User login
Updated analysis from JAVELIN Renal 101 to be presented at ESMO 2018
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
Entospletinib falls short in relapsed/refractory DLBCL
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The rate of progression-free survival at 16 weeks was 3.6% with a median PFS of 1.5 months.
Study details: An analysis of 43 relapsed/refractory DLBCL patients who received single-agent entospletinib.
Disclosures: The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
Source: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Level of Serum Neurofilament Light Enables Treatment Monitoring
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
COPD: Triple trumps dual therapy regardless of baseline reversibility
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
REPORTING FROM CHEST 2018
Key clinical point: Triple therapy with fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) is superior to UMEC/VI in COPD patients regardless of baseline bronchodilator reversibility.
Major finding: Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction.
Study details: Retrospective analysis of IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
Disclosures: Study authors reported disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
Source: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j/chest.2018.08.662.
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
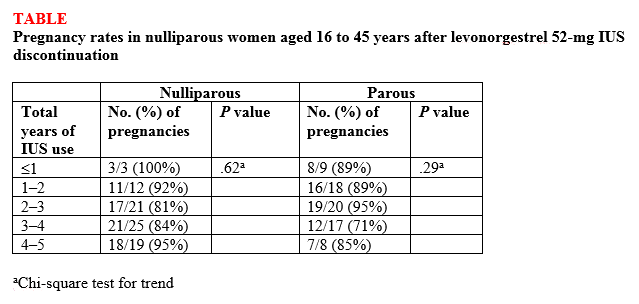
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
ICU infections: Chlorhexidine wipes tame MRSA, CRE
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
REPORTING FROM ID WEEK 2018
Key clinical point: For high rates of MRSA and CRE in the ICU, consider decolonization instead of contact precautions.
Major finding: Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6; infection rates fell from 3.9 isolates to 2 per 10,000 patient-days.
Study details: Review of ICU quality improvement initiative
Disclosures: There was no funding for the work, and the investigators had no disclosures.
Source: Moss J et al. ID Week 2018, Abstract 32.
Acute Superior Mesenteric Venous Thrombosis in a Young Patient Without Risk Factors
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
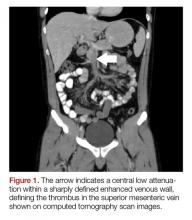
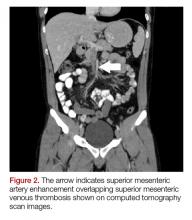
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
Crizanlizumab relieves sickle cell crises across subgroups
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
FROM THE AMERICAN JOURNAL OF HEMATOLOGY
Key clinical point:
Major finding: Crizanlizumab eliminated vaso-occlusive crises about seven times more frequently than did placebo in patients with numerous crises (28.0% vs. 4.2%).
Study details: A post hoc analysis of 132 patients from the phase 2 SUSTAIN trial.
Disclosures: The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Source: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Updated ThroLy system predicts need for thromboprophylaxis
DUBROVNIK, CROATIA – An updated scoring system can more accurately identify lymphoma patients who may require thromboprophylaxis, according to researchers.
The revised scoring system, ThroLy, proved more effective than other systems for predicting thromboembolic events in lymphoma patients, with a positive predictive value of 22%-25%, a negative predictive value of 96%, sensitivity of 56%-57%, and specificity of 85%-87%.
Darko Antic, MD, PhD, of the University of Belgrade in Serbia, presented these findings at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Antic said that he and his colleagues developed ThroLy because other systems used to predict venous thromboembolism (VTE) are not quite right for lymphoma. He noted that the Padua score is not designed for cancer patients and the Khorana score is predominantly used for solid tumor malignancies.
The ThroLy scoring system is based on variables used in the Padua and Khorana systems, as well as variables that are specific to lymphoma patients.
In a previous study, the researchers found several variables that were independently associated with risk for VTE in lymphoma, including previous VTE, previous acute MI or stroke, mediastinal involvement, high body mass index, reduced mobility, extranodal localization, neutropenia, and hemoglobin less than 100 g/L (Am J Hematol. 2016 Oct;91[10]:1014-9).
In an initial version of the ThroLy scoring system, previous VTE, previous acute MI/stroke, obesity, and mediastinal involvement were all worth two points, and the other factors were worth a single point in the ThroLy system.
Patients with scores of 0 to 1 were considered low risk, patients with scores of 2 to 3 were considered intermediate risk, and patients with scores of 4 or greater were considered high risk.
To validate and refine ThroLy, Dr. Antic and his colleagues used it to assess 1,723 lymphoma patients treated at eight institutions in Austria, Croatia, France, Jordan, Macedonia, Spain, Switzerland, and the United States.
