User login
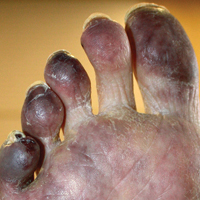
Cyanosis of the Foot
The Diagnosis: Antiphospholipid Antibody Syndrome
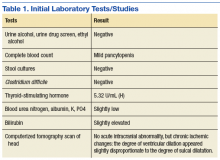
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

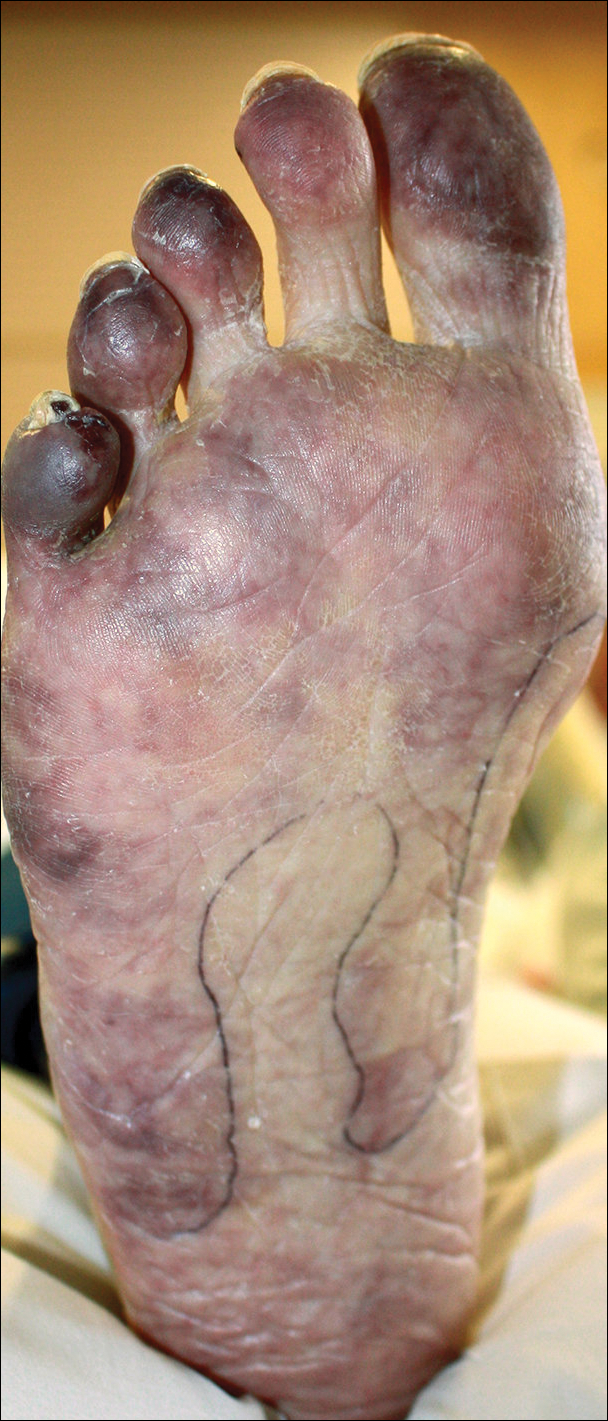
The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).
Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
The Diagnosis: Antiphospholipid Antibody Syndrome
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).

Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
The Diagnosis: Antiphospholipid Antibody Syndrome
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).

Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
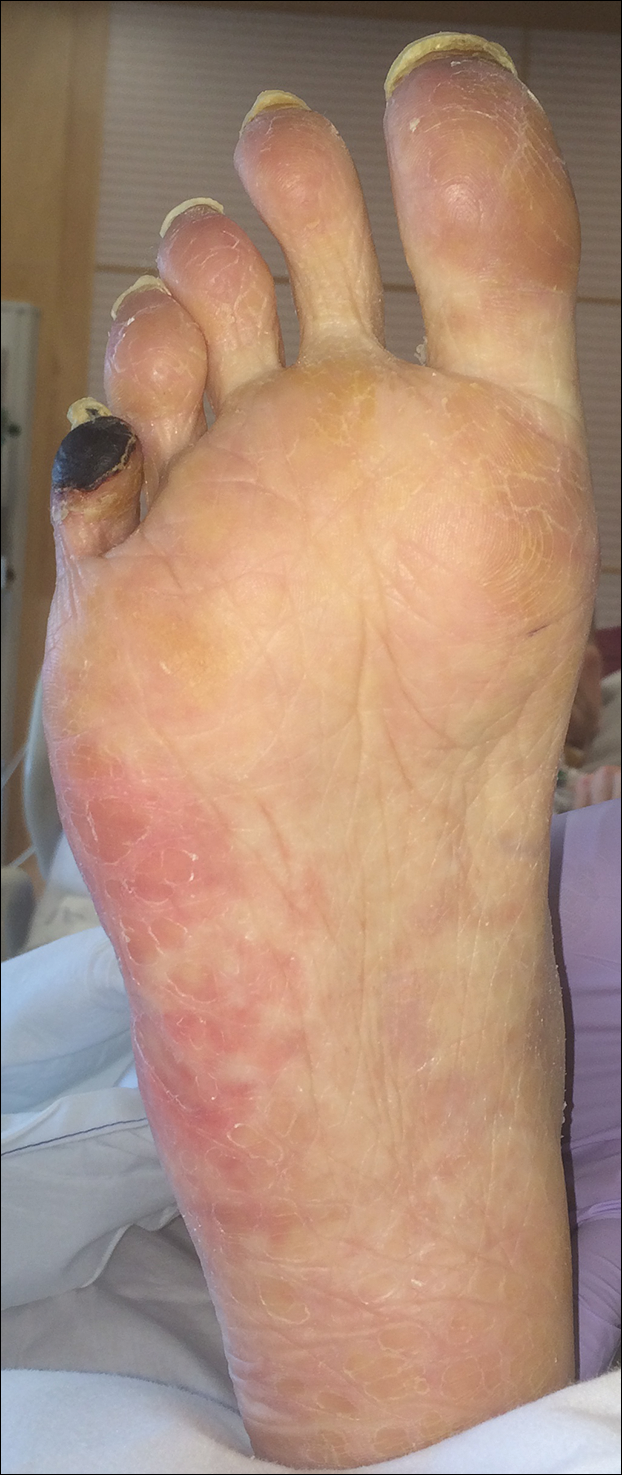
A man in his 50s with a medical history of arterial thrombosis of the right arm, multiple deep vein thromboses (DVTs) of the legs on long-term warfarin, ischemic stroke, atrial fibrillation, and peripheral arterial disease presented with discoloration of the right foot and increasing tenderness of 1 month's duration. There was no history of trauma or recent change in outpatient medications. A family history was notable for an aunt and 2 cousins with DVTs and protein S deficiency. Physical examination revealed livedo reticularis on the sole and lateral aspect of the right foot. There was violaceous discoloration of the volar aspects of all 5 toes and a focal area of ulceration on the fifth toe. Pulses were palpable bilaterally. Initial laboratory evaluation was notable for thrombocytopenia, and preliminary blood cultures revealed no growth of bacterial or fungal organisms. Imaging studies revealed increased arterial stenosis of the right leg as well as DVT of the right great saphenous vein. A punch biopsy of the right medial foot was performed for hematoxylin and eosin stain as well as tissue culture.
