User login
Can Probiotics Help Treat MS?
ORLANDO—Probiotic treatment is associated with the induction of an anti-inflammatory peripheral immune profile in patients with multiple sclerosis (MS), and discontinuing probiotics is associated with a decrease in immune regulatory cells, according to pilot study results presented at the ACTRIMS 2017 Forum. The results suggest that probiotics may be applicable as a therapy for MS. More studies, including controlled clinical trials, are needed to guide treatment decisions, said Howard L. Weiner, MD, Director of the MS Program at Brigham and Women’s Hospital in Newton, Massachusetts, and Robert L. Kroc Professor of Neurology at Harvard Medical School in Boston.
The gut microbiome can modulate neuroimmune function, and studies have found that patients with MS have alterations in the gut microbiome, compared with healthy controls. These findings have led researchers to ask, “Can you treat MS by modulating the microbiome?” Dr. Weiner said. One way to modulate the microbiome is with probiotics, which can be taken as an oral, nontoxic treatment in combination with current therapies. He and his research colleagues conducted a pilot study to investigate the effect of probiotics in patients with MS. “We did not feel that we could do a clinically related study,” he said. Instead, the investigators focused on what happens to the gut microbiome and immune profiles in the blood of patients who receive probiotics.
Two Months of Treatment
The study included nine patients with MS and 13 controls. The investigators gave study participants the probiotic VSL#3. Some formulations of VSL#3 are available in stores. The company that markets the probiotic, Sigma-Tau HealthScience USA, based in Gaithersburg, Maryland, requires a prescription for VSL#3 DS, the double-strength formulation used in the study.
Prior studies of the probiotic have found that VSL#3 provides benefit in animal models of diabetes, colitis, and allergy. Kigerl et al reported that, in mice with spinal cord injury, dysbiosis induced by antibiotics exacerbates neurologic impairment and spinal cord pathology, whereas feeding mice VSL#3 enriched with lactic-acid-producing bacteria triggers a protective immune response, confers neuroprotection, and improves walking. In addition, some doctors prescribe VSL#3 to patients with ulcerative colitis, irritable bowel syndrome, and pouchitis, Dr. Weiner said.
In the pilot study, participants took VSL#3 DS sachets twice daily for two months. Researchers took blood and stool samples from participants before administering the probiotic, at discontinuation of therapy, and three months thereafter.
VSL#3 had no effect on alpha or phylogenetic diversity, but did change the gut microbiome composition. Researchers observed an increase in the relative abundance of specific organisms contained in the probiotic. Methanogens, which are increased in MS, decreased when patients received the probiotic. Administration of VSL#3 also was associated with a decrease in proinflammatory monocytes and decreased expression of activation markers on monocytes and dendritic cells.
When patients stopped probiotic treatment, the numbers of CD39 and IL10 T regulatory cells decreased, and proinflammatory monocytes increased. “We did see changes when you stop treatment, almost as if there is a rebound,” he said. “We are seeing an immune effect in the blood.”
The treatment was well tolerated. “I myself took some,” Dr. Weiner said. “It tastes a little chalky, but otherwise it is fine.” Some patients said they felt better after taking the probiotic. The study was not placebo-controlled, however. Future randomized clinical studies will include MRI scans, he said.
What should neurologists tell patients who want to take a probiotic and ask which probiotic they should take? “We are all asked those questions,” Dr. Weiner said. “I tell them, we really don’t know, but if you would like to take a probiotic, that is fine. But we have no particular recommendations at this time. We need a lot of science before we know what we are doing, as far as treatment, in this regard.”
Changes in the Gut Microbiome in MS
Prior to conducting the pilot study, Sushrut Jangi, MD, research fellow at Brigham and Women’s Hospital, Dr. Weiner, and colleagues studied the gut microbiome in 60 patients with MS and 43 healthy controls. They found that patients with MS had increases in Methanobrevibacter and Akkermansia and decreases in Butyricimonas, compared with controls. Methanobrevibacter produces methane, and in another cohort, the researchers detected more methane in the breath of patients with MS than in controls.
In addition, Prevotella and Sutterella were increased, and Sarcina was decreased in patients with MS who were treated with disease-modifying therapy, compared with untreated patients with MS. “It gives us a whole series of targets to try to understand,” he said. The gut microbiome alterations in patients with MS correlated with variations in the expression of genes in circulating T cells and monocytes that have been implicated in MS pathogenesis.
How Are the Gut Microbiome and MS Related?
The nature of the relationship between the gut microbiome and MS is unclear. One possibility is that “the gut could be the key to MS,” Dr. Weiner said. An organism in the gut may trigger MS, and that organism potentially could be altered or used in vaccinations to treat or prevent the disease. Alternatively, the gut microbiome might relate to MS susceptibility. Environmental factors, including diet and antibiotics, could affect the microbiome and MS risk. It is also possible that MS disease-modifying therapies act in part through the gut, Dr. Weiner said.
Various treatments targeting the microbiome are under investigation. For example, researchers are asking whether a particular bacteria or fecal microbiome transplants might help treat MS. “I am very interested in the concept of oral tolerance, where we trigger immune responses in the mucosa,” said Dr. Weiner. “We are beginning a trial of anti-CD3 [monoclonal antibodies], which will be given mucosally to stimulate the gut immune response.”
—Jake Remaly
Suggested Reading
Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015.
Kigerl KA, Hall JC, Wang L, et al. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213(12):2603-2620.
Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2016 Sep 16 [Epub ahead of print].
Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429.
Tremlett H, Waubant E. The multiple sclerosis microbiome? Ann Transl Med. 2017;5(3):53.
ORLANDO—Probiotic treatment is associated with the induction of an anti-inflammatory peripheral immune profile in patients with multiple sclerosis (MS), and discontinuing probiotics is associated with a decrease in immune regulatory cells, according to pilot study results presented at the ACTRIMS 2017 Forum. The results suggest that probiotics may be applicable as a therapy for MS. More studies, including controlled clinical trials, are needed to guide treatment decisions, said Howard L. Weiner, MD, Director of the MS Program at Brigham and Women’s Hospital in Newton, Massachusetts, and Robert L. Kroc Professor of Neurology at Harvard Medical School in Boston.
The gut microbiome can modulate neuroimmune function, and studies have found that patients with MS have alterations in the gut microbiome, compared with healthy controls. These findings have led researchers to ask, “Can you treat MS by modulating the microbiome?” Dr. Weiner said. One way to modulate the microbiome is with probiotics, which can be taken as an oral, nontoxic treatment in combination with current therapies. He and his research colleagues conducted a pilot study to investigate the effect of probiotics in patients with MS. “We did not feel that we could do a clinically related study,” he said. Instead, the investigators focused on what happens to the gut microbiome and immune profiles in the blood of patients who receive probiotics.
Two Months of Treatment
The study included nine patients with MS and 13 controls. The investigators gave study participants the probiotic VSL#3. Some formulations of VSL#3 are available in stores. The company that markets the probiotic, Sigma-Tau HealthScience USA, based in Gaithersburg, Maryland, requires a prescription for VSL#3 DS, the double-strength formulation used in the study.
Prior studies of the probiotic have found that VSL#3 provides benefit in animal models of diabetes, colitis, and allergy. Kigerl et al reported that, in mice with spinal cord injury, dysbiosis induced by antibiotics exacerbates neurologic impairment and spinal cord pathology, whereas feeding mice VSL#3 enriched with lactic-acid-producing bacteria triggers a protective immune response, confers neuroprotection, and improves walking. In addition, some doctors prescribe VSL#3 to patients with ulcerative colitis, irritable bowel syndrome, and pouchitis, Dr. Weiner said.
In the pilot study, participants took VSL#3 DS sachets twice daily for two months. Researchers took blood and stool samples from participants before administering the probiotic, at discontinuation of therapy, and three months thereafter.
VSL#3 had no effect on alpha or phylogenetic diversity, but did change the gut microbiome composition. Researchers observed an increase in the relative abundance of specific organisms contained in the probiotic. Methanogens, which are increased in MS, decreased when patients received the probiotic. Administration of VSL#3 also was associated with a decrease in proinflammatory monocytes and decreased expression of activation markers on monocytes and dendritic cells.
When patients stopped probiotic treatment, the numbers of CD39 and IL10 T regulatory cells decreased, and proinflammatory monocytes increased. “We did see changes when you stop treatment, almost as if there is a rebound,” he said. “We are seeing an immune effect in the blood.”
The treatment was well tolerated. “I myself took some,” Dr. Weiner said. “It tastes a little chalky, but otherwise it is fine.” Some patients said they felt better after taking the probiotic. The study was not placebo-controlled, however. Future randomized clinical studies will include MRI scans, he said.
What should neurologists tell patients who want to take a probiotic and ask which probiotic they should take? “We are all asked those questions,” Dr. Weiner said. “I tell them, we really don’t know, but if you would like to take a probiotic, that is fine. But we have no particular recommendations at this time. We need a lot of science before we know what we are doing, as far as treatment, in this regard.”
