User login
Hot Threads in ACS Communities
Here are the top discussion threads in ACS Communities this week. (All of these threads are from the General Surgery community except where indicated.)
- Texas SB 1148 The MOC Bill
- Low back pain (Women Surgeons)
- Domestic volunteerism
- Hour limits for staff/attending surgeons?
- Health Care Reform
- A day on Capitol Hill
- Reactions to the “Replacement” of our Surgeon General?
- Any input on this article?
- Another tough case
- Rectal prolapse (Colon and Rectal Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Here are the top discussion threads in ACS Communities this week. (All of these threads are from the General Surgery community except where indicated.)
- Texas SB 1148 The MOC Bill
- Low back pain (Women Surgeons)
- Domestic volunteerism
- Hour limits for staff/attending surgeons?
- Health Care Reform
- A day on Capitol Hill
- Reactions to the “Replacement” of our Surgeon General?
- Any input on this article?
- Another tough case
- Rectal prolapse (Colon and Rectal Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Here are the top discussion threads in ACS Communities this week. (All of these threads are from the General Surgery community except where indicated.)
- Texas SB 1148 The MOC Bill
- Low back pain (Women Surgeons)
- Domestic volunteerism
- Hour limits for staff/attending surgeons?
- Health Care Reform
- A day on Capitol Hill
- Reactions to the “Replacement” of our Surgeon General?
- Any input on this article?
- Another tough case
- Rectal prolapse (Colon and Rectal Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Nurse practitioner urges advocacy for HPV vaccination
"IT IS TIME FOR HPV VACCINATION TO BE CONSIDERED PART OF ROUTINE PREVENTIVE HEALTH CARE"
BARBARA S. LEVY, MD (MARCH 2017)
Nurse practitioner urges advocacy for HPV vaccination
I could not agree more with Dr. Levy's view on human papillomavirus (HPV) vaccination. I am a Doctor of Nursing Practice student and improving HPV vaccination rates in adolescents is the focus of my research project for the next year. Based on the current literature, the most significant factors for increasing vaccination rates are patient education and provider recommendation. As the article mentions, "special" attention should not be given to the HPV vaccine, because this raises questions with families presenting to the office for routine well-child care. There have been many missed opportunities for vaccination of our young people over the past 10 years. As a result, we will continue to see increases in HPV-related cancers. We have a vaccine that has the potential to significantly decrease these cases, but it is underutilized. The recent recommendation of a 2-dose series (before the age of 15) should make completing the series easier. I urge all providers to be better advocates for their patients and make appropriate changes to their current practice in order to reduce the significant burden this disease carries.
Tiffany Edwards, MSN, APRN, FNP-BC
Seaford, Delaware
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
"IT IS TIME FOR HPV VACCINATION TO BE CONSIDERED PART OF ROUTINE PREVENTIVE HEALTH CARE"
BARBARA S. LEVY, MD (MARCH 2017)
Nurse practitioner urges advocacy for HPV vaccination
I could not agree more with Dr. Levy's view on human papillomavirus (HPV) vaccination. I am a Doctor of Nursing Practice student and improving HPV vaccination rates in adolescents is the focus of my research project for the next year. Based on the current literature, the most significant factors for increasing vaccination rates are patient education and provider recommendation. As the article mentions, "special" attention should not be given to the HPV vaccine, because this raises questions with families presenting to the office for routine well-child care. There have been many missed opportunities for vaccination of our young people over the past 10 years. As a result, we will continue to see increases in HPV-related cancers. We have a vaccine that has the potential to significantly decrease these cases, but it is underutilized. The recent recommendation of a 2-dose series (before the age of 15) should make completing the series easier. I urge all providers to be better advocates for their patients and make appropriate changes to their current practice in order to reduce the significant burden this disease carries.
Tiffany Edwards, MSN, APRN, FNP-BC
Seaford, Delaware
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
"IT IS TIME FOR HPV VACCINATION TO BE CONSIDERED PART OF ROUTINE PREVENTIVE HEALTH CARE"
BARBARA S. LEVY, MD (MARCH 2017)
Nurse practitioner urges advocacy for HPV vaccination
I could not agree more with Dr. Levy's view on human papillomavirus (HPV) vaccination. I am a Doctor of Nursing Practice student and improving HPV vaccination rates in adolescents is the focus of my research project for the next year. Based on the current literature, the most significant factors for increasing vaccination rates are patient education and provider recommendation. As the article mentions, "special" attention should not be given to the HPV vaccine, because this raises questions with families presenting to the office for routine well-child care. There have been many missed opportunities for vaccination of our young people over the past 10 years. As a result, we will continue to see increases in HPV-related cancers. We have a vaccine that has the potential to significantly decrease these cases, but it is underutilized. The recent recommendation of a 2-dose series (before the age of 15) should make completing the series easier. I urge all providers to be better advocates for their patients and make appropriate changes to their current practice in order to reduce the significant burden this disease carries.
Tiffany Edwards, MSN, APRN, FNP-BC
Seaford, Delaware
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Leadership hacks: The drama triangle
There are plenty of leadership books, seminars, podcasts, and websites available for the aspiring physician leader. However, not all of them are pertinent to the health care environment. As chairman of a large academic department, I have sifted through some of these resources and have found a few that have been particularly helpful to me. One of the more useful is the drama triangle.
Here’s an example: Pat, the administrator, just sent Sam an email stating that, starting today, all computers will be automatically turned off at 5 p.m. and cannot be restarted until 8 a.m. the next day. The reasons are many but unimportant to Sam because they pale in comparison to the inconvenience this places on Sam’s ability to finish work in the evening. No one asked about this change or even gave advance warning.
Anger builds in Sam, especially toward Pat. Something has to be done about this! That something becomes a petition to Kelly, a supervisor/manager/director/chairman with authority. Sam recounts the surprise of the order, details the inefficiencies created, expresses indignation at not being consulted first, lists the personal failings of Pat, and demands an immediate resolution to the problem. Kelly wants to help Sam and approaches Pat to reverse the order. Kelly feels good about helping Sam, and Sam feels great about going to Kelly. Pat doesn’t feel good at all.
Sam started, and Kelly completed, the drama triangle.
Breaking a drama triangle requires uncomfortable work building trust and a more functional relationship between the three players. Ideally, the victim becomes challenged, not threatened. The rescuer becomes a coach, not an enabler. The persecutor raises the bar rather than creating obstacles. With roles redefined, trust is restored. There are many methods and techniques to develop psychological safety and interdependent trust, but the work is the critical first step toward improving the function of a team (Lencioni, P. (2002). The Five Dysfunctions of a Team. Jossey-Bass).
What could Kelly have done to stop a triangle from forming? Sam wanted help, and Kelly’s first instinct is to do just that. However, by completing the triangle with a visit to Pat, Kelly creates more tension between Pat and Sam. To help both Sam and Pat, Kelly has to resist the temptation to rescue Sam from Pat and instead leverage Sam’s trust to begin asking Sam questions that lead Sam toward resolution of Sam’s own problem.
Aware of drama triangles, Kelly asks Sam what the ideal situation would be. Sam replies that all the work would get done by the end of the day. Kelly then asks what the current situation is. According to Pat, the computers turn off at 5, but the work is not done by then. Then, Kelly asks Sam what could be done before 5 that would help get the work done. Sam considers the question and then begins to list some changes to work flow that could allow greater efficiency during the day. Sam also decides to meet with Pat to explain how the two of them can improve communication in the future. Through coaching, Kelly defuses Sam’s anger, avoids a drama triangle, and gets Sam to start considering previously unknown solutions. The model Kelly uses to begin the work of breaking the triangle is sometimes called structural, or dynamic, tension.
Drama triangles compromise trust. The workplace culture can either enable simmering tensions and resentments to persist or it can foster psychological safety that allows for open and honest debate whereby all are heard and validated when change inevitably occurs. Exploring structural tension can build a sense of team and restore trust. A more robust discussion of structural tension will have to wait until my next column. In the meantime, as yourselves, Where do you see drama triangles? What worked to break them? What failed?
For more reading: Emerald, D. (2010). The Power of Ted* (The Empowerment Dynamic). Bainbridge Island, WA: Polaris Publishing.
