User login
CPT and relative value changes that may affect reimbursement to your ObGyn practice
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
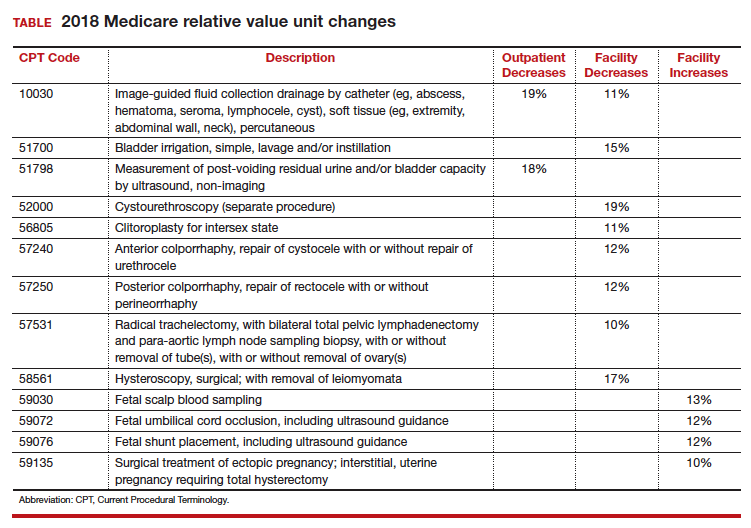
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Another year brings changes to Current Procedural Terminology (CPT) codes (which are developed and copyrighted by the American Medical Association) in the form of additions and revisions, and payments related to resource-based relative value scale (RBRVS) revisions for selected services. As of January 1, 2018, 2 new Category I codes pertain to laparoscopic treatments for gynecologic cancer, and the 4 existing codes for colporrhaphy have been revised to include cystourethroscopy. New Category III codes include 4 for fetal magnetocardiography and 1 for transvaginal tactile imaging. Medicare also has reevaluated certain relative value units (RVUs) in outpatient and facility settings.
New and revised Category I codes
Laparoscopic treatments for gynecologic cancer. Technologic advances in performing laparoscopic procedures have allowed for more extensive laparoscopic surgery for various gynecologic cancers and, to this end, 2 new codes have been added.
First, a new code was added to capture comprehensive laparoscopic surgical staging for gynecologic cancer. This new code, 38573, Laparoscopy, surgical; with bilateral total pelvic lymphadenectomy and peri-aortic lymph node sampling, peritoneal washings, peritoneal biopsy(ies), omentectomy, and diaphragmatic washings, including diaphragmatic and other serosal biopsy(ies), when performed, may not be reported with any other code that includes lymphadenectomy, omentectomy, or hysterectomy. It is intended primarily for a stand-alone staging procedure after an initial biopsy shows a gynecologic malignancy such as ovarian cancer. This new code has been valued at 33.59 RVUs.
Second, a new code was added to capture laparoscopic debulking in conjunction with hysterectomy. The new code, 58575, Laparoscopy, surgical, total hysterectomy for resection of malignancy (tumor debulking), with omentectomy including salpingo-oophorectomy, unilateral or bilateral, when performed, has been valued at 53.62 RVUs. The open equivalent to this new code is 58953, Bilateral salpingo-oophorectomy with omentectomy, total abdominal hysterectomy and radical dissection for debulking.
Cystourethroscopy. The revisions involve no longer permitting separate reporting of 52000, Cystourethroscopy (separate procedure), with the colporrhaphy codes 57240−57265. The rationale behind this change was that surgeons were routinely performing cystoscopy at the time of these procedures and therefore it should become part of the surgical procedure. Currently the Medicare National Correct Coding Initiative (NCCI) bundles 52000 with these 4 codes, but only code 57250 allows for the use of a modifier -59 to bypass the edit if the purpose of the cystoscopy was evaluation of a distinct complaint or problem (such as evaluating patient-expressed urinary symptoms prior to the surgery that were investigated at the time of the prolapse surgery). When codes 57240, 57260, or 57265 are billed along with 52000, the cystoscopy will be denied and a modifier -59 cannot be reported to bypass this edit.
New Category III codes
The new Category III codes represent emerging technology, and it is important to report them, rather than an unlisted code, if the procedures described are performed so that data can be collected for later consideration to make these Category I CPT codes. Since these codes are not assigned relative values, the provider will need to let the payer know which existing CPT Category I code most closely represents the work involved.
Fetal magnetocardiography. The new Category III codes for fetal magnetocardiography describe essentially a fetal electrocardiogram (ECG) that would be performed to assess fetal arrhythmias by placing up to 3 leads on the mother’s abdomen. Possible comparison codes for physician work might include 59050, fetal monitoring by consultant during labor; 93000−93010, 12-lead ECG, or 93040−93042, rhythm strip up to 3 leads. However, because the equipment is very expensive, these codes would not capture practice expense and the physician would have to negotiate a reasonable reimbursement level with the payer, if the magnetocardiography was a covered service. The new codes are as follows:
- 0475T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording and storage, data scanning with signal extraction, technical analysis and result, as well as supervision, review, and interpretation of report by a physician or other qualified health care professional
- 0476T, Recording of fetal magnetic cardiac signal using at least 3 channels; patient recording, data scanning, with raw electronic signal transfer of data and storage
- 0477T, Recording of fetal magnetic cardiac signal using at least 3 channels; signal extraction, technical analysis, and result.
Transvaginal tactile imaging. The new Category III code, 0487T, Biomechanical mapping, transvaginal, with report, describes the use of a pressure sensor probe inserted into the vaginal canal to measure and collect data on pelvic muscle strength, elasticity, tissue integrity, and tone. These data produce images in real time that are mapped to produce a report for physician review, interpretation, and report. The data allow quantification of pelvic floor dysfunction and may be useful in determining the most appropriate treatment (whether surgical or medical) for this gynecologic condition. The procedure uses a transvaginal probe like an ultrasound, so using 76830, transvaginal ultrasound, would not be unreasonable as a comparison code as a start.
Medicare relative value changes
Every year, Medicare reevaluates potentially misvalued CPT codes and this year was no exception. The TABLE represents the winners and losers for codes in the outpatient and facility settings that have increased or decreased RVUs by more than 10%.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Alcohol dependence may accelerate aging, frontal cortical deficits
Alcoholism compounds age-associated volume deficits in the frontal cortex, independent of the additional effects of drug dependence or hepatitis C infection, suggests new research published March 14 in JAMA Psychiatry.
Edith V. Sullivan, PhD, and her coauthors reported the results of a 14-year longitudinal study that used magnetic resonance imaging to examine the brains of 116 participants with alcohol dependence and 96 age-matched controls.
They found that participants with alcohol dependence as defined by the DSM-IV had significantly greater gray matter volume deficits in their frontal, temporal, parietal, cingulate and insular cortices, compared with controls – most prominently in the frontal subregions – with the only exception being the occipital lobe. When age was taken into account, age-related volume deficits were seen in the control group in five of the six cortical regions, but the alcoholism group showed a significantly greater deficit in the precentral and superior frontal cortex.
Dr. Sullivan, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and her coauthors said the presence of age-alcoholism interactions puts older alcohol-dependent individuals at greater risk of age-associated functional compromise, even if their excessive drinking starts later in life.
More than half of individuals in the alcoholism group (54.5%) also reported drug dependence. The imaging showed that participants with alcohol use disorder who also reported opiate or cocaine use had smaller frontal cortex volumes compared with those who were not drug users. However, the non–drug-dependent participants in the group still showed deficits in precentral, supplementary motor and medial cortices volumes, compared with controls.
“These findings in alcohol-dependent and control participants, examined 1 to 8 times or more during intervals of 1 week to 12.5 years, representing, to our knowledge, the largest and longest-studied group to date, support our study hypotheses regarding alcoholism-associated accelerated aging and cortical volume deficits independent of drug dependence or HCV infection comorbidity,” the authors wrote.
“We observed a selectivity of frontal cortex to age-alcoholism interaction beyond normal aging effects and independent of deficits related to drug dependence.”
, compared with those with alcoholism alone and compared with controls. “Thus, HCV infection, while having focal effects on frontal brain systems, targeted frontally based systems also vulnerable to chronic and extensive alcohol consumption,” the authors wrote. “Whether the compounded untoward effects of alcoholism and HCV infection on brain structure can be ameliorated with successful treatment of the infection remains to be determined.”
Dr. Sullivan and her coauthors cited several limitations. For example, non–alcohol-dependent or HCV-infected comparison groups were not available for analysis.
The study was supported by the National Institute on Alcohol Abuse and Alcoholism, and the Moldow Women’s Hope and Heal Fund. No conflicts of interest were declared.
SOURCE: Sullivan EV et al. JAMA Psychiatry. 2018 Mar 14. doi: 10.1001/jamapsychiatry.2018.0021.
With an aging population that is also showing significant increases in alcohol use and misuse, studies of the interaction between alcohol and aging and the brain are highly significant. The most compelling finding of this study is the impact of that interaction on the frontal cortex volume, because of the key role this region of the brain plays in executive function.
Deficits in frontal cortical volume associated with aging are hypothesized to also result in impulsivity and compulsivity, which opens up the possibility of greater vulnerability to alcohol use disorder later in life. So as excessive alcohol consumption contributes to this aging process, this aging process also might contribute to excessive drinking in the elderly as a form of self-medication of the negative emotional states associated with aging.
“The study ... provides compelling evidence that alcohol misuse during later adulthood could confer a greater risk of deficits in frontal lobe function beyond the deficits that typically occur with aging,” wrote George F. Koob, PhD.
Given this, it is critical that strategies be explored and implemented aimed at addressing the misuse of alcohol by older drinkers. “As Yoda might say, ‘Protect their brains, we must.’ ”
George F. Koob, PhD, is affiliated with the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, Rockville, Md. These comments are taken from an editorial (JAMA Psychiatry. 2018 March 14. doi: 10.1001/jamapsychiatry.2018.0009). No conflicts of interest were declared.
With an aging population that is also showing significant increases in alcohol use and misuse, studies of the interaction between alcohol and aging and the brain are highly significant. The most compelling finding of this study is the impact of that interaction on the frontal cortex volume, because of the key role this region of the brain plays in executive function.
Deficits in frontal cortical volume associated with aging are hypothesized to also result in impulsivity and compulsivity, which opens up the possibility of greater vulnerability to alcohol use disorder later in life. So as excessive alcohol consumption contributes to this aging process, this aging process also might contribute to excessive drinking in the elderly as a form of self-medication of the negative emotional states associated with aging.
“The study ... provides compelling evidence that alcohol misuse during later adulthood could confer a greater risk of deficits in frontal lobe function beyond the deficits that typically occur with aging,” wrote George F. Koob, PhD.
Given this, it is critical that strategies be explored and implemented aimed at addressing the misuse of alcohol by older drinkers. “As Yoda might say, ‘Protect their brains, we must.’ ”
George F. Koob, PhD, is affiliated with the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, Rockville, Md. These comments are taken from an editorial (JAMA Psychiatry. 2018 March 14. doi: 10.1001/jamapsychiatry.2018.0009). No conflicts of interest were declared.
With an aging population that is also showing significant increases in alcohol use and misuse, studies of the interaction between alcohol and aging and the brain are highly significant. The most compelling finding of this study is the impact of that interaction on the frontal cortex volume, because of the key role this region of the brain plays in executive function.
Deficits in frontal cortical volume associated with aging are hypothesized to also result in impulsivity and compulsivity, which opens up the possibility of greater vulnerability to alcohol use disorder later in life. So as excessive alcohol consumption contributes to this aging process, this aging process also might contribute to excessive drinking in the elderly as a form of self-medication of the negative emotional states associated with aging.
“The study ... provides compelling evidence that alcohol misuse during later adulthood could confer a greater risk of deficits in frontal lobe function beyond the deficits that typically occur with aging,” wrote George F. Koob, PhD.
Given this, it is critical that strategies be explored and implemented aimed at addressing the misuse of alcohol by older drinkers. “As Yoda might say, ‘Protect their brains, we must.’ ”
George F. Koob, PhD, is affiliated with the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, Rockville, Md. These comments are taken from an editorial (JAMA Psychiatry. 2018 March 14. doi: 10.1001/jamapsychiatry.2018.0009). No conflicts of interest were declared.
Alcoholism compounds age-associated volume deficits in the frontal cortex, independent of the additional effects of drug dependence or hepatitis C infection, suggests new research published March 14 in JAMA Psychiatry.
Edith V. Sullivan, PhD, and her coauthors reported the results of a 14-year longitudinal study that used magnetic resonance imaging to examine the brains of 116 participants with alcohol dependence and 96 age-matched controls.
They found that participants with alcohol dependence as defined by the DSM-IV had significantly greater gray matter volume deficits in their frontal, temporal, parietal, cingulate and insular cortices, compared with controls – most prominently in the frontal subregions – with the only exception being the occipital lobe. When age was taken into account, age-related volume deficits were seen in the control group in five of the six cortical regions, but the alcoholism group showed a significantly greater deficit in the precentral and superior frontal cortex.
Dr. Sullivan, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and her coauthors said the presence of age-alcoholism interactions puts older alcohol-dependent individuals at greater risk of age-associated functional compromise, even if their excessive drinking starts later in life.
More than half of individuals in the alcoholism group (54.5%) also reported drug dependence. The imaging showed that participants with alcohol use disorder who also reported opiate or cocaine use had smaller frontal cortex volumes compared with those who were not drug users. However, the non–drug-dependent participants in the group still showed deficits in precentral, supplementary motor and medial cortices volumes, compared with controls.
“These findings in alcohol-dependent and control participants, examined 1 to 8 times or more during intervals of 1 week to 12.5 years, representing, to our knowledge, the largest and longest-studied group to date, support our study hypotheses regarding alcoholism-associated accelerated aging and cortical volume deficits independent of drug dependence or HCV infection comorbidity,” the authors wrote.
“We observed a selectivity of frontal cortex to age-alcoholism interaction beyond normal aging effects and independent of deficits related to drug dependence.”
, compared with those with alcoholism alone and compared with controls. “Thus, HCV infection, while having focal effects on frontal brain systems, targeted frontally based systems also vulnerable to chronic and extensive alcohol consumption,” the authors wrote. “Whether the compounded untoward effects of alcoholism and HCV infection on brain structure can be ameliorated with successful treatment of the infection remains to be determined.”
Dr. Sullivan and her coauthors cited several limitations. For example, non–alcohol-dependent or HCV-infected comparison groups were not available for analysis.
The study was supported by the National Institute on Alcohol Abuse and Alcoholism, and the Moldow Women’s Hope and Heal Fund. No conflicts of interest were declared.
SOURCE: Sullivan EV et al. JAMA Psychiatry. 2018 Mar 14. doi: 10.1001/jamapsychiatry.2018.0021.
Alcoholism compounds age-associated volume deficits in the frontal cortex, independent of the additional effects of drug dependence or hepatitis C infection, suggests new research published March 14 in JAMA Psychiatry.
Edith V. Sullivan, PhD, and her coauthors reported the results of a 14-year longitudinal study that used magnetic resonance imaging to examine the brains of 116 participants with alcohol dependence and 96 age-matched controls.
They found that participants with alcohol dependence as defined by the DSM-IV had significantly greater gray matter volume deficits in their frontal, temporal, parietal, cingulate and insular cortices, compared with controls – most prominently in the frontal subregions – with the only exception being the occipital lobe. When age was taken into account, age-related volume deficits were seen in the control group in five of the six cortical regions, but the alcoholism group showed a significantly greater deficit in the precentral and superior frontal cortex.
Dr. Sullivan, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and her coauthors said the presence of age-alcoholism interactions puts older alcohol-dependent individuals at greater risk of age-associated functional compromise, even if their excessive drinking starts later in life.
More than half of individuals in the alcoholism group (54.5%) also reported drug dependence. The imaging showed that participants with alcohol use disorder who also reported opiate or cocaine use had smaller frontal cortex volumes compared with those who were not drug users. However, the non–drug-dependent participants in the group still showed deficits in precentral, supplementary motor and medial cortices volumes, compared with controls.
“These findings in alcohol-dependent and control participants, examined 1 to 8 times or more during intervals of 1 week to 12.5 years, representing, to our knowledge, the largest and longest-studied group to date, support our study hypotheses regarding alcoholism-associated accelerated aging and cortical volume deficits independent of drug dependence or HCV infection comorbidity,” the authors wrote.
“We observed a selectivity of frontal cortex to age-alcoholism interaction beyond normal aging effects and independent of deficits related to drug dependence.”
, compared with those with alcoholism alone and compared with controls. “Thus, HCV infection, while having focal effects on frontal brain systems, targeted frontally based systems also vulnerable to chronic and extensive alcohol consumption,” the authors wrote. “Whether the compounded untoward effects of alcoholism and HCV infection on brain structure can be ameliorated with successful treatment of the infection remains to be determined.”
Dr. Sullivan and her coauthors cited several limitations. For example, non–alcohol-dependent or HCV-infected comparison groups were not available for analysis.
The study was supported by the National Institute on Alcohol Abuse and Alcoholism, and the Moldow Women’s Hope and Heal Fund. No conflicts of interest were declared.
SOURCE: Sullivan EV et al. JAMA Psychiatry. 2018 Mar 14. doi: 10.1001/jamapsychiatry.2018.0021.
FROM JAMA PSYCHIATRY
Key clinical point: Older alcohol-dependent patients are at greater risk of functional compromise, even if their excessive drinking starts later in life.
Major finding: Individuals with alcohol use disorder show significantly greater deficits in the precentral and superior frontal cortex, compared with age-matched controls.
Data source: Longitudinal study of 116 participants with alcohol dependence and 96 age-matched controls.
Disclosures: The U.S. National Institute on Alcohol Abuse and Alcoholism, and the Moldow Women’s Hope and Heal Fund supported the study. No conflicts of interest were declared.
Source: Sullivan EV et al. JAMA Psychiatry. 2018 Mar 14. doi: 10.1001/jamapsychiatry.2018.0021.
FDA approves continuous glucose monitor with AI assistant
between the ages of 14 and 75 years old.
The first CGM to use artificial intelligence for this purpose, the Guardian Connect system is intended to help people with diabetes who use multiple daily injections of insulin to manage their diabetes by helping them prevent hyperglycemia and hypoglycemia, according to a statement from Medtronic.
Guardian Connect utilizes a predictive algorithm that alerts patients of significant swings in blood glucose levels up to 60 minutes prior to the event. When combined with the Guardian Sensor 3, which is placed on the abdomen to monitor blood glucose levels, the Guardian Connect system was accurate and was able to alert patients about 98.5% of hypoglycemic events, according to the results of a clinical trial. This information can also be shared and monitored with care takers and family member in real time or via text message.
Perhaps the most intriguing aspect of using the Guardian Connect system is exclusive access to the Sugar.IQ smart diabetes assistant. Utilizing artificial intelligence technology from IBM Watson Health, the Sugar.IQ assistant continually analyzes how patient’s blood glucose levels respond to factors like food intake, insulin dosages, and daily routines. The combination of continuous monitoring and real-time analysis may be able to identify patterns and lead to personalized insights that will help patients with diabetes keep their blood glucose levels under control.
“Despite proven benefits and advances in technology, only a minority of insulin-using people with diabetes currently use continuous glucose monitors,” Timothy Bailey, MD, the director of the AMCR Institute and a clinical associate professor at University of California, San Diego, said in a statement. “Newer sensors paired with intelligent algorithms that help to both predict and understand glucose excursions, particularly hypoglycemia, will make diabetes safer and more comprehensible for people who inject insulin. Greater utilization of smarter CGM systems promises to allow our patients to achieve more glycemic time-in-range and to further reduce the risk of hypoglycemia,” he said.
Similar products have been approved in the last 6 months, but they have not utilized artificial intelligence and continuous analysis.
No serious adverse events were reported in the clinical trial of Guardian Connect, but less serious adverse events were observed, including gastroenteritis, upper respiratory infection, worsening of benign prostatic hyperplasia, rash, and blisters.
Guardian Connect should be available commercially sometime this summer.
between the ages of 14 and 75 years old.
The first CGM to use artificial intelligence for this purpose, the Guardian Connect system is intended to help people with diabetes who use multiple daily injections of insulin to manage their diabetes by helping them prevent hyperglycemia and hypoglycemia, according to a statement from Medtronic.
Guardian Connect utilizes a predictive algorithm that alerts patients of significant swings in blood glucose levels up to 60 minutes prior to the event. When combined with the Guardian Sensor 3, which is placed on the abdomen to monitor blood glucose levels, the Guardian Connect system was accurate and was able to alert patients about 98.5% of hypoglycemic events, according to the results of a clinical trial. This information can also be shared and monitored with care takers and family member in real time or via text message.
Perhaps the most intriguing aspect of using the Guardian Connect system is exclusive access to the Sugar.IQ smart diabetes assistant. Utilizing artificial intelligence technology from IBM Watson Health, the Sugar.IQ assistant continually analyzes how patient’s blood glucose levels respond to factors like food intake, insulin dosages, and daily routines. The combination of continuous monitoring and real-time analysis may be able to identify patterns and lead to personalized insights that will help patients with diabetes keep their blood glucose levels under control.
“Despite proven benefits and advances in technology, only a minority of insulin-using people with diabetes currently use continuous glucose monitors,” Timothy Bailey, MD, the director of the AMCR Institute and a clinical associate professor at University of California, San Diego, said in a statement. “Newer sensors paired with intelligent algorithms that help to both predict and understand glucose excursions, particularly hypoglycemia, will make diabetes safer and more comprehensible for people who inject insulin. Greater utilization of smarter CGM systems promises to allow our patients to achieve more glycemic time-in-range and to further reduce the risk of hypoglycemia,” he said.
Similar products have been approved in the last 6 months, but they have not utilized artificial intelligence and continuous analysis.
No serious adverse events were reported in the clinical trial of Guardian Connect, but less serious adverse events were observed, including gastroenteritis, upper respiratory infection, worsening of benign prostatic hyperplasia, rash, and blisters.
Guardian Connect should be available commercially sometime this summer.
between the ages of 14 and 75 years old.
The first CGM to use artificial intelligence for this purpose, the Guardian Connect system is intended to help people with diabetes who use multiple daily injections of insulin to manage their diabetes by helping them prevent hyperglycemia and hypoglycemia, according to a statement from Medtronic.
Guardian Connect utilizes a predictive algorithm that alerts patients of significant swings in blood glucose levels up to 60 minutes prior to the event. When combined with the Guardian Sensor 3, which is placed on the abdomen to monitor blood glucose levels, the Guardian Connect system was accurate and was able to alert patients about 98.5% of hypoglycemic events, according to the results of a clinical trial. This information can also be shared and monitored with care takers and family member in real time or via text message.
Perhaps the most intriguing aspect of using the Guardian Connect system is exclusive access to the Sugar.IQ smart diabetes assistant. Utilizing artificial intelligence technology from IBM Watson Health, the Sugar.IQ assistant continually analyzes how patient’s blood glucose levels respond to factors like food intake, insulin dosages, and daily routines. The combination of continuous monitoring and real-time analysis may be able to identify patterns and lead to personalized insights that will help patients with diabetes keep their blood glucose levels under control.
“Despite proven benefits and advances in technology, only a minority of insulin-using people with diabetes currently use continuous glucose monitors,” Timothy Bailey, MD, the director of the AMCR Institute and a clinical associate professor at University of California, San Diego, said in a statement. “Newer sensors paired with intelligent algorithms that help to both predict and understand glucose excursions, particularly hypoglycemia, will make diabetes safer and more comprehensible for people who inject insulin. Greater utilization of smarter CGM systems promises to allow our patients to achieve more glycemic time-in-range and to further reduce the risk of hypoglycemia,” he said.
Similar products have been approved in the last 6 months, but they have not utilized artificial intelligence and continuous analysis.
No serious adverse events were reported in the clinical trial of Guardian Connect, but less serious adverse events were observed, including gastroenteritis, upper respiratory infection, worsening of benign prostatic hyperplasia, rash, and blisters.
Guardian Connect should be available commercially sometime this summer.
CAR T before transplant yields durable remission in B-cell malignancies
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 20 patients receiving CAR T before HCT, 15 (60%) were in ongoing remission of a median 35 months.
Study details: Systematic analysis of 42 patients with B-cell malignancies receiving CAR T-cell therapy at the National Cancer Institute.
Disclosures: The study was conducted at the National Cancer Institute, where Dr. Shalabi is employed.
Source: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
Emerging data help inform immunotherapy for urothelial cancer
SAN FRANCISCO – Emerging data from phase 3 clinical trials are better clarifying the efficacy and safety of immunotherapy in advanced urothelial cancer and helping identify patients most likely to benefit.
An updated analysis of the KEYNOTE-045 trial showed that, compared with chemotherapy, pembrolizumab (Keytruda), an antibody to programmed death-1 (PD-1), almost doubled the 2-year survival rate in patients with recurrent or advanced urothelial cancer. No cumulative toxicity was seen.
Biomarker analyses from the IMvigor 211 trial showed that, compared with chemotherapy, atezolizumab (Tecentriq), an antibody to programmed cell death ligand 1 (PD-L1), prolonged survival by more than 7 months in patients with platinum-treated locally advanced or metastatic disease whose tumors were positive for this ligand and had a high mutational burden. The difference translated to a halving of the risk of death.
Results of both trials were reported at the 2018 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“It is clear that PD-1 and PD-L1 targeted immunotherapy now has a role in most patients with advanced urothelial cancer,” Robert J. Jones, MBChB, PhD, professor of clinical cancer research, University of Glasgow, Beatson West of Scotland Cancer Centre, commented in an invited discussion. “It is also true that cytotoxic chemotherapy maintains a role for many, and that both modalities are often ineffective and may be toxic.”
KEYNOTE-045 trial update
Initial results of KEYNOTE-045, at a median follow-up of 14.1 months, provided level I evidence for the safety and efficacy of pembrolizumab over chemotherapy as second-line therapy for recurrent, advanced urothelial cancer (N Engl J Med. 2017;376:1015-26), leading to approval for that indication. (Pembrolizumab is also approved as first-line therapy for cisplatin-ineligible patients).
Lead investigator Joaquim Bellmunt, MD, PhD, an associate professor of medicine at Harvard Medical School and director of the Bladder Cancer Center, Dana-Farber Cancer Institute, both in Boston, reported a trial update, now at a median follow-up of 27.7 months.
Results among 542 patients showed that the initial overall survival benefit of pembrolizumab over chemotherapy (vinflunine, paclitaxel, or docetaxel) (hazard ratio, 0.73; P = .0022) was sustained and in fact now somewhat greater with longer follow-up (HR, 0.70; P = .00017).
Median overall survival was 10.3 months and 7.3 months, respectively. Corresponding 1-year survival rates were 44.4% and 29.8%, and corresponding 2-year survival rates, 27.0% and 14.3%.
At 24 months, 60.6% of patients in the chemotherapy arm had received an immunotherapy agent, including some who had received pembrolizumab as part of crossover, Dr. Bellmunt noted.
Overall survival benefit was generally similar across subgroups, including patients with PD-L1–positive tumors (defined as a combined positive score of 10% or higher) (HR, 0.56; P = .00153) and patients with PD-L1–negative tumors (HR, 0.75; P = .00859).
The lack of a progression-free survival benefit of pembrolizumab over chemotherapy in the initial analysis (HR, 0.98; P = .420) persisted in the updated analysis (HR, 0.96; P = .317).
The overall response rate now was 21.1% with pembrolizumab and 11.0% with chemotherapy. In the former group, the rate of complete response had increased from 7.0% to 9.3% with the longer follow-up. Median time to response was identical, at 2.1 months, but duration of response was longer with pembrolizumab (not reached vs. 4.4 months).
“We haven’t seen any signals of cumulative toxicity with subsequent follow-up,” Dr. Bellmunt reported. Similar to findings of the initial analysis, the most common grade 3-5 treatment-related adverse events were pruritus, fatigue, and diarrhea with pembrolizumab, and neutropenia, anemia, and fatigue with chemotherapy. As expected, the pembrolizumab group had higher rates of hypothyroidism, pneumonitis, hyperthyroidism, and colitis.
“The overall survival benefit and superior safety of pembrolizumab versus chemotherapy in this second-line patient population is maintained after 2 years of follow-up. At 24 months, 27% of patients are alive, and this is similar to what we are seeing with other immune-sensitive diseases like melanoma,” he concluded. “Results in patients with PD-L1–positive or –negative tumors were consistent with the intent-to-treat population. We have seen some hints with this biomarker, but they are not very striking.”
IMvigor 211 trial biomarker analyses
The IMvigor 211 trial enrolled 931 patients with locally advanced or metastatic urothelial carcinoma who had experienced progression during or after platinum-based chemotherapy and had received at most two prior lines of therapy.
At a median follow-up of 17.3 months, the trial did not meet its primary endpoint of significantly better overall survival with atezolizumab versus chemotherapy (vinflunine, docetaxel, or paclitaxel) in patients having PD-L1–positive tumors, defined as immunohistochemical staining of 2 or 3 (HR, 0.87; 95% confidence interval, 0.63-1.21; P = .41) (Lancet. 2018;391:748-57).
Findings were similar in the entire intention-to-treat population (HR, 0.85; 95% CI, 0.73-0.99) and in the subset with tumors having PD-L1–negative tumors, defined as immunohistochemical staining of 0 or 1 (HR, 0.84; 0.71-1.00). In an unexpected finding, PD-L1 positivity was associated with better outcome in both treatment arms.
“So PD-L1 is a classic prognostic, not predictive, biomarker,” commented lead investigator Thomas Powles, MBBS, MRCP, MD, a clinical professor of genitourinary oncology with Barts Health NHS Trust, St. Bartholomew’s Hospital, London. The investigators therefore conducted a series of analyses in the intent-to-treat population to identify predictive biomarkers.
Results were essentially the same with the tumor gene expression 3 (tGE3) signature, an RNA signature that captures expression of the genes encoding interferon gamma, a chemokine ligand, and PD-L1, and that is a marker for preexisting T-cell immunity. And they were also similar with the DNA damage response (DDR) biomarker.
However, a different pattern was seen with tumor mutational burden (TMB), a FoundationOne panel. Patients with TMB-high tumors had a substantial gain in overall survival from atezolizumab versus chemotherapy (HR, 0.68; 95% CI, 0.51-0.90), whereas those with TMB-low tumors did not (HR, 1.00; 95% CI, 0.75-1.32).
“TMB appears to be a predictive but not a prognostic biomarker,” Dr. Powles said. “It’s not perfect. Complete and partial responses and long overall survival were seen in both arms. Nevertheless, it seems like a step in the right direction.”
Finally, with insight on the nature and relationships of the various biomarkers, the investigators assessed the combination of TMB with PD-L1, finding that atezolizumab had a marked overall survival benefit in patients with TMB-high and PD-L1–positive tumors (17.8 vs. 10.6 months; HR, 0.50; 95% CI, 0.29-0.86).
“I think by using combinations of biomarkers, first-generation and second-generation, we may actually be able to better select patients for treatment in the future,” Dr. Powles concluded.
New data, new insights
“We already know the KEYNOTE-045 trial is positive,” commented the invited discussant, Dr. Jones. “This longer follow-up data is important because we need to better describe the magnitude of benefit.”
The updated survival findings are exciting because they resemble those seen with ipilimumab in melanoma, with about a fifth of patients still alive several years out, he said. Longer follow-up is needed, but “the data we see today are in keeping with the possibility that we might be seeing a similar long-term tail – the so-called immuno-oncology tail – of survival for patients with urothelial cancer as well.”
Although not statistically significant, the difference in progression-free survival is clinically important, according to Dr. Jones. This benefit is driven by both higher response rate and longer duration of response with pembrolizumab.
Long-term toxicity, especially immune-related toxicity, is also a consideration. “There is a small increment in the number of some of these toxicities, but essentially it hasn’t changed. So it would appear that there is certainly not an unexpected peak of latent immunotoxicity with this treatment,” he noted.
“Our confidence in the data for second-line pembrolizumab, if it needed to be further increased, is increased. This does help our patients make an informed decision about whether or not to accept this treatment,” he summarized.
Turning to the IMvigor 211 biomarker study, Dr. Jones said, “I would argue that the choice of second-line therapy in advanced urothelial cancer is not a clinically important decision. The reason for saying that is the case for second-line chemotherapy is poor. We’ve all used it, but we’ve never had high level evidence of benefit. … But it’s important because when we consider moving into the first-line setting, where there are active alternatives, or maybe even more into the perioperative setting, it actually could be vitally important.”
Studies of immunotherapies and targeted therapies in other cancers suggest that a clinically useful predictive biomarker will identify patients who will derive at least a doubling of favorable outcome or a halving of unfavorable outcome when given the drug as compared with a control. “So it appears that the bar has been set quite high,” Dr. Jones said.
“These IMvigor 211 data are exploratory, and they would need further independent validation, in my view,” he said. Nevertheless, “this may provide us the opportunity to use combinations of biomarkers where now we are seeing a hazard ratio of 0.50 [for risk of death] combining PD-L1 with high tumor mutational burden (TMB). That hazard ratio is bringing it into the area where it may be of a magnitude big enough to use in clinical practice, if this could be validated.”
“I would say that none of these data support a role for second-line cytotoxics after failure of platinum, at least not in preference to a checkpoint inhibitor,” Dr. Jones concluded. “There is still no biomarker to inform a choice in second-line treatment. However, TMB, either alone or in combination with other markers, shows promise, which we need to validate in future randomized trials.”
SOURCE: Bellmunt J et al. GUC 2018 Abstract 410; Powles T et al. GUC 2018, Abstract 409.
SAN FRANCISCO – Emerging data from phase 3 clinical trials are better clarifying the efficacy and safety of immunotherapy in advanced urothelial cancer and helping identify patients most likely to benefit.
An updated analysis of the KEYNOTE-045 trial showed that, compared with chemotherapy, pembrolizumab (Keytruda), an antibody to programmed death-1 (PD-1), almost doubled the 2-year survival rate in patients with recurrent or advanced urothelial cancer. No cumulative toxicity was seen.
Biomarker analyses from the IMvigor 211 trial showed that, compared with chemotherapy, atezolizumab (Tecentriq), an antibody to programmed cell death ligand 1 (PD-L1), prolonged survival by more than 7 months in patients with platinum-treated locally advanced or metastatic disease whose tumors were positive for this ligand and had a high mutational burden. The difference translated to a halving of the risk of death.
Results of both trials were reported at the 2018 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“It is clear that PD-1 and PD-L1 targeted immunotherapy now has a role in most patients with advanced urothelial cancer,” Robert J. Jones, MBChB, PhD, professor of clinical cancer research, University of Glasgow, Beatson West of Scotland Cancer Centre, commented in an invited discussion. “It is also true that cytotoxic chemotherapy maintains a role for many, and that both modalities are often ineffective and may be toxic.”
KEYNOTE-045 trial update
Initial results of KEYNOTE-045, at a median follow-up of 14.1 months, provided level I evidence for the safety and efficacy of pembrolizumab over chemotherapy as second-line therapy for recurrent, advanced urothelial cancer (N Engl J Med. 2017;376:1015-26), leading to approval for that indication. (Pembrolizumab is also approved as first-line therapy for cisplatin-ineligible patients).
Lead investigator Joaquim Bellmunt, MD, PhD, an associate professor of medicine at Harvard Medical School and director of the Bladder Cancer Center, Dana-Farber Cancer Institute, both in Boston, reported a trial update, now at a median follow-up of 27.7 months.
Results among 542 patients showed that the initial overall survival benefit of pembrolizumab over chemotherapy (vinflunine, paclitaxel, or docetaxel) (hazard ratio, 0.73; P = .0022) was sustained and in fact now somewhat greater with longer follow-up (HR, 0.70; P = .00017).
Median overall survival was 10.3 months and 7.3 months, respectively. Corresponding 1-year survival rates were 44.4% and 29.8%, and corresponding 2-year survival rates, 27.0% and 14.3%.
At 24 months, 60.6% of patients in the chemotherapy arm had received an immunotherapy agent, including some who had received pembrolizumab as part of crossover, Dr. Bellmunt noted.
Overall survival benefit was generally similar across subgroups, including patients with PD-L1–positive tumors (defined as a combined positive score of 10% or higher) (HR, 0.56; P = .00153) and patients with PD-L1–negative tumors (HR, 0.75; P = .00859).
The lack of a progression-free survival benefit of pembrolizumab over chemotherapy in the initial analysis (HR, 0.98; P = .420) persisted in the updated analysis (HR, 0.96; P = .317).
The overall response rate now was 21.1% with pembrolizumab and 11.0% with chemotherapy. In the former group, the rate of complete response had increased from 7.0% to 9.3% with the longer follow-up. Median time to response was identical, at 2.1 months, but duration of response was longer with pembrolizumab (not reached vs. 4.4 months).
“We haven’t seen any signals of cumulative toxicity with subsequent follow-up,” Dr. Bellmunt reported. Similar to findings of the initial analysis, the most common grade 3-5 treatment-related adverse events were pruritus, fatigue, and diarrhea with pembrolizumab, and neutropenia, anemia, and fatigue with chemotherapy. As expected, the pembrolizumab group had higher rates of hypothyroidism, pneumonitis, hyperthyroidism, and colitis.
“The overall survival benefit and superior safety of pembrolizumab versus chemotherapy in this second-line patient population is maintained after 2 years of follow-up. At 24 months, 27% of patients are alive, and this is similar to what we are seeing with other immune-sensitive diseases like melanoma,” he concluded. “Results in patients with PD-L1–positive or –negative tumors were consistent with the intent-to-treat population. We have seen some hints with this biomarker, but they are not very striking.”
IMvigor 211 trial biomarker analyses
The IMvigor 211 trial enrolled 931 patients with locally advanced or metastatic urothelial carcinoma who had experienced progression during or after platinum-based chemotherapy and had received at most two prior lines of therapy.
At a median follow-up of 17.3 months, the trial did not meet its primary endpoint of significantly better overall survival with atezolizumab versus chemotherapy (vinflunine, docetaxel, or paclitaxel) in patients having PD-L1–positive tumors, defined as immunohistochemical staining of 2 or 3 (HR, 0.87; 95% confidence interval, 0.63-1.21; P = .41) (Lancet. 2018;391:748-57).
Findings were similar in the entire intention-to-treat population (HR, 0.85; 95% CI, 0.73-0.99) and in the subset with tumors having PD-L1–negative tumors, defined as immunohistochemical staining of 0 or 1 (HR, 0.84; 0.71-1.00). In an unexpected finding, PD-L1 positivity was associated with better outcome in both treatment arms.
“So PD-L1 is a classic prognostic, not predictive, biomarker,” commented lead investigator Thomas Powles, MBBS, MRCP, MD, a clinical professor of genitourinary oncology with Barts Health NHS Trust, St. Bartholomew’s Hospital, London. The investigators therefore conducted a series of analyses in the intent-to-treat population to identify predictive biomarkers.
Results were essentially the same with the tumor gene expression 3 (tGE3) signature, an RNA signature that captures expression of the genes encoding interferon gamma, a chemokine ligand, and PD-L1, and that is a marker for preexisting T-cell immunity. And they were also similar with the DNA damage response (DDR) biomarker.
However, a different pattern was seen with tumor mutational burden (TMB), a FoundationOne panel. Patients with TMB-high tumors had a substantial gain in overall survival from atezolizumab versus chemotherapy (HR, 0.68; 95% CI, 0.51-0.90), whereas those with TMB-low tumors did not (HR, 1.00; 95% CI, 0.75-1.32).
“TMB appears to be a predictive but not a prognostic biomarker,” Dr. Powles said. “It’s not perfect. Complete and partial responses and long overall survival were seen in both arms. Nevertheless, it seems like a step in the right direction.”
Finally, with insight on the nature and relationships of the various biomarkers, the investigators assessed the combination of TMB with PD-L1, finding that atezolizumab had a marked overall survival benefit in patients with TMB-high and PD-L1–positive tumors (17.8 vs. 10.6 months; HR, 0.50; 95% CI, 0.29-0.86).
“I think by using combinations of biomarkers, first-generation and second-generation, we may actually be able to better select patients for treatment in the future,” Dr. Powles concluded.
New data, new insights
“We already know the KEYNOTE-045 trial is positive,” commented the invited discussant, Dr. Jones. “This longer follow-up data is important because we need to better describe the magnitude of benefit.”
The updated survival findings are exciting because they resemble those seen with ipilimumab in melanoma, with about a fifth of patients still alive several years out, he said. Longer follow-up is needed, but “the data we see today are in keeping with the possibility that we might be seeing a similar long-term tail – the so-called immuno-oncology tail – of survival for patients with urothelial cancer as well.”
Although not statistically significant, the difference in progression-free survival is clinically important, according to Dr. Jones. This benefit is driven by both higher response rate and longer duration of response with pembrolizumab.
Long-term toxicity, especially immune-related toxicity, is also a consideration. “There is a small increment in the number of some of these toxicities, but essentially it hasn’t changed. So it would appear that there is certainly not an unexpected peak of latent immunotoxicity with this treatment,” he noted.
“Our confidence in the data for second-line pembrolizumab, if it needed to be further increased, is increased. This does help our patients make an informed decision about whether or not to accept this treatment,” he summarized.
Turning to the IMvigor 211 biomarker study, Dr. Jones said, “I would argue that the choice of second-line therapy in advanced urothelial cancer is not a clinically important decision. The reason for saying that is the case for second-line chemotherapy is poor. We’ve all used it, but we’ve never had high level evidence of benefit. … But it’s important because when we consider moving into the first-line setting, where there are active alternatives, or maybe even more into the perioperative setting, it actually could be vitally important.”
Studies of immunotherapies and targeted therapies in other cancers suggest that a clinically useful predictive biomarker will identify patients who will derive at least a doubling of favorable outcome or a halving of unfavorable outcome when given the drug as compared with a control. “So it appears that the bar has been set quite high,” Dr. Jones said.
“These IMvigor 211 data are exploratory, and they would need further independent validation, in my view,” he said. Nevertheless, “this may provide us the opportunity to use combinations of biomarkers where now we are seeing a hazard ratio of 0.50 [for risk of death] combining PD-L1 with high tumor mutational burden (TMB). That hazard ratio is bringing it into the area where it may be of a magnitude big enough to use in clinical practice, if this could be validated.”
“I would say that none of these data support a role for second-line cytotoxics after failure of platinum, at least not in preference to a checkpoint inhibitor,” Dr. Jones concluded. “There is still no biomarker to inform a choice in second-line treatment. However, TMB, either alone or in combination with other markers, shows promise, which we need to validate in future randomized trials.”
SOURCE: Bellmunt J et al. GUC 2018 Abstract 410; Powles T et al. GUC 2018, Abstract 409.
SAN FRANCISCO – Emerging data from phase 3 clinical trials are better clarifying the efficacy and safety of immunotherapy in advanced urothelial cancer and helping identify patients most likely to benefit.
An updated analysis of the KEYNOTE-045 trial showed that, compared with chemotherapy, pembrolizumab (Keytruda), an antibody to programmed death-1 (PD-1), almost doubled the 2-year survival rate in patients with recurrent or advanced urothelial cancer. No cumulative toxicity was seen.
Biomarker analyses from the IMvigor 211 trial showed that, compared with chemotherapy, atezolizumab (Tecentriq), an antibody to programmed cell death ligand 1 (PD-L1), prolonged survival by more than 7 months in patients with platinum-treated locally advanced or metastatic disease whose tumors were positive for this ligand and had a high mutational burden. The difference translated to a halving of the risk of death.
Results of both trials were reported at the 2018 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“It is clear that PD-1 and PD-L1 targeted immunotherapy now has a role in most patients with advanced urothelial cancer,” Robert J. Jones, MBChB, PhD, professor of clinical cancer research, University of Glasgow, Beatson West of Scotland Cancer Centre, commented in an invited discussion. “It is also true that cytotoxic chemotherapy maintains a role for many, and that both modalities are often ineffective and may be toxic.”
KEYNOTE-045 trial update
Initial results of KEYNOTE-045, at a median follow-up of 14.1 months, provided level I evidence for the safety and efficacy of pembrolizumab over chemotherapy as second-line therapy for recurrent, advanced urothelial cancer (N Engl J Med. 2017;376:1015-26), leading to approval for that indication. (Pembrolizumab is also approved as first-line therapy for cisplatin-ineligible patients).
Lead investigator Joaquim Bellmunt, MD, PhD, an associate professor of medicine at Harvard Medical School and director of the Bladder Cancer Center, Dana-Farber Cancer Institute, both in Boston, reported a trial update, now at a median follow-up of 27.7 months.
Results among 542 patients showed that the initial overall survival benefit of pembrolizumab over chemotherapy (vinflunine, paclitaxel, or docetaxel) (hazard ratio, 0.73; P = .0022) was sustained and in fact now somewhat greater with longer follow-up (HR, 0.70; P = .00017).
Median overall survival was 10.3 months and 7.3 months, respectively. Corresponding 1-year survival rates were 44.4% and 29.8%, and corresponding 2-year survival rates, 27.0% and 14.3%.
At 24 months, 60.6% of patients in the chemotherapy arm had received an immunotherapy agent, including some who had received pembrolizumab as part of crossover, Dr. Bellmunt noted.
Overall survival benefit was generally similar across subgroups, including patients with PD-L1–positive tumors (defined as a combined positive score of 10% or higher) (HR, 0.56; P = .00153) and patients with PD-L1–negative tumors (HR, 0.75; P = .00859).
The lack of a progression-free survival benefit of pembrolizumab over chemotherapy in the initial analysis (HR, 0.98; P = .420) persisted in the updated analysis (HR, 0.96; P = .317).
The overall response rate now was 21.1% with pembrolizumab and 11.0% with chemotherapy. In the former group, the rate of complete response had increased from 7.0% to 9.3% with the longer follow-up. Median time to response was identical, at 2.1 months, but duration of response was longer with pembrolizumab (not reached vs. 4.4 months).
“We haven’t seen any signals of cumulative toxicity with subsequent follow-up,” Dr. Bellmunt reported. Similar to findings of the initial analysis, the most common grade 3-5 treatment-related adverse events were pruritus, fatigue, and diarrhea with pembrolizumab, and neutropenia, anemia, and fatigue with chemotherapy. As expected, the pembrolizumab group had higher rates of hypothyroidism, pneumonitis, hyperthyroidism, and colitis.
“The overall survival benefit and superior safety of pembrolizumab versus chemotherapy in this second-line patient population is maintained after 2 years of follow-up. At 24 months, 27% of patients are alive, and this is similar to what we are seeing with other immune-sensitive diseases like melanoma,” he concluded. “Results in patients with PD-L1–positive or –negative tumors were consistent with the intent-to-treat population. We have seen some hints with this biomarker, but they are not very striking.”
IMvigor 211 trial biomarker analyses
The IMvigor 211 trial enrolled 931 patients with locally advanced or metastatic urothelial carcinoma who had experienced progression during or after platinum-based chemotherapy and had received at most two prior lines of therapy.
At a median follow-up of 17.3 months, the trial did not meet its primary endpoint of significantly better overall survival with atezolizumab versus chemotherapy (vinflunine, docetaxel, or paclitaxel) in patients having PD-L1–positive tumors, defined as immunohistochemical staining of 2 or 3 (HR, 0.87; 95% confidence interval, 0.63-1.21; P = .41) (Lancet. 2018;391:748-57).
Findings were similar in the entire intention-to-treat population (HR, 0.85; 95% CI, 0.73-0.99) and in the subset with tumors having PD-L1–negative tumors, defined as immunohistochemical staining of 0 or 1 (HR, 0.84; 0.71-1.00). In an unexpected finding, PD-L1 positivity was associated with better outcome in both treatment arms.
“So PD-L1 is a classic prognostic, not predictive, biomarker,” commented lead investigator Thomas Powles, MBBS, MRCP, MD, a clinical professor of genitourinary oncology with Barts Health NHS Trust, St. Bartholomew’s Hospital, London. The investigators therefore conducted a series of analyses in the intent-to-treat population to identify predictive biomarkers.
Results were essentially the same with the tumor gene expression 3 (tGE3) signature, an RNA signature that captures expression of the genes encoding interferon gamma, a chemokine ligand, and PD-L1, and that is a marker for preexisting T-cell immunity. And they were also similar with the DNA damage response (DDR) biomarker.
However, a different pattern was seen with tumor mutational burden (TMB), a FoundationOne panel. Patients with TMB-high tumors had a substantial gain in overall survival from atezolizumab versus chemotherapy (HR, 0.68; 95% CI, 0.51-0.90), whereas those with TMB-low tumors did not (HR, 1.00; 95% CI, 0.75-1.32).
“TMB appears to be a predictive but not a prognostic biomarker,” Dr. Powles said. “It’s not perfect. Complete and partial responses and long overall survival were seen in both arms. Nevertheless, it seems like a step in the right direction.”
Finally, with insight on the nature and relationships of the various biomarkers, the investigators assessed the combination of TMB with PD-L1, finding that atezolizumab had a marked overall survival benefit in patients with TMB-high and PD-L1–positive tumors (17.8 vs. 10.6 months; HR, 0.50; 95% CI, 0.29-0.86).
“I think by using combinations of biomarkers, first-generation and second-generation, we may actually be able to better select patients for treatment in the future,” Dr. Powles concluded.
New data, new insights
“We already know the KEYNOTE-045 trial is positive,” commented the invited discussant, Dr. Jones. “This longer follow-up data is important because we need to better describe the magnitude of benefit.”
The updated survival findings are exciting because they resemble those seen with ipilimumab in melanoma, with about a fifth of patients still alive several years out, he said. Longer follow-up is needed, but “the data we see today are in keeping with the possibility that we might be seeing a similar long-term tail – the so-called immuno-oncology tail – of survival for patients with urothelial cancer as well.”
Although not statistically significant, the difference in progression-free survival is clinically important, according to Dr. Jones. This benefit is driven by both higher response rate and longer duration of response with pembrolizumab.
Long-term toxicity, especially immune-related toxicity, is also a consideration. “There is a small increment in the number of some of these toxicities, but essentially it hasn’t changed. So it would appear that there is certainly not an unexpected peak of latent immunotoxicity with this treatment,” he noted.
“Our confidence in the data for second-line pembrolizumab, if it needed to be further increased, is increased. This does help our patients make an informed decision about whether or not to accept this treatment,” he summarized.
Turning to the IMvigor 211 biomarker study, Dr. Jones said, “I would argue that the choice of second-line therapy in advanced urothelial cancer is not a clinically important decision. The reason for saying that is the case for second-line chemotherapy is poor. We’ve all used it, but we’ve never had high level evidence of benefit. … But it’s important because when we consider moving into the first-line setting, where there are active alternatives, or maybe even more into the perioperative setting, it actually could be vitally important.”
Studies of immunotherapies and targeted therapies in other cancers suggest that a clinically useful predictive biomarker will identify patients who will derive at least a doubling of favorable outcome or a halving of unfavorable outcome when given the drug as compared with a control. “So it appears that the bar has been set quite high,” Dr. Jones said.
“These IMvigor 211 data are exploratory, and they would need further independent validation, in my view,” he said. Nevertheless, “this may provide us the opportunity to use combinations of biomarkers where now we are seeing a hazard ratio of 0.50 [for risk of death] combining PD-L1 with high tumor mutational burden (TMB). That hazard ratio is bringing it into the area where it may be of a magnitude big enough to use in clinical practice, if this could be validated.”
“I would say that none of these data support a role for second-line cytotoxics after failure of platinum, at least not in preference to a checkpoint inhibitor,” Dr. Jones concluded. “There is still no biomarker to inform a choice in second-line treatment. However, TMB, either alone or in combination with other markers, shows promise, which we need to validate in future randomized trials.”
SOURCE: Bellmunt J et al. GUC 2018 Abstract 410; Powles T et al. GUC 2018, Abstract 409.
REPORTING FROM GUCS 2018
Key clinical point:
Major finding: Pembrolizumab yielded a higher 2-year rate of survival vs. chemotherapy (27.0% vs. 14.3%). Atezolizumab had a marked survival benefit vs. chemotherapy in patients with TMB-high, PD-L1–positive tumors (17.8 vs. 10.6 months; HR, 0.50).
Data source: Two-year follow-up of a phase 3 randomized trial among 542 patients with recurrent, advanced urothelial cancer (KEYNOTE-045 trial). Biomarker analyses of a phase 3 randomized trial among 931 patients with platinum-treated locally advanced or metastatic urothelial cancer (IMvigor 211 trial).
Disclosures: Dr. Bellmunt disclosed that he has a consulting or advisory role with Astellas Pharma, AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech, Merck, Novartis, Pfizer, and Pierre Fabre; that his institution receives research funding from Millennium and Sanofi; and that he receives travel, accommodations, and/or expenses from MSD Oncology and Pfizer. KEYNOTE-045 was funded by Merck & Co. Dr. Powles disclosed that he receives honoraria from Bristol-Myers Squibb, Merck, and Roche/Genentech; has a consulting or advisory role with AstraZeneca; Bristol-Myers Squibb, Roche/Genentech, Merck, and Novartis; receives research funding from AstraZeneca/MedImmune and Roche/Genentech; and has another relationship with Bristol-Myers Squibb and Ipsen. IMvigor 211 was sponsored by Hoffmann-La Roche.
Sources: Bellmunt J et al. GUC 2018 Abstract 410; Powles T et al. GUC 2018, Abstract 409.
Easing into laparoscopic colectomy
LAS VEGAS – , including reduced hospital length of stay, less pain, faster return to work, and reduced incidence of adhesive small bowel obstructions. But the methods are underutilized, in part because they can be challenging to learn.
There are some tools and methods that can help surgeons perform surgeries laparoscopically, including hand-assisted laparoscopy and the use of single-incision laparoscopic colectomy (SILC). These methods were the subject of a talk at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Between 2009 and 2012, a little over 30% of colon cancer cases in the United States and about half of diverticulitis cases were performed laparoscopically. The percentage of laparoscopic procedures ticked up slightly from 2009 to 2012, but only by a few percentage points. “The fact is, we’re just not doing [minimally invasive procedures],” Ian M. Paquette, MD, FACS, associate professor of surgery at the University of Cincinnati, said during his talk.
When a laparoscopic procedure proves challenging, one option is the hand-assist technique. The literature hasn’t been very supportive of the method, with one study of data from the American College of Surgeons National Surgical Quality Improvement Program showing that it offers no improvement in operation time, compared with standard laparoscopy, and that it has slightly higher complication rates (J Gastrointest Surg. 2016 Nov;20[11]:1854-60). “They say you should do everything laparoscopically,” said Dr. Paquette.
One high-volume surgeon showed what happened over time when his practice employed hand-assisted laparoscopy for diverticulitis cases (Dis Colon Rectum. 2014;57[9]1090-7). Initially, most cases were done with the hand-assist technique. Over time, that percentage dropped precipitously and the number that were performed laparoscopically rose sharply. Throughout the study period, the percentage of open procedures remained very low, dropping to almost zero near the conclusion. “They were able to keep their experience of open surgery quite low by using hand-assisted techniques to help with the tough cases of diverticular disease and get over that learning curve,” said Dr. Paquette.
In colon cancer, the study found that laparoscopic procedures hovered around 50%, while open and hand-assisted techniques tended to be around 20%. The higher incidence of open procedures was probably due to the desire of surgeons to be certain that the entire tumor has been excised. “If you’re in doubt, you don’t compromise on oncology. You do an open procedure if you need to,” said Dr. Paquette.
Another tool available to surgeons is single-incision laparoscopic colectomy, as opposed to conventional multiport laparoscopic colectomy. The method results in a very small incision, but can be challenging because the instruments are closer together and it is tricky when the surgeon has to cross the instrument, he said.
Dr. Paquette’s own group also looked at extraction in hand-assisted sigmoid colectomy, in which the surgeon went in laparoscopically, mobilized the colon laterally as would be done in an open procedure, and then performed the colectomy through a small extraction incision. The length of stay was about 15% shorter, there was a lower readmission rate, and gastrointestinal function returned more quickly (Surg Endosc. 2016 Aug;30[8]:3567-72).
Can patients who previously underwent a midline laparotomy be treated laparoscopically? “The answer is possibly yes,” said Dr. Paquette. He noted one study that showed higher rates of minor morbidity, ileotomy, and longer length of stay with laparoscopic treatment (Surg Endosc. 2015;29[3]537-42). “It’s a worth a try if you carefully plan where you’re going to go in through your ports, get in off the midline somewhere if you have to, and just take a look. If you have some adhesions of the omentum to the abdominal wall, it’s really no problem and you can proceed. If you have a frozen abdomen, just do the right thing and open the patient,” said Dr. Paquette.
When it comes to extraction options, Pfannenstiel incisions have the lowest rates of incisional hernias, at 1.9%, according to a survey of 2,148 cases at the Cleveland Clinic. The periumbilical midline incision had the highest frequency at 16.2% (Dis Colon Rectum. 2016 Aug;59[8]:743-50).
“If I’m doing a laparoscopic sigmoid or a laparoscopic low anterior, I do prefer to do a Pfannenstiel incision if I can. If you think that you may need to convert that patient for some reason, don’t do the Pfannenstiel first – nobody wants that big T-shaped incision,” said Dr. Paquette.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Paquette has consulted for Ethicon.
LAS VEGAS – , including reduced hospital length of stay, less pain, faster return to work, and reduced incidence of adhesive small bowel obstructions. But the methods are underutilized, in part because they can be challenging to learn.
There are some tools and methods that can help surgeons perform surgeries laparoscopically, including hand-assisted laparoscopy and the use of single-incision laparoscopic colectomy (SILC). These methods were the subject of a talk at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Between 2009 and 2012, a little over 30% of colon cancer cases in the United States and about half of diverticulitis cases were performed laparoscopically. The percentage of laparoscopic procedures ticked up slightly from 2009 to 2012, but only by a few percentage points. “The fact is, we’re just not doing [minimally invasive procedures],” Ian M. Paquette, MD, FACS, associate professor of surgery at the University of Cincinnati, said during his talk.
When a laparoscopic procedure proves challenging, one option is the hand-assist technique. The literature hasn’t been very supportive of the method, with one study of data from the American College of Surgeons National Surgical Quality Improvement Program showing that it offers no improvement in operation time, compared with standard laparoscopy, and that it has slightly higher complication rates (J Gastrointest Surg. 2016 Nov;20[11]:1854-60). “They say you should do everything laparoscopically,” said Dr. Paquette.
One high-volume surgeon showed what happened over time when his practice employed hand-assisted laparoscopy for diverticulitis cases (Dis Colon Rectum. 2014;57[9]1090-7). Initially, most cases were done with the hand-assist technique. Over time, that percentage dropped precipitously and the number that were performed laparoscopically rose sharply. Throughout the study period, the percentage of open procedures remained very low, dropping to almost zero near the conclusion. “They were able to keep their experience of open surgery quite low by using hand-assisted techniques to help with the tough cases of diverticular disease and get over that learning curve,” said Dr. Paquette.
In colon cancer, the study found that laparoscopic procedures hovered around 50%, while open and hand-assisted techniques tended to be around 20%. The higher incidence of open procedures was probably due to the desire of surgeons to be certain that the entire tumor has been excised. “If you’re in doubt, you don’t compromise on oncology. You do an open procedure if you need to,” said Dr. Paquette.
Another tool available to surgeons is single-incision laparoscopic colectomy, as opposed to conventional multiport laparoscopic colectomy. The method results in a very small incision, but can be challenging because the instruments are closer together and it is tricky when the surgeon has to cross the instrument, he said.
Dr. Paquette’s own group also looked at extraction in hand-assisted sigmoid colectomy, in which the surgeon went in laparoscopically, mobilized the colon laterally as would be done in an open procedure, and then performed the colectomy through a small extraction incision. The length of stay was about 15% shorter, there was a lower readmission rate, and gastrointestinal function returned more quickly (Surg Endosc. 2016 Aug;30[8]:3567-72).
Can patients who previously underwent a midline laparotomy be treated laparoscopically? “The answer is possibly yes,” said Dr. Paquette. He noted one study that showed higher rates of minor morbidity, ileotomy, and longer length of stay with laparoscopic treatment (Surg Endosc. 2015;29[3]537-42). “It’s a worth a try if you carefully plan where you’re going to go in through your ports, get in off the midline somewhere if you have to, and just take a look. If you have some adhesions of the omentum to the abdominal wall, it’s really no problem and you can proceed. If you have a frozen abdomen, just do the right thing and open the patient,” said Dr. Paquette.
When it comes to extraction options, Pfannenstiel incisions have the lowest rates of incisional hernias, at 1.9%, according to a survey of 2,148 cases at the Cleveland Clinic. The periumbilical midline incision had the highest frequency at 16.2% (Dis Colon Rectum. 2016 Aug;59[8]:743-50).
“If I’m doing a laparoscopic sigmoid or a laparoscopic low anterior, I do prefer to do a Pfannenstiel incision if I can. If you think that you may need to convert that patient for some reason, don’t do the Pfannenstiel first – nobody wants that big T-shaped incision,” said Dr. Paquette.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Paquette has consulted for Ethicon.
LAS VEGAS – , including reduced hospital length of stay, less pain, faster return to work, and reduced incidence of adhesive small bowel obstructions. But the methods are underutilized, in part because they can be challenging to learn.
There are some tools and methods that can help surgeons perform surgeries laparoscopically, including hand-assisted laparoscopy and the use of single-incision laparoscopic colectomy (SILC). These methods were the subject of a talk at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Between 2009 and 2012, a little over 30% of colon cancer cases in the United States and about half of diverticulitis cases were performed laparoscopically. The percentage of laparoscopic procedures ticked up slightly from 2009 to 2012, but only by a few percentage points. “The fact is, we’re just not doing [minimally invasive procedures],” Ian M. Paquette, MD, FACS, associate professor of surgery at the University of Cincinnati, said during his talk.
When a laparoscopic procedure proves challenging, one option is the hand-assist technique. The literature hasn’t been very supportive of the method, with one study of data from the American College of Surgeons National Surgical Quality Improvement Program showing that it offers no improvement in operation time, compared with standard laparoscopy, and that it has slightly higher complication rates (J Gastrointest Surg. 2016 Nov;20[11]:1854-60). “They say you should do everything laparoscopically,” said Dr. Paquette.
One high-volume surgeon showed what happened over time when his practice employed hand-assisted laparoscopy for diverticulitis cases (Dis Colon Rectum. 2014;57[9]1090-7). Initially, most cases were done with the hand-assist technique. Over time, that percentage dropped precipitously and the number that were performed laparoscopically rose sharply. Throughout the study period, the percentage of open procedures remained very low, dropping to almost zero near the conclusion. “They were able to keep their experience of open surgery quite low by using hand-assisted techniques to help with the tough cases of diverticular disease and get over that learning curve,” said Dr. Paquette.
In colon cancer, the study found that laparoscopic procedures hovered around 50%, while open and hand-assisted techniques tended to be around 20%. The higher incidence of open procedures was probably due to the desire of surgeons to be certain that the entire tumor has been excised. “If you’re in doubt, you don’t compromise on oncology. You do an open procedure if you need to,” said Dr. Paquette.
Another tool available to surgeons is single-incision laparoscopic colectomy, as opposed to conventional multiport laparoscopic colectomy. The method results in a very small incision, but can be challenging because the instruments are closer together and it is tricky when the surgeon has to cross the instrument, he said.
Dr. Paquette’s own group also looked at extraction in hand-assisted sigmoid colectomy, in which the surgeon went in laparoscopically, mobilized the colon laterally as would be done in an open procedure, and then performed the colectomy through a small extraction incision. The length of stay was about 15% shorter, there was a lower readmission rate, and gastrointestinal function returned more quickly (Surg Endosc. 2016 Aug;30[8]:3567-72).
Can patients who previously underwent a midline laparotomy be treated laparoscopically? “The answer is possibly yes,” said Dr. Paquette. He noted one study that showed higher rates of minor morbidity, ileotomy, and longer length of stay with laparoscopic treatment (Surg Endosc. 2015;29[3]537-42). “It’s a worth a try if you carefully plan where you’re going to go in through your ports, get in off the midline somewhere if you have to, and just take a look. If you have some adhesions of the omentum to the abdominal wall, it’s really no problem and you can proceed. If you have a frozen abdomen, just do the right thing and open the patient,” said Dr. Paquette.
When it comes to extraction options, Pfannenstiel incisions have the lowest rates of incisional hernias, at 1.9%, according to a survey of 2,148 cases at the Cleveland Clinic. The periumbilical midline incision had the highest frequency at 16.2% (Dis Colon Rectum. 2016 Aug;59[8]:743-50).
“If I’m doing a laparoscopic sigmoid or a laparoscopic low anterior, I do prefer to do a Pfannenstiel incision if I can. If you think that you may need to convert that patient for some reason, don’t do the Pfannenstiel first – nobody wants that big T-shaped incision,” said Dr. Paquette.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Paquette has consulted for Ethicon.
REPORTING FROM MISS
Olopatadine/mometasone combo is safe and effective
ORLANDO – Twice-daily treatment with a combination of olopatadine and mometasone showed significant clinical benefit and demonstrated safety for patients with seasonal allergic rhinitis, according to the results of a phase 3 trial.
The combination, known as GSP301, is a fixed-dose nasal spray containing olopatadine, a Food and Drug Adminstration–approved antihistamine, and mometasone, an FDA-approved corticosteroid.
The combination therapy demonstrated statistically significant improvement in scores, compared with those associated with placebo (P less than .001) and olopatadine alone (P = .003). It also showed benefit when compared with mometasone alone (P = .059), Dr. Hampel reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Allergy Organization.
“You expect this outcome,” Dr. Hampel said in an interview. “Olopatadine is a good drug, mometasone is a good drug, so you’d expect the combination to be good, and it was.”
Dr. Hampel and his colleagues also analyzed changes in baseline scores for patients in the olopatadine group and the mometasone group and compared those changes with those seen in the placebo group. Patients in the mometasone group experienced statistically significant improvement, compared with those in the placebo group (P = .004), and patients in the olopatadine group also appeared to experienced benefit (P = .076).
Adverse events were similar across all treatment groups, although a slightly higher percentage of patients in the GSP301 group (12.9%) and olopatadine groups (12.5%) experienced adverse events, Dr. Hampel said.
Glenmark Pharmaceuticals sponsored the study. Dr. Hampel reported funding from Glenmark Pharmaceuticals and other pharmaceutical companies
SOURCE: Hampel F et al. AAAAI/WAO Joint Congress, Abstract 546.
ORLANDO – Twice-daily treatment with a combination of olopatadine and mometasone showed significant clinical benefit and demonstrated safety for patients with seasonal allergic rhinitis, according to the results of a phase 3 trial.
The combination, known as GSP301, is a fixed-dose nasal spray containing olopatadine, a Food and Drug Adminstration–approved antihistamine, and mometasone, an FDA-approved corticosteroid.
The combination therapy demonstrated statistically significant improvement in scores, compared with those associated with placebo (P less than .001) and olopatadine alone (P = .003). It also showed benefit when compared with mometasone alone (P = .059), Dr. Hampel reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Allergy Organization.
“You expect this outcome,” Dr. Hampel said in an interview. “Olopatadine is a good drug, mometasone is a good drug, so you’d expect the combination to be good, and it was.”
Dr. Hampel and his colleagues also analyzed changes in baseline scores for patients in the olopatadine group and the mometasone group and compared those changes with those seen in the placebo group. Patients in the mometasone group experienced statistically significant improvement, compared with those in the placebo group (P = .004), and patients in the olopatadine group also appeared to experienced benefit (P = .076).
Adverse events were similar across all treatment groups, although a slightly higher percentage of patients in the GSP301 group (12.9%) and olopatadine groups (12.5%) experienced adverse events, Dr. Hampel said.
Glenmark Pharmaceuticals sponsored the study. Dr. Hampel reported funding from Glenmark Pharmaceuticals and other pharmaceutical companies
SOURCE: Hampel F et al. AAAAI/WAO Joint Congress, Abstract 546.
ORLANDO – Twice-daily treatment with a combination of olopatadine and mometasone showed significant clinical benefit and demonstrated safety for patients with seasonal allergic rhinitis, according to the results of a phase 3 trial.
The combination, known as GSP301, is a fixed-dose nasal spray containing olopatadine, a Food and Drug Adminstration–approved antihistamine, and mometasone, an FDA-approved corticosteroid.
The combination therapy demonstrated statistically significant improvement in scores, compared with those associated with placebo (P less than .001) and olopatadine alone (P = .003). It also showed benefit when compared with mometasone alone (P = .059), Dr. Hampel reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Allergy Organization.
“You expect this outcome,” Dr. Hampel said in an interview. “Olopatadine is a good drug, mometasone is a good drug, so you’d expect the combination to be good, and it was.”
Dr. Hampel and his colleagues also analyzed changes in baseline scores for patients in the olopatadine group and the mometasone group and compared those changes with those seen in the placebo group. Patients in the mometasone group experienced statistically significant improvement, compared with those in the placebo group (P = .004), and patients in the olopatadine group also appeared to experienced benefit (P = .076).
Adverse events were similar across all treatment groups, although a slightly higher percentage of patients in the GSP301 group (12.9%) and olopatadine groups (12.5%) experienced adverse events, Dr. Hampel said.
Glenmark Pharmaceuticals sponsored the study. Dr. Hampel reported funding from Glenmark Pharmaceuticals and other pharmaceutical companies
SOURCE: Hampel F et al. AAAAI/WAO Joint Congress, Abstract 546.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point: Twice-daily combination therapy improved reflective total nasal symptom scores better than either component alone.
Major finding: Combination therapy significantly improved total nasal symptom scores (P less than .001).
Study details: Phase 3, double-blind, randomized, parallel-group study of 1,180 patients aged 12 years and older with seasonal allergic rhinitis.
Disclosures: Glenmark Pharmaceuticals sponsored the study. Dr. Hampel reported funding from Glenmark Pharmaceuticals and other pharmaceutical companies.
Source: Hampel F et al. AAAAI/WAO Joint Congress, Abstract 546.
Tofacitinib: FDA panel recommends ulcerative colitis indication
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study; two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2); a 53-week, phase 3 maintenance trial (OCTAVE Sustain); and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.
Patients who did not achieve remission but showed some clinical response (decrease in Mayo score of at least 3 points) were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5-mg and 10-mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior tumor necrosis factor–blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks, as part of an open-label extension study. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated patients, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection, “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair Jean-Pierre Raufman, MD, and vice chair Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
Visit www.gastro.org/IBD for patient education guides that you can share with your ulcerative colitis patients.
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study; two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2); a 53-week, phase 3 maintenance trial (OCTAVE Sustain); and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.
Patients who did not achieve remission but showed some clinical response (decrease in Mayo score of at least 3 points) were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5-mg and 10-mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior tumor necrosis factor–blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks, as part of an open-label extension study. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated patients, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection, “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair Jean-Pierre Raufman, MD, and vice chair Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
Visit www.gastro.org/IBD for patient education guides that you can share with your ulcerative colitis patients.
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study; two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2); a 53-week, phase 3 maintenance trial (OCTAVE Sustain); and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.
Patients who did not achieve remission but showed some clinical response (decrease in Mayo score of at least 3 points) were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5-mg and 10-mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior tumor necrosis factor–blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks, as part of an open-label extension study. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated patients, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection, “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair Jean-Pierre Raufman, MD, and vice chair Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
Visit www.gastro.org/IBD for patient education guides that you can share with your ulcerative colitis patients.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Beware the con
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As I stepped from an exam room one recent busy morning, my office manager pulled me aside. “Someone from the county courthouse is on the phone and needs to talk to you,” she whispered.
“You know better than that,” I said. “While I’m seeing patients, I don’t take calls from anyone except colleagues and immediate family.”
I took the call.
“You failed to appear for jury duty,” the official-sounding voice said. “That’s a violation of state law, as you were warned when you received your summons. You’ll have to come down here and surrender yourself immediately, or else we’ll have to send deputies to your office. I don’t think you’ll want to be led through your waiting room in handcuffs.”
“Wait a minute,” I replied nervously. “I haven’t received a jury summons for 2 years, at least. There must be some mistake.”
“Perhaps we’ve confused you with a citizen with the same or a similar name,” he said. “Let me have your Social Security number and birth date.”
Alarm bells! “You should have that information already,” I replied. “Why don’t you read me what you have?”
A short silence, and then … click.
I immediately called the courthouse. “Citizens who fail to appear receive a warning letter and a new questionnaire, not a phone call,” said the jury manager. “And we use driver license numbers to keep track of jurors.”
The phone company traced the call, which dead-ended at a VoIP circuit, to no one’s surprise. The downside of VoIP (Voice over Internet Protocol) and similar technologies is that unscrupulous individuals can use them to appear to be calling you from a legitimate business when they are not.
Those of us of a certain age remember phony office calls offering great deals on supplies or waiting room magazine subscriptions. Those capers eventually disappeared; but scam artists are endlessly creative. This is especially true since the Internet took over, well, everything. There’s a real dark side to the information age.
The jury duty scheme, I learned, is an increasingly popular one. Others involve calls or e-mails from the “fraud department” of your bank, claiming to be investigating a breach of your account, or one of your credit or debit cards. Another purports to be a “Customs official” informing you that you owe a big duty payment on an overseas shipment. Victims of power outages due to natural disasters are hearing from crooks claiming to be from the local power company; the power won’t be restored, they say, without an advance payment.
In most cases, the common denominator – and the biggest red flag – is a request for a social security number, a birth date, a credit card number, or other private information that could be used to steal your identity or empty your accounts.
Here’s a summary of what my recent experience taught (or reminded) me:
- Never give out a bank account, social security, or credit card number online or over the telephone if you didn’t initiate the contact, no matter how legitimate the caller sounds. This is true of anyone claiming to be from a bank, a service company, or a government office, as well as anyone trying to sell you anything.
- No federal or state court will call to say you’ve missed jury duty – or that they are assembling jury pools and need to “prescreen” those who might be selected to serve on them. The jury manager I spoke with said she knew of no reason why anyone in my state would ever be called about jury service before mailing back a completed questionnaire, and even then, such a call would be extraordinary.
- Never send anyone a “commission” or “finder’s fee” as a condition of receiving funds. In legitimate transactions, such fees are merely deducted from the money being paid out.
- Examine your credit card and bank account statements each month. Immediately challenge any charges you don’t recognize.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Jump-starting the day
I’ve never been a fan of delayed school start times for high school students. The data just don’t impress me. But mostly I think delayed start times should be just one component of a broad community-wide initiative to address sleep hygiene that includes discussions about bedtimes, after-school schedules, and overuse of electronic devices. And I don’t see those discussions happening.
In most communities, delaying start times for adolescents will mean that younger children will be starting their school days earlier. Buses and drivers are finite and expensive resources that must be shared. Although I have heard it used as an argument against delayed school starts for high schoolers, an earlier start time for grade-school age children is not one of the downsides I include on my list of negatives. In fact, from my perspective, getting youngsters to school early is one of the few advantages of a delayed school start program for high school.
Underwritten by the Reebok athletic footwear manufacturer, the BOKS (Build Our Kids’ Success) program began in 2009 when a group of mothers in Massachusetts organized a before-school activity program in their local grade school (“A before-school exercise program may help children thrive,” by Gretchen Reynolds, New York Times, Feb. 14, 2018). They may have been motivated primarily by the need to survive those difficult morning hours, but clearly they weren’t alone in their concerns, and the concept has spread to include 3,000 schools worldwide.
Hoping to document the anecdotal observations of the program’s success, researchers from Harvard and the Massachusetts General Hospital surveyed children in 24 schools (“Effects of Before-School Physical Activity on Obesity Prevention and Wellness,” Am J Prev Med. 2018. Feb 12. doi: 10.1016/j.amepre.2018.01.017). Participation in the program was voluntary, and the control group consisted of children whose families chose not to participate. Those children in the before-school activity program 3 mornings per week were more likely to have lower body mass index z scores and “demonstrated improvement in their student engagement scores.” The children who participated only 2 days per week had no significant changes in their body mass index scores. However, they did demonstrate “significant improvements in positive affect and vitality/energy.”
The early-morning energy of youth is a given. The problem is that many children find themselves in home environments in which that energy is squandered or at least misdirected. School can be the environment in which that physical exuberance is allowed to run its natural course. We simply need the will to invest in what needs to be done to make it happen.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
I’ve never been a fan of delayed school start times for high school students. The data just don’t impress me. But mostly I think delayed start times should be just one component of a broad community-wide initiative to address sleep hygiene that includes discussions about bedtimes, after-school schedules, and overuse of electronic devices. And I don’t see those discussions happening.
In most communities, delaying start times for adolescents will mean that younger children will be starting their school days earlier. Buses and drivers are finite and expensive resources that must be shared. Although I have heard it used as an argument against delayed school starts for high schoolers, an earlier start time for grade-school age children is not one of the downsides I include on my list of negatives. In fact, from my perspective, getting youngsters to school early is one of the few advantages of a delayed school start program for high school.
Underwritten by the Reebok athletic footwear manufacturer, the BOKS (Build Our Kids’ Success) program began in 2009 when a group of mothers in Massachusetts organized a before-school activity program in their local grade school (“A before-school exercise program may help children thrive,” by Gretchen Reynolds, New York Times, Feb. 14, 2018). They may have been motivated primarily by the need to survive those difficult morning hours, but clearly they weren’t alone in their concerns, and the concept has spread to include 3,000 schools worldwide.
Hoping to document the anecdotal observations of the program’s success, researchers from Harvard and the Massachusetts General Hospital surveyed children in 24 schools (“Effects of Before-School Physical Activity on Obesity Prevention and Wellness,” Am J Prev Med. 2018. Feb 12. doi: 10.1016/j.amepre.2018.01.017). Participation in the program was voluntary, and the control group consisted of children whose families chose not to participate. Those children in the before-school activity program 3 mornings per week were more likely to have lower body mass index z scores and “demonstrated improvement in their student engagement scores.” The children who participated only 2 days per week had no significant changes in their body mass index scores. However, they did demonstrate “significant improvements in positive affect and vitality/energy.”
The early-morning energy of youth is a given. The problem is that many children find themselves in home environments in which that energy is squandered or at least misdirected. School can be the environment in which that physical exuberance is allowed to run its natural course. We simply need the will to invest in what needs to be done to make it happen.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
I’ve never been a fan of delayed school start times for high school students. The data just don’t impress me. But mostly I think delayed start times should be just one component of a broad community-wide initiative to address sleep hygiene that includes discussions about bedtimes, after-school schedules, and overuse of electronic devices. And I don’t see those discussions happening.
In most communities, delaying start times for adolescents will mean that younger children will be starting their school days earlier. Buses and drivers are finite and expensive resources that must be shared. Although I have heard it used as an argument against delayed school starts for high schoolers, an earlier start time for grade-school age children is not one of the downsides I include on my list of negatives. In fact, from my perspective, getting youngsters to school early is one of the few advantages of a delayed school start program for high school.
Underwritten by the Reebok athletic footwear manufacturer, the BOKS (Build Our Kids’ Success) program began in 2009 when a group of mothers in Massachusetts organized a before-school activity program in their local grade school (“A before-school exercise program may help children thrive,” by Gretchen Reynolds, New York Times, Feb. 14, 2018). They may have been motivated primarily by the need to survive those difficult morning hours, but clearly they weren’t alone in their concerns, and the concept has spread to include 3,000 schools worldwide.
Hoping to document the anecdotal observations of the program’s success, researchers from Harvard and the Massachusetts General Hospital surveyed children in 24 schools (“Effects of Before-School Physical Activity on Obesity Prevention and Wellness,” Am J Prev Med. 2018. Feb 12. doi: 10.1016/j.amepre.2018.01.017). Participation in the program was voluntary, and the control group consisted of children whose families chose not to participate. Those children in the before-school activity program 3 mornings per week were more likely to have lower body mass index z scores and “demonstrated improvement in their student engagement scores.” The children who participated only 2 days per week had no significant changes in their body mass index scores. However, they did demonstrate “significant improvements in positive affect and vitality/energy.”
The early-morning energy of youth is a given. The problem is that many children find themselves in home environments in which that energy is squandered or at least misdirected. School can be the environment in which that physical exuberance is allowed to run its natural course. We simply need the will to invest in what needs to be done to make it happen.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].