Changes in the Gut Microbiome in MS
Prior to conducting the pilot study, Sushrut Jangi, MD, research fellow at Brigham and Women’s Hospital, Dr. Weiner, and colleagues studied the gut microbiome in 60 patients with MS and 43 healthy controls. They found that patients with MS had increases in Methanobrevibacter and Akkermansia and decreases in Butyricimonas, compared with controls. Methanobrevibacter produces methane, and in another cohort, the researchers detected more methane in the breath of patients with MS than in controls.
In addition, Prevotella and Sutterella were increased, and Sarcina was decreased in patients with MS who were treated with disease-modifying therapy, compared with untreated patients with MS. “It gives us a whole series of targets to try to understand,” he said. The gut microbiome alterations in patients with MS correlated with variations in the expression of genes in circulating T cells and monocytes that have been implicated in MS pathogenesis.
How Are the Gut Microbiome and MS Related?
The nature of the relationship between the gut microbiome and MS is unclear. One possibility is that “the gut could be the key to MS,” Dr. Weiner said. An organism in the gut may trigger MS, and that organism potentially could be altered or used in vaccinations to treat or prevent the disease. Alternatively, the gut microbiome might relate to MS susceptibility. Environmental factors, including diet and antibiotics, could affect the microbiome and MS risk. It is also possible that MS disease-modifying therapies act in part through the gut, Dr. Weiner said.
Various treatments targeting the microbiome are under investigation. For example, researchers are asking whether a particular bacteria or fecal microbiome transplants might help treat MS. “I am very interested in the concept of oral tolerance, where we trigger immune responses in the mucosa,” said Dr. Weiner. “We are beginning a trial of anti-CD3 [monoclonal antibodies], which will be given mucosally to stimulate the gut immune response.”
—Jake Remaly
Suggested Reading
Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015.
Kigerl KA, Hall JC, Wang L, et al. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213(12):2603-2620.
Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2016 Sep 16 [Epub ahead of print].
Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429.
Tremlett H, Waubant E. The multiple sclerosis microbiome? Ann Transl Med. 2017;5(3):53.
ORLANDO—Probiotic treatment is associated with the induction of an anti-inflammatory peripheral immune profile in patients with multiple sclerosis (MS), and discontinuing probiotics is associated with a decrease in immune regulatory cells, according to pilot study results presented at the ACTRIMS 2017 Forum. The results suggest that probiotics may be applicable as a therapy for MS. More studies, including controlled clinical trials, are needed to guide treatment decisions, said Howard L. Weiner, MD, Director of the MS Program at Brigham and Women’s Hospital in Newton, Massachusetts, and Robert L. Kroc Professor of Neurology at Harvard Medical School in Boston.
The gut microbiome can modulate neuroimmune function, and studies have found that patients with MS have alterations in the gut microbiome, compared with healthy controls. These findings have led researchers to ask, “Can you treat MS by modulating the microbiome?” Dr. Weiner said. One way to modulate the microbiome is with probiotics, which can be taken as an oral, nontoxic treatment in combination with current therapies. He and his research colleagues conducted a pilot study to investigate the effect of probiotics in patients with MS. “We did not feel that we could do a clinically related study,” he said. Instead, the investigators focused on what happens to the gut microbiome and immune profiles in the blood of patients who receive probiotics.
Two Months of Treatment
The study included nine patients with MS and 13 controls. The investigators gave study participants the probiotic VSL#3. Some formulations of VSL#3 are available in stores. The company that markets the probiotic, Sigma-Tau HealthScience USA, based in Gaithersburg, Maryland, requires a prescription for VSL#3 DS, the double-strength formulation used in the study.
Prior studies of the probiotic have found that VSL#3 provides benefit in animal models of diabetes, colitis, and allergy. Kigerl et al reported that, in mice with spinal cord injury, dysbiosis induced by antibiotics exacerbates neurologic impairment and spinal cord pathology, whereas feeding mice VSL#3 enriched with lactic-acid-producing bacteria triggers a protective immune response, confers neuroprotection, and improves walking. In addition, some doctors prescribe VSL#3 to patients with ulcerative colitis, irritable bowel syndrome, and pouchitis, Dr. Weiner said.
In the pilot study, participants took VSL#3 DS sachets twice daily for two months. Researchers took blood and stool samples from participants before administering the probiotic, at discontinuation of therapy, and three months thereafter.
VSL#3 had no effect on alpha or phylogenetic diversity, but did change the gut microbiome composition. Researchers observed an increase in the relative abundance of specific organisms contained in the probiotic. Methanogens, which are increased in MS, decreased when patients received the probiotic. Administration of VSL#3 also was associated with a decrease in proinflammatory monocytes and decreased expression of activation markers on monocytes and dendritic cells.
When patients stopped probiotic treatment, the numbers of CD39 and IL10 T regulatory cells decreased, and proinflammatory monocytes increased. “We did see changes when you stop treatment, almost as if there is a rebound,” he said. “We are seeing an immune effect in the blood.”
The treatment was well tolerated. “I myself took some,” Dr. Weiner said. “It tastes a little chalky, but otherwise it is fine.” Some patients said they felt better after taking the probiotic. The study was not placebo-controlled, however. Future randomized clinical studies will include MRI scans, he said.
What should neurologists tell patients who want to take a probiotic and ask which probiotic they should take? “We are all asked those questions,” Dr. Weiner said. “I tell them, we really don’t know, but if you would like to take a probiotic, that is fine. But we have no particular recommendations at this time. We need a lot of science before we know what we are doing, as far as treatment, in this regard.”
Changes in the Gut Microbiome in MS
Prior to conducting the pilot study, Sushrut Jangi, MD, research fellow at Brigham and Women’s Hospital, Dr. Weiner, and colleagues studied the gut microbiome in 60 patients with MS and 43 healthy controls. They found that patients with MS had increases in Methanobrevibacter and Akkermansia and decreases in Butyricimonas, compared with controls. Methanobrevibacter produces methane, and in another cohort, the researchers detected more methane in the breath of patients with MS than in controls.
In addition, Prevotella and Sutterella were increased, and Sarcina was decreased in patients with MS who were treated with disease-modifying therapy, compared with untreated patients with MS. “It gives us a whole series of targets to try to understand,” he said. The gut microbiome alterations in patients with MS correlated with variations in the expression of genes in circulating T cells and monocytes that have been implicated in MS pathogenesis.
How Are the Gut Microbiome and MS Related?
The nature of the relationship between the gut microbiome and MS is unclear. One possibility is that “the gut could be the key to MS,” Dr. Weiner said. An organism in the gut may trigger MS, and that organism potentially could be altered or used in vaccinations to treat or prevent the disease. Alternatively, the gut microbiome might relate to MS susceptibility. Environmental factors, including diet and antibiotics, could affect the microbiome and MS risk. It is also possible that MS disease-modifying therapies act in part through the gut, Dr. Weiner said.
Various treatments targeting the microbiome are under investigation. For example, researchers are asking whether a particular bacteria or fecal microbiome transplants might help treat MS. “I am very interested in the concept of oral tolerance, where we trigger immune responses in the mucosa,” said Dr. Weiner. “We are beginning a trial of anti-CD3 [monoclonal antibodies], which will be given mucosally to stimulate the gut immune response.”
—Jake Remaly
Suggested Reading
Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015.
Kigerl KA, Hall JC, Wang L, et al. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213(12):2603-2620.
Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2016 Sep 16 [Epub ahead of print].
Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429.
Tremlett H, Waubant E. The multiple sclerosis microbiome? Ann Transl Med. 2017;5(3):53.
