User login
At Opening Reception: Vendors, Posters, Raffle
Meet up with friends, visit vendor booths to see the latest and greatest in all things vascular, peruse the posters.
And enter a high-tech raffle to win a high-flying prize: two airline tickets to anywhere in the continental United States AND complimentary registration for the 2018 Vascular Annual Meeting. While traversing the Exhibit Hall, be sure to look for placards with QR codes in select exhibitor booths; scan the QR code and be automatically entered into the raffle. No pens, no paper required, just a smart phone and the QR code.
Industry support is vital to VAM, permitting registration fees considerably lower than those assessed at comparable annual meetings. Please show your support by visiting our vendors.
The reception coincides with the end of the Interactive Poster Session, set for 5:00 to 6:30 p.m. in the same location.
Thursday, June 1
5:00 – 6:30 p.m.
Exhibit Hall B
Interactive Poster Session
5:30 – 6:30 p.m.
Exhibit Hall B
Opening Reception
Meet up with friends, visit vendor booths to see the latest and greatest in all things vascular, peruse the posters.
And enter a high-tech raffle to win a high-flying prize: two airline tickets to anywhere in the continental United States AND complimentary registration for the 2018 Vascular Annual Meeting. While traversing the Exhibit Hall, be sure to look for placards with QR codes in select exhibitor booths; scan the QR code and be automatically entered into the raffle. No pens, no paper required, just a smart phone and the QR code.
Industry support is vital to VAM, permitting registration fees considerably lower than those assessed at comparable annual meetings. Please show your support by visiting our vendors.
The reception coincides with the end of the Interactive Poster Session, set for 5:00 to 6:30 p.m. in the same location.
Thursday, June 1
5:00 – 6:30 p.m.
Exhibit Hall B
Interactive Poster Session
5:30 – 6:30 p.m.
Exhibit Hall B
Opening Reception
Meet up with friends, visit vendor booths to see the latest and greatest in all things vascular, peruse the posters.
And enter a high-tech raffle to win a high-flying prize: two airline tickets to anywhere in the continental United States AND complimentary registration for the 2018 Vascular Annual Meeting. While traversing the Exhibit Hall, be sure to look for placards with QR codes in select exhibitor booths; scan the QR code and be automatically entered into the raffle. No pens, no paper required, just a smart phone and the QR code.
Industry support is vital to VAM, permitting registration fees considerably lower than those assessed at comparable annual meetings. Please show your support by visiting our vendors.
The reception coincides with the end of the Interactive Poster Session, set for 5:00 to 6:30 p.m. in the same location.
Thursday, June 1
5:00 – 6:30 p.m.
Exhibit Hall B
Interactive Poster Session
5:30 – 6:30 p.m.
Exhibit Hall B
Opening Reception
Does laparoscopic versus open abdominal surgery for stage I endometrial cancer affect oncologic outcomes?
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
The objective of the study by Janda and colleagues (known as the “LACE” trial) was to evaluate the equivalency of total laparoscopic hysterectomy (TLH) with staging versus the standard procedure, which is total abdominal hysterectomy (TAH) with staging, for surgical management of women with presumed low-risk, early-stage endometrial cancer.
Related Article:
2016 Update on cancer
Details of the study
This nonblinded, randomized controlled multicenter equivalency trial included 760 women from Australia, New Zealand, and Hong Kong undergoing surgical management of presumed stage I uterine endometrioid adenocarcinoma. All surgeries were performed or supervised by trained gynecologic oncologists. Pelvic lymph node sampling was required but omission was permitted for: morbid obesity, low risk of metastasis based on frozen section results, medically unfit status, or institutional guidelines prohibiting the procedure. Patients were excluded for preoperative nonendometrioid histology, suspected ultimate FIGO stage II–IV based on preoperative imaging, or uterine size greater than 10 weeks’ gestation.
The primary outcome was disease-free survival, defined as the time from surgery to the date of first recurrence, which included disease progression, development of a new primary malignancy, or death. Secondary outcomes included disease recurrence, patterns of recurrence, and overall survival. A 7% difference in disease-free survival at 4.5 years postoperatively was prespecified and determined based on previously published literature.1–4
By Kaplan-Meier estimates, disease-free survival at 4.5 years was 81.3% in the TAH group and 81.6% in the TLH group, a 0.3% difference. In addition, there were no differences noted in secondary outcomes, further supporting equivalency of the surgical modalities. The only significantly different surgical findings included decreased operative time in the TAH group and decreased lymph node dissection completion in the TLH group.
Related Article:
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Study strengths and weaknesses
The largest previous trial of more than 2,000 patients examining the method of surgical management was the Gynecologic Oncology Group’s (GOG) noninferiority LAP2 trial.3 This trial has been used widely to promote a minimally invasive approach, but did not actually reach the prespecified statistical goals. The LACE trial, however, successfully reached its statistical targets and is now the largest randomized trial supporting an equivalence in oncologic outcomes.
It is important to recognize the limitations of the LACE trial in the current medical environment. The study population was a very specific group of low-risk women without high-risk histologic subtypes or even moderately enlarged uteri; many institutions would consider offering a minimally invasive approach to these women. In addition, this study did not include robotic minimally invasive surgery, which in many regions of the country is rapidly becoming accepted as the first choice procedure over traditional laparoscopy.5 Furthermore, the FIRES trial and others6–8 have demonstrated that utilizing a minimally invasive approach that includes sentinel lymph node identification and removal may be as diagnostic as a full dissection, adding considerations to surgical modality selection.
--Kathryn A. Mills, MD, and David G. Mutch, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355.
- Stevenson AR, Solomon MJ, Lumley JW, et al; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700.
- Creutzberg CL, van Putten WL, Koper PC, et al; Post Operative Radiation Therapy in Endometrial Carcinoma. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Lancet. 2000;355(9213):1404–1411.
- Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. 2016;34(10):1087–1096.
- Rossi EC, Kowalski LD, Scalici JS, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392.
- Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;12(3):531–535.
- Darai E, Dubernard G, Bats AS, et al. Sentinel node biopsy for the management of early stage endometrial cancer: long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136(1):54-59.
Caring for the transgender patient: The role of the gynecologist
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Transgender man consults gynecologist for fertility options
A 36-year-old transgender man considering the possibility of having his own biological children presents to the gynecology office to discuss hysterectomy as gender dysphoria treatment as well as his fertility preservation options. He has never had a gynecologic examination. Since age 24, he has been on testosterone therapy. Although his menses initially ceased, each month over the past 2 years he has had breakthrough spotting lasting 2 to 4 days, sometimes accompanied by pelvic pain and cramping. These symptoms have caused him distress and anxiety, which have led to his missing work 1 to 3 days each month. On presentation, he appears anxious and makes little eye contact. His girlfriend of 6 years has come in with him and is very supportive.
Over the past decade, transgender health care has moved to the forefront of the medical conversation. At many prominent medical centers across the United States, clinicians are forming multidisciplinary teams to help improve the health care of this patient population. Outcomes are being studied, and the literature is becoming more robust.
People tend to think of transgender women—male-assigned persons who self-identify as female—as the typical prototype for transgender people, but this focus is skewed in both society and the medical community. Transgender men—female-assigned persons who self-identify as male—remain underrepresented, mostly because they want to stay “under the radar,” especially with respect to medical care and, more specifically, routine gynecologic care.
Although the transgender woman has unique health needs and may present to a gynecologist for care after gender-affirmationsurgery, the transgender man’s many health care needs and their subtleties can be addressed only by a gynecologist. In this article, I review these intricacies of care to help increase clinician comfort in treating these patients.
Clinicians can take steps to:
- ensure all patients have the correct identifiers in their medical records
- provide staff with the proper education and diversity training
- instruct staff in proper use of pronouns
- set up unisex or gender-nonbinary restrooms with appropriate signage
- make the decor gender nonspecific.
Beth Cronin, MD, a practicing general gynecologist in Providence, Rhode Island, says that you also should consider a general sign, placed in a highly visible area, that represents your nondiscrimination policy. The AMA offers this wording: "This office appreciates the diversity of human beings and does not discriminate based on race, age, religion, ability, marital status, sexual orientation, sex or gender identity." She also recommends having education and marketing materials with affirmative imagery and content and providing educational brochures on transgender health topics.