I invite you to reply to [email protected] to initiate a broader discussion of physician leadership. Responses will be posted to hematologynews.com. Dr. Kalaycio is editor in chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
There are plenty of leadership books, seminars, podcasts, and websites available for the aspiring physician leader. However, not all of them are pertinent to the health care environment. As chairman of a large academic department, I have sifted through some of these resources and have found a few that have been particularly helpful to me. One of the more useful is the drama triangle.
Here’s an example: Pat, the administrator, just sent Sam an email stating that, starting today, all computers will be automatically turned off at 5 p.m. and cannot be restarted until 8 a.m. the next day. The reasons are many but unimportant to Sam because they pale in comparison to the inconvenience this places on Sam’s ability to finish work in the evening. No one asked about this change or even gave advance warning.
Anger builds in Sam, especially toward Pat. Something has to be done about this! That something becomes a petition to Kelly, a supervisor/manager/director/chairman with authority. Sam recounts the surprise of the order, details the inefficiencies created, expresses indignation at not being consulted first, lists the personal failings of Pat, and demands an immediate resolution to the problem. Kelly wants to help Sam and approaches Pat to reverse the order. Kelly feels good about helping Sam, and Sam feels great about going to Kelly. Pat doesn’t feel good at all.
Sam started, and Kelly completed, the drama triangle.
Breaking a drama triangle requires uncomfortable work building trust and a more functional relationship between the three players. Ideally, the victim becomes challenged, not threatened. The rescuer becomes a coach, not an enabler. The persecutor raises the bar rather than creating obstacles. With roles redefined, trust is restored. There are many methods and techniques to develop psychological safety and interdependent trust, but the work is the critical first step toward improving the function of a team (Lencioni, P. (2002). The Five Dysfunctions of a Team. Jossey-Bass).
What could Kelly have done to stop a triangle from forming? Sam wanted help, and Kelly’s first instinct is to do just that. However, by completing the triangle with a visit to Pat, Kelly creates more tension between Pat and Sam. To help both Sam and Pat, Kelly has to resist the temptation to rescue Sam from Pat and instead leverage Sam’s trust to begin asking Sam questions that lead Sam toward resolution of Sam’s own problem.
Aware of drama triangles, Kelly asks Sam what the ideal situation would be. Sam replies that all the work would get done by the end of the day. Kelly then asks what the current situation is. According to Pat, the computers turn off at 5, but the work is not done by then. Then, Kelly asks Sam what could be done before 5 that would help get the work done. Sam considers the question and then begins to list some changes to work flow that could allow greater efficiency during the day. Sam also decides to meet with Pat to explain how the two of them can improve communication in the future. Through coaching, Kelly defuses Sam’s anger, avoids a drama triangle, and gets Sam to start considering previously unknown solutions. The model Kelly uses to begin the work of breaking the triangle is sometimes called structural, or dynamic, tension.
Drama triangles compromise trust. The workplace culture can either enable simmering tensions and resentments to persist or it can foster psychological safety that allows for open and honest debate whereby all are heard and validated when change inevitably occurs. Exploring structural tension can build a sense of team and restore trust. A more robust discussion of structural tension will have to wait until my next column. In the meantime, as yourselves, Where do you see drama triangles? What worked to break them? What failed?
For more reading: Emerald, D. (2010). The Power of Ted* (The Empowerment Dynamic). Bainbridge Island, WA: Polaris Publishing.
I invite you to reply to [email protected] to initiate a broader discussion of physician leadership. Responses will be posted to hematologynews.com. Dr. Kalaycio is editor in chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
There are plenty of leadership books, seminars, podcasts, and websites available for the aspiring physician leader. However, not all of them are pertinent to the health care environment. As chairman of a large academic department, I have sifted through some of these resources and have found a few that have been particularly helpful to me. One of the more useful is the drama triangle.
Here’s an example: Pat, the administrator, just sent Sam an email stating that, starting today, all computers will be automatically turned off at 5 p.m. and cannot be restarted until 8 a.m. the next day. The reasons are many but unimportant to Sam because they pale in comparison to the inconvenience this places on Sam’s ability to finish work in the evening. No one asked about this change or even gave advance warning.
Anger builds in Sam, especially toward Pat. Something has to be done about this! That something becomes a petition to Kelly, a supervisor/manager/director/chairman with authority. Sam recounts the surprise of the order, details the inefficiencies created, expresses indignation at not being consulted first, lists the personal failings of Pat, and demands an immediate resolution to the problem. Kelly wants to help Sam and approaches Pat to reverse the order. Kelly feels good about helping Sam, and Sam feels great about going to Kelly. Pat doesn’t feel good at all.
Sam started, and Kelly completed, the drama triangle.
Breaking a drama triangle requires uncomfortable work building trust and a more functional relationship between the three players. Ideally, the victim becomes challenged, not threatened. The rescuer becomes a coach, not an enabler. The persecutor raises the bar rather than creating obstacles. With roles redefined, trust is restored. There are many methods and techniques to develop psychological safety and interdependent trust, but the work is the critical first step toward improving the function of a team (Lencioni, P. (2002). The Five Dysfunctions of a Team. Jossey-Bass).
What could Kelly have done to stop a triangle from forming? Sam wanted help, and Kelly’s first instinct is to do just that. However, by completing the triangle with a visit to Pat, Kelly creates more tension between Pat and Sam. To help both Sam and Pat, Kelly has to resist the temptation to rescue Sam from Pat and instead leverage Sam’s trust to begin asking Sam questions that lead Sam toward resolution of Sam’s own problem.
Aware of drama triangles, Kelly asks Sam what the ideal situation would be. Sam replies that all the work would get done by the end of the day. Kelly then asks what the current situation is. According to Pat, the computers turn off at 5, but the work is not done by then. Then, Kelly asks Sam what could be done before 5 that would help get the work done. Sam considers the question and then begins to list some changes to work flow that could allow greater efficiency during the day. Sam also decides to meet with Pat to explain how the two of them can improve communication in the future. Through coaching, Kelly defuses Sam’s anger, avoids a drama triangle, and gets Sam to start considering previously unknown solutions. The model Kelly uses to begin the work of breaking the triangle is sometimes called structural, or dynamic, tension.
Drama triangles compromise trust. The workplace culture can either enable simmering tensions and resentments to persist or it can foster psychological safety that allows for open and honest debate whereby all are heard and validated when change inevitably occurs. Exploring structural tension can build a sense of team and restore trust. A more robust discussion of structural tension will have to wait until my next column. In the meantime, as yourselves, Where do you see drama triangles? What worked to break them? What failed?
For more reading: Emerald, D. (2010). The Power of Ted* (The Empowerment Dynamic). Bainbridge Island, WA: Polaris Publishing.
I invite you to reply to [email protected] to initiate a broader discussion of physician leadership. Responses will be posted to hematologynews.com. Dr. Kalaycio is editor in chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Prepping the vagina before cesarean delivery
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
Longer metronidazole treatment is better than 1-day dose for women with trichomoniasis
"SHOULD THE LENGTH OF TREATMENT FOR TRICHOMONIASIS IN WOMEN BE RECONSIDERED?"
PATRICK DUFF, MD (MARCH 2017)
Longer metronidazole treatment is better than 1-day dose for women with trichomoniasis
From 37 years of experience as a Women's Healthcare Nurse Practitioner, I have found it is always better to prescribe metronidazole 500 mg bid for 7 days rather than 1-day treatment for women. I will prescribe 1-day treatment for men. I have been treating men and women using these regimens in a sexually transmitted diseases clinic for nearly 5 years. Colleagues have used the 1-time dose for women and it rarely works as well as the 7-day dose. However, I am always concerned about men taking the medication for 7 days, because often they are not symptomatic and they may stop taking their medication early if given the 1-week regimen, so I usually prescribe the 1-day dose for men. I wish more prescribers would offer treatment for the male partners, as they may not be symptomatic or may not want to spend the money to visit a provider. In my state, it is legal to prescribe for the partner without seeing him, and the Centers for Disease Control and Prevention suggests doing so. We encourage the men to come in but if the partner says he is unlikely to, we will treat without seeing him.
Carol Glascock, WHNP-BC
Columbia, Missouri
Dr. Duff responds
I appreciate Ms. Glascock's thoughtful comments. I am pleased that her years of clinical experience support the main conclusion reached by Howe and Kissinger that, in general, patients do better when they receive multidose therapy for trichomonas infection.1 I agree with Ms. Glascock's observation that single-dose therapy still has a role in situations in which patients may not be adherent with multidose therapy, such as the asymptomatic male partner of an infected woman. I also agree wholeheartedly that women will have less likelihood of recurrence when their partner receives adequate antibiotic treatment. I concur that, in states where this practice is legally permissible, we should be willing to offer antibiotic therapy to the partner of our female patient.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29-24.
"SHOULD THE LENGTH OF TREATMENT FOR TRICHOMONIASIS IN WOMEN BE RECONSIDERED?"
PATRICK DUFF, MD (MARCH 2017)
Longer metronidazole treatment is better than 1-day dose for women with trichomoniasis
From 37 years of experience as a Women's Healthcare Nurse Practitioner, I have found it is always better to prescribe metronidazole 500 mg bid for 7 days rather than 1-day treatment for women. I will prescribe 1-day treatment for men. I have been treating men and women using these regimens in a sexually transmitted diseases clinic for nearly 5 years. Colleagues have used the 1-time dose for women and it rarely works as well as the 7-day dose. However, I am always concerned about men taking the medication for 7 days, because often they are not symptomatic and they may stop taking their medication early if given the 1-week regimen, so I usually prescribe the 1-day dose for men. I wish more prescribers would offer treatment for the male partners, as they may not be symptomatic or may not want to spend the money to visit a provider. In my state, it is legal to prescribe for the partner without seeing him, and the Centers for Disease Control and Prevention suggests doing so. We encourage the men to come in but if the partner says he is unlikely to, we will treat without seeing him.
Carol Glascock, WHNP-BC
Columbia, Missouri
Dr. Duff responds
I appreciate Ms. Glascock's thoughtful comments. I am pleased that her years of clinical experience support the main conclusion reached by Howe and Kissinger that, in general, patients do better when they receive multidose therapy for trichomonas infection.1 I agree with Ms. Glascock's observation that single-dose therapy still has a role in situations in which patients may not be adherent with multidose therapy, such as the asymptomatic male partner of an infected woman. I also agree wholeheartedly that women will have less likelihood of recurrence when their partner receives adequate antibiotic treatment. I concur that, in states where this practice is legally permissible, we should be willing to offer antibiotic therapy to the partner of our female patient.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
"SHOULD THE LENGTH OF TREATMENT FOR TRICHOMONIASIS IN WOMEN BE RECONSIDERED?"
PATRICK DUFF, MD (MARCH 2017)
Longer metronidazole treatment is better than 1-day dose for women with trichomoniasis
From 37 years of experience as a Women's Healthcare Nurse Practitioner, I have found it is always better to prescribe metronidazole 500 mg bid for 7 days rather than 1-day treatment for women. I will prescribe 1-day treatment for men. I have been treating men and women using these regimens in a sexually transmitted diseases clinic for nearly 5 years. Colleagues have used the 1-time dose for women and it rarely works as well as the 7-day dose. However, I am always concerned about men taking the medication for 7 days, because often they are not symptomatic and they may stop taking their medication early if given the 1-week regimen, so I usually prescribe the 1-day dose for men. I wish more prescribers would offer treatment for the male partners, as they may not be symptomatic or may not want to spend the money to visit a provider. In my state, it is legal to prescribe for the partner without seeing him, and the Centers for Disease Control and Prevention suggests doing so. We encourage the men to come in but if the partner says he is unlikely to, we will treat without seeing him.
Carol Glascock, WHNP-BC
Columbia, Missouri
Dr. Duff responds
I appreciate Ms. Glascock's thoughtful comments. I am pleased that her years of clinical experience support the main conclusion reached by Howe and Kissinger that, in general, patients do better when they receive multidose therapy for trichomonas infection.1 I agree with Ms. Glascock's observation that single-dose therapy still has a role in situations in which patients may not be adherent with multidose therapy, such as the asymptomatic male partner of an infected woman. I also agree wholeheartedly that women will have less likelihood of recurrence when their partner receives adequate antibiotic treatment. I concur that, in states where this practice is legally permissible, we should be willing to offer antibiotic therapy to the partner of our female patient.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29-24.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29-24.
Hydralazine-Associated Cutaneous Vasculitis Presenting With Aerodigestive Tract Involvement
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus
Case Report
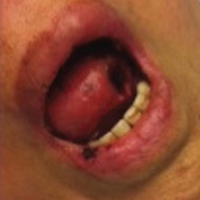
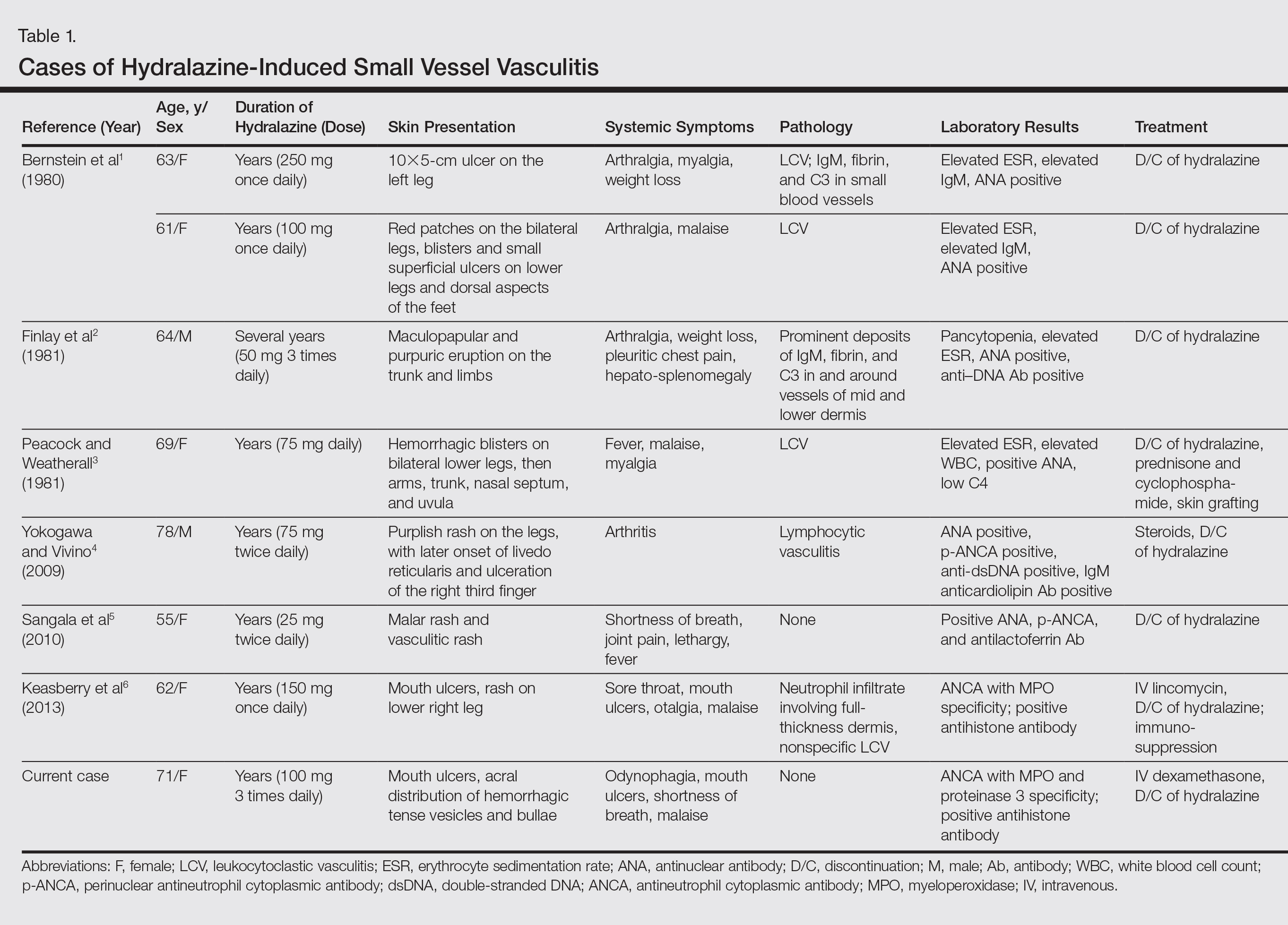
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.