Patients had indolent non-Hodgkin lymphoma, aggressive non-Hodgkin lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and Hodgkin lymphoma. Most subjects (84%) were outpatients. A total of 9%of patients had thrombosis, with 7% having VTE.
ThroLy had a positive predictive value of 17%, compared with 11% with Khorana and 13% with Padua. The negative predictive value was 93%, 92%, and 95%, respectively. The sensitivity was 51% with ThroLy, 42% with Khorana, and 70% with Padua; specificity was 72%, 64%, and 52%, respectively.
“The positive predictive value was low [with ThroLy] but definitely higher than the positive predictive value of the other two [scoring systems],” Dr. Antic noted.
Updated models
To further improve ThroLy, the researchers updated the system, creating two new models. Model 1 included the type of lymphoma/clinical stage (1 point), previous VTE (5 points), reduced mobility (2 points), hemoglobin less than 100 g/L (1 point), and the presence of vascular devices (1 point). Model 2 included all of the variables in Model 1 plus the thrombophilic condition, which was worth 1 point.
Patients were considered low risk if they scored 2 points or lower and high risk if they scored more than 2 points.
For Model 1, the positive predictive value was 22%, the negative predictive value was 96%, the sensitivity was 56%, and the specificity was 85%. For Model 2, the positive predictive value was 25%, the negative predictive value was 96%, the sensitivity was 57%, and the specificity was 87%.
There were no major differences in model discrimination and calibration based on the country in which a patient was treated or whether the patient was treated in an inpatient or outpatient setting.
Dr. Antic did not report any conflicts of interest. The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – An updated scoring system can more accurately identify lymphoma patients who may require thromboprophylaxis, according to researchers.
The revised scoring system, ThroLy, proved more effective than other systems for predicting thromboembolic events in lymphoma patients, with a positive predictive value of 22%-25%, a negative predictive value of 96%, sensitivity of 56%-57%, and specificity of 85%-87%.
Darko Antic, MD, PhD, of the University of Belgrade in Serbia, presented these findings at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Antic said that he and his colleagues developed ThroLy because other systems used to predict venous thromboembolism (VTE) are not quite right for lymphoma. He noted that the Padua score is not designed for cancer patients and the Khorana score is predominantly used for solid tumor malignancies.
The ThroLy scoring system is based on variables used in the Padua and Khorana systems, as well as variables that are specific to lymphoma patients.
In a previous study, the researchers found several variables that were independently associated with risk for VTE in lymphoma, including previous VTE, previous acute MI or stroke, mediastinal involvement, high body mass index, reduced mobility, extranodal localization, neutropenia, and hemoglobin less than 100 g/L (Am J Hematol. 2016 Oct;91[10]:1014-9).
In an initial version of the ThroLy scoring system, previous VTE, previous acute MI/stroke, obesity, and mediastinal involvement were all worth two points, and the other factors were worth a single point in the ThroLy system.
Patients with scores of 0 to 1 were considered low risk, patients with scores of 2 to 3 were considered intermediate risk, and patients with scores of 4 or greater were considered high risk.
To validate and refine ThroLy, Dr. Antic and his colleagues used it to assess 1,723 lymphoma patients treated at eight institutions in Austria, Croatia, France, Jordan, Macedonia, Spain, Switzerland, and the United States.
Patients had indolent non-Hodgkin lymphoma, aggressive non-Hodgkin lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and Hodgkin lymphoma. Most subjects (84%) were outpatients. A total of 9%of patients had thrombosis, with 7% having VTE.
ThroLy had a positive predictive value of 17%, compared with 11% with Khorana and 13% with Padua. The negative predictive value was 93%, 92%, and 95%, respectively. The sensitivity was 51% with ThroLy, 42% with Khorana, and 70% with Padua; specificity was 72%, 64%, and 52%, respectively.
“The positive predictive value was low [with ThroLy] but definitely higher than the positive predictive value of the other two [scoring systems],” Dr. Antic noted.
Updated models
To further improve ThroLy, the researchers updated the system, creating two new models. Model 1 included the type of lymphoma/clinical stage (1 point), previous VTE (5 points), reduced mobility (2 points), hemoglobin less than 100 g/L (1 point), and the presence of vascular devices (1 point). Model 2 included all of the variables in Model 1 plus the thrombophilic condition, which was worth 1 point.
Patients were considered low risk if they scored 2 points or lower and high risk if they scored more than 2 points.
For Model 1, the positive predictive value was 22%, the negative predictive value was 96%, the sensitivity was 56%, and the specificity was 85%. For Model 2, the positive predictive value was 25%, the negative predictive value was 96%, the sensitivity was 57%, and the specificity was 87%.
There were no major differences in model discrimination and calibration based on the country in which a patient was treated or whether the patient was treated in an inpatient or outpatient setting.
Dr. Antic did not report any conflicts of interest. The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – An updated scoring system can more accurately identify lymphoma patients who may require thromboprophylaxis, according to researchers.
The revised scoring system, ThroLy, proved more effective than other systems for predicting thromboembolic events in lymphoma patients, with a positive predictive value of 22%-25%, a negative predictive value of 96%, sensitivity of 56%-57%, and specificity of 85%-87%.
Darko Antic, MD, PhD, of the University of Belgrade in Serbia, presented these findings at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Antic said that he and his colleagues developed ThroLy because other systems used to predict venous thromboembolism (VTE) are not quite right for lymphoma. He noted that the Padua score is not designed for cancer patients and the Khorana score is predominantly used for solid tumor malignancies.
The ThroLy scoring system is based on variables used in the Padua and Khorana systems, as well as variables that are specific to lymphoma patients.
In a previous study, the researchers found several variables that were independently associated with risk for VTE in lymphoma, including previous VTE, previous acute MI or stroke, mediastinal involvement, high body mass index, reduced mobility, extranodal localization, neutropenia, and hemoglobin less than 100 g/L (Am J Hematol. 2016 Oct;91[10]:1014-9).
In an initial version of the ThroLy scoring system, previous VTE, previous acute MI/stroke, obesity, and mediastinal involvement were all worth two points, and the other factors were worth a single point in the ThroLy system.
Patients with scores of 0 to 1 were considered low risk, patients with scores of 2 to 3 were considered intermediate risk, and patients with scores of 4 or greater were considered high risk.
To validate and refine ThroLy, Dr. Antic and his colleagues used it to assess 1,723 lymphoma patients treated at eight institutions in Austria, Croatia, France, Jordan, Macedonia, Spain, Switzerland, and the United States.
Patients had indolent non-Hodgkin lymphoma, aggressive non-Hodgkin lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and Hodgkin lymphoma. Most subjects (84%) were outpatients. A total of 9%of patients had thrombosis, with 7% having VTE.
ThroLy had a positive predictive value of 17%, compared with 11% with Khorana and 13% with Padua. The negative predictive value was 93%, 92%, and 95%, respectively. The sensitivity was 51% with ThroLy, 42% with Khorana, and 70% with Padua; specificity was 72%, 64%, and 52%, respectively.
“The positive predictive value was low [with ThroLy] but definitely higher than the positive predictive value of the other two [scoring systems],” Dr. Antic noted.
Updated models
To further improve ThroLy, the researchers updated the system, creating two new models. Model 1 included the type of lymphoma/clinical stage (1 point), previous VTE (5 points), reduced mobility (2 points), hemoglobin less than 100 g/L (1 point), and the presence of vascular devices (1 point). Model 2 included all of the variables in Model 1 plus the thrombophilic condition, which was worth 1 point.
Patients were considered low risk if they scored 2 points or lower and high risk if they scored more than 2 points.
For Model 1, the positive predictive value was 22%, the negative predictive value was 96%, the sensitivity was 56%, and the specificity was 85%. For Model 2, the positive predictive value was 25%, the negative predictive value was 96%, the sensitivity was 57%, and the specificity was 87%.
There were no major differences in model discrimination and calibration based on the country in which a patient was treated or whether the patient was treated in an inpatient or outpatient setting.
Dr. Antic did not report any conflicts of interest. The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
REPORTING FROM LEUKEMIA AND LYMPHOMA 2018
Key clinical point:
Major finding: The updated ThroLy had a positive predictive value of 22%-25%, a negative predictive value of 96%, sensitivity of 56%-57%, and specificity of 85%-87%.
Study details: The scoring system was validated on 1,723 lymphoma patients treated at eight institutions worldwide.
Disclosures: Dr. Antic reported having no conflicts of interest.
How can we best use diagnostic brain imaging in pregnant women with severe headache?
WHAT DOES THIS MEAN FOR PRACTICE?
- Acute, severe headache in pregnancy needs immediate attention when it includes:
- seizures
- altered sensorium, or
- loss of consciousness
- An appropriate threshold utilizing history and clinical diagnosis must be set for obtaining neurologic consultation and for the consultant to obtain imaging
- Brain scans can identify symptomatic pathologic results (27.6% in this study)
- Theoretical concerns about imaging call for the OB to be very involved in evaluation and management
- OB and neurologist should discuss risks and benefits of imaging throughout care