2017 Update on abnormal uterine bleeding
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1
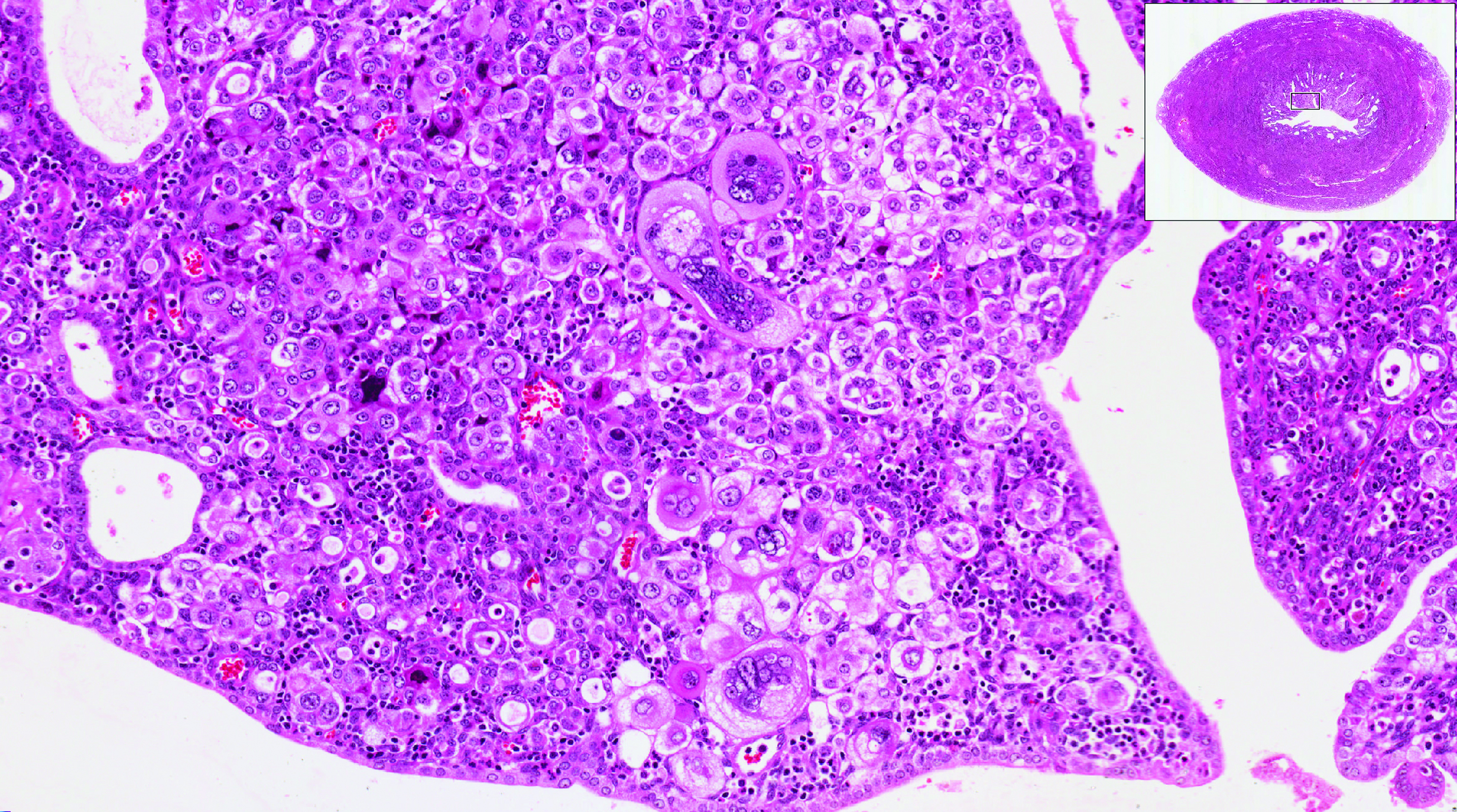
Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.
Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1

Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.

Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1

Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.

Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
Two free, comprehensive drug reference apps for your practice
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
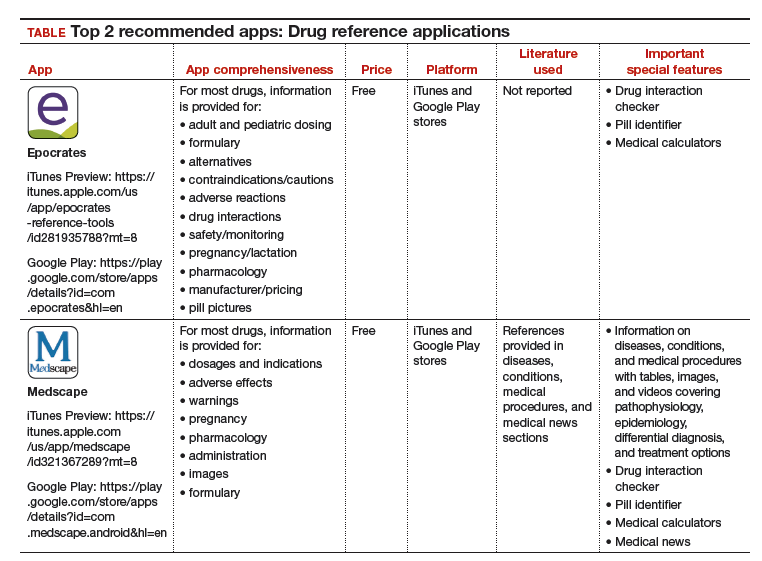
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.
Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.

Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.

Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
Venetoclax produces durable effects in relapsed/refractory CLL
ORLANDO – In patients with relapsed or refractory chronic lymphocytic leukemia (CLL), the results with venetoclax continue to be encouraging, and recent findings from a multicenter phase Ib study hint that venetoclax may also provide durable responses – even with treatment discontinuation.
Venetoclax is an orally bioavailable selective BCL2 inhibitor that is typically given in an open-ended fashion. Toxicity – including tumor lysis syndrome – is always a concern, however, and the issue of whether open-ended administration is necessary is an important question, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
In one phase II multicenter study of venetoclax monotherapy in 107 patients with relapsed/refractory CLL with del(17p) at 31 centers in the United States, Canada, and Europe, the overall response rate was 79.4% based on an independent review committee assessment, (Lancet Oncol. 2016[17]:768-78).
Treatment included once-daily venetoclax beginning with a dose of 20 mg that was ramped up to 50, 100, 200, and 400 mg over 4-5 weeks, followed by daily 400 mg continuous dosing until disease progression or discontinuation for another reason.
Notably, 18 of 85 patients from the study who achieved an objective response were minimal residual disease (MRD)–negative in peripheral blood samples – an outcome that has not been seen with tyrosine kinase inhibitors, noted Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
The durability of venetoclax’s activity in the study was 84.7% at 12 months in all responders; 100% in those who achieved complete response, complete response with incomplete recovery of blood counts, or nodular partial remission; and 94.4% in the MRD-negative patients.
The authors concluded that venetoclax monotherapy is active and well tolerated in patients with relapsed or refractory del(17p) chronic lymphocytic leukemia, and that given the distinct mechanism of action of this new therapeutic option for these very poor-prognosis patients, it deserves further investigation as part of a combination or as part of sequential treatment with other novel targeted agents.
The finding that patients on venetoclax can achieve MRD negativity also raised the question of whether treatment can be stopped, Dr. Zelenetz said.
“Because who wants to be on a drug forever? Nobody,” he added.
This question was explored in a study published in February by Seymour et al., which provided early evidence that stopping treatment may indeed be feasible in some patients (Lancet Oncol. 2017;18[2]:230-40).
Venetoclax in this study was given in a dose-escalating fashion to target doses of 200-600 mg daily, in 49 patients with relapsed or refractory “moderately heavily pretreated” CLL. Rituximab was added at a dose of 375 mg/m2 in month 1 and 500 mg/m2 in months 2-6.
Patients had the option of stopping treatment if they achieved a complete response.
The overall response rate was 86%, including a complete response in 51% of patients. Two-year estimates for progression-free survival and ongoing response were 82% and 89% respectively.
MRD negativity was attained in 80% of complete responders and 57% of patients overall. Thirteen responders discontinued all treatment, and at the time of publication, 11 MRD-negative responders who discontinued therapy remained progression free off therapy. Two MRD-positive patients who achieved complete response and discontinued therapy progressed after 2 years, but were able to recapture response once they restarted the drug.
“So it’s really quite interesting. We might have durable responses after discontinuation of this drug in an MRD-negative state,” Dr. Zelenetz said.
Of note, the latest update to the NCCN guidelines for the treatment of CLL/SLL (small lymphocytic lymphoma) included the addition of “+/– rituximab” as part of the “suggested treatment regimen” of venetoclax in the relapsed/refractory disease setting. This recommendation is category 2A, meaning it is based on lower level evidence with uniform NCCN consensus that the intervention is appropriate.
Venetoclax: adverse events of special interest
In these studies, venetoclax was considered well tolerated, but attention to adverse events and their prevention and management – particularly with respect to tumor lysis syndrome – is essential, Dr. Zelenetz said.
In the phase II study by Stilgenbauer et al., adverse events of special interest included grade 3/4 neutropenia, which occurred in 40% of patients. This was manageable with dose interruptions (five patients) or reductions (four patients), or with granulocyte–colony stimulating factor and antibiotics (six patients, including one who received only antibiotics). None of the patients permanently discontinued treatment.
Infections occurred in 72% of patients, and grade 3 or greater infections occurred in 20% of patients. The most common overall were upper respiratory infections (15%), nasopharyngitis (14%), and urinary tract infections (9%).
Serious infections occurring in two or more patients were pneumonia, lower respiratory tract infection, and upper respiratory tract infection. One patient died from septic shock, 10 had infections leading to venetoclax interruption, and 2 had infections leading to dose reduction.
No mandated infection prophylaxis was used in this study.
Serious adverse events occurred in 59 patients (55%). The most common, occurring in at least 5% of patients, were pyrexia, autoimmune hemolytic anemia, pneumonia, and febrile neutropenia. Thirteen had adverse events leading to dose reductions, most commonly due to neutropenia, thrombocytopenia, and febrile neutropenia.
Laboratory-confirmed tumor lysis syndrome was reported in five patients during the ramp-up period, including four who developed the syndrome within the first 2 days of treatment. All cases resolved without clinical sequelae. Treatment was continued without interruption in three patients with tumor lysis syndrome, who received only electrolyte management, and two patients had a 1-day treatment interruption. Both resumed dosing the next day.
In the phase Ib study by Seymour et al., common grade 1-2 toxicities included upper respiratory tract infections, diarrhea, and nausea occurring in 57%, 55%, and 53% of patients, respectively. Grade 3-4 adverse events occurred in 76% of 49 patients, and most often included neutropenia (12% of patients), thrombocytopenia (16%), anemia (14%), febrile neutropenia (12%), and leukopenia (12%). The most common serious adverse events were pyrexia (12%), febrile neutropenia (10%), lower respiratory tract infection (6%), and pneumonia (6%). Clinical tumor lysis syndrome occurred in two patients who initiated venetoclax at 50 mg, one of whom died as a result.
After enhancement of tumor lysis syndrome prophylaxis measures and reduction of the starting dose of venetoclax to 20 mg, no additional cases occurred, the authors reported.
Mitigating tumor lysis syndrome risk
General measures for mitigating the risk of tumor lysis syndrome include identification of patients at increased risk, initiation of prophylaxis with hydration and a uric acid reducing agent, and initiation of venetoclax at a 20 mg dose for 1 week, with gradual step-wise ramp-up over 5 weeks to the target dose, Dr. Zelenetz noted.
As reported by Stilgenbauer, et al., patients with a nodal mass less than 5 cm and absolute lymphocyte count (ALC) of 25,000 or less are considered at low risk for tumor lysis syndrome, those with a nodal mass of 5 cm to less than 10 cm or ALC greater than 25,000 are at medium risk, and those with a nodal mass of 10 cm or greater or nodal mass of 5 cm or greater and ALC of greater than 25,000 are considered to be at high risk.
High-risk patients in the study received inpatient venetoclax dosing and monitoring at 4, 8, 12, and 24 hours with 20 mg and 50 mg dosing, as well as outpatient intravenous hydration at 100 mg if there was no indication to hospitalize, and post-dose 8-24 hour laboratory monitoring at 100 mg and above.
Medium-risk patients received IV hydration at 20 and 50 mg dosing, inpatient care if creatinine clearance was less than 80 mL/min or if there was a high tumor burden, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose escalations. Low-risk patients received outpatient dosing at all dose levels in the absence of an indication to hospitalize, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose increases.
“Unfortunately, the dose-limiting toxicity of venetoclax is fatal tumor lysis,” Dr. Zelenetz said, adding that by increasing the dose slowly over time according to current treatment recommendations – from 20 mg, to 50, 100, 200, and 400 mg at weekly intervals, this complication can be avoided.
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Genentech, the maker of venetoclax (Venclexta), and a wide variety of other drug companies.
ORLANDO – In patients with relapsed or refractory chronic lymphocytic leukemia (CLL), the results with venetoclax continue to be encouraging, and recent findings from a multicenter phase Ib study hint that venetoclax may also provide durable responses – even with treatment discontinuation.
Venetoclax is an orally bioavailable selective BCL2 inhibitor that is typically given in an open-ended fashion. Toxicity – including tumor lysis syndrome – is always a concern, however, and the issue of whether open-ended administration is necessary is an important question, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
In one phase II multicenter study of venetoclax monotherapy in 107 patients with relapsed/refractory CLL with del(17p) at 31 centers in the United States, Canada, and Europe, the overall response rate was 79.4% based on an independent review committee assessment, (Lancet Oncol. 2016[17]:768-78).
Treatment included once-daily venetoclax beginning with a dose of 20 mg that was ramped up to 50, 100, 200, and 400 mg over 4-5 weeks, followed by daily 400 mg continuous dosing until disease progression or discontinuation for another reason.
Notably, 18 of 85 patients from the study who achieved an objective response were minimal residual disease (MRD)–negative in peripheral blood samples – an outcome that has not been seen with tyrosine kinase inhibitors, noted Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
The durability of venetoclax’s activity in the study was 84.7% at 12 months in all responders; 100% in those who achieved complete response, complete response with incomplete recovery of blood counts, or nodular partial remission; and 94.4% in the MRD-negative patients.
The authors concluded that venetoclax monotherapy is active and well tolerated in patients with relapsed or refractory del(17p) chronic lymphocytic leukemia, and that given the distinct mechanism of action of this new therapeutic option for these very poor-prognosis patients, it deserves further investigation as part of a combination or as part of sequential treatment with other novel targeted agents.
The finding that patients on venetoclax can achieve MRD negativity also raised the question of whether treatment can be stopped, Dr. Zelenetz said.
“Because who wants to be on a drug forever? Nobody,” he added.
This question was explored in a study published in February by Seymour et al., which provided early evidence that stopping treatment may indeed be feasible in some patients (Lancet Oncol. 2017;18[2]:230-40).
Venetoclax in this study was given in a dose-escalating fashion to target doses of 200-600 mg daily, in 49 patients with relapsed or refractory “moderately heavily pretreated” CLL. Rituximab was added at a dose of 375 mg/m2 in month 1 and 500 mg/m2 in months 2-6.
Patients had the option of stopping treatment if they achieved a complete response.
The overall response rate was 86%, including a complete response in 51% of patients. Two-year estimates for progression-free survival and ongoing response were 82% and 89% respectively.
MRD negativity was attained in 80% of complete responders and 57% of patients overall. Thirteen responders discontinued all treatment, and at the time of publication, 11 MRD-negative responders who discontinued therapy remained progression free off therapy. Two MRD-positive patients who achieved complete response and discontinued therapy progressed after 2 years, but were able to recapture response once they restarted the drug.
“So it’s really quite interesting. We might have durable responses after discontinuation of this drug in an MRD-negative state,” Dr. Zelenetz said.
Of note, the latest update to the NCCN guidelines for the treatment of CLL/SLL (small lymphocytic lymphoma) included the addition of “+/– rituximab” as part of the “suggested treatment regimen” of venetoclax in the relapsed/refractory disease setting. This recommendation is category 2A, meaning it is based on lower level evidence with uniform NCCN consensus that the intervention is appropriate.
Venetoclax: adverse events of special interest
In these studies, venetoclax was considered well tolerated, but attention to adverse events and their prevention and management – particularly with respect to tumor lysis syndrome – is essential, Dr. Zelenetz said.
In the phase II study by Stilgenbauer et al., adverse events of special interest included grade 3/4 neutropenia, which occurred in 40% of patients. This was manageable with dose interruptions (five patients) or reductions (four patients), or with granulocyte–colony stimulating factor and antibiotics (six patients, including one who received only antibiotics). None of the patients permanently discontinued treatment.
Infections occurred in 72% of patients, and grade 3 or greater infections occurred in 20% of patients. The most common overall were upper respiratory infections (15%), nasopharyngitis (14%), and urinary tract infections (9%).
Serious infections occurring in two or more patients were pneumonia, lower respiratory tract infection, and upper respiratory tract infection. One patient died from septic shock, 10 had infections leading to venetoclax interruption, and 2 had infections leading to dose reduction.
No mandated infection prophylaxis was used in this study.
Serious adverse events occurred in 59 patients (55%). The most common, occurring in at least 5% of patients, were pyrexia, autoimmune hemolytic anemia, pneumonia, and febrile neutropenia. Thirteen had adverse events leading to dose reductions, most commonly due to neutropenia, thrombocytopenia, and febrile neutropenia.
Laboratory-confirmed tumor lysis syndrome was reported in five patients during the ramp-up period, including four who developed the syndrome within the first 2 days of treatment. All cases resolved without clinical sequelae. Treatment was continued without interruption in three patients with tumor lysis syndrome, who received only electrolyte management, and two patients had a 1-day treatment interruption. Both resumed dosing the next day.
In the phase Ib study by Seymour et al., common grade 1-2 toxicities included upper respiratory tract infections, diarrhea, and nausea occurring in 57%, 55%, and 53% of patients, respectively. Grade 3-4 adverse events occurred in 76% of 49 patients, and most often included neutropenia (12% of patients), thrombocytopenia (16%), anemia (14%), febrile neutropenia (12%), and leukopenia (12%). The most common serious adverse events were pyrexia (12%), febrile neutropenia (10%), lower respiratory tract infection (6%), and pneumonia (6%). Clinical tumor lysis syndrome occurred in two patients who initiated venetoclax at 50 mg, one of whom died as a result.
After enhancement of tumor lysis syndrome prophylaxis measures and reduction of the starting dose of venetoclax to 20 mg, no additional cases occurred, the authors reported.
Mitigating tumor lysis syndrome risk
General measures for mitigating the risk of tumor lysis syndrome include identification of patients at increased risk, initiation of prophylaxis with hydration and a uric acid reducing agent, and initiation of venetoclax at a 20 mg dose for 1 week, with gradual step-wise ramp-up over 5 weeks to the target dose, Dr. Zelenetz noted.
As reported by Stilgenbauer, et al., patients with a nodal mass less than 5 cm and absolute lymphocyte count (ALC) of 25,000 or less are considered at low risk for tumor lysis syndrome, those with a nodal mass of 5 cm to less than 10 cm or ALC greater than 25,000 are at medium risk, and those with a nodal mass of 10 cm or greater or nodal mass of 5 cm or greater and ALC of greater than 25,000 are considered to be at high risk.
High-risk patients in the study received inpatient venetoclax dosing and monitoring at 4, 8, 12, and 24 hours with 20 mg and 50 mg dosing, as well as outpatient intravenous hydration at 100 mg if there was no indication to hospitalize, and post-dose 8-24 hour laboratory monitoring at 100 mg and above.
Medium-risk patients received IV hydration at 20 and 50 mg dosing, inpatient care if creatinine clearance was less than 80 mL/min or if there was a high tumor burden, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose escalations. Low-risk patients received outpatient dosing at all dose levels in the absence of an indication to hospitalize, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose increases.
“Unfortunately, the dose-limiting toxicity of venetoclax is fatal tumor lysis,” Dr. Zelenetz said, adding that by increasing the dose slowly over time according to current treatment recommendations – from 20 mg, to 50, 100, 200, and 400 mg at weekly intervals, this complication can be avoided.
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Genentech, the maker of venetoclax (Venclexta), and a wide variety of other drug companies.
ORLANDO – In patients with relapsed or refractory chronic lymphocytic leukemia (CLL), the results with venetoclax continue to be encouraging, and recent findings from a multicenter phase Ib study hint that venetoclax may also provide durable responses – even with treatment discontinuation.
Venetoclax is an orally bioavailable selective BCL2 inhibitor that is typically given in an open-ended fashion. Toxicity – including tumor lysis syndrome – is always a concern, however, and the issue of whether open-ended administration is necessary is an important question, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
In one phase II multicenter study of venetoclax monotherapy in 107 patients with relapsed/refractory CLL with del(17p) at 31 centers in the United States, Canada, and Europe, the overall response rate was 79.4% based on an independent review committee assessment, (Lancet Oncol. 2016[17]:768-78).
Treatment included once-daily venetoclax beginning with a dose of 20 mg that was ramped up to 50, 100, 200, and 400 mg over 4-5 weeks, followed by daily 400 mg continuous dosing until disease progression or discontinuation for another reason.
Notably, 18 of 85 patients from the study who achieved an objective response were minimal residual disease (MRD)–negative in peripheral blood samples – an outcome that has not been seen with tyrosine kinase inhibitors, noted Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York.
The durability of venetoclax’s activity in the study was 84.7% at 12 months in all responders; 100% in those who achieved complete response, complete response with incomplete recovery of blood counts, or nodular partial remission; and 94.4% in the MRD-negative patients.
The authors concluded that venetoclax monotherapy is active and well tolerated in patients with relapsed or refractory del(17p) chronic lymphocytic leukemia, and that given the distinct mechanism of action of this new therapeutic option for these very poor-prognosis patients, it deserves further investigation as part of a combination or as part of sequential treatment with other novel targeted agents.
The finding that patients on venetoclax can achieve MRD negativity also raised the question of whether treatment can be stopped, Dr. Zelenetz said.
“Because who wants to be on a drug forever? Nobody,” he added.
This question was explored in a study published in February by Seymour et al., which provided early evidence that stopping treatment may indeed be feasible in some patients (Lancet Oncol. 2017;18[2]:230-40).
Venetoclax in this study was given in a dose-escalating fashion to target doses of 200-600 mg daily, in 49 patients with relapsed or refractory “moderately heavily pretreated” CLL. Rituximab was added at a dose of 375 mg/m2 in month 1 and 500 mg/m2 in months 2-6.
Patients had the option of stopping treatment if they achieved a complete response.
The overall response rate was 86%, including a complete response in 51% of patients. Two-year estimates for progression-free survival and ongoing response were 82% and 89% respectively.
MRD negativity was attained in 80% of complete responders and 57% of patients overall. Thirteen responders discontinued all treatment, and at the time of publication, 11 MRD-negative responders who discontinued therapy remained progression free off therapy. Two MRD-positive patients who achieved complete response and discontinued therapy progressed after 2 years, but were able to recapture response once they restarted the drug.
“So it’s really quite interesting. We might have durable responses after discontinuation of this drug in an MRD-negative state,” Dr. Zelenetz said.
Of note, the latest update to the NCCN guidelines for the treatment of CLL/SLL (small lymphocytic lymphoma) included the addition of “+/– rituximab” as part of the “suggested treatment regimen” of venetoclax in the relapsed/refractory disease setting. This recommendation is category 2A, meaning it is based on lower level evidence with uniform NCCN consensus that the intervention is appropriate.
Venetoclax: adverse events of special interest
In these studies, venetoclax was considered well tolerated, but attention to adverse events and their prevention and management – particularly with respect to tumor lysis syndrome – is essential, Dr. Zelenetz said.
In the phase II study by Stilgenbauer et al., adverse events of special interest included grade 3/4 neutropenia, which occurred in 40% of patients. This was manageable with dose interruptions (five patients) or reductions (four patients), or with granulocyte–colony stimulating factor and antibiotics (six patients, including one who received only antibiotics). None of the patients permanently discontinued treatment.
Infections occurred in 72% of patients, and grade 3 or greater infections occurred in 20% of patients. The most common overall were upper respiratory infections (15%), nasopharyngitis (14%), and urinary tract infections (9%).
Serious infections occurring in two or more patients were pneumonia, lower respiratory tract infection, and upper respiratory tract infection. One patient died from septic shock, 10 had infections leading to venetoclax interruption, and 2 had infections leading to dose reduction.
No mandated infection prophylaxis was used in this study.
Serious adverse events occurred in 59 patients (55%). The most common, occurring in at least 5% of patients, were pyrexia, autoimmune hemolytic anemia, pneumonia, and febrile neutropenia. Thirteen had adverse events leading to dose reductions, most commonly due to neutropenia, thrombocytopenia, and febrile neutropenia.
Laboratory-confirmed tumor lysis syndrome was reported in five patients during the ramp-up period, including four who developed the syndrome within the first 2 days of treatment. All cases resolved without clinical sequelae. Treatment was continued without interruption in three patients with tumor lysis syndrome, who received only electrolyte management, and two patients had a 1-day treatment interruption. Both resumed dosing the next day.
In the phase Ib study by Seymour et al., common grade 1-2 toxicities included upper respiratory tract infections, diarrhea, and nausea occurring in 57%, 55%, and 53% of patients, respectively. Grade 3-4 adverse events occurred in 76% of 49 patients, and most often included neutropenia (12% of patients), thrombocytopenia (16%), anemia (14%), febrile neutropenia (12%), and leukopenia (12%). The most common serious adverse events were pyrexia (12%), febrile neutropenia (10%), lower respiratory tract infection (6%), and pneumonia (6%). Clinical tumor lysis syndrome occurred in two patients who initiated venetoclax at 50 mg, one of whom died as a result.
After enhancement of tumor lysis syndrome prophylaxis measures and reduction of the starting dose of venetoclax to 20 mg, no additional cases occurred, the authors reported.
Mitigating tumor lysis syndrome risk
General measures for mitigating the risk of tumor lysis syndrome include identification of patients at increased risk, initiation of prophylaxis with hydration and a uric acid reducing agent, and initiation of venetoclax at a 20 mg dose for 1 week, with gradual step-wise ramp-up over 5 weeks to the target dose, Dr. Zelenetz noted.
As reported by Stilgenbauer, et al., patients with a nodal mass less than 5 cm and absolute lymphocyte count (ALC) of 25,000 or less are considered at low risk for tumor lysis syndrome, those with a nodal mass of 5 cm to less than 10 cm or ALC greater than 25,000 are at medium risk, and those with a nodal mass of 10 cm or greater or nodal mass of 5 cm or greater and ALC of greater than 25,000 are considered to be at high risk.
High-risk patients in the study received inpatient venetoclax dosing and monitoring at 4, 8, 12, and 24 hours with 20 mg and 50 mg dosing, as well as outpatient intravenous hydration at 100 mg if there was no indication to hospitalize, and post-dose 8-24 hour laboratory monitoring at 100 mg and above.
Medium-risk patients received IV hydration at 20 and 50 mg dosing, inpatient care if creatinine clearance was less than 80 mL/min or if there was a high tumor burden, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose escalations. Low-risk patients received outpatient dosing at all dose levels in the absence of an indication to hospitalize, and postdose 8- and 24-hour laboratory monitoring after the initial dose and at dose increases.
“Unfortunately, the dose-limiting toxicity of venetoclax is fatal tumor lysis,” Dr. Zelenetz said, adding that by increasing the dose slowly over time according to current treatment recommendations – from 20 mg, to 50, 100, 200, and 400 mg at weekly intervals, this complication can be avoided.
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Genentech, the maker of venetoclax (Venclexta), and a wide variety of other drug companies.
EXPERT ANALYSIS AT THE NCCN ANNUAL CONFERENCE
Support for ObGyn versus “evidence” for attorney
“TRUST: How to build a support net for ObGyns affected by a medical error”
PATRICE M. WEISS, MD (JANUARY 2017)
Support for ObGyn versus “evidence” for attorney
While every clinician recognizes the need to support the practi- tioner involved in a significant medical error, I found it puzzling that Dr. Weiss’ article did not mention our constant after-the-event associate, the personal injury attorney. How are we to provide the needed relief for the practitioner’s emotional distress without handing ammunition to the plaintiff’s lawyer?
E. Darryl Barnes, MD
Mechanicsville, Virginia
Experienced being the second victim
As Dr. Weiss states in her article, patients and their families, the first victims, are not the only ones affected by medical errors. I was involved in a medication error on a labor and delivery unit more than 20 years ago, and I was the second victim. There were also countless others. You are correct when you state that physicians, and others in medicine, do not support colleagues who have experienced a medical error. I agree with Dr. Wu’s observation that lack of empathy by peers is distressing. Symptoms of depression, burnout, decreased quality of life, and feelings of distress, guilt, and shame can occur in the second victim. I hope more people will get on board to use The Joint Commission toolkit to assist health care organizations in developing a second-victim program.
Carol Permiceo, RN
Long Island, New York
Dr. Weiss responds
I thank Dr. Barnes for his comments. The purpose of this article was mainly to assist people in establishing institutional support systems for providers when medical errors occur. Often we are not aware of litigation until some time well after the event. The TRUST second-victim support program and other programs are for immediate first aid for the provider and the team. Concerning the plaintiff’s ammunition, please remember that the purpose of these support systems, whether immediate or ongoing, is to discuss the emotional impact of the case on the provider, not the clinical details of the case.
I appreciate Ms. Permiceo sharing her story. As you probably have figured out, my interest in this area stems from my own experiences with medical errors (one in particular) and unanticipated outcomes. I hope by talking about it and validating our feelings (we are only human, after all) others will suffer less and come forward.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TRUST: How to build a support net for ObGyns affected by a medical error”
PATRICE M. WEISS, MD (JANUARY 2017)
Support for ObGyn versus “evidence” for attorney
While every clinician recognizes the need to support the practi- tioner involved in a significant medical error, I found it puzzling that Dr. Weiss’ article did not mention our constant after-the-event associate, the personal injury attorney. How are we to provide the needed relief for the practitioner’s emotional distress without handing ammunition to the plaintiff’s lawyer?
E. Darryl Barnes, MD
Mechanicsville, Virginia
Experienced being the second victim
As Dr. Weiss states in her article, patients and their families, the first victims, are not the only ones affected by medical errors. I was involved in a medication error on a labor and delivery unit more than 20 years ago, and I was the second victim. There were also countless others. You are correct when you state that physicians, and others in medicine, do not support colleagues who have experienced a medical error. I agree with Dr. Wu’s observation that lack of empathy by peers is distressing. Symptoms of depression, burnout, decreased quality of life, and feelings of distress, guilt, and shame can occur in the second victim. I hope more people will get on board to use The Joint Commission toolkit to assist health care organizations in developing a second-victim program.
Carol Permiceo, RN
Long Island, New York
Dr. Weiss responds
I thank Dr. Barnes for his comments. The purpose of this article was mainly to assist people in establishing institutional support systems for providers when medical errors occur. Often we are not aware of litigation until some time well after the event. The TRUST second-victim support program and other programs are for immediate first aid for the provider and the team. Concerning the plaintiff’s ammunition, please remember that the purpose of these support systems, whether immediate or ongoing, is to discuss the emotional impact of the case on the provider, not the clinical details of the case.
I appreciate Ms. Permiceo sharing her story. As you probably have figured out, my interest in this area stems from my own experiences with medical errors (one in particular) and unanticipated outcomes. I hope by talking about it and validating our feelings (we are only human, after all) others will suffer less and come forward.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TRUST: How to build a support net for ObGyns affected by a medical error”
PATRICE M. WEISS, MD (JANUARY 2017)
Support for ObGyn versus “evidence” for attorney
While every clinician recognizes the need to support the practi- tioner involved in a significant medical error, I found it puzzling that Dr. Weiss’ article did not mention our constant after-the-event associate, the personal injury attorney. How are we to provide the needed relief for the practitioner’s emotional distress without handing ammunition to the plaintiff’s lawyer?
E. Darryl Barnes, MD
Mechanicsville, Virginia
Experienced being the second victim
As Dr. Weiss states in her article, patients and their families, the first victims, are not the only ones affected by medical errors. I was involved in a medication error on a labor and delivery unit more than 20 years ago, and I was the second victim. There were also countless others. You are correct when you state that physicians, and others in medicine, do not support colleagues who have experienced a medical error. I agree with Dr. Wu’s observation that lack of empathy by peers is distressing. Symptoms of depression, burnout, decreased quality of life, and feelings of distress, guilt, and shame can occur in the second victim. I hope more people will get on board to use The Joint Commission toolkit to assist health care organizations in developing a second-victim program.
Carol Permiceo, RN
Long Island, New York
Dr. Weiss responds
I thank Dr. Barnes for his comments. The purpose of this article was mainly to assist people in establishing institutional support systems for providers when medical errors occur. Often we are not aware of litigation until some time well after the event. The TRUST second-victim support program and other programs are for immediate first aid for the provider and the team. Concerning the plaintiff’s ammunition, please remember that the purpose of these support systems, whether immediate or ongoing, is to discuss the emotional impact of the case on the provider, not the clinical details of the case.
I appreciate Ms. Permiceo sharing her story. As you probably have figured out, my interest in this area stems from my own experiences with medical errors (one in particular) and unanticipated outcomes. I hope by talking about it and validating our feelings (we are only human, after all) others will suffer less and come forward.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Delivering clinician should be seated
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
Montgomery maneuver for shoulder dystocia
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas
Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas
Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“SHOULDER DYSTOCIA: TAKING THE FEAR OUT OF MANAGEMENT”
JOHN T. REPKE, MD, AND RONALD T. BURKMAN, MD (WEB EXCLUSIVE; APRIL 2016)
Montgomery maneuver for shoulder dystocia
In managing shoulder dystocia, my maneuver is to use my elbow to maximize mechanical advantage when applying suprapubic pressure to push the trapped shoulder down. It works well and is more efficient than having a nurse standing off to the side.
J.S. Montgomery, MD
Cypress, Texas
Dr. Barbieri responds
I thank Dr. Montgomery for sharing his maneuver for dislodging the trapped anterior shoulder by using his elbow to apply suprapubic pressure. There is vast knowledge and experience in our clinical community, and sharing insights is helpful to all our readers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Manage acne with spironolactone for women on LARC
“LONG-ACTING REVERSIBLE CONTRACEPTIVES AND ACNE IN ADOLESCENTS”
ROBERT L. BARBIERI, MD, AND ANDREA H. ROE, MD (EDITORIAL; JANUARY 2017)
Manage acne with spironolactone for women on LARC
Dr. Barbieri’s editorial with Dr. Roe addressed the very important theme of proactively talking about acne before a patient starts long-acting reversible contraception (LARC), especially when switching from a birth control pill that had controlled the acne to a levonorgestrel intrauterine device (LNG-IUD). It missed the mark, however, in not mentioning a very important presenting feature of adolescent polycystic ovary syndrome (PCOS)—cystic acne. I highly recommend obtaining baseline testosterone levels and using spironolactone, 50 to 200 mg daily, to treat acne while on LARC, especially an LNG-IUD. I learned this trick a few years ago from a Canadian endocrinologist.
John Lewis, MD
Waterbury, Connecticut
Dr. Barbieri responds
I thank Dr. Lewis for the important clinical pearl to use spironolactone to prevent and treat acne when inserting a progestin-releasing LARC in an adolescent or young woman. Spironolactone blocks testosterone action in the pilosebaceous unit, thereby decreasing sebum production and reducing acne activity. I frequently use spironolactone in my practice, especially for women with PCOS who have hirsutism and acne (see my editorial on page 8 of this issue). However, authors of a recent systematic review reported that there is minimal evidence from clinical trials to support the use of spironolactone to treat acne vulgaris.1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
“LONG-ACTING REVERSIBLE CONTRACEPTIVES AND ACNE IN ADOLESCENTS”
ROBERT L. BARBIERI, MD, AND ANDREA H. ROE, MD (EDITORIAL; JANUARY 2017)
Manage acne with spironolactone for women on LARC
Dr. Barbieri’s editorial with Dr. Roe addressed the very important theme of proactively talking about acne before a patient starts long-acting reversible contraception (LARC), especially when switching from a birth control pill that had controlled the acne to a levonorgestrel intrauterine device (LNG-IUD). It missed the mark, however, in not mentioning a very important presenting feature of adolescent polycystic ovary syndrome (PCOS)—cystic acne. I highly recommend obtaining baseline testosterone levels and using spironolactone, 50 to 200 mg daily, to treat acne while on LARC, especially an LNG-IUD. I learned this trick a few years ago from a Canadian endocrinologist.
