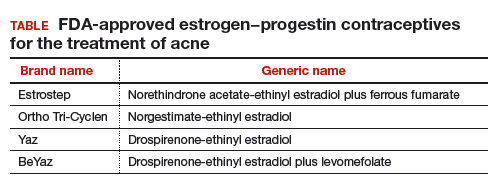
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.
The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.

Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.


The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.

Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.


The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
Moving or starting a practice? Consider Iowa
The federal government may or may not believe in global warming, but when it comes to states’ medical practice climates, Iowa trumps them all, according to the personal finance website WalletHub.
The Hawkeye State came out on top of WalletHub’s list of the Best States to Practice Medicine for 2017 with 68.7 out of a possible 100 points, while New York finished 51st (Washington, D.C., was 50th) with 28.5 points. Minnesota is the second-best state for physicians, followed by Idaho, Wisconsin, and Kansas. The rest of the bottom five included New Jersey at 49th, Maryland at 48th, and Rhode Island at 47th, WalletHub reported.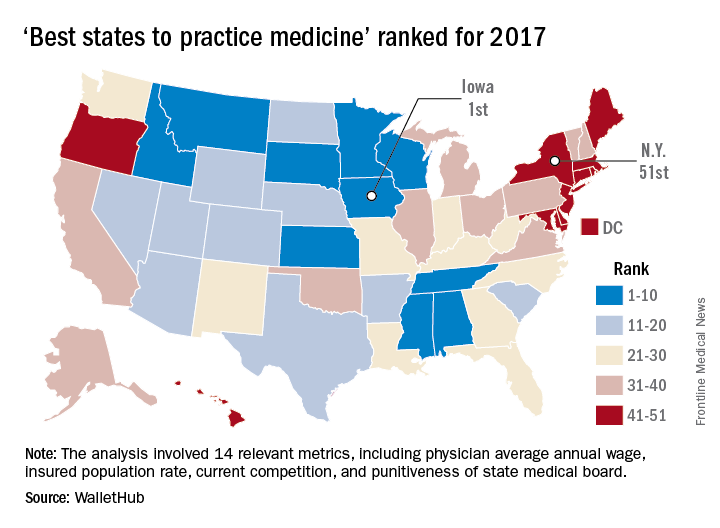
WalletHub compared the 50 states and Washington using 14 different metrics across two broad categories: “opportunity and competition” (70 points) and “medical environment” (30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), hospitals per capita, quality of public hospital system, and annual malpractice liability insurance rate.
The federal government may or may not believe in global warming, but when it comes to states’ medical practice climates, Iowa trumps them all, according to the personal finance website WalletHub.
The Hawkeye State came out on top of WalletHub’s list of the Best States to Practice Medicine for 2017 with 68.7 out of a possible 100 points, while New York finished 51st (Washington, D.C., was 50th) with 28.5 points. Minnesota is the second-best state for physicians, followed by Idaho, Wisconsin, and Kansas. The rest of the bottom five included New Jersey at 49th, Maryland at 48th, and Rhode Island at 47th, WalletHub reported.
WalletHub compared the 50 states and Washington using 14 different metrics across two broad categories: “opportunity and competition” (70 points) and “medical environment” (30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), hospitals per capita, quality of public hospital system, and annual malpractice liability insurance rate.
The federal government may or may not believe in global warming, but when it comes to states’ medical practice climates, Iowa trumps them all, according to the personal finance website WalletHub.
The Hawkeye State came out on top of WalletHub’s list of the Best States to Practice Medicine for 2017 with 68.7 out of a possible 100 points, while New York finished 51st (Washington, D.C., was 50th) with 28.5 points. Minnesota is the second-best state for physicians, followed by Idaho, Wisconsin, and Kansas. The rest of the bottom five included New Jersey at 49th, Maryland at 48th, and Rhode Island at 47th, WalletHub reported.
WalletHub compared the 50 states and Washington using 14 different metrics across two broad categories: “opportunity and competition” (70 points) and “medical environment” (30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), hospitals per capita, quality of public hospital system, and annual malpractice liability insurance rate.
Vascular involvement may signify worse outcomes in lupus nephritis
MELBOURNE – Vascular involvement in patients with lupus nephritis is associated with poorer outcomes and could be a trigger for a more aggressive treatment approach, according to observational study results reported at an international congress on systemic lupus erythematosus.
Manish Rathi, MD, a nephrologist at the Postgraduate Institution of Medical Education & Research in Chandigarh, India, reported the results of a 5-year prospective cohort study in 241 patients with biopsy-proven lupus nephritis.
Researchers found that patients with vascular involvement had significantly higher serum creatinine at baseline than did those without it. At follow-up, they also had significantly higher proteinuria and serum creatinine, as well as significantly lower serum albumin.
This group was also less likely to achieve complete remission, compared with patients without vascular involvement (38.2% vs. 61.9%; P = .006), and had treatment-refractory disease almost twice as often (26.3% vs. 14.3%; P = .02).
Overall, vascular involvement was seen in 32.3% of patients, with the most common form being arteriosclerosis (22.8%), followed by vascular thrombotic microangiopathy (11.2%), asymptomatic vascular immune deposits (5.3%), vasculopathy (2%), and vasculitis (0.8%).
Three-quarters of all patients had nephrotic syndrome, and 41.9% were identified as Class IV, 18.7% as Class V, 10.4% as Class III, and 3.7% as Class II.
When researchers examined the presentation and outcomes among these subgroups, they found that patients with vascular thrombotic microangiopathy had a significantly higher serum creatinine and were less likely to respond to treatment, compared with patients without vascular thrombotic microangiopathy (60% vs. 79.1%).
Similarly, patients with arteriosclerosis had significantly lower incidence of complete remission, compared with those without arteriosclerosis (37.7% vs. 58.8%) although they had significantly higher rates of partial remission (35.8% vs. 19.4%).
“Lupus patients, if they had involvement of vascular compartment, they had more severe presentation at the time of presentation as well as poorer outcomes despite giving the standard therapy,” Dr. Rathi said.
In an interview, Dr. Rathi said the results had already influenced their own treatment approach with these patients.
“What we have started doing now is – if there is vascular involvement, particularly the thrombotic microangiopathy – we treat them as severe lupus nephritis [patients], so even if their class of lupus nephritis is less severe, we’ll be treating them as severe,” he said.
Commenting on the presentation, Frederic Houssiau, MD, PhD, a professor of rheumatology at the Cliniques Universitaires Saint-Luc in Brussels, said he agreed that vascular involvement was neglected in the current classification of lupus nephritis and that it should be taken into account.
“Maybe we should not only consider the class but also look in more detail to the pathophysiological findings,” Dr. Houssiau said in an interview. “When you have a lot of inflammation in the vessels, for instance, maybe we should use cyclophosphamide.”
No conflicts of interest were disclosed.
MELBOURNE – Vascular involvement in patients with lupus nephritis is associated with poorer outcomes and could be a trigger for a more aggressive treatment approach, according to observational study results reported at an international congress on systemic lupus erythematosus.
Manish Rathi, MD, a nephrologist at the Postgraduate Institution of Medical Education & Research in Chandigarh, India, reported the results of a 5-year prospective cohort study in 241 patients with biopsy-proven lupus nephritis.
Researchers found that patients with vascular involvement had significantly higher serum creatinine at baseline than did those without it. At follow-up, they also had significantly higher proteinuria and serum creatinine, as well as significantly lower serum albumin.
This group was also less likely to achieve complete remission, compared with patients without vascular involvement (38.2% vs. 61.9%; P = .006), and had treatment-refractory disease almost twice as often (26.3% vs. 14.3%; P = .02).
Overall, vascular involvement was seen in 32.3% of patients, with the most common form being arteriosclerosis (22.8%), followed by vascular thrombotic microangiopathy (11.2%), asymptomatic vascular immune deposits (5.3%), vasculopathy (2%), and vasculitis (0.8%).
Three-quarters of all patients had nephrotic syndrome, and 41.9% were identified as Class IV, 18.7% as Class V, 10.4% as Class III, and 3.7% as Class II.
When researchers examined the presentation and outcomes among these subgroups, they found that patients with vascular thrombotic microangiopathy had a significantly higher serum creatinine and were less likely to respond to treatment, compared with patients without vascular thrombotic microangiopathy (60% vs. 79.1%).
Similarly, patients with arteriosclerosis had significantly lower incidence of complete remission, compared with those without arteriosclerosis (37.7% vs. 58.8%) although they had significantly higher rates of partial remission (35.8% vs. 19.4%).
“Lupus patients, if they had involvement of vascular compartment, they had more severe presentation at the time of presentation as well as poorer outcomes despite giving the standard therapy,” Dr. Rathi said.
In an interview, Dr. Rathi said the results had already influenced their own treatment approach with these patients.
“What we have started doing now is – if there is vascular involvement, particularly the thrombotic microangiopathy – we treat them as severe lupus nephritis [patients], so even if their class of lupus nephritis is less severe, we’ll be treating them as severe,” he said.
Commenting on the presentation, Frederic Houssiau, MD, PhD, a professor of rheumatology at the Cliniques Universitaires Saint-Luc in Brussels, said he agreed that vascular involvement was neglected in the current classification of lupus nephritis and that it should be taken into account.
“Maybe we should not only consider the class but also look in more detail to the pathophysiological findings,” Dr. Houssiau said in an interview. “When you have a lot of inflammation in the vessels, for instance, maybe we should use cyclophosphamide.”
No conflicts of interest were disclosed.
MELBOURNE – Vascular involvement in patients with lupus nephritis is associated with poorer outcomes and could be a trigger for a more aggressive treatment approach, according to observational study results reported at an international congress on systemic lupus erythematosus.
Manish Rathi, MD, a nephrologist at the Postgraduate Institution of Medical Education & Research in Chandigarh, India, reported the results of a 5-year prospective cohort study in 241 patients with biopsy-proven lupus nephritis.
Researchers found that patients with vascular involvement had significantly higher serum creatinine at baseline than did those without it. At follow-up, they also had significantly higher proteinuria and serum creatinine, as well as significantly lower serum albumin.
This group was also less likely to achieve complete remission, compared with patients without vascular involvement (38.2% vs. 61.9%; P = .006), and had treatment-refractory disease almost twice as often (26.3% vs. 14.3%; P = .02).
Overall, vascular involvement was seen in 32.3% of patients, with the most common form being arteriosclerosis (22.8%), followed by vascular thrombotic microangiopathy (11.2%), asymptomatic vascular immune deposits (5.3%), vasculopathy (2%), and vasculitis (0.8%).
Three-quarters of all patients had nephrotic syndrome, and 41.9% were identified as Class IV, 18.7% as Class V, 10.4% as Class III, and 3.7% as Class II.
When researchers examined the presentation and outcomes among these subgroups, they found that patients with vascular thrombotic microangiopathy had a significantly higher serum creatinine and were less likely to respond to treatment, compared with patients without vascular thrombotic microangiopathy (60% vs. 79.1%).
Similarly, patients with arteriosclerosis had significantly lower incidence of complete remission, compared with those without arteriosclerosis (37.7% vs. 58.8%) although they had significantly higher rates of partial remission (35.8% vs. 19.4%).
“Lupus patients, if they had involvement of vascular compartment, they had more severe presentation at the time of presentation as well as poorer outcomes despite giving the standard therapy,” Dr. Rathi said.
In an interview, Dr. Rathi said the results had already influenced their own treatment approach with these patients.
“What we have started doing now is – if there is vascular involvement, particularly the thrombotic microangiopathy – we treat them as severe lupus nephritis [patients], so even if their class of lupus nephritis is less severe, we’ll be treating them as severe,” he said.
Commenting on the presentation, Frederic Houssiau, MD, PhD, a professor of rheumatology at the Cliniques Universitaires Saint-Luc in Brussels, said he agreed that vascular involvement was neglected in the current classification of lupus nephritis and that it should be taken into account.
“Maybe we should not only consider the class but also look in more detail to the pathophysiological findings,” Dr. Houssiau said in an interview. “When you have a lot of inflammation in the vessels, for instance, maybe we should use cyclophosphamide.”
No conflicts of interest were disclosed.
AT LUPUS 2017
Key clinical point:
Major finding: Patients with vascular involvement in lupus nephritis are significantly less likely to achieve complete remission and have higher rates of treatment-refractory disease.
Data source: An observational cohort study in 241 patients with biopsy-proven lupus nephritis.
Disclosures: No conflicts of interest were declared.
Associate Fellows: Apply now for ACS Fellowship
Associate Fellows who are interested in pursuing the next level of membership and who meet the criteria for Fellowship are encouraged to start the application process now.
Applications for American College of Surgeons (ACS) Fellowship for induction at the 2018 Clinical Congress in Boston, MA, are due December 1, 2017.
ACS Fellowship is granted to physicians who devote their practice entirely to surgical services and who agree to practice in accordance with the College’s professional and ethical standards.
The College’s Fellowship Pledge and Statements on Principles, found on the ACS website at facs.org, outline the ACS standards of practice. All ACS Fellows and applicants for Fellowship are expected to adhere to these standards.
Surgeons voluntarily submit applications for Fellowship, thereby inviting an evaluation of their practice by their peers. In evaluating the eligibility of Fellowship applicants, the College investigates each applicant’s entire surgical practice. Applicants for Fellowship are required to provide to the appointed committees of the College all information deemed necessary for the investigation and evaluation of their surgical practice.
It is our intention that all Associate Fellows consider applying for Fellowship within the first six years of their surgical practice. To encourage that transition, Associate Fellowship is limited to surgeons who have been in practice less than six years.
Requirements
The basic requirements for Domestic (U.S. and Canada) Fellowship are as follows:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American Osteopathic Association surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital with no reportable action pending
A full list of the domestic requirements can be accessed at facs.org/member-services/join/fellows. The list of requirements for International Fellowship is online at facs.org/member-services/join/international.
Associate Fellows who are current with their membership dues may apply online for free by visiting facs.org/member-services/join and clicking on the link for either Fellow or International Fellow. You will need your log-in information to access the application. If you do not have your log-in information, contact the College’s Member Services staff at 800-293-9623 or via e-mail at [email protected].
The application requests basic information regarding licensure, certification, education, and hospital affiliations. Applicants also are asked to provide the names of five Fellows of the College, preferably from their current practice location, to serve as references. Applicants do not need to request letters of recommendation; simply list the names in your application, and the College staff will contact your references.
If you need assistance finding ACS Fellows in your area, go to facs.org and click on the “Find a Surgeon” button.
When your application is processed, you will receive an e-mail notification providing details about the application timeline along with a request for your surgical case list.
All Fellowship applicants are required to participate in a personal interview by an ACS committee in their local area. Exceptions are made for military applicants. Following the interview, you will receive notification by July 15 of the action taken on your application. Approved applicants are designated as Initiates to be inducted as Fellows during the Convocation Ceremony at the Clinical Congress.
Contact Member Services with questions at any time throughout the application process. We look forward to you becoming a Fellow of the American College of Surgeons.
Associate Fellows who are interested in pursuing the next level of membership and who meet the criteria for Fellowship are encouraged to start the application process now.
Applications for American College of Surgeons (ACS) Fellowship for induction at the 2018 Clinical Congress in Boston, MA, are due December 1, 2017.
ACS Fellowship is granted to physicians who devote their practice entirely to surgical services and who agree to practice in accordance with the College’s professional and ethical standards.
The College’s Fellowship Pledge and Statements on Principles, found on the ACS website at facs.org, outline the ACS standards of practice. All ACS Fellows and applicants for Fellowship are expected to adhere to these standards.
Surgeons voluntarily submit applications for Fellowship, thereby inviting an evaluation of their practice by their peers. In evaluating the eligibility of Fellowship applicants, the College investigates each applicant’s entire surgical practice. Applicants for Fellowship are required to provide to the appointed committees of the College all information deemed necessary for the investigation and evaluation of their surgical practice.
It is our intention that all Associate Fellows consider applying for Fellowship within the first six years of their surgical practice. To encourage that transition, Associate Fellowship is limited to surgeons who have been in practice less than six years.
Requirements
The basic requirements for Domestic (U.S. and Canada) Fellowship are as follows:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American Osteopathic Association surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital with no reportable action pending
A full list of the domestic requirements can be accessed at facs.org/member-services/join/fellows. The list of requirements for International Fellowship is online at facs.org/member-services/join/international.
Associate Fellows who are current with their membership dues may apply online for free by visiting facs.org/member-services/join and clicking on the link for either Fellow or International Fellow. You will need your log-in information to access the application. If you do not have your log-in information, contact the College’s Member Services staff at 800-293-9623 or via e-mail at [email protected].
The application requests basic information regarding licensure, certification, education, and hospital affiliations. Applicants also are asked to provide the names of five Fellows of the College, preferably from their current practice location, to serve as references. Applicants do not need to request letters of recommendation; simply list the names in your application, and the College staff will contact your references.
If you need assistance finding ACS Fellows in your area, go to facs.org and click on the “Find a Surgeon” button.
When your application is processed, you will receive an e-mail notification providing details about the application timeline along with a request for your surgical case list.
All Fellowship applicants are required to participate in a personal interview by an ACS committee in their local area. Exceptions are made for military applicants. Following the interview, you will receive notification by July 15 of the action taken on your application. Approved applicants are designated as Initiates to be inducted as Fellows during the Convocation Ceremony at the Clinical Congress.
Contact Member Services with questions at any time throughout the application process. We look forward to you becoming a Fellow of the American College of Surgeons.
Associate Fellows who are interested in pursuing the next level of membership and who meet the criteria for Fellowship are encouraged to start the application process now.
Applications for American College of Surgeons (ACS) Fellowship for induction at the 2018 Clinical Congress in Boston, MA, are due December 1, 2017.
ACS Fellowship is granted to physicians who devote their practice entirely to surgical services and who agree to practice in accordance with the College’s professional and ethical standards.
The College’s Fellowship Pledge and Statements on Principles, found on the ACS website at facs.org, outline the ACS standards of practice. All ACS Fellows and applicants for Fellowship are expected to adhere to these standards.
Surgeons voluntarily submit applications for Fellowship, thereby inviting an evaluation of their practice by their peers. In evaluating the eligibility of Fellowship applicants, the College investigates each applicant’s entire surgical practice. Applicants for Fellowship are required to provide to the appointed committees of the College all information deemed necessary for the investigation and evaluation of their surgical practice.
It is our intention that all Associate Fellows consider applying for Fellowship within the first six years of their surgical practice. To encourage that transition, Associate Fellowship is limited to surgeons who have been in practice less than six years.
Requirements
The basic requirements for Domestic (U.S. and Canada) Fellowship are as follows:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American Osteopathic Association surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital with no reportable action pending
A full list of the domestic requirements can be accessed at facs.org/member-services/join/fellows. The list of requirements for International Fellowship is online at facs.org/member-services/join/international.
Associate Fellows who are current with their membership dues may apply online for free by visiting facs.org/member-services/join and clicking on the link for either Fellow or International Fellow. You will need your log-in information to access the application. If you do not have your log-in information, contact the College’s Member Services staff at 800-293-9623 or via e-mail at [email protected].
The application requests basic information regarding licensure, certification, education, and hospital affiliations. Applicants also are asked to provide the names of five Fellows of the College, preferably from their current practice location, to serve as references. Applicants do not need to request letters of recommendation; simply list the names in your application, and the College staff will contact your references.
If you need assistance finding ACS Fellows in your area, go to facs.org and click on the “Find a Surgeon” button.
When your application is processed, you will receive an e-mail notification providing details about the application timeline along with a request for your surgical case list.
All Fellowship applicants are required to participate in a personal interview by an ACS committee in their local area. Exceptions are made for military applicants. Following the interview, you will receive notification by July 15 of the action taken on your application. Approved applicants are designated as Initiates to be inducted as Fellows during the Convocation Ceremony at the Clinical Congress.
Contact Member Services with questions at any time throughout the application process. We look forward to you becoming a Fellow of the American College of Surgeons.
Psychiatry Innovation Lab aimed at transforming mental health
“Often, innovation is a product of desperation. I have seen too many of my patients die from opioid overdoses, and I’ve decided to create something that can stop this.”
This is the opening description of an innovative idea that Joseph Insler, MD, an early–career psychiatrist in Boston, pitched to the judges last October.
As one of the judges, this is how I described the item: “It’s like a Fitbit for people addicted to opioids, who are at risk of overdose. But, instead of tracking your footsteps and your sleep movements, it tracks your blood oxygen level, heart rate, and lack of movement. Based on an algorithm tuned to identify signs of an overdose, the Opioid Overdose Recovery Bracelet would give you a shot of medicine in your wrist. If you have accidentally overdosed, it will give you a premeasured dose of naloxone from its reservoir, likely saving your life.”
The goal of the Psychiatry Innovation Lab is to catalyze the formation of innovative ventures to transform mental health. “We nurture early stage ideas and ventures by investing in them with mentorship, education, funding, and collaboration opportunities with our community of mental health innovators,” Dr. Vasan said. At its core, the lab is an interactive exercise in experiential learning, where participants learn how to develop and pitch an entrepreneurial idea and then work together with experts in real time to improve their idea so that they leave with a solid plan for improving mental health. A panel of judges and leaders in innovation collaborate by providing feedback and mentoring. The competition event uses a “Shark Tank” style of winnowing out competitors but is a friendlier format than that of the TV show.
“There’s been a real call to action for using entrepreneurship to change the future, and the Psychiatry Innovation Lab is our answer to that call,” Dr. Vasan said. “We’ve had finalists ranging from high school students to emeritus professors. We’ve seen ideas for [anything from] advancing human rights all the way to using technology to improve access to care.”
Access to mental health and addiction care is one of the driving forces behind a recent wave of investment in behavioral health. There is a lot of interest now in how newer technologies can be leveraged in to improve access, screening, prevention, analytics, and treatments. Younger people coming into the field now have a much shorter path between idea and action. “Think of the lab as a place where people turn their idealism into impact. They learn how to create change that reflects our values: effective, measurable, collaborative, affordable, and sustainable.”
New lab will set records
On May 21, at the APA annual meeting in San Diego, the third Innovation Lab event will take place with record sponsorship and funding. More than $30,000 in prizes will be awarded to winning teams in the following categories: Grand Prize, Audience Choice, Outstanding Progress, Most Promising Innovation, and Most Disruptive Innovation. New this year, the Accelerator Prize will be awarded to the alumni team that has made the most progress since its participation in a previous Innovation Lab. A special prize from Google, worth $20,000, will be given to the innovation that best uses the potential of Cloud services, including Web applications, software, and machine learning.
Also, on May 21, the live Innovation Lab event will begin with the seven finalists giving initial pitches about their innovative ideas for improving mental health care delivery and how psychiatrists are diagnosing, treating, or managing patients. In addition, 10 semifinalists will be selected to deliver rapid pitches. Audience members will then vote from their devices, and the top semifinalist will proceed as a finalist. The event will end with an evening networking session aimed at building community and collaborations among mental health innovators, including clinicians, entrepreneurs, engineers, investors, and patients.
To learn more or watch videos about these innovators, go to www.psychiatryinnovation.com, or search for “APA innovation lab.”
Dr. Daviss is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology.
Psychiatry Innovation Lab alumni
Entrepreneurs from the October 2016 competition created products that addressed addiction, autism, Alzheimer’s, posttraumatic stress disorder, and other mental disorders.
Finalists
- Overdose Recovery Bracelet – “A novel solution to the opioid epidemic” – Joseph Insler
- Spectrum – “An app to encourage facial processing and emotion recognition in autism spectrum disorder” – Swathi Krishna
- Spring – “Enabling personalized behavioral healthcare using machine learning and big data” – April Koh
- Alzhelp – “Using augmented reality and intelligent personal assistant software to keep Alzheimer’s patients safe” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- MiHelper – “Identifying patterns of distress and determining optimal periods for real time mental health interventions” – Kammarauche Isuzu and Mackenzie Drazan
- WEmbrace – “A mobile application for foreign-background psychiatric patients to effectively provide critical care” – Ellen Oh
Semifinalists
- Broadleaf Mental Health –“Reaching school-aged children in the rural eastern Himalayas” – Michael Matergia
- TechLink – “Connecting students and tech” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- Beacon – “Smarter therapy. Together” – Shrenik Jain and Ravi Shah
- Muse – “Assisted meditation in mental health” – Graeme Moffat
- MiResource – “Helping adolescents find the right therapeutic fit” – Gabriela Asturias and Mackenzie Drazen
- BraVe Reality – “Virtual treatment for PTSD patients” – Monica Kullar
- SKNR – “A user-centric psychotherapy tool for the digital age” – Hyun-Hee Kim
- We2Link – “Connect better” – Michael Malone PRISM – “Helping patients gain insight through digital art mobile app” – Kenechi Ejebe and Whitney McFadden
SOURCE: Dr. Daviss
“Often, innovation is a product of desperation. I have seen too many of my patients die from opioid overdoses, and I’ve decided to create something that can stop this.”
This is the opening description of an innovative idea that Joseph Insler, MD, an early–career psychiatrist in Boston, pitched to the judges last October.
As one of the judges, this is how I described the item: “It’s like a Fitbit for people addicted to opioids, who are at risk of overdose. But, instead of tracking your footsteps and your sleep movements, it tracks your blood oxygen level, heart rate, and lack of movement. Based on an algorithm tuned to identify signs of an overdose, the Opioid Overdose Recovery Bracelet would give you a shot of medicine in your wrist. If you have accidentally overdosed, it will give you a premeasured dose of naloxone from its reservoir, likely saving your life.”
The goal of the Psychiatry Innovation Lab is to catalyze the formation of innovative ventures to transform mental health. “We nurture early stage ideas and ventures by investing in them with mentorship, education, funding, and collaboration opportunities with our community of mental health innovators,” Dr. Vasan said. At its core, the lab is an interactive exercise in experiential learning, where participants learn how to develop and pitch an entrepreneurial idea and then work together with experts in real time to improve their idea so that they leave with a solid plan for improving mental health. A panel of judges and leaders in innovation collaborate by providing feedback and mentoring. The competition event uses a “Shark Tank” style of winnowing out competitors but is a friendlier format than that of the TV show.
“There’s been a real call to action for using entrepreneurship to change the future, and the Psychiatry Innovation Lab is our answer to that call,” Dr. Vasan said. “We’ve had finalists ranging from high school students to emeritus professors. We’ve seen ideas for [anything from] advancing human rights all the way to using technology to improve access to care.”
Access to mental health and addiction care is one of the driving forces behind a recent wave of investment in behavioral health. There is a lot of interest now in how newer technologies can be leveraged in to improve access, screening, prevention, analytics, and treatments. Younger people coming into the field now have a much shorter path between idea and action. “Think of the lab as a place where people turn their idealism into impact. They learn how to create change that reflects our values: effective, measurable, collaborative, affordable, and sustainable.”
New lab will set records
On May 21, at the APA annual meeting in San Diego, the third Innovation Lab event will take place with record sponsorship and funding. More than $30,000 in prizes will be awarded to winning teams in the following categories: Grand Prize, Audience Choice, Outstanding Progress, Most Promising Innovation, and Most Disruptive Innovation. New this year, the Accelerator Prize will be awarded to the alumni team that has made the most progress since its participation in a previous Innovation Lab. A special prize from Google, worth $20,000, will be given to the innovation that best uses the potential of Cloud services, including Web applications, software, and machine learning.
Also, on May 21, the live Innovation Lab event will begin with the seven finalists giving initial pitches about their innovative ideas for improving mental health care delivery and how psychiatrists are diagnosing, treating, or managing patients. In addition, 10 semifinalists will be selected to deliver rapid pitches. Audience members will then vote from their devices, and the top semifinalist will proceed as a finalist. The event will end with an evening networking session aimed at building community and collaborations among mental health innovators, including clinicians, entrepreneurs, engineers, investors, and patients.
To learn more or watch videos about these innovators, go to www.psychiatryinnovation.com, or search for “APA innovation lab.”
Dr. Daviss is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology.
Psychiatry Innovation Lab alumni
Entrepreneurs from the October 2016 competition created products that addressed addiction, autism, Alzheimer’s, posttraumatic stress disorder, and other mental disorders.
Finalists
- Overdose Recovery Bracelet – “A novel solution to the opioid epidemic” – Joseph Insler
- Spectrum – “An app to encourage facial processing and emotion recognition in autism spectrum disorder” – Swathi Krishna
- Spring – “Enabling personalized behavioral healthcare using machine learning and big data” – April Koh
- Alzhelp – “Using augmented reality and intelligent personal assistant software to keep Alzheimer’s patients safe” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- MiHelper – “Identifying patterns of distress and determining optimal periods for real time mental health interventions” – Kammarauche Isuzu and Mackenzie Drazan
- WEmbrace – “A mobile application for foreign-background psychiatric patients to effectively provide critical care” – Ellen Oh
Semifinalists
- Broadleaf Mental Health –“Reaching school-aged children in the rural eastern Himalayas” – Michael Matergia
- TechLink – “Connecting students and tech” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- Beacon – “Smarter therapy. Together” – Shrenik Jain and Ravi Shah
- Muse – “Assisted meditation in mental health” – Graeme Moffat
- MiResource – “Helping adolescents find the right therapeutic fit” – Gabriela Asturias and Mackenzie Drazen
- BraVe Reality – “Virtual treatment for PTSD patients” – Monica Kullar
- SKNR – “A user-centric psychotherapy tool for the digital age” – Hyun-Hee Kim
- We2Link – “Connect better” – Michael Malone PRISM – “Helping patients gain insight through digital art mobile app” – Kenechi Ejebe and Whitney McFadden
SOURCE: Dr. Daviss
“Often, innovation is a product of desperation. I have seen too many of my patients die from opioid overdoses, and I’ve decided to create something that can stop this.”
This is the opening description of an innovative idea that Joseph Insler, MD, an early–career psychiatrist in Boston, pitched to the judges last October.
As one of the judges, this is how I described the item: “It’s like a Fitbit for people addicted to opioids, who are at risk of overdose. But, instead of tracking your footsteps and your sleep movements, it tracks your blood oxygen level, heart rate, and lack of movement. Based on an algorithm tuned to identify signs of an overdose, the Opioid Overdose Recovery Bracelet would give you a shot of medicine in your wrist. If you have accidentally overdosed, it will give you a premeasured dose of naloxone from its reservoir, likely saving your life.”
The goal of the Psychiatry Innovation Lab is to catalyze the formation of innovative ventures to transform mental health. “We nurture early stage ideas and ventures by investing in them with mentorship, education, funding, and collaboration opportunities with our community of mental health innovators,” Dr. Vasan said. At its core, the lab is an interactive exercise in experiential learning, where participants learn how to develop and pitch an entrepreneurial idea and then work together with experts in real time to improve their idea so that they leave with a solid plan for improving mental health. A panel of judges and leaders in innovation collaborate by providing feedback and mentoring. The competition event uses a “Shark Tank” style of winnowing out competitors but is a friendlier format than that of the TV show.
“There’s been a real call to action for using entrepreneurship to change the future, and the Psychiatry Innovation Lab is our answer to that call,” Dr. Vasan said. “We’ve had finalists ranging from high school students to emeritus professors. We’ve seen ideas for [anything from] advancing human rights all the way to using technology to improve access to care.”
