User login
FDA grants drug orphan designation for PNH
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Hand pain and rash
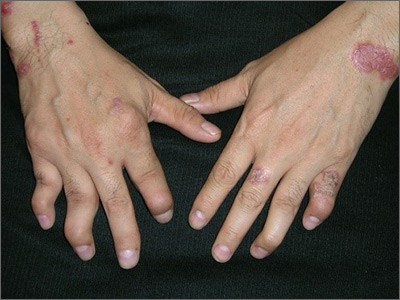
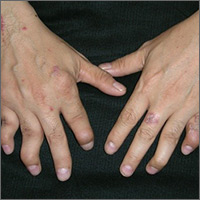
The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Slow and Steady: A Worrisome Pace
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su

For more than two years, a 43-year-old man has had an asymptomatic lesion on his right deltoid area. His primary care provider has repeatedly assured him of its benignancy “because it is flat.” But its slow, steady, horizontal growth concerns the patient, who is otherwise healthy.
He does admit to a significant history of sun exposure, explaining that he burns easily but acquires a tan by summer’s end each year. Several members of his immediate family have had skin cancers removed.
Examination reveals a round, dark macule, measuring 1.1 cm, on the right lateral deltoid area. The lesion has poorly defined borders and a variety of colors—mostly black and brown, with flecks of red and pink. There is no palpable component, and no nodes can be detected in the area.
The rest of his type II skin has abundant evidence of UV overexposure, including solar lentigines and weathering, especially around the neck.
The lesion is anesthetized, removed by deep shave technique, and submitted to pathology. The report shows a superficial, spreading melanoma with a Breslow depth of 0.75 mm. Malignant cells extend to the dermoepidermal junction (Clark level II). Only a few mitotic cells can be seen, with no intravascular invasion or signs of ulceration.
Syncope Guidelines
Title: 2017 ACC/AHA/HRS guidelines for patients with syncope
Clinical Question: What are the key points from the 2017 ACC/AHA/HRS guidelines for the evaluation and management of adult patients with syncope?
Background: Syncope is a common condition for which patients present to a hospital setting. Updated guidance and recommendations on the evaluation and management of syncope are provided.
Study Design: Evidence-based guidelines.
Setting: Panel of experts.
Synopsis: A detailed history and physical should be performed. A 12-lead ECG should be obtained. Short- and long-term morbidity and mortality risk of syncope should be assessed. Inpatient evaluation and treatment is recommended for patients presenting with syncope and who have serious medical condition relevant to the cause of syncope. Lab tests are not useful. Routine cardiac imaging is not useful unless a cardiac etiology of syncope is suspected. Carotid artery imaging is not useful in the absence of focal neurologic findings. Continuous telemetry is indicated for inpatients suspected of syncope due to a cardiac etiology.
The most common cause of syncope is vasovagal. Medication therapy has modest effect, and patient education is recommended. Dual chamber pacing may be reasonable in select patients over the age of 40 with recurrent vasovagal syncope and prolonged spontaneous pauses. If orthostatic hypotension is suspected as the cause of syncope due to dehydration, then fluid resuscitation is recommended. Removing medications causing hypotension may be appropriate for select patients with syncope.
Cardiac syncope requires expert directed care and may include life-style changes, medication therapy and/or procedural intervention.
Bottom Line: The 2017 syncope guidelines provide updated and concise recommendations on the management of syncope.
Citation: Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary. Journal of the American College of Cardiology. 2017; doi: 10.1016/ j.jacc.2017.03.002.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: 2017 ACC/AHA/HRS guidelines for patients with syncope
Clinical Question: What are the key points from the 2017 ACC/AHA/HRS guidelines for the evaluation and management of adult patients with syncope?
Background: Syncope is a common condition for which patients present to a hospital setting. Updated guidance and recommendations on the evaluation and management of syncope are provided.
Study Design: Evidence-based guidelines.
Setting: Panel of experts.
Synopsis: A detailed history and physical should be performed. A 12-lead ECG should be obtained. Short- and long-term morbidity and mortality risk of syncope should be assessed. Inpatient evaluation and treatment is recommended for patients presenting with syncope and who have serious medical condition relevant to the cause of syncope. Lab tests are not useful. Routine cardiac imaging is not useful unless a cardiac etiology of syncope is suspected. Carotid artery imaging is not useful in the absence of focal neurologic findings. Continuous telemetry is indicated for inpatients suspected of syncope due to a cardiac etiology.
The most common cause of syncope is vasovagal. Medication therapy has modest effect, and patient education is recommended. Dual chamber pacing may be reasonable in select patients over the age of 40 with recurrent vasovagal syncope and prolonged spontaneous pauses. If orthostatic hypotension is suspected as the cause of syncope due to dehydration, then fluid resuscitation is recommended. Removing medications causing hypotension may be appropriate for select patients with syncope.
Cardiac syncope requires expert directed care and may include life-style changes, medication therapy and/or procedural intervention.
Bottom Line: The 2017 syncope guidelines provide updated and concise recommendations on the management of syncope.
Citation: Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary. Journal of the American College of Cardiology. 2017; doi: 10.1016/ j.jacc.2017.03.002.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: 2017 ACC/AHA/HRS guidelines for patients with syncope
Clinical Question: What are the key points from the 2017 ACC/AHA/HRS guidelines for the evaluation and management of adult patients with syncope?
Background: Syncope is a common condition for which patients present to a hospital setting. Updated guidance and recommendations on the evaluation and management of syncope are provided.
Study Design: Evidence-based guidelines.
Setting: Panel of experts.
Synopsis: A detailed history and physical should be performed. A 12-lead ECG should be obtained. Short- and long-term morbidity and mortality risk of syncope should be assessed. Inpatient evaluation and treatment is recommended for patients presenting with syncope and who have serious medical condition relevant to the cause of syncope. Lab tests are not useful. Routine cardiac imaging is not useful unless a cardiac etiology of syncope is suspected. Carotid artery imaging is not useful in the absence of focal neurologic findings. Continuous telemetry is indicated for inpatients suspected of syncope due to a cardiac etiology.
The most common cause of syncope is vasovagal. Medication therapy has modest effect, and patient education is recommended. Dual chamber pacing may be reasonable in select patients over the age of 40 with recurrent vasovagal syncope and prolonged spontaneous pauses. If orthostatic hypotension is suspected as the cause of syncope due to dehydration, then fluid resuscitation is recommended. Removing medications causing hypotension may be appropriate for select patients with syncope.
Cardiac syncope requires expert directed care and may include life-style changes, medication therapy and/or procedural intervention.
Bottom Line: The 2017 syncope guidelines provide updated and concise recommendations on the management of syncope.
Citation: Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary. Journal of the American College of Cardiology. 2017; doi: 10.1016/ j.jacc.2017.03.002.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
FDA committee rejects sirukumab approval on safety concerns
Citing safety concerns, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly against recommending FDA approval of the interleukin-6 inhibitor sirukumab for refractory rheumatoid arthritis.
Janssen Biotech submitted a biologics license application (BLA) for the monoclonal antibody, seeking an indication for adults with moderately to severely active rheumatoid arthritis (RA) who had an inadequate response or intolerance to one or more prior disease-modifying antirheumatic drugs, but despite agreeing unanimously that the data presented by the applicant provided substantial evidence of efficacy for this indication, the committee voted 12-1 against approval at an Aug. 2 meeting.
“I’m not sure whether the safety signal is of concern or not. I don’t think there’s enough data here to know that. It’s concerning, and it may be just noise, but it may also be real and I’m not willing to ... be supportive of the notion that it’s safe enough to take its place along with other biologicals,” said temporary voting member David T. Felson, MD, professor of medicine and public health at Boston University.
Similarly, temporary voting member Erica Brittain, PhD, said the mortality concerns swayed her vote.
“It was a very close call for me. I do think there’s a real argument to be made about bias in the analysis that shows some possibility of a difference. On the other hand, I just couldn’t get past the uncertainty, and when we’re talking about mortality it’s hard to dismiss that,” said Dr. Brittain, a mathematical statistician and deputy chief of the Biostatistics Research Branch at the National Institute of Allergy and Infectious Diseases.
Michael H. Weisman, MD, also a temporary voting member and chair of rheumatology at Cedars-Sinai Medical Center in Los Angeles, said that if the indication had been narrowed – perhaps to those who showed a biologic response – he would have voted “yes.”
Temporary voting member James Katz, MD, was the only committee member to vote in favor of approval.
“I actually voted yes because this drug doesn’t scare me any more than all the other drugs I use. I’m very scared by all the biological agents, and this is no different,” said Dr. Katz, director of the Rheumatology Fellowship and Training Branch at the National Institute of Arthritis and Musculoskeletal Diseases.
Sirukumab differs from two other approved monoclonal antibodies that target the IL-6 pathway for the treatment of patients with RA – tocilizumab (Actemra) and sarilumab (Kevzara) – in that it targets IL-6, while the others target the IL-6 receptor. This slight difference could make a difference for some highly refractory patients who had failed to respond to prior treatments, according to applicant presentations at the meeting.
The efficacy and safety of the agent were assessed in a phase 2 dose-ranging study, as well in three pivotal phase 3 studies. Two of the phase 3 studies compared 100 mg twice weekly and 50 mg four times weekly doses of subcutaneous sirukumab to placebo – which were shown to have similar efficacy for reducing the signs and symptoms of RA – and a third compared those two doses against adalimumab (Humira) and showed that it was not superior to adalimumab for efficacy.
One phase 3 placebo-controlled study involving 878 refractory patients was published in February in The Lancet and showed that sirukumab was associated with rapid and sustained improvements in RA signs and symptoms, physical function, and health status, as well as improvement in physical and mental well-being.
However, a safety signal – a trend of increased overall mortality with sirukumab vs. placebo – emerged from the studies. The mortality was mainly associated with major adverse cardiovascular events, infection, and malignancy.
Three speakers, including RA patients or patient representatives, participated in the open public hearing portion of the committee meeting, and all spoke in favor of approval of sirukumab, but ultimately the committee agreed that the limited benefits of the agent – given that it does not involve an entirely new mechanism of action – did not outweigh the unknowns regarding safety.
Committee chairperson Daniel H. Solomon, MD, professor of medicine at Harvard Medical School and chief of the section of clinical sciences at Brigham and Women’s Hospital, both in Boston, said longer-term outcomes data with a clear comparator are needed.
The FDA will now consider the committee’s recommendations in making its final determination regarding the BLA.
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Citing safety concerns, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly against recommending FDA approval of the interleukin-6 inhibitor sirukumab for refractory rheumatoid arthritis.
Janssen Biotech submitted a biologics license application (BLA) for the monoclonal antibody, seeking an indication for adults with moderately to severely active rheumatoid arthritis (RA) who had an inadequate response or intolerance to one or more prior disease-modifying antirheumatic drugs, but despite agreeing unanimously that the data presented by the applicant provided substantial evidence of efficacy for this indication, the committee voted 12-1 against approval at an Aug. 2 meeting.
“I’m not sure whether the safety signal is of concern or not. I don’t think there’s enough data here to know that. It’s concerning, and it may be just noise, but it may also be real and I’m not willing to ... be supportive of the notion that it’s safe enough to take its place along with other biologicals,” said temporary voting member David T. Felson, MD, professor of medicine and public health at Boston University.
Similarly, temporary voting member Erica Brittain, PhD, said the mortality concerns swayed her vote.
“It was a very close call for me. I do think there’s a real argument to be made about bias in the analysis that shows some possibility of a difference. On the other hand, I just couldn’t get past the uncertainty, and when we’re talking about mortality it’s hard to dismiss that,” said Dr. Brittain, a mathematical statistician and deputy chief of the Biostatistics Research Branch at the National Institute of Allergy and Infectious Diseases.
Michael H. Weisman, MD, also a temporary voting member and chair of rheumatology at Cedars-Sinai Medical Center in Los Angeles, said that if the indication had been narrowed – perhaps to those who showed a biologic response – he would have voted “yes.”
Temporary voting member James Katz, MD, was the only committee member to vote in favor of approval.
“I actually voted yes because this drug doesn’t scare me any more than all the other drugs I use. I’m very scared by all the biological agents, and this is no different,” said Dr. Katz, director of the Rheumatology Fellowship and Training Branch at the National Institute of Arthritis and Musculoskeletal Diseases.
Sirukumab differs from two other approved monoclonal antibodies that target the IL-6 pathway for the treatment of patients with RA – tocilizumab (Actemra) and sarilumab (Kevzara) – in that it targets IL-6, while the others target the IL-6 receptor. This slight difference could make a difference for some highly refractory patients who had failed to respond to prior treatments, according to applicant presentations at the meeting.
The efficacy and safety of the agent were assessed in a phase 2 dose-ranging study, as well in three pivotal phase 3 studies. Two of the phase 3 studies compared 100 mg twice weekly and 50 mg four times weekly doses of subcutaneous sirukumab to placebo – which were shown to have similar efficacy for reducing the signs and symptoms of RA – and a third compared those two doses against adalimumab (Humira) and showed that it was not superior to adalimumab for efficacy.
One phase 3 placebo-controlled study involving 878 refractory patients was published in February in The Lancet and showed that sirukumab was associated with rapid and sustained improvements in RA signs and symptoms, physical function, and health status, as well as improvement in physical and mental well-being.
However, a safety signal – a trend of increased overall mortality with sirukumab vs. placebo – emerged from the studies. The mortality was mainly associated with major adverse cardiovascular events, infection, and malignancy.
Three speakers, including RA patients or patient representatives, participated in the open public hearing portion of the committee meeting, and all spoke in favor of approval of sirukumab, but ultimately the committee agreed that the limited benefits of the agent – given that it does not involve an entirely new mechanism of action – did not outweigh the unknowns regarding safety.
Committee chairperson Daniel H. Solomon, MD, professor of medicine at Harvard Medical School and chief of the section of clinical sciences at Brigham and Women’s Hospital, both in Boston, said longer-term outcomes data with a clear comparator are needed.
The FDA will now consider the committee’s recommendations in making its final determination regarding the BLA.
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Citing safety concerns, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly against recommending FDA approval of the interleukin-6 inhibitor sirukumab for refractory rheumatoid arthritis.
Janssen Biotech submitted a biologics license application (BLA) for the monoclonal antibody, seeking an indication for adults with moderately to severely active rheumatoid arthritis (RA) who had an inadequate response or intolerance to one or more prior disease-modifying antirheumatic drugs, but despite agreeing unanimously that the data presented by the applicant provided substantial evidence of efficacy for this indication, the committee voted 12-1 against approval at an Aug. 2 meeting.
“I’m not sure whether the safety signal is of concern or not. I don’t think there’s enough data here to know that. It’s concerning, and it may be just noise, but it may also be real and I’m not willing to ... be supportive of the notion that it’s safe enough to take its place along with other biologicals,” said temporary voting member David T. Felson, MD, professor of medicine and public health at Boston University.
Similarly, temporary voting member Erica Brittain, PhD, said the mortality concerns swayed her vote.
“It was a very close call for me. I do think there’s a real argument to be made about bias in the analysis that shows some possibility of a difference. On the other hand, I just couldn’t get past the uncertainty, and when we’re talking about mortality it’s hard to dismiss that,” said Dr. Brittain, a mathematical statistician and deputy chief of the Biostatistics Research Branch at the National Institute of Allergy and Infectious Diseases.
Michael H. Weisman, MD, also a temporary voting member and chair of rheumatology at Cedars-Sinai Medical Center in Los Angeles, said that if the indication had been narrowed – perhaps to those who showed a biologic response – he would have voted “yes.”
Temporary voting member James Katz, MD, was the only committee member to vote in favor of approval.
“I actually voted yes because this drug doesn’t scare me any more than all the other drugs I use. I’m very scared by all the biological agents, and this is no different,” said Dr. Katz, director of the Rheumatology Fellowship and Training Branch at the National Institute of Arthritis and Musculoskeletal Diseases.
Sirukumab differs from two other approved monoclonal antibodies that target the IL-6 pathway for the treatment of patients with RA – tocilizumab (Actemra) and sarilumab (Kevzara) – in that it targets IL-6, while the others target the IL-6 receptor. This slight difference could make a difference for some highly refractory patients who had failed to respond to prior treatments, according to applicant presentations at the meeting.
The efficacy and safety of the agent were assessed in a phase 2 dose-ranging study, as well in three pivotal phase 3 studies. Two of the phase 3 studies compared 100 mg twice weekly and 50 mg four times weekly doses of subcutaneous sirukumab to placebo – which were shown to have similar efficacy for reducing the signs and symptoms of RA – and a third compared those two doses against adalimumab (Humira) and showed that it was not superior to adalimumab for efficacy.
One phase 3 placebo-controlled study involving 878 refractory patients was published in February in The Lancet and showed that sirukumab was associated with rapid and sustained improvements in RA signs and symptoms, physical function, and health status, as well as improvement in physical and mental well-being.
However, a safety signal – a trend of increased overall mortality with sirukumab vs. placebo – emerged from the studies. The mortality was mainly associated with major adverse cardiovascular events, infection, and malignancy.
Three speakers, including RA patients or patient representatives, participated in the open public hearing portion of the committee meeting, and all spoke in favor of approval of sirukumab, but ultimately the committee agreed that the limited benefits of the agent – given that it does not involve an entirely new mechanism of action – did not outweigh the unknowns regarding safety.
Committee chairperson Daniel H. Solomon, MD, professor of medicine at Harvard Medical School and chief of the section of clinical sciences at Brigham and Women’s Hospital, both in Boston, said longer-term outcomes data with a clear comparator are needed.
The FDA will now consider the committee’s recommendations in making its final determination regarding the BLA.
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Abilify Maintena OK’d by FDA for adults with bipolar I disorder
The Food and Drug Administration has approved a monthly injectable formulation of aripiprazole, (Abilify Maintena) , for a maintenance monotherapy treatment of bipolar I disorder for adults, Otsuka and Lundbeck have announced.
Patients treated with the injectable formulation of the atypical antipsychotic must continue to take a daily oral antipsychotic for the first 14 days. After that, however, the long-acting injectable (LAI) – which must be administered by a health care professional – can replace the daily medication.