John Lewis, MD
Waterbury, Connecticut
Dr. Barbieri responds
I thank Dr. Lewis for the important clinical pearl to use spironolactone to prevent and treat acne when inserting a progestin-releasing LARC in an adolescent or young woman. Spironolactone blocks testosterone action in the pilosebaceous unit, thereby decreasing sebum production and reducing acne activity. I frequently use spironolactone in my practice, especially for women with PCOS who have hirsutism and acne (see my editorial on page 8 of this issue). However, authors of a recent systematic review reported that there is minimal evidence from clinical trials to support the use of spironolactone to treat acne vulgaris.1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“LONG-ACTING REVERSIBLE CONTRACEPTIVES AND ACNE IN ADOLESCENTS”
ROBERT L. BARBIERI, MD, AND ANDREA H. ROE, MD (EDITORIAL; JANUARY 2017)
Manage acne with spironolactone for women on LARC
Dr. Barbieri’s editorial with Dr. Roe addressed the very important theme of proactively talking about acne before a patient starts long-acting reversible contraception (LARC), especially when switching from a birth control pill that had controlled the acne to a levonorgestrel intrauterine device (LNG-IUD). It missed the mark, however, in not mentioning a very important presenting feature of adolescent polycystic ovary syndrome (PCOS)—cystic acne. I highly recommend obtaining baseline testosterone levels and using spironolactone, 50 to 200 mg daily, to treat acne while on LARC, especially an LNG-IUD. I learned this trick a few years ago from a Canadian endocrinologist.
John Lewis, MD
Waterbury, Connecticut
Dr. Barbieri responds
I thank Dr. Lewis for the important clinical pearl to use spironolactone to prevent and treat acne when inserting a progestin-releasing LARC in an adolescent or young woman. Spironolactone blocks testosterone action in the pilosebaceous unit, thereby decreasing sebum production and reducing acne activity. I frequently use spironolactone in my practice, especially for women with PCOS who have hirsutism and acne (see my editorial on page 8 of this issue). However, authors of a recent systematic review reported that there is minimal evidence from clinical trials to support the use of spironolactone to treat acne vulgaris.1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
Forceful use of forceps, infant dies: $10.2M verdict
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Forceful use of forceps, infant dies: $10.2M verdict
A woman in her mid-20s went to the hospital in labor. After several hours, fetal heart-rate (FHR) monitor results became nonreassuring. The ObGyn and the nurse in charge disagreed on the interpretation of the FHR monitor strips. The nurse went to her supervisor, who confronted the ObGyn 2 hours later, saying that fetal distress was a serious concern and necessitated the cessation of oxytocin. The ObGyn disagreed and ordered another nurse to increase the oxytocin dose.
Three hours later, when the FHR monitoring strips showed severe distress, the ObGyn decided to undertake an operative vaginal delivery. During a 17-minute period, the ObGyn unsuccessfully used forceps 3 times. On the second attempt, a cracking noise was heard. Then a cesarean delivery was ordered; the baby was born limp, lifeless, and unresponsive. She was found to have hypoxic ischemic encephalopathy, was removed from life support, and died.
PARENTS’ CLAIM:
Oxytocin should not have been continued when the baby was clearly in distress. The supervising nurse should have contacted her supervisor and continued up the chain of command until the ObGyn was forced to stop the oxytocin.
Physicians are prohibited from using their leg muscles when applying forceps; gentle action is critical. During one attempt, the ObGyn had his leg on the bed to increase the force with which he pulled on the forceps. The ObGyn’s reckless use of forceps caused a skull fracture to depress into the brain. The ObGyn also tried to turn the baby using forceps, which is outside the standard of care because of the risk of rotational injury. A mother’s pushing rarely causes such severe damage to the baby.
DEFENDANTS’ DEFENSE:
There was no negligence. The hypoxia was due to a hemorrhage. Natural forces of a long delivery caused the skull injury.
VERDICT:
A $10,200,575 Texas verdict was returned.
After long labor, baby has CP: $8.4M settlement
Early on March 20, a 30-year-old woman who weighed 300 lbs was admitted for delivery at 40 weeks’ gestation. Labor was induced with oxytocin. Within 30 minutes, FHR monitoring showed that the baby’s baseline began to climb, accelerations ceased, and late decelerations commenced. The oxytocin dose was steadily increased throughout the day. A nurse decided that the baby was not tolerating the contractions and discontinued oxytocin. The attending ObGyn ordered oxytocin be restarted after giving the baby a chance to recover. The mother requested a cesarean delivery, but the ObGyn refused, saying that he was concerned with the risk due to her excessive weight and prior heart surgery. When his shift ended, his partner took over.
On March 21, a nurse reported that the FHR had climbed to 160 bpm although labor had not progressed. The ObGyn ordered terbutaline to slow contractions but he did not examine the mother. An hour after terbutaline administration, the FHR showed a deceleration. An emergency cesarean delivery was performed. The baby, born severely depressed, was resuscitated. Magnetic resonance imaging performed at 23 days of life showed that the child had a hypoxic ischemic injury. She has cerebral palsy and is nonambulatory with significant cognitive deficits.
PARENTS’ CLAIM:
The care provided by 2 ObGyns, nursing staff, and hospital was negligent. A cesarean delivery should have been performed on March 20 when the nurse identified fetal distress. The nurses should have been more assertive in recommending cesarean delivery. The injury occurred 30 minutes prior to delivery and could have been prevented by an earlier cesarean delivery.
DEFENDANTS’ DEFENSE:
FHR strips on March 20 were not as nonreassuring as claimed and did not warrant cesarean delivery, which was performed when needed.
VERDICT:
An $8.4 million Wisconsin settlement was reached by mediation.
Eclamptic seizure, twins stillborn: $4.25M
A 29-year-old woman pregnant with twins had an eclamptic seizure at 33 4/7 weeks’ gestation. The babies were stillborn.
PARENTS’ CLAIM:
The ObGyn failed to properly treat the patient’s preeclampsia for more than 11 weeks. The seizure caused hypovolemic shock, tachycardia, and massive hemorrhaging and required an emergency hysterectomy and bilateral salpingo-oophorectomy. The patient has no children and has been rendered unable to conceive. She sought to apportion 60% of the settlement proceeds to her distress claim and 20% each to wrongful-death and survival claims. She also sought to bar the twins’ biological father from sharing in the recovery due to abandonment.
HOSPITAL'S DEFENSE:
The case was settled during the trial.
VERDICT:
The mother agreed to receive 65% of the wrongful-death and survival funds, with 35% going to the father. A Pennsylvania settlement of $4.25 million was reached.
Brachial plexus injury: $4.8M verdict
A woman gave birth with assistance from a midwife. During delivery, shoulder dystocia was encountered. The baby has a permanent brachial plexus injury.
PARENTS’ CLAIM:
The midwife mismanaged shoulder dystocia by applying excessive traction to the baby’s head. The ObGyn in charge of the mother’s care did not provide adequate supervision.
DEFENDANTS’ DEFENSE:
The hospital settled prior to trial. The midwife and ObGyn denied negligence during delivery and contended that the child’s injury occurred as a result of the natural forces of labor.
VERDICT:
The jury found the midwife 60% negligent and the ObGyn 40% negligent. A $4.82 million Florida verdict was returned.
What caused infant's death?
During prenatal care, a woman underwent weekly nonstress tests due to excessive amniotic fluid until the level returned to normal. Near the end of her pregnancy, the patient noticed a decrease in fetal movement and called her ObGyn group. She was told to perform a fetal kick count and go to the emergency department (ED) if the count was abnormal, but she fell asleep. In the morning, she presented to the ObGyns’ office and was sent to the hospital for emergency cesarean delivery, which was performed 2.5 hrs after her arrival. The infant was born in distress and died 8 hours later.
PARENTS’ CLAIM:
The ObGyns should have continued weekly tests even after the amniotic fluid level returned to normal. She should have been sent to the ED when she initially reported decreased fetal movement. Cesarean delivery should have been performed immediately upon her arrival at the hospital.
PHYSICIAN’S DEFENSE:
Further prenatal testing for amniotic fluid levels was unwarranted. Telephone advice to count fetal kicks was appropriate. The delay in performing a cesarean delivery was beyond the ObGyns’ control. The outcome would have been the same regardless of their actions.
VERDICT:
A Michigan defense verdict was returned.
Perineal laceration during vaginal delivery
During vaginal delivery, a 27-year-old woman suffered a 4th-degree perineal laceration. She developed a retrovaginal fistula and has permanent fecal incontinence.
PARENTS’ CLAIM:
The ObGyn’s care was negligent. She failed to perform a rectal examination to assess the severity of the perineal laceration. The laceration was improperly repaired, and, as a result, the patient developed a retrovaginal fistula that persisted for 6 months until it was surgically repaired. A divot in her anal canal causes fecal incontinence.
PHYSICIAN’S DEFENSE:
The ObGyn contended she correctly diagnosed and repaired a 3rd-degree laceration. The wound later broke down for unknown reasons.
VERDICT:
An Arizona defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Biomarker combination may forecast remission in lupus nephritis
MELBOURNE – A reduction in urinary protein creatinine ratio and normalization of inflammatory biomarkers early in treatment of lupus nephritis may predict response rates at 24 and 48 weeks, according to data from the AURION study presented at an international congress on systemic lupus erythematosus.
The AURION study was a single-arm exploratory study assessing the value of an early reduction in proteinuria in 10 patients with active lupus nephritis. The patients were treated with novel calcineurin inhibitor voclosporin (23.7 mg twice daily) in addition to usual care with mycophenolate mofetil and low-dose steroids. The treatment protocol also included a forced steroid taper from 0.5 g at day 0 to 2.5 mg from week 12.