Why transgender patients may delay seeking health care
Transgender patients remain underserved because of the health care barriers they encounter. Factors contributing to poor access include lack of health insurance, inability to pay for services, clinician insensitivity and hostility, and fear of exposure of transgender status during health care encounters.1 In a recent large survey study, 30% of transgender respondents indicated that they delayed or did not seek medical care as a result of discrimination, and those who had needed to teach their clinicians about transgenderism were 4 times more likely to postpone or not seek care.2
In a 2015 survey of ObGyns’ current knowledge and practice regarding LGBT (lesbian, gay, bisexual, transgender) care, only one-third of respondents indicated they were comfortable caring for transgender patients.3 In addition, only one-third indicated being knowledgeable about the steps transgender patients must take to transition to their self-identified gender, and less than half were familiar with the recommendations for the routine health maintenance and screening of these patients.
Much of this discomfort derives from the lack of incorporation of LGBT-specific topics in medical curricula. In 2011, Obedin-Maliver and colleagues found that, at 176 US and Canadian allopathic and osteopathic medical schools, the median time dedicated to LGBT health care needs and related topics was unsatisfactory.4 This deficiency is slowly being reduced with changes in the curricula of many health care specialties. In ObGyn residency programs, for example, transgender-specific questions have been added to annual in-service examinations. The hope is that, as education initiatives improve, clinicians will become more comfortable caring for gender-minority patients, who with improved access to care will no longer need to seek subspecialists in transgender services.
Read about the need for gyn exams, managing benign disorders, and cervical cancer screening
Considerations for the gynecologic visit and examination
Transgender men visit the gynecology office for many reasons, including routine gynecologic care and health maintenance, care for acute and chronic gynecologic conditions (abnormal bleeding, pelvic pain, vaginitis), evaluation and management of pelvic floor disorders, consultation on hysterectomy for gender transition, and fertility counseling.
However, transgender men who reach their third, fourth, or fifth decade without having had a pelvic examination cite many reasons for avoiding the gynecology office. Most commonly, gynecologic visits and genital examination can severely exacerbate these patients’ gender dysphoria. In addition, many patients who do not engage in penetrative vaginal sex think their health risks are so low that they can forgo or delay pelvic exams. Patients who have stopped menstruating while on testosterone therapy may think there is no need for routine gynecologic care. Other reasons for avoiding pelvic exams are pain and traumatic sexual memories.5
Related Article:
Four pillars of a successful practice: 4. Motivate your staff
Transgender men need to receive the regular guideline-recommended pelvic exams and screenings used for cisgender women. (Cisgender refers to a person whose sense of gender identity corresponds with their birth sex.) We need to educate patients in this regard and to discuss several issues before performing an examination. First, take a thorough history and avoid making assumptions about sexual orientation and sex practices. Some patients have penetrative vaginal intercourse with either men or women. For some patients, the exam may cause dysphoria symptoms, and we need to validate patients’ fears. Discussing these issues ahead of time helps patients get used to the idea of undergoing an exam and assures them that the clinician is experienced in performing these exams for transgender men. In my practice, we explain the exam’s purpose (screening or diagnosis) and importance. We also counsel patients that they may experience some normal, and temporary, spotting after the exam. For those who experience severe dysphoria with vaginal bleeding of any kind, we acknowledge that postexam spotting may cause some anxiety. Patients with severe anxiety before the exam may be premedicated with an anxiolytic agent as long as someone can transport them to and from the office.
The bimanual exam should be performed with care and efficiency and with the patient given as much control as possible. In most cases, we ask patients to undress only from the waist down, and their genitals stay covered. Patients uncomfortable in stirrups are asked to show us the position that suits them best, and we try to accommodate them. Although speed is a goal, remember that many patients are nulliparous, have had limited or no vaginal penetration, or are on testosterone and have significant vaginal dryness. Use the smallest speculum possible, a pediatric or long and narrow adult speculum, and apply lubricant copiously. Pre-exam application of topical lidocaine jelly to the introitus can help reduce pain. To help a patient relax the pelvic floor muscles and habituate to the presence of a foreign object in the vagina, start the exam by inserting a single digit. In addition, ask the patient about speculum placement inside the vagina: Does he want to place the speculum himself or guide the clinician’s hand? Open the speculum only as much as needed to adequately visualize the cervix and then remove it with care.
Managing benign gynecologic disorders
The same algorithms are used to evaluate abnormal bleeding in all patients, but the differential diagnosis expands for those on testosterone therapy. Testosterone may no longer be suppressing their cycles, and abnormal bleeding could simply be the return of menses, which would present as regular cyclic bleeding. Increasing the testosterone dosing or changing the testosterone formulation may help, and the gynecologist should discuss these options with the patient’s prescribing clinician. In addition, progesterone in any form (for example, medroxyprogesterone acetate 5 to 30 mg daily) can be added to testosterone regimens to help suppress menses. The levonorgestrel-releasing intrauterine device (LNG-IUD) can be very effective, but placement can induce anxiety, and some patients decline this treatment option.
In patients with intermenstrual spotting, assess the vagina for atrophy. Both over-the-counter vaginal moisturizers and DHEA (dehydroepiandrosterone) suppositories (1% compounded) can help treat atrophy, but not all patients are comfortable using them. Most patients decline vaginal estrogen products for symptomatic vaginal atrophy even though the systemic effects are minimal.
The historic literature suggests that female-to-male patients’ long-term exposure to androgens leads to atrophic changes in the endometrium and myometrium, and clinical studies of menopausal women who take exogenous androgens have confirmed this effect.6 However, new data point to a different histologic scenario. A recent study found a possible association between long-term testosterone use in transgender men of reproductive age and a low proliferative active endometrium, as well as hypertrophic changes in the myometrium.7 The causes may be peripheral aromatization of androgens and expression and up-regulation of androgen receptors within the endometrial stroma and myometrial cells.8 Given these emerging data and anecdotal cases reported by clinicians who perform hysterectomies for transgender men, imaging and tissue sampling should be used to evaluate abnormal uterine bleeding, particularly in patients previously amenorrheic on testosterone. Be aware that transvaginal ultrasound or endometrial biopsy are challenging procedures for these patients. Counsel patients to ensure that they adhere to follow-up.
Related Article:
2017 Update on cervical disease
The ongoing need for cervical cancer screening
The concept of “original gender surveillance” was presented in a 2-case series of transgender men with uterine and cervical cancer that might have been detected earlier with better screening and routine care.9 There is no evidence, however, that long-term high-dose androgen therapy causes endometrial or cervical cancer,10 and the data on endometrial cancer in patients on cross-sex hormone therapy are limited such that a causal relationship between testosterone and these malignancies cannot be established.9,11–14
The rate of unsatisfactory Pap smears is higher in transgender men than in cisgender women. The difference was anecdotally noted by clinicians who routinely cared for transgender patients over time and was confirmed with a retrospective chart review.15
Peitzmeier and colleagues reviewed the records of 233 transgender men and 3,625 cisgender women with Pap tests performed at an urban community health center over 6 years.15 The transgender cohort, with its prevalence rate of 10%, was 10 times more likely to have an unsatisfactory or inadequate Pap smear. Moreover, the transgender patients were more likely to have longer latency to follow-up for a repeat Pap test. In addition, testosterone therapy was more likely associated with inadequate Pap smears, and time on testosterone therapy was associated with higher odds of Pap smear inadequacy. Besides the exogenous hormone therapy, clinician comfort level and experience may have contributed to the high prevalence of inadequate Pap smears.
As mentioned earlier, it is important to become comfortable performing pelvic exams for transgender men and to prepare patients for the possibility that a Pap smear might be inadequate, making a follow-up visit and repeat Pap test necessary.16
Read about hysterectomy, oophorectomy, and vaginectomy choices
Consultation for hysterectomy: Perioperative considerations
Transgender men may undergo hysterectomy, oophorectomy, and/or vaginectomy. The TABLE summarizes the indications and perioperative considerations for each procedure.

Some transgender men undergo hysterectomy for benign gynecologic disease. Counseling and perioperative planning are the same for these patients as for cisgender women, although some of the considerations discussed here remain important.
Other patients undergo hysterectomy as part of transitioning to their self-affirmed gender. The World Professional Association for Transgender Health (WPATH) Standards of Care should be used to guide counseling and treatment.17 These guidelines were designed as a framework for performing hysterectomy and other gender-affirming procedures. According to the WPATH standards, the criteria for hysterectomy and oophorectomy are:
- 2 referral letters from qualified mental health professionals
- well-documented persistent gender dysphoria
- capacity to make fully informed decisions and to consent to treatment
- age of majority in given country
- good control of any concurrent medical or mental health concerns, and
- hormone therapy for 12 continuous months, as appropriate to gender goals, unless the patient has a medical contraindication or is otherwise unable or unwilling to take hormones.