Access to mental health and addiction care is one of the driving forces behind a recent wave of investment in behavioral health. There is a lot of interest now in how newer technologies can be leveraged in to improve access, screening, prevention, analytics, and treatments. Younger people coming into the field now have a much shorter path between idea and action. “Think of the lab as a place where people turn their idealism into impact. They learn how to create change that reflects our values: effective, measurable, collaborative, affordable, and sustainable.”
New lab will set records
On May 21, at the APA annual meeting in San Diego, the third Innovation Lab event will take place with record sponsorship and funding. More than $30,000 in prizes will be awarded to winning teams in the following categories: Grand Prize, Audience Choice, Outstanding Progress, Most Promising Innovation, and Most Disruptive Innovation. New this year, the Accelerator Prize will be awarded to the alumni team that has made the most progress since its participation in a previous Innovation Lab. A special prize from Google, worth $20,000, will be given to the innovation that best uses the potential of Cloud services, including Web applications, software, and machine learning.
Also, on May 21, the live Innovation Lab event will begin with the seven finalists giving initial pitches about their innovative ideas for improving mental health care delivery and how psychiatrists are diagnosing, treating, or managing patients. In addition, 10 semifinalists will be selected to deliver rapid pitches. Audience members will then vote from their devices, and the top semifinalist will proceed as a finalist. The event will end with an evening networking session aimed at building community and collaborations among mental health innovators, including clinicians, entrepreneurs, engineers, investors, and patients.
To learn more or watch videos about these innovators, go to www.psychiatryinnovation.com, or search for “APA innovation lab.”
Dr. Daviss is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology.
Psychiatry Innovation Lab alumni
Entrepreneurs from the October 2016 competition created products that addressed addiction, autism, Alzheimer’s, posttraumatic stress disorder, and other mental disorders.
Finalists
- Overdose Recovery Bracelet – “A novel solution to the opioid epidemic” – Joseph Insler
- Spectrum – “An app to encourage facial processing and emotion recognition in autism spectrum disorder” – Swathi Krishna
- Spring – “Enabling personalized behavioral healthcare using machine learning and big data” – April Koh
- Alzhelp – “Using augmented reality and intelligent personal assistant software to keep Alzheimer’s patients safe” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- MiHelper – “Identifying patterns of distress and determining optimal periods for real time mental health interventions” – Kammarauche Isuzu and Mackenzie Drazan
- WEmbrace – “A mobile application for foreign-background psychiatric patients to effectively provide critical care” – Ellen Oh
Semifinalists
- Broadleaf Mental Health –“Reaching school-aged children in the rural eastern Himalayas” – Michael Matergia
- TechLink – “Connecting students and tech” – Akanksha Jain, Michelle Koh, and Priscilla Siow
- Beacon – “Smarter therapy. Together” – Shrenik Jain and Ravi Shah
- Muse – “Assisted meditation in mental health” – Graeme Moffat
- MiResource – “Helping adolescents find the right therapeutic fit” – Gabriela Asturias and Mackenzie Drazen
- BraVe Reality – “Virtual treatment for PTSD patients” – Monica Kullar
- SKNR – “A user-centric psychotherapy tool for the digital age” – Hyun-Hee Kim
- We2Link – “Connect better” – Michael Malone PRISM – “Helping patients gain insight through digital art mobile app” – Kenechi Ejebe and Whitney McFadden
SOURCE: Dr. Daviss
Outpatient Visits Involving CNS Polypharmacy Rising Among Elderly
The number of outpatient visits involving CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, according to research published online ahead of print February 13 in JAMA Internal Medicine.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” said Donovan T. Maust, MD, Assistant Professor of Geriatric Psychiatry at the University of Michigan in Ann Arbor. “Opioids have recently been included in a Beers measure of CNS polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the US Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants,” he said.
Dr. Maust and his colleagues analyzed data on 97,910 outpatients age 65 and older from the National Ambulatory Medical Care Survey (NAMCS) from 2004 through 2013. Patients met Beers CNS polypharmacy criteria if three or more of the following medications were initiated or continued: antipsychotics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonists, tricyclic antidepressants, selective serotonin reuptake inhibitors, and opioids. The researchers recorded as many as three visit diagnoses and included information collected from NAMCS such as chronic medical conditions, whether psychotherapy was provided or ordered, whether stress management or other mental health counseling services were provided or ordered, and time spent with physician.
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults age 65 or older increased from 1.50 million in 2004 to 3.68 million in 2013, or from 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio [AOR], 3.12). The largest increases were observed among rural visits and among visits with no mental health or pain diagnoses (AOR, 4.99 and 2.65, respectively).
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by individuals who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
—Doug Brunk
Suggested Reading
Maust DT, Gerlach LB, Gibson A, et al. Trends in central nervous system-active polypharmacy among older adults seen in outpatient care in the United States. JAMA Intern Med. 2017 Feb 13 [Epub ahead of print].
The number of outpatient visits involving CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, according to research published online ahead of print February 13 in JAMA Internal Medicine.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” said Donovan T. Maust, MD, Assistant Professor of Geriatric Psychiatry at the University of Michigan in Ann Arbor. “Opioids have recently been included in a Beers measure of CNS polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the US Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants,” he said.
Dr. Maust and his colleagues analyzed data on 97,910 outpatients age 65 and older from the National Ambulatory Medical Care Survey (NAMCS) from 2004 through 2013. Patients met Beers CNS polypharmacy criteria if three or more of the following medications were initiated or continued: antipsychotics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonists, tricyclic antidepressants, selective serotonin reuptake inhibitors, and opioids. The researchers recorded as many as three visit diagnoses and included information collected from NAMCS such as chronic medical conditions, whether psychotherapy was provided or ordered, whether stress management or other mental health counseling services were provided or ordered, and time spent with physician.
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults age 65 or older increased from 1.50 million in 2004 to 3.68 million in 2013, or from 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio [AOR], 3.12). The largest increases were observed among rural visits and among visits with no mental health or pain diagnoses (AOR, 4.99 and 2.65, respectively).
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by individuals who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
—Doug Brunk
Suggested Reading
Maust DT, Gerlach LB, Gibson A, et al. Trends in central nervous system-active polypharmacy among older adults seen in outpatient care in the United States. JAMA Intern Med. 2017 Feb 13 [Epub ahead of print].
The number of outpatient visits involving CNS polypharmacy by adults aged 65 and older more than doubled between 2004 and 2013, especially among those who reside in rural areas, according to research published online ahead of print February 13 in JAMA Internal Medicine.
“With each new revision of the Beers Criteria, the list of psychotropic medications considered potentially inappropriate in the elderly has grown,” said Donovan T. Maust, MD, Assistant Professor of Geriatric Psychiatry at the University of Michigan in Ann Arbor. “Opioids have recently been included in a Beers measure of CNS polypharmacy. Prescribing related drug combinations also received increased regulatory attention when the US Food and Drug Administration recently ordered a black box warning to alert patients of serious risks, including death, caused by opioids coprescribed with CNS depressants,” he said.
Dr. Maust and his colleagues analyzed data on 97,910 outpatients age 65 and older from the National Ambulatory Medical Care Survey (NAMCS) from 2004 through 2013. Patients met Beers CNS polypharmacy criteria if three or more of the following medications were initiated or continued: antipsychotics, benzodiazepines, nonbenzodiazepine benzodiazepine receptor agonists, tricyclic antidepressants, selective serotonin reuptake inhibitors, and opioids. The researchers recorded as many as three visit diagnoses and included information collected from NAMCS such as chronic medical conditions, whether psychotherapy was provided or ordered, whether stress management or other mental health counseling services were provided or ordered, and time spent with physician.
Dr. Maust and his associates found that annual CNS polypharmacy visits by adults age 65 or older increased from 1.50 million in 2004 to 3.68 million in 2013, or from 0.6% of visits in 2004 to 1.4% in 2013 (adjusted odds ratio [AOR], 3.12). The largest increases were observed among rural visits and among visits with no mental health or pain diagnoses (AOR, 4.99 and 2.65, respectively).
More than two-thirds of polypharmacy visits (68%) were by women, and 17% were by individuals who lived in rural areas. In addition, nearly half of polypharmacy visits studied (46%) included neither mental health nor pain diagnoses. No significant demographic differences were observed between polypharmacy visits with and without opioids. “Older adults have become more open to mental health treatment,” the researchers concluded. “Because of limited access to specialty care and a preference to receive treatment in primary care settings, it is unsurprising that mental health treatment has expanded in nonpsychiatric settings.”
—Doug Brunk
Suggested Reading
Maust DT, Gerlach LB, Gibson A, et al. Trends in central nervous system-active polypharmacy among older adults seen in outpatient care in the United States. JAMA Intern Med. 2017 Feb 13 [Epub ahead of print].
Rate of heroin use in U.S. soars, especially among white individuals
Rates of heroin use and heroin use disorder rose dramatically between 2001-2002 and 2012-2013, and the trend was greatest among the white population. The rise among white individuals could be tied to the opioid epidemic, because nonmedical opioid also rose disproportionately in that group, according to research published online March 29.
The findings come from an analysis of 43,093 people who responded to the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), and 36,309 respondents to the 2012-2013 NESARC-III.
In addition, Dr. Martins and her associates found a significant rise in the number of white heroin users who had started nonmedical prescription opioid (NMPO) use before heroin (35.83% to 52.83%; P =.01). In contrast, the percentage of nonwhite individuals who started off with NMPO use dropped from 44.12% to 26.20% (P = .04).
The increase in heroin use was larger among individuals at less than 100% of the poverty level (0.44% to 2.42%; P less than .001), as well as among people with education levels of less than high school (heroin use, 0.41% to 2.01%; P = .03; heroin use disorder, 0.24% to 0.87%; P = .08) and among those with no more than high school education (heroin use, 0.39% to 2.15%; P =.003; heroin use disorder, 0.29% to 1.11%; P = .003). The absolute values of the findings may be inexact, because the methods of the two surveys differed slightly. In addition, the investigators did not include homeless and incarcerated individuals.
Based on their analysis, Dr. Martins and her associates offered strategies aimed at addressing the crisis. “To curb the heroin epidemic, particularly among younger adults, collective prevention and intervention efforts may be most effective,” they wrote. “Promising examples include expansion of access to medication-assisted treatment (including methadone hydrochloride, buprenorphine hydrochloride, or injectable naltrexone hydrochloride), educational programs in schools and community settings, overdose prevention training in concert with comprehensive naloxone hydrochloride distribution programs, and consistent use of prescription drug–monitoring programs that implement best practices by prescribers.”
NESARC and NESARC-III were funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The authors received funding from several sources, including the National Institute on Drug Abuse, the New York State Psychiatric Institute, and the J. William Fulbright and the Colciencias doctoral scholarships. One of the study authors, Deborah S. Hasin, PhD, was a principal investigator on a study that was funded by InVentiv Health Consulting, which pool funds from nine pharmaceutical companies.
Opioid misuse can be prevented by the medical community with a change in prescribing practices aimed at limiting the supply of prescription opioids, Bertha K. Madras, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Mar 29. doi: 10.1001/jamapsychiatry.2017.0163). Also, medical training “should include awareness of the risks posed by high opioid doses, immediate-release formulations, use combined with alcohol and/or benzodiazepines, history of overdoses, and other factors,” she wrote.
The United States has more than 14,000 drug treatment programs, but many are staffed with clinicians who are not licensed. One way to foster comprehensive services would be to develop an integrated medical and behavioral treatment system that would be supervised by a physician and substance abuse specialist. “Resources, training, and workforce issues are a concern, but the benefits of integrated health care and behavioral treatment conceivably outweigh the risks of maintaining the status quo,” she wrote.
Dr. Madras is affiliated with the department of psychiatry at Harvard Medical School in Boston, and McLean Hospital in Belmont, Mass. She also serves on the scientific advisory board of RiverMend Health and consults for Guidepoint.
Opioid misuse can be prevented by the medical community with a change in prescribing practices aimed at limiting the supply of prescription opioids, Bertha K. Madras, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Mar 29. doi: 10.1001/jamapsychiatry.2017.0163). Also, medical training “should include awareness of the risks posed by high opioid doses, immediate-release formulations, use combined with alcohol and/or benzodiazepines, history of overdoses, and other factors,” she wrote.
The United States has more than 14,000 drug treatment programs, but many are staffed with clinicians who are not licensed. One way to foster comprehensive services would be to develop an integrated medical and behavioral treatment system that would be supervised by a physician and substance abuse specialist. “Resources, training, and workforce issues are a concern, but the benefits of integrated health care and behavioral treatment conceivably outweigh the risks of maintaining the status quo,” she wrote.
Dr. Madras is affiliated with the department of psychiatry at Harvard Medical School in Boston, and McLean Hospital in Belmont, Mass. She also serves on the scientific advisory board of RiverMend Health and consults for Guidepoint.
Opioid misuse can be prevented by the medical community with a change in prescribing practices aimed at limiting the supply of prescription opioids, Bertha K. Madras, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Mar 29. doi: 10.1001/jamapsychiatry.2017.0163). Also, medical training “should include awareness of the risks posed by high opioid doses, immediate-release formulations, use combined with alcohol and/or benzodiazepines, history of overdoses, and other factors,” she wrote.
The United States has more than 14,000 drug treatment programs, but many are staffed with clinicians who are not licensed. One way to foster comprehensive services would be to develop an integrated medical and behavioral treatment system that would be supervised by a physician and substance abuse specialist. “Resources, training, and workforce issues are a concern, but the benefits of integrated health care and behavioral treatment conceivably outweigh the risks of maintaining the status quo,” she wrote.
Dr. Madras is affiliated with the department of psychiatry at Harvard Medical School in Boston, and McLean Hospital in Belmont, Mass. She also serves on the scientific advisory board of RiverMend Health and consults for Guidepoint.
Rates of heroin use and heroin use disorder rose dramatically between 2001-2002 and 2012-2013, and the trend was greatest among the white population. The rise among white individuals could be tied to the opioid epidemic, because nonmedical opioid also rose disproportionately in that group, according to research published online March 29.
The findings come from an analysis of 43,093 people who responded to the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), and 36,309 respondents to the 2012-2013 NESARC-III.
In addition, Dr. Martins and her associates found a significant rise in the number of white heroin users who had started nonmedical prescription opioid (NMPO) use before heroin (35.83% to 52.83%; P =.01). In contrast, the percentage of nonwhite individuals who started off with NMPO use dropped from 44.12% to 26.20% (P = .04).
The increase in heroin use was larger among individuals at less than 100% of the poverty level (0.44% to 2.42%; P less than .001), as well as among people with education levels of less than high school (heroin use, 0.41% to 2.01%; P = .03; heroin use disorder, 0.24% to 0.87%; P = .08) and among those with no more than high school education (heroin use, 0.39% to 2.15%; P =.003; heroin use disorder, 0.29% to 1.11%; P = .003). The absolute values of the findings may be inexact, because the methods of the two surveys differed slightly. In addition, the investigators did not include homeless and incarcerated individuals.
Based on their analysis, Dr. Martins and her associates offered strategies aimed at addressing the crisis. “To curb the heroin epidemic, particularly among younger adults, collective prevention and intervention efforts may be most effective,” they wrote. “Promising examples include expansion of access to medication-assisted treatment (including methadone hydrochloride, buprenorphine hydrochloride, or injectable naltrexone hydrochloride), educational programs in schools and community settings, overdose prevention training in concert with comprehensive naloxone hydrochloride distribution programs, and consistent use of prescription drug–monitoring programs that implement best practices by prescribers.”
NESARC and NESARC-III were funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The authors received funding from several sources, including the National Institute on Drug Abuse, the New York State Psychiatric Institute, and the J. William Fulbright and the Colciencias doctoral scholarships. One of the study authors, Deborah S. Hasin, PhD, was a principal investigator on a study that was funded by InVentiv Health Consulting, which pool funds from nine pharmaceutical companies.
Rates of heroin use and heroin use disorder rose dramatically between 2001-2002 and 2012-2013, and the trend was greatest among the white population. The rise among white individuals could be tied to the opioid epidemic, because nonmedical opioid also rose disproportionately in that group, according to research published online March 29.
The findings come from an analysis of 43,093 people who responded to the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), and 36,309 respondents to the 2012-2013 NESARC-III.
In addition, Dr. Martins and her associates found a significant rise in the number of white heroin users who had started nonmedical prescription opioid (NMPO) use before heroin (35.83% to 52.83%; P =.01). In contrast, the percentage of nonwhite individuals who started off with NMPO use dropped from 44.12% to 26.20% (P = .04).
The increase in heroin use was larger among individuals at less than 100% of the poverty level (0.44% to 2.42%; P less than .001), as well as among people with education levels of less than high school (heroin use, 0.41% to 2.01%; P = .03; heroin use disorder, 0.24% to 0.87%; P = .08) and among those with no more than high school education (heroin use, 0.39% to 2.15%; P =.003; heroin use disorder, 0.29% to 1.11%; P = .003). The absolute values of the findings may be inexact, because the methods of the two surveys differed slightly. In addition, the investigators did not include homeless and incarcerated individuals.
Based on their analysis, Dr. Martins and her associates offered strategies aimed at addressing the crisis. “To curb the heroin epidemic, particularly among younger adults, collective prevention and intervention efforts may be most effective,” they wrote. “Promising examples include expansion of access to medication-assisted treatment (including methadone hydrochloride, buprenorphine hydrochloride, or injectable naltrexone hydrochloride), educational programs in schools and community settings, overdose prevention training in concert with comprehensive naloxone hydrochloride distribution programs, and consistent use of prescription drug–monitoring programs that implement best practices by prescribers.”
NESARC and NESARC-III were funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The authors received funding from several sources, including the National Institute on Drug Abuse, the New York State Psychiatric Institute, and the J. William Fulbright and the Colciencias doctoral scholarships. One of the study authors, Deborah S. Hasin, PhD, was a principal investigator on a study that was funded by InVentiv Health Consulting, which pool funds from nine pharmaceutical companies.
FROM JAMA PSYCHIATRY
Key clinical point: Campaigns are needed to educate the public about harms tied to heroin use, and access should be expanded to populations at risk for both heroin use and heroin use disorder.
Major finding: Rates of lifetime heroin use rose from 0.33% to 1.61%.
Data source: Retrospective analysis of 43,093 respondents to the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), and 36,309 respondents to the 2012-2013 NESARC-III.
Disclosures: NESARC and NESARC-III were funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The authors received funding from several sources, including the National Institute on Drug Abuse, the New York State Psychiatric Institute, and the J. William Fulbright and the Colciencias doctoral scholarships. One of the study authors, Deborah S. Hasin, PhD, was a principal investigator on a study that was funded by InVentiv Health Consulting, which pools funds from nine pharmaceutical companies.