SVS Coding Workshop is Oct. 13-14
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Read SVS Foundation Annual Report
The SVS Foundation highlights innovation and the connection between research bench and patient bedside in its just-published 2017 Annual Report, now available online.
Read about the Foundation’s expanded mission, emphasizing disease prevent and patient education along with the core commitment to fund basic and clinical research. Read why Michael C. Dalsing, MD, gives and how Ulka Sachdev, MD, has utilized her Foundation awards to try to unlock the suffering caused by chronic venous insufficiency. Read about this year’s grant recipients, Foundation financial details, lists of donors to not only the Foundation but also the new Alexander Clowes Lecture Fund, award opportunities and how every gift helps. Read, and donate today.
The SVS Foundation highlights innovation and the connection between research bench and patient bedside in its just-published 2017 Annual Report, now available online.
Read about the Foundation’s expanded mission, emphasizing disease prevent and patient education along with the core commitment to fund basic and clinical research. Read why Michael C. Dalsing, MD, gives and how Ulka Sachdev, MD, has utilized her Foundation awards to try to unlock the suffering caused by chronic venous insufficiency. Read about this year’s grant recipients, Foundation financial details, lists of donors to not only the Foundation but also the new Alexander Clowes Lecture Fund, award opportunities and how every gift helps. Read, and donate today.
The SVS Foundation highlights innovation and the connection between research bench and patient bedside in its just-published 2017 Annual Report, now available online.
Read about the Foundation’s expanded mission, emphasizing disease prevent and patient education along with the core commitment to fund basic and clinical research. Read why Michael C. Dalsing, MD, gives and how Ulka Sachdev, MD, has utilized her Foundation awards to try to unlock the suffering caused by chronic venous insufficiency. Read about this year’s grant recipients, Foundation financial details, lists of donors to not only the Foundation but also the new Alexander Clowes Lecture Fund, award opportunities and how every gift helps. Read, and donate today.
Keep Up to Date with VESAP4
Don’t forget how valuable the Vascular Educational and Self-Assessment Program can be in keeping with all things vascular-related.
The fourth edition – which will soon include a mobile app (Apple products only) for off-line – launched just two months ago. Besides the app, VESAP4 also offers syncing between the companion app and desktop version; expanded bookmarking and annotation, easier navigation and simplified tracking of CME/MOC certificates.
Costs is $450 for candidates, $550 for members and $650 for non-members. A total of 75 CME (7.5 for each of the 10 sections) will be available. For information, email [email protected] or call 800-258-7188.
Don’t forget how valuable the Vascular Educational and Self-Assessment Program can be in keeping with all things vascular-related.
The fourth edition – which will soon include a mobile app (Apple products only) for off-line – launched just two months ago. Besides the app, VESAP4 also offers syncing between the companion app and desktop version; expanded bookmarking and annotation, easier navigation and simplified tracking of CME/MOC certificates.
Costs is $450 for candidates, $550 for members and $650 for non-members. A total of 75 CME (7.5 for each of the 10 sections) will be available. For information, email [email protected] or call 800-258-7188.
Don’t forget how valuable the Vascular Educational and Self-Assessment Program can be in keeping with all things vascular-related.
The fourth edition – which will soon include a mobile app (Apple products only) for off-line – launched just two months ago. Besides the app, VESAP4 also offers syncing between the companion app and desktop version; expanded bookmarking and annotation, easier navigation and simplified tracking of CME/MOC certificates.
Costs is $450 for candidates, $550 for members and $650 for non-members. A total of 75 CME (7.5 for each of the 10 sections) will be available. For information, email [email protected] or call 800-258-7188.
Assessing the Effectiveness of Knowledge-Based Interventions in Increasing Skin Cancer Awareness, Knowledge, and Protective Behaviors in Skin of Color Populations
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
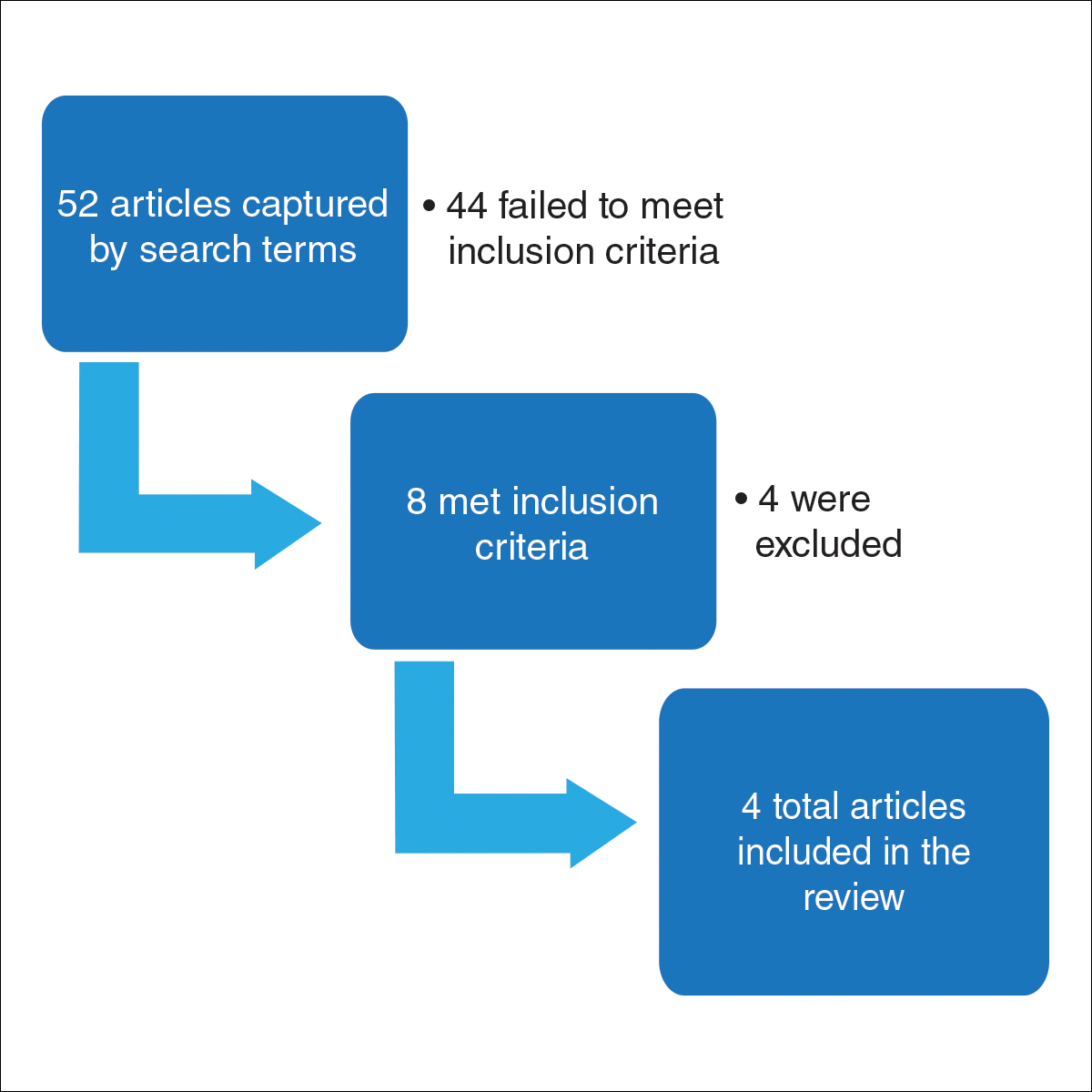
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).
Results
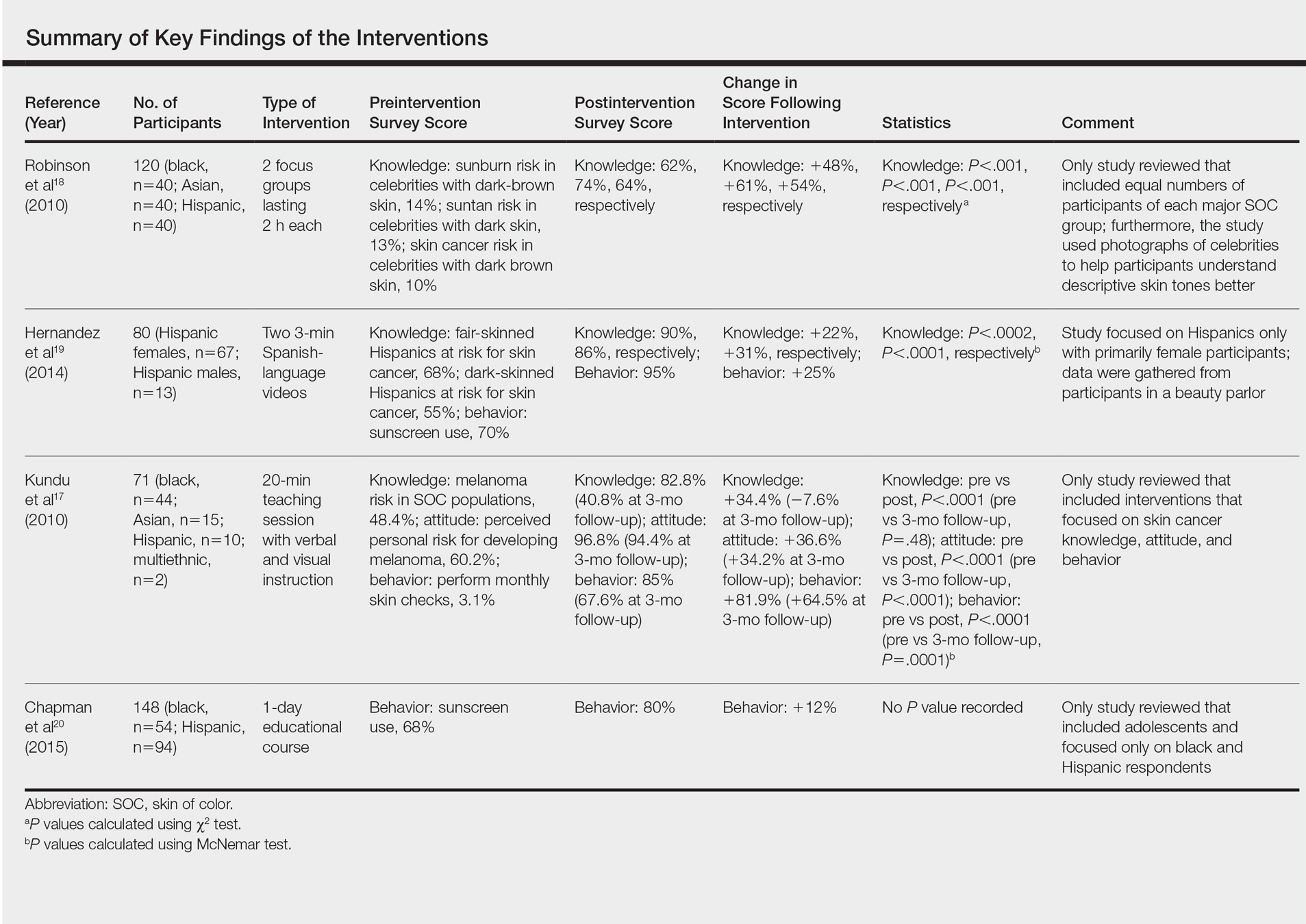
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Practice Points
- Patients of color should be informed that they are at risk for skin cancer including melanoma.
- Patients of color should be taught to identify suspicious skin lesions including the ABCDEs of melanoma.
- Patients of color should be instructed to perform self-body skin examinations, especially of the palms and soles, for any evolving skin lesions. Patients should be instructed on the importance of visiting a physician for an evolving or suspicious mole or lesion.
MACRA Monday: Try this measure
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
A Severe Case of Paliperidone Palmitate-Induced Parkinsonism Leading to Prolonged Hospitalization: Opportunities for Improvement
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
Primary care may be inadequate for cancer survivors
Primary care may not meet the healthcare needs of cancer survivors in the US, according to research published in JAMA Internal Medicine.
Researchers examined 12 advanced primary care practices selected from a national registry of “workforce innovators” and found that none of these practices had a comprehensive survivorship care program in place.
In addition, there were 3 main barriers to survivorship care—not treating cancer survivors as a distinct population, limitations of electronic health records, and a lack of information and guidance for clinicians.
“This is troubling because these are highly innovative practices that have a national reputation,” said study author Benjamin Crabtree, PhD, of Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey.
Dr Crabtree and his colleagues evaluated survivorship care* at the 12 practices, which were based in Colorado, Illinois, Maine, New York, Pennsylvania, and Washington.
Over nearly 2 years, the team spent 10 to 12 days observing each of the practices and interviewing clinicians and administrators.
In this way, the researchers identified 3 main barriers to integrating survivorship care into primary medicine.
Barrier 1
The first barrier was that clinicians did not treat cancer survivors as a distinct population or clinical category.
“There is no diagnosis code for ‘cancer survivor’ that can be entered into the medical record, which is important if you want physicians to pay attention,” Dr Crabtree said.
Some of the clinicians interviewed said their care was comprehensive enough to address the needs of all patients. Other clinicians did not understand what survivorship care entails.
Barrier 2
The second barrier was that electronic health record systems didn’t support survivorship care.
Clinicians reported an inability to identify patients with a history of cancer. Even if a patient’s cancer history was included in his or her record, it might take searching through multiple screens to find the information.
In addition, medical records were sometimes lost as patients changed clinicians over the years, which left it up to patients to report their cancer histories.
Barrier 3
The third barrier was that clinicians did not receive adequate information or guidance for follow-up care of cancer survivors.
Although some of the practices received cancer-related information about their patients, it was considered “inadequate” or “not actionable.”
Clinicians expressed concerns about their knowledge gaps in cancer care and the need to monitor changing information in oncology.
“There is nothing in the residency curriculum about cancer survivorship,” Dr Crabtree said. “There is also nothing in Continuing Medical Education courses. It’s just not there.”
Dr Crabtree and his colleagues believe these barriers must be addressed so that comprehensive cancer survivorship services can move to the forefront of primary care. ![]()
* Survivorship care includes checking for cancer recurrence, monitoring long-term effects of radiation and chemotherapy, and assessing a patient’s psychological well-being.
Primary care may not meet the healthcare needs of cancer survivors in the US, according to research published in JAMA Internal Medicine.
Researchers examined 12 advanced primary care practices selected from a national registry of “workforce innovators” and found that none of these practices had a comprehensive survivorship care program in place.
In addition, there were 3 main barriers to survivorship care—not treating cancer survivors as a distinct population, limitations of electronic health records, and a lack of information and guidance for clinicians.
“This is troubling because these are highly innovative practices that have a national reputation,” said study author Benjamin Crabtree, PhD, of Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey.
Dr Crabtree and his colleagues evaluated survivorship care* at the 12 practices, which were based in Colorado, Illinois, Maine, New York, Pennsylvania, and Washington.
Over nearly 2 years, the team spent 10 to 12 days observing each of the practices and interviewing clinicians and administrators.
In this way, the researchers identified 3 main barriers to integrating survivorship care into primary medicine.
Barrier 1
The first barrier was that clinicians did not treat cancer survivors as a distinct population or clinical category.
“There is no diagnosis code for ‘cancer survivor’ that can be entered into the medical record, which is important if you want physicians to pay attention,” Dr Crabtree said.
Some of the clinicians interviewed said their care was comprehensive enough to address the needs of all patients. Other clinicians did not understand what survivorship care entails.
Barrier 2
The second barrier was that electronic health record systems didn’t support survivorship care.
Clinicians reported an inability to identify patients with a history of cancer. Even if a patient’s cancer history was included in his or her record, it might take searching through multiple screens to find the information.
In addition, medical records were sometimes lost as patients changed clinicians over the years, which left it up to patients to report their cancer histories.
Barrier 3
The third barrier was that clinicians did not receive adequate information or guidance for follow-up care of cancer survivors.
Although some of the practices received cancer-related information about their patients, it was considered “inadequate” or “not actionable.”
Clinicians expressed concerns about their knowledge gaps in cancer care and the need to monitor changing information in oncology.
“There is nothing in the residency curriculum about cancer survivorship,” Dr Crabtree said. “There is also nothing in Continuing Medical Education courses. It’s just not there.”
Dr Crabtree and his colleagues believe these barriers must be addressed so that comprehensive cancer survivorship services can move to the forefront of primary care. ![]()
* Survivorship care includes checking for cancer recurrence, monitoring long-term effects of radiation and chemotherapy, and assessing a patient’s psychological well-being.
Primary care may not meet the healthcare needs of cancer survivors in the US, according to research published in JAMA Internal Medicine.
Researchers examined 12 advanced primary care practices selected from a national registry of “workforce innovators” and found that none of these practices had a comprehensive survivorship care program in place.
In addition, there were 3 main barriers to survivorship care—not treating cancer survivors as a distinct population, limitations of electronic health records, and a lack of information and guidance for clinicians.
“This is troubling because these are highly innovative practices that have a national reputation,” said study author Benjamin Crabtree, PhD, of Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey.
Dr Crabtree and his colleagues evaluated survivorship care* at the 12 practices, which were based in Colorado, Illinois, Maine, New York, Pennsylvania, and Washington.
Over nearly 2 years, the team spent 10 to 12 days observing each of the practices and interviewing clinicians and administrators.
In this way, the researchers identified 3 main barriers to integrating survivorship care into primary medicine.
Barrier 1
The first barrier was that clinicians did not treat cancer survivors as a distinct population or clinical category.
“There is no diagnosis code for ‘cancer survivor’ that can be entered into the medical record, which is important if you want physicians to pay attention,” Dr Crabtree said.
Some of the clinicians interviewed said their care was comprehensive enough to address the needs of all patients. Other clinicians did not understand what survivorship care entails.
Barrier 2
The second barrier was that electronic health record systems didn’t support survivorship care.
Clinicians reported an inability to identify patients with a history of cancer. Even if a patient’s cancer history was included in his or her record, it might take searching through multiple screens to find the information.
In addition, medical records were sometimes lost as patients changed clinicians over the years, which left it up to patients to report their cancer histories.
Barrier 3
The third barrier was that clinicians did not receive adequate information or guidance for follow-up care of cancer survivors.
Although some of the practices received cancer-related information about their patients, it was considered “inadequate” or “not actionable.”
Clinicians expressed concerns about their knowledge gaps in cancer care and the need to monitor changing information in oncology.
“There is nothing in the residency curriculum about cancer survivorship,” Dr Crabtree said. “There is also nothing in Continuing Medical Education courses. It’s just not there.”
