User login
Cancer groups offer guidance on immune-related adverse events
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
Flu activity continues to decline
The 2017-2018 flu season continued to loosen its grip on the country as both outpatient activity and pediatric deaths dropped during the week ending March 3, according to the Centers for Disease Control and Prevention.
After five consecutive weeks of double-digit pediatric deaths related to influenza-like illness (ILI), five deaths were reported for the week ending March 3, four of which occurred in previous weeks. The total for the 2017-2018 season is now 119, the CDC said in its weekly surveillance report.
The proportion of outpatient visits for ILI was 3.7% for the week, which is down from 4.9% the week before and less than half of the seasonal high of 7.5% that was recorded for the week of Feb. 3, CDC data show. The national baseline level of outpatient activity is 2.2%.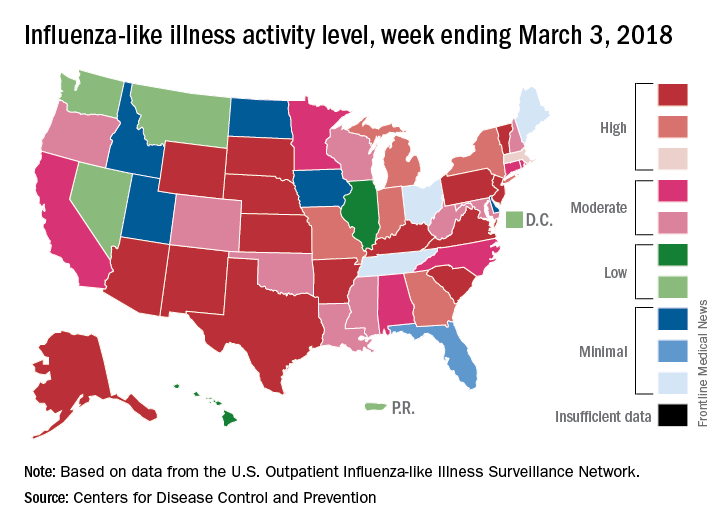
The cumulative hospitalization rate for the 2017-2018 flu season climbed from 84.2 the previous week to 86.3 per 100,000 population – well above the rate of 57.2 per 100,000 that was recorded for the corresponding week of the hospitalization-record-setting 2014-2015 season, FluView data show.
The 2017-2018 flu season continued to loosen its grip on the country as both outpatient activity and pediatric deaths dropped during the week ending March 3, according to the Centers for Disease Control and Prevention.
After five consecutive weeks of double-digit pediatric deaths related to influenza-like illness (ILI), five deaths were reported for the week ending March 3, four of which occurred in previous weeks. The total for the 2017-2018 season is now 119, the CDC said in its weekly surveillance report.
The proportion of outpatient visits for ILI was 3.7% for the week, which is down from 4.9% the week before and less than half of the seasonal high of 7.5% that was recorded for the week of Feb. 3, CDC data show. The national baseline level of outpatient activity is 2.2%.
The cumulative hospitalization rate for the 2017-2018 flu season climbed from 84.2 the previous week to 86.3 per 100,000 population – well above the rate of 57.2 per 100,000 that was recorded for the corresponding week of the hospitalization-record-setting 2014-2015 season, FluView data show.
The 2017-2018 flu season continued to loosen its grip on the country as both outpatient activity and pediatric deaths dropped during the week ending March 3, according to the Centers for Disease Control and Prevention.
After five consecutive weeks of double-digit pediatric deaths related to influenza-like illness (ILI), five deaths were reported for the week ending March 3, four of which occurred in previous weeks. The total for the 2017-2018 season is now 119, the CDC said in its weekly surveillance report.
The proportion of outpatient visits for ILI was 3.7% for the week, which is down from 4.9% the week before and less than half of the seasonal high of 7.5% that was recorded for the week of Feb. 3, CDC data show. The national baseline level of outpatient activity is 2.2%.
The cumulative hospitalization rate for the 2017-2018 flu season climbed from 84.2 the previous week to 86.3 per 100,000 population – well above the rate of 57.2 per 100,000 that was recorded for the corresponding week of the hospitalization-record-setting 2014-2015 season, FluView data show.
IBD: When to operate and when to punt
LAS VEGAS – Patients with inflammatory bowel disease who are in need of a surgical intervention can pose a special challenge to surgeons who encounter these patients only occasionally.
The question of whether to perform surgery or refer a patient to a higher-volume specialty center can depend on proximity. In some cases, a specialty center isn’t close, or the patient can’t tolerate the required travel. In fact, a recent study showed that 85.8% of IBD patients are treated surgically in hospitals that treat fewer than 50 patients per year (Am J Gastroenterol. 2008;103:2789-98).
In a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education, Larry Whelan, MD, FACS, discussed some of the challenges these patients pose and offered guidance on which cases are best referred to high-volume centers, and the best way to proceed in emergencies.
IBD patients at high-volume centers have lower mortality than do those in low-volume centers, but patients treated at lower-volume centers tend to be sicker, and studies have shown no significant difference in complication rate. This suggests that surgeons shouldn’t be afraid to tackle these cases, according to Dr. Whelan, chief of colorectal surgery at Mount Sinai West, New York.
"If you get an IBD case and you don’t see a lot of those, how do you decide what to do about it, and should you just refer it to a high-volume center?” said Dr. Whelan.
These patients are often under complex medical management, frequently spanning years, and this is an important factor in surgical decisions. They are often on multiple medications, including steroids, and most patients these days are taking monoclonal antibodies, said Dr. Whelan. The latter in particular can lead patients to be susceptible to infections. “These things can all affect decision making,” said Dr. Whelan.
Sometimes the nutritional status of IBD patients is poor, and most of the time, surgery is elective in these patients. So surgery can often be delayed for a month or more to allow time for nutritional status to improve, and this gives time for a patient to go off monoclonal antibodies, and for the physician to arrange for a referral to a high-volume center, if that seems the wisest course.
Surgery should not be considered without a gastrointestinal specialist who is comfortable in managing these patients. “Having someone who knows when to operate and not to operate, and how to handle medication, is really important,” said Dr. Whelan.
Certain cases should definitely be referred out. Ileal pouches are one. Another is a Crohn’s disease patient with multiple points of obstruction. “That may be one that you’re better off to punt,” said Dr. Whelan. Other cases include patients under complex medical management, when there is no experienced GI specialist available to help.
Emergencies require quicker decisions. In ulcerative colitis, emergency cases may include toxic megacolon, perforated colon, or obstruction from either a stricture or cancer, as well as bleeding in rare cases. Scenarios in Crohn’s disease include perforation with sepsis, inaccessible abscess, and, most commonly, obstruction resulting from fibrous stricture or acute inflammation.
When surgery is required, what’s the best choice? Dr. Whelan emphasized keeping it simple. Redo ileal pouches and ileal pouch excisions should generally be avoided. “Even if you do [pouches] often. It’s not the smartest way to go. These patients are almost all on immunosuppressive medications … to make an operation that’s already big even bigger often doesn’t work out well,” he said.
In emergency chronic ulcerative colitis cases, the safest choice is total abdominal colectomy plus end ileostomy. Dr. Whelan discourages surgeons from considering proctectomy and ileal pouch in emergency cases. A number of studies have shown that delaying pouch surgery is associated with fewer minor and major adverse events, and lower reoperation rates, he said. “If you do these operations on an immunosuppressed population, they don’t do as well,” said Dr. Whelan.
Crohn’s disease emergencies can often be managed nonsurgically. Most patients have phlegmon, fistulae, or a partial obstruction. Intravenous antibiotics, percutaneous drainage, hydration, and boosting nutritional status are good options. In cases where an obstruction requires surgery, and the surgeon isn’t comfortable performing stricturoplasty, “you want to limit the resection as best you can,” he said.
Dr. Whelan disclosed financial relationships with Ethicon Endosurgery and Olympus Corporation. Global Academy for Medical Education and this news organization are owned by the same parent company.”
LAS VEGAS – Patients with inflammatory bowel disease who are in need of a surgical intervention can pose a special challenge to surgeons who encounter these patients only occasionally.
The question of whether to perform surgery or refer a patient to a higher-volume specialty center can depend on proximity. In some cases, a specialty center isn’t close, or the patient can’t tolerate the required travel. In fact, a recent study showed that 85.8% of IBD patients are treated surgically in hospitals that treat fewer than 50 patients per year (Am J Gastroenterol. 2008;103:2789-98).
In a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education, Larry Whelan, MD, FACS, discussed some of the challenges these patients pose and offered guidance on which cases are best referred to high-volume centers, and the best way to proceed in emergencies.
IBD patients at high-volume centers have lower mortality than do those in low-volume centers, but patients treated at lower-volume centers tend to be sicker, and studies have shown no significant difference in complication rate. This suggests that surgeons shouldn’t be afraid to tackle these cases, according to Dr. Whelan, chief of colorectal surgery at Mount Sinai West, New York.
"If you get an IBD case and you don’t see a lot of those, how do you decide what to do about it, and should you just refer it to a high-volume center?” said Dr. Whelan.
These patients are often under complex medical management, frequently spanning years, and this is an important factor in surgical decisions. They are often on multiple medications, including steroids, and most patients these days are taking monoclonal antibodies, said Dr. Whelan. The latter in particular can lead patients to be susceptible to infections. “These things can all affect decision making,” said Dr. Whelan.
Sometimes the nutritional status of IBD patients is poor, and most of the time, surgery is elective in these patients. So surgery can often be delayed for a month or more to allow time for nutritional status to improve, and this gives time for a patient to go off monoclonal antibodies, and for the physician to arrange for a referral to a high-volume center, if that seems the wisest course.
Surgery should not be considered without a gastrointestinal specialist who is comfortable in managing these patients. “Having someone who knows when to operate and not to operate, and how to handle medication, is really important,” said Dr. Whelan.
Certain cases should definitely be referred out. Ileal pouches are one. Another is a Crohn’s disease patient with multiple points of obstruction. “That may be one that you’re better off to punt,” said Dr. Whelan. Other cases include patients under complex medical management, when there is no experienced GI specialist available to help.
Emergencies require quicker decisions. In ulcerative colitis, emergency cases may include toxic megacolon, perforated colon, or obstruction from either a stricture or cancer, as well as bleeding in rare cases. Scenarios in Crohn’s disease include perforation with sepsis, inaccessible abscess, and, most commonly, obstruction resulting from fibrous stricture or acute inflammation.
When surgery is required, what’s the best choice? Dr. Whelan emphasized keeping it simple. Redo ileal pouches and ileal pouch excisions should generally be avoided. “Even if you do [pouches] often. It’s not the smartest way to go. These patients are almost all on immunosuppressive medications … to make an operation that’s already big even bigger often doesn’t work out well,” he said.
In emergency chronic ulcerative colitis cases, the safest choice is total abdominal colectomy plus end ileostomy. Dr. Whelan discourages surgeons from considering proctectomy and ileal pouch in emergency cases. A number of studies have shown that delaying pouch surgery is associated with fewer minor and major adverse events, and lower reoperation rates, he said. “If you do these operations on an immunosuppressed population, they don’t do as well,” said Dr. Whelan.
Crohn’s disease emergencies can often be managed nonsurgically. Most patients have phlegmon, fistulae, or a partial obstruction. Intravenous antibiotics, percutaneous drainage, hydration, and boosting nutritional status are good options. In cases where an obstruction requires surgery, and the surgeon isn’t comfortable performing stricturoplasty, “you want to limit the resection as best you can,” he said.
Dr. Whelan disclosed financial relationships with Ethicon Endosurgery and Olympus Corporation. Global Academy for Medical Education and this news organization are owned by the same parent company.”
LAS VEGAS – Patients with inflammatory bowel disease who are in need of a surgical intervention can pose a special challenge to surgeons who encounter these patients only occasionally.
The question of whether to perform surgery or refer a patient to a higher-volume specialty center can depend on proximity. In some cases, a specialty center isn’t close, or the patient can’t tolerate the required travel. In fact, a recent study showed that 85.8% of IBD patients are treated surgically in hospitals that treat fewer than 50 patients per year (Am J Gastroenterol. 2008;103:2789-98).
In a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education, Larry Whelan, MD, FACS, discussed some of the challenges these patients pose and offered guidance on which cases are best referred to high-volume centers, and the best way to proceed in emergencies.
IBD patients at high-volume centers have lower mortality than do those in low-volume centers, but patients treated at lower-volume centers tend to be sicker, and studies have shown no significant difference in complication rate. This suggests that surgeons shouldn’t be afraid to tackle these cases, according to Dr. Whelan, chief of colorectal surgery at Mount Sinai West, New York.
"If you get an IBD case and you don’t see a lot of those, how do you decide what to do about it, and should you just refer it to a high-volume center?” said Dr. Whelan.
These patients are often under complex medical management, frequently spanning years, and this is an important factor in surgical decisions. They are often on multiple medications, including steroids, and most patients these days are taking monoclonal antibodies, said Dr. Whelan. The latter in particular can lead patients to be susceptible to infections. “These things can all affect decision making,” said Dr. Whelan.
Sometimes the nutritional status of IBD patients is poor, and most of the time, surgery is elective in these patients. So surgery can often be delayed for a month or more to allow time for nutritional status to improve, and this gives time for a patient to go off monoclonal antibodies, and for the physician to arrange for a referral to a high-volume center, if that seems the wisest course.
Surgery should not be considered without a gastrointestinal specialist who is comfortable in managing these patients. “Having someone who knows when to operate and not to operate, and how to handle medication, is really important,” said Dr. Whelan.
Certain cases should definitely be referred out. Ileal pouches are one. Another is a Crohn’s disease patient with multiple points of obstruction. “That may be one that you’re better off to punt,” said Dr. Whelan. Other cases include patients under complex medical management, when there is no experienced GI specialist available to help.
Emergencies require quicker decisions. In ulcerative colitis, emergency cases may include toxic megacolon, perforated colon, or obstruction from either a stricture or cancer, as well as bleeding in rare cases. Scenarios in Crohn’s disease include perforation with sepsis, inaccessible abscess, and, most commonly, obstruction resulting from fibrous stricture or acute inflammation.
When surgery is required, what’s the best choice? Dr. Whelan emphasized keeping it simple. Redo ileal pouches and ileal pouch excisions should generally be avoided. “Even if you do [pouches] often. It’s not the smartest way to go. These patients are almost all on immunosuppressive medications … to make an operation that’s already big even bigger often doesn’t work out well,” he said.
In emergency chronic ulcerative colitis cases, the safest choice is total abdominal colectomy plus end ileostomy. Dr. Whelan discourages surgeons from considering proctectomy and ileal pouch in emergency cases. A number of studies have shown that delaying pouch surgery is associated with fewer minor and major adverse events, and lower reoperation rates, he said. “If you do these operations on an immunosuppressed population, they don’t do as well,” said Dr. Whelan.
Crohn’s disease emergencies can often be managed nonsurgically. Most patients have phlegmon, fistulae, or a partial obstruction. Intravenous antibiotics, percutaneous drainage, hydration, and boosting nutritional status are good options. In cases where an obstruction requires surgery, and the surgeon isn’t comfortable performing stricturoplasty, “you want to limit the resection as best you can,” he said.
Dr. Whelan disclosed financial relationships with Ethicon Endosurgery and Olympus Corporation. Global Academy for Medical Education and this news organization are owned by the same parent company.”
EXPERT ANALYSIS FROM MISS
A health plan ‘down payment’ is one way states are retooling individual mandate
As President Donald Trump and congressional Republicans tirelessly try to dismantle the Affordable Care Act, a number of states are scrambling to enact laws that safeguard its central provisions.
The GOP tax plan approved by Congress in the last days of 2017 repealed the ACA penalty for people who fail to carry health insurance, a provision called the “individual mandate.” On Jan. 30, in Trump’s first State of the Union address, he claimed victory in killing off this part of the health law, saying Obamacare was effectively dead without it.
But before that federal action kicks in next year, some states are enacting measures to preserve the effects of the mandate by creating their own versions of it.
Maryland is on the cutting edge with legislation moving through both chambers of the Statehouse.
“We’ve been just struggling since Trump became president with how to protect the ACA in our state,” said Vincent DeMarco, president of the Maryland Citizens’ Health Initiative, a nonprofit organization that has been instrumental in pushing the measure.
Proposals have been discussed or advanced in at least nine states, including California, Washington, Connecticut, and the District of Columbia.
Creating an individual mandate is just one way that states – generally blue states where Democrats control the legislature – seek to ensure that what many lawmakers view as key advances made by the ACA don’t disappear.
They’re looking to one another as test cases to see how state-level legislation can either buttress or alter the ACA, according to Trish Riley, the executive director of the National Academy for State Health Policy.
“One state will try one approach; others will try it,” Riley said. “It’s an experiment, and an important one.”
Time is short, since most states have limited legislative calendars and are fast approaching the deadlines for insurers to file their 2019 rate plans.
Passing and implementing these kinds of measures will be tough, said Sabrina Corlette, a research professor at Georgetown University’s Health Policy Institute. But “I think there’s still a window of opportunity for states to do something and have an impact on 2019 premiums,” she said.
Maryland’s take on the individual mandate
Maryland’s effort began last April when the state legislature created the Maryland Health Insurance Coverage Protection Commission “both in response to and in anticipation of efforts at the federal level to repeal and replace the ACA,” according to a report by the state’s legislative services department and the commission itself.
The commission, chartered for 3 years, is charged with studying how federal action could affect the state’s health insurance market and Medicaid program and offering recommendations to mitigate any negative impacts. The panel began meeting months before the Maryland General Assembly started its 90-day session in January.
Based on the commission’s initial recommendations, Sen. Brian Feldman and House Del. Joseline Peña-Melnyk introduced the Protect Maryland Health Care Act of 2018, which lays out a framework for preserving an individual mandate in the state.
The federal individual mandate was put in place to make sure that younger, healthier people joined the insurance risk pool, helping to stabilize the market. The idea is that those relatively healthy customers help cover the insurers’ costs for sicker customers’ care, which keeps premium costs manageable for everyone.
The Congressional Budget Office estimated that 13 million people nationwide would become uninsured without the individual mandate. Some will choose to go without insurance or will not be able to find an affordable plan. Insurers could opt to leave local markets because they could not make money covering only sick patients.
Feldman said insurers and health care experts testified before the commission that Maryland’s insurance exchange would collapse in 2019 if the state didn’t act.
“Because of uncertainty at the federal level, it’s going to be up to states in this arena to pick up the slack and to enact legislation that responds to that uncertainty,” he said.
The federal mandate imposed a tax penalty on people who could afford to but chose not to buy insurance, depositing the money in a general Treasury fund.
In Maryland, the penalty fee will effectively be used, according to advocates, as a “down payment” on an insurance policy.
Beginning in 2020, if someone indicates on their taxes that they’re uninsured, the state would use the fine, plus any tax credits from the federal government, to buy an insurance plan for them.
Maryland would match its residents only with plans that cost nothing more than the fine plus the federal subsidy. So, if such a plan isn’t available in a person’s area, the state will hold on to the money in an interest-bearing account until the next open enrollment season. Then, the person has another chance to buy insurance. If at this time they don’t purchase a plan, the state will deposit the money into an insurance stabilization fund.
Politics and policy on the ground
Maryland is fertile ground for such health care experiments. The ACA remains popular within the state. Polling commissioned by Mr. DeMarco’s group puts the law’s support at 62%.
In addition, about 52% of Marylanders favored a state-based individual mandate, to make up for the federal provision that was repealed.
Democrats control the general assembly, but Gov. Larry Hogan, a Republican, has not offered a specific position on the issue – rather, he alluded to health reform efforts in his State of the State address. “Let’s develop bipartisan solutions to stabilize [health insurance] rates,” he said.
Ed Haislmaier, a senior research fellow at the Heritage Foundation, expressed skepticism about whether this approach will make a difference. The people who are targeted, he argued, are younger, healthier, and generally lower income. They don’t have insurance because they don’t want it, he suggested.
Jason Levitis, a senior fellow at Yale Law School’s Solomon Center for Health Law and Policy who has been instrumental in helping states craft their own versions of the individual mandate, warned that Maryland’s approach could face administrative challenges.
States that follow an approach more closely modeled after the federal mandate, he said, will have an easier time implementing it because regulators have already had 5 years of experience enforcing it.
Still, Mr. Levitis praised the Maryland plan: “There’s something attractive about the idea there, that you put this money … towards coverage.”
And a sampling of state proposals highlight a common theme.
“All the mandate efforts are based on the federal one,” Mr. Levitis said. “The variations are what you put on top, [how states] individually keep track of the money people pay and use it for health care services.”
He pointed to Connecticut as an example. It has two bills pending in its legislature – one that closely mirrors the federal mandate, but with slightly lower fines, and another in which the fines would be deposited into health savings accounts for the individuals.
In New Jersey, a Senate panel advanced a two-bill approach on March 5 that would collect a fee from residents who opt against buying health insurance. These fines would then be used to help pay the health care claims of people who are catastrophically ill.
In the District of Columbia, a health care working group recommended an individual mandate nearly identical to the federal one. The plan would require City Council and congressional approval to become law.
Washington state has convened a group to study how to enforce a mandate, and no legislation has been introduced yet in California.
Meanwhile, Maryland officials also hope to learn from the experiences of other states.
For instance, lawmakers in Maryland are considering the creation of a state-based, basic, low-cost health plan, as well as a fund to help insurers cope with the burden of very high-cost patients.
These efforts also come from the work of the commission.
Stan Dorn, a senior fellow with the pro-Obamacare group Families USA, said Maryland “had the foresight to see threats coming and to try to be proactive about it.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
As President Donald Trump and congressional Republicans tirelessly try to dismantle the Affordable Care Act, a number of states are scrambling to enact laws that safeguard its central provisions.
The GOP tax plan approved by Congress in the last days of 2017 repealed the ACA penalty for people who fail to carry health insurance, a provision called the “individual mandate.” On Jan. 30, in Trump’s first State of the Union address, he claimed victory in killing off this part of the health law, saying Obamacare was effectively dead without it.
But before that federal action kicks in next year, some states are enacting measures to preserve the effects of the mandate by creating their own versions of it.
Maryland is on the cutting edge with legislation moving through both chambers of the Statehouse.
“We’ve been just struggling since Trump became president with how to protect the ACA in our state,” said Vincent DeMarco, president of the Maryland Citizens’ Health Initiative, a nonprofit organization that has been instrumental in pushing the measure.
Proposals have been discussed or advanced in at least nine states, including California, Washington, Connecticut, and the District of Columbia.
Creating an individual mandate is just one way that states – generally blue states where Democrats control the legislature – seek to ensure that what many lawmakers view as key advances made by the ACA don’t disappear.
They’re looking to one another as test cases to see how state-level legislation can either buttress or alter the ACA, according to Trish Riley, the executive director of the National Academy for State Health Policy.
“One state will try one approach; others will try it,” Riley said. “It’s an experiment, and an important one.”
Time is short, since most states have limited legislative calendars and are fast approaching the deadlines for insurers to file their 2019 rate plans.
Passing and implementing these kinds of measures will be tough, said Sabrina Corlette, a research professor at Georgetown University’s Health Policy Institute. But “I think there’s still a window of opportunity for states to do something and have an impact on 2019 premiums,” she said.
Maryland’s take on the individual mandate
Maryland’s effort began last April when the state legislature created the Maryland Health Insurance Coverage Protection Commission “both in response to and in anticipation of efforts at the federal level to repeal and replace the ACA,” according to a report by the state’s legislative services department and the commission itself.
The commission, chartered for 3 years, is charged with studying how federal action could affect the state’s health insurance market and Medicaid program and offering recommendations to mitigate any negative impacts. The panel began meeting months before the Maryland General Assembly started its 90-day session in January.
Based on the commission’s initial recommendations, Sen. Brian Feldman and House Del. Joseline Peña-Melnyk introduced the Protect Maryland Health Care Act of 2018, which lays out a framework for preserving an individual mandate in the state.
The federal individual mandate was put in place to make sure that younger, healthier people joined the insurance risk pool, helping to stabilize the market. The idea is that those relatively healthy customers help cover the insurers’ costs for sicker customers’ care, which keeps premium costs manageable for everyone.
The Congressional Budget Office estimated that 13 million people nationwide would become uninsured without the individual mandate. Some will choose to go without insurance or will not be able to find an affordable plan. Insurers could opt to leave local markets because they could not make money covering only sick patients.
Feldman said insurers and health care experts testified before the commission that Maryland’s insurance exchange would collapse in 2019 if the state didn’t act.
“Because of uncertainty at the federal level, it’s going to be up to states in this arena to pick up the slack and to enact legislation that responds to that uncertainty,” he said.
The federal mandate imposed a tax penalty on people who could afford to but chose not to buy insurance, depositing the money in a general Treasury fund.
In Maryland, the penalty fee will effectively be used, according to advocates, as a “down payment” on an insurance policy.
Beginning in 2020, if someone indicates on their taxes that they’re uninsured, the state would use the fine, plus any tax credits from the federal government, to buy an insurance plan for them.
Maryland would match its residents only with plans that cost nothing more than the fine plus the federal subsidy. So, if such a plan isn’t available in a person’s area, the state will hold on to the money in an interest-bearing account until the next open enrollment season. Then, the person has another chance to buy insurance. If at this time they don’t purchase a plan, the state will deposit the money into an insurance stabilization fund.
Politics and policy on the ground
Maryland is fertile ground for such health care experiments. The ACA remains popular within the state. Polling commissioned by Mr. DeMarco’s group puts the law’s support at 62%.
In addition, about 52% of Marylanders favored a state-based individual mandate, to make up for the federal provision that was repealed.
Democrats control the general assembly, but Gov. Larry Hogan, a Republican, has not offered a specific position on the issue – rather, he alluded to health reform efforts in his State of the State address. “Let’s develop bipartisan solutions to stabilize [health insurance] rates,” he said.
Ed Haislmaier, a senior research fellow at the Heritage Foundation, expressed skepticism about whether this approach will make a difference. The people who are targeted, he argued, are younger, healthier, and generally lower income. They don’t have insurance because they don’t want it, he suggested.
Jason Levitis, a senior fellow at Yale Law School’s Solomon Center for Health Law and Policy who has been instrumental in helping states craft their own versions of the individual mandate, warned that Maryland’s approach could face administrative challenges.
States that follow an approach more closely modeled after the federal mandate, he said, will have an easier time implementing it because regulators have already had 5 years of experience enforcing it.
Still, Mr. Levitis praised the Maryland plan: “There’s something attractive about the idea there, that you put this money … towards coverage.”
And a sampling of state proposals highlight a common theme.
“All the mandate efforts are based on the federal one,” Mr. Levitis said. “The variations are what you put on top, [how states] individually keep track of the money people pay and use it for health care services.”
He pointed to Connecticut as an example. It has two bills pending in its legislature – one that closely mirrors the federal mandate, but with slightly lower fines, and another in which the fines would be deposited into health savings accounts for the individuals.
In New Jersey, a Senate panel advanced a two-bill approach on March 5 that would collect a fee from residents who opt against buying health insurance. These fines would then be used to help pay the health care claims of people who are catastrophically ill.
In the District of Columbia, a health care working group recommended an individual mandate nearly identical to the federal one. The plan would require City Council and congressional approval to become law.
Washington state has convened a group to study how to enforce a mandate, and no legislation has been introduced yet in California.
Meanwhile, Maryland officials also hope to learn from the experiences of other states.
For instance, lawmakers in Maryland are considering the creation of a state-based, basic, low-cost health plan, as well as a fund to help insurers cope with the burden of very high-cost patients.
These efforts also come from the work of the commission.
Stan Dorn, a senior fellow with the pro-Obamacare group Families USA, said Maryland “had the foresight to see threats coming and to try to be proactive about it.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
As President Donald Trump and congressional Republicans tirelessly try to dismantle the Affordable Care Act, a number of states are scrambling to enact laws that safeguard its central provisions.
The GOP tax plan approved by Congress in the last days of 2017 repealed the ACA penalty for people who fail to carry health insurance, a provision called the “individual mandate.” On Jan. 30, in Trump’s first State of the Union address, he claimed victory in killing off this part of the health law, saying Obamacare was effectively dead without it.
But before that federal action kicks in next year, some states are enacting measures to preserve the effects of the mandate by creating their own versions of it.
Maryland is on the cutting edge with legislation moving through both chambers of the Statehouse.
“We’ve been just struggling since Trump became president with how to protect the ACA in our state,” said Vincent DeMarco, president of the Maryland Citizens’ Health Initiative, a nonprofit organization that has been instrumental in pushing the measure.
Proposals have been discussed or advanced in at least nine states, including California, Washington, Connecticut, and the District of Columbia.
Creating an individual mandate is just one way that states – generally blue states where Democrats control the legislature – seek to ensure that what many lawmakers view as key advances made by the ACA don’t disappear.
They’re looking to one another as test cases to see how state-level legislation can either buttress or alter the ACA, according to Trish Riley, the executive director of the National Academy for State Health Policy.
“One state will try one approach; others will try it,” Riley said. “It’s an experiment, and an important one.”
Time is short, since most states have limited legislative calendars and are fast approaching the deadlines for insurers to file their 2019 rate plans.
Passing and implementing these kinds of measures will be tough, said Sabrina Corlette, a research professor at Georgetown University’s Health Policy Institute. But “I think there’s still a window of opportunity for states to do something and have an impact on 2019 premiums,” she said.
Maryland’s take on the individual mandate
Maryland’s effort began last April when the state legislature created the Maryland Health Insurance Coverage Protection Commission “both in response to and in anticipation of efforts at the federal level to repeal and replace the ACA,” according to a report by the state’s legislative services department and the commission itself.
The commission, chartered for 3 years, is charged with studying how federal action could affect the state’s health insurance market and Medicaid program and offering recommendations to mitigate any negative impacts. The panel began meeting months before the Maryland General Assembly started its 90-day session in January.
Based on the commission’s initial recommendations, Sen. Brian Feldman and House Del. Joseline Peña-Melnyk introduced the Protect Maryland Health Care Act of 2018, which lays out a framework for preserving an individual mandate in the state.
The federal individual mandate was put in place to make sure that younger, healthier people joined the insurance risk pool, helping to stabilize the market. The idea is that those relatively healthy customers help cover the insurers’ costs for sicker customers’ care, which keeps premium costs manageable for everyone.
The Congressional Budget Office estimated that 13 million people nationwide would become uninsured without the individual mandate. Some will choose to go without insurance or will not be able to find an affordable plan. Insurers could opt to leave local markets because they could not make money covering only sick patients.
Feldman said insurers and health care experts testified before the commission that Maryland’s insurance exchange would collapse in 2019 if the state didn’t act.
“Because of uncertainty at the federal level, it’s going to be up to states in this arena to pick up the slack and to enact legislation that responds to that uncertainty,” he said.
The federal mandate imposed a tax penalty on people who could afford to but chose not to buy insurance, depositing the money in a general Treasury fund.
In Maryland, the penalty fee will effectively be used, according to advocates, as a “down payment” on an insurance policy.
Beginning in 2020, if someone indicates on their taxes that they’re uninsured, the state would use the fine, plus any tax credits from the federal government, to buy an insurance plan for them.
Maryland would match its residents only with plans that cost nothing more than the fine plus the federal subsidy. So, if such a plan isn’t available in a person’s area, the state will hold on to the money in an interest-bearing account until the next open enrollment season. Then, the person has another chance to buy insurance. If at this time they don’t purchase a plan, the state will deposit the money into an insurance stabilization fund.
Politics and policy on the ground
Maryland is fertile ground for such health care experiments. The ACA remains popular within the state. Polling commissioned by Mr. DeMarco’s group puts the law’s support at 62%.
In addition, about 52% of Marylanders favored a state-based individual mandate, to make up for the federal provision that was repealed.
Democrats control the general assembly, but Gov. Larry Hogan, a Republican, has not offered a specific position on the issue – rather, he alluded to health reform efforts in his State of the State address. “Let’s develop bipartisan solutions to stabilize [health insurance] rates,” he said.
Ed Haislmaier, a senior research fellow at the Heritage Foundation, expressed skepticism about whether this approach will make a difference. The people who are targeted, he argued, are younger, healthier, and generally lower income. They don’t have insurance because they don’t want it, he suggested.
Jason Levitis, a senior fellow at Yale Law School’s Solomon Center for Health Law and Policy who has been instrumental in helping states craft their own versions of the individual mandate, warned that Maryland’s approach could face administrative challenges.
States that follow an approach more closely modeled after the federal mandate, he said, will have an easier time implementing it because regulators have already had 5 years of experience enforcing it.
Still, Mr. Levitis praised the Maryland plan: “There’s something attractive about the idea there, that you put this money … towards coverage.”
And a sampling of state proposals highlight a common theme.
“All the mandate efforts are based on the federal one,” Mr. Levitis said. “The variations are what you put on top, [how states] individually keep track of the money people pay and use it for health care services.”
He pointed to Connecticut as an example. It has two bills pending in its legislature – one that closely mirrors the federal mandate, but with slightly lower fines, and another in which the fines would be deposited into health savings accounts for the individuals.
In New Jersey, a Senate panel advanced a two-bill approach on March 5 that would collect a fee from residents who opt against buying health insurance. These fines would then be used to help pay the health care claims of people who are catastrophically ill.
In the District of Columbia, a health care working group recommended an individual mandate nearly identical to the federal one. The plan would require City Council and congressional approval to become law.
Washington state has convened a group to study how to enforce a mandate, and no legislation has been introduced yet in California.
Meanwhile, Maryland officials also hope to learn from the experiences of other states.
For instance, lawmakers in Maryland are considering the creation of a state-based, basic, low-cost health plan, as well as a fund to help insurers cope with the burden of very high-cost patients.
These efforts also come from the work of the commission.
Stan Dorn, a senior fellow with the pro-Obamacare group Families USA, said Maryland “had the foresight to see threats coming and to try to be proactive about it.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Nonendoscopic nonmalignant polyp surgery increasing despite greater risk
Rate of nonendoscopic surgeries for nonmalignant colorectal polyps significantly increased from 5.9 to 9.4 per 100,000 people from 2000 to 2014, according to a study in Gastroenterology.
These surgeries are not only associated with a much higher risk to patients than endoscopic procedures, but they are significantly less cost effective, confusing investigators as to the cause of the increase.
“The literature to date is clear that endoscopic resection is the preferred management of nonmalignant colorectal polyps,” Anne Peery, MD, gastroenterologist at the University of North Carolina at Chapel Hill, and colleagues explained. “Among patients who have surgery for a nonmalignant colorectal polyp, 14% will have at least one major short-term postoperative event.”
Data from 1,230,458 surgeries conducted during 2000-2014 and recorded in the Healthcare Cost and Utilization Project National Inpatients Sample were included in this study. Patients who underwent a nonendoscopic procedure for nonmalignant polyps were predominantly non-Hispanic white, covered by Medicare, from the highest household income range, and an average age of 66 years.
While non-Hispanic white patients had the highest overall rate increase by ethnicity, rising from 5.6 to 10.5 per 100,000 population, rates in non-Hispanic black and Hispanic patients also rose significantly, increasing from 3.5 to 5.8 per 100,000 population, and from 1.1 to 3.7 per 100,000 population, respectively.
Regionally, rates of surgery were higher in the Midwest (10.8 per 100,000) and the South (10.6 per 100,000) than in the Northeast (7.8 per 100,000) and West (7.5 per 100,000). Incidence rates rose equally during the study period for both men and women.
Large urban teaching hospitals were found to have the largest rate increase when data were stratified by teaching status, a finding which caught Dr. Peery and fellow investigators by surprise.
“We had hypothesized that surgery for nonmalignant colorectal polyps would be both uncommon and declining in teaching hospitals where providers are more likely to be familiar with current guidelines and to have access to endoscopic mucosal resection,” wrote the investigators. “Instead, we found that surgery for nonmalignant colorectal polyps is both common and significantly increasing in teaching hospitals.”
The investigators first hypothesized the increased rate in teaching hospitals could be due to a higher concentration of case referrals to these high-volume centers, following a trend of centralizing cancer procedures. However, there has been no other sign that colon and rectal cancer procedures are following this trend.
Another option considered by Dr. Peery and her colleagues was that increased procedures may stem from a rise in colorectal cancer screening; however, the data indicate screenings did not change from 2010 to 2015, leaving investigators with few final guesses to go on.
“It is also conceivable that increasing production pressure and inadequate reimbursement for endoscopic mucosal resection may persuade endoscopists to refer patients with complex nonmalignant colorectal polyps for surgery,” said Dr. Peery and fellow investigators. “Finally, there is the issue of risk ... for endoscopists without additional training in advanced endoscopic resection, these risks may be perceived as too great, especially when they have the option of referring for a surgical resection.”
There is a possibility that the incidence of surgery was over- or underestimated, as investigators were using ICD-9 codes to identify cases, and patients with diverticulitis were also excluded, which may have affected results.
The investigators reported no relevant financial disclosures.
SOURCE: Peery A et al. Gastroenterology. 2018 Jan 6. doi: 10.1053/j.gastro.2018.01.003.
In this comprehensive analysis, Peery et al. found a rising incidence of surgery for nonmalignant colorectal polyps despite relatively stable colorectal cancer screening rates and with decreasing incidence of colorectal cancer surgery.
In a separate study, the authors found that 14% of patients who underwent surgical resection of nonmalignant colorectal polyps had a major postoperative event. Other population-based studies have reported similar incidence of surgical complications.
This report thus raises concern for inappropriate surgical referral. While reimbursement models may play a role, many factors are involved with surgical referral. Complex polypectomy, often using endoscopic mucosal resection techniques to remove large polyps, is associated with higher rates of bleeding, perforation, and incomplete resection, compared with standard polypectomies. The decision to refer to surgery or to attempt endoscopic resection is based on provider experience and polyp characteristics, including suspicion for malignancy. Current literature suggests that surgical removal is recommended less frequently by specialists in complex polypectomy, compared with nonspecialists.
Given this study’s findings, health systems should consider including surgical referral rates in their quality measures. Thus, high-quality endoscopy centers would ensure that complex polyps are appropriately characterized and initially managed by endoscopists experienced in complex polypectomy. This is especially important with the increasing repertoire of endoscopic alternatives to surgery that we can offer our patients.
Gyanprakash A. Ketwaroo, MD, MSc, is an an assistant professor, division of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts.
In this comprehensive analysis, Peery et al. found a rising incidence of surgery for nonmalignant colorectal polyps despite relatively stable colorectal cancer screening rates and with decreasing incidence of colorectal cancer surgery.
In a separate study, the authors found that 14% of patients who underwent surgical resection of nonmalignant colorectal polyps had a major postoperative event. Other population-based studies have reported similar incidence of surgical complications.
This report thus raises concern for inappropriate surgical referral. While reimbursement models may play a role, many factors are involved with surgical referral. Complex polypectomy, often using endoscopic mucosal resection techniques to remove large polyps, is associated with higher rates of bleeding, perforation, and incomplete resection, compared with standard polypectomies. The decision to refer to surgery or to attempt endoscopic resection is based on provider experience and polyp characteristics, including suspicion for malignancy. Current literature suggests that surgical removal is recommended less frequently by specialists in complex polypectomy, compared with nonspecialists.
Given this study’s findings, health systems should consider including surgical referral rates in their quality measures. Thus, high-quality endoscopy centers would ensure that complex polyps are appropriately characterized and initially managed by endoscopists experienced in complex polypectomy. This is especially important with the increasing repertoire of endoscopic alternatives to surgery that we can offer our patients.
Gyanprakash A. Ketwaroo, MD, MSc, is an an assistant professor, division of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts.
In this comprehensive analysis, Peery et al. found a rising incidence of surgery for nonmalignant colorectal polyps despite relatively stable colorectal cancer screening rates and with decreasing incidence of colorectal cancer surgery.
In a separate study, the authors found that 14% of patients who underwent surgical resection of nonmalignant colorectal polyps had a major postoperative event. Other population-based studies have reported similar incidence of surgical complications.
This report thus raises concern for inappropriate surgical referral. While reimbursement models may play a role, many factors are involved with surgical referral. Complex polypectomy, often using endoscopic mucosal resection techniques to remove large polyps, is associated with higher rates of bleeding, perforation, and incomplete resection, compared with standard polypectomies. The decision to refer to surgery or to attempt endoscopic resection is based on provider experience and polyp characteristics, including suspicion for malignancy. Current literature suggests that surgical removal is recommended less frequently by specialists in complex polypectomy, compared with nonspecialists.
Given this study’s findings, health systems should consider including surgical referral rates in their quality measures. Thus, high-quality endoscopy centers would ensure that complex polyps are appropriately characterized and initially managed by endoscopists experienced in complex polypectomy. This is especially important with the increasing repertoire of endoscopic alternatives to surgery that we can offer our patients.
Gyanprakash A. Ketwaroo, MD, MSc, is an an assistant professor, division of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts.
Rate of nonendoscopic surgeries for nonmalignant colorectal polyps significantly increased from 5.9 to 9.4 per 100,000 people from 2000 to 2014, according to a study in Gastroenterology.
These surgeries are not only associated with a much higher risk to patients than endoscopic procedures, but they are significantly less cost effective, confusing investigators as to the cause of the increase.
“The literature to date is clear that endoscopic resection is the preferred management of nonmalignant colorectal polyps,” Anne Peery, MD, gastroenterologist at the University of North Carolina at Chapel Hill, and colleagues explained. “Among patients who have surgery for a nonmalignant colorectal polyp, 14% will have at least one major short-term postoperative event.”
Data from 1,230,458 surgeries conducted during 2000-2014 and recorded in the Healthcare Cost and Utilization Project National Inpatients Sample were included in this study. Patients who underwent a nonendoscopic procedure for nonmalignant polyps were predominantly non-Hispanic white, covered by Medicare, from the highest household income range, and an average age of 66 years.
While non-Hispanic white patients had the highest overall rate increase by ethnicity, rising from 5.6 to 10.5 per 100,000 population, rates in non-Hispanic black and Hispanic patients also rose significantly, increasing from 3.5 to 5.8 per 100,000 population, and from 1.1 to 3.7 per 100,000 population, respectively.
Regionally, rates of surgery were higher in the Midwest (10.8 per 100,000) and the South (10.6 per 100,000) than in the Northeast (7.8 per 100,000) and West (7.5 per 100,000). Incidence rates rose equally during the study period for both men and women.
Large urban teaching hospitals were found to have the largest rate increase when data were stratified by teaching status, a finding which caught Dr. Peery and fellow investigators by surprise.
“We had hypothesized that surgery for nonmalignant colorectal polyps would be both uncommon and declining in teaching hospitals where providers are more likely to be familiar with current guidelines and to have access to endoscopic mucosal resection,” wrote the investigators. “Instead, we found that surgery for nonmalignant colorectal polyps is both common and significantly increasing in teaching hospitals.”
The investigators first hypothesized the increased rate in teaching hospitals could be due to a higher concentration of case referrals to these high-volume centers, following a trend of centralizing cancer procedures. However, there has been no other sign that colon and rectal cancer procedures are following this trend.
Another option considered by Dr. Peery and her colleagues was that increased procedures may stem from a rise in colorectal cancer screening; however, the data indicate screenings did not change from 2010 to 2015, leaving investigators with few final guesses to go on.
“It is also conceivable that increasing production pressure and inadequate reimbursement for endoscopic mucosal resection may persuade endoscopists to refer patients with complex nonmalignant colorectal polyps for surgery,” said Dr. Peery and fellow investigators. “Finally, there is the issue of risk ... for endoscopists without additional training in advanced endoscopic resection, these risks may be perceived as too great, especially when they have the option of referring for a surgical resection.”
There is a possibility that the incidence of surgery was over- or underestimated, as investigators were using ICD-9 codes to identify cases, and patients with diverticulitis were also excluded, which may have affected results.
The investigators reported no relevant financial disclosures.
SOURCE: Peery A et al. Gastroenterology. 2018 Jan 6. doi: 10.1053/j.gastro.2018.01.003.
Rate of nonendoscopic surgeries for nonmalignant colorectal polyps significantly increased from 5.9 to 9.4 per 100,000 people from 2000 to 2014, according to a study in Gastroenterology.
These surgeries are not only associated with a much higher risk to patients than endoscopic procedures, but they are significantly less cost effective, confusing investigators as to the cause of the increase.
“The literature to date is clear that endoscopic resection is the preferred management of nonmalignant colorectal polyps,” Anne Peery, MD, gastroenterologist at the University of North Carolina at Chapel Hill, and colleagues explained. “Among patients who have surgery for a nonmalignant colorectal polyp, 14% will have at least one major short-term postoperative event.”
Data from 1,230,458 surgeries conducted during 2000-2014 and recorded in the Healthcare Cost and Utilization Project National Inpatients Sample were included in this study. Patients who underwent a nonendoscopic procedure for nonmalignant polyps were predominantly non-Hispanic white, covered by Medicare, from the highest household income range, and an average age of 66 years.
While non-Hispanic white patients had the highest overall rate increase by ethnicity, rising from 5.6 to 10.5 per 100,000 population, rates in non-Hispanic black and Hispanic patients also rose significantly, increasing from 3.5 to 5.8 per 100,000 population, and from 1.1 to 3.7 per 100,000 population, respectively.
Regionally, rates of surgery were higher in the Midwest (10.8 per 100,000) and the South (10.6 per 100,000) than in the Northeast (7.8 per 100,000) and West (7.5 per 100,000). Incidence rates rose equally during the study period for both men and women.
Large urban teaching hospitals were found to have the largest rate increase when data were stratified by teaching status, a finding which caught Dr. Peery and fellow investigators by surprise.
“We had hypothesized that surgery for nonmalignant colorectal polyps would be both uncommon and declining in teaching hospitals where providers are more likely to be familiar with current guidelines and to have access to endoscopic mucosal resection,” wrote the investigators. “Instead, we found that surgery for nonmalignant colorectal polyps is both common and significantly increasing in teaching hospitals.”
The investigators first hypothesized the increased rate in teaching hospitals could be due to a higher concentration of case referrals to these high-volume centers, following a trend of centralizing cancer procedures. However, there has been no other sign that colon and rectal cancer procedures are following this trend.
Another option considered by Dr. Peery and her colleagues was that increased procedures may stem from a rise in colorectal cancer screening; however, the data indicate screenings did not change from 2010 to 2015, leaving investigators with few final guesses to go on.
“It is also conceivable that increasing production pressure and inadequate reimbursement for endoscopic mucosal resection may persuade endoscopists to refer patients with complex nonmalignant colorectal polyps for surgery,” said Dr. Peery and fellow investigators. “Finally, there is the issue of risk ... for endoscopists without additional training in advanced endoscopic resection, these risks may be perceived as too great, especially when they have the option of referring for a surgical resection.”
There is a possibility that the incidence of surgery was over- or underestimated, as investigators were using ICD-9 codes to identify cases, and patients with diverticulitis were also excluded, which may have affected results.
The investigators reported no relevant financial disclosures.
SOURCE: Peery A et al. Gastroenterology. 2018 Jan 6. doi: 10.1053/j.gastro.2018.01.003.
FROM GASTROENTEROLOGY
Key clinical point: Surgical resections for nonmalignant colorectal polyps are increasing while safer endoscopic procedures are available.
Major finding: Incidence rate of surgery for nonmalignant polyps has increased from 5.9 to 9.4 per 100,000 adults from 2000 to 2014.
Study details: A retrospective study of 1,230,458 surgeries recorded in the Healthcare Cost and Utilization Project National Inpatient Sample from 2000 to 2014.
Disclosures: The authors reported no relevant financial disclosures.
Source: Peery A et al. Gastroenterology. 2018 Jan 6. doi: 10.1053/j.gastro.2018.01.003.
TFR achievable with second-line nilotinib for chronic CML
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: In total, 58% of patients who switched to nilotinib experienced treatment-free remission at 48 weeks.
Study details: A single-group, open-label phase 2 study.
Disclosures: Novartis Pharmaceuticals funded the study. The researchers reported financial ties to Novartis and other pharmaceutical companies.
Source: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
Peanut is most prevalent culprit in anaphylaxis PICU admits
ORLANDO – Food was found to be the most commonly identified trigger, with peanuts the most prevalent food cause, in what researchers say is the largest comprehensive review of anaphylaxis episodes in North America that led to pediatric intensive-care unit stays.
Researchers examined the Virtual Pediatrics Systems database, an international database of pediatric intensive care unit (PICU) information, said Carla M. Davis, MD, a pediatrician at Baylor College of Medicine, Houston. During 2010-2015, there were 1,989 pediatric anaphylaxis admissions to these units in North America, she reported at the joint congress of the American Academy of Allergy, Asthma and Immunology and the World Asthma Organization.
“Because anaphylaxis is one of the most severe consequences of allergic disease, we decided that this study needed to be done to see really what the landscape was in the most critically ill children,” she said.
Peanuts accounted for 45% of the food triggers, followed by tree nuts and seeds at 19%, and milk at 10%.
Common causes aside from food included drug, blood products, and venom, Dr. Davis said.
Anaphylaxis accounted for 0.3% of all PICU admissions over the 5-year period, researchers found. Dr. Davis said this was “higher than what we anticipated.”
The overall mortality rate was 1%, and researchers found that peanuts and dairy were main causes of death of all the food-induced cases.
Anaphylaxis occurred more often in children ages 6-18 years than in kids of other ages and was least common among those aged 2-5 years. Asian children were disproportionately represented among the PICU anaphylaxis patients, but the mortality rate didn’t vary by any demographic factors.
Admissions were most likely to happen in the fall and were more common in the Northeast and Western regions of the United States, Dr. Davis reported.
She said the deep look at the causes of these severe cases should help drive home the importance of counseling patients and families about prevention.
“For patients that have had a history of an allergic reaction to food or medication, but specifically food, I think really stressing avoidance measures will be something that will be very helpful, as well as counseling about epinephrine injectors and carrying them is going to help,” she said. “I think having a little more knowledge, pediatricians should be able to counsel and refer to allergists when they don’t feel they have all the necessary skills.”
Dr. Davis reports financial relationships with Aimmune Therapeutics and DBV Technologies.
SOURCE: Davis CM et al. 2018 AAAAI/WAO Joint Congress Abstract 775.
ORLANDO – Food was found to be the most commonly identified trigger, with peanuts the most prevalent food cause, in what researchers say is the largest comprehensive review of anaphylaxis episodes in North America that led to pediatric intensive-care unit stays.
Researchers examined the Virtual Pediatrics Systems database, an international database of pediatric intensive care unit (PICU) information, said Carla M. Davis, MD, a pediatrician at Baylor College of Medicine, Houston. During 2010-2015, there were 1,989 pediatric anaphylaxis admissions to these units in North America, she reported at the joint congress of the American Academy of Allergy, Asthma and Immunology and the World Asthma Organization.
“Because anaphylaxis is one of the most severe consequences of allergic disease, we decided that this study needed to be done to see really what the landscape was in the most critically ill children,” she said.
Peanuts accounted for 45% of the food triggers, followed by tree nuts and seeds at 19%, and milk at 10%.
Common causes aside from food included drug, blood products, and venom, Dr. Davis said.
Anaphylaxis accounted for 0.3% of all PICU admissions over the 5-year period, researchers found. Dr. Davis said this was “higher than what we anticipated.”
The overall mortality rate was 1%, and researchers found that peanuts and dairy were main causes of death of all the food-induced cases.
Anaphylaxis occurred more often in children ages 6-18 years than in kids of other ages and was least common among those aged 2-5 years. Asian children were disproportionately represented among the PICU anaphylaxis patients, but the mortality rate didn’t vary by any demographic factors.
Admissions were most likely to happen in the fall and were more common in the Northeast and Western regions of the United States, Dr. Davis reported.
She said the deep look at the causes of these severe cases should help drive home the importance of counseling patients and families about prevention.
“For patients that have had a history of an allergic reaction to food or medication, but specifically food, I think really stressing avoidance measures will be something that will be very helpful, as well as counseling about epinephrine injectors and carrying them is going to help,” she said. “I think having a little more knowledge, pediatricians should be able to counsel and refer to allergists when they don’t feel they have all the necessary skills.”
Dr. Davis reports financial relationships with Aimmune Therapeutics and DBV Technologies.
SOURCE: Davis CM et al. 2018 AAAAI/WAO Joint Congress Abstract 775.
ORLANDO – Food was found to be the most commonly identified trigger, with peanuts the most prevalent food cause, in what researchers say is the largest comprehensive review of anaphylaxis episodes in North America that led to pediatric intensive-care unit stays.
Researchers examined the Virtual Pediatrics Systems database, an international database of pediatric intensive care unit (PICU) information, said Carla M. Davis, MD, a pediatrician at Baylor College of Medicine, Houston. During 2010-2015, there were 1,989 pediatric anaphylaxis admissions to these units in North America, she reported at the joint congress of the American Academy of Allergy, Asthma and Immunology and the World Asthma Organization.
“Because anaphylaxis is one of the most severe consequences of allergic disease, we decided that this study needed to be done to see really what the landscape was in the most critically ill children,” she said.
Peanuts accounted for 45% of the food triggers, followed by tree nuts and seeds at 19%, and milk at 10%.
Common causes aside from food included drug, blood products, and venom, Dr. Davis said.
Anaphylaxis accounted for 0.3% of all PICU admissions over the 5-year period, researchers found. Dr. Davis said this was “higher than what we anticipated.”
The overall mortality rate was 1%, and researchers found that peanuts and dairy were main causes of death of all the food-induced cases.
Anaphylaxis occurred more often in children ages 6-18 years than in kids of other ages and was least common among those aged 2-5 years. Asian children were disproportionately represented among the PICU anaphylaxis patients, but the mortality rate didn’t vary by any demographic factors.
Admissions were most likely to happen in the fall and were more common in the Northeast and Western regions of the United States, Dr. Davis reported.
She said the deep look at the causes of these severe cases should help drive home the importance of counseling patients and families about prevention.
“For patients that have had a history of an allergic reaction to food or medication, but specifically food, I think really stressing avoidance measures will be something that will be very helpful, as well as counseling about epinephrine injectors and carrying them is going to help,” she said. “I think having a little more knowledge, pediatricians should be able to counsel and refer to allergists when they don’t feel they have all the necessary skills.”
Dr. Davis reports financial relationships with Aimmune Therapeutics and DBV Technologies.
SOURCE: Davis CM et al. 2018 AAAAI/WAO Joint Congress Abstract 775.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point:
Major finding: Researchers found that 45% of these PICU stays from food were caused by reactions to peanuts.
Study details: A review of 1,989 cases during 2010-2015 in the Virtual Pediatrics Systems database, which collects international PICU information.
Disclosures: Dr. Davis reports financial relationships with Aimmune Therapeutics and DBV Technologies.
Source: Davis CM et al. 2018 AAAAI/WAO Joint Congress, Abstract 775.
Phosphodiesterase-5 inhibitors often prescribed inappropriately
While most veterans with pulmonary hypertension are treated in accordance with clinical guidelines, almost two-thirds who are prescribed therapy are being treated with pulmonary vasodilators inappropriately, an analysis of veteran prescription data reveals.
Little was known about how pulmonary vasodilators were used in practice prior to the publication of this study. While pulmonary vasodilators are considered effective for group 1 pulmonary hypertension (PH), clinical guidelines and advice from the Choosing Wisely campaign recommend against their routine use for PH patients classified into the most common types of PH – groups 2 and 3 – because of a lack of benefit, potential for harm, and high cost, the authors wrote. The report was published in Annals of the American Thoracic Society.
The new analysis shows that patients with PH are potentially being exposed to unnecessary harm, according to study author Renda Soylemez Wiener, MD, MPH, of the Center for Healthcare Organization & Implementation Research at Bedford (Mass.) Veterans Affairs Medical Center, and her colleagues. Their findings also reveal that inappropriate prescribing of pulmonary vasodilators, mostly by specialist clinicians, is contributing to the financial burden of an already stretched health system.
The research team looked at prescription data for veterans prescribed a phosphodiesterase-5 inhibitor (PDE5i), which causes pulmonary vasodilation, between 2005 and 2012 at any VA site. The primary outcome of the study was the proportion of patients who received potentially inappropriate PDE5i as classified in guideline recommendations. Patients with group 1 PH were deemed to have been treated appropriately, while those with group 2 and 3 PH were deemed to have been potentially treated inappropriately. Those with groups 4 and 5 PH were thought to have received treatment of “uncertain value.”
In a chart abstraction analysis from a randomly selected subset of PDE5i-treated patients, half (110/230, 47.8% [41.3%-54.5%]) had documented right heart catheterization to confirm the presence of PH. After factoring this into their algorithm, the investigators determined that only 11.7% [8.0%-16.8%] of these patients received clearly appropriate treatment.
Over the 8-year study period, the number of patients with PH group 2 or 3 prescribed PDE5i rose more than 14-fold, the researchers said. They speculated that this figure was likely to continue to rise with the increasing use of echocardiography and detection of PH.
According to the authors, the cost of treating one PH patient for 1 year with PDE5i therapy was between $10,000 and $13,000.
The 1,711 PH patients classified as being treated inappropriately in the study translated into a cost of over $20 million, if each patient were treated for only 1 year, but many of the patients were treated for a longer period of time.
The researchers suggested that there were several reasons why clinicians might choose to deviate from the guidelines, including lacking familiarity with them or disagreeing with them.
“While guidelines do allow trials of PDE5i in treatment for groups 2 or 3 PH on a case-by-case basis after consultation with a PH expert and a confirmatory [right heart catheterization], even PH experts disagree about whether a trial of PDE5i therapy is reasonable and appropriate for patients with group 3 PH,” they wrote.
They may also overestimate the potential benefits of treatment and/or underestimate potential harm.
Clinicians may believe that guidelines developed for a general population do not apply to the patients they are treating.
“It is understandable why clinicians may offer unproven therapies like PDE5i in hopes of providing relief to very sick patients with groups 2 or 3 PH, especially if they do not believe the recommendation applies to their individual patient or they are not convinced about the potential harms of pulmonary vasodilators,” they said.
The authors expressed concern about VA clinicians’ allowing patients to take PDE5i therapy that had been initially prescribed by clinicians outside of VA hospitals. The researchers said such drugs, which potentially had been prescribed inappropriately, “were continued by VA clinicians without much apparent scrutiny.”
The chart abstraction analysis also showed that specialists prescribed the majority of potentially inappropriate PDE5i treatment, suggesting “that other interventions to prevent inappropriate use may be required.”
The researchers concluded that “[the] time has come to develop interventions to optimize prescribing for PH in order to improve the value, quality, and safety of care.”
One potential intervention suggested by the researchers was to require patients with PH to be evaluated at a PH expert center, as recommended by treatment guidelines.
The study was funded by the Department of Veterans Affairs with resources from the Edith Nourse Rogers Memorial VA Hospital. Elizabeth S. Klings, MD, one of the study’s authors, declared receiving research support from several pharmaceutical companies.
SOURCE: Wiener RS et al. Ann Am Thorac Soc. 2018 Feb 27. doi: 10.1513/AnnalsATS.201710-762OC.
While most veterans with pulmonary hypertension are treated in accordance with clinical guidelines, almost two-thirds who are prescribed therapy are being treated with pulmonary vasodilators inappropriately, an analysis of veteran prescription data reveals.
Little was known about how pulmonary vasodilators were used in practice prior to the publication of this study. While pulmonary vasodilators are considered effective for group 1 pulmonary hypertension (PH), clinical guidelines and advice from the Choosing Wisely campaign recommend against their routine use for PH patients classified into the most common types of PH – groups 2 and 3 – because of a lack of benefit, potential for harm, and high cost, the authors wrote. The report was published in Annals of the American Thoracic Society.
The new analysis shows that patients with PH are potentially being exposed to unnecessary harm, according to study author Renda Soylemez Wiener, MD, MPH, of the Center for Healthcare Organization & Implementation Research at Bedford (Mass.) Veterans Affairs Medical Center, and her colleagues. Their findings also reveal that inappropriate prescribing of pulmonary vasodilators, mostly by specialist clinicians, is contributing to the financial burden of an already stretched health system.
The research team looked at prescription data for veterans prescribed a phosphodiesterase-5 inhibitor (PDE5i), which causes pulmonary vasodilation, between 2005 and 2012 at any VA site. The primary outcome of the study was the proportion of patients who received potentially inappropriate PDE5i as classified in guideline recommendations. Patients with group 1 PH were deemed to have been treated appropriately, while those with group 2 and 3 PH were deemed to have been potentially treated inappropriately. Those with groups 4 and 5 PH were thought to have received treatment of “uncertain value.”
In a chart abstraction analysis from a randomly selected subset of PDE5i-treated patients, half (110/230, 47.8% [41.3%-54.5%]) had documented right heart catheterization to confirm the presence of PH. After factoring this into their algorithm, the investigators determined that only 11.7% [8.0%-16.8%] of these patients received clearly appropriate treatment.
Over the 8-year study period, the number of patients with PH group 2 or 3 prescribed PDE5i rose more than 14-fold, the researchers said. They speculated that this figure was likely to continue to rise with the increasing use of echocardiography and detection of PH.
According to the authors, the cost of treating one PH patient for 1 year with PDE5i therapy was between $10,000 and $13,000.
The 1,711 PH patients classified as being treated inappropriately in the study translated into a cost of over $20 million, if each patient were treated for only 1 year, but many of the patients were treated for a longer period of time.
The researchers suggested that there were several reasons why clinicians might choose to deviate from the guidelines, including lacking familiarity with them or disagreeing with them.
“While guidelines do allow trials of PDE5i in treatment for groups 2 or 3 PH on a case-by-case basis after consultation with a PH expert and a confirmatory [right heart catheterization], even PH experts disagree about whether a trial of PDE5i therapy is reasonable and appropriate for patients with group 3 PH,” they wrote.
They may also overestimate the potential benefits of treatment and/or underestimate potential harm.
Clinicians may believe that guidelines developed for a general population do not apply to the patients they are treating.
“It is understandable why clinicians may offer unproven therapies like PDE5i in hopes of providing relief to very sick patients with groups 2 or 3 PH, especially if they do not believe the recommendation applies to their individual patient or they are not convinced about the potential harms of pulmonary vasodilators,” they said.
The authors expressed concern about VA clinicians’ allowing patients to take PDE5i therapy that had been initially prescribed by clinicians outside of VA hospitals. The researchers said such drugs, which potentially had been prescribed inappropriately, “were continued by VA clinicians without much apparent scrutiny.”
The chart abstraction analysis also showed that specialists prescribed the majority of potentially inappropriate PDE5i treatment, suggesting “that other interventions to prevent inappropriate use may be required.”
The researchers concluded that “[the] time has come to develop interventions to optimize prescribing for PH in order to improve the value, quality, and safety of care.”
One potential intervention suggested by the researchers was to require patients with PH to be evaluated at a PH expert center, as recommended by treatment guidelines.
The study was funded by the Department of Veterans Affairs with resources from the Edith Nourse Rogers Memorial VA Hospital. Elizabeth S. Klings, MD, one of the study’s authors, declared receiving research support from several pharmaceutical companies.
SOURCE: Wiener RS et al. Ann Am Thorac Soc. 2018 Feb 27. doi: 10.1513/AnnalsATS.201710-762OC.
While most veterans with pulmonary hypertension are treated in accordance with clinical guidelines, almost two-thirds who are prescribed therapy are being treated with pulmonary vasodilators inappropriately, an analysis of veteran prescription data reveals.
Little was known about how pulmonary vasodilators were used in practice prior to the publication of this study. While pulmonary vasodilators are considered effective for group 1 pulmonary hypertension (PH), clinical guidelines and advice from the Choosing Wisely campaign recommend against their routine use for PH patients classified into the most common types of PH – groups 2 and 3 – because of a lack of benefit, potential for harm, and high cost, the authors wrote. The report was published in Annals of the American Thoracic Society.
The new analysis shows that patients with PH are potentially being exposed to unnecessary harm, according to study author Renda Soylemez Wiener, MD, MPH, of the Center for Healthcare Organization & Implementation Research at Bedford (Mass.) Veterans Affairs Medical Center, and her colleagues. Their findings also reveal that inappropriate prescribing of pulmonary vasodilators, mostly by specialist clinicians, is contributing to the financial burden of an already stretched health system.
The research team looked at prescription data for veterans prescribed a phosphodiesterase-5 inhibitor (PDE5i), which causes pulmonary vasodilation, between 2005 and 2012 at any VA site. The primary outcome of the study was the proportion of patients who received potentially inappropriate PDE5i as classified in guideline recommendations. Patients with group 1 PH were deemed to have been treated appropriately, while those with group 2 and 3 PH were deemed to have been potentially treated inappropriately. Those with groups 4 and 5 PH were thought to have received treatment of “uncertain value.”
In a chart abstraction analysis from a randomly selected subset of PDE5i-treated patients, half (110/230, 47.8% [41.3%-54.5%]) had documented right heart catheterization to confirm the presence of PH. After factoring this into their algorithm, the investigators determined that only 11.7% [8.0%-16.8%] of these patients received clearly appropriate treatment.
Over the 8-year study period, the number of patients with PH group 2 or 3 prescribed PDE5i rose more than 14-fold, the researchers said. They speculated that this figure was likely to continue to rise with the increasing use of echocardiography and detection of PH.
According to the authors, the cost of treating one PH patient for 1 year with PDE5i therapy was between $10,000 and $13,000.
The 1,711 PH patients classified as being treated inappropriately in the study translated into a cost of over $20 million, if each patient were treated for only 1 year, but many of the patients were treated for a longer period of time.
The researchers suggested that there were several reasons why clinicians might choose to deviate from the guidelines, including lacking familiarity with them or disagreeing with them.
“While guidelines do allow trials of PDE5i in treatment for groups 2 or 3 PH on a case-by-case basis after consultation with a PH expert and a confirmatory [right heart catheterization], even PH experts disagree about whether a trial of PDE5i therapy is reasonable and appropriate for patients with group 3 PH,” they wrote.
They may also overestimate the potential benefits of treatment and/or underestimate potential harm.
Clinicians may believe that guidelines developed for a general population do not apply to the patients they are treating.
“It is understandable why clinicians may offer unproven therapies like PDE5i in hopes of providing relief to very sick patients with groups 2 or 3 PH, especially if they do not believe the recommendation applies to their individual patient or they are not convinced about the potential harms of pulmonary vasodilators,” they said.
The authors expressed concern about VA clinicians’ allowing patients to take PDE5i therapy that had been initially prescribed by clinicians outside of VA hospitals. The researchers said such drugs, which potentially had been prescribed inappropriately, “were continued by VA clinicians without much apparent scrutiny.”
The chart abstraction analysis also showed that specialists prescribed the majority of potentially inappropriate PDE5i treatment, suggesting “that other interventions to prevent inappropriate use may be required.”
The researchers concluded that “[the] time has come to develop interventions to optimize prescribing for PH in order to improve the value, quality, and safety of care.”
One potential intervention suggested by the researchers was to require patients with PH to be evaluated at a PH expert center, as recommended by treatment guidelines.
The study was funded by the Department of Veterans Affairs with resources from the Edith Nourse Rogers Memorial VA Hospital. Elizabeth S. Klings, MD, one of the study’s authors, declared receiving research support from several pharmaceutical companies.
SOURCE: Wiener RS et al. Ann Am Thorac Soc. 2018 Feb 27. doi: 10.1513/AnnalsATS.201710-762OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point: Inappropriate prescribing of phosphodiesterase-5 inhibitor (PDE5i) therapy has risen 14-fold in 8 years, at a cost of $20 million per year.
Major finding:
Study details: A retrospective analysis of veterans prescribed phosphodiesterase-5 inhibitors for pulmonary hypertension between 2005 and 2012.
Disclosures: The study was funded by the Department of Veterans Affairs with resources from the Edith Nourse Rogers Memorial Veterans Hospital. Elizabeth S. Klings, MD, one of the study’s authors, declared receiving research support from several pharmaceutical companies.
Source: Wiener RS et al. Ann Am Thorac Soc. 2018 Feb 27. doi: 10.1513/AnnalsATS.201710-762OC.
Product News: 03 2018
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène TriXera
Pierre Fabre Dermo-Cosmetique launches the Avène TriXera line consisting of 3 products. The TriXera Nutrition Nutri-fluid Cleanser gently cleanses and nourishes dry to very dry, sensitive skin. TriXera Nutrition Nutri-fluid Lotion offers 48-hour hydration for dry, sensitive skin. These nourishing effects last up to 6 hours after application. TriXera Nutrition Nutri-fluid Balm has a fluidlike texture and also offers 48-hour hydration. All 3 products contain Avène Thermal Spring Water to soothe and soften as it restores the skin’s balance, as well as evening primrose oil and soy extract to rebuild the skin barrier and prevent moisture loss. For more information, visit www.aveneusa.com.
CoolSculpting
Allergan plc announces US Food and Drug Administration clearance for the CoolSculpting treatment, a nonsurgical fat reduction technology for improved appearance of lax tissue in conjunction with submental fat. CoolSculpting works by gently cooling targeted fat cells in the body to induce natural, controlled elimination of fat cells without affecting surrounding tissue. For more information, visit www.coolsculpting.com.
Discoloration Defense
SkinCeuticals introduces Discoloration Defense, a high-potency treatment serum that prevents and corrects multiple types of discoloration. This product contains tranexamic acid and niacinamide for pigmentation, hepes for hydration, and kojic acid to brighten skin and help to reduce the amount of melanin produced. These ingredients work together to help break up existing melanin clusters, inhibit the formation of melanocytes, and deactivate inflammatory mediators. The serum provides a reduction in visible and stubborn pigmentation for a revitalized and even complexion with refined texture and clarity. For more information, visit www.skinceuticals.com.
Eskata
Aclaris Therapeutics, Inc, announces US Food and Drug Administration approval of Eskata (hydrogen peroxide) topical solution 40% for the treatment of raised seborrheic keratoses. This product is a targeted, in-office treatment applied directly to raised seborrheic keratoses using a penlike applicator. Clinical studies showed clearing of seborrheic keratoses after 2 treatments. Eskata is expected to be commercially available in the spring of 2018. For more information, visit www.eskata.com.
Ixifi
Pfizer Inc announces US Food and Drug Administration approval of Ixifi (infliximab-qbtx), a chimeric human-murine monoclonal antibody against tumor necrosis factor, as a biosimilar to Remicade (infliximab) for all eligible indications of the reference product. Ixifi has been approved in the United States as a treatment for rheumatoid arthritis, Crohn disease, ulcerative colitis, psoriatic arthritis, and plaque psoriasis. For more information, visit www.pfizer.com.
Jemdel
Ortho Dermatologics announces US Food and Drug Administration acceptance of the New Drug Application for Jemdel (halobetasol propionate 0.01%), a high-potency topical steroid for the treatment of plaque psoriasis with dosing for as long as 8 weeks. Jemdel has a Prescription Drug User Fee Act action date of October 5, 2018. For more information, visit www.ortho-dermatologics.com.
Retin-A Micro
Ortho Dermatologics announces that Retin-A Micro (tretinoin) gel microsphere 0.06%, a topical treatment for acne vulgaris, will be available commercially to health care professionals. The US Food and Drug Administration previously approved the Supplemental New Drug Application for this product in October 2017. Retin-A Micro features a microsponge delivery system technology that helps control the release of tretinoin and improves photostability, even when used in conjunction with benzoyl peroxide. This product also features a pump delivery system for controlled dispensing and consistent dosing. Caution should be exercised when prescribing to eczema patients and nursing mothers. For more information, visit www.retinamicro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
U.S. adolescent malignant melanoma nearly halved during 2000-2014
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
REPORTING FROM AAD 18
Key clinical point: U.S. incident malignant melanoma in adolescents dropped by nearly 50% during 2000-2014.
Major finding: Malignant melanoma occurred in about 11 adolescents per million in 2001 and about 6 per million in 2013.
Study details: Review of data collected in the SEER database of the National Cancer Institute.
Disclosures: Mr. Kelm had no disclosures.
Source: Kelm RC et al. AAD 18, Abstract 6722.