The 48-week study examined the biomarkers of a 25% reduction in urinary protein creatinine ratio and normalization of inflammatory lupus biomarkers C3, C4, and anti–double stranded DNA at 8 weeks, and explored their relationship to remission rates at 24 and 48 weeks. Overall, by week 24, 70% of patients had achieved complete remission, and by week 48, 5 of 7 (71%) had achieved complete remission. Three patients dropped out of the study before week 48: one because of decreased estimated glomerular filtration rate, one because of a systemic lupus erythematosus flare, and one at the discretion of an investigator.
All patients showed a 25% reduction in urinary protein creatinine ratio at week 8, with a mean decrease of 61% by week 24. Researchers also saw a 22% increase in mean C3 levels and a 58% increase in mean C4 from baseline to week 24. There was also a decrease in anti–double stranded DNA.
When researchers examined the specificity and sensitivities of these biomarkers at week 8 in predicting renal response at weeks 24 and 48, they found that a 25% reduction in urinary protein creatinine ratio had a good sensitivity (71% and 75% at 24 and 48 weeks, respectively), but low specificity (33% and 24%).
In contrast, normalization of C4 and C3 by week 8 both showed a specificity of 100% by week 24 and 75% by week 48, but a sensitivity of 29% for week 24 and 25% for week 48.
Normalization of anti–double stranded DNA had a sensitivity of 57% at week 24 and 50% at week 48, and a specificity of 100% at week 24 and 50% at week 48.
“Certainly if you use C3, C4, and urinary protein creatinine ratio, you can say to the patient after week 8, ‘I don’t think this is going to work for you. You need to come off this therapy and move to something else,’ ” he said in an interview.
Mr. Huizinga said the company, which recently released 48-week data from the larger AURA-LV study of the same regimen in 265 patients, was now building this week-8 analysis into its studies, and hoped it would also provide an early predictive marker for other clinical trials.
Commenting on the findings, Brad Rovin, MD, professor of nephrology and pathology at Ohio State University, Columbus, and also an adviser to Aurinia, said this predictive ability would be extremely useful for clinicians.
“If a patient isn’t responding appropriately and you can really know that with some degree of certainty at 8 weeks, then instead of waiting 6 months to change therapy, maybe you should change earlier,” Dr. Rovin said in an interview.
This was particularly important in lupus nephritis, as the longer inflammation is allowed to continue, the greater the likelihood that it might tip over into fibrosis, he noted.
The study was funded by Aurinia.
MELBOURNE – A reduction in urinary protein creatinine ratio and normalization of inflammatory biomarkers early in treatment of lupus nephritis may predict response rates at 24 and 48 weeks, according to data from the AURION study presented at an international congress on systemic lupus erythematosus.
The AURION study was a single-arm exploratory study assessing the value of an early reduction in proteinuria in 10 patients with active lupus nephritis. The patients were treated with novel calcineurin inhibitor voclosporin (23.7 mg twice daily) in addition to usual care with mycophenolate mofetil and low-dose steroids. The treatment protocol also included a forced steroid taper from 0.5 g at day 0 to 2.5 mg from week 12.
The 48-week study examined the biomarkers of a 25% reduction in urinary protein creatinine ratio and normalization of inflammatory lupus biomarkers C3, C4, and anti–double stranded DNA at 8 weeks, and explored their relationship to remission rates at 24 and 48 weeks. Overall, by week 24, 70% of patients had achieved complete remission, and by week 48, 5 of 7 (71%) had achieved complete remission. Three patients dropped out of the study before week 48: one because of decreased estimated glomerular filtration rate, one because of a systemic lupus erythematosus flare, and one at the discretion of an investigator.
All patients showed a 25% reduction in urinary protein creatinine ratio at week 8, with a mean decrease of 61% by week 24. Researchers also saw a 22% increase in mean C3 levels and a 58% increase in mean C4 from baseline to week 24. There was also a decrease in anti–double stranded DNA.
When researchers examined the specificity and sensitivities of these biomarkers at week 8 in predicting renal response at weeks 24 and 48, they found that a 25% reduction in urinary protein creatinine ratio had a good sensitivity (71% and 75% at 24 and 48 weeks, respectively), but low specificity (33% and 24%).
In contrast, normalization of C4 and C3 by week 8 both showed a specificity of 100% by week 24 and 75% by week 48, but a sensitivity of 29% for week 24 and 25% for week 48.
Normalization of anti–double stranded DNA had a sensitivity of 57% at week 24 and 50% at week 48, and a specificity of 100% at week 24 and 50% at week 48.
“Certainly if you use C3, C4, and urinary protein creatinine ratio, you can say to the patient after week 8, ‘I don’t think this is going to work for you. You need to come off this therapy and move to something else,’ ” he said in an interview.
Mr. Huizinga said the company, which recently released 48-week data from the larger AURA-LV study of the same regimen in 265 patients, was now building this week-8 analysis into its studies, and hoped it would also provide an early predictive marker for other clinical trials.
Commenting on the findings, Brad Rovin, MD, professor of nephrology and pathology at Ohio State University, Columbus, and also an adviser to Aurinia, said this predictive ability would be extremely useful for clinicians.
“If a patient isn’t responding appropriately and you can really know that with some degree of certainty at 8 weeks, then instead of waiting 6 months to change therapy, maybe you should change earlier,” Dr. Rovin said in an interview.
This was particularly important in lupus nephritis, as the longer inflammation is allowed to continue, the greater the likelihood that it might tip over into fibrosis, he noted.
The study was funded by Aurinia.
MELBOURNE – A reduction in urinary protein creatinine ratio and normalization of inflammatory biomarkers early in treatment of lupus nephritis may predict response rates at 24 and 48 weeks, according to data from the AURION study presented at an international congress on systemic lupus erythematosus.
The AURION study was a single-arm exploratory study assessing the value of an early reduction in proteinuria in 10 patients with active lupus nephritis. The patients were treated with novel calcineurin inhibitor voclosporin (23.7 mg twice daily) in addition to usual care with mycophenolate mofetil and low-dose steroids. The treatment protocol also included a forced steroid taper from 0.5 g at day 0 to 2.5 mg from week 12.
The 48-week study examined the biomarkers of a 25% reduction in urinary protein creatinine ratio and normalization of inflammatory lupus biomarkers C3, C4, and anti–double stranded DNA at 8 weeks, and explored their relationship to remission rates at 24 and 48 weeks. Overall, by week 24, 70% of patients had achieved complete remission, and by week 48, 5 of 7 (71%) had achieved complete remission. Three patients dropped out of the study before week 48: one because of decreased estimated glomerular filtration rate, one because of a systemic lupus erythematosus flare, and one at the discretion of an investigator.
All patients showed a 25% reduction in urinary protein creatinine ratio at week 8, with a mean decrease of 61% by week 24. Researchers also saw a 22% increase in mean C3 levels and a 58% increase in mean C4 from baseline to week 24. There was also a decrease in anti–double stranded DNA.
When researchers examined the specificity and sensitivities of these biomarkers at week 8 in predicting renal response at weeks 24 and 48, they found that a 25% reduction in urinary protein creatinine ratio had a good sensitivity (71% and 75% at 24 and 48 weeks, respectively), but low specificity (33% and 24%).
In contrast, normalization of C4 and C3 by week 8 both showed a specificity of 100% by week 24 and 75% by week 48, but a sensitivity of 29% for week 24 and 25% for week 48.
Normalization of anti–double stranded DNA had a sensitivity of 57% at week 24 and 50% at week 48, and a specificity of 100% at week 24 and 50% at week 48.
“Certainly if you use C3, C4, and urinary protein creatinine ratio, you can say to the patient after week 8, ‘I don’t think this is going to work for you. You need to come off this therapy and move to something else,’ ” he said in an interview.
Mr. Huizinga said the company, which recently released 48-week data from the larger AURA-LV study of the same regimen in 265 patients, was now building this week-8 analysis into its studies, and hoped it would also provide an early predictive marker for other clinical trials.
Commenting on the findings, Brad Rovin, MD, professor of nephrology and pathology at Ohio State University, Columbus, and also an adviser to Aurinia, said this predictive ability would be extremely useful for clinicians.
“If a patient isn’t responding appropriately and you can really know that with some degree of certainty at 8 weeks, then instead of waiting 6 months to change therapy, maybe you should change earlier,” Dr. Rovin said in an interview.
This was particularly important in lupus nephritis, as the longer inflammation is allowed to continue, the greater the likelihood that it might tip over into fibrosis, he noted.
The study was funded by Aurinia.
AT LUPUS 2017
Key clinical point: Early reduction in urinary protein creatinine ratio and normalization of inflammatory biomarkers may predict 24- and 48-week lupus nephritis treatment response rates.
Major finding: A 25% reduction in urine protein creatinine ratio, and normalization of C3 or C4 levels at 8 weeks may be predictive of complete remission at 48 weeks.
Data source: The single-center exploratory AURION study of 10 patients with active lupus nephritis.
Disclosures: Mr. Huizinga is vice president of clinical affairs for Aurinia Pharmaceuticals, which funded the study.