User login
Lymphoma, breast cancer survivors have greater risk of CHF
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
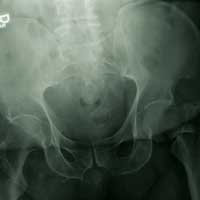
His Old Pain Is Back
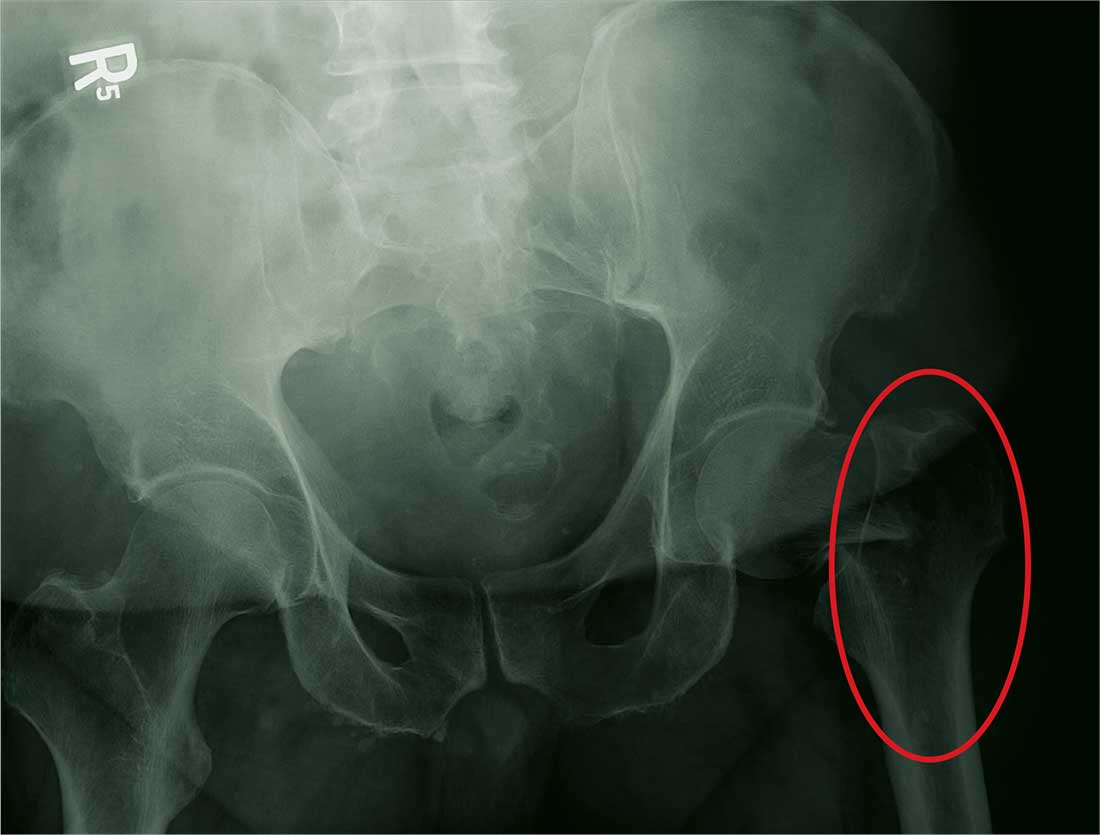
ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.

ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.

ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.
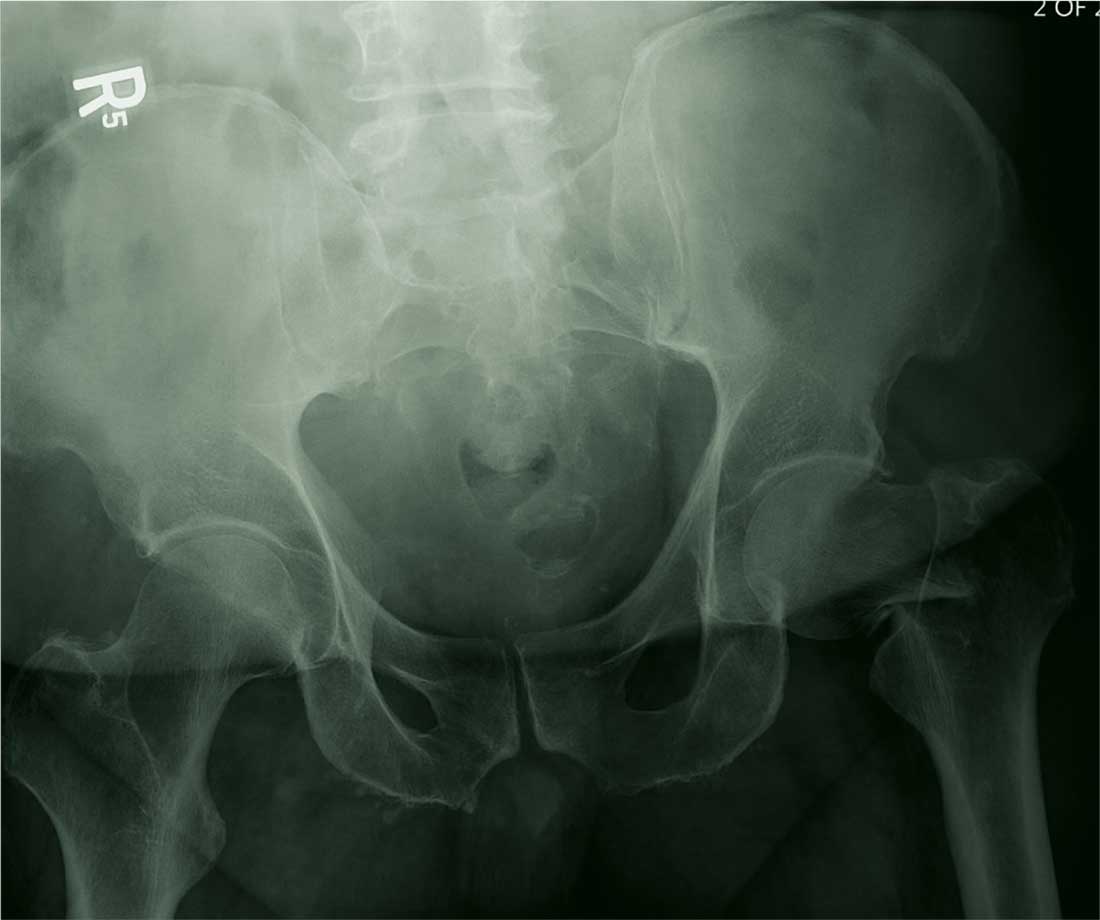
You are called in for a neurosurgical consult of a 60-year-old man who underwent a lumbar decompression approximately 18 months ago. For the past several weeks, he has been experiencing worsening back pain that radiates down his left leg; at times, he has to use his cane and walker. The patient denies any acute injury or trauma preceding the pain; however, last night he slipped and fell in his bedroom, which made it much worse.
Medical history is significant for hypertension and hyperlipidemia. The patient responded well to his previous back surgery. The emergency department clinician is concerned that his symptoms may be caused by a new lumbar stenosis at an adjacent level.
On exam, you note a mildly obese male who appears uncomfortable but is in no obvious distress. His vital signs are stable. He has some tenderness within his left hip. Distally, his strength is good, and there is no evidence of neurovascular compromise. Internal and external rotation causes some discomfort.
A portable pelvic radiograph is obtained (shown). What is your impression?
PASI responses with biologics similar among white, nonwhite individuals, study finds
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
REPORTING FROM ODAC 2018
Key clinical point: Responses to brodalumab and ustekinumab were comparable in nonwhite and white patients with psoriasis.
Major finding: At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved PASI 75, a nonsignificant difference.
Study details: An ad hoc comparison of week 12 phase 3 study data in 1,849 patients including 182 patients with skin of color.
Disclosures: Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed; and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
Source: McMichael A et al. ODAC 2018.
Is there a resilience deficit?
Even if you have never experienced a symptom of burnout, you probably have at least one colleague who has. In the last decade, collateral damage from physician burnout has earned it a place on the agenda of the American Academy of Pediatrics and most other physician organizations.
When one steps back and takes a longer view, burnout is simply a poor fit between physicians and their roles. An increasing number of physicians are finding themselves in jobs in which – for a variety of reasons – they feel uncomfortable. Eventually, the discomfort resulting from that poor fit becomes so unbearable the only solution is to change jobs or retire.
However, an article in Pediatrics entitled “Seeking professional resilience” addresses burnout from the perspective that physician vulnerability is a major contributor to the problem (Pediatrics. 2018, Feb 1. doi: 10.1542/peds.2017-2388). The author, Abby R. Rosenberg, MD, suggests that one solution to burnout is helping physicians learn how “to maintain physical and emotional well-being in the face of adversity,” that is, “resilience.”
It turns out that the recent buzz surrounding “resilience” has drawn a throng of theorists. I guess if we can have chaos theory, we can have resilience theories. Dr. Rosenberg sorts these theories into three categories based on whether they consider resilience an intrinsic trait, a process of adaptation, or an outcome. She offers an alternative description in which resilience is conceived as “a process of harnessing the resources we need to sustain well-being.” Dr. Rosenberg’s suggestions of how this harnessing process can be achieved are certainly worth reading, but I fear that most physicians threatened with burnout won’t have the time or the composure to follow her recommendations. Fifty years of watching physicians both thrive and flame out has convinced me that in most cases, resilience is an intrinsic trait gifted to the recipient at birth.
I am sure there are older physicians who believe that burnout is just another case of “they-don’t-make-’em-like-they-used-to” and would claim that young physicians just don’t have the same grit that we had a generation ago. I guess it is possible that the shift away from the owner/operator model toward one in which a physician has become a cog in the wheel of a large corporation has selected for physicians who are less resilient by nature. But I suspect that the number of resilient physicians is unchanged over the last hundred years. It is more likely that even those blessed with a resilient nature enter their training challenged by a burden of debt significantly greater than my peers and I faced 50 years ago.
The problem isn’t the resiliency deficit. Burnout is the result of a job that has evolved into one with challenges that even the more resilient physicians struggle to tolerate. Under a litigious cloud, hunched over a computer for half the day, the modern physician must struggle to find relevance in a situation in which he has relinquished control to a system that may not share his values.
Refining the selection process to find even more resilient candidates for medical school might lower the burnout rate by a point or two. However, the real answer requires a major overhaul of medical delivery system so that providers can once again feel that every hour they invest is meaningful. The privilege to practice medicine always has required sacrifices on the part of the physician. However, without a sense of purpose, these sacrifices can become intolerable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Even if you have never experienced a symptom of burnout, you probably have at least one colleague who has. In the last decade, collateral damage from physician burnout has earned it a place on the agenda of the American Academy of Pediatrics and most other physician organizations.
When one steps back and takes a longer view, burnout is simply a poor fit between physicians and their roles. An increasing number of physicians are finding themselves in jobs in which – for a variety of reasons – they feel uncomfortable. Eventually, the discomfort resulting from that poor fit becomes so unbearable the only solution is to change jobs or retire.
However, an article in Pediatrics entitled “Seeking professional resilience” addresses burnout from the perspective that physician vulnerability is a major contributor to the problem (Pediatrics. 2018, Feb 1. doi: 10.1542/peds.2017-2388). The author, Abby R. Rosenberg, MD, suggests that one solution to burnout is helping physicians learn how “to maintain physical and emotional well-being in the face of adversity,” that is, “resilience.”
It turns out that the recent buzz surrounding “resilience” has drawn a throng of theorists. I guess if we can have chaos theory, we can have resilience theories. Dr. Rosenberg sorts these theories into three categories based on whether they consider resilience an intrinsic trait, a process of adaptation, or an outcome. She offers an alternative description in which resilience is conceived as “a process of harnessing the resources we need to sustain well-being.” Dr. Rosenberg’s suggestions of how this harnessing process can be achieved are certainly worth reading, but I fear that most physicians threatened with burnout won’t have the time or the composure to follow her recommendations. Fifty years of watching physicians both thrive and flame out has convinced me that in most cases, resilience is an intrinsic trait gifted to the recipient at birth.
I am sure there are older physicians who believe that burnout is just another case of “they-don’t-make-’em-like-they-used-to” and would claim that young physicians just don’t have the same grit that we had a generation ago. I guess it is possible that the shift away from the owner/operator model toward one in which a physician has become a cog in the wheel of a large corporation has selected for physicians who are less resilient by nature. But I suspect that the number of resilient physicians is unchanged over the last hundred years. It is more likely that even those blessed with a resilient nature enter their training challenged by a burden of debt significantly greater than my peers and I faced 50 years ago.
The problem isn’t the resiliency deficit. Burnout is the result of a job that has evolved into one with challenges that even the more resilient physicians struggle to tolerate. Under a litigious cloud, hunched over a computer for half the day, the modern physician must struggle to find relevance in a situation in which he has relinquished control to a system that may not share his values.
Refining the selection process to find even more resilient candidates for medical school might lower the burnout rate by a point or two. However, the real answer requires a major overhaul of medical delivery system so that providers can once again feel that every hour they invest is meaningful. The privilege to practice medicine always has required sacrifices on the part of the physician. However, without a sense of purpose, these sacrifices can become intolerable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Even if you have never experienced a symptom of burnout, you probably have at least one colleague who has. In the last decade, collateral damage from physician burnout has earned it a place on the agenda of the American Academy of Pediatrics and most other physician organizations.
When one steps back and takes a longer view, burnout is simply a poor fit between physicians and their roles. An increasing number of physicians are finding themselves in jobs in which – for a variety of reasons – they feel uncomfortable. Eventually, the discomfort resulting from that poor fit becomes so unbearable the only solution is to change jobs or retire.
However, an article in Pediatrics entitled “Seeking professional resilience” addresses burnout from the perspective that physician vulnerability is a major contributor to the problem (Pediatrics. 2018, Feb 1. doi: 10.1542/peds.2017-2388). The author, Abby R. Rosenberg, MD, suggests that one solution to burnout is helping physicians learn how “to maintain physical and emotional well-being in the face of adversity,” that is, “resilience.”
It turns out that the recent buzz surrounding “resilience” has drawn a throng of theorists. I guess if we can have chaos theory, we can have resilience theories. Dr. Rosenberg sorts these theories into three categories based on whether they consider resilience an intrinsic trait, a process of adaptation, or an outcome. She offers an alternative description in which resilience is conceived as “a process of harnessing the resources we need to sustain well-being.” Dr. Rosenberg’s suggestions of how this harnessing process can be achieved are certainly worth reading, but I fear that most physicians threatened with burnout won’t have the time or the composure to follow her recommendations. Fifty years of watching physicians both thrive and flame out has convinced me that in most cases, resilience is an intrinsic trait gifted to the recipient at birth.
I am sure there are older physicians who believe that burnout is just another case of “they-don’t-make-’em-like-they-used-to” and would claim that young physicians just don’t have the same grit that we had a generation ago. I guess it is possible that the shift away from the owner/operator model toward one in which a physician has become a cog in the wheel of a large corporation has selected for physicians who are less resilient by nature. But I suspect that the number of resilient physicians is unchanged over the last hundred years. It is more likely that even those blessed with a resilient nature enter their training challenged by a burden of debt significantly greater than my peers and I faced 50 years ago.
The problem isn’t the resiliency deficit. Burnout is the result of a job that has evolved into one with challenges that even the more resilient physicians struggle to tolerate. Under a litigious cloud, hunched over a computer for half the day, the modern physician must struggle to find relevance in a situation in which he has relinquished control to a system that may not share his values.
Refining the selection process to find even more resilient candidates for medical school might lower the burnout rate by a point or two. However, the real answer requires a major overhaul of medical delivery system so that providers can once again feel that every hour they invest is meaningful. The privilege to practice medicine always has required sacrifices on the part of the physician. However, without a sense of purpose, these sacrifices can become intolerable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
VIDEO: Patient vouchers prompt physicians to prescribe top antiplatelet drugs
ORLANDO – Patients who received vouchers to cover copayments were more likely to receive prescriptions for more effective antiplatelet medication, according to data from a multicenter, randomized trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We know that guidelines are very clear; we need to treat patients with antiplatelet therapy for 12 months,” and that the most potent drug, ticagrelor, should be used, Tracy Wang, MD, of Duke University, Durham, N.C., said in a video interview at the annual meeting of the American College of Cardiology. However, in the United States, clopidogrel, though less effective, is prescribed much more often, and many patients discontinue their P2Y12 inhibitor therapy within the first year because of cost, she added.
“We hypothesized that, by reducing the out of pocket costs, treatment would be more evidence driven, rather than driven by what patients could afford,” she said.
The Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study (ARTEMIS) included 11,001 MI patients at 301 hospitals across the United States. Patients in the treatment hospital group received a voucher to use at a pharmacy or through a mail-order pharmacy to reduce out of pocket costs. Randomization occurred at the hospital level, and hospital characteristics were similar between the groups.
Overall, patients in the treatment group were significantly more likely to receive a prescription for ticagrelor than clopidogrel (60% vs. 36%); 55% and 32% of patients in the usual care group were prescribed ticagrelor and clopidogrel, respectively. Nonpersistence, defined as a gap in P2Y12-inhibitor use of at least 30 days within 1 year, was significantly lower in the treatment group than it was in the usual care group based on patient reported analysis (13% vs. 16%).
However, the incidence of major adverse cardiac events was roughly 10% in both groups. The similar outcomes may stem from the fact that 28% of patients with vouchers did not fill their prescriptions for reasons that the study did not explore, said Dr. Wang.
All patients had health insurance: 64% private, 42% Medicare, 9% Medicaid. The average age of the patients was 62 years, and 31% were women. Patient demographics and clinical characteristics were similar between the groups.
The vouchers affected choice of treatment but didn’t help clinical outcomes, which suggests that copayment reduction should be part of a broader strategy to help patients with adherence over time, said Dr. Wang.
Next steps for research include taking a subset of patients who are more likely to be nonadherent and at high risk for adverse events and targeting them for additional intervention, she noted.
Discussant Craig J. Beavers, PharmD, of the University of Kentucky College of Pharmacy, Lexington, agreed that a multipronged approach is needed to get patients to take their medicines. “We have to figure out what other barriers there are,” he said. “The real trick is, even if you lead a horse to water, how to get them to drink it,” he said.
The study was funded by AstraZeneca. Dr. Wang disclosed relationships with companies including Gilead Sciences, Merck, and Sanofi Pasteur. Dr. Beavers had no financial conflicts to disclose.
SOURCE: Wang T. ACC 18.
ORLANDO – Patients who received vouchers to cover copayments were more likely to receive prescriptions for more effective antiplatelet medication, according to data from a multicenter, randomized trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We know that guidelines are very clear; we need to treat patients with antiplatelet therapy for 12 months,” and that the most potent drug, ticagrelor, should be used, Tracy Wang, MD, of Duke University, Durham, N.C., said in a video interview at the annual meeting of the American College of Cardiology. However, in the United States, clopidogrel, though less effective, is prescribed much more often, and many patients discontinue their P2Y12 inhibitor therapy within the first year because of cost, she added.
“We hypothesized that, by reducing the out of pocket costs, treatment would be more evidence driven, rather than driven by what patients could afford,” she said.
The Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study (ARTEMIS) included 11,001 MI patients at 301 hospitals across the United States. Patients in the treatment hospital group received a voucher to use at a pharmacy or through a mail-order pharmacy to reduce out of pocket costs. Randomization occurred at the hospital level, and hospital characteristics were similar between the groups.
Overall, patients in the treatment group were significantly more likely to receive a prescription for ticagrelor than clopidogrel (60% vs. 36%); 55% and 32% of patients in the usual care group were prescribed ticagrelor and clopidogrel, respectively. Nonpersistence, defined as a gap in P2Y12-inhibitor use of at least 30 days within 1 year, was significantly lower in the treatment group than it was in the usual care group based on patient reported analysis (13% vs. 16%).
However, the incidence of major adverse cardiac events was roughly 10% in both groups. The similar outcomes may stem from the fact that 28% of patients with vouchers did not fill their prescriptions for reasons that the study did not explore, said Dr. Wang.
All patients had health insurance: 64% private, 42% Medicare, 9% Medicaid. The average age of the patients was 62 years, and 31% were women. Patient demographics and clinical characteristics were similar between the groups.
The vouchers affected choice of treatment but didn’t help clinical outcomes, which suggests that copayment reduction should be part of a broader strategy to help patients with adherence over time, said Dr. Wang.
Next steps for research include taking a subset of patients who are more likely to be nonadherent and at high risk for adverse events and targeting them for additional intervention, she noted.
Discussant Craig J. Beavers, PharmD, of the University of Kentucky College of Pharmacy, Lexington, agreed that a multipronged approach is needed to get patients to take their medicines. “We have to figure out what other barriers there are,” he said. “The real trick is, even if you lead a horse to water, how to get them to drink it,” he said.
The study was funded by AstraZeneca. Dr. Wang disclosed relationships with companies including Gilead Sciences, Merck, and Sanofi Pasteur. Dr. Beavers had no financial conflicts to disclose.
SOURCE: Wang T. ACC 18.
ORLANDO – Patients who received vouchers to cover copayments were more likely to receive prescriptions for more effective antiplatelet medication, according to data from a multicenter, randomized trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We know that guidelines are very clear; we need to treat patients with antiplatelet therapy for 12 months,” and that the most potent drug, ticagrelor, should be used, Tracy Wang, MD, of Duke University, Durham, N.C., said in a video interview at the annual meeting of the American College of Cardiology. However, in the United States, clopidogrel, though less effective, is prescribed much more often, and many patients discontinue their P2Y12 inhibitor therapy within the first year because of cost, she added.
“We hypothesized that, by reducing the out of pocket costs, treatment would be more evidence driven, rather than driven by what patients could afford,” she said.
The Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study (ARTEMIS) included 11,001 MI patients at 301 hospitals across the United States. Patients in the treatment hospital group received a voucher to use at a pharmacy or through a mail-order pharmacy to reduce out of pocket costs. Randomization occurred at the hospital level, and hospital characteristics were similar between the groups.
Overall, patients in the treatment group were significantly more likely to receive a prescription for ticagrelor than clopidogrel (60% vs. 36%); 55% and 32% of patients in the usual care group were prescribed ticagrelor and clopidogrel, respectively. Nonpersistence, defined as a gap in P2Y12-inhibitor use of at least 30 days within 1 year, was significantly lower in the treatment group than it was in the usual care group based on patient reported analysis (13% vs. 16%).
However, the incidence of major adverse cardiac events was roughly 10% in both groups. The similar outcomes may stem from the fact that 28% of patients with vouchers did not fill their prescriptions for reasons that the study did not explore, said Dr. Wang.
All patients had health insurance: 64% private, 42% Medicare, 9% Medicaid. The average age of the patients was 62 years, and 31% were women. Patient demographics and clinical characteristics were similar between the groups.
The vouchers affected choice of treatment but didn’t help clinical outcomes, which suggests that copayment reduction should be part of a broader strategy to help patients with adherence over time, said Dr. Wang.
Next steps for research include taking a subset of patients who are more likely to be nonadherent and at high risk for adverse events and targeting them for additional intervention, she noted.
Discussant Craig J. Beavers, PharmD, of the University of Kentucky College of Pharmacy, Lexington, agreed that a multipronged approach is needed to get patients to take their medicines. “We have to figure out what other barriers there are,” he said. “The real trick is, even if you lead a horse to water, how to get them to drink it,” he said.
The study was funded by AstraZeneca. Dr. Wang disclosed relationships with companies including Gilead Sciences, Merck, and Sanofi Pasteur. Dr. Beavers had no financial conflicts to disclose.
SOURCE: Wang T. ACC 18.
REPORTING FROM ACC 18
Key clinical point: Physicians were more likely to prescribe ticagrelor after an MI when patients received vouchers.
Major finding: Patients with vouchers received prescriptions for ticagrelor significantly more than clopidogrel (60% vs. 36%).
Study details: The data come from a randomized trial of 301 hospitals in the United States and included 11,001 MI patients.
Disclosures: ARTEMIS was funded by AstraZeneca. Dr. Wang disclosed relationships with companies including Gilead Sciences, Merck, and Sanofi Pasteur. Dr. Beavers had no financial conflicts to disclose.
Source: Wang T. ACC 2018.
Barbershop intervention cuts blood pressure in black men
ORLANDO – Black men who received a pharmacist-led intervention in their local barbershops showed significantly improved blood pressure after 6 months, compared with controls, in a randomized trial of 319 individuals.
“Non-Hispanic black men still have the highest hypertension death rate of any group in the country. Something like 60% of black men have blood pressure of 140/90 or higher,” but they have relatively low rates of physician interaction for blood pressure management, compared with other groups, Ronald G. Victor, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a video interview at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Health outreach to barbershops has been well established in the lay press, but they only scratch the surface in terms of a scientific evaluation, and that’s what we did,” he noted.
The primary outcome was a change in systolic blood pressure at 6 months. The average decrease was 27.0 mm Hg in the intervention group, compared with 9.3 mm Hg in the control group.
Dr. Victor and colleagues identified a study population of non-Hispanic black men aged 35-79 years with a baseline blood pressure of at least 140 mm Hg who were regular patrons of their local barbershops. Of these, 139 were randomized to a pharmacist-led intervention in 28 barbershops, and 180 served as controls in 24 barbershops.
The intervention included monthly checkups with a pharmacist in the barbershop setting, along with blood pressure readings, medication management, electrolyte monitoring, and progress notes sent to each man’s primary care provider. In addition, the barbers encouraged blood pressure management and a healthy lifestyle during the men’s regular haircut visits, occurring about every 2 weeks. The control group received encouragement from their barbers and usual care from their primary care providers.
The average baseline systolic blood pressure was 152.8 mm Hg in the intervention group, which dropped to 125.8 mm Hg at 6 months. The controls’ average systolic blood pressure was 154.6 mm Hg at baseline and 145.4 at 6 months.
Dr. Victor said he was thrilled with the results, and that the intervention group’s improvement was roughly three times that seen in many blood pressure intervention studies. “We lost very few men to follow-up,” Dr. Victor said. “I can’t underestimate how important the buy-in of the barbers was,” he emphasized. The primary analysis included 132 intervention men and 171 controls with complete 6 months data.
The between-group difference for the primary outcome was 21.6 mm Hg in favor of the intervention,” Dr. Victor said. As a secondary outcome, the between-group difference in diastolic blood pressure was 14.9 mm Hg in favor of the intervention.
In addition, 64% and 12% of the intervention and control groups, respectively, achieved the blood pressure target of 130/80.
“We think the intervention effect is multifaceted,” said Dr. Victor. The pharmacists were doctorate level with specialty training, and prescribed more intense therapy than did a community clinic. In addition, the convenience and comfort of the community barbershop setting, and the endorsement by the barbers, who are significant figures in the community, contributed to the success of the study, he said.
“We think the whole package was important,” he emphasized.
The intervention was safe and well tolerated, with no adverse events. A total of three cases of reversible acute kidney injury occurred in the intervention group that were related to indapamide and resolved when it was discontinued.
“This [study] is a home run,” discussant Eileen Handberg, MD, said in a press conference, “This is taking care where patients live; this is ‘high-touch’ medicine,” she said. Also, the 9-mm Hg improvement in the control group was comparable with improvements in many previous blood pressure control trials, she noted.
Dr. Victor said he plans to expand the study by establishing similar protocols in other communities. Additional next steps for research include extending the current study for another 6 months, expanding the research criteria to include men with mild hypertension, and conducting a cost analysis, he said.
The study was funded by the National Heart, Lung, and Blood Institute and others. Dr. Victor had no financial conflicts to disclose. Dr. Handberg disclosed relationships with multiple companies including Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Ionis, and Relypsa.
The findings were published online simultaneously with Dr. Victor’s report (N Engl J Med. 2018 Mar 11; doi: 10.1056/NEJMoa1717250).
SOURCE: Victor R et al. ACC 2018.
ORLANDO – Black men who received a pharmacist-led intervention in their local barbershops showed significantly improved blood pressure after 6 months, compared with controls, in a randomized trial of 319 individuals.
“Non-Hispanic black men still have the highest hypertension death rate of any group in the country. Something like 60% of black men have blood pressure of 140/90 or higher,” but they have relatively low rates of physician interaction for blood pressure management, compared with other groups, Ronald G. Victor, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a video interview at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Health outreach to barbershops has been well established in the lay press, but they only scratch the surface in terms of a scientific evaluation, and that’s what we did,” he noted.
The primary outcome was a change in systolic blood pressure at 6 months. The average decrease was 27.0 mm Hg in the intervention group, compared with 9.3 mm Hg in the control group.
Dr. Victor and colleagues identified a study population of non-Hispanic black men aged 35-79 years with a baseline blood pressure of at least 140 mm Hg who were regular patrons of their local barbershops. Of these, 139 were randomized to a pharmacist-led intervention in 28 barbershops, and 180 served as controls in 24 barbershops.
The intervention included monthly checkups with a pharmacist in the barbershop setting, along with blood pressure readings, medication management, electrolyte monitoring, and progress notes sent to each man’s primary care provider. In addition, the barbers encouraged blood pressure management and a healthy lifestyle during the men’s regular haircut visits, occurring about every 2 weeks. The control group received encouragement from their barbers and usual care from their primary care providers.
The average baseline systolic blood pressure was 152.8 mm Hg in the intervention group, which dropped to 125.8 mm Hg at 6 months. The controls’ average systolic blood pressure was 154.6 mm Hg at baseline and 145.4 at 6 months.
Dr. Victor said he was thrilled with the results, and that the intervention group’s improvement was roughly three times that seen in many blood pressure intervention studies. “We lost very few men to follow-up,” Dr. Victor said. “I can’t underestimate how important the buy-in of the barbers was,” he emphasized. The primary analysis included 132 intervention men and 171 controls with complete 6 months data.
The between-group difference for the primary outcome was 21.6 mm Hg in favor of the intervention,” Dr. Victor said. As a secondary outcome, the between-group difference in diastolic blood pressure was 14.9 mm Hg in favor of the intervention.
In addition, 64% and 12% of the intervention and control groups, respectively, achieved the blood pressure target of 130/80.
“We think the intervention effect is multifaceted,” said Dr. Victor. The pharmacists were doctorate level with specialty training, and prescribed more intense therapy than did a community clinic. In addition, the convenience and comfort of the community barbershop setting, and the endorsement by the barbers, who are significant figures in the community, contributed to the success of the study, he said.
“We think the whole package was important,” he emphasized.
The intervention was safe and well tolerated, with no adverse events. A total of three cases of reversible acute kidney injury occurred in the intervention group that were related to indapamide and resolved when it was discontinued.
“This [study] is a home run,” discussant Eileen Handberg, MD, said in a press conference, “This is taking care where patients live; this is ‘high-touch’ medicine,” she said. Also, the 9-mm Hg improvement in the control group was comparable with improvements in many previous blood pressure control trials, she noted.
Dr. Victor said he plans to expand the study by establishing similar protocols in other communities. Additional next steps for research include extending the current study for another 6 months, expanding the research criteria to include men with mild hypertension, and conducting a cost analysis, he said.
The study was funded by the National Heart, Lung, and Blood Institute and others. Dr. Victor had no financial conflicts to disclose. Dr. Handberg disclosed relationships with multiple companies including Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Ionis, and Relypsa.
The findings were published online simultaneously with Dr. Victor’s report (N Engl J Med. 2018 Mar 11; doi: 10.1056/NEJMoa1717250).
SOURCE: Victor R et al. ACC 2018.
ORLANDO – Black men who received a pharmacist-led intervention in their local barbershops showed significantly improved blood pressure after 6 months, compared with controls, in a randomized trial of 319 individuals.
“Non-Hispanic black men still have the highest hypertension death rate of any group in the country. Something like 60% of black men have blood pressure of 140/90 or higher,” but they have relatively low rates of physician interaction for blood pressure management, compared with other groups, Ronald G. Victor, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a video interview at the annual meeting of the American College of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Health outreach to barbershops has been well established in the lay press, but they only scratch the surface in terms of a scientific evaluation, and that’s what we did,” he noted.
The primary outcome was a change in systolic blood pressure at 6 months. The average decrease was 27.0 mm Hg in the intervention group, compared with 9.3 mm Hg in the control group.
Dr. Victor and colleagues identified a study population of non-Hispanic black men aged 35-79 years with a baseline blood pressure of at least 140 mm Hg who were regular patrons of their local barbershops. Of these, 139 were randomized to a pharmacist-led intervention in 28 barbershops, and 180 served as controls in 24 barbershops.
The intervention included monthly checkups with a pharmacist in the barbershop setting, along with blood pressure readings, medication management, electrolyte monitoring, and progress notes sent to each man’s primary care provider. In addition, the barbers encouraged blood pressure management and a healthy lifestyle during the men’s regular haircut visits, occurring about every 2 weeks. The control group received encouragement from their barbers and usual care from their primary care providers.
The average baseline systolic blood pressure was 152.8 mm Hg in the intervention group, which dropped to 125.8 mm Hg at 6 months. The controls’ average systolic blood pressure was 154.6 mm Hg at baseline and 145.4 at 6 months.
Dr. Victor said he was thrilled with the results, and that the intervention group’s improvement was roughly three times that seen in many blood pressure intervention studies. “We lost very few men to follow-up,” Dr. Victor said. “I can’t underestimate how important the buy-in of the barbers was,” he emphasized. The primary analysis included 132 intervention men and 171 controls with complete 6 months data.
The between-group difference for the primary outcome was 21.6 mm Hg in favor of the intervention,” Dr. Victor said. As a secondary outcome, the between-group difference in diastolic blood pressure was 14.9 mm Hg in favor of the intervention.
In addition, 64% and 12% of the intervention and control groups, respectively, achieved the blood pressure target of 130/80.
“We think the intervention effect is multifaceted,” said Dr. Victor. The pharmacists were doctorate level with specialty training, and prescribed more intense therapy than did a community clinic. In addition, the convenience and comfort of the community barbershop setting, and the endorsement by the barbers, who are significant figures in the community, contributed to the success of the study, he said.
“We think the whole package was important,” he emphasized.
The intervention was safe and well tolerated, with no adverse events. A total of three cases of reversible acute kidney injury occurred in the intervention group that were related to indapamide and resolved when it was discontinued.
“This [study] is a home run,” discussant Eileen Handberg, MD, said in a press conference, “This is taking care where patients live; this is ‘high-touch’ medicine,” she said. Also, the 9-mm Hg improvement in the control group was comparable with improvements in many previous blood pressure control trials, she noted.
Dr. Victor said he plans to expand the study by establishing similar protocols in other communities. Additional next steps for research include extending the current study for another 6 months, expanding the research criteria to include men with mild hypertension, and conducting a cost analysis, he said.
The study was funded by the National Heart, Lung, and Blood Institute and others. Dr. Victor had no financial conflicts to disclose. Dr. Handberg disclosed relationships with multiple companies including Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Ionis, and Relypsa.
The findings were published online simultaneously with Dr. Victor’s report (N Engl J Med. 2018 Mar 11; doi: 10.1056/NEJMoa1717250).
SOURCE: Victor R et al. ACC 2018.
REPORTING FROM ACC 18
Key clinical point: Major finding: After 6 months, mean systolic blood pressure among men who received intervention dropped an average of 27 mm Hg, compared with 9 mm Hg in controls.
Study details: The data come from a cluster randomized trial including 319 black men who visited 52 barbershops.
Disclosures: The study was funded by the National Heart, Lung, and Blood Institute and others.
Source: Victor R et al. ACC 2018.
VIDEO: Dabigatran effective for myocardial injury after noncardiac surgery
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
REPORTING FROM ACC 18
Key clinical point: Dabigatran is the first intervention proven to benefit patients with MINS.
Major finding: Major vascular complications occurred in 11% of patients on dabigatran and 15% on placebo.
Study details: MANAGE, a multicenter, randomized trial with 1,754 patients.
Disclosures: MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
Source: Devereaux P et al. ACC 18.
Gaps exist in receipt of clinically indicated genetic counseling after breast cancer diagnosis
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: There exists a large gap between mandates for timely pretest formal genetic counseling of higher-risk, breast cancer patients and its implementation in clinical practice.
Major finding: Almost half (47.4%) of patients diagnosed with early breast cancer with an indication for genetic risk evaluation did not get genetic tests. Of those who got genetic testing, 43.5% received formal counseling and 31.1% received physician-directed discussion.
Study details: Data on 5,080 women aged 20-79 years diagnosed with early stage breast during 2013-2015, reported to National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County.
Disclosures: Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
Source: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
Fruquintinib promising agent for advanced NSCLC
Investigational agent fruquintinib holds promise as a third- or fourth-line treatment option for patients with advanced non–small-cell lung cancer (NSCLC), according to new findings published in the Journal of Clinical Oncology.
The median progression free survival (PFS) as evaluated by a blinded image central review committee and the study’s primary endpoint, was more than threefold higher than observed with placebo; 3.8 months (95% confidence interval, 2.8 to 4.6 months) with fruquintinib versus 1.1 months (95% CI, 1.0 to 1.9 months) with placebo (stratified HR was 0.34 (95% CI,0.20 to 0.57; P less than .001). PFS assessed by investigators was nearly identical.
Median overall survival, however, was numerically longer for patients in the placebo arm versus the fruquintinib arm (7.7 and 9.7 months in the fruquintinib and placebo groups; stratified HR, 0.70; 95% CI, 0.43 to 1.15; P = .152). The authors point out that overall survival is a secondary endpoint and the study was insufficiently powered to assess differences in overall survival.
“In patients with NSCLC who experienced treatment failure with two standard chemotherapies, fruquintinib may provide a clinically meaningful benefit, and further evidence of a statistically significant OS benefit of fruquintinib is expected from a phase 3 randomized study in this target population,” wrote Dr. Shun Lu of Shanghai Lung Cancer Center, Shanghai Chest Hospital, Jiao Tong University, Shanghai, China, and colleagues.
Fruquintinib is a highly selective inhibitor of VEGFR-1, -2 and -3 kinases, and showed promising activity against solid tumors, including NSCLC, in a phase 1 trial. The current phase 2 trial evaluated the efficacy and safety of single-agent fruquintinib in 91 patients with advanced nonsquamous NSCLC. All patients had experienced disease progression after two lines of standard chemotherapy, and were randomly assigned to be treated with either fruquintinib (n = 61) or placebo (n = 30).
At data cutoff for PFS analysis, a total of 46 patients (75.4%) in the fruquintinib group and 25 (83.3%) in the placebo group had experienced a PFS event. The median follow-up for survival was 28.0 months for fruquintinib and 25.4 months for the placebo group.
Both the 3- and 6-month survival rates were numerically higher for patients receiving fruquintinib group versus placebo (90.2% vs. 73.3% for 3-month survival and 67.2% vs. 58.8% for 6-month survival).
The results for other secondary endpoints showed a more favorable overall response rate for fruquintinib vs placebo (13.1% vs 0%; P = .041), as was the disease control rate (60.7% vs 13.3%; P less than .001).
Subgroup analyses favored PFS with fruquintinib, as it was significantly longer vs. placebo for all patient subsets except for those with brain metastases. The median overall survival also was numerically greater with fruquintinib among patients positive for EGFR mutations (8.4 vs. 5.5 months; HR, 0.58; 95% CI, 0.30-1.141 P =.11).
Treatment emergent adverse events were higher in the fruquintinib arm (100% vs. 90%), but most of these were grade 1/2 in both groups. The most common grade 3 and higher events observed with fruquintinib were hypertension (8.2%), hand-foot syndrome (4.9%), and proteinuria (4.9%).
SOURCE: Lu S. et al. J Clin Oncol. 2018 Mar. 12 doi: 10.1200/JCO.2017.76.7145.
Investigational agent fruquintinib holds promise as a third- or fourth-line treatment option for patients with advanced non–small-cell lung cancer (NSCLC), according to new findings published in the Journal of Clinical Oncology.
The median progression free survival (PFS) as evaluated by a blinded image central review committee and the study’s primary endpoint, was more than threefold higher than observed with placebo; 3.8 months (95% confidence interval, 2.8 to 4.6 months) with fruquintinib versus 1.1 months (95% CI, 1.0 to 1.9 months) with placebo (stratified HR was 0.34 (95% CI,0.20 to 0.57; P less than .001). PFS assessed by investigators was nearly identical.
Median overall survival, however, was numerically longer for patients in the placebo arm versus the fruquintinib arm (7.7 and 9.7 months in the fruquintinib and placebo groups; stratified HR, 0.70; 95% CI, 0.43 to 1.15; P = .152). The authors point out that overall survival is a secondary endpoint and the study was insufficiently powered to assess differences in overall survival.
“In patients with NSCLC who experienced treatment failure with two standard chemotherapies, fruquintinib may provide a clinically meaningful benefit, and further evidence of a statistically significant OS benefit of fruquintinib is expected from a phase 3 randomized study in this target population,” wrote Dr. Shun Lu of Shanghai Lung Cancer Center, Shanghai Chest Hospital, Jiao Tong University, Shanghai, China, and colleagues.
Fruquintinib is a highly selective inhibitor of VEGFR-1, -2 and -3 kinases, and showed promising activity against solid tumors, including NSCLC, in a phase 1 trial. The current phase 2 trial evaluated the efficacy and safety of single-agent fruquintinib in 91 patients with advanced nonsquamous NSCLC. All patients had experienced disease progression after two lines of standard chemotherapy, and were randomly assigned to be treated with either fruquintinib (n = 61) or placebo (n = 30).
At data cutoff for PFS analysis, a total of 46 patients (75.4%) in the fruquintinib group and 25 (83.3%) in the placebo group had experienced a PFS event. The median follow-up for survival was 28.0 months for fruquintinib and 25.4 months for the placebo group.
Both the 3- and 6-month survival rates were numerically higher for patients receiving fruquintinib group versus placebo (90.2% vs. 73.3% for 3-month survival and 67.2% vs. 58.8% for 6-month survival).
The results for other secondary endpoints showed a more favorable overall response rate for fruquintinib vs placebo (13.1% vs 0%; P = .041), as was the disease control rate (60.7% vs 13.3%; P less than .001).
Subgroup analyses favored PFS with fruquintinib, as it was significantly longer vs. placebo for all patient subsets except for those with brain metastases. The median overall survival also was numerically greater with fruquintinib among patients positive for EGFR mutations (8.4 vs. 5.5 months; HR, 0.58; 95% CI, 0.30-1.141 P =.11).
Treatment emergent adverse events were higher in the fruquintinib arm (100% vs. 90%), but most of these were grade 1/2 in both groups. The most common grade 3 and higher events observed with fruquintinib were hypertension (8.2%), hand-foot syndrome (4.9%), and proteinuria (4.9%).
SOURCE: Lu S. et al. J Clin Oncol. 2018 Mar. 12 doi: 10.1200/JCO.2017.76.7145.
Investigational agent fruquintinib holds promise as a third- or fourth-line treatment option for patients with advanced non–small-cell lung cancer (NSCLC), according to new findings published in the Journal of Clinical Oncology.
The median progression free survival (PFS) as evaluated by a blinded image central review committee and the study’s primary endpoint, was more than threefold higher than observed with placebo; 3.8 months (95% confidence interval, 2.8 to 4.6 months) with fruquintinib versus 1.1 months (95% CI, 1.0 to 1.9 months) with placebo (stratified HR was 0.34 (95% CI,0.20 to 0.57; P less than .001). PFS assessed by investigators was nearly identical.
Median overall survival, however, was numerically longer for patients in the placebo arm versus the fruquintinib arm (7.7 and 9.7 months in the fruquintinib and placebo groups; stratified HR, 0.70; 95% CI, 0.43 to 1.15; P = .152). The authors point out that overall survival is a secondary endpoint and the study was insufficiently powered to assess differences in overall survival.
“In patients with NSCLC who experienced treatment failure with two standard chemotherapies, fruquintinib may provide a clinically meaningful benefit, and further evidence of a statistically significant OS benefit of fruquintinib is expected from a phase 3 randomized study in this target population,” wrote Dr. Shun Lu of Shanghai Lung Cancer Center, Shanghai Chest Hospital, Jiao Tong University, Shanghai, China, and colleagues.
Fruquintinib is a highly selective inhibitor of VEGFR-1, -2 and -3 kinases, and showed promising activity against solid tumors, including NSCLC, in a phase 1 trial. The current phase 2 trial evaluated the efficacy and safety of single-agent fruquintinib in 91 patients with advanced nonsquamous NSCLC. All patients had experienced disease progression after two lines of standard chemotherapy, and were randomly assigned to be treated with either fruquintinib (n = 61) or placebo (n = 30).
At data cutoff for PFS analysis, a total of 46 patients (75.4%) in the fruquintinib group and 25 (83.3%) in the placebo group had experienced a PFS event. The median follow-up for survival was 28.0 months for fruquintinib and 25.4 months for the placebo group.
Both the 3- and 6-month survival rates were numerically higher for patients receiving fruquintinib group versus placebo (90.2% vs. 73.3% for 3-month survival and 67.2% vs. 58.8% for 6-month survival).
The results for other secondary endpoints showed a more favorable overall response rate for fruquintinib vs placebo (13.1% vs 0%; P = .041), as was the disease control rate (60.7% vs 13.3%; P less than .001).
Subgroup analyses favored PFS with fruquintinib, as it was significantly longer vs. placebo for all patient subsets except for those with brain metastases. The median overall survival also was numerically greater with fruquintinib among patients positive for EGFR mutations (8.4 vs. 5.5 months; HR, 0.58; 95% CI, 0.30-1.141 P =.11).
Treatment emergent adverse events were higher in the fruquintinib arm (100% vs. 90%), but most of these were grade 1/2 in both groups. The most common grade 3 and higher events observed with fruquintinib were hypertension (8.2%), hand-foot syndrome (4.9%), and proteinuria (4.9%).
SOURCE: Lu S. et al. J Clin Oncol. 2018 Mar. 12 doi: 10.1200/JCO.2017.76.7145.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Fruquintinib is a potential option for heavily pretreated patients with advanced non–small-cell lung cancer.
Major finding: Median progression free survival was 3.8 months (95% CI, 2.8 to 4.6 months) with fruquintinib versus 1.1 months (95% CI, 1.0 to 1.9 months) with placebo (stratified HR was 0.34 (95% CI,0.20 to 0.57; P less than .001).
Data source: Phase II multicenter, randomized, placebo controlled trial of 91 patients with advanced NSCLC.
Disclosures: Hutchison MediPharma supported the study. Dr. Lu and several coauthors report relationships with industry including Hutchison MediPharma.
Source: Lu S. et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.7145.
Teleconference is effective in assessing penicillin allergy
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
ORLANDO – and resulted in almost every patient having their allergy label removed, researchers reported.
In what the researchers said was the first study showing the utility of telemedicine in evaluating patient-reported penicillin allergies, allergy and immunology physicians did a secure telemedicine consultation with patients after they underwent penicillin skin testing with a physician assistant; an approach which, on average, took 123 minutes fewer each time than if the physician had done the consultation face-to-face. The teleconference can be done on a laptop or smartphone.
She said the approach is sensible and effective, and it is a good alternative to the traditional way of doing these tests. “What this takes out of that is the travel part. Someone else is doing the travel and the technique of testing,” added that people often are labeled in childhood after getting a rash that was thought to be related to penicillin, but actually was just a coincidence that was unrelated. Then the false allergy label is attached to them for life.
“This is so overlabeled,” Dr. Ramsey said. “Ten percent of the population thinks they’re allergic to penicillin, and 90% of them are not.”
A stark difference was found in the types of medicines administered before and after the evaluations, with aminopenicillin therapy jumping from 0 days of use to 188 days, and vancomycin – a more potent, but more costly alternative – dropping from 130 days of use to 16 days (P less than .05 for both).
Dr. Ramsey noted that, in part because of the time-consuming nature of the penicillin skin tests, they often are simply not done, so the false allergy labels are not caught, leading to pointless costs and exposure to more potent and potentially harmful forms of antibiotic therapy.
“Some hospitals don’t have allergists who will come in to do testing,” she said. “Sometimes patients are on medications that may interfere. And then a lot of times it’s just underrecognized – the implications of a penicillin allergy label. That is a very hot topic in our field and also in infectious disease.”
She hopes the telemedicine approach catches on more widely, which would help minimize the multitude of problems linked to penicillin allergy labels.
“Patients that avoid penicillin, they’re on more costly second-line antibiotics that are, in general, less effective, depending on which infection you’re talking about,” Dr. Ramsey said. “The [second-line antibiotics] have more side effects. And there’s data to show that patients with [a] penicillin allergy label have longer hospital stays, more costly hospital stays, are at risk for more resistant infections. And it breeds antimicrobial resistance in the long-term.”
Dr. Ramsey had no relevant financial disclosures.
SOURCE: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point: Teleconferencing to assess patient-reported penicillin allergies saves time and results in 9 out of 10 patients being delabeled.
Major finding: Of 50 patients prospectively assessed with this approach over a 4-month period last year, 46 were delabeled, with $23,000 in direct antibiotic cost savings, or $360 per patient.
Study details: A prospective study conducted over 4 months in 2017.
Disclosures: Dr. Ramsey had no relevant financial disclosures.
Source: Ramsey AC et al. AAAAI/WAO Joint Congress, Abstract 104.