User login
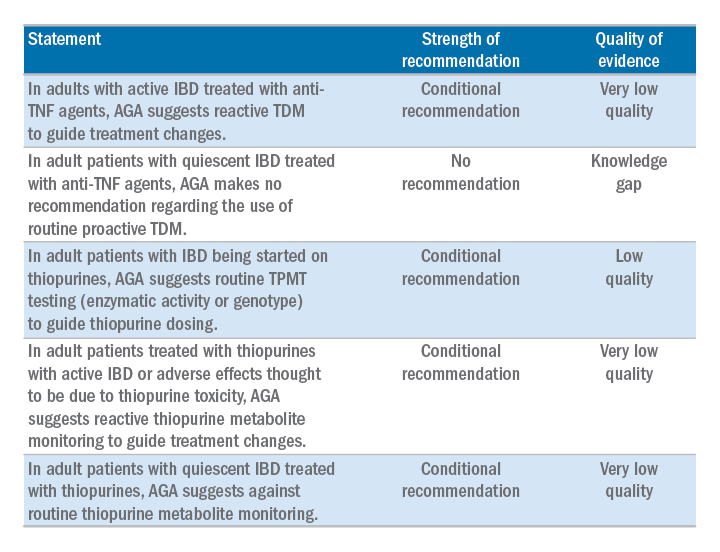
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:
The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.