As the guidelines emphasize, these criteria do not apply to patients undergoing either procedure for medical indications other than gender dysphoria.
Hysterectomy approach. Most surgeons perform gender-affirming hysterectomies laparoscopically. Many clinicians hesitate to perform these hysterectomies vaginally, as the patients are often nulliparous. In general, the best operative route is the one the surgeon feels most comfortable performing safely and efficiently. For a nulliparous patient with minimal pelvic organ descensus and a narrow pelvis, the laparoscopic approach is reasonable. A recent study in a small cohort of transgender men found that vaginal hysterectomy was successful in only 1 in 4 patients.18 Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) recommends vaginal hysterectomy, when appropriate, for limiting complications and morbidity while maximizing cost-effectiveness.19 Although data are limited, vaginal hysterectomy seems feasible and should be considered in a subset of patients who pre‑sent for gender-affirming hysterectomy.
Related Article:
Total laparoscopic versus laparoscopic supracervical hysterectomy
The oophorectomy debate
Oophorectomy concurrent with hysterectomy remains a topic of debate among gynecologists who perform hysterectomy for gender transition. Some clinicians think gonadectomy poses a significant risk for bone health compromise at an early age. The long-term effects of testosterone on bone have not been well studied. Although bone metabolism is thought to increase over the short term, there are no major changes in bone density over the long term. In fact, in the setting of long-term testosterone therapy, cortical bone was found to be larger in transgender men than in cisgender women.20 The issue is for patients who stop taking exogenous testosterone after oophorectomy. This subset of patients has not been well studied but clearly needs bone health surveillance and supplementation.
Another concern about oophorectomy is its effect on fertility. Because it is important to discuss fertility-preserving options, during consultation for a hysterectomy I spend a large portion of time addressing fertility goals. Patients who want to become a parent but do not want to carry a child (they want a current or future partner or surrogate to carry) are candidates for hysterectomy; those who do not want a genetic child are candidates for oophorectomy; and those who do not want to preserve their fertility (or have already ended it) and who meet the WPATH criteria for surgery are candidates for oophorectomy concurrent with hysterectomy. The discussion can be particularly challenging with young transgender men, since their ability to project their family planning goals may be compromised by their gender dysphoria. Clinicians can counsel patients about another option: isolated hysterectomy with subsequent staged oophorectomy.
Similar to cisgender women with polycystic ovary syndrome, transgender men on exogenous testosterone therapy are at risk for ovarian cysts,7 which can cause pain and should be evaluated and managed. As mentioned, these patients may find it difficult to visit a gynecologist and tolerate a vaginal examination, and many fear presenting to an emergency room, as they will need to disclose their transgender status and risk being discriminated against or, worse, not being triaged or cared for properly. Patients should be thoroughly counseled about the risks and benefits of having oophorectomy performed concurrently with hysterectomy.
Related Article:
Vaginal hysterectomy with basic instrumentation
The question of vaginectomy
Patients and clinicians often ask about concurrent vaginectomy procedures. In some cases, patients with severe gender dysphoria and absence of penetrative vaginal activity request excision or obliteration of the vagina. There is no standard of care, however. Vaginectomy can be done transvaginally or abdominally: open, laparoscopically, or robotically. It therefore should be performed by surgeons experienced in the procedure. Patients should be advised that a portion of the vaginal epithelium is sometimes used for certain phalloplasty procedures and that, if they are considering genital reconstruction in the future, it may be beneficial to preserve the vagina until that time.
There are no guidelines on stopping or continuing testosterone therapy perioperatively. Some clinicians are concerned about possible venous thromboembolic events related to perioperative use of testosterone, but there are no data supporting increased risk. The risk of postoperative vaginal cuff bleeding in patients on and off testosterone has not been well studied. Since patients who stop taking testosterone may develop severe mood swings and malaise, they should be counseled on recognizing and managing such changes. There are also no data on the risk of vaginal cuff dehiscence in this patient population. Testosterone usually causes the vagina to become very atrophic, so proper closure should be ensured to avoid cuff evisceration. In my practice, the vaginal cuff is closed in 2 layers using at least 1 layer of delayed absorbable suture.
Read about addressing fertility, contraception, OB care, and your role
Addressing fertility, contraception, and obstetric care
Most transgender men are able to conceive a child.21 Data in this area, however, are sparse. Most of the literature on reproductive health in this patient population is focused on human immunodeficiency virus (HIV) and other sexually transmitted infections.22 Nevertheless, patient-physician dialogue on fertility and reproductive health has increased since more patients started seeking surgical transition services (likely a result of improved coverage for these surgeries). In addition, we are learning more about patients’ ability and desire to conceive after long-term use of cross-sex hormone therapy. The importance of this dialogue is becoming apparent. One survey study found that more than half of the transgender men who had undergone affirmation surgery wanted to become parents.23
Before initiating cross-sex hormone therapy or before undergoing hysterectomy and/or oophorectomy, patients must be counseled about their fertility options. Testosterone may affect fertility and fecundity, but there are case reports of successful pregnancy after discontinuation of testosterone.21 Reproductive endocrinology and fertility specialists have begun to recognize the importance of fertility preservation in this patient population and to apply the principles of oncofertility care beyond patients with cancer. In a 2015 opinion paper on access to fertility services by transgender persons, the Ethics Committee of the American Society for Reproductive Medicine focused on this population’s unique fertility needs.24 Currently, oocyte and embryo cryopreservation are options for transgender men planning to start cross-sex hormones or undergo surgery.25 Other methods being investigated may become options in the future.25
There are even fewer data on transgender men’s contraceptive needs. Many clinicians mistakenly think these patients are at low risk for pregnancy. Some patients have male partners and engage in penetrative penile-vaginal intercourse; others are not on testosterone therapy; and still others, despite taking testosterone, are not always amenorrheic and may be ovulating. In a small cross-sectional study, Light and colleagues found that 12% of transgender men who were surveyed after conceiving had been amenorrheic on testosterone therapy, and 24% of these pregnancies were not planned.21
In a study by Cipres and colleagues, half of the 26 transgender men were considered at risk for pregnancy: These patients still had a uterus, not all were on testosterone, not all on testosterone were amenorrheic, they were having vaginal intercourse with cisgender men, and none were using condoms or other contraception.26 The authors noted several potential underlying reasons for poor counseling on contraceptive needs: patients feel stigmatized, clinicians assume these patients are not candidates for “female” hormone therapy, patients fear these modalities may feminize them and compromise their affirmed identities, patients poorly understand how testosterone works and have mistaken ideas about its contraceptive properties, and clinician discomfort with broaching fertility and reproductive health discussions.
Data are also limited on pregnancy in transgender men. We do know that clinicians are not well equipped to help patients during the peripartum period and better resources are needed.21 Gender dysphoria can worsen during and immediately after pregnancy, and patients may be at significant risk for postpartum depression. More research is needed.
Related Article:
Care of the transgender patient: What is the gynecologist's role?
Gynecologists play key role in transgender care
Transgender men’s unique health care needs can be addressed only by gynecologists.It is important to become comfortable with and educated about these needs and their subtleties. This starts with understanding transgender patients’ gender dysphoria associated with the gynecologic visit and examination. Learning more about these patients and their needs will improve health care delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
- Xavier JM, Simmons R. The Washington Transgender Needs Assessment Survey, 2000. http://www.glaa.org/archive/2000/tgneedsassessment1112.shtml. Accessed January 2, 2017.
- Jaffee KD, Shires DA, Stroumsa D. Discrimination and delayed health care among transgender women and men: implications for improving medical education and health care delivery. Med Care. 2016;54(11):1010–1016.
- Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health. 2015;24(2):114–118.
- Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306(9):971–977.
- Feldman J. Medical and surgical management of the transgender patient: what the primary care clinician needs to know. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. Fenway Guide to Lesbian, Gay, Bisexual, and Transgender Health. Philadelphia, PA: American College of Physicians; 2008:365–392.
- Hickok LR, Toomey C, Speroff L. A comparison of esterified estrogens with and without methyltestosterone: effects on endometrial histology and serum lipoproteins in postmenopausal women. Obstet Gynecol. 1993;82(6):919–924.
- Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691.
