User login
2023 Update on minimally invasive gynecologic surgery
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
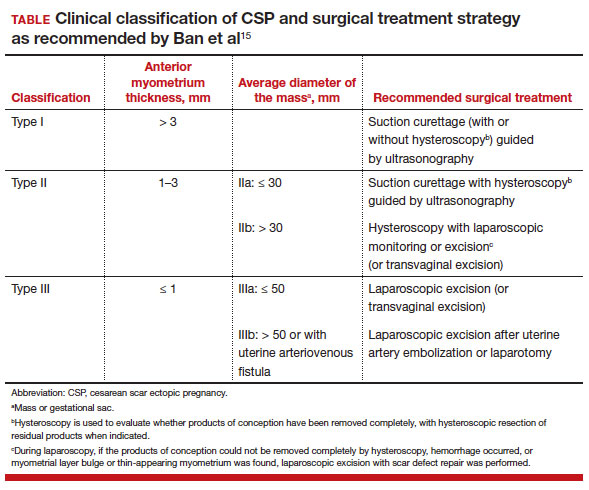
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15
Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
CGRP in migraine prodrome can stop headache, reduce severity
BARCELONA, SPAIN — In the randomized, placebo-controlled crossover PRODROME trial, treatment with ubrogepant (Ubrelvy) 100 mg, one of the new CGRP receptor antagonists, during the prodrome prevented the development of moderate/severe headache at both 24 hours and 48 hours post-dose. The medication also reduced headache of any intensity within 24 hours and functional disability compared with placebo.
“This represents a totally different way of treating a migraine attack – to treat it before the headache starts. This is a paradigm shift in the way we approach the acute treatment of migraine,” study investigator Peter Goadsby, MBBS, MD, PhD, professor of neurology at Kings College London, UK, said in an interview.
The findings were presented at 17th European Headache Congress (EHC) and were also recently published online in The Lancet.
A New Way to Manage Migraine?
The prodrome is usually the earliest phase of a migraine attack and is believed to be experienced by the vast majority of patients with migraine. It consists of various symptoms, including sensitivity to light, fatigue, mood changes, cognitive dysfunction, craving certain foods, and neck pain, which can occur several hours or days before onset.
Dr. Goadsby notes that, at present, there isn’t very much a patient can do about the prodrome.
“We advise patients if they feel an attack is coming not to do anything that might make it worse and make sure they have their acute treatment available for when the headache phase starts. So, we just advise people to prepare for the attack rather than doing anything specific to stop it. But with new data from this study, we now have something that can be done. Patients have an option,” he said.
Dr. Goadsby explained that currently patients are not encouraged to use acute migraine medications such as triptans in the prodrome phase.
“There is actually no evidence that taking a triptan during the prodromal phase works. The advice is to take a triptan as soon as the headache starts, but not before the headache starts.”
He noted that there is also the problem of medication overuse that is seen with triptans, and most other medications used to treat acute migraine, which leads to medication overuse headache, “so we don’t like to encourage patients to increase the frequency of taking triptans for this reason.”
But ubrogepant and other members of the “gepant” class do not seem to have the propensity for medication overuse problems. “Rather, the more a patient takes the less likely they are to get a headache as these drugs also have a preventative effect,” Dr. Goadsby said.
Major Reduction in Severity
The PRODROME trial was conducted at 75 sites in the United States in 518 patients who had at least a 1-year history of migraine with or without aura and a history of two to eight migraine attacks per month with moderate to severe headache in each of the 3 months before study entry.
Participants underwent a rigorous screening period during which they were required to show that they could identify prodromal symptoms that were reliably followed by migraine headache within 1-6 hours.
They were randomly assigned to receive either placebo to treat the first qualifying prodrome event and ubrogepant 100 mg to treat the second qualifying prodrome event or vice versa, with instructions to take the study drug at the onset of the prodrome event.
Efficacy assessments during the double-blind treatment period were recorded by the participant in an electronic diary. On identifying a qualifying prodrome, the patient recorded prodromal symptoms, and was then required to report the absence or presence of a headache at regular intervals up to 48 hours after the study drug dose. If a headache was reported, participants rated the intensity as mild, moderate, or severe and reported whether rescue medication was taken to treat it.
The primary endpoint was absence of moderate or severe intensity headache within 24 hours after study-drug dose. This occurred after 46% of 418 qualifying prodrome events that had been treated with ubrogepant and after 29% of 423 qualifying prodrome events that had been treated with placebo (odds ratio, 2.09; 95% CI, 1.63 - 2.69; P < .0001).
“The incidence of moderate to severe headache was almost halved when ubrogepant was taken in the prodrome,” Dr. Goadsby reported.
Ubrogepant also showed similar impressive results for the secondary endpoints in the absence of moderate to severe headache within 48 hours post-dose and the absence of any headache of any intensity at 24 hours.
Little to No Disability
The researchers also evaluated functional ability, and more participants reported “no disability or able to function normally” during the 24 hours after treatment with ubrogepant than after placebo (OR, 1.66; P < .0001).
Other findings showed that the prodromal symptoms themselves, such as light sensitivity and cognitive dysfunction, were also reduced with ubrogepant.
Dr. Goadsby said he was pleased but not surprised by the results, as the “gepant” class of drugs are used in both the acute treatment of migraine and as preventive agents, although different agents have been approved for different indications in this regard.
“The ‘gepants’ are a class of medication that can be used in almost any way in migraine — to treat an acute migraine headache, to prevent migraine if taken chronically, and now we see that they can also stop a migraine from developing if taken during the initial prodromal phase. That’s unique for a migraine medication,” he said.
While the current study was conducted with ubrogepant, Dr. Goadsby suspects that any of the “gepants” would probably have a similar effect.
He noted that the prodromal phase of migraine has only just started to be explored, with functional imaging studies showing that structural brain changes occur during this phase.
Dr. Goadsby said the current study opens up a whole new area of interest, emphasizing the clinical value of identifying the prodrome in individuals with migraine, better characterizing the symptomology of the prodrome and understanding more about how to treat it.
“It’s the ultimate way of treating migraine early, and by taking this type of medication in the prodromal phase, patients may be able to stop having pain. That’s quite an implication,” he concluded.
The PRODROME study was funded by AbbVie. Dr. Goadsby reports personal fees from AbbVie.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — In the randomized, placebo-controlled crossover PRODROME trial, treatment with ubrogepant (Ubrelvy) 100 mg, one of the new CGRP receptor antagonists, during the prodrome prevented the development of moderate/severe headache at both 24 hours and 48 hours post-dose. The medication also reduced headache of any intensity within 24 hours and functional disability compared with placebo.
“This represents a totally different way of treating a migraine attack – to treat it before the headache starts. This is a paradigm shift in the way we approach the acute treatment of migraine,” study investigator Peter Goadsby, MBBS, MD, PhD, professor of neurology at Kings College London, UK, said in an interview.
The findings were presented at 17th European Headache Congress (EHC) and were also recently published online in The Lancet.
A New Way to Manage Migraine?
The prodrome is usually the earliest phase of a migraine attack and is believed to be experienced by the vast majority of patients with migraine. It consists of various symptoms, including sensitivity to light, fatigue, mood changes, cognitive dysfunction, craving certain foods, and neck pain, which can occur several hours or days before onset.
Dr. Goadsby notes that, at present, there isn’t very much a patient can do about the prodrome.
“We advise patients if they feel an attack is coming not to do anything that might make it worse and make sure they have their acute treatment available for when the headache phase starts. So, we just advise people to prepare for the attack rather than doing anything specific to stop it. But with new data from this study, we now have something that can be done. Patients have an option,” he said.
Dr. Goadsby explained that currently patients are not encouraged to use acute migraine medications such as triptans in the prodrome phase.
“There is actually no evidence that taking a triptan during the prodromal phase works. The advice is to take a triptan as soon as the headache starts, but not before the headache starts.”
He noted that there is also the problem of medication overuse that is seen with triptans, and most other medications used to treat acute migraine, which leads to medication overuse headache, “so we don’t like to encourage patients to increase the frequency of taking triptans for this reason.”
But ubrogepant and other members of the “gepant” class do not seem to have the propensity for medication overuse problems. “Rather, the more a patient takes the less likely they are to get a headache as these drugs also have a preventative effect,” Dr. Goadsby said.
Major Reduction in Severity
The PRODROME trial was conducted at 75 sites in the United States in 518 patients who had at least a 1-year history of migraine with or without aura and a history of two to eight migraine attacks per month with moderate to severe headache in each of the 3 months before study entry.
Participants underwent a rigorous screening period during which they were required to show that they could identify prodromal symptoms that were reliably followed by migraine headache within 1-6 hours.
They were randomly assigned to receive either placebo to treat the first qualifying prodrome event and ubrogepant 100 mg to treat the second qualifying prodrome event or vice versa, with instructions to take the study drug at the onset of the prodrome event.
Efficacy assessments during the double-blind treatment period were recorded by the participant in an electronic diary. On identifying a qualifying prodrome, the patient recorded prodromal symptoms, and was then required to report the absence or presence of a headache at regular intervals up to 48 hours after the study drug dose. If a headache was reported, participants rated the intensity as mild, moderate, or severe and reported whether rescue medication was taken to treat it.
The primary endpoint was absence of moderate or severe intensity headache within 24 hours after study-drug dose. This occurred after 46% of 418 qualifying prodrome events that had been treated with ubrogepant and after 29% of 423 qualifying prodrome events that had been treated with placebo (odds ratio, 2.09; 95% CI, 1.63 - 2.69; P < .0001).
“The incidence of moderate to severe headache was almost halved when ubrogepant was taken in the prodrome,” Dr. Goadsby reported.
Ubrogepant also showed similar impressive results for the secondary endpoints in the absence of moderate to severe headache within 48 hours post-dose and the absence of any headache of any intensity at 24 hours.
Little to No Disability
The researchers also evaluated functional ability, and more participants reported “no disability or able to function normally” during the 24 hours after treatment with ubrogepant than after placebo (OR, 1.66; P < .0001).
Other findings showed that the prodromal symptoms themselves, such as light sensitivity and cognitive dysfunction, were also reduced with ubrogepant.
Dr. Goadsby said he was pleased but not surprised by the results, as the “gepant” class of drugs are used in both the acute treatment of migraine and as preventive agents, although different agents have been approved for different indications in this regard.
“The ‘gepants’ are a class of medication that can be used in almost any way in migraine — to treat an acute migraine headache, to prevent migraine if taken chronically, and now we see that they can also stop a migraine from developing if taken during the initial prodromal phase. That’s unique for a migraine medication,” he said.
While the current study was conducted with ubrogepant, Dr. Goadsby suspects that any of the “gepants” would probably have a similar effect.
He noted that the prodromal phase of migraine has only just started to be explored, with functional imaging studies showing that structural brain changes occur during this phase.
Dr. Goadsby said the current study opens up a whole new area of interest, emphasizing the clinical value of identifying the prodrome in individuals with migraine, better characterizing the symptomology of the prodrome and understanding more about how to treat it.
“It’s the ultimate way of treating migraine early, and by taking this type of medication in the prodromal phase, patients may be able to stop having pain. That’s quite an implication,” he concluded.
The PRODROME study was funded by AbbVie. Dr. Goadsby reports personal fees from AbbVie.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — In the randomized, placebo-controlled crossover PRODROME trial, treatment with ubrogepant (Ubrelvy) 100 mg, one of the new CGRP receptor antagonists, during the prodrome prevented the development of moderate/severe headache at both 24 hours and 48 hours post-dose. The medication also reduced headache of any intensity within 24 hours and functional disability compared with placebo.
“This represents a totally different way of treating a migraine attack – to treat it before the headache starts. This is a paradigm shift in the way we approach the acute treatment of migraine,” study investigator Peter Goadsby, MBBS, MD, PhD, professor of neurology at Kings College London, UK, said in an interview.
The findings were presented at 17th European Headache Congress (EHC) and were also recently published online in The Lancet.
A New Way to Manage Migraine?
The prodrome is usually the earliest phase of a migraine attack and is believed to be experienced by the vast majority of patients with migraine. It consists of various symptoms, including sensitivity to light, fatigue, mood changes, cognitive dysfunction, craving certain foods, and neck pain, which can occur several hours or days before onset.
Dr. Goadsby notes that, at present, there isn’t very much a patient can do about the prodrome.
“We advise patients if they feel an attack is coming not to do anything that might make it worse and make sure they have their acute treatment available for when the headache phase starts. So, we just advise people to prepare for the attack rather than doing anything specific to stop it. But with new data from this study, we now have something that can be done. Patients have an option,” he said.
Dr. Goadsby explained that currently patients are not encouraged to use acute migraine medications such as triptans in the prodrome phase.
“There is actually no evidence that taking a triptan during the prodromal phase works. The advice is to take a triptan as soon as the headache starts, but not before the headache starts.”
He noted that there is also the problem of medication overuse that is seen with triptans, and most other medications used to treat acute migraine, which leads to medication overuse headache, “so we don’t like to encourage patients to increase the frequency of taking triptans for this reason.”
But ubrogepant and other members of the “gepant” class do not seem to have the propensity for medication overuse problems. “Rather, the more a patient takes the less likely they are to get a headache as these drugs also have a preventative effect,” Dr. Goadsby said.
Major Reduction in Severity
The PRODROME trial was conducted at 75 sites in the United States in 518 patients who had at least a 1-year history of migraine with or without aura and a history of two to eight migraine attacks per month with moderate to severe headache in each of the 3 months before study entry.
Participants underwent a rigorous screening period during which they were required to show that they could identify prodromal symptoms that were reliably followed by migraine headache within 1-6 hours.
They were randomly assigned to receive either placebo to treat the first qualifying prodrome event and ubrogepant 100 mg to treat the second qualifying prodrome event or vice versa, with instructions to take the study drug at the onset of the prodrome event.
Efficacy assessments during the double-blind treatment period were recorded by the participant in an electronic diary. On identifying a qualifying prodrome, the patient recorded prodromal symptoms, and was then required to report the absence or presence of a headache at regular intervals up to 48 hours after the study drug dose. If a headache was reported, participants rated the intensity as mild, moderate, or severe and reported whether rescue medication was taken to treat it.
The primary endpoint was absence of moderate or severe intensity headache within 24 hours after study-drug dose. This occurred after 46% of 418 qualifying prodrome events that had been treated with ubrogepant and after 29% of 423 qualifying prodrome events that had been treated with placebo (odds ratio, 2.09; 95% CI, 1.63 - 2.69; P < .0001).
“The incidence of moderate to severe headache was almost halved when ubrogepant was taken in the prodrome,” Dr. Goadsby reported.
Ubrogepant also showed similar impressive results for the secondary endpoints in the absence of moderate to severe headache within 48 hours post-dose and the absence of any headache of any intensity at 24 hours.
Little to No Disability
The researchers also evaluated functional ability, and more participants reported “no disability or able to function normally” during the 24 hours after treatment with ubrogepant than after placebo (OR, 1.66; P < .0001).
Other findings showed that the prodromal symptoms themselves, such as light sensitivity and cognitive dysfunction, were also reduced with ubrogepant.
Dr. Goadsby said he was pleased but not surprised by the results, as the “gepant” class of drugs are used in both the acute treatment of migraine and as preventive agents, although different agents have been approved for different indications in this regard.
“The ‘gepants’ are a class of medication that can be used in almost any way in migraine — to treat an acute migraine headache, to prevent migraine if taken chronically, and now we see that they can also stop a migraine from developing if taken during the initial prodromal phase. That’s unique for a migraine medication,” he said.
While the current study was conducted with ubrogepant, Dr. Goadsby suspects that any of the “gepants” would probably have a similar effect.
He noted that the prodromal phase of migraine has only just started to be explored, with functional imaging studies showing that structural brain changes occur during this phase.
Dr. Goadsby said the current study opens up a whole new area of interest, emphasizing the clinical value of identifying the prodrome in individuals with migraine, better characterizing the symptomology of the prodrome and understanding more about how to treat it.
“It’s the ultimate way of treating migraine early, and by taking this type of medication in the prodromal phase, patients may be able to stop having pain. That’s quite an implication,” he concluded.
The PRODROME study was funded by AbbVie. Dr. Goadsby reports personal fees from AbbVie.
A version of this article appeared on Medscape.com.
FROM EHC 2023
Why Are Prion Diseases on the Rise?
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 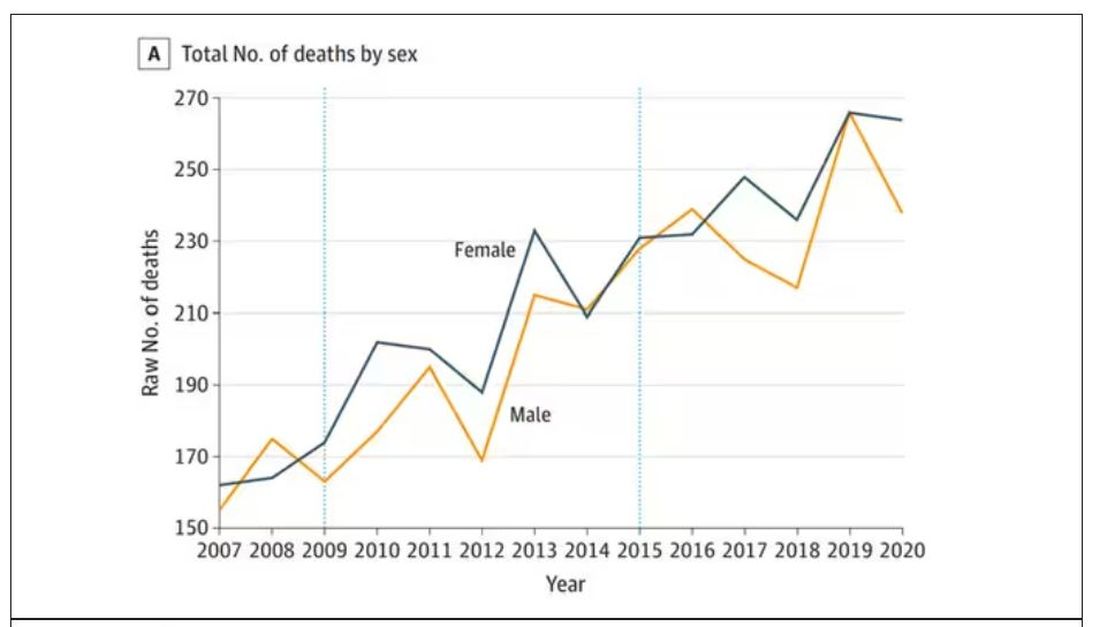
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.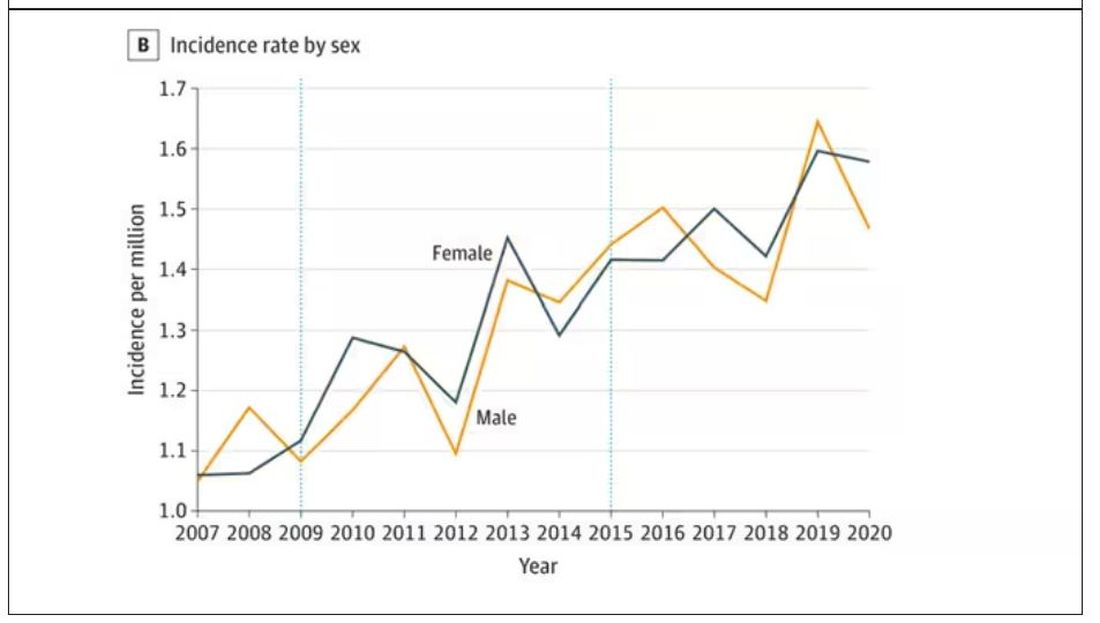
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Is migraine really a female disorder?
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
FROM EHC 2023
Distinct toxicity profiles for anti-BCMA myeloma therapies
Among 1803 patients with multiple myeloma treated with either chimeric antigen receptor (CAR) T-cell constructs or a bispecific antibody, CAR T-cell therapy was associated with a “prominent” risk for both cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, while the antibody was associated with a high risk for infection-related mortality, reported Zimu Gong, MD, PhD, from the Cancer Center at Houston Methodist Hospital.“When we are selecting or sequencing these agents, because they are approved for almost identical indications, we need to carefully consider their unique toxicity profile,” he said in an oral abstract session at the annual meeting of the American Society of Hematology (ASH) here.
Going to the FAERS
Dr. Gong and colleagues drew on the FDA Adverse Event Reporting System (FAERS) database for data on toxicities associated with three BCMA-directed therapies: CAR T-cell treatments idecabtagene vicleucel (ide-cel; Abecma) and ciltacabtagene autoleucel (cilta-cel; Carvykti), and the bispecific antibody teclistamab (Tecvayli).
They identified a total of 1803 cases with a total of 4423 reported adverse events.
The authors calculated a reporting odds ratio (ROR) by dividing the odds of a specific event occurring with an agent by the odds of the same event occurring with all other BCMA-directed agents in the FAERS database.
They found that the highest ROR for cytokine release syndrome was with ide-cel, at 1.8, compared with 0.74 with cilta-cel, and 0.63 with teclistamab. Ide-cel was also most strongly associated with risk for both immune effector cell-associated neurotoxicity syndrome, with an ROR of 1.38, compared with 1.04 with cilta-cel and 0.69 with teclistamab, and with non-immune effector cell-associated neurotoxicity, with an ROR of 2.19 vs 0.83 and 0.4, respectively.
There were 14 reported cases of Bell’s palsy, 13 of which were associated with cilta-cel and 1 with teclistamab, and 11 cases of Parkinsonism, including 7 occurring with cilta-cel, 4 with ide-cel, and none with teclistamab.
In contrast, risk for infection was highest with teclistamab, with an ROR of 4.38 compared with 1.3 with cilta-cel and 0.12 with ide-cel. The infections most commonly reported with teclistamab included pneumonia, sepsis, COVID-19 pneumonia, pneumocystis jirovecii pneumonia, cytomegalovirus reactive and cytomegalovirus pneumonia.
The antibody was also associated with the highest risk for nonrelapse mortality, with an ROR of 1.73 compared with 1.28 with cilta-cel and 0.13 with ide-cel.
There were 309 reported deaths. The investigators calculated nonrelapse mortality by excluding disease progress from cases with death as the final reported outcome. Ide-cell had the lowest odds ratio for non-relapse mortality, at 0.53, compared with 0.99 for cilta-cel, and 1.72 for teclistamab. The most common cause of nonrelapse deaths was toxicities associated with CAR T-cell therapy, and infections, Dr. Gong said.
Dr. Gong acknowledged that one of the major limitations of the study is the nature of the FAERS database itself, which includes both mandatory reports on adverse events, medication errors, and product quality complaints submitted as required by law by manufacturers, but also voluntarily reported by healthcare professionals and consumers.
In an interview with this news organization, David Miklos, MD, PhD, chief of the blood and marrow transplantation and cellular therapy division at Stanford University, who attended the session but was not involved in the study, commented that although the study showed differences among various anti-BCMA products in terms of adverse events, the analysis is only one of several that show different values.
“The concern I have about the FAERS database is simply the lack of validation, and maybe, with no disrespect to the institution, this is kind of like review scores on Amazon.com: not validated, nobody knows who put them out there, and we don’t even know if it’s true,” he said.
He noted that whatever the system, data collection and reporting is both time- and resource-consuming, and given the potential of cellular therapies to significantly improve survival may burden clinicians with requirements for decades of follow-up and reporting.
“Self-reporting isn’t the answer either,” said Dr. Miklos. Perhaps, he suggested, there is a role for apps with “patients self-reporting” and medical practitioners validating the reports.
Dr. Gong and colleagues did not report a study funding source. Dr. Gong had no conflict of interest disclosures. Dr. Miklos has disclosed serving as a director, officer, partner, employee, advisor, consultant, or trustee for: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen; received research funding from: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclic; patents, royalties, or other intellectual property from Pharmacyclics, and travel support from Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen.
A version of this article first appeared on Medscape.com.
Among 1803 patients with multiple myeloma treated with either chimeric antigen receptor (CAR) T-cell constructs or a bispecific antibody, CAR T-cell therapy was associated with a “prominent” risk for both cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, while the antibody was associated with a high risk for infection-related mortality, reported Zimu Gong, MD, PhD, from the Cancer Center at Houston Methodist Hospital.“When we are selecting or sequencing these agents, because they are approved for almost identical indications, we need to carefully consider their unique toxicity profile,” he said in an oral abstract session at the annual meeting of the American Society of Hematology (ASH) here.
Going to the FAERS
Dr. Gong and colleagues drew on the FDA Adverse Event Reporting System (FAERS) database for data on toxicities associated with three BCMA-directed therapies: CAR T-cell treatments idecabtagene vicleucel (ide-cel; Abecma) and ciltacabtagene autoleucel (cilta-cel; Carvykti), and the bispecific antibody teclistamab (Tecvayli).
They identified a total of 1803 cases with a total of 4423 reported adverse events.
The authors calculated a reporting odds ratio (ROR) by dividing the odds of a specific event occurring with an agent by the odds of the same event occurring with all other BCMA-directed agents in the FAERS database.
They found that the highest ROR for cytokine release syndrome was with ide-cel, at 1.8, compared with 0.74 with cilta-cel, and 0.63 with teclistamab. Ide-cel was also most strongly associated with risk for both immune effector cell-associated neurotoxicity syndrome, with an ROR of 1.38, compared with 1.04 with cilta-cel and 0.69 with teclistamab, and with non-immune effector cell-associated neurotoxicity, with an ROR of 2.19 vs 0.83 and 0.4, respectively.
There were 14 reported cases of Bell’s palsy, 13 of which were associated with cilta-cel and 1 with teclistamab, and 11 cases of Parkinsonism, including 7 occurring with cilta-cel, 4 with ide-cel, and none with teclistamab.
In contrast, risk for infection was highest with teclistamab, with an ROR of 4.38 compared with 1.3 with cilta-cel and 0.12 with ide-cel. The infections most commonly reported with teclistamab included pneumonia, sepsis, COVID-19 pneumonia, pneumocystis jirovecii pneumonia, cytomegalovirus reactive and cytomegalovirus pneumonia.
The antibody was also associated with the highest risk for nonrelapse mortality, with an ROR of 1.73 compared with 1.28 with cilta-cel and 0.13 with ide-cel.
There were 309 reported deaths. The investigators calculated nonrelapse mortality by excluding disease progress from cases with death as the final reported outcome. Ide-cell had the lowest odds ratio for non-relapse mortality, at 0.53, compared with 0.99 for cilta-cel, and 1.72 for teclistamab. The most common cause of nonrelapse deaths was toxicities associated with CAR T-cell therapy, and infections, Dr. Gong said.
Dr. Gong acknowledged that one of the major limitations of the study is the nature of the FAERS database itself, which includes both mandatory reports on adverse events, medication errors, and product quality complaints submitted as required by law by manufacturers, but also voluntarily reported by healthcare professionals and consumers.
In an interview with this news organization, David Miklos, MD, PhD, chief of the blood and marrow transplantation and cellular therapy division at Stanford University, who attended the session but was not involved in the study, commented that although the study showed differences among various anti-BCMA products in terms of adverse events, the analysis is only one of several that show different values.
“The concern I have about the FAERS database is simply the lack of validation, and maybe, with no disrespect to the institution, this is kind of like review scores on Amazon.com: not validated, nobody knows who put them out there, and we don’t even know if it’s true,” he said.
He noted that whatever the system, data collection and reporting is both time- and resource-consuming, and given the potential of cellular therapies to significantly improve survival may burden clinicians with requirements for decades of follow-up and reporting.
“Self-reporting isn’t the answer either,” said Dr. Miklos. Perhaps, he suggested, there is a role for apps with “patients self-reporting” and medical practitioners validating the reports.
Dr. Gong and colleagues did not report a study funding source. Dr. Gong had no conflict of interest disclosures. Dr. Miklos has disclosed serving as a director, officer, partner, employee, advisor, consultant, or trustee for: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen; received research funding from: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclic; patents, royalties, or other intellectual property from Pharmacyclics, and travel support from Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen.
A version of this article first appeared on Medscape.com.
Among 1803 patients with multiple myeloma treated with either chimeric antigen receptor (CAR) T-cell constructs or a bispecific antibody, CAR T-cell therapy was associated with a “prominent” risk for both cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, while the antibody was associated with a high risk for infection-related mortality, reported Zimu Gong, MD, PhD, from the Cancer Center at Houston Methodist Hospital.“When we are selecting or sequencing these agents, because they are approved for almost identical indications, we need to carefully consider their unique toxicity profile,” he said in an oral abstract session at the annual meeting of the American Society of Hematology (ASH) here.
Going to the FAERS
Dr. Gong and colleagues drew on the FDA Adverse Event Reporting System (FAERS) database for data on toxicities associated with three BCMA-directed therapies: CAR T-cell treatments idecabtagene vicleucel (ide-cel; Abecma) and ciltacabtagene autoleucel (cilta-cel; Carvykti), and the bispecific antibody teclistamab (Tecvayli).
They identified a total of 1803 cases with a total of 4423 reported adverse events.
The authors calculated a reporting odds ratio (ROR) by dividing the odds of a specific event occurring with an agent by the odds of the same event occurring with all other BCMA-directed agents in the FAERS database.
They found that the highest ROR for cytokine release syndrome was with ide-cel, at 1.8, compared with 0.74 with cilta-cel, and 0.63 with teclistamab. Ide-cel was also most strongly associated with risk for both immune effector cell-associated neurotoxicity syndrome, with an ROR of 1.38, compared with 1.04 with cilta-cel and 0.69 with teclistamab, and with non-immune effector cell-associated neurotoxicity, with an ROR of 2.19 vs 0.83 and 0.4, respectively.
There were 14 reported cases of Bell’s palsy, 13 of which were associated with cilta-cel and 1 with teclistamab, and 11 cases of Parkinsonism, including 7 occurring with cilta-cel, 4 with ide-cel, and none with teclistamab.
In contrast, risk for infection was highest with teclistamab, with an ROR of 4.38 compared with 1.3 with cilta-cel and 0.12 with ide-cel. The infections most commonly reported with teclistamab included pneumonia, sepsis, COVID-19 pneumonia, pneumocystis jirovecii pneumonia, cytomegalovirus reactive and cytomegalovirus pneumonia.
The antibody was also associated with the highest risk for nonrelapse mortality, with an ROR of 1.73 compared with 1.28 with cilta-cel and 0.13 with ide-cel.
There were 309 reported deaths. The investigators calculated nonrelapse mortality by excluding disease progress from cases with death as the final reported outcome. Ide-cell had the lowest odds ratio for non-relapse mortality, at 0.53, compared with 0.99 for cilta-cel, and 1.72 for teclistamab. The most common cause of nonrelapse deaths was toxicities associated with CAR T-cell therapy, and infections, Dr. Gong said.
Dr. Gong acknowledged that one of the major limitations of the study is the nature of the FAERS database itself, which includes both mandatory reports on adverse events, medication errors, and product quality complaints submitted as required by law by manufacturers, but also voluntarily reported by healthcare professionals and consumers.
In an interview with this news organization, David Miklos, MD, PhD, chief of the blood and marrow transplantation and cellular therapy division at Stanford University, who attended the session but was not involved in the study, commented that although the study showed differences among various anti-BCMA products in terms of adverse events, the analysis is only one of several that show different values.
“The concern I have about the FAERS database is simply the lack of validation, and maybe, with no disrespect to the institution, this is kind of like review scores on Amazon.com: not validated, nobody knows who put them out there, and we don’t even know if it’s true,” he said.
He noted that whatever the system, data collection and reporting is both time- and resource-consuming, and given the potential of cellular therapies to significantly improve survival may burden clinicians with requirements for decades of follow-up and reporting.
“Self-reporting isn’t the answer either,” said Dr. Miklos. Perhaps, he suggested, there is a role for apps with “patients self-reporting” and medical practitioners validating the reports.
Dr. Gong and colleagues did not report a study funding source. Dr. Gong had no conflict of interest disclosures. Dr. Miklos has disclosed serving as a director, officer, partner, employee, advisor, consultant, or trustee for: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen; received research funding from: Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclic; patents, royalties, or other intellectual property from Pharmacyclics, and travel support from Kite-Gilead, Novartis, Juno-Celgene-Bristol-Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen.
A version of this article first appeared on Medscape.com.
FROM ASH 2023
ADA issues new screening, obesity management recommendations
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
Acne stigma persists across social and professional settings
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
FROM JAMA DERMATOLOGY
Breastfeeding by patients with serious mental illness: An ethical approach
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21
Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.
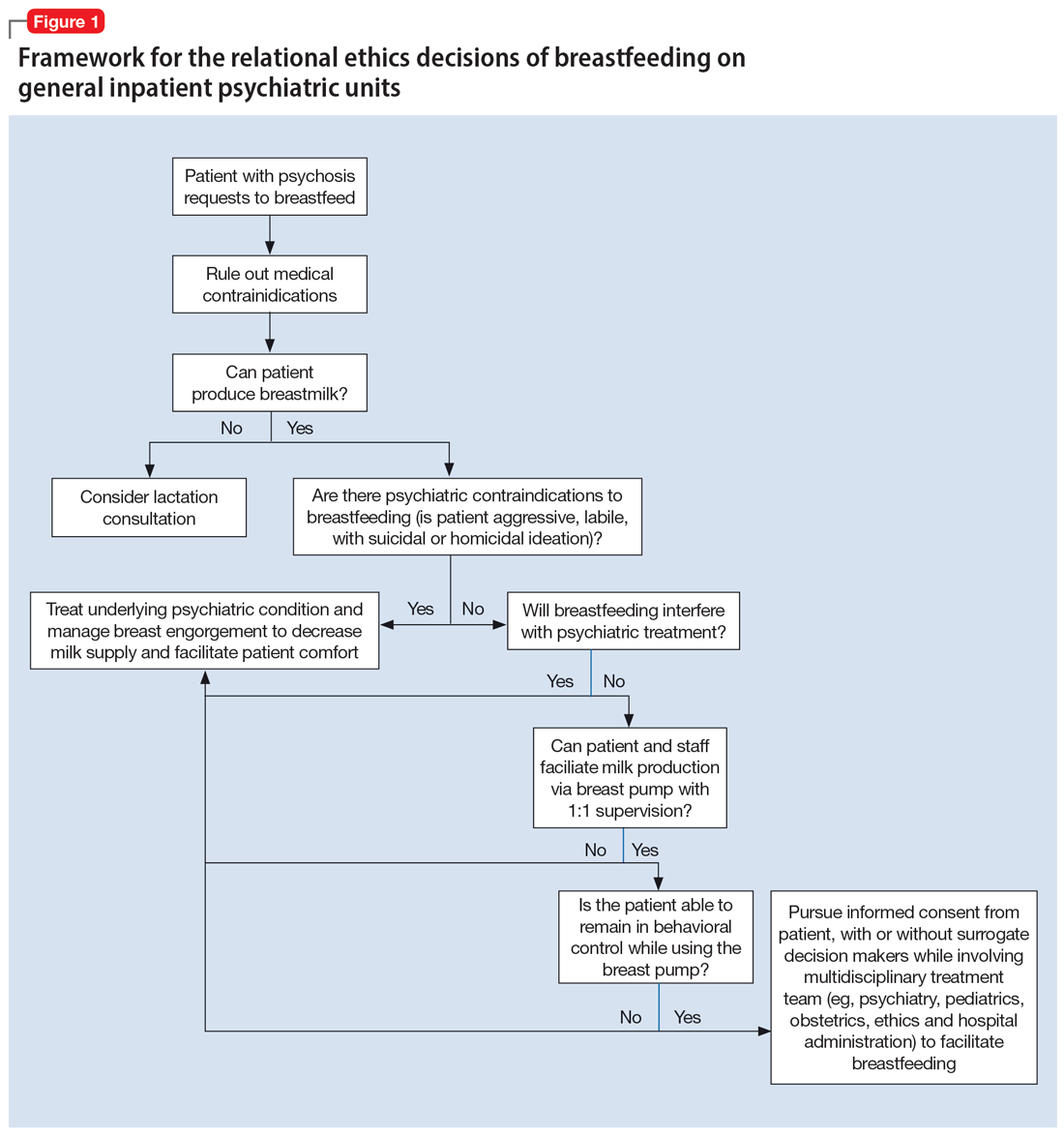
In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.
Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Opioid use disorder in pregnancy: A strategy for using methadone
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.
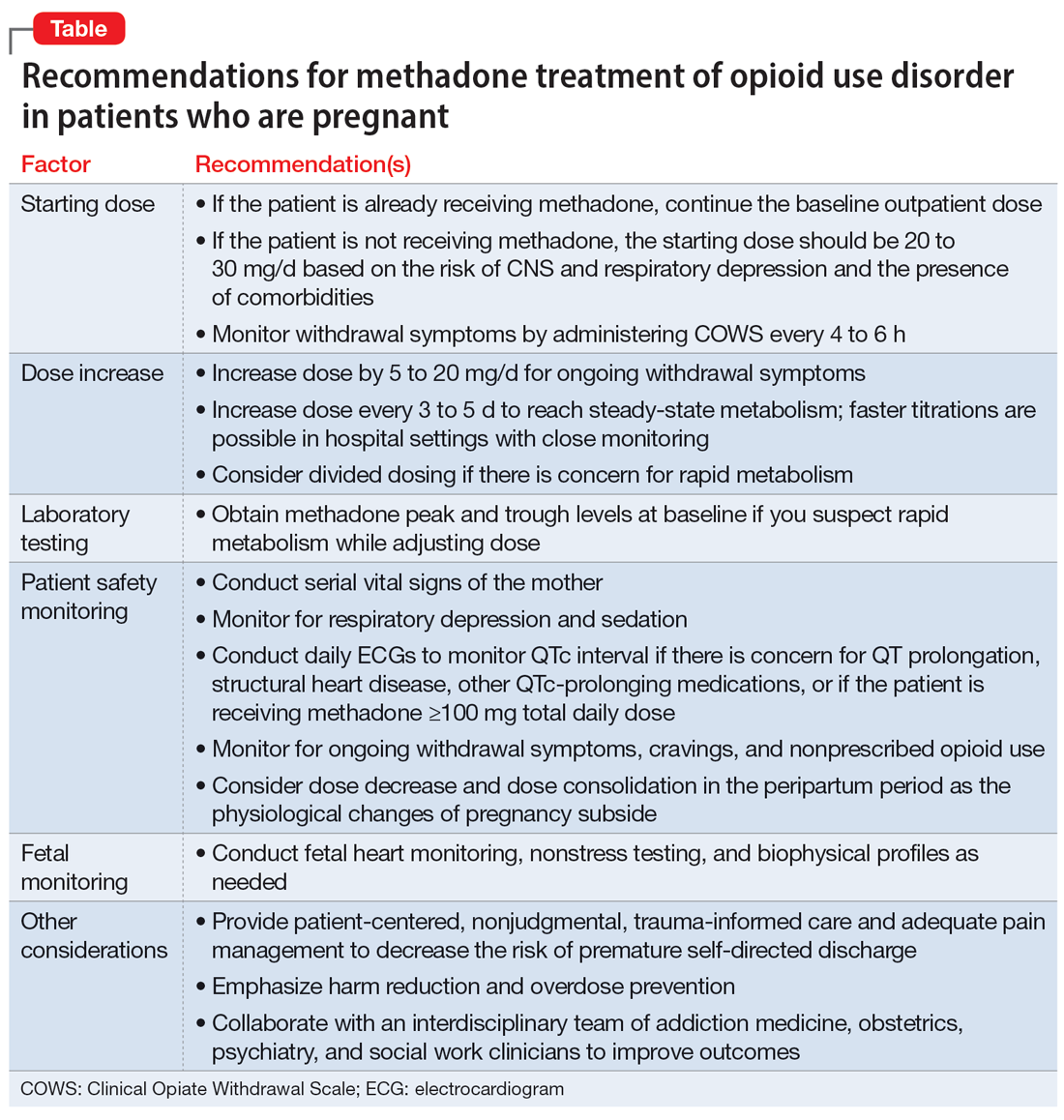
Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
Is there a new role for metformin in the management of gestational diabetes?
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660

