User login
MDedge Daily News: Why prior authorization may get easier
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A mitochondrial protein may help explain gender differences in cardiovascular risk, PPIs may boost the risk of C. difficile infections in hospitals, topical retinoids and benzoyl peroxide are tops for inflammatory acne, and the grueling prior authorization process may get easier.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Alex Azar confirmed as HHS Secretary
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Alex M. Azar II has been confirmed as secretary of the Department of Health & Human Services following a Jan. 24 vote in the U.S. Senate.
His nomination passed by a vote of 55-43.
Mr. Azar has previously been confirmed to two posts at HHS, first as general counsel and later as deputy HHS secretary during the administration of President George W. Bush between 2001 and 2007. Both appointments were confirmed by unanimous consent in the Senate.
Democrats on the Senate Finance Committee challenged Mr. Azar on drug prices and other issues, but their objections to his pharmaceutical industry past were not enough to stop his confirmation.
“Mr. Azar was part of this broken system [of high drug pricing], and despite the cheerful overtures that he has made to senators on the other side of the aisle over the last few weeks on how he wants to work on the issue, he has not given a single concrete example of how he would actually change the system, change the system that he said is broken,” Senate Finance Committee Ranking Member Ron Wyden (D-Ore.) said on the Senate floor. “He won’t give us an example of how he would change it to make it better.”
In a statement, Senate Health, Education, Labor, and Pensions Chairman Lamar Alexander (R-Tenn.) said that Mr. Azar “has the broad perspective necessary to address the opioid crisis.”
However, Sen. Maggie Hassan (D-N.H.) expressed concern about whether he could effectively address the opioid epidemic.
“I was disappointed that Mr. Azar would not commit to advocating for new funding during his confirmation hearing. Considering his tenure as a top executive at a major pharmaceutical company, I also continue to have serious doubts that Mr. Azar can be a leader in addressing the skyrocketing cost of prescription drugs,” she said.
Gene therapy moves from promise to reality
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
Uncovering Clues That Explain the Ototoxicity of Cisplatin
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Cisplatin and other platinum-based drugs are prescribed to 10% to 20% of patients with cancer. The drugs cause permanent hearing loss in as many as 80% of adult patients and at least half of children. Why is cisplatin so toxic to the inner ear?
Researchers from the National Institute on Deafness and other Communications Disorders may have found the answer. The inner ear readily takes up cisplatin but has little ability to remove the drug. In most areas of the body, cisplatin is eliminated within days or weeks after treatment; in the inner ear it remains much longer.
Using a mouse model, the researchers found cisplatin remained in the inner ear much longer than in most other body tissues and built up with each treatment. They also studied inner ear tissue donated by deceased patients who had been treated with cisplatin and found cisplatin remained in the inner ear many months or even years after treatment. They also examined inner ear tissue from one child, and found cisplatin buildup even higher than that seen in adults.
The highest buildup of cisplatin was in the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound, the researchers say. They believe that accumulation contributed to the hearing loss. “If we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment,” Lisa Cunningham, PhD, lead investigator says, “we may be able to protect cancer patients from developing cisplatin-induced hearing loss.”
Technique could aid treatment of CLL
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
Researchers say they have developed a new technique for assessing chromosomal abnormalities in chronic lymphocytic leukemia (CLL).
The team believes their method, called immuno-flowFISH, could be used at the time of CLL diagnosis for disease stratification and after treatment to assess residual disease.
Kathryn A. Fuller, PhD, of The University of Western Australia in Crawley, Australia, and her colleagues described immuno-flowFISH in the journal Methods.
The name “immuno-flowFISH” acknowledges what has been incorporated into this technology.
“Immuno” recognizes that immunology testing is used to identify the CLL cells. “Flow” is used because the machine is an imaging flow cytometer. And “FISH” is the test that identifies the chromosomes inside the cells.
The researchers said they found that immuno-flowFISH could detect trisomic chromosomal abnormalities in cells with the phenotype of CLL.
And immuno-flowFISH provided greater specificity and sensitivity than standard FISH.
In particular, the researchers were able to analyze 10,000 to 20,000 cells in each sample, which is 100 to 200 times greater than traditional FISH methods.
“The imaging cytometer can analyze samples at a rate of up to 2000 cells per second, which means we can investigate a large number of cells in a relatively short amount of time, giving us greater sensitivity,” Dr Fuller said.
“This immuno-flowFISH method is an exciting development in personalizing pathology testing for leukemia,” added study author Wendy N. Erber, MD, DPhil, PhD, of The University of Western Australia.
Dr Erber and her colleagues are now expanding immuno-flowFISH so it can be applied to other malignancies as well. ![]()
FDA approves test to diagnose MPNs
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
Adjunct to HSCT receives orphan designation
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
The European Commission (EC) has granted orphan designation to NLA101 as an adjunct to hematopoietic stem cell transplant (HSCT).
NLA101 is a universal, off-the-shelf, stem and progenitor cell therapy intended to provide a short-term bridge for hematopoietic recovery while also providing long-term immunologic and clinical benefits in HSCT recipients.
NLA101 is a product of Nohla Therapeutics Inc.
The company says more than 125 infusions of NLA101 have been administered since 2009. The therapy is under investigation in patients receiving intensive chemotherapy as well as in HSCT recipients.
Results from a pilot study of NLA101 in HSCT recipients were presented at the 2014 ASH Annual Meeting.
In this study, 15 patients with hematologic malignancies underwent myeloablative cord blood transplant, with or without NLA101.
Patients who received NLA101 had a significantly reduced median time to platelet and neutrophil recovery, compared to controls. At 5 years, disease-free survival was 86% in the NLA101 group and 67% in the control group.
The rate of grade 3-4 acute graft-versus-host disease was 0% in the NLA101 group and 29% in the control group. The rate of transplant-related mortality was 0% and 22%, respectively.
Phase 2 studies of NLA101 in chemotherapy and HSCT recipients are ongoing.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
EHR application doubles hypertension recognition rate
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
The rate of children and adolescents with elevated blood pressure going unrecognized is an increasingly concerning issue – one that is complicated because physicians lack a simple, single BP value system. Some have tried to fill the gap, creating simplified tables of BP values or automated displays of BP values in EHRs, but having a table that only takes age and sex into consideration for screening does not cover the complexities needed to identify hypertension.
Previous studies have looked into utilizing a clinical decision support (CDS) application, which has great potential as a digital multitool to improve quality of care, increase efficiency, and reduce medical errors. However, to be an effective CDS, it must fill the “CDS Five Rights” framework. This guideline states that a CDS tool needs to provide: “the right information, to the right people, through the right channels, in the right intervention formats, at the right points in the work flow.”
The TeenBP CDS developed by Kharbanda et al. fulfills these requirements and goes beyond any CDS previously designed. Even so, 45% of children with elevated BP or hypertension were not recognized, emphasizing the need for additional strategies outside of relying on new technology.
Visit summaries should be given to parents with BP readings so that they can monitor their children’s levels, for example.
Recognition of abnormal BP in teens is the first step toward preventing cardiovascular disease as an adult, and hopefully, the development of new tools, including this CDS, will help physicians find those children who have been overlooked.
Ari H. Pollack, MD, MSIM, is a pediatric nephrologist at the Seattle Children’s Hospital and an assistant professor of pediatrics at the University of Washington, Seattle. Joseph T. Flynn, MD, MS, is the division chief of nephrology in prenatal diagnosis and treatment at the Seattle Children’s Hospital and a professor of pediatrics at the same university. Dr. Pollack and Dr. Flynn reported no relevant financial disclosures in their commentary in Pediatrics (2018. doi: 10.1542/peds.2017-3756).
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
Hypertension discovery in pediatric patients more than doubled for physicians using a clinical decision support (CDS) tool connected to the EHR, results of a study found.
Elyse O. Kharbanda, MD, MPH, a researcher at the HealthPartners Institute, Minneapolis, and her fellow investigators assert that using such a tool will help rectify the trend of underreported hypertension in adolescents, which remains a serious concern despite providers’ routinely taking blood pressure measurements during outpatient visits.
“Among patients with multiple visits, electronic health records should contain sufficient information to diagnose hypertension,” Dr. Kharbanda and her associates reported in their article published in Pediatrics. “However, even when EHRs are configured to display BP percentiles, information on the patterns of BP percentiles over time, previous diagnoses, and medications is not presented in a format that is useful for clinicians.”
With TeenBP, providers are first prompted to take an initial BP reading, as well as height and weight measurements.
If the first measure is above the 95th percentile, the CDS requests an additional reading, which is then averaged with the first. If average of the two is above or within the 95th percentile, the provider is notified and sent a list of recommendations, including a diagnosis of hypertension, lipid screening, and nutrition referral.
The 2-year trial included 522 pediatric patients with incident hypertension; the data were gathered from 20 primary care clinics within one health system between April 2014 and April 2016.
The rate of clinical recognition of patients’ hypertension in the clinics utilizing the CDS tool was more than double the rate seen in the clinics that weren’t (55% and 21%, respectively; P less than .001).
More of the children seen in CDS clinics were referred to dietitians or weight loss programs, compared with those seen in the control clinics (17% and 4%, respectively; P = .001).
Those who used the tool reported high levels of satisfaction, which is likely partly because investigators consulted physicians to help design the application.
“The CDS tool was based on the guidelines for BP management in children and adolescents in effect at the time of the study with local input from clinical and operational leaders within the medical group, and thus it contained the so-called right information,” according to Dr. Kharbanda and her fellow investigators.
Of the 55 physicians who remembered using the tool, 92% thought is was useful in identifying hypertension, 94% considered the CDS a good use of time, and 95% believed is was a useful shared-decision making tool.
When designing TeenBP, investigators tailored the application to the work flow and culture of the health system used for the study, which may limit the generalizability of the findings.
The study was funded by the National Institutes of Health. Dr. Kharbanda and her associates reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
FROM PEDIATRICS
Key clinical point: Using a clinical decision support (CDS) tool doubles the rate of hypertension detection in children.
Major finding: Providers in clinics that used CDS recognized hypertension in 55% of patients, compared with 21% of patients in usual care (P less than .001).
Study details: Cluster-randomized trial of 522 pediatric patients across 20 primary care clinics who received care between April 2014 and April 2016.
Disclosures: The study was funded by the National Institutes of Health. The investigators reported no relevant financial disclosures.
Source: Kharbanda EO et al. Pediatrics. 2018. doi: 10.1542/peds.2017- 2954.
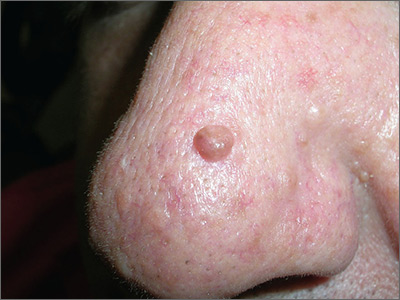
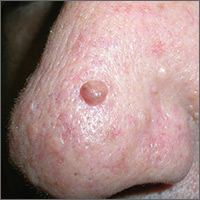
Hyperpigmented growth on nose
At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
At follow-up 2 weeks later, pathology revealed a diagnosis of sebaceous hyperplasia (SH). On close examination of the face and nose, the FP noted other nonpigmented SHs and telangiectasias, which confirmed an overall diagnosis of rosacea.
The FP explained to the patient that SH is benign and that there are treatments for rosacea. In this case, the FP prescribed 1% metronidazole gel for the rosacea. At follow-up one month later, the shave biopsy had effectively removed the SH and the site had healed well. The metronidazole gel was also improving the appearance of the patient’s face. The patient indicated that he’d been avoiding the sun and drinking less alcohol, which was helping the rosacea and was, of course, good for his overall health.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Sebaceous hyperplasia. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 931-934.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Three in 10 diabetic patients may have liver fibrosis
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
LOS ANGELES – For every 10 adult patients with type 2 diabetes, three are likely to have moderate to severe liver fibrosis, according to Kenneth Cusi, MD, FACP, FACE.
“The question is, How are we going to tackle this problem? My academic goal is that we incorporate screening for NASH [nonalcoholic steatohepatitis], or for fibrosis more specifically, in the same way we do for retinopathy or nephropathy [in diabetes], because we do have a way to treat it,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Dr. Cusi, chief of the division of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, predicted that obesity will become the No. 1 cause of liver transplantation. “It’s a real epidemic; you’re not seeing it because the inflexion of obesity happened just 2 decades ago,” he said. “Patients with diabetes face the greatest risk of fatty liver and of fibrosis. Untreated, it’s the equivalent of having macroalbuminuria. If you do nothing and they don’t die of cardiovascular disease, they’re going to have a good chance of getting fibrosis.”
As part of the large population-based Rotterdam study of individuals aged 45 years and older, researchers found that liver stiffness of 8 kPa or more by transient elastography was present in 5.6% of the study participants and was strongly associated with steatosis and diabetes (Hepatology. 2016;63:138-47). According to Dr. Cusi, individuals who have steatosis without diabetes face a 5%-10% risk of fibrosis, while those with steatosis and diabetes face a 15%-20% risk. “It’s well established in a number of studies that if you have fibrosis, you’re at high risk not only of cirrhosis, but also of hepatocellular carcinoma,” he said. “The key thing is not detecting fat, which is not really the target. The target is if there’s fibrosis or not.” Three ways to assess for fibrosis include MR elastography, transient elastography (which is the most commonly used), and fibrosis marker panels.
Liver fibrosis likely starts with adipose tissue dysfunction, said Dr. Cusi, who authored a review on the pathophysiology of interactions between adipose tissue and target organs in obesity and the resulting clinical implications for the management of nonalcoholic steatohepatitis (Gastroenterology. 2012;142[4]:711-25.e6). “When you have insulin-resistant, sick adipose tissue, that leads to the accumulation of fat in the liver,” he said. “. Even if you get people who are matched for BMIs [body mass indexes] between 30 and 35 kg/m2, there is a spectrum in which some individuals have very insulin-resistant adipose tissue and others less so. I would say that 1 out of 10 are metabolically healthy, and we don’t understand exactly why.”
In a recent cross-sectional analysis of 352 healthy individuals, Dr. Cusi and his associates found that intrahepatic triglyceride (IHTG) accumulation is strongly associated with adipose tissue insulin resistance, supporting the current theory of lipotoxicity as a driver of IHTG accumulation (Hepatology. 2017;65[4]:1132-44). The researchers observed that once IHTG accumulation reaches about 6%, skeletal muscle insulin resistance, hypertriglyceridemia, and low HDL cholesterol become fully established. “The next question is, How does this correlate with NASH?” Dr. Cusi said. “Our take is that there is a threshold effect. Once you have a critical amount of triglycerides in your liver, some individuals are going to activate pathways that are harmful. NASH is not something exclusive to individuals who are obese. Lean people can also develop NASH. The key feature is insulin resistance, not metabolic syndrome. Once you develop a fatty liver, your chances of NASH are comparable to that of an obese individual. The paradox is that lean individuals get a fatty liver, but when they get a fatty liver, they are at risk for NASH and for fibrosis.”
Why lean individuals develop NASH is not fully understood, but Dr. Cusi said he suspects that the problem develops at the mitochondrial level. Results from an unpublished animal model in which mice were fed a high–trans-fat diet for 24 weeks showed that the mice developed steatosis by week 8 and NASH by week 24. The mice had an increase in the tricarboxylic acid (TCA) cycle, which is typical of the NASH period, as well as an increase in ceramides. “Perhaps a unifying hypothesis would be that the development of NASH is linked to inflammation and to insulin signaling,” Dr. Cusi said. “Not surprisingly, it had a number of effects on the mitochondria, and in this animal model it decreases the TCA.” He noted that the biology of fibrosis remains unknown in humans. “What we have been familiar with is the high-triglyceride, low-HDL pattern,” he said. “If you look at how that correlates with the amount of liver fat, it is basically a threshold effect. Once you have steatosis, you don’t see much worse dyslipidemia, which is typical of these patients.”
Recently published guidance from the American Association for the Study of Liver Diseases on the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) suggests that patients require a weight loss of 3%-5% to improve steatosis, but a loss of 7%-10% to improve most histologic features of NASH, including fibrosis (Hepatology. 2018;67[1]:328-57). Exercise alone may prevent or reduce steatosis, but its ability to improve other aspects of liver histology remains unknown. Bariatric surgery can be considered in otherwise eligible obese individuals with NAFLD or NASH. The procedure’s impact on fibrosis is unknown.
The AASLD practice guideline notes that metformin is not recommended for treating NASH in adult patients, but pioglitazone improves liver histology in patients with and without type 2 diabetes with biopsy-proven NASH. “Pioglitazone has had the greatest benefit in terms of treatment effect, compared to placebo,” Dr. Cusi said. “It’s a generic drug; at the VA [Veterans Affairs], it costs 8 cents per tablet. I think that pioglitazone will be to NASH what metformin has been to type 2 diabetes. The most common side effect is weight gain, typically between 4 and 9 lb. Risks and benefits should be discussed with each patient. It should not be used for NAFLD without biopsy-proven NASH.” The guideline goes on to say that it’s currently premature to consider GLP-1 (glucagonlike peptide–1) agonists for treating liver disease in patients with NAFLD or NASH. Meanwhile, vitamin E at 800 IU has been shown to improve liver histology in nondiabetic adults with NASH, but the risks and benefits should be discussed with each patient. Vitamin E is not recommended for NASH in diabetic patients, NAFLD without a liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.
The AASLD practice guideline also states that the best evidence for using SGLT2 (sodium-glucose cotransporter–2) inhibitors in NAFLD comes from animal studies, which report a reduction in steatosis with and without weight loss. Clinical studies reporting a reduction in steatosis are limited. There are positive observational studies with a reduction in alanine aminotransferase and some studies that have shown a reduction in liver fat. “For me, the best option is to tailor treatment to the pathophysiology of the disease,” Dr. Cusi said. “You reduce fat by weight loss in some way, or you change the biology of fat with a thiazolidinedione.”
Dr. Cusi reported that he has received grant support from the Burroughs Wellcome Fund, the American Diabetes Association, and the National Institutes of Health.
EXPERT ANALYSIS FROM WCIRDC 2017