- Mertens HJ, Heineman MJ, Koudstaal J, Theunissen P, Evers JL. Androgen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):11–13.
- Urban RR, Teng NN, Kapp DS. Gynecologic malignancies in female-to-male transgender patients: the need of original gender surveillance. Am J Obstet Gynecol. 2011;204(5):e9–e12.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159(3):197–202.
- Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497.
- Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76(3):413–415.
- Dizon DS, Tejada-Berges T, Keolliker S, Steinhoff M, Grania CO. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Oncol Invest. 2006;62(4):226–228.
- Schenck TL, Holzbach T, Zantl N, et al. Vaginal carcinoma in a female-to-male transsexual. J Sex Med. 2010;7(8):2899–2902.
- Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784.
- Potter J, Peitzmeier SM, Bernstein I, et al. Cervical cancer screening for patients on the female-to-male spectrum: a narrative review and guide for clinicians. J Gen Intern Med. 2015;30(12):1857–1864.
- Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Version 7. https://s3.amazonaws.com/amo_hub_content/Association140/files/Standards_of_Care_V7_2011_WPATH(2)(1).pdf. Published 2011. Accessed January 21, 2017.
- Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of vaginal hysterectomy for female-to-male transgender men. Obstet Gynecol. 2017;129(3):457–463.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466.
- Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127.
- Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS Behav. 2011;15(3):683–686.
- Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487.
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104(5):1111–1115.
- Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871.
- Cipres D, Seidman D, Cloniger C 3rd, Nova C, O’Shea A, Obedin-Maliver J. Contraceptive use and pregnancy intentions among transgender men presenting to a clinic for sex workers and their families in San Francisco. Contraception. 2016;95(2):186–189.
Pregnancy test missed before IUD placement? Your liability.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.
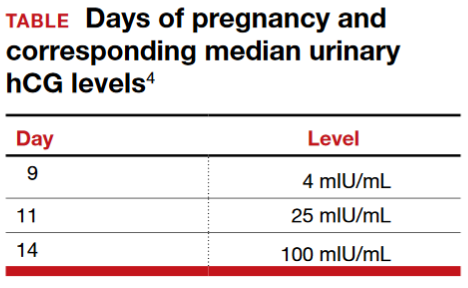
Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.

Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.

Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
Weight loss and dietary management for PCOS
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Weight loss and dietary management for PCOS
I enjoyed Dr. Barbieri’s editorial on polycystic ovary syndrome (PCOS), but I feel that first-line management for PCOS should be weight loss and diet modifications that include instructions on decreasing carbohydrates and i
Luis Linan, MD
El Paso, Texas
Metformin and progesterone for PCOS-related infertility
I have been using Beyaz and Yaz for several years in my PCOS patients for the lower androgenic activity of the drospirenone based on the same assumption and its similarity to spironolactone. I have gotten great results with metformin 1,500 mg daily and, for those who desire fertility, cycling once a month for 10 days with progesterone. My own daughter was able to conceive in just 3 months of therapy. PCOS is extremely common in our region, probably due to the high obesity rate. I saw many more cases here than I ever thought I would when I was training.
Lisa Gowan, CNM, WHNP-BC
Albany, Georgia
Check insulin levels in PCOS patients
Thank you for the very nice article regarding PCOS treatment. Does Dr. Barbieri routinely check insulin levels on patients before treating with metformin and does he require abnormal insulin levels to be present before initiating treatment? The article suggested that using the listed risk factors is sufficient. Additionally, does he perform glucose-insulin testing? If so, what is the protocol used? I have used fasting levels and 2-hour post 75-g glucose-drink testing as well. What is the best approach?
Scott A. Beckman, MD
Jasper, Indiana
Contraception and spironolactone
As usual, Dr. Barbieri has provided a thorough, concise, and practical overview on the medical management of PCOS. I would add just one small point. Another reason for using an oral estrogen-progestin pill concomitantly with spironolactone is due to the potential teratogenicity of this medication.
Bryan R. Hecht, MD
Cleveland, Ohio
Low-carb diet helps mitigate metformin side effects
Thank you for the article on PCOS. I have been treating PCOS this way for about 15 years and have been following lipids and seen dramatic improvements with that as well. I wish we as a medical community would focus on the low carbohydrate diet to help avert metformin side effects as well as treat the metabolic issues. You can get many people back on metformin by just adjusting their diet. I hope you can spread this word.
Steven Foley, MD
Lamar, Colorado
Appreciates Dr. Barbieri’s editorials
G’Day from Australia. I am a big fan of your editorials and opinions and enjoy reading
Kanapathippillai Sivanesan, MD
Brisbane, Australia
Dr. Barbieri responds
I thank Dr. Linan, Dr. Foley, and Ms. Gowan for sharing their important insights with our readers. I agree with Dr. Linan that I should have highlighted the important guidance that women with PCOS and a body mass index (BMI) above the normal range should be encouraged to reduce their weight by 5% to 10% with diet and exercise. Dr. Foley offers a clinical pearl that a low carbohydrate diet will reduce the gastrointestinal symptoms that may occur with metformin therapy. Ms. Gowan notes that the combination of metformin plus cyclic progesterone may help to initiate more frequent ovulatory cycles in women with PCOS, thereby improving fertility. Dr. Hecht reminds us that spironolactone is a teratogen and using effective contraception can help reduce the risk of exposing a pregnancy to the medication.
Dr. Beckman raises the important clinical issue of whether it is helpful to measure insulin concentration. Measuring insulin and glucose is especially helpful in understanding the causes of hypoglycemia. An elevated insulin level at the time of an abnormally low glucose level is very worrisome. However, for women with PCOS, in whom insulin resistance is common, measuring insulin is of minimal clinical value. A normal or elevated insulin level is consistent with the diagnosis of PCOS. Assessing BMI, waist circumference, HDL-cholesterol, fasting triglyceride level, and blood pressure— components of the metabolic syndrome—are much more useful clinically. The dermatologic skin lesion acanthosis nigricans is also a sign consistent with insulin resistance. I do not measure insulin levels in my patients with PCOS. Metformin is a useful agent in the treatment of PCOS whether or not insulin resistance is present. Metformin may have direct actions on the ovary to reduce androgen production, in addition to its beneficial effects in the liver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Weight loss and dietary management for PCOS
I enjoyed Dr. Barbieri’s editorial on polycystic ovary syndrome (PCOS), but I feel that first-line management for PCOS should be weight loss and diet modifications that include instructions on decreasing carbohydrates and i
Luis Linan, MD
El Paso, Texas
Metformin and progesterone for PCOS-related infertility
I have been using Beyaz and Yaz for several years in my PCOS patients for the lower androgenic activity of the drospirenone based on the same assumption and its similarity to spironolactone. I have gotten great results with metformin 1,500 mg daily and, for those who desire fertility, cycling once a month for 10 days with progesterone. My own daughter was able to conceive in just 3 months of therapy. PCOS is extremely common in our region, probably due to the high obesity rate. I saw many more cases here than I ever thought I would when I was training.
Lisa Gowan, CNM, WHNP-BC
Albany, Georgia
Check insulin levels in PCOS patients
Thank you for the very nice article regarding PCOS treatment. Does Dr. Barbieri routinely check insulin levels on patients before treating with metformin and does he require abnormal insulin levels to be present before initiating treatment? The article suggested that using the listed risk factors is sufficient. Additionally, does he perform glucose-insulin testing? If so, what is the protocol used? I have used fasting levels and 2-hour post 75-g glucose-drink testing as well. What is the best approach?
Scott A. Beckman, MD
Jasper, Indiana
Contraception and spironolactone
As usual, Dr. Barbieri has provided a thorough, concise, and practical overview on the medical management of PCOS. I would add just one small point. Another reason for using an oral estrogen-progestin pill concomitantly with spironolactone is due to the potential teratogenicity of this medication.
Bryan R. Hecht, MD
Cleveland, Ohio
Low-carb diet helps mitigate metformin side effects
Thank you for the article on PCOS. I have been treating PCOS this way for about 15 years and have been following lipids and seen dramatic improvements with that as well. I wish we as a medical community would focus on the low carbohydrate diet to help avert metformin side effects as well as treat the metabolic issues. You can get many people back on metformin by just adjusting their diet. I hope you can spread this word.
Steven Foley, MD
Lamar, Colorado
Appreciates Dr. Barbieri’s editorials
G’Day from Australia. I am a big fan of your editorials and opinions and enjoy reading
Kanapathippillai Sivanesan, MD
Brisbane, Australia
Dr. Barbieri responds
I thank Dr. Linan, Dr. Foley, and Ms. Gowan for sharing their important insights with our readers. I agree with Dr. Linan that I should have highlighted the important guidance that women with PCOS and a body mass index (BMI) above the normal range should be encouraged to reduce their weight by 5% to 10% with diet and exercise. Dr. Foley offers a clinical pearl that a low carbohydrate diet will reduce the gastrointestinal symptoms that may occur with metformin therapy. Ms. Gowan notes that the combination of metformin plus cyclic progesterone may help to initiate more frequent ovulatory cycles in women with PCOS, thereby improving fertility. Dr. Hecht reminds us that spironolactone is a teratogen and using effective contraception can help reduce the risk of exposing a pregnancy to the medication.
Dr. Beckman raises the important clinical issue of whether it is helpful to measure insulin concentration. Measuring insulin and glucose is especially helpful in understanding the causes of hypoglycemia. An elevated insulin level at the time of an abnormally low glucose level is very worrisome. However, for women with PCOS, in whom insulin resistance is common, measuring insulin is of minimal clinical value. A normal or elevated insulin level is consistent with the diagnosis of PCOS. Assessing BMI, waist circumference, HDL-cholesterol, fasting triglyceride level, and blood pressure— components of the metabolic syndrome—are much more useful clinically. The dermatologic skin lesion acanthosis nigricans is also a sign consistent with insulin resistance. I do not measure insulin levels in my patients with PCOS. Metformin is a useful agent in the treatment of PCOS whether or not insulin resistance is present. Metformin may have direct actions on the ovary to reduce androgen production, in addition to its beneficial effects in the liver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Weight loss and dietary management for PCOS
I enjoyed Dr. Barbieri’s editorial on polycystic ovary syndrome (PCOS), but I feel that first-line management for PCOS should be weight loss and diet modifications that include instructions on decreasing carbohydrates and i
Luis Linan, MD
El Paso, Texas
Metformin and progesterone for PCOS-related infertility
I have been using Beyaz and Yaz for several years in my PCOS patients for the lower androgenic activity of the drospirenone based on the same assumption and its similarity to spironolactone. I have gotten great results with metformin 1,500 mg daily and, for those who desire fertility, cycling once a month for 10 days with progesterone. My own daughter was able to conceive in just 3 months of therapy. PCOS is extremely common in our region, probably due to the high obesity rate. I saw many more cases here than I ever thought I would when I was training.
Lisa Gowan, CNM, WHNP-BC
Albany, Georgia
Check insulin levels in PCOS patients
Thank you for the very nice article regarding PCOS treatment. Does Dr. Barbieri routinely check insulin levels on patients before treating with metformin and does he require abnormal insulin levels to be present before initiating treatment? The article suggested that using the listed risk factors is sufficient. Additionally, does he perform glucose-insulin testing? If so, what is the protocol used? I have used fasting levels and 2-hour post 75-g glucose-drink testing as well. What is the best approach?
Scott A. Beckman, MD
Jasper, Indiana
Contraception and spironolactone
As usual, Dr. Barbieri has provided a thorough, concise, and practical overview on the medical management of PCOS. I would add just one small point. Another reason for using an oral estrogen-progestin pill concomitantly with spironolactone is due to the potential teratogenicity of this medication.
Bryan R. Hecht, MD
Cleveland, Ohio
Low-carb diet helps mitigate metformin side effects
Thank you for the article on PCOS. I have been treating PCOS this way for about 15 years and have been following lipids and seen dramatic improvements with that as well. I wish we as a medical community would focus on the low carbohydrate diet to help avert metformin side effects as well as treat the metabolic issues. You can get many people back on metformin by just adjusting their diet. I hope you can spread this word.
Steven Foley, MD
Lamar, Colorado
Appreciates Dr. Barbieri’s editorials
G’Day from Australia. I am a big fan of your editorials and opinions and enjoy reading
Kanapathippillai Sivanesan, MD
Brisbane, Australia
Dr. Barbieri responds
I thank Dr. Linan, Dr. Foley, and Ms. Gowan for sharing their important insights with our readers. I agree with Dr. Linan that I should have highlighted the important guidance that women with PCOS and a body mass index (BMI) above the normal range should be encouraged to reduce their weight by 5% to 10% with diet and exercise. Dr. Foley offers a clinical pearl that a low carbohydrate diet will reduce the gastrointestinal symptoms that may occur with metformin therapy. Ms. Gowan notes that the combination of metformin plus cyclic progesterone may help to initiate more frequent ovulatory cycles in women with PCOS, thereby improving fertility. Dr. Hecht reminds us that spironolactone is a teratogen and using effective contraception can help reduce the risk of exposing a pregnancy to the medication.
Dr. Beckman raises the important clinical issue of whether it is helpful to measure insulin concentration. Measuring insulin and glucose is especially helpful in understanding the causes of hypoglycemia. An elevated insulin level at the time of an abnormally low glucose level is very worrisome. However, for women with PCOS, in whom insulin resistance is common, measuring insulin is of minimal clinical value. A normal or elevated insulin level is consistent with the diagnosis of PCOS. Assessing BMI, waist circumference, HDL-cholesterol, fasting triglyceride level, and blood pressure— components of the metabolic syndrome—are much more useful clinically. The dermatologic skin lesion acanthosis nigricans is also a sign consistent with insulin resistance. I do not measure insulin levels in my patients with PCOS. Metformin is a useful agent in the treatment of PCOS whether or not insulin resistance is present. Metformin may have direct actions on the ovary to reduce androgen production, in addition to its beneficial effects in the liver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Universal cervical length screening–saving babies lives
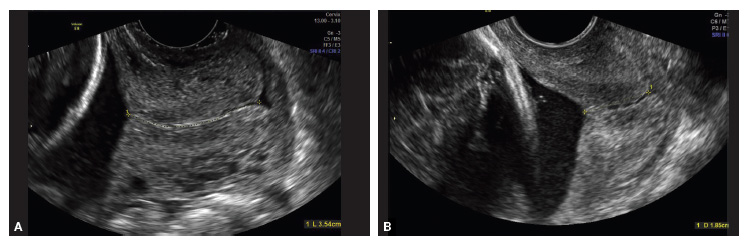
Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).
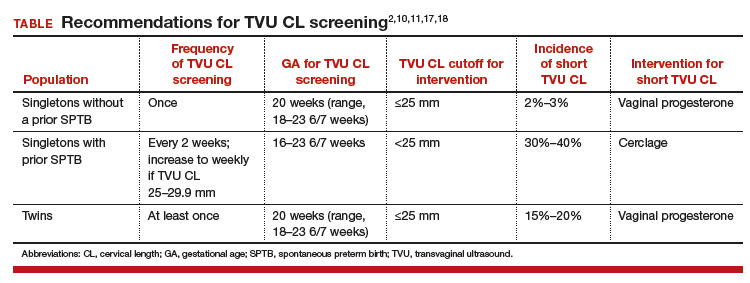
Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.

Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).

Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).

Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
LARCs are underutilized, even where Zika risk is high
SAN DIEGO – Los Angeles County officials report that few women surveyed are using the most effective contraceptive measures, a fact that concerns public health officials in an area at potential risk for local Zika virus infection.
With close to half of the births in Los Angeles County being unplanned and more than 59% of women reporting use of less effective contraceptive measures, educating providers on the why and the how of placing the most effective contraceptive measures could make a big difference, said Diana Ramos, MD, of the Los Angeles County Department of Public Health.
Los Angeles-area health care providers and public health officials are bracing themselves for a summer mosquito population explosion brought on by the West Coast’s very wet winter and spring of 2016-2017, Dr. Ramos said during a press briefing at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This sets up the very real possibility of local transmission in the Los Angeles area in the summer of 2017, Dr. Ramos said, adding that the county has both Aedes aegypti and Aedes albopictus mosquitoes, two species capable of transmitting Zika virus.
Dr. Ramos and her colleagues drew from the Los Angeles Mommy and Baby (LAMB) project, a population-based survey of women who have recently given birth. As part of ongoing surveillance to assess whether the county is meeting the CDC’s Healthy People 2020 goals, the 2012 LAMB survey asked about preconception and perinatal experiences, including family planning methods used. From the 2012 survey, the investigators could then identify women who had not had a subsequent pregnancy. They excluded women who did not complete the family planning portion of the 2012 survey. A total of 3,175 women were queried in 2014 about their current family planning practices.
Overall, 28% of women said that they were using not using any form of birth control. The remaining women (n = 2,400) used a variety of methods, with condoms being the most common, used by 38.1%. Oral contraceptives were used by 15.6% of respondents, but nearly as many (14.8%) reported using the withdrawal method, and 6.1% said they used the rhythm method. An additional 15% reported that either they or their partner had undergone a permanent sterilization procedure. Vaginal rings were used by 1.7%.
Of the remaining women who were using birth control, 14.5% were using intrauterine devices, and 6.1% were using depot medroxyprogesterone acetate. These two methods of long-acting reversible contraception (LARC) represent some of the most effective methods to prevent conception, Dr. Ramos said. The fact that only one in five women is using these methods leaves room for provider and public education, she said.
Though some women used a combination of methods, the researchers estimated that about 59% of the women using any birth control were using methods proven to be less effective in real-world studies, including condoms, withdrawal, and the rhythm method.
Accordingly, she said her department is working with providers to expand awareness of the high efficacy rates and good safety profiles of LARCs, and also to educate the public that “the most effective contraceptive methods can decrease neonatal Zika complications by preventing unplanned pregnancies.” The hope, Dr. Ramos said, is to decrease the number of neonatal Zika cases.
Dr. Ramos and her coauthors reported no external sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN DIEGO – Los Angeles County officials report that few women surveyed are using the most effective contraceptive measures, a fact that concerns public health officials in an area at potential risk for local Zika virus infection.
With close to half of the births in Los Angeles County being unplanned and more than 59% of women reporting use of less effective contraceptive measures, educating providers on the why and the how of placing the most effective contraceptive measures could make a big difference, said Diana Ramos, MD, of the Los Angeles County Department of Public Health.
Los Angeles-area health care providers and public health officials are bracing themselves for a summer mosquito population explosion brought on by the West Coast’s very wet winter and spring of 2016-2017, Dr. Ramos said during a press briefing at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This sets up the very real possibility of local transmission in the Los Angeles area in the summer of 2017, Dr. Ramos said, adding that the county has both Aedes aegypti and Aedes albopictus mosquitoes, two species capable of transmitting Zika virus.
Dr. Ramos and her colleagues drew from the Los Angeles Mommy and Baby (LAMB) project, a population-based survey of women who have recently given birth. As part of ongoing surveillance to assess whether the county is meeting the CDC’s Healthy People 2020 goals, the 2012 LAMB survey asked about preconception and perinatal experiences, including family planning methods used. From the 2012 survey, the investigators could then identify women who had not had a subsequent pregnancy. They excluded women who did not complete the family planning portion of the 2012 survey. A total of 3,175 women were queried in 2014 about their current family planning practices.
Overall, 28% of women said that they were using not using any form of birth control. The remaining women (n = 2,400) used a variety of methods, with condoms being the most common, used by 38.1%. Oral contraceptives were used by 15.6% of respondents, but nearly as many (14.8%) reported using the withdrawal method, and 6.1% said they used the rhythm method. An additional 15% reported that either they or their partner had undergone a permanent sterilization procedure. Vaginal rings were used by 1.7%.
Of the remaining women who were using birth control, 14.5% were using intrauterine devices, and 6.1% were using depot medroxyprogesterone acetate. These two methods of long-acting reversible contraception (LARC) represent some of the most effective methods to prevent conception, Dr. Ramos said. The fact that only one in five women is using these methods leaves room for provider and public education, she said.
Though some women used a combination of methods, the researchers estimated that about 59% of the women using any birth control were using methods proven to be less effective in real-world studies, including condoms, withdrawal, and the rhythm method.
Accordingly, she said her department is working with providers to expand awareness of the high efficacy rates and good safety profiles of LARCs, and also to educate the public that “the most effective contraceptive methods can decrease neonatal Zika complications by preventing unplanned pregnancies.” The hope, Dr. Ramos said, is to decrease the number of neonatal Zika cases.
Dr. Ramos and her coauthors reported no external sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN DIEGO – Los Angeles County officials report that few women surveyed are using the most effective contraceptive measures, a fact that concerns public health officials in an area at potential risk for local Zika virus infection.
With close to half of the births in Los Angeles County being unplanned and more than 59% of women reporting use of less effective contraceptive measures, educating providers on the why and the how of placing the most effective contraceptive measures could make a big difference, said Diana Ramos, MD, of the Los Angeles County Department of Public Health.
Los Angeles-area health care providers and public health officials are bracing themselves for a summer mosquito population explosion brought on by the West Coast’s very wet winter and spring of 2016-2017, Dr. Ramos said during a press briefing at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This sets up the very real possibility of local transmission in the Los Angeles area in the summer of 2017, Dr. Ramos said, adding that the county has both Aedes aegypti and Aedes albopictus mosquitoes, two species capable of transmitting Zika virus.
Dr. Ramos and her colleagues drew from the Los Angeles Mommy and Baby (LAMB) project, a population-based survey of women who have recently given birth. As part of ongoing surveillance to assess whether the county is meeting the CDC’s Healthy People 2020 goals, the 2012 LAMB survey asked about preconception and perinatal experiences, including family planning methods used. From the 2012 survey, the investigators could then identify women who had not had a subsequent pregnancy. They excluded women who did not complete the family planning portion of the 2012 survey. A total of 3,175 women were queried in 2014 about their current family planning practices.
Overall, 28% of women said that they were using not using any form of birth control. The remaining women (n = 2,400) used a variety of methods, with condoms being the most common, used by 38.1%. Oral contraceptives were used by 15.6% of respondents, but nearly as many (14.8%) reported using the withdrawal method, and 6.1% said they used the rhythm method. An additional 15% reported that either they or their partner had undergone a permanent sterilization procedure. Vaginal rings were used by 1.7%.
Of the remaining women who were using birth control, 14.5% were using intrauterine devices, and 6.1% were using depot medroxyprogesterone acetate. These two methods of long-acting reversible contraception (LARC) represent some of the most effective methods to prevent conception, Dr. Ramos said. The fact that only one in five women is using these methods leaves room for provider and public education, she said.
Though some women used a combination of methods, the researchers estimated that about 59% of the women using any birth control were using methods proven to be less effective in real-world studies, including condoms, withdrawal, and the rhythm method.
Accordingly, she said her department is working with providers to expand awareness of the high efficacy rates and good safety profiles of LARCs, and also to educate the public that “the most effective contraceptive methods can decrease neonatal Zika complications by preventing unplanned pregnancies.” The hope, Dr. Ramos said, is to decrease the number of neonatal Zika cases.
Dr. Ramos and her coauthors reported no external sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
AT ACOG 2017
Key clinical point:
Major finding: Of women surveyed who were using contraception, 20.6% were using a highly effective long-acting reversible contraceptive.
Data source: A population-based survey of 3,175 women in Los Angeles County who had previously given birth.
Disclosures: The study authors reported no outside sources of funding and no conflicts of interest.
Gastric bands hit with high reoperation rates, rising costs
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).
The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).

The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).

The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
FROM JAMA SURGERY
Key clinical point: Reoperations after gastric band placement are common and raise concerns about the safety, effectiveness, and value of the device.
Major finding: During the study period, reoperations accounted for 47.6% of Medicare payments for laparoscopic gastric band procedures.
Data source: Medicare Provider Analysis and Review file of 25,042 beneficiaries who had gastric band procedures between 2006 and 2013.
Disclosures: Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
iFCG achieves high MRD-negative remission in untreated CLL
NEW YORK – Three courses of treatment with the novel combination of ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) was well tolerated and associated with a high rate of minimal residual disease (MRD)–negative remission in the bone marrow of favorable-risk, treatment-naive patients with chronic lymphocytic leukemia, based on early results from an ongoing investigator-initiated phase II trial.
Of 29 patients, 24 had completed treatment and been followed for a median of 8.3 months. All 24 had an overall response rate (42% complete response/complete remission with incomplete blood count recovery and 58% partial response), and 83% of patients achieved MRD negativity (100% with complete response and 71% with partial response), Nitin Jain, MD, reported at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
All nine patients who reached the 12-month time point are off therapy and are being monitored, he said.
Patients with IGHV mutations generally have favorable long-term outcomes with 10-year progression-free survival rates greater than 60% after receiving standard first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR). However, ibrutinib is approved for patients with CLL, and obinutuzumab, a glycoengineered type II CD20 monoclonal antibody, was superior to rituximab in the CLL11 trial, Dr. Jain said.
Further, data from the HELIOS trial indicated that combining targeted therapies with chemoimmunotherapy is safe and effective.
iFCG was developed with the intent to limit fludarabine and cyclophosphamide to three courses, potentially reducing short- and long-term toxicity, while maintaining efficacy through the addition of ibrutinib and obinutuzumab, he explained.
Of note, higher pretreatment levels of beta-2 microglobulin were associated with a lower MRD-negativity rate after 3 cycles of iFCG, he said.
In six patients with beta-2 microglobulin of 4 mg/dL or greater, the rate was 50%, compared with 94% in 18 patients with beta-2 microglobulin less than 4 mg/dL.
The patients in the current analysis had a median age of 60 years and adequate organ function. All had IGHV mutation and did not have del(17p) or TP53 mutation. They received three courses of iFCG, including ibrutinib at 420 mg once daily continuously starting at day 1 of course 1 (C1D1); obinutuzumab at 100 mg C1D1, 900 mg C1D2, 1000 mg C1D8, 1000 mg C1D15, 1000 mg C2D1, and 1000 mg C3D1; fludarabine at 25 mg/m2 daily for 3 days each course; and cyclophosphamide at 250mg/m2 daily for 3 days each course.
Per study protocol, all patients receive ibrutinib with obinutuzumab for courses 4-6. Patients meeting the primary endpoint of complete response/complete remission with incomplete blood count recovery and bone marrow MRD negativity received ibrutinib monotherapy for courses 7-12. Those who did not achieve the primary endpoint received six more courses of ibrutinib and obinutuzumab. All patients who are MRD negative at 1 year stop all therapy, including ibrutinib, while those who are MRD positive at 1 year may continue ibrutinib monotherapy until disease progression.
The target bone marrow MRD-negative rate after 3 cycles of iFCG is 45%. The historic C3 bone marrow MRD-negative rate with standard FCR therapy in patients with IGHV mutation is 26%, Dr. Jain said, noting that the rate in the current analysis compared favorably with both.
The treatment thus far has been generally well tolerated. Toxicities included neutropenia (grade 3 and 4 occurring in 9 and 12 patients, respectively), thrombocytopenia (grade 3 and 4 occurring in 12 and 1 patients, respectively), ALT/AST (grade 3 and 4 occurring in 3 and 1 patients respectively), atrial fibrillation (grade 3 occurring in 1 patient), arthralgia (grade 3 occurring in 1 patient), and infusion-related reaction (grade 2 and 3 occurring in 9 patients and 1 patient, respectively).
Infections included herpes zoster, acute cholecystitis, pulmonary mycobacterium avium complex infection, and pneumocystis pneumonia, which occurred in 1 patient each, and neutropenic fever, which occurred in 4 patients.
“Notably, no patient has progressed or died in the study so far,” Dr. Jain said.
The trial continues to enroll patients, with plans for enrolling a total of 45.
Dr. Jain has received research support from and/or served on an advisory board for Pharmacyclics, Genentech, Abbvie, Prizer, Incyte, BMS, Infinity, ADC Therapeutics, Seattle Genetics, Celgene, Servier, Novartis Novimmune, and Adaptive Biotechnologies.
NEW YORK – Three courses of treatment with the novel combination of ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) was well tolerated and associated with a high rate of minimal residual disease (MRD)–negative remission in the bone marrow of favorable-risk, treatment-naive patients with chronic lymphocytic leukemia, based on early results from an ongoing investigator-initiated phase II trial.
Of 29 patients, 24 had completed treatment and been followed for a median of 8.3 months. All 24 had an overall response rate (42% complete response/complete remission with incomplete blood count recovery and 58% partial response), and 83% of patients achieved MRD negativity (100% with complete response and 71% with partial response), Nitin Jain, MD, reported at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
All nine patients who reached the 12-month time point are off therapy and are being monitored, he said.
Patients with IGHV mutations generally have favorable long-term outcomes with 10-year progression-free survival rates greater than 60% after receiving standard first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR). However, ibrutinib is approved for patients with CLL, and obinutuzumab, a glycoengineered type II CD20 monoclonal antibody, was superior to rituximab in the CLL11 trial, Dr. Jain said.
Further, data from the HELIOS trial indicated that combining targeted therapies with chemoimmunotherapy is safe and effective.
iFCG was developed with the intent to limit fludarabine and cyclophosphamide to three courses, potentially reducing short- and long-term toxicity, while maintaining efficacy through the addition of ibrutinib and obinutuzumab, he explained.
Of note, higher pretreatment levels of beta-2 microglobulin were associated with a lower MRD-negativity rate after 3 cycles of iFCG, he said.
In six patients with beta-2 microglobulin of 4 mg/dL or greater, the rate was 50%, compared with 94% in 18 patients with beta-2 microglobulin less than 4 mg/dL.
The patients in the current analysis had a median age of 60 years and adequate organ function. All had IGHV mutation and did not have del(17p) or TP53 mutation. They received three courses of iFCG, including ibrutinib at 420 mg once daily continuously starting at day 1 of course 1 (C1D1); obinutuzumab at 100 mg C1D1, 900 mg C1D2, 1000 mg C1D8, 1000 mg C1D15, 1000 mg C2D1, and 1000 mg C3D1; fludarabine at 25 mg/m2 daily for 3 days each course; and cyclophosphamide at 250mg/m2 daily for 3 days each course.
Per study protocol, all patients receive ibrutinib with obinutuzumab for courses 4-6. Patients meeting the primary endpoint of complete response/complete remission with incomplete blood count recovery and bone marrow MRD negativity received ibrutinib monotherapy for courses 7-12. Those who did not achieve the primary endpoint received six more courses of ibrutinib and obinutuzumab. All patients who are MRD negative at 1 year stop all therapy, including ibrutinib, while those who are MRD positive at 1 year may continue ibrutinib monotherapy until disease progression.
The target bone marrow MRD-negative rate after 3 cycles of iFCG is 45%. The historic C3 bone marrow MRD-negative rate with standard FCR therapy in patients with IGHV mutation is 26%, Dr. Jain said, noting that the rate in the current analysis compared favorably with both.
The treatment thus far has been generally well tolerated. Toxicities included neutropenia (grade 3 and 4 occurring in 9 and 12 patients, respectively), thrombocytopenia (grade 3 and 4 occurring in 12 and 1 patients, respectively), ALT/AST (grade 3 and 4 occurring in 3 and 1 patients respectively), atrial fibrillation (grade 3 occurring in 1 patient), arthralgia (grade 3 occurring in 1 patient), and infusion-related reaction (grade 2 and 3 occurring in 9 patients and 1 patient, respectively).
Infections included herpes zoster, acute cholecystitis, pulmonary mycobacterium avium complex infection, and pneumocystis pneumonia, which occurred in 1 patient each, and neutropenic fever, which occurred in 4 patients.
“Notably, no patient has progressed or died in the study so far,” Dr. Jain said.
The trial continues to enroll patients, with plans for enrolling a total of 45.
Dr. Jain has received research support from and/or served on an advisory board for Pharmacyclics, Genentech, Abbvie, Prizer, Incyte, BMS, Infinity, ADC Therapeutics, Seattle Genetics, Celgene, Servier, Novartis Novimmune, and Adaptive Biotechnologies.
NEW YORK – Three courses of treatment with the novel combination of ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) was well tolerated and associated with a high rate of minimal residual disease (MRD)–negative remission in the bone marrow of favorable-risk, treatment-naive patients with chronic lymphocytic leukemia, based on early results from an ongoing investigator-initiated phase II trial.
Of 29 patients, 24 had completed treatment and been followed for a median of 8.3 months. All 24 had an overall response rate (42% complete response/complete remission with incomplete blood count recovery and 58% partial response), and 83% of patients achieved MRD negativity (100% with complete response and 71% with partial response), Nitin Jain, MD, reported at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
All nine patients who reached the 12-month time point are off therapy and are being monitored, he said.
Patients with IGHV mutations generally have favorable long-term outcomes with 10-year progression-free survival rates greater than 60% after receiving standard first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR). However, ibrutinib is approved for patients with CLL, and obinutuzumab, a glycoengineered type II CD20 monoclonal antibody, was superior to rituximab in the CLL11 trial, Dr. Jain said.
Further, data from the HELIOS trial indicated that combining targeted therapies with chemoimmunotherapy is safe and effective.
iFCG was developed with the intent to limit fludarabine and cyclophosphamide to three courses, potentially reducing short- and long-term toxicity, while maintaining efficacy through the addition of ibrutinib and obinutuzumab, he explained.
Of note, higher pretreatment levels of beta-2 microglobulin were associated with a lower MRD-negativity rate after 3 cycles of iFCG, he said.
In six patients with beta-2 microglobulin of 4 mg/dL or greater, the rate was 50%, compared with 94% in 18 patients with beta-2 microglobulin less than 4 mg/dL.
The patients in the current analysis had a median age of 60 years and adequate organ function. All had IGHV mutation and did not have del(17p) or TP53 mutation. They received three courses of iFCG, including ibrutinib at 420 mg once daily continuously starting at day 1 of course 1 (C1D1); obinutuzumab at 100 mg C1D1, 900 mg C1D2, 1000 mg C1D8, 1000 mg C1D15, 1000 mg C2D1, and 1000 mg C3D1; fludarabine at 25 mg/m2 daily for 3 days each course; and cyclophosphamide at 250mg/m2 daily for 3 days each course.
Per study protocol, all patients receive ibrutinib with obinutuzumab for courses 4-6. Patients meeting the primary endpoint of complete response/complete remission with incomplete blood count recovery and bone marrow MRD negativity received ibrutinib monotherapy for courses 7-12. Those who did not achieve the primary endpoint received six more courses of ibrutinib and obinutuzumab. All patients who are MRD negative at 1 year stop all therapy, including ibrutinib, while those who are MRD positive at 1 year may continue ibrutinib monotherapy until disease progression.
The target bone marrow MRD-negative rate after 3 cycles of iFCG is 45%. The historic C3 bone marrow MRD-negative rate with standard FCR therapy in patients with IGHV mutation is 26%, Dr. Jain said, noting that the rate in the current analysis compared favorably with both.
The treatment thus far has been generally well tolerated. Toxicities included neutropenia (grade 3 and 4 occurring in 9 and 12 patients, respectively), thrombocytopenia (grade 3 and 4 occurring in 12 and 1 patients, respectively), ALT/AST (grade 3 and 4 occurring in 3 and 1 patients respectively), atrial fibrillation (grade 3 occurring in 1 patient), arthralgia (grade 3 occurring in 1 patient), and infusion-related reaction (grade 2 and 3 occurring in 9 patients and 1 patient, respectively).
Infections included herpes zoster, acute cholecystitis, pulmonary mycobacterium avium complex infection, and pneumocystis pneumonia, which occurred in 1 patient each, and neutropenic fever, which occurred in 4 patients.
“Notably, no patient has progressed or died in the study so far,” Dr. Jain said.
The trial continues to enroll patients, with plans for enrolling a total of 45.
Dr. Jain has received research support from and/or served on an advisory board for Pharmacyclics, Genentech, Abbvie, Prizer, Incyte, BMS, Infinity, ADC Therapeutics, Seattle Genetics, Celgene, Servier, Novartis Novimmune, and Adaptive Biotechnologies.
AT THE IWCLL MEETING
Key clinical point:
Major finding: The overall response rate was 100%, and 83% of patients achieved MRD-negativity after three courses.
Data source: 29 patients from an investigator-initiated phase II trial.
Disclosures: Dr. Jain has received research support from and/or served on an advisory board for Pharmacyclics, Genentech, Abbvie, Prizer, Incyte, BMS, Infinity, ADC Therapeutics, Seattle Genetics, Celgene, Servier, Novartis, Novimmune, and Adaptive Biotechnologies.
The ‘monster note’ in EHR systems rarely helps
Recently, the hospital I take call at switched to Epic as its electronic health record system.
Overall, I don’t have too many complaints about it. It does some things better and some things worse than other systems I’ve used. That’s to be expected.
But with Epic has come an alarming new trend: the monster note.
Rarely does it ever give you a hint into the thought process or what’s going on that (at least to me) is so critical to medicine.
In a recent example of the insanity, one of my office patients was in the hospital overnight for a transient ischemic attack. When I went to get the discharge summary, it was 97 pages long! (Really, it was.) All of it was auto-filled in with test results, vital signs, MRI screening forms, medication administration records, and nurse, therapy, and respiratory notes. Most of it was far from the stuff that discharge summaries are supposed to contain. What part of “summary” are people not understanding anymore?
Of course, this isn’t Epic’s fault. It’s just a tool. It’s how humans use it that becomes the problem. This misuse of the system has made routine notes, as Shakespeare’s Macbeth said, “a tale told by an idiot, full of sound and fury, signifying nothing.”
For better or worse, I deliberately don’t do this. I let Epic put in the patient’s name, birthday, and most recent vital signs ... and nothing else. I’ll fill in the test results when needed, in a concise form that I can grasp. (It’s my note, after all.) To me, writing (or typing) the note is part of the thought process. As I enter results, I turn over what they mean, in a way that just seeing five paragraphs auto-pasted in doesn’t do. It also allows me to boil them down to one or two sentences.
After all, brevity is the soul of wit. And while I’m not trying to be witty in my notes, I am trying solve the problem in front of me. Taking the time write it out in my own words is essential to my thought process and letting others understand how I came to my plan. And, as a result, it is what’s best for the patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, the hospital I take call at switched to Epic as its electronic health record system.
Overall, I don’t have too many complaints about it. It does some things better and some things worse than other systems I’ve used. That’s to be expected.
But with Epic has come an alarming new trend: the monster note.
Rarely does it ever give you a hint into the thought process or what’s going on that (at least to me) is so critical to medicine.
In a recent example of the insanity, one of my office patients was in the hospital overnight for a transient ischemic attack. When I went to get the discharge summary, it was 97 pages long! (Really, it was.) All of it was auto-filled in with test results, vital signs, MRI screening forms, medication administration records, and nurse, therapy, and respiratory notes. Most of it was far from the stuff that discharge summaries are supposed to contain. What part of “summary” are people not understanding anymore?
Of course, this isn’t Epic’s fault. It’s just a tool. It’s how humans use it that becomes the problem. This misuse of the system has made routine notes, as Shakespeare’s Macbeth said, “a tale told by an idiot, full of sound and fury, signifying nothing.”
For better or worse, I deliberately don’t do this. I let Epic put in the patient’s name, birthday, and most recent vital signs ... and nothing else. I’ll fill in the test results when needed, in a concise form that I can grasp. (It’s my note, after all.) To me, writing (or typing) the note is part of the thought process. As I enter results, I turn over what they mean, in a way that just seeing five paragraphs auto-pasted in doesn’t do. It also allows me to boil them down to one or two sentences.
After all, brevity is the soul of wit. And while I’m not trying to be witty in my notes, I am trying solve the problem in front of me. Taking the time write it out in my own words is essential to my thought process and letting others understand how I came to my plan. And, as a result, it is what’s best for the patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, the hospital I take call at switched to Epic as its electronic health record system.
Overall, I don’t have too many complaints about it. It does some things better and some things worse than other systems I’ve used. That’s to be expected.
But with Epic has come an alarming new trend: the monster note.
Rarely does it ever give you a hint into the thought process or what’s going on that (at least to me) is so critical to medicine.
In a recent example of the insanity, one of my office patients was in the hospital overnight for a transient ischemic attack. When I went to get the discharge summary, it was 97 pages long! (Really, it was.) All of it was auto-filled in with test results, vital signs, MRI screening forms, medication administration records, and nurse, therapy, and respiratory notes. Most of it was far from the stuff that discharge summaries are supposed to contain. What part of “summary” are people not understanding anymore?
Of course, this isn’t Epic’s fault. It’s just a tool. It’s how humans use it that becomes the problem. This misuse of the system has made routine notes, as Shakespeare’s Macbeth said, “a tale told by an idiot, full of sound and fury, signifying nothing.”
For better or worse, I deliberately don’t do this. I let Epic put in the patient’s name, birthday, and most recent vital signs ... and nothing else. I’ll fill in the test results when needed, in a concise form that I can grasp. (It’s my note, after all.) To me, writing (or typing) the note is part of the thought process. As I enter results, I turn over what they mean, in a way that just seeing five paragraphs auto-pasted in doesn’t do. It also allows me to boil them down to one or two sentences.
After all, brevity is the soul of wit. And while I’m not trying to be witty in my notes, I am trying solve the problem in front of me. Taking the time write it out in my own words is essential to my thought process and letting others understand how I came to my plan. And, as a result, it is what’s best for the patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.