User login
Fivefold Increase in Vaping During Adolescent Pregnancies
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
AI-Aided Stethoscope Beats PCP in Detecting Valvular HD
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Report: CKD Severity Linked to Thinning of Retina, Choroid Layers
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Slow-to-moderate weight loss better than rapid with antiobesity drugs in OA
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Progressive joint pain and swelling
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
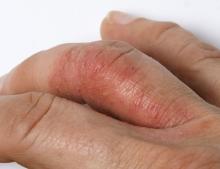
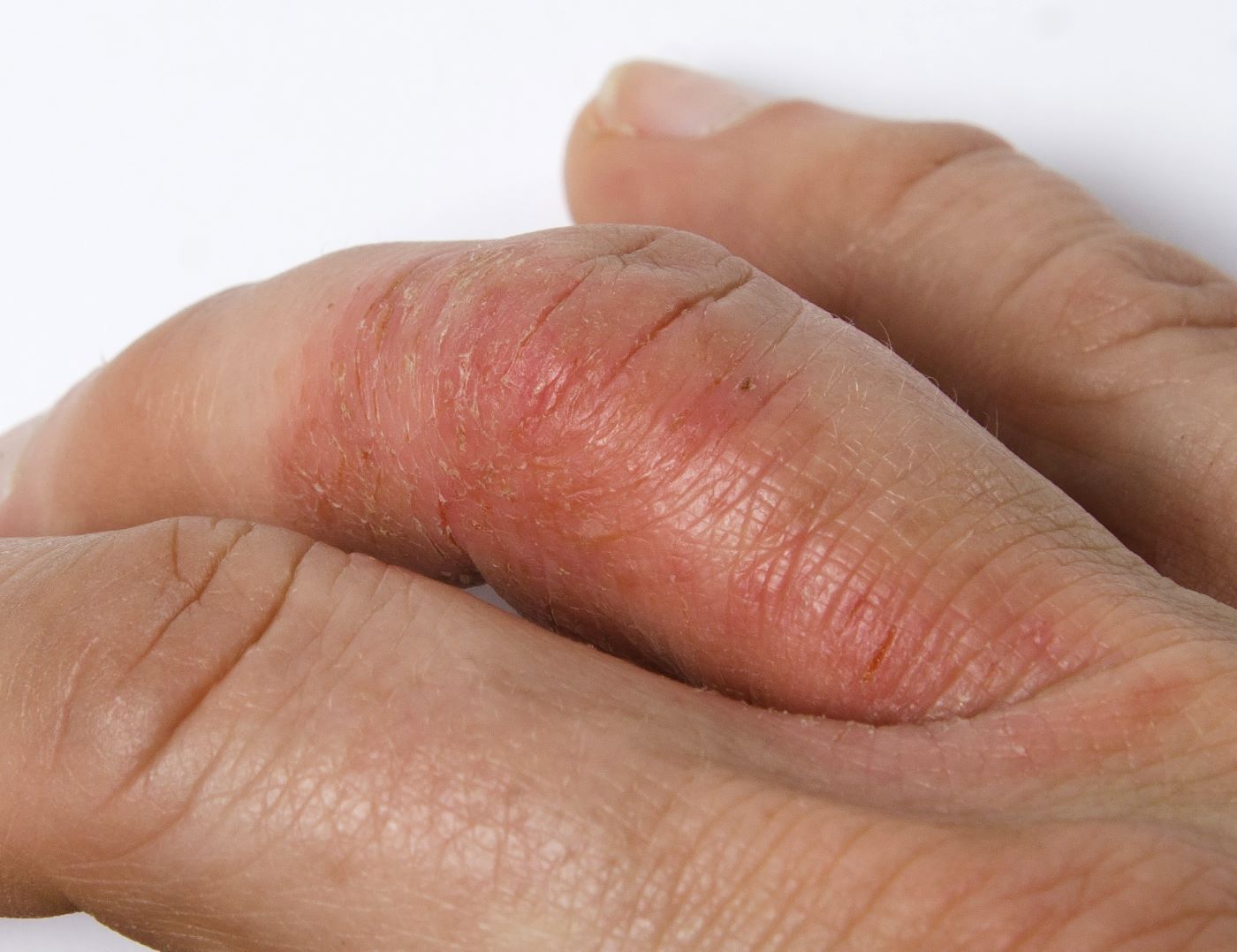
A 45-year-old man visited the rheumatology clinic with a 6-month history of progressive joint pain and swelling. He described experiencing morning stiffness that lasted about an hour, with the pain showing improvement with activity. Interestingly, he also mentioned having rashes for the past 10 years, which he initially attributed to eczema and managed with over-the-counter creams.
On physical examination, there was noticeable swelling and tenderness in the distal interphalangeal (DIP) joints of both hands. The fourth finger on the right hand exhibited dactylitis with a well-circumscribed, erythematous, scaly lesion (see image). Physical exam suggested enthesitis at the insertion of the Achilles tendon. Skin examination revealed plaques with a characteristic silver scaling on the elbows and knees. Laboratory tests indicated elevated C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR). Notably, both the rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) tests returned negative results. Radiography of the hands showed periarticular erosions and a "pencil-in-cup" deformity at the DIP joints.
Teen and young adult rheumatology patients report gaps in sexual health counseling
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2023
No Benefit to Salvage Transplant in R/R Multiple Myeloma
Patients receiving a second, salvage-autologous stem cell transplant alongside lenalidomide-dexamethasone maintenance therapy did not demonstrate improved progression-free survival (PFS) or overall survival compared with patients who continued the two-drug regimen without salvage transplant, according to research presented at the American Society of Hematology annual meeting.
The primary phase 3 analysis, published in 2021, showed no survival benefit following salvage transplant at the time of relapse, though it only followed patients for a median of 37 months.
However, because a significant fraction of patients in the transplant arm — about 29% — did not undergo the planned salvage transplant before dropping out of the study, the researchers performed further analyses that “suggested a survival benefit in patients who actually received the transplant,” first author Marc-Andrea Baertsch, MD, of the German Cancer Research Center and University Hospital Heidelberg, reported at ASH.
Now, the latest analysis, which followed patients for a median of 99 months (8.25 years), confirmed the initial 2021 findings, Dr. Baertsch explained.
“The writing on the wall is clear: Don’t repeat a transplant at the time of relapse for those who have already gotten a transplant,” said Manni Mohyuddin, MD, of the University of Utah in Salt Lake City, who was not involved in the research. Dr. Mohyuddin added, however, that this finding doesn’t apply to those who haven’t yet gotten a transplant. “Data from other trials suggests a role of transplant in this situation, depending on the unique circumstances.”
The current trial included 282 adult patients, aged 75 years or younger, with relapsed or refractory multiple myeloma. Between 2010 and 2016, patients in the intention-to-treat analysis (n = 277) were randomized to lenalidomide-dexamethasone reinduction and maintenance, along with salvage high-dose chemotherapy with melphalan and autologous stem cell transplantation (n = 139) or just continuous lenalidomide-dexamethasone until progression (n = 138).
Patients in both arms received three cycles of lenalidomide-dexamethasone up front: 25 mg of lenalidomide on days 1 through 21, and 40 mg of dexamethasone on days 1, 8, 15, and 22 in 4-week cycles. Those in the salvage transplant arm then received high-dose chemotherapy with 200 mg/m2 of melphalan followed by transplant and 10 mg of lenalidomide maintenance therapy daily, while those in the control arm continued with receiving lenalidomide-dexamethasone.
All patients had received one to three prior lines of therapy, had good performance status, and had a time-to-disease-progression of at least 12 months after frontline autologous stem cell transplant.
In the primary 2021 study, patients in the salvage transplant group did not demonstrate a survival benefit (hazard ratio [HR] for PFS, 0.87; HR for overall survival, 0.81).
In the latest analysis, no survival benefit emerged after following patients for a median of about 8 years. Patients in the salvage transplant arm had a median PFS of 20.5 months vs 19.3 months in the continuous therapy arms (HR, 0.98; 95% CI, 0.76-1.27; P = .9). Median overall survival was 67.1 months in the salvage transplant arm and 62.7 months in the continuous treatment arm (HR, 0.89; 95% CI, 0.66 - 1.20; P = .44).
Time to first progression after frontline transplant was associated with a PFS benefit but did not predict an overall survival benefit, Dr. Baertsch noted.
When evaluating outcomes from the time of salvage transplant to account for the high number of dropouts, the PFS and overall survival findings held. Patients who received salvage transplant did not experience significantly improved PFS (HR, 0.91) or overall survival (76.3 months in the salvage group vs 65.9 months in the continuous treatment arm; HR, 0.80).
The lack of PFS and overall survival benefit occurred across all myeloma subgroups, Dr. Baertsch said.
Overall, the results indicate that “ a repeat transplant at the time of relapse for patients who had already gotten a transplant previously was no better than continuing a two-drug regimen,” Dr. Mohyuddin said.
However, he noted, “a lot has changed for myeloma care” since this trial was initially conducted. “We now have better regimens available that do not involve a transplant. If a repeat transplant couldn’t beat a two-drug regimen, it surely cannot beat a three drug or four drug regimen.”
Dr. Baertsch reported no disclosures.
A version of this article first appeared on Medscape.com.
Patients receiving a second, salvage-autologous stem cell transplant alongside lenalidomide-dexamethasone maintenance therapy did not demonstrate improved progression-free survival (PFS) or overall survival compared with patients who continued the two-drug regimen without salvage transplant, according to research presented at the American Society of Hematology annual meeting.
The primary phase 3 analysis, published in 2021, showed no survival benefit following salvage transplant at the time of relapse, though it only followed patients for a median of 37 months.
However, because a significant fraction of patients in the transplant arm — about 29% — did not undergo the planned salvage transplant before dropping out of the study, the researchers performed further analyses that “suggested a survival benefit in patients who actually received the transplant,” first author Marc-Andrea Baertsch, MD, of the German Cancer Research Center and University Hospital Heidelberg, reported at ASH.
Now, the latest analysis, which followed patients for a median of 99 months (8.25 years), confirmed the initial 2021 findings, Dr. Baertsch explained.
“The writing on the wall is clear: Don’t repeat a transplant at the time of relapse for those who have already gotten a transplant,” said Manni Mohyuddin, MD, of the University of Utah in Salt Lake City, who was not involved in the research. Dr. Mohyuddin added, however, that this finding doesn’t apply to those who haven’t yet gotten a transplant. “Data from other trials suggests a role of transplant in this situation, depending on the unique circumstances.”
The current trial included 282 adult patients, aged 75 years or younger, with relapsed or refractory multiple myeloma. Between 2010 and 2016, patients in the intention-to-treat analysis (n = 277) were randomized to lenalidomide-dexamethasone reinduction and maintenance, along with salvage high-dose chemotherapy with melphalan and autologous stem cell transplantation (n = 139) or just continuous lenalidomide-dexamethasone until progression (n = 138).
Patients in both arms received three cycles of lenalidomide-dexamethasone up front: 25 mg of lenalidomide on days 1 through 21, and 40 mg of dexamethasone on days 1, 8, 15, and 22 in 4-week cycles. Those in the salvage transplant arm then received high-dose chemotherapy with 200 mg/m2 of melphalan followed by transplant and 10 mg of lenalidomide maintenance therapy daily, while those in the control arm continued with receiving lenalidomide-dexamethasone.
All patients had received one to three prior lines of therapy, had good performance status, and had a time-to-disease-progression of at least 12 months after frontline autologous stem cell transplant.
In the primary 2021 study, patients in the salvage transplant group did not demonstrate a survival benefit (hazard ratio [HR] for PFS, 0.87; HR for overall survival, 0.81).
In the latest analysis, no survival benefit emerged after following patients for a median of about 8 years. Patients in the salvage transplant arm had a median PFS of 20.5 months vs 19.3 months in the continuous therapy arms (HR, 0.98; 95% CI, 0.76-1.27; P = .9). Median overall survival was 67.1 months in the salvage transplant arm and 62.7 months in the continuous treatment arm (HR, 0.89; 95% CI, 0.66 - 1.20; P = .44).
Time to first progression after frontline transplant was associated with a PFS benefit but did not predict an overall survival benefit, Dr. Baertsch noted.
When evaluating outcomes from the time of salvage transplant to account for the high number of dropouts, the PFS and overall survival findings held. Patients who received salvage transplant did not experience significantly improved PFS (HR, 0.91) or overall survival (76.3 months in the salvage group vs 65.9 months in the continuous treatment arm; HR, 0.80).
The lack of PFS and overall survival benefit occurred across all myeloma subgroups, Dr. Baertsch said.
Overall, the results indicate that “ a repeat transplant at the time of relapse for patients who had already gotten a transplant previously was no better than continuing a two-drug regimen,” Dr. Mohyuddin said.
However, he noted, “a lot has changed for myeloma care” since this trial was initially conducted. “We now have better regimens available that do not involve a transplant. If a repeat transplant couldn’t beat a two-drug regimen, it surely cannot beat a three drug or four drug regimen.”
Dr. Baertsch reported no disclosures.
A version of this article first appeared on Medscape.com.
Patients receiving a second, salvage-autologous stem cell transplant alongside lenalidomide-dexamethasone maintenance therapy did not demonstrate improved progression-free survival (PFS) or overall survival compared with patients who continued the two-drug regimen without salvage transplant, according to research presented at the American Society of Hematology annual meeting.
The primary phase 3 analysis, published in 2021, showed no survival benefit following salvage transplant at the time of relapse, though it only followed patients for a median of 37 months.
However, because a significant fraction of patients in the transplant arm — about 29% — did not undergo the planned salvage transplant before dropping out of the study, the researchers performed further analyses that “suggested a survival benefit in patients who actually received the transplant,” first author Marc-Andrea Baertsch, MD, of the German Cancer Research Center and University Hospital Heidelberg, reported at ASH.
Now, the latest analysis, which followed patients for a median of 99 months (8.25 years), confirmed the initial 2021 findings, Dr. Baertsch explained.
“The writing on the wall is clear: Don’t repeat a transplant at the time of relapse for those who have already gotten a transplant,” said Manni Mohyuddin, MD, of the University of Utah in Salt Lake City, who was not involved in the research. Dr. Mohyuddin added, however, that this finding doesn’t apply to those who haven’t yet gotten a transplant. “Data from other trials suggests a role of transplant in this situation, depending on the unique circumstances.”
The current trial included 282 adult patients, aged 75 years or younger, with relapsed or refractory multiple myeloma. Between 2010 and 2016, patients in the intention-to-treat analysis (n = 277) were randomized to lenalidomide-dexamethasone reinduction and maintenance, along with salvage high-dose chemotherapy with melphalan and autologous stem cell transplantation (n = 139) or just continuous lenalidomide-dexamethasone until progression (n = 138).
Patients in both arms received three cycles of lenalidomide-dexamethasone up front: 25 mg of lenalidomide on days 1 through 21, and 40 mg of dexamethasone on days 1, 8, 15, and 22 in 4-week cycles. Those in the salvage transplant arm then received high-dose chemotherapy with 200 mg/m2 of melphalan followed by transplant and 10 mg of lenalidomide maintenance therapy daily, while those in the control arm continued with receiving lenalidomide-dexamethasone.
All patients had received one to three prior lines of therapy, had good performance status, and had a time-to-disease-progression of at least 12 months after frontline autologous stem cell transplant.
In the primary 2021 study, patients in the salvage transplant group did not demonstrate a survival benefit (hazard ratio [HR] for PFS, 0.87; HR for overall survival, 0.81).
In the latest analysis, no survival benefit emerged after following patients for a median of about 8 years. Patients in the salvage transplant arm had a median PFS of 20.5 months vs 19.3 months in the continuous therapy arms (HR, 0.98; 95% CI, 0.76-1.27; P = .9). Median overall survival was 67.1 months in the salvage transplant arm and 62.7 months in the continuous treatment arm (HR, 0.89; 95% CI, 0.66 - 1.20; P = .44).
Time to first progression after frontline transplant was associated with a PFS benefit but did not predict an overall survival benefit, Dr. Baertsch noted.
When evaluating outcomes from the time of salvage transplant to account for the high number of dropouts, the PFS and overall survival findings held. Patients who received salvage transplant did not experience significantly improved PFS (HR, 0.91) or overall survival (76.3 months in the salvage group vs 65.9 months in the continuous treatment arm; HR, 0.80).
The lack of PFS and overall survival benefit occurred across all myeloma subgroups, Dr. Baertsch said.
Overall, the results indicate that “ a repeat transplant at the time of relapse for patients who had already gotten a transplant previously was no better than continuing a two-drug regimen,” Dr. Mohyuddin said.
However, he noted, “a lot has changed for myeloma care” since this trial was initially conducted. “We now have better regimens available that do not involve a transplant. If a repeat transplant couldn’t beat a two-drug regimen, it surely cannot beat a three drug or four drug regimen.”
Dr. Baertsch reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ASH 2023
Diffuse Capillary Malformation With Undergrowth of a Limb in a Boy
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.
An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.
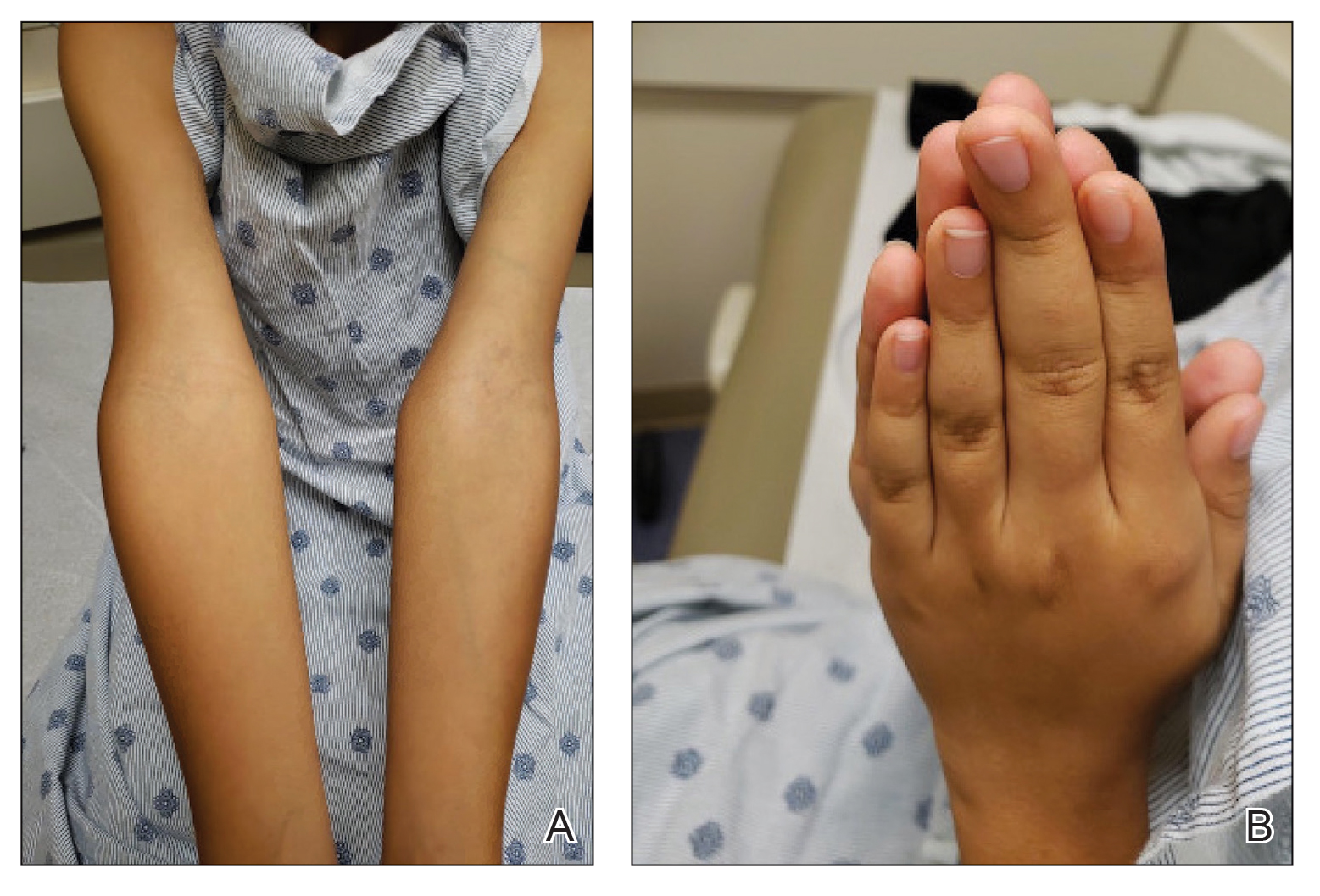
It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
Practice Points
- The term diffuse capillary malformation with undergrowth (DCMU) describes a distinct counterpart to diffuse capillary malformation with overgrowth. It can be challenging to distinguish from other vascular malformations associated with congenital structural abnormalities.
- The vascular patterning of DCMU may fade over time, but patients should continue to be monitored for their structural incongruity.
Exercise plan cost-effective in post-stroke cognitive rehab
A multicomponent exercise program that includes strength, aerobic, agility, and balance training exercises is cost-effective and results in improved cognition among stroke survivors, compared with a balance and tone control group, according to a new analysis.
On the other hand, a program consisting of cognitive and social enrichment activities that includes memory, brain training, and group social games entailed higher costs, compared with the balance and tone group, which included stretches, deep breathing and relaxation techniques, posture education, and core control exercises.
“Cognitive impairment is experienced in approximately one-third of stroke survivors,” study author Jennifer Davis, PhD, a Canada research chair in applied health economics and assistant professor of management at the University of British Columbia in Kelowna, said in an interview.
“The economic evaluation of the exercise intervention demonstrated that the multicomponent exercise program provided good value for the money when comparing costs and cognitive outcomes,” she said. However, “impacts on health-related quality of life were not observed.”
The study was published online November 30 in JAMA Network Open.
Comparing Three Approaches
Despite improved care, patients with stroke often face challenges with physical function, cognitive abilities, and quality of life, the authors wrote. Among older adults, in particular, cognitive deficits remain prevalent and are associated with increased risks for dementia, mortality, and increased burdens for patients, caregivers, and health systems.
Numerous interventions have shown promise for post-stroke cognitive rehabilitation, including exercise and cognitive training, the authors wrote. Research hasn’t indicated which programs offer the most efficient or cost-effective options, however.
Dr. Davis and colleagues conducted an economic evaluation alongside the Vitality study, a three-group randomized clinical trial that examined the efficacy of improving cognitive function among patients with chronic stroke through a multicomponent exercise program, cognitive and social enrichment activities, or a control group with balance and tone activities.
The economic evaluation team included a cost-effectiveness analysis (based on incremental cost per cognitive function change) and a cost-utility analysis (incremental cost per quality-adjusted life-year [QALY] gained). The researchers used a cost-effectiveness threshold of CAD $50,000 (Canadian dollars) per QALY for the cost-utility analysis, which was based on precedent treatment in Canada.
The clinical trial included 120 community-dwelling adults aged 55 years and older who had a stroke at least 12 months before the study. Based in the Vancouver metropolitan area, participants were randomly assigned to twice-weekly, 60-minute classes led by trained instructors for 26 weeks. The mean age was 71 years, and 62% of participants were men.
Exercise Effective
Overall, the balance and tone control group had the lowest delivery cost at CAD $777 per person, followed by CAD $1090 per person for the exercise group and CAD $1492 per person for the cognitive and social enrichment group.
After the 6-month intervention, the mean cognitive scores were –0.192 for the exercise group, –0.184 for the cognitive and social enrichment group, and –0.171 for the balance and tone group, indicating better cognitive function across all three groups.
In the cost-effectiveness analysis, the exercise intervention was costlier but more effective than the control group, with an incremental cost-effectiveness ratio (ICER) of CAD –$8823.
In the cost-utility analysis, the exercise intervention was cost saving (less costly and more effective), compared with the control group, with an ICER of CAD –$3381 per QALY gained at the end of the intervention and an ICER of CAD –$154,198 per QALY gained at the end of the 12-month follow-up period. The cognitive and social enrichment program was more costly and more effective than the control group, with an ICER of CAD $101,687 per QALY gained at the end of the intervention and an ICER of CAD $331,306 per QALY gained at the end of the follow-up period.
In additional analyses, the exercise group had the lowest healthcare resource utilization due to lower healthcare costs for physician visits and lab tests.
“This study provides initial data that suggests multicomponent exercise may be a cost-effective solution for combating cognitive decline among stroke survivors,” said Dr. Davis.
Overall, exercise was cost-effective for improving cognitive function but not quality of life among participants. The clinical trial was powered to detect changes in cognitive function rather than quality of life, so it lacked statistical power to detect differences in quality of life, said Dr. Davis.
Exercise programs and cognitive and social enrichment programs show promise for improving cognitive function after stroke, the authors wrote, though future research should focus on optimizing cost-effectiveness and enhancing health-related quality of life.
Considering Additional Benefits
Commenting on the study, Alan Tam, MD, a physiatrist at the Toronto Rehabilitation Institute’s Brain Rehabilitation Program, said, “The authors show that within the timeframe of their analysis, there is a trend to cost-effectiveness for the cognitive intervention being offered.” Dr. Tam did not participate in the research.
“However, the finding is not robust, as less than 50% of their simulations would meet their acceptability level they have defined,” he said. “Given that most of the cost of the intervention is up front, but the benefits are likely lifelong, potentially taking the 12-month analysis to a lifetime analysis would show more significant findings.”
Dr. Tam researches factors associated with brain injury rehabilitation and has explored the cost-effectiveness of a high-intensity outpatient stroke rehabilitation program.
“Presenting this type of work is important,” he said. “While there are interventions that do not meet our definition of statistical significance, especially in the rehabilitation world, there can still be a benefit for patients and health systems.”
The primary study was funded by the Canadian Institutes of Health Research (CIHR) and the Jack Brown and Family Alzheimer Research Foundation Society. Dr. Davis reported receiving grants from the CIHR and Michael Smith Health Research BC during the conduct of the study. Dr. Tam reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
A multicomponent exercise program that includes strength, aerobic, agility, and balance training exercises is cost-effective and results in improved cognition among stroke survivors, compared with a balance and tone control group, according to a new analysis.
On the other hand, a program consisting of cognitive and social enrichment activities that includes memory, brain training, and group social games entailed higher costs, compared with the balance and tone group, which included stretches, deep breathing and relaxation techniques, posture education, and core control exercises.
“Cognitive impairment is experienced in approximately one-third of stroke survivors,” study author Jennifer Davis, PhD, a Canada research chair in applied health economics and assistant professor of management at the University of British Columbia in Kelowna, said in an interview.
“The economic evaluation of the exercise intervention demonstrated that the multicomponent exercise program provided good value for the money when comparing costs and cognitive outcomes,” she said. However, “impacts on health-related quality of life were not observed.”
The study was published online November 30 in JAMA Network Open.
Comparing Three Approaches
Despite improved care, patients with stroke often face challenges with physical function, cognitive abilities, and quality of life, the authors wrote. Among older adults, in particular, cognitive deficits remain prevalent and are associated with increased risks for dementia, mortality, and increased burdens for patients, caregivers, and health systems.
Numerous interventions have shown promise for post-stroke cognitive rehabilitation, including exercise and cognitive training, the authors wrote. Research hasn’t indicated which programs offer the most efficient or cost-effective options, however.
Dr. Davis and colleagues conducted an economic evaluation alongside the Vitality study, a three-group randomized clinical trial that examined the efficacy of improving cognitive function among patients with chronic stroke through a multicomponent exercise program, cognitive and social enrichment activities, or a control group with balance and tone activities.
The economic evaluation team included a cost-effectiveness analysis (based on incremental cost per cognitive function change) and a cost-utility analysis (incremental cost per quality-adjusted life-year [QALY] gained). The researchers used a cost-effectiveness threshold of CAD $50,000 (Canadian dollars) per QALY for the cost-utility analysis, which was based on precedent treatment in Canada.
The clinical trial included 120 community-dwelling adults aged 55 years and older who had a stroke at least 12 months before the study. Based in the Vancouver metropolitan area, participants were randomly assigned to twice-weekly, 60-minute classes led by trained instructors for 26 weeks. The mean age was 71 years, and 62% of participants were men.
Exercise Effective
Overall, the balance and tone control group had the lowest delivery cost at CAD $777 per person, followed by CAD $1090 per person for the exercise group and CAD $1492 per person for the cognitive and social enrichment group.
After the 6-month intervention, the mean cognitive scores were –0.192 for the exercise group, –0.184 for the cognitive and social enrichment group, and –0.171 for the balance and tone group, indicating better cognitive function across all three groups.
In the cost-effectiveness analysis, the exercise intervention was costlier but more effective than the control group, with an incremental cost-effectiveness ratio (ICER) of CAD –$8823.
In the cost-utility analysis, the exercise intervention was cost saving (less costly and more effective), compared with the control group, with an ICER of CAD –$3381 per QALY gained at the end of the intervention and an ICER of CAD –$154,198 per QALY gained at the end of the 12-month follow-up period. The cognitive and social enrichment program was more costly and more effective than the control group, with an ICER of CAD $101,687 per QALY gained at the end of the intervention and an ICER of CAD $331,306 per QALY gained at the end of the follow-up period.
In additional analyses, the exercise group had the lowest healthcare resource utilization due to lower healthcare costs for physician visits and lab tests.
“This study provides initial data that suggests multicomponent exercise may be a cost-effective solution for combating cognitive decline among stroke survivors,” said Dr. Davis.
Overall, exercise was cost-effective for improving cognitive function but not quality of life among participants. The clinical trial was powered to detect changes in cognitive function rather than quality of life, so it lacked statistical power to detect differences in quality of life, said Dr. Davis.
Exercise programs and cognitive and social enrichment programs show promise for improving cognitive function after stroke, the authors wrote, though future research should focus on optimizing cost-effectiveness and enhancing health-related quality of life.
Considering Additional Benefits
Commenting on the study, Alan Tam, MD, a physiatrist at the Toronto Rehabilitation Institute’s Brain Rehabilitation Program, said, “The authors show that within the timeframe of their analysis, there is a trend to cost-effectiveness for the cognitive intervention being offered.” Dr. Tam did not participate in the research.
“However, the finding is not robust, as less than 50% of their simulations would meet their acceptability level they have defined,” he said. “Given that most of the cost of the intervention is up front, but the benefits are likely lifelong, potentially taking the 12-month analysis to a lifetime analysis would show more significant findings.”
Dr. Tam researches factors associated with brain injury rehabilitation and has explored the cost-effectiveness of a high-intensity outpatient stroke rehabilitation program.
“Presenting this type of work is important,” he said. “While there are interventions that do not meet our definition of statistical significance, especially in the rehabilitation world, there can still be a benefit for patients and health systems.”
The primary study was funded by the Canadian Institutes of Health Research (CIHR) and the Jack Brown and Family Alzheimer Research Foundation Society. Dr. Davis reported receiving grants from the CIHR and Michael Smith Health Research BC during the conduct of the study. Dr. Tam reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
A multicomponent exercise program that includes strength, aerobic, agility, and balance training exercises is cost-effective and results in improved cognition among stroke survivors, compared with a balance and tone control group, according to a new analysis.
On the other hand, a program consisting of cognitive and social enrichment activities that includes memory, brain training, and group social games entailed higher costs, compared with the balance and tone group, which included stretches, deep breathing and relaxation techniques, posture education, and core control exercises.
“Cognitive impairment is experienced in approximately one-third of stroke survivors,” study author Jennifer Davis, PhD, a Canada research chair in applied health economics and assistant professor of management at the University of British Columbia in Kelowna, said in an interview.
“The economic evaluation of the exercise intervention demonstrated that the multicomponent exercise program provided good value for the money when comparing costs and cognitive outcomes,” she said. However, “impacts on health-related quality of life were not observed.”
The study was published online November 30 in JAMA Network Open.
Comparing Three Approaches
Despite improved care, patients with stroke often face challenges with physical function, cognitive abilities, and quality of life, the authors wrote. Among older adults, in particular, cognitive deficits remain prevalent and are associated with increased risks for dementia, mortality, and increased burdens for patients, caregivers, and health systems.
Numerous interventions have shown promise for post-stroke cognitive rehabilitation, including exercise and cognitive training, the authors wrote. Research hasn’t indicated which programs offer the most efficient or cost-effective options, however.
Dr. Davis and colleagues conducted an economic evaluation alongside the Vitality study, a three-group randomized clinical trial that examined the efficacy of improving cognitive function among patients with chronic stroke through a multicomponent exercise program, cognitive and social enrichment activities, or a control group with balance and tone activities.
The economic evaluation team included a cost-effectiveness analysis (based on incremental cost per cognitive function change) and a cost-utility analysis (incremental cost per quality-adjusted life-year [QALY] gained). The researchers used a cost-effectiveness threshold of CAD $50,000 (Canadian dollars) per QALY for the cost-utility analysis, which was based on precedent treatment in Canada.
The clinical trial included 120 community-dwelling adults aged 55 years and older who had a stroke at least 12 months before the study. Based in the Vancouver metropolitan area, participants were randomly assigned to twice-weekly, 60-minute classes led by trained instructors for 26 weeks. The mean age was 71 years, and 62% of participants were men.
Exercise Effective
Overall, the balance and tone control group had the lowest delivery cost at CAD $777 per person, followed by CAD $1090 per person for the exercise group and CAD $1492 per person for the cognitive and social enrichment group.
After the 6-month intervention, the mean cognitive scores were –0.192 for the exercise group, –0.184 for the cognitive and social enrichment group, and –0.171 for the balance and tone group, indicating better cognitive function across all three groups.
In the cost-effectiveness analysis, the exercise intervention was costlier but more effective than the control group, with an incremental cost-effectiveness ratio (ICER) of CAD –$8823.
In the cost-utility analysis, the exercise intervention was cost saving (less costly and more effective), compared with the control group, with an ICER of CAD –$3381 per QALY gained at the end of the intervention and an ICER of CAD –$154,198 per QALY gained at the end of the 12-month follow-up period. The cognitive and social enrichment program was more costly and more effective than the control group, with an ICER of CAD $101,687 per QALY gained at the end of the intervention and an ICER of CAD $331,306 per QALY gained at the end of the follow-up period.
In additional analyses, the exercise group had the lowest healthcare resource utilization due to lower healthcare costs for physician visits and lab tests.
“This study provides initial data that suggests multicomponent exercise may be a cost-effective solution for combating cognitive decline among stroke survivors,” said Dr. Davis.
Overall, exercise was cost-effective for improving cognitive function but not quality of life among participants. The clinical trial was powered to detect changes in cognitive function rather than quality of life, so it lacked statistical power to detect differences in quality of life, said Dr. Davis.
Exercise programs and cognitive and social enrichment programs show promise for improving cognitive function after stroke, the authors wrote, though future research should focus on optimizing cost-effectiveness and enhancing health-related quality of life.
Considering Additional Benefits
Commenting on the study, Alan Tam, MD, a physiatrist at the Toronto Rehabilitation Institute’s Brain Rehabilitation Program, said, “The authors show that within the timeframe of their analysis, there is a trend to cost-effectiveness for the cognitive intervention being offered.” Dr. Tam did not participate in the research.
“However, the finding is not robust, as less than 50% of their simulations would meet their acceptability level they have defined,” he said. “Given that most of the cost of the intervention is up front, but the benefits are likely lifelong, potentially taking the 12-month analysis to a lifetime analysis would show more significant findings.”
Dr. Tam researches factors associated with brain injury rehabilitation and has explored the cost-effectiveness of a high-intensity outpatient stroke rehabilitation program.
“Presenting this type of work is important,” he said. “While there are interventions that do not meet our definition of statistical significance, especially in the rehabilitation world, there can still be a benefit for patients and health systems.”
The primary study was funded by the Canadian Institutes of Health Research (CIHR) and the Jack Brown and Family Alzheimer Research Foundation Society. Dr. Davis reported receiving grants from the CIHR and Michael Smith Health Research BC during the conduct of the study. Dr. Tam reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adequate disease control elusive for many patients on systemic AD therapies, study finds
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
FROM RAD 2023