Dr Crabtree and his colleagues believe these barriers must be addressed so that comprehensive cancer survivorship services can move to the forefront of primary care. ![]()
* Survivorship care includes checking for cancer recurrence, monitoring long-term effects of radiation and chemotherapy, and assessing a patient’s psychological well-being.
If You Were Surgeon General ...
This past August, President Trump, with the advice and consent of the United States Senate, nominated Indiana Health Commissioner Jerome M. Adams, MD, MPH, as the nation’s 20th Surgeon General (SG). As the country’s “doctor,” the SG has access to the best available scientific information to advise Americans of ways to improve their health and decrease risk for illness and injury. Overseen by the Office of the Assistant Secretary for Health, the Office of the Surgeon General has no budget or line authority. As a political appointee, the SG ranks three levels below a presidential cabinet member and reports to an assistant health secretary.1
The SG nominally oversees the US Public Health Service Commissioned Corps, an exclusive group of more than 6,700 public health professionals working throughout the federal government with the mission to protect, promote, and advance the health of our nation.2 In 2010, under the Affordable Care Act, the SG was designated as Chair of the National Prevention Council, which coordinates and leads 20 executive departments encouraging prevention, wellness, and health promotion activities.
Past SGs, during their time in office, have quietly gone about their duties, although some have used their platform to raise awareness of specific public health concerns. C. Everett Koop (a Reagan appointee) is among the most-remembered SGs, thanks in part to his use of the media to promote his causes, specifically smoking cessation and AIDS prevention. Newly appointed Dr. Adams, an anesthesiologist by training, is known for his focus on the opioid epidemic, tobacco use, and infant mortality.
As we’ve seen over the years, the limitations of the SG’s role equate to a mixed bag of “results.” For every C. Everett Koop (whether you agree with his views or not, he was prolific!), there are several SGs who came and went from office without making a blip on the public’s radar. Since health care remains at the forefront of our national conversation, the burning question is: If you were a consultant to the SG, which health issues would you prioritize?
I posed this question to 30 of my PA and NP colleagues. While certainly not an official survey—rather, a straw poll—I was nonetheless surprised by the overlap in responses. Here are some of the highlights.
Substance abuse/opioid crisis. Globally, one in every eight deaths result from the use of tobacco, alcohol, and other drugs. According to Humphreys et al, the implications of addiction are clear: It will do massive and increasing damage to humanity if not addressed emergently and at the source.3 But to do so, we must understand the root cause. Neuroscientific research has shown that repeated addictive drug use can rewire the brain’s motivational and reward circuits and influence decision-making.3
This is evidenced by the startling fact that every day, an average of 91 Americans die of opioid overdose.3 In March, the Director-General of the World Health Organization called for more scientifically informed public policies regarding addiction.4 The President’s Commission on Combating Drug Addiction and the Opioid Crisis released a preliminary report in August recommending next steps, which included the declaration of a federal state of emergency.5
Mental health. As you may recall from my December 2016 editorial, mental health is a forgotten facet of primary care—and one that is imperative to address.6 Only 43% of family physicians in this country provide mental health care; furthermore, half of Americans with mental health conditions go without essential care, and those with intellectual and developmental disabilities are significantly underserved.6 Perhaps increased efforts to attend to mental health in primary care will subsequently reduce addiction and substance abuse rates, resulting in a physically and mentally healthier America.
Oral health. Dr. Koop was known for his quote, “You can’t be healthy without good oral health.” Unfortunately, the major challenge expressed in Oral Health in America: A Report of the Surgeon General—“not all Americans have achieved the same level of oral health and well-being”—is as relevant today as it was when the report was released in 2000.7 We must therefore accelerate efforts toward achieving this goal. We must also address the need for a more diverse and well-distributed oral health workforce.
The CDC Division of Oral Health has made oral health an integral component of public health programs, with a goal of eliminating disparities and improving oral health for all. Continued investment in research, such as that undertaken by the National Institute of Dental and Craniofacial Research Centers for Research to Reduce Disparities in Oral Health, is critical.8 Lastly, we must continue to expand initiatives to prevent tobacco use and promote better dietary choices.
Obesity. Literature on the health consequences of obesity in both adults and children has increased exponentially in recent decades, due to the condition’s alarming prevalence in the US and other industrialized countries. The CDC reports significant racial and ethnic disparities in obesity, specifically among Hispanics/Latinos compared to non-Hispanic whites. Because obesity significantly contributes to acute and chronic diseases and has a direct relationship to morbidity and mortality, public health officials should target health prevention messages and interventions to those populations with the greatest need.9
Kidney disease. An estimated 31 million people in the US have chronic kidney disease (CKD), and it is the eighth leading cause of death in this country. Shockingly, 9 out of 10 people who have stage 3 CKD are not even aware they have it.10 The cost of CKD in the US is extortionate; research in this area is horrifically underfunded compared to that for other chronic diseases. The entire NIH budget for CKD is $31 billion, while the expense to the patient population is $32 billion—and every five minutes, someone’s kidneys fail.11
The seemingly obvious question is, what can we do to prevent CKD? As far back as 1948, SG Thomas Parran Jr. advocated for preventive and curative services.12 Alas, I recently came across an excellent poem by Joseph Malins (circa 1855) that speaks volumes about prevention versus cure (see box).
Environmental threats/emerging viruses. Particular attention should be paid to newly identified infectious agents, both locally and internationally, to prevent public health problems—for example, the Zika virus epidemic. In 1896, William Osler said, “Humanity has but three great enemies: infection, famine, and war; of these, by far the greatest, by far the most terrible is infection.”13 We know the factors that contribute to the emergence of new infections: the evolution of pathogenic infectious agents (microbial adaptation and change), development of drug resistance, and vector resistance to pesticides. Other important factors include climate and changing ecosystems, economic development and land use (eg, urbanization, deforestation), and technology and industry (eg, food processing and handling).14
Access to health care. Last but certainly not least, every respondent emphasized that we need to prioritize access to health care and eliminate socioeconomic disparities. But when we recognize that these disparities exist across all populations, we see that this is not an easy task. Lack of health insurance, lack of financial resources, irregular sources of care, legal obstacles, structural barriers, lack of health care providers, language barriers, socioeconomic disparity, and age are all contributing factors that are also obstacles.15 But in order for any of the previously discussed changes in health care to be influential, they must be accessible.
And so I open the floor to you, colleagues: What do you think should be done to improve access to health care? What are your pressing public health issues? Share your thoughts with me at [email protected].
1. Scutti S. Dr. Jerome Adams confirmed as Surgeon General. www.cnn.com/2017/08/04/health/jerome-adams-surgeon-general-confirmation/index.html. Accessed September 1, 2017.
2. U.S. Department of Health and Human Services. About the Office of the Surgeon General. www.surgeongeneral.gov/about/index.html. Accessed September 1, 2017.
3. Humphreys K, Malenka RC, Knutson B, MacCoun RJ. Brains, environments, and policy responses to addiction. Science. 2017;356(6344):1237-1238.
4. Chan M. Opening remarks at the 60th session of the Commission on Narcotic Drugs. www.who.int/dg/speeches/2017/commission-narcotic-drugs/en/. Accessed September 1, 2017.
5. Burns J. Opioid Task Force recommends state of emergency and (sort of) bold treatment agenda. www.forbes.com/sites/janetwburns/2017/08/02/opioid-task-force-recommends-state-of-emergency-and-sort-of-bold-treatment-agenda/#378810163956. Accessed September 1, 2017.
6. Danielsen RD. Mental health: a forgotten facet of primary care. Clinician Reviews. 2016;26(12):7,46.
7. NIH National Institute of Dental and Craniofacial Research. Oral health in America: report of the Surgeon General (executive summary). Rockville, MD: National Institute of Dental and Craniofacial Research; 2000. Page 287.
8. Satcher D, Nottingham JH. Revisiting oral health in America: a report of the Surgeon General. AJPH. 2017;107(suppl 1):S32-S33.
9. Hu F. Obesity Epidemiology. 2008; New York: Oxford University Press. 5-7.
10. American Kidney Fund. 2015 kidney disease statistics. www.kidneyfund.org/assets/pdf/kidney-disease-statistics.pdf. Accessed September 7, 2017.
11. Tyler. Statistics on chronic kidney disease (CKD) that may shock you. www.dietitiansathome.com/post/statistics-on-chronic-kidney-disease-ckd.
12. Sledge D. Linking public health and individual medicine: the health policy approach of Surgeon General Thomas Parran. Am J Public Health. 2017;107(4):509-516.
13. Osler W. The study of the fevers of the south. JAMA. 1896;XXVI(21):999-1004.
14. Fauci AS, Touchette NA, Folkers GK. Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerging Infectious Diseases. 2005;11(4):519-525.
15. Mandal A. Disparities in access to health care. www.news-medical.net/health/Disparities-in-Access-to-Health-Care.aspx. Accessed September 1, 2017.
This past August, President Trump, with the advice and consent of the United States Senate, nominated Indiana Health Commissioner Jerome M. Adams, MD, MPH, as the nation’s 20th Surgeon General (SG). As the country’s “doctor,” the SG has access to the best available scientific information to advise Americans of ways to improve their health and decrease risk for illness and injury. Overseen by the Office of the Assistant Secretary for Health, the Office of the Surgeon General has no budget or line authority. As a political appointee, the SG ranks three levels below a presidential cabinet member and reports to an assistant health secretary.1
The SG nominally oversees the US Public Health Service Commissioned Corps, an exclusive group of more than 6,700 public health professionals working throughout the federal government with the mission to protect, promote, and advance the health of our nation.2 In 2010, under the Affordable Care Act, the SG was designated as Chair of the National Prevention Council, which coordinates and leads 20 executive departments encouraging prevention, wellness, and health promotion activities.
Past SGs, during their time in office, have quietly gone about their duties, although some have used their platform to raise awareness of specific public health concerns. C. Everett Koop (a Reagan appointee) is among the most-remembered SGs, thanks in part to his use of the media to promote his causes, specifically smoking cessation and AIDS prevention. Newly appointed Dr. Adams, an anesthesiologist by training, is known for his focus on the opioid epidemic, tobacco use, and infant mortality.
As we’ve seen over the years, the limitations of the SG’s role equate to a mixed bag of “results.” For every C. Everett Koop (whether you agree with his views or not, he was prolific!), there are several SGs who came and went from office without making a blip on the public’s radar. Since health care remains at the forefront of our national conversation, the burning question is: If you were a consultant to the SG, which health issues would you prioritize?
I posed this question to 30 of my PA and NP colleagues. While certainly not an official survey—rather, a straw poll—I was nonetheless surprised by the overlap in responses. Here are some of the highlights.
Substance abuse/opioid crisis. Globally, one in every eight deaths result from the use of tobacco, alcohol, and other drugs. According to Humphreys et al, the implications of addiction are clear: It will do massive and increasing damage to humanity if not addressed emergently and at the source.3 But to do so, we must understand the root cause. Neuroscientific research has shown that repeated addictive drug use can rewire the brain’s motivational and reward circuits and influence decision-making.3
This is evidenced by the startling fact that every day, an average of 91 Americans die of opioid overdose.3 In March, the Director-General of the World Health Organization called for more scientifically informed public policies regarding addiction.4 The President’s Commission on Combating Drug Addiction and the Opioid Crisis released a preliminary report in August recommending next steps, which included the declaration of a federal state of emergency.5
Mental health. As you may recall from my December 2016 editorial, mental health is a forgotten facet of primary care—and one that is imperative to address.6 Only 43% of family physicians in this country provide mental health care; furthermore, half of Americans with mental health conditions go without essential care, and those with intellectual and developmental disabilities are significantly underserved.6 Perhaps increased efforts to attend to mental health in primary care will subsequently reduce addiction and substance abuse rates, resulting in a physically and mentally healthier America.
Oral health. Dr. Koop was known for his quote, “You can’t be healthy without good oral health.” Unfortunately, the major challenge expressed in Oral Health in America: A Report of the Surgeon General—“not all Americans have achieved the same level of oral health and well-being”—is as relevant today as it was when the report was released in 2000.7 We must therefore accelerate efforts toward achieving this goal. We must also address the need for a more diverse and well-distributed oral health workforce.
The CDC Division of Oral Health has made oral health an integral component of public health programs, with a goal of eliminating disparities and improving oral health for all. Continued investment in research, such as that undertaken by the National Institute of Dental and Craniofacial Research Centers for Research to Reduce Disparities in Oral Health, is critical.8 Lastly, we must continue to expand initiatives to prevent tobacco use and promote better dietary choices.
Obesity. Literature on the health consequences of obesity in both adults and children has increased exponentially in recent decades, due to the condition’s alarming prevalence in the US and other industrialized countries. The CDC reports significant racial and ethnic disparities in obesity, specifically among Hispanics/Latinos compared to non-Hispanic whites. Because obesity significantly contributes to acute and chronic diseases and has a direct relationship to morbidity and mortality, public health officials should target health prevention messages and interventions to those populations with the greatest need.9
Kidney disease. An estimated 31 million people in the US have chronic kidney disease (CKD), and it is the eighth leading cause of death in this country. Shockingly, 9 out of 10 people who have stage 3 CKD are not even aware they have it.10 The cost of CKD in the US is extortionate; research in this area is horrifically underfunded compared to that for other chronic diseases. The entire NIH budget for CKD is $31 billion, while the expense to the patient population is $32 billion—and every five minutes, someone’s kidneys fail.11
The seemingly obvious question is, what can we do to prevent CKD? As far back as 1948, SG Thomas Parran Jr. advocated for preventive and curative services.12 Alas, I recently came across an excellent poem by Joseph Malins (circa 1855) that speaks volumes about prevention versus cure (see box).

Environmental threats/emerging viruses. Particular attention should be paid to newly identified infectious agents, both locally and internationally, to prevent public health problems—for example, the Zika virus epidemic. In 1896, William Osler said, “Humanity has but three great enemies: infection, famine, and war; of these, by far the greatest, by far the most terrible is infection.”13 We know the factors that contribute to the emergence of new infections: the evolution of pathogenic infectious agents (microbial adaptation and change), development of drug resistance, and vector resistance to pesticides. Other important factors include climate and changing ecosystems, economic development and land use (eg, urbanization, deforestation), and technology and industry (eg, food processing and handling).14
Access to health care. Last but certainly not least, every respondent emphasized that we need to prioritize access to health care and eliminate socioeconomic disparities. But when we recognize that these disparities exist across all populations, we see that this is not an easy task. Lack of health insurance, lack of financial resources, irregular sources of care, legal obstacles, structural barriers, lack of health care providers, language barriers, socioeconomic disparity, and age are all contributing factors that are also obstacles.15 But in order for any of the previously discussed changes in health care to be influential, they must be accessible.
And so I open the floor to you, colleagues: What do you think should be done to improve access to health care? What are your pressing public health issues? Share your thoughts with me at [email protected].
This past August, President Trump, with the advice and consent of the United States Senate, nominated Indiana Health Commissioner Jerome M. Adams, MD, MPH, as the nation’s 20th Surgeon General (SG). As the country’s “doctor,” the SG has access to the best available scientific information to advise Americans of ways to improve their health and decrease risk for illness and injury. Overseen by the Office of the Assistant Secretary for Health, the Office of the Surgeon General has no budget or line authority. As a political appointee, the SG ranks three levels below a presidential cabinet member and reports to an assistant health secretary.1
The SG nominally oversees the US Public Health Service Commissioned Corps, an exclusive group of more than 6,700 public health professionals working throughout the federal government with the mission to protect, promote, and advance the health of our nation.2 In 2010, under the Affordable Care Act, the SG was designated as Chair of the National Prevention Council, which coordinates and leads 20 executive departments encouraging prevention, wellness, and health promotion activities.
Past SGs, during their time in office, have quietly gone about their duties, although some have used their platform to raise awareness of specific public health concerns. C. Everett Koop (a Reagan appointee) is among the most-remembered SGs, thanks in part to his use of the media to promote his causes, specifically smoking cessation and AIDS prevention. Newly appointed Dr. Adams, an anesthesiologist by training, is known for his focus on the opioid epidemic, tobacco use, and infant mortality.
As we’ve seen over the years, the limitations of the SG’s role equate to a mixed bag of “results.” For every C. Everett Koop (whether you agree with his views or not, he was prolific!), there are several SGs who came and went from office without making a blip on the public’s radar. Since health care remains at the forefront of our national conversation, the burning question is: If you were a consultant to the SG, which health issues would you prioritize?
I posed this question to 30 of my PA and NP colleagues. While certainly not an official survey—rather, a straw poll—I was nonetheless surprised by the overlap in responses. Here are some of the highlights.
Substance abuse/opioid crisis. Globally, one in every eight deaths result from the use of tobacco, alcohol, and other drugs. According to Humphreys et al, the implications of addiction are clear: It will do massive and increasing damage to humanity if not addressed emergently and at the source.3 But to do so, we must understand the root cause. Neuroscientific research has shown that repeated addictive drug use can rewire the brain’s motivational and reward circuits and influence decision-making.3
This is evidenced by the startling fact that every day, an average of 91 Americans die of opioid overdose.3 In March, the Director-General of the World Health Organization called for more scientifically informed public policies regarding addiction.4 The President’s Commission on Combating Drug Addiction and the Opioid Crisis released a preliminary report in August recommending next steps, which included the declaration of a federal state of emergency.5
Mental health. As you may recall from my December 2016 editorial, mental health is a forgotten facet of primary care—and one that is imperative to address.6 Only 43% of family physicians in this country provide mental health care; furthermore, half of Americans with mental health conditions go without essential care, and those with intellectual and developmental disabilities are significantly underserved.6 Perhaps increased efforts to attend to mental health in primary care will subsequently reduce addiction and substance abuse rates, resulting in a physically and mentally healthier America.
Oral health. Dr. Koop was known for his quote, “You can’t be healthy without good oral health.” Unfortunately, the major challenge expressed in Oral Health in America: A Report of the Surgeon General—“not all Americans have achieved the same level of oral health and well-being”—is as relevant today as it was when the report was released in 2000.7 We must therefore accelerate efforts toward achieving this goal. We must also address the need for a more diverse and well-distributed oral health workforce.
The CDC Division of Oral Health has made oral health an integral component of public health programs, with a goal of eliminating disparities and improving oral health for all. Continued investment in research, such as that undertaken by the National Institute of Dental and Craniofacial Research Centers for Research to Reduce Disparities in Oral Health, is critical.8 Lastly, we must continue to expand initiatives to prevent tobacco use and promote better dietary choices.
Obesity. Literature on the health consequences of obesity in both adults and children has increased exponentially in recent decades, due to the condition’s alarming prevalence in the US and other industrialized countries. The CDC reports significant racial and ethnic disparities in obesity, specifically among Hispanics/Latinos compared to non-Hispanic whites. Because obesity significantly contributes to acute and chronic diseases and has a direct relationship to morbidity and mortality, public health officials should target health prevention messages and interventions to those populations with the greatest need.9
Kidney disease. An estimated 31 million people in the US have chronic kidney disease (CKD), and it is the eighth leading cause of death in this country. Shockingly, 9 out of 10 people who have stage 3 CKD are not even aware they have it.10 The cost of CKD in the US is extortionate; research in this area is horrifically underfunded compared to that for other chronic diseases. The entire NIH budget for CKD is $31 billion, while the expense to the patient population is $32 billion—and every five minutes, someone’s kidneys fail.11
The seemingly obvious question is, what can we do to prevent CKD? As far back as 1948, SG Thomas Parran Jr. advocated for preventive and curative services.12 Alas, I recently came across an excellent poem by Joseph Malins (circa 1855) that speaks volumes about prevention versus cure (see box).

Environmental threats/emerging viruses. Particular attention should be paid to newly identified infectious agents, both locally and internationally, to prevent public health problems—for example, the Zika virus epidemic. In 1896, William Osler said, “Humanity has but three great enemies: infection, famine, and war; of these, by far the greatest, by far the most terrible is infection.”13 We know the factors that contribute to the emergence of new infections: the evolution of pathogenic infectious agents (microbial adaptation and change), development of drug resistance, and vector resistance to pesticides. Other important factors include climate and changing ecosystems, economic development and land use (eg, urbanization, deforestation), and technology and industry (eg, food processing and handling).14
Access to health care. Last but certainly not least, every respondent emphasized that we need to prioritize access to health care and eliminate socioeconomic disparities. But when we recognize that these disparities exist across all populations, we see that this is not an easy task. Lack of health insurance, lack of financial resources, irregular sources of care, legal obstacles, structural barriers, lack of health care providers, language barriers, socioeconomic disparity, and age are all contributing factors that are also obstacles.15 But in order for any of the previously discussed changes in health care to be influential, they must be accessible.
And so I open the floor to you, colleagues: What do you think should be done to improve access to health care? What are your pressing public health issues? Share your thoughts with me at [email protected].
1. Scutti S. Dr. Jerome Adams confirmed as Surgeon General. www.cnn.com/2017/08/04/health/jerome-adams-surgeon-general-confirmation/index.html. Accessed September 1, 2017.
2. U.S. Department of Health and Human Services. About the Office of the Surgeon General. www.surgeongeneral.gov/about/index.html. Accessed September 1, 2017.
3. Humphreys K, Malenka RC, Knutson B, MacCoun RJ. Brains, environments, and policy responses to addiction. Science. 2017;356(6344):1237-1238.
4. Chan M. Opening remarks at the 60th session of the Commission on Narcotic Drugs. www.who.int/dg/speeches/2017/commission-narcotic-drugs/en/. Accessed September 1, 2017.
5. Burns J. Opioid Task Force recommends state of emergency and (sort of) bold treatment agenda. www.forbes.com/sites/janetwburns/2017/08/02/opioid-task-force-recommends-state-of-emergency-and-sort-of-bold-treatment-agenda/#378810163956. Accessed September 1, 2017.
6. Danielsen RD. Mental health: a forgotten facet of primary care. Clinician Reviews. 2016;26(12):7,46.
7. NIH National Institute of Dental and Craniofacial Research. Oral health in America: report of the Surgeon General (executive summary). Rockville, MD: National Institute of Dental and Craniofacial Research; 2000. Page 287.
8. Satcher D, Nottingham JH. Revisiting oral health in America: a report of the Surgeon General. AJPH. 2017;107(suppl 1):S32-S33.
9. Hu F. Obesity Epidemiology. 2008; New York: Oxford University Press. 5-7.
10. American Kidney Fund. 2015 kidney disease statistics. www.kidneyfund.org/assets/pdf/kidney-disease-statistics.pdf. Accessed September 7, 2017.
11. Tyler. Statistics on chronic kidney disease (CKD) that may shock you. www.dietitiansathome.com/post/statistics-on-chronic-kidney-disease-ckd.
12. Sledge D. Linking public health and individual medicine: the health policy approach of Surgeon General Thomas Parran. Am J Public Health. 2017;107(4):509-516.
13. Osler W. The study of the fevers of the south. JAMA. 1896;XXVI(21):999-1004.
14. Fauci AS, Touchette NA, Folkers GK. Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerging Infectious Diseases. 2005;11(4):519-525.
15. Mandal A. Disparities in access to health care. www.news-medical.net/health/Disparities-in-Access-to-Health-Care.aspx. Accessed September 1, 2017.
1. Scutti S. Dr. Jerome Adams confirmed as Surgeon General. www.cnn.com/2017/08/04/health/jerome-adams-surgeon-general-confirmation/index.html. Accessed September 1, 2017.
2. U.S. Department of Health and Human Services. About the Office of the Surgeon General. www.surgeongeneral.gov/about/index.html. Accessed September 1, 2017.
3. Humphreys K, Malenka RC, Knutson B, MacCoun RJ. Brains, environments, and policy responses to addiction. Science. 2017;356(6344):1237-1238.
4. Chan M. Opening remarks at the 60th session of the Commission on Narcotic Drugs. www.who.int/dg/speeches/2017/commission-narcotic-drugs/en/. Accessed September 1, 2017.
5. Burns J. Opioid Task Force recommends state of emergency and (sort of) bold treatment agenda. www.forbes.com/sites/janetwburns/2017/08/02/opioid-task-force-recommends-state-of-emergency-and-sort-of-bold-treatment-agenda/#378810163956. Accessed September 1, 2017.
6. Danielsen RD. Mental health: a forgotten facet of primary care. Clinician Reviews. 2016;26(12):7,46.
7. NIH National Institute of Dental and Craniofacial Research. Oral health in America: report of the Surgeon General (executive summary). Rockville, MD: National Institute of Dental and Craniofacial Research; 2000. Page 287.
8. Satcher D, Nottingham JH. Revisiting oral health in America: a report of the Surgeon General. AJPH. 2017;107(suppl 1):S32-S33.
9. Hu F. Obesity Epidemiology. 2008; New York: Oxford University Press. 5-7.
10. American Kidney Fund. 2015 kidney disease statistics. www.kidneyfund.org/assets/pdf/kidney-disease-statistics.pdf. Accessed September 7, 2017.
11. Tyler. Statistics on chronic kidney disease (CKD) that may shock you. www.dietitiansathome.com/post/statistics-on-chronic-kidney-disease-ckd.
12. Sledge D. Linking public health and individual medicine: the health policy approach of Surgeon General Thomas Parran. Am J Public Health. 2017;107(4):509-516.
13. Osler W. The study of the fevers of the south. JAMA. 1896;XXVI(21):999-1004.
14. Fauci AS, Touchette NA, Folkers GK. Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerging Infectious Diseases. 2005;11(4):519-525.
15. Mandal A. Disparities in access to health care. www.news-medical.net/health/Disparities-in-Access-to-Health-Care.aspx. Accessed September 1, 2017.
Many new cancer drugs lack evidence of survival or QoL benefit
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
From BMJ
Key clinical point: Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) lacked conclusive evidence that they can extend or improve quality of life.
Major finding: With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA.
Data source: Retrospective cohort study of regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013.
Disclosures: The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation.