User login
Slow-wave sleep linked to Parkinson’s disease cognition
SAN DIEGO – Slow-wave sleep improves cognition in Parkinson’s disease, according to University of Alabama at Birmingham investigators.
After sleep studies, they compared the cognitive performance of 16 patients who spent more than 14% of their sleep time in slow-wave (SW) sleep with the cognitive performance of 16 who spent less than 14% in SW sleep; 13 of the patients in the low SW group (81%) met the criteria for mild Parkinson’s disease (PD) cognitive impairment versus 7 of the patients in the higher SW sleep group (44%); the patients were well matched for age, sex, disease duration, and other variables.
The normative value for SW sleep is about 15%-20%, although people spend less time in SW sleep as they age. The study subjects were in their mid-60s on average, so the 14% cut point wasn’t too far off from what might be considered typical.
More SW sleep in PD was associated with better performance on measures of global function, attention/working memory, executive function, and language comprehension. Patients in the low SW group, for instance, performed 0.25 standard deviations below the normative mean on attention/working memory tests, while subjects in the high SW group performed 0.5 standard deviations above, meaning that they outperformed people in their age group who didn’t have PD. These differences were statistically significant.
It raises the question of what can be done to help PD patients sleep better. “I’m interested in exercise to improve sleep, and we have some primary data that show it’s helpful for sleep in general but also SW sleep,” said Dr. Amara. Sodium oxybate (Xyrem) might also help, but for now, it’s a controlled substance approved only for narcolepsy. “We might have to branch out and try something novel,” she said.
The next step is to “see if we can use SW sleep to predict who might have cognitive declines and find interventions to [prevent] it,” she said.
Patients in the study had been diagnosed with PD for a mean of about 7 years. People in the high SW group were on lower amounts of levodopa equivalents, however, the investigators controlled for that, as well as for the fact that women tend to spend more time in SW sleep than men. There were no differences between the groups in measures of movement problems and of subjective scores of sleep quality and daytime sleepiness.
People with high SW sleep fell asleep sooner than did those in the low SW groups, about 9 minutes versus 20 minutes. There was no correlation between visual-spatial function and SW sleep, but visual-spatial function did correlate with the amount of time spent in rapid eye movement sleep, suggesting that dreaming might be important for visual-spatial function, Dr. Amara said.
The work was funded by the National Institutes of Health. Dr. Amara had no relevant disclosures.
SAN DIEGO – Slow-wave sleep improves cognition in Parkinson’s disease, according to University of Alabama at Birmingham investigators.
After sleep studies, they compared the cognitive performance of 16 patients who spent more than 14% of their sleep time in slow-wave (SW) sleep with the cognitive performance of 16 who spent less than 14% in SW sleep; 13 of the patients in the low SW group (81%) met the criteria for mild Parkinson’s disease (PD) cognitive impairment versus 7 of the patients in the higher SW sleep group (44%); the patients were well matched for age, sex, disease duration, and other variables.
The normative value for SW sleep is about 15%-20%, although people spend less time in SW sleep as they age. The study subjects were in their mid-60s on average, so the 14% cut point wasn’t too far off from what might be considered typical.
More SW sleep in PD was associated with better performance on measures of global function, attention/working memory, executive function, and language comprehension. Patients in the low SW group, for instance, performed 0.25 standard deviations below the normative mean on attention/working memory tests, while subjects in the high SW group performed 0.5 standard deviations above, meaning that they outperformed people in their age group who didn’t have PD. These differences were statistically significant.
It raises the question of what can be done to help PD patients sleep better. “I’m interested in exercise to improve sleep, and we have some primary data that show it’s helpful for sleep in general but also SW sleep,” said Dr. Amara. Sodium oxybate (Xyrem) might also help, but for now, it’s a controlled substance approved only for narcolepsy. “We might have to branch out and try something novel,” she said.
The next step is to “see if we can use SW sleep to predict who might have cognitive declines and find interventions to [prevent] it,” she said.
Patients in the study had been diagnosed with PD for a mean of about 7 years. People in the high SW group were on lower amounts of levodopa equivalents, however, the investigators controlled for that, as well as for the fact that women tend to spend more time in SW sleep than men. There were no differences between the groups in measures of movement problems and of subjective scores of sleep quality and daytime sleepiness.
People with high SW sleep fell asleep sooner than did those in the low SW groups, about 9 minutes versus 20 minutes. There was no correlation between visual-spatial function and SW sleep, but visual-spatial function did correlate with the amount of time spent in rapid eye movement sleep, suggesting that dreaming might be important for visual-spatial function, Dr. Amara said.
The work was funded by the National Institutes of Health. Dr. Amara had no relevant disclosures.
SAN DIEGO – Slow-wave sleep improves cognition in Parkinson’s disease, according to University of Alabama at Birmingham investigators.
After sleep studies, they compared the cognitive performance of 16 patients who spent more than 14% of their sleep time in slow-wave (SW) sleep with the cognitive performance of 16 who spent less than 14% in SW sleep; 13 of the patients in the low SW group (81%) met the criteria for mild Parkinson’s disease (PD) cognitive impairment versus 7 of the patients in the higher SW sleep group (44%); the patients were well matched for age, sex, disease duration, and other variables.
The normative value for SW sleep is about 15%-20%, although people spend less time in SW sleep as they age. The study subjects were in their mid-60s on average, so the 14% cut point wasn’t too far off from what might be considered typical.
More SW sleep in PD was associated with better performance on measures of global function, attention/working memory, executive function, and language comprehension. Patients in the low SW group, for instance, performed 0.25 standard deviations below the normative mean on attention/working memory tests, while subjects in the high SW group performed 0.5 standard deviations above, meaning that they outperformed people in their age group who didn’t have PD. These differences were statistically significant.
It raises the question of what can be done to help PD patients sleep better. “I’m interested in exercise to improve sleep, and we have some primary data that show it’s helpful for sleep in general but also SW sleep,” said Dr. Amara. Sodium oxybate (Xyrem) might also help, but for now, it’s a controlled substance approved only for narcolepsy. “We might have to branch out and try something novel,” she said.
The next step is to “see if we can use SW sleep to predict who might have cognitive declines and find interventions to [prevent] it,” she said.
Patients in the study had been diagnosed with PD for a mean of about 7 years. People in the high SW group were on lower amounts of levodopa equivalents, however, the investigators controlled for that, as well as for the fact that women tend to spend more time in SW sleep than men. There were no differences between the groups in measures of movement problems and of subjective scores of sleep quality and daytime sleepiness.
People with high SW sleep fell asleep sooner than did those in the low SW groups, about 9 minutes versus 20 minutes. There was no correlation between visual-spatial function and SW sleep, but visual-spatial function did correlate with the amount of time spent in rapid eye movement sleep, suggesting that dreaming might be important for visual-spatial function, Dr. Amara said.
The work was funded by the National Institutes of Health. Dr. Amara had no relevant disclosures.
AT ANA 2017
Key clinical point:
Major finding: Thirteen patients in the low SW group (81%) met criteria for mild Parkinson’s disease (PD) cognitive impairment versus seven (44%) in the higher SW sleep group.
Data source: Cognitive testing of 32 patients with PD stratified by amount of SW sleep
Disclosures: The work was funded by the National Institutes of Health. The presenter had no relevant disclosures.
Post-Ebola syndrome includes neurologic sequelae
SAN DIEGO – Add neurologic issues to the growing list of medical problems faced by survivors of Ebola virus.
Among 153 Liberian patients about a year out from their acute illness, “there were only a handful who didn’t have” some lingering neurologic problem. “The most commonly reported ongoing symptoms were headache and memory loss. A couple of people had seizures possibly related to Ebola.” Depression, anxiety, and posttraumatic stress disorder were common, said neurologist Jeanne Billioux, MD, a clinical fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md.
Almost two-thirds of the patients had abnormal neurologic exams. The most common findings were tremors, pathological reflexes, mild dysmetria, and abnormalities of eye pursuits and saccades, plus nystagmus. The findings were statistically significant, compared with 81 close contacts, generally household members, who served as controls in the ongoing natural history study, which was presented at the annual meeting of the American Neurological Association.
What’s become clear in the wake of the recent outbreak in West Africa, by far the worst to date with over 28,000 cases and more than 11,000 deaths, is that there is a post-Ebola syndrome that includes ophthalmologic, cardiac, and rheumatologic problems. It now appears that “neurologic sequelae are a part of it,” as well, she said.
The natural history study – dubbed the Partnership for Research on Ebola Virus in Liberia (PREVAIL III) – is a collaboration between the National Institutes of Health and the Ministry of Health of Liberia, one of the hardest-hit countries; the neurology investigation is just one component of the study, which includes about 1,500 patients overall.
Serology testing confirmed that cases truly did have Ebola, and the controls did not. After the first evaluation a year or so after the acute illness, patients have been followed up every 6 months, with some out to about 3 years.
Although patients aren’t back to normal, the good news is that their symptoms and exams are improving. “We started with only 6 who had no symptoms; now we have 15. Headaches are getting better; memory is getting better. It’s wonderful,” Dr. Billioux said.
The patients were asked to recall their acute symptoms during their first study visit. Many reported headaches, weakness, altered mental status, and cranial nerve symptoms. About 2% described convulsions or strokelike symptoms, and about 25% described symptoms consistent with meningitis.
It’s unclear how the virus affected the CNS, which isn’t considered to be a target organ. Perhaps fluid loss from severe diarrhea led to cerebral hypoperfusion. The cytokine storm during the acute phase might also have played a role. The virus has, however, been isolated from cerebral spinal fluid and, although uncommon, there are the reports of meningitis symptoms, so perhaps it does have direct CNS effects. Much remains to be learned.
Both cases and controls were a mean of about 35 years old, and evenly split between the sexes; 108 patients (70.6%) spent more than 2 weeks in an Ebola treatment unit.
The work was funded by the National Institutes of Health. Dr. Billioux had no disclosures.
SAN DIEGO – Add neurologic issues to the growing list of medical problems faced by survivors of Ebola virus.
Among 153 Liberian patients about a year out from their acute illness, “there were only a handful who didn’t have” some lingering neurologic problem. “The most commonly reported ongoing symptoms were headache and memory loss. A couple of people had seizures possibly related to Ebola.” Depression, anxiety, and posttraumatic stress disorder were common, said neurologist Jeanne Billioux, MD, a clinical fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md.
Almost two-thirds of the patients had abnormal neurologic exams. The most common findings were tremors, pathological reflexes, mild dysmetria, and abnormalities of eye pursuits and saccades, plus nystagmus. The findings were statistically significant, compared with 81 close contacts, generally household members, who served as controls in the ongoing natural history study, which was presented at the annual meeting of the American Neurological Association.
What’s become clear in the wake of the recent outbreak in West Africa, by far the worst to date with over 28,000 cases and more than 11,000 deaths, is that there is a post-Ebola syndrome that includes ophthalmologic, cardiac, and rheumatologic problems. It now appears that “neurologic sequelae are a part of it,” as well, she said.
The natural history study – dubbed the Partnership for Research on Ebola Virus in Liberia (PREVAIL III) – is a collaboration between the National Institutes of Health and the Ministry of Health of Liberia, one of the hardest-hit countries; the neurology investigation is just one component of the study, which includes about 1,500 patients overall.
Serology testing confirmed that cases truly did have Ebola, and the controls did not. After the first evaluation a year or so after the acute illness, patients have been followed up every 6 months, with some out to about 3 years.
Although patients aren’t back to normal, the good news is that their symptoms and exams are improving. “We started with only 6 who had no symptoms; now we have 15. Headaches are getting better; memory is getting better. It’s wonderful,” Dr. Billioux said.
The patients were asked to recall their acute symptoms during their first study visit. Many reported headaches, weakness, altered mental status, and cranial nerve symptoms. About 2% described convulsions or strokelike symptoms, and about 25% described symptoms consistent with meningitis.
It’s unclear how the virus affected the CNS, which isn’t considered to be a target organ. Perhaps fluid loss from severe diarrhea led to cerebral hypoperfusion. The cytokine storm during the acute phase might also have played a role. The virus has, however, been isolated from cerebral spinal fluid and, although uncommon, there are the reports of meningitis symptoms, so perhaps it does have direct CNS effects. Much remains to be learned.
Both cases and controls were a mean of about 35 years old, and evenly split between the sexes; 108 patients (70.6%) spent more than 2 weeks in an Ebola treatment unit.
The work was funded by the National Institutes of Health. Dr. Billioux had no disclosures.
SAN DIEGO – Add neurologic issues to the growing list of medical problems faced by survivors of Ebola virus.
Among 153 Liberian patients about a year out from their acute illness, “there were only a handful who didn’t have” some lingering neurologic problem. “The most commonly reported ongoing symptoms were headache and memory loss. A couple of people had seizures possibly related to Ebola.” Depression, anxiety, and posttraumatic stress disorder were common, said neurologist Jeanne Billioux, MD, a clinical fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md.
Almost two-thirds of the patients had abnormal neurologic exams. The most common findings were tremors, pathological reflexes, mild dysmetria, and abnormalities of eye pursuits and saccades, plus nystagmus. The findings were statistically significant, compared with 81 close contacts, generally household members, who served as controls in the ongoing natural history study, which was presented at the annual meeting of the American Neurological Association.
What’s become clear in the wake of the recent outbreak in West Africa, by far the worst to date with over 28,000 cases and more than 11,000 deaths, is that there is a post-Ebola syndrome that includes ophthalmologic, cardiac, and rheumatologic problems. It now appears that “neurologic sequelae are a part of it,” as well, she said.
The natural history study – dubbed the Partnership for Research on Ebola Virus in Liberia (PREVAIL III) – is a collaboration between the National Institutes of Health and the Ministry of Health of Liberia, one of the hardest-hit countries; the neurology investigation is just one component of the study, which includes about 1,500 patients overall.
Serology testing confirmed that cases truly did have Ebola, and the controls did not. After the first evaluation a year or so after the acute illness, patients have been followed up every 6 months, with some out to about 3 years.
Although patients aren’t back to normal, the good news is that their symptoms and exams are improving. “We started with only 6 who had no symptoms; now we have 15. Headaches are getting better; memory is getting better. It’s wonderful,” Dr. Billioux said.
The patients were asked to recall their acute symptoms during their first study visit. Many reported headaches, weakness, altered mental status, and cranial nerve symptoms. About 2% described convulsions or strokelike symptoms, and about 25% described symptoms consistent with meningitis.
It’s unclear how the virus affected the CNS, which isn’t considered to be a target organ. Perhaps fluid loss from severe diarrhea led to cerebral hypoperfusion. The cytokine storm during the acute phase might also have played a role. The virus has, however, been isolated from cerebral spinal fluid and, although uncommon, there are the reports of meningitis symptoms, so perhaps it does have direct CNS effects. Much remains to be learned.
Both cases and controls were a mean of about 35 years old, and evenly split between the sexes; 108 patients (70.6%) spent more than 2 weeks in an Ebola treatment unit.
The work was funded by the National Institutes of Health. Dr. Billioux had no disclosures.
AT ANA 2017
Key clinical point:
Major finding: A year after their acute infection, almost two-thirds of Ebola survivors had abnormal neurologic exams.
Data source: Natural history study involving 153 patients
Disclosures: The work was funded by the National Institutes of Health. The lead investigator had no disclosures.
Higher stroke risk found for TAVR versus SAVR
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
AT ANA 2017
Key clinical point:
Major finding: There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke.
Data source: Database review of more than 44,000 patients
Disclosures: Three was no industry funding for the work, and the lead investigator did not have any relevant disclosures.
CHMP recommends new formulation of pegaspargase
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) is recommending marketing authorization for lyophilized pegaspargase (ONCASPAR).
If approved, the product would be used as a component of antineoplastic therapy in patients of all ages who have acute lymphoblastic leukemia (ALL).
The product is a freeze-dried formulation of liquid pegaspargase, which is already approved for the aforementioned indication.
The CHMP’s recommendation regarding lyophilized pegaspargase will be submitted to the European Commission (EC).
The EC typically adheres to the CHMP’s recommendations and delivers its final decision within 67 days of the CHMP’s recommendation.
The EC’s decision will be applicable to the entire European Economic Area—all member states of the European Union plus Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation regarding lyophilized pegaspargase is based on analytical and nonclinical studies, which indicate that lyophilized pegaspargase is comparable to the liquid formulation.
Once reconstituted, lyophilized pegaspargase demonstrates similar pharmacokinetics and pharmacodynamics as liquid pegaspargase.
“Lyophilized ONCASPAR builds on more than a decade of data and research with liquid ONCASPAR, and, with no change in dosing regimen, it offers a 3-times longer shelf life,” said Howard B. Mayer, MD, of Shire, the company that developed lyophilized pegaspargase.
“Prolonging shelf life to 24 months for this critically important therapy facilitates management of product inventory by enabling greater flexibility and longer-term planning. Once approved, with the extended shelf life of lyophilized ONCASPAR, we also hope to improve access to the medicine for ALL patients in countries currently not offering liquid ONCASPAR.”
Lyophilized pegaspargase works in the same way as the liquid formulation. It rapidly depletes serum L-asparagine levels and interferes with protein synthesis, thereby depriving lymphoblasts of asparaginase and resulting in cell death. ![]()
Ciprofloxacin cured gyrA wild-type Neisseria gonorrhoeae infections
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
AT IDWEEK 2017
Key clinical point:
Major finding: The cure rate was 100% among 25 patients who received ciprofloxacin for wild-type gyrA gonorrhea.
Data source: A single-center study of 582 patients with gonorrhea.
Disclosures: The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
Rectal swabs concurred with stool tests in children with GI illness
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
AT IDWEEK 2017
Key clinical point: and cannot immediately obtain a bulk stool sample.
Major finding: Diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively.
Data source: A multicenter retrospective cohort study of 1,519 children and adolescents with acute-onset diarrhea, vomiting, or both.
Disclosures: Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
Steroids underused in bacterial meningitis despite low risk
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
AT IDWEEK 2017
Key clinical point:
Major finding: Five of 120 (4%) of patients developed delayed cerebral thrombosis. Only 40% received steroids within the maximum recommended time frame.
Data source: A retrospective multicenter study of 120 adults with culture-confirmed bacterial meningitis.
Disclosures: The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
C. auris: ‘A yeast that acts like a bacteria’
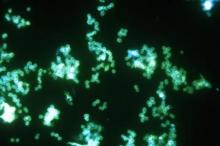
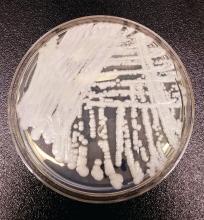
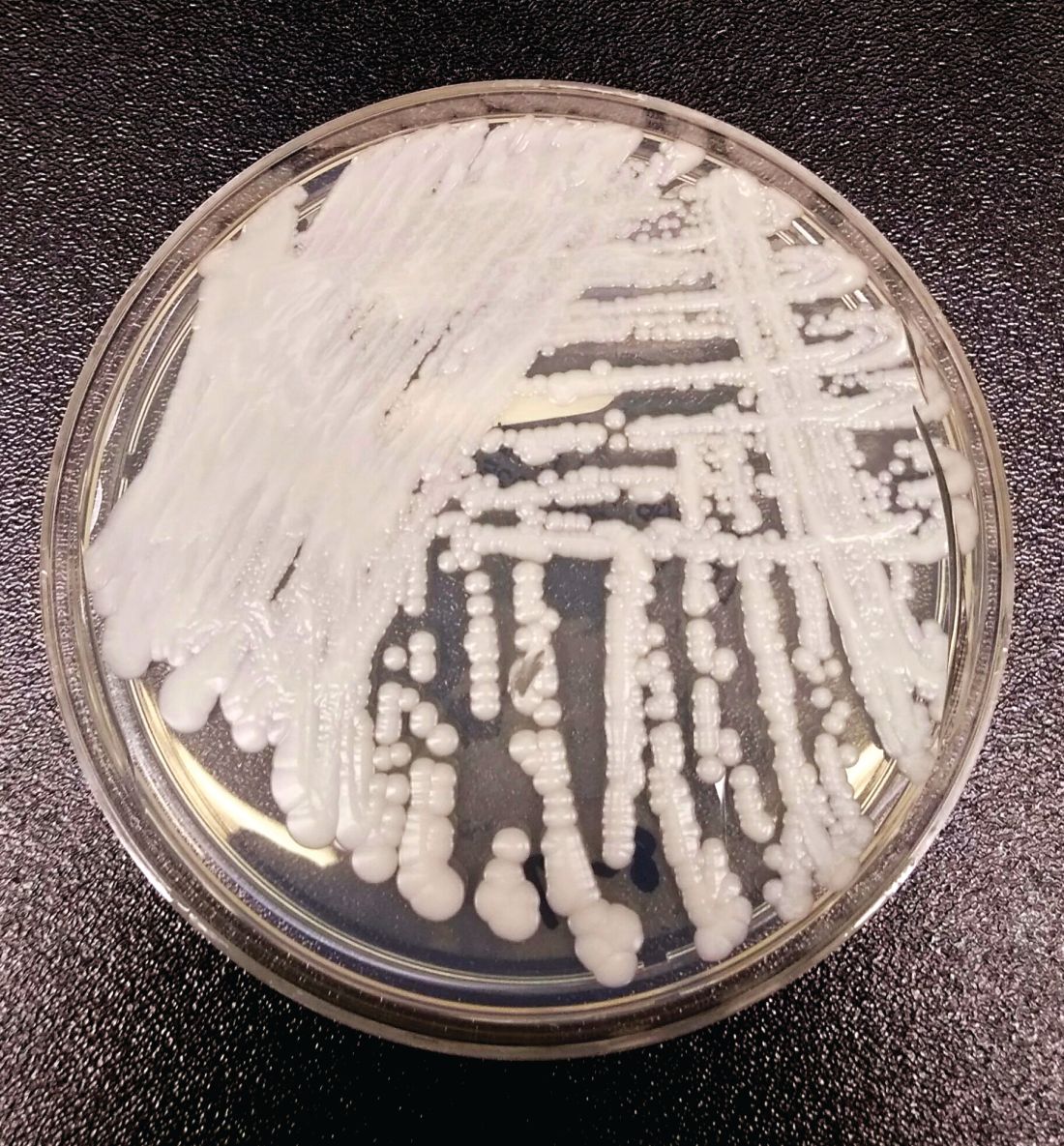
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Pediatric psoriasis carries sharply increased risk of selected autoimmune comorbidities
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.

Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.

Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Pediatric psoriasis patients were at an adjusted 6.6-fold increased risk of comorbid rheumatoid arthritis, 4.8-fold risk of vitiligo, and significantly increased risks of several other autoimmune diseases, compared with matched youths without psoriasis.
Data source: A cross-sectional study of all children and adolescents living in Denmark at the end of 2012.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
Metals may surprise as sources of contact dermatitis
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR