User login
Insurance coverage gainers outnumber coverage losers
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.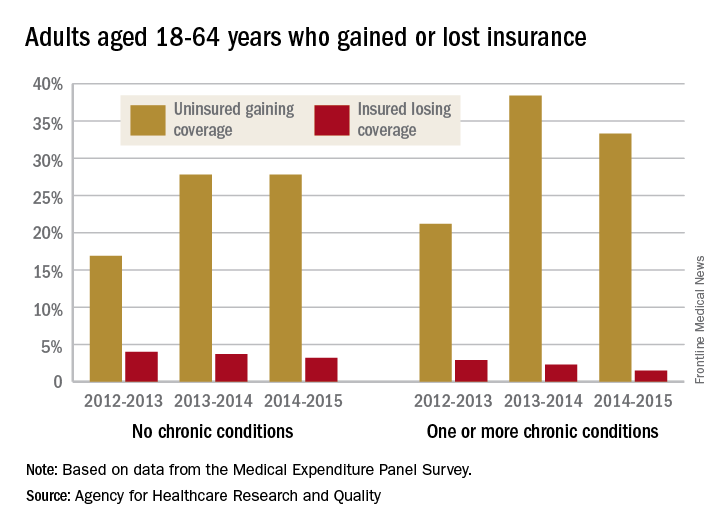
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Fewer nonelderly adults lost their health insurance in 2015 than in 2013, while more gained coverage, according to the Agency for Healthcare Research and Quality.
The presence of chronic conditions played a part for those who lost coverage. From 2012 to 2013, 2.9% of adults aged 18-64 years with one or more chronic conditions lost their insurance, compared with 1.5% who lost coverage from 2014 to 2015. Those with no chronic conditions saw a corresponding drop from 4% to 3.2%, but that change was not significant, AHRQ investigators reported.
For this analysis, the chronic conditions were active asthma, arthritis, diabetes, emphysema, heart disease, high blood pressure, high cholesterol, bronchitis, and stroke. The source of the data was the Medical Expenditure Panel Survey.
Noninvasive NASH test could help monitor hepatotoxicity in patients on methotrexate
A noninvasive test for nonalcoholic steatohepatitis (NASH) and hepatic fibrosis could be used to help detect worsening hepatic fibrosis in psoriasis patients who are on long-term methotrexate therapy, according to the authors of a retrospective study published online Aug. 23.
To evaluate the use of the noninvasive test to monitor for hepatic fibrosis in this group of patients and guide the management of methotrexate (MTX) without a liver biopsy, investigators conducted an analysis of 107 patients with psoriasis who were on long-term MTX treatment, for whom the NASH FibroSure test was used between January 2007 and December 2013. All the patients were white, fifty were men, the mean age was 83 years, and almost all of the patients had a body mass index of 28 or more (16% had a BMI between 28 and 30, and 81% had a BMI over 30).
The NASH FibroSure test, which was developed for use in patients suspected of having nonalcoholic fatty liver disease (NAFLD), combines analyses of 10 biochemical markers combined with age, sex, height, and weight to calculate the degree of hepatic fibrosis. The test has a reported sensitivity of 83% and a specificity of 78% in detecting risk of significant fibrosis, according to Bruce Bauer, MD, of Pariser Dermatology Specialists, Norfolk, Va., and his coinvestigators (JAMA Dermatol. 2017 Aug 23. doi: 10.1001/jamadermatol.2017.2083).
Among the 107 patients, the investigators found a statistically significant correlation “between worsening fibrosis scores and cumulative methotrexate” dose among women (P = .02), but not among men (P = .11). In addition, women with a BMI of 28 or more were more likely to have worsening fibrosis scores (P = .03). “There were no differences between men and women with regard to prevalence of a BMI of 28 or more, diabetes, age older than 65 years, or chronic kidney disease,” which they said, suggests that among women, “obesity influences the progression of fibrosis scores.”
The investigators advised providers not to disregard any potential warning signs when using FibroSure on men. “No differences between the cohorts were observed that would explain the association of worsening fibrosis scores and cumulative MTX dose among women but not men,” they wrote. “However, given the implications of the progression of hepatic fibrosis, we still recommend discontinuing MTX for male patients who demonstrate worsening fibrosis scores.”
While the test may not be able to replace liver biopsy, based on these results, the investigators noted that noninvasive tests such as this one could help significantly reduce the number of liver biopsies needed.
They pointed out that because the study was conducted at one center, the next step is to conduct “a prospective, randomized, multi-institutional analysis of NASH FibroSure and liver biopsies for patients with psoriasis receiving MTX vs. other treatments, including a larger cohort of men and women with different racial and ethnic backgrounds.”
One of the four authors reported receiving research funding from T2 Biosystems. Dr. Bauer and the two other authors reported no relevant financial disclosures. The study was funded by the Marshfield Clinic Resident Research Program.
[email protected]
On Twitter @eaztweets
A noninvasive test for nonalcoholic steatohepatitis (NASH) and hepatic fibrosis could be used to help detect worsening hepatic fibrosis in psoriasis patients who are on long-term methotrexate therapy, according to the authors of a retrospective study published online Aug. 23.
To evaluate the use of the noninvasive test to monitor for hepatic fibrosis in this group of patients and guide the management of methotrexate (MTX) without a liver biopsy, investigators conducted an analysis of 107 patients with psoriasis who were on long-term MTX treatment, for whom the NASH FibroSure test was used between January 2007 and December 2013. All the patients were white, fifty were men, the mean age was 83 years, and almost all of the patients had a body mass index of 28 or more (16% had a BMI between 28 and 30, and 81% had a BMI over 30).
The NASH FibroSure test, which was developed for use in patients suspected of having nonalcoholic fatty liver disease (NAFLD), combines analyses of 10 biochemical markers combined with age, sex, height, and weight to calculate the degree of hepatic fibrosis. The test has a reported sensitivity of 83% and a specificity of 78% in detecting risk of significant fibrosis, according to Bruce Bauer, MD, of Pariser Dermatology Specialists, Norfolk, Va., and his coinvestigators (JAMA Dermatol. 2017 Aug 23. doi: 10.1001/jamadermatol.2017.2083).
Among the 107 patients, the investigators found a statistically significant correlation “between worsening fibrosis scores and cumulative methotrexate” dose among women (P = .02), but not among men (P = .11). In addition, women with a BMI of 28 or more were more likely to have worsening fibrosis scores (P = .03). “There were no differences between men and women with regard to prevalence of a BMI of 28 or more, diabetes, age older than 65 years, or chronic kidney disease,” which they said, suggests that among women, “obesity influences the progression of fibrosis scores.”
The investigators advised providers not to disregard any potential warning signs when using FibroSure on men. “No differences between the cohorts were observed that would explain the association of worsening fibrosis scores and cumulative MTX dose among women but not men,” they wrote. “However, given the implications of the progression of hepatic fibrosis, we still recommend discontinuing MTX for male patients who demonstrate worsening fibrosis scores.”
While the test may not be able to replace liver biopsy, based on these results, the investigators noted that noninvasive tests such as this one could help significantly reduce the number of liver biopsies needed.
They pointed out that because the study was conducted at one center, the next step is to conduct “a prospective, randomized, multi-institutional analysis of NASH FibroSure and liver biopsies for patients with psoriasis receiving MTX vs. other treatments, including a larger cohort of men and women with different racial and ethnic backgrounds.”
One of the four authors reported receiving research funding from T2 Biosystems. Dr. Bauer and the two other authors reported no relevant financial disclosures. The study was funded by the Marshfield Clinic Resident Research Program.
[email protected]
On Twitter @eaztweets
A noninvasive test for nonalcoholic steatohepatitis (NASH) and hepatic fibrosis could be used to help detect worsening hepatic fibrosis in psoriasis patients who are on long-term methotrexate therapy, according to the authors of a retrospective study published online Aug. 23.
To evaluate the use of the noninvasive test to monitor for hepatic fibrosis in this group of patients and guide the management of methotrexate (MTX) without a liver biopsy, investigators conducted an analysis of 107 patients with psoriasis who were on long-term MTX treatment, for whom the NASH FibroSure test was used between January 2007 and December 2013. All the patients were white, fifty were men, the mean age was 83 years, and almost all of the patients had a body mass index of 28 or more (16% had a BMI between 28 and 30, and 81% had a BMI over 30).
The NASH FibroSure test, which was developed for use in patients suspected of having nonalcoholic fatty liver disease (NAFLD), combines analyses of 10 biochemical markers combined with age, sex, height, and weight to calculate the degree of hepatic fibrosis. The test has a reported sensitivity of 83% and a specificity of 78% in detecting risk of significant fibrosis, according to Bruce Bauer, MD, of Pariser Dermatology Specialists, Norfolk, Va., and his coinvestigators (JAMA Dermatol. 2017 Aug 23. doi: 10.1001/jamadermatol.2017.2083).
Among the 107 patients, the investigators found a statistically significant correlation “between worsening fibrosis scores and cumulative methotrexate” dose among women (P = .02), but not among men (P = .11). In addition, women with a BMI of 28 or more were more likely to have worsening fibrosis scores (P = .03). “There were no differences between men and women with regard to prevalence of a BMI of 28 or more, diabetes, age older than 65 years, or chronic kidney disease,” which they said, suggests that among women, “obesity influences the progression of fibrosis scores.”
The investigators advised providers not to disregard any potential warning signs when using FibroSure on men. “No differences between the cohorts were observed that would explain the association of worsening fibrosis scores and cumulative MTX dose among women but not men,” they wrote. “However, given the implications of the progression of hepatic fibrosis, we still recommend discontinuing MTX for male patients who demonstrate worsening fibrosis scores.”
While the test may not be able to replace liver biopsy, based on these results, the investigators noted that noninvasive tests such as this one could help significantly reduce the number of liver biopsies needed.
They pointed out that because the study was conducted at one center, the next step is to conduct “a prospective, randomized, multi-institutional analysis of NASH FibroSure and liver biopsies for patients with psoriasis receiving MTX vs. other treatments, including a larger cohort of men and women with different racial and ethnic backgrounds.”
One of the four authors reported receiving research funding from T2 Biosystems. Dr. Bauer and the two other authors reported no relevant financial disclosures. The study was funded by the Marshfield Clinic Resident Research Program.
[email protected]
On Twitter @eaztweets
FROM JAMA DERMATOLOGY
Key clinical point:
Major finding: There was a significant correlation between worsening fibrosis scores on the test and cumulative methotrexate dose among women (P = .02), but not among men (P = .11).
Data source: A retrospective single-center study analyzing the test in 107 psoriasis patients on methotrexate, collected between January 2007 and December 2013.
Disclosures: One of the four authors received research funding from T2 Biosystems. There were no other financial disclosures.
National Trends (2007-2013) of Clostridium difficile Infection in Patients with Septic Shock: Impact on Outcome
Clostridium difficile infection (CDI) is the most common infectious cause of healthcare-associated diarrhea.1 Development of a CDI during hospitalization is associated with increases in morbidity, mortality, length of stay (LOS), and cost.2-5 The prevalence of CDI in hospitalized patients has increased dramatically from the mid-1990s to the mid-2000s to almost 9 cases per 1000 discharges; however, the CDI rate since 2007 appears to have plateaued.6,7 Antibiotic use has historically been the most important risk factor for acquiring CDI; however, use of acid-suppressing agents, chemotherapy, chronic comorbidities, and healthcare exposure all also increase the risk of CDI.7-10 The elderly (> 65 years of age) are particularly at risk for developing CDI and having worse clinical outcomes with CDI.6,7
Patients with septic shock (SS) often have multiple CDI risk factors (in particular, extensive antibiotic exposure) and thus, represent a population at a particularly high risk for acquiring a CDI during hospitalization. However, little data are available on the prevalence of CDI acquired in patients hospitalized with SS. We sought to determine the national-level temporal trends in the prevalence of CDI in patients with SS and the impact of CDI complicating SS on clinical outcomes between 2007 and 2013.
METHODS
Data Source
We used the National Inpatient Sample (NIS) and Nationwide Readmissions Database (NRD) for this study. The NIS is a database developed by the Agency of Healthcare Research and Quality for the Healthcare Cost and Utilization Project (HCUP).11 It is the largest all-payer inpatient database in the United States and has been used by researchers and policy makers to analyze national trends in outcomes and healthcare utilization. The NIS database now approximates a 20% stratified sample of all discharges from all participating US hospitals. Sampling weights are provided by the manufacturer and can be used to produce national-level estimates. Following the redesign of the NIS in 2012, new sampling weights were provided for trend analysis for the years prior to 2012 to account for the new design. Every hospitalization is deidentified and converted into one unique entry that provides information on demographics, hospital characteristics, 1 primary and up to 24 secondary discharge diagnoses, comorbidities, LOS, in-hospital mortality, and procedures performed during stay. The discharge diagnoses are provided in the form of the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) codes.
The NRD is a database developed for HCUP that contains about 35 million discharges each year and supports readmission data analyses. In 2013, the NRD contained data from 21 geographically diverse states, accounting for 49.1% of all US hospitalizations. Diagnosis, comorbidities, and outcomes are presented in a similar manner to NIS.
Study Design
This was a retrospective cohort study. Data from the NIS between 2007 and 2013 were used for the analysis. Demographic data obtained included age, gender, race, Charlson-Deyo Comorbidity Index,12 hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Cases with information missing on key demographic variables (age, gender, and race) were excluded. Only adults (>18 years of age) were included for the analysis.
SS was identified by either (1) ICD-9-CM diagnosis code for SS (785.52) or (2) presence of vasopressor use (00.17) along with ICD-9-CM codes of sepsis, severe sepsis, septicemia, bacteremia, or fungemia. This approach is consistent with what has been utilized in other studies to identify cases of sepsis or SS from administrative databases.13-15 The appendix provides a complete list of ICD-9-CM codes used in the study. CDI was identified by ICD-9-CM code 008.45 among the secondary diagnosis. This code has been shown to have good accuracy for identifying CDI using administrative data.16 To minimize the inclusion of cases in which a CDI was present at admission, hospitalizations with a primary diagnosis of CDI were not included as cases of CDI complicating SS.
We used NRD 2013 for estimating the effect of CDI on 30-day readmission after initial hospitalizations with SS. We used the criteria for index admissions and 30-day readmissions as defined by the Centers for Medicare and Medicaid Services. We excluded patients who died during their index admission, patients with index discharges in December due to a lack of sufficient time to capture 30-day readmissions, and patients with missing information on key variables. We also excluded patients who were not a resident of the state of index hospitalization since readmission across state boundaries could not be identified in NRD. Manufacturer provided sampling weights were used to produce national level estimates. The cases of SS and CDI were identified by ICD-9-CM codes using the methodology described above.
Outcomes
Our primary outcome of interest was the total and yearly prevalence of CDI in patients with SS from 2007 to 2013. The secondary outcomes were mortality, LOS, and 30-day readmissions in patients with SS with and without CDI.
Statistical Analysis
Weighted data from NIS were used for all analyses. Demographics, hospital characteristics, and outcomes of all patients with SS were obtained. The prevalence of CDI was calculated for each calendar year. The temporal trends of outcomes (LOS and in-hospital mortality) of patients were plotted for patients with SS with and without CDI. A χ2 test of trend for proportions was used with the Cochran-Armitage test to calculate statistical significance of changes in prevalence. To test for statistical significance of the temporal trends of LOS, a univariate linear regression was used, with calendar year as a covariate. Independent samples t test, a Mann-Whitney U test, and a χ2 test were used to determine statistical significance of parameters between the group with CDI and the group without CDI.
Prolonged LOS was defined either as a LOS > 75th or > 90th percentile of LOS among all patients with SS. To identify if CDI was associated with a prolonged LOS after adjusting for patient and hospital characteristics, a multivariate logistic regression analysis was used. Variables included in the regression model were age, gender, race, Charlson-Deyo Comorbidity Index, hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Data on cases were available for all the above covariates except hospital characteristics, such as teaching status, location, and bed size (these were missing for 0.7% of hospitals).
Stata 13.1.0 (Stata Corp, College Station, TX) and SPSS 23.0 (SPSS Inc., Chicago, IL) were used to perform statistical analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Demographics
A total of 2,031,739 hospitalizations of adults with SS were identified between 2007 and 2013. CDI was present in 166,432 (8.2%) of these patients. Demographic data are displayed in Table 1. CDI was more commonly observed in elderly patients (> 65 years) with SS; 9.3% among the elderly versus 6.6% among individuals < 65 years; P < 0.001. The prevalence of CDI was greater in urban than in rural hospitals (8.4% vs 5.4%; P < 0.001) and greater in teaching than in nonteaching hospitals (8.7% vs 7.7%; P < 0.001). The prevalence of CDI in SS remained stable between 2007 and 2013 (Table 2).
Mortality
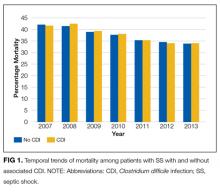
In the overall study cohort, the in-hospital mortality for SS was 37%. The in-hospital mortality rate of patients with SS complicated by a CDI was comparable to the mortality rate of patients without a CDI (37.1% vs 37.0%; P = 0.48). The mortality of patients with SS, with or without CDI, progressively decreased from 2007 to 2013 (P value for trend < 0.001 for each group; Figure 1).
Length of Stay
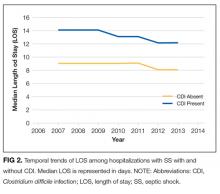
The median LOS for all patients with SS was 9 days. Patients with CDI had a longer median LOS than did those without CDI (13 vs 9 days; P < 0.001). Between 2007 and 2013, the median LOS of CDI group decreased from 14 to 12 days (P < 0.001) while that of non-CDI group decreased from 9 to 8 days (P < 0.001; Figure 2). We also examined LOS among subgroups who were discharged alive and those who died during hospitalization. For patients who were discharged alive, the LOS with and without CDI was 15 days versus 10 days, respectively (P < 0.001). For patients who died during hospitalization, LOS with and without CDI was 10 days versus 6 days, respectively (P < 0.001).
The 75th percentile of LOS of the total SS cohort was 17 days. An LOS > 17 days was observed in 36.9% of SS patients with CDI versus 22.7% without CDI (P < 0.001). After adjusting for patient and provider level variables, the odds of a LOS > 17 days were significantly greater for SS patients with CDI (odds ratio [OR] 2.11; 95% confidence interval [CI], 2.06-2.15; P < 0.001).
The 90th percentile of LOS of the total SS cohort was 29 days. An LOS > 29 days was observed in 17.5% of SS patients with a CDI versus 9.1% without a CDI (P < 0.001). After adjustment for patient and provider level variables, the odds of a LOS > 29 days were significantly greater for SS patients with a CDI (OR 2.25; 95% CI, 2.22-2.28; P < 0.001).
Hospital Readmission
In 2013, patients with SS and CDI had a higher rate of 30-day readmission as compared to patients with SS without CDI (9.8% vs 7.4% respectively; P < 0.001). The multivariate adjusted OR for 30-day readmission for patients with SS and a CDI was 1.26 (95% CI, 1.22-1.31; P < 0.001).
Additional Analyses
Lastly, we performed an additional analysis to confirm our hypothesis that a CDI by itself is rarely a cause of SS, and that CDI as the principal diagnosis would constitute an extremely low number of patients with SS in an administrative dataset. In NIS 2013, there were 105,750 cases with CDI as the primary diagnosis. A total of 4470 (4.2%) had a secondary diagnosis of sepsis and only 930 (0.9%) cases had a secondary diagnosis of SS.
DISCUSSION
This is the first study to report on the prevalence and outcome of CDI complicating SS. By using a large nationally representative sample, we found CDI was very prevalent among individuals hospitalized with SS and, at a level in excess of that seen in other populations. Of interest, we did not observe an increase in mortality of SS when complicated by CDI. On the other hand, patients with SS complicated by CDI were more much likely to have a prolonged hospital LOS and a higher risk of 30-day hospital readmission.
The prevalence of CDI exploded between the mid-1990s and mid-2000s, including community, hospital, and intensive care unit (ICU)–related disease.6,7,17-20 Patients with SS often have multiple risk factors associated with CDI and thus represent a high-risk population for developing CDI.7 Our findings are consistent with the suggestion that individuals with SS are at a higher risk of developing CDI. Compared to the rate of CDI in all hospitalized patients, our data suggest an almost 10-fold increase in CDI rate for patients with SS.6 Patients with SS and CDI may account for as much as 10% of total CDIs.6,7 As has been reported for CDI in general, we observed that CDI complicating SS was more common in those > 65 years of age.4,21 The prevalence of CDI we observed in patients with SS was also higher than has been reported in ICU patients in general (1%), and higher than reported for patients requiring mechanical ventilation (6.6%), including prolonged mechanical ventilation (5.3%); further supporting the conclusion that patients with SS are a particularly high-risk group for acquiring CDI, even compared with other ICU patients.20,22,23 Similarly, the rate of CDI among SS was 8 times higher than that of recently reported hospital-onset CDI among patients with sepsis in general (incidence 1.08%).24 We have no data regarding why patients with SS have a higher rate of CDI; however, the intensity and duration of antibiotic treatment of these patients may certainly play a role.25 It has recently been reported that CDI in itself can be a precursor leading to intestinal dysbiosis that can increase the risk of subsequent sepsis. Similarly, patients with SS may have higher prevalence of dysbiosis that, in turn, might predispose them to CDI at a higher rate than other individuals.
Following the increase in CDIs in the mid-1990s and the mid-2000s, since 2007 the overall prevalence of CDIs has been stable, albeit at the higher rate. More recently, the Centers for Disease Control and Prevention (CDC) has reported a decrease in hospital onset CDI after 2011.26
The finding that CDI in SS patients was not associated with an increase in mortality is consistent with other reports of CDI in ICU patients in general as well as higher-risk ICU populations such as patients requiring mechanical ventilation, including those on long-term mechanical ventilator support.17,18,20,22,23 Why the mortality of ICU patients with CDI is not increased is not completely clear. It has been suggested that this may be related to early recognition and treatment of CDI developing in the ICU.22 Along these lines, it has been previously observed that for patients with CDI on mechanical ventilation, patients who were transferred to the ICU from the ward had worse clinical outcomes compared to patients directly admitted to the ICU, likely due to delayed recognition and treatment in the former.22 Similarly, ICU patients in whom CDI was identified prior to ICU admission had more severe CDI, and mortality that was directly related to CDI was only observed in patients who had CDI identified pre-ICU transfer.18 The increase in mortality observed in patients with sepsis in general with CDI may reflect similar factors.24 We observed a trend of decreasing mortality in SS patients with or without CDI during 2007 to 2013 consistent to what has been generally reported in SS.13,14
The increase in LOS observed in SS patients with CDI is also consistent with what has been observed in other ICU populations, as well as in patients with sepsis in general.17,22-24 Of note, in addition to the increase in median LOS, we found a significant increase in the number of patients with a prolonged LOS associated with having SS with CDI. It is important to note that development of CDI during hospitalization is affected by pre-CDI hospital LOS, so prolonged LOS may not be solely attributable to CDI. The interaction between LOS and CDI remains complex in which higher LOS might be associated with higher incidence of CDI occurrence, and once established, CDI might be associated with changes in LOS for the remaining hospitalization.
Hospitalized patients with CDI have an overall higher resource utilization than those without CDI.27 A recent review has estimated the overall attributable cost of CDI to be $6.3 billion; the attributable cost per case of hospital acquired CDI being 1.5 times the cost of community-acquired CDI.5 We did not look at cost directly. However, in the high-CDI risk ICU population requiring prolonged mechanical ventilation, those with CDI had a substantial increase in total costs.23 Given the substantial increase in LOS associated with CDI complicating SS, there would likely be a significant increase in hospital costs related to providing care for these patients. Further adding to the potential burden of CDI is our finding that CDI and SS was associated with an increase in 30-day hospital readmission rate. This is consistent with a recent report that ICU patients with CDI who are discharged from the hospital have a 25% 30-day hospital readmission rate.28 However, we do not have data either as to the reason for hospital readmission or whether the initial CDI or CDI recurrence played a role. This suggests that, in addition to intervention directed toward preventing CDI, efforts should be directed towards identifying factors that can be modified in CDI patients prior to or after hospital discharge.
This study has several limitations. Using an administrative database (such as NIS) has an inherent limitation of coding errors and reporting bias can lead to misclassification of cohort definition (SS) and outcome (CDI). To minimize bias due to coding errors, we used previously validated ICD-9-CM codes and approach to identify individuals with SS and CDI.13-15 Although the SS population was identified with ICD-9-CM codes using an administrative database, the in-hospital mortality for our septic population was similar to previously reported mortality of SS, suggesting the population selected was appropriate.13 SS due to CDI could not be identified; however, CDI by itself causing SS is rare, as described in recent literature.29,30 An important potential bias that needs to be acknowledged is the immortal time bias. The occurrence of CDI in itself can be influenced by pre-CDI hospital LOS. Patients who were extremely sick could have died early in their hospital course before they could acquire CDI, which would influence the mortality difference between the group with CDI and group without CDI. Furthermore, we did not have information on either the treatment of CDI or SS or any measures of severity of illness, which could lead to residual confounding despite adjusting for multiple variables. In terms of readmission data, it was necessary to exclude nonresidents of a state for the 30-day readmission analysis, as readmissions could not be tracked across state boundaries by using the NRD. This might have resulted in an underrepresentation of the readmission burden. Lastly, it was not possible to identify mortality after hospital discharge as the NIS provides only in-hospital mortality.
In conclusion, CDI is more prevalent in SS than are other ICU populations or the hospital population in general, and CDI complicating SS is associated with significant increase in LOS and risk of 30-day hospital readmission. How much of the increase in resource utilization and cost are in fact attributable to CDI in this population remains to be studied. Our finding of high prevalence of CDI in the SS population further emphasizes the importance of maintaining and furthering approaches to reduce incidence of hospital acquired CDI. While reducing unnecessary antibiotics is important, a multipronged approach that includes education and infection control interventions has also been shown to reduce the incidence of CDI in the ICU.31 Given the economic burden of CDI, implementing these strategies to reduce CDI is warranted. Similarly, the risk of 30-day hospital readmission with CDI highlights the importance of identifying the factors that contribute to hospital readmission prior to initial hospital discharge. Programs to reduce CDI will not only improve outcomes directly attributable to CDI but also decrease the reservoir of CDI. Finally, to the extent that CDI can be reduced in the ICU, the utilization of ICU resources will be more effective.
Disclosure
No conflicts of interest or financial disclosures to report. Author Contributions: KC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. KC, AG, AC, KK, and HC contributed to study design, data analysis, interpretation, and the writing of the manuscript. Guarantor statement: Kshitij Chatterjee takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis.
1. Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis. 2012;55(7):982-989. Doi: 10.1093/cid/cis551. PubMed
2. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3):346-353. Doi: 10.1086/338260. PubMed
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88-S92. Doi: 10.1093/cid/cis335. PubMed
4. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. Doi: 10.1056/NEJMoa1408913. PubMed
5. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. Doi: 10.1186/s12879-016-1786-6. PubMed
6. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70. Doi: 10.1093/cid/cis319. PubMed
7. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-475. Doi: 10.1177/0897190013499521. PubMed
8. Dial S., Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995. Doi: 10.1001/jama.294.23.2989. PubMed
9. Aseeri M., Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313. Doi: 10.1111/j.1572-0241.2008.01975.x. PubMed
10. Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect. 2008;70(1):1-6. Doi: 10.1016/j.jhin.2008.04.023. PubMed
11. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on April 23, 2016.
12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
13. Goto T, Yoshida K, Tsugawa Y, Filbin MR, Camargo CA, Hasegawa K. Mortality trends in U.S. adults with septic shock, 2005-2011: a serial cross-sectional analysis of nationally-representative data. BMC Infect Dis. 2016;16:294. Doi: 10.1186/s12879-016-1620-1. PubMed
14. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. 2011;140(5):1223-1231. Doi: 10.1378/chest.11-0352. PubMed
15. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554. Doi: 10.1056/NEJMoa022139. PubMed
16. Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol Infect. 2007;135(6):1010-1013. Doi: 10.1017/S0950268806007655. PubMed
17. Dodek PM, Norena M, Ayas NT, Romney M, Wong H. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care. 2013;28(4):335-340. Doi: 10.1016/j.jcrc.2012.11.008. PubMed
18. Bouza E, Rodríguez-Créixems M, Alcalá L, et al. Is Clostridium difficile infection an increasingly common severe disease in adult intensive care units? A 10-year experience. J Crit Care. 2015;30(3):543-549. Doi: 10.1016/j.jcrc.2015.02.011. PubMed
19. Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and clinical outcomes of Clostridium difficile infection in the intensive care unit: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3(1):ofv186. Doi: 10.1093/ofid/ofv186. PubMed
20. Zahar JR, Schwebel C, Adrie C, et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care. 2012;16(6):R215. Doi: 10.1186/cc11852. PubMed
21. Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and costs in clostridium difficile infection among the elderly in the United States. Infect Control Hosp Epidemiol. 2016;37(11):1331-1336. Doi: 10.1017/ice.2016.188. PubMed
22. Micek ST, Schramm G, Morrow L, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41(8):1968-1975. Doi: 10.1097/CCM.0b013e31828a40d5. PubMed
23. Zilberberg MD, Nathanson BH, Sadigov S, Higgins TL, Kollef MH, Shorr AF. Epidemiology and outcomes of clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136(3):752-758. Doi: 10.1378/chest.09-0596. PubMed
24. Lagu T, Stefan MS, Haessler S, et al. The impact of hospital-onset Clostridium difficile infection on outcomes of hospitalized patients with sepsis. J Hosp Med. 2014;9(7):411-417. Doi: 10.1002/jhm.2199. PubMed
25. Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192(5):581-588. Doi: 10.1164/rccm.201503-0483OC. PubMed
26. Healthcare-associated Infections (HAI) Progress Report. Centers for Disease Control and Prevention. http://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed on July 29, 2017.
27. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828. Doi: 10.1086/588756. PubMed
28. Zilberberg MD, Shorr AF, Micek ST, et al. Clostridium difficile recurrence is a strong predictor of 30-day rehospitalization among patients in intensive care. Infect Control Hosp Epidemiol. 2015;36(3):273-279. Doi: 10.1017/ice.2014.47. PubMed
29. Loftus KV, Wilson PM. A curiously rare case of septic shock from Clostridium difficile colitis. Pediatr Emerg Care. 2015. [Epub ahead of print]. Doi: 10.1097/PEC.0000000000000496. PubMed
30. Bermejo C, Maseda E, Salgado P, Gabilondo G., Gilsanz F. Septic shock due to a community acquired Clostridium difficile infection. A case study and a review of the literature. Rev Esp Anestesiol Reanimvol. 2014;61(4):219-222. PubMed
31. You E, Song H, Cho J, Lee J. Reduction in the incidence of hospital-acquired Clostridium difficile infection through infection control interventions other than the restriction of antimicrobial use. Int J Infect Dis. 2014;22:9-10. 2014. PubMed
Clostridium difficile infection (CDI) is the most common infectious cause of healthcare-associated diarrhea.1 Development of a CDI during hospitalization is associated with increases in morbidity, mortality, length of stay (LOS), and cost.2-5 The prevalence of CDI in hospitalized patients has increased dramatically from the mid-1990s to the mid-2000s to almost 9 cases per 1000 discharges; however, the CDI rate since 2007 appears to have plateaued.6,7 Antibiotic use has historically been the most important risk factor for acquiring CDI; however, use of acid-suppressing agents, chemotherapy, chronic comorbidities, and healthcare exposure all also increase the risk of CDI.7-10 The elderly (> 65 years of age) are particularly at risk for developing CDI and having worse clinical outcomes with CDI.6,7
Patients with septic shock (SS) often have multiple CDI risk factors (in particular, extensive antibiotic exposure) and thus, represent a population at a particularly high risk for acquiring a CDI during hospitalization. However, little data are available on the prevalence of CDI acquired in patients hospitalized with SS. We sought to determine the national-level temporal trends in the prevalence of CDI in patients with SS and the impact of CDI complicating SS on clinical outcomes between 2007 and 2013.
METHODS
Data Source
We used the National Inpatient Sample (NIS) and Nationwide Readmissions Database (NRD) for this study. The NIS is a database developed by the Agency of Healthcare Research and Quality for the Healthcare Cost and Utilization Project (HCUP).11 It is the largest all-payer inpatient database in the United States and has been used by researchers and policy makers to analyze national trends in outcomes and healthcare utilization. The NIS database now approximates a 20% stratified sample of all discharges from all participating US hospitals. Sampling weights are provided by the manufacturer and can be used to produce national-level estimates. Following the redesign of the NIS in 2012, new sampling weights were provided for trend analysis for the years prior to 2012 to account for the new design. Every hospitalization is deidentified and converted into one unique entry that provides information on demographics, hospital characteristics, 1 primary and up to 24 secondary discharge diagnoses, comorbidities, LOS, in-hospital mortality, and procedures performed during stay. The discharge diagnoses are provided in the form of the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) codes.
The NRD is a database developed for HCUP that contains about 35 million discharges each year and supports readmission data analyses. In 2013, the NRD contained data from 21 geographically diverse states, accounting for 49.1% of all US hospitalizations. Diagnosis, comorbidities, and outcomes are presented in a similar manner to NIS.
Study Design
This was a retrospective cohort study. Data from the NIS between 2007 and 2013 were used for the analysis. Demographic data obtained included age, gender, race, Charlson-Deyo Comorbidity Index,12 hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Cases with information missing on key demographic variables (age, gender, and race) were excluded. Only adults (>18 years of age) were included for the analysis.
SS was identified by either (1) ICD-9-CM diagnosis code for SS (785.52) or (2) presence of vasopressor use (00.17) along with ICD-9-CM codes of sepsis, severe sepsis, septicemia, bacteremia, or fungemia. This approach is consistent with what has been utilized in other studies to identify cases of sepsis or SS from administrative databases.13-15 The appendix provides a complete list of ICD-9-CM codes used in the study. CDI was identified by ICD-9-CM code 008.45 among the secondary diagnosis. This code has been shown to have good accuracy for identifying CDI using administrative data.16 To minimize the inclusion of cases in which a CDI was present at admission, hospitalizations with a primary diagnosis of CDI were not included as cases of CDI complicating SS.
We used NRD 2013 for estimating the effect of CDI on 30-day readmission after initial hospitalizations with SS. We used the criteria for index admissions and 30-day readmissions as defined by the Centers for Medicare and Medicaid Services. We excluded patients who died during their index admission, patients with index discharges in December due to a lack of sufficient time to capture 30-day readmissions, and patients with missing information on key variables. We also excluded patients who were not a resident of the state of index hospitalization since readmission across state boundaries could not be identified in NRD. Manufacturer provided sampling weights were used to produce national level estimates. The cases of SS and CDI were identified by ICD-9-CM codes using the methodology described above.
Outcomes
Our primary outcome of interest was the total and yearly prevalence of CDI in patients with SS from 2007 to 2013. The secondary outcomes were mortality, LOS, and 30-day readmissions in patients with SS with and without CDI.
Statistical Analysis
Weighted data from NIS were used for all analyses. Demographics, hospital characteristics, and outcomes of all patients with SS were obtained. The prevalence of CDI was calculated for each calendar year. The temporal trends of outcomes (LOS and in-hospital mortality) of patients were plotted for patients with SS with and without CDI. A χ2 test of trend for proportions was used with the Cochran-Armitage test to calculate statistical significance of changes in prevalence. To test for statistical significance of the temporal trends of LOS, a univariate linear regression was used, with calendar year as a covariate. Independent samples t test, a Mann-Whitney U test, and a χ2 test were used to determine statistical significance of parameters between the group with CDI and the group without CDI.
Prolonged LOS was defined either as a LOS > 75th or > 90th percentile of LOS among all patients with SS. To identify if CDI was associated with a prolonged LOS after adjusting for patient and hospital characteristics, a multivariate logistic regression analysis was used. Variables included in the regression model were age, gender, race, Charlson-Deyo Comorbidity Index, hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Data on cases were available for all the above covariates except hospital characteristics, such as teaching status, location, and bed size (these were missing for 0.7% of hospitals).
Stata 13.1.0 (Stata Corp, College Station, TX) and SPSS 23.0 (SPSS Inc., Chicago, IL) were used to perform statistical analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Demographics
A total of 2,031,739 hospitalizations of adults with SS were identified between 2007 and 2013. CDI was present in 166,432 (8.2%) of these patients. Demographic data are displayed in Table 1. CDI was more commonly observed in elderly patients (> 65 years) with SS; 9.3% among the elderly versus 6.6% among individuals < 65 years; P < 0.001. The prevalence of CDI was greater in urban than in rural hospitals (8.4% vs 5.4%; P < 0.001) and greater in teaching than in nonteaching hospitals (8.7% vs 7.7%; P < 0.001). The prevalence of CDI in SS remained stable between 2007 and 2013 (Table 2).
Mortality
In the overall study cohort, the in-hospital mortality for SS was 37%. The in-hospital mortality rate of patients with SS complicated by a CDI was comparable to the mortality rate of patients without a CDI (37.1% vs 37.0%; P = 0.48). The mortality of patients with SS, with or without CDI, progressively decreased from 2007 to 2013 (P value for trend < 0.001 for each group; Figure 1).
Length of Stay
The median LOS for all patients with SS was 9 days. Patients with CDI had a longer median LOS than did those without CDI (13 vs 9 days; P < 0.001). Between 2007 and 2013, the median LOS of CDI group decreased from 14 to 12 days (P < 0.001) while that of non-CDI group decreased from 9 to 8 days (P < 0.001; Figure 2). We also examined LOS among subgroups who were discharged alive and those who died during hospitalization. For patients who were discharged alive, the LOS with and without CDI was 15 days versus 10 days, respectively (P < 0.001). For patients who died during hospitalization, LOS with and without CDI was 10 days versus 6 days, respectively (P < 0.001).
The 75th percentile of LOS of the total SS cohort was 17 days. An LOS > 17 days was observed in 36.9% of SS patients with CDI versus 22.7% without CDI (P < 0.001). After adjusting for patient and provider level variables, the odds of a LOS > 17 days were significantly greater for SS patients with CDI (odds ratio [OR] 2.11; 95% confidence interval [CI], 2.06-2.15; P < 0.001).
The 90th percentile of LOS of the total SS cohort was 29 days. An LOS > 29 days was observed in 17.5% of SS patients with a CDI versus 9.1% without a CDI (P < 0.001). After adjustment for patient and provider level variables, the odds of a LOS > 29 days were significantly greater for SS patients with a CDI (OR 2.25; 95% CI, 2.22-2.28; P < 0.001).
Hospital Readmission
In 2013, patients with SS and CDI had a higher rate of 30-day readmission as compared to patients with SS without CDI (9.8% vs 7.4% respectively; P < 0.001). The multivariate adjusted OR for 30-day readmission for patients with SS and a CDI was 1.26 (95% CI, 1.22-1.31; P < 0.001).
Additional Analyses
Lastly, we performed an additional analysis to confirm our hypothesis that a CDI by itself is rarely a cause of SS, and that CDI as the principal diagnosis would constitute an extremely low number of patients with SS in an administrative dataset. In NIS 2013, there were 105,750 cases with CDI as the primary diagnosis. A total of 4470 (4.2%) had a secondary diagnosis of sepsis and only 930 (0.9%) cases had a secondary diagnosis of SS.
DISCUSSION
This is the first study to report on the prevalence and outcome of CDI complicating SS. By using a large nationally representative sample, we found CDI was very prevalent among individuals hospitalized with SS and, at a level in excess of that seen in other populations. Of interest, we did not observe an increase in mortality of SS when complicated by CDI. On the other hand, patients with SS complicated by CDI were more much likely to have a prolonged hospital LOS and a higher risk of 30-day hospital readmission.
The prevalence of CDI exploded between the mid-1990s and mid-2000s, including community, hospital, and intensive care unit (ICU)–related disease.6,7,17-20 Patients with SS often have multiple risk factors associated with CDI and thus represent a high-risk population for developing CDI.7 Our findings are consistent with the suggestion that individuals with SS are at a higher risk of developing CDI. Compared to the rate of CDI in all hospitalized patients, our data suggest an almost 10-fold increase in CDI rate for patients with SS.6 Patients with SS and CDI may account for as much as 10% of total CDIs.6,7 As has been reported for CDI in general, we observed that CDI complicating SS was more common in those > 65 years of age.4,21 The prevalence of CDI we observed in patients with SS was also higher than has been reported in ICU patients in general (1%), and higher than reported for patients requiring mechanical ventilation (6.6%), including prolonged mechanical ventilation (5.3%); further supporting the conclusion that patients with SS are a particularly high-risk group for acquiring CDI, even compared with other ICU patients.20,22,23 Similarly, the rate of CDI among SS was 8 times higher than that of recently reported hospital-onset CDI among patients with sepsis in general (incidence 1.08%).24 We have no data regarding why patients with SS have a higher rate of CDI; however, the intensity and duration of antibiotic treatment of these patients may certainly play a role.25 It has recently been reported that CDI in itself can be a precursor leading to intestinal dysbiosis that can increase the risk of subsequent sepsis. Similarly, patients with SS may have higher prevalence of dysbiosis that, in turn, might predispose them to CDI at a higher rate than other individuals.
Following the increase in CDIs in the mid-1990s and the mid-2000s, since 2007 the overall prevalence of CDIs has been stable, albeit at the higher rate. More recently, the Centers for Disease Control and Prevention (CDC) has reported a decrease in hospital onset CDI after 2011.26
The finding that CDI in SS patients was not associated with an increase in mortality is consistent with other reports of CDI in ICU patients in general as well as higher-risk ICU populations such as patients requiring mechanical ventilation, including those on long-term mechanical ventilator support.17,18,20,22,23 Why the mortality of ICU patients with CDI is not increased is not completely clear. It has been suggested that this may be related to early recognition and treatment of CDI developing in the ICU.22 Along these lines, it has been previously observed that for patients with CDI on mechanical ventilation, patients who were transferred to the ICU from the ward had worse clinical outcomes compared to patients directly admitted to the ICU, likely due to delayed recognition and treatment in the former.22 Similarly, ICU patients in whom CDI was identified prior to ICU admission had more severe CDI, and mortality that was directly related to CDI was only observed in patients who had CDI identified pre-ICU transfer.18 The increase in mortality observed in patients with sepsis in general with CDI may reflect similar factors.24 We observed a trend of decreasing mortality in SS patients with or without CDI during 2007 to 2013 consistent to what has been generally reported in SS.13,14
The increase in LOS observed in SS patients with CDI is also consistent with what has been observed in other ICU populations, as well as in patients with sepsis in general.17,22-24 Of note, in addition to the increase in median LOS, we found a significant increase in the number of patients with a prolonged LOS associated with having SS with CDI. It is important to note that development of CDI during hospitalization is affected by pre-CDI hospital LOS, so prolonged LOS may not be solely attributable to CDI. The interaction between LOS and CDI remains complex in which higher LOS might be associated with higher incidence of CDI occurrence, and once established, CDI might be associated with changes in LOS for the remaining hospitalization.
Hospitalized patients with CDI have an overall higher resource utilization than those without CDI.27 A recent review has estimated the overall attributable cost of CDI to be $6.3 billion; the attributable cost per case of hospital acquired CDI being 1.5 times the cost of community-acquired CDI.5 We did not look at cost directly. However, in the high-CDI risk ICU population requiring prolonged mechanical ventilation, those with CDI had a substantial increase in total costs.23 Given the substantial increase in LOS associated with CDI complicating SS, there would likely be a significant increase in hospital costs related to providing care for these patients. Further adding to the potential burden of CDI is our finding that CDI and SS was associated with an increase in 30-day hospital readmission rate. This is consistent with a recent report that ICU patients with CDI who are discharged from the hospital have a 25% 30-day hospital readmission rate.28 However, we do not have data either as to the reason for hospital readmission or whether the initial CDI or CDI recurrence played a role. This suggests that, in addition to intervention directed toward preventing CDI, efforts should be directed towards identifying factors that can be modified in CDI patients prior to or after hospital discharge.
This study has several limitations. Using an administrative database (such as NIS) has an inherent limitation of coding errors and reporting bias can lead to misclassification of cohort definition (SS) and outcome (CDI). To minimize bias due to coding errors, we used previously validated ICD-9-CM codes and approach to identify individuals with SS and CDI.13-15 Although the SS population was identified with ICD-9-CM codes using an administrative database, the in-hospital mortality for our septic population was similar to previously reported mortality of SS, suggesting the population selected was appropriate.13 SS due to CDI could not be identified; however, CDI by itself causing SS is rare, as described in recent literature.29,30 An important potential bias that needs to be acknowledged is the immortal time bias. The occurrence of CDI in itself can be influenced by pre-CDI hospital LOS. Patients who were extremely sick could have died early in their hospital course before they could acquire CDI, which would influence the mortality difference between the group with CDI and group without CDI. Furthermore, we did not have information on either the treatment of CDI or SS or any measures of severity of illness, which could lead to residual confounding despite adjusting for multiple variables. In terms of readmission data, it was necessary to exclude nonresidents of a state for the 30-day readmission analysis, as readmissions could not be tracked across state boundaries by using the NRD. This might have resulted in an underrepresentation of the readmission burden. Lastly, it was not possible to identify mortality after hospital discharge as the NIS provides only in-hospital mortality.
In conclusion, CDI is more prevalent in SS than are other ICU populations or the hospital population in general, and CDI complicating SS is associated with significant increase in LOS and risk of 30-day hospital readmission. How much of the increase in resource utilization and cost are in fact attributable to CDI in this population remains to be studied. Our finding of high prevalence of CDI in the SS population further emphasizes the importance of maintaining and furthering approaches to reduce incidence of hospital acquired CDI. While reducing unnecessary antibiotics is important, a multipronged approach that includes education and infection control interventions has also been shown to reduce the incidence of CDI in the ICU.31 Given the economic burden of CDI, implementing these strategies to reduce CDI is warranted. Similarly, the risk of 30-day hospital readmission with CDI highlights the importance of identifying the factors that contribute to hospital readmission prior to initial hospital discharge. Programs to reduce CDI will not only improve outcomes directly attributable to CDI but also decrease the reservoir of CDI. Finally, to the extent that CDI can be reduced in the ICU, the utilization of ICU resources will be more effective.
Disclosure
No conflicts of interest or financial disclosures to report. Author Contributions: KC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. KC, AG, AC, KK, and HC contributed to study design, data analysis, interpretation, and the writing of the manuscript. Guarantor statement: Kshitij Chatterjee takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis.
Clostridium difficile infection (CDI) is the most common infectious cause of healthcare-associated diarrhea.1 Development of a CDI during hospitalization is associated with increases in morbidity, mortality, length of stay (LOS), and cost.2-5 The prevalence of CDI in hospitalized patients has increased dramatically from the mid-1990s to the mid-2000s to almost 9 cases per 1000 discharges; however, the CDI rate since 2007 appears to have plateaued.6,7 Antibiotic use has historically been the most important risk factor for acquiring CDI; however, use of acid-suppressing agents, chemotherapy, chronic comorbidities, and healthcare exposure all also increase the risk of CDI.7-10 The elderly (> 65 years of age) are particularly at risk for developing CDI and having worse clinical outcomes with CDI.6,7
Patients with septic shock (SS) often have multiple CDI risk factors (in particular, extensive antibiotic exposure) and thus, represent a population at a particularly high risk for acquiring a CDI during hospitalization. However, little data are available on the prevalence of CDI acquired in patients hospitalized with SS. We sought to determine the national-level temporal trends in the prevalence of CDI in patients with SS and the impact of CDI complicating SS on clinical outcomes between 2007 and 2013.
METHODS
Data Source
We used the National Inpatient Sample (NIS) and Nationwide Readmissions Database (NRD) for this study. The NIS is a database developed by the Agency of Healthcare Research and Quality for the Healthcare Cost and Utilization Project (HCUP).11 It is the largest all-payer inpatient database in the United States and has been used by researchers and policy makers to analyze national trends in outcomes and healthcare utilization. The NIS database now approximates a 20% stratified sample of all discharges from all participating US hospitals. Sampling weights are provided by the manufacturer and can be used to produce national-level estimates. Following the redesign of the NIS in 2012, new sampling weights were provided for trend analysis for the years prior to 2012 to account for the new design. Every hospitalization is deidentified and converted into one unique entry that provides information on demographics, hospital characteristics, 1 primary and up to 24 secondary discharge diagnoses, comorbidities, LOS, in-hospital mortality, and procedures performed during stay. The discharge diagnoses are provided in the form of the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) codes.
The NRD is a database developed for HCUP that contains about 35 million discharges each year and supports readmission data analyses. In 2013, the NRD contained data from 21 geographically diverse states, accounting for 49.1% of all US hospitalizations. Diagnosis, comorbidities, and outcomes are presented in a similar manner to NIS.
Study Design
This was a retrospective cohort study. Data from the NIS between 2007 and 2013 were used for the analysis. Demographic data obtained included age, gender, race, Charlson-Deyo Comorbidity Index,12 hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Cases with information missing on key demographic variables (age, gender, and race) were excluded. Only adults (>18 years of age) were included for the analysis.
SS was identified by either (1) ICD-9-CM diagnosis code for SS (785.52) or (2) presence of vasopressor use (00.17) along with ICD-9-CM codes of sepsis, severe sepsis, septicemia, bacteremia, or fungemia. This approach is consistent with what has been utilized in other studies to identify cases of sepsis or SS from administrative databases.13-15 The appendix provides a complete list of ICD-9-CM codes used in the study. CDI was identified by ICD-9-CM code 008.45 among the secondary diagnosis. This code has been shown to have good accuracy for identifying CDI using administrative data.16 To minimize the inclusion of cases in which a CDI was present at admission, hospitalizations with a primary diagnosis of CDI were not included as cases of CDI complicating SS.
We used NRD 2013 for estimating the effect of CDI on 30-day readmission after initial hospitalizations with SS. We used the criteria for index admissions and 30-day readmissions as defined by the Centers for Medicare and Medicaid Services. We excluded patients who died during their index admission, patients with index discharges in December due to a lack of sufficient time to capture 30-day readmissions, and patients with missing information on key variables. We also excluded patients who were not a resident of the state of index hospitalization since readmission across state boundaries could not be identified in NRD. Manufacturer provided sampling weights were used to produce national level estimates. The cases of SS and CDI were identified by ICD-9-CM codes using the methodology described above.
Outcomes
Our primary outcome of interest was the total and yearly prevalence of CDI in patients with SS from 2007 to 2013. The secondary outcomes were mortality, LOS, and 30-day readmissions in patients with SS with and without CDI.
Statistical Analysis
Weighted data from NIS were used for all analyses. Demographics, hospital characteristics, and outcomes of all patients with SS were obtained. The prevalence of CDI was calculated for each calendar year. The temporal trends of outcomes (LOS and in-hospital mortality) of patients were plotted for patients with SS with and without CDI. A χ2 test of trend for proportions was used with the Cochran-Armitage test to calculate statistical significance of changes in prevalence. To test for statistical significance of the temporal trends of LOS, a univariate linear regression was used, with calendar year as a covariate. Independent samples t test, a Mann-Whitney U test, and a χ2 test were used to determine statistical significance of parameters between the group with CDI and the group without CDI.
Prolonged LOS was defined either as a LOS > 75th or > 90th percentile of LOS among all patients with SS. To identify if CDI was associated with a prolonged LOS after adjusting for patient and hospital characteristics, a multivariate logistic regression analysis was used. Variables included in the regression model were age, gender, race, Charlson-Deyo Comorbidity Index, hospital characteristics (hospital region, hospital-bed size, urban versus rural location, and teaching status), calendar year, and use of mechanical ventilation. Data on cases were available for all the above covariates except hospital characteristics, such as teaching status, location, and bed size (these were missing for 0.7% of hospitals).
Stata 13.1.0 (Stata Corp, College Station, TX) and SPSS 23.0 (SPSS Inc., Chicago, IL) were used to perform statistical analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Demographics
A total of 2,031,739 hospitalizations of adults with SS were identified between 2007 and 2013. CDI was present in 166,432 (8.2%) of these patients. Demographic data are displayed in Table 1. CDI was more commonly observed in elderly patients (> 65 years) with SS; 9.3% among the elderly versus 6.6% among individuals < 65 years; P < 0.001. The prevalence of CDI was greater in urban than in rural hospitals (8.4% vs 5.4%; P < 0.001) and greater in teaching than in nonteaching hospitals (8.7% vs 7.7%; P < 0.001). The prevalence of CDI in SS remained stable between 2007 and 2013 (Table 2).
Mortality
In the overall study cohort, the in-hospital mortality for SS was 37%. The in-hospital mortality rate of patients with SS complicated by a CDI was comparable to the mortality rate of patients without a CDI (37.1% vs 37.0%; P = 0.48). The mortality of patients with SS, with or without CDI, progressively decreased from 2007 to 2013 (P value for trend < 0.001 for each group; Figure 1).
Length of Stay
The median LOS for all patients with SS was 9 days. Patients with CDI had a longer median LOS than did those without CDI (13 vs 9 days; P < 0.001). Between 2007 and 2013, the median LOS of CDI group decreased from 14 to 12 days (P < 0.001) while that of non-CDI group decreased from 9 to 8 days (P < 0.001; Figure 2). We also examined LOS among subgroups who were discharged alive and those who died during hospitalization. For patients who were discharged alive, the LOS with and without CDI was 15 days versus 10 days, respectively (P < 0.001). For patients who died during hospitalization, LOS with and without CDI was 10 days versus 6 days, respectively (P < 0.001).
The 75th percentile of LOS of the total SS cohort was 17 days. An LOS > 17 days was observed in 36.9% of SS patients with CDI versus 22.7% without CDI (P < 0.001). After adjusting for patient and provider level variables, the odds of a LOS > 17 days were significantly greater for SS patients with CDI (odds ratio [OR] 2.11; 95% confidence interval [CI], 2.06-2.15; P < 0.001).
The 90th percentile of LOS of the total SS cohort was 29 days. An LOS > 29 days was observed in 17.5% of SS patients with a CDI versus 9.1% without a CDI (P < 0.001). After adjustment for patient and provider level variables, the odds of a LOS > 29 days were significantly greater for SS patients with a CDI (OR 2.25; 95% CI, 2.22-2.28; P < 0.001).
Hospital Readmission
In 2013, patients with SS and CDI had a higher rate of 30-day readmission as compared to patients with SS without CDI (9.8% vs 7.4% respectively; P < 0.001). The multivariate adjusted OR for 30-day readmission for patients with SS and a CDI was 1.26 (95% CI, 1.22-1.31; P < 0.001).
Additional Analyses
Lastly, we performed an additional analysis to confirm our hypothesis that a CDI by itself is rarely a cause of SS, and that CDI as the principal diagnosis would constitute an extremely low number of patients with SS in an administrative dataset. In NIS 2013, there were 105,750 cases with CDI as the primary diagnosis. A total of 4470 (4.2%) had a secondary diagnosis of sepsis and only 930 (0.9%) cases had a secondary diagnosis of SS.
DISCUSSION
This is the first study to report on the prevalence and outcome of CDI complicating SS. By using a large nationally representative sample, we found CDI was very prevalent among individuals hospitalized with SS and, at a level in excess of that seen in other populations. Of interest, we did not observe an increase in mortality of SS when complicated by CDI. On the other hand, patients with SS complicated by CDI were more much likely to have a prolonged hospital LOS and a higher risk of 30-day hospital readmission.
The prevalence of CDI exploded between the mid-1990s and mid-2000s, including community, hospital, and intensive care unit (ICU)–related disease.6,7,17-20 Patients with SS often have multiple risk factors associated with CDI and thus represent a high-risk population for developing CDI.7 Our findings are consistent with the suggestion that individuals with SS are at a higher risk of developing CDI. Compared to the rate of CDI in all hospitalized patients, our data suggest an almost 10-fold increase in CDI rate for patients with SS.6 Patients with SS and CDI may account for as much as 10% of total CDIs.6,7 As has been reported for CDI in general, we observed that CDI complicating SS was more common in those > 65 years of age.4,21 The prevalence of CDI we observed in patients with SS was also higher than has been reported in ICU patients in general (1%), and higher than reported for patients requiring mechanical ventilation (6.6%), including prolonged mechanical ventilation (5.3%); further supporting the conclusion that patients with SS are a particularly high-risk group for acquiring CDI, even compared with other ICU patients.20,22,23 Similarly, the rate of CDI among SS was 8 times higher than that of recently reported hospital-onset CDI among patients with sepsis in general (incidence 1.08%).24 We have no data regarding why patients with SS have a higher rate of CDI; however, the intensity and duration of antibiotic treatment of these patients may certainly play a role.25 It has recently been reported that CDI in itself can be a precursor leading to intestinal dysbiosis that can increase the risk of subsequent sepsis. Similarly, patients with SS may have higher prevalence of dysbiosis that, in turn, might predispose them to CDI at a higher rate than other individuals.
Following the increase in CDIs in the mid-1990s and the mid-2000s, since 2007 the overall prevalence of CDIs has been stable, albeit at the higher rate. More recently, the Centers for Disease Control and Prevention (CDC) has reported a decrease in hospital onset CDI after 2011.26
The finding that CDI in SS patients was not associated with an increase in mortality is consistent with other reports of CDI in ICU patients in general as well as higher-risk ICU populations such as patients requiring mechanical ventilation, including those on long-term mechanical ventilator support.17,18,20,22,23 Why the mortality of ICU patients with CDI is not increased is not completely clear. It has been suggested that this may be related to early recognition and treatment of CDI developing in the ICU.22 Along these lines, it has been previously observed that for patients with CDI on mechanical ventilation, patients who were transferred to the ICU from the ward had worse clinical outcomes compared to patients directly admitted to the ICU, likely due to delayed recognition and treatment in the former.22 Similarly, ICU patients in whom CDI was identified prior to ICU admission had more severe CDI, and mortality that was directly related to CDI was only observed in patients who had CDI identified pre-ICU transfer.18 The increase in mortality observed in patients with sepsis in general with CDI may reflect similar factors.24 We observed a trend of decreasing mortality in SS patients with or without CDI during 2007 to 2013 consistent to what has been generally reported in SS.13,14
The increase in LOS observed in SS patients with CDI is also consistent with what has been observed in other ICU populations, as well as in patients with sepsis in general.17,22-24 Of note, in addition to the increase in median LOS, we found a significant increase in the number of patients with a prolonged LOS associated with having SS with CDI. It is important to note that development of CDI during hospitalization is affected by pre-CDI hospital LOS, so prolonged LOS may not be solely attributable to CDI. The interaction between LOS and CDI remains complex in which higher LOS might be associated with higher incidence of CDI occurrence, and once established, CDI might be associated with changes in LOS for the remaining hospitalization.
Hospitalized patients with CDI have an overall higher resource utilization than those without CDI.27 A recent review has estimated the overall attributable cost of CDI to be $6.3 billion; the attributable cost per case of hospital acquired CDI being 1.5 times the cost of community-acquired CDI.5 We did not look at cost directly. However, in the high-CDI risk ICU population requiring prolonged mechanical ventilation, those with CDI had a substantial increase in total costs.23 Given the substantial increase in LOS associated with CDI complicating SS, there would likely be a significant increase in hospital costs related to providing care for these patients. Further adding to the potential burden of CDI is our finding that CDI and SS was associated with an increase in 30-day hospital readmission rate. This is consistent with a recent report that ICU patients with CDI who are discharged from the hospital have a 25% 30-day hospital readmission rate.28 However, we do not have data either as to the reason for hospital readmission or whether the initial CDI or CDI recurrence played a role. This suggests that, in addition to intervention directed toward preventing CDI, efforts should be directed towards identifying factors that can be modified in CDI patients prior to or after hospital discharge.
This study has several limitations. Using an administrative database (such as NIS) has an inherent limitation of coding errors and reporting bias can lead to misclassification of cohort definition (SS) and outcome (CDI). To minimize bias due to coding errors, we used previously validated ICD-9-CM codes and approach to identify individuals with SS and CDI.13-15 Although the SS population was identified with ICD-9-CM codes using an administrative database, the in-hospital mortality for our septic population was similar to previously reported mortality of SS, suggesting the population selected was appropriate.13 SS due to CDI could not be identified; however, CDI by itself causing SS is rare, as described in recent literature.29,30 An important potential bias that needs to be acknowledged is the immortal time bias. The occurrence of CDI in itself can be influenced by pre-CDI hospital LOS. Patients who were extremely sick could have died early in their hospital course before they could acquire CDI, which would influence the mortality difference between the group with CDI and group without CDI. Furthermore, we did not have information on either the treatment of CDI or SS or any measures of severity of illness, which could lead to residual confounding despite adjusting for multiple variables. In terms of readmission data, it was necessary to exclude nonresidents of a state for the 30-day readmission analysis, as readmissions could not be tracked across state boundaries by using the NRD. This might have resulted in an underrepresentation of the readmission burden. Lastly, it was not possible to identify mortality after hospital discharge as the NIS provides only in-hospital mortality.
In conclusion, CDI is more prevalent in SS than are other ICU populations or the hospital population in general, and CDI complicating SS is associated with significant increase in LOS and risk of 30-day hospital readmission. How much of the increase in resource utilization and cost are in fact attributable to CDI in this population remains to be studied. Our finding of high prevalence of CDI in the SS population further emphasizes the importance of maintaining and furthering approaches to reduce incidence of hospital acquired CDI. While reducing unnecessary antibiotics is important, a multipronged approach that includes education and infection control interventions has also been shown to reduce the incidence of CDI in the ICU.31 Given the economic burden of CDI, implementing these strategies to reduce CDI is warranted. Similarly, the risk of 30-day hospital readmission with CDI highlights the importance of identifying the factors that contribute to hospital readmission prior to initial hospital discharge. Programs to reduce CDI will not only improve outcomes directly attributable to CDI but also decrease the reservoir of CDI. Finally, to the extent that CDI can be reduced in the ICU, the utilization of ICU resources will be more effective.
Disclosure
No conflicts of interest or financial disclosures to report. Author Contributions: KC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. KC, AG, AC, KK, and HC contributed to study design, data analysis, interpretation, and the writing of the manuscript. Guarantor statement: Kshitij Chatterjee takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis.
1. Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis. 2012;55(7):982-989. Doi: 10.1093/cid/cis551. PubMed
2. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3):346-353. Doi: 10.1086/338260. PubMed
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88-S92. Doi: 10.1093/cid/cis335. PubMed
4. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. Doi: 10.1056/NEJMoa1408913. PubMed
5. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. Doi: 10.1186/s12879-016-1786-6. PubMed
6. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70. Doi: 10.1093/cid/cis319. PubMed
7. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-475. Doi: 10.1177/0897190013499521. PubMed
8. Dial S., Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995. Doi: 10.1001/jama.294.23.2989. PubMed
9. Aseeri M., Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313. Doi: 10.1111/j.1572-0241.2008.01975.x. PubMed
10. Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect. 2008;70(1):1-6. Doi: 10.1016/j.jhin.2008.04.023. PubMed
11. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on April 23, 2016.
12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
13. Goto T, Yoshida K, Tsugawa Y, Filbin MR, Camargo CA, Hasegawa K. Mortality trends in U.S. adults with septic shock, 2005-2011: a serial cross-sectional analysis of nationally-representative data. BMC Infect Dis. 2016;16:294. Doi: 10.1186/s12879-016-1620-1. PubMed
14. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. 2011;140(5):1223-1231. Doi: 10.1378/chest.11-0352. PubMed
15. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554. Doi: 10.1056/NEJMoa022139. PubMed
16. Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol Infect. 2007;135(6):1010-1013. Doi: 10.1017/S0950268806007655. PubMed
17. Dodek PM, Norena M, Ayas NT, Romney M, Wong H. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care. 2013;28(4):335-340. Doi: 10.1016/j.jcrc.2012.11.008. PubMed
18. Bouza E, Rodríguez-Créixems M, Alcalá L, et al. Is Clostridium difficile infection an increasingly common severe disease in adult intensive care units? A 10-year experience. J Crit Care. 2015;30(3):543-549. Doi: 10.1016/j.jcrc.2015.02.011. PubMed
19. Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and clinical outcomes of Clostridium difficile infection in the intensive care unit: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3(1):ofv186. Doi: 10.1093/ofid/ofv186. PubMed
20. Zahar JR, Schwebel C, Adrie C, et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care. 2012;16(6):R215. Doi: 10.1186/cc11852. PubMed
21. Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and costs in clostridium difficile infection among the elderly in the United States. Infect Control Hosp Epidemiol. 2016;37(11):1331-1336. Doi: 10.1017/ice.2016.188. PubMed
22. Micek ST, Schramm G, Morrow L, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41(8):1968-1975. Doi: 10.1097/CCM.0b013e31828a40d5. PubMed
23. Zilberberg MD, Nathanson BH, Sadigov S, Higgins TL, Kollef MH, Shorr AF. Epidemiology and outcomes of clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136(3):752-758. Doi: 10.1378/chest.09-0596. PubMed
24. Lagu T, Stefan MS, Haessler S, et al. The impact of hospital-onset Clostridium difficile infection on outcomes of hospitalized patients with sepsis. J Hosp Med. 2014;9(7):411-417. Doi: 10.1002/jhm.2199. PubMed
25. Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192(5):581-588. Doi: 10.1164/rccm.201503-0483OC. PubMed
26. Healthcare-associated Infections (HAI) Progress Report. Centers for Disease Control and Prevention. http://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed on July 29, 2017.
27. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828. Doi: 10.1086/588756. PubMed
28. Zilberberg MD, Shorr AF, Micek ST, et al. Clostridium difficile recurrence is a strong predictor of 30-day rehospitalization among patients in intensive care. Infect Control Hosp Epidemiol. 2015;36(3):273-279. Doi: 10.1017/ice.2014.47. PubMed
29. Loftus KV, Wilson PM. A curiously rare case of septic shock from Clostridium difficile colitis. Pediatr Emerg Care. 2015. [Epub ahead of print]. Doi: 10.1097/PEC.0000000000000496. PubMed
30. Bermejo C, Maseda E, Salgado P, Gabilondo G., Gilsanz F. Septic shock due to a community acquired Clostridium difficile infection. A case study and a review of the literature. Rev Esp Anestesiol Reanimvol. 2014;61(4):219-222. PubMed
31. You E, Song H, Cho J, Lee J. Reduction in the incidence of hospital-acquired Clostridium difficile infection through infection control interventions other than the restriction of antimicrobial use. Int J Infect Dis. 2014;22:9-10. 2014. PubMed
1. Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis. 2012;55(7):982-989. Doi: 10.1093/cid/cis551. PubMed
2. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3):346-353. Doi: 10.1086/338260. PubMed
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88-S92. Doi: 10.1093/cid/cis335. PubMed
4. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. Doi: 10.1056/NEJMoa1408913. PubMed
5. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. Doi: 10.1186/s12879-016-1786-6. PubMed
6. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70. Doi: 10.1093/cid/cis319. PubMed
7. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-475. Doi: 10.1177/0897190013499521. PubMed
8. Dial S., Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995. Doi: 10.1001/jama.294.23.2989. PubMed
9. Aseeri M., Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313. Doi: 10.1111/j.1572-0241.2008.01975.x. PubMed
10. Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect. 2008;70(1):1-6. Doi: 10.1016/j.jhin.2008.04.023. PubMed
11. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on April 23, 2016.
12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
13. Goto T, Yoshida K, Tsugawa Y, Filbin MR, Camargo CA, Hasegawa K. Mortality trends in U.S. adults with septic shock, 2005-2011: a serial cross-sectional analysis of nationally-representative data. BMC Infect Dis. 2016;16:294. Doi: 10.1186/s12879-016-1620-1. PubMed
14. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. 2011;140(5):1223-1231. Doi: 10.1378/chest.11-0352. PubMed
15. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554. Doi: 10.1056/NEJMoa022139. PubMed
16. Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol Infect. 2007;135(6):1010-1013. Doi: 10.1017/S0950268806007655. PubMed
17. Dodek PM, Norena M, Ayas NT, Romney M, Wong H. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care. 2013;28(4):335-340. Doi: 10.1016/j.jcrc.2012.11.008. PubMed
18. Bouza E, Rodríguez-Créixems M, Alcalá L, et al. Is Clostridium difficile infection an increasingly common severe disease in adult intensive care units? A 10-year experience. J Crit Care. 2015;30(3):543-549. Doi: 10.1016/j.jcrc.2015.02.011. PubMed
19. Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and clinical outcomes of Clostridium difficile infection in the intensive care unit: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3(1):ofv186. Doi: 10.1093/ofid/ofv186. PubMed
20. Zahar JR, Schwebel C, Adrie C, et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care. 2012;16(6):R215. Doi: 10.1186/cc11852. PubMed
21. Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and costs in clostridium difficile infection among the elderly in the United States. Infect Control Hosp Epidemiol. 2016;37(11):1331-1336. Doi: 10.1017/ice.2016.188. PubMed
22. Micek ST, Schramm G, Morrow L, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41(8):1968-1975. Doi: 10.1097/CCM.0b013e31828a40d5. PubMed
23. Zilberberg MD, Nathanson BH, Sadigov S, Higgins TL, Kollef MH, Shorr AF. Epidemiology and outcomes of clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136(3):752-758. Doi: 10.1378/chest.09-0596. PubMed
24. Lagu T, Stefan MS, Haessler S, et al. The impact of hospital-onset Clostridium difficile infection on outcomes of hospitalized patients with sepsis. J Hosp Med. 2014;9(7):411-417. Doi: 10.1002/jhm.2199. PubMed
25. Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192(5):581-588. Doi: 10.1164/rccm.201503-0483OC. PubMed
26. Healthcare-associated Infections (HAI) Progress Report. Centers for Disease Control and Prevention. http://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed on July 29, 2017.
27. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828. Doi: 10.1086/588756. PubMed
28. Zilberberg MD, Shorr AF, Micek ST, et al. Clostridium difficile recurrence is a strong predictor of 30-day rehospitalization among patients in intensive care. Infect Control Hosp Epidemiol. 2015;36(3):273-279. Doi: 10.1017/ice.2014.47. PubMed
29. Loftus KV, Wilson PM. A curiously rare case of septic shock from Clostridium difficile colitis. Pediatr Emerg Care. 2015. [Epub ahead of print]. Doi: 10.1097/PEC.0000000000000496. PubMed
30. Bermejo C, Maseda E, Salgado P, Gabilondo G., Gilsanz F. Septic shock due to a community acquired Clostridium difficile infection. A case study and a review of the literature. Rev Esp Anestesiol Reanimvol. 2014;61(4):219-222. PubMed
31. You E, Song H, Cho J, Lee J. Reduction in the incidence of hospital-acquired Clostridium difficile infection through infection control interventions other than the restriction of antimicrobial use. Int J Infect Dis. 2014;22:9-10. 2014. PubMed
© 2017 Society of Hospital Medicine
No overall survival benefit from early SIRT for liver metastases from colorectal cancer
Combining first-line chemotherapy with selective internal radiotherapy (SIRT) using Y-90 resin microspheres in patients with liver metastases from colorectal cancer does not result in greater overall survival compared with first-line chemotherapy alone, according to an analysis of three randomized trials.
The liver is the most common site of metastases in colorectal cancer, and liver metastases are the most common cause of death in these patients. Data from previous trials suggest SIRT has a clinical benefit as a third-line or subsequent therapy in patients with colorectal liver metastases with liver-dominant disease after chemotherapy.
In the current analysis, the largest so far to look at first-line SIRT plus chemotherapy’s effects on overall survival in these patients, researchers assessed data from FOXFIRE, SIRFLOX, and FOXFIRE-Global – phase 3 trials conducted in 14 countries. Patients were randomized to either the oxaliplatin-based chemotherapy FOLFOX (leucovorin, fluorouracil, and oxaliplatin) plus SIRT, or FOLFOX alone. In SIRFLOX and FOXFIRE-Global the chemotherapy regimen was modified, compared with FOXFIRE. (The Lancet. 2017 Aug 3. doi: 10.1016/S1470-2045[17]30457-6).
The randomizing was tweaked using the “minimization” technique to keep the treatment groups balanced for liver-only versus liver plus extrahepatic involvement, the extent of tumor involvement, anticipated use of a biologic agent, and the investigational center.
With a median follow-up of 43 months, there was no difference in overall survival, with a median survival time of 22.6 months in the FOLFOX plus SIRT group and 23.3 months in the FOLFOX alone group, said Harpreet Wasan, MBBS, MRCP, head of the gastrointestinal clinical research program at Imperial College London.
Serious adverse events occurred in 54% of the FOLFOX plus SIRT group and 43% of the FOLFOX alone group. There were eight treatment-related deaths in the FOLFOX plus SIRT group and three in the FOLFOX alone group.
Dr. Wasan said the lack of a benefit from SIRT could be partially explained by a high proportion of patients who developed first progression at an extrahepatic site.
“The absence of an overall survival benefit,” he said, “suggests that early use of SIRT in combination with first-line oxaliplatin-based chemotherapy cannot be recommended in unselected patients with metastatic colorectal cancer.”
SIRFLOX and FOXFIRE-Global were sponsored by Sirtex, the manufacturer of the resin microspheres used for the SIRT therapy in the trials that were analyzed. Study authors reported receiving grants, personal fees, speakers fees, and other financial relationships with Sirtex, Merck, Pfizer, Roche, and other companies.
Combining first-line chemotherapy with selective internal radiotherapy (SIRT) using Y-90 resin microspheres in patients with liver metastases from colorectal cancer does not result in greater overall survival compared with first-line chemotherapy alone, according to an analysis of three randomized trials.
The liver is the most common site of metastases in colorectal cancer, and liver metastases are the most common cause of death in these patients. Data from previous trials suggest SIRT has a clinical benefit as a third-line or subsequent therapy in patients with colorectal liver metastases with liver-dominant disease after chemotherapy.
In the current analysis, the largest so far to look at first-line SIRT plus chemotherapy’s effects on overall survival in these patients, researchers assessed data from FOXFIRE, SIRFLOX, and FOXFIRE-Global – phase 3 trials conducted in 14 countries. Patients were randomized to either the oxaliplatin-based chemotherapy FOLFOX (leucovorin, fluorouracil, and oxaliplatin) plus SIRT, or FOLFOX alone. In SIRFLOX and FOXFIRE-Global the chemotherapy regimen was modified, compared with FOXFIRE. (The Lancet. 2017 Aug 3. doi: 10.1016/S1470-2045[17]30457-6).
The randomizing was tweaked using the “minimization” technique to keep the treatment groups balanced for liver-only versus liver plus extrahepatic involvement, the extent of tumor involvement, anticipated use of a biologic agent, and the investigational center.
With a median follow-up of 43 months, there was no difference in overall survival, with a median survival time of 22.6 months in the FOLFOX plus SIRT group and 23.3 months in the FOLFOX alone group, said Harpreet Wasan, MBBS, MRCP, head of the gastrointestinal clinical research program at Imperial College London.
Serious adverse events occurred in 54% of the FOLFOX plus SIRT group and 43% of the FOLFOX alone group. There were eight treatment-related deaths in the FOLFOX plus SIRT group and three in the FOLFOX alone group.
Dr. Wasan said the lack of a benefit from SIRT could be partially explained by a high proportion of patients who developed first progression at an extrahepatic site.
“The absence of an overall survival benefit,” he said, “suggests that early use of SIRT in combination with first-line oxaliplatin-based chemotherapy cannot be recommended in unselected patients with metastatic colorectal cancer.”
SIRFLOX and FOXFIRE-Global were sponsored by Sirtex, the manufacturer of the resin microspheres used for the SIRT therapy in the trials that were analyzed. Study authors reported receiving grants, personal fees, speakers fees, and other financial relationships with Sirtex, Merck, Pfizer, Roche, and other companies.
Combining first-line chemotherapy with selective internal radiotherapy (SIRT) using Y-90 resin microspheres in patients with liver metastases from colorectal cancer does not result in greater overall survival compared with first-line chemotherapy alone, according to an analysis of three randomized trials.
The liver is the most common site of metastases in colorectal cancer, and liver metastases are the most common cause of death in these patients. Data from previous trials suggest SIRT has a clinical benefit as a third-line or subsequent therapy in patients with colorectal liver metastases with liver-dominant disease after chemotherapy.
In the current analysis, the largest so far to look at first-line SIRT plus chemotherapy’s effects on overall survival in these patients, researchers assessed data from FOXFIRE, SIRFLOX, and FOXFIRE-Global – phase 3 trials conducted in 14 countries. Patients were randomized to either the oxaliplatin-based chemotherapy FOLFOX (leucovorin, fluorouracil, and oxaliplatin) plus SIRT, or FOLFOX alone. In SIRFLOX and FOXFIRE-Global the chemotherapy regimen was modified, compared with FOXFIRE. (The Lancet. 2017 Aug 3. doi: 10.1016/S1470-2045[17]30457-6).
The randomizing was tweaked using the “minimization” technique to keep the treatment groups balanced for liver-only versus liver plus extrahepatic involvement, the extent of tumor involvement, anticipated use of a biologic agent, and the investigational center.
With a median follow-up of 43 months, there was no difference in overall survival, with a median survival time of 22.6 months in the FOLFOX plus SIRT group and 23.3 months in the FOLFOX alone group, said Harpreet Wasan, MBBS, MRCP, head of the gastrointestinal clinical research program at Imperial College London.
Serious adverse events occurred in 54% of the FOLFOX plus SIRT group and 43% of the FOLFOX alone group. There were eight treatment-related deaths in the FOLFOX plus SIRT group and three in the FOLFOX alone group.
Dr. Wasan said the lack of a benefit from SIRT could be partially explained by a high proportion of patients who developed first progression at an extrahepatic site.
“The absence of an overall survival benefit,” he said, “suggests that early use of SIRT in combination with first-line oxaliplatin-based chemotherapy cannot be recommended in unselected patients with metastatic colorectal cancer.”
SIRFLOX and FOXFIRE-Global were sponsored by Sirtex, the manufacturer of the resin microspheres used for the SIRT therapy in the trials that were analyzed. Study authors reported receiving grants, personal fees, speakers fees, and other financial relationships with Sirtex, Merck, Pfizer, Roche, and other companies.
FROM THE LANCET
Key clinical point:
Major finding: There was a median survival time of 22.6 months in the FOLFOX plus SIRT group and 23.3 months in the FOLFOX alone group, which was not a significant difference, after a median follow-up of 43 months.
Data source: An analysis of three phase 3 randomized trials across 14 countries from 2006 to 2014.
Disclosures: SIRFLOX and FOXFIRE-Global were sponsored by Sirtex, the manufacturer of the resin microspheres used for the SIRT therapy in the trials that were analyzed. Study authors reported receiving grants, personal fees, speakers fees, and other financial relationships with Sirtex, Merck, Pfizer, Roche, and other companies.
Room for improvement with HPV vaccine coverage
Six in ten teens now have had at least one dose of the human papillomavirus (HPV) vaccine, but many still are not receiving the second dose, according to 2016 data on vaccination coverage among adolescents published in the Morbidity and Mortality Weekly Report.
Researchers analyzed data from 20,475 adolescents aged 13-17 years in the 2016 National Immunization Survey–Teen, which showed that coverage for at least one dose of the HPV vaccine had increased from 56% in 2015 to 60% in 2016. However only 43% of adolescents – 50% of females and 38% of males – were up to date with the full two-dose vaccination series, in accordance with the updated vaccine recommendations (MMWR. 2017 Aug 25;66:874-82).
“Since HPV vaccine was introduced for females in 2006 and for males in 2011, coverage has increased gradually among females and more rapidly among males,” wrote Tanja Y. Walker and her colleagues from the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. “Coverage with 1 or more doses HPV vaccine among males continues to approach that among females, particularly for adolescents aged 13 years, suggesting that HPV vaccination of both female and male adolescents has been integrated into vaccination practices.”
HPV vaccine coverage with at least one dose was highest in central cities (66%) and lowest among adolescents in nonmetropolitan areas (50%). However, HPV coverage also was higher among adolescents living below the federal poverty line, compared with those living at or above the poverty line.
There was also significant geographic variation in HPV vaccine coverage, ranging from coverage with at least one dose among 90% of females and 88% of males in Rhode Island, to just 37% of males in Indiana and Wyoming and 48% of females in Mississippi. New York City showed the greatest average annual increase in coverage of one or more doses of the HPV vaccine from 2015 to 2016 (7.7 percentage point).
The analysis also looked at coverage for the Tdap vaccine, which increased for one or more doses from 86% to 88% from 2015 to 2016, respectively, while coverage with two or more doses of the meningococcal conjugate vaccine among 17-year-olds increased from 33% to 39%, respectively.
The authors pointed out that coverage for HPV immunization was still 22-28 percentage points below coverage for Tdap and the meningococcal conjugate vaccine, suggesting there was substantial opportunity for improvement.
“Potential contributing factors might include differences in parental acceptance of certain vaccines and provider participation in, and adolescents’ eligibility for, the Vaccines for Children program,” Ms. Walker and her colleagues said.
No conflicts of interest were declared.
Six in ten teens now have had at least one dose of the human papillomavirus (HPV) vaccine, but many still are not receiving the second dose, according to 2016 data on vaccination coverage among adolescents published in the Morbidity and Mortality Weekly Report.
Researchers analyzed data from 20,475 adolescents aged 13-17 years in the 2016 National Immunization Survey–Teen, which showed that coverage for at least one dose of the HPV vaccine had increased from 56% in 2015 to 60% in 2016. However only 43% of adolescents – 50% of females and 38% of males – were up to date with the full two-dose vaccination series, in accordance with the updated vaccine recommendations (MMWR. 2017 Aug 25;66:874-82).
“Since HPV vaccine was introduced for females in 2006 and for males in 2011, coverage has increased gradually among females and more rapidly among males,” wrote Tanja Y. Walker and her colleagues from the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. “Coverage with 1 or more doses HPV vaccine among males continues to approach that among females, particularly for adolescents aged 13 years, suggesting that HPV vaccination of both female and male adolescents has been integrated into vaccination practices.”
HPV vaccine coverage with at least one dose was highest in central cities (66%) and lowest among adolescents in nonmetropolitan areas (50%). However, HPV coverage also was higher among adolescents living below the federal poverty line, compared with those living at or above the poverty line.
There was also significant geographic variation in HPV vaccine coverage, ranging from coverage with at least one dose among 90% of females and 88% of males in Rhode Island, to just 37% of males in Indiana and Wyoming and 48% of females in Mississippi. New York City showed the greatest average annual increase in coverage of one or more doses of the HPV vaccine from 2015 to 2016 (7.7 percentage point).
The analysis also looked at coverage for the Tdap vaccine, which increased for one or more doses from 86% to 88% from 2015 to 2016, respectively, while coverage with two or more doses of the meningococcal conjugate vaccine among 17-year-olds increased from 33% to 39%, respectively.
The authors pointed out that coverage for HPV immunization was still 22-28 percentage points below coverage for Tdap and the meningococcal conjugate vaccine, suggesting there was substantial opportunity for improvement.
“Potential contributing factors might include differences in parental acceptance of certain vaccines and provider participation in, and adolescents’ eligibility for, the Vaccines for Children program,” Ms. Walker and her colleagues said.
No conflicts of interest were declared.
Six in ten teens now have had at least one dose of the human papillomavirus (HPV) vaccine, but many still are not receiving the second dose, according to 2016 data on vaccination coverage among adolescents published in the Morbidity and Mortality Weekly Report.
Researchers analyzed data from 20,475 adolescents aged 13-17 years in the 2016 National Immunization Survey–Teen, which showed that coverage for at least one dose of the HPV vaccine had increased from 56% in 2015 to 60% in 2016. However only 43% of adolescents – 50% of females and 38% of males – were up to date with the full two-dose vaccination series, in accordance with the updated vaccine recommendations (MMWR. 2017 Aug 25;66:874-82).
“Since HPV vaccine was introduced for females in 2006 and for males in 2011, coverage has increased gradually among females and more rapidly among males,” wrote Tanja Y. Walker and her colleagues from the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. “Coverage with 1 or more doses HPV vaccine among males continues to approach that among females, particularly for adolescents aged 13 years, suggesting that HPV vaccination of both female and male adolescents has been integrated into vaccination practices.”
HPV vaccine coverage with at least one dose was highest in central cities (66%) and lowest among adolescents in nonmetropolitan areas (50%). However, HPV coverage also was higher among adolescents living below the federal poverty line, compared with those living at or above the poverty line.
There was also significant geographic variation in HPV vaccine coverage, ranging from coverage with at least one dose among 90% of females and 88% of males in Rhode Island, to just 37% of males in Indiana and Wyoming and 48% of females in Mississippi. New York City showed the greatest average annual increase in coverage of one or more doses of the HPV vaccine from 2015 to 2016 (7.7 percentage point).
The analysis also looked at coverage for the Tdap vaccine, which increased for one or more doses from 86% to 88% from 2015 to 2016, respectively, while coverage with two or more doses of the meningococcal conjugate vaccine among 17-year-olds increased from 33% to 39%, respectively.
The authors pointed out that coverage for HPV immunization was still 22-28 percentage points below coverage for Tdap and the meningococcal conjugate vaccine, suggesting there was substantial opportunity for improvement.
“Potential contributing factors might include differences in parental acceptance of certain vaccines and provider participation in, and adolescents’ eligibility for, the Vaccines for Children program,” Ms. Walker and her colleagues said.
No conflicts of interest were declared.
FROM MMWR
Key clinical point: More than half of teens now have had at least one dose of the HPV vaccine, but many still are not receiving the second dose and immunization rates still are well below those of other vaccines.
Major finding:
Data source: Data from 20,475 adolescents aged 13-17 years in the 2016 National Immunization Survey–Teen.
Disclosures: No conflicts of interest were declared.
Cardiology News brings breaking ESC news to you
AT THE ESC CONGRESS 2017
Consider routine penicillin allergy testing in obstetrics
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
AT IDSOG
Key clinical point:
Major finding: Among 81 women with documented penicillin allergies who received gentamicin and clindamycin, 16% developed surgical site infections. In contrast, among the 864 women who received cefazolin, the infection rate was 7%.
Data source: A single-center review at Duke University.
Disclosures: The investigators reported having no relevant financial disclosures.
Communication tools improve patient experience and satisfaction
How hospitalists and other clinicians communicate with patients impacts a patient’s overall experience and satisfaction. But according to the authors of “Communication the Cleveland Way,”1 a book about how the clinic created and applied communication skills training, “in a culture prioritizing clinical outcomes above all, there can be a tendency to lose sight of one of the most critical aspects of providing effective care: the communication skills that build and foster physician-patient relationships.”
“Studies2,3 have shown that good communication between doctors and patients and among all caregivers who interface with patients directly results in better clinical outcomes, reduced costs, greater patient satisfaction, and lower rates of physician burnout,” the authors wrote.
“It places a special focus on empathy in relationships, and in our case, the provider-patient relationship rather than patient-centered care. The former acknowledges that the thoughts and feelings in both sides of a relationship are important. We know that clinicians, too, can suffer as a result of the care they provide,” Dr. Velez wrote in “Communication the Cleveland Way.”1
“Healthy relationships are based on balance and mutual respect,” Dr Velez said. “Courses made a strong point to practice empathy in order to teach empathy. Clinician participants were gifted with a safe space, an opportunity to share their own skills and expertise, and a chance to be appreciated for what they already do effectively. Most of all, activities were designed to be fun and engaging.” For example, CEHC encouraged and fostered an attitude of exploration, experimentation, and adventure. Various warm-up activities effectively helped the participants enter a more playful space and get into character portrayal.
Dr. Velez credits the CEHC model’s sustainability and success to the early realization that an appreciative approach is effective. In a study3 about the strategy, hospital-employed attending physicians participated in the 8-hour experiential communication skills training course on REDE. The study compared approximately 1,500 “intervention” physicians who attended and 1,900 “nonintervention” physicians who did not attend.
Following the course, scores for physician communication and respect were higher for intervention physicians. Furthermore, physicians showed significant improvement in self-perceptions of empathy and burnout. Some of these gains were sustained for at least 3 months. “This is especially important because in the current health care climate, physicians experience increased burnout,” Dr. Velez notes.
How it works
Because a provider’s connection with a patient occurs when a relationship is established, the REDE course focuses on the beginning of the conversation. “It’s important for clinicians to exhibit value and respect through words and actions when welcoming patients,” Dr. Velez said. “Further, instead of guiding the medical interview with a series of close-ended questions like an interrogator would, we invite the use of open-ended questions and setting an agenda for the visit early on, by asking the patient what they wish to discuss.”
Another key component is empathy, which plays a huge role in patient satisfaction. “Learning how to express empathy is very important,” Dr. Velez said. “A patient may not remember all of the medical details discussed, but human interactions, rapport, expressions of care, support, validation, and acknowledgment of emotions tend to be more indelible.”
Dr. Velez notes that decades of literature regarding effective communication have demonstrated improved outcomes. “If trust in a therapeutic relationship is strong, a patient is more likely to follow instructions and have better engagement with their care plan,” he said. “If a clinician ensures that the patient understands the diagnosis and recommendations, then compliance will increase, especially if the plan is tailored to the patient’s goals and perspective.”
One surprising effect of the REDE course was how it improved relationships among professionals. “Many participants have shared that having a day out of one’s normal schedule, not only to learn, but also to share their own experiences, is quite therapeutic,” Dr. Velez said. “We can extend the same communication strategies to team building, interprofessional interactions, and challenging encounters.”
Study focuses on comportment and communication
In an effort to define optimal care in hospital medicine, a team from Johns Hopkins Health System set out to establish a metric that would comprehensively assess hospitalists’ comportment (which includes behavior as well as general demeanor) and communication to establish norms and expectations when they saw patients at the bedside.
To perform the study,4 chiefs of hospital medicine divisions at five independent hospitals located in Baltimore and Washington identified their most clinically excellent hospitalists. Then, an investigator observed each hospitalist during a routine clinical shift and recorded behaviors believed to be associated with excellent behavior and communication using the hospital medicine comportment and communication observation tool (HMCCOT), said Susrutha Kotwal, MD, assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, and lead author. The investigators collected basic demographic information while observing hospitalists for an average of 280 minutes; 26 physicians were observed for 181 separate clinical encounters. Each provider’s mean HMCCOT score was compared with patient satisfaction surveys such as Press Ganey (PG) scores.
Noteworthy is the fact that the distribution of HMCCOT scores were similar when analyzed by age, gender, race, amount of clinical experience, the hospitalist’s clinical workload, hospital, or time spent observing the hospitalist. But the distribution of HMCCOT scores was quite different in new patient encounters, compared with follow-ups (68.1% versus 39.7%). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes versus 8.7 minutes). The physicians’ HMCCOT scores were also associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might also translate into enhanced patient satisfaction.
As a result of the study, a comportment and communication tool was established and validated by following clinically excellent hospitalists at the bedside. “Even among clinically respected hospitalists, the results reveal that there is wide variability in behaviors and communication practices at the bedside,” Dr. Kotwal said.
Employing the tool
Hospitalists can choose whether to perform behaviors in the HMCCOT themselves, while others may wish to watch other hospitalists to give them feedback tied to specific behaviors. “These simple behaviors are intimately linked to excellent communication and comportment, which can serve as the foundation for delivering patient-centered care,” Dr. Kotwal said.
A positive correlation was found between spending more time with patients and higher HMCCOT scores. “Patients’ complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that they did not convey information in a clear manner,” Dr. Kotwal said. “When successfully achieved, patient-centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self-management of chronic disease. Many of the components of the HMCCOT described in our study are at the heart of patient-centered care.”
Dr. Kotwal believes HMCCOT is a better strategy to improve patient satisfaction than patient satisfaction surveys because patients can’t always recall which specific provider saw them. In addition, patients’ recall about the provider may be poor because surveys are sent to patients days after they return home. In addition, patients’ recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Therefore, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.5
The study authors conclude that, “Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies are then needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital. Because hospitalists spend only a small proportion of their clinical time in direct patient care, it is imperative that excellent comportment and communication be established as a goal for every encounter.”
The effectiveness of care team rounds at the bedside
Investigators at the UMass Memorial Medical Center, in Worcester, Mass., studied the effectiveness of assembling the entire care team (i.e., physicians, including residents and attendings, nursing, and clinical pharmacy) to round at the patient’s bedside each morning – in lieu of its traditionally separate rounding strategies – on one unit of its academic hospitalist service for an internal quality program, said Patricia Seymour, MD, FAAFP, assistant professor and family medicine hospitalist education director.
Additionally, academic presentations and discussions were all done in front of patients and their families (with a few exceptions) rather than traditional hallway rounds or sit rounds. Over the course of the project, the hospital also offered residents training around physician behaviors that improve patient satisfaction; provided incentives for nurses and residents to work as a team; and created a welcome visit template for the nursing manager and instruments for patients to enhance engagement. Through all of these cycles, the collaborative rounding strategy continued.
Because Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores yielded low response rates for the singular test unit and service, the investigators used a validated patient satisfaction instrument and surveyed patients from the intervention group and patients on the same unit who did not experience this collaborative rounding on their day of discharge. The intervention group had higher satisfaction scores at most of the time points. The unit-based HCAHPS scores (not just study patients) improved during this time period.
“We think the strategy of collaborative rounding yielded positive results for obvious reasons – the entire team was on the same page and the information given to the patient was consistent,” said Dr. Seymour, who notes that the study’s findings weren’t published and the project was completed for an internal quality program. “Doctors had an increased understanding about nursing concerns and the nursing staff expressed improved understanding of patients’ care plans.”
Certainly, face time with the patient was extended because much of the academic discussion occurred at the bedside instead of at another physical location without patient awareness, Dr. Seymour said. She believes the strategy boosted patient satisfaction because it was patient centered. “While this rounding strategy is not the most convenient rounding strategy for nurses or doctors, it consolidates the discussion about the patient’s clinical condition and the plan for the day. The patient experiences a strong sense of being cared for by a unified team and receives consistent messaging,” she said.
Also noteworthy is that job satisfaction for residents and nurses improved on the unit over the study time period because of the expected collaboration that was built into the work flow.
Although the facility is no longer using this communication strategy to the same degree, teaching attendings have seen the value of true bedside rounding and continue to teach this skill to learners. “We have had some challenges with geographic cohorting at our institution, which is essential for this type of team-based strategy,” Dr. Seymour said. “Sustainability requires constant encouragement, oversight, and auditing from team leaders which is also challenging and fluctuates with competing demands.”
The results of this study, and others, show that employing tools to improve communication can also result in improved patient satisfaction and experience.
Karen Appold is a medical writer in Pennsylvania.
References
1. Boissy A, Gilligan T. “Communication the Cleveland Clinic Way: How to drive a relationship-centered strategy for superior patient experience.” New York: McGraw-Hill Education. 2016.
2. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45:835-42.
3. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016 Jul;31(7):755-761. doi: 10.1007/s11606-016-3597-2. Epub 2016 Feb 26.
4. Kotwal S, Khaliq W, Landis R, Wright S. Developing a comportment and communication tool for use in hospital medicine. J Hosp Med. 2016 Dec;11(12):853-858. doi: 10.1002/jhm.2647. Epub 2016 Aug 13.
5. Fong Ha J, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010 Spring; 10(1):38-43.
6. Bodenheimer T, Sinsky C. From Triple to Quadruple Aim: care of the patient requires care of the provider. Ann Fam Med. 2014 Nov;12(6): 573-6. doi: 10.1370/afm.1713.
Bonus Content
Clinicians wary of course's worthiness
Before clinicians took Cleveland Clinic’s Relationship Establishment, Development, and Engagement (REDE) course, only 20% strongly agreed that the course would be valuable, whereas afterward 58% strongly agreed that it was indeed valuable. Less than 1% said it wasn’t valuable.4 “Most likely clinicians had a preconceived notion about how communication courses go, but they were probably surprised at how much these sessions were equally about them as providers as they were about caring for patients,” said Vicente J. Velez, MD, FACP, FHM, a hospitalist who serves as the director of faculty enrichment for the leadership team of CEHC. “This is the power of relationship-centered care, and also why I think the model has been sustainable.”
Physicians also reported that before taking the course, they had moderate levels of burnout and low levels of empathy. After taking it, burnout metrics (i.e., emotional exhaustion, depersonalization, and personal achievement) and empathy improved significantly. “I observed that most are surprised to find out that empathy is a discreet set of skills that can be learned, practiced, observed, measured, and improved upon,” Dr. Velez said. “If taught in a safe and validating environment and if principles of adult learning are followed, improvement can be optimized and sustained.”
Since the REDE course rolled out in 2012, all attending physicians and medical staff members have been trained in it.
Why empathy is preferred over patient-centered care
The Cleveland Clinic intentionally puts a focus on relationship-centered care.
“When there’s an emphasis on patient-centered care, some physicians have a hard time figuring out what to do when the patient wants something that the physician doesn’t feel is appropriate,” said Katie Neuendorf, MD, director for the Center for Excellence in Healthcare Communication. “Patient-centered implies that the patient is always right and that their opinion should win out over the physician’s opinion. In that same scenario, relationship-centered care implies that the relationship should be prioritized, even when there’s disagreement in the plan of care. I can tell my patients that I hear what they are saying, that I empathize with their struggles, that I care about the way the illness is affecting their lives, and that I am here to support them. I can do all of that and still not prescribe a treatment that I feel is inappropriate just because it happens to be what the patient wants.”
The Cleveland Clinic’s Relationship Establishment, Development and Engagement (REDE) course helps clinicians to see the individual that exists beyond a diagnosis. “Having empathy, or putting yourself in the other person’s shoes, is a key step in that process,” Dr. Neuendorf said. “Once a physician understands the patient’s perspective, the treatment for the diagnosis is more meaningful to both the patient and physician. Finding meaning in their work addresses the Quadruple Aim.”
How hospitalists and other clinicians communicate with patients impacts a patient’s overall experience and satisfaction. But according to the authors of “Communication the Cleveland Way,”1 a book about how the clinic created and applied communication skills training, “in a culture prioritizing clinical outcomes above all, there can be a tendency to lose sight of one of the most critical aspects of providing effective care: the communication skills that build and foster physician-patient relationships.”
“Studies2,3 have shown that good communication between doctors and patients and among all caregivers who interface with patients directly results in better clinical outcomes, reduced costs, greater patient satisfaction, and lower rates of physician burnout,” the authors wrote.
“It places a special focus on empathy in relationships, and in our case, the provider-patient relationship rather than patient-centered care. The former acknowledges that the thoughts and feelings in both sides of a relationship are important. We know that clinicians, too, can suffer as a result of the care they provide,” Dr. Velez wrote in “Communication the Cleveland Way.”1
“Healthy relationships are based on balance and mutual respect,” Dr Velez said. “Courses made a strong point to practice empathy in order to teach empathy. Clinician participants were gifted with a safe space, an opportunity to share their own skills and expertise, and a chance to be appreciated for what they already do effectively. Most of all, activities were designed to be fun and engaging.” For example, CEHC encouraged and fostered an attitude of exploration, experimentation, and adventure. Various warm-up activities effectively helped the participants enter a more playful space and get into character portrayal.
Dr. Velez credits the CEHC model’s sustainability and success to the early realization that an appreciative approach is effective. In a study3 about the strategy, hospital-employed attending physicians participated in the 8-hour experiential communication skills training course on REDE. The study compared approximately 1,500 “intervention” physicians who attended and 1,900 “nonintervention” physicians who did not attend.
Following the course, scores for physician communication and respect were higher for intervention physicians. Furthermore, physicians showed significant improvement in self-perceptions of empathy and burnout. Some of these gains were sustained for at least 3 months. “This is especially important because in the current health care climate, physicians experience increased burnout,” Dr. Velez notes.
How it works
Because a provider’s connection with a patient occurs when a relationship is established, the REDE course focuses on the beginning of the conversation. “It’s important for clinicians to exhibit value and respect through words and actions when welcoming patients,” Dr. Velez said. “Further, instead of guiding the medical interview with a series of close-ended questions like an interrogator would, we invite the use of open-ended questions and setting an agenda for the visit early on, by asking the patient what they wish to discuss.”
Another key component is empathy, which plays a huge role in patient satisfaction. “Learning how to express empathy is very important,” Dr. Velez said. “A patient may not remember all of the medical details discussed, but human interactions, rapport, expressions of care, support, validation, and acknowledgment of emotions tend to be more indelible.”
Dr. Velez notes that decades of literature regarding effective communication have demonstrated improved outcomes. “If trust in a therapeutic relationship is strong, a patient is more likely to follow instructions and have better engagement with their care plan,” he said. “If a clinician ensures that the patient understands the diagnosis and recommendations, then compliance will increase, especially if the plan is tailored to the patient’s goals and perspective.”
One surprising effect of the REDE course was how it improved relationships among professionals. “Many participants have shared that having a day out of one’s normal schedule, not only to learn, but also to share their own experiences, is quite therapeutic,” Dr. Velez said. “We can extend the same communication strategies to team building, interprofessional interactions, and challenging encounters.”
Study focuses on comportment and communication
In an effort to define optimal care in hospital medicine, a team from Johns Hopkins Health System set out to establish a metric that would comprehensively assess hospitalists’ comportment (which includes behavior as well as general demeanor) and communication to establish norms and expectations when they saw patients at the bedside.
To perform the study,4 chiefs of hospital medicine divisions at five independent hospitals located in Baltimore and Washington identified their most clinically excellent hospitalists. Then, an investigator observed each hospitalist during a routine clinical shift and recorded behaviors believed to be associated with excellent behavior and communication using the hospital medicine comportment and communication observation tool (HMCCOT), said Susrutha Kotwal, MD, assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, and lead author. The investigators collected basic demographic information while observing hospitalists for an average of 280 minutes; 26 physicians were observed for 181 separate clinical encounters. Each provider’s mean HMCCOT score was compared with patient satisfaction surveys such as Press Ganey (PG) scores.
Noteworthy is the fact that the distribution of HMCCOT scores were similar when analyzed by age, gender, race, amount of clinical experience, the hospitalist’s clinical workload, hospital, or time spent observing the hospitalist. But the distribution of HMCCOT scores was quite different in new patient encounters, compared with follow-ups (68.1% versus 39.7%). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes versus 8.7 minutes). The physicians’ HMCCOT scores were also associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might also translate into enhanced patient satisfaction.
As a result of the study, a comportment and communication tool was established and validated by following clinically excellent hospitalists at the bedside. “Even among clinically respected hospitalists, the results reveal that there is wide variability in behaviors and communication practices at the bedside,” Dr. Kotwal said.
Employing the tool
Hospitalists can choose whether to perform behaviors in the HMCCOT themselves, while others may wish to watch other hospitalists to give them feedback tied to specific behaviors. “These simple behaviors are intimately linked to excellent communication and comportment, which can serve as the foundation for delivering patient-centered care,” Dr. Kotwal said.
A positive correlation was found between spending more time with patients and higher HMCCOT scores. “Patients’ complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that they did not convey information in a clear manner,” Dr. Kotwal said. “When successfully achieved, patient-centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self-management of chronic disease. Many of the components of the HMCCOT described in our study are at the heart of patient-centered care.”
Dr. Kotwal believes HMCCOT is a better strategy to improve patient satisfaction than patient satisfaction surveys because patients can’t always recall which specific provider saw them. In addition, patients’ recall about the provider may be poor because surveys are sent to patients days after they return home. In addition, patients’ recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Therefore, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.5
The study authors conclude that, “Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies are then needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital. Because hospitalists spend only a small proportion of their clinical time in direct patient care, it is imperative that excellent comportment and communication be established as a goal for every encounter.”
The effectiveness of care team rounds at the bedside
Investigators at the UMass Memorial Medical Center, in Worcester, Mass., studied the effectiveness of assembling the entire care team (i.e., physicians, including residents and attendings, nursing, and clinical pharmacy) to round at the patient’s bedside each morning – in lieu of its traditionally separate rounding strategies – on one unit of its academic hospitalist service for an internal quality program, said Patricia Seymour, MD, FAAFP, assistant professor and family medicine hospitalist education director.
Additionally, academic presentations and discussions were all done in front of patients and their families (with a few exceptions) rather than traditional hallway rounds or sit rounds. Over the course of the project, the hospital also offered residents training around physician behaviors that improve patient satisfaction; provided incentives for nurses and residents to work as a team; and created a welcome visit template for the nursing manager and instruments for patients to enhance engagement. Through all of these cycles, the collaborative rounding strategy continued.
Because Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores yielded low response rates for the singular test unit and service, the investigators used a validated patient satisfaction instrument and surveyed patients from the intervention group and patients on the same unit who did not experience this collaborative rounding on their day of discharge. The intervention group had higher satisfaction scores at most of the time points. The unit-based HCAHPS scores (not just study patients) improved during this time period.
“We think the strategy of collaborative rounding yielded positive results for obvious reasons – the entire team was on the same page and the information given to the patient was consistent,” said Dr. Seymour, who notes that the study’s findings weren’t published and the project was completed for an internal quality program. “Doctors had an increased understanding about nursing concerns and the nursing staff expressed improved understanding of patients’ care plans.”
Certainly, face time with the patient was extended because much of the academic discussion occurred at the bedside instead of at another physical location without patient awareness, Dr. Seymour said. She believes the strategy boosted patient satisfaction because it was patient centered. “While this rounding strategy is not the most convenient rounding strategy for nurses or doctors, it consolidates the discussion about the patient’s clinical condition and the plan for the day. The patient experiences a strong sense of being cared for by a unified team and receives consistent messaging,” she said.
Also noteworthy is that job satisfaction for residents and nurses improved on the unit over the study time period because of the expected collaboration that was built into the work flow.
Although the facility is no longer using this communication strategy to the same degree, teaching attendings have seen the value of true bedside rounding and continue to teach this skill to learners. “We have had some challenges with geographic cohorting at our institution, which is essential for this type of team-based strategy,” Dr. Seymour said. “Sustainability requires constant encouragement, oversight, and auditing from team leaders which is also challenging and fluctuates with competing demands.”
The results of this study, and others, show that employing tools to improve communication can also result in improved patient satisfaction and experience.
Karen Appold is a medical writer in Pennsylvania.
References
1. Boissy A, Gilligan T. “Communication the Cleveland Clinic Way: How to drive a relationship-centered strategy for superior patient experience.” New York: McGraw-Hill Education. 2016.
2. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45:835-42.
3. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016 Jul;31(7):755-761. doi: 10.1007/s11606-016-3597-2. Epub 2016 Feb 26.
4. Kotwal S, Khaliq W, Landis R, Wright S. Developing a comportment and communication tool for use in hospital medicine. J Hosp Med. 2016 Dec;11(12):853-858. doi: 10.1002/jhm.2647. Epub 2016 Aug 13.
5. Fong Ha J, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010 Spring; 10(1):38-43.
6. Bodenheimer T, Sinsky C. From Triple to Quadruple Aim: care of the patient requires care of the provider. Ann Fam Med. 2014 Nov;12(6): 573-6. doi: 10.1370/afm.1713.
Bonus Content
Clinicians wary of course's worthiness
Before clinicians took Cleveland Clinic’s Relationship Establishment, Development, and Engagement (REDE) course, only 20% strongly agreed that the course would be valuable, whereas afterward 58% strongly agreed that it was indeed valuable. Less than 1% said it wasn’t valuable.4 “Most likely clinicians had a preconceived notion about how communication courses go, but they were probably surprised at how much these sessions were equally about them as providers as they were about caring for patients,” said Vicente J. Velez, MD, FACP, FHM, a hospitalist who serves as the director of faculty enrichment for the leadership team of CEHC. “This is the power of relationship-centered care, and also why I think the model has been sustainable.”
Physicians also reported that before taking the course, they had moderate levels of burnout and low levels of empathy. After taking it, burnout metrics (i.e., emotional exhaustion, depersonalization, and personal achievement) and empathy improved significantly. “I observed that most are surprised to find out that empathy is a discreet set of skills that can be learned, practiced, observed, measured, and improved upon,” Dr. Velez said. “If taught in a safe and validating environment and if principles of adult learning are followed, improvement can be optimized and sustained.”
Since the REDE course rolled out in 2012, all attending physicians and medical staff members have been trained in it.
Why empathy is preferred over patient-centered care
The Cleveland Clinic intentionally puts a focus on relationship-centered care.
“When there’s an emphasis on patient-centered care, some physicians have a hard time figuring out what to do when the patient wants something that the physician doesn’t feel is appropriate,” said Katie Neuendorf, MD, director for the Center for Excellence in Healthcare Communication. “Patient-centered implies that the patient is always right and that their opinion should win out over the physician’s opinion. In that same scenario, relationship-centered care implies that the relationship should be prioritized, even when there’s disagreement in the plan of care. I can tell my patients that I hear what they are saying, that I empathize with their struggles, that I care about the way the illness is affecting their lives, and that I am here to support them. I can do all of that and still not prescribe a treatment that I feel is inappropriate just because it happens to be what the patient wants.”
The Cleveland Clinic’s Relationship Establishment, Development and Engagement (REDE) course helps clinicians to see the individual that exists beyond a diagnosis. “Having empathy, or putting yourself in the other person’s shoes, is a key step in that process,” Dr. Neuendorf said. “Once a physician understands the patient’s perspective, the treatment for the diagnosis is more meaningful to both the patient and physician. Finding meaning in their work addresses the Quadruple Aim.”
How hospitalists and other clinicians communicate with patients impacts a patient’s overall experience and satisfaction. But according to the authors of “Communication the Cleveland Way,”1 a book about how the clinic created and applied communication skills training, “in a culture prioritizing clinical outcomes above all, there can be a tendency to lose sight of one of the most critical aspects of providing effective care: the communication skills that build and foster physician-patient relationships.”
“Studies2,3 have shown that good communication between doctors and patients and among all caregivers who interface with patients directly results in better clinical outcomes, reduced costs, greater patient satisfaction, and lower rates of physician burnout,” the authors wrote.
“It places a special focus on empathy in relationships, and in our case, the provider-patient relationship rather than patient-centered care. The former acknowledges that the thoughts and feelings in both sides of a relationship are important. We know that clinicians, too, can suffer as a result of the care they provide,” Dr. Velez wrote in “Communication the Cleveland Way.”1
“Healthy relationships are based on balance and mutual respect,” Dr Velez said. “Courses made a strong point to practice empathy in order to teach empathy. Clinician participants were gifted with a safe space, an opportunity to share their own skills and expertise, and a chance to be appreciated for what they already do effectively. Most of all, activities were designed to be fun and engaging.” For example, CEHC encouraged and fostered an attitude of exploration, experimentation, and adventure. Various warm-up activities effectively helped the participants enter a more playful space and get into character portrayal.
Dr. Velez credits the CEHC model’s sustainability and success to the early realization that an appreciative approach is effective. In a study3 about the strategy, hospital-employed attending physicians participated in the 8-hour experiential communication skills training course on REDE. The study compared approximately 1,500 “intervention” physicians who attended and 1,900 “nonintervention” physicians who did not attend.
Following the course, scores for physician communication and respect were higher for intervention physicians. Furthermore, physicians showed significant improvement in self-perceptions of empathy and burnout. Some of these gains were sustained for at least 3 months. “This is especially important because in the current health care climate, physicians experience increased burnout,” Dr. Velez notes.
How it works
Because a provider’s connection with a patient occurs when a relationship is established, the REDE course focuses on the beginning of the conversation. “It’s important for clinicians to exhibit value and respect through words and actions when welcoming patients,” Dr. Velez said. “Further, instead of guiding the medical interview with a series of close-ended questions like an interrogator would, we invite the use of open-ended questions and setting an agenda for the visit early on, by asking the patient what they wish to discuss.”
Another key component is empathy, which plays a huge role in patient satisfaction. “Learning how to express empathy is very important,” Dr. Velez said. “A patient may not remember all of the medical details discussed, but human interactions, rapport, expressions of care, support, validation, and acknowledgment of emotions tend to be more indelible.”
Dr. Velez notes that decades of literature regarding effective communication have demonstrated improved outcomes. “If trust in a therapeutic relationship is strong, a patient is more likely to follow instructions and have better engagement with their care plan,” he said. “If a clinician ensures that the patient understands the diagnosis and recommendations, then compliance will increase, especially if the plan is tailored to the patient’s goals and perspective.”
One surprising effect of the REDE course was how it improved relationships among professionals. “Many participants have shared that having a day out of one’s normal schedule, not only to learn, but also to share their own experiences, is quite therapeutic,” Dr. Velez said. “We can extend the same communication strategies to team building, interprofessional interactions, and challenging encounters.”
Study focuses on comportment and communication
In an effort to define optimal care in hospital medicine, a team from Johns Hopkins Health System set out to establish a metric that would comprehensively assess hospitalists’ comportment (which includes behavior as well as general demeanor) and communication to establish norms and expectations when they saw patients at the bedside.
To perform the study,4 chiefs of hospital medicine divisions at five independent hospitals located in Baltimore and Washington identified their most clinically excellent hospitalists. Then, an investigator observed each hospitalist during a routine clinical shift and recorded behaviors believed to be associated with excellent behavior and communication using the hospital medicine comportment and communication observation tool (HMCCOT), said Susrutha Kotwal, MD, assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, and lead author. The investigators collected basic demographic information while observing hospitalists for an average of 280 minutes; 26 physicians were observed for 181 separate clinical encounters. Each provider’s mean HMCCOT score was compared with patient satisfaction surveys such as Press Ganey (PG) scores.
Noteworthy is the fact that the distribution of HMCCOT scores were similar when analyzed by age, gender, race, amount of clinical experience, the hospitalist’s clinical workload, hospital, or time spent observing the hospitalist. But the distribution of HMCCOT scores was quite different in new patient encounters, compared with follow-ups (68.1% versus 39.7%). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes versus 8.7 minutes). The physicians’ HMCCOT scores were also associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might also translate into enhanced patient satisfaction.
As a result of the study, a comportment and communication tool was established and validated by following clinically excellent hospitalists at the bedside. “Even among clinically respected hospitalists, the results reveal that there is wide variability in behaviors and communication practices at the bedside,” Dr. Kotwal said.
Employing the tool
Hospitalists can choose whether to perform behaviors in the HMCCOT themselves, while others may wish to watch other hospitalists to give them feedback tied to specific behaviors. “These simple behaviors are intimately linked to excellent communication and comportment, which can serve as the foundation for delivering patient-centered care,” Dr. Kotwal said.
A positive correlation was found between spending more time with patients and higher HMCCOT scores. “Patients’ complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that they did not convey information in a clear manner,” Dr. Kotwal said. “When successfully achieved, patient-centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self-management of chronic disease. Many of the components of the HMCCOT described in our study are at the heart of patient-centered care.”
Dr. Kotwal believes HMCCOT is a better strategy to improve patient satisfaction than patient satisfaction surveys because patients can’t always recall which specific provider saw them. In addition, patients’ recall about the provider may be poor because surveys are sent to patients days after they return home. In addition, patients’ recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Therefore, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.5
The study authors conclude that, “Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies are then needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital. Because hospitalists spend only a small proportion of their clinical time in direct patient care, it is imperative that excellent comportment and communication be established as a goal for every encounter.”
The effectiveness of care team rounds at the bedside
Investigators at the UMass Memorial Medical Center, in Worcester, Mass., studied the effectiveness of assembling the entire care team (i.e., physicians, including residents and attendings, nursing, and clinical pharmacy) to round at the patient’s bedside each morning – in lieu of its traditionally separate rounding strategies – on one unit of its academic hospitalist service for an internal quality program, said Patricia Seymour, MD, FAAFP, assistant professor and family medicine hospitalist education director.
Additionally, academic presentations and discussions were all done in front of patients and their families (with a few exceptions) rather than traditional hallway rounds or sit rounds. Over the course of the project, the hospital also offered residents training around physician behaviors that improve patient satisfaction; provided incentives for nurses and residents to work as a team; and created a welcome visit template for the nursing manager and instruments for patients to enhance engagement. Through all of these cycles, the collaborative rounding strategy continued.
Because Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey scores yielded low response rates for the singular test unit and service, the investigators used a validated patient satisfaction instrument and surveyed patients from the intervention group and patients on the same unit who did not experience this collaborative rounding on their day of discharge. The intervention group had higher satisfaction scores at most of the time points. The unit-based HCAHPS scores (not just study patients) improved during this time period.
“We think the strategy of collaborative rounding yielded positive results for obvious reasons – the entire team was on the same page and the information given to the patient was consistent,” said Dr. Seymour, who notes that the study’s findings weren’t published and the project was completed for an internal quality program. “Doctors had an increased understanding about nursing concerns and the nursing staff expressed improved understanding of patients’ care plans.”
Certainly, face time with the patient was extended because much of the academic discussion occurred at the bedside instead of at another physical location without patient awareness, Dr. Seymour said. She believes the strategy boosted patient satisfaction because it was patient centered. “While this rounding strategy is not the most convenient rounding strategy for nurses or doctors, it consolidates the discussion about the patient’s clinical condition and the plan for the day. The patient experiences a strong sense of being cared for by a unified team and receives consistent messaging,” she said.
Also noteworthy is that job satisfaction for residents and nurses improved on the unit over the study time period because of the expected collaboration that was built into the work flow.
Although the facility is no longer using this communication strategy to the same degree, teaching attendings have seen the value of true bedside rounding and continue to teach this skill to learners. “We have had some challenges with geographic cohorting at our institution, which is essential for this type of team-based strategy,” Dr. Seymour said. “Sustainability requires constant encouragement, oversight, and auditing from team leaders which is also challenging and fluctuates with competing demands.”
The results of this study, and others, show that employing tools to improve communication can also result in improved patient satisfaction and experience.
Karen Appold is a medical writer in Pennsylvania.
References
1. Boissy A, Gilligan T. “Communication the Cleveland Clinic Way: How to drive a relationship-centered strategy for superior patient experience.” New York: McGraw-Hill Education. 2016.
2. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45:835-42.
3. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016 Jul;31(7):755-761. doi: 10.1007/s11606-016-3597-2. Epub 2016 Feb 26.
4. Kotwal S, Khaliq W, Landis R, Wright S. Developing a comportment and communication tool for use in hospital medicine. J Hosp Med. 2016 Dec;11(12):853-858. doi: 10.1002/jhm.2647. Epub 2016 Aug 13.
5. Fong Ha J, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010 Spring; 10(1):38-43.
6. Bodenheimer T, Sinsky C. From Triple to Quadruple Aim: care of the patient requires care of the provider. Ann Fam Med. 2014 Nov;12(6): 573-6. doi: 10.1370/afm.1713.
Bonus Content
Clinicians wary of course's worthiness
Before clinicians took Cleveland Clinic’s Relationship Establishment, Development, and Engagement (REDE) course, only 20% strongly agreed that the course would be valuable, whereas afterward 58% strongly agreed that it was indeed valuable. Less than 1% said it wasn’t valuable.4 “Most likely clinicians had a preconceived notion about how communication courses go, but they were probably surprised at how much these sessions were equally about them as providers as they were about caring for patients,” said Vicente J. Velez, MD, FACP, FHM, a hospitalist who serves as the director of faculty enrichment for the leadership team of CEHC. “This is the power of relationship-centered care, and also why I think the model has been sustainable.”
Physicians also reported that before taking the course, they had moderate levels of burnout and low levels of empathy. After taking it, burnout metrics (i.e., emotional exhaustion, depersonalization, and personal achievement) and empathy improved significantly. “I observed that most are surprised to find out that empathy is a discreet set of skills that can be learned, practiced, observed, measured, and improved upon,” Dr. Velez said. “If taught in a safe and validating environment and if principles of adult learning are followed, improvement can be optimized and sustained.”
Since the REDE course rolled out in 2012, all attending physicians and medical staff members have been trained in it.
Why empathy is preferred over patient-centered care
The Cleveland Clinic intentionally puts a focus on relationship-centered care.
“When there’s an emphasis on patient-centered care, some physicians have a hard time figuring out what to do when the patient wants something that the physician doesn’t feel is appropriate,” said Katie Neuendorf, MD, director for the Center for Excellence in Healthcare Communication. “Patient-centered implies that the patient is always right and that their opinion should win out over the physician’s opinion. In that same scenario, relationship-centered care implies that the relationship should be prioritized, even when there’s disagreement in the plan of care. I can tell my patients that I hear what they are saying, that I empathize with their struggles, that I care about the way the illness is affecting their lives, and that I am here to support them. I can do all of that and still not prescribe a treatment that I feel is inappropriate just because it happens to be what the patient wants.”
The Cleveland Clinic’s Relationship Establishment, Development and Engagement (REDE) course helps clinicians to see the individual that exists beyond a diagnosis. “Having empathy, or putting yourself in the other person’s shoes, is a key step in that process,” Dr. Neuendorf said. “Once a physician understands the patient’s perspective, the treatment for the diagnosis is more meaningful to both the patient and physician. Finding meaning in their work addresses the Quadruple Aim.”
Despite global decline, rheumatic heart disease persists in poorest regions
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Rheumatic heart disease ranks as one of the most serious cardiovascular scourges of the past century. As a result of improvements in living conditions and the introduction of penicillin, the disease was almost eradicated in the developed world by the 1980s. However, it remains a force to be reckoned with in the developing world, as demonstrated by an assessment from the 2015 Global Burden of Disease study (GBD 2015), painstakingly performed by Dr. Watkins and his colleagues.
Several key messages emerge from this important study. It confirms the marked global heterogeneity of the burden of rheumatic heart disease, with near-zero prevalence in developed countries sharply contrasting with substantial prevalence and mortality in developing areas. In addition, however, the study documents the scarcity of accurately measured data in many locations, especially in areas with the highest prevalence (such as sub-Saharan Africa).
Although the “headline news” of a global decline in the prevalence of rheumatic heart disease described by Watkins et al. may give cause for optimism, the burden remains great for those parts of the world least able to afford it. Without sustained re-engagement of clinicians, researchers, funders, and public health bodies, the menace of rheumatic heart disease is unlikely to be eliminated in the near future. Rheumatic heart disease remains a problematic iceberg, yet undissolved, in warm tropical waters.
Eloi Marijon, MD, PhD, and Xavier Jouven, MD, PhD, are at European Georges Pompidou Hospital, Paris. David S. Celermajer, PhD, is at Sydney (Australia) Medical School. They reported having no conflicts of interest. Their editorial accompanied the report by Dr. Watkins and his colleagues (N Engl J Med. 2017;377:780-1).
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
Global mortality due to rheumatic heart disease fell by about 48% during a recent 25-year-period, but some of the poorest areas of the world were left behind, according to a report in the New England Journal of Medicine.
Those regions included Oceania, South Asia, and central sub-Saharan Africa, where rheumatic heart disease remains endemic, wrote David A. Watkins, MD, MPH, of the University of Washington, Seattle, and his coinvestigators. “We estimate that 10 persons per 1,000 population living in South Asia and central sub-Saharan Africa and 15 persons per 1,000 population in Oceania were living with rheumatic heart disease in the year 2015,” they wrote. “Improvements in the measurement of the burden of rheumatic heart disease will assist in planning for its control and will help identify countries where further investments are needed.”
To better define the problem, Dr. Watkins and his associates analyzed epidemiologic studies of rheumatic heart disease from 1990 through 2015. They used the Cause of Death Ensemble model, which estimates mortality more reliably than older methods, and DisMod-MR (version 2.1), which sums epidemiologic data from multiple sources and corrects for gaps and inconsistencies (N Engl J Med. 2017;377:713-22).
Worldwide, about 319,400 individuals died of rheumatic heart disease in 2015, the researchers reported. Age-adjusted death rates fell by about 48% (95% confidence interval, 45%-51%), from 9.2 deaths per 100,000 population in 1990 to 4.8 deaths per 100,000 population in 2015. But this global trend masked striking regional disparities. In 1990, 77% of deaths from rheumatic heart disease occurred in endemic areas of Africa, South Asia, Oceania, and the Caribbean; by 2015, 82% of deaths occurred in endemic regions. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates and were the only regions where the 95% confidence intervals for 1990 and 2015 overlapped, the investigators noted.
In 2015, age-standardized death rates exceeded 10 deaths per 100,000 population in the Solomon Islands, Pakistan, Papua New Guinea, Kiribati, Vanuatu, Fiji, India, Federated States of Micronesia, Marshall Islands, Central African Republic, and Lesotho, they reported. Estimated fatalities were highest in India (119,100 deaths), China (72,600), and Pakistan (18,900). They estimated that in 2015, there were 33.2 million cases of rheumatic heart disease and 10.5 million associated disability-adjusted life-years globally.
The study excluded “borderline” or subclinical rheumatic heart disease, which is detected by echocardiography and whose management remains unclear. “Better data for low-income and middle-income countries are needed to guide policies for the control of rheumatic heart disease,” the investigators wrote. They recommended studying death certificate misclassifications, disease prevalence among adults, and longitudinal trends in nonfatal outcomes and excess mortality.
Funders of the study included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Globally, age-adjusted death rates fell by about 48% between 1990 and 2015. Oceania, South Asia, and central sub-Saharan Africa had the highest death rates in 2015, and were the only regions where the 95% confidence intervals overlapped with those for 1990.
Data source: A systematic review and analysis of morbidity and mortality data from 1990 through 2015.
Disclosures: Funders included the Bill and Melinda Gates Foundation and the Medtronic Foundation. Dr. Watkins disclosed grants from the Medtronic Foundation during the conduct of the study and grants from the Bill and Melinda Gates Foundation outside the submitted work.
Dermatologists have a role in managing GVHD
NEW YORK – Dermatologists have an important role to play in caring for patients with chronic graft versus host disease (GVHD), a condition whose cutaneous manifestations are many, stubborn, and often disabling.
Although a wide range of systemic therapies are available, topical and intralesional treatment with such agents as potent steroids and calcineurin inhibitors can also help with cutaneous manifestations of GVHD in some instances, said Kathryn Martires, MD, at the American Academy of Dermatology summer meeting. However, she noted, “there are no studies or series examining the use of topical steroids alone in these patients, partly speaking to the complexity of these patients and required other care, but partly also due to the lack of dermatologists’ involvement in the care of these patients on a wide scale.”
“The types of GVHD that are particularly amenable to high dose steroids are predominantly the epidermal types,” she said. These include ichthyotic and eczematous as well as lichen planus–like cutaneous GVHD. “We also use topical steroids frequently in the papulosquamous type, though this is a rare variant,” she added.
Topical steroids can be used for dermal skin changes of GVHD as well, including lichen sclerosus–like and focal morphea–like plaques, according to Dr. Martires of the department of dermatology at Stanford (Calif.) University. These lesions are often first seen in the skin folds of the neck.
Even for patients with more diffuse dermal sclerosis, topical steroids have a role in quieting specific areas where active flares are occurring, she noted. These flares can look like erythematous, scaly patches and are “particularly amenable” to spot treatment with topical steroids.
“Just like in vitiligo that’s not associated with GVHD, certainly, topical steroids have their role in treating vitiligo that’s associated with chronic GVHD,” Dr. Martires said. This scenario stands in contrast to the situation where a patient has postinflammatory hyperpigmentation, for example, further along in the course of epidermal GVHD. Steroids should be avoided in situations where there’s hyperpigmentation.
Topical steroids are not usually useful for chronic poikilodermatous GVHD, or, generally, when patients have little epidermal change and the GVHD-associated changes are mostly dermal or subcutaneous, she said.
“Intralesional steroids have their role” in GVHD, although this is another instance where there are no studies to back up their efficacy, and recommendations are based on consensus, Dr. Martires pointed out. Nodular sclerotic GVHD is a rare manifestation, with firm, keloid-like lesions. These can flatten with intralesional injections, said Dr. Martires.
Intralesional injections have also been described in the literature as a treatment for ulcerative oral GVHD, she noted. Other therapy options for oral mucosal GVHD are fluocinonide gel 0.05% or clobetasol gel 0.05%, with spot application to the lesions. When there’s more diffuse lichenoid GVHD of the mouth, dexamethasone or prednisolone oral rinses can also be used, but should be combined with nystatin to prevent thrush, she advised. Triamcinolone 0.1% can be used with topical benzocaine dental paste (Orabase).
Calcineurin inhibitors are another option for oral lesions. Patients generally have a good comfort level with starting topical calcineurin inhibitors, said Dr. Martires, because they’ve likely had exposure to the systemic formulation. Case series have reported improvement “primarily in lichenoid GVHD” with the adjunctive use of topical calcineurin inhibitors, she said. In the mouth, tacrolimus 0.1% can be put in dental paste for focal lesions, and cyclosporine and azathioprine oral solutions can also be used.
Dry mouth is common in GVHD. “Remember, in patients who have other skin symptoms like pruritus, to ask about oral sicca symptoms in order to avoid things that might exacerbate it, like antihistamines and [tricyclic antidepressants],” she added.
Genital mucosal GVHD can respond to topical steroids, with ointment as the preferred vehicle, said Dr. Martires, noting that clobetasol 0.05% ointment and fluocinolone 0.025% ointment are good options, and tacrolimus 0.1% ointment is a logical nonsteroidal topical choice for the genital mucosa.
“Intralesionals are also first-line therapy here,” and “may prevent progression and permanent scarring if initiated early,” she pointed out. However, these injections are quite painful, so “patients have to be quite motivated” to be on board with this line of therapy, she said, adding that numbing prior to injections can help with pain.
Genital discomfort in women may not all be GVHD-related. “Remember, in patients who have undergone several cycles of chemotherapy prior to transplant, that they often have been experiencing menopausal symptoms, sometimes for years, so estrogen cream can sometimes go a long way,” said Dr. Martires, adding, “Certainly, a reminder about lubrication during intercourse is appropriate.”
Also, she said, dermatologists can help patients understand how important it is to be vigilant in preserving skin integrity by, for example, keeping skin well moisturized, avoiding aggressive nail care, and wearing gloves for wet work.
Dr. Martires reported no relevant financial relationships.
[email protected]
On Twitter @karioakes
NEW YORK – Dermatologists have an important role to play in caring for patients with chronic graft versus host disease (GVHD), a condition whose cutaneous manifestations are many, stubborn, and often disabling.
Although a wide range of systemic therapies are available, topical and intralesional treatment with such agents as potent steroids and calcineurin inhibitors can also help with cutaneous manifestations of GVHD in some instances, said Kathryn Martires, MD, at the American Academy of Dermatology summer meeting. However, she noted, “there are no studies or series examining the use of topical steroids alone in these patients, partly speaking to the complexity of these patients and required other care, but partly also due to the lack of dermatologists’ involvement in the care of these patients on a wide scale.”
“The types of GVHD that are particularly amenable to high dose steroids are predominantly the epidermal types,” she said. These include ichthyotic and eczematous as well as lichen planus–like cutaneous GVHD. “We also use topical steroids frequently in the papulosquamous type, though this is a rare variant,” she added.
Topical steroids can be used for dermal skin changes of GVHD as well, including lichen sclerosus–like and focal morphea–like plaques, according to Dr. Martires of the department of dermatology at Stanford (Calif.) University. These lesions are often first seen in the skin folds of the neck.
Even for patients with more diffuse dermal sclerosis, topical steroids have a role in quieting specific areas where active flares are occurring, she noted. These flares can look like erythematous, scaly patches and are “particularly amenable” to spot treatment with topical steroids.
“Just like in vitiligo that’s not associated with GVHD, certainly, topical steroids have their role in treating vitiligo that’s associated with chronic GVHD,” Dr. Martires said. This scenario stands in contrast to the situation where a patient has postinflammatory hyperpigmentation, for example, further along in the course of epidermal GVHD. Steroids should be avoided in situations where there’s hyperpigmentation.
Topical steroids are not usually useful for chronic poikilodermatous GVHD, or, generally, when patients have little epidermal change and the GVHD-associated changes are mostly dermal or subcutaneous, she said.
“Intralesional steroids have their role” in GVHD, although this is another instance where there are no studies to back up their efficacy, and recommendations are based on consensus, Dr. Martires pointed out. Nodular sclerotic GVHD is a rare manifestation, with firm, keloid-like lesions. These can flatten with intralesional injections, said Dr. Martires.
Intralesional injections have also been described in the literature as a treatment for ulcerative oral GVHD, she noted. Other therapy options for oral mucosal GVHD are fluocinonide gel 0.05% or clobetasol gel 0.05%, with spot application to the lesions. When there’s more diffuse lichenoid GVHD of the mouth, dexamethasone or prednisolone oral rinses can also be used, but should be combined with nystatin to prevent thrush, she advised. Triamcinolone 0.1% can be used with topical benzocaine dental paste (Orabase).
Calcineurin inhibitors are another option for oral lesions. Patients generally have a good comfort level with starting topical calcineurin inhibitors, said Dr. Martires, because they’ve likely had exposure to the systemic formulation. Case series have reported improvement “primarily in lichenoid GVHD” with the adjunctive use of topical calcineurin inhibitors, she said. In the mouth, tacrolimus 0.1% can be put in dental paste for focal lesions, and cyclosporine and azathioprine oral solutions can also be used.
Dry mouth is common in GVHD. “Remember, in patients who have other skin symptoms like pruritus, to ask about oral sicca symptoms in order to avoid things that might exacerbate it, like antihistamines and [tricyclic antidepressants],” she added.
Genital mucosal GVHD can respond to topical steroids, with ointment as the preferred vehicle, said Dr. Martires, noting that clobetasol 0.05% ointment and fluocinolone 0.025% ointment are good options, and tacrolimus 0.1% ointment is a logical nonsteroidal topical choice for the genital mucosa.
“Intralesionals are also first-line therapy here,” and “may prevent progression and permanent scarring if initiated early,” she pointed out. However, these injections are quite painful, so “patients have to be quite motivated” to be on board with this line of therapy, she said, adding that numbing prior to injections can help with pain.
Genital discomfort in women may not all be GVHD-related. “Remember, in patients who have undergone several cycles of chemotherapy prior to transplant, that they often have been experiencing menopausal symptoms, sometimes for years, so estrogen cream can sometimes go a long way,” said Dr. Martires, adding, “Certainly, a reminder about lubrication during intercourse is appropriate.”
Also, she said, dermatologists can help patients understand how important it is to be vigilant in preserving skin integrity by, for example, keeping skin well moisturized, avoiding aggressive nail care, and wearing gloves for wet work.
Dr. Martires reported no relevant financial relationships.
[email protected]
On Twitter @karioakes
NEW YORK – Dermatologists have an important role to play in caring for patients with chronic graft versus host disease (GVHD), a condition whose cutaneous manifestations are many, stubborn, and often disabling.
Although a wide range of systemic therapies are available, topical and intralesional treatment with such agents as potent steroids and calcineurin inhibitors can also help with cutaneous manifestations of GVHD in some instances, said Kathryn Martires, MD, at the American Academy of Dermatology summer meeting. However, she noted, “there are no studies or series examining the use of topical steroids alone in these patients, partly speaking to the complexity of these patients and required other care, but partly also due to the lack of dermatologists’ involvement in the care of these patients on a wide scale.”
“The types of GVHD that are particularly amenable to high dose steroids are predominantly the epidermal types,” she said. These include ichthyotic and eczematous as well as lichen planus–like cutaneous GVHD. “We also use topical steroids frequently in the papulosquamous type, though this is a rare variant,” she added.
Topical steroids can be used for dermal skin changes of GVHD as well, including lichen sclerosus–like and focal morphea–like plaques, according to Dr. Martires of the department of dermatology at Stanford (Calif.) University. These lesions are often first seen in the skin folds of the neck.
Even for patients with more diffuse dermal sclerosis, topical steroids have a role in quieting specific areas where active flares are occurring, she noted. These flares can look like erythematous, scaly patches and are “particularly amenable” to spot treatment with topical steroids.
“Just like in vitiligo that’s not associated with GVHD, certainly, topical steroids have their role in treating vitiligo that’s associated with chronic GVHD,” Dr. Martires said. This scenario stands in contrast to the situation where a patient has postinflammatory hyperpigmentation, for example, further along in the course of epidermal GVHD. Steroids should be avoided in situations where there’s hyperpigmentation.
Topical steroids are not usually useful for chronic poikilodermatous GVHD, or, generally, when patients have little epidermal change and the GVHD-associated changes are mostly dermal or subcutaneous, she said.
“Intralesional steroids have their role” in GVHD, although this is another instance where there are no studies to back up their efficacy, and recommendations are based on consensus, Dr. Martires pointed out. Nodular sclerotic GVHD is a rare manifestation, with firm, keloid-like lesions. These can flatten with intralesional injections, said Dr. Martires.
Intralesional injections have also been described in the literature as a treatment for ulcerative oral GVHD, she noted. Other therapy options for oral mucosal GVHD are fluocinonide gel 0.05% or clobetasol gel 0.05%, with spot application to the lesions. When there’s more diffuse lichenoid GVHD of the mouth, dexamethasone or prednisolone oral rinses can also be used, but should be combined with nystatin to prevent thrush, she advised. Triamcinolone 0.1% can be used with topical benzocaine dental paste (Orabase).
Calcineurin inhibitors are another option for oral lesions. Patients generally have a good comfort level with starting topical calcineurin inhibitors, said Dr. Martires, because they’ve likely had exposure to the systemic formulation. Case series have reported improvement “primarily in lichenoid GVHD” with the adjunctive use of topical calcineurin inhibitors, she said. In the mouth, tacrolimus 0.1% can be put in dental paste for focal lesions, and cyclosporine and azathioprine oral solutions can also be used.
Dry mouth is common in GVHD. “Remember, in patients who have other skin symptoms like pruritus, to ask about oral sicca symptoms in order to avoid things that might exacerbate it, like antihistamines and [tricyclic antidepressants],” she added.
Genital mucosal GVHD can respond to topical steroids, with ointment as the preferred vehicle, said Dr. Martires, noting that clobetasol 0.05% ointment and fluocinolone 0.025% ointment are good options, and tacrolimus 0.1% ointment is a logical nonsteroidal topical choice for the genital mucosa.
“Intralesionals are also first-line therapy here,” and “may prevent progression and permanent scarring if initiated early,” she pointed out. However, these injections are quite painful, so “patients have to be quite motivated” to be on board with this line of therapy, she said, adding that numbing prior to injections can help with pain.
Genital discomfort in women may not all be GVHD-related. “Remember, in patients who have undergone several cycles of chemotherapy prior to transplant, that they often have been experiencing menopausal symptoms, sometimes for years, so estrogen cream can sometimes go a long way,” said Dr. Martires, adding, “Certainly, a reminder about lubrication during intercourse is appropriate.”
Also, she said, dermatologists can help patients understand how important it is to be vigilant in preserving skin integrity by, for example, keeping skin well moisturized, avoiding aggressive nail care, and wearing gloves for wet work.
Dr. Martires reported no relevant financial relationships.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING