User login
One in four ‘control’ mothers in NAS study tests positive for drug use
CHICAGO – A study that compares babies who develop neonatal abstinence syndrome (NAS) with a control group of healthy newborns found largely what researchers expected to see – lower gestational ages, lower birth weights, and substance use in 100% of the mothers in the affected group. It’s when they looked at the control group of healthy newborns and their mothers that they got a surprise.
“Something that was very alarming in our study is [that] one in four of the control mothers was also positive for illicit drugs. That is something we definitely didn’t expect,” said Pallavi Karunakaran MD, a pediatric resident at Children’s Hospital of Michigan in Detroit.

The investigators also looked at maternal characteristics in both groups, with data available on 198 mothers whose babies developed the syndrome and 490 controls. “Interestingly enough, we expected that the mothers of the NAS babies would have some kind of drug use, [but] a lot of the NAS cohort mothers tested positive for additional drugs, other than methadone, which is usually how babies end up developing neonatal abstinence syndrome,” Dr. Karunakaran said at the annual meeting of the American Academy of Pediatrics. Drug use in mothers of affected babies included opioids among 81% (including methadone for 70%); heroin, 42%; cocaine, 29%; marijuana, 26%; benzodiazepines, 18%; buprenorphine, 6%; and barbiturates, 2%.
Among the control group mothers, drug use included marijuana among 20.4%; opioids, 1.8%; cocaine, 1.2%; benzodiazepines, 0.6%; heroin, 0.2%; and barbiturates, 0.2%.
Mean gestational age for affected infants was 38 weeks versus 39 weeks in controls, a significant difference (P less than .001). Mean birth weight for affected infants was 2,930 grams versus 3,145 grams in controls (P = .018). The average length of hospital stay was 21 days for infants who developed NAS, compared with 3 days among control infants (P less than .001). Mean Apgar scores were not significantly different between groups.
“The next step in terms of taking care of our population – is we’re trying to see how we can catch those one in four control mothers who are having well babies because we want to make sure we’re also taking care of them while they’re pregnant,” Dr. Karunakaran said. “If they’re involved in drug use, we want to get them the right resources and the right doctors they may need.”
Dr. Karunakaran had no relevant financial disclosures.
CHICAGO – A study that compares babies who develop neonatal abstinence syndrome (NAS) with a control group of healthy newborns found largely what researchers expected to see – lower gestational ages, lower birth weights, and substance use in 100% of the mothers in the affected group. It’s when they looked at the control group of healthy newborns and their mothers that they got a surprise.
“Something that was very alarming in our study is [that] one in four of the control mothers was also positive for illicit drugs. That is something we definitely didn’t expect,” said Pallavi Karunakaran MD, a pediatric resident at Children’s Hospital of Michigan in Detroit.

The investigators also looked at maternal characteristics in both groups, with data available on 198 mothers whose babies developed the syndrome and 490 controls. “Interestingly enough, we expected that the mothers of the NAS babies would have some kind of drug use, [but] a lot of the NAS cohort mothers tested positive for additional drugs, other than methadone, which is usually how babies end up developing neonatal abstinence syndrome,” Dr. Karunakaran said at the annual meeting of the American Academy of Pediatrics. Drug use in mothers of affected babies included opioids among 81% (including methadone for 70%); heroin, 42%; cocaine, 29%; marijuana, 26%; benzodiazepines, 18%; buprenorphine, 6%; and barbiturates, 2%.
Among the control group mothers, drug use included marijuana among 20.4%; opioids, 1.8%; cocaine, 1.2%; benzodiazepines, 0.6%; heroin, 0.2%; and barbiturates, 0.2%.
Mean gestational age for affected infants was 38 weeks versus 39 weeks in controls, a significant difference (P less than .001). Mean birth weight for affected infants was 2,930 grams versus 3,145 grams in controls (P = .018). The average length of hospital stay was 21 days for infants who developed NAS, compared with 3 days among control infants (P less than .001). Mean Apgar scores were not significantly different between groups.
“The next step in terms of taking care of our population – is we’re trying to see how we can catch those one in four control mothers who are having well babies because we want to make sure we’re also taking care of them while they’re pregnant,” Dr. Karunakaran said. “If they’re involved in drug use, we want to get them the right resources and the right doctors they may need.”
Dr. Karunakaran had no relevant financial disclosures.
CHICAGO – A study that compares babies who develop neonatal abstinence syndrome (NAS) with a control group of healthy newborns found largely what researchers expected to see – lower gestational ages, lower birth weights, and substance use in 100% of the mothers in the affected group. It’s when they looked at the control group of healthy newborns and their mothers that they got a surprise.
“Something that was very alarming in our study is [that] one in four of the control mothers was also positive for illicit drugs. That is something we definitely didn’t expect,” said Pallavi Karunakaran MD, a pediatric resident at Children’s Hospital of Michigan in Detroit.

The investigators also looked at maternal characteristics in both groups, with data available on 198 mothers whose babies developed the syndrome and 490 controls. “Interestingly enough, we expected that the mothers of the NAS babies would have some kind of drug use, [but] a lot of the NAS cohort mothers tested positive for additional drugs, other than methadone, which is usually how babies end up developing neonatal abstinence syndrome,” Dr. Karunakaran said at the annual meeting of the American Academy of Pediatrics. Drug use in mothers of affected babies included opioids among 81% (including methadone for 70%); heroin, 42%; cocaine, 29%; marijuana, 26%; benzodiazepines, 18%; buprenorphine, 6%; and barbiturates, 2%.
Among the control group mothers, drug use included marijuana among 20.4%; opioids, 1.8%; cocaine, 1.2%; benzodiazepines, 0.6%; heroin, 0.2%; and barbiturates, 0.2%.
Mean gestational age for affected infants was 38 weeks versus 39 weeks in controls, a significant difference (P less than .001). Mean birth weight for affected infants was 2,930 grams versus 3,145 grams in controls (P = .018). The average length of hospital stay was 21 days for infants who developed NAS, compared with 3 days among control infants (P less than .001). Mean Apgar scores were not significantly different between groups.
“The next step in terms of taking care of our population – is we’re trying to see how we can catch those one in four control mothers who are having well babies because we want to make sure we’re also taking care of them while they’re pregnant,” Dr. Karunakaran said. “If they’re involved in drug use, we want to get them the right resources and the right doctors they may need.”
Dr. Karunakaran had no relevant financial disclosures.
AT AAP 2017
Key clinical point: Investigators studied mothers whose babies developed NAS and were surprised when almost 25% of women in the control group also tested positive for illicit drug use.
Major finding: Among the control mothers, drug use included marijuana 20.4%, opioids 1.8%, cocaine 1.2%, benzodiazepines 0.6%, heroin 0.2%, and barbiturates 0.2%.
Data source: Chart review of 222 infants who developed neonatal abstinence syndrome, compared with 490 controls.
Disclosures: Dr. Karunakaran had no relevant financial disclosures.
Patients with severe obesity may have difficulty grading pain
Patients with severe obesity may have greater difficulty perceiving and grading pain than do average-weight patients. Those with severe obesity display hypoalgesia when exposed to random noxious stimuli, according to Bart Torensma and his associates.
In the study, 43 patients with severe obesity and 38 controls were enrolled, of whom 41 and 35 participated. Results found the penalty scores, a tool developed to assess perception and grading of pain, differed significantly with higher penalty scores in patients with obesity for both nociceptive assays (heat pain; P = .01, electrical pain; P = .03). “We observed that participants with severe obesity had higher electrical pain threshold and tolerance values, compared with control patients, an indication of lower sensitivity to electrical pain,” the investigators wrote.
In patients with obesity the penalty scores ranged from 1.5 to 13.5 (heat pain) and from 1.0 to 12.5 (electrical pain). But penalty score distribution differed significantly between study groups for electrical pain, with penalty scores greater than 3.5 in 47.3% in patients with obesity versus 22.9% of controls (P = .049). For heat pain, 46.2% of patients with obesity versus 28.6% of control participants had penalty scores greater than 3.5 (P = .15).
“Compared with patients without obesity, patients with obesity displayed hypoalgesia to noxious electrical stimuli together with difficulty in grading experimental noxious thermal and electrical stimuli in between pain threshold and tolerance,” researchers concluded. “We argue that the latter may have a significant effect on pain treatment and consequently needs to be taken into account when treating patients with obesity for acute or chronic pain.”
Read the full study in Surgery for Obesity and Related Diseases (doi: 10.1016/j.soard.2017.01.015).
Patients with severe obesity may have greater difficulty perceiving and grading pain than do average-weight patients. Those with severe obesity display hypoalgesia when exposed to random noxious stimuli, according to Bart Torensma and his associates.
In the study, 43 patients with severe obesity and 38 controls were enrolled, of whom 41 and 35 participated. Results found the penalty scores, a tool developed to assess perception and grading of pain, differed significantly with higher penalty scores in patients with obesity for both nociceptive assays (heat pain; P = .01, electrical pain; P = .03). “We observed that participants with severe obesity had higher electrical pain threshold and tolerance values, compared with control patients, an indication of lower sensitivity to electrical pain,” the investigators wrote.
In patients with obesity the penalty scores ranged from 1.5 to 13.5 (heat pain) and from 1.0 to 12.5 (electrical pain). But penalty score distribution differed significantly between study groups for electrical pain, with penalty scores greater than 3.5 in 47.3% in patients with obesity versus 22.9% of controls (P = .049). For heat pain, 46.2% of patients with obesity versus 28.6% of control participants had penalty scores greater than 3.5 (P = .15).
“Compared with patients without obesity, patients with obesity displayed hypoalgesia to noxious electrical stimuli together with difficulty in grading experimental noxious thermal and electrical stimuli in between pain threshold and tolerance,” researchers concluded. “We argue that the latter may have a significant effect on pain treatment and consequently needs to be taken into account when treating patients with obesity for acute or chronic pain.”
Read the full study in Surgery for Obesity and Related Diseases (doi: 10.1016/j.soard.2017.01.015).
Patients with severe obesity may have greater difficulty perceiving and grading pain than do average-weight patients. Those with severe obesity display hypoalgesia when exposed to random noxious stimuli, according to Bart Torensma and his associates.
In the study, 43 patients with severe obesity and 38 controls were enrolled, of whom 41 and 35 participated. Results found the penalty scores, a tool developed to assess perception and grading of pain, differed significantly with higher penalty scores in patients with obesity for both nociceptive assays (heat pain; P = .01, electrical pain; P = .03). “We observed that participants with severe obesity had higher electrical pain threshold and tolerance values, compared with control patients, an indication of lower sensitivity to electrical pain,” the investigators wrote.
In patients with obesity the penalty scores ranged from 1.5 to 13.5 (heat pain) and from 1.0 to 12.5 (electrical pain). But penalty score distribution differed significantly between study groups for electrical pain, with penalty scores greater than 3.5 in 47.3% in patients with obesity versus 22.9% of controls (P = .049). For heat pain, 46.2% of patients with obesity versus 28.6% of control participants had penalty scores greater than 3.5 (P = .15).
“Compared with patients without obesity, patients with obesity displayed hypoalgesia to noxious electrical stimuli together with difficulty in grading experimental noxious thermal and electrical stimuli in between pain threshold and tolerance,” researchers concluded. “We argue that the latter may have a significant effect on pain treatment and consequently needs to be taken into account when treating patients with obesity for acute or chronic pain.”
Read the full study in Surgery for Obesity and Related Diseases (doi: 10.1016/j.soard.2017.01.015).
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Teriparatide reduces fractures over risedronate in all subgroups with osteoporosis
DENVER – The anabolic agent teriparatide reduced the risk of clinical vertebral fractures in women with osteoporosis when compared with the antiresorptive agent risedronate, and the results held up across groups of women with varying fracture histories in a post hoc analysis of data from the VERO trial.
VERO, which pitted teriparatide (Forteo) against risedronate in women with at least two moderate or one severe vertebral fracture, as well as a bone mineral density (BMD) T-score of -1.5 or less, was the first study to compare an anabolic treatment to antiresorptive therapy using fractures as an endpoint, according to Astrid Fahrleitner-Pammer, MD, of the departments of internal medicine, endocrinology, and diabetology at Medical University of Graz (Austria), who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
“I think it’s a great thing to show that [teriparatide] is superior, and the point is we always talk about who is the right patient for an anabolic treatment. There was no difference between the subgroup categories,” Dr. Fahrleitner-Pammer said.
There were no statistically significant between-group differences based on the number of vertebral fractures, severity of vertebral fractures, prior nonvertebral fracture, glucocorticoid use, prior use of osteoporosis drugs, BMD T-score, age, recent bisphosphonate use, or recent clinical vertebral fractures.
“In patients with really established osteoporosis, mainly osteoporosis with two moderate fractures or one severe fracture, they all profit from an anabolic treatment,” Dr. Fahrleitner-Pammer said.
The work underscores the utility of anabolic agents. “I don’t think it’s a question of if, but when to use an anabolic therapy,” Dr. Fahrleitner-Pammer said.
The therapies are limited to 24 months of lifetime exposure because of their warning labels, and patient characteristics should help physicians to decide when to use them. “I wouldn’t start a 50-year-old patient with low BMD without any prevalent fractures. I would try to conserve the bone with an antiresorptive therapy, and if this isn’t enough, the patient will sustain a fracture, and sooner or later then I think it’s a time to switch to an anabolic therapy. If we have an old lady with already prevalent vertebral fractures, the best way is to go for the anabolic therapy first and then conserve the newly formed bone with an antiresorptive therapy,” Dr. Fahrleitner-Pammer said.
The study was funded by Eli Lilly. Dr. Fahrleitner-Pammer has received research funding and unrestricted grants from Eli Lilly and several other pharmaceutical companies and has also been a speaker for Eli Lilly and nine other pharmaceutical companies.
DENVER – The anabolic agent teriparatide reduced the risk of clinical vertebral fractures in women with osteoporosis when compared with the antiresorptive agent risedronate, and the results held up across groups of women with varying fracture histories in a post hoc analysis of data from the VERO trial.
VERO, which pitted teriparatide (Forteo) against risedronate in women with at least two moderate or one severe vertebral fracture, as well as a bone mineral density (BMD) T-score of -1.5 or less, was the first study to compare an anabolic treatment to antiresorptive therapy using fractures as an endpoint, according to Astrid Fahrleitner-Pammer, MD, of the departments of internal medicine, endocrinology, and diabetology at Medical University of Graz (Austria), who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
“I think it’s a great thing to show that [teriparatide] is superior, and the point is we always talk about who is the right patient for an anabolic treatment. There was no difference between the subgroup categories,” Dr. Fahrleitner-Pammer said.
There were no statistically significant between-group differences based on the number of vertebral fractures, severity of vertebral fractures, prior nonvertebral fracture, glucocorticoid use, prior use of osteoporosis drugs, BMD T-score, age, recent bisphosphonate use, or recent clinical vertebral fractures.
“In patients with really established osteoporosis, mainly osteoporosis with two moderate fractures or one severe fracture, they all profit from an anabolic treatment,” Dr. Fahrleitner-Pammer said.
The work underscores the utility of anabolic agents. “I don’t think it’s a question of if, but when to use an anabolic therapy,” Dr. Fahrleitner-Pammer said.
The therapies are limited to 24 months of lifetime exposure because of their warning labels, and patient characteristics should help physicians to decide when to use them. “I wouldn’t start a 50-year-old patient with low BMD without any prevalent fractures. I would try to conserve the bone with an antiresorptive therapy, and if this isn’t enough, the patient will sustain a fracture, and sooner or later then I think it’s a time to switch to an anabolic therapy. If we have an old lady with already prevalent vertebral fractures, the best way is to go for the anabolic therapy first and then conserve the newly formed bone with an antiresorptive therapy,” Dr. Fahrleitner-Pammer said.
The study was funded by Eli Lilly. Dr. Fahrleitner-Pammer has received research funding and unrestricted grants from Eli Lilly and several other pharmaceutical companies and has also been a speaker for Eli Lilly and nine other pharmaceutical companies.
DENVER – The anabolic agent teriparatide reduced the risk of clinical vertebral fractures in women with osteoporosis when compared with the antiresorptive agent risedronate, and the results held up across groups of women with varying fracture histories in a post hoc analysis of data from the VERO trial.
VERO, which pitted teriparatide (Forteo) against risedronate in women with at least two moderate or one severe vertebral fracture, as well as a bone mineral density (BMD) T-score of -1.5 or less, was the first study to compare an anabolic treatment to antiresorptive therapy using fractures as an endpoint, according to Astrid Fahrleitner-Pammer, MD, of the departments of internal medicine, endocrinology, and diabetology at Medical University of Graz (Austria), who presented the study at the annual meeting of the American Society for Bone and Mineral Research.
“I think it’s a great thing to show that [teriparatide] is superior, and the point is we always talk about who is the right patient for an anabolic treatment. There was no difference between the subgroup categories,” Dr. Fahrleitner-Pammer said.
There were no statistically significant between-group differences based on the number of vertebral fractures, severity of vertebral fractures, prior nonvertebral fracture, glucocorticoid use, prior use of osteoporosis drugs, BMD T-score, age, recent bisphosphonate use, or recent clinical vertebral fractures.
“In patients with really established osteoporosis, mainly osteoporosis with two moderate fractures or one severe fracture, they all profit from an anabolic treatment,” Dr. Fahrleitner-Pammer said.
The work underscores the utility of anabolic agents. “I don’t think it’s a question of if, but when to use an anabolic therapy,” Dr. Fahrleitner-Pammer said.
The therapies are limited to 24 months of lifetime exposure because of their warning labels, and patient characteristics should help physicians to decide when to use them. “I wouldn’t start a 50-year-old patient with low BMD without any prevalent fractures. I would try to conserve the bone with an antiresorptive therapy, and if this isn’t enough, the patient will sustain a fracture, and sooner or later then I think it’s a time to switch to an anabolic therapy. If we have an old lady with already prevalent vertebral fractures, the best way is to go for the anabolic therapy first and then conserve the newly formed bone with an antiresorptive therapy,” Dr. Fahrleitner-Pammer said.
The study was funded by Eli Lilly. Dr. Fahrleitner-Pammer has received research funding and unrestricted grants from Eli Lilly and several other pharmaceutical companies and has also been a speaker for Eli Lilly and nine other pharmaceutical companies.
AT ASBMR
Key clinical point:
Major finding: There was no statistically significant difference in fracture risk reduction across any subgroups.
Data source: Post hoc analysis of the randomized, controlled VERO trial (n = 1,360).
Disclosures: The study was funded by Eli Lilly. Dr. Fahrleitner-Pammer has received research funding and unrestricted grants from Eli Lilly and several other pharmaceutical companies and has also been a speaker for Eli Lilly and nine other pharmaceutical companies.
ACS releases quality and safety manual
The American College of Surgeons (ACS) is pleased to announce that the Optimal Resources for Surgical Quality and Safety manual is now available for purchase. This manual is intended to serve as a trusted resource for surgical leaders seeking to improve patient care in their institutions, departments, and practices. It introduces key concepts in quality, safety, and reliability and explores the essential elements that all hospitals should have in place to ensure the delivery of patient-centered care.
Specific topics covered include the following: the domains and phases of surgical care, peer and case review, responsibilities of the surgical quality officer, institutional infrastructure, privileging and credentialing, high reliability, applications to the unique surgical disciplines, data analytics, clinical practice guidelines, quality collaboratives, and education and training. The manual also includes a look at some of the “soft skills” that influence quality and safety in health care, as well as the individual surgeon’s responsibility to patients, colleagues, and the next generation of surgeons.
Optimal Resources for Surgical Quality and Safety is available on the ACS website at facs.org/quality-programs/about/optimal-resources-manual for $44.95 (includes shipping) for single copies (up to a quantity of nine) or $39.95 (includes shipping) per copy for 10 copies or more.
The American College of Surgeons (ACS) is pleased to announce that the Optimal Resources for Surgical Quality and Safety manual is now available for purchase. This manual is intended to serve as a trusted resource for surgical leaders seeking to improve patient care in their institutions, departments, and practices. It introduces key concepts in quality, safety, and reliability and explores the essential elements that all hospitals should have in place to ensure the delivery of patient-centered care.
Specific topics covered include the following: the domains and phases of surgical care, peer and case review, responsibilities of the surgical quality officer, institutional infrastructure, privileging and credentialing, high reliability, applications to the unique surgical disciplines, data analytics, clinical practice guidelines, quality collaboratives, and education and training. The manual also includes a look at some of the “soft skills” that influence quality and safety in health care, as well as the individual surgeon’s responsibility to patients, colleagues, and the next generation of surgeons.
Optimal Resources for Surgical Quality and Safety is available on the ACS website at facs.org/quality-programs/about/optimal-resources-manual for $44.95 (includes shipping) for single copies (up to a quantity of nine) or $39.95 (includes shipping) per copy for 10 copies or more.
The American College of Surgeons (ACS) is pleased to announce that the Optimal Resources for Surgical Quality and Safety manual is now available for purchase. This manual is intended to serve as a trusted resource for surgical leaders seeking to improve patient care in their institutions, departments, and practices. It introduces key concepts in quality, safety, and reliability and explores the essential elements that all hospitals should have in place to ensure the delivery of patient-centered care.
Specific topics covered include the following: the domains and phases of surgical care, peer and case review, responsibilities of the surgical quality officer, institutional infrastructure, privileging and credentialing, high reliability, applications to the unique surgical disciplines, data analytics, clinical practice guidelines, quality collaboratives, and education and training. The manual also includes a look at some of the “soft skills” that influence quality and safety in health care, as well as the individual surgeon’s responsibility to patients, colleagues, and the next generation of surgeons.
Optimal Resources for Surgical Quality and Safety is available on the ACS website at facs.org/quality-programs/about/optimal-resources-manual for $44.95 (includes shipping) for single copies (up to a quantity of nine) or $39.95 (includes shipping) per copy for 10 copies or more.
A call for psychiatrists with clozapine expertise in schizophrenia
The CURESZ Foundation was founded in 2016 to bring hope to people suffering from schizophrenia and those who love and care for them. CURESZ was established by Bethany Yeiser and her psychiatrist, Henry Nasrallah, MD, and was inspired by Bethany's complete recovery from schizophrenia after 4 years of delusions, hallucinations, homelessness, and disability. Bethany returned to her normal life and graduated from college with honors, thanks to clozapine, which cured her symptoms when several other medications did not work (for more of Bethany’s story, see From the Editor, Current Psychiatry. October 2014, p. 21,24-25).
One of the major initiatives of CURESZ is to promote the use of clozapine, which is vastly underused despite the fact that it is the only medication approved by the FDA for schizophrenia that fails to improve with other antipsychotics, and for schizophrenia patients with a history of suicidality. It is estimated that of the 2.5 million persons living with schizophrenia in the United States, about 500,000 have not had a chance to receive clozapine to possibly recover and overcome their vocational and social disability. To increase the chance that a patient suffering from treatment-resistant schizophrenia can receive a trial of clozapine, CURESZ is assembling a panel of clozapine experts around the United States to serve as a referral base.
This is a call for readers of Current Psychiatry who are prescribing clozapine to their patients and who practice in settings that can accommodate additional patients seeking clozapine treatment. We hope to get up to 100 experts to join this national panel, which we are calling CLOSZE (Clozapine Schizophrenia Experts). The mission is to “close” the door on disability among persons living with schizophrenia. Jonathan Meyer, MD, Deputy Editor of Current Psychiatry, a member of the Board of Trustees of the CURESZ Foundation, and a national clozapine expert, will serve as the Chair of the CLOSZE Panel.
If you would like to join CLOSZE, please go to https://curesz.org/closze-panel and enter your name, email, work address, and office phone number. We will later organize the list by state and city so that patients and families around the country can contact the nearest expert to get an evaluation for a possible clinical trial.
Thank you and we look forward to working with the Clozapine Experts who say “YESZ” to joining the CLOSZE Panel.
The CURESZ Foundation was founded in 2016 to bring hope to people suffering from schizophrenia and those who love and care for them. CURESZ was established by Bethany Yeiser and her psychiatrist, Henry Nasrallah, MD, and was inspired by Bethany's complete recovery from schizophrenia after 4 years of delusions, hallucinations, homelessness, and disability. Bethany returned to her normal life and graduated from college with honors, thanks to clozapine, which cured her symptoms when several other medications did not work (for more of Bethany’s story, see From the Editor, Current Psychiatry. October 2014, p. 21,24-25).
One of the major initiatives of CURESZ is to promote the use of clozapine, which is vastly underused despite the fact that it is the only medication approved by the FDA for schizophrenia that fails to improve with other antipsychotics, and for schizophrenia patients with a history of suicidality. It is estimated that of the 2.5 million persons living with schizophrenia in the United States, about 500,000 have not had a chance to receive clozapine to possibly recover and overcome their vocational and social disability. To increase the chance that a patient suffering from treatment-resistant schizophrenia can receive a trial of clozapine, CURESZ is assembling a panel of clozapine experts around the United States to serve as a referral base.
This is a call for readers of Current Psychiatry who are prescribing clozapine to their patients and who practice in settings that can accommodate additional patients seeking clozapine treatment. We hope to get up to 100 experts to join this national panel, which we are calling CLOSZE (Clozapine Schizophrenia Experts). The mission is to “close” the door on disability among persons living with schizophrenia. Jonathan Meyer, MD, Deputy Editor of Current Psychiatry, a member of the Board of Trustees of the CURESZ Foundation, and a national clozapine expert, will serve as the Chair of the CLOSZE Panel.
If you would like to join CLOSZE, please go to https://curesz.org/closze-panel and enter your name, email, work address, and office phone number. We will later organize the list by state and city so that patients and families around the country can contact the nearest expert to get an evaluation for a possible clinical trial.
Thank you and we look forward to working with the Clozapine Experts who say “YESZ” to joining the CLOSZE Panel.
The CURESZ Foundation was founded in 2016 to bring hope to people suffering from schizophrenia and those who love and care for them. CURESZ was established by Bethany Yeiser and her psychiatrist, Henry Nasrallah, MD, and was inspired by Bethany's complete recovery from schizophrenia after 4 years of delusions, hallucinations, homelessness, and disability. Bethany returned to her normal life and graduated from college with honors, thanks to clozapine, which cured her symptoms when several other medications did not work (for more of Bethany’s story, see From the Editor, Current Psychiatry. October 2014, p. 21,24-25).
One of the major initiatives of CURESZ is to promote the use of clozapine, which is vastly underused despite the fact that it is the only medication approved by the FDA for schizophrenia that fails to improve with other antipsychotics, and for schizophrenia patients with a history of suicidality. It is estimated that of the 2.5 million persons living with schizophrenia in the United States, about 500,000 have not had a chance to receive clozapine to possibly recover and overcome their vocational and social disability. To increase the chance that a patient suffering from treatment-resistant schizophrenia can receive a trial of clozapine, CURESZ is assembling a panel of clozapine experts around the United States to serve as a referral base.
This is a call for readers of Current Psychiatry who are prescribing clozapine to their patients and who practice in settings that can accommodate additional patients seeking clozapine treatment. We hope to get up to 100 experts to join this national panel, which we are calling CLOSZE (Clozapine Schizophrenia Experts). The mission is to “close” the door on disability among persons living with schizophrenia. Jonathan Meyer, MD, Deputy Editor of Current Psychiatry, a member of the Board of Trustees of the CURESZ Foundation, and a national clozapine expert, will serve as the Chair of the CLOSZE Panel.
If you would like to join CLOSZE, please go to https://curesz.org/closze-panel and enter your name, email, work address, and office phone number. We will later organize the list by state and city so that patients and families around the country can contact the nearest expert to get an evaluation for a possible clinical trial.
Thank you and we look forward to working with the Clozapine Experts who say “YESZ” to joining the CLOSZE Panel.
Reverse Shoulder Arthroplasty and Latissimus Dorsi Tendon Transfer
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
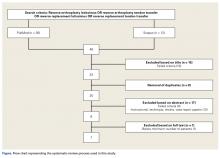
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
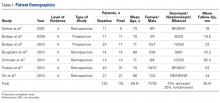
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
FDA warns of risks of excessive dosing of obeticholic acid
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
Motesanib flops again in lung cancer
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Belying a previous subgroup analysis, motesanib, an investigational oral vascular endothelial growth factor inhibitor, did not significantly improve progression-free survival when added to paclitaxel/carboplatin in East Asian patients with advanced nonsquamous non–small-cell lung cancer.
Major finding: After a median follow-up of 10 months, median PFS was 6.1 months among motesanib recipients and 5.6 months in the placebo group (hazard ratio, 0.81; P = .08).
Data source: A double-blind, phase III trial of 401 patients.
Disclosures: Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
Electronic Medical Record: Friend or Foe?
The Resident Viewpoint:
As a recent cardiothoracic surgery residency graduate, and having trained at multiple hospitals (those with electronic medical records and those with paper medical records), I believe I have a unique perspective on electronic medical records (EMRs). With our tech-savvy culture, it is no surprise that the medical record followed suit, evolving from a paper to electronic system. It is important to note that mandates were initiated years prior, which tie electronic medical record use to Medicare and Medicaid reimbursement. Much like the old adage, “bigger is not always better,” I have found myself questioning: “Is electronic better?” This topic has been much of a debate at my training institution as well, and it deserves more attention. As such, below are outlined what I believe to be the top three positives and negatives of the EMR. Maybe this will help settle the debate: Is the EMR our friend or foe?
Votes for “Friend”:
Portability
Centralized data
Having easily accessible, legible data is vital in caring for our patient population. You never have to guess what a providers are trying to write in their notes in the EMR (no hand-written notes). Most EMRs allow laboratory data, imaging, and notes to be viewed using one platform. Capabilities such as trends of vitals and laboratory values are useful to providers as well. As noted above, providers no longer have to physically gather laboratory slips or imaging films. Having centralized data for each patient saves on a provider’s time.
Standardization of orders
This has to be one of the greatest attributes of the EMR, especially when considering a teaching institution where trainees are constantly learning. Whether a provider orders Tylenol, a thoracic angiogram, echocardiogram, or a diet for a patient, the order is standardized. With medication orders, the usual dose is provided, along with other important information (renal dosing suggestions, etc.). Pharmacists and nursing staff do not have to worry about the legibility when reading and performing medication orders, for example.
Votes for “Foe”:
Cumbersome
There is no question that there is a considerable amount of time that adds up when logging into the EMR, entering orders, and writing notes. That time does not include any restarts or rebooting that can occur, not to mention the various “warnings” that pop up during each of the processes (e.g. creatinine level for all contrasted imaging studies or patient allergies). Depending on the type of note a provider writes (free text versus standardized format) a note can seem to require endless “clicks” to get to the end. Sometimes you find yourself wishing to just have a pen and paper.
Reliance on technology
When data gathering and documentation is centered on a computer, focus often shifts from the patient’s bedside to a desk. Although “computers on wheels” and computer stations positioned throughout the intensive care unit and ward have helped move providers closer to the bedside, a significant amount of time is still tied to the computer itself. Further, review of patient information on a computer is absolutely no substitute to bedside evaluation of a patient, especially in our line of work.
Lack of single EMR
I have lost track of the number of times I have been frustrated by this fact. At tertiary care centers, we often receive patients that had initial treatment or surgery at another institution. Full detail and information from those prior visits is usually not available. As a result, many tests (laboratory and imaging) have to be repeated. As stewards of health care cost, we do no justice by simply repeating each test. Unfortunately, given the “business” aspects of EMRs, a single EMR, which all providers can access, is not in our near future. This is unfortunate, as I strongly feel it would improve communication among providers across cities/states, and reduce health care costs (one example being the elimination of repeating tests).
As one can see, EMR offers a great deal but still has serious shortcomings. In my mind, the jury is still out. Hopefully as we refine the current models of EMR we will get closer to the ideal EMR: one that continues to be portable, can house centralized data, and allows for standardization of orders but at the same time offers efficient use, thereby decreasing the time tied to a computer, and is accessible to all providers.
Dr. Eilers is a general thoracic surgeon at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
The Attending Viewpoint:
Some of us embraced the idea of the EMR while others resisted until failure to use one led to financial penalty. Even so, it is absolutely clear that EMRs are here to stay. As is true of all technology, there are pros and cons to their deployment.
Pro EMR
Notes can be read. We have all suffered the indignity of trying to read illegible handwriting to guess what a consultant has recommended, or as the consultant trying to understand the question you are being asked. Even more than handwriting issues, with the paper chart there was always the challenge of finding the chart or the note walking around in a resident’s pocket awaiting rounds with the attending. With the EMR, what is written is legible and it can be found/reviewed remotely before going in search of the patient. This has the potential to save time and offers the consultant the advantage of knowing about the patient prior to the visit. In the academic setting, the EMR affords the attending opportunity to review and amend a resident’s note for accuracy that sometimes leads to a “teachable moment.”
Communication among providers can be shared more efficiently. To the extent that the patient’s providers access the same record this is very useful. On completion of any procedure or note, the outcome can be promptly sent electronically or by fax to other providers with the need to know. This can really optimize health care in many ways.
Con EMR
The worst thing about the EMR is the separation of patient from provider at all levels! In the “old days” we were all at the patient’s bedside. Today I often find decisions made based on limited data in the record without benefit of seeing the patient. Failing to observe whether the patient is breathing comfortably, feel whether the feet are warm, or notice whether the pressure transducers are appropriately placed in relation to the patient takes away the real art of medicine. Worse, decisions are made and interventions selected that may be quite inappropriate. It seems that nurses spend more time in the hallway on their workstations clicking away to satisfy documentation demands than spending time assessing and knowing their patients. The patient becomes “Room 920” not “Mr. Smith.” I see this as a real tragedy of our reliance on electronic media.
Data can be reviewed remotely sometimes. This is indeed a two-edged sword in that it separates us from our patients too easily. The ultimate time saver of the computer too often becomes a time sink with the need to negotiate multiple securities to access the EMR at all. Also, we must often seek information in more than one source to review relevant data from the in- or outpatient environment, from a referring doctor in another system, or to review an actual image rather than just the report. It becomes an expensive endeavor when we need additional staff to track down data, images, and other records.
Overall, I am very happy to be practicing with the great technology we have today. I am excited to watch as all these technologies evolve to make better our health care delivery. In reality, the EMR is not our enemy. It is a friend with a bad temper.
Dr. Carpenter is an adult cardiac surgeon and program director, Thoracic Surgery Residency, at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
The Resident Viewpoint:
As a recent cardiothoracic surgery residency graduate, and having trained at multiple hospitals (those with electronic medical records and those with paper medical records), I believe I have a unique perspective on electronic medical records (EMRs). With our tech-savvy culture, it is no surprise that the medical record followed suit, evolving from a paper to electronic system. It is important to note that mandates were initiated years prior, which tie electronic medical record use to Medicare and Medicaid reimbursement. Much like the old adage, “bigger is not always better,” I have found myself questioning: “Is electronic better?” This topic has been much of a debate at my training institution as well, and it deserves more attention. As such, below are outlined what I believe to be the top three positives and negatives of the EMR. Maybe this will help settle the debate: Is the EMR our friend or foe?
Votes for “Friend”:
Portability
Centralized data
Having easily accessible, legible data is vital in caring for our patient population. You never have to guess what a providers are trying to write in their notes in the EMR (no hand-written notes). Most EMRs allow laboratory data, imaging, and notes to be viewed using one platform. Capabilities such as trends of vitals and laboratory values are useful to providers as well. As noted above, providers no longer have to physically gather laboratory slips or imaging films. Having centralized data for each patient saves on a provider’s time.
Standardization of orders
This has to be one of the greatest attributes of the EMR, especially when considering a teaching institution where trainees are constantly learning. Whether a provider orders Tylenol, a thoracic angiogram, echocardiogram, or a diet for a patient, the order is standardized. With medication orders, the usual dose is provided, along with other important information (renal dosing suggestions, etc.). Pharmacists and nursing staff do not have to worry about the legibility when reading and performing medication orders, for example.
Votes for “Foe”:
Cumbersome
There is no question that there is a considerable amount of time that adds up when logging into the EMR, entering orders, and writing notes. That time does not include any restarts or rebooting that can occur, not to mention the various “warnings” that pop up during each of the processes (e.g. creatinine level for all contrasted imaging studies or patient allergies). Depending on the type of note a provider writes (free text versus standardized format) a note can seem to require endless “clicks” to get to the end. Sometimes you find yourself wishing to just have a pen and paper.
Reliance on technology
When data gathering and documentation is centered on a computer, focus often shifts from the patient’s bedside to a desk. Although “computers on wheels” and computer stations positioned throughout the intensive care unit and ward have helped move providers closer to the bedside, a significant amount of time is still tied to the computer itself. Further, review of patient information on a computer is absolutely no substitute to bedside evaluation of a patient, especially in our line of work.
Lack of single EMR
I have lost track of the number of times I have been frustrated by this fact. At tertiary care centers, we often receive patients that had initial treatment or surgery at another institution. Full detail and information from those prior visits is usually not available. As a result, many tests (laboratory and imaging) have to be repeated. As stewards of health care cost, we do no justice by simply repeating each test. Unfortunately, given the “business” aspects of EMRs, a single EMR, which all providers can access, is not in our near future. This is unfortunate, as I strongly feel it would improve communication among providers across cities/states, and reduce health care costs (one example being the elimination of repeating tests).
As one can see, EMR offers a great deal but still has serious shortcomings. In my mind, the jury is still out. Hopefully as we refine the current models of EMR we will get closer to the ideal EMR: one that continues to be portable, can house centralized data, and allows for standardization of orders but at the same time offers efficient use, thereby decreasing the time tied to a computer, and is accessible to all providers.
Dr. Eilers is a general thoracic surgeon at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
The Attending Viewpoint:
Some of us embraced the idea of the EMR while others resisted until failure to use one led to financial penalty. Even so, it is absolutely clear that EMRs are here to stay. As is true of all technology, there are pros and cons to their deployment.
Pro EMR
Notes can be read. We have all suffered the indignity of trying to read illegible handwriting to guess what a consultant has recommended, or as the consultant trying to understand the question you are being asked. Even more than handwriting issues, with the paper chart there was always the challenge of finding the chart or the note walking around in a resident’s pocket awaiting rounds with the attending. With the EMR, what is written is legible and it can be found/reviewed remotely before going in search of the patient. This has the potential to save time and offers the consultant the advantage of knowing about the patient prior to the visit. In the academic setting, the EMR affords the attending opportunity to review and amend a resident’s note for accuracy that sometimes leads to a “teachable moment.”
Communication among providers can be shared more efficiently. To the extent that the patient’s providers access the same record this is very useful. On completion of any procedure or note, the outcome can be promptly sent electronically or by fax to other providers with the need to know. This can really optimize health care in many ways.
Con EMR
The worst thing about the EMR is the separation of patient from provider at all levels! In the “old days” we were all at the patient’s bedside. Today I often find decisions made based on limited data in the record without benefit of seeing the patient. Failing to observe whether the patient is breathing comfortably, feel whether the feet are warm, or notice whether the pressure transducers are appropriately placed in relation to the patient takes away the real art of medicine. Worse, decisions are made and interventions selected that may be quite inappropriate. It seems that nurses spend more time in the hallway on their workstations clicking away to satisfy documentation demands than spending time assessing and knowing their patients. The patient becomes “Room 920” not “Mr. Smith.” I see this as a real tragedy of our reliance on electronic media.
Data can be reviewed remotely sometimes. This is indeed a two-edged sword in that it separates us from our patients too easily. The ultimate time saver of the computer too often becomes a time sink with the need to negotiate multiple securities to access the EMR at all. Also, we must often seek information in more than one source to review relevant data from the in- or outpatient environment, from a referring doctor in another system, or to review an actual image rather than just the report. It becomes an expensive endeavor when we need additional staff to track down data, images, and other records.
Overall, I am very happy to be practicing with the great technology we have today. I am excited to watch as all these technologies evolve to make better our health care delivery. In reality, the EMR is not our enemy. It is a friend with a bad temper.
Dr. Carpenter is an adult cardiac surgeon and program director, Thoracic Surgery Residency, at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
The Resident Viewpoint:
As a recent cardiothoracic surgery residency graduate, and having trained at multiple hospitals (those with electronic medical records and those with paper medical records), I believe I have a unique perspective on electronic medical records (EMRs). With our tech-savvy culture, it is no surprise that the medical record followed suit, evolving from a paper to electronic system. It is important to note that mandates were initiated years prior, which tie electronic medical record use to Medicare and Medicaid reimbursement. Much like the old adage, “bigger is not always better,” I have found myself questioning: “Is electronic better?” This topic has been much of a debate at my training institution as well, and it deserves more attention. As such, below are outlined what I believe to be the top three positives and negatives of the EMR. Maybe this will help settle the debate: Is the EMR our friend or foe?
Votes for “Friend”:
Portability
Centralized data
Having easily accessible, legible data is vital in caring for our patient population. You never have to guess what a providers are trying to write in their notes in the EMR (no hand-written notes). Most EMRs allow laboratory data, imaging, and notes to be viewed using one platform. Capabilities such as trends of vitals and laboratory values are useful to providers as well. As noted above, providers no longer have to physically gather laboratory slips or imaging films. Having centralized data for each patient saves on a provider’s time.
Standardization of orders
This has to be one of the greatest attributes of the EMR, especially when considering a teaching institution where trainees are constantly learning. Whether a provider orders Tylenol, a thoracic angiogram, echocardiogram, or a diet for a patient, the order is standardized. With medication orders, the usual dose is provided, along with other important information (renal dosing suggestions, etc.). Pharmacists and nursing staff do not have to worry about the legibility when reading and performing medication orders, for example.
Votes for “Foe”:
Cumbersome
There is no question that there is a considerable amount of time that adds up when logging into the EMR, entering orders, and writing notes. That time does not include any restarts or rebooting that can occur, not to mention the various “warnings” that pop up during each of the processes (e.g. creatinine level for all contrasted imaging studies or patient allergies). Depending on the type of note a provider writes (free text versus standardized format) a note can seem to require endless “clicks” to get to the end. Sometimes you find yourself wishing to just have a pen and paper.
Reliance on technology
When data gathering and documentation is centered on a computer, focus often shifts from the patient’s bedside to a desk. Although “computers on wheels” and computer stations positioned throughout the intensive care unit and ward have helped move providers closer to the bedside, a significant amount of time is still tied to the computer itself. Further, review of patient information on a computer is absolutely no substitute to bedside evaluation of a patient, especially in our line of work.
Lack of single EMR
I have lost track of the number of times I have been frustrated by this fact. At tertiary care centers, we often receive patients that had initial treatment or surgery at another institution. Full detail and information from those prior visits is usually not available. As a result, many tests (laboratory and imaging) have to be repeated. As stewards of health care cost, we do no justice by simply repeating each test. Unfortunately, given the “business” aspects of EMRs, a single EMR, which all providers can access, is not in our near future. This is unfortunate, as I strongly feel it would improve communication among providers across cities/states, and reduce health care costs (one example being the elimination of repeating tests).
As one can see, EMR offers a great deal but still has serious shortcomings. In my mind, the jury is still out. Hopefully as we refine the current models of EMR we will get closer to the ideal EMR: one that continues to be portable, can house centralized data, and allows for standardization of orders but at the same time offers efficient use, thereby decreasing the time tied to a computer, and is accessible to all providers.
Dr. Eilers is a general thoracic surgeon at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
The Attending Viewpoint:
Some of us embraced the idea of the EMR while others resisted until failure to use one led to financial penalty. Even so, it is absolutely clear that EMRs are here to stay. As is true of all technology, there are pros and cons to their deployment.
Pro EMR
Notes can be read. We have all suffered the indignity of trying to read illegible handwriting to guess what a consultant has recommended, or as the consultant trying to understand the question you are being asked. Even more than handwriting issues, with the paper chart there was always the challenge of finding the chart or the note walking around in a resident’s pocket awaiting rounds with the attending. With the EMR, what is written is legible and it can be found/reviewed remotely before going in search of the patient. This has the potential to save time and offers the consultant the advantage of knowing about the patient prior to the visit. In the academic setting, the EMR affords the attending opportunity to review and amend a resident’s note for accuracy that sometimes leads to a “teachable moment.”
Communication among providers can be shared more efficiently. To the extent that the patient’s providers access the same record this is very useful. On completion of any procedure or note, the outcome can be promptly sent electronically or by fax to other providers with the need to know. This can really optimize health care in many ways.
Con EMR
The worst thing about the EMR is the separation of patient from provider at all levels! In the “old days” we were all at the patient’s bedside. Today I often find decisions made based on limited data in the record without benefit of seeing the patient. Failing to observe whether the patient is breathing comfortably, feel whether the feet are warm, or notice whether the pressure transducers are appropriately placed in relation to the patient takes away the real art of medicine. Worse, decisions are made and interventions selected that may be quite inappropriate. It seems that nurses spend more time in the hallway on their workstations clicking away to satisfy documentation demands than spending time assessing and knowing their patients. The patient becomes “Room 920” not “Mr. Smith.” I see this as a real tragedy of our reliance on electronic media.
Data can be reviewed remotely sometimes. This is indeed a two-edged sword in that it separates us from our patients too easily. The ultimate time saver of the computer too often becomes a time sink with the need to negotiate multiple securities to access the EMR at all. Also, we must often seek information in more than one source to review relevant data from the in- or outpatient environment, from a referring doctor in another system, or to review an actual image rather than just the report. It becomes an expensive endeavor when we need additional staff to track down data, images, and other records.
Overall, I am very happy to be practicing with the great technology we have today. I am excited to watch as all these technologies evolve to make better our health care delivery. In reality, the EMR is not our enemy. It is a friend with a bad temper.
Dr. Carpenter is an adult cardiac surgeon and program director, Thoracic Surgery Residency, at the University of Texas Health Science Center at San Antonio, Division of Thoracic Surgery.
IN.PACT Global: Promising 2-year data for drug-coated balloon performance for femoropopliteal PAD
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
FROM VIVA 17
Key clinical point:
Major finding: The freedom from clinically driven target lesion revascularization (CD-TLR) rate at 2 years was 83%.
Data source: The prospective, multicenter, single-arm, IN.PACT Global Clinical Study.
Disclosures: The IN.PACT Global Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.