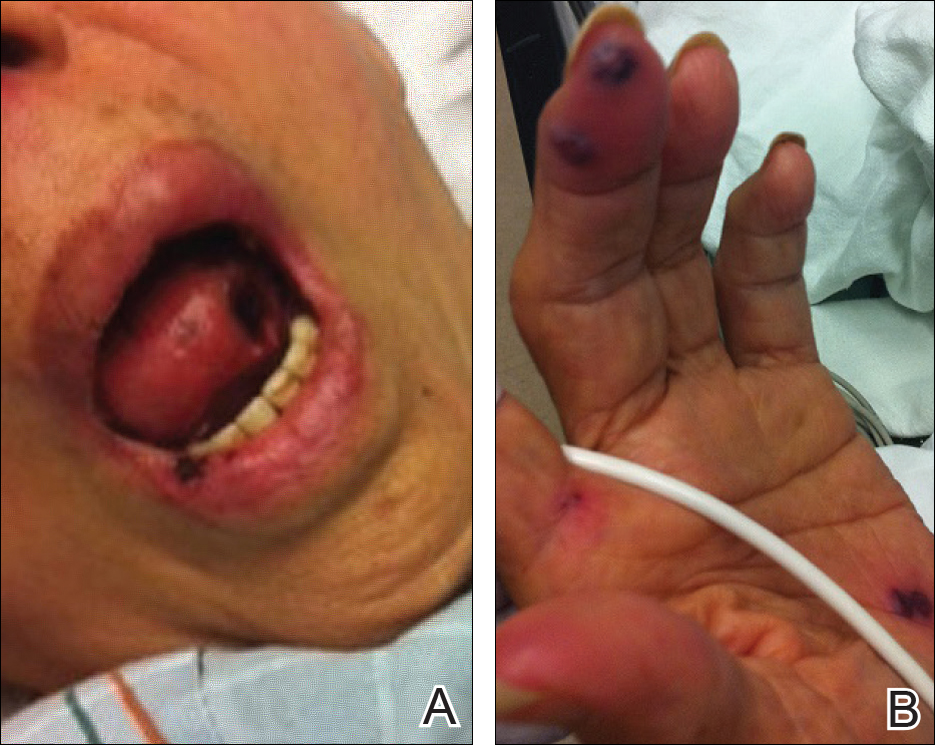
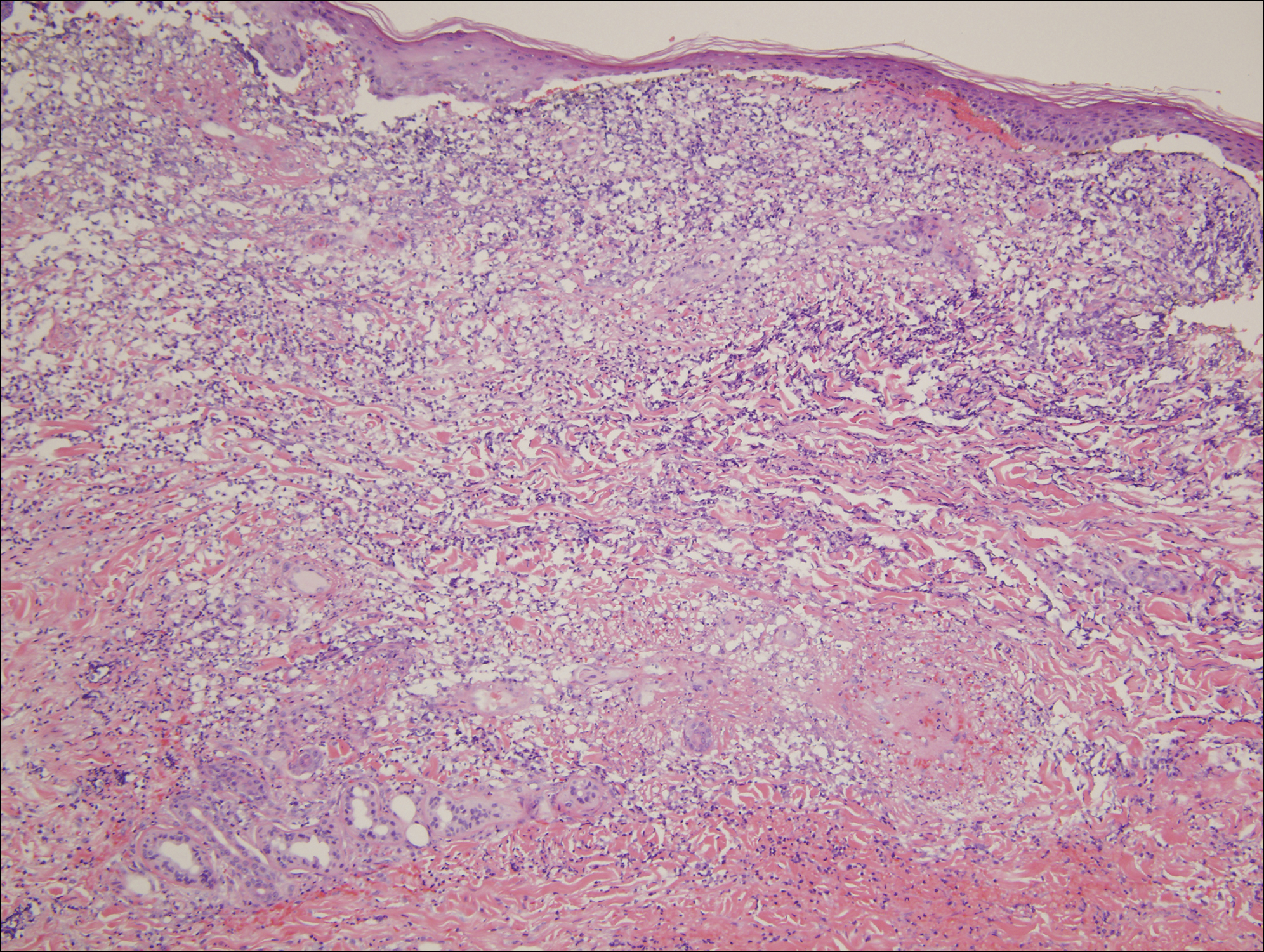
An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.

Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus

Case Report
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.

An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.


Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
Hydralazine-induced antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis is a complex entity characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement. Although it is an established entity, a PubMed search of articles indexed for MEDLINE using the terms hydralazine vasculitis, ANCA positive vasculitis, and hydralazine associated vasculitis revealed a limited number of cases reported in the dermatologic literature (Table 1).1-6 We report a rare case of hydralazine-induced vasculitis associated with airway compromise and severe gastrointestinal tract bleeding.
RELATED ARTICLE: Sweet Syndrome Associated With Hydralazine-Induced Lupus Erythematosus

Case Report
A 71-year-old woman with a history of end-stage renal disease treated with hemodialysis, as well as hypertension, diabetes mellitus, and ischemic cardiomyopathy, presented to our emergency department with odynophagia, muscle weakness, shortness of breath, and a distinctive mucocutaneous eruption on the left eyelid, lips, and tongue of 2 days’ duration. Physical examination revealed an ill-appearing, afebrile, dyspneic woman with swelling of the left upper eyelid, conjunctival injection, ulcerations on the lips and tongue, and tense hemorrhagic vesicles, as well as vesiculopustules on the elbows, palms, fingers, lower legs, and toes (Figure 1). Given her dyspnea, flexible laryngoscopy was performed and revealed ulceration and edema involving the epiglottis, aryepiglottic folds, and arytenoids. The patient was intubated for airway protection and started on intravenous dexamethasone.

An extensive diagnostic workup commenced. Bacterial, viral, and fungal cultures of blood, skin tissue, and respiratory secretions, as well as human immunodeficiency virus screening, were all negative. Specifically, a tissue culture was performed on skin from the left thigh, viral culture and direct fluorescent antibody were performed on a vesicle on the right knee for herpes simplex virus and herpes zoster, and a superficial wound culture was taken from the left arm, all showing no growth. The patient’s home medications were reviewed and revealed she was currently taking hydralazine (100 mg 3 times daily), which was started approximately 2 years prior. Laboratory results revealed a positive antinuclear antibody titer of 1:320 (diffuse pattern), positive antihistone antibody, and positive ANCA with cytoplasmic and perinuclear accentuation (Table 2). Enzyme-linked immunosorbent assays showed IgG antibodies to myeloperoxidase (MPO) and proteinase 3 (Table 2). Skin biopsies from the right lower leg and right upper arm were compatible with necrotizing leukocytoclastic vasculitis characterized by mural and luminal fibrin deposition involving capillaries and venules of the superficial and deep dermis (Figure 2). The vessel walls were infiltrated by neutrophils with concomitant leukocytoclasia. Vessels in the mid dermis were occluded by cellular fibrin thrombi. Foci of neutrophilic interface dermatitis with subepidermal bulla formation were observed. Infectious stains were negative. On direct immunofluorescence, striking homogeneous mantles of staining of IgG were present within the cutaneous vasculature.


Because the infectious workup was negative and there was no other known instigating factor of vasculitis, concern for a drug-induced process prompted thorough review of the patient’s home medications and discontinuation of hydralazine. A diagnosis of hydralazine-associated cutaneous vasculitis was made when laboratory workup confirmed no underlying infectious process or rheumatologic condition and the medication known to cause her symptoms was on her medication list. The dexamethasone dose was increased, leading to rapid improvement of her mucocutaneous findings; however, on initiation of a steroid taper, she developed substantial gastrointestinal tract bleeding. An esophageal biopsy revealed a neutrophil-rich necrotizing process that essentially mirrored the cutaneous biopsy consistent with vasculitic involvement of the gastrointestinal tract. Steroids were again increased with resolution in gastrointestinal tract bleeding.
Comment
Our case highlights a distinct clinical presentation of hydralazine-induced ANCA-positive cutaneous vasculitis associated with severe involvement of the aerodigestive tract with gastrointestinal tract bleeding and airway compromise requiring intubation. Although discontinuation of hydralazine and in certain cases the addition of immunosuppressive agents may be adequate for resolution of symptoms, some cases progress despite treatment, leading to skin grafting, amputation, and death.3,4 Therefore, early recognition of hydralazine-induced cutaneous vasculitis and discontinuation of hydralazine are of paramount importance.
Reporting hydralazine-induced vasculitis is valuable because of its unique cutaneous, extracutaneous, and serologic findings. In our case, the cutaneous vasculitis presented clinically with acral hemorrhagic vesiculopustules and necrotic ulcerations resembling septic emboli, as opposed to classic lesions of palpable purpura typical of drug-induced leukocytoclastic vasculitis. Similar cutaneous findings have been described in other cases of hydralazine-induced vasculitis, indicating that this pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration is characteristic of this entity.1,3,6 In addition, involvement of the oral cavity, larynx, and gastrointestinal tract have been reported in cases of hydralazine-induced vasculitis, indicating mucosal involvement is an important feature of this disease.3,6 Although involvement of the oral mucosa, larynx, and acral sites appears to be characteristic, the exact basis for this site localization remains elusive. A precedent has been established for a similar pattern of intraoral and laryngeal involvement in other ANCA-positive vasculitic syndromes, most notably Wegener granulomatosis.7 Similarly, there are certain occlusive vasculitic syndromes that show acral localization including chronic septic vasculitis and vasculitis of collagen vascular disease.
Serologic trends can aid in diagnosing hydralazine-induced vasculitis. In theory, the nonspecific cutaneous findings, often in association with joint pain and positive antinuclear antibodies, may lead clinicians to the misdiagnosis of a connective tissue disease, such as systemic lupus erythematosus (SLE). However, unlike SLE, hydralazine-induced vasculitis is associated with positive ANCAs, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.8,9 Our patient had both positive perinuclear ANCA with cytoplasmic ANCA as well as a positive antihistone antibodies, a combination highly suggestive of a drug-induced process.
Despite the often acute presentation of hydralazine-induced ANCA-positive vasculitis, afflicted patients have characteristically been on the drug for a long period of time. Our patient is exemplary of most reported cases, as the time from initiation of hydralazine to onset of vasculitis was 2 years.4
The mechanism by which hydralazine causes this reaction is still a matter of debate. It seems clear that there are certain at-risk populations, such as slow acetylators and patients with an underlying hypercoagulable state. There are several theories by which hydralazine induces autoantibody formation. The first involves hydralazine metabolization by MPO released from activated neutrophils to form reactive intermediate metabolites. Such metabolites can be cytotoxic and may cause abnormal degradation of chromatin in susceptible individuals, leading to an autoimmune response against histone-DNA complexes. Alternatively, hydralazine may act as a hapten and bind to MPO, inducing an immune response against the hydralazine-MPO complex, with resultant formation of anti-MPO antibodies in susceptible individuals.10
Conclusion
Hydralazine-induced ANCA-positive vasculitis is a syndromic complex characterized by a distinctive clinical presentation comprising acral hemorrhagic vesiculopustules and necrotic ulcerations, at times with severe mucosal involvement along with a characteristic ANCA-positive serologic profile. Drug withdrawal is the cornerstone of therapy, and depending on the severity of symptoms, additional immunosuppressive treatment such as corticosteroids may be necessary. Older age of onset, female gender, and underlying autoimmune diatheses likely define important risk factors. With more recognition and reporting of this disease, further trends in both clinical and serological presentation will emerge.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
- Bernstein RM, Egerton-Vernon J, Webster J. Hydrallazine-induced cutaneous vasculitis. Br Med J. 1980;280:156-157.
- Finlay AY, Statham B, Knight AG. Hydrallazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1703-1704.
- Peacock A, Weatherall D. Hydralazine-induced necrotising vasculitis. Br Med J (Clin Res Ed). 1981;282:1121-1122.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophil cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- Wojciechowska J, Krajewski W, Krajewski P, et al. Granulomatosis with polyangiitis in otolaryngologist practice: a review of current knowledge. Clin Exp Otorhinolaryngol. 2016;9:8-13.
- Short AK, Lockwood CM. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88:775-783.
- Nässberger L, Hultquist R, Sturfelt G. Occurrence of anti-lactoferrin antibodies in patients with systemic lupus erythematosus, hydralazine-induced lupus, and rheumatoid arthritis. Scand J Rheumatol. 1994;23:206-210.
- Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109-114.
Practice Points
- Hydralazine-induced small vessel vasculitis has a characteristic pattern of acral pseudoembolic vesiculopustules with necrosis and ulceration, along with involvement of the aerodigestive tract.
- Unlike systemic lupus erythematosus (SLE), hydralazine-induced vasculitis is associated with positive antineutrophil cytoplasmic antibodies, while antibodies against double-stranded DNA, a highly specific antibody for SLE, are uncommon.
- Increased recognition of the clinical and serological features of hydralazine-induced small vessel vasculitis may lead to earlier recognition of this disease and decreased time to discontinuation of hydralazine when appropriate.
Modern estrogen ‘microdoses’ in contraceptives did not increase risk of melanoma
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
AT SID 2017
Key clinical point: Long-term exposure to modern “microdoses” of ethinyl estradiol in contraceptives was not associated with malignant melanoma.
Major finding: Rates were 0.1% in the exposed group and 0.3% in the unexposed group (P = .3).
Data source: A retrospective cohort study of 77,293 women.
Disclosures: The National Institutes of Health helps support the Northwestern Medicine Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
Tweaking CBT may boost outcomes in hoarding disorder
SAN FRANCISCO – Cognitive-behavioral therapy for hoarding disorder leaves substantial room for improvement in efficacy, and additional therapeutic attention to maladaptive beliefs regarding perfectionism just might be the answer, Hannah C. Levy, PhD, said at the annual conference of the Anxiety and Depression Association of America.
She presented a secondary analysis of the relationship between cognitive-behavioral therapy (CBT) outcomes and baseline maladaptive beliefs in a wait list–controlled study of 36 patients with hoarding disorder (HD) who were not on psychiatric medication. Her purpose was to identify which cognitive domains predicted treatment outcome. This, in turn, could point the way to new treatment targets.
In contrast, the baseline strength of Saving Cognitions Inventory maladaptive beliefs about saving, such as emotional attachment to hoarded objects or a belief that keeping those objects is the only way to be able to remember an important event, proved unrelated to CBT outcomes. And this finding may help explain CBT’s limited effectiveness in HD.
“I think traditionally our CBT interventions are more focused on the maladaptive saving beliefs. We’re currently not doing a whole lot about the perfectionism ideas that people may be bringing in,” said Dr. Levy of the anxiety disorders center at the Institute of Living in Hartford, Conn.
A strong unmet need exists for novel targets for CBT in hoarding disorder to improve current less-than-stellar outcomes, she said. A recent meta-analysis demonstrated that while CBT did provide a statistically significant benefit for this common and often disabling disease, outcomes were far from optimal. Indeed, after completing their course of CBT, patients still scored an average of 3 standard deviations above mean normal for scores on the SI-R (Depress Anxiety. 2015 Mar;32[3]:158-66).
Other investigators have recognized the limitations of current CBT for HD and tried tweaking the therapy to boost efficacy. These efforts have included formal studies incorporating home visits, adding cognitive remediation to reduce the neuropsychological deficits often present in patients with HD, and/or extending the treatment duration to 26 weekly sessions from the current standard of 15 or 16.
Unfortunately, none of these innovations has really panned out when tested, Dr. Levy said. For example, in the 36-patient study analyzed by Dr. Levy, clinically significant improvement was seen in only 41% of subjects following 26 weeks of CBT (Depress Anxiety. 2010 May;27[5]:476-84). In contrast, published response rates for CBT in patients with obsessive-compulsive disorder – a related condition – are in the 85% range.
She described the perfectionism that often figures prominently in HD as a maladaptive belief that if something can’t be done perfectly, it’s not worth doing at all.
“People will say to me, ‘I can’t start discarding until I’ve got my organizational system down.’ They’re completely stymied. They can’t make any progress because their system isn’t fully coordinated yet,” the psychologist explained.
One way to potentially target this perfectionism more explicitly might be to incorporate cognitive restructuring or behavioral experiments that enable patients to test out and perhaps discard those beliefs, according to Dr. Levy.
She reported no financial conflicts regarding her analysis, which was based upon data collected in an earlier study sponsored by the National Institute of Mental Health.
SAN FRANCISCO – Cognitive-behavioral therapy for hoarding disorder leaves substantial room for improvement in efficacy, and additional therapeutic attention to maladaptive beliefs regarding perfectionism just might be the answer, Hannah C. Levy, PhD, said at the annual conference of the Anxiety and Depression Association of America.
She presented a secondary analysis of the relationship between cognitive-behavioral therapy (CBT) outcomes and baseline maladaptive beliefs in a wait list–controlled study of 36 patients with hoarding disorder (HD) who were not on psychiatric medication. Her purpose was to identify which cognitive domains predicted treatment outcome. This, in turn, could point the way to new treatment targets.
In contrast, the baseline strength of Saving Cognitions Inventory maladaptive beliefs about saving, such as emotional attachment to hoarded objects or a belief that keeping those objects is the only way to be able to remember an important event, proved unrelated to CBT outcomes. And this finding may help explain CBT’s limited effectiveness in HD.
“I think traditionally our CBT interventions are more focused on the maladaptive saving beliefs. We’re currently not doing a whole lot about the perfectionism ideas that people may be bringing in,” said Dr. Levy of the anxiety disorders center at the Institute of Living in Hartford, Conn.
A strong unmet need exists for novel targets for CBT in hoarding disorder to improve current less-than-stellar outcomes, she said. A recent meta-analysis demonstrated that while CBT did provide a statistically significant benefit for this common and often disabling disease, outcomes were far from optimal. Indeed, after completing their course of CBT, patients still scored an average of 3 standard deviations above mean normal for scores on the SI-R (Depress Anxiety. 2015 Mar;32[3]:158-66).
Other investigators have recognized the limitations of current CBT for HD and tried tweaking the therapy to boost efficacy. These efforts have included formal studies incorporating home visits, adding cognitive remediation to reduce the neuropsychological deficits often present in patients with HD, and/or extending the treatment duration to 26 weekly sessions from the current standard of 15 or 16.
Unfortunately, none of these innovations has really panned out when tested, Dr. Levy said. For example, in the 36-patient study analyzed by Dr. Levy, clinically significant improvement was seen in only 41% of subjects following 26 weeks of CBT (Depress Anxiety. 2010 May;27[5]:476-84). In contrast, published response rates for CBT in patients with obsessive-compulsive disorder – a related condition – are in the 85% range.
She described the perfectionism that often figures prominently in HD as a maladaptive belief that if something can’t be done perfectly, it’s not worth doing at all.
“People will say to me, ‘I can’t start discarding until I’ve got my organizational system down.’ They’re completely stymied. They can’t make any progress because their system isn’t fully coordinated yet,” the psychologist explained.
One way to potentially target this perfectionism more explicitly might be to incorporate cognitive restructuring or behavioral experiments that enable patients to test out and perhaps discard those beliefs, according to Dr. Levy.
She reported no financial conflicts regarding her analysis, which was based upon data collected in an earlier study sponsored by the National Institute of Mental Health.
SAN FRANCISCO – Cognitive-behavioral therapy for hoarding disorder leaves substantial room for improvement in efficacy, and additional therapeutic attention to maladaptive beliefs regarding perfectionism just might be the answer, Hannah C. Levy, PhD, said at the annual conference of the Anxiety and Depression Association of America.
She presented a secondary analysis of the relationship between cognitive-behavioral therapy (CBT) outcomes and baseline maladaptive beliefs in a wait list–controlled study of 36 patients with hoarding disorder (HD) who were not on psychiatric medication. Her purpose was to identify which cognitive domains predicted treatment outcome. This, in turn, could point the way to new treatment targets.
In contrast, the baseline strength of Saving Cognitions Inventory maladaptive beliefs about saving, such as emotional attachment to hoarded objects or a belief that keeping those objects is the only way to be able to remember an important event, proved unrelated to CBT outcomes. And this finding may help explain CBT’s limited effectiveness in HD.
“I think traditionally our CBT interventions are more focused on the maladaptive saving beliefs. We’re currently not doing a whole lot about the perfectionism ideas that people may be bringing in,” said Dr. Levy of the anxiety disorders center at the Institute of Living in Hartford, Conn.
A strong unmet need exists for novel targets for CBT in hoarding disorder to improve current less-than-stellar outcomes, she said. A recent meta-analysis demonstrated that while CBT did provide a statistically significant benefit for this common and often disabling disease, outcomes were far from optimal. Indeed, after completing their course of CBT, patients still scored an average of 3 standard deviations above mean normal for scores on the SI-R (Depress Anxiety. 2015 Mar;32[3]:158-66).
Other investigators have recognized the limitations of current CBT for HD and tried tweaking the therapy to boost efficacy. These efforts have included formal studies incorporating home visits, adding cognitive remediation to reduce the neuropsychological deficits often present in patients with HD, and/or extending the treatment duration to 26 weekly sessions from the current standard of 15 or 16.
Unfortunately, none of these innovations has really panned out when tested, Dr. Levy said. For example, in the 36-patient study analyzed by Dr. Levy, clinically significant improvement was seen in only 41% of subjects following 26 weeks of CBT (Depress Anxiety. 2010 May;27[5]:476-84). In contrast, published response rates for CBT in patients with obsessive-compulsive disorder – a related condition – are in the 85% range.
She described the perfectionism that often figures prominently in HD as a maladaptive belief that if something can’t be done perfectly, it’s not worth doing at all.
“People will say to me, ‘I can’t start discarding until I’ve got my organizational system down.’ They’re completely stymied. They can’t make any progress because their system isn’t fully coordinated yet,” the psychologist explained.
One way to potentially target this perfectionism more explicitly might be to incorporate cognitive restructuring or behavioral experiments that enable patients to test out and perhaps discard those beliefs, according to Dr. Levy.
She reported no financial conflicts regarding her analysis, which was based upon data collected in an earlier study sponsored by the National Institute of Mental Health.
AT ANXIETY AND DEPRESSION CONFERENCE 2017
Key clinical point:
Major finding: High baseline levels of perfectionism and rigidity of thinking were associated with lack of response to 26 weeks of CBT for hoarding disorder.
Data source: A secondary analysis of data from a prospective study of 36 patients with primary hoarding disorder.
Disclosures: The presenter reported no financial conflicts regarding her analysis, which was based upon data collected in an earlier study sponsored by the National Institute of Mental Health.
Practice management skills more relevant than ever
LAS VEGAS – Babatunde Akinsete, MD, took a new job about 18 months ago as a lead hospitalist within Adventist Health System of Florida. The role has the expected leadership responsibilities, but those folks he’s now partly supervising are the same ones who used to be his peers.
The same people he spent time “in the trenches” with, complaining about the problems they saw – issues that are now partly his job to help fix.
“It’s tough,” Dr. Akinsete said at the annual meeting of the Society of Hospital Medicine. “How do you motivate people?”
Welcome to managing a practice, circa 2017. The day-to-day doings of an HM group – recruiting, retention, compensation, scheduling and more – are the backbone of the specialty. And SHM’s annual meeting makes the topics a principal point, from a dedicated precourse to dozens of presentations to networking opportunities introducing experienced leaders to nascent ones.
The subject is more relevant than ever these days as the maturing specialty now has three generations of hospitalists practicing side by side, including those who founded the society and laid the groundwork for the specialty some 20 years ago and those who will now infuse it with new blood for the next 20 years, said Jerome Siy, MD, SFHM, an HM17 faculty member and chair of SHM’s Practice Management Committee.
“We’re heading into a cycle of a lot of change,” he said. “Being able to manage change is going to be pretty key.”
The first step in building or bettering a “healthy practice” is building a “culture of ownership,” Dr. Siy said.
“You must have the right culture first if you’re going to tackle any of these issues, whether it’s things like schedules to finances to negotiations,” he added. “Second is this openness and innovation to think outside the box and to allow yourself to hear things that might not work for you. Be open to it because whether you hear something that doesn’t work or not, it may inspire you to figure out … what is the key element you were missing before.”
That’s what Liza Rodriguez Jimenez, MD, is taking away from the meeting. She is moving into a codirector position for her medical group at St. Luke’s in Boise, Idaho. A crash course in alternative-payment models, full-time equivalents (FTEs), relative value units (RVUs) and scheduling was an eye-opener for her.
But to Dr. Siy’s point, it wasn’t the specific examples of how other people do what they do that intrigued Dr. Rodriguez Jimenez. It was more so that people just did it differently.
“It’s just helpful to know that there are other choices,” Dr. Rodriguez Jimenez said. “In other words, why do we do 7 on, 7 off? I don’t know. We just do. If you don’t know that you don’t know, then how do you know to change it? You get exposed to so much stuff here now that you can theoretically go back and say, ‘why do we do 7 on, 7 off? … And then let the group say we want 5 on, 10 off, 4 on, 3 off. Whatever people decide.”
Nasim Afsar, MD, SFHM, chief quality officer of the department of medicine at UCLA Health in Los Angeles, said that idea of just framing the question differently is a big deal, and a leadership skill in and of itself. For example, say a hospital medicine group’s leaders are trying to discuss whether the practice should continue its comanagement focus.
“If you frame a decision as, ‘We are going to lose this comanagement,’ there’s just something, like a gut feeling, you don’t want to lose stuff,” she said. “As opposed to, if you say, ‘Gosh, think about the gains. That we will have all this free time that we now have where we can develop other aspects of our hospital medicine group.’ So when you frame the same exact thing in terms of loss, it becomes so much more difficult for us to actually let go of that.”
Leadership is more than just framing, of course. Dr. Afsar and former SHM president Eric Howell, MD, MHM, said that leadership traits include using standardized processes to make decisions, as well as getting group members involved in those decisions when necessary and using feedback and motivation properly.
But, at day’s end, practice management is managing the needs of your practice.
For Abdul-Hady Kheder, MD, of Hamilton Hospitalists LLC in Central New Jersey, the meeting opened his eyes to techniques he could use to deal with lower reimbursement figures and less patients.
“What can help my situation will be increasing the volume of the practice,” he said. “Right now, we admit 30%-40% of the patients admitted into the hospital. National average is 60%-90% of total hospital admissions. I think that most probably will balance my financial dilemma.”
For Rodney Hollis, practice administrator for Eskenazi Health of Indianapolis, the meeting was a way to glean tips on improving his practice. One nugget he’s excited about: pairing an experienced hospitalist with a new hire for a year. As a nonclinical administrator, Hollis said he views his role as helping clinicians work on the things they are best at, while he handle the rest.
“The more clinical time that the clinical directors can spend, that’s more advantageous to the group,” Hollis said. “Allowing the nonclinical activities to be done by an administrator helps. We want more responsibility and if there’s something that our clinical is doing that I can do, why not have me do it?”
For Dr. Rodriguez Jimenez, open-ended questions like that one are among the most “insightful” takeaways from the meeting.
“There is no right or wrong way, so maybe we’ve been doing it this way ‘just because,’ ” she said. “Now we need to look at it and say, ‘Can we do it a different way? Can we adapt it? Can we change it?’”
She’s starting to sound like a practice manager already.
LAS VEGAS – Babatunde Akinsete, MD, took a new job about 18 months ago as a lead hospitalist within Adventist Health System of Florida. The role has the expected leadership responsibilities, but those folks he’s now partly supervising are the same ones who used to be his peers.
The same people he spent time “in the trenches” with, complaining about the problems they saw – issues that are now partly his job to help fix.
“It’s tough,” Dr. Akinsete said at the annual meeting of the Society of Hospital Medicine. “How do you motivate people?”
Welcome to managing a practice, circa 2017. The day-to-day doings of an HM group – recruiting, retention, compensation, scheduling and more – are the backbone of the specialty. And SHM’s annual meeting makes the topics a principal point, from a dedicated precourse to dozens of presentations to networking opportunities introducing experienced leaders to nascent ones.
The subject is more relevant than ever these days as the maturing specialty now has three generations of hospitalists practicing side by side, including those who founded the society and laid the groundwork for the specialty some 20 years ago and those who will now infuse it with new blood for the next 20 years, said Jerome Siy, MD, SFHM, an HM17 faculty member and chair of SHM’s Practice Management Committee.
“We’re heading into a cycle of a lot of change,” he said. “Being able to manage change is going to be pretty key.”
The first step in building or bettering a “healthy practice” is building a “culture of ownership,” Dr. Siy said.
“You must have the right culture first if you’re going to tackle any of these issues, whether it’s things like schedules to finances to negotiations,” he added. “Second is this openness and innovation to think outside the box and to allow yourself to hear things that might not work for you. Be open to it because whether you hear something that doesn’t work or not, it may inspire you to figure out … what is the key element you were missing before.”
That’s what Liza Rodriguez Jimenez, MD, is taking away from the meeting. She is moving into a codirector position for her medical group at St. Luke’s in Boise, Idaho. A crash course in alternative-payment models, full-time equivalents (FTEs), relative value units (RVUs) and scheduling was an eye-opener for her.
But to Dr. Siy’s point, it wasn’t the specific examples of how other people do what they do that intrigued Dr. Rodriguez Jimenez. It was more so that people just did it differently.
“It’s just helpful to know that there are other choices,” Dr. Rodriguez Jimenez said. “In other words, why do we do 7 on, 7 off? I don’t know. We just do. If you don’t know that you don’t know, then how do you know to change it? You get exposed to so much stuff here now that you can theoretically go back and say, ‘why do we do 7 on, 7 off? … And then let the group say we want 5 on, 10 off, 4 on, 3 off. Whatever people decide.”
Nasim Afsar, MD, SFHM, chief quality officer of the department of medicine at UCLA Health in Los Angeles, said that idea of just framing the question differently is a big deal, and a leadership skill in and of itself. For example, say a hospital medicine group’s leaders are trying to discuss whether the practice should continue its comanagement focus.
“If you frame a decision as, ‘We are going to lose this comanagement,’ there’s just something, like a gut feeling, you don’t want to lose stuff,” she said. “As opposed to, if you say, ‘Gosh, think about the gains. That we will have all this free time that we now have where we can develop other aspects of our hospital medicine group.’ So when you frame the same exact thing in terms of loss, it becomes so much more difficult for us to actually let go of that.”
Leadership is more than just framing, of course. Dr. Afsar and former SHM president Eric Howell, MD, MHM, said that leadership traits include using standardized processes to make decisions, as well as getting group members involved in those decisions when necessary and using feedback and motivation properly.
But, at day’s end, practice management is managing the needs of your practice.
For Abdul-Hady Kheder, MD, of Hamilton Hospitalists LLC in Central New Jersey, the meeting opened his eyes to techniques he could use to deal with lower reimbursement figures and less patients.
“What can help my situation will be increasing the volume of the practice,” he said. “Right now, we admit 30%-40% of the patients admitted into the hospital. National average is 60%-90% of total hospital admissions. I think that most probably will balance my financial dilemma.”
For Rodney Hollis, practice administrator for Eskenazi Health of Indianapolis, the meeting was a way to glean tips on improving his practice. One nugget he’s excited about: pairing an experienced hospitalist with a new hire for a year. As a nonclinical administrator, Hollis said he views his role as helping clinicians work on the things they are best at, while he handle the rest.
“The more clinical time that the clinical directors can spend, that’s more advantageous to the group,” Hollis said. “Allowing the nonclinical activities to be done by an administrator helps. We want more responsibility and if there’s something that our clinical is doing that I can do, why not have me do it?”
For Dr. Rodriguez Jimenez, open-ended questions like that one are among the most “insightful” takeaways from the meeting.
“There is no right or wrong way, so maybe we’ve been doing it this way ‘just because,’ ” she said. “Now we need to look at it and say, ‘Can we do it a different way? Can we adapt it? Can we change it?’”
She’s starting to sound like a practice manager already.
LAS VEGAS – Babatunde Akinsete, MD, took a new job about 18 months ago as a lead hospitalist within Adventist Health System of Florida. The role has the expected leadership responsibilities, but those folks he’s now partly supervising are the same ones who used to be his peers.
The same people he spent time “in the trenches” with, complaining about the problems they saw – issues that are now partly his job to help fix.
“It’s tough,” Dr. Akinsete said at the annual meeting of the Society of Hospital Medicine. “How do you motivate people?”
Welcome to managing a practice, circa 2017. The day-to-day doings of an HM group – recruiting, retention, compensation, scheduling and more – are the backbone of the specialty. And SHM’s annual meeting makes the topics a principal point, from a dedicated precourse to dozens of presentations to networking opportunities introducing experienced leaders to nascent ones.
The subject is more relevant than ever these days as the maturing specialty now has three generations of hospitalists practicing side by side, including those who founded the society and laid the groundwork for the specialty some 20 years ago and those who will now infuse it with new blood for the next 20 years, said Jerome Siy, MD, SFHM, an HM17 faculty member and chair of SHM’s Practice Management Committee.
“We’re heading into a cycle of a lot of change,” he said. “Being able to manage change is going to be pretty key.”
The first step in building or bettering a “healthy practice” is building a “culture of ownership,” Dr. Siy said.
“You must have the right culture first if you’re going to tackle any of these issues, whether it’s things like schedules to finances to negotiations,” he added. “Second is this openness and innovation to think outside the box and to allow yourself to hear things that might not work for you. Be open to it because whether you hear something that doesn’t work or not, it may inspire you to figure out … what is the key element you were missing before.”
That’s what Liza Rodriguez Jimenez, MD, is taking away from the meeting. She is moving into a codirector position for her medical group at St. Luke’s in Boise, Idaho. A crash course in alternative-payment models, full-time equivalents (FTEs), relative value units (RVUs) and scheduling was an eye-opener for her.
But to Dr. Siy’s point, it wasn’t the specific examples of how other people do what they do that intrigued Dr. Rodriguez Jimenez. It was more so that people just did it differently.
“It’s just helpful to know that there are other choices,” Dr. Rodriguez Jimenez said. “In other words, why do we do 7 on, 7 off? I don’t know. We just do. If you don’t know that you don’t know, then how do you know to change it? You get exposed to so much stuff here now that you can theoretically go back and say, ‘why do we do 7 on, 7 off? … And then let the group say we want 5 on, 10 off, 4 on, 3 off. Whatever people decide.”
Nasim Afsar, MD, SFHM, chief quality officer of the department of medicine at UCLA Health in Los Angeles, said that idea of just framing the question differently is a big deal, and a leadership skill in and of itself. For example, say a hospital medicine group’s leaders are trying to discuss whether the practice should continue its comanagement focus.
“If you frame a decision as, ‘We are going to lose this comanagement,’ there’s just something, like a gut feeling, you don’t want to lose stuff,” she said. “As opposed to, if you say, ‘Gosh, think about the gains. That we will have all this free time that we now have where we can develop other aspects of our hospital medicine group.’ So when you frame the same exact thing in terms of loss, it becomes so much more difficult for us to actually let go of that.”
Leadership is more than just framing, of course. Dr. Afsar and former SHM president Eric Howell, MD, MHM, said that leadership traits include using standardized processes to make decisions, as well as getting group members involved in those decisions when necessary and using feedback and motivation properly.
But, at day’s end, practice management is managing the needs of your practice.
For Abdul-Hady Kheder, MD, of Hamilton Hospitalists LLC in Central New Jersey, the meeting opened his eyes to techniques he could use to deal with lower reimbursement figures and less patients.
“What can help my situation will be increasing the volume of the practice,” he said. “Right now, we admit 30%-40% of the patients admitted into the hospital. National average is 60%-90% of total hospital admissions. I think that most probably will balance my financial dilemma.”
For Rodney Hollis, practice administrator for Eskenazi Health of Indianapolis, the meeting was a way to glean tips on improving his practice. One nugget he’s excited about: pairing an experienced hospitalist with a new hire for a year. As a nonclinical administrator, Hollis said he views his role as helping clinicians work on the things they are best at, while he handle the rest.
“The more clinical time that the clinical directors can spend, that’s more advantageous to the group,” Hollis said. “Allowing the nonclinical activities to be done by an administrator helps. We want more responsibility and if there’s something that our clinical is doing that I can do, why not have me do it?”
For Dr. Rodriguez Jimenez, open-ended questions like that one are among the most “insightful” takeaways from the meeting.
“There is no right or wrong way, so maybe we’ve been doing it this way ‘just because,’ ” she said. “Now we need to look at it and say, ‘Can we do it a different way? Can we adapt it? Can we change it?’”
She’s starting to sound like a practice manager already.
Statins: No benefit as primary prevention in elderly
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statins are commonly prescribed for primary prevention to older patients, particularly those older than age 75 years. And the prevalence of use in this patient population is increasing.
But statins are associated with a variety of musculoskeletal disorders – including myopathy, myalgias, muscle weakness, back conditions, injuries, and arthropathies – which may be particularly harmful in older people and contribute to their physical deconditioning and frailty. The drugs also have been linked to cognitive dysfunction, which can further raise the risk of falls and disability.
Physicians should take into consideration these multiple risks, in addition to the ALLHAT data showing no mortality benefit in this age group, before prescribing or continuing statin therapy in such patients.
Gregory Curfman, MD, is at Harvard Medical School, Boston, and is also the health care policy and law editor for JAMA Internal Medicine. Dr. Curfman reported having no relevant financial disclosures. He made these remarks in an Editor’s Note accompanying Dr. Han’s report.
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes, according to post-hoc analysis of the ALLHAT trial published online May 22 in JAMA Internal Medicine.
Many older Americans take statins for primary cardiovascular prevention even though the evidence supporting such use is both sparse and conflicting, particularly for patients aged 75 years and older, said Benjamin H. Han, MD, of the division of geriatric medicine and palliative care, New York University, and his associates.
The primary outcome, all-cause mortality, was slightly higher in the statin group. For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care, for a hazard ratio of 1.08, and for those aged 75 and older, there were 92 deaths with pravastatin vs. 65 with usual care, for an HR of 1.34. This represents a nonsignificant trend toward increased mortality with statin therapy. The results were similar, with pravastatin providing no significant benefit, regarding coronary heart disease, stroke, heart failure, and cancer events.
These findings indicate that “statins may be producing untoward effects in the function or health of older adults that could offset any possible cardiovascular benefit,” Dr. Han and his associates said (JAMA Intern Med. 2017 May 22. doi: 10.1001/jamainternmed.2017.1442).
One important limitation of this secondary analysis is that patients were allowed to cross over to the other study group during follow-up, at their own or their physicians’ discretion. By the end of the study, 29% of the control group were taking lipid-lowering drugs while 22% of the active-treatment group had discontinued pravastatin. “This level of crossover should be considered when interpreting our findings,” the investigators noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Statin therapy given as primary prevention to adults aged 65 years and older produced no benefit in any primary or secondary outcomes.
Major finding: For patients aged 65-74 years, there were 141 deaths with pravastatin vs. 130 with usual care (HR, 1.08), and for those aged 75 years and older, there were 92 deaths with pravastatin vs. 65 with usual care (HR, 1.34).
Data source: A secondary analysis of data in the randomized, open-label ALLHAT trial, focusing on 2,867 elderly participants followed for 6 years.
Disclosures: This work was supported by the National Institutes of Health. Pfizer, AstraZeneca, and Bristol-Myers Squibb provided study medications free of charge. Dr. Han and his associates reported having no relevant financial disclosures.