Joseph R. Calabrese, MD, director of the mood disorders program at University Hospitals Cleveland Medical Center, said in the July 28 announcement that the LAI is a new treatment option for bipolar I patients “who have established tolerability with oral aripiprazole.”
The drug label includes a warning that elderly patients with dementia-related psychosis who are treated with antipsychotics are at a higher mortality risk. Adverse reactions that have been associated with treatment with aripiprazole include weight gain, akathisia, injection site pain, sedation, and certain compulsive behaviors.
Created by Otsuka, and marketed by Otsuka and Lundbeck, the LAI was approved in the United States for treating adults with schizophrenia in 2013.
The Food and Drug Administration has approved a monthly injectable formulation of aripiprazole, (Abilify Maintena) , for a maintenance monotherapy treatment of bipolar I disorder for adults, Otsuka and Lundbeck have announced.
Patients treated with the injectable formulation of the atypical antipsychotic must continue to take a daily oral antipsychotic for the first 14 days. After that, however, the long-acting injectable (LAI) – which must be administered by a health care professional – can replace the daily medication.
Joseph R. Calabrese, MD, director of the mood disorders program at University Hospitals Cleveland Medical Center, said in the July 28 announcement that the LAI is a new treatment option for bipolar I patients “who have established tolerability with oral aripiprazole.”
The drug label includes a warning that elderly patients with dementia-related psychosis who are treated with antipsychotics are at a higher mortality risk. Adverse reactions that have been associated with treatment with aripiprazole include weight gain, akathisia, injection site pain, sedation, and certain compulsive behaviors.
Created by Otsuka, and marketed by Otsuka and Lundbeck, the LAI was approved in the United States for treating adults with schizophrenia in 2013.
The Food and Drug Administration has approved a monthly injectable formulation of aripiprazole, (Abilify Maintena) , for a maintenance monotherapy treatment of bipolar I disorder for adults, Otsuka and Lundbeck have announced.
Patients treated with the injectable formulation of the atypical antipsychotic must continue to take a daily oral antipsychotic for the first 14 days. After that, however, the long-acting injectable (LAI) – which must be administered by a health care professional – can replace the daily medication.
Joseph R. Calabrese, MD, director of the mood disorders program at University Hospitals Cleveland Medical Center, said in the July 28 announcement that the LAI is a new treatment option for bipolar I patients “who have established tolerability with oral aripiprazole.”
The drug label includes a warning that elderly patients with dementia-related psychosis who are treated with antipsychotics are at a higher mortality risk. Adverse reactions that have been associated with treatment with aripiprazole include weight gain, akathisia, injection site pain, sedation, and certain compulsive behaviors.
Created by Otsuka, and marketed by Otsuka and Lundbeck, the LAI was approved in the United States for treating adults with schizophrenia in 2013.
Forced commitments still low under California’s AOT law
Critics have feared that California’s “Laura’s Law,” designed to prevent violence by making it easier for officials to force outpatient care upon people with mental illness who have resisted treatment, would violate the civil rights of Golden State residents.
But early statistics gathered by a Northern California psychiatrist suggest that few people have been forced involuntarily into outpatient care in large counties that have adopted the tougher approach in recent years. Instead, Californians targeted by the law appear to be choosing – under pressure – the alternative of accepting outpatient treatment.
California enacted Laura’s Law in 2003 after the death of a 19-year-old woman named Laura Wilcox at a mental health clinic in Northern California. Ms. Wilcox, who worked at the clinic, and two others were shot to death by a patient whose family had failed to persuade officials to force him to get treatment.
As California’s version of assisted outpatient treatment, or AOT, Laura’s Law, allows courts to order people into outpatient treatment in cases that meet certain criteria. Among other things, the person must have a mental illness, be considered “unlikely to survive safely in the community without supervision,” and have a “history of lack of compliance with treatment.” The law “also obligates any county that implements and accepts the law to provide excellent and comprehensive community-based services, not only for court-ordered patients but anybody who wants care,” Dr. Nelson said in an interview after his presentation.
As a state law, the measure must be adopted on a county-by-county basis. Tiny and remote Nevada County, where the shootings occurred, adopted the policy first when it was forced to do so by a lawsuit, Dr. Nelson said. But other counties were slow to sign on.
“Unlike Kendra’s Law, which passed earlier in New York, and both mandated and funded assisted outpatient treatment for all counties, Laura’s Law was completely unfunded. It was left to the discretion of each county to decide for itself whether or not to implement,” said Dr. Nelson, of Marin County, just north of San Francisco, who has served on the councils of the Northern California Psychiatric Society and California Psychiatric Association.
But things changed in 2013, when the state allowed money from a special tax on wealthy residents to be used for AOT programs, Dr. Nelson said. Counties began implementing Laura’s Law in 2014, and the number has now reached 17, representing about two-thirds of the state’s population, he said.
Dr. Nelson gathered statistics from several of California’s 58 counties. He found that over a period of 5 months of implementation, 59 people were referred to the AOT program, and 12 voluntarily accepted outpatient therapy in San Francisco County, which encompasses the city of San Francisco only and has 864,000 residents.
Over a period of 5 months in San Diego County, which has 3.3 million residents, there were 376 referrals, including those to a program that uses home visits to urge people to accept outpatient care. Twenty people accepted voluntary outpatient treatment. No court-ordered treatment was required for anyone in either San Diego or San Francisco counties.
Over a period of 12 months in Orange County, with 3.2 million residents, there were 389 referrals, and 126 people voluntarily accepted AOT. Only three were ordered into treatment by courts. Over 6 months in Placer County, with just 375,000 residents, eight patients voluntarily accepted AOT, and one was ordered into treatment by a court but refused it.
(The Orange County Register reports that refusal to abide by court-ordered outpatient treatment results in no civil or criminal penalties.)
Los Angeles County, by far the most populous in the state with 10.1 million residents, reported 805 AOT referrals, 30 involuntary commitments to outpatient care, and 239 voluntary agreements to outpatient care. The numbers are from 2016 to 2017, but Dr. Nelson did not have details about the exact period covered.
Dr. Nelson also looked at 7 years of statistics from Nevada County, which was forced into adopting an AOT program by a lawsuit. The county, which has fewer than 100,000 residents, reported 67 referrals to the AOT program and 30 cases of court-ordered treatment.
A report estimates that over the first 30 months of the program, Nevada County saved $1.80 for every $1 spent on its AOT program by preventing acute psychiatric hospitalizations and imprisonment.
The low numbers of forced commitments to outpatient care across the state make sense, Dr. Nelson said, since the law wasn’t intended to “force a bunch of folks with typically high recidivism with mental illness into involuntary treatment.”
Instead, he said, the law has promoted a so-called black robe effect – essentially, a form of intimidation by judge.
Dr. Nelson said he has tracked the debate over forced outpatient care for years and has mixed feelings about the law.
There’s value to coaxing people with mental illness into care when they are unable to recognize their illness, he said. “But there are way too many gaps and holes for people to fall into,” he said. “They have the potential to be mistreated, poorly treated, poorly diagnosed.”
While Laura’s Law requires counties to offer comprehensive outpatient mental health care, “it’s really difficult to know whether the programs are meeting all of the conditions of the law,” Dr. Nelson said.
Still, he said, Laura’s Law is forcing counties to discuss how to care for people with mental illness who are targeted by the legislation, he said. “They are beginning to have a lively conversation about how we are going to reach out to these people and help them, even to just avoid the implementation of Laura’s Law.”
Dr. Nelson has no relevant disclosures.
Critics have feared that California’s “Laura’s Law,” designed to prevent violence by making it easier for officials to force outpatient care upon people with mental illness who have resisted treatment, would violate the civil rights of Golden State residents.
But early statistics gathered by a Northern California psychiatrist suggest that few people have been forced involuntarily into outpatient care in large counties that have adopted the tougher approach in recent years. Instead, Californians targeted by the law appear to be choosing – under pressure – the alternative of accepting outpatient treatment.
California enacted Laura’s Law in 2003 after the death of a 19-year-old woman named Laura Wilcox at a mental health clinic in Northern California. Ms. Wilcox, who worked at the clinic, and two others were shot to death by a patient whose family had failed to persuade officials to force him to get treatment.
As California’s version of assisted outpatient treatment, or AOT, Laura’s Law, allows courts to order people into outpatient treatment in cases that meet certain criteria. Among other things, the person must have a mental illness, be considered “unlikely to survive safely in the community without supervision,” and have a “history of lack of compliance with treatment.” The law “also obligates any county that implements and accepts the law to provide excellent and comprehensive community-based services, not only for court-ordered patients but anybody who wants care,” Dr. Nelson said in an interview after his presentation.
As a state law, the measure must be adopted on a county-by-county basis. Tiny and remote Nevada County, where the shootings occurred, adopted the policy first when it was forced to do so by a lawsuit, Dr. Nelson said. But other counties were slow to sign on.
“Unlike Kendra’s Law, which passed earlier in New York, and both mandated and funded assisted outpatient treatment for all counties, Laura’s Law was completely unfunded. It was left to the discretion of each county to decide for itself whether or not to implement,” said Dr. Nelson, of Marin County, just north of San Francisco, who has served on the councils of the Northern California Psychiatric Society and California Psychiatric Association.
But things changed in 2013, when the state allowed money from a special tax on wealthy residents to be used for AOT programs, Dr. Nelson said. Counties began implementing Laura’s Law in 2014, and the number has now reached 17, representing about two-thirds of the state’s population, he said.
Dr. Nelson gathered statistics from several of California’s 58 counties. He found that over a period of 5 months of implementation, 59 people were referred to the AOT program, and 12 voluntarily accepted outpatient therapy in San Francisco County, which encompasses the city of San Francisco only and has 864,000 residents.
Over a period of 5 months in San Diego County, which has 3.3 million residents, there were 376 referrals, including those to a program that uses home visits to urge people to accept outpatient care. Twenty people accepted voluntary outpatient treatment. No court-ordered treatment was required for anyone in either San Diego or San Francisco counties.
Over a period of 12 months in Orange County, with 3.2 million residents, there were 389 referrals, and 126 people voluntarily accepted AOT. Only three were ordered into treatment by courts. Over 6 months in Placer County, with just 375,000 residents, eight patients voluntarily accepted AOT, and one was ordered into treatment by a court but refused it.
(The Orange County Register reports that refusal to abide by court-ordered outpatient treatment results in no civil or criminal penalties.)
Los Angeles County, by far the most populous in the state with 10.1 million residents, reported 805 AOT referrals, 30 involuntary commitments to outpatient care, and 239 voluntary agreements to outpatient care. The numbers are from 2016 to 2017, but Dr. Nelson did not have details about the exact period covered.
Dr. Nelson also looked at 7 years of statistics from Nevada County, which was forced into adopting an AOT program by a lawsuit. The county, which has fewer than 100,000 residents, reported 67 referrals to the AOT program and 30 cases of court-ordered treatment.
A report estimates that over the first 30 months of the program, Nevada County saved $1.80 for every $1 spent on its AOT program by preventing acute psychiatric hospitalizations and imprisonment.
The low numbers of forced commitments to outpatient care across the state make sense, Dr. Nelson said, since the law wasn’t intended to “force a bunch of folks with typically high recidivism with mental illness into involuntary treatment.”
Instead, he said, the law has promoted a so-called black robe effect – essentially, a form of intimidation by judge.
Dr. Nelson said he has tracked the debate over forced outpatient care for years and has mixed feelings about the law.
There’s value to coaxing people with mental illness into care when they are unable to recognize their illness, he said. “But there are way too many gaps and holes for people to fall into,” he said. “They have the potential to be mistreated, poorly treated, poorly diagnosed.”
While Laura’s Law requires counties to offer comprehensive outpatient mental health care, “it’s really difficult to know whether the programs are meeting all of the conditions of the law,” Dr. Nelson said.
Still, he said, Laura’s Law is forcing counties to discuss how to care for people with mental illness who are targeted by the legislation, he said. “They are beginning to have a lively conversation about how we are going to reach out to these people and help them, even to just avoid the implementation of Laura’s Law.”
Dr. Nelson has no relevant disclosures.
Critics have feared that California’s “Laura’s Law,” designed to prevent violence by making it easier for officials to force outpatient care upon people with mental illness who have resisted treatment, would violate the civil rights of Golden State residents.
But early statistics gathered by a Northern California psychiatrist suggest that few people have been forced involuntarily into outpatient care in large counties that have adopted the tougher approach in recent years. Instead, Californians targeted by the law appear to be choosing – under pressure – the alternative of accepting outpatient treatment.
California enacted Laura’s Law in 2003 after the death of a 19-year-old woman named Laura Wilcox at a mental health clinic in Northern California. Ms. Wilcox, who worked at the clinic, and two others were shot to death by a patient whose family had failed to persuade officials to force him to get treatment.
As California’s version of assisted outpatient treatment, or AOT, Laura’s Law, allows courts to order people into outpatient treatment in cases that meet certain criteria. Among other things, the person must have a mental illness, be considered “unlikely to survive safely in the community without supervision,” and have a “history of lack of compliance with treatment.” The law “also obligates any county that implements and accepts the law to provide excellent and comprehensive community-based services, not only for court-ordered patients but anybody who wants care,” Dr. Nelson said in an interview after his presentation.
As a state law, the measure must be adopted on a county-by-county basis. Tiny and remote Nevada County, where the shootings occurred, adopted the policy first when it was forced to do so by a lawsuit, Dr. Nelson said. But other counties were slow to sign on.
“Unlike Kendra’s Law, which passed earlier in New York, and both mandated and funded assisted outpatient treatment for all counties, Laura’s Law was completely unfunded. It was left to the discretion of each county to decide for itself whether or not to implement,” said Dr. Nelson, of Marin County, just north of San Francisco, who has served on the councils of the Northern California Psychiatric Society and California Psychiatric Association.
But things changed in 2013, when the state allowed money from a special tax on wealthy residents to be used for AOT programs, Dr. Nelson said. Counties began implementing Laura’s Law in 2014, and the number has now reached 17, representing about two-thirds of the state’s population, he said.
Dr. Nelson gathered statistics from several of California’s 58 counties. He found that over a period of 5 months of implementation, 59 people were referred to the AOT program, and 12 voluntarily accepted outpatient therapy in San Francisco County, which encompasses the city of San Francisco only and has 864,000 residents.
Over a period of 5 months in San Diego County, which has 3.3 million residents, there were 376 referrals, including those to a program that uses home visits to urge people to accept outpatient care. Twenty people accepted voluntary outpatient treatment. No court-ordered treatment was required for anyone in either San Diego or San Francisco counties.
Over a period of 12 months in Orange County, with 3.2 million residents, there were 389 referrals, and 126 people voluntarily accepted AOT. Only three were ordered into treatment by courts. Over 6 months in Placer County, with just 375,000 residents, eight patients voluntarily accepted AOT, and one was ordered into treatment by a court but refused it.
(The Orange County Register reports that refusal to abide by court-ordered outpatient treatment results in no civil or criminal penalties.)
Los Angeles County, by far the most populous in the state with 10.1 million residents, reported 805 AOT referrals, 30 involuntary commitments to outpatient care, and 239 voluntary agreements to outpatient care. The numbers are from 2016 to 2017, but Dr. Nelson did not have details about the exact period covered.
Dr. Nelson also looked at 7 years of statistics from Nevada County, which was forced into adopting an AOT program by a lawsuit. The county, which has fewer than 100,000 residents, reported 67 referrals to the AOT program and 30 cases of court-ordered treatment.
A report estimates that over the first 30 months of the program, Nevada County saved $1.80 for every $1 spent on its AOT program by preventing acute psychiatric hospitalizations and imprisonment.
The low numbers of forced commitments to outpatient care across the state make sense, Dr. Nelson said, since the law wasn’t intended to “force a bunch of folks with typically high recidivism with mental illness into involuntary treatment.”
Instead, he said, the law has promoted a so-called black robe effect – essentially, a form of intimidation by judge.
Dr. Nelson said he has tracked the debate over forced outpatient care for years and has mixed feelings about the law.
There’s value to coaxing people with mental illness into care when they are unable to recognize their illness, he said. “But there are way too many gaps and holes for people to fall into,” he said. “They have the potential to be mistreated, poorly treated, poorly diagnosed.”
While Laura’s Law requires counties to offer comprehensive outpatient mental health care, “it’s really difficult to know whether the programs are meeting all of the conditions of the law,” Dr. Nelson said.
Still, he said, Laura’s Law is forcing counties to discuss how to care for people with mental illness who are targeted by the legislation, he said. “They are beginning to have a lively conversation about how we are going to reach out to these people and help them, even to just avoid the implementation of Laura’s Law.”
Dr. Nelson has no relevant disclosures.
EXPERT ANALYSIS FROM APA
Alopecia may be permanent in one in four pediatric HSCT patients
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
AT WCPD 2017
Key clinical point:
Major finding: Permanent alopecia was seen in 25% of patients, and 16% had psoriasis a median of 3.6 years after transplant.
Data source: A single-center prospective study of 85 children in routine post-HSCT follow-up.
Disclosures: Dr. Song reported no conflicts of interest.
Stopping statins after stroke may up recurrent stroke risk
Discontinuing statin therapy 3-6 months after a stroke may increase the risk of recurrent stroke within a year, according to findings from a large retrospective Taiwanese cohort study.
And being older or male in the Stroke Belt increases the likelihood that statins won’t be prescribed at all at the time of hospital discharge after a stroke, a separate cohort study from the United States suggests.
Both studies were reported online in the Journal of the American Heart Association.
Of 45,151 ischemic stroke patients from the Taiwan National Health Insurance Research Database who were on a moderate- or high-intensity statin within 90 days of discharge after an ischemic stroke between 2001 and 2012, 3,175 (7%) were on a reduced dosage by the 90- to 180-day period, and 8,353 (18.5%) were not on any statin by that period. After adjustment for numerous factors including age, sex, several comorbid conditions, and stroke severity index, discontinuation of statins was associated with an increased hazard of recurrent ischemic or hemorrhagic stroke (6.2% vs. 4.4%; adjusted hazard ratio [HR], 1.42), Meng Lee, MD, of Chang Gung Memorial Hospital, Chiayi Branch, Puzy, Taiwan and colleagues reported.
Discontinuation of statins was also linked to higher risks of ischemic stroke (5.6% vs. 3.9%, adjusted HR, 1.45), all-cause mortality (1.4% vs. 1%; adjusted HR, 1.37), all major events (7.8% vs. 5.6%, adjusted HR 1.38), and any hospitalization (31.7% vs. 27.1%; adjusted HR, 1.19), but had neutral effects on intracerebral hemorrhage and myocardial infarction.
“Reduced dosage statin therapy was not associated with increased risks of ischemic stroke, intracerebral hemorrhage, all-cause mortality, myocardial infarction, or all major events,” they said.
The findings support and extend those of some prior studies, and the study “affirms the deleterious effect of stopping statins after the initial period following a stroke,” they noted, adding: “Our study has both clinical and policy implications ... After a stroke due to large or small vessel atherosclerosis, patients are at high risk of recurrent stroke and should be treated aggressively in the absence of clear contraindications. Physicians also need to increase awareness among stroke patients about the potential risk of discontinuing their medications and to encourage greater adherence.”
Statin therapy should not be discontinued in the absence of a “highly compelling reason for doing so,” they added, concluding that prospective studies are needed to clarify the mechanisms underlying the association between statin discontinuation and higher recurrent stroke risk.
In a press statement, Dr. Lee said that based on these “real world” findings, statins should be a lifelong therapy for ischemic stroke patients who need a statin to lower cholesterol.
“Discontinuation of statin treatment in patients with ischemic stroke should be strongly discouraged in any stage, acute or chronic, of stroke,” Dr. Lee said. “Shifting to low-intensity statin therapy could be an alternative for stroke patients not able to tolerate moderate or high-intensity statin therapy in the years following a stroke.”
As for the findings regarding starting statin therapy at the time of discharge, an analysis of discharge prescriptions for U.S. ischemic stroke patients from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study showed that 48.7% of 323 patients who were not statin users at the time of admission and who had no history of atrial fibrillation were prescribed a statin at discharge.
Overall, after adjustment for age, race, sex, numerous comorbid conditions, impaired cognition, current smoking status, stroke buckle or Stroke Belt residence, and other factors, patients aged 65 years and older were less likely to receive a statin prescription at discharge (risk ratio, 0.75), and those with dyslipidemia were more likely to be prescribed a stain at discharge (risk ratio, 1.67), Karen C. Albright, PhD, DO, of the Birmingham (Ala.) VA Medical Center, and her colleagues found.
Further, there were no significant overall differences in statin prescribing by race (black:white risk ratio, 1.13), or by sex (male:female risk ratio, 0.97).
When subjects were analyzed based on Stroke Belt residence (defined as residence in Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia), adults aged 65 and older in the Stroke Belt were 47% less likely to be discharged on a statin, compared with younger patients (risk ratio, 0.53). This association was not observed in non–Stroke Belt residents (risk ratio, 1.14), the investigators found (J Am Heart Assoc. 2017 Aug 2. doi: 10.1161/JAHA.117.005523).
Also, among non–Stroke Belt residents, blacks were more likely to be discharged on a statin (risk ratio, 1.42), but this association was not seen in the Stroke Belt (risk ratio, 0.93).
Male Stroke Belt residents were 31% less likely than were female Stroke Belt residents to be discharged on a statin (risk ratio, 0.69), while men who were non–Stroke Belt residents were more likely than were female non–Stroke Belt residents to be discharged on a statin (risk ratio 1.38).
“Although statin prescribing increased over time in the current study, statins were prescribed at discharge to only 49% of patients with ischemic stroke. This represents a treatment gap given current American College of Cardiology/American Heart Association recommendations,” the investigators wrote.
This gap was particularly seen in men and those over aged 65 years – but not among blacks – in the Stroke Belt.
This leaves the reasons for higher rates of recurrent stroke in blacks unresolved, they noted.
“A next step in our efforts to understand the reasons for the higher rate of recurrent stroke in blacks is to evaluate statin adherence in ischemic stroke survivors,” they said.
The Taiwanese cohort study was funded by the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Taiwan. Dr. Lee and colleagues reported having no disclosures. The REGARDS study was funded by the National Institutes of Health and the Department of Health and Human Services. Dr. Albright reported having no disclosures, as did all other authors except Paul Muntner, PhD, who receives research support from Amgen Inc.
Discontinuing statin therapy 3-6 months after a stroke may increase the risk of recurrent stroke within a year, according to findings from a large retrospective Taiwanese cohort study.
And being older or male in the Stroke Belt increases the likelihood that statins won’t be prescribed at all at the time of hospital discharge after a stroke, a separate cohort study from the United States suggests.
Both studies were reported online in the Journal of the American Heart Association.
Of 45,151 ischemic stroke patients from the Taiwan National Health Insurance Research Database who were on a moderate- or high-intensity statin within 90 days of discharge after an ischemic stroke between 2001 and 2012, 3,175 (7%) were on a reduced dosage by the 90- to 180-day period, and 8,353 (18.5%) were not on any statin by that period. After adjustment for numerous factors including age, sex, several comorbid conditions, and stroke severity index, discontinuation of statins was associated with an increased hazard of recurrent ischemic or hemorrhagic stroke (6.2% vs. 4.4%; adjusted hazard ratio [HR], 1.42), Meng Lee, MD, of Chang Gung Memorial Hospital, Chiayi Branch, Puzy, Taiwan and colleagues reported.
Discontinuation of statins was also linked to higher risks of ischemic stroke (5.6% vs. 3.9%, adjusted HR, 1.45), all-cause mortality (1.4% vs. 1%; adjusted HR, 1.37), all major events (7.8% vs. 5.6%, adjusted HR 1.38), and any hospitalization (31.7% vs. 27.1%; adjusted HR, 1.19), but had neutral effects on intracerebral hemorrhage and myocardial infarction.
“Reduced dosage statin therapy was not associated with increased risks of ischemic stroke, intracerebral hemorrhage, all-cause mortality, myocardial infarction, or all major events,” they said.
The findings support and extend those of some prior studies, and the study “affirms the deleterious effect of stopping statins after the initial period following a stroke,” they noted, adding: “Our study has both clinical and policy implications ... After a stroke due to large or small vessel atherosclerosis, patients are at high risk of recurrent stroke and should be treated aggressively in the absence of clear contraindications. Physicians also need to increase awareness among stroke patients about the potential risk of discontinuing their medications and to encourage greater adherence.”
Statin therapy should not be discontinued in the absence of a “highly compelling reason for doing so,” they added, concluding that prospective studies are needed to clarify the mechanisms underlying the association between statin discontinuation and higher recurrent stroke risk.
In a press statement, Dr. Lee said that based on these “real world” findings, statins should be a lifelong therapy for ischemic stroke patients who need a statin to lower cholesterol.
“Discontinuation of statin treatment in patients with ischemic stroke should be strongly discouraged in any stage, acute or chronic, of stroke,” Dr. Lee said. “Shifting to low-intensity statin therapy could be an alternative for stroke patients not able to tolerate moderate or high-intensity statin therapy in the years following a stroke.”
As for the findings regarding starting statin therapy at the time of discharge, an analysis of discharge prescriptions for U.S. ischemic stroke patients from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study showed that 48.7% of 323 patients who were not statin users at the time of admission and who had no history of atrial fibrillation were prescribed a statin at discharge.
Overall, after adjustment for age, race, sex, numerous comorbid conditions, impaired cognition, current smoking status, stroke buckle or Stroke Belt residence, and other factors, patients aged 65 years and older were less likely to receive a statin prescription at discharge (risk ratio, 0.75), and those with dyslipidemia were more likely to be prescribed a stain at discharge (risk ratio, 1.67), Karen C. Albright, PhD, DO, of the Birmingham (Ala.) VA Medical Center, and her colleagues found.
Further, there were no significant overall differences in statin prescribing by race (black:white risk ratio, 1.13), or by sex (male:female risk ratio, 0.97).
When subjects were analyzed based on Stroke Belt residence (defined as residence in Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia), adults aged 65 and older in the Stroke Belt were 47% less likely to be discharged on a statin, compared with younger patients (risk ratio, 0.53). This association was not observed in non–Stroke Belt residents (risk ratio, 1.14), the investigators found (J Am Heart Assoc. 2017 Aug 2. doi: 10.1161/JAHA.117.005523).
Also, among non–Stroke Belt residents, blacks were more likely to be discharged on a statin (risk ratio, 1.42), but this association was not seen in the Stroke Belt (risk ratio, 0.93).
Male Stroke Belt residents were 31% less likely than were female Stroke Belt residents to be discharged on a statin (risk ratio, 0.69), while men who were non–Stroke Belt residents were more likely than were female non–Stroke Belt residents to be discharged on a statin (risk ratio 1.38).
“Although statin prescribing increased over time in the current study, statins were prescribed at discharge to only 49% of patients with ischemic stroke. This represents a treatment gap given current American College of Cardiology/American Heart Association recommendations,” the investigators wrote.
This gap was particularly seen in men and those over aged 65 years – but not among blacks – in the Stroke Belt.
This leaves the reasons for higher rates of recurrent stroke in blacks unresolved, they noted.
“A next step in our efforts to understand the reasons for the higher rate of recurrent stroke in blacks is to evaluate statin adherence in ischemic stroke survivors,” they said.
The Taiwanese cohort study was funded by the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Taiwan. Dr. Lee and colleagues reported having no disclosures. The REGARDS study was funded by the National Institutes of Health and the Department of Health and Human Services. Dr. Albright reported having no disclosures, as did all other authors except Paul Muntner, PhD, who receives research support from Amgen Inc.
Discontinuing statin therapy 3-6 months after a stroke may increase the risk of recurrent stroke within a year, according to findings from a large retrospective Taiwanese cohort study.
And being older or male in the Stroke Belt increases the likelihood that statins won’t be prescribed at all at the time of hospital discharge after a stroke, a separate cohort study from the United States suggests.
Both studies were reported online in the Journal of the American Heart Association.
Of 45,151 ischemic stroke patients from the Taiwan National Health Insurance Research Database who were on a moderate- or high-intensity statin within 90 days of discharge after an ischemic stroke between 2001 and 2012, 3,175 (7%) were on a reduced dosage by the 90- to 180-day period, and 8,353 (18.5%) were not on any statin by that period. After adjustment for numerous factors including age, sex, several comorbid conditions, and stroke severity index, discontinuation of statins was associated with an increased hazard of recurrent ischemic or hemorrhagic stroke (6.2% vs. 4.4%; adjusted hazard ratio [HR], 1.42), Meng Lee, MD, of Chang Gung Memorial Hospital, Chiayi Branch, Puzy, Taiwan and colleagues reported.
Discontinuation of statins was also linked to higher risks of ischemic stroke (5.6% vs. 3.9%, adjusted HR, 1.45), all-cause mortality (1.4% vs. 1%; adjusted HR, 1.37), all major events (7.8% vs. 5.6%, adjusted HR 1.38), and any hospitalization (31.7% vs. 27.1%; adjusted HR, 1.19), but had neutral effects on intracerebral hemorrhage and myocardial infarction.
“Reduced dosage statin therapy was not associated with increased risks of ischemic stroke, intracerebral hemorrhage, all-cause mortality, myocardial infarction, or all major events,” they said.
The findings support and extend those of some prior studies, and the study “affirms the deleterious effect of stopping statins after the initial period following a stroke,” they noted, adding: “Our study has both clinical and policy implications ... After a stroke due to large or small vessel atherosclerosis, patients are at high risk of recurrent stroke and should be treated aggressively in the absence of clear contraindications. Physicians also need to increase awareness among stroke patients about the potential risk of discontinuing their medications and to encourage greater adherence.”
Statin therapy should not be discontinued in the absence of a “highly compelling reason for doing so,” they added, concluding that prospective studies are needed to clarify the mechanisms underlying the association between statin discontinuation and higher recurrent stroke risk.
In a press statement, Dr. Lee said that based on these “real world” findings, statins should be a lifelong therapy for ischemic stroke patients who need a statin to lower cholesterol.
“Discontinuation of statin treatment in patients with ischemic stroke should be strongly discouraged in any stage, acute or chronic, of stroke,” Dr. Lee said. “Shifting to low-intensity statin therapy could be an alternative for stroke patients not able to tolerate moderate or high-intensity statin therapy in the years following a stroke.”
As for the findings regarding starting statin therapy at the time of discharge, an analysis of discharge prescriptions for U.S. ischemic stroke patients from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study showed that 48.7% of 323 patients who were not statin users at the time of admission and who had no history of atrial fibrillation were prescribed a statin at discharge.
Overall, after adjustment for age, race, sex, numerous comorbid conditions, impaired cognition, current smoking status, stroke buckle or Stroke Belt residence, and other factors, patients aged 65 years and older were less likely to receive a statin prescription at discharge (risk ratio, 0.75), and those with dyslipidemia were more likely to be prescribed a stain at discharge (risk ratio, 1.67), Karen C. Albright, PhD, DO, of the Birmingham (Ala.) VA Medical Center, and her colleagues found.
Further, there were no significant overall differences in statin prescribing by race (black:white risk ratio, 1.13), or by sex (male:female risk ratio, 0.97).
When subjects were analyzed based on Stroke Belt residence (defined as residence in Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia), adults aged 65 and older in the Stroke Belt were 47% less likely to be discharged on a statin, compared with younger patients (risk ratio, 0.53). This association was not observed in non–Stroke Belt residents (risk ratio, 1.14), the investigators found (J Am Heart Assoc. 2017 Aug 2. doi: 10.1161/JAHA.117.005523).
Also, among non–Stroke Belt residents, blacks were more likely to be discharged on a statin (risk ratio, 1.42), but this association was not seen in the Stroke Belt (risk ratio, 0.93).
Male Stroke Belt residents were 31% less likely than were female Stroke Belt residents to be discharged on a statin (risk ratio, 0.69), while men who were non–Stroke Belt residents were more likely than were female non–Stroke Belt residents to be discharged on a statin (risk ratio 1.38).
“Although statin prescribing increased over time in the current study, statins were prescribed at discharge to only 49% of patients with ischemic stroke. This represents a treatment gap given current American College of Cardiology/American Heart Association recommendations,” the investigators wrote.
This gap was particularly seen in men and those over aged 65 years – but not among blacks – in the Stroke Belt.
This leaves the reasons for higher rates of recurrent stroke in blacks unresolved, they noted.
“A next step in our efforts to understand the reasons for the higher rate of recurrent stroke in blacks is to evaluate statin adherence in ischemic stroke survivors,” they said.
The Taiwanese cohort study was funded by the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Taiwan. Dr. Lee and colleagues reported having no disclosures. The REGARDS study was funded by the National Institutes of Health and the Department of Health and Human Services. Dr. Albright reported having no disclosures, as did all other authors except Paul Muntner, PhD, who receives research support from Amgen Inc.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point:
Major finding: Discontinuation of statins 90-180 days after ischemic stroke was associated with an increased hazard of recurrent ischemic or hemorrhagic stroke (6.2% vs. 4.4%; adjusted hazard ratio [HR], 1.42).
Data source: Cohort studies in Taiwan and the United States involving 45,151 and 323 patients, respectively.
Disclosures: The Taiwanese cohort study was funded by the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Taiwan. Dr. Lee and colleagues reported having no disclosures. The REGARDS study was funded by the National Institutes of Health and the Department of Health and Human Services. Dr. Albright reported having no disclosures, as did all other authors except Paul Muntner, PhD, who receives research support from Amgen Inc.
Novel device aims to make cervical cancer screening more accessible
A novel vaginal inserter currently in development at Duke University for use with a miniature colposcope proved feasible for comfortable, speculum-free cervical image capture in a recent study.
Along with allowing for more comfortable – and possibly self-facilitated – cervical cancer screening, the device could have important implications for providing care and improving screening rates in underserved, low-resource communities in the United States and around the world, according to Mercy N. Asiedu, a graduate student research assistant in the Duke University department of biomedical engineering.
Ms. Asiedu is part of a team that has been working to develop effective, low-cost methods for diagnosing cervical and breast cancer, particularly in low-resource regions.
A device such as the integrated inserter and colposcope could potentially be purchased over the counter at a local pharmacy and used for “self checkups,” or used in clinics to facilitate a more comfortable, patient-friendly approach to exams here in the United States, she said in an interview.
“But in other countries, one of the bigger benefits [relates to] cultural factors that deter women from going in for gynecological exams,” Ms. Asiedu said.
Overcoming screening barriers
Invasive cervical cancer ranks as the second most common female cancer in low and middle-income countries and the seventh most common in high-income countries, she and her colleagues wrote in a study published in PLoS ONE (12[5]:e0177782. doi: 10.1371/journal.pone.0177782).
The discrepancies between high- and low-resource areas largely result from the fact that visualization of the cervix via colposcopy using a standard approach requires a highly-trained professional and expensive equipment that is not easily accessible in underserved regions.
But even in the United states, the use of a standard speculum to expand the vaginal canal has been identified as a factor in the resistance of some women to undergo screening. Embarrassment and fear of pain have also been reported as potential barriers to screening.
The ability to self-insert can also help reduce pain associated with the exam, as it allows the user to adjust positioning when there is discomfort, she said.
A speculum alternative
The inserter is a tampon-sized tubular device designed as an alternative to a standard speculum, and is used with the Pocket Colposcope, a miniature pen-sized colposcope also developed by Duke University researchers and validated by physicians worldwide as providing image quality comparable to high-end, state-of-the-art digital colposcopes.
The current rendition of the inserter device has a funnel-like, curved tip measuring approximately 2.6 cm in diameter, a channel for insertion of a 2-megapixel mini-USB camera with LED illumination for the cervical image capture (as opposed to the 5-megapixel camera typically used with the Pocket Colposcope in the setting of standard speculum use), and can connect to a number of devices such as smartphones and laptop computers for image display.
“The curved tip enables easy manipulation of the cervix, especially in cases where the patient has a tilted uterus,” Ms Asiedu and her colleagues wrote in PLoS. “Our design also enables manipulation of the cervix for cervical imaging of women with tilted uteri, a condition that affects about 20% of women and is difficult and painful when using the standard speculum for manipulation.”
Using a custom-made vaginal phantom, the researchers tested various designs and demonstrated that it was able to withstand a range of supine vaginal pressures. In addition, 12 of 15 healthy volunteers achieved adequate cervical image capture after self-insertion of the device, and 14 of the 15 women expressed an overall preference for the inserter over the speculum (based on past experience). All of the volunteers said the inserter was slightly more, or much more, comfortable than a standard speculum, and noted that comfort was a particular benefit.
Prior to self-insertion, the volunteers typically received about 5 minutes of instruction using a pelvic mannequin. Images were captured and displayed on a mobile device once the cervix was in view.
From a patient perspective, the volunteer study “was almost universally positive,” John W. Schmitt, MD, professor of obstetrics and gynecology, as well as global health, at Duke, said in an interview. He conducted the clinical portion of the research.
From the clinician perspective, there is a bit of a learning curve, he added.
“I’ve approached it with what I’d call healthy skepticism,” he said, noting that traditional specula have been around for ages, and are easy to use. “I’ve used them for a long time and I know how, so there’s a learning curve to get it right [with the new device], but as I’ve done more and more, it’s becoming really obvious that this can be used, and ... it’s got real potential to make for a more comfortable internal exam.”
That is particularly true if the size and design can be maintained while also allowing channels to be added to allow solutions, such as acetic acid or Lugol’s solution, to be applied to the cervix to help with visualization – all while keeping the camera in place and allowing the insertion of swabs for obtaining samples from the cervix, he said.
Future directions
Efforts are underway to further improve the design for better visualization, ease of use, and comfort. The next phase of study will involve comparing visualization between the device and a standard speculum for routine exams and more advanced cancer screening exams. Ms. Asiedu said the researchers are also seeking to modify the inserter to accommodate the original Pocket Colposcope with a 5-megapixel camera for better image quality.
Should the inserter and colposcope be validated in further studies, the potential benefits are numerous, she said, explaining that the device’s potential for expanding access to screening are driven by its portability and low cost; the 2-megapixel camera currently used with the device costs about $54, compared with $20,000 for a standard-of-care digital colposcope. The estimated cost of the inserter under mass production would be less than $1.
It may be possible to produce the inserter in varying sizes to match cervix size. There are other potential uses for the device, including at-home viewing of the cervical os to gauge cervical dilation during labor and to detect false labor, she and her colleagues noted.
One limitation, which is also a problem with current methods of visual inspection of the cervix, is lack of ability to see the squamocolumnar junction.
“It is therefore not a replacement for gold standard pathology, where cells from the squamocolumnar junction are removed for pathology. However, our device can aid in guiding biopsies and has merit for places that lack gold standard biopsy,” they wrote, noting that future studies will compare cervix samples collected via the inserter versus a standard speculum to explore the possibility of self-sampling.
Efforts also are underway to develop an algorithm for making a diagnosis based on such samples, Ms. Asiedu said.
Given that 11 of the 15 volunteers in the study said that self-screening with the device was more difficult than having a physician perform the exam, another longitudinal study will look at whether ease of use improves over time, she said.
“There’s a lot of work to be done to get it where it could be routinely used,” Dr. Schmitt said. “Even for someone like me who does have this healthy skepticism, this might really be a profound change in the way we do pelvic exams.”
A word of caution
But Michael R. Caudle, MD, an ob.gyn. with Cherokee Health Systems in Knoxville, Tenn., urged caution, at least among rural U.S. health centers, with any approach that involves only visualizing the cervix. His concern is that this type of device could give women “false reassurances.”
“The promulgation of Pap smears and HPV testing would be the direction rural sites should go,” he said. “Colposcopy requires biopsies to rule out serious disease.”
He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) provides training for mid-level providers to do these evaluations.
“I think this is better than looking only. We see tragedies from women not getting adequate follow-up for abnormal paps, so making this even more removed from proper oversight is a concern. There have been attempts in the past to send photos of the cervix for evaluation and one problem again was the lack of biopsies.
“Rural health departments do a good job at triaging abnormal paps, and expansion of this, in my opinion, is a better idea,” he said.
The Duke University study was supported by the National Institutes of Health. Ms. Asiedu reported having no disclosures, but two of her coauthors founded companies and developed technologies related to this work where the investigators or Duke may benefit financially if the system is sold commercially. Ms. Asiedu and her coauthors have filed a provisional patent application for the concept of the system.
A novel vaginal inserter currently in development at Duke University for use with a miniature colposcope proved feasible for comfortable, speculum-free cervical image capture in a recent study.
Along with allowing for more comfortable – and possibly self-facilitated – cervical cancer screening, the device could have important implications for providing care and improving screening rates in underserved, low-resource communities in the United States and around the world, according to Mercy N. Asiedu, a graduate student research assistant in the Duke University department of biomedical engineering.
Ms. Asiedu is part of a team that has been working to develop effective, low-cost methods for diagnosing cervical and breast cancer, particularly in low-resource regions.
A device such as the integrated inserter and colposcope could potentially be purchased over the counter at a local pharmacy and used for “self checkups,” or used in clinics to facilitate a more comfortable, patient-friendly approach to exams here in the United States, she said in an interview.
“But in other countries, one of the bigger benefits [relates to] cultural factors that deter women from going in for gynecological exams,” Ms. Asiedu said.
Overcoming screening barriers
Invasive cervical cancer ranks as the second most common female cancer in low and middle-income countries and the seventh most common in high-income countries, she and her colleagues wrote in a study published in PLoS ONE (12[5]:e0177782. doi: 10.1371/journal.pone.0177782).
The discrepancies between high- and low-resource areas largely result from the fact that visualization of the cervix via colposcopy using a standard approach requires a highly-trained professional and expensive equipment that is not easily accessible in underserved regions.
But even in the United states, the use of a standard speculum to expand the vaginal canal has been identified as a factor in the resistance of some women to undergo screening. Embarrassment and fear of pain have also been reported as potential barriers to screening.
The ability to self-insert can also help reduce pain associated with the exam, as it allows the user to adjust positioning when there is discomfort, she said.
A speculum alternative
The inserter is a tampon-sized tubular device designed as an alternative to a standard speculum, and is used with the Pocket Colposcope, a miniature pen-sized colposcope also developed by Duke University researchers and validated by physicians worldwide as providing image quality comparable to high-end, state-of-the-art digital colposcopes.
The current rendition of the inserter device has a funnel-like, curved tip measuring approximately 2.6 cm in diameter, a channel for insertion of a 2-megapixel mini-USB camera with LED illumination for the cervical image capture (as opposed to the 5-megapixel camera typically used with the Pocket Colposcope in the setting of standard speculum use), and can connect to a number of devices such as smartphones and laptop computers for image display.
“The curved tip enables easy manipulation of the cervix, especially in cases where the patient has a tilted uterus,” Ms Asiedu and her colleagues wrote in PLoS. “Our design also enables manipulation of the cervix for cervical imaging of women with tilted uteri, a condition that affects about 20% of women and is difficult and painful when using the standard speculum for manipulation.”
Using a custom-made vaginal phantom, the researchers tested various designs and demonstrated that it was able to withstand a range of supine vaginal pressures. In addition, 12 of 15 healthy volunteers achieved adequate cervical image capture after self-insertion of the device, and 14 of the 15 women expressed an overall preference for the inserter over the speculum (based on past experience). All of the volunteers said the inserter was slightly more, or much more, comfortable than a standard speculum, and noted that comfort was a particular benefit.
Prior to self-insertion, the volunteers typically received about 5 minutes of instruction using a pelvic mannequin. Images were captured and displayed on a mobile device once the cervix was in view.
From a patient perspective, the volunteer study “was almost universally positive,” John W. Schmitt, MD, professor of obstetrics and gynecology, as well as global health, at Duke, said in an interview. He conducted the clinical portion of the research.
From the clinician perspective, there is a bit of a learning curve, he added.
“I’ve approached it with what I’d call healthy skepticism,” he said, noting that traditional specula have been around for ages, and are easy to use. “I’ve used them for a long time and I know how, so there’s a learning curve to get it right [with the new device], but as I’ve done more and more, it’s becoming really obvious that this can be used, and ... it’s got real potential to make for a more comfortable internal exam.”
That is particularly true if the size and design can be maintained while also allowing channels to be added to allow solutions, such as acetic acid or Lugol’s solution, to be applied to the cervix to help with visualization – all while keeping the camera in place and allowing the insertion of swabs for obtaining samples from the cervix, he said.
Future directions
Efforts are underway to further improve the design for better visualization, ease of use, and comfort. The next phase of study will involve comparing visualization between the device and a standard speculum for routine exams and more advanced cancer screening exams. Ms. Asiedu said the researchers are also seeking to modify the inserter to accommodate the original Pocket Colposcope with a 5-megapixel camera for better image quality.
Should the inserter and colposcope be validated in further studies, the potential benefits are numerous, she said, explaining that the device’s potential for expanding access to screening are driven by its portability and low cost; the 2-megapixel camera currently used with the device costs about $54, compared with $20,000 for a standard-of-care digital colposcope. The estimated cost of the inserter under mass production would be less than $1.
It may be possible to produce the inserter in varying sizes to match cervix size. There are other potential uses for the device, including at-home viewing of the cervical os to gauge cervical dilation during labor and to detect false labor, she and her colleagues noted.
One limitation, which is also a problem with current methods of visual inspection of the cervix, is lack of ability to see the squamocolumnar junction.
“It is therefore not a replacement for gold standard pathology, where cells from the squamocolumnar junction are removed for pathology. However, our device can aid in guiding biopsies and has merit for places that lack gold standard biopsy,” they wrote, noting that future studies will compare cervix samples collected via the inserter versus a standard speculum to explore the possibility of self-sampling.
Efforts also are underway to develop an algorithm for making a diagnosis based on such samples, Ms. Asiedu said.
Given that 11 of the 15 volunteers in the study said that self-screening with the device was more difficult than having a physician perform the exam, another longitudinal study will look at whether ease of use improves over time, she said.
“There’s a lot of work to be done to get it where it could be routinely used,” Dr. Schmitt said. “Even for someone like me who does have this healthy skepticism, this might really be a profound change in the way we do pelvic exams.”
A word of caution
But Michael R. Caudle, MD, an ob.gyn. with Cherokee Health Systems in Knoxville, Tenn., urged caution, at least among rural U.S. health centers, with any approach that involves only visualizing the cervix. His concern is that this type of device could give women “false reassurances.”
“The promulgation of Pap smears and HPV testing would be the direction rural sites should go,” he said. “Colposcopy requires biopsies to rule out serious disease.”
He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) provides training for mid-level providers to do these evaluations.
“I think this is better than looking only. We see tragedies from women not getting adequate follow-up for abnormal paps, so making this even more removed from proper oversight is a concern. There have been attempts in the past to send photos of the cervix for evaluation and one problem again was the lack of biopsies.
“Rural health departments do a good job at triaging abnormal paps, and expansion of this, in my opinion, is a better idea,” he said.
The Duke University study was supported by the National Institutes of Health. Ms. Asiedu reported having no disclosures, but two of her coauthors founded companies and developed technologies related to this work where the investigators or Duke may benefit financially if the system is sold commercially. Ms. Asiedu and her coauthors have filed a provisional patent application for the concept of the system.
A novel vaginal inserter currently in development at Duke University for use with a miniature colposcope proved feasible for comfortable, speculum-free cervical image capture in a recent study.
Along with allowing for more comfortable – and possibly self-facilitated – cervical cancer screening, the device could have important implications for providing care and improving screening rates in underserved, low-resource communities in the United States and around the world, according to Mercy N. Asiedu, a graduate student research assistant in the Duke University department of biomedical engineering.
Ms. Asiedu is part of a team that has been working to develop effective, low-cost methods for diagnosing cervical and breast cancer, particularly in low-resource regions.
A device such as the integrated inserter and colposcope could potentially be purchased over the counter at a local pharmacy and used for “self checkups,” or used in clinics to facilitate a more comfortable, patient-friendly approach to exams here in the United States, she said in an interview.
“But in other countries, one of the bigger benefits [relates to] cultural factors that deter women from going in for gynecological exams,” Ms. Asiedu said.
Overcoming screening barriers
Invasive cervical cancer ranks as the second most common female cancer in low and middle-income countries and the seventh most common in high-income countries, she and her colleagues wrote in a study published in PLoS ONE (12[5]:e0177782. doi: 10.1371/journal.pone.0177782).
The discrepancies between high- and low-resource areas largely result from the fact that visualization of the cervix via colposcopy using a standard approach requires a highly-trained professional and expensive equipment that is not easily accessible in underserved regions.
But even in the United states, the use of a standard speculum to expand the vaginal canal has been identified as a factor in the resistance of some women to undergo screening. Embarrassment and fear of pain have also been reported as potential barriers to screening.
The ability to self-insert can also help reduce pain associated with the exam, as it allows the user to adjust positioning when there is discomfort, she said.
A speculum alternative
The inserter is a tampon-sized tubular device designed as an alternative to a standard speculum, and is used with the Pocket Colposcope, a miniature pen-sized colposcope also developed by Duke University researchers and validated by physicians worldwide as providing image quality comparable to high-end, state-of-the-art digital colposcopes.
The current rendition of the inserter device has a funnel-like, curved tip measuring approximately 2.6 cm in diameter, a channel for insertion of a 2-megapixel mini-USB camera with LED illumination for the cervical image capture (as opposed to the 5-megapixel camera typically used with the Pocket Colposcope in the setting of standard speculum use), and can connect to a number of devices such as smartphones and laptop computers for image display.
“The curved tip enables easy manipulation of the cervix, especially in cases where the patient has a tilted uterus,” Ms Asiedu and her colleagues wrote in PLoS. “Our design also enables manipulation of the cervix for cervical imaging of women with tilted uteri, a condition that affects about 20% of women and is difficult and painful when using the standard speculum for manipulation.”
Using a custom-made vaginal phantom, the researchers tested various designs and demonstrated that it was able to withstand a range of supine vaginal pressures. In addition, 12 of 15 healthy volunteers achieved adequate cervical image capture after self-insertion of the device, and 14 of the 15 women expressed an overall preference for the inserter over the speculum (based on past experience). All of the volunteers said the inserter was slightly more, or much more, comfortable than a standard speculum, and noted that comfort was a particular benefit.
Prior to self-insertion, the volunteers typically received about 5 minutes of instruction using a pelvic mannequin. Images were captured and displayed on a mobile device once the cervix was in view.
From a patient perspective, the volunteer study “was almost universally positive,” John W. Schmitt, MD, professor of obstetrics and gynecology, as well as global health, at Duke, said in an interview. He conducted the clinical portion of the research.
From the clinician perspective, there is a bit of a learning curve, he added.
“I’ve approached it with what I’d call healthy skepticism,” he said, noting that traditional specula have been around for ages, and are easy to use. “I’ve used them for a long time and I know how, so there’s a learning curve to get it right [with the new device], but as I’ve done more and more, it’s becoming really obvious that this can be used, and ... it’s got real potential to make for a more comfortable internal exam.”
That is particularly true if the size and design can be maintained while also allowing channels to be added to allow solutions, such as acetic acid or Lugol’s solution, to be applied to the cervix to help with visualization – all while keeping the camera in place and allowing the insertion of swabs for obtaining samples from the cervix, he said.
Future directions
Efforts are underway to further improve the design for better visualization, ease of use, and comfort. The next phase of study will involve comparing visualization between the device and a standard speculum for routine exams and more advanced cancer screening exams. Ms. Asiedu said the researchers are also seeking to modify the inserter to accommodate the original Pocket Colposcope with a 5-megapixel camera for better image quality.
Should the inserter and colposcope be validated in further studies, the potential benefits are numerous, she said, explaining that the device’s potential for expanding access to screening are driven by its portability and low cost; the 2-megapixel camera currently used with the device costs about $54, compared with $20,000 for a standard-of-care digital colposcope. The estimated cost of the inserter under mass production would be less than $1.
It may be possible to produce the inserter in varying sizes to match cervix size. There are other potential uses for the device, including at-home viewing of the cervical os to gauge cervical dilation during labor and to detect false labor, she and her colleagues noted.
One limitation, which is also a problem with current methods of visual inspection of the cervix, is lack of ability to see the squamocolumnar junction.
“It is therefore not a replacement for gold standard pathology, where cells from the squamocolumnar junction are removed for pathology. However, our device can aid in guiding biopsies and has merit for places that lack gold standard biopsy,” they wrote, noting that future studies will compare cervix samples collected via the inserter versus a standard speculum to explore the possibility of self-sampling.
Efforts also are underway to develop an algorithm for making a diagnosis based on such samples, Ms. Asiedu said.
Given that 11 of the 15 volunteers in the study said that self-screening with the device was more difficult than having a physician perform the exam, another longitudinal study will look at whether ease of use improves over time, she said.
“There’s a lot of work to be done to get it where it could be routinely used,” Dr. Schmitt said. “Even for someone like me who does have this healthy skepticism, this might really be a profound change in the way we do pelvic exams.”
A word of caution
But Michael R. Caudle, MD, an ob.gyn. with Cherokee Health Systems in Knoxville, Tenn., urged caution, at least among rural U.S. health centers, with any approach that involves only visualizing the cervix. His concern is that this type of device could give women “false reassurances.”
“The promulgation of Pap smears and HPV testing would be the direction rural sites should go,” he said. “Colposcopy requires biopsies to rule out serious disease.”
He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) provides training for mid-level providers to do these evaluations.
“I think this is better than looking only. We see tragedies from women not getting adequate follow-up for abnormal paps, so making this even more removed from proper oversight is a concern. There have been attempts in the past to send photos of the cervix for evaluation and one problem again was the lack of biopsies.
“Rural health departments do a good job at triaging abnormal paps, and expansion of this, in my opinion, is a better idea,” he said.
The Duke University study was supported by the National Institutes of Health. Ms. Asiedu reported having no disclosures, but two of her coauthors founded companies and developed technologies related to this work where the investigators or Duke may benefit financially if the system is sold commercially. Ms. Asiedu and her coauthors have filed a provisional patent application for the concept of the system.