User login
Baseline lab data is class specific for biologics to treat psoriasis patients
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
New psoriasis therapies coming of age
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM
New treatments up the ante against acne
Acne remains “an equal opportunity annoyance,” according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, Newark, NJ.
However, acne medications also work equally well across age, gender, and skin type groups, and new systemic and topical options are emerging, said Dr. Baldwin, who serves as medical director of The Acne Treatment and Research Center in Morristown, NJ.
Several products that entered the market in 2016 have demonstrated success, she said in a presentation on acne at the annual Coastal Dermatology Symposium. She cited data on dapsone 7.5% gel (Aczone) applied daily, which showed significant improvements in moderate facial acne and lesion counts compared with vehicle.
A noteworthy new potential acne treatment combines 200 mg doxycycline with topical adapalene 0.3%/benzoyl peroxide 2.5%, Dr. Baldwin said. In a small but promising 12-week open-label study of patients aged 12 years and older with severe facial acne considered candidates for isotretinoin, inflammatory, noninflammatory, and overall total lesion counts reduced significantly from baseline, she said.
Other acne treatments in the pipeline include a nitric oxide gel, a topical sebum inhibitor, and minocycline gel and foam formulations.
Sarecycline, a tetracycline class antibiotic, has generated some excitement after a phase 2 dose ranging study presented at the 2017 American Academy of Dermatology’s annual meeting showed significant improvement in inflammatory lesion counts among acne patients who received 1.5 mg/kg or 3 mg/kg once a day compared with placebo patients, after 12 weeks. Noninflammatory lesion counts were not significantly improved compared with placebo. Potential advantages of sarecycline include improved efficacy with fewer side effects and possibly, a lower risk of antibiotic resistance, Dr. Baldwin said. Phase 3 study results of the 1.5 mg/kg dose are pending.
Data on the potential role of diet in acne continue to evolve, she noted. A recent study of 225 teens with acne suggested that skim and/or low-fat dairy products are associated with acne and that reducing consumption of these products might help (J Am Acad Dermatol. 2016 Aug;75[2]:318-22). Another small study of 64 adults involving a nutritional survey showed that those with moderate to severe acne consumed significantly more carbohydrates than did those without acne, an indication that clinicians could consider recommending that acne patients reduce their carbohydrate intake to see whether it makes a difference.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
Acne remains “an equal opportunity annoyance,” according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, Newark, NJ.
However, acne medications also work equally well across age, gender, and skin type groups, and new systemic and topical options are emerging, said Dr. Baldwin, who serves as medical director of The Acne Treatment and Research Center in Morristown, NJ.
Several products that entered the market in 2016 have demonstrated success, she said in a presentation on acne at the annual Coastal Dermatology Symposium. She cited data on dapsone 7.5% gel (Aczone) applied daily, which showed significant improvements in moderate facial acne and lesion counts compared with vehicle.
A noteworthy new potential acne treatment combines 200 mg doxycycline with topical adapalene 0.3%/benzoyl peroxide 2.5%, Dr. Baldwin said. In a small but promising 12-week open-label study of patients aged 12 years and older with severe facial acne considered candidates for isotretinoin, inflammatory, noninflammatory, and overall total lesion counts reduced significantly from baseline, she said.
Other acne treatments in the pipeline include a nitric oxide gel, a topical sebum inhibitor, and minocycline gel and foam formulations.
Sarecycline, a tetracycline class antibiotic, has generated some excitement after a phase 2 dose ranging study presented at the 2017 American Academy of Dermatology’s annual meeting showed significant improvement in inflammatory lesion counts among acne patients who received 1.5 mg/kg or 3 mg/kg once a day compared with placebo patients, after 12 weeks. Noninflammatory lesion counts were not significantly improved compared with placebo. Potential advantages of sarecycline include improved efficacy with fewer side effects and possibly, a lower risk of antibiotic resistance, Dr. Baldwin said. Phase 3 study results of the 1.5 mg/kg dose are pending.
Data on the potential role of diet in acne continue to evolve, she noted. A recent study of 225 teens with acne suggested that skim and/or low-fat dairy products are associated with acne and that reducing consumption of these products might help (J Am Acad Dermatol. 2016 Aug;75[2]:318-22). Another small study of 64 adults involving a nutritional survey showed that those with moderate to severe acne consumed significantly more carbohydrates than did those without acne, an indication that clinicians could consider recommending that acne patients reduce their carbohydrate intake to see whether it makes a difference.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
Acne remains “an equal opportunity annoyance,” according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, Newark, NJ.
However, acne medications also work equally well across age, gender, and skin type groups, and new systemic and topical options are emerging, said Dr. Baldwin, who serves as medical director of The Acne Treatment and Research Center in Morristown, NJ.
Several products that entered the market in 2016 have demonstrated success, she said in a presentation on acne at the annual Coastal Dermatology Symposium. She cited data on dapsone 7.5% gel (Aczone) applied daily, which showed significant improvements in moderate facial acne and lesion counts compared with vehicle.
A noteworthy new potential acne treatment combines 200 mg doxycycline with topical adapalene 0.3%/benzoyl peroxide 2.5%, Dr. Baldwin said. In a small but promising 12-week open-label study of patients aged 12 years and older with severe facial acne considered candidates for isotretinoin, inflammatory, noninflammatory, and overall total lesion counts reduced significantly from baseline, she said.
Other acne treatments in the pipeline include a nitric oxide gel, a topical sebum inhibitor, and minocycline gel and foam formulations.
Sarecycline, a tetracycline class antibiotic, has generated some excitement after a phase 2 dose ranging study presented at the 2017 American Academy of Dermatology’s annual meeting showed significant improvement in inflammatory lesion counts among acne patients who received 1.5 mg/kg or 3 mg/kg once a day compared with placebo patients, after 12 weeks. Noninflammatory lesion counts were not significantly improved compared with placebo. Potential advantages of sarecycline include improved efficacy with fewer side effects and possibly, a lower risk of antibiotic resistance, Dr. Baldwin said. Phase 3 study results of the 1.5 mg/kg dose are pending.
Data on the potential role of diet in acne continue to evolve, she noted. A recent study of 225 teens with acne suggested that skim and/or low-fat dairy products are associated with acne and that reducing consumption of these products might help (J Am Acad Dermatol. 2016 Aug;75[2]:318-22). Another small study of 64 adults involving a nutritional survey showed that those with moderate to severe acne consumed significantly more carbohydrates than did those without acne, an indication that clinicians could consider recommending that acne patients reduce their carbohydrate intake to see whether it makes a difference.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM
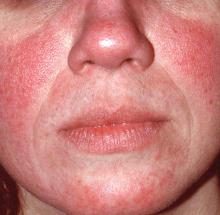
Rosacea: Expert recommends treating erythema, papules/pustules simultaneously
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Old and newer systemic therapies benefit patients with chronic eczema
Atopic dermatitis (AD) that becomes chronic and persists into adulthood often becomes less responsive to topical treatment with mid- to high-potency corticosteroids and calcineurin inhibitors, necessitating a different approach.
Joseph F. Fowler Jr., MD, discussed these treatment options at the annual Coastal Dermatology Symposium.
Older systemic medications
These include methotrexate, mycophenolate mofetil, cyclosporine, azathioprine, and retinoids. Methotrexate is predictably effective, and dermatologists generally are comfortable with it. The drug requires monitoring for adverse effects along with other precautions, similar to its use in psoriasis.
Mycophenolate mofetil is useful when the adverse event profiles of azathioprine, methotrexate, and cyclosporin A eliminate them from consideration, but it tends to confer slower improvement and has less efficacy overall.
Cyclosporin A led to successful outcomes in 77% of patients and mild improvement in 16% of patients in one trial, with milder side effects than those commonly seen in transplant patients. There was no increased risk of nephrotoxicity or hypertension over 6 months of treatment. The drug is useful for short-term control of flares and in contact dermatitis when corticosteroids are contraindicated, according to Dr. Fowler of the department of dermatology and director of occupational dermatitis at the University of Louisville (Ky.). It is the only drug other than corticosteroids that offers rapid improvement.
Newer drug options
One is dupilumab (Dupixent), an antibody that blocks interleukin (IL)–4 and IL-13. It received Food and Drug Administration approval in March 2017 for moderate to severe atopic dermatitis that doesn’t respond to topical treatment. Most patients get at least some benefit from the injectable drug, and some get a strong benefit, according to Dr. Fowler, although he pointed out that it can take 12 weeks before it achieves maximum effect. The initial dose is 600 mg administered subcutaneously, followed by 300 mg every 2 weeks. At 16 weeks, it reduced Eczema Area and Severity Index (EASI) scores by about 75% in patients taking a 300 mg dose every other week, compared with about a 20% decline in placebo.
The IL12/23 inhibitor ustekinumab (Stelara), approved for psoriasis and psoriatic arthritis, was effective in a case series of three patients. It led to a greater than 50% reduction in EASI score at week 16, following doses with 45 mg at weeks 0, 4, and 12. Biopsies revealed reductions in Th22 cells and cytokine levels. But, as a caveat, Dr. Fowler reported personal communication with two eczema experts who said they had seen little or no effect with ustekinumab in 10 patients.
The Janus kinase inhibitor tofacitinib, approved for rheumatoid arthritis, also is under investigation for AD. A trial in six patients who had not achieved adequate control with methotrexate or azathioprine showed a 67% improvement in the SCORAD (Scoring Atopic Dermatitis) index at doses of 5 mg of tofacitinib twice per day in five patients and 5 mg every other day in one patient, he said.
The PDE-4 inhibitor apremilast, approved for psoriasis and psoriatic arthritis, has been reported to improve AD symptoms in individual patients. Celgene demonstrated some improvement in a clinical trial of apremilast at doses of 30 or 40 mg twice per day, but the company isn’t pursuing AD as an indication, he noted.
Dr. Fowler has consulted for Abbvie, IntraDerm, and SmartPractice. He is on the speaker’s bureaus of SmartPractice and Regeneron/Sanofi. He has been a research investigator for Abbvie, Allergan, Amgen, Bayer, Dow, Galderma, Genentech, InnovaDerm, Johnson & Johnson, Lilly, Merck, Novartis, Pfizer, Precision Dermatology, Regeneron, SmartPractice, Taro, and Valeant. This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Atopic dermatitis (AD) that becomes chronic and persists into adulthood often becomes less responsive to topical treatment with mid- to high-potency corticosteroids and calcineurin inhibitors, necessitating a different approach.
Joseph F. Fowler Jr., MD, discussed these treatment options at the annual Coastal Dermatology Symposium.
Older systemic medications
These include methotrexate, mycophenolate mofetil, cyclosporine, azathioprine, and retinoids. Methotrexate is predictably effective, and dermatologists generally are comfortable with it. The drug requires monitoring for adverse effects along with other precautions, similar to its use in psoriasis.
Mycophenolate mofetil is useful when the adverse event profiles of azathioprine, methotrexate, and cyclosporin A eliminate them from consideration, but it tends to confer slower improvement and has less efficacy overall.
Cyclosporin A led to successful outcomes in 77% of patients and mild improvement in 16% of patients in one trial, with milder side effects than those commonly seen in transplant patients. There was no increased risk of nephrotoxicity or hypertension over 6 months of treatment. The drug is useful for short-term control of flares and in contact dermatitis when corticosteroids are contraindicated, according to Dr. Fowler of the department of dermatology and director of occupational dermatitis at the University of Louisville (Ky.). It is the only drug other than corticosteroids that offers rapid improvement.
Newer drug options
One is dupilumab (Dupixent), an antibody that blocks interleukin (IL)–4 and IL-13. It received Food and Drug Administration approval in March 2017 for moderate to severe atopic dermatitis that doesn’t respond to topical treatment. Most patients get at least some benefit from the injectable drug, and some get a strong benefit, according to Dr. Fowler, although he pointed out that it can take 12 weeks before it achieves maximum effect. The initial dose is 600 mg administered subcutaneously, followed by 300 mg every 2 weeks. At 16 weeks, it reduced Eczema Area and Severity Index (EASI) scores by about 75% in patients taking a 300 mg dose every other week, compared with about a 20% decline in placebo.
The IL12/23 inhibitor ustekinumab (Stelara), approved for psoriasis and psoriatic arthritis, was effective in a case series of three patients. It led to a greater than 50% reduction in EASI score at week 16, following doses with 45 mg at weeks 0, 4, and 12. Biopsies revealed reductions in Th22 cells and cytokine levels. But, as a caveat, Dr. Fowler reported personal communication with two eczema experts who said they had seen little or no effect with ustekinumab in 10 patients.
The Janus kinase inhibitor tofacitinib, approved for rheumatoid arthritis, also is under investigation for AD. A trial in six patients who had not achieved adequate control with methotrexate or azathioprine showed a 67% improvement in the SCORAD (Scoring Atopic Dermatitis) index at doses of 5 mg of tofacitinib twice per day in five patients and 5 mg every other day in one patient, he said.
The PDE-4 inhibitor apremilast, approved for psoriasis and psoriatic arthritis, has been reported to improve AD symptoms in individual patients. Celgene demonstrated some improvement in a clinical trial of apremilast at doses of 30 or 40 mg twice per day, but the company isn’t pursuing AD as an indication, he noted.
Dr. Fowler has consulted for Abbvie, IntraDerm, and SmartPractice. He is on the speaker’s bureaus of SmartPractice and Regeneron/Sanofi. He has been a research investigator for Abbvie, Allergan, Amgen, Bayer, Dow, Galderma, Genentech, InnovaDerm, Johnson & Johnson, Lilly, Merck, Novartis, Pfizer, Precision Dermatology, Regeneron, SmartPractice, Taro, and Valeant. This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Atopic dermatitis (AD) that becomes chronic and persists into adulthood often becomes less responsive to topical treatment with mid- to high-potency corticosteroids and calcineurin inhibitors, necessitating a different approach.
Joseph F. Fowler Jr., MD, discussed these treatment options at the annual Coastal Dermatology Symposium.
Older systemic medications
These include methotrexate, mycophenolate mofetil, cyclosporine, azathioprine, and retinoids. Methotrexate is predictably effective, and dermatologists generally are comfortable with it. The drug requires monitoring for adverse effects along with other precautions, similar to its use in psoriasis.
Mycophenolate mofetil is useful when the adverse event profiles of azathioprine, methotrexate, and cyclosporin A eliminate them from consideration, but it tends to confer slower improvement and has less efficacy overall.
Cyclosporin A led to successful outcomes in 77% of patients and mild improvement in 16% of patients in one trial, with milder side effects than those commonly seen in transplant patients. There was no increased risk of nephrotoxicity or hypertension over 6 months of treatment. The drug is useful for short-term control of flares and in contact dermatitis when corticosteroids are contraindicated, according to Dr. Fowler of the department of dermatology and director of occupational dermatitis at the University of Louisville (Ky.). It is the only drug other than corticosteroids that offers rapid improvement.
Newer drug options
One is dupilumab (Dupixent), an antibody that blocks interleukin (IL)–4 and IL-13. It received Food and Drug Administration approval in March 2017 for moderate to severe atopic dermatitis that doesn’t respond to topical treatment. Most patients get at least some benefit from the injectable drug, and some get a strong benefit, according to Dr. Fowler, although he pointed out that it can take 12 weeks before it achieves maximum effect. The initial dose is 600 mg administered subcutaneously, followed by 300 mg every 2 weeks. At 16 weeks, it reduced Eczema Area and Severity Index (EASI) scores by about 75% in patients taking a 300 mg dose every other week, compared with about a 20% decline in placebo.
The IL12/23 inhibitor ustekinumab (Stelara), approved for psoriasis and psoriatic arthritis, was effective in a case series of three patients. It led to a greater than 50% reduction in EASI score at week 16, following doses with 45 mg at weeks 0, 4, and 12. Biopsies revealed reductions in Th22 cells and cytokine levels. But, as a caveat, Dr. Fowler reported personal communication with two eczema experts who said they had seen little or no effect with ustekinumab in 10 patients.
The Janus kinase inhibitor tofacitinib, approved for rheumatoid arthritis, also is under investigation for AD. A trial in six patients who had not achieved adequate control with methotrexate or azathioprine showed a 67% improvement in the SCORAD (Scoring Atopic Dermatitis) index at doses of 5 mg of tofacitinib twice per day in five patients and 5 mg every other day in one patient, he said.
The PDE-4 inhibitor apremilast, approved for psoriasis and psoriatic arthritis, has been reported to improve AD symptoms in individual patients. Celgene demonstrated some improvement in a clinical trial of apremilast at doses of 30 or 40 mg twice per day, but the company isn’t pursuing AD as an indication, he noted.
Dr. Fowler has consulted for Abbvie, IntraDerm, and SmartPractice. He is on the speaker’s bureaus of SmartPractice and Regeneron/Sanofi. He has been a research investigator for Abbvie, Allergan, Amgen, Bayer, Dow, Galderma, Genentech, InnovaDerm, Johnson & Johnson, Lilly, Merck, Novartis, Pfizer, Precision Dermatology, Regeneron, SmartPractice, Taro, and Valeant. This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Working up patients with allergic contact dermatitis
When working up patients with allergic contact dermatitis (ACD), the patch test used may depend on how frequently testing is performed in the practice, and the type of allergies that are being evaluated, according to Joseph Fowler Jr., MD, of the department of dermatology at the University of Louisville (Ky).
T.R.U.E. TEST is more convenient than standard patch testing is but misses allergic contact dermatitis up to 40% of the time, he pointed out. The benefit of the T.R.U.E. TEST is that it’s easy to use, allergens come preapplied to a gel-based tape so there’s very little prep time, and they are well standardized with the same quantity on each patch every time.
T.R.U.E. TEST seems to work well for when testing for metal allergies, as well as allergies to topical antibiotics, steroids, and rubber, but not as well for dental implants, fragrances, newer preservatives, surfactants, acrylates, and some industrial and cosmetic allergens. It’s not so effective in many occupational settings, but even so, T.R.U.E. TEST is a good option when testing is performed infrequently, and “is much better than no patch testing at all,” according to Dr. Fowler, who spoke at the Annual Coastal Dermatology Symposium, jointly presented by the University of Louisville and Global Academy for Medical Education.
In a presentation on contact dermatitis and itch, he pointed out that what appears to be atopic dermatitis (AD) in a patient might actually be ACD and that ACD is common in patients with AD and complicates its treatment. Metals, fragrances, and topical components – namely lanolin and neomycin – are the most likely allergens to cause trouble in AD. Nickel allergy can be particularly problematic, causing severe lesions beyond the point of contact (Dermatitis. 2012 Nov-Dec;23[6]:275-80).
“Strongly consider patch testing any chronic, difficult to control atopic patient,” especially when AD is not affecting the typical areas – or spreads beyond them – and when it doesn’t respond to the usual treatments. The onset of AD beyond age 5 years is another clue that contact dermatitis might be at work. Patch testing atopic patients is “more likely to be helpful in disease management than scratch or RAST [radioallergosorbent] testing,” Dr. Fowler said.
It’s best if patch testing is done while patients are off immunosuppressants, but current immunosuppressive therapy should not be an absolute contraindication to testing, he said. Not all of them throw off the results. “You do not need to worry about patch testing a patient who is on antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.” However, when it comes to patch testing a patient on cyclosporine, tacrolimus, azathioprine, and mycophenolate mofetil, he said, “probably not” (Dermatitis. 2012 Nov-Dec;23[6]:301-3).
Pruritus might or might not be related to the skin issues. For itch caused by skin diseases such as scabies, dermatitis, or psoriasis, “treat the dermatosis to treat the itch,” Dr. Fowler said.
Several topicals can help while the skin problems are being tamed, including hypochlorous acid to stabilize mast cells; strontium 4% hydrogel; and compounded topical ketamine, amitriptyline, and lidocaine, which seems to be particularly helpful (J Am Acad Dermatol. 2017 Apr;76[4]:760-1). Other than for urticaria, antihistamines are of little use, except to provide sedation.
Renal disease, liver disease, lymphoma, and neurologic abnormalities are among the systemic problems that can cause itch; the giveaway is that there’s no primary skin disease, Dr. Fowler said. While systemic problems are being addressed, gabapentin, tricyclic antidepressants, and anxiolytics can help. For generalized pruritus, with no primary skin disease, a referral to a neurologist is essential, he said.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News. Dr. Fowler is a consultant, speaker, and/or researcher for a number of companies, including AbbVie, Regeneron/Sanofi, Allergan, Galderma, and Merck.
When working up patients with allergic contact dermatitis (ACD), the patch test used may depend on how frequently testing is performed in the practice, and the type of allergies that are being evaluated, according to Joseph Fowler Jr., MD, of the department of dermatology at the University of Louisville (Ky).
T.R.U.E. TEST is more convenient than standard patch testing is but misses allergic contact dermatitis up to 40% of the time, he pointed out. The benefit of the T.R.U.E. TEST is that it’s easy to use, allergens come preapplied to a gel-based tape so there’s very little prep time, and they are well standardized with the same quantity on each patch every time.
T.R.U.E. TEST seems to work well for when testing for metal allergies, as well as allergies to topical antibiotics, steroids, and rubber, but not as well for dental implants, fragrances, newer preservatives, surfactants, acrylates, and some industrial and cosmetic allergens. It’s not so effective in many occupational settings, but even so, T.R.U.E. TEST is a good option when testing is performed infrequently, and “is much better than no patch testing at all,” according to Dr. Fowler, who spoke at the Annual Coastal Dermatology Symposium, jointly presented by the University of Louisville and Global Academy for Medical Education.
In a presentation on contact dermatitis and itch, he pointed out that what appears to be atopic dermatitis (AD) in a patient might actually be ACD and that ACD is common in patients with AD and complicates its treatment. Metals, fragrances, and topical components – namely lanolin and neomycin – are the most likely allergens to cause trouble in AD. Nickel allergy can be particularly problematic, causing severe lesions beyond the point of contact (Dermatitis. 2012 Nov-Dec;23[6]:275-80).
“Strongly consider patch testing any chronic, difficult to control atopic patient,” especially when AD is not affecting the typical areas – or spreads beyond them – and when it doesn’t respond to the usual treatments. The onset of AD beyond age 5 years is another clue that contact dermatitis might be at work. Patch testing atopic patients is “more likely to be helpful in disease management than scratch or RAST [radioallergosorbent] testing,” Dr. Fowler said.
It’s best if patch testing is done while patients are off immunosuppressants, but current immunosuppressive therapy should not be an absolute contraindication to testing, he said. Not all of them throw off the results. “You do not need to worry about patch testing a patient who is on antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.” However, when it comes to patch testing a patient on cyclosporine, tacrolimus, azathioprine, and mycophenolate mofetil, he said, “probably not” (Dermatitis. 2012 Nov-Dec;23[6]:301-3).
Pruritus might or might not be related to the skin issues. For itch caused by skin diseases such as scabies, dermatitis, or psoriasis, “treat the dermatosis to treat the itch,” Dr. Fowler said.
Several topicals can help while the skin problems are being tamed, including hypochlorous acid to stabilize mast cells; strontium 4% hydrogel; and compounded topical ketamine, amitriptyline, and lidocaine, which seems to be particularly helpful (J Am Acad Dermatol. 2017 Apr;76[4]:760-1). Other than for urticaria, antihistamines are of little use, except to provide sedation.
Renal disease, liver disease, lymphoma, and neurologic abnormalities are among the systemic problems that can cause itch; the giveaway is that there’s no primary skin disease, Dr. Fowler said. While systemic problems are being addressed, gabapentin, tricyclic antidepressants, and anxiolytics can help. For generalized pruritus, with no primary skin disease, a referral to a neurologist is essential, he said.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News. Dr. Fowler is a consultant, speaker, and/or researcher for a number of companies, including AbbVie, Regeneron/Sanofi, Allergan, Galderma, and Merck.
When working up patients with allergic contact dermatitis (ACD), the patch test used may depend on how frequently testing is performed in the practice, and the type of allergies that are being evaluated, according to Joseph Fowler Jr., MD, of the department of dermatology at the University of Louisville (Ky).
T.R.U.E. TEST is more convenient than standard patch testing is but misses allergic contact dermatitis up to 40% of the time, he pointed out. The benefit of the T.R.U.E. TEST is that it’s easy to use, allergens come preapplied to a gel-based tape so there’s very little prep time, and they are well standardized with the same quantity on each patch every time.
T.R.U.E. TEST seems to work well for when testing for metal allergies, as well as allergies to topical antibiotics, steroids, and rubber, but not as well for dental implants, fragrances, newer preservatives, surfactants, acrylates, and some industrial and cosmetic allergens. It’s not so effective in many occupational settings, but even so, T.R.U.E. TEST is a good option when testing is performed infrequently, and “is much better than no patch testing at all,” according to Dr. Fowler, who spoke at the Annual Coastal Dermatology Symposium, jointly presented by the University of Louisville and Global Academy for Medical Education.
In a presentation on contact dermatitis and itch, he pointed out that what appears to be atopic dermatitis (AD) in a patient might actually be ACD and that ACD is common in patients with AD and complicates its treatment. Metals, fragrances, and topical components – namely lanolin and neomycin – are the most likely allergens to cause trouble in AD. Nickel allergy can be particularly problematic, causing severe lesions beyond the point of contact (Dermatitis. 2012 Nov-Dec;23[6]:275-80).
“Strongly consider patch testing any chronic, difficult to control atopic patient,” especially when AD is not affecting the typical areas – or spreads beyond them – and when it doesn’t respond to the usual treatments. The onset of AD beyond age 5 years is another clue that contact dermatitis might be at work. Patch testing atopic patients is “more likely to be helpful in disease management than scratch or RAST [radioallergosorbent] testing,” Dr. Fowler said.
It’s best if patch testing is done while patients are off immunosuppressants, but current immunosuppressive therapy should not be an absolute contraindication to testing, he said. Not all of them throw off the results. “You do not need to worry about patch testing a patient who is on antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.” However, when it comes to patch testing a patient on cyclosporine, tacrolimus, azathioprine, and mycophenolate mofetil, he said, “probably not” (Dermatitis. 2012 Nov-Dec;23[6]:301-3).
Pruritus might or might not be related to the skin issues. For itch caused by skin diseases such as scabies, dermatitis, or psoriasis, “treat the dermatosis to treat the itch,” Dr. Fowler said.
Several topicals can help while the skin problems are being tamed, including hypochlorous acid to stabilize mast cells; strontium 4% hydrogel; and compounded topical ketamine, amitriptyline, and lidocaine, which seems to be particularly helpful (J Am Acad Dermatol. 2017 Apr;76[4]:760-1). Other than for urticaria, antihistamines are of little use, except to provide sedation.
Renal disease, liver disease, lymphoma, and neurologic abnormalities are among the systemic problems that can cause itch; the giveaway is that there’s no primary skin disease, Dr. Fowler said. While systemic problems are being addressed, gabapentin, tricyclic antidepressants, and anxiolytics can help. For generalized pruritus, with no primary skin disease, a referral to a neurologist is essential, he said.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News. Dr. Fowler is a consultant, speaker, and/or researcher for a number of companies, including AbbVie, Regeneron/Sanofi, Allergan, Galderma